User login
As cord blood inventory increases, use declines
Recently, the US has seen a decline in the use of cord blood in transplants, according to a study by the RAND Corporation.
The research revealed a significant increase in the national cord blood inventory but also suggested many cord blood units may go unused because they have a total nucleated cell (TNC) count of less than 1.25 billion cells per unit.
RAND researchers conducted this study by examining past research about cord blood banks, analyzing data on public cord blood bank collection and use, and interviewing stakeholders such as blood bank leaders and transplant center physicians.
The data showed the number of hematopoietic stem cell transplants (HSCTs) performed in the US has increased in recent years.
However, the percentage of HSCTs in which cord blood is used has declined, from about 12% (n=822) of all HSCTs in 2010 to about 8% (n=718) of all HSCTs in 2015.
At the same time, public cord blood banks in the US have greatly increased their inventory. This is, at least in part, because the federal government began offering subsidies to public contractor banks with the goal of helping them obtain 150,000 new units of high-quality cord blood.
The numeric goal has been met, as the inventory currently exceeds 200,000 units. However, many of the units cannot be considered high-quality because they have a TNC count of less than 1.25 billion cells.
The researchers noted that the government subsidies are not structured to discourage the processing and banking of cord blood units with lower TNC counts.
In fact, the team found that about half of the national inventory is made up of cord blood units with TNC counts of less than 1.25 billion cells. They said the probability that such a unit will be used in a given year is about 0.1%, and there’s an 11% chance it will ever be used.
On the other hand, there is a 1% to 3% chance a cord blood unit with a TNC count of more than 1.5 billion will be used in a given year, and there’s a 61% chance it will ever be used.
Therefore, the researchers recommend the federal government find ways to encourage public cord blood banks to collect samples with higher TNC counts, as they will help expand the utility of the national inventory.
Support for this research was provided by the US Department of Health and Human Services, Office of the Assistant Secretary of Health. ![]()
Recently, the US has seen a decline in the use of cord blood in transplants, according to a study by the RAND Corporation.
The research revealed a significant increase in the national cord blood inventory but also suggested many cord blood units may go unused because they have a total nucleated cell (TNC) count of less than 1.25 billion cells per unit.
RAND researchers conducted this study by examining past research about cord blood banks, analyzing data on public cord blood bank collection and use, and interviewing stakeholders such as blood bank leaders and transplant center physicians.
The data showed the number of hematopoietic stem cell transplants (HSCTs) performed in the US has increased in recent years.
However, the percentage of HSCTs in which cord blood is used has declined, from about 12% (n=822) of all HSCTs in 2010 to about 8% (n=718) of all HSCTs in 2015.
At the same time, public cord blood banks in the US have greatly increased their inventory. This is, at least in part, because the federal government began offering subsidies to public contractor banks with the goal of helping them obtain 150,000 new units of high-quality cord blood.
The numeric goal has been met, as the inventory currently exceeds 200,000 units. However, many of the units cannot be considered high-quality because they have a TNC count of less than 1.25 billion cells.
The researchers noted that the government subsidies are not structured to discourage the processing and banking of cord blood units with lower TNC counts.
In fact, the team found that about half of the national inventory is made up of cord blood units with TNC counts of less than 1.25 billion cells. They said the probability that such a unit will be used in a given year is about 0.1%, and there’s an 11% chance it will ever be used.
On the other hand, there is a 1% to 3% chance a cord blood unit with a TNC count of more than 1.5 billion will be used in a given year, and there’s a 61% chance it will ever be used.
Therefore, the researchers recommend the federal government find ways to encourage public cord blood banks to collect samples with higher TNC counts, as they will help expand the utility of the national inventory.
Support for this research was provided by the US Department of Health and Human Services, Office of the Assistant Secretary of Health. ![]()
Recently, the US has seen a decline in the use of cord blood in transplants, according to a study by the RAND Corporation.
The research revealed a significant increase in the national cord blood inventory but also suggested many cord blood units may go unused because they have a total nucleated cell (TNC) count of less than 1.25 billion cells per unit.
RAND researchers conducted this study by examining past research about cord blood banks, analyzing data on public cord blood bank collection and use, and interviewing stakeholders such as blood bank leaders and transplant center physicians.
The data showed the number of hematopoietic stem cell transplants (HSCTs) performed in the US has increased in recent years.
However, the percentage of HSCTs in which cord blood is used has declined, from about 12% (n=822) of all HSCTs in 2010 to about 8% (n=718) of all HSCTs in 2015.
At the same time, public cord blood banks in the US have greatly increased their inventory. This is, at least in part, because the federal government began offering subsidies to public contractor banks with the goal of helping them obtain 150,000 new units of high-quality cord blood.
The numeric goal has been met, as the inventory currently exceeds 200,000 units. However, many of the units cannot be considered high-quality because they have a TNC count of less than 1.25 billion cells.
The researchers noted that the government subsidies are not structured to discourage the processing and banking of cord blood units with lower TNC counts.
In fact, the team found that about half of the national inventory is made up of cord blood units with TNC counts of less than 1.25 billion cells. They said the probability that such a unit will be used in a given year is about 0.1%, and there’s an 11% chance it will ever be used.
On the other hand, there is a 1% to 3% chance a cord blood unit with a TNC count of more than 1.5 billion will be used in a given year, and there’s a 61% chance it will ever be used.
Therefore, the researchers recommend the federal government find ways to encourage public cord blood banks to collect samples with higher TNC counts, as they will help expand the utility of the national inventory.
Support for this research was provided by the US Department of Health and Human Services, Office of the Assistant Secretary of Health. ![]()
Older RBCs may sometimes be better
New research suggests that, overall, the age of transfused red blood cells (RBCs) does not significantly impact outcomes in critically ill adults, but, in some cases, older RBCs may be the better choice.
The study showed no significant difference in 90-day mortality whether patients received RBCs stored for a mean of 11.8 days or 22.4 days.
Likewise, there were no significant differences in most other study endpoints.
However, febrile nonhemolytic transfusion reactions were more frequent in the short-term storage group.
And among the most severely ill patients, the transfusion of older RBCs was associated with fewer deaths at 90 days.
“Older blood appears to be like a good red wine—better with some age,” said study author D. James Cooper, MD, of Monash University and Alfred Hospital in Melbourne, Victoria, Australia.
“The findings of our trial confirm that the current duration of storage of red blood cells for transfusion is both safe and optimal.”
Dr Cooper and his colleagues reported their findings in NEJM.
The researchers conducted this trial from November 2012 through December 2016 at 59 centers in 5 countries—Australia, New Zealand, Ireland, Finland, and Saudi Arabia.
The study included nearly 5000 critically ill adults who were randomized to receive either the freshest available RBCs or the oldest available RBCs.
There were 2457 patients in the short-term storage group, where the mean RBC storage duration was 11.8 ± 5.3 days. And there were 2462 patients in the long-term storage group, where the mean RBC storage duration was 22.4 ± 7.5 days.
Baseline characteristics were largely similar between the 2 groups. However, patients were significantly older in the short-term storage group, with a mean age of 62.5 ± 16.8 years, compared to 61.4 ± 17.3 years in the long-term storage group (P=0.02).
The median time from randomization to first RBC transfusion was similar between the groups—1.6 hours in the short-term group and 1.5 hours in the long-term group. The mean number of RBC units was also similar—4.1 ± 6.0 and 4.0 ± 6.2, respectively. The use of other blood products was similar as well.
90-day mortality
The study’s primary endpoint was 90-day mortality, which was 24.8% (n=610) in the short-term storage group and 24.1% (n=594) in the long-term storage group. The unadjusted (u) odds ratio (OR) was 1.04 (P=0.57).
When the researchers adjusted for APACHE III risk of death, patient age, hemoglobin at randomization, blood group, and site, the adjusted (a) OR was 1.04 (P=0.59).
When the researchers looked at patient subgroups, they found a significant difference between the storage groups when it came to 90-day mortality according to APACHE III risk of death.
Patients with an APACHE III predicted risk of death at hospital discharge at a median of 21.5% or higher had a significantly higher rate of 90-day mortality if they received the freshest available RBCs rather than the oldest available RBCs—37.7% and 34.0%, respectively (uOR=1.18, P=0.05).
There were no significant differences in 90-day mortality in the other subgroups.
Secondary endpoints
There were no significant between-group differences in secondary endpoints, with the exception of febrile nonhemolytic transfusion reaction. The incidence of this outcome was 5.0% in the short-term storage group and 3.6% in the long-term group (uOR=1.42, P=0.01; aOR=1.45, P=0.01).
