User login
Make the Diagnosis - October 2016
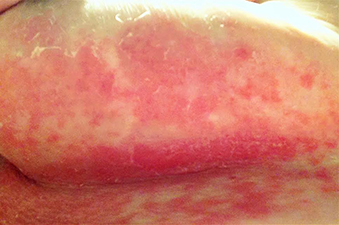
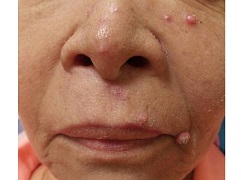
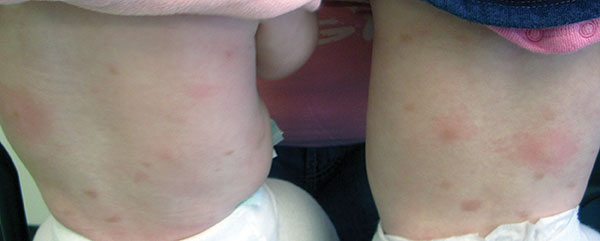
Erythema multiforme
Erythema multiforme (EM) is a hypersensitivity reaction. Cutaneous lesions generally occur symmetrically on the extensor surfaces of the acral extremities and spread centripetally. Lesions are polymorphous and may be macular, papular, or bullous, with or without mucosal involvement. Classic targetoid lesions have a dusky central area, a paler edematous ring, and an erythematous outermost ring. EM can be triggered by multiple causes. 90% of cases are infectious, with herpes simplex virus infection most common and mycoplasma pneumoniae second. Medications (sulfonamides, anticonvulsants, etc), malignancy, autoimmune disease, immunizations, radiation, and sarcoidosis may also cause EM.
The differential diagnosis for EM includes urticaria, Stevens-Johnson syndrome, fixed drug eruption, bullous pemphigoid, paraneoplastic pemphigus, acute febrile neutophilic dermatosis (Sweet's syndrome), polymorphous light eruption (PMLE), and cutaneous small vessel vasculitis. The lesions of EM are fixed and all arise within 72 hours of onset, which can help distinguish between urticaria. The distribution of the lesions can help rule out Stevens-Johnson syndrome, which classically begins centrally and spreads distally and often will involve mucosal surfaces. Fixed drug eruption generally presents with fewer lesions and a preceding medication history. Bullous pemphigoid and paraneoplastic pemphigus are both chronic in their course and have distinguishing histopathologic characteristics that will not be present in erythema multiforme. Sweet's syndrome is also associated with infection; however, histology reveals a dense neutrophilic infiltrate with dermal edema on biopsy. History can be useful in distinguishing EM from PMLE: preceding infection strongly suggests EM, while ultraviolet radiation exposure is more likely to result in PMLE. Lastly, EM differs from cutaneous small vessel vasculitis through both histopathologic examination and direct immunofluorescence.
EM is usually a self-limited condition of about two weeks without significant sequelae, however, it may vary in both the length and severity of its course. A small subset of patients may experience episodic eruptions over a period of years, in what is known as recurrent EM. An even smaller subset of patients may continuously suffer from the lesions for months or even years, which is termed persistent EM. Treatment is aimed at the underlying condition or cause. Offending medications should be discontinued. Patients with mild cases of EM generally fare well with symptomatic treatment alone; however, more severe cases may require hospitalization for hydration, analgesia, and antiviral therapy. The use of systemic corticosteroids for EM is controversial and is generally reserved for severe cases with mucosal involvement. Prophylactic antiviral therapy is indicated for those with recurrent erythema multiforme of viral origin who suffer from six or more episodes per year. Very rarely, erythema multiforme may include ocular involvement leading to serious long-term sequelae, so patients with ocular symptoms should be immediately referred to an ophthalmologist for evaluation.
The patient's biopsy revealed necrotic keratinocytes and exocytosis of lymphocytes and was consistent with EM. She improved with oral antiviral therapy and topical steroid cream.
Erythema multiforme
Erythema multiforme (EM) is a hypersensitivity reaction. Cutaneous lesions generally occur symmetrically on the extensor surfaces of the acral extremities and spread centripetally. Lesions are polymorphous and may be macular, papular, or bullous, with or without mucosal involvement. Classic targetoid lesions have a dusky central area, a paler edematous ring, and an erythematous outermost ring. EM can be triggered by multiple causes. 90% of cases are infectious, with herpes simplex virus infection most common and mycoplasma pneumoniae second. Medications (sulfonamides, anticonvulsants, etc), malignancy, autoimmune disease, immunizations, radiation, and sarcoidosis may also cause EM.
The differential diagnosis for EM includes urticaria, Stevens-Johnson syndrome, fixed drug eruption, bullous pemphigoid, paraneoplastic pemphigus, acute febrile neutophilic dermatosis (Sweet's syndrome), polymorphous light eruption (PMLE), and cutaneous small vessel vasculitis. The lesions of EM are fixed and all arise within 72 hours of onset, which can help distinguish between urticaria. The distribution of the lesions can help rule out Stevens-Johnson syndrome, which classically begins centrally and spreads distally and often will involve mucosal surfaces. Fixed drug eruption generally presents with fewer lesions and a preceding medication history. Bullous pemphigoid and paraneoplastic pemphigus are both chronic in their course and have distinguishing histopathologic characteristics that will not be present in erythema multiforme. Sweet's syndrome is also associated with infection; however, histology reveals a dense neutrophilic infiltrate with dermal edema on biopsy. History can be useful in distinguishing EM from PMLE: preceding infection strongly suggests EM, while ultraviolet radiation exposure is more likely to result in PMLE. Lastly, EM differs from cutaneous small vessel vasculitis through both histopathologic examination and direct immunofluorescence.
EM is usually a self-limited condition of about two weeks without significant sequelae, however, it may vary in both the length and severity of its course. A small subset of patients may experience episodic eruptions over a period of years, in what is known as recurrent EM. An even smaller subset of patients may continuously suffer from the lesions for months or even years, which is termed persistent EM. Treatment is aimed at the underlying condition or cause. Offending medications should be discontinued. Patients with mild cases of EM generally fare well with symptomatic treatment alone; however, more severe cases may require hospitalization for hydration, analgesia, and antiviral therapy. The use of systemic corticosteroids for EM is controversial and is generally reserved for severe cases with mucosal involvement. Prophylactic antiviral therapy is indicated for those with recurrent erythema multiforme of viral origin who suffer from six or more episodes per year. Very rarely, erythema multiforme may include ocular involvement leading to serious long-term sequelae, so patients with ocular symptoms should be immediately referred to an ophthalmologist for evaluation.
The patient's biopsy revealed necrotic keratinocytes and exocytosis of lymphocytes and was consistent with EM. She improved with oral antiviral therapy and topical steroid cream.
Erythema multiforme
Erythema multiforme (EM) is a hypersensitivity reaction. Cutaneous lesions generally occur symmetrically on the extensor surfaces of the acral extremities and spread centripetally. Lesions are polymorphous and may be macular, papular, or bullous, with or without mucosal involvement. Classic targetoid lesions have a dusky central area, a paler edematous ring, and an erythematous outermost ring. EM can be triggered by multiple causes. 90% of cases are infectious, with herpes simplex virus infection most common and mycoplasma pneumoniae second. Medications (sulfonamides, anticonvulsants, etc), malignancy, autoimmune disease, immunizations, radiation, and sarcoidosis may also cause EM.
The differential diagnosis for EM includes urticaria, Stevens-Johnson syndrome, fixed drug eruption, bullous pemphigoid, paraneoplastic pemphigus, acute febrile neutophilic dermatosis (Sweet's syndrome), polymorphous light eruption (PMLE), and cutaneous small vessel vasculitis. The lesions of EM are fixed and all arise within 72 hours of onset, which can help distinguish between urticaria. The distribution of the lesions can help rule out Stevens-Johnson syndrome, which classically begins centrally and spreads distally and often will involve mucosal surfaces. Fixed drug eruption generally presents with fewer lesions and a preceding medication history. Bullous pemphigoid and paraneoplastic pemphigus are both chronic in their course and have distinguishing histopathologic characteristics that will not be present in erythema multiforme. Sweet's syndrome is also associated with infection; however, histology reveals a dense neutrophilic infiltrate with dermal edema on biopsy. History can be useful in distinguishing EM from PMLE: preceding infection strongly suggests EM, while ultraviolet radiation exposure is more likely to result in PMLE. Lastly, EM differs from cutaneous small vessel vasculitis through both histopathologic examination and direct immunofluorescence.
EM is usually a self-limited condition of about two weeks without significant sequelae, however, it may vary in both the length and severity of its course. A small subset of patients may experience episodic eruptions over a period of years, in what is known as recurrent EM. An even smaller subset of patients may continuously suffer from the lesions for months or even years, which is termed persistent EM. Treatment is aimed at the underlying condition or cause. Offending medications should be discontinued. Patients with mild cases of EM generally fare well with symptomatic treatment alone; however, more severe cases may require hospitalization for hydration, analgesia, and antiviral therapy. The use of systemic corticosteroids for EM is controversial and is generally reserved for severe cases with mucosal involvement. Prophylactic antiviral therapy is indicated for those with recurrent erythema multiforme of viral origin who suffer from six or more episodes per year. Very rarely, erythema multiforme may include ocular involvement leading to serious long-term sequelae, so patients with ocular symptoms should be immediately referred to an ophthalmologist for evaluation.
The patient's biopsy revealed necrotic keratinocytes and exocytosis of lymphocytes and was consistent with EM. She improved with oral antiviral therapy and topical steroid cream.
A healthy 30 year old female with a history of herpes simplex virus on the lips presented with three days of pruritic targetoid lesions on the arms and legs. She reports similar episodes in the past.
Make the Diagnosis - July 2016
Diagnosis: Lichen sclerosus et atrophicus with morphea
Lichen sclerosus et atrophicus (LSetA) is a chronic inflammatory condition mediated primarily by lymphocytic infiltration. It is up to 10 times more likely to occur in women than men. There is a genetic component, as approximately 12% of affected patients also have relatives with the condition. Human leukocyte antigen subtypes DQ7, DQ8, and DQ9 have been associated with LSetA, which is thought to be primarily autoimmune in nature. There is also a strong association with other autoimmune diseases, predominantly thyroid disease, vitiligo, alopecia areata, and pernicious anemia.
LSetA is most commonly seen in the vulvar region, but can also present extragenitally. Vulvar LSetA has a bimodal distribution, peaking in prepubertal and postmenopausal women. The most common symptoms are pruritus and pain. On physical examination, LSetA appears as thick white plaques with a thin epidermis resembling “cigarette paper.” Chronic inflammation can also lead to permanent scarring. Pruritus or pain can result, which can significantly affect quality of life.
Morphea is characterized by thickened skin as a result of deep dermal fibrosis. Also known as localized scleroderma, morphea presents with the cutaneous findings of scleroderma without the systemic complications. Like LSetA, morphea is more common in females than males, although the ratio is not as high. Plaque morphea is the most prevalent type and presents as circumscribed, edematous plaques on the trunk or extremities. Morphea has been reported as a consequence of localized injury or infection, but is also commonly associated with various autoimmune conditions.
Histologic changes of LSetA include epidermal atrophy and homogenization of collagen limited to the superficial dermis. In contrast, morphea histologically consists of deep dermal fibrosis with associated epidermal hyperkeratosis. While LSetA and morphea are usually distinct entities, they can rarely occur together. The diagnosis of LSetA with morphea can be confirmed when the deep and superficial histologic findings are consistent with both entities.
Treatment options for LSetA include short-term, high-potency topical corticosteroids followed by maintenance therapy with emollients. Reducing exposure to irritants is also crucial for a sustained response. Other modalities such as topical calcineurin inhibitors, photodynamic therapy, surgery, and methotrexate are potential alternatives in recalcitrant cases. Morphea can be treated with methotrexate and systemic glucocorticoids, which are usually given in combination. Systemic glucocorticoids can be tapered to avoid long-term side effects, while tapering the methotrexate based on clinical response. In general, the evidence supports the use of systemic therapy for extensive plaquetype morphea over topical treatments; however, there is some evidence to support the use of topical calcineurin inhibitors in resistant cases.
