User login
Blood Eosinophil Counts Might Predict Childhood Asthma, Treatment Response
VIENNA — Simply relying on clinical symptoms is insufficient to predict which children with wheezing will develop asthma and respond to treatments.
Sejal Saglani, MD, PhD, a professor of pediatric respiratory medicine at the National Heart and Lung Institute, Imperial College, London, England, said that preschool wheezing has long-term adverse consequences through to adulthood. “We need to prevent that downward trajectory of low lung function,” she said, presenting the latest research in the field at the annual European Respiratory Society International Congress.
Wheezing affects up to one third of all infants and preschool children, with one third developing asthma later in life. “It’s important to identify those kids because then we can treat them with the right medication,” said Mariëlle W.H. Pijnenburg, MD, PhD, a pulmonary specialist at Erasmus University Rotterdam in the Netherlands.
“We cannot just use clinical phenotype to decide what treatment a child should get. We need to run tests to identify the endotype of preschool wheeze and intervene appropriately,” Dr. Saglani added.
Eosinophilia as a Biomarker for Predicting Exacerbations and Steroid Responsiveness
In a cluster analysis, Dr. Saglani and colleagues classified preschool children with wheezing into two main subgroups: Those who experience frequent exacerbations and those who experience sporadic attacks. Frequent exacerbators were more likely to develop asthma, use asthma medications, and show signs of reduced lung function and airway inflammation, such as higher fractional exhaled nitric oxide and allergic sensitization. “Severe and frequent exacerbators are the kids that get in trouble,” she said. “They’re the ones we must identify at preschool age and really try to minimize their exacerbations.”
Research has shown that eosinophilia is a valuable biomarker in predicting both asthma exacerbations and responsiveness to inhaled corticosteroids. Children with elevated blood eosinophils are more likely to experience frequent and severe exacerbations. These children often demonstrate an inflammatory profile more responsive to corticosteroids, making eosinophilia a predictor of treatment success. Children with eosinophilia are also more likely to have underlying allergic sensitizations, which further supports the use of corticosteroids as part of their management strategy.
Dr. Saglani said a simple blood test can provide a window into the child’s inflammatory status, allowing physicians to make more targeted and personalized treatment plans.
Traditionally, identifying eosinophilia required venipuncture and laboratory analysis, which can be time consuming and impractical in a busy clinical setting. Dr. Saglani’s research group is developing a point-of-care test designed to quickly and efficiently measure blood eosinophil levels in children with asthma or wheezing symptoms from a finger-prick test. Preliminary data presented at the congress show that children with higher eosinophil counts in the clinic were more likely to experience an asthma attack within 3 months.
“The problem is the majority of the children we see are either not atopic or do not have high blood eosinophils. What are we going to do with those?”
How to Treat Those Who Don’t Have Eosinophilia
Most children with wheezing are not atopic and do not exhibit eosinophilic inflammation, and these children may not respond as effectively to corticosteroids. How to treat them remains the “1-billion-dollar question,” Dr. Saglani said.
Respiratory syncytial virus and rhinovirus play a crucial role in triggering wheezing episodes in these children. Research has shown that viral-induced wheezing is a common feature in this phenotype, and repeated viral infections can lead to an increased severity and frequency of exacerbations. However, there are currently no effective antiviral therapies or vaccines for rhinovirus, which limits the ability to address the viral component of the disease directly.
Up to 50% of children with severe, recurrent wheezing also have bacterial pathogens like Moraxella catarrhalis and Haemophilus influenzae in their lower airways. For these children, addressing the bacterial infection is the best treatment option to mitigate the wheezing. “We now have something that we can target with antibiotics for those who don’t respond to corticosteroids,” Dr. Saglani said.
Dr. Pijnenburg said that this body of research is helping pulmonary specialists and general pediatricians navigate the complexity of childhood wheezing beyond phenotyping and symptoms. “We need to dive more deeply into those kids with preschool wheezing to see what’s happening in their lungs.”
Dr. Pijnenburg and Dr. Saglani reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
VIENNA — Simply relying on clinical symptoms is insufficient to predict which children with wheezing will develop asthma and respond to treatments.
Sejal Saglani, MD, PhD, a professor of pediatric respiratory medicine at the National Heart and Lung Institute, Imperial College, London, England, said that preschool wheezing has long-term adverse consequences through to adulthood. “We need to prevent that downward trajectory of low lung function,” she said, presenting the latest research in the field at the annual European Respiratory Society International Congress.
Wheezing affects up to one third of all infants and preschool children, with one third developing asthma later in life. “It’s important to identify those kids because then we can treat them with the right medication,” said Mariëlle W.H. Pijnenburg, MD, PhD, a pulmonary specialist at Erasmus University Rotterdam in the Netherlands.
“We cannot just use clinical phenotype to decide what treatment a child should get. We need to run tests to identify the endotype of preschool wheeze and intervene appropriately,” Dr. Saglani added.
Eosinophilia as a Biomarker for Predicting Exacerbations and Steroid Responsiveness
In a cluster analysis, Dr. Saglani and colleagues classified preschool children with wheezing into two main subgroups: Those who experience frequent exacerbations and those who experience sporadic attacks. Frequent exacerbators were more likely to develop asthma, use asthma medications, and show signs of reduced lung function and airway inflammation, such as higher fractional exhaled nitric oxide and allergic sensitization. “Severe and frequent exacerbators are the kids that get in trouble,” she said. “They’re the ones we must identify at preschool age and really try to minimize their exacerbations.”
Research has shown that eosinophilia is a valuable biomarker in predicting both asthma exacerbations and responsiveness to inhaled corticosteroids. Children with elevated blood eosinophils are more likely to experience frequent and severe exacerbations. These children often demonstrate an inflammatory profile more responsive to corticosteroids, making eosinophilia a predictor of treatment success. Children with eosinophilia are also more likely to have underlying allergic sensitizations, which further supports the use of corticosteroids as part of their management strategy.
Dr. Saglani said a simple blood test can provide a window into the child’s inflammatory status, allowing physicians to make more targeted and personalized treatment plans.
Traditionally, identifying eosinophilia required venipuncture and laboratory analysis, which can be time consuming and impractical in a busy clinical setting. Dr. Saglani’s research group is developing a point-of-care test designed to quickly and efficiently measure blood eosinophil levels in children with asthma or wheezing symptoms from a finger-prick test. Preliminary data presented at the congress show that children with higher eosinophil counts in the clinic were more likely to experience an asthma attack within 3 months.
“The problem is the majority of the children we see are either not atopic or do not have high blood eosinophils. What are we going to do with those?”
How to Treat Those Who Don’t Have Eosinophilia
Most children with wheezing are not atopic and do not exhibit eosinophilic inflammation, and these children may not respond as effectively to corticosteroids. How to treat them remains the “1-billion-dollar question,” Dr. Saglani said.
Respiratory syncytial virus and rhinovirus play a crucial role in triggering wheezing episodes in these children. Research has shown that viral-induced wheezing is a common feature in this phenotype, and repeated viral infections can lead to an increased severity and frequency of exacerbations. However, there are currently no effective antiviral therapies or vaccines for rhinovirus, which limits the ability to address the viral component of the disease directly.
Up to 50% of children with severe, recurrent wheezing also have bacterial pathogens like Moraxella catarrhalis and Haemophilus influenzae in their lower airways. For these children, addressing the bacterial infection is the best treatment option to mitigate the wheezing. “We now have something that we can target with antibiotics for those who don’t respond to corticosteroids,” Dr. Saglani said.
Dr. Pijnenburg said that this body of research is helping pulmonary specialists and general pediatricians navigate the complexity of childhood wheezing beyond phenotyping and symptoms. “We need to dive more deeply into those kids with preschool wheezing to see what’s happening in their lungs.”
Dr. Pijnenburg and Dr. Saglani reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
VIENNA — Simply relying on clinical symptoms is insufficient to predict which children with wheezing will develop asthma and respond to treatments.
Sejal Saglani, MD, PhD, a professor of pediatric respiratory medicine at the National Heart and Lung Institute, Imperial College, London, England, said that preschool wheezing has long-term adverse consequences through to adulthood. “We need to prevent that downward trajectory of low lung function,” she said, presenting the latest research in the field at the annual European Respiratory Society International Congress.
Wheezing affects up to one third of all infants and preschool children, with one third developing asthma later in life. “It’s important to identify those kids because then we can treat them with the right medication,” said Mariëlle W.H. Pijnenburg, MD, PhD, a pulmonary specialist at Erasmus University Rotterdam in the Netherlands.
“We cannot just use clinical phenotype to decide what treatment a child should get. We need to run tests to identify the endotype of preschool wheeze and intervene appropriately,” Dr. Saglani added.
Eosinophilia as a Biomarker for Predicting Exacerbations and Steroid Responsiveness
In a cluster analysis, Dr. Saglani and colleagues classified preschool children with wheezing into two main subgroups: Those who experience frequent exacerbations and those who experience sporadic attacks. Frequent exacerbators were more likely to develop asthma, use asthma medications, and show signs of reduced lung function and airway inflammation, such as higher fractional exhaled nitric oxide and allergic sensitization. “Severe and frequent exacerbators are the kids that get in trouble,” she said. “They’re the ones we must identify at preschool age and really try to minimize their exacerbations.”
Research has shown that eosinophilia is a valuable biomarker in predicting both asthma exacerbations and responsiveness to inhaled corticosteroids. Children with elevated blood eosinophils are more likely to experience frequent and severe exacerbations. These children often demonstrate an inflammatory profile more responsive to corticosteroids, making eosinophilia a predictor of treatment success. Children with eosinophilia are also more likely to have underlying allergic sensitizations, which further supports the use of corticosteroids as part of their management strategy.
Dr. Saglani said a simple blood test can provide a window into the child’s inflammatory status, allowing physicians to make more targeted and personalized treatment plans.
Traditionally, identifying eosinophilia required venipuncture and laboratory analysis, which can be time consuming and impractical in a busy clinical setting. Dr. Saglani’s research group is developing a point-of-care test designed to quickly and efficiently measure blood eosinophil levels in children with asthma or wheezing symptoms from a finger-prick test. Preliminary data presented at the congress show that children with higher eosinophil counts in the clinic were more likely to experience an asthma attack within 3 months.