Other secondary endpoints included (data in the short-term and long-term groups, respectively):
- Death at day 28 (19.4% and 18.8%, uOR=1.04, P=0.61)
- Death at day 180 (28.5% and 28.1%, uOR=1.02, P=0.75)
- Persistent organ dysfunction or death at day 28 (23.3% and 22.3%, uOR=1.06, P=0.39)
- New bloodstream infection (1.4% and 1.6%, uOR=0.90, P=0.65)
- Duration of hospital stay (median 14.5 days and 14.7 days, P=0.42)
- Duration of stay in the intensive care unit (median 4.2 days for both, P=0.86)
- Invasive mechanical ventilation (58.6% and 59.3%, uOR=0.97, P=0.64)
- Renal-replacement therapy (13.9% and 14.6%, uOR=0.97, P=0.48).

New research suggests that, overall, the age of transfused red blood cells (RBCs) does not significantly impact outcomes in critically ill adults, but, in some cases, older RBCs may be the better choice.
The study showed no significant difference in 90-day mortality whether patients received RBCs stored for a mean of 11.8 days or 22.4 days.
Likewise, there were no significant differences in most other study endpoints.
However, febrile nonhemolytic transfusion reactions were more frequent in the short-term storage group.
And among the most severely ill patients, the transfusion of older RBCs was associated with fewer deaths at 90 days.
“Older blood appears to be like a good red wine—better with some age,” said study author D. James Cooper, MD, of Monash University and Alfred Hospital in Melbourne, Victoria, Australia.
“The findings of our trial confirm that the current duration of storage of red blood cells for transfusion is both safe and optimal.”
Dr Cooper and his colleagues reported their findings in NEJM.
The researchers conducted this trial from November 2012 through December 2016 at 59 centers in 5 countries—Australia, New Zealand, Ireland, Finland, and Saudi Arabia.
The study included nearly 5000 critically ill adults who were randomized to receive either the freshest available RBCs or the oldest available RBCs.
There were 2457 patients in the short-term storage group, where the mean RBC storage duration was 11.8 ± 5.3 days. And there were 2462 patients in the long-term storage group, where the mean RBC storage duration was 22.4 ± 7.5 days.
Baseline characteristics were largely similar between the 2 groups. However, patients were significantly older in the short-term storage group, with a mean age of 62.5 ± 16.8 years, compared to 61.4 ± 17.3 years in the long-term storage group (P=0.02).
The median time from randomization to first RBC transfusion was similar between the groups—1.6 hours in the short-term group and 1.5 hours in the long-term group. The mean number of RBC units was also similar—4.1 ± 6.0 and 4.0 ± 6.2, respectively. The use of other blood products was similar as well.
90-day mortality
The study’s primary endpoint was 90-day mortality, which was 24.8% (n=610) in the short-term storage group and 24.1% (n=594) in the long-term storage group. The unadjusted (u) odds ratio (OR) was 1.04 (P=0.57).
When the researchers adjusted for APACHE III risk of death, patient age, hemoglobin at randomization, blood group, and site, the adjusted (a) OR was 1.04 (P=0.59).
When the researchers looked at patient subgroups, they found a significant difference between the storage groups when it came to 90-day mortality according to APACHE III risk of death.
Patients with an APACHE III predicted risk of death at hospital discharge at a median of 21.5% or higher had a significantly higher rate of 90-day mortality if they received the freshest available RBCs rather than the oldest available RBCs—37.7% and 34.0%, respectively (uOR=1.18, P=0.05).
There were no significant differences in 90-day mortality in the other subgroups.
Secondary endpoints
There were no significant between-group differences in secondary endpoints, with the exception of febrile nonhemolytic transfusion reaction. The incidence of this outcome was 5.0% in the short-term storage group and 3.6% in the long-term group (uOR=1.42, P=0.01; aOR=1.45, P=0.01).
Other secondary endpoints included (data in the short-term and long-term groups, respectively):
- Death at day 28 (19.4% and 18.8%, uOR=1.04, P=0.61)
- Death at day 180 (28.5% and 28.1%, uOR=1.02, P=0.75)
- Persistent organ dysfunction or death at day 28 (23.3% and 22.3%, uOR=1.06, P=0.39)
- New bloodstream infection (1.4% and 1.6%, uOR=0.90, P=0.65)
- Duration of hospital stay (median 14.5 days and 14.7 days, P=0.42)
- Duration of stay in the intensive care unit (median 4.2 days for both, P=0.86)
- Invasive mechanical ventilation (58.6% and 59.3%, uOR=0.97, P=0.64)
- Renal-replacement therapy (13.9% and 14.6%, uOR=0.97, P=0.48).

New research suggests that, overall, the age of transfused red blood cells (RBCs) does not significantly impact outcomes in critically ill adults, but, in some cases, older RBCs may be the better choice.
The study showed no significant difference in 90-day mortality whether patients received RBCs stored for a mean of 11.8 days or 22.4 days.
Likewise, there were no significant differences in most other study endpoints.
However, febrile nonhemolytic transfusion reactions were more frequent in the short-term storage group.
And among the most severely ill patients, the transfusion of older RBCs was associated with fewer deaths at 90 days.
“Older blood appears to be like a good red wine—better with some age,” said study author D. James Cooper, MD, of Monash University and Alfred Hospital in Melbourne, Victoria, Australia.
“The findings of our trial confirm that the current duration of storage of red blood cells for transfusion is both safe and optimal.”
Dr Cooper and his colleagues reported their findings in NEJM.
The researchers conducted this trial from November 2012 through December 2016 at 59 centers in 5 countries—Australia, New Zealand, Ireland, Finland, and Saudi Arabia.
The study included nearly 5000 critically ill adults who were randomized to receive either the freshest available RBCs or the oldest available RBCs.
There were 2457 patients in the short-term storage group, where the mean RBC storage duration was 11.8 ± 5.3 days. And there were 2462 patients in the long-term storage group, where the mean RBC storage duration was 22.4 ± 7.5 days.
Baseline characteristics were largely similar between the 2 groups. However, patients were significantly older in the short-term storage group, with a mean age of 62.5 ± 16.8 years, compared to 61.4 ± 17.3 years in the long-term storage group (P=0.02).
The median time from randomization to first RBC transfusion was similar between the groups—1.6 hours in the short-term group and 1.5 hours in the long-term group. The mean number of RBC units was also similar—4.1 ± 6.0 and 4.0 ± 6.2, respectively. The use of other blood products was similar as well.
90-day mortality
The study’s primary endpoint was 90-day mortality, which was 24.8% (n=610) in the short-term storage group and 24.1% (n=594) in the long-term storage group. The unadjusted (u) odds ratio (OR) was 1.04 (P=0.57).
When the researchers adjusted for APACHE III risk of death, patient age, hemoglobin at randomization, blood group, and site, the adjusted (a) OR was 1.04 (P=0.59).
When the researchers looked at patient subgroups, they found a significant difference between the storage groups when it came to 90-day mortality according to APACHE III risk of death.
Patients with an APACHE III predicted risk of death at hospital discharge at a median of 21.5% or higher had a significantly higher rate of 90-day mortality if they received the freshest available RBCs rather than the oldest available RBCs—37.7% and 34.0%, respectively (uOR=1.18, P=0.05).
There were no significant differences in 90-day mortality in the other subgroups.
Secondary endpoints
There were no significant between-group differences in secondary endpoints, with the exception of febrile nonhemolytic transfusion reaction. The incidence of this outcome was 5.0% in the short-term storage group and 3.6% in the long-term group (uOR=1.42, P=0.01; aOR=1.45, P=0.01).
Other secondary endpoints included (data in the short-term and long-term groups, respectively):
- Death at day 28 (19.4% and 18.8%, uOR=1.04, P=0.61)
- Death at day 180 (28.5% and 28.1%, uOR=1.02, P=0.75)
- Persistent organ dysfunction or death at day 28 (23.3% and 22.3%, uOR=1.06, P=0.39)
- New bloodstream infection (1.4% and 1.6%, uOR=0.90, P=0.65)
- Duration of hospital stay (median 14.5 days and 14.7 days, P=0.42)
- Duration of stay in the intensive care unit (median 4.2 days for both, P=0.86)
- Invasive mechanical ventilation (58.6% and 59.3%, uOR=0.97, P=0.64)
- Renal-replacement therapy (13.9% and 14.6%, uOR=0.97, P=0.48).

FDA issues EUA for Zika virus test
The US Food and Drug Administration (FDA) has issued an emergency use authorization (EUA) for Thermo Fisher Scientific’s TaqPath Zika Virus Kit.
