User login
Low intensity bridging may be best path to CAR T in adult ALL
CHICAGO – A low intensity chemotherapy regimen may be the best approach to bridge patients waiting for chimeric antigen receptor (CAR) T-cell therapy, according to a retrospective analysis of adults with acute lymphoblastic leukemia (ALL).
Investigators found that high intensity bridging regimens provided no clear outcome benefit, but did produce a greater number of infections.
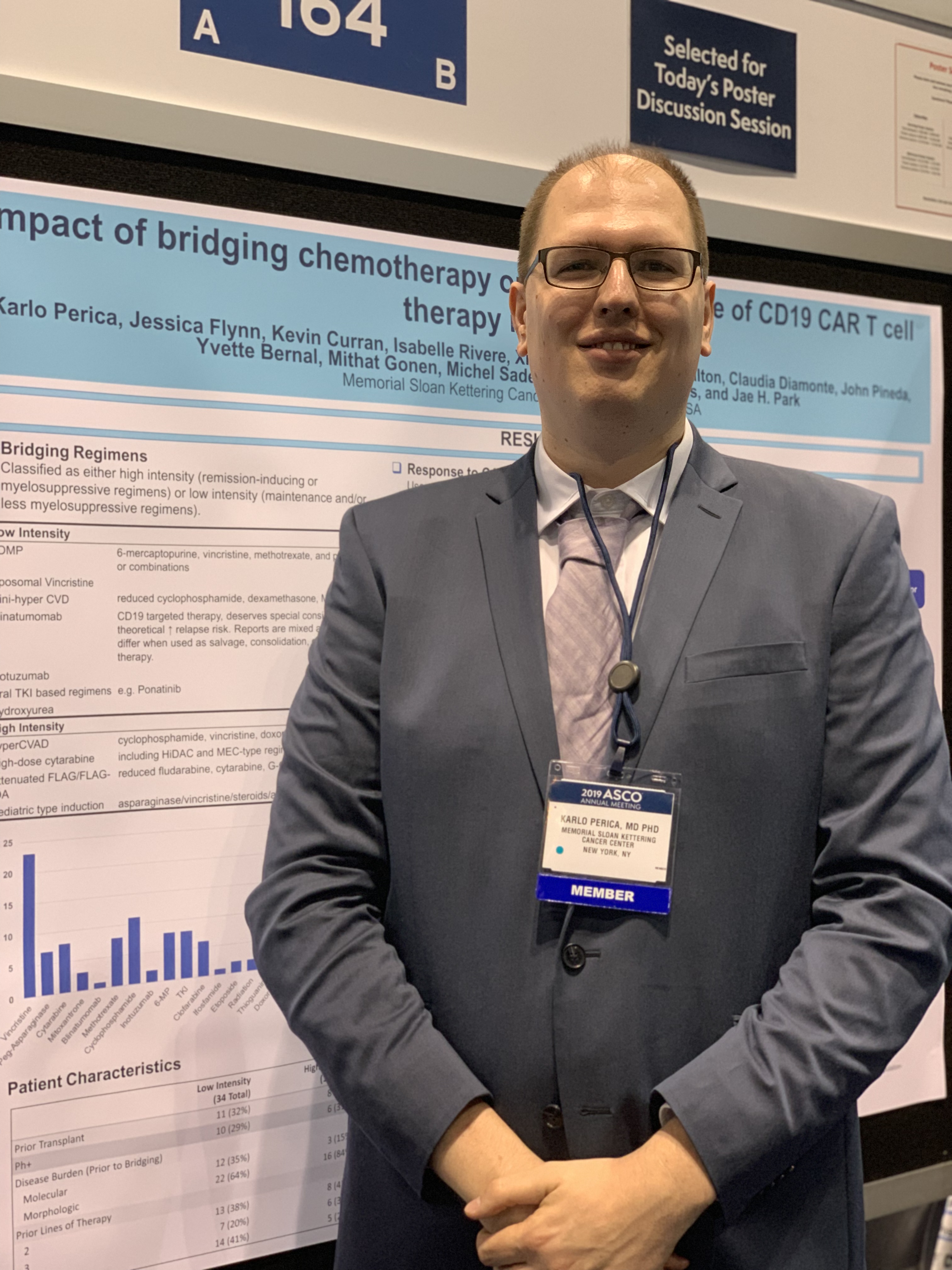
But the decision on the type of regimen is very much dependent on the individual patient, Karlo Perica, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, said at the annual meeting of the American Society of Clinical Oncology.
Dr. Perica and his colleagues at Memorial Sloan Kettering examined the effectiveness and toxicity of bridging therapies provided to relapsed or refractory ALL patients waiting to receive CD19 CAR T-cell therapy as part of a phase 1 trial (N Engl J Med. 2018 Feb 1;378[5]:449-59).
Bridging therapy was defined as any therapy given from leukapheresis to cell infusion.
The low-intensity regimens included POMP (6-mercaptopurine, vincristine, methotrexate, and prednisone, or combinations), liposomal vincristine, mini-hyper CVD (reduced cyclophosphamide, dexamethasone, methotrexate, Ara-C), blinatumomab, inotuzumab, oral tyrosine kinase inhibitor-based regimens, or hydroxyurea.
The high-intensity regimens included hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone), high-dose cytarabine, attenuated FLAG/FLAG-IDA (reduced fludarabine, cytarabine, G-CSF plus or minus idarubicin), and pediatric-type induction.
Of the 53 patients who were ultimately infused with CAR T cells, 19 received some type of high intensity regimen, 29 received low intensity regimens, and 5 received no bridging treatment. The group overall was heavily pretreated. Nearly a third of the low intensity and no bridging patients and 42% of the high intensity patients had previously undergone transplant. More than 40% of the low intensity and no bridging patients and about a quarter of the high intensity bridging group had four or more prior lines of therapy.
The use of high intensity bridging therapy was not associated with improved overall response or relapse-free survival to CAR T-cell therapy, the investigators reported. In a subgroup with 23 high disease burden patients with greater than 20% blasts, there was no difference in MRD-negative complete response by intensity (75% versus 60%, Fisher’s P = .65).
High intensity bridging was also not associated with successful CAR T-cell infusion, versus low intensity regimens (63% versus 79%, P greater than .05) or a combined endpoint of CAR T-cell infusion plus transplant or alternative treatment (80% versus 86%, P greater than .05).
In terms of toxicity, the high intensity bridging regimens were associated with a higher rate of grade 3 or 4 infections – 15 versus 11 infections (Fisher’s P = .002). But there was no association with post-infusion grade 3 or 4 cytokine release syndrome or neurotoxicity.
Dr. Perica said the results reflect that the real goal of bridging is not to reduce disease burden but instead to successfully bring patients to the next phase of their treatment. “The goal of the bridging therapy is to get the patient to the CAR infusion,” he said.
Due to the retrospective nature of the study, Dr. Perica said he can’t recommend any single bridging regimen and he emphasized that the decisions are patient-specific.
The original study was funded by several foundations and Juno Therapeutics. Dr. Perica reported royalties from technology licensed to Neximmune.
SOURCE: Perica K et al. ASCO 2019, Abstract 2520.
CHICAGO – A low intensity chemotherapy regimen may be the best approach to bridge patients waiting for chimeric antigen receptor (CAR) T-cell therapy, according to a retrospective analysis of adults with acute lymphoblastic leukemia (ALL).
Investigators found that high intensity bridging regimens provided no clear outcome benefit, but did produce a greater number of infections.
But the decision on the type of regimen is very much dependent on the individual patient, Karlo Perica, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, said at the annual meeting of the American Society of Clinical Oncology.
Dr. Perica and his colleagues at Memorial Sloan Kettering examined the effectiveness and toxicity of bridging therapies provided to relapsed or refractory ALL patients waiting to receive CD19 CAR T-cell therapy as part of a phase 1 trial (N Engl J Med. 2018 Feb 1;378[5]:449-59).
Bridging therapy was defined as any therapy given from leukapheresis to cell infusion.
The low-intensity regimens included POMP (6-mercaptopurine, vincristine, methotrexate, and prednisone, or combinations), liposomal vincristine, mini-hyper CVD (reduced cyclophosphamide, dexamethasone, methotrexate, Ara-C), blinatumomab, inotuzumab, oral tyrosine kinase inhibitor-based regimens, or hydroxyurea.
The high-intensity regimens included hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone), high-dose cytarabine, attenuated FLAG/FLAG-IDA (reduced fludarabine, cytarabine, G-CSF plus or minus idarubicin), and pediatric-type induction.
Of the 53 patients who were ultimately infused with CAR T cells, 19 received some type of high intensity regimen, 29 received low intensity regimens, and 5 received no bridging treatment. The group overall was heavily pretreated. Nearly a third of the low intensity and no bridging patients and 42% of the high intensity patients had previously undergone transplant. More than 40% of the low intensity and no bridging patients and about a quarter of the high intensity bridging group had four or more prior lines of therapy.
The use of high intensity bridging therapy was not associated with improved overall response or relapse-free survival to CAR T-cell therapy, the investigators reported. In a subgroup with 23 high disease burden patients with greater than 20% blasts, there was no difference in MRD-negative complete response by intensity (75% versus 60%, Fisher’s P = .65).
High intensity bridging was also not associated with successful CAR T-cell infusion, versus low intensity regimens (63% versus 79%, P greater than .05) or a combined endpoint of CAR T-cell infusion plus transplant or alternative treatment (80% versus 86%, P greater than .05).
In terms of toxicity, the high intensity bridging regimens were associated with a higher rate of grade 3 or 4 infections – 15 versus 11 infections (Fisher’s P = .002). But there was no association with post-infusion grade 3 or 4 cytokine release syndrome or neurotoxicity.
Dr. Perica said the results reflect that the real goal of bridging is not to reduce disease burden but instead to successfully bring patients to the next phase of their treatment. “The goal of the bridging therapy is to get the patient to the CAR infusion,” he said.
Due to the retrospective nature of the study, Dr. Perica said he can’t recommend any single bridging regimen and he emphasized that the decisions are patient-specific.
The original study was funded by several foundations and Juno Therapeutics. Dr. Perica reported royalties from technology licensed to Neximmune.
SOURCE: Perica K et al. ASCO 2019, Abstract 2520.
CHICAGO – A low intensity chemotherapy regimen may be the best approach to bridge patients waiting for chimeric antigen receptor (CAR) T-cell therapy, according to a retrospective analysis of adults with acute lymphoblastic leukemia (ALL).
Investigators found that high intensity bridging regimens provided no clear outcome benefit, but did produce a greater number of infections.
But the decision on the type of regimen is very much dependent on the individual patient, Karlo Perica, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, said at the annual meeting of the American Society of Clinical Oncology.
Dr. Perica and his colleagues at Memorial Sloan Kettering examined the effectiveness and toxicity of bridging therapies provided to relapsed or refractory ALL patients waiting to receive CD19 CAR T-cell therapy as part of a phase 1 trial (N Engl J Med. 2018 Feb 1;378[5]:449-59).
Bridging therapy was defined as any therapy given from leukapheresis to cell infusion.
The low-intensity regimens included POMP (6-mercaptopurine, vincristine, methotrexate, and prednisone, or combinations), liposomal vincristine, mini-hyper CVD (reduced cyclophosphamide, dexamethasone, methotrexate, Ara-C), blinatumomab, inotuzumab, oral tyrosine kinase inhibitor-based regimens, or hydroxyurea.
The high-intensity regimens included hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone), high-dose cytarabine, attenuated FLAG/FLAG-IDA (reduced fludarabine, cytarabine, G-CSF plus or minus idarubicin), and pediatric-type induction.
Of the 53 patients who were ultimately infused with CAR T cells, 19 received some type of high intensity regimen, 29 received low intensity regimens, and 5 received no bridging treatment. The group overall was heavily pretreated. Nearly a third of the low intensity and no bridging patients and 42% of the high intensity patients had previously undergone transplant. More than 40% of the low intensity and no bridging patients and about a quarter of the high intensity bridging group had four or more prior lines of therapy.
The use of high intensity bridging therapy was not associated with improved overall response or relapse-free survival to CAR T-cell therapy, the investigators reported. In a subgroup with 23 high disease burden patients with greater than 20% blasts, there was no difference in MRD-negative complete response by intensity (75% versus 60%, Fisher’s P = .65).
High intensity bridging was also not associated with successful CAR T-cell infusion, versus low intensity regimens (63% versus 79%, P greater than .05) or a combined endpoint of CAR T-cell infusion plus transplant or alternative treatment (80% versus 86%, P greater than .05).
In terms of toxicity, the high intensity bridging regimens were associated with a higher rate of grade 3 or 4 infections – 15 versus 11 infections (Fisher’s P = .002). But there was no association with post-infusion grade 3 or 4 cytokine release syndrome or neurotoxicity.
Dr. Perica said the results reflect that the real goal of bridging is not to reduce disease burden but instead to successfully bring patients to the next phase of their treatment. “The goal of the bridging therapy is to get the patient to the CAR infusion,” he said.
Due to the retrospective nature of the study, Dr. Perica said he can’t recommend any single bridging regimen and he emphasized that the decisions are patient-specific.
The original study was funded by several foundations and Juno Therapeutics. Dr. Perica reported royalties from technology licensed to Neximmune.
SOURCE: Perica K et al. ASCO 2019, Abstract 2520.
