User login
Can Vitamin D Supplements Help With Hypertension?
Q) One of my patients came in and said he had read that vitamin D supplementation will help with hypertension. Now he wants to quit his blood pressure meds and use vitamin D instead. Do you have any background on this?
Vitamin D is critical for utilization of calcium, a vital nutrient for multiple metabolic and cellular processes; deficiency is associated with worsening of autoimmune disorders, osteoporosis, and certain cardiovascular conditions, among others.7 An association between vitamin D level and blood pressure has been recognized for some time, but the pathophysiology is not well understood.
A literature review of studies from 1988 to 2013 found contradictory results regarding vitamin D deficiency and concurrent elevated blood pressure (systolic and/or diastolic), as well as the impact on blood pressure with restoration of vitamin D levels. The findings were limited by several factors, including differences in study design, variables evaluated, and type of vitamin D compound used. The results suggested a link between the renin-angiotensin-aldosterone system, fibroblast growth factor 23/klotho axis, and vitamin D level.8
A study of 158 subjects (98 with newly diagnosed essential hypertension, 60 with normal blood pressure) found significantly lower 25(OH)D3 serum levels in hypertensive patients. Furthermore, the 25(OH)D3 level was significantly correlated with both systolic (r = –0.33) and diastolic blood pressure (r = –0.26). Using multiple regression analysis, after adjustment for age, smoking status, and BMI, the impact of 25(OH)D3 level accounted for 10% of the variation in systolic blood pressure.9
In a mendelian randomization study of 108,173 subjects from 35 studies, an inverse association between vitamin D level and systolic blood pressure (P = .0003) was found. A reduced risk for essential hypertension with increased vitamin D level (P = .0003) was also noted. However, no association was found between increasing vitamin D level and a reduction in diastolic blood pressure
(P = .37).10
With the ever-increasing access to health information from sources such as “Doctor Google,” it can be difficult for a non–health care professional to separate hype from evidence-based recommendations. While current evidence suggests optimal vitamin D levels may be beneficial for improving blood pressure control and may be a useful adjunctive therapy, there is no evidence to support discontinuing antihypertensive therapy and replacing it with vitamin D therapy.
Cynthia A. Smith, DNP, APRN, FNP-BC
Renal Consultants, South Charleston, West Virginia
REFERENCES
1. Monfared A, Heidarzadeh A, Ghaffari M, Akbarpour M. Effect of megestrol acetate on serum albumin level in malnourished dialysis patients. J Renal Nutr. 2009;19(2):167-171.
2. Byham-Gray L, Stover J, Wiesen K. A clinical guide to nutrition care in kidney disease. Acad Nutr Diet. 2013.
3. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; ASPEN Malnutrition Task Force; ASPEN Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) [erratum appears in J Acad Nutr Diet. 2012 Nov;112(11):1899].
J Acad Nutr Diet. 2012;112(5):730-738.
4. Rammohan M, Kalantar-Zedeh K, Liang A, Ghossein C. Megestrol acetate in a moderate dose for the treatment of malnutrition-inflammation complex in maintenance dialysis patients. J Ren Nutr. 2005;15(3):345-355.
5. Yeh S, Marandi M, Thode H Jr, et al. Report of a pilot, double blind, placebo-controlled study of megestrol acetate in elderly dialysis patients with cachexia. J Ren Nutr. 2010; 20(1):52-62.
6. Golebiewska JE, Lichodziejewska-Niemierko M, Aleksandrowicz-Wrona E, et al. Megestrol acetate use in hypoalbuminemic dialysis patients [comment]. J Ren Nutr. 2011;21(2): 200-202.
7. Bendik I, Friedel A, Roos FF, et al. Vitamin D: a critical and necessary micronutrient for human health. Front Physiol. 2014;5:248.
8. Cabone F, Mach F, Vuilleumier N, Montecucco F. Potential pathophysiological role for the vitamin D deficiency in essential hypertension. World J Cardiol. 2014;6(5):260-276.
9. Sypniewska G, Pollak J, Strozecki P, et al. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am J Hypertens. 2014;27(1):114-121.
10. Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719-729.
Q) One of my patients came in and said he had read that vitamin D supplementation will help with hypertension. Now he wants to quit his blood pressure meds and use vitamin D instead. Do you have any background on this?
Vitamin D is critical for utilization of calcium, a vital nutrient for multiple metabolic and cellular processes; deficiency is associated with worsening of autoimmune disorders, osteoporosis, and certain cardiovascular conditions, among others.7 An association between vitamin D level and blood pressure has been recognized for some time, but the pathophysiology is not well understood.
A literature review of studies from 1988 to 2013 found contradictory results regarding vitamin D deficiency and concurrent elevated blood pressure (systolic and/or diastolic), as well as the impact on blood pressure with restoration of vitamin D levels. The findings were limited by several factors, including differences in study design, variables evaluated, and type of vitamin D compound used. The results suggested a link between the renin-angiotensin-aldosterone system, fibroblast growth factor 23/klotho axis, and vitamin D level.8
A study of 158 subjects (98 with newly diagnosed essential hypertension, 60 with normal blood pressure) found significantly lower 25(OH)D3 serum levels in hypertensive patients. Furthermore, the 25(OH)D3 level was significantly correlated with both systolic (r = –0.33) and diastolic blood pressure (r = –0.26). Using multiple regression analysis, after adjustment for age, smoking status, and BMI, the impact of 25(OH)D3 level accounted for 10% of the variation in systolic blood pressure.9
In a mendelian randomization study of 108,173 subjects from 35 studies, an inverse association between vitamin D level and systolic blood pressure (P = .0003) was found. A reduced risk for essential hypertension with increased vitamin D level (P = .0003) was also noted. However, no association was found between increasing vitamin D level and a reduction in diastolic blood pressure
(P = .37).10
With the ever-increasing access to health information from sources such as “Doctor Google,” it can be difficult for a non–health care professional to separate hype from evidence-based recommendations. While current evidence suggests optimal vitamin D levels may be beneficial for improving blood pressure control and may be a useful adjunctive therapy, there is no evidence to support discontinuing antihypertensive therapy and replacing it with vitamin D therapy.
Cynthia A. Smith, DNP, APRN, FNP-BC
Renal Consultants, South Charleston, West Virginia
REFERENCES
1. Monfared A, Heidarzadeh A, Ghaffari M, Akbarpour M. Effect of megestrol acetate on serum albumin level in malnourished dialysis patients. J Renal Nutr. 2009;19(2):167-171.
2. Byham-Gray L, Stover J, Wiesen K. A clinical guide to nutrition care in kidney disease. Acad Nutr Diet. 2013.
3. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; ASPEN Malnutrition Task Force; ASPEN Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) [erratum appears in J Acad Nutr Diet. 2012 Nov;112(11):1899].
J Acad Nutr Diet. 2012;112(5):730-738.
4. Rammohan M, Kalantar-Zedeh K, Liang A, Ghossein C. Megestrol acetate in a moderate dose for the treatment of malnutrition-inflammation complex in maintenance dialysis patients. J Ren Nutr. 2005;15(3):345-355.
5. Yeh S, Marandi M, Thode H Jr, et al. Report of a pilot, double blind, placebo-controlled study of megestrol acetate in elderly dialysis patients with cachexia. J Ren Nutr. 2010; 20(1):52-62.
6. Golebiewska JE, Lichodziejewska-Niemierko M, Aleksandrowicz-Wrona E, et al. Megestrol acetate use in hypoalbuminemic dialysis patients [comment]. J Ren Nutr. 2011;21(2): 200-202.
7. Bendik I, Friedel A, Roos FF, et al. Vitamin D: a critical and necessary micronutrient for human health. Front Physiol. 2014;5:248.
8. Cabone F, Mach F, Vuilleumier N, Montecucco F. Potential pathophysiological role for the vitamin D deficiency in essential hypertension. World J Cardiol. 2014;6(5):260-276.
9. Sypniewska G, Pollak J, Strozecki P, et al. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am J Hypertens. 2014;27(1):114-121.
10. Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719-729.
Q) One of my patients came in and said he had read that vitamin D supplementation will help with hypertension. Now he wants to quit his blood pressure meds and use vitamin D instead. Do you have any background on this?
Vitamin D is critical for utilization of calcium, a vital nutrient for multiple metabolic and cellular processes; deficiency is associated with worsening of autoimmune disorders, osteoporosis, and certain cardiovascular conditions, among others.7 An association between vitamin D level and blood pressure has been recognized for some time, but the pathophysiology is not well understood.
A literature review of studies from 1988 to 2013 found contradictory results regarding vitamin D deficiency and concurrent elevated blood pressure (systolic and/or diastolic), as well as the impact on blood pressure with restoration of vitamin D levels. The findings were limited by several factors, including differences in study design, variables evaluated, and type of vitamin D compound used. The results suggested a link between the renin-angiotensin-aldosterone system, fibroblast growth factor 23/klotho axis, and vitamin D level.8
A study of 158 subjects (98 with newly diagnosed essential hypertension, 60 with normal blood pressure) found significantly lower 25(OH)D3 serum levels in hypertensive patients. Furthermore, the 25(OH)D3 level was significantly correlated with both systolic (r = –0.33) and diastolic blood pressure (r = –0.26). Using multiple regression analysis, after adjustment for age, smoking status, and BMI, the impact of 25(OH)D3 level accounted for 10% of the variation in systolic blood pressure.9
In a mendelian randomization study of 108,173 subjects from 35 studies, an inverse association between vitamin D level and systolic blood pressure (P = .0003) was found. A reduced risk for essential hypertension with increased vitamin D level (P = .0003) was also noted. However, no association was found between increasing vitamin D level and a reduction in diastolic blood pressure
(P = .37).10
With the ever-increasing access to health information from sources such as “Doctor Google,” it can be difficult for a non–health care professional to separate hype from evidence-based recommendations. While current evidence suggests optimal vitamin D levels may be beneficial for improving blood pressure control and may be a useful adjunctive therapy, there is no evidence to support discontinuing antihypertensive therapy and replacing it with vitamin D therapy.
Cynthia A. Smith, DNP, APRN, FNP-BC
Renal Consultants, South Charleston, West Virginia
REFERENCES
1. Monfared A, Heidarzadeh A, Ghaffari M, Akbarpour M. Effect of megestrol acetate on serum albumin level in malnourished dialysis patients. J Renal Nutr. 2009;19(2):167-171.
2. Byham-Gray L, Stover J, Wiesen K. A clinical guide to nutrition care in kidney disease. Acad Nutr Diet. 2013.
3. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; ASPEN Malnutrition Task Force; ASPEN Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) [erratum appears in J Acad Nutr Diet. 2012 Nov;112(11):1899].
J Acad Nutr Diet. 2012;112(5):730-738.
4. Rammohan M, Kalantar-Zedeh K, Liang A, Ghossein C. Megestrol acetate in a moderate dose for the treatment of malnutrition-inflammation complex in maintenance dialysis patients. J Ren Nutr. 2005;15(3):345-355.
5. Yeh S, Marandi M, Thode H Jr, et al. Report of a pilot, double blind, placebo-controlled study of megestrol acetate in elderly dialysis patients with cachexia. J Ren Nutr. 2010; 20(1):52-62.
6. Golebiewska JE, Lichodziejewska-Niemierko M, Aleksandrowicz-Wrona E, et al. Megestrol acetate use in hypoalbuminemic dialysis patients [comment]. J Ren Nutr. 2011;21(2): 200-202.
7. Bendik I, Friedel A, Roos FF, et al. Vitamin D: a critical and necessary micronutrient for human health. Front Physiol. 2014;5:248.
8. Cabone F, Mach F, Vuilleumier N, Montecucco F. Potential pathophysiological role for the vitamin D deficiency in essential hypertension. World J Cardiol. 2014;6(5):260-276.
9. Sypniewska G, Pollak J, Strozecki P, et al. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am J Hypertens. 2014;27(1):114-121.
10. Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719-729.
Megestrol Acetate for CKD and Dialysis Patients
Q) Some of my CKD patients are malnourished; in fact, some of those on dialysis do not eat well and have low albumin levels. Previously in this column, it was stated that higher albumin levels (> 4 g/dL) confer survival benefits to dialysis patients. Should I consider prescribing megestrol acetate to improve appetite? If I do prescribe it, what dose is safe for CKD and dialysis patients?
Malnutrition affects one-third of dialysis patients,1 and malnutrition-inflammation complex syndrome (MICS) is common in those with stage 5 CKD. Albumin is used as an indicator of MICS in dialysis patients; however, since other factors (stress, infection, inflammation, comorbidities) affect nutritional status,2 serum albumin alone may not be sufficient to assess it.
In fact, a recent consensus statement on malnutrition from the Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition excluded serum albumin as a diagnostic characteristic; the criteria included percentage of energy requirement, percentage of weight loss and time frame, loss of body fat and muscle mass, presence of edema, and reduced grip strength.3 These may be better measures of malnutrition in dialysis patients and could be used as criteria for determining when to prescribe an appetite stimulant, such as megestrol acetate.