This patient was started on a prednisone taper in addition to weekly methotrexate. Gabapentin was prescribed for pain control. The patient returned to the clinic during the next several months with improvement in her symptoms and reduction in the size and induration of the lesions.
This case and photo were submitted by Adam Aldahan, Dr. Alyx Rosen, and Dr. Anne Burdick of the University of Miami department of dermatology.
Diagnosis: Lichen sclerosus et atrophicus with morphea
Lichen sclerosus et atrophicus (LSetA) is a chronic inflammatory condition mediated primarily by lymphocytic infiltration. It is up to 10 times more likely to occur in women than men. There is a genetic component, as approximately 12% of affected patients also have relatives with the condition. Human leukocyte antigen subtypes DQ7, DQ8, and DQ9 have been associated with LSetA, which is thought to be primarily autoimmune in nature. There is also a strong association with other autoimmune diseases, predominantly thyroid disease, vitiligo, alopecia areata, and pernicious anemia.
LSetA is most commonly seen in the vulvar region, but can also present extragenitally. Vulvar LSetA has a bimodal distribution, peaking in prepubertal and postmenopausal women. The most common symptoms are pruritus and pain. On physical examination, LSetA appears as thick white plaques with a thin epidermis resembling “cigarette paper.” Chronic inflammation can also lead to permanent scarring. Pruritus or pain can result, which can significantly affect quality of life.
Morphea is characterized by thickened skin as a result of deep dermal fibrosis. Also known as localized scleroderma, morphea presents with the cutaneous findings of scleroderma without the systemic complications. Like LSetA, morphea is more common in females than males, although the ratio is not as high. Plaque morphea is the most prevalent type and presents as circumscribed, edematous plaques on the trunk or extremities. Morphea has been reported as a consequence of localized injury or infection, but is also commonly associated with various autoimmune conditions.
Histologic changes of LSetA include epidermal atrophy and homogenization of collagen limited to the superficial dermis. In contrast, morphea histologically consists of deep dermal fibrosis with associated epidermal hyperkeratosis. While LSetA and morphea are usually distinct entities, they can rarely occur together. The diagnosis of LSetA with morphea can be confirmed when the deep and superficial histologic findings are consistent with both entities.
Treatment options for LSetA include short-term, high-potency topical corticosteroids followed by maintenance therapy with emollients. Reducing exposure to irritants is also crucial for a sustained response. Other modalities such as topical calcineurin inhibitors, photodynamic therapy, surgery, and methotrexate are potential alternatives in recalcitrant cases. Morphea can be treated with methotrexate and systemic glucocorticoids, which are usually given in combination. Systemic glucocorticoids can be tapered to avoid long-term side effects, while tapering the methotrexate based on clinical response. In general, the evidence supports the use of systemic therapy for extensive plaquetype morphea over topical treatments; however, there is some evidence to support the use of topical calcineurin inhibitors in resistant cases.
This patient was started on a prednisone taper in addition to weekly methotrexate. Gabapentin was prescribed for pain control. The patient returned to the clinic during the next several months with improvement in her symptoms and reduction in the size and induration of the lesions.
This case and photo were submitted by Adam Aldahan, Dr. Alyx Rosen, and Dr. Anne Burdick of the University of Miami department of dermatology.
Diagnosis: Lichen sclerosus et atrophicus with morphea
Lichen sclerosus et atrophicus (LSetA) is a chronic inflammatory condition mediated primarily by lymphocytic infiltration. It is up to 10 times more likely to occur in women than men. There is a genetic component, as approximately 12% of affected patients also have relatives with the condition. Human leukocyte antigen subtypes DQ7, DQ8, and DQ9 have been associated with LSetA, which is thought to be primarily autoimmune in nature. There is also a strong association with other autoimmune diseases, predominantly thyroid disease, vitiligo, alopecia areata, and pernicious anemia.
LSetA is most commonly seen in the vulvar region, but can also present extragenitally. Vulvar LSetA has a bimodal distribution, peaking in prepubertal and postmenopausal women. The most common symptoms are pruritus and pain. On physical examination, LSetA appears as thick white plaques with a thin epidermis resembling “cigarette paper.” Chronic inflammation can also lead to permanent scarring. Pruritus or pain can result, which can significantly affect quality of life.
Morphea is characterized by thickened skin as a result of deep dermal fibrosis. Also known as localized scleroderma, morphea presents with the cutaneous findings of scleroderma without the systemic complications. Like LSetA, morphea is more common in females than males, although the ratio is not as high. Plaque morphea is the most prevalent type and presents as circumscribed, edematous plaques on the trunk or extremities. Morphea has been reported as a consequence of localized injury or infection, but is also commonly associated with various autoimmune conditions.
Histologic changes of LSetA include epidermal atrophy and homogenization of collagen limited to the superficial dermis. In contrast, morphea histologically consists of deep dermal fibrosis with associated epidermal hyperkeratosis. While LSetA and morphea are usually distinct entities, they can rarely occur together. The diagnosis of LSetA with morphea can be confirmed when the deep and superficial histologic findings are consistent with both entities.
Treatment options for LSetA include short-term, high-potency topical corticosteroids followed by maintenance therapy with emollients. Reducing exposure to irritants is also crucial for a sustained response. Other modalities such as topical calcineurin inhibitors, photodynamic therapy, surgery, and methotrexate are potential alternatives in recalcitrant cases. Morphea can be treated with methotrexate and systemic glucocorticoids, which are usually given in combination. Systemic glucocorticoids can be tapered to avoid long-term side effects, while tapering the methotrexate based on clinical response. In general, the evidence supports the use of systemic therapy for extensive plaquetype morphea over topical treatments; however, there is some evidence to support the use of topical calcineurin inhibitors in resistant cases.
This patient was started on a prednisone taper in addition to weekly methotrexate. Gabapentin was prescribed for pain control. The patient returned to the clinic during the next several months with improvement in her symptoms and reduction in the size and induration of the lesions.
This case and photo were submitted by Adam Aldahan, Dr. Alyx Rosen, and Dr. Anne Burdick of the University of Miami department of dermatology.
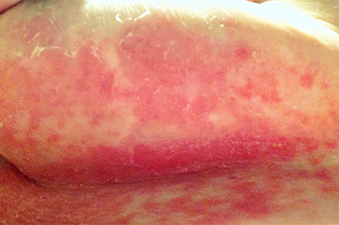
A 63-year-old woman presented with extremely painful plaques on the breasts, inframammary folds, and chest, and under the abdomen, for 3 years. The lesions had progressively increased in size with worsening pain over the past several months. She also reported painful raw areas on her genitals. Physical examination revealed multiple well-demarcated oval and geographic plaques with central white induration with areas of superficial erosion and pink peripheral borders on the breasts, inframammary folds, midchest, and abdomen that were exquisitely tender to light palpation. Genital examination revealed a vulvar white plaque with superficial erosions and an atrophic labia minora.
Make the Diagnosis - June 2016
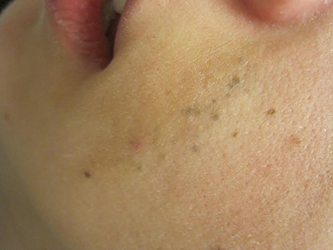
Diagnosis: Agminated blue nevus
Agminated blue nevi are aggregated clusters of nevi that appear blue due to their deep dermal location and the subsequent scattering of short wavelengths of light (Tyndall effect). The clusters are typically contained within a 10 cm area. The intervening background skin is usually without hyperpigmentation or skin discoloration, although speckled pigmentation can occur. Generally, all forms of agminated nevi likely result from a loss of heterozygosity (LOH) mutation in the somatic line of an embryo, inducing a localized predilection for the development of nevi.
The differential diagnosis for agminated blue nevi includes segmental melanocytic nevi, melanoma, and other agminated melanocytic nevi: benign melanocytic nevi, atypical or dysplastic nevi, and speckled lentiginous nevi (nevus spilus). The distribution of the nevi can help narrow the differential, as segmental melanocytic nevi are less clustered and spread over a larger area than agminated blue nevi. Congenital nevi are present at birth or within a few months and often have unique features, including perifollicular pigmentary changes, hypertrichosis, and focal hypopigmentation. Acquired nevi (blue nevi, benign melanocytic nevi, and atypical or dysplastic nevi) usually descend over time from the dermal-epidermal junction to the dermis and typically become a lighter color. Lastly, the presence or absence of background pigmentation can help distinguish between agminated blue nevi and nevus spilus. Nevus spilus is a subtype of congenital melanocytic nevi with a similar aggregated appearance but has a characteristic background hyperpigmentation between the discrete nevi.
Agminated blue nevi and nevus spilus can be differentiated in the clinic by using a Wood’s lamp or performing a biopsy, which is not indicated unless atypia is present. If a non-speckled background hyperpigmentation is present on Wood’s lamp illumination, the diagnosis of nevus spilus may be more appropriate, as agminated blue nevi typically show no background pigmentation. A biopsy of the skin would allow for distinguishing between nevus spilus and agminated nevi based on the pigment within the surrounding skin. The presence of melanocytic hyperplasia within the skin surrounding the discrete nevi would support the diagnosis of nevus spilus.
The main concern with any melanocytic proliferation is the potential for transformation into a melanoma. In general, agminated nevi carry an increased melanoma risk. Specifically, congenital melanocytic nevi result in a <1-5% risk of melanoma depending on the lesion size. The risk of melanoma associated with agminated blue nevi is currently unknown, but it should be noted that individual blue nevi rarely become malignant. Since more than 50% of cutaneous melanomas develop without a preceding nevus, removal of nevi to reduce melanoma risk is not typically indicated, except in the case of a large congenital melanocytic nevi (>20cm). However, long-term surveillance is crucial for agminated blue nevi, especially if the individual has dysplastic nevus syndrome. If the involved nevi develop any features of atypia, a biopsy should be performed to assess for the development of a melanoma.
This case and photo were submitted by Dr. Damon McClain of Three Rivers Dermatology in Coraopolis, Pa., and Mark Ash, a medical student at East Carolina University, Greenville, N.C.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to dermnews@frontlinemedcom.com.
References:
- Ashfaq A. Marghoob, Robin Blum, Robert Nossa, Klaus J. Busam, Dana Sachs, Allan Halpern. Agminated Atypical (Dysplastic) Nevi Case Report and Review of the Literature. Arch Dermatol. 2001;137(7):917-920.
- Belinda Tan, Noah Craft, Lindy P. Fox, Lowell A. Goldsmith, Michael D. Tharp. Visual Dx. Blue Nevus. Accessed 2/11/16. Camila Roos Mariano da Rocha, Thaís Corsetti Grazziotin, Maria Carolina Widholzer Rey, Laura Luzzatto, Renan Rangel Bonamigo. Congenital agminated melanocytic nevus - Case report. An Bras Dermatol. 2013;88(6 Suppl 1):170-2.
- Chris G. Adigun, Jeffrey D. Bernhard, Sarah Stein, Karen Wiss, Sheila Galbraith, Craig N. Burkhart, Dean Morrell, Lynn Garfunkel, Nancy Esterly. Visual Dx. Agminated Nevus. Accessed 2/11/16.
- Julie V Schaffer and Jean L Bolognia. Acquired melanocytic nevi (moles). In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. (Accessed on February 11, 2016.)
- Julie V Schaffer and Jean L Bolognia. Congenital melanocytic nevi. In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. (Accessed on February 11, 2016.)
- Maria A. Pizzichetta, H. Peter Soyer, Cesare Massone, Lorenzo Cerroni. Clinical and Dermoscopic Features of Agminated Blue Nevus. Arch Dermatol. 2007;143(9):1209-1226.
Diagnosis: Agminated blue nevus
Agminated blue nevi are aggregated clusters of nevi that appear blue due to their deep dermal location and the subsequent scattering of short wavelengths of light (Tyndall effect). The clusters are typically contained within a 10 cm area. The intervening background skin is usually without hyperpigmentation or skin discoloration, although speckled pigmentation can occur. Generally, all forms of agminated nevi likely result from a loss of heterozygosity (LOH) mutation in the somatic line of an embryo, inducing a localized predilection for the development of nevi.
