User login
Can TEE find septal defects in conotruncal repair?
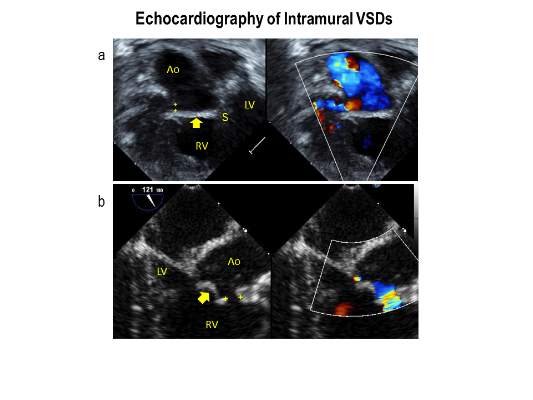
Intramural ventricular septal defects (VSD), residual defects that can occur after repair of conotruncal defects in newborns, increase the risk of complications and death if they’re not detected and closed during the index operation. While various methods have been tried to find these defects during surgery, researchers from Children’s Hospital of Philadelphia (CHOP) reported that the use of transesophageal echocardiography (TEE) has a good chance of finding VSDs and giving cardiac surgeons the opportunity to correct these residual defects.
“TEE has modest sensitivity but high specificity for identifying intramural VSDs and can identify most defects requiring reinterventions,” Jyoti Patel, MD, and her coauthors reported in a study published in the September issue of the Journal of Thoracic and Cardiovascular Surgery (2016;152:688-95).
Previous studies have shown that intraoperative TEE is safe for evaluating operations in congenital heart disease, but this is the first study to evaluate the modality for detecting intramural VSDs, Dr. Patel and her colleagues said.
Dr. Patel and her coinvestigators analyzed results of TEE and postoperative transthoracic echocardiography (TTE) in patients who had biventricular repair of conotruncal anomalies at CHOP from January 2006 through June 2013. Intramural VSDs occurred in 34 of 337 patients who met the inclusion criteria out of a total population of 903. Actually, 462 patients had biventricular repairs of conotruncal defects involving baffle closure of a VSD, but 125 were excluded for various reasons, including 105 for inadequate intraoperative TEE.
TTE identified a total of 177 residual VSDs, 34 of which were intramural in nature. Among the evaluated procedures, both TEE at the end of the index operation and TTE detected VSD in 19 patients; TTE alone found VSD in 15. “Sensitivity was 56% and specificity was 100% for TEE to identify intramural VSDs,” Dr. Patel and her colleagues said.
What’s more, both TTE and TEE combined identified peripatch VSDs in 90 patients, while TTE only in 53 and TEE only in 15, “yielding a sensitivity of 63% and specificity of 92%,” Dr. Patel and her colleagues said.
Of the VSDs that required catheterization or reintervention during surgery, intraoperative TEE detected six of seven intramural VSDs and all five peripatch VSDs, the study found.
“In this study, TEE identified most intramural VSDs and all peripatch VSDs that required subsequent reintervention,” Dr. Patel and her colleagues said.
“This finding underscores the importance of adequate imaging of the superior aspect of the VSD patch during intraoperative TEE for conotruncal anomalies, given that many intramural defects may be repaired during the initial operation.”
Coauthor Andrew Glatz, MD, disclosed receiving consulting fees from Bristol-Myers Squibb, and coauthor Chitra Ravishankar, MD, disclosed lecture fees from Danone Medical. Dr. Patel and the remaining coauthors had no financial relationships to disclose.
Because of the clinical importance of intramural VSDs, cardiac surgeons need to be highly suspicious in any operation to repair conotruncal defects where the VSD margins are close to the trabeculae, Edward Buratto, MBBS, Philip Naimo, MD, and Igor Konstantinov, MD, PhD, of Royal Children’s Hospital at the University of Melbourne said in their invited commentary (J Thorac Cardiovasc Surg. 2016;152:696-7). “The best way to resolve the problem would be to prevent it,” they said.
While intraoperative TEE can detect VSDs preemptively, the imaging technique is “not without its flaws,” the commentators said, as evidenced by the 105 subjects the CHOP study excluded because of inadequate TEE imaging. Those excluded cases comprised patients aged 30 days and younger with lower body weight and higher early death rates. “It is these patients who would benefit most from intraoperative identification of intramural VSD,” the commentators said.
They also noted that TEE in detecting intramural and peripatch VSD in children aged 30 days and older “was not perfect either,” with sensitivities of 56% and 63%, respectively. In the CHOP study, TEE was more likely to detect intramural VSD in patients older than 30 days with higher body weight, Dr. Buratto and his colleagues said.
The favored approach at Royal Children’s Hospital in Melbourne is routine epicardial echocardiograms in conotruncal repair. This imaging technique provides “superb imaging quality,” they said. “This is of particular importance in small children.” They advocate closing a significant VSD once it’s identified.
“After all, failure to close intramural VSD occurs when surgeons do not realize how close they were to success when they gave up,” the commentators said. Precise echocardiographic guidance would “dramatically facilitate” that strategy.
Dr. Buratto, Dr. Naimo, and Dr. Konstantinov had no financial relationships to disclose.
Because of the clinical importance of intramural VSDs, cardiac surgeons need to be highly suspicious in any operation to repair conotruncal defects where the VSD margins are close to the trabeculae, Edward Buratto, MBBS, Philip Naimo, MD, and Igor Konstantinov, MD, PhD, of Royal Children’s Hospital at the University of Melbourne said in their invited commentary (J Thorac Cardiovasc Surg. 2016;152:696-7). “The best way to resolve the problem would be to prevent it,” they said.
While intraoperative TEE can detect VSDs preemptively, the imaging technique is “not without its flaws,” the commentators said, as evidenced by the 105 subjects the CHOP study excluded because of inadequate TEE imaging. Those excluded cases comprised patients aged 30 days and younger with lower body weight and higher early death rates. “It is these patients who would benefit most from intraoperative identification of intramural VSD,” the commentators said.
They also noted that TEE in detecting intramural and peripatch VSD in children aged 30 days and older “was not perfect either,” with sensitivities of 56% and 63%, respectively. In the CHOP study, TEE was more likely to detect intramural VSD in patients older than 30 days with higher body weight, Dr. Buratto and his colleagues said.
The favored approach at Royal Children’s Hospital in Melbourne is routine epicardial echocardiograms in conotruncal repair. This imaging technique provides “superb imaging quality,” they said. “This is of particular importance in small children.” They advocate closing a significant VSD once it’s identified.
“After all, failure to close intramural VSD occurs when surgeons do not realize how close they were to success when they gave up,” the commentators said. Precise echocardiographic guidance would “dramatically facilitate” that strategy.
Dr. Buratto, Dr. Naimo, and Dr. Konstantinov had no financial relationships to disclose.
Because of the clinical importance of intramural VSDs, cardiac surgeons need to be highly suspicious in any operation to repair conotruncal defects where the VSD margins are close to the trabeculae, Edward Buratto, MBBS, Philip Naimo, MD, and Igor Konstantinov, MD, PhD, of Royal Children’s Hospital at the University of Melbourne said in their invited commentary (J Thorac Cardiovasc Surg. 2016;152:696-7). “The best way to resolve the problem would be to prevent it,” they said.
While intraoperative TEE can detect VSDs preemptively, the imaging technique is “not without its flaws,” the commentators said, as evidenced by the 105 subjects the CHOP study excluded because of inadequate TEE imaging. Those excluded cases comprised patients aged 30 days and younger with lower body weight and higher early death rates. “It is these patients who would benefit most from intraoperative identification of intramural VSD,” the commentators said.
They also noted that TEE in detecting intramural and peripatch VSD in children aged 30 days and older “was not perfect either,” with sensitivities of 56% and 63%, respectively. In the CHOP study, TEE was more likely to detect intramural VSD in patients older than 30 days with higher body weight, Dr. Buratto and his colleagues said.
The favored approach at Royal Children’s Hospital in Melbourne is routine epicardial echocardiograms in conotruncal repair. This imaging technique provides “superb imaging quality,” they said. “This is of particular importance in small children.” They advocate closing a significant VSD once it’s identified.
“After all, failure to close intramural VSD occurs when surgeons do not realize how close they were to success when they gave up,” the commentators said. Precise echocardiographic guidance would “dramatically facilitate” that strategy.
Dr. Buratto, Dr. Naimo, and Dr. Konstantinov had no financial relationships to disclose.
Intramural ventricular septal defects (VSD), residual defects that can occur after repair of conotruncal defects in newborns, increase the risk of complications and death if they’re not detected and closed during the index operation. While various methods have been tried to find these defects during surgery, researchers from Children’s Hospital of Philadelphia (CHOP) reported that the use of transesophageal echocardiography (TEE) has a good chance of finding VSDs and giving cardiac surgeons the opportunity to correct these residual defects.
“TEE has modest sensitivity but high specificity for identifying intramural VSDs and can identify most defects requiring reinterventions,” Jyoti Patel, MD, and her coauthors reported in a study published in the September issue of the Journal of Thoracic and Cardiovascular Surgery (2016;152:688-95).
Previous studies have shown that intraoperative TEE is safe for evaluating operations in congenital heart disease, but this is the first study to evaluate the modality for detecting intramural VSDs, Dr. Patel and her colleagues said.
Dr. Patel and her coinvestigators analyzed results of TEE and postoperative transthoracic echocardiography (TTE) in patients who had biventricular repair of conotruncal anomalies at CHOP from January 2006 through June 2013. Intramural VSDs occurred in 34 of 337 patients who met the inclusion criteria out of a total population of 903. Actually, 462 patients had biventricular repairs of conotruncal defects involving baffle closure of a VSD, but 125 were excluded for various reasons, including 105 for inadequate intraoperative TEE.
TTE identified a total of 177 residual VSDs, 34 of which were intramural in nature. Among the evaluated procedures, both TEE at the end of the index operation and TTE detected VSD in 19 patients; TTE alone found VSD in 15. “Sensitivity was 56% and specificity was 100% for TEE to identify intramural VSDs,” Dr. Patel and her colleagues said.
What’s more, both TTE and TEE combined identified peripatch VSDs in 90 patients, while TTE only in 53 and TEE only in 15, “yielding a sensitivity of 63% and specificity of 92%,” Dr. Patel and her colleagues said.
Of the VSDs that required catheterization or reintervention during surgery, intraoperative TEE detected six of seven intramural VSDs and all five peripatch VSDs, the study found.
“In this study, TEE identified most intramural VSDs and all peripatch VSDs that required subsequent reintervention,” Dr. Patel and her colleagues said.
“This finding underscores the importance of adequate imaging of the superior aspect of the VSD patch during intraoperative TEE for conotruncal anomalies, given that many intramural defects may be repaired during the initial operation.”
Coauthor Andrew Glatz, MD, disclosed receiving consulting fees from Bristol-Myers Squibb, and coauthor Chitra Ravishankar, MD, disclosed lecture fees from Danone Medical. Dr. Patel and the remaining coauthors had no financial relationships to disclose.
Intramural ventricular septal defects (VSD), residual defects that can occur after repair of conotruncal defects in newborns, increase the risk of complications and death if they’re not detected and closed during the index operation. While various methods have been tried to find these defects during surgery, researchers from Children’s Hospital of Philadelphia (CHOP) reported that the use of transesophageal echocardiography (TEE) has a good chance of finding VSDs and giving cardiac surgeons the opportunity to correct these residual defects.
“TEE has modest sensitivity but high specificity for identifying intramural VSDs and can identify most defects requiring reinterventions,” Jyoti Patel, MD, and her coauthors reported in a study published in the September issue of the Journal of Thoracic and Cardiovascular Surgery (2016;152:688-95).
Previous studies have shown that intraoperative TEE is safe for evaluating operations in congenital heart disease, but this is the first study to evaluate the modality for detecting intramural VSDs, Dr. Patel and her colleagues said.
Dr. Patel and her coinvestigators analyzed results of TEE and postoperative transthoracic echocardiography (TTE) in patients who had biventricular repair of conotruncal anomalies at CHOP from January 2006 through June 2013. Intramural VSDs occurred in 34 of 337 patients who met the inclusion criteria out of a total population of 903. Actually, 462 patients had biventricular repairs of conotruncal defects involving baffle closure of a VSD, but 125 were excluded for various reasons, including 105 for inadequate intraoperative TEE.
TTE identified a total of 177 residual VSDs, 34 of which were intramural in nature. Among the evaluated procedures, both TEE at the end of the index operation and TTE detected VSD in 19 patients; TTE alone found VSD in 15. “Sensitivity was 56% and specificity was 100% for TEE to identify intramural VSDs,” Dr. Patel and her colleagues said.
What’s more, both TTE and TEE combined identified peripatch VSDs in 90 patients, while TTE only in 53 and TEE only in 15, “yielding a sensitivity of 63% and specificity of 92%,” Dr. Patel and her colleagues said.
Of the VSDs that required catheterization or reintervention during surgery, intraoperative TEE detected six of seven intramural VSDs and all five peripatch VSDs, the study found.
“In this study, TEE identified most intramural VSDs and all peripatch VSDs that required subsequent reintervention,” Dr. Patel and her colleagues said.
“This finding underscores the importance of adequate imaging of the superior aspect of the VSD patch during intraoperative TEE for conotruncal anomalies, given that many intramural defects may be repaired during the initial operation.”
Coauthor Andrew Glatz, MD, disclosed receiving consulting fees from Bristol-Myers Squibb, and coauthor Chitra Ravishankar, MD, disclosed lecture fees from Danone Medical. Dr. Patel and the remaining coauthors had no financial relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Intraoperative transesophageal echocardiography has modest sensitivity but high specificity for detecting ventricular septal defects after repair of conotruncal anomalies.
Major finding: TEE is useful for identifying most VSDs during the index operation, providing the opportunity to repair the defects during the index operation.
Data source: A single-institution database of 337 patients who had operations to repair conotruncal anomalies between January 2006 and June 2013.
Disclosures: Coauthor Andrew Glatz, MD, disclosed receiving consulting fees from Bristol-Myers Squibb, and coauthor Chitra Ravishankar, MD, disclosed lecture fees from Danone Medical. Dr. Patel and the remaining coauthors had no financial relationships to disclose.
Inadequate diversity snags hypertrophic cardiomyopathy genetic linkages
The genetic tests used for more than a decade to identify patients or family members who carry genetic mutations linked to hypertrophic cardiomyopathy are seriously flawed.
The tests have been erroneously flagging people as genetically positive for hypertrophic cardiomyopathy (HC) when they actually carried benign genetic variants, according to a new reassessment of the genetic linkages by researchers using a more genetically diverse database. Five genetic variants now reclassified as benign were collectively responsible for flagging 74% of people flagged at genetic risk for HC in the more than 8,500 cases examined.
The results call into question any genetic diagnosis of HC made since genetic testing entered the mainstream in 2003, especially among African Americans who seem to have been disproportionately affected by these mislabeled genetic markers because of inadequate population diversity when the markers were first established.
In addition, the results more broadly cast a shadow over the full spectrum of genetic tests for disease-linked variants now in routine medical practice because of the possibility that other linkage determinations derived from an inadequately-representative reference population, reported Arjun K. Manrai, PhD, and his associates (N Engl J Med. 2016 Aug 18;375[7]:655-65). The researchers call the HC experience they document a “cautionary tale of broad relevance to genetic diagnosis.”
The findings “powerfully illustrate the importance of racial and genetic diversity” when running linkage studies aimed at validating genetic markers for widespread clinical use, Isaac S. Kohane, MD, senior author of the study, said in a written statement. “Racial and ethnic inclusiveness improves the validity and accuracy” of genetic tests, said Dr. Kohane, professor of biomedical informatics and pediatrics at Harvard Medical School in Boston.
“We believe that what we’re seeing in the case of hypertrophic cardiomyopathy may be the tip of the iceberg of a larger problem that transcends a single genetic disease,” Dr. Manrai, a biomedical informatics researcher at Harvard, said in the same statement. “Much genetic assessment today relies on historical links between a disorder and variant, sometimes decades old. We believe our findings illustrate the critical need to systematically reevaluate prior assertions about genetic variants,” Dr. Manrai added in an interview.
