User login
Product update: Cervical dilator, hair rejuvenation cap, pelvic floor SUI treatment
NEW CERVICAL DILATOR
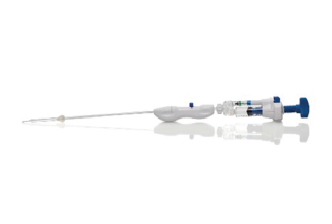
Hologic, Inc. announces the launch of the Definity Cervical Dilator, allowing tenaculum-free access to the uterine cavity, which lessens patient discomfort and reduces the risk of perforation during dilation, says Hologic. The dilator uses SureAccess—an expandable balloon technology designed to eliminate multiple passes and ensure safety during insertion, even for patients with complex or challenging cervical anatomy. Definity Cervical Dilator is not intended for use during induction of labor; some examples for its use include treatment of cervical stenosis, intrauterine device placement and removal, uterine tissue removal, diagnostic hysteroscopy, and operative hysteroscopy. Use of the system is contraindicated in patients with active genital tract infection, pelvic structure abnormality preventing device passage, or invasive cervical cancer. The Definity Cervical Dilator is now available across the United States in 5 mm, 7 mm, and 9 mm sizes.
FOR MORE INFORMATION, VISIT: https://www.hologic.com/
LIGHT DEVICE FOR HAIR REJUVENATION
Pattern hair loss (androgenetic alopecia) affects about 80 million men and women in the United States. In women, the hair loss typically occurs as thinning or widening of the midline. Revian, Inc. is focused on improving overall scalp health by stimulating the body’s natural processes for hair and skin rejuvenation with light therapy. REVIAN RED, a dual-wavelength LED hair growth cap, has successfully demonstrated the ability to stop hair loss and subsequently grow new hair, says Revian. In a recent clinical trial, women who were at least 80% compliant with wearing REVIAN RED (versus a placebo cap with no light therapy) for 10 minutes per day had a mean improvement of 26.3 hairs/cm2. Scalp irritations were assessed during the trial, with patient-reported itching and burning/stinging treated with at-home therapies.
The REVIAN RED wireless cap system is controlled by a mobile app and is US Food and Drug Administration (FDA) cleared as a hair loss treatment for men and women. The mechanism of action for improved scalp symptoms, says Revian, is the patented dual-wavelength light, which releases nitric oxide and is proposed to be anti-inflammatory.
The REVIAN RED system is indicated to treat androgenetic alopecia and to promote hair growth in men with Norwood-Hamilton classifications of IIa–V patterns of hair loss and to treat androgenetic alopecia and promote hair growth in women with Ludwig-Savin Scale I-1 to I-4, II-1, II-2 or frontal patterns of hair loss; both with Fitzpatrick Skin Types I–IV.
FOR MORE INFORMATION, VISIT: https://www.revian.com/
SUI TREATMENT
ELITONE from Elidah is the first transcutaneous pelvic floor muscle stimulation treatment for stress urinary incontinence (SUI). It is FDA cleared and works to train women to perform Kegel techniques by naturally contracting the correct pelvic floor muscles and surrounding tissues. The device is placed where a pad would go, according to Elidah, with conductive gel areas within the device delivering electrical muscle stimulation. Twenty-minute treatments 4 to 5 times per week are recommended, with improved SUI symptoms, depending on severity, for many women in 6 weeks. Three-quarters of women had significant reduction in daily leaks after 6 weeks of self-administered treatment with ELITONE, reports the manufacturer.
Elidah says that ELITONE is ideal for women with mild to moderate SUI symptoms who would benefit from pelvic floor muscle training, including those who are resistant to intravaginal treatments, need postpartum care, or have limited access to physical therapy. The device is contraindicated in women who have an implanted cardiac device, cancer, epilepsy, or recent pelvic floor surgery.