In recent years, megestrol acetate (an antineoplastic drug) has been used to improve appetite, weight, albumin levels, and MICS in patients receiving maintenance dialysis.1,4-6 Rammohan et al found significant increases in weight, BMI, body fat, triceps skinfold thickness, protein/energy intake, and serum albumin in 10 dialysis patients who took megestrol acetate (400 mg/d) for 16 weeks.4
Continue for megestrol acetate's effects >>
In a 20-week randomized, double-blind, placebo-controlled trial, Yeh et al found significant increases in weight, body fat, and fat-free mass in elderly hemodialysis patients receiving megestrol acetate (800 mg/d). The treatment group also demonstrated greater improvement in ability to exercise.5
Monfared and colleagues looked specifically at megestrol acetate’s effect on serum albumin levels in dialysis patients.1 Using a much lower dose (40 mg bid for two months), they found a significant increase in serum albumin in the treatment group. Although an increase in appetite was noted, the researchers did not observe any significant change in total weight following treatment.1
In a letter to the editor of the Journal of Renal Nutrition, Golebiewska et al reported their use of megestrol acetate in maintenance hemodialysis and peritoneal dialysis patients.6 Hypoalbuminemic patients were given megestrol acetate (160 mg/d). Significant increases in weight, BMI, subjective global assessment scores (a measure of nutritional status based on clinical indices such as weight, appetite, muscle, and fat mass), and serum albumin levels were seen. Only 12 of the 32 patients completed the study; the others dropped out due to adverse effects, including high intradialytic weight gain (the amount of fluid gained between dialysis sessions), dyspnea, diarrhea, and nausea.6
Currently, there is no consensus in the literature regarding the most effective dosage of megestrol acetate. Furthermore, evidence is lacking as to whether megestrol acetate–induced increases in appetite, oral intake, weight, and serum albumin level bestow any survival benefit or affect outcomes in dialysis patients.4 However, the increased sense of well-being a patient experiences when appetite returns and weight is restored may be worth the effort.
Luanne DiGuglielmo, MS, RD, CSR
DaVita Summit Renal Center
Mountainside, New Jersey
REFERENCES
1. Monfared A, Heidarzadeh A, Ghaffari M, Akbarpour M. Effect of megestrol acetate on serum albumin level in malnourished dialysis patients. J Renal Nutr. 2009;19(2):167-171.
2. Byham-Gray L, Stover J, Wiesen K. A clinical guide to nutrition care in kidney disease. Acad Nutr Diet. 2013.
3. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; ASPEN Malnutrition Task Force; ASPEN Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) [erratum appears in J Acad Nutr Diet. 2012 Nov;112(11):1899].
J Acad Nutr Diet. 2012;112(5):730-738.
4. Rammohan M, Kalantar-Zedeh K, Liang A, Ghossein C. Megestrol acetate in a moderate dose for the treatment of malnutrition-inflammation complex in maintenance dialysis patients. J Ren Nutr. 2005;15(3):345-355.
5. Yeh S, Marandi M, Thode H Jr, et al. Report of a pilot, double blind, placebo-controlled study of megestrol acetate in elderly dialysis patients with cachexia. J Ren Nutr. 2010; 20(1):52-62.
6. Golebiewska JE, Lichodziejewska-Niemierko M, Aleksandrowicz-Wrona E, et al. Megestrol acetate use in hypoalbuminemic dialysis patients [comment]. J Ren Nutr. 2011;21(2): 200-202.
7. Bendik I, Friedel A, Roos FF, et al. Vitamin D: a critical and necessary micronutrient for human health. Front Physiol. 2014;5:248.
8. Cabone F, Mach F, Vuilleumier N, Montecucco F. Potential pathophysiological role for the vitamin D deficiency in essential hypertension. World J Cardiol. 2014;6(5):260-276.
9. Sypniewska G, Pollak J, Strozecki P, et al. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am J Hypertens. 2014;27(1):114-121.
10. Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719-729.
Q) Some of my CKD patients are malnourished; in fact, some of those on dialysis do not eat well and have low albumin levels. Previously in this column, it was stated that higher albumin levels (> 4 g/dL) confer survival benefits to dialysis patients. Should I consider prescribing megestrol acetate to improve appetite? If I do prescribe it, what dose is safe for CKD and dialysis patients?
Malnutrition affects one-third of dialysis patients,1 and malnutrition-inflammation complex syndrome (MICS) is common in those with stage 5 CKD. Albumin is used as an indicator of MICS in dialysis patients; however, since other factors (stress, infection, inflammation, comorbidities) affect nutritional status,2 serum albumin alone may not be sufficient to assess it.
In fact, a recent consensus statement on malnutrition from the Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition excluded serum albumin as a diagnostic characteristic; the criteria included percentage of energy requirement, percentage of weight loss and time frame, loss of body fat and muscle mass, presence of edema, and reduced grip strength.3 These may be better measures of malnutrition in dialysis patients and could be used as criteria for determining when to prescribe an appetite stimulant, such as megestrol acetate.
In recent years, megestrol acetate (an antineoplastic drug) has been used to improve appetite, weight, albumin levels, and MICS in patients receiving maintenance dialysis.1,4-6 Rammohan et al found significant increases in weight, BMI, body fat, triceps skinfold thickness, protein/energy intake, and serum albumin in 10 dialysis patients who took megestrol acetate (400 mg/d) for 16 weeks.4
Continue for megestrol acetate's effects >>
In a 20-week randomized, double-blind, placebo-controlled trial, Yeh et al found significant increases in weight, body fat, and fat-free mass in elderly hemodialysis patients receiving megestrol acetate (800 mg/d). The treatment group also demonstrated greater improvement in ability to exercise.5
Monfared and colleagues looked specifically at megestrol acetate’s effect on serum albumin levels in dialysis patients.1 Using a much lower dose (40 mg bid for two months), they found a significant increase in serum albumin in the treatment group. Although an increase in appetite was noted, the researchers did not observe any significant change in total weight following treatment.1
In a letter to the editor of the Journal of Renal Nutrition, Golebiewska et al reported their use of megestrol acetate in maintenance hemodialysis and peritoneal dialysis patients.6 Hypoalbuminemic patients were given megestrol acetate (160 mg/d). Significant increases in weight, BMI, subjective global assessment scores (a measure of nutritional status based on clinical indices such as weight, appetite, muscle, and fat mass), and serum albumin levels were seen. Only 12 of the 32 patients completed the study; the others dropped out due to adverse effects, including high intradialytic weight gain (the amount of fluid gained between dialysis sessions), dyspnea, diarrhea, and nausea.6
Currently, there is no consensus in the literature regarding the most effective dosage of megestrol acetate. Furthermore, evidence is lacking as to whether megestrol acetate–induced increases in appetite, oral intake, weight, and serum albumin level bestow any survival benefit or affect outcomes in dialysis patients.4 However, the increased sense of well-being a patient experiences when appetite returns and weight is restored may be worth the effort.
Luanne DiGuglielmo, MS, RD, CSR
DaVita Summit Renal Center
Mountainside, New Jersey
REFERENCES
1. Monfared A, Heidarzadeh A, Ghaffari M, Akbarpour M. Effect of megestrol acetate on serum albumin level in malnourished dialysis patients. J Renal Nutr. 2009;19(2):167-171.
2. Byham-Gray L, Stover J, Wiesen K. A clinical guide to nutrition care in kidney disease. Acad Nutr Diet. 2013.
3. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; ASPEN Malnutrition Task Force; ASPEN Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) [erratum appears in J Acad Nutr Diet. 2012 Nov;112(11):1899].
J Acad Nutr Diet. 2012;112(5):730-738.
4. Rammohan M, Kalantar-Zedeh K, Liang A, Ghossein C. Megestrol acetate in a moderate dose for the treatment of malnutrition-inflammation complex in maintenance dialysis patients. J Ren Nutr. 2005;15(3):345-355.
5. Yeh S, Marandi M, Thode H Jr, et al. Report of a pilot, double blind, placebo-controlled study of megestrol acetate in elderly dialysis patients with cachexia. J Ren Nutr. 2010; 20(1):52-62.
6. Golebiewska JE, Lichodziejewska-Niemierko M, Aleksandrowicz-Wrona E, et al. Megestrol acetate use in hypoalbuminemic dialysis patients [comment]. J Ren Nutr. 2011;21(2): 200-202.
7. Bendik I, Friedel A, Roos FF, et al. Vitamin D: a critical and necessary micronutrient for human health. Front Physiol. 2014;5:248.
8. Cabone F, Mach F, Vuilleumier N, Montecucco F. Potential pathophysiological role for the vitamin D deficiency in essential hypertension. World J Cardiol. 2014;6(5):260-276.
9. Sypniewska G, Pollak J, Strozecki P, et al. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am J Hypertens. 2014;27(1):114-121.
10. Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719-729.
Q) Some of my CKD patients are malnourished; in fact, some of those on dialysis do not eat well and have low albumin levels. Previously in this column, it was stated that higher albumin levels (> 4 g/dL) confer survival benefits to dialysis patients. Should I consider prescribing megestrol acetate to improve appetite? If I do prescribe it, what dose is safe for CKD and dialysis patients?
Malnutrition affects one-third of dialysis patients,1 and malnutrition-inflammation complex syndrome (MICS) is common in those with stage 5 CKD. Albumin is used as an indicator of MICS in dialysis patients; however, since other factors (stress, infection, inflammation, comorbidities) affect nutritional status,2 serum albumin alone may not be sufficient to assess it.
In fact, a recent consensus statement on malnutrition from the Academy of Nutrition and Dietetics and the American Society for Parenteral and Enteral Nutrition excluded serum albumin as a diagnostic characteristic; the criteria included percentage of energy requirement, percentage of weight loss and time frame, loss of body fat and muscle mass, presence of edema, and reduced grip strength.3 These may be better measures of malnutrition in dialysis patients and could be used as criteria for determining when to prescribe an appetite stimulant, such as megestrol acetate.
In recent years, megestrol acetate (an antineoplastic drug) has been used to improve appetite, weight, albumin levels, and MICS in patients receiving maintenance dialysis.1,4-6 Rammohan et al found significant increases in weight, BMI, body fat, triceps skinfold thickness, protein/energy intake, and serum albumin in 10 dialysis patients who took megestrol acetate (400 mg/d) for 16 weeks.4
Continue for megestrol acetate's effects >>
In a 20-week randomized, double-blind, placebo-controlled trial, Yeh et al found significant increases in weight, body fat, and fat-free mass in elderly hemodialysis patients receiving megestrol acetate (800 mg/d). The treatment group also demonstrated greater improvement in ability to exercise.5
Monfared and colleagues looked specifically at megestrol acetate’s effect on serum albumin levels in dialysis patients.1 Using a much lower dose (40 mg bid for two months), they found a significant increase in serum albumin in the treatment group. Although an increase in appetite was noted, the researchers did not observe any significant change in total weight following treatment.1
In a letter to the editor of the Journal of Renal Nutrition, Golebiewska et al reported their use of megestrol acetate in maintenance hemodialysis and peritoneal dialysis patients.6 Hypoalbuminemic patients were given megestrol acetate (160 mg/d). Significant increases in weight, BMI, subjective global assessment scores (a measure of nutritional status based on clinical indices such as weight, appetite, muscle, and fat mass), and serum albumin levels were seen. Only 12 of the 32 patients completed the study; the others dropped out due to adverse effects, including high intradialytic weight gain (the amount of fluid gained between dialysis sessions), dyspnea, diarrhea, and nausea.6
Currently, there is no consensus in the literature regarding the most effective dosage of megestrol acetate. Furthermore, evidence is lacking as to whether megestrol acetate–induced increases in appetite, oral intake, weight, and serum albumin level bestow any survival benefit or affect outcomes in dialysis patients.4 However, the increased sense of well-being a patient experiences when appetite returns and weight is restored may be worth the effort.
Luanne DiGuglielmo, MS, RD, CSR
DaVita Summit Renal Center
Mountainside, New Jersey
REFERENCES
1. Monfared A, Heidarzadeh A, Ghaffari M, Akbarpour M. Effect of megestrol acetate on serum albumin level in malnourished dialysis patients. J Renal Nutr. 2009;19(2):167-171.
2. Byham-Gray L, Stover J, Wiesen K. A clinical guide to nutrition care in kidney disease. Acad Nutr Diet. 2013.
3. White JV, Guenter P, Jensen G, Malone A, Schofield M; Academy of Nutrition and Dietetics Malnutrition Work Group; ASPEN Malnutrition Task Force; ASPEN Board of Directors. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) [erratum appears in J Acad Nutr Diet. 2012 Nov;112(11):1899].
J Acad Nutr Diet. 2012;112(5):730-738.
4. Rammohan M, Kalantar-Zedeh K, Liang A, Ghossein C. Megestrol acetate in a moderate dose for the treatment of malnutrition-inflammation complex in maintenance dialysis patients. J Ren Nutr. 2005;15(3):345-355.
5. Yeh S, Marandi M, Thode H Jr, et al. Report of a pilot, double blind, placebo-controlled study of megestrol acetate in elderly dialysis patients with cachexia. J Ren Nutr. 2010; 20(1):52-62.
6. Golebiewska JE, Lichodziejewska-Niemierko M, Aleksandrowicz-Wrona E, et al. Megestrol acetate use in hypoalbuminemic dialysis patients [comment]. J Ren Nutr. 2011;21(2): 200-202.
7. Bendik I, Friedel A, Roos FF, et al. Vitamin D: a critical and necessary micronutrient for human health. Front Physiol. 2014;5:248.
8. Cabone F, Mach F, Vuilleumier N, Montecucco F. Potential pathophysiological role for the vitamin D deficiency in essential hypertension. World J Cardiol. 2014;6(5):260-276.
9. Sypniewska G, Pollak J, Strozecki P, et al. 25-hydroxyvitamin D, biomarkers of endothelial dysfunction and subclinical organ damage in adults with hypertension. Am J Hypertens. 2014;27(1):114-121.
10. Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719-729.
Early Intervention Could Lead to Reduction in CKD Cases
Q) When I see a patient for an annual physical or gynecologic exam (or even just to give a flu shot), I try to encourage healthy living. Are there any CKD statistics I can use to “encourage” my hypertensive or overweight patients to follow a better plan of care?
According to a recent study by McMahon et al, risk factors for chronic kidney disease (CKD)—including hypertension, dyslipidemia, and diabetes—may be present up to 30 years prior to diagnosis of CKD.1 Since these risk factors are modifiable, the researchers concluded that early intervention could lead to a reduction in new CKD cases.1
Using data from the Framingham Offspring Study, the researchers identified 441 patients with incident CKD and then matched them with a control group of 882 patients who did not develop CKD during the 30-year study period. Subjects who eventually developed CKD were more likely than their counterparts to have hypertension (odds ratio [OR], 1.76), obesity (OR, 1.71), and elevated triglyceride levels (OR, 1.43) 30 years prior to CKD diagnosis. Having diabetes nearly tripled a patient’s likelihood of developing CKD within 20 years (OR, 2.90).1
Early identification of these risk factors and treatment of affected patients is imperative to help prevent kidney disease. Regular screening of young and middle-aged adult patients, as well as early intervention when risk factors are identified, should slow not only the progression of these detrimental conditions but also the development of CKD.
