User login
Bone Health in Kidney Disease
Q) What are the current recommendations for the use of DXA and bisphosphonates in patients with chronic kidney disease and end-stage renal disease?
For patients with kidney disease, mineral and bone disorder (MBD) is a common complication, affecting the majority of those with moderate to severe chronic kidney disease (CKD; see Table 1).1,2 CKD-MBD is a systemic disorder that encompasses abnormalities in mineral metabolism, skeletal health, and soft-tissue calcifications.1,2 It manifests as one or more of the following:
- Abnormalities of calcium, phosphorus, parathyroid hormone (PTH), or vitamin D metabolism
- Abnormalities in bone turnover, mineralization, volume, linear growth, or strength
- Vascular or other soft-tissue calcification.2
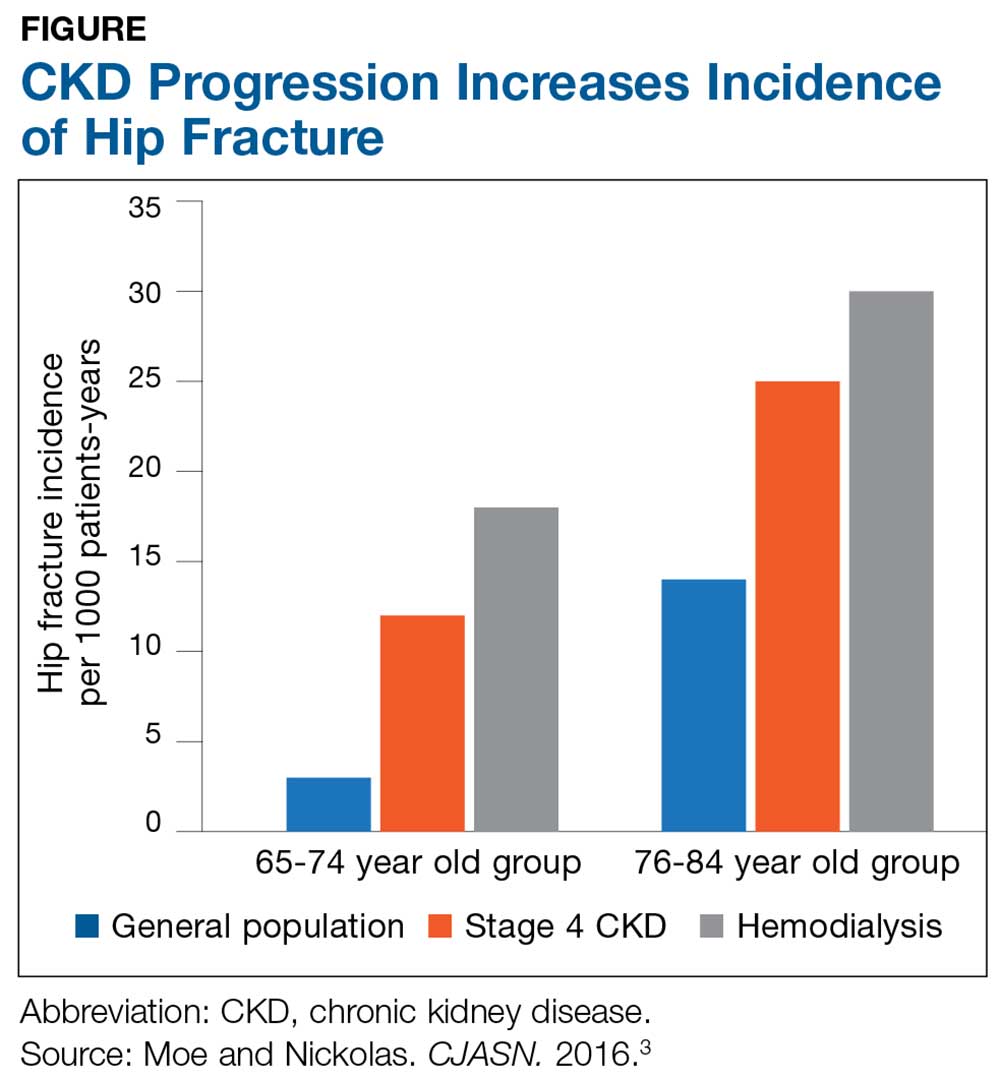
The Figure provides an illustration of the effect of CKD on bone health: In the general population, risk for hip fracture increases with age; risk is further exacerbated in those who have CKD.3
To assess for fracture risk in patients with advanced stages of CKD (3-5) who have evidence of CKD-MBD and/or risk factors for osteoporosis, the Kidney Disease: Improving Global Outcomes (KDIGO) group recommends bone mineral density testing with dual-energy X-ray absorptiometry (DXA).2 Bone biopsy—the gold standard for diagnosis of renal osteodystrophy, a form of osteoporosis and one type of bone abnormality seen in CKD-MBD—is “reasonable” to perform in cases in which knowing the type of renal osteodystrophy would inform treatment choices.2 KDIGO also recognizes limitations in the ability to perform a bone biopsy and therefore recommends monitoring serial PTH and bone-specific alkaline phosphatase to evaluate for bone disease.2
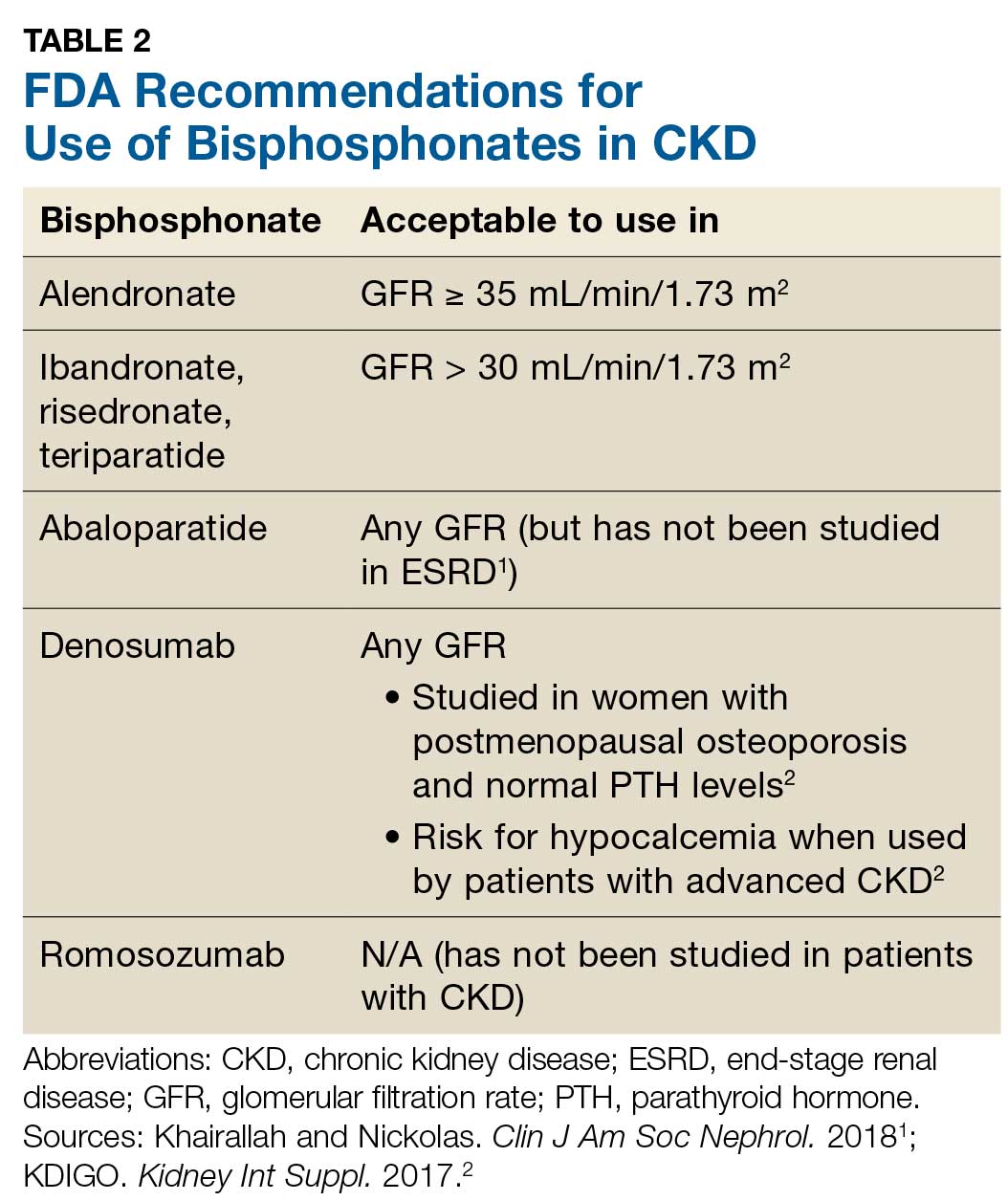
Prevention of fractures and treatment of patients with CKD-MDB has historically been challenging, since many of the available pharmacologic agents have not been developed for or studied in patients with CKD.1 According to KDIGO, it is acceptable for patients with CKD stages 1 and 2 to receive the same osteoporosis/fracture risk management as recommended for the general population.2 Patients with CKD stages 3a and 3b can also receive treatment as recommended for the general population, as long as the patient’s PTH level is in normal range.2 Table 2 outlines the FDA-approved glomerular filtration rate cutoffs for some bisphosphonates commonly used to treat osteoporosis.
Before initiating treatment for CKD-associated osteoporosis, no matter what the stage, it is important to manage vitamin D deficiency, hyperphosphatemia, and hyperparathyroidism.1 In CKD patients with abnormalities of calcium, phosphorus, PTH, and/or vitamin D, involve the nephrology team to assist in providing MBD care. Different approaches to treatment may include, but are not limited to, adjusting phosphorus binders; using vitamin D supplements or analogs; using calcimimetics; prescribing dialysis; providing dietary education; and addressing medication costs.
1. Khairallah P, Nickolas TL. Management of osteoporosis in CKD. Clin J Am Soc Nephrol. 2018;13(6):962-969.
2. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2017;7:1-59.
3. Moe SM, Nickolas TL. Fractures in patients with CKD: time for action. Clin J Am Soc Nephrol. 2016;11(11):1929-1931.
Q) What are the current recommendations for the use of DXA and bisphosphonates in patients with chronic kidney disease and end-stage renal disease?
For patients with kidney disease, mineral and bone disorder (MBD) is a common complication, affecting the majority of those with moderate to severe chronic kidney disease (CKD; see Table 1).1,2 CKD-MBD is a systemic disorder that encompasses abnormalities in mineral metabolism, skeletal health, and soft-tissue calcifications.1,2 It manifests as one or more of the following:
- Abnormalities of calcium, phosphorus, parathyroid hormone (PTH), or vitamin D metabolism
- Abnormalities in bone turnover, mineralization, volume, linear growth, or strength
- Vascular or other soft-tissue calcification.2
The Figure provides an illustration of the effect of CKD on bone health: In the general population, risk for hip fracture increases with age; risk is further exacerbated in those who have CKD.3
To assess for fracture risk in patients with advanced stages of CKD (3-5) who have evidence of CKD-MBD and/or risk factors for osteoporosis, the Kidney Disease: Improving Global Outcomes (KDIGO) group recommends bone mineral density testing with dual-energy X-ray absorptiometry (DXA).2 Bone biopsy—the gold standard for diagnosis of renal osteodystrophy, a form of osteoporosis and one type of bone abnormality seen in CKD-MBD—is “reasonable” to perform in cases in which knowing the type of renal osteodystrophy would inform treatment choices.2 KDIGO also recognizes limitations in the ability to perform a bone biopsy and therefore recommends monitoring serial PTH and bone-specific alkaline phosphatase to evaluate for bone disease.2
Prevention of fractures and treatment of patients with CKD-MDB has historically been challenging, since many of the available pharmacologic agents have not been developed for or studied in patients with CKD.1 According to KDIGO, it is acceptable for patients with CKD stages 1 and 2 to receive the same osteoporosis/fracture risk management as recommended for the general population.2 Patients with CKD stages 3a and 3b can also receive treatment as recommended for the general population, as long as the patient’s PTH level is in normal range.2 Table 2 outlines the FDA-approved glomerular filtration rate cutoffs for some bisphosphonates commonly used to treat osteoporosis.
Before initiating treatment for CKD-associated osteoporosis, no matter what the stage, it is important to manage vitamin D deficiency, hyperphosphatemia, and hyperparathyroidism.1 In CKD patients with abnormalities of calcium, phosphorus, PTH, and/or vitamin D, involve the nephrology team to assist in providing MBD care. Different approaches to treatment may include, but are not limited to, adjusting phosphorus binders; using vitamin D supplements or analogs; using calcimimetics; prescribing dialysis; providing dietary education; and addressing medication costs.
Q) What are the current recommendations for the use of DXA and bisphosphonates in patients with chronic kidney disease and end-stage renal disease?
For patients with kidney disease, mineral and bone disorder (MBD) is a common complication, affecting the majority of those with moderate to severe chronic kidney disease (CKD; see Table 1).1,2 CKD-MBD is a systemic disorder that encompasses abnormalities in mineral metabolism, skeletal health, and soft-tissue calcifications.1,2 It manifests as one or more of the following:
- Abnormalities of calcium, phosphorus, parathyroid hormone (PTH), or vitamin D metabolism
- Abnormalities in bone turnover, mineralization, volume, linear growth, or strength
- Vascular or other soft-tissue calcification.2
The Figure provides an illustration of the effect of CKD on bone health: In the general population, risk for hip fracture increases with age; risk is further exacerbated in those who have CKD.3
To assess for fracture risk in patients with advanced stages of CKD (3-5) who have evidence of CKD-MBD and/or risk factors for osteoporosis, the Kidney Disease: Improving Global Outcomes (KDIGO) group recommends bone mineral density testing with dual-energy X-ray absorptiometry (DXA).2 Bone biopsy—the gold standard for diagnosis of renal osteodystrophy, a form of osteoporosis and one type of bone abnormality seen in CKD-MBD—is “reasonable” to perform in cases in which knowing the type of renal osteodystrophy would inform treatment choices.2 KDIGO also recognizes limitations in the ability to perform a bone biopsy and therefore recommends monitoring serial PTH and bone-specific alkaline phosphatase to evaluate for bone disease.2
Prevention of fractures and treatment of patients with CKD-MDB has historically been challenging, since many of the available pharmacologic agents have not been developed for or studied in patients with CKD.1 According to KDIGO, it is acceptable for patients with CKD stages 1 and 2 to receive the same osteoporosis/fracture risk management as recommended for the general population.2 Patients with CKD stages 3a and 3b can also receive treatment as recommended for the general population, as long as the patient’s PTH level is in normal range.2 Table 2 outlines the FDA-approved glomerular filtration rate cutoffs for some bisphosphonates commonly used to treat osteoporosis.
Before initiating treatment for CKD-associated osteoporosis, no matter what the stage, it is important to manage vitamin D deficiency, hyperphosphatemia, and hyperparathyroidism.1 In CKD patients with abnormalities of calcium, phosphorus, PTH, and/or vitamin D, involve the nephrology team to assist in providing MBD care. Different approaches to treatment may include, but are not limited to, adjusting phosphorus binders; using vitamin D supplements or analogs; using calcimimetics; prescribing dialysis; providing dietary education; and addressing medication costs.
1. Khairallah P, Nickolas TL. Management of osteoporosis in CKD. Clin J Am Soc Nephrol. 2018;13(6):962-969.
2. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2017;7:1-59.
3. Moe SM, Nickolas TL. Fractures in patients with CKD: time for action. Clin J Am Soc Nephrol. 2016;11(11):1929-1931.
1. Khairallah P, Nickolas TL. Management of osteoporosis in CKD. Clin J Am Soc Nephrol. 2018;13(6):962-969.
2. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2017;7:1-59.
3. Moe SM, Nickolas TL. Fractures in patients with CKD: time for action. Clin J Am Soc Nephrol. 2016;11(11):1929-1931.
Screening for Endocrine Hypertension
Hypertension is one of the most common reasons for patient visits.1 According to the US Preventive Services Task Force, more than 70 million individuals older than 20 have hypertension, which is defined as a blood pressure (BP) of ≥ 130/85 mm Hg.2 Essential hypertension is the most common form of this condition; most affected patients will show improvement with evidence-based pharmacologic treatment, lifestyle modifications, and risk factor reductions.
For patients with refractory hypertension, however, identifying what steps to take in screening and diagnosis can be daunting for clinicians. It is important to identify cases of secondary hypertension, because if it is left undiagnosed and untreated, serious complications—such as cardiovascular and renal disease—are likely to occur.3,4
Secondary hypertension can be caused by myriad disease states and disorders, including endocrine disorders, renal disease, neurologic disorders, acute stress, and drug-induced hypertension.5 Endocrine hypertension is most commonly caused by adrenal gland disorders, including primary aldosteronism, Cushing syndrome, and pheochromocytoma. (Of note, Cushing syndrome is caused by glucocorticoid-secreting adrenal tumors, while Cushing disease is a condition in which there is glucocorticoid excess caused by oversecretion of pituitary adrenocorticotropic hormone.6 Cushing disease is more common than Cushing syndrome, which is rare.7) While nonadrenal endocrine disorders are not as common, they pose significant health issues, including growth hormone excess or deficiency, thyroid disorders, testosterone deficiency, obesity, insulin resistance, and metabolic syndrome.8
Understanding the endocrine causes of hypertension is a valuable resource for clinicians to have in their toolbox. Although the negative consequences of endocrine disorders are significant, these conditions are often recognizable, and pharmacologic treatment and/or surgical interventions can potentially resolve or improve hypertension and reduce risk for other comorbidities. This article summarizes screening and diagnosis guidelines for several possible causes of endocrine hypertension: primary aldosteronism, Cushing syndrome, and pheochromocytoma.
PRIMARY ALDOSTERONISM
Primary aldosteronism occurs in 5% to 10% of all hypertensive patients and is a common cause of secondary and endocrine hypertension (although in younger—particularly female—patients, it most commonly causes renal artery stenosis).9,10 Historically, primary aldosteronism was considered rare and not generally included in a differential diagnosis for patients presenting with resistant hypertension. However, clinical investigations have indicated that primary aldosteronism is more prevalent than previously thought.11
Patients develop this condition when there is increased aldosterone production independent of the renin-angiotensin system. The resulting sodium retention can lead to hypertension, hypokalemia, and high plasma aldosterone/renin ratio (ARR).12 Clinical findings and symptoms can be vague, increasing the difficulty in identifying primary aldosteronism as the diagnosis. Patients may be asymptomatic, with the only abnormal lab finding being hypokalemia (an infrequent finding, affecting < 25% of patients).13 If hypokalemia is present, symptoms can include nocturia, polyuria, muscle weakness, cramps, paresthesias, and palpitations.11
The Endocrine Society has identified 8 characteristics that increase the likelihood of primary aldosteronism. Patients require further screening if they
- Have a sustained elevated BP (≥ 150 mm Hg [systolic] and/or 100 mm Hg [diastolic])
- Have hypertension (BP > 140/90 mm Hg) that is resistant to 3 conventional antihypertensive drugs, including a diuretic
- Have controlled BP (BP < 140/90 mm Hg) with ≥ 4 antihypertensive drugs
- Have hypertension and spontaneous or diuretic-induced hypokalemia
- Have hypertension and adrenal incidentaloma
- Have hypertension and obstructive sleep apnea
- Have hypertension and a family history of early-onset hypertension or a cerebrovascular accident at a young age (< 40 years)
- Are hypertensive and a first-degree relative of a patient with primary aldosteronism.14
Continue to: The most reliable screening test...
The most reliable screening test for primary aldosterone is the ARR, although false-negative and false-positive results are possible.11 False-negative results can be caused by dietary salt restriction, hypokalemia, and use of medications including diuretics, calcium channel blockers, ACE inhibitors, and angiotensin receptor antagonists. Use of ß-adrenergic blockers, α-methyldopa, or NSAIDs can cause false-positive results.15 Patients should be encouraged to follow a liberal sodium diet before ARR testing, and efforts to correct hypokalemia should be implemented. Before ARR is measured, diuretics (specifically spironolactone) should be stopped for at least 4 weeks; other possible interfering medications should be stopped for at least 2 weeks.16
The ARR should be obtained multiple times to confirm elevated readings.16 Reference ranges vary, but generally plasma aldosterone concentrations > 20 ng/dL and plasma renin activity < 1 ng/mL/h indicate whether confirmatory testing should be completed.14 Further confirmatory testing can be achieved with efforts to suppress plasma aldosterone to < 10 ng/dL after an IV infusion of 2 L isotonic saline over 4 hours.12 Oral sodium load is used as well and usually before IV infusion.