This means the TaqPath Zika Virus Kit is authorized for the qualitative detection of RNA from Zika virus and diagnosis of Zika virus infection in human serum and urine (collected alongside a patient-matched serum specimen) from individuals meeting the US Centers for Disease Control and Prevention’s criteria for testing.
The EUA does not mean the TaqPath Zika Virus Kit is FDA cleared or approved.
An EUA allows for the use of unapproved medical products or unapproved uses of approved medical products in an emergency. The products must be used to diagnose, treat, or prevent serious or life-threatening conditions caused by chemical, biological, radiological, or nuclear threat agents, when there are no adequate alternatives.
The EUA for the TaqPath Zika Virus Kit means the test is only authorized as long as circumstances exist to justify the emergency use of in vitro diagnostics for the detection of Zika virus, unless the authorization is terminated or revoked sooner.
Testing using the TaqPath Zika Virus Kit is authorized to be conducted by laboratories in the US that are certified under the Clinical Laboratory Improvement Amendments of 1988, 42 U.S.C. § 263a, to perform high complexity tests, or by similarly qualified non-US laboratories.
More information on the TaqPath Zika Virus Kit and other Zika tests granted EUAs can be found on the FDA’s EUA page. ![]()
The US Food and Drug Administration (FDA) has issued an emergency use authorization (EUA) for Thermo Fisher Scientific’s TaqPath Zika Virus Kit.
This means the TaqPath Zika Virus Kit is authorized for the qualitative detection of RNA from Zika virus and diagnosis of Zika virus infection in human serum and urine (collected alongside a patient-matched serum specimen) from individuals meeting the US Centers for Disease Control and Prevention’s criteria for testing.
The EUA does not mean the TaqPath Zika Virus Kit is FDA cleared or approved.
An EUA allows for the use of unapproved medical products or unapproved uses of approved medical products in an emergency. The products must be used to diagnose, treat, or prevent serious or life-threatening conditions caused by chemical, biological, radiological, or nuclear threat agents, when there are no adequate alternatives.
The EUA for the TaqPath Zika Virus Kit means the test is only authorized as long as circumstances exist to justify the emergency use of in vitro diagnostics for the detection of Zika virus, unless the authorization is terminated or revoked sooner.
Testing using the TaqPath Zika Virus Kit is authorized to be conducted by laboratories in the US that are certified under the Clinical Laboratory Improvement Amendments of 1988, 42 U.S.C. § 263a, to perform high complexity tests, or by similarly qualified non-US laboratories.
More information on the TaqPath Zika Virus Kit and other Zika tests granted EUAs can be found on the FDA’s EUA page. ![]()
The US Food and Drug Administration (FDA) has issued an emergency use authorization (EUA) for Thermo Fisher Scientific’s TaqPath Zika Virus Kit.
This means the TaqPath Zika Virus Kit is authorized for the qualitative detection of RNA from Zika virus and diagnosis of Zika virus infection in human serum and urine (collected alongside a patient-matched serum specimen) from individuals meeting the US Centers for Disease Control and Prevention’s criteria for testing.
The EUA does not mean the TaqPath Zika Virus Kit is FDA cleared or approved.
An EUA allows for the use of unapproved medical products or unapproved uses of approved medical products in an emergency. The products must be used to diagnose, treat, or prevent serious or life-threatening conditions caused by chemical, biological, radiological, or nuclear threat agents, when there are no adequate alternatives.
The EUA for the TaqPath Zika Virus Kit means the test is only authorized as long as circumstances exist to justify the emergency use of in vitro diagnostics for the detection of Zika virus, unless the authorization is terminated or revoked sooner.
Testing using the TaqPath Zika Virus Kit is authorized to be conducted by laboratories in the US that are certified under the Clinical Laboratory Improvement Amendments of 1988, 42 U.S.C. § 263a, to perform high complexity tests, or by similarly qualified non-US laboratories.
More information on the TaqPath Zika Virus Kit and other Zika tests granted EUAs can be found on the FDA’s EUA page. ![]()
Program reduces transfusions in leukemia, HSCT patients
New research suggests a patient blood management (PBM) program can safely reduce transfusion use in patients with acute leukemia and those undergoing hematopoietic stem cell transplant (HSCT).
The program significantly reduced the use of red blood cell (RBC) and platelet transfusions without increasing morbidity or mortality in patients who were receiving intensive chemotherapy to treat acute leukemia and in patients receiving an allogeneic or autologous HSCT.
“There has been a long-standing belief among hematologists that patients with leukemia undergoing chemotherapy should have a transfusion of red blood cells if their hemoglobin level drops below about 9 g/dL to help avoid adverse outcomes,” said study author Michael Leahy, MB ChB, a consultant hematologist at the University of Western Australia in Perth.
“Findings in this real-world, non-clinical trial setting challenge that belief.”
Dr Leahy and his colleagues published their findings in Transfusion.
The researchers said the PBM program used in this study was built around the “3 pillars” concept of PBM, which are:
- Optimize the patient’s RBC mass
- Minimize blood loss
- Harness and optimize the patient’s physiologic anemia reserve.
No specific transfusion thresholds were established. However, the hospitals did adopt a single-unit RBC transfusion policy for symptomatic anemic patients who were not actively bleeding.
Results
The study included 695 admissions to 2 major hospitals in Western Australia. Patients were admitted between July 2010 and December 2014 for treatment of acute leukemia or for autologous or allogeneic HSCT.
During this time, the patients received 3384 RBC units and 3639 units of platelets.
The mean number of platelet units transfused per hospital admission decreased 35% from baseline to the end of the study period, from 6.3 to 4.1 units (P<0.001).
The mean number of RBC units transfused decreased 39%, from 6.1 to 3.7 (P<0.001). Meanwhile, the use of single-unit RBC transfusions increased from 39% to 67% (P<0.001).
And the mean hemoglobin level prior to RBC transfusion decreased from 8.0 g/dL to 6.8 g/dL (P<0.001).
“This study suggests that patients undergoing chemotherapy with hematological disease may tolerate much lower levels of hemoglobin than previously thought,” said Shannon Farmer, an adjunct research fellow at the University of Western Australia.
“The transfusion threshold, the hemoglobin value at which a transfusion is given, dropped significantly from 8.0 g/dL at the beginning of the study to 6.8 g/dL at the end. This was associated with significant reductions in transfusion and substantial costs savings without evidence of harm to the patients. In fact, it was associated with a trend toward improved survival.”
The reduction in blood products over the study period resulted in a cost savings of AU$694,886 (US$654,007)—AU$389,537 (US$364,177) for RBCs and AU$305,349 (US$289,830) for platelets.
There were no significant changes over the study period in length of hospital stay, serious bleeding events, or in-hospital mortality.
There was a non-significant reduction in the mean length of hospital stay, from 24.5 days to 22.6 days (P=0.338). The difference was still not significant after the researchers adjusted for patient age, patient group, and comorbidities (incident rate ratio=0.88; 95% CI, 0.75-1.04).
The rate of serious bleeding increased over the study period, from 5.3% to 7.0% (P=0.582). After adjustment, the odds ratio was 1.14 (95% CI, 0.38-3.44; P=0.811).
There was a non-significant decrease in in-hospital mortality, from 5.3% to 2.0% (P=0.218). After adjustment, the odds ratio was 0.31 (95% CI, 0.06-1.56; P=0.154).
Based on these results, the researchers concluded that PBM programs could have a substantial impact in this patient population, reducing blood utilization and healthcare costs. ![]()
New research suggests a patient blood management (PBM) program can safely reduce transfusion use in patients with acute leukemia and those undergoing hematopoietic stem cell transplant (HSCT).
The program significantly reduced the use of red blood cell (RBC) and platelet transfusions without increasing morbidity or mortality in patients who were receiving intensive chemotherapy to treat acute leukemia and in patients receiving an allogeneic or autologous HSCT.
“There has been a long-standing belief among hematologists that patients with leukemia undergoing chemotherapy should have a transfusion of red blood cells if their hemoglobin level drops below about 9 g/dL to help avoid adverse outcomes,” said study author Michael Leahy, MB ChB, a consultant hematologist at the University of Western Australia in Perth.
“Findings in this real-world, non-clinical trial setting challenge that belief.”
Dr Leahy and his colleagues published their findings in Transfusion.
The researchers said the PBM program used in this study was built around the “3 pillars” concept of PBM, which are:
- Optimize the patient’s RBC mass
- Minimize blood loss
- Harness and optimize the patient’s physiologic anemia reserve.
No specific transfusion thresholds were established. However, the hospitals did adopt a single-unit RBC transfusion policy for symptomatic anemic patients who were not actively bleeding.
