User login
Artificial platelets halt bleeding faster

Credit: Andre E.X. Brown
Artificial platelet mimics can halt bleeding in mouse models 65% faster than nature can on its own, a new study has shown.
These platelet-like nanoparticles (PLNs) mimic the shape, size, flexibility, and surface chemistry of real blood platelets.
Researchers believe these design factors together are important for inducing clots to form faster at vascular injury sites while preventing harmful clots from forming indiscriminately elsewhere in the body.
The new technology is reported in ACS Nano.
Study investigator Anirban Sen Gupta, PhD, of Case Western Reserve in Cleveland, Ohio, previously designed peptide-based surface chemistries that mimic the clot-relevant activities of real platelets.
Building on this work, Dr Sen Gupta has now focused on incorporating morphological and mechanical cues that are naturally present in platelets to further refine the design.
“Morphological and mechanical factors influence the margination of natural platelets to the blood vessel wall, and only when they are near the wall can the critical clot-promoting chemical interactions take place,” he said.
These natural cues motivated Dr Sen Gupta to team up with Samir Mitragotri, PhD, of the University of California Santa Barbara, whose lab recently developed albumin-based technologies to make particles that mimic the geometry and mechanical properties of red blood cells and platelets.
Together, the researchers developed PLNs that combine morphological, mechanical, and surface chemical properties of natural platelets.
The team believes this refined design will be able to simulate natural platelet’s ability to collide effectively with larger and softer red blood cells in systemic blood flow. The collisions cause margination—pushing the platelets out of the main flow and closer to the blood vessel wall—increasing the probability of interacting with an injury site.
The surface coatings enable the PLNs to anchor to injury-site-specific proteins, von Willebrand factor and collagen, while inducing the natural and artificial platelets to aggregate faster at the injury site.
When the researchers injected the PLNs in mice, the artificial platelets formed clots at the site of injury 3 times faster than natural platelets alone in control mice.
The ability to interact selectively with injury site proteins, as well as the ability to remain mechanically flexible like natural platelets, enables these PLNs to safely ride through the smallest of blood vessels without causing unwanted clots. PLNs that don’t aggregate in a clot and continue to circulate are metabolized in 1 to 2 days.
The researchers believe their new artificial platelet design may be even more effective in larger-volume blood flows, where margination to the blood vessel wall is more prominent. They expect to soon begin testing those capabilities.
If the PLNs prove effective in humans, they could be used to stem bleeding in patients with clotting disorders, those suffering from traumatic injury, and patients undergoing surgeries.
The technology might also be used to deliver drugs to target sites in patients suffering from atherosclerosis, thrombosis, or other platelet-involved pathologic conditions. Dr Sen Gupta believes the PLNs could even be used to deliver cancer drugs to metastatic tumors that have high platelet interactions. ![]()

Credit: Andre E.X. Brown
Artificial platelet mimics can halt bleeding in mouse models 65% faster than nature can on its own, a new study has shown.
These platelet-like nanoparticles (PLNs) mimic the shape, size, flexibility, and surface chemistry of real blood platelets.
Researchers believe these design factors together are important for inducing clots to form faster at vascular injury sites while preventing harmful clots from forming indiscriminately elsewhere in the body.
The new technology is reported in ACS Nano.
Study investigator Anirban Sen Gupta, PhD, of Case Western Reserve in Cleveland, Ohio, previously designed peptide-based surface chemistries that mimic the clot-relevant activities of real platelets.
Building on this work, Dr Sen Gupta has now focused on incorporating morphological and mechanical cues that are naturally present in platelets to further refine the design.
“Morphological and mechanical factors influence the margination of natural platelets to the blood vessel wall, and only when they are near the wall can the critical clot-promoting chemical interactions take place,” he said.
These natural cues motivated Dr Sen Gupta to team up with Samir Mitragotri, PhD, of the University of California Santa Barbara, whose lab recently developed albumin-based technologies to make particles that mimic the geometry and mechanical properties of red blood cells and platelets.
Together, the researchers developed PLNs that combine morphological, mechanical, and surface chemical properties of natural platelets.
The team believes this refined design will be able to simulate natural platelet’s ability to collide effectively with larger and softer red blood cells in systemic blood flow. The collisions cause margination—pushing the platelets out of the main flow and closer to the blood vessel wall—increasing the probability of interacting with an injury site.
The surface coatings enable the PLNs to anchor to injury-site-specific proteins, von Willebrand factor and collagen, while inducing the natural and artificial platelets to aggregate faster at the injury site.
When the researchers injected the PLNs in mice, the artificial platelets formed clots at the site of injury 3 times faster than natural platelets alone in control mice.
The ability to interact selectively with injury site proteins, as well as the ability to remain mechanically flexible like natural platelets, enables these PLNs to safely ride through the smallest of blood vessels without causing unwanted clots. PLNs that don’t aggregate in a clot and continue to circulate are metabolized in 1 to 2 days.
The researchers believe their new artificial platelet design may be even more effective in larger-volume blood flows, where margination to the blood vessel wall is more prominent. They expect to soon begin testing those capabilities.
If the PLNs prove effective in humans, they could be used to stem bleeding in patients with clotting disorders, those suffering from traumatic injury, and patients undergoing surgeries.
The technology might also be used to deliver drugs to target sites in patients suffering from atherosclerosis, thrombosis, or other platelet-involved pathologic conditions. Dr Sen Gupta believes the PLNs could even be used to deliver cancer drugs to metastatic tumors that have high platelet interactions. ![]()

Credit: Andre E.X. Brown
Artificial platelet mimics can halt bleeding in mouse models 65% faster than nature can on its own, a new study has shown.
These platelet-like nanoparticles (PLNs) mimic the shape, size, flexibility, and surface chemistry of real blood platelets.
Researchers believe these design factors together are important for inducing clots to form faster at vascular injury sites while preventing harmful clots from forming indiscriminately elsewhere in the body.
The new technology is reported in ACS Nano.
Study investigator Anirban Sen Gupta, PhD, of Case Western Reserve in Cleveland, Ohio, previously designed peptide-based surface chemistries that mimic the clot-relevant activities of real platelets.
Building on this work, Dr Sen Gupta has now focused on incorporating morphological and mechanical cues that are naturally present in platelets to further refine the design.
“Morphological and mechanical factors influence the margination of natural platelets to the blood vessel wall, and only when they are near the wall can the critical clot-promoting chemical interactions take place,” he said.
These natural cues motivated Dr Sen Gupta to team up with Samir Mitragotri, PhD, of the University of California Santa Barbara, whose lab recently developed albumin-based technologies to make particles that mimic the geometry and mechanical properties of red blood cells and platelets.
Together, the researchers developed PLNs that combine morphological, mechanical, and surface chemical properties of natural platelets.
The team believes this refined design will be able to simulate natural platelet’s ability to collide effectively with larger and softer red blood cells in systemic blood flow. The collisions cause margination—pushing the platelets out of the main flow and closer to the blood vessel wall—increasing the probability of interacting with an injury site.
The surface coatings enable the PLNs to anchor to injury-site-specific proteins, von Willebrand factor and collagen, while inducing the natural and artificial platelets to aggregate faster at the injury site.
When the researchers injected the PLNs in mice, the artificial platelets formed clots at the site of injury 3 times faster than natural platelets alone in control mice.
The ability to interact selectively with injury site proteins, as well as the ability to remain mechanically flexible like natural platelets, enables these PLNs to safely ride through the smallest of blood vessels without causing unwanted clots. PLNs that don’t aggregate in a clot and continue to circulate are metabolized in 1 to 2 days.
The researchers believe their new artificial platelet design may be even more effective in larger-volume blood flows, where margination to the blood vessel wall is more prominent. They expect to soon begin testing those capabilities.
If the PLNs prove effective in humans, they could be used to stem bleeding in patients with clotting disorders, those suffering from traumatic injury, and patients undergoing surgeries.
The technology might also be used to deliver drugs to target sites in patients suffering from atherosclerosis, thrombosis, or other platelet-involved pathologic conditions. Dr Sen Gupta believes the PLNs could even be used to deliver cancer drugs to metastatic tumors that have high platelet interactions. ![]()
Study reveals gap in patient blood management
PHILADELPHIA—Healthcare professionals may not be using blood management interventions in a majority of patients receiving red blood cell (RBC) transfusions, a large study suggests.
The research showed that 72 US hospitals have made strides in reducing the use of RBCs in patients undergoing orthopedic and cardiac surgery.
And smaller reductions have occurred in patients with gastrointestinal bleeding, obstetric patients, and those receiving bone marrow transplants or inpatient chemotherapy.
However, more than 60% of the transfusions studied were given to patients who did not belong to any of the aforementioned groups.
“So when we target our [patient blood management] interventions to these kind of surgical procedures, in fact, we’re looking at only about 40% of the red cell use, and 60% goes to a myriad of other patients,” said Robert L. Thurer, MD, of Haemonetics in Braintree, Massachusetts.
He added that the groups of specific patient populations within that 60% were so small—“2% of patients here and 3% of patients there”—that it was too difficult to examine them individually in this study.
Dr Thurer presented this research at the AABB Annual Meeting 2014 (abstract S65-030K).
He and his colleagues wanted to determine which specific groups of patients have been most affected by patient blood management, understand further opportunities for decreasing transfusion use, and project future blood needs.
So the researchers analyzed data from 3,946,428 inpatients at 72 US hospitals, comparing the use of RBC transfusions in 2009/2010 to use in 2013.
In 2009/2010, there were 1,378,581 patients admitted to the hospitals, the RBC transfusion rate was 11.5.%, and the utilization (total units/total patients) was 0.41. In 2013, there were 861,804 patients, the transfusion rate was 10%, and the utilization was 0.34.
So from 2009/2010 to 2013, there was a 13% reduction in transfusion rate and a 17% reduction in utilization.
The greatest decrease was in orthopedic surgery patients. In those undergoing hip and knee joint replacement surgery, there was a 45% reduction in transfusion rate and a 43% reduction in utilization. In patients with a hip fracture, there was a 20% decrease in transfusion rate and a 20% decrease in utilization.
There was a smaller, though still sizable, reduction in transfusion use among patients undergoing cardiac surgery—a 15% decrease in transfusion rate and an 18% decrease in utilization.
“My personal thoughts about this is that it represents, certainly, lower transfusion triggers, which are becoming more widely adopted, [and] the use of antifibrinolytic drugs, particularly in orthopedic surgeries,” Dr Thurer said.
“We like to think that comprehensive coagulation testing and better matching of coagulation abnormalities contributes to this. Perhaps correction of preoperative anemia [contributes] for elective patients. And, clearly, surgical techniques have evolved, and, as they do, blood loss goes down.”
Reductions in RBC use were also seen in patients with gastrointestinal bleeding, where there was a 3% decrease in transfusion rate and a 13% decrease in utilization.
“For gastrointestinal bleeding, I think the lower transfusion triggers [have made an impact], but there’s also . . . more interest in timely interventions to stop bleeding,” Dr Thurer said.
“So rather than the gastroenterologist saying, ‘Correct the hematocrit and the coagulation factors, and I’ll stop the bleeding in the morning,’ we’re seeing now much more interest in very prompt endoscopy to stop bleeding. And as you know, the way to stop giving transfusions is to stop the patient from bleeding.”
Obstetric patients saw a 5% reduction in transfusion rate and an 8% reduction in utilization. And patients undergoing bone marrow transplant or inpatient chemotherapy saw a 6% decrease in transfusion rate and an 8% decrease in utilization.
Other transplant patients actually saw an increase in RBC transfusions. In liver transplant recipients, there was a 2.2% increase in transfusion rate and a 6% increase in utilization. And in kidney transplant recipients, there was a 0.2% increase in transfusion rate and a 19% increase in utilization.
However, Dr Thurer noted that the majority of RBC transfusions are administered to patients outside of these groups. In 2013, 60.6% of transfusions went to patients who did not fit into any of the aforementioned categories.
“So clearly,” he concluded, “further studies are needed to determine whether these reductions that we’ve seen in some areas can be implemented in a wider variety of patients.” ![]()
PHILADELPHIA—Healthcare professionals may not be using blood management interventions in a majority of patients receiving red blood cell (RBC) transfusions, a large study suggests.
The research showed that 72 US hospitals have made strides in reducing the use of RBCs in patients undergoing orthopedic and cardiac surgery.
And smaller reductions have occurred in patients with gastrointestinal bleeding, obstetric patients, and those receiving bone marrow transplants or inpatient chemotherapy.
However, more than 60% of the transfusions studied were given to patients who did not belong to any of the aforementioned groups.