FOR OTHER CONTRAINDICATIONS AND MORE INFORMATION, VISIT: https:/elitone.com/
NEW CERVICAL DILATOR

Hologic, Inc. announces the launch of the Definity Cervical Dilator, allowing tenaculum-free access to the uterine cavity, which lessens patient discomfort and reduces the risk of perforation during dilation, says Hologic. The dilator uses SureAccess—an expandable balloon technology designed to eliminate multiple passes and ensure safety during insertion, even for patients with complex or challenging cervical anatomy. Definity Cervical Dilator is not intended for use during induction of labor; some examples for its use include treatment of cervical stenosis, intrauterine device placement and removal, uterine tissue removal, diagnostic hysteroscopy, and operative hysteroscopy. Use of the system is contraindicated in patients with active genital tract infection, pelvic structure abnormality preventing device passage, or invasive cervical cancer. The Definity Cervical Dilator is now available across the United States in 5 mm, 7 mm, and 9 mm sizes.
FOR MORE INFORMATION, VISIT: https://www.hologic.com/
LIGHT DEVICE FOR HAIR REJUVENATION
Pattern hair loss (androgenetic alopecia) affects about 80 million men and women in the United States. In women, the hair loss typically occurs as thinning or widening of the midline. Revian, Inc. is focused on improving overall scalp health by stimulating the body’s natural processes for hair and skin rejuvenation with light therapy. REVIAN RED, a dual-wavelength LED hair growth cap, has successfully demonstrated the ability to stop hair loss and subsequently grow new hair, says Revian. In a recent clinical trial, women who were at least 80% compliant with wearing REVIAN RED (versus a placebo cap with no light therapy) for 10 minutes per day had a mean improvement of 26.3 hairs/cm2. Scalp irritations were assessed during the trial, with patient-reported itching and burning/stinging treated with at-home therapies.
The REVIAN RED wireless cap system is controlled by a mobile app and is US Food and Drug Administration (FDA) cleared as a hair loss treatment for men and women. The mechanism of action for improved scalp symptoms, says Revian, is the patented dual-wavelength light, which releases nitric oxide and is proposed to be anti-inflammatory.
The REVIAN RED system is indicated to treat androgenetic alopecia and to promote hair growth in men with Norwood-Hamilton classifications of IIa–V patterns of hair loss and to treat androgenetic alopecia and promote hair growth in women with Ludwig-Savin Scale I-1 to I-4, II-1, II-2 or frontal patterns of hair loss; both with Fitzpatrick Skin Types I–IV.
FOR MORE INFORMATION, VISIT: https://www.revian.com/
SUI TREATMENT
ELITONE from Elidah is the first transcutaneous pelvic floor muscle stimulation treatment for stress urinary incontinence (SUI). It is FDA cleared and works to train women to perform Kegel techniques by naturally contracting the correct pelvic floor muscles and surrounding tissues. The device is placed where a pad would go, according to Elidah, with conductive gel areas within the device delivering electrical muscle stimulation. Twenty-minute treatments 4 to 5 times per week are recommended, with improved SUI symptoms, depending on severity, for many women in 6 weeks. Three-quarters of women had significant reduction in daily leaks after 6 weeks of self-administered treatment with ELITONE, reports the manufacturer.
Elidah says that ELITONE is ideal for women with mild to moderate SUI symptoms who would benefit from pelvic floor muscle training, including those who are resistant to intravaginal treatments, need postpartum care, or have limited access to physical therapy. The device is contraindicated in women who have an implanted cardiac device, cancer, epilepsy, or recent pelvic floor surgery.
