User login
Diffusely Scattered Macules Following Radiation Therapy
The Diagnosis: Cutaneous Mastocytosis
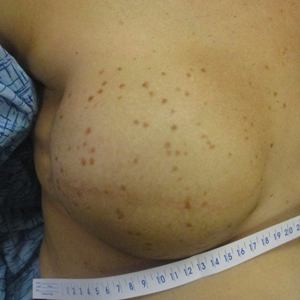
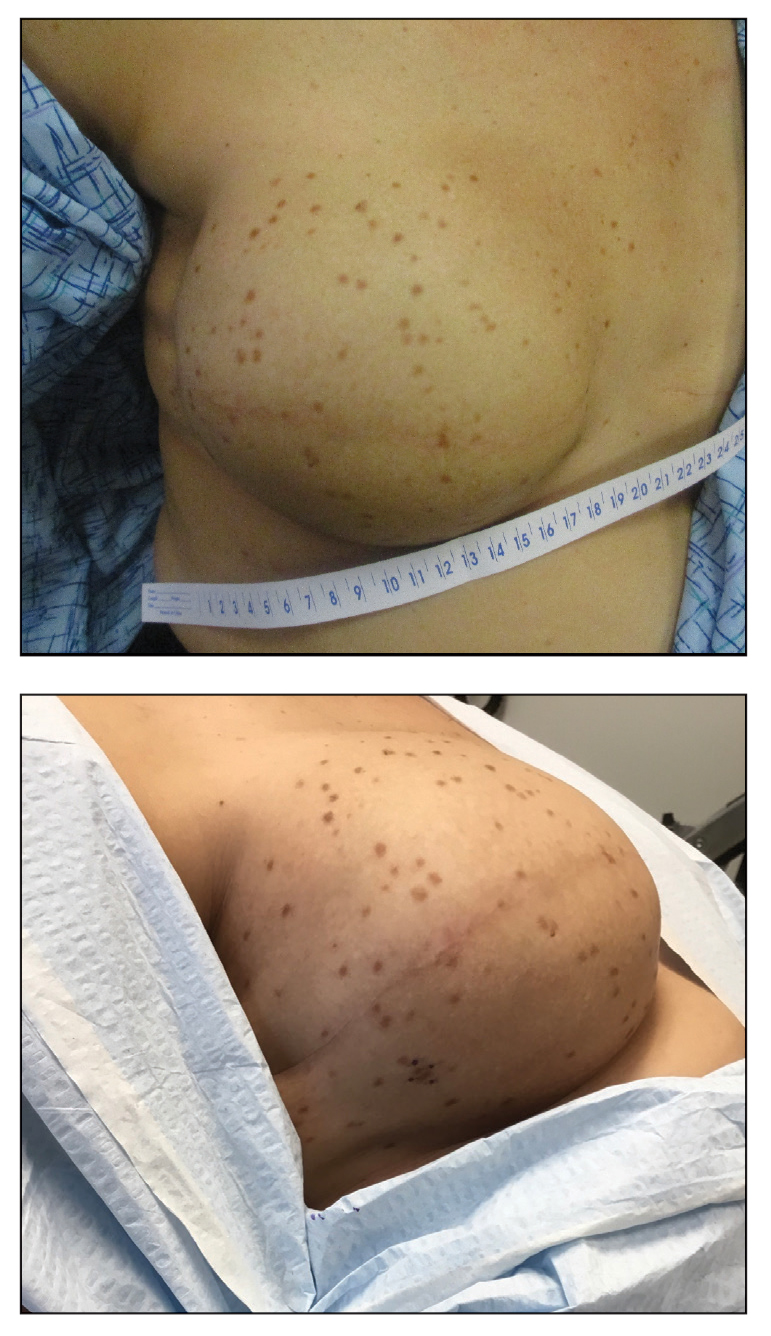
A shave skin biopsy from the right lateral breast and a punch skin biopsy from the right thigh showed similar histopathology. There were dermal predominantly perivascular aggregates of cells demonstrating basophilic granular cytoplasm and round to oval nuclei (Figure, A and B). These cells were highlighted by CD117 immunohistochemical stain (Figure, C), consistent with mastocytes. Additionally, occasional lymphocytes and rare eosinophils were noted. These histopathologic findings confirmed the diagnosis of cutaneous mastocytosis (CM). The patient’s complete blood cell count was within reference range, but serum tryptase was elevated at 15.7 μg/L (reference range, <11.0 μg/L), which prompted a bone marrow biopsy to rule out systemic mastocytosis (SM). The result showed normocellular bone marrow with no evidence of dysplasia or increased blasts, granuloma, lymphoproliferative disorder, or malignancy. Fluorescence in situ hybridization for PDGFRA (platelet-derived growth factor receptor alpha) and KIT mutation was negative. Because CM developed predominantly on the right breast where the patient previously had received radiation therapy, we concluded that this reaction was triggered by exposure to ionizing radiation.
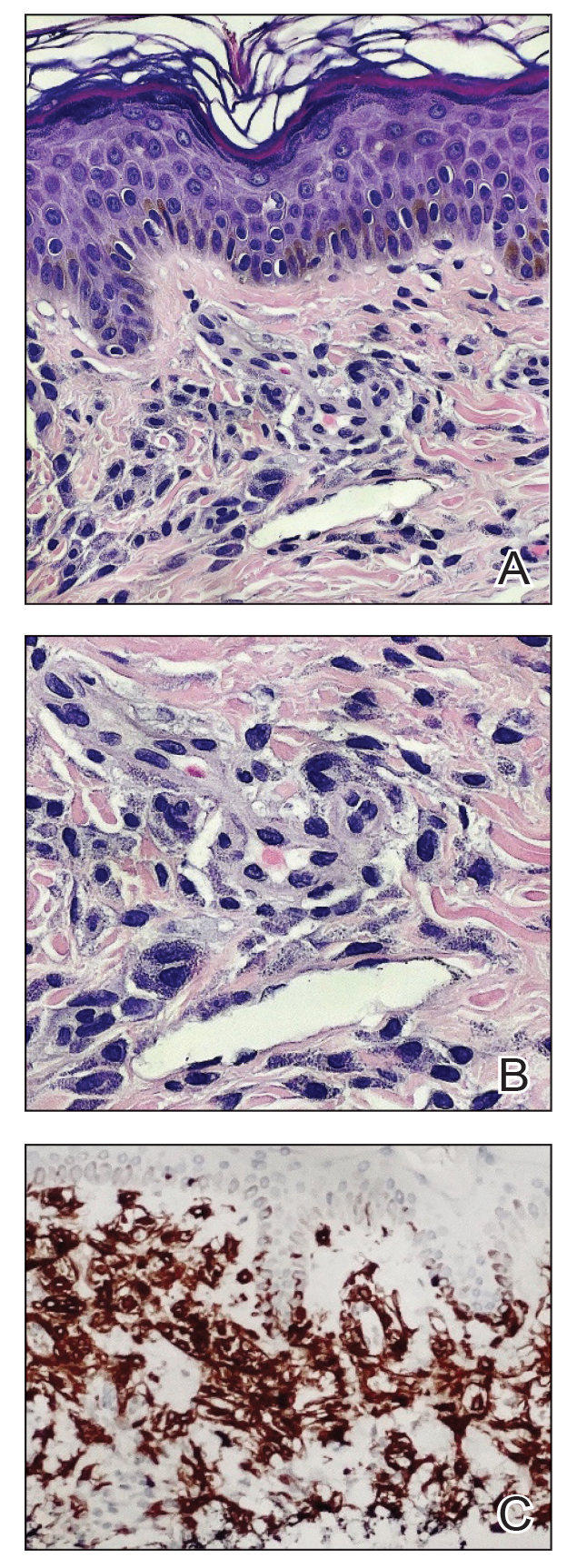
Mastocytosis can be divided into 2 groups: CM and SM.1 The histologic differential diagnosis of CM includes solitary mastocytoma, urticaria pigmentosa, telangiectasia macularis eruptiva perstans, and diffuse mastocytosis.2 Clinicopathologic correlation is of crucial importance to render the final diagnosis in these disorders. Immunohistochemically, mast cells express CD177, CD5, CD68, tryptase, and chymase. Unlike normal mast cells, neoplastic cells express CD2 and/or CD25; CD25 is commonly expressed in cutaneous involvement by SM.2
Macdonald and Feiwel3 reported the first case of CM following ionizing radiation. Cutaneous mastocytosis is most common in female patients and presents with redbrown macules originating at the site of radiation therapy. Prior literature suggests that radiation-associated CM has a predilection for White patients4; however, our patient was Hispanic. It also is important to note that the presentation of this rash may differ in individuals with skin of color. In one case it spread beyond the radiation site.2 Systemic mast call–mediated symptoms can occur in both CM and SM. The macules manifest as blanching with pressure.5 Typically these macules also are asymptomatic, though a positive Darier sign has been reported.6,7 The interval between radiotherapy and CM has ranged from 3 to 24 months.2
Patients with CM should have a serum tryptase evaluation along with a complete blood cell count, serum biochemistry, and liver function tests. Elevated serum tryptase has a high positive predictive value for SM and should prompt a bone marrow biopsy. Our patient’s bone marrow biopsy results failed to establish SM; however, her serum tryptase levels will be carefully monitored going forward. At the time of publication, the skin macules were still persistent but not worsening or symptomatic.
Treatment is focused on symptomatic relief of cutaneous symptoms, if present; avoiding triggers of mast cell degranulation; and implementing the use of oral antihistamines and leukotriene antagonists as needed. Because our patient was completely asymptomatic, we did not recommend any topical or oral treatment. However, we do counsel patients on avoiding triggers of mast cell degranulation including nonsteroidal anti-inflammatory drugs, morphine and codeine derivatives, alcohol, certain anesthetics, and anticholinergic medications.8
Additional diagnoses were ruled out for the following reasons: Although lichen planus pigmentosus presents with ill-defined, oval, gray-brown macules, histopathology shows a bandlike lymphocytic infiltrate at the dermoepidermal junction. Solar lentiginosis is characterized by grouped tan macules in a sun-exposed distribution. A fixed drug eruption is a delayed hypersensitivity reaction, usually to an ingested medication, characterized by violaceous or hyperpigmented patches, with histopathology showing interface dermatitis with a lymphoeosinophilic infiltrate. Eruptive seborrheic keratoses can result from sunburn or dermatitis but does not show mastocytes on histopathology.8
In conclusion, dermatologists should be reminded of the rare possibility of CM when evaluating an atypical eruption in a prior radiation field.