“So when we target our [patient blood management] interventions to these kind of surgical procedures, in fact, we’re looking at only about 40% of the red cell use, and 60% goes to a myriad of other patients,” said Robert L. Thurer, MD, of Haemonetics in Braintree, Massachusetts.
He added that the groups of specific patient populations within that 60% were so small—“2% of patients here and 3% of patients there”—that it was too difficult to examine them individually in this study.
Dr Thurer presented this research at the AABB Annual Meeting 2014 (abstract S65-030K).
He and his colleagues wanted to determine which specific groups of patients have been most affected by patient blood management, understand further opportunities for decreasing transfusion use, and project future blood needs.
So the researchers analyzed data from 3,946,428 inpatients at 72 US hospitals, comparing the use of RBC transfusions in 2009/2010 to use in 2013.
In 2009/2010, there were 1,378,581 patients admitted to the hospitals, the RBC transfusion rate was 11.5.%, and the utilization (total units/total patients) was 0.41. In 2013, there were 861,804 patients, the transfusion rate was 10%, and the utilization was 0.34.
So from 2009/2010 to 2013, there was a 13% reduction in transfusion rate and a 17% reduction in utilization.
The greatest decrease was in orthopedic surgery patients. In those undergoing hip and knee joint replacement surgery, there was a 45% reduction in transfusion rate and a 43% reduction in utilization. In patients with a hip fracture, there was a 20% decrease in transfusion rate and a 20% decrease in utilization.
There was a smaller, though still sizable, reduction in transfusion use among patients undergoing cardiac surgery—a 15% decrease in transfusion rate and an 18% decrease in utilization.
“My personal thoughts about this is that it represents, certainly, lower transfusion triggers, which are becoming more widely adopted, [and] the use of antifibrinolytic drugs, particularly in orthopedic surgeries,” Dr Thurer said.
“We like to think that comprehensive coagulation testing and better matching of coagulation abnormalities contributes to this. Perhaps correction of preoperative anemia [contributes] for elective patients. And, clearly, surgical techniques have evolved, and, as they do, blood loss goes down.”
Reductions in RBC use were also seen in patients with gastrointestinal bleeding, where there was a 3% decrease in transfusion rate and a 13% decrease in utilization.
“For gastrointestinal bleeding, I think the lower transfusion triggers [have made an impact], but there’s also . . . more interest in timely interventions to stop bleeding,” Dr Thurer said.
“So rather than the gastroenterologist saying, ‘Correct the hematocrit and the coagulation factors, and I’ll stop the bleeding in the morning,’ we’re seeing now much more interest in very prompt endoscopy to stop bleeding. And as you know, the way to stop giving transfusions is to stop the patient from bleeding.”
Obstetric patients saw a 5% reduction in transfusion rate and an 8% reduction in utilization. And patients undergoing bone marrow transplant or inpatient chemotherapy saw a 6% decrease in transfusion rate and an 8% decrease in utilization.
Other transplant patients actually saw an increase in RBC transfusions. In liver transplant recipients, there was a 2.2% increase in transfusion rate and a 6% increase in utilization. And in kidney transplant recipients, there was a 0.2% increase in transfusion rate and a 19% increase in utilization.
However, Dr Thurer noted that the majority of RBC transfusions are administered to patients outside of these groups. In 2013, 60.6% of transfusions went to patients who did not fit into any of the aforementioned categories.
“So clearly,” he concluded, “further studies are needed to determine whether these reductions that we’ve seen in some areas can be implemented in a wider variety of patients.” ![]()
PHILADELPHIA—Healthcare professionals may not be using blood management interventions in a majority of patients receiving red blood cell (RBC) transfusions, a large study suggests.
The research showed that 72 US hospitals have made strides in reducing the use of RBCs in patients undergoing orthopedic and cardiac surgery.
And smaller reductions have occurred in patients with gastrointestinal bleeding, obstetric patients, and those receiving bone marrow transplants or inpatient chemotherapy.
However, more than 60% of the transfusions studied were given to patients who did not belong to any of the aforementioned groups.
“So when we target our [patient blood management] interventions to these kind of surgical procedures, in fact, we’re looking at only about 40% of the red cell use, and 60% goes to a myriad of other patients,” said Robert L. Thurer, MD, of Haemonetics in Braintree, Massachusetts.
He added that the groups of specific patient populations within that 60% were so small—“2% of patients here and 3% of patients there”—that it was too difficult to examine them individually in this study.
Dr Thurer presented this research at the AABB Annual Meeting 2014 (abstract S65-030K).
He and his colleagues wanted to determine which specific groups of patients have been most affected by patient blood management, understand further opportunities for decreasing transfusion use, and project future blood needs.
So the researchers analyzed data from 3,946,428 inpatients at 72 US hospitals, comparing the use of RBC transfusions in 2009/2010 to use in 2013.
In 2009/2010, there were 1,378,581 patients admitted to the hospitals, the RBC transfusion rate was 11.5.%, and the utilization (total units/total patients) was 0.41. In 2013, there were 861,804 patients, the transfusion rate was 10%, and the utilization was 0.34.
So from 2009/2010 to 2013, there was a 13% reduction in transfusion rate and a 17% reduction in utilization.
The greatest decrease was in orthopedic surgery patients. In those undergoing hip and knee joint replacement surgery, there was a 45% reduction in transfusion rate and a 43% reduction in utilization. In patients with a hip fracture, there was a 20% decrease in transfusion rate and a 20% decrease in utilization.
There was a smaller, though still sizable, reduction in transfusion use among patients undergoing cardiac surgery—a 15% decrease in transfusion rate and an 18% decrease in utilization.
“My personal thoughts about this is that it represents, certainly, lower transfusion triggers, which are becoming more widely adopted, [and] the use of antifibrinolytic drugs, particularly in orthopedic surgeries,” Dr Thurer said.
“We like to think that comprehensive coagulation testing and better matching of coagulation abnormalities contributes to this. Perhaps correction of preoperative anemia [contributes] for elective patients. And, clearly, surgical techniques have evolved, and, as they do, blood loss goes down.”
Reductions in RBC use were also seen in patients with gastrointestinal bleeding, where there was a 3% decrease in transfusion rate and a 13% decrease in utilization.
“For gastrointestinal bleeding, I think the lower transfusion triggers [have made an impact], but there’s also . . . more interest in timely interventions to stop bleeding,” Dr Thurer said.
“So rather than the gastroenterologist saying, ‘Correct the hematocrit and the coagulation factors, and I’ll stop the bleeding in the morning,’ we’re seeing now much more interest in very prompt endoscopy to stop bleeding. And as you know, the way to stop giving transfusions is to stop the patient from bleeding.”
Obstetric patients saw a 5% reduction in transfusion rate and an 8% reduction in utilization. And patients undergoing bone marrow transplant or inpatient chemotherapy saw a 6% decrease in transfusion rate and an 8% decrease in utilization.
Other transplant patients actually saw an increase in RBC transfusions. In liver transplant recipients, there was a 2.2% increase in transfusion rate and a 6% increase in utilization. And in kidney transplant recipients, there was a 0.2% increase in transfusion rate and a 19% increase in utilization.
However, Dr Thurer noted that the majority of RBC transfusions are administered to patients outside of these groups. In 2013, 60.6% of transfusions went to patients who did not fit into any of the aforementioned categories.
“So clearly,” he concluded, “further studies are needed to determine whether these reductions that we’ve seen in some areas can be implemented in a wider variety of patients.” ![]()
Results support transfusing with caution in TTP, HIT
PHILADELPHIA—Results of a large study support the recommendation that patients with platelet consumptive disorders only receive platelet transfusions if they exhibit severe or life-threatening bleeding that is refractory to other therapies.
The research indicated that platelet transfusions may increase the risk of arterial thrombosis and mortality among hospitalized patients with thrombotic thrombocytopenic purpura (TTP) and those with heparin-induced thrombocytopenia (HIT).
Platelet transfusions were also associated with a greater risk of acute myocardial infarction in TTP patients.
However, transfused patients with immune thrombocytopenia (ITP) did not have an increased risk of such complications.
The study did not establish a causal link between transfusions and complications, as it was retrospective and the researchers did not know the exact timing of events.
However, the complications and the transfusions did occur during the same hospital admission, noted Ruchika Goel, MD, of Johns Hopkins University in Baltimore, Maryland. She presented these findings at the AABB Annual Meeting 2014 (abstract S41-030G).
Dr Goel and her colleagues conducted this study to assess current platelet transfusion practices in the US in hospitalized patients with TTP, HIT, and ITP. The team wanted to explore any associations between transfusions and bleeding, venous and arterial thrombotic events, acute myocardial infarction, stroke, and in-hospital mortality in these patients.
“Currently, very little data are available on the risks and benefits associated with platelet transfusions in various platelet consumptive or disruptive disorders,” Dr Goel said. “Thus, evidence-based platelet transfusion guidelines in these disorders are either non-existent or they’re based on consensus statements, with not much supportive data.”
With this in mind, the researchers analyzed data from the Nationwide Inpatient Sample, a stratified probability sample of 20% of all discharges at community hospitals in the US, which covers more than 1100 hospitals across 47 states. The team looked at 5 years of data spanning the period from 2007 through 2011.
They included patients in whom TTP and ITP were the primary admitting diagnoses and patients in whom HIT was 1 of the top 3 diagnoses. Hospitalizations in which patients had a prior history of thrombosis were excluded, as were hospitalizations with any thrombosis/thromboembolism listed as the primary admitting diagnosis (implying that it was already present at admission).
So the analysis included 10,624 patients with TTP, 6332 with HIT, and 79,980 with ITP. The median ages were 47.4, 61.8, and 47.5, respectively. And platelet transfusions were given to 10.1%, 7.1%, and 25.8% of patients, respectively.
When the researchers adjusted their analysis for age and gender, they discovered a significantly increased risk of bleeding among all transfused patients. The odds ratios (ORs) were 2.3 for TTP, 5.5 for HIT, and 5.1 for ITP patients.
“The odds of platelet transfusion were significantly higher in patients who had bleeding, thus implying that . . . that was the indication for the transfusion—an actual bleeding complication,” Dr Goel said.
The results also showed that none of the transfused patients had a significantly increased risk of venous thrombosis or stroke. The ORs for venous thrombosis were 1.1 for TTP, 0.8 for HIT, and 1.3 for ITP patients. And the ORs for stroke were 1.6, 0.5, and 1.3, respectively.
However, both TTP patients and HIT patients had a significantly increased risk of arterial thrombosis. The ORs were 5.8 for TTP, 3.4 for HIT, and 0.3 for ITP patients.
TTP patients also had a significantly increased risk of acute myocardial infarction. The ORs were 2.0 for TTP, 1.9 for HIT, and 1.3 for ITP patients.
And patients with TTP and HIT had a significantly increased risk of in-hospital mortality. The ORs were 2.0 for TTP, 5.2 for HIT, and 1.1 for ITP patients.
Dr Goel noted that this study had several limitations. The temporality of events was not reported, there was no information on platelet thresholds for transfusion or disease severity and the effect on outcomes, and accuracy was limited by the precision of discharge coding.
Therefore, further studies are needed to assess whether platelet transfusions are directly responsible for complications or if they serve as a surrogate marker for the severity of illness.
“We propose that, until such studies or trials are indeed available, which are very hard [to conduct in] these rare disorders, platelets should continue to be considered relatively contraindicated and used only for severe or life-threatening bleeding which is refractory to other therapies,” Dr Goel concluded. ![]()
PHILADELPHIA—Results of a large study support the recommendation that patients with platelet consumptive disorders only receive platelet transfusions if they exhibit severe or life-threatening bleeding that is refractory to other therapies.
The research indicated that platelet transfusions may increase the risk of arterial thrombosis and mortality among hospitalized patients with thrombotic thrombocytopenic purpura (TTP) and those with heparin-induced thrombocytopenia (HIT).
Platelet transfusions were also associated with a greater risk of acute myocardial infarction in TTP patients.
However, transfused patients with immune thrombocytopenia (ITP) did not have an increased risk of such complications.
The study did not establish a causal link between transfusions and complications, as it was retrospective and the researchers did not know the exact timing of events.
However, the complications and the transfusions did occur during the same hospital admission, noted Ruchika Goel, MD, of Johns Hopkins University in Baltimore, Maryland. She presented these findings at the AABB Annual Meeting 2014 (abstract S41-030G).
Dr Goel and her colleagues conducted this study to assess current platelet transfusion practices in the US in hospitalized patients with TTP, HIT, and ITP. The team wanted to explore any associations between transfusions and bleeding, venous and arterial thrombotic events, acute myocardial infarction, stroke, and in-hospital mortality in these patients.
