User login
Researchers generate RBCs to treat SCD
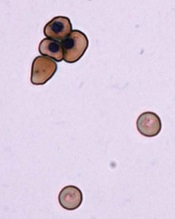
Image by Ying Wang–
Johns Hopkins Medicine
Researchers say they have devised a technique for generating normal, mature red blood cells (RBCs) from patients with sickle cell disease (SCD).
The team hopes that, ultimately, the RBCs could be transfused back into the patients from which they are derived and eliminate the need for donor transfusion in SCD.
Linzhao Cheng, PhD, of the Johns Hopkins School of Medicine in Baltimore, Maryland, and his colleagues described the technique in Stem Cells.
Dr Cheng noted that SCD patients often require RBC transfusions, but over time, their bodies may begin to mount an immune response against the foreign blood.
“Their bodies quickly kill off the blood cells,” Dr Cheng said. “So they have to get transfusions more and more frequently.”
A solution, Dr Cheng and his colleagues thought, would be to generate RBCs for transfusion using a patient’s own cells.
To do this, the researchers first took hematopoietic cells from an SCD patient and generated induced pluripotent stem cells (iPSCs).
Then, the team used the gene-editing technique CRISPR/Cas9 to target the homozygous SCD mutation (nt. 69A>T) in the HBB gene and ensure the RBCs they generated would not be sickled.
Finally, they coaxed the iPSCs into mature RBCs that expressed the corrected HBB gene. The edited iPSCs generated RBCs just as efficiently as iPSCs that hadn’t been subjected to CRISPR/Cas9.
And the level of HBB protein expression in the RBCs derived from edited iPSCs was similar to that of RBCs generated from unedited iPSCs.
Dr Cheng noted that, to become medically useful, this method will have to be made more efficient and scaled up significantly. And the cells would need to be tested for safety.
“[Nevertheless,] this study shows it may be possible in the not-too-distant future to provide patients with sickle cell disease with an exciting new treatment option,” Dr Cheng said.
He and his colleagues believe this method of RBC generation may also be applicable for other blood disorders. And they think it might be possible to edit cells from healthy individuals so they can resist malaria and other infectious agents.
Another research group has reported the ability to correct the SCD mutation using zinc-finger nucleases. ![]()

Image by Ying Wang–
Johns Hopkins Medicine
Researchers say they have devised a technique for generating normal, mature red blood cells (RBCs) from patients with sickle cell disease (SCD).
The team hopes that, ultimately, the RBCs could be transfused back into the patients from which they are derived and eliminate the need for donor transfusion in SCD.
Linzhao Cheng, PhD, of the Johns Hopkins School of Medicine in Baltimore, Maryland, and his colleagues described the technique in Stem Cells.
Dr Cheng noted that SCD patients often require RBC transfusions, but over time, their bodies may begin to mount an immune response against the foreign blood.
“Their bodies quickly kill off the blood cells,” Dr Cheng said. “So they have to get transfusions more and more frequently.”
A solution, Dr Cheng and his colleagues thought, would be to generate RBCs for transfusion using a patient’s own cells.
To do this, the researchers first took hematopoietic cells from an SCD patient and generated induced pluripotent stem cells (iPSCs).
Then, the team used the gene-editing technique CRISPR/Cas9 to target the homozygous SCD mutation (nt. 69A>T) in the HBB gene and ensure the RBCs they generated would not be sickled.
Finally, they coaxed the iPSCs into mature RBCs that expressed the corrected HBB gene. The edited iPSCs generated RBCs just as efficiently as iPSCs that hadn’t been subjected to CRISPR/Cas9.
And the level of HBB protein expression in the RBCs derived from edited iPSCs was similar to that of RBCs generated from unedited iPSCs.
Dr Cheng noted that, to become medically useful, this method will have to be made more efficient and scaled up significantly. And the cells would need to be tested for safety.
“[Nevertheless,] this study shows it may be possible in the not-too-distant future to provide patients with sickle cell disease with an exciting new treatment option,” Dr Cheng said.
He and his colleagues believe this method of RBC generation may also be applicable for other blood disorders. And they think it might be possible to edit cells from healthy individuals so they can resist malaria and other infectious agents.
Another research group has reported the ability to correct the SCD mutation using zinc-finger nucleases. ![]()

Image by Ying Wang–
Johns Hopkins Medicine
Researchers say they have devised a technique for generating normal, mature red blood cells (RBCs) from patients with sickle cell disease (SCD).
The team hopes that, ultimately, the RBCs could be transfused back into the patients from which they are derived and eliminate the need for donor transfusion in SCD.
Linzhao Cheng, PhD, of the Johns Hopkins School of Medicine in Baltimore, Maryland, and his colleagues described the technique in Stem Cells.
Dr Cheng noted that SCD patients often require RBC transfusions, but over time, their bodies may begin to mount an immune response against the foreign blood.
“Their bodies quickly kill off the blood cells,” Dr Cheng said. “So they have to get transfusions more and more frequently.”
A solution, Dr Cheng and his colleagues thought, would be to generate RBCs for transfusion using a patient’s own cells.
To do this, the researchers first took hematopoietic cells from an SCD patient and generated induced pluripotent stem cells (iPSCs).
Then, the team used the gene-editing technique CRISPR/Cas9 to target the homozygous SCD mutation (nt. 69A>T) in the HBB gene and ensure the RBCs they generated would not be sickled.
Finally, they coaxed the iPSCs into mature RBCs that expressed the corrected HBB gene. The edited iPSCs generated RBCs just as efficiently as iPSCs that hadn’t been subjected to CRISPR/Cas9.
And the level of HBB protein expression in the RBCs derived from edited iPSCs was similar to that of RBCs generated from unedited iPSCs.
Dr Cheng noted that, to become medically useful, this method will have to be made more efficient and scaled up significantly. And the cells would need to be tested for safety.
“[Nevertheless,] this study shows it may be possible in the not-too-distant future to provide patients with sickle cell disease with an exciting new treatment option,” Dr Cheng said.
He and his colleagues believe this method of RBC generation may also be applicable for other blood disorders. And they think it might be possible to edit cells from healthy individuals so they can resist malaria and other infectious agents.
Another research group has reported the ability to correct the SCD mutation using zinc-finger nucleases. ![]()
ASH advocates use of systems-based hematologists

Photo courtesy of CDC
The American Society of Hematology (ASH) has released a report proposing a new role for hematologists specializing in non-malignant blood disorders.
ASH partnered with the healthcare consulting firm The Lewin Group to identify emerging career opportunities for health system- and hospital-based hematologists and to provide guidance on pursuing those opportunities.
The resulting report, published in Blood, outlines a few models for a systems-based clinical hematologist.
The report’s authors noted that demand for hematology expertise remains high nationwide. However, ASH and its members are concerned that changes to academic training will hinder both the recruitment of new talent to the field and the retention of seasoned experts.
The authors said that today’s hematology trainees are unlikely to receive the same non-malignant training as many “classic” hematologists trained in prior decades. And training shortfalls are further compounded by the fact that primary care physicians do not have the expertise to manage common blood disorders, which increases referrals to hematologists.
This results in higher demand for a smaller pool of hematologists entering the field with adequate training to effectively and efficiently manage non-malignant disorders.
“Given the rapid evolution and complexity of the field, the time is appropriate to identify career pathways that attract and enable physicians to practice non-malignant hematology in a sustainable manner,” said author Janis L. Abkowitz, MD, of the University of Washington in Seattle.
She and her colleagues noted that, in response to these challenges, US hematologists are defining new paths and assuming more centralized positions in large and small healthcare systems.
These systems-based hematologists are specialty-trained physicians—employed by a hospital, medical center, or health system—who optimize individual patient care as well as the overall system of healthcare delivery for patients with blood disorders.
For example, a systems-based hematologist could work closely with surgeons to minimize perioperative bleeding and could manage care pathways for patients with chronic blood diseases.
The report offered 4 examples where the involvement of a systems-based hematologist would lead to cost-effective decision-making. These were based upon interviews with 14 early adoptors of the systems-based approach to hematology.
The first example was heparin-induced thrombocytopenia (HIT). A systems-based hematologist could implement care pathways that focus on HIT by working to reduce unnecessary heparin exposure, optimizing laboratory testing for suspected HIT, and reducing unnecessary procedures in patients.
The second example was thrombotic thrombocytopenic purpura (TTP). A systems-based hematologist could optimize testing for TTP, which may reduce system-wide plasma use.
The third example was a medical director for hemostasis and thrombosis. A systems-based hematologist could foster appropriate and safe practices, including the implementation of and adherence to preventive care for thrombotic events and the optimal use of anticoagulant medications.
The fourth example was non-malignant hematology consultation in an accountable care organization (ACO) environment. The authors noted that ACOs have enabled more patients to be served by a health system, but there are fewer incentives for physicians to manage common hematology-related issues. A funded systems-based hematologist could ensure that patients have more timely access to hematology consultations.
“A systems-based hematologist position presents a unique opportunity for hematologists to design new models for care delivery and demonstrate their ability to improve clinical outcomes while maintaining or reducing costs,” Dr Abkowitz said. “Just as blood must flow throughout the body, the expertise of hematology must flow throughout the healthcare system.”
As a next step, ASH has invited its members to share practice models they have developed and examples of how they have collaborated with others to improve healthcare outcomes, reduce complications, and eliminate unnecessary spending. ![]()