The two researchers and their associates reexamined the link between genetic variants and HC in three genetic databases that involved a total of more than 8,500 people. One database included 4,300 white Americans and 2,203 black Americans. A second database included genetic data from 1,092 people from 14 worldwide populations, and the third had genetic data from 938 people from 51 worldwide populations.
The analysis showed that although 94 distinct genetic variants that had previously been reported as associated with HC were confirmed as linked, just 5 met the study’s definition of a “high-frequency” variant with an allele frequent of more than 1%. These five variants together accounted for 74% of the overall total of linkages seen in these 8,533 people. Further analysis classified all five of these high-frequency genetic variants as benign with no discernible link to HC.
These five high-frequency variants occurred disproportionately higher among black Americans, and the consequences of this showed up in the patient records the researchers reviewed from one large U.S. genetic testing laboratory. They examined in detail HC genetic test results during 2004-2013 from 2,912 unrelated people. The records showed seven people had been labeled as carrying either a pathogenic or “presumed pathogenic” variant when in fact they had one of the five high-frequency variants now declared benign. Five of the seven mislabeled people were of African ancestry; the other two had unknown ancestry.
The researchers called for reevaluating known variants for all genetic diseases in more diverse populations and to immediately release the results of updated linkage assessments. This has the potential to meaningfully rewrite current gospel for many genetic variants and linkages.
“Our findings point to the value of patients staying in contact with their genetic counselors and physicians, even years after genetic testing,” Dr. Manrai said. Reassessments using more diverse populations will take time, he acknowledged, but tools are available to allow clinical geneticists to update old variants and apply new ones in real time, as soon as a new assessment completes.
Dr. Manrai and Dr. Kohane had no disclosures.
On Twitter @mitchelzoler
The genetic tests used for more than a decade to identify patients or family members who carry genetic mutations linked to hypertrophic cardiomyopathy are seriously flawed.
The tests have been erroneously flagging people as genetically positive for hypertrophic cardiomyopathy (HC) when they actually carried benign genetic variants, according to a new reassessment of the genetic linkages by researchers using a more genetically diverse database. Five genetic variants now reclassified as benign were collectively responsible for flagging 74% of people flagged at genetic risk for HC in the more than 8,500 cases examined.
The results call into question any genetic diagnosis of HC made since genetic testing entered the mainstream in 2003, especially among African Americans who seem to have been disproportionately affected by these mislabeled genetic markers because of inadequate population diversity when the markers were first established.
In addition, the results more broadly cast a shadow over the full spectrum of genetic tests for disease-linked variants now in routine medical practice because of the possibility that other linkage determinations derived from an inadequately-representative reference population, reported Arjun K. Manrai, PhD, and his associates (N Engl J Med. 2016 Aug 18;375[7]:655-65). The researchers call the HC experience they document a “cautionary tale of broad relevance to genetic diagnosis.”
The findings “powerfully illustrate the importance of racial and genetic diversity” when running linkage studies aimed at validating genetic markers for widespread clinical use, Isaac S. Kohane, MD, senior author of the study, said in a written statement. “Racial and ethnic inclusiveness improves the validity and accuracy” of genetic tests, said Dr. Kohane, professor of biomedical informatics and pediatrics at Harvard Medical School in Boston.
“We believe that what we’re seeing in the case of hypertrophic cardiomyopathy may be the tip of the iceberg of a larger problem that transcends a single genetic disease,” Dr. Manrai, a biomedical informatics researcher at Harvard, said in the same statement. “Much genetic assessment today relies on historical links between a disorder and variant, sometimes decades old. We believe our findings illustrate the critical need to systematically reevaluate prior assertions about genetic variants,” Dr. Manrai added in an interview.
The two researchers and their associates reexamined the link between genetic variants and HC in three genetic databases that involved a total of more than 8,500 people. One database included 4,300 white Americans and 2,203 black Americans. A second database included genetic data from 1,092 people from 14 worldwide populations, and the third had genetic data from 938 people from 51 worldwide populations.
The analysis showed that although 94 distinct genetic variants that had previously been reported as associated with HC were confirmed as linked, just 5 met the study’s definition of a “high-frequency” variant with an allele frequent of more than 1%. These five variants together accounted for 74% of the overall total of linkages seen in these 8,533 people. Further analysis classified all five of these high-frequency genetic variants as benign with no discernible link to HC.
These five high-frequency variants occurred disproportionately higher among black Americans, and the consequences of this showed up in the patient records the researchers reviewed from one large U.S. genetic testing laboratory. They examined in detail HC genetic test results during 2004-2013 from 2,912 unrelated people. The records showed seven people had been labeled as carrying either a pathogenic or “presumed pathogenic” variant when in fact they had one of the five high-frequency variants now declared benign. Five of the seven mislabeled people were of African ancestry; the other two had unknown ancestry.
The researchers called for reevaluating known variants for all genetic diseases in more diverse populations and to immediately release the results of updated linkage assessments. This has the potential to meaningfully rewrite current gospel for many genetic variants and linkages.
“Our findings point to the value of patients staying in contact with their genetic counselors and physicians, even years after genetic testing,” Dr. Manrai said. Reassessments using more diverse populations will take time, he acknowledged, but tools are available to allow clinical geneticists to update old variants and apply new ones in real time, as soon as a new assessment completes.
Dr. Manrai and Dr. Kohane had no disclosures.
On Twitter @mitchelzoler
The genetic tests used for more than a decade to identify patients or family members who carry genetic mutations linked to hypertrophic cardiomyopathy are seriously flawed.
The tests have been erroneously flagging people as genetically positive for hypertrophic cardiomyopathy (HC) when they actually carried benign genetic variants, according to a new reassessment of the genetic linkages by researchers using a more genetically diverse database. Five genetic variants now reclassified as benign were collectively responsible for flagging 74% of people flagged at genetic risk for HC in the more than 8,500 cases examined.
The results call into question any genetic diagnosis of HC made since genetic testing entered the mainstream in 2003, especially among African Americans who seem to have been disproportionately affected by these mislabeled genetic markers because of inadequate population diversity when the markers were first established.
In addition, the results more broadly cast a shadow over the full spectrum of genetic tests for disease-linked variants now in routine medical practice because of the possibility that other linkage determinations derived from an inadequately-representative reference population, reported Arjun K. Manrai, PhD, and his associates (N Engl J Med. 2016 Aug 18;375[7]:655-65). The researchers call the HC experience they document a “cautionary tale of broad relevance to genetic diagnosis.”
The findings “powerfully illustrate the importance of racial and genetic diversity” when running linkage studies aimed at validating genetic markers for widespread clinical use, Isaac S. Kohane, MD, senior author of the study, said in a written statement. “Racial and ethnic inclusiveness improves the validity and accuracy” of genetic tests, said Dr. Kohane, professor of biomedical informatics and pediatrics at Harvard Medical School in Boston.
“We believe that what we’re seeing in the case of hypertrophic cardiomyopathy may be the tip of the iceberg of a larger problem that transcends a single genetic disease,” Dr. Manrai, a biomedical informatics researcher at Harvard, said in the same statement. “Much genetic assessment today relies on historical links between a disorder and variant, sometimes decades old. We believe our findings illustrate the critical need to systematically reevaluate prior assertions about genetic variants,” Dr. Manrai added in an interview.
The two researchers and their associates reexamined the link between genetic variants and HC in three genetic databases that involved a total of more than 8,500 people. One database included 4,300 white Americans and 2,203 black Americans. A second database included genetic data from 1,092 people from 14 worldwide populations, and the third had genetic data from 938 people from 51 worldwide populations.
The analysis showed that although 94 distinct genetic variants that had previously been reported as associated with HC were confirmed as linked, just 5 met the study’s definition of a “high-frequency” variant with an allele frequent of more than 1%. These five variants together accounted for 74% of the overall total of linkages seen in these 8,533 people. Further analysis classified all five of these high-frequency genetic variants as benign with no discernible link to HC.
These five high-frequency variants occurred disproportionately higher among black Americans, and the consequences of this showed up in the patient records the researchers reviewed from one large U.S. genetic testing laboratory. They examined in detail HC genetic test results during 2004-2013 from 2,912 unrelated people. The records showed seven people had been labeled as carrying either a pathogenic or “presumed pathogenic” variant when in fact they had one of the five high-frequency variants now declared benign. Five of the seven mislabeled people were of African ancestry; the other two had unknown ancestry.
The researchers called for reevaluating known variants for all genetic diseases in more diverse populations and to immediately release the results of updated linkage assessments. This has the potential to meaningfully rewrite current gospel for many genetic variants and linkages.
“Our findings point to the value of patients staying in contact with their genetic counselors and physicians, even years after genetic testing,” Dr. Manrai said. Reassessments using more diverse populations will take time, he acknowledged, but tools are available to allow clinical geneticists to update old variants and apply new ones in real time, as soon as a new assessment completes.
Dr. Manrai and Dr. Kohane had no disclosures.
On Twitter @mitchelzoler
Key clinical point: Five high-frequency genetic variants that collectively had been linked to 74% of hypertrophic cardiomyopathy cases are actually benign with no detectable pathologic linkage.
Major finding: Inaccurate linkage data mislabeled seven people as having a hypertrophic cardiomyopathy–causing genetic variant.
Data source: Three genomic sequence databases that included 8,533 people and a genetic laboratory’s records for 2,912 clients.
Disclosures: Dr. Manrai and Dr. Kohane had no disclosures.
Can anesthesia in infants affect IQ scores?
About 10,000 newborns receive general anesthesia for congenital heart defects every year, and the more exposure they have to inhaled anesthetic agents, the greater effect it may have on their neurologic development, investigators at Children’s Hospital of Philadelphia reported in a study of newborns with hypoplastic left heart syndrome.
While previous studies have linked worse neurodevelopment to patient factors like prematurity and genetics, this is the first study to show a consistent relationship between neurodevelopment outcomes and modifiable factors during cardiac surgery in infants, Laura K. Diaz, MD, and her colleagues reported in the August issue of the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2016;152:482-9).
They studied 96 patients with hypoplastic left heart syndrome (HLHS) or similar syndromes who received volatile anesthetic agents (VAA) at their institution from 1998 to 2003. The patients underwent a battery of neurodevelopmental tests between the ages of 4 and 5 years that included full-scale IQ (FSIQ), verbal IQ (VIQ), performance IQ (PIQ), and processing speed.
“This study provides evidence that in children undergoing staged reconstructive surgery for HLHS, increasing cumulative exposure to VAAs beginning in infancy is associated with worse performance for FSIQ and VIQ, suggesting that VAA exposure may be a modifiable risk factor for adverse neurodevelopment outcomes,” Dr. Diaz and her colleagues wrote.
While survival has improved significantly in recent years for infants with hypoplastic left heart syndrome, physicians have harbored concerns that these children encounter neurodevelopmental issues later on. Dr. Diaz and her colleagues acknowledged that previous studies have shown factors, such as the use of cardiopulmonary bypass (CPB) and hospital length of stay, that could affect neurodevelopment in these children, but the findings have been inconsistent. Instead, those studies have shown such patient-specific factors as birth weight, ethnicity, and hereditary disorders were strong determinants of neurodevelopment in infants who have cardiac surgery, Dr. Diaz and her coauthors pointed out.
Their own previous study of patients with single-ventricle congenital heart disease concurred with the findings of those other studies, but it did not evaluate exposure to anesthesia (J. Thorac. Cardiovasc. Surg. 2014;147:1276-82). That was the focus of their current study.
Among the study group, 94 patients had an initial operation with CPB in their first 30 days of life. All 96 infants in the study group had additional operations, whether cardiac or noncardiac. The study tracked all anesthetic exposures up until the neurodevelopment evaluation in February 2008. All but 2 patients had initial VAA exposure at less than 1 year of age, and 45 at less than 1 month of age. Deep hypothermic circulatory arrest was used uniformly for aortic arch reconstruction.
The study used four different generalized linear models to evaluate anesthesia exposure and neurodevelopment.
For both FSIQ and PIQ, total minimum alveolar concentration hours were deemed to be statistically significant factors for lower scores. For PIQ, birth weight and length of postoperative hospital stay were statistically significant. For processing speed, gestational age and length of hospital stay were statistically significant.
Dr. Diaz and her colleagues said their findings are preliminary and do not justify a change in practice. “Prospective randomized, controlled multicenter clinical trials are indicated to continue to clarify the effects of early and repetitive exposure to VAA in this and other pediatric populations,” the study authors concluded.
Dr. Diaz and the study authors had no financial relationships to disclose.
The study by Dr. Diaz and her colleagues makes all the more clear the need for a prospective randomized trial on the effect inhaled anesthetic agents in infants can have on their neurologic development, Richard A. Jonas, MD, of Children’s National Heart Institute, Children’s National Medical Center, Washington, said in his invited commentary (J. Thorac. Cardiovasc. Surg. 2016;152:490).
 |
Dr. Richard A. Jonas |
However, besides the study limitations that Dr. Diaz and her colleagues pointed out in their study, another “problem” Dr. Jonas noted with the study subjects was that they had staged reconstruction for hypoplastic left heart syndrome. “Not only is this group of patients at risk for prenatal effects of their abnormal in utero circulation, but in addition, they all underwent additional cardiac or noncardiac procedures after their initial cardiac surgery,” he said. These factors, along with some degree of cyanosis in their formative years, may help explain why this study is an outlier in that it did not implicate nonoperative factors that other studies implicated, Dr. Jonas said.
Nonetheless, the study is “an important contribution that adds further evidence to the observation that volatile agents can affect neurodevelopmental outcome,” Dr. Jonas said. Hence the need for a prospective randomized trial.
Dr. Jonas had no financial relationships to disclose.
The study by Dr. Diaz and her colleagues makes all the more clear the need for a prospective randomized trial on the effect inhaled anesthetic agents in infants can have on their neurologic development, Richard A. Jonas, MD, of Children’s National Heart Institute, Children’s National Medical Center, Washington, said in his invited commentary (J. Thorac. Cardiovasc. Surg. 2016;152:490).
 |
Dr. Richard A. Jonas |
However, besides the study limitations that Dr. Diaz and her colleagues pointed out in their study, another “problem” Dr. Jonas noted with the study subjects was that they had staged reconstruction for hypoplastic left heart syndrome. “Not only is this group of patients at risk for prenatal effects of their abnormal in utero circulation, but in addition, they all underwent additional cardiac or noncardiac procedures after their initial cardiac surgery,” he said. These factors, along with some degree of cyanosis in their formative years, may help explain why this study is an outlier in that it did not implicate nonoperative factors that other studies implicated, Dr. Jonas said.
Nonetheless, the study is “an important contribution that adds further evidence to the observation that volatile agents can affect neurodevelopmental outcome,” Dr. Jonas said. Hence the need for a prospective randomized trial.
Dr. Jonas had no financial relationships to disclose.
The study by Dr. Diaz and her colleagues makes all the more clear the need for a prospective randomized trial on the effect inhaled anesthetic agents in infants can have on their neurologic development, Richard A. Jonas, MD, of Children’s National Heart Institute, Children’s National Medical Center, Washington, said in his invited commentary (J. Thorac. Cardiovasc. Surg. 2016;152:490).
 |
Dr. Richard A. Jonas |
However, besides the study limitations that Dr. Diaz and her colleagues pointed out in their study, another “problem” Dr. Jonas noted with the study subjects was that they had staged reconstruction for hypoplastic left heart syndrome. “Not only is this group of patients at risk for prenatal effects of their abnormal in utero circulation, but in addition, they all underwent additional cardiac or noncardiac procedures after their initial cardiac surgery,” he said. These factors, along with some degree of cyanosis in their formative years, may help explain why this study is an outlier in that it did not implicate nonoperative factors that other studies implicated, Dr. Jonas said.
Nonetheless, the study is “an important contribution that adds further evidence to the observation that volatile agents can affect neurodevelopmental outcome,” Dr. Jonas said. Hence the need for a prospective randomized trial.
Dr. Jonas had no financial relationships to disclose.
About 10,000 newborns receive general anesthesia for congenital heart defects every year, and the more exposure they have to inhaled anesthetic agents, the greater effect it may have on their neurologic development, investigators at Children’s Hospital of Philadelphia reported in a study of newborns with hypoplastic left heart syndrome.
While previous studies have linked worse neurodevelopment to patient factors like prematurity and genetics, this is the first study to show a consistent relationship between neurodevelopment outcomes and modifiable factors during cardiac surgery in infants, Laura K. Diaz, MD, and her colleagues reported in the August issue of the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2016;152:482-9).