“Currently, very little data are available on the risks and benefits associated with platelet transfusions in various platelet consumptive or disruptive disorders,” Dr Goel said. “Thus, evidence-based platelet transfusion guidelines in these disorders are either non-existent or they’re based on consensus statements, with not much supportive data.”
With this in mind, the researchers analyzed data from the Nationwide Inpatient Sample, a stratified probability sample of 20% of all discharges at community hospitals in the US, which covers more than 1100 hospitals across 47 states. The team looked at 5 years of data spanning the period from 2007 through 2011.
They included patients in whom TTP and ITP were the primary admitting diagnoses and patients in whom HIT was 1 of the top 3 diagnoses. Hospitalizations in which patients had a prior history of thrombosis were excluded, as were hospitalizations with any thrombosis/thromboembolism listed as the primary admitting diagnosis (implying that it was already present at admission).
So the analysis included 10,624 patients with TTP, 6332 with HIT, and 79,980 with ITP. The median ages were 47.4, 61.8, and 47.5, respectively. And platelet transfusions were given to 10.1%, 7.1%, and 25.8% of patients, respectively.
When the researchers adjusted their analysis for age and gender, they discovered a significantly increased risk of bleeding among all transfused patients. The odds ratios (ORs) were 2.3 for TTP, 5.5 for HIT, and 5.1 for ITP patients.
“The odds of platelet transfusion were significantly higher in patients who had bleeding, thus implying that . . . that was the indication for the transfusion—an actual bleeding complication,” Dr Goel said.
The results also showed that none of the transfused patients had a significantly increased risk of venous thrombosis or stroke. The ORs for venous thrombosis were 1.1 for TTP, 0.8 for HIT, and 1.3 for ITP patients. And the ORs for stroke were 1.6, 0.5, and 1.3, respectively.
However, both TTP patients and HIT patients had a significantly increased risk of arterial thrombosis. The ORs were 5.8 for TTP, 3.4 for HIT, and 0.3 for ITP patients.
TTP patients also had a significantly increased risk of acute myocardial infarction. The ORs were 2.0 for TTP, 1.9 for HIT, and 1.3 for ITP patients.
And patients with TTP and HIT had a significantly increased risk of in-hospital mortality. The ORs were 2.0 for TTP, 5.2 for HIT, and 1.1 for ITP patients.
Dr Goel noted that this study had several limitations. The temporality of events was not reported, there was no information on platelet thresholds for transfusion or disease severity and the effect on outcomes, and accuracy was limited by the precision of discharge coding.
Therefore, further studies are needed to assess whether platelet transfusions are directly responsible for complications or if they serve as a surrogate marker for the severity of illness.
“We propose that, until such studies or trials are indeed available, which are very hard [to conduct in] these rare disorders, platelets should continue to be considered relatively contraindicated and used only for severe or life-threatening bleeding which is refractory to other therapies,” Dr Goel concluded. ![]()
PHILADELPHIA—Results of a large study support the recommendation that patients with platelet consumptive disorders only receive platelet transfusions if they exhibit severe or life-threatening bleeding that is refractory to other therapies.
The research indicated that platelet transfusions may increase the risk of arterial thrombosis and mortality among hospitalized patients with thrombotic thrombocytopenic purpura (TTP) and those with heparin-induced thrombocytopenia (HIT).
Platelet transfusions were also associated with a greater risk of acute myocardial infarction in TTP patients.
However, transfused patients with immune thrombocytopenia (ITP) did not have an increased risk of such complications.
The study did not establish a causal link between transfusions and complications, as it was retrospective and the researchers did not know the exact timing of events.
However, the complications and the transfusions did occur during the same hospital admission, noted Ruchika Goel, MD, of Johns Hopkins University in Baltimore, Maryland. She presented these findings at the AABB Annual Meeting 2014 (abstract S41-030G).
Dr Goel and her colleagues conducted this study to assess current platelet transfusion practices in the US in hospitalized patients with TTP, HIT, and ITP. The team wanted to explore any associations between transfusions and bleeding, venous and arterial thrombotic events, acute myocardial infarction, stroke, and in-hospital mortality in these patients.
“Currently, very little data are available on the risks and benefits associated with platelet transfusions in various platelet consumptive or disruptive disorders,” Dr Goel said. “Thus, evidence-based platelet transfusion guidelines in these disorders are either non-existent or they’re based on consensus statements, with not much supportive data.”
With this in mind, the researchers analyzed data from the Nationwide Inpatient Sample, a stratified probability sample of 20% of all discharges at community hospitals in the US, which covers more than 1100 hospitals across 47 states. The team looked at 5 years of data spanning the period from 2007 through 2011.
They included patients in whom TTP and ITP were the primary admitting diagnoses and patients in whom HIT was 1 of the top 3 diagnoses. Hospitalizations in which patients had a prior history of thrombosis were excluded, as were hospitalizations with any thrombosis/thromboembolism listed as the primary admitting diagnosis (implying that it was already present at admission).
So the analysis included 10,624 patients with TTP, 6332 with HIT, and 79,980 with ITP. The median ages were 47.4, 61.8, and 47.5, respectively. And platelet transfusions were given to 10.1%, 7.1%, and 25.8% of patients, respectively.
When the researchers adjusted their analysis for age and gender, they discovered a significantly increased risk of bleeding among all transfused patients. The odds ratios (ORs) were 2.3 for TTP, 5.5 for HIT, and 5.1 for ITP patients.
“The odds of platelet transfusion were significantly higher in patients who had bleeding, thus implying that . . . that was the indication for the transfusion—an actual bleeding complication,” Dr Goel said.
The results also showed that none of the transfused patients had a significantly increased risk of venous thrombosis or stroke. The ORs for venous thrombosis were 1.1 for TTP, 0.8 for HIT, and 1.3 for ITP patients. And the ORs for stroke were 1.6, 0.5, and 1.3, respectively.
However, both TTP patients and HIT patients had a significantly increased risk of arterial thrombosis. The ORs were 5.8 for TTP, 3.4 for HIT, and 0.3 for ITP patients.
TTP patients also had a significantly increased risk of acute myocardial infarction. The ORs were 2.0 for TTP, 1.9 for HIT, and 1.3 for ITP patients.
And patients with TTP and HIT had a significantly increased risk of in-hospital mortality. The ORs were 2.0 for TTP, 5.2 for HIT, and 1.1 for ITP patients.
Dr Goel noted that this study had several limitations. The temporality of events was not reported, there was no information on platelet thresholds for transfusion or disease severity and the effect on outcomes, and accuracy was limited by the precision of discharge coding.
Therefore, further studies are needed to assess whether platelet transfusions are directly responsible for complications or if they serve as a surrogate marker for the severity of illness.
“We propose that, until such studies or trials are indeed available, which are very hard [to conduct in] these rare disorders, platelets should continue to be considered relatively contraindicated and used only for severe or life-threatening bleeding which is refractory to other therapies,” Dr Goel concluded. ![]()
Transfusions benefit adults with sickle cell disease
PHILADELPHIA—Blood transfusions can provide pain relief in adults with sickle cell disease (SCD) who have failed treatment with hydroxyurea, a pilot study suggests.
Patients had fewer visits to the emergency department (ED) and fewer hospital admissions for pain control after they received chronic transfusions for pain prophylaxis than they did prior to receiving transfusions.
Matthew S. Karafin, MD, of the Blood Center of Wisconsin in Milwaukee, presented these results at the AABB Annual Meeting 2014 (abstract S42-030G).
“Pain in adults with sickle cell disease is probably one of the most important things that we deal with in our clinics,” he began. “It is the leading cause of morbidity in this population.”
Dr Karafin also noted that adults with SCD seem to experience pain differently from children, reporting more of a constant pain, as opposed to the episodic pain observed in kids. And although previous studies have suggested that transfusions do provide pain relief in SCD, most of those studies have focused on children.
So Dr Karafin and his colleagues set out to determine the impact of prophylactic transfusions on the rate of serious pain episodes in adults with SCD. The team retrospectively analyzed a cohort of patients who received chronic transfusions at 3- to 8-week intervals from January 2009 to October 2013.
The researchers defined chronic transfusions as receiving blood—either simple transfusions or red cell exchanges—in an outpatient setting 3 days a week with the goal of controlling hemoglobin (Hb) S percentage, maintaining it at less than 30%.
Patients had to have at least 1 ED or hospital visit for severe pain per month prior to starting transfusions, they were required to have failed hydroxyurea therapy, and they had to have at least 3 months both on and off chronic transfusions. The patients could have no other reason for receiving chronic transfusions (ie, no previous stroke).
So the study included 17 patients, 12 of whom were female. Fifteen (88.1%) had Hb SS disease, and 2 had Hb SC disease. Their median age was 26 (range, 20-54).
“We were able to record 541 total ED admissions over the study period and 404 total hospital admissions,” Dr Karafin said. “The median study evaluation period pre-transfusion was about 3.5 years, and we were able to study [patients for] a median of more than a year for the post-transfusion protocol period.”
Dr Karafin also noted that most of the patients were not transfusion-naïve, but they received significantly more units after being placed on the transfusion protocol.
The median number of red cell units received per 100 days was 1.2 (range, 0-7.2) pre-transfusion and 10.2 (range, 6.7-24.3) post-transfusion (P=0.0003). Nine of the patients received simple transfusions, and 8 received red cell exchanges.
There was a significant difference in the median Hb S pre- and post-transfusion—79% (range, 26.5%-89.6%) and 30.2% (range, 10.9%-57.4%), respectively (P=0.0003).
But there was no significant difference in median ferritin levels—1128.2 ng/mL (range, 65.4-11,130) and 2632.8 ng/mL (range, 16.7-8023.6), respectively (P=0.18). Dr Karafin said this could be explained by the fact that patients were not transfusion-naïve prior to starting the protocol.
Similarly, the median new alloantibody rate per 100 units was 0 both pre- and post-transfusion. This may be due to the fact that all patients received C-, E-, and KEL-matched blood, as well as the freshest available units, Dr Karafin said.
He and his colleagues also found that the median ED admission rate was significantly lower post-transfusion compared to pre-transfusion—0.79 (range, 0-6.6) and 2 (range, 0.4-11) visits every 100 days, respectively (P=0.04).
Thirteen patients (76.5%) had a reduced ED visit rate after chronic transfusion, and there was a 60.5% reduction in the ED visit rate overall.
Likewise, the median hospital admission rate decreased from 1.7 per 100 days (range, 0.05-5.8) pre-transfusion to 1.3 per 100 days (range, 0.2-3.2) post-transfusion (P=0.004).
Fifteen patients (88.2%) had reduced hospital admissions after chronic transfusion, and there was a 20.3% reduction in hospital admissions overall.
Dr Karafin noted that this study had a number of limitations, including a small number of patients, its retrospective nature, and the fact that it was conducted at a comprehensive SCD clinic.
“However, limitations aside, we found significant evidence to support that the findings observed in children seem to be similar in the adult population,” he said.
Namely, chronic transfusions can prevent serious pain episodes in adults with SCD who have failed treatment with hydroxyurea. ![]()
PHILADELPHIA—Blood transfusions can provide pain relief in adults with sickle cell disease (SCD) who have failed treatment with hydroxyurea, a pilot study suggests.
Patients had fewer visits to the emergency department (ED) and fewer hospital admissions for pain control after they received chronic transfusions for pain prophylaxis than they did prior to receiving transfusions.
Matthew S. Karafin, MD, of the Blood Center of Wisconsin in Milwaukee, presented these results at the AABB Annual Meeting 2014 (abstract S42-030G).
“Pain in adults with sickle cell disease is probably one of the most important things that we deal with in our clinics,” he began. “It is the leading cause of morbidity in this population.”
Dr Karafin also noted that adults with SCD seem to experience pain differently from children, reporting more of a constant pain, as opposed to the episodic pain observed in kids. And although previous studies have suggested that transfusions do provide pain relief in SCD, most of those studies have focused on children.
So Dr Karafin and his colleagues set out to determine the impact of prophylactic transfusions on the rate of serious pain episodes in adults with SCD. The team retrospectively analyzed a cohort of patients who received chronic transfusions at 3- to 8-week intervals from January 2009 to October 2013.
The researchers defined chronic transfusions as receiving blood—either simple transfusions or red cell exchanges—in an outpatient setting 3 days a week with the goal of controlling hemoglobin (Hb) S percentage, maintaining it at less than 30%.
Patients had to have at least 1 ED or hospital visit for severe pain per month prior to starting transfusions, they were required to have failed hydroxyurea therapy, and they had to have at least 3 months both on and off chronic transfusions. The patients could have no other reason for receiving chronic transfusions (ie, no previous stroke).