CUSHING SYNDROME
Cushing syndrome is caused by excess circulating levels of glucocorticoids and affects < 0.1% of the world population.17 Signs and symptoms include centripetal obesity, moon facies, facial plethora, easy bruising, buffalo hump (or posterior cervical fat pad), hirsutism, and wide-purple striae.18 Up to 80% of these patients also have hypertension.19 If these patients have chronic exposure to high levels of glucocorticoid (the most common source being therapeutic administration of exogenous glucocorticoids), multiple complications can occur.6,20
The Endocrine Society Clinical Practice Guideline recommends the following patient groups be tested for Cushing syndrome:
- Young patients with unusual medical conditions, such as osteoporosis and resistant hypertension
- Patients with classic signs and symptoms, such as easy bruising, weight gain, facial plethora, and purple striae
- Children with decreasing height percentile and increasing weight
- Patients with adrenal incidentaloma compatible with adenoma.18
If Cushing syndrome is suspected, 1 of the following 3 initial tests can be completed: 24-hour, urine-free cortisol and creatinine; late-night salivary cortisol; or 1-mg overnight dexamethasone suppression test. Two of these tests must have abnormal results for confirmation before appropriate pituitary or adrenal imaging. If a patient has clinical features indicating Cushing syndrome but test results are normal, he or she should be referred to an endocrinologist. If a patient has ≥ 2 normal tests and probability of Cushing syndrome is unlikely, patients should be recommended for follow-up in 6 months to evaluate for any worsening of symptoms.18
Continue to: PHEOCHROMOCYTOMA
PHEOCHROMOCYTOMA
Pheochromocytoma is a condition in which there is secretion of excess catecholamines, epinephrine, norepinephrine, and dopamine due to a tumor of the adrenal medulla.21 This is a rare disease and accounts for only 0.2% to 0.6% of all causes of hypertension.22 Hypertension (persistent or paroxysmal) is the most common finding for patients with pheochromocytoma, with 80% to 90% presenting with this finding.23 It is important to note that approximately 10% of these patients will be normotensive. Three of the condition’s classic symptoms are headache, sweating, and palpitations.24 Additional symptoms include anxiety, sense of impending doom, fever, nausea, or vomiting.21
If left untreated, there is risk for hypertensive retinopathy, nephropathy, myocardial infarction, stroke from cerebral infarction, intracranial hemorrhage, or embolism.25 Due to the high rate of morbidity and mortality with untreated pheochromocytoma, laboratory testing should be initiated immediately upon suspicion of this diagnosis or if the patient has relevant family history.11
Patients should be screened for pheochromocytoma if they have ≥ 1 of the following factors:
- Resistant hypertension and hyperadrenergic symptoms (palpitations, perspiration, pallor, or headache)
- Family history of pheochromocytoma
- Any genetic syndrome with a known association to pheochromocytoma
- An adrenal mass that is > 4 cm, is cystic, or has hemorrhagic changes.19
Pheochromocytoma is diagnosed by identifying high concentrations of plasma-free metanephrines or 24-hour fractionated metanephrines and catecholamines. Some medications can interfere with the accuracy of lab results and therefore may need to be temporarily stopped; it is important to check the specific lab guidelines and review the patient’s medication lists before tests are ordered and conducted.25
ALWAYS SCREEN THE PATIENT
Although the causes of endocrine-related hypertension are very rare, screening for endocrine hypertension in patients who present with signs and symptoms of these conditions can greatly improve their lives. The endocrine disorders discussed in this article can be treated or controlled with appropriate diagnosis and treatment. In addition, resolving uncontrolled hypertension by addressing endocrine disorders can reduce the risk for long-term sequelae. It is important for clinicians to consider referral to an endocrine specialist if a patient has endocrine-related hypertension. In particular, patients with pheochromocytoma require quick referral due to a risk for high morbidity and mortality if left untreated.11
1. Smith MA, Schrager S, WinklerPrins V. Essentials of Family Medicine. 7th ed. Baltimore, MD: Lipincott Williams & Wilkins; 2019.
2. US Preventive Services Task Force. High blood pressure in adults: screening [final recommendation statement]. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed May 20, 2019.
3. Puar T, Mok Y, Debajyoti R, et al. Secondary hypertension in adults. Singapore Med J. 2016;57:228-232.
4. Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet. 2015;386:801-812.
5. Faselis C, Doumas M, Papademetriou V. Common secondary causes of resistant hypertension and rational for treatment. Int J Hypertens. 2010;2011: doi: 10.4061/2011/236239.
6. Else T, Hammer GD. Disorders of the Adrenal Cortex. In: Hammer GD, McPhee SJ, eds. Pathophysiology of Disease: An Introduction to Clinical Medicine. 8th ed. New York, NY: McGraw-Hill; 2014.
7. Nieman L, Swearingen B; the Pituitary Society. Cushing’s syndrome and Cushing’s disease: your questions answered. www.pituitarysociety.org/sites/all/pdfs/Pituitary_Society_Cushings_brochure.pdf. Accessed May 20, 2019.
8. Koch, C. Chrousos, G. Overview of endocrine hypertension. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. South Dartmouth, MA: MDText.com; 2016.
9. Barlow M, Abdel-Latif A. The forgotten cause of hypertension: a case report and literature review of the prevalence, diagnosis and management of primary aldosteronism. Case Rep Intern Med. 2018;5:4-7.
10. Viera A, Neutze D. Diagnosis of secondary hypertension: an age-based approach. Am Fam Physician. 2010;82:1471-1478.
11. Young WF, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society scientific statement. Endocr Rev. 2017;38:103-122.
12. Kotchen TA. Hypertensive vascular disease. In: Jameson JL, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 20th ed. New York, NY: McGraw-Hill Education; 2018.
13. Rossi GP, Bernini G, Caliumi C, et al; PAPY Study Investigators. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293-2300.
14. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889-1916.
15. Stowasser M, Taylor PJ, Pimenta E, et al. Laboratory investigation of primary aldosteronism. Clin Biochem Rev. 2010;31:39-56.
16. Stowasser M, Gordon RD. The aldosterone-renin ratio for screening for primary aldosteronism. Endocrinologist. 2004;14:267-276.
17. Newell-Price J, Bertagna X, Grossman AB, et al. Cushing’s syndrome. Lancet. 2006;367:1605-1617.
18. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93: 1526-1540.
19. Rimoldi S, Scherrer U, Messerli F. Secondary arterial hypertension: when, who, and how to screen? Eur Heart J. 2014;35:1245-1254.
20. Kirk L, Hash R, Harold K. Cushing’s syndrome and Cushing’s disease. Am Fam Physician. 2000;62:1133-1134.
21. Thomas RM, Ruel E, Shantavasinkul PC. Endocrine hypertension: an overview on the current etiopathogenesis and management options. World J Hypertens. 2015;5:14-27.
22. Ariton M, Juan CS, AvRuskin TW. Pheochromocytoma: clinical observations from a Brooklyn tertiary hospital. Endocr Pract. 2000;6:249-252.
23. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403-1419.
24. Lenders JW, Eisenhofer G, Mannelli M, et al. Pheochromocytoma. Lancet. 2005;366:665-675.
25. Fishbein L, Else T. Disorders of the adrenal medulla. In: Hammer GD, McPhee SJ, eds. Pathophysiology of Disease: An Introduction to Clinical Medicine. 8th ed. New York, NY: McGraw-Hill; 2014.
Hypertension is one of the most common reasons for patient visits.1 According to the US Preventive Services Task Force, more than 70 million individuals older than 20 have hypertension, which is defined as a blood pressure (BP) of ≥ 130/85 mm Hg.2 Essential hypertension is the most common form of this condition; most affected patients will show improvement with evidence-based pharmacologic treatment, lifestyle modifications, and risk factor reductions.
For patients with refractory hypertension, however, identifying what steps to take in screening and diagnosis can be daunting for clinicians. It is important to identify cases of secondary hypertension, because if it is left undiagnosed and untreated, serious complications—such as cardiovascular and renal disease—are likely to occur.3,4
Secondary hypertension can be caused by myriad disease states and disorders, including endocrine disorders, renal disease, neurologic disorders, acute stress, and drug-induced hypertension.5 Endocrine hypertension is most commonly caused by adrenal gland disorders, including primary aldosteronism, Cushing syndrome, and pheochromocytoma. (Of note, Cushing syndrome is caused by glucocorticoid-secreting adrenal tumors, while Cushing disease is a condition in which there is glucocorticoid excess caused by oversecretion of pituitary adrenocorticotropic hormone.6 Cushing disease is more common than Cushing syndrome, which is rare.7) While nonadrenal endocrine disorders are not as common, they pose significant health issues, including growth hormone excess or deficiency, thyroid disorders, testosterone deficiency, obesity, insulin resistance, and metabolic syndrome.8
Understanding the endocrine causes of hypertension is a valuable resource for clinicians to have in their toolbox. Although the negative consequences of endocrine disorders are significant, these conditions are often recognizable, and pharmacologic treatment and/or surgical interventions can potentially resolve or improve hypertension and reduce risk for other comorbidities. This article summarizes screening and diagnosis guidelines for several possible causes of endocrine hypertension: primary aldosteronism, Cushing syndrome, and pheochromocytoma.
PRIMARY ALDOSTERONISM
Primary aldosteronism occurs in 5% to 10% of all hypertensive patients and is a common cause of secondary and endocrine hypertension (although in younger—particularly female—patients, it most commonly causes renal artery stenosis).9,10 Historically, primary aldosteronism was considered rare and not generally included in a differential diagnosis for patients presenting with resistant hypertension. However, clinical investigations have indicated that primary aldosteronism is more prevalent than previously thought.11
Patients develop this condition when there is increased aldosterone production independent of the renin-angiotensin system. The resulting sodium retention can lead to hypertension, hypokalemia, and high plasma aldosterone/renin ratio (ARR).12 Clinical findings and symptoms can be vague, increasing the difficulty in identifying primary aldosteronism as the diagnosis. Patients may be asymptomatic, with the only abnormal lab finding being hypokalemia (an infrequent finding, affecting < 25% of patients).13 If hypokalemia is present, symptoms can include nocturia, polyuria, muscle weakness, cramps, paresthesias, and palpitations.11
The Endocrine Society has identified 8 characteristics that increase the likelihood of primary aldosteronism. Patients require further screening if they
- Have a sustained elevated BP (≥ 150 mm Hg [systolic] and/or 100 mm Hg [diastolic])
- Have hypertension (BP > 140/90 mm Hg) that is resistant to 3 conventional antihypertensive drugs, including a diuretic
- Have controlled BP (BP < 140/90 mm Hg) with ≥ 4 antihypertensive drugs
- Have hypertension and spontaneous or diuretic-induced hypokalemia
- Have hypertension and adrenal incidentaloma
- Have hypertension and obstructive sleep apnea
- Have hypertension and a family history of early-onset hypertension or a cerebrovascular accident at a young age (< 40 years)
- Are hypertensive and a first-degree relative of a patient with primary aldosteronism.14
Continue to: The most reliable screening test...
The most reliable screening test for primary aldosterone is the ARR, although false-negative and false-positive results are possible.11 False-negative results can be caused by dietary salt restriction, hypokalemia, and use of medications including diuretics, calcium channel blockers, ACE inhibitors, and angiotensin receptor antagonists. Use of ß-adrenergic blockers, α-methyldopa, or NSAIDs can cause false-positive results.15 Patients should be encouraged to follow a liberal sodium diet before ARR testing, and efforts to correct hypokalemia should be implemented. Before ARR is measured, diuretics (specifically spironolactone) should be stopped for at least 4 weeks; other possible interfering medications should be stopped for at least 2 weeks.16
The ARR should be obtained multiple times to confirm elevated readings.16 Reference ranges vary, but generally plasma aldosterone concentrations > 20 ng/dL and plasma renin activity < 1 ng/mL/h indicate whether confirmatory testing should be completed.14 Further confirmatory testing can be achieved with efforts to suppress plasma aldosterone to < 10 ng/dL after an IV infusion of 2 L isotonic saline over 4 hours.12 Oral sodium load is used as well and usually before IV infusion.
CUSHING SYNDROME
Cushing syndrome is caused by excess circulating levels of glucocorticoids and affects < 0.1% of the world population.17 Signs and symptoms include centripetal obesity, moon facies, facial plethora, easy bruising, buffalo hump (or posterior cervical fat pad), hirsutism, and wide-purple striae.18 Up to 80% of these patients also have hypertension.19 If these patients have chronic exposure to high levels of glucocorticoid (the most common source being therapeutic administration of exogenous glucocorticoids), multiple complications can occur.6,20
The Endocrine Society Clinical Practice Guideline recommends the following patient groups be tested for Cushing syndrome:
- Young patients with unusual medical conditions, such as osteoporosis and resistant hypertension
- Patients with classic signs and symptoms, such as easy bruising, weight gain, facial plethora, and purple striae
- Children with decreasing height percentile and increasing weight
- Patients with adrenal incidentaloma compatible with adenoma.18
If Cushing syndrome is suspected, 1 of the following 3 initial tests can be completed: 24-hour, urine-free cortisol and creatinine; late-night salivary cortisol; or 1-mg overnight dexamethasone suppression test. Two of these tests must have abnormal results for confirmation before appropriate pituitary or adrenal imaging. If a patient has clinical features indicating Cushing syndrome but test results are normal, he or she should be referred to an endocrinologist. If a patient has ≥ 2 normal tests and probability of Cushing syndrome is unlikely, patients should be recommended for follow-up in 6 months to evaluate for any worsening of symptoms.18
Continue to: PHEOCHROMOCYTOMA
PHEOCHROMOCYTOMA
Pheochromocytoma is a condition in which there is secretion of excess catecholamines, epinephrine, norepinephrine, and dopamine due to a tumor of the adrenal medulla.21 This is a rare disease and accounts for only 0.2% to 0.6% of all causes of hypertension.22 Hypertension (persistent or paroxysmal) is the most common finding for patients with pheochromocytoma, with 80% to 90% presenting with this finding.23 It is important to note that approximately 10% of these patients will be normotensive. Three of the condition’s classic symptoms are headache, sweating, and palpitations.24 Additional symptoms include anxiety, sense of impending doom, fever, nausea, or vomiting.21
If left untreated, there is risk for hypertensive retinopathy, nephropathy, myocardial infarction, stroke from cerebral infarction, intracranial hemorrhage, or embolism.25 Due to the high rate of morbidity and mortality with untreated pheochromocytoma, laboratory testing should be initiated immediately upon suspicion of this diagnosis or if the patient has relevant family history.11
Patients should be screened for pheochromocytoma if they have ≥ 1 of the following factors:
- Resistant hypertension and hyperadrenergic symptoms (palpitations, perspiration, pallor, or headache)
- Family history of pheochromocytoma
- Any genetic syndrome with a known association to pheochromocytoma
- An adrenal mass that is > 4 cm, is cystic, or has hemorrhagic changes.19
Pheochromocytoma is diagnosed by identifying high concentrations of plasma-free metanephrines or 24-hour fractionated metanephrines and catecholamines. Some medications can interfere with the accuracy of lab results and therefore may need to be temporarily stopped; it is important to check the specific lab guidelines and review the patient’s medication lists before tests are ordered and conducted.25
ALWAYS SCREEN THE PATIENT
Although the causes of endocrine-related hypertension are very rare, screening for endocrine hypertension in patients who present with signs and symptoms of these conditions can greatly improve their lives. The endocrine disorders discussed in this article can be treated or controlled with appropriate diagnosis and treatment. In addition, resolving uncontrolled hypertension by addressing endocrine disorders can reduce the risk for long-term sequelae. It is important for clinicians to consider referral to an endocrine specialist if a patient has endocrine-related hypertension. In particular, patients with pheochromocytoma require quick referral due to a risk for high morbidity and mortality if left untreated.11
Hypertension is one of the most common reasons for patient visits.1 According to the US Preventive Services Task Force, more than 70 million individuals older than 20 have hypertension, which is defined as a blood pressure (BP) of ≥ 130/85 mm Hg.2 Essential hypertension is the most common form of this condition; most affected patients will show improvement with evidence-based pharmacologic treatment, lifestyle modifications, and risk factor reductions.
For patients with refractory hypertension, however, identifying what steps to take in screening and diagnosis can be daunting for clinicians. It is important to identify cases of secondary hypertension, because if it is left undiagnosed and untreated, serious complications—such as cardiovascular and renal disease—are likely to occur.3,4
Secondary hypertension can be caused by myriad disease states and disorders, including endocrine disorders, renal disease, neurologic disorders, acute stress, and drug-induced hypertension.5 Endocrine hypertension is most commonly caused by adrenal gland disorders, including primary aldosteronism, Cushing syndrome, and pheochromocytoma. (Of note, Cushing syndrome is caused by glucocorticoid-secreting adrenal tumors, while Cushing disease is a condition in which there is glucocorticoid excess caused by oversecretion of pituitary adrenocorticotropic hormone.6 Cushing disease is more common than Cushing syndrome, which is rare.7) While nonadrenal endocrine disorders are not as common, they pose significant health issues, including growth hormone excess or deficiency, thyroid disorders, testosterone deficiency, obesity, insulin resistance, and metabolic syndrome.8
Understanding the endocrine causes of hypertension is a valuable resource for clinicians to have in their toolbox. Although the negative consequences of endocrine disorders are significant, these conditions are often recognizable, and pharmacologic treatment and/or surgical interventions can potentially resolve or improve hypertension and reduce risk for other comorbidities. This article summarizes screening and diagnosis guidelines for several possible causes of endocrine hypertension: primary aldosteronism, Cushing syndrome, and pheochromocytoma.
PRIMARY ALDOSTERONISM
Primary aldosteronism occurs in 5% to 10% of all hypertensive patients and is a common cause of secondary and endocrine hypertension (although in younger—particularly female—patients, it most commonly causes renal artery stenosis).9,10 Historically, primary aldosteronism was considered rare and not generally included in a differential diagnosis for patients presenting with resistant hypertension. However, clinical investigations have indicated that primary aldosteronism is more prevalent than previously thought.11
Patients develop this condition when there is increased aldosterone production independent of the renin-angiotensin system. The resulting sodium retention can lead to hypertension, hypokalemia, and high plasma aldosterone/renin ratio (ARR).12 Clinical findings and symptoms can be vague, increasing the difficulty in identifying primary aldosteronism as the diagnosis. Patients may be asymptomatic, with the only abnormal lab finding being hypokalemia (an infrequent finding, affecting < 25% of patients).13 If hypokalemia is present, symptoms can include nocturia, polyuria, muscle weakness, cramps, paresthesias, and palpitations.11
The Endocrine Society has identified 8 characteristics that increase the likelihood of primary aldosteronism. Patients require further screening if they
- Have a sustained elevated BP (≥ 150 mm Hg [systolic] and/or 100 mm Hg [diastolic])
- Have hypertension (BP > 140/90 mm Hg) that is resistant to 3 conventional antihypertensive drugs, including a diuretic
- Have controlled BP (BP < 140/90 mm Hg) with ≥ 4 antihypertensive drugs
- Have hypertension and spontaneous or diuretic-induced hypokalemia
- Have hypertension and adrenal incidentaloma
- Have hypertension and obstructive sleep apnea
- Have hypertension and a family history of early-onset hypertension or a cerebrovascular accident at a young age (< 40 years)
- Are hypertensive and a first-degree relative of a patient with primary aldosteronism.14
Continue to: The most reliable screening test...
The most reliable screening test for primary aldosterone is the ARR, although false-negative and false-positive results are possible.11 False-negative results can be caused by dietary salt restriction, hypokalemia, and use of medications including diuretics, calcium channel blockers, ACE inhibitors, and angiotensin receptor antagonists. Use of ß-adrenergic blockers, α-methyldopa, or NSAIDs can cause false-positive results.15 Patients should be encouraged to follow a liberal sodium diet before ARR testing, and efforts to correct hypokalemia should be implemented. Before ARR is measured, diuretics (specifically spironolactone) should be stopped for at least 4 weeks; other possible interfering medications should be stopped for at least 2 weeks.16
The ARR should be obtained multiple times to confirm elevated readings.16 Reference ranges vary, but generally plasma aldosterone concentrations > 20 ng/dL and plasma renin activity < 1 ng/mL/h indicate whether confirmatory testing should be completed.14 Further confirmatory testing can be achieved with efforts to suppress plasma aldosterone to < 10 ng/dL after an IV infusion of 2 L isotonic saline over 4 hours.12 Oral sodium load is used as well and usually before IV infusion.
