User login
New myeloma drugs improve response and extend survival
In this interview, Dr David Henry, the Editor-in-Chief of The Journal of Community and Supportive Oncology, and Dr Ken Anderson, the Kraft Family Professor of Medicine at Harvard Medical School and an international thought leader and investigator in myeloma, discuss three cases of patients with myeloma that are indicative of the remarkable therapeutic advances in oncology in general, and in myeloma in particular.
Listen to the podcast below
In this interview, Dr David Henry, the Editor-in-Chief of The Journal of Community and Supportive Oncology, and Dr Ken Anderson, the Kraft Family Professor of Medicine at Harvard Medical School and an international thought leader and investigator in myeloma, discuss three cases of patients with myeloma that are indicative of the remarkable therapeutic advances in oncology in general, and in myeloma in particular.
Listen to the podcast below
In this interview, Dr David Henry, the Editor-in-Chief of The Journal of Community and Supportive Oncology, and Dr Ken Anderson, the Kraft Family Professor of Medicine at Harvard Medical School and an international thought leader and investigator in myeloma, discuss three cases of patients with myeloma that are indicative of the remarkable therapeutic advances in oncology in general, and in myeloma in particular.
Listen to the podcast below
New myeloma drugs improve response and extend survival
DR HENRY I thought we might discuss some cases of patients with myeloma, starting with a relatively simple case and ending with one that is a little more complicated. For the first case, we have a 56-year-old healthy man with IgG kappa myeloma whose work-up shows he has multiple lytic bone lesions. He has normal renal function, normal calcium, and he’s transplant-eligible by other health issues. I’ll leave the cytogenetics up to you if that changes your approach. How would you develop or pose some options for this man’s treatment to begin with?
DR ANDERSON It’s important to start out by saying that we, in myeloma, have many new classes of drugs and many new opportunities to choose from to treat this patient.1 As you know, we have proteasome inhibitors, the first-generation bortezomib, then carfilzomib and ixazomib. We have the immunomodulatory drugs (IMiDs), thalidomide, and now lenalidomide and pomalidomide. We have a histone deacetylase (HDAC) inhibitor approved called panobinostat, and we have 2 monoclonal antibodies approved, elotuzumab and daratumumab. These classes of medicine have made it possible for 20 different Food and Drug Administration (FDA) approvals in the last 10-15 years. These agents, having been tested in advanced myeloma, have moved toward initial management.
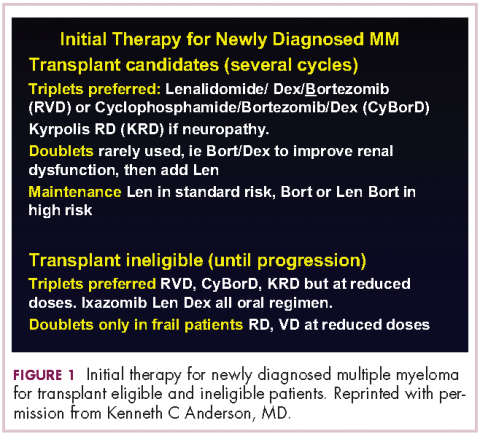
This person is 50 years old. He has adequate liver, heart, lung, and kidney function, so he would be eligible for high-dose therapy and stem-cell transplantation. In terms of initial management, there are many options (Figure 1). We strongly recommend that triplet therapy be used initially. The most common triplets would be lenalidomide, bortezomib, and dexamethasone (RVD)2,3 or cyclophosphamide, bortezomib, and dexamethasone (CyBorD).4 If this man had neuropathy, perhaps carfilzomib, the second-generation proteasome inhibitor, with lenalidomide and dexamethasone could have been used. Why do we use these? The extent and frequency of response with these triplets is nearly universal overall response rate, with three-quarters very good partial and half-complete responses, including minimal residual disease negative responses. In this patient, we would therefore recommend treatment with either RVD or CyBorD for several cycles to maximal response.
He would then have autologous stem cells collected, and it is still the standard of care to proceed to high-dose melphalan and a single high-dose therapy and stem-cell transplantation. The cytogenetics are important: if this patient has standard-risk multiple myeloma, then lenalidomide maintenance would be given after transplant. It is now FDA-approved for this purpose because it can prolong both progression-free and – most importantly – overall survival.5 Standard-risk cytogenetics might, for example, include hyperdiploidy or translocation 11;14. On the other hand, if his myeloma were high-risk and characterized, for example, by 17p deletion, we would carry out the same induction and transplantation, but we would alter the maintenance to incorporate a proteasome inhibitor. Lenalidomide and bortezomib, for example, could be combined. Early data show that using combined maintenance therapy with lenalidomide and bortezomib, can overcome the early relapses that are characteristic of high-risk disease.6
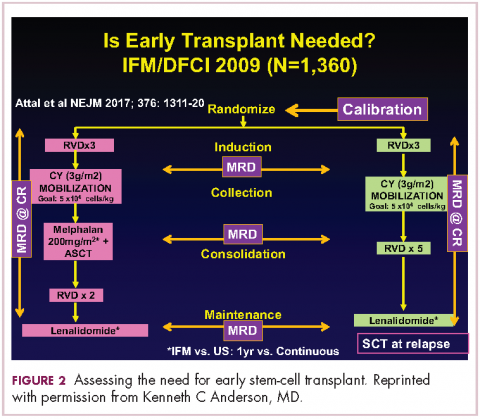
Because of the extent and frequency of response to combination novel therapies, we have undertaken with our French colleagues a clinical trial of RVD in newly diagnosed patients – such as this patient – followed by stem-cell collection in all patients (Figure 2). Then there is a randomization to either early high-dose therapy, melphalan, and autologous stem-cell transplantation, followed by lenalidomide maintenance; or in the other cohort, harvesting of stem cells, additional RVD, and then maintenance with lenalidomide, saving the stem-cell transplant for later.
The French portion of this trial was reported in the New England Journal of Medicine earlier in 2017.7 It showed that patients who received RVD, high-dose melphalan, stem-cell transplant, and had 1 year of lenalidomide maintenance, had a progression-free survival advantage of about 1 year, without an overall survival advantage; compared with those patients who received RVD and lenalidomide maintenance, saving the transplant for later. I would hasten to add that lenalidomide maintenance was given for only 1 year in this trial, and patients in the RVD-only or RVD-and-transplant arms of this trial relapsed after the lenalidomide maintenance was discontinued.
The American portion of this trial is identical. That is, RVD induction is being given and all patients have a stem-cell collection. Half of the patients then go to high-dose melphalan and stem-cell transplant early, and half of them have the transplant only later at the time of relapse. A major difference, however, is that in both the RVD-only and RVD-and-transplant cohorts, patients receive lenalidomide maintenance until progression. This trial has been ongoing since 2009 and is still ongoing, which tells us that patients in both arms – the RVD-only as well as the RVD-and-transplant arms – are doing well.
In the recent STAMINA trial, all patients underwent a single high-dose therapy and transplant. Then there was a randomization to lenalidomide maintenance only in 1 cohort; a randomization to consolidation with RVD posttransplant followed by lenalidomide maintenance in the second cohort; or a randomization to a second high-dose melphalan and stem-cell transplant followed by lenalidomide maintenance in the third cohort.8 I mention this because the outcomes in all 3 cohorts was similar.
I believe this tells us strongly that high-dose therapy and stem-cell transplantation twice – so-called tandem transplant – is no longer a major option in multiple myeloma. For now, however, in this patient, the standard of care would be to undergo induction therapy with triplet, novel combination treatment. Then, stem cells would be collected and high-dose therapy stem-cell transplant would be done, followed either by lenalidomide maintenance for standard disease or lenalidomide and bortezomib maintenance for high-risk disease. We won’t really know if we can delay transplant until the trials I’ve mentioned totally read out. In my clinical practice, if patients have had a major response to their induction therapy and have stem cells harvested, we can then offer them the opportunity to use maintenance therapy and save the transplant as a potential option for later, when myeloma relapses.
DR HENRY In summary then, this would be, in 2017, off-protocol while the data is pending: it’s reasonable to get a deep induction response, collect stem cells, have a discussion with the patient, and then consider high-dose therapy or not.
DR ANDERSON Yes. I think it’s reasonable to discuss it. We need to be open and honest with patients that the standard of care remains transplant, that you incorporate novel treatments before the transplant and novel treatments as maintenance after the transplant. The happy news is that the outcome, especially for patients who have standard-risk myeloma, is at least a decade or longer progression-free survival. It’s an optimistic picture. The data in terms of transplant being needed or not, will come within the next several years.
For now, it is a standard of care to use 1 high-dose melphalan and stem-cell transplant in this setting. I will add into our discussion with patients – besides the opportunity to harvest stem cells and think about whether one needs to do a transplant early on or not – is the issue of toxicity. High-dose melphalan by itself has a small but real secondary incidence of cancer, myelodysplasia, or leukemia. If one uses lenalidomide maintenance after melphalan transplantation treatment, that risk of secondary cancer is slightly increased.
In my experience, if patients have achieved a complete response with induction therapy only, it’s not unreasonable to offer early transplant and be clear that’s the standard of care. The alternative is maintenance with lenalidomide, knowing once the stem cells have been harvested, that transplantation can be an option to treat relapsed myeloma. We have many other options available as well. Time will tell in terms of the ongoing randomized trials as to whether transplant remains central to our treatment paradigm.
DR HENRY This leads us to our second patient. Here we have an older man of 74 years. He’s a professional piano player, so we want to try to avoid peripheral neuropathy in him. He has some mild renal insufficiency and some coronary artery disease, so he’s deemed transplant-ineligible. He has IgG kappa myeloma, and he’s brand new. What would you consider to be options for him for treatment?
DR ANDERSON This brings up the issue of a transplant-ineligible patient. He has significant comorbidity that would make transplantation an increased risk. What we would recommend in such a patient is still triplet induction therapy incorporating novel agents (Figure 1). Lenalidomide, the immunomodulatory drug, can safely be given in the context of neuropathy because it does not cause significant neuropathy. It would need to be dose modified, depending on the degree of renal insufficiency. We would recommend also including proteasome inhibitors. Bortezomib, the first-generation proteasome inhibitor, would be contraindicated because it does have a small but real attendant neuropathy. If, however, it is given weekly and subcutaneously, the risk of attendant neuropathy is quite low. In this patient, therefore, one could start with lenalidomide and bortezomib weekly and subcutaneously,1,2 with a very early and vigilant follow-up for the earliest signs of neuropathy, so as not to allow it to develop and compromise his career.
Alternatively, one could use a proteasome inhibitor that does not have attendant neuropathy. Carfilzomib, the second-generation proteasome inhibitor, does not have neuropathy.9 But we would need to have caution here, because this patient has a history of coronary artery disease, and carfilzomib has a very small, but real, incidence of cardiac toxicity so would need to be used judiciously in this setting. The third proteasome inhibitor, ixazomib, is the next-generation bortezomib-class proteasome inhibitor, and it’s oral.10 It has less neuropathy than does bortezomib, so in my view is a very realistic option for him together with lenalidomide. It does have a small incidence of neuropathy, so close monitoring for neuropathy would be indicated. We could use lenalidomide–dexamethasone as a doublet and avoid neuropathy,11 but usually doublets are reserved only for frail patients.
My recommendation, therefore, would be RVD with the bortezomib weekly or subcutaneously, or alternatively, lenalidomide, ixazomib, dexamethasone as an all-oral regimen as induction therapy. In my view, this 74-year-old patient with comorbidity is not a transplant candidate. However, one can be very optimistic with this patient. The likelihood that he could have myeloma as a chronic illness and die from something else is quite high. Initial induction triplet therapy would achieve a very high response extent and frequency. The durability would be long, especially with lenalidomide maintenance if it’s standard-risk myeloma or lenalidomide and a proteasome inhibitor, probably ixazomib in this setting, if he were to have high-risk myeloma.
If myeloma relapses, then there are many options that could be used in this patient and achieve years of progression-free and overall survival. Indeed, he is 74 years old and will respond very well to induction triplet therapy, with many years’ duration of response due to continuous lenalidomide or lenalidomide and proteasome inhibitor maintenance. Then there are many effective options to treat relapsed therapy using triplet novel agents. Therefore, his lifespan is unlikely to be shortened by multiple myeloma.
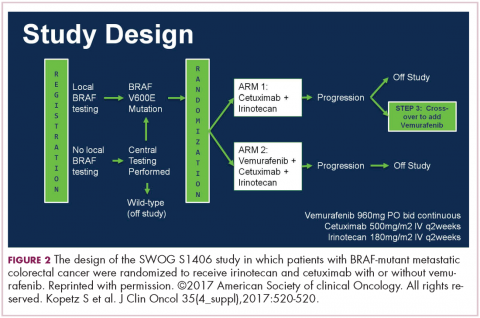
DR HENRY It’s so incredible compared with what it was when I trained. The next patient, a 45-year-old woman with IgG lambda myeloma, has had RVD induction and responded. She had lenalidomide maintenance, but then she progressed, and she got her stem-cell transplant, and she’s progressing after that. I guess we’re looking here to fold in some of the newer agents. How you would you do that in this patient?DR ANDERSON Yes. I think one of the most remarkable and exciting developments with myeloma is the rapid approval of the novel classes of agents that I mentioned earlier – the proteasome inhibitors, the immunomodulatory drugs, the HDAC inhibitor, and the monoclonal antibodies.1 They’re particularly relevant in a patient such as this one, whose myeloma has relapsed after what would be considered standard therapy for a young person with standard-risk myeloma. This patient had RVD and maintenance therapy, and then progressed. The transplant was given for relapsed myeloma. The opportunity to use stem-cell transplant in patients when myeloma becomes active after maintenance should not be forgotten as it can be very effective. In all the trials done to date in which early versus late transplant are compared, there have been similar outcomes. Therefore, if the transplant isn’t done early, don’t forget that it’s an option at the time the myeloma progresses. I do want to mention, that there are lots of options for relapsed myeloma (Figures 3 and 4). I mentioned RVD or CyBorD as initial triplet therapies.2-4 In North America, those are the 2 most common regimens. If myeloma then relapses and is resistant to RVD or to CyBorD, then we need to identify alternatives.
We also need to think about the comorbidities in the patient – issues such as age, neuropathy, presence of renal dysfunction, and other clinical factors. And we need to think about what treatment they’ve had in the past. This patient has had RVD, maintenance with lenalidomide, and a stem-cell transplant. We can offer patients a variety of therapies, but in the context of resistance to the first-generation proteasome inhibitor bortezomib and the first-generation immunomodulatory drug lenalidomide, we would strongly recommend the second-generation immunomodulatory drug pomalidomide12 together with a second-generation proteasome inhibitor, be that carfilzomib13 or ixazomib.14 When one uses the second-generation IMiDs and proteasome inhibitors together, there’s a very high frequency of response in the order of 70%-80%, which lasts years.
Besides carfilzomib and ixazomib proteasome inhibitors, we also have elotuzumab and daratumumab, the monoclonal antibodies.15-17 These agents have been FDA approved to treat patients such as this one who has had 1-3 previous therapies for their myeloma. All of them have been approved in randomized phase 3 trials compared with lenalidomide-dexamethasone in the control arm.13-15,17 They’ve all been found to be superior. Although lenalidomide-dexamethasone combined with daratumumab, ixazomib, elotuzumab, or carfilzomib is superior to lenalidomide in relapsed myeloma, the situation in North America, as in this patient, is usually that patients have had lenalidomide-dexamethasone as part of their initial treatment and their myeloma is refractory to lenalidomide.