Results
The study included 695 admissions to 2 major hospitals in Western Australia. Patients were admitted between July 2010 and December 2014 for treatment of acute leukemia or for autologous or allogeneic HSCT.
During this time, the patients received 3384 RBC units and 3639 units of platelets.
The mean number of platelet units transfused per hospital admission decreased 35% from baseline to the end of the study period, from 6.3 to 4.1 units (P<0.001).
The mean number of RBC units transfused decreased 39%, from 6.1 to 3.7 (P<0.001). Meanwhile, the use of single-unit RBC transfusions increased from 39% to 67% (P<0.001).
And the mean hemoglobin level prior to RBC transfusion decreased from 8.0 g/dL to 6.8 g/dL (P<0.001).
“This study suggests that patients undergoing chemotherapy with hematological disease may tolerate much lower levels of hemoglobin than previously thought,” said Shannon Farmer, an adjunct research fellow at the University of Western Australia.
“The transfusion threshold, the hemoglobin value at which a transfusion is given, dropped significantly from 8.0 g/dL at the beginning of the study to 6.8 g/dL at the end. This was associated with significant reductions in transfusion and substantial costs savings without evidence of harm to the patients. In fact, it was associated with a trend toward improved survival.”
The reduction in blood products over the study period resulted in a cost savings of AU$694,886 (US$654,007)—AU$389,537 (US$364,177) for RBCs and AU$305,349 (US$289,830) for platelets.
There were no significant changes over the study period in length of hospital stay, serious bleeding events, or in-hospital mortality.
There was a non-significant reduction in the mean length of hospital stay, from 24.5 days to 22.6 days (P=0.338). The difference was still not significant after the researchers adjusted for patient age, patient group, and comorbidities (incident rate ratio=0.88; 95% CI, 0.75-1.04).
The rate of serious bleeding increased over the study period, from 5.3% to 7.0% (P=0.582). After adjustment, the odds ratio was 1.14 (95% CI, 0.38-3.44; P=0.811).
There was a non-significant decrease in in-hospital mortality, from 5.3% to 2.0% (P=0.218). After adjustment, the odds ratio was 0.31 (95% CI, 0.06-1.56; P=0.154).
Based on these results, the researchers concluded that PBM programs could have a substantial impact in this patient population, reducing blood utilization and healthcare costs. ![]()
New research suggests a patient blood management (PBM) program can safely reduce transfusion use in patients with acute leukemia and those undergoing hematopoietic stem cell transplant (HSCT).
The program significantly reduced the use of red blood cell (RBC) and platelet transfusions without increasing morbidity or mortality in patients who were receiving intensive chemotherapy to treat acute leukemia and in patients receiving an allogeneic or autologous HSCT.
“There has been a long-standing belief among hematologists that patients with leukemia undergoing chemotherapy should have a transfusion of red blood cells if their hemoglobin level drops below about 9 g/dL to help avoid adverse outcomes,” said study author Michael Leahy, MB ChB, a consultant hematologist at the University of Western Australia in Perth.
“Findings in this real-world, non-clinical trial setting challenge that belief.”
Dr Leahy and his colleagues published their findings in Transfusion.
The researchers said the PBM program used in this study was built around the “3 pillars” concept of PBM, which are:
- Optimize the patient’s RBC mass
- Minimize blood loss
- Harness and optimize the patient’s physiologic anemia reserve.
No specific transfusion thresholds were established. However, the hospitals did adopt a single-unit RBC transfusion policy for symptomatic anemic patients who were not actively bleeding.
Results
The study included 695 admissions to 2 major hospitals in Western Australia. Patients were admitted between July 2010 and December 2014 for treatment of acute leukemia or for autologous or allogeneic HSCT.
During this time, the patients received 3384 RBC units and 3639 units of platelets.
The mean number of platelet units transfused per hospital admission decreased 35% from baseline to the end of the study period, from 6.3 to 4.1 units (P<0.001).
The mean number of RBC units transfused decreased 39%, from 6.1 to 3.7 (P<0.001). Meanwhile, the use of single-unit RBC transfusions increased from 39% to 67% (P<0.001).
And the mean hemoglobin level prior to RBC transfusion decreased from 8.0 g/dL to 6.8 g/dL (P<0.001).
“This study suggests that patients undergoing chemotherapy with hematological disease may tolerate much lower levels of hemoglobin than previously thought,” said Shannon Farmer, an adjunct research fellow at the University of Western Australia.
“The transfusion threshold, the hemoglobin value at which a transfusion is given, dropped significantly from 8.0 g/dL at the beginning of the study to 6.8 g/dL at the end. This was associated with significant reductions in transfusion and substantial costs savings without evidence of harm to the patients. In fact, it was associated with a trend toward improved survival.”
The reduction in blood products over the study period resulted in a cost savings of AU$694,886 (US$654,007)—AU$389,537 (US$364,177) for RBCs and AU$305,349 (US$289,830) for platelets.
There were no significant changes over the study period in length of hospital stay, serious bleeding events, or in-hospital mortality.
There was a non-significant reduction in the mean length of hospital stay, from 24.5 days to 22.6 days (P=0.338). The difference was still not significant after the researchers adjusted for patient age, patient group, and comorbidities (incident rate ratio=0.88; 95% CI, 0.75-1.04).
The rate of serious bleeding increased over the study period, from 5.3% to 7.0% (P=0.582). After adjustment, the odds ratio was 1.14 (95% CI, 0.38-3.44; P=0.811).
There was a non-significant decrease in in-hospital mortality, from 5.3% to 2.0% (P=0.218). After adjustment, the odds ratio was 0.31 (95% CI, 0.06-1.56; P=0.154).
Based on these results, the researchers concluded that PBM programs could have a substantial impact in this patient population, reducing blood utilization and healthcare costs. ![]()
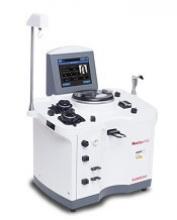
FDA clears new plasmapheresis system
The US Food and Drug Administration (FDA) has granted 510(k) clearance for Haemonetics Corporation’s NexSys PCS™ plasmapheresis system.
The open architecture of NexSys PCS facilitates bi-directional connectivity to donor management systems, enabling automated collection procedure programming and automated end-of-procedure documentation.
The guided operation, large touch screen, and on-screen troubleshooting assistance on NexSys PCS are designed to improve plasma center efficiency. The goal is to reduce donors’ time in centers and improve the centers’ collection capacity.
“NexSys PCS is designed to increase productivity and improve quality and compliance in plasma collection centers,” said Christopher Simon, CEO of Haemonetics.
“Each of these benefits is noteworthy and, when combined, we believe will unlock meaningful value for our customers.”
Haemonetics plans to begin limited production of NexSys PCS devices immediately and to pursue further regulatory clearances for additional enhancements to the overall product offering.
NexSys PCS was previously referred to as PCS 300. ![]()
The US Food and Drug Administration (FDA) has granted 510(k) clearance for Haemonetics Corporation’s NexSys PCS™ plasmapheresis system.
The open architecture of NexSys PCS facilitates bi-directional connectivity to donor management systems, enabling automated collection procedure programming and automated end-of-procedure documentation.
The guided operation, large touch screen, and on-screen troubleshooting assistance on NexSys PCS are designed to improve plasma center efficiency. The goal is to reduce donors’ time in centers and improve the centers’ collection capacity.
“NexSys PCS is designed to increase productivity and improve quality and compliance in plasma collection centers,” said Christopher Simon, CEO of Haemonetics.
“Each of these benefits is noteworthy and, when combined, we believe will unlock meaningful value for our customers.”
Haemonetics plans to begin limited production of NexSys PCS devices immediately and to pursue further regulatory clearances for additional enhancements to the overall product offering.
NexSys PCS was previously referred to as PCS 300. ![]()
The US Food and Drug Administration (FDA) has granted 510(k) clearance for Haemonetics Corporation’s NexSys PCS™ plasmapheresis system.
The open architecture of NexSys PCS facilitates bi-directional connectivity to donor management systems, enabling automated collection procedure programming and automated end-of-procedure documentation.
The guided operation, large touch screen, and on-screen troubleshooting assistance on NexSys PCS are designed to improve plasma center efficiency. The goal is to reduce donors’ time in centers and improve the centers’ collection capacity.
“NexSys PCS is designed to increase productivity and improve quality and compliance in plasma collection centers,” said Christopher Simon, CEO of Haemonetics.
“Each of these benefits is noteworthy and, when combined, we believe will unlock meaningful value for our customers.”
