User login
ACOG recommends that annual screening mammograms begin at age 40
Annual mammography screening should be offered to women when they reach 40 years, says a new Practice Bulletin on breast cancer screening from ACOG.1 Previous ACOG guidelines recommended mammograms every 1 to 2 years starting at age 40 and annually beginning at age 50.
According to Jennifer Griffin, MD, MPH, who coauthored the ACOG guidelines, the change in mammography screening for women beginning at age 40 is based on three variables:
- Incidence of breast cancer. The malignancy remains the second-leading cause of all cancer-related deaths.
- “Sojourn time” for breast cancer growth (the time it takes for a tumor that is identifiable by mammography to grow big enough to cause symptoms)
- Potential to reduce the number of deaths from breast cancer.
Although the sojourn time of individual cancers varies, the greatest predictor is age. Women 40 to 49 years old have the shortest average sojourn time (2–2.4 years), whereas women 70 to 74 years old have the longest average sojourn time (4–4.1 years).
“Although women in their 40s have a lower overall incidence of breast cancer compared with older women, the window to detect tumors before they become symptomatic is shorter, on average,” said Dr. Griffin. The 5-year survival rate is 98% for women whose breast cancer tumors are discovered at their earliest stage, before they are palpable and when they are small and confined to the breast. “If women in their 40s have annual mammograms, there is a better chance of detecting and treating the cancer before it has time to spread than if they wait 2 years between mammograms.”
The College continues to recommend annual clinical breast exams (CBE) for women 40 years and older, and CBE every 1 to 3 years for women ages 20 to 39. Additionally, ACOG encourages “breast self-awareness” for women 20 years and older. Breast self-awareness is a woman’s understanding of the normal appearance and feel of her breasts.
“The goal here is for women to be alert to any changes, no matter how small, in their breasts, and report them to their doctor,” said Dr. Griffin. “Although we’ve moved away from routinely recommending [breast self-exams], some women will want to continue doing them and that’s OK.”
Enhanced breast cancer screening, such as more frequent CBEs, annual magnetic resonance imaging (MRI), or mammograms before 40 years of age, may be recommended for women at high risk of breast cancer. Breast MRI is not recommended for women at average risk.
The “downside” of annual screening
“More frequent screening is associated with more false-positive findings,” says Andrew M. Kaunitz, MD, professor and associate chairman of obstetrics and gynecology at the University of Florida College of Medicine in Jacksonville, Fla.
“Due to the high rate of false-positive screens and the large number of screening mammograms needed to prevent the death of one woman in her 40s from breast cancer, the US Preventive Services Task Force recommended in late 2009 that routine screening mammography be deferred until age 50 and that screens be biennial,” he explains.2
“The new ACOG guidance acknowledges these concerns, as well as the potential for anxiety associated with false-positive findings. However, ACOG clearly concluded that, in general, US women cope well with such anxiety.
“When discussing mammograms with women in their 40s, it’s appropriate to acknowledge the high rate of false positives in this age group, which may lead to additional imaging, as well as biopsies,” Dr. Kaunitz suggests. “In my view, we can best serve our patients by making recommendations regarding screening mammograms in a manner that is sensitive to women’s personal values as they attempt to weigh the benefits of earlier and more frequent screening against the risks associated with false-positive screens.”
We want to hear from you! Tell us what you think.
1. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Practice Bulletin No. 122: Breast cancer screening. Obstet Gynecol. 2011;118(2 Part 1):372-382.
2. Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151(10):727-737.
Annual mammography screening should be offered to women when they reach 40 years, says a new Practice Bulletin on breast cancer screening from ACOG.1 Previous ACOG guidelines recommended mammograms every 1 to 2 years starting at age 40 and annually beginning at age 50.
According to Jennifer Griffin, MD, MPH, who coauthored the ACOG guidelines, the change in mammography screening for women beginning at age 40 is based on three variables:
- Incidence of breast cancer. The malignancy remains the second-leading cause of all cancer-related deaths.
- “Sojourn time” for breast cancer growth (the time it takes for a tumor that is identifiable by mammography to grow big enough to cause symptoms)
- Potential to reduce the number of deaths from breast cancer.
Although the sojourn time of individual cancers varies, the greatest predictor is age. Women 40 to 49 years old have the shortest average sojourn time (2–2.4 years), whereas women 70 to 74 years old have the longest average sojourn time (4–4.1 years).
“Although women in their 40s have a lower overall incidence of breast cancer compared with older women, the window to detect tumors before they become symptomatic is shorter, on average,” said Dr. Griffin. The 5-year survival rate is 98% for women whose breast cancer tumors are discovered at their earliest stage, before they are palpable and when they are small and confined to the breast. “If women in their 40s have annual mammograms, there is a better chance of detecting and treating the cancer before it has time to spread than if they wait 2 years between mammograms.”
The College continues to recommend annual clinical breast exams (CBE) for women 40 years and older, and CBE every 1 to 3 years for women ages 20 to 39. Additionally, ACOG encourages “breast self-awareness” for women 20 years and older. Breast self-awareness is a woman’s understanding of the normal appearance and feel of her breasts.
“The goal here is for women to be alert to any changes, no matter how small, in their breasts, and report them to their doctor,” said Dr. Griffin. “Although we’ve moved away from routinely recommending [breast self-exams], some women will want to continue doing them and that’s OK.”
Enhanced breast cancer screening, such as more frequent CBEs, annual magnetic resonance imaging (MRI), or mammograms before 40 years of age, may be recommended for women at high risk of breast cancer. Breast MRI is not recommended for women at average risk.
The “downside” of annual screening
“More frequent screening is associated with more false-positive findings,” says Andrew M. Kaunitz, MD, professor and associate chairman of obstetrics and gynecology at the University of Florida College of Medicine in Jacksonville, Fla.
“Due to the high rate of false-positive screens and the large number of screening mammograms needed to prevent the death of one woman in her 40s from breast cancer, the US Preventive Services Task Force recommended in late 2009 that routine screening mammography be deferred until age 50 and that screens be biennial,” he explains.2
“The new ACOG guidance acknowledges these concerns, as well as the potential for anxiety associated with false-positive findings. However, ACOG clearly concluded that, in general, US women cope well with such anxiety.
“When discussing mammograms with women in their 40s, it’s appropriate to acknowledge the high rate of false positives in this age group, which may lead to additional imaging, as well as biopsies,” Dr. Kaunitz suggests. “In my view, we can best serve our patients by making recommendations regarding screening mammograms in a manner that is sensitive to women’s personal values as they attempt to weigh the benefits of earlier and more frequent screening against the risks associated with false-positive screens.”
We want to hear from you! Tell us what you think.
Annual mammography screening should be offered to women when they reach 40 years, says a new Practice Bulletin on breast cancer screening from ACOG.1 Previous ACOG guidelines recommended mammograms every 1 to 2 years starting at age 40 and annually beginning at age 50.
According to Jennifer Griffin, MD, MPH, who coauthored the ACOG guidelines, the change in mammography screening for women beginning at age 40 is based on three variables:
- Incidence of breast cancer. The malignancy remains the second-leading cause of all cancer-related deaths.
- “Sojourn time” for breast cancer growth (the time it takes for a tumor that is identifiable by mammography to grow big enough to cause symptoms)
- Potential to reduce the number of deaths from breast cancer.
Although the sojourn time of individual cancers varies, the greatest predictor is age. Women 40 to 49 years old have the shortest average sojourn time (2–2.4 years), whereas women 70 to 74 years old have the longest average sojourn time (4–4.1 years).
“Although women in their 40s have a lower overall incidence of breast cancer compared with older women, the window to detect tumors before they become symptomatic is shorter, on average,” said Dr. Griffin. The 5-year survival rate is 98% for women whose breast cancer tumors are discovered at their earliest stage, before they are palpable and when they are small and confined to the breast. “If women in their 40s have annual mammograms, there is a better chance of detecting and treating the cancer before it has time to spread than if they wait 2 years between mammograms.”
The College continues to recommend annual clinical breast exams (CBE) for women 40 years and older, and CBE every 1 to 3 years for women ages 20 to 39. Additionally, ACOG encourages “breast self-awareness” for women 20 years and older. Breast self-awareness is a woman’s understanding of the normal appearance and feel of her breasts.
“The goal here is for women to be alert to any changes, no matter how small, in their breasts, and report them to their doctor,” said Dr. Griffin. “Although we’ve moved away from routinely recommending [breast self-exams], some women will want to continue doing them and that’s OK.”
Enhanced breast cancer screening, such as more frequent CBEs, annual magnetic resonance imaging (MRI), or mammograms before 40 years of age, may be recommended for women at high risk of breast cancer. Breast MRI is not recommended for women at average risk.
The “downside” of annual screening
“More frequent screening is associated with more false-positive findings,” says Andrew M. Kaunitz, MD, professor and associate chairman of obstetrics and gynecology at the University of Florida College of Medicine in Jacksonville, Fla.
“Due to the high rate of false-positive screens and the large number of screening mammograms needed to prevent the death of one woman in her 40s from breast cancer, the US Preventive Services Task Force recommended in late 2009 that routine screening mammography be deferred until age 50 and that screens be biennial,” he explains.2
“The new ACOG guidance acknowledges these concerns, as well as the potential for anxiety associated with false-positive findings. However, ACOG clearly concluded that, in general, US women cope well with such anxiety.
“When discussing mammograms with women in their 40s, it’s appropriate to acknowledge the high rate of false positives in this age group, which may lead to additional imaging, as well as biopsies,” Dr. Kaunitz suggests. “In my view, we can best serve our patients by making recommendations regarding screening mammograms in a manner that is sensitive to women’s personal values as they attempt to weigh the benefits of earlier and more frequent screening against the risks associated with false-positive screens.”
We want to hear from you! Tell us what you think.
1. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Practice Bulletin No. 122: Breast cancer screening. Obstet Gynecol. 2011;118(2 Part 1):372-382.
2. Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151(10):727-737.
1. American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG Practice Bulletin No. 122: Breast cancer screening. Obstet Gynecol. 2011;118(2 Part 1):372-382.
2. Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L. US Preventive Services Task Force. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151(10):727-737.
Take this simplified approach to correcting exposure of vaginal mesh
CASE: Pain during intercourse, well after mesh implantation
Your patient, 61 years old, para 3, has come to your office by referral with a complaint of dyspareunia. The history includes placement of a synthetic vaginal mesh kit 14 months earlier for prolapse.
The medical record shows that the referring physician performed a “mesh excision” 1 year after the original procedure.
The woman reports that she is “very frustrated” that she is still dealing with this problem so long after the original procedure.
On examination, you note a 2.5-cm diameter area of exposed mesh in the anterior vagina, with healthy surrounding tissue and without inflammation or purulence (FIGURE 1). You are unable to reproduce her complaint of pain on vaginal examination.
What options can you offer to this woman? And will those options meet her therapeutic expectations?
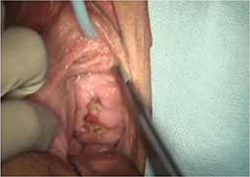
FIGURE 1 Examination of your referred patient: Mesh is noticeably exposedThe recent increase in the use of mesh grafts to reconstruct pelvic anatomy has been directed mainly at improving surgical outcomes. Yet, at the same time, gynecologic surgeons find themselves facing a rise in associated complications of such surgery that they did not see previously.
Among the most troublesome and concerning of those complications are 1) exposure of mesh through the vaginal epithelium and 2) contraction or hardening of mesh (or both) that can result in dyspareunia and chronic pelvic pain. Other, rare complications include infection and fistula.
Our goal in this article is to address the management of graft-healing abnormalities in which a segment of the mesh is palpable or visible, or both, within the vaginal canal. Our focus is on simple abnormalities that can be managed by most generalist gynecologists; to be clear, more complex abnormalities, and those that provoke more serious or lasting symptoms, belong under the care of a specialist.
A recent shift in terminology is significant
Early on, this complication was called “erosion” as understanding of the mechanism of its development grew, however, terminology applied to the problem has changed.
In fact, mesh itself very rarely erodes into the vagina or an underlying viscus. Instead, the complication occurs most commonly as a result of disruption of a suture line—most likely the result of a hematoma or localized inflammation that develops postoperatively.
“Exposure” (our preference here) and “extrusion” are now the recommended terms, based on a consensus terminology document published this year jointly by the International Urogynecological Association and the International Continence Society.1
Exposure of implanted mesh is considered a “simple” healing abnormality because it typically
- occurs along the suture line and early in the course of healing
- is not associated with infection of the graft.2
The typical physical appearance is one of visible mesh along an open suture line without granulation tissue or purulence—again, see FIGURE 1. The mesh is firmly adherent to the vaginal epithelial edges and underlying fascia.
The reported incidence of mesh exposures—in regard to currently used meshes, which are all Type-1, monofilament, macroporous polypropylene grafts—is approximately 10% but as high as 15% to 20% in some reported series.3,4 The higher rates of exposure are usually seen in series in which some patients have had a synthetic graft implanted as an overlay to fascial midline plication. When the graft is implanted in the subfascial layer of the vaginal wall (i.e., without midline plication), however, the reported rate of exposure falls—to 5% to 10%.5-7
Recommendations for management
Initially, recommendations for “erosion” management were based on concerns about underlying mesh infection or rejection, and included a need to remove the entire graft. That recommendation still applies to multifilament, microporous grafts that present with inflammatory infiltrates, granulation tissue, and purulence. Although these kinds of grafts (known as “Type-2/3 grafts”—e.g., GoreTex, IVS) have not been marketed for pelvic reconstruction over the past 3 to 5 years, their behavior post-implantation is less predictable—and patients who have delayed healing abnormalities are, therefore, still being seen. It’s fortunate that development of an overlying biofilm prevents tissue incorporation into these types of graft, allowing them to be removed easily.
Exposures related to Type-1 mesh—currently used in pelvic reconstruction—that occur without surrounding infection do not require extensive removal. Rather, they can be managed conservatively or, when necessary, with outpatient surgery. In patients who are not sexually active, exposures are usually asymptomatic; they might only be observed by the physician on vaginal examination and are amenable to simple monitoring. In sexually active patients, exposure of Type-1 mesh usually results in dyspareunia or a complaint that the partner “can feel the mesh.” Depending on the size and the nature of symptoms and the extent of the defect, these commonly seen exposures can be managed by following a simple algorithm.
Palpable or visible mesh fibrils can be trimmed in the office; they might even respond to local estrogen alone. Consider these options if the patient displays vaginal atrophy.
Typically, vaginal estrogen is prescribed as 1 g nightly for 2 weeks and then 1 g two or three nights a week. Re-examine the patient in 3 months; if symptoms of mesh exposure persist, it’s unlikely that continued conservative therapy will be successful, and outpatient surgery is recommended.
When exposure is asymptomatic, you can simply monitor the condition for 3 to 6 months; if complaints or findings arise, consider intervention.
Small (<0.5 cm in diameter) exposures can also be managed in the office, including excision of exposed mesh and local estrogen. If the exposure is easily reachable, we recommend grasping the exposed area with pick-ups or a hemostat and with gentle traction, using Metzenbaum scissors to trim exposed mesh as close to the vaginal epithelium as possible. Local topical or injected anesthesia may be needed. Bleeding should be minimal because no dissection is necessary. Silver nitrate can be applied for any minor bleeding. Larger (0.5–4.0 cm) exposures are unlikely to heal on their own. They require outpatient excision in the operating room.
Preoperative tissue preparation with local estrogen is key to successful repair of these exposures. Vaginal estrogen increases blood flow to the epithelium; as tissue becomes well-estrogenized, risk of recurrence diminishes.
The technique we employ includes:
- circumferential infiltration of vaginal epithelium surrounding the exposed mesh with 1% lidocaine with epinephrine
- sharp circumscription of the area of exposure, using a scalpel, with a 0.5-cm margin of vaginal epithelium (FIGURE 2)
- wide dissection, with undermining and mobilization of surrounding healthy vaginal epithelium around the exposure (FIGURE 3)
- excision of the exposed mesh and attached vaginal mucosa, with careful dissection of the mesh off underlying tissues with Metzenbaum scissors—being careful to avoid injury to underlying bladder or rectum (FIGURE 4)
- reapproximation of mesh edges, using 2-0 polypropylene suture to close the resulting defect so that prolapse does not recur (FIGURE 5)
- closing of the previously mobilized vaginal epithelium with 2-0 Vicryl suture, without tension, to cover the reapproximated mesh edges—after irrigation and assurance of adequate hemostasis (FIGURE 6).
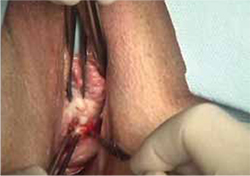
FIGURE 2 Incision of vaginal epithelium
Allow for a 0.5-cm margin.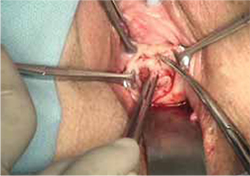
FIGURE 3 Undermining and mobilization of epithelium
Perform wide dissection.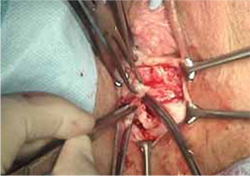
FIGURE 4 Dissection of mesh from underlying tissue
Keep clear of underlying bladder and rectum!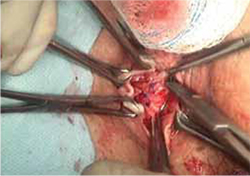
FIGURE 5 Reapproximation of edges to re-establish support
Our choice of suture is 2-0 polypropylene.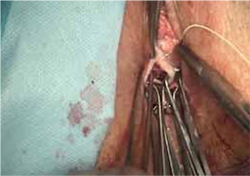
FIGURE 6 Irrigation of vaginal epithelium, followed by closure
Before you close, ensure that hemostasis is adequate.The choice of closure—vertical or horizontal—depends on the nature of the original defect.