Joanne Hindlet, ACNP, CNN-NP
Houston Nephrology Group
REFERENCES
1. McMahon GM, Preis SR, Hwang S-J, Fox CS. Mid-adulthood risk factor profiles for CKD. J Am Soc Nephrol. 2014 Jun 26; [Epub ahead of print].
2. Byham-Gray L, Stover J, Wiesen K. A Clinical Guide to Nutrition Care in Kidney Disease. 2nd ed. The Academy of Nutrition and Dietetics; 2013.
3. Crews DC. Chronic kidney disease and access to healthful foods. ASN Kidney News. 2014;6(5):11.
4. Moe SM. Phosphate additives in food: you are what you eat—but shouldn’t you know that? ASN Kidney News. 2014;6(5):8.
5. Narva A, Norton J. Medical nutrition therapy for CKD. ASN Kidney News. 2014;6(5):7.
Q) When I see a patient for an annual physical or gynecologic exam (or even just to give a flu shot), I try to encourage healthy living. Are there any CKD statistics I can use to “encourage” my hypertensive or overweight patients to follow a better plan of care?
According to a recent study by McMahon et al, risk factors for chronic kidney disease (CKD)—including hypertension, dyslipidemia, and diabetes—may be present up to 30 years prior to diagnosis of CKD.1 Since these risk factors are modifiable, the researchers concluded that early intervention could lead to a reduction in new CKD cases.1
Using data from the Framingham Offspring Study, the researchers identified 441 patients with incident CKD and then matched them with a control group of 882 patients who did not develop CKD during the 30-year study period. Subjects who eventually developed CKD were more likely than their counterparts to have hypertension (odds ratio [OR], 1.76), obesity (OR, 1.71), and elevated triglyceride levels (OR, 1.43) 30 years prior to CKD diagnosis. Having diabetes nearly tripled a patient’s likelihood of developing CKD within 20 years (OR, 2.90).1
Early identification of these risk factors and treatment of affected patients is imperative to help prevent kidney disease. Regular screening of young and middle-aged adult patients, as well as early intervention when risk factors are identified, should slow not only the progression of these detrimental conditions but also the development of CKD.
Joanne Hindlet, ACNP, CNN-NP
Houston Nephrology Group
REFERENCES
1. McMahon GM, Preis SR, Hwang S-J, Fox CS. Mid-adulthood risk factor profiles for CKD. J Am Soc Nephrol. 2014 Jun 26; [Epub ahead of print].
2. Byham-Gray L, Stover J, Wiesen K. A Clinical Guide to Nutrition Care in Kidney Disease. 2nd ed. The Academy of Nutrition and Dietetics; 2013.
3. Crews DC. Chronic kidney disease and access to healthful foods. ASN Kidney News. 2014;6(5):11.
4. Moe SM. Phosphate additives in food: you are what you eat—but shouldn’t you know that? ASN Kidney News. 2014;6(5):8.
5. Narva A, Norton J. Medical nutrition therapy for CKD. ASN Kidney News. 2014;6(5):7.
Q) When I see a patient for an annual physical or gynecologic exam (or even just to give a flu shot), I try to encourage healthy living. Are there any CKD statistics I can use to “encourage” my hypertensive or overweight patients to follow a better plan of care?
According to a recent study by McMahon et al, risk factors for chronic kidney disease (CKD)—including hypertension, dyslipidemia, and diabetes—may be present up to 30 years prior to diagnosis of CKD.1 Since these risk factors are modifiable, the researchers concluded that early intervention could lead to a reduction in new CKD cases.1
Using data from the Framingham Offspring Study, the researchers identified 441 patients with incident CKD and then matched them with a control group of 882 patients who did not develop CKD during the 30-year study period. Subjects who eventually developed CKD were more likely than their counterparts to have hypertension (odds ratio [OR], 1.76), obesity (OR, 1.71), and elevated triglyceride levels (OR, 1.43) 30 years prior to CKD diagnosis. Having diabetes nearly tripled a patient’s likelihood of developing CKD within 20 years (OR, 2.90).1
Early identification of these risk factors and treatment of affected patients is imperative to help prevent kidney disease. Regular screening of young and middle-aged adult patients, as well as early intervention when risk factors are identified, should slow not only the progression of these detrimental conditions but also the development of CKD.
Joanne Hindlet, ACNP, CNN-NP
Houston Nephrology Group
REFERENCES
1. McMahon GM, Preis SR, Hwang S-J, Fox CS. Mid-adulthood risk factor profiles for CKD. J Am Soc Nephrol. 2014 Jun 26; [Epub ahead of print].
2. Byham-Gray L, Stover J, Wiesen K. A Clinical Guide to Nutrition Care in Kidney Disease. 2nd ed. The Academy of Nutrition and Dietetics; 2013.
3. Crews DC. Chronic kidney disease and access to healthful foods. ASN Kidney News. 2014;6(5):11.
4. Moe SM. Phosphate additives in food: you are what you eat—but shouldn’t you know that? ASN Kidney News. 2014;6(5):8.
5. Narva A, Norton J. Medical nutrition therapy for CKD. ASN Kidney News. 2014;6(5):7.
CKD: Risk Before, Diet After
Q) In my admitting orders for a CKD patient, I wrote for a “renal diet.” However, the nephrology practitioners changed it to a DASH diet. What is the difference? Why would they not want a “renal diet”?
The answer to this question is: It depends—on the patient, his/her comorbidities, and the need for dialysis treatments (and if so, what type he/she is receiving). Renal diet is a general term used to refer to medical nutrition therapy (MNT) given to a patient with CKD. Each of the five stages of CKD has its own specific MNT requirements.
The MNT for CKD patients often involves modification of the following nutrients: protein, sodium, potassium, phosphorus, and sometimes fluid. The complexity of this therapy often confuses health care professionals when a CKD patient is admitted to the hospital. Let’s examine each nutrient modification to understand optimal nutrition for CKD patients.
Protein. As kidneys fail to excrete urea, protein metabolism is compromised. Thus, in CKD stages 1 and 2, the general recommendations for protein intake are 0.8 to 1.4 g/kg/d. As a patient progresses into CKD stages 3 and 4, these recommendations decrease to 0.6 to 0.8 g/kg/d. In addition, the Kidney Disease Outcomes Quality Initiative (KDOQI) and the Academy of Nutrition and Dietetics recommend that at least 50% of that protein intake be of high biological value (eg, foods of animal origin, soy proteins, dairy, legumes, and nuts and nut butters).2
Why the wide range in protein intake? Needs vary depending on the patient’s comorbidities and nutritional status. Patients with greater need (eg, documented malnutrition, infections, or wounds) will require more protein than those without documented catabolic stress. Additionally, protein needs are based on weight, making it crucial to obtain an accurate weight. When managing a very overweight or underweight patient, an appropriate standard body weight must be calculated. This assessment should be done by a registered dietitian (RD).
Also, renal replacement therapies, once introduced, sharply increase protein needs. Hemodialysis (HD) can account for free amino acid losses of 5 to 20 g per treatment. Peritoneal dialysis (PD) can result in albumin losses of 5 to 15 g/d.2 As a result, protein needs in HD and PD patients are about 1.2 g/kg/d of standard body weight. It has been reported that 30% to 50% of patients are not consuming these amounts, placing them at risk for malnutrition and a higher incidence of morbidity and mortality.2
Sodium. In CKD stages 1 to 4, dietary sodium intake should be less than 2,400 mg/d. The Dietary Approaches to Stop Hypertension (DASH) diet and a Mediterranean diet have been associated with reduced risk for decline in glomerular filtration rate (GFR) and better blood pressure control.3 Both of these diets can be employed, especially in the beginning stages of CKD. But as CKD progresses and urine output declines, recommendations for sodium intake for both HD and PD patients decrease to 2,000 mg/d. In an anuric patient, 2,000 mg/d is the maximum.2
Potassium. As kidney function declines, potassium retention occurs. In CKD stages 1 to 4, potassium restriction is not employed unless the serum level rises above normal.2 The addition of an ACE inhibitor or an angiotensin II receptor blocker to the medication regimen necessitates close monitoring of potassium levels. Potassium allowance for HD varies according to the patient’s urine output and can range from 2 to 4 g/d. PD patients generally can tolerate 3 to 4 g/d of potassium without becoming hyperkalemic, as potassium is well cleared with PD.2
Continue for further examinations >>
Phosphorus. Mineral bone abnormalities begin early in the course of CKD and lead to high-turnover bone disease, adynamic bone disease, fractures, and soft-tissue calcification. Careful monitoring of calcium, intact parathyroid hormone, and phosphorus levels is required throughout all stages of CKD, with hyperphosphatemia of particular concern.
In CKD stages 1 and 2, dietary phosphorus should be limited to maintain a normal serum level.2 As CKD progresses and phosphorus retention increases, 800 to 1,000 mg/d or 10 to 12 mg of phosphorus per gram of protein should be prescribed.
Even with the limitation of dietary phosphorus, phosphate-binding medications may be required to control serum phosphorus in later CKD stages and in HD and PD patients.2 Limiting dietary phosphorus can be difficult for patients because of inorganic phosphate salt additives widely found in canned and processed foods; they are also added to dark colas and to meats and poultry to act as preservatives and improve flavor and texture. Phosphorus additives are 100% bioavailable and therefore more readily absorbed than organic phosphorus.4
Fluid. Lastly, CKD patients need to think about their fluid intake. HD patients with a urine output of > 1,000 mL/24-h period will be allowed up to 2,000 mL/d of fluid. (A 12-oz canned drink is 355 mL.) Those with less than 1,000 mL of urine output will be allowed 1,000 to 1,500 mL/d, with anuric patients capped at 1,000 mL/d. PD patients are allowed 1,000 to 3,000 mL/d depending on urine output and overall status.2 Patients should also be reminded that foods such as soup and gelatin are counted in their fluid allowance.
The complexities of the “renal diet” make patient education by an RD critical. However, a recent article suggested that MNT for CKD patients is underutilized, with limited referrals and lack of education for primary care providers and RDs cited as reasons.5 This is mystifying considering that Medicare will pay for RD services for CKD patients.
The National Kidney Disease Education Program, in association with the Academy of Nutrition and Dietetics, has developed free professional and patient education materials to address this need; they are available at http://nkdep.nih.gov/.
Luanne DiGuglielmo, MS, RD, CSR
DaVita Summit Renal Center
Mountainside, New Jersey
REFERENCES
1. McMahon GM, Preis SR, Hwang S-J, Fox CS. Mid-adulthood risk factor profiles for CKD. J Am Soc Nephrol. 2014 Jun 26; [Epub ahead of print].
2. Byham-Gray L, Stover J, Wiesen K. A Clinical Guide to Nutrition Care in Kidney Disease. 2nd ed. The Academy of Nutrition and Dietetics; 2013.
3. Crews DC. Chronic kidney disease and access to healthful foods. ASN Kidney News. 2014;6(5):11.
4. Moe SM. Phosphate additives in food: you are what you eat—but shouldn’t you know that? ASN Kidney News. 2014;6(5):8.
5. Narva A, Norton J. Medical nutrition therapy for CKD. ASN Kidney News. 2014;6(5):7.
Q) In my admitting orders for a CKD patient, I wrote for a “renal diet.” However, the nephrology practitioners changed it to a DASH diet. What is the difference? Why would they not want a “renal diet”?
The answer to this question is: It depends—on the patient, his/her comorbidities, and the need for dialysis treatments (and if so, what type he/she is receiving). Renal diet is a general term used to refer to medical nutrition therapy (MNT) given to a patient with CKD. Each of the five stages of CKD has its own specific MNT requirements.
The MNT for CKD patients often involves modification of the following nutrients: protein, sodium, potassium, phosphorus, and sometimes fluid. The complexity of this therapy often confuses health care professionals when a CKD patient is admitted to the hospital. Let’s examine each nutrient modification to understand optimal nutrition for CKD patients.
Protein. As kidneys fail to excrete urea, protein metabolism is compromised. Thus, in CKD stages 1 and 2, the general recommendations for protein intake are 0.8 to 1.4 g/kg/d. As a patient progresses into CKD stages 3 and 4, these recommendations decrease to 0.6 to 0.8 g/kg/d. In addition, the Kidney Disease Outcomes Quality Initiative (KDOQI) and the Academy of Nutrition and Dietetics recommend that at least 50% of that protein intake be of high biological value (eg, foods of animal origin, soy proteins, dairy, legumes, and nuts and nut butters).2
Why the wide range in protein intake? Needs vary depending on the patient’s comorbidities and nutritional status. Patients with greater need (eg, documented malnutrition, infections, or wounds) will require more protein than those without documented catabolic stress. Additionally, protein needs are based on weight, making it crucial to obtain an accurate weight. When managing a very overweight or underweight patient, an appropriate standard body weight must be calculated. This assessment should be done by a registered dietitian (RD).