“The problem is the majority of the children we see are either not atopic or do not have high blood eosinophils. What are we going to do with those?”
How to Treat Those Who Don’t Have Eosinophilia
Most children with wheezing are not atopic and do not exhibit eosinophilic inflammation, and these children may not respond as effectively to corticosteroids. How to treat them remains the “1-billion-dollar question,” Dr. Saglani said.
Respiratory syncytial virus and rhinovirus play a crucial role in triggering wheezing episodes in these children. Research has shown that viral-induced wheezing is a common feature in this phenotype, and repeated viral infections can lead to an increased severity and frequency of exacerbations. However, there are currently no effective antiviral therapies or vaccines for rhinovirus, which limits the ability to address the viral component of the disease directly.
Up to 50% of children with severe, recurrent wheezing also have bacterial pathogens like Moraxella catarrhalis and Haemophilus influenzae in their lower airways. For these children, addressing the bacterial infection is the best treatment option to mitigate the wheezing. “We now have something that we can target with antibiotics for those who don’t respond to corticosteroids,” Dr. Saglani said.
Dr. Pijnenburg said that this body of research is helping pulmonary specialists and general pediatricians navigate the complexity of childhood wheezing beyond phenotyping and symptoms. “We need to dive more deeply into those kids with preschool wheezing to see what’s happening in their lungs.”
Dr. Pijnenburg and Dr. Saglani reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Pulmonology Data Trends 2024
Pulmonology Data Trends 2024 is a supplement to CHEST Physician highlighting the latest breakthroughs in pulmonology research and treatments through a series of infographics.
Read more:
Artificial Intelligence in Sleep Apnea
Ritwick Agrawal, MD, MS, FCCP
RSV Updates: Prophylaxis Approval and Hospitalization for Severe RSV
Riddhi Upadhyay, MD
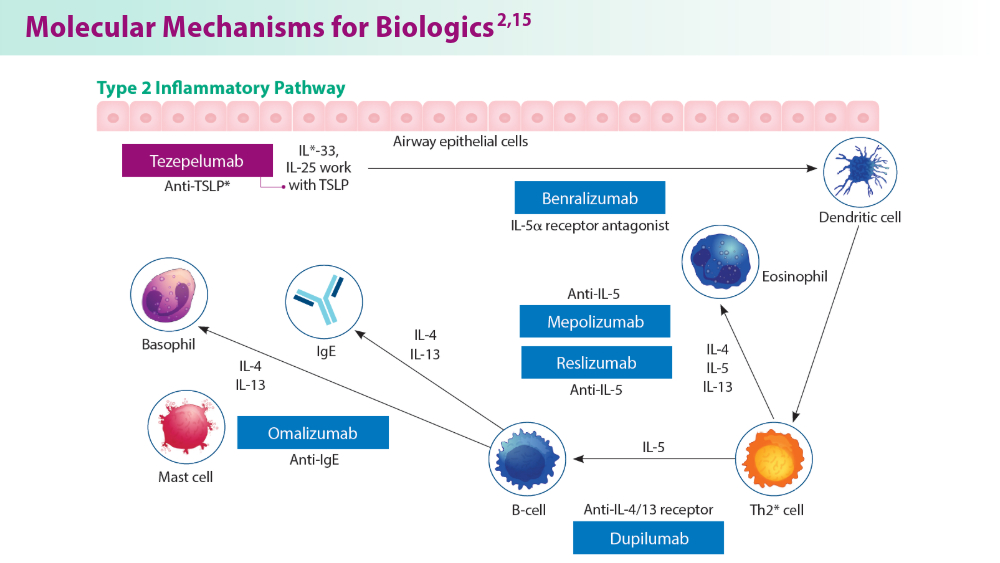
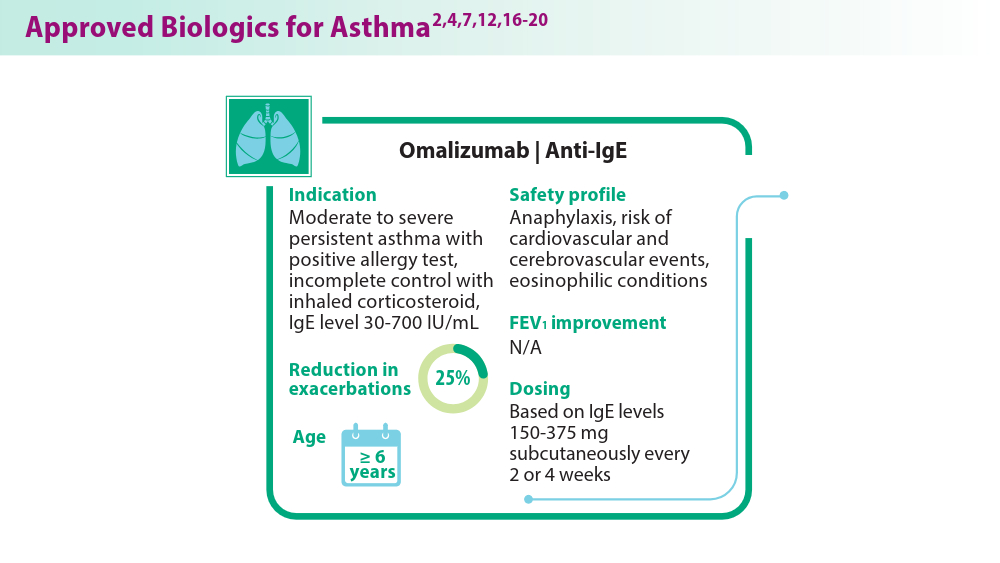
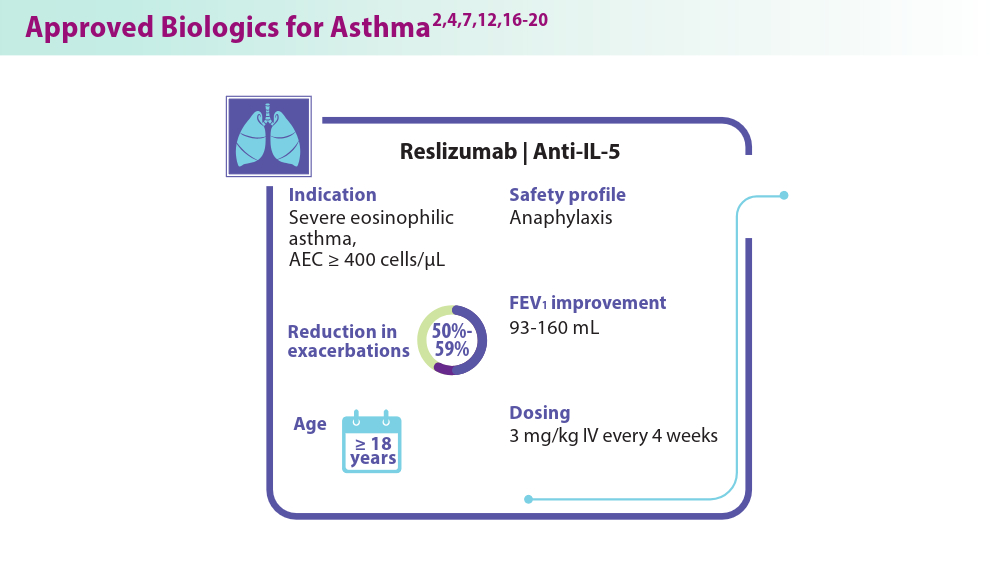
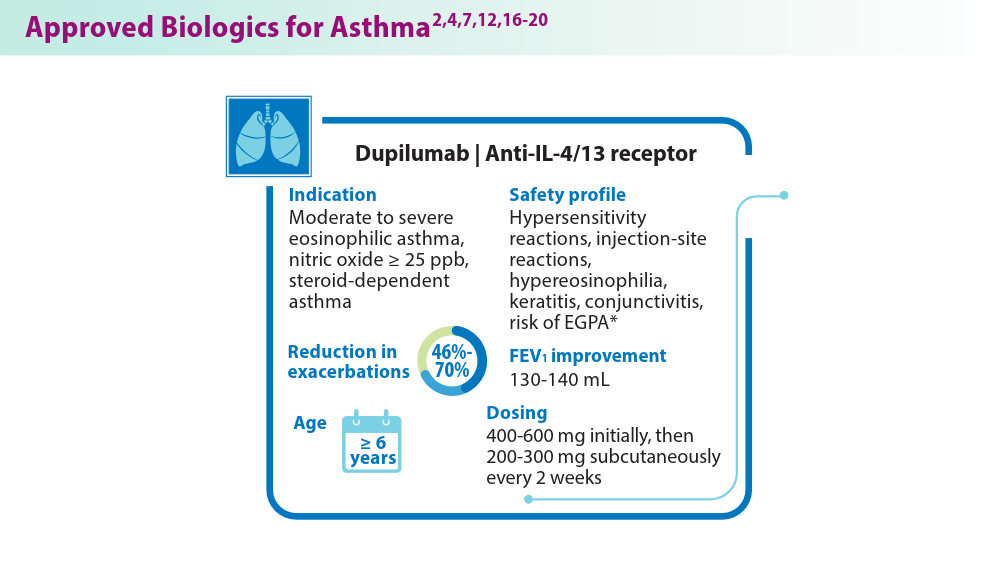
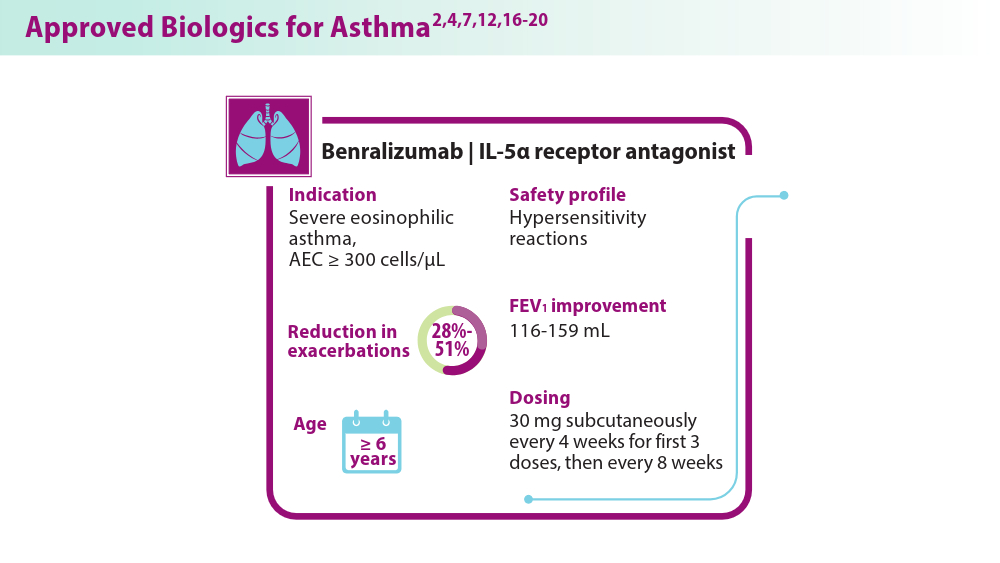
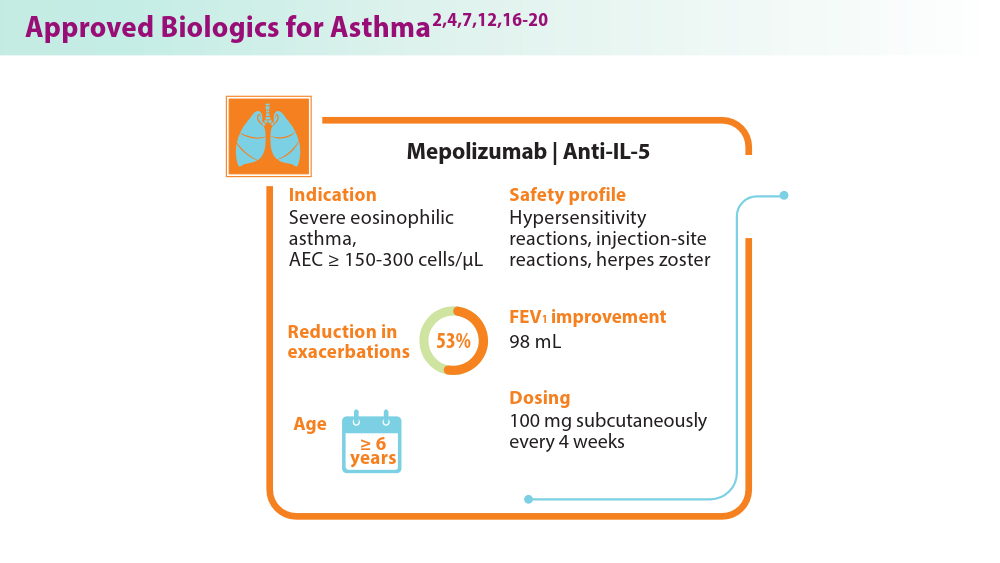
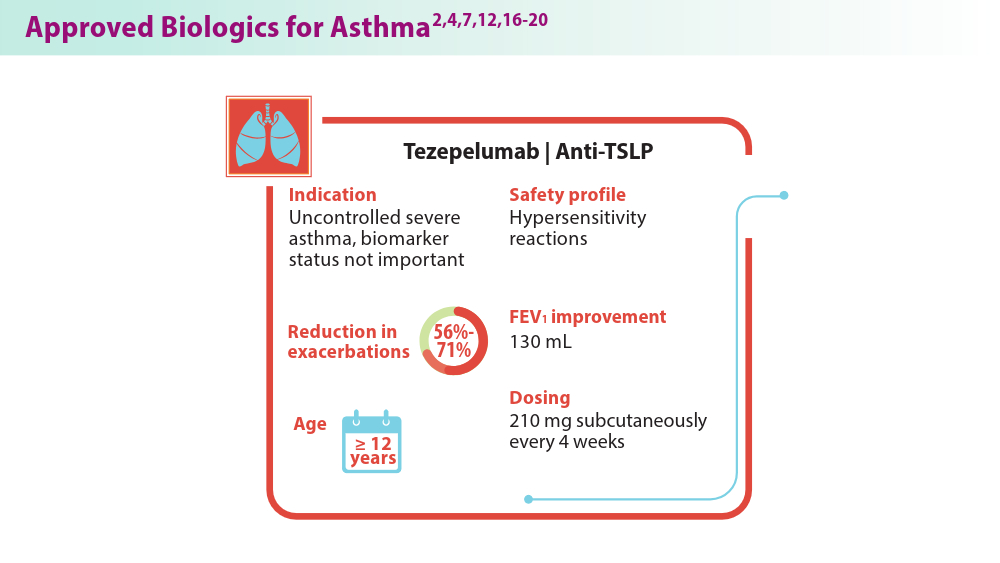
Biologics in Asthma: Changing the Severe Asthma Paradigm
Shyam Subramanian, MD, FCCP
Updates in COPD Guidelines and Treatment
Dharani K. Narendra, MD, FCCP
Targeted Therapies and Surgical Resection for Lung Cancer: Evolving Treatment Options
Saadia A. Faiz, MD, FCCP
Closing the GAP in Idiopathic Pulmonary Fibrosis
Humayun Anjum, MD, FCCP
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
Sujith V. Cherian, MD, FCCP
Pulmonary Hypertension: Comorbidities and Novel Therapies
Mary Jo S. Farmer, MD, PhD, FCCP
The Genetic Side of Interstitial Lung Disease
Priya Balakrishnan, MD, MS, FCCP
Noninvasive Ventilation in Neuromuscular Disease
Sreelatha Naik, MD, FCCP, and Kelly Lobrutto, CRNP
Pulmonology Data Trends 2024 is a supplement to CHEST Physician highlighting the latest breakthroughs in pulmonology research and treatments through a series of infographics.
Read more:
Artificial Intelligence in Sleep Apnea
Ritwick Agrawal, MD, MS, FCCP
RSV Updates: Prophylaxis Approval and Hospitalization for Severe RSV
Riddhi Upadhyay, MD
Biologics in Asthma: Changing the Severe Asthma Paradigm
Shyam Subramanian, MD, FCCP
Updates in COPD Guidelines and Treatment
Dharani K. Narendra, MD, FCCP
Targeted Therapies and Surgical Resection for Lung Cancer: Evolving Treatment Options
Saadia A. Faiz, MD, FCCP
Closing the GAP in Idiopathic Pulmonary Fibrosis
Humayun Anjum, MD, FCCP
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
Sujith V. Cherian, MD, FCCP
Pulmonary Hypertension: Comorbidities and Novel Therapies
Mary Jo S. Farmer, MD, PhD, FCCP
The Genetic Side of Interstitial Lung Disease
Priya Balakrishnan, MD, MS, FCCP
Noninvasive Ventilation in Neuromuscular Disease
Sreelatha Naik, MD, FCCP, and Kelly Lobrutto, CRNP
Pulmonology Data Trends 2024 is a supplement to CHEST Physician highlighting the latest breakthroughs in pulmonology research and treatments through a series of infographics.
Read more:
Artificial Intelligence in Sleep Apnea
Ritwick Agrawal, MD, MS, FCCP
RSV Updates: Prophylaxis Approval and Hospitalization for Severe RSV
Riddhi Upadhyay, MD
Biologics in Asthma: Changing the Severe Asthma Paradigm
Shyam Subramanian, MD, FCCP
Updates in COPD Guidelines and Treatment
Dharani K. Narendra, MD, FCCP
Targeted Therapies and Surgical Resection for Lung Cancer: Evolving Treatment Options
Saadia A. Faiz, MD, FCCP
Closing the GAP in Idiopathic Pulmonary Fibrosis
Humayun Anjum, MD, FCCP
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
Sujith V. Cherian, MD, FCCP
Pulmonary Hypertension: Comorbidities and Novel Therapies
Mary Jo S. Farmer, MD, PhD, FCCP
The Genetic Side of Interstitial Lung Disease
Priya Balakrishnan, MD, MS, FCCP
Noninvasive Ventilation in Neuromuscular Disease
Sreelatha Naik, MD, FCCP, and Kelly Lobrutto, CRNP
Biologics in Asthma: Changing the Severe Asthma Paradigm
- Shah PA, Brightling C. Biologics for severe asthma—which, when and why? Respirology. 2023;28(8):709-721. doi:10.1111/resp.14520
- Rogers L, Jesenak M, Bjermer L, Hanania NA, Seys SF, Diamant Z. Biologics in severe asthma: a pragmatic approach for choosing the right treatment for the right patient. Respir Med. 2023;218:107414. doi:10.1016/j.rmed.2023.107414
- Frøssing L, Silberbrandt A, Von Bülow A, Backer V, Porsbjerg C. The Prevalence of Subtypes of Type 2 Inflammation in an Unselected Population of Patients with Severe Asthma. J Allergy Clin Immunol Pract. 2021;9(3):1267-1275. doi:10.1016/j.jaip.2020.09.051
- McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199(4):433-445. doi:10.1164/rccm.201810-1944CI
- d'Ancona G, Kavanagh J, Roxas C, et al. Adherence to corticosteroids and clinical outcomes in mepolizumab therapy for severe asthma. Eur Respir J. 2020;55(5):1902259. Published 2020 May 7. doi:10.1183/13993003.02259-2019
- Exacerbation reduction & other clinical information | TEZSPIRE® (tezepelumab-Ekko) for hcps. Accessed July 25, 2024. https://www.tezspirehcp.com/efficacy-and-clinical-data/exacerbation-reductions-and-clinical-in-formation.html
- Exacerbation reduction in patients 12+ years. DUPIXENT® (dupilumab) for healthcare providers. Accessed June 18, 2024. https://www.dupixenthcp.com/asthma/efficacy/exacerbations
- Korn S, Bourdin A, Chupp G, et al. Integrated Safety and Efficacy Among Patients Receiving Benralizumab for Up to 5 Years. J Allergy Clin Immunol Pract. 2021;9(12):4381-4392.e4. doi:10.1016/j.jaip.2021.07.058
- Jackson DJ, Heaney LG, Humbert M, et al; for the SHAMAL Investigators. Reduction of daily maintenance inhaled corticosteroids in patients with severe eosinophilic asthma treated with benralizumab (SHAMAL): a randomised, multicentre, open-label, phase 4 study [published correction appears in Lancet. 2024;403(10432):1140]. Lancet. 2024;403(10423):271-281. doi:10.1016/S0140-6736(23)02284-5
- Thomas D, McDonald VM, Stevens S, et al. Biologics (mepolizumab and omalizumab) induced remission in severe asthma patients. Allergy. 2024;79(2):384-392. doi:10.1111/all.15867
- Hansen S, Baastrup Søndergaard M, von Bülow A, et al. Clinical response and remission in patients with severe asthma treated with biologic therapies. Chest. 2024;165(2):253-266. doi:10.1016/j.chest.2023.10.046
- Bagnasco D, Savarino EV, Yacoub MR, et al. Personalized and precision medicine in asthma and eosinophilic esophagitis: the role of T2 target therapy. Pharmaceutics. 2023;15(9):2359. doi:10.3390/pharmaceutics15092359
- Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry [published correction appears in Chest. 2021;160(5):1989.]. Chest. 2020;157(4):790-804. doi:10.1016/j.chest.2019.10.053
- Inselman JW, Jeffery MM, Maddux JT, Shah NS, Rank MA. Trends and Disparities in Asthma Biologic Use in the United States. J Allergy Clin Immunol Pract. 2020;8(2):549-554.e1. doi:10.1016/j.jaip.2019.08.024
- Pelaia C, Crimi C, Vatrella A, Tinello C, Terracciano R, Pelaia G. Molecular targets for biological therapies of severe asthma. Front Immunol. 2020;11:603312. doi:10.3389/fimmu.2020.603312
- Biologics for the treatment of asthma. Asthma and Allergy Foundation of America. Reviewed November 2023. Accessed June 18, 2024. https://aafa.org/asthma/asthma-treatment/biologics-asthma-treatment/
- Safety profile. TEZSPIRE® (tezepelumab-ekko) for healthcare providers. Accessed June 18, 2024. https://www.tezspirehcp.com/safety-profile.html
- Nucala (mepolizumab) for hcps. Severe Eosinophilic Asthma | NUCALA (mepolizumab) for HCPs. Accessed August 1, 2024. https://nucalahcp.com/severe-eosinophilic-asthma/.
- Xolair® (omalizumab). xolair. Accessed August 1, 2024. https://www.xolairhcp.com/allergic-asthma/side-effects/summary.html.
- Cinqair. Cinqairhcp.com. Accessed August 1, 2024. https://www.cinqairhcp.com/efficacy-and-safety-profiles/.
- Shah PA, Brightling C. Biologics for severe asthma—which, when and why? Respirology. 2023;28(8):709-721. doi:10.1111/resp.14520
- Rogers L, Jesenak M, Bjermer L, Hanania NA, Seys SF, Diamant Z. Biologics in severe asthma: a pragmatic approach for choosing the right treatment for the right patient. Respir Med. 2023;218:107414. doi:10.1016/j.rmed.2023.107414
- Frøssing L, Silberbrandt A, Von Bülow A, Backer V, Porsbjerg C. The Prevalence of Subtypes of Type 2 Inflammation in an Unselected Population of Patients with Severe Asthma. J Allergy Clin Immunol Pract. 2021;9(3):1267-1275. doi:10.1016/j.jaip.2020.09.051
- McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199(4):433-445. doi:10.1164/rccm.201810-1944CI
- d'Ancona G, Kavanagh J, Roxas C, et al. Adherence to corticosteroids and clinical outcomes in mepolizumab therapy for severe asthma. Eur Respir J. 2020;55(5):1902259. Published 2020 May 7. doi:10.1183/13993003.02259-2019
- Exacerbation reduction & other clinical information | TEZSPIRE® (tezepelumab-Ekko) for hcps. Accessed July 25, 2024. https://www.tezspirehcp.com/efficacy-and-clinical-data/exacerbation-reductions-and-clinical-in-formation.html
- Exacerbation reduction in patients 12+ years. DUPIXENT® (dupilumab) for healthcare providers. Accessed June 18, 2024. https://www.dupixenthcp.com/asthma/efficacy/exacerbations
- Korn S, Bourdin A, Chupp G, et al. Integrated Safety and Efficacy Among Patients Receiving Benralizumab for Up to 5 Years. J Allergy Clin Immunol Pract. 2021;9(12):4381-4392.e4. doi:10.1016/j.jaip.2021.07.058
- Jackson DJ, Heaney LG, Humbert M, et al; for the SHAMAL Investigators. Reduction of daily maintenance inhaled corticosteroids in patients with severe eosinophilic asthma treated with benralizumab (SHAMAL): a randomised, multicentre, open-label, phase 4 study [published correction appears in Lancet. 2024;403(10432):1140]. Lancet. 2024;403(10423):271-281. doi:10.1016/S0140-6736(23)02284-5
- Thomas D, McDonald VM, Stevens S, et al. Biologics (mepolizumab and omalizumab) induced remission in severe asthma patients. Allergy. 2024;79(2):384-392. doi:10.1111/all.15867
- Hansen S, Baastrup Søndergaard M, von Bülow A, et al. Clinical response and remission in patients with severe asthma treated with biologic therapies. Chest. 2024;165(2):253-266. doi:10.1016/j.chest.2023.10.046
- Bagnasco D, Savarino EV, Yacoub MR, et al. Personalized and precision medicine in asthma and eosinophilic esophagitis: the role of T2 target therapy. Pharmaceutics. 2023;15(9):2359. doi:10.3390/pharmaceutics15092359
- Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry [published correction appears in Chest. 2021;160(5):1989.]. Chest. 2020;157(4):790-804. doi:10.1016/j.chest.2019.10.053
- Inselman JW, Jeffery MM, Maddux JT, Shah NS, Rank MA. Trends and Disparities in Asthma Biologic Use in the United States. J Allergy Clin Immunol Pract. 2020;8(2):549-554.e1. doi:10.1016/j.jaip.2019.08.024
- Pelaia C, Crimi C, Vatrella A, Tinello C, Terracciano R, Pelaia G. Molecular targets for biological therapies of severe asthma. Front Immunol. 2020;11:603312. doi:10.3389/fimmu.2020.603312
- Biologics for the treatment of asthma. Asthma and Allergy Foundation of America. Reviewed November 2023. Accessed June 18, 2024. https://aafa.org/asthma/asthma-treatment/biologics-asthma-treatment/
- Safety profile. TEZSPIRE® (tezepelumab-ekko) for healthcare providers. Accessed June 18, 2024. https://www.tezspirehcp.com/safety-profile.html
- Nucala (mepolizumab) for hcps. Severe Eosinophilic Asthma | NUCALA (mepolizumab) for HCPs. Accessed August 1, 2024. https://nucalahcp.com/severe-eosinophilic-asthma/.