The differential diagnosis for agminated blue nevi includes segmental melanocytic nevi, melanoma, and other agminated melanocytic nevi: benign melanocytic nevi, atypical or dysplastic nevi, and speckled lentiginous nevi (nevus spilus). The distribution of the nevi can help narrow the differential, as segmental melanocytic nevi are less clustered and spread over a larger area than agminated blue nevi. Congenital nevi are present at birth or within a few months and often have unique features, including perifollicular pigmentary changes, hypertrichosis, and focal hypopigmentation. Acquired nevi (blue nevi, benign melanocytic nevi, and atypical or dysplastic nevi) usually descend over time from the dermal-epidermal junction to the dermis and typically become a lighter color. Lastly, the presence or absence of background pigmentation can help distinguish between agminated blue nevi and nevus spilus. Nevus spilus is a subtype of congenital melanocytic nevi with a similar aggregated appearance but has a characteristic background hyperpigmentation between the discrete nevi.
Agminated blue nevi and nevus spilus can be differentiated in the clinic by using a Wood’s lamp or performing a biopsy, which is not indicated unless atypia is present. If a non-speckled background hyperpigmentation is present on Wood’s lamp illumination, the diagnosis of nevus spilus may be more appropriate, as agminated blue nevi typically show no background pigmentation. A biopsy of the skin would allow for distinguishing between nevus spilus and agminated nevi based on the pigment within the surrounding skin. The presence of melanocytic hyperplasia within the skin surrounding the discrete nevi would support the diagnosis of nevus spilus.
The main concern with any melanocytic proliferation is the potential for transformation into a melanoma. In general, agminated nevi carry an increased melanoma risk. Specifically, congenital melanocytic nevi result in a <1-5% risk of melanoma depending on the lesion size. The risk of melanoma associated with agminated blue nevi is currently unknown, but it should be noted that individual blue nevi rarely become malignant. Since more than 50% of cutaneous melanomas develop without a preceding nevus, removal of nevi to reduce melanoma risk is not typically indicated, except in the case of a large congenital melanocytic nevi (>20cm). However, long-term surveillance is crucial for agminated blue nevi, especially if the individual has dysplastic nevus syndrome. If the involved nevi develop any features of atypia, a biopsy should be performed to assess for the development of a melanoma.
This case and photo were submitted by Dr. Damon McClain of Three Rivers Dermatology in Coraopolis, Pa., and Mark Ash, a medical student at East Carolina University, Greenville, N.C.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to dermnews@frontlinemedcom.com.
References:
- Ashfaq A. Marghoob, Robin Blum, Robert Nossa, Klaus J. Busam, Dana Sachs, Allan Halpern. Agminated Atypical (Dysplastic) Nevi Case Report and Review of the Literature. Arch Dermatol. 2001;137(7):917-920.
- Belinda Tan, Noah Craft, Lindy P. Fox, Lowell A. Goldsmith, Michael D. Tharp. Visual Dx. Blue Nevus. Accessed 2/11/16. Camila Roos Mariano da Rocha, Thaís Corsetti Grazziotin, Maria Carolina Widholzer Rey, Laura Luzzatto, Renan Rangel Bonamigo. Congenital agminated melanocytic nevus - Case report. An Bras Dermatol. 2013;88(6 Suppl 1):170-2.
- Chris G. Adigun, Jeffrey D. Bernhard, Sarah Stein, Karen Wiss, Sheila Galbraith, Craig N. Burkhart, Dean Morrell, Lynn Garfunkel, Nancy Esterly. Visual Dx. Agminated Nevus. Accessed 2/11/16.
- Julie V Schaffer and Jean L Bolognia. Acquired melanocytic nevi (moles). In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. (Accessed on February 11, 2016.)
- Julie V Schaffer and Jean L Bolognia. Congenital melanocytic nevi. In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. (Accessed on February 11, 2016.)
- Maria A. Pizzichetta, H. Peter Soyer, Cesare Massone, Lorenzo Cerroni. Clinical and Dermoscopic Features of Agminated Blue Nevus. Arch Dermatol. 2007;143(9):1209-1226.
Diagnosis: Agminated blue nevus
Agminated blue nevi are aggregated clusters of nevi that appear blue due to their deep dermal location and the subsequent scattering of short wavelengths of light (Tyndall effect). The clusters are typically contained within a 10 cm area. The intervening background skin is usually without hyperpigmentation or skin discoloration, although speckled pigmentation can occur. Generally, all forms of agminated nevi likely result from a loss of heterozygosity (LOH) mutation in the somatic line of an embryo, inducing a localized predilection for the development of nevi.
The differential diagnosis for agminated blue nevi includes segmental melanocytic nevi, melanoma, and other agminated melanocytic nevi: benign melanocytic nevi, atypical or dysplastic nevi, and speckled lentiginous nevi (nevus spilus). The distribution of the nevi can help narrow the differential, as segmental melanocytic nevi are less clustered and spread over a larger area than agminated blue nevi. Congenital nevi are present at birth or within a few months and often have unique features, including perifollicular pigmentary changes, hypertrichosis, and focal hypopigmentation. Acquired nevi (blue nevi, benign melanocytic nevi, and atypical or dysplastic nevi) usually descend over time from the dermal-epidermal junction to the dermis and typically become a lighter color. Lastly, the presence or absence of background pigmentation can help distinguish between agminated blue nevi and nevus spilus. Nevus spilus is a subtype of congenital melanocytic nevi with a similar aggregated appearance but has a characteristic background hyperpigmentation between the discrete nevi.
Agminated blue nevi and nevus spilus can be differentiated in the clinic by using a Wood’s lamp or performing a biopsy, which is not indicated unless atypia is present. If a non-speckled background hyperpigmentation is present on Wood’s lamp illumination, the diagnosis of nevus spilus may be more appropriate, as agminated blue nevi typically show no background pigmentation. A biopsy of the skin would allow for distinguishing between nevus spilus and agminated nevi based on the pigment within the surrounding skin. The presence of melanocytic hyperplasia within the skin surrounding the discrete nevi would support the diagnosis of nevus spilus.
The main concern with any melanocytic proliferation is the potential for transformation into a melanoma. In general, agminated nevi carry an increased melanoma risk. Specifically, congenital melanocytic nevi result in a <1-5% risk of melanoma depending on the lesion size. The risk of melanoma associated with agminated blue nevi is currently unknown, but it should be noted that individual blue nevi rarely become malignant. Since more than 50% of cutaneous melanomas develop without a preceding nevus, removal of nevi to reduce melanoma risk is not typically indicated, except in the case of a large congenital melanocytic nevi (>20cm). However, long-term surveillance is crucial for agminated blue nevi, especially if the individual has dysplastic nevus syndrome. If the involved nevi develop any features of atypia, a biopsy should be performed to assess for the development of a melanoma.
This case and photo were submitted by Dr. Damon McClain of Three Rivers Dermatology in Coraopolis, Pa., and Mark Ash, a medical student at East Carolina University, Greenville, N.C.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to dermnews@frontlinemedcom.com.
References:
- Ashfaq A. Marghoob, Robin Blum, Robert Nossa, Klaus J. Busam, Dana Sachs, Allan Halpern. Agminated Atypical (Dysplastic) Nevi Case Report and Review of the Literature. Arch Dermatol. 2001;137(7):917-920.
- Belinda Tan, Noah Craft, Lindy P. Fox, Lowell A. Goldsmith, Michael D. Tharp. Visual Dx. Blue Nevus. Accessed 2/11/16. Camila Roos Mariano da Rocha, Thaís Corsetti Grazziotin, Maria Carolina Widholzer Rey, Laura Luzzatto, Renan Rangel Bonamigo. Congenital agminated melanocytic nevus - Case report. An Bras Dermatol. 2013;88(6 Suppl 1):170-2.
- Chris G. Adigun, Jeffrey D. Bernhard, Sarah Stein, Karen Wiss, Sheila Galbraith, Craig N. Burkhart, Dean Morrell, Lynn Garfunkel, Nancy Esterly. Visual Dx. Agminated Nevus. Accessed 2/11/16.
- Julie V Schaffer and Jean L Bolognia. Acquired melanocytic nevi (moles). In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. (Accessed on February 11, 2016.)
- Julie V Schaffer and Jean L Bolognia. Congenital melanocytic nevi. In: UpToDate, Post TW (Ed), UpToDate, Waltham, MA. (Accessed on February 11, 2016.)
- Maria A. Pizzichetta, H. Peter Soyer, Cesare Massone, Lorenzo Cerroni. Clinical and Dermoscopic Features of Agminated Blue Nevus. Arch Dermatol. 2007;143(9):1209-1226.
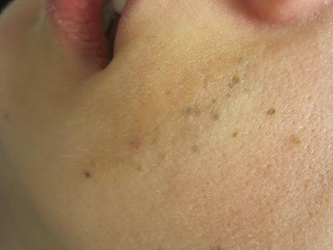
A 23-year-old healthy female presented with a several-year history of asymptomatic dark spots on her left cheek. The lesions seemed to be darkening. Incidentally, she was in a four-wheeler accident in middle school and suffered facial trauma, which resulted in the replacement of two superior left front teeth, but she denied significant cutaneous injury at that time. On physical examination, there were several clustered 1-mm blue-gray macules in linear formation with a subtle underlying tan patch on her left cheek.
Make the Diagnosis - May 2016
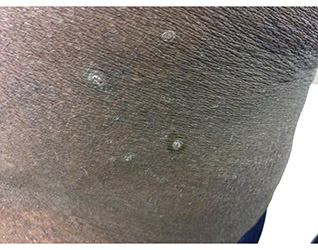
Diagnosis: Perforating disorder
Histology on this patient revealed compact hyperkeratosis and parakeratosis with an entrapped neutrophilic crust and invaginated epidermis. The epidermis showed collagen fibers and the underlying dermis was fibrotic with inflammatory cells. PAS was negative. A Verhoeff-Van Gieson (VVG) stain failed to reveal elastic tissue fibers within the crusted stratum corneum. The results were consistent with a perforating disorder.
Perforating dermatoses result from the extrusion of dermal substances through epidermal channels without any alteration of nearby histologic structures. The substances in the upper dermis induce epidermal hyperplasia that results in the epidermis surrounding the dermal materials, with eventual elimination secondary to keratinocyte maturation and sloughing. Several different forms of perforating dermatoses exist, including reactive perforating collagenosis and the acquired perforating dermatosis diagnostic group (often associated with diabetes mellitus or chronic renal disease), which includes acquired reactive perforating collagenosis (RPC), elastosis perforans serpiginosa, perforating folliculitis, and Kyrle’s disease. Both RPC and perforating folliculitis may demonstrate koebnerization (a phenomenon in which superficial trauma induces lesion formation), which may help a clinician further delineate the specific perforating dermatosis diagnosis.
Reactive perforating collagenosis (RPC) consists of both a familial and an acquired form. The familial form is typically autosomal recessive and presents in childhood but may persist into adulthood. The acquired form develops in adulthood. Clinically, RPC presents as pruritic papules (1-3 mm) with a central dark keratotic plug, which enlarge but often regress after 2 months. Lesions often occur on the extremities and face with onset after superficial trauma (associated with scratching, arthropod bites, etc.). With acquired RPC, underlying systemic diseases are often present, including renal failure, diabetes, and occasionally liver disease or malignant neoplasm. Laboratory tests to screen for these conditions should be ordered as appropriate. In managing RPC, avoiding skin trauma and controlling itching appear to be the most important interventions. Lesions may resolve without treatment, but topical retinoids also may be useful.
Elastosis perforans serpiginosa (EPS) results from changes in both elastic and collagen fibers within the dermis, resulting in a foreign body reaction with transepidermal elimination. Clinically, EPS presents around the second decade of life as erythematous umbilicated papules (2-3 mm) with a central keratin plug arranged in an annular or serpiginous pattern. Lesions usually only involve one anatomic area (frequently the neck and upper extremities). Some of the associated conditions can be remembered with the mnemonic MADPPORES: Marfan syndrome, Acrogeria, Down syndrome, Penicillamine, Pseudoxanthoma elasticum, Osteogenesis imperfecta, Rothmund-Thomson syndrome, Ehlers-Danlos (some forms), and Scleroderma. Regression of lesions often leaves hypopigmented scars. Treatment with cryotherapy may be useful, but electrocautery, dermabrasion, and surgery should be avoided.