So the study included 17 patients, 12 of whom were female. Fifteen (88.1%) had Hb SS disease, and 2 had Hb SC disease. Their median age was 26 (range, 20-54).
“We were able to record 541 total ED admissions over the study period and 404 total hospital admissions,” Dr Karafin said. “The median study evaluation period pre-transfusion was about 3.5 years, and we were able to study [patients for] a median of more than a year for the post-transfusion protocol period.”
Dr Karafin also noted that most of the patients were not transfusion-naïve, but they received significantly more units after being placed on the transfusion protocol.
The median number of red cell units received per 100 days was 1.2 (range, 0-7.2) pre-transfusion and 10.2 (range, 6.7-24.3) post-transfusion (P=0.0003). Nine of the patients received simple transfusions, and 8 received red cell exchanges.
There was a significant difference in the median Hb S pre- and post-transfusion—79% (range, 26.5%-89.6%) and 30.2% (range, 10.9%-57.4%), respectively (P=0.0003).
But there was no significant difference in median ferritin levels—1128.2 ng/mL (range, 65.4-11,130) and 2632.8 ng/mL (range, 16.7-8023.6), respectively (P=0.18). Dr Karafin said this could be explained by the fact that patients were not transfusion-naïve prior to starting the protocol.
Similarly, the median new alloantibody rate per 100 units was 0 both pre- and post-transfusion. This may be due to the fact that all patients received C-, E-, and KEL-matched blood, as well as the freshest available units, Dr Karafin said.
He and his colleagues also found that the median ED admission rate was significantly lower post-transfusion compared to pre-transfusion—0.79 (range, 0-6.6) and 2 (range, 0.4-11) visits every 100 days, respectively (P=0.04).
Thirteen patients (76.5%) had a reduced ED visit rate after chronic transfusion, and there was a 60.5% reduction in the ED visit rate overall.
Likewise, the median hospital admission rate decreased from 1.7 per 100 days (range, 0.05-5.8) pre-transfusion to 1.3 per 100 days (range, 0.2-3.2) post-transfusion (P=0.004).
Fifteen patients (88.2%) had reduced hospital admissions after chronic transfusion, and there was a 20.3% reduction in hospital admissions overall.
Dr Karafin noted that this study had a number of limitations, including a small number of patients, its retrospective nature, and the fact that it was conducted at a comprehensive SCD clinic.
“However, limitations aside, we found significant evidence to support that the findings observed in children seem to be similar in the adult population,” he said.
Namely, chronic transfusions can prevent serious pain episodes in adults with SCD who have failed treatment with hydroxyurea. ![]()
PHILADELPHIA—Blood transfusions can provide pain relief in adults with sickle cell disease (SCD) who have failed treatment with hydroxyurea, a pilot study suggests.
Patients had fewer visits to the emergency department (ED) and fewer hospital admissions for pain control after they received chronic transfusions for pain prophylaxis than they did prior to receiving transfusions.
Matthew S. Karafin, MD, of the Blood Center of Wisconsin in Milwaukee, presented these results at the AABB Annual Meeting 2014 (abstract S42-030G).
“Pain in adults with sickle cell disease is probably one of the most important things that we deal with in our clinics,” he began. “It is the leading cause of morbidity in this population.”
Dr Karafin also noted that adults with SCD seem to experience pain differently from children, reporting more of a constant pain, as opposed to the episodic pain observed in kids. And although previous studies have suggested that transfusions do provide pain relief in SCD, most of those studies have focused on children.
So Dr Karafin and his colleagues set out to determine the impact of prophylactic transfusions on the rate of serious pain episodes in adults with SCD. The team retrospectively analyzed a cohort of patients who received chronic transfusions at 3- to 8-week intervals from January 2009 to October 2013.
The researchers defined chronic transfusions as receiving blood—either simple transfusions or red cell exchanges—in an outpatient setting 3 days a week with the goal of controlling hemoglobin (Hb) S percentage, maintaining it at less than 30%.
Patients had to have at least 1 ED or hospital visit for severe pain per month prior to starting transfusions, they were required to have failed hydroxyurea therapy, and they had to have at least 3 months both on and off chronic transfusions. The patients could have no other reason for receiving chronic transfusions (ie, no previous stroke).
So the study included 17 patients, 12 of whom were female. Fifteen (88.1%) had Hb SS disease, and 2 had Hb SC disease. Their median age was 26 (range, 20-54).
“We were able to record 541 total ED admissions over the study period and 404 total hospital admissions,” Dr Karafin said. “The median study evaluation period pre-transfusion was about 3.5 years, and we were able to study [patients for] a median of more than a year for the post-transfusion protocol period.”
Dr Karafin also noted that most of the patients were not transfusion-naïve, but they received significantly more units after being placed on the transfusion protocol.
The median number of red cell units received per 100 days was 1.2 (range, 0-7.2) pre-transfusion and 10.2 (range, 6.7-24.3) post-transfusion (P=0.0003). Nine of the patients received simple transfusions, and 8 received red cell exchanges.
There was a significant difference in the median Hb S pre- and post-transfusion—79% (range, 26.5%-89.6%) and 30.2% (range, 10.9%-57.4%), respectively (P=0.0003).
But there was no significant difference in median ferritin levels—1128.2 ng/mL (range, 65.4-11,130) and 2632.8 ng/mL (range, 16.7-8023.6), respectively (P=0.18). Dr Karafin said this could be explained by the fact that patients were not transfusion-naïve prior to starting the protocol.
Similarly, the median new alloantibody rate per 100 units was 0 both pre- and post-transfusion. This may be due to the fact that all patients received C-, E-, and KEL-matched blood, as well as the freshest available units, Dr Karafin said.
He and his colleagues also found that the median ED admission rate was significantly lower post-transfusion compared to pre-transfusion—0.79 (range, 0-6.6) and 2 (range, 0.4-11) visits every 100 days, respectively (P=0.04).
Thirteen patients (76.5%) had a reduced ED visit rate after chronic transfusion, and there was a 60.5% reduction in the ED visit rate overall.
Likewise, the median hospital admission rate decreased from 1.7 per 100 days (range, 0.05-5.8) pre-transfusion to 1.3 per 100 days (range, 0.2-3.2) post-transfusion (P=0.004).
Fifteen patients (88.2%) had reduced hospital admissions after chronic transfusion, and there was a 20.3% reduction in hospital admissions overall.
Dr Karafin noted that this study had a number of limitations, including a small number of patients, its retrospective nature, and the fact that it was conducted at a comprehensive SCD clinic.
“However, limitations aside, we found significant evidence to support that the findings observed in children seem to be similar in the adult population,” he said.
Namely, chronic transfusions can prevent serious pain episodes in adults with SCD who have failed treatment with hydroxyurea. ![]()
RECESS suggests RBC age doesn’t affect outcomes

Credit: UAB Hospital
PHILADELPHIA—As the medical community continues to debate whether transfusing older blood has a negative outcome on patients, results of the RECESS trial add fuel to the fire.
The study showed no significant differences in clinical outcomes between cardiac surgery patients who received newer red blood cells (RBCs) and those who received older RBCs.
There were no differences in multi-organ dysfunction scores (MODS), mortality rates, or the incidence of serious adverse events.
Marie E. Stein, MD, of the University of Minnesota in Minneapolis, presented the results of RECESS at the AABB Annual Meeting 2014 (abstract P2-020A).
The findings contradict results from another recent study presented at the Canadian Cardiovascular Congress.
“There are many studies of the effects of red blood cell storage duration on clinical outcomes, most of which are observational and include only a few randomized trials to date,” Dr Stein noted. “When studying cardiac surgery patients, some studies have found significant adverse outcomes of subjects who are transfused with red cells stored for a longer duration compared to shorter duration, and other studies have not.”
“Based on this equipoise, the primary hypothesis for RECESS was that there would be an important difference between the effect of transfusing shorter-storage-age-duration red cells compared to transfusing longer-storage-age-duration red cells on clinical outcomes in cardiac surgery patients.”
Patient characteristics
Dr Stein and her colleagues enrolled 1481 patients who were 12 years of age and older, weighed 40 kg or more, were undergoing complex cardiac surgery, and were considered “highly likely” to be transfused. They had to have a TRUST score of 3 or greater if they were older than 18 years of age, but this was not required for children.
Patients were split into two groups: those set to receive RBCs stored for 10 days or fewer and those set to receive RBCs stored for 21 days or more. Patients were stratified by age (those 12 to 17 years vs patients 18 and older) and according to whether they were in the intensive care unit prior to surgery. They were balanced by site as well.
In all, there were 538 subjects evaluable for the newer RBC arm and 560 subjects evaluable for the older RBC arm. (Subjects were evaluable if they underwent surgery and received at least 1 RBC unit.) The median patient age was 73 and 72 years, respectively, and males made up 42% and 44% of the patients, respectively.
The same percentage of patients in each arm—96%—underwent cardiopulmonary bypass, and 23% of patients in each arm underwent coronary artery bypass grafting. Seventeen percent of patients in the newer RBC arm and 14% in the older RBC arm underwent valve surgery only.
“Red cells were leukoreduced, stored in additive solution, and provided according to storage duration arm assignment for all pre-op, post-op, and intra-operative transfusions through day 28, discharge, or death, whichever occurred first,” Dr Stein noted.
The number of RBC units did not differ significantly between the two arms (P=0.80), and there was a comparable number of highly transfused subjects in each arm (P=0.81).
Eighty-seven percent of patients in the newer RBC arm and 89% in the older RBC arm received all their RBC units as assigned (P=0.35).
Six percent and 8%, respectively, received 1 or more unit aged 11 to 20 days but none belonging to the opposite arm to which they were assigned. Five percent and 4%, respectively, received 1 or more RBC unit from the opposite arm to which they were assigned.
“There was a 20-day difference in the mean storage duration between the two arms: 8 vs 28 days,” Dr Stein pointed out.
Outcomes
The study’s primary outcome was the change in multi-organ dysfunction score (ΔMODS) at 7 days. The MODS system includes assessments of respiratory, renal, hepatic, cardiovascular, hematologic, and neurologic function.
“The MODS system was chosen as the primary endpoint because the data elements are objective and readily available,” Dr Stein said. “MODS also incorporates organ dysfunction and not just frank organ failure. It correlates with mortality, with length of stay, and does incorporate death. MODS has been validated in other studies and has been used in other transfusion trials, including TRICC.”
To calculate 7-day ΔMODS, the researchers identified the worst score for each organ system through day 7 after surgery, discharge, or death. The 7-day MODS was the sum of the worst score for each organ system, and the 7-day ΔMODS was the pre-surgery MODS subtracted from the 7-day MODS.
Secondary outcomes were the 28-day ΔMODS, 28-day mortality, and the incidence of serious adverse events.
There was no significant difference between the arms with regard to 7-day or 28-day ΔMODS.
For the 7-day ΔMODS, the mean was 8.5±3.6 in the newer RBC arm and 8.7±3.6 in the older RBC arm. The unadjusted difference and the difference adjusted for baseline MODS were both -0.02.
For the 28-day ΔMODS, the mean was 8.7±4.0 in the newer RBC arm and 9.1±4.2 in the older RBC arm. The unadjusted difference and the difference adjusted for baseline MODS were both -0.3.
There was no significant difference between the arms in time to death (P=0.50), 7-day mortality (P=0.43), or 28-day mortality (P=0.57). The rate of 7-day mortality was 2.8% in the newer RBC arm and 2.0% in the older RBC arm. The 28-day mortality was 4.4% and 5.3%, respectively.
There was no significant difference in the percentage of subjects with 1 or more serious adverse events. The rate was 53% in the newer RBC arm and 51% in the older RBC arm (P=0.72).
Taking these results together, Dr Stein concluded that differences in the storage duration of RBCs did not translate to significant differences in “key clinical outcomes.” ![]()

Credit: UAB Hospital
PHILADELPHIA—As the medical community continues to debate whether transfusing older blood has a negative outcome on patients, results of the RECESS trial add fuel to the fire.
The study showed no significant differences in clinical outcomes between cardiac surgery patients who received newer red blood cells (RBCs) and those who received older RBCs.
There were no differences in multi-organ dysfunction scores (MODS), mortality rates, or the incidence of serious adverse events.
Marie E. Stein, MD, of the University of Minnesota in Minneapolis, presented the results of RECESS at the AABB Annual Meeting 2014 (abstract P2-020A).
The findings contradict results from another recent study presented at the Canadian Cardiovascular Congress.
