User login
NEW CERVICAL DILATOR
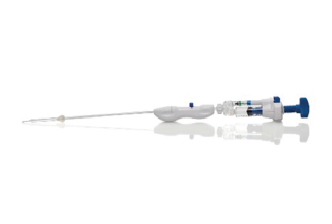
Hologic, Inc. announces the launch of the Definity Cervical Dilator, allowing tenaculum-free access to the uterine cavity, which lessens patient discomfort and reduces the risk of perforation during dilation, says Hologic. The dilator uses SureAccess—an expandable balloon technology designed to eliminate multiple passes and ensure safety during insertion, even for patients with complex or challenging cervical anatomy. Definity Cervical Dilator is not intended for use during induction of labor; some examples for its use include treatment of cervical stenosis, intrauterine device placement and removal, uterine tissue removal, diagnostic hysteroscopy, and operative hysteroscopy. Use of the system is contraindicated in patients with active genital tract infection, pelvic structure abnormality preventing device passage, or invasive cervical cancer. The Definity Cervical Dilator is now available across the United States in 5 mm, 7 mm, and 9 mm sizes.
FOR MORE INFORMATION, VISIT: https://www.hologic.com/
LIGHT DEVICE FOR HAIR REJUVENATION
Pattern hair loss (androgenetic alopecia) affects about 80 million men and women in the United States. In women, the hair loss typically occurs as thinning or widening of the midline. Revian, Inc. is focused on improving overall scalp health by stimulating the body’s natural processes for hair and skin rejuvenation with light therapy. REVIAN RED, a dual-wavelength LED hair growth cap, has successfully demonstrated the ability to stop hair loss and subsequently grow new hair, says Revian. In a recent clinical trial, women who were at least 80% compliant with wearing REVIAN RED (versus a placebo cap with no light therapy) for 10 minutes per day had a mean improvement of 26.3 hairs/cm2. Scalp irritations were assessed during the trial, with patient-reported itching and burning/stinging treated with at-home therapies.
The REVIAN RED wireless cap system is controlled by a mobile app and is US Food and Drug Administration (FDA) cleared as a hair loss treatment for men and women. The mechanism of action for improved scalp symptoms, says Revian, is the patented dual-wavelength light, which releases nitric oxide and is proposed to be anti-inflammatory.
The REVIAN RED system is indicated to treat androgenetic alopecia and to promote hair growth in men with Norwood-Hamilton classifications of IIa–V patterns of hair loss and to treat androgenetic alopecia and promote hair growth in women with Ludwig-Savin Scale I-1 to I-4, II-1, II-2 or frontal patterns of hair loss; both with Fitzpatrick Skin Types I–IV.
FOR MORE INFORMATION, VISIT: https://www.revian.com/
SUI TREATMENT
ELITONE from Elidah is the first transcutaneous pelvic floor muscle stimulation treatment for stress urinary incontinence (SUI). It is FDA cleared and works to train women to perform Kegel techniques by naturally contracting the correct pelvic floor muscles and surrounding tissues. The device is placed where a pad would go, according to Elidah, with conductive gel areas within the device delivering electrical muscle stimulation. Twenty-minute treatments 4 to 5 times per week are recommended, with improved SUI symptoms, depending on severity, for many women in 6 weeks. Three-quarters of women had significant reduction in daily leaks after 6 weeks of self-administered treatment with ELITONE, reports the manufacturer.
Elidah says that ELITONE is ideal for women with mild to moderate SUI symptoms who would benefit from pelvic floor muscle training, including those who are resistant to intravaginal treatments, need postpartum care, or have limited access to physical therapy. The device is contraindicated in women who have an implanted cardiac device, cancer, epilepsy, or recent pelvic floor surgery.
FOR OTHER CONTRAINDICATIONS AND MORE INFORMATION, VISIT: https:/elitone.com/
NEW CERVICAL DILATOR

Hologic, Inc. announces the launch of the Definity Cervical Dilator, allowing tenaculum-free access to the uterine cavity, which lessens patient discomfort and reduces the risk of perforation during dilation, says Hologic. The dilator uses SureAccess—an expandable balloon technology designed to eliminate multiple passes and ensure safety during insertion, even for patients with complex or challenging cervical anatomy. Definity Cervical Dilator is not intended for use during induction of labor; some examples for its use include treatment of cervical stenosis, intrauterine device placement and removal, uterine tissue removal, diagnostic hysteroscopy, and operative hysteroscopy. Use of the system is contraindicated in patients with active genital tract infection, pelvic structure abnormality preventing device passage, or invasive cervical cancer. The Definity Cervical Dilator is now available across the United States in 5 mm, 7 mm, and 9 mm sizes.
FOR MORE INFORMATION, VISIT: https://www.hologic.com/
LIGHT DEVICE FOR HAIR REJUVENATION
Pattern hair loss (androgenetic alopecia) affects about 80 million men and women in the United States. In women, the hair loss typically occurs as thinning or widening of the midline. Revian, Inc. is focused on improving overall scalp health by stimulating the body’s natural processes for hair and skin rejuvenation with light therapy. REVIAN RED, a dual-wavelength LED hair growth cap, has successfully demonstrated the ability to stop hair loss and subsequently grow new hair, says Revian. In a recent clinical trial, women who were at least 80% compliant with wearing REVIAN RED (versus a placebo cap with no light therapy) for 10 minutes per day had a mean improvement of 26.3 hairs/cm2. Scalp irritations were assessed during the trial, with patient-reported itching and burning/stinging treated with at-home therapies.