- Landy RE, Stross WC, May JM, et al. Idiopathic mast cell activation syndrome and radiation therapy: a case study, literature review, and discussion of mast cell disorders and radiotherapy [published online December 9, 2019]. Radiat Oncol. 2019;14:222. doi:10.1186 /s13014-019-1434-6
- Easwaralingam N, Wu Y, Cheung D, et al. Radiotherapy for breast cancer associated with a cutaneous presentation of systemic mastocytosis—a case report and literature review. J Surg Case Rep. 2018;2018:1-3. doi:10.1093/jscr/rjy317
- Macdonald A, Feiwel M. Cutaneous mastocytosis: an unusual radiation dermatitis. Proc R Soc Med. 1971;64:29-30.
- Kirshenbaum AS, Abuhay H, Bolan H, et al. Maculopapular cutaneous mastocytosis in a diverse population. J Allergy Clin Immunol Pract. 2019;7:2845-2847. doi:10.1016/j.jaip.2019.04.003
- Soilleux EJ, Brown VL, Bowling J. Cutaneous mastocytosis localized to a radiotherapy field. Clin Exp Dermatol. 2008;34:111-112. doi:10.1111 /j.1365-2230.2008.02931.x
- Comte C, Bessis D, Dereure O, et al. Urticaria pigmentosa localized on radiation field. Eur J Dermatol. 2003;13:408-409.
- Davidson SJ, Coates D. Cutaneous mastocytosis extending beyond a radiotherapy site: a form of radiodermatitis or a neoplastic phenomenon? Australas J Dermatol. 2012;54:E85-E87. doi:10.1111 /j.1440-0960.2012.00961.x
- Bolognia J, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2022.
The Diagnosis: Cutaneous Mastocytosis
A shave skin biopsy from the right lateral breast and a punch skin biopsy from the right thigh showed similar histopathology. There were dermal predominantly perivascular aggregates of cells demonstrating basophilic granular cytoplasm and round to oval nuclei (Figure, A and B). These cells were highlighted by CD117 immunohistochemical stain (Figure, C), consistent with mastocytes. Additionally, occasional lymphocytes and rare eosinophils were noted. These histopathologic findings confirmed the diagnosis of cutaneous mastocytosis (CM). The patient’s complete blood cell count was within reference range, but serum tryptase was elevated at 15.7 μg/L (reference range, <11.0 μg/L), which prompted a bone marrow biopsy to rule out systemic mastocytosis (SM). The result showed normocellular bone marrow with no evidence of dysplasia or increased blasts, granuloma, lymphoproliferative disorder, or malignancy. Fluorescence in situ hybridization for PDGFRA (platelet-derived growth factor receptor alpha) and KIT mutation was negative. Because CM developed predominantly on the right breast where the patient previously had received radiation therapy, we concluded that this reaction was triggered by exposure to ionizing radiation.

Mastocytosis can be divided into 2 groups: CM and SM.1 The histologic differential diagnosis of CM includes solitary mastocytoma, urticaria pigmentosa, telangiectasia macularis eruptiva perstans, and diffuse mastocytosis.2 Clinicopathologic correlation is of crucial importance to render the final diagnosis in these disorders. Immunohistochemically, mast cells express CD177, CD5, CD68, tryptase, and chymase. Unlike normal mast cells, neoplastic cells express CD2 and/or CD25; CD25 is commonly expressed in cutaneous involvement by SM.2
Macdonald and Feiwel3 reported the first case of CM following ionizing radiation. Cutaneous mastocytosis is most common in female patients and presents with redbrown macules originating at the site of radiation therapy. Prior literature suggests that radiation-associated CM has a predilection for White patients4; however, our patient was Hispanic. It also is important to note that the presentation of this rash may differ in individuals with skin of color. In one case it spread beyond the radiation site.2 Systemic mast call–mediated symptoms can occur in both CM and SM. The macules manifest as blanching with pressure.5 Typically these macules also are asymptomatic, though a positive Darier sign has been reported.6,7 The interval between radiotherapy and CM has ranged from 3 to 24 months.2
Patients with CM should have a serum tryptase evaluation along with a complete blood cell count, serum biochemistry, and liver function tests. Elevated serum tryptase has a high positive predictive value for SM and should prompt a bone marrow biopsy. Our patient’s bone marrow biopsy results failed to establish SM; however, her serum tryptase levels will be carefully monitored going forward. At the time of publication, the skin macules were still persistent but not worsening or symptomatic.
Treatment is focused on symptomatic relief of cutaneous symptoms, if present; avoiding triggers of mast cell degranulation; and implementing the use of oral antihistamines and leukotriene antagonists as needed. Because our patient was completely asymptomatic, we did not recommend any topical or oral treatment. However, we do counsel patients on avoiding triggers of mast cell degranulation including nonsteroidal anti-inflammatory drugs, morphine and codeine derivatives, alcohol, certain anesthetics, and anticholinergic medications.8
Additional diagnoses were ruled out for the following reasons: Although lichen planus pigmentosus presents with ill-defined, oval, gray-brown macules, histopathology shows a bandlike lymphocytic infiltrate at the dermoepidermal junction. Solar lentiginosis is characterized by grouped tan macules in a sun-exposed distribution. A fixed drug eruption is a delayed hypersensitivity reaction, usually to an ingested medication, characterized by violaceous or hyperpigmented patches, with histopathology showing interface dermatitis with a lymphoeosinophilic infiltrate. Eruptive seborrheic keratoses can result from sunburn or dermatitis but does not show mastocytes on histopathology.8
In conclusion, dermatologists should be reminded of the rare possibility of CM when evaluating an atypical eruption in a prior radiation field.
The Diagnosis: Cutaneous Mastocytosis
A shave skin biopsy from the right lateral breast and a punch skin biopsy from the right thigh showed similar histopathology. There were dermal predominantly perivascular aggregates of cells demonstrating basophilic granular cytoplasm and round to oval nuclei (Figure, A and B). These cells were highlighted by CD117 immunohistochemical stain (Figure, C), consistent with mastocytes. Additionally, occasional lymphocytes and rare eosinophils were noted. These histopathologic findings confirmed the diagnosis of cutaneous mastocytosis (CM). The patient’s complete blood cell count was within reference range, but serum tryptase was elevated at 15.7 μg/L (reference range, <11.0 μg/L), which prompted a bone marrow biopsy to rule out systemic mastocytosis (SM). The result showed normocellular bone marrow with no evidence of dysplasia or increased blasts, granuloma, lymphoproliferative disorder, or malignancy. Fluorescence in situ hybridization for PDGFRA (platelet-derived growth factor receptor alpha) and KIT mutation was negative. Because CM developed predominantly on the right breast where the patient previously had received radiation therapy, we concluded that this reaction was triggered by exposure to ionizing radiation.

Mastocytosis can be divided into 2 groups: CM and SM.1 The histologic differential diagnosis of CM includes solitary mastocytoma, urticaria pigmentosa, telangiectasia macularis eruptiva perstans, and diffuse mastocytosis.2 Clinicopathologic correlation is of crucial importance to render the final diagnosis in these disorders. Immunohistochemically, mast cells express CD177, CD5, CD68, tryptase, and chymase. Unlike normal mast cells, neoplastic cells express CD2 and/or CD25; CD25 is commonly expressed in cutaneous involvement by SM.2
Macdonald and Feiwel3 reported the first case of CM following ionizing radiation. Cutaneous mastocytosis is most common in female patients and presents with redbrown macules originating at the site of radiation therapy. Prior literature suggests that radiation-associated CM has a predilection for White patients4; however, our patient was Hispanic. It also is important to note that the presentation of this rash may differ in individuals with skin of color. In one case it spread beyond the radiation site.2 Systemic mast call–mediated symptoms can occur in both CM and SM. The macules manifest as blanching with pressure.5 Typically these macules also are asymptomatic, though a positive Darier sign has been reported.6,7 The interval between radiotherapy and CM has ranged from 3 to 24 months.2
Patients with CM should have a serum tryptase evaluation along with a complete blood cell count, serum biochemistry, and liver function tests. Elevated serum tryptase has a high positive predictive value for SM and should prompt a bone marrow biopsy. Our patient’s bone marrow biopsy results failed to establish SM; however, her serum tryptase levels will be carefully monitored going forward. At the time of publication, the skin macules were still persistent but not worsening or symptomatic.
Treatment is focused on symptomatic relief of cutaneous symptoms, if present; avoiding triggers of mast cell degranulation; and implementing the use of oral antihistamines and leukotriene antagonists as needed. Because our patient was completely asymptomatic, we did not recommend any topical or oral treatment. However, we do counsel patients on avoiding triggers of mast cell degranulation including nonsteroidal anti-inflammatory drugs, morphine and codeine derivatives, alcohol, certain anesthetics, and anticholinergic medications.8
Additional diagnoses were ruled out for the following reasons: Although lichen planus pigmentosus presents with ill-defined, oval, gray-brown macules, histopathology shows a bandlike lymphocytic infiltrate at the dermoepidermal junction. Solar lentiginosis is characterized by grouped tan macules in a sun-exposed distribution. A fixed drug eruption is a delayed hypersensitivity reaction, usually to an ingested medication, characterized by violaceous or hyperpigmented patches, with histopathology showing interface dermatitis with a lymphoeosinophilic infiltrate. Eruptive seborrheic keratoses can result from sunburn or dermatitis but does not show mastocytes on histopathology.8
In conclusion, dermatologists should be reminded of the rare possibility of CM when evaluating an atypical eruption in a prior radiation field.
- Landy RE, Stross WC, May JM, et al. Idiopathic mast cell activation syndrome and radiation therapy: a case study, literature review, and discussion of mast cell disorders and radiotherapy [published online December 9, 2019]. Radiat Oncol. 2019;14:222. doi:10.1186 /s13014-019-1434-6
- Easwaralingam N, Wu Y, Cheung D, et al. Radiotherapy for breast cancer associated with a cutaneous presentation of systemic mastocytosis—a case report and literature review. J Surg Case Rep. 2018;2018:1-3. doi:10.1093/jscr/rjy317
- Macdonald A, Feiwel M. Cutaneous mastocytosis: an unusual radiation dermatitis. Proc R Soc Med. 1971;64:29-30.