- Xolair® (omalizumab). xolair. Accessed August 1, 2024. https://www.xolairhcp.com/allergic-asthma/side-effects/summary.html.
- Cinqair. Cinqairhcp.com. Accessed August 1, 2024. https://www.cinqairhcp.com/efficacy-and-safety-profiles/.
- Shah PA, Brightling C. Biologics for severe asthma—which, when and why? Respirology. 2023;28(8):709-721. doi:10.1111/resp.14520
- Rogers L, Jesenak M, Bjermer L, Hanania NA, Seys SF, Diamant Z. Biologics in severe asthma: a pragmatic approach for choosing the right treatment for the right patient. Respir Med. 2023;218:107414. doi:10.1016/j.rmed.2023.107414
- Frøssing L, Silberbrandt A, Von Bülow A, Backer V, Porsbjerg C. The Prevalence of Subtypes of Type 2 Inflammation in an Unselected Population of Patients with Severe Asthma. J Allergy Clin Immunol Pract. 2021;9(3):1267-1275. doi:10.1016/j.jaip.2020.09.051
- McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199(4):433-445. doi:10.1164/rccm.201810-1944CI
- d'Ancona G, Kavanagh J, Roxas C, et al. Adherence to corticosteroids and clinical outcomes in mepolizumab therapy for severe asthma. Eur Respir J. 2020;55(5):1902259. Published 2020 May 7. doi:10.1183/13993003.02259-2019
- Exacerbation reduction & other clinical information | TEZSPIRE® (tezepelumab-Ekko) for hcps. Accessed July 25, 2024. https://www.tezspirehcp.com/efficacy-and-clinical-data/exacerbation-reductions-and-clinical-in-formation.html
- Exacerbation reduction in patients 12+ years. DUPIXENT® (dupilumab) for healthcare providers. Accessed June 18, 2024. https://www.dupixenthcp.com/asthma/efficacy/exacerbations
- Korn S, Bourdin A, Chupp G, et al. Integrated Safety and Efficacy Among Patients Receiving Benralizumab for Up to 5 Years. J Allergy Clin Immunol Pract. 2021;9(12):4381-4392.e4. doi:10.1016/j.jaip.2021.07.058
- Jackson DJ, Heaney LG, Humbert M, et al; for the SHAMAL Investigators. Reduction of daily maintenance inhaled corticosteroids in patients with severe eosinophilic asthma treated with benralizumab (SHAMAL): a randomised, multicentre, open-label, phase 4 study [published correction appears in Lancet. 2024;403(10432):1140]. Lancet. 2024;403(10423):271-281. doi:10.1016/S0140-6736(23)02284-5
- Thomas D, McDonald VM, Stevens S, et al. Biologics (mepolizumab and omalizumab) induced remission in severe asthma patients. Allergy. 2024;79(2):384-392. doi:10.1111/all.15867
- Hansen S, Baastrup Søndergaard M, von Bülow A, et al. Clinical response and remission in patients with severe asthma treated with biologic therapies. Chest. 2024;165(2):253-266. doi:10.1016/j.chest.2023.10.046
- Bagnasco D, Savarino EV, Yacoub MR, et al. Personalized and precision medicine in asthma and eosinophilic esophagitis: the role of T2 target therapy. Pharmaceutics. 2023;15(9):2359. doi:10.3390/pharmaceutics15092359
- Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry [published correction appears in Chest. 2021;160(5):1989.]. Chest. 2020;157(4):790-804. doi:10.1016/j.chest.2019.10.053
- Inselman JW, Jeffery MM, Maddux JT, Shah NS, Rank MA. Trends and Disparities in Asthma Biologic Use in the United States. J Allergy Clin Immunol Pract. 2020;8(2):549-554.e1. doi:10.1016/j.jaip.2019.08.024
- Pelaia C, Crimi C, Vatrella A, Tinello C, Terracciano R, Pelaia G. Molecular targets for biological therapies of severe asthma. Front Immunol. 2020;11:603312. doi:10.3389/fimmu.2020.603312
- Biologics for the treatment of asthma. Asthma and Allergy Foundation of America. Reviewed November 2023. Accessed June 18, 2024. https://aafa.org/asthma/asthma-treatment/biologics-asthma-treatment/
- Safety profile. TEZSPIRE® (tezepelumab-ekko) for healthcare providers. Accessed June 18, 2024. https://www.tezspirehcp.com/safety-profile.html
- Nucala (mepolizumab) for hcps. Severe Eosinophilic Asthma | NUCALA (mepolizumab) for HCPs. Accessed August 1, 2024. https://nucalahcp.com/severe-eosinophilic-asthma/.
- Xolair® (omalizumab). xolair. Accessed August 1, 2024. https://www.xolairhcp.com/allergic-asthma/side-effects/summary.html.
- Cinqair. Cinqairhcp.com. Accessed August 1, 2024. https://www.cinqairhcp.com/efficacy-and-safety-profiles/.
Wildfire Pollution May Increase Asthma Hospitalizations
Short-term increases in fine particulate matter (PM2.5) resulting from wildfire smoke are becoming a greater global problem and have been associated with poor asthma and COPD outcomes, wrote Benjamin D. Horne, PhD, of the Intermountain Medical Center Heart Institute, Salt Lake City, Utah, and colleagues. However, the effect of short-term increases in PM2.5 on hospitalizations for asthma and COPD has not been well studied, they noted.
“Our primary reason for studying the association of air pollution in the summer/fall wildfire season separately from the winter is that the drought conditions in the western United States from 2012-2022 resulted in more wildfires and increasingly large wildfires across the west,” Dr. Horne said in an interview. “In part, this provided a chance to measure an increase of fine particulate matter (PM2.5) air pollution from wildfires and also to track what happened to their health when people were exposed to the PM2.5 from wildfire,” he said.
During 2020-2022, the PM2.5 produced during the wildfire season exceeded the PM2.5 levels measured in the winter for the first time, Dr. Horne said. In the part of Utah where the study was conducted, PM2.5 increases in winter because of a combination of concentrated PM2.5 from cars and industry and a weather phenomenon known as a temperature inversion, he said.
A temperature inversion occurs when mountain topography traps pollutants near the ground where the people are, but only during times of cold and snowy weather, Dr. Horne said.
“Past studies in the region were conducted with the assumption that the winter inversion was the primary source of pollution-related health risks, and public and healthcare guidance for health was based on avoiding winter air pollution,” Dr. Horne noted. However, “it may be that the smoke from wildfires requires people to also anticipate how to avoid exposure to PM2.5 during the summer,” he said.
In a study published in CHEST Pulmonary, the researchers reviewed data from 63,976 patients hospitalized with asthma and 18,514 hospitalized with COPD between January 1999 and March 2022 who lived in an area of Utah in which PM2.5 and ozone are measured by the Environmental Protection Agency. The average age of the asthma patients was 22.6 years; 51.0% were women, 16.0% had hypertension, and 10.1% had a history of smoking. The average age of the COPD patients was 63.5 years, 50.3% were women, 69.1% had hypertension, and 42.3% had a history of smoking.
In a regression analysis, the risk for asthma was significantly associated with days of increased PM2.5 during wildfire season and similar to the winter inversion (when cold air traps air pollutants), with odds ratios (ORs) of 1.057 and 1.023 for every 10 µg per m3 of particulate matter, respectively.
Although the risk for asthma hospitalization decreased after a week, a rebound occurred during wildfire season after a 4-week lag, with an OR of 1.098 for every 10 µg per m3 of particulate matter, the researchers wrote. A review of all months showed a significant association between a concurrent day increase in PM2.5 and asthma hospitalization (OR, 1.020 per every 10 µg per m3 of particulate matter, P = .0006).
By contrast, PM2.5 increases had only a weak association with hospitalizations for COPD during either wildfire season or winter inversion season, and ozone was not associated with increased risks for patients with asthma or COPD.
The findings were limited by several factors including the observational design, potential for confounding, and relatively homogeneous study population, the researchers noted.
However, “these findings suggest that people should be aware of the risks from wildfire-generated PM2.5 during the summer and fall, including following best practices for people with asthma such as anticipating symptoms in warm months, carrying medications during summer activities, and expecting to stay indoors to avoid smoke exposure when wildfires have polluted the outdoor air,” Dr. Horne told this news organization.
In the current study, Dr. Horne and colleagues expected to see increases in the risk for asthma and COPD during summer wildfire season. “What was surprising was that the size of the risk of needing care of asthma appeared to occur just as rapidly after the PM2.5 became elevated during wildfire events as it did in the winter,” said Dr. Horne. “Further, the risk in the summer appeared to be greater than during the winter. Increases in hospitalization for asthma occurred on the same day and throughout the first week after a rise in air pollution in summer and early fall, and especially in children that risk remained increased for up to a month after the rise in air pollution,” he said.
Clinicians should be aware of environmental sources of respiratory declines caused by wildfire smoke that may prompt patients to seek care during wildfire events, said Horne. Finally, the general population should recognize the smell of smoke during warm months as an alert that leads to greater caution about spending time outdoors during wildfire events, he said. “Short-term PM2.5 elevations may affect respiratory health and have other effects such as on heart health,” Dr. Horne said. “In general, people should avoid outdoor exercise when air pollution is elevated, since the amount of air that is breathed in during exercise is substantially increased,” he added.
“Further research is needed regarding the mechanisms of effect from PM2.5 on health risk, including effects on respiratory and cardiovascular health,” said Dr. Horne. “This includes evaluating what biomarkers in the blood are changed by air pollution such as inflammatory factors, determining whether some medications may block or reduce the adverse effects of air pollution, and examining whether masks or indoor air purifiers have a meaningful benefit in protecting health during short-term air pollution elevations,” he said.
Data Reveal Respiratory Impact of Wildfires
“Fine particle air pollution has been linked to poor respiratory health outcomes, but relatively little is known about the specific impact of wildfire particulate pollution on patients living in urban population centers,” Alexander S. Rabin, MD, of the University of Michigan, Ann Arbor, said in an interview.
“Although it is known that wildfire risk is increasing throughout the western United States, the increase in the number of days per month with elevated fine particulate matter from 1999 to 2022 was striking,” said Dr. Rabin, who was not involved in the current study. “Over the same period, there was a decrease in the number of high fine particulate matter air pollution days related to the wintertime temperature inversion phenomenon when air pollutants are trapped in Utah’s valleys,” he said. “These data underscore the increased risk of wildfire-related air pollution relative to ‘traditional sources of air pollution from industrial and transportation sources,” he added.
Although the adverse effects of exposure to wildfire smoke and inversion season pollution on asthma were not unexpected, the degree of the effect size of wildfire smoke relative to inversion season was surprising, said Dr. Rabin.
“Why the wildfire smoke seems to have a worse impact on asthma outcomes could not be determined from this study, but there may be something inherently more dangerous about the cocktail of pollutants released when large wildfires burn uncontrolled,” he said. “I was surprised by the lack of association between wildfire smoke and adverse COPD outcomes; whether this relates to physiological differences or variations in healthcare-seeking behaviors between patients with asthma vs COPD is unknown,” he added.
The current study underscores the harmful effects of fine particulate pollution from wildfire smoke on health, and the increased risk for hospitalization for those with asthma even in urban environments far from the source of the fire, Dr. Rabin said.
However, limitations include the use of estimates of fine particulate pollution taken from monitoring stations that were an average of 14 km from the participants’ primary residences, and air quality measurements may not have accurately reflected exposure, Dr. Rabin noted. “Additionally, the population studied was not reflective of the US population, with approximately 80% of study participants described as non-Hispanic white,” he said. “Patients of color may have increased vulnerability to adverse outcomes from air pollution and therefore additional study is needed in these populations,” Dr. Rabin added.
The study was supported in part by the AIRHEALTH program project and by internal institutional funds. Dr. Horne disclosed serving on the advisory board of Opsis Health, previously consulting for Pfizer regarding risk scores and serving as site principal investigator of a grant funded by the Task Force for Global Health and a grant from the Patient-Centered Outcomes Research Institute and the NIH-funded RECOVER initiative. Dr. Rabin had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Short-term increases in fine particulate matter (PM2.5) resulting from wildfire smoke are becoming a greater global problem and have been associated with poor asthma and COPD outcomes, wrote Benjamin D. Horne, PhD, of the Intermountain Medical Center Heart Institute, Salt Lake City, Utah, and colleagues. However, the effect of short-term increases in PM2.5 on hospitalizations for asthma and COPD has not been well studied, they noted.
“Our primary reason for studying the association of air pollution in the summer/fall wildfire season separately from the winter is that the drought conditions in the western United States from 2012-2022 resulted in more wildfires and increasingly large wildfires across the west,” Dr. Horne said in an interview. “In part, this provided a chance to measure an increase of fine particulate matter (PM2.5) air pollution from wildfires and also to track what happened to their health when people were exposed to the PM2.5 from wildfire,” he said.
During 2020-2022, the PM2.5 produced during the wildfire season exceeded the PM2.5 levels measured in the winter for the first time, Dr. Horne said. In the part of Utah where the study was conducted, PM2.5 increases in winter because of a combination of concentrated PM2.5 from cars and industry and a weather phenomenon known as a temperature inversion, he said.
A temperature inversion occurs when mountain topography traps pollutants near the ground where the people are, but only during times of cold and snowy weather, Dr. Horne said.
“Past studies in the region were conducted with the assumption that the winter inversion was the primary source of pollution-related health risks, and public and healthcare guidance for health was based on avoiding winter air pollution,” Dr. Horne noted. However, “it may be that the smoke from wildfires requires people to also anticipate how to avoid exposure to PM2.5 during the summer,” he said.
In a study published in CHEST Pulmonary, the researchers reviewed data from 63,976 patients hospitalized with asthma and 18,514 hospitalized with COPD between January 1999 and March 2022 who lived in an area of Utah in which PM2.5 and ozone are measured by the Environmental Protection Agency. The average age of the asthma patients was 22.6 years; 51.0% were women, 16.0% had hypertension, and 10.1% had a history of smoking. The average age of the COPD patients was 63.5 years, 50.3% were women, 69.1% had hypertension, and 42.3% had a history of smoking.
In a regression analysis, the risk for asthma was significantly associated with days of increased PM2.5 during wildfire season and similar to the winter inversion (when cold air traps air pollutants), with odds ratios (ORs) of 1.057 and 1.023 for every 10 µg per m3 of particulate matter, respectively.
Although the risk for asthma hospitalization decreased after a week, a rebound occurred during wildfire season after a 4-week lag, with an OR of 1.098 for every 10 µg per m3 of particulate matter, the researchers wrote. A review of all months showed a significant association between a concurrent day increase in PM2.5 and asthma hospitalization (OR, 1.020 per every 10 µg per m3 of particulate matter, P = .0006).
By contrast, PM2.5 increases had only a weak association with hospitalizations for COPD during either wildfire season or winter inversion season, and ozone was not associated with increased risks for patients with asthma or COPD.
The findings were limited by several factors including the observational design, potential for confounding, and relatively homogeneous study population, the researchers noted.
However, “these findings suggest that people should be aware of the risks from wildfire-generated PM2.5 during the summer and fall, including following best practices for people with asthma such as anticipating symptoms in warm months, carrying medications during summer activities, and expecting to stay indoors to avoid smoke exposure when wildfires have polluted the outdoor air,” Dr. Horne told this news organization.
In the current study, Dr. Horne and colleagues expected to see increases in the risk for asthma and COPD during summer wildfire season. “What was surprising was that the size of the risk of needing care of asthma appeared to occur just as rapidly after the PM2.5 became elevated during wildfire events as it did in the winter,” said Dr. Horne. “Further, the risk in the summer appeared to be greater than during the winter. Increases in hospitalization for asthma occurred on the same day and throughout the first week after a rise in air pollution in summer and early fall, and especially in children that risk remained increased for up to a month after the rise in air pollution,” he said.
Clinicians should be aware of environmental sources of respiratory declines caused by wildfire smoke that may prompt patients to seek care during wildfire events, said Horne. Finally, the general population should recognize the smell of smoke during warm months as an alert that leads to greater caution about spending time outdoors during wildfire events, he said. “Short-term PM2.5 elevations may affect respiratory health and have other effects such as on heart health,” Dr. Horne said. “In general, people should avoid outdoor exercise when air pollution is elevated, since the amount of air that is breathed in during exercise is substantially increased,” he added.
“Further research is needed regarding the mechanisms of effect from PM2.5 on health risk, including effects on respiratory and cardiovascular health,” said Dr. Horne. “This includes evaluating what biomarkers in the blood are changed by air pollution such as inflammatory factors, determining whether some medications may block or reduce the adverse effects of air pollution, and examining whether masks or indoor air purifiers have a meaningful benefit in protecting health during short-term air pollution elevations,” he said.
Data Reveal Respiratory Impact of Wildfires
“Fine particle air pollution has been linked to poor respiratory health outcomes, but relatively little is known about the specific impact of wildfire particulate pollution on patients living in urban population centers,” Alexander S. Rabin, MD, of the University of Michigan, Ann Arbor, said in an interview.
“Although it is known that wildfire risk is increasing throughout the western United States, the increase in the number of days per month with elevated fine particulate matter from 1999 to 2022 was striking,” said Dr. Rabin, who was not involved in the current study. “Over the same period, there was a decrease in the number of high fine particulate matter air pollution days related to the wintertime temperature inversion phenomenon when air pollutants are trapped in Utah’s valleys,” he said. “These data underscore the increased risk of wildfire-related air pollution relative to ‘traditional sources of air pollution from industrial and transportation sources,” he added.
Although the adverse effects of exposure to wildfire smoke and inversion season pollution on asthma were not unexpected, the degree of the effect size of wildfire smoke relative to inversion season was surprising, said Dr. Rabin.
“Why the wildfire smoke seems to have a worse impact on asthma outcomes could not be determined from this study, but there may be something inherently more dangerous about the cocktail of pollutants released when large wildfires burn uncontrolled,” he said. “I was surprised by the lack of association between wildfire smoke and adverse COPD outcomes; whether this relates to physiological differences or variations in healthcare-seeking behaviors between patients with asthma vs COPD is unknown,” he added.