You can watch a video of this technique that we’ve provided.
Several cautions should be taken with this technique, including:
- avoiding narrowing the vaginal canal
- minimizing trauma to healthy vaginal epithelium that will be used for closure
- maintaining hemostasis to avoid formation of hematomas.
Largest (>4 cm) exposures are likely the result of devascularized sloughing of vaginal epithelium. They are, fortunately, uncommon.
It’s unlikely that, after excision of exposed mesh, the vaginal epithelial edges can be approximated without significantly narrowing or shortening the vaginal canal. Proposed techniques for managing these large exposures include covering the defect with a biologic graft, such as small intestinal submucosa, to allow epithelium to re-grow. Regrettably, prolapse is likely to recur in the unprotected area that results.
Contraction and localized pain
Hardening and contraction typically occur along the fixation arms of the mesh. These complications might result from mesh shrinkage or from mesh being placed too tight, so to speak, at implantation. Rarely does the entire implanted mesh contract.
Severe mesh contraction can result in localized pain and de novo dyspareunia. Symptoms usually resolve after identification of the painful area and removal of the involved mesh segment.8
Diagnostic maneuver. In-office trigger-point injection of bupivacaine with triamcinolone is useful to accurately identify the location of pain that is causing dyspareunia. After injection, the patient is asked to return home and resume sexual intercourse; if dyspareunia diminishes significantly, surgical removal of the involved mesh segment is likely to ameliorate symptoms.
If dyspareunia persists after injection, however, the problem either 1) originates in a different location along the graft or 2) may not be related to the mesh—that is, it may be introital pain or preexisting vaginal pain.
The findings of trigger-point injection and a subsequent trial of sexual intercourse are useful for counseling the patient and developing realistic expectations that surgery will be successful.
Management note: Mesh contraction should be managed by a surgeon who is experienced in extensive deep pelvic dissection, which is necessary to remove the mesh arms.
Chronic pain
Diffuse vaginal pain after mesh implantation is unusual; typically, the patient’s report of pain has been preceded by recognition of another, underlying pelvic pain syndrome. Management of such pain is controversial, and many patients will not be satisfied until the entire graft is removed. Whether such drastic intervention actually resolves the pain is unclear; again, work with the patient to create realistic expectations before surgery—including the risk that prolapse will recur and that reoperation will be necessary.
Management note: An existing pelvic pain syndrome should be considered a relative contraindication to implantation of mesh.
Infection of the graft
Rarely, infection has been reported after implantation of Type-1 mesh—the result of either multi-microbial colonization or isolated infection by Bacteriodes melaninogenicus, Actinomyces spp, or Staphylococcus aureus. Untreated preoperative bacterial vaginitis is likely the underlying cause, and should be considered a contraindication to mesh implantation.
Typically, these patients complain of vaginal discharge and bleeding early postoperatively. Vaginal exposure of the mesh results from local inflammation and necrosis of tissue.
Management note: In these cases, it is necessary to 1) prescribe antimicrobial therapy that covers gram-negative and anaerobic bacteria and 2) undertake surgical removal of the exposed mesh, as we outlined above.9
Visceral erosion or fistula
Many experts believe that what is recorded as “erosion” of synthetic mesh into bladder or rectum is, in fact, a result of unrecognized visceral perforation at original implantation. This is a rare complication of mesh implantation.
Patients who experience mesh erosion into the bladder may have lower urinary-tract symptoms (LUTS) of urgency, frequency, dysuria, and hematuria. Any patient who reports de novo LUTS in the early postoperative period after a vaginal mesh procedure should receive office cystourethroscopy to ensure that no foreign body is present in the bladder or urethra.
Management note: Operative cystourethroscopy, with removal of exposed mesh, is the management of choice when mesh is found in the bladder or urethra.
Patients who have constant urinary or fecal incontinence immediately after surgery should be evaluated for vesicovaginal or rectovaginal fistula.
The presence of any of these complications necessitates removal of the involved mesh in its entirety, with concomitant repair of fistula. Typically, the procedures are performed by a specialist.
Our experience with correcting simple mesh exposures
During the past year at our tertiary referral center, 26 patients have undergone mesh revision because of exposure, using the technique we described above (FIGURE 2-6). The problem resolved in all; none had persistent dyspareunia. Many of these patients had already undergone attempts at correction of the exposure elsewhere—mostly, in the office, using techniques appropriate for that setting. Prolapse has not recurred in the 10 patients who required reapproximation of mesh edges because of a defect >2.5 cm.
CASE RESOLVED: Treatment, improvement
Under your care, the patient undergoes simplified outpatient excision of the exposed area of mesh. Mesh edges are reapproximated to support the resulting 3-cm defect.
At a 12-week postop visit, you note complete resolution of the exposure and normal vaginal caliber. The patient continues to apply estrogen cream and reports sustained improvement in sexual function.
- Preoperatively, prepare the vaginal epithelium with local estrogen cream (recommended dosage: 1 g, two nights every week for a trial of at least 6 weeks)
- Use hydrodissection to facilitate placement of the graft deep to the vaginal epithelial fibromuscular fascial layer
- Do not place a synthetic mesh as an overlay to a midline fascial plication
- Be fastidious about hemostasis
- Close the vaginal epithelium without tension
- Leave vaginal packing in place for 24 hours
- Consider using biologic grafts when appropriate (as an overlay to midline plication when used on the anterior vaginal wall).
For simple presentations, success is within reach
Simple mesh exposure can (as in the case we described) be managed by most gynecologists, utilizing the simple stepwise approach that we outlined above (for additional tips based on our experience, see “Pearls for avoiding mesh exposures”). In the case of more significant symptoms, de novo dyspareunia, visceral erosion, or fistula, however, referral to a specialist is warranted.
Transvaginal mesh surgery reduces pelvic organ prolapse
But dyspareunia may develop in premenopausal women
Transvaginal mesh (TVM) surgery is effective in treating pelvic organ prolapse (POP) in both pre- and postmenopausal women but dyspareunia may worsen in premenopausal women, according to a study published online May 23 in the Journal of Sexual Medicine.
Cheng-Yu Long, MD, PhD, from Kaohsiung Medical University in Taiwan, and colleagues compared the changes in sexual function of premenopausal and postmenopausal women after TVM surgery. A total of 68 sexually active women, categorized as premenopausal (36) and postmenopausal (32), with symptomatic POP stages II to IV were referred for TVM surgery. Preoperative and postoperative assessments included pelvic examination using the POP quantification (POP-Q) system, and completing the Female Sexual Function Index (FSFI), Urogenital Distress Inventory (UDI-6), and Incontinence Impact Questionnaire (IIQ-7).
The investigators found significant improvement in the POP-Q analysis at points Aa, Ba, C, Ap, and Bp in both groups but not in total vaginal length. The UDI-6 and IIQ-7 scores decreased significantly after TVM surgery. The dyspareunia domain score decreased significantly after surgery only in the premenopausal group. Reports of diminished scores of the dyspareunia domain and total scores were more common among women in the premenopausal group, but there were no significant differences in FSFI domains or total scores between the groups.
Copyright © 2011 HealthDay. All rights reserved.
We want to hear from you! Tell us what you think.
1. Haylen BT, Freeman RM, Swift SE, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prosthesis (meshes, implants, tapes) and grafts in female pelvic floor surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2011;22(1):3-15.
2. Davila GW, Drutz H, Deprest J. Clinical implications of the biology of grafts: conclusions of the 2005 IUGA Grafts Roundtable. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(suppl 1):S51-55.
3. Iglesia CB, Sokol AI, Sokol ER, et al. Vaginal mesh for prolapse: a randomized controlled trial. Obstet Gynecol. 2010;116(2 pt 1):293-303.
4. Hiltunen R, Nieminen K, Takala T, et al. Low-weight polypropylene mesh for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol. 2007;110(2 pt 2):455-462.
5. Fatton B, Amblard J, Debodiance P, Cosson M, Jacquetin B. Transvaginal repair of genital prolapse: preliminary results of a new tension-free vaginal mesh (Prolift technique)—a case series multicentric study. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(7):743-752.
6. Diwadkar GB, Barber MD, Feiner B, Maher C, Jelovsek JE. Complication and reoperation rates after apical vaginal prolapse surgical repair. Obstet Gynecol. 2009;113(2):367-373.
7. Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008;111(4):891-898.
8. Feiner B, Maher C. Vaginal mesh contraction: definition clinical presentation, and management. Obstet Gynecol. 2010;115(2 pt 2):325-330.
9. Athanasiou S, Matthaiou DK, Falagas ME. Vaginal mesh infection due to Bacteroides melaninogenicus: a case report of another emerging foreign body related infection. Scand J Infect Dis. 2006;38(11-12):1108-1110.
CASE: Pain during intercourse, well after mesh implantation
Your patient, 61 years old, para 3, has come to your office by referral with a complaint of dyspareunia. The history includes placement of a synthetic vaginal mesh kit 14 months earlier for prolapse.
The medical record shows that the referring physician performed a “mesh excision” 1 year after the original procedure.
The woman reports that she is “very frustrated” that she is still dealing with this problem so long after the original procedure.
On examination, you note a 2.5-cm diameter area of exposed mesh in the anterior vagina, with healthy surrounding tissue and without inflammation or purulence (FIGURE 1). You are unable to reproduce her complaint of pain on vaginal examination.
What options can you offer to this woman? And will those options meet her therapeutic expectations?

FIGURE 1 Examination of your referred patient: Mesh is noticeably exposedThe recent increase in the use of mesh grafts to reconstruct pelvic anatomy has been directed mainly at improving surgical outcomes. Yet, at the same time, gynecologic surgeons find themselves facing a rise in associated complications of such surgery that they did not see previously.
Among the most troublesome and concerning of those complications are 1) exposure of mesh through the vaginal epithelium and 2) contraction or hardening of mesh (or both) that can result in dyspareunia and chronic pelvic pain. Other, rare complications include infection and fistula.
Our goal in this article is to address the management of graft-healing abnormalities in which a segment of the mesh is palpable or visible, or both, within the vaginal canal. Our focus is on simple abnormalities that can be managed by most generalist gynecologists; to be clear, more complex abnormalities, and those that provoke more serious or lasting symptoms, belong under the care of a specialist.
A recent shift in terminology is significant
Early on, this complication was called “erosion” as understanding of the mechanism of its development grew, however, terminology applied to the problem has changed.
In fact, mesh itself very rarely erodes into the vagina or an underlying viscus. Instead, the complication occurs most commonly as a result of disruption of a suture line—most likely the result of a hematoma or localized inflammation that develops postoperatively.
“Exposure” (our preference here) and “extrusion” are now the recommended terms, based on a consensus terminology document published this year jointly by the International Urogynecological Association and the International Continence Society.1
Exposure of implanted mesh is considered a “simple” healing abnormality because it typically
- occurs along the suture line and early in the course of healing
- is not associated with infection of the graft.2
The typical physical appearance is one of visible mesh along an open suture line without granulation tissue or purulence—again, see FIGURE 1. The mesh is firmly adherent to the vaginal epithelial edges and underlying fascia.
The reported incidence of mesh exposures—in regard to currently used meshes, which are all Type-1, monofilament, macroporous polypropylene grafts—is approximately 10% but as high as 15% to 20% in some reported series.3,4 The higher rates of exposure are usually seen in series in which some patients have had a synthetic graft implanted as an overlay to fascial midline plication. When the graft is implanted in the subfascial layer of the vaginal wall (i.e., without midline plication), however, the reported rate of exposure falls—to 5% to 10%.5-7
Recommendations for management
Initially, recommendations for “erosion” management were based on concerns about underlying mesh infection or rejection, and included a need to remove the entire graft. That recommendation still applies to multifilament, microporous grafts that present with inflammatory infiltrates, granulation tissue, and purulence. Although these kinds of grafts (known as “Type-2/3 grafts”—e.g., GoreTex, IVS) have not been marketed for pelvic reconstruction over the past 3 to 5 years, their behavior post-implantation is less predictable—and patients who have delayed healing abnormalities are, therefore, still being seen. It’s fortunate that development of an overlying biofilm prevents tissue incorporation into these types of graft, allowing them to be removed easily.
Exposures related to Type-1 mesh—currently used in pelvic reconstruction—that occur without surrounding infection do not require extensive removal. Rather, they can be managed conservatively or, when necessary, with outpatient surgery. In patients who are not sexually active, exposures are usually asymptomatic; they might only be observed by the physician on vaginal examination and are amenable to simple monitoring. In sexually active patients, exposure of Type-1 mesh usually results in dyspareunia or a complaint that the partner “can feel the mesh.” Depending on the size and the nature of symptoms and the extent of the defect, these commonly seen exposures can be managed by following a simple algorithm.
Palpable or visible mesh fibrils can be trimmed in the office; they might even respond to local estrogen alone. Consider these options if the patient displays vaginal atrophy.
Typically, vaginal estrogen is prescribed as 1 g nightly for 2 weeks and then 1 g two or three nights a week. Re-examine the patient in 3 months; if symptoms of mesh exposure persist, it’s unlikely that continued conservative therapy will be successful, and outpatient surgery is recommended.
When exposure is asymptomatic, you can simply monitor the condition for 3 to 6 months; if complaints or findings arise, consider intervention.
Small (<0.5 cm in diameter) exposures can also be managed in the office, including excision of exposed mesh and local estrogen. If the exposure is easily reachable, we recommend grasping the exposed area with pick-ups or a hemostat and with gentle traction, using Metzenbaum scissors to trim exposed mesh as close to the vaginal epithelium as possible. Local topical or injected anesthesia may be needed. Bleeding should be minimal because no dissection is necessary. Silver nitrate can be applied for any minor bleeding. Larger (0.5–4.0 cm) exposures are unlikely to heal on their own. They require outpatient excision in the operating room.
Preoperative tissue preparation with local estrogen is key to successful repair of these exposures. Vaginal estrogen increases blood flow to the epithelium; as tissue becomes well-estrogenized, risk of recurrence diminishes.
The technique we employ includes:
- circumferential infiltration of vaginal epithelium surrounding the exposed mesh with 1% lidocaine with epinephrine
- sharp circumscription of the area of exposure, using a scalpel, with a 0.5-cm margin of vaginal epithelium (FIGURE 2)
- wide dissection, with undermining and mobilization of surrounding healthy vaginal epithelium around the exposure (FIGURE 3)
- excision of the exposed mesh and attached vaginal mucosa, with careful dissection of the mesh off underlying tissues with Metzenbaum scissors—being careful to avoid injury to underlying bladder or rectum (FIGURE 4)
- reapproximation of mesh edges, using 2-0 polypropylene suture to close the resulting defect so that prolapse does not recur (FIGURE 5)
- closing of the previously mobilized vaginal epithelium with 2-0 Vicryl suture, without tension, to cover the reapproximated mesh edges—after irrigation and assurance of adequate hemostasis (FIGURE 6).

FIGURE 2 Incision of vaginal epithelium
Allow for a 0.5-cm margin.
FIGURE 3 Undermining and mobilization of epithelium
Perform wide dissection.
FIGURE 4 Dissection of mesh from underlying tissue
Keep clear of underlying bladder and rectum!
FIGURE 5 Reapproximation of edges to re-establish support
Our choice of suture is 2-0 polypropylene.
FIGURE 6 Irrigation of vaginal epithelium, followed by closure
Before you close, ensure that hemostasis is adequate.The choice of closure—vertical or horizontal—depends on the nature of the original defect.
You can watch a video of this technique that we’ve provided.
Several cautions should be taken with this technique, including:
- avoiding narrowing the vaginal canal
- minimizing trauma to healthy vaginal epithelium that will be used for closure
- maintaining hemostasis to avoid formation of hematomas.
Largest (>4 cm) exposures are likely the result of devascularized sloughing of vaginal epithelium. They are, fortunately, uncommon.
It’s unlikely that, after excision of exposed mesh, the vaginal epithelial edges can be approximated without significantly narrowing or shortening the vaginal canal. Proposed techniques for managing these large exposures include covering the defect with a biologic graft, such as small intestinal submucosa, to allow epithelium to re-grow. Regrettably, prolapse is likely to recur in the unprotected area that results.
Contraction and localized pain
Hardening and contraction typically occur along the fixation arms of the mesh. These complications might result from mesh shrinkage or from mesh being placed too tight, so to speak, at implantation. Rarely does the entire implanted mesh contract.
Severe mesh contraction can result in localized pain and de novo dyspareunia. Symptoms usually resolve after identification of the painful area and removal of the involved mesh segment.8
Diagnostic maneuver. In-office trigger-point injection of bupivacaine with triamcinolone is useful to accurately identify the location of pain that is causing dyspareunia. After injection, the patient is asked to return home and resume sexual intercourse; if dyspareunia diminishes significantly, surgical removal of the involved mesh segment is likely to ameliorate symptoms.