- Kirshenbaum AS, Abuhay H, Bolan H, et al. Maculopapular cutaneous mastocytosis in a diverse population. J Allergy Clin Immunol Pract. 2019;7:2845-2847. doi:10.1016/j.jaip.2019.04.003
- Soilleux EJ, Brown VL, Bowling J. Cutaneous mastocytosis localized to a radiotherapy field. Clin Exp Dermatol. 2008;34:111-112. doi:10.1111 /j.1365-2230.2008.02931.x
- Comte C, Bessis D, Dereure O, et al. Urticaria pigmentosa localized on radiation field. Eur J Dermatol. 2003;13:408-409.
- Davidson SJ, Coates D. Cutaneous mastocytosis extending beyond a radiotherapy site: a form of radiodermatitis or a neoplastic phenomenon? Australas J Dermatol. 2012;54:E85-E87. doi:10.1111 /j.1440-0960.2012.00961.x
- Bolognia J, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2022.
- Landy RE, Stross WC, May JM, et al. Idiopathic mast cell activation syndrome and radiation therapy: a case study, literature review, and discussion of mast cell disorders and radiotherapy [published online December 9, 2019]. Radiat Oncol. 2019;14:222. doi:10.1186 /s13014-019-1434-6
- Easwaralingam N, Wu Y, Cheung D, et al. Radiotherapy for breast cancer associated with a cutaneous presentation of systemic mastocytosis—a case report and literature review. J Surg Case Rep. 2018;2018:1-3. doi:10.1093/jscr/rjy317
- Macdonald A, Feiwel M. Cutaneous mastocytosis: an unusual radiation dermatitis. Proc R Soc Med. 1971;64:29-30.
- Kirshenbaum AS, Abuhay H, Bolan H, et al. Maculopapular cutaneous mastocytosis in a diverse population. J Allergy Clin Immunol Pract. 2019;7:2845-2847. doi:10.1016/j.jaip.2019.04.003
- Soilleux EJ, Brown VL, Bowling J. Cutaneous mastocytosis localized to a radiotherapy field. Clin Exp Dermatol. 2008;34:111-112. doi:10.1111 /j.1365-2230.2008.02931.x
- Comte C, Bessis D, Dereure O, et al. Urticaria pigmentosa localized on radiation field. Eur J Dermatol. 2003;13:408-409.
- Davidson SJ, Coates D. Cutaneous mastocytosis extending beyond a radiotherapy site: a form of radiodermatitis or a neoplastic phenomenon? Australas J Dermatol. 2012;54:E85-E87. doi:10.1111 /j.1440-0960.2012.00961.x
- Bolognia J, Schaffer JV, Duncan KO, et al, eds. Dermatology Essentials. 2nd ed. Elsevier; 2022.
A 41-year-old woman was referred to dermatology by her radiation oncologist for evaluation of a rash on the right breast at the site of prior radiation therapy of 4 to 6 weeks’ duration. Approximately 2 years prior, the patient was diagnosed with triple-negative invasive ductal carcinoma of the right breast. She was treated with neoadjuvant chemotherapy, bilateral simple mastectomies, and 28 doses of adjuvant radiation therapy. Thirteen months after completing radiation therapy, the patient noted the onset of asymptomatic freckles on the right breast that had appeared over weeks and seemed to be multiplying. Physical examination at the time of dermatology consultation revealed multiple diffusely scattered, brownishred, 3- to 5-mm macules concentrated on the right breast but also involving the right supraclavicular and right axillary areas, abdomen, and thighs.
Commentary: Drug Comparisons and Contact Allergy in AD, February 2024
But here's the thing: We should not be making clinical judgments on the basis of differences in relative risk; clinical decisions should be based on absolute risks. Should we worry about VTE risk when treating patients with AD? This paper did not focus on absolute risk, but we can get an idea of the absolute risk by looking at the data presented in the figures in the paper. The risk for VTE in patients without AD was about 1 in 400, whereas with AD the risk was about 1 in 300, even before controlling for risk factors. This rate is sufficiently low for both groups that it doesn't seem like this risk would affect whether we would use a drug that might be associated with some minimal or theoretical increased risk for VTE.
The bottom line is that the findings of this study are reassuring, at least to me.
I'm already convinced that dupilumab is a very safe treatment for our patients with AD. The study by Simpson and colleagues looked at data from a registry of patients followed in real-life practice. The 2-year study showed no new concerns for dupilumab treatment of AD. The most common adverse event was conjunctivitis, and that was seen in only 2.4% of the patients. Perhaps the most interesting finding was that 83% of the patients who started in the study were still on dupilumab treatment at the end of 2 years. Dupilumab has a good level of efficacy and safety such that the great majority of patients who start on it seem to do well.
Dupilumab is a highly effective, very safe treatment for AD. Rademikibart Is another interleukin-4 receptor alpha-chain blocker. Not surprisingly, rademikibart also seems to be an effective, safe treatment for AD (Silverberg et al). Rademikibart may serve as another option for AD, and I imagine that it could be used if a patient on dupilumab were to develop an anti-drug antibody and lose effectiveness.
The very interesting analysis by Silverberg and colleagues looks at a new way to compare the effectiveness of different drugs for AD. They use this new approach to compare upadacitinib and dupilumab. What they found, not surprisingly, was that upadacitinib was generally more effective for AD than dupilumab. I used to think I would never see anything more effective for AD than dupilumab, but, clearly, based on head-to-head trials, upadacitinib is more effective for AD than is dupilumab. But does that greater efficacy mean that we should use upadacitinib first? We need to consider safety, too. Dupilumab works well enough for the great majority of patients and is extremely safe. I think upadacitinib is a great choice for patients who did not respond to dupilumab and could also be considered for those patients who want to take the most effective treatment option.
Trimeche and colleagues' study of contact allergens in patients with AD may change how I practice. In this study, 60% of the AD patients had positive patch test results of which 71% were considered relevant. The most frequent allergens included textile dye mix (25%), nickel (20%), cobalt (13%), isothiazolinone (9%), quanterium-15 (4%), and balsam of Peru (4%). Two patients were allergic to corticosteroids. Avoidance of relevant allergens resulted in improvement. I need to warn my AD patients to be on the lookout for contact allergens that may be causing or exacerbating their skin disease.
But here's the thing: We should not be making clinical judgments on the basis of differences in relative risk; clinical decisions should be based on absolute risks. Should we worry about VTE risk when treating patients with AD? This paper did not focus on absolute risk, but we can get an idea of the absolute risk by looking at the data presented in the figures in the paper. The risk for VTE in patients without AD was about 1 in 400, whereas with AD the risk was about 1 in 300, even before controlling for risk factors. This rate is sufficiently low for both groups that it doesn't seem like this risk would affect whether we would use a drug that might be associated with some minimal or theoretical increased risk for VTE.
The bottom line is that the findings of this study are reassuring, at least to me.
I'm already convinced that dupilumab is a very safe treatment for our patients with AD. The study by Simpson and colleagues looked at data from a registry of patients followed in real-life practice. The 2-year study showed no new concerns for dupilumab treatment of AD. The most common adverse event was conjunctivitis, and that was seen in only 2.4% of the patients. Perhaps the most interesting finding was that 83% of the patients who started in the study were still on dupilumab treatment at the end of 2 years. Dupilumab has a good level of efficacy and safety such that the great majority of patients who start on it seem to do well.
Dupilumab is a highly effective, very safe treatment for AD. Rademikibart Is another interleukin-4 receptor alpha-chain blocker. Not surprisingly, rademikibart also seems to be an effective, safe treatment for AD (Silverberg et al). Rademikibart may serve as another option for AD, and I imagine that it could be used if a patient on dupilumab were to develop an anti-drug antibody and lose effectiveness.
The very interesting analysis by Silverberg and colleagues looks at a new way to compare the effectiveness of different drugs for AD. They use this new approach to compare upadacitinib and dupilumab. What they found, not surprisingly, was that upadacitinib was generally more effective for AD than dupilumab. I used to think I would never see anything more effective for AD than dupilumab, but, clearly, based on head-to-head trials, upadacitinib is more effective for AD than is dupilumab. But does that greater efficacy mean that we should use upadacitinib first? We need to consider safety, too. Dupilumab works well enough for the great majority of patients and is extremely safe. I think upadacitinib is a great choice for patients who did not respond to dupilumab and could also be considered for those patients who want to take the most effective treatment option.
Trimeche and colleagues' study of contact allergens in patients with AD may change how I practice. In this study, 60% of the AD patients had positive patch test results of which 71% were considered relevant. The most frequent allergens included textile dye mix (25%), nickel (20%), cobalt (13%), isothiazolinone (9%), quanterium-15 (4%), and balsam of Peru (4%). Two patients were allergic to corticosteroids. Avoidance of relevant allergens resulted in improvement. I need to warn my AD patients to be on the lookout for contact allergens that may be causing or exacerbating their skin disease.
But here's the thing: We should not be making clinical judgments on the basis of differences in relative risk; clinical decisions should be based on absolute risks. Should we worry about VTE risk when treating patients with AD? This paper did not focus on absolute risk, but we can get an idea of the absolute risk by looking at the data presented in the figures in the paper. The risk for VTE in patients without AD was about 1 in 400, whereas with AD the risk was about 1 in 300, even before controlling for risk factors. This rate is sufficiently low for both groups that it doesn't seem like this risk would affect whether we would use a drug that might be associated with some minimal or theoretical increased risk for VTE.
The bottom line is that the findings of this study are reassuring, at least to me.
I'm already convinced that dupilumab is a very safe treatment for our patients with AD. The study by Simpson and colleagues looked at data from a registry of patients followed in real-life practice. The 2-year study showed no new concerns for dupilumab treatment of AD. The most common adverse event was conjunctivitis, and that was seen in only 2.4% of the patients. Perhaps the most interesting finding was that 83% of the patients who started in the study were still on dupilumab treatment at the end of 2 years. Dupilumab has a good level of efficacy and safety such that the great majority of patients who start on it seem to do well.
Dupilumab is a highly effective, very safe treatment for AD. Rademikibart Is another interleukin-4 receptor alpha-chain blocker. Not surprisingly, rademikibart also seems to be an effective, safe treatment for AD (Silverberg et al). Rademikibart may serve as another option for AD, and I imagine that it could be used if a patient on dupilumab were to develop an anti-drug antibody and lose effectiveness.
The very interesting analysis by Silverberg and colleagues looks at a new way to compare the effectiveness of different drugs for AD. They use this new approach to compare upadacitinib and dupilumab. What they found, not surprisingly, was that upadacitinib was generally more effective for AD than dupilumab. I used to think I would never see anything more effective for AD than dupilumab, but, clearly, based on head-to-head trials, upadacitinib is more effective for AD than is dupilumab. But does that greater efficacy mean that we should use upadacitinib first? We need to consider safety, too. Dupilumab works well enough for the great majority of patients and is extremely safe. I think upadacitinib is a great choice for patients who did not respond to dupilumab and could also be considered for those patients who want to take the most effective treatment option.