Hence, we recommend, that we go to the second-generation pomalidomide and second-generation proteasome inhibitors, either carfilzomib or ixazomib. Having said that, the treatment paradigm is evolving. For example, the monoclonal antibody daratumumab was initially approved by the FDA in multiply relapsed disease as a single agent because it achieves a 30% response rate.16 It now has been moved earlier into the first relapse of multiple myeloma, where it achieves much higher response rates when combined with lenalidomide–dexamethasone or combined with bortezomib–dexamethasone.17,18 Response rates of 70%-80% can be achieved, including minimal residual disease negative complete responses.
Today, in a patient who has had RVD transplant and myeloma has returned, we would recommend second-generation IMiDs, pomalidomide, and second-generation proteasome inhibitors, either carfilzomib or ixazomib. Data for daratumumab combined with lenalidomide-dexamethasone or with bortezomib-dexamethasone, look very promising. We need, however, to see more experience of daratumumab together with lenalidomide-dexamethasone or daratumumab together with bortezomib-dexamethasone in patients whose myeloma is refractory to RVD, that is, patients whose myeloma has returned after RVD induction treatment. Of note, pomalidomide, dexamethasone, and daratumumab have just been approved by the FDA and may also be active even in myeloma recurring after RVD treatment.19
Daratumumab in combination will be moving earlier and earlier and may be appropriate to treat the first relapse. I do want to stress, however, that at present I save daratumumab for the second or greater relapse. Daratumumab is active even when relapse occurs after treatment with second-generation IMiDs and proteasome inhibitors.
DR HENRY Before we close, I have a couple practical questions with these antibodies. Daratumumab has the track record of first-treatment severe reactions and long infusion times. How long are you anticipating the first daratumumab treatment takes? There has been some talk that maybe splitting it in half and going over 2 days is easier on the patient and the infusion center. Have you done that?
DR ANDERSON Yes, I think that’s a very important point. We need to be thinking – first and foremost – about efficacy of our therapy. Equally important, however, are the safety profile and the user-friendliness for the patient. Daratumumab infusions are quite long – on the order of 8 hours or longer on day 1 of infusion. And to date, all the clinical trials have used daratumumab infusions weekly for 8 treatments, followed by 8 treatments given every 2 weeks. Then monthly daratumumab is given as a maintenance therapy. Thus, there is a requirement for multiple outpatient clinic visits that can be prolonged.
One of the opportunities that’s being tested is to give daratumumab subcutaneously. While this is being evaluated in protocols now, the results that have been reported at our national meetings look to be quite promising in terms of efficacy, similar to results with the intravenous administration. Obviously, this would allow for a much more convenient clinic visit and shorter time for the patients being treated.
I should mention that the other antibody, elotuzumab, has been approved in combination with lenalidomide and dexamethasone.15 The infusions with lenalidomide, dexamethasone, and the antibody elotuzumab are much shorter, on the order of 2- or 3-hour visits. The place for elotuzumab in the management of relapsed myeloma is yet to be totally defined. We tend to use it now in the setting of more indolent relapses, where patients might have a slowly rising monoclonal protein. Elotuzumab-lenalidomide-dexamethasone has maintained an overall survival advantage at 4 years compared with lenalidomide-dexamethasone when used in relapsed myeloma.
We are quite excited about both antibodies. Daratumumab tends to get most of the activity, as it achieves responses as a single agent,16 and the depth of the responses are markedly increased when it’s combined with lenalidomide-dexamethasone or bortezomib-dexamethasone.17,18 However, one shouldn’t forget elotuzumab15 based on its tolerability and the survival advantage I mentioned at 4 years.
The final point is that we think about myeloma genetically at the time of diagnosis and relapse in terms of standard or high-risk disease. One of the hallmarks of high-risk disease has been 17P deletion or P53 dysfunction. One of the most exciting outcomes of the development of monoclonal antibodies has been the responses observed even in the context of P53 deletion. Clearly, antibody-mediated cellular cytotoxicity, complement-mediated cytotoxicity, and other mechanisms of action of these antibodies do not require normal P53 function. The important point, therefore, is that what has previously been thought of as high-risk disease can nowadays be effectively treated with these new immune treatments, correlating with the marked improvement in survival and overall outcome.
DR HENRY We have outlined 3 kinds of myeloma patients we see, and especially interesting is the last patient, who has relapsed and then progressed, and in whom newer drugs have a role. Thank you for such a complete and thorough discussion, Dr Anderson.
1. Kumar SK, Callander NS, Alsina M, et al. Multiple Myeloma, Version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:230-269.
2. Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly-diagnosed multiple myeloma. Blood. 2010;116:679-686.
3. Durie BG, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777: a randomized, open-label, phase 3 trial. Lancet. 2017;389(10068):519-527.
4. Moreau P, Hulin C, Macro M, et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood. 2016;127:2569-2574.
5. McCarthy PL, Owzar K, Hofmeister CC, et al. A phase III study of lenalidomide after transplant for multiple myeloma. N Engl J Med. 2012;366:1770-1781.
6. Nooka AK, Kaufman JL, Muppidi S, et al. Consolidation and maintenance therapy with lenalidomide, bortezomib, and dexamethasone (RVD) in high risk myeloma patients. Leukemia. 2014;28:690-693.
7. Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, bortezomib and dexamethasone with transplantation in myeloma. N Engl J Med. 2017;376:1311-1320.
8. Stadtmauer EA, Pasquini MC, Blackwell B, et al. Comparison of autologous hematopoietic cell transplant (autoHCT), bortezomib, lenalidomide (len) and dexamethasone (RVD) consolidation with len maintenance (ACM), tandem autohct with len maintenance (TAM) and autohct with len maintenance (AM) for up-front treatment of patients with multiple myeloma (MM): primary results from the randomized phase III trial of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 0702 – StaMINA Trial). Abstract LBA-1. Presented at the 2016 ASH Annual Meeting, December 6, 2016; San Diego, CA.
9. Dytfeld D, Jasielec J, Griffith KA, et al: Carfilzomib, lenalidomide, and low-dose dexamethasone in elderly patients with newly diagnosed multiple myeloma. Haematologica. 2014;99:e162-164.
10. Kumar SK, Berdeja JG, Niesvizky R, et al. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 2014;15:1503-1512
11. Benboubker L, Dimopoulos MA, Dispenzieri A, et al. for the FIRST Trial Team. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906-917.
12. Richardson P, Siegel D, Vij R, et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood. 2014;123:1826-1832.
13. Stewart AK, Rajkumar SV, Dimopoulos MA, et al. for the ASPIRE Investigators. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142-152
14. Moreau P, Masszi T, Grzasko N, et al. for the TOURMALINE-MM1 Study Group. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;28;374:1621-1634.
15. Lonial S, Dimopoulos M, Palumbo A, et al. for the ELOQUENT-2 Investigators. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621-631.
16. Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD 38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373:1207-1219.
17. Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319-1331.
18. Palumbo A, Chanan-Khan A, Weisel K, et al. for the CASTOR Investigators. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754-766.
19. Chari A, Suvannasankha A, Fay JW, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130(8):974-981.
DR HENRY I thought we might discuss some cases of patients with myeloma, starting with a relatively simple case and ending with one that is a little more complicated. For the first case, we have a 56-year-old healthy man with IgG kappa myeloma whose work-up shows he has multiple lytic bone lesions. He has normal renal function, normal calcium, and he’s transplant-eligible by other health issues. I’ll leave the cytogenetics up to you if that changes your approach. How would you develop or pose some options for this man’s treatment to begin with?
DR ANDERSON It’s important to start out by saying that we, in myeloma, have many new classes of drugs and many new opportunities to choose from to treat this patient.1 As you know, we have proteasome inhibitors, the first-generation bortezomib, then carfilzomib and ixazomib. We have the immunomodulatory drugs (IMiDs), thalidomide, and now lenalidomide and pomalidomide. We have a histone deacetylase (HDAC) inhibitor approved called panobinostat, and we have 2 monoclonal antibodies approved, elotuzumab and daratumumab. These classes of medicine have made it possible for 20 different Food and Drug Administration (FDA) approvals in the last 10-15 years. These agents, having been tested in advanced myeloma, have moved toward initial management.
This person is 50 years old. He has adequate liver, heart, lung, and kidney function, so he would be eligible for high-dose therapy and stem-cell transplantation. In terms of initial management, there are many options (Figure 1). We strongly recommend that triplet therapy be used initially. The most common triplets would be lenalidomide, bortezomib, and dexamethasone (RVD)2,3 or cyclophosphamide, bortezomib, and dexamethasone (CyBorD).4 If this man had neuropathy, perhaps carfilzomib, the second-generation proteasome inhibitor, with lenalidomide and dexamethasone could have been used. Why do we use these? The extent and frequency of response with these triplets is nearly universal overall response rate, with three-quarters very good partial and half-complete responses, including minimal residual disease negative responses. In this patient, we would therefore recommend treatment with either RVD or CyBorD for several cycles to maximal response.
He would then have autologous stem cells collected, and it is still the standard of care to proceed to high-dose melphalan and a single high-dose therapy and stem-cell transplantation. The cytogenetics are important: if this patient has standard-risk multiple myeloma, then lenalidomide maintenance would be given after transplant. It is now FDA-approved for this purpose because it can prolong both progression-free and – most importantly – overall survival.5 Standard-risk cytogenetics might, for example, include hyperdiploidy or translocation 11;14. On the other hand, if his myeloma were high-risk and characterized, for example, by 17p deletion, we would carry out the same induction and transplantation, but we would alter the maintenance to incorporate a proteasome inhibitor. Lenalidomide and bortezomib, for example, could be combined. Early data show that using combined maintenance therapy with lenalidomide and bortezomib, can overcome the early relapses that are characteristic of high-risk disease.6
Because of the extent and frequency of response to combination novel therapies, we have undertaken with our French colleagues a clinical trial of RVD in newly diagnosed patients – such as this patient – followed by stem-cell collection in all patients (Figure 2). Then there is a randomization to either early high-dose therapy, melphalan, and autologous stem-cell transplantation, followed by lenalidomide maintenance; or in the other cohort, harvesting of stem cells, additional RVD, and then maintenance with lenalidomide, saving the stem-cell transplant for later.
The French portion of this trial was reported in the New England Journal of Medicine earlier in 2017.7 It showed that patients who received RVD, high-dose melphalan, stem-cell transplant, and had 1 year of lenalidomide maintenance, had a progression-free survival advantage of about 1 year, without an overall survival advantage; compared with those patients who received RVD and lenalidomide maintenance, saving the transplant for later. I would hasten to add that lenalidomide maintenance was given for only 1 year in this trial, and patients in the RVD-only or RVD-and-transplant arms of this trial relapsed after the lenalidomide maintenance was discontinued.
The American portion of this trial is identical. That is, RVD induction is being given and all patients have a stem-cell collection. Half of the patients then go to high-dose melphalan and stem-cell transplant early, and half of them have the transplant only later at the time of relapse. A major difference, however, is that in both the RVD-only and RVD-and-transplant cohorts, patients receive lenalidomide maintenance until progression. This trial has been ongoing since 2009 and is still ongoing, which tells us that patients in both arms – the RVD-only as well as the RVD-and-transplant arms – are doing well.
In the recent STAMINA trial, all patients underwent a single high-dose therapy and transplant. Then there was a randomization to lenalidomide maintenance only in 1 cohort; a randomization to consolidation with RVD posttransplant followed by lenalidomide maintenance in the second cohort; or a randomization to a second high-dose melphalan and stem-cell transplant followed by lenalidomide maintenance in the third cohort.8 I mention this because the outcomes in all 3 cohorts was similar.
I believe this tells us strongly that high-dose therapy and stem-cell transplantation twice – so-called tandem transplant – is no longer a major option in multiple myeloma. For now, however, in this patient, the standard of care would be to undergo induction therapy with triplet, novel combination treatment. Then, stem cells would be collected and high-dose therapy stem-cell transplant would be done, followed either by lenalidomide maintenance for standard disease or lenalidomide and bortezomib maintenance for high-risk disease. We won’t really know if we can delay transplant until the trials I’ve mentioned totally read out. In my clinical practice, if patients have had a major response to their induction therapy and have stem cells harvested, we can then offer them the opportunity to use maintenance therapy and save the transplant as a potential option for later, when myeloma relapses.
DR HENRY In summary then, this would be, in 2017, off-protocol while the data is pending: it’s reasonable to get a deep induction response, collect stem cells, have a discussion with the patient, and then consider high-dose therapy or not.
DR ANDERSON Yes. I think it’s reasonable to discuss it. We need to be open and honest with patients that the standard of care remains transplant, that you incorporate novel treatments before the transplant and novel treatments as maintenance after the transplant. The happy news is that the outcome, especially for patients who have standard-risk myeloma, is at least a decade or longer progression-free survival. It’s an optimistic picture. The data in terms of transplant being needed or not, will come within the next several years.
For now, it is a standard of care to use 1 high-dose melphalan and stem-cell transplant in this setting. I will add into our discussion with patients – besides the opportunity to harvest stem cells and think about whether one needs to do a transplant early on or not – is the issue of toxicity. High-dose melphalan by itself has a small but real secondary incidence of cancer, myelodysplasia, or leukemia. If one uses lenalidomide maintenance after melphalan transplantation treatment, that risk of secondary cancer is slightly increased.
In my experience, if patients have achieved a complete response with induction therapy only, it’s not unreasonable to offer early transplant and be clear that’s the standard of care. The alternative is maintenance with lenalidomide, knowing once the stem cells have been harvested, that transplantation can be an option to treat relapsed myeloma. We have many other options available as well. Time will tell in terms of the ongoing randomized trials as to whether transplant remains central to our treatment paradigm.
DR HENRY This leads us to our second patient. Here we have an older man of 74 years. He’s a professional piano player, so we want to try to avoid peripheral neuropathy in him. He has some mild renal insufficiency and some coronary artery disease, so he’s deemed transplant-ineligible. He has IgG kappa myeloma, and he’s brand new. What would you consider to be options for him for treatment?
DR ANDERSON This brings up the issue of a transplant-ineligible patient. He has significant comorbidity that would make transplantation an increased risk. What we would recommend in such a patient is still triplet induction therapy incorporating novel agents (Figure 1). Lenalidomide, the immunomodulatory drug, can safely be given in the context of neuropathy because it does not cause significant neuropathy. It would need to be dose modified, depending on the degree of renal insufficiency. We would recommend also including proteasome inhibitors. Bortezomib, the first-generation proteasome inhibitor, would be contraindicated because it does have a small but real attendant neuropathy. If, however, it is given weekly and subcutaneously, the risk of attendant neuropathy is quite low. In this patient, therefore, one could start with lenalidomide and bortezomib weekly and subcutaneously,1,2 with a very early and vigilant follow-up for the earliest signs of neuropathy, so as not to allow it to develop and compromise his career.