CUSHING SYNDROME
Cushing syndrome is caused by excess circulating levels of glucocorticoids and affects < 0.1% of the world population.17 Signs and symptoms include centripetal obesity, moon facies, facial plethora, easy bruising, buffalo hump (or posterior cervical fat pad), hirsutism, and wide-purple striae.18 Up to 80% of these patients also have hypertension.19 If these patients have chronic exposure to high levels of glucocorticoid (the most common source being therapeutic administration of exogenous glucocorticoids), multiple complications can occur.6,20
The Endocrine Society Clinical Practice Guideline recommends the following patient groups be tested for Cushing syndrome:
- Young patients with unusual medical conditions, such as osteoporosis and resistant hypertension
- Patients with classic signs and symptoms, such as easy bruising, weight gain, facial plethora, and purple striae
- Children with decreasing height percentile and increasing weight
- Patients with adrenal incidentaloma compatible with adenoma.18
If Cushing syndrome is suspected, 1 of the following 3 initial tests can be completed: 24-hour, urine-free cortisol and creatinine; late-night salivary cortisol; or 1-mg overnight dexamethasone suppression test. Two of these tests must have abnormal results for confirmation before appropriate pituitary or adrenal imaging. If a patient has clinical features indicating Cushing syndrome but test results are normal, he or she should be referred to an endocrinologist. If a patient has ≥ 2 normal tests and probability of Cushing syndrome is unlikely, patients should be recommended for follow-up in 6 months to evaluate for any worsening of symptoms.18
Continue to: PHEOCHROMOCYTOMA
PHEOCHROMOCYTOMA
Pheochromocytoma is a condition in which there is secretion of excess catecholamines, epinephrine, norepinephrine, and dopamine due to a tumor of the adrenal medulla.21 This is a rare disease and accounts for only 0.2% to 0.6% of all causes of hypertension.22 Hypertension (persistent or paroxysmal) is the most common finding for patients with pheochromocytoma, with 80% to 90% presenting with this finding.23 It is important to note that approximately 10% of these patients will be normotensive. Three of the condition’s classic symptoms are headache, sweating, and palpitations.24 Additional symptoms include anxiety, sense of impending doom, fever, nausea, or vomiting.21
If left untreated, there is risk for hypertensive retinopathy, nephropathy, myocardial infarction, stroke from cerebral infarction, intracranial hemorrhage, or embolism.25 Due to the high rate of morbidity and mortality with untreated pheochromocytoma, laboratory testing should be initiated immediately upon suspicion of this diagnosis or if the patient has relevant family history.11
Patients should be screened for pheochromocytoma if they have ≥ 1 of the following factors:
- Resistant hypertension and hyperadrenergic symptoms (palpitations, perspiration, pallor, or headache)
- Family history of pheochromocytoma
- Any genetic syndrome with a known association to pheochromocytoma
- An adrenal mass that is > 4 cm, is cystic, or has hemorrhagic changes.19
Pheochromocytoma is diagnosed by identifying high concentrations of plasma-free metanephrines or 24-hour fractionated metanephrines and catecholamines. Some medications can interfere with the accuracy of lab results and therefore may need to be temporarily stopped; it is important to check the specific lab guidelines and review the patient’s medication lists before tests are ordered and conducted.25
ALWAYS SCREEN THE PATIENT
Although the causes of endocrine-related hypertension are very rare, screening for endocrine hypertension in patients who present with signs and symptoms of these conditions can greatly improve their lives. The endocrine disorders discussed in this article can be treated or controlled with appropriate diagnosis and treatment. In addition, resolving uncontrolled hypertension by addressing endocrine disorders can reduce the risk for long-term sequelae. It is important for clinicians to consider referral to an endocrine specialist if a patient has endocrine-related hypertension. In particular, patients with pheochromocytoma require quick referral due to a risk for high morbidity and mortality if left untreated.11
1. Smith MA, Schrager S, WinklerPrins V. Essentials of Family Medicine. 7th ed. Baltimore, MD: Lipincott Williams & Wilkins; 2019.
2. US Preventive Services Task Force. High blood pressure in adults: screening [final recommendation statement]. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed May 20, 2019.
3. Puar T, Mok Y, Debajyoti R, et al. Secondary hypertension in adults. Singapore Med J. 2016;57:228-232.
4. Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet. 2015;386:801-812.
5. Faselis C, Doumas M, Papademetriou V. Common secondary causes of resistant hypertension and rational for treatment. Int J Hypertens. 2010;2011: doi: 10.4061/2011/236239.
6. Else T, Hammer GD. Disorders of the Adrenal Cortex. In: Hammer GD, McPhee SJ, eds. Pathophysiology of Disease: An Introduction to Clinical Medicine. 8th ed. New York, NY: McGraw-Hill; 2014.
7. Nieman L, Swearingen B; the Pituitary Society. Cushing’s syndrome and Cushing’s disease: your questions answered. www.pituitarysociety.org/sites/all/pdfs/Pituitary_Society_Cushings_brochure.pdf. Accessed May 20, 2019.
8. Koch, C. Chrousos, G. Overview of endocrine hypertension. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. South Dartmouth, MA: MDText.com; 2016.
9. Barlow M, Abdel-Latif A. The forgotten cause of hypertension: a case report and literature review of the prevalence, diagnosis and management of primary aldosteronism. Case Rep Intern Med. 2018;5:4-7.
10. Viera A, Neutze D. Diagnosis of secondary hypertension: an age-based approach. Am Fam Physician. 2010;82:1471-1478.
11. Young WF, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society scientific statement. Endocr Rev. 2017;38:103-122.
12. Kotchen TA. Hypertensive vascular disease. In: Jameson JL, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 20th ed. New York, NY: McGraw-Hill Education; 2018.
13. Rossi GP, Bernini G, Caliumi C, et al; PAPY Study Investigators. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293-2300.
14. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889-1916.
15. Stowasser M, Taylor PJ, Pimenta E, et al. Laboratory investigation of primary aldosteronism. Clin Biochem Rev. 2010;31:39-56.
16. Stowasser M, Gordon RD. The aldosterone-renin ratio for screening for primary aldosteronism. Endocrinologist. 2004;14:267-276.
17. Newell-Price J, Bertagna X, Grossman AB, et al. Cushing’s syndrome. Lancet. 2006;367:1605-1617.
18. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93: 1526-1540.
19. Rimoldi S, Scherrer U, Messerli F. Secondary arterial hypertension: when, who, and how to screen? Eur Heart J. 2014;35:1245-1254.
20. Kirk L, Hash R, Harold K. Cushing’s syndrome and Cushing’s disease. Am Fam Physician. 2000;62:1133-1134.
21. Thomas RM, Ruel E, Shantavasinkul PC. Endocrine hypertension: an overview on the current etiopathogenesis and management options. World J Hypertens. 2015;5:14-27.
22. Ariton M, Juan CS, AvRuskin TW. Pheochromocytoma: clinical observations from a Brooklyn tertiary hospital. Endocr Pract. 2000;6:249-252.
23. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403-1419.
24. Lenders JW, Eisenhofer G, Mannelli M, et al. Pheochromocytoma. Lancet. 2005;366:665-675.
25. Fishbein L, Else T. Disorders of the adrenal medulla. In: Hammer GD, McPhee SJ, eds. Pathophysiology of Disease: An Introduction to Clinical Medicine. 8th ed. New York, NY: McGraw-Hill; 2014.
1. Smith MA, Schrager S, WinklerPrins V. Essentials of Family Medicine. 7th ed. Baltimore, MD: Lipincott Williams & Wilkins; 2019.
2. US Preventive Services Task Force. High blood pressure in adults: screening [final recommendation statement]. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/high-blood-pressure-in-adults-screening. Accessed May 20, 2019.
3. Puar T, Mok Y, Debajyoti R, et al. Secondary hypertension in adults. Singapore Med J. 2016;57:228-232.
4. Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet. 2015;386:801-812.
5. Faselis C, Doumas M, Papademetriou V. Common secondary causes of resistant hypertension and rational for treatment. Int J Hypertens. 2010;2011: doi: 10.4061/2011/236239.
6. Else T, Hammer GD. Disorders of the Adrenal Cortex. In: Hammer GD, McPhee SJ, eds. Pathophysiology of Disease: An Introduction to Clinical Medicine. 8th ed. New York, NY: McGraw-Hill; 2014.
7. Nieman L, Swearingen B; the Pituitary Society. Cushing’s syndrome and Cushing’s disease: your questions answered. www.pituitarysociety.org/sites/all/pdfs/Pituitary_Society_Cushings_brochure.pdf. Accessed May 20, 2019.
8. Koch, C. Chrousos, G. Overview of endocrine hypertension. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. South Dartmouth, MA: MDText.com; 2016.
9. Barlow M, Abdel-Latif A. The forgotten cause of hypertension: a case report and literature review of the prevalence, diagnosis and management of primary aldosteronism. Case Rep Intern Med. 2018;5:4-7.
10. Viera A, Neutze D. Diagnosis of secondary hypertension: an age-based approach. Am Fam Physician. 2010;82:1471-1478.
11. Young WF, Calhoun DA, Lenders JWM, et al. Screening for endocrine hypertension: an Endocrine Society scientific statement. Endocr Rev. 2017;38:103-122.
12. Kotchen TA. Hypertensive vascular disease. In: Jameson JL, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 20th ed. New York, NY: McGraw-Hill Education; 2018.
13. Rossi GP, Bernini G, Caliumi C, et al; PAPY Study Investigators. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293-2300.
14. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889-1916.
15. Stowasser M, Taylor PJ, Pimenta E, et al. Laboratory investigation of primary aldosteronism. Clin Biochem Rev. 2010;31:39-56.
16. Stowasser M, Gordon RD. The aldosterone-renin ratio for screening for primary aldosteronism. Endocrinologist. 2004;14:267-276.
17. Newell-Price J, Bertagna X, Grossman AB, et al. Cushing’s syndrome. Lancet. 2006;367:1605-1617.
18. Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2008;93: 1526-1540.
19. Rimoldi S, Scherrer U, Messerli F. Secondary arterial hypertension: when, who, and how to screen? Eur Heart J. 2014;35:1245-1254.
20. Kirk L, Hash R, Harold K. Cushing’s syndrome and Cushing’s disease. Am Fam Physician. 2000;62:1133-1134.
21. Thomas RM, Ruel E, Shantavasinkul PC. Endocrine hypertension: an overview on the current etiopathogenesis and management options. World J Hypertens. 2015;5:14-27.
22. Ariton M, Juan CS, AvRuskin TW. Pheochromocytoma: clinical observations from a Brooklyn tertiary hospital. Endocr Pract. 2000;6:249-252.
23. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403-1419.
24. Lenders JW, Eisenhofer G, Mannelli M, et al. Pheochromocytoma. Lancet. 2005;366:665-675.
25. Fishbein L, Else T. Disorders of the adrenal medulla. In: Hammer GD, McPhee SJ, eds. Pathophysiology of Disease: An Introduction to Clinical Medicine. 8th ed. New York, NY: McGraw-Hill; 2014.
MS: Partnering With Patients to Improve Health
Sharon, a 19-year-old woman, has a history of right optic neuritis and paraparesis that occurred 2 years ago. At that time, the diagnosis of multiple sclerosis (MS) was confirmed by a brain MRI and lumbar puncture. She has been taking disease-modifying therapy for 2 years and rarely misses a dose. Lately, however, she has experienced worsening symptoms and feels that her MS is progressing. Her neurologist doesn’t agree; he informs her that a recent MRI shows no changes, and her neurologic examination is within normal limits. At his suggestion, she presents to her primary care provider for an annual check-up.
HISTORY & PHYSICAL EXAM
Sharon’s height is 5 ft 2 in and her weight, 170 lb. Her blood pressure is 140/88 mm Hg and pulse, 80 beats/min and regular. Review of systems is remarkable for fatigue, visual changes when she is overheated, and weight gain of about 50 lb during the past year. Her lungs are clear to percussion and auscultation.
Her current medications include oral disease-modifying therapy, which she takes daily; an oral contraceptive (for regulation of her menstrual cycle; she says she is not sexually active); and an occasional pain reliever for headache.
CLINICAL IMPRESSION
Following history-taking and examination, the clinician notes the following impressions about Sharon’s health status:
Obesity: Examination reveals an overweight female with a BMI of 31.1.
Physical inactivity: As a legal secretary, Sharon sits at her desk most of the day. Her exercise is limited to walking to and from the bus to get to work. She has limited time for social activities due to fatigue. She spends most of her time watching television or visiting her parents.
Heat intolerance: While describing her lifestyle, Sharon notes that she does not participate in outdoor activity due to heat intolerance.
Continue to: Ambulation difficulty
Ambulation difficulty: Sharon’s walking and balance are worse than they were 6 months ago—a problem she relates to her MS, not her increased weight. She walks with a wide-based ataxic gait and transfers with difficulty, using the arms of her chair to stand up.
Poor nutritional habits: Sharon reports an irregular diet with an occasional breakfast, a sandwich for lunch, and a microwavable meal for dinner. Between meals, she snacks on nutrition bars, chocolate, and hot and cold coffee.
Smoking: Sharon smokes 1 pack of cigarettes daily.
Headache: As noted, Sharon reports occasional analgesic use for relief of headache pain.
The clinician’s impression is as follows: relapsing MS treated with disease-modifying therapy; obesity; ambulation difficulty; heat intolerance; sedentary lifestyle; and headache. In addition, the patient has the following risk factors: smoking; suboptimal activity and exercise; and poor nutritional habits.
Continue to: DISCUSSION
DISCUSSION
Sharon has relapsing MS treated with disease-modifying therapy. But she also demonstrates or reports several independent risk factors, including borderline hypertension; obesity; inadequate diet; lack of activity and exercise; and possible lack of insight into her disease.1
The plan of care for Sharon should include a review of her MS disease course. As this is explained, it is important to emphasize how adherence to the care plan will yield positive outcomes from the treatment. For example, the patient should understand that the underlying cause of damage in MS is related to the immune system. Providing this education might involve 1 or 2 sessions with written material, simple graphics, and explanation on how disease-modifying therapies work. Even a simple statement such as
The next step is to review Sharon’s risk factors for worsening MS, along with the impact these have on her general health. This might entail a long discussion focusing on the patient’s diet, minimal activity and exercise, and smoking. Sharon’s provider explained how all 3 factors can contribute to poor general health and have been shown to negatively affect MS. There is a general impression that wellness and neurologic diseases such as MS are disconnected. The clinician must “reconnect” the 2 through encouragement, education, and coaching.
By working closely with the patient and providing the education to help her make informed decisions about her health, the clinician can develop a plan to implement that has the patient’s full support. For a patient like Sharon, this includes
- Dietary modifications to improve nutrition and promote healthy weight loss
- A program of daily walking to improve stamina and support the patient’s weight loss program2
- Smoking cessation, including participation in a local support group of former smokers.3
Continue to: In Sharon's case...
In Sharon’s case, both she and her clinician agreed that it was important to meet regularly to assess progress toward their mutually agreed-upon goals. It is not enough to devise a plan—providers need to support patients in their efforts to improve their health. Meeting regularly can motivate patients to stay on track, and it gives providers an opportunity to address problems or concerns that might interfere with the patient’s progress.
1. Dalgas U, Stenager E. Exercise and disease progression in multiple sclerosis: can exercise slow down the progression of multiple sclerosis? Ther Adv Neurol Disord. 2012;5(2):81-95.
2. Gianfrancesco MA, Barcellos LF. Obesity and multiple sclerosis susceptibility: a review. J Neurol Neuromedicine. 2016:1(7):1-5.
3. Healy BC, Eman A, Guttmann CRG, et al. Smoking and disease progression in multiple sclerosis. Arch Neurol. 2009;66(7):858-864.
Sharon, a 19-year-old woman, has a history of right optic neuritis and paraparesis that occurred 2 years ago. At that time, the diagnosis of multiple sclerosis (MS) was confirmed by a brain MRI and lumbar puncture. She has been taking disease-modifying therapy for 2 years and rarely misses a dose. Lately, however, she has experienced worsening symptoms and feels that her MS is progressing. Her neurologist doesn’t agree; he informs her that a recent MRI shows no changes, and her neurologic examination is within normal limits. At his suggestion, she presents to her primary care provider for an annual check-up.
HISTORY & PHYSICAL EXAM
Sharon’s height is 5 ft 2 in and her weight, 170 lb. Her blood pressure is 140/88 mm Hg and pulse, 80 beats/min and regular. Review of systems is remarkable for fatigue, visual changes when she is overheated, and weight gain of about 50 lb during the past year. Her lungs are clear to percussion and auscultation.
Her current medications include oral disease-modifying therapy, which she takes daily; an oral contraceptive (for regulation of her menstrual cycle; she says she is not sexually active); and an occasional pain reliever for headache.
CLINICAL IMPRESSION
Following history-taking and examination, the clinician notes the following impressions about Sharon’s health status:
Obesity: Examination reveals an overweight female with a BMI of 31.1.
Physical inactivity: As a legal secretary, Sharon sits at her desk most of the day. Her exercise is limited to walking to and from the bus to get to work. She has limited time for social activities due to fatigue. She spends most of her time watching television or visiting her parents.
Heat intolerance: While describing her lifestyle, Sharon notes that she does not participate in outdoor activity due to heat intolerance.
Continue to: Ambulation difficulty
Ambulation difficulty: Sharon’s walking and balance are worse than they were 6 months ago—a problem she relates to her MS, not her increased weight. She walks with a wide-based ataxic gait and transfers with difficulty, using the arms of her chair to stand up.
Poor nutritional habits: Sharon reports an irregular diet with an occasional breakfast, a sandwich for lunch, and a microwavable meal for dinner. Between meals, she snacks on nutrition bars, chocolate, and hot and cold coffee.
Smoking: Sharon smokes 1 pack of cigarettes daily.
Headache: As noted, Sharon reports occasional analgesic use for relief of headache pain.
The clinician’s impression is as follows: relapsing MS treated with disease-modifying therapy; obesity; ambulation difficulty; heat intolerance; sedentary lifestyle; and headache. In addition, the patient has the following risk factors: smoking; suboptimal activity and exercise; and poor nutritional habits.
Continue to: DISCUSSION
DISCUSSION
Sharon has relapsing MS treated with disease-modifying therapy. But she also demonstrates or reports several independent risk factors, including borderline hypertension; obesity; inadequate diet; lack of activity and exercise; and possible lack of insight into her disease.1
The plan of care for Sharon should include a review of her MS disease course. As this is explained, it is important to emphasize how adherence to the care plan will yield positive outcomes from the treatment. For example, the patient should understand that the underlying cause of damage in MS is related to the immune system. Providing this education might involve 1 or 2 sessions with written material, simple graphics, and explanation on how disease-modifying therapies work. Even a simple statement such as
The next step is to review Sharon’s risk factors for worsening MS, along with the impact these have on her general health. This might entail a long discussion focusing on the patient’s diet, minimal activity and exercise, and smoking. Sharon’s provider explained how all 3 factors can contribute to poor general health and have been shown to negatively affect MS. There is a general impression that wellness and neurologic diseases such as MS are disconnected. The clinician must “reconnect” the 2 through encouragement, education, and coaching.
By working closely with the patient and providing the education to help her make informed decisions about her health, the clinician can develop a plan to implement that has the patient’s full support. For a patient like Sharon, this includes
- Dietary modifications to improve nutrition and promote healthy weight loss
- A program of daily walking to improve stamina and support the patient’s weight loss program2
- Smoking cessation, including participation in a local support group of former smokers.3
Continue to: In Sharon's case...
In Sharon’s case, both she and her clinician agreed that it was important to meet regularly to assess progress toward their mutually agreed-upon goals. It is not enough to devise a plan—providers need to support patients in their efforts to improve their health. Meeting regularly can motivate patients to stay on track, and it gives providers an opportunity to address problems or concerns that might interfere with the patient’s progress.
Sharon, a 19-year-old woman, has a history of right optic neuritis and paraparesis that occurred 2 years ago. At that time, the diagnosis of multiple sclerosis (MS) was confirmed by a brain MRI and lumbar puncture. She has been taking disease-modifying therapy for 2 years and rarely misses a dose. Lately, however, she has experienced worsening symptoms and feels that her MS is progressing. Her neurologist doesn’t agree; he informs her that a recent MRI shows no changes, and her neurologic examination is within normal limits. At his suggestion, she presents to her primary care provider for an annual check-up.
HISTORY & PHYSICAL EXAM
Sharon’s height is 5 ft 2 in and her weight, 170 lb. Her blood pressure is 140/88 mm Hg and pulse, 80 beats/min and regular. Review of systems is remarkable for fatigue, visual changes when she is overheated, and weight gain of about 50 lb during the past year. Her lungs are clear to percussion and auscultation.
Her current medications include oral disease-modifying therapy, which she takes daily; an oral contraceptive (for regulation of her menstrual cycle; she says she is not sexually active); and an occasional pain reliever for headache.
CLINICAL IMPRESSION
Following history-taking and examination, the clinician notes the following impressions about Sharon’s health status:
Obesity: Examination reveals an overweight female with a BMI of 31.1.
Physical inactivity: As a legal secretary, Sharon sits at her desk most of the day. Her exercise is limited to walking to and from the bus to get to work. She has limited time for social activities due to fatigue. She spends most of her time watching television or visiting her parents.