Perforating folliculitis is characterized by adult-onset scaly follicular papules with a white central keratotic plug (may contain curled hair) on the extensor surfaces of the extremities and buttocks. Lesions may be pruritic but are more often asymptomatic. The pathophysiology includes the transfollicular extrusion of dermal debris (keratin, cellular debris, degenerated collagen, or degenerated elastic fibers), which may be expressed when the papule is compressed. Perforating folliculitis is often associated with chronic renal failure, diabetes mellitus, and other systemic diseases. The lesions may resolve after stabilization of the underlying disease, including renal transplantation. Management focuses on controlling pruritus with oral antihistamines and topical soothing lotions (menthol, phenol, or camphor), but other unsubstantiated treatments have been tried, including UVB, oral retinoids, and topical retinoids.
Kyrle’s disease (hyperkeratosis folliculitis) may have both an acquired and autosomal recessive form. In the inherited form, a genetic disturbance may accelerate epidermal keratinization, resulting in dyskeratotic foci and parakeratosis that penetrate the dermis and induce a granulomatous inflammatory reaction. The acquired form may be associated with diabetic nephropathy, hemodialysis secondary to diabetic nephropathy, congestive heart failure, and liver disease. In contrast to other perforating disorders, the extruded material is predominantly keratin rather than elastic fibers or collagen. Clinically, Kyrle’s disease presents around age 30 years as discrete, pruritic, and scaly folliculocentric papules (1-8 mm) with a hyperkeratotic plug (one-third of diameter). With removal of the plug, a cutaneous depression remains (Kyrle’s phenomenon). The lesions also may coalesce into plaques. Typically, the bilateral lower extremities and extensor surfaces are involved, but lesions may present on any area except for plantar, palmar, and mucosal surfaces. Management should focus on treating the associated medical condition, but UVB and tretinoin also have been used. Other treatment options include allopurinol, psoralen plus UVA, acitretin, phenol, doxycycline, surgical debridement, and topical corticosteroids. Regardless, lesions may resolve and leave atrophic scars.
This case and photo were submitted by Dr. Damon McClain of Three Rivers Dermatology in Coraopolis, Pa., and Mark Ash, a medical student at East Carolina University, Greenville, N.C.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to dermnews@frontlinemedcom.com.
Diagnosis: Perforating disorder
Histology on this patient revealed compact hyperkeratosis and parakeratosis with an entrapped neutrophilic crust and invaginated epidermis. The epidermis showed collagen fibers and the underlying dermis was fibrotic with inflammatory cells. PAS was negative. A Verhoeff-Van Gieson (VVG) stain failed to reveal elastic tissue fibers within the crusted stratum corneum. The results were consistent with a perforating disorder.
Perforating dermatoses result from the extrusion of dermal substances through epidermal channels without any alteration of nearby histologic structures. The substances in the upper dermis induce epidermal hyperplasia that results in the epidermis surrounding the dermal materials, with eventual elimination secondary to keratinocyte maturation and sloughing. Several different forms of perforating dermatoses exist, including reactive perforating collagenosis and the acquired perforating dermatosis diagnostic group (often associated with diabetes mellitus or chronic renal disease), which includes acquired reactive perforating collagenosis (RPC), elastosis perforans serpiginosa, perforating folliculitis, and Kyrle’s disease. Both RPC and perforating folliculitis may demonstrate koebnerization (a phenomenon in which superficial trauma induces lesion formation), which may help a clinician further delineate the specific perforating dermatosis diagnosis.
Reactive perforating collagenosis (RPC) consists of both a familial and an acquired form. The familial form is typically autosomal recessive and presents in childhood but may persist into adulthood. The acquired form develops in adulthood. Clinically, RPC presents as pruritic papules (1-3 mm) with a central dark keratotic plug, which enlarge but often regress after 2 months. Lesions often occur on the extremities and face with onset after superficial trauma (associated with scratching, arthropod bites, etc.). With acquired RPC, underlying systemic diseases are often present, including renal failure, diabetes, and occasionally liver disease or malignant neoplasm. Laboratory tests to screen for these conditions should be ordered as appropriate. In managing RPC, avoiding skin trauma and controlling itching appear to be the most important interventions. Lesions may resolve without treatment, but topical retinoids also may be useful.
Elastosis perforans serpiginosa (EPS) results from changes in both elastic and collagen fibers within the dermis, resulting in a foreign body reaction with transepidermal elimination. Clinically, EPS presents around the second decade of life as erythematous umbilicated papules (2-3 mm) with a central keratin plug arranged in an annular or serpiginous pattern. Lesions usually only involve one anatomic area (frequently the neck and upper extremities). Some of the associated conditions can be remembered with the mnemonic MADPPORES: Marfan syndrome, Acrogeria, Down syndrome, Penicillamine, Pseudoxanthoma elasticum, Osteogenesis imperfecta, Rothmund-Thomson syndrome, Ehlers-Danlos (some forms), and Scleroderma. Regression of lesions often leaves hypopigmented scars. Treatment with cryotherapy may be useful, but electrocautery, dermabrasion, and surgery should be avoided.
Perforating folliculitis is characterized by adult-onset scaly follicular papules with a white central keratotic plug (may contain curled hair) on the extensor surfaces of the extremities and buttocks. Lesions may be pruritic but are more often asymptomatic. The pathophysiology includes the transfollicular extrusion of dermal debris (keratin, cellular debris, degenerated collagen, or degenerated elastic fibers), which may be expressed when the papule is compressed. Perforating folliculitis is often associated with chronic renal failure, diabetes mellitus, and other systemic diseases. The lesions may resolve after stabilization of the underlying disease, including renal transplantation. Management focuses on controlling pruritus with oral antihistamines and topical soothing lotions (menthol, phenol, or camphor), but other unsubstantiated treatments have been tried, including UVB, oral retinoids, and topical retinoids.
Kyrle’s disease (hyperkeratosis folliculitis) may have both an acquired and autosomal recessive form. In the inherited form, a genetic disturbance may accelerate epidermal keratinization, resulting in dyskeratotic foci and parakeratosis that penetrate the dermis and induce a granulomatous inflammatory reaction. The acquired form may be associated with diabetic nephropathy, hemodialysis secondary to diabetic nephropathy, congestive heart failure, and liver disease. In contrast to other perforating disorders, the extruded material is predominantly keratin rather than elastic fibers or collagen. Clinically, Kyrle’s disease presents around age 30 years as discrete, pruritic, and scaly folliculocentric papules (1-8 mm) with a hyperkeratotic plug (one-third of diameter). With removal of the plug, a cutaneous depression remains (Kyrle’s phenomenon). The lesions also may coalesce into plaques. Typically, the bilateral lower extremities and extensor surfaces are involved, but lesions may present on any area except for plantar, palmar, and mucosal surfaces. Management should focus on treating the associated medical condition, but UVB and tretinoin also have been used. Other treatment options include allopurinol, psoralen plus UVA, acitretin, phenol, doxycycline, surgical debridement, and topical corticosteroids. Regardless, lesions may resolve and leave atrophic scars.
This case and photo were submitted by Dr. Damon McClain of Three Rivers Dermatology in Coraopolis, Pa., and Mark Ash, a medical student at East Carolina University, Greenville, N.C.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to dermnews@frontlinemedcom.com.
Diagnosis: Perforating disorder
Histology on this patient revealed compact hyperkeratosis and parakeratosis with an entrapped neutrophilic crust and invaginated epidermis. The epidermis showed collagen fibers and the underlying dermis was fibrotic with inflammatory cells. PAS was negative. A Verhoeff-Van Gieson (VVG) stain failed to reveal elastic tissue fibers within the crusted stratum corneum. The results were consistent with a perforating disorder.
Perforating dermatoses result from the extrusion of dermal substances through epidermal channels without any alteration of nearby histologic structures. The substances in the upper dermis induce epidermal hyperplasia that results in the epidermis surrounding the dermal materials, with eventual elimination secondary to keratinocyte maturation and sloughing. Several different forms of perforating dermatoses exist, including reactive perforating collagenosis and the acquired perforating dermatosis diagnostic group (often associated with diabetes mellitus or chronic renal disease), which includes acquired reactive perforating collagenosis (RPC), elastosis perforans serpiginosa, perforating folliculitis, and Kyrle’s disease. Both RPC and perforating folliculitis may demonstrate koebnerization (a phenomenon in which superficial trauma induces lesion formation), which may help a clinician further delineate the specific perforating dermatosis diagnosis.
Reactive perforating collagenosis (RPC) consists of both a familial and an acquired form. The familial form is typically autosomal recessive and presents in childhood but may persist into adulthood. The acquired form develops in adulthood. Clinically, RPC presents as pruritic papules (1-3 mm) with a central dark keratotic plug, which enlarge but often regress after 2 months. Lesions often occur on the extremities and face with onset after superficial trauma (associated with scratching, arthropod bites, etc.). With acquired RPC, underlying systemic diseases are often present, including renal failure, diabetes, and occasionally liver disease or malignant neoplasm. Laboratory tests to screen for these conditions should be ordered as appropriate. In managing RPC, avoiding skin trauma and controlling itching appear to be the most important interventions. Lesions may resolve without treatment, but topical retinoids also may be useful.
Elastosis perforans serpiginosa (EPS) results from changes in both elastic and collagen fibers within the dermis, resulting in a foreign body reaction with transepidermal elimination. Clinically, EPS presents around the second decade of life as erythematous umbilicated papules (2-3 mm) with a central keratin plug arranged in an annular or serpiginous pattern. Lesions usually only involve one anatomic area (frequently the neck and upper extremities). Some of the associated conditions can be remembered with the mnemonic MADPPORES: Marfan syndrome, Acrogeria, Down syndrome, Penicillamine, Pseudoxanthoma elasticum, Osteogenesis imperfecta, Rothmund-Thomson syndrome, Ehlers-Danlos (some forms), and Scleroderma. Regression of lesions often leaves hypopigmented scars. Treatment with cryotherapy may be useful, but electrocautery, dermabrasion, and surgery should be avoided.
Perforating folliculitis is characterized by adult-onset scaly follicular papules with a white central keratotic plug (may contain curled hair) on the extensor surfaces of the extremities and buttocks. Lesions may be pruritic but are more often asymptomatic. The pathophysiology includes the transfollicular extrusion of dermal debris (keratin, cellular debris, degenerated collagen, or degenerated elastic fibers), which may be expressed when the papule is compressed. Perforating folliculitis is often associated with chronic renal failure, diabetes mellitus, and other systemic diseases. The lesions may resolve after stabilization of the underlying disease, including renal transplantation. Management focuses on controlling pruritus with oral antihistamines and topical soothing lotions (menthol, phenol, or camphor), but other unsubstantiated treatments have been tried, including UVB, oral retinoids, and topical retinoids.
Kyrle’s disease (hyperkeratosis folliculitis) may have both an acquired and autosomal recessive form. In the inherited form, a genetic disturbance may accelerate epidermal keratinization, resulting in dyskeratotic foci and parakeratosis that penetrate the dermis and induce a granulomatous inflammatory reaction. The acquired form may be associated with diabetic nephropathy, hemodialysis secondary to diabetic nephropathy, congestive heart failure, and liver disease. In contrast to other perforating disorders, the extruded material is predominantly keratin rather than elastic fibers or collagen. Clinically, Kyrle’s disease presents around age 30 years as discrete, pruritic, and scaly folliculocentric papules (1-8 mm) with a hyperkeratotic plug (one-third of diameter). With removal of the plug, a cutaneous depression remains (Kyrle’s phenomenon). The lesions also may coalesce into plaques. Typically, the bilateral lower extremities and extensor surfaces are involved, but lesions may present on any area except for plantar, palmar, and mucosal surfaces. Management should focus on treating the associated medical condition, but UVB and tretinoin also have been used. Other treatment options include allopurinol, psoralen plus UVA, acitretin, phenol, doxycycline, surgical debridement, and topical corticosteroids. Regardless, lesions may resolve and leave atrophic scars.