Also, renal replacement therapies, once introduced, sharply increase protein needs. Hemodialysis (HD) can account for free amino acid losses of 5 to 20 g per treatment. Peritoneal dialysis (PD) can result in albumin losses of 5 to 15 g/d.2 As a result, protein needs in HD and PD patients are about 1.2 g/kg/d of standard body weight. It has been reported that 30% to 50% of patients are not consuming these amounts, placing them at risk for malnutrition and a higher incidence of morbidity and mortality.2
Sodium. In CKD stages 1 to 4, dietary sodium intake should be less than 2,400 mg/d. The Dietary Approaches to Stop Hypertension (DASH) diet and a Mediterranean diet have been associated with reduced risk for decline in glomerular filtration rate (GFR) and better blood pressure control.3 Both of these diets can be employed, especially in the beginning stages of CKD. But as CKD progresses and urine output declines, recommendations for sodium intake for both HD and PD patients decrease to 2,000 mg/d. In an anuric patient, 2,000 mg/d is the maximum.2
Potassium. As kidney function declines, potassium retention occurs. In CKD stages 1 to 4, potassium restriction is not employed unless the serum level rises above normal.2 The addition of an ACE inhibitor or an angiotensin II receptor blocker to the medication regimen necessitates close monitoring of potassium levels. Potassium allowance for HD varies according to the patient’s urine output and can range from 2 to 4 g/d. PD patients generally can tolerate 3 to 4 g/d of potassium without becoming hyperkalemic, as potassium is well cleared with PD.2
Continue for further examinations >>
Phosphorus. Mineral bone abnormalities begin early in the course of CKD and lead to high-turnover bone disease, adynamic bone disease, fractures, and soft-tissue calcification. Careful monitoring of calcium, intact parathyroid hormone, and phosphorus levels is required throughout all stages of CKD, with hyperphosphatemia of particular concern.
In CKD stages 1 and 2, dietary phosphorus should be limited to maintain a normal serum level.2 As CKD progresses and phosphorus retention increases, 800 to 1,000 mg/d or 10 to 12 mg of phosphorus per gram of protein should be prescribed.
Even with the limitation of dietary phosphorus, phosphate-binding medications may be required to control serum phosphorus in later CKD stages and in HD and PD patients.2 Limiting dietary phosphorus can be difficult for patients because of inorganic phosphate salt additives widely found in canned and processed foods; they are also added to dark colas and to meats and poultry to act as preservatives and improve flavor and texture. Phosphorus additives are 100% bioavailable and therefore more readily absorbed than organic phosphorus.4
Fluid. Lastly, CKD patients need to think about their fluid intake. HD patients with a urine output of > 1,000 mL/24-h period will be allowed up to 2,000 mL/d of fluid. (A 12-oz canned drink is 355 mL.) Those with less than 1,000 mL of urine output will be allowed 1,000 to 1,500 mL/d, with anuric patients capped at 1,000 mL/d. PD patients are allowed 1,000 to 3,000 mL/d depending on urine output and overall status.2 Patients should also be reminded that foods such as soup and gelatin are counted in their fluid allowance.
The complexities of the “renal diet” make patient education by an RD critical. However, a recent article suggested that MNT for CKD patients is underutilized, with limited referrals and lack of education for primary care providers and RDs cited as reasons.5 This is mystifying considering that Medicare will pay for RD services for CKD patients.
The National Kidney Disease Education Program, in association with the Academy of Nutrition and Dietetics, has developed free professional and patient education materials to address this need; they are available at http://nkdep.nih.gov/.
Luanne DiGuglielmo, MS, RD, CSR
DaVita Summit Renal Center
Mountainside, New Jersey
REFERENCES
1. McMahon GM, Preis SR, Hwang S-J, Fox CS. Mid-adulthood risk factor profiles for CKD. J Am Soc Nephrol. 2014 Jun 26; [Epub ahead of print].
2. Byham-Gray L, Stover J, Wiesen K. A Clinical Guide to Nutrition Care in Kidney Disease. 2nd ed. The Academy of Nutrition and Dietetics; 2013.
3. Crews DC. Chronic kidney disease and access to healthful foods. ASN Kidney News. 2014;6(5):11.
4. Moe SM. Phosphate additives in food: you are what you eat—but shouldn’t you know that? ASN Kidney News. 2014;6(5):8.
5. Narva A, Norton J. Medical nutrition therapy for CKD. ASN Kidney News. 2014;6(5):7.
Q) In my admitting orders for a CKD patient, I wrote for a “renal diet.” However, the nephrology practitioners changed it to a DASH diet. What is the difference? Why would they not want a “renal diet”?
The answer to this question is: It depends—on the patient, his/her comorbidities, and the need for dialysis treatments (and if so, what type he/she is receiving). Renal diet is a general term used to refer to medical nutrition therapy (MNT) given to a patient with CKD. Each of the five stages of CKD has its own specific MNT requirements.
The MNT for CKD patients often involves modification of the following nutrients: protein, sodium, potassium, phosphorus, and sometimes fluid. The complexity of this therapy often confuses health care professionals when a CKD patient is admitted to the hospital. Let’s examine each nutrient modification to understand optimal nutrition for CKD patients.
Protein. As kidneys fail to excrete urea, protein metabolism is compromised. Thus, in CKD stages 1 and 2, the general recommendations for protein intake are 0.8 to 1.4 g/kg/d. As a patient progresses into CKD stages 3 and 4, these recommendations decrease to 0.6 to 0.8 g/kg/d. In addition, the Kidney Disease Outcomes Quality Initiative (KDOQI) and the Academy of Nutrition and Dietetics recommend that at least 50% of that protein intake be of high biological value (eg, foods of animal origin, soy proteins, dairy, legumes, and nuts and nut butters).2
Why the wide range in protein intake? Needs vary depending on the patient’s comorbidities and nutritional status. Patients with greater need (eg, documented malnutrition, infections, or wounds) will require more protein than those without documented catabolic stress. Additionally, protein needs are based on weight, making it crucial to obtain an accurate weight. When managing a very overweight or underweight patient, an appropriate standard body weight must be calculated. This assessment should be done by a registered dietitian (RD).
Also, renal replacement therapies, once introduced, sharply increase protein needs. Hemodialysis (HD) can account for free amino acid losses of 5 to 20 g per treatment. Peritoneal dialysis (PD) can result in albumin losses of 5 to 15 g/d.2 As a result, protein needs in HD and PD patients are about 1.2 g/kg/d of standard body weight. It has been reported that 30% to 50% of patients are not consuming these amounts, placing them at risk for malnutrition and a higher incidence of morbidity and mortality.2
Sodium. In CKD stages 1 to 4, dietary sodium intake should be less than 2,400 mg/d. The Dietary Approaches to Stop Hypertension (DASH) diet and a Mediterranean diet have been associated with reduced risk for decline in glomerular filtration rate (GFR) and better blood pressure control.3 Both of these diets can be employed, especially in the beginning stages of CKD. But as CKD progresses and urine output declines, recommendations for sodium intake for both HD and PD patients decrease to 2,000 mg/d. In an anuric patient, 2,000 mg/d is the maximum.2
Potassium. As kidney function declines, potassium retention occurs. In CKD stages 1 to 4, potassium restriction is not employed unless the serum level rises above normal.2 The addition of an ACE inhibitor or an angiotensin II receptor blocker to the medication regimen necessitates close monitoring of potassium levels. Potassium allowance for HD varies according to the patient’s urine output and can range from 2 to 4 g/d. PD patients generally can tolerate 3 to 4 g/d of potassium without becoming hyperkalemic, as potassium is well cleared with PD.2
Continue for further examinations >>
Phosphorus. Mineral bone abnormalities begin early in the course of CKD and lead to high-turnover bone disease, adynamic bone disease, fractures, and soft-tissue calcification. Careful monitoring of calcium, intact parathyroid hormone, and phosphorus levels is required throughout all stages of CKD, with hyperphosphatemia of particular concern.
In CKD stages 1 and 2, dietary phosphorus should be limited to maintain a normal serum level.2 As CKD progresses and phosphorus retention increases, 800 to 1,000 mg/d or 10 to 12 mg of phosphorus per gram of protein should be prescribed.
Even with the limitation of dietary phosphorus, phosphate-binding medications may be required to control serum phosphorus in later CKD stages and in HD and PD patients.2 Limiting dietary phosphorus can be difficult for patients because of inorganic phosphate salt additives widely found in canned and processed foods; they are also added to dark colas and to meats and poultry to act as preservatives and improve flavor and texture. Phosphorus additives are 100% bioavailable and therefore more readily absorbed than organic phosphorus.4
Fluid. Lastly, CKD patients need to think about their fluid intake. HD patients with a urine output of > 1,000 mL/24-h period will be allowed up to 2,000 mL/d of fluid. (A 12-oz canned drink is 355 mL.) Those with less than 1,000 mL of urine output will be allowed 1,000 to 1,500 mL/d, with anuric patients capped at 1,000 mL/d. PD patients are allowed 1,000 to 3,000 mL/d depending on urine output and overall status.2 Patients should also be reminded that foods such as soup and gelatin are counted in their fluid allowance.
The complexities of the “renal diet” make patient education by an RD critical. However, a recent article suggested that MNT for CKD patients is underutilized, with limited referrals and lack of education for primary care providers and RDs cited as reasons.5 This is mystifying considering that Medicare will pay for RD services for CKD patients.
The National Kidney Disease Education Program, in association with the Academy of Nutrition and Dietetics, has developed free professional and patient education materials to address this need; they are available at http://nkdep.nih.gov/.
Luanne DiGuglielmo, MS, RD, CSR
DaVita Summit Renal Center
Mountainside, New Jersey
REFERENCES
1. McMahon GM, Preis SR, Hwang S-J, Fox CS. Mid-adulthood risk factor profiles for CKD. J Am Soc Nephrol. 2014 Jun 26; [Epub ahead of print].
2. Byham-Gray L, Stover J, Wiesen K. A Clinical Guide to Nutrition Care in Kidney Disease. 2nd ed. The Academy of Nutrition and Dietetics; 2013.
3. Crews DC. Chronic kidney disease and access to healthful foods. ASN Kidney News. 2014;6(5):11.
4. Moe SM. Phosphate additives in food: you are what you eat—but shouldn’t you know that? ASN Kidney News. 2014;6(5):8.
5. Narva A, Norton J. Medical nutrition therapy for CKD. ASN Kidney News. 2014;6(5):7.
Genetics of Renal Disease: APOL1 Variations
Q) I have heard about a gene that causes high blood pressure. Did I hear that right? Is testing for this gene available now?
African-Americans have a higher risk for chronic kidney disease (CKD), including end-stage renal disease (ESRD; defined as kidney failure requiring dialysis or transplant), than any other racial or ethnic group in the United States.1 Previously, this has been attributed to poorly controlled hypertension and diabetes, as well as socioeconomic factors such as limited access to health care.
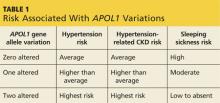
Research now shows that autosomal recessive genetic variations on chromosome 22q, the gene that encodes apolipoprotein-1 (APOL1; an HDL protein), promote hypertension. This subsequently increases the risk for and progression of CKD in black patients (who have up to 29x higher risk than white patients without this genetic variation).2
The APOL1 gene has two alleles. Having at least one of them provides resistance to Trypanosoma brucei, the cause of “sleeping sickness” transmitted by the tsetse fly, but increases risk for CKD and ESRD (see Table 1).2,3 Black patients descending from the southern and western portions of Africa are most likely to have two alleles, putting them at the highest risk for hypertension and associated CKD.
Foster et al reported that black patients with two altered alleles had a 31% higher risk for CKD and ESRD, compared with individuals with hypertension-induced nephrosclerosis who had zero to one altered alleles.4 Nondiabetic black patients with CKD who have two altered alleles are at highest risk for focal segmental glomerulosclerosis, HIV nephropathy, and CKD attributable to hypertension.2 The African-American Study of Kidney Disease and Hypertension found that black patients with hypertension controlled by ACE inhibitors had slower progression of CKD, regardless of allele variation.5 Currently, there is no treatment for this genetic alteration.4
One could posit that black patients undergoing renal transplant would have a higher risk for renal failure in the transplanted kidney due to APOL1-related hypertension, compared to nonblack renal transplant recipients. Additionally, a donor kidney with an altered APOL1 gene may have a higher risk for failure.6
Genotyping for APOL1 (CPT code: 81479) is available in select laboratories at a cost of approximately $400.7 For a family that has a member affected by kidney failure at a young age, knowing whether the APOL1 gene is carried in the family would allow early aggressive hypertension management to help prevent a lifetime of severe CKD.
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personalizedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Disease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.
Q) I have heard about a gene that causes high blood pressure. Did I hear that right? Is testing for this gene available now?
African-Americans have a higher risk for chronic kidney disease (CKD), including end-stage renal disease (ESRD; defined as kidney failure requiring dialysis or transplant), than any other racial or ethnic group in the United States.1 Previously, this has been attributed to poorly controlled hypertension and diabetes, as well as socioeconomic factors such as limited access to health care.
Research now shows that autosomal recessive genetic variations on chromosome 22q, the gene that encodes apolipoprotein-1 (APOL1; an HDL protein), promote hypertension. This subsequently increases the risk for and progression of CKD in black patients (who have up to 29x higher risk than white patients without this genetic variation).2
The APOL1 gene has two alleles. Having at least one of them provides resistance to Trypanosoma brucei, the cause of “sleeping sickness” transmitted by the tsetse fly, but increases risk for CKD and ESRD (see Table 1).2,3 Black patients descending from the southern and western portions of Africa are most likely to have two alleles, putting them at the highest risk for hypertension and associated CKD.
Foster et al reported that black patients with two altered alleles had a 31% higher risk for CKD and ESRD, compared with individuals with hypertension-induced nephrosclerosis who had zero to one altered alleles.4 Nondiabetic black patients with CKD who have two altered alleles are at highest risk for focal segmental glomerulosclerosis, HIV nephropathy, and CKD attributable to hypertension.2 The African-American Study of Kidney Disease and Hypertension found that black patients with hypertension controlled by ACE inhibitors had slower progression of CKD, regardless of allele variation.5 Currently, there is no treatment for this genetic alteration.4
One could posit that black patients undergoing renal transplant would have a higher risk for renal failure in the transplanted kidney due to APOL1-related hypertension, compared to nonblack renal transplant recipients. Additionally, a donor kidney with an altered APOL1 gene may have a higher risk for failure.6
Genotyping for APOL1 (CPT code: 81479) is available in select laboratories at a cost of approximately $400.7 For a family that has a member affected by kidney failure at a young age, knowing whether the APOL1 gene is carried in the family would allow early aggressive hypertension management to help prevent a lifetime of severe CKD.