FROM ASCO 2019
NGS comparable to FC for minimal residual disease assessment
NEW ORLEANS – Next-generation sequencing of peripheral blood is at least as effective as flow cytometry of bone marrow for assessing minimal residual disease, according to a new study.
Researchers compared bone marrow flow cytometry (FC) and peripheral blood next-generation sequencing (NGS) for minimal residual disease (MRD) assessment in pediatric and young adult patients with B-cell acute lymphoblastic leukemia (B-ALL) who received treatment with tisagenlecleucel. There was a high level of concordance between the assays, but the NGS assay detected more MRD-positive samples and NGS results provided a longer lead time to relapse.
Michael A. Pulsipher, MD, of the Children’s Hospital Los Angeles, presented these results at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The researchers analyzed samples from pediatric and young adult patients aged 2-25 years who had relapsed or refractory B-ALL and received treatment with tisagenlecleucel on the ELIANA or ENSIGN trials.
The patients had received at least two prior lines of therapy and were ineligible for allogeneic transplant. They received a single dose of tisagenlecleucel. MRD was assessed before tisagenlecleucel infusion, at various time points after infusion, and at relapse.
Dr. Pulsipher and his colleagues compared MRD results from an NGS assay – Adaptive Biotechnologies’ clonoSEQ – using peripheral blood and results from FC of bone marrow. NGS and FC results were available for 237 samples from 83 patients.
After treatment, NGS detected more MRD-positive samples at each sensitivity level tested (10-4, 10-5, and 10-6). At 10-6, NGS detected 18% more MRD-positive samples than did FC – 50% and 32%, respectively.
Detection of MRD positivity prior to relapse was faster with NGS than with FC. In 17 of 34 patients with morphological relapse, NGS provided a median lead time of 67 days. FC provided a median lead time of 39 days in 11 of the 34 patients.
About 80% of patients who had an MRD status of zero by NGS at day 28 remained relapse-free for up to 3 years.
Among complete responders (n = 50), the duration of response was significantly longer in patients who had an MRD status of zero at day 28 by NGS than in patients who had an MRD status greater than zero (P = .0003). Overall survival was significantly better among patients with an MRD status of zero as well (P = .0004).
Dr. Pulsipher said additional studies are needed to confirm these findings and determine the best way to know if a patient has been cured or needs additional therapy after tisagenlecleucel.
Dr. Pulsipher reported relationships with Adaptive Biotech, Novartis, Incyte, Amgen, Bellicum Pharmaceuticals, Medac Pharma, and Miltenyi Biotec. ELIANA and ENSIGN were funded by Novartis, which markets tisagenlecleucel as Kymriah.
SOURCE: Pulsipher MA et al. ASPHO 2019, Abstract 2001.
NEW ORLEANS – Next-generation sequencing of peripheral blood is at least as effective as flow cytometry of bone marrow for assessing minimal residual disease, according to a new study.
Researchers compared bone marrow flow cytometry (FC) and peripheral blood next-generation sequencing (NGS) for minimal residual disease (MRD) assessment in pediatric and young adult patients with B-cell acute lymphoblastic leukemia (B-ALL) who received treatment with tisagenlecleucel. There was a high level of concordance between the assays, but the NGS assay detected more MRD-positive samples and NGS results provided a longer lead time to relapse.
Michael A. Pulsipher, MD, of the Children’s Hospital Los Angeles, presented these results at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The researchers analyzed samples from pediatric and young adult patients aged 2-25 years who had relapsed or refractory B-ALL and received treatment with tisagenlecleucel on the ELIANA or ENSIGN trials.
The patients had received at least two prior lines of therapy and were ineligible for allogeneic transplant. They received a single dose of tisagenlecleucel. MRD was assessed before tisagenlecleucel infusion, at various time points after infusion, and at relapse.
Dr. Pulsipher and his colleagues compared MRD results from an NGS assay – Adaptive Biotechnologies’ clonoSEQ – using peripheral blood and results from FC of bone marrow. NGS and FC results were available for 237 samples from 83 patients.
After treatment, NGS detected more MRD-positive samples at each sensitivity level tested (10-4, 10-5, and 10-6). At 10-6, NGS detected 18% more MRD-positive samples than did FC – 50% and 32%, respectively.
Detection of MRD positivity prior to relapse was faster with NGS than with FC. In 17 of 34 patients with morphological relapse, NGS provided a median lead time of 67 days. FC provided a median lead time of 39 days in 11 of the 34 patients.
About 80% of patients who had an MRD status of zero by NGS at day 28 remained relapse-free for up to 3 years.
Among complete responders (n = 50), the duration of response was significantly longer in patients who had an MRD status of zero at day 28 by NGS than in patients who had an MRD status greater than zero (P = .0003). Overall survival was significantly better among patients with an MRD status of zero as well (P = .0004).
Dr. Pulsipher said additional studies are needed to confirm these findings and determine the best way to know if a patient has been cured or needs additional therapy after tisagenlecleucel.
Dr. Pulsipher reported relationships with Adaptive Biotech, Novartis, Incyte, Amgen, Bellicum Pharmaceuticals, Medac Pharma, and Miltenyi Biotec. ELIANA and ENSIGN were funded by Novartis, which markets tisagenlecleucel as Kymriah.
SOURCE: Pulsipher MA et al. ASPHO 2019, Abstract 2001.
NEW ORLEANS – Next-generation sequencing of peripheral blood is at least as effective as flow cytometry of bone marrow for assessing minimal residual disease, according to a new study.
Researchers compared bone marrow flow cytometry (FC) and peripheral blood next-generation sequencing (NGS) for minimal residual disease (MRD) assessment in pediatric and young adult patients with B-cell acute lymphoblastic leukemia (B-ALL) who received treatment with tisagenlecleucel. There was a high level of concordance between the assays, but the NGS assay detected more MRD-positive samples and NGS results provided a longer lead time to relapse.
Michael A. Pulsipher, MD, of the Children’s Hospital Los Angeles, presented these results at the annual meeting of the American Society of Pediatric Hematology/Oncology.
The researchers analyzed samples from pediatric and young adult patients aged 2-25 years who had relapsed or refractory B-ALL and received treatment with tisagenlecleucel on the ELIANA or ENSIGN trials.
The patients had received at least two prior lines of therapy and were ineligible for allogeneic transplant. They received a single dose of tisagenlecleucel. MRD was assessed before tisagenlecleucel infusion, at various time points after infusion, and at relapse.
Dr. Pulsipher and his colleagues compared MRD results from an NGS assay – Adaptive Biotechnologies’ clonoSEQ – using peripheral blood and results from FC of bone marrow. NGS and FC results were available for 237 samples from 83 patients.
After treatment, NGS detected more MRD-positive samples at each sensitivity level tested (10-4, 10-5, and 10-6). At 10-6, NGS detected 18% more MRD-positive samples than did FC – 50% and 32%, respectively.
Detection of MRD positivity prior to relapse was faster with NGS than with FC. In 17 of 34 patients with morphological relapse, NGS provided a median lead time of 67 days. FC provided a median lead time of 39 days in 11 of the 34 patients.
About 80% of patients who had an MRD status of zero by NGS at day 28 remained relapse-free for up to 3 years.
Among complete responders (n = 50), the duration of response was significantly longer in patients who had an MRD status of zero at day 28 by NGS than in patients who had an MRD status greater than zero (P = .0003). Overall survival was significantly better among patients with an MRD status of zero as well (P = .0004).
Dr. Pulsipher said additional studies are needed to confirm these findings and determine the best way to know if a patient has been cured or needs additional therapy after tisagenlecleucel.
Dr. Pulsipher reported relationships with Adaptive Biotech, Novartis, Incyte, Amgen, Bellicum Pharmaceuticals, Medac Pharma, and Miltenyi Biotec. ELIANA and ENSIGN were funded by Novartis, which markets tisagenlecleucel as Kymriah.
SOURCE: Pulsipher MA et al. ASPHO 2019, Abstract 2001.
REPORTING FROM 2019 ASPHO CONFERENCE
Key clinical point: Major finding: At the highest sensitivity level tested, next-generation sequencing detected 18% more minimal residual disease–positive samples than did flow cytometry – 50% and 32%, respectively.
Study details: An analysis of samples from pediatric and young adult patients with B-cell acute lymphoblastic leukemia who received treatment with tisagenlecleucel on the ELIANA and ENSIGN trials.
Disclosures: The speaker reported relationships with Adaptive Biotech, Novartis, Incyte, Amgen, Bellicum Pharmaceuticals, Medac Pharma, and Miltenyi Biotec. The ELIANA and ENSIGN trials were funded by Novartis, which markets tisagenlecleucel as Kymriah.
Source: Pulsipher MA et al. ASPHO 2019, Abstract 2001.
Tagraxofusp produces high response rate in BPDCN
Tagraxofusp demonstrated efficacy in a phase 2 trial of patients with previously treated or untreated blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The overall response rate was 90% in previously untreated patients who received the highest dose of tagraxofusp and 67% in patients with relapsed/refractory BPDCN.
The researchers wrote that capillary leak syndrome (CLS) was an important adverse event in this trial, as it caused two deaths. However, the researchers developed strategies that appear to mitigate the risk of CLS in patients taking tagraxofusp.
Naveen Pemmaraju, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues conducted the trial and reported the results in the New England Journal of Medicine.
The trial included 47 patients – 32 with previously untreated BPDCN and 15 with relapsed/refractory BPDCN. The patients’ median age at baseline was 70 years and 83% were men.
Three patients (all previously untreated) received tagraxofusp at 7 mcg/kg per day, and 44 patients received a 12 mcg/kg per day dose. All patients were treated on days 1-5 of a 21-day cycle.
Response and survival
In the 29 previously untreated patients who received the 12 mcg/kg dose of tagraxofusp, the overall response rate was 90%. The rate of complete response plus clinical complete response in these patients was 72%.
In the 15 patients with relapsed/refractory BPDCN, the overall response rate was 67%, and the rate of complete response plus clinical complete response was 33%.
A total of 14 patients, 13 of whom had previously untreated BPDCN, went on to transplant.
In the 29 previously untreated patients, the median overall survival was not reached at a median follow-up of 25 months. The overall survival rate was 62% at 12 months, 59% at 18 months, and 52% at 24 months.
In the 15 previously treated patients, the median overall survival was 8.5 months.
Safety
Common adverse events in this trial were ALT increase (64%), AST increase (60%), hypoalbuminemia (55%), peripheral edema (51%), thrombocytopenia (49%), nausea (45%), pyrexia (45%), and fatigue (45%).
Among the 44 patients who received the 12 mcg/kg dose of tagraxofusp, 8 (18%) developed CLS. Six patients had grade 2 CLS, one had grade 4, and one had grade 5. There was an additional CLS-related death in a patient who received tagraxofusp at 7 mcg/kg.
After the first death, the trial protocol was amended to reduce CLS risk. Inclusion criteria were changed so that patients must have normal cardiac function, adequate kidney function, and serum albumin of at least 3.2 g/dL. Additionally, the researchers began monitoring patients’ weight, albumin levels, and kidney function. The team withheld tagraxofusp if patients experienced rapid weight gain or if their serum albumin or systolic blood pressure fell too low.
The trial was sponsored by Stemline Therapeutics. The researchers reported relationships with Stemline and other companies.
Tagraxofusp demonstrated efficacy in a phase 2 trial of patients with previously treated or untreated blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The overall response rate was 90% in previously untreated patients who received the highest dose of tagraxofusp and 67% in patients with relapsed/refractory BPDCN.
The researchers wrote that capillary leak syndrome (CLS) was an important adverse event in this trial, as it caused two deaths. However, the researchers developed strategies that appear to mitigate the risk of CLS in patients taking tagraxofusp.
Naveen Pemmaraju, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues conducted the trial and reported the results in the New England Journal of Medicine.
The trial included 47 patients – 32 with previously untreated BPDCN and 15 with relapsed/refractory BPDCN. The patients’ median age at baseline was 70 years and 83% were men.
Three patients (all previously untreated) received tagraxofusp at 7 mcg/kg per day, and 44 patients received a 12 mcg/kg per day dose. All patients were treated on days 1-5 of a 21-day cycle.
Response and survival
In the 29 previously untreated patients who received the 12 mcg/kg dose of tagraxofusp, the overall response rate was 90%. The rate of complete response plus clinical complete response in these patients was 72%.
In the 15 patients with relapsed/refractory BPDCN, the overall response rate was 67%, and the rate of complete response plus clinical complete response was 33%.
A total of 14 patients, 13 of whom had previously untreated BPDCN, went on to transplant.
In the 29 previously untreated patients, the median overall survival was not reached at a median follow-up of 25 months. The overall survival rate was 62% at 12 months, 59% at 18 months, and 52% at 24 months.
In the 15 previously treated patients, the median overall survival was 8.5 months.
Safety
Common adverse events in this trial were ALT increase (64%), AST increase (60%), hypoalbuminemia (55%), peripheral edema (51%), thrombocytopenia (49%), nausea (45%), pyrexia (45%), and fatigue (45%).
Among the 44 patients who received the 12 mcg/kg dose of tagraxofusp, 8 (18%) developed CLS. Six patients had grade 2 CLS, one had grade 4, and one had grade 5. There was an additional CLS-related death in a patient who received tagraxofusp at 7 mcg/kg.