“There are many studies of the effects of red blood cell storage duration on clinical outcomes, most of which are observational and include only a few randomized trials to date,” Dr Stein noted. “When studying cardiac surgery patients, some studies have found significant adverse outcomes of subjects who are transfused with red cells stored for a longer duration compared to shorter duration, and other studies have not.”
“Based on this equipoise, the primary hypothesis for RECESS was that there would be an important difference between the effect of transfusing shorter-storage-age-duration red cells compared to transfusing longer-storage-age-duration red cells on clinical outcomes in cardiac surgery patients.”
Patient characteristics
Dr Stein and her colleagues enrolled 1481 patients who were 12 years of age and older, weighed 40 kg or more, were undergoing complex cardiac surgery, and were considered “highly likely” to be transfused. They had to have a TRUST score of 3 or greater if they were older than 18 years of age, but this was not required for children.
Patients were split into two groups: those set to receive RBCs stored for 10 days or fewer and those set to receive RBCs stored for 21 days or more. Patients were stratified by age (those 12 to 17 years vs patients 18 and older) and according to whether they were in the intensive care unit prior to surgery. They were balanced by site as well.
In all, there were 538 subjects evaluable for the newer RBC arm and 560 subjects evaluable for the older RBC arm. (Subjects were evaluable if they underwent surgery and received at least 1 RBC unit.) The median patient age was 73 and 72 years, respectively, and males made up 42% and 44% of the patients, respectively.
The same percentage of patients in each arm—96%—underwent cardiopulmonary bypass, and 23% of patients in each arm underwent coronary artery bypass grafting. Seventeen percent of patients in the newer RBC arm and 14% in the older RBC arm underwent valve surgery only.
“Red cells were leukoreduced, stored in additive solution, and provided according to storage duration arm assignment for all pre-op, post-op, and intra-operative transfusions through day 28, discharge, or death, whichever occurred first,” Dr Stein noted.
The number of RBC units did not differ significantly between the two arms (P=0.80), and there was a comparable number of highly transfused subjects in each arm (P=0.81).
Eighty-seven percent of patients in the newer RBC arm and 89% in the older RBC arm received all their RBC units as assigned (P=0.35).
Six percent and 8%, respectively, received 1 or more unit aged 11 to 20 days but none belonging to the opposite arm to which they were assigned. Five percent and 4%, respectively, received 1 or more RBC unit from the opposite arm to which they were assigned.
“There was a 20-day difference in the mean storage duration between the two arms: 8 vs 28 days,” Dr Stein pointed out.
Outcomes
The study’s primary outcome was the change in multi-organ dysfunction score (ΔMODS) at 7 days. The MODS system includes assessments of respiratory, renal, hepatic, cardiovascular, hematologic, and neurologic function.
“The MODS system was chosen as the primary endpoint because the data elements are objective and readily available,” Dr Stein said. “MODS also incorporates organ dysfunction and not just frank organ failure. It correlates with mortality, with length of stay, and does incorporate death. MODS has been validated in other studies and has been used in other transfusion trials, including TRICC.”
To calculate 7-day ΔMODS, the researchers identified the worst score for each organ system through day 7 after surgery, discharge, or death. The 7-day MODS was the sum of the worst score for each organ system, and the 7-day ΔMODS was the pre-surgery MODS subtracted from the 7-day MODS.
Secondary outcomes were the 28-day ΔMODS, 28-day mortality, and the incidence of serious adverse events.
There was no significant difference between the arms with regard to 7-day or 28-day ΔMODS.
For the 7-day ΔMODS, the mean was 8.5±3.6 in the newer RBC arm and 8.7±3.6 in the older RBC arm. The unadjusted difference and the difference adjusted for baseline MODS were both -0.02.
For the 28-day ΔMODS, the mean was 8.7±4.0 in the newer RBC arm and 9.1±4.2 in the older RBC arm. The unadjusted difference and the difference adjusted for baseline MODS were both -0.3.
There was no significant difference between the arms in time to death (P=0.50), 7-day mortality (P=0.43), or 28-day mortality (P=0.57). The rate of 7-day mortality was 2.8% in the newer RBC arm and 2.0% in the older RBC arm. The 28-day mortality was 4.4% and 5.3%, respectively.
There was no significant difference in the percentage of subjects with 1 or more serious adverse events. The rate was 53% in the newer RBC arm and 51% in the older RBC arm (P=0.72).
Taking these results together, Dr Stein concluded that differences in the storage duration of RBCs did not translate to significant differences in “key clinical outcomes.” ![]()

Credit: UAB Hospital
PHILADELPHIA—As the medical community continues to debate whether transfusing older blood has a negative outcome on patients, results of the RECESS trial add fuel to the fire.
The study showed no significant differences in clinical outcomes between cardiac surgery patients who received newer red blood cells (RBCs) and those who received older RBCs.
There were no differences in multi-organ dysfunction scores (MODS), mortality rates, or the incidence of serious adverse events.
Marie E. Stein, MD, of the University of Minnesota in Minneapolis, presented the results of RECESS at the AABB Annual Meeting 2014 (abstract P2-020A).
The findings contradict results from another recent study presented at the Canadian Cardiovascular Congress.
“There are many studies of the effects of red blood cell storage duration on clinical outcomes, most of which are observational and include only a few randomized trials to date,” Dr Stein noted. “When studying cardiac surgery patients, some studies have found significant adverse outcomes of subjects who are transfused with red cells stored for a longer duration compared to shorter duration, and other studies have not.”
“Based on this equipoise, the primary hypothesis for RECESS was that there would be an important difference between the effect of transfusing shorter-storage-age-duration red cells compared to transfusing longer-storage-age-duration red cells on clinical outcomes in cardiac surgery patients.”
Patient characteristics
Dr Stein and her colleagues enrolled 1481 patients who were 12 years of age and older, weighed 40 kg or more, were undergoing complex cardiac surgery, and were considered “highly likely” to be transfused. They had to have a TRUST score of 3 or greater if they were older than 18 years of age, but this was not required for children.
Patients were split into two groups: those set to receive RBCs stored for 10 days or fewer and those set to receive RBCs stored for 21 days or more. Patients were stratified by age (those 12 to 17 years vs patients 18 and older) and according to whether they were in the intensive care unit prior to surgery. They were balanced by site as well.
In all, there were 538 subjects evaluable for the newer RBC arm and 560 subjects evaluable for the older RBC arm. (Subjects were evaluable if they underwent surgery and received at least 1 RBC unit.) The median patient age was 73 and 72 years, respectively, and males made up 42% and 44% of the patients, respectively.
The same percentage of patients in each arm—96%—underwent cardiopulmonary bypass, and 23% of patients in each arm underwent coronary artery bypass grafting. Seventeen percent of patients in the newer RBC arm and 14% in the older RBC arm underwent valve surgery only.
“Red cells were leukoreduced, stored in additive solution, and provided according to storage duration arm assignment for all pre-op, post-op, and intra-operative transfusions through day 28, discharge, or death, whichever occurred first,” Dr Stein noted.
The number of RBC units did not differ significantly between the two arms (P=0.80), and there was a comparable number of highly transfused subjects in each arm (P=0.81).
Eighty-seven percent of patients in the newer RBC arm and 89% in the older RBC arm received all their RBC units as assigned (P=0.35).
Six percent and 8%, respectively, received 1 or more unit aged 11 to 20 days but none belonging to the opposite arm to which they were assigned. Five percent and 4%, respectively, received 1 or more RBC unit from the opposite arm to which they were assigned.
“There was a 20-day difference in the mean storage duration between the two arms: 8 vs 28 days,” Dr Stein pointed out.
Outcomes
The study’s primary outcome was the change in multi-organ dysfunction score (ΔMODS) at 7 days. The MODS system includes assessments of respiratory, renal, hepatic, cardiovascular, hematologic, and neurologic function.
“The MODS system was chosen as the primary endpoint because the data elements are objective and readily available,” Dr Stein said. “MODS also incorporates organ dysfunction and not just frank organ failure. It correlates with mortality, with length of stay, and does incorporate death. MODS has been validated in other studies and has been used in other transfusion trials, including TRICC.”
To calculate 7-day ΔMODS, the researchers identified the worst score for each organ system through day 7 after surgery, discharge, or death. The 7-day MODS was the sum of the worst score for each organ system, and the 7-day ΔMODS was the pre-surgery MODS subtracted from the 7-day MODS.
Secondary outcomes were the 28-day ΔMODS, 28-day mortality, and the incidence of serious adverse events.
There was no significant difference between the arms with regard to 7-day or 28-day ΔMODS.
For the 7-day ΔMODS, the mean was 8.5±3.6 in the newer RBC arm and 8.7±3.6 in the older RBC arm. The unadjusted difference and the difference adjusted for baseline MODS were both -0.02.
For the 28-day ΔMODS, the mean was 8.7±4.0 in the newer RBC arm and 9.1±4.2 in the older RBC arm. The unadjusted difference and the difference adjusted for baseline MODS were both -0.3.
There was no significant difference between the arms in time to death (P=0.50), 7-day mortality (P=0.43), or 28-day mortality (P=0.57). The rate of 7-day mortality was 2.8% in the newer RBC arm and 2.0% in the older RBC arm. The 28-day mortality was 4.4% and 5.3%, respectively.
There was no significant difference in the percentage of subjects with 1 or more serious adverse events. The rate was 53% in the newer RBC arm and 51% in the older RBC arm (P=0.72).
Taking these results together, Dr Stein concluded that differences in the storage duration of RBCs did not translate to significant differences in “key clinical outcomes.” ![]()
Study supports 2:1 ratio for transfusion in pregnancy
PHILADELPHIA—Results of a single-center study suggest that, when it comes to massive transfusion in pregnancy, a 1:1 ratio of red blood cells (RBCs) to plasma is not needed to maintain adequate hemostasis.
A 2:1 ratio produces prothrombin times (PTs), activated partial thromboplastin times (PTTs), and fibrinogen levels within references ranges.
Vanessa Plasencia, MLS (ASCP)CM, of Texas Children’s Hospital in Houston, presented these findings at the AABB Annual Meeting 2014 (abstract S43-030G).
She noted that hospital staff perform approximately 4500 to 5000 deliveries per year, and they define massive transfusion as 4 or more RBC units in 1 hour or 10 or more RBC units in 24 hours.
The hospital’s initial obstetric massive transfusion protocol was 4 units of RBCs and 4 units of plasma to be issued in a cooler. Four units of group AB thawed plasma or liquid plasma were always available.
To determine if this protocol is optimal, Plasencia and her colleagues conducted a retrospective review of patient records from April 2012 to June 2014. During this time, there were 28 cases of massive transfusion.
Two of these patients died and were excluded from the study. One, who had placental abruption, received 131 RBC units and 48 plasma units (ratio=2.7:1). The other, who had placenta percreta, received 90 RBC units and 52 plasma units (ratio=1.7:1).
The median age of the remaining 26 patients was 34 years (range, 24-44). Four of these patients had placenta accreta, 2 had placenta increta, 14 had placenta percreta, and 6 had other complications (such as placental abruption, diabetes, and risks due to advanced-age pregnancy).
A median of 12 RBC units (range, 9-20) and 9 plasma units (range, 5-19) were issued. And a median of 8 RBC units (range, 6-12) and 5 plasma units (range, 4-8) were actually transfused. That translates to RBC-to-plasma ratios of 1.4:1 (range, 1.0-2.0) and 1.7:1 (1.3-2.5), respectively.
So despite the hospital’s protocol of a 1:1 RBC-to-plasma ratio, the actual ratio of transfusion in practice was approximately 2:1, Plasencia noted. And the patients had PT, PTT, and fibrinogen values within reference ranges.
Coagulation data were collected after transfusions took place, once patients were stable. The median PT was 14.8 seconds (range, 14.1-15.2), the median PTT was 29.9 seconds (range, 27.6-33.3), and the median fibrinogen was 283 mg/dL (range, 225-325).
Because of these results, Texas Children’s Hospital decided to change its massive transfusion protocol for obstetrics to a 2:1 RBC-to-plasma ratio. Now, the hospital issues 4 units of RBCs and 2 units of plasma in its initial blood package.
PHILADELPHIA—Results of a single-center study suggest that, when it comes to massive transfusion in pregnancy, a 1:1 ratio of red blood cells (RBCs) to plasma is not needed to maintain adequate hemostasis.
A 2:1 ratio produces prothrombin times (PTs), activated partial thromboplastin times (PTTs), and fibrinogen levels within references ranges.
Vanessa Plasencia, MLS (ASCP)CM, of Texas Children’s Hospital in Houston, presented these findings at the AABB Annual Meeting 2014 (abstract S43-030G).