The current study underscores the harmful effects of fine particulate pollution from wildfire smoke on health, and the increased risk for hospitalization for those with asthma even in urban environments far from the source of the fire, Dr. Rabin said.
However, limitations include the use of estimates of fine particulate pollution taken from monitoring stations that were an average of 14 km from the participants’ primary residences, and air quality measurements may not have accurately reflected exposure, Dr. Rabin noted. “Additionally, the population studied was not reflective of the US population, with approximately 80% of study participants described as non-Hispanic white,” he said. “Patients of color may have increased vulnerability to adverse outcomes from air pollution and therefore additional study is needed in these populations,” Dr. Rabin added.
The study was supported in part by the AIRHEALTH program project and by internal institutional funds. Dr. Horne disclosed serving on the advisory board of Opsis Health, previously consulting for Pfizer regarding risk scores and serving as site principal investigator of a grant funded by the Task Force for Global Health and a grant from the Patient-Centered Outcomes Research Institute and the NIH-funded RECOVER initiative. Dr. Rabin had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Short-term increases in fine particulate matter (PM2.5) resulting from wildfire smoke are becoming a greater global problem and have been associated with poor asthma and COPD outcomes, wrote Benjamin D. Horne, PhD, of the Intermountain Medical Center Heart Institute, Salt Lake City, Utah, and colleagues. However, the effect of short-term increases in PM2.5 on hospitalizations for asthma and COPD has not been well studied, they noted.
“Our primary reason for studying the association of air pollution in the summer/fall wildfire season separately from the winter is that the drought conditions in the western United States from 2012-2022 resulted in more wildfires and increasingly large wildfires across the west,” Dr. Horne said in an interview. “In part, this provided a chance to measure an increase of fine particulate matter (PM2.5) air pollution from wildfires and also to track what happened to their health when people were exposed to the PM2.5 from wildfire,” he said.
During 2020-2022, the PM2.5 produced during the wildfire season exceeded the PM2.5 levels measured in the winter for the first time, Dr. Horne said. In the part of Utah where the study was conducted, PM2.5 increases in winter because of a combination of concentrated PM2.5 from cars and industry and a weather phenomenon known as a temperature inversion, he said.
A temperature inversion occurs when mountain topography traps pollutants near the ground where the people are, but only during times of cold and snowy weather, Dr. Horne said.
“Past studies in the region were conducted with the assumption that the winter inversion was the primary source of pollution-related health risks, and public and healthcare guidance for health was based on avoiding winter air pollution,” Dr. Horne noted. However, “it may be that the smoke from wildfires requires people to also anticipate how to avoid exposure to PM2.5 during the summer,” he said.
In a study published in CHEST Pulmonary, the researchers reviewed data from 63,976 patients hospitalized with asthma and 18,514 hospitalized with COPD between January 1999 and March 2022 who lived in an area of Utah in which PM2.5 and ozone are measured by the Environmental Protection Agency. The average age of the asthma patients was 22.6 years; 51.0% were women, 16.0% had hypertension, and 10.1% had a history of smoking. The average age of the COPD patients was 63.5 years, 50.3% were women, 69.1% had hypertension, and 42.3% had a history of smoking.
In a regression analysis, the risk for asthma was significantly associated with days of increased PM2.5 during wildfire season and similar to the winter inversion (when cold air traps air pollutants), with odds ratios (ORs) of 1.057 and 1.023 for every 10 µg per m3 of particulate matter, respectively.
Although the risk for asthma hospitalization decreased after a week, a rebound occurred during wildfire season after a 4-week lag, with an OR of 1.098 for every 10 µg per m3 of particulate matter, the researchers wrote. A review of all months showed a significant association between a concurrent day increase in PM2.5 and asthma hospitalization (OR, 1.020 per every 10 µg per m3 of particulate matter, P = .0006).
By contrast, PM2.5 increases had only a weak association with hospitalizations for COPD during either wildfire season or winter inversion season, and ozone was not associated with increased risks for patients with asthma or COPD.
The findings were limited by several factors including the observational design, potential for confounding, and relatively homogeneous study population, the researchers noted.
However, “these findings suggest that people should be aware of the risks from wildfire-generated PM2.5 during the summer and fall, including following best practices for people with asthma such as anticipating symptoms in warm months, carrying medications during summer activities, and expecting to stay indoors to avoid smoke exposure when wildfires have polluted the outdoor air,” Dr. Horne told this news organization.
In the current study, Dr. Horne and colleagues expected to see increases in the risk for asthma and COPD during summer wildfire season. “What was surprising was that the size of the risk of needing care of asthma appeared to occur just as rapidly after the PM2.5 became elevated during wildfire events as it did in the winter,” said Dr. Horne. “Further, the risk in the summer appeared to be greater than during the winter. Increases in hospitalization for asthma occurred on the same day and throughout the first week after a rise in air pollution in summer and early fall, and especially in children that risk remained increased for up to a month after the rise in air pollution,” he said.
Clinicians should be aware of environmental sources of respiratory declines caused by wildfire smoke that may prompt patients to seek care during wildfire events, said Horne. Finally, the general population should recognize the smell of smoke during warm months as an alert that leads to greater caution about spending time outdoors during wildfire events, he said. “Short-term PM2.5 elevations may affect respiratory health and have other effects such as on heart health,” Dr. Horne said. “In general, people should avoid outdoor exercise when air pollution is elevated, since the amount of air that is breathed in during exercise is substantially increased,” he added.
“Further research is needed regarding the mechanisms of effect from PM2.5 on health risk, including effects on respiratory and cardiovascular health,” said Dr. Horne. “This includes evaluating what biomarkers in the blood are changed by air pollution such as inflammatory factors, determining whether some medications may block or reduce the adverse effects of air pollution, and examining whether masks or indoor air purifiers have a meaningful benefit in protecting health during short-term air pollution elevations,” he said.
Data Reveal Respiratory Impact of Wildfires
“Fine particle air pollution has been linked to poor respiratory health outcomes, but relatively little is known about the specific impact of wildfire particulate pollution on patients living in urban population centers,” Alexander S. Rabin, MD, of the University of Michigan, Ann Arbor, said in an interview.
“Although it is known that wildfire risk is increasing throughout the western United States, the increase in the number of days per month with elevated fine particulate matter from 1999 to 2022 was striking,” said Dr. Rabin, who was not involved in the current study. “Over the same period, there was a decrease in the number of high fine particulate matter air pollution days related to the wintertime temperature inversion phenomenon when air pollutants are trapped in Utah’s valleys,” he said. “These data underscore the increased risk of wildfire-related air pollution relative to ‘traditional sources of air pollution from industrial and transportation sources,” he added.
Although the adverse effects of exposure to wildfire smoke and inversion season pollution on asthma were not unexpected, the degree of the effect size of wildfire smoke relative to inversion season was surprising, said Dr. Rabin.
“Why the wildfire smoke seems to have a worse impact on asthma outcomes could not be determined from this study, but there may be something inherently more dangerous about the cocktail of pollutants released when large wildfires burn uncontrolled,” he said. “I was surprised by the lack of association between wildfire smoke and adverse COPD outcomes; whether this relates to physiological differences or variations in healthcare-seeking behaviors between patients with asthma vs COPD is unknown,” he added.
The current study underscores the harmful effects of fine particulate pollution from wildfire smoke on health, and the increased risk for hospitalization for those with asthma even in urban environments far from the source of the fire, Dr. Rabin said.
However, limitations include the use of estimates of fine particulate pollution taken from monitoring stations that were an average of 14 km from the participants’ primary residences, and air quality measurements may not have accurately reflected exposure, Dr. Rabin noted. “Additionally, the population studied was not reflective of the US population, with approximately 80% of study participants described as non-Hispanic white,” he said. “Patients of color may have increased vulnerability to adverse outcomes from air pollution and therefore additional study is needed in these populations,” Dr. Rabin added.
The study was supported in part by the AIRHEALTH program project and by internal institutional funds. Dr. Horne disclosed serving on the advisory board of Opsis Health, previously consulting for Pfizer regarding risk scores and serving as site principal investigator of a grant funded by the Task Force for Global Health and a grant from the Patient-Centered Outcomes Research Institute and the NIH-funded RECOVER initiative. Dr. Rabin had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
‘Gift That Keeps Giving’: The Impact of GLP-1 in Asthma
This transcript has been edited for clarity.
Akshay B. Jain, MD: Welcome back to Medscape at ADA 2024, where Dr. James Kim, primary care physician from Calgary, Alberta, will be joining me in deciphering the key highlights at the ADA conference and bringing our own clinical twist into what the relevance would be for people like you and I to take back to our clinics.
Welcome back, Dr. Kim.
James Kim, MBBCh, PgDip, MScCH: Thank you very much. It’s nice to be back.
Dr. Jain: This was a diabetes conference, so obviously we are very pancreas focused. At this conference, we went outside our general area of territory, going outside of the pancreas and delving into other organ states. What I found fascinating were some data regarding the effects of incretin therapy on the lung, and in particular, some of the restrictive lung disorders.
Dr. Kim, you attended these sessions as well. Can you tell us a little bit more about the results that were discussed?
Dr. Kim: This is an interesting field. The moderator of the session went up and said that there has been no time in any previous ADA sessions where the lung issue was actually discussed. This was the first time ever.
They had some of the world leaders in this field, so it was really awesome to see them. Just to paint a picture of these obese asthmatic patients, they are challenging cases because, as you know, the main therapy for any asthmatic patient is inhaled corticosteroid.
Patients who are obese have quite a bit of a steroid resistance. Therefore, they end up being on many medications that sometimes are off label, and many end up on biologics as well. Therefore, the respiratory world has been seeking therapies for these obese asthmatic patients who are likely to be steroid resistant because these people are also likely to end up on an oral steroid as well.
Dr. Jain, you know the effect of the steroids much better than I do, and it’s like a laundry list. We really don’t want our patients to be on oral steroids.
In the past few years, GLP-1 has been studied quite extensively in the lung, especially in the world of asthma, and also in COPD. What’s really fascinating is that the GLP-1 receptors have been found to be quite abundant in the airway. Some studies show that the highest concentration of GLP-1 lies in the airway, whereas some studies have said that it’s the third most common area to find the GLP-1.
It is not a surprise that GLP-1 is being studied in managing the airway, especially airway inflammation in asthma and COPD patients. The preliminary data have been quite encouraging. They also discussed that there are new medications coming out that seem to be incretin based, so we’ll wait to see what those studies show.
There are two current phase 3 trials being held at the moment. One is using semaglutide 2.4 mg subcutaneous and another one is using metformin to reduce the airway inflammation in these asthmatic patients and also in some COPD patients. We’ll look forward to these results.
Dr. Jain: That’s really important to note because we see that there is a high density of these receptors in the airways, and hitherto we had no idea about the overall effect. Now, we’re looking at, as you mentioned, individuals with obesity who have asthma, so there are both the restrictive and obstructive components in the lung coming into play here.
From an endocrinology perspective, I’m thinking that this could be multiple effects of the GLP-1 receptor agonists, where on one hand you’re managing the obesity and you’re working along that line, and on the other hand, it could have local anti-inflammatory effects in the lung. Hence, there could be potential improvement in the overall pulmonary function of these individuals.
Dr. Kim: We are seeing this in primary care. Ever since I found out this information, I have started numerous patients, who are obese, asthmatic patients who do not have diabetes, on GLP-1 therapies, and their pulmonary function tests have improved significantly.
As a matter of fact, one of my personal friends is a severe asthmatic patient. She ends up being on oral steroids about three times a year. There was even one day when I saw her in one of my classes and she was dyspneic. She was short of breath.
I introduced her to one of my colleagues who’s a respirologist and very much into the impact of the incretins and asthma, and she was started on a GLP-1 receptor agonist. She lost about 30 pounds of weight, but now she is labeled as a mild asthmatic. Her pulmonary function test is completely normal. She hasn’t touched an oral steroid for a couple of years now.
That is a huge success story and I’m seeing that even in my own clinic as well. It’s a huge win for the respiratory world.
Dr. Jain: I think from an endocrinology perspective as well, if we are initiating GLP-1 receptor agonists or medications in that class, where we use it for management of obesity, sooner or later we do hit a stage where people will plateau with their weight loss. They won’t have any additional weight loss.
We tell individuals at that time that the fact that they’re able to maintain the weight loss still means that the medication is working from the obesity perspective. For individuals who also have asthma, it would be a good point to tell them that it could still have potential effects on reducing inflammation ongoing. Hence, even though they may not be losing any additional weight, it would still be helpful to continue on these medications from a pulmonary perspective.
Dr. Kim: Right now these pleiotropic effects of GLP-1 agents are absolutely mind-blowing. I mentioned in one of my respiratory presentations to a bunch of respirologists that diabetes is taking over the world, including the respiratory world. Well, you can imagine what their faces were like. However, they were quite impressed at that, and they were very excited with what these two phase 3 trials will show.
Dr. Jain: I think, based on the ADA 2024 conference, GLP-1 receptor agonists continue to be the gift that keeps giving. We have the effects on diabetes, obesity, kidney function, liver protection, lungs, and Alzheimer’s. We saw some sessions about potential use in people with alcohol misuse disorder or gambling problems. Clearly, there’s a large amount of research that›s being done with these agents.
Perhaps when you and I talk about ADA 2025, we might be able to talk about some more pleiotropic benefits outside the pancreas. Until then, please do check out our other videos from ADA 2024. Thanks for joining us again, Dr. Kim.
Dr. Kim: Thank you very much for having me.
Dr. Jain, clinical instructor, Department of Endocrinology, University of British Columbia, and endocrinologist, TLC Diabetes and Endocrinology, Vancouver, British Columbia, Canada, has disclosed ties with Abbott, Acerus, AstraZeneca, Amgen, Bausch Healthcare, Bayer, Boehringer Ingelheim, Care to Know, CCRN, Connected in Motion, CPD Network, Dexcom, Diabetes Canada, Eli Lilly, GSK, HLS Therapeutics, Janssen, Master Clinician Alliance, MDBriefcase, Merck, Medtronic, Moderna, Novartis, Novo Nordisk, Partners in Progressive Medical Education, Pfizer, Sanofi Aventis, Timed Right, WebMD, Gilead Sciences, Insulet, PocketPills, Roche, and Takeda. Dr. Kim, clinical assistant professor, Department of Family Medicine, University of Calgary, Alberta, has disclosed ties with Abbott, AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Eisai, Embecta, Eli Lilly, GSK, Janssen, Linpharma, Novo Nordisk, Miravo, Otsuka, Pfizer, Teva, Takeda, and Sanofi, and Partners in Progressive Medical Education.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Akshay B. Jain, MD: Welcome back to Medscape at ADA 2024, where Dr. James Kim, primary care physician from Calgary, Alberta, will be joining me in deciphering the key highlights at the ADA conference and bringing our own clinical twist into what the relevance would be for people like you and I to take back to our clinics.
Welcome back, Dr. Kim.
James Kim, MBBCh, PgDip, MScCH: Thank you very much. It’s nice to be back.
Dr. Jain: This was a diabetes conference, so obviously we are very pancreas focused. At this conference, we went outside our general area of territory, going outside of the pancreas and delving into other organ states. What I found fascinating were some data regarding the effects of incretin therapy on the lung, and in particular, some of the restrictive lung disorders.
Dr. Kim, you attended these sessions as well. Can you tell us a little bit more about the results that were discussed?
Dr. Kim: This is an interesting field. The moderator of the session went up and said that there has been no time in any previous ADA sessions where the lung issue was actually discussed. This was the first time ever.
They had some of the world leaders in this field, so it was really awesome to see them. Just to paint a picture of these obese asthmatic patients, they are challenging cases because, as you know, the main therapy for any asthmatic patient is inhaled corticosteroid.
Patients who are obese have quite a bit of a steroid resistance. Therefore, they end up being on many medications that sometimes are off label, and many end up on biologics as well. Therefore, the respiratory world has been seeking therapies for these obese asthmatic patients who are likely to be steroid resistant because these people are also likely to end up on an oral steroid as well.
Dr. Jain, you know the effect of the steroids much better than I do, and it’s like a laundry list. We really don’t want our patients to be on oral steroids.
In the past few years, GLP-1 has been studied quite extensively in the lung, especially in the world of asthma, and also in COPD. What’s really fascinating is that the GLP-1 receptors have been found to be quite abundant in the airway. Some studies show that the highest concentration of GLP-1 lies in the airway, whereas some studies have said that it’s the third most common area to find the GLP-1.
It is not a surprise that GLP-1 is being studied in managing the airway, especially airway inflammation in asthma and COPD patients. The preliminary data have been quite encouraging. They also discussed that there are new medications coming out that seem to be incretin based, so we’ll wait to see what those studies show.
There are two current phase 3 trials being held at the moment. One is using semaglutide 2.4 mg subcutaneous and another one is using metformin to reduce the airway inflammation in these asthmatic patients and also in some COPD patients. We’ll look forward to these results.
Dr. Jain: That’s really important to note because we see that there is a high density of these receptors in the airways, and hitherto we had no idea about the overall effect. Now, we’re looking at, as you mentioned, individuals with obesity who have asthma, so there are both the restrictive and obstructive components in the lung coming into play here.
From an endocrinology perspective, I’m thinking that this could be multiple effects of the GLP-1 receptor agonists, where on one hand you’re managing the obesity and you’re working along that line, and on the other hand, it could have local anti-inflammatory effects in the lung. Hence, there could be potential improvement in the overall pulmonary function of these individuals.
Dr. Kim: We are seeing this in primary care. Ever since I found out this information, I have started numerous patients, who are obese, asthmatic patients who do not have diabetes, on GLP-1 therapies, and their pulmonary function tests have improved significantly.
As a matter of fact, one of my personal friends is a severe asthmatic patient. She ends up being on oral steroids about three times a year. There was even one day when I saw her in one of my classes and she was dyspneic. She was short of breath.
I introduced her to one of my colleagues who’s a respirologist and very much into the impact of the incretins and asthma, and she was started on a GLP-1 receptor agonist. She lost about 30 pounds of weight, but now she is labeled as a mild asthmatic. Her pulmonary function test is completely normal. She hasn’t touched an oral steroid for a couple of years now.
That is a huge success story and I’m seeing that even in my own clinic as well. It’s a huge win for the respiratory world.
Dr. Jain: I think from an endocrinology perspective as well, if we are initiating GLP-1 receptor agonists or medications in that class, where we use it for management of obesity, sooner or later we do hit a stage where people will plateau with their weight loss. They won’t have any additional weight loss.
We tell individuals at that time that the fact that they’re able to maintain the weight loss still means that the medication is working from the obesity perspective. For individuals who also have asthma, it would be a good point to tell them that it could still have potential effects on reducing inflammation ongoing. Hence, even though they may not be losing any additional weight, it would still be helpful to continue on these medications from a pulmonary perspective.
Dr. Kim: Right now these pleiotropic effects of GLP-1 agents are absolutely mind-blowing. I mentioned in one of my respiratory presentations to a bunch of respirologists that diabetes is taking over the world, including the respiratory world. Well, you can imagine what their faces were like. However, they were quite impressed at that, and they were very excited with what these two phase 3 trials will show.
Dr. Jain: I think, based on the ADA 2024 conference, GLP-1 receptor agonists continue to be the gift that keeps giving. We have the effects on diabetes, obesity, kidney function, liver protection, lungs, and Alzheimer’s. We saw some sessions about potential use in people with alcohol misuse disorder or gambling problems. Clearly, there’s a large amount of research that›s being done with these agents.
Perhaps when you and I talk about ADA 2025, we might be able to talk about some more pleiotropic benefits outside the pancreas. Until then, please do check out our other videos from ADA 2024. Thanks for joining us again, Dr. Kim.
Dr. Kim: Thank you very much for having me.
Dr. Jain, clinical instructor, Department of Endocrinology, University of British Columbia, and endocrinologist, TLC Diabetes and Endocrinology, Vancouver, British Columbia, Canada, has disclosed ties with Abbott, Acerus, AstraZeneca, Amgen, Bausch Healthcare, Bayer, Boehringer Ingelheim, Care to Know, CCRN, Connected in Motion, CPD Network, Dexcom, Diabetes Canada, Eli Lilly, GSK, HLS Therapeutics, Janssen, Master Clinician Alliance, MDBriefcase, Merck, Medtronic, Moderna, Novartis, Novo Nordisk, Partners in Progressive Medical Education, Pfizer, Sanofi Aventis, Timed Right, WebMD, Gilead Sciences, Insulet, PocketPills, Roche, and Takeda. Dr. Kim, clinical assistant professor, Department of Family Medicine, University of Calgary, Alberta, has disclosed ties with Abbott, AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Eisai, Embecta, Eli Lilly, GSK, Janssen, Linpharma, Novo Nordisk, Miravo, Otsuka, Pfizer, Teva, Takeda, and Sanofi, and Partners in Progressive Medical Education.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Akshay B. Jain, MD: Welcome back to Medscape at ADA 2024, where Dr. James Kim, primary care physician from Calgary, Alberta, will be joining me in deciphering the key highlights at the ADA conference and bringing our own clinical twist into what the relevance would be for people like you and I to take back to our clinics.
Welcome back, Dr. Kim.
James Kim, MBBCh, PgDip, MScCH: Thank you very much. It’s nice to be back.
Dr. Jain: This was a diabetes conference, so obviously we are very pancreas focused. At this conference, we went outside our general area of territory, going outside of the pancreas and delving into other organ states. What I found fascinating were some data regarding the effects of incretin therapy on the lung, and in particular, some of the restrictive lung disorders.
Dr. Kim, you attended these sessions as well. Can you tell us a little bit more about the results that were discussed?
Dr. Kim: This is an interesting field. The moderator of the session went up and said that there has been no time in any previous ADA sessions where the lung issue was actually discussed. This was the first time ever.
They had some of the world leaders in this field, so it was really awesome to see them. Just to paint a picture of these obese asthmatic patients, they are challenging cases because, as you know, the main therapy for any asthmatic patient is inhaled corticosteroid.
Patients who are obese have quite a bit of a steroid resistance. Therefore, they end up being on many medications that sometimes are off label, and many end up on biologics as well. Therefore, the respiratory world has been seeking therapies for these obese asthmatic patients who are likely to be steroid resistant because these people are also likely to end up on an oral steroid as well.
Dr. Jain, you know the effect of the steroids much better than I do, and it’s like a laundry list. We really don’t want our patients to be on oral steroids.
In the past few years, GLP-1 has been studied quite extensively in the lung, especially in the world of asthma, and also in COPD. What’s really fascinating is that the GLP-1 receptors have been found to be quite abundant in the airway. Some studies show that the highest concentration of GLP-1 lies in the airway, whereas some studies have said that it’s the third most common area to find the GLP-1.
It is not a surprise that GLP-1 is being studied in managing the airway, especially airway inflammation in asthma and COPD patients. The preliminary data have been quite encouraging. They also discussed that there are new medications coming out that seem to be incretin based, so we’ll wait to see what those studies show.
There are two current phase 3 trials being held at the moment. One is using semaglutide 2.4 mg subcutaneous and another one is using metformin to reduce the airway inflammation in these asthmatic patients and also in some COPD patients. We’ll look forward to these results.
Dr. Jain: That’s really important to note because we see that there is a high density of these receptors in the airways, and hitherto we had no idea about the overall effect. Now, we’re looking at, as you mentioned, individuals with obesity who have asthma, so there are both the restrictive and obstructive components in the lung coming into play here.
From an endocrinology perspective, I’m thinking that this could be multiple effects of the GLP-1 receptor agonists, where on one hand you’re managing the obesity and you’re working along that line, and on the other hand, it could have local anti-inflammatory effects in the lung. Hence, there could be potential improvement in the overall pulmonary function of these individuals.
Dr. Kim: We are seeing this in primary care. Ever since I found out this information, I have started numerous patients, who are obese, asthmatic patients who do not have diabetes, on GLP-1 therapies, and their pulmonary function tests have improved significantly.
As a matter of fact, one of my personal friends is a severe asthmatic patient. She ends up being on oral steroids about three times a year. There was even one day when I saw her in one of my classes and she was dyspneic. She was short of breath.
I introduced her to one of my colleagues who’s a respirologist and very much into the impact of the incretins and asthma, and she was started on a GLP-1 receptor agonist. She lost about 30 pounds of weight, but now she is labeled as a mild asthmatic. Her pulmonary function test is completely normal. She hasn’t touched an oral steroid for a couple of years now.
That is a huge success story and I’m seeing that even in my own clinic as well. It’s a huge win for the respiratory world.
Dr. Jain: I think from an endocrinology perspective as well, if we are initiating GLP-1 receptor agonists or medications in that class, where we use it for management of obesity, sooner or later we do hit a stage where people will plateau with their weight loss. They won’t have any additional weight loss.
We tell individuals at that time that the fact that they’re able to maintain the weight loss still means that the medication is working from the obesity perspective. For individuals who also have asthma, it would be a good point to tell them that it could still have potential effects on reducing inflammation ongoing. Hence, even though they may not be losing any additional weight, it would still be helpful to continue on these medications from a pulmonary perspective.
Dr. Kim: Right now these pleiotropic effects of GLP-1 agents are absolutely mind-blowing. I mentioned in one of my respiratory presentations to a bunch of respirologists that diabetes is taking over the world, including the respiratory world. Well, you can imagine what their faces were like. However, they were quite impressed at that, and they were very excited with what these two phase 3 trials will show.
Dr. Jain: I think, based on the ADA 2024 conference, GLP-1 receptor agonists continue to be the gift that keeps giving. We have the effects on diabetes, obesity, kidney function, liver protection, lungs, and Alzheimer’s. We saw some sessions about potential use in people with alcohol misuse disorder or gambling problems. Clearly, there’s a large amount of research that›s being done with these agents.
Perhaps when you and I talk about ADA 2025, we might be able to talk about some more pleiotropic benefits outside the pancreas. Until then, please do check out our other videos from ADA 2024. Thanks for joining us again, Dr. Kim.
Dr. Kim: Thank you very much for having me.
Dr. Jain, clinical instructor, Department of Endocrinology, University of British Columbia, and endocrinologist, TLC Diabetes and Endocrinology, Vancouver, British Columbia, Canada, has disclosed ties with Abbott, Acerus, AstraZeneca, Amgen, Bausch Healthcare, Bayer, Boehringer Ingelheim, Care to Know, CCRN, Connected in Motion, CPD Network, Dexcom, Diabetes Canada, Eli Lilly, GSK, HLS Therapeutics, Janssen, Master Clinician Alliance, MDBriefcase, Merck, Medtronic, Moderna, Novartis, Novo Nordisk, Partners in Progressive Medical Education, Pfizer, Sanofi Aventis, Timed Right, WebMD, Gilead Sciences, Insulet, PocketPills, Roche, and Takeda. Dr. Kim, clinical assistant professor, Department of Family Medicine, University of Calgary, Alberta, has disclosed ties with Abbott, AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Eisai, Embecta, Eli Lilly, GSK, Janssen, Linpharma, Novo Nordisk, Miravo, Otsuka, Pfizer, Teva, Takeda, and Sanofi, and Partners in Progressive Medical Education.
A version of this article first appeared on Medscape.com.
Alert System Could Warn of Impact of Severe Weather on Health
As more data show potentially dangerous effects of climate and weather on individuals with chronic medical conditions, CVS Health has introduced an initiative that uses technology to provide weather alerts and targeted outreach to those at increased risk, according to a press release from the company. Ultimately, the goals of the initiative are to improve health, reduce emergency department visits, hospital stays, and medical costs, according to the press release.
Extreme weather events such as heat waves are becoming more frequent and severe, but most heat-related deaths are preventable with outreach and intervention, Dan Knecht, MD, vice president and chief clinical innovation officer for CVS Caremark, a division of CVS Health, said in an interview. The approach will combine the company’s services, including care managers, health centers, and data, to aid patients vulnerable to severe weather.
The initiative is starting with a focus on extreme heat events and will expand this fall with alerts about high levels of air pollution for individuals with vulnerability to reduced lung function, asthma, and cardiac problems as a result of exposure to high air-pollution levels, according to Dr. Knecht.
For now, the initiative is available to members of Aetna Medicare, according to Dr. Knecht. “Our goal is to expand to other consumers, including those who visit MinuteClinic and CVS Pharmacy locations, where we can provide timely environment-related recommendations at time of care,” he said.
The alert system uses environmental data analytics to pair highly localized forecasts and real-time insights about air quality, wildfires, and high heat with medical and pharmacy data for high-risk patients in areas affected by extreme weather.
For example, for individuals who are at risk and living in areas facing extreme heat, “registered nurse care managers proactively reach out to vulnerable patients up to several days in advance of an extreme weather event and provide them personalized tips and resources,” said Dr. Knecht.
In addition, he added, “we talk to patients about how to manage their medications during periods of extreme heat and, when delivering medications, take weather data into account to determine appropriate packaging materials for shipments.”
These interventions direct patients to CVS Health–linked resources, such as Oak Street Health clinics available as cooling centers, health services provided at MinuteClinic locations, and medication management at CVS pharmacies. Other interventions include virtual or in-person mental health counseling through MinuteClinic.
Dr. Knecht offered additional guidance for clinicians and patients to help manage heat waves. “Heat and certain medications can impair heat tolerance and the ability to regulate body temperature,” he told this news organization. Extreme heat may affect the performance of some medications and their devices, such as inhalers and diabetes supplies, he added.
Health Alerts Have Potential, But Comprehensive Approach is Needed
“Patients with chronic lung conditions are highly susceptible to the impact of climate change,” MeiLan K. Han, MD, a pulmonologist and professor of internal medicine at the University of Michigan, Ann Arbor, said in an interview. “Increasing dust, hotter temperatures, and higher levels of air pollution make it more difficult for patients to breathe,” she said. Data also suggest that higher levels of air pollution may not only cause chronic lung disease but also cause worsening symptoms among those with existing disease, she added.
A weather-related health alert could be useful for patients so they can be prepared, Dr. Han told this news organization.
“For a patient with chronic lung disease, a hot weather alert may mean that it will be harder for patients to breathe, and [they] may [be] more susceptible to heat stroke and dehydration if they do not have access to air conditioning,” she said. “At a minimum, patients should ensure they are on their controller medications, which often means a daily inhaler for patients with conditions such as asthma and chronic obstructive pulmonary disease (COPD). However, patients also should have access to their short-term reliever medications so they can be prepared for increased shortness of breath that may accompany a hot weather day,” Dr. Han explained.
However, not all patients have access to technology such as smartphones or other devices that will alert them to impending weather events, such as heat waves, said Dr. Han. “For these patients, a standard phone call may be beneficial,” she said.
Looking ahead, “programs for weather-related health alerts will need to be comprehensive, focusing not only on access to needed medications but also climate-controlled settings for temporary relief of heat,” said Dr. Han. “For some of our most vulnerable patients, while they may have air conditioning, they may not be able to afford to run it, so this needs to be considered in developing a comprehensive program,” she emphasized.
Dr. Knecht had no financial conflicts to disclose. Dr. Han disclosed ties with Aerogen, Altesa BioSciences, American Lung Association, Amgen, Apreo Health, AstraZeneca, Biodesix, Boehringer Ingelheim, Chiesi, Cipla, COPD Foundation, DevPro, Gala Therapeutics, Genentech, GlaxoSmithKline, Integrity, MDBriefcase, Medscape, Medtronic, Medwiz, Meissa Vaccines, Merck, Mylan, NACE, National Institutes of Health, Novartis, Nuvaira, Polarian, Pulmonx, Regeneron, Roche, RS Biotherapeutics, Sanofi, Sunovion, Teva, UpToDate, and Verona..
A version of this article first appeared on Medscape.com.
As more data show potentially dangerous effects of climate and weather on individuals with chronic medical conditions, CVS Health has introduced an initiative that uses technology to provide weather alerts and targeted outreach to those at increased risk, according to a press release from the company. Ultimately, the goals of the initiative are to improve health, reduce emergency department visits, hospital stays, and medical costs, according to the press release.
Extreme weather events such as heat waves are becoming more frequent and severe, but most heat-related deaths are preventable with outreach and intervention, Dan Knecht, MD, vice president and chief clinical innovation officer for CVS Caremark, a division of CVS Health, said in an interview. The approach will combine the company’s services, including care managers, health centers, and data, to aid patients vulnerable to severe weather.
The initiative is starting with a focus on extreme heat events and will expand this fall with alerts about high levels of air pollution for individuals with vulnerability to reduced lung function, asthma, and cardiac problems as a result of exposure to high air-pollution levels, according to Dr. Knecht.
For now, the initiative is available to members of Aetna Medicare, according to Dr. Knecht. “Our goal is to expand to other consumers, including those who visit MinuteClinic and CVS Pharmacy locations, where we can provide timely environment-related recommendations at time of care,” he said.
The alert system uses environmental data analytics to pair highly localized forecasts and real-time insights about air quality, wildfires, and high heat with medical and pharmacy data for high-risk patients in areas affected by extreme weather.
For example, for individuals who are at risk and living in areas facing extreme heat, “registered nurse care managers proactively reach out to vulnerable patients up to several days in advance of an extreme weather event and provide them personalized tips and resources,” said Dr. Knecht.
In addition, he added, “we talk to patients about how to manage their medications during periods of extreme heat and, when delivering medications, take weather data into account to determine appropriate packaging materials for shipments.”
These interventions direct patients to CVS Health–linked resources, such as Oak Street Health clinics available as cooling centers, health services provided at MinuteClinic locations, and medication management at CVS pharmacies. Other interventions include virtual or in-person mental health counseling through MinuteClinic.
Dr. Knecht offered additional guidance for clinicians and patients to help manage heat waves. “Heat and certain medications can impair heat tolerance and the ability to regulate body temperature,” he told this news organization. Extreme heat may affect the performance of some medications and their devices, such as inhalers and diabetes supplies, he added.
Health Alerts Have Potential, But Comprehensive Approach is Needed
“Patients with chronic lung conditions are highly susceptible to the impact of climate change,” MeiLan K. Han, MD, a pulmonologist and professor of internal medicine at the University of Michigan, Ann Arbor, said in an interview. “Increasing dust, hotter temperatures, and higher levels of air pollution make it more difficult for patients to breathe,” she said. Data also suggest that higher levels of air pollution may not only cause chronic lung disease but also cause worsening symptoms among those with existing disease, she added.
A weather-related health alert could be useful for patients so they can be prepared, Dr. Han told this news organization.
“For a patient with chronic lung disease, a hot weather alert may mean that it will be harder for patients to breathe, and [they] may [be] more susceptible to heat stroke and dehydration if they do not have access to air conditioning,” she said. “At a minimum, patients should ensure they are on their controller medications, which often means a daily inhaler for patients with conditions such as asthma and chronic obstructive pulmonary disease (COPD). However, patients also should have access to their short-term reliever medications so they can be prepared for increased shortness of breath that may accompany a hot weather day,” Dr. Han explained.
However, not all patients have access to technology such as smartphones or other devices that will alert them to impending weather events, such as heat waves, said Dr. Han. “For these patients, a standard phone call may be beneficial,” she said.
Looking ahead, “programs for weather-related health alerts will need to be comprehensive, focusing not only on access to needed medications but also climate-controlled settings for temporary relief of heat,” said Dr. Han. “For some of our most vulnerable patients, while they may have air conditioning, they may not be able to afford to run it, so this needs to be considered in developing a comprehensive program,” she emphasized.
Dr. Knecht had no financial conflicts to disclose. Dr. Han disclosed ties with Aerogen, Altesa BioSciences, American Lung Association, Amgen, Apreo Health, AstraZeneca, Biodesix, Boehringer Ingelheim, Chiesi, Cipla, COPD Foundation, DevPro, Gala Therapeutics, Genentech, GlaxoSmithKline, Integrity, MDBriefcase, Medscape, Medtronic, Medwiz, Meissa Vaccines, Merck, Mylan, NACE, National Institutes of Health, Novartis, Nuvaira, Polarian, Pulmonx, Regeneron, Roche, RS Biotherapeutics, Sanofi, Sunovion, Teva, UpToDate, and Verona..
A version of this article first appeared on Medscape.com.
As more data show potentially dangerous effects of climate and weather on individuals with chronic medical conditions, CVS Health has introduced an initiative that uses technology to provide weather alerts and targeted outreach to those at increased risk, according to a press release from the company. Ultimately, the goals of the initiative are to improve health, reduce emergency department visits, hospital stays, and medical costs, according to the press release.
Extreme weather events such as heat waves are becoming more frequent and severe, but most heat-related deaths are preventable with outreach and intervention, Dan Knecht, MD, vice president and chief clinical innovation officer for CVS Caremark, a division of CVS Health, said in an interview. The approach will combine the company’s services, including care managers, health centers, and data, to aid patients vulnerable to severe weather.
The initiative is starting with a focus on extreme heat events and will expand this fall with alerts about high levels of air pollution for individuals with vulnerability to reduced lung function, asthma, and cardiac problems as a result of exposure to high air-pollution levels, according to Dr. Knecht.
For now, the initiative is available to members of Aetna Medicare, according to Dr. Knecht. “Our goal is to expand to other consumers, including those who visit MinuteClinic and CVS Pharmacy locations, where we can provide timely environment-related recommendations at time of care,” he said.
The alert system uses environmental data analytics to pair highly localized forecasts and real-time insights about air quality, wildfires, and high heat with medical and pharmacy data for high-risk patients in areas affected by extreme weather.
For example, for individuals who are at risk and living in areas facing extreme heat, “registered nurse care managers proactively reach out to vulnerable patients up to several days in advance of an extreme weather event and provide them personalized tips and resources,” said Dr. Knecht.
In addition, he added, “we talk to patients about how to manage their medications during periods of extreme heat and, when delivering medications, take weather data into account to determine appropriate packaging materials for shipments.”
These interventions direct patients to CVS Health–linked resources, such as Oak Street Health clinics available as cooling centers, health services provided at MinuteClinic locations, and medication management at CVS pharmacies. Other interventions include virtual or in-person mental health counseling through MinuteClinic.
Dr. Knecht offered additional guidance for clinicians and patients to help manage heat waves. “Heat and certain medications can impair heat tolerance and the ability to regulate body temperature,” he told this news organization. Extreme heat may affect the performance of some medications and their devices, such as inhalers and diabetes supplies, he added.
Health Alerts Have Potential, But Comprehensive Approach is Needed
“Patients with chronic lung conditions are highly susceptible to the impact of climate change,” MeiLan K. Han, MD, a pulmonologist and professor of internal medicine at the University of Michigan, Ann Arbor, said in an interview. “Increasing dust, hotter temperatures, and higher levels of air pollution make it more difficult for patients to breathe,” she said. Data also suggest that higher levels of air pollution may not only cause chronic lung disease but also cause worsening symptoms among those with existing disease, she added.
A weather-related health alert could be useful for patients so they can be prepared, Dr. Han told this news organization.
“For a patient with chronic lung disease, a hot weather alert may mean that it will be harder for patients to breathe, and [they] may [be] more susceptible to heat stroke and dehydration if they do not have access to air conditioning,” she said. “At a minimum, patients should ensure they are on their controller medications, which often means a daily inhaler for patients with conditions such as asthma and chronic obstructive pulmonary disease (COPD). However, patients also should have access to their short-term reliever medications so they can be prepared for increased shortness of breath that may accompany a hot weather day,” Dr. Han explained.
However, not all patients have access to technology such as smartphones or other devices that will alert them to impending weather events, such as heat waves, said Dr. Han. “For these patients, a standard phone call may be beneficial,” she said.
Looking ahead, “programs for weather-related health alerts will need to be comprehensive, focusing not only on access to needed medications but also climate-controlled settings for temporary relief of heat,” said Dr. Han. “For some of our most vulnerable patients, while they may have air conditioning, they may not be able to afford to run it, so this needs to be considered in developing a comprehensive program,” she emphasized.
Dr. Knecht had no financial conflicts to disclose. Dr. Han disclosed ties with Aerogen, Altesa BioSciences, American Lung Association, Amgen, Apreo Health, AstraZeneca, Biodesix, Boehringer Ingelheim, Chiesi, Cipla, COPD Foundation, DevPro, Gala Therapeutics, Genentech, GlaxoSmithKline, Integrity, MDBriefcase, Medscape, Medtronic, Medwiz, Meissa Vaccines, Merck, Mylan, NACE, National Institutes of Health, Novartis, Nuvaira, Polarian, Pulmonx, Regeneron, Roche, RS Biotherapeutics, Sanofi, Sunovion, Teva, UpToDate, and Verona..
A version of this article first appeared on Medscape.com.
Pediatric Studies Produce Mixed Messages on Relationship Between COVID and Asthma
In one of several recently published studies on the relationship between COVID-19 infection and asthma, according to data drawn from the National Survey of Children’s Health (NSCH).
The inverse correlation between symptoms and vaccination was strong and statistically significant, according to investigators led by Matthew M. Davis, MD, Physician in Chief and Chief Scientific Officer, Nemours Children’s Health, Wilmington, Delaware.
“With each increase of 10 percentage points in COVID-19 vaccination coverage, the parent-reported child asthma symptoms prevalence decreased by 0.36 percentage points (P < .05),” Dr. Davis and his coinvestigators reported in a research letter published in JAMA Network Open.
Studies Explore Relationship of COVID and Asthma
The reduced risk of asthma symptoms with COVID-19 vaccination in children at the population level is just one of several recently published studies exploring the interaction between COVID-19 infection and asthma, but two studies that posed the same question did not reach the same conclusion.
In one, COVID-19 infection in children was not found to be a trigger for new-onset asthma, but the second found that it was. In a third study, the preponderance of evidence from a meta-analysis found that patients with asthma – whether children or adults – did not necessarily experience a more severe course of COVID-19 infection than in those without asthma.
The NSCH database study calculated state-level change in scores for patient-reported childhood asthma symptoms in the years in the years 2018-2019, which preceded the pandemic and the years 2020-2021, when the pandemic began. The hypothesis was that the proportion of the population 5 years of age or older who completed the COVID-19 primary vaccination would be inversely related to asthma symptom prevalence.
Relative to the 2018-2019 years, the mean rate of parent-reported asthma symptoms was 0.85% lower (6.93% vs 7.77%; P < .001) in 2020-2021, when the mean primary series COVID-19 vaccination rate was 72.3%.
The study was not able to evaluate the impact of COVID-19 vaccination specifically in children with asthma, because history of asthma is not captured in the NSCH data, but Dr. Davis contended that the reduction in symptomatic asthma among children with increased vaccination offers validation for the state-level findings.
“Moreover, the absence of an association of COVID-19 vaccination administered predominantly in 2021 with population-level COVID-19 mortality in 2020 serves as a negative control,” he and his colleagues wrote in their research letter.
Protection from Respiratory Viruses Seen for Asthma Patients
In an interview, Dr. Davis reported that these data are consistent with previous evidence that immunization against influenza also reduces risk of asthma symptoms. In a meta-analysis published in 2017, it was estimated that live vaccines reduced risk of influenza by 81% and prevented 59%-72% of asthma attacks leading to hospitalizations or emergency room visits.
“The similarity of our findings regarding COVID-19 vaccination to prior data regarding influenza vaccination underscores the importance of preventing viral illnesses in individuals with a history of asthma,” Dr. Davis said. It is not yet clear if this is true of respiratory syncytial virus (RSV). Because of the short time that the RSV vaccine has been available, it is too soon to conduct an analysis.
One message from this study is that “clinicians should continue to encourage COVID-19 vaccination for children because of its general benefits in preventing coronavirus-related illness and the apparent specific benefits for children with a history of asthma,” he said.
While vaccination appears to reduce asthmatic symptoms related to COVID-19 infection, one study suggests that COVID-19 does not trigger new-onset asthma. In a retrospective study published in Pediatrics, no association between COVID-19 infection and new-onset asthma could be made in an analysis of 27,423 children (ages, 1-16 years) from the Children’s Hospital of Philadelphia (CHOP) Care Network.
Across all the pediatric age groups evaluated, the consistent finding was “SARS-CoV-2 positivity does not confer an additional risk for asthma diagnosis at least within the first 18 months after a [polymerase chain reaction] test,” concluded the investigators, led by David A. Hill, MD, PhD, Division of Allergy and Immunology, CHOP, Philadelphia, Pennsylvania.
Risk of Asthma Doubled After COVID-19 Infection
However, the opposite conclusion was reached by investigators evaluating data from two cohorts of children ages 5-18 drawn from the TriNetX database, a global health research network with data on more than 250 million individuals. Cohort 1 included more than 250,000 children. These children had never received COVID-19 vaccination. The 50,000 patients in cohort 2 had all received COVID19 vaccination.
To compare the impact of COVID-19 infection on new-onset asthma, the patients who were infected with COVID-19 were compared with those who were not infected after propensity score matching over 18 months of follow-up.
In cohort 1, the rate of new onset asthma was more than twofold greater among those with COVID-19 infection (4.7% vs 2.0%). The hazard ratio (HR) of 2.25 had tight confidence intervals (95% CI, 2.158-2.367).
In cohort 2, the risk of new-onset asthma at 18 months among those who had a COVID-19 infection relative to those without was even greater (8.3% vs 3.1%). The relative risk approached a 3-fold increase (HR 2.745; 95% CI, 2.521-2.99).
The conclusion of these investigators, led by Chia-Chi Lung, PhD, Department of Public Health, Chung Shan Medical University, Taichung City, Taiwan, was that there is “a critical need for ongoing monitoring and customized healthcare strategies to mitigate the long-term respiratory impacts of COVID-19 in children.”
These health risks might not be as significant as once feared. In the recently published study from Environmental Health Insights, the goal of a meta-analysis was to determine if patients with asthma relative to those without asthma face a higher risk of serious disease from COVID-19 infection. The meta-analysis included studies of children and adults. The answer, according an in-depth analysis of 21 articles in a “scoping review,” was a qualified no.
Of the 21 articles, 4 concluded that asthma is a risk factor for serious COVID-19 infection, but 17 did not, according to Chukwudi S. Ubah, PhD, Department of Public Health, Brody School of Medicine, East Caroline University, Greenville, North Carolina.
None of These Questions are Fully Resolved
However, given the disparity in the results and the fact that many of the studies included in this analysis had small sample sizes, Dr. Ubah called for larger studies and studies with better controls. He noted, for example, that the studies did not consistently evaluate mitigating factors, such as used of inhaled or oral corticosteroids, which might affect risk of the severity of a COVID-19 infection.
Rather, “our findings pointed out that the type of medication prescribed for asthma may have implications for the severity of COVID-19 infection in these patients,” Dr. Ubah said in an interview.
Overall, the data do not support a major interaction between asthma and COVID-19, even if the data are not conclusive. Each of the senior authors of these studies called for larger and better investigations to further explore whether COVID-19 infection and preexisting asthma interact. So far, the data indicate that if COVID-19 infection poses a risk of precipitating new-onset asthma or inducing a more severe infection in children with asthma, it is low, but the degree of risk, if any, remains unresolved in subgroups defined by asthma treatment or asthma severity.
Dr. Davis, Dr. Hill, Dr. Lung, and Dr. Ubah reported no potential conflicts of interest. None of these studies received funding from commercial interests.
In one of several recently published studies on the relationship between COVID-19 infection and asthma, according to data drawn from the National Survey of Children’s Health (NSCH).
The inverse correlation between symptoms and vaccination was strong and statistically significant, according to investigators led by Matthew M. Davis, MD, Physician in Chief and Chief Scientific Officer, Nemours Children’s Health, Wilmington, Delaware.
“With each increase of 10 percentage points in COVID-19 vaccination coverage, the parent-reported child asthma symptoms prevalence decreased by 0.36 percentage points (P < .05),” Dr. Davis and his coinvestigators reported in a research letter published in JAMA Network Open.
Studies Explore Relationship of COVID and Asthma
The reduced risk of asthma symptoms with COVID-19 vaccination in children at the population level is just one of several recently published studies exploring the interaction between COVID-19 infection and asthma, but two studies that posed the same question did not reach the same conclusion.
In one, COVID-19 infection in children was not found to be a trigger for new-onset asthma, but the second found that it was. In a third study, the preponderance of evidence from a meta-analysis found that patients with asthma – whether children or adults – did not necessarily experience a more severe course of COVID-19 infection than in those without asthma.
The NSCH database study calculated state-level change in scores for patient-reported childhood asthma symptoms in the years in the years 2018-2019, which preceded the pandemic and the years 2020-2021, when the pandemic began. The hypothesis was that the proportion of the population 5 years of age or older who completed the COVID-19 primary vaccination would be inversely related to asthma symptom prevalence.
Relative to the 2018-2019 years, the mean rate of parent-reported asthma symptoms was 0.85% lower (6.93% vs 7.77%; P < .001) in 2020-2021, when the mean primary series COVID-19 vaccination rate was 72.3%.
The study was not able to evaluate the impact of COVID-19 vaccination specifically in children with asthma, because history of asthma is not captured in the NSCH data, but Dr. Davis contended that the reduction in symptomatic asthma among children with increased vaccination offers validation for the state-level findings.
“Moreover, the absence of an association of COVID-19 vaccination administered predominantly in 2021 with population-level COVID-19 mortality in 2020 serves as a negative control,” he and his colleagues wrote in their research letter.
Protection from Respiratory Viruses Seen for Asthma Patients
In an interview, Dr. Davis reported that these data are consistent with previous evidence that immunization against influenza also reduces risk of asthma symptoms. In a meta-analysis published in 2017, it was estimated that live vaccines reduced risk of influenza by 81% and prevented 59%-72% of asthma attacks leading to hospitalizations or emergency room visits.
“The similarity of our findings regarding COVID-19 vaccination to prior data regarding influenza vaccination underscores the importance of preventing viral illnesses in individuals with a history of asthma,” Dr. Davis said. It is not yet clear if this is true of respiratory syncytial virus (RSV). Because of the short time that the RSV vaccine has been available, it is too soon to conduct an analysis.
One message from this study is that “clinicians should continue to encourage COVID-19 vaccination for children because of its general benefits in preventing coronavirus-related illness and the apparent specific benefits for children with a history of asthma,” he said.
While vaccination appears to reduce asthmatic symptoms related to COVID-19 infection, one study suggests that COVID-19 does not trigger new-onset asthma. In a retrospective study published in Pediatrics, no association between COVID-19 infection and new-onset asthma could be made in an analysis of 27,423 children (ages, 1-16 years) from the Children’s Hospital of Philadelphia (CHOP) Care Network.
Across all the pediatric age groups evaluated, the consistent finding was “SARS-CoV-2 positivity does not confer an additional risk for asthma diagnosis at least within the first 18 months after a [polymerase chain reaction] test,” concluded the investigators, led by David A. Hill, MD, PhD, Division of Allergy and Immunology, CHOP, Philadelphia, Pennsylvania.
Risk of Asthma Doubled After COVID-19 Infection
However, the opposite conclusion was reached by investigators evaluating data from two cohorts of children ages 5-18 drawn from the TriNetX database, a global health research network with data on more than 250 million individuals. Cohort 1 included more than 250,000 children. These children had never received COVID-19 vaccination. The 50,000 patients in cohort 2 had all received COVID19 vaccination.
To compare the impact of COVID-19 infection on new-onset asthma, the patients who were infected with COVID-19 were compared with those who were not infected after propensity score matching over 18 months of follow-up.
In cohort 1, the rate of new onset asthma was more than twofold greater among those with COVID-19 infection (4.7% vs 2.0%). The hazard ratio (HR) of 2.25 had tight confidence intervals (95% CI, 2.158-2.367).
In cohort 2, the risk of new-onset asthma at 18 months among those who had a COVID-19 infection relative to those without was even greater (8.3% vs 3.1%). The relative risk approached a 3-fold increase (HR 2.745; 95% CI, 2.521-2.99).
The conclusion of these investigators, led by Chia-Chi Lung, PhD, Department of Public Health, Chung Shan Medical University, Taichung City, Taiwan, was that there is “a critical need for ongoing monitoring and customized healthcare strategies to mitigate the long-term respiratory impacts of COVID-19 in children.”
These health risks might not be as significant as once feared. In the recently published study from Environmental Health Insights, the goal of a meta-analysis was to determine if patients with asthma relative to those without asthma face a higher risk of serious disease from COVID-19 infection. The meta-analysis included studies of children and adults. The answer, according an in-depth analysis of 21 articles in a “scoping review,” was a qualified no.
Of the 21 articles, 4 concluded that asthma is a risk factor for serious COVID-19 infection, but 17 did not, according to Chukwudi S. Ubah, PhD, Department of Public Health, Brody School of Medicine, East Caroline University, Greenville, North Carolina.
None of These Questions are Fully Resolved
However, given the disparity in the results and the fact that many of the studies included in this analysis had small sample sizes, Dr. Ubah called for larger studies and studies with better controls. He noted, for example, that the studies did not consistently evaluate mitigating factors, such as used of inhaled or oral corticosteroids, which might affect risk of the severity of a COVID-19 infection.
Rather, “our findings pointed out that the type of medication prescribed for asthma may have implications for the severity of COVID-19 infection in these patients,” Dr. Ubah said in an interview.
Overall, the data do not support a major interaction between asthma and COVID-19, even if the data are not conclusive. Each of the senior authors of these studies called for larger and better investigations to further explore whether COVID-19 infection and preexisting asthma interact. So far, the data indicate that if COVID-19 infection poses a risk of precipitating new-onset asthma or inducing a more severe infection in children with asthma, it is low, but the degree of risk, if any, remains unresolved in subgroups defined by asthma treatment or asthma severity.
Dr. Davis, Dr. Hill, Dr. Lung, and Dr. Ubah reported no potential conflicts of interest. None of these studies received funding from commercial interests.
In one of several recently published studies on the relationship between COVID-19 infection and asthma, according to data drawn from the National Survey of Children’s Health (NSCH).
The inverse correlation between symptoms and vaccination was strong and statistically significant, according to investigators led by Matthew M. Davis, MD, Physician in Chief and Chief Scientific Officer, Nemours Children’s Health, Wilmington, Delaware.
“With each increase of 10 percentage points in COVID-19 vaccination coverage, the parent-reported child asthma symptoms prevalence decreased by 0.36 percentage points (P < .05),” Dr. Davis and his coinvestigators reported in a research letter published in JAMA Network Open.
Studies Explore Relationship of COVID and Asthma
The reduced risk of asthma symptoms with COVID-19 vaccination in children at the population level is just one of several recently published studies exploring the interaction between COVID-19 infection and asthma, but two studies that posed the same question did not reach the same conclusion.
In one, COVID-19 infection in children was not found to be a trigger for new-onset asthma, but the second found that it was. In a third study, the preponderance of evidence from a meta-analysis found that patients with asthma – whether children or adults – did not necessarily experience a more severe course of COVID-19 infection than in those without asthma.
The NSCH database study calculated state-level change in scores for patient-reported childhood asthma symptoms in the years in the years 2018-2019, which preceded the pandemic and the years 2020-2021, when the pandemic began. The hypothesis was that the proportion of the population 5 years of age or older who completed the COVID-19 primary vaccination would be inversely related to asthma symptom prevalence.
Relative to the 2018-2019 years, the mean rate of parent-reported asthma symptoms was 0.85% lower (6.93% vs 7.77%; P < .001) in 2020-2021, when the mean primary series COVID-19 vaccination rate was 72.3%.
The study was not able to evaluate the impact of COVID-19 vaccination specifically in children with asthma, because history of asthma is not captured in the NSCH data, but Dr. Davis contended that the reduction in symptomatic asthma among children with increased vaccination offers validation for the state-level findings.
“Moreover, the absence of an association of COVID-19 vaccination administered predominantly in 2021 with population-level COVID-19 mortality in 2020 serves as a negative control,” he and his colleagues wrote in their research letter.
Protection from Respiratory Viruses Seen for Asthma Patients
In an interview, Dr. Davis reported that these data are consistent with previous evidence that immunization against influenza also reduces risk of asthma symptoms. In a meta-analysis published in 2017, it was estimated that live vaccines reduced risk of influenza by 81% and prevented 59%-72% of asthma attacks leading to hospitalizations or emergency room visits.
“The similarity of our findings regarding COVID-19 vaccination to prior data regarding influenza vaccination underscores the importance of preventing viral illnesses in individuals with a history of asthma,” Dr. Davis said. It is not yet clear if this is true of respiratory syncytial virus (RSV). Because of the short time that the RSV vaccine has been available, it is too soon to conduct an analysis.
One message from this study is that “clinicians should continue to encourage COVID-19 vaccination for children because of its general benefits in preventing coronavirus-related illness and the apparent specific benefits for children with a history of asthma,” he said.
While vaccination appears to reduce asthmatic symptoms related to COVID-19 infection, one study suggests that COVID-19 does not trigger new-onset asthma. In a retrospective study published in Pediatrics, no association between COVID-19 infection and new-onset asthma could be made in an analysis of 27,423 children (ages, 1-16 years) from the Children’s Hospital of Philadelphia (CHOP) Care Network.
Across all the pediatric age groups evaluated, the consistent finding was “SARS-CoV-2 positivity does not confer an additional risk for asthma diagnosis at least within the first 18 months after a [polymerase chain reaction] test,” concluded the investigators, led by David A. Hill, MD, PhD, Division of Allergy and Immunology, CHOP, Philadelphia, Pennsylvania.
Risk of Asthma Doubled After COVID-19 Infection
However, the opposite conclusion was reached by investigators evaluating data from two cohorts of children ages 5-18 drawn from the TriNetX database, a global health research network with data on more than 250 million individuals. Cohort 1 included more than 250,000 children. These children had never received COVID-19 vaccination. The 50,000 patients in cohort 2 had all received COVID19 vaccination.
To compare the impact of COVID-19 infection on new-onset asthma, the patients who were infected with COVID-19 were compared with those who were not infected after propensity score matching over 18 months of follow-up.
In cohort 1, the rate of new onset asthma was more than twofold greater among those with COVID-19 infection (4.7% vs 2.0%). The hazard ratio (HR) of 2.25 had tight confidence intervals (95% CI, 2.158-2.367).
In cohort 2, the risk of new-onset asthma at 18 months among those who had a COVID-19 infection relative to those without was even greater (8.3% vs 3.1%). The relative risk approached a 3-fold increase (HR 2.745; 95% CI, 2.521-2.99).
The conclusion of these investigators, led by Chia-Chi Lung, PhD, Department of Public Health, Chung Shan Medical University, Taichung City, Taiwan, was that there is “a critical need for ongoing monitoring and customized healthcare strategies to mitigate the long-term respiratory impacts of COVID-19 in children.”
These health risks might not be as significant as once feared. In the recently published study from Environmental Health Insights, the goal of a meta-analysis was to determine if patients with asthma relative to those without asthma face a higher risk of serious disease from COVID-19 infection. The meta-analysis included studies of children and adults. The answer, according an in-depth analysis of 21 articles in a “scoping review,” was a qualified no.
Of the 21 articles, 4 concluded that asthma is a risk factor for serious COVID-19 infection, but 17 did not, according to Chukwudi S. Ubah, PhD, Department of Public Health, Brody School of Medicine, East Caroline University, Greenville, North Carolina.
None of These Questions are Fully Resolved
However, given the disparity in the results and the fact that many of the studies included in this analysis had small sample sizes, Dr. Ubah called for larger studies and studies with better controls. He noted, for example, that the studies did not consistently evaluate mitigating factors, such as used of inhaled or oral corticosteroids, which might affect risk of the severity of a COVID-19 infection.
Rather, “our findings pointed out that the type of medication prescribed for asthma may have implications for the severity of COVID-19 infection in these patients,” Dr. Ubah said in an interview.
Overall, the data do not support a major interaction between asthma and COVID-19, even if the data are not conclusive. Each of the senior authors of these studies called for larger and better investigations to further explore whether COVID-19 infection and preexisting asthma interact. So far, the data indicate that if COVID-19 infection poses a risk of precipitating new-onset asthma or inducing a more severe infection in children with asthma, it is low, but the degree of risk, if any, remains unresolved in subgroups defined by asthma treatment or asthma severity.
Dr. Davis, Dr. Hill, Dr. Lung, and Dr. Ubah reported no potential conflicts of interest. None of these studies received funding from commercial interests.
FROM JAMA NETWORK OPEN
Children on Medicaid With Asthma Receive Less Specialty Care
Primary care clinicians successfully manage many children with asthma, but data on specialist care according to insurance coverage are lacking, wrote Kimberley H. Geissler, PhD, of the University of Massachusetts Chan Medical School–Baystate, Springfield, Massachusetts, and colleagues.
Despite many interventions over time, “low-income children insured by Medicaid, many of whom are from minoritized racial and ethnic groups, continue to have worse outcomes and higher rates of poorly controlled asthma than children who are privately insured,” Dr. Geissler said in an interview.
“Because differences in whether a child sees an asthma specialist could contribute to these disparities, better understanding specialist use among both groups of kids may help inform potential solutions,” she said.
In a study published in JAMA Network Open, the researchers identified children with asthma aged 2-17 years using data from the Massachusetts All-Payer Claims Database for the years 2015-2020. The study population included 198,101 children and 432,455 child-year observations from children with asthma during a year when they met at least one of three criteria with any asthma diagnosis: One or more hospital visits, two or more outpatient visits, or at least one outpatient visit and at least one asthma medication.
Outpatient Visit Outcome
The primary outcome of asthma specialist care was defined as at least one outpatient visit with any asthma diagnosis to a clinician with a code of allergy and immunology, pulmonology, or otolaryngology.
A total of 66.2% of the child-year observations involved Medicaid and 33.8% involved private insurance. Approximately 15% of the children received asthma specialist care. However, nearly twice as many children with private insurance received asthma specialty care compared with those with Medicaid (20.6% vs 11.9%). In a full logistic regression analysis, children with Medicaid insurance were 55% less likely to receive asthma specialist treatment than children with private insurance.
Allergy and immunology was the most common specialty used, and the child-years for this specialty among children with Medicaid were less than half of those among children with private insurance (7.1% vs 15.9%).
Rates of persistent asthma were 20.0% and 16.9% in children with Medicaid and private insurance, respectively. Overall, children with persistent asthma were nearly four times as likely to receive asthma specialist care (adjusted odds ratio, 3.96). However, the difference in odds of receiving specialty care based on insurance type in favor of private insurance was greater among children with persistent asthma than among those without persistent asthma (−24.0 percentage points vs −20.8 percentage points).
The researchers found a similar pattern of difference in asthma specialty care in a sensitivity analysis limiting the results to child-year observations with at least one outpatient visit with any asthma diagnosis in a calendar year, although they also found a slight narrowing of the difference between the groups over time.
“Contrary to expectations, disparities in specialist care by insurance type were even more striking in children with persistent asthma,” the researchers wrote in their discussion. Notably, the growth of specialty drugs such as biologics for moderate to severe asthma are mainly prescribed by specialists, and ensuring access to specialists for children with Medicaid may reduce disparities in asthma control for those with severe or poorly controlled disease, they added.
The study findings were limited by several factors including the use only of data from Massachusetts, which may not generalize to other states, and the use of completed specialist visits without data on referrals, the researchers noted. Other limitations included a lack of data on asthma symptom frequency or control and on the setting in which an asthma diagnosis was made.
However, the results suggest a need for more attention to disparities in asthma care by insurance type, and more research is needed to determine whether these disparities persist in subsets of children with asthma, such as those with allergies or chronic medical conditions, they concluded.
Takeaways and Next Steps
“Perhaps unsurprisingly, children with private insurance were more likely to receive asthma specialist care than children with Medicaid,” Dr. Geissler told this news organization. The researchers expected a smaller gap between insurance types among children with persistent asthma, a marker for asthma severity, she said. However, “we found that the gap between those with Medicaid and those with private insurance is actually larger” for children with persistent asthma, she added.
As improved treatments for hard-to-control asthma become more available, pediatricians and primary care clinicians should follow the latest clinical guidelines for referring children to specialists for asthma care, said Dr. Geissler.
“Additionally, asthma specialists should ensure that their practices are accessible to children with Medicaid, as these families may face higher barriers to care; for example, transportation needs or scheduling challenges,” she said. Other strategies to overcome barriers to care might include electronic consultations with specialists or primary care–oriented interdisciplinary asthma clinics, which may be useful for all children with asthma but may particularly benefit those insured by Medicaid, she noted.
“Based on data limitations, we could not examine why we observed such big differences in specialist use by insurance type; for example, whether pediatricians were referring to specialists less for Medicaid-insured kids, or whether kids with Medicaid were less likely to see a specialist after a referral was made,” Dr. Geissler said. More research is needed to examine not only these factors but also the appropriateness of specialty care based on clinical guidelines to ensure high-quality evidence-based care for children with asthma who are insured by Medicaid, she said.
Improve Access and Expand Analysis
Asthma is a chronic and prevalent disease and requires a comprehensive approach that sometimes calls for specialist care, Anne Coates, MD, a pediatric pulmonologist in Portland, Maine, said in an interview.
Dr. Coates said she was surprised by the results of the current study but commended the authors for highlighting the limitations of the study, which illustrate areas for additional research. Notably, “the authors couldn’t observe referrals to specialists from primary care physicians; they used completed visits as a proxy,” Dr. Coates said.
More studies are needed to assess the completion of referral visits regardless of children’s insurance in order to better understand and address the barriers to specialty care, she added.
The current study is important because of the extent of asthma coupled with the significant number of children across the United States who are insured by Medicaid, especially underserved populations, she said.
“The burden of asthma differentially affects people of color who are living in lower resourced areas, and it is important in further research to understanding the barriers to helping people get the care they need,” Dr. Coates told this news organization. Some alternatives might include telehealth visits or even a hybrid visit to a primary care provider (PCP) who has high-speed internet, and the specialist could then conduct a telehealth visit from the PCP’s office, with the PCP acting as on-site eyes and ears, said Dr. Coates, who has used this strategy in her practice in Maine, where many patients live far from specialist care.
The study was supported by the National Heart, Lung, and Blood Institute and the University of Massachusetts Center for Clinical and Translational Science-Biostatistics, Epidemiology & Research Design Component. Dr. Geissler and Dr. Coates had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Primary care clinicians successfully manage many children with asthma, but data on specialist care according to insurance coverage are lacking, wrote Kimberley H. Geissler, PhD, of the University of Massachusetts Chan Medical School–Baystate, Springfield, Massachusetts, and colleagues.
Despite many interventions over time, “low-income children insured by Medicaid, many of whom are from minoritized racial and ethnic groups, continue to have worse outcomes and higher rates of poorly controlled asthma than children who are privately insured,” Dr. Geissler said in an interview.
“Because differences in whether a child sees an asthma specialist could contribute to these disparities, better understanding specialist use among both groups of kids may help inform potential solutions,” she said.
In a study published in JAMA Network Open, the researchers identified children with asthma aged 2-17 years using data from the Massachusetts All-Payer Claims Database for the years 2015-2020. The study population included 198,101 children and 432,455 child-year observations from children with asthma during a year when they met at least one of three criteria with any asthma diagnosis: One or more hospital visits, two or more outpatient visits, or at least one outpatient visit and at least one asthma medication.
Outpatient Visit Outcome
The primary outcome of asthma specialist care was defined as at least one outpatient visit with any asthma diagnosis to a clinician with a code of allergy and immunology, pulmonology, or otolaryngology.
A total of 66.2% of the child-year observations involved Medicaid and 33.8% involved private insurance. Approximately 15% of the children received asthma specialist care. However, nearly twice as many children with private insurance received asthma specialty care compared with those with Medicaid (20.6% vs 11.9%). In a full logistic regression analysis, children with Medicaid insurance were 55% less likely to receive asthma specialist treatment than children with private insurance.
Allergy and immunology was the most common specialty used, and the child-years for this specialty among children with Medicaid were less than half of those among children with private insurance (7.1% vs 15.9%).
Rates of persistent asthma were 20.0% and 16.9% in children with Medicaid and private insurance, respectively. Overall, children with persistent asthma were nearly four times as likely to receive asthma specialist care (adjusted odds ratio, 3.96). However, the difference in odds of receiving specialty care based on insurance type in favor of private insurance was greater among children with persistent asthma than among those without persistent asthma (−24.0 percentage points vs −20.8 percentage points).
The researchers found a similar pattern of difference in asthma specialty care in a sensitivity analysis limiting the results to child-year observations with at least one outpatient visit with any asthma diagnosis in a calendar year, although they also found a slight narrowing of the difference between the groups over time.
“Contrary to expectations, disparities in specialist care by insurance type were even more striking in children with persistent asthma,” the researchers wrote in their discussion. Notably, the growth of specialty drugs such as biologics for moderate to severe asthma are mainly prescribed by specialists, and ensuring access to specialists for children with Medicaid may reduce disparities in asthma control for those with severe or poorly controlled disease, they added.
The study findings were limited by several factors including the use only of data from Massachusetts, which may not generalize to other states, and the use of completed specialist visits without data on referrals, the researchers noted. Other limitations included a lack of data on asthma symptom frequency or control and on the setting in which an asthma diagnosis was made.
However, the results suggest a need for more attention to disparities in asthma care by insurance type, and more research is needed to determine whether these disparities persist in subsets of children with asthma, such as those with allergies or chronic medical conditions, they concluded.
Takeaways and Next Steps
“Perhaps unsurprisingly, children with private insurance were more likely to receive asthma specialist care than children with Medicaid,” Dr. Geissler told this news organization. The researchers expected a smaller gap between insurance types among children with persistent asthma, a marker for asthma severity, she said. However, “we found that the gap between those with Medicaid and those with private insurance is actually larger” for children with persistent asthma, she added.
As improved treatments for hard-to-control asthma become more available, pediatricians and primary care clinicians should follow the latest clinical guidelines for referring children to specialists for asthma care, said Dr. Geissler.
“Additionally, asthma specialists should ensure that their practices are accessible to children with Medicaid, as these families may face higher barriers to care; for example, transportation needs or scheduling challenges,” she said. Other strategies to overcome barriers to care might include electronic consultations with specialists or primary care–oriented interdisciplinary asthma clinics, which may be useful for all children with asthma but may particularly benefit those insured by Medicaid, she noted.
“Based on data limitations, we could not examine why we observed such big differences in specialist use by insurance type; for example, whether pediatricians were referring to specialists less for Medicaid-insured kids, or whether kids with Medicaid were less likely to see a specialist after a referral was made,” Dr. Geissler said. More research is needed to examine not only these factors but also the appropriateness of specialty care based on clinical guidelines to ensure high-quality evidence-based care for children with asthma who are insured by Medicaid, she said.
Improve Access and Expand Analysis
Asthma is a chronic and prevalent disease and requires a comprehensive approach that sometimes calls for specialist care, Anne Coates, MD, a pediatric pulmonologist in Portland, Maine, said in an interview.
Dr. Coates said she was surprised by the results of the current study but commended the authors for highlighting the limitations of the study, which illustrate areas for additional research. Notably, “the authors couldn’t observe referrals to specialists from primary care physicians; they used completed visits as a proxy,” Dr. Coates said.
More studies are needed to assess the completion of referral visits regardless of children’s insurance in order to better understand and address the barriers to specialty care, she added.
The current study is important because of the extent of asthma coupled with the significant number of children across the United States who are insured by Medicaid, especially underserved populations, she said.
“The burden of asthma differentially affects people of color who are living in lower resourced areas, and it is important in further research to understanding the barriers to helping people get the care they need,” Dr. Coates told this news organization. Some alternatives might include telehealth visits or even a hybrid visit to a primary care provider (PCP) who has high-speed internet, and the specialist could then conduct a telehealth visit from the PCP’s office, with the PCP acting as on-site eyes and ears, said Dr. Coates, who has used this strategy in her practice in Maine, where many patients live far from specialist care.
The study was supported by the National Heart, Lung, and Blood Institute and the University of Massachusetts Center for Clinical and Translational Science-Biostatistics, Epidemiology & Research Design Component. Dr. Geissler and Dr. Coates had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Primary care clinicians successfully manage many children with asthma, but data on specialist care according to insurance coverage are lacking, wrote Kimberley H. Geissler, PhD, of the University of Massachusetts Chan Medical School–Baystate, Springfield, Massachusetts, and colleagues.
Despite many interventions over time, “low-income children insured by Medicaid, many of whom are from minoritized racial and ethnic groups, continue to have worse outcomes and higher rates of poorly controlled asthma than children who are privately insured,” Dr. Geissler said in an interview.
“Because differences in whether a child sees an asthma specialist could contribute to these disparities, better understanding specialist use among both groups of kids may help inform potential solutions,” she said.
In a study published in JAMA Network Open, the researchers identified children with asthma aged 2-17 years using data from the Massachusetts All-Payer Claims Database for the years 2015-2020. The study population included 198,101 children and 432,455 child-year observations from children with asthma during a year when they met at least one of three criteria with any asthma diagnosis: One or more hospital visits, two or more outpatient visits, or at least one outpatient visit and at least one asthma medication.
Outpatient Visit Outcome
The primary outcome of asthma specialist care was defined as at least one outpatient visit with any asthma diagnosis to a clinician with a code of allergy and immunology, pulmonology, or otolaryngology.
A total of 66.2% of the child-year observations involved Medicaid and 33.8% involved private insurance. Approximately 15% of the children received asthma specialist care. However, nearly twice as many children with private insurance received asthma specialty care compared with those with Medicaid (20.6% vs 11.9%). In a full logistic regression analysis, children with Medicaid insurance were 55% less likely to receive asthma specialist treatment than children with private insurance.
Allergy and immunology was the most common specialty used, and the child-years for this specialty among children with Medicaid were less than half of those among children with private insurance (7.1% vs 15.9%).
Rates of persistent asthma were 20.0% and 16.9% in children with Medicaid and private insurance, respectively. Overall, children with persistent asthma were nearly four times as likely to receive asthma specialist care (adjusted odds ratio, 3.96). However, the difference in odds of receiving specialty care based on insurance type in favor of private insurance was greater among children with persistent asthma than among those without persistent asthma (−24.0 percentage points vs −20.8 percentage points).
The researchers found a similar pattern of difference in asthma specialty care in a sensitivity analysis limiting the results to child-year observations with at least one outpatient visit with any asthma diagnosis in a calendar year, although they also found a slight narrowing of the difference between the groups over time.
“Contrary to expectations, disparities in specialist care by insurance type were even more striking in children with persistent asthma,” the researchers wrote in their discussion. Notably, the growth of specialty drugs such as biologics for moderate to severe asthma are mainly prescribed by specialists, and ensuring access to specialists for children with Medicaid may reduce disparities in asthma control for those with severe or poorly controlled disease, they added.
The study findings were limited by several factors including the use only of data from Massachusetts, which may not generalize to other states, and the use of completed specialist visits without data on referrals, the researchers noted. Other limitations included a lack of data on asthma symptom frequency or control and on the setting in which an asthma diagnosis was made.
However, the results suggest a need for more attention to disparities in asthma care by insurance type, and more research is needed to determine whether these disparities persist in subsets of children with asthma, such as those with allergies or chronic medical conditions, they concluded.
Takeaways and Next Steps
“Perhaps unsurprisingly, children with private insurance were more likely to receive asthma specialist care than children with Medicaid,” Dr. Geissler told this news organization. The researchers expected a smaller gap between insurance types among children with persistent asthma, a marker for asthma severity, she said. However, “we found that the gap between those with Medicaid and those with private insurance is actually larger” for children with persistent asthma, she added.
As improved treatments for hard-to-control asthma become more available, pediatricians and primary care clinicians should follow the latest clinical guidelines for referring children to specialists for asthma care, said Dr. Geissler.
“Additionally, asthma specialists should ensure that their practices are accessible to children with Medicaid, as these families may face higher barriers to care; for example, transportation needs or scheduling challenges,” she said. Other strategies to overcome barriers to care might include electronic consultations with specialists or primary care–oriented interdisciplinary asthma clinics, which may be useful for all children with asthma but may particularly benefit those insured by Medicaid, she noted.
“Based on data limitations, we could not examine why we observed such big differences in specialist use by insurance type; for example, whether pediatricians were referring to specialists less for Medicaid-insured kids, or whether kids with Medicaid were less likely to see a specialist after a referral was made,” Dr. Geissler said. More research is needed to examine not only these factors but also the appropriateness of specialty care based on clinical guidelines to ensure high-quality evidence-based care for children with asthma who are insured by Medicaid, she said.
Improve Access and Expand Analysis
Asthma is a chronic and prevalent disease and requires a comprehensive approach that sometimes calls for specialist care, Anne Coates, MD, a pediatric pulmonologist in Portland, Maine, said in an interview.
Dr. Coates said she was surprised by the results of the current study but commended the authors for highlighting the limitations of the study, which illustrate areas for additional research. Notably, “the authors couldn’t observe referrals to specialists from primary care physicians; they used completed visits as a proxy,” Dr. Coates said.
More studies are needed to assess the completion of referral visits regardless of children’s insurance in order to better understand and address the barriers to specialty care, she added.
The current study is important because of the extent of asthma coupled with the significant number of children across the United States who are insured by Medicaid, especially underserved populations, she said.
“The burden of asthma differentially affects people of color who are living in lower resourced areas, and it is important in further research to understanding the barriers to helping people get the care they need,” Dr. Coates told this news organization. Some alternatives might include telehealth visits or even a hybrid visit to a primary care provider (PCP) who has high-speed internet, and the specialist could then conduct a telehealth visit from the PCP’s office, with the PCP acting as on-site eyes and ears, said Dr. Coates, who has used this strategy in her practice in Maine, where many patients live far from specialist care.
The study was supported by the National Heart, Lung, and Blood Institute and the University of Massachusetts Center for Clinical and Translational Science-Biostatistics, Epidemiology & Research Design Component. Dr. Geissler and Dr. Coates had no financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
Expanding recommendations for RSV vaccination
AIRWAYS DISORDERS NETWORK
Asthma and COPD Section
Respiratory syncytial virus (RSV) has been increasingly recognized as a prevalent cause of lower respiratory tract infection (LRTI) among adults in the United States. The risk of hospitalization and mortality from RSV-associated respiratory failure is higher in those with chronic lung disease. In adults aged 65 years or older, RSV has shown to cause up to 160,000 hospitalizations and 10,000 deaths annually.
RSV has been well established as a major cause of LRTI and morbidity among infants. Maternal vaccination with RSVPreF in patients who are pregnant is suggested between 32 0/7 and 36 6/7 weeks of gestation if the date of delivery falls during RSV season to prevent severe illness in young infants in their first months of life. At present, there are no data supporting vaccine administration to patients who are pregnant delivering outside of the RSV season.
What about the rest of the patients? A phase 3b clinical trial to assess the safety and immunogenicity of the RSVPreF3 vaccine in individuals 18 to 49 years of age at increased risk for RSV LRTI, including those with chronic respiratory diseases, is currently underway with projected completion in April 2025 (clinical trials.gov; ID NCT06389487). Additional studies examining safety and immunogenicity combining RSV vaccines with PCV20, influenza, COVID, or Tdap vaccines are also underway. These outcomes will be significant for future recommendations to further lower the risk of developing LRTI, hospitalization, and death among patients less than the age of 60 with chronic lung diseases.
Resources
1. Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices - United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(29):793-801.
2. Healthcare Providers: RSV Vaccination for Adults 60 Years of Age and Over. Centers for Disease Control and Prevention. Updated March 1, 2024. https://www.cdc.gov/vaccines/vpd/rsv/hcp/older-adults.html
3. Ault KA, Hughes BL, Riley LE. Maternal Respiratory Syncytial Virus Vaccination. The American College of Obstetricians and Gynecologists. Updated December 11, 2023. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2023/09/maternal-respiratory-syncytial-virus-vaccination
AIRWAYS DISORDERS NETWORK
Asthma and COPD Section
Respiratory syncytial virus (RSV) has been increasingly recognized as a prevalent cause of lower respiratory tract infection (LRTI) among adults in the United States. The risk of hospitalization and mortality from RSV-associated respiratory failure is higher in those with chronic lung disease. In adults aged 65 years or older, RSV has shown to cause up to 160,000 hospitalizations and 10,000 deaths annually.
RSV has been well established as a major cause of LRTI and morbidity among infants. Maternal vaccination with RSVPreF in patients who are pregnant is suggested between 32 0/7 and 36 6/7 weeks of gestation if the date of delivery falls during RSV season to prevent severe illness in young infants in their first months of life. At present, there are no data supporting vaccine administration to patients who are pregnant delivering outside of the RSV season.
What about the rest of the patients? A phase 3b clinical trial to assess the safety and immunogenicity of the RSVPreF3 vaccine in individuals 18 to 49 years of age at increased risk for RSV LRTI, including those with chronic respiratory diseases, is currently underway with projected completion in April 2025 (clinical trials.gov; ID NCT06389487). Additional studies examining safety and immunogenicity combining RSV vaccines with PCV20, influenza, COVID, or Tdap vaccines are also underway. These outcomes will be significant for future recommendations to further lower the risk of developing LRTI, hospitalization, and death among patients less than the age of 60 with chronic lung diseases.
Resources
1. Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices - United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(29):793-801.
2. Healthcare Providers: RSV Vaccination for Adults 60 Years of Age and Over. Centers for Disease Control and Prevention. Updated March 1, 2024. https://www.cdc.gov/vaccines/vpd/rsv/hcp/older-adults.html
3. Ault KA, Hughes BL, Riley LE. Maternal Respiratory Syncytial Virus Vaccination. The American College of Obstetricians and Gynecologists. Updated December 11, 2023. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2023/09/maternal-respiratory-syncytial-virus-vaccination
AIRWAYS DISORDERS NETWORK
Asthma and COPD Section
Respiratory syncytial virus (RSV) has been increasingly recognized as a prevalent cause of lower respiratory tract infection (LRTI) among adults in the United States. The risk of hospitalization and mortality from RSV-associated respiratory failure is higher in those with chronic lung disease. In adults aged 65 years or older, RSV has shown to cause up to 160,000 hospitalizations and 10,000 deaths annually.
RSV has been well established as a major cause of LRTI and morbidity among infants. Maternal vaccination with RSVPreF in patients who are pregnant is suggested between 32 0/7 and 36 6/7 weeks of gestation if the date of delivery falls during RSV season to prevent severe illness in young infants in their first months of life. At present, there are no data supporting vaccine administration to patients who are pregnant delivering outside of the RSV season.
What about the rest of the patients? A phase 3b clinical trial to assess the safety and immunogenicity of the RSVPreF3 vaccine in individuals 18 to 49 years of age at increased risk for RSV LRTI, including those with chronic respiratory diseases, is currently underway with projected completion in April 2025 (clinical trials.gov; ID NCT06389487). Additional studies examining safety and immunogenicity combining RSV vaccines with PCV20, influenza, COVID, or Tdap vaccines are also underway. These outcomes will be significant for future recommendations to further lower the risk of developing LRTI, hospitalization, and death among patients less than the age of 60 with chronic lung diseases.
Resources
1. Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices - United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(29):793-801.
2. Healthcare Providers: RSV Vaccination for Adults 60 Years of Age and Over. Centers for Disease Control and Prevention. Updated March 1, 2024. https://www.cdc.gov/vaccines/vpd/rsv/hcp/older-adults.html
3. Ault KA, Hughes BL, Riley LE. Maternal Respiratory Syncytial Virus Vaccination. The American College of Obstetricians and Gynecologists. Updated December 11, 2023. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2023/09/maternal-respiratory-syncytial-virus-vaccination
Pediatric Atopic Dermatitis: Study Suggests Treatment May Impact Atopic March
TOPLINE:
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from the US Collaborative Network, focusing on pediatric patients aged 18 years and younger with two AD diagnoses at least 30 days apart.
- Patients were divided into two cohorts: Those treated with dupilumab (n = 2192) and those who received conventional therapies (n = 2192), including systemic corticosteroids or conventional immunomodulators. They were stratified into three age groups: Preschoolers (< 6 years), school-aged children (6 to < 12 years), and adolescents (12-18 years).
- Both cohorts underwent 1:1 propensity score matching based on current age, age at index (first prescription of dupilumab or conventional therapy), sex, race, comorbidities, laboratory measurements, and prior medications. The primary outcome was atopic march progression, defined by incident asthma or allergic rhinitis.
TAKEAWAY:
- Over 3 years, the dupilumab-treated cohort had a significantly lower cumulative incidence of atopic march progression (20.09% vs 27.22%; P < .001), asthma (9.43% vs 14.64%; P = .001), and allergic rhinitis (13.57% vs 20.52%; P = .003) than the conventional therapy cohort.
- The risk for atopic march progression, asthma, and allergic rhinitis was also significantly reduced by 32%, 40%, and 31%, respectively, in the dupilumab vs conventional therapy cohort.
- Age-specific analyses found that the protective effect of dupilumab against allergic rhinitis was the most pronounced in adolescents (hazard ratio [HR], 0.503; 95% CI, 0.322-0.784), followed by school-aged children (HR, 0.577; 95% CI, 0.399-0.834), and preschoolers (HR, 0.623; 95% CI, 0.412-0.942).
- However, dupilumab was associated with reduced risk for asthma only in preschoolers (HR, 0.427; 95% CI, 0.247-0.738) and not in school-aged children or adolescents.
IN PRACTICE:
“Dupilumab in AD not only treats the disease but may influence atopic march mechanisms, suggesting its role as a disease-modifying atopic march drug,” the authors wrote, adding that more research “with extended follow-up and proof-of-concept is warranted.”
SOURCE:
The study was led by Teng-Li Lin, MD, Department of Dermatology, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Chiayi, Taiwan, and was published online on June 13, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The observational nature of the study limited the ability to infer direct causality between dupilumab use and reduced atopic march risk. Lack of detailed information on AD severity, total dosage, and duration of medication treatment may affect the interpretation of the study’s findings. The demographic data suggest that the dupilumab cohort had more severe AD, so the observed risk reduction may be greater than that reported in this study.
DISCLOSURES:
The study was supported in part by the National Science and Technology Council, Taiwan, and Taichung Veterans General Hospital. The authors had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com .
TOPLINE:
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from the US Collaborative Network, focusing on pediatric patients aged 18 years and younger with two AD diagnoses at least 30 days apart.
- Patients were divided into two cohorts: Those treated with dupilumab (n = 2192) and those who received conventional therapies (n = 2192), including systemic corticosteroids or conventional immunomodulators. They were stratified into three age groups: Preschoolers (< 6 years), school-aged children (6 to < 12 years), and adolescents (12-18 years).
- Both cohorts underwent 1:1 propensity score matching based on current age, age at index (first prescription of dupilumab or conventional therapy), sex, race, comorbidities, laboratory measurements, and prior medications. The primary outcome was atopic march progression, defined by incident asthma or allergic rhinitis.
TAKEAWAY:
- Over 3 years, the dupilumab-treated cohort had a significantly lower cumulative incidence of atopic march progression (20.09% vs 27.22%; P < .001), asthma (9.43% vs 14.64%; P = .001), and allergic rhinitis (13.57% vs 20.52%; P = .003) than the conventional therapy cohort.
- The risk for atopic march progression, asthma, and allergic rhinitis was also significantly reduced by 32%, 40%, and 31%, respectively, in the dupilumab vs conventional therapy cohort.
- Age-specific analyses found that the protective effect of dupilumab against allergic rhinitis was the most pronounced in adolescents (hazard ratio [HR], 0.503; 95% CI, 0.322-0.784), followed by school-aged children (HR, 0.577; 95% CI, 0.399-0.834), and preschoolers (HR, 0.623; 95% CI, 0.412-0.942).
- However, dupilumab was associated with reduced risk for asthma only in preschoolers (HR, 0.427; 95% CI, 0.247-0.738) and not in school-aged children or adolescents.
IN PRACTICE:
“Dupilumab in AD not only treats the disease but may influence atopic march mechanisms, suggesting its role as a disease-modifying atopic march drug,” the authors wrote, adding that more research “with extended follow-up and proof-of-concept is warranted.”
SOURCE:
The study was led by Teng-Li Lin, MD, Department of Dermatology, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Chiayi, Taiwan, and was published online on June 13, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The observational nature of the study limited the ability to infer direct causality between dupilumab use and reduced atopic march risk. Lack of detailed information on AD severity, total dosage, and duration of medication treatment may affect the interpretation of the study’s findings. The demographic data suggest that the dupilumab cohort had more severe AD, so the observed risk reduction may be greater than that reported in this study.
DISCLOSURES:
The study was supported in part by the National Science and Technology Council, Taiwan, and Taichung Veterans General Hospital. The authors had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com .
TOPLINE:
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from the US Collaborative Network, focusing on pediatric patients aged 18 years and younger with two AD diagnoses at least 30 days apart.
- Patients were divided into two cohorts: Those treated with dupilumab (n = 2192) and those who received conventional therapies (n = 2192), including systemic corticosteroids or conventional immunomodulators. They were stratified into three age groups: Preschoolers (< 6 years), school-aged children (6 to < 12 years), and adolescents (12-18 years).
- Both cohorts underwent 1:1 propensity score matching based on current age, age at index (first prescription of dupilumab or conventional therapy), sex, race, comorbidities, laboratory measurements, and prior medications. The primary outcome was atopic march progression, defined by incident asthma or allergic rhinitis.
TAKEAWAY:
- Over 3 years, the dupilumab-treated cohort had a significantly lower cumulative incidence of atopic march progression (20.09% vs 27.22%; P < .001), asthma (9.43% vs 14.64%; P = .001), and allergic rhinitis (13.57% vs 20.52%; P = .003) than the conventional therapy cohort.
- The risk for atopic march progression, asthma, and allergic rhinitis was also significantly reduced by 32%, 40%, and 31%, respectively, in the dupilumab vs conventional therapy cohort.
- Age-specific analyses found that the protective effect of dupilumab against allergic rhinitis was the most pronounced in adolescents (hazard ratio [HR], 0.503; 95% CI, 0.322-0.784), followed by school-aged children (HR, 0.577; 95% CI, 0.399-0.834), and preschoolers (HR, 0.623; 95% CI, 0.412-0.942).
- However, dupilumab was associated with reduced risk for asthma only in preschoolers (HR, 0.427; 95% CI, 0.247-0.738) and not in school-aged children or adolescents.
IN PRACTICE:
“Dupilumab in AD not only treats the disease but may influence atopic march mechanisms, suggesting its role as a disease-modifying atopic march drug,” the authors wrote, adding that more research “with extended follow-up and proof-of-concept is warranted.”
SOURCE:
The study was led by Teng-Li Lin, MD, Department of Dermatology, Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Chiayi, Taiwan, and was published online on June 13, 2024, in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The observational nature of the study limited the ability to infer direct causality between dupilumab use and reduced atopic march risk. Lack of detailed information on AD severity, total dosage, and duration of medication treatment may affect the interpretation of the study’s findings. The demographic data suggest that the dupilumab cohort had more severe AD, so the observed risk reduction may be greater than that reported in this study.
DISCLOSURES:
The study was supported in part by the National Science and Technology Council, Taiwan, and Taichung Veterans General Hospital. The authors had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article appeared on Medscape.com .