After the first death, the trial protocol was amended to reduce CLS risk. Inclusion criteria were changed so that patients must have normal cardiac function, adequate kidney function, and serum albumin of at least 3.2 g/dL. Additionally, the researchers began monitoring patients’ weight, albumin levels, and kidney function. The team withheld tagraxofusp if patients experienced rapid weight gain or if their serum albumin or systolic blood pressure fell too low.
The trial was sponsored by Stemline Therapeutics. The researchers reported relationships with Stemline and other companies.
Tagraxofusp demonstrated efficacy in a phase 2 trial of patients with previously treated or untreated blastic plasmacytoid dendritic cell neoplasm (BPDCN).
The overall response rate was 90% in previously untreated patients who received the highest dose of tagraxofusp and 67% in patients with relapsed/refractory BPDCN.
The researchers wrote that capillary leak syndrome (CLS) was an important adverse event in this trial, as it caused two deaths. However, the researchers developed strategies that appear to mitigate the risk of CLS in patients taking tagraxofusp.
Naveen Pemmaraju, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues conducted the trial and reported the results in the New England Journal of Medicine.
The trial included 47 patients – 32 with previously untreated BPDCN and 15 with relapsed/refractory BPDCN. The patients’ median age at baseline was 70 years and 83% were men.
Three patients (all previously untreated) received tagraxofusp at 7 mcg/kg per day, and 44 patients received a 12 mcg/kg per day dose. All patients were treated on days 1-5 of a 21-day cycle.
Response and survival
In the 29 previously untreated patients who received the 12 mcg/kg dose of tagraxofusp, the overall response rate was 90%. The rate of complete response plus clinical complete response in these patients was 72%.
In the 15 patients with relapsed/refractory BPDCN, the overall response rate was 67%, and the rate of complete response plus clinical complete response was 33%.
A total of 14 patients, 13 of whom had previously untreated BPDCN, went on to transplant.
In the 29 previously untreated patients, the median overall survival was not reached at a median follow-up of 25 months. The overall survival rate was 62% at 12 months, 59% at 18 months, and 52% at 24 months.
In the 15 previously treated patients, the median overall survival was 8.5 months.
Safety
Common adverse events in this trial were ALT increase (64%), AST increase (60%), hypoalbuminemia (55%), peripheral edema (51%), thrombocytopenia (49%), nausea (45%), pyrexia (45%), and fatigue (45%).
Among the 44 patients who received the 12 mcg/kg dose of tagraxofusp, 8 (18%) developed CLS. Six patients had grade 2 CLS, one had grade 4, and one had grade 5. There was an additional CLS-related death in a patient who received tagraxofusp at 7 mcg/kg.
After the first death, the trial protocol was amended to reduce CLS risk. Inclusion criteria were changed so that patients must have normal cardiac function, adequate kidney function, and serum albumin of at least 3.2 g/dL. Additionally, the researchers began monitoring patients’ weight, albumin levels, and kidney function. The team withheld tagraxofusp if patients experienced rapid weight gain or if their serum albumin or systolic blood pressure fell too low.
The trial was sponsored by Stemline Therapeutics. The researchers reported relationships with Stemline and other companies.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Tagraxofusp produced responses in patients with blastic plasmacytoid dendritic cell neoplasm (BPDCN).
Major finding: The overall response rate was 90% in previously untreated patients who received the highest dose of tagraxofusp and 67% in patients with relapsed/refractory BPDCN.
Study details: A phase 2 trial of 47 patients, 32 with previously untreated BPDCN and 15 with relapsed/refractory BPDCN.
Disclosures: The trial was sponsored by Stemline Therapeutics. The researchers reported relationships with Stemline and other companies.
Source: Pemmaraju N et al. N Engl J Med. 2019;380:1628-37.
Factors emerge for mitigating CD19 CAR T toxicity
HOUSTON – Cytokine release syndrome and neurotoxicity frequently occur with CD19-directed chimeric antigen receptor (CAR) T-cell immunotherapies, but targetable factors for mitigating the risk and effects of these complications are emerging, according to Cameron Turtle, MBBS, PhD.
These factors include infused CAR T-cell dose, bone marrow disease burden, immune response, and the lymphodepletion regimen used, Dr. Turtle, of Fred Hutchinson Cancer Research Center, Seattle, said at the Transplantation & Cellular Therapies Meetings. This list is based on an analysis of several studies that included a total of 195 patients with B-cell malignancies who were treated with defined-composition CD19 CAR T cells.
In a 2016 study included in the analysis, for instance, Dr. Turtle and his colleagues found that CD19 CAR T cells administered to adults with B-cell acute lymphoblastic leukemia (B-ALL) after lymphodepletion chemotherapy were “remarkably potent.” Remission was achieved in 27 of 29 patients (J Clin Invest. 2016 Jun 1;126[6]:2123-38).
However, the study also established that high CAR T-cell doses and tumor burden increased the risk of severe cytokine release syndrome (CRS) and neurotoxicity, Dr. Turtle said at the meeting, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
“Importantly, we identified serum biomarkers that allow testing of early intervention strategies in the patients who have the highest risk of toxicity,” he said.
Dr. Turtle explained that significantly higher peak interleuken-6 (IL-6) and interferon (IFN)-gamma levels were seen after CAR T-cell infusion in patients with high bone marrow tumor burden and in patients requiring treatment in an intensive care unit (ICU).
ICU care correlated with a higher percentage of bone marrow blasts before lymphodepletion chemotherapy, he added.
Elevations of serum C-reactive protein (CRP) and ferritin also correlated with bone marrow disease burden and with the occurrence of severe CRS requiring ICU care, he said, noting that ferritin and CRP levels declined after tocilizumab or corticosteroid therapy.
In addition, all patients in the study who developed neurotoxicity had evidence of CRS. Peak levels of IL-6, IFN-gamma, ferritin, and CRP were significantly higher in those who developed grade 3 or higher neurotoxicity. Further, serum IL-6 and IFN-gamma concentrations on day 1 after infusion were significantly higher in those who required ICU care and in those who subsequently developed grade 4 neurotoxicity than in patients who developed grade 3 neurotoxicity.
Multivariate analysis indicated that serum IL-6 concentration of more than 30 pg/mL on day 1 and the total number of CD19+ cells in bone marrow before therapy were independent predictors of subsequent development of grade 3 or higher neurotoxicity.
Notably, serum IL-6 of more than 30 pg/mL on day 1 identified all patients in the study who subsequently developed grade 4 or higher neurotoxicity, Dr. Turtle and his colleagues noted.
“The findings suggested that evaluation of serum IL-6 concentration early after CAR T-cell infusion might be useful for identifying patients at high risk of severe neurotoxicity and to evaluate early intervention approaches,” he said.
Neurotoxicity
In a 2017 study from Juliane Gust, MD, PhD, and her colleagues, bone marrow disease burden, lymphodepletion regimen, and CAR T-cell dose were found to be significantly associated with neurotoxicity during multivariate analysis (Cancer Discov. 2017 Dec;7[12]:1404-19).
Patients with severe neurotoxicity in that study demonstrated evidence of endothelial activation, including disseminated intravascular coagulation, capillary leak, and increased blood-brain barrier permeability – with the latter leading to a failure to protect the cerebrospinal fluid from high concentrations of systemic cytokines, including IFN-gamma. These high levels of cytokines may cause vascular pericyte activation and stress, Dr. Turtle explained.
Patients who subsequently developed grade 4 or higher neurotoxicity had higher pretreatment levels of endothelial activation biomarkers.
“Endothelial cells and pericytes contribute to the integrity of the blood-brain barrier; this suggests a potential role for IL-6 and vascular endothelial growth factor from pericytes to augment endothelial permeability,” Dr. Turtle said.
CRS
In another 2017 study, from Kevin A. Hay, MD, and his colleagues, similar factors were found to be associated with CRS (Blood. 2017 Nov 23;130[21]:2295-306).
Multivariable analysis identified high marrow tumor burden, lymphodepletion using cyclophosphamide and fludarabine, higher CAR T-cell dose, thrombocytopenia before lymphodepletion, and manufacturing of CAR T cells without selection of CD8+ central memory T cells as independent predictors of CRS.
Severe CRS was characterized by hemodynamic instability, capillary leak, and consumptive coagulopathy. As in the study by Dr. Gust and her colleagues, biomarkers of endothelial activation, including angiopoietin-2 and von Willebrand factor, were increased during severe CRS and before lymphodepletion in patients who subsequently developed CRS.
Potential modifications
The findings to date suggest that risk stratification, prophylaxis, early intervention and therapeutic intervention are among potential strategies for mitigating the risk of CD19-directed CAR T toxicity, Dr. Turtle said. Steroids, tocilizumab, siltuximab, anakinra, anti-GM-CSF, small molecules, plasma exchange, angiopoietin-1, and hypertransfusion are among candidates under consideration for such interventions, he noted.
Other approaches that have been tested in small studies, and which may reduce toxicity and improve the therapeutic index of CD19 CAR T-cell therapy for B-ALL, include split dosing and risk-adapted dosing.
“These approaches do appear to mitigate toxicity, but larger studies are needed to confirm that treatment efficacy is maintained,” Dr. Turtle said.
Toxicity prediction and early intervention to maintain the CAR T-cell dose while avoiding grade 4 or greater toxicities would be helpful and is within reach, he said, noting that the findings by Dr. Hay and his colleagues led to the development of “day-1 cytokine combination algorithms that predict grade 4-5 CRS and could direct preemptive intervention.”
One algorithm based on three cytokines had high sensitivity and specificity, but would require screening of all patients.
Early intervention in patients in whom toxicity is predicted has not been extensively evaluated in clinical studies, he said.
Dr. Hay and his colleagues did, however, develop a “classification tree model of early intervention strategies” using their findings.
A complicating factor in predicting risk and intervening is that each CAR T-cell product is associated with differing levels of toxicity risk. The varying rates of toxicity suggest that promising approaches for addressing CAR T toxicity require validation for each product with respect to cutpoints, efficacy, and maintenance of response, Dr. Turtle said.
“The findings to date are encouraging and show that potentially targetable factors for mitigating the toxicity of CAR T-cell therapy can be identified,” he said. “But clinical studies have yet to convincingly establish the best approach.”
Dr. Turtle has served on advisory boards for Juno/Celgene, Kite/Gilead, Novartis, Precision Biosciences, Eureka Therapeutics, Caribou Biosciences, Nektar Therapeutics, Humanigen, and Aptevo; has intellectual property rights licensed to Juno; has stock options with Precision Biosciences, Eureka Therapeutics, and Caribou Biosciences; and has received research funding from Juno and Nektar Therapeutics.
HOUSTON – Cytokine release syndrome and neurotoxicity frequently occur with CD19-directed chimeric antigen receptor (CAR) T-cell immunotherapies, but targetable factors for mitigating the risk and effects of these complications are emerging, according to Cameron Turtle, MBBS, PhD.
These factors include infused CAR T-cell dose, bone marrow disease burden, immune response, and the lymphodepletion regimen used, Dr. Turtle, of Fred Hutchinson Cancer Research Center, Seattle, said at the Transplantation & Cellular Therapies Meetings. This list is based on an analysis of several studies that included a total of 195 patients with B-cell malignancies who were treated with defined-composition CD19 CAR T cells.
In a 2016 study included in the analysis, for instance, Dr. Turtle and his colleagues found that CD19 CAR T cells administered to adults with B-cell acute lymphoblastic leukemia (B-ALL) after lymphodepletion chemotherapy were “remarkably potent.” Remission was achieved in 27 of 29 patients (J Clin Invest. 2016 Jun 1;126[6]:2123-38).
However, the study also established that high CAR T-cell doses and tumor burden increased the risk of severe cytokine release syndrome (CRS) and neurotoxicity, Dr. Turtle said at the meeting, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
“Importantly, we identified serum biomarkers that allow testing of early intervention strategies in the patients who have the highest risk of toxicity,” he said.
Dr. Turtle explained that significantly higher peak interleuken-6 (IL-6) and interferon (IFN)-gamma levels were seen after CAR T-cell infusion in patients with high bone marrow tumor burden and in patients requiring treatment in an intensive care unit (ICU).
ICU care correlated with a higher percentage of bone marrow blasts before lymphodepletion chemotherapy, he added.
Elevations of serum C-reactive protein (CRP) and ferritin also correlated with bone marrow disease burden and with the occurrence of severe CRS requiring ICU care, he said, noting that ferritin and CRP levels declined after tocilizumab or corticosteroid therapy.
In addition, all patients in the study who developed neurotoxicity had evidence of CRS. Peak levels of IL-6, IFN-gamma, ferritin, and CRP were significantly higher in those who developed grade 3 or higher neurotoxicity. Further, serum IL-6 and IFN-gamma concentrations on day 1 after infusion were significantly higher in those who required ICU care and in those who subsequently developed grade 4 neurotoxicity than in patients who developed grade 3 neurotoxicity.
Multivariate analysis indicated that serum IL-6 concentration of more than 30 pg/mL on day 1 and the total number of CD19+ cells in bone marrow before therapy were independent predictors of subsequent development of grade 3 or higher neurotoxicity.
Notably, serum IL-6 of more than 30 pg/mL on day 1 identified all patients in the study who subsequently developed grade 4 or higher neurotoxicity, Dr. Turtle and his colleagues noted.
“The findings suggested that evaluation of serum IL-6 concentration early after CAR T-cell infusion might be useful for identifying patients at high risk of severe neurotoxicity and to evaluate early intervention approaches,” he said.