This case and photo were submitted by Dr. Damon McClain of Three Rivers Dermatology in Coraopolis, Pa., and Mark Ash, a medical student at East Carolina University, Greenville, N.C.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to dermnews@frontlinemedcom.com.
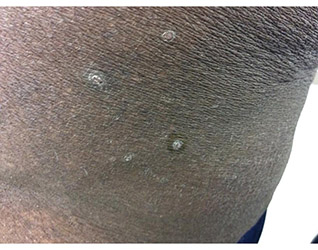
A 41-year-old black male with a past medical history of a kidney transplant, currently on dialysis and waiting for another kidney transplant, presented with a 2-month history of a pruritic rash on the upper body consisting of erythematous papules with central erosions.
Make the Diagnosis - April 2016
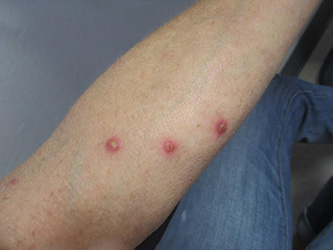
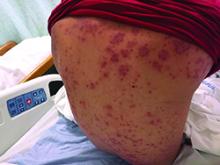
Diagnosis: Lymphomatoid papulosis
Lymphomatoid papulosis is a rare, chronic, and benign papulonodular or papulonecrotic skin disorder. LyP affects people of all ages, and its peak incidence occurs in the 5th decade. It generally has no predilection for a particular sex; however, some have reported a slight predominance in males. Patients of all races may be diagnosed with LyP, although it is less common in black patients. In addition, 10% of LyP cases are associated with anaplastic large cell lymphoma, cutaneous T-cell lymphoma (mycosis fungoides), or Hodgkin’s lymphoma.
Patients typically present with multiple erythematous papules that evolve into red-brown papulopustular or papulovesicular lesions. The papules may be mildly pruritic or asymptomatic and can be few in number to hundreds at presentation. The lesions usually appear in crops that resolve within 2-8 weeks with or without scarring, and can continue this cyclic process for months to years. The arms, legs, and trunk are most commonly affected, although LyP can present anywhere on the body.
The diagnosis of LyP is classically based upon histopathologic examination. Hematoxylin and eosin staining reveals a dense dermal infiltrate of atypical lymphocytes along with numerous eosinophils and neutrophils; lymphocytes are CD30+. Vessels in the dermis also appear with fibrin deposition, endothelial edema, and red blood cell extravasation. In addition, LyP can be classified as type A, type B, type C, and/or type D. These subtypes are determined by the size of atypical lymphocytes, type of atypical cells, location and amount of infiltrate, and CD30 and CD8 staining.
The differential diagnosis of LyP includes anaplastic large cell lymphoma, cutaneous T-cell lymphoma, folliculitis, insect bites, Langerhans cell histiocytosis, leukemia cutis, milia, miliaria, and scabies.
The etiology of LyP is unknown. It is unclear whether the proliferation of T cells is a benign and chronic disorder initiated by a stimulus or an indolent T-cell malignancy that the immune system monitors and only allows for localized, cutaneous involvement.
Mild forms of LyP can often be managed with topical corticosteroids. However, other therapies such as intralesional corticosteroids, phototherapy (UVB or PUVA), tetracycline antibiotics, and methotrexate are effective in treating cases of more persistent and widespread disease.
Our patient’s biopsy showed an irregular epidermis with scale, focal ulceration, scattered eosinophils, and dermal lymphocytes and histiocytes present in a perivascular pattern. Many of the lymphoid cells were enlarged, hyperchromatic, and irregular. Immunohistochemical staining was CD30+. These histologic changes were most consistent with lymphomatoid papulosis.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to dermnews@frontlinemedcom.com.
Diagnosis: Lymphomatoid papulosis
Lymphomatoid papulosis is a rare, chronic, and benign papulonodular or papulonecrotic skin disorder. LyP affects people of all ages, and its peak incidence occurs in the 5th decade. It generally has no predilection for a particular sex; however, some have reported a slight predominance in males. Patients of all races may be diagnosed with LyP, although it is less common in black patients. In addition, 10% of LyP cases are associated with anaplastic large cell lymphoma, cutaneous T-cell lymphoma (mycosis fungoides), or Hodgkin’s lymphoma.
Patients typically present with multiple erythematous papules that evolve into red-brown papulopustular or papulovesicular lesions. The papules may be mildly pruritic or asymptomatic and can be few in number to hundreds at presentation. The lesions usually appear in crops that resolve within 2-8 weeks with or without scarring, and can continue this cyclic process for months to years. The arms, legs, and trunk are most commonly affected, although LyP can present anywhere on the body.
The diagnosis of LyP is classically based upon histopathologic examination. Hematoxylin and eosin staining reveals a dense dermal infiltrate of atypical lymphocytes along with numerous eosinophils and neutrophils; lymphocytes are CD30+. Vessels in the dermis also appear with fibrin deposition, endothelial edema, and red blood cell extravasation. In addition, LyP can be classified as type A, type B, type C, and/or type D. These subtypes are determined by the size of atypical lymphocytes, type of atypical cells, location and amount of infiltrate, and CD30 and CD8 staining.
The differential diagnosis of LyP includes anaplastic large cell lymphoma, cutaneous T-cell lymphoma, folliculitis, insect bites, Langerhans cell histiocytosis, leukemia cutis, milia, miliaria, and scabies.
The etiology of LyP is unknown. It is unclear whether the proliferation of T cells is a benign and chronic disorder initiated by a stimulus or an indolent T-cell malignancy that the immune system monitors and only allows for localized, cutaneous involvement.
Mild forms of LyP can often be managed with topical corticosteroids. However, other therapies such as intralesional corticosteroids, phototherapy (UVB or PUVA), tetracycline antibiotics, and methotrexate are effective in treating cases of more persistent and widespread disease.
Our patient’s biopsy showed an irregular epidermis with scale, focal ulceration, scattered eosinophils, and dermal lymphocytes and histiocytes present in a perivascular pattern. Many of the lymphoid cells were enlarged, hyperchromatic, and irregular. Immunohistochemical staining was CD30+. These histologic changes were most consistent with lymphomatoid papulosis.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to dermnews@frontlinemedcom.com.
Diagnosis: Lymphomatoid papulosis
Lymphomatoid papulosis is a rare, chronic, and benign papulonodular or papulonecrotic skin disorder. LyP affects people of all ages, and its peak incidence occurs in the 5th decade. It generally has no predilection for a particular sex; however, some have reported a slight predominance in males. Patients of all races may be diagnosed with LyP, although it is less common in black patients. In addition, 10% of LyP cases are associated with anaplastic large cell lymphoma, cutaneous T-cell lymphoma (mycosis fungoides), or Hodgkin’s lymphoma.
Patients typically present with multiple erythematous papules that evolve into red-brown papulopustular or papulovesicular lesions. The papules may be mildly pruritic or asymptomatic and can be few in number to hundreds at presentation. The lesions usually appear in crops that resolve within 2-8 weeks with or without scarring, and can continue this cyclic process for months to years. The arms, legs, and trunk are most commonly affected, although LyP can present anywhere on the body.
The diagnosis of LyP is classically based upon histopathologic examination. Hematoxylin and eosin staining reveals a dense dermal infiltrate of atypical lymphocytes along with numerous eosinophils and neutrophils; lymphocytes are CD30+. Vessels in the dermis also appear with fibrin deposition, endothelial edema, and red blood cell extravasation. In addition, LyP can be classified as type A, type B, type C, and/or type D. These subtypes are determined by the size of atypical lymphocytes, type of atypical cells, location and amount of infiltrate, and CD30 and CD8 staining.
The differential diagnosis of LyP includes anaplastic large cell lymphoma, cutaneous T-cell lymphoma, folliculitis, insect bites, Langerhans cell histiocytosis, leukemia cutis, milia, miliaria, and scabies.
The etiology of LyP is unknown. It is unclear whether the proliferation of T cells is a benign and chronic disorder initiated by a stimulus or an indolent T-cell malignancy that the immune system monitors and only allows for localized, cutaneous involvement.
Mild forms of LyP can often be managed with topical corticosteroids. However, other therapies such as intralesional corticosteroids, phototherapy (UVB or PUVA), tetracycline antibiotics, and methotrexate are effective in treating cases of more persistent and widespread disease.
Our patient’s biopsy showed an irregular epidermis with scale, focal ulceration, scattered eosinophils, and dermal lymphocytes and histiocytes present in a perivascular pattern. Many of the lymphoid cells were enlarged, hyperchromatic, and irregular. Immunohistochemical staining was CD30+. These histologic changes were most consistent with lymphomatoid papulosis.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to dermnews@frontlinemedcom.com.
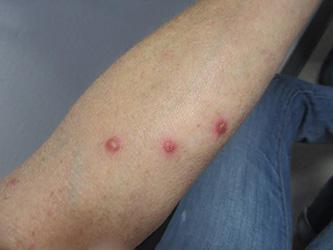
A 61-year-old woman with no significant past medical history presented with a rash on her arms. The lesions were pruritic and showed mild improvement after she took an oral antihistamine. The patient stated that she had similar outbreaks in the past that were treated with minocycline by an outside dermatologist. At that time, one lesion was cultured, showing no evidence of bacteria or herpes. She denied any history of gardening. On physical exam, she had erythematous papules and pustules in a sporotrichoid pattern on the arms, axilla, upper back, and antecubital fossa. A biopsy was performed.
Make the Diagnosis - March 2016
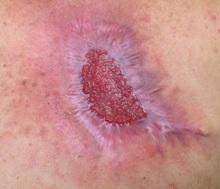
Diagnosis: Eruptive keratoacanthomas
Keratoacanthomas (KAs) most commonly affect people between the ages of 50 and 69 years old, although there have been reports in all age groups, including children. Studies have additionally revealed an equal distribution in prevalence between the sexes.
KAs are common, frequently self-limiting, epidermal tumors that consist of keratinizing squamous cells, thought to arise from the seboglandular part of the hair follicle. KAs have been divided into two general categories consisting of solitary and multiple types. Although the solitary type is most commonly observed, the multiple KAs category may be further subdivided to include the Ferguson-Smith type, which involves multiple self-healing KAs, generalized eruptive KA, which involves both skin and mucosa, multiple familial KA, multiple KA in association with Muir-Torre syndrome, and multiple KA centrifugum marginatum.
There are numerous factors implicated in the development of KAs, including trauma, light, exogenous carcinogens, impaired cell-mediated immunity, and immunosuppressive medications. A KA, which may be asymptomatic, slightly tender, or pruritic, initially forms as a small red macule and then evolves into a rapidly-growing (2 to 8 weeks) firm papule with scale. The papule then becomes a round, firm and raised skin-colored to pink nodule with a central keratin plug at the peak.
Histopathology varies depending upon the developmental stage of the lesion when biopsied. KA formation is comprised of 3 stages that may be recognized clinically and histologically, including the early-growing phase, the fully developed (stationary) phase and the senescent phase. Although not unique to KAs, histology may commonly show reactive proliferation of eccrine gland ducts beneath the tumor lobules. The ducts may adopt an adenomatoid appearance, as they lose their two-layer cellular construct.
The controversy regarding KA’s benign or malignant nature remains. Therefore, diagnosis is frequently confirmed through biopsy, in order to rule out squamous cell carcinoma. Although most KAs may resolve spontaneously, patients who find the lesions cosmetically unacceptable or painful may seek treatment. Nonsurgical modalities should be utilized before surgery, as surgical removal may leave scarring. Nonsurgical treatment options include local and systemic therapies, as well as electrodessication and curettage and laser therapy. A promising agent emerging in the treatment of KAs is 5-fluorouracil, which may be used as an intralesional injection, topically, or combined with lasers, leading to optimal cosmetic results with rapid clearance. The patient and family reported a noticeable improvement in appearance two weeks after discontinuing the new therapy.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to dermnews@frontlinemedcom.com.
Diagnosis: Eruptive keratoacanthomas
Keratoacanthomas (KAs) most commonly affect people between the ages of 50 and 69 years old, although there have been reports in all age groups, including children. Studies have additionally revealed an equal distribution in prevalence between the sexes.