She noted that hospital staff perform approximately 4500 to 5000 deliveries per year, and they define massive transfusion as 4 or more RBC units in 1 hour or 10 or more RBC units in 24 hours.
The hospital’s initial obstetric massive transfusion protocol was 4 units of RBCs and 4 units of plasma to be issued in a cooler. Four units of group AB thawed plasma or liquid plasma were always available.
To determine if this protocol is optimal, Plasencia and her colleagues conducted a retrospective review of patient records from April 2012 to June 2014. During this time, there were 28 cases of massive transfusion.
Two of these patients died and were excluded from the study. One, who had placental abruption, received 131 RBC units and 48 plasma units (ratio=2.7:1). The other, who had placenta percreta, received 90 RBC units and 52 plasma units (ratio=1.7:1).
The median age of the remaining 26 patients was 34 years (range, 24-44). Four of these patients had placenta accreta, 2 had placenta increta, 14 had placenta percreta, and 6 had other complications (such as placental abruption, diabetes, and risks due to advanced-age pregnancy).
A median of 12 RBC units (range, 9-20) and 9 plasma units (range, 5-19) were issued. And a median of 8 RBC units (range, 6-12) and 5 plasma units (range, 4-8) were actually transfused. That translates to RBC-to-plasma ratios of 1.4:1 (range, 1.0-2.0) and 1.7:1 (1.3-2.5), respectively.
So despite the hospital’s protocol of a 1:1 RBC-to-plasma ratio, the actual ratio of transfusion in practice was approximately 2:1, Plasencia noted. And the patients had PT, PTT, and fibrinogen values within reference ranges.
Coagulation data were collected after transfusions took place, once patients were stable. The median PT was 14.8 seconds (range, 14.1-15.2), the median PTT was 29.9 seconds (range, 27.6-33.3), and the median fibrinogen was 283 mg/dL (range, 225-325).
Because of these results, Texas Children’s Hospital decided to change its massive transfusion protocol for obstetrics to a 2:1 RBC-to-plasma ratio. Now, the hospital issues 4 units of RBCs and 2 units of plasma in its initial blood package.
PHILADELPHIA—Results of a single-center study suggest that, when it comes to massive transfusion in pregnancy, a 1:1 ratio of red blood cells (RBCs) to plasma is not needed to maintain adequate hemostasis.
A 2:1 ratio produces prothrombin times (PTs), activated partial thromboplastin times (PTTs), and fibrinogen levels within references ranges.
Vanessa Plasencia, MLS (ASCP)CM, of Texas Children’s Hospital in Houston, presented these findings at the AABB Annual Meeting 2014 (abstract S43-030G).
She noted that hospital staff perform approximately 4500 to 5000 deliveries per year, and they define massive transfusion as 4 or more RBC units in 1 hour or 10 or more RBC units in 24 hours.
The hospital’s initial obstetric massive transfusion protocol was 4 units of RBCs and 4 units of plasma to be issued in a cooler. Four units of group AB thawed plasma or liquid plasma were always available.
To determine if this protocol is optimal, Plasencia and her colleagues conducted a retrospective review of patient records from April 2012 to June 2014. During this time, there were 28 cases of massive transfusion.
Two of these patients died and were excluded from the study. One, who had placental abruption, received 131 RBC units and 48 plasma units (ratio=2.7:1). The other, who had placenta percreta, received 90 RBC units and 52 plasma units (ratio=1.7:1).
The median age of the remaining 26 patients was 34 years (range, 24-44). Four of these patients had placenta accreta, 2 had placenta increta, 14 had placenta percreta, and 6 had other complications (such as placental abruption, diabetes, and risks due to advanced-age pregnancy).
A median of 12 RBC units (range, 9-20) and 9 plasma units (range, 5-19) were issued. And a median of 8 RBC units (range, 6-12) and 5 plasma units (range, 4-8) were actually transfused. That translates to RBC-to-plasma ratios of 1.4:1 (range, 1.0-2.0) and 1.7:1 (1.3-2.5), respectively.
So despite the hospital’s protocol of a 1:1 RBC-to-plasma ratio, the actual ratio of transfusion in practice was approximately 2:1, Plasencia noted. And the patients had PT, PTT, and fibrinogen values within reference ranges.
Coagulation data were collected after transfusions took place, once patients were stable. The median PT was 14.8 seconds (range, 14.1-15.2), the median PTT was 29.9 seconds (range, 27.6-33.3), and the median fibrinogen was 283 mg/dL (range, 225-325).
Because of these results, Texas Children’s Hospital decided to change its massive transfusion protocol for obstetrics to a 2:1 RBC-to-plasma ratio. Now, the hospital issues 4 units of RBCs and 2 units of plasma in its initial blood package.
Number of cord blood units doesn’t affect survival

Credit: NHS
Single and double cord blood transplants produce similar outcomes, according to a study of young patients with hematologic disorders.
Researchers found that rates of overall and disease-free survival were not significantly different in patients who received a single unit of cord blood and those who received two units.
Other outcome measures, such as neutrophil recovery, relapse, and transplant-related death, were similar between the two groups as well.
However, patients who received a single cord blood unit showed improved platelet recovery, a lower incidence of grade 3-4 acute graft-vs-host disease (GVHD), and a lower rate of extensive chronic GVHD.
John Wagner, Jr, MD, of the University of Minnesota in Minneapolis, and his colleagues reported these results in NEJM. Dr Wagner previously presented results from this study at ASH 2012.
“Based on promising early studies using two cord blood units in adults for whom one unit is often not sufficient, we designed this study in order to determine if the higher number of blood-forming stem cells in two cord blood units might improve survival,” Dr Wagner said. “What we found, however, was that both treatment arms performed very well, with similar rates of white blood cell recovery and survival.”
The researchers enrolled 224 patients, ages 1 to 21 years, with hematologic disorders, including acute and chronic leukemias as well as myelodysplastic syndromes.
Patients were randomized to receive double-unit (n=111) or single-unit (n=113) cord blood transplants after a uniform myeloablative conditioning regimen and immunoprophylaxis for GVHD.
The researchers matched the treatment arms for age, sex, self-reported race, performance status, degree of donor-recipient HLA matching, disease type, and disease status at transplant.
Survival and relapse
The study’s primary endpoint was 1-year survival, which was 65% in the double-unit arm and 73% in the single-unit arm (P=0.17). In a multivariate analysis, the risk of death did not differ significantly between the arms (hazard ratio=1.34, P=0.20).
Similarly, there was no significant difference in 1-year disease-free survival between the double- and single-unit arms—64% and 70%, respectively (P=0.11). In a multivariate analysis, the risk of relapse or death did not differ significantly between arms (hazard ratio=1.48, P=0.08).
It therefore follows that rates of relapse and transplant-related death were similar at 1 year as well. The incidence of relapse was 14% in the double-unit arm and 12% in the single-unit arm (P=0.12). And rates of transplant-related death were 22% and 19%, respectively (P=0.43).
Recovery and GVHD
The incidence of neutrophil recovery was similar between treatment arms—88% in the double-unit arm and 89% in the single-unit arm (P=0.29) at a median of 23 days (range, 11 to 133) and 21 days (range, 11 to 62) after transplant, respectively.
However, the rate of platelet recovery was significantly higher in the single-unit arm—76% vs 65% (P=0.04). Furthermore, the median time to platelet recovery was 58 days (range, 28 to 295) in the single-unit arm and 84 days (range, 22 to 716) in the double-unit arm.
The rate of grade 2-4 acute GVHD was similar between the treatment arms (P=0.78), but patients in the double-unit arm had a higher incidence of grade 3-4 acute GVHD—23% vs 13% (P=0.02).
There was no difference in the incidence of any chronic GVHD at 1 year after transplant—32% in the double-unit arm and 30% in the single-unit arm (P=0.51). But there was a higher incidence of extensive chronic GVHD after double-unit transplant—15% vs 9% (P=0.05).
“This is helpful news for physicians considering the best treatment options for their patients,” said Joanne Kurtzberg, MD, of Duke University Medical Center in Durham, North Carolina.
“We found children who have a cord blood unit with an adequate number of cells do not benefit from receiving two units. This reduces the cost of a cord blood transplant for the majority of pediatric patients needing the procedure. However, for larger children without an adequately dosed single cord blood unit, using two units will provide access to a potentially life-saving transplant.” ![]()

Credit: NHS
Single and double cord blood transplants produce similar outcomes, according to a study of young patients with hematologic disorders.
Researchers found that rates of overall and disease-free survival were not significantly different in patients who received a single unit of cord blood and those who received two units.
Other outcome measures, such as neutrophil recovery, relapse, and transplant-related death, were similar between the two groups as well.
However, patients who received a single cord blood unit showed improved platelet recovery, a lower incidence of grade 3-4 acute graft-vs-host disease (GVHD), and a lower rate of extensive chronic GVHD.
John Wagner, Jr, MD, of the University of Minnesota in Minneapolis, and his colleagues reported these results in NEJM. Dr Wagner previously presented results from this study at ASH 2012.
“Based on promising early studies using two cord blood units in adults for whom one unit is often not sufficient, we designed this study in order to determine if the higher number of blood-forming stem cells in two cord blood units might improve survival,” Dr Wagner said. “What we found, however, was that both treatment arms performed very well, with similar rates of white blood cell recovery and survival.”
The researchers enrolled 224 patients, ages 1 to 21 years, with hematologic disorders, including acute and chronic leukemias as well as myelodysplastic syndromes.
Patients were randomized to receive double-unit (n=111) or single-unit (n=113) cord blood transplants after a uniform myeloablative conditioning regimen and immunoprophylaxis for GVHD.
The researchers matched the treatment arms for age, sex, self-reported race, performance status, degree of donor-recipient HLA matching, disease type, and disease status at transplant.
Survival and relapse
The study’s primary endpoint was 1-year survival, which was 65% in the double-unit arm and 73% in the single-unit arm (P=0.17). In a multivariate analysis, the risk of death did not differ significantly between the arms (hazard ratio=1.34, P=0.20).
Similarly, there was no significant difference in 1-year disease-free survival between the double- and single-unit arms—64% and 70%, respectively (P=0.11). In a multivariate analysis, the risk of relapse or death did not differ significantly between arms (hazard ratio=1.48, P=0.08).
It therefore follows that rates of relapse and transplant-related death were similar at 1 year as well. The incidence of relapse was 14% in the double-unit arm and 12% in the single-unit arm (P=0.12). And rates of transplant-related death were 22% and 19%, respectively (P=0.43).
Recovery and GVHD
The incidence of neutrophil recovery was similar between treatment arms—88% in the double-unit arm and 89% in the single-unit arm (P=0.29) at a median of 23 days (range, 11 to 133) and 21 days (range, 11 to 62) after transplant, respectively.
However, the rate of platelet recovery was significantly higher in the single-unit arm—76% vs 65% (P=0.04). Furthermore, the median time to platelet recovery was 58 days (range, 28 to 295) in the single-unit arm and 84 days (range, 22 to 716) in the double-unit arm.
The rate of grade 2-4 acute GVHD was similar between the treatment arms (P=0.78), but patients in the double-unit arm had a higher incidence of grade 3-4 acute GVHD—23% vs 13% (P=0.02).
There was no difference in the incidence of any chronic GVHD at 1 year after transplant—32% in the double-unit arm and 30% in the single-unit arm (P=0.51). But there was a higher incidence of extensive chronic GVHD after double-unit transplant—15% vs 9% (P=0.05).
“This is helpful news for physicians considering the best treatment options for their patients,” said Joanne Kurtzberg, MD, of Duke University Medical Center in Durham, North Carolina.
“We found children who have a cord blood unit with an adequate number of cells do not benefit from receiving two units. This reduces the cost of a cord blood transplant for the majority of pediatric patients needing the procedure. However, for larger children without an adequately dosed single cord blood unit, using two units will provide access to a potentially life-saving transplant.” ![]()

Credit: NHS
Single and double cord blood transplants produce similar outcomes, according to a study of young patients with hematologic disorders.
Researchers found that rates of overall and disease-free survival were not significantly different in patients who received a single unit of cord blood and those who received two units.
Other outcome measures, such as neutrophil recovery, relapse, and transplant-related death, were similar between the two groups as well.
However, patients who received a single cord blood unit showed improved platelet recovery, a lower incidence of grade 3-4 acute graft-vs-host disease (GVHD), and a lower rate of extensive chronic GVHD.
John Wagner, Jr, MD, of the University of Minnesota in Minneapolis, and his colleagues reported these results in NEJM. Dr Wagner previously presented results from this study at ASH 2012.