Neurotoxicity
In a 2017 study from Juliane Gust, MD, PhD, and her colleagues, bone marrow disease burden, lymphodepletion regimen, and CAR T-cell dose were found to be significantly associated with neurotoxicity during multivariate analysis (Cancer Discov. 2017 Dec;7[12]:1404-19).
Patients with severe neurotoxicity in that study demonstrated evidence of endothelial activation, including disseminated intravascular coagulation, capillary leak, and increased blood-brain barrier permeability – with the latter leading to a failure to protect the cerebrospinal fluid from high concentrations of systemic cytokines, including IFN-gamma. These high levels of cytokines may cause vascular pericyte activation and stress, Dr. Turtle explained.
Patients who subsequently developed grade 4 or higher neurotoxicity had higher pretreatment levels of endothelial activation biomarkers.
“Endothelial cells and pericytes contribute to the integrity of the blood-brain barrier; this suggests a potential role for IL-6 and vascular endothelial growth factor from pericytes to augment endothelial permeability,” Dr. Turtle said.
CRS
In another 2017 study, from Kevin A. Hay, MD, and his colleagues, similar factors were found to be associated with CRS (Blood. 2017 Nov 23;130[21]:2295-306).
Multivariable analysis identified high marrow tumor burden, lymphodepletion using cyclophosphamide and fludarabine, higher CAR T-cell dose, thrombocytopenia before lymphodepletion, and manufacturing of CAR T cells without selection of CD8+ central memory T cells as independent predictors of CRS.
Severe CRS was characterized by hemodynamic instability, capillary leak, and consumptive coagulopathy. As in the study by Dr. Gust and her colleagues, biomarkers of endothelial activation, including angiopoietin-2 and von Willebrand factor, were increased during severe CRS and before lymphodepletion in patients who subsequently developed CRS.
Potential modifications
The findings to date suggest that risk stratification, prophylaxis, early intervention and therapeutic intervention are among potential strategies for mitigating the risk of CD19-directed CAR T toxicity, Dr. Turtle said. Steroids, tocilizumab, siltuximab, anakinra, anti-GM-CSF, small molecules, plasma exchange, angiopoietin-1, and hypertransfusion are among candidates under consideration for such interventions, he noted.
Other approaches that have been tested in small studies, and which may reduce toxicity and improve the therapeutic index of CD19 CAR T-cell therapy for B-ALL, include split dosing and risk-adapted dosing.
“These approaches do appear to mitigate toxicity, but larger studies are needed to confirm that treatment efficacy is maintained,” Dr. Turtle said.
Toxicity prediction and early intervention to maintain the CAR T-cell dose while avoiding grade 4 or greater toxicities would be helpful and is within reach, he said, noting that the findings by Dr. Hay and his colleagues led to the development of “day-1 cytokine combination algorithms that predict grade 4-5 CRS and could direct preemptive intervention.”
One algorithm based on three cytokines had high sensitivity and specificity, but would require screening of all patients.
Early intervention in patients in whom toxicity is predicted has not been extensively evaluated in clinical studies, he said.
Dr. Hay and his colleagues did, however, develop a “classification tree model of early intervention strategies” using their findings.
A complicating factor in predicting risk and intervening is that each CAR T-cell product is associated with differing levels of toxicity risk. The varying rates of toxicity suggest that promising approaches for addressing CAR T toxicity require validation for each product with respect to cutpoints, efficacy, and maintenance of response, Dr. Turtle said.
“The findings to date are encouraging and show that potentially targetable factors for mitigating the toxicity of CAR T-cell therapy can be identified,” he said. “But clinical studies have yet to convincingly establish the best approach.”
Dr. Turtle has served on advisory boards for Juno/Celgene, Kite/Gilead, Novartis, Precision Biosciences, Eureka Therapeutics, Caribou Biosciences, Nektar Therapeutics, Humanigen, and Aptevo; has intellectual property rights licensed to Juno; has stock options with Precision Biosciences, Eureka Therapeutics, and Caribou Biosciences; and has received research funding from Juno and Nektar Therapeutics.
HOUSTON – Cytokine release syndrome and neurotoxicity frequently occur with CD19-directed chimeric antigen receptor (CAR) T-cell immunotherapies, but targetable factors for mitigating the risk and effects of these complications are emerging, according to Cameron Turtle, MBBS, PhD.
These factors include infused CAR T-cell dose, bone marrow disease burden, immune response, and the lymphodepletion regimen used, Dr. Turtle, of Fred Hutchinson Cancer Research Center, Seattle, said at the Transplantation & Cellular Therapies Meetings. This list is based on an analysis of several studies that included a total of 195 patients with B-cell malignancies who were treated with defined-composition CD19 CAR T cells.
In a 2016 study included in the analysis, for instance, Dr. Turtle and his colleagues found that CD19 CAR T cells administered to adults with B-cell acute lymphoblastic leukemia (B-ALL) after lymphodepletion chemotherapy were “remarkably potent.” Remission was achieved in 27 of 29 patients (J Clin Invest. 2016 Jun 1;126[6]:2123-38).
However, the study also established that high CAR T-cell doses and tumor burden increased the risk of severe cytokine release syndrome (CRS) and neurotoxicity, Dr. Turtle said at the meeting, held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research. At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
“Importantly, we identified serum biomarkers that allow testing of early intervention strategies in the patients who have the highest risk of toxicity,” he said.
Dr. Turtle explained that significantly higher peak interleuken-6 (IL-6) and interferon (IFN)-gamma levels were seen after CAR T-cell infusion in patients with high bone marrow tumor burden and in patients requiring treatment in an intensive care unit (ICU).
ICU care correlated with a higher percentage of bone marrow blasts before lymphodepletion chemotherapy, he added.
Elevations of serum C-reactive protein (CRP) and ferritin also correlated with bone marrow disease burden and with the occurrence of severe CRS requiring ICU care, he said, noting that ferritin and CRP levels declined after tocilizumab or corticosteroid therapy.
In addition, all patients in the study who developed neurotoxicity had evidence of CRS. Peak levels of IL-6, IFN-gamma, ferritin, and CRP were significantly higher in those who developed grade 3 or higher neurotoxicity. Further, serum IL-6 and IFN-gamma concentrations on day 1 after infusion were significantly higher in those who required ICU care and in those who subsequently developed grade 4 neurotoxicity than in patients who developed grade 3 neurotoxicity.
Multivariate analysis indicated that serum IL-6 concentration of more than 30 pg/mL on day 1 and the total number of CD19+ cells in bone marrow before therapy were independent predictors of subsequent development of grade 3 or higher neurotoxicity.
Notably, serum IL-6 of more than 30 pg/mL on day 1 identified all patients in the study who subsequently developed grade 4 or higher neurotoxicity, Dr. Turtle and his colleagues noted.
“The findings suggested that evaluation of serum IL-6 concentration early after CAR T-cell infusion might be useful for identifying patients at high risk of severe neurotoxicity and to evaluate early intervention approaches,” he said.
Neurotoxicity
In a 2017 study from Juliane Gust, MD, PhD, and her colleagues, bone marrow disease burden, lymphodepletion regimen, and CAR T-cell dose were found to be significantly associated with neurotoxicity during multivariate analysis (Cancer Discov. 2017 Dec;7[12]:1404-19).
Patients with severe neurotoxicity in that study demonstrated evidence of endothelial activation, including disseminated intravascular coagulation, capillary leak, and increased blood-brain barrier permeability – with the latter leading to a failure to protect the cerebrospinal fluid from high concentrations of systemic cytokines, including IFN-gamma. These high levels of cytokines may cause vascular pericyte activation and stress, Dr. Turtle explained.
Patients who subsequently developed grade 4 or higher neurotoxicity had higher pretreatment levels of endothelial activation biomarkers.
“Endothelial cells and pericytes contribute to the integrity of the blood-brain barrier; this suggests a potential role for IL-6 and vascular endothelial growth factor from pericytes to augment endothelial permeability,” Dr. Turtle said.
CRS
In another 2017 study, from Kevin A. Hay, MD, and his colleagues, similar factors were found to be associated with CRS (Blood. 2017 Nov 23;130[21]:2295-306).
Multivariable analysis identified high marrow tumor burden, lymphodepletion using cyclophosphamide and fludarabine, higher CAR T-cell dose, thrombocytopenia before lymphodepletion, and manufacturing of CAR T cells without selection of CD8+ central memory T cells as independent predictors of CRS.
Severe CRS was characterized by hemodynamic instability, capillary leak, and consumptive coagulopathy. As in the study by Dr. Gust and her colleagues, biomarkers of endothelial activation, including angiopoietin-2 and von Willebrand factor, were increased during severe CRS and before lymphodepletion in patients who subsequently developed CRS.
Potential modifications
The findings to date suggest that risk stratification, prophylaxis, early intervention and therapeutic intervention are among potential strategies for mitigating the risk of CD19-directed CAR T toxicity, Dr. Turtle said. Steroids, tocilizumab, siltuximab, anakinra, anti-GM-CSF, small molecules, plasma exchange, angiopoietin-1, and hypertransfusion are among candidates under consideration for such interventions, he noted.
Other approaches that have been tested in small studies, and which may reduce toxicity and improve the therapeutic index of CD19 CAR T-cell therapy for B-ALL, include split dosing and risk-adapted dosing.
“These approaches do appear to mitigate toxicity, but larger studies are needed to confirm that treatment efficacy is maintained,” Dr. Turtle said.
Toxicity prediction and early intervention to maintain the CAR T-cell dose while avoiding grade 4 or greater toxicities would be helpful and is within reach, he said, noting that the findings by Dr. Hay and his colleagues led to the development of “day-1 cytokine combination algorithms that predict grade 4-5 CRS and could direct preemptive intervention.”
One algorithm based on three cytokines had high sensitivity and specificity, but would require screening of all patients.
Early intervention in patients in whom toxicity is predicted has not been extensively evaluated in clinical studies, he said.
Dr. Hay and his colleagues did, however, develop a “classification tree model of early intervention strategies” using their findings.
A complicating factor in predicting risk and intervening is that each CAR T-cell product is associated with differing levels of toxicity risk. The varying rates of toxicity suggest that promising approaches for addressing CAR T toxicity require validation for each product with respect to cutpoints, efficacy, and maintenance of response, Dr. Turtle said.
“The findings to date are encouraging and show that potentially targetable factors for mitigating the toxicity of CAR T-cell therapy can be identified,” he said. “But clinical studies have yet to convincingly establish the best approach.”
Dr. Turtle has served on advisory boards for Juno/Celgene, Kite/Gilead, Novartis, Precision Biosciences, Eureka Therapeutics, Caribou Biosciences, Nektar Therapeutics, Humanigen, and Aptevo; has intellectual property rights licensed to Juno; has stock options with Precision Biosciences, Eureka Therapeutics, and Caribou Biosciences; and has received research funding from Juno and Nektar Therapeutics.
REPORTING FROM TCT 2019
Creating CAR T-cell therapies for T-cell malignancies
NEWPORT BEACH, CALIF. – Preclinical research has revealed workarounds that may make chimeric antigen receptor (CAR) T-cell therapy feasible for patients with T-cell malignancies.
Researchers have found that using allogeneic cells for CAR T-cell therapy can eliminate contamination by malignant T cells, and editing those allogeneic T cells to delete the target antigen and the T-cell receptor alpha chain (TRAC) can prevent fratricide and graft-versus-host disease (GVHD).
Additionally, an interleukin-7 molecule called NT-I7 has been shown to enhance CAR T-cell proliferation, differentiation, and tumor killing in a mouse model of a T-cell malignancy.
John F. DiPersio, MD, PhD, of Washington University in St. Louis, described this work in a presentation at the Acute Leukemia Forum of Hemedicus.
Obstacles to development
“The primary obstacle for targeting T-cell malignancies with a T cell is that all of the targets that are on the [malignant] T cells are also expressed on the normal T cells,” Dr. DiPersio said. “So when you put a CAR into a normal T cell, it just kills itself. It’s called fratricide.”
A second issue that has limited development is that the phenotype of the malignant T cell in the blood is similar to a normal T cell, so they can’t be separated, he explained.
“So if you were to do anything to a normal T cell, you would also be doing it, in theory, to the malignant T cell – in theory, making it resistant to therapy,” he said.
A third obstacle, which has been seen in patients with B-cell malignancies as well, is the inability to harvest enough T cells to generate effective CAR T-cell therapy.
And a fourth obstacle is that T cells from patients with malignancies may not function normally because they have been exposed to prior therapies.
Dr. DiPersio and his colleagues believe these obstacles can be overcome by creating CAR T-cell therapies using T cells derived from healthy donors or cord blood, using gene editing to remove the target antigen and TRAC, and using NT-I7 to enhance the efficacy of these universal, “off-the-shelf” CAR T cells.
The researchers have tested these theories, and achieved successes, in preclinical models. The team is now planning a clinical trial in patients at Washington University. Dr. DiPersio and his colleagues also created a company called WUGEN that will develop the universal CAR T-cell therapies if the initial proof-of-principle trial proves successful.
UCART7
One of the universal CAR T-cell therapies Dr. DiPersio and his colleagues have tested is UCART7, which targets CD7. Dr. DiPersio noted that CD7 is expressed on 98% of T-cell acute lymphoblastic leukemias (T-ALLs), 24% of acute myeloid leukemias, natural killer (NK) cells, and T cells.