They studied 96 patients with hypoplastic left heart syndrome (HLHS) or similar syndromes who received volatile anesthetic agents (VAA) at their institution from 1998 to 2003. The patients underwent a battery of neurodevelopmental tests between the ages of 4 and 5 years that included full-scale IQ (FSIQ), verbal IQ (VIQ), performance IQ (PIQ), and processing speed.
“This study provides evidence that in children undergoing staged reconstructive surgery for HLHS, increasing cumulative exposure to VAAs beginning in infancy is associated with worse performance for FSIQ and VIQ, suggesting that VAA exposure may be a modifiable risk factor for adverse neurodevelopment outcomes,” Dr. Diaz and her colleagues wrote.
While survival has improved significantly in recent years for infants with hypoplastic left heart syndrome, physicians have harbored concerns that these children encounter neurodevelopmental issues later on. Dr. Diaz and her colleagues acknowledged that previous studies have shown factors, such as the use of cardiopulmonary bypass (CPB) and hospital length of stay, that could affect neurodevelopment in these children, but the findings have been inconsistent. Instead, those studies have shown such patient-specific factors as birth weight, ethnicity, and hereditary disorders were strong determinants of neurodevelopment in infants who have cardiac surgery, Dr. Diaz and her coauthors pointed out.
Their own previous study of patients with single-ventricle congenital heart disease concurred with the findings of those other studies, but it did not evaluate exposure to anesthesia (J. Thorac. Cardiovasc. Surg. 2014;147:1276-82). That was the focus of their current study.
Among the study group, 94 patients had an initial operation with CPB in their first 30 days of life. All 96 infants in the study group had additional operations, whether cardiac or noncardiac. The study tracked all anesthetic exposures up until the neurodevelopment evaluation in February 2008. All but 2 patients had initial VAA exposure at less than 1 year of age, and 45 at less than 1 month of age. Deep hypothermic circulatory arrest was used uniformly for aortic arch reconstruction.
The study used four different generalized linear models to evaluate anesthesia exposure and neurodevelopment.
For both FSIQ and PIQ, total minimum alveolar concentration hours were deemed to be statistically significant factors for lower scores. For PIQ, birth weight and length of postoperative hospital stay were statistically significant. For processing speed, gestational age and length of hospital stay were statistically significant.
Dr. Diaz and her colleagues said their findings are preliminary and do not justify a change in practice. “Prospective randomized, controlled multicenter clinical trials are indicated to continue to clarify the effects of early and repetitive exposure to VAA in this and other pediatric populations,” the study authors concluded.
Dr. Diaz and the study authors had no financial relationships to disclose.
About 10,000 newborns receive general anesthesia for congenital heart defects every year, and the more exposure they have to inhaled anesthetic agents, the greater effect it may have on their neurologic development, investigators at Children’s Hospital of Philadelphia reported in a study of newborns with hypoplastic left heart syndrome.
While previous studies have linked worse neurodevelopment to patient factors like prematurity and genetics, this is the first study to show a consistent relationship between neurodevelopment outcomes and modifiable factors during cardiac surgery in infants, Laura K. Diaz, MD, and her colleagues reported in the August issue of the Journal of Thoracic and Cardiovascular Surgery (J Thorac Cardiovasc Surg. 2016;152:482-9).
They studied 96 patients with hypoplastic left heart syndrome (HLHS) or similar syndromes who received volatile anesthetic agents (VAA) at their institution from 1998 to 2003. The patients underwent a battery of neurodevelopmental tests between the ages of 4 and 5 years that included full-scale IQ (FSIQ), verbal IQ (VIQ), performance IQ (PIQ), and processing speed.
“This study provides evidence that in children undergoing staged reconstructive surgery for HLHS, increasing cumulative exposure to VAAs beginning in infancy is associated with worse performance for FSIQ and VIQ, suggesting that VAA exposure may be a modifiable risk factor for adverse neurodevelopment outcomes,” Dr. Diaz and her colleagues wrote.
While survival has improved significantly in recent years for infants with hypoplastic left heart syndrome, physicians have harbored concerns that these children encounter neurodevelopmental issues later on. Dr. Diaz and her colleagues acknowledged that previous studies have shown factors, such as the use of cardiopulmonary bypass (CPB) and hospital length of stay, that could affect neurodevelopment in these children, but the findings have been inconsistent. Instead, those studies have shown such patient-specific factors as birth weight, ethnicity, and hereditary disorders were strong determinants of neurodevelopment in infants who have cardiac surgery, Dr. Diaz and her coauthors pointed out.
Their own previous study of patients with single-ventricle congenital heart disease concurred with the findings of those other studies, but it did not evaluate exposure to anesthesia (J. Thorac. Cardiovasc. Surg. 2014;147:1276-82). That was the focus of their current study.
Among the study group, 94 patients had an initial operation with CPB in their first 30 days of life. All 96 infants in the study group had additional operations, whether cardiac or noncardiac. The study tracked all anesthetic exposures up until the neurodevelopment evaluation in February 2008. All but 2 patients had initial VAA exposure at less than 1 year of age, and 45 at less than 1 month of age. Deep hypothermic circulatory arrest was used uniformly for aortic arch reconstruction.
The study used four different generalized linear models to evaluate anesthesia exposure and neurodevelopment.
For both FSIQ and PIQ, total minimum alveolar concentration hours were deemed to be statistically significant factors for lower scores. For PIQ, birth weight and length of postoperative hospital stay were statistically significant. For processing speed, gestational age and length of hospital stay were statistically significant.
Dr. Diaz and her colleagues said their findings are preliminary and do not justify a change in practice. “Prospective randomized, controlled multicenter clinical trials are indicated to continue to clarify the effects of early and repetitive exposure to VAA in this and other pediatric populations,” the study authors concluded.
Dr. Diaz and the study authors had no financial relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Volatile inhaled anesthesia may affect neurodevelopment in infants with hypoplastic left heart syndrome.
Major finding: Different generalized linear models determined an association between minimum alveolar concentration hours and hospital length of stay with lower IQ scores and processing speed.
Data source: Meta-analysis reviewed a subgroup of 96 patients with hypoplastic left heart syndrome who had neurodevelopmental testing at a single center between 1998 and 2003.
Disclosures: The authors have no financial relationships to disclose.
Anatomic repair of ccTGA did not yield superior survival
BALTIMORE – Anatomic repair did not outperform physiologic repair in patients with congenitally corrected transposition of the great arteries (ccTGA), according to a study presented by Maryam Al-Omair, M.D., of the University of Toronto at the annual meeting of the American Association for Thoracic Surgery.
Dr. Al-Omair and her colleagues hypothesized that patients undergoing anatomic repair for ccTGA would have superior systemic ventricular function and survival. However, their results showed that anatomic repair of ccTGA did not yield superior survival, compared with physiologic repair, and the long-term impact on systemic ventricular function was not certain.
Because of early evidence showing better outcomes of anatomic over physiologic repair for ccTGA, the surgical trend over time greatly favored the use of anatomic repair: At her team’s institution, anatomic repair went from 2.3% in the 1982-1989 period to 92.3% in the 2010-2015 period, Dr. Al-Omair said.
Their study assessed 200 patients (165 with biventricular ccTGA and 35 Fontan patients) who were managed from 1982 to 2015 at the Hospital for Sick Children, Toronto. The patient treatment groups were anatomic repair (38 patients), physiologic repair (89), single-ventricle (Fontan) repair (35), and palliated (no intracardiac repair) patients (38). The median follow-up was 3.4 years for anatomic repair, 13.5 years for physiologic repair, 7.5 years for single-ventricle repair, and 11.8 years with no repair (11.8 years), reflecting their change in practice.
The investigators followed the primary outcome of transplant-free survival and secondary outcomes of late systemic ventricular function and systemic atrioventricular valve function.
They found no significant difference in transplant-free survival at 20 years in the three repair groups assessed from 1892 to 2105: anatomic repair (58%), physiologic repair (71%), and single-ventricle (Fontan) repair (78%). Looking at the latter period of 2000-2015 for 10-year transplant-free survival, they found similar results: anatomic repair (77%), physiologic repair (85%), and single-ventricle (Fontan) repair (100%).
They also found that transplant-free survival in patients who required no intracadiac repair and had no associated lesions such as ventral septal defect or ventral septal defect with pulmonary stenosis was nearly 95% at 25 years.
A multivariate analysis showed no independent predictors of mortality among the three treatments, patient age at index operation, or period of treatment, as well as the need for a permanent pacemaker, or moderately to severely reduced ventricular function or moderate to severe valve regurgitation after the index operation, according to Dr. Al-Omair.
For the secondary outcome of late systemic ventricular function, a multivariate analysis showed that two of the variables were independent predictors: Index operation at or after 2000 was shown to be protective (hazard ratio, 0.152), while a negative association was seen with moderately to severely reduced ventricular function after the index operation (HR, 12.4).
For the secondary outcome of late systemic valve function, a multivariate analysis showed that three of the variables were independent predictors: Fontan operation (HR, 0.124) and index operation at or after 2000 (HR, 0.258) were shown to be protective, while a negative association was seen with moderately to severely reduced valve regurgitation after the index operation (HR, 9.00).
The researchers concluded that midterm Fontan survival was relatively favorable, pushing borderline repair may not be necessary, and “prophylactic banding” and the double-switch procedure should be looked on with caution for lower-risk patients.
“Our study also showed that survival was best in those having no associated lesions requiring operation, indicating that performing an anatomic repair for those not having associated lesions could be counterproductive,” Dr. Al-Omair concluded.
The webcast of the annual meeting presentation is available at www.aats.org.
Dr. Al-Omair reported that she and her colleagues had no relevant financial disclosures.
The choice of anatomic vs. physiologic repair of congenitally corrected transposition of the great arteries is a controversial area, with many well-known surgeons and centers advocating for anatomic repair (a much tougher and more challenging operation) as opposed to physiologic repair. The Toronto group is to be applauded for this honest conclusion, which goes a bit against the currently fashionable “more is better” approach.
Robert Jaquiss, M.D., of Duke University, Durham, N.C., is the congenital heart disease associate medical editor for Thoracic Surgery News.
The choice of anatomic vs. physiologic repair of congenitally corrected transposition of the great arteries is a controversial area, with many well-known surgeons and centers advocating for anatomic repair (a much tougher and more challenging operation) as opposed to physiologic repair. The Toronto group is to be applauded for this honest conclusion, which goes a bit against the currently fashionable “more is better” approach.
Robert Jaquiss, M.D., of Duke University, Durham, N.C., is the congenital heart disease associate medical editor for Thoracic Surgery News.
The choice of anatomic vs. physiologic repair of congenitally corrected transposition of the great arteries is a controversial area, with many well-known surgeons and centers advocating for anatomic repair (a much tougher and more challenging operation) as opposed to physiologic repair. The Toronto group is to be applauded for this honest conclusion, which goes a bit against the currently fashionable “more is better” approach.
Robert Jaquiss, M.D., of Duke University, Durham, N.C., is the congenital heart disease associate medical editor for Thoracic Surgery News.
BALTIMORE – Anatomic repair did not outperform physiologic repair in patients with congenitally corrected transposition of the great arteries (ccTGA), according to a study presented by Maryam Al-Omair, M.D., of the University of Toronto at the annual meeting of the American Association for Thoracic Surgery.
Dr. Al-Omair and her colleagues hypothesized that patients undergoing anatomic repair for ccTGA would have superior systemic ventricular function and survival. However, their results showed that anatomic repair of ccTGA did not yield superior survival, compared with physiologic repair, and the long-term impact on systemic ventricular function was not certain.
Because of early evidence showing better outcomes of anatomic over physiologic repair for ccTGA, the surgical trend over time greatly favored the use of anatomic repair: At her team’s institution, anatomic repair went from 2.3% in the 1982-1989 period to 92.3% in the 2010-2015 period, Dr. Al-Omair said.
Their study assessed 200 patients (165 with biventricular ccTGA and 35 Fontan patients) who were managed from 1982 to 2015 at the Hospital for Sick Children, Toronto. The patient treatment groups were anatomic repair (38 patients), physiologic repair (89), single-ventricle (Fontan) repair (35), and palliated (no intracardiac repair) patients (38). The median follow-up was 3.4 years for anatomic repair, 13.5 years for physiologic repair, 7.5 years for single-ventricle repair, and 11.8 years with no repair (11.8 years), reflecting their change in practice.
The investigators followed the primary outcome of transplant-free survival and secondary outcomes of late systemic ventricular function and systemic atrioventricular valve function.
They found no significant difference in transplant-free survival at 20 years in the three repair groups assessed from 1892 to 2105: anatomic repair (58%), physiologic repair (71%), and single-ventricle (Fontan) repair (78%). Looking at the latter period of 2000-2015 for 10-year transplant-free survival, they found similar results: anatomic repair (77%), physiologic repair (85%), and single-ventricle (Fontan) repair (100%).
They also found that transplant-free survival in patients who required no intracadiac repair and had no associated lesions such as ventral septal defect or ventral septal defect with pulmonary stenosis was nearly 95% at 25 years.
A multivariate analysis showed no independent predictors of mortality among the three treatments, patient age at index operation, or period of treatment, as well as the need for a permanent pacemaker, or moderately to severely reduced ventricular function or moderate to severe valve regurgitation after the index operation, according to Dr. Al-Omair.
For the secondary outcome of late systemic ventricular function, a multivariate analysis showed that two of the variables were independent predictors: Index operation at or after 2000 was shown to be protective (hazard ratio, 0.152), while a negative association was seen with moderately to severely reduced ventricular function after the index operation (HR, 12.4).
For the secondary outcome of late systemic valve function, a multivariate analysis showed that three of the variables were independent predictors: Fontan operation (HR, 0.124) and index operation at or after 2000 (HR, 0.258) were shown to be protective, while a negative association was seen with moderately to severely reduced valve regurgitation after the index operation (HR, 9.00).
The researchers concluded that midterm Fontan survival was relatively favorable, pushing borderline repair may not be necessary, and “prophylactic banding” and the double-switch procedure should be looked on with caution for lower-risk patients.
“Our study also showed that survival was best in those having no associated lesions requiring operation, indicating that performing an anatomic repair for those not having associated lesions could be counterproductive,” Dr. Al-Omair concluded.
The webcast of the annual meeting presentation is available at www.aats.org.
Dr. Al-Omair reported that she and her colleagues had no relevant financial disclosures.
BALTIMORE – Anatomic repair did not outperform physiologic repair in patients with congenitally corrected transposition of the great arteries (ccTGA), according to a study presented by Maryam Al-Omair, M.D., of the University of Toronto at the annual meeting of the American Association for Thoracic Surgery.
Dr. Al-Omair and her colleagues hypothesized that patients undergoing anatomic repair for ccTGA would have superior systemic ventricular function and survival. However, their results showed that anatomic repair of ccTGA did not yield superior survival, compared with physiologic repair, and the long-term impact on systemic ventricular function was not certain.
Because of early evidence showing better outcomes of anatomic over physiologic repair for ccTGA, the surgical trend over time greatly favored the use of anatomic repair: At her team’s institution, anatomic repair went from 2.3% in the 1982-1989 period to 92.3% in the 2010-2015 period, Dr. Al-Omair said.
Their study assessed 200 patients (165 with biventricular ccTGA and 35 Fontan patients) who were managed from 1982 to 2015 at the Hospital for Sick Children, Toronto. The patient treatment groups were anatomic repair (38 patients), physiologic repair (89), single-ventricle (Fontan) repair (35), and palliated (no intracardiac repair) patients (38). The median follow-up was 3.4 years for anatomic repair, 13.5 years for physiologic repair, 7.5 years for single-ventricle repair, and 11.8 years with no repair (11.8 years), reflecting their change in practice.
The investigators followed the primary outcome of transplant-free survival and secondary outcomes of late systemic ventricular function and systemic atrioventricular valve function.
They found no significant difference in transplant-free survival at 20 years in the three repair groups assessed from 1892 to 2105: anatomic repair (58%), physiologic repair (71%), and single-ventricle (Fontan) repair (78%). Looking at the latter period of 2000-2015 for 10-year transplant-free survival, they found similar results: anatomic repair (77%), physiologic repair (85%), and single-ventricle (Fontan) repair (100%).