Haemonetics plans to begin limited production of NexSys PCS devices immediately and to pursue further regulatory clearances for additional enhancements to the overall product offering.
NexSys PCS was previously referred to as PCS 300. ![]()
Decreasing RBC transfusions saves center $1 million
Researchers have reported that interventions designed to decrease the need for red blood cell (RBC) transfusions proved effective when implemented at an academic medical center, and they resulted in an annual cost savings of more than $1 million.
The interventions included educational tools, a “best practice advisory,” and changes to the computerized provider order entry system.
They enabled the medical center to reduce multi-unit transfusions by 67.1% and transfusions for patients with hemoglobin (Hb) values of at least 7 g/dL by 47.4%.
Ian Jenkins, MD, of University of California San Diego (UCSD) Health, and his colleagues reported these results in The Joint Commission Journal on Quality and Patient Safety.
This work began with a multidisciplinary team at UCSD Health reviewing the transfusion literature on clinical trials, meta-analyses, guidelines, and improvement efforts.
The team used the information gleaned from this review and implemented the following interventions in an attempt to reduce unnecessary RBC transfusions:
- Educational tools (handouts, a video, PowerPoint presentations, etc)
- Enhancements to the health system’s computerized provider order entry system (eg, changing default RBC dose from 2 units to 1 unit)
- A “best practice advisory” intended to reduce unnecessary blood product use and costs by using real-time clinical decision support, a process for providing information at the point of care to help inform decisions about a patient’s care.
To assess the impact of their interventions, the researchers evaluated data on most non-infant, inpatient RBC transfusions given at UCSD Health between January 1, 2014, and September 30, 2016.
They excluded units given to patients with gastrointestinal bleeding and units given within 12 hours of a surgical procedure. The team said they excluded these transfusions because of the higher probability of unstable blood volume or special circumstances that might justify off-protocol transfusion.
The researchers then calculated the rate of inpatient RBC units transfused per 1000 patient-days (without exclusions), the percentage of inpatient RBC units transfused for patients with Hb ≥ 7 g/dL, and the percentage of multi-unit RBC transfusions, divided into 3 periods:
- Pre-intervention or baseline (January 1, 2014–September 30, 2014)
- During the intervention (October 1, 2014–April 30, 2015)
- Post-intervention (May 1, 2015–September 30, 2016).
There were 36,386 RBC units administered during the study period, which was 464,424 patient days.
Multi-unit transfusions decreased from 59.9% at baseline to 41.7% during the intervention period and 19.7% during the post-intervention period (P<0.0001).
Transfusions in patients with Hb values ≥ 7 g/dL decreased from 72.3% at baseline to 57.8% during the intervention period and 38.0% during the post-intervention period (P<0.0001).
The total RBC transfusion rate (units per 1000 patient-days) decreased from 89.8 at baseline to 78.1 during the intervention period and 72.7 during the post-intervention period (P<0.0001).
The estimated savings, based on a cost of $250 per RBC unit, was about $1,050,750 per year. ![]()
Researchers have reported that interventions designed to decrease the need for red blood cell (RBC) transfusions proved effective when implemented at an academic medical center, and they resulted in an annual cost savings of more than $1 million.
The interventions included educational tools, a “best practice advisory,” and changes to the computerized provider order entry system.
They enabled the medical center to reduce multi-unit transfusions by 67.1% and transfusions for patients with hemoglobin (Hb) values of at least 7 g/dL by 47.4%.
Ian Jenkins, MD, of University of California San Diego (UCSD) Health, and his colleagues reported these results in The Joint Commission Journal on Quality and Patient Safety.
This work began with a multidisciplinary team at UCSD Health reviewing the transfusion literature on clinical trials, meta-analyses, guidelines, and improvement efforts.
The team used the information gleaned from this review and implemented the following interventions in an attempt to reduce unnecessary RBC transfusions:
- Educational tools (handouts, a video, PowerPoint presentations, etc)
- Enhancements to the health system’s computerized provider order entry system (eg, changing default RBC dose from 2 units to 1 unit)
- A “best practice advisory” intended to reduce unnecessary blood product use and costs by using real-time clinical decision support, a process for providing information at the point of care to help inform decisions about a patient’s care.
To assess the impact of their interventions, the researchers evaluated data on most non-infant, inpatient RBC transfusions given at UCSD Health between January 1, 2014, and September 30, 2016.
They excluded units given to patients with gastrointestinal bleeding and units given within 12 hours of a surgical procedure. The team said they excluded these transfusions because of the higher probability of unstable blood volume or special circumstances that might justify off-protocol transfusion.
The researchers then calculated the rate of inpatient RBC units transfused per 1000 patient-days (without exclusions), the percentage of inpatient RBC units transfused for patients with Hb ≥ 7 g/dL, and the percentage of multi-unit RBC transfusions, divided into 3 periods:
- Pre-intervention or baseline (January 1, 2014–September 30, 2014)
- During the intervention (October 1, 2014–April 30, 2015)
- Post-intervention (May 1, 2015–September 30, 2016).
There were 36,386 RBC units administered during the study period, which was 464,424 patient days.
Multi-unit transfusions decreased from 59.9% at baseline to 41.7% during the intervention period and 19.7% during the post-intervention period (P<0.0001).
Transfusions in patients with Hb values ≥ 7 g/dL decreased from 72.3% at baseline to 57.8% during the intervention period and 38.0% during the post-intervention period (P<0.0001).
The total RBC transfusion rate (units per 1000 patient-days) decreased from 89.8 at baseline to 78.1 during the intervention period and 72.7 during the post-intervention period (P<0.0001).
The estimated savings, based on a cost of $250 per RBC unit, was about $1,050,750 per year. ![]()
Researchers have reported that interventions designed to decrease the need for red blood cell (RBC) transfusions proved effective when implemented at an academic medical center, and they resulted in an annual cost savings of more than $1 million.
The interventions included educational tools, a “best practice advisory,” and changes to the computerized provider order entry system.
They enabled the medical center to reduce multi-unit transfusions by 67.1% and transfusions for patients with hemoglobin (Hb) values of at least 7 g/dL by 47.4%.
Ian Jenkins, MD, of University of California San Diego (UCSD) Health, and his colleagues reported these results in The Joint Commission Journal on Quality and Patient Safety.
This work began with a multidisciplinary team at UCSD Health reviewing the transfusion literature on clinical trials, meta-analyses, guidelines, and improvement efforts.
The team used the information gleaned from this review and implemented the following interventions in an attempt to reduce unnecessary RBC transfusions:
- Educational tools (handouts, a video, PowerPoint presentations, etc)
- Enhancements to the health system’s computerized provider order entry system (eg, changing default RBC dose from 2 units to 1 unit)
- A “best practice advisory” intended to reduce unnecessary blood product use and costs by using real-time clinical decision support, a process for providing information at the point of care to help inform decisions about a patient’s care.
To assess the impact of their interventions, the researchers evaluated data on most non-infant, inpatient RBC transfusions given at UCSD Health between January 1, 2014, and September 30, 2016.
They excluded units given to patients with gastrointestinal bleeding and units given within 12 hours of a surgical procedure. The team said they excluded these transfusions because of the higher probability of unstable blood volume or special circumstances that might justify off-protocol transfusion.
The researchers then calculated the rate of inpatient RBC units transfused per 1000 patient-days (without exclusions), the percentage of inpatient RBC units transfused for patients with Hb ≥ 7 g/dL, and the percentage of multi-unit RBC transfusions, divided into 3 periods:
- Pre-intervention or baseline (January 1, 2014–September 30, 2014)
- During the intervention (October 1, 2014–April 30, 2015)
- Post-intervention (May 1, 2015–September 30, 2016).
There were 36,386 RBC units administered during the study period, which was 464,424 patient days.
Multi-unit transfusions decreased from 59.9% at baseline to 41.7% during the intervention period and 19.7% during the post-intervention period (P<0.0001).
Transfusions in patients with Hb values ≥ 7 g/dL decreased from 72.3% at baseline to 57.8% during the intervention period and 38.0% during the post-intervention period (P<0.0001).
The total RBC transfusion rate (units per 1000 patient-days) decreased from 89.8 at baseline to 78.1 during the intervention period and 72.7 during the post-intervention period (P<0.0001).
The estimated savings, based on a cost of $250 per RBC unit, was about $1,050,750 per year. ![]()
England, Scotland to shorten deferral for high-risk blood donors
England and Scotland are changing some of their policies regarding blood donation, shortening the deferral periods for donors who engage in “high-risk” sexual behavior.
Wales is considering making the same changes to its blood donation policies but has not yet made a commitment to do so.