If dyspareunia persists after injection, however, the problem either 1) originates in a different location along the graft or 2) may not be related to the mesh—that is, it may be introital pain or preexisting vaginal pain.
The findings of trigger-point injection and a subsequent trial of sexual intercourse are useful for counseling the patient and developing realistic expectations that surgery will be successful.
Management note: Mesh contraction should be managed by a surgeon who is experienced in extensive deep pelvic dissection, which is necessary to remove the mesh arms.
Chronic pain
Diffuse vaginal pain after mesh implantation is unusual; typically, the patient’s report of pain has been preceded by recognition of another, underlying pelvic pain syndrome. Management of such pain is controversial, and many patients will not be satisfied until the entire graft is removed. Whether such drastic intervention actually resolves the pain is unclear; again, work with the patient to create realistic expectations before surgery—including the risk that prolapse will recur and that reoperation will be necessary.
Management note: An existing pelvic pain syndrome should be considered a relative contraindication to implantation of mesh.
Infection of the graft
Rarely, infection has been reported after implantation of Type-1 mesh—the result of either multi-microbial colonization or isolated infection by Bacteriodes melaninogenicus, Actinomyces spp, or Staphylococcus aureus. Untreated preoperative bacterial vaginitis is likely the underlying cause, and should be considered a contraindication to mesh implantation.
Typically, these patients complain of vaginal discharge and bleeding early postoperatively. Vaginal exposure of the mesh results from local inflammation and necrosis of tissue.
Management note: In these cases, it is necessary to 1) prescribe antimicrobial therapy that covers gram-negative and anaerobic bacteria and 2) undertake surgical removal of the exposed mesh, as we outlined above.9
Visceral erosion or fistula
Many experts believe that what is recorded as “erosion” of synthetic mesh into bladder or rectum is, in fact, a result of unrecognized visceral perforation at original implantation. This is a rare complication of mesh implantation.
Patients who experience mesh erosion into the bladder may have lower urinary-tract symptoms (LUTS) of urgency, frequency, dysuria, and hematuria. Any patient who reports de novo LUTS in the early postoperative period after a vaginal mesh procedure should receive office cystourethroscopy to ensure that no foreign body is present in the bladder or urethra.
Management note: Operative cystourethroscopy, with removal of exposed mesh, is the management of choice when mesh is found in the bladder or urethra.
Patients who have constant urinary or fecal incontinence immediately after surgery should be evaluated for vesicovaginal or rectovaginal fistula.
The presence of any of these complications necessitates removal of the involved mesh in its entirety, with concomitant repair of fistula. Typically, the procedures are performed by a specialist.
Our experience with correcting simple mesh exposures
During the past year at our tertiary referral center, 26 patients have undergone mesh revision because of exposure, using the technique we described above (FIGURE 2-6). The problem resolved in all; none had persistent dyspareunia. Many of these patients had already undergone attempts at correction of the exposure elsewhere—mostly, in the office, using techniques appropriate for that setting. Prolapse has not recurred in the 10 patients who required reapproximation of mesh edges because of a defect >2.5 cm.
CASE RESOLVED: Treatment, improvement
Under your care, the patient undergoes simplified outpatient excision of the exposed area of mesh. Mesh edges are reapproximated to support the resulting 3-cm defect.
At a 12-week postop visit, you note complete resolution of the exposure and normal vaginal caliber. The patient continues to apply estrogen cream and reports sustained improvement in sexual function.
- Preoperatively, prepare the vaginal epithelium with local estrogen cream (recommended dosage: 1 g, two nights every week for a trial of at least 6 weeks)
- Use hydrodissection to facilitate placement of the graft deep to the vaginal epithelial fibromuscular fascial layer
- Do not place a synthetic mesh as an overlay to a midline fascial plication
- Be fastidious about hemostasis
- Close the vaginal epithelium without tension
- Leave vaginal packing in place for 24 hours
- Consider using biologic grafts when appropriate (as an overlay to midline plication when used on the anterior vaginal wall).
For simple presentations, success is within reach
Simple mesh exposure can (as in the case we described) be managed by most gynecologists, utilizing the simple stepwise approach that we outlined above (for additional tips based on our experience, see “Pearls for avoiding mesh exposures”). In the case of more significant symptoms, de novo dyspareunia, visceral erosion, or fistula, however, referral to a specialist is warranted.
Transvaginal mesh surgery reduces pelvic organ prolapse
But dyspareunia may develop in premenopausal women
Transvaginal mesh (TVM) surgery is effective in treating pelvic organ prolapse (POP) in both pre- and postmenopausal women but dyspareunia may worsen in premenopausal women, according to a study published online May 23 in the Journal of Sexual Medicine.
Cheng-Yu Long, MD, PhD, from Kaohsiung Medical University in Taiwan, and colleagues compared the changes in sexual function of premenopausal and postmenopausal women after TVM surgery. A total of 68 sexually active women, categorized as premenopausal (36) and postmenopausal (32), with symptomatic POP stages II to IV were referred for TVM surgery. Preoperative and postoperative assessments included pelvic examination using the POP quantification (POP-Q) system, and completing the Female Sexual Function Index (FSFI), Urogenital Distress Inventory (UDI-6), and Incontinence Impact Questionnaire (IIQ-7).
The investigators found significant improvement in the POP-Q analysis at points Aa, Ba, C, Ap, and Bp in both groups but not in total vaginal length. The UDI-6 and IIQ-7 scores decreased significantly after TVM surgery. The dyspareunia domain score decreased significantly after surgery only in the premenopausal group. Reports of diminished scores of the dyspareunia domain and total scores were more common among women in the premenopausal group, but there were no significant differences in FSFI domains or total scores between the groups.
Copyright © 2011 HealthDay. All rights reserved.
We want to hear from you! Tell us what you think.
CASE: Pain during intercourse, well after mesh implantation
Your patient, 61 years old, para 3, has come to your office by referral with a complaint of dyspareunia. The history includes placement of a synthetic vaginal mesh kit 14 months earlier for prolapse.
The medical record shows that the referring physician performed a “mesh excision” 1 year after the original procedure.
The woman reports that she is “very frustrated” that she is still dealing with this problem so long after the original procedure.
On examination, you note a 2.5-cm diameter area of exposed mesh in the anterior vagina, with healthy surrounding tissue and without inflammation or purulence (FIGURE 1). You are unable to reproduce her complaint of pain on vaginal examination.
What options can you offer to this woman? And will those options meet her therapeutic expectations?

FIGURE 1 Examination of your referred patient: Mesh is noticeably exposedThe recent increase in the use of mesh grafts to reconstruct pelvic anatomy has been directed mainly at improving surgical outcomes. Yet, at the same time, gynecologic surgeons find themselves facing a rise in associated complications of such surgery that they did not see previously.
Among the most troublesome and concerning of those complications are 1) exposure of mesh through the vaginal epithelium and 2) contraction or hardening of mesh (or both) that can result in dyspareunia and chronic pelvic pain. Other, rare complications include infection and fistula.
Our goal in this article is to address the management of graft-healing abnormalities in which a segment of the mesh is palpable or visible, or both, within the vaginal canal. Our focus is on simple abnormalities that can be managed by most generalist gynecologists; to be clear, more complex abnormalities, and those that provoke more serious or lasting symptoms, belong under the care of a specialist.
A recent shift in terminology is significant
Early on, this complication was called “erosion” as understanding of the mechanism of its development grew, however, terminology applied to the problem has changed.
In fact, mesh itself very rarely erodes into the vagina or an underlying viscus. Instead, the complication occurs most commonly as a result of disruption of a suture line—most likely the result of a hematoma or localized inflammation that develops postoperatively.
“Exposure” (our preference here) and “extrusion” are now the recommended terms, based on a consensus terminology document published this year jointly by the International Urogynecological Association and the International Continence Society.1
Exposure of implanted mesh is considered a “simple” healing abnormality because it typically
- occurs along the suture line and early in the course of healing
- is not associated with infection of the graft.2
The typical physical appearance is one of visible mesh along an open suture line without granulation tissue or purulence—again, see FIGURE 1. The mesh is firmly adherent to the vaginal epithelial edges and underlying fascia.
The reported incidence of mesh exposures—in regard to currently used meshes, which are all Type-1, monofilament, macroporous polypropylene grafts—is approximately 10% but as high as 15% to 20% in some reported series.3,4 The higher rates of exposure are usually seen in series in which some patients have had a synthetic graft implanted as an overlay to fascial midline plication. When the graft is implanted in the subfascial layer of the vaginal wall (i.e., without midline plication), however, the reported rate of exposure falls—to 5% to 10%.5-7
Recommendations for management
Initially, recommendations for “erosion” management were based on concerns about underlying mesh infection or rejection, and included a need to remove the entire graft. That recommendation still applies to multifilament, microporous grafts that present with inflammatory infiltrates, granulation tissue, and purulence. Although these kinds of grafts (known as “Type-2/3 grafts”—e.g., GoreTex, IVS) have not been marketed for pelvic reconstruction over the past 3 to 5 years, their behavior post-implantation is less predictable—and patients who have delayed healing abnormalities are, therefore, still being seen. It’s fortunate that development of an overlying biofilm prevents tissue incorporation into these types of graft, allowing them to be removed easily.
Exposures related to Type-1 mesh—currently used in pelvic reconstruction—that occur without surrounding infection do not require extensive removal. Rather, they can be managed conservatively or, when necessary, with outpatient surgery. In patients who are not sexually active, exposures are usually asymptomatic; they might only be observed by the physician on vaginal examination and are amenable to simple monitoring. In sexually active patients, exposure of Type-1 mesh usually results in dyspareunia or a complaint that the partner “can feel the mesh.” Depending on the size and the nature of symptoms and the extent of the defect, these commonly seen exposures can be managed by following a simple algorithm.
Palpable or visible mesh fibrils can be trimmed in the office; they might even respond to local estrogen alone. Consider these options if the patient displays vaginal atrophy.
Typically, vaginal estrogen is prescribed as 1 g nightly for 2 weeks and then 1 g two or three nights a week. Re-examine the patient in 3 months; if symptoms of mesh exposure persist, it’s unlikely that continued conservative therapy will be successful, and outpatient surgery is recommended.
When exposure is asymptomatic, you can simply monitor the condition for 3 to 6 months; if complaints or findings arise, consider intervention.
Small (<0.5 cm in diameter) exposures can also be managed in the office, including excision of exposed mesh and local estrogen. If the exposure is easily reachable, we recommend grasping the exposed area with pick-ups or a hemostat and with gentle traction, using Metzenbaum scissors to trim exposed mesh as close to the vaginal epithelium as possible. Local topical or injected anesthesia may be needed. Bleeding should be minimal because no dissection is necessary. Silver nitrate can be applied for any minor bleeding. Larger (0.5–4.0 cm) exposures are unlikely to heal on their own. They require outpatient excision in the operating room.
Preoperative tissue preparation with local estrogen is key to successful repair of these exposures. Vaginal estrogen increases blood flow to the epithelium; as tissue becomes well-estrogenized, risk of recurrence diminishes.
The technique we employ includes:
- circumferential infiltration of vaginal epithelium surrounding the exposed mesh with 1% lidocaine with epinephrine
- sharp circumscription of the area of exposure, using a scalpel, with a 0.5-cm margin of vaginal epithelium (FIGURE 2)
- wide dissection, with undermining and mobilization of surrounding healthy vaginal epithelium around the exposure (FIGURE 3)
- excision of the exposed mesh and attached vaginal mucosa, with careful dissection of the mesh off underlying tissues with Metzenbaum scissors—being careful to avoid injury to underlying bladder or rectum (FIGURE 4)
- reapproximation of mesh edges, using 2-0 polypropylene suture to close the resulting defect so that prolapse does not recur (FIGURE 5)
- closing of the previously mobilized vaginal epithelium with 2-0 Vicryl suture, without tension, to cover the reapproximated mesh edges—after irrigation and assurance of adequate hemostasis (FIGURE 6).

FIGURE 2 Incision of vaginal epithelium
Allow for a 0.5-cm margin.
FIGURE 3 Undermining and mobilization of epithelium
Perform wide dissection.
FIGURE 4 Dissection of mesh from underlying tissue
Keep clear of underlying bladder and rectum!
FIGURE 5 Reapproximation of edges to re-establish support
Our choice of suture is 2-0 polypropylene.
FIGURE 6 Irrigation of vaginal epithelium, followed by closure
Before you close, ensure that hemostasis is adequate.The choice of closure—vertical or horizontal—depends on the nature of the original defect.
You can watch a video of this technique that we’ve provided.
Several cautions should be taken with this technique, including:
- avoiding narrowing the vaginal canal
- minimizing trauma to healthy vaginal epithelium that will be used for closure
- maintaining hemostasis to avoid formation of hematomas.
Largest (>4 cm) exposures are likely the result of devascularized sloughing of vaginal epithelium. They are, fortunately, uncommon.
It’s unlikely that, after excision of exposed mesh, the vaginal epithelial edges can be approximated without significantly narrowing or shortening the vaginal canal. Proposed techniques for managing these large exposures include covering the defect with a biologic graft, such as small intestinal submucosa, to allow epithelium to re-grow. Regrettably, prolapse is likely to recur in the unprotected area that results.
Contraction and localized pain
Hardening and contraction typically occur along the fixation arms of the mesh. These complications might result from mesh shrinkage or from mesh being placed too tight, so to speak, at implantation. Rarely does the entire implanted mesh contract.
Severe mesh contraction can result in localized pain and de novo dyspareunia. Symptoms usually resolve after identification of the painful area and removal of the involved mesh segment.8
Diagnostic maneuver. In-office trigger-point injection of bupivacaine with triamcinolone is useful to accurately identify the location of pain that is causing dyspareunia. After injection, the patient is asked to return home and resume sexual intercourse; if dyspareunia diminishes significantly, surgical removal of the involved mesh segment is likely to ameliorate symptoms.
If dyspareunia persists after injection, however, the problem either 1) originates in a different location along the graft or 2) may not be related to the mesh—that is, it may be introital pain or preexisting vaginal pain.
The findings of trigger-point injection and a subsequent trial of sexual intercourse are useful for counseling the patient and developing realistic expectations that surgery will be successful.
Management note: Mesh contraction should be managed by a surgeon who is experienced in extensive deep pelvic dissection, which is necessary to remove the mesh arms.
Chronic pain
Diffuse vaginal pain after mesh implantation is unusual; typically, the patient’s report of pain has been preceded by recognition of another, underlying pelvic pain syndrome. Management of such pain is controversial, and many patients will not be satisfied until the entire graft is removed. Whether such drastic intervention actually resolves the pain is unclear; again, work with the patient to create realistic expectations before surgery—including the risk that prolapse will recur and that reoperation will be necessary.
Management note: An existing pelvic pain syndrome should be considered a relative contraindication to implantation of mesh.
Infection of the graft
Rarely, infection has been reported after implantation of Type-1 mesh—the result of either multi-microbial colonization or isolated infection by Bacteriodes melaninogenicus, Actinomyces spp, or Staphylococcus aureus. Untreated preoperative bacterial vaginitis is likely the underlying cause, and should be considered a contraindication to mesh implantation.
Typically, these patients complain of vaginal discharge and bleeding early postoperatively. Vaginal exposure of the mesh results from local inflammation and necrosis of tissue.
Management note: In these cases, it is necessary to 1) prescribe antimicrobial therapy that covers gram-negative and anaerobic bacteria and 2) undertake surgical removal of the exposed mesh, as we outlined above.9
Visceral erosion or fistula
Many experts believe that what is recorded as “erosion” of synthetic mesh into bladder or rectum is, in fact, a result of unrecognized visceral perforation at original implantation. This is a rare complication of mesh implantation.
Patients who experience mesh erosion into the bladder may have lower urinary-tract symptoms (LUTS) of urgency, frequency, dysuria, and hematuria. Any patient who reports de novo LUTS in the early postoperative period after a vaginal mesh procedure should receive office cystourethroscopy to ensure that no foreign body is present in the bladder or urethra.
Management note: Operative cystourethroscopy, with removal of exposed mesh, is the management of choice when mesh is found in the bladder or urethra.
Patients who have constant urinary or fecal incontinence immediately after surgery should be evaluated for vesicovaginal or rectovaginal fistula.
The presence of any of these complications necessitates removal of the involved mesh in its entirety, with concomitant repair of fistula. Typically, the procedures are performed by a specialist.
Our experience with correcting simple mesh exposures
During the past year at our tertiary referral center, 26 patients have undergone mesh revision because of exposure, using the technique we described above (FIGURE 2-6). The problem resolved in all; none had persistent dyspareunia. Many of these patients had already undergone attempts at correction of the exposure elsewhere—mostly, in the office, using techniques appropriate for that setting. Prolapse has not recurred in the 10 patients who required reapproximation of mesh edges because of a defect >2.5 cm.
CASE RESOLVED: Treatment, improvement
Under your care, the patient undergoes simplified outpatient excision of the exposed area of mesh. Mesh edges are reapproximated to support the resulting 3-cm defect.
At a 12-week postop visit, you note complete resolution of the exposure and normal vaginal caliber. The patient continues to apply estrogen cream and reports sustained improvement in sexual function.