The researchers created UCART7 by using CRISPR/Cas9 to delete CD7 and TRAC from allogeneic T cells and following this with lentiviral transduction with a third-generation CD7-CAR. The team found a way to delete both TRAC and CD7 in a single day with 95% efficiency, Dr. DiPersio noted.
“Knocking out CD7 doesn’t seem to have any impact on the expansion or trafficking of these T cells in vivo,” Dr. DiPersio said. “So we think that deleting that target in a normal T cell will not affect its overall ability to kill a target when we put a CAR into those T cells.”
In fact, the researchers’ experiments showed that UCART7 can kill T-ALL cells in vitro and target primary T-ALL in vivo without inducing GVHD (Leukemia. 2018 Sep;32[9]:1970-83.)
UCART2 and NT-I7
Dr. DiPersio and his colleagues have also tested UCART2, an allogeneic CAR T-cell therapy in which CD2 and TRAC are deleted. The therapy targets CD2 because this antigen is expressed on T-ALL and other T-cell and NK-cell malignancies. Experiments showed that UCART2 targets T-cell malignancies, including T-ALL and cutaneous T-cell lymphoma, in vitro.
The researchers also tested UCART2 in a mouse model of Sézary syndrome. In these experiments, UCART2 was combined with NT-I7.
NT-I7 enhanced the proliferation, persistence, and tumor killing ability of UCART2. Sézary mice that received UCART2 and NT-I7 had “virtually no tumor burden,” according to researchers, and survived longer than mice treated with UCART2 alone (Blood. 2018;132:340).
Dr. DiPersio noted that there was no cytokine release syndrome because these were immunodeficient mice. However, cytokine release syndrome may be a side effect of NT-I7 in patients as NT-I7 induces rapid expansion of CAR T cells.
Dr. DiPersio reported ownership and investment in WUGEN and Magenta Therapeutics. He also has relationships with Cellworks Group, Tioma Therapeutics, RiverVest Venture Partners, Bioline, Asterias Biotherapeutics, Amphivena Therapeutics, Bluebird Bio, Celgene, Incyte, NeoImuneTech, and MacroGenics.
The Acute Leukemia Forum is organized by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – Preclinical research has revealed workarounds that may make chimeric antigen receptor (CAR) T-cell therapy feasible for patients with T-cell malignancies.
Researchers have found that using allogeneic cells for CAR T-cell therapy can eliminate contamination by malignant T cells, and editing those allogeneic T cells to delete the target antigen and the T-cell receptor alpha chain (TRAC) can prevent fratricide and graft-versus-host disease (GVHD).
Additionally, an interleukin-7 molecule called NT-I7 has been shown to enhance CAR T-cell proliferation, differentiation, and tumor killing in a mouse model of a T-cell malignancy.
John F. DiPersio, MD, PhD, of Washington University in St. Louis, described this work in a presentation at the Acute Leukemia Forum of Hemedicus.
Obstacles to development
“The primary obstacle for targeting T-cell malignancies with a T cell is that all of the targets that are on the [malignant] T cells are also expressed on the normal T cells,” Dr. DiPersio said. “So when you put a CAR into a normal T cell, it just kills itself. It’s called fratricide.”
A second issue that has limited development is that the phenotype of the malignant T cell in the blood is similar to a normal T cell, so they can’t be separated, he explained.
“So if you were to do anything to a normal T cell, you would also be doing it, in theory, to the malignant T cell – in theory, making it resistant to therapy,” he said.
A third obstacle, which has been seen in patients with B-cell malignancies as well, is the inability to harvest enough T cells to generate effective CAR T-cell therapy.
And a fourth obstacle is that T cells from patients with malignancies may not function normally because they have been exposed to prior therapies.
Dr. DiPersio and his colleagues believe these obstacles can be overcome by creating CAR T-cell therapies using T cells derived from healthy donors or cord blood, using gene editing to remove the target antigen and TRAC, and using NT-I7 to enhance the efficacy of these universal, “off-the-shelf” CAR T cells.
The researchers have tested these theories, and achieved successes, in preclinical models. The team is now planning a clinical trial in patients at Washington University. Dr. DiPersio and his colleagues also created a company called WUGEN that will develop the universal CAR T-cell therapies if the initial proof-of-principle trial proves successful.
UCART7
One of the universal CAR T-cell therapies Dr. DiPersio and his colleagues have tested is UCART7, which targets CD7. Dr. DiPersio noted that CD7 is expressed on 98% of T-cell acute lymphoblastic leukemias (T-ALLs), 24% of acute myeloid leukemias, natural killer (NK) cells, and T cells.
The researchers created UCART7 by using CRISPR/Cas9 to delete CD7 and TRAC from allogeneic T cells and following this with lentiviral transduction with a third-generation CD7-CAR. The team found a way to delete both TRAC and CD7 in a single day with 95% efficiency, Dr. DiPersio noted.
“Knocking out CD7 doesn’t seem to have any impact on the expansion or trafficking of these T cells in vivo,” Dr. DiPersio said. “So we think that deleting that target in a normal T cell will not affect its overall ability to kill a target when we put a CAR into those T cells.”
In fact, the researchers’ experiments showed that UCART7 can kill T-ALL cells in vitro and target primary T-ALL in vivo without inducing GVHD (Leukemia. 2018 Sep;32[9]:1970-83.)
UCART2 and NT-I7
Dr. DiPersio and his colleagues have also tested UCART2, an allogeneic CAR T-cell therapy in which CD2 and TRAC are deleted. The therapy targets CD2 because this antigen is expressed on T-ALL and other T-cell and NK-cell malignancies. Experiments showed that UCART2 targets T-cell malignancies, including T-ALL and cutaneous T-cell lymphoma, in vitro.
The researchers also tested UCART2 in a mouse model of Sézary syndrome. In these experiments, UCART2 was combined with NT-I7.
NT-I7 enhanced the proliferation, persistence, and tumor killing ability of UCART2. Sézary mice that received UCART2 and NT-I7 had “virtually no tumor burden,” according to researchers, and survived longer than mice treated with UCART2 alone (Blood. 2018;132:340).
Dr. DiPersio noted that there was no cytokine release syndrome because these were immunodeficient mice. However, cytokine release syndrome may be a side effect of NT-I7 in patients as NT-I7 induces rapid expansion of CAR T cells.
Dr. DiPersio reported ownership and investment in WUGEN and Magenta Therapeutics. He also has relationships with Cellworks Group, Tioma Therapeutics, RiverVest Venture Partners, Bioline, Asterias Biotherapeutics, Amphivena Therapeutics, Bluebird Bio, Celgene, Incyte, NeoImuneTech, and MacroGenics.
The Acute Leukemia Forum is organized by Hemedicus, which is owned by the same company as this news organization.
NEWPORT BEACH, CALIF. – Preclinical research has revealed workarounds that may make chimeric antigen receptor (CAR) T-cell therapy feasible for patients with T-cell malignancies.
Researchers have found that using allogeneic cells for CAR T-cell therapy can eliminate contamination by malignant T cells, and editing those allogeneic T cells to delete the target antigen and the T-cell receptor alpha chain (TRAC) can prevent fratricide and graft-versus-host disease (GVHD).
Additionally, an interleukin-7 molecule called NT-I7 has been shown to enhance CAR T-cell proliferation, differentiation, and tumor killing in a mouse model of a T-cell malignancy.
John F. DiPersio, MD, PhD, of Washington University in St. Louis, described this work in a presentation at the Acute Leukemia Forum of Hemedicus.
Obstacles to development
“The primary obstacle for targeting T-cell malignancies with a T cell is that all of the targets that are on the [malignant] T cells are also expressed on the normal T cells,” Dr. DiPersio said. “So when you put a CAR into a normal T cell, it just kills itself. It’s called fratricide.”
A second issue that has limited development is that the phenotype of the malignant T cell in the blood is similar to a normal T cell, so they can’t be separated, he explained.
“So if you were to do anything to a normal T cell, you would also be doing it, in theory, to the malignant T cell – in theory, making it resistant to therapy,” he said.
A third obstacle, which has been seen in patients with B-cell malignancies as well, is the inability to harvest enough T cells to generate effective CAR T-cell therapy.
And a fourth obstacle is that T cells from patients with malignancies may not function normally because they have been exposed to prior therapies.
Dr. DiPersio and his colleagues believe these obstacles can be overcome by creating CAR T-cell therapies using T cells derived from healthy donors or cord blood, using gene editing to remove the target antigen and TRAC, and using NT-I7 to enhance the efficacy of these universal, “off-the-shelf” CAR T cells.
The researchers have tested these theories, and achieved successes, in preclinical models. The team is now planning a clinical trial in patients at Washington University. Dr. DiPersio and his colleagues also created a company called WUGEN that will develop the universal CAR T-cell therapies if the initial proof-of-principle trial proves successful.
UCART7
One of the universal CAR T-cell therapies Dr. DiPersio and his colleagues have tested is UCART7, which targets CD7. Dr. DiPersio noted that CD7 is expressed on 98% of T-cell acute lymphoblastic leukemias (T-ALLs), 24% of acute myeloid leukemias, natural killer (NK) cells, and T cells.
The researchers created UCART7 by using CRISPR/Cas9 to delete CD7 and TRAC from allogeneic T cells and following this with lentiviral transduction with a third-generation CD7-CAR. The team found a way to delete both TRAC and CD7 in a single day with 95% efficiency, Dr. DiPersio noted.
“Knocking out CD7 doesn’t seem to have any impact on the expansion or trafficking of these T cells in vivo,” Dr. DiPersio said. “So we think that deleting that target in a normal T cell will not affect its overall ability to kill a target when we put a CAR into those T cells.”
In fact, the researchers’ experiments showed that UCART7 can kill T-ALL cells in vitro and target primary T-ALL in vivo without inducing GVHD (Leukemia. 2018 Sep;32[9]:1970-83.)
UCART2 and NT-I7
Dr. DiPersio and his colleagues have also tested UCART2, an allogeneic CAR T-cell therapy in which CD2 and TRAC are deleted. The therapy targets CD2 because this antigen is expressed on T-ALL and other T-cell and NK-cell malignancies. Experiments showed that UCART2 targets T-cell malignancies, including T-ALL and cutaneous T-cell lymphoma, in vitro.
The researchers also tested UCART2 in a mouse model of Sézary syndrome. In these experiments, UCART2 was combined with NT-I7.
NT-I7 enhanced the proliferation, persistence, and tumor killing ability of UCART2. Sézary mice that received UCART2 and NT-I7 had “virtually no tumor burden,” according to researchers, and survived longer than mice treated with UCART2 alone (Blood. 2018;132:340).
Dr. DiPersio noted that there was no cytokine release syndrome because these were immunodeficient mice. However, cytokine release syndrome may be a side effect of NT-I7 in patients as NT-I7 induces rapid expansion of CAR T cells.
Dr. DiPersio reported ownership and investment in WUGEN and Magenta Therapeutics. He also has relationships with Cellworks Group, Tioma Therapeutics, RiverVest Venture Partners, Bioline, Asterias Biotherapeutics, Amphivena Therapeutics, Bluebird Bio, Celgene, Incyte, NeoImuneTech, and MacroGenics.
The Acute Leukemia Forum is organized by Hemedicus, which is owned by the same company as this news organization.
EXPERT ANALYSIS FROM ALF 2019
Whole-genome sequencing demonstrates clinical relevance
GLASGOW – Whole genome sequencing (WGS) appears capable of replacing cytogenetic testing and next generation sequencing (NGS) for the detection of clinically relevant molecular abnormalities in hematological malignancies, according to investigators.
A comparison of WGS with fluorescence in situ hybridization (FISH) showed that WGS caught all the same significant structural variants, plus some abnormalities that FISH had not detected, reported lead author Shirley Henderson, PhD, lead for cancer molecular diagnostics at Genomics England in Oxford.
Although further validation is needed, these findings, reported at the annual meeting of the British Society for Haematology, support an ongoing effort to validate the clinical reliability of WGS, which is currently reserved for research purposes.
“It’s vitally important that the clinical community engage with this and understand both the power and the limitations of this technique and how this work is going to be interpreted for the benefit of patients,” said Adele Fielding, PhD, session chair from University College London’s Cancer Institute.
The investigators compared WGS with FISH for detection of clinically significant structural variants (SVs) and copy number variants (CNVs) in tumor samples from 34 patients with acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
The 252 standard of care FISH tests – conducted at three separate clinical diagnostic centers in the United Kingdom – included 138 SVs and 114 CNVs. WGS relied on a combination of bioinformatics and visual inspection of Circos plots. WGS confirmed all of the SVs detected by FISH with high confidence; WGS detected four additional SVs, also with high confidence, including an ETV6-RUNX1 fusion not detected by FISH because of probe limitations.
Results for CNVs were similar, with WGS detecting 78 out of 85 positive CNVs. Six of the missed positives were associated with low quality samples or low level mutations in the FISH test, suggesting that at least some positives may have been detected with better samples. Only one negative CNV from FISH was missed by WGS.
Overall, WGS had a false positive rate of less than 5% and a positive percentage agreement with FISH that exceeded 90%.
“Further work is required to fully validate all aspects of the WGS analysis pipeline,” Dr. Henderson said. “But these results indicate that WGS has the potential to reliably detect SVs and CNVs in these conditions while offering the advantage of detecting all SVs and CNVs present without the need for additional interrogation of the sample by multiple tests or probes.”