“Based on promising early studies using two cord blood units in adults for whom one unit is often not sufficient, we designed this study in order to determine if the higher number of blood-forming stem cells in two cord blood units might improve survival,” Dr Wagner said. “What we found, however, was that both treatment arms performed very well, with similar rates of white blood cell recovery and survival.”
The researchers enrolled 224 patients, ages 1 to 21 years, with hematologic disorders, including acute and chronic leukemias as well as myelodysplastic syndromes.
Patients were randomized to receive double-unit (n=111) or single-unit (n=113) cord blood transplants after a uniform myeloablative conditioning regimen and immunoprophylaxis for GVHD.
The researchers matched the treatment arms for age, sex, self-reported race, performance status, degree of donor-recipient HLA matching, disease type, and disease status at transplant.
Survival and relapse
The study’s primary endpoint was 1-year survival, which was 65% in the double-unit arm and 73% in the single-unit arm (P=0.17). In a multivariate analysis, the risk of death did not differ significantly between the arms (hazard ratio=1.34, P=0.20).
Similarly, there was no significant difference in 1-year disease-free survival between the double- and single-unit arms—64% and 70%, respectively (P=0.11). In a multivariate analysis, the risk of relapse or death did not differ significantly between arms (hazard ratio=1.48, P=0.08).
It therefore follows that rates of relapse and transplant-related death were similar at 1 year as well. The incidence of relapse was 14% in the double-unit arm and 12% in the single-unit arm (P=0.12). And rates of transplant-related death were 22% and 19%, respectively (P=0.43).
Recovery and GVHD
The incidence of neutrophil recovery was similar between treatment arms—88% in the double-unit arm and 89% in the single-unit arm (P=0.29) at a median of 23 days (range, 11 to 133) and 21 days (range, 11 to 62) after transplant, respectively.
However, the rate of platelet recovery was significantly higher in the single-unit arm—76% vs 65% (P=0.04). Furthermore, the median time to platelet recovery was 58 days (range, 28 to 295) in the single-unit arm and 84 days (range, 22 to 716) in the double-unit arm.
The rate of grade 2-4 acute GVHD was similar between the treatment arms (P=0.78), but patients in the double-unit arm had a higher incidence of grade 3-4 acute GVHD—23% vs 13% (P=0.02).
There was no difference in the incidence of any chronic GVHD at 1 year after transplant—32% in the double-unit arm and 30% in the single-unit arm (P=0.51). But there was a higher incidence of extensive chronic GVHD after double-unit transplant—15% vs 9% (P=0.05).
“This is helpful news for physicians considering the best treatment options for their patients,” said Joanne Kurtzberg, MD, of Duke University Medical Center in Durham, North Carolina.
“We found children who have a cord blood unit with an adequate number of cells do not benefit from receiving two units. This reduces the cost of a cord blood transplant for the majority of pediatric patients needing the procedure. However, for larger children without an adequately dosed single cord blood unit, using two units will provide access to a potentially life-saving transplant.” ![]()
Residents arrange transfusions despite poor knowledge
PHILADELPHIA—Internal medicine residents are obtaining transfusion consent from patients despite having poor knowledge of transfusion medicine, according to a study of nearly 500 residents in 9 countries.
On an exam assessing transfusion knowledge, the residents’ mean score was 45.7%.
And in a survey, an overwhelming majority of residents said they had “beginner” or “intermediate” transfusion knowledge.
Still, 89% said they had obtained patient consent for a transfusion.
Richard Haspel, MD, PhD, of Beth Israel Deacon Medical Center and Harvard Medical School in Boston, presented these data at the AABB Annual Meeting 2014 (abstract S45-030G).
“We all know there’s a problem with clinicians not knowing how to transfuse blood,” Dr Haspel began. “I would argue, though, that there are a lot of questions we don’t know the answer to. How prevalent is this problem? Are there some places that do it better than others? What areas need improvement?”
With these questions in mind, Dr Haspel and his colleagues used a 23-question survey and a 20-question exam (validated by the BEST Collaborative) to assess 474 internal medicine residents from 23 sites in 9 countries: Australia, Canada, England, Ireland, Italy, Germany, The Netherlands, Spain, and the US.
The mean score of correct responses in the exam was 45.7%. The mean score was significantly lower for first-year residents (43.9%) than for third- (47.1%; P=0.02) and fourth-year residents (50.6%, P=0.002).
However, as 50.6% was the highest mean score, exam scores were poor regardless of a resident’s time served, Dr Haspel noted. Scores were poor across the different study sites as well, ranging from about 32% to 55%.
The exam included questions on red cells, platelets, plasma, allergic reactions, transfusion-related acute lung injury (TRALI), and transfusion-associated circulatory overload (TACO), among other topics.
As an example, Dr Haspel pointed out that, for the 3 questions on TRALI, the percentage of correct responses did not exceed 15%. This was the topic about which residents seemed the least informed.
Dr Haspel noted that, in general, residents with more medical school hours spent learning about transfusion medicine and those with better perceived quality of their training tended to score higher on the exam. Still, there wasn’t much of a difference in exam scores between residents who said they had beginner, intermediate, or advanced knowledge of transfusion medicine.
Twelve percent of residents said they did not receive any transfusion medicine training in medical school, and 28% said they didn’t receive any training during their residency. About 35% said they received more than 2 hours of training in medical school, and 18% said they received more than 2 hours of training during their residency.
“In terms of the quality of the training, most rated it ‘slightly’ or ‘moderately’ effective,” Dr Haspel said. “In terms of attitudes and perceptions, most of them considered themselves a beginner [48%] or intermediate [48%] in regard to transfusion medicine knowledge.”
Ninety-seven percent of residents said they know how to contact the blood bank, and 72% said they know how to contact a transfusion medicine doctor. But 14% percent of residents did not know if their hospital had transfusion guidelines, and 1% wrongly said their hospital did not have guidelines.
Yet 89% of residents said they had obtained consent for a transfusion from a patient.
On the other hand, most residents (77%) said knowledge of transfusion medicine is “very” or “extremely” important in providing appropriate patient care. And 65% said they would find additional training “very” or “extremely” helpful. ![]()
PHILADELPHIA—Internal medicine residents are obtaining transfusion consent from patients despite having poor knowledge of transfusion medicine, according to a study of nearly 500 residents in 9 countries.
On an exam assessing transfusion knowledge, the residents’ mean score was 45.7%.
And in a survey, an overwhelming majority of residents said they had “beginner” or “intermediate” transfusion knowledge.
Still, 89% said they had obtained patient consent for a transfusion.
Richard Haspel, MD, PhD, of Beth Israel Deacon Medical Center and Harvard Medical School in Boston, presented these data at the AABB Annual Meeting 2014 (abstract S45-030G).
“We all know there’s a problem with clinicians not knowing how to transfuse blood,” Dr Haspel began. “I would argue, though, that there are a lot of questions we don’t know the answer to. How prevalent is this problem? Are there some places that do it better than others? What areas need improvement?”
With these questions in mind, Dr Haspel and his colleagues used a 23-question survey and a 20-question exam (validated by the BEST Collaborative) to assess 474 internal medicine residents from 23 sites in 9 countries: Australia, Canada, England, Ireland, Italy, Germany, The Netherlands, Spain, and the US.
The mean score of correct responses in the exam was 45.7%. The mean score was significantly lower for first-year residents (43.9%) than for third- (47.1%; P=0.02) and fourth-year residents (50.6%, P=0.002).
However, as 50.6% was the highest mean score, exam scores were poor regardless of a resident’s time served, Dr Haspel noted. Scores were poor across the different study sites as well, ranging from about 32% to 55%.
The exam included questions on red cells, platelets, plasma, allergic reactions, transfusion-related acute lung injury (TRALI), and transfusion-associated circulatory overload (TACO), among other topics.
As an example, Dr Haspel pointed out that, for the 3 questions on TRALI, the percentage of correct responses did not exceed 15%. This was the topic about which residents seemed the least informed.
Dr Haspel noted that, in general, residents with more medical school hours spent learning about transfusion medicine and those with better perceived quality of their training tended to score higher on the exam. Still, there wasn’t much of a difference in exam scores between residents who said they had beginner, intermediate, or advanced knowledge of transfusion medicine.
Twelve percent of residents said they did not receive any transfusion medicine training in medical school, and 28% said they didn’t receive any training during their residency. About 35% said they received more than 2 hours of training in medical school, and 18% said they received more than 2 hours of training during their residency.
“In terms of the quality of the training, most rated it ‘slightly’ or ‘moderately’ effective,” Dr Haspel said. “In terms of attitudes and perceptions, most of them considered themselves a beginner [48%] or intermediate [48%] in regard to transfusion medicine knowledge.”
Ninety-seven percent of residents said they know how to contact the blood bank, and 72% said they know how to contact a transfusion medicine doctor. But 14% percent of residents did not know if their hospital had transfusion guidelines, and 1% wrongly said their hospital did not have guidelines.
Yet 89% of residents said they had obtained consent for a transfusion from a patient.
On the other hand, most residents (77%) said knowledge of transfusion medicine is “very” or “extremely” important in providing appropriate patient care. And 65% said they would find additional training “very” or “extremely” helpful. ![]()
PHILADELPHIA—Internal medicine residents are obtaining transfusion consent from patients despite having poor knowledge of transfusion medicine, according to a study of nearly 500 residents in 9 countries.
On an exam assessing transfusion knowledge, the residents’ mean score was 45.7%.
And in a survey, an overwhelming majority of residents said they had “beginner” or “intermediate” transfusion knowledge.
Still, 89% said they had obtained patient consent for a transfusion.
Richard Haspel, MD, PhD, of Beth Israel Deacon Medical Center and Harvard Medical School in Boston, presented these data at the AABB Annual Meeting 2014 (abstract S45-030G).
“We all know there’s a problem with clinicians not knowing how to transfuse blood,” Dr Haspel began. “I would argue, though, that there are a lot of questions we don’t know the answer to. How prevalent is this problem? Are there some places that do it better than others? What areas need improvement?”
With these questions in mind, Dr Haspel and his colleagues used a 23-question survey and a 20-question exam (validated by the BEST Collaborative) to assess 474 internal medicine residents from 23 sites in 9 countries: Australia, Canada, England, Ireland, Italy, Germany, The Netherlands, Spain, and the US.
The mean score of correct responses in the exam was 45.7%. The mean score was significantly lower for first-year residents (43.9%) than for third- (47.1%; P=0.02) and fourth-year residents (50.6%, P=0.002).
However, as 50.6% was the highest mean score, exam scores were poor regardless of a resident’s time served, Dr Haspel noted. Scores were poor across the different study sites as well, ranging from about 32% to 55%.
The exam included questions on red cells, platelets, plasma, allergic reactions, transfusion-related acute lung injury (TRALI), and transfusion-associated circulatory overload (TACO), among other topics.
As an example, Dr Haspel pointed out that, for the 3 questions on TRALI, the percentage of correct responses did not exceed 15%. This was the topic about which residents seemed the least informed.
Dr Haspel noted that, in general, residents with more medical school hours spent learning about transfusion medicine and those with better perceived quality of their training tended to score higher on the exam. Still, there wasn’t much of a difference in exam scores between residents who said they had beginner, intermediate, or advanced knowledge of transfusion medicine.
Twelve percent of residents said they did not receive any transfusion medicine training in medical school, and 28% said they didn’t receive any training during their residency. About 35% said they received more than 2 hours of training in medical school, and 18% said they received more than 2 hours of training during their residency.
“In terms of the quality of the training, most rated it ‘slightly’ or ‘moderately’ effective,” Dr Haspel said. “In terms of attitudes and perceptions, most of them considered themselves a beginner [48%] or intermediate [48%] in regard to transfusion medicine knowledge.”
Ninety-seven percent of residents said they know how to contact the blood bank, and 72% said they know how to contact a transfusion medicine doctor. But 14% percent of residents did not know if their hospital had transfusion guidelines, and 1% wrongly said their hospital did not have guidelines.
Yet 89% of residents said they had obtained consent for a transfusion from a patient.
On the other hand, most residents (77%) said knowledge of transfusion medicine is “very” or “extremely” important in providing appropriate patient care. And 65% said they would find additional training “very” or “extremely” helpful. ![]()
Newer blood linked to fewer complications from heart surgery
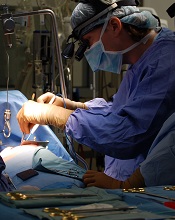
Credit: University of Ottawa
Heart Institute
VANCOUVER—In a large study, heart surgery patients who received recently donated blood had significantly fewer post-operative complications than those who received blood stored for more than 2 weeks.
Patients who received newer blood had a lower rate of mortality, infection, and renal failure.