Heat intolerance: While describing her lifestyle, Sharon notes that she does not participate in outdoor activity due to heat intolerance.
Continue to: Ambulation difficulty
Ambulation difficulty: Sharon’s walking and balance are worse than they were 6 months ago—a problem she relates to her MS, not her increased weight. She walks with a wide-based ataxic gait and transfers with difficulty, using the arms of her chair to stand up.
Poor nutritional habits: Sharon reports an irregular diet with an occasional breakfast, a sandwich for lunch, and a microwavable meal for dinner. Between meals, she snacks on nutrition bars, chocolate, and hot and cold coffee.
Smoking: Sharon smokes 1 pack of cigarettes daily.
Headache: As noted, Sharon reports occasional analgesic use for relief of headache pain.
The clinician’s impression is as follows: relapsing MS treated with disease-modifying therapy; obesity; ambulation difficulty; heat intolerance; sedentary lifestyle; and headache. In addition, the patient has the following risk factors: smoking; suboptimal activity and exercise; and poor nutritional habits.
Continue to: DISCUSSION
DISCUSSION
Sharon has relapsing MS treated with disease-modifying therapy. But she also demonstrates or reports several independent risk factors, including borderline hypertension; obesity; inadequate diet; lack of activity and exercise; and possible lack of insight into her disease.1
The plan of care for Sharon should include a review of her MS disease course. As this is explained, it is important to emphasize how adherence to the care plan will yield positive outcomes from the treatment. For example, the patient should understand that the underlying cause of damage in MS is related to the immune system. Providing this education might involve 1 or 2 sessions with written material, simple graphics, and explanation on how disease-modifying therapies work. Even a simple statement such as
The next step is to review Sharon’s risk factors for worsening MS, along with the impact these have on her general health. This might entail a long discussion focusing on the patient’s diet, minimal activity and exercise, and smoking. Sharon’s provider explained how all 3 factors can contribute to poor general health and have been shown to negatively affect MS. There is a general impression that wellness and neurologic diseases such as MS are disconnected. The clinician must “reconnect” the 2 through encouragement, education, and coaching.
By working closely with the patient and providing the education to help her make informed decisions about her health, the clinician can develop a plan to implement that has the patient’s full support. For a patient like Sharon, this includes
- Dietary modifications to improve nutrition and promote healthy weight loss
- A program of daily walking to improve stamina and support the patient’s weight loss program2
- Smoking cessation, including participation in a local support group of former smokers.3
Continue to: In Sharon's case...
In Sharon’s case, both she and her clinician agreed that it was important to meet regularly to assess progress toward their mutually agreed-upon goals. It is not enough to devise a plan—providers need to support patients in their efforts to improve their health. Meeting regularly can motivate patients to stay on track, and it gives providers an opportunity to address problems or concerns that might interfere with the patient’s progress.
1. Dalgas U, Stenager E. Exercise and disease progression in multiple sclerosis: can exercise slow down the progression of multiple sclerosis? Ther Adv Neurol Disord. 2012;5(2):81-95.
2. Gianfrancesco MA, Barcellos LF. Obesity and multiple sclerosis susceptibility: a review. J Neurol Neuromedicine. 2016:1(7):1-5.
3. Healy BC, Eman A, Guttmann CRG, et al. Smoking and disease progression in multiple sclerosis. Arch Neurol. 2009;66(7):858-864.
1. Dalgas U, Stenager E. Exercise and disease progression in multiple sclerosis: can exercise slow down the progression of multiple sclerosis? Ther Adv Neurol Disord. 2012;5(2):81-95.
2. Gianfrancesco MA, Barcellos LF. Obesity and multiple sclerosis susceptibility: a review. J Neurol Neuromedicine. 2016:1(7):1-5.
3. Healy BC, Eman A, Guttmann CRG, et al. Smoking and disease progression in multiple sclerosis. Arch Neurol. 2009;66(7):858-864.
When Can You Stop Dialysis?
Q) When my patient was told that she needed dialysis, one of her first questions was, "For how long?" Which got me thinking: How often do dialysis patients regain kidney function? Are some more likely than others to be able to stop dialysis?
Diagnosis with end-stage renal disease (ESRD), which requires dialysis, is a life-changing event. Inevitably, patients ask about their chance of recovery and the likelihood of stopping dialysis. Studies have consistently demonstrated low rates of kidney recovery, ranging from 0.9% to 2.4%.1
According to the United States Renal Data System (USRDS), from 1995-2006 only 0.9% of ESRD patients regained kidney function resulting in the discontinuation of dialysis.2 In one study, Agraharkar and colleagues reviewed the medical records and lab results of patients discharged from a chronic dialysis unit and reported a 1% to 2% rate of kidney recovery. The researchers concluded that closer monitoring of residual kidney function was key to identification of patients with a greater chance of recovery.3 Chu and Folkert noted a recovery rate of 1.0% to 2.4% in a review of large observational studies, concluding that the underlying etiology of the kidney failure was the single most important predictor.4
Another study of approximately 194,000 patients who started dialysis between 2008-2009 demonstrated much higher rates of sustained recovery: up to 5%. This study showed that patients with kidney failure associated with acute kidney injury (AKI) were more likely to achieve recovery; patients with the AKI diagnosis of acute tubular necrosis had the highest rate of recovery.1
Similar studies of pediatric patients are rare. One European study followed 6,574 children who started dialysis before age 15. Within 2 years of dialysis initiation, just 2% showed kidney function recovery. This study also identified underlying etiology as an important predictor of recovery; ischemic kidney failure, hemolytic uremic syndrome, and vasculitis were associated with the greatest chance of recovery.5
Despite these recent findings, the prospect of discontinuation of dialysis with a diagnosis of ESRD remains very low. A patient's underlying etiology influences the possibility of recovery; those with AKI tend to have the greatest chance, making close monitoring of residual kidney function essential in this population.3 — MSG
Marlene Shaw-Gallagher, PA-C
Nephrology Division of Michigan Medicine
Assistant Professor at University of Detroit Mercy
1. Mohan S, Huff E, Wish J, et al. Recovery of renal function among ESRD patients in the US Medicare program. PLoS One. 2013;8(12):e83447.
2. United States Renal Data System. 2018 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018.
3. Agraharkar M, Nair V, Patlovany M. Recovery of renal function in dialysis patients. BMC Nephrol. 2003;4:9.
4. Chu JK, Folkert VW. Renal function recovery in chronic dialysis patients. Semin Dial. 2010;23(6):606-613.
5. Bonthius M, Harambat J, Berard E, et al. Recovery of kidney function in children treated with maintenance dialysis. Clin J Am Soc Nephrol. 2018;13(10):1510-1516.
Q) When my patient was told that she needed dialysis, one of her first questions was, "For how long?" Which got me thinking: How often do dialysis patients regain kidney function? Are some more likely than others to be able to stop dialysis?
Diagnosis with end-stage renal disease (ESRD), which requires dialysis, is a life-changing event. Inevitably, patients ask about their chance of recovery and the likelihood of stopping dialysis. Studies have consistently demonstrated low rates of kidney recovery, ranging from 0.9% to 2.4%.1
According to the United States Renal Data System (USRDS), from 1995-2006 only 0.9% of ESRD patients regained kidney function resulting in the discontinuation of dialysis.2 In one study, Agraharkar and colleagues reviewed the medical records and lab results of patients discharged from a chronic dialysis unit and reported a 1% to 2% rate of kidney recovery. The researchers concluded that closer monitoring of residual kidney function was key to identification of patients with a greater chance of recovery.3 Chu and Folkert noted a recovery rate of 1.0% to 2.4% in a review of large observational studies, concluding that the underlying etiology of the kidney failure was the single most important predictor.4
Another study of approximately 194,000 patients who started dialysis between 2008-2009 demonstrated much higher rates of sustained recovery: up to 5%. This study showed that patients with kidney failure associated with acute kidney injury (AKI) were more likely to achieve recovery; patients with the AKI diagnosis of acute tubular necrosis had the highest rate of recovery.1
Similar studies of pediatric patients are rare. One European study followed 6,574 children who started dialysis before age 15. Within 2 years of dialysis initiation, just 2% showed kidney function recovery. This study also identified underlying etiology as an important predictor of recovery; ischemic kidney failure, hemolytic uremic syndrome, and vasculitis were associated with the greatest chance of recovery.5
Despite these recent findings, the prospect of discontinuation of dialysis with a diagnosis of ESRD remains very low. A patient's underlying etiology influences the possibility of recovery; those with AKI tend to have the greatest chance, making close monitoring of residual kidney function essential in this population.3 — MSG
Marlene Shaw-Gallagher, PA-C
Nephrology Division of Michigan Medicine
Assistant Professor at University of Detroit Mercy
Q) When my patient was told that she needed dialysis, one of her first questions was, "For how long?" Which got me thinking: How often do dialysis patients regain kidney function? Are some more likely than others to be able to stop dialysis?
Diagnosis with end-stage renal disease (ESRD), which requires dialysis, is a life-changing event. Inevitably, patients ask about their chance of recovery and the likelihood of stopping dialysis. Studies have consistently demonstrated low rates of kidney recovery, ranging from 0.9% to 2.4%.1
According to the United States Renal Data System (USRDS), from 1995-2006 only 0.9% of ESRD patients regained kidney function resulting in the discontinuation of dialysis.2 In one study, Agraharkar and colleagues reviewed the medical records and lab results of patients discharged from a chronic dialysis unit and reported a 1% to 2% rate of kidney recovery. The researchers concluded that closer monitoring of residual kidney function was key to identification of patients with a greater chance of recovery.3 Chu and Folkert noted a recovery rate of 1.0% to 2.4% in a review of large observational studies, concluding that the underlying etiology of the kidney failure was the single most important predictor.4
Another study of approximately 194,000 patients who started dialysis between 2008-2009 demonstrated much higher rates of sustained recovery: up to 5%. This study showed that patients with kidney failure associated with acute kidney injury (AKI) were more likely to achieve recovery; patients with the AKI diagnosis of acute tubular necrosis had the highest rate of recovery.1
Similar studies of pediatric patients are rare. One European study followed 6,574 children who started dialysis before age 15. Within 2 years of dialysis initiation, just 2% showed kidney function recovery. This study also identified underlying etiology as an important predictor of recovery; ischemic kidney failure, hemolytic uremic syndrome, and vasculitis were associated with the greatest chance of recovery.5
Despite these recent findings, the prospect of discontinuation of dialysis with a diagnosis of ESRD remains very low. A patient's underlying etiology influences the possibility of recovery; those with AKI tend to have the greatest chance, making close monitoring of residual kidney function essential in this population.3 — MSG
Marlene Shaw-Gallagher, PA-C
Nephrology Division of Michigan Medicine
Assistant Professor at University of Detroit Mercy
1. Mohan S, Huff E, Wish J, et al. Recovery of renal function among ESRD patients in the US Medicare program. PLoS One. 2013;8(12):e83447.
2. United States Renal Data System. 2018 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018.
3. Agraharkar M, Nair V, Patlovany M. Recovery of renal function in dialysis patients. BMC Nephrol. 2003;4:9.
4. Chu JK, Folkert VW. Renal function recovery in chronic dialysis patients. Semin Dial. 2010;23(6):606-613.
5. Bonthius M, Harambat J, Berard E, et al. Recovery of kidney function in children treated with maintenance dialysis. Clin J Am Soc Nephrol. 2018;13(10):1510-1516.
1. Mohan S, Huff E, Wish J, et al. Recovery of renal function among ESRD patients in the US Medicare program. PLoS One. 2013;8(12):e83447.
2. United States Renal Data System. 2018 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018.
3. Agraharkar M, Nair V, Patlovany M. Recovery of renal function in dialysis patients. BMC Nephrol. 2003;4:9.
4. Chu JK, Folkert VW. Renal function recovery in chronic dialysis patients. Semin Dial. 2010;23(6):606-613.
5. Bonthius M, Harambat J, Berard E, et al. Recovery of kidney function in children treated with maintenance dialysis. Clin J Am Soc Nephrol. 2018;13(10):1510-1516.
When to Start Dialysis
Q) I sent a patient with a glomerular filtration rate (GFR) of 15 mL/min to nephrology to start dialysis. He came back to me and said they don’t start dialysis at 15. When do you start? Why?
There is considerable variation in the timing of dialysis initiation. Research suggests that sometimes earlier is not better.
IDEAL, a randomized controlled trial conducted in Australia and New Zealand, evaluated the advantages and disadvantages of earlier versus later dialysis initiation.1 Patients were randomly assigned to start any type of dialysis when their GFR was 8 or 11 mL/min. The results indicated that starting dialysis in a patient with a higher GFR did not lower the mortality or morbidity rate but did increase costs and complications (mostly for vascular access).1
Based on these findings, most of us start dialysis in a patient who has a GFR < 10 mL/min and symptoms of kidney failure. These include a metallic taste in mouth, weight gain (usually due to edema) or loss (cachexia), feeling “poorly,” hard-to-control hypertension, shortness of breath, confusion (uremic brain), odor, skin color changes, and insomnia. Symptomatic patients can be started on dialysis at a higher GFR (usually ≤ 18 mL/min), but there are many hoops to jump through with Medicare.
However, IDEAL was conducted outside the United States and included very few elderly (age > 75) patients with chronic kidney disease. In 2018, Kurella and colleagues published a study that analyzed age and kidney function in a US veteran population.2 Their results showed that age should be included in the “when to start dialysis” calculation. For older veterans, starting dialysis earlier—at a GFR of 10 mL/min—increased survival. However, the researchers pointed out that in this age group, survival is in months (not years) and does not necessarily equate to quality of life.
In conclusion, there is no compelling evidence that initiation of dialysis based solely on measurement of kidney function leads to improvement in clinical outcomes. In otherwise asymptomatic patients, there is no reason to begin dialysis based solely on GFR; age and fragility need to be considered in the equation. Earlier is not always better, and for the elderly patient with multiple comorbidities, dialysis is not always a better choice. —TH
Tricia Howard, MHS, PA-C, DFAAPA
Georgia Regional Medical Team, Savannah
1. Cooper BA, Branley P, Bulfone L, et al; for the IDEAL Trial. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609-619.
2. Kurella Tamura M, Desai M, Kapphahn KI, et al. Dialysis versus medical management at different ages and levels of kidney function in veterans with advanced CKD. J Am Soc Nephrol. 2018;29(8):2169-2177.
Q) I sent a patient with a glomerular filtration rate (GFR) of 15 mL/min to nephrology to start dialysis. He came back to me and said they don’t start dialysis at 15. When do you start? Why?
There is considerable variation in the timing of dialysis initiation. Research suggests that sometimes earlier is not better.
IDEAL, a randomized controlled trial conducted in Australia and New Zealand, evaluated the advantages and disadvantages of earlier versus later dialysis initiation.1 Patients were randomly assigned to start any type of dialysis when their GFR was 8 or 11 mL/min. The results indicated that starting dialysis in a patient with a higher GFR did not lower the mortality or morbidity rate but did increase costs and complications (mostly for vascular access).1
Based on these findings, most of us start dialysis in a patient who has a GFR < 10 mL/min and symptoms of kidney failure. These include a metallic taste in mouth, weight gain (usually due to edema) or loss (cachexia), feeling “poorly,” hard-to-control hypertension, shortness of breath, confusion (uremic brain), odor, skin color changes, and insomnia. Symptomatic patients can be started on dialysis at a higher GFR (usually ≤ 18 mL/min), but there are many hoops to jump through with Medicare.
However, IDEAL was conducted outside the United States and included very few elderly (age > 75) patients with chronic kidney disease. In 2018, Kurella and colleagues published a study that analyzed age and kidney function in a US veteran population.2 Their results showed that age should be included in the “when to start dialysis” calculation. For older veterans, starting dialysis earlier—at a GFR of 10 mL/min—increased survival. However, the researchers pointed out that in this age group, survival is in months (not years) and does not necessarily equate to quality of life.
In conclusion, there is no compelling evidence that initiation of dialysis based solely on measurement of kidney function leads to improvement in clinical outcomes. In otherwise asymptomatic patients, there is no reason to begin dialysis based solely on GFR; age and fragility need to be considered in the equation. Earlier is not always better, and for the elderly patient with multiple comorbidities, dialysis is not always a better choice. —TH
Tricia Howard, MHS, PA-C, DFAAPA
Georgia Regional Medical Team, Savannah
Q) I sent a patient with a glomerular filtration rate (GFR) of 15 mL/min to nephrology to start dialysis. He came back to me and said they don’t start dialysis at 15. When do you start? Why?
There is considerable variation in the timing of dialysis initiation. Research suggests that sometimes earlier is not better.
IDEAL, a randomized controlled trial conducted in Australia and New Zealand, evaluated the advantages and disadvantages of earlier versus later dialysis initiation.1 Patients were randomly assigned to start any type of dialysis when their GFR was 8 or 11 mL/min. The results indicated that starting dialysis in a patient with a higher GFR did not lower the mortality or morbidity rate but did increase costs and complications (mostly for vascular access).1
Based on these findings, most of us start dialysis in a patient who has a GFR < 10 mL/min and symptoms of kidney failure. These include a metallic taste in mouth, weight gain (usually due to edema) or loss (cachexia), feeling “poorly,” hard-to-control hypertension, shortness of breath, confusion (uremic brain), odor, skin color changes, and insomnia. Symptomatic patients can be started on dialysis at a higher GFR (usually ≤ 18 mL/min), but there are many hoops to jump through with Medicare.
However, IDEAL was conducted outside the United States and included very few elderly (age > 75) patients with chronic kidney disease. In 2018, Kurella and colleagues published a study that analyzed age and kidney function in a US veteran population.2 Their results showed that age should be included in the “when to start dialysis” calculation. For older veterans, starting dialysis earlier—at a GFR of 10 mL/min—increased survival. However, the researchers pointed out that in this age group, survival is in months (not years) and does not necessarily equate to quality of life.
In conclusion, there is no compelling evidence that initiation of dialysis based solely on measurement of kidney function leads to improvement in clinical outcomes. In otherwise asymptomatic patients, there is no reason to begin dialysis based solely on GFR; age and fragility need to be considered in the equation. Earlier is not always better, and for the elderly patient with multiple comorbidities, dialysis is not always a better choice. —TH
Tricia Howard, MHS, PA-C, DFAAPA
Georgia Regional Medical Team, Savannah
1. Cooper BA, Branley P, Bulfone L, et al; for the IDEAL Trial. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609-619.
2. Kurella Tamura M, Desai M, Kapphahn KI, et al. Dialysis versus medical management at different ages and levels of kidney function in veterans with advanced CKD. J Am Soc Nephrol. 2018;29(8):2169-2177.
1. Cooper BA, Branley P, Bulfone L, et al; for the IDEAL Trial. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609-619.
2. Kurella Tamura M, Desai M, Kapphahn KI, et al. Dialysis versus medical management at different ages and levels of kidney function in veterans with advanced CKD. J Am Soc Nephrol. 2018;29(8):2169-2177.
Diabetes and the Commercial Motor Vehicle Driver
A 60-year-old man is sent by his new employer to your urgent care for a pre-employment Department of Transportation (DOT) physical to obtain clearance to drive a commercial motor vehicle. His medical history is significant for hypertension, for which he takes lisinopril. Otherwise, he is healthy, with normal vital signs. His physical exam is unremarkable, but the urine sample is notably positive for glucose. A fingerstick glucose test yields a measurement of 212 m
Commercial motor vehicle (CMV) drivers are mandated by the Federal Motor Carrier Safety Administration (FMCSA) to receive a DOT physical examination by a licensed medical examiner. To qualify to perform the exam, physician assistants, advanced practice nurses, physicians, and chiropractors must complete an educational program and pass a written certification examination.1 Subsequently, the examiners are placed on a national registry—the National Registry of Certified Medical Examiners—with the mission to improve highway safety by determining whether a CMV driver’s health meets standards and guidelines set by the FMCSA.2
Under current guidelines, a DOT physical exam for a healthy CMV driver is considered valid for a maximum of 24 months. However, some diseases and medications require frequent follow-up, which can shorten the length of time a driver can be medically cleared to operate a CMV. Furthermore, certain conditions can disqualify the driver from meeting the necessary standards required for medical certification.