Trimeche and colleagues' study of contact allergens in patients with AD may change how I practice. In this study, 60% of the AD patients had positive patch test results of which 71% were considered relevant. The most frequent allergens included textile dye mix (25%), nickel (20%), cobalt (13%), isothiazolinone (9%), quanterium-15 (4%), and balsam of Peru (4%). Two patients were allergic to corticosteroids. Avoidance of relevant allergens resulted in improvement. I need to warn my AD patients to be on the lookout for contact allergens that may be causing or exacerbating their skin disease.
A Look at the Evidence Linking Diet to Skin Conditions
ORLANDO, FLORIDA — Amid all the hype, claims, and confusion, there is evidence linking some foods and drinks to an increased risk for acne, psoriasis, atopic dermatitis, rosacea, and other common skin conditions. So, what is the connection in each case? And how can people with any of these skin conditions potentially improve their health and quality of life with dietary changes?
What is clear is that there has been an explosion of interest in learning which foods can improve or worsen skin issues in recent years. It’s a good idea to familiarize yourself with the research and also to Google ‘diet’ and ‘skin’, said Vivian Shi, MD, associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock. “As practitioners, we should be well prepared to talk about what patients want to talk about.”
Acne
One of the major areas of interest is diet and acne. “We’ve all heard sugar and dairy are bad, and the Western diet is high in sugar and dairy,” Dr. Shi said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Dairy, red meat, and carbohydrates can break down into leucine, an essential amino acid found in protein. Leucine and sugar together, in turn, can produce insulin and insulin-like growth factor 1 (IGF-1), which, through different pathways, can reach the androgen receptors throughout the body, including the skin. This results in sebogenesis, lipogenesis, and keratinization, which triggers follicular inflammation and results in more of the acne-causing bacteria Cutibacterium acnes.
Milk and other dairy products also can increase IGF-1 levels, which can alter hormonal mediators and increase acne.
Not all types of dairy milk are created equal, however, when it comes to acne. Dr. Shi wondered why 2% milk has overall color and nutritional content very similar to that of whole milk. “I looked into this.” She discovered that when milk manufacturers remove the fat, they often add whey proteins to restore some nutrients. Whey protein can increase acne, Dr. Shi added.
“So, if you’re going to choose any milk to drink, I think from an acne perspective, it’s better to use whole milk. If you can get it organic, even better.” Skim milk is the most acnegenic, she said.
Psoriasis
A systematic review of 55 studies evaluating diet and psoriasis found obesity can be an exacerbating factor. The strongest evidence for dietary weight reduction points to a hypocaloric diet in people with overweight or obesity, according to the review. Other evidence suggests alcohol can lower response to treatment and is linked with more severe psoriasis. Furthermore, a gluten-free diet or vitamin D supplements can help some subpopulations of people with psoriasis.
“An overwhelming majority of our psoriasis patients are vitamin D deficient,” Dr. Shi said.
The National Psoriasis Foundation (NPF) publishes dietary modification guidelines, updated as recently as November 2023. The NPF states that “there is no diet that will cure psoriatic disease, but there are many ways in which eating healthful food may lessen the severity of symptoms and play a role in lowering the likelihood of developing comorbidities.”
Healthier choices include fruits, vegetables, whole grains, and fat-free or low-fat dairy products. Include lean meats, poultry, fish, beans, eggs, and nuts. Adherence to a Mediterranean diet has been linked to a lower severity of psoriasis.
Atopic Dermatitis
Atopic dermatitis (AD) is “one of the prototypical diseases related to diet,” Dr. Shi said. A different meta-analysis looked at randomized controlled trials of synbiotics (a combination of prebiotics and probiotics) for treatment of AD.
These researchers found that synbiotics do not prevent AD, but they can help treat it in adults and children older than 1 year. In addition, synbiotics are more beneficial than probiotics in treating the condition, although there are no head-to-head comparison studies. In addition, the meta-analysis found that prebiotics alone can lower AD severity.
However, Dr. Shi said, there are no recommendations from the American Academy of Dermatology (AAD) on prebiotics or probiotics for AD, and the AAD does not recommend any supplement or essential oil for AD.
In a 2022 review, investigators ranked the efficacy of different supplements for AD based on available evidence. They found the greatest benefit associated with vitamin D supplementation, followed by vitamin E, probiotics, hemp seed oil, histidine, and oolong tea. They also noted the ‘Six Food Elimination Diet and Autoimmune Protocol’ featured the least amount of evidence to back it up.
Rosacea
Rosacea appears to be caused by “all the fun things in life” like sunlight, alcohol, chocolate, spicy foods, and caffeine, Dr. Shi said. In people with rosacea, they can cause facial flushing, edema, burning, and an inflammatory response.
Certain foods can activate skin receptors and sensory neurons, which can release neuropeptides that act on mast cells in blood that lead to flushing. The skin-gut axis may also be involved, evidence suggests. “And that is why food has a pretty profound impact on rosacea,” Dr. Shi said.
Dr. Shi reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
ORLANDO, FLORIDA — Amid all the hype, claims, and confusion, there is evidence linking some foods and drinks to an increased risk for acne, psoriasis, atopic dermatitis, rosacea, and other common skin conditions. So, what is the connection in each case? And how can people with any of these skin conditions potentially improve their health and quality of life with dietary changes?
What is clear is that there has been an explosion of interest in learning which foods can improve or worsen skin issues in recent years. It’s a good idea to familiarize yourself with the research and also to Google ‘diet’ and ‘skin’, said Vivian Shi, MD, associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock. “As practitioners, we should be well prepared to talk about what patients want to talk about.”
Acne
One of the major areas of interest is diet and acne. “We’ve all heard sugar and dairy are bad, and the Western diet is high in sugar and dairy,” Dr. Shi said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Dairy, red meat, and carbohydrates can break down into leucine, an essential amino acid found in protein. Leucine and sugar together, in turn, can produce insulin and insulin-like growth factor 1 (IGF-1), which, through different pathways, can reach the androgen receptors throughout the body, including the skin. This results in sebogenesis, lipogenesis, and keratinization, which triggers follicular inflammation and results in more of the acne-causing bacteria Cutibacterium acnes.
Milk and other dairy products also can increase IGF-1 levels, which can alter hormonal mediators and increase acne.
Not all types of dairy milk are created equal, however, when it comes to acne. Dr. Shi wondered why 2% milk has overall color and nutritional content very similar to that of whole milk. “I looked into this.” She discovered that when milk manufacturers remove the fat, they often add whey proteins to restore some nutrients. Whey protein can increase acne, Dr. Shi added.
“So, if you’re going to choose any milk to drink, I think from an acne perspective, it’s better to use whole milk. If you can get it organic, even better.” Skim milk is the most acnegenic, she said.
Psoriasis
A systematic review of 55 studies evaluating diet and psoriasis found obesity can be an exacerbating factor. The strongest evidence for dietary weight reduction points to a hypocaloric diet in people with overweight or obesity, according to the review. Other evidence suggests alcohol can lower response to treatment and is linked with more severe psoriasis. Furthermore, a gluten-free diet or vitamin D supplements can help some subpopulations of people with psoriasis.
“An overwhelming majority of our psoriasis patients are vitamin D deficient,” Dr. Shi said.
The National Psoriasis Foundation (NPF) publishes dietary modification guidelines, updated as recently as November 2023. The NPF states that “there is no diet that will cure psoriatic disease, but there are many ways in which eating healthful food may lessen the severity of symptoms and play a role in lowering the likelihood of developing comorbidities.”
Healthier choices include fruits, vegetables, whole grains, and fat-free or low-fat dairy products. Include lean meats, poultry, fish, beans, eggs, and nuts. Adherence to a Mediterranean diet has been linked to a lower severity of psoriasis.
Atopic Dermatitis
Atopic dermatitis (AD) is “one of the prototypical diseases related to diet,” Dr. Shi said. A different meta-analysis looked at randomized controlled trials of synbiotics (a combination of prebiotics and probiotics) for treatment of AD.
These researchers found that synbiotics do not prevent AD, but they can help treat it in adults and children older than 1 year. In addition, synbiotics are more beneficial than probiotics in treating the condition, although there are no head-to-head comparison studies. In addition, the meta-analysis found that prebiotics alone can lower AD severity.
However, Dr. Shi said, there are no recommendations from the American Academy of Dermatology (AAD) on prebiotics or probiotics for AD, and the AAD does not recommend any supplement or essential oil for AD.
In a 2022 review, investigators ranked the efficacy of different supplements for AD based on available evidence. They found the greatest benefit associated with vitamin D supplementation, followed by vitamin E, probiotics, hemp seed oil, histidine, and oolong tea. They also noted the ‘Six Food Elimination Diet and Autoimmune Protocol’ featured the least amount of evidence to back it up.
Rosacea
Rosacea appears to be caused by “all the fun things in life” like sunlight, alcohol, chocolate, spicy foods, and caffeine, Dr. Shi said. In people with rosacea, they can cause facial flushing, edema, burning, and an inflammatory response.
Certain foods can activate skin receptors and sensory neurons, which can release neuropeptides that act on mast cells in blood that lead to flushing. The skin-gut axis may also be involved, evidence suggests. “And that is why food has a pretty profound impact on rosacea,” Dr. Shi said.
Dr. Shi reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
ORLANDO, FLORIDA — Amid all the hype, claims, and confusion, there is evidence linking some foods and drinks to an increased risk for acne, psoriasis, atopic dermatitis, rosacea, and other common skin conditions. So, what is the connection in each case? And how can people with any of these skin conditions potentially improve their health and quality of life with dietary changes?
What is clear is that there has been an explosion of interest in learning which foods can improve or worsen skin issues in recent years. It’s a good idea to familiarize yourself with the research and also to Google ‘diet’ and ‘skin’, said Vivian Shi, MD, associate professor of dermatology at the University of Arkansas for Medical Sciences, Little Rock. “As practitioners, we should be well prepared to talk about what patients want to talk about.”
Acne
One of the major areas of interest is diet and acne. “We’ve all heard sugar and dairy are bad, and the Western diet is high in sugar and dairy,” Dr. Shi said at the ODAC Dermatology, Aesthetic & Surgical Conference.
