User login
Later-life PTSD boosts vascular risk, study finds
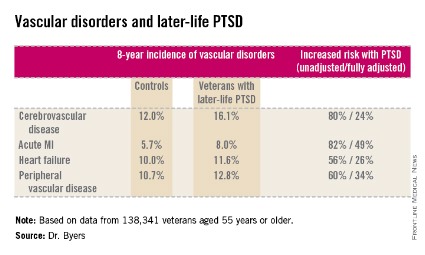
ORLANDO – Military veterans aged 55 years or older with current posttraumatic stress disorder are at significantly higher risk of developing new-onset vascular disease than are those without PTSD, according to a very large national longitudinal study.
"This study suggests the need for greater monitoring and treatment of PTSD in older veterans to assist in the prevention of vascular disorders," Amy L. Byers, Ph.D., said at the annual meeting of the American Association for Geriatric Psychiatry.
She reported on 138,341 veterans aged 55 years or older who were free of known vascular disease at baseline. During 8 years of follow-up, those with PTSD had significantly higher rates of incident cerebrovascular disease, acute MI, heart failure, and peripheral vascular disease than did those without PTSD, even after adjustment for demographics, comorbid diabetes, hypertension, cancer, chronic obstructive pulmonary disease, renal disease, traumatic brain injury, dementia, substance use disorders, and psychiatric diagnoses. The fully adjusted increased risk of each of the forms of vascular disease under study still remained significant at P less than .001, noted Dr. Byers, an epidemiologist in the psychiatry department at the University of California, San Francisco.
In a separate study led by Dr. Byers, PTSD in the general population with onset prior to and persistence beyond age 55 was a powerful independent predictor of global disability.
Dr. Byers’ study of older veterans was funded by the Department of Defense. She had no disclosures.
This paper continues to strengthen a link between PTSD and inflammatory markers. Dewleen Baker of the VA health care system in San Diego reported that there was a 10-fold increase in C-reactive protein (CRP) post deployment as compared with these same soldiers predeployment CRP levels. After adjustment for battlefield experience scores and combat exposures, those patients with PTSD symptoms had elevated CRP levels of 1.0 ng/mL versus 0.7 ng/mL without postdeployment symptoms (JAMA Psychiatry. 2014;71:423-31). So it seems that there may be a link between PTSD negative cardiovascular outcomes. And there may be a link between PTSD and elevated CRP. So, this leaves us with at least two questions: Is elevated CRP related to increased incidence of negative cardiovascular outcomes? And, which came first, the chicken (PTSD) or the egg (elevated CRP)?
Dr. Mark A. Adelman is chief of vascular and endovascular surgery at NYU Langone Medical Center, New York, and an associate medical editor for Vascular Specialist.
This paper continues to strengthen a link between PTSD and inflammatory markers. Dewleen Baker of the VA health care system in San Diego reported that there was a 10-fold increase in C-reactive protein (CRP) post deployment as compared with these same soldiers predeployment CRP levels. After adjustment for battlefield experience scores and combat exposures, those patients with PTSD symptoms had elevated CRP levels of 1.0 ng/mL versus 0.7 ng/mL without postdeployment symptoms (JAMA Psychiatry. 2014;71:423-31). So it seems that there may be a link between PTSD negative cardiovascular outcomes. And there may be a link between PTSD and elevated CRP. So, this leaves us with at least two questions: Is elevated CRP related to increased incidence of negative cardiovascular outcomes? And, which came first, the chicken (PTSD) or the egg (elevated CRP)?
Dr. Mark A. Adelman is chief of vascular and endovascular surgery at NYU Langone Medical Center, New York, and an associate medical editor for Vascular Specialist.
This paper continues to strengthen a link between PTSD and inflammatory markers. Dewleen Baker of the VA health care system in San Diego reported that there was a 10-fold increase in C-reactive protein (CRP) post deployment as compared with these same soldiers predeployment CRP levels. After adjustment for battlefield experience scores and combat exposures, those patients with PTSD symptoms had elevated CRP levels of 1.0 ng/mL versus 0.7 ng/mL without postdeployment symptoms (JAMA Psychiatry. 2014;71:423-31). So it seems that there may be a link between PTSD negative cardiovascular outcomes. And there may be a link between PTSD and elevated CRP. So, this leaves us with at least two questions: Is elevated CRP related to increased incidence of negative cardiovascular outcomes? And, which came first, the chicken (PTSD) or the egg (elevated CRP)?
Dr. Mark A. Adelman is chief of vascular and endovascular surgery at NYU Langone Medical Center, New York, and an associate medical editor for Vascular Specialist.
ORLANDO – Military veterans aged 55 years or older with current posttraumatic stress disorder are at significantly higher risk of developing new-onset vascular disease than are those without PTSD, according to a very large national longitudinal study.
"This study suggests the need for greater monitoring and treatment of PTSD in older veterans to assist in the prevention of vascular disorders," Amy L. Byers, Ph.D., said at the annual meeting of the American Association for Geriatric Psychiatry.
She reported on 138,341 veterans aged 55 years or older who were free of known vascular disease at baseline. During 8 years of follow-up, those with PTSD had significantly higher rates of incident cerebrovascular disease, acute MI, heart failure, and peripheral vascular disease than did those without PTSD, even after adjustment for demographics, comorbid diabetes, hypertension, cancer, chronic obstructive pulmonary disease, renal disease, traumatic brain injury, dementia, substance use disorders, and psychiatric diagnoses. The fully adjusted increased risk of each of the forms of vascular disease under study still remained significant at P less than .001, noted Dr. Byers, an epidemiologist in the psychiatry department at the University of California, San Francisco.
In a separate study led by Dr. Byers, PTSD in the general population with onset prior to and persistence beyond age 55 was a powerful independent predictor of global disability.
Dr. Byers’ study of older veterans was funded by the Department of Defense. She had no disclosures.
ORLANDO – Military veterans aged 55 years or older with current posttraumatic stress disorder are at significantly higher risk of developing new-onset vascular disease than are those without PTSD, according to a very large national longitudinal study.
"This study suggests the need for greater monitoring and treatment of PTSD in older veterans to assist in the prevention of vascular disorders," Amy L. Byers, Ph.D., said at the annual meeting of the American Association for Geriatric Psychiatry.
She reported on 138,341 veterans aged 55 years or older who were free of known vascular disease at baseline. During 8 years of follow-up, those with PTSD had significantly higher rates of incident cerebrovascular disease, acute MI, heart failure, and peripheral vascular disease than did those without PTSD, even after adjustment for demographics, comorbid diabetes, hypertension, cancer, chronic obstructive pulmonary disease, renal disease, traumatic brain injury, dementia, substance use disorders, and psychiatric diagnoses. The fully adjusted increased risk of each of the forms of vascular disease under study still remained significant at P less than .001, noted Dr. Byers, an epidemiologist in the psychiatry department at the University of California, San Francisco.
In a separate study led by Dr. Byers, PTSD in the general population with onset prior to and persistence beyond age 55 was a powerful independent predictor of global disability.
Dr. Byers’ study of older veterans was funded by the Department of Defense. She had no disclosures.
Major finding: Military veterans with late-life posttraumatic stress disorder were 80% more likely to develop new-onset cerebrovascular disease during 8 years of follow-up than were those without PTSD. They were also 82% more likely to have a first acute myocardial infarction, 56% more likely to develop heart failure, and 60% more likely to be diagnosed with peripheral vascular disease.
Data source: This was a longitudinal observational study in 138,341 veterans aged 55 years or older who were free of known vascular disease at baseline and were followed for 8 years.
Disclosures: Dr. Byers’ study of older veterans was funded by the Department of Defense. She reported having no financial conflicts.
CEA vs. stenting in the elderly: The debate continues
For elderly patients with carotid disease, carotid endarterectomy carries a lower risk of perioperative stroke or transient ischemic attack, the same risk of perioperative MI, and a slightly higher risk of perioperative death compared with carotid artery stenting, according to a meta-analysis.
However, the individual elderly patient’s vascular anatomy plays a crucial role in determining perioperative risk, as does his or her overall health and clinical profile.
"The results of [our] analysis suggest that careful consideration of a constellation of clinical and anatomic factors is required before an appropriate treatment of carotid disease in elderly patients is selected. The cardiovascular disease burden and general health of the individual patient should be meticulously evaluated before interventional instead of optimal medical treatment is applied," said Dr. George A. Antoniou of the department of vascular surgery, Hellenic Red Cross Hospital, Athens, and his associates.
Which treatment is the most appropriate for elderly patients with carotid disease is still much debated. Dr. Antoniou and his colleagues performed a comprehensive review of the medical literature since 1986 and a meta-analysis of 44 articles that directly compared outcomes in elderly patients with those of younger patients after carotid endarterectomy (39 studies) or carotid stenting (18 articles).
"Elderly" was defined as older than 80 years in most of these studies, and as older than 75 years in many, but there was great variability among the studies, and some even considered "older than 65 years" to be elderly.
Overall, the meta-analysis included 269,596 endarterectomies in elderly patients against 243,089 in younger patients, and 38,751 carotid stenting procedures in elderly patients against 36,450 in younger patients.
For endarterectomy, the rate of perioperative stroke was not significantly different between elderly (0.9%) and younger (1.2%) patients, nor was the rate of TIA (1.9% vs 1.8%, respectively). However, perioperative mortality was significantly higher in elderly (0.5%) than in younger (0.4%) patients.
In contrast, for carotid stenting, the rate of perioperative stroke was significantly higher for elderly patients (2.4%) than for younger patients (1.7%), as was the rate of TIA (3.6% vs 2.1%). And mortality was not significantly different between elderly patients (0.6%) and younger patients (0.7%), the researchers wrote (JAMA Surg. 2013 Oct. 23 [doi:10.1001/jamasurg.2013.4135]).
Both procedures were associated with an increased rate of perioperative MI in elderly patients, compared with younger patients. These rates were 2.2% in elderly patients, compared with 1.4% in younger patients undergoing endarterectomy; and 2.3% in elderly patients, compared with 1.5% in younger patients undergoing carotid stenting.
These findings remained robust in sensitivity analyses.
"It seems that endarterectomy is associated with improved neurologic outcomes compared with carotid stenting in elderly patients, at the expense of increased perioperative mortality." However, the small increase in mortality seen with endarterectomy – one-tenth of 1% – may not be clinically significant, Dr. Antoniou and his associates said.
Moreover, neurologic risk is closely tied to vascular anatomy. Elderly patients tend to have more unfavorable anatomy than do younger patients, but should be assessed on an individual basis. Unfavorable traits include heavily calcified and tortuous supra-aortic branches, as well as adverse morphology of the aortic arch such as elongation, distortion, and stenosis.
Manipulating the stenting instruments through such features may in itself raise the risk of neurologic sequelae. It also makes the procedure more technically difficult, which increases the risk of endothelial trauma, thrombus dislodgment, and thromboembolic events.
"In addition, elderly patients with significant extracranial atherosclerotic disease are likely to have a compromised cerebrovascular reserve, which makes them more susceptible to ischemic events from cerebral microembolization," the researchers said.
They reported having no conflicts.
This study’s conclusions are not surprising, given that most clinicians have already seen them both in randomized prospective studies and in their own practices, said Dr. R. Clement Darling III.
However, the variation in the definition of "elderly" among the trials in this meta-analysis is a real concern: 64% used 80 years as the cutoff, 31% used 75 years, and some used 70 or even 65 years as the cutoff.
"The bottom line is, carotid endarterectomy and carotid stenting seem to work equally well in younger patients, in expert hands. However, in the ‘elderly’ (at any age), endarterectomy has better outcomes with low morbidity, mortality, and stroke rate, and it remains the standard of care."
Dr. Darling of the Vascular Group, Albany, N.Y., made these remarks in an invited commentary (JAMA Surgery 2013 Oct. 23 [doi:10.1001/jamasurg.2013.4160]). He reported no financial conflicts of interest.
This study’s conclusions are not surprising, given that most clinicians have already seen them both in randomized prospective studies and in their own practices, said Dr. R. Clement Darling III.
However, the variation in the definition of "elderly" among the trials in this meta-analysis is a real concern: 64% used 80 years as the cutoff, 31% used 75 years, and some used 70 or even 65 years as the cutoff.
"The bottom line is, carotid endarterectomy and carotid stenting seem to work equally well in younger patients, in expert hands. However, in the ‘elderly’ (at any age), endarterectomy has better outcomes with low morbidity, mortality, and stroke rate, and it remains the standard of care."
Dr. Darling of the Vascular Group, Albany, N.Y., made these remarks in an invited commentary (JAMA Surgery 2013 Oct. 23 [doi:10.1001/jamasurg.2013.4160]). He reported no financial conflicts of interest.
This study’s conclusions are not surprising, given that most clinicians have already seen them both in randomized prospective studies and in their own practices, said Dr. R. Clement Darling III.
However, the variation in the definition of "elderly" among the trials in this meta-analysis is a real concern: 64% used 80 years as the cutoff, 31% used 75 years, and some used 70 or even 65 years as the cutoff.
"The bottom line is, carotid endarterectomy and carotid stenting seem to work equally well in younger patients, in expert hands. However, in the ‘elderly’ (at any age), endarterectomy has better outcomes with low morbidity, mortality, and stroke rate, and it remains the standard of care."
Dr. Darling of the Vascular Group, Albany, N.Y., made these remarks in an invited commentary (JAMA Surgery 2013 Oct. 23 [doi:10.1001/jamasurg.2013.4160]). He reported no financial conflicts of interest.
For elderly patients with carotid disease, carotid endarterectomy carries a lower risk of perioperative stroke or transient ischemic attack, the same risk of perioperative MI, and a slightly higher risk of perioperative death compared with carotid artery stenting, according to a meta-analysis.
However, the individual elderly patient’s vascular anatomy plays a crucial role in determining perioperative risk, as does his or her overall health and clinical profile.
"The results of [our] analysis suggest that careful consideration of a constellation of clinical and anatomic factors is required before an appropriate treatment of carotid disease in elderly patients is selected. The cardiovascular disease burden and general health of the individual patient should be meticulously evaluated before interventional instead of optimal medical treatment is applied," said Dr. George A. Antoniou of the department of vascular surgery, Hellenic Red Cross Hospital, Athens, and his associates.
Which treatment is the most appropriate for elderly patients with carotid disease is still much debated. Dr. Antoniou and his colleagues performed a comprehensive review of the medical literature since 1986 and a meta-analysis of 44 articles that directly compared outcomes in elderly patients with those of younger patients after carotid endarterectomy (39 studies) or carotid stenting (18 articles).
"Elderly" was defined as older than 80 years in most of these studies, and as older than 75 years in many, but there was great variability among the studies, and some even considered "older than 65 years" to be elderly.
Overall, the meta-analysis included 269,596 endarterectomies in elderly patients against 243,089 in younger patients, and 38,751 carotid stenting procedures in elderly patients against 36,450 in younger patients.
For endarterectomy, the rate of perioperative stroke was not significantly different between elderly (0.9%) and younger (1.2%) patients, nor was the rate of TIA (1.9% vs 1.8%, respectively). However, perioperative mortality was significantly higher in elderly (0.5%) than in younger (0.4%) patients.
In contrast, for carotid stenting, the rate of perioperative stroke was significantly higher for elderly patients (2.4%) than for younger patients (1.7%), as was the rate of TIA (3.6% vs 2.1%). And mortality was not significantly different between elderly patients (0.6%) and younger patients (0.7%), the researchers wrote (JAMA Surg. 2013 Oct. 23 [doi:10.1001/jamasurg.2013.4135]).
Both procedures were associated with an increased rate of perioperative MI in elderly patients, compared with younger patients. These rates were 2.2% in elderly patients, compared with 1.4% in younger patients undergoing endarterectomy; and 2.3% in elderly patients, compared with 1.5% in younger patients undergoing carotid stenting.
These findings remained robust in sensitivity analyses.
"It seems that endarterectomy is associated with improved neurologic outcomes compared with carotid stenting in elderly patients, at the expense of increased perioperative mortality." However, the small increase in mortality seen with endarterectomy – one-tenth of 1% – may not be clinically significant, Dr. Antoniou and his associates said.
Moreover, neurologic risk is closely tied to vascular anatomy. Elderly patients tend to have more unfavorable anatomy than do younger patients, but should be assessed on an individual basis. Unfavorable traits include heavily calcified and tortuous supra-aortic branches, as well as adverse morphology of the aortic arch such as elongation, distortion, and stenosis.
Manipulating the stenting instruments through such features may in itself raise the risk of neurologic sequelae. It also makes the procedure more technically difficult, which increases the risk of endothelial trauma, thrombus dislodgment, and thromboembolic events.
"In addition, elderly patients with significant extracranial atherosclerotic disease are likely to have a compromised cerebrovascular reserve, which makes them more susceptible to ischemic events from cerebral microembolization," the researchers said.
They reported having no conflicts.
For elderly patients with carotid disease, carotid endarterectomy carries a lower risk of perioperative stroke or transient ischemic attack, the same risk of perioperative MI, and a slightly higher risk of perioperative death compared with carotid artery stenting, according to a meta-analysis.
However, the individual elderly patient’s vascular anatomy plays a crucial role in determining perioperative risk, as does his or her overall health and clinical profile.
"The results of [our] analysis suggest that careful consideration of a constellation of clinical and anatomic factors is required before an appropriate treatment of carotid disease in elderly patients is selected. The cardiovascular disease burden and general health of the individual patient should be meticulously evaluated before interventional instead of optimal medical treatment is applied," said Dr. George A. Antoniou of the department of vascular surgery, Hellenic Red Cross Hospital, Athens, and his associates.