This case presentation offers the opportunity to review the requirements for evaluation and certification of a CMV driver with new-onset hyperglycemia and, ultimately, diabetes. In the United States, types 1 and 2 diabetes are estimated to affect 30.3 million people.3 About 33% of CMV drivers have been diagnosed with diabetes, which is significant since research has demonstrated an increased risk for crashes in individuals with diabetes, due to potential incapacitation from hypoglycemia.4-6
Thus, for practitioners and medical examiners, it is prudent to screen and manage diabetes in CMV drivers. In fact, over the past 15 years, federal regulations have stipulated that any driver with diabetes requiring insulin for control was disqualified from this type of work.7 This standard was developed in response to the increased risk for hypoglycemic reactions with the use of insulin. However, in September 2018, the FMCSA revised this regulation, permitting individuals with a stable insulin regimen and properly controlled diabetes to be qualified to operate a CMV. As a result, for drivers requiring insulin, the treating clinician must complete a standardized form within 45 days of the DOT exam, documenting management of the patient’s diabetes.8 For drivers with diabetes who do not require insulin, determinations are made on a case-by-case basis, with discernment of the driver’s ability to manage the disease and concurrently meet other standards for qualification.
HEALTH HISTORY AND EXAMINATION
Each CMV driver completes a standard medical history form that asks about specific medications, surgeries, or medical conditions, including diabetes or blood glucose problems. Subsequently, the driver and, ultimately, the medical examiner must expand upon and discuss every “yes” response to this questionnaire.
Regarding diabetes, the examiner should determine whether the disease is controlled by diet, pills, and/or insulin, with clarification of the doses, frequency, and prescriber. In addition, the examiner should review and document glucose control, blood glucose monitoring, history of hypoglycemic episodes, and episodes of fainting, dizziness, or loss of consciousness.7
Continue to: The physical exam should focus on...
The physical exam should focus on identifying signs of complications from diabetes, such as retinopathy, nephropathy, or peripheral neuropathy. At each certification visit, the examiner should assess the patient’s height and weight, BMI, vision, hearing, blood pressure, and heart rate, and perform urinalysis to screen for proteinuria or glycosuria. A fingerstick test to obtain a random blood glucose reading is often performed in a driver with glycosuria.
Likewise, the A1C level should be documented in every patient with new-onset or known diabetes, with the recommendation from the FMCSA that a level >10% is an indicator of poor glucose control.7 It is important to note that an A1C level up to 10% is not the glycemic target recognized by the American Diabetes Association and the American Association of Clinical Endocrinologists. The FMCSA is focused more on hypoglycemic concerns than on providing management guidelines.
DETERMINING CERTIFICATION
Currently, the recertification time recommended for CMV drivers with diabetes and documented glucose control is 1 year. This is based on the assumption that the driver is under medical care with a treatment plan and that he/she is not currently experiencing any complications from the disease. Furthermore, insulin secretagogues (eg, sulfonylureas) can be used for glucose control as long as adverse effects (eg, hypoglycemia) do not interfere with safe driving. However, the FMCSA does not recommend certifying any driver who
- In the past 12 months has experienced a hypoglycemic reaction resulting in seizure; loss of consciousness; need of assistance from another person; or period of impaired cognitive function that occurred without warning.
- In the past 5 years has had recurring (≥ 2) disqualifying hypoglycemic reactions.
- Has received a formal diagnosis of peripheral neuropathy, loss of position, or pedal sensation.
- Has resting tachycardia or orthostatic hypotension.
- Has severe diabetic nephropathy requiring dialysis.
- Has severe nonproliferative or proliferative retinopathy.8
In drivers with new-onset hyperglycemia, it is appropriate for the medical examiner to refer the driver to his/her primary care provider for further testing (eg, A1C), determination of treatment, a copy of the diabetes medical standard for driving, and written opinion of the driver’s medical fitness for duty. Subsequently, the medical examiner can utilize this information from the primary care provider to determine certification for the driver. While there are no specific guidelines on the waiting period for certification, the driver should demonstrate glucose control with treatment that is adequate, effective, safe, and stable.7
Overall, while living with diabetes can be challenging, patients who demonstrate control of the disease can maintain their occupation as a CMV driver. The role of the medical examiner is to evaluate the driver’s risk to safely operate a CMV—in particular, considering the possibilities of a severe hypoglycemic episode or target organ dysfunction—whereas the clinician treating the driver’s diabetes is focused on minimizing the complications associated with hyperglycemia.
Continue to: As a reminder...
As a reminder, due to the progressive nature of the disease, recertification is recommended annually for drivers.7 Nevertheless, it is reassuring that the DOT has implemented safeguards designed to keep our citizens safe while travelling the highways and byways of the United States.
Given the patient’s elevated glucose, more information is needed to safely provide clearance for driving a CMV. The patient would be disqualified until he could provide documentation of glucose control. Therefore, this patient would benefit from a referral to his primary care provider to obtain a list of medications used to manage his disease, documentation of an A1C level <10% and no evidence of complications from diabetes, and a written opinion from the primary care provider indicating the driver is medically fit for duty. Accordingly, the primary care provider can ensure the patient demonstrates compliance in managing diabetes and can safely operate a CMV.
1. Federal Motor Carrier Safety Administration. DOT Medical Exam and Commercial Motor Vehicle Certification. www.fmcsa.dot.gov/medical/driver-medical-requirements/dot-medical-exam-and-commercial-motor-vehicle-certification. Accessed February 22, 2019.
2. Federal Motor Carrier Safety Administration. National Registry of Certified Medical Examiners. www.fmcsa.dot.gov/medical/driver-medical-requirements/national-registry-certified-medical-examiners. Accessed February 22, 2019.
3. CDC. National Diabetes Statistics Report, 2017: estimates of diabetes and its burden in the United States. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed February 22, 2019.
4. Abu Dabrh AM, Firwana B, Cowl CT, et al. Health assessment of commercial drivers: a meta-narrative systematic review. BMJ Open. 2014;4:e003434.
5. Laberge-Nadeau C, Dionne G, Maag U, et al. Medical conditions and the severity of commercial motor vehicle drivers’ road accidents. Accid Anal Prev. 1996;28:43-51.
6. Redelmeier DA, Kenshole AB, Ray JG. Motor vehicle crashes in diabetic patients with tight glycemic control: a population-based case control analysis. PLoS Med. 2009;6:e1000192.
7. Federal Motor Carrier Safety Administration. Medical Examiner Handbook. www.fmcsa.dot.gov/sites/fmcsa.dot.gov/files/docs/mission/advisory-committees/mrb/83401/fmcsamedicalexaminerhandbook.pdf. Accessed February 22, 2019.
8. Federal Motor Carrier Safety Administration. Qualifications of Drivers; Diabetes Standard. Federal Register. September 19, 2018. www.federalregister.gov/documents/2018/09/19/2018-20161/qualifications-of-drivers-diabetes-standard. Accessed February 25, 2019.
A 60-year-old man is sent by his new employer to your urgent care for a pre-employment Department of Transportation (DOT) physical to obtain clearance to drive a commercial motor vehicle. His medical history is significant for hypertension, for which he takes lisinopril. Otherwise, he is healthy, with normal vital signs. His physical exam is unremarkable, but the urine sample is notably positive for glucose. A fingerstick glucose test yields a measurement of 212 m
Commercial motor vehicle (CMV) drivers are mandated by the Federal Motor Carrier Safety Administration (FMCSA) to receive a DOT physical examination by a licensed medical examiner. To qualify to perform the exam, physician assistants, advanced practice nurses, physicians, and chiropractors must complete an educational program and pass a written certification examination.1 Subsequently, the examiners are placed on a national registry—the National Registry of Certified Medical Examiners—with the mission to improve highway safety by determining whether a CMV driver’s health meets standards and guidelines set by the FMCSA.2
Under current guidelines, a DOT physical exam for a healthy CMV driver is considered valid for a maximum of 24 months. However, some diseases and medications require frequent follow-up, which can shorten the length of time a driver can be medically cleared to operate a CMV. Furthermore, certain conditions can disqualify the driver from meeting the necessary standards required for medical certification.
This case presentation offers the opportunity to review the requirements for evaluation and certification of a CMV driver with new-onset hyperglycemia and, ultimately, diabetes. In the United States, types 1 and 2 diabetes are estimated to affect 30.3 million people.3 About 33% of CMV drivers have been diagnosed with diabetes, which is significant since research has demonstrated an increased risk for crashes in individuals with diabetes, due to potential incapacitation from hypoglycemia.4-6
Thus, for practitioners and medical examiners, it is prudent to screen and manage diabetes in CMV drivers. In fact, over the past 15 years, federal regulations have stipulated that any driver with diabetes requiring insulin for control was disqualified from this type of work.7 This standard was developed in response to the increased risk for hypoglycemic reactions with the use of insulin. However, in September 2018, the FMCSA revised this regulation, permitting individuals with a stable insulin regimen and properly controlled diabetes to be qualified to operate a CMV. As a result, for drivers requiring insulin, the treating clinician must complete a standardized form within 45 days of the DOT exam, documenting management of the patient’s diabetes.8 For drivers with diabetes who do not require insulin, determinations are made on a case-by-case basis, with discernment of the driver’s ability to manage the disease and concurrently meet other standards for qualification.
HEALTH HISTORY AND EXAMINATION
Each CMV driver completes a standard medical history form that asks about specific medications, surgeries, or medical conditions, including diabetes or blood glucose problems. Subsequently, the driver and, ultimately, the medical examiner must expand upon and discuss every “yes” response to this questionnaire.
Regarding diabetes, the examiner should determine whether the disease is controlled by diet, pills, and/or insulin, with clarification of the doses, frequency, and prescriber. In addition, the examiner should review and document glucose control, blood glucose monitoring, history of hypoglycemic episodes, and episodes of fainting, dizziness, or loss of consciousness.7
Continue to: The physical exam should focus on...
The physical exam should focus on identifying signs of complications from diabetes, such as retinopathy, nephropathy, or peripheral neuropathy. At each certification visit, the examiner should assess the patient’s height and weight, BMI, vision, hearing, blood pressure, and heart rate, and perform urinalysis to screen for proteinuria or glycosuria. A fingerstick test to obtain a random blood glucose reading is often performed in a driver with glycosuria.
Likewise, the A1C level should be documented in every patient with new-onset or known diabetes, with the recommendation from the FMCSA that a level >10% is an indicator of poor glucose control.7 It is important to note that an A1C level up to 10% is not the glycemic target recognized by the American Diabetes Association and the American Association of Clinical Endocrinologists. The FMCSA is focused more on hypoglycemic concerns than on providing management guidelines.
DETERMINING CERTIFICATION
Currently, the recertification time recommended for CMV drivers with diabetes and documented glucose control is 1 year. This is based on the assumption that the driver is under medical care with a treatment plan and that he/she is not currently experiencing any complications from the disease. Furthermore, insulin secretagogues (eg, sulfonylureas) can be used for glucose control as long as adverse effects (eg, hypoglycemia) do not interfere with safe driving. However, the FMCSA does not recommend certifying any driver who
- In the past 12 months has experienced a hypoglycemic reaction resulting in seizure; loss of consciousness; need of assistance from another person; or period of impaired cognitive function that occurred without warning.
- In the past 5 years has had recurring (≥ 2) disqualifying hypoglycemic reactions.
- Has received a formal diagnosis of peripheral neuropathy, loss of position, or pedal sensation.
- Has resting tachycardia or orthostatic hypotension.
- Has severe diabetic nephropathy requiring dialysis.
- Has severe nonproliferative or proliferative retinopathy.8
In drivers with new-onset hyperglycemia, it is appropriate for the medical examiner to refer the driver to his/her primary care provider for further testing (eg, A1C), determination of treatment, a copy of the diabetes medical standard for driving, and written opinion of the driver’s medical fitness for duty. Subsequently, the medical examiner can utilize this information from the primary care provider to determine certification for the driver. While there are no specific guidelines on the waiting period for certification, the driver should demonstrate glucose control with treatment that is adequate, effective, safe, and stable.7
Overall, while living with diabetes can be challenging, patients who demonstrate control of the disease can maintain their occupation as a CMV driver. The role of the medical examiner is to evaluate the driver’s risk to safely operate a CMV—in particular, considering the possibilities of a severe hypoglycemic episode or target organ dysfunction—whereas the clinician treating the driver’s diabetes is focused on minimizing the complications associated with hyperglycemia.
Continue to: As a reminder...
As a reminder, due to the progressive nature of the disease, recertification is recommended annually for drivers.7 Nevertheless, it is reassuring that the DOT has implemented safeguards designed to keep our citizens safe while travelling the highways and byways of the United States.
Given the patient’s elevated glucose, more information is needed to safely provide clearance for driving a CMV. The patient would be disqualified until he could provide documentation of glucose control. Therefore, this patient would benefit from a referral to his primary care provider to obtain a list of medications used to manage his disease, documentation of an A1C level <10% and no evidence of complications from diabetes, and a written opinion from the primary care provider indicating the driver is medically fit for duty. Accordingly, the primary care provider can ensure the patient demonstrates compliance in managing diabetes and can safely operate a CMV.
A 60-year-old man is sent by his new employer to your urgent care for a pre-employment Department of Transportation (DOT) physical to obtain clearance to drive a commercial motor vehicle. His medical history is significant for hypertension, for which he takes lisinopril. Otherwise, he is healthy, with normal vital signs. His physical exam is unremarkable, but the urine sample is notably positive for glucose. A fingerstick glucose test yields a measurement of 212 m
Commercial motor vehicle (CMV) drivers are mandated by the Federal Motor Carrier Safety Administration (FMCSA) to receive a DOT physical examination by a licensed medical examiner. To qualify to perform the exam, physician assistants, advanced practice nurses, physicians, and chiropractors must complete an educational program and pass a written certification examination.1 Subsequently, the examiners are placed on a national registry—the National Registry of Certified Medical Examiners—with the mission to improve highway safety by determining whether a CMV driver’s health meets standards and guidelines set by the FMCSA.2
Under current guidelines, a DOT physical exam for a healthy CMV driver is considered valid for a maximum of 24 months. However, some diseases and medications require frequent follow-up, which can shorten the length of time a driver can be medically cleared to operate a CMV. Furthermore, certain conditions can disqualify the driver from meeting the necessary standards required for medical certification.
This case presentation offers the opportunity to review the requirements for evaluation and certification of a CMV driver with new-onset hyperglycemia and, ultimately, diabetes. In the United States, types 1 and 2 diabetes are estimated to affect 30.3 million people.3 About 33% of CMV drivers have been diagnosed with diabetes, which is significant since research has demonstrated an increased risk for crashes in individuals with diabetes, due to potential incapacitation from hypoglycemia.4-6
Thus, for practitioners and medical examiners, it is prudent to screen and manage diabetes in CMV drivers. In fact, over the past 15 years, federal regulations have stipulated that any driver with diabetes requiring insulin for control was disqualified from this type of work.7 This standard was developed in response to the increased risk for hypoglycemic reactions with the use of insulin. However, in September 2018, the FMCSA revised this regulation, permitting individuals with a stable insulin regimen and properly controlled diabetes to be qualified to operate a CMV. As a result, for drivers requiring insulin, the treating clinician must complete a standardized form within 45 days of the DOT exam, documenting management of the patient’s diabetes.8 For drivers with diabetes who do not require insulin, determinations are made on a case-by-case basis, with discernment of the driver’s ability to manage the disease and concurrently meet other standards for qualification.
HEALTH HISTORY AND EXAMINATION
Each CMV driver completes a standard medical history form that asks about specific medications, surgeries, or medical conditions, including diabetes or blood glucose problems. Subsequently, the driver and, ultimately, the medical examiner must expand upon and discuss every “yes” response to this questionnaire.
Regarding diabetes, the examiner should determine whether the disease is controlled by diet, pills, and/or insulin, with clarification of the doses, frequency, and prescriber. In addition, the examiner should review and document glucose control, blood glucose monitoring, history of hypoglycemic episodes, and episodes of fainting, dizziness, or loss of consciousness.7
Continue to: The physical exam should focus on...
The physical exam should focus on identifying signs of complications from diabetes, such as retinopathy, nephropathy, or peripheral neuropathy. At each certification visit, the examiner should assess the patient’s height and weight, BMI, vision, hearing, blood pressure, and heart rate, and perform urinalysis to screen for proteinuria or glycosuria. A fingerstick test to obtain a random blood glucose reading is often performed in a driver with glycosuria.
Likewise, the A1C level should be documented in every patient with new-onset or known diabetes, with the recommendation from the FMCSA that a level >10% is an indicator of poor glucose control.7 It is important to note that an A1C level up to 10% is not the glycemic target recognized by the American Diabetes Association and the American Association of Clinical Endocrinologists. The FMCSA is focused more on hypoglycemic concerns than on providing management guidelines.
DETERMINING CERTIFICATION
Currently, the recertification time recommended for CMV drivers with diabetes and documented glucose control is 1 year. This is based on the assumption that the driver is under medical care with a treatment plan and that he/she is not currently experiencing any complications from the disease. Furthermore, insulin secretagogues (eg, sulfonylureas) can be used for glucose control as long as adverse effects (eg, hypoglycemia) do not interfere with safe driving. However, the FMCSA does not recommend certifying any driver who
- In the past 12 months has experienced a hypoglycemic reaction resulting in seizure; loss of consciousness; need of assistance from another person; or period of impaired cognitive function that occurred without warning.
- In the past 5 years has had recurring (≥ 2) disqualifying hypoglycemic reactions.
- Has received a formal diagnosis of peripheral neuropathy, loss of position, or pedal sensation.
- Has resting tachycardia or orthostatic hypotension.
- Has severe diabetic nephropathy requiring dialysis.
- Has severe nonproliferative or proliferative retinopathy.8
In drivers with new-onset hyperglycemia, it is appropriate for the medical examiner to refer the driver to his/her primary care provider for further testing (eg, A1C), determination of treatment, a copy of the diabetes medical standard for driving, and written opinion of the driver’s medical fitness for duty. Subsequently, the medical examiner can utilize this information from the primary care provider to determine certification for the driver. While there are no specific guidelines on the waiting period for certification, the driver should demonstrate glucose control with treatment that is adequate, effective, safe, and stable.7
Overall, while living with diabetes can be challenging, patients who demonstrate control of the disease can maintain their occupation as a CMV driver. The role of the medical examiner is to evaluate the driver’s risk to safely operate a CMV—in particular, considering the possibilities of a severe hypoglycemic episode or target organ dysfunction—whereas the clinician treating the driver’s diabetes is focused on minimizing the complications associated with hyperglycemia.
Continue to: As a reminder...
As a reminder, due to the progressive nature of the disease, recertification is recommended annually for drivers.7 Nevertheless, it is reassuring that the DOT has implemented safeguards designed to keep our citizens safe while travelling the highways and byways of the United States.
Given the patient’s elevated glucose, more information is needed to safely provide clearance for driving a CMV. The patient would be disqualified until he could provide documentation of glucose control. Therefore, this patient would benefit from a referral to his primary care provider to obtain a list of medications used to manage his disease, documentation of an A1C level <10% and no evidence of complications from diabetes, and a written opinion from the primary care provider indicating the driver is medically fit for duty. Accordingly, the primary care provider can ensure the patient demonstrates compliance in managing diabetes and can safely operate a CMV.
1. Federal Motor Carrier Safety Administration. DOT Medical Exam and Commercial Motor Vehicle Certification. www.fmcsa.dot.gov/medical/driver-medical-requirements/dot-medical-exam-and-commercial-motor-vehicle-certification. Accessed February 22, 2019.
2. Federal Motor Carrier Safety Administration. National Registry of Certified Medical Examiners. www.fmcsa.dot.gov/medical/driver-medical-requirements/national-registry-certified-medical-examiners. Accessed February 22, 2019.
3. CDC. National Diabetes Statistics Report, 2017: estimates of diabetes and its burden in the United States. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed February 22, 2019.
4. Abu Dabrh AM, Firwana B, Cowl CT, et al. Health assessment of commercial drivers: a meta-narrative systematic review. BMJ Open. 2014;4:e003434.
5. Laberge-Nadeau C, Dionne G, Maag U, et al. Medical conditions and the severity of commercial motor vehicle drivers’ road accidents. Accid Anal Prev. 1996;28:43-51.
6. Redelmeier DA, Kenshole AB, Ray JG. Motor vehicle crashes in diabetic patients with tight glycemic control: a population-based case control analysis. PLoS Med. 2009;6:e1000192.
7. Federal Motor Carrier Safety Administration. Medical Examiner Handbook. www.fmcsa.dot.gov/sites/fmcsa.dot.gov/files/docs/mission/advisory-committees/mrb/83401/fmcsamedicalexaminerhandbook.pdf. Accessed February 22, 2019.
8. Federal Motor Carrier Safety Administration. Qualifications of Drivers; Diabetes Standard. Federal Register. September 19, 2018. www.federalregister.gov/documents/2018/09/19/2018-20161/qualifications-of-drivers-diabetes-standard. Accessed February 25, 2019.
1. Federal Motor Carrier Safety Administration. DOT Medical Exam and Commercial Motor Vehicle Certification. www.fmcsa.dot.gov/medical/driver-medical-requirements/dot-medical-exam-and-commercial-motor-vehicle-certification. Accessed February 22, 2019.