FOR OTHER CONTRAINDICATIONS AND MORE INFORMATION, VISIT: https:/elitone.com/
NEW CERVICAL DILATOR

Hologic, Inc. announces the launch of the Definity Cervical Dilator, allowing tenaculum-free access to the uterine cavity, which lessens patient discomfort and reduces the risk of perforation during dilation, says Hologic. The dilator uses SureAccess—an expandable balloon technology designed to eliminate multiple passes and ensure safety during insertion, even for patients with complex or challenging cervical anatomy. Definity Cervical Dilator is not intended for use during induction of labor; some examples for its use include treatment of cervical stenosis, intrauterine device placement and removal, uterine tissue removal, diagnostic hysteroscopy, and operative hysteroscopy. Use of the system is contraindicated in patients with active genital tract infection, pelvic structure abnormality preventing device passage, or invasive cervical cancer. The Definity Cervical Dilator is now available across the United States in 5 mm, 7 mm, and 9 mm sizes.
FOR MORE INFORMATION, VISIT: https://www.hologic.com/
LIGHT DEVICE FOR HAIR REJUVENATION
Pattern hair loss (androgenetic alopecia) affects about 80 million men and women in the United States. In women, the hair loss typically occurs as thinning or widening of the midline. Revian, Inc. is focused on improving overall scalp health by stimulating the body’s natural processes for hair and skin rejuvenation with light therapy. REVIAN RED, a dual-wavelength LED hair growth cap, has successfully demonstrated the ability to stop hair loss and subsequently grow new hair, says Revian. In a recent clinical trial, women who were at least 80% compliant with wearing REVIAN RED (versus a placebo cap with no light therapy) for 10 minutes per day had a mean improvement of 26.3 hairs/cm2. Scalp irritations were assessed during the trial, with patient-reported itching and burning/stinging treated with at-home therapies.
The REVIAN RED wireless cap system is controlled by a mobile app and is US Food and Drug Administration (FDA) cleared as a hair loss treatment for men and women. The mechanism of action for improved scalp symptoms, says Revian, is the patented dual-wavelength light, which releases nitric oxide and is proposed to be anti-inflammatory.
The REVIAN RED system is indicated to treat androgenetic alopecia and to promote hair growth in men with Norwood-Hamilton classifications of IIa–V patterns of hair loss and to treat androgenetic alopecia and promote hair growth in women with Ludwig-Savin Scale I-1 to I-4, II-1, II-2 or frontal patterns of hair loss; both with Fitzpatrick Skin Types I–IV.
FOR MORE INFORMATION, VISIT: https://www.revian.com/
SUI TREATMENT
ELITONE from Elidah is the first transcutaneous pelvic floor muscle stimulation treatment for stress urinary incontinence (SUI). It is FDA cleared and works to train women to perform Kegel techniques by naturally contracting the correct pelvic floor muscles and surrounding tissues. The device is placed where a pad would go, according to Elidah, with conductive gel areas within the device delivering electrical muscle stimulation. Twenty-minute treatments 4 to 5 times per week are recommended, with improved SUI symptoms, depending on severity, for many women in 6 weeks. Three-quarters of women had significant reduction in daily leaks after 6 weeks of self-administered treatment with ELITONE, reports the manufacturer.
Elidah says that ELITONE is ideal for women with mild to moderate SUI symptoms who would benefit from pelvic floor muscle training, including those who are resistant to intravaginal treatments, need postpartum care, or have limited access to physical therapy. The device is contraindicated in women who have an implanted cardiac device, cancer, epilepsy, or recent pelvic floor surgery.
FOR OTHER CONTRAINDICATIONS AND MORE INFORMATION, VISIT: https:/elitone.com/
Product News August 2020
FDA Approves Wynzora Cream for Plaque Psoriasis
MC2 Therapeutics announces US Food and Drug Administration (FDA) approval of Wynzora Cream (calcipotriene 0.005% and betamethasone dipropionate 0.064%) for once-daily treatment of plaque psoriasis in adults.
Wynzora Cream is based on PAD Technology, which enables stability of calcipotriene and betamethasone dipropionate in an aqueous formulation. Key features of PAD Technology formulations are high penetration of active ingredients to the target tissue, improved solubility and stability of active ingredients, high tolerability, and excellent treatment convenience. In the phase 3 trials conducted at multiple sites in the United States and the European Union, Wynzora Cream has demonstrated a combination of clinical efficacy, a favorable safety profile, and high convenience, offering overall better patient satisfaction in the topical treatment of plaque psoriasis in the real-world setting.