Photo courtesy of CDC
The American Society of Hematology (ASH) has released a report proposing a new role for hematologists specializing in non-malignant blood disorders.
ASH partnered with the healthcare consulting firm The Lewin Group to identify emerging career opportunities for health system- and hospital-based hematologists and to provide guidance on pursuing those opportunities.
The resulting report, published in Blood, outlines a few models for a systems-based clinical hematologist.
The report’s authors noted that demand for hematology expertise remains high nationwide. However, ASH and its members are concerned that changes to academic training will hinder both the recruitment of new talent to the field and the retention of seasoned experts.
The authors said that today’s hematology trainees are unlikely to receive the same non-malignant training as many “classic” hematologists trained in prior decades. And training shortfalls are further compounded by the fact that primary care physicians do not have the expertise to manage common blood disorders, which increases referrals to hematologists.
This results in higher demand for a smaller pool of hematologists entering the field with adequate training to effectively and efficiently manage non-malignant disorders.
“Given the rapid evolution and complexity of the field, the time is appropriate to identify career pathways that attract and enable physicians to practice non-malignant hematology in a sustainable manner,” said author Janis L. Abkowitz, MD, of the University of Washington in Seattle.
She and her colleagues noted that, in response to these challenges, US hematologists are defining new paths and assuming more centralized positions in large and small healthcare systems.
These systems-based hematologists are specialty-trained physicians—employed by a hospital, medical center, or health system—who optimize individual patient care as well as the overall system of healthcare delivery for patients with blood disorders.
For example, a systems-based hematologist could work closely with surgeons to minimize perioperative bleeding and could manage care pathways for patients with chronic blood diseases.
The report offered 4 examples where the involvement of a systems-based hematologist would lead to cost-effective decision-making. These were based upon interviews with 14 early adoptors of the systems-based approach to hematology.
The first example was heparin-induced thrombocytopenia (HIT). A systems-based hematologist could implement care pathways that focus on HIT by working to reduce unnecessary heparin exposure, optimizing laboratory testing for suspected HIT, and reducing unnecessary procedures in patients.
The second example was thrombotic thrombocytopenic purpura (TTP). A systems-based hematologist could optimize testing for TTP, which may reduce system-wide plasma use.
The third example was a medical director for hemostasis and thrombosis. A systems-based hematologist could foster appropriate and safe practices, including the implementation of and adherence to preventive care for thrombotic events and the optimal use of anticoagulant medications.
The fourth example was non-malignant hematology consultation in an accountable care organization (ACO) environment. The authors noted that ACOs have enabled more patients to be served by a health system, but there are fewer incentives for physicians to manage common hematology-related issues. A funded systems-based hematologist could ensure that patients have more timely access to hematology consultations.
“A systems-based hematologist position presents a unique opportunity for hematologists to design new models for care delivery and demonstrate their ability to improve clinical outcomes while maintaining or reducing costs,” Dr Abkowitz said. “Just as blood must flow throughout the body, the expertise of hematology must flow throughout the healthcare system.”
As a next step, ASH has invited its members to share practice models they have developed and examples of how they have collaborated with others to improve healthcare outcomes, reduce complications, and eliminate unnecessary spending. ![]()

Photo courtesy of CDC
The American Society of Hematology (ASH) has released a report proposing a new role for hematologists specializing in non-malignant blood disorders.
ASH partnered with the healthcare consulting firm The Lewin Group to identify emerging career opportunities for health system- and hospital-based hematologists and to provide guidance on pursuing those opportunities.
The resulting report, published in Blood, outlines a few models for a systems-based clinical hematologist.
The report’s authors noted that demand for hematology expertise remains high nationwide. However, ASH and its members are concerned that changes to academic training will hinder both the recruitment of new talent to the field and the retention of seasoned experts.
The authors said that today’s hematology trainees are unlikely to receive the same non-malignant training as many “classic” hematologists trained in prior decades. And training shortfalls are further compounded by the fact that primary care physicians do not have the expertise to manage common blood disorders, which increases referrals to hematologists.
This results in higher demand for a smaller pool of hematologists entering the field with adequate training to effectively and efficiently manage non-malignant disorders.
“Given the rapid evolution and complexity of the field, the time is appropriate to identify career pathways that attract and enable physicians to practice non-malignant hematology in a sustainable manner,” said author Janis L. Abkowitz, MD, of the University of Washington in Seattle.
She and her colleagues noted that, in response to these challenges, US hematologists are defining new paths and assuming more centralized positions in large and small healthcare systems.
These systems-based hematologists are specialty-trained physicians—employed by a hospital, medical center, or health system—who optimize individual patient care as well as the overall system of healthcare delivery for patients with blood disorders.
For example, a systems-based hematologist could work closely with surgeons to minimize perioperative bleeding and could manage care pathways for patients with chronic blood diseases.
The report offered 4 examples where the involvement of a systems-based hematologist would lead to cost-effective decision-making. These were based upon interviews with 14 early adoptors of the systems-based approach to hematology.
The first example was heparin-induced thrombocytopenia (HIT). A systems-based hematologist could implement care pathways that focus on HIT by working to reduce unnecessary heparin exposure, optimizing laboratory testing for suspected HIT, and reducing unnecessary procedures in patients.
The second example was thrombotic thrombocytopenic purpura (TTP). A systems-based hematologist could optimize testing for TTP, which may reduce system-wide plasma use.
The third example was a medical director for hemostasis and thrombosis. A systems-based hematologist could foster appropriate and safe practices, including the implementation of and adherence to preventive care for thrombotic events and the optimal use of anticoagulant medications.
The fourth example was non-malignant hematology consultation in an accountable care organization (ACO) environment. The authors noted that ACOs have enabled more patients to be served by a health system, but there are fewer incentives for physicians to manage common hematology-related issues. A funded systems-based hematologist could ensure that patients have more timely access to hematology consultations.
“A systems-based hematologist position presents a unique opportunity for hematologists to design new models for care delivery and demonstrate their ability to improve clinical outcomes while maintaining or reducing costs,” Dr Abkowitz said. “Just as blood must flow throughout the body, the expertise of hematology must flow throughout the healthcare system.”
As a next step, ASH has invited its members to share practice models they have developed and examples of how they have collaborated with others to improve healthcare outcomes, reduce complications, and eliminate unnecessary spending. ![]()
Too many blood tests can lead to anemia, transfusions

Photo by Juan D. Alfonso
A single-center study has shown that laboratory testing among patients undergoing cardiac surgery can lead to excessive bloodletting.
This can increase the risk of hospital-acquired anemia and, therefore, the need for blood transfusions.
Among cardiac surgery patients, transfusions have been associated with an increased risk of infection, more time spent on a ventilator, and a higher likelihood of death, said Colleen G. Koch, MD, of the Cleveland Clinic in Ohio.
She and her colleagues conducted this research and published their findings in The Annals of Thoracic Surgery.
The researchers recorded every laboratory test performed on 1894 patients who underwent cardiac surgery at the Cleveland Clinic from January to June 2012.
The team evaluated the number and type of blood tests performed from the time patients met their surgeons until hospital discharge, tallying up the total amount of blood taken from each patient.
‘Astonishing’ amount of blood drawn
There were 221,498 laboratory tests performed during the study period, or an average of 115 tests per patient. The most common tests were blood gas analyses (n=88,068), coagulation tests (n=39,535), complete blood counts (n=30,421), and metabolic panels (n=29,374).
The cumulative median phlebotomy volume for the entire hospital stay was 454 mL per patient. Patients tended to have more blood drawn if they were in the intensive care unit as compared to other hospital floors, with median phlebotomy volumes of 332 mL and 118 mL, respectively.
“We were astonished by the amount of blood taken from our patients for laboratory testing,” Dr Koch said. “Total phlebotomy volumes approached 1 to 2 units of red blood cells, which is roughly equivalent to 1 to 2 cans of soda.”
More complex procedures were associated with higher overall phlebotomy volume. Patients undergoing combined coronary artery bypass grafting surgery (CABG) and valve procedures had the highest median cumulative phlebotomy volume. The median volume was 653 mL for CABG-valve procedures, 448 mL for CABG alone, and 338 mL for valve procedures alone.
Transfusion need
The researchers also found that an increase in cumulative phlebotomy volume was linked to an increased need for blood products. Similarly, the longer a patient was hospitalized, the more blood was taken, which increased the subsequent need for a transfusion.
Overall, 49% of patients received red blood cells (RBCs), 25% fresh-frozen plasma (FFP), 33% platelets, and 15% cryoprecipitate.
Patients in the lowest phlebotomy volume quartile (0%-25th%) were much less likely to receive transfusions than patients in the highest quartile (75th% to 100th%).
In the lowest quartile, 2% of patients received cryoprecipitate, 3% FFP, 7% platelets, and 12% RBCs. In the highest quartile, 31% of patients received cryoprecipitate, 54% FFP, 61% platelets, and 87% RBCs.
So to reduce the use of transfusions, we must curb the use of blood tests, Dr Koch said, noting that patients can help.
“Patients should feel empowered to ask their doctors whether a specific test is necessary—’What is the indication for the test?,’ ‘Will it change my care?,’ and ‘If so, do you need to do it every day?,’” Dr Koch said.
“They should inquire whether smaller-volume test tubes could be used for the tests that are deemed necessary. Every attempt should be made to conserve the patient’s own blood. Every drop of blood counts.”
In an invited commentary, Milo Engoren, MD, of the University of Michigan in Ann Arbor, emphasized the importance of reducing blood loss to decrease possible complications during surgery.
“We make efforts to minimize intraoperative blood loss,” he noted. “Now, we need to make similar efforts postoperatively. While some may argue that transfusion itself is not harmful, but only a marker of a sicker patient, most would agree that avoiding anemia and transfusion is the best course for patients.” ![]()