2. Federal Motor Carrier Safety Administration. National Registry of Certified Medical Examiners. www.fmcsa.dot.gov/medical/driver-medical-requirements/national-registry-certified-medical-examiners. Accessed February 22, 2019.
3. CDC. National Diabetes Statistics Report, 2017: estimates of diabetes and its burden in the United States. www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed February 22, 2019.
4. Abu Dabrh AM, Firwana B, Cowl CT, et al. Health assessment of commercial drivers: a meta-narrative systematic review. BMJ Open. 2014;4:e003434.
5. Laberge-Nadeau C, Dionne G, Maag U, et al. Medical conditions and the severity of commercial motor vehicle drivers’ road accidents. Accid Anal Prev. 1996;28:43-51.
6. Redelmeier DA, Kenshole AB, Ray JG. Motor vehicle crashes in diabetic patients with tight glycemic control: a population-based case control analysis. PLoS Med. 2009;6:e1000192.
7. Federal Motor Carrier Safety Administration. Medical Examiner Handbook. www.fmcsa.dot.gov/sites/fmcsa.dot.gov/files/docs/mission/advisory-committees/mrb/83401/fmcsamedicalexaminerhandbook.pdf. Accessed February 22, 2019.
8. Federal Motor Carrier Safety Administration. Qualifications of Drivers; Diabetes Standard. Federal Register. September 19, 2018. www.federalregister.gov/documents/2018/09/19/2018-20161/qualifications-of-drivers-diabetes-standard. Accessed February 25, 2019.
Cognition and MS
Cognitive changes related to multiple sclerosis (MS) were first mentioned by Jean-Martin Charcot in 1877; however, it is only within the past 25-30 years that cognitive impairment in MS has received significant clinical study. Despite a growing body of research, though, formal screening of cognitive function is not always part of routine MS clinical care.
Q)How common are cognitive symptoms in MS?
Cognitive changes affect up to 65% of patients in MS clinic samples and about one-third of pediatric MS patients.1 Cognitive deficits occur in all the MS disease courses, including clinically isolated syndrome, although they are most prevalent in secondary progressive and primary progressive disease.1 Cognitive changes have even been observed in radiographically isolated syndrome, in which MRI changes consistent with MS are observed without any neurologic symptoms or signs.2
Q)What cognitive domains are affected in MS?
Strong correlations have been demonstrated between cognitive impairment and MRI findings, including whole brain atrophy and, to some degree, overall white matter lesion burden. Cognitive changes also result from damage in specific areas, including deep gray matter and the corpus callosum, cerebral cortex, and mesial temporal lobe.3-5
The type and severity of cognitive deficits vary widely among people with MS. However, difficulties with information processing speed and short-term memory are the symptoms most commonly seen in this population. Processing speed problems affect new learning and impact memory and executive function. Other domains that can be affected are complex attention, verbal fluency, and visuospatial perception.1
Q)Are cognitive symptoms in MS progressive?
Not everyone with cognitive symptoms related to MS will show progressive changes. However, in a longitudinal study, increasing age and degree of physical disability were predictive of worsening cognitive symptoms. Also, people who demonstrate early cognitive symptoms may experience greater worsening.6
Q)What impact do cognitive symptoms have?
Changes in cognition are a common reason for someone to experience performance issues in the workplace and as such significantly affect a person’s ability to maintain employment. Impaired cognition is a primary cause of early departure from the workforceand has significant implications for self-image and self-esteem.7
Furthermore, cognitive symptoms can impact adherence to medications. They also can negatively affect daily life, through increased risk for motor vehicle accidents, difficulties with routine household tasks, and significant challenges to relationships (particularly but not exclusively those with caregivers).
Continue to: How are cognitive symptoms assessed?
Q)How are cognitive symptoms assessed?
There are several screening tools that take very little time to administer and can be used in the clinic setting. The Symbol Digit Modalities Test (SDMT; www.wpspublish.com/store/p/2955/sdmt-symbol-digit-modalities-test) is validated in MS and takes approximately 90 s to complete. This screening instrument is proprietary and has a small fee associated with its use.8
Other possible causes of cognitive dysfunction should be investigated as well. These include an examination of medications being used—such as anticholinergics, benzodiazepines, other sedatives, cannabis, topiramate, and opioids—and consideration of other diseases and conditions, including vascular conditions, metabolic deficiencies, infection, tumor, substance abuse, early dementia, or hypothyroidism, which may contribute to or cause cognitive impairment.
Should cognitive problems be identified—either through the history, during the clinic visit, or via screening tests—more formal testing, usually performed by a neuropsychologist, may be useful in identifying the domains of function that are impaired. This information can help to identify and implement appropriate compensatory strategies, plan cognitive rehabilitation interventions, and (in the United States) assist the individual to obtain Social Security disability benefits.
Q)How are cognitive symptoms managed?
Multiple clinical trials of cognitive rehabilitation strategies have demonstrated the efficacy of computer-based programs in improving new learning, short-term memory, processing speed, and attention.9 Cognitive rehabilitation programs should be administered and/or supervised by a health care professional who is knowledgeable about MS as well as cognitive rehabilitation. Professionals such as neuropsychologists, occupational therapists, and speech language pathologists often direct cognitive training programs.
Medications that stimulate the central nervous system have been used to improve mental alertness. However, clinical trials are few and have yielded mixed results.
Continue to: In clinical trials...
In clinical trials, physical exercise has been shown to improve processing speed. More research is needed to demonstrate the type of exercise that is most beneficial and the extent of improvement in cognitive function that results.
SUMMARY
Cognitive function can be negatively impacted by MS. Activities of daily living, including employment and relationships, can be negatively impacted by changes in cognition. Regular screening of cognition is recommended by the National MS Society, using validated screening tools such as the SDMT. Additional testing is warranted for individuals reporting cognitive difficulties at home or work, or those who score below controls on screening tests. Cognitive rehabilitation may help some individuals improve their cognitive function. More research is needed to identify additional cognitive training techniques, better understand the role of physical exercise, and identify medications that may be of benefit to maintain cognitive function.
1. Amato MP, Zipoli V, Portaccio E. Cognitive changes in multiple sclerosis. Expert Rev Neurother. 2008;8(10):1585-1596.
2. Labiano-Fontcuberta A, Martínez-Ginés ML, Aladro Y, et al. A comparison study of cognitive deficits in radiologically and clinically isolated syndromes. Mult Scler. 2016;22(2):250-253.
3. Benedict RH, Ramasamy D, Munschauer F, et al. Memory impairment in multiple sclerosis: correlation with deep grey matter and mesial temporal atrophy. J Neurol Neurosurg Psychiatry. 2009;80(2):201-206.
4. Rocca MA, Amato MP, De Stefano N, et al; MAGNIMS Study Group. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015;14(3):302-317.
5. Rovaris M, Comi G, Filippi M. MRI markers of destructive pathology in multiple sclerosis-related cognitive dysfunction. J Neurol Sci. 2006;245(1-2):111-116.
6. Johnen A, Landmeyer NC, Bürkner PC, et al. Distinct cognitive impairments in different disease courses of multiple sclerosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2017;83:568-578.
7. Rao SM, Leo GJ, Ellington L, et al. Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology. 1991;41(5):692-696.
8. Parmenter BA, Weinstock-Guttman B, Garg N, et al. Screening for cognitive impairment in multiple sclerosis using the symbol digit modalities test. Mult Scler. 2007;13(1):52-57.
9. Goverover Y, Chiaravalloti ND, O’Brien AR, DeLuca J. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: an updated review of the literature from 2007 to 2016. Arch Phys Med Rehabil. 2018;99(2):390-407.
Cognitive changes related to multiple sclerosis (MS) were first mentioned by Jean-Martin Charcot in 1877; however, it is only within the past 25-30 years that cognitive impairment in MS has received significant clinical study. Despite a growing body of research, though, formal screening of cognitive function is not always part of routine MS clinical care.
Q)How common are cognitive symptoms in MS?
Cognitive changes affect up to 65% of patients in MS clinic samples and about one-third of pediatric MS patients.1 Cognitive deficits occur in all the MS disease courses, including clinically isolated syndrome, although they are most prevalent in secondary progressive and primary progressive disease.1 Cognitive changes have even been observed in radiographically isolated syndrome, in which MRI changes consistent with MS are observed without any neurologic symptoms or signs.2
Q)What cognitive domains are affected in MS?
Strong correlations have been demonstrated between cognitive impairment and MRI findings, including whole brain atrophy and, to some degree, overall white matter lesion burden. Cognitive changes also result from damage in specific areas, including deep gray matter and the corpus callosum, cerebral cortex, and mesial temporal lobe.3-5
The type and severity of cognitive deficits vary widely among people with MS. However, difficulties with information processing speed and short-term memory are the symptoms most commonly seen in this population. Processing speed problems affect new learning and impact memory and executive function. Other domains that can be affected are complex attention, verbal fluency, and visuospatial perception.1
Q)Are cognitive symptoms in MS progressive?
Not everyone with cognitive symptoms related to MS will show progressive changes. However, in a longitudinal study, increasing age and degree of physical disability were predictive of worsening cognitive symptoms. Also, people who demonstrate early cognitive symptoms may experience greater worsening.6
Q)What impact do cognitive symptoms have?
Changes in cognition are a common reason for someone to experience performance issues in the workplace and as such significantly affect a person’s ability to maintain employment. Impaired cognition is a primary cause of early departure from the workforceand has significant implications for self-image and self-esteem.7
Furthermore, cognitive symptoms can impact adherence to medications. They also can negatively affect daily life, through increased risk for motor vehicle accidents, difficulties with routine household tasks, and significant challenges to relationships (particularly but not exclusively those with caregivers).
Continue to: How are cognitive symptoms assessed?
Q)How are cognitive symptoms assessed?
There are several screening tools that take very little time to administer and can be used in the clinic setting. The Symbol Digit Modalities Test (SDMT; www.wpspublish.com/store/p/2955/sdmt-symbol-digit-modalities-test) is validated in MS and takes approximately 90 s to complete. This screening instrument is proprietary and has a small fee associated with its use.8
Other possible causes of cognitive dysfunction should be investigated as well. These include an examination of medications being used—such as anticholinergics, benzodiazepines, other sedatives, cannabis, topiramate, and opioids—and consideration of other diseases and conditions, including vascular conditions, metabolic deficiencies, infection, tumor, substance abuse, early dementia, or hypothyroidism, which may contribute to or cause cognitive impairment.
Should cognitive problems be identified—either through the history, during the clinic visit, or via screening tests—more formal testing, usually performed by a neuropsychologist, may be useful in identifying the domains of function that are impaired. This information can help to identify and implement appropriate compensatory strategies, plan cognitive rehabilitation interventions, and (in the United States) assist the individual to obtain Social Security disability benefits.
Q)How are cognitive symptoms managed?
Multiple clinical trials of cognitive rehabilitation strategies have demonstrated the efficacy of computer-based programs in improving new learning, short-term memory, processing speed, and attention.9 Cognitive rehabilitation programs should be administered and/or supervised by a health care professional who is knowledgeable about MS as well as cognitive rehabilitation. Professionals such as neuropsychologists, occupational therapists, and speech language pathologists often direct cognitive training programs.
Medications that stimulate the central nervous system have been used to improve mental alertness. However, clinical trials are few and have yielded mixed results.
Continue to: In clinical trials...
In clinical trials, physical exercise has been shown to improve processing speed. More research is needed to demonstrate the type of exercise that is most beneficial and the extent of improvement in cognitive function that results.
SUMMARY
Cognitive function can be negatively impacted by MS. Activities of daily living, including employment and relationships, can be negatively impacted by changes in cognition. Regular screening of cognition is recommended by the National MS Society, using validated screening tools such as the SDMT. Additional testing is warranted for individuals reporting cognitive difficulties at home or work, or those who score below controls on screening tests. Cognitive rehabilitation may help some individuals improve their cognitive function. More research is needed to identify additional cognitive training techniques, better understand the role of physical exercise, and identify medications that may be of benefit to maintain cognitive function.
Cognitive changes related to multiple sclerosis (MS) were first mentioned by Jean-Martin Charcot in 1877; however, it is only within the past 25-30 years that cognitive impairment in MS has received significant clinical study. Despite a growing body of research, though, formal screening of cognitive function is not always part of routine MS clinical care.
Q)How common are cognitive symptoms in MS?
Cognitive changes affect up to 65% of patients in MS clinic samples and about one-third of pediatric MS patients.1 Cognitive deficits occur in all the MS disease courses, including clinically isolated syndrome, although they are most prevalent in secondary progressive and primary progressive disease.1 Cognitive changes have even been observed in radiographically isolated syndrome, in which MRI changes consistent with MS are observed without any neurologic symptoms or signs.2
Q)What cognitive domains are affected in MS?
Strong correlations have been demonstrated between cognitive impairment and MRI findings, including whole brain atrophy and, to some degree, overall white matter lesion burden. Cognitive changes also result from damage in specific areas, including deep gray matter and the corpus callosum, cerebral cortex, and mesial temporal lobe.3-5
The type and severity of cognitive deficits vary widely among people with MS. However, difficulties with information processing speed and short-term memory are the symptoms most commonly seen in this population. Processing speed problems affect new learning and impact memory and executive function. Other domains that can be affected are complex attention, verbal fluency, and visuospatial perception.1
Q)Are cognitive symptoms in MS progressive?
Not everyone with cognitive symptoms related to MS will show progressive changes. However, in a longitudinal study, increasing age and degree of physical disability were predictive of worsening cognitive symptoms. Also, people who demonstrate early cognitive symptoms may experience greater worsening.6
Q)What impact do cognitive symptoms have?
Changes in cognition are a common reason for someone to experience performance issues in the workplace and as such significantly affect a person’s ability to maintain employment. Impaired cognition is a primary cause of early departure from the workforceand has significant implications for self-image and self-esteem.7
Furthermore, cognitive symptoms can impact adherence to medications. They also can negatively affect daily life, through increased risk for motor vehicle accidents, difficulties with routine household tasks, and significant challenges to relationships (particularly but not exclusively those with caregivers).
Continue to: How are cognitive symptoms assessed?
Q)How are cognitive symptoms assessed?
There are several screening tools that take very little time to administer and can be used in the clinic setting. The Symbol Digit Modalities Test (SDMT; www.wpspublish.com/store/p/2955/sdmt-symbol-digit-modalities-test) is validated in MS and takes approximately 90 s to complete. This screening instrument is proprietary and has a small fee associated with its use.8
Other possible causes of cognitive dysfunction should be investigated as well. These include an examination of medications being used—such as anticholinergics, benzodiazepines, other sedatives, cannabis, topiramate, and opioids—and consideration of other diseases and conditions, including vascular conditions, metabolic deficiencies, infection, tumor, substance abuse, early dementia, or hypothyroidism, which may contribute to or cause cognitive impairment.
Should cognitive problems be identified—either through the history, during the clinic visit, or via screening tests—more formal testing, usually performed by a neuropsychologist, may be useful in identifying the domains of function that are impaired. This information can help to identify and implement appropriate compensatory strategies, plan cognitive rehabilitation interventions, and (in the United States) assist the individual to obtain Social Security disability benefits.
Q)How are cognitive symptoms managed?
Multiple clinical trials of cognitive rehabilitation strategies have demonstrated the efficacy of computer-based programs in improving new learning, short-term memory, processing speed, and attention.9 Cognitive rehabilitation programs should be administered and/or supervised by a health care professional who is knowledgeable about MS as well as cognitive rehabilitation. Professionals such as neuropsychologists, occupational therapists, and speech language pathologists often direct cognitive training programs.
Medications that stimulate the central nervous system have been used to improve mental alertness. However, clinical trials are few and have yielded mixed results.
Continue to: In clinical trials...
In clinical trials, physical exercise has been shown to improve processing speed. More research is needed to demonstrate the type of exercise that is most beneficial and the extent of improvement in cognitive function that results.
SUMMARY
Cognitive function can be negatively impacted by MS. Activities of daily living, including employment and relationships, can be negatively impacted by changes in cognition. Regular screening of cognition is recommended by the National MS Society, using validated screening tools such as the SDMT. Additional testing is warranted for individuals reporting cognitive difficulties at home or work, or those who score below controls on screening tests. Cognitive rehabilitation may help some individuals improve their cognitive function. More research is needed to identify additional cognitive training techniques, better understand the role of physical exercise, and identify medications that may be of benefit to maintain cognitive function.
1. Amato MP, Zipoli V, Portaccio E. Cognitive changes in multiple sclerosis. Expert Rev Neurother. 2008;8(10):1585-1596.
2. Labiano-Fontcuberta A, Martínez-Ginés ML, Aladro Y, et al. A comparison study of cognitive deficits in radiologically and clinically isolated syndromes. Mult Scler. 2016;22(2):250-253.
3. Benedict RH, Ramasamy D, Munschauer F, et al. Memory impairment in multiple sclerosis: correlation with deep grey matter and mesial temporal atrophy. J Neurol Neurosurg Psychiatry. 2009;80(2):201-206.
4. Rocca MA, Amato MP, De Stefano N, et al; MAGNIMS Study Group. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015;14(3):302-317.
5. Rovaris M, Comi G, Filippi M. MRI markers of destructive pathology in multiple sclerosis-related cognitive dysfunction. J Neurol Sci. 2006;245(1-2):111-116.
6. Johnen A, Landmeyer NC, Bürkner PC, et al. Distinct cognitive impairments in different disease courses of multiple sclerosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2017;83:568-578.
7. Rao SM, Leo GJ, Ellington L, et al. Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology. 1991;41(5):692-696.
8. Parmenter BA, Weinstock-Guttman B, Garg N, et al. Screening for cognitive impairment in multiple sclerosis using the symbol digit modalities test. Mult Scler. 2007;13(1):52-57.
9. Goverover Y, Chiaravalloti ND, O’Brien AR, DeLuca J. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: an updated review of the literature from 2007 to 2016. Arch Phys Med Rehabil. 2018;99(2):390-407.
1. Amato MP, Zipoli V, Portaccio E. Cognitive changes in multiple sclerosis. Expert Rev Neurother. 2008;8(10):1585-1596.
2. Labiano-Fontcuberta A, Martínez-Ginés ML, Aladro Y, et al. A comparison study of cognitive deficits in radiologically and clinically isolated syndromes. Mult Scler. 2016;22(2):250-253.
3. Benedict RH, Ramasamy D, Munschauer F, et al. Memory impairment in multiple sclerosis: correlation with deep grey matter and mesial temporal atrophy. J Neurol Neurosurg Psychiatry. 2009;80(2):201-206.
4. Rocca MA, Amato MP, De Stefano N, et al; MAGNIMS Study Group. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015;14(3):302-317.
5. Rovaris M, Comi G, Filippi M. MRI markers of destructive pathology in multiple sclerosis-related cognitive dysfunction. J Neurol Sci. 2006;245(1-2):111-116.
6. Johnen A, Landmeyer NC, Bürkner PC, et al. Distinct cognitive impairments in different disease courses of multiple sclerosis: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2017;83:568-578.
7. Rao SM, Leo GJ, Ellington L, et al. Cognitive dysfunction in multiple sclerosis. II. Impact on employment and social functioning. Neurology. 1991;41(5):692-696.
8. Parmenter BA, Weinstock-Guttman B, Garg N, et al. Screening for cognitive impairment in multiple sclerosis using the symbol digit modalities test. Mult Scler. 2007;13(1):52-57.
9. Goverover Y, Chiaravalloti ND, O’Brien AR, DeLuca J. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: an updated review of the literature from 2007 to 2016. Arch Phys Med Rehabil. 2018;99(2):390-407.
Osteoporosis: Breaking Down the Treatment Options
Ms. B, a 72-year-old woman, presents with new-onset low back pain. A comprehensive workup is performed, and a radiograph reveals compression fractures of the L1 and L2 vertebral bodies. The patient recalls no trauma to account for her fractures. Dual-energy x-ray absorptiometry (DXA) is ordered; the results show evidence of osteoporosis. Ms. B asks about initiating longterm treatment.
Osteoporosis is a disease of significant public health concern.1 According to the NIH Osteoporosis and Related Bone Diseases National Resource Center, more than 53 million people in the United States either have osteoporosis or are at high risk for it.2 The total cost of osteoporosis-related fractures is expected to reach $25.3 billion by 2025.3 It is estimated that one in three women (and one in five men) older than 50 will sustain osteoporotic fractures.4 The morbidity and mortality associated with these fractures must be recognized by health care providers in all medical specialties. Appropriate preventive and treatment modalities should be employed when providing care to persons with or at risk for osteoporosis. Advances in medical science have yielded multiple options for the prevention and treatment of osteoporosis.
CASE CONTINUED Ms. B’s medical history includes hypertension and GERD, for which she uses twice-daily dosing of a proton pump inhibitor (PPI). At age 53, she was diagnosed with left breast cancer, which required surgical excision and radiation therapy. She took tamoxifen for a total of five years, and the cancer did not recur. She takes no OTC products, including vitamins. She has no history of systemic inflammatory conditions, kidney stones, or extended treatment with corticosteroids. No history of gastrointestinal surgeries is reported. Ms. B has never smoked cigarettes and has never consumed two or more alcoholic beverages a day. She has no family history of osteoporosis in first-degree relatives. She is otherwise healthy but is physically inactive, with no regular weight-bearing exercise routine. It is also notable that she experienced an uneventful early menopause at age 41 and did not take estrogen replacement therapy.
NONPHARMACOLOGIC OPTIONS
Regular weight-bearing exercise, adequate calcium and vitamin D intake, smoking cessation, avoidance of heavy alcohol use, and education in fall prevention are vital. Recommended calcium intake varies by age, ranging from 1,000 mg/d to 1,200 mg/d in divided doses.2 Vitamin D intake is recommended at 600 IU/d until age 70; 800 IU/d after age 70;and additional units if deficiency is noted.2 Avoidance of medications that contribute to bone loss (eg, corticosteroids) is also encouraged, if possible. Patient education should include balance training and a home safety assessment.
CASE POINT Nonpharmacologic strategies should be encouraged for every patient to promote optimal bone health and to prevent or treat osteoporosis.
PHARMACOLOGIC OPTIONS
Oral bisphosphonates are considered firstline treatment for osteoporosis; currently available options include alendronate, risedronate, and ibandronate. Bisphosphonates work by inhibiting osteoclast function, thereby reducing bone resorption.5
Oral bisphosphonates have been clinically available since the 1990s and have demonstrated their efficacy, safety, and cost-effectiveness.6-8 However, a thoughtful approach should be taken to their use in specific patient populations: those with esophageal disorders, chronic kidney disease, and/or a history of bariatric gastrointestinal procedures. Bisphosphonates of any form should be avoided in a patient with chronic kidney disease with a glomerular filtration rate ≤ 30 mL/min or ≤ 35 mL/min (based on the package insert for the specific product).7 Patients with a recent or upcoming tooth extraction should also avoid using bisphosphonates until they have healed, due to concerns for osteonecrosis of the jaw.
Continue to: Administration of oral bisphosphonates requires...
Administration of oral bisphosphonates requires special attention. Oral bisphosphonates must be taken first thing in the morning with water; for the next 30 to 60 minutes, the patient must stay upright and not have any food, drink, or additional medications by mouth. These specifications may affect patient adherence to treatment.
Intravenous bisphosphonates. Depending on the IV bisphosphonate chosen—ibandronate and zoledronic acid are the currently available options—administration is recommended either every three or 12 months. A common adverse effect of IV bisphosphonates is flulike symptoms, which are generally brief in duration. Hypocalcemia has also been associated with IV administration, more so than with oral bisphosphonate use. Osteonecrosis of the jaw, while rare, must also be considered.
CASE POINT Because of Ms. B’s GERD requiring PPI use, oral bisphosphonates are not the most ideal treatment for her osteoporosis; they could exacerbate her gastrointestinal symptoms. IV bisphosphonates are a potential option for her, as this method of administration would eliminate the gastrointestinal risk associated with oral bisphosphonates.
Selective estrogen receptor modulators (SERMs), which are administered orally, are another option for osteoporosis treatment for vertebral fractures. One medication in this class, raloxifene, selectively acts on estrogen receptors—it works as an agonist in bone estrogen receptors (preventing bone loss) and an estrogen antagonist in other tissue (eg, breast, uterine). SERMs are not considered firstline treatment for osteoporosis because they appear to be less potent than other currently available agents. However, a postmenopausal patient with a high risk for invasive breast cancer without a history of fragility fracture might consider this option, as raloxifene can reduce the risk for invasive breast cancer.9 SERMs have been associated with an increase in thromboembolic events and hot flashes.
Calcitonin nasal spray is used much less commonly now because its effect on bone mineral density is weaker than other currently available options. Calcitonin nasal spray is administered as one spray in one nostril each day. There has been some concern regarding calcitonin use and its association with malignancy.10
Continue to: CASE POINT
CASE POINT Ms. B’s history of compression fractures suggests the need for potent pharmacologic options to treat her osteoporosis. SERMS and calcitonin nasal spray are felt to be less potent and therefore are not the preferred treatment recommendations for her
Parathyroid hormone analogs. The availability of the parathyroid hormone analogs teriparatide and abaloparatide gives patients and health care providers another treatment option for osteoporosis.11 These potent stimulators of bone remodeling help reduce future fracture risk. Teriparatide and abaloparatide are considered anabolic bone agents, rather than antiresorptive medications. These medications are administered subcutaneously daily for no more than two years. Many health care providers use parathyroid hormone analogs for patients with severe osteoporosis (T score, ≤ –3.5 without fragility fracture history or ≤ –2.5 with fragility fracture history).12 The cost of these agents must be considered when recommending them to eligible patients.8
Parathyroid hormone analogs do carry a black box warning because of an increased risk for osteosarcoma observed in rat studies.13,14 These products should therefore be avoided in patients with increased risk for osteosarcoma: those who have Paget disease of the bone or unexplained elevations of alkaline phosphatase; pediatric and young adult patients with open epiphyses; or those who have had external beam or implant radiation therapy involving the skeleton.13,14
CASE POINT Because of Ms. B’s prior history of breast cancer requiring radiation treatment, parathyroid hormone analogs are not recommended.
Denosumab is a human monoclonal antibody, a RANKL inhibitor, that works by preventing the development of osteoclasts. This medication is administered subcutaneously every six months. There are no dosing adjustments recommended for hepatic impairment.11 The denosumab package insert does not specify a dosage adjustment for patients with renal impairment; however, clinical studies have indicated that patients who have a creatine clearance < 30 mL/min or who are on dialysis are more likely to experience hypocalcemia with denosumab use.15 As with other newer osteoporosis treatments, cost considerations should be discussed with patients.
Continue to: One unique consideration...
One unique consideration is that clinical trials have shown an increased fracture risk and the return of bone mineral density to predenosumab treatment levels within 18 months of discontinuing the medication.15 Health care providers should be prepared to recommend alternative treatment options if denosumab is discontinued.
CASE CONCLUDED After a discussion of the risks, benefits, and expectations associated with each of the available treatment options, Ms. B and her health care provider narrow down her options to use of an IV bisphosphonate or denosumab for her osteoporosis. She ultimately chooses denosumab, based on her preference for an injectable medication.
CONCLUSION
The morbidity and mortality associated with osteoporosis can be improved with an appropriate balance of nonpharmacologic and pharmacologic approaches. The varying mechanisms of action, administration methods, and documented efficacy of the available medications provide an opportunity for patient education and informed decision-making when choosing treatment. For additional guidance, the American College of Physicians, the American Association of Clinical Endocrinologists, and American College of Endocrinology have published guidelines that can help in the decision-making process.16,17
1. Cauley JA. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci. 2013;68(10):1243-1251.
2. NIH Osteoporosis and Related Bone Diseases National Resource Center. Osteoporosis overview. February 2017. www.bones.nih.gov/health-info/bone/osteoporosis/overview. Accessed October 1, 2018.
3. Dempster DW. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. 2011;17: S164-S169.
4. International Osteoporosis Foundation. Osteoporosis facts and statistics. www.iofbonehealth.org/facts-and-statistics/calcium-studies-map. Accessed October 1, 2018.
5. Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med. 2009;360(1):53-62.
6. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122(2):S14-S21.
7. Miller PD. Long-term extension trials to prove the efficacy of and safety of bisphosphonates. Clin Invest. 2014;4(1):35-43.
8. Hiligsmann M, Evers SM, Sedrine B, et al. A systematic review of cost-effectiveness analyses of drugs for postmenopausal osteoporosis. Pharmacoeconomics. 2015;33(3):205-224.
9. Raloxifene [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
10. Wells G, Chernoff J, Gilligan JP, Krause DS. Does salmon calcitonin cause cancer? A review and meta-analysis. Osteoporos Int. 2016;27(1):13-19.
11. Leder BZ. Parathyroid hormone and parathyroid hormone-related protein analogs in osteoporosis therapy. Curr Osteoporos Rep. 2017;15:110-119.
12. Kendler DL, Marin F, Zerbini CAF, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391:230-240.
13. Teriparatide [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
14. Abaloparatide [package insert]. Waltham, MA: Radius Health, Inc; 2017.
15. Denosumab [package insert]. Thousand Oaks, CA: Amgen Inc; 2018.
16. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2016. Endocr Pract. 2016;22(4):1-42.
17. Qaseem A, Forciea MA, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(11):818-839.
Ms. B, a 72-year-old woman, presents with new-onset low back pain. A comprehensive workup is performed, and a radiograph reveals compression fractures of the L1 and L2 vertebral bodies. The patient recalls no trauma to account for her fractures. Dual-energy x-ray absorptiometry (DXA) is ordered; the results show evidence of osteoporosis. Ms. B asks about initiating longterm treatment.
Osteoporosis is a disease of significant public health concern.1 According to the NIH Osteoporosis and Related Bone Diseases National Resource Center, more than 53 million people in the United States either have osteoporosis or are at high risk for it.2 The total cost of osteoporosis-related fractures is expected to reach $25.3 billion by 2025.3 It is estimated that one in three women (and one in five men) older than 50 will sustain osteoporotic fractures.4 The morbidity and mortality associated with these fractures must be recognized by health care providers in all medical specialties. Appropriate preventive and treatment modalities should be employed when providing care to persons with or at risk for osteoporosis. Advances in medical science have yielded multiple options for the prevention and treatment of osteoporosis.
CASE CONTINUED Ms. B’s medical history includes hypertension and GERD, for which she uses twice-daily dosing of a proton pump inhibitor (PPI). At age 53, she was diagnosed with left breast cancer, which required surgical excision and radiation therapy. She took tamoxifen for a total of five years, and the cancer did not recur. She takes no OTC products, including vitamins. She has no history of systemic inflammatory conditions, kidney stones, or extended treatment with corticosteroids. No history of gastrointestinal surgeries is reported. Ms. B has never smoked cigarettes and has never consumed two or more alcoholic beverages a day. She has no family history of osteoporosis in first-degree relatives. She is otherwise healthy but is physically inactive, with no regular weight-bearing exercise routine. It is also notable that she experienced an uneventful early menopause at age 41 and did not take estrogen replacement therapy.
NONPHARMACOLOGIC OPTIONS
Regular weight-bearing exercise, adequate calcium and vitamin D intake, smoking cessation, avoidance of heavy alcohol use, and education in fall prevention are vital. Recommended calcium intake varies by age, ranging from 1,000 mg/d to 1,200 mg/d in divided doses.2 Vitamin D intake is recommended at 600 IU/d until age 70; 800 IU/d after age 70;and additional units if deficiency is noted.2 Avoidance of medications that contribute to bone loss (eg, corticosteroids) is also encouraged, if possible. Patient education should include balance training and a home safety assessment.
CASE POINT Nonpharmacologic strategies should be encouraged for every patient to promote optimal bone health and to prevent or treat osteoporosis.
PHARMACOLOGIC OPTIONS
Oral bisphosphonates are considered firstline treatment for osteoporosis; currently available options include alendronate, risedronate, and ibandronate. Bisphosphonates work by inhibiting osteoclast function, thereby reducing bone resorption.5
Oral bisphosphonates have been clinically available since the 1990s and have demonstrated their efficacy, safety, and cost-effectiveness.6-8 However, a thoughtful approach should be taken to their use in specific patient populations: those with esophageal disorders, chronic kidney disease, and/or a history of bariatric gastrointestinal procedures. Bisphosphonates of any form should be avoided in a patient with chronic kidney disease with a glomerular filtration rate ≤ 30 mL/min or ≤ 35 mL/min (based on the package insert for the specific product).7 Patients with a recent or upcoming tooth extraction should also avoid using bisphosphonates until they have healed, due to concerns for osteonecrosis of the jaw.
Continue to: Administration of oral bisphosphonates requires...
Administration of oral bisphosphonates requires special attention. Oral bisphosphonates must be taken first thing in the morning with water; for the next 30 to 60 minutes, the patient must stay upright and not have any food, drink, or additional medications by mouth. These specifications may affect patient adherence to treatment.
Intravenous bisphosphonates. Depending on the IV bisphosphonate chosen—ibandronate and zoledronic acid are the currently available options—administration is recommended either every three or 12 months. A common adverse effect of IV bisphosphonates is flulike symptoms, which are generally brief in duration. Hypocalcemia has also been associated with IV administration, more so than with oral bisphosphonate use. Osteonecrosis of the jaw, while rare, must also be considered.
CASE POINT Because of Ms. B’s GERD requiring PPI use, oral bisphosphonates are not the most ideal treatment for her osteoporosis; they could exacerbate her gastrointestinal symptoms. IV bisphosphonates are a potential option for her, as this method of administration would eliminate the gastrointestinal risk associated with oral bisphosphonates.
Selective estrogen receptor modulators (SERMs), which are administered orally, are another option for osteoporosis treatment for vertebral fractures. One medication in this class, raloxifene, selectively acts on estrogen receptors—it works as an agonist in bone estrogen receptors (preventing bone loss) and an estrogen antagonist in other tissue (eg, breast, uterine). SERMs are not considered firstline treatment for osteoporosis because they appear to be less potent than other currently available agents. However, a postmenopausal patient with a high risk for invasive breast cancer without a history of fragility fracture might consider this option, as raloxifene can reduce the risk for invasive breast cancer.9 SERMs have been associated with an increase in thromboembolic events and hot flashes.
Calcitonin nasal spray is used much less commonly now because its effect on bone mineral density is weaker than other currently available options. Calcitonin nasal spray is administered as one spray in one nostril each day. There has been some concern regarding calcitonin use and its association with malignancy.10
Continue to: CASE POINT
CASE POINT Ms. B’s history of compression fractures suggests the need for potent pharmacologic options to treat her osteoporosis. SERMS and calcitonin nasal spray are felt to be less potent and therefore are not the preferred treatment recommendations for her
Parathyroid hormone analogs. The availability of the parathyroid hormone analogs teriparatide and abaloparatide gives patients and health care providers another treatment option for osteoporosis.11 These potent stimulators of bone remodeling help reduce future fracture risk. Teriparatide and abaloparatide are considered anabolic bone agents, rather than antiresorptive medications. These medications are administered subcutaneously daily for no more than two years. Many health care providers use parathyroid hormone analogs for patients with severe osteoporosis (T score, ≤ –3.5 without fragility fracture history or ≤ –2.5 with fragility fracture history).12 The cost of these agents must be considered when recommending them to eligible patients.8
Parathyroid hormone analogs do carry a black box warning because of an increased risk for osteosarcoma observed in rat studies.13,14 These products should therefore be avoided in patients with increased risk for osteosarcoma: those who have Paget disease of the bone or unexplained elevations of alkaline phosphatase; pediatric and young adult patients with open epiphyses; or those who have had external beam or implant radiation therapy involving the skeleton.13,14
CASE POINT Because of Ms. B’s prior history of breast cancer requiring radiation treatment, parathyroid hormone analogs are not recommended.
Denosumab is a human monoclonal antibody, a RANKL inhibitor, that works by preventing the development of osteoclasts. This medication is administered subcutaneously every six months. There are no dosing adjustments recommended for hepatic impairment.11 The denosumab package insert does not specify a dosage adjustment for patients with renal impairment; however, clinical studies have indicated that patients who have a creatine clearance < 30 mL/min or who are on dialysis are more likely to experience hypocalcemia with denosumab use.15 As with other newer osteoporosis treatments, cost considerations should be discussed with patients.
Continue to: One unique consideration...
One unique consideration is that clinical trials have shown an increased fracture risk and the return of bone mineral density to predenosumab treatment levels within 18 months of discontinuing the medication.15 Health care providers should be prepared to recommend alternative treatment options if denosumab is discontinued.
CASE CONCLUDED After a discussion of the risks, benefits, and expectations associated with each of the available treatment options, Ms. B and her health care provider narrow down her options to use of an IV bisphosphonate or denosumab for her osteoporosis. She ultimately chooses denosumab, based on her preference for an injectable medication.
CONCLUSION
The morbidity and mortality associated with osteoporosis can be improved with an appropriate balance of nonpharmacologic and pharmacologic approaches. The varying mechanisms of action, administration methods, and documented efficacy of the available medications provide an opportunity for patient education and informed decision-making when choosing treatment. For additional guidance, the American College of Physicians, the American Association of Clinical Endocrinologists, and American College of Endocrinology have published guidelines that can help in the decision-making process.16,17
Ms. B, a 72-year-old woman, presents with new-onset low back pain. A comprehensive workup is performed, and a radiograph reveals compression fractures of the L1 and L2 vertebral bodies. The patient recalls no trauma to account for her fractures. Dual-energy x-ray absorptiometry (DXA) is ordered; the results show evidence of osteoporosis. Ms. B asks about initiating longterm treatment.
Osteoporosis is a disease of significant public health concern.1 According to the NIH Osteoporosis and Related Bone Diseases National Resource Center, more than 53 million people in the United States either have osteoporosis or are at high risk for it.2 The total cost of osteoporosis-related fractures is expected to reach $25.3 billion by 2025.3 It is estimated that one in three women (and one in five men) older than 50 will sustain osteoporotic fractures.4 The morbidity and mortality associated with these fractures must be recognized by health care providers in all medical specialties. Appropriate preventive and treatment modalities should be employed when providing care to persons with or at risk for osteoporosis. Advances in medical science have yielded multiple options for the prevention and treatment of osteoporosis.
CASE CONTINUED Ms. B’s medical history includes hypertension and GERD, for which she uses twice-daily dosing of a proton pump inhibitor (PPI). At age 53, she was diagnosed with left breast cancer, which required surgical excision and radiation therapy. She took tamoxifen for a total of five years, and the cancer did not recur. She takes no OTC products, including vitamins. She has no history of systemic inflammatory conditions, kidney stones, or extended treatment with corticosteroids. No history of gastrointestinal surgeries is reported. Ms. B has never smoked cigarettes and has never consumed two or more alcoholic beverages a day. She has no family history of osteoporosis in first-degree relatives. She is otherwise healthy but is physically inactive, with no regular weight-bearing exercise routine. It is also notable that she experienced an uneventful early menopause at age 41 and did not take estrogen replacement therapy.
NONPHARMACOLOGIC OPTIONS
Regular weight-bearing exercise, adequate calcium and vitamin D intake, smoking cessation, avoidance of heavy alcohol use, and education in fall prevention are vital. Recommended calcium intake varies by age, ranging from 1,000 mg/d to 1,200 mg/d in divided doses.2 Vitamin D intake is recommended at 600 IU/d until age 70; 800 IU/d after age 70;and additional units if deficiency is noted.2 Avoidance of medications that contribute to bone loss (eg, corticosteroids) is also encouraged, if possible. Patient education should include balance training and a home safety assessment.
CASE POINT Nonpharmacologic strategies should be encouraged for every patient to promote optimal bone health and to prevent or treat osteoporosis.
PHARMACOLOGIC OPTIONS
Oral bisphosphonates are considered firstline treatment for osteoporosis; currently available options include alendronate, risedronate, and ibandronate. Bisphosphonates work by inhibiting osteoclast function, thereby reducing bone resorption.5
Oral bisphosphonates have been clinically available since the 1990s and have demonstrated their efficacy, safety, and cost-effectiveness.6-8 However, a thoughtful approach should be taken to their use in specific patient populations: those with esophageal disorders, chronic kidney disease, and/or a history of bariatric gastrointestinal procedures. Bisphosphonates of any form should be avoided in a patient with chronic kidney disease with a glomerular filtration rate ≤ 30 mL/min or ≤ 35 mL/min (based on the package insert for the specific product).7 Patients with a recent or upcoming tooth extraction should also avoid using bisphosphonates until they have healed, due to concerns for osteonecrosis of the jaw.
Continue to: Administration of oral bisphosphonates requires...
Administration of oral bisphosphonates requires special attention. Oral bisphosphonates must be taken first thing in the morning with water; for the next 30 to 60 minutes, the patient must stay upright and not have any food, drink, or additional medications by mouth. These specifications may affect patient adherence to treatment.
Intravenous bisphosphonates. Depending on the IV bisphosphonate chosen—ibandronate and zoledronic acid are the currently available options—administration is recommended either every three or 12 months. A common adverse effect of IV bisphosphonates is flulike symptoms, which are generally brief in duration. Hypocalcemia has also been associated with IV administration, more so than with oral bisphosphonate use. Osteonecrosis of the jaw, while rare, must also be considered.
CASE POINT Because of Ms. B’s GERD requiring PPI use, oral bisphosphonates are not the most ideal treatment for her osteoporosis; they could exacerbate her gastrointestinal symptoms. IV bisphosphonates are a potential option for her, as this method of administration would eliminate the gastrointestinal risk associated with oral bisphosphonates.