Alternatively, one could use a proteasome inhibitor that does not have attendant neuropathy. Carfilzomib, the second-generation proteasome inhibitor, does not have neuropathy.9 But we would need to have caution here, because this patient has a history of coronary artery disease, and carfilzomib has a very small, but real, incidence of cardiac toxicity so would need to be used judiciously in this setting. The third proteasome inhibitor, ixazomib, is the next-generation bortezomib-class proteasome inhibitor, and it’s oral.10 It has less neuropathy than does bortezomib, so in my view is a very realistic option for him together with lenalidomide. It does have a small incidence of neuropathy, so close monitoring for neuropathy would be indicated. We could use lenalidomide–dexamethasone as a doublet and avoid neuropathy,11 but usually doublets are reserved only for frail patients.
My recommendation, therefore, would be RVD with the bortezomib weekly or subcutaneously, or alternatively, lenalidomide, ixazomib, dexamethasone as an all-oral regimen as induction therapy. In my view, this 74-year-old patient with comorbidity is not a transplant candidate. However, one can be very optimistic with this patient. The likelihood that he could have myeloma as a chronic illness and die from something else is quite high. Initial induction triplet therapy would achieve a very high response extent and frequency. The durability would be long, especially with lenalidomide maintenance if it’s standard-risk myeloma or lenalidomide and a proteasome inhibitor, probably ixazomib in this setting, if he were to have high-risk myeloma.
If myeloma relapses, then there are many options that could be used in this patient and achieve years of progression-free and overall survival. Indeed, he is 74 years old and will respond very well to induction triplet therapy, with many years’ duration of response due to continuous lenalidomide or lenalidomide and proteasome inhibitor maintenance. Then there are many effective options to treat relapsed therapy using triplet novel agents. Therefore, his lifespan is unlikely to be shortened by multiple myeloma.
DR HENRY It’s so incredible compared with what it was when I trained. The next patient, a 45-year-old woman with IgG lambda myeloma, has had RVD induction and responded. She had lenalidomide maintenance, but then she progressed, and she got her stem-cell transplant, and she’s progressing after that. I guess we’re looking here to fold in some of the newer agents. How you would you do that in this patient?DR ANDERSON Yes. I think one of the most remarkable and exciting developments with myeloma is the rapid approval of the novel classes of agents that I mentioned earlier – the proteasome inhibitors, the immunomodulatory drugs, the HDAC inhibitor, and the monoclonal antibodies.1 They’re particularly relevant in a patient such as this one, whose myeloma has relapsed after what would be considered standard therapy for a young person with standard-risk myeloma. This patient had RVD and maintenance therapy, and then progressed. The transplant was given for relapsed myeloma. The opportunity to use stem-cell transplant in patients when myeloma becomes active after maintenance should not be forgotten as it can be very effective. In all the trials done to date in which early versus late transplant are compared, there have been similar outcomes. Therefore, if the transplant isn’t done early, don’t forget that it’s an option at the time the myeloma progresses. I do want to mention, that there are lots of options for relapsed myeloma (Figures 3 and 4). I mentioned RVD or CyBorD as initial triplet therapies.2-4 In North America, those are the 2 most common regimens. If myeloma then relapses and is resistant to RVD or to CyBorD, then we need to identify alternatives.
We also need to think about the comorbidities in the patient – issues such as age, neuropathy, presence of renal dysfunction, and other clinical factors. And we need to think about what treatment they’ve had in the past. This patient has had RVD, maintenance with lenalidomide, and a stem-cell transplant. We can offer patients a variety of therapies, but in the context of resistance to the first-generation proteasome inhibitor bortezomib and the first-generation immunomodulatory drug lenalidomide, we would strongly recommend the second-generation immunomodulatory drug pomalidomide12 together with a second-generation proteasome inhibitor, be that carfilzomib13 or ixazomib.14 When one uses the second-generation IMiDs and proteasome inhibitors together, there’s a very high frequency of response in the order of 70%-80%, which lasts years.
Besides carfilzomib and ixazomib proteasome inhibitors, we also have elotuzumab and daratumumab, the monoclonal antibodies.15-17 These agents have been FDA approved to treat patients such as this one who has had 1-3 previous therapies for their myeloma. All of them have been approved in randomized phase 3 trials compared with lenalidomide-dexamethasone in the control arm.13-15,17 They’ve all been found to be superior. Although lenalidomide-dexamethasone combined with daratumumab, ixazomib, elotuzumab, or carfilzomib is superior to lenalidomide in relapsed myeloma, the situation in North America, as in this patient, is usually that patients have had lenalidomide-dexamethasone as part of their initial treatment and their myeloma is refractory to lenalidomide.
Hence, we recommend, that we go to the second-generation pomalidomide and second-generation proteasome inhibitors, either carfilzomib or ixazomib. Having said that, the treatment paradigm is evolving. For example, the monoclonal antibody daratumumab was initially approved by the FDA in multiply relapsed disease as a single agent because it achieves a 30% response rate.16 It now has been moved earlier into the first relapse of multiple myeloma, where it achieves much higher response rates when combined with lenalidomide–dexamethasone or combined with bortezomib–dexamethasone.17,18 Response rates of 70%-80% can be achieved, including minimal residual disease negative complete responses.
Today, in a patient who has had RVD transplant and myeloma has returned, we would recommend second-generation IMiDs, pomalidomide, and second-generation proteasome inhibitors, either carfilzomib or ixazomib. Data for daratumumab combined with lenalidomide-dexamethasone or with bortezomib-dexamethasone, look very promising. We need, however, to see more experience of daratumumab together with lenalidomide-dexamethasone or daratumumab together with bortezomib-dexamethasone in patients whose myeloma is refractory to RVD, that is, patients whose myeloma has returned after RVD induction treatment. Of note, pomalidomide, dexamethasone, and daratumumab have just been approved by the FDA and may also be active even in myeloma recurring after RVD treatment.19
Daratumumab in combination will be moving earlier and earlier and may be appropriate to treat the first relapse. I do want to stress, however, that at present I save daratumumab for the second or greater relapse. Daratumumab is active even when relapse occurs after treatment with second-generation IMiDs and proteasome inhibitors.
DR HENRY Before we close, I have a couple practical questions with these antibodies. Daratumumab has the track record of first-treatment severe reactions and long infusion times. How long are you anticipating the first daratumumab treatment takes? There has been some talk that maybe splitting it in half and going over 2 days is easier on the patient and the infusion center. Have you done that?
DR ANDERSON Yes, I think that’s a very important point. We need to be thinking – first and foremost – about efficacy of our therapy. Equally important, however, are the safety profile and the user-friendliness for the patient. Daratumumab infusions are quite long – on the order of 8 hours or longer on day 1 of infusion. And to date, all the clinical trials have used daratumumab infusions weekly for 8 treatments, followed by 8 treatments given every 2 weeks. Then monthly daratumumab is given as a maintenance therapy. Thus, there is a requirement for multiple outpatient clinic visits that can be prolonged.
One of the opportunities that’s being tested is to give daratumumab subcutaneously. While this is being evaluated in protocols now, the results that have been reported at our national meetings look to be quite promising in terms of efficacy, similar to results with the intravenous administration. Obviously, this would allow for a much more convenient clinic visit and shorter time for the patients being treated.
I should mention that the other antibody, elotuzumab, has been approved in combination with lenalidomide and dexamethasone.15 The infusions with lenalidomide, dexamethasone, and the antibody elotuzumab are much shorter, on the order of 2- or 3-hour visits. The place for elotuzumab in the management of relapsed myeloma is yet to be totally defined. We tend to use it now in the setting of more indolent relapses, where patients might have a slowly rising monoclonal protein. Elotuzumab-lenalidomide-dexamethasone has maintained an overall survival advantage at 4 years compared with lenalidomide-dexamethasone when used in relapsed myeloma.
We are quite excited about both antibodies. Daratumumab tends to get most of the activity, as it achieves responses as a single agent,16 and the depth of the responses are markedly increased when it’s combined with lenalidomide-dexamethasone or bortezomib-dexamethasone.17,18 However, one shouldn’t forget elotuzumab15 based on its tolerability and the survival advantage I mentioned at 4 years.
The final point is that we think about myeloma genetically at the time of diagnosis and relapse in terms of standard or high-risk disease. One of the hallmarks of high-risk disease has been 17P deletion or P53 dysfunction. One of the most exciting outcomes of the development of monoclonal antibodies has been the responses observed even in the context of P53 deletion. Clearly, antibody-mediated cellular cytotoxicity, complement-mediated cytotoxicity, and other mechanisms of action of these antibodies do not require normal P53 function. The important point, therefore, is that what has previously been thought of as high-risk disease can nowadays be effectively treated with these new immune treatments, correlating with the marked improvement in survival and overall outcome.
DR HENRY We have outlined 3 kinds of myeloma patients we see, and especially interesting is the last patient, who has relapsed and then progressed, and in whom newer drugs have a role. Thank you for such a complete and thorough discussion, Dr Anderson.
DR HENRY I thought we might discuss some cases of patients with myeloma, starting with a relatively simple case and ending with one that is a little more complicated. For the first case, we have a 56-year-old healthy man with IgG kappa myeloma whose work-up shows he has multiple lytic bone lesions. He has normal renal function, normal calcium, and he’s transplant-eligible by other health issues. I’ll leave the cytogenetics up to you if that changes your approach. How would you develop or pose some options for this man’s treatment to begin with?
DR ANDERSON It’s important to start out by saying that we, in myeloma, have many new classes of drugs and many new opportunities to choose from to treat this patient.1 As you know, we have proteasome inhibitors, the first-generation bortezomib, then carfilzomib and ixazomib. We have the immunomodulatory drugs (IMiDs), thalidomide, and now lenalidomide and pomalidomide. We have a histone deacetylase (HDAC) inhibitor approved called panobinostat, and we have 2 monoclonal antibodies approved, elotuzumab and daratumumab. These classes of medicine have made it possible for 20 different Food and Drug Administration (FDA) approvals in the last 10-15 years. These agents, having been tested in advanced myeloma, have moved toward initial management.
This person is 50 years old. He has adequate liver, heart, lung, and kidney function, so he would be eligible for high-dose therapy and stem-cell transplantation. In terms of initial management, there are many options (Figure 1). We strongly recommend that triplet therapy be used initially. The most common triplets would be lenalidomide, bortezomib, and dexamethasone (RVD)2,3 or cyclophosphamide, bortezomib, and dexamethasone (CyBorD).4 If this man had neuropathy, perhaps carfilzomib, the second-generation proteasome inhibitor, with lenalidomide and dexamethasone could have been used. Why do we use these? The extent and frequency of response with these triplets is nearly universal overall response rate, with three-quarters very good partial and half-complete responses, including minimal residual disease negative responses. In this patient, we would therefore recommend treatment with either RVD or CyBorD for several cycles to maximal response.
He would then have autologous stem cells collected, and it is still the standard of care to proceed to high-dose melphalan and a single high-dose therapy and stem-cell transplantation. The cytogenetics are important: if this patient has standard-risk multiple myeloma, then lenalidomide maintenance would be given after transplant. It is now FDA-approved for this purpose because it can prolong both progression-free and – most importantly – overall survival.5 Standard-risk cytogenetics might, for example, include hyperdiploidy or translocation 11;14. On the other hand, if his myeloma were high-risk and characterized, for example, by 17p deletion, we would carry out the same induction and transplantation, but we would alter the maintenance to incorporate a proteasome inhibitor. Lenalidomide and bortezomib, for example, could be combined. Early data show that using combined maintenance therapy with lenalidomide and bortezomib, can overcome the early relapses that are characteristic of high-risk disease.6
Because of the extent and frequency of response to combination novel therapies, we have undertaken with our French colleagues a clinical trial of RVD in newly diagnosed patients – such as this patient – followed by stem-cell collection in all patients (Figure 2). Then there is a randomization to either early high-dose therapy, melphalan, and autologous stem-cell transplantation, followed by lenalidomide maintenance; or in the other cohort, harvesting of stem cells, additional RVD, and then maintenance with lenalidomide, saving the stem-cell transplant for later.
The French portion of this trial was reported in the New England Journal of Medicine earlier in 2017.7 It showed that patients who received RVD, high-dose melphalan, stem-cell transplant, and had 1 year of lenalidomide maintenance, had a progression-free survival advantage of about 1 year, without an overall survival advantage; compared with those patients who received RVD and lenalidomide maintenance, saving the transplant for later. I would hasten to add that lenalidomide maintenance was given for only 1 year in this trial, and patients in the RVD-only or RVD-and-transplant arms of this trial relapsed after the lenalidomide maintenance was discontinued.
The American portion of this trial is identical. That is, RVD induction is being given and all patients have a stem-cell collection. Half of the patients then go to high-dose melphalan and stem-cell transplant early, and half of them have the transplant only later at the time of relapse. A major difference, however, is that in both the RVD-only and RVD-and-transplant cohorts, patients receive lenalidomide maintenance until progression. This trial has been ongoing since 2009 and is still ongoing, which tells us that patients in both arms – the RVD-only as well as the RVD-and-transplant arms – are doing well.
In the recent STAMINA trial, all patients underwent a single high-dose therapy and transplant. Then there was a randomization to lenalidomide maintenance only in 1 cohort; a randomization to consolidation with RVD posttransplant followed by lenalidomide maintenance in the second cohort; or a randomization to a second high-dose melphalan and stem-cell transplant followed by lenalidomide maintenance in the third cohort.8 I mention this because the outcomes in all 3 cohorts was similar.
I believe this tells us strongly that high-dose therapy and stem-cell transplantation twice – so-called tandem transplant – is no longer a major option in multiple myeloma. For now, however, in this patient, the standard of care would be to undergo induction therapy with triplet, novel combination treatment. Then, stem cells would be collected and high-dose therapy stem-cell transplant would be done, followed either by lenalidomide maintenance for standard disease or lenalidomide and bortezomib maintenance for high-risk disease. We won’t really know if we can delay transplant until the trials I’ve mentioned totally read out. In my clinical practice, if patients have had a major response to their induction therapy and have stem cells harvested, we can then offer them the opportunity to use maintenance therapy and save the transplant as a potential option for later, when myeloma relapses.
DR HENRY In summary then, this would be, in 2017, off-protocol while the data is pending: it’s reasonable to get a deep induction response, collect stem cells, have a discussion with the patient, and then consider high-dose therapy or not.
DR ANDERSON Yes. I think it’s reasonable to discuss it. We need to be open and honest with patients that the standard of care remains transplant, that you incorporate novel treatments before the transplant and novel treatments as maintenance after the transplant. The happy news is that the outcome, especially for patients who have standard-risk myeloma, is at least a decade or longer progression-free survival. It’s an optimistic picture. The data in terms of transplant being needed or not, will come within the next several years.
For now, it is a standard of care to use 1 high-dose melphalan and stem-cell transplant in this setting. I will add into our discussion with patients – besides the opportunity to harvest stem cells and think about whether one needs to do a transplant early on or not – is the issue of toxicity. High-dose melphalan by itself has a small but real secondary incidence of cancer, myelodysplasia, or leukemia. If one uses lenalidomide maintenance after melphalan transplantation treatment, that risk of secondary cancer is slightly increased.
In my experience, if patients have achieved a complete response with induction therapy only, it’s not unreasonable to offer early transplant and be clear that’s the standard of care. The alternative is maintenance with lenalidomide, knowing once the stem cells have been harvested, that transplantation can be an option to treat relapsed myeloma. We have many other options available as well. Time will tell in terms of the ongoing randomized trials as to whether transplant remains central to our treatment paradigm.