They were also less likely to require prolonged ventilation or re-exploration for bleeding.
Ansar Hassan, MD, PhD, of Saint John Regional Hospital in New Brunswick, Canada, and his colleagues presented these results at the Canadian Cardiovascular Congress as abstract 562.
The researchers examined records at the New Brunswick Heart Centre in Saint John for non-emergency heart surgeries performed from January 2005 to September 2013 on patients who received red blood cells during or after surgery and who stayed in the hospital less than 30 days.
Of 2015 patients, slightly more than half (n=1052) received only blood that was donated within 14 days of the transfusion. The rest of the patients received some or only blood that was donated more than 14 days before transfusion. Canadian protocols allow blood to be stored and used up to 6 weeks after donation.
Patients who received newer blood were more likely to be female, have unstable angina, to have undergone isolated coronary artery bypass graft or valve surgery, to have experienced shorter bypass and cross-clamp times, and to have left the operating room on inotropes.
After surgery, patients who received newer blood had a lower rate of mortality (1.7% vs 3.3%, P=0.02), infection (3.2% vs 5.4%, P=0.02), atrial fibrillation (43.8% vs 47.3%, P=0.12), and renal failure (12.8% vs 17.7%, P=0.0003).
In addition, they were less likely to require ventilation for more than 24 hours (3% vs 7.7%, P<0.0001) or re-exploration for bleeding (1.5% vs 3.1%, P=0.02).
After the researchers adjusted for differences in baseline and intra-operative characteristics, receiving newer blood was associated with a significant reduction in a composite of the aforementioned outcomes (odds ratio=0.79, P=0.01).
“The findings show that we need to pay attention to the age of the blood we give cardiac surgery patients,” Dr Hassan said. “Perhaps more importantly, we need new studies to determine what is driving this relationship between the age of blood and the outcomes we are seeing.”
Dr Hassan noted that previous studies have reached contradictory conclusions on this subject, which was a reason this study was conducted. ![]()

Credit: University of Ottawa
Heart Institute
VANCOUVER—In a large study, heart surgery patients who received recently donated blood had significantly fewer post-operative complications than those who received blood stored for more than 2 weeks.
Patients who received newer blood had a lower rate of mortality, infection, and renal failure.
They were also less likely to require prolonged ventilation or re-exploration for bleeding.
Ansar Hassan, MD, PhD, of Saint John Regional Hospital in New Brunswick, Canada, and his colleagues presented these results at the Canadian Cardiovascular Congress as abstract 562.
The researchers examined records at the New Brunswick Heart Centre in Saint John for non-emergency heart surgeries performed from January 2005 to September 2013 on patients who received red blood cells during or after surgery and who stayed in the hospital less than 30 days.
Of 2015 patients, slightly more than half (n=1052) received only blood that was donated within 14 days of the transfusion. The rest of the patients received some or only blood that was donated more than 14 days before transfusion. Canadian protocols allow blood to be stored and used up to 6 weeks after donation.
Patients who received newer blood were more likely to be female, have unstable angina, to have undergone isolated coronary artery bypass graft or valve surgery, to have experienced shorter bypass and cross-clamp times, and to have left the operating room on inotropes.
After surgery, patients who received newer blood had a lower rate of mortality (1.7% vs 3.3%, P=0.02), infection (3.2% vs 5.4%, P=0.02), atrial fibrillation (43.8% vs 47.3%, P=0.12), and renal failure (12.8% vs 17.7%, P=0.0003).
In addition, they were less likely to require ventilation for more than 24 hours (3% vs 7.7%, P<0.0001) or re-exploration for bleeding (1.5% vs 3.1%, P=0.02).
After the researchers adjusted for differences in baseline and intra-operative characteristics, receiving newer blood was associated with a significant reduction in a composite of the aforementioned outcomes (odds ratio=0.79, P=0.01).
“The findings show that we need to pay attention to the age of the blood we give cardiac surgery patients,” Dr Hassan said. “Perhaps more importantly, we need new studies to determine what is driving this relationship between the age of blood and the outcomes we are seeing.”
Dr Hassan noted that previous studies have reached contradictory conclusions on this subject, which was a reason this study was conducted. ![]()

Credit: University of Ottawa
Heart Institute
VANCOUVER—In a large study, heart surgery patients who received recently donated blood had significantly fewer post-operative complications than those who received blood stored for more than 2 weeks.
Patients who received newer blood had a lower rate of mortality, infection, and renal failure.
They were also less likely to require prolonged ventilation or re-exploration for bleeding.
Ansar Hassan, MD, PhD, of Saint John Regional Hospital in New Brunswick, Canada, and his colleagues presented these results at the Canadian Cardiovascular Congress as abstract 562.
The researchers examined records at the New Brunswick Heart Centre in Saint John for non-emergency heart surgeries performed from January 2005 to September 2013 on patients who received red blood cells during or after surgery and who stayed in the hospital less than 30 days.
Of 2015 patients, slightly more than half (n=1052) received only blood that was donated within 14 days of the transfusion. The rest of the patients received some or only blood that was donated more than 14 days before transfusion. Canadian protocols allow blood to be stored and used up to 6 weeks after donation.
Patients who received newer blood were more likely to be female, have unstable angina, to have undergone isolated coronary artery bypass graft or valve surgery, to have experienced shorter bypass and cross-clamp times, and to have left the operating room on inotropes.
After surgery, patients who received newer blood had a lower rate of mortality (1.7% vs 3.3%, P=0.02), infection (3.2% vs 5.4%, P=0.02), atrial fibrillation (43.8% vs 47.3%, P=0.12), and renal failure (12.8% vs 17.7%, P=0.0003).
In addition, they were less likely to require ventilation for more than 24 hours (3% vs 7.7%, P<0.0001) or re-exploration for bleeding (1.5% vs 3.1%, P=0.02).
After the researchers adjusted for differences in baseline and intra-operative characteristics, receiving newer blood was associated with a significant reduction in a composite of the aforementioned outcomes (odds ratio=0.79, P=0.01).
“The findings show that we need to pay attention to the age of the blood we give cardiac surgery patients,” Dr Hassan said. “Perhaps more importantly, we need new studies to determine what is driving this relationship between the age of blood and the outcomes we are seeing.”
Dr Hassan noted that previous studies have reached contradictory conclusions on this subject, which was a reason this study was conducted.
Group creates universal platelets using iPSCs

Credit: Salk Institute
Researchers say they can use induced pluripotent stem cells (iPSCs) to produce large-scale quantities of universal donor platelets.
The team generated megakaryocytes and platelets from iPSCs under feeder-free conditions.
They were able to produce universal platelets by removing a gene essential to expression of the major histocompatibility antigens.
The resulting platelets were functional and behaved like normal human platelets.
The researchers described this method of platelet production, owned by Advanced Cell Technology, Inc., in Stem Cell Reports.
“Unlike other sources of platelets, human induced pluripotent stem cells can be propagated indefinitely, providing a potentially unlimited source of cells for therapeutic purposes,” said Robert Lanza, MD, Chief Scientific Officer at Advanced Cell Technology.
“This study shows that platelets may be produced from [iPSCs] without the need for serum and feeders and, thus, removes potential risks associated with contaminants and pathogens.”
Dr Lanza and his colleagues used a 3-step protocol to differentiate human iPSCs into megakaryocytes and functional platelets in less than 20 days. The method incorporates several discrete intermediate cells, including proprietary hemogenic endothelium-like cells.
The technique allows for long-term storage of megakaryocyte progenitors so they can be available within a few days when needed to produce large quantities of platelets for transfusion.
In addition, by knocking out the β2-microglobulin gene, the researchers were able to generate platelets that are negative for the major histocompatibility antigens.
This suggests the platelets could be transfused into almost any patient, and the method might even prevent platelet refractoriness, according to the researchers.
The team found no major differences in the iPSC platelets and normal human platelets. The iPSC platelets formed aggregates, lamellipodia, and filopodia after activation, just like normal platelets.
Also like normal platelets, the iPSC platelets circulated for at least 8 hours in macrophage-depleted NOD/SCID mice, with a time to reach maximal accumulation of 30 minutes to an hour.
In another murine experiment, iPSC platelets incorporated into a growing thrombus just like normal human platelets, with an average number of 9.0 ± 1.8 platelets per thrombus.
“The platelets generated with our technology are functional and behave like normal human platelets,” Dr Lanza said. “This technology and these results represent an important step towards generating unlimited supplies of universal donor platelets for transfusion.”

Credit: Salk Institute
Researchers say they can use induced pluripotent stem cells (iPSCs) to produce large-scale quantities of universal donor platelets.
The team generated megakaryocytes and platelets from iPSCs under feeder-free conditions.
They were able to produce universal platelets by removing a gene essential to expression of the major histocompatibility antigens.
The resulting platelets were functional and behaved like normal human platelets.
The researchers described this method of platelet production, owned by Advanced Cell Technology, Inc., in Stem Cell Reports.
“Unlike other sources of platelets, human induced pluripotent stem cells can be propagated indefinitely, providing a potentially unlimited source of cells for therapeutic purposes,” said Robert Lanza, MD, Chief Scientific Officer at Advanced Cell Technology.
“This study shows that platelets may be produced from [iPSCs] without the need for serum and feeders and, thus, removes potential risks associated with contaminants and pathogens.”
Dr Lanza and his colleagues used a 3-step protocol to differentiate human iPSCs into megakaryocytes and functional platelets in less than 20 days. The method incorporates several discrete intermediate cells, including proprietary hemogenic endothelium-like cells.
The technique allows for long-term storage of megakaryocyte progenitors so they can be available within a few days when needed to produce large quantities of platelets for transfusion.
In addition, by knocking out the β2-microglobulin gene, the researchers were able to generate platelets that are negative for the major histocompatibility antigens.
This suggests the platelets could be transfused into almost any patient, and the method might even prevent platelet refractoriness, according to the researchers.
The team found no major differences in the iPSC platelets and normal human platelets. The iPSC platelets formed aggregates, lamellipodia, and filopodia after activation, just like normal platelets.
Also like normal platelets, the iPSC platelets circulated for at least 8 hours in macrophage-depleted NOD/SCID mice, with a time to reach maximal accumulation of 30 minutes to an hour.
In another murine experiment, iPSC platelets incorporated into a growing thrombus just like normal human platelets, with an average number of 9.0 ± 1.8 platelets per thrombus.
“The platelets generated with our technology are functional and behave like normal human platelets,” Dr Lanza said. “This technology and these results represent an important step towards generating unlimited supplies of universal donor platelets for transfusion.”

Credit: Salk Institute
Researchers say they can use induced pluripotent stem cells (iPSCs) to produce large-scale quantities of universal donor platelets.
The team generated megakaryocytes and platelets from iPSCs under feeder-free conditions.
They were able to produce universal platelets by removing a gene essential to expression of the major histocompatibility antigens.
The resulting platelets were functional and behaved like normal human platelets.
The researchers described this method of platelet production, owned by Advanced Cell Technology, Inc., in Stem Cell Reports.
“Unlike other sources of platelets, human induced pluripotent stem cells can be propagated indefinitely, providing a potentially unlimited source of cells for therapeutic purposes,” said Robert Lanza, MD, Chief Scientific Officer at Advanced Cell Technology.
“This study shows that platelets may be produced from [iPSCs] without the need for serum and feeders and, thus, removes potential risks associated with contaminants and pathogens.”
Dr Lanza and his colleagues used a 3-step protocol to differentiate human iPSCs into megakaryocytes and functional platelets in less than 20 days. The method incorporates several discrete intermediate cells, including proprietary hemogenic endothelium-like cells.
The technique allows for long-term storage of megakaryocyte progenitors so they can be available within a few days when needed to produce large quantities of platelets for transfusion.
In addition, by knocking out the β2-microglobulin gene, the researchers were able to generate platelets that are negative for the major histocompatibility antigens.
This suggests the platelets could be transfused into almost any patient, and the method might even prevent platelet refractoriness, according to the researchers.
The team found no major differences in the iPSC platelets and normal human platelets. The iPSC platelets formed aggregates, lamellipodia, and filopodia after activation, just like normal platelets.
Also like normal platelets, the iPSC platelets circulated for at least 8 hours in macrophage-depleted NOD/SCID mice, with a time to reach maximal accumulation of 30 minutes to an hour.
In another murine experiment, iPSC platelets incorporated into a growing thrombus just like normal human platelets, with an average number of 9.0 ± 1.8 platelets per thrombus.
“The platelets generated with our technology are functional and behave like normal human platelets,” Dr Lanza said. “This technology and these results represent an important step towards generating unlimited supplies of universal donor platelets for transfusion.”