Selective estrogen receptor modulators (SERMs), which are administered orally, are another option for osteoporosis treatment for vertebral fractures. One medication in this class, raloxifene, selectively acts on estrogen receptors—it works as an agonist in bone estrogen receptors (preventing bone loss) and an estrogen antagonist in other tissue (eg, breast, uterine). SERMs are not considered firstline treatment for osteoporosis because they appear to be less potent than other currently available agents. However, a postmenopausal patient with a high risk for invasive breast cancer without a history of fragility fracture might consider this option, as raloxifene can reduce the risk for invasive breast cancer.9 SERMs have been associated with an increase in thromboembolic events and hot flashes.
Calcitonin nasal spray is used much less commonly now because its effect on bone mineral density is weaker than other currently available options. Calcitonin nasal spray is administered as one spray in one nostril each day. There has been some concern regarding calcitonin use and its association with malignancy.10
Continue to: CASE POINT
CASE POINT Ms. B’s history of compression fractures suggests the need for potent pharmacologic options to treat her osteoporosis. SERMS and calcitonin nasal spray are felt to be less potent and therefore are not the preferred treatment recommendations for her
Parathyroid hormone analogs. The availability of the parathyroid hormone analogs teriparatide and abaloparatide gives patients and health care providers another treatment option for osteoporosis.11 These potent stimulators of bone remodeling help reduce future fracture risk. Teriparatide and abaloparatide are considered anabolic bone agents, rather than antiresorptive medications. These medications are administered subcutaneously daily for no more than two years. Many health care providers use parathyroid hormone analogs for patients with severe osteoporosis (T score, ≤ –3.5 without fragility fracture history or ≤ –2.5 with fragility fracture history).12 The cost of these agents must be considered when recommending them to eligible patients.8
Parathyroid hormone analogs do carry a black box warning because of an increased risk for osteosarcoma observed in rat studies.13,14 These products should therefore be avoided in patients with increased risk for osteosarcoma: those who have Paget disease of the bone or unexplained elevations of alkaline phosphatase; pediatric and young adult patients with open epiphyses; or those who have had external beam or implant radiation therapy involving the skeleton.13,14
CASE POINT Because of Ms. B’s prior history of breast cancer requiring radiation treatment, parathyroid hormone analogs are not recommended.
Denosumab is a human monoclonal antibody, a RANKL inhibitor, that works by preventing the development of osteoclasts. This medication is administered subcutaneously every six months. There are no dosing adjustments recommended for hepatic impairment.11 The denosumab package insert does not specify a dosage adjustment for patients with renal impairment; however, clinical studies have indicated that patients who have a creatine clearance < 30 mL/min or who are on dialysis are more likely to experience hypocalcemia with denosumab use.15 As with other newer osteoporosis treatments, cost considerations should be discussed with patients.
Continue to: One unique consideration...
One unique consideration is that clinical trials have shown an increased fracture risk and the return of bone mineral density to predenosumab treatment levels within 18 months of discontinuing the medication.15 Health care providers should be prepared to recommend alternative treatment options if denosumab is discontinued.
CASE CONCLUDED After a discussion of the risks, benefits, and expectations associated with each of the available treatment options, Ms. B and her health care provider narrow down her options to use of an IV bisphosphonate or denosumab for her osteoporosis. She ultimately chooses denosumab, based on her preference for an injectable medication.
CONCLUSION
The morbidity and mortality associated with osteoporosis can be improved with an appropriate balance of nonpharmacologic and pharmacologic approaches. The varying mechanisms of action, administration methods, and documented efficacy of the available medications provide an opportunity for patient education and informed decision-making when choosing treatment. For additional guidance, the American College of Physicians, the American Association of Clinical Endocrinologists, and American College of Endocrinology have published guidelines that can help in the decision-making process.16,17
1. Cauley JA. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci. 2013;68(10):1243-1251.
2. NIH Osteoporosis and Related Bone Diseases National Resource Center. Osteoporosis overview. February 2017. www.bones.nih.gov/health-info/bone/osteoporosis/overview. Accessed October 1, 2018.
3. Dempster DW. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. 2011;17: S164-S169.
4. International Osteoporosis Foundation. Osteoporosis facts and statistics. www.iofbonehealth.org/facts-and-statistics/calcium-studies-map. Accessed October 1, 2018.
5. Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med. 2009;360(1):53-62.
6. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122(2):S14-S21.
7. Miller PD. Long-term extension trials to prove the efficacy of and safety of bisphosphonates. Clin Invest. 2014;4(1):35-43.
8. Hiligsmann M, Evers SM, Sedrine B, et al. A systematic review of cost-effectiveness analyses of drugs for postmenopausal osteoporosis. Pharmacoeconomics. 2015;33(3):205-224.
9. Raloxifene [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
10. Wells G, Chernoff J, Gilligan JP, Krause DS. Does salmon calcitonin cause cancer? A review and meta-analysis. Osteoporos Int. 2016;27(1):13-19.
11. Leder BZ. Parathyroid hormone and parathyroid hormone-related protein analogs in osteoporosis therapy. Curr Osteoporos Rep. 2017;15:110-119.
12. Kendler DL, Marin F, Zerbini CAF, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391:230-240.
13. Teriparatide [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
14. Abaloparatide [package insert]. Waltham, MA: Radius Health, Inc; 2017.
15. Denosumab [package insert]. Thousand Oaks, CA: Amgen Inc; 2018.
16. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2016. Endocr Pract. 2016;22(4):1-42.
17. Qaseem A, Forciea MA, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(11):818-839.
1. Cauley JA. Public health impact of osteoporosis. J Gerontol A Biol Sci Med Sci. 2013;68(10):1243-1251.
2. NIH Osteoporosis and Related Bone Diseases National Resource Center. Osteoporosis overview. February 2017. www.bones.nih.gov/health-info/bone/osteoporosis/overview. Accessed October 1, 2018.
3. Dempster DW. Osteoporosis and the burden of osteoporosis-related fractures. Am J Manag Care. 2011;17: S164-S169.
4. International Osteoporosis Foundation. Osteoporosis facts and statistics. www.iofbonehealth.org/facts-and-statistics/calcium-studies-map. Accessed October 1, 2018.
5. Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med. 2009;360(1):53-62.
6. Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122(2):S14-S21.
7. Miller PD. Long-term extension trials to prove the efficacy of and safety of bisphosphonates. Clin Invest. 2014;4(1):35-43.
8. Hiligsmann M, Evers SM, Sedrine B, et al. A systematic review of cost-effectiveness analyses of drugs for postmenopausal osteoporosis. Pharmacoeconomics. 2015;33(3):205-224.
9. Raloxifene [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
10. Wells G, Chernoff J, Gilligan JP, Krause DS. Does salmon calcitonin cause cancer? A review and meta-analysis. Osteoporos Int. 2016;27(1):13-19.
11. Leder BZ. Parathyroid hormone and parathyroid hormone-related protein analogs in osteoporosis therapy. Curr Osteoporos Rep. 2017;15:110-119.
12. Kendler DL, Marin F, Zerbini CAF, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391:230-240.
13. Teriparatide [package insert]. Indianapolis, IN: Lilly USA, LLC; 2018.
14. Abaloparatide [package insert]. Waltham, MA: Radius Health, Inc; 2017.
15. Denosumab [package insert]. Thousand Oaks, CA: Amgen Inc; 2018.
16. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2016. Endocr Pract. 2016;22(4):1-42.
17. Qaseem A, Forciea MA, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(11):818-839.
How Do I Use the New Cholesterol Guidelines?
Q) I’m still confused by the change in approach to use of statin therapy for cardiovascular disease. How do I determine which patients need statins?
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death in adults in the United States.1 Statins have long been recommended in the management of individuals with ASCVD.
Historically, statin use was guided by an LDL cholesterol (LDL-C) target, per the Adult Treatment Panel (ATP III) guidelines. Therapy was intensified based on whether patients met these targets. Newer guidelines from the American Heart Association/American College of Cardiology (AHA/ACC) base statin therapy not on an LDL-C number but rather on risk stratification that considers several factors.1-3
Continue to: The AHA/ACC guidelines classify statins as...
The AHA/ACC guidelines classify statins as high-, moderate-, or low-intensity.2 They also identify four major groups in whom the benefits of statin therapy for reducing ASCVD risk outweigh the risks of therapy. These include patients with
- Clinical ASCVD (eg, coronary heart disease, stroke, transient ischemic attack, or atherosclerotic peripheral arterial disease)
- Primary elevated LDL-C ≥ 190 mg/dL
- Diabetes (specifically, in those ages 40-75 with an LDL-C of 70-189 mg/dL)
- An estimated 10-year ASCVD risk ≥ 7.5%.2,3 (A risk calculator can be found at www.cvriskcalculator.com).
Recommended statin regimens for patients meeting these criteria are outlined in the Table.
These new guidelines significantly increase the number of adults who are eligible for statin therapy. The number of adults ages 60 to 75 without cardiovascular disease who now qualify for statin therapy has substantially increased (from 30% to 87% in men and from 21% to 54% among women).4 The bulk of this increase is in adults needing primary prevention based on their 10-year cardiovascular risk.4 Evidence as to whether expanded use of statins will improve clinical outcomes is still pending. —AF
Ashley Fort
PA Program at Louisiana State University Health Science Center
1. Gencer B, Auer R, Nanchen D, et al. Expected impact of applying new 2013 AHA/ACC cholesterol guidelines criteria on the recommended lipid target achievement after acute coronary syndromes. Atherosclerosis. 2015; 239(1):118-124.
2. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25):2889-2934.
3. Adhyaru B, Jacobson T. New cholesterol guidelines for the management of atherosclerotic cardiovascular disease risk: a comparison of the 2013 American College of Cardiology/American Heart Association cholesterol guidelines with the 2014 National Lipid Association recommendations for patient-centered management of dyslipidemia. Cardiol Clin. 2015;33(15):181-196.
4. Pencina MJ, Navar-Boggan AM, D’Agostino RB Sr, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014;370(15):1422-1431.
Q) I’m still confused by the change in approach to use of statin therapy for cardiovascular disease. How do I determine which patients need statins?
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death in adults in the United States.1 Statins have long been recommended in the management of individuals with ASCVD.
Historically, statin use was guided by an LDL cholesterol (LDL-C) target, per the Adult Treatment Panel (ATP III) guidelines. Therapy was intensified based on whether patients met these targets. Newer guidelines from the American Heart Association/American College of Cardiology (AHA/ACC) base statin therapy not on an LDL-C number but rather on risk stratification that considers several factors.1-3
Continue to: The AHA/ACC guidelines classify statins as...
The AHA/ACC guidelines classify statins as high-, moderate-, or low-intensity.2 They also identify four major groups in whom the benefits of statin therapy for reducing ASCVD risk outweigh the risks of therapy. These include patients with
- Clinical ASCVD (eg, coronary heart disease, stroke, transient ischemic attack, or atherosclerotic peripheral arterial disease)
- Primary elevated LDL-C ≥ 190 mg/dL
- Diabetes (specifically, in those ages 40-75 with an LDL-C of 70-189 mg/dL)
- An estimated 10-year ASCVD risk ≥ 7.5%.2,3 (A risk calculator can be found at www.cvriskcalculator.com).
Recommended statin regimens for patients meeting these criteria are outlined in the Table.
These new guidelines significantly increase the number of adults who are eligible for statin therapy. The number of adults ages 60 to 75 without cardiovascular disease who now qualify for statin therapy has substantially increased (from 30% to 87% in men and from 21% to 54% among women).4 The bulk of this increase is in adults needing primary prevention based on their 10-year cardiovascular risk.4 Evidence as to whether expanded use of statins will improve clinical outcomes is still pending. —AF
Ashley Fort
PA Program at Louisiana State University Health Science Center
Q) I’m still confused by the change in approach to use of statin therapy for cardiovascular disease. How do I determine which patients need statins?
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death in adults in the United States.1 Statins have long been recommended in the management of individuals with ASCVD.
Historically, statin use was guided by an LDL cholesterol (LDL-C) target, per the Adult Treatment Panel (ATP III) guidelines. Therapy was intensified based on whether patients met these targets. Newer guidelines from the American Heart Association/American College of Cardiology (AHA/ACC) base statin therapy not on an LDL-C number but rather on risk stratification that considers several factors.1-3
Continue to: The AHA/ACC guidelines classify statins as...
The AHA/ACC guidelines classify statins as high-, moderate-, or low-intensity.2 They also identify four major groups in whom the benefits of statin therapy for reducing ASCVD risk outweigh the risks of therapy. These include patients with
- Clinical ASCVD (eg, coronary heart disease, stroke, transient ischemic attack, or atherosclerotic peripheral arterial disease)
- Primary elevated LDL-C ≥ 190 mg/dL
- Diabetes (specifically, in those ages 40-75 with an LDL-C of 70-189 mg/dL)
- An estimated 10-year ASCVD risk ≥ 7.5%.2,3 (A risk calculator can be found at www.cvriskcalculator.com).
Recommended statin regimens for patients meeting these criteria are outlined in the Table.
These new guidelines significantly increase the number of adults who are eligible for statin therapy. The number of adults ages 60 to 75 without cardiovascular disease who now qualify for statin therapy has substantially increased (from 30% to 87% in men and from 21% to 54% among women).4 The bulk of this increase is in adults needing primary prevention based on their 10-year cardiovascular risk.4 Evidence as to whether expanded use of statins will improve clinical outcomes is still pending. —AF
Ashley Fort
PA Program at Louisiana State University Health Science Center
1. Gencer B, Auer R, Nanchen D, et al. Expected impact of applying new 2013 AHA/ACC cholesterol guidelines criteria on the recommended lipid target achievement after acute coronary syndromes. Atherosclerosis. 2015; 239(1):118-124.
2. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25):2889-2934.
3. Adhyaru B, Jacobson T. New cholesterol guidelines for the management of atherosclerotic cardiovascular disease risk: a comparison of the 2013 American College of Cardiology/American Heart Association cholesterol guidelines with the 2014 National Lipid Association recommendations for patient-centered management of dyslipidemia. Cardiol Clin. 2015;33(15):181-196.
4. Pencina MJ, Navar-Boggan AM, D’Agostino RB Sr, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014;370(15):1422-1431.
1. Gencer B, Auer R, Nanchen D, et al. Expected impact of applying new 2013 AHA/ACC cholesterol guidelines criteria on the recommended lipid target achievement after acute coronary syndromes. Atherosclerosis. 2015; 239(1):118-124.
2. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25):2889-2934.
3. Adhyaru B, Jacobson T. New cholesterol guidelines for the management of atherosclerotic cardiovascular disease risk: a comparison of the 2013 American College of Cardiology/American Heart Association cholesterol guidelines with the 2014 National Lipid Association recommendations for patient-centered management of dyslipidemia. Cardiol Clin. 2015;33(15):181-196.
4. Pencina MJ, Navar-Boggan AM, D’Agostino RB Sr, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med. 2014;370(15):1422-1431.
The Unsaid Dangers of NSAIDs
Q) Many total joint replacements and other orthopedic procedures are performed at the surgical center where I work. To decrease the use of narcotics, the anesthesiology department often uses IV push ketorolac postop. Our nephrology colleagues in the community are unhappy about this—but we think they’re overreacting, since these patients are often generally healthy. Is there any data on the use of ketorolac and orthopedic surgery?
All medications have associated risks. For example, while therapeutic dosages for a limited time are considered safe and effective, prolonged use of any NSAID can increase the risk for acute kidney injury (AKI) or chronic kidney disease (CKD) progression. We tend to associate these issues only with patients who are at higher risk for CKD: those who are older or who have diabetes or hypertension.
Thus, it was shocking to read a clinical report on four previously healthy young adults who were admitted for AKI three to four days after postoperative administration of ketorolac. None of these patients had risk factors that would predispose them to kidney disease. All had complained of gastrointestinal symptoms along with mild dehydration and flank pain; one young man even required a kidney biopsy and dialysis. All four did eventually recover kidney function. 1
Continue to: Ketorolac—like most NSAIDs...
Ketorolac—like most NSAIDs—can affect kidney function, decreasing renal plasma flow and causing a dysfunction in salt and water balance. Postoperative patients may have activity limitations (eg, the young healthy patient on crutches). Factor in kidney damage from presurgical/outpatient
With the opioid crisis at the forefront of national health news, nonnarcotic alternatives for pain control are much in demand. This puts a whole new population at risk for AKI. Educate patients and their families about preventive measures, such as controlling nausea, maintaining hydration, and monitoring urine output. Fever, flank pain, or any untoward symptoms should be reported. Remember, AKI may be more common in the older patient with diabetes—but it can occur in anyone. —EA
Ellen Apple
Dickson Schools Family Clinic, Tennessee
1. Mariano F, Cogno C, Giaretta F, et al. Urinary protein profiles in ketorolac-associated acute kidney injury in patients undergoing orthopedic day surgery. Int J Nephrol Renovasc Dis. 2017;10:269-274.
Q) Many total joint replacements and other orthopedic procedures are performed at the surgical center where I work. To decrease the use of narcotics, the anesthesiology department often uses IV push ketorolac postop. Our nephrology colleagues in the community are unhappy about this—but we think they’re overreacting, since these patients are often generally healthy. Is there any data on the use of ketorolac and orthopedic surgery?
All medications have associated risks. For example, while therapeutic dosages for a limited time are considered safe and effective, prolonged use of any NSAID can increase the risk for acute kidney injury (AKI) or chronic kidney disease (CKD) progression. We tend to associate these issues only with patients who are at higher risk for CKD: those who are older or who have diabetes or hypertension.
Thus, it was shocking to read a clinical report on four previously healthy young adults who were admitted for AKI three to four days after postoperative administration of ketorolac. None of these patients had risk factors that would predispose them to kidney disease. All had complained of gastrointestinal symptoms along with mild dehydration and flank pain; one young man even required a kidney biopsy and dialysis. All four did eventually recover kidney function. 1
Continue to: Ketorolac—like most NSAIDs...
Ketorolac—like most NSAIDs—can affect kidney function, decreasing renal plasma flow and causing a dysfunction in salt and water balance. Postoperative patients may have activity limitations (eg, the young healthy patient on crutches). Factor in kidney damage from presurgical/outpatient
With the opioid crisis at the forefront of national health news, nonnarcotic alternatives for pain control are much in demand. This puts a whole new population at risk for AKI. Educate patients and their families about preventive measures, such as controlling nausea, maintaining hydration, and monitoring urine output. Fever, flank pain, or any untoward symptoms should be reported. Remember, AKI may be more common in the older patient with diabetes—but it can occur in anyone. —EA
Ellen Apple
Dickson Schools Family Clinic, Tennessee
Q) Many total joint replacements and other orthopedic procedures are performed at the surgical center where I work. To decrease the use of narcotics, the anesthesiology department often uses IV push ketorolac postop. Our nephrology colleagues in the community are unhappy about this—but we think they’re overreacting, since these patients are often generally healthy. Is there any data on the use of ketorolac and orthopedic surgery?
All medications have associated risks. For example, while therapeutic dosages for a limited time are considered safe and effective, prolonged use of any NSAID can increase the risk for acute kidney injury (AKI) or chronic kidney disease (CKD) progression. We tend to associate these issues only with patients who are at higher risk for CKD: those who are older or who have diabetes or hypertension.
Thus, it was shocking to read a clinical report on four previously healthy young adults who were admitted for AKI three to four days after postoperative administration of ketorolac. None of these patients had risk factors that would predispose them to kidney disease. All had complained of gastrointestinal symptoms along with mild dehydration and flank pain; one young man even required a kidney biopsy and dialysis. All four did eventually recover kidney function. 1
Continue to: Ketorolac—like most NSAIDs...
Ketorolac—like most NSAIDs—can affect kidney function, decreasing renal plasma flow and causing a dysfunction in salt and water balance. Postoperative patients may have activity limitations (eg, the young healthy patient on crutches). Factor in kidney damage from presurgical/outpatient
With the opioid crisis at the forefront of national health news, nonnarcotic alternatives for pain control are much in demand. This puts a whole new population at risk for AKI. Educate patients and their families about preventive measures, such as controlling nausea, maintaining hydration, and monitoring urine output. Fever, flank pain, or any untoward symptoms should be reported. Remember, AKI may be more common in the older patient with diabetes—but it can occur in anyone. —EA
Ellen Apple
Dickson Schools Family Clinic, Tennessee
1. Mariano F, Cogno C, Giaretta F, et al. Urinary protein profiles in ketorolac-associated acute kidney injury in patients undergoing orthopedic day surgery. Int J Nephrol Renovasc Dis. 2017;10:269-274.
1. Mariano F, Cogno C, Giaretta F, et al. Urinary protein profiles in ketorolac-associated acute kidney injury in patients undergoing orthopedic day surgery. Int J Nephrol Renovasc Dis. 2017;10:269-274.