KAs are common, frequently self-limiting, epidermal tumors that consist of keratinizing squamous cells, thought to arise from the seboglandular part of the hair follicle. KAs have been divided into two general categories consisting of solitary and multiple types. Although the solitary type is most commonly observed, the multiple KAs category may be further subdivided to include the Ferguson-Smith type, which involves multiple self-healing KAs, generalized eruptive KA, which involves both skin and mucosa, multiple familial KA, multiple KA in association with Muir-Torre syndrome, and multiple KA centrifugum marginatum.
There are numerous factors implicated in the development of KAs, including trauma, light, exogenous carcinogens, impaired cell-mediated immunity, and immunosuppressive medications. A KA, which may be asymptomatic, slightly tender, or pruritic, initially forms as a small red macule and then evolves into a rapidly-growing (2 to 8 weeks) firm papule with scale. The papule then becomes a round, firm and raised skin-colored to pink nodule with a central keratin plug at the peak.
Histopathology varies depending upon the developmental stage of the lesion when biopsied. KA formation is comprised of 3 stages that may be recognized clinically and histologically, including the early-growing phase, the fully developed (stationary) phase and the senescent phase. Although not unique to KAs, histology may commonly show reactive proliferation of eccrine gland ducts beneath the tumor lobules. The ducts may adopt an adenomatoid appearance, as they lose their two-layer cellular construct.
The controversy regarding KA’s benign or malignant nature remains. Therefore, diagnosis is frequently confirmed through biopsy, in order to rule out squamous cell carcinoma. Although most KAs may resolve spontaneously, patients who find the lesions cosmetically unacceptable or painful may seek treatment. Nonsurgical modalities should be utilized before surgery, as surgical removal may leave scarring. Nonsurgical treatment options include local and systemic therapies, as well as electrodessication and curettage and laser therapy. A promising agent emerging in the treatment of KAs is 5-fluorouracil, which may be used as an intralesional injection, topically, or combined with lasers, leading to optimal cosmetic results with rapid clearance. The patient and family reported a noticeable improvement in appearance two weeks after discontinuing the new therapy.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to dermnews@frontlinemedcom.com.
Diagnosis: Eruptive keratoacanthomas
Keratoacanthomas (KAs) most commonly affect people between the ages of 50 and 69 years old, although there have been reports in all age groups, including children. Studies have additionally revealed an equal distribution in prevalence between the sexes.
KAs are common, frequently self-limiting, epidermal tumors that consist of keratinizing squamous cells, thought to arise from the seboglandular part of the hair follicle. KAs have been divided into two general categories consisting of solitary and multiple types. Although the solitary type is most commonly observed, the multiple KAs category may be further subdivided to include the Ferguson-Smith type, which involves multiple self-healing KAs, generalized eruptive KA, which involves both skin and mucosa, multiple familial KA, multiple KA in association with Muir-Torre syndrome, and multiple KA centrifugum marginatum.
There are numerous factors implicated in the development of KAs, including trauma, light, exogenous carcinogens, impaired cell-mediated immunity, and immunosuppressive medications. A KA, which may be asymptomatic, slightly tender, or pruritic, initially forms as a small red macule and then evolves into a rapidly-growing (2 to 8 weeks) firm papule with scale. The papule then becomes a round, firm and raised skin-colored to pink nodule with a central keratin plug at the peak.
Histopathology varies depending upon the developmental stage of the lesion when biopsied. KA formation is comprised of 3 stages that may be recognized clinically and histologically, including the early-growing phase, the fully developed (stationary) phase and the senescent phase. Although not unique to KAs, histology may commonly show reactive proliferation of eccrine gland ducts beneath the tumor lobules. The ducts may adopt an adenomatoid appearance, as they lose their two-layer cellular construct.
The controversy regarding KA’s benign or malignant nature remains. Therefore, diagnosis is frequently confirmed through biopsy, in order to rule out squamous cell carcinoma. Although most KAs may resolve spontaneously, patients who find the lesions cosmetically unacceptable or painful may seek treatment. Nonsurgical modalities should be utilized before surgery, as surgical removal may leave scarring. Nonsurgical treatment options include local and systemic therapies, as well as electrodessication and curettage and laser therapy. A promising agent emerging in the treatment of KAs is 5-fluorouracil, which may be used as an intralesional injection, topically, or combined with lasers, leading to optimal cosmetic results with rapid clearance. The patient and family reported a noticeable improvement in appearance two weeks after discontinuing the new therapy.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to dermnews@frontlinemedcom.com.
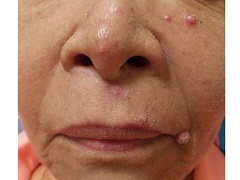
A 67 year-old female with a past medical history significant for metastatic carcinoma of the lung and synovial sarcoma presented with a 6 week history of multiple verrucous scaly and acneiform papules scattered diffusely across her face and trunk. The lesions began one month post cancer treatment with a Notch inhibitor.
Make the Diagnosis - February 2016
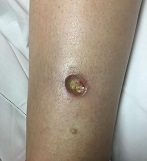
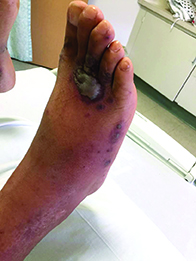
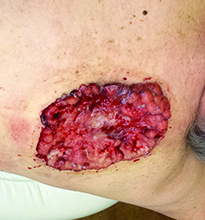
Diagnosis: Pyoderma gangrenosum
Pyoderma gangrenosum (PG) is an uncommon, noninfectious neutrophilic dermatosis that results in chronic ulcerative lesions. This disease process favors adult women and can be associated with systemic diseases in the majority of cases. The most common underlying systemic ailments include inflammatory bowel disease, arthritis, infection, and hematologic malignancy; it can also be drug induced.
Typically, the lesions begin as an erythematous pustule or nodule on an extremity. As was the case with our patient, a history of a "spider bite" or other arthropod assault may be elicited in the history as patients try to attribute a cause to the development of the initial ulceration. The pustule then develops into an ulcer with a characteristic necrotic, violaceous undermined border with a purulent base. Also, this disease process is associated with pathergy, in which minor trauma can induce additional lesions at remote sites.
There are four well-known types of pyoderma gangrenosum including the classic ulcerative lesions, pustular, bullous, and superficial granulomatous type, also known as vegetative PG. The pustular type may be seen more frequently in patients with inflammatory bowel disease, the bullous type may predominate in hematologic disorders, and the superficial granulomatous type is known to occur following surgery or other trauma.
The pathology of lesions can be nonspecific. However, in untreated lesions, widespread infiltration of neutrophils can be demonstrated at the base of the ulcers with accompanying necrosis at the periphery of lesions.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to dermnews@frontlinemedcom.com.
Diagnosis: Pyoderma gangrenosum
Pyoderma gangrenosum (PG) is an uncommon, noninfectious neutrophilic dermatosis that results in chronic ulcerative lesions. This disease process favors adult women and can be associated with systemic diseases in the majority of cases. The most common underlying systemic ailments include inflammatory bowel disease, arthritis, infection, and hematologic malignancy; it can also be drug induced.
Typically, the lesions begin as an erythematous pustule or nodule on an extremity. As was the case with our patient, a history of a "spider bite" or other arthropod assault may be elicited in the history as patients try to attribute a cause to the development of the initial ulceration. The pustule then develops into an ulcer with a characteristic necrotic, violaceous undermined border with a purulent base. Also, this disease process is associated with pathergy, in which minor trauma can induce additional lesions at remote sites.
There are four well-known types of pyoderma gangrenosum including the classic ulcerative lesions, pustular, bullous, and superficial granulomatous type, also known as vegetative PG. The pustular type may be seen more frequently in patients with inflammatory bowel disease, the bullous type may predominate in hematologic disorders, and the superficial granulomatous type is known to occur following surgery or other trauma.
The pathology of lesions can be nonspecific. However, in untreated lesions, widespread infiltration of neutrophils can be demonstrated at the base of the ulcers with accompanying necrosis at the periphery of lesions.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to dermnews@frontlinemedcom.com.
Diagnosis: Pyoderma gangrenosum
Pyoderma gangrenosum (PG) is an uncommon, noninfectious neutrophilic dermatosis that results in chronic ulcerative lesions. This disease process favors adult women and can be associated with systemic diseases in the majority of cases. The most common underlying systemic ailments include inflammatory bowel disease, arthritis, infection, and hematologic malignancy; it can also be drug induced.
Typically, the lesions begin as an erythematous pustule or nodule on an extremity. As was the case with our patient, a history of a "spider bite" or other arthropod assault may be elicited in the history as patients try to attribute a cause to the development of the initial ulceration. The pustule then develops into an ulcer with a characteristic necrotic, violaceous undermined border with a purulent base. Also, this disease process is associated with pathergy, in which minor trauma can induce additional lesions at remote sites.
There are four well-known types of pyoderma gangrenosum including the classic ulcerative lesions, pustular, bullous, and superficial granulomatous type, also known as vegetative PG. The pustular type may be seen more frequently in patients with inflammatory bowel disease, the bullous type may predominate in hematologic disorders, and the superficial granulomatous type is known to occur following surgery or other trauma.
The pathology of lesions can be nonspecific. However, in untreated lesions, widespread infiltration of neutrophils can be demonstrated at the base of the ulcers with accompanying necrosis at the periphery of lesions.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to dermnews@frontlinemedcom.com.
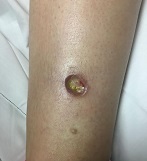
A 42-year-old woman with a 10-year history of Crohn's disease, treated with weekly subcutaneous injections of adalimumab, and hypertension presented with ulcerations on the lower extremities. She stated that the ulcerations began after she had been camping and reported being bitten by several ants during the trip, approximately 3 months earlier.
Make the Diagnosis - January 2016
Diagnosis: Urticaria pigmentosa
Urticaria pigmentosa (UP), also known as cutaneous mastocytosis, is characterized by the presence of pigmented macular and/or papular lesions associated with severe pruritis that can appear on any part of the body. With increased scratching and/or exposure to heat, the lesions become elevated. This phenomenon is known as Darier’s sign. The urticarial lesions can progress to become fluid-filled blisters. UP can rarely progress to systemic mastocytosis and present with systemic symptoms that are more common in adults, including headache, fatigue, abdominal pain, diarrhea, and tachycardia.
UP has been associated with increased inflammatory mast cells that abnormally collect in the skin. Mast cells specialize in producing histamine. In UP, the overproliferation of mast cells, secondary to point mutations in proto-oncogene c-kit binding to mast cell growth factor (MCGF), leads to an abundance of inflammatory chemicals, which produces the characteristic itching and presenting symptoms.
Although the pathophysiology of UP is known, the exact etiology is unclear. Certain medications that can cause mast-cell degranulation have been implicated, such as aspirin, NSAIDs, narcotics, alcohol, and anticholinergics. Children with allergies such as asthma are known to have an increased predisposition to UP, which typically presents in the first year of life and is self-limited by adolescence. Some cases sporadically appear, but others may be secondary to genetic inheritance as an autosomal dominant trait.
Diagnosis of UP is made by the presence of the characteristic skin lesions but can be confirmed by microscopic evaluation. A positive Darier’s sign on physical exam and lab testing for elevated histamine can aid in the diagnosis. UP is often mistaken for moles or insect bites on initial presentation; however, the persistence of the lesions for months to years is a distinguishing factor.
Given the self-limiting nature of UP, the treatment is symptomatic and supportive. Topical steroids and antihistamines can be useful to treat severe pruritis. In addition, PUVA has been shown to be an effective treatment for UP in adults.
This case and photo were submitted by Dr. Parteek Singla and Dr. Damon McClain of Naval Hospital Camp Lejeune, N.C.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to dermnews@frontlinemedcom.com.
Diagnosis: Urticaria pigmentosa
Urticaria pigmentosa (UP), also known as cutaneous mastocytosis, is characterized by the presence of pigmented macular and/or papular lesions associated with severe pruritis that can appear on any part of the body. With increased scratching and/or exposure to heat, the lesions become elevated. This phenomenon is known as Darier’s sign. The urticarial lesions can progress to become fluid-filled blisters. UP can rarely progress to systemic mastocytosis and present with systemic symptoms that are more common in adults, including headache, fatigue, abdominal pain, diarrhea, and tachycardia.