Dairy, red meat, and carbohydrates can break down into leucine, an essential amino acid found in protein. Leucine and sugar together, in turn, can produce insulin and insulin-like growth factor 1 (IGF-1), which, through different pathways, can reach the androgen receptors throughout the body, including the skin. This results in sebogenesis, lipogenesis, and keratinization, which triggers follicular inflammation and results in more of the acne-causing bacteria Cutibacterium acnes.
Milk and other dairy products also can increase IGF-1 levels, which can alter hormonal mediators and increase acne.
Not all types of dairy milk are created equal, however, when it comes to acne. Dr. Shi wondered why 2% milk has overall color and nutritional content very similar to that of whole milk. “I looked into this.” She discovered that when milk manufacturers remove the fat, they often add whey proteins to restore some nutrients. Whey protein can increase acne, Dr. Shi added.
“So, if you’re going to choose any milk to drink, I think from an acne perspective, it’s better to use whole milk. If you can get it organic, even better.” Skim milk is the most acnegenic, she said.
Psoriasis
A systematic review of 55 studies evaluating diet and psoriasis found obesity can be an exacerbating factor. The strongest evidence for dietary weight reduction points to a hypocaloric diet in people with overweight or obesity, according to the review. Other evidence suggests alcohol can lower response to treatment and is linked with more severe psoriasis. Furthermore, a gluten-free diet or vitamin D supplements can help some subpopulations of people with psoriasis.
“An overwhelming majority of our psoriasis patients are vitamin D deficient,” Dr. Shi said.
The National Psoriasis Foundation (NPF) publishes dietary modification guidelines, updated as recently as November 2023. The NPF states that “there is no diet that will cure psoriatic disease, but there are many ways in which eating healthful food may lessen the severity of symptoms and play a role in lowering the likelihood of developing comorbidities.”
Healthier choices include fruits, vegetables, whole grains, and fat-free or low-fat dairy products. Include lean meats, poultry, fish, beans, eggs, and nuts. Adherence to a Mediterranean diet has been linked to a lower severity of psoriasis.
Atopic Dermatitis
Atopic dermatitis (AD) is “one of the prototypical diseases related to diet,” Dr. Shi said. A different meta-analysis looked at randomized controlled trials of synbiotics (a combination of prebiotics and probiotics) for treatment of AD.
These researchers found that synbiotics do not prevent AD, but they can help treat it in adults and children older than 1 year. In addition, synbiotics are more beneficial than probiotics in treating the condition, although there are no head-to-head comparison studies. In addition, the meta-analysis found that prebiotics alone can lower AD severity.
However, Dr. Shi said, there are no recommendations from the American Academy of Dermatology (AAD) on prebiotics or probiotics for AD, and the AAD does not recommend any supplement or essential oil for AD.
In a 2022 review, investigators ranked the efficacy of different supplements for AD based on available evidence. They found the greatest benefit associated with vitamin D supplementation, followed by vitamin E, probiotics, hemp seed oil, histidine, and oolong tea. They also noted the ‘Six Food Elimination Diet and Autoimmune Protocol’ featured the least amount of evidence to back it up.
Rosacea
Rosacea appears to be caused by “all the fun things in life” like sunlight, alcohol, chocolate, spicy foods, and caffeine, Dr. Shi said. In people with rosacea, they can cause facial flushing, edema, burning, and an inflammatory response.
Certain foods can activate skin receptors and sensory neurons, which can release neuropeptides that act on mast cells in blood that lead to flushing. The skin-gut axis may also be involved, evidence suggests. “And that is why food has a pretty profound impact on rosacea,” Dr. Shi said.
Dr. Shi reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Novel Clinic Resulted in ‘Impressive’ Outcomes for Patients With Moderate to Severe Eczema
, results from a single-center study showed.
“A significant challenge in caring for patients with atopic dermatitis is lack of collaboration between healthcare providers, leading to disjointed care, inconsistent treatment plans, and conflicting dialogue with patients,” first author Alexis Tracy, MD, a combined allergy and dermatology research fellow at Rady Children’s Hospital, San Diego, and colleagues wrote in the study, which was published online January 14, 2024, in Pediatric Dermatology.
Launched in 2019, the clinic, which is called the Multidisciplinary Atopic Dermatitis Program (MADP), is a collaborative effort between with Rady Children’s Hospital and the University of California San Diego Health division of dermatology, division of allergy & immunology, and the hospital’s clinical pharmacy. Patients referred to the MADP undergo a concurrent, comprehensive evaluation by a dermatologist, allergist, clinical pharmacist, and others who help to assess AD severity, provide family education about the disease, and form a care plan using the model of shared decision-making (SDM). Visits take about two hours, and the frequency of follow-up visits varies.
In the dermatology realm, tools used to compare the extent and severity of AD between visits include the Eczema Area and Severity Index (EASI), Patient-Oriented Eczema Measure (POEM), the Children’s Dermatology Life Quality Index (CDLQI), Validated Investigator Global Assessment (vIGA), Body Surface Area (BSA), and the Numerical Rating Scale (NRS).To investigate the MADP’s success to date, Dr. Tracy and colleagues evaluated 44 patients with a history of moderate to severe, persistent AD who were referred to the clinic between April 3, 2019, and October 22, 2022, and had between one and three follow-up visits. The patients ranged from age 4 months to 18 years (mean, 7.74 years).
Compared with baseline, EASI scores of patients decreased significantly, with an average mean improvement of 9.61 by the second visit, 15.12 by the third visit, and 17.42 by the fourth visit (P <.001 for all three). These represent an average decreases of 44.20%, 63.26%, 74.35%, respectively.
At the seventh visit, the EASI score decreased by a mean of 33.48 (P = .008), which represents an average decrease of 91.52% from baseline. Of the 44 patients, 32 achieved an EASI 50 and 21 achieved an EASI 75.
In other findings, the mean vIGA improved with each visit, with the largest observed improvement at the seventh visit (a mean of 2.25 points; P = .009) and the greatest mean improvement in the POEM score was seen at the sixth visit (a mean of 11.13 points; P < .001). The mean difference in CDLQI scores also increased with each visit, with the largest improvement seen at the sixth visit (an increase of 12 points; P < .001).
Similarly, BSA progressively improved at each clinic visit, from a mean decrease of 16.02% at the second visit to a mean decrease of 56.04% at the seventh visit (P < .001 for both). Meanwhile, the largest mean improvement in pruritus was seen at the sixth visit (a mean of 4.10 points; P = .001).
In an interview, MADP’s codirector, Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, said that the consistency of data showing rapid, consistent improvement with a varied set of physician assessed scores and patient-reported outcomes “was very impressive, especially given the variation in severity, extent and difficult course of many of the patients we saw, and spectrum of interventions – from topical regimens to advanced systemic therapies,” he said. “As clinicians we tend to remember the ‘tough cases,’ and it was tremendous to see the impact and utility of the clinic.”
He noted that he and Bob Geng, MD, an allergist/immunologist at Rady Children’s who co-directs the MADP, regularly discuss how much they have learned from the program. “Some take-aways are simple, like ‘do body surface area assessment in pediatric patients with moderate to serve atopic dermatitis,’ ” Dr. Eichenfield said. “These help us show the severity to the patient and family, and everyone loves to see the objective improvement measures over time.”
The MADP providers and personnel have become better at explaining AD “and understanding how families come in with broad differences in understanding of the disease, therapies and prior treatments,” he added. “And I have learned that discussing environmental allergies and food allergies, even if they might not be triggers of the AD, is appreciated by patients and families, as they are part of the family experience and they appreciate our ‘broadly caring’ beyond our narrow niches of intervention.”
Important model of care
Asked to comment on the results, pediatric dermatologist Moise L. Levy, MD, professor of internal medicine and pediatrics at the University of Texas at Austin, who was not involved with the study, characterized the MADP as an important model of care. “Multi-interdisciplinary care of such conditions is well-known to be of great help for patients and their families,” he told this news organization.
“A key part of the ‘team’ is the family/patient engagement and shared decision-making. The use of visual aides to highlight components of care was likely of great use, as well,” he said. “All such interventions impact the disease, as well as associated problems, such as itch, sleep, and mental health. Importantly, such interventions, while known to be useful as demonstrated by the authors, take time, and relate to improved outcomes as noted by the date outlined by the authors.”
The study authors acknowledged certain limitations of the study, including the lack of a control group with single-specialty visits. “The real take-away is that taking the time to do more holistic assessments of health — with skin and allergy issues being discussed, and consistent education and messaging — helps make our medical interventions more successful, with both objective disease improvement and patient/family satisfaction,” Dr. Eichenfield said in the interview.
Pfizer and Sanofi provided financial support to MADP, and for the study. Dr. Eichenfield disclosed that he serves as a scientific adviser, consultant, and/or clinical trial investigator for AbbVie, Amgen, Aslan, Castle Biosciences, Dermavant, Eli Lilly and Company, Forté, Galderma, Incyte, Janssen, LEO Pharma, Novartis, Ortho Dermatologics, Pfizer, Regeneron Pharmaceuticals, Sanofi-Genzyme, Trialspark, and UCB. Dr. Geng disclosed ties with Sanofi, Regeneron, Pfizer, and AbbVie, and is an adviser to Incyte, Galderma, Eli-Lilly, and LEO. The other authors reported having no disclosures. Dr. Levy disclosed ties with Abeona, Amgen, Arcutis, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi Genzyme. He is also an investigator for Janssen.
, results from a single-center study showed.
“A significant challenge in caring for patients with atopic dermatitis is lack of collaboration between healthcare providers, leading to disjointed care, inconsistent treatment plans, and conflicting dialogue with patients,” first author Alexis Tracy, MD, a combined allergy and dermatology research fellow at Rady Children’s Hospital, San Diego, and colleagues wrote in the study, which was published online January 14, 2024, in Pediatric Dermatology.
Launched in 2019, the clinic, which is called the Multidisciplinary Atopic Dermatitis Program (MADP), is a collaborative effort between with Rady Children’s Hospital and the University of California San Diego Health division of dermatology, division of allergy & immunology, and the hospital’s clinical pharmacy. Patients referred to the MADP undergo a concurrent, comprehensive evaluation by a dermatologist, allergist, clinical pharmacist, and others who help to assess AD severity, provide family education about the disease, and form a care plan using the model of shared decision-making (SDM). Visits take about two hours, and the frequency of follow-up visits varies.