- Preoperatively, prepare the vaginal epithelium with local estrogen cream (recommended dosage: 1 g, two nights every week for a trial of at least 6 weeks)
- Use hydrodissection to facilitate placement of the graft deep to the vaginal epithelial fibromuscular fascial layer
- Do not place a synthetic mesh as an overlay to a midline fascial plication
- Be fastidious about hemostasis
- Close the vaginal epithelium without tension
- Leave vaginal packing in place for 24 hours
- Consider using biologic grafts when appropriate (as an overlay to midline plication when used on the anterior vaginal wall).
For simple presentations, success is within reach
Simple mesh exposure can (as in the case we described) be managed by most gynecologists, utilizing the simple stepwise approach that we outlined above (for additional tips based on our experience, see “Pearls for avoiding mesh exposures”). In the case of more significant symptoms, de novo dyspareunia, visceral erosion, or fistula, however, referral to a specialist is warranted.
Transvaginal mesh surgery reduces pelvic organ prolapse
But dyspareunia may develop in premenopausal women
Transvaginal mesh (TVM) surgery is effective in treating pelvic organ prolapse (POP) in both pre- and postmenopausal women but dyspareunia may worsen in premenopausal women, according to a study published online May 23 in the Journal of Sexual Medicine.
Cheng-Yu Long, MD, PhD, from Kaohsiung Medical University in Taiwan, and colleagues compared the changes in sexual function of premenopausal and postmenopausal women after TVM surgery. A total of 68 sexually active women, categorized as premenopausal (36) and postmenopausal (32), with symptomatic POP stages II to IV were referred for TVM surgery. Preoperative and postoperative assessments included pelvic examination using the POP quantification (POP-Q) system, and completing the Female Sexual Function Index (FSFI), Urogenital Distress Inventory (UDI-6), and Incontinence Impact Questionnaire (IIQ-7).
The investigators found significant improvement in the POP-Q analysis at points Aa, Ba, C, Ap, and Bp in both groups but not in total vaginal length. The UDI-6 and IIQ-7 scores decreased significantly after TVM surgery. The dyspareunia domain score decreased significantly after surgery only in the premenopausal group. Reports of diminished scores of the dyspareunia domain and total scores were more common among women in the premenopausal group, but there were no significant differences in FSFI domains or total scores between the groups.
Copyright © 2011 HealthDay. All rights reserved.
We want to hear from you! Tell us what you think.
1. Haylen BT, Freeman RM, Swift SE, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prosthesis (meshes, implants, tapes) and grafts in female pelvic floor surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2011;22(1):3-15.
2. Davila GW, Drutz H, Deprest J. Clinical implications of the biology of grafts: conclusions of the 2005 IUGA Grafts Roundtable. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(suppl 1):S51-55.
3. Iglesia CB, Sokol AI, Sokol ER, et al. Vaginal mesh for prolapse: a randomized controlled trial. Obstet Gynecol. 2010;116(2 pt 1):293-303.
4. Hiltunen R, Nieminen K, Takala T, et al. Low-weight polypropylene mesh for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol. 2007;110(2 pt 2):455-462.
5. Fatton B, Amblard J, Debodiance P, Cosson M, Jacquetin B. Transvaginal repair of genital prolapse: preliminary results of a new tension-free vaginal mesh (Prolift technique)—a case series multicentric study. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(7):743-752.
6. Diwadkar GB, Barber MD, Feiner B, Maher C, Jelovsek JE. Complication and reoperation rates after apical vaginal prolapse surgical repair. Obstet Gynecol. 2009;113(2):367-373.
7. Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008;111(4):891-898.
8. Feiner B, Maher C. Vaginal mesh contraction: definition clinical presentation, and management. Obstet Gynecol. 2010;115(2 pt 2):325-330.
9. Athanasiou S, Matthaiou DK, Falagas ME. Vaginal mesh infection due to Bacteroides melaninogenicus: a case report of another emerging foreign body related infection. Scand J Infect Dis. 2006;38(11-12):1108-1110.
1. Haylen BT, Freeman RM, Swift SE, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint terminology and classification of the complications related directly to the insertion of prosthesis (meshes, implants, tapes) and grafts in female pelvic floor surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2011;22(1):3-15.
2. Davila GW, Drutz H, Deprest J. Clinical implications of the biology of grafts: conclusions of the 2005 IUGA Grafts Roundtable. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(suppl 1):S51-55.
3. Iglesia CB, Sokol AI, Sokol ER, et al. Vaginal mesh for prolapse: a randomized controlled trial. Obstet Gynecol. 2010;116(2 pt 1):293-303.
4. Hiltunen R, Nieminen K, Takala T, et al. Low-weight polypropylene mesh for anterior vaginal wall prolapse: a randomized controlled trial. Obstet Gynecol. 2007;110(2 pt 2):455-462.
5. Fatton B, Amblard J, Debodiance P, Cosson M, Jacquetin B. Transvaginal repair of genital prolapse: preliminary results of a new tension-free vaginal mesh (Prolift technique)—a case series multicentric study. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(7):743-752.
6. Diwadkar GB, Barber MD, Feiner B, Maher C, Jelovsek JE. Complication and reoperation rates after apical vaginal prolapse surgical repair. Obstet Gynecol. 2009;113(2):367-373.
7. Nguyen JN, Burchette RJ. Outcome after anterior vaginal prolapse repair: a randomized controlled trial. Obstet Gynecol. 2008;111(4):891-898.
8. Feiner B, Maher C. Vaginal mesh contraction: definition clinical presentation, and management. Obstet Gynecol. 2010;115(2 pt 2):325-330.
9. Athanasiou S, Matthaiou DK, Falagas ME. Vaginal mesh infection due to Bacteroides melaninogenicus: a case report of another emerging foreign body related infection. Scand J Infect Dis. 2006;38(11-12):1108-1110.
Cancer survivors have many complaints not addressed by their physicians
![]()
How to talk to patients about sex
Barbara S. Levy, MD (September 2010)
Sex talk is ubiquitous in American culture—except in the doctor’s office.
So observed psychologist Sharon L. Bober, PhD, at the Cancer Survivorship and Sexual Health Symposium, held June 17–19, 2011, in Washington, DC. Dr. Bober is director of the Sexual Health Program in Pediatric Oncology at Dana-Farber Cancer Institute in Boston.
The survivorship symposium, sponsored by the International Society for Sexual Medicine and the Sexual Medicine Society of North America, offered background on the most common problems encountered by cancer survivors, as well as guidance on how to care for them in gynecology, primary care, oncology, and other fields. Sexual dysfunction, urinary incontinence, and fatigue figured prominently among those problems, yet few clinicians are asking about these conditions among cancer survivors—and even fewer are treating them, Dr. Bober said.
One in three women will be diagnosed with cancer in her lifetime, Dr. Bober noted, and the number of cancer survivors will double—from the current number of approximately 12 million—by the year 2016. The bulk of survivors seek care in the community after treatment, she added.
One reason sexual dysfunction is so widespread among cancer survivors: 64% of all cancer patients have a malignancy that directly affects sexual organs, said Stacy T. Lindau, MD, another speaker at the symposium. Dr. Lindau is associate professor of obstetrics and gynecology and of medicine and geriatrics at the University of Chicago.
According to a 2010 survey by the Lance Armstrong Foundation cited at the symposium, 43% of respondents reported problems with sexual function following treatment, with 29% of that group reporting “a lot” of functional impairment.1 Yet, only 13% of respondents who reported problems with sexual function received care. When respondents were asked why they did not receive care for their problem:
- 55% said they had learned to live with it
- 37% said they had been told it was a side effect of treatment
- 20% said they addressed the problem on their own
- 19% said they were told nothing could be done
- 14% said they expected to get care in the future.
“Discomfort around human sexuality is the main reason the issue doesn’t get raised by health-care providers,” Dr. Bober noted.
“If you’re going to wait for your doctor to bring up this topic, it’s like waiting for Godot.”
We want to hear from you! Tell us what you think.
Reference
1. 2010 LIVESTRONG Survey for People Affected by Cancer. Austin, Tex: Lance Armstrong Foundation; 2011.
![]()
How to talk to patients about sex
Barbara S. Levy, MD (September 2010)
Sex talk is ubiquitous in American culture—except in the doctor’s office.
So observed psychologist Sharon L. Bober, PhD, at the Cancer Survivorship and Sexual Health Symposium, held June 17–19, 2011, in Washington, DC. Dr. Bober is director of the Sexual Health Program in Pediatric Oncology at Dana-Farber Cancer Institute in Boston.
The survivorship symposium, sponsored by the International Society for Sexual Medicine and the Sexual Medicine Society of North America, offered background on the most common problems encountered by cancer survivors, as well as guidance on how to care for them in gynecology, primary care, oncology, and other fields. Sexual dysfunction, urinary incontinence, and fatigue figured prominently among those problems, yet few clinicians are asking about these conditions among cancer survivors—and even fewer are treating them, Dr. Bober said.
One in three women will be diagnosed with cancer in her lifetime, Dr. Bober noted, and the number of cancer survivors will double—from the current number of approximately 12 million—by the year 2016. The bulk of survivors seek care in the community after treatment, she added.
One reason sexual dysfunction is so widespread among cancer survivors: 64% of all cancer patients have a malignancy that directly affects sexual organs, said Stacy T. Lindau, MD, another speaker at the symposium. Dr. Lindau is associate professor of obstetrics and gynecology and of medicine and geriatrics at the University of Chicago.
According to a 2010 survey by the Lance Armstrong Foundation cited at the symposium, 43% of respondents reported problems with sexual function following treatment, with 29% of that group reporting “a lot” of functional impairment.1 Yet, only 13% of respondents who reported problems with sexual function received care. When respondents were asked why they did not receive care for their problem:
- 55% said they had learned to live with it
- 37% said they had been told it was a side effect of treatment
- 20% said they addressed the problem on their own
- 19% said they were told nothing could be done
- 14% said they expected to get care in the future.
“Discomfort around human sexuality is the main reason the issue doesn’t get raised by health-care providers,” Dr. Bober noted.
“If you’re going to wait for your doctor to bring up this topic, it’s like waiting for Godot.”
We want to hear from you! Tell us what you think.
![]()
How to talk to patients about sex
Barbara S. Levy, MD (September 2010)
Sex talk is ubiquitous in American culture—except in the doctor’s office.
So observed psychologist Sharon L. Bober, PhD, at the Cancer Survivorship and Sexual Health Symposium, held June 17–19, 2011, in Washington, DC. Dr. Bober is director of the Sexual Health Program in Pediatric Oncology at Dana-Farber Cancer Institute in Boston.
The survivorship symposium, sponsored by the International Society for Sexual Medicine and the Sexual Medicine Society of North America, offered background on the most common problems encountered by cancer survivors, as well as guidance on how to care for them in gynecology, primary care, oncology, and other fields. Sexual dysfunction, urinary incontinence, and fatigue figured prominently among those problems, yet few clinicians are asking about these conditions among cancer survivors—and even fewer are treating them, Dr. Bober said.
One in three women will be diagnosed with cancer in her lifetime, Dr. Bober noted, and the number of cancer survivors will double—from the current number of approximately 12 million—by the year 2016. The bulk of survivors seek care in the community after treatment, she added.
One reason sexual dysfunction is so widespread among cancer survivors: 64% of all cancer patients have a malignancy that directly affects sexual organs, said Stacy T. Lindau, MD, another speaker at the symposium. Dr. Lindau is associate professor of obstetrics and gynecology and of medicine and geriatrics at the University of Chicago.
According to a 2010 survey by the Lance Armstrong Foundation cited at the symposium, 43% of respondents reported problems with sexual function following treatment, with 29% of that group reporting “a lot” of functional impairment.1 Yet, only 13% of respondents who reported problems with sexual function received care. When respondents were asked why they did not receive care for their problem:
- 55% said they had learned to live with it
- 37% said they had been told it was a side effect of treatment
- 20% said they addressed the problem on their own
- 19% said they were told nothing could be done
- 14% said they expected to get care in the future.
“Discomfort around human sexuality is the main reason the issue doesn’t get raised by health-care providers,” Dr. Bober noted.
“If you’re going to wait for your doctor to bring up this topic, it’s like waiting for Godot.”
We want to hear from you! Tell us what you think.
Reference
1. 2010 LIVESTRONG Survey for People Affected by Cancer. Austin, Tex: Lance Armstrong Foundation; 2011.
Reference
1. 2010 LIVESTRONG Survey for People Affected by Cancer. Austin, Tex: Lance Armstrong Foundation; 2011.
Most OB malpractice claims involve cascading events, not isolated errors
- Read Medical Verdicts, Notable Judgments and Settlements
every month
(June 2011)
Errors in clinical judgment were cited in 77% of more than 800 clinically coded obstetric malpractice cases analyzed by CRICO Strategies, a division of CRICO. CRICO is the patient safety and medical malpractice insurance company owned by the Harvard medical community since 1976. The findings of the analysis were published in a 2010 report entitled Malpractice Risks in Obstetrics.1 The cases on which the report is based were asserted from 2005 to 2009.
According to the report, other prevalent areas of causation were:
- miscommunication (36% of cases)
- technical error (26%)
- inadequate documentation (26%)
- administrative failures (23%)
- ineffective supervision (15%).
The report also reveals the top three most common OB risks or allegations:
- delay in treatment of fetal distress
- improper performance of vaginal delivery
- improper management of pregnancy.
In the CRICO analysis, OB malpractice issues were rarely the result of a single act or omission by a single clinician. Rather, cases typically involved a series of missteps and mishandled decisions by a team of physicians and nurses who converged too late to resolve a rapidly devolving crisis.
“Obstetrics has some unique vulnerabilities, most often involving situations in which a sequence of errors or oversights cascade into a crisis that can put mother and baby in jeopardy,” said Robert Hanscom, senior vice president of CRICO Strategies. “Because there is rarely that standout ‘single event,’ it is absolutely paramount that OB practices understand how these missteps unfold, and then focus on education and training initiatives designed specifically to help clinicians avert those mistakes.”
Although the rate of OB claims is relatively infrequent—less than one case for every 1,000 births—the average malpractice payment is approximately $947,000. That figure is more than twice that of other clinical areas, and second only to surgery in total indemnity payments.
We want to hear from you! Tell us what you think.
Reference
1. 2010 Annual Benchmarking Report: Malpractice Risks in Obstetrics. Boston, Mass: CRICO Strategies; 2010.
- Read Medical Verdicts, Notable Judgments and Settlements
every month
(June 2011)
Errors in clinical judgment were cited in 77% of more than 800 clinically coded obstetric malpractice cases analyzed by CRICO Strategies, a division of CRICO. CRICO is the patient safety and medical malpractice insurance company owned by the Harvard medical community since 1976. The findings of the analysis were published in a 2010 report entitled Malpractice Risks in Obstetrics.1 The cases on which the report is based were asserted from 2005 to 2009.
According to the report, other prevalent areas of causation were:
- miscommunication (36% of cases)
- technical error (26%)
- inadequate documentation (26%)
- administrative failures (23%)
- ineffective supervision (15%).
The report also reveals the top three most common OB risks or allegations:
- delay in treatment of fetal distress
- improper performance of vaginal delivery
- improper management of pregnancy.
In the CRICO analysis, OB malpractice issues were rarely the result of a single act or omission by a single clinician. Rather, cases typically involved a series of missteps and mishandled decisions by a team of physicians and nurses who converged too late to resolve a rapidly devolving crisis.
“Obstetrics has some unique vulnerabilities, most often involving situations in which a sequence of errors or oversights cascade into a crisis that can put mother and baby in jeopardy,” said Robert Hanscom, senior vice president of CRICO Strategies. “Because there is rarely that standout ‘single event,’ it is absolutely paramount that OB practices understand how these missteps unfold, and then focus on education and training initiatives designed specifically to help clinicians avert those mistakes.”
Although the rate of OB claims is relatively infrequent—less than one case for every 1,000 births—the average malpractice payment is approximately $947,000. That figure is more than twice that of other clinical areas, and second only to surgery in total indemnity payments.
We want to hear from you! Tell us what you think.
- Read Medical Verdicts, Notable Judgments and Settlements
every month
(June 2011)
Errors in clinical judgment were cited in 77% of more than 800 clinically coded obstetric malpractice cases analyzed by CRICO Strategies, a division of CRICO. CRICO is the patient safety and medical malpractice insurance company owned by the Harvard medical community since 1976. The findings of the analysis were published in a 2010 report entitled Malpractice Risks in Obstetrics.1 The cases on which the report is based were asserted from 2005 to 2009.
According to the report, other prevalent areas of causation were:
- miscommunication (36% of cases)
- technical error (26%)
- inadequate documentation (26%)
- administrative failures (23%)
- ineffective supervision (15%).
The report also reveals the top three most common OB risks or allegations:
- delay in treatment of fetal distress
- improper performance of vaginal delivery
- improper management of pregnancy.
In the CRICO analysis, OB malpractice issues were rarely the result of a single act or omission by a single clinician. Rather, cases typically involved a series of missteps and mishandled decisions by a team of physicians and nurses who converged too late to resolve a rapidly devolving crisis.