UP has been associated with increased inflammatory mast cells that abnormally collect in the skin. Mast cells specialize in producing histamine. In UP, the overproliferation of mast cells, secondary to point mutations in proto-oncogene c-kit binding to mast cell growth factor (MCGF), leads to an abundance of inflammatory chemicals, which produces the characteristic itching and presenting symptoms.
Although the pathophysiology of UP is known, the exact etiology is unclear. Certain medications that can cause mast-cell degranulation have been implicated, such as aspirin, NSAIDs, narcotics, alcohol, and anticholinergics. Children with allergies such as asthma are known to have an increased predisposition to UP, which typically presents in the first year of life and is self-limited by adolescence. Some cases sporadically appear, but others may be secondary to genetic inheritance as an autosomal dominant trait.
Diagnosis of UP is made by the presence of the characteristic skin lesions but can be confirmed by microscopic evaluation. A positive Darier’s sign on physical exam and lab testing for elevated histamine can aid in the diagnosis. UP is often mistaken for moles or insect bites on initial presentation; however, the persistence of the lesions for months to years is a distinguishing factor.
Given the self-limiting nature of UP, the treatment is symptomatic and supportive. Topical steroids and antihistamines can be useful to treat severe pruritis. In addition, PUVA has been shown to be an effective treatment for UP in adults.
This case and photo were submitted by Dr. Parteek Singla and Dr. Damon McClain of Naval Hospital Camp Lejeune, N.C.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to dermnews@frontlinemedcom.com.
Diagnosis: Urticaria pigmentosa
Urticaria pigmentosa (UP), also known as cutaneous mastocytosis, is characterized by the presence of pigmented macular and/or papular lesions associated with severe pruritis that can appear on any part of the body. With increased scratching and/or exposure to heat, the lesions become elevated. This phenomenon is known as Darier’s sign. The urticarial lesions can progress to become fluid-filled blisters. UP can rarely progress to systemic mastocytosis and present with systemic symptoms that are more common in adults, including headache, fatigue, abdominal pain, diarrhea, and tachycardia.
UP has been associated with increased inflammatory mast cells that abnormally collect in the skin. Mast cells specialize in producing histamine. In UP, the overproliferation of mast cells, secondary to point mutations in proto-oncogene c-kit binding to mast cell growth factor (MCGF), leads to an abundance of inflammatory chemicals, which produces the characteristic itching and presenting symptoms.
Although the pathophysiology of UP is known, the exact etiology is unclear. Certain medications that can cause mast-cell degranulation have been implicated, such as aspirin, NSAIDs, narcotics, alcohol, and anticholinergics. Children with allergies such as asthma are known to have an increased predisposition to UP, which typically presents in the first year of life and is self-limited by adolescence. Some cases sporadically appear, but others may be secondary to genetic inheritance as an autosomal dominant trait.
Diagnosis of UP is made by the presence of the characteristic skin lesions but can be confirmed by microscopic evaluation. A positive Darier’s sign on physical exam and lab testing for elevated histamine can aid in the diagnosis. UP is often mistaken for moles or insect bites on initial presentation; however, the persistence of the lesions for months to years is a distinguishing factor.
Given the self-limiting nature of UP, the treatment is symptomatic and supportive. Topical steroids and antihistamines can be useful to treat severe pruritis. In addition, PUVA has been shown to be an effective treatment for UP in adults.
This case and photo were submitted by Dr. Parteek Singla and Dr. Damon McClain of Naval Hospital Camp Lejeune, N.C.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to dermnews@frontlinemedcom.com.
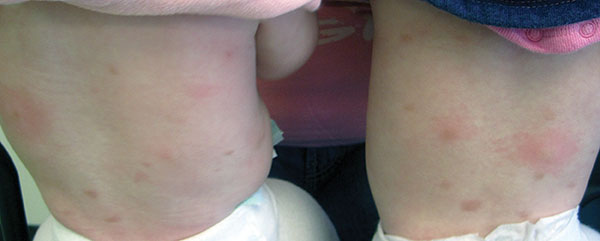
Otherwise healthy 11-month-old female twins presented with a mildly pruritic rash that had been present since birth. There was no history of prolonged nausea, diarrhea, or vomiting. There was no family history of mast cell diseases. The girls were not taking any medications. On exam, there were hyperpigmented macules, patches, and papules mostly on the trunk but also on the extremities. A Darier’s sign was noted when firmly scratching with a tongue depressor
Make the Diagnosis - December 2015
Diagnosis: Henoch-Schönlein purpura
Henoch-Schönlein purpura (HSP), also known as immunoglobulin A (IgA) vasculitis, is characterized by palpable purpura, abdominal pain, renal disease, arthritis and/or arthralgia. HSP affects all ages, but is most common in children (the mean age of onset is between six and seven years of age). It also has a slight predilection for males, as well as whites and Asians. Patients are more likely to present during the fall, winter, and spring. It is thought that HSP may be associated with infections that are more prevalent during these times of the year.
Patients typically have purpura and joint or abdominal pain, although some may present without significant skin manifestations or pain. The rash associated with HSP may appear as erythematous macules or papules that coalesce and evolve into palpable purpura with petechiae, ecchymoses, and/or subcutaneous edema. The skin is typically affected in areas that are more dependent or experience more pressure, such as the lower extremities or buttocks. A more diffuse distribution across the entire body can be seen in those who are nonambulatory.
The arthritis associated with HSP usually affects the lower extremities, and it is oligoarticular, nondeforming, and transient, with prominent swelling and tenderness. Gastrointestinal involvement may manifest as nausea, vomiting, abdominal pain, transient paralytic ileus, hemorrhage, bowel ischemia, intussusception (rare in adults), and/or bowel perforation. These symptoms tend to develop 1 week after the rash, but there are reports of gastrointestinal complaints preceding all other manifestations of HSP.
Additionally, there can be renal involvement, which is more common in adults. These patients may experience hematuria with or without red blood cell casts, nephrotic range proteinuria, and/or elevated serum creatinine. Other less common symptoms that have been reported in patients with HSP include scrotal pain and swelling, headaches, seizures, ataxia, central and peripheral neuropathies, impaired lung diffusion capacity, keratitis, and uveitis.
The diagnosis of HSP is classically based upon clinical presentation. The diagnosis may be complicated in cases in which patients do not present with classic signs, such as palpable purpura of the lower extremities and buttocks. In these situations, a biopsy of the skin or kidney may aid in the diagnosis.
The differential diagnosis for HSP includes acute hemorrhagic edema of infancy, coagulopathies, hemolytic uremic syndrome, hypersensitivity vasculitis, IgA nephropathy, immune thrombocytopenia purpura, juvenile idiopathic arthritis, reactive arthritis, rheumatic fever, small vessel vasculitis, and systemic lupus erythematosus.
The etiology of HSP is unclear. Most believe that it is an immune-mediated vasculitis with genetic and environmental influences. In HSP, there is leukocytoclastic vasculitis with deposition of IgA immune complexes, complement component 3 (C3), and fibrin in the affected vessel walls. Small vessels within the papillary dermis are usually present with an inflammatory infiltrate of neutrophils and monocytes.
The treatment of HSP usually consists of supportive care, since most patients will recover spontaneously. Therefore, hydration, rest, and pain relief are essential. Other treatment modalities may be required for more severe or extensive HSP and its complications.
Our patient’s biopsy results were consistent with a leukocytoclastic vasculitis. His biopsy revealed an inflammatory infiltrate of lymphocytes, neutrophils, and nuclear dust with extravasation of erythrocytes and fibrin in the walls of small blood vessels, along with prominent papillary dermal edema and subepidermal vesiculation. Direct immunofluorescence was positive for IgA, C3, and fibrinogen in upper dermal blood vessels. The patient also had renal involvement that was confirmed with a renal biopsy.
This case and photos are courtesy of Tanya Greywal, University of California, San Diego, and Dr. Brooke Resh Sateesh, San Diego Family Dermatology.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to dermnews@frontlinemedcom.com.
Diagnosis: Henoch-Schönlein purpura
Henoch-Schönlein purpura (HSP), also known as immunoglobulin A (IgA) vasculitis, is characterized by palpable purpura, abdominal pain, renal disease, arthritis and/or arthralgia. HSP affects all ages, but is most common in children (the mean age of onset is between six and seven years of age). It also has a slight predilection for males, as well as whites and Asians. Patients are more likely to present during the fall, winter, and spring. It is thought that HSP may be associated with infections that are more prevalent during these times of the year.
Patients typically have purpura and joint or abdominal pain, although some may present without significant skin manifestations or pain. The rash associated with HSP may appear as erythematous macules or papules that coalesce and evolve into palpable purpura with petechiae, ecchymoses, and/or subcutaneous edema. The skin is typically affected in areas that are more dependent or experience more pressure, such as the lower extremities or buttocks. A more diffuse distribution across the entire body can be seen in those who are nonambulatory.
The arthritis associated with HSP usually affects the lower extremities, and it is oligoarticular, nondeforming, and transient, with prominent swelling and tenderness. Gastrointestinal involvement may manifest as nausea, vomiting, abdominal pain, transient paralytic ileus, hemorrhage, bowel ischemia, intussusception (rare in adults), and/or bowel perforation. These symptoms tend to develop 1 week after the rash, but there are reports of gastrointestinal complaints preceding all other manifestations of HSP.
Additionally, there can be renal involvement, which is more common in adults. These patients may experience hematuria with or without red blood cell casts, nephrotic range proteinuria, and/or elevated serum creatinine. Other less common symptoms that have been reported in patients with HSP include scrotal pain and swelling, headaches, seizures, ataxia, central and peripheral neuropathies, impaired lung diffusion capacity, keratitis, and uveitis.
The diagnosis of HSP is classically based upon clinical presentation. The diagnosis may be complicated in cases in which patients do not present with classic signs, such as palpable purpura of the lower extremities and buttocks. In these situations, a biopsy of the skin or kidney may aid in the diagnosis.
The differential diagnosis for HSP includes acute hemorrhagic edema of infancy, coagulopathies, hemolytic uremic syndrome, hypersensitivity vasculitis, IgA nephropathy, immune thrombocytopenia purpura, juvenile idiopathic arthritis, reactive arthritis, rheumatic fever, small vessel vasculitis, and systemic lupus erythematosus.
The etiology of HSP is unclear. Most believe that it is an immune-mediated vasculitis with genetic and environmental influences. In HSP, there is leukocytoclastic vasculitis with deposition of IgA immune complexes, complement component 3 (C3), and fibrin in the affected vessel walls. Small vessels within the papillary dermis are usually present with an inflammatory infiltrate of neutrophils and monocytes.
The treatment of HSP usually consists of supportive care, since most patients will recover spontaneously. Therefore, hydration, rest, and pain relief are essential. Other treatment modalities may be required for more severe or extensive HSP and its complications.
Our patient’s biopsy results were consistent with a leukocytoclastic vasculitis. His biopsy revealed an inflammatory infiltrate of lymphocytes, neutrophils, and nuclear dust with extravasation of erythrocytes and fibrin in the walls of small blood vessels, along with prominent papillary dermal edema and subepidermal vesiculation. Direct immunofluorescence was positive for IgA, C3, and fibrinogen in upper dermal blood vessels. The patient also had renal involvement that was confirmed with a renal biopsy.
This case and photos are courtesy of Tanya Greywal, University of California, San Diego, and Dr. Brooke Resh Sateesh, San Diego Family Dermatology.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to dermnews@frontlinemedcom.com.
Diagnosis: Henoch-Schönlein purpura
Henoch-Schönlein purpura (HSP), also known as immunoglobulin A (IgA) vasculitis, is characterized by palpable purpura, abdominal pain, renal disease, arthritis and/or arthralgia. HSP affects all ages, but is most common in children (the mean age of onset is between six and seven years of age). It also has a slight predilection for males, as well as whites and Asians. Patients are more likely to present during the fall, winter, and spring. It is thought that HSP may be associated with infections that are more prevalent during these times of the year.