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personalizedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Disease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.
Q) I have heard about a gene that causes high blood pressure. Did I hear that right? Is testing for this gene available now?
African-Americans have a higher risk for chronic kidney disease (CKD), including end-stage renal disease (ESRD; defined as kidney failure requiring dialysis or transplant), than any other racial or ethnic group in the United States.1 Previously, this has been attributed to poorly controlled hypertension and diabetes, as well as socioeconomic factors such as limited access to health care.
Research now shows that autosomal recessive genetic variations on chromosome 22q, the gene that encodes apolipoprotein-1 (APOL1; an HDL protein), promote hypertension. This subsequently increases the risk for and progression of CKD in black patients (who have up to 29x higher risk than white patients without this genetic variation).2
The APOL1 gene has two alleles. Having at least one of them provides resistance to Trypanosoma brucei, the cause of “sleeping sickness” transmitted by the tsetse fly, but increases risk for CKD and ESRD (see Table 1).2,3 Black patients descending from the southern and western portions of Africa are most likely to have two alleles, putting them at the highest risk for hypertension and associated CKD.
Foster et al reported that black patients with two altered alleles had a 31% higher risk for CKD and ESRD, compared with individuals with hypertension-induced nephrosclerosis who had zero to one altered alleles.4 Nondiabetic black patients with CKD who have two altered alleles are at highest risk for focal segmental glomerulosclerosis, HIV nephropathy, and CKD attributable to hypertension.2 The African-American Study of Kidney Disease and Hypertension found that black patients with hypertension controlled by ACE inhibitors had slower progression of CKD, regardless of allele variation.5 Currently, there is no treatment for this genetic alteration.4
One could posit that black patients undergoing renal transplant would have a higher risk for renal failure in the transplanted kidney due to APOL1-related hypertension, compared to nonblack renal transplant recipients. Additionally, a donor kidney with an altered APOL1 gene may have a higher risk for failure.6
Genotyping for APOL1 (CPT code: 81479) is available in select laboratories at a cost of approximately $400.7 For a family that has a member affected by kidney failure at a young age, knowing whether the APOL1 gene is carried in the family would allow early aggressive hypertension management to help prevent a lifetime of severe CKD.
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personalizedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Disease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.
The Risk and Treatment for Wilms Tumors
Q) In school, they always emphasized the abdominal exam to rule out Wilms tumors. Are Wilms tumors still with us? Has treatment and evaluation changed?
Wilms tumor is a renal cancer found most commonly in children younger than 9 and represents approximately 7% of all malignancies in children.8,9 It can occur in one or both kidneys, with earlier diagnosis noted with bilateral involvement. Risk is highest among non-Hispanic white persons and African-Americans and lowest among Asians.8
Wilms tumor develops due to a genetic mutation in the WT1 gene located on the 11p13 chromosome. Defects are also noted on the 11p15 chromosome and the p53 tumor suppressor gene.10 Urbach et al recently identified a relationship between the LIN28 gene and Wilms tumor.11 Tumors develop when embryonic renal cells that should cease growing at the time of birth continue to grow in the postnatal period. Wilms tumor can be familial or sporadic. It can also be associated with various congenital anomalies manifested within various syndromes (see Table 2), as well as isolated genitourinary abnormalities, especially in boys.10
Most children present with a palpable, smooth, firm, generally painless mass in the abdomen; those who have bilateral renal involvement usually present earlier than those with unilateral involvement. Palpation of the abdomen during examination, if vigorous, can result in rupture of the renal capsule and tumor spillage. Additional symptoms include hematuria, fever, and hypertension. Referral to pediatric oncology is imperative.12
Definitive diagnosis is made by histologic evaluation following biopsy or surgical excision.13 Other possible diagnostic tests include but are not limited to abdominal ultrasound or CT; chest CT (to rule out metastatic lung disease); urinalysis (to evaluate for hematuria and proteinuria); liver function studies (to evaluate for hepatic involvement); and laboratory studies to measure coagulation, serum calcium, blood urea nitrogen, creatinine, and complete blood count.
Histologic examination for staging (I-V) occurs following surgical excision of the tumor. There are two staging systems available: the National Wilms Tumor Study, based on postoperative tumor evaluation, and the International Society of Pediatric Oncology, based on postchemotherapy evaluation.13
Treatment options include surgical excision (including complete nephrectomy of the affected kidney), chemotherapy based on tumor staging, and internal and/or external radiation therapy.13
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personal izedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Dis ease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.
Q) In school, they always emphasized the abdominal exam to rule out Wilms tumors. Are Wilms tumors still with us? Has treatment and evaluation changed?
Wilms tumor is a renal cancer found most commonly in children younger than 9 and represents approximately 7% of all malignancies in children.8,9 It can occur in one or both kidneys, with earlier diagnosis noted with bilateral involvement. Risk is highest among non-Hispanic white persons and African-Americans and lowest among Asians.8
Wilms tumor develops due to a genetic mutation in the WT1 gene located on the 11p13 chromosome. Defects are also noted on the 11p15 chromosome and the p53 tumor suppressor gene.10 Urbach et al recently identified a relationship between the LIN28 gene and Wilms tumor.11 Tumors develop when embryonic renal cells that should cease growing at the time of birth continue to grow in the postnatal period. Wilms tumor can be familial or sporadic. It can also be associated with various congenital anomalies manifested within various syndromes (see Table 2), as well as isolated genitourinary abnormalities, especially in boys.10
Most children present with a palpable, smooth, firm, generally painless mass in the abdomen; those who have bilateral renal involvement usually present earlier than those with unilateral involvement. Palpation of the abdomen during examination, if vigorous, can result in rupture of the renal capsule and tumor spillage. Additional symptoms include hematuria, fever, and hypertension. Referral to pediatric oncology is imperative.12
Definitive diagnosis is made by histologic evaluation following biopsy or surgical excision.13 Other possible diagnostic tests include but are not limited to abdominal ultrasound or CT; chest CT (to rule out metastatic lung disease); urinalysis (to evaluate for hematuria and proteinuria); liver function studies (to evaluate for hepatic involvement); and laboratory studies to measure coagulation, serum calcium, blood urea nitrogen, creatinine, and complete blood count.
Histologic examination for staging (I-V) occurs following surgical excision of the tumor. There are two staging systems available: the National Wilms Tumor Study, based on postoperative tumor evaluation, and the International Society of Pediatric Oncology, based on postchemotherapy evaluation.13
Treatment options include surgical excision (including complete nephrectomy of the affected kidney), chemotherapy based on tumor staging, and internal and/or external radiation therapy.13
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personal izedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Dis ease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.
Q) In school, they always emphasized the abdominal exam to rule out Wilms tumors. Are Wilms tumors still with us? Has treatment and evaluation changed?
Wilms tumor is a renal cancer found most commonly in children younger than 9 and represents approximately 7% of all malignancies in children.8,9 It can occur in one or both kidneys, with earlier diagnosis noted with bilateral involvement. Risk is highest among non-Hispanic white persons and African-Americans and lowest among Asians.8
Wilms tumor develops due to a genetic mutation in the WT1 gene located on the 11p13 chromosome. Defects are also noted on the 11p15 chromosome and the p53 tumor suppressor gene.10 Urbach et al recently identified a relationship between the LIN28 gene and Wilms tumor.11 Tumors develop when embryonic renal cells that should cease growing at the time of birth continue to grow in the postnatal period. Wilms tumor can be familial or sporadic. It can also be associated with various congenital anomalies manifested within various syndromes (see Table 2), as well as isolated genitourinary abnormalities, especially in boys.10
Most children present with a palpable, smooth, firm, generally painless mass in the abdomen; those who have bilateral renal involvement usually present earlier than those with unilateral involvement. Palpation of the abdomen during examination, if vigorous, can result in rupture of the renal capsule and tumor spillage. Additional symptoms include hematuria, fever, and hypertension. Referral to pediatric oncology is imperative.12
Definitive diagnosis is made by histologic evaluation following biopsy or surgical excision.13 Other possible diagnostic tests include but are not limited to abdominal ultrasound or CT; chest CT (to rule out metastatic lung disease); urinalysis (to evaluate for hematuria and proteinuria); liver function studies (to evaluate for hepatic involvement); and laboratory studies to measure coagulation, serum calcium, blood urea nitrogen, creatinine, and complete blood count.
Histologic examination for staging (I-V) occurs following surgical excision of the tumor. There are two staging systems available: the National Wilms Tumor Study, based on postoperative tumor evaluation, and the International Society of Pediatric Oncology, based on postchemotherapy evaluation.13
Treatment options include surgical excision (including complete nephrectomy of the affected kidney), chemotherapy based on tumor staging, and internal and/or external radiation therapy.13
Susan E. Brown, MS, ARNP,
ACNP-BC, CCRN
Great River Nephrology,
West Burlington, Iowa
REFERENCES
1. United States Renal Data System. Annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States (2012). www.usrds.org/2012/view/v1_01.aspx. Accessed October 19, 2014.
2. Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy.
J Am Soc Nephrol. 2011;22(11):2129-2137.
3. Parsa A, Kao L, Xie D, et al; AASK and CRIC Study Investigators. APOL1 risk variants, race and progression of chronic kidney disease.
N Engl J Med. 2013;369:2183-2196.
4. Foster MC, Coresh J, Fornage M, et al. APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol. 2013;24(9):1484-1491.
5. Lipkowitz MS, Freedman BI, Langefeld CD, et al; AASK Investigators. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83(1):114–120.
6. Reeves-Daniel AM, DePalma JA, Bleyer AJ, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11(5):1025-1030.
7. Partners Healthcare Personalized Medicine. Order APOL1 genotyping test for non-diabetic nephropathy kidney disease. http://personal izedmedicine.partners.org/Laboratory-For-Molecular-Medicine/Ordering/Kidney-Dis ease/APOL1-Gene-Sequencing.aspx. Accessed October 19, 2014.
8. Grovas A, Fremgen A, Rauck A, et al. The National Cancer Data Base report on patterns of childhood cancers in the United States. Cancer. 1997;80(12):2321-2332.
9. Johns Hopkins Medicine. Wilm’s tumor. www.hopkinsmedicine.org/kimmel_cancer_center/centers/pediatric_oncology/cancer_types/wilms_tumor.html. Accessed October 19, 2014.
10. Dome JS, Huff V. Wilms tumor overview. In: Pagon RA, Adam MP, Ardinger HH, et al (eds). GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. www.ncbi.nlm.nih.gov/books/NBK1294/. Accessed October 19, 2014.
11. Urbach A, Yermalovich A, Zhang J, et al. Lin28 sustains early renal progenitors and induces Wilms tumor. Genes & Dev. 2014;28:971-982.
12. Fernandez C, Geller JI, Ehrlich PF, et al. Renal tumors. In: Pizzo P, Poplack D (eds). Principles and Practice of Pediatric Oncology. 6th ed, St Louis, MO: Lippincott Williams & Wilkins. 2011; 861.
13. Metzger ML, Dome JS. Current therapy for Wilms’ tumor. Oncologist. 2005;10(10):815-826.
Overcorrection of Hyponatremia
Q) A clinic patient of mine was recently admitted to the hospital with hyponatremia (serum sodium, 115 mEq/L). She was treated with 2 L of normal saline and discharged home 48 hours later, at her baseline mental status with a serum sodium level of 132 mEq/L. Two days later, she was readmitted for mental status changes, and MRI showed brain swelling. The neurologist stated this was a result of the initial treatment for her hyponatremia. How is this possible?
The cause-and-effect relationship between rapid correction of chronic hyponatremia and subsequent development of neurologic problems was discovered in the late 1970s. Central pontine and extrapontine myelinolysis (known as osmotic demyelination syndrome or ODS) is a neurologic condition that can occur from rapid sodium correction. It is diagnosed by MRI, which shows hyperintense lesions on T2-weighted images. Clinical signs include upper motor neuron signs, pseudobulbar palsy, spastic quadriparesis, and mental status changes ranging from mild confusion to coma.2
Treatment for hyponatremia should be guided by symptom management.2,3 If a patient is asymptomatic, a simple and effective strategy is to keep NPO for 24 hours, except for medications. Simple food and fluid restriction will likely increase the serum sodium level because of obligate solute losses and urinary electrolyte free water loss.2,4 While the first instinct is to feed these patients, as they often appear malnourished, this can cause a solute load leading to a too-rapid sodium correction. After 24 hours, if intake restriction is not effective, use 0.5% normal saline but with limited dosing orders, as usual saline dosing can cause too rapid a correction.2
For symptomatic patients (confusion, seizures, coma), the goal is to initially elevate sodium by 1 to 2 mEq/L per hour for the first two to three hours. Do not exceed 10 mEq/L in 24 hours or 18 mEq/L in 48 hours. Exceeding these limits puts patients at high risk for ODS. In fact, even when staying within these parameters, there is some risk for overcorrection. It is always better to go slowly.2,3
In the patient with hyponatremia due to low solute intake (eg, beer potomania), diuresis can start spontaneously after a period of food and fluid restriction. It can also be initiated with just a small amount of solute. For example, administering an IV antibiotic with a base solution of 100 mL of normal saline or a “banana bag” (an IV solution containing 0.5 to 1 L of normal saline with multivitamins/minerals that cause the fluid to be yellow) can produce several liters of diuresis.2 Once you open the floodgate, you can unintentionally cause too-rapid correction that could lead to ODS.
In chronic hyponatremic patients, low antidiuretic hormone (ADH) levels are often found; thus when a solute is introduced, there is little ADH in the system to protect against excessive water loss and electrolyte imbalance. At the same time, excessive water loss can translate to higher sodium levels and increase the risk for cerebral edema. If rapid diuresis occurs, an infusion of D5W (5% dextrose in water) to match the rate of urine output may prevent a rapid serum sodium level rise. Frequent monitoring of serum sodium levels is often necessary. In instances where ODS is already present, there are case studies of improved neurologic outcomes with reduction of serum sodium levels.2,3
While the treatment of hyponatremia at first glance seems straightforward—replace that which is lost—it can actually transform a seemingly simple problem into a complicated clinical course requiring intensive care, due to the need for frequent monitoring and intervention.