“Obstetrics has some unique vulnerabilities, most often involving situations in which a sequence of errors or oversights cascade into a crisis that can put mother and baby in jeopardy,” said Robert Hanscom, senior vice president of CRICO Strategies. “Because there is rarely that standout ‘single event,’ it is absolutely paramount that OB practices understand how these missteps unfold, and then focus on education and training initiatives designed specifically to help clinicians avert those mistakes.”
Although the rate of OB claims is relatively infrequent—less than one case for every 1,000 births—the average malpractice payment is approximately $947,000. That figure is more than twice that of other clinical areas, and second only to surgery in total indemnity payments.
We want to hear from you! Tell us what you think.
Reference
1. 2010 Annual Benchmarking Report: Malpractice Risks in Obstetrics. Boston, Mass: CRICO Strategies; 2010.
Reference
1. 2010 Annual Benchmarking Report: Malpractice Risks in Obstetrics. Boston, Mass: CRICO Strategies; 2010.
Is the MOC process driving some physicians into early retirement?
Send us an e-mail and let us know your thoughts about the process, at obg@qhc.com
A year has passed since the straightforwardly named organization Change Board Recertification was launched as a call to arms against Maintenance of Certification (MOC), but the debate among physicians over recertification shows no signs of being resolved any time soon. In fact, the summer 2011 issue of The Journal of American Physicians and Surgeons presents not one but two editorials that address the topic—challenging the utility, necessity, and cost of MOC. And a press release from the Association of American Physicians and Surgeons (AAPS) avers that the recertification process is so onerous that it may “drive our most seasoned, experienced physicians into early retirement”—or so AAPS Executive Director Jane M. Orient, MD, is quoted.
In one editorial, Martin Dubravec, MD, minces no words, calling board certification, recertification, and MOC “a malignant growth.”1 And in the other, Lee D. Hieb, MD, president of AAPS, writes that the process of recertification “benefits neither patients nor physicians, and certainly adds nothing to the time-honored practice of medicine.”2
In March 2010, the New England Journal of Medicine ran a clinical vignette and asked readers to vote on whether the internist described in the scenario should enroll in the current MOC program or not.3 Almost two thirds (63%) voted no. And an ongoing poll—admittedly unscientific—at the Change Board Recertification Web site reveals that 92.5% of respondents would abolish the process of recertification altogether.
In addition, a 2009 survey of 100 randomly selected members of AAPS found that only 30% felt that the process of recertification had improved their performance as a physician, and only 22% would voluntarily go through it again.
The American Board of Obstetrics and Gynecology has no figures on how many ObGyns choose not to pursue MOC. One reason for the lack of data: “It’s a 6-year process, and we’re only in year 4,” said MOC administrator Marsha Markham. She did confirm that more than 24,000 physicians are going through the MOC process.
“Right now, our numbers keep increasing each year,” she said.
We want to hear from you! Tell us what you think.
1. Dubravec M. Board certification/recertification/ maintenance of certification: a malignant growth. J Am Physicians Surgeons. 2011;16(2):52-53.
2. Hieb LD. Down the rabbit hole of recertification. J Am Physicians Surgeons. 2011;16(2):36-37.
3. Clinical decisions: American Board of Internal Medicine Maintenance of Certification program. N Engl J Med. 2010;362(10):948-952.
Send us an e-mail and let us know your thoughts about the process, at obg@qhc.com
A year has passed since the straightforwardly named organization Change Board Recertification was launched as a call to arms against Maintenance of Certification (MOC), but the debate among physicians over recertification shows no signs of being resolved any time soon. In fact, the summer 2011 issue of The Journal of American Physicians and Surgeons presents not one but two editorials that address the topic—challenging the utility, necessity, and cost of MOC. And a press release from the Association of American Physicians and Surgeons (AAPS) avers that the recertification process is so onerous that it may “drive our most seasoned, experienced physicians into early retirement”—or so AAPS Executive Director Jane M. Orient, MD, is quoted.
In one editorial, Martin Dubravec, MD, minces no words, calling board certification, recertification, and MOC “a malignant growth.”1 And in the other, Lee D. Hieb, MD, president of AAPS, writes that the process of recertification “benefits neither patients nor physicians, and certainly adds nothing to the time-honored practice of medicine.”2
In March 2010, the New England Journal of Medicine ran a clinical vignette and asked readers to vote on whether the internist described in the scenario should enroll in the current MOC program or not.3 Almost two thirds (63%) voted no. And an ongoing poll—admittedly unscientific—at the Change Board Recertification Web site reveals that 92.5% of respondents would abolish the process of recertification altogether.
In addition, a 2009 survey of 100 randomly selected members of AAPS found that only 30% felt that the process of recertification had improved their performance as a physician, and only 22% would voluntarily go through it again.
The American Board of Obstetrics and Gynecology has no figures on how many ObGyns choose not to pursue MOC. One reason for the lack of data: “It’s a 6-year process, and we’re only in year 4,” said MOC administrator Marsha Markham. She did confirm that more than 24,000 physicians are going through the MOC process.
“Right now, our numbers keep increasing each year,” she said.
We want to hear from you! Tell us what you think.
Send us an e-mail and let us know your thoughts about the process, at obg@qhc.com
A year has passed since the straightforwardly named organization Change Board Recertification was launched as a call to arms against Maintenance of Certification (MOC), but the debate among physicians over recertification shows no signs of being resolved any time soon. In fact, the summer 2011 issue of The Journal of American Physicians and Surgeons presents not one but two editorials that address the topic—challenging the utility, necessity, and cost of MOC. And a press release from the Association of American Physicians and Surgeons (AAPS) avers that the recertification process is so onerous that it may “drive our most seasoned, experienced physicians into early retirement”—or so AAPS Executive Director Jane M. Orient, MD, is quoted.
In one editorial, Martin Dubravec, MD, minces no words, calling board certification, recertification, and MOC “a malignant growth.”1 And in the other, Lee D. Hieb, MD, president of AAPS, writes that the process of recertification “benefits neither patients nor physicians, and certainly adds nothing to the time-honored practice of medicine.”2
In March 2010, the New England Journal of Medicine ran a clinical vignette and asked readers to vote on whether the internist described in the scenario should enroll in the current MOC program or not.3 Almost two thirds (63%) voted no. And an ongoing poll—admittedly unscientific—at the Change Board Recertification Web site reveals that 92.5% of respondents would abolish the process of recertification altogether.
In addition, a 2009 survey of 100 randomly selected members of AAPS found that only 30% felt that the process of recertification had improved their performance as a physician, and only 22% would voluntarily go through it again.
The American Board of Obstetrics and Gynecology has no figures on how many ObGyns choose not to pursue MOC. One reason for the lack of data: “It’s a 6-year process, and we’re only in year 4,” said MOC administrator Marsha Markham. She did confirm that more than 24,000 physicians are going through the MOC process.
“Right now, our numbers keep increasing each year,” she said.
We want to hear from you! Tell us what you think.
1. Dubravec M. Board certification/recertification/ maintenance of certification: a malignant growth. J Am Physicians Surgeons. 2011;16(2):52-53.
2. Hieb LD. Down the rabbit hole of recertification. J Am Physicians Surgeons. 2011;16(2):36-37.
3. Clinical decisions: American Board of Internal Medicine Maintenance of Certification program. N Engl J Med. 2010;362(10):948-952.
1. Dubravec M. Board certification/recertification/ maintenance of certification: a malignant growth. J Am Physicians Surgeons. 2011;16(2):52-53.
2. Hieb LD. Down the rabbit hole of recertification. J Am Physicians Surgeons. 2011;16(2):36-37.
3. Clinical decisions: American Board of Internal Medicine Maintenance of Certification program. N Engl J Med. 2010;362(10):948-952.
In women under 50 years, mammography detects smaller tumors than clinical examination does
- “Just how much does screening mammography reduce mortality from breast cancer?”
Andrew M. Kaunitz, MD (Examining the Evidence; November 2010) - “Access to screening mammography: Priceless”
Robert L. Barbieri, MD (Editorial; January 2010) - “Confused about mammography guidelines? 7 questions answered”
Janelle Yates (January 2010) - “I’ve been rethinking my zeal for breast cancer screening”
Andrew M. Kaunitz, MD (Editorial; December 2009) - “Does the clinical breast exam boost the sensitivity of mammography?”
Jennifer Griffin, MD, and Mark Pearlman, MD (Examining the Evidence; December 2009)
In women younger than 50 years, screening mammography detects smaller tumors that have less nodal involvement than the tumors detected by clinical exam. That’s a principal finding of a study presented this spring at the annual meeting of the American Society of Breast Surgeons in Washington, DC. The study also found that women whose tumors were identified through mammography generally had better outcomes after treatment than women whose tumors were found through a clinical exam, said researcher Paul Dale, MD, chief of surgical oncology at the Ellis Fischel Cancer Center at the University of Missouri School of Medicine in Columbia, Missouri.
The 10-year retrospective study, conducted at the University of Missouri, involved patients treated for breast cancer at the Ellis Fischel Cancer Center between 1998 and 2008. Of these 1,581 women, 20% were 40 to 49 years old. Forty-seven percent of these patients were given a diagnosis on the basis of mammography, and 53% were given a diagnosis on the basis of a clinical exam or other non-mammographic method.
In the group whose diagnosis was based on mammography, the mean tumor diameter was 20 mm; tumors identified by non-mammographic methods, on the other hand, had a mean diameter of 30 mm. This tumor size differential is “highly significant,” said Dr. Dale.
The study also found that lymph-node involvement among women whose diagnosis was based on non-mammographic methods was about twice as common as that of patients whose tumors were found by mammography.
Five-year disease-free survival was estimated to be 94% for the women receiving mammograms; 78%, for those who did not.
“This study found that 20% of women diagnosed with breast cancer in our institution are under age 50, and almost half of their tumors were detected through mammography,” said Dr. Dale. Under the new US Preventive Services Task Force (USPSTF) guidelines, which recommend against routine mammography screening in women under 50, “younger breast cancer patients not undergoing screening and early detection may miss out on important therapy that could significantly impact their survival.”
Many of the studies the USPSTF evaluated before issuing the new guidelines were published before the availability of digital mammography, observed Dr. Dale. He noted that full-field digital mammography has recognized benefits over plain film exams in younger patients who have dense breast tissue that may be difficult to image.
“One concern underlying the new recommendations is that mammography in younger women is less effective and results in too many biopsies that are unnecessary and potentially traumatic for patients,” said Dr. Dale. “Perhaps today’s advancing imaging technologies, as well as less invasive biopsies, will help to eliminate those concerns.”
should start at 40
A new Harris Interactive/HealthDay poll found that women in their 40s want their mammograms, regardless of the latest USPSTF guidelines, which recommend against routine screening mammography in this population. In fact, two thirds of women polled were unaware of the task force’s recommendation.
About 57% of women surveyed believe screening mammography should start at age 40, according to the poll of 1,083 US women 18 years and older. Just 12% thought 50 years was the right age to begin imaging.
When women were apprised of the USPSTF guidelines, 45% of them said the task force had pushed back the recommended age to begin screening to reduce health-care costs and avoid administering unnecessary tests. Thirty percent believed the task force made the recommendation because excessive screening produces too many so-called false-positive results, leading women to think they had cancer when they did not.
New recommendations notwithstanding, many women in their 40s are still getting mammograms—77% of women in their 40s have already had at least one mammogram, and 64% report annual screening, the poll found.
The American Cancer Society continues to recommend annual mammograms for women starting at 40 years.
We want to hear from you! Tell us what you think.
- “Just how much does screening mammography reduce mortality from breast cancer?”
Andrew M. Kaunitz, MD (Examining the Evidence; November 2010) - “Access to screening mammography: Priceless”
Robert L. Barbieri, MD (Editorial; January 2010) - “Confused about mammography guidelines? 7 questions answered”
Janelle Yates (January 2010) - “I’ve been rethinking my zeal for breast cancer screening”
Andrew M. Kaunitz, MD (Editorial; December 2009) - “Does the clinical breast exam boost the sensitivity of mammography?”
Jennifer Griffin, MD, and Mark Pearlman, MD (Examining the Evidence; December 2009)
In women younger than 50 years, screening mammography detects smaller tumors that have less nodal involvement than the tumors detected by clinical exam. That’s a principal finding of a study presented this spring at the annual meeting of the American Society of Breast Surgeons in Washington, DC. The study also found that women whose tumors were identified through mammography generally had better outcomes after treatment than women whose tumors were found through a clinical exam, said researcher Paul Dale, MD, chief of surgical oncology at the Ellis Fischel Cancer Center at the University of Missouri School of Medicine in Columbia, Missouri.
The 10-year retrospective study, conducted at the University of Missouri, involved patients treated for breast cancer at the Ellis Fischel Cancer Center between 1998 and 2008. Of these 1,581 women, 20% were 40 to 49 years old. Forty-seven percent of these patients were given a diagnosis on the basis of mammography, and 53% were given a diagnosis on the basis of a clinical exam or other non-mammographic method.
In the group whose diagnosis was based on mammography, the mean tumor diameter was 20 mm; tumors identified by non-mammographic methods, on the other hand, had a mean diameter of 30 mm. This tumor size differential is “highly significant,” said Dr. Dale.
The study also found that lymph-node involvement among women whose diagnosis was based on non-mammographic methods was about twice as common as that of patients whose tumors were found by mammography.
Five-year disease-free survival was estimated to be 94% for the women receiving mammograms; 78%, for those who did not.
“This study found that 20% of women diagnosed with breast cancer in our institution are under age 50, and almost half of their tumors were detected through mammography,” said Dr. Dale. Under the new US Preventive Services Task Force (USPSTF) guidelines, which recommend against routine mammography screening in women under 50, “younger breast cancer patients not undergoing screening and early detection may miss out on important therapy that could significantly impact their survival.”
Many of the studies the USPSTF evaluated before issuing the new guidelines were published before the availability of digital mammography, observed Dr. Dale. He noted that full-field digital mammography has recognized benefits over plain film exams in younger patients who have dense breast tissue that may be difficult to image.
“One concern underlying the new recommendations is that mammography in younger women is less effective and results in too many biopsies that are unnecessary and potentially traumatic for patients,” said Dr. Dale. “Perhaps today’s advancing imaging technologies, as well as less invasive biopsies, will help to eliminate those concerns.”
should start at 40
A new Harris Interactive/HealthDay poll found that women in their 40s want their mammograms, regardless of the latest USPSTF guidelines, which recommend against routine screening mammography in this population. In fact, two thirds of women polled were unaware of the task force’s recommendation.
About 57% of women surveyed believe screening mammography should start at age 40, according to the poll of 1,083 US women 18 years and older. Just 12% thought 50 years was the right age to begin imaging.
When women were apprised of the USPSTF guidelines, 45% of them said the task force had pushed back the recommended age to begin screening to reduce health-care costs and avoid administering unnecessary tests. Thirty percent believed the task force made the recommendation because excessive screening produces too many so-called false-positive results, leading women to think they had cancer when they did not.
New recommendations notwithstanding, many women in their 40s are still getting mammograms—77% of women in their 40s have already had at least one mammogram, and 64% report annual screening, the poll found.
The American Cancer Society continues to recommend annual mammograms for women starting at 40 years.
We want to hear from you! Tell us what you think.
- “Just how much does screening mammography reduce mortality from breast cancer?”
Andrew M. Kaunitz, MD (Examining the Evidence; November 2010) - “Access to screening mammography: Priceless”
Robert L. Barbieri, MD (Editorial; January 2010) - “Confused about mammography guidelines? 7 questions answered”
Janelle Yates (January 2010) - “I’ve been rethinking my zeal for breast cancer screening”
Andrew M. Kaunitz, MD (Editorial; December 2009) - “Does the clinical breast exam boost the sensitivity of mammography?”
Jennifer Griffin, MD, and Mark Pearlman, MD (Examining the Evidence; December 2009)
In women younger than 50 years, screening mammography detects smaller tumors that have less nodal involvement than the tumors detected by clinical exam. That’s a principal finding of a study presented this spring at the annual meeting of the American Society of Breast Surgeons in Washington, DC. The study also found that women whose tumors were identified through mammography generally had better outcomes after treatment than women whose tumors were found through a clinical exam, said researcher Paul Dale, MD, chief of surgical oncology at the Ellis Fischel Cancer Center at the University of Missouri School of Medicine in Columbia, Missouri.
The 10-year retrospective study, conducted at the University of Missouri, involved patients treated for breast cancer at the Ellis Fischel Cancer Center between 1998 and 2008. Of these 1,581 women, 20% were 40 to 49 years old. Forty-seven percent of these patients were given a diagnosis on the basis of mammography, and 53% were given a diagnosis on the basis of a clinical exam or other non-mammographic method.
In the group whose diagnosis was based on mammography, the mean tumor diameter was 20 mm; tumors identified by non-mammographic methods, on the other hand, had a mean diameter of 30 mm. This tumor size differential is “highly significant,” said Dr. Dale.
The study also found that lymph-node involvement among women whose diagnosis was based on non-mammographic methods was about twice as common as that of patients whose tumors were found by mammography.
Five-year disease-free survival was estimated to be 94% for the women receiving mammograms; 78%, for those who did not.