DR HENRY This leads us to our second patient. Here we have an older man of 74 years. He’s a professional piano player, so we want to try to avoid peripheral neuropathy in him. He has some mild renal insufficiency and some coronary artery disease, so he’s deemed transplant-ineligible. He has IgG kappa myeloma, and he’s brand new. What would you consider to be options for him for treatment?
DR ANDERSON This brings up the issue of a transplant-ineligible patient. He has significant comorbidity that would make transplantation an increased risk. What we would recommend in such a patient is still triplet induction therapy incorporating novel agents (Figure 1). Lenalidomide, the immunomodulatory drug, can safely be given in the context of neuropathy because it does not cause significant neuropathy. It would need to be dose modified, depending on the degree of renal insufficiency. We would recommend also including proteasome inhibitors. Bortezomib, the first-generation proteasome inhibitor, would be contraindicated because it does have a small but real attendant neuropathy. If, however, it is given weekly and subcutaneously, the risk of attendant neuropathy is quite low. In this patient, therefore, one could start with lenalidomide and bortezomib weekly and subcutaneously,1,2 with a very early and vigilant follow-up for the earliest signs of neuropathy, so as not to allow it to develop and compromise his career.
Alternatively, one could use a proteasome inhibitor that does not have attendant neuropathy. Carfilzomib, the second-generation proteasome inhibitor, does not have neuropathy.9 But we would need to have caution here, because this patient has a history of coronary artery disease, and carfilzomib has a very small, but real, incidence of cardiac toxicity so would need to be used judiciously in this setting. The third proteasome inhibitor, ixazomib, is the next-generation bortezomib-class proteasome inhibitor, and it’s oral.10 It has less neuropathy than does bortezomib, so in my view is a very realistic option for him together with lenalidomide. It does have a small incidence of neuropathy, so close monitoring for neuropathy would be indicated. We could use lenalidomide–dexamethasone as a doublet and avoid neuropathy,11 but usually doublets are reserved only for frail patients.
My recommendation, therefore, would be RVD with the bortezomib weekly or subcutaneously, or alternatively, lenalidomide, ixazomib, dexamethasone as an all-oral regimen as induction therapy. In my view, this 74-year-old patient with comorbidity is not a transplant candidate. However, one can be very optimistic with this patient. The likelihood that he could have myeloma as a chronic illness and die from something else is quite high. Initial induction triplet therapy would achieve a very high response extent and frequency. The durability would be long, especially with lenalidomide maintenance if it’s standard-risk myeloma or lenalidomide and a proteasome inhibitor, probably ixazomib in this setting, if he were to have high-risk myeloma.
If myeloma relapses, then there are many options that could be used in this patient and achieve years of progression-free and overall survival. Indeed, he is 74 years old and will respond very well to induction triplet therapy, with many years’ duration of response due to continuous lenalidomide or lenalidomide and proteasome inhibitor maintenance. Then there are many effective options to treat relapsed therapy using triplet novel agents. Therefore, his lifespan is unlikely to be shortened by multiple myeloma.
DR HENRY It’s so incredible compared with what it was when I trained. The next patient, a 45-year-old woman with IgG lambda myeloma, has had RVD induction and responded. She had lenalidomide maintenance, but then she progressed, and she got her stem-cell transplant, and she’s progressing after that. I guess we’re looking here to fold in some of the newer agents. How you would you do that in this patient?DR ANDERSON Yes. I think one of the most remarkable and exciting developments with myeloma is the rapid approval of the novel classes of agents that I mentioned earlier – the proteasome inhibitors, the immunomodulatory drugs, the HDAC inhibitor, and the monoclonal antibodies.1 They’re particularly relevant in a patient such as this one, whose myeloma has relapsed after what would be considered standard therapy for a young person with standard-risk myeloma. This patient had RVD and maintenance therapy, and then progressed. The transplant was given for relapsed myeloma. The opportunity to use stem-cell transplant in patients when myeloma becomes active after maintenance should not be forgotten as it can be very effective. In all the trials done to date in which early versus late transplant are compared, there have been similar outcomes. Therefore, if the transplant isn’t done early, don’t forget that it’s an option at the time the myeloma progresses. I do want to mention, that there are lots of options for relapsed myeloma (Figures 3 and 4). I mentioned RVD or CyBorD as initial triplet therapies.2-4 In North America, those are the 2 most common regimens. If myeloma then relapses and is resistant to RVD or to CyBorD, then we need to identify alternatives.
We also need to think about the comorbidities in the patient – issues such as age, neuropathy, presence of renal dysfunction, and other clinical factors. And we need to think about what treatment they’ve had in the past. This patient has had RVD, maintenance with lenalidomide, and a stem-cell transplant. We can offer patients a variety of therapies, but in the context of resistance to the first-generation proteasome inhibitor bortezomib and the first-generation immunomodulatory drug lenalidomide, we would strongly recommend the second-generation immunomodulatory drug pomalidomide12 together with a second-generation proteasome inhibitor, be that carfilzomib13 or ixazomib.14 When one uses the second-generation IMiDs and proteasome inhibitors together, there’s a very high frequency of response in the order of 70%-80%, which lasts years.
Besides carfilzomib and ixazomib proteasome inhibitors, we also have elotuzumab and daratumumab, the monoclonal antibodies.15-17 These agents have been FDA approved to treat patients such as this one who has had 1-3 previous therapies for their myeloma. All of them have been approved in randomized phase 3 trials compared with lenalidomide-dexamethasone in the control arm.13-15,17 They’ve all been found to be superior. Although lenalidomide-dexamethasone combined with daratumumab, ixazomib, elotuzumab, or carfilzomib is superior to lenalidomide in relapsed myeloma, the situation in North America, as in this patient, is usually that patients have had lenalidomide-dexamethasone as part of their initial treatment and their myeloma is refractory to lenalidomide.
Hence, we recommend, that we go to the second-generation pomalidomide and second-generation proteasome inhibitors, either carfilzomib or ixazomib. Having said that, the treatment paradigm is evolving. For example, the monoclonal antibody daratumumab was initially approved by the FDA in multiply relapsed disease as a single agent because it achieves a 30% response rate.16 It now has been moved earlier into the first relapse of multiple myeloma, where it achieves much higher response rates when combined with lenalidomide–dexamethasone or combined with bortezomib–dexamethasone.17,18 Response rates of 70%-80% can be achieved, including minimal residual disease negative complete responses.
Today, in a patient who has had RVD transplant and myeloma has returned, we would recommend second-generation IMiDs, pomalidomide, and second-generation proteasome inhibitors, either carfilzomib or ixazomib. Data for daratumumab combined with lenalidomide-dexamethasone or with bortezomib-dexamethasone, look very promising. We need, however, to see more experience of daratumumab together with lenalidomide-dexamethasone or daratumumab together with bortezomib-dexamethasone in patients whose myeloma is refractory to RVD, that is, patients whose myeloma has returned after RVD induction treatment. Of note, pomalidomide, dexamethasone, and daratumumab have just been approved by the FDA and may also be active even in myeloma recurring after RVD treatment.19
Daratumumab in combination will be moving earlier and earlier and may be appropriate to treat the first relapse. I do want to stress, however, that at present I save daratumumab for the second or greater relapse. Daratumumab is active even when relapse occurs after treatment with second-generation IMiDs and proteasome inhibitors.
DR HENRY Before we close, I have a couple practical questions with these antibodies. Daratumumab has the track record of first-treatment severe reactions and long infusion times. How long are you anticipating the first daratumumab treatment takes? There has been some talk that maybe splitting it in half and going over 2 days is easier on the patient and the infusion center. Have you done that?
DR ANDERSON Yes, I think that’s a very important point. We need to be thinking – first and foremost – about efficacy of our therapy. Equally important, however, are the safety profile and the user-friendliness for the patient. Daratumumab infusions are quite long – on the order of 8 hours or longer on day 1 of infusion. And to date, all the clinical trials have used daratumumab infusions weekly for 8 treatments, followed by 8 treatments given every 2 weeks. Then monthly daratumumab is given as a maintenance therapy. Thus, there is a requirement for multiple outpatient clinic visits that can be prolonged.
One of the opportunities that’s being tested is to give daratumumab subcutaneously. While this is being evaluated in protocols now, the results that have been reported at our national meetings look to be quite promising in terms of efficacy, similar to results with the intravenous administration. Obviously, this would allow for a much more convenient clinic visit and shorter time for the patients being treated.
I should mention that the other antibody, elotuzumab, has been approved in combination with lenalidomide and dexamethasone.15 The infusions with lenalidomide, dexamethasone, and the antibody elotuzumab are much shorter, on the order of 2- or 3-hour visits. The place for elotuzumab in the management of relapsed myeloma is yet to be totally defined. We tend to use it now in the setting of more indolent relapses, where patients might have a slowly rising monoclonal protein. Elotuzumab-lenalidomide-dexamethasone has maintained an overall survival advantage at 4 years compared with lenalidomide-dexamethasone when used in relapsed myeloma.
We are quite excited about both antibodies. Daratumumab tends to get most of the activity, as it achieves responses as a single agent,16 and the depth of the responses are markedly increased when it’s combined with lenalidomide-dexamethasone or bortezomib-dexamethasone.17,18 However, one shouldn’t forget elotuzumab15 based on its tolerability and the survival advantage I mentioned at 4 years.
The final point is that we think about myeloma genetically at the time of diagnosis and relapse in terms of standard or high-risk disease. One of the hallmarks of high-risk disease has been 17P deletion or P53 dysfunction. One of the most exciting outcomes of the development of monoclonal antibodies has been the responses observed even in the context of P53 deletion. Clearly, antibody-mediated cellular cytotoxicity, complement-mediated cytotoxicity, and other mechanisms of action of these antibodies do not require normal P53 function. The important point, therefore, is that what has previously been thought of as high-risk disease can nowadays be effectively treated with these new immune treatments, correlating with the marked improvement in survival and overall outcome.
DR HENRY We have outlined 3 kinds of myeloma patients we see, and especially interesting is the last patient, who has relapsed and then progressed, and in whom newer drugs have a role. Thank you for such a complete and thorough discussion, Dr Anderson.
1. Kumar SK, Callander NS, Alsina M, et al. Multiple Myeloma, Version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:230-269.
2. Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly-diagnosed multiple myeloma. Blood. 2010;116:679-686.
3. Durie BG, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777: a randomized, open-label, phase 3 trial. Lancet. 2017;389(10068):519-527.
4. Moreau P, Hulin C, Macro M, et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood. 2016;127:2569-2574.
5. McCarthy PL, Owzar K, Hofmeister CC, et al. A phase III study of lenalidomide after transplant for multiple myeloma. N Engl J Med. 2012;366:1770-1781.
6. Nooka AK, Kaufman JL, Muppidi S, et al. Consolidation and maintenance therapy with lenalidomide, bortezomib, and dexamethasone (RVD) in high risk myeloma patients. Leukemia. 2014;28:690-693.
7. Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, bortezomib and dexamethasone with transplantation in myeloma. N Engl J Med. 2017;376:1311-1320.
8. Stadtmauer EA, Pasquini MC, Blackwell B, et al. Comparison of autologous hematopoietic cell transplant (autoHCT), bortezomib, lenalidomide (len) and dexamethasone (RVD) consolidation with len maintenance (ACM), tandem autohct with len maintenance (TAM) and autohct with len maintenance (AM) for up-front treatment of patients with multiple myeloma (MM): primary results from the randomized phase III trial of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 0702 – StaMINA Trial). Abstract LBA-1. Presented at the 2016 ASH Annual Meeting, December 6, 2016; San Diego, CA.
9. Dytfeld D, Jasielec J, Griffith KA, et al: Carfilzomib, lenalidomide, and low-dose dexamethasone in elderly patients with newly diagnosed multiple myeloma. Haematologica. 2014;99:e162-164.
10. Kumar SK, Berdeja JG, Niesvizky R, et al. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 2014;15:1503-1512
11. Benboubker L, Dimopoulos MA, Dispenzieri A, et al. for the FIRST Trial Team. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906-917.
12. Richardson P, Siegel D, Vij R, et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood. 2014;123:1826-1832.
13. Stewart AK, Rajkumar SV, Dimopoulos MA, et al. for the ASPIRE Investigators. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142-152
14. Moreau P, Masszi T, Grzasko N, et al. for the TOURMALINE-MM1 Study Group. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;28;374:1621-1634.
15. Lonial S, Dimopoulos M, Palumbo A, et al. for the ELOQUENT-2 Investigators. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621-631.
16. Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD 38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373:1207-1219.
17. Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319-1331.
18. Palumbo A, Chanan-Khan A, Weisel K, et al. for the CASTOR Investigators. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754-766.
19. Chari A, Suvannasankha A, Fay JW, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130(8):974-981.
1. Kumar SK, Callander NS, Alsina M, et al. Multiple Myeloma, Version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:230-269.
2. Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly-diagnosed multiple myeloma. Blood. 2010;116:679-686.
3. Durie BG, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777: a randomized, open-label, phase 3 trial. Lancet. 2017;389(10068):519-527.
4. Moreau P, Hulin C, Macro M, et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood. 2016;127:2569-2574.
5. McCarthy PL, Owzar K, Hofmeister CC, et al. A phase III study of lenalidomide after transplant for multiple myeloma. N Engl J Med. 2012;366:1770-1781.
6. Nooka AK, Kaufman JL, Muppidi S, et al. Consolidation and maintenance therapy with lenalidomide, bortezomib, and dexamethasone (RVD) in high risk myeloma patients. Leukemia. 2014;28:690-693.
7. Attal M, Lauwers-Cances V, Hulin C, et al. Lenalidomide, bortezomib and dexamethasone with transplantation in myeloma. N Engl J Med. 2017;376:1311-1320.
8. Stadtmauer EA, Pasquini MC, Blackwell B, et al. Comparison of autologous hematopoietic cell transplant (autoHCT), bortezomib, lenalidomide (len) and dexamethasone (RVD) consolidation with len maintenance (ACM), tandem autohct with len maintenance (TAM) and autohct with len maintenance (AM) for up-front treatment of patients with multiple myeloma (MM): primary results from the randomized phase III trial of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 0702 – StaMINA Trial). Abstract LBA-1. Presented at the 2016 ASH Annual Meeting, December 6, 2016; San Diego, CA.
9. Dytfeld D, Jasielec J, Griffith KA, et al: Carfilzomib, lenalidomide, and low-dose dexamethasone in elderly patients with newly diagnosed multiple myeloma. Haematologica. 2014;99:e162-164.
10. Kumar SK, Berdeja JG, Niesvizky R, et al. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 2014;15:1503-1512
11. Benboubker L, Dimopoulos MA, Dispenzieri A, et al. for the FIRST Trial Team. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906-917.
12. Richardson P, Siegel D, Vij R, et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood. 2014;123:1826-1832.
13. Stewart AK, Rajkumar SV, Dimopoulos MA, et al. for the ASPIRE Investigators. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142-152
14. Moreau P, Masszi T, Grzasko N, et al. for the TOURMALINE-MM1 Study Group. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;28;374:1621-1634.
15. Lonial S, Dimopoulos M, Palumbo A, et al. for the ELOQUENT-2 Investigators. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621-631.
16. Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD 38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 2015;373:1207-1219.
17. Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319-1331.
18. Palumbo A, Chanan-Khan A, Weisel K, et al. for the CASTOR Investigators. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754-766.