Patients typically have purpura and joint or abdominal pain, although some may present without significant skin manifestations or pain. The rash associated with HSP may appear as erythematous macules or papules that coalesce and evolve into palpable purpura with petechiae, ecchymoses, and/or subcutaneous edema. The skin is typically affected in areas that are more dependent or experience more pressure, such as the lower extremities or buttocks. A more diffuse distribution across the entire body can be seen in those who are nonambulatory.
The arthritis associated with HSP usually affects the lower extremities, and it is oligoarticular, nondeforming, and transient, with prominent swelling and tenderness. Gastrointestinal involvement may manifest as nausea, vomiting, abdominal pain, transient paralytic ileus, hemorrhage, bowel ischemia, intussusception (rare in adults), and/or bowel perforation. These symptoms tend to develop 1 week after the rash, but there are reports of gastrointestinal complaints preceding all other manifestations of HSP.
Additionally, there can be renal involvement, which is more common in adults. These patients may experience hematuria with or without red blood cell casts, nephrotic range proteinuria, and/or elevated serum creatinine. Other less common symptoms that have been reported in patients with HSP include scrotal pain and swelling, headaches, seizures, ataxia, central and peripheral neuropathies, impaired lung diffusion capacity, keratitis, and uveitis.
The diagnosis of HSP is classically based upon clinical presentation. The diagnosis may be complicated in cases in which patients do not present with classic signs, such as palpable purpura of the lower extremities and buttocks. In these situations, a biopsy of the skin or kidney may aid in the diagnosis.
The differential diagnosis for HSP includes acute hemorrhagic edema of infancy, coagulopathies, hemolytic uremic syndrome, hypersensitivity vasculitis, IgA nephropathy, immune thrombocytopenia purpura, juvenile idiopathic arthritis, reactive arthritis, rheumatic fever, small vessel vasculitis, and systemic lupus erythematosus.
The etiology of HSP is unclear. Most believe that it is an immune-mediated vasculitis with genetic and environmental influences. In HSP, there is leukocytoclastic vasculitis with deposition of IgA immune complexes, complement component 3 (C3), and fibrin in the affected vessel walls. Small vessels within the papillary dermis are usually present with an inflammatory infiltrate of neutrophils and monocytes.
The treatment of HSP usually consists of supportive care, since most patients will recover spontaneously. Therefore, hydration, rest, and pain relief are essential. Other treatment modalities may be required for more severe or extensive HSP and its complications.
Our patient’s biopsy results were consistent with a leukocytoclastic vasculitis. His biopsy revealed an inflammatory infiltrate of lymphocytes, neutrophils, and nuclear dust with extravasation of erythrocytes and fibrin in the walls of small blood vessels, along with prominent papillary dermal edema and subepidermal vesiculation. Direct immunofluorescence was positive for IgA, C3, and fibrinogen in upper dermal blood vessels. The patient also had renal involvement that was confirmed with a renal biopsy.
This case and photos are courtesy of Tanya Greywal, University of California, San Diego, and Dr. Brooke Resh Sateesh, San Diego Family Dermatology.
Dr. Bilu Martin is in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit your case for possible publication, send an email to dermnews@frontlinemedcom.com.
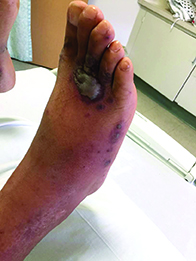
A 40-year-old male with no significant past medical history presented for progressive asymptomatic blisters on the feet, and erythematous lesions on the arms, legs, and trunk. His symptoms began with edema of the feet after a long flight to the Philippines. After a few days, erosions and bullae began forming on his dorsal feet. He was initially evaluated in the Philippines by his primary care physician,who treated him with antibiotics. The patient soon developed pruritic red bumps on his forearms and trunk. The patient was later seen in the emergency department in the United States. At that time, his physical exam was significant for large bullae on a dusky base on the bilateral dorsal feet, and scattered erythematous papules and violaceous macules with dusky centers on the legs, arms, and trunk.
Make the Diagnosis - November 2015
Diagnosis: Basal cell carcinoma
Basal cell carcinoma (BCC) is the most common skin cancer diagnosed in the United States, with approximately 2.8 million cases diagnosed annually, according to the Skin Cancer Foundation. While a minority of cases are linked to genetic syndromes (e.g., basal cell nevus syndrome), most cases result from ultraviolet sun exposure. A power law model was recently described linking ultraviolet exposure and incidence of basal cell carcinoma.
Many diagnosed BCC cases are small (< 1cm in size) and easily treated in the clinic. In cases where treatment is delayed for years due to financial, psychological, or psychiatric reasons, tumors can cause significant local tissue destruction and grow to alarming sizes.
The differential diagnosis of BCC may vary depending on the clinical sub-type (e.g., superficial, nodular, infiltrative, etc). Nodular BCC may mimic adnexal neoplasms, intradermal melanocytic nevi, Merkel cell carcinoma, or even amelanotic melanoma. Superficial BCC may mimic a lichenoid keratosis, Bowen’s disease, or other inflammatory conditions such as psoriasis and dermatitis. A larger lesion may mimic chronic infections such as mycetoma, or distant metastases from another primary carcinoma (breast or renal). Location and history can assist with teasing out the probable cause.
While diagnosis of this lesion can be made based on history and clinical appearance, this is best assisted with a biopsy, preferably a punch or incisional biopsy as chronic scarring and pseudoepitheliomatous hyperplasia may affect the pathologic diagnosis. Bacteria often colonize these large tumors and can lead to secondary infections. In this patient, maggots were noted in the skin.
While there are multiple modalities available for treating small tumors (e.g., topical imiquimod, 5-fluorouracil, electrodessication and curettage, excision, Mohs), larger tumors are often handled differently. While some might consider radiation therapy in this case, typically Mohs surgery is the treatment of choice. Oral vismodegib, a smoothened inhibitor, has been marketed for locally advanced basal cell carcinoma and has been used as a primary or adjunctive therapy with Mohs in tumors of this size. One important factor determining the choice of treatment is whether the primary tumor has spread. In tumors who have been left untreated for a long time, it is reasonable to image the patient to evaluate for metastases.
We present this case as an example where Mohs provided immediate definitive treatment. This tumor was cleared in 1 stage, with 67 slides read to evaluate the entire peripheral and deep margin. The Mohs surgery was performed under local anesthesia with no additional sedatives and 3-0 nylon sutures were used to approximate the wound. A two month follow-up photo is shown showing an almost healed surgical wound with an acceptable cosmetic result.
Diagnosis: Basal cell carcinoma
Basal cell carcinoma (BCC) is the most common skin cancer diagnosed in the United States, with approximately 2.8 million cases diagnosed annually, according to the Skin Cancer Foundation. While a minority of cases are linked to genetic syndromes (e.g., basal cell nevus syndrome), most cases result from ultraviolet sun exposure. A power law model was recently described linking ultraviolet exposure and incidence of basal cell carcinoma.
Many diagnosed BCC cases are small (< 1cm in size) and easily treated in the clinic. In cases where treatment is delayed for years due to financial, psychological, or psychiatric reasons, tumors can cause significant local tissue destruction and grow to alarming sizes.
The differential diagnosis of BCC may vary depending on the clinical sub-type (e.g., superficial, nodular, infiltrative, etc). Nodular BCC may mimic adnexal neoplasms, intradermal melanocytic nevi, Merkel cell carcinoma, or even amelanotic melanoma. Superficial BCC may mimic a lichenoid keratosis, Bowen’s disease, or other inflammatory conditions such as psoriasis and dermatitis. A larger lesion may mimic chronic infections such as mycetoma, or distant metastases from another primary carcinoma (breast or renal). Location and history can assist with teasing out the probable cause.
While diagnosis of this lesion can be made based on history and clinical appearance, this is best assisted with a biopsy, preferably a punch or incisional biopsy as chronic scarring and pseudoepitheliomatous hyperplasia may affect the pathologic diagnosis. Bacteria often colonize these large tumors and can lead to secondary infections. In this patient, maggots were noted in the skin.
While there are multiple modalities available for treating small tumors (e.g., topical imiquimod, 5-fluorouracil, electrodessication and curettage, excision, Mohs), larger tumors are often handled differently. While some might consider radiation therapy in this case, typically Mohs surgery is the treatment of choice. Oral vismodegib, a smoothened inhibitor, has been marketed for locally advanced basal cell carcinoma and has been used as a primary or adjunctive therapy with Mohs in tumors of this size. One important factor determining the choice of treatment is whether the primary tumor has spread. In tumors who have been left untreated for a long time, it is reasonable to image the patient to evaluate for metastases.
We present this case as an example where Mohs provided immediate definitive treatment. This tumor was cleared in 1 stage, with 67 slides read to evaluate the entire peripheral and deep margin. The Mohs surgery was performed under local anesthesia with no additional sedatives and 3-0 nylon sutures were used to approximate the wound. A two month follow-up photo is shown showing an almost healed surgical wound with an acceptable cosmetic result.
Diagnosis: Basal cell carcinoma
Basal cell carcinoma (BCC) is the most common skin cancer diagnosed in the United States, with approximately 2.8 million cases diagnosed annually, according to the Skin Cancer Foundation. While a minority of cases are linked to genetic syndromes (e.g., basal cell nevus syndrome), most cases result from ultraviolet sun exposure. A power law model was recently described linking ultraviolet exposure and incidence of basal cell carcinoma.
Many diagnosed BCC cases are small (< 1cm in size) and easily treated in the clinic. In cases where treatment is delayed for years due to financial, psychological, or psychiatric reasons, tumors can cause significant local tissue destruction and grow to alarming sizes.
The differential diagnosis of BCC may vary depending on the clinical sub-type (e.g., superficial, nodular, infiltrative, etc). Nodular BCC may mimic adnexal neoplasms, intradermal melanocytic nevi, Merkel cell carcinoma, or even amelanotic melanoma. Superficial BCC may mimic a lichenoid keratosis, Bowen’s disease, or other inflammatory conditions such as psoriasis and dermatitis. A larger lesion may mimic chronic infections such as mycetoma, or distant metastases from another primary carcinoma (breast or renal). Location and history can assist with teasing out the probable cause.
While diagnosis of this lesion can be made based on history and clinical appearance, this is best assisted with a biopsy, preferably a punch or incisional biopsy as chronic scarring and pseudoepitheliomatous hyperplasia may affect the pathologic diagnosis. Bacteria often colonize these large tumors and can lead to secondary infections. In this patient, maggots were noted in the skin.
While there are multiple modalities available for treating small tumors (e.g., topical imiquimod, 5-fluorouracil, electrodessication and curettage, excision, Mohs), larger tumors are often handled differently. While some might consider radiation therapy in this case, typically Mohs surgery is the treatment of choice. Oral vismodegib, a smoothened inhibitor, has been marketed for locally advanced basal cell carcinoma and has been used as a primary or adjunctive therapy with Mohs in tumors of this size. One important factor determining the choice of treatment is whether the primary tumor has spread. In tumors who have been left untreated for a long time, it is reasonable to image the patient to evaluate for metastases.
We present this case as an example where Mohs provided immediate definitive treatment. This tumor was cleared in 1 stage, with 67 slides read to evaluate the entire peripheral and deep margin. The Mohs surgery was performed under local anesthesia with no additional sedatives and 3-0 nylon sutures were used to approximate the wound. A two month follow-up photo is shown showing an almost healed surgical wound with an acceptable cosmetic result.
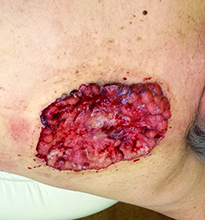
Case and photo courtesy of: Andrew R. Styperek MD; Houston Methodist Hospital and DermSurgery Associates, Houston TX Arash Kimyai-Asadi MD; DermSurgery Associates, Houston TX Dr. Bilu Martin is in private practice at Premier Dermatology, MD in Aventura, Fla. To submit your case for possible publication, send an e-mail to dermnews@frontlinemedcom.com. A 65 year old Caucasian male arrived with a decades-long history of a lesion on his back measuring 25 x 21 centimeters. In the last few years he has noticed discharge and an unpleasant smell. He denied any fatigue, shortness of breath, lymph node enlargement, or any other systemic symptoms. He had a history of hyperlipidemia and hypertension, which were controlled with daily oral medications. The patient was not taking aspirin or any other anticoagulant therapy.