Dr. Henderson noted that there is really no “perfect method” for identifying structural and copy number variants at the present time.
Small variants are relatively easy to detect with techniques such as karyotyping and gene banding, but these tests have shortcomings, namely, that they require live cells and have “fairly high failure rates for various reasons,” Dr. Henderson said.
“FISH is an incredibly useful test and it has higher resolution than gene banding, but the problem with FISH is that you only find what you’re looking at,” Dr. Henderson said. “It’s not genome wide; it’s very targeted.”
Similarly, polymerase chain reaction (PCR), including next generation sequencing (NGS), can detect molecular abnormalities, but only those that are targeted, which may necessitate multiple tests, she said.
“If you start looking for all of the structural variants [with existing techniques], then you’re going to be doing an awful lot of tests,” Dr. Henderson said.
Another potential benefit of WGS is that it is “future resistant,” Dr. Henderson said. “As new biomarkers are discovered, you don’t have to redesign a new targeted test. It will also detect emerging biomarkers, such as mutational signatures and burden.”
The study was sponsored by NHS England. The investigators reported having no conflicts of interest.
SOURCE: Henderson S et al. BSH 2019, Abstract OR-002.
GLASGOW – Whole genome sequencing (WGS) appears capable of replacing cytogenetic testing and next generation sequencing (NGS) for the detection of clinically relevant molecular abnormalities in hematological malignancies, according to investigators.
A comparison of WGS with fluorescence in situ hybridization (FISH) showed that WGS caught all the same significant structural variants, plus some abnormalities that FISH had not detected, reported lead author Shirley Henderson, PhD, lead for cancer molecular diagnostics at Genomics England in Oxford.
Although further validation is needed, these findings, reported at the annual meeting of the British Society for Haematology, support an ongoing effort to validate the clinical reliability of WGS, which is currently reserved for research purposes.
“It’s vitally important that the clinical community engage with this and understand both the power and the limitations of this technique and how this work is going to be interpreted for the benefit of patients,” said Adele Fielding, PhD, session chair from University College London’s Cancer Institute.
The investigators compared WGS with FISH for detection of clinically significant structural variants (SVs) and copy number variants (CNVs) in tumor samples from 34 patients with acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
The 252 standard of care FISH tests – conducted at three separate clinical diagnostic centers in the United Kingdom – included 138 SVs and 114 CNVs. WGS relied on a combination of bioinformatics and visual inspection of Circos plots. WGS confirmed all of the SVs detected by FISH with high confidence; WGS detected four additional SVs, also with high confidence, including an ETV6-RUNX1 fusion not detected by FISH because of probe limitations.
Results for CNVs were similar, with WGS detecting 78 out of 85 positive CNVs. Six of the missed positives were associated with low quality samples or low level mutations in the FISH test, suggesting that at least some positives may have been detected with better samples. Only one negative CNV from FISH was missed by WGS.
Overall, WGS had a false positive rate of less than 5% and a positive percentage agreement with FISH that exceeded 90%.
“Further work is required to fully validate all aspects of the WGS analysis pipeline,” Dr. Henderson said. “But these results indicate that WGS has the potential to reliably detect SVs and CNVs in these conditions while offering the advantage of detecting all SVs and CNVs present without the need for additional interrogation of the sample by multiple tests or probes.”
Dr. Henderson noted that there is really no “perfect method” for identifying structural and copy number variants at the present time.
Small variants are relatively easy to detect with techniques such as karyotyping and gene banding, but these tests have shortcomings, namely, that they require live cells and have “fairly high failure rates for various reasons,” Dr. Henderson said.
“FISH is an incredibly useful test and it has higher resolution than gene banding, but the problem with FISH is that you only find what you’re looking at,” Dr. Henderson said. “It’s not genome wide; it’s very targeted.”
Similarly, polymerase chain reaction (PCR), including next generation sequencing (NGS), can detect molecular abnormalities, but only those that are targeted, which may necessitate multiple tests, she said.
“If you start looking for all of the structural variants [with existing techniques], then you’re going to be doing an awful lot of tests,” Dr. Henderson said.
Another potential benefit of WGS is that it is “future resistant,” Dr. Henderson said. “As new biomarkers are discovered, you don’t have to redesign a new targeted test. It will also detect emerging biomarkers, such as mutational signatures and burden.”
The study was sponsored by NHS England. The investigators reported having no conflicts of interest.
SOURCE: Henderson S et al. BSH 2019, Abstract OR-002.
GLASGOW – Whole genome sequencing (WGS) appears capable of replacing cytogenetic testing and next generation sequencing (NGS) for the detection of clinically relevant molecular abnormalities in hematological malignancies, according to investigators.
A comparison of WGS with fluorescence in situ hybridization (FISH) showed that WGS caught all the same significant structural variants, plus some abnormalities that FISH had not detected, reported lead author Shirley Henderson, PhD, lead for cancer molecular diagnostics at Genomics England in Oxford.
Although further validation is needed, these findings, reported at the annual meeting of the British Society for Haematology, support an ongoing effort to validate the clinical reliability of WGS, which is currently reserved for research purposes.
“It’s vitally important that the clinical community engage with this and understand both the power and the limitations of this technique and how this work is going to be interpreted for the benefit of patients,” said Adele Fielding, PhD, session chair from University College London’s Cancer Institute.
The investigators compared WGS with FISH for detection of clinically significant structural variants (SVs) and copy number variants (CNVs) in tumor samples from 34 patients with acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL).
The 252 standard of care FISH tests – conducted at three separate clinical diagnostic centers in the United Kingdom – included 138 SVs and 114 CNVs. WGS relied on a combination of bioinformatics and visual inspection of Circos plots. WGS confirmed all of the SVs detected by FISH with high confidence; WGS detected four additional SVs, also with high confidence, including an ETV6-RUNX1 fusion not detected by FISH because of probe limitations.
Results for CNVs were similar, with WGS detecting 78 out of 85 positive CNVs. Six of the missed positives were associated with low quality samples or low level mutations in the FISH test, suggesting that at least some positives may have been detected with better samples. Only one negative CNV from FISH was missed by WGS.
Overall, WGS had a false positive rate of less than 5% and a positive percentage agreement with FISH that exceeded 90%.
“Further work is required to fully validate all aspects of the WGS analysis pipeline,” Dr. Henderson said. “But these results indicate that WGS has the potential to reliably detect SVs and CNVs in these conditions while offering the advantage of detecting all SVs and CNVs present without the need for additional interrogation of the sample by multiple tests or probes.”
Dr. Henderson noted that there is really no “perfect method” for identifying structural and copy number variants at the present time.
Small variants are relatively easy to detect with techniques such as karyotyping and gene banding, but these tests have shortcomings, namely, that they require live cells and have “fairly high failure rates for various reasons,” Dr. Henderson said.
“FISH is an incredibly useful test and it has higher resolution than gene banding, but the problem with FISH is that you only find what you’re looking at,” Dr. Henderson said. “It’s not genome wide; it’s very targeted.”
Similarly, polymerase chain reaction (PCR), including next generation sequencing (NGS), can detect molecular abnormalities, but only those that are targeted, which may necessitate multiple tests, she said.
“If you start looking for all of the structural variants [with existing techniques], then you’re going to be doing an awful lot of tests,” Dr. Henderson said.
Another potential benefit of WGS is that it is “future resistant,” Dr. Henderson said. “As new biomarkers are discovered, you don’t have to redesign a new targeted test. It will also detect emerging biomarkers, such as mutational signatures and burden.”
The study was sponsored by NHS England. The investigators reported having no conflicts of interest.
SOURCE: Henderson S et al. BSH 2019, Abstract OR-002.
REPORTING FROM BSH 2019
MRD status at transplant predicts outcomes in ALL patients
HOUSTON – Acute lymphoblastic leukemia patients with measurable residual disease (MRD) negativity prior to hematopoietic cell transplantation achieve better outcomes than do those who are MRD positive, particularly when total body irradiation (TBI)–based conditioning is used, a large retrospective study suggests.
Of 2,780 ALL patients who underwent hematopoietic cell transplantation (HCT) in first or second complete remission (CR), and who were included in the study, 1,816 were MRD negative before transplantation and 964 were MRD positive.
Overall, with follow-up of 40-44 months, MRD positivity was a significant independent predictor of lower overall survival (OS; hazard ratio, 1.19), leukemia-free survival (LFS; HR, 1.26), and higher relapse incidence (RI; 1.51), Arnon Nagler, MD, reported at the Transplantation & Cellular Therapy Meetings.
Conditioning was TBI-based in 76% of the patients; when these patients were compared with those who received chemotherapy-based conditioning, they were found to have better OS, LFS, and RI (HRs, 0.75, 0.70, and 0.60, respectively), said Dr. Nagler, director of both the division of hematology and the bone marrow transplantation and cord blood bank at the Chaim Sheba Medical Center, Tel-Hashomer, and professor of medicine at Tel Aviv University, both in Israel.
“There was no significant interaction between the MRD status and the conditioning,” he said.
On multivariate analysis, MRD positivity was found to be associated with lower OS and LFS (HRs, 1.26 and 1.3), and higher RI (HR, 1.53) in the TBI group, and with higher RI (HR 1.58) in the chemotherapy group, he said. There was no significant association between MRD and other outcomes in this last cohort, he added, noting that TBI-based conditioning was associated with improved OS, LFS, and RI in both MRD-negative and MRD-positive patients.
“MRD is an extremely important prognostic factor for ALL,” he said, noting that its prognostic value in this setting has been established in multiple studies, and that MRD measured at the end of induction is increasingly used to guide further therapy.
However, although MRD detectable immediately before HCT is known to be associated with poor outcomes, it has been unclear if – or to what extent – this differs with different types of conditioning, he added.
“So the aim of this study was to explore if MRD detectable before allogeneic HCT for ALL is associated with different outcomes in adult patients receiving myeloablative conditioning, either TBI or chemotherapy based,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Patients included in the analysis had a median age of 38 years and underwent HCT between 2000 and 2017 using sibling or unrelated 9/10 or 10/10 matched donors. None received blinatumomab or inotuzumab, Dr. Nagler said, adding that more patients are likely to achieve MRD negativity with these agents.
It will be interesting to see if the prognostic value of MRD will remain as strong with the new agents, and if TBI will be “a strong factor in overall survival and disease-free survival” with modern immunotherapy, he concluded.
The study was conducted on behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT).
Dr. Nagler reported having no relevant financial disclosures.
SOURCE: Nagler A et al. TCT 2019, Abstract 7.
HOUSTON – Acute lymphoblastic leukemia patients with measurable residual disease (MRD) negativity prior to hematopoietic cell transplantation achieve better outcomes than do those who are MRD positive, particularly when total body irradiation (TBI)–based conditioning is used, a large retrospective study suggests.
Of 2,780 ALL patients who underwent hematopoietic cell transplantation (HCT) in first or second complete remission (CR), and who were included in the study, 1,816 were MRD negative before transplantation and 964 were MRD positive.
Overall, with follow-up of 40-44 months, MRD positivity was a significant independent predictor of lower overall survival (OS; hazard ratio, 1.19), leukemia-free survival (LFS; HR, 1.26), and higher relapse incidence (RI; 1.51), Arnon Nagler, MD, reported at the Transplantation & Cellular Therapy Meetings.
Conditioning was TBI-based in 76% of the patients; when these patients were compared with those who received chemotherapy-based conditioning, they were found to have better OS, LFS, and RI (HRs, 0.75, 0.70, and 0.60, respectively), said Dr. Nagler, director of both the division of hematology and the bone marrow transplantation and cord blood bank at the Chaim Sheba Medical Center, Tel-Hashomer, and professor of medicine at Tel Aviv University, both in Israel.
“There was no significant interaction between the MRD status and the conditioning,” he said.
On multivariate analysis, MRD positivity was found to be associated with lower OS and LFS (HRs, 1.26 and 1.3), and higher RI (HR, 1.53) in the TBI group, and with higher RI (HR 1.58) in the chemotherapy group, he said. There was no significant association between MRD and other outcomes in this last cohort, he added, noting that TBI-based conditioning was associated with improved OS, LFS, and RI in both MRD-negative and MRD-positive patients.
“MRD is an extremely important prognostic factor for ALL,” he said, noting that its prognostic value in this setting has been established in multiple studies, and that MRD measured at the end of induction is increasingly used to guide further therapy.
However, although MRD detectable immediately before HCT is known to be associated with poor outcomes, it has been unclear if – or to what extent – this differs with different types of conditioning, he added.
“So the aim of this study was to explore if MRD detectable before allogeneic HCT for ALL is associated with different outcomes in adult patients receiving myeloablative conditioning, either TBI or chemotherapy based,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Patients included in the analysis had a median age of 38 years and underwent HCT between 2000 and 2017 using sibling or unrelated 9/10 or 10/10 matched donors. None received blinatumomab or inotuzumab, Dr. Nagler said, adding that more patients are likely to achieve MRD negativity with these agents.
It will be interesting to see if the prognostic value of MRD will remain as strong with the new agents, and if TBI will be “a strong factor in overall survival and disease-free survival” with modern immunotherapy, he concluded.
The study was conducted on behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT).
Dr. Nagler reported having no relevant financial disclosures.
SOURCE: Nagler A et al. TCT 2019, Abstract 7.