They also found that transplant-free survival in patients who required no intracadiac repair and had no associated lesions such as ventral septal defect or ventral septal defect with pulmonary stenosis was nearly 95% at 25 years.
A multivariate analysis showed no independent predictors of mortality among the three treatments, patient age at index operation, or period of treatment, as well as the need for a permanent pacemaker, or moderately to severely reduced ventricular function or moderate to severe valve regurgitation after the index operation, according to Dr. Al-Omair.
For the secondary outcome of late systemic ventricular function, a multivariate analysis showed that two of the variables were independent predictors: Index operation at or after 2000 was shown to be protective (hazard ratio, 0.152), while a negative association was seen with moderately to severely reduced ventricular function after the index operation (HR, 12.4).
For the secondary outcome of late systemic valve function, a multivariate analysis showed that three of the variables were independent predictors: Fontan operation (HR, 0.124) and index operation at or after 2000 (HR, 0.258) were shown to be protective, while a negative association was seen with moderately to severely reduced valve regurgitation after the index operation (HR, 9.00).
The researchers concluded that midterm Fontan survival was relatively favorable, pushing borderline repair may not be necessary, and “prophylactic banding” and the double-switch procedure should be looked on with caution for lower-risk patients.
“Our study also showed that survival was best in those having no associated lesions requiring operation, indicating that performing an anatomic repair for those not having associated lesions could be counterproductive,” Dr. Al-Omair concluded.
The webcast of the annual meeting presentation is available at www.aats.org.
Dr. Al-Omair reported that she and her colleagues had no relevant financial disclosures.
AT THE AATS ANNUAL MEETING
Key clinical point: Performing an anatomic repair for ccTGA in patients without associated lesions could be counterproductive.
Major finding: There was no significant difference in transplant-free survival at 20 years among anatomic repair (58%), physiologic repair (71%), and single-ventricle repair (78%).
Data source: A single-institution study assessing 200 patients with ccGTA/Fontan who were managed from 1982 to 2015.
Disclosures: Dr. Al-Omair reported that she and her colleagues had no relevant financial disclosures.
MRI-VA improves view of anomalous coronary arteries
Failure to achieve a rounded and unobstructed ostia in children who have surgery to repair anomalous coronary arteries can put these children at continued risk for sudden death, but cardiac MRI with virtual angioscopy (VA) before and after the operation can give cardiologists a clear picture of a patient’s risk for sudden death and help direct ongoing management, according to a study in the July issue of the Journal of Thoracic and Cardiovascular Surgery (2016;152:205-10).
“Cardiac MRI with virtual angioscopy is an important tool for evaluating anomalous coronary anatomy, myocardial function, and ischemia and should be considered for initial and postoperative assessment of children with anomalous coronary arteries,” lead author Julie A. Brothers, MD, and her coauthors said in reporting their findings.
Anomalous coronary artery is a rare congenital condition in which the left coronary artery (LCA) originates from the right sinus or the right coronary artery (RCA) originates from the left coronary sinus. Dr. Brothers, a pediatric cardiologist, and her colleagues from the Children’s Hospital of Philadelphia and the University of Pennsylvania, also in Philadelphia, studied nine male patients who had operations for anomalous coronary arteries during Feb. 2009-May 2015 in what they said is the first study to document anomalous coronary artery anatomy both before and after surgery. The patients’ average age was 14.1 years; seven had right anomalous coronary arteries and two had left anomalous arteries. After the operations, MRI-VA revealed that two patients still had narrowing in the neo-orifices.
Previous reports recommend surgical repair for all patients with anomalous LCA and for symptomatic patients with anomalous RCA anatomy (Ann Thorac Surg. 2011;92:691-7; Ann Thorac Surg. 2014;98:941-5). MRI-VA allows the surgical team to survey the ostial stenosis before the operation “as if standing within the vessel itself,” Dr. Brothers and her coauthors wrote. Afterward, MRI-VA lets the surgeon and team see if the operation succeeded in repairing the orifices.
In the study population, VA before surgery confirmed elliptical, slit-like orifices in all patients. The operations involved unroofing procedures; two patients also had detachment and resuspension procedures during surgery. After surgery, VA showed that seven patients had round, patent, unobstructed repaired orifices; but two had orifices that were still narrow and somewhat stenotic, Dr. Brothers and her coauthors said. The study group had postoperative MRI-VA an average of 8.6 months after surgery.
“The significance of these findings is unknown; however, if the proposed mechanism of ischemia is due to a slit-like orifice, a continued stenotic orifice may place subjects at risk for sudden death,” the researchers said. The two study patients with the narrowed, stenotic orifices have remained symptom free, with no evidence of ischemia on exercise stress test or cardiac MRI. “These subjects will need to be followed up in the future to monitor for progression or resolution,” the study authors wrote.
Sudden cardiac death (SCD) is more common in anomalous aortic origin of the LCA than the RCA, Dr. Brothers and her colleagues said. Thus, an elliptical, slit-like neo-orifice is a concern because it can become blocked during exercise, possibly leading to lethal ventricular arrhythmia, they said. Ischemia in patients with anomalous coronary artery seems to result from a cumulative effect of exercise.
Patients who undergo the modified unroofing procedure typically have electrocardiography and echocardiography afterward and then get cleared to return to competitive sports in about 3 months if their stress test indicates it. Dr. Brothers and her colleagues said this activity recommendation may need alteration for those patients who have had a heart attack or sudden cardiac arrest, because they may remain at increased risk of SCD after surgery. “At the very least, additional imaging, such as with MRI-VA, should be used in this population,” the study authors said.
While Dr. Brothers and her colleagues acknowledged the small sample size is a limitation of the study, they also pointed out that anomalous coronary artery is a rare disease. They also noted that high-quality VA images can be difficult to obtain in noncompliant patients or those have arrhythmia or irregular breathing. “The images obtained in this study were acquired at an institution very familiar with pediatric cardiac coronary MRI and would be appropriate for assessing the coronary ostia with VA,” they said.
Dr. Brothers and her coauthors had no financial disclosures.
The MRI technique that Dr. Brothers and her colleagues reported on can provide important details of the anomalous coronary anatomy and about myocardial function, Philip S. Naimo, MD, Edward Buratto, MBBS, and Igor Konstantinov, MD, PhD, FRACS, of the Royal Children’s Hospital, University of Melbourne, wrote in their invited commentary. But, the ability to evaluate the neo-ostium after surgery had “particular value,” the commentators said (J. Thorac. Cardiovasc. Surg. 2016 Jul;152:211-12).
MRI with virtual angioscopy can fill help fill in the gaps where the significance of a narrowed neo-ostium is unknown, the commentators said. “The combination of anatomic information on the ostium size, shape, and location, as well as functional information on wall motion and myocardial perfusion, which can be provided by MRI-VA, would be particularly valuable in these patients,” they said.
They also pointed out that MRI-VA could be used in patients who have ongoing but otherwise undetected narrowing of the ostia after the unroofing procedure. At the same time, the technique will also require sufficient caseloads to maintain expertise. “It is safe to say that MRI-VA is here to stay,” Dr. Naimo, Dr. Buratto, and Dr. Konstantinov wrote. “The actual application of this virtual modality will need further refinement to be used routinely.”
The commentary authors had no financial relationships to disclose.
The MRI technique that Dr. Brothers and her colleagues reported on can provide important details of the anomalous coronary anatomy and about myocardial function, Philip S. Naimo, MD, Edward Buratto, MBBS, and Igor Konstantinov, MD, PhD, FRACS, of the Royal Children’s Hospital, University of Melbourne, wrote in their invited commentary. But, the ability to evaluate the neo-ostium after surgery had “particular value,” the commentators said (J. Thorac. Cardiovasc. Surg. 2016 Jul;152:211-12).
MRI with virtual angioscopy can fill help fill in the gaps where the significance of a narrowed neo-ostium is unknown, the commentators said. “The combination of anatomic information on the ostium size, shape, and location, as well as functional information on wall motion and myocardial perfusion, which can be provided by MRI-VA, would be particularly valuable in these patients,” they said.
They also pointed out that MRI-VA could be used in patients who have ongoing but otherwise undetected narrowing of the ostia after the unroofing procedure. At the same time, the technique will also require sufficient caseloads to maintain expertise. “It is safe to say that MRI-VA is here to stay,” Dr. Naimo, Dr. Buratto, and Dr. Konstantinov wrote. “The actual application of this virtual modality will need further refinement to be used routinely.”
The commentary authors had no financial relationships to disclose.
The MRI technique that Dr. Brothers and her colleagues reported on can provide important details of the anomalous coronary anatomy and about myocardial function, Philip S. Naimo, MD, Edward Buratto, MBBS, and Igor Konstantinov, MD, PhD, FRACS, of the Royal Children’s Hospital, University of Melbourne, wrote in their invited commentary. But, the ability to evaluate the neo-ostium after surgery had “particular value,” the commentators said (J. Thorac. Cardiovasc. Surg. 2016 Jul;152:211-12).
MRI with virtual angioscopy can fill help fill in the gaps where the significance of a narrowed neo-ostium is unknown, the commentators said. “The combination of anatomic information on the ostium size, shape, and location, as well as functional information on wall motion and myocardial perfusion, which can be provided by MRI-VA, would be particularly valuable in these patients,” they said.
They also pointed out that MRI-VA could be used in patients who have ongoing but otherwise undetected narrowing of the ostia after the unroofing procedure. At the same time, the technique will also require sufficient caseloads to maintain expertise. “It is safe to say that MRI-VA is here to stay,” Dr. Naimo, Dr. Buratto, and Dr. Konstantinov wrote. “The actual application of this virtual modality will need further refinement to be used routinely.”
The commentary authors had no financial relationships to disclose.
Failure to achieve a rounded and unobstructed ostia in children who have surgery to repair anomalous coronary arteries can put these children at continued risk for sudden death, but cardiac MRI with virtual angioscopy (VA) before and after the operation can give cardiologists a clear picture of a patient’s risk for sudden death and help direct ongoing management, according to a study in the July issue of the Journal of Thoracic and Cardiovascular Surgery (2016;152:205-10).
“Cardiac MRI with virtual angioscopy is an important tool for evaluating anomalous coronary anatomy, myocardial function, and ischemia and should be considered for initial and postoperative assessment of children with anomalous coronary arteries,” lead author Julie A. Brothers, MD, and her coauthors said in reporting their findings.
Anomalous coronary artery is a rare congenital condition in which the left coronary artery (LCA) originates from the right sinus or the right coronary artery (RCA) originates from the left coronary sinus. Dr. Brothers, a pediatric cardiologist, and her colleagues from the Children’s Hospital of Philadelphia and the University of Pennsylvania, also in Philadelphia, studied nine male patients who had operations for anomalous coronary arteries during Feb. 2009-May 2015 in what they said is the first study to document anomalous coronary artery anatomy both before and after surgery. The patients’ average age was 14.1 years; seven had right anomalous coronary arteries and two had left anomalous arteries. After the operations, MRI-VA revealed that two patients still had narrowing in the neo-orifices.
Previous reports recommend surgical repair for all patients with anomalous LCA and for symptomatic patients with anomalous RCA anatomy (Ann Thorac Surg. 2011;92:691-7; Ann Thorac Surg. 2014;98:941-5). MRI-VA allows the surgical team to survey the ostial stenosis before the operation “as if standing within the vessel itself,” Dr. Brothers and her coauthors wrote. Afterward, MRI-VA lets the surgeon and team see if the operation succeeded in repairing the orifices.
In the study population, VA before surgery confirmed elliptical, slit-like orifices in all patients. The operations involved unroofing procedures; two patients also had detachment and resuspension procedures during surgery. After surgery, VA showed that seven patients had round, patent, unobstructed repaired orifices; but two had orifices that were still narrow and somewhat stenotic, Dr. Brothers and her coauthors said. The study group had postoperative MRI-VA an average of 8.6 months after surgery.
“The significance of these findings is unknown; however, if the proposed mechanism of ischemia is due to a slit-like orifice, a continued stenotic orifice may place subjects at risk for sudden death,” the researchers said. The two study patients with the narrowed, stenotic orifices have remained symptom free, with no evidence of ischemia on exercise stress test or cardiac MRI. “These subjects will need to be followed up in the future to monitor for progression or resolution,” the study authors wrote.
Sudden cardiac death (SCD) is more common in anomalous aortic origin of the LCA than the RCA, Dr. Brothers and her colleagues said. Thus, an elliptical, slit-like neo-orifice is a concern because it can become blocked during exercise, possibly leading to lethal ventricular arrhythmia, they said. Ischemia in patients with anomalous coronary artery seems to result from a cumulative effect of exercise.
Patients who undergo the modified unroofing procedure typically have electrocardiography and echocardiography afterward and then get cleared to return to competitive sports in about 3 months if their stress test indicates it. Dr. Brothers and her colleagues said this activity recommendation may need alteration for those patients who have had a heart attack or sudden cardiac arrest, because they may remain at increased risk of SCD after surgery. “At the very least, additional imaging, such as with MRI-VA, should be used in this population,” the study authors said.
While Dr. Brothers and her colleagues acknowledged the small sample size is a limitation of the study, they also pointed out that anomalous coronary artery is a rare disease. They also noted that high-quality VA images can be difficult to obtain in noncompliant patients or those have arrhythmia or irregular breathing. “The images obtained in this study were acquired at an institution very familiar with pediatric cardiac coronary MRI and would be appropriate for assessing the coronary ostia with VA,” they said.
Dr. Brothers and her coauthors had no financial disclosures.
Failure to achieve a rounded and unobstructed ostia in children who have surgery to repair anomalous coronary arteries can put these children at continued risk for sudden death, but cardiac MRI with virtual angioscopy (VA) before and after the operation can give cardiologists a clear picture of a patient’s risk for sudden death and help direct ongoing management, according to a study in the July issue of the Journal of Thoracic and Cardiovascular Surgery (2016;152:205-10).
“Cardiac MRI with virtual angioscopy is an important tool for evaluating anomalous coronary anatomy, myocardial function, and ischemia and should be considered for initial and postoperative assessment of children with anomalous coronary arteries,” lead author Julie A. Brothers, MD, and her coauthors said in reporting their findings.
Anomalous coronary artery is a rare congenital condition in which the left coronary artery (LCA) originates from the right sinus or the right coronary artery (RCA) originates from the left coronary sinus. Dr. Brothers, a pediatric cardiologist, and her colleagues from the Children’s Hospital of Philadelphia and the University of Pennsylvania, also in Philadelphia, studied nine male patients who had operations for anomalous coronary arteries during Feb. 2009-May 2015 in what they said is the first study to document anomalous coronary artery anatomy both before and after surgery. The patients’ average age was 14.1 years; seven had right anomalous coronary arteries and two had left anomalous arteries. After the operations, MRI-VA revealed that two patients still had narrowing in the neo-orifices.
Previous reports recommend surgical repair for all patients with anomalous LCA and for symptomatic patients with anomalous RCA anatomy (Ann Thorac Surg. 2011;92:691-7; Ann Thorac Surg. 2014;98:941-5). MRI-VA allows the surgical team to survey the ostial stenosis before the operation “as if standing within the vessel itself,” Dr. Brothers and her coauthors wrote. Afterward, MRI-VA lets the surgeon and team see if the operation succeeded in repairing the orifices.
In the study population, VA before surgery confirmed elliptical, slit-like orifices in all patients. The operations involved unroofing procedures; two patients also had detachment and resuspension procedures during surgery. After surgery, VA showed that seven patients had round, patent, unobstructed repaired orifices; but two had orifices that were still narrow and somewhat stenotic, Dr. Brothers and her coauthors said. The study group had postoperative MRI-VA an average of 8.6 months after surgery.
“The significance of these findings is unknown; however, if the proposed mechanism of ischemia is due to a slit-like orifice, a continued stenotic orifice may place subjects at risk for sudden death,” the researchers said. The two study patients with the narrowed, stenotic orifices have remained symptom free, with no evidence of ischemia on exercise stress test or cardiac MRI. “These subjects will need to be followed up in the future to monitor for progression or resolution,” the study authors wrote.
Sudden cardiac death (SCD) is more common in anomalous aortic origin of the LCA than the RCA, Dr. Brothers and her colleagues said. Thus, an elliptical, slit-like neo-orifice is a concern because it can become blocked during exercise, possibly leading to lethal ventricular arrhythmia, they said. Ischemia in patients with anomalous coronary artery seems to result from a cumulative effect of exercise.