WynzoraCream is applied to affected areas once daily for up to 8 weeks and not more than 100 g per week. Patients should stop treatment when the plaque psoriasis is under control, unless a health care provider gives other instructions.
MC2 Therapeutics also has submitted a Marketing Authorization Application in the European Union for Wynzora Cream (50 µg/g calcipotriol and 0.5 mg/g betamethasone [as dipropionate]) for the treatment of plaque psoriasis. For more information, visit www.mc2therapeutics.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@mdedge.com.
FDA Approves Wynzora Cream for Plaque Psoriasis
MC2 Therapeutics announces US Food and Drug Administration (FDA) approval of Wynzora Cream (calcipotriene 0.005% and betamethasone dipropionate 0.064%) for once-daily treatment of plaque psoriasis in adults.
Wynzora Cream is based on PAD Technology, which enables stability of calcipotriene and betamethasone dipropionate in an aqueous formulation. Key features of PAD Technology formulations are high penetration of active ingredients to the target tissue, improved solubility and stability of active ingredients, high tolerability, and excellent treatment convenience. In the phase 3 trials conducted at multiple sites in the United States and the European Union, Wynzora Cream has demonstrated a combination of clinical efficacy, a favorable safety profile, and high convenience, offering overall better patient satisfaction in the topical treatment of plaque psoriasis in the real-world setting.
WynzoraCream is applied to affected areas once daily for up to 8 weeks and not more than 100 g per week. Patients should stop treatment when the plaque psoriasis is under control, unless a health care provider gives other instructions.
MC2 Therapeutics also has submitted a Marketing Authorization Application in the European Union for Wynzora Cream (50 µg/g calcipotriol and 0.5 mg/g betamethasone [as dipropionate]) for the treatment of plaque psoriasis. For more information, visit www.mc2therapeutics.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@mdedge.com.
FDA Approves Wynzora Cream for Plaque Psoriasis
MC2 Therapeutics announces US Food and Drug Administration (FDA) approval of Wynzora Cream (calcipotriene 0.005% and betamethasone dipropionate 0.064%) for once-daily treatment of plaque psoriasis in adults.
Wynzora Cream is based on PAD Technology, which enables stability of calcipotriene and betamethasone dipropionate in an aqueous formulation. Key features of PAD Technology formulations are high penetration of active ingredients to the target tissue, improved solubility and stability of active ingredients, high tolerability, and excellent treatment convenience. In the phase 3 trials conducted at multiple sites in the United States and the European Union, Wynzora Cream has demonstrated a combination of clinical efficacy, a favorable safety profile, and high convenience, offering overall better patient satisfaction in the topical treatment of plaque psoriasis in the real-world setting.
WynzoraCream is applied to affected areas once daily for up to 8 weeks and not more than 100 g per week. Patients should stop treatment when the plaque psoriasis is under control, unless a health care provider gives other instructions.
MC2 Therapeutics also has submitted a Marketing Authorization Application in the European Union for Wynzora Cream (50 µg/g calcipotriol and 0.5 mg/g betamethasone [as dipropionate]) for the treatment of plaque psoriasis. For more information, visit www.mc2therapeutics.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@mdedge.com.
Product News July 2020
Arazlo Now Available in the United States
Ortho Dermatologics launches Arazlo (tazarotene) Lotion 0.045% to health care professionals in the United States. Arazlo was approved by the US Food and Drug Administration in December 2019 for the treatment of acne in patients 9 years and older. Clinical trials indicated that Arazlo Lotion offers a tolerable formulation without sacrificing efficacy. For more information, visit arazlo.com/hcp/.