“This study found that 20% of women diagnosed with breast cancer in our institution are under age 50, and almost half of their tumors were detected through mammography,” said Dr. Dale. Under the new US Preventive Services Task Force (USPSTF) guidelines, which recommend against routine mammography screening in women under 50, “younger breast cancer patients not undergoing screening and early detection may miss out on important therapy that could significantly impact their survival.”
Many of the studies the USPSTF evaluated before issuing the new guidelines were published before the availability of digital mammography, observed Dr. Dale. He noted that full-field digital mammography has recognized benefits over plain film exams in younger patients who have dense breast tissue that may be difficult to image.
“One concern underlying the new recommendations is that mammography in younger women is less effective and results in too many biopsies that are unnecessary and potentially traumatic for patients,” said Dr. Dale. “Perhaps today’s advancing imaging technologies, as well as less invasive biopsies, will help to eliminate those concerns.”
should start at 40
A new Harris Interactive/HealthDay poll found that women in their 40s want their mammograms, regardless of the latest USPSTF guidelines, which recommend against routine screening mammography in this population. In fact, two thirds of women polled were unaware of the task force’s recommendation.
About 57% of women surveyed believe screening mammography should start at age 40, according to the poll of 1,083 US women 18 years and older. Just 12% thought 50 years was the right age to begin imaging.
When women were apprised of the USPSTF guidelines, 45% of them said the task force had pushed back the recommended age to begin screening to reduce health-care costs and avoid administering unnecessary tests. Thirty percent believed the task force made the recommendation because excessive screening produces too many so-called false-positive results, leading women to think they had cancer when they did not.
New recommendations notwithstanding, many women in their 40s are still getting mammograms—77% of women in their 40s have already had at least one mammogram, and 64% report annual screening, the poll found.
The American Cancer Society continues to recommend annual mammograms for women starting at 40 years.
We want to hear from you! Tell us what you think.
New group B strep guidelines clarify management of key groups
- Neonatal death from group B strep
(Medical Verdicts, March 2011)
Before widespread intrapartum prophylaxis against group B Streptococcus (GBS) was initiated in the late 1990s, roughly 7,500 newborns developed invasive GBS disease every year in the United States, and the case-fatality rate reached an astonishing—and disheartening—50%.1 Now that all pregnant women undergo culture-based screening at 35 to 37 weeks’ gestation, the incidence of early-onset neonatal GBS disease has declined precipitously.
According to a report issued late last year by the Centers for Disease Control and Prevention (CDC), GBS now causes roughly 1,200 cases of early-onset invasive disease every year, approximately 70% of them among infants born at or after 37 weeks’ gestation, and the case-fatality rate is 4% to 6%.2 Mortality is higher among preterm infants, with a case-fatality rate of 20% to 30% for infants born at or before 33 weeks’ gestation, compared with 2% to 3% for full-term infants.2
Despite progress, GBS remains the leading cause of early-onset neonatal sepsis in the United States. In November 2010, to spur further improvement, the CDC updated its guidelines on prevention of perinatal GBS, and ACOG and other professional organizations endorsed the new recommendations. This article highlights changes to the guidelines—the first since 2002—in four critical areas:
- clarification of who should receive GBS prophylaxis, and when
- updated algorithms for screening and intrapartum prophylaxis for women who experience preterm labor or pre-term premature rupture of membranes (pPROM)
- new recommended dosage of penicillin G for prophylaxis
- updated regimens for prophylaxis among women who are allergic to penicillin.2
When is intrapartum antibiotic prophylaxis indicated? When is it not?
| Indicated | Not indicated |
|---|---|
Previous infant with invasive GBS disease GBS bacteriuria during any trimester of the current pregnancy* Positive GBS vaginal-rectal screening culture in late gestation† during current pregnancy* Unknown GBS status at the onset of labor (culture not done, incomplete, or results unknown) and any of the following:
| Colonization with GBS during a previous pregnancy (unless an indication for GBS prophylaxis is present for current pregnancy) GBS bacteriuria during previous pregnancy (unless an indication for GBS prophylaxis is present for current pregnancy) Negative vaginal and rectal GBS screening culture in late gestation† during the current pregnancy, regardless of intrapartum risk factors Cesarean delivery performed before onset of labor on a woman who has intact amniotic membranes, regardless of GBS colonization status or gestational age |
| SOURCE: CDC2 * Intrapartum antibiotic prophylaxis is not indicated in this circumstance if a cesarean delivery is performed before onset of labor on a woman who has intact amniotic membranes. † Optimal timing for prenatal GBS screening is at 35–37 weeks’ gestation. § Recommendations for the use of intrapartum antibiotics for prevention of early-onset GBS disease in the setting of threatened preterm delivery are presented in FIGURES 1 and 2. ¶ If amnionitis is suspected, broad-spectrum antibiotic therapy that includes an agent known to be active against GBS should replace GBS prophylaxis. ** NAAT testing for GBS is optional and might not be available in all settings. If intrapartum NAAT is negative for GBS but any other intrapartum risk factor (delivery at <37 weeks’ gestation, amniotic membrane rupture at ≥18 hours, or temperature ≥100.4°F [≥38.0°C]) is present, then intrapartum antibiotic prophylaxis is indicated. | |
Who should receive prophylaxis?
In its report, the CDC reiterated the indications and “nonindications” for intrapartum prophylaxis (TABLE). Among the clarifications:
- Women who have GBS isolated from the urine at any time during pregnancy should undergo intrapartum prophylaxis. They do not need third-trimester screening for GBS.
- Women who had a previous infant with invasive GBS disease should also undergo intrapartum prophylaxis, with no need for third-trimester screening
- All other pregnant women should undergo screening at 35 to 37 weeks’ gestation. If results are positive, intrapartum prophylaxis is indicated.
FIGURE 1 Recommended management when a patient experiences preterm labor*
SOURCE: CDC2
*At <37 weeks and 0 days’ gestation.
† If patient has undergone vaginal-rectal GBS culture within the preceding 5 weeks, the results of that culture should guide management. GBS-colonized women should receive intrapartum antibiotic prophylaxis. No antibiotics are indicated for GBS prophylaxis if a vaginal-rectal screen within 5 weeks was negative.
§ Patient should be regularly assessed for progression to true labor; if the patient is considered not to be in true labor, discontinue GBS prophylaxis.
¶ If GBS culture results become available prior to delivery and are negative, discontinue GBS prophylaxis.
** Unless subsequent GBS culture prior to delivery is positive.
†† A negative GBS screen is considered valid for 5 weeks. If a patient with a history of preterm labor is readmitted with signs and symptoms of preterm labor and had a negative GBS screen >5 weeks earlier, she should be rescreened and managed according to this algorithm at that time.
CDC now offers distinct algorithms for preterm labor and pPROM
To clarify the management of two distinct groups of women, the CDC developed separate algorithms for GBS prophylaxis in the setting of threatened preterm delivery—one for spontaneous preterm labor (FIGURE 1) and another for pPROM (FIGURE 2). In addition, it now recommends:
- When GBS prophylaxis is given to a woman who has signs and symptoms of preterm labor, it should be discontinued if it is later determined that she is not in true labor
- If antibiotics given to prolong latency for pPROM include adequate coverage for GBS (i.e., 2 g intravenous [IV] ampicillin followed by 1 g IV ampicillin every 6 hours for 48 hours), no additional prophylaxis for GBS is necessary, provided delivery occurs during administration of that antibiotic regimen. Oral antibiotics alone are not adequate for GBS prophylaxis.
- When a woman who has pPROM is not in labor and is receiving antibiotics with adequate GBS coverage to prolong latency, she should be managed according to the standard of care for pPROM. GBS testing results should not affect the duration of antibiotics.
- When a woman who has pPROM is not in labor and is not receiving antibiotics to prolong latency (or is receiving antibiotics that do not have adequate GBS coverage), she should undergo GBS prophylaxis for 48 hours unless a GBS screen performed within 5 weeks was negative.
FIGURE 2 GBS screening and prophylaxis for preterm premature rupture of membranes (pPROM)*
SOURCE: CDC2
* At <37 weeks and 0 days’ gestation.
† If patient has undergone vaginal-rectal GBS culture within the preceding 5 weeks, the results of that culture should guide management. GBS-colonized women should receive intrapartum antibiotic prophylaxis. No antibiotics are indicated for GBS prophylaxis if a vaginal-rectal screen within 5 weeks was negative.
§ Antibiotics given for latency in the setting of pPROM that include ampicillin 2 g IV once, followed by 1 g IV every 6 hours for at least 48 hours are adequate for GBS prophylaxis. If other regimens are used, GBS prophylaxis should be initiated in addition.
¶ GBS prophylaxis should be discontinued at 48 hours for women with pPROM who are not in labor. If results from a GBS screen performed at admission become available during the 48-hour period and are negative, GBS prophylaxis should be discontinued at that time.
** Unless subsequent GBS culture prior to delivery is positive.
†† A negative GBS screen is considered valid for 5 weeks. If a patient with pPROM is entering labor and had a negative GBS screen >5 weeks earlier, she should be rescreened and managed according to this algorithm at that time.
New dosage allows room for flexibility
The CDC now recommends a dosage of 5 million units of IV penicillin G for GBS prophylaxis, followed by 2.5 to 3.0 million units IV every 4 hours. The range of 2.5 to 3.0 million units is recommended to ensure that the drug reaches an adequate concentration in the fetal circulation and amniotic fluid without being neurotoxic. The choice of dosage within that range should be guided by which formulations of penicillin G are readily available, says the CDC.
Penicillin remains the agent of choice for intrapartum prophylaxis, but ampicillin is an acceptable alternative.
If a woman is allergic to penicillin but has no history of anaphylaxis, angioedema, respiratory distress, or urticaria following administration of a penicillin or cephalosporin, she should be given 2 g IV cefazolin, followed by 1 g IV cefazolin every 8 hours until delivery. If she does have a history of anaphylaxis or is at high risk for anaphylaxis, ask the laboratory for antimicrobial susceptibility testing on the antenatal GBS culture. If the isolate is susceptible to clindamycin, give her 900 mg IV clindamycin every 8 hours until delivery. If it is not susceptible to clindamycin, give her 1 g IV vancomycin every 12 hours until the time of delivery.
The CDC no longer considers erythromycin to be an acceptable alternative for intrapartum GBS prophylaxis for penicillin-allergic women at high risk of anaphylaxis.
Where we go from here
Although early-onset GBS disease has become relatively uncommon, the rate of maternal GBS colonization remains unchanged since the 1970s. Therefore, it is important to continue efforts to sustain and improve on the progress that has been made. There is also a need to monitor for potential adverse consequences of intrapartum antibiotic prophylaxis, such as emergence of bacterial antimicrobial resistance or an increased incidence or severity of nonGBS neonatal pathogens, the CDC observes. “In the absence of a licensed GBS vaccine, universal screening and intrapartum antibiotic prophylaxis continue to be the cornerstones of early-onset GBS disease prevention."
We want to hear from you! Tell us what you think.
1. Baker CJ, Barrett FF. Group B streptococcal infections in infants. The importance of the various serotypes. JAMA. 1974;230(8):1158-1160.
2. Verani JR, McGee L, Schrag SJ. Centers for Disease Control and Prevention. Prevention of Perinatal Group B Streptococcal Disease: Revised Guidelines from CDC, 2010. MMWR. 2010;59(RR-10):1-36.
- Neonatal death from group B strep
(Medical Verdicts, March 2011)
Before widespread intrapartum prophylaxis against group B Streptococcus (GBS) was initiated in the late 1990s, roughly 7,500 newborns developed invasive GBS disease every year in the United States, and the case-fatality rate reached an astonishing—and disheartening—50%.1 Now that all pregnant women undergo culture-based screening at 35 to 37 weeks’ gestation, the incidence of early-onset neonatal GBS disease has declined precipitously.
According to a report issued late last year by the Centers for Disease Control and Prevention (CDC), GBS now causes roughly 1,200 cases of early-onset invasive disease every year, approximately 70% of them among infants born at or after 37 weeks’ gestation, and the case-fatality rate is 4% to 6%.2 Mortality is higher among preterm infants, with a case-fatality rate of 20% to 30% for infants born at or before 33 weeks’ gestation, compared with 2% to 3% for full-term infants.2
Despite progress, GBS remains the leading cause of early-onset neonatal sepsis in the United States. In November 2010, to spur further improvement, the CDC updated its guidelines on prevention of perinatal GBS, and ACOG and other professional organizations endorsed the new recommendations. This article highlights changes to the guidelines—the first since 2002—in four critical areas:
- clarification of who should receive GBS prophylaxis, and when
- updated algorithms for screening and intrapartum prophylaxis for women who experience preterm labor or pre-term premature rupture of membranes (pPROM)
- new recommended dosage of penicillin G for prophylaxis
- updated regimens for prophylaxis among women who are allergic to penicillin.2
When is intrapartum antibiotic prophylaxis indicated? When is it not?
| Indicated | Not indicated |
|---|---|
Previous infant with invasive GBS disease GBS bacteriuria during any trimester of the current pregnancy* Positive GBS vaginal-rectal screening culture in late gestation† during current pregnancy* Unknown GBS status at the onset of labor (culture not done, incomplete, or results unknown) and any of the following:
| Colonization with GBS during a previous pregnancy (unless an indication for GBS prophylaxis is present for current pregnancy) GBS bacteriuria during previous pregnancy (unless an indication for GBS prophylaxis is present for current pregnancy) Negative vaginal and rectal GBS screening culture in late gestation† during the current pregnancy, regardless of intrapartum risk factors Cesarean delivery performed before onset of labor on a woman who has intact amniotic membranes, regardless of GBS colonization status or gestational age |
| SOURCE: CDC2 * Intrapartum antibiotic prophylaxis is not indicated in this circumstance if a cesarean delivery is performed before onset of labor on a woman who has intact amniotic membranes. † Optimal timing for prenatal GBS screening is at 35–37 weeks’ gestation. § Recommendations for the use of intrapartum antibiotics for prevention of early-onset GBS disease in the setting of threatened preterm delivery are presented in FIGURES 1 and 2. ¶ If amnionitis is suspected, broad-spectrum antibiotic therapy that includes an agent known to be active against GBS should replace GBS prophylaxis. ** NAAT testing for GBS is optional and might not be available in all settings. If intrapartum NAAT is negative for GBS but any other intrapartum risk factor (delivery at <37 weeks’ gestation, amniotic membrane rupture at ≥18 hours, or temperature ≥100.4°F [≥38.0°C]) is present, then intrapartum antibiotic prophylaxis is indicated. | |
Who should receive prophylaxis?
In its report, the CDC reiterated the indications and “nonindications” for intrapartum prophylaxis (TABLE). Among the clarifications:
- Women who have GBS isolated from the urine at any time during pregnancy should undergo intrapartum prophylaxis. They do not need third-trimester screening for GBS.
- Women who had a previous infant with invasive GBS disease should also undergo intrapartum prophylaxis, with no need for third-trimester screening
- All other pregnant women should undergo screening at 35 to 37 weeks’ gestation. If results are positive, intrapartum prophylaxis is indicated.

FIGURE 1 Recommended management when a patient experiences preterm labor*
SOURCE: CDC2
*At <37 weeks and 0 days’ gestation.
† If patient has undergone vaginal-rectal GBS culture within the preceding 5 weeks, the results of that culture should guide management. GBS-colonized women should receive intrapartum antibiotic prophylaxis. No antibiotics are indicated for GBS prophylaxis if a vaginal-rectal screen within 5 weeks was negative.
§ Patient should be regularly assessed for progression to true labor; if the patient is considered not to be in true labor, discontinue GBS prophylaxis.
¶ If GBS culture results become available prior to delivery and are negative, discontinue GBS prophylaxis.
** Unless subsequent GBS culture prior to delivery is positive.
†† A negative GBS screen is considered valid for 5 weeks. If a patient with a history of preterm labor is readmitted with signs and symptoms of preterm labor and had a negative GBS screen >5 weeks earlier, she should be rescreened and managed according to this algorithm at that time.
CDC now offers distinct algorithms for preterm labor and pPROM
To clarify the management of two distinct groups of women, the CDC developed separate algorithms for GBS prophylaxis in the setting of threatened preterm delivery—one for spontaneous preterm labor (FIGURE 1) and another for pPROM (FIGURE 2). In addition, it now recommends:
- When GBS prophylaxis is given to a woman who has signs and symptoms of preterm labor, it should be discontinued if it is later determined that she is not in true labor
- If antibiotics given to prolong latency for pPROM include adequate coverage for GBS (i.e., 2 g intravenous [IV] ampicillin followed by 1 g IV ampicillin every 6 hours for 48 hours), no additional prophylaxis for GBS is necessary, provided delivery occurs during administration of that antibiotic regimen. Oral antibiotics alone are not adequate for GBS prophylaxis.
- When a woman who has pPROM is not in labor and is receiving antibiotics with adequate GBS coverage to prolong latency, she should be managed according to the standard of care for pPROM. GBS testing results should not affect the duration of antibiotics.
- When a woman who has pPROM is not in labor and is not receiving antibiotics to prolong latency (or is receiving antibiotics that do not have adequate GBS coverage), she should undergo GBS prophylaxis for 48 hours unless a GBS screen performed within 5 weeks was negative.

FIGURE 2 GBS screening and prophylaxis for preterm premature rupture of membranes (pPROM)*
SOURCE: CDC2
* At <37 weeks and 0 days’ gestation.