Patients who undergo the modified unroofing procedure typically have electrocardiography and echocardiography afterward and then get cleared to return to competitive sports in about 3 months if their stress test indicates it. Dr. Brothers and her colleagues said this activity recommendation may need alteration for those patients who have had a heart attack or sudden cardiac arrest, because they may remain at increased risk of SCD after surgery. “At the very least, additional imaging, such as with MRI-VA, should be used in this population,” the study authors said.
While Dr. Brothers and her colleagues acknowledged the small sample size is a limitation of the study, they also pointed out that anomalous coronary artery is a rare disease. They also noted that high-quality VA images can be difficult to obtain in noncompliant patients or those have arrhythmia or irregular breathing. “The images obtained in this study were acquired at an institution very familiar with pediatric cardiac coronary MRI and would be appropriate for assessing the coronary ostia with VA,” they said.
Dr. Brothers and her coauthors had no financial disclosures.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Cardiac MRI with virtual angioscopy (VA) can perform pre- and postoperative assessment in pediatric patients with anomalous coronary arteries.
Major finding: MRI-VA showed that neo-ostium in seven patients were round and unobstructed after surgery, but remained elliptical and somewhat stenotic in two patients.
Data source: Nine male patients aged 5-19 years who had modified unroofing procedure for anomalous coronary artery anatomy at a single institution between February 2009 and May 2015.
Disclosures: Dr. Brothers and coauthors had no financial relationships to disclose.
Syndecan-1 may predict kidney injury after ped heart surgery
Acute kidney injury is a common complication after pediatric cardiac surgery, but measuring for a specific genetic protein immediately after cardiac surgery may improve cardiac surgeons’ ability to predict patients at higher risk of AKI, according to researchers from Brazil. The study results are in the July issue of the Journal of Thoracic and Cardiovascular Surgery (2016;152-178-86).
“Plasma syndecan-1 levels measured early in the postoperative period were independently associated with severe acute kidney injury,” wrote Candice Torres de Melo Bezerra Cavalcante, MD, of Heart Hospital of Messejana and Federal University of Ceará.
Their prospective cohort study involved 289 pediatric patients who had cardiac surgery at their institution between September 2013 and December 2014.
Dr. Cavalcante and colleagues acknowledged that the traditional biomarker for renal function, serum creatinine, only increases appreciably after the glomerular filtration rate declines 50%, impairing physicians’ ability to detect AKI early enough to treat it. “This delay can explain, in part the, negative results in AKI therapeutic clinical trials,” they wrote.
They evaluated two different endothelial biomarkers in addition to syndecan-1 with regard to their capacity for predicting severe AKI: plasma ICAM-1, a marker of endothelial cell activation; and E-selectin, an endothelial cell adhesion molecule. Syndecan-1 works as a biomarker of injury to the glycocalyx protein that surrounds endothelial cell membranes that acts as a permeability barrier and prevents the cells from adhering to blood. They found that median syndecan-1 levels soon after surgery were higher in patients with severe AKI, 103.6 vs. 42.3 ng/mL.
“Although syndecan-1 is not a renal-specific biomarker, there has been recent increasing evidence that endothelial injury has an important role in AKI pathophysiology,” the researchers noted.
Study results showed the higher the level of syndecan-1, the greater the adjusted odds ratio (OR) for severe AKI. Levels of less than 17 ng/mL were considered normal; 17.1-46.7 ng/mL carried an adjusted OR of 1.42; 47.4-93.1 ng/mL had an adjusted OR of 2.05; and levels 96.3 or greater had an OR of 8.87.
“Maintenance of endothelial glycocalyx integrity can be a therapeutic target to reduce AKI in this setting,” the researchers wrote.
The authors acknowledged that the study was done at a single center that had dialysis and death rates three and five times higher, respectively, than those of developed countries; and it measured syndecan-1 at only one time point almost immediately after the operation.
“Adding postoperative syndecan-1, even when using a clinical model that already incorporates variables from renal angina index, results in significant improvement in the capacity to predict severe AKI,” Dr. Cavalcante and colleagues concluded.
They had no financial relationships to disclose.
Results of AKI in heart surgery patients have been “sobering,” with up to 56% of these patients being diagnosed with AKI, but research such as that by Dr. Cavalcante and colleagues represents a new approach to improving outcomes by combining clinical risk factors with specific biomarkers to identify patients at risk, Petros V. Anagnostopoulos, MD, of American Family Children’s Hospital, University of Wisconsin, said in his invited commentary (J Thorac Cardiovasc Surg. 2016;152[1]:187-8).
Dr. Anagnostopoulos acknowledged problems with traditional markers for renal function. “An ideal biomarker should be sensitive, easy to measure, reproducible, and inexpensive,” he said. “Finally, when combined with clinical prediction models, it should potentiate the discrimination of these models.”
Syndecan-1 answers that call, he said. “It peaks early and is cheap, fast, and easy to measure with readily available methods, which makes it an ideal early biomarker of AKI,” Dr. Anagnostopoulos said. Even so, he pointed out potential shortcomings of syndecan-1: It is not renal specific and it does not increase before the operation.
But he applauded Dr. Cavalcante and colleagues for pursuing research to combine clinical risk factors with specific biomarkers. “It is very likely that this type of clinical research will become prevalent in the near future and will hopefully produce results that will allow better individual patient-specific risk stratification,” Dr. Anagnostopoulos said.
He had no financial relationships to disclose.
Results of AKI in heart surgery patients have been “sobering,” with up to 56% of these patients being diagnosed with AKI, but research such as that by Dr. Cavalcante and colleagues represents a new approach to improving outcomes by combining clinical risk factors with specific biomarkers to identify patients at risk, Petros V. Anagnostopoulos, MD, of American Family Children’s Hospital, University of Wisconsin, said in his invited commentary (J Thorac Cardiovasc Surg. 2016;152[1]:187-8).
Dr. Anagnostopoulos acknowledged problems with traditional markers for renal function. “An ideal biomarker should be sensitive, easy to measure, reproducible, and inexpensive,” he said. “Finally, when combined with clinical prediction models, it should potentiate the discrimination of these models.”
Syndecan-1 answers that call, he said. “It peaks early and is cheap, fast, and easy to measure with readily available methods, which makes it an ideal early biomarker of AKI,” Dr. Anagnostopoulos said. Even so, he pointed out potential shortcomings of syndecan-1: It is not renal specific and it does not increase before the operation.
But he applauded Dr. Cavalcante and colleagues for pursuing research to combine clinical risk factors with specific biomarkers. “It is very likely that this type of clinical research will become prevalent in the near future and will hopefully produce results that will allow better individual patient-specific risk stratification,” Dr. Anagnostopoulos said.
He had no financial relationships to disclose.
Results of AKI in heart surgery patients have been “sobering,” with up to 56% of these patients being diagnosed with AKI, but research such as that by Dr. Cavalcante and colleagues represents a new approach to improving outcomes by combining clinical risk factors with specific biomarkers to identify patients at risk, Petros V. Anagnostopoulos, MD, of American Family Children’s Hospital, University of Wisconsin, said in his invited commentary (J Thorac Cardiovasc Surg. 2016;152[1]:187-8).
Dr. Anagnostopoulos acknowledged problems with traditional markers for renal function. “An ideal biomarker should be sensitive, easy to measure, reproducible, and inexpensive,” he said. “Finally, when combined with clinical prediction models, it should potentiate the discrimination of these models.”
Syndecan-1 answers that call, he said. “It peaks early and is cheap, fast, and easy to measure with readily available methods, which makes it an ideal early biomarker of AKI,” Dr. Anagnostopoulos said. Even so, he pointed out potential shortcomings of syndecan-1: It is not renal specific and it does not increase before the operation.
But he applauded Dr. Cavalcante and colleagues for pursuing research to combine clinical risk factors with specific biomarkers. “It is very likely that this type of clinical research will become prevalent in the near future and will hopefully produce results that will allow better individual patient-specific risk stratification,” Dr. Anagnostopoulos said.
He had no financial relationships to disclose.
Acute kidney injury is a common complication after pediatric cardiac surgery, but measuring for a specific genetic protein immediately after cardiac surgery may improve cardiac surgeons’ ability to predict patients at higher risk of AKI, according to researchers from Brazil. The study results are in the July issue of the Journal of Thoracic and Cardiovascular Surgery (2016;152-178-86).
“Plasma syndecan-1 levels measured early in the postoperative period were independently associated with severe acute kidney injury,” wrote Candice Torres de Melo Bezerra Cavalcante, MD, of Heart Hospital of Messejana and Federal University of Ceará.
Their prospective cohort study involved 289 pediatric patients who had cardiac surgery at their institution between September 2013 and December 2014.
Dr. Cavalcante and colleagues acknowledged that the traditional biomarker for renal function, serum creatinine, only increases appreciably after the glomerular filtration rate declines 50%, impairing physicians’ ability to detect AKI early enough to treat it. “This delay can explain, in part the, negative results in AKI therapeutic clinical trials,” they wrote.
They evaluated two different endothelial biomarkers in addition to syndecan-1 with regard to their capacity for predicting severe AKI: plasma ICAM-1, a marker of endothelial cell activation; and E-selectin, an endothelial cell adhesion molecule. Syndecan-1 works as a biomarker of injury to the glycocalyx protein that surrounds endothelial cell membranes that acts as a permeability barrier and prevents the cells from adhering to blood. They found that median syndecan-1 levels soon after surgery were higher in patients with severe AKI, 103.6 vs. 42.3 ng/mL.
“Although syndecan-1 is not a renal-specific biomarker, there has been recent increasing evidence that endothelial injury has an important role in AKI pathophysiology,” the researchers noted.
Study results showed the higher the level of syndecan-1, the greater the adjusted odds ratio (OR) for severe AKI. Levels of less than 17 ng/mL were considered normal; 17.1-46.7 ng/mL carried an adjusted OR of 1.42; 47.4-93.1 ng/mL had an adjusted OR of 2.05; and levels 96.3 or greater had an OR of 8.87.
“Maintenance of endothelial glycocalyx integrity can be a therapeutic target to reduce AKI in this setting,” the researchers wrote.
The authors acknowledged that the study was done at a single center that had dialysis and death rates three and five times higher, respectively, than those of developed countries; and it measured syndecan-1 at only one time point almost immediately after the operation.
“Adding postoperative syndecan-1, even when using a clinical model that already incorporates variables from renal angina index, results in significant improvement in the capacity to predict severe AKI,” Dr. Cavalcante and colleagues concluded.
They had no financial relationships to disclose.
Acute kidney injury is a common complication after pediatric cardiac surgery, but measuring for a specific genetic protein immediately after cardiac surgery may improve cardiac surgeons’ ability to predict patients at higher risk of AKI, according to researchers from Brazil. The study results are in the July issue of the Journal of Thoracic and Cardiovascular Surgery (2016;152-178-86).
“Plasma syndecan-1 levels measured early in the postoperative period were independently associated with severe acute kidney injury,” wrote Candice Torres de Melo Bezerra Cavalcante, MD, of Heart Hospital of Messejana and Federal University of Ceará.
Their prospective cohort study involved 289 pediatric patients who had cardiac surgery at their institution between September 2013 and December 2014.
Dr. Cavalcante and colleagues acknowledged that the traditional biomarker for renal function, serum creatinine, only increases appreciably after the glomerular filtration rate declines 50%, impairing physicians’ ability to detect AKI early enough to treat it. “This delay can explain, in part the, negative results in AKI therapeutic clinical trials,” they wrote.
They evaluated two different endothelial biomarkers in addition to syndecan-1 with regard to their capacity for predicting severe AKI: plasma ICAM-1, a marker of endothelial cell activation; and E-selectin, an endothelial cell adhesion molecule. Syndecan-1 works as a biomarker of injury to the glycocalyx protein that surrounds endothelial cell membranes that acts as a permeability barrier and prevents the cells from adhering to blood. They found that median syndecan-1 levels soon after surgery were higher in patients with severe AKI, 103.6 vs. 42.3 ng/mL.
“Although syndecan-1 is not a renal-specific biomarker, there has been recent increasing evidence that endothelial injury has an important role in AKI pathophysiology,” the researchers noted.
Study results showed the higher the level of syndecan-1, the greater the adjusted odds ratio (OR) for severe AKI. Levels of less than 17 ng/mL were considered normal; 17.1-46.7 ng/mL carried an adjusted OR of 1.42; 47.4-93.1 ng/mL had an adjusted OR of 2.05; and levels 96.3 or greater had an OR of 8.87.
“Maintenance of endothelial glycocalyx integrity can be a therapeutic target to reduce AKI in this setting,” the researchers wrote.
The authors acknowledged that the study was done at a single center that had dialysis and death rates three and five times higher, respectively, than those of developed countries; and it measured syndecan-1 at only one time point almost immediately after the operation.
“Adding postoperative syndecan-1, even when using a clinical model that already incorporates variables from renal angina index, results in significant improvement in the capacity to predict severe AKI,” Dr. Cavalcante and colleagues concluded.
They had no financial relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: The biomarker syndecan-1 may aid in determining acute kidney injury risk for children having cardiac surgery.
Major finding: Children with elevated levels of syndecan-1 had a two- to ninefold greater risk of acute kidney injury.
Data source: Single-institution, prospective cohort study of 289 pediatric patients who had cardiac surgery from September 2013 to December 2014.
Disclosures: Dr. Cavalcante and coauthors had no financial relationships to disclose.
Does congenital cardiac surgery training need a makeover?
Trainees in congenital cardiac surgery fellowship programs are doing more operations since the programs became accredited in 2007, but no clear parameters have emerged to determine if certification has improved the quality of training, according to an evaluation of fellowship training programs published in the June issue of the Journal of Thoracic and Cardiovascular Surgery (2016 Jun;151:1488-95).
Overall, the training has become standardized, the fellows’ operative experience is “robust,” and fellows are mostly satisfied since the Accreditation Council of Graduate Medical Education (ACGME) recognized congenital cardiac surgery as a fellowship in 2007, lead study author Dr. Brian Kogon of Emory University, Atlanta, said.
However, Dr. Kogon and his colleagues also found some shortcomings in fellowship training. They received survey responses from 36 of 44 fellows in 12 accredited programs nationwide. To determine if fellows were meeting minimum case requirements, they also reviewed operative logs of 38 of the 44 fellows. They compared their findings to a study of congenital cardiac surgery fellowship programs they did pre-ACGME accreditation (J Thorac Cardiovasc Surg. 2006 Dec;132:1280). “The number of operations performed by the fellows during their training was underwhelming, and most of the fellows were dissatisfied with their operative experience,” Dr. Kogon and his colleagues wrote in the earlier study.
The study found that all fellows achieved the minimum number of 75 total cases the standards require for graduation, with a median of 136; and the minimum standard of 36 specific qualifying cases with a median of 63. However, seven did not meet the minimum of five complex neonate cases. Among other types of operations for which fellows failed to meet the minimum cases were atrioventricular septal defect repair, arch reconstruction including coarctation procedures and systemic-to-pulmonary artery shunt procedures.
The comparative lack of adult cardiac surgery operations was also considered a potential problem, the authors noted, pointing out that “the number of adults who have congenital heart disease now exceeds the number of children who have the disease, and many of these patients will require an operation.”
Another shortcoming the study found was a drop-off in international fellowships since 2007. “This change places us at risk of becoming intellectually isolated and losing international relationships that are critical to the future of our specialty,” Dr. Kogon and his colleagues wrote. Graduated fellows also acknowledged dissatisfaction with their lack of exposure to neonate surgery.
The study also determined the following demographics of the fellows: 83% are men and the median age at graduation was 40 years, with a range of 35-48 years. Only 25% of graduates participated in nonsurgical rotations such as cardiac catheterization and echocardiography.
“Although the operative experience seems to be much more robust, and this finding has been corroborated in other surgical disciplines after the advent of ACGME accreditation, comparing training before and after the accreditation process came into existence is difficult,” Dr. Kogon and his colleagues said.
The study also noted that the Thoracic Surgery Directors Association developed a congenital curriculum for congenital cardiothoracic surgery fellows, but only 28% used that curriculum and only 61% used any formal curriculum. “Unfortunately, regardless of the curriculum, only 50% of the graduates found it helpful,” Dr. Kogon and his colleagues said.
And regardless of the curriculum, only half of the graduates have passed the written qualifying and oral certifying examinations after completing their fellowship. “Although the curriculum is quite robust, the latter statistic suggests that we need either more emphasis on education by the program directors or a better and/or different curriculum,” Dr. Kogon and his colleagues said. However, they added that “after training, former fellows have adequate case volumes and mixes and seem to be thriving in the field.”
Dr. Kogon and his study coauthors had no financial disclosures.
In his invited commentary, Dr. Charles D. Fraser Jr. of Texas Children’s Hospital, Baylor University, Houston, called the study findings that only 50% of congenital cardiac surgery fellowship graduates had passed the congenital examination “quite disturbing” and the demographic data and surgical and nonsurgical experience of the trainees “thought provoking” (J Thorac Cardiovasc Surg. 2016;151:1496-7)
“Is the bar too high or too low?” Dr. Fraser asked. He suggested the fellowship training system for congenital cardiac surgeons may be a work in progress. “For one, having a median age of 40 years for graduates is unacceptable,” he said. For half of trainees to not pass the examination “at this advanced age is tragic.” That 25% of fellows participate in nonsurgical rotations “also is concerning.”
 |
Dr. Charles D. Fraser |
A challenge is that after fellows complete their training in general and cardiothoracic surgery, opportunities to operate on newborns in a new fellowship setting are extremely limited, Dr. Fraser said. “To expect someone to be able to perform complex newborn heart surgery with excellent outcomes in a brand-new environment after just learning how to perform adult cardiac surgery is unrealistic,” he said.
Dr. Fraser said 1 formal year of training for congenital cardiac surgery fellows may not be enough. “Our colleagues in general pediatric surgery have a 2-year fellowship, and our specialty is every bit as complex as theirs,” he said. The basic American Board of Thoracic Surgery thoracic fellowship should have more latitude in its congenital heart surgery rotations, including exposure to pediatrics, neonatal/pediatric critical care, and the nonsurgical rotations the study referred to. Congenital heart surgery fellowships should also embrace adult congenital heart surgery with a more formalized experience requirement, he said.
“As a specialty, we owe it to our fine young surgeon candidates to offer the most robust and fair pathway to success while never compromising on the public trust and patient well-being,” Dr. Fraser said.
Dr. Fraser is chief of the division of congenital heart surgery at Baylor and codirector of the Texas Children’s Heart Center. He had no financial relationships to disclose.
In his invited commentary, Dr. Charles D. Fraser Jr. of Texas Children’s Hospital, Baylor University, Houston, called the study findings that only 50% of congenital cardiac surgery fellowship graduates had passed the congenital examination “quite disturbing” and the demographic data and surgical and nonsurgical experience of the trainees “thought provoking” (J Thorac Cardiovasc Surg. 2016;151:1496-7)
“Is the bar too high or too low?” Dr. Fraser asked. He suggested the fellowship training system for congenital cardiac surgeons may be a work in progress. “For one, having a median age of 40 years for graduates is unacceptable,” he said. For half of trainees to not pass the examination “at this advanced age is tragic.” That 25% of fellows participate in nonsurgical rotations “also is concerning.”
 |
Dr. Charles D. Fraser |
A challenge is that after fellows complete their training in general and cardiothoracic surgery, opportunities to operate on newborns in a new fellowship setting are extremely limited, Dr. Fraser said. “To expect someone to be able to perform complex newborn heart surgery with excellent outcomes in a brand-new environment after just learning how to perform adult cardiac surgery is unrealistic,” he said.
Dr. Fraser said 1 formal year of training for congenital cardiac surgery fellows may not be enough. “Our colleagues in general pediatric surgery have a 2-year fellowship, and our specialty is every bit as complex as theirs,” he said. The basic American Board of Thoracic Surgery thoracic fellowship should have more latitude in its congenital heart surgery rotations, including exposure to pediatrics, neonatal/pediatric critical care, and the nonsurgical rotations the study referred to. Congenital heart surgery fellowships should also embrace adult congenital heart surgery with a more formalized experience requirement, he said.
“As a specialty, we owe it to our fine young surgeon candidates to offer the most robust and fair pathway to success while never compromising on the public trust and patient well-being,” Dr. Fraser said.
Dr. Fraser is chief of the division of congenital heart surgery at Baylor and codirector of the Texas Children’s Heart Center. He had no financial relationships to disclose.
In his invited commentary, Dr. Charles D. Fraser Jr. of Texas Children’s Hospital, Baylor University, Houston, called the study findings that only 50% of congenital cardiac surgery fellowship graduates had passed the congenital examination “quite disturbing” and the demographic data and surgical and nonsurgical experience of the trainees “thought provoking” (J Thorac Cardiovasc Surg. 2016;151:1496-7)
“Is the bar too high or too low?” Dr. Fraser asked. He suggested the fellowship training system for congenital cardiac surgeons may be a work in progress. “For one, having a median age of 40 years for graduates is unacceptable,” he said. For half of trainees to not pass the examination “at this advanced age is tragic.” That 25% of fellows participate in nonsurgical rotations “also is concerning.”
 |
Dr. Charles D. Fraser |
A challenge is that after fellows complete their training in general and cardiothoracic surgery, opportunities to operate on newborns in a new fellowship setting are extremely limited, Dr. Fraser said. “To expect someone to be able to perform complex newborn heart surgery with excellent outcomes in a brand-new environment after just learning how to perform adult cardiac surgery is unrealistic,” he said.
Dr. Fraser said 1 formal year of training for congenital cardiac surgery fellows may not be enough. “Our colleagues in general pediatric surgery have a 2-year fellowship, and our specialty is every bit as complex as theirs,” he said. The basic American Board of Thoracic Surgery thoracic fellowship should have more latitude in its congenital heart surgery rotations, including exposure to pediatrics, neonatal/pediatric critical care, and the nonsurgical rotations the study referred to. Congenital heart surgery fellowships should also embrace adult congenital heart surgery with a more formalized experience requirement, he said.
“As a specialty, we owe it to our fine young surgeon candidates to offer the most robust and fair pathway to success while never compromising on the public trust and patient well-being,” Dr. Fraser said.
Dr. Fraser is chief of the division of congenital heart surgery at Baylor and codirector of the Texas Children’s Heart Center. He had no financial relationships to disclose.
Trainees in congenital cardiac surgery fellowship programs are doing more operations since the programs became accredited in 2007, but no clear parameters have emerged to determine if certification has improved the quality of training, according to an evaluation of fellowship training programs published in the June issue of the Journal of Thoracic and Cardiovascular Surgery (2016 Jun;151:1488-95).
Overall, the training has become standardized, the fellows’ operative experience is “robust,” and fellows are mostly satisfied since the Accreditation Council of Graduate Medical Education (ACGME) recognized congenital cardiac surgery as a fellowship in 2007, lead study author Dr. Brian Kogon of Emory University, Atlanta, said.
However, Dr. Kogon and his colleagues also found some shortcomings in fellowship training. They received survey responses from 36 of 44 fellows in 12 accredited programs nationwide. To determine if fellows were meeting minimum case requirements, they also reviewed operative logs of 38 of the 44 fellows. They compared their findings to a study of congenital cardiac surgery fellowship programs they did pre-ACGME accreditation (J Thorac Cardiovasc Surg. 2006 Dec;132:1280). “The number of operations performed by the fellows during their training was underwhelming, and most of the fellows were dissatisfied with their operative experience,” Dr. Kogon and his colleagues wrote in the earlier study.
The study found that all fellows achieved the minimum number of 75 total cases the standards require for graduation, with a median of 136; and the minimum standard of 36 specific qualifying cases with a median of 63. However, seven did not meet the minimum of five complex neonate cases. Among other types of operations for which fellows failed to meet the minimum cases were atrioventricular septal defect repair, arch reconstruction including coarctation procedures and systemic-to-pulmonary artery shunt procedures.
The comparative lack of adult cardiac surgery operations was also considered a potential problem, the authors noted, pointing out that “the number of adults who have congenital heart disease now exceeds the number of children who have the disease, and many of these patients will require an operation.”
Another shortcoming the study found was a drop-off in international fellowships since 2007. “This change places us at risk of becoming intellectually isolated and losing international relationships that are critical to the future of our specialty,” Dr. Kogon and his colleagues wrote. Graduated fellows also acknowledged dissatisfaction with their lack of exposure to neonate surgery.
The study also determined the following demographics of the fellows: 83% are men and the median age at graduation was 40 years, with a range of 35-48 years. Only 25% of graduates participated in nonsurgical rotations such as cardiac catheterization and echocardiography.
“Although the operative experience seems to be much more robust, and this finding has been corroborated in other surgical disciplines after the advent of ACGME accreditation, comparing training before and after the accreditation process came into existence is difficult,” Dr. Kogon and his colleagues said.
The study also noted that the Thoracic Surgery Directors Association developed a congenital curriculum for congenital cardiothoracic surgery fellows, but only 28% used that curriculum and only 61% used any formal curriculum. “Unfortunately, regardless of the curriculum, only 50% of the graduates found it helpful,” Dr. Kogon and his colleagues said.
And regardless of the curriculum, only half of the graduates have passed the written qualifying and oral certifying examinations after completing their fellowship. “Although the curriculum is quite robust, the latter statistic suggests that we need either more emphasis on education by the program directors or a better and/or different curriculum,” Dr. Kogon and his colleagues said. However, they added that “after training, former fellows have adequate case volumes and mixes and seem to be thriving in the field.”
Dr. Kogon and his study coauthors had no financial disclosures.
Trainees in congenital cardiac surgery fellowship programs are doing more operations since the programs became accredited in 2007, but no clear parameters have emerged to determine if certification has improved the quality of training, according to an evaluation of fellowship training programs published in the June issue of the Journal of Thoracic and Cardiovascular Surgery (2016 Jun;151:1488-95).
Overall, the training has become standardized, the fellows’ operative experience is “robust,” and fellows are mostly satisfied since the Accreditation Council of Graduate Medical Education (ACGME) recognized congenital cardiac surgery as a fellowship in 2007, lead study author Dr. Brian Kogon of Emory University, Atlanta, said.
However, Dr. Kogon and his colleagues also found some shortcomings in fellowship training. They received survey responses from 36 of 44 fellows in 12 accredited programs nationwide. To determine if fellows were meeting minimum case requirements, they also reviewed operative logs of 38 of the 44 fellows. They compared their findings to a study of congenital cardiac surgery fellowship programs they did pre-ACGME accreditation (J Thorac Cardiovasc Surg. 2006 Dec;132:1280). “The number of operations performed by the fellows during their training was underwhelming, and most of the fellows were dissatisfied with their operative experience,” Dr. Kogon and his colleagues wrote in the earlier study.
The study found that all fellows achieved the minimum number of 75 total cases the standards require for graduation, with a median of 136; and the minimum standard of 36 specific qualifying cases with a median of 63. However, seven did not meet the minimum of five complex neonate cases. Among other types of operations for which fellows failed to meet the minimum cases were atrioventricular septal defect repair, arch reconstruction including coarctation procedures and systemic-to-pulmonary artery shunt procedures.
The comparative lack of adult cardiac surgery operations was also considered a potential problem, the authors noted, pointing out that “the number of adults who have congenital heart disease now exceeds the number of children who have the disease, and many of these patients will require an operation.”
Another shortcoming the study found was a drop-off in international fellowships since 2007. “This change places us at risk of becoming intellectually isolated and losing international relationships that are critical to the future of our specialty,” Dr. Kogon and his colleagues wrote. Graduated fellows also acknowledged dissatisfaction with their lack of exposure to neonate surgery.
The study also determined the following demographics of the fellows: 83% are men and the median age at graduation was 40 years, with a range of 35-48 years. Only 25% of graduates participated in nonsurgical rotations such as cardiac catheterization and echocardiography.
“Although the operative experience seems to be much more robust, and this finding has been corroborated in other surgical disciplines after the advent of ACGME accreditation, comparing training before and after the accreditation process came into existence is difficult,” Dr. Kogon and his colleagues said.
The study also noted that the Thoracic Surgery Directors Association developed a congenital curriculum for congenital cardiothoracic surgery fellows, but only 28% used that curriculum and only 61% used any formal curriculum. “Unfortunately, regardless of the curriculum, only 50% of the graduates found it helpful,” Dr. Kogon and his colleagues said.
And regardless of the curriculum, only half of the graduates have passed the written qualifying and oral certifying examinations after completing their fellowship. “Although the curriculum is quite robust, the latter statistic suggests that we need either more emphasis on education by the program directors or a better and/or different curriculum,” Dr. Kogon and his colleagues said. However, they added that “after training, former fellows have adequate case volumes and mixes and seem to be thriving in the field.”
Dr. Kogon and his study coauthors had no financial disclosures.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Since congenital cardiac fellowship programs became accredited in 2007, training requirements have been standardized and the surgical experience robust.
Major finding: Recent graduates of fellowship programs are thriving in practice, but shortcomings with existing fellowship training exist, including only 50% gaining certification by passing the written and oral exams.
Data source: The study drew on survey responses from 36 of 44 fellows in 12 accredited programs and a review of operative logs of 38 of the 44 fellows.
Disclosures: Dr. Kogon and his study coauthors had no financial disclosures.
VIDEO: Del Nido cardioplegic solution receives high grade
BALTIMORE – A cardioplegic solution developed by a noted cardiac surgeon has been deemed a “simple and safe” cardio protective strategy in a study presented at the annual meeting of the American Association for Thoracic Surgery.
In an interview at the event, Pedro J. del Nido, MD, chief of cardiac surgery at Boston Children’s Hospital, and inventor of the long-acting Del Nido solution, explained how and why he and his colleagues developed the new cardioplegic solution. He praised the study as uniquely important, given that there have been few prospective, randomized, and controlled comparative studies of cardioplegia targeted at pediatric patients.
“Baby hearts have a slightly different metabolism than adult hearts,” Dr. del Nido said. “Typically in pediatric procedures we not only cool the heart but we lower the whole body temperature... In the adult world we don’t drop the temperature so much.” He also said the method and flow rate of cardioplegia delivery was unique for pediatric patients, given the fragility of the endothelial cells lining their blood vessels.
The study compared the Del Nido solution with the St. Thomas cardioplegia solution. The investigators praised the Del Nido solution’s performance for “better cardiac index profile, lesser troponin-I release, and decreased morbidity.”
Dr. del Nido developed the Del Nido cardioplegic solution, but reported no other relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @richpizzi
BALTIMORE – A cardioplegic solution developed by a noted cardiac surgeon has been deemed a “simple and safe” cardio protective strategy in a study presented at the annual meeting of the American Association for Thoracic Surgery.
In an interview at the event, Pedro J. del Nido, MD, chief of cardiac surgery at Boston Children’s Hospital, and inventor of the long-acting Del Nido solution, explained how and why he and his colleagues developed the new cardioplegic solution. He praised the study as uniquely important, given that there have been few prospective, randomized, and controlled comparative studies of cardioplegia targeted at pediatric patients.
“Baby hearts have a slightly different metabolism than adult hearts,” Dr. del Nido said. “Typically in pediatric procedures we not only cool the heart but we lower the whole body temperature... In the adult world we don’t drop the temperature so much.” He also said the method and flow rate of cardioplegia delivery was unique for pediatric patients, given the fragility of the endothelial cells lining their blood vessels.
The study compared the Del Nido solution with the St. Thomas cardioplegia solution. The investigators praised the Del Nido solution’s performance for “better cardiac index profile, lesser troponin-I release, and decreased morbidity.”
Dr. del Nido developed the Del Nido cardioplegic solution, but reported no other relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @richpizzi
BALTIMORE – A cardioplegic solution developed by a noted cardiac surgeon has been deemed a “simple and safe” cardio protective strategy in a study presented at the annual meeting of the American Association for Thoracic Surgery.
In an interview at the event, Pedro J. del Nido, MD, chief of cardiac surgery at Boston Children’s Hospital, and inventor of the long-acting Del Nido solution, explained how and why he and his colleagues developed the new cardioplegic solution. He praised the study as uniquely important, given that there have been few prospective, randomized, and controlled comparative studies of cardioplegia targeted at pediatric patients.
“Baby hearts have a slightly different metabolism than adult hearts,” Dr. del Nido said. “Typically in pediatric procedures we not only cool the heart but we lower the whole body temperature... In the adult world we don’t drop the temperature so much.” He also said the method and flow rate of cardioplegia delivery was unique for pediatric patients, given the fragility of the endothelial cells lining their blood vessels.
The study compared the Del Nido solution with the St. Thomas cardioplegia solution. The investigators praised the Del Nido solution’s performance for “better cardiac index profile, lesser troponin-I release, and decreased morbidity.”
Dr. del Nido developed the Del Nido cardioplegic solution, but reported no other relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @richpizzi
AT THE AATS ANNUAL MEETING
Hybrid option ‘reasonable’ for HLHS?
Although the classic Norwood palliation for infants with hypoplastic left heart syndrome (HLHS) has been well established, the procedure has had its drawbacks, namely the need for cardiopulmonary bypass with hypothermia and a because it rules out biventricular correction months later. A hybrid procedure avoids the need for bypass and accommodates short-term biventricular correction, but it has lacked strong evidence.
Researchers from Justus-Liebig University Giessen, Germany, reported on 182 patients with HLHS who had the three-stage Giessen hybrid procedure, noting 10-year survival of almost 80% with almost a third of patients requiring no artery intervention in that time (J Thorac Cardiovasc Surg. 2016 April;151:1112-23).
“In view of the early results and long-term outcome after Giessen hybrid palliation, the hybrid approach has become a reasonable alternative to the conventional strategy to treat neonates with HLHS and variants,” wrote Dr. Can Yerebakan and colleagues. “Further refinements are warranted to decrease patient morbidity.”
The Giessen hybrid procedure uses a technique to control pulmonary blood flow that is different from the Norwood procedure. The hybrid approach involves stenting of the arterial duct or prostaglandin therapy to maintain systemic perfusion combined with off-pump bilateral banding of the pulmonary arteries (bPAB) in the neonatal period. The Giessen hybrid operation defers the Norwood-type palliation using cardiopulmonary bypass that involves an aortic arch reconstruction, including a superior cavopulmonary connection or a biventricular correction, if indicated, until the infant is 4-8 months of age.
“In recent years, hybrid treatment has moved from a myth to an alternative modality in a growing number of institutions globally,” Dr. Yerebakan and colleagues said. The hybrid procedure has been used in high-risk patients. One report claimed higher morbidity in the hybrid procedure due to bPAB (Ann Thorac Surg. 2013;96:1382-8). Another study raised concerns about an adequate pulmonary artery rehabilitation at the time of the Fontan operation, the third stage in the hybrid strategy (J Thorac Cardiovasc Surg. 2014;147:706-12).
But with the hybrid approach, patients retain the potential to receive a biventricular correction up to 8 months later without compromising survival, “postponing an immediate definitive decision in the newborn period in comparison with the classic Norwood palliation,” Dr. Yerebakan and coauthors noted.
The doctors at the Pediatric Heart Center Giessen treat all types and variants of HLHS with the modified Giessen hybrid strategy. Between 1998 and 2015, 182 patients with HLHS had the Giessen hybrid stage I operation, including 126 patients who received univentricular palliation or a heart transplant. The median age of stage I recipients was 6 days, and median weight 3.2 kg. The stage II operation was performed at 4.5 months, with a range of 2.9 to 39.5 months, and Fontan completion was established at 33.7 months, with a range of 21 to 108 months.
Median follow-up after the stage I procedure was 4.6 years, and the death rate was 2.5%. After stage II, mortality was 4.9%; no deaths were reported after Fontan completion. Body weight less than 2.5 kg and aortic atresia had no significant effect on survival. Mortality rates were 8.9% between stages I and II and 5.3% between stage II and Fontan completion. “Cumulative interstage mortality was 14.2%,” Dr. Yerebakan and colleagues noted. “At 10 years, the probability of survival is 77.8%.”
Also at 10 years, 32.2% of patients were free from further pulmonary artery intervention, and 16.7% needed aortic arch reconstruction. Two patients required reoperations for aortic arch reconstruction.
Dr. Yerebakan and colleagues suggested several steps to improve outcomes with the hybrid approach: “intense collaboration” with anesthesiology and pediatric cardiology during and after the procedure to risk stratify individual patients; implementation of standards for management of all stages, including out-of-hospital care, in all departments that participate in a case; and liberalized indications for use of MRI before the stage II and Fontan completion.
Among the limitations of the study the authors noted were its retrospective nature and a median follow-up of only 5 years when the center has some cases with up to 15 years of follow-up. But Dr. Yerebakan and coauthors said they could not determine if the patients benefit from the hybrid treatment in the long-term.
The researchers had no disclosures.
The study by Dr. Yerebakan and colleagues is one of the largest single-center series of patients with HLHS who routinely undergo a hybrid palliation to date, and while the study is open to criticisms, “the authors should be applauded,” Dr. Ralph S. Mosca of New York University said in his invited commentary (J Thorac Cardiovasc Surg. 2016;151:1123-25).
Among the criticisms Dr. Mosca mentioned are that the hybrid approach requires a more extensive stage II reconstruction, “often further complicated by the presence of significant branch PA stenosis and a difficult aortic arch reconstruction”; that there is “appreciable” interstage mortality at 12.2%; and that there is an absence of data on renal or respiratory insufficiency, infection rates, and neurologic outcomes.
Dr. Mosca cited the cause for applause, however: “Through their persistence and collective experience, [the authors] have achieved commendable results in this difficult patient population.”
Yet, Dr. Mosca also noted a number of “potential problems” with the hybrid approach: bilateral banding of the pulmonary artery is a “crude procedure”; arterial duct stenting can lead to retrograde aortic arch reduction; and the interstage mortality “remains significant.”
Results of the hybrid and Norwood procedures are “strikingly similar,” Dr. Mosca said. While the hybrid approach may lower neonatal mortality, it may also carry longer-term consequences “predicated upon the need to closely observe and intervene,” he said. Clinicians need more information on hybrid outcomes, but in time it will likely take its place as an option for HLHS alongside the Norwood procedure, Dr. Mosca said.
The study by Dr. Yerebakan and colleagues is one of the largest single-center series of patients with HLHS who routinely undergo a hybrid palliation to date, and while the study is open to criticisms, “the authors should be applauded,” Dr. Ralph S. Mosca of New York University said in his invited commentary (J Thorac Cardiovasc Surg. 2016;151:1123-25).
Among the criticisms Dr. Mosca mentioned are that the hybrid approach requires a more extensive stage II reconstruction, “often further complicated by the presence of significant branch PA stenosis and a difficult aortic arch reconstruction”; that there is “appreciable” interstage mortality at 12.2%; and that there is an absence of data on renal or respiratory insufficiency, infection rates, and neurologic outcomes.
Dr. Mosca cited the cause for applause, however: “Through their persistence and collective experience, [the authors] have achieved commendable results in this difficult patient population.”
Yet, Dr. Mosca also noted a number of “potential problems” with the hybrid approach: bilateral banding of the pulmonary artery is a “crude procedure”; arterial duct stenting can lead to retrograde aortic arch reduction; and the interstage mortality “remains significant.”
Results of the hybrid and Norwood procedures are “strikingly similar,” Dr. Mosca said. While the hybrid approach may lower neonatal mortality, it may also carry longer-term consequences “predicated upon the need to closely observe and intervene,” he said. Clinicians need more information on hybrid outcomes, but in time it will likely take its place as an option for HLHS alongside the Norwood procedure, Dr. Mosca said.
The study by Dr. Yerebakan and colleagues is one of the largest single-center series of patients with HLHS who routinely undergo a hybrid palliation to date, and while the study is open to criticisms, “the authors should be applauded,” Dr. Ralph S. Mosca of New York University said in his invited commentary (J Thorac Cardiovasc Surg. 2016;151:1123-25).
Among the criticisms Dr. Mosca mentioned are that the hybrid approach requires a more extensive stage II reconstruction, “often further complicated by the presence of significant branch PA stenosis and a difficult aortic arch reconstruction”; that there is “appreciable” interstage mortality at 12.2%; and that there is an absence of data on renal or respiratory insufficiency, infection rates, and neurologic outcomes.
Dr. Mosca cited the cause for applause, however: “Through their persistence and collective experience, [the authors] have achieved commendable results in this difficult patient population.”
Yet, Dr. Mosca also noted a number of “potential problems” with the hybrid approach: bilateral banding of the pulmonary artery is a “crude procedure”; arterial duct stenting can lead to retrograde aortic arch reduction; and the interstage mortality “remains significant.”
Results of the hybrid and Norwood procedures are “strikingly similar,” Dr. Mosca said. While the hybrid approach may lower neonatal mortality, it may also carry longer-term consequences “predicated upon the need to closely observe and intervene,” he said. Clinicians need more information on hybrid outcomes, but in time it will likely take its place as an option for HLHS alongside the Norwood procedure, Dr. Mosca said.
Although the classic Norwood palliation for infants with hypoplastic left heart syndrome (HLHS) has been well established, the procedure has had its drawbacks, namely the need for cardiopulmonary bypass with hypothermia and a because it rules out biventricular correction months later. A hybrid procedure avoids the need for bypass and accommodates short-term biventricular correction, but it has lacked strong evidence.
Researchers from Justus-Liebig University Giessen, Germany, reported on 182 patients with HLHS who had the three-stage Giessen hybrid procedure, noting 10-year survival of almost 80% with almost a third of patients requiring no artery intervention in that time (J Thorac Cardiovasc Surg. 2016 April;151:1112-23).
“In view of the early results and long-term outcome after Giessen hybrid palliation, the hybrid approach has become a reasonable alternative to the conventional strategy to treat neonates with HLHS and variants,” wrote Dr. Can Yerebakan and colleagues. “Further refinements are warranted to decrease patient morbidity.”
The Giessen hybrid procedure uses a technique to control pulmonary blood flow that is different from the Norwood procedure. The hybrid approach involves stenting of the arterial duct or prostaglandin therapy to maintain systemic perfusion combined with off-pump bilateral banding of the pulmonary arteries (bPAB) in the neonatal period. The Giessen hybrid operation defers the Norwood-type palliation using cardiopulmonary bypass that involves an aortic arch reconstruction, including a superior cavopulmonary connection or a biventricular correction, if indicated, until the infant is 4-8 months of age.
“In recent years, hybrid treatment has moved from a myth to an alternative modality in a growing number of institutions globally,” Dr. Yerebakan and colleagues said. The hybrid procedure has been used in high-risk patients. One report claimed higher morbidity in the hybrid procedure due to bPAB (Ann Thorac Surg. 2013;96:1382-8). Another study raised concerns about an adequate pulmonary artery rehabilitation at the time of the Fontan operation, the third stage in the hybrid strategy (J Thorac Cardiovasc Surg. 2014;147:706-12).
But with the hybrid approach, patients retain the potential to receive a biventricular correction up to 8 months later without compromising survival, “postponing an immediate definitive decision in the newborn period in comparison with the classic Norwood palliation,” Dr. Yerebakan and coauthors noted.
The doctors at the Pediatric Heart Center Giessen treat all types and variants of HLHS with the modified Giessen hybrid strategy. Between 1998 and 2015, 182 patients with HLHS had the Giessen hybrid stage I operation, including 126 patients who received univentricular palliation or a heart transplant. The median age of stage I recipients was 6 days, and median weight 3.2 kg. The stage II operation was performed at 4.5 months, with a range of 2.9 to 39.5 months, and Fontan completion was established at 33.7 months, with a range of 21 to 108 months.
Median follow-up after the stage I procedure was 4.6 years, and the death rate was 2.5%. After stage II, mortality was 4.9%; no deaths were reported after Fontan completion. Body weight less than 2.5 kg and aortic atresia had no significant effect on survival. Mortality rates were 8.9% between stages I and II and 5.3% between stage II and Fontan completion. “Cumulative interstage mortality was 14.2%,” Dr. Yerebakan and colleagues noted. “At 10 years, the probability of survival is 77.8%.”
Also at 10 years, 32.2% of patients were free from further pulmonary artery intervention, and 16.7% needed aortic arch reconstruction. Two patients required reoperations for aortic arch reconstruction.
Dr. Yerebakan and colleagues suggested several steps to improve outcomes with the hybrid approach: “intense collaboration” with anesthesiology and pediatric cardiology during and after the procedure to risk stratify individual patients; implementation of standards for management of all stages, including out-of-hospital care, in all departments that participate in a case; and liberalized indications for use of MRI before the stage II and Fontan completion.
Among the limitations of the study the authors noted were its retrospective nature and a median follow-up of only 5 years when the center has some cases with up to 15 years of follow-up. But Dr. Yerebakan and coauthors said they could not determine if the patients benefit from the hybrid treatment in the long-term.
The researchers had no disclosures.
Although the classic Norwood palliation for infants with hypoplastic left heart syndrome (HLHS) has been well established, the procedure has had its drawbacks, namely the need for cardiopulmonary bypass with hypothermia and a because it rules out biventricular correction months later. A hybrid procedure avoids the need for bypass and accommodates short-term biventricular correction, but it has lacked strong evidence.
Researchers from Justus-Liebig University Giessen, Germany, reported on 182 patients with HLHS who had the three-stage Giessen hybrid procedure, noting 10-year survival of almost 80% with almost a third of patients requiring no artery intervention in that time (J Thorac Cardiovasc Surg. 2016 April;151:1112-23).
“In view of the early results and long-term outcome after Giessen hybrid palliation, the hybrid approach has become a reasonable alternative to the conventional strategy to treat neonates with HLHS and variants,” wrote Dr. Can Yerebakan and colleagues. “Further refinements are warranted to decrease patient morbidity.”
The Giessen hybrid procedure uses a technique to control pulmonary blood flow that is different from the Norwood procedure. The hybrid approach involves stenting of the arterial duct or prostaglandin therapy to maintain systemic perfusion combined with off-pump bilateral banding of the pulmonary arteries (bPAB) in the neonatal period. The Giessen hybrid operation defers the Norwood-type palliation using cardiopulmonary bypass that involves an aortic arch reconstruction, including a superior cavopulmonary connection or a biventricular correction, if indicated, until the infant is 4-8 months of age.
“In recent years, hybrid treatment has moved from a myth to an alternative modality in a growing number of institutions globally,” Dr. Yerebakan and colleagues said. The hybrid procedure has been used in high-risk patients. One report claimed higher morbidity in the hybrid procedure due to bPAB (Ann Thorac Surg. 2013;96:1382-8). Another study raised concerns about an adequate pulmonary artery rehabilitation at the time of the Fontan operation, the third stage in the hybrid strategy (J Thorac Cardiovasc Surg. 2014;147:706-12).
But with the hybrid approach, patients retain the potential to receive a biventricular correction up to 8 months later without compromising survival, “postponing an immediate definitive decision in the newborn period in comparison with the classic Norwood palliation,” Dr. Yerebakan and coauthors noted.
The doctors at the Pediatric Heart Center Giessen treat all types and variants of HLHS with the modified Giessen hybrid strategy. Between 1998 and 2015, 182 patients with HLHS had the Giessen hybrid stage I operation, including 126 patients who received univentricular palliation or a heart transplant. The median age of stage I recipients was 6 days, and median weight 3.2 kg. The stage II operation was performed at 4.5 months, with a range of 2.9 to 39.5 months, and Fontan completion was established at 33.7 months, with a range of 21 to 108 months.
Median follow-up after the stage I procedure was 4.6 years, and the death rate was 2.5%. After stage II, mortality was 4.9%; no deaths were reported after Fontan completion. Body weight less than 2.5 kg and aortic atresia had no significant effect on survival. Mortality rates were 8.9% between stages I and II and 5.3% between stage II and Fontan completion. “Cumulative interstage mortality was 14.2%,” Dr. Yerebakan and colleagues noted. “At 10 years, the probability of survival is 77.8%.”
Also at 10 years, 32.2% of patients were free from further pulmonary artery intervention, and 16.7% needed aortic arch reconstruction. Two patients required reoperations for aortic arch reconstruction.
Dr. Yerebakan and colleagues suggested several steps to improve outcomes with the hybrid approach: “intense collaboration” with anesthesiology and pediatric cardiology during and after the procedure to risk stratify individual patients; implementation of standards for management of all stages, including out-of-hospital care, in all departments that participate in a case; and liberalized indications for use of MRI before the stage II and Fontan completion.