HOUSTON – Acute lymphoblastic leukemia patients with measurable residual disease (MRD) negativity prior to hematopoietic cell transplantation achieve better outcomes than do those who are MRD positive, particularly when total body irradiation (TBI)–based conditioning is used, a large retrospective study suggests.
Of 2,780 ALL patients who underwent hematopoietic cell transplantation (HCT) in first or second complete remission (CR), and who were included in the study, 1,816 were MRD negative before transplantation and 964 were MRD positive.
Overall, with follow-up of 40-44 months, MRD positivity was a significant independent predictor of lower overall survival (OS; hazard ratio, 1.19), leukemia-free survival (LFS; HR, 1.26), and higher relapse incidence (RI; 1.51), Arnon Nagler, MD, reported at the Transplantation & Cellular Therapy Meetings.
Conditioning was TBI-based in 76% of the patients; when these patients were compared with those who received chemotherapy-based conditioning, they were found to have better OS, LFS, and RI (HRs, 0.75, 0.70, and 0.60, respectively), said Dr. Nagler, director of both the division of hematology and the bone marrow transplantation and cord blood bank at the Chaim Sheba Medical Center, Tel-Hashomer, and professor of medicine at Tel Aviv University, both in Israel.
“There was no significant interaction between the MRD status and the conditioning,” he said.
On multivariate analysis, MRD positivity was found to be associated with lower OS and LFS (HRs, 1.26 and 1.3), and higher RI (HR, 1.53) in the TBI group, and with higher RI (HR 1.58) in the chemotherapy group, he said. There was no significant association between MRD and other outcomes in this last cohort, he added, noting that TBI-based conditioning was associated with improved OS, LFS, and RI in both MRD-negative and MRD-positive patients.
“MRD is an extremely important prognostic factor for ALL,” he said, noting that its prognostic value in this setting has been established in multiple studies, and that MRD measured at the end of induction is increasingly used to guide further therapy.
However, although MRD detectable immediately before HCT is known to be associated with poor outcomes, it has been unclear if – or to what extent – this differs with different types of conditioning, he added.
“So the aim of this study was to explore if MRD detectable before allogeneic HCT for ALL is associated with different outcomes in adult patients receiving myeloablative conditioning, either TBI or chemotherapy based,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Patients included in the analysis had a median age of 38 years and underwent HCT between 2000 and 2017 using sibling or unrelated 9/10 or 10/10 matched donors. None received blinatumomab or inotuzumab, Dr. Nagler said, adding that more patients are likely to achieve MRD negativity with these agents.
It will be interesting to see if the prognostic value of MRD will remain as strong with the new agents, and if TBI will be “a strong factor in overall survival and disease-free survival” with modern immunotherapy, he concluded.
The study was conducted on behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT).
Dr. Nagler reported having no relevant financial disclosures.
SOURCE: Nagler A et al. TCT 2019, Abstract 7.
REPORTING FROM TCT 2019
Haplo-HSCT bests chemotherapy for MRD-positive adult ALL
HOUSTON – Haploidentical stem cell transplantation (Haplo-HSCT) outperforms chemotherapy for the treatment of adults with acute lymphoblastic leukemia (ALL) in first complete remission, findings from a prospective multicenter trial suggest.
The 2-year leukemia-free survival (LFS) was about 70% in 49 patients in first remission who received haplo-HSCT vs. 40% in 40 patients who received chemotherapy, and 2-year overall survival (OS) was about 80% vs. 50% in the groups, respectively, Meng Lv, MD, PhD, of Peking University People’s Hospital in Beijing reported at the Transplantation & Cellular Therapy Meetings.
“This result is comparable to results of our previous reports,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
He noted that the findings also support those from other institutions.
Study subjects initially included 112 newly diagnosed standard-risk ALL patients aged 18-39 years without high-risk features who achieved complete remission (CR) after one or two cycles of induction. They were consecutively enrolled at five centers in China, including high-volume centers, between July 2014 and June 2017 and were followed for a median of 24.6 months.
Subjects without a suitable HLA-matched sibling donor (MSD) or HLA-matched unrelated donor after two cycles of consolidation with hyper-CVAD chemotherapy were eligible for haplo-HSCT or further hyper-CVAD chemotherapy.
The final analysis included 89 patients after 23 were excluded because of early relapse (6 patients) or a decision to undergo MSD HSCT (16 patients), or unrelated donor-HSCT (1 patient), Dr. Lv said, noting that landmark analysis was used when comparing the outcomes of patients receiving haplo-HSCT with those receiving chemotherapy.
Multivariate analysis with adjustment for a propensity score calculated for each patient showed that treatment (haplo-HSCT vs. chemotherapy) independently predicted LFS (hazard ratio, 0.388), OS (HR, 0.346), and cumulative incidence of relapse (CIR; HR, 0.247). Minimal residual disease (MRD) positivity after the first consolidation was an independent risk factor for LFS (HR, 2.162) and CIR (HR, 3.667). Additionally, diagnosis (T- vs. B-cell) was an independent risk factor for OS (HR, 2.267), Dr. Lv said, adding that nonrelapse mortality was similar in the groups in the propensity score–adjusted analysis.
The findings overall show that haplo-HSCT has variable impact on survival in standard-risk ALL, when compared with traditional chemotherapy, with subgroup analyses showing MRD-positive patients deriving the greatest benefit, he said. Future studies are planned to look more closely at MRD-positive disease and the possible benefits of postponing transplant until the second CR.
At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Lv reported having no financial disclosures.
SOURCE: Lv M et al. TCT 2019, Abstract 8.
HOUSTON – Haploidentical stem cell transplantation (Haplo-HSCT) outperforms chemotherapy for the treatment of adults with acute lymphoblastic leukemia (ALL) in first complete remission, findings from a prospective multicenter trial suggest.
The 2-year leukemia-free survival (LFS) was about 70% in 49 patients in first remission who received haplo-HSCT vs. 40% in 40 patients who received chemotherapy, and 2-year overall survival (OS) was about 80% vs. 50% in the groups, respectively, Meng Lv, MD, PhD, of Peking University People’s Hospital in Beijing reported at the Transplantation & Cellular Therapy Meetings.
“This result is comparable to results of our previous reports,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
He noted that the findings also support those from other institutions.
Study subjects initially included 112 newly diagnosed standard-risk ALL patients aged 18-39 years without high-risk features who achieved complete remission (CR) after one or two cycles of induction. They were consecutively enrolled at five centers in China, including high-volume centers, between July 2014 and June 2017 and were followed for a median of 24.6 months.
Subjects without a suitable HLA-matched sibling donor (MSD) or HLA-matched unrelated donor after two cycles of consolidation with hyper-CVAD chemotherapy were eligible for haplo-HSCT or further hyper-CVAD chemotherapy.
The final analysis included 89 patients after 23 were excluded because of early relapse (6 patients) or a decision to undergo MSD HSCT (16 patients), or unrelated donor-HSCT (1 patient), Dr. Lv said, noting that landmark analysis was used when comparing the outcomes of patients receiving haplo-HSCT with those receiving chemotherapy.
Multivariate analysis with adjustment for a propensity score calculated for each patient showed that treatment (haplo-HSCT vs. chemotherapy) independently predicted LFS (hazard ratio, 0.388), OS (HR, 0.346), and cumulative incidence of relapse (CIR; HR, 0.247). Minimal residual disease (MRD) positivity after the first consolidation was an independent risk factor for LFS (HR, 2.162) and CIR (HR, 3.667). Additionally, diagnosis (T- vs. B-cell) was an independent risk factor for OS (HR, 2.267), Dr. Lv said, adding that nonrelapse mortality was similar in the groups in the propensity score–adjusted analysis.
The findings overall show that haplo-HSCT has variable impact on survival in standard-risk ALL, when compared with traditional chemotherapy, with subgroup analyses showing MRD-positive patients deriving the greatest benefit, he said. Future studies are planned to look more closely at MRD-positive disease and the possible benefits of postponing transplant until the second CR.
At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Lv reported having no financial disclosures.
SOURCE: Lv M et al. TCT 2019, Abstract 8.
HOUSTON – Haploidentical stem cell transplantation (Haplo-HSCT) outperforms chemotherapy for the treatment of adults with acute lymphoblastic leukemia (ALL) in first complete remission, findings from a prospective multicenter trial suggest.
The 2-year leukemia-free survival (LFS) was about 70% in 49 patients in first remission who received haplo-HSCT vs. 40% in 40 patients who received chemotherapy, and 2-year overall survival (OS) was about 80% vs. 50% in the groups, respectively, Meng Lv, MD, PhD, of Peking University People’s Hospital in Beijing reported at the Transplantation & Cellular Therapy Meetings.
“This result is comparable to results of our previous reports,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
He noted that the findings also support those from other institutions.
Study subjects initially included 112 newly diagnosed standard-risk ALL patients aged 18-39 years without high-risk features who achieved complete remission (CR) after one or two cycles of induction. They were consecutively enrolled at five centers in China, including high-volume centers, between July 2014 and June 2017 and were followed for a median of 24.6 months.
Subjects without a suitable HLA-matched sibling donor (MSD) or HLA-matched unrelated donor after two cycles of consolidation with hyper-CVAD chemotherapy were eligible for haplo-HSCT or further hyper-CVAD chemotherapy.
The final analysis included 89 patients after 23 were excluded because of early relapse (6 patients) or a decision to undergo MSD HSCT (16 patients), or unrelated donor-HSCT (1 patient), Dr. Lv said, noting that landmark analysis was used when comparing the outcomes of patients receiving haplo-HSCT with those receiving chemotherapy.
Multivariate analysis with adjustment for a propensity score calculated for each patient showed that treatment (haplo-HSCT vs. chemotherapy) independently predicted LFS (hazard ratio, 0.388), OS (HR, 0.346), and cumulative incidence of relapse (CIR; HR, 0.247). Minimal residual disease (MRD) positivity after the first consolidation was an independent risk factor for LFS (HR, 2.162) and CIR (HR, 3.667). Additionally, diagnosis (T- vs. B-cell) was an independent risk factor for OS (HR, 2.267), Dr. Lv said, adding that nonrelapse mortality was similar in the groups in the propensity score–adjusted analysis.
The findings overall show that haplo-HSCT has variable impact on survival in standard-risk ALL, when compared with traditional chemotherapy, with subgroup analyses showing MRD-positive patients deriving the greatest benefit, he said. Future studies are planned to look more closely at MRD-positive disease and the possible benefits of postponing transplant until the second CR.
At its meeting, the American Society for Blood and Marrow Transplantation announced a new name for the society: American Society for Transplantation and Cellular Therapy (ASTCT).
Dr. Lv reported having no financial disclosures.
SOURCE: Lv M et al. TCT 2019, Abstract 8.
REPORTING FROM TCT 2019
EC approves dasatinib plus chemo for kids with Ph+ ALL
The European Commission has approved dasatinib (Sprycel) for use in combination with chemotherapy for the treatment of pediatric patients with newly diagnosed, Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL).
Dasatinib will be available in tablet form and as a powder for oral suspension, Bristol-Myers Squib said in a press release.
The approval was based on an event-free survival rate of 65.5% (95% confidence interval, 57.7-73.7) and an overall survival rate of 91.5% (95% CI, 84.2-95.5) in a phase 2 trial that evaluated the addition of dasatinib to a chemotherapy regimen modeled on a Berlin-Frankfurt-Münster high-risk backbone in pediatric patients with newly diagnosed Ph+ ALL.
Patients treated in the study (n = 106) were all aged younger than 18 years and received dasatinib at a daily dose of 60 mg/m2 on a continuous dosing regimen for up to 24 months, in combination with chemotherapy. About 77 % of patients (n = 82) received tablets exclusively; 23% of patients (n = 24) received the powder for oral suspension at least once.
Hematologic adverse events included grade 3 or 4 febrile neutropenia (75.5%), sepsis (23.6%), and bacteremia (24.5%). Nonhematologic, noninfectious grade 3 or 4 adverse events attributed to dasatinib and reported in more than 10% of patients included elevated ALT (21.7%) and AST (10.4%). Additional grade 3 or 4 adverse events attributed to dasatinib were pleural effusion (3.8%), edema (2.8%), hemorrhage (5.7%), and cardiac failure (0.8%). No events of pulmonary hypertension or pulmonary arterial hypertension were reported, the company said in the press release.
Dasatinib is already approved by the European Commission to treat children with Ph+ chronic myeloid leukemia in the chronic phase, which includes newly diagnosed patients and those with resistance or intolerance to imatinib.
The European Commission has approved dasatinib (Sprycel) for use in combination with chemotherapy for the treatment of pediatric patients with newly diagnosed, Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL).
Dasatinib will be available in tablet form and as a powder for oral suspension, Bristol-Myers Squib said in a press release.
The approval was based on an event-free survival rate of 65.5% (95% confidence interval, 57.7-73.7) and an overall survival rate of 91.5% (95% CI, 84.2-95.5) in a phase 2 trial that evaluated the addition of dasatinib to a chemotherapy regimen modeled on a Berlin-Frankfurt-Münster high-risk backbone in pediatric patients with newly diagnosed Ph+ ALL.
Patients treated in the study (n = 106) were all aged younger than 18 years and received dasatinib at a daily dose of 60 mg/m2 on a continuous dosing regimen for up to 24 months, in combination with chemotherapy. About 77 % of patients (n = 82) received tablets exclusively; 23% of patients (n = 24) received the powder for oral suspension at least once.