In the dermatology realm, tools used to compare the extent and severity of AD between visits include the Eczema Area and Severity Index (EASI), Patient-Oriented Eczema Measure (POEM), the Children’s Dermatology Life Quality Index (CDLQI), Validated Investigator Global Assessment (vIGA), Body Surface Area (BSA), and the Numerical Rating Scale (NRS).To investigate the MADP’s success to date, Dr. Tracy and colleagues evaluated 44 patients with a history of moderate to severe, persistent AD who were referred to the clinic between April 3, 2019, and October 22, 2022, and had between one and three follow-up visits. The patients ranged from age 4 months to 18 years (mean, 7.74 years).
Compared with baseline, EASI scores of patients decreased significantly, with an average mean improvement of 9.61 by the second visit, 15.12 by the third visit, and 17.42 by the fourth visit (P <.001 for all three). These represent an average decreases of 44.20%, 63.26%, 74.35%, respectively.
At the seventh visit, the EASI score decreased by a mean of 33.48 (P = .008), which represents an average decrease of 91.52% from baseline. Of the 44 patients, 32 achieved an EASI 50 and 21 achieved an EASI 75.
In other findings, the mean vIGA improved with each visit, with the largest observed improvement at the seventh visit (a mean of 2.25 points; P = .009) and the greatest mean improvement in the POEM score was seen at the sixth visit (a mean of 11.13 points; P < .001). The mean difference in CDLQI scores also increased with each visit, with the largest improvement seen at the sixth visit (an increase of 12 points; P < .001).
Similarly, BSA progressively improved at each clinic visit, from a mean decrease of 16.02% at the second visit to a mean decrease of 56.04% at the seventh visit (P < .001 for both). Meanwhile, the largest mean improvement in pruritus was seen at the sixth visit (a mean of 4.10 points; P = .001).
In an interview, MADP’s codirector, Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, said that the consistency of data showing rapid, consistent improvement with a varied set of physician assessed scores and patient-reported outcomes “was very impressive, especially given the variation in severity, extent and difficult course of many of the patients we saw, and spectrum of interventions – from topical regimens to advanced systemic therapies,” he said. “As clinicians we tend to remember the ‘tough cases,’ and it was tremendous to see the impact and utility of the clinic.”
He noted that he and Bob Geng, MD, an allergist/immunologist at Rady Children’s who co-directs the MADP, regularly discuss how much they have learned from the program. “Some take-aways are simple, like ‘do body surface area assessment in pediatric patients with moderate to serve atopic dermatitis,’ ” Dr. Eichenfield said. “These help us show the severity to the patient and family, and everyone loves to see the objective improvement measures over time.”
The MADP providers and personnel have become better at explaining AD “and understanding how families come in with broad differences in understanding of the disease, therapies and prior treatments,” he added. “And I have learned that discussing environmental allergies and food allergies, even if they might not be triggers of the AD, is appreciated by patients and families, as they are part of the family experience and they appreciate our ‘broadly caring’ beyond our narrow niches of intervention.”
Important model of care
Asked to comment on the results, pediatric dermatologist Moise L. Levy, MD, professor of internal medicine and pediatrics at the University of Texas at Austin, who was not involved with the study, characterized the MADP as an important model of care. “Multi-interdisciplinary care of such conditions is well-known to be of great help for patients and their families,” he told this news organization.
“A key part of the ‘team’ is the family/patient engagement and shared decision-making. The use of visual aides to highlight components of care was likely of great use, as well,” he said. “All such interventions impact the disease, as well as associated problems, such as itch, sleep, and mental health. Importantly, such interventions, while known to be useful as demonstrated by the authors, take time, and relate to improved outcomes as noted by the date outlined by the authors.”
The study authors acknowledged certain limitations of the study, including the lack of a control group with single-specialty visits. “The real take-away is that taking the time to do more holistic assessments of health — with skin and allergy issues being discussed, and consistent education and messaging — helps make our medical interventions more successful, with both objective disease improvement and patient/family satisfaction,” Dr. Eichenfield said in the interview.
Pfizer and Sanofi provided financial support to MADP, and for the study. Dr. Eichenfield disclosed that he serves as a scientific adviser, consultant, and/or clinical trial investigator for AbbVie, Amgen, Aslan, Castle Biosciences, Dermavant, Eli Lilly and Company, Forté, Galderma, Incyte, Janssen, LEO Pharma, Novartis, Ortho Dermatologics, Pfizer, Regeneron Pharmaceuticals, Sanofi-Genzyme, Trialspark, and UCB. Dr. Geng disclosed ties with Sanofi, Regeneron, Pfizer, and AbbVie, and is an adviser to Incyte, Galderma, Eli-Lilly, and LEO. The other authors reported having no disclosures. Dr. Levy disclosed ties with Abeona, Amgen, Arcutis, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi Genzyme. He is also an investigator for Janssen.
, results from a single-center study showed.
“A significant challenge in caring for patients with atopic dermatitis is lack of collaboration between healthcare providers, leading to disjointed care, inconsistent treatment plans, and conflicting dialogue with patients,” first author Alexis Tracy, MD, a combined allergy and dermatology research fellow at Rady Children’s Hospital, San Diego, and colleagues wrote in the study, which was published online January 14, 2024, in Pediatric Dermatology.
Launched in 2019, the clinic, which is called the Multidisciplinary Atopic Dermatitis Program (MADP), is a collaborative effort between with Rady Children’s Hospital and the University of California San Diego Health division of dermatology, division of allergy & immunology, and the hospital’s clinical pharmacy. Patients referred to the MADP undergo a concurrent, comprehensive evaluation by a dermatologist, allergist, clinical pharmacist, and others who help to assess AD severity, provide family education about the disease, and form a care plan using the model of shared decision-making (SDM). Visits take about two hours, and the frequency of follow-up visits varies.
In the dermatology realm, tools used to compare the extent and severity of AD between visits include the Eczema Area and Severity Index (EASI), Patient-Oriented Eczema Measure (POEM), the Children’s Dermatology Life Quality Index (CDLQI), Validated Investigator Global Assessment (vIGA), Body Surface Area (BSA), and the Numerical Rating Scale (NRS).To investigate the MADP’s success to date, Dr. Tracy and colleagues evaluated 44 patients with a history of moderate to severe, persistent AD who were referred to the clinic between April 3, 2019, and October 22, 2022, and had between one and three follow-up visits. The patients ranged from age 4 months to 18 years (mean, 7.74 years).
Compared with baseline, EASI scores of patients decreased significantly, with an average mean improvement of 9.61 by the second visit, 15.12 by the third visit, and 17.42 by the fourth visit (P <.001 for all three). These represent an average decreases of 44.20%, 63.26%, 74.35%, respectively.
At the seventh visit, the EASI score decreased by a mean of 33.48 (P = .008), which represents an average decrease of 91.52% from baseline. Of the 44 patients, 32 achieved an EASI 50 and 21 achieved an EASI 75.
In other findings, the mean vIGA improved with each visit, with the largest observed improvement at the seventh visit (a mean of 2.25 points; P = .009) and the greatest mean improvement in the POEM score was seen at the sixth visit (a mean of 11.13 points; P < .001). The mean difference in CDLQI scores also increased with each visit, with the largest improvement seen at the sixth visit (an increase of 12 points; P < .001).
Similarly, BSA progressively improved at each clinic visit, from a mean decrease of 16.02% at the second visit to a mean decrease of 56.04% at the seventh visit (P < .001 for both). Meanwhile, the largest mean improvement in pruritus was seen at the sixth visit (a mean of 4.10 points; P = .001).
In an interview, MADP’s codirector, Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, said that the consistency of data showing rapid, consistent improvement with a varied set of physician assessed scores and patient-reported outcomes “was very impressive, especially given the variation in severity, extent and difficult course of many of the patients we saw, and spectrum of interventions – from topical regimens to advanced systemic therapies,” he said. “As clinicians we tend to remember the ‘tough cases,’ and it was tremendous to see the impact and utility of the clinic.”
He noted that he and Bob Geng, MD, an allergist/immunologist at Rady Children’s who co-directs the MADP, regularly discuss how much they have learned from the program. “Some take-aways are simple, like ‘do body surface area assessment in pediatric patients with moderate to serve atopic dermatitis,’ ” Dr. Eichenfield said. “These help us show the severity to the patient and family, and everyone loves to see the objective improvement measures over time.”
The MADP providers and personnel have become better at explaining AD “and understanding how families come in with broad differences in understanding of the disease, therapies and prior treatments,” he added. “And I have learned that discussing environmental allergies and food allergies, even if they might not be triggers of the AD, is appreciated by patients and families, as they are part of the family experience and they appreciate our ‘broadly caring’ beyond our narrow niches of intervention.”
Important model of care
Asked to comment on the results, pediatric dermatologist Moise L. Levy, MD, professor of internal medicine and pediatrics at the University of Texas at Austin, who was not involved with the study, characterized the MADP as an important model of care. “Multi-interdisciplinary care of such conditions is well-known to be of great help for patients and their families,” he told this news organization.
“A key part of the ‘team’ is the family/patient engagement and shared decision-making. The use of visual aides to highlight components of care was likely of great use, as well,” he said. “All such interventions impact the disease, as well as associated problems, such as itch, sleep, and mental health. Importantly, such interventions, while known to be useful as demonstrated by the authors, take time, and relate to improved outcomes as noted by the date outlined by the authors.”
The study authors acknowledged certain limitations of the study, including the lack of a control group with single-specialty visits. “The real take-away is that taking the time to do more holistic assessments of health — with skin and allergy issues being discussed, and consistent education and messaging — helps make our medical interventions more successful, with both objective disease improvement and patient/family satisfaction,” Dr. Eichenfield said in the interview.
Pfizer and Sanofi provided financial support to MADP, and for the study. Dr. Eichenfield disclosed that he serves as a scientific adviser, consultant, and/or clinical trial investigator for AbbVie, Amgen, Aslan, Castle Biosciences, Dermavant, Eli Lilly and Company, Forté, Galderma, Incyte, Janssen, LEO Pharma, Novartis, Ortho Dermatologics, Pfizer, Regeneron Pharmaceuticals, Sanofi-Genzyme, Trialspark, and UCB. Dr. Geng disclosed ties with Sanofi, Regeneron, Pfizer, and AbbVie, and is an adviser to Incyte, Galderma, Eli-Lilly, and LEO. The other authors reported having no disclosures. Dr. Levy disclosed ties with Abeona, Amgen, Arcutis, Castle Creek, Dusa Pharma, Krystal Bio, Novan, Regeneron, and Sanofi Genzyme. He is also an investigator for Janssen.
FROM PEDIATRIC DERMATOLOGY
Men with atopic dermatitis more likely to have poorer cognitive function
Key clinical point: A significant association was observed between atopic dermatitis (AD) and poorer cognitive function in men, and familial characteristics exerted a confounding effect on this association.
Major finding: After effectively controlling for familial environmental confounding factors and addressing genetic influences, AD in men was significantly associated with poorer cognitive function (regression coefficient −0.04; 95% CI −0.07 to −0.003).
Study details: This sibling-comparison study included 1,687,038 men who underwent a military conscription examination at 17-22 years of age, of which 25,995 were diagnosed with AD.
Disclosures: This study was sponsored by grants from the Swedish Research Council for Health, Working Life, and Welfare (Forte) and the UK Economic and Social Research Council. L von Kobyletzki declared being a consultant for and receiving research funding from various organizations. The other authors declared no conflicts of interest.
Source: Smith KA et al. Atopic dermatitis and cognitive function: A sibling comparison study among males in Sweden. Br J Dermatol. 2024 (Jan 3). doi: 10.1093/bjd/ljae004
Key clinical point: A significant association was observed between atopic dermatitis (AD) and poorer cognitive function in men, and familial characteristics exerted a confounding effect on this association.
Major finding: After effectively controlling for familial environmental confounding factors and addressing genetic influences, AD in men was significantly associated with poorer cognitive function (regression coefficient −0.04; 95% CI −0.07 to −0.003).
Study details: This sibling-comparison study included 1,687,038 men who underwent a military conscription examination at 17-22 years of age, of which 25,995 were diagnosed with AD.
Disclosures: This study was sponsored by grants from the Swedish Research Council for Health, Working Life, and Welfare (Forte) and the UK Economic and Social Research Council. L von Kobyletzki declared being a consultant for and receiving research funding from various organizations. The other authors declared no conflicts of interest.
Source: Smith KA et al. Atopic dermatitis and cognitive function: A sibling comparison study among males in Sweden. Br J Dermatol. 2024 (Jan 3). doi: 10.1093/bjd/ljae004
Key clinical point: A significant association was observed between atopic dermatitis (AD) and poorer cognitive function in men, and familial characteristics exerted a confounding effect on this association.
Major finding: After effectively controlling for familial environmental confounding factors and addressing genetic influences, AD in men was significantly associated with poorer cognitive function (regression coefficient −0.04; 95% CI −0.07 to −0.003).
Study details: This sibling-comparison study included 1,687,038 men who underwent a military conscription examination at 17-22 years of age, of which 25,995 were diagnosed with AD.
Disclosures: This study was sponsored by grants from the Swedish Research Council for Health, Working Life, and Welfare (Forte) and the UK Economic and Social Research Council. L von Kobyletzki declared being a consultant for and receiving research funding from various organizations. The other authors declared no conflicts of interest.
Source: Smith KA et al. Atopic dermatitis and cognitive function: A sibling comparison study among males in Sweden. Br J Dermatol. 2024 (Jan 3). doi: 10.1093/bjd/ljae004
Atopic dermatitis is associated with increased prevalence of inflammatory bowel disease
Key clinical point: Patients with atopic dermatitis (AD), especially moderate-to-severe AD, had an increased prevalence of inflammatory bowel disease (IBD).
Major finding: A significant association was observed between IBD and AD (adjusted odds ratio [aOR] 3.89; P = .0169); however, when stratified by AD severity, only moderate-to-severe AD was found to be associated with IBD (aOR 4.45; P = .0102).
Study details: Findings are from a retrospective observational study including 364 patients with AD and 725 matched control individuals without AD.
Disclosures: This study was sponsored by an independent investigator grant from AbbVie. Two authors declared serving as investigators for or receiving honoraria or fees as consultants or advisory board members from various organizations, including AbbVie. The other authors declared no conflicts of interest.
Source: Rom H et al. The association between atopic dermatitis and inflammatory bowel disease in adults: A cross-sectional study in a specialized atopic dermatitis clinic. J Eur Acad Dermatol Venereol. 2023 (Dec 21). doi: 10.1111/jdv.19769
Key clinical point: Patients with atopic dermatitis (AD), especially moderate-to-severe AD, had an increased prevalence of inflammatory bowel disease (IBD).
Major finding: A significant association was observed between IBD and AD (adjusted odds ratio [aOR] 3.89; P = .0169); however, when stratified by AD severity, only moderate-to-severe AD was found to be associated with IBD (aOR 4.45; P = .0102).
Study details: Findings are from a retrospective observational study including 364 patients with AD and 725 matched control individuals without AD.
Disclosures: This study was sponsored by an independent investigator grant from AbbVie. Two authors declared serving as investigators for or receiving honoraria or fees as consultants or advisory board members from various organizations, including AbbVie. The other authors declared no conflicts of interest.
Source: Rom H et al. The association between atopic dermatitis and inflammatory bowel disease in adults: A cross-sectional study in a specialized atopic dermatitis clinic. J Eur Acad Dermatol Venereol. 2023 (Dec 21). doi: 10.1111/jdv.19769
Key clinical point: Patients with atopic dermatitis (AD), especially moderate-to-severe AD, had an increased prevalence of inflammatory bowel disease (IBD).
Major finding: A significant association was observed between IBD and AD (adjusted odds ratio [aOR] 3.89; P = .0169); however, when stratified by AD severity, only moderate-to-severe AD was found to be associated with IBD (aOR 4.45; P = .0102).
Study details: Findings are from a retrospective observational study including 364 patients with AD and 725 matched control individuals without AD.
Disclosures: This study was sponsored by an independent investigator grant from AbbVie. Two authors declared serving as investigators for or receiving honoraria or fees as consultants or advisory board members from various organizations, including AbbVie. The other authors declared no conflicts of interest.
Source: Rom H et al. The association between atopic dermatitis and inflammatory bowel disease in adults: A cross-sectional study in a specialized atopic dermatitis clinic. J Eur Acad Dermatol Venereol. 2023 (Dec 21). doi: 10.1111/jdv.19769
Real-world study confirms the multidimensional efficacy of tralokinumab in atopic dermatitis
Key clinical point: The majority of tralokinumab-treated patients with moderate-to-severe atopic dermatitis (AD) attained physician- and patient-reported outcomes over 32 weeks of observation, highlighting the multidimensional efficacy of tralokinumab in real-world settings.
Major finding: The proportion of patients achieving a ≥75% improvement in the baseline Eczema Area and Severity Index (EASI) score increased significantly from 42% at week 4 to 76% at week 32 (P = .0075). A similar trend was observed for patient-reported outcomes. At week 16, at least one real-world therapeutic endpoint was achieved by 88% of patients treated with tralokinumab.
Study details: Findings are from a multicenter real-world retrospective cohort study including 194 patients with moderate-to-severe AD who were treated with tralokinumab for ≥16 weeks.
Disclosures: This study did not receive any funding. Several authors declared serving as speakers, consultants, or scientific advisors; receiving personal fees, speaker’s honoraria, or travel support, or having other ties with various pharmaceutical companies.
Source: Chiricozzi A et al for the MEDaCoTRA Study Group. Current treatment goals are achieved by the majority of patients with atopic dermatitis treated with tralokinumab: Results from a multicentric, multinational, retrospective, cohort study. Expert Opin Biol Ther. 2023;23(12):1307-1315 (Dec 18). doi: 10.1080/14712598.2023.2292627
Key clinical point: The majority of tralokinumab-treated patients with moderate-to-severe atopic dermatitis (AD) attained physician- and patient-reported outcomes over 32 weeks of observation, highlighting the multidimensional efficacy of tralokinumab in real-world settings.
Major finding: The proportion of patients achieving a ≥75% improvement in the baseline Eczema Area and Severity Index (EASI) score increased significantly from 42% at week 4 to 76% at week 32 (P = .0075). A similar trend was observed for patient-reported outcomes. At week 16, at least one real-world therapeutic endpoint was achieved by 88% of patients treated with tralokinumab.
Study details: Findings are from a multicenter real-world retrospective cohort study including 194 patients with moderate-to-severe AD who were treated with tralokinumab for ≥16 weeks.
Disclosures: This study did not receive any funding. Several authors declared serving as speakers, consultants, or scientific advisors; receiving personal fees, speaker’s honoraria, or travel support, or having other ties with various pharmaceutical companies.
Source: Chiricozzi A et al for the MEDaCoTRA Study Group. Current treatment goals are achieved by the majority of patients with atopic dermatitis treated with tralokinumab: Results from a multicentric, multinational, retrospective, cohort study. Expert Opin Biol Ther. 2023;23(12):1307-1315 (Dec 18). doi: 10.1080/14712598.2023.2292627
Key clinical point: The majority of tralokinumab-treated patients with moderate-to-severe atopic dermatitis (AD) attained physician- and patient-reported outcomes over 32 weeks of observation, highlighting the multidimensional efficacy of tralokinumab in real-world settings.
Major finding: The proportion of patients achieving a ≥75% improvement in the baseline Eczema Area and Severity Index (EASI) score increased significantly from 42% at week 4 to 76% at week 32 (P = .0075). A similar trend was observed for patient-reported outcomes. At week 16, at least one real-world therapeutic endpoint was achieved by 88% of patients treated with tralokinumab.
Study details: Findings are from a multicenter real-world retrospective cohort study including 194 patients with moderate-to-severe AD who were treated with tralokinumab for ≥16 weeks.
Disclosures: This study did not receive any funding. Several authors declared serving as speakers, consultants, or scientific advisors; receiving personal fees, speaker’s honoraria, or travel support, or having other ties with various pharmaceutical companies.
Source: Chiricozzi A et al for the MEDaCoTRA Study Group. Current treatment goals are achieved by the majority of patients with atopic dermatitis treated with tralokinumab: Results from a multicentric, multinational, retrospective, cohort study. Expert Opin Biol Ther. 2023;23(12):1307-1315 (Dec 18). doi: 10.1080/14712598.2023.2292627
Abrocitinib downregulates genes associated with atopic dermatitis pathology
Key clinical point: Abrocitinib treatment over 12 weeks significantly decreased the cutaneous expression of selected genes involved in inflammation, epidermal hyperplasia, and T-helper (Th) 2 and Th22 immune responses in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: Compared with placebo, 12-week abrocitinib treatment led to a dose-dependent reduction in the cutaneous expression of genes involved in inflammation (MMP-12), epidermal hyperplasia (KRT16), Th2 (CCL17 and CCL18), and Th22 (S100A8, S100A9, and S100A12) responses (all P < .05).
Study details: Findings are from the phase 2a JADE MOA trial including patients with moderate-to-severe AD who were randomly assigned to receive 100 mg (n = 16) or 200 mg (n = 14) abrocitinib monotherapy or placebo (n = 16) daily for 12 weeks.
Disclosures: This study was sponsored by Pfizer Inc. Several authors declared being on the advisory board of; serving as consultants, advisors, or speakers for; or receiving honoraria or grants from Pfizer or others. Seven authors declared being current or former employees and shareholders of Pfizer.
Source: Guttman-Yassky E et al. Effect of abrocitinib on skin biomarkers in patients with moderate-to-severe atopic dermatitis. Allergy. 2023 (Dec 18). doi: 10.1111/all.15969
Key clinical point: Abrocitinib treatment over 12 weeks significantly decreased the cutaneous expression of selected genes involved in inflammation, epidermal hyperplasia, and T-helper (Th) 2 and Th22 immune responses in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: Compared with placebo, 12-week abrocitinib treatment led to a dose-dependent reduction in the cutaneous expression of genes involved in inflammation (MMP-12), epidermal hyperplasia (KRT16), Th2 (CCL17 and CCL18), and Th22 (S100A8, S100A9, and S100A12) responses (all P < .05).
Study details: Findings are from the phase 2a JADE MOA trial including patients with moderate-to-severe AD who were randomly assigned to receive 100 mg (n = 16) or 200 mg (n = 14) abrocitinib monotherapy or placebo (n = 16) daily for 12 weeks.
Disclosures: This study was sponsored by Pfizer Inc. Several authors declared being on the advisory board of; serving as consultants, advisors, or speakers for; or receiving honoraria or grants from Pfizer or others. Seven authors declared being current or former employees and shareholders of Pfizer.
Source: Guttman-Yassky E et al. Effect of abrocitinib on skin biomarkers in patients with moderate-to-severe atopic dermatitis. Allergy. 2023 (Dec 18). doi: 10.1111/all.15969
Key clinical point: Abrocitinib treatment over 12 weeks significantly decreased the cutaneous expression of selected genes involved in inflammation, epidermal hyperplasia, and T-helper (Th) 2 and Th22 immune responses in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: Compared with placebo, 12-week abrocitinib treatment led to a dose-dependent reduction in the cutaneous expression of genes involved in inflammation (MMP-12), epidermal hyperplasia (KRT16), Th2 (CCL17 and CCL18), and Th22 (S100A8, S100A9, and S100A12) responses (all P < .05).
Study details: Findings are from the phase 2a JADE MOA trial including patients with moderate-to-severe AD who were randomly assigned to receive 100 mg (n = 16) or 200 mg (n = 14) abrocitinib monotherapy or placebo (n = 16) daily for 12 weeks.
Disclosures: This study was sponsored by Pfizer Inc. Several authors declared being on the advisory board of; serving as consultants, advisors, or speakers for; or receiving honoraria or grants from Pfizer or others. Seven authors declared being current or former employees and shareholders of Pfizer.
Source: Guttman-Yassky E et al. Effect of abrocitinib on skin biomarkers in patients with moderate-to-severe atopic dermatitis. Allergy. 2023 (Dec 18). doi: 10.1111/all.15969
Rapid and sustained improvement in skin pain with abrocitinib in atopic dermatitis
Key clinical point: Abrocitinib as monotherapy or in combination with topical therapy improves skin pain in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: Abrocitinib vs placebo led to a significantly greater dose-dependent least squares mean change in Pruritus and Symptoms Assessment for AD (PSAAD) skin pain score from baseline to as early as week 1 that were sustained through week 12 or 16 (nominal P < .05). A greater proportion of patients achieved a stringent threshold of skin pain improvement (PSAAD skin pain score < 2) with abrocitinib vs placebo (nominal P < .05).
Study details: This post hoc analysis of five phase 2/3 trials included 1822 patients with moderate-to-severe AD (age ≥ 12 years) treated with 100 mg or 200 mg abrocitinib as monotherapy or in combination with topical therapy or placebo for 12 or 16 weeks.
Disclosures: This study was funded by Pfizer Inc., USA. Six authors declared being employees and stockholders of Pfizer. The other authors declared receiving research or travel grants or having other ties with various sources, including Pfizer.
Source: Thyssen JP et al. Abrocitinib provides rapid and sustained improvement in skin pain and is associated with improved quality of life outcomes in adult and adolescent patients with moderate-to-severe atopic dermatitis. Dermatology. 2023 (Dec 11). doi: 10.1159/000535285
Key clinical point: Abrocitinib as monotherapy or in combination with topical therapy improves skin pain in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: Abrocitinib vs placebo led to a significantly greater dose-dependent least squares mean change in Pruritus and Symptoms Assessment for AD (PSAAD) skin pain score from baseline to as early as week 1 that were sustained through week 12 or 16 (nominal P < .05). A greater proportion of patients achieved a stringent threshold of skin pain improvement (PSAAD skin pain score < 2) with abrocitinib vs placebo (nominal P < .05).
Study details: This post hoc analysis of five phase 2/3 trials included 1822 patients with moderate-to-severe AD (age ≥ 12 years) treated with 100 mg or 200 mg abrocitinib as monotherapy or in combination with topical therapy or placebo for 12 or 16 weeks.
Disclosures: This study was funded by Pfizer Inc., USA. Six authors declared being employees and stockholders of Pfizer. The other authors declared receiving research or travel grants or having other ties with various sources, including Pfizer.
Source: Thyssen JP et al. Abrocitinib provides rapid and sustained improvement in skin pain and is associated with improved quality of life outcomes in adult and adolescent patients with moderate-to-severe atopic dermatitis. Dermatology. 2023 (Dec 11). doi: 10.1159/000535285
Key clinical point: Abrocitinib as monotherapy or in combination with topical therapy improves skin pain in patients with moderate-to-severe atopic dermatitis (AD).
Major finding: Abrocitinib vs placebo led to a significantly greater dose-dependent least squares mean change in Pruritus and Symptoms Assessment for AD (PSAAD) skin pain score from baseline to as early as week 1 that were sustained through week 12 or 16 (nominal P < .05). A greater proportion of patients achieved a stringent threshold of skin pain improvement (PSAAD skin pain score < 2) with abrocitinib vs placebo (nominal P < .05).
Study details: This post hoc analysis of five phase 2/3 trials included 1822 patients with moderate-to-severe AD (age ≥ 12 years) treated with 100 mg or 200 mg abrocitinib as monotherapy or in combination with topical therapy or placebo for 12 or 16 weeks.
Disclosures: This study was funded by Pfizer Inc., USA. Six authors declared being employees and stockholders of Pfizer. The other authors declared receiving research or travel grants or having other ties with various sources, including Pfizer.
Source: Thyssen JP et al. Abrocitinib provides rapid and sustained improvement in skin pain and is associated with improved quality of life outcomes in adult and adolescent patients with moderate-to-severe atopic dermatitis. Dermatology. 2023 (Dec 11). doi: 10.1159/000535285
Allergic contact dermatitis a crucial comorbidity in atopic dermatitis
Key clinical point: Allergic contact dermatitis (ACD) is an important comorbidity in patients with atopic dermatitis (AD) and leads to the maintenance and aggravation of their dermatosis, with a high frequency of ACD observed to textile dyes, isothiazolinones, and fragrances.
Major finding: Contact sensitization was significantly associated with facial involvement (P = .04) and a longer duration of AD (P = .005). The most frequent allergen was textile dye mix (24.70%) followed by nickel (20.21%), cobalt (12.70%), and methylchlorisothiazolinone+methylisothiazolinone (8.50%). The avoidance of relevant allergens led to a significant reduction in the Scoring of Atopic Dermatitis (SCORAD) scores at 6 months (P < .001).
Study details: This longitudinal prospective study included 93 patients with AD (age > 2 years) who were patch-tested with the 2019 European baseline series and the corticosteroid series, 60.2% of whom had positive patch test results.
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Trimeche K et al. Contact allergy in atopic dermatitis: A prospective study on prevalence, incriminated allergens and clinical insights. Contact Dermatitis. 2023 (Dec 27). doi: 10.1111/cod.14494
Key clinical point: Allergic contact dermatitis (ACD) is an important comorbidity in patients with atopic dermatitis (AD) and leads to the maintenance and aggravation of their dermatosis, with a high frequency of ACD observed to textile dyes, isothiazolinones, and fragrances.
Major finding: Contact sensitization was significantly associated with facial involvement (P = .04) and a longer duration of AD (P = .005). The most frequent allergen was textile dye mix (24.70%) followed by nickel (20.21%), cobalt (12.70%), and methylchlorisothiazolinone+methylisothiazolinone (8.50%). The avoidance of relevant allergens led to a significant reduction in the Scoring of Atopic Dermatitis (SCORAD) scores at 6 months (P < .001).
Study details: This longitudinal prospective study included 93 patients with AD (age > 2 years) who were patch-tested with the 2019 European baseline series and the corticosteroid series, 60.2% of whom had positive patch test results.
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Trimeche K et al. Contact allergy in atopic dermatitis: A prospective study on prevalence, incriminated allergens and clinical insights. Contact Dermatitis. 2023 (Dec 27). doi: 10.1111/cod.14494
Key clinical point: Allergic contact dermatitis (ACD) is an important comorbidity in patients with atopic dermatitis (AD) and leads to the maintenance and aggravation of their dermatosis, with a high frequency of ACD observed to textile dyes, isothiazolinones, and fragrances.
Major finding: Contact sensitization was significantly associated with facial involvement (P = .04) and a longer duration of AD (P = .005). The most frequent allergen was textile dye mix (24.70%) followed by nickel (20.21%), cobalt (12.70%), and methylchlorisothiazolinone+methylisothiazolinone (8.50%). The avoidance of relevant allergens led to a significant reduction in the Scoring of Atopic Dermatitis (SCORAD) scores at 6 months (P < .001).
Study details: This longitudinal prospective study included 93 patients with AD (age > 2 years) who were patch-tested with the 2019 European baseline series and the corticosteroid series, 60.2% of whom had positive patch test results.
Disclosures: This study did not disclose any funding source. The authors declared no conflicts of interest.
Source: Trimeche K et al. Contact allergy in atopic dermatitis: A prospective study on prevalence, incriminated allergens and clinical insights. Contact Dermatitis. 2023 (Dec 27). doi: 10.1111/cod.14494