Kristina Unterseher, MSN, FNP, CNN-NP
Peacehealth St. John
Medical Center
Longview, WA
REFERENCES
1. Hilden T, Swensen TL. Electrolyte disturbances in beer drinkers: a specific “hypo-osmolaity syndrome.” Lancet. 1975;2(7928):245-246.
2. Sanghvi SR, Kellerman PS, Nanovic L. Beer potomania: an unusual cause of hyponatremia at high risk of complications from rapid correction. Am J Kidney Dis. 2007;50(4):673-680.
3. Bhattarai N, Poonam K, Panda M. Beer potomania: a case report. BMJ Case Rep. 2010; 2010: bcr10.2009.2414.
4. Campbell M. Hyponatremia and central pontine myelinolysis as a result of beer potomania: a case report. Prim Care Companion J Clin Psychiatry. 2010;12(4):PCC.09100936.
5. Thaler SM, Teitelbaum I, Beri T. “Beer potomania” in non-beer drinkers: effect of low dietary solute intake. Am J Kidney Dis. 1998;31(6):1028-1031.
Q) A clinic patient of mine was recently admitted to the hospital with hyponatremia (serum sodium, 115 mEq/L). She was treated with 2 L of normal saline and discharged home 48 hours later, at her baseline mental status with a serum sodium level of 132 mEq/L. Two days later, she was readmitted for mental status changes, and MRI showed brain swelling. The neurologist stated this was a result of the initial treatment for her hyponatremia. How is this possible?
The cause-and-effect relationship between rapid correction of chronic hyponatremia and subsequent development of neurologic problems was discovered in the late 1970s. Central pontine and extrapontine myelinolysis (known as osmotic demyelination syndrome or ODS) is a neurologic condition that can occur from rapid sodium correction. It is diagnosed by MRI, which shows hyperintense lesions on T2-weighted images. Clinical signs include upper motor neuron signs, pseudobulbar palsy, spastic quadriparesis, and mental status changes ranging from mild confusion to coma.2
Treatment for hyponatremia should be guided by symptom management.2,3 If a patient is asymptomatic, a simple and effective strategy is to keep NPO for 24 hours, except for medications. Simple food and fluid restriction will likely increase the serum sodium level because of obligate solute losses and urinary electrolyte free water loss.2,4 While the first instinct is to feed these patients, as they often appear malnourished, this can cause a solute load leading to a too-rapid sodium correction. After 24 hours, if intake restriction is not effective, use 0.5% normal saline but with limited dosing orders, as usual saline dosing can cause too rapid a correction.2
For symptomatic patients (confusion, seizures, coma), the goal is to initially elevate sodium by 1 to 2 mEq/L per hour for the first two to three hours. Do not exceed 10 mEq/L in 24 hours or 18 mEq/L in 48 hours. Exceeding these limits puts patients at high risk for ODS. In fact, even when staying within these parameters, there is some risk for overcorrection. It is always better to go slowly.2,3
In the patient with hyponatremia due to low solute intake (eg, beer potomania), diuresis can start spontaneously after a period of food and fluid restriction. It can also be initiated with just a small amount of solute. For example, administering an IV antibiotic with a base solution of 100 mL of normal saline or a “banana bag” (an IV solution containing 0.5 to 1 L of normal saline with multivitamins/minerals that cause the fluid to be yellow) can produce several liters of diuresis.2 Once you open the floodgate, you can unintentionally cause too-rapid correction that could lead to ODS.
In chronic hyponatremic patients, low antidiuretic hormone (ADH) levels are often found; thus when a solute is introduced, there is little ADH in the system to protect against excessive water loss and electrolyte imbalance. At the same time, excessive water loss can translate to higher sodium levels and increase the risk for cerebral edema. If rapid diuresis occurs, an infusion of D5W (5% dextrose in water) to match the rate of urine output may prevent a rapid serum sodium level rise. Frequent monitoring of serum sodium levels is often necessary. In instances where ODS is already present, there are case studies of improved neurologic outcomes with reduction of serum sodium levels.2,3
While the treatment of hyponatremia at first glance seems straightforward—replace that which is lost—it can actually transform a seemingly simple problem into a complicated clinical course requiring intensive care, due to the need for frequent monitoring and intervention.
Kristina Unterseher, MSN, FNP, CNN-NP
Peacehealth St. John
Medical Center
Longview, WA
REFERENCES
1. Hilden T, Swensen TL. Electrolyte disturbances in beer drinkers: a specific “hypo-osmolaity syndrome.” Lancet. 1975;2(7928):245-246.
2. Sanghvi SR, Kellerman PS, Nanovic L. Beer potomania: an unusual cause of hyponatremia at high risk of complications from rapid correction. Am J Kidney Dis. 2007;50(4):673-680.
3. Bhattarai N, Poonam K, Panda M. Beer potomania: a case report. BMJ Case Rep. 2010; 2010: bcr10.2009.2414.
4. Campbell M. Hyponatremia and central pontine myelinolysis as a result of beer potomania: a case report. Prim Care Companion J Clin Psychiatry. 2010;12(4):PCC.09100936.
5. Thaler SM, Teitelbaum I, Beri T. “Beer potomania” in non-beer drinkers: effect of low dietary solute intake. Am J Kidney Dis. 1998;31(6):1028-1031.
Q) A clinic patient of mine was recently admitted to the hospital with hyponatremia (serum sodium, 115 mEq/L). She was treated with 2 L of normal saline and discharged home 48 hours later, at her baseline mental status with a serum sodium level of 132 mEq/L. Two days later, she was readmitted for mental status changes, and MRI showed brain swelling. The neurologist stated this was a result of the initial treatment for her hyponatremia. How is this possible?
The cause-and-effect relationship between rapid correction of chronic hyponatremia and subsequent development of neurologic problems was discovered in the late 1970s. Central pontine and extrapontine myelinolysis (known as osmotic demyelination syndrome or ODS) is a neurologic condition that can occur from rapid sodium correction. It is diagnosed by MRI, which shows hyperintense lesions on T2-weighted images. Clinical signs include upper motor neuron signs, pseudobulbar palsy, spastic quadriparesis, and mental status changes ranging from mild confusion to coma.2
Treatment for hyponatremia should be guided by symptom management.2,3 If a patient is asymptomatic, a simple and effective strategy is to keep NPO for 24 hours, except for medications. Simple food and fluid restriction will likely increase the serum sodium level because of obligate solute losses and urinary electrolyte free water loss.2,4 While the first instinct is to feed these patients, as they often appear malnourished, this can cause a solute load leading to a too-rapid sodium correction. After 24 hours, if intake restriction is not effective, use 0.5% normal saline but with limited dosing orders, as usual saline dosing can cause too rapid a correction.2
For symptomatic patients (confusion, seizures, coma), the goal is to initially elevate sodium by 1 to 2 mEq/L per hour for the first two to three hours. Do not exceed 10 mEq/L in 24 hours or 18 mEq/L in 48 hours. Exceeding these limits puts patients at high risk for ODS. In fact, even when staying within these parameters, there is some risk for overcorrection. It is always better to go slowly.2,3
In the patient with hyponatremia due to low solute intake (eg, beer potomania), diuresis can start spontaneously after a period of food and fluid restriction. It can also be initiated with just a small amount of solute. For example, administering an IV antibiotic with a base solution of 100 mL of normal saline or a “banana bag” (an IV solution containing 0.5 to 1 L of normal saline with multivitamins/minerals that cause the fluid to be yellow) can produce several liters of diuresis.2 Once you open the floodgate, you can unintentionally cause too-rapid correction that could lead to ODS.
In chronic hyponatremic patients, low antidiuretic hormone (ADH) levels are often found; thus when a solute is introduced, there is little ADH in the system to protect against excessive water loss and electrolyte imbalance. At the same time, excessive water loss can translate to higher sodium levels and increase the risk for cerebral edema. If rapid diuresis occurs, an infusion of D5W (5% dextrose in water) to match the rate of urine output may prevent a rapid serum sodium level rise. Frequent monitoring of serum sodium levels is often necessary. In instances where ODS is already present, there are case studies of improved neurologic outcomes with reduction of serum sodium levels.2,3
While the treatment of hyponatremia at first glance seems straightforward—replace that which is lost—it can actually transform a seemingly simple problem into a complicated clinical course requiring intensive care, due to the need for frequent monitoring and intervention.
Kristina Unterseher, MSN, FNP, CNN-NP
Peacehealth St. John
Medical Center
Longview, WA
REFERENCES
1. Hilden T, Swensen TL. Electrolyte disturbances in beer drinkers: a specific “hypo-osmolaity syndrome.” Lancet. 1975;2(7928):245-246.
2. Sanghvi SR, Kellerman PS, Nanovic L. Beer potomania: an unusual cause of hyponatremia at high risk of complications from rapid correction. Am J Kidney Dis. 2007;50(4):673-680.
3. Bhattarai N, Poonam K, Panda M. Beer potomania: a case report. BMJ Case Rep. 2010; 2010: bcr10.2009.2414.
4. Campbell M. Hyponatremia and central pontine myelinolysis as a result of beer potomania: a case report. Prim Care Companion J Clin Psychiatry. 2010;12(4):PCC.09100936.
5. Thaler SM, Teitelbaum I, Beri T. “Beer potomania” in non-beer drinkers: effect of low dietary solute intake. Am J Kidney Dis. 1998;31(6):1028-1031.
Hyponatremia: Beer Potomania
Q) Recently, we had a patient admitted for hyponatremia with
a serum sodium level of 117 mEq/L. One of the hospitalists mentioned “beer potomania” in the differential. Not wanting to look dumb, I just agreed. What is beer potomania, and how is it related to low serum sodium?
Potomania is the excessive consumption of alcoholic beverages; beer potomania is used to refer to a dilutional hyponatremia caused by excessive consumption of beer.1 First recognized in 1971, this cause of hyponatremia is not the most common but should be in the differential if the patient is a heavy alcohol imbiber who presents with encephalopathy and low serum sodium.
When considering this diagnosis, keep in mind that hyponatremia is common among chronic alcoholics and can be due to conditions such as cirrhosis, congestive heart failure, syndrome of inappropriate antidiuretic hormone (SIADH) secretion, and hypovolemia. Less common but still belonging in the differential are pseudohyponatremia secondary to alcohol-induced severe hypertriglyceridemia and cerebral salt wasting syndrome.2,3
Beer potomania usually manifests as altered mental status, weakness, and gait disturbance with an average serum sodium concentration of 108 mEq/L.3 Other abnormal lab results consistent with this diagnosis include hypokalemia (mean potassium, 3 mEq/L) and low blood urea nitrogen and urine sodium levels.2,3 Another fairly consistent finding is a recent personal history of binge drinking (more than about 5 L, or 14 cans of beer, in 24 hours) and/or history of illness (vomiting, diarrhea) that predisposed the patient to a rapid drop in serum sodium levels.2
Based on the information presented thus far, you may ask, “Why haven’t I seen this diagnosed more often? There are a lot of beer bingers out there!” Good question. Let’s review the pathophysiology of beer potomania. When patients have poor protein and solute (food, electrolytes) intake, they can experience water intoxication with smaller-than-usual volumes of fluid. The kidneys need a certain amount of solute to facilitate free water clearance (the ability to clear excess fluid from the body). A lack of adequate solute results in a buildup of free water in the vascular system, leading to a dilutional hyponatremia.3
Free water clearance is dependent on both solute excretion and the ability to dilute urine. Someone consuming an average diet will excrete 600 to 900 mOsm/d of solute. This osmolar load in-cludes urea generated from protein (10 g of protein produces about 50 mOsm of urea), along with dietary sodium and potassium. The maximum capacity for urinary dilution is 50 mOsm/L. In a nutritionally sound person, a lot of fluid—about 20 L—would be required to overwhelm the body’s capacity for urinary dilution.2
However, when you don’t eat, the body starts to break down tissue to create energy to survive. This catabolism creates 100 to 150 mOsm/d of urea, allowing you to continue to appropriately excrete a moderate amount of fluid in spite of poor solute intake ... as long as you are not drinking excessive amounts of water.5
Alcoholics get a moderate amount of their calories via beer consumption and do not experience this endogenous protein breakdown or its resultant low urea/solute level. With low solute intake, dramatically lower fluid intake (about 14 cans of beer) will overwhelm the kidneys’ ability to clear excess free water in the body.2 Fortunately, most heavy beer drinkers continue to eat at least modestly, which is sufficient to avoid this rare type of hyponatremia. Chronic alcoholics who go on a drinking binge beyond their normal baseline alcohol consumption, or who develop a flulike illness that causes electrolyte depletion (via diarrhea or vomiting), are at higher risk for beer potomania.
Kristina Unterseher, MSN, FNP, CNN-NP
Peacehealth St. John
Medical Center
Longview, WA
REFERENCES
1. Hilden T, Swensen TL. Electrolyte disturbances in beer drinkers: a specific “hypo-osmolaity syndrome.” Lancet. 1975;2(7928):245-246.
2. Sanghvi SR, Kellerman PS, Nanovic L. Beer potomania: an unusual cause of hyponatremia at high risk of complications from rapid correction. Am J Kidney Dis. 2007;50(4):673-680.
3. Bhattarai N, Poonam K, Panda M. Beer potomania: a case report. BMJ Case Rep. 2010; 2010: bcr10.2009.2414.
4. Campbell M. Hyponatremia and central pontine myelinolysis as a result of beer potomania: a case report. Prim Care Companion J Clin Psychiatry. 2010;12(4):PCC.09100936.
5. Thaler SM, Teitelbaum I, Beri T. “Beer potomania” in non-beer drinkers: effect of low dietary solute intake. Am J Kidney Dis. 1998;31(6):1028-1031.
Q) Recently, we had a patient admitted for hyponatremia with
a serum sodium level of 117 mEq/L. One of the hospitalists mentioned “beer potomania” in the differential. Not wanting to look dumb, I just agreed. What is beer potomania, and how is it related to low serum sodium?
Potomania is the excessive consumption of alcoholic beverages; beer potomania is used to refer to a dilutional hyponatremia caused by excessive consumption of beer.1 First recognized in 1971, this cause of hyponatremia is not the most common but should be in the differential if the patient is a heavy alcohol imbiber who presents with encephalopathy and low serum sodium.
When considering this diagnosis, keep in mind that hyponatremia is common among chronic alcoholics and can be due to conditions such as cirrhosis, congestive heart failure, syndrome of inappropriate antidiuretic hormone (SIADH) secretion, and hypovolemia. Less common but still belonging in the differential are pseudohyponatremia secondary to alcohol-induced severe hypertriglyceridemia and cerebral salt wasting syndrome.2,3
Beer potomania usually manifests as altered mental status, weakness, and gait disturbance with an average serum sodium concentration of 108 mEq/L.3 Other abnormal lab results consistent with this diagnosis include hypokalemia (mean potassium, 3 mEq/L) and low blood urea nitrogen and urine sodium levels.2,3 Another fairly consistent finding is a recent personal history of binge drinking (more than about 5 L, or 14 cans of beer, in 24 hours) and/or history of illness (vomiting, diarrhea) that predisposed the patient to a rapid drop in serum sodium levels.2
Based on the information presented thus far, you may ask, “Why haven’t I seen this diagnosed more often? There are a lot of beer bingers out there!” Good question. Let’s review the pathophysiology of beer potomania. When patients have poor protein and solute (food, electrolytes) intake, they can experience water intoxication with smaller-than-usual volumes of fluid. The kidneys need a certain amount of solute to facilitate free water clearance (the ability to clear excess fluid from the body). A lack of adequate solute results in a buildup of free water in the vascular system, leading to a dilutional hyponatremia.3
Free water clearance is dependent on both solute excretion and the ability to dilute urine. Someone consuming an average diet will excrete 600 to 900 mOsm/d of solute. This osmolar load in-cludes urea generated from protein (10 g of protein produces about 50 mOsm of urea), along with dietary sodium and potassium. The maximum capacity for urinary dilution is 50 mOsm/L. In a nutritionally sound person, a lot of fluid—about 20 L—would be required to overwhelm the body’s capacity for urinary dilution.2
However, when you don’t eat, the body starts to break down tissue to create energy to survive. This catabolism creates 100 to 150 mOsm/d of urea, allowing you to continue to appropriately excrete a moderate amount of fluid in spite of poor solute intake ... as long as you are not drinking excessive amounts of water.5
Alcoholics get a moderate amount of their calories via beer consumption and do not experience this endogenous protein breakdown or its resultant low urea/solute level. With low solute intake, dramatically lower fluid intake (about 14 cans of beer) will overwhelm the kidneys’ ability to clear excess free water in the body.2 Fortunately, most heavy beer drinkers continue to eat at least modestly, which is sufficient to avoid this rare type of hyponatremia. Chronic alcoholics who go on a drinking binge beyond their normal baseline alcohol consumption, or who develop a flulike illness that causes electrolyte depletion (via diarrhea or vomiting), are at higher risk for beer potomania.
Kristina Unterseher, MSN, FNP, CNN-NP
Peacehealth St. John
Medical Center
Longview, WA
REFERENCES
1. Hilden T, Swensen TL. Electrolyte disturbances in beer drinkers: a specific “hypo-osmolaity syndrome.” Lancet. 1975;2(7928):245-246.
2. Sanghvi SR, Kellerman PS, Nanovic L. Beer potomania: an unusual cause of hyponatremia at high risk of complications from rapid correction. Am J Kidney Dis. 2007;50(4):673-680.
3. Bhattarai N, Poonam K, Panda M. Beer potomania: a case report. BMJ Case Rep. 2010; 2010: bcr10.2009.2414.
4. Campbell M. Hyponatremia and central pontine myelinolysis as a result of beer potomania: a case report. Prim Care Companion J Clin Psychiatry. 2010;12(4):PCC.09100936.
5. Thaler SM, Teitelbaum I, Beri T. “Beer potomania” in non-beer drinkers: effect of low dietary solute intake. Am J Kidney Dis. 1998;31(6):1028-1031.
Q) Recently, we had a patient admitted for hyponatremia with
a serum sodium level of 117 mEq/L. One of the hospitalists mentioned “beer potomania” in the differential. Not wanting to look dumb, I just agreed. What is beer potomania, and how is it related to low serum sodium?
Potomania is the excessive consumption of alcoholic beverages; beer potomania is used to refer to a dilutional hyponatremia caused by excessive consumption of beer.1 First recognized in 1971, this cause of hyponatremia is not the most common but should be in the differential if the patient is a heavy alcohol imbiber who presents with encephalopathy and low serum sodium.
When considering this diagnosis, keep in mind that hyponatremia is common among chronic alcoholics and can be due to conditions such as cirrhosis, congestive heart failure, syndrome of inappropriate antidiuretic hormone (SIADH) secretion, and hypovolemia. Less common but still belonging in the differential are pseudohyponatremia secondary to alcohol-induced severe hypertriglyceridemia and cerebral salt wasting syndrome.2,3
Beer potomania usually manifests as altered mental status, weakness, and gait disturbance with an average serum sodium concentration of 108 mEq/L.3 Other abnormal lab results consistent with this diagnosis include hypokalemia (mean potassium, 3 mEq/L) and low blood urea nitrogen and urine sodium levels.2,3 Another fairly consistent finding is a recent personal history of binge drinking (more than about 5 L, or 14 cans of beer, in 24 hours) and/or history of illness (vomiting, diarrhea) that predisposed the patient to a rapid drop in serum sodium levels.2
Based on the information presented thus far, you may ask, “Why haven’t I seen this diagnosed more often? There are a lot of beer bingers out there!” Good question. Let’s review the pathophysiology of beer potomania. When patients have poor protein and solute (food, electrolytes) intake, they can experience water intoxication with smaller-than-usual volumes of fluid. The kidneys need a certain amount of solute to facilitate free water clearance (the ability to clear excess fluid from the body). A lack of adequate solute results in a buildup of free water in the vascular system, leading to a dilutional hyponatremia.3
Free water clearance is dependent on both solute excretion and the ability to dilute urine. Someone consuming an average diet will excrete 600 to 900 mOsm/d of solute. This osmolar load in-cludes urea generated from protein (10 g of protein produces about 50 mOsm of urea), along with dietary sodium and potassium. The maximum capacity for urinary dilution is 50 mOsm/L. In a nutritionally sound person, a lot of fluid—about 20 L—would be required to overwhelm the body’s capacity for urinary dilution.2
However, when you don’t eat, the body starts to break down tissue to create energy to survive. This catabolism creates 100 to 150 mOsm/d of urea, allowing you to continue to appropriately excrete a moderate amount of fluid in spite of poor solute intake ... as long as you are not drinking excessive amounts of water.5
Alcoholics get a moderate amount of their calories via beer consumption and do not experience this endogenous protein breakdown or its resultant low urea/solute level. With low solute intake, dramatically lower fluid intake (about 14 cans of beer) will overwhelm the kidneys’ ability to clear excess free water in the body.2 Fortunately, most heavy beer drinkers continue to eat at least modestly, which is sufficient to avoid this rare type of hyponatremia. Chronic alcoholics who go on a drinking binge beyond their normal baseline alcohol consumption, or who develop a flulike illness that causes electrolyte depletion (via diarrhea or vomiting), are at higher risk for beer potomania.
Kristina Unterseher, MSN, FNP, CNN-NP
Peacehealth St. John
Medical Center
Longview, WA
REFERENCES
1. Hilden T, Swensen TL. Electrolyte disturbances in beer drinkers: a specific “hypo-osmolaity syndrome.” Lancet. 1975;2(7928):245-246.
2. Sanghvi SR, Kellerman PS, Nanovic L. Beer potomania: an unusual cause of hyponatremia at high risk of complications from rapid correction. Am J Kidney Dis. 2007;50(4):673-680.
3. Bhattarai N, Poonam K, Panda M. Beer potomania: a case report. BMJ Case Rep. 2010; 2010: bcr10.2009.2414.
4. Campbell M. Hyponatremia and central pontine myelinolysis as a result of beer potomania: a case report. Prim Care Companion J Clin Psychiatry. 2010;12(4):PCC.09100936.
5. Thaler SM, Teitelbaum I, Beri T. “Beer potomania” in non-beer drinkers: effect of low dietary solute intake. Am J Kidney Dis. 1998;31(6):1028-1031.
Updates on Kidney Donation
Q) A good friend was diagnosed with chronic kidney disease (CKD) and is presently undergoing workup for a transplant. He is 60 and otherwise healthy; his glomerular filtration rate (GFR) is 14, and he has no uremic symptoms. If I volunteer to give him a kidney, are there any long-term risks for me?
Kidney failure, dialysis, and kidney transplant are terms that can invoke stress and uncertainty in patients with end-stage renal disease (ESRD) and among their family members and friends. In addition to adjusting to the changes wrought by ESRD, patients may also be burdened by the prospect of a family member or friend donating a kidney to them and the concern that the donation will lead to complications for their donor. Family members or friends who volunteer may also experience stress, uncertain of their own risk for ESRD in the future.
Past research improperly compared relative risk for ESRD in donors with that in the general population (without accounting for higher propensity for complications in donors with preexisting conditions). In an effort to correct this misperception, a study recently published in JAMA compared the risk for ESRD in donors with that in a healthy group of nondonors.1 The nondonor pool was taken from the National Health and Nutrition Examination Survey (NHANES III), which assesses the health and nutritional status of adults and children in the United States.
The JAMA study included a cohort of 96,217 kidney donors in the US in a 17-year period and a cohort of 20,024 participants in a six-year period of the NHANES III trial. This data was then compared to Centers for Medicare & Medicaid Services (CMS) data to determine the development of ESRD in kidney donors. ESRD was defined by CMS as the initiation of dialysis, placement on the kidney transplant waiting list, or receipt of a living or deceased donor kidney transplant.
In addition to comparing risk for ESRD in kidney donors with that of a healthy population of nondonors, the researchers also stratified their results demographically. Thus, the lifetime rate of kidney failure in donors is 90 per 10,000, compared with 326 per 10,000 in the general population of nondonors. In healthy nondonors, the risk for kidney failure was 14 per 10,000. After 15 years, the risk for kidney failure associated with donating a kidney was 51 per 10,000 in African-American donors and 23 per 10,000 in white donors. So while the study did reveal an increased risk associated with kidney donation, the degree of risk is considered small.
These findings demonstrate the importance of understanding the facts surrounding inherent risk for ESRD in kidney donation. Overall, a donor’s lifetime risk is considered minuscule. So, to answer the question, yes, there is a slight increase in risk for kidney failure if you donate to your friend. That said, the risk is 0.014 x a standardized risk of 1. This increases at 15 years to 0.51 for African-American and 0.23 for white donors. With such tiny increases, you can safely feel good about donating a kidney to your friend.
Donna Reesman, MSN, CNP
VP Clinical & Quality Management
St Clair Specialty Physicians Detroit
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Q) A good friend was diagnosed with chronic kidney disease (CKD) and is presently undergoing workup for a transplant. He is 60 and otherwise healthy; his glomerular filtration rate (GFR) is 14, and he has no uremic symptoms. If I volunteer to give him a kidney, are there any long-term risks for me?
Kidney failure, dialysis, and kidney transplant are terms that can invoke stress and uncertainty in patients with end-stage renal disease (ESRD) and among their family members and friends. In addition to adjusting to the changes wrought by ESRD, patients may also be burdened by the prospect of a family member or friend donating a kidney to them and the concern that the donation will lead to complications for their donor. Family members or friends who volunteer may also experience stress, uncertain of their own risk for ESRD in the future.
Past research improperly compared relative risk for ESRD in donors with that in the general population (without accounting for higher propensity for complications in donors with preexisting conditions). In an effort to correct this misperception, a study recently published in JAMA compared the risk for ESRD in donors with that in a healthy group of nondonors.1 The nondonor pool was taken from the National Health and Nutrition Examination Survey (NHANES III), which assesses the health and nutritional status of adults and children in the United States.
The JAMA study included a cohort of 96,217 kidney donors in the US in a 17-year period and a cohort of 20,024 participants in a six-year period of the NHANES III trial. This data was then compared to Centers for Medicare & Medicaid Services (CMS) data to determine the development of ESRD in kidney donors. ESRD was defined by CMS as the initiation of dialysis, placement on the kidney transplant waiting list, or receipt of a living or deceased donor kidney transplant.
In addition to comparing risk for ESRD in kidney donors with that of a healthy population of nondonors, the researchers also stratified their results demographically. Thus, the lifetime rate of kidney failure in donors is 90 per 10,000, compared with 326 per 10,000 in the general population of nondonors. In healthy nondonors, the risk for kidney failure was 14 per 10,000. After 15 years, the risk for kidney failure associated with donating a kidney was 51 per 10,000 in African-American donors and 23 per 10,000 in white donors. So while the study did reveal an increased risk associated with kidney donation, the degree of risk is considered small.
These findings demonstrate the importance of understanding the facts surrounding inherent risk for ESRD in kidney donation. Overall, a donor’s lifetime risk is considered minuscule. So, to answer the question, yes, there is a slight increase in risk for kidney failure if you donate to your friend. That said, the risk is 0.014 x a standardized risk of 1. This increases at 15 years to 0.51 for African-American and 0.23 for white donors. With such tiny increases, you can safely feel good about donating a kidney to your friend.