The REVIAN RED wireless cap system is controlled by a mobile app and is US Food and Drug Administration (FDA) cleared as a hair loss treatment for men and women. The mechanism of action for improved scalp symptoms, says Revian, is the patented dual-wavelength light, which releases nitric oxide and is proposed to be anti-inflammatory.
The REVIAN RED system is indicated to treat androgenetic alopecia and to promote hair growth in men with Norwood-Hamilton classifications of IIa–V patterns of hair loss and to treat androgenetic alopecia and promote hair growth in women with Ludwig-Savin Scale I-1 to I-4, II-1, II-2 or frontal patterns of hair loss; both with Fitzpatrick Skin Types I–IV.
FOR MORE INFORMATION, VISIT: https://www.revian.com/
SUI TREATMENT
ELITONE from Elidah is the first transcutaneous pelvic floor muscle stimulation treatment for stress urinary incontinence (SUI). It is FDA cleared and works to train women to perform Kegel techniques by naturally contracting the correct pelvic floor muscles and surrounding tissues. The device is placed where a pad would go, according to Elidah, with conductive gel areas within the device delivering electrical muscle stimulation. Twenty-minute treatments 4 to 5 times per week are recommended, with improved SUI symptoms, depending on severity, for many women in 6 weeks. Three-quarters of women had significant reduction in daily leaks after 6 weeks of self-administered treatment with ELITONE, reports the manufacturer.
Elidah says that ELITONE is ideal for women with mild to moderate SUI symptoms who would benefit from pelvic floor muscle training, including those who are resistant to intravaginal treatments, need postpartum care, or have limited access to physical therapy. The device is contraindicated in women who have an implanted cardiac device, cancer, epilepsy, or recent pelvic floor surgery.
FOR OTHER CONTRAINDICATIONS AND MORE INFORMATION, VISIT: https:/elitone.com/
NEW CERVICAL DILATOR

Hologic, Inc. announces the launch of the Definity Cervical Dilator, allowing tenaculum-free access to the uterine cavity, which lessens patient discomfort and reduces the risk of perforation during dilation, says Hologic. The dilator uses SureAccess—an expandable balloon technology designed to eliminate multiple passes and ensure safety during insertion, even for patients with complex or challenging cervical anatomy. Definity Cervical Dilator is not intended for use during induction of labor; some examples for its use include treatment of cervical stenosis, intrauterine device placement and removal, uterine tissue removal, diagnostic hysteroscopy, and operative hysteroscopy. Use of the system is contraindicated in patients with active genital tract infection, pelvic structure abnormality preventing device passage, or invasive cervical cancer. The Definity Cervical Dilator is now available across the United States in 5 mm, 7 mm, and 9 mm sizes.
FOR MORE INFORMATION, VISIT: https://www.hologic.com/
LIGHT DEVICE FOR HAIR REJUVENATION
Pattern hair loss (androgenetic alopecia) affects about 80 million men and women in the United States. In women, the hair loss typically occurs as thinning or widening of the midline. Revian, Inc. is focused on improving overall scalp health by stimulating the body’s natural processes for hair and skin rejuvenation with light therapy. REVIAN RED, a dual-wavelength LED hair growth cap, has successfully demonstrated the ability to stop hair loss and subsequently grow new hair, says Revian. In a recent clinical trial, women who were at least 80% compliant with wearing REVIAN RED (versus a placebo cap with no light therapy) for 10 minutes per day had a mean improvement of 26.3 hairs/cm2. Scalp irritations were assessed during the trial, with patient-reported itching and burning/stinging treated with at-home therapies.
The REVIAN RED wireless cap system is controlled by a mobile app and is US Food and Drug Administration (FDA) cleared as a hair loss treatment for men and women. The mechanism of action for improved scalp symptoms, says Revian, is the patented dual-wavelength light, which releases nitric oxide and is proposed to be anti-inflammatory.
The REVIAN RED system is indicated to treat androgenetic alopecia and to promote hair growth in men with Norwood-Hamilton classifications of IIa–V patterns of hair loss and to treat androgenetic alopecia and promote hair growth in women with Ludwig-Savin Scale I-1 to I-4, II-1, II-2 or frontal patterns of hair loss; both with Fitzpatrick Skin Types I–IV.
FOR MORE INFORMATION, VISIT: https://www.revian.com/
SUI TREATMENT
ELITONE from Elidah is the first transcutaneous pelvic floor muscle stimulation treatment for stress urinary incontinence (SUI). It is FDA cleared and works to train women to perform Kegel techniques by naturally contracting the correct pelvic floor muscles and surrounding tissues. The device is placed where a pad would go, according to Elidah, with conductive gel areas within the device delivering electrical muscle stimulation. Twenty-minute treatments 4 to 5 times per week are recommended, with improved SUI symptoms, depending on severity, for many women in 6 weeks. Three-quarters of women had significant reduction in daily leaks after 6 weeks of self-administered treatment with ELITONE, reports the manufacturer.
Elidah says that ELITONE is ideal for women with mild to moderate SUI symptoms who would benefit from pelvic floor muscle training, including those who are resistant to intravaginal treatments, need postpartum care, or have limited access to physical therapy. The device is contraindicated in women who have an implanted cardiac device, cancer, epilepsy, or recent pelvic floor surgery.
FOR OTHER CONTRAINDICATIONS AND MORE INFORMATION, VISIT: https:/elitone.com/