The policy changes will mean that men who have sex with men (MSM), commercial sex workers, and people with sexual partners who have a high risk of sexually transmitted infections (including those who have been sexually active in areas where HIV is common) will be able to donate blood after 3 months have passed since their last sexual activity.
At present, MSMs and individuals with high-risk sexual partners can only donate blood after 12 months have passed since their last sexual activity, and commercial sex workers are permanently banned from giving blood.
The change in deferral periods will begin to take effect in England in early 2018 and in Scotland in November 2017. Until then, the existing rules still apply.
The Advisory Committee on the Safety of Blood, Tissues and Organs (SaBTO), which advises UK ministers and health departments, recommended the aforementioned policy changes following a review of blood donor criteria and risk assessment of certain behaviors.
SaBTO also recommended shortening the deferral period for potential donors who have undergone acupuncture, piercing, tattooing, and endoscopy, as well as those with a history of non-prescribed injectable drug use.
However, this change requires changing UK legislation, in addition to a derogation from or amendments to current European Union legislation.
“We welcome the review by SaBTO and the recommendations,” said Moira Carter, Associate Director of Donor Services and Transport for the Scottish National Blood Transfusion Service.
“The updates for donor eligibility will allow more people the opportunity to give blood. The changes take into account the latest available medical and scientific evidence about the risk of acquiring infections that can be passed on in blood, along with evidence supporting the reliability of the blood screening tests we use.”
“We’re pleased that the lifetime ban on former and current sex workers has been lifted, and the deferral period is now in line with other deferrals based on sexual behavior,” said Alex Phillips, Blood Donations Policy Lead at Terrence Higgins Trust in London.
“We know from our research that the majority of sex workers take great care of their sexual health, with 98% of sex workers we asked rating their sexual health as very important, 76% having a sexual health check up every 3 months, and 98% knowing their HIV status.”
“Medical evidence is, of course, constantly and quickly being updated, so it’s important that the deferral periods are regularly reviewed in line with the latest evidence. We therefore hope that today’s changes will pave the way for more progress as further evidence becomes available.” ![]()
England and Scotland are changing some of their policies regarding blood donation, shortening the deferral periods for donors who engage in “high-risk” sexual behavior.
Wales is considering making the same changes to its blood donation policies but has not yet made a commitment to do so.
The policy changes will mean that men who have sex with men (MSM), commercial sex workers, and people with sexual partners who have a high risk of sexually transmitted infections (including those who have been sexually active in areas where HIV is common) will be able to donate blood after 3 months have passed since their last sexual activity.
At present, MSMs and individuals with high-risk sexual partners can only donate blood after 12 months have passed since their last sexual activity, and commercial sex workers are permanently banned from giving blood.
The change in deferral periods will begin to take effect in England in early 2018 and in Scotland in November 2017. Until then, the existing rules still apply.
The Advisory Committee on the Safety of Blood, Tissues and Organs (SaBTO), which advises UK ministers and health departments, recommended the aforementioned policy changes following a review of blood donor criteria and risk assessment of certain behaviors.
SaBTO also recommended shortening the deferral period for potential donors who have undergone acupuncture, piercing, tattooing, and endoscopy, as well as those with a history of non-prescribed injectable drug use.
However, this change requires changing UK legislation, in addition to a derogation from or amendments to current European Union legislation.
“We welcome the review by SaBTO and the recommendations,” said Moira Carter, Associate Director of Donor Services and Transport for the Scottish National Blood Transfusion Service.
“The updates for donor eligibility will allow more people the opportunity to give blood. The changes take into account the latest available medical and scientific evidence about the risk of acquiring infections that can be passed on in blood, along with evidence supporting the reliability of the blood screening tests we use.”
“We’re pleased that the lifetime ban on former and current sex workers has been lifted, and the deferral period is now in line with other deferrals based on sexual behavior,” said Alex Phillips, Blood Donations Policy Lead at Terrence Higgins Trust in London.
“We know from our research that the majority of sex workers take great care of their sexual health, with 98% of sex workers we asked rating their sexual health as very important, 76% having a sexual health check up every 3 months, and 98% knowing their HIV status.”
“Medical evidence is, of course, constantly and quickly being updated, so it’s important that the deferral periods are regularly reviewed in line with the latest evidence. We therefore hope that today’s changes will pave the way for more progress as further evidence becomes available.” ![]()
England and Scotland are changing some of their policies regarding blood donation, shortening the deferral periods for donors who engage in “high-risk” sexual behavior.
Wales is considering making the same changes to its blood donation policies but has not yet made a commitment to do so.
The policy changes will mean that men who have sex with men (MSM), commercial sex workers, and people with sexual partners who have a high risk of sexually transmitted infections (including those who have been sexually active in areas where HIV is common) will be able to donate blood after 3 months have passed since their last sexual activity.
At present, MSMs and individuals with high-risk sexual partners can only donate blood after 12 months have passed since their last sexual activity, and commercial sex workers are permanently banned from giving blood.
The change in deferral periods will begin to take effect in England in early 2018 and in Scotland in November 2017. Until then, the existing rules still apply.
The Advisory Committee on the Safety of Blood, Tissues and Organs (SaBTO), which advises UK ministers and health departments, recommended the aforementioned policy changes following a review of blood donor criteria and risk assessment of certain behaviors.
SaBTO also recommended shortening the deferral period for potential donors who have undergone acupuncture, piercing, tattooing, and endoscopy, as well as those with a history of non-prescribed injectable drug use.
However, this change requires changing UK legislation, in addition to a derogation from or amendments to current European Union legislation.
“We welcome the review by SaBTO and the recommendations,” said Moira Carter, Associate Director of Donor Services and Transport for the Scottish National Blood Transfusion Service.
“The updates for donor eligibility will allow more people the opportunity to give blood. The changes take into account the latest available medical and scientific evidence about the risk of acquiring infections that can be passed on in blood, along with evidence supporting the reliability of the blood screening tests we use.”
“We’re pleased that the lifetime ban on former and current sex workers has been lifted, and the deferral period is now in line with other deferrals based on sexual behavior,” said Alex Phillips, Blood Donations Policy Lead at Terrence Higgins Trust in London.
“We know from our research that the majority of sex workers take great care of their sexual health, with 98% of sex workers we asked rating their sexual health as very important, 76% having a sexual health check up every 3 months, and 98% knowing their HIV status.”
“Medical evidence is, of course, constantly and quickly being updated, so it’s important that the deferral periods are regularly reviewed in line with the latest evidence. We therefore hope that today’s changes will pave the way for more progress as further evidence becomes available.”
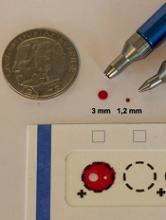
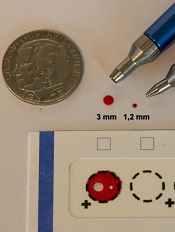
Study supports wider use of dried blood samples
Researchers have found evidence to suggest that dried blood samples may sometimes be a suitable alternative to conventional blood sampling.
The team measured levels of 92 proteins in millimeter-sized circles punched out of dried blood samples.
They found that, in many cases, little happens to these proteins when they are allowed to dry.
Most of the proteins remain unaltered after 30 years, or they change only minimally.
However, the proteins can be affected by storage temperatures.
Still, the researchers believe these results suggest dried blood samples could be used more widely—for routine health checks and to set up large-scale biobanks, with patients collecting the blood samples themselves.
“[Y]ou can prick your own finger and send in a dried blood spot by post,” study author Ulf Landegren, MD, PhD, of Uppsala University in Sweden.
“[A]t a minimal cost, it will be possible to build gigantic biobanks of samples obtained on a routine clinical basis. This means that samples can be taken before the clinical debut of a disease to identify markers of value for early diagnosis, improving the scope for curative treatment.”
Dr Landegren and his colleagues discussed these possibilities in a paper published in Molecular and Cellular Proteomics.
The researchers analyzed dried blood samples, measuring levels of 92 proteins that are relevant in oncology. To determine the effects of long-term storage, the team examined what happens to protein detection as an effect of the drying process.
Some of the dried blood samples analyzed had been collected recently, while others had been preserved for up to 30 years in biobanks in Sweden and Denmark. These 2 biobanks keep their dried blood samples at different temperatures: the Swedish one at +4°C and the Danish one at -24°C.
The researchers also looked at wet plasma samples kept at -70°C for corresponding periods of time.
“Our conclusion is that we can measure levels of 92 proteins with very high precision and sensitivity using PEA [proximity extension assay] technology in the tiny, punched-out discs from a dried blood spot,” said study author Johan Björkesten, a doctoral student at Uppsala University.