FDA Approves Updated Label for Seysara Tablets
Almirall, LLC, receives US Food and Drug Administration (FDA) approval for an update to the Seysara (sarecycline) Tablets label stating that Propionibacterium acnes strains displayed a low propensity for the development of resistance to sarecycline, which is important when considering the appropriate use of antibiotics. The FDA approved Seysara in 2018 for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 9 years and older. For more information, visit seysara.com.
Jublia Labeling Extends to Pediatric Patients
Ortho Dermatologics announces the US Food and Drug Administration has approved a supplemental New Drug Application for Jublia (efinaconazole) Solution 10% for the treatment of onychomycosis in patients 6 years and older. Jublia was initially approved in 2014 for patients 18 years and older. With its safety and efficacy profile demonstrated in real-world use over the last 6 years, Jublia can now be a valuable treatment option for children with toenail fungal infections. For more information, visit jubliarx.com.
Revance and Mylan to Develop Biosimilar to Botox
Revance Therapeutics, Inc, and Mylan N.V. announce they are moving forward with a 351(k) development plan for a proposed biosimilar to Botox and Botox Cosmetic (onabotulinumtoxinA), which would allow Revance to financially participate in the short-acting neurotoxin space while focusing commercial efforts in the long-acting neuromodulator category. Mylan will handle the commercialization of the new biosimilar in the United States, Europe, and other markets worldwide. For more information, visit revance.com and mylan.com.
The Skin Cancer Foundation Awards $125,000 in Research Grants
The Skin Cancer Foundation awards $125,000 in grants to 3 separate researchers who submitted proposals to further prevention, early detection, and treatment of skin cancer. Matthew Hangauer, PhD (La Jolla, California), received $50,000 for “Targeting Immunotherapy-Tolerant Melanoma Persister Cells”; Lee E. Wheless, MD, PhD (Nashville, Tennessee), received $50,000 for “Using Bioinformatics to Stratify Skin Cancer Risk in Organ Transplant Recipients”; and Vishal Patel, MD (Washington, DC), received $25,000 for “Delphi Consensus Determination of a Tumor Stage Based Approach to High-Risk Cutaneous Squamous Cell Carcinoma.” The Skin Cancer Foundation’s Research Grants program helps provide funding for studies that have led to lifesaving breakthroughs. The 2021 research grants application period will open soon. For more information, visit SkinCancer.org/research.
Arazlo Now Available in the United States
Ortho Dermatologics launches Arazlo (tazarotene) Lotion 0.045% to health care professionals in the United States. Arazlo was approved by the US Food and Drug Administration in December 2019 for the treatment of acne in patients 9 years and older. Clinical trials indicated that Arazlo Lotion offers a tolerable formulation without sacrificing efficacy. For more information, visit arazlo.com/hcp/.
FDA Approves Updated Label for Seysara Tablets
Almirall, LLC, receives US Food and Drug Administration (FDA) approval for an update to the Seysara (sarecycline) Tablets label stating that Propionibacterium acnes strains displayed a low propensity for the development of resistance to sarecycline, which is important when considering the appropriate use of antibiotics. The FDA approved Seysara in 2018 for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 9 years and older. For more information, visit seysara.com.
Jublia Labeling Extends to Pediatric Patients
Ortho Dermatologics announces the US Food and Drug Administration has approved a supplemental New Drug Application for Jublia (efinaconazole) Solution 10% for the treatment of onychomycosis in patients 6 years and older. Jublia was initially approved in 2014 for patients 18 years and older. With its safety and efficacy profile demonstrated in real-world use over the last 6 years, Jublia can now be a valuable treatment option for children with toenail fungal infections. For more information, visit jubliarx.com.
Revance and Mylan to Develop Biosimilar to Botox
Revance Therapeutics, Inc, and Mylan N.V. announce they are moving forward with a 351(k) development plan for a proposed biosimilar to Botox and Botox Cosmetic (onabotulinumtoxinA), which would allow Revance to financially participate in the short-acting neurotoxin space while focusing commercial efforts in the long-acting neuromodulator category. Mylan will handle the commercialization of the new biosimilar in the United States, Europe, and other markets worldwide. For more information, visit revance.com and mylan.com.