19. Chari A, Suvannasankha A, Fay JW, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130(8):974-981.
Treating immunotherapy-related AEs in the emergency department
In this interview, Dr David Henry and Dr Maura Sammon discuss some of the most common immunotherapy-related side effects – lung, gastrointestinal, rash, and endocrine-related problems – and Dr Sammon describes in detail how physicians in the ED would triage and treat the patient. However, the overarching takeaway is the importance of communication: first, between the oncologist and patient, so that the patient is aware of these nuances in advance of an emergency, and second, between the ED physician and the treating oncologist soon after the patient has presented and undergone an initial assessment.
Dr Henry is Editor-in-Chief of the JCSO, and Dr Sammon is at the Lewis Katz School of Medicine, Temple University, in Philadelphia, Pennsylvania
Listen here:
In this interview, Dr David Henry and Dr Maura Sammon discuss some of the most common immunotherapy-related side effects – lung, gastrointestinal, rash, and endocrine-related problems – and Dr Sammon describes in detail how physicians in the ED would triage and treat the patient. However, the overarching takeaway is the importance of communication: first, between the oncologist and patient, so that the patient is aware of these nuances in advance of an emergency, and second, between the ED physician and the treating oncologist soon after the patient has presented and undergone an initial assessment.
Dr Henry is Editor-in-Chief of the JCSO, and Dr Sammon is at the Lewis Katz School of Medicine, Temple University, in Philadelphia, Pennsylvania
Listen here:
In this interview, Dr David Henry and Dr Maura Sammon discuss some of the most common immunotherapy-related side effects – lung, gastrointestinal, rash, and endocrine-related problems – and Dr Sammon describes in detail how physicians in the ED would triage and treat the patient. However, the overarching takeaway is the importance of communication: first, between the oncologist and patient, so that the patient is aware of these nuances in advance of an emergency, and second, between the ED physician and the treating oncologist soon after the patient has presented and undergone an initial assessment.
Dr Henry is Editor-in-Chief of the JCSO, and Dr Sammon is at the Lewis Katz School of Medicine, Temple University, in Philadelphia, Pennsylvania
Listen here:
Gastrointestinal cancers: new standards of care from landmark trials
In this interview, Dr David Henry, the Editor-in-Chief of JCSO, and Dr Daniel Haller, of the Abramson Cancer Center, University of Pennsylvania Perelman School of Medicine in Philadelphia, discuss the findings from recent groundbreaking studies in gastrointestinal cancers.
Listen to the podcast below.
In this interview, Dr David Henry, the Editor-in-Chief of JCSO, and Dr Daniel Haller, of the Abramson Cancer Center, University of Pennsylvania Perelman School of Medicine in Philadelphia, discuss the findings from recent groundbreaking studies in gastrointestinal cancers.
Listen to the podcast below.
In this interview, Dr David Henry, the Editor-in-Chief of JCSO, and Dr Daniel Haller, of the Abramson Cancer Center, University of Pennsylvania Perelman School of Medicine in Philadelphia, discuss the findings from recent groundbreaking studies in gastrointestinal cancers.
Listen to the podcast below.
Gastrointestinal cancers: new standards of care from landmark trials
DR HENRY I am Dr David Henry, the Editor-in-Chief of
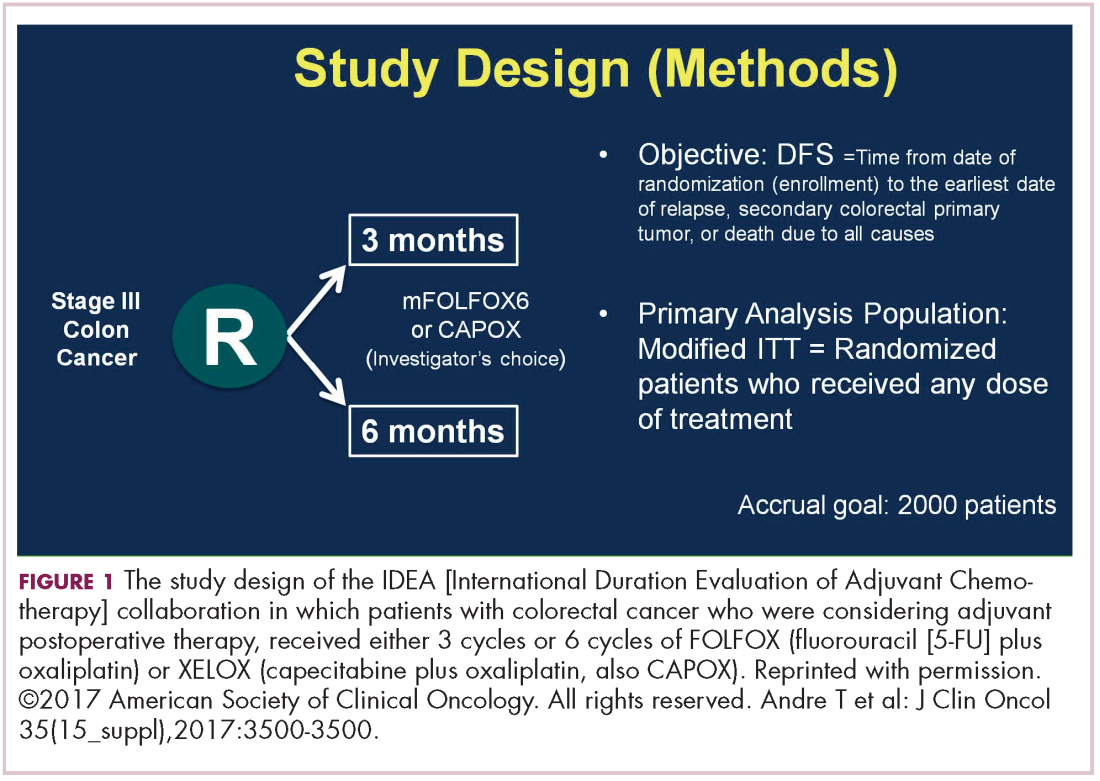
DR HALLER The IDEA collaboration was the brainchild of the late Dan Sargent, a biostatistician who was at the Mayo Clinic. It was his idea, since 6 international groups were all testing the same question of 3 months for oxaliplatin to 6 months of oxaliplatin, to combine the data in an individual patient database – which is the best way to do it – so there were these six trials that were all completed.
Three of them were individually reported at ASCO this year, and then the totality was presented at the plenary session – the first time in 12 years that a gastrointestinal (GI) cancer trial made the plenary session. The whole point, obviously, is neuropathy. With 6 months of FOLFOX or XELOX, about 13% or more patients will develop grade 3 neuropathy, even if people stop short of the full-cycle length, and that is a big deal for the 50,000 patients or so who get adjuvant therapy. At the plenary session, the data were presented and the next day three individual trials were presented and discussed by Jeff Meyerhardt (of Dana-Farber Cancer Institute, Boston).
There were 6 different trials: a few included rectum, some included stage II, some used CAPOX and FOLFOX-4 or 6. The only trial that used only FOLFOX was the Cancer and Leukemia Group B (CALGB) trial in the United States (US). There was a lot of heterogeneity, but when Dan was around, I asked him whether that was a problem, and he said on the contrary, was a better thing because it allowed for real-life practice.
The primary endpoint of the study was to look for noninferiority of 3 months versus 6 months of treatment. The noninferiority margin was at a hazard ratio of 1.12, so they were willing to barter down a few percentage points from benefit. If you looked at the primary disease-free survival analysis, the hazard ratio was 1.07, which was an absolute difference of 0.9%, favoring 3 months of therapy. But because the hazard ratio crossed the 1.12 boundary, it was considered inconclusive and not proven.
If you looked at the regimens, CAPOX outperformed FOLFOX. That’s a regimen we don’t do much in the US. We tend to use more FOLFOX, but CAPOX looked better. What they then did was look at the different subsets of patients, and the subsets that it was obviously as good in was the group that had T1-3N1 disease, where 3 months of therapy was clearly just as good as 6 months of therapy, with only a 3% risk of grade 3 neuropathy.
DR HENRY That would be one to three nodes?
DR HALLER Exactly. That’s about 50% of patients. In the T4N2 patients, neither regimen did very well and the 3-year disease-free survival was in the range of 50%, which is clearly unacceptable. Jeff discussed two things. Why could CAPOX be better? If you do the math, when you do CAPOX, you get more oxaliplatin during the first few months of therapy, because it’s 130 mg every 3 weeks, rather than 85 mg every 2 weeks. His conclusion was, “for my next patient who has T4N2 disease, I’ll offer 6 months of FOLFOX.” The study that really need
He discussed the two new trials. One is a study called ARGO, which is being done by the National Surgical Adjuvant Breast and Bowel Project, where people get standard adjuvant chemotherapy, and they’re then randomized to either 24 months of regorafenib 120 mg per day or a placebo. This is an attempt to recreate the transient benefit from bevacizumab in the NSABP C-08 trial. It’s accruing slowly because regorafenib has some toxicity associated with it, but it probably will be completed. Will it continue the benefit as seen in the 12 months of bevacizumab and C-08? We’ll see.
The other, more interesting study is being done in the cooperative groups looking at FOLFOX plus atezolizumab, one of the checkpoint inhibitors. The difficulty here is that only 15% of people with stage III disease have microsatellite instability (MSI)-high tumors, but it’s certainly compelling. This is a straight up comparison. It’s 6 months of FOLFOX in the control arm, or 6 months of FOLFOX plus atezolizumab concurrently for 6 months, and then an additional 6 months of atezolizumab. These are both very fascinating ideas.
DR HENRY To go back to one of your original points, this 3 versus 6 months: the neuropathy is significantly less in those getting the 3 months?
DR HALLER It went to 3%.
DR HENRY We all see that is very bothersome to patients. Before we leave colorectal, I must ask about the right-sided versus left-sided colorectal cancer that we hear a lot about now. Could you comment on how right-sided is worse than left-sided, and do we understand why?
DR HALLER There are two things to consider. If you look back even to simple trials of 5-FU or biochemical modulated 5-FU from 20 years ago, there were clear differences showing worse prognosis in patients with right-sided tumors, so that’s one point to be made. It’s been consistently seen but never acted upon. Then, the explanation for it, possibly, is that the right colon and left colon are two biologically different organs – and they are. Embryologically, the right colon comes from the midgut and the left colon comes from the hindgut, and there were several presentations at ASCO and at prior meetings showing that when you look at different mutations, they differ between the right and left colons. The right-sided tumors are more MSI-high and more BRAF-mutated, left-sided mutations less so.
Then, people started analyzing many of the very large colon cancer trials, including the US trial CALB/SWOG C80405 and the FIRE-3 trials in Europe, where backbone chemotherapy of FOLFIRI or FOLFOX was given with either cetuximab or bevacizumab in RAS wild-type patients. For one study, C80405, they saw that for cetuximab, on the right side, the median survival was 16.7 months and on the left side, it’s 36 months – a 20-month difference. In fact, if you look at the totality of the data, 16.7 almost looks like cetuximab is harming them, as if you were giving it to a RAS-mutated patient, but they were not. They were all RAS wild-type.
For bevacizumab, the right side was 24 months; the left side was 31.4 months. If you look at the left, cetuximab was 36 months and bevacizumab was 31.4 months, so it appears left-sided tumors should get more cetuximab than they are now getting in the US with a 5-month difference, but that decrement is much different on the right, where there’s an 8-month benefit for bevacizumab compared with cetuximab. There is a very good review by Dirk Arnold, who looked at a totality of 6 studies to really examine this more carefully.2
The National Comprehensive Cancer Network has chimed in on this, and is suggesting that for the 25% of people who have right-sided tumors, epidermal growth factor receptor (EGFR) agents not be considered in first-line therapy. NCCN did not go as far to say that EGFR agents should be given on the left side. As I said, the differences are much more impressive in the right, so this is a real sea change for people to consider which side of the tumor affects outcome.
Deb Schrag (Dana-Farber Cancer Institute) presented data at last year’s ASCO not only for stage IV disease showing the same thing, but also stage III disease where there are also right-versus-left differences in terms of recurrence, with a hazard ratio on the right side of about 1.4 compared with the left-sided tumors. Maybe it should be true that 3 months is especially good if you’re treating left-sided tumors, and maybe the right-sided tumor needs to be also calculated with the factors we just talked about. These are two big changes in an area in which we literally haven’t made any change since FOLFOX was introduced a decade ago.
DR HENRY That’s really fascinating, and if not practice changing, then practice challenging. Staying with the mutations idea, in my patients, I’m checking the RAS family and the BRAF mutation, where I’ve learned that’s a particularly bad mutation. I wonder if you might comment on the Kopetz trial, which took a cohort of BRAF mutants and treated them (Figure 2).3 How did that turn out?
DR HALLER It turned out well. We’re turning colon cancer into non–small cell lung cancer in that we’re getting small groups of patients who now have very dedicated care. The backstory here is that there was some thought that you should be treating mutations, not tumor sites. Drugs such as vemurafenib, for example, which is a BRAF inhibitor, worked well in melanoma for the same mutation that’s in colon cancer, V600E. But when vemurafenib was used in the BRAF-mutant patients – these are 10% of the population – median survivorship was one-third that of the rest of the patients, so roughly 12 months. People looked like they were doing worse when vemurafenib was used. They had no benefit.
Scott Kopetz at MD Anderson (Houston, Texas) is a very good bench-to-bed-and-back sort of doc. He looked at this in cell lines and found that when you give a BRAF inhibitor, you upregulate EGFR so you add an EGFR inhibitor. He did a phase 1 and 1B study, and then in the co-operative groups, a study was done – a randomized phase 2 trial for people who had the BRAF-V600E mutation failing first-line therapy, and then went on to receive either irinotecan single agent or irinotecan plus cetuximab or a triple arm of irinotecan, cetuximab, and vemurafenib. There was a crossover, and so the primary endpoint was progression-free survival. It accrued rapidly.
Again, small study, about 100 patients, but for the double-agent arm, or cetuximab–irinotecan, the median survivorship was 2 months. It was 4.4 months for the combination, so more than double. The response rate quadrupled from 4% to 16%, and the people who had disease control tripled, from 22% to 67%. Many of these patients had bulky disease, BRAF mutations. They need response, so this is a very important endpoint.
Overall survival was not different, in part because it was a crossover, and the crossover patients did pretty well. This is going to move more toward first-line therapy, because we don’t talk about fourth- and fifth-line therapies, TAS-102 or regorafenib. These patients don’t make it to even third line. We’re chipping away at what we think is a very homogenous group of peoples’ metastatic disease. They’re obviously not.
DR HENRY In the BRAF-mutant patient, the vemurafenib might drive them toward EGFR, and then the cetuximab could come in and handle that diversion of the pathway. Fascinating.
DR HALLER The preferred regimen in first-line therapy for a BRAF mutant might be FOLFIRI, cetuximab, and vemurafenib, especially on the left side.
DR HENRY Certainly makes sense. We’ll continue the theme at ASCO of “new standard of care.” Let’s move to gastroesophageal junction. There was a so-called FLOT (5-FU, leucovorin, oxaliplatin, Taxotere) presentation in the neoadjuvant/adjuvant setting, 4 cycles preoperatively and 4 cycles postoperatively. Could you comment on that study?
DR HALLER Gastric cancer for metastatic disease has a very large buffet of treatment regimens, and some just become entrenched, like the ECF regimen with epirubicin (epirubicin, cisplatin, 5-FU), where most people don’t exactly know what the contribution of that drug is, and so some people use EOX (epirubicin, oxaliplatin, capecitabine), some people use FOLFOX, some people use FOLFIRI. It gets a little bit confusing as to whether you use taxanes, platinums, or 5-FU or capecitabine.
The Germans came up with a regimen called FLOT – it’s sort of like FOLFOX with Taxotere attached. They did a very large study comparing it with ECF or ECX (epirubicin, cisplatin, capecitabine; Figure 3).4 The overall endpoint with over 700 patients was survival. This is an adjuvant regimen. Only 37% of people got ECF or ECX postoperatively, and 50% of the FLOT patients got the regimens postoperatively.
One of the reasons FLOT might be more beneficial is that more people were given postoperative treatment, and it’s one reason why many adjuvant regimens are being moved completely preoperatively, because so few people get the planned treatment. The FLOT regimen improved overall survival with a P value of .0112 and a hazard ratio of 0.77. The difference was 35 months versus 50 months. With the uncertainty as to what epirubicin actually does and the fact that it’s been around for a while and that fewer people receive postoperative treatment, with that 15-month benefit, if you’re using chemotherapy alone, and there’s no radiotherapy component for true gastric cancer, this is a new standard of care.
DR HENRY I struggle with this in my patients as well. This concept of getting more therapy preoperatively to those who can’t get it postoperatively certainly resonates with most of us in practice.
DR HALLER If I were redesigning the trial, I would probably say just give 4-6 cycles of treatment, and give it all preoperatively. In rectal cancer, there’s the total neoadjuvant approach, where it’s being tested in people who get all their chemotherapy first, then chemoradiotherapy, then surgery, and you’re done.
DR HENRY Yes, right. Thank you for mentioning that. Staying with the gastric GE junction, you couldn’t get away from ASCO this year without hearing about the checkpoint inhibitor immunotherapies in this population. In the CHECKMATE-142 trial with nivolumab versus placebo, response rates were good, especially in the MSI-high (microsatellite instability). Could you comment on that study?
DR HALLER We already know that in May and July 2017, pembrolizumab and nivolumab were both approved for any MSI-high solid tumor based on phase 2 data only, and based on response. That’s the first time we’ve seen that happen. It’s remarkable. For nivolumab, the approval was based on 53 patients with MSI-high metastatic colon cancer. So these were people who failed standard therapy and got nivolumab by standard infusion every 2 weeks. The overall response rate was almost 30% in this population, which is typically quite resistant to any treatment, so one expects much lower response rates with anything in that setting – chemotherapy, TAS-102, regorafenib, et cetera (Table).5
More importantly, as we’re seeing with Jimmy Carter with checkpoint treatment (for melanoma that had metastasized to the brain), responses lasted for more than 6 months in about two-thirds of patients, even a complete response, so this is just off the wall. I mean, this is not what you would expect with almost any other treatment. The data are the same for atezolizumab and for pembrolizumab. What seems to be true is that in the GI tumors and colon cancer, MSI-high seems more important than expression of PD-1 or PD-L1 (programmed cell death protein-1 or programmed cell death protein-ligand 1).
In different tumor sites, PD-1 or PD-L1 measurement may be important, but in these tumors, and in colorectal cancer, it looks as if MSI-high is the preferred measurement. Recently ASCO, together with the American Society for Clinical Pathology, College of American Pathologists, and Association for Molecular Pathology, came out with guidelines on what you should measure in colorectal cancer specimens. Obviously, one is extended RAS. They say you should get BRAF for prognosis, but it may also be a prognostic factor that leads you to treat, which ultimately makes it a predictive factor, so the data from Kopetz might suggest that will move up to something you also must measure. If patients have the BRAF mutation, it’s important they know that it’s a poor prognostic sign. But if they come in with literature saying they might live 36 months when their actual outcome is about a third of that, you need to frame your discussion in that regard and make sure they understand it.
The guidelines also suggested getting MSI-high, and certainly prognostically in early-stage disease, but now it’s going to be a predictive factor, so in the month in which these recommendations are made, two of them are already out of date. They also didn’t include human epidermal growth factor receptor 2 (HER2), and what we’ve heard from the HERACLES (HER2 Amplification for Colorectal Cancer Enhanced Stratification) trial is that for those patients who got the trastuzumab and pertuzumab combination – and this is another 5% of patients – almost the same data was seen as in the MSI-high patients with checkpoint inhibitors. That is double-digit response rates and durable responses. As I said, we’re very much nearing in colorectal cancer what’s now being done in non-small cell lung cancer.
DR HENRY Indeed. Could you comment on the BILCAP study and adjuvant capecitabine for biliary tract cancer?
DR HALLER There are large meta-analyses looking at adjuvant therapy for biliary tract cancers typically from fairly small, fairly old studies that all suggest that in certain stages of resected biliary tumors, either bile duct or gall bladder, adjuvant treatment works, and typically either chemotherapy and radiotherapy, or chemotherapy alone, but not radiotherapy alone.
Capecitabine has been used for metastatic disease for years, mostly by default, and because most GI tumors have some response to fluoropyridines. But we’re finally able now to do large trials in biliary tumors, so this trial was a very large study with almost 450 patients from the United Kingdom over an 8-year period. About 20% were gallbladder, so the R0 surgery was about 60%, R1 at about 40% (Figure 4).6
The endpoint of the study was survival advantage, and when they did the protocol analysis, the survival for the treated population was 53 months and for the observation arm, 36 months, so that was a hazard ratio of 0.75, which is acceptable in an adjuvant study. It’s simple drug to give, and usually tolerable, so this will represent a new standard of care. Of course, in the advanced disease setting, the gemcitabine–cisplatin combination is the standard of care for metastatic disease. It’s a little more toxic combination, but we know that’s standard. There’s an ongoing study in Europe called the ACTICCA-1 trial, and this is gemcitabine–cisplatin for 6 months versus not capecitabine, but a control arm. My guess is if the capecitabine study was positive, that this also will be a positive trial, because gemcitabine–cisplatin is probably more active. Then, we’ll have 2 standards, and I don’t think anyone is going to compare capecitabine with gemcitabine–cisplatin.
What you’ll have are two regimens for two different populations of patients. Perhaps for the elderly and people who have renal problems, capecitabine alone will give them benefit, and then you’ll have gemcitabine–cisplatin, which may be just a more toxic regimen, but also more effective for the younger, healthier people with fewer comorbidities.
DR HENRY Great data and a small population, but a population in need. That moves us on to pancreatic cancer, and I don’t know if this is happening nationwide, but in my practice, I’m seeing more. These patients tend to present beyond surgery, so they have metastatic or advanced pancreatic cancer. Any comment on where you think this field is going?
DR HALLER We were a bit bereft of new pancreatic cancer studies at ASCO this year. We’re certainly looking more at neoadjuvant therapy for pancreatic cancer, primarily because of ease of administration and the increased ability to tolerate treatments in the preoperative setting. There aren’t many people that get downstaged, but some are. Unfortunately, even in the MSI-high pancreas, which is a small subset, they don’t seem to get as big a bang out of the checkpoint inhibitors as in other tumor sites, so I’m afraid I didn’t come home with much new about this subset of patients.
DR HENRY We’ve covered a nice group of studies and practice-changing new standard-of-care comments from ASCO and other studies. Thank Dr Dan Haller for being with us and commenting. This podcast and discussion are brought to you from
1. Andre T, Bonnetain F, Mineur L, et al. Oxaliplatin-based chemotherapy for patients with stage III colon cancer: disease free survival results of the three versus six months adjuvant IDEA France trial. Abstract presented at: 2017 American Society of Clinical Oncology Annual Meeting; June 2-6, 2017; Chicago, IL. Abstract 3500.
2. Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28(8):1713-1729.
3. Kopetz S, McDonough SL, Lenz H-J, et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406). Abstract presented at: 2017 American Society of Clinical Oncology Annual Meeting; June 2-6, 2017; Chicago, IL. Abstract 3505.
4. Al-Batran S-E, Homann N, Schmalenberg H, et al. Perioperative chemotherapy with docetaxel, oxaliplatin. Abstract presented at: 2017 American Society of Clinical Oncology Annu, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): a multicenter, randomized phase 3 trial. Abstract presented at: 2017 American Society of Clinical Oncology Annual Meeting; June 2-6, 2017; Chicago, IL. Abstract 4004.
5. Kopetz S, Lonardi S, McDermott RS, et al. Concordance of DNA mismatch repair deficient (dMMR)/microsatellite instability (MSI) assessment by local and central testing in patients with metastatic CRC (mCRC) receiving nivolumab (nivo) in Checkmate 142 study. Abstract presented at: 2017 American Society of Clinical Oncology Annual Meeting; June 2-6, 2017; Chicago, IL. Abstract 3548.
6. Primrose JN, Fox R, Palmer DH, et al. Adjuvant capecitabine for biliary tract cancer: the BILCAP randomized study. Abstract presented at: 2017 American Society of Clinical Oncology Annual Meeting; June 2-6, 2017; Chicago, IL. Abstract 4006.
DR HENRY I am Dr David Henry, the Editor-in-Chief of
DR HALLER The IDEA collaboration was the brainchild of the late Dan Sargent, a biostatistician who was at the Mayo Clinic. It was his idea, since 6 international groups were all testing the same question of 3 months for oxaliplatin to 6 months of oxaliplatin, to combine the data in an individual patient database – which is the best way to do it – so there were these six trials that were all completed.
Three of them were individually reported at ASCO this year, and then the totality was presented at the plenary session – the first time in 12 years that a gastrointestinal (GI) cancer trial made the plenary session. The whole point, obviously, is neuropathy. With 6 months of FOLFOX or XELOX, about 13% or more patients will develop grade 3 neuropathy, even if people stop short of the full-cycle length, and that is a big deal for the 50,000 patients or so who get adjuvant therapy. At the plenary session, the data were presented and the next day three individual trials were presented and discussed by Jeff Meyerhardt (of Dana-Farber Cancer Institute, Boston).
There were 6 different trials: a few included rectum, some included stage II, some used CAPOX and FOLFOX-4 or 6. The only trial that used only FOLFOX was the Cancer and Leukemia Group B (CALGB) trial in the United States (US). There was a lot of heterogeneity, but when Dan was around, I asked him whether that was a problem, and he said on the contrary, was a better thing because it allowed for real-life practice.
The primary endpoint of the study was to look for noninferiority of 3 months versus 6 months of treatment. The noninferiority margin was at a hazard ratio of 1.12, so they were willing to barter down a few percentage points from benefit. If you looked at the primary disease-free survival analysis, the hazard ratio was 1.07, which was an absolute difference of 0.9%, favoring 3 months of therapy. But because the hazard ratio crossed the 1.12 boundary, it was considered inconclusive and not proven.
If you looked at the regimens, CAPOX outperformed FOLFOX. That’s a regimen we don’t do much in the US. We tend to use more FOLFOX, but CAPOX looked better. What they then did was look at the different subsets of patients, and the subsets that it was obviously as good in was the group that had T1-3N1 disease, where 3 months of therapy was clearly just as good as 6 months of therapy, with only a 3% risk of grade 3 neuropathy.
DR HENRY That would be one to three nodes?
DR HALLER Exactly. That’s about 50% of patients. In the T4N2 patients, neither regimen did very well and the 3-year disease-free survival was in the range of 50%, which is clearly unacceptable. Jeff discussed two things. Why could CAPOX be better? If you do the math, when you do CAPOX, you get more oxaliplatin during the first few months of therapy, because it’s 130 mg every 3 weeks, rather than 85 mg every 2 weeks. His conclusion was, “for my next patient who has T4N2 disease, I’ll offer 6 months of FOLFOX.” The study that really need
He discussed the two new trials. One is a study called ARGO, which is being done by the National Surgical Adjuvant Breast and Bowel Project, where people get standard adjuvant chemotherapy, and they’re then randomized to either 24 months of regorafenib 120 mg per day or a placebo. This is an attempt to recreate the transient benefit from bevacizumab in the NSABP C-08 trial. It’s accruing slowly because regorafenib has some toxicity associated with it, but it probably will be completed. Will it continue the benefit as seen in the 12 months of bevacizumab and C-08? We’ll see.
The other, more interesting study is being done in the cooperative groups looking at FOLFOX plus atezolizumab, one of the checkpoint inhibitors. The difficulty here is that only 15% of people with stage III disease have microsatellite instability (MSI)-high tumors, but it’s certainly compelling. This is a straight up comparison. It’s 6 months of FOLFOX in the control arm, or 6 months of FOLFOX plus atezolizumab concurrently for 6 months, and then an additional 6 months of atezolizumab. These are both very fascinating ideas.
DR HENRY To go back to one of your original points, this 3 versus 6 months: the neuropathy is significantly less in those getting the 3 months?
DR HALLER It went to 3%.
DR HENRY We all see that is very bothersome to patients. Before we leave colorectal, I must ask about the right-sided versus left-sided colorectal cancer that we hear a lot about now. Could you comment on how right-sided is worse than left-sided, and do we understand why?
DR HALLER There are two things to consider. If you look back even to simple trials of 5-FU or biochemical modulated 5-FU from 20 years ago, there were clear differences showing worse prognosis in patients with right-sided tumors, so that’s one point to be made. It’s been consistently seen but never acted upon. Then, the explanation for it, possibly, is that the right colon and left colon are two biologically different organs – and they are. Embryologically, the right colon comes from the midgut and the left colon comes from the hindgut, and there were several presentations at ASCO and at prior meetings showing that when you look at different mutations, they differ between the right and left colons. The right-sided tumors are more MSI-high and more BRAF-mutated, left-sided mutations less so.
Then, people started analyzing many of the very large colon cancer trials, including the US trial CALB/SWOG C80405 and the FIRE-3 trials in Europe, where backbone chemotherapy of FOLFIRI or FOLFOX was given with either cetuximab or bevacizumab in RAS wild-type patients. For one study, C80405, they saw that for cetuximab, on the right side, the median survival was 16.7 months and on the left side, it’s 36 months – a 20-month difference. In fact, if you look at the totality of the data, 16.7 almost looks like cetuximab is harming them, as if you were giving it to a RAS-mutated patient, but they were not. They were all RAS wild-type.
For bevacizumab, the right side was 24 months; the left side was 31.4 months. If you look at the left, cetuximab was 36 months and bevacizumab was 31.4 months, so it appears left-sided tumors should get more cetuximab than they are now getting in the US with a 5-month difference, but that decrement is much different on the right, where there’s an 8-month benefit for bevacizumab compared with cetuximab. There is a very good review by Dirk Arnold, who looked at a totality of 6 studies to really examine this more carefully.2
The National Comprehensive Cancer Network has chimed in on this, and is suggesting that for the 25% of people who have right-sided tumors, epidermal growth factor receptor (EGFR) agents not be considered in first-line therapy. NCCN did not go as far to say that EGFR agents should be given on the left side. As I said, the differences are much more impressive in the right, so this is a real sea change for people to consider which side of the tumor affects outcome.
Deb Schrag (Dana-Farber Cancer Institute) presented data at last year’s ASCO not only for stage IV disease showing the same thing, but also stage III disease where there are also right-versus-left differences in terms of recurrence, with a hazard ratio on the right side of about 1.4 compared with the left-sided tumors. Maybe it should be true that 3 months is especially good if you’re treating left-sided tumors, and maybe the right-sided tumor needs to be also calculated with the factors we just talked about. These are two big changes in an area in which we literally haven’t made any change since FOLFOX was introduced a decade ago.
DR HENRY That’s really fascinating, and if not practice changing, then practice challenging. Staying with the mutations idea, in my patients, I’m checking the RAS family and the BRAF mutation, where I’ve learned that’s a particularly bad mutation. I wonder if you might comment on the Kopetz trial, which took a cohort of BRAF mutants and treated them (Figure 2).3 How did that turn out?
DR HALLER It turned out well. We’re turning colon cancer into non–small cell lung cancer in that we’re getting small groups of patients who now have very dedicated care. The backstory here is that there was some thought that you should be treating mutations, not tumor sites. Drugs such as vemurafenib, for example, which is a BRAF inhibitor, worked well in melanoma for the same mutation that’s in colon cancer, V600E. But when vemurafenib was used in the BRAF-mutant patients – these are 10% of the population – median survivorship was one-third that of the rest of the patients, so roughly 12 months. People looked like they were doing worse when vemurafenib was used. They had no benefit.
Scott Kopetz at MD Anderson (Houston, Texas) is a very good bench-to-bed-and-back sort of doc. He looked at this in cell lines and found that when you give a BRAF inhibitor, you upregulate EGFR so you add an EGFR inhibitor. He did a phase 1 and 1B study, and then in the co-operative groups, a study was done – a randomized phase 2 trial for people who had the BRAF-V600E mutation failing first-line therapy, and then went on to receive either irinotecan single agent or irinotecan plus cetuximab or a triple arm of irinotecan, cetuximab, and vemurafenib. There was a crossover, and so the primary endpoint was progression-free survival. It accrued rapidly.
Again, small study, about 100 patients, but for the double-agent arm, or cetuximab–irinotecan, the median survivorship was 2 months. It was 4.4 months for the combination, so more than double. The response rate quadrupled from 4% to 16%, and the people who had disease control tripled, from 22% to 67%. Many of these patients had bulky disease, BRAF mutations. They need response, so this is a very important endpoint.
Overall survival was not different, in part because it was a crossover, and the crossover patients did pretty well. This is going to move more toward first-line therapy, because we don’t talk about fourth- and fifth-line therapies, TAS-102 or regorafenib. These patients don’t make it to even third line. We’re chipping away at what we think is a very homogenous group of peoples’ metastatic disease. They’re obviously not.
DR HENRY In the BRAF-mutant patient, the vemurafenib might drive them toward EGFR, and then the cetuximab could come in and handle that diversion of the pathway. Fascinating.
DR HALLER The preferred regimen in first-line therapy for a BRAF mutant might be FOLFIRI, cetuximab, and vemurafenib, especially on the left side.
DR HENRY Certainly makes sense. We’ll continue the theme at ASCO of “new standard of care.” Let’s move to gastroesophageal junction. There was a so-called FLOT (5-FU, leucovorin, oxaliplatin, Taxotere) presentation in the neoadjuvant/adjuvant setting, 4 cycles preoperatively and 4 cycles postoperatively. Could you comment on that study?
DR HALLER Gastric cancer for metastatic disease has a very large buffet of treatment regimens, and some just become entrenched, like the ECF regimen with epirubicin (epirubicin, cisplatin, 5-FU), where most people don’t exactly know what the contribution of that drug is, and so some people use EOX (epirubicin, oxaliplatin, capecitabine), some people use FOLFOX, some people use FOLFIRI. It gets a little bit confusing as to whether you use taxanes, platinums, or 5-FU or capecitabine.
The Germans came up with a regimen called FLOT – it’s sort of like FOLFOX with Taxotere attached. They did a very large study comparing it with ECF or ECX (epirubicin, cisplatin, capecitabine; Figure 3).4 The overall endpoint with over 700 patients was survival. This is an adjuvant regimen. Only 37% of people got ECF or ECX postoperatively, and 50% of the FLOT patients got the regimens postoperatively.
One of the reasons FLOT might be more beneficial is that more people were given postoperative treatment, and it’s one reason why many adjuvant regimens are being moved completely preoperatively, because so few people get the planned treatment. The FLOT regimen improved overall survival with a P value of .0112 and a hazard ratio of 0.77. The difference was 35 months versus 50 months. With the uncertainty as to what epirubicin actually does and the fact that it’s been around for a while and that fewer people receive postoperative treatment, with that 15-month benefit, if you’re using chemotherapy alone, and there’s no radiotherapy component for true gastric cancer, this is a new standard of care.
DR HENRY I struggle with this in my patients as well. This concept of getting more therapy preoperatively to those who can’t get it postoperatively certainly resonates with most of us in practice.
DR HALLER If I were redesigning the trial, I would probably say just give 4-6 cycles of treatment, and give it all preoperatively. In rectal cancer, there’s the total neoadjuvant approach, where it’s being tested in people who get all their chemotherapy first, then chemoradiotherapy, then surgery, and you’re done.
DR HENRY Yes, right. Thank you for mentioning that. Staying with the gastric GE junction, you couldn’t get away from ASCO this year without hearing about the checkpoint inhibitor immunotherapies in this population. In the CHECKMATE-142 trial with nivolumab versus placebo, response rates were good, especially in the MSI-high (microsatellite instability). Could you comment on that study?
DR HALLER We already know that in May and July 2017, pembrolizumab and nivolumab were both approved for any MSI-high solid tumor based on phase 2 data only, and based on response. That’s the first time we’ve seen that happen. It’s remarkable. For nivolumab, the approval was based on 53 patients with MSI-high metastatic colon cancer. So these were people who failed standard therapy and got nivolumab by standard infusion every 2 weeks. The overall response rate was almost 30% in this population, which is typically quite resistant to any treatment, so one expects much lower response rates with anything in that setting – chemotherapy, TAS-102, regorafenib, et cetera (Table).5
More importantly, as we’re seeing with Jimmy Carter with checkpoint treatment (for melanoma that had metastasized to the brain), responses lasted for more than 6 months in about two-thirds of patients, even a complete response, so this is just off the wall. I mean, this is not what you would expect with almost any other treatment. The data are the same for atezolizumab and for pembrolizumab. What seems to be true is that in the GI tumors and colon cancer, MSI-high seems more important than expression of PD-1 or PD-L1 (programmed cell death protein-1 or programmed cell death protein-ligand 1).
In different tumor sites, PD-1 or PD-L1 measurement may be important, but in these tumors, and in colorectal cancer, it looks as if MSI-high is the preferred measurement. Recently ASCO, together with the American Society for Clinical Pathology, College of American Pathologists, and Association for Molecular Pathology, came out with guidelines on what you should measure in colorectal cancer specimens. Obviously, one is extended RAS. They say you should get BRAF for prognosis, but it may also be a prognostic factor that leads you to treat, which ultimately makes it a predictive factor, so the data from Kopetz might suggest that will move up to something you also must measure. If patients have the BRAF mutation, it’s important they know that it’s a poor prognostic sign. But if they come in with literature saying they might live 36 months when their actual outcome is about a third of that, you need to frame your discussion in that regard and make sure they understand it.
The guidelines also suggested getting MSI-high, and certainly prognostically in early-stage disease, but now it’s going to be a predictive factor, so in the month in which these recommendations are made, two of them are already out of date. They also didn’t include human epidermal growth factor receptor 2 (HER2), and what we’ve heard from the HERACLES (HER2 Amplification for Colorectal Cancer Enhanced Stratification) trial is that for those patients who got the trastuzumab and pertuzumab combination – and this is another 5% of patients – almost the same data was seen as in the MSI-high patients with checkpoint inhibitors. That is double-digit response rates and durable responses. As I said, we’re very much nearing in colorectal cancer what’s now being done in non-small cell lung cancer.
DR HENRY Indeed. Could you comment on the BILCAP study and adjuvant capecitabine for biliary tract cancer?
DR HALLER There are large meta-analyses looking at adjuvant therapy for biliary tract cancers typically from fairly small, fairly old studies that all suggest that in certain stages of resected biliary tumors, either bile duct or gall bladder, adjuvant treatment works, and typically either chemotherapy and radiotherapy, or chemotherapy alone, but not radiotherapy alone.
Capecitabine has been used for metastatic disease for years, mostly by default, and because most GI tumors have some response to fluoropyridines. But we’re finally able now to do large trials in biliary tumors, so this trial was a very large study with almost 450 patients from the United Kingdom over an 8-year period. About 20% were gallbladder, so the R0 surgery was about 60%, R1 at about 40% (Figure 4).6
The endpoint of the study was survival advantage, and when they did the protocol analysis, the survival for the treated population was 53 months and for the observation arm, 36 months, so that was a hazard ratio of 0.75, which is acceptable in an adjuvant study. It’s simple drug to give, and usually tolerable, so this will represent a new standard of care. Of course, in the advanced disease setting, the gemcitabine–cisplatin combination is the standard of care for metastatic disease. It’s a little more toxic combination, but we know that’s standard. There’s an ongoing study in Europe called the ACTICCA-1 trial, and this is gemcitabine–cisplatin for 6 months versus not capecitabine, but a control arm. My guess is if the capecitabine study was positive, that this also will be a positive trial, because gemcitabine–cisplatin is probably more active. Then, we’ll have 2 standards, and I don’t think anyone is going to compare capecitabine with gemcitabine–cisplatin.
What you’ll have are two regimens for two different populations of patients. Perhaps for the elderly and people who have renal problems, capecitabine alone will give them benefit, and then you’ll have gemcitabine–cisplatin, which may be just a more toxic regimen, but also more effective for the younger, healthier people with fewer comorbidities.
DR HENRY Great data and a small population, but a population in need. That moves us on to pancreatic cancer, and I don’t know if this is happening nationwide, but in my practice, I’m seeing more. These patients tend to present beyond surgery, so they have metastatic or advanced pancreatic cancer. Any comment on where you think this field is going?
DR HALLER We were a bit bereft of new pancreatic cancer studies at ASCO this year. We’re certainly looking more at neoadjuvant therapy for pancreatic cancer, primarily because of ease of administration and the increased ability to tolerate treatments in the preoperative setting. There aren’t many people that get downstaged, but some are. Unfortunately, even in the MSI-high pancreas, which is a small subset, they don’t seem to get as big a bang out of the checkpoint inhibitors as in other tumor sites, so I’m afraid I didn’t come home with much new about this subset of patients.
DR HENRY We’ve covered a nice group of studies and practice-changing new standard-of-care comments from ASCO and other studies. Thank Dr Dan Haller for being with us and commenting. This podcast and discussion are brought to you from
DR HENRY I am Dr David Henry, the Editor-in-Chief of
DR HALLER The IDEA collaboration was the brainchild of the late Dan Sargent, a biostatistician who was at the Mayo Clinic. It was his idea, since 6 international groups were all testing the same question of 3 months for oxaliplatin to 6 months of oxaliplatin, to combine the data in an individual patient database – which is the best way to do it – so there were these six trials that were all completed.
Three of them were individually reported at ASCO this year, and then the totality was presented at the plenary session – the first time in 12 years that a gastrointestinal (GI) cancer trial made the plenary session. The whole point, obviously, is neuropathy. With 6 months of FOLFOX or XELOX, about 13% or more patients will develop grade 3 neuropathy, even if people stop short of the full-cycle length, and that is a big deal for the 50,000 patients or so who get adjuvant therapy. At the plenary session, the data were presented and the next day three individual trials were presented and discussed by Jeff Meyerhardt (of Dana-Farber Cancer Institute, Boston).
There were 6 different trials: a few included rectum, some included stage II, some used CAPOX and FOLFOX-4 or 6. The only trial that used only FOLFOX was the Cancer and Leukemia Group B (CALGB) trial in the United States (US). There was a lot of heterogeneity, but when Dan was around, I asked him whether that was a problem, and he said on the contrary, was a better thing because it allowed for real-life practice.
The primary endpoint of the study was to look for noninferiority of 3 months versus 6 months of treatment. The noninferiority margin was at a hazard ratio of 1.12, so they were willing to barter down a few percentage points from benefit. If you looked at the primary disease-free survival analysis, the hazard ratio was 1.07, which was an absolute difference of 0.9%, favoring 3 months of therapy. But because the hazard ratio crossed the 1.12 boundary, it was considered inconclusive and not proven.
If you looked at the regimens, CAPOX outperformed FOLFOX. That’s a regimen we don’t do much in the US. We tend to use more FOLFOX, but CAPOX looked better. What they then did was look at the different subsets of patients, and the subsets that it was obviously as good in was the group that had T1-3N1 disease, where 3 months of therapy was clearly just as good as 6 months of therapy, with only a 3% risk of grade 3 neuropathy.
DR HENRY That would be one to three nodes?
DR HALLER Exactly. That’s about 50% of patients. In the T4N2 patients, neither regimen did very well and the 3-year disease-free survival was in the range of 50%, which is clearly unacceptable. Jeff discussed two things. Why could CAPOX be better? If you do the math, when you do CAPOX, you get more oxaliplatin during the first few months of therapy, because it’s 130 mg every 3 weeks, rather than 85 mg every 2 weeks. His conclusion was, “for my next patient who has T4N2 disease, I’ll offer 6 months of FOLFOX.” The study that really need
He discussed the two new trials. One is a study called ARGO, which is being done by the National Surgical Adjuvant Breast and Bowel Project, where people get standard adjuvant chemotherapy, and they’re then randomized to either 24 months of regorafenib 120 mg per day or a placebo. This is an attempt to recreate the transient benefit from bevacizumab in the NSABP C-08 trial. It’s accruing slowly because regorafenib has some toxicity associated with it, but it probably will be completed. Will it continue the benefit as seen in the 12 months of bevacizumab and C-08? We’ll see.
The other, more interesting study is being done in the cooperative groups looking at FOLFOX plus atezolizumab, one of the checkpoint inhibitors. The difficulty here is that only 15% of people with stage III disease have microsatellite instability (MSI)-high tumors, but it’s certainly compelling. This is a straight up comparison. It’s 6 months of FOLFOX in the control arm, or 6 months of FOLFOX plus atezolizumab concurrently for 6 months, and then an additional 6 months of atezolizumab. These are both very fascinating ideas.
DR HENRY To go back to one of your original points, this 3 versus 6 months: the neuropathy is significantly less in those getting the 3 months?
DR HALLER It went to 3%.
DR HENRY We all see that is very bothersome to patients. Before we leave colorectal, I must ask about the right-sided versus left-sided colorectal cancer that we hear a lot about now. Could you comment on how right-sided is worse than left-sided, and do we understand why?
DR HALLER There are two things to consider. If you look back even to simple trials of 5-FU or biochemical modulated 5-FU from 20 years ago, there were clear differences showing worse prognosis in patients with right-sided tumors, so that’s one point to be made. It’s been consistently seen but never acted upon. Then, the explanation for it, possibly, is that the right colon and left colon are two biologically different organs – and they are. Embryologically, the right colon comes from the midgut and the left colon comes from the hindgut, and there were several presentations at ASCO and at prior meetings showing that when you look at different mutations, they differ between the right and left colons. The right-sided tumors are more MSI-high and more BRAF-mutated, left-sided mutations less so.
Then, people started analyzing many of the very large colon cancer trials, including the US trial CALB/SWOG C80405 and the FIRE-3 trials in Europe, where backbone chemotherapy of FOLFIRI or FOLFOX was given with either cetuximab or bevacizumab in RAS wild-type patients. For one study, C80405, they saw that for cetuximab, on the right side, the median survival was 16.7 months and on the left side, it’s 36 months – a 20-month difference. In fact, if you look at the totality of the data, 16.7 almost looks like cetuximab is harming them, as if you were giving it to a RAS-mutated patient, but they were not. They were all RAS wild-type.
For bevacizumab, the right side was 24 months; the left side was 31.4 months. If you look at the left, cetuximab was 36 months and bevacizumab was 31.4 months, so it appears left-sided tumors should get more cetuximab than they are now getting in the US with a 5-month difference, but that decrement is much different on the right, where there’s an 8-month benefit for bevacizumab compared with cetuximab. There is a very good review by Dirk Arnold, who looked at a totality of 6 studies to really examine this more carefully.2
The National Comprehensive Cancer Network has chimed in on this, and is suggesting that for the 25% of people who have right-sided tumors, epidermal growth factor receptor (EGFR) agents not be considered in first-line therapy. NCCN did not go as far to say that EGFR agents should be given on the left side. As I said, the differences are much more impressive in the right, so this is a real sea change for people to consider which side of the tumor affects outcome.
Deb Schrag (Dana-Farber Cancer Institute) presented data at last year’s ASCO not only for stage IV disease showing the same thing, but also stage III disease where there are also right-versus-left differences in terms of recurrence, with a hazard ratio on the right side of about 1.4 compared with the left-sided tumors. Maybe it should be true that 3 months is especially good if you’re treating left-sided tumors, and maybe the right-sided tumor needs to be also calculated with the factors we just talked about. These are two big changes in an area in which we literally haven’t made any change since FOLFOX was introduced a decade ago.
DR HENRY That’s really fascinating, and if not practice changing, then practice challenging. Staying with the mutations idea, in my patients, I’m checking the RAS family and the BRAF mutation, where I’ve learned that’s a particularly bad mutation. I wonder if you might comment on the Kopetz trial, which took a cohort of BRAF mutants and treated them (Figure 2).3 How did that turn out?
DR HALLER It turned out well. We’re turning colon cancer into non–small cell lung cancer in that we’re getting small groups of patients who now have very dedicated care. The backstory here is that there was some thought that you should be treating mutations, not tumor sites. Drugs such as vemurafenib, for example, which is a BRAF inhibitor, worked well in melanoma for the same mutation that’s in colon cancer, V600E. But when vemurafenib was used in the BRAF-mutant patients – these are 10% of the population – median survivorship was one-third that of the rest of the patients, so roughly 12 months. People looked like they were doing worse when vemurafenib was used. They had no benefit.
Scott Kopetz at MD Anderson (Houston, Texas) is a very good bench-to-bed-and-back sort of doc. He looked at this in cell lines and found that when you give a BRAF inhibitor, you upregulate EGFR so you add an EGFR inhibitor. He did a phase 1 and 1B study, and then in the co-operative groups, a study was done – a randomized phase 2 trial for people who had the BRAF-V600E mutation failing first-line therapy, and then went on to receive either irinotecan single agent or irinotecan plus cetuximab or a triple arm of irinotecan, cetuximab, and vemurafenib. There was a crossover, and so the primary endpoint was progression-free survival. It accrued rapidly.
Again, small study, about 100 patients, but for the double-agent arm, or cetuximab–irinotecan, the median survivorship was 2 months. It was 4.4 months for the combination, so more than double. The response rate quadrupled from 4% to 16%, and the people who had disease control tripled, from 22% to 67%. Many of these patients had bulky disease, BRAF mutations. They need response, so this is a very important endpoint.
Overall survival was not different, in part because it was a crossover, and the crossover patients did pretty well. This is going to move more toward first-line therapy, because we don’t talk about fourth- and fifth-line therapies, TAS-102 or regorafenib. These patients don’t make it to even third line. We’re chipping away at what we think is a very homogenous group of peoples’ metastatic disease. They’re obviously not.
DR HENRY In the BRAF-mutant patient, the vemurafenib might drive them toward EGFR, and then the cetuximab could come in and handle that diversion of the pathway. Fascinating.
DR HALLER The preferred regimen in first-line therapy for a BRAF mutant might be FOLFIRI, cetuximab, and vemurafenib, especially on the left side.
DR HENRY Certainly makes sense. We’ll continue the theme at ASCO of “new standard of care.” Let’s move to gastroesophageal junction. There was a so-called FLOT (5-FU, leucovorin, oxaliplatin, Taxotere) presentation in the neoadjuvant/adjuvant setting, 4 cycles preoperatively and 4 cycles postoperatively. Could you comment on that study?
DR HALLER Gastric cancer for metastatic disease has a very large buffet of treatment regimens, and some just become entrenched, like the ECF regimen with epirubicin (epirubicin, cisplatin, 5-FU), where most people don’t exactly know what the contribution of that drug is, and so some people use EOX (epirubicin, oxaliplatin, capecitabine), some people use FOLFOX, some people use FOLFIRI. It gets a little bit confusing as to whether you use taxanes, platinums, or 5-FU or capecitabine.
The Germans came up with a regimen called FLOT – it’s sort of like FOLFOX with Taxotere attached. They did a very large study comparing it with ECF or ECX (epirubicin, cisplatin, capecitabine; Figure 3).4 The overall endpoint with over 700 patients was survival. This is an adjuvant regimen. Only 37% of people got ECF or ECX postoperatively, and 50% of the FLOT patients got the regimens postoperatively.
One of the reasons FLOT might be more beneficial is that more people were given postoperative treatment, and it’s one reason why many adjuvant regimens are being moved completely preoperatively, because so few people get the planned treatment. The FLOT regimen improved overall survival with a P value of .0112 and a hazard ratio of 0.77. The difference was 35 months versus 50 months. With the uncertainty as to what epirubicin actually does and the fact that it’s been around for a while and that fewer people receive postoperative treatment, with that 15-month benefit, if you’re using chemotherapy alone, and there’s no radiotherapy component for true gastric cancer, this is a new standard of care.
DR HENRY I struggle with this in my patients as well. This concept of getting more therapy preoperatively to those who can’t get it postoperatively certainly resonates with most of us in practice.
DR HALLER If I were redesigning the trial, I would probably say just give 4-6 cycles of treatment, and give it all preoperatively. In rectal cancer, there’s the total neoadjuvant approach, where it’s being tested in people who get all their chemotherapy first, then chemoradiotherapy, then surgery, and you’re done.
DR HENRY Yes, right. Thank you for mentioning that. Staying with the gastric GE junction, you couldn’t get away from ASCO this year without hearing about the checkpoint inhibitor immunotherapies in this population. In the CHECKMATE-142 trial with nivolumab versus placebo, response rates were good, especially in the MSI-high (microsatellite instability). Could you comment on that study?
DR HALLER We already know that in May and July 2017, pembrolizumab and nivolumab were both approved for any MSI-high solid tumor based on phase 2 data only, and based on response. That’s the first time we’ve seen that happen. It’s remarkable. For nivolumab, the approval was based on 53 patients with MSI-high metastatic colon cancer. So these were people who failed standard therapy and got nivolumab by standard infusion every 2 weeks. The overall response rate was almost 30% in this population, which is typically quite resistant to any treatment, so one expects much lower response rates with anything in that setting – chemotherapy, TAS-102, regorafenib, et cetera (Table).5
More importantly, as we’re seeing with Jimmy Carter with checkpoint treatment (for melanoma that had metastasized to the brain), responses lasted for more than 6 months in about two-thirds of patients, even a complete response, so this is just off the wall. I mean, this is not what you would expect with almost any other treatment. The data are the same for atezolizumab and for pembrolizumab. What seems to be true is that in the GI tumors and colon cancer, MSI-high seems more important than expression of PD-1 or PD-L1 (programmed cell death protein-1 or programmed cell death protein-ligand 1).
In different tumor sites, PD-1 or PD-L1 measurement may be important, but in these tumors, and in colorectal cancer, it looks as if MSI-high is the preferred measurement. Recently ASCO, together with the American Society for Clinical Pathology, College of American Pathologists, and Association for Molecular Pathology, came out with guidelines on what you should measure in colorectal cancer specimens. Obviously, one is extended RAS. They say you should get BRAF for prognosis, but it may also be a prognostic factor that leads you to treat, which ultimately makes it a predictive factor, so the data from Kopetz might suggest that will move up to something you also must measure. If patients have the BRAF mutation, it’s important they know that it’s a poor prognostic sign. But if they come in with literature saying they might live 36 months when their actual outcome is about a third of that, you need to frame your discussion in that regard and make sure they understand it.
The guidelines also suggested getting MSI-high, and certainly prognostically in early-stage disease, but now it’s going to be a predictive factor, so in the month in which these recommendations are made, two of them are already out of date. They also didn’t include human epidermal growth factor receptor 2 (HER2), and what we’ve heard from the HERACLES (HER2 Amplification for Colorectal Cancer Enhanced Stratification) trial is that for those patients who got the trastuzumab and pertuzumab combination – and this is another 5% of patients – almost the same data was seen as in the MSI-high patients with checkpoint inhibitors. That is double-digit response rates and durable responses. As I said, we’re very much nearing in colorectal cancer what’s now being done in non-small cell lung cancer.
DR HENRY Indeed. Could you comment on the BILCAP study and adjuvant capecitabine for biliary tract cancer?
DR HALLER There are large meta-analyses looking at adjuvant therapy for biliary tract cancers typically from fairly small, fairly old studies that all suggest that in certain stages of resected biliary tumors, either bile duct or gall bladder, adjuvant treatment works, and typically either chemotherapy and radiotherapy, or chemotherapy alone, but not radiotherapy alone.
Capecitabine has been used for metastatic disease for years, mostly by default, and because most GI tumors have some response to fluoropyridines. But we’re finally able now to do large trials in biliary tumors, so this trial was a very large study with almost 450 patients from the United Kingdom over an 8-year period. About 20% were gallbladder, so the R0 surgery was about 60%, R1 at about 40% (Figure 4).6
The endpoint of the study was survival advantage, and when they did the protocol analysis, the survival for the treated population was 53 months and for the observation arm, 36 months, so that was a hazard ratio of 0.75, which is acceptable in an adjuvant study. It’s simple drug to give, and usually tolerable, so this will represent a new standard of care. Of course, in the advanced disease setting, the gemcitabine–cisplatin combination is the standard of care for metastatic disease. It’s a little more toxic combination, but we know that’s standard. There’s an ongoing study in Europe called the ACTICCA-1 trial, and this is gemcitabine–cisplatin for 6 months versus not capecitabine, but a control arm. My guess is if the capecitabine study was positive, that this also will be a positive trial, because gemcitabine–cisplatin is probably more active. Then, we’ll have 2 standards, and I don’t think anyone is going to compare capecitabine with gemcitabine–cisplatin.
What you’ll have are two regimens for two different populations of patients. Perhaps for the elderly and people who have renal problems, capecitabine alone will give them benefit, and then you’ll have gemcitabine–cisplatin, which may be just a more toxic regimen, but also more effective for the younger, healthier people with fewer comorbidities.
DR HENRY Great data and a small population, but a population in need. That moves us on to pancreatic cancer, and I don’t know if this is happening nationwide, but in my practice, I’m seeing more. These patients tend to present beyond surgery, so they have metastatic or advanced pancreatic cancer. Any comment on where you think this field is going?
DR HALLER We were a bit bereft of new pancreatic cancer studies at ASCO this year. We’re certainly looking more at neoadjuvant therapy for pancreatic cancer, primarily because of ease of administration and the increased ability to tolerate treatments in the preoperative setting. There aren’t many people that get downstaged, but some are. Unfortunately, even in the MSI-high pancreas, which is a small subset, they don’t seem to get as big a bang out of the checkpoint inhibitors as in other tumor sites, so I’m afraid I didn’t come home with much new about this subset of patients.
DR HENRY We’ve covered a nice group of studies and practice-changing new standard-of-care comments from ASCO and other studies. Thank Dr Dan Haller for being with us and commenting. This podcast and discussion are brought to you from
1. Andre T, Bonnetain F, Mineur L, et al. Oxaliplatin-based chemotherapy for patients with stage III colon cancer: disease free survival results of the three versus six months adjuvant IDEA France trial. Abstract presented at: 2017 American Society of Clinical Oncology Annual Meeting; June 2-6, 2017; Chicago, IL. Abstract 3500.
2. Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28(8):1713-1729.
3. Kopetz S, McDonough SL, Lenz H-J, et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406). Abstract presented at: 2017 American Society of Clinical Oncology Annual Meeting; June 2-6, 2017; Chicago, IL. Abstract 3505.
4. Al-Batran S-E, Homann N, Schmalenberg H, et al. Perioperative chemotherapy with docetaxel, oxaliplatin. Abstract presented at: 2017 American Society of Clinical Oncology Annu, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): a multicenter, randomized phase 3 trial. Abstract presented at: 2017 American Society of Clinical Oncology Annual Meeting; June 2-6, 2017; Chicago, IL. Abstract 4004.
5. Kopetz S, Lonardi S, McDermott RS, et al. Concordance of DNA mismatch repair deficient (dMMR)/microsatellite instability (MSI) assessment by local and central testing in patients with metastatic CRC (mCRC) receiving nivolumab (nivo) in Checkmate 142 study. Abstract presented at: 2017 American Society of Clinical Oncology Annual Meeting; June 2-6, 2017; Chicago, IL. Abstract 3548.
6. Primrose JN, Fox R, Palmer DH, et al. Adjuvant capecitabine for biliary tract cancer: the BILCAP randomized study. Abstract presented at: 2017 American Society of Clinical Oncology Annual Meeting; June 2-6, 2017; Chicago, IL. Abstract 4006.
1. Andre T, Bonnetain F, Mineur L, et al. Oxaliplatin-based chemotherapy for patients with stage III colon cancer: disease free survival results of the three versus six months adjuvant IDEA France trial. Abstract presented at: 2017 American Society of Clinical Oncology Annual Meeting; June 2-6, 2017; Chicago, IL. Abstract 3500.
2. Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28(8):1713-1729.
3. Kopetz S, McDonough SL, Lenz H-J, et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406). Abstract presented at: 2017 American Society of Clinical Oncology Annual Meeting; June 2-6, 2017; Chicago, IL. Abstract 3505.
4. Al-Batran S-E, Homann N, Schmalenberg H, et al. Perioperative chemotherapy with docetaxel, oxaliplatin. Abstract presented at: 2017 American Society of Clinical Oncology Annu, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): a multicenter, randomized phase 3 trial. Abstract presented at: 2017 American Society of Clinical Oncology Annual Meeting; June 2-6, 2017; Chicago, IL. Abstract 4004.
5. Kopetz S, Lonardi S, McDermott RS, et al. Concordance of DNA mismatch repair deficient (dMMR)/microsatellite instability (MSI) assessment by local and central testing in patients with metastatic CRC (mCRC) receiving nivolumab (nivo) in Checkmate 142 study. Abstract presented at: 2017 American Society of Clinical Oncology Annual Meeting; June 2-6, 2017; Chicago, IL. Abstract 3548.
6. Primrose JN, Fox R, Palmer DH, et al. Adjuvant capecitabine for biliary tract cancer: the BILCAP randomized study. Abstract presented at: 2017 American Society of Clinical Oncology Annual Meeting; June 2-6, 2017; Chicago, IL. Abstract 4006.