User login
Intrathecal methotrexate dosing in acute leukemia falls short
STOCKHOLM – Although intrathecal chemoprophylaxis for prevention of central nervous system involvement is an essential component of modern regimens to treat acute lymphoblastic leukemia (ALL), patients don’t always receive the recommended number of doses, potentially compromising remissions.
But as a large-scale audit of health care delivery in the United Kingdom has suggested, many of the possible causes for suboptimal delivery of intrathecal methotrexate (IT MTX) appear to be modifiable, reported Sven A. Sommerfeld, MD, and his colleagues from the Christie Hospital in Manchester, England.
“In our clinical observations, and confirmed in this audit, patients on intensive chemotherapy, including induction and consolidation protocols for acute lymphoblastic leukemia, can develop a number of issues and toxicities, which can interfere with the administration of IT MTX,” they wrote in a poster presented at the annual congress of the European Hematology Association.
Reasons for canceling or postponing scheduled doses of IT MTX range from “fairly compelling clinical reasons affecting patient safety or tolerance” to more mundane issues, such as scheduling and staffing problems, the investigators found.
“I think there are a number of factors that can improve compliance, including having your cancellation rate as low as possible. You have already prescribed the treatment, the patient is supposed to be attending [clinic], yet for some reason you cannot go ahead,” Dr. Sommerfeld said in an interview. “I think blood product support is important, but there are capacity issues. Organization of complex treatment protocols that involve systemic chemotherapy and concurrent intrathecal administration can be difficult as different people oversee both of these treatments.”
For example, protocols specify delivery of intrathecal chemoprophylaxis on specific days and induction chemotherapy on other days, and it may be difficult to coordinate the care so that the doses don’t overlap, he said.
Dr. Sommerfeld and his colleagues conducted an audit of standard of care for patients aged 16-60 years who were diagnosed with B-cell or T-cell ALL and received IT MTX under one of two clinical protocols: UKALL 2011 or UKALL 14.
The investigators examined data on IT MTX compliance and assigned each patient a compliance score calculated by the number of administered doses divided by protocol-defined number of doses. They also examined pharmacy records of cancellations of protocol-scheduled IT MTX from January 2016 through July 2017.
They found that the total number of IT MTX prescriptions delivered as a proportion of the number prescribed ranged from a low of 61.8% in 2009 to a high of 84.2% in 2007.
When the investigators looked at records for individual patients, including 29 adolescent and young adults receiving IT MTX under the UKALL 2011 protocol and 27 adults receiving it under the UKALL 14 protocol, they found that failure to maintain coagulation levels within parameters accounted for 23% of cancellations. The next most common reasons for cancellation were rescheduling for administrative or clinical reasons in 20% of canceled doses, low platelet levels in 19% of cases, and patient nonattendance or communication failure in 16% of cases. Other factors included problems with lumbar access, vincristine schedule for the same day, recent anticoagulation, and delay of blood results.
“Notable is the variable approach to IT MTX in patients requiring therapeutic dose anticoagulation, reflecting clinical decisions of different physicians on a case-by-case basis. Whilst a full discussion of this topic and management of asparaginase-associated [venous thromboembolism] is beyond the scope of this audit, we generally recognize today that many affected patients can be managed to continue therapy as per protocol,” the investigators wrote.
Integrating relevant prescriptions into a single information system, monitoring clinics for treatment backlogs, and improving clinical resources, such as staffing, could help to improve the efficacy of IT MTX therapy, they suggested.
The study was supported by the National Health Service Foundation Trust. Dr. Sommerfeld reported having no relevant financial disclosures.
SOURCE: Sommerfeld SA et al. EHA Congress, Abstract PS930.
STOCKHOLM – Although intrathecal chemoprophylaxis for prevention of central nervous system involvement is an essential component of modern regimens to treat acute lymphoblastic leukemia (ALL), patients don’t always receive the recommended number of doses, potentially compromising remissions.
But as a large-scale audit of health care delivery in the United Kingdom has suggested, many of the possible causes for suboptimal delivery of intrathecal methotrexate (IT MTX) appear to be modifiable, reported Sven A. Sommerfeld, MD, and his colleagues from the Christie Hospital in Manchester, England.
“In our clinical observations, and confirmed in this audit, patients on intensive chemotherapy, including induction and consolidation protocols for acute lymphoblastic leukemia, can develop a number of issues and toxicities, which can interfere with the administration of IT MTX,” they wrote in a poster presented at the annual congress of the European Hematology Association.
Reasons for canceling or postponing scheduled doses of IT MTX range from “fairly compelling clinical reasons affecting patient safety or tolerance” to more mundane issues, such as scheduling and staffing problems, the investigators found.
“I think there are a number of factors that can improve compliance, including having your cancellation rate as low as possible. You have already prescribed the treatment, the patient is supposed to be attending [clinic], yet for some reason you cannot go ahead,” Dr. Sommerfeld said in an interview. “I think blood product support is important, but there are capacity issues. Organization of complex treatment protocols that involve systemic chemotherapy and concurrent intrathecal administration can be difficult as different people oversee both of these treatments.”
For example, protocols specify delivery of intrathecal chemoprophylaxis on specific days and induction chemotherapy on other days, and it may be difficult to coordinate the care so that the doses don’t overlap, he said.
Dr. Sommerfeld and his colleagues conducted an audit of standard of care for patients aged 16-60 years who were diagnosed with B-cell or T-cell ALL and received IT MTX under one of two clinical protocols: UKALL 2011 or UKALL 14.
The investigators examined data on IT MTX compliance and assigned each patient a compliance score calculated by the number of administered doses divided by protocol-defined number of doses. They also examined pharmacy records of cancellations of protocol-scheduled IT MTX from January 2016 through July 2017.
They found that the total number of IT MTX prescriptions delivered as a proportion of the number prescribed ranged from a low of 61.8% in 2009 to a high of 84.2% in 2007.
When the investigators looked at records for individual patients, including 29 adolescent and young adults receiving IT MTX under the UKALL 2011 protocol and 27 adults receiving it under the UKALL 14 protocol, they found that failure to maintain coagulation levels within parameters accounted for 23% of cancellations. The next most common reasons for cancellation were rescheduling for administrative or clinical reasons in 20% of canceled doses, low platelet levels in 19% of cases, and patient nonattendance or communication failure in 16% of cases. Other factors included problems with lumbar access, vincristine schedule for the same day, recent anticoagulation, and delay of blood results.
“Notable is the variable approach to IT MTX in patients requiring therapeutic dose anticoagulation, reflecting clinical decisions of different physicians on a case-by-case basis. Whilst a full discussion of this topic and management of asparaginase-associated [venous thromboembolism] is beyond the scope of this audit, we generally recognize today that many affected patients can be managed to continue therapy as per protocol,” the investigators wrote.
Integrating relevant prescriptions into a single information system, monitoring clinics for treatment backlogs, and improving clinical resources, such as staffing, could help to improve the efficacy of IT MTX therapy, they suggested.
The study was supported by the National Health Service Foundation Trust. Dr. Sommerfeld reported having no relevant financial disclosures.
SOURCE: Sommerfeld SA et al. EHA Congress, Abstract PS930.
STOCKHOLM – Although intrathecal chemoprophylaxis for prevention of central nervous system involvement is an essential component of modern regimens to treat acute lymphoblastic leukemia (ALL), patients don’t always receive the recommended number of doses, potentially compromising remissions.
But as a large-scale audit of health care delivery in the United Kingdom has suggested, many of the possible causes for suboptimal delivery of intrathecal methotrexate (IT MTX) appear to be modifiable, reported Sven A. Sommerfeld, MD, and his colleagues from the Christie Hospital in Manchester, England.
“In our clinical observations, and confirmed in this audit, patients on intensive chemotherapy, including induction and consolidation protocols for acute lymphoblastic leukemia, can develop a number of issues and toxicities, which can interfere with the administration of IT MTX,” they wrote in a poster presented at the annual congress of the European Hematology Association.
Reasons for canceling or postponing scheduled doses of IT MTX range from “fairly compelling clinical reasons affecting patient safety or tolerance” to more mundane issues, such as scheduling and staffing problems, the investigators found.
“I think there are a number of factors that can improve compliance, including having your cancellation rate as low as possible. You have already prescribed the treatment, the patient is supposed to be attending [clinic], yet for some reason you cannot go ahead,” Dr. Sommerfeld said in an interview. “I think blood product support is important, but there are capacity issues. Organization of complex treatment protocols that involve systemic chemotherapy and concurrent intrathecal administration can be difficult as different people oversee both of these treatments.”
For example, protocols specify delivery of intrathecal chemoprophylaxis on specific days and induction chemotherapy on other days, and it may be difficult to coordinate the care so that the doses don’t overlap, he said.
Dr. Sommerfeld and his colleagues conducted an audit of standard of care for patients aged 16-60 years who were diagnosed with B-cell or T-cell ALL and received IT MTX under one of two clinical protocols: UKALL 2011 or UKALL 14.
The investigators examined data on IT MTX compliance and assigned each patient a compliance score calculated by the number of administered doses divided by protocol-defined number of doses. They also examined pharmacy records of cancellations of protocol-scheduled IT MTX from January 2016 through July 2017.
They found that the total number of IT MTX prescriptions delivered as a proportion of the number prescribed ranged from a low of 61.8% in 2009 to a high of 84.2% in 2007.
When the investigators looked at records for individual patients, including 29 adolescent and young adults receiving IT MTX under the UKALL 2011 protocol and 27 adults receiving it under the UKALL 14 protocol, they found that failure to maintain coagulation levels within parameters accounted for 23% of cancellations. The next most common reasons for cancellation were rescheduling for administrative or clinical reasons in 20% of canceled doses, low platelet levels in 19% of cases, and patient nonattendance or communication failure in 16% of cases. Other factors included problems with lumbar access, vincristine schedule for the same day, recent anticoagulation, and delay of blood results.
“Notable is the variable approach to IT MTX in patients requiring therapeutic dose anticoagulation, reflecting clinical decisions of different physicians on a case-by-case basis. Whilst a full discussion of this topic and management of asparaginase-associated [venous thromboembolism] is beyond the scope of this audit, we generally recognize today that many affected patients can be managed to continue therapy as per protocol,” the investigators wrote.
Integrating relevant prescriptions into a single information system, monitoring clinics for treatment backlogs, and improving clinical resources, such as staffing, could help to improve the efficacy of IT MTX therapy, they suggested.
The study was supported by the National Health Service Foundation Trust. Dr. Sommerfeld reported having no relevant financial disclosures.
SOURCE: Sommerfeld SA et al. EHA Congress, Abstract PS930.
REPORTING FROM THE EHA CONGRESS
Key clinical point:
Major finding: The total number of intrathecal methotrexate prescriptions delivered as a proportion of the number prescribed ranged from a low of 61.8% in 2009 to a high of 84.2% in 2007.
Study details: Audit of standard of care delivery of intrathecal methotrexate in the United Kingdom.
Disclosures: The study was supported by the National Health Service Foundation Trust. Dr. Sommerfeld reported having no relevant financial disclosures.
Source: Sommerfeld SA et al. EHA Congress, Abstract PS930.
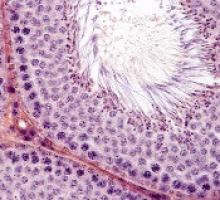
Treatments, disease affect spermatogonia in boys
Alkylating agents, hydroxyurea (HU), and certain non-malignant diseases can significantly deplete spermatogonial cell counts in young boys, according to research published in Human Reproduction.
Boys who received alkylating agents to treat cancer had significantly lower spermatogonial cell counts than control subjects or boys with malignant/nonmalignant diseases treated with non-alkylating agents.
Five of 6 SCD patients treated with HU had a totally depleted spermatogonial pool, and the remaining patient had a low spermatogonial cell count.
Five boys with non-malignant diseases who were not exposed to chemotherapy had significantly lower spermatogonial cell counts than controls.
“Our findings of a dramatic decrease in germ cell numbers in boys treated with alkylating agents and in sickle cell disease patients treated with hydroxyurea suggest that storing frozen testicular tissue from these boys should be performed before these treatments are initiated,” said study author Cecilia Petersen, MD, PhD, of Karolinska Institutet and University Hospital in Stockholm, Sweden.
“This needs to be communicated to physicians as well as patients and their parents or carers. However, until sperm that are able to fertilize eggs are produced from stored testicular tissue, we cannot confirm that germ cell quantity might determine the success of transplantation of the tissue in adulthood. Further research on this is needed to establish a realistic fertility preservation technique.”
Dr Petersen and her colleagues also noted that preserving testicular tissue may not be a viable option for boys who have low spermatogonial cell counts prior to treatment.
Patients and controls
For this study, the researchers analyzed testicular tissue from 32 boys facing treatments that carried a high risk of infertility—testicular irradiation, chemotherapy, or radiotherapy in advance of stem cell transplant.
Twenty boys had the tissue taken after initial chemotherapy, and 12 had it taken before starting any treatment.1
Eight patients had received chemotherapy with non-alkylating agents, 6 (all with malignancies) had received alkylating agents, and 6 (all with SCD) had received HU.
Diseases included acute lymphoblastic leukemia (n=6), SCD (n=6), acute myeloid leukemia (n=3), thalassemia major (n=3), neuroblastoma (n=2), juvenile myelomonocytic leukemia (n=2), myelodysplastic syndromes (n=2), primary immunodeficiency (n=2), Wilms tumor (n=1), adrenoleukodystrophy (n=1), hepatoblastoma (n=1), primitive neuroectodermal tumor (n=1), severe aplastic anemia (n=1), and Fanconi anemia (n=1).
The researchers compared samples from these 32 patients to 14 healthy testicular tissue samples stored in the biobank at the Karolinska University Hospital.
For both sample types, the team counted the number of spermatogonial cells found in a cross-section of seminiferous tubules.
“We could compare the number of spermatogonia with those found in the healthy boys as a way to estimate the effect of medical treatment or the disease itself on the future fertility of a patient,” explained study author Jan-Bernd Stukenborg, PhD, of Karolinska Institutet and University Hospital.
Impact of treatment
There was no significant difference in the mean quantity of spermatogonia per transverse tubular cross-section (S/T) between patients exposed to non-alkylating agents (1.7 ± 1.0, n=8) and biobank controls (4.1 ± 4.6, n=14).
However, samples from patients who received alkylating agents had a significantly lower mean S/T value (0.2 ± 0.3, n=6) than samples from patients treated with non-alkylating agents (P=0.003) and biobank controls (P<0.001).
“We found that the numbers of germ cells present in the cross-sections of the seminiferous tubules were significantly depleted and close to 0 in patients treated with alkylating agents,” Dr Stukenborg said.
Samples from the SCD patients also had a significantly lower mean S/T value (0.3 ± 0.6, n=6) than biobank controls (P=0.003).
Dr Stukenborg noted that the germ cell pool was totally depleted in 5 of the boys with SCD, and the pool was “very low” in the sixth SCD patient.
“This was not seen in patients who had not started treatment or were treated with non-alkylating agents or in the biobank tissues,” Dr Stukenborg said.2
He and his colleagues noted that it is possible for germ cells to recover to normal levels after treatment that is highly toxic to the testes, but high doses of alkylating agents and radiotherapy to the testicles are strongly associated with permanent or long-term infertility.
“The first group of boys who received bone marrow transplants are now reaching their thirties,” said study author Kirsi Jahnukainen, MD, PhD, of Helsinki University Central Hospital in Finland.
“Recent data suggest they may have a high chance of their sperm production recovering, even if they received high-dose alkylating therapies, so long as they had no testicular irradiation.”
Impact of disease
The researchers also found evidence to suggest that, for some boys, their disease may have affected spermatogonial cell counts before any treatment began.
Five patients with non-malignant disease who had not been exposed to chemotherapy (3 with thalassemia major, 1 with Fanconi anemia, and 1 with primary immunodeficiency) had a significantly lower mean S/T value (0.4 ± 0.5) than controls (P=0.006).
“Among patients who had not been treated previously with chemotherapy, there were several boys with a low number of germ cells for their age,” Dr Jahnukainen said.
“This suggests that some non-malignant diseases that require bone marrow transplants may affect the fertility of young boys even before exposure to therapy that is toxic for the testes.”
The researchers noted that a limitation of this study was that biobank samples had no detailed information regarding previous medical treatments and testicular volumes.
1. Testicular tissue is taken from patients under general anesthesia. The surgeon removes approximately 20% of the tissue from the testicular capsule in one of the testicles. For this study, a third of the tissue was taken to the Karolinska Institutet for analysis.
2. A recent meta-analysis showed that normal testicular tissue samples of newborns contain approximately 2.5 germ cells per tubular cross-section. This number decreases to approximately 1.2 within the first 3 years of age, followed by an increase up to 2.6 germ cells per tubular cross-section at 6 to 7 years, reaching a plateau until the age of 11. At the onset of puberty, an increase of up to 7 spermatogonia per tubular cross-section could be observed.
Alkylating agents, hydroxyurea (HU), and certain non-malignant diseases can significantly deplete spermatogonial cell counts in young boys, according to research published in Human Reproduction.
Boys who received alkylating agents to treat cancer had significantly lower spermatogonial cell counts than control subjects or boys with malignant/nonmalignant diseases treated with non-alkylating agents.
Five of 6 SCD patients treated with HU had a totally depleted spermatogonial pool, and the remaining patient had a low spermatogonial cell count.
Five boys with non-malignant diseases who were not exposed to chemotherapy had significantly lower spermatogonial cell counts than controls.
“Our findings of a dramatic decrease in germ cell numbers in boys treated with alkylating agents and in sickle cell disease patients treated with hydroxyurea suggest that storing frozen testicular tissue from these boys should be performed before these treatments are initiated,” said study author Cecilia Petersen, MD, PhD, of Karolinska Institutet and University Hospital in Stockholm, Sweden.
“This needs to be communicated to physicians as well as patients and their parents or carers. However, until sperm that are able to fertilize eggs are produced from stored testicular tissue, we cannot confirm that germ cell quantity might determine the success of transplantation of the tissue in adulthood. Further research on this is needed to establish a realistic fertility preservation technique.”
Dr Petersen and her colleagues also noted that preserving testicular tissue may not be a viable option for boys who have low spermatogonial cell counts prior to treatment.
Patients and controls
For this study, the researchers analyzed testicular tissue from 32 boys facing treatments that carried a high risk of infertility—testicular irradiation, chemotherapy, or radiotherapy in advance of stem cell transplant.
Twenty boys had the tissue taken after initial chemotherapy, and 12 had it taken before starting any treatment.1
Eight patients had received chemotherapy with non-alkylating agents, 6 (all with malignancies) had received alkylating agents, and 6 (all with SCD) had received HU.
Diseases included acute lymphoblastic leukemia (n=6), SCD (n=6), acute myeloid leukemia (n=3), thalassemia major (n=3), neuroblastoma (n=2), juvenile myelomonocytic leukemia (n=2), myelodysplastic syndromes (n=2), primary immunodeficiency (n=2), Wilms tumor (n=1), adrenoleukodystrophy (n=1), hepatoblastoma (n=1), primitive neuroectodermal tumor (n=1), severe aplastic anemia (n=1), and Fanconi anemia (n=1).
The researchers compared samples from these 32 patients to 14 healthy testicular tissue samples stored in the biobank at the Karolinska University Hospital.
For both sample types, the team counted the number of spermatogonial cells found in a cross-section of seminiferous tubules.
“We could compare the number of spermatogonia with those found in the healthy boys as a way to estimate the effect of medical treatment or the disease itself on the future fertility of a patient,” explained study author Jan-Bernd Stukenborg, PhD, of Karolinska Institutet and University Hospital.
Impact of treatment
There was no significant difference in the mean quantity of spermatogonia per transverse tubular cross-section (S/T) between patients exposed to non-alkylating agents (1.7 ± 1.0, n=8) and biobank controls (4.1 ± 4.6, n=14).
However, samples from patients who received alkylating agents had a significantly lower mean S/T value (0.2 ± 0.3, n=6) than samples from patients treated with non-alkylating agents (P=0.003) and biobank controls (P<0.001).
“We found that the numbers of germ cells present in the cross-sections of the seminiferous tubules were significantly depleted and close to 0 in patients treated with alkylating agents,” Dr Stukenborg said.
Samples from the SCD patients also had a significantly lower mean S/T value (0.3 ± 0.6, n=6) than biobank controls (P=0.003).
Dr Stukenborg noted that the germ cell pool was totally depleted in 5 of the boys with SCD, and the pool was “very low” in the sixth SCD patient.
“This was not seen in patients who had not started treatment or were treated with non-alkylating agents or in the biobank tissues,” Dr Stukenborg said.2
He and his colleagues noted that it is possible for germ cells to recover to normal levels after treatment that is highly toxic to the testes, but high doses of alkylating agents and radiotherapy to the testicles are strongly associated with permanent or long-term infertility.
“The first group of boys who received bone marrow transplants are now reaching their thirties,” said study author Kirsi Jahnukainen, MD, PhD, of Helsinki University Central Hospital in Finland.
“Recent data suggest they may have a high chance of their sperm production recovering, even if they received high-dose alkylating therapies, so long as they had no testicular irradiation.”
Impact of disease
The researchers also found evidence to suggest that, for some boys, their disease may have affected spermatogonial cell counts before any treatment began.
Five patients with non-malignant disease who had not been exposed to chemotherapy (3 with thalassemia major, 1 with Fanconi anemia, and 1 with primary immunodeficiency) had a significantly lower mean S/T value (0.4 ± 0.5) than controls (P=0.006).
“Among patients who had not been treated previously with chemotherapy, there were several boys with a low number of germ cells for their age,” Dr Jahnukainen said.
“This suggests that some non-malignant diseases that require bone marrow transplants may affect the fertility of young boys even before exposure to therapy that is toxic for the testes.”
The researchers noted that a limitation of this study was that biobank samples had no detailed information regarding previous medical treatments and testicular volumes.
1. Testicular tissue is taken from patients under general anesthesia. The surgeon removes approximately 20% of the tissue from the testicular capsule in one of the testicles. For this study, a third of the tissue was taken to the Karolinska Institutet for analysis.
2. A recent meta-analysis showed that normal testicular tissue samples of newborns contain approximately 2.5 germ cells per tubular cross-section. This number decreases to approximately 1.2 within the first 3 years of age, followed by an increase up to 2.6 germ cells per tubular cross-section at 6 to 7 years, reaching a plateau until the age of 11. At the onset of puberty, an increase of up to 7 spermatogonia per tubular cross-section could be observed.
Alkylating agents, hydroxyurea (HU), and certain non-malignant diseases can significantly deplete spermatogonial cell counts in young boys, according to research published in Human Reproduction.
Boys who received alkylating agents to treat cancer had significantly lower spermatogonial cell counts than control subjects or boys with malignant/nonmalignant diseases treated with non-alkylating agents.
Five of 6 SCD patients treated with HU had a totally depleted spermatogonial pool, and the remaining patient had a low spermatogonial cell count.
Five boys with non-malignant diseases who were not exposed to chemotherapy had significantly lower spermatogonial cell counts than controls.
“Our findings of a dramatic decrease in germ cell numbers in boys treated with alkylating agents and in sickle cell disease patients treated with hydroxyurea suggest that storing frozen testicular tissue from these boys should be performed before these treatments are initiated,” said study author Cecilia Petersen, MD, PhD, of Karolinska Institutet and University Hospital in Stockholm, Sweden.
“This needs to be communicated to physicians as well as patients and their parents or carers. However, until sperm that are able to fertilize eggs are produced from stored testicular tissue, we cannot confirm that germ cell quantity might determine the success of transplantation of the tissue in adulthood. Further research on this is needed to establish a realistic fertility preservation technique.”
Dr Petersen and her colleagues also noted that preserving testicular tissue may not be a viable option for boys who have low spermatogonial cell counts prior to treatment.
Patients and controls
For this study, the researchers analyzed testicular tissue from 32 boys facing treatments that carried a high risk of infertility—testicular irradiation, chemotherapy, or radiotherapy in advance of stem cell transplant.
Twenty boys had the tissue taken after initial chemotherapy, and 12 had it taken before starting any treatment.1
Eight patients had received chemotherapy with non-alkylating agents, 6 (all with malignancies) had received alkylating agents, and 6 (all with SCD) had received HU.
Diseases included acute lymphoblastic leukemia (n=6), SCD (n=6), acute myeloid leukemia (n=3), thalassemia major (n=3), neuroblastoma (n=2), juvenile myelomonocytic leukemia (n=2), myelodysplastic syndromes (n=2), primary immunodeficiency (n=2), Wilms tumor (n=1), adrenoleukodystrophy (n=1), hepatoblastoma (n=1), primitive neuroectodermal tumor (n=1), severe aplastic anemia (n=1), and Fanconi anemia (n=1).
The researchers compared samples from these 32 patients to 14 healthy testicular tissue samples stored in the biobank at the Karolinska University Hospital.
For both sample types, the team counted the number of spermatogonial cells found in a cross-section of seminiferous tubules.
“We could compare the number of spermatogonia with those found in the healthy boys as a way to estimate the effect of medical treatment or the disease itself on the future fertility of a patient,” explained study author Jan-Bernd Stukenborg, PhD, of Karolinska Institutet and University Hospital.
Impact of treatment
There was no significant difference in the mean quantity of spermatogonia per transverse tubular cross-section (S/T) between patients exposed to non-alkylating agents (1.7 ± 1.0, n=8) and biobank controls (4.1 ± 4.6, n=14).
However, samples from patients who received alkylating agents had a significantly lower mean S/T value (0.2 ± 0.3, n=6) than samples from patients treated with non-alkylating agents (P=0.003) and biobank controls (P<0.001).
“We found that the numbers of germ cells present in the cross-sections of the seminiferous tubules were significantly depleted and close to 0 in patients treated with alkylating agents,” Dr Stukenborg said.
Samples from the SCD patients also had a significantly lower mean S/T value (0.3 ± 0.6, n=6) than biobank controls (P=0.003).
Dr Stukenborg noted that the germ cell pool was totally depleted in 5 of the boys with SCD, and the pool was “very low” in the sixth SCD patient.
“This was not seen in patients who had not started treatment or were treated with non-alkylating agents or in the biobank tissues,” Dr Stukenborg said.2
He and his colleagues noted that it is possible for germ cells to recover to normal levels after treatment that is highly toxic to the testes, but high doses of alkylating agents and radiotherapy to the testicles are strongly associated with permanent or long-term infertility.
“The first group of boys who received bone marrow transplants are now reaching their thirties,” said study author Kirsi Jahnukainen, MD, PhD, of Helsinki University Central Hospital in Finland.
“Recent data suggest they may have a high chance of their sperm production recovering, even if they received high-dose alkylating therapies, so long as they had no testicular irradiation.”
Impact of disease
The researchers also found evidence to suggest that, for some boys, their disease may have affected spermatogonial cell counts before any treatment began.
Five patients with non-malignant disease who had not been exposed to chemotherapy (3 with thalassemia major, 1 with Fanconi anemia, and 1 with primary immunodeficiency) had a significantly lower mean S/T value (0.4 ± 0.5) than controls (P=0.006).
“Among patients who had not been treated previously with chemotherapy, there were several boys with a low number of germ cells for their age,” Dr Jahnukainen said.
“This suggests that some non-malignant diseases that require bone marrow transplants may affect the fertility of young boys even before exposure to therapy that is toxic for the testes.”
The researchers noted that a limitation of this study was that biobank samples had no detailed information regarding previous medical treatments and testicular volumes.
1. Testicular tissue is taken from patients under general anesthesia. The surgeon removes approximately 20% of the tissue from the testicular capsule in one of the testicles. For this study, a third of the tissue was taken to the Karolinska Institutet for analysis.
2. A recent meta-analysis showed that normal testicular tissue samples of newborns contain approximately 2.5 germ cells per tubular cross-section. This number decreases to approximately 1.2 within the first 3 years of age, followed by an increase up to 2.6 germ cells per tubular cross-section at 6 to 7 years, reaching a plateau until the age of 11. At the onset of puberty, an increase of up to 7 spermatogonia per tubular cross-section could be observed.
Team finds potential therapeutic targets for T-ALL
Researchers have found the NOTCH1 pathway “hijacks” heat shock transcription factor 1 (HSF1) signaling in T-cell acute lymphoblastic leukemia (T-ALL).
Therefore, blocking one or more genes in the HSF1 pathway could represent a new approach to treating T-ALL.
An experimental drug, PU-H71, is already in development against one of these targets, heat shock protein 90 (HSP90).
The researchers found that PU-H71 was active against T-ALL in vitro and in vivo.
The team recounted these findings in Nature Medicine.
“Our study shows how the NOTCH1 pathway hijacks the heat shock transcription factor 1 pathway to promote tumor growth,” said study author Iannis Aifantis, PhD, of NYU School of Medicine in New York, New York.
“The cancer cells are sending into overdrive a system that helps healthy cells respond to stress.”
Dr Aifantis and his colleagues found that HSF1 is involved in the pathogenesis of T-ALL. When they knocked down HSF1 in T-ALL cell lines, the researchers observed an increase in apoptosis, defective proteostasis, and a decrease in the growth of leukemic cells.
Similarly, HSF1 was deemed necessary for disease progression in mouse models of T-ALL. When the researchers deleted HSF1, mice experienced “striking” reductions in leukemic burden and “dramatic” improvements in survival. However, HSF1 deletion did not affect normal hematopoiesis.
Dr Aifantis and his colleagues also showed that NOTCH1 regulates the epichaperome in T-ALL. The team said previous studies have shown that, in the presence of oncogenic stress, heat shock proteins participate in a network nucleated by HSP90 and HSP70 chaperones—the epichaperome.
The researchers found that an intact epichaperome was critical for T-ALL by showing that pharmacologic inhibition of HSP90 and HSP70 significantly hindered the growth of human T-ALL in vitro. The team also found the HSP90 inhibitor PU-H71 reduced leukemic burden and extended survival in a NOTCH1-inducible T-ALL mouse model.
Then, the researchers found NOTCH1 levels could predict response to HSP90 inhibition in vitro. T-ALL patient samples expressing high levels of nuclear NOTCH1 and high levels of epichaperome were significantly more sensitive to treatment with PU-H71.
PU-H71 is already in early clinical trials of patients with breast cancer. If further testing proves successful, PU-H71 could be quickly adapted for trials in T-ALL patients, according to Dr Aifantis.
In the meantime, he and his colleagues plan to evaluate the effects of another 8 proteins produced by genes active in the HSF1 pathway to see if any show promising anticancer activity in T-ALL.
Researchers have found the NOTCH1 pathway “hijacks” heat shock transcription factor 1 (HSF1) signaling in T-cell acute lymphoblastic leukemia (T-ALL).
Therefore, blocking one or more genes in the HSF1 pathway could represent a new approach to treating T-ALL.
An experimental drug, PU-H71, is already in development against one of these targets, heat shock protein 90 (HSP90).
The researchers found that PU-H71 was active against T-ALL in vitro and in vivo.
The team recounted these findings in Nature Medicine.
“Our study shows how the NOTCH1 pathway hijacks the heat shock transcription factor 1 pathway to promote tumor growth,” said study author Iannis Aifantis, PhD, of NYU School of Medicine in New York, New York.
“The cancer cells are sending into overdrive a system that helps healthy cells respond to stress.”
Dr Aifantis and his colleagues found that HSF1 is involved in the pathogenesis of T-ALL. When they knocked down HSF1 in T-ALL cell lines, the researchers observed an increase in apoptosis, defective proteostasis, and a decrease in the growth of leukemic cells.
Similarly, HSF1 was deemed necessary for disease progression in mouse models of T-ALL. When the researchers deleted HSF1, mice experienced “striking” reductions in leukemic burden and “dramatic” improvements in survival. However, HSF1 deletion did not affect normal hematopoiesis.
Dr Aifantis and his colleagues also showed that NOTCH1 regulates the epichaperome in T-ALL. The team said previous studies have shown that, in the presence of oncogenic stress, heat shock proteins participate in a network nucleated by HSP90 and HSP70 chaperones—the epichaperome.
The researchers found that an intact epichaperome was critical for T-ALL by showing that pharmacologic inhibition of HSP90 and HSP70 significantly hindered the growth of human T-ALL in vitro. The team also found the HSP90 inhibitor PU-H71 reduced leukemic burden and extended survival in a NOTCH1-inducible T-ALL mouse model.
Then, the researchers found NOTCH1 levels could predict response to HSP90 inhibition in vitro. T-ALL patient samples expressing high levels of nuclear NOTCH1 and high levels of epichaperome were significantly more sensitive to treatment with PU-H71.
PU-H71 is already in early clinical trials of patients with breast cancer. If further testing proves successful, PU-H71 could be quickly adapted for trials in T-ALL patients, according to Dr Aifantis.
In the meantime, he and his colleagues plan to evaluate the effects of another 8 proteins produced by genes active in the HSF1 pathway to see if any show promising anticancer activity in T-ALL.
Researchers have found the NOTCH1 pathway “hijacks” heat shock transcription factor 1 (HSF1) signaling in T-cell acute lymphoblastic leukemia (T-ALL).
Therefore, blocking one or more genes in the HSF1 pathway could represent a new approach to treating T-ALL.
An experimental drug, PU-H71, is already in development against one of these targets, heat shock protein 90 (HSP90).
The researchers found that PU-H71 was active against T-ALL in vitro and in vivo.
The team recounted these findings in Nature Medicine.
“Our study shows how the NOTCH1 pathway hijacks the heat shock transcription factor 1 pathway to promote tumor growth,” said study author Iannis Aifantis, PhD, of NYU School of Medicine in New York, New York.
“The cancer cells are sending into overdrive a system that helps healthy cells respond to stress.”
Dr Aifantis and his colleagues found that HSF1 is involved in the pathogenesis of T-ALL. When they knocked down HSF1 in T-ALL cell lines, the researchers observed an increase in apoptosis, defective proteostasis, and a decrease in the growth of leukemic cells.
Similarly, HSF1 was deemed necessary for disease progression in mouse models of T-ALL. When the researchers deleted HSF1, mice experienced “striking” reductions in leukemic burden and “dramatic” improvements in survival. However, HSF1 deletion did not affect normal hematopoiesis.
Dr Aifantis and his colleagues also showed that NOTCH1 regulates the epichaperome in T-ALL. The team said previous studies have shown that, in the presence of oncogenic stress, heat shock proteins participate in a network nucleated by HSP90 and HSP70 chaperones—the epichaperome.
The researchers found that an intact epichaperome was critical for T-ALL by showing that pharmacologic inhibition of HSP90 and HSP70 significantly hindered the growth of human T-ALL in vitro. The team also found the HSP90 inhibitor PU-H71 reduced leukemic burden and extended survival in a NOTCH1-inducible T-ALL mouse model.
Then, the researchers found NOTCH1 levels could predict response to HSP90 inhibition in vitro. T-ALL patient samples expressing high levels of nuclear NOTCH1 and high levels of epichaperome were significantly more sensitive to treatment with PU-H71.
PU-H71 is already in early clinical trials of patients with breast cancer. If further testing proves successful, PU-H71 could be quickly adapted for trials in T-ALL patients, according to Dr Aifantis.
In the meantime, he and his colleagues plan to evaluate the effects of another 8 proteins produced by genes active in the HSF1 pathway to see if any show promising anticancer activity in T-ALL.
FDA approves biosimilar filgrastim
The US Food and Drug Administration (FDA) has approved the leukocyte growth factor Nivestym™ (filgrastim-aafi), a biosimilar to Neupogen (filgrastim).
Nivestym is approved to treat patients with nonmyeloid malignancies who are receiving myelosuppressive chemotherapy or undergoing bone marrow transplant, acute myeloid leukemia patients receiving induction or consolidation chemotherapy, patients undergoing autologous peripheral blood progenitor cell collection, and patients with severe chronic neutropenia.
The FDA’s approval of Nivestym was based on a review of evidence suggesting the drug is highly similar to Neupogen, according to Pfizer, the company developing Nivestym.
The full approved indication for Nivestym is as follows:
- To decrease the incidence of infection, as manifested by febrile neutropenia, in patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a significant incidence of severe neutropenia with fever
- To reduce the time to neutrophil recovery and the duration of fever following induction or consolidation chemotherapy in patients with acute myeloid leukemia
- To reduce the duration of neutropenia and neutropenia-related clinical sequelae (eg, febrile neutropenia) in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by bone marrow transplant
- For the mobilization of autologous hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis
- For chronic administration to reduce the incidence and duration of sequelae of severe neutropenia (eg, fever, infections, oropharyngeal ulcers) in symptomatic patients with congenital neutropenia, cyclic neutropenia, or idiopathic neutropenia.
For more details on Nivestym, see the full prescribing information.
The US Food and Drug Administration (FDA) has approved the leukocyte growth factor Nivestym™ (filgrastim-aafi), a biosimilar to Neupogen (filgrastim).
Nivestym is approved to treat patients with nonmyeloid malignancies who are receiving myelosuppressive chemotherapy or undergoing bone marrow transplant, acute myeloid leukemia patients receiving induction or consolidation chemotherapy, patients undergoing autologous peripheral blood progenitor cell collection, and patients with severe chronic neutropenia.
The FDA’s approval of Nivestym was based on a review of evidence suggesting the drug is highly similar to Neupogen, according to Pfizer, the company developing Nivestym.
The full approved indication for Nivestym is as follows:
- To decrease the incidence of infection, as manifested by febrile neutropenia, in patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a significant incidence of severe neutropenia with fever
- To reduce the time to neutrophil recovery and the duration of fever following induction or consolidation chemotherapy in patients with acute myeloid leukemia
- To reduce the duration of neutropenia and neutropenia-related clinical sequelae (eg, febrile neutropenia) in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by bone marrow transplant
- For the mobilization of autologous hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis
- For chronic administration to reduce the incidence and duration of sequelae of severe neutropenia (eg, fever, infections, oropharyngeal ulcers) in symptomatic patients with congenital neutropenia, cyclic neutropenia, or idiopathic neutropenia.
For more details on Nivestym, see the full prescribing information.
The US Food and Drug Administration (FDA) has approved the leukocyte growth factor Nivestym™ (filgrastim-aafi), a biosimilar to Neupogen (filgrastim).
Nivestym is approved to treat patients with nonmyeloid malignancies who are receiving myelosuppressive chemotherapy or undergoing bone marrow transplant, acute myeloid leukemia patients receiving induction or consolidation chemotherapy, patients undergoing autologous peripheral blood progenitor cell collection, and patients with severe chronic neutropenia.
The FDA’s approval of Nivestym was based on a review of evidence suggesting the drug is highly similar to Neupogen, according to Pfizer, the company developing Nivestym.
The full approved indication for Nivestym is as follows:
- To decrease the incidence of infection, as manifested by febrile neutropenia, in patients with nonmyeloid malignancies receiving myelosuppressive anticancer drugs associated with a significant incidence of severe neutropenia with fever
- To reduce the time to neutrophil recovery and the duration of fever following induction or consolidation chemotherapy in patients with acute myeloid leukemia
- To reduce the duration of neutropenia and neutropenia-related clinical sequelae (eg, febrile neutropenia) in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by bone marrow transplant
- For the mobilization of autologous hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis
- For chronic administration to reduce the incidence and duration of sequelae of severe neutropenia (eg, fever, infections, oropharyngeal ulcers) in symptomatic patients with congenital neutropenia, cyclic neutropenia, or idiopathic neutropenia.
For more details on Nivestym, see the full prescribing information.
Diabetics have higher risk of hematologic, other cancers
A review of data from more than 19 million people indicates that diabetes significantly raises a person’s risk of developing cancer.
When researchers compared patients with diabetes and without, both male and female diabetics had an increased risk of leukemias and lymphomas as well as certain solid tumors.
Researchers also found that diabetes conferred a higher cancer risk for women than men, both for all cancers combined and for some specific cancers, including leukemia.
“The link between diabetes and the risk of developing cancer is now firmly established,” said Toshiaki Ohkuma, PhD, of The George Institute for Global Health at the University of New South Wales in Australia.
“We have also demonstrated, for the first time, that women with diabetes are more likely to develop any form of cancer and have a significantly higher chance of developing kidney, oral, and stomach cancers and leukemia.”
Dr Ohkuma and his colleagues reported these findings in Diabetologia.
The researchers conducted a systematic search in PubMed MEDLINE to identify reports on the links between diabetes and cancer. Additional reports were identified from the reference lists of the relevant studies.
Only those cohort studies providing relative risks (RRs) for the association between diabetes and cancer for both women and men were included. In total, 107 relevant articles were identified, along with 36 cohorts of individual participant data.
RRs for cancer were obtained for patients with diabetes (types 1 and 2 combined) versus those without diabetes, for both men and women. The women-to-men ratios of these relative risk ratios (RRRs) were then calculated to determine the excess risk in women if present.
Data on all-site cancer was available from 47 studies, involving 121 cohorts and 19,239,302 individuals.
Diabetics vs non-diabetics
Women with diabetes had a 27% higher risk of all-site cancer compared to women without diabetes (RR=1.27; 95% CI 1.21, 1.32; P<0.001).
For men, the risk of all-site cancer was 19% higher among those with diabetes than those without (RR=1.19; 95% CI 1.13, 1.25; P<0.001).
There were several hematologic malignancies for which diabetics had an increased risk, as shown in the following table.
| Cancer type | RR for women (99% CI) | RR for men (99% CI) |
| Lymphatic and hematopoietic tissue | 1.24 (1.05, 1.46)* | 1.21 (0.98, 1.48) |
| Leukemia | 1.53 (1.00, 2.33) | 1.22 (0.80, 1.85) |
| Myeloid leukemia | 0.83 (0.39, 1.76) | 1.12 (0.77, 1.62) |
| Acute myeloid leukemia | 1.33 (1.12, 1.57)* | 1.14 (0.56, 2.33) |
| Chronic myeloid leukemia | 1.67 (1.27, 2.20)* | 1.62 (1.32, 1.98)* |
| Lymphoid leukemia | 1.74 (0.31, 9.79) | 1.20 (0.86, 1.68) |
| Lymphoma | 2.31 (0.57, 9.30) | 1.80 (0.68, 4.75) |
| Non-Hodgkin lymphoma | 1.16 (1.02, 1.32)* | 1.20 (1.08, 1.34)* |
| Hodgkin lymphoma | 1.20 (0.61, 2.38) | 1.36 (1.05, 1.77)* |
| Multiple myeloma | 1.19 (0.97, 1.47) | 1.12 (0.90, 1.41) |
| *denotes statistical significance with a P value < 0.01 | ||
Sex comparison
Calculation of the women-to-men ratio revealed that women with diabetes had a 6% greater excess risk of all-site cancer compared to men with diabetes (RRR=1.06; 95% CI 1.03, 1.09; P<0.001).
The women-to-men ratios also showed significantly higher risks for female diabetics for:
- Kidney cancer—RRR=1.11 (99% CI 1.04, 1.18; P<0.001)
- Oral cancer—RRR=1.13 (99% CI 1.00, 1.28; P=0.009)
- Stomach cancer—RRR=1.14 (99% CI 1.07, 1.22; P<0.001)
- Leukemia—RRR=1.15 (99% CI 1.02, 1.28; P=0.002).
However, women had a significantly lower risk of liver cancer (RRR=0.88; 99% CI 0.79, 0.99; P=0.005).
There are several possible reasons for the excess cancer risk observed in women, according to study author Sanne Peters, PhD, of The George Institute for Global Health at the University of Oxford in the UK.
For example, on average, women are in the pre-diabetic state of impaired glucose tolerance 2 years longer than men.
“Historically, we know that women are often under-treated when they first present with symptoms of diabetes, are less likely to receive intensive care, and are not taking the same levels of medications as men,” Dr Peters said. “All of these could go some way into explaining why women are at greater risk of developing cancer, but, without more research, we can’t be certain.”
A review of data from more than 19 million people indicates that diabetes significantly raises a person’s risk of developing cancer.
When researchers compared patients with diabetes and without, both male and female diabetics had an increased risk of leukemias and lymphomas as well as certain solid tumors.
Researchers also found that diabetes conferred a higher cancer risk for women than men, both for all cancers combined and for some specific cancers, including leukemia.
“The link between diabetes and the risk of developing cancer is now firmly established,” said Toshiaki Ohkuma, PhD, of The George Institute for Global Health at the University of New South Wales in Australia.
“We have also demonstrated, for the first time, that women with diabetes are more likely to develop any form of cancer and have a significantly higher chance of developing kidney, oral, and stomach cancers and leukemia.”
Dr Ohkuma and his colleagues reported these findings in Diabetologia.
The researchers conducted a systematic search in PubMed MEDLINE to identify reports on the links between diabetes and cancer. Additional reports were identified from the reference lists of the relevant studies.
Only those cohort studies providing relative risks (RRs) for the association between diabetes and cancer for both women and men were included. In total, 107 relevant articles were identified, along with 36 cohorts of individual participant data.
RRs for cancer were obtained for patients with diabetes (types 1 and 2 combined) versus those without diabetes, for both men and women. The women-to-men ratios of these relative risk ratios (RRRs) were then calculated to determine the excess risk in women if present.
Data on all-site cancer was available from 47 studies, involving 121 cohorts and 19,239,302 individuals.
Diabetics vs non-diabetics
Women with diabetes had a 27% higher risk of all-site cancer compared to women without diabetes (RR=1.27; 95% CI 1.21, 1.32; P<0.001).
For men, the risk of all-site cancer was 19% higher among those with diabetes than those without (RR=1.19; 95% CI 1.13, 1.25; P<0.001).
There were several hematologic malignancies for which diabetics had an increased risk, as shown in the following table.
| Cancer type | RR for women (99% CI) | RR for men (99% CI) |
| Lymphatic and hematopoietic tissue | 1.24 (1.05, 1.46)* | 1.21 (0.98, 1.48) |
| Leukemia | 1.53 (1.00, 2.33) | 1.22 (0.80, 1.85) |
| Myeloid leukemia | 0.83 (0.39, 1.76) | 1.12 (0.77, 1.62) |
| Acute myeloid leukemia | 1.33 (1.12, 1.57)* | 1.14 (0.56, 2.33) |
| Chronic myeloid leukemia | 1.67 (1.27, 2.20)* | 1.62 (1.32, 1.98)* |
| Lymphoid leukemia | 1.74 (0.31, 9.79) | 1.20 (0.86, 1.68) |
| Lymphoma | 2.31 (0.57, 9.30) | 1.80 (0.68, 4.75) |
| Non-Hodgkin lymphoma | 1.16 (1.02, 1.32)* | 1.20 (1.08, 1.34)* |
| Hodgkin lymphoma | 1.20 (0.61, 2.38) | 1.36 (1.05, 1.77)* |
| Multiple myeloma | 1.19 (0.97, 1.47) | 1.12 (0.90, 1.41) |
| *denotes statistical significance with a P value < 0.01 | ||
Sex comparison
Calculation of the women-to-men ratio revealed that women with diabetes had a 6% greater excess risk of all-site cancer compared to men with diabetes (RRR=1.06; 95% CI 1.03, 1.09; P<0.001).
The women-to-men ratios also showed significantly higher risks for female diabetics for:
- Kidney cancer—RRR=1.11 (99% CI 1.04, 1.18; P<0.001)
- Oral cancer—RRR=1.13 (99% CI 1.00, 1.28; P=0.009)
- Stomach cancer—RRR=1.14 (99% CI 1.07, 1.22; P<0.001)
- Leukemia—RRR=1.15 (99% CI 1.02, 1.28; P=0.002).
However, women had a significantly lower risk of liver cancer (RRR=0.88; 99% CI 0.79, 0.99; P=0.005).
There are several possible reasons for the excess cancer risk observed in women, according to study author Sanne Peters, PhD, of The George Institute for Global Health at the University of Oxford in the UK.
For example, on average, women are in the pre-diabetic state of impaired glucose tolerance 2 years longer than men.
“Historically, we know that women are often under-treated when they first present with symptoms of diabetes, are less likely to receive intensive care, and are not taking the same levels of medications as men,” Dr Peters said. “All of these could go some way into explaining why women are at greater risk of developing cancer, but, without more research, we can’t be certain.”
A review of data from more than 19 million people indicates that diabetes significantly raises a person’s risk of developing cancer.
When researchers compared patients with diabetes and without, both male and female diabetics had an increased risk of leukemias and lymphomas as well as certain solid tumors.
Researchers also found that diabetes conferred a higher cancer risk for women than men, both for all cancers combined and for some specific cancers, including leukemia.
“The link between diabetes and the risk of developing cancer is now firmly established,” said Toshiaki Ohkuma, PhD, of The George Institute for Global Health at the University of New South Wales in Australia.
“We have also demonstrated, for the first time, that women with diabetes are more likely to develop any form of cancer and have a significantly higher chance of developing kidney, oral, and stomach cancers and leukemia.”
Dr Ohkuma and his colleagues reported these findings in Diabetologia.
The researchers conducted a systematic search in PubMed MEDLINE to identify reports on the links between diabetes and cancer. Additional reports were identified from the reference lists of the relevant studies.
Only those cohort studies providing relative risks (RRs) for the association between diabetes and cancer for both women and men were included. In total, 107 relevant articles were identified, along with 36 cohorts of individual participant data.
RRs for cancer were obtained for patients with diabetes (types 1 and 2 combined) versus those without diabetes, for both men and women. The women-to-men ratios of these relative risk ratios (RRRs) were then calculated to determine the excess risk in women if present.
Data on all-site cancer was available from 47 studies, involving 121 cohorts and 19,239,302 individuals.
Diabetics vs non-diabetics
Women with diabetes had a 27% higher risk of all-site cancer compared to women without diabetes (RR=1.27; 95% CI 1.21, 1.32; P<0.001).
For men, the risk of all-site cancer was 19% higher among those with diabetes than those without (RR=1.19; 95% CI 1.13, 1.25; P<0.001).
There were several hematologic malignancies for which diabetics had an increased risk, as shown in the following table.
| Cancer type | RR for women (99% CI) | RR for men (99% CI) |
| Lymphatic and hematopoietic tissue | 1.24 (1.05, 1.46)* | 1.21 (0.98, 1.48) |
| Leukemia | 1.53 (1.00, 2.33) | 1.22 (0.80, 1.85) |
| Myeloid leukemia | 0.83 (0.39, 1.76) | 1.12 (0.77, 1.62) |
| Acute myeloid leukemia | 1.33 (1.12, 1.57)* | 1.14 (0.56, 2.33) |
| Chronic myeloid leukemia | 1.67 (1.27, 2.20)* | 1.62 (1.32, 1.98)* |
| Lymphoid leukemia | 1.74 (0.31, 9.79) | 1.20 (0.86, 1.68) |
| Lymphoma | 2.31 (0.57, 9.30) | 1.80 (0.68, 4.75) |
| Non-Hodgkin lymphoma | 1.16 (1.02, 1.32)* | 1.20 (1.08, 1.34)* |
| Hodgkin lymphoma | 1.20 (0.61, 2.38) | 1.36 (1.05, 1.77)* |
| Multiple myeloma | 1.19 (0.97, 1.47) | 1.12 (0.90, 1.41) |
| *denotes statistical significance with a P value < 0.01 | ||
Sex comparison
Calculation of the women-to-men ratio revealed that women with diabetes had a 6% greater excess risk of all-site cancer compared to men with diabetes (RRR=1.06; 95% CI 1.03, 1.09; P<0.001).
The women-to-men ratios also showed significantly higher risks for female diabetics for:
- Kidney cancer—RRR=1.11 (99% CI 1.04, 1.18; P<0.001)
- Oral cancer—RRR=1.13 (99% CI 1.00, 1.28; P=0.009)
- Stomach cancer—RRR=1.14 (99% CI 1.07, 1.22; P<0.001)
- Leukemia—RRR=1.15 (99% CI 1.02, 1.28; P=0.002).
However, women had a significantly lower risk of liver cancer (RRR=0.88; 99% CI 0.79, 0.99; P=0.005).
There are several possible reasons for the excess cancer risk observed in women, according to study author Sanne Peters, PhD, of The George Institute for Global Health at the University of Oxford in the UK.
For example, on average, women are in the pre-diabetic state of impaired glucose tolerance 2 years longer than men.
“Historically, we know that women are often under-treated when they first present with symptoms of diabetes, are less likely to receive intensive care, and are not taking the same levels of medications as men,” Dr Peters said. “All of these could go some way into explaining why women are at greater risk of developing cancer, but, without more research, we can’t be certain.”
How ALL invades the CNS
Researchers believe they have solved the mystery of how acute lymphoblastic leukemia (ALL) infiltrates the central nervous system (CNS).
Experiments in mice suggested that ALL enters the CNS not by breaching the blood-brain barrier but by evading it.
The researchers said they found that expression of the laminin receptor α6 integrin, which is common in ALL, allows cells to use neural migratory pathways to invade the CNS.
“It’s a very unexpected way for cells to travel into the central nervous system,” said Dorothy Sipkins, MD, PhD, of Duke University in Durham, North Carolina.
She and her colleagues described the cells’ journey in Nature.
The researchers said they found that α6 integrin–laminin interactions mediate the migration of ALL cells toward the cerebrospinal fluid.
The team noted that α6 integrin is expressed in most cases of ALL, and laminin surrounds blood vessels that pass directly through the vertebrae to the meninges tissue that lines the spinal cord and brain.
Experiments indicated that ALL cells latch onto the laminin surrounding these blood vessels and travel down into the meninges region where cerebral spinal fluid circulates.
“Understanding how ALL gets into the central nervous system arms us with new ways to target this pathway and hopefully shut it down,” Dr Sipkins noted.
She and her colleagues found that treatment with a PI3Kδ inhibitor may be one way to do that.
The team tested the PI3Kδ inhibitor GS-649443 in a mouse model of CNS ALL (Nalm-6) and found the drug decreased α6 integrin expression on ALL cells.
Mice treated with the inhibitor had a 50% decrease in CNS disease burden compared to vehicle-treated controls. However, there was no significant difference between treated mice and controls when it came to bone marrow or splenic Nalm-6 disease burden or peripheral blood cell counts.
The researchers observed similar results in another model of CNS disease (RCH-ACV ALL).
The team also tested α6 integrin-neutralizing antibodies in Nalm-6-engrafted mice. There was no difference in peripheral disease burden between targeted and isotype control antibody-treated mice. However, anti-α6 integrin-treated mice had a reduction in cerebrospinal fluid blast counts.
This research was supported by the Duke Cancer Institute and Gilead Sciences, Inc., which provided the PI3Kδ inhibitor.
Researchers believe they have solved the mystery of how acute lymphoblastic leukemia (ALL) infiltrates the central nervous system (CNS).
Experiments in mice suggested that ALL enters the CNS not by breaching the blood-brain barrier but by evading it.
The researchers said they found that expression of the laminin receptor α6 integrin, which is common in ALL, allows cells to use neural migratory pathways to invade the CNS.
“It’s a very unexpected way for cells to travel into the central nervous system,” said Dorothy Sipkins, MD, PhD, of Duke University in Durham, North Carolina.
She and her colleagues described the cells’ journey in Nature.
The researchers said they found that α6 integrin–laminin interactions mediate the migration of ALL cells toward the cerebrospinal fluid.
The team noted that α6 integrin is expressed in most cases of ALL, and laminin surrounds blood vessels that pass directly through the vertebrae to the meninges tissue that lines the spinal cord and brain.
Experiments indicated that ALL cells latch onto the laminin surrounding these blood vessels and travel down into the meninges region where cerebral spinal fluid circulates.
“Understanding how ALL gets into the central nervous system arms us with new ways to target this pathway and hopefully shut it down,” Dr Sipkins noted.
She and her colleagues found that treatment with a PI3Kδ inhibitor may be one way to do that.
The team tested the PI3Kδ inhibitor GS-649443 in a mouse model of CNS ALL (Nalm-6) and found the drug decreased α6 integrin expression on ALL cells.
Mice treated with the inhibitor had a 50% decrease in CNS disease burden compared to vehicle-treated controls. However, there was no significant difference between treated mice and controls when it came to bone marrow or splenic Nalm-6 disease burden or peripheral blood cell counts.
The researchers observed similar results in another model of CNS disease (RCH-ACV ALL).
The team also tested α6 integrin-neutralizing antibodies in Nalm-6-engrafted mice. There was no difference in peripheral disease burden between targeted and isotype control antibody-treated mice. However, anti-α6 integrin-treated mice had a reduction in cerebrospinal fluid blast counts.
This research was supported by the Duke Cancer Institute and Gilead Sciences, Inc., which provided the PI3Kδ inhibitor.
Researchers believe they have solved the mystery of how acute lymphoblastic leukemia (ALL) infiltrates the central nervous system (CNS).
Experiments in mice suggested that ALL enters the CNS not by breaching the blood-brain barrier but by evading it.
The researchers said they found that expression of the laminin receptor α6 integrin, which is common in ALL, allows cells to use neural migratory pathways to invade the CNS.
“It’s a very unexpected way for cells to travel into the central nervous system,” said Dorothy Sipkins, MD, PhD, of Duke University in Durham, North Carolina.
She and her colleagues described the cells’ journey in Nature.
The researchers said they found that α6 integrin–laminin interactions mediate the migration of ALL cells toward the cerebrospinal fluid.
The team noted that α6 integrin is expressed in most cases of ALL, and laminin surrounds blood vessels that pass directly through the vertebrae to the meninges tissue that lines the spinal cord and brain.
Experiments indicated that ALL cells latch onto the laminin surrounding these blood vessels and travel down into the meninges region where cerebral spinal fluid circulates.
“Understanding how ALL gets into the central nervous system arms us with new ways to target this pathway and hopefully shut it down,” Dr Sipkins noted.
She and her colleagues found that treatment with a PI3Kδ inhibitor may be one way to do that.
The team tested the PI3Kδ inhibitor GS-649443 in a mouse model of CNS ALL (Nalm-6) and found the drug decreased α6 integrin expression on ALL cells.
Mice treated with the inhibitor had a 50% decrease in CNS disease burden compared to vehicle-treated controls. However, there was no significant difference between treated mice and controls when it came to bone marrow or splenic Nalm-6 disease burden or peripheral blood cell counts.
The researchers observed similar results in another model of CNS disease (RCH-ACV ALL).
The team also tested α6 integrin-neutralizing antibodies in Nalm-6-engrafted mice. There was no difference in peripheral disease burden between targeted and isotype control antibody-treated mice. However, anti-α6 integrin-treated mice had a reduction in cerebrospinal fluid blast counts.
This research was supported by the Duke Cancer Institute and Gilead Sciences, Inc., which provided the PI3Kδ inhibitor.
Lab results may help predict complications in ALL treatment
(ALL) who were treated with four-drug induction therapy.
Kasper Warrick, MD, and his colleagues at Indiana University in Indianapolis reported findings from a retrospective study of 73 ALL patients at their hospital. They performed chart reviews comparing a cohort of 42 patients who were discharged on day 4 of their induction treatment with 31 similar patients who had a longer hospital stay or admission to the intensive care unit. The report was published in Leukemia Research.
Univariate analysis found that patients with a longer length of stay were more likely to have a fever, pretransfusion hemoglobin of less than 8 g/dL, lower serum bicarbonate values, abnormal serum calcium, and abnormal serum phosphate. Multivariate stepwise logistic regression found that low serum bicarbonate and a lower platelet count on day 4 of admission was predictive of a prolonged hospital stay. About a third of patients from each group had an unplanned readmission within 30 days.
The researchers concluded that early discharge is safe in only a subgroup of high-risk ALL patients undergoing induction chemotherapy. “Treating physicians could opt for a discharge only after normalization of electrolyte abnormalities and renal functions, and when no transfusion support is needed (stable hematocrit and platelet count),” they wrote. Even in those cases, they recommended “aggressive and close outpatient follow” since patients are vulnerable to complications and readmissions.
SOURCE: Warrick K et al. Leuk Res. 2018 Jun 30:71:36-42.
(ALL) who were treated with four-drug induction therapy.
Kasper Warrick, MD, and his colleagues at Indiana University in Indianapolis reported findings from a retrospective study of 73 ALL patients at their hospital. They performed chart reviews comparing a cohort of 42 patients who were discharged on day 4 of their induction treatment with 31 similar patients who had a longer hospital stay or admission to the intensive care unit. The report was published in Leukemia Research.
Univariate analysis found that patients with a longer length of stay were more likely to have a fever, pretransfusion hemoglobin of less than 8 g/dL, lower serum bicarbonate values, abnormal serum calcium, and abnormal serum phosphate. Multivariate stepwise logistic regression found that low serum bicarbonate and a lower platelet count on day 4 of admission was predictive of a prolonged hospital stay. About a third of patients from each group had an unplanned readmission within 30 days.
The researchers concluded that early discharge is safe in only a subgroup of high-risk ALL patients undergoing induction chemotherapy. “Treating physicians could opt for a discharge only after normalization of electrolyte abnormalities and renal functions, and when no transfusion support is needed (stable hematocrit and platelet count),” they wrote. Even in those cases, they recommended “aggressive and close outpatient follow” since patients are vulnerable to complications and readmissions.
SOURCE: Warrick K et al. Leuk Res. 2018 Jun 30:71:36-42.
(ALL) who were treated with four-drug induction therapy.
Kasper Warrick, MD, and his colleagues at Indiana University in Indianapolis reported findings from a retrospective study of 73 ALL patients at their hospital. They performed chart reviews comparing a cohort of 42 patients who were discharged on day 4 of their induction treatment with 31 similar patients who had a longer hospital stay or admission to the intensive care unit. The report was published in Leukemia Research.
Univariate analysis found that patients with a longer length of stay were more likely to have a fever, pretransfusion hemoglobin of less than 8 g/dL, lower serum bicarbonate values, abnormal serum calcium, and abnormal serum phosphate. Multivariate stepwise logistic regression found that low serum bicarbonate and a lower platelet count on day 4 of admission was predictive of a prolonged hospital stay. About a third of patients from each group had an unplanned readmission within 30 days.
The researchers concluded that early discharge is safe in only a subgroup of high-risk ALL patients undergoing induction chemotherapy. “Treating physicians could opt for a discharge only after normalization of electrolyte abnormalities and renal functions, and when no transfusion support is needed (stable hematocrit and platelet count),” they wrote. Even in those cases, they recommended “aggressive and close outpatient follow” since patients are vulnerable to complications and readmissions.
SOURCE: Warrick K et al. Leuk Res. 2018 Jun 30:71:36-42.
FROM LEUKEMIA RESEARCH
CAR T Therapy: From Bench to Bedside and Back
Release Date: July 15, 2018
Expiration Date: July 14, 2019
Note: This activity is no longer available for credit
Introductory Comments: (Duration: 9 minutes)
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, MD
Presentation: (Duration: 39 minutes)
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Provided by:

Learning Objectives
• Review clinical data and individual case studies to determine where CAR T-cell therapy might be appropriate in the treatment of adult and pediatric patients with leukemia, lymphoma, and multiple myeloma.
• Discuss the management of cytotoxicity of CAR T-cell therapy.
Target Audience
Hematologists, oncologists, and other members of the healthcare team who treat or manage patients with hematologic malignancies.
Statement of Need
It is critical that clinicians managing patients with acute leukemia and other hematologic malignancies are cognizant of exciting breakthroughs and are also able to integrate recent progress into practice. However, given the overwhelming influx of data, it is no surprise that many hematology professionals face difficulties in identifying the most relevant findings for clinical practice. Hematologists are unable to stay abreast of the latest evidence on investigational agents. Educational programs are thus crucial to address this important professional practice gap.
Faculty
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Disclosures: Consultant: Novartis; Grant/Research support and royalties/IPR: Novartis
Stockholder: Tmunity Therapeutics, Inc.
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, Maryland
Disclosures: No relevant financial relationships with a commercial supporter
Permissions
- Slide 3: Complex tumor, host and environmental factors govern the strength and timing of anti-cancer immune responses
- Reprinted from Immunity, Vol 39/No 1, Chen DS, Mellman I, Oncology meets immunology: the cancer-immunity cycle, pp 1-10, 2013, with permission from Elsevier
- Slide 9: Genes differentially expressed in CART19 cellular infusion products from CLL patients
- From Fraietta JA, Lacey SF, Orlando EJ, . . . June CH, Melenhorst JJ. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 2018; 24:563-571
- Slide 10: Characterization of CLL CAR T cells in NSG CLL model
- Same as slide 9
- Slide 15: First adult ALL patient
- Photos originally published in Kaiser Health News/Photo courtesy of Dr Keith Eaton. Available at: https://khn.org/news/cascade-of-costs-could-push-new-gene-therapy-above-1-million-per-patient/
- Slide 21: Efficient trafficking of CTL019 T Cells to CNS in ALL
- From N Engl J Med, Grupp SA, Kalos M, Barrett D, . . V. June CH, Chimeric antigen receptor-modified T cells for acute lymphoid leukemia, Volume No 368, pp 1509-1518. Copyright © 2013 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
- Slide 26: Long-term persistence and expression of CTL019 is associated with durable remission in leukemia: Predictive Biomarker
- From Porter DL, Hwang WT, Frey NV . . . June CH. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015; 7(303):303ra139. Reprinted with permission from AAAS.
- Slide 28: Rapid massive expansion of clonal CART cell population in patient #10
- Initially published in Fraietta JA, Nobles CL, Sammons MA, . . . June CH, Melenhors JJ. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 2018; 558(7709):307-312
- Slide 29: Mapping CAR integration site in Pt #10
- Same as slide 28.
- Slide 31: Long-term stable persistence of TET2-deficient CAR T cells in Pt #10
- Same as slide 28
- Slide 32: Epigenetic and genetic changes uncovered by ATAC-seq in Pt #10
- Same as slide 28.
- Slide 33: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 34: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 36: CAR T for myeloma: BCMA
- From Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev 2011; 244(1):115-33. Reprinted with permission from John Wiley and Sons.
- Slide 38: CAR T for myeloma: Patient #1
- Photo originally published by UT Southwestern Medical Center. Available at: https://www.utsouthwestern.edu/newsroom/articles/year-2018/wright-car-t.html
- Slide 39: Autoimmunity is the “Achilles’ Heel” of immunotherapy
- First published in June CH, Warshauer JT, and Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med 2017;23(5):540-7
- Slide 41: Multiplex CRISPR /Cas9 editing: Universal T cells TCR, HLA, PD-1, CTLA-4 and Fas
- From Ren J, Zhang X, Liu X, Fang C, Jiang S, June CH, Zhao Y. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget 2017; 8:17002-17011.
- Slide 45: CAR T-cell trials for cancer are now global
- From June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018; 359:1361-1365. Reprinted with permission from AAAS.
Disclaimer
The content and views presented in this educational activity are those of the author and do not necessarily reflect those of Hemedicus or Frontline Medical Communications. This material is prepared based upon a review of multiple sources of information, but it is not exhaustive of the subject matter. Therefore, healthcare professionals and other individuals should review and consider other publications and materials on the subject matter before relying solely upon the information contained within this educational activity.
Release Date: July 15, 2018
Expiration Date: July 14, 2019
Note: This activity is no longer available for credit
Introductory Comments: (Duration: 9 minutes)
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, MD
Presentation: (Duration: 39 minutes)
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Provided by:

Learning Objectives
• Review clinical data and individual case studies to determine where CAR T-cell therapy might be appropriate in the treatment of adult and pediatric patients with leukemia, lymphoma, and multiple myeloma.
• Discuss the management of cytotoxicity of CAR T-cell therapy.
Target Audience
Hematologists, oncologists, and other members of the healthcare team who treat or manage patients with hematologic malignancies.
Statement of Need
It is critical that clinicians managing patients with acute leukemia and other hematologic malignancies are cognizant of exciting breakthroughs and are also able to integrate recent progress into practice. However, given the overwhelming influx of data, it is no surprise that many hematology professionals face difficulties in identifying the most relevant findings for clinical practice. Hematologists are unable to stay abreast of the latest evidence on investigational agents. Educational programs are thus crucial to address this important professional practice gap.
Faculty
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Disclosures: Consultant: Novartis; Grant/Research support and royalties/IPR: Novartis
Stockholder: Tmunity Therapeutics, Inc.
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, Maryland
Disclosures: No relevant financial relationships with a commercial supporter
Permissions
- Slide 3: Complex tumor, host and environmental factors govern the strength and timing of anti-cancer immune responses
- Reprinted from Immunity, Vol 39/No 1, Chen DS, Mellman I, Oncology meets immunology: the cancer-immunity cycle, pp 1-10, 2013, with permission from Elsevier
- Slide 9: Genes differentially expressed in CART19 cellular infusion products from CLL patients
- From Fraietta JA, Lacey SF, Orlando EJ, . . . June CH, Melenhorst JJ. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 2018; 24:563-571
- Slide 10: Characterization of CLL CAR T cells in NSG CLL model
- Same as slide 9
- Slide 15: First adult ALL patient
- Photos originally published in Kaiser Health News/Photo courtesy of Dr Keith Eaton. Available at: https://khn.org/news/cascade-of-costs-could-push-new-gene-therapy-above-1-million-per-patient/
- Slide 21: Efficient trafficking of CTL019 T Cells to CNS in ALL
- From N Engl J Med, Grupp SA, Kalos M, Barrett D, . . V. June CH, Chimeric antigen receptor-modified T cells for acute lymphoid leukemia, Volume No 368, pp 1509-1518. Copyright © 2013 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
- Slide 26: Long-term persistence and expression of CTL019 is associated with durable remission in leukemia: Predictive Biomarker
- From Porter DL, Hwang WT, Frey NV . . . June CH. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015; 7(303):303ra139. Reprinted with permission from AAAS.
- Slide 28: Rapid massive expansion of clonal CART cell population in patient #10
- Initially published in Fraietta JA, Nobles CL, Sammons MA, . . . June CH, Melenhors JJ. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 2018; 558(7709):307-312
- Slide 29: Mapping CAR integration site in Pt #10
- Same as slide 28.
- Slide 31: Long-term stable persistence of TET2-deficient CAR T cells in Pt #10
- Same as slide 28
- Slide 32: Epigenetic and genetic changes uncovered by ATAC-seq in Pt #10
- Same as slide 28.
- Slide 33: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 34: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 36: CAR T for myeloma: BCMA
- From Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev 2011; 244(1):115-33. Reprinted with permission from John Wiley and Sons.
- Slide 38: CAR T for myeloma: Patient #1
- Photo originally published by UT Southwestern Medical Center. Available at: https://www.utsouthwestern.edu/newsroom/articles/year-2018/wright-car-t.html
- Slide 39: Autoimmunity is the “Achilles’ Heel” of immunotherapy
- First published in June CH, Warshauer JT, and Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med 2017;23(5):540-7
- Slide 41: Multiplex CRISPR /Cas9 editing: Universal T cells TCR, HLA, PD-1, CTLA-4 and Fas
- From Ren J, Zhang X, Liu X, Fang C, Jiang S, June CH, Zhao Y. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget 2017; 8:17002-17011.
- Slide 45: CAR T-cell trials for cancer are now global
- From June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018; 359:1361-1365. Reprinted with permission from AAAS.
Disclaimer
The content and views presented in this educational activity are those of the author and do not necessarily reflect those of Hemedicus or Frontline Medical Communications. This material is prepared based upon a review of multiple sources of information, but it is not exhaustive of the subject matter. Therefore, healthcare professionals and other individuals should review and consider other publications and materials on the subject matter before relying solely upon the information contained within this educational activity.
Release Date: July 15, 2018
Expiration Date: July 14, 2019
Note: This activity is no longer available for credit
Introductory Comments: (Duration: 9 minutes)
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, MD
Presentation: (Duration: 39 minutes)
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Provided by:

Learning Objectives
• Review clinical data and individual case studies to determine where CAR T-cell therapy might be appropriate in the treatment of adult and pediatric patients with leukemia, lymphoma, and multiple myeloma.
• Discuss the management of cytotoxicity of CAR T-cell therapy.
Target Audience
Hematologists, oncologists, and other members of the healthcare team who treat or manage patients with hematologic malignancies.
Statement of Need
It is critical that clinicians managing patients with acute leukemia and other hematologic malignancies are cognizant of exciting breakthroughs and are also able to integrate recent progress into practice. However, given the overwhelming influx of data, it is no surprise that many hematology professionals face difficulties in identifying the most relevant findings for clinical practice. Hematologists are unable to stay abreast of the latest evidence on investigational agents. Educational programs are thus crucial to address this important professional practice gap.
Faculty
Carl H. June, MD
Richard W. Vague Professor in Immunotherapy
Perelman School of Medicine
University of Pennsylvania
Philadelphia, PA
Disclosures: Consultant: Novartis; Grant/Research support and royalties/IPR: Novartis
Stockholder: Tmunity Therapeutics, Inc.
Aaron P. Rapoport, MD
Bone Marrow Transplant Program
University of Maryland School of Medicine
Baltimore, Maryland
Disclosures: No relevant financial relationships with a commercial supporter
Permissions
- Slide 3: Complex tumor, host and environmental factors govern the strength and timing of anti-cancer immune responses
- Reprinted from Immunity, Vol 39/No 1, Chen DS, Mellman I, Oncology meets immunology: the cancer-immunity cycle, pp 1-10, 2013, with permission from Elsevier
- Slide 9: Genes differentially expressed in CART19 cellular infusion products from CLL patients
- From Fraietta JA, Lacey SF, Orlando EJ, . . . June CH, Melenhorst JJ. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 2018; 24:563-571
- Slide 10: Characterization of CLL CAR T cells in NSG CLL model
- Same as slide 9
- Slide 15: First adult ALL patient
- Photos originally published in Kaiser Health News/Photo courtesy of Dr Keith Eaton. Available at: https://khn.org/news/cascade-of-costs-could-push-new-gene-therapy-above-1-million-per-patient/
- Slide 21: Efficient trafficking of CTL019 T Cells to CNS in ALL
- From N Engl J Med, Grupp SA, Kalos M, Barrett D, . . V. June CH, Chimeric antigen receptor-modified T cells for acute lymphoid leukemia, Volume No 368, pp 1509-1518. Copyright © 2013 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
- Slide 26: Long-term persistence and expression of CTL019 is associated with durable remission in leukemia: Predictive Biomarker
- From Porter DL, Hwang WT, Frey NV . . . June CH. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015; 7(303):303ra139. Reprinted with permission from AAAS.
- Slide 28: Rapid massive expansion of clonal CART cell population in patient #10
- Initially published in Fraietta JA, Nobles CL, Sammons MA, . . . June CH, Melenhors JJ. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 2018; 558(7709):307-312
- Slide 29: Mapping CAR integration site in Pt #10
- Same as slide 28.
- Slide 31: Long-term stable persistence of TET2-deficient CAR T cells in Pt #10
- Same as slide 28
- Slide 32: Epigenetic and genetic changes uncovered by ATAC-seq in Pt #10
- Same as slide 28.
- Slide 33: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 34: TET2 knock down in healthy donor T cells
- Same as slide 28.
- Slide 36: CAR T for myeloma: BCMA
- From Rickert RC, Jellusova J, Miletic AV. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev 2011; 244(1):115-33. Reprinted with permission from John Wiley and Sons.
- Slide 38: CAR T for myeloma: Patient #1
- Photo originally published by UT Southwestern Medical Center. Available at: https://www.utsouthwestern.edu/newsroom/articles/year-2018/wright-car-t.html
- Slide 39: Autoimmunity is the “Achilles’ Heel” of immunotherapy
- First published in June CH, Warshauer JT, and Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med 2017;23(5):540-7
- Slide 41: Multiplex CRISPR /Cas9 editing: Universal T cells TCR, HLA, PD-1, CTLA-4 and Fas
- From Ren J, Zhang X, Liu X, Fang C, Jiang S, June CH, Zhao Y. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget 2017; 8:17002-17011.
- Slide 45: CAR T-cell trials for cancer are now global
- From June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science 2018; 359:1361-1365. Reprinted with permission from AAAS.
Disclaimer
The content and views presented in this educational activity are those of the author and do not necessarily reflect those of Hemedicus or Frontline Medical Communications. This material is prepared based upon a review of multiple sources of information, but it is not exhaustive of the subject matter. Therefore, healthcare professionals and other individuals should review and consider other publications and materials on the subject matter before relying solely upon the information contained within this educational activity.
Concomitant drugs may explain PEG-ASP liver toxicities in ALL
STOCKHOLM – Liver toxicities in adults with acute lymphoblastic leukemia (ALL) treated with a pediatric-type regimen containing pegylated asparaginase (PEG-ASP) may be related to concomitant use of other hepatotoxic drugs, investigators cautioned.
A retrospective review of records on 26 adult ALL patients treated with PEG-ASP since 2013 showed that concomitant use of vincristine, idarubicin, and vancomycin was associated with an increased risk for grade 3 or 4 hepatotoxicity, reported Fabio Guolo, MD, from the University of Genoa (Italy) and his colleagues.
In contrast, patients who received other chemotherapy drugs or antimicrobial agents did not have significant liver toxicities, Dr. Guolo said in an interview at the annual congress of the European Hematology Association.
“Increased toxicity from therapy prevents delivery of the most active therapy, and asparaginase is one of the keys to the success of pediatric trials in ALL, so we have tried to push the dose of asparaginase as high as we could in adult patients,” he said.
“We asked why some patients will develop toxicity while receiving a relatively low dose of asparaginase, whereas other patients who received higher doses did not,” Dr. Guolo added.
In recent years, investigators have found that adults with ALL tend to have better outcomes when they were treated with standard pediatric ALL regimens, which includes high-dose PEG-ASP.
To identify factors related to potential PEG-ASP toxicity in adults with ALL, the investigators combed through records of 26 adults patients, 19 of whom had received PEG-ASP in the frontline setting, and 7 of whom received it during treatment of relapsed or refractory disease.
The investigators looked at each course of PEG-ASP as an independent event (51 total episodes), paying special attention to concomitant chemotherapy and the use of both antimycotic and antibiotic agents.
Five of the patients had grade 3 hepatotoxicity, and three had grade 4 hepatotoxicity. The patients with grade 4 events had unexplained severe weight gain and painful hepatomegaly. Ultrasonography in these patients revealed acute steatosis similar to that seen with sinusoidal occlusive disease. All three patients had received concomitant idarubicin, vincristine, and vancomycin.
In univariate analysis, neither being older than 45 years, administration of PEG-ASP during an active leukemia phase, nor having a body mass index greater than 25 kg/m2 were significantly associated with increased incidence of grade 3 or 4 hepatotoxicity.
When the investigators looked at concomitant chemotherapy drugs, however, they found that liver toxicity was significantly higher with idarubicin cumulative doses of 20 mg/m2 or greater (hazard ratio, 1.49; P = .047) and that vincristine doses of 2 mg/m2 or greater were associated with a borderline increase in risk (HR, 4.75; P = .055).
There was no increased risk for liver toxicities with either steroids, daunorubicin, cyclophosphamide, cytarabine, methotrexate, or 6-mercaptopurine.
Additionally, concomitant vancomycin was also linked to increased hepatotoxicity (HR, 1.86; P =.009). In contrast, neither carbapenem-class anti-infectives nor azole were significantly associated with liver toxicities.
“Notably, none of the patients undergoing full pediatric induction, which contains higher cumulative doses of PEG-ASP, experienced grade 4 hepatotoxicity regardless of age,” Dr. Guolo and his colleagues wrote in their poster presentation.
In multivariate analysis controlling for age, BMI, drug regimen, and concomitant therapies, idarubicin remained a significant risk factor for severe hepatotoxicity (P = .004), and vancomycin remained as a borderline risk (P = .054).
Dr. Guolo acknowledged that the investigators could not account for the potential contribution of over-the-counter medications with known risk for hepatotoxicity, such as acetaminophen.
He noted that in his group’s experience, the toxicity profile of PEG-ASP in adults, including high-dose regimens, was manageable without excess toxicities as long as clinicians paid close attention to the use of concomitant agents.
The study was internally funded. The authors reported having no relevant conflicts of interest.
SOURCE: Minetto P et al. EHA Congress, Abstract PS934.
STOCKHOLM – Liver toxicities in adults with acute lymphoblastic leukemia (ALL) treated with a pediatric-type regimen containing pegylated asparaginase (PEG-ASP) may be related to concomitant use of other hepatotoxic drugs, investigators cautioned.
A retrospective review of records on 26 adult ALL patients treated with PEG-ASP since 2013 showed that concomitant use of vincristine, idarubicin, and vancomycin was associated with an increased risk for grade 3 or 4 hepatotoxicity, reported Fabio Guolo, MD, from the University of Genoa (Italy) and his colleagues.
In contrast, patients who received other chemotherapy drugs or antimicrobial agents did not have significant liver toxicities, Dr. Guolo said in an interview at the annual congress of the European Hematology Association.
“Increased toxicity from therapy prevents delivery of the most active therapy, and asparaginase is one of the keys to the success of pediatric trials in ALL, so we have tried to push the dose of asparaginase as high as we could in adult patients,” he said.
“We asked why some patients will develop toxicity while receiving a relatively low dose of asparaginase, whereas other patients who received higher doses did not,” Dr. Guolo added.
In recent years, investigators have found that adults with ALL tend to have better outcomes when they were treated with standard pediatric ALL regimens, which includes high-dose PEG-ASP.
To identify factors related to potential PEG-ASP toxicity in adults with ALL, the investigators combed through records of 26 adults patients, 19 of whom had received PEG-ASP in the frontline setting, and 7 of whom received it during treatment of relapsed or refractory disease.
The investigators looked at each course of PEG-ASP as an independent event (51 total episodes), paying special attention to concomitant chemotherapy and the use of both antimycotic and antibiotic agents.
Five of the patients had grade 3 hepatotoxicity, and three had grade 4 hepatotoxicity. The patients with grade 4 events had unexplained severe weight gain and painful hepatomegaly. Ultrasonography in these patients revealed acute steatosis similar to that seen with sinusoidal occlusive disease. All three patients had received concomitant idarubicin, vincristine, and vancomycin.
In univariate analysis, neither being older than 45 years, administration of PEG-ASP during an active leukemia phase, nor having a body mass index greater than 25 kg/m2 were significantly associated with increased incidence of grade 3 or 4 hepatotoxicity.
When the investigators looked at concomitant chemotherapy drugs, however, they found that liver toxicity was significantly higher with idarubicin cumulative doses of 20 mg/m2 or greater (hazard ratio, 1.49; P = .047) and that vincristine doses of 2 mg/m2 or greater were associated with a borderline increase in risk (HR, 4.75; P = .055).
There was no increased risk for liver toxicities with either steroids, daunorubicin, cyclophosphamide, cytarabine, methotrexate, or 6-mercaptopurine.
Additionally, concomitant vancomycin was also linked to increased hepatotoxicity (HR, 1.86; P =.009). In contrast, neither carbapenem-class anti-infectives nor azole were significantly associated with liver toxicities.
“Notably, none of the patients undergoing full pediatric induction, which contains higher cumulative doses of PEG-ASP, experienced grade 4 hepatotoxicity regardless of age,” Dr. Guolo and his colleagues wrote in their poster presentation.
In multivariate analysis controlling for age, BMI, drug regimen, and concomitant therapies, idarubicin remained a significant risk factor for severe hepatotoxicity (P = .004), and vancomycin remained as a borderline risk (P = .054).
Dr. Guolo acknowledged that the investigators could not account for the potential contribution of over-the-counter medications with known risk for hepatotoxicity, such as acetaminophen.
He noted that in his group’s experience, the toxicity profile of PEG-ASP in adults, including high-dose regimens, was manageable without excess toxicities as long as clinicians paid close attention to the use of concomitant agents.
The study was internally funded. The authors reported having no relevant conflicts of interest.
SOURCE: Minetto P et al. EHA Congress, Abstract PS934.
STOCKHOLM – Liver toxicities in adults with acute lymphoblastic leukemia (ALL) treated with a pediatric-type regimen containing pegylated asparaginase (PEG-ASP) may be related to concomitant use of other hepatotoxic drugs, investigators cautioned.
A retrospective review of records on 26 adult ALL patients treated with PEG-ASP since 2013 showed that concomitant use of vincristine, idarubicin, and vancomycin was associated with an increased risk for grade 3 or 4 hepatotoxicity, reported Fabio Guolo, MD, from the University of Genoa (Italy) and his colleagues.
In contrast, patients who received other chemotherapy drugs or antimicrobial agents did not have significant liver toxicities, Dr. Guolo said in an interview at the annual congress of the European Hematology Association.
“Increased toxicity from therapy prevents delivery of the most active therapy, and asparaginase is one of the keys to the success of pediatric trials in ALL, so we have tried to push the dose of asparaginase as high as we could in adult patients,” he said.
“We asked why some patients will develop toxicity while receiving a relatively low dose of asparaginase, whereas other patients who received higher doses did not,” Dr. Guolo added.
In recent years, investigators have found that adults with ALL tend to have better outcomes when they were treated with standard pediatric ALL regimens, which includes high-dose PEG-ASP.
To identify factors related to potential PEG-ASP toxicity in adults with ALL, the investigators combed through records of 26 adults patients, 19 of whom had received PEG-ASP in the frontline setting, and 7 of whom received it during treatment of relapsed or refractory disease.
The investigators looked at each course of PEG-ASP as an independent event (51 total episodes), paying special attention to concomitant chemotherapy and the use of both antimycotic and antibiotic agents.
Five of the patients had grade 3 hepatotoxicity, and three had grade 4 hepatotoxicity. The patients with grade 4 events had unexplained severe weight gain and painful hepatomegaly. Ultrasonography in these patients revealed acute steatosis similar to that seen with sinusoidal occlusive disease. All three patients had received concomitant idarubicin, vincristine, and vancomycin.
In univariate analysis, neither being older than 45 years, administration of PEG-ASP during an active leukemia phase, nor having a body mass index greater than 25 kg/m2 were significantly associated with increased incidence of grade 3 or 4 hepatotoxicity.
When the investigators looked at concomitant chemotherapy drugs, however, they found that liver toxicity was significantly higher with idarubicin cumulative doses of 20 mg/m2 or greater (hazard ratio, 1.49; P = .047) and that vincristine doses of 2 mg/m2 or greater were associated with a borderline increase in risk (HR, 4.75; P = .055).
There was no increased risk for liver toxicities with either steroids, daunorubicin, cyclophosphamide, cytarabine, methotrexate, or 6-mercaptopurine.
Additionally, concomitant vancomycin was also linked to increased hepatotoxicity (HR, 1.86; P =.009). In contrast, neither carbapenem-class anti-infectives nor azole were significantly associated with liver toxicities.
“Notably, none of the patients undergoing full pediatric induction, which contains higher cumulative doses of PEG-ASP, experienced grade 4 hepatotoxicity regardless of age,” Dr. Guolo and his colleagues wrote in their poster presentation.
In multivariate analysis controlling for age, BMI, drug regimen, and concomitant therapies, idarubicin remained a significant risk factor for severe hepatotoxicity (P = .004), and vancomycin remained as a borderline risk (P = .054).
Dr. Guolo acknowledged that the investigators could not account for the potential contribution of over-the-counter medications with known risk for hepatotoxicity, such as acetaminophen.
He noted that in his group’s experience, the toxicity profile of PEG-ASP in adults, including high-dose regimens, was manageable without excess toxicities as long as clinicians paid close attention to the use of concomitant agents.
The study was internally funded. The authors reported having no relevant conflicts of interest.
SOURCE: Minetto P et al. EHA Congress, Abstract PS934.
REPORTING FROM EHA CONGRESS
Key clinical point:
Major finding: Idarubicin was associated with a higher risk of grade 3 or 4 hepatotoxicity, and vincristine was associated with a borderline risk.
Study details: Retrospective review of 51 PEG-ASP dosing events in 26 adult patients with ALL.
Disclosures: The study was internally funded. The authors reported having no relevant conflicts of interest.
Source: Minetto P et al. EHA Congress, Abstract PS934.
CHMP recommends CAR T for ALL, DLBCL
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended the approval of tisagenlecleucel (Kymriah®, formerly CTL019) for 2 indications.
According to the CHMP, the chimeric antigen receptor (CAR) T-cell therapy should be approved to treat adults with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) who have received 2 or more lines of systemic therapy and patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s recommendation is based on results from a pair of phase 2 trials—ELIANA and JULIET.
JULIET trial
Updated results from JULIET were presented at the recent 23rd Annual Congress of the European Hematology Association (EHA) as abstract S799.
The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so due to disease progression or clinical deterioration. The patients’ median age at baseline was 56 (range, 22-76).
Ninety-two percent of patients received bridging therapy, and 93% received lymphodepleting chemotherapy prior to tisagenlecleucel.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. Of the patients in CR at month 3, 83% remained in CR at month 12. The median duration of response was not reached.
At the time of data cutoff, none of the responders had proceeded to stem cell transplant.
For all infused patients (n=111), the 12-month overall survival (OS) rate was 49%, and the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome (CRS). Fifteen percent of patients received tocilizumab for CRS, including 3% of patients with grade 2 CRS and 50% of patients with grade 3 CRS.
Other adverse events (AEs) of interest included grade 3/4 neurologic events (12%), grade 3/4 cytopenias lasting more than 28 days (32%), grade 3/4 infections (20%), and grade 3/4 febrile neutropenia (15%).
ELIANA trial
Updated results from ELIANA were published in NEJM in February.
The trial included 75 children and young adults with relapsed/refractory ALL. The patients’ median age was 11 (range, 3 to 23).
All 75 patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CR with incomplete hematologic recovery (CRi) within 3 months.
The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to transplant while in remission. At last follow-up, 4 were still in remission, and 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least 1 AE, and 95% had AEs thought to be related to tisagenlecleucel. The rate of grade 3/4 AEs was 88%, and the rate of related grade 3/4 AEs was 73%.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended the approval of tisagenlecleucel (Kymriah®, formerly CTL019) for 2 indications.
According to the CHMP, the chimeric antigen receptor (CAR) T-cell therapy should be approved to treat adults with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) who have received 2 or more lines of systemic therapy and patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s recommendation is based on results from a pair of phase 2 trials—ELIANA and JULIET.
JULIET trial
Updated results from JULIET were presented at the recent 23rd Annual Congress of the European Hematology Association (EHA) as abstract S799.
The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so due to disease progression or clinical deterioration. The patients’ median age at baseline was 56 (range, 22-76).
Ninety-two percent of patients received bridging therapy, and 93% received lymphodepleting chemotherapy prior to tisagenlecleucel.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. Of the patients in CR at month 3, 83% remained in CR at month 12. The median duration of response was not reached.
At the time of data cutoff, none of the responders had proceeded to stem cell transplant.
For all infused patients (n=111), the 12-month overall survival (OS) rate was 49%, and the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome (CRS). Fifteen percent of patients received tocilizumab for CRS, including 3% of patients with grade 2 CRS and 50% of patients with grade 3 CRS.
Other adverse events (AEs) of interest included grade 3/4 neurologic events (12%), grade 3/4 cytopenias lasting more than 28 days (32%), grade 3/4 infections (20%), and grade 3/4 febrile neutropenia (15%).
ELIANA trial
Updated results from ELIANA were published in NEJM in February.
The trial included 75 children and young adults with relapsed/refractory ALL. The patients’ median age was 11 (range, 3 to 23).
All 75 patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CR with incomplete hematologic recovery (CRi) within 3 months.
The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to transplant while in remission. At last follow-up, 4 were still in remission, and 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least 1 AE, and 95% had AEs thought to be related to tisagenlecleucel. The rate of grade 3/4 AEs was 88%, and the rate of related grade 3/4 AEs was 73%.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended the approval of tisagenlecleucel (Kymriah®, formerly CTL019) for 2 indications.
According to the CHMP, the chimeric antigen receptor (CAR) T-cell therapy should be approved to treat adults with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) who have received 2 or more lines of systemic therapy and patients up to 25 years of age who have B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
The CHMP’s recommendation will be reviewed by the European Commission, which has the authority to approve medicines for use in the European Union, Norway, Iceland, and Liechtenstein.
The European Commission usually makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s recommendation is based on results from a pair of phase 2 trials—ELIANA and JULIET.
JULIET trial
Updated results from JULIET were presented at the recent 23rd Annual Congress of the European Hematology Association (EHA) as abstract S799.
The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so due to disease progression or clinical deterioration. The patients’ median age at baseline was 56 (range, 22-76).
Ninety-two percent of patients received bridging therapy, and 93% received lymphodepleting chemotherapy prior to tisagenlecleucel.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. Of the patients in CR at month 3, 83% remained in CR at month 12. The median duration of response was not reached.
At the time of data cutoff, none of the responders had proceeded to stem cell transplant.
For all infused patients (n=111), the 12-month overall survival (OS) rate was 49%, and the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome (CRS). Fifteen percent of patients received tocilizumab for CRS, including 3% of patients with grade 2 CRS and 50% of patients with grade 3 CRS.
Other adverse events (AEs) of interest included grade 3/4 neurologic events (12%), grade 3/4 cytopenias lasting more than 28 days (32%), grade 3/4 infections (20%), and grade 3/4 febrile neutropenia (15%).
ELIANA trial
Updated results from ELIANA were published in NEJM in February.
The trial included 75 children and young adults with relapsed/refractory ALL. The patients’ median age was 11 (range, 3 to 23).
All 75 patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CR with incomplete hematologic recovery (CRi) within 3 months.
The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to transplant while in remission. At last follow-up, 4 were still in remission, and 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least 1 AE, and 95% had AEs thought to be related to tisagenlecleucel. The rate of grade 3/4 AEs was 88%, and the rate of related grade 3/4 AEs was 73%.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).