“The actual drying process has a negligible effect on the various proteins, and the effect is reproducible, which means that it can be included in the calculation.”
The researchers did find that long-term storage affects the detectability of certain proteins more than others.
Most proteins remain completely intact after 30 years or exhibit minimal changes. However, levels of some proteins decrease so that half the quantity remains after a period of between 10 and 50 years.
The researchers also found that a relatively low storage temperature is preferable for proteins that are affected by storage.
Protein detection was less affected when dried blood samples were stored at -24°C than when they were stored at +4°C. Over the 30-year period, detectability was not affected for 34% of proteins stored at +4°C and 76% of proteins stored at -24°C.
However, storing wet plasma at -70°C preserved proteins better than dried blood sample storage at -24°C. Detectability decreased for 5% of the proteins stored wet at -70°C for 45 years, compared to 24% for proteins in dried samples stored at -24°C for 30 years.
The researchers did note, though, that this part of their analysis was complicated by some confounding factors, so this was not a clear, direct comparison between wet and dry samples.
Researchers have found evidence to suggest that dried blood samples may sometimes be a suitable alternative to conventional blood sampling.
The team measured levels of 92 proteins in millimeter-sized circles punched out of dried blood samples.
They found that, in many cases, little happens to these proteins when they are allowed to dry.
Most of the proteins remain unaltered after 30 years, or they change only minimally.
However, the proteins can be affected by storage temperatures.
Still, the researchers believe these results suggest dried blood samples could be used more widely—for routine health checks and to set up large-scale biobanks, with patients collecting the blood samples themselves.
“[Y]ou can prick your own finger and send in a dried blood spot by post,” study author Ulf Landegren, MD, PhD, of Uppsala University in Sweden.
“[A]t a minimal cost, it will be possible to build gigantic biobanks of samples obtained on a routine clinical basis. This means that samples can be taken before the clinical debut of a disease to identify markers of value for early diagnosis, improving the scope for curative treatment.”
Dr Landegren and his colleagues discussed these possibilities in a paper published in Molecular and Cellular Proteomics.
The researchers analyzed dried blood samples, measuring levels of 92 proteins that are relevant in oncology. To determine the effects of long-term storage, the team examined what happens to protein detection as an effect of the drying process.
Some of the dried blood samples analyzed had been collected recently, while others had been preserved for up to 30 years in biobanks in Sweden and Denmark. These 2 biobanks keep their dried blood samples at different temperatures: the Swedish one at +4°C and the Danish one at -24°C.
The researchers also looked at wet plasma samples kept at -70°C for corresponding periods of time.
“Our conclusion is that we can measure levels of 92 proteins with very high precision and sensitivity using PEA [proximity extension assay] technology in the tiny, punched-out discs from a dried blood spot,” said study author Johan Björkesten, a doctoral student at Uppsala University.
“The actual drying process has a negligible effect on the various proteins, and the effect is reproducible, which means that it can be included in the calculation.”
The researchers did find that long-term storage affects the detectability of certain proteins more than others.
Most proteins remain completely intact after 30 years or exhibit minimal changes. However, levels of some proteins decrease so that half the quantity remains after a period of between 10 and 50 years.
The researchers also found that a relatively low storage temperature is preferable for proteins that are affected by storage.
Protein detection was less affected when dried blood samples were stored at -24°C than when they were stored at +4°C. Over the 30-year period, detectability was not affected for 34% of proteins stored at +4°C and 76% of proteins stored at -24°C.
However, storing wet plasma at -70°C preserved proteins better than dried blood sample storage at -24°C. Detectability decreased for 5% of the proteins stored wet at -70°C for 45 years, compared to 24% for proteins in dried samples stored at -24°C for 30 years.
The researchers did note, though, that this part of their analysis was complicated by some confounding factors, so this was not a clear, direct comparison between wet and dry samples.
Researchers have found evidence to suggest that dried blood samples may sometimes be a suitable alternative to conventional blood sampling.
The team measured levels of 92 proteins in millimeter-sized circles punched out of dried blood samples.
They found that, in many cases, little happens to these proteins when they are allowed to dry.
Most of the proteins remain unaltered after 30 years, or they change only minimally.
However, the proteins can be affected by storage temperatures.
Still, the researchers believe these results suggest dried blood samples could be used more widely—for routine health checks and to set up large-scale biobanks, with patients collecting the blood samples themselves.
“[Y]ou can prick your own finger and send in a dried blood spot by post,” study author Ulf Landegren, MD, PhD, of Uppsala University in Sweden.
“[A]t a minimal cost, it will be possible to build gigantic biobanks of samples obtained on a routine clinical basis. This means that samples can be taken before the clinical debut of a disease to identify markers of value for early diagnosis, improving the scope for curative treatment.”
Dr Landegren and his colleagues discussed these possibilities in a paper published in Molecular and Cellular Proteomics.
The researchers analyzed dried blood samples, measuring levels of 92 proteins that are relevant in oncology. To determine the effects of long-term storage, the team examined what happens to protein detection as an effect of the drying process.
Some of the dried blood samples analyzed had been collected recently, while others had been preserved for up to 30 years in biobanks in Sweden and Denmark. These 2 biobanks keep their dried blood samples at different temperatures: the Swedish one at +4°C and the Danish one at -24°C.
The researchers also looked at wet plasma samples kept at -70°C for corresponding periods of time.
“Our conclusion is that we can measure levels of 92 proteins with very high precision and sensitivity using PEA [proximity extension assay] technology in the tiny, punched-out discs from a dried blood spot,” said study author Johan Björkesten, a doctoral student at Uppsala University.
“The actual drying process has a negligible effect on the various proteins, and the effect is reproducible, which means that it can be included in the calculation.”
The researchers did find that long-term storage affects the detectability of certain proteins more than others.
Most proteins remain completely intact after 30 years or exhibit minimal changes. However, levels of some proteins decrease so that half the quantity remains after a period of between 10 and 50 years.
The researchers also found that a relatively low storage temperature is preferable for proteins that are affected by storage.
Protein detection was less affected when dried blood samples were stored at -24°C than when they were stored at +4°C. Over the 30-year period, detectability was not affected for 34% of proteins stored at +4°C and 76% of proteins stored at -24°C.
However, storing wet plasma at -70°C preserved proteins better than dried blood sample storage at -24°C. Detectability decreased for 5% of the proteins stored wet at -70°C for 45 years, compared to 24% for proteins in dried samples stored at -24°C for 30 years.
The researchers did note, though, that this part of their analysis was complicated by some confounding factors, so this was not a clear, direct comparison between wet and dry samples.
Assay available to screen donated blood for Zika
Blood banks in countries that accept the CE mark can now use the Procleix Zika Virus Assay to screen blood donations for the presence of Zika virus.
CE marking means a product conforms to relevant legislation for sale in the European economic area.
The Procleix Zika Virus Assay, which was developed by Hologic, Inc. and Grifols, is designed to run on the Procleix Panther System, an automated, nucleic acid technology (NAT) blood screening platform.
NAT enables detection of infectious agents in blood and plasma donations.
The Procleix Panther system automates all aspects of NAT-based blood screening on a single, integrated platform.
The system has received regulatory approvals in countries around the world, and it is in development for the US market.
In the US, the Procleix Zika Virus Assay is being used under an investigational new drug protocol in response to the US Food and Drug Administration’s recommendation to screen all US blood donations for Zika virus.
“The CE marking of the Procleix Zika virus assay is a further step in our mission to support safer blood donations, the result of our passion for innovation and the role we play as market leaders in transfusion medicine,” said Grifols Diagnostic Division President Carsten Schroeder.
About Zika virus
Zika is a mosquito-borne virus that was first identified in rhesus monkeys in Uganda in 1947 and in humans in 1952. Outbreaks of Zika virus have been recorded in Africa, the Americas, Asia, and the Pacific.
Zika virus is transmitted to humans primarily through the bite of an infected mosquito from the Aedes genus, mainly Aedes aegypti, in tropical regions.
Sexual transmission of Zika virus is also possible, and reports have suggested Zika can be transmitted via transfusion of blood products. Other modes of transmission are being investigated as well.
In total, 64 countries and territories have reported transmission of Zika virus since January 1, 2007.
Blood banks in countries that accept the CE mark can now use the Procleix Zika Virus Assay to screen blood donations for the presence of Zika virus.
CE marking means a product conforms to relevant legislation for sale in the European economic area.
The Procleix Zika Virus Assay, which was developed by Hologic, Inc. and Grifols, is designed to run on the Procleix Panther System, an automated, nucleic acid technology (NAT) blood screening platform.