Hematologic adverse events included grade 3 or 4 febrile neutropenia (75.5%), sepsis (23.6%), and bacteremia (24.5%). Nonhematologic, noninfectious grade 3 or 4 adverse events attributed to dasatinib and reported in more than 10% of patients included elevated ALT (21.7%) and AST (10.4%). Additional grade 3 or 4 adverse events attributed to dasatinib were pleural effusion (3.8%), edema (2.8%), hemorrhage (5.7%), and cardiac failure (0.8%). No events of pulmonary hypertension or pulmonary arterial hypertension were reported, the company said in the press release.
Dasatinib is already approved by the European Commission to treat children with Ph+ chronic myeloid leukemia in the chronic phase, which includes newly diagnosed patients and those with resistance or intolerance to imatinib.
The European Commission has approved dasatinib (Sprycel) for use in combination with chemotherapy for the treatment of pediatric patients with newly diagnosed, Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL).
Dasatinib will be available in tablet form and as a powder for oral suspension, Bristol-Myers Squib said in a press release.
The approval was based on an event-free survival rate of 65.5% (95% confidence interval, 57.7-73.7) and an overall survival rate of 91.5% (95% CI, 84.2-95.5) in a phase 2 trial that evaluated the addition of dasatinib to a chemotherapy regimen modeled on a Berlin-Frankfurt-Münster high-risk backbone in pediatric patients with newly diagnosed Ph+ ALL.
Patients treated in the study (n = 106) were all aged younger than 18 years and received dasatinib at a daily dose of 60 mg/m2 on a continuous dosing regimen for up to 24 months, in combination with chemotherapy. About 77 % of patients (n = 82) received tablets exclusively; 23% of patients (n = 24) received the powder for oral suspension at least once.
Hematologic adverse events included grade 3 or 4 febrile neutropenia (75.5%), sepsis (23.6%), and bacteremia (24.5%). Nonhematologic, noninfectious grade 3 or 4 adverse events attributed to dasatinib and reported in more than 10% of patients included elevated ALT (21.7%) and AST (10.4%). Additional grade 3 or 4 adverse events attributed to dasatinib were pleural effusion (3.8%), edema (2.8%), hemorrhage (5.7%), and cardiac failure (0.8%). No events of pulmonary hypertension or pulmonary arterial hypertension were reported, the company said in the press release.
Dasatinib is already approved by the European Commission to treat children with Ph+ chronic myeloid leukemia in the chronic phase, which includes newly diagnosed patients and those with resistance or intolerance to imatinib.
PD-1 blockade plus CD19 CAR T boosts CAR T-cell persistence
SAN DIEGO – Checkpoint inhibition can be used safely and effectively with CD19-directed chimeric antigen receptor (CAR) T-cell therapy in children with relapsed B-cell acute lymphoblastic leukemia (ALL), and it may bolster CAR T-cell effects and persistence, suggest the findings in a series of 14 patients at the Children’s Hospital of Philadelphia.
Combined programmed death-1 (PD-1) blockade and CAR T-cell therapy appeared to have particular benefit in patients with early B-cell recovery and in those with bulky extramedullary disease, Shannon Maude, MD, PhD, reported during a press conference at the annual meeting of the American Society of Hematology.
The patients, aged 4-17 years with heavily pretreated relapsed B-ALL (13 patients) or B lymphoblastic lymphoma (1 patient), were treated with CD19-directed CAR T-cell therapy, including CTL019 in 4 patients and CTL119 in 10 patients, followed by pembrolizumab (in 13 patients) or nivolumab (in 1 patient).
Six patients received the combination therapy because of early B-cell recovery after initial CAR T-cell infusion, four patients had relapsed or refractory (R/R) bulky extramedullary disease, and four patients had failed to respond or relapsed after initial CAR T-cell therapy.
Three of the six with poor persistence of response reestablished B-cell aplasia (a reflection of CAR T-cell function) after reinfusion of the CAR T-cell product followed by infusion with PD-1 blockade, and they have “sustained CR [complete response] with B-cell aplasia, showing continued persistence of their CAR T cells,” said Dr. Maude, an attending physician in the Cancer Center at Children’s Hospital of Philadelphia.
Of the four patients with R/R bulky extramedullary disease, two patients had a partial response and two patients had CR, she said, explaining that it was hypothesized that the “PD-1 checkpoint pathway may be activated through the microenvironment in that extramedullary situation.”
However, all four patients who had partial or no response to initial CAR T-cell therapy progressed after PD-1 administration, she said, noting that “in one patient, this progression was marked by reduced CD19 expression, which was probably the mode of escape from CD19 CAR T cells.”
Prior studies have shown that patients who respond to CAR T-cell therapy have persistence of CD19 CAR T cells, whereas those with loss of CD19 CAR T cells within 6 months of infusion have a higher rate of relapse, Dr. Maude explained.
“Our hypothesis was that T cells, upon activation, may become exhausted through activation of immune checkpoint pathways, that one such pathway – PD-1 – may be involved in early loss of CD19 CAR T cells and therefore that the combination [of CD19 CAR T-cell therapy] with PD-1 checkpoint blockade may improve the function of the CAR T cells and their persistence,” she said.
The combined approach was well tolerated in this study, she said, noting that mild cytokine release syndrome symptoms and fever typical of CAR T-cell proliferative responses were observed in three patients within 2 days of starting pembrolizumab.
Other adverse effects associated with PD-1 inhibition, including acute pancreatitis, hypothyroidism, arthralgias, and urticaria, occurred in one patient each. There were four cases of grade 3-4 cytopenias that were deemed tolerable or reversible upon discontinuation.
“We show that PD-1 checkpoint inhibitors can be safely combined with CD19 CAR T-cell therapy and that this mechanism may be useful to improve CAR T-cell persistence,” Dr. Maude said.
These findings, which showed particular benefit in patients with poor persistence marked by early B-cell recovery and in those with R/R bulky extramedullary disease, should help inform future use of checkpoint inhibitors after CAR T-cell therapy, she added.
Dr. Maude reported financial ties to Novartis.
SOURCE: Li AM et al. ASH 2018, Abstract 556.
SAN DIEGO – Checkpoint inhibition can be used safely and effectively with CD19-directed chimeric antigen receptor (CAR) T-cell therapy in children with relapsed B-cell acute lymphoblastic leukemia (ALL), and it may bolster CAR T-cell effects and persistence, suggest the findings in a series of 14 patients at the Children’s Hospital of Philadelphia.
Combined programmed death-1 (PD-1) blockade and CAR T-cell therapy appeared to have particular benefit in patients with early B-cell recovery and in those with bulky extramedullary disease, Shannon Maude, MD, PhD, reported during a press conference at the annual meeting of the American Society of Hematology.
The patients, aged 4-17 years with heavily pretreated relapsed B-ALL (13 patients) or B lymphoblastic lymphoma (1 patient), were treated with CD19-directed CAR T-cell therapy, including CTL019 in 4 patients and CTL119 in 10 patients, followed by pembrolizumab (in 13 patients) or nivolumab (in 1 patient).
Six patients received the combination therapy because of early B-cell recovery after initial CAR T-cell infusion, four patients had relapsed or refractory (R/R) bulky extramedullary disease, and four patients had failed to respond or relapsed after initial CAR T-cell therapy.
Three of the six with poor persistence of response reestablished B-cell aplasia (a reflection of CAR T-cell function) after reinfusion of the CAR T-cell product followed by infusion with PD-1 blockade, and they have “sustained CR [complete response] with B-cell aplasia, showing continued persistence of their CAR T cells,” said Dr. Maude, an attending physician in the Cancer Center at Children’s Hospital of Philadelphia.
Of the four patients with R/R bulky extramedullary disease, two patients had a partial response and two patients had CR, she said, explaining that it was hypothesized that the “PD-1 checkpoint pathway may be activated through the microenvironment in that extramedullary situation.”
However, all four patients who had partial or no response to initial CAR T-cell therapy progressed after PD-1 administration, she said, noting that “in one patient, this progression was marked by reduced CD19 expression, which was probably the mode of escape from CD19 CAR T cells.”
Prior studies have shown that patients who respond to CAR T-cell therapy have persistence of CD19 CAR T cells, whereas those with loss of CD19 CAR T cells within 6 months of infusion have a higher rate of relapse, Dr. Maude explained.
“Our hypothesis was that T cells, upon activation, may become exhausted through activation of immune checkpoint pathways, that one such pathway – PD-1 – may be involved in early loss of CD19 CAR T cells and therefore that the combination [of CD19 CAR T-cell therapy] with PD-1 checkpoint blockade may improve the function of the CAR T cells and their persistence,” she said.
The combined approach was well tolerated in this study, she said, noting that mild cytokine release syndrome symptoms and fever typical of CAR T-cell proliferative responses were observed in three patients within 2 days of starting pembrolizumab.
Other adverse effects associated with PD-1 inhibition, including acute pancreatitis, hypothyroidism, arthralgias, and urticaria, occurred in one patient each. There were four cases of grade 3-4 cytopenias that were deemed tolerable or reversible upon discontinuation.
“We show that PD-1 checkpoint inhibitors can be safely combined with CD19 CAR T-cell therapy and that this mechanism may be useful to improve CAR T-cell persistence,” Dr. Maude said.
These findings, which showed particular benefit in patients with poor persistence marked by early B-cell recovery and in those with R/R bulky extramedullary disease, should help inform future use of checkpoint inhibitors after CAR T-cell therapy, she added.
Dr. Maude reported financial ties to Novartis.
SOURCE: Li AM et al. ASH 2018, Abstract 556.
SAN DIEGO – Checkpoint inhibition can be used safely and effectively with CD19-directed chimeric antigen receptor (CAR) T-cell therapy in children with relapsed B-cell acute lymphoblastic leukemia (ALL), and it may bolster CAR T-cell effects and persistence, suggest the findings in a series of 14 patients at the Children’s Hospital of Philadelphia.
Combined programmed death-1 (PD-1) blockade and CAR T-cell therapy appeared to have particular benefit in patients with early B-cell recovery and in those with bulky extramedullary disease, Shannon Maude, MD, PhD, reported during a press conference at the annual meeting of the American Society of Hematology.
The patients, aged 4-17 years with heavily pretreated relapsed B-ALL (13 patients) or B lymphoblastic lymphoma (1 patient), were treated with CD19-directed CAR T-cell therapy, including CTL019 in 4 patients and CTL119 in 10 patients, followed by pembrolizumab (in 13 patients) or nivolumab (in 1 patient).
Six patients received the combination therapy because of early B-cell recovery after initial CAR T-cell infusion, four patients had relapsed or refractory (R/R) bulky extramedullary disease, and four patients had failed to respond or relapsed after initial CAR T-cell therapy.
Three of the six with poor persistence of response reestablished B-cell aplasia (a reflection of CAR T-cell function) after reinfusion of the CAR T-cell product followed by infusion with PD-1 blockade, and they have “sustained CR [complete response] with B-cell aplasia, showing continued persistence of their CAR T cells,” said Dr. Maude, an attending physician in the Cancer Center at Children’s Hospital of Philadelphia.
Of the four patients with R/R bulky extramedullary disease, two patients had a partial response and two patients had CR, she said, explaining that it was hypothesized that the “PD-1 checkpoint pathway may be activated through the microenvironment in that extramedullary situation.”
However, all four patients who had partial or no response to initial CAR T-cell therapy progressed after PD-1 administration, she said, noting that “in one patient, this progression was marked by reduced CD19 expression, which was probably the mode of escape from CD19 CAR T cells.”
Prior studies have shown that patients who respond to CAR T-cell therapy have persistence of CD19 CAR T cells, whereas those with loss of CD19 CAR T cells within 6 months of infusion have a higher rate of relapse, Dr. Maude explained.
“Our hypothesis was that T cells, upon activation, may become exhausted through activation of immune checkpoint pathways, that one such pathway – PD-1 – may be involved in early loss of CD19 CAR T cells and therefore that the combination [of CD19 CAR T-cell therapy] with PD-1 checkpoint blockade may improve the function of the CAR T cells and their persistence,” she said.
The combined approach was well tolerated in this study, she said, noting that mild cytokine release syndrome symptoms and fever typical of CAR T-cell proliferative responses were observed in three patients within 2 days of starting pembrolizumab.
Other adverse effects associated with PD-1 inhibition, including acute pancreatitis, hypothyroidism, arthralgias, and urticaria, occurred in one patient each. There were four cases of grade 3-4 cytopenias that were deemed tolerable or reversible upon discontinuation.
“We show that PD-1 checkpoint inhibitors can be safely combined with CD19 CAR T-cell therapy and that this mechanism may be useful to improve CAR T-cell persistence,” Dr. Maude said.
These findings, which showed particular benefit in patients with poor persistence marked by early B-cell recovery and in those with R/R bulky extramedullary disease, should help inform future use of checkpoint inhibitors after CAR T-cell therapy, she added.
Dr. Maude reported financial ties to Novartis.
SOURCE: Li AM et al. ASH 2018, Abstract 556.
REPORTING FROM ASH 2018
Key clinical point: Major finding: Three of six patients with poor CAR T-cell persistence had a complete response after treatment with the combination therapy. Study details: A clinical study of 14 patients with relapsed B-ALL.
Disclosures: Dr. Maude reported financial relationships with Novartis.
Source: Li AM et al. ASH 2018, Abstract 556.