Donna Reesman, MSN, CNP
VP Clinical & Quality Management
St Clair Specialty Physicians Detroit
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Q) A good friend was diagnosed with chronic kidney disease (CKD) and is presently undergoing workup for a transplant. He is 60 and otherwise healthy; his glomerular filtration rate (GFR) is 14, and he has no uremic symptoms. If I volunteer to give him a kidney, are there any long-term risks for me?
Kidney failure, dialysis, and kidney transplant are terms that can invoke stress and uncertainty in patients with end-stage renal disease (ESRD) and among their family members and friends. In addition to adjusting to the changes wrought by ESRD, patients may also be burdened by the prospect of a family member or friend donating a kidney to them and the concern that the donation will lead to complications for their donor. Family members or friends who volunteer may also experience stress, uncertain of their own risk for ESRD in the future.
Past research improperly compared relative risk for ESRD in donors with that in the general population (without accounting for higher propensity for complications in donors with preexisting conditions). In an effort to correct this misperception, a study recently published in JAMA compared the risk for ESRD in donors with that in a healthy group of nondonors.1 The nondonor pool was taken from the National Health and Nutrition Examination Survey (NHANES III), which assesses the health and nutritional status of adults and children in the United States.
The JAMA study included a cohort of 96,217 kidney donors in the US in a 17-year period and a cohort of 20,024 participants in a six-year period of the NHANES III trial. This data was then compared to Centers for Medicare & Medicaid Services (CMS) data to determine the development of ESRD in kidney donors. ESRD was defined by CMS as the initiation of dialysis, placement on the kidney transplant waiting list, or receipt of a living or deceased donor kidney transplant.
In addition to comparing risk for ESRD in kidney donors with that of a healthy population of nondonors, the researchers also stratified their results demographically. Thus, the lifetime rate of kidney failure in donors is 90 per 10,000, compared with 326 per 10,000 in the general population of nondonors. In healthy nondonors, the risk for kidney failure was 14 per 10,000. After 15 years, the risk for kidney failure associated with donating a kidney was 51 per 10,000 in African-American donors and 23 per 10,000 in white donors. So while the study did reveal an increased risk associated with kidney donation, the degree of risk is considered small.
These findings demonstrate the importance of understanding the facts surrounding inherent risk for ESRD in kidney donation. Overall, a donor’s lifetime risk is considered minuscule. So, to answer the question, yes, there is a slight increase in risk for kidney failure if you donate to your friend. That said, the risk is 0.014 x a standardized risk of 1. This increases at 15 years to 0.51 for African-American and 0.23 for white donors. With such tiny increases, you can safely feel good about donating a kidney to your friend.
Donna Reesman, MSN, CNP
VP Clinical & Quality Management
St Clair Specialty Physicians Detroit
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Kidney Donation & HIV
Q) Now that patients are living with HIV/AIDS, can they donate kidneys or receive a kidney transplant?
Kidney disease often has multiple causes, including hypertension, diabetes, inherited conditions, and viral illnesses. The latter include primarily HIV, hepatitis C, and hepatitis B. With advances in the treatment of viral illnesses, the question of whether patients with these viruses can donate or receive a kidney transplant is being discussed not only in the United States but also worldwide.
The most recent CDC figures estimate that more than 1.1 million people in the US are living with HIV, of whom one in six (or nearly 16%) are undiagnosed. There are approximately 50,000 new infections reported annually.2
The Organ Transplant Amendments Act of 1988 banned HIV-positive people from donating organs. However, with the introduction of highly active antiretroviral therapy (HAART, now often referred to as active antiretroviral therapy) and the effective prophylaxis and management of opportunistic infections, mortality has been reduced. HIV/AIDS is often seen as a chronic disease and not the death sentence it once was.3 Since the development of HAART, there have been successful transplants to HIV-positive recipients from non–HIV-infected donors.
In November 2013, President Obama signed the HIV Organ Policy Equity (HOPE) Act, which lifted the ban on using organs from HIV-infected donors. The legislation directs the Department of Health and Human Services and the Organ Procurement and Transplantation Network to develop standards to make these transplants possible.4
Although there have not been any documented cases of transplants from HIV-infected donors to HIV-infected recipients in this country, such transplants have been very successful in South Africa.5 There, to qualify for kidney transplant, all recipients must have proven adherence, virologic suppression, and immune constitution. Donor suitability is defined as HIV infection (confirmed with the use of enzyme-linked immunosorbent assay), absence of proteinuria, and a normal kidney as assessed with post hoc renal biopsy.5
One of the chief concerns has been the effect of further immunosuppression on the recipients and the possibility of disease progression. Although the sample size is limited (four transplants), data from the available cases indicate no evidence of organ rejection at 12 months post-transplantation. In addition, the recipients’ CD4 counts remained lower than baseline due to immunosuppressive therapy. All four patients maintained a viral load of less than 50 copies, which suggested that any virus transplanted along with the kidney had not affected control of HIV infection.5 However, it should be noted that many of the agents used for posttransplant maintenance immunosuppression (mycophenolate mofetil, cyclosporine, tacrolimus, and sirolimus) have antiretroviral properties.3
HIV patients in the US must meet the following criteria to be listed for a transplant:
• Diagnosis of ESRD with at least a five-year life-expectancy
• CD4 count of > 200 cells/ μL for at least six months
• Undetectable HIV viremia (< 50 HIV-1 RNA copies/mL)
• Demonstrated adherence to stable antiviral regimen for at least six months
• Absence of AIDS-defining illness following successful immune reconstitution6
A prospective trial of 150 patients in 19 US transplant centers who met the above criteria demonstrated patient survival and graft survival rates comparable to those in patients ages 65 and older.6
While awaiting the donation, HIV patients can continue hemodialysis and peritoneal dialysis. With the improved antiviral drugs, HIV patients have a survival rate similar to the non–HIV-infected population.
Transplantation is the goal and certainly the hope of many advanced-stage kidney patients, but in reality, the need far exceeds the resources. The HOPE Act opens the door for many patients who were previously excluded from the possibility of a life without dialysis. Taking care of these patients will be a team effort, encompassing HIV and infectious disease specialists, pharmacists, nephrologists, transplant surgeons and coordinators, and primary care providers—including, of course, advanced practitioners.
Shelly Levinstein, MSN, CRNP
Nephrology Associates of York
York, PA
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Q) Now that patients are living with HIV/AIDS, can they donate kidneys or receive a kidney transplant?
Kidney disease often has multiple causes, including hypertension, diabetes, inherited conditions, and viral illnesses. The latter include primarily HIV, hepatitis C, and hepatitis B. With advances in the treatment of viral illnesses, the question of whether patients with these viruses can donate or receive a kidney transplant is being discussed not only in the United States but also worldwide.
The most recent CDC figures estimate that more than 1.1 million people in the US are living with HIV, of whom one in six (or nearly 16%) are undiagnosed. There are approximately 50,000 new infections reported annually.2
The Organ Transplant Amendments Act of 1988 banned HIV-positive people from donating organs. However, with the introduction of highly active antiretroviral therapy (HAART, now often referred to as active antiretroviral therapy) and the effective prophylaxis and management of opportunistic infections, mortality has been reduced. HIV/AIDS is often seen as a chronic disease and not the death sentence it once was.3 Since the development of HAART, there have been successful transplants to HIV-positive recipients from non–HIV-infected donors.
In November 2013, President Obama signed the HIV Organ Policy Equity (HOPE) Act, which lifted the ban on using organs from HIV-infected donors. The legislation directs the Department of Health and Human Services and the Organ Procurement and Transplantation Network to develop standards to make these transplants possible.4
Although there have not been any documented cases of transplants from HIV-infected donors to HIV-infected recipients in this country, such transplants have been very successful in South Africa.5 There, to qualify for kidney transplant, all recipients must have proven adherence, virologic suppression, and immune constitution. Donor suitability is defined as HIV infection (confirmed with the use of enzyme-linked immunosorbent assay), absence of proteinuria, and a normal kidney as assessed with post hoc renal biopsy.5
One of the chief concerns has been the effect of further immunosuppression on the recipients and the possibility of disease progression. Although the sample size is limited (four transplants), data from the available cases indicate no evidence of organ rejection at 12 months post-transplantation. In addition, the recipients’ CD4 counts remained lower than baseline due to immunosuppressive therapy. All four patients maintained a viral load of less than 50 copies, which suggested that any virus transplanted along with the kidney had not affected control of HIV infection.5 However, it should be noted that many of the agents used for posttransplant maintenance immunosuppression (mycophenolate mofetil, cyclosporine, tacrolimus, and sirolimus) have antiretroviral properties.3
HIV patients in the US must meet the following criteria to be listed for a transplant:
• Diagnosis of ESRD with at least a five-year life-expectancy
• CD4 count of > 200 cells/ μL for at least six months
• Undetectable HIV viremia (< 50 HIV-1 RNA copies/mL)
• Demonstrated adherence to stable antiviral regimen for at least six months
• Absence of AIDS-defining illness following successful immune reconstitution6
A prospective trial of 150 patients in 19 US transplant centers who met the above criteria demonstrated patient survival and graft survival rates comparable to those in patients ages 65 and older.6
While awaiting the donation, HIV patients can continue hemodialysis and peritoneal dialysis. With the improved antiviral drugs, HIV patients have a survival rate similar to the non–HIV-infected population.
Transplantation is the goal and certainly the hope of many advanced-stage kidney patients, but in reality, the need far exceeds the resources. The HOPE Act opens the door for many patients who were previously excluded from the possibility of a life without dialysis. Taking care of these patients will be a team effort, encompassing HIV and infectious disease specialists, pharmacists, nephrologists, transplant surgeons and coordinators, and primary care providers—including, of course, advanced practitioners.
Shelly Levinstein, MSN, CRNP
Nephrology Associates of York
York, PA
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.
Q) Now that patients are living with HIV/AIDS, can they donate kidneys or receive a kidney transplant?
Kidney disease often has multiple causes, including hypertension, diabetes, inherited conditions, and viral illnesses. The latter include primarily HIV, hepatitis C, and hepatitis B. With advances in the treatment of viral illnesses, the question of whether patients with these viruses can donate or receive a kidney transplant is being discussed not only in the United States but also worldwide.
The most recent CDC figures estimate that more than 1.1 million people in the US are living with HIV, of whom one in six (or nearly 16%) are undiagnosed. There are approximately 50,000 new infections reported annually.2
The Organ Transplant Amendments Act of 1988 banned HIV-positive people from donating organs. However, with the introduction of highly active antiretroviral therapy (HAART, now often referred to as active antiretroviral therapy) and the effective prophylaxis and management of opportunistic infections, mortality has been reduced. HIV/AIDS is often seen as a chronic disease and not the death sentence it once was.3 Since the development of HAART, there have been successful transplants to HIV-positive recipients from non–HIV-infected donors.
In November 2013, President Obama signed the HIV Organ Policy Equity (HOPE) Act, which lifted the ban on using organs from HIV-infected donors. The legislation directs the Department of Health and Human Services and the Organ Procurement and Transplantation Network to develop standards to make these transplants possible.4
Although there have not been any documented cases of transplants from HIV-infected donors to HIV-infected recipients in this country, such transplants have been very successful in South Africa.5 There, to qualify for kidney transplant, all recipients must have proven adherence, virologic suppression, and immune constitution. Donor suitability is defined as HIV infection (confirmed with the use of enzyme-linked immunosorbent assay), absence of proteinuria, and a normal kidney as assessed with post hoc renal biopsy.5
One of the chief concerns has been the effect of further immunosuppression on the recipients and the possibility of disease progression. Although the sample size is limited (four transplants), data from the available cases indicate no evidence of organ rejection at 12 months post-transplantation. In addition, the recipients’ CD4 counts remained lower than baseline due to immunosuppressive therapy. All four patients maintained a viral load of less than 50 copies, which suggested that any virus transplanted along with the kidney had not affected control of HIV infection.5 However, it should be noted that many of the agents used for posttransplant maintenance immunosuppression (mycophenolate mofetil, cyclosporine, tacrolimus, and sirolimus) have antiretroviral properties.3
HIV patients in the US must meet the following criteria to be listed for a transplant:
• Diagnosis of ESRD with at least a five-year life-expectancy
• CD4 count of > 200 cells/ μL for at least six months
• Undetectable HIV viremia (< 50 HIV-1 RNA copies/mL)
• Demonstrated adherence to stable antiviral regimen for at least six months
• Absence of AIDS-defining illness following successful immune reconstitution6
A prospective trial of 150 patients in 19 US transplant centers who met the above criteria demonstrated patient survival and graft survival rates comparable to those in patients ages 65 and older.6
While awaiting the donation, HIV patients can continue hemodialysis and peritoneal dialysis. With the improved antiviral drugs, HIV patients have a survival rate similar to the non–HIV-infected population.
Transplantation is the goal and certainly the hope of many advanced-stage kidney patients, but in reality, the need far exceeds the resources. The HOPE Act opens the door for many patients who were previously excluded from the possibility of a life without dialysis. Taking care of these patients will be a team effort, encompassing HIV and infectious disease specialists, pharmacists, nephrologists, transplant surgeons and coordinators, and primary care providers—including, of course, advanced practitioners.
Shelly Levinstein, MSN, CRNP
Nephrology Associates of York
York, PA
REFERENCES
1. Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA. 2014;311(6):579-586.
2. CDC. HIV in the United States: at a glance (2013). www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed June 16, 2014.
3. Frassetto LA, Tan-Tam C, Stock PG. Renal transplantation in patients with HIV. Nat Rev Nephrol. 2009;5(10):582-589.
4. Malani PN. New law allows organ transplants from deceased HIV-infected donors to HIV-infected recipients. JAMA. 2013;310(23): 2492-2493.
5. Muller E, Kahn D, Mendelson M. Renal transplantation between HIV-positive donors and recipients. N Engl J Med. 2010;362(24):2336-2337.
6. Mariani LH, Berns JS. Viral nephropathies. In: Gilbert SJ, Weiner DE, eds. National Kidney Foundation’s Primer on Kidney Diseases. 6th ed. Elsevier; 2014:253-261.