NAT enables detection of infectious agents in blood and plasma donations.
The Procleix Panther system automates all aspects of NAT-based blood screening on a single, integrated platform.
The system has received regulatory approvals in countries around the world, and it is in development for the US market.
In the US, the Procleix Zika Virus Assay is being used under an investigational new drug protocol in response to the US Food and Drug Administration’s recommendation to screen all US blood donations for Zika virus.
“The CE marking of the Procleix Zika virus assay is a further step in our mission to support safer blood donations, the result of our passion for innovation and the role we play as market leaders in transfusion medicine,” said Grifols Diagnostic Division President Carsten Schroeder.
About Zika virus
Zika is a mosquito-borne virus that was first identified in rhesus monkeys in Uganda in 1947 and in humans in 1952. Outbreaks of Zika virus have been recorded in Africa, the Americas, Asia, and the Pacific.
Zika virus is transmitted to humans primarily through the bite of an infected mosquito from the Aedes genus, mainly Aedes aegypti, in tropical regions.
Sexual transmission of Zika virus is also possible, and reports have suggested Zika can be transmitted via transfusion of blood products. Other modes of transmission are being investigated as well.
In total, 64 countries and territories have reported transmission of Zika virus since January 1, 2007.
Blood banks in countries that accept the CE mark can now use the Procleix Zika Virus Assay to screen blood donations for the presence of Zika virus.
CE marking means a product conforms to relevant legislation for sale in the European economic area.
The Procleix Zika Virus Assay, which was developed by Hologic, Inc. and Grifols, is designed to run on the Procleix Panther System, an automated, nucleic acid technology (NAT) blood screening platform.
NAT enables detection of infectious agents in blood and plasma donations.
The Procleix Panther system automates all aspects of NAT-based blood screening on a single, integrated platform.
The system has received regulatory approvals in countries around the world, and it is in development for the US market.
In the US, the Procleix Zika Virus Assay is being used under an investigational new drug protocol in response to the US Food and Drug Administration’s recommendation to screen all US blood donations for Zika virus.
“The CE marking of the Procleix Zika virus assay is a further step in our mission to support safer blood donations, the result of our passion for innovation and the role we play as market leaders in transfusion medicine,” said Grifols Diagnostic Division President Carsten Schroeder.
About Zika virus
Zika is a mosquito-borne virus that was first identified in rhesus monkeys in Uganda in 1947 and in humans in 1952. Outbreaks of Zika virus have been recorded in Africa, the Americas, Asia, and the Pacific.
Zika virus is transmitted to humans primarily through the bite of an infected mosquito from the Aedes genus, mainly Aedes aegypti, in tropical regions.
Sexual transmission of Zika virus is also possible, and reports have suggested Zika can be transmitted via transfusion of blood products. Other modes of transmission are being investigated as well.
In total, 64 countries and territories have reported transmission of Zika virus since January 1, 2007.
Device reduces blood draw contamination
A novel device can significantly reduce contamination of blood cultures, according to research published in Clinical Infectious Diseases.
The SteriPath initial specimen diversion device (ISDD) is a blood collection system that diverts and sequesters the first 1.5 mL to 2 mL of blood drawn, which often carries contaminating skin cells and microbes.
By allowing for the disposal of this blood, the ISDD reduced blood culture contamination by 88%, when compared to standard phlebotomy procedures.
In reducing contamination, the SteriPath ISDD could reduce the unnecessary use of antibiotics, according to researchers.
“A lot of people think this is a minor problem,” said study author Mark Rupp, MD, of the University of Nebraska Medical Center in Omaha.
“However, contaminated blood cultures are a big deal. Physicians can be led astray, and patients may be harmed by additional tests and unnecessary antimicrobial therapy.”
For this study, Dr Rupp and his colleagues compared the ISDD and standard blood draw procedures, collecting a total of 1808 blood samples from 904 patients.
The researchers observed a significantly lower rate of blood culture contamination with the ISDD than with standard procedures—0.22% (2/904) and 1.78% (16/904), respectively (P=0.001).
“The 1.78% baseline rate of contamination may seem small, but we should strive to decrease adverse events to the lowest possible level because of the impact to the patient and the burden to our healthcare system,” Dr Rupp said. “We quite clearly showed the rate of contamination was significantly reduced, and that decrease has a very big impact.”
Dr Rupp and his colleagues also noted that the ISDD was about as sensitive as standard procedures. The ISDD detected true bacteremia in 7.2% (65/904) of patients, and standard procedures detected true bacteremia in 7.6% (69/904) of patients (P=0.41).
“What is important about this device is it can greatly limit the blood culture from being contaminated, so physicians are rarely fooled by false-positive results,” Dr Rupp said. “It gives clinicians confidence that results are accurate.”
This research was supported by Magnolia Medical Technologies, Inc., the company marketing the SteriPath ISDD.
A novel device can significantly reduce contamination of blood cultures, according to research published in Clinical Infectious Diseases.
The SteriPath initial specimen diversion device (ISDD) is a blood collection system that diverts and sequesters the first 1.5 mL to 2 mL of blood drawn, which often carries contaminating skin cells and microbes.
By allowing for the disposal of this blood, the ISDD reduced blood culture contamination by 88%, when compared to standard phlebotomy procedures.
In reducing contamination, the SteriPath ISDD could reduce the unnecessary use of antibiotics, according to researchers.
“A lot of people think this is a minor problem,” said study author Mark Rupp, MD, of the University of Nebraska Medical Center in Omaha.
“However, contaminated blood cultures are a big deal. Physicians can be led astray, and patients may be harmed by additional tests and unnecessary antimicrobial therapy.”
For this study, Dr Rupp and his colleagues compared the ISDD and standard blood draw procedures, collecting a total of 1808 blood samples from 904 patients.
The researchers observed a significantly lower rate of blood culture contamination with the ISDD than with standard procedures—0.22% (2/904) and 1.78% (16/904), respectively (P=0.001).
“The 1.78% baseline rate of contamination may seem small, but we should strive to decrease adverse events to the lowest possible level because of the impact to the patient and the burden to our healthcare system,” Dr Rupp said. “We quite clearly showed the rate of contamination was significantly reduced, and that decrease has a very big impact.”
Dr Rupp and his colleagues also noted that the ISDD was about as sensitive as standard procedures. The ISDD detected true bacteremia in 7.2% (65/904) of patients, and standard procedures detected true bacteremia in 7.6% (69/904) of patients (P=0.41).
“What is important about this device is it can greatly limit the blood culture from being contaminated, so physicians are rarely fooled by false-positive results,” Dr Rupp said. “It gives clinicians confidence that results are accurate.”
This research was supported by Magnolia Medical Technologies, Inc., the company marketing the SteriPath ISDD.
A novel device can significantly reduce contamination of blood cultures, according to research published in Clinical Infectious Diseases.
The SteriPath initial specimen diversion device (ISDD) is a blood collection system that diverts and sequesters the first 1.5 mL to 2 mL of blood drawn, which often carries contaminating skin cells and microbes.
By allowing for the disposal of this blood, the ISDD reduced blood culture contamination by 88%, when compared to standard phlebotomy procedures.
In reducing contamination, the SteriPath ISDD could reduce the unnecessary use of antibiotics, according to researchers.
“A lot of people think this is a minor problem,” said study author Mark Rupp, MD, of the University of Nebraska Medical Center in Omaha.
“However, contaminated blood cultures are a big deal. Physicians can be led astray, and patients may be harmed by additional tests and unnecessary antimicrobial therapy.”
For this study, Dr Rupp and his colleagues compared the ISDD and standard blood draw procedures, collecting a total of 1808 blood samples from 904 patients.
The researchers observed a significantly lower rate of blood culture contamination with the ISDD than with standard procedures—0.22% (2/904) and 1.78% (16/904), respectively (P=0.001).
“The 1.78% baseline rate of contamination may seem small, but we should strive to decrease adverse events to the lowest possible level because of the impact to the patient and the burden to our healthcare system,” Dr Rupp said. “We quite clearly showed the rate of contamination was significantly reduced, and that decrease has a very big impact.”
Dr Rupp and his colleagues also noted that the ISDD was about as sensitive as standard procedures. The ISDD detected true bacteremia in 7.2% (65/904) of patients, and standard procedures detected true bacteremia in 7.6% (69/904) of patients (P=0.41).
“What is important about this device is it can greatly limit the blood culture from being contaminated, so physicians are rarely fooled by false-positive results,” Dr Rupp said. “It gives clinicians confidence that results are accurate.”
This research was supported by Magnolia Medical Technologies, Inc., the company marketing the SteriPath ISDD.