Which treatment is the most appropriate for elderly patients with carotid disease is still much debated. Dr. Antoniou and his colleagues performed a comprehensive review of the medical literature since 1986 and a meta-analysis of 44 articles that directly compared outcomes in elderly patients with those of younger patients after carotid endarterectomy (39 studies) or carotid stenting (18 articles).
"Elderly" was defined as older than 80 years in most of these studies, and as older than 75 years in many, but there was great variability among the studies, and some even considered "older than 65 years" to be elderly.
Overall, the meta-analysis included 269,596 endarterectomies in elderly patients against 243,089 in younger patients, and 38,751 carotid stenting procedures in elderly patients against 36,450 in younger patients.
For endarterectomy, the rate of perioperative stroke was not significantly different between elderly (0.9%) and younger (1.2%) patients, nor was the rate of TIA (1.9% vs 1.8%, respectively). However, perioperative mortality was significantly higher in elderly (0.5%) than in younger (0.4%) patients.
In contrast, for carotid stenting, the rate of perioperative stroke was significantly higher for elderly patients (2.4%) than for younger patients (1.7%), as was the rate of TIA (3.6% vs 2.1%). And mortality was not significantly different between elderly patients (0.6%) and younger patients (0.7%), the researchers wrote (JAMA Surg. 2013 Oct. 23 [doi:10.1001/jamasurg.2013.4135]).
Both procedures were associated with an increased rate of perioperative MI in elderly patients, compared with younger patients. These rates were 2.2% in elderly patients, compared with 1.4% in younger patients undergoing endarterectomy; and 2.3% in elderly patients, compared with 1.5% in younger patients undergoing carotid stenting.
These findings remained robust in sensitivity analyses.
"It seems that endarterectomy is associated with improved neurologic outcomes compared with carotid stenting in elderly patients, at the expense of increased perioperative mortality." However, the small increase in mortality seen with endarterectomy – one-tenth of 1% – may not be clinically significant, Dr. Antoniou and his associates said.
Moreover, neurologic risk is closely tied to vascular anatomy. Elderly patients tend to have more unfavorable anatomy than do younger patients, but should be assessed on an individual basis. Unfavorable traits include heavily calcified and tortuous supra-aortic branches, as well as adverse morphology of the aortic arch such as elongation, distortion, and stenosis.
Manipulating the stenting instruments through such features may in itself raise the risk of neurologic sequelae. It also makes the procedure more technically difficult, which increases the risk of endothelial trauma, thrombus dislodgment, and thromboembolic events.
"In addition, elderly patients with significant extracranial atherosclerotic disease are likely to have a compromised cerebrovascular reserve, which makes them more susceptible to ischemic events from cerebral microembolization," the researchers said.
They reported having no conflicts.
No sex differences in carotid revascularization outcomes?
SAN FRANCISCO – There were no significant differences in endpoints between men and women after carotid endarterectomy or carotid artery stenting, results from a large registry study showed.
"These data suggest that, contrary to previous reports, women do not have a higher risk of adverse events after carotid revascularization," Dr. Jeffrey Jim said at the Society for Vascular Surgery Annual Meeting. "As such, women may derive similar benefits as men from carotid revascularization."
Carotid endarterectomy is considered by many as the gold standard treatment option for patients with severe internal carotid artery stenosis, said Dr. Jim, a vascular surgeon at Washington University School of Medicine, St. Louis. "Its benefit over best medical therapy has been proven by several landmark randomized controlled trials," he said. "While the efficacy of carotid artery stenting compared with carotid endarterectomy remains highly debated, there is clear utility in patients with select high risk criteria. However, it’s important to remember that gender plays an important role in cardiovascular disease. Epidemiologic studies clearly show that males have a higher stroke incidence as well as prevalence rate compared with women. However, when strokes do happen in women they tend to be more severe."
In terms of revascularization, he continued, available data suggested that women have a higher risk of perioperative adverse events compared with men, "suggesting that they may not benefit as much from revascularization compared with men."
He and other members of the SVS Outcomes Committee set out to evaluate the impact of gender on the outcomes after carotid revascularization (CAE and CAS), with the primary endpoint being composite risk of death, stroke, or MI at 30 days. They used data from 9,865 patients in the SVS Vascular Registry, which was developed in 2005 as a response to CMS approval of CAS. The registry was available to all clinical facilities and individual providers. "There are no specific inclusion or exclusion criteria because it aims to capture real-world results," Dr. Jim said. The registry is closed "but it remains one of the largest databases on carotid revascularization in the country."
Of the 9,865 patients 59% were men. There was no difference in age between sexes (both had a mean age of 71 years), but men were more likely to be symptomatic compared with women (42% vs. 39%, respectively).
For disease etiology in CAS, restenosis was higher in women compared with men (29% vs. 20%), while a greater proportion of men were being treated with radiation compared with women (6.2% vs. 2.6%). For CEA, more men were symptomatic compared with women (39% vs. 36%). "This was primarily driven by the fact that slightly more men than women had a stroke in the past (22% vs. 19%)," he said.
"Among patients overall, men had a slightly higher prevalence of coronary artery disease as well as MI, while women had a higher prevalence of hypertension as well as COPD," Dr. Jim said.
The researchers found no statistically significant differences in the composite endpoint of death, stroke, and MI at 30 days between men and women for either CEA (4.06% vs. 4.07%, respectively) or CAS (6.80% vs. 6.69%). The findings remained similar even after stratification by symptomatology and multivariate risk adjustment.
"In all the different ways we looked at it men and women had similar outcomes," Dr. Jim said. "This data is important. It’s representative of real-world outcomes, but there are limitations. It was an observational study done in a retrospective manner, and there is the potential for reporting bias. The most important limitation is that there is no comparison group of patients treated with best medical therapy. That’s an investigation that needs to be done going forward."
Dr. Jim said that he had no relevant financial conflicts to disclose.
SAN FRANCISCO – There were no significant differences in endpoints between men and women after carotid endarterectomy or carotid artery stenting, results from a large registry study showed.
"These data suggest that, contrary to previous reports, women do not have a higher risk of adverse events after carotid revascularization," Dr. Jeffrey Jim said at the Society for Vascular Surgery Annual Meeting. "As such, women may derive similar benefits as men from carotid revascularization."
Carotid endarterectomy is considered by many as the gold standard treatment option for patients with severe internal carotid artery stenosis, said Dr. Jim, a vascular surgeon at Washington University School of Medicine, St. Louis. "Its benefit over best medical therapy has been proven by several landmark randomized controlled trials," he said. "While the efficacy of carotid artery stenting compared with carotid endarterectomy remains highly debated, there is clear utility in patients with select high risk criteria. However, it’s important to remember that gender plays an important role in cardiovascular disease. Epidemiologic studies clearly show that males have a higher stroke incidence as well as prevalence rate compared with women. However, when strokes do happen in women they tend to be more severe."
In terms of revascularization, he continued, available data suggested that women have a higher risk of perioperative adverse events compared with men, "suggesting that they may not benefit as much from revascularization compared with men."
He and other members of the SVS Outcomes Committee set out to evaluate the impact of gender on the outcomes after carotid revascularization (CAE and CAS), with the primary endpoint being composite risk of death, stroke, or MI at 30 days. They used data from 9,865 patients in the SVS Vascular Registry, which was developed in 2005 as a response to CMS approval of CAS. The registry was available to all clinical facilities and individual providers. "There are no specific inclusion or exclusion criteria because it aims to capture real-world results," Dr. Jim said. The registry is closed "but it remains one of the largest databases on carotid revascularization in the country."
Of the 9,865 patients 59% were men. There was no difference in age between sexes (both had a mean age of 71 years), but men were more likely to be symptomatic compared with women (42% vs. 39%, respectively).
For disease etiology in CAS, restenosis was higher in women compared with men (29% vs. 20%), while a greater proportion of men were being treated with radiation compared with women (6.2% vs. 2.6%). For CEA, more men were symptomatic compared with women (39% vs. 36%). "This was primarily driven by the fact that slightly more men than women had a stroke in the past (22% vs. 19%)," he said.
"Among patients overall, men had a slightly higher prevalence of coronary artery disease as well as MI, while women had a higher prevalence of hypertension as well as COPD," Dr. Jim said.
The researchers found no statistically significant differences in the composite endpoint of death, stroke, and MI at 30 days between men and women for either CEA (4.06% vs. 4.07%, respectively) or CAS (6.80% vs. 6.69%). The findings remained similar even after stratification by symptomatology and multivariate risk adjustment.
"In all the different ways we looked at it men and women had similar outcomes," Dr. Jim said. "This data is important. It’s representative of real-world outcomes, but there are limitations. It was an observational study done in a retrospective manner, and there is the potential for reporting bias. The most important limitation is that there is no comparison group of patients treated with best medical therapy. That’s an investigation that needs to be done going forward."
Dr. Jim said that he had no relevant financial conflicts to disclose.
SAN FRANCISCO – There were no significant differences in endpoints between men and women after carotid endarterectomy or carotid artery stenting, results from a large registry study showed.
"These data suggest that, contrary to previous reports, women do not have a higher risk of adverse events after carotid revascularization," Dr. Jeffrey Jim said at the Society for Vascular Surgery Annual Meeting. "As such, women may derive similar benefits as men from carotid revascularization."
Carotid endarterectomy is considered by many as the gold standard treatment option for patients with severe internal carotid artery stenosis, said Dr. Jim, a vascular surgeon at Washington University School of Medicine, St. Louis. "Its benefit over best medical therapy has been proven by several landmark randomized controlled trials," he said. "While the efficacy of carotid artery stenting compared with carotid endarterectomy remains highly debated, there is clear utility in patients with select high risk criteria. However, it’s important to remember that gender plays an important role in cardiovascular disease. Epidemiologic studies clearly show that males have a higher stroke incidence as well as prevalence rate compared with women. However, when strokes do happen in women they tend to be more severe."
In terms of revascularization, he continued, available data suggested that women have a higher risk of perioperative adverse events compared with men, "suggesting that they may not benefit as much from revascularization compared with men."
He and other members of the SVS Outcomes Committee set out to evaluate the impact of gender on the outcomes after carotid revascularization (CAE and CAS), with the primary endpoint being composite risk of death, stroke, or MI at 30 days. They used data from 9,865 patients in the SVS Vascular Registry, which was developed in 2005 as a response to CMS approval of CAS. The registry was available to all clinical facilities and individual providers. "There are no specific inclusion or exclusion criteria because it aims to capture real-world results," Dr. Jim said. The registry is closed "but it remains one of the largest databases on carotid revascularization in the country."
Of the 9,865 patients 59% were men. There was no difference in age between sexes (both had a mean age of 71 years), but men were more likely to be symptomatic compared with women (42% vs. 39%, respectively).
For disease etiology in CAS, restenosis was higher in women compared with men (29% vs. 20%), while a greater proportion of men were being treated with radiation compared with women (6.2% vs. 2.6%). For CEA, more men were symptomatic compared with women (39% vs. 36%). "This was primarily driven by the fact that slightly more men than women had a stroke in the past (22% vs. 19%)," he said.
"Among patients overall, men had a slightly higher prevalence of coronary artery disease as well as MI, while women had a higher prevalence of hypertension as well as COPD," Dr. Jim said.
The researchers found no statistically significant differences in the composite endpoint of death, stroke, and MI at 30 days between men and women for either CEA (4.06% vs. 4.07%, respectively) or CAS (6.80% vs. 6.69%). The findings remained similar even after stratification by symptomatology and multivariate risk adjustment.
"In all the different ways we looked at it men and women had similar outcomes," Dr. Jim said. "This data is important. It’s representative of real-world outcomes, but there are limitations. It was an observational study done in a retrospective manner, and there is the potential for reporting bias. The most important limitation is that there is no comparison group of patients treated with best medical therapy. That’s an investigation that needs to be done going forward."
Dr. Jim said that he had no relevant financial conflicts to disclose.
Local anesthesia aids hemodynamic stability for CEA
CHICAGO – Patients undergoing carotid endarterectomy with cervical block anesthesia had fewer hemodynamic fluctuations and required less vasoactive medications than those under general anesthesia in a retrospective evaluation.
"Under cervical block anesthesia, carotid endarterectomy can be performed with a better hemodynamic profile," Dr. Marika Y. Gassner, a resident with Henry Ford Macomb Hospital, Clinton Township, Mich., said at the annual meeting of the Midwestern Vascular Surgical Society.
The practice switched in 2003 from using general anesthesia for the majority of carotid endarterectomy to performing more than 90% of cases under local cervical block anesthesia (CBA). Exceptions include patients who are extremely nervous, unable to communicate in English, or who have plaque extending above C2.
The investigators organized the retrospective cohort study after initial observations suggested patients under CBA had less intraoperative hypotension or fluctuations in mean arterial pressure below 65 mm Hg. Vasoactive therapy demands were also lower. For example, anesthesia records showed that several doses of beta-blockers and ephedrine were required for a patient under general anesthesia, while a patient under CBA had only a single dose of midazolam (Versed) early in the procedure, she said.
Other advantages of CBA include continuous feedback on neurologic status/cerebral perfusion, endotracheal intubation not required, shorter operative times, and reduced use of shunts, Dr. Gassner said.
The analysis included 651 patients who underwent carotid endarterectomy by a single surgeon at two suburban teaching hospitals, with 397 under general anesthesia (GA) and 254 under CBA.
The CBA and GA groups were similar in age (71.26 vs. 70.97 years) and incidence of coronary artery disease (57% vs. 56%), hypertension (77% vs. 75%), and renal failure (3.5% vs. 4.0%). The GA group, however, had significantly more females (39% vs. 46.6%), and a higher incidence of chronic obstructive pulmonary disease (16% vs. 23%), nicotine abuse, (50% vs. 63%), and symptomatic patients (41.3% vs. 54%).
The incidence of intraoperative hypotension (systolic BP less than 100 mm HG) was 0.52% with CBA and 17.84% with GA (P less than .001), Dr. Gassner said.
Mean arterial pressure changes of 20% or more per patient occurred in 20% and 41%, respectively (P less than .001).
Vasopressors were required during surgery in 51.13% of the GA group and 36.22% of the CBA group (P = .0002), and antihypertensive medications in 64% and 73.6% (P = .0085). Drugs from both categories were required by significantly fewer CBA patients (22.5% vs. 33.75%; P = .045), she said.
There were no deaths in either group. Postoperative complications were higher in the GA than the CBA group including myocardial infarction (4 vs. 0 events), stroke (6 vs. 0 events), hematoma (7 vs. 2 events), and return to the OR (7 vs. 0 events). The difference did not reach statistical significance because of the sample size, Dr. Gassner said.
Earlier in the presentation, she observed that there was no difference in the primary composite endpoint of stroke, myocardial infarction, or death at 30 days in the randomized GALA (general anaesthesia vs. local anaesthesia for carotid surgery) trial conducted at 95 centers in 24 countries (Lancet 2008;372:2132-42).
"If they couldn’t find it [a survival advantage] in GALA with 3,500 patients, we couldn’t find it here," Dr. Gassner said, adding that a randomized trial powered to look at late mortality is needed.
The group has no current plans to conduct such a study or a cost analysis, although a subsequent analysis of the GALA data revealed a cost savings of about $283 favoring carotid endarterectomy using local anesthesia (Br. J. Surg. 2010;97:1218-25).
Dr. Gassner and her coauthors reported having no financial disclosures.
CHICAGO – Patients undergoing carotid endarterectomy with cervical block anesthesia had fewer hemodynamic fluctuations and required less vasoactive medications than those under general anesthesia in a retrospective evaluation.
"Under cervical block anesthesia, carotid endarterectomy can be performed with a better hemodynamic profile," Dr. Marika Y. Gassner, a resident with Henry Ford Macomb Hospital, Clinton Township, Mich., said at the annual meeting of the Midwestern Vascular Surgical Society.
The practice switched in 2003 from using general anesthesia for the majority of carotid endarterectomy to performing more than 90% of cases under local cervical block anesthesia (CBA). Exceptions include patients who are extremely nervous, unable to communicate in English, or who have plaque extending above C2.
The investigators organized the retrospective cohort study after initial observations suggested patients under CBA had less intraoperative hypotension or fluctuations in mean arterial pressure below 65 mm Hg. Vasoactive therapy demands were also lower. For example, anesthesia records showed that several doses of beta-blockers and ephedrine were required for a patient under general anesthesia, while a patient under CBA had only a single dose of midazolam (Versed) early in the procedure, she said.
Other advantages of CBA include continuous feedback on neurologic status/cerebral perfusion, endotracheal intubation not required, shorter operative times, and reduced use of shunts, Dr. Gassner said.
The analysis included 651 patients who underwent carotid endarterectomy by a single surgeon at two suburban teaching hospitals, with 397 under general anesthesia (GA) and 254 under CBA.
The CBA and GA groups were similar in age (71.26 vs. 70.97 years) and incidence of coronary artery disease (57% vs. 56%), hypertension (77% vs. 75%), and renal failure (3.5% vs. 4.0%). The GA group, however, had significantly more females (39% vs. 46.6%), and a higher incidence of chronic obstructive pulmonary disease (16% vs. 23%), nicotine abuse, (50% vs. 63%), and symptomatic patients (41.3% vs. 54%).
The incidence of intraoperative hypotension (systolic BP less than 100 mm HG) was 0.52% with CBA and 17.84% with GA (P less than .001), Dr. Gassner said.
Mean arterial pressure changes of 20% or more per patient occurred in 20% and 41%, respectively (P less than .001).
Vasopressors were required during surgery in 51.13% of the GA group and 36.22% of the CBA group (P = .0002), and antihypertensive medications in 64% and 73.6% (P = .0085). Drugs from both categories were required by significantly fewer CBA patients (22.5% vs. 33.75%; P = .045), she said.
There were no deaths in either group. Postoperative complications were higher in the GA than the CBA group including myocardial infarction (4 vs. 0 events), stroke (6 vs. 0 events), hematoma (7 vs. 2 events), and return to the OR (7 vs. 0 events). The difference did not reach statistical significance because of the sample size, Dr. Gassner said.
Earlier in the presentation, she observed that there was no difference in the primary composite endpoint of stroke, myocardial infarction, or death at 30 days in the randomized GALA (general anaesthesia vs. local anaesthesia for carotid surgery) trial conducted at 95 centers in 24 countries (Lancet 2008;372:2132-42).
"If they couldn’t find it [a survival advantage] in GALA with 3,500 patients, we couldn’t find it here," Dr. Gassner said, adding that a randomized trial powered to look at late mortality is needed.
The group has no current plans to conduct such a study or a cost analysis, although a subsequent analysis of the GALA data revealed a cost savings of about $283 favoring carotid endarterectomy using local anesthesia (Br. J. Surg. 2010;97:1218-25).
Dr. Gassner and her coauthors reported having no financial disclosures.
CHICAGO – Patients undergoing carotid endarterectomy with cervical block anesthesia had fewer hemodynamic fluctuations and required less vasoactive medications than those under general anesthesia in a retrospective evaluation.
"Under cervical block anesthesia, carotid endarterectomy can be performed with a better hemodynamic profile," Dr. Marika Y. Gassner, a resident with Henry Ford Macomb Hospital, Clinton Township, Mich., said at the annual meeting of the Midwestern Vascular Surgical Society.
The practice switched in 2003 from using general anesthesia for the majority of carotid endarterectomy to performing more than 90% of cases under local cervical block anesthesia (CBA). Exceptions include patients who are extremely nervous, unable to communicate in English, or who have plaque extending above C2.
The investigators organized the retrospective cohort study after initial observations suggested patients under CBA had less intraoperative hypotension or fluctuations in mean arterial pressure below 65 mm Hg. Vasoactive therapy demands were also lower. For example, anesthesia records showed that several doses of beta-blockers and ephedrine were required for a patient under general anesthesia, while a patient under CBA had only a single dose of midazolam (Versed) early in the procedure, she said.
Other advantages of CBA include continuous feedback on neurologic status/cerebral perfusion, endotracheal intubation not required, shorter operative times, and reduced use of shunts, Dr. Gassner said.
The analysis included 651 patients who underwent carotid endarterectomy by a single surgeon at two suburban teaching hospitals, with 397 under general anesthesia (GA) and 254 under CBA.
The CBA and GA groups were similar in age (71.26 vs. 70.97 years) and incidence of coronary artery disease (57% vs. 56%), hypertension (77% vs. 75%), and renal failure (3.5% vs. 4.0%). The GA group, however, had significantly more females (39% vs. 46.6%), and a higher incidence of chronic obstructive pulmonary disease (16% vs. 23%), nicotine abuse, (50% vs. 63%), and symptomatic patients (41.3% vs. 54%).
The incidence of intraoperative hypotension (systolic BP less than 100 mm HG) was 0.52% with CBA and 17.84% with GA (P less than .001), Dr. Gassner said.
Mean arterial pressure changes of 20% or more per patient occurred in 20% and 41%, respectively (P less than .001).
Vasopressors were required during surgery in 51.13% of the GA group and 36.22% of the CBA group (P = .0002), and antihypertensive medications in 64% and 73.6% (P = .0085). Drugs from both categories were required by significantly fewer CBA patients (22.5% vs. 33.75%; P = .045), she said.
There were no deaths in either group. Postoperative complications were higher in the GA than the CBA group including myocardial infarction (4 vs. 0 events), stroke (6 vs. 0 events), hematoma (7 vs. 2 events), and return to the OR (7 vs. 0 events). The difference did not reach statistical significance because of the sample size, Dr. Gassner said.
Earlier in the presentation, she observed that there was no difference in the primary composite endpoint of stroke, myocardial infarction, or death at 30 days in the randomized GALA (general anaesthesia vs. local anaesthesia for carotid surgery) trial conducted at 95 centers in 24 countries (Lancet 2008;372:2132-42).
"If they couldn’t find it [a survival advantage] in GALA with 3,500 patients, we couldn’t find it here," Dr. Gassner said, adding that a randomized trial powered to look at late mortality is needed.
The group has no current plans to conduct such a study or a cost analysis, although a subsequent analysis of the GALA data revealed a cost savings of about $283 favoring carotid endarterectomy using local anesthesia (Br. J. Surg. 2010;97:1218-25).
Dr. Gassner and her coauthors reported having no financial disclosures.
Study IDs predictors of unplanned hospital readmission after CEA
SAN FRANCISCO – The 30-day unplanned readmission rate following carotid endarterectomy was 6.5% in a single-center study.
In addition, four variables were significantly associated with unplanned readmission: in-hospital postoperative congestive heart failure (CHF) exacerbation; in-hospital postoperative stroke; in-hospital postoperative hematoma; and prior coronary artery bypass graft (CABG).
"Whether these complications are completely avoidable is unknown, but we do identify a group of patients who would probably benefit from more comprehensive discharge planning and careful postdischarge care," Dr. Karen J. Ho said at the Society for Vascular Surgery annual meeting earlier this year.
According to a study of Medicare claims data from 2003 to 2004, 20% of Medicare beneficiaries discharged from a hospital were rehospitalized within 30 days (N. Eng. J. Med. 2009;360:1418-28). The 30-day rehospitalization rate after vascular surgery was 24%, "the highest of all surgical specialties examined in the study," said Dr. Ho of the surgery department at Brigham and Women’s Hospital, Boston, who was not involved with the published study. "Medicare has started to decrease reimbursements for hospitals with excess readmissions after acute MI, heart failure, and pneumonia. Hip and knee replacements and chronic obstructive pulmonary disease will be added in 2014, and we anticipate that additional surgical procedures will be added thereafter," she said.
In an effort to determine the rate of 30-day unplanned readmission after carotid endarterectomy (CEA), Dr. Ho and her associates conducted a retrospective study of a prospectively collected vascular surgery database at Brigham and Women’s Hospital. The cohort included 896 consecutive CEAs performed between 2002 and 2011. Combined CABG/CEA procedures were excluded.
The primary endpoint was unplanned readmission within 30 days, defined as "any unanticipated, nonelective hospital readmission," she said. The secondary endpoint was 1-year survival.
The mean age of the patients was 70 years, 60% were male, and 95% were white. More than half (65%) had asymptomatic evidence of carotid artery disease.
Dr. Ho reported that the median postoperative length of stay was 1 day and that 9.9% of patients had at least one in-hospital complication. The most frequent in-hospital complication was bleeding/hematoma (4.1%), followed by arrhythmia (2.1%), dysphagia (1.7%), stroke (1.3%), and myocardial infarction (1.2%). Only 3% of patients required a reoperation, while most (94%) were discharged to home. The 30-day stroke rate was 1.7%, while the 30-day death rate was 0.6%.
The overall 30-day readmission rate was 8.6%, while the unplanned 30-day readmission rate was 6.5%. "Most of the overall readmissions (80%) occurred in the first 10 days, and the median time to unplanned readmission was 4 days," Dr. Ho said.
The most common reason for an unplanned readmission was a cardiac complication, followed by headache, bleeding/hematoma, stroke/transient ischemic attack/intracerebral hemorrhage, or other medical emergency. More than one-quarter of patients (27.5%) had more than one reason for an unplanned readmission, while 87.9% of patients had a CEA-related unplanned readmission.
When the researchers performed a univariate analysis followed by analysis with a multivariable Cox model for unplanned readmission, four variables were independently associated with unplanned readmission: in-hospital postoperative CHF exacerbation (hazard ratio, 15.1), in-hospital postoperative stroke (HR, 5.0), in-hospital postoperative hematoma (HR, 3.1), and prior CABG (HR, 2.0).
They also observed a significant difference in survival at 1 year between patients who had an unplanned readmission and those who did not (91% vs. 96%, respectively; P less than .01.) "It’s unclear whether these deaths in the unplanned readmission group were preventable or if they were related to carotid disease or to a procedure-related complication," Dr. Ho said. "Our guess is that the increased overall burden of comorbid disease in these patients, rather than the readmission itself, predicted decreased survival."
Limitations of the study included its retrospective design and the fact that it was conducted at a single center, she said, "but we do know that our unplanned readmission rate is comparable to estimates from recent Medicare data."
Dr. Ho said she had no relevant financial disclosures.
Over the past several years, the role of carotid endarterectomy (CEA) for asymptomatic carotid stenosis has, again, come under the microscope; with many proponents still advocating CEA as the treatment of choice for asymptomatic patients with greater than or equal to 60% stenosis, while some propose greater than or equal to 70-80% stenosis in good surgical risk patients. Meanwhile, others oppose this philosophy because of the advances in modern medical therapy for patients with atherosclerosis, in general, with emphasis on risk modification. The findings of this article are quite disturbing, since the authors concluded that the 30-day unplanned re-admission rate after CEA was 6.5%; this is especially surprising to me, coming from this institution.
| Dr. AbuRahma |
The authors also concluded that unplanned re-admission rate was influenced by congestive heart failure, in-hospital postoperative stroke, in-hospital postoperative hematoma, and prior coronary artery bypass grafting. This emphasizes the importance of selection, selection, selection for asymptomatic carotid artery stenosis, if the outcome is to be acceptable to those who still advocate carotid endarterectomy for asymptomatic carotid disease.
Perhaps this procedure should not be encouraged for patients with congestive heart failure or those with severe coronary artery disease, unstable angina. Today, Level I evidence still supports carotid endarterectomy for patients with severe carotid artery stenosis, provided the patient is a good surgical risk, with relatively good longevity; with perioperative stroke and/or death rates of less than 3%. Several modern clinical series have concluded that CEA can be done in these patients with a stroke and/or death rate of less than 1-2%, which was produced most recently in the CREST trial. For those clinicians who cannot keep these numbers down, perhaps this procedure should not be done for asymptomatic carotid disease. What’s also surprising to me, is the in-hospital postoperative hematomas, which I presume necessitated the re-admission and, perhaps, reoperation. This should highlight the fact that, perhaps, we need to look further as to whether or not these patients should be on a combined regimen of aspirin and Plavix, preoperatively and postoperatively, as prescribed by many clinicians.
There is no Level I evidence to support that the combination of aspirin and Plavix, postoperatvely, for these patients would yield a better outcome than simple aspirin daily. It is difficult to determine from this study whether a significant portion of their patients were on dual antiplatelet therapy.
It is also interesting to notice that the authors found that almost 10% of patients had at least one in-hospital complication; some of which were major complications, e.g. stroke, MI, and dysphagia. Including bleeding/hematoma in these complications, which may not have necessitated surgery, may have inflated this number. A similar observation can be made regarding postoperative arrhythmias, particularly if they did not necessitate extra therapy. However, the fact of the matter is that it should be emphasized that the selection of patients for carotid endarterectomy in asymptomatic patients is extremely critical if this procedure is to be continued or blessed.
Dr. Ali F. AbuRahma is Professor of Surgery and Chief, Vascular & Endovascular Surgery, and Director, Vascular Surgery Fellowship and Residency Programs, and Medical Director, Vascular Laboratory, Co-Director, Vascular Center of Excellence, Robert C. Byrd Health Sciences Center, West Virginia University, Charleston Area Medical Center, Charleston. He is a also an associate medical editor of Vascular Specialist.
Over the past several years, the role of carotid endarterectomy (CEA) for asymptomatic carotid stenosis has, again, come under the microscope; with many proponents still advocating CEA as the treatment of choice for asymptomatic patients with greater than or equal to 60% stenosis, while some propose greater than or equal to 70-80% stenosis in good surgical risk patients. Meanwhile, others oppose this philosophy because of the advances in modern medical therapy for patients with atherosclerosis, in general, with emphasis on risk modification. The findings of this article are quite disturbing, since the authors concluded that the 30-day unplanned re-admission rate after CEA was 6.5%; this is especially surprising to me, coming from this institution.
| Dr. AbuRahma |
The authors also concluded that unplanned re-admission rate was influenced by congestive heart failure, in-hospital postoperative stroke, in-hospital postoperative hematoma, and prior coronary artery bypass grafting. This emphasizes the importance of selection, selection, selection for asymptomatic carotid artery stenosis, if the outcome is to be acceptable to those who still advocate carotid endarterectomy for asymptomatic carotid disease.
Perhaps this procedure should not be encouraged for patients with congestive heart failure or those with severe coronary artery disease, unstable angina. Today, Level I evidence still supports carotid endarterectomy for patients with severe carotid artery stenosis, provided the patient is a good surgical risk, with relatively good longevity; with perioperative stroke and/or death rates of less than 3%. Several modern clinical series have concluded that CEA can be done in these patients with a stroke and/or death rate of less than 1-2%, which was produced most recently in the CREST trial. For those clinicians who cannot keep these numbers down, perhaps this procedure should not be done for asymptomatic carotid disease. What’s also surprising to me, is the in-hospital postoperative hematomas, which I presume necessitated the re-admission and, perhaps, reoperation. This should highlight the fact that, perhaps, we need to look further as to whether or not these patients should be on a combined regimen of aspirin and Plavix, preoperatively and postoperatively, as prescribed by many clinicians.
There is no Level I evidence to support that the combination of aspirin and Plavix, postoperatvely, for these patients would yield a better outcome than simple aspirin daily. It is difficult to determine from this study whether a significant portion of their patients were on dual antiplatelet therapy.
It is also interesting to notice that the authors found that almost 10% of patients had at least one in-hospital complication; some of which were major complications, e.g. stroke, MI, and dysphagia. Including bleeding/hematoma in these complications, which may not have necessitated surgery, may have inflated this number. A similar observation can be made regarding postoperative arrhythmias, particularly if they did not necessitate extra therapy. However, the fact of the matter is that it should be emphasized that the selection of patients for carotid endarterectomy in asymptomatic patients is extremely critical if this procedure is to be continued or blessed.
Dr. Ali F. AbuRahma is Professor of Surgery and Chief, Vascular & Endovascular Surgery, and Director, Vascular Surgery Fellowship and Residency Programs, and Medical Director, Vascular Laboratory, Co-Director, Vascular Center of Excellence, Robert C. Byrd Health Sciences Center, West Virginia University, Charleston Area Medical Center, Charleston. He is a also an associate medical editor of Vascular Specialist.
Over the past several years, the role of carotid endarterectomy (CEA) for asymptomatic carotid stenosis has, again, come under the microscope; with many proponents still advocating CEA as the treatment of choice for asymptomatic patients with greater than or equal to 60% stenosis, while some propose greater than or equal to 70-80% stenosis in good surgical risk patients. Meanwhile, others oppose this philosophy because of the advances in modern medical therapy for patients with atherosclerosis, in general, with emphasis on risk modification. The findings of this article are quite disturbing, since the authors concluded that the 30-day unplanned re-admission rate after CEA was 6.5%; this is especially surprising to me, coming from this institution.
| Dr. AbuRahma |
The authors also concluded that unplanned re-admission rate was influenced by congestive heart failure, in-hospital postoperative stroke, in-hospital postoperative hematoma, and prior coronary artery bypass grafting. This emphasizes the importance of selection, selection, selection for asymptomatic carotid artery stenosis, if the outcome is to be acceptable to those who still advocate carotid endarterectomy for asymptomatic carotid disease.
Perhaps this procedure should not be encouraged for patients with congestive heart failure or those with severe coronary artery disease, unstable angina. Today, Level I evidence still supports carotid endarterectomy for patients with severe carotid artery stenosis, provided the patient is a good surgical risk, with relatively good longevity; with perioperative stroke and/or death rates of less than 3%. Several modern clinical series have concluded that CEA can be done in these patients with a stroke and/or death rate of less than 1-2%, which was produced most recently in the CREST trial. For those clinicians who cannot keep these numbers down, perhaps this procedure should not be done for asymptomatic carotid disease. What’s also surprising to me, is the in-hospital postoperative hematomas, which I presume necessitated the re-admission and, perhaps, reoperation. This should highlight the fact that, perhaps, we need to look further as to whether or not these patients should be on a combined regimen of aspirin and Plavix, preoperatively and postoperatively, as prescribed by many clinicians.
There is no Level I evidence to support that the combination of aspirin and Plavix, postoperatvely, for these patients would yield a better outcome than simple aspirin daily. It is difficult to determine from this study whether a significant portion of their patients were on dual antiplatelet therapy.
It is also interesting to notice that the authors found that almost 10% of patients had at least one in-hospital complication; some of which were major complications, e.g. stroke, MI, and dysphagia. Including bleeding/hematoma in these complications, which may not have necessitated surgery, may have inflated this number. A similar observation can be made regarding postoperative arrhythmias, particularly if they did not necessitate extra therapy. However, the fact of the matter is that it should be emphasized that the selection of patients for carotid endarterectomy in asymptomatic patients is extremely critical if this procedure is to be continued or blessed.
Dr. Ali F. AbuRahma is Professor of Surgery and Chief, Vascular & Endovascular Surgery, and Director, Vascular Surgery Fellowship and Residency Programs, and Medical Director, Vascular Laboratory, Co-Director, Vascular Center of Excellence, Robert C. Byrd Health Sciences Center, West Virginia University, Charleston Area Medical Center, Charleston. He is a also an associate medical editor of Vascular Specialist.
SAN FRANCISCO – The 30-day unplanned readmission rate following carotid endarterectomy was 6.5% in a single-center study.
In addition, four variables were significantly associated with unplanned readmission: in-hospital postoperative congestive heart failure (CHF) exacerbation; in-hospital postoperative stroke; in-hospital postoperative hematoma; and prior coronary artery bypass graft (CABG).
"Whether these complications are completely avoidable is unknown, but we do identify a group of patients who would probably benefit from more comprehensive discharge planning and careful postdischarge care," Dr. Karen J. Ho said at the Society for Vascular Surgery annual meeting earlier this year.
According to a study of Medicare claims data from 2003 to 2004, 20% of Medicare beneficiaries discharged from a hospital were rehospitalized within 30 days (N. Eng. J. Med. 2009;360:1418-28). The 30-day rehospitalization rate after vascular surgery was 24%, "the highest of all surgical specialties examined in the study," said Dr. Ho of the surgery department at Brigham and Women’s Hospital, Boston, who was not involved with the published study. "Medicare has started to decrease reimbursements for hospitals with excess readmissions after acute MI, heart failure, and pneumonia. Hip and knee replacements and chronic obstructive pulmonary disease will be added in 2014, and we anticipate that additional surgical procedures will be added thereafter," she said.
In an effort to determine the rate of 30-day unplanned readmission after carotid endarterectomy (CEA), Dr. Ho and her associates conducted a retrospective study of a prospectively collected vascular surgery database at Brigham and Women’s Hospital. The cohort included 896 consecutive CEAs performed between 2002 and 2011. Combined CABG/CEA procedures were excluded.
The primary endpoint was unplanned readmission within 30 days, defined as "any unanticipated, nonelective hospital readmission," she said. The secondary endpoint was 1-year survival.
The mean age of the patients was 70 years, 60% were male, and 95% were white. More than half (65%) had asymptomatic evidence of carotid artery disease.
Dr. Ho reported that the median postoperative length of stay was 1 day and that 9.9% of patients had at least one in-hospital complication. The most frequent in-hospital complication was bleeding/hematoma (4.1%), followed by arrhythmia (2.1%), dysphagia (1.7%), stroke (1.3%), and myocardial infarction (1.2%). Only 3% of patients required a reoperation, while most (94%) were discharged to home. The 30-day stroke rate was 1.7%, while the 30-day death rate was 0.6%.
The overall 30-day readmission rate was 8.6%, while the unplanned 30-day readmission rate was 6.5%. "Most of the overall readmissions (80%) occurred in the first 10 days, and the median time to unplanned readmission was 4 days," Dr. Ho said.
The most common reason for an unplanned readmission was a cardiac complication, followed by headache, bleeding/hematoma, stroke/transient ischemic attack/intracerebral hemorrhage, or other medical emergency. More than one-quarter of patients (27.5%) had more than one reason for an unplanned readmission, while 87.9% of patients had a CEA-related unplanned readmission.
When the researchers performed a univariate analysis followed by analysis with a multivariable Cox model for unplanned readmission, four variables were independently associated with unplanned readmission: in-hospital postoperative CHF exacerbation (hazard ratio, 15.1), in-hospital postoperative stroke (HR, 5.0), in-hospital postoperative hematoma (HR, 3.1), and prior CABG (HR, 2.0).
They also observed a significant difference in survival at 1 year between patients who had an unplanned readmission and those who did not (91% vs. 96%, respectively; P less than .01.) "It’s unclear whether these deaths in the unplanned readmission group were preventable or if they were related to carotid disease or to a procedure-related complication," Dr. Ho said. "Our guess is that the increased overall burden of comorbid disease in these patients, rather than the readmission itself, predicted decreased survival."
Limitations of the study included its retrospective design and the fact that it was conducted at a single center, she said, "but we do know that our unplanned readmission rate is comparable to estimates from recent Medicare data."
Dr. Ho said she had no relevant financial disclosures.
SAN FRANCISCO – The 30-day unplanned readmission rate following carotid endarterectomy was 6.5% in a single-center study.
In addition, four variables were significantly associated with unplanned readmission: in-hospital postoperative congestive heart failure (CHF) exacerbation; in-hospital postoperative stroke; in-hospital postoperative hematoma; and prior coronary artery bypass graft (CABG).
"Whether these complications are completely avoidable is unknown, but we do identify a group of patients who would probably benefit from more comprehensive discharge planning and careful postdischarge care," Dr. Karen J. Ho said at the Society for Vascular Surgery annual meeting earlier this year.
According to a study of Medicare claims data from 2003 to 2004, 20% of Medicare beneficiaries discharged from a hospital were rehospitalized within 30 days (N. Eng. J. Med. 2009;360:1418-28). The 30-day rehospitalization rate after vascular surgery was 24%, "the highest of all surgical specialties examined in the study," said Dr. Ho of the surgery department at Brigham and Women’s Hospital, Boston, who was not involved with the published study. "Medicare has started to decrease reimbursements for hospitals with excess readmissions after acute MI, heart failure, and pneumonia. Hip and knee replacements and chronic obstructive pulmonary disease will be added in 2014, and we anticipate that additional surgical procedures will be added thereafter," she said.
In an effort to determine the rate of 30-day unplanned readmission after carotid endarterectomy (CEA), Dr. Ho and her associates conducted a retrospective study of a prospectively collected vascular surgery database at Brigham and Women’s Hospital. The cohort included 896 consecutive CEAs performed between 2002 and 2011. Combined CABG/CEA procedures were excluded.
The primary endpoint was unplanned readmission within 30 days, defined as "any unanticipated, nonelective hospital readmission," she said. The secondary endpoint was 1-year survival.
The mean age of the patients was 70 years, 60% were male, and 95% were white. More than half (65%) had asymptomatic evidence of carotid artery disease.
Dr. Ho reported that the median postoperative length of stay was 1 day and that 9.9% of patients had at least one in-hospital complication. The most frequent in-hospital complication was bleeding/hematoma (4.1%), followed by arrhythmia (2.1%), dysphagia (1.7%), stroke (1.3%), and myocardial infarction (1.2%). Only 3% of patients required a reoperation, while most (94%) were discharged to home. The 30-day stroke rate was 1.7%, while the 30-day death rate was 0.6%.
The overall 30-day readmission rate was 8.6%, while the unplanned 30-day readmission rate was 6.5%. "Most of the overall readmissions (80%) occurred in the first 10 days, and the median time to unplanned readmission was 4 days," Dr. Ho said.
The most common reason for an unplanned readmission was a cardiac complication, followed by headache, bleeding/hematoma, stroke/transient ischemic attack/intracerebral hemorrhage, or other medical emergency. More than one-quarter of patients (27.5%) had more than one reason for an unplanned readmission, while 87.9% of patients had a CEA-related unplanned readmission.
When the researchers performed a univariate analysis followed by analysis with a multivariable Cox model for unplanned readmission, four variables were independently associated with unplanned readmission: in-hospital postoperative CHF exacerbation (hazard ratio, 15.1), in-hospital postoperative stroke (HR, 5.0), in-hospital postoperative hematoma (HR, 3.1), and prior CABG (HR, 2.0).
They also observed a significant difference in survival at 1 year between patients who had an unplanned readmission and those who did not (91% vs. 96%, respectively; P less than .01.) "It’s unclear whether these deaths in the unplanned readmission group were preventable or if they were related to carotid disease or to a procedure-related complication," Dr. Ho said. "Our guess is that the increased overall burden of comorbid disease in these patients, rather than the readmission itself, predicted decreased survival."
Limitations of the study included its retrospective design and the fact that it was conducted at a single center, she said, "but we do know that our unplanned readmission rate is comparable to estimates from recent Medicare data."
Dr. Ho said she had no relevant financial disclosures.
Major finding: Four variables were independently associated with unplanned readmission: in-hospital postoperative CHF exacerbation (hazard ratio, 15.1), in-hospital postoperative stroke (HR, 5.0), in-hospital postoperative hematoma (HR, 3.1), and prior CABG (HR, 2.0).
Data source: A study of 896 consecutive CEAs performed between 2002 and 2011 at Brigham and Women’s Hospital, Boston.
Disclosures: Dr. Ho said she had no relevant financial disclosures.
Postop troponin elevation, MI impact 5-year survival
SAN FRANCISCO – Postoperative troponin elevation and myocardial infarction both impact 5-year survival following vascular surgery procedures, the results of a large long-term study showed.
In fact, troponin elevation increased the hazard of death by 50% while myocardial infarction increased the hazard of death by nearly threefold, Dr. Jessica P. Simons reported at the annual meeting of the Society for Vascular Surgery. "Future studies are needed to assess the nature of this association as well as the utility of routine postoperative screening for myocardial ischemia," said Dr. Simons of the division of vascular and endovascular surgery at the University of Massachusetts, Worcester.
In a study that she presented on behalf of the Vascular Study Group of New England (VSGNE), Dr. Simons and her associates set out to determine the association of postoperative troponin elevation with long-term survival in patients undergoing vascular surgical procedures. "Postoperative myocardial infarction has been shown to impact short- and long-term mortality," she said. "In addition, troponin elevations have also been shown to negatively impact survival for a wide range of diagnoses. This has been seen in critical care medical literature and also in the general surgical population."
The researchers identified 16,363 VSGNE patients who underwent carotid revascularization, open AAA repair, endovascular AAA repair, or lower-extremity bypass between 2003 and 2011. The exposure variable of interest was postoperative myocardial ischemia, which was categorized as either no ischemia, troponin elevation, or myocardial infarction. The primary end point was survival during the first 5 years postoperatively. They used Kaplan-Meier analyses and Cox proportional hazards models to evaluate the effect of postoperative troponin elevation and myocardial infarction.
Of the 16,363 patients, 15,888 (97.1%) had no ischemia, 211 (1.3%) had troponin elevation, and 264 (1.6%) had myocardial infarction. When this was broken down by procedure type, open AAA had the highest rates of postoperative myocardial ischemia (9%), troponin elevation (3.9%), and myocardial infarction (5.1%), compared with carotid revascularization, endovascular aneurysm repair, and lower-extremity bypass.
The rate of 5-year survival for all procedures was 73% among those with no ischemia, 54% among those with troponin elevation, and 33% among those with myocardial infarction. This difference reached statistical significance with a P value of less than .0001. After adjusting for covariates, the researchers found a similar trend. In this analysis the rate of 5-year survival was 78% among those with no ischemia, 48% among those with troponin elevation, and 35% among those with myocardial infarction. This also reached statistical significance with a P value of less than .0001.
"We performed a subgroup analysis by procedure type, and the trend was the same across all procedure types," Dr. Simons said.
In Cox modeling the researchers found that postoperative ischemia in the form of a troponin elevation increased the hazard of death at 5 years by 45% (HR, 1.45; P =.01) while myocardial infarction nearly tripled the hazard of death (HR, 2.93; P =.0001).
"We have shown an association between postoperative myocardial ischemia and worse survival, but does postoperative myocardial ischemia worsen long-term survival, or does postoperative myocardial ischemia simply identify a high-risk subset of patients?" Dr. Simons asked. "If postoperative myocardial ischemia worsens long-term survival, then efforts should focus on better preoperative medical optimization and perioperative prevention of ischemia. If postoperative myocardial ischemia is simply identifying a high-risk subset of patients, then efforts should focus on better preoperative risk stratification and postoperative medical surveillance."
She concluded that postoperative myocardial ischemia, "whether a troponin elevation or a myocardial infarction, is associated with lower survival. This effect is seen across all procedure types and persists out to 5 years postoperatively."
Dr. Simons said she had no relevant financial disclosures.
The publication of the CREST landmark study at the New England Journal of Medicine in 2010 showed that the outcomes of carotid stenting and carotid endarterectomy (CEA) for patients with =70% carotid stenosis were not statistically significant when the combined 30-day endpoints of stroke, death, and MI were considered (4.5% for CEA versus 5.2% for stenting).
| Dr. AbuRahma |
The rate for minor stroke in symptomatic patients was more frequent after carotid stenting (4.3% versus 2.3% for CEA, p=0.042); and for periprocedural MI, the results were somewhat opposite – 1% versus 2.3%, p=0.083). MI was an important endpoint from a prognostic standpoint, since the 4-year mortality rate for patients who sustained an MI was 19.5% versus 6.7% for patients without an MI. This led many interventionalists to claim equivalency between the two interventions and also to claim that perioperative MI had a larger impact on late mortality than stroke. However, the 4-year mortality rate for patients suffering a stroke was 20% versus 11% for patients who were stroke-free, i.e. the 4-year survival rate was equivalent for both procedures but with the additional disadvantage of increased disability in patients with stents who sustained strokes. When using a quality of life SF36 form, it was concluded that both physical and mental aspects of life one year after the procedure were more highly impacted following a stroke, whether major or minor, than an MI.
This present study only highlighted one aspect of the findings from the VSGNE of over 16,000, emphasizing the impact of MI (clinical or chemical) and survival. However, if we take the CREST data into consideration, if all of these patients had undergone perioperative monitoring, including troponin and EKG analyses, would this have impacted the long-term survival rates differently?
Dr. Ali F. AbuRahma is Professor of Surgery and Chief, Vascular & Endovascular Surgery at West Virginia University,Charleston, WV. He is also an associate editor for Vascular Specialist.
The publication of the CREST landmark study at the New England Journal of Medicine in 2010 showed that the outcomes of carotid stenting and carotid endarterectomy (CEA) for patients with =70% carotid stenosis were not statistically significant when the combined 30-day endpoints of stroke, death, and MI were considered (4.5% for CEA versus 5.2% for stenting).
| Dr. AbuRahma |
The rate for minor stroke in symptomatic patients was more frequent after carotid stenting (4.3% versus 2.3% for CEA, p=0.042); and for periprocedural MI, the results were somewhat opposite – 1% versus 2.3%, p=0.083). MI was an important endpoint from a prognostic standpoint, since the 4-year mortality rate for patients who sustained an MI was 19.5% versus 6.7% for patients without an MI. This led many interventionalists to claim equivalency between the two interventions and also to claim that perioperative MI had a larger impact on late mortality than stroke. However, the 4-year mortality rate for patients suffering a stroke was 20% versus 11% for patients who were stroke-free, i.e. the 4-year survival rate was equivalent for both procedures but with the additional disadvantage of increased disability in patients with stents who sustained strokes. When using a quality of life SF36 form, it was concluded that both physical and mental aspects of life one year after the procedure were more highly impacted following a stroke, whether major or minor, than an MI.
This present study only highlighted one aspect of the findings from the VSGNE of over 16,000, emphasizing the impact of MI (clinical or chemical) and survival. However, if we take the CREST data into consideration, if all of these patients had undergone perioperative monitoring, including troponin and EKG analyses, would this have impacted the long-term survival rates differently?
Dr. Ali F. AbuRahma is Professor of Surgery and Chief, Vascular & Endovascular Surgery at West Virginia University,Charleston, WV. He is also an associate editor for Vascular Specialist.
The publication of the CREST landmark study at the New England Journal of Medicine in 2010 showed that the outcomes of carotid stenting and carotid endarterectomy (CEA) for patients with =70% carotid stenosis were not statistically significant when the combined 30-day endpoints of stroke, death, and MI were considered (4.5% for CEA versus 5.2% for stenting).
| Dr. AbuRahma |
The rate for minor stroke in symptomatic patients was more frequent after carotid stenting (4.3% versus 2.3% for CEA, p=0.042); and for periprocedural MI, the results were somewhat opposite – 1% versus 2.3%, p=0.083). MI was an important endpoint from a prognostic standpoint, since the 4-year mortality rate for patients who sustained an MI was 19.5% versus 6.7% for patients without an MI. This led many interventionalists to claim equivalency between the two interventions and also to claim that perioperative MI had a larger impact on late mortality than stroke. However, the 4-year mortality rate for patients suffering a stroke was 20% versus 11% for patients who were stroke-free, i.e. the 4-year survival rate was equivalent for both procedures but with the additional disadvantage of increased disability in patients with stents who sustained strokes. When using a quality of life SF36 form, it was concluded that both physical and mental aspects of life one year after the procedure were more highly impacted following a stroke, whether major or minor, than an MI.
This present study only highlighted one aspect of the findings from the VSGNE of over 16,000, emphasizing the impact of MI (clinical or chemical) and survival. However, if we take the CREST data into consideration, if all of these patients had undergone perioperative monitoring, including troponin and EKG analyses, would this have impacted the long-term survival rates differently?
Dr. Ali F. AbuRahma is Professor of Surgery and Chief, Vascular & Endovascular Surgery at West Virginia University,Charleston, WV. He is also an associate editor for Vascular Specialist.
SAN FRANCISCO – Postoperative troponin elevation and myocardial infarction both impact 5-year survival following vascular surgery procedures, the results of a large long-term study showed.
In fact, troponin elevation increased the hazard of death by 50% while myocardial infarction increased the hazard of death by nearly threefold, Dr. Jessica P. Simons reported at the annual meeting of the Society for Vascular Surgery. "Future studies are needed to assess the nature of this association as well as the utility of routine postoperative screening for myocardial ischemia," said Dr. Simons of the division of vascular and endovascular surgery at the University of Massachusetts, Worcester.
In a study that she presented on behalf of the Vascular Study Group of New England (VSGNE), Dr. Simons and her associates set out to determine the association of postoperative troponin elevation with long-term survival in patients undergoing vascular surgical procedures. "Postoperative myocardial infarction has been shown to impact short- and long-term mortality," she said. "In addition, troponin elevations have also been shown to negatively impact survival for a wide range of diagnoses. This has been seen in critical care medical literature and also in the general surgical population."
The researchers identified 16,363 VSGNE patients who underwent carotid revascularization, open AAA repair, endovascular AAA repair, or lower-extremity bypass between 2003 and 2011. The exposure variable of interest was postoperative myocardial ischemia, which was categorized as either no ischemia, troponin elevation, or myocardial infarction. The primary end point was survival during the first 5 years postoperatively. They used Kaplan-Meier analyses and Cox proportional hazards models to evaluate the effect of postoperative troponin elevation and myocardial infarction.
Of the 16,363 patients, 15,888 (97.1%) had no ischemia, 211 (1.3%) had troponin elevation, and 264 (1.6%) had myocardial infarction. When this was broken down by procedure type, open AAA had the highest rates of postoperative myocardial ischemia (9%), troponin elevation (3.9%), and myocardial infarction (5.1%), compared with carotid revascularization, endovascular aneurysm repair, and lower-extremity bypass.
The rate of 5-year survival for all procedures was 73% among those with no ischemia, 54% among those with troponin elevation, and 33% among those with myocardial infarction. This difference reached statistical significance with a P value of less than .0001. After adjusting for covariates, the researchers found a similar trend. In this analysis the rate of 5-year survival was 78% among those with no ischemia, 48% among those with troponin elevation, and 35% among those with myocardial infarction. This also reached statistical significance with a P value of less than .0001.
"We performed a subgroup analysis by procedure type, and the trend was the same across all procedure types," Dr. Simons said.
In Cox modeling the researchers found that postoperative ischemia in the form of a troponin elevation increased the hazard of death at 5 years by 45% (HR, 1.45; P =.01) while myocardial infarction nearly tripled the hazard of death (HR, 2.93; P =.0001).
"We have shown an association between postoperative myocardial ischemia and worse survival, but does postoperative myocardial ischemia worsen long-term survival, or does postoperative myocardial ischemia simply identify a high-risk subset of patients?" Dr. Simons asked. "If postoperative myocardial ischemia worsens long-term survival, then efforts should focus on better preoperative medical optimization and perioperative prevention of ischemia. If postoperative myocardial ischemia is simply identifying a high-risk subset of patients, then efforts should focus on better preoperative risk stratification and postoperative medical surveillance."
She concluded that postoperative myocardial ischemia, "whether a troponin elevation or a myocardial infarction, is associated with lower survival. This effect is seen across all procedure types and persists out to 5 years postoperatively."
Dr. Simons said she had no relevant financial disclosures.
SAN FRANCISCO – Postoperative troponin elevation and myocardial infarction both impact 5-year survival following vascular surgery procedures, the results of a large long-term study showed.
In fact, troponin elevation increased the hazard of death by 50% while myocardial infarction increased the hazard of death by nearly threefold, Dr. Jessica P. Simons reported at the annual meeting of the Society for Vascular Surgery. "Future studies are needed to assess the nature of this association as well as the utility of routine postoperative screening for myocardial ischemia," said Dr. Simons of the division of vascular and endovascular surgery at the University of Massachusetts, Worcester.
In a study that she presented on behalf of the Vascular Study Group of New England (VSGNE), Dr. Simons and her associates set out to determine the association of postoperative troponin elevation with long-term survival in patients undergoing vascular surgical procedures. "Postoperative myocardial infarction has been shown to impact short- and long-term mortality," she said. "In addition, troponin elevations have also been shown to negatively impact survival for a wide range of diagnoses. This has been seen in critical care medical literature and also in the general surgical population."
The researchers identified 16,363 VSGNE patients who underwent carotid revascularization, open AAA repair, endovascular AAA repair, or lower-extremity bypass between 2003 and 2011. The exposure variable of interest was postoperative myocardial ischemia, which was categorized as either no ischemia, troponin elevation, or myocardial infarction. The primary end point was survival during the first 5 years postoperatively. They used Kaplan-Meier analyses and Cox proportional hazards models to evaluate the effect of postoperative troponin elevation and myocardial infarction.
Of the 16,363 patients, 15,888 (97.1%) had no ischemia, 211 (1.3%) had troponin elevation, and 264 (1.6%) had myocardial infarction. When this was broken down by procedure type, open AAA had the highest rates of postoperative myocardial ischemia (9%), troponin elevation (3.9%), and myocardial infarction (5.1%), compared with carotid revascularization, endovascular aneurysm repair, and lower-extremity bypass.
The rate of 5-year survival for all procedures was 73% among those with no ischemia, 54% among those with troponin elevation, and 33% among those with myocardial infarction. This difference reached statistical significance with a P value of less than .0001. After adjusting for covariates, the researchers found a similar trend. In this analysis the rate of 5-year survival was 78% among those with no ischemia, 48% among those with troponin elevation, and 35% among those with myocardial infarction. This also reached statistical significance with a P value of less than .0001.
"We performed a subgroup analysis by procedure type, and the trend was the same across all procedure types," Dr. Simons said.
In Cox modeling the researchers found that postoperative ischemia in the form of a troponin elevation increased the hazard of death at 5 years by 45% (HR, 1.45; P =.01) while myocardial infarction nearly tripled the hazard of death (HR, 2.93; P =.0001).
"We have shown an association between postoperative myocardial ischemia and worse survival, but does postoperative myocardial ischemia worsen long-term survival, or does postoperative myocardial ischemia simply identify a high-risk subset of patients?" Dr. Simons asked. "If postoperative myocardial ischemia worsens long-term survival, then efforts should focus on better preoperative medical optimization and perioperative prevention of ischemia. If postoperative myocardial ischemia is simply identifying a high-risk subset of patients, then efforts should focus on better preoperative risk stratification and postoperative medical surveillance."
She concluded that postoperative myocardial ischemia, "whether a troponin elevation or a myocardial infarction, is associated with lower survival. This effect is seen across all procedure types and persists out to 5 years postoperatively."
Dr. Simons said she had no relevant financial disclosures.
AT THE SVS ANNUAL MEETING
Major finding: Postoperative ischemia in the form of a troponin elevation increased the hazard of death at 5 years by 45% (HR, 1.45; P =.01) while myocardial infarction nearly tripled the hazard of death (HR, 2.93; P =.0001).
Data source: A study of 16,363 Vascular Study Group of New England patients who underwent carotid revascularization, open AAA repair, endovascular AAA repair, or lower-extremity bypass between 2003 and 2011.
Disclosures: Dr. Simons said she had no relevant financial disclosures.
No benefit of endovascular therapy added to TPA for stroke
Functional outcomes in patients treated with intravenous tissue plasminogen activator with or without endovascular therapy after a moderate to severe acute ischemic stroke were not significantly different, and safety outcomes were similar, in a study that was stopped early because of these results.
In the IMS (Interventional Management of Stroke) III study, 40.8% of patients randomized to receive endovascular therapy plus intravenous TPA met the primary endpoint, a measure of functional independence -- a modified Rankin score of 2 or less at 90 days -- compared with 38.7% among those who had intravenous TPA alone, a difference that was not statistically significant, reported Dr. Joseph Broderick of the University of Cincinnati Neuroscience Institute, and the other IMS III investigators.
Mortality and other safety outcomes were also not significantly different between the two groups of patients in the study, which was stopped early because of futility after 656 of the planned 900 patients had been randomized.
The study was published online to coincide with the presentation of the results at the International Stroke Conference (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1214300]).
Referring to the lack of randomized clinical trial data, the authors pointed out that it is uncertain whether endovascular therapy (which includes endovascular pharmacologic thrombolysis and, more recently, the use of stent retrievers) alone or combined with intravenous TPA is a more effective treatment of acute stroke than intravenous TPA alone, "the only proven reperfusion therapy for acute ischemic stroke."
In the study, conducted at 58 centers in the United States, Canada, Australia, and Europe, 434 patients were randomized to endovascular therapy plus intravenous TPA and 222 were randomized to standard treatment with intravenous TPA alone (started within 3 hours of stroke onset). The median age of those enrolled was 68-69 years (range, 23-89 years), a little over half were men, about 14% were black or Hispanic, and the median the National Institute of Health Stroke Scale (NIHSS) score was 16-17 (8-19 is a moderately severe stroke and 20 or greater is a severe stroke At the beginning of the study, only one thrombectomy device had been cleared by the Food and Drug Administration and, as the trial continued, other devices were used as they became cleared for use in the different countries.
In addition to the main finding, there were no differences in the primary outcome among those patients with an NIHSS score of 20 or more, and those with a score of 19 or lower, said the authors, who had hypothesized that endovascular therapy would have greater efficacy in patients with more-severe strokes since they "have the highest likelihood of occlusion in a major intracranial artery and the greatest volume of ischemic brain at risk."
They had also hypothesized that receiving endovascular therapy earlier would be associated with a greater benefit, but this was also not a significant factor in outcomes.
Mortality at 90 days was 19.1% in the endovascular therapy group and 21.6% in the intravenous TPA–alone group. Within 30 hours of TPA initiation, 6.2% of those on endovascular therapy and 5.9% of those on TPA alone had a symptomatic intracerebral hemorrhage. The differences in mortality at 7 days and in parenchymal hematoma rates were also not significantly different between the two groups. The rate of asymptomatic intracerebral hemorrhage, however, was significantly higher in the endovascular group.
Outcomes consistently trended better with combined therapy in patients with strokes involving larger artery occlusions and those with the shortest times from stroke onset to initiation of treatment, although because of small patient numbers the differences didn’t achieve statistical significance. These will be the subgroups that ought to be the focus of future clinical trials, Dr. Broderick said in a press briefing at the conference.
The underlying rationale for combined therapy is that intravenous TPA can quickly be started in the emergency department while the endovascular device therapy team is assembling, often at another hospital, which entails time-consuming patient transfer.
Intravenous TPA is the only proven therapy for acute ischemic stroke, but endovascular therapy is more effective at achieving recanalization. The study results bore this out: for example, the rate of partial or complete recanalization at 24 hours for an occlusion in the internal carotid artery was 81% with combined therapy compared to 35% with intravenous TPA alone. Yet this higher recanalization rate bore no clinical benefit, possibly because recanalization occurred too late, after ischemia had turned into infarction, Dr. Broderick explained.
"IMS III is going to be disappointing for a lot of people who are proponents of endovascular therapy. However, there is a light at the end of the tunnel in that there are these subgroups who may benefit," Dr. Brian Silver, who was not involved in the trial, said in an interview.
"The most critical feature is to treat the patients as soon as possible when they arrive in the emergency department, perhaps within 90 minutes. I think that’s the best chance for recovery. We are nowhere near what’s being done in cardiology, where there are door-to-balloon times of an hour. We need to do that in stroke. Since we're dealing with an organ that's more sensitive than the heart to ischemia, we probably need to be even faster than what's being done in cardiology. There is definitely room for improvement in our systems, perhaps by having the endovascular team stay in the hospital. Expense will be the limitation," according to Dr. Silver, director of the stroke center at Brown University, Providence, R.I.
IMS III investigator and interventional neuroradiologist Dr. Thomas A. Tomsick said in an interview that the study results won't change his own clinical practice.
"IMS III is by no means the final word on combined therapy. In Cincinnati tomorrow, if a patient with a large NIH Stroke Severity score shows up and we're treating him with IV TPA at 2 hours from stroke onset, we're not going to do a CT angiogram to evaluate that patient. He's going to the cath lab for angiography to see if there's a clot suitable for endovascular therapy," said Dr. Tomsick, professor of radiology at the University of Cincinnati.
Five different endovascular device therapies were utilized in IMS III. As new devices reached clinical practice, their use was allowed by investigators in order to keep the randomized trial clinically relevant. But recruitment for the study was slow because so many clinicians were already convinced by anecdotal experience that combined therapy is better. So the endovascular therapies used most frequently in IMS III aren't the ones widely used in clinical practice today. Major new randomized trials are now getting underway comparing combined therapy using state-of-the-art, more effective stent clot retriever devices to intravenous TPA alone, he added.
In the New England Journal of Medicine report, the authors noted that "the use of randomization in ongoing and future stroke trials, rather than the treatment of eligible patients with endovascular therapy outside any trial, and minimization of the time to treatment will be essential for assessing the potential benefit of endovascular therapy for acute ischemic stroke."
No matter how future trials of combined therapy turn out, endovascular therapy is not going away, Dr. Broderick observed.
"It's a very good tool. The reason why is there are patients who can't get TPA. For example, roughly 5% of patients who undergo coronary artery bypass surgery have a stroke. If you have somebody with a big stroke 2 days after having their chest cracked, you can't use TPA. In that case, those endovascular devices are the way we can get up in there and get rid of the clot," he explained.
In an editorial accompanying the report in the New England Journal of Medicine (doi: 10.1056/NEJMe1215730), Dr. Marc I. Chimowitz declared that the clinical implication of IMS III is that endovascular therapy remains unproven and intravenous TPA should continue to be the first-line treatment for patients with acute ischemic stroke within 4.5 hours after stroke onset.
While new clinical trials featuring more effective IV clot busters, such as tenecteplase, and next-generation endovascular devices are urgently needed in an effort to improve stroke outcomes, patient recruitment is likely to continue to be a challenge in the current environment. This could be overcome if Medicare were to place a moratorium on reimbursement for endovascular therapy of acute ischemic stroke except as part of a randomized trial, according to Dr. Chimowitz, professor of neurology at the Medical University of South Carolina, Charleston.
The study was supported with grants from NIH and the National Institute of Neurological Disorders and Stroke; and by Genentech (which supplied the TPA); and EKOS, Concentric Medical, and Cordis Neurovascular (which supplied catheters); and Actilyse (alteplase) manufacturer Boehringer Ingelheim (which, along with Genentech and EKOS, provided support for investigator meetings). Dr. Broderick disclosed consulting fees from PhotoThera. Of the 28 other authors, disclosures for 14 were listed and included having received consulting fees, grant support, and/or lecture fees from a variety of device and pharmaceutical companies that include Genentech. Dr. Chimowitz, Dr. Silver, and Dr. Tomsick reported having no financial conflicts.
The key to understanding the results of this study is the difference in the recanalization rates between the two groups and their lack of relationship to outcome. In the IV TPA plus catheter directed TPA group, the recanalization rate was higher than in the IV alone group, but the clinical outcomes were not better. Undoubtedly this is due to the fact that the recanalization was accomplished after brain tissue death had already occurred.
Hence the next and critical question is....what would the result be if we could administer the catheter directed TPA in a timely fashion, as is done with acute myocardial infarction? If the study had been performed in centers where this therapy was available without delay, would the results have been different? I think almost certainly. While it is true that this therapy is not available as widely as coronary interventions, it is critical for us to know whether patients who are fortunate enough to be treated in an institution where the therapy is available should receive it. Another disappointing aspect of the study design was the inability to perform subgroup analysis. While it appeared that patients with larger stroke distribution might benefit, the study was apparently not powered to detect this difference. Lastly, it is puzzling that the investigators themselves do not appear to believe the results of their own study, with two of them indicating the results would not change their own practice. If that's the case, why spend all this time and money designing a trial that doesn't answer the questions?
Dr. Cynthia K. Shortell is Professor and Chief, Division of Vascular Surgery, Duke University Medical Center, and an associate medical editor for Vascular Specialist.
The key to understanding the results of this study is the difference in the recanalization rates between the two groups and their lack of relationship to outcome. In the IV TPA plus catheter directed TPA group, the recanalization rate was higher than in the IV alone group, but the clinical outcomes were not better. Undoubtedly this is due to the fact that the recanalization was accomplished after brain tissue death had already occurred.
Hence the next and critical question is....what would the result be if we could administer the catheter directed TPA in a timely fashion, as is done with acute myocardial infarction? If the study had been performed in centers where this therapy was available without delay, would the results have been different? I think almost certainly. While it is true that this therapy is not available as widely as coronary interventions, it is critical for us to know whether patients who are fortunate enough to be treated in an institution where the therapy is available should receive it. Another disappointing aspect of the study design was the inability to perform subgroup analysis. While it appeared that patients with larger stroke distribution might benefit, the study was apparently not powered to detect this difference. Lastly, it is puzzling that the investigators themselves do not appear to believe the results of their own study, with two of them indicating the results would not change their own practice. If that's the case, why spend all this time and money designing a trial that doesn't answer the questions?
Dr. Cynthia K. Shortell is Professor and Chief, Division of Vascular Surgery, Duke University Medical Center, and an associate medical editor for Vascular Specialist.
The key to understanding the results of this study is the difference in the recanalization rates between the two groups and their lack of relationship to outcome. In the IV TPA plus catheter directed TPA group, the recanalization rate was higher than in the IV alone group, but the clinical outcomes were not better. Undoubtedly this is due to the fact that the recanalization was accomplished after brain tissue death had already occurred.
Hence the next and critical question is....what would the result be if we could administer the catheter directed TPA in a timely fashion, as is done with acute myocardial infarction? If the study had been performed in centers where this therapy was available without delay, would the results have been different? I think almost certainly. While it is true that this therapy is not available as widely as coronary interventions, it is critical for us to know whether patients who are fortunate enough to be treated in an institution where the therapy is available should receive it. Another disappointing aspect of the study design was the inability to perform subgroup analysis. While it appeared that patients with larger stroke distribution might benefit, the study was apparently not powered to detect this difference. Lastly, it is puzzling that the investigators themselves do not appear to believe the results of their own study, with two of them indicating the results would not change their own practice. If that's the case, why spend all this time and money designing a trial that doesn't answer the questions?
Dr. Cynthia K. Shortell is Professor and Chief, Division of Vascular Surgery, Duke University Medical Center, and an associate medical editor for Vascular Specialist.
Functional outcomes in patients treated with intravenous tissue plasminogen activator with or without endovascular therapy after a moderate to severe acute ischemic stroke were not significantly different, and safety outcomes were similar, in a study that was stopped early because of these results.
In the IMS (Interventional Management of Stroke) III study, 40.8% of patients randomized to receive endovascular therapy plus intravenous TPA met the primary endpoint, a measure of functional independence -- a modified Rankin score of 2 or less at 90 days -- compared with 38.7% among those who had intravenous TPA alone, a difference that was not statistically significant, reported Dr. Joseph Broderick of the University of Cincinnati Neuroscience Institute, and the other IMS III investigators.
Mortality and other safety outcomes were also not significantly different between the two groups of patients in the study, which was stopped early because of futility after 656 of the planned 900 patients had been randomized.
The study was published online to coincide with the presentation of the results at the International Stroke Conference (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1214300]).
Referring to the lack of randomized clinical trial data, the authors pointed out that it is uncertain whether endovascular therapy (which includes endovascular pharmacologic thrombolysis and, more recently, the use of stent retrievers) alone or combined with intravenous TPA is a more effective treatment of acute stroke than intravenous TPA alone, "the only proven reperfusion therapy for acute ischemic stroke."
In the study, conducted at 58 centers in the United States, Canada, Australia, and Europe, 434 patients were randomized to endovascular therapy plus intravenous TPA and 222 were randomized to standard treatment with intravenous TPA alone (started within 3 hours of stroke onset). The median age of those enrolled was 68-69 years (range, 23-89 years), a little over half were men, about 14% were black or Hispanic, and the median the National Institute of Health Stroke Scale (NIHSS) score was 16-17 (8-19 is a moderately severe stroke and 20 or greater is a severe stroke At the beginning of the study, only one thrombectomy device had been cleared by the Food and Drug Administration and, as the trial continued, other devices were used as they became cleared for use in the different countries.
In addition to the main finding, there were no differences in the primary outcome among those patients with an NIHSS score of 20 or more, and those with a score of 19 or lower, said the authors, who had hypothesized that endovascular therapy would have greater efficacy in patients with more-severe strokes since they "have the highest likelihood of occlusion in a major intracranial artery and the greatest volume of ischemic brain at risk."
They had also hypothesized that receiving endovascular therapy earlier would be associated with a greater benefit, but this was also not a significant factor in outcomes.
Mortality at 90 days was 19.1% in the endovascular therapy group and 21.6% in the intravenous TPA–alone group. Within 30 hours of TPA initiation, 6.2% of those on endovascular therapy and 5.9% of those on TPA alone had a symptomatic intracerebral hemorrhage. The differences in mortality at 7 days and in parenchymal hematoma rates were also not significantly different between the two groups. The rate of asymptomatic intracerebral hemorrhage, however, was significantly higher in the endovascular group.
Outcomes consistently trended better with combined therapy in patients with strokes involving larger artery occlusions and those with the shortest times from stroke onset to initiation of treatment, although because of small patient numbers the differences didn’t achieve statistical significance. These will be the subgroups that ought to be the focus of future clinical trials, Dr. Broderick said in a press briefing at the conference.
The underlying rationale for combined therapy is that intravenous TPA can quickly be started in the emergency department while the endovascular device therapy team is assembling, often at another hospital, which entails time-consuming patient transfer.
Intravenous TPA is the only proven therapy for acute ischemic stroke, but endovascular therapy is more effective at achieving recanalization. The study results bore this out: for example, the rate of partial or complete recanalization at 24 hours for an occlusion in the internal carotid artery was 81% with combined therapy compared to 35% with intravenous TPA alone. Yet this higher recanalization rate bore no clinical benefit, possibly because recanalization occurred too late, after ischemia had turned into infarction, Dr. Broderick explained.
"IMS III is going to be disappointing for a lot of people who are proponents of endovascular therapy. However, there is a light at the end of the tunnel in that there are these subgroups who may benefit," Dr. Brian Silver, who was not involved in the trial, said in an interview.
"The most critical feature is to treat the patients as soon as possible when they arrive in the emergency department, perhaps within 90 minutes. I think that’s the best chance for recovery. We are nowhere near what’s being done in cardiology, where there are door-to-balloon times of an hour. We need to do that in stroke. Since we're dealing with an organ that's more sensitive than the heart to ischemia, we probably need to be even faster than what's being done in cardiology. There is definitely room for improvement in our systems, perhaps by having the endovascular team stay in the hospital. Expense will be the limitation," according to Dr. Silver, director of the stroke center at Brown University, Providence, R.I.
IMS III investigator and interventional neuroradiologist Dr. Thomas A. Tomsick said in an interview that the study results won't change his own clinical practice.
"IMS III is by no means the final word on combined therapy. In Cincinnati tomorrow, if a patient with a large NIH Stroke Severity score shows up and we're treating him with IV TPA at 2 hours from stroke onset, we're not going to do a CT angiogram to evaluate that patient. He's going to the cath lab for angiography to see if there's a clot suitable for endovascular therapy," said Dr. Tomsick, professor of radiology at the University of Cincinnati.
Five different endovascular device therapies were utilized in IMS III. As new devices reached clinical practice, their use was allowed by investigators in order to keep the randomized trial clinically relevant. But recruitment for the study was slow because so many clinicians were already convinced by anecdotal experience that combined therapy is better. So the endovascular therapies used most frequently in IMS III aren't the ones widely used in clinical practice today. Major new randomized trials are now getting underway comparing combined therapy using state-of-the-art, more effective stent clot retriever devices to intravenous TPA alone, he added.
In the New England Journal of Medicine report, the authors noted that "the use of randomization in ongoing and future stroke trials, rather than the treatment of eligible patients with endovascular therapy outside any trial, and minimization of the time to treatment will be essential for assessing the potential benefit of endovascular therapy for acute ischemic stroke."
No matter how future trials of combined therapy turn out, endovascular therapy is not going away, Dr. Broderick observed.
"It's a very good tool. The reason why is there are patients who can't get TPA. For example, roughly 5% of patients who undergo coronary artery bypass surgery have a stroke. If you have somebody with a big stroke 2 days after having their chest cracked, you can't use TPA. In that case, those endovascular devices are the way we can get up in there and get rid of the clot," he explained.
In an editorial accompanying the report in the New England Journal of Medicine (doi: 10.1056/NEJMe1215730), Dr. Marc I. Chimowitz declared that the clinical implication of IMS III is that endovascular therapy remains unproven and intravenous TPA should continue to be the first-line treatment for patients with acute ischemic stroke within 4.5 hours after stroke onset.
While new clinical trials featuring more effective IV clot busters, such as tenecteplase, and next-generation endovascular devices are urgently needed in an effort to improve stroke outcomes, patient recruitment is likely to continue to be a challenge in the current environment. This could be overcome if Medicare were to place a moratorium on reimbursement for endovascular therapy of acute ischemic stroke except as part of a randomized trial, according to Dr. Chimowitz, professor of neurology at the Medical University of South Carolina, Charleston.
The study was supported with grants from NIH and the National Institute of Neurological Disorders and Stroke; and by Genentech (which supplied the TPA); and EKOS, Concentric Medical, and Cordis Neurovascular (which supplied catheters); and Actilyse (alteplase) manufacturer Boehringer Ingelheim (which, along with Genentech and EKOS, provided support for investigator meetings). Dr. Broderick disclosed consulting fees from PhotoThera. Of the 28 other authors, disclosures for 14 were listed and included having received consulting fees, grant support, and/or lecture fees from a variety of device and pharmaceutical companies that include Genentech. Dr. Chimowitz, Dr. Silver, and Dr. Tomsick reported having no financial conflicts.
Functional outcomes in patients treated with intravenous tissue plasminogen activator with or without endovascular therapy after a moderate to severe acute ischemic stroke were not significantly different, and safety outcomes were similar, in a study that was stopped early because of these results.
In the IMS (Interventional Management of Stroke) III study, 40.8% of patients randomized to receive endovascular therapy plus intravenous TPA met the primary endpoint, a measure of functional independence -- a modified Rankin score of 2 or less at 90 days -- compared with 38.7% among those who had intravenous TPA alone, a difference that was not statistically significant, reported Dr. Joseph Broderick of the University of Cincinnati Neuroscience Institute, and the other IMS III investigators.
Mortality and other safety outcomes were also not significantly different between the two groups of patients in the study, which was stopped early because of futility after 656 of the planned 900 patients had been randomized.
The study was published online to coincide with the presentation of the results at the International Stroke Conference (N. Engl. J. Med. 2013 [doi:10.1056/NEJMoa1214300]).
Referring to the lack of randomized clinical trial data, the authors pointed out that it is uncertain whether endovascular therapy (which includes endovascular pharmacologic thrombolysis and, more recently, the use of stent retrievers) alone or combined with intravenous TPA is a more effective treatment of acute stroke than intravenous TPA alone, "the only proven reperfusion therapy for acute ischemic stroke."
In the study, conducted at 58 centers in the United States, Canada, Australia, and Europe, 434 patients were randomized to endovascular therapy plus intravenous TPA and 222 were randomized to standard treatment with intravenous TPA alone (started within 3 hours of stroke onset). The median age of those enrolled was 68-69 years (range, 23-89 years), a little over half were men, about 14% were black or Hispanic, and the median the National Institute of Health Stroke Scale (NIHSS) score was 16-17 (8-19 is a moderately severe stroke and 20 or greater is a severe stroke At the beginning of the study, only one thrombectomy device had been cleared by the Food and Drug Administration and, as the trial continued, other devices were used as they became cleared for use in the different countries.
In addition to the main finding, there were no differences in the primary outcome among those patients with an NIHSS score of 20 or more, and those with a score of 19 or lower, said the authors, who had hypothesized that endovascular therapy would have greater efficacy in patients with more-severe strokes since they "have the highest likelihood of occlusion in a major intracranial artery and the greatest volume of ischemic brain at risk."
They had also hypothesized that receiving endovascular therapy earlier would be associated with a greater benefit, but this was also not a significant factor in outcomes.
Mortality at 90 days was 19.1% in the endovascular therapy group and 21.6% in the intravenous TPA–alone group. Within 30 hours of TPA initiation, 6.2% of those on endovascular therapy and 5.9% of those on TPA alone had a symptomatic intracerebral hemorrhage. The differences in mortality at 7 days and in parenchymal hematoma rates were also not significantly different between the two groups. The rate of asymptomatic intracerebral hemorrhage, however, was significantly higher in the endovascular group.
Outcomes consistently trended better with combined therapy in patients with strokes involving larger artery occlusions and those with the shortest times from stroke onset to initiation of treatment, although because of small patient numbers the differences didn’t achieve statistical significance. These will be the subgroups that ought to be the focus of future clinical trials, Dr. Broderick said in a press briefing at the conference.
The underlying rationale for combined therapy is that intravenous TPA can quickly be started in the emergency department while the endovascular device therapy team is assembling, often at another hospital, which entails time-consuming patient transfer.
Intravenous TPA is the only proven therapy for acute ischemic stroke, but endovascular therapy is more effective at achieving recanalization. The study results bore this out: for example, the rate of partial or complete recanalization at 24 hours for an occlusion in the internal carotid artery was 81% with combined therapy compared to 35% with intravenous TPA alone. Yet this higher recanalization rate bore no clinical benefit, possibly because recanalization occurred too late, after ischemia had turned into infarction, Dr. Broderick explained.
"IMS III is going to be disappointing for a lot of people who are proponents of endovascular therapy. However, there is a light at the end of the tunnel in that there are these subgroups who may benefit," Dr. Brian Silver, who was not involved in the trial, said in an interview.
"The most critical feature is to treat the patients as soon as possible when they arrive in the emergency department, perhaps within 90 minutes. I think that’s the best chance for recovery. We are nowhere near what’s being done in cardiology, where there are door-to-balloon times of an hour. We need to do that in stroke. Since we're dealing with an organ that's more sensitive than the heart to ischemia, we probably need to be even faster than what's being done in cardiology. There is definitely room for improvement in our systems, perhaps by having the endovascular team stay in the hospital. Expense will be the limitation," according to Dr. Silver, director of the stroke center at Brown University, Providence, R.I.
IMS III investigator and interventional neuroradiologist Dr. Thomas A. Tomsick said in an interview that the study results won't change his own clinical practice.
"IMS III is by no means the final word on combined therapy. In Cincinnati tomorrow, if a patient with a large NIH Stroke Severity score shows up and we're treating him with IV TPA at 2 hours from stroke onset, we're not going to do a CT angiogram to evaluate that patient. He's going to the cath lab for angiography to see if there's a clot suitable for endovascular therapy," said Dr. Tomsick, professor of radiology at the University of Cincinnati.
Five different endovascular device therapies were utilized in IMS III. As new devices reached clinical practice, their use was allowed by investigators in order to keep the randomized trial clinically relevant. But recruitment for the study was slow because so many clinicians were already convinced by anecdotal experience that combined therapy is better. So the endovascular therapies used most frequently in IMS III aren't the ones widely used in clinical practice today. Major new randomized trials are now getting underway comparing combined therapy using state-of-the-art, more effective stent clot retriever devices to intravenous TPA alone, he added.
In the New England Journal of Medicine report, the authors noted that "the use of randomization in ongoing and future stroke trials, rather than the treatment of eligible patients with endovascular therapy outside any trial, and minimization of the time to treatment will be essential for assessing the potential benefit of endovascular therapy for acute ischemic stroke."
No matter how future trials of combined therapy turn out, endovascular therapy is not going away, Dr. Broderick observed.
"It's a very good tool. The reason why is there are patients who can't get TPA. For example, roughly 5% of patients who undergo coronary artery bypass surgery have a stroke. If you have somebody with a big stroke 2 days after having their chest cracked, you can't use TPA. In that case, those endovascular devices are the way we can get up in there and get rid of the clot," he explained.
In an editorial accompanying the report in the New England Journal of Medicine (doi: 10.1056/NEJMe1215730), Dr. Marc I. Chimowitz declared that the clinical implication of IMS III is that endovascular therapy remains unproven and intravenous TPA should continue to be the first-line treatment for patients with acute ischemic stroke within 4.5 hours after stroke onset.
While new clinical trials featuring more effective IV clot busters, such as tenecteplase, and next-generation endovascular devices are urgently needed in an effort to improve stroke outcomes, patient recruitment is likely to continue to be a challenge in the current environment. This could be overcome if Medicare were to place a moratorium on reimbursement for endovascular therapy of acute ischemic stroke except as part of a randomized trial, according to Dr. Chimowitz, professor of neurology at the Medical University of South Carolina, Charleston.
The study was supported with grants from NIH and the National Institute of Neurological Disorders and Stroke; and by Genentech (which supplied the TPA); and EKOS, Concentric Medical, and Cordis Neurovascular (which supplied catheters); and Actilyse (alteplase) manufacturer Boehringer Ingelheim (which, along with Genentech and EKOS, provided support for investigator meetings). Dr. Broderick disclosed consulting fees from PhotoThera. Of the 28 other authors, disclosures for 14 were listed and included having received consulting fees, grant support, and/or lecture fees from a variety of device and pharmaceutical companies that include Genentech. Dr. Chimowitz, Dr. Silver, and Dr. Tomsick reported having no financial conflicts.
FROM THE INTERNATIONAL STROKE CONFERENCE
Major Finding: Endovascular therapy plus intravenous TPA showed no added outcome benefits, compared with TPA alone, with 40.8% and 38.7% of patients, respectively, reaching functional independence at 90 days.
Data Source: An international, phase III study of 656 patients with an acute moderate to severe ischemic stroke randomized 2:1 to IV TPA with endovascular therapy or IV TPA alone.
Disclosures: The study was supported with grants from NIH and the National Institute of Neurological Disorders and Stroke, and by Genentech (supplier of TPA), and EKOS, Concentric Medical, and Cordis Neurovascular (which supplied catheters). Dr. Broderick disclosed consulting fees from PhotoThera. Of the 28 other authors, disclosures for 14 were listed and included having received consulting fees, grant support, and/or lecture fees from a variety of device and pharmaceutical companies that included Genentech. Dr. Chimowitz, Dr. Silver, and Dr. Tomsick reported having no financial conflicts.
New stroke guidelines stress rtPA
Expanded use of clot-busting therapy is strongly endorsed for patients with acute ischemic stroke, while mechanical thrombectomy devices garner only lukewarm support in updated acute ischemic stroke guidelines from the American Heart Association and the American Stroke Association.
The "door-to-needle time" for intravenous administration of recombinant tissue plasminogen activator (rtPA) should be within 60 minutes from hospital arrival, according to a new class I, evidence level A recommendation.
Clinicians are advised to consider a noncontrast brain CT or MRI and a series of blood tests in all patients with suspected ischemic stroke before administering rtPA, but ultimately, the guidelines state that, "The only laboratory result required in all patients before fibrinolytics therapy is initiated is a glucose determination; use of finger-stick measurement devices is acceptable"(Stroke 2013 [doi:10.1161/STR.0b013e318284056a]).
The treatment window for rtPA therapy is also extended from 3 hours to 4.5 hours after stroke onset – as recommended in the AHA/ASA 2009 update on the extended time window for administration of fibrinolytic agents (Stroke 2009;40:2945-8).
"It’s clear that time is brain," lead guideline author Dr. Edward Jauch, director of emergency medicine at the Medical University of South Carolina in Charleston, said in an interview. "We are making a much greater emphasis that patients should be evaluated as quickly as possible and get treated as quickly as possible to give them the maximum opportunity for benefit."
Dr. Jauch acknowledges that the recommendations could reignite the long-standing controversy over the use of rtPA in stroke patients, particularly in light of the Food and Drug Administration’s recent decision not to expand approval of rtPA to include treatment up to 4.5 hours, as the European Medicines Agency has done.
"The FDA makes decisions largely based on American data, and we make guidelines based on all available data," he said, noting that safety data were also obtained from Genentech, maker of the rtPA activase (Alteplase).
Two European trials – the third International Stroke Trial (Lancet 2012;379:2352-63) and a British meta-analysis (Lancet 2012;379:2364-72) – reported last year that rtPA therapy within 6 hours of symptom onset increased the proportion of people who were alive and independent on follow-up.
Dr. Patrick Lyden, director of the stroke program at Cedars-Sinai Medical Center in Los Angeles, said reducing the battery of blood tests prior to rtPA administration is particularly important and pointed out that when the FDA first approved rtPA to treat stroke on the basis of a National Institutes of Health study, it "took the research protocol and turned it into a package insert.
"It’s taken the intervening 16 years for people to do studies and realize that you don’t need to do all the things in the package insert," he said. "So the American Heart Association, for the first time, is endorsing a much more practical, a much more optimal use of tPA for stroke."
The arrival of new classes of anticoagulants has prompted the AHA/ASA to add a new recommendation that the use of intravenous or intra-arterial rtPA in patients taking direct thrombin inhibitors like dabigatran (Pradaxa) or direct factor Xa inhibitors like rivaroxaban (Xarelto) "may be harmful" and is not recommended unless specialized testing is normal, or the patient has been off the drug for more than 2 days.
"I think that’s overreaching; I don’t think the data support that," said Dr. Lyden, who was not a member of the guidelines writing committee. He added that his team has had "no safety issues whatsoever" when administering the anticoagulant argatroban in patients on rtPA.
Dr. Jauch counters that data are lacking to support the safety of rtPA in patients on the new anticoagulants. Common blood tests – such as the international normalized ratio used for warfarin – do not register the anticoagulant effects of these drugs and reversal strategies are not yet known.
"As a community, we have a ways to go to figure out the optimal way to manage stroke in patients who come in on these drugs," he said.
When mechanical thrombectomy is pursued, stent retrievers are generally preferred to coil retrievers. The guidelines acknowledge that the Merci embolus retrieval system, Penumbra System, Solitaire FR, and TREVO thrombectomy devices "can be useful" in achieving recanalization alone or in combination with fibrinolytics in carefully selected patients, but that "their ability to improve patient outcomes has not been established" and continued study in randomized trials is warranted.
While these devices can restore blood flow very quickly, part of the problem in evaluating them is that the time from when the patient develops their stroke to when they get to the catheterization lab continues to increase, Dr. Jauch said.
"One of the challenges we have is, yes, we have a great device and if you happen to have your stroke on the cath table, you’re in great luck," he said.
"But if you transfer multiple times or there’s a delay in getting the patient evaluated sufficiently, then it diminishes the chance of getting a good outcome."
If feasible, patients should be transported to the closest available certified primary care stroke center or comprehensive stroke center, which in some cases may involve air transport or hospital bypass.
An estimated 40% of Americans, however, live in remote or rural areas without direct access to a comprehensive stroke center. For these patients, the updated guidelines emphasize the use of telemedicine to extend expert stroke care and optimize the use of intravenous rtPA, said guideline coauthor Dr. Bart M. Demaerschalk, professor of neurology at Mayo Clinic in Phoenix, which serves as a hub for 12 hospitals across Arizona with limited or no neurologic support.
"Even if air transport is available, the patients generally arrive when the respective treatment window is already closed," he said. "So telemedicine often means the difference between no treatment whatsoever, which is the usual case, and treatment."
The guidelines recommend tele-radiology systems approved by the FDA or "an equivalent organization" for sites without in-house imaging expertise for prompt review of brain CT and MRI scans in patients with suspected acute stroke.
Many guidelines committee members had financial ties with drug manufacturers and device makers.
Carotid endarterectomy is the most commonly performed open arterial procedure in the US and the most effective treatment available for appropriate stroke/TIA patients. It is accepted, however, that extracranial arterial disease accounts for less than half of all stroke/TIA patients. Three stories in this month's issue dealing with stroke and TIA and their accompanying comments by our editors underscore the need for all treating doctors, including vascular surgeons, to be alert for nonextracranial causes of neuro-ischemic events, both acute and chronic. Likewise, a proper work-up should not overlook evaluation of the cervical extracranial arteries which could deny the effectiveness of CEA to those who stand to benefit from it.
Dr. George Andros is Medical Editor of Vascular Specialist.
Carotid endarterectomy is the most commonly performed open arterial procedure in the US and the most effective treatment available for appropriate stroke/TIA patients. It is accepted, however, that extracranial arterial disease accounts for less than half of all stroke/TIA patients. Three stories in this month's issue dealing with stroke and TIA and their accompanying comments by our editors underscore the need for all treating doctors, including vascular surgeons, to be alert for nonextracranial causes of neuro-ischemic events, both acute and chronic. Likewise, a proper work-up should not overlook evaluation of the cervical extracranial arteries which could deny the effectiveness of CEA to those who stand to benefit from it.
Dr. George Andros is Medical Editor of Vascular Specialist.
Carotid endarterectomy is the most commonly performed open arterial procedure in the US and the most effective treatment available for appropriate stroke/TIA patients. It is accepted, however, that extracranial arterial disease accounts for less than half of all stroke/TIA patients. Three stories in this month's issue dealing with stroke and TIA and their accompanying comments by our editors underscore the need for all treating doctors, including vascular surgeons, to be alert for nonextracranial causes of neuro-ischemic events, both acute and chronic. Likewise, a proper work-up should not overlook evaluation of the cervical extracranial arteries which could deny the effectiveness of CEA to those who stand to benefit from it.
Dr. George Andros is Medical Editor of Vascular Specialist.
Expanded use of clot-busting therapy is strongly endorsed for patients with acute ischemic stroke, while mechanical thrombectomy devices garner only lukewarm support in updated acute ischemic stroke guidelines from the American Heart Association and the American Stroke Association.
The "door-to-needle time" for intravenous administration of recombinant tissue plasminogen activator (rtPA) should be within 60 minutes from hospital arrival, according to a new class I, evidence level A recommendation.
Clinicians are advised to consider a noncontrast brain CT or MRI and a series of blood tests in all patients with suspected ischemic stroke before administering rtPA, but ultimately, the guidelines state that, "The only laboratory result required in all patients before fibrinolytics therapy is initiated is a glucose determination; use of finger-stick measurement devices is acceptable"(Stroke 2013 [doi:10.1161/STR.0b013e318284056a]).
The treatment window for rtPA therapy is also extended from 3 hours to 4.5 hours after stroke onset – as recommended in the AHA/ASA 2009 update on the extended time window for administration of fibrinolytic agents (Stroke 2009;40:2945-8).
"It’s clear that time is brain," lead guideline author Dr. Edward Jauch, director of emergency medicine at the Medical University of South Carolina in Charleston, said in an interview. "We are making a much greater emphasis that patients should be evaluated as quickly as possible and get treated as quickly as possible to give them the maximum opportunity for benefit."
Dr. Jauch acknowledges that the recommendations could reignite the long-standing controversy over the use of rtPA in stroke patients, particularly in light of the Food and Drug Administration’s recent decision not to expand approval of rtPA to include treatment up to 4.5 hours, as the European Medicines Agency has done.
"The FDA makes decisions largely based on American data, and we make guidelines based on all available data," he said, noting that safety data were also obtained from Genentech, maker of the rtPA activase (Alteplase).
Two European trials – the third International Stroke Trial (Lancet 2012;379:2352-63) and a British meta-analysis (Lancet 2012;379:2364-72) – reported last year that rtPA therapy within 6 hours of symptom onset increased the proportion of people who were alive and independent on follow-up.
Dr. Patrick Lyden, director of the stroke program at Cedars-Sinai Medical Center in Los Angeles, said reducing the battery of blood tests prior to rtPA administration is particularly important and pointed out that when the FDA first approved rtPA to treat stroke on the basis of a National Institutes of Health study, it "took the research protocol and turned it into a package insert.
"It’s taken the intervening 16 years for people to do studies and realize that you don’t need to do all the things in the package insert," he said. "So the American Heart Association, for the first time, is endorsing a much more practical, a much more optimal use of tPA for stroke."
The arrival of new classes of anticoagulants has prompted the AHA/ASA to add a new recommendation that the use of intravenous or intra-arterial rtPA in patients taking direct thrombin inhibitors like dabigatran (Pradaxa) or direct factor Xa inhibitors like rivaroxaban (Xarelto) "may be harmful" and is not recommended unless specialized testing is normal, or the patient has been off the drug for more than 2 days.
"I think that’s overreaching; I don’t think the data support that," said Dr. Lyden, who was not a member of the guidelines writing committee. He added that his team has had "no safety issues whatsoever" when administering the anticoagulant argatroban in patients on rtPA.
Dr. Jauch counters that data are lacking to support the safety of rtPA in patients on the new anticoagulants. Common blood tests – such as the international normalized ratio used for warfarin – do not register the anticoagulant effects of these drugs and reversal strategies are not yet known.
"As a community, we have a ways to go to figure out the optimal way to manage stroke in patients who come in on these drugs," he said.
When mechanical thrombectomy is pursued, stent retrievers are generally preferred to coil retrievers. The guidelines acknowledge that the Merci embolus retrieval system, Penumbra System, Solitaire FR, and TREVO thrombectomy devices "can be useful" in achieving recanalization alone or in combination with fibrinolytics in carefully selected patients, but that "their ability to improve patient outcomes has not been established" and continued study in randomized trials is warranted.
While these devices can restore blood flow very quickly, part of the problem in evaluating them is that the time from when the patient develops their stroke to when they get to the catheterization lab continues to increase, Dr. Jauch said.
"One of the challenges we have is, yes, we have a great device and if you happen to have your stroke on the cath table, you’re in great luck," he said.
"But if you transfer multiple times or there’s a delay in getting the patient evaluated sufficiently, then it diminishes the chance of getting a good outcome."
If feasible, patients should be transported to the closest available certified primary care stroke center or comprehensive stroke center, which in some cases may involve air transport or hospital bypass.
An estimated 40% of Americans, however, live in remote or rural areas without direct access to a comprehensive stroke center. For these patients, the updated guidelines emphasize the use of telemedicine to extend expert stroke care and optimize the use of intravenous rtPA, said guideline coauthor Dr. Bart M. Demaerschalk, professor of neurology at Mayo Clinic in Phoenix, which serves as a hub for 12 hospitals across Arizona with limited or no neurologic support.
"Even if air transport is available, the patients generally arrive when the respective treatment window is already closed," he said. "So telemedicine often means the difference between no treatment whatsoever, which is the usual case, and treatment."
The guidelines recommend tele-radiology systems approved by the FDA or "an equivalent organization" for sites without in-house imaging expertise for prompt review of brain CT and MRI scans in patients with suspected acute stroke.
Many guidelines committee members had financial ties with drug manufacturers and device makers.
Expanded use of clot-busting therapy is strongly endorsed for patients with acute ischemic stroke, while mechanical thrombectomy devices garner only lukewarm support in updated acute ischemic stroke guidelines from the American Heart Association and the American Stroke Association.
The "door-to-needle time" for intravenous administration of recombinant tissue plasminogen activator (rtPA) should be within 60 minutes from hospital arrival, according to a new class I, evidence level A recommendation.
Clinicians are advised to consider a noncontrast brain CT or MRI and a series of blood tests in all patients with suspected ischemic stroke before administering rtPA, but ultimately, the guidelines state that, "The only laboratory result required in all patients before fibrinolytics therapy is initiated is a glucose determination; use of finger-stick measurement devices is acceptable"(Stroke 2013 [doi:10.1161/STR.0b013e318284056a]).
The treatment window for rtPA therapy is also extended from 3 hours to 4.5 hours after stroke onset – as recommended in the AHA/ASA 2009 update on the extended time window for administration of fibrinolytic agents (Stroke 2009;40:2945-8).
"It’s clear that time is brain," lead guideline author Dr. Edward Jauch, director of emergency medicine at the Medical University of South Carolina in Charleston, said in an interview. "We are making a much greater emphasis that patients should be evaluated as quickly as possible and get treated as quickly as possible to give them the maximum opportunity for benefit."
Dr. Jauch acknowledges that the recommendations could reignite the long-standing controversy over the use of rtPA in stroke patients, particularly in light of the Food and Drug Administration’s recent decision not to expand approval of rtPA to include treatment up to 4.5 hours, as the European Medicines Agency has done.
"The FDA makes decisions largely based on American data, and we make guidelines based on all available data," he said, noting that safety data were also obtained from Genentech, maker of the rtPA activase (Alteplase).
Two European trials – the third International Stroke Trial (Lancet 2012;379:2352-63) and a British meta-analysis (Lancet 2012;379:2364-72) – reported last year that rtPA therapy within 6 hours of symptom onset increased the proportion of people who were alive and independent on follow-up.
Dr. Patrick Lyden, director of the stroke program at Cedars-Sinai Medical Center in Los Angeles, said reducing the battery of blood tests prior to rtPA administration is particularly important and pointed out that when the FDA first approved rtPA to treat stroke on the basis of a National Institutes of Health study, it "took the research protocol and turned it into a package insert.
"It’s taken the intervening 16 years for people to do studies and realize that you don’t need to do all the things in the package insert," he said. "So the American Heart Association, for the first time, is endorsing a much more practical, a much more optimal use of tPA for stroke."
The arrival of new classes of anticoagulants has prompted the AHA/ASA to add a new recommendation that the use of intravenous or intra-arterial rtPA in patients taking direct thrombin inhibitors like dabigatran (Pradaxa) or direct factor Xa inhibitors like rivaroxaban (Xarelto) "may be harmful" and is not recommended unless specialized testing is normal, or the patient has been off the drug for more than 2 days.
"I think that’s overreaching; I don’t think the data support that," said Dr. Lyden, who was not a member of the guidelines writing committee. He added that his team has had "no safety issues whatsoever" when administering the anticoagulant argatroban in patients on rtPA.
Dr. Jauch counters that data are lacking to support the safety of rtPA in patients on the new anticoagulants. Common blood tests – such as the international normalized ratio used for warfarin – do not register the anticoagulant effects of these drugs and reversal strategies are not yet known.
"As a community, we have a ways to go to figure out the optimal way to manage stroke in patients who come in on these drugs," he said.
When mechanical thrombectomy is pursued, stent retrievers are generally preferred to coil retrievers. The guidelines acknowledge that the Merci embolus retrieval system, Penumbra System, Solitaire FR, and TREVO thrombectomy devices "can be useful" in achieving recanalization alone or in combination with fibrinolytics in carefully selected patients, but that "their ability to improve patient outcomes has not been established" and continued study in randomized trials is warranted.
While these devices can restore blood flow very quickly, part of the problem in evaluating them is that the time from when the patient develops their stroke to when they get to the catheterization lab continues to increase, Dr. Jauch said.
"One of the challenges we have is, yes, we have a great device and if you happen to have your stroke on the cath table, you’re in great luck," he said.
"But if you transfer multiple times or there’s a delay in getting the patient evaluated sufficiently, then it diminishes the chance of getting a good outcome."
If feasible, patients should be transported to the closest available certified primary care stroke center or comprehensive stroke center, which in some cases may involve air transport or hospital bypass.
An estimated 40% of Americans, however, live in remote or rural areas without direct access to a comprehensive stroke center. For these patients, the updated guidelines emphasize the use of telemedicine to extend expert stroke care and optimize the use of intravenous rtPA, said guideline coauthor Dr. Bart M. Demaerschalk, professor of neurology at Mayo Clinic in Phoenix, which serves as a hub for 12 hospitals across Arizona with limited or no neurologic support.
"Even if air transport is available, the patients generally arrive when the respective treatment window is already closed," he said. "So telemedicine often means the difference between no treatment whatsoever, which is the usual case, and treatment."
The guidelines recommend tele-radiology systems approved by the FDA or "an equivalent organization" for sites without in-house imaging expertise for prompt review of brain CT and MRI scans in patients with suspected acute stroke.
Many guidelines committee members had financial ties with drug manufacturers and device makers.
Carotid Stent Cell Design May Affect Outcomes
SAN DIEGO – The 30-day periprocedural outcomes in patients who underwent carotid artery stenting with closed-cell design stents were not significantly inferior to outcomes of those treated with carotid endarterectomy, a large meta-analysis demonstrated.
However, patients who underwent carotid endarterectomy (CEA) had significantly better 30-day periprocedural outcomes, compared with those who underwent carotid artery stenting (CAS) with open-cell design stents.
"A number of randomized clinical trials and meta-analyses have consistently showed the higher risk of periprocedural stroke in patients undergoing stenting when compared to endarterectomy," Dr. Mohammed A. Almekhlafi said at the annual meeting of the Society of Neurointerventional Surgery.
"One of the factors that has been implicated as a determinant of periprocedural neurological events is the stent cell design. The small free-cell area between the struts of a closed-cell stent theoretically provides better scaffolding of the vessel wall and superior plaque stabilization compared to the larger uncovered gaps in open-cell stents."
Dr. Almekhlafi, an interventional neurology fellow at the University of Calgary (Alta.), and his associates set out to investigate the impact of stent cell design on the outcome of randomized controlled trials comparing CAS vs. CEA. The stent cell design was divided into closed (meaning all stent struts are interconnected) or open (meaning not all stent-struts are interconnected). The primary outcome was a composite of the 30-day risk of stroke or death.
The final analysis included 4,949 patients from nine randomized clinical trials. Of these, 807 underwent CAS with closed-cell stenting, 1,657 underwent CAS with open-cell stenting, and 2,485 underwent CEA.
Dr. Almekhlafi reported that the primary outcome was significantly lower among patients in the CEA arm, compared with those in the CAS open-cell design arm (odds ratio, 1.84; P = .003). The primary outcome was lower among patients in the CEA arm, compared with those in the CAS closed-cell design arm, although this difference did not reach statistical significance (OR, 1.54; P = .29).
When the researchers limited their analysis to risk of 30-day periprocedural stroke, this outcome remained nonsignificant among patients in the CEA arm, compared with those in the CAS closed-cell design arm (OR 2.92; P = .22). However, the risk of 30-day periprocedural stroke remained significantly higher among patients in the CAS open-cell design arm, compared with those in the CEA arm (OR, 1.97; P = .0007).
"Uncertainty still exists regarding the impact of stent characteristics on CAS outcome," Dr. Almekhlafi said. "The size of the emboli might also be relevant."
He acknowledged certain limitations of the study, including the fact that trials included in this analysis did not randomize patients to open vs. closed stents, and that trials using the closed-design stents recruited fewer patients than did those using open-cell stents.
Dr. Almekhlafi said that he had no relevant financial disclosures to make.
Several randomized carotid trials have compared the outcome of carotid endarterectomy (CEA) and carotid artery stenting (CAS) over the past several years, with the majority, if not all, concluding that CAS had a higher rate of stroke than CEA, particularly in symptomatic patients. This has been thought to be secondary to the excessive microembolic burden during CAS. Several embolic protection devices have been designed to improve the safety profile of CAS, which have also been shown to induce new hyperintensities on diffusion-weighted magnetic resonance imaging (DWMRI) of the brain. There is also compelling evidence to suggest that distal filter protection devices are suboptimal, and that these embolic devices may actually increase the microembolization, compared with unprotected CAS procedures. Therefore, many authorities are advocating the use of proximal embolic protection devices (flow reversal system).
| Dr. AbuRhama |
In a recent systemic review (Stroke 2008;39:1911-19), comprised of 32 studies including a mix of CEA and CAS cases (incorporating 1,363 CAS procedures), demonstrated that closed-cell stents significantly reduced the new white lesion rate on DWMRI compared to open-cell stents. A recent study by Carlos Timaran of a randomized clinical trial of open- versus closed-cell stents for carotid stenting showed that cerebral embolization as detected by TCD and DWMRI occurred with similar frequency after CAS with open- and closed-cell stents.
The author did not support the superiority of any stent design in respect to cerebral embolization. Meanwhile, a recent study presented by Fritz Wodarg at the European Stroke Conference found that a primary outcome event of any stroke or death within 30 days of CAS occurred significantly less often in patients treated with closed-cell stents (6.1%) than in those with open-cell stents (10.1%, P = 0.003). Other trials, including the SPACE trial, showed that the use of closed-cell stents was associated with a significantly better outcome than open-cell stents. This present study will only add to the controversy of CAS and whether the stent design, open versus closed, has an impact on the outcome of this procedure and its future acceptance.
Dr. Ali F. AbuRahma is Professor of Surgery and Chief, Vascular & Endovascular Surgery, Robert C. Byrd Health Sciences Center, West Virginia University, Charleston Area Medical Center, Charleston W.V., and an associate medical editor for Vascular Specialist.
Several randomized carotid trials have compared the outcome of carotid endarterectomy (CEA) and carotid artery stenting (CAS) over the past several years, with the majority, if not all, concluding that CAS had a higher rate of stroke than CEA, particularly in symptomatic patients. This has been thought to be secondary to the excessive microembolic burden during CAS. Several embolic protection devices have been designed to improve the safety profile of CAS, which have also been shown to induce new hyperintensities on diffusion-weighted magnetic resonance imaging (DWMRI) of the brain. There is also compelling evidence to suggest that distal filter protection devices are suboptimal, and that these embolic devices may actually increase the microembolization, compared with unprotected CAS procedures. Therefore, many authorities are advocating the use of proximal embolic protection devices (flow reversal system).
| Dr. AbuRhama |
In a recent systemic review (Stroke 2008;39:1911-19), comprised of 32 studies including a mix of CEA and CAS cases (incorporating 1,363 CAS procedures), demonstrated that closed-cell stents significantly reduced the new white lesion rate on DWMRI compared to open-cell stents. A recent study by Carlos Timaran of a randomized clinical trial of open- versus closed-cell stents for carotid stenting showed that cerebral embolization as detected by TCD and DWMRI occurred with similar frequency after CAS with open- and closed-cell stents.
The author did not support the superiority of any stent design in respect to cerebral embolization. Meanwhile, a recent study presented by Fritz Wodarg at the European Stroke Conference found that a primary outcome event of any stroke or death within 30 days of CAS occurred significantly less often in patients treated with closed-cell stents (6.1%) than in those with open-cell stents (10.1%, P = 0.003). Other trials, including the SPACE trial, showed that the use of closed-cell stents was associated with a significantly better outcome than open-cell stents. This present study will only add to the controversy of CAS and whether the stent design, open versus closed, has an impact on the outcome of this procedure and its future acceptance.
Dr. Ali F. AbuRahma is Professor of Surgery and Chief, Vascular & Endovascular Surgery, Robert C. Byrd Health Sciences Center, West Virginia University, Charleston Area Medical Center, Charleston W.V., and an associate medical editor for Vascular Specialist.
Several randomized carotid trials have compared the outcome of carotid endarterectomy (CEA) and carotid artery stenting (CAS) over the past several years, with the majority, if not all, concluding that CAS had a higher rate of stroke than CEA, particularly in symptomatic patients. This has been thought to be secondary to the excessive microembolic burden during CAS. Several embolic protection devices have been designed to improve the safety profile of CAS, which have also been shown to induce new hyperintensities on diffusion-weighted magnetic resonance imaging (DWMRI) of the brain. There is also compelling evidence to suggest that distal filter protection devices are suboptimal, and that these embolic devices may actually increase the microembolization, compared with unprotected CAS procedures. Therefore, many authorities are advocating the use of proximal embolic protection devices (flow reversal system).
| Dr. AbuRhama |
In a recent systemic review (Stroke 2008;39:1911-19), comprised of 32 studies including a mix of CEA and CAS cases (incorporating 1,363 CAS procedures), demonstrated that closed-cell stents significantly reduced the new white lesion rate on DWMRI compared to open-cell stents. A recent study by Carlos Timaran of a randomized clinical trial of open- versus closed-cell stents for carotid stenting showed that cerebral embolization as detected by TCD and DWMRI occurred with similar frequency after CAS with open- and closed-cell stents.
The author did not support the superiority of any stent design in respect to cerebral embolization. Meanwhile, a recent study presented by Fritz Wodarg at the European Stroke Conference found that a primary outcome event of any stroke or death within 30 days of CAS occurred significantly less often in patients treated with closed-cell stents (6.1%) than in those with open-cell stents (10.1%, P = 0.003). Other trials, including the SPACE trial, showed that the use of closed-cell stents was associated with a significantly better outcome than open-cell stents. This present study will only add to the controversy of CAS and whether the stent design, open versus closed, has an impact on the outcome of this procedure and its future acceptance.
Dr. Ali F. AbuRahma is Professor of Surgery and Chief, Vascular & Endovascular Surgery, Robert C. Byrd Health Sciences Center, West Virginia University, Charleston Area Medical Center, Charleston W.V., and an associate medical editor for Vascular Specialist.
SAN DIEGO – The 30-day periprocedural outcomes in patients who underwent carotid artery stenting with closed-cell design stents were not significantly inferior to outcomes of those treated with carotid endarterectomy, a large meta-analysis demonstrated.
However, patients who underwent carotid endarterectomy (CEA) had significantly better 30-day periprocedural outcomes, compared with those who underwent carotid artery stenting (CAS) with open-cell design stents.
"A number of randomized clinical trials and meta-analyses have consistently showed the higher risk of periprocedural stroke in patients undergoing stenting when compared to endarterectomy," Dr. Mohammed A. Almekhlafi said at the annual meeting of the Society of Neurointerventional Surgery.
"One of the factors that has been implicated as a determinant of periprocedural neurological events is the stent cell design. The small free-cell area between the struts of a closed-cell stent theoretically provides better scaffolding of the vessel wall and superior plaque stabilization compared to the larger uncovered gaps in open-cell stents."
Dr. Almekhlafi, an interventional neurology fellow at the University of Calgary (Alta.), and his associates set out to investigate the impact of stent cell design on the outcome of randomized controlled trials comparing CAS vs. CEA. The stent cell design was divided into closed (meaning all stent struts are interconnected) or open (meaning not all stent-struts are interconnected). The primary outcome was a composite of the 30-day risk of stroke or death.
The final analysis included 4,949 patients from nine randomized clinical trials. Of these, 807 underwent CAS with closed-cell stenting, 1,657 underwent CAS with open-cell stenting, and 2,485 underwent CEA.
Dr. Almekhlafi reported that the primary outcome was significantly lower among patients in the CEA arm, compared with those in the CAS open-cell design arm (odds ratio, 1.84; P = .003). The primary outcome was lower among patients in the CEA arm, compared with those in the CAS closed-cell design arm, although this difference did not reach statistical significance (OR, 1.54; P = .29).
When the researchers limited their analysis to risk of 30-day periprocedural stroke, this outcome remained nonsignificant among patients in the CEA arm, compared with those in the CAS closed-cell design arm (OR 2.92; P = .22). However, the risk of 30-day periprocedural stroke remained significantly higher among patients in the CAS open-cell design arm, compared with those in the CEA arm (OR, 1.97; P = .0007).
"Uncertainty still exists regarding the impact of stent characteristics on CAS outcome," Dr. Almekhlafi said. "The size of the emboli might also be relevant."
He acknowledged certain limitations of the study, including the fact that trials included in this analysis did not randomize patients to open vs. closed stents, and that trials using the closed-design stents recruited fewer patients than did those using open-cell stents.
Dr. Almekhlafi said that he had no relevant financial disclosures to make.
SAN DIEGO – The 30-day periprocedural outcomes in patients who underwent carotid artery stenting with closed-cell design stents were not significantly inferior to outcomes of those treated with carotid endarterectomy, a large meta-analysis demonstrated.
However, patients who underwent carotid endarterectomy (CEA) had significantly better 30-day periprocedural outcomes, compared with those who underwent carotid artery stenting (CAS) with open-cell design stents.
"A number of randomized clinical trials and meta-analyses have consistently showed the higher risk of periprocedural stroke in patients undergoing stenting when compared to endarterectomy," Dr. Mohammed A. Almekhlafi said at the annual meeting of the Society of Neurointerventional Surgery.
"One of the factors that has been implicated as a determinant of periprocedural neurological events is the stent cell design. The small free-cell area between the struts of a closed-cell stent theoretically provides better scaffolding of the vessel wall and superior plaque stabilization compared to the larger uncovered gaps in open-cell stents."
Dr. Almekhlafi, an interventional neurology fellow at the University of Calgary (Alta.), and his associates set out to investigate the impact of stent cell design on the outcome of randomized controlled trials comparing CAS vs. CEA. The stent cell design was divided into closed (meaning all stent struts are interconnected) or open (meaning not all stent-struts are interconnected). The primary outcome was a composite of the 30-day risk of stroke or death.
The final analysis included 4,949 patients from nine randomized clinical trials. Of these, 807 underwent CAS with closed-cell stenting, 1,657 underwent CAS with open-cell stenting, and 2,485 underwent CEA.
Dr. Almekhlafi reported that the primary outcome was significantly lower among patients in the CEA arm, compared with those in the CAS open-cell design arm (odds ratio, 1.84; P = .003). The primary outcome was lower among patients in the CEA arm, compared with those in the CAS closed-cell design arm, although this difference did not reach statistical significance (OR, 1.54; P = .29).
When the researchers limited their analysis to risk of 30-day periprocedural stroke, this outcome remained nonsignificant among patients in the CEA arm, compared with those in the CAS closed-cell design arm (OR 2.92; P = .22). However, the risk of 30-day periprocedural stroke remained significantly higher among patients in the CAS open-cell design arm, compared with those in the CEA arm (OR, 1.97; P = .0007).
"Uncertainty still exists regarding the impact of stent characteristics on CAS outcome," Dr. Almekhlafi said. "The size of the emboli might also be relevant."
He acknowledged certain limitations of the study, including the fact that trials included in this analysis did not randomize patients to open vs. closed stents, and that trials using the closed-design stents recruited fewer patients than did those using open-cell stents.
Dr. Almekhlafi said that he had no relevant financial disclosures to make.
Major Finding: The 30-day risk of stroke or death was significantly lower among patients who underwent CEA, compared with those who underwent CAS with open-cell design stents (OR, 1.84; P = .003). The risk was also lower among patients who underwent CEA, compared with those who underwent CAS with closed-cell design stents, but this difference did not reach statistical significance (OR, 1.54; P = .29).
Data Source: Data are from a meta-analysis of 4,949 patients from nine randomized controlled trials comparing CAS vs. CEA.
Disclosures: Dr. Almekhlafi said that he had no relevant financial conflicts to disclose.
Mechanical Embolectomy Device Cleared for Acute Stroke Treatment
A thrombus retrieval device for use in patients experiencing an acute ischemic stroke has been cleared for marketing in the United States by the Food and Drug Administration, the manufacturer announced on Aug. 13.
The device will be marketed as the "Trevo Pro Retriever," according to the statement issued by Stryker Neurovascular.
In May, the company announced the results of the TREVO 2 study, a randomized, clinical trial that compared the Trevo device with another Stryker device, the Merci Retriever, in patients experiencing an acute ischemic stroke. The inclusion criteria were patients aged 18-85 years presenting with clinical signs and symptoms consistent with an acute ischemic stroke, who had either failed ; tissue plasminogen activator (TPA) therapy or in whom TPA was contraindicated, according to the description of the study.
The postprocedure revascularization rate was 92% among patients randomized to treatment with the Trevo device, compared with 77% among those treated with the Merci Retriever device, a significant difference, according to the statement announcing the device’s premarket notification clearance. Performance measures that included shorter hospital stays and improvement in the National Institutes of Health Stroke Scale also favored the Trevo Pro Retriever device, the statement said.
The device was developed by Concentric Medical, which was acquired by Stryker in October 2011.
Courtesy Stryker Neurovascular
An illustration of the Trevo Pro Retriever engaging a thrombus.
A thrombus retrieval device for use in patients experiencing an acute ischemic stroke has been cleared for marketing in the United States by the Food and Drug Administration, the manufacturer announced on Aug. 13.
The device will be marketed as the "Trevo Pro Retriever," according to the statement issued by Stryker Neurovascular.
In May, the company announced the results of the TREVO 2 study, a randomized, clinical trial that compared the Trevo device with another Stryker device, the Merci Retriever, in patients experiencing an acute ischemic stroke. The inclusion criteria were patients aged 18-85 years presenting with clinical signs and symptoms consistent with an acute ischemic stroke, who had either failed ; tissue plasminogen activator (TPA) therapy or in whom TPA was contraindicated, according to the description of the study.
The postprocedure revascularization rate was 92% among patients randomized to treatment with the Trevo device, compared with 77% among those treated with the Merci Retriever device, a significant difference, according to the statement announcing the device’s premarket notification clearance. Performance measures that included shorter hospital stays and improvement in the National Institutes of Health Stroke Scale also favored the Trevo Pro Retriever device, the statement said.
The device was developed by Concentric Medical, which was acquired by Stryker in October 2011.
Courtesy Stryker Neurovascular
An illustration of the Trevo Pro Retriever engaging a thrombus.
A thrombus retrieval device for use in patients experiencing an acute ischemic stroke has been cleared for marketing in the United States by the Food and Drug Administration, the manufacturer announced on Aug. 13.
The device will be marketed as the "Trevo Pro Retriever," according to the statement issued by Stryker Neurovascular.
In May, the company announced the results of the TREVO 2 study, a randomized, clinical trial that compared the Trevo device with another Stryker device, the Merci Retriever, in patients experiencing an acute ischemic stroke. The inclusion criteria were patients aged 18-85 years presenting with clinical signs and symptoms consistent with an acute ischemic stroke, who had either failed ; tissue plasminogen activator (TPA) therapy or in whom TPA was contraindicated, according to the description of the study.
The postprocedure revascularization rate was 92% among patients randomized to treatment with the Trevo device, compared with 77% among those treated with the Merci Retriever device, a significant difference, according to the statement announcing the device’s premarket notification clearance. Performance measures that included shorter hospital stays and improvement in the National Institutes of Health Stroke Scale also favored the Trevo Pro Retriever device, the statement said.
The device was developed by Concentric Medical, which was acquired by Stryker in October 2011.
Courtesy Stryker Neurovascular
An illustration of the Trevo Pro Retriever engaging a thrombus.