Photo by Juan D. Alfonso
A single-center study has shown that laboratory testing among patients undergoing cardiac surgery can lead to excessive bloodletting.
This can increase the risk of hospital-acquired anemia and, therefore, the need for blood transfusions.
Among cardiac surgery patients, transfusions have been associated with an increased risk of infection, more time spent on a ventilator, and a higher likelihood of death, said Colleen G. Koch, MD, of the Cleveland Clinic in Ohio.
She and her colleagues conducted this research and published their findings in The Annals of Thoracic Surgery.
The researchers recorded every laboratory test performed on 1894 patients who underwent cardiac surgery at the Cleveland Clinic from January to June 2012.
The team evaluated the number and type of blood tests performed from the time patients met their surgeons until hospital discharge, tallying up the total amount of blood taken from each patient.
‘Astonishing’ amount of blood drawn
There were 221,498 laboratory tests performed during the study period, or an average of 115 tests per patient. The most common tests were blood gas analyses (n=88,068), coagulation tests (n=39,535), complete blood counts (n=30,421), and metabolic panels (n=29,374).
The cumulative median phlebotomy volume for the entire hospital stay was 454 mL per patient. Patients tended to have more blood drawn if they were in the intensive care unit as compared to other hospital floors, with median phlebotomy volumes of 332 mL and 118 mL, respectively.
“We were astonished by the amount of blood taken from our patients for laboratory testing,” Dr Koch said. “Total phlebotomy volumes approached 1 to 2 units of red blood cells, which is roughly equivalent to 1 to 2 cans of soda.”
More complex procedures were associated with higher overall phlebotomy volume. Patients undergoing combined coronary artery bypass grafting surgery (CABG) and valve procedures had the highest median cumulative phlebotomy volume. The median volume was 653 mL for CABG-valve procedures, 448 mL for CABG alone, and 338 mL for valve procedures alone.
Transfusion need
The researchers also found that an increase in cumulative phlebotomy volume was linked to an increased need for blood products. Similarly, the longer a patient was hospitalized, the more blood was taken, which increased the subsequent need for a transfusion.
Overall, 49% of patients received red blood cells (RBCs), 25% fresh-frozen plasma (FFP), 33% platelets, and 15% cryoprecipitate.
Patients in the lowest phlebotomy volume quartile (0%-25th%) were much less likely to receive transfusions than patients in the highest quartile (75th% to 100th%).
In the lowest quartile, 2% of patients received cryoprecipitate, 3% FFP, 7% platelets, and 12% RBCs. In the highest quartile, 31% of patients received cryoprecipitate, 54% FFP, 61% platelets, and 87% RBCs.
So to reduce the use of transfusions, we must curb the use of blood tests, Dr Koch said, noting that patients can help.
“Patients should feel empowered to ask their doctors whether a specific test is necessary—’What is the indication for the test?,’ ‘Will it change my care?,’ and ‘If so, do you need to do it every day?,’” Dr Koch said.
“They should inquire whether smaller-volume test tubes could be used for the tests that are deemed necessary. Every attempt should be made to conserve the patient’s own blood. Every drop of blood counts.”
In an invited commentary, Milo Engoren, MD, of the University of Michigan in Ann Arbor, emphasized the importance of reducing blood loss to decrease possible complications during surgery.
“We make efforts to minimize intraoperative blood loss,” he noted. “Now, we need to make similar efforts postoperatively. While some may argue that transfusion itself is not harmful, but only a marker of a sicker patient, most would agree that avoiding anemia and transfusion is the best course for patients.” ![]()

Photo by Juan D. Alfonso
A single-center study has shown that laboratory testing among patients undergoing cardiac surgery can lead to excessive bloodletting.
This can increase the risk of hospital-acquired anemia and, therefore, the need for blood transfusions.
Among cardiac surgery patients, transfusions have been associated with an increased risk of infection, more time spent on a ventilator, and a higher likelihood of death, said Colleen G. Koch, MD, of the Cleveland Clinic in Ohio.
She and her colleagues conducted this research and published their findings in The Annals of Thoracic Surgery.
The researchers recorded every laboratory test performed on 1894 patients who underwent cardiac surgery at the Cleveland Clinic from January to June 2012.
The team evaluated the number and type of blood tests performed from the time patients met their surgeons until hospital discharge, tallying up the total amount of blood taken from each patient.
‘Astonishing’ amount of blood drawn
There were 221,498 laboratory tests performed during the study period, or an average of 115 tests per patient. The most common tests were blood gas analyses (n=88,068), coagulation tests (n=39,535), complete blood counts (n=30,421), and metabolic panels (n=29,374).
The cumulative median phlebotomy volume for the entire hospital stay was 454 mL per patient. Patients tended to have more blood drawn if they were in the intensive care unit as compared to other hospital floors, with median phlebotomy volumes of 332 mL and 118 mL, respectively.
“We were astonished by the amount of blood taken from our patients for laboratory testing,” Dr Koch said. “Total phlebotomy volumes approached 1 to 2 units of red blood cells, which is roughly equivalent to 1 to 2 cans of soda.”
More complex procedures were associated with higher overall phlebotomy volume. Patients undergoing combined coronary artery bypass grafting surgery (CABG) and valve procedures had the highest median cumulative phlebotomy volume. The median volume was 653 mL for CABG-valve procedures, 448 mL for CABG alone, and 338 mL for valve procedures alone.
Transfusion need
The researchers also found that an increase in cumulative phlebotomy volume was linked to an increased need for blood products. Similarly, the longer a patient was hospitalized, the more blood was taken, which increased the subsequent need for a transfusion.
Overall, 49% of patients received red blood cells (RBCs), 25% fresh-frozen plasma (FFP), 33% platelets, and 15% cryoprecipitate.
Patients in the lowest phlebotomy volume quartile (0%-25th%) were much less likely to receive transfusions than patients in the highest quartile (75th% to 100th%).
In the lowest quartile, 2% of patients received cryoprecipitate, 3% FFP, 7% platelets, and 12% RBCs. In the highest quartile, 31% of patients received cryoprecipitate, 54% FFP, 61% platelets, and 87% RBCs.
So to reduce the use of transfusions, we must curb the use of blood tests, Dr Koch said, noting that patients can help.
“Patients should feel empowered to ask their doctors whether a specific test is necessary—’What is the indication for the test?,’ ‘Will it change my care?,’ and ‘If so, do you need to do it every day?,’” Dr Koch said.
“They should inquire whether smaller-volume test tubes could be used for the tests that are deemed necessary. Every attempt should be made to conserve the patient’s own blood. Every drop of blood counts.”
In an invited commentary, Milo Engoren, MD, of the University of Michigan in Ann Arbor, emphasized the importance of reducing blood loss to decrease possible complications during surgery.
“We make efforts to minimize intraoperative blood loss,” he noted. “Now, we need to make similar efforts postoperatively. While some may argue that transfusion itself is not harmful, but only a marker of a sicker patient, most would agree that avoiding anemia and transfusion is the best course for patients.” ![]()
FDA grants T-cell therapy breakthrough designation

Photo by Charles Haymond
The US Food and Drug Administration (FDA) has granted breakthrough designation to a therapy consisting of cytotoxic T lymphocytes activated against Epstein-Barr virus (EBV-CTLs).
The treatment is intended for use in patients with rituximab-refractory, EBV-associated lymphoproliferative disease (EBV-LPD), which occurs after allogeneic hematopoietic stem cell transplant.
EBV-CTLs consist of T cells collected from third-party donors.
The T cells are exposed to antigens, expanded, characterized, and stored for future use in an appropriate, partially HLA-matched patient.
In the context of EBV-LPD, the EBV-CTLs are able to target and destroy cancer cells expressing EBV.
“The receipt of breakthrough therapy designation brings us one step closer to our ultimate goal of making EBV-CTL available to all patients with EBV-LPD, a serious and life-threatening condition with limited treatment options,” said Richard O’Reilly, MD, Chair of the Department of Pediatrics and Chief of the Pediatric Bone Marrow Transplant Service at Memorial Sloan Kettering Cancer Center (MSKCC) in New York, New York.
MSKCC is developing EBV-CTLs in conjunction with Atara Biotherapeutics, Inc.
Breakthrough therapy designation for EBV-CTLs was based on data from 2 clinical trials of EBV-CTLs conducted at MSKCC.
Data from these studies have been submitted for presentation at an upcoming medical conference. Results of a phase 1/2 study of EBV-CTLs were previously presented at the APHON 37th Annual Conference and Exhibit in 2013.
The FDA’s breakthrough therapy designation is designed to expedite the development and review of new drugs for the treatment of serious or life-threatening conditions.
To qualify for this designation, a drug must show credible evidence of a substantial improvement on a clinically significant endpoint over available therapies, or over placebo if there is no available therapy, or in a study that compares the new treatment plus the standard of care to the standard of care alone.
The designation confers several benefits, including intensive FDA guidance and eligibility for submission of a rolling biologic license application. ![]()

Photo by Charles Haymond
The US Food and Drug Administration (FDA) has granted breakthrough designation to a therapy consisting of cytotoxic T lymphocytes activated against Epstein-Barr virus (EBV-CTLs).
The treatment is intended for use in patients with rituximab-refractory, EBV-associated lymphoproliferative disease (EBV-LPD), which occurs after allogeneic hematopoietic stem cell transplant.
EBV-CTLs consist of T cells collected from third-party donors.
The T cells are exposed to antigens, expanded, characterized, and stored for future use in an appropriate, partially HLA-matched patient.
In the context of EBV-LPD, the EBV-CTLs are able to target and destroy cancer cells expressing EBV.
“The receipt of breakthrough therapy designation brings us one step closer to our ultimate goal of making EBV-CTL available to all patients with EBV-LPD, a serious and life-threatening condition with limited treatment options,” said Richard O’Reilly, MD, Chair of the Department of Pediatrics and Chief of the Pediatric Bone Marrow Transplant Service at Memorial Sloan Kettering Cancer Center (MSKCC) in New York, New York.
MSKCC is developing EBV-CTLs in conjunction with Atara Biotherapeutics, Inc.
Breakthrough therapy designation for EBV-CTLs was based on data from 2 clinical trials of EBV-CTLs conducted at MSKCC.
Data from these studies have been submitted for presentation at an upcoming medical conference. Results of a phase 1/2 study of EBV-CTLs were previously presented at the APHON 37th Annual Conference and Exhibit in 2013.
The FDA’s breakthrough therapy designation is designed to expedite the development and review of new drugs for the treatment of serious or life-threatening conditions.
To qualify for this designation, a drug must show credible evidence of a substantial improvement on a clinically significant endpoint over available therapies, or over placebo if there is no available therapy, or in a study that compares the new treatment plus the standard of care to the standard of care alone.
The designation confers several benefits, including intensive FDA guidance and eligibility for submission of a rolling biologic license application. ![]()