† If patient has undergone vaginal-rectal GBS culture within the preceding 5 weeks, the results of that culture should guide management. GBS-colonized women should receive intrapartum antibiotic prophylaxis. No antibiotics are indicated for GBS prophylaxis if a vaginal-rectal screen within 5 weeks was negative.
§ Antibiotics given for latency in the setting of pPROM that include ampicillin 2 g IV once, followed by 1 g IV every 6 hours for at least 48 hours are adequate for GBS prophylaxis. If other regimens are used, GBS prophylaxis should be initiated in addition.
¶ GBS prophylaxis should be discontinued at 48 hours for women with pPROM who are not in labor. If results from a GBS screen performed at admission become available during the 48-hour period and are negative, GBS prophylaxis should be discontinued at that time.
** Unless subsequent GBS culture prior to delivery is positive.
†† A negative GBS screen is considered valid for 5 weeks. If a patient with pPROM is entering labor and had a negative GBS screen >5 weeks earlier, she should be rescreened and managed according to this algorithm at that time.
New dosage allows room for flexibility
The CDC now recommends a dosage of 5 million units of IV penicillin G for GBS prophylaxis, followed by 2.5 to 3.0 million units IV every 4 hours. The range of 2.5 to 3.0 million units is recommended to ensure that the drug reaches an adequate concentration in the fetal circulation and amniotic fluid without being neurotoxic. The choice of dosage within that range should be guided by which formulations of penicillin G are readily available, says the CDC.
Penicillin remains the agent of choice for intrapartum prophylaxis, but ampicillin is an acceptable alternative.
If a woman is allergic to penicillin but has no history of anaphylaxis, angioedema, respiratory distress, or urticaria following administration of a penicillin or cephalosporin, she should be given 2 g IV cefazolin, followed by 1 g IV cefazolin every 8 hours until delivery. If she does have a history of anaphylaxis or is at high risk for anaphylaxis, ask the laboratory for antimicrobial susceptibility testing on the antenatal GBS culture. If the isolate is susceptible to clindamycin, give her 900 mg IV clindamycin every 8 hours until delivery. If it is not susceptible to clindamycin, give her 1 g IV vancomycin every 12 hours until the time of delivery.
The CDC no longer considers erythromycin to be an acceptable alternative for intrapartum GBS prophylaxis for penicillin-allergic women at high risk of anaphylaxis.
Where we go from here
Although early-onset GBS disease has become relatively uncommon, the rate of maternal GBS colonization remains unchanged since the 1970s. Therefore, it is important to continue efforts to sustain and improve on the progress that has been made. There is also a need to monitor for potential adverse consequences of intrapartum antibiotic prophylaxis, such as emergence of bacterial antimicrobial resistance or an increased incidence or severity of nonGBS neonatal pathogens, the CDC observes. “In the absence of a licensed GBS vaccine, universal screening and intrapartum antibiotic prophylaxis continue to be the cornerstones of early-onset GBS disease prevention."
We want to hear from you! Tell us what you think.
- Neonatal death from group B strep
(Medical Verdicts, March 2011)
Before widespread intrapartum prophylaxis against group B Streptococcus (GBS) was initiated in the late 1990s, roughly 7,500 newborns developed invasive GBS disease every year in the United States, and the case-fatality rate reached an astonishing—and disheartening—50%.1 Now that all pregnant women undergo culture-based screening at 35 to 37 weeks’ gestation, the incidence of early-onset neonatal GBS disease has declined precipitously.
According to a report issued late last year by the Centers for Disease Control and Prevention (CDC), GBS now causes roughly 1,200 cases of early-onset invasive disease every year, approximately 70% of them among infants born at or after 37 weeks’ gestation, and the case-fatality rate is 4% to 6%.2 Mortality is higher among preterm infants, with a case-fatality rate of 20% to 30% for infants born at or before 33 weeks’ gestation, compared with 2% to 3% for full-term infants.2
Despite progress, GBS remains the leading cause of early-onset neonatal sepsis in the United States. In November 2010, to spur further improvement, the CDC updated its guidelines on prevention of perinatal GBS, and ACOG and other professional organizations endorsed the new recommendations. This article highlights changes to the guidelines—the first since 2002—in four critical areas:
- clarification of who should receive GBS prophylaxis, and when
- updated algorithms for screening and intrapartum prophylaxis for women who experience preterm labor or pre-term premature rupture of membranes (pPROM)
- new recommended dosage of penicillin G for prophylaxis
- updated regimens for prophylaxis among women who are allergic to penicillin.2
When is intrapartum antibiotic prophylaxis indicated? When is it not?
| Indicated | Not indicated |
|---|---|
Previous infant with invasive GBS disease GBS bacteriuria during any trimester of the current pregnancy* Positive GBS vaginal-rectal screening culture in late gestation† during current pregnancy* Unknown GBS status at the onset of labor (culture not done, incomplete, or results unknown) and any of the following:
| Colonization with GBS during a previous pregnancy (unless an indication for GBS prophylaxis is present for current pregnancy) GBS bacteriuria during previous pregnancy (unless an indication for GBS prophylaxis is present for current pregnancy) Negative vaginal and rectal GBS screening culture in late gestation† during the current pregnancy, regardless of intrapartum risk factors Cesarean delivery performed before onset of labor on a woman who has intact amniotic membranes, regardless of GBS colonization status or gestational age |
| SOURCE: CDC2 * Intrapartum antibiotic prophylaxis is not indicated in this circumstance if a cesarean delivery is performed before onset of labor on a woman who has intact amniotic membranes. † Optimal timing for prenatal GBS screening is at 35–37 weeks’ gestation. § Recommendations for the use of intrapartum antibiotics for prevention of early-onset GBS disease in the setting of threatened preterm delivery are presented in FIGURES 1 and 2. ¶ If amnionitis is suspected, broad-spectrum antibiotic therapy that includes an agent known to be active against GBS should replace GBS prophylaxis. ** NAAT testing for GBS is optional and might not be available in all settings. If intrapartum NAAT is negative for GBS but any other intrapartum risk factor (delivery at <37 weeks’ gestation, amniotic membrane rupture at ≥18 hours, or temperature ≥100.4°F [≥38.0°C]) is present, then intrapartum antibiotic prophylaxis is indicated. | |
Who should receive prophylaxis?
In its report, the CDC reiterated the indications and “nonindications” for intrapartum prophylaxis (TABLE). Among the clarifications:
- Women who have GBS isolated from the urine at any time during pregnancy should undergo intrapartum prophylaxis. They do not need third-trimester screening for GBS.
- Women who had a previous infant with invasive GBS disease should also undergo intrapartum prophylaxis, with no need for third-trimester screening
- All other pregnant women should undergo screening at 35 to 37 weeks’ gestation. If results are positive, intrapartum prophylaxis is indicated.

FIGURE 1 Recommended management when a patient experiences preterm labor*
SOURCE: CDC2
*At <37 weeks and 0 days’ gestation.
† If patient has undergone vaginal-rectal GBS culture within the preceding 5 weeks, the results of that culture should guide management. GBS-colonized women should receive intrapartum antibiotic prophylaxis. No antibiotics are indicated for GBS prophylaxis if a vaginal-rectal screen within 5 weeks was negative.
§ Patient should be regularly assessed for progression to true labor; if the patient is considered not to be in true labor, discontinue GBS prophylaxis.
¶ If GBS culture results become available prior to delivery and are negative, discontinue GBS prophylaxis.
** Unless subsequent GBS culture prior to delivery is positive.
†† A negative GBS screen is considered valid for 5 weeks. If a patient with a history of preterm labor is readmitted with signs and symptoms of preterm labor and had a negative GBS screen >5 weeks earlier, she should be rescreened and managed according to this algorithm at that time.
CDC now offers distinct algorithms for preterm labor and pPROM
To clarify the management of two distinct groups of women, the CDC developed separate algorithms for GBS prophylaxis in the setting of threatened preterm delivery—one for spontaneous preterm labor (FIGURE 1) and another for pPROM (FIGURE 2). In addition, it now recommends:
- When GBS prophylaxis is given to a woman who has signs and symptoms of preterm labor, it should be discontinued if it is later determined that she is not in true labor
- If antibiotics given to prolong latency for pPROM include adequate coverage for GBS (i.e., 2 g intravenous [IV] ampicillin followed by 1 g IV ampicillin every 6 hours for 48 hours), no additional prophylaxis for GBS is necessary, provided delivery occurs during administration of that antibiotic regimen. Oral antibiotics alone are not adequate for GBS prophylaxis.
- When a woman who has pPROM is not in labor and is receiving antibiotics with adequate GBS coverage to prolong latency, she should be managed according to the standard of care for pPROM. GBS testing results should not affect the duration of antibiotics.
- When a woman who has pPROM is not in labor and is not receiving antibiotics to prolong latency (or is receiving antibiotics that do not have adequate GBS coverage), she should undergo GBS prophylaxis for 48 hours unless a GBS screen performed within 5 weeks was negative.

FIGURE 2 GBS screening and prophylaxis for preterm premature rupture of membranes (pPROM)*
SOURCE: CDC2
* At <37 weeks and 0 days’ gestation.
† If patient has undergone vaginal-rectal GBS culture within the preceding 5 weeks, the results of that culture should guide management. GBS-colonized women should receive intrapartum antibiotic prophylaxis. No antibiotics are indicated for GBS prophylaxis if a vaginal-rectal screen within 5 weeks was negative.
§ Antibiotics given for latency in the setting of pPROM that include ampicillin 2 g IV once, followed by 1 g IV every 6 hours for at least 48 hours are adequate for GBS prophylaxis. If other regimens are used, GBS prophylaxis should be initiated in addition.
¶ GBS prophylaxis should be discontinued at 48 hours for women with pPROM who are not in labor. If results from a GBS screen performed at admission become available during the 48-hour period and are negative, GBS prophylaxis should be discontinued at that time.
** Unless subsequent GBS culture prior to delivery is positive.
†† A negative GBS screen is considered valid for 5 weeks. If a patient with pPROM is entering labor and had a negative GBS screen >5 weeks earlier, she should be rescreened and managed according to this algorithm at that time.
New dosage allows room for flexibility
The CDC now recommends a dosage of 5 million units of IV penicillin G for GBS prophylaxis, followed by 2.5 to 3.0 million units IV every 4 hours. The range of 2.5 to 3.0 million units is recommended to ensure that the drug reaches an adequate concentration in the fetal circulation and amniotic fluid without being neurotoxic. The choice of dosage within that range should be guided by which formulations of penicillin G are readily available, says the CDC.
Penicillin remains the agent of choice for intrapartum prophylaxis, but ampicillin is an acceptable alternative.
If a woman is allergic to penicillin but has no history of anaphylaxis, angioedema, respiratory distress, or urticaria following administration of a penicillin or cephalosporin, she should be given 2 g IV cefazolin, followed by 1 g IV cefazolin every 8 hours until delivery. If she does have a history of anaphylaxis or is at high risk for anaphylaxis, ask the laboratory for antimicrobial susceptibility testing on the antenatal GBS culture. If the isolate is susceptible to clindamycin, give her 900 mg IV clindamycin every 8 hours until delivery. If it is not susceptible to clindamycin, give her 1 g IV vancomycin every 12 hours until the time of delivery.
The CDC no longer considers erythromycin to be an acceptable alternative for intrapartum GBS prophylaxis for penicillin-allergic women at high risk of anaphylaxis.
Where we go from here
Although early-onset GBS disease has become relatively uncommon, the rate of maternal GBS colonization remains unchanged since the 1970s. Therefore, it is important to continue efforts to sustain and improve on the progress that has been made. There is also a need to monitor for potential adverse consequences of intrapartum antibiotic prophylaxis, such as emergence of bacterial antimicrobial resistance or an increased incidence or severity of nonGBS neonatal pathogens, the CDC observes. “In the absence of a licensed GBS vaccine, universal screening and intrapartum antibiotic prophylaxis continue to be the cornerstones of early-onset GBS disease prevention."
We want to hear from you! Tell us what you think.
1. Baker CJ, Barrett FF. Group B streptococcal infections in infants. The importance of the various serotypes. JAMA. 1974;230(8):1158-1160.
2. Verani JR, McGee L, Schrag SJ. Centers for Disease Control and Prevention. Prevention of Perinatal Group B Streptococcal Disease: Revised Guidelines from CDC, 2010. MMWR. 2010;59(RR-10):1-36.
1. Baker CJ, Barrett FF. Group B streptococcal infections in infants. The importance of the various serotypes. JAMA. 1974;230(8):1158-1160.
2. Verani JR, McGee L, Schrag SJ. Centers for Disease Control and Prevention. Prevention of Perinatal Group B Streptococcal Disease: Revised Guidelines from CDC, 2010. MMWR. 2010;59(RR-10):1-36.
Quadrivalent HPV vaccine now FDA-approved to prevent anal cancer
2 HPV vaccines, 7 questions that you need answered
Neal M. Lonky, MD, MPH, and an expert panel
The FDA recently approved the quadrivalent formulation of the human papillomavirus (HPV) vaccine (Merck’s Gardasil) for prevention of anal cancer and associated precancerous lesions caused by HPV types 6, 11, 16, and 18 in people 9 to 26 years old.
The quadrivalent HPV vaccine is already approved for the same age population for the prevention of cervical, vulvar, and vaginal cancer and the associated precancerous lesions caused by HPV types 6, 11, 16, and 18 in females. In addition, it is approved for the prevention of genital warts caused by types 6 and 11 in both males and females.
The indication for anal cancer prevention does not extend to the other FDA-approved HPV vaccine (GlaxoSmithKline’s bivalent [types 16 and 18] formulation, Cervarix).
“Treatment for anal cancer is challenging; the use of Gardasil as a method of prevention is important, as it may result in fewer diagnoses and the subsequent surgery, radiation or chemotherapy that individuals need to endure,” said Karen Midthun, MD, director of the FDA’s Center for Biologics Evaluation and Research.
Anal cancer is uncommon in the general population, but incidence is increasing. HPV is associated with approximately 90% of anal cancer cases. The American Cancer Society estimates that approximately 5,300 people are given a diagnosis of anal cancer each year in the United States—more often women than men.
Gardasil will not prevent development of anal precancerous lesions associated with HPV infection that is already present at the time of vaccination. Its full potential for benefit—across all FDA-approved indications—is obtained by people who are vaccinated before they are exposed to HPV strains contained in the vaccine.
People who undergo regular screening for anal cancer on the recommendation of their health care provider should not discontinue screening after they receive the quadrivalent HPV vaccine.
2 HPV vaccines, 7 questions that you need answered
Neal M. Lonky, MD, MPH, and an expert panel
The FDA recently approved the quadrivalent formulation of the human papillomavirus (HPV) vaccine (Merck’s Gardasil) for prevention of anal cancer and associated precancerous lesions caused by HPV types 6, 11, 16, and 18 in people 9 to 26 years old.
The quadrivalent HPV vaccine is already approved for the same age population for the prevention of cervical, vulvar, and vaginal cancer and the associated precancerous lesions caused by HPV types 6, 11, 16, and 18 in females. In addition, it is approved for the prevention of genital warts caused by types 6 and 11 in both males and females.
The indication for anal cancer prevention does not extend to the other FDA-approved HPV vaccine (GlaxoSmithKline’s bivalent [types 16 and 18] formulation, Cervarix).
“Treatment for anal cancer is challenging; the use of Gardasil as a method of prevention is important, as it may result in fewer diagnoses and the subsequent surgery, radiation or chemotherapy that individuals need to endure,” said Karen Midthun, MD, director of the FDA’s Center for Biologics Evaluation and Research.
Anal cancer is uncommon in the general population, but incidence is increasing. HPV is associated with approximately 90% of anal cancer cases. The American Cancer Society estimates that approximately 5,300 people are given a diagnosis of anal cancer each year in the United States—more often women than men.
Gardasil will not prevent development of anal precancerous lesions associated with HPV infection that is already present at the time of vaccination. Its full potential for benefit—across all FDA-approved indications—is obtained by people who are vaccinated before they are exposed to HPV strains contained in the vaccine.
People who undergo regular screening for anal cancer on the recommendation of their health care provider should not discontinue screening after they receive the quadrivalent HPV vaccine.
2 HPV vaccines, 7 questions that you need answered
Neal M. Lonky, MD, MPH, and an expert panel
The FDA recently approved the quadrivalent formulation of the human papillomavirus (HPV) vaccine (Merck’s Gardasil) for prevention of anal cancer and associated precancerous lesions caused by HPV types 6, 11, 16, and 18 in people 9 to 26 years old.
The quadrivalent HPV vaccine is already approved for the same age population for the prevention of cervical, vulvar, and vaginal cancer and the associated precancerous lesions caused by HPV types 6, 11, 16, and 18 in females. In addition, it is approved for the prevention of genital warts caused by types 6 and 11 in both males and females.
The indication for anal cancer prevention does not extend to the other FDA-approved HPV vaccine (GlaxoSmithKline’s bivalent [types 16 and 18] formulation, Cervarix).
“Treatment for anal cancer is challenging; the use of Gardasil as a method of prevention is important, as it may result in fewer diagnoses and the subsequent surgery, radiation or chemotherapy that individuals need to endure,” said Karen Midthun, MD, director of the FDA’s Center for Biologics Evaluation and Research.