The Skin Cancer Foundation Awards $125,000 in Research Grants
The Skin Cancer Foundation awards $125,000 in grants to 3 separate researchers who submitted proposals to further prevention, early detection, and treatment of skin cancer. Matthew Hangauer, PhD (La Jolla, California), received $50,000 for “Targeting Immunotherapy-Tolerant Melanoma Persister Cells”; Lee E. Wheless, MD, PhD (Nashville, Tennessee), received $50,000 for “Using Bioinformatics to Stratify Skin Cancer Risk in Organ Transplant Recipients”; and Vishal Patel, MD (Washington, DC), received $25,000 for “Delphi Consensus Determination of a Tumor Stage Based Approach to High-Risk Cutaneous Squamous Cell Carcinoma.” The Skin Cancer Foundation’s Research Grants program helps provide funding for studies that have led to lifesaving breakthroughs. The 2021 research grants application period will open soon. For more information, visit SkinCancer.org/research.
Arazlo Now Available in the United States
Ortho Dermatologics launches Arazlo (tazarotene) Lotion 0.045% to health care professionals in the United States. Arazlo was approved by the US Food and Drug Administration in December 2019 for the treatment of acne in patients 9 years and older. Clinical trials indicated that Arazlo Lotion offers a tolerable formulation without sacrificing efficacy. For more information, visit arazlo.com/hcp/.
FDA Approves Updated Label for Seysara Tablets
Almirall, LLC, receives US Food and Drug Administration (FDA) approval for an update to the Seysara (sarecycline) Tablets label stating that Propionibacterium acnes strains displayed a low propensity for the development of resistance to sarecycline, which is important when considering the appropriate use of antibiotics. The FDA approved Seysara in 2018 for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 9 years and older. For more information, visit seysara.com.
Jublia Labeling Extends to Pediatric Patients
Ortho Dermatologics announces the US Food and Drug Administration has approved a supplemental New Drug Application for Jublia (efinaconazole) Solution 10% for the treatment of onychomycosis in patients 6 years and older. Jublia was initially approved in 2014 for patients 18 years and older. With its safety and efficacy profile demonstrated in real-world use over the last 6 years, Jublia can now be a valuable treatment option for children with toenail fungal infections. For more information, visit jubliarx.com.
Revance and Mylan to Develop Biosimilar to Botox
Revance Therapeutics, Inc, and Mylan N.V. announce they are moving forward with a 351(k) development plan for a proposed biosimilar to Botox and Botox Cosmetic (onabotulinumtoxinA), which would allow Revance to financially participate in the short-acting neurotoxin space while focusing commercial efforts in the long-acting neuromodulator category. Mylan will handle the commercialization of the new biosimilar in the United States, Europe, and other markets worldwide. For more information, visit revance.com and mylan.com.
The Skin Cancer Foundation Awards $125,000 in Research Grants
The Skin Cancer Foundation awards $125,000 in grants to 3 separate researchers who submitted proposals to further prevention, early detection, and treatment of skin cancer. Matthew Hangauer, PhD (La Jolla, California), received $50,000 for “Targeting Immunotherapy-Tolerant Melanoma Persister Cells”; Lee E. Wheless, MD, PhD (Nashville, Tennessee), received $50,000 for “Using Bioinformatics to Stratify Skin Cancer Risk in Organ Transplant Recipients”; and Vishal Patel, MD (Washington, DC), received $25,000 for “Delphi Consensus Determination of a Tumor Stage Based Approach to High-Risk Cutaneous Squamous Cell Carcinoma.” The Skin Cancer Foundation’s Research Grants program helps provide funding for studies that have led to lifesaving breakthroughs. The 2021 research grants application period will open soon. For more information, visit SkinCancer.org/research.