Photo by Charles Haymond
The US Food and Drug Administration (FDA) has granted breakthrough designation to a therapy consisting of cytotoxic T lymphocytes activated against Epstein-Barr virus (EBV-CTLs).
The treatment is intended for use in patients with rituximab-refractory, EBV-associated lymphoproliferative disease (EBV-LPD), which occurs after allogeneic hematopoietic stem cell transplant.
EBV-CTLs consist of T cells collected from third-party donors.
The T cells are exposed to antigens, expanded, characterized, and stored for future use in an appropriate, partially HLA-matched patient.
In the context of EBV-LPD, the EBV-CTLs are able to target and destroy cancer cells expressing EBV.
“The receipt of breakthrough therapy designation brings us one step closer to our ultimate goal of making EBV-CTL available to all patients with EBV-LPD, a serious and life-threatening condition with limited treatment options,” said Richard O’Reilly, MD, Chair of the Department of Pediatrics and Chief of the Pediatric Bone Marrow Transplant Service at Memorial Sloan Kettering Cancer Center (MSKCC) in New York, New York.
MSKCC is developing EBV-CTLs in conjunction with Atara Biotherapeutics, Inc.
Breakthrough therapy designation for EBV-CTLs was based on data from 2 clinical trials of EBV-CTLs conducted at MSKCC.
Data from these studies have been submitted for presentation at an upcoming medical conference. Results of a phase 1/2 study of EBV-CTLs were previously presented at the APHON 37th Annual Conference and Exhibit in 2013.
The FDA’s breakthrough therapy designation is designed to expedite the development and review of new drugs for the treatment of serious or life-threatening conditions.
To qualify for this designation, a drug must show credible evidence of a substantial improvement on a clinically significant endpoint over available therapies, or over placebo if there is no available therapy, or in a study that compares the new treatment plus the standard of care to the standard of care alone.
The designation confers several benefits, including intensive FDA guidance and eligibility for submission of a rolling biologic license application. ![]()
Product ‘solves engraftment problem’ with UCBT

Photo courtesy of NHS
SAN DIEGO—A product that promotes CD34 expansion has solved the problem of poor engraftment associated with umbilical cord blood transplant (UCBT), according to a speaker at the 2015 BMT Tandem Meetings.
John Wagner, MD, of the University of Minnesota in Minneapolis, supported this statement with early results from trials of HSC835, cord
blood units in which CD34 cells were expanded using the aryl hydrocarbon
receptor antagonist StemRegenin 1.
Dr Wagner and his colleagues conducted phase 1 and 2 trials of HSC835, which were supported by Novartis, the company developing the product.
Dr Wagner presented results of these trials at the BMT Tandem Meetings as abstract 29.*
The phase 1 trial included 18 patients who received double UCBT after myeloablative conditioning. They received an unmanipulated cord blood unit and an HSC835 unit.
In the phase 2 trial, 3 patients received a single HSC835 unit after myeloablative conditioning, and 3 received an HSC835 unit after non-myeloablative conditioning.
Manufacturing HSC835 took 15 days. Following expansion, the median CD34 cell dose increased 346-fold, and the median total nucleated cell (TNC) dose increased 848-fold.
Twenty-eight HSC835 products were produced, but 4 of them were not infused. Two products were contaminated, 1 was not infused due to patient relapse, and 1 product failed to expand. Dr Wagner noted that the failed unit started out at 50% viability, whereas the other units started at about 90% viability.
Phase 1
Eighteen patients were treated in the phase 1 trial. Eleven had acute lymphoblastic leukemia, 5 had acute myeloid leukemia, and 2 had myelodysplastic syndromes. The median age was 28 (range, 12 to 53).
The median TNC dose was 2.5 x 107/kg for the unmanipulated cord blood unit and 5.1 x 107/kg for the HSC835 unit. The median CD34 count was 0.4 x 106/kg and 17.4 x 106/kg, respectively. And the median CD3 count was 8.5 x 106/kg and 2.5 x 106/kg, respectively.
Most units were a 5/6 HLA match—50% for the unmanipulated unit and 67% for the HSC835 unit. Forty-four percent and 22% of the units, respectively, were 4/6 matches. And 6% and 11%, respectively, were 6/6 matches.
Neutrophil recovery was 100%, and the median time to recovery was 14.5 days.
Dr Wagner compared this to results in 121 matched historical controls who received UCBT with 2 unmanipulated units. Of those patients, 86% achieved neutrophil engraftment, and the median time to engraftment was 25 days (P<0.001).
In the current study, 6 patients had complete chimerism with HSC835, 6 had complete chimerism with the unmanipulated unit, and 6 had dual chimerism. In the dual-chimerism patients, both units were present, but all the T cells were derived from the unmanipulated unit.
“When we look at those patients who had engraftment of HSC835, whether it be this dual chimerism or complete chimerism, you see there is a very rapid recovery of 10.5 days, as compared to the historical control [recovery time] of 25 days,” Dr Wagner said. “And those that engrafted with the unmanipulated unit fall right where you’d expect them; that is, with the historical controls [23 days].”
Dr Wagner noted that the CD34 dose correlated with the pace of neutrophil recovery in patients who engrafted with the HSC835 unit, and patients with dual chimerism had the fastest neutrophil recovery.
Furthermore, the HSC835 unit predominated more than expected (P=0.05), winning out over the cord blood unit with a higher CD3 dose a disproportionate amount of time. The unit with a higher CD3 dose typically predominates two-thirds of the time in double UCBT.
“[These results] gave us enough information to say that [HSC835] could be a stand-alone product,” Dr Wagner said.
Phase 2
For phase 2, the researchers used a single HSC835 unit. They chose the lesser of 2 cord blood units (keeping the better unit as a backup), expanded it, and infused the resulting HSC835 unit into 3 patients who received myeloablative conditioning and 3 patients who did not.
In patients who received myeloablative conditioning, chimerism was complete at days 8, 12, and 14. Among the patients who received non-myeloablative conditioning, 1 had mixed chimerism at day 6. The other 2 had complete chimerism at days 5 and 7.
“So in conclusion, we believe that HSC835 is safe and effective in speeding neutrophil recovery after cord blood transplant,” Dr Wagner said. “These are very promising early results that compel us now to explore the single expanded product.”
Dr Wagner added that other considerations for HSC835 are that it may reduce the unit selection threshold, improve HLA match, enable re-cryopreservation (for transplant delays, backup, or multiple dosing), and perhaps allow for reduced-intensity conditioning with “mega-dose” grafts. ![]()
*Information in the abstract differs from that presented at the meeting.

Photo courtesy of NHS
SAN DIEGO—A product that promotes CD34 expansion has solved the problem of poor engraftment associated with umbilical cord blood transplant (UCBT), according to a speaker at the 2015 BMT Tandem Meetings.
John Wagner, MD, of the University of Minnesota in Minneapolis, supported this statement with early results from trials of HSC835, cord
blood units in which CD34 cells were expanded using the aryl hydrocarbon
receptor antagonist StemRegenin 1.
Dr Wagner and his colleagues conducted phase 1 and 2 trials of HSC835, which were supported by Novartis, the company developing the product.
Dr Wagner presented results of these trials at the BMT Tandem Meetings as abstract 29.*
The phase 1 trial included 18 patients who received double UCBT after myeloablative conditioning. They received an unmanipulated cord blood unit and an HSC835 unit.
In the phase 2 trial, 3 patients received a single HSC835 unit after myeloablative conditioning, and 3 received an HSC835 unit after non-myeloablative conditioning.
Manufacturing HSC835 took 15 days. Following expansion, the median CD34 cell dose increased 346-fold, and the median total nucleated cell (TNC) dose increased 848-fold.
Twenty-eight HSC835 products were produced, but 4 of them were not infused. Two products were contaminated, 1 was not infused due to patient relapse, and 1 product failed to expand. Dr Wagner noted that the failed unit started out at 50% viability, whereas the other units started at about 90% viability.
Phase 1
Eighteen patients were treated in the phase 1 trial. Eleven had acute lymphoblastic leukemia, 5 had acute myeloid leukemia, and 2 had myelodysplastic syndromes. The median age was 28 (range, 12 to 53).
The median TNC dose was 2.5 x 107/kg for the unmanipulated cord blood unit and 5.1 x 107/kg for the HSC835 unit. The median CD34 count was 0.4 x 106/kg and 17.4 x 106/kg, respectively. And the median CD3 count was 8.5 x 106/kg and 2.5 x 106/kg, respectively.
Most units were a 5/6 HLA match—50% for the unmanipulated unit and 67% for the HSC835 unit. Forty-four percent and 22% of the units, respectively, were 4/6 matches. And 6% and 11%, respectively, were 6/6 matches.
Neutrophil recovery was 100%, and the median time to recovery was 14.5 days.
Dr Wagner compared this to results in 121 matched historical controls who received UCBT with 2 unmanipulated units. Of those patients, 86% achieved neutrophil engraftment, and the median time to engraftment was 25 days (P<0.001).
In the current study, 6 patients had complete chimerism with HSC835, 6 had complete chimerism with the unmanipulated unit, and 6 had dual chimerism. In the dual-chimerism patients, both units were present, but all the T cells were derived from the unmanipulated unit.
“When we look at those patients who had engraftment of HSC835, whether it be this dual chimerism or complete chimerism, you see there is a very rapid recovery of 10.5 days, as compared to the historical control [recovery time] of 25 days,” Dr Wagner said. “And those that engrafted with the unmanipulated unit fall right where you’d expect them; that is, with the historical controls [23 days].”
Dr Wagner noted that the CD34 dose correlated with the pace of neutrophil recovery in patients who engrafted with the HSC835 unit, and patients with dual chimerism had the fastest neutrophil recovery.
Furthermore, the HSC835 unit predominated more than expected (P=0.05), winning out over the cord blood unit with a higher CD3 dose a disproportionate amount of time. The unit with a higher CD3 dose typically predominates two-thirds of the time in double UCBT.
“[These results] gave us enough information to say that [HSC835] could be a stand-alone product,” Dr Wagner said.
Phase 2
For phase 2, the researchers used a single HSC835 unit. They chose the lesser of 2 cord blood units (keeping the better unit as a backup), expanded it, and infused the resulting HSC835 unit into 3 patients who received myeloablative conditioning and 3 patients who did not.
In patients who received myeloablative conditioning, chimerism was complete at days 8, 12, and 14. Among the patients who received non-myeloablative conditioning, 1 had mixed chimerism at day 6. The other 2 had complete chimerism at days 5 and 7.
“So in conclusion, we believe that HSC835 is safe and effective in speeding neutrophil recovery after cord blood transplant,” Dr Wagner said. “These are very promising early results that compel us now to explore the single expanded product.”
Dr Wagner added that other considerations for HSC835 are that it may reduce the unit selection threshold, improve HLA match, enable re-cryopreservation (for transplant delays, backup, or multiple dosing), and perhaps allow for reduced-intensity conditioning with “mega-dose” grafts. ![]()
*Information in the abstract differs from that presented at the meeting.

Photo courtesy of NHS
SAN DIEGO—A product that promotes CD34 expansion has solved the problem of poor engraftment associated with umbilical cord blood transplant (UCBT), according to a speaker at the 2015 BMT Tandem Meetings.
John Wagner, MD, of the University of Minnesota in Minneapolis, supported this statement with early results from trials of HSC835, cord
blood units in which CD34 cells were expanded using the aryl hydrocarbon
receptor antagonist StemRegenin 1.
Dr Wagner and his colleagues conducted phase 1 and 2 trials of HSC835, which were supported by Novartis, the company developing the product.
Dr Wagner presented results of these trials at the BMT Tandem Meetings as abstract 29.*
The phase 1 trial included 18 patients who received double UCBT after myeloablative conditioning. They received an unmanipulated cord blood unit and an HSC835 unit.
In the phase 2 trial, 3 patients received a single HSC835 unit after myeloablative conditioning, and 3 received an HSC835 unit after non-myeloablative conditioning.
Manufacturing HSC835 took 15 days. Following expansion, the median CD34 cell dose increased 346-fold, and the median total nucleated cell (TNC) dose increased 848-fold.
Twenty-eight HSC835 products were produced, but 4 of them were not infused. Two products were contaminated, 1 was not infused due to patient relapse, and 1 product failed to expand. Dr Wagner noted that the failed unit started out at 50% viability, whereas the other units started at about 90% viability.
Phase 1
Eighteen patients were treated in the phase 1 trial. Eleven had acute lymphoblastic leukemia, 5 had acute myeloid leukemia, and 2 had myelodysplastic syndromes. The median age was 28 (range, 12 to 53).
The median TNC dose was 2.5 x 107/kg for the unmanipulated cord blood unit and 5.1 x 107/kg for the HSC835 unit. The median CD34 count was 0.4 x 106/kg and 17.4 x 106/kg, respectively. And the median CD3 count was 8.5 x 106/kg and 2.5 x 106/kg, respectively.
Most units were a 5/6 HLA match—50% for the unmanipulated unit and 67% for the HSC835 unit. Forty-four percent and 22% of the units, respectively, were 4/6 matches. And 6% and 11%, respectively, were 6/6 matches.
Neutrophil recovery was 100%, and the median time to recovery was 14.5 days.
Dr Wagner compared this to results in 121 matched historical controls who received UCBT with 2 unmanipulated units. Of those patients, 86% achieved neutrophil engraftment, and the median time to engraftment was 25 days (P<0.001).
In the current study, 6 patients had complete chimerism with HSC835, 6 had complete chimerism with the unmanipulated unit, and 6 had dual chimerism. In the dual-chimerism patients, both units were present, but all the T cells were derived from the unmanipulated unit.
“When we look at those patients who had engraftment of HSC835, whether it be this dual chimerism or complete chimerism, you see there is a very rapid recovery of 10.5 days, as compared to the historical control [recovery time] of 25 days,” Dr Wagner said. “And those that engrafted with the unmanipulated unit fall right where you’d expect them; that is, with the historical controls [23 days].”
Dr Wagner noted that the CD34 dose correlated with the pace of neutrophil recovery in patients who engrafted with the HSC835 unit, and patients with dual chimerism had the fastest neutrophil recovery.
Furthermore, the HSC835 unit predominated more than expected (P=0.05), winning out over the cord blood unit with a higher CD3 dose a disproportionate amount of time. The unit with a higher CD3 dose typically predominates two-thirds of the time in double UCBT.
“[These results] gave us enough information to say that [HSC835] could be a stand-alone product,” Dr Wagner said.
Phase 2
For phase 2, the researchers used a single HSC835 unit. They chose the lesser of 2 cord blood units (keeping the better unit as a backup), expanded it, and infused the resulting HSC835 unit into 3 patients who received myeloablative conditioning and 3 patients who did not.
In patients who received myeloablative conditioning, chimerism was complete at days 8, 12, and 14. Among the patients who received non-myeloablative conditioning, 1 had mixed chimerism at day 6. The other 2 had complete chimerism at days 5 and 7.
“So in conclusion, we believe that HSC835 is safe and effective in speeding neutrophil recovery after cord blood transplant,” Dr Wagner said. “These are very promising early results that compel us now to explore the single expanded product.”
Dr Wagner added that other considerations for HSC835 are that it may reduce the unit selection threshold, improve HLA match, enable re-cryopreservation (for transplant delays, backup, or multiple dosing), and perhaps allow for reduced-intensity conditioning with “mega-dose” grafts. ![]()
*Information in the abstract differs from that presented at the meeting.
In-flight transfusions enable better outcomes in trauma patients

Photo by Elise Amendola
In-flight red blood cell (RBC) transfusions can improve outcomes in trauma patients, according to a study published in the Journal of the American College of Surgeons.
The research showed that air-lifted trauma victims who received blood transfusions in the helicopter had higher one-day survival rates and a lower risk of shock than air-lifted patients who did not receive transfusions until they arrived at the trauma unit.
Patients who received in-flight transfusions also required fewer RBCs once they arrived at the hospital.
Joshua Brown, MD, and his colleagues at the University of Pittsburgh Medical Center (UPMC) conducted this research, evaluating the air medical evacuation strategy at UPMC, which has a network of 18 helicopter bases in Pennsylvania, Ohio, and Maryland.
The STAT MedEvac helicopter teams have been carrying blood for transfusion on their flights for about 2 decades, but this is the first study that evaluated the use of transfused blood in civilian trauma victims air-evacuated directly from the injury scene and compared them with air-evacuated trauma victims who did not receive transfused blood.
It is also the largest study to date of a civilian in-flight trauma resuscitation protocol that has been used by the military in Iraq and Afghanistan.
The researchers evaluated 240 patients who received in-flight RBC transfusions and 480 patients who were not transfused until they reached the trauma center.
Receiving an in-flight transfusion was associated with better odds of 24-hour survival (adjusted odds ratio=4.92, P=0.01), decreased odds of shock (adjusted odds ratio=0.28, P=0.03), and lower 24-hour RBC requirement (coef -3.6 RBC units, P=0.04).
Based on these data, the UPMC may modify its protocol, Dr Brown said.
“It used to be the paramedics had to give the patient 2 liters of saline before giving them blood, and we dropped that down to only 1 liter of saline,” he noted. “Now, based on this study, we’re actually looking at giving patients blood without any saline who meet the criteria of low blood pressure and elevated heart rate and are clearly in shock.”
The UPMC protocol involves giving guidelines on when to administer transfusions to the paramedics and nurses onboard flights. All the STAT MedEvac flights at the institution carry 2 units of RBCs for transfusion.
Helicopter staff can communicate with the medical command doctor at the trauma center to get the go-ahead order to give blood to patients who may not meet the guidelines for transfusion but still may benefit from receiving it.
However, there are regulatory issues that may prevent such a protocol from being adopted universally, Dr Brown noted. In Pennsylvania, paramedics who have had additional training are allowed to start a blood transfusion without a physician present, but not all states allow this.
Dr Brown also explained the logistics and challenges of storing blood away from the blood bank.
“The blood needs to be refrigerated, the helicopter base must have a freezer, and the helicopters must have coolers when they’re actually out on a mission to keep the blood at an appropriate temperature,” he said.
Meeting these requirements involves close coordination with the blood bank and having a way to return unused blood after it expires in 30 days. The University of Pittsburgh has registered all of its helicopter bases as satellite blood banks to comply with the regulations. ![]()

Photo by Elise Amendola
In-flight red blood cell (RBC) transfusions can improve outcomes in trauma patients, according to a study published in the Journal of the American College of Surgeons.
The research showed that air-lifted trauma victims who received blood transfusions in the helicopter had higher one-day survival rates and a lower risk of shock than air-lifted patients who did not receive transfusions until they arrived at the trauma unit.
Patients who received in-flight transfusions also required fewer RBCs once they arrived at the hospital.
Joshua Brown, MD, and his colleagues at the University of Pittsburgh Medical Center (UPMC) conducted this research, evaluating the air medical evacuation strategy at UPMC, which has a network of 18 helicopter bases in Pennsylvania, Ohio, and Maryland.
The STAT MedEvac helicopter teams have been carrying blood for transfusion on their flights for about 2 decades, but this is the first study that evaluated the use of transfused blood in civilian trauma victims air-evacuated directly from the injury scene and compared them with air-evacuated trauma victims who did not receive transfused blood.
It is also the largest study to date of a civilian in-flight trauma resuscitation protocol that has been used by the military in Iraq and Afghanistan.
The researchers evaluated 240 patients who received in-flight RBC transfusions and 480 patients who were not transfused until they reached the trauma center.
Receiving an in-flight transfusion was associated with better odds of 24-hour survival (adjusted odds ratio=4.92, P=0.01), decreased odds of shock (adjusted odds ratio=0.28, P=0.03), and lower 24-hour RBC requirement (coef -3.6 RBC units, P=0.04).
Based on these data, the UPMC may modify its protocol, Dr Brown said.
“It used to be the paramedics had to give the patient 2 liters of saline before giving them blood, and we dropped that down to only 1 liter of saline,” he noted. “Now, based on this study, we’re actually looking at giving patients blood without any saline who meet the criteria of low blood pressure and elevated heart rate and are clearly in shock.”
The UPMC protocol involves giving guidelines on when to administer transfusions to the paramedics and nurses onboard flights. All the STAT MedEvac flights at the institution carry 2 units of RBCs for transfusion.
Helicopter staff can communicate with the medical command doctor at the trauma center to get the go-ahead order to give blood to patients who may not meet the guidelines for transfusion but still may benefit from receiving it.
However, there are regulatory issues that may prevent such a protocol from being adopted universally, Dr Brown noted. In Pennsylvania, paramedics who have had additional training are allowed to start a blood transfusion without a physician present, but not all states allow this.
Dr Brown also explained the logistics and challenges of storing blood away from the blood bank.
“The blood needs to be refrigerated, the helicopter base must have a freezer, and the helicopters must have coolers when they’re actually out on a mission to keep the blood at an appropriate temperature,” he said.
Meeting these requirements involves close coordination with the blood bank and having a way to return unused blood after it expires in 30 days. The University of Pittsburgh has registered all of its helicopter bases as satellite blood banks to comply with the regulations. ![]()

Photo by Elise Amendola
In-flight red blood cell (RBC) transfusions can improve outcomes in trauma patients, according to a study published in the Journal of the American College of Surgeons.
The research showed that air-lifted trauma victims who received blood transfusions in the helicopter had higher one-day survival rates and a lower risk of shock than air-lifted patients who did not receive transfusions until they arrived at the trauma unit.
Patients who received in-flight transfusions also required fewer RBCs once they arrived at the hospital.
Joshua Brown, MD, and his colleagues at the University of Pittsburgh Medical Center (UPMC) conducted this research, evaluating the air medical evacuation strategy at UPMC, which has a network of 18 helicopter bases in Pennsylvania, Ohio, and Maryland.
The STAT MedEvac helicopter teams have been carrying blood for transfusion on their flights for about 2 decades, but this is the first study that evaluated the use of transfused blood in civilian trauma victims air-evacuated directly from the injury scene and compared them with air-evacuated trauma victims who did not receive transfused blood.
It is also the largest study to date of a civilian in-flight trauma resuscitation protocol that has been used by the military in Iraq and Afghanistan.
The researchers evaluated 240 patients who received in-flight RBC transfusions and 480 patients who were not transfused until they reached the trauma center.
Receiving an in-flight transfusion was associated with better odds of 24-hour survival (adjusted odds ratio=4.92, P=0.01), decreased odds of shock (adjusted odds ratio=0.28, P=0.03), and lower 24-hour RBC requirement (coef -3.6 RBC units, P=0.04).
Based on these data, the UPMC may modify its protocol, Dr Brown said.
“It used to be the paramedics had to give the patient 2 liters of saline before giving them blood, and we dropped that down to only 1 liter of saline,” he noted. “Now, based on this study, we’re actually looking at giving patients blood without any saline who meet the criteria of low blood pressure and elevated heart rate and are clearly in shock.”
The UPMC protocol involves giving guidelines on when to administer transfusions to the paramedics and nurses onboard flights. All the STAT MedEvac flights at the institution carry 2 units of RBCs for transfusion.
Helicopter staff can communicate with the medical command doctor at the trauma center to get the go-ahead order to give blood to patients who may not meet the guidelines for transfusion but still may benefit from receiving it.
However, there are regulatory issues that may prevent such a protocol from being adopted universally, Dr Brown noted. In Pennsylvania, paramedics who have had additional training are allowed to start a blood transfusion without a physician present, but not all states allow this.
Dr Brown also explained the logistics and challenges of storing blood away from the blood bank.
“The blood needs to be refrigerated, the helicopter base must have a freezer, and the helicopters must have coolers when they’re actually out on a mission to keep the blood at an appropriate temperature,” he said.
Meeting these requirements involves close coordination with the blood bank and having a way to return unused blood after it expires in 30 days. The University of Pittsburgh has registered all of its helicopter bases as satellite blood banks to comply with the regulations. ![]()
Study supports iron supplementation after blood donation

Photo by Charles Haymond
Low-dose oral iron supplementation can reduce the time to hemoglobin recovery after blood donation, according to a study published in JAMA.
Researchers found that a daily dose of ferrous gluconate (37.5 mg of elemental iron) reduced blood donors’ time to 80% hemoglobin recovery, whether the donors were iron-depleted (with ferritin levels of 26 ng/mL or lower) or iron-replete (with ferritin levels higher than 26 ng/mL).
Joseph E. Kiss, MD, of the Institute for Transfusion Medicine in Pittsburgh, Pennsylvania, and his colleagues conducted this study at 4 regional blood centers in the US.
The researchers randomized 215 subjects (who had not donated whole blood or red blood cells within 4 months) to receive one tablet of ferrous gluconate daily or no iron for 24 weeks after donating a unit of whole blood (500 mL).
The study’s primary outcomes were time to recovery of 80% of the post-donation decrease in hemoglobin and recovery of baseline ferritin levels.
The researchers found that subjects who received iron supplementation achieved 80% hemoglobin recovery more quickly than subjects who did not receive iron, regardless of ferritin levels.
In the low-ferritin group, the mean time to 80% hemoglobin recovery was 32 days in subjects who received iron and 158 days in those who did not (P<0.001). In the higher-ferritin group, the mean time to 80% hemoglobin recovery was 31 days in subjects who received iron and 78 days in those who did not (P=0.02).
In the low-ferritin group, the median time to recovery of baseline ferritin levels was 21 days in subjects who received iron and more than 168 days in subjects who did not. In the higher-ferritin group, the median time to recovery of baseline ferritin levels was 107 days in subjects who received iron and more than 168 days in those who did not.
The median time to recovery of iron stores was 76 days in all subjects who received iron supplements and more than 168 days in those who did not (P<0.001). Sixty-seven percent of subjects who did not receive iron had failed to recover their iron stores by 168 days.
The researchers said these findings raise important considerations regarding the 8-week minimum waiting period between blood donations that is required in the US and Canada. This is a shorter period than those allowed in many other countries.
The study suggests that hemoglobin recovery differs according to a donor’s pre-donation ferritin level, but, even in iron-replete donors, the mean recovery was only 70% at 8 weeks.
Prolonging the minimum wait time between blood donations would allow for more thorough recovery, the researchers noted, but it could also compromise the blood supply. Furthermore, increasing the waiting period might not be adequate for donors who do not take iron supplements. ![]()

Photo by Charles Haymond
Low-dose oral iron supplementation can reduce the time to hemoglobin recovery after blood donation, according to a study published in JAMA.
Researchers found that a daily dose of ferrous gluconate (37.5 mg of elemental iron) reduced blood donors’ time to 80% hemoglobin recovery, whether the donors were iron-depleted (with ferritin levels of 26 ng/mL or lower) or iron-replete (with ferritin levels higher than 26 ng/mL).
Joseph E. Kiss, MD, of the Institute for Transfusion Medicine in Pittsburgh, Pennsylvania, and his colleagues conducted this study at 4 regional blood centers in the US.
The researchers randomized 215 subjects (who had not donated whole blood or red blood cells within 4 months) to receive one tablet of ferrous gluconate daily or no iron for 24 weeks after donating a unit of whole blood (500 mL).
The study’s primary outcomes were time to recovery of 80% of the post-donation decrease in hemoglobin and recovery of baseline ferritin levels.
The researchers found that subjects who received iron supplementation achieved 80% hemoglobin recovery more quickly than subjects who did not receive iron, regardless of ferritin levels.
In the low-ferritin group, the mean time to 80% hemoglobin recovery was 32 days in subjects who received iron and 158 days in those who did not (P<0.001). In the higher-ferritin group, the mean time to 80% hemoglobin recovery was 31 days in subjects who received iron and 78 days in those who did not (P=0.02).
In the low-ferritin group, the median time to recovery of baseline ferritin levels was 21 days in subjects who received iron and more than 168 days in subjects who did not. In the higher-ferritin group, the median time to recovery of baseline ferritin levels was 107 days in subjects who received iron and more than 168 days in those who did not.
The median time to recovery of iron stores was 76 days in all subjects who received iron supplements and more than 168 days in those who did not (P<0.001). Sixty-seven percent of subjects who did not receive iron had failed to recover their iron stores by 168 days.
The researchers said these findings raise important considerations regarding the 8-week minimum waiting period between blood donations that is required in the US and Canada. This is a shorter period than those allowed in many other countries.
The study suggests that hemoglobin recovery differs according to a donor’s pre-donation ferritin level, but, even in iron-replete donors, the mean recovery was only 70% at 8 weeks.
Prolonging the minimum wait time between blood donations would allow for more thorough recovery, the researchers noted, but it could also compromise the blood supply. Furthermore, increasing the waiting period might not be adequate for donors who do not take iron supplements. ![]()

Photo by Charles Haymond
Low-dose oral iron supplementation can reduce the time to hemoglobin recovery after blood donation, according to a study published in JAMA.
Researchers found that a daily dose of ferrous gluconate (37.5 mg of elemental iron) reduced blood donors’ time to 80% hemoglobin recovery, whether the donors were iron-depleted (with ferritin levels of 26 ng/mL or lower) or iron-replete (with ferritin levels higher than 26 ng/mL).
Joseph E. Kiss, MD, of the Institute for Transfusion Medicine in Pittsburgh, Pennsylvania, and his colleagues conducted this study at 4 regional blood centers in the US.
The researchers randomized 215 subjects (who had not donated whole blood or red blood cells within 4 months) to receive one tablet of ferrous gluconate daily or no iron for 24 weeks after donating a unit of whole blood (500 mL).
The study’s primary outcomes were time to recovery of 80% of the post-donation decrease in hemoglobin and recovery of baseline ferritin levels.
The researchers found that subjects who received iron supplementation achieved 80% hemoglobin recovery more quickly than subjects who did not receive iron, regardless of ferritin levels.
In the low-ferritin group, the mean time to 80% hemoglobin recovery was 32 days in subjects who received iron and 158 days in those who did not (P<0.001). In the higher-ferritin group, the mean time to 80% hemoglobin recovery was 31 days in subjects who received iron and 78 days in those who did not (P=0.02).
In the low-ferritin group, the median time to recovery of baseline ferritin levels was 21 days in subjects who received iron and more than 168 days in subjects who did not. In the higher-ferritin group, the median time to recovery of baseline ferritin levels was 107 days in subjects who received iron and more than 168 days in those who did not.
The median time to recovery of iron stores was 76 days in all subjects who received iron supplements and more than 168 days in those who did not (P<0.001). Sixty-seven percent of subjects who did not receive iron had failed to recover their iron stores by 168 days.
The researchers said these findings raise important considerations regarding the 8-week minimum waiting period between blood donations that is required in the US and Canada. This is a shorter period than those allowed in many other countries.
The study suggests that hemoglobin recovery differs according to a donor’s pre-donation ferritin level, but, even in iron-replete donors, the mean recovery was only 70% at 8 weeks.
Prolonging the minimum wait time between blood donations would allow for more thorough recovery, the researchers noted, but it could also compromise the blood supply. Furthermore, increasing the waiting period might not be adequate for donors who do not take iron supplements.
Balanced transfusion strategy may be better

Credit: UAB Hospital
A new study indicates that transfusing a balanced ratio of plasma, platelets, and red blood cells (RBCs) can decrease bleeding better than blood products with a higher ratio of RBCs, but this does not seem to affect mortality rates.
Patients who received blood products with a plasma-platelet-RBC ratio of 1:1:1 were more likely to achieve hemostasis and less likely to experience exsanguination than patients who received blood products with a ratio of 1:1:2.
However, there was no significant difference between the two transfusion strategies in overall death rates at 24 hours or at 30 days.
John B. Holcomb, MD, of the University of Texas Health Science Center at Houston, and his colleagues reported these results in JAMA.
The team conducted a study of 680 severely injured patients who arrived at 1 of 12 Level 1 trauma centers. The patients were predicted to require massive transfusion and were randomly assigned to receive blood products with ratios of 1:1:1 or 1:1:2 during active resuscitation, in addition to all local standard-of-care interventions.
The researchers found no significant differences for the primary outcomes of the study: mortality at 24 hours—12.7% in the 1:1:1 group and 17.0% in the 1:1:2 group (P=0.12)—or at 30 days—22.4% and 26.1%, respectively (P=0.26).
However, the incidence of exsanguination, which was the predominant cause of death within the first 24 hours, was significantly lower in the 1:1:1 group than in the 1:1:2 group—9.2% and 14.6%, respectively (P=0.03).
And more patients in the 1:1:1 group achieved hemostasis than in the 1:1:2 group—86% and 78%, respectively (P=0.006).
On the other hand, there were no significant differences between the two groups for rates of multiple inflammatory-mediated complications, such as acute respiratory distress syndrome, multiple organ failure, infection, sepsis, venous thromboembolism, and transfusion-related complications.
Based on these results, Dr Holcomb and his colleagues recommended that clinicians consider using a 1:1:1 transfusion protocol, starting with the initial units transfused while patients are actively bleeding, and then transitioning to laboratory-guided treatment once they’ve achieved hemorrhage control.
The team added that future studies should concentrate on the physiologically relevant period of active bleeding after injury and use acute complications and later deaths as safety endpoints.

Credit: UAB Hospital
A new study indicates that transfusing a balanced ratio of plasma, platelets, and red blood cells (RBCs) can decrease bleeding better than blood products with a higher ratio of RBCs, but this does not seem to affect mortality rates.
Patients who received blood products with a plasma-platelet-RBC ratio of 1:1:1 were more likely to achieve hemostasis and less likely to experience exsanguination than patients who received blood products with a ratio of 1:1:2.
However, there was no significant difference between the two transfusion strategies in overall death rates at 24 hours or at 30 days.
John B. Holcomb, MD, of the University of Texas Health Science Center at Houston, and his colleagues reported these results in JAMA.
The team conducted a study of 680 severely injured patients who arrived at 1 of 12 Level 1 trauma centers. The patients were predicted to require massive transfusion and were randomly assigned to receive blood products with ratios of 1:1:1 or 1:1:2 during active resuscitation, in addition to all local standard-of-care interventions.
The researchers found no significant differences for the primary outcomes of the study: mortality at 24 hours—12.7% in the 1:1:1 group and 17.0% in the 1:1:2 group (P=0.12)—or at 30 days—22.4% and 26.1%, respectively (P=0.26).
However, the incidence of exsanguination, which was the predominant cause of death within the first 24 hours, was significantly lower in the 1:1:1 group than in the 1:1:2 group—9.2% and 14.6%, respectively (P=0.03).
And more patients in the 1:1:1 group achieved hemostasis than in the 1:1:2 group—86% and 78%, respectively (P=0.006).
On the other hand, there were no significant differences between the two groups for rates of multiple inflammatory-mediated complications, such as acute respiratory distress syndrome, multiple organ failure, infection, sepsis, venous thromboembolism, and transfusion-related complications.
Based on these results, Dr Holcomb and his colleagues recommended that clinicians consider using a 1:1:1 transfusion protocol, starting with the initial units transfused while patients are actively bleeding, and then transitioning to laboratory-guided treatment once they’ve achieved hemorrhage control.
The team added that future studies should concentrate on the physiologically relevant period of active bleeding after injury and use acute complications and later deaths as safety endpoints.

Credit: UAB Hospital
A new study indicates that transfusing a balanced ratio of plasma, platelets, and red blood cells (RBCs) can decrease bleeding better than blood products with a higher ratio of RBCs, but this does not seem to affect mortality rates.
Patients who received blood products with a plasma-platelet-RBC ratio of 1:1:1 were more likely to achieve hemostasis and less likely to experience exsanguination than patients who received blood products with a ratio of 1:1:2.
However, there was no significant difference between the two transfusion strategies in overall death rates at 24 hours or at 30 days.
John B. Holcomb, MD, of the University of Texas Health Science Center at Houston, and his colleagues reported these results in JAMA.
The team conducted a study of 680 severely injured patients who arrived at 1 of 12 Level 1 trauma centers. The patients were predicted to require massive transfusion and were randomly assigned to receive blood products with ratios of 1:1:1 or 1:1:2 during active resuscitation, in addition to all local standard-of-care interventions.
The researchers found no significant differences for the primary outcomes of the study: mortality at 24 hours—12.7% in the 1:1:1 group and 17.0% in the 1:1:2 group (P=0.12)—or at 30 days—22.4% and 26.1%, respectively (P=0.26).
However, the incidence of exsanguination, which was the predominant cause of death within the first 24 hours, was significantly lower in the 1:1:1 group than in the 1:1:2 group—9.2% and 14.6%, respectively (P=0.03).
And more patients in the 1:1:1 group achieved hemostasis than in the 1:1:2 group—86% and 78%, respectively (P=0.006).
On the other hand, there were no significant differences between the two groups for rates of multiple inflammatory-mediated complications, such as acute respiratory distress syndrome, multiple organ failure, infection, sepsis, venous thromboembolism, and transfusion-related complications.
Based on these results, Dr Holcomb and his colleagues recommended that clinicians consider using a 1:1:1 transfusion protocol, starting with the initial units transfused while patients are actively bleeding, and then transitioning to laboratory-guided treatment once they’ve achieved hemorrhage control.
The team added that future studies should concentrate on the physiologically relevant period of active bleeding after injury and use acute complications and later deaths as safety endpoints.
Platelet transfusions ineffective for TBI

Platelet transfusions may not be appropriate treatment for patients with traumatic brain injury (TBI), new research suggests.
More than 1.7 million people in the US suffer a TBI every year, and as many as half of them experience progression of bleeding inside or around the brain, which is associated with an increased risk of death.
Platelet transfusions and desmopressin (DDAVP) are commonly used to prevent bleeding—and therefore death—in these patients.
But a new study published in the Journal of Neurotrauma suggests neither treatment is effective.
“Previous studies of platelet transfusion have looked only at mortality, and few studies have addressed the effect of DDAVP on bleeding in patients with TBI,” said study author Dennis Yong Kim, MD, of LA BioMed in Los Angeles, California.
“Our study found that the administration of platelets and DDAVP is no more effective in preventing progression of hemorrhage or death than was the use of none of these medications, irrespective of whether or not patients were on antiplatelet medications, such as aspirin, prior to their TBI.”
“Given the limited availability and potential for complications associated with transfusion of blood products like platelets, we believe that physicians should take a step back and re-think the necessity and efficacy of such treatments in patients with TBI.”
Dr Kim and his colleagues conducted a 3-year retrospective study of patients admitted to a Level 1 trauma center with TBI between January 1, 2010, and December 31, 2012. Of 408 patients, 126 received platelet transfusions and DDAVP, and 282 did not.
Overall, 37% of the patients demonstrated progression of traumatic intracranial hemorrhage within 4 hours of admission.
The researchers compared outcomes for the patients who received platelet transfusions and DDAVP to patients who did not receive the therapies.
A univariate analysis showed no difference in the incidence of hemorrhage progression, which occurred in 43.7% of patients who received transfusions and DDAVP and 34.2% of patients who received neither treatment (P=0.07).
And multivariate analyses showed that platelet transfusions and DDAVP were not associated with a decreased risk of hemorrhage progression (odds ratio=1.40, P=0.2) or mortality (odds ratio=1.50, P=0.4).

Platelet transfusions may not be appropriate treatment for patients with traumatic brain injury (TBI), new research suggests.
More than 1.7 million people in the US suffer a TBI every year, and as many as half of them experience progression of bleeding inside or around the brain, which is associated with an increased risk of death.
Platelet transfusions and desmopressin (DDAVP) are commonly used to prevent bleeding—and therefore death—in these patients.
But a new study published in the Journal of Neurotrauma suggests neither treatment is effective.
“Previous studies of platelet transfusion have looked only at mortality, and few studies have addressed the effect of DDAVP on bleeding in patients with TBI,” said study author Dennis Yong Kim, MD, of LA BioMed in Los Angeles, California.
“Our study found that the administration of platelets and DDAVP is no more effective in preventing progression of hemorrhage or death than was the use of none of these medications, irrespective of whether or not patients were on antiplatelet medications, such as aspirin, prior to their TBI.”
“Given the limited availability and potential for complications associated with transfusion of blood products like platelets, we believe that physicians should take a step back and re-think the necessity and efficacy of such treatments in patients with TBI.”
Dr Kim and his colleagues conducted a 3-year retrospective study of patients admitted to a Level 1 trauma center with TBI between January 1, 2010, and December 31, 2012. Of 408 patients, 126 received platelet transfusions and DDAVP, and 282 did not.
Overall, 37% of the patients demonstrated progression of traumatic intracranial hemorrhage within 4 hours of admission.
The researchers compared outcomes for the patients who received platelet transfusions and DDAVP to patients who did not receive the therapies.
A univariate analysis showed no difference in the incidence of hemorrhage progression, which occurred in 43.7% of patients who received transfusions and DDAVP and 34.2% of patients who received neither treatment (P=0.07).
And multivariate analyses showed that platelet transfusions and DDAVP were not associated with a decreased risk of hemorrhage progression (odds ratio=1.40, P=0.2) or mortality (odds ratio=1.50, P=0.4).

Platelet transfusions may not be appropriate treatment for patients with traumatic brain injury (TBI), new research suggests.
More than 1.7 million people in the US suffer a TBI every year, and as many as half of them experience progression of bleeding inside or around the brain, which is associated with an increased risk of death.
Platelet transfusions and desmopressin (DDAVP) are commonly used to prevent bleeding—and therefore death—in these patients.
But a new study published in the Journal of Neurotrauma suggests neither treatment is effective.
“Previous studies of platelet transfusion have looked only at mortality, and few studies have addressed the effect of DDAVP on bleeding in patients with TBI,” said study author Dennis Yong Kim, MD, of LA BioMed in Los Angeles, California.
“Our study found that the administration of platelets and DDAVP is no more effective in preventing progression of hemorrhage or death than was the use of none of these medications, irrespective of whether or not patients were on antiplatelet medications, such as aspirin, prior to their TBI.”
“Given the limited availability and potential for complications associated with transfusion of blood products like platelets, we believe that physicians should take a step back and re-think the necessity and efficacy of such treatments in patients with TBI.”
Dr Kim and his colleagues conducted a 3-year retrospective study of patients admitted to a Level 1 trauma center with TBI between January 1, 2010, and December 31, 2012. Of 408 patients, 126 received platelet transfusions and DDAVP, and 282 did not.
Overall, 37% of the patients demonstrated progression of traumatic intracranial hemorrhage within 4 hours of admission.
The researchers compared outcomes for the patients who received platelet transfusions and DDAVP to patients who did not receive the therapies.
A univariate analysis showed no difference in the incidence of hemorrhage progression, which occurred in 43.7% of patients who received transfusions and DDAVP and 34.2% of patients who received neither treatment (P=0.07).
And multivariate analyses showed that platelet transfusions and DDAVP were not associated with a decreased risk of hemorrhage progression (odds ratio=1.40, P=0.2) or mortality (odds ratio=1.50, P=0.4).
RBC transfusions during CABG increase risk of pneumonia

Credit: Elise Amendola
SAN DIEGO—Patients who receive red blood cell (RBC) transfusions during coronary artery bypass grafting (CABG) surgery are at an increased risk of
developing pneumonia, according to research presented at the 51st Annual Meeting of The Society of Thoracic Surgeons.
And the risk appears to increase with the volume of RBCs transfused. Patients who received 6 or more units had a 14 times higher risk of developing pneumonia than their untransfused peers.
“Pneumonia is a known risk following CABG surgery, and developing it has been shown to significantly increase a patient’s risk of morbidity and mortality,” said study investigator Donald S. Likosky, PhD, of the University of Michigan Health System in Ann Arbor.
“Previous research has shown that 1 in every 20 CABG patients develop a major infection, with pneumonia being the most common type of infection.”
For this study, Dr Liksoky and his colleagues examined data on 16,182 patients who underwent CABG between 2011 and 2013 at any of the 33 hospitals participating in the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative.
They used propensity scoring to match the 4585 patients (32.3%) who received RBCs to the 9612 who did not (total=14,197). The team matched patients based on age, sex, body mass index, history of smoking, congestive heart failure, chronic obstructive pulmonary disease, diabetes, prior cardiac surgery, vascular disease, ejection fraction, preoperative hematocrit, and preoperative pneumonia.
The researchers then calculated adjusted odds ratios (ORs) reflecting the association between the number of RBC units transfused (0 to 6+) and postoperative pneumonia.
In all, 450 patients (3.2%) developed pneumonia. And the analysis revealed a significant association between any RBC transfusion and pneumonia (OR=4.0, P<0.001), as well as associations between the number of units transfused and the odds of developing pneumonia.
The OR was 1.6 (P=0.02) for patients who received 1 RBC unit, 2.1 for those who received 2 units (P<0.001), 4.9 for those who received 3 units (P<0.001), 5.5 for those who received 4 units (P<0.001), 8.9 for those who received 5 units (P<0.001), and 14.4 for patients who received 6 or more units (P<0.001).
“The ability to store and transfuse blood is one of medicine’s greatest accomplishments, but we are continuing to see that receiving a blood transfusion may alter a patient’s ability to fight infection,” said James R. Edgerton, MD, from The Heart Hospital Baylor Plano in Texas, who was not affiliated with this study.
“In their study, Dr Likosky and colleagues have identified an increased risk of pneumonia after transfusion, which is an important breakthrough because it allows physicians to remain vigilant for the onset of pneumonia and initiate therapy early in hopes of shortening its course and severity. It also enables physicians to initiate preventive therapies in patients who have been transfused, which will contribute to better care of our patients.”
“Patients should receive red blood cell transfusions based on clinical need,” Dr Likosky added. “Surgical teams may have opportunities to reduce the need for transfusions among patients, thereby reducing the risk of secondary complications.”

Credit: Elise Amendola
SAN DIEGO—Patients who receive red blood cell (RBC) transfusions during coronary artery bypass grafting (CABG) surgery are at an increased risk of
developing pneumonia, according to research presented at the 51st Annual Meeting of The Society of Thoracic Surgeons.
And the risk appears to increase with the volume of RBCs transfused. Patients who received 6 or more units had a 14 times higher risk of developing pneumonia than their untransfused peers.
“Pneumonia is a known risk following CABG surgery, and developing it has been shown to significantly increase a patient’s risk of morbidity and mortality,” said study investigator Donald S. Likosky, PhD, of the University of Michigan Health System in Ann Arbor.
“Previous research has shown that 1 in every 20 CABG patients develop a major infection, with pneumonia being the most common type of infection.”
For this study, Dr Liksoky and his colleagues examined data on 16,182 patients who underwent CABG between 2011 and 2013 at any of the 33 hospitals participating in the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative.
They used propensity scoring to match the 4585 patients (32.3%) who received RBCs to the 9612 who did not (total=14,197). The team matched patients based on age, sex, body mass index, history of smoking, congestive heart failure, chronic obstructive pulmonary disease, diabetes, prior cardiac surgery, vascular disease, ejection fraction, preoperative hematocrit, and preoperative pneumonia.
The researchers then calculated adjusted odds ratios (ORs) reflecting the association between the number of RBC units transfused (0 to 6+) and postoperative pneumonia.
In all, 450 patients (3.2%) developed pneumonia. And the analysis revealed a significant association between any RBC transfusion and pneumonia (OR=4.0, P<0.001), as well as associations between the number of units transfused and the odds of developing pneumonia.
The OR was 1.6 (P=0.02) for patients who received 1 RBC unit, 2.1 for those who received 2 units (P<0.001), 4.9 for those who received 3 units (P<0.001), 5.5 for those who received 4 units (P<0.001), 8.9 for those who received 5 units (P<0.001), and 14.4 for patients who received 6 or more units (P<0.001).
“The ability to store and transfuse blood is one of medicine’s greatest accomplishments, but we are continuing to see that receiving a blood transfusion may alter a patient’s ability to fight infection,” said James R. Edgerton, MD, from The Heart Hospital Baylor Plano in Texas, who was not affiliated with this study.
“In their study, Dr Likosky and colleagues have identified an increased risk of pneumonia after transfusion, which is an important breakthrough because it allows physicians to remain vigilant for the onset of pneumonia and initiate therapy early in hopes of shortening its course and severity. It also enables physicians to initiate preventive therapies in patients who have been transfused, which will contribute to better care of our patients.”
“Patients should receive red blood cell transfusions based on clinical need,” Dr Likosky added. “Surgical teams may have opportunities to reduce the need for transfusions among patients, thereby reducing the risk of secondary complications.”

Credit: Elise Amendola
SAN DIEGO—Patients who receive red blood cell (RBC) transfusions during coronary artery bypass grafting (CABG) surgery are at an increased risk of
developing pneumonia, according to research presented at the 51st Annual Meeting of The Society of Thoracic Surgeons.
And the risk appears to increase with the volume of RBCs transfused. Patients who received 6 or more units had a 14 times higher risk of developing pneumonia than their untransfused peers.
“Pneumonia is a known risk following CABG surgery, and developing it has been shown to significantly increase a patient’s risk of morbidity and mortality,” said study investigator Donald S. Likosky, PhD, of the University of Michigan Health System in Ann Arbor.
“Previous research has shown that 1 in every 20 CABG patients develop a major infection, with pneumonia being the most common type of infection.”
For this study, Dr Liksoky and his colleagues examined data on 16,182 patients who underwent CABG between 2011 and 2013 at any of the 33 hospitals participating in the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative.
They used propensity scoring to match the 4585 patients (32.3%) who received RBCs to the 9612 who did not (total=14,197). The team matched patients based on age, sex, body mass index, history of smoking, congestive heart failure, chronic obstructive pulmonary disease, diabetes, prior cardiac surgery, vascular disease, ejection fraction, preoperative hematocrit, and preoperative pneumonia.
The researchers then calculated adjusted odds ratios (ORs) reflecting the association between the number of RBC units transfused (0 to 6+) and postoperative pneumonia.
In all, 450 patients (3.2%) developed pneumonia. And the analysis revealed a significant association between any RBC transfusion and pneumonia (OR=4.0, P<0.001), as well as associations between the number of units transfused and the odds of developing pneumonia.
The OR was 1.6 (P=0.02) for patients who received 1 RBC unit, 2.1 for those who received 2 units (P<0.001), 4.9 for those who received 3 units (P<0.001), 5.5 for those who received 4 units (P<0.001), 8.9 for those who received 5 units (P<0.001), and 14.4 for patients who received 6 or more units (P<0.001).
“The ability to store and transfuse blood is one of medicine’s greatest accomplishments, but we are continuing to see that receiving a blood transfusion may alter a patient’s ability to fight infection,” said James R. Edgerton, MD, from The Heart Hospital Baylor Plano in Texas, who was not affiliated with this study.
“In their study, Dr Likosky and colleagues have identified an increased risk of pneumonia after transfusion, which is an important breakthrough because it allows physicians to remain vigilant for the onset of pneumonia and initiate therapy early in hopes of shortening its course and severity. It also enables physicians to initiate preventive therapies in patients who have been transfused, which will contribute to better care of our patients.”
“Patients should receive red blood cell transfusions based on clinical need,” Dr Likosky added. “Surgical teams may have opportunities to reduce the need for transfusions among patients, thereby reducing the risk of secondary complications.”