Anal cancer is uncommon in the general population, but incidence is increasing. HPV is associated with approximately 90% of anal cancer cases. The American Cancer Society estimates that approximately 5,300 people are given a diagnosis of anal cancer each year in the United States—more often women than men.
Gardasil will not prevent development of anal precancerous lesions associated with HPV infection that is already present at the time of vaccination. Its full potential for benefit—across all FDA-approved indications—is obtained by people who are vaccinated before they are exposed to HPV strains contained in the vaccine.
People who undergo regular screening for anal cancer on the recommendation of their health care provider should not discontinue screening after they receive the quadrivalent HPV vaccine.
Cease the practice of early elective delivery, says March of Dimes
“39 weeks is the rule, provided delivery is truly elective”
“If you give magnesium sulfate for fetal neuroprotection, adhere to a protocol”
(Update on Obstetrics; January 2011)
John T. Repke, MD, and Jaimey M. Pauli, MD
“More strategies to avoid malpractice hazards on labor and delivery”
(Second of two parts; Focus on Professional Liability; January 2011)
Martin L. Gimovsky, MD, and Alexis C. Gimovsky, MD
Obstetricians and other providers of intrapartum care can improve birth outcomes significantly by eliminating the practice of elective delivery before 39 full weeks of gestation. That’s a key recommendation in a report issued by the March of Dimes at the end of 2010.1
In tandem with the report and accompanying formal recommendations for clinical care, the March of Dimes is expanding a quality improvement program to reduce unnecessary inductions and cesarean deliveries, noted Scott D. Berns, MD, MPH, at a presentation by the organization in New York on December 15. Dr. Berns is senior vice president for Chapter Programs of the March of Dimes and editor of the report, Toward Improving the Outcome of Pregnancy III (TIOP III). He is also clinical professor of pediatrics at the Warren Alpert Medical School at Brown University.
“It’s about babies being born at the right time for the right reasons,” Dr. Berns said.
Medical inductions are too common
A key focus of TIOP III is the need to curtail the practice of elective “term” delivery at 37 and 38 weeks of gestation. As the report notes, although “there are many valid medical and obstetric indications for delivery before 39 weeks of gestation, medical justification for a significant proportion of early deliveries is questionable.”1
Of particular concern is the use of medical induction of labor at 37 to 39 weeks without a legitimate indication—a practice that has increased dramatically over the past decade and that raises the rate of cesarean delivery, said Mark R. Chassin, MD, MPP, MPH, who spoke at the New York release of the report. Dr. Chassin is President of the Joint Commission.
Morbidity rises with early delivery
Early term delivery is widespread. As many as 30% of all births in the United States are performed electively (“without identifiable medical or obstetric indication”) before 39 weeks’ gestation, said Dr. Berns. This statistic includes elective induction of labor and elective primary and repeat cesarean delivery, he added.
The morbidity associated with these early births is significant:
- The rate of admission to a newborn intensive care unit (NICU) doubles in infants born electively at 38 to 39 weeks of gestation, compared with those delivered at or beyond 39 weeks
- “Infants born before 39 completed weeks of gestation also have a higher incidence of respiratory distress syndrome and infant death than those delivered later”1
- There is evidence that neonatal morbidity increases even after fetal lung maturity is confirmed when elective delivery takes place before 39 weeks.1
ACOG has also warned against early elective delivery.2
The March of Dimes Foundation offers a toolkit on its Web site for clinicians to use to reduce the rate of elective delivery before 39 full weeks of gestation. It’s available at http://www.marchofdimes.com/professionals/less-than-39-weeks-toolkit.aspx.
Other intrapartum actions can boost outcomes
Other recommendations for improving intrapartum care and pregnancy outcomes included in TIOP III:
- Introduce facility-based protocols and develop effective leadership to eliminate elective deliveries before 39 weeks’ gestation
- Use standardized, low-dose oxytocin protocols for induction and augmentation of labor. (According to TIOP III: “Oxtyocin is the drug most commonly associated with preventable adverse events during childbirth and is also the drug most frequently implicated in professional liability claims.”1
- Uniformly implement unambiguous protocols for monitoring oxytocin infusion
- Avoid “inappropriate” cesarean delivery and de-emphasize the cesarean delivery rate as a primary quality indicator
- Adopt protocols for administration of magnesium sulfate for fetal neuroprotection in preterm infants
- Enhance and support a team approach to obstetric emergencies, and promote clinician understanding of intermediate and abnormal fetal heart rate patterns
- Use available checklists to document maneuvers—including those avoided—in the management of shoulder dystocia
- Develop a “robust quality improvement program” for intrapartum care processes.1
“39 weeks is the rule, provided delivery is truly elective”
“If you give magnesium sulfate for fetal neuroprotection, adhere to a protocol”
(Update on Obstetrics; January 2011)
John T. Repke, MD, and Jaimey M. Pauli, MD
“More strategies to avoid malpractice hazards on labor and delivery”
(Second of two parts; Focus on Professional Liability; January 2011)
Martin L. Gimovsky, MD, and Alexis C. Gimovsky, MD
Obstetricians and other providers of intrapartum care can improve birth outcomes significantly by eliminating the practice of elective delivery before 39 full weeks of gestation. That’s a key recommendation in a report issued by the March of Dimes at the end of 2010.1
In tandem with the report and accompanying formal recommendations for clinical care, the March of Dimes is expanding a quality improvement program to reduce unnecessary inductions and cesarean deliveries, noted Scott D. Berns, MD, MPH, at a presentation by the organization in New York on December 15. Dr. Berns is senior vice president for Chapter Programs of the March of Dimes and editor of the report, Toward Improving the Outcome of Pregnancy III (TIOP III). He is also clinical professor of pediatrics at the Warren Alpert Medical School at Brown University.
“It’s about babies being born at the right time for the right reasons,” Dr. Berns said.
Medical inductions are too common
A key focus of TIOP III is the need to curtail the practice of elective “term” delivery at 37 and 38 weeks of gestation. As the report notes, although “there are many valid medical and obstetric indications for delivery before 39 weeks of gestation, medical justification for a significant proportion of early deliveries is questionable.”1
Of particular concern is the use of medical induction of labor at 37 to 39 weeks without a legitimate indication—a practice that has increased dramatically over the past decade and that raises the rate of cesarean delivery, said Mark R. Chassin, MD, MPP, MPH, who spoke at the New York release of the report. Dr. Chassin is President of the Joint Commission.
Morbidity rises with early delivery
Early term delivery is widespread. As many as 30% of all births in the United States are performed electively (“without identifiable medical or obstetric indication”) before 39 weeks’ gestation, said Dr. Berns. This statistic includes elective induction of labor and elective primary and repeat cesarean delivery, he added.
The morbidity associated with these early births is significant:
- The rate of admission to a newborn intensive care unit (NICU) doubles in infants born electively at 38 to 39 weeks of gestation, compared with those delivered at or beyond 39 weeks
- “Infants born before 39 completed weeks of gestation also have a higher incidence of respiratory distress syndrome and infant death than those delivered later”1
- There is evidence that neonatal morbidity increases even after fetal lung maturity is confirmed when elective delivery takes place before 39 weeks.1
ACOG has also warned against early elective delivery.2
The March of Dimes Foundation offers a toolkit on its Web site for clinicians to use to reduce the rate of elective delivery before 39 full weeks of gestation. It’s available at http://www.marchofdimes.com/professionals/less-than-39-weeks-toolkit.aspx.
Other intrapartum actions can boost outcomes
Other recommendations for improving intrapartum care and pregnancy outcomes included in TIOP III:
- Introduce facility-based protocols and develop effective leadership to eliminate elective deliveries before 39 weeks’ gestation
- Use standardized, low-dose oxytocin protocols for induction and augmentation of labor. (According to TIOP III: “Oxtyocin is the drug most commonly associated with preventable adverse events during childbirth and is also the drug most frequently implicated in professional liability claims.”1
- Uniformly implement unambiguous protocols for monitoring oxytocin infusion
- Avoid “inappropriate” cesarean delivery and de-emphasize the cesarean delivery rate as a primary quality indicator
- Adopt protocols for administration of magnesium sulfate for fetal neuroprotection in preterm infants
- Enhance and support a team approach to obstetric emergencies, and promote clinician understanding of intermediate and abnormal fetal heart rate patterns
- Use available checklists to document maneuvers—including those avoided—in the management of shoulder dystocia
- Develop a “robust quality improvement program” for intrapartum care processes.1
“39 weeks is the rule, provided delivery is truly elective”
“If you give magnesium sulfate for fetal neuroprotection, adhere to a protocol”
(Update on Obstetrics; January 2011)
John T. Repke, MD, and Jaimey M. Pauli, MD
“More strategies to avoid malpractice hazards on labor and delivery”
(Second of two parts; Focus on Professional Liability; January 2011)
Martin L. Gimovsky, MD, and Alexis C. Gimovsky, MD
Obstetricians and other providers of intrapartum care can improve birth outcomes significantly by eliminating the practice of elective delivery before 39 full weeks of gestation. That’s a key recommendation in a report issued by the March of Dimes at the end of 2010.1
In tandem with the report and accompanying formal recommendations for clinical care, the March of Dimes is expanding a quality improvement program to reduce unnecessary inductions and cesarean deliveries, noted Scott D. Berns, MD, MPH, at a presentation by the organization in New York on December 15. Dr. Berns is senior vice president for Chapter Programs of the March of Dimes and editor of the report, Toward Improving the Outcome of Pregnancy III (TIOP III). He is also clinical professor of pediatrics at the Warren Alpert Medical School at Brown University.
“It’s about babies being born at the right time for the right reasons,” Dr. Berns said.
Medical inductions are too common
A key focus of TIOP III is the need to curtail the practice of elective “term” delivery at 37 and 38 weeks of gestation. As the report notes, although “there are many valid medical and obstetric indications for delivery before 39 weeks of gestation, medical justification for a significant proportion of early deliveries is questionable.”1
Of particular concern is the use of medical induction of labor at 37 to 39 weeks without a legitimate indication—a practice that has increased dramatically over the past decade and that raises the rate of cesarean delivery, said Mark R. Chassin, MD, MPP, MPH, who spoke at the New York release of the report. Dr. Chassin is President of the Joint Commission.
Morbidity rises with early delivery
Early term delivery is widespread. As many as 30% of all births in the United States are performed electively (“without identifiable medical or obstetric indication”) before 39 weeks’ gestation, said Dr. Berns. This statistic includes elective induction of labor and elective primary and repeat cesarean delivery, he added.
The morbidity associated with these early births is significant:
- The rate of admission to a newborn intensive care unit (NICU) doubles in infants born electively at 38 to 39 weeks of gestation, compared with those delivered at or beyond 39 weeks
- “Infants born before 39 completed weeks of gestation also have a higher incidence of respiratory distress syndrome and infant death than those delivered later”1
- There is evidence that neonatal morbidity increases even after fetal lung maturity is confirmed when elective delivery takes place before 39 weeks.1
ACOG has also warned against early elective delivery.2
The March of Dimes Foundation offers a toolkit on its Web site for clinicians to use to reduce the rate of elective delivery before 39 full weeks of gestation. It’s available at http://www.marchofdimes.com/professionals/less-than-39-weeks-toolkit.aspx.
Other intrapartum actions can boost outcomes
Other recommendations for improving intrapartum care and pregnancy outcomes included in TIOP III:
- Introduce facility-based protocols and develop effective leadership to eliminate elective deliveries before 39 weeks’ gestation
- Use standardized, low-dose oxytocin protocols for induction and augmentation of labor. (According to TIOP III: “Oxtyocin is the drug most commonly associated with preventable adverse events during childbirth and is also the drug most frequently implicated in professional liability claims.”1
- Uniformly implement unambiguous protocols for monitoring oxytocin infusion
- Avoid “inappropriate” cesarean delivery and de-emphasize the cesarean delivery rate as a primary quality indicator
- Adopt protocols for administration of magnesium sulfate for fetal neuroprotection in preterm infants
- Enhance and support a team approach to obstetric emergencies, and promote clinician understanding of intermediate and abnormal fetal heart rate patterns
- Use available checklists to document maneuvers—including those avoided—in the management of shoulder dystocia
- Develop a “robust quality improvement program” for intrapartum care processes.1
Women who have low sexual arousal may respond to simple measures
- Update on Sexual Dysfunction: What can you do for your patients?
Barbara S. Levy, MD (September 2010)
A new study reveals that women who have low sexual arousal may experience a clinically significant change in symptoms after taking a placebo.
Does this finding suggest that ObGyns should use placebo when managing women who have sexual dysfunction?
No.
But it does suggest that even simple interventions can make a difference in the management of these women, the authors say.
Andrea Bradford, PhD, a psychologist at Baylor College of Medicine, and coauthor Cindy Meston, PhD, a psychologist at the University of Texas at Austin, analyzed the behaviors and symptoms of 50 women who were randomly assigned to receive placebo in a large clinical trial of a drug treatment for low sexual arousal. Neither the women nor the study physicians knew whether they were taking the real drug or placebo.
After 12 weeks of treatment, symptoms in about one in three of these women improved to a degree that most clinicians would consider a meaningful change. Most of that improvement seemed to happen during the first four weeks of the study. Results were published online in the September 2010 issue of the Journal of Sexual Medicine.
The most important predictor of symptom change was an increase in the frequency of satisfying sexual encounters during the treatment. Many women even reported that they received more stimulation during sexual activity while they participated in the trial, even though their partners were not given any special instructions.
“It’s important to note that, even though these women received placebo, they all had an opportunity to talk to a health provider about their difficulties and were asked to closely monitor their sexual behavior and feelings over a 12-week period. Just taking part in this study probably started some meaningful conversations,” said Bradford. “Our study shows that even a limited intervention can have a positive effect in many women with sexual dysfunction. This comes as no surprise to sex therapists, but it does suggest a need to investigate behavioral factors more closely in clinical trials.”
We want to hear from you! Tell us what you think.
- Update on Sexual Dysfunction: What can you do for your patients?
Barbara S. Levy, MD (September 2010)
A new study reveals that women who have low sexual arousal may experience a clinically significant change in symptoms after taking a placebo.
Does this finding suggest that ObGyns should use placebo when managing women who have sexual dysfunction?
No.
But it does suggest that even simple interventions can make a difference in the management of these women, the authors say.
Andrea Bradford, PhD, a psychologist at Baylor College of Medicine, and coauthor Cindy Meston, PhD, a psychologist at the University of Texas at Austin, analyzed the behaviors and symptoms of 50 women who were randomly assigned to receive placebo in a large clinical trial of a drug treatment for low sexual arousal. Neither the women nor the study physicians knew whether they were taking the real drug or placebo.
After 12 weeks of treatment, symptoms in about one in three of these women improved to a degree that most clinicians would consider a meaningful change. Most of that improvement seemed to happen during the first four weeks of the study. Results were published online in the September 2010 issue of the Journal of Sexual Medicine.
The most important predictor of symptom change was an increase in the frequency of satisfying sexual encounters during the treatment. Many women even reported that they received more stimulation during sexual activity while they participated in the trial, even though their partners were not given any special instructions.
“It’s important to note that, even though these women received placebo, they all had an opportunity to talk to a health provider about their difficulties and were asked to closely monitor their sexual behavior and feelings over a 12-week period. Just taking part in this study probably started some meaningful conversations,” said Bradford. “Our study shows that even a limited intervention can have a positive effect in many women with sexual dysfunction. This comes as no surprise to sex therapists, but it does suggest a need to investigate behavioral factors more closely in clinical trials.”
We want to hear from you! Tell us what you think.
- Update on Sexual Dysfunction: What can you do for your patients?
Barbara S. Levy, MD (September 2010)
A new study reveals that women who have low sexual arousal may experience a clinically significant change in symptoms after taking a placebo.
Does this finding suggest that ObGyns should use placebo when managing women who have sexual dysfunction?
No.
But it does suggest that even simple interventions can make a difference in the management of these women, the authors say.
Andrea Bradford, PhD, a psychologist at Baylor College of Medicine, and coauthor Cindy Meston, PhD, a psychologist at the University of Texas at Austin, analyzed the behaviors and symptoms of 50 women who were randomly assigned to receive placebo in a large clinical trial of a drug treatment for low sexual arousal. Neither the women nor the study physicians knew whether they were taking the real drug or placebo.
After 12 weeks of treatment, symptoms in about one in three of these women improved to a degree that most clinicians would consider a meaningful change. Most of that improvement seemed to happen during the first four weeks of the study. Results were published online in the September 2010 issue of the Journal of Sexual Medicine.
The most important predictor of symptom change was an increase in the frequency of satisfying sexual encounters during the treatment. Many women even reported that they received more stimulation during sexual activity while they participated in the trial, even though their partners were not given any special instructions.
“It’s important to note that, even though these women received placebo, they all had an opportunity to talk to a health provider about their difficulties and were asked to closely monitor their sexual behavior and feelings over a 12-week period. Just taking part in this study probably started some meaningful conversations,” said Bradford. “Our study shows that even a limited intervention can have a positive effect in many women with sexual dysfunction. This comes as no surprise to sex therapists, but it does suggest a need to investigate behavioral factors more closely in clinical trials.”
We want to hear from you! Tell us what you think.