Among the limitations of the study the authors noted were its retrospective nature and a median follow-up of only 5 years when the center has some cases with up to 15 years of follow-up. But Dr. Yerebakan and coauthors said they could not determine if the patients benefit from the hybrid treatment in the long-term.
The researchers had no disclosures.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: A hybrid operation for hypoplastic left heart syndrome (HLHS) and variants in neonates is emerging as an alternative to the Norwood palliation.
Major finding: At 10 years, the probability of survival with the hybrid procedure was 77.8%. Low body weight (less than 2.5 kg) and aortic atresia had no significant impact on survival.
Data source: Retrospective study of 182 patients who had the hybrid procedure at a single center between June 1998 and February 2015.
Disclosures: The study investigators had no relationships to disclose.
MRI assessment of pulmonary vein stenosis predicts outcomes
A retrospective analysis of children who underwent pulmonary vein stenosis repair with preoperative computed tomography and magnetic resonance imaging from 1990 to 2012 showed that smaller upstream or downstream total cross-sectional area indexed (TCSAi) for body surface area led to poorer survival.
The study of 31 patients at a single institution also indicated that early survival seemed especially poor for patients with a greater number of stenotic veins and upstream pulmonary vein (PV) involvement. The study was published in the March issue of the Journal of Thoracic and Cardiovascular Surgery.
Dr. Mauro Lo Rito and his colleagues at The Hospital for Sick Children, Toronto, retrospectively assessed the 31 patients out of 145 who underwent surgical repair who had had preoperative CT and MRI imaging. Complete sutureless repair was done in 18 (58%), single-side sutureless repair in 12 (39%), and pericardial patch reconstruction in 1 (3%). The mean follow-up was 4.3 years; the median patient age at time of operation was 226 days. Stenosis was bilateral in 45% of patients and unilateral in 55 (J Thorac Cardiovasc Surg. 2016;151:657-66).
In-hospital mortality was 9.7%, with an overall survival of 75%, 69%, and 64% at 1, 3, and 5 years, respectively. Univariate analysis showed that a younger age at operation, lower body surface area, smaller upstream TCSAi, and greater number of PV with stenosis/occlusion were associated with an increased risk of death.
Multivariate analysis showed that smaller upstream TCSAi for body surface area (P = .030) and greater number of stenotic PVs (P = .007) were associated with poor early (less than 1 year) survival. There was a nonsignificant tendency for smaller downstream TCSAi to be associated with poor late survival (greater than 1 year). None of the different PV morphologies were found to influence survival, according to Dr. Lo Rito and his colleagues.
Among the 28 hospital survivors, restenosis occurred in 10 patients, 7 of whom did not undergo further surgery (3 of these were alive at last follow-up and 4 died secondary to disease progression). Of the 3 patients who underwent subsequent intervention, 2 were alive at last follow-up.
“Risk stratification for patients with PV stenosis is currently challenging because of the variability in the anatomic configuration and the unknown relationship between these anatomic variants and survival. Our study demonstrates that by using cross-sectional areas, pulmonary vein cross-sectional area indexed to body surface area (PVCSAi) and TCSAi and tabulating the number of stenotic PVs, we can identify high-risk subsets of patients with high predicted mortality.” Dr. Lo Rito and his colleagues stated.
“The upstream total cross-sectional area and the number of stenotic PVs influence early survival and can be used to guide counseling. Smaller downstream cross-sectional area influences late survival, and those patients should be monitored with close follow-up. This methodology could aid in risk stratification for future clinical trials of pharmacologic agents designed to target upstream pulmonary vasculopathy,” the investigators concluded.
The authors reported that they had no conflicts of interest.
A webcast of the original presentation of these results at the 95th American Association for Thoracic Surgery Annual Meeting is available online (http://webcast.aats.org/2015/Video/Tuesday/04-28-15_6A_1615_Lo_Rito.mp4).
“The Toronto group has contributed significantly to our knowledge and management of pulmonary vein stenosis during the past decade. This article by Dr. Lo Rito and coworkers continues that contribution by reinforcing the values of MRI in imaging PVs before intervention and providing a valuable “hint” that preoperative PV size measurements are related to outcome,” Dr. William M. DeCampli wrote in his invited commentary (J Thorac Cardiovasc Surg. 2016;1510:667-8).
“The task of definitively demonstrating this relationship is daunting for any single institution, however, because 1) PVS is relatively rare, 2) MRI and computed tomography are relatively recently used diagnostic modalities, and 3) MRI is not easily used in an important subset of the cohort, small infants.” This limited the study to a small number of covariates,” noted Dr. DeCampli, and prevented the researchers from taking into account a myriad of additional covariates commonly associated with survival in complex congenital heart disease.
 |
Dr. William M. DeCampli |
Such covariates included in a sufficiently large model could significantly alter the observed odds ratios otherwise calculated for the included variables in this study, he added, citing a study of PVS by Boston Children’s Hospital (J Thorac Cardiovasc Surg. 2015;150:911-7), which found a different set of covariates associated with death; in that case, age younger than 6 months at operation, weight less than 3 kg at operation, and lesser preoperative right ventricular systolic pressure.
“The challenges in studying PVS encountered by these two high-volume, research-oriented programs leads us to suggest that PVS should be studied in a different way. Perhaps it is time to consider a multi-institutional, mixed or inception cohort registry for PVS. The spring 2015 Society of Thoracic Surgeons Congenital Heart Database report lists 506 cases of PVS repair as the primary procedure between January 2011 and December 2014. If a study were to enroll just one-third of these subjects it would accrue more than 40 subjects per year. Five years hence with an anticipated 50-80 events (deaths), it would be possible to carry out more robust risk-hazard analyses,” Dr. DeCampli suggested.
Dr. DeCampli is a congenital heart surgeon at the department of clinical sciences, University of Central Florida, and the Heart Center at Arnold Palmer Hospital for Children, both in Orlando. He reported having no conflicts.
“The Toronto group has contributed significantly to our knowledge and management of pulmonary vein stenosis during the past decade. This article by Dr. Lo Rito and coworkers continues that contribution by reinforcing the values of MRI in imaging PVs before intervention and providing a valuable “hint” that preoperative PV size measurements are related to outcome,” Dr. William M. DeCampli wrote in his invited commentary (J Thorac Cardiovasc Surg. 2016;1510:667-8).
“The task of definitively demonstrating this relationship is daunting for any single institution, however, because 1) PVS is relatively rare, 2) MRI and computed tomography are relatively recently used diagnostic modalities, and 3) MRI is not easily used in an important subset of the cohort, small infants.” This limited the study to a small number of covariates,” noted Dr. DeCampli, and prevented the researchers from taking into account a myriad of additional covariates commonly associated with survival in complex congenital heart disease.
 |
Dr. William M. DeCampli |
Such covariates included in a sufficiently large model could significantly alter the observed odds ratios otherwise calculated for the included variables in this study, he added, citing a study of PVS by Boston Children’s Hospital (J Thorac Cardiovasc Surg. 2015;150:911-7), which found a different set of covariates associated with death; in that case, age younger than 6 months at operation, weight less than 3 kg at operation, and lesser preoperative right ventricular systolic pressure.
“The challenges in studying PVS encountered by these two high-volume, research-oriented programs leads us to suggest that PVS should be studied in a different way. Perhaps it is time to consider a multi-institutional, mixed or inception cohort registry for PVS. The spring 2015 Society of Thoracic Surgeons Congenital Heart Database report lists 506 cases of PVS repair as the primary procedure between January 2011 and December 2014. If a study were to enroll just one-third of these subjects it would accrue more than 40 subjects per year. Five years hence with an anticipated 50-80 events (deaths), it would be possible to carry out more robust risk-hazard analyses,” Dr. DeCampli suggested.
Dr. DeCampli is a congenital heart surgeon at the department of clinical sciences, University of Central Florida, and the Heart Center at Arnold Palmer Hospital for Children, both in Orlando. He reported having no conflicts.
“The Toronto group has contributed significantly to our knowledge and management of pulmonary vein stenosis during the past decade. This article by Dr. Lo Rito and coworkers continues that contribution by reinforcing the values of MRI in imaging PVs before intervention and providing a valuable “hint” that preoperative PV size measurements are related to outcome,” Dr. William M. DeCampli wrote in his invited commentary (J Thorac Cardiovasc Surg. 2016;1510:667-8).
“The task of definitively demonstrating this relationship is daunting for any single institution, however, because 1) PVS is relatively rare, 2) MRI and computed tomography are relatively recently used diagnostic modalities, and 3) MRI is not easily used in an important subset of the cohort, small infants.” This limited the study to a small number of covariates,” noted Dr. DeCampli, and prevented the researchers from taking into account a myriad of additional covariates commonly associated with survival in complex congenital heart disease.
 |
Dr. William M. DeCampli |
Such covariates included in a sufficiently large model could significantly alter the observed odds ratios otherwise calculated for the included variables in this study, he added, citing a study of PVS by Boston Children’s Hospital (J Thorac Cardiovasc Surg. 2015;150:911-7), which found a different set of covariates associated with death; in that case, age younger than 6 months at operation, weight less than 3 kg at operation, and lesser preoperative right ventricular systolic pressure.
“The challenges in studying PVS encountered by these two high-volume, research-oriented programs leads us to suggest that PVS should be studied in a different way. Perhaps it is time to consider a multi-institutional, mixed or inception cohort registry for PVS. The spring 2015 Society of Thoracic Surgeons Congenital Heart Database report lists 506 cases of PVS repair as the primary procedure between January 2011 and December 2014. If a study were to enroll just one-third of these subjects it would accrue more than 40 subjects per year. Five years hence with an anticipated 50-80 events (deaths), it would be possible to carry out more robust risk-hazard analyses,” Dr. DeCampli suggested.
Dr. DeCampli is a congenital heart surgeon at the department of clinical sciences, University of Central Florida, and the Heart Center at Arnold Palmer Hospital for Children, both in Orlando. He reported having no conflicts.
A retrospective analysis of children who underwent pulmonary vein stenosis repair with preoperative computed tomography and magnetic resonance imaging from 1990 to 2012 showed that smaller upstream or downstream total cross-sectional area indexed (TCSAi) for body surface area led to poorer survival.
The study of 31 patients at a single institution also indicated that early survival seemed especially poor for patients with a greater number of stenotic veins and upstream pulmonary vein (PV) involvement. The study was published in the March issue of the Journal of Thoracic and Cardiovascular Surgery.
Dr. Mauro Lo Rito and his colleagues at The Hospital for Sick Children, Toronto, retrospectively assessed the 31 patients out of 145 who underwent surgical repair who had had preoperative CT and MRI imaging. Complete sutureless repair was done in 18 (58%), single-side sutureless repair in 12 (39%), and pericardial patch reconstruction in 1 (3%). The mean follow-up was 4.3 years; the median patient age at time of operation was 226 days. Stenosis was bilateral in 45% of patients and unilateral in 55 (J Thorac Cardiovasc Surg. 2016;151:657-66).
In-hospital mortality was 9.7%, with an overall survival of 75%, 69%, and 64% at 1, 3, and 5 years, respectively. Univariate analysis showed that a younger age at operation, lower body surface area, smaller upstream TCSAi, and greater number of PV with stenosis/occlusion were associated with an increased risk of death.
Multivariate analysis showed that smaller upstream TCSAi for body surface area (P = .030) and greater number of stenotic PVs (P = .007) were associated with poor early (less than 1 year) survival. There was a nonsignificant tendency for smaller downstream TCSAi to be associated with poor late survival (greater than 1 year). None of the different PV morphologies were found to influence survival, according to Dr. Lo Rito and his colleagues.
Among the 28 hospital survivors, restenosis occurred in 10 patients, 7 of whom did not undergo further surgery (3 of these were alive at last follow-up and 4 died secondary to disease progression). Of the 3 patients who underwent subsequent intervention, 2 were alive at last follow-up.
“Risk stratification for patients with PV stenosis is currently challenging because of the variability in the anatomic configuration and the unknown relationship between these anatomic variants and survival. Our study demonstrates that by using cross-sectional areas, pulmonary vein cross-sectional area indexed to body surface area (PVCSAi) and TCSAi and tabulating the number of stenotic PVs, we can identify high-risk subsets of patients with high predicted mortality.” Dr. Lo Rito and his colleagues stated.
“The upstream total cross-sectional area and the number of stenotic PVs influence early survival and can be used to guide counseling. Smaller downstream cross-sectional area influences late survival, and those patients should be monitored with close follow-up. This methodology could aid in risk stratification for future clinical trials of pharmacologic agents designed to target upstream pulmonary vasculopathy,” the investigators concluded.
The authors reported that they had no conflicts of interest.
A webcast of the original presentation of these results at the 95th American Association for Thoracic Surgery Annual Meeting is available online (http://webcast.aats.org/2015/Video/Tuesday/04-28-15_6A_1615_Lo_Rito.mp4).
A retrospective analysis of children who underwent pulmonary vein stenosis repair with preoperative computed tomography and magnetic resonance imaging from 1990 to 2012 showed that smaller upstream or downstream total cross-sectional area indexed (TCSAi) for body surface area led to poorer survival.
The study of 31 patients at a single institution also indicated that early survival seemed especially poor for patients with a greater number of stenotic veins and upstream pulmonary vein (PV) involvement. The study was published in the March issue of the Journal of Thoracic and Cardiovascular Surgery.
Dr. Mauro Lo Rito and his colleagues at The Hospital for Sick Children, Toronto, retrospectively assessed the 31 patients out of 145 who underwent surgical repair who had had preoperative CT and MRI imaging. Complete sutureless repair was done in 18 (58%), single-side sutureless repair in 12 (39%), and pericardial patch reconstruction in 1 (3%). The mean follow-up was 4.3 years; the median patient age at time of operation was 226 days. Stenosis was bilateral in 45% of patients and unilateral in 55 (J Thorac Cardiovasc Surg. 2016;151:657-66).
In-hospital mortality was 9.7%, with an overall survival of 75%, 69%, and 64% at 1, 3, and 5 years, respectively. Univariate analysis showed that a younger age at operation, lower body surface area, smaller upstream TCSAi, and greater number of PV with stenosis/occlusion were associated with an increased risk of death.
Multivariate analysis showed that smaller upstream TCSAi for body surface area (P = .030) and greater number of stenotic PVs (P = .007) were associated with poor early (less than 1 year) survival. There was a nonsignificant tendency for smaller downstream TCSAi to be associated with poor late survival (greater than 1 year). None of the different PV morphologies were found to influence survival, according to Dr. Lo Rito and his colleagues.
Among the 28 hospital survivors, restenosis occurred in 10 patients, 7 of whom did not undergo further surgery (3 of these were alive at last follow-up and 4 died secondary to disease progression). Of the 3 patients who underwent subsequent intervention, 2 were alive at last follow-up.
“Risk stratification for patients with PV stenosis is currently challenging because of the variability in the anatomic configuration and the unknown relationship between these anatomic variants and survival. Our study demonstrates that by using cross-sectional areas, pulmonary vein cross-sectional area indexed to body surface area (PVCSAi) and TCSAi and tabulating the number of stenotic PVs, we can identify high-risk subsets of patients with high predicted mortality.” Dr. Lo Rito and his colleagues stated.
“The upstream total cross-sectional area and the number of stenotic PVs influence early survival and can be used to guide counseling. Smaller downstream cross-sectional area influences late survival, and those patients should be monitored with close follow-up. This methodology could aid in risk stratification for future clinical trials of pharmacologic agents designed to target upstream pulmonary vasculopathy,” the investigators concluded.
The authors reported that they had no conflicts of interest.
A webcast of the original presentation of these results at the 95th American Association for Thoracic Surgery Annual Meeting is available online (http://webcast.aats.org/2015/Video/Tuesday/04-28-15_6A_1615_Lo_Rito.mp4).
FROM JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Survival after pulmonary vein stenosis repair was adversely affected by smaller upstream cross-sectional area indexed to body surface area.
Major finding: Smaller upstream total cross-sectional area indexed for body surface area (P = .30) and greater number of stenotic pulmonary veins (P = .007) were associated with increased early risk of death.
Data source: Researchers reviewed the outcomes of 31/145 patients who underwent surgical repair of pulmonary stenosis who had preoperative computed tomography and magnetic resonance imaging between 1990 and 2012.
Disclosures: The authors reported that they had no conflicts of interest.