User login
Managing Eating Disorders on a General Pediatrics Unit: A Centralized Video Monitoring Pilot
Hospitalizations for nutritional rehabilitation of patients with restrictive eating disorders are increasing.1 Among primary mental health admissions at free-standing children’s hospitals, eating disorders represent 5.5% of hospitalizations and are associated with the longest length of stay (LOS; mean 14.3 days) and costliest care (mean $46,130).2 Admission is necessary to ensure initial weight restoration and monitoring for symptoms of refeeding syndrome, including electrolyte shifts and vital sign abnormalities.3-5
Supervision is generally considered an essential element of caring for hospitalized patients with eating disorders, who may experience difficulty adhering to nutritional treatment, perform excessive movement or exercise, or demonstrate purging or self-harming behaviors. Supervision is presumed to prevent counterproductive behaviors, facilitating weight gain and earlier discharge to psychiatric treatment. Best practices for patient supervision to address these challenges have not been established but often include meal time or continuous one-to-one supervision by nursing assistants (NAs) or other staff.6,7 While meal supervision has been shown to decrease medical LOS, it is costly, reduces staff availability for the care of other patient care, and can be a barrier to caring for patients with eating disorders in many institutions.8
Although not previously used in patients with eating disorders, centralized video monitoring (CVM) may provide an additional mode of supervision. CVM is an emerging technology consisting of real-time video streaming, without video recording, enabling tracking of patient movement, redirection of behaviors, and communication with unit nurses when necessary. CVM has been used in multiple patient safety initiatives to reduce falls, address staffing shortages, reduce costs,9,10 supervise patients at risk for self-harm or elopement, and prevent controlled medication diversion.10,11
We sought to pilot a novel use of CVM to replace our institution’s standard practice of continuous one-to-one nursing assistant (NA) supervision of patients admitted for medical stabilization of an eating disorder. Our objective was to evaluate the supervision cost and feasibility of CVM, using LOS and days to weight gain as balancing measures.
METHODS
Setting and Participants
This retrospective cohort study included patients 12-18 years old admitted to the pediatric hospital medicine service on a general unit of an academic quaternary care children’s hospital for medical stabilization of an eating disorder between September 2013 and March 2017. Patients were identified using administrative data based on primary or secondary diagnosis of anorexia nervosa, eating disorder not other wise specified, or another specified eating disorder (ICD 9 3071, 20759, or ICD 10 f5000, 5001, f5089, f509).12,13 This research study was considered exempt by the University of Wisconsin School of Medicine and Public Health’s Institutional Review Board.
Supervision Interventions
A standard medical stabilization protocol was used for patients admitted with an eating disorder throughout the study period (Appendix). All patients received continuous one-to-one NA supervision until they reached the target calorie intake and demonstrated the ability to follow the nutritional meal protocol. Beginning July 2015, patients received continuous CVM supervision unless they expressed suicidal ideation (SI), which triggered one-to-one NA supervision until they no longer endorsed suicidality.
Centralized Video Monitoring Implementation
Institutional CVM technology was AvaSys TeleSitter Solution (AvaSure, Inc). Our institution purchased CVM devices for use in adult settings, and one was assigned for pediatric CVM. Mobile CVM video carts were deployed to patient rooms and generated live video streams, without recorded capture, which were supervised by CVM technicians. These technicians were NAs hired and trained specifically for this role; worked four-, eight-, and 12-hour shifts; and observed up to eight camera feeds on a single monitor in a centralized room. Patients and family members could refuse CVM, which would trigger one-to-one NA supervision. Patients were not observed by CVM while in the restroom; staff were notified by either the patient or technician, and one-to-one supervision was provided. CVM had two-way audio communication, which allowed technicians to redirect patients verbally. Technicians could contact nursing staff directly by phone when additional intervention was needed.
Supervision Costs
NA supervision costs were estimated at $19/hour, based upon institutional human resources average NA salaries at that time. No additional mealtime supervision was included, as in-person supervision was already occurring.
CVM supervision costs were defined as the sum of the device cost plus CVM technician costs and two hours of one-to-one NA mealtime supervision per day. The CVM device cost was estimated at $2.10/hour, assuming a 10-year machine life expectancy (single unit cost $82,893 in 2015, 3,944 hours of use in fiscal year of 2018). CVM technician costs were $19/hour, based upon institutional human resources average CVM technician salaries at that time. Because technicians monitored an average of six patients simultaneously during this study, one-sixth of a CVM technician’s salary (ie, $3.17/hour) was used for each hour of CVM monitoring. Patients with mixed (NA and CVM) supervision were analyzed with those having CVM supervision. These patients’ costs were the sum of their NA supervision costs plus their CVM supervision costs.
Data Collection
Descriptive variables including age, gender, race/ethnicity, insurance, and LOS were collected from administrative data. The duration and type of supervision for all patients were collected from daily staffing logs. The eating disorder protocol standardized the process of obtaining daily weights (Appendix). Days to weight gain following admission were defined as the total number of days from admission to the first day of weight gain that was followed by another day of weight gain or maintaining the same weight
Data Analysis
Patient and hospitalization characteristics were summarized. A sample size of at least 14 in each group was estimated as necessary to detect a 50% reduction in supervision cost between the groups using alpha = 0.05, a power of 80%, a mean cost of $4,400 in the NA group, and a standard deviation of $1,600.Wilcoxon rank-sum tests were used to assess differences in median supervision cost between NA and CVM use. Differences in mean LOS and days to weight gain between NA and CVM use were assessed with t-tests because these data were normally distributed.
RESULTS
Patient Characteristics and Supervision Costs
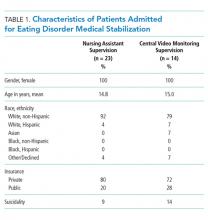
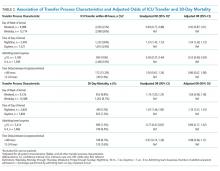
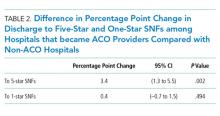
The study included 37 consecutive admissions (NA = 23 and CVM = 14) with 35 unique patients. Patients were female, primarily non-Hispanic White, and privately insured (Table 1). Median supervision cost for the NA was statistically significantly more expensive at $4,104/admission versus $1,166/admission for CVM (P < .001, Table 2).
Balancing Measures, Acceptability, and Feasibility
Mean LOS was 11.7 days for NA and 9.8 days for CVM (P = .27; Table 2). The mean number of days to weight gain was 3.1 and 3.6 days, respectively (P = .28). No patients converted from CVM to NA supervision. One patient with SI converted to CVM after SI resolved and two patients required ongoing NA supervision due to continued SI. There were no reported refusals, technology failures, or unplanned discontinuations of CVM. One patient/family reported excessive CVM redirection of behavior.
DISCUSSION
This is the first description of CVM use in adolescent patients or patients with eating disorders. Our results suggest that CVM appears feasible and less costly in this population than one-to-one NA supervision, without statistically significant differences in LOS or time to weight gain. Patients with CVM with any NA supervision (except mealtime alone) were analyzed in the CVM group; therefore, this study may underestimate cost savings from CVM supervision. This innovative use of CVM may represent an opportunity for hospitals to repurpose monitoring technology for more efficient supervision of patients with eating disorders.
This pediatric pilot study adds to the growing body of literature in adult patients suggesting CVM supervision may be a feasible inpatient cost-reduction strategy.9,10 One single-center study demonstrated that the use of CVM with adult inpatients led to fewer unsafe behaviors, eg, patient removal of intravenous catheters and oxygen therapy. Personnel savings exceeded the original investment cost of the monitor within one fiscal quarter.9 Results of another study suggest that CVM use with hospitalized adults who required supervision to prevent falls was associated with improved patient and family satisfaction.14 In the absence of a gold standard for supervision of patients hospitalized with eating disorders, CVM technology is a tool that may balance cost, care quality, and patient experience. Given the upfront investment in CVM units, this technology may be most appropriate for institutions already using CVM for other inpatient indications.
Although our institutional cost of CVM use was similar to that reported by other institutions,11,15 the single-center design of this pilot study limits the generalizability of our findings. Unadjusted results of this observational study may be confounded by indication bias. As this was a pilot study, it was powered to detect a clinically significant difference in cost between NA and CVM supervision. While statistically significant differences were not seen in LOS or weight gain, this pilot study was not powered to detect potential differences or to adjust for all potential confounders (eg, other mental health conditions or comorbidities, eating disorder type, previous hospitalizations). Future studies should include these considerations in estimating sample sizes. The ability to conduct a robust cost-effectiveness analysis was also limited by cost data availability and reliance on staffing assumptions to calculate supervision costs. However, these findings will be important for valid effect size estimates for future interventional studies that rigorously evaluate CVM effectiveness and safety. Patients and families were not formally surveyed about their experiences with CVM, and the patient and family experience is another important outcome to consider in future studies.
CONCLUSION
The results of this pilot study suggest that supervision costs for patients admitted for medical stabilization of eating disorders were statistically significantly lower with CVM when compared with one-to-one NA supervision, without a change in hospitalization LOS or time to weight gain. These findings are particularly important as hospitals seek opportunities to reduce costs while providing safe and effective care. Future efforts should focus on evaluating clinical outcomes and patient experiences with this technology and strategies to maximize efficiency to offset the initial device cost.
Disclosures
The authors have no financial relationships relevant to this article to disclose. The authors have no conflicts of interest relevant to this article to disclose.
1. Zhao Y, Encinosa W. An update on hospitalizations for eating disorders, 1999 to 2009: statistical brief #120. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality (US); 2006. PubMed
2. Bardach NS, Coker TR, Zima BT, et al. Common and costly hospitalizations for pediatric mental health disorders. Pediatrics. 2014;133(4):602-609. doi: 10.1542/peds.2013-3165. PubMed
3. Society for Adolescent H, Medicine, Golden NH, et al. Position Paper of the Society for Adolescent Health and Medicine: medical management of restrictive eating disorders in adolescents and young adults. J Adolesc Health. 2015;56(1):121-125. doi: 10.1016/j.jadohealth.2014.10.259. PubMed
4. Katzman DK. Medical complications in adolescents with anorexia nervosa: a review of the literature. Int J Eat Disord. 2005;37(S1):S52-S59; discussion S87-S59. doi: 10.1002/eat.20118. PubMed
5. Strandjord SE, Sieke EH, Richmond M, Khadilkar A, Rome ES. Medical stabilization of adolescents with nutritional insufficiency: a clinical care path. Eat Weight Disord. 2016;21(3):403-410. doi: 10.1007/s40519-015-0245-5. PubMed
6. Kells M, Davidson K, Hitchko L, O’Neil K, Schubert-Bob P, McCabe M. Examining supervised meals in patients with restrictive eating disorders. Appl Nurs Res. 2013;26(2):76-79. doi: 10.1016/j.apnr.2012.06.003. PubMed
7. Leclerc A, Turrini T, Sherwood K, Katzman DK. Evaluation of a nutrition rehabilitation protocol in hospitalized adolescents with restrictive eating disorders. J Adolesc Health. 2013;53(5):585-589. doi: 10.1016/j.jadohealth.2013.06.001. PubMed
8. Kells M, Schubert-Bob P, Nagle K, et al. Meal supervision during medical hospitalization for eating disorders. Clin Nurs Res. 2017;26(4):525-537. doi: 10.1177/1054773816637598. PubMed
9. Jeffers S, Searcey P, Boyle K, et al. Centralized video monitoring for patient safety: a Denver Health Lean journey. Nurs Econ. 2013;31(6):298-306. PubMed
10. Sand-Jecklin K, Johnson JR, Tylka S. Protecting patient safety: can video monitoring prevent falls in high-risk patient populations? J Nurs Care Qual. 2016;31(2):131-138. doi: 10.1097/NCQ.0000000000000163. PubMed
11. Burtson PL, Vento L. Sitter reduction through mobile video monitoring: a nurse-driven sitter protocol and administrative oversight. J Nurs Adm. 2015;45(7-8):363-369. doi: 10.1097/NNA.0000000000000216. PubMed
12. Prevention CfDCa. ICD-9-CM Guidelines, 9th ed. https://www.cdc.gov/nchs/data/icd/icd9cm_guidelines_2011.pdf. Accessed April 11, 2018.
13. Prevention CfDca. IDC-9-CM Code Conversion Table. https://www.cdc.gov/nchs/data/icd/icd-9-cm_fy14_cnvtbl_final.pdf. Accessed April 11, 2018.
14. Cournan M, Fusco-Gessick B, Wright L. Improving patient safety through video monitoring. Rehabil Nurs. 2016. doi: 10.1002/rnj.308. PubMed
15. Rochefort CM, Ward L, Ritchie JA, Girard N, Tamblyn RM. Patient and nurse staffing characteristics associated with high sitter use costs. J Adv Nurs. 2012;68(8):1758-1767. doi: 10.1111/j.1365-2648.2011.05864.x. PubMed
Hospitalizations for nutritional rehabilitation of patients with restrictive eating disorders are increasing.1 Among primary mental health admissions at free-standing children’s hospitals, eating disorders represent 5.5% of hospitalizations and are associated with the longest length of stay (LOS; mean 14.3 days) and costliest care (mean $46,130).2 Admission is necessary to ensure initial weight restoration and monitoring for symptoms of refeeding syndrome, including electrolyte shifts and vital sign abnormalities.3-5
Supervision is generally considered an essential element of caring for hospitalized patients with eating disorders, who may experience difficulty adhering to nutritional treatment, perform excessive movement or exercise, or demonstrate purging or self-harming behaviors. Supervision is presumed to prevent counterproductive behaviors, facilitating weight gain and earlier discharge to psychiatric treatment. Best practices for patient supervision to address these challenges have not been established but often include meal time or continuous one-to-one supervision by nursing assistants (NAs) or other staff.6,7 While meal supervision has been shown to decrease medical LOS, it is costly, reduces staff availability for the care of other patient care, and can be a barrier to caring for patients with eating disorders in many institutions.8
Although not previously used in patients with eating disorders, centralized video monitoring (CVM) may provide an additional mode of supervision. CVM is an emerging technology consisting of real-time video streaming, without video recording, enabling tracking of patient movement, redirection of behaviors, and communication with unit nurses when necessary. CVM has been used in multiple patient safety initiatives to reduce falls, address staffing shortages, reduce costs,9,10 supervise patients at risk for self-harm or elopement, and prevent controlled medication diversion.10,11
We sought to pilot a novel use of CVM to replace our institution’s standard practice of continuous one-to-one nursing assistant (NA) supervision of patients admitted for medical stabilization of an eating disorder. Our objective was to evaluate the supervision cost and feasibility of CVM, using LOS and days to weight gain as balancing measures.
METHODS
Setting and Participants
This retrospective cohort study included patients 12-18 years old admitted to the pediatric hospital medicine service on a general unit of an academic quaternary care children’s hospital for medical stabilization of an eating disorder between September 2013 and March 2017. Patients were identified using administrative data based on primary or secondary diagnosis of anorexia nervosa, eating disorder not other wise specified, or another specified eating disorder (ICD 9 3071, 20759, or ICD 10 f5000, 5001, f5089, f509).12,13 This research study was considered exempt by the University of Wisconsin School of Medicine and Public Health’s Institutional Review Board.
Supervision Interventions
A standard medical stabilization protocol was used for patients admitted with an eating disorder throughout the study period (Appendix). All patients received continuous one-to-one NA supervision until they reached the target calorie intake and demonstrated the ability to follow the nutritional meal protocol. Beginning July 2015, patients received continuous CVM supervision unless they expressed suicidal ideation (SI), which triggered one-to-one NA supervision until they no longer endorsed suicidality.
Centralized Video Monitoring Implementation
Institutional CVM technology was AvaSys TeleSitter Solution (AvaSure, Inc). Our institution purchased CVM devices for use in adult settings, and one was assigned for pediatric CVM. Mobile CVM video carts were deployed to patient rooms and generated live video streams, without recorded capture, which were supervised by CVM technicians. These technicians were NAs hired and trained specifically for this role; worked four-, eight-, and 12-hour shifts; and observed up to eight camera feeds on a single monitor in a centralized room. Patients and family members could refuse CVM, which would trigger one-to-one NA supervision. Patients were not observed by CVM while in the restroom; staff were notified by either the patient or technician, and one-to-one supervision was provided. CVM had two-way audio communication, which allowed technicians to redirect patients verbally. Technicians could contact nursing staff directly by phone when additional intervention was needed.
Supervision Costs
NA supervision costs were estimated at $19/hour, based upon institutional human resources average NA salaries at that time. No additional mealtime supervision was included, as in-person supervision was already occurring.
CVM supervision costs were defined as the sum of the device cost plus CVM technician costs and two hours of one-to-one NA mealtime supervision per day. The CVM device cost was estimated at $2.10/hour, assuming a 10-year machine life expectancy (single unit cost $82,893 in 2015, 3,944 hours of use in fiscal year of 2018). CVM technician costs were $19/hour, based upon institutional human resources average CVM technician salaries at that time. Because technicians monitored an average of six patients simultaneously during this study, one-sixth of a CVM technician’s salary (ie, $3.17/hour) was used for each hour of CVM monitoring. Patients with mixed (NA and CVM) supervision were analyzed with those having CVM supervision. These patients’ costs were the sum of their NA supervision costs plus their CVM supervision costs.
Data Collection
Descriptive variables including age, gender, race/ethnicity, insurance, and LOS were collected from administrative data. The duration and type of supervision for all patients were collected from daily staffing logs. The eating disorder protocol standardized the process of obtaining daily weights (Appendix). Days to weight gain following admission were defined as the total number of days from admission to the first day of weight gain that was followed by another day of weight gain or maintaining the same weight
Data Analysis
Patient and hospitalization characteristics were summarized. A sample size of at least 14 in each group was estimated as necessary to detect a 50% reduction in supervision cost between the groups using alpha = 0.05, a power of 80%, a mean cost of $4,400 in the NA group, and a standard deviation of $1,600.Wilcoxon rank-sum tests were used to assess differences in median supervision cost between NA and CVM use. Differences in mean LOS and days to weight gain between NA and CVM use were assessed with t-tests because these data were normally distributed.
RESULTS
Patient Characteristics and Supervision Costs
The study included 37 consecutive admissions (NA = 23 and CVM = 14) with 35 unique patients. Patients were female, primarily non-Hispanic White, and privately insured (Table 1). Median supervision cost for the NA was statistically significantly more expensive at $4,104/admission versus $1,166/admission for CVM (P < .001, Table 2).
Balancing Measures, Acceptability, and Feasibility
Mean LOS was 11.7 days for NA and 9.8 days for CVM (P = .27; Table 2). The mean number of days to weight gain was 3.1 and 3.6 days, respectively (P = .28). No patients converted from CVM to NA supervision. One patient with SI converted to CVM after SI resolved and two patients required ongoing NA supervision due to continued SI. There were no reported refusals, technology failures, or unplanned discontinuations of CVM. One patient/family reported excessive CVM redirection of behavior.
DISCUSSION
This is the first description of CVM use in adolescent patients or patients with eating disorders. Our results suggest that CVM appears feasible and less costly in this population than one-to-one NA supervision, without statistically significant differences in LOS or time to weight gain. Patients with CVM with any NA supervision (except mealtime alone) were analyzed in the CVM group; therefore, this study may underestimate cost savings from CVM supervision. This innovative use of CVM may represent an opportunity for hospitals to repurpose monitoring technology for more efficient supervision of patients with eating disorders.
This pediatric pilot study adds to the growing body of literature in adult patients suggesting CVM supervision may be a feasible inpatient cost-reduction strategy.9,10 One single-center study demonstrated that the use of CVM with adult inpatients led to fewer unsafe behaviors, eg, patient removal of intravenous catheters and oxygen therapy. Personnel savings exceeded the original investment cost of the monitor within one fiscal quarter.9 Results of another study suggest that CVM use with hospitalized adults who required supervision to prevent falls was associated with improved patient and family satisfaction.14 In the absence of a gold standard for supervision of patients hospitalized with eating disorders, CVM technology is a tool that may balance cost, care quality, and patient experience. Given the upfront investment in CVM units, this technology may be most appropriate for institutions already using CVM for other inpatient indications.
Although our institutional cost of CVM use was similar to that reported by other institutions,11,15 the single-center design of this pilot study limits the generalizability of our findings. Unadjusted results of this observational study may be confounded by indication bias. As this was a pilot study, it was powered to detect a clinically significant difference in cost between NA and CVM supervision. While statistically significant differences were not seen in LOS or weight gain, this pilot study was not powered to detect potential differences or to adjust for all potential confounders (eg, other mental health conditions or comorbidities, eating disorder type, previous hospitalizations). Future studies should include these considerations in estimating sample sizes. The ability to conduct a robust cost-effectiveness analysis was also limited by cost data availability and reliance on staffing assumptions to calculate supervision costs. However, these findings will be important for valid effect size estimates for future interventional studies that rigorously evaluate CVM effectiveness and safety. Patients and families were not formally surveyed about their experiences with CVM, and the patient and family experience is another important outcome to consider in future studies.
CONCLUSION
The results of this pilot study suggest that supervision costs for patients admitted for medical stabilization of eating disorders were statistically significantly lower with CVM when compared with one-to-one NA supervision, without a change in hospitalization LOS or time to weight gain. These findings are particularly important as hospitals seek opportunities to reduce costs while providing safe and effective care. Future efforts should focus on evaluating clinical outcomes and patient experiences with this technology and strategies to maximize efficiency to offset the initial device cost.
Disclosures
The authors have no financial relationships relevant to this article to disclose. The authors have no conflicts of interest relevant to this article to disclose.
Hospitalizations for nutritional rehabilitation of patients with restrictive eating disorders are increasing.1 Among primary mental health admissions at free-standing children’s hospitals, eating disorders represent 5.5% of hospitalizations and are associated with the longest length of stay (LOS; mean 14.3 days) and costliest care (mean $46,130).2 Admission is necessary to ensure initial weight restoration and monitoring for symptoms of refeeding syndrome, including electrolyte shifts and vital sign abnormalities.3-5
Supervision is generally considered an essential element of caring for hospitalized patients with eating disorders, who may experience difficulty adhering to nutritional treatment, perform excessive movement or exercise, or demonstrate purging or self-harming behaviors. Supervision is presumed to prevent counterproductive behaviors, facilitating weight gain and earlier discharge to psychiatric treatment. Best practices for patient supervision to address these challenges have not been established but often include meal time or continuous one-to-one supervision by nursing assistants (NAs) or other staff.6,7 While meal supervision has been shown to decrease medical LOS, it is costly, reduces staff availability for the care of other patient care, and can be a barrier to caring for patients with eating disorders in many institutions.8
Although not previously used in patients with eating disorders, centralized video monitoring (CVM) may provide an additional mode of supervision. CVM is an emerging technology consisting of real-time video streaming, without video recording, enabling tracking of patient movement, redirection of behaviors, and communication with unit nurses when necessary. CVM has been used in multiple patient safety initiatives to reduce falls, address staffing shortages, reduce costs,9,10 supervise patients at risk for self-harm or elopement, and prevent controlled medication diversion.10,11
We sought to pilot a novel use of CVM to replace our institution’s standard practice of continuous one-to-one nursing assistant (NA) supervision of patients admitted for medical stabilization of an eating disorder. Our objective was to evaluate the supervision cost and feasibility of CVM, using LOS and days to weight gain as balancing measures.
METHODS
Setting and Participants
This retrospective cohort study included patients 12-18 years old admitted to the pediatric hospital medicine service on a general unit of an academic quaternary care children’s hospital for medical stabilization of an eating disorder between September 2013 and March 2017. Patients were identified using administrative data based on primary or secondary diagnosis of anorexia nervosa, eating disorder not other wise specified, or another specified eating disorder (ICD 9 3071, 20759, or ICD 10 f5000, 5001, f5089, f509).12,13 This research study was considered exempt by the University of Wisconsin School of Medicine and Public Health’s Institutional Review Board.
Supervision Interventions
A standard medical stabilization protocol was used for patients admitted with an eating disorder throughout the study period (Appendix). All patients received continuous one-to-one NA supervision until they reached the target calorie intake and demonstrated the ability to follow the nutritional meal protocol. Beginning July 2015, patients received continuous CVM supervision unless they expressed suicidal ideation (SI), which triggered one-to-one NA supervision until they no longer endorsed suicidality.
Centralized Video Monitoring Implementation
Institutional CVM technology was AvaSys TeleSitter Solution (AvaSure, Inc). Our institution purchased CVM devices for use in adult settings, and one was assigned for pediatric CVM. Mobile CVM video carts were deployed to patient rooms and generated live video streams, without recorded capture, which were supervised by CVM technicians. These technicians were NAs hired and trained specifically for this role; worked four-, eight-, and 12-hour shifts; and observed up to eight camera feeds on a single monitor in a centralized room. Patients and family members could refuse CVM, which would trigger one-to-one NA supervision. Patients were not observed by CVM while in the restroom; staff were notified by either the patient or technician, and one-to-one supervision was provided. CVM had two-way audio communication, which allowed technicians to redirect patients verbally. Technicians could contact nursing staff directly by phone when additional intervention was needed.
Supervision Costs
NA supervision costs were estimated at $19/hour, based upon institutional human resources average NA salaries at that time. No additional mealtime supervision was included, as in-person supervision was already occurring.
CVM supervision costs were defined as the sum of the device cost plus CVM technician costs and two hours of one-to-one NA mealtime supervision per day. The CVM device cost was estimated at $2.10/hour, assuming a 10-year machine life expectancy (single unit cost $82,893 in 2015, 3,944 hours of use in fiscal year of 2018). CVM technician costs were $19/hour, based upon institutional human resources average CVM technician salaries at that time. Because technicians monitored an average of six patients simultaneously during this study, one-sixth of a CVM technician’s salary (ie, $3.17/hour) was used for each hour of CVM monitoring. Patients with mixed (NA and CVM) supervision were analyzed with those having CVM supervision. These patients’ costs were the sum of their NA supervision costs plus their CVM supervision costs.
Data Collection
Descriptive variables including age, gender, race/ethnicity, insurance, and LOS were collected from administrative data. The duration and type of supervision for all patients were collected from daily staffing logs. The eating disorder protocol standardized the process of obtaining daily weights (Appendix). Days to weight gain following admission were defined as the total number of days from admission to the first day of weight gain that was followed by another day of weight gain or maintaining the same weight
Data Analysis
Patient and hospitalization characteristics were summarized. A sample size of at least 14 in each group was estimated as necessary to detect a 50% reduction in supervision cost between the groups using alpha = 0.05, a power of 80%, a mean cost of $4,400 in the NA group, and a standard deviation of $1,600.Wilcoxon rank-sum tests were used to assess differences in median supervision cost between NA and CVM use. Differences in mean LOS and days to weight gain between NA and CVM use were assessed with t-tests because these data were normally distributed.
RESULTS
Patient Characteristics and Supervision Costs
The study included 37 consecutive admissions (NA = 23 and CVM = 14) with 35 unique patients. Patients were female, primarily non-Hispanic White, and privately insured (Table 1). Median supervision cost for the NA was statistically significantly more expensive at $4,104/admission versus $1,166/admission for CVM (P < .001, Table 2).
Balancing Measures, Acceptability, and Feasibility
Mean LOS was 11.7 days for NA and 9.8 days for CVM (P = .27; Table 2). The mean number of days to weight gain was 3.1 and 3.6 days, respectively (P = .28). No patients converted from CVM to NA supervision. One patient with SI converted to CVM after SI resolved and two patients required ongoing NA supervision due to continued SI. There were no reported refusals, technology failures, or unplanned discontinuations of CVM. One patient/family reported excessive CVM redirection of behavior.
DISCUSSION
This is the first description of CVM use in adolescent patients or patients with eating disorders. Our results suggest that CVM appears feasible and less costly in this population than one-to-one NA supervision, without statistically significant differences in LOS or time to weight gain. Patients with CVM with any NA supervision (except mealtime alone) were analyzed in the CVM group; therefore, this study may underestimate cost savings from CVM supervision. This innovative use of CVM may represent an opportunity for hospitals to repurpose monitoring technology for more efficient supervision of patients with eating disorders.
This pediatric pilot study adds to the growing body of literature in adult patients suggesting CVM supervision may be a feasible inpatient cost-reduction strategy.9,10 One single-center study demonstrated that the use of CVM with adult inpatients led to fewer unsafe behaviors, eg, patient removal of intravenous catheters and oxygen therapy. Personnel savings exceeded the original investment cost of the monitor within one fiscal quarter.9 Results of another study suggest that CVM use with hospitalized adults who required supervision to prevent falls was associated with improved patient and family satisfaction.14 In the absence of a gold standard for supervision of patients hospitalized with eating disorders, CVM technology is a tool that may balance cost, care quality, and patient experience. Given the upfront investment in CVM units, this technology may be most appropriate for institutions already using CVM for other inpatient indications.
Although our institutional cost of CVM use was similar to that reported by other institutions,11,15 the single-center design of this pilot study limits the generalizability of our findings. Unadjusted results of this observational study may be confounded by indication bias. As this was a pilot study, it was powered to detect a clinically significant difference in cost between NA and CVM supervision. While statistically significant differences were not seen in LOS or weight gain, this pilot study was not powered to detect potential differences or to adjust for all potential confounders (eg, other mental health conditions or comorbidities, eating disorder type, previous hospitalizations). Future studies should include these considerations in estimating sample sizes. The ability to conduct a robust cost-effectiveness analysis was also limited by cost data availability and reliance on staffing assumptions to calculate supervision costs. However, these findings will be important for valid effect size estimates for future interventional studies that rigorously evaluate CVM effectiveness and safety. Patients and families were not formally surveyed about their experiences with CVM, and the patient and family experience is another important outcome to consider in future studies.
CONCLUSION
The results of this pilot study suggest that supervision costs for patients admitted for medical stabilization of eating disorders were statistically significantly lower with CVM when compared with one-to-one NA supervision, without a change in hospitalization LOS or time to weight gain. These findings are particularly important as hospitals seek opportunities to reduce costs while providing safe and effective care. Future efforts should focus on evaluating clinical outcomes and patient experiences with this technology and strategies to maximize efficiency to offset the initial device cost.
Disclosures
The authors have no financial relationships relevant to this article to disclose. The authors have no conflicts of interest relevant to this article to disclose.
1. Zhao Y, Encinosa W. An update on hospitalizations for eating disorders, 1999 to 2009: statistical brief #120. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality (US); 2006. PubMed
2. Bardach NS, Coker TR, Zima BT, et al. Common and costly hospitalizations for pediatric mental health disorders. Pediatrics. 2014;133(4):602-609. doi: 10.1542/peds.2013-3165. PubMed
3. Society for Adolescent H, Medicine, Golden NH, et al. Position Paper of the Society for Adolescent Health and Medicine: medical management of restrictive eating disorders in adolescents and young adults. J Adolesc Health. 2015;56(1):121-125. doi: 10.1016/j.jadohealth.2014.10.259. PubMed
4. Katzman DK. Medical complications in adolescents with anorexia nervosa: a review of the literature. Int J Eat Disord. 2005;37(S1):S52-S59; discussion S87-S59. doi: 10.1002/eat.20118. PubMed
5. Strandjord SE, Sieke EH, Richmond M, Khadilkar A, Rome ES. Medical stabilization of adolescents with nutritional insufficiency: a clinical care path. Eat Weight Disord. 2016;21(3):403-410. doi: 10.1007/s40519-015-0245-5. PubMed
6. Kells M, Davidson K, Hitchko L, O’Neil K, Schubert-Bob P, McCabe M. Examining supervised meals in patients with restrictive eating disorders. Appl Nurs Res. 2013;26(2):76-79. doi: 10.1016/j.apnr.2012.06.003. PubMed
7. Leclerc A, Turrini T, Sherwood K, Katzman DK. Evaluation of a nutrition rehabilitation protocol in hospitalized adolescents with restrictive eating disorders. J Adolesc Health. 2013;53(5):585-589. doi: 10.1016/j.jadohealth.2013.06.001. PubMed
8. Kells M, Schubert-Bob P, Nagle K, et al. Meal supervision during medical hospitalization for eating disorders. Clin Nurs Res. 2017;26(4):525-537. doi: 10.1177/1054773816637598. PubMed
9. Jeffers S, Searcey P, Boyle K, et al. Centralized video monitoring for patient safety: a Denver Health Lean journey. Nurs Econ. 2013;31(6):298-306. PubMed
10. Sand-Jecklin K, Johnson JR, Tylka S. Protecting patient safety: can video monitoring prevent falls in high-risk patient populations? J Nurs Care Qual. 2016;31(2):131-138. doi: 10.1097/NCQ.0000000000000163. PubMed
11. Burtson PL, Vento L. Sitter reduction through mobile video monitoring: a nurse-driven sitter protocol and administrative oversight. J Nurs Adm. 2015;45(7-8):363-369. doi: 10.1097/NNA.0000000000000216. PubMed
12. Prevention CfDCa. ICD-9-CM Guidelines, 9th ed. https://www.cdc.gov/nchs/data/icd/icd9cm_guidelines_2011.pdf. Accessed April 11, 2018.
13. Prevention CfDca. IDC-9-CM Code Conversion Table. https://www.cdc.gov/nchs/data/icd/icd-9-cm_fy14_cnvtbl_final.pdf. Accessed April 11, 2018.
14. Cournan M, Fusco-Gessick B, Wright L. Improving patient safety through video monitoring. Rehabil Nurs. 2016. doi: 10.1002/rnj.308. PubMed
15. Rochefort CM, Ward L, Ritchie JA, Girard N, Tamblyn RM. Patient and nurse staffing characteristics associated with high sitter use costs. J Adv Nurs. 2012;68(8):1758-1767. doi: 10.1111/j.1365-2648.2011.05864.x. PubMed
1. Zhao Y, Encinosa W. An update on hospitalizations for eating disorders, 1999 to 2009: statistical brief #120. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality (US); 2006. PubMed
2. Bardach NS, Coker TR, Zima BT, et al. Common and costly hospitalizations for pediatric mental health disorders. Pediatrics. 2014;133(4):602-609. doi: 10.1542/peds.2013-3165. PubMed
3. Society for Adolescent H, Medicine, Golden NH, et al. Position Paper of the Society for Adolescent Health and Medicine: medical management of restrictive eating disorders in adolescents and young adults. J Adolesc Health. 2015;56(1):121-125. doi: 10.1016/j.jadohealth.2014.10.259. PubMed
4. Katzman DK. Medical complications in adolescents with anorexia nervosa: a review of the literature. Int J Eat Disord. 2005;37(S1):S52-S59; discussion S87-S59. doi: 10.1002/eat.20118. PubMed
5. Strandjord SE, Sieke EH, Richmond M, Khadilkar A, Rome ES. Medical stabilization of adolescents with nutritional insufficiency: a clinical care path. Eat Weight Disord. 2016;21(3):403-410. doi: 10.1007/s40519-015-0245-5. PubMed
6. Kells M, Davidson K, Hitchko L, O’Neil K, Schubert-Bob P, McCabe M. Examining supervised meals in patients with restrictive eating disorders. Appl Nurs Res. 2013;26(2):76-79. doi: 10.1016/j.apnr.2012.06.003. PubMed
7. Leclerc A, Turrini T, Sherwood K, Katzman DK. Evaluation of a nutrition rehabilitation protocol in hospitalized adolescents with restrictive eating disorders. J Adolesc Health. 2013;53(5):585-589. doi: 10.1016/j.jadohealth.2013.06.001. PubMed
8. Kells M, Schubert-Bob P, Nagle K, et al. Meal supervision during medical hospitalization for eating disorders. Clin Nurs Res. 2017;26(4):525-537. doi: 10.1177/1054773816637598. PubMed
9. Jeffers S, Searcey P, Boyle K, et al. Centralized video monitoring for patient safety: a Denver Health Lean journey. Nurs Econ. 2013;31(6):298-306. PubMed
10. Sand-Jecklin K, Johnson JR, Tylka S. Protecting patient safety: can video monitoring prevent falls in high-risk patient populations? J Nurs Care Qual. 2016;31(2):131-138. doi: 10.1097/NCQ.0000000000000163. PubMed
11. Burtson PL, Vento L. Sitter reduction through mobile video monitoring: a nurse-driven sitter protocol and administrative oversight. J Nurs Adm. 2015;45(7-8):363-369. doi: 10.1097/NNA.0000000000000216. PubMed
12. Prevention CfDCa. ICD-9-CM Guidelines, 9th ed. https://www.cdc.gov/nchs/data/icd/icd9cm_guidelines_2011.pdf. Accessed April 11, 2018.
13. Prevention CfDca. IDC-9-CM Code Conversion Table. https://www.cdc.gov/nchs/data/icd/icd-9-cm_fy14_cnvtbl_final.pdf. Accessed April 11, 2018.
14. Cournan M, Fusco-Gessick B, Wright L. Improving patient safety through video monitoring. Rehabil Nurs. 2016. doi: 10.1002/rnj.308. PubMed
15. Rochefort CM, Ward L, Ritchie JA, Girard N, Tamblyn RM. Patient and nurse staffing characteristics associated with high sitter use costs. J Adv Nurs. 2012;68(8):1758-1767. doi: 10.1111/j.1365-2648.2011.05864.x. PubMed
© 2019 Society of Hospital Medicine
Interhospital Transfer: Transfer Processes and Patient Outcomes
The transfer of patients between acute care hospitals (interhospital transfer [IHT]) occurs regularly among patients with a variety of diagnoses, in theory, to gain access to unique specialty services and/or a higher level of care, among other reasons.1,2
However, the practice of IHT is variable and nonstandardized,3,4 and existing data largely suggests that transferred patients experience worse outcomes, including longer length of stay, higher hospitalization costs, longer ICU time, and greater mortality, even with rigorous adjustment for confounding by indication.5,6 Though there are many possible reasons for these findings, existing literature suggests that there may be aspects of the transfer process itself which contribute to these outcomes.2,6,7
Understanding which aspects of the transfer process contribute to poor patient outcomes is a key first step toward the development of targeted quality improvement initiatives to improve this process of care. In this study, we aim to examine the association between select characteristics of the transfer process, including the timing of transfer and workload of the admitting physician team, and clinical outcomes among patients undergoing IHT.
METHODS
Data and Study Population
We performed a retrospective analysis of patients ≥age 18 years who transferred to Brigham and Women’s Hospital (BWH), a 777-bed tertiary care hospital, from another acute care hospital between January 2005, and September 2013. Dates of inclusion were purposefully chosen prior to BWH implementation of a new electronic health records system to avoid potential information bias. As at most academic medical centers, night coverage at BWH differs by service and includes a combination of long-call admitting teams and night float coverage. On weekends, many services are less well staffed, and some procedures may only be available if needed emergently. Some services have caps on the daily number of admissions or total patient census, but none have caps on the number of discharges per day. Patients were excluded from analysis if they left BWH against medical advice, were transferred from closely affiliated hospitals with shared personnel and electronic health records (Brigham and Women’s Faulkner Hospital, Dana Farber Cancer Institute), transferred from inpatient psychiatric or inpatient hospice facilities, or transferred to obstetrics or nursery services. Data were obtained from administrative sources and the research patient data repository (RPDR), a centralized clinical data repository that gathers data from various hospital legacy systems and stores them in one data warehouse.8 Our study was approved by the Partners Institutional Review Board (IRB) with a waiver of patient consent.
Transfer Process Characteristics
Predictors included select characteristics of the transfer process, including (1) Day of week of transfer, dichotomized into Friday through Sunday (“weekend”), versus Monday through Thursday (“weekday”);9 Friday was included with “weekend” given the suggestion of increased volume of transfers in advance of the weekend; (2) Time of arrival of the transferred patient, categorized into “daytime” (7
Outcomes
Outcomes included transfer to the intensive care unit (ICU) within 48 hours of arrival and 30-day mortality from date of index admission.5,6
Patient Characteristics
Covariates for adjustment included: patient age, sex, race, Elixhauser comorbidity score,11 Diagnosis-Related Group (DRG)-weight, insurance status, year of admission, number of preadmission medications, and service of admission.
Statistical Analyses
We used descriptive statistics to display baseline characteristics and performed a series of univariable and multivariable logistic regression models to obtain the adjusted odds of each transfer process characteristic on each outcome, adjusting for all covariates (proc logistic, SAS Statistical Software, Cary, North Carolina). For analyses of ICU transfer within 48 hours of arrival, all patients initially admitted to the ICU at time of transfer were excluded.
In the secondary analyses, we used a combined day-of-week and time-of-day variable (ie, Monday day, Monday evening, Monday night, Tuesday day, and so on, with Monday day as the reference group) to obtain a more detailed evaluation of timing of transfer on patient outcomes. We also performed stratified analyses to evaluate each transfer process characteristic on adjusted odds of 30-day mortality stratified by service of admission (ie, at the time of transfer to BWH), adjusting for all covariates. For all analyses, two-sided P values < .05 were considered significant.
RESULTS
Overall, 24,352 patients met our inclusion criteria and underwent IHT, of whom 2,174 (8.9%) died within 30 days. Of the 22,910 transferred patients originally admitted to a non-ICU service, 5,464 (23.8%) underwent ICU transfer within 48 hours of arrival. Cohort characteristics are shown in Table 1.
Multivariable regression analyses demonstrated no significant association between weekend (versus weekday) transfer or increased time delay between patient acceptance and arrival (>48 hours) and adjusted odds of ICU transfer within 48 hours or 30-day mortality. However, they did demonstrate that nighttime (versus daytime) transfer was associated with greater adjusted odds of both ICU transfer and 30-day mortality. Increased admitting team busyness was associated with lower adjusted odds of ICU transfer but was not significantly associated with adjusted odds of 30-day mortality (Table 2). As expected, decreased time delay between patient acceptance and arrival (0-12 hours) was associated with increased adjusted odds of both ICU transfer (adjusted OR 2.68; 95% CI 2.29, 3.15) and 30-day mortality (adjusted OR 1.25; 95% CI 1.03, 1.53) compared with 12-24 hours (results not shown). Time delay >48 hours was not associated with either outcome.
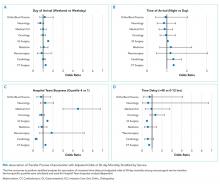
Regression analyses with the combined day/time variable demonstrated that compared with Monday daytime transfer, Sunday night transfer was significantly associated with increased adjusted odds of 30-day mortality, and Friday night transfer was associated with a trend toward increased 30-day mortality (adjusted OR [aOR] 1.88; 95% CI 1.25, 2.82, and aOR 1.43; 95% CI 0.99, 2.06, respectively). We also found that all nighttime transfers (ie, Monday through Sunday night) were associated with increased adjusted odds of ICU transfer within 48 hours (as compared with Monday daytime transfer). Other days/time analyses were not significant.
Univariable and multivariable analyses stratified by service were performed (Appendix). Multivariable stratified analyses demonstrated that weekend transfer, nighttime transfer, and increased admitting team busyness were associated with increased adjusted odds of 30-day mortality among cardiothoracic (CT) and gastrointestinal (GI) surgical service patients. Increased admitting team busyness was also associated with increased mortality among ICU service patients but was associated with decreased mortality among cardiology service patients. An increased time delay between patient acceptance and arrival was associated with decreased mortality among CT and GI surgical service patients (Figure; Appendix). Other adjusted stratified outcomes were not significant.
DISCUSSION
In this study of 24,352 patients undergoing IHT, we found no significant association between weekend transfer or increased time delay between transfer acceptance and arrival and patient outcomes in the cohort as a whole; but we found that nighttime transfer is associated with increased adjusted odds of both ICU transfer within 48 hours and 30-day mortality. Our analyses combining day-of-week and time-of-day demonstrate that Sunday night transfer is particularly associated with increased adjusted odds of 30-day mortality (as compared with Monday daytime transfer), and show a trend toward increased mortality with Friday night transfers. These detailed analyses otherwise reinforce that nighttime transfer across all nights of the week is associated with increased adjusted odds of ICU transfer within 48 hours. We also found that increased admitting team busyness on the day of patient transfer is associated with decreased odds of ICU transfer, though this may solely be reflective of higher turnover services (ie, cardiology) caring for lower acuity patients, as suggested by secondary analyses stratified by service. In addition, secondary analyses demonstrated differential associations between weekend transfers, nighttime transfers, and increased team busyness on the odds of 30-day mortality based on service of transfer. These analyses showed that patients transferred to higher acuity services requiring procedural care, including CT surgery, GI surgery, and Medical ICU, do worse under all three circumstances as compared with patients transferred to other services. Secondary analyses also demonstrated that increased time delay between patient acceptance and arrival is inversely associated with 30-day mortality among CT and GI surgery service patients, likely reflecting lower acuity patients (ie, less sick patients are less rapidly transferred).
There are several possible explanations for these findings. Patients transferred to surgical services at night may reflect a more urgent need for surgery and include a sicker cohort of patients, possibly explaining these findings. Alternatively, or in addition, both weekend and nighttime hospital admission expose patients to similar potential risks, ie, limited resources available during off-peak hours. Our findings could, therefore, reflect the possibility that patients transferred to higher acuity services in need of procedural care are most vulnerable to off-peak timing of transfer. Similar data looking at patients admitted through the emergency room (ER) find the strongest effect of off-peak admissions on patients in need of procedures, including GI hemorrhage,12 atrial fibrillation13 and acute myocardial infarction (AMI),14 arguably because of the limited availability of necessary interventions. Patients undergoing IHT are a sicker cohort of patients than those admitted through the ER, and, therefore, may be even more vulnerable to these issues.3,5 This is supported by our findings that Sunday night transfers (and trend toward Friday night transfers) are associated with greater mortality compared with Monday daytime transfers, when at-the-ready resources and/or specialty personnel may be less available (Sunday night), and delays until receipt of necessary procedures may be longer (Friday night). Though we did not observe similar results among cardiology service transfers, as may be expected based on existing literature,13,14 this subset of patients includes more heterogeneous diagnoses, (ie, not solely those that require acute intervention) and exhibited a low level of acuity (low Elixhauser score and DRG-weight, data not shown).
We also found that increased admitting team busyness on the day of patient transfer is associated with increased odds of 30-day mortality among CT surgery, GI surgery, and ICU service transfers. As above, there are several possible explanations for this finding. It is possible that among these services, only the sickest/neediest patients are accepted for transfer when teams are busiest, explaining our findings. Though this explanation is possible, the measure of team “busyness” includes patient discharge, thereby increasing, not decreasing, availability for incoming patients, making this explanation less likely. Alternatively, it is possible that this finding is reflective of reverse causation, ie, that teams have less ability to discharge/admit new patients when caring for particularly sick/unstable patient transfers, though this assumes that transferred patients arrive earlier in the day, (eg, in time to influence discharge decisions), which infrequently occurs (Table 1). Lastly, it is possible that this subset of patients will be more vulnerable to the workload of the team that is caring for them at the time of their arrival. With high patient turnover (admissions/discharges), the time allocated to each patient’s care may be diminished (ie, “work compression,” trying to do the same amount of work in less time), and may result in decreased time to care for the transferred patient. This has been shown to influence patient outcomes at the time of patient discharge.10
In trying to understand why we observed an inverse relationship between admitting team busyness and odds of ICU transfer within 48 hours, we believe this finding is largely driven by cardiology service transfers, which comprise the highest volume of transferred patients in our cohort (Table 1), and are low acuity patients. Within this population of patients, admitting team busyness is likely a surrogate variable for high turnover/low acuity. This idea is supported by our findings that admitting team busyness is associated with decreased adjusted odds of 30-day mortality in this group (and only in this group).
Similarly, our observed inverse relationship between increased time delay and 30-day mortality among CT and GI surgical service patients is also likely reflective of lower acuity patients. We anticipated that decreased time delay (0-12 hours) would be reflective of greater patient acuity (supported by our findings that decreased time delay is associated with increased odds of ICU transfer and 30-day mortality). However, our findings also suggest that increased time delay (>48 hours) is similarly representative of lower patient acuity and therefore an imperfect measure of discontinuity and/or harmful delays in care during IHT (see limitations below).
Our study is subject to several limitations. This is a single site study; given known variation in transfer practices between hospitals,3 it is possible that our findings are not generalizable. However, given similar existing data on patients admitted through the ER, it is likely our findings may be reflective of IHT to similar tertiary referral hospitals. Second, although we adjusted for patient characteristics, there remains the possibility of unmeasured confounding and other bias that account for our results, as discussed. Third, although the definition of “busyness” used in this study was chosen based on prior data demonstrating an effect on patient outcomes,10 we did not include other measures of busyness that may influence outcomes of transferred patients such as overall team census or hospital busyness. However, the workload associated with a high volume of patient admissions and discharges is arguably a greater reflection of “work compression” for the admitting team compared with overall team census, which may reflect a more static workload with less impact on the care of a newly transferred patient. Also, although hospital census may influence the ability to transfer (ie, lower volume of transferred patients during times of high hospital census), this likely has less of an impact on the direct care of transferred patients than the admitting team’s workload. It is more likely that it would serve as a confounder (eg, sicker patients are accepted for transfer despite high hospital census, while lower risk patients are not).
Nevertheless, future studies should further evaluate the association with other measures of busyness/workload and outcomes of transferred patients. Lastly, though we anticipated time delay between transfer acceptance and arrival would be correlated with patient acuity, we hypothesized that longer delay might affect patient continuity and communication and impact patient outcomes. However, our results demonstrate that our measurement of this variable was unsuccessful in unraveling patient acuity from our intended evaluation of these vulnerable aspects of IHT. It is likely that a more detailed evaluation is required to explore potential challenges more fully that may occur with greater time delays (eg, suboptimal communication regarding changes in clinical status during this time period, delays in treatment). Similarly, though our study evaluates the association between nighttime and weekend transfer (and the interaction between these) with patient outcomes, we did not evaluate other intermediate outcomes that may be more affected by the timing of transfer, such as diagnostic errors or delays in procedural care, which warrant further investigation. We do not directly examine the underlying reasons that explain our observed associations, and thus more research is needed to identify these as well as design and evaluate solutions.
Collectively, our findings suggest that high acuity patients in need of procedural care experience worse outcomes during off-peak times of transfer, and during times of high care-team workload. Though further research is needed to identify underlying reasons to explain our findings, both the timing of patient transfer (when modifiable) and workload of the team caring for the patient on arrival may serve as potential targets for interventions to improve the quality and safety of IHT for patients at greatest risk.
Disclosures
Dr. Mueller and Dr. Schnipper have nothing to disclose. Ms. Fiskio has nothing to disclose. Dr. Schnipper is the recipient of grant funding from Mallinckrodt Pharmaceuticals to conduct an investigator-initiated study of predictors and impact of opioid-related adverse drug events.
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2 012;40(8):2470-2478. https://doi.org/10.1097/CCM.0b013e318254516f.
2. Mueller SK, Shannon E, Dalal A, Schnipper JL, Dykes P. Patient and physician experience with interhospital transfer: a qualitative study. J Patient Saf. 2018. https://doi.org/10.1097/PTS.0000000000000501
3. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Rates, predictors and variability of interhospital transfers: a national evaluation. J Hosp Med. 2017;12(6):435-442.https://doi.org/10.12788/jhm.2747.
4. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. https://doi.org/10.1097/MLR.0b013e31820fb71b.
5. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: characteristics and outcomes. J Hosp Med. 2016;11(4):245-50. https://doi.org/10.1002/jhm.2515.
6. Mueller S, Zheng J, Orav EJP, Schnipper JL. Inter-hospital transfer and patient outcomes: a retrospective cohort study. BMJ Qual Saf. 2018. https://doi.org/10.1136/bmjqs-2018-008087.
7. Mueller SK, Schnipper JL. Physician perspectives on interhospital transfers. J Patient Saf. 2016. https://doi.org/10.1097/PTS.0000000000000312.
8. Research Patient Data Registry (RPDR). http://rc.partners.org/rpdr. Accessed April 20, 2018.
9. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. https://doi.org/10.1056/NEJMsa003376
10. Mueller SK, Donze J, Schnipper JL. Intern workload and discontinuity of care on 30-day readmission. Am J Med. 2013;126(1):81-88. https://doi.org/10.1016/j.amjmed.2012.09.003.
11. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. PubMed
12. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol. 2009;7(3):296-302e1. https://doi.org/10.1016/j.cgh.2008.08.013.
13. Deshmukh A, Pant S, Kumar G, Bursac Z, Paydak H, Mehta JL. Comparison of outcomes of weekend versus weekday admissions for atrial fibrillation. Am J Cardiol. 2012;110(2):208-211. https://doi.org/10.1016/j.amjcard.2012.03.011.
14. Clarke MS, Wills RA, Bowman RV, et al. Exploratory study of the ‘weekend effect’ for acute medical admissions to public hospitals in Queensland, Australia. Intern Med J. 2010;40(11):777-783. https://doi.org/-10.1111/j.1445-5994.2009.02067.x.
The transfer of patients between acute care hospitals (interhospital transfer [IHT]) occurs regularly among patients with a variety of diagnoses, in theory, to gain access to unique specialty services and/or a higher level of care, among other reasons.1,2
However, the practice of IHT is variable and nonstandardized,3,4 and existing data largely suggests that transferred patients experience worse outcomes, including longer length of stay, higher hospitalization costs, longer ICU time, and greater mortality, even with rigorous adjustment for confounding by indication.5,6 Though there are many possible reasons for these findings, existing literature suggests that there may be aspects of the transfer process itself which contribute to these outcomes.2,6,7
Understanding which aspects of the transfer process contribute to poor patient outcomes is a key first step toward the development of targeted quality improvement initiatives to improve this process of care. In this study, we aim to examine the association between select characteristics of the transfer process, including the timing of transfer and workload of the admitting physician team, and clinical outcomes among patients undergoing IHT.
METHODS
Data and Study Population
We performed a retrospective analysis of patients ≥age 18 years who transferred to Brigham and Women’s Hospital (BWH), a 777-bed tertiary care hospital, from another acute care hospital between January 2005, and September 2013. Dates of inclusion were purposefully chosen prior to BWH implementation of a new electronic health records system to avoid potential information bias. As at most academic medical centers, night coverage at BWH differs by service and includes a combination of long-call admitting teams and night float coverage. On weekends, many services are less well staffed, and some procedures may only be available if needed emergently. Some services have caps on the daily number of admissions or total patient census, but none have caps on the number of discharges per day. Patients were excluded from analysis if they left BWH against medical advice, were transferred from closely affiliated hospitals with shared personnel and electronic health records (Brigham and Women’s Faulkner Hospital, Dana Farber Cancer Institute), transferred from inpatient psychiatric or inpatient hospice facilities, or transferred to obstetrics or nursery services. Data were obtained from administrative sources and the research patient data repository (RPDR), a centralized clinical data repository that gathers data from various hospital legacy systems and stores them in one data warehouse.8 Our study was approved by the Partners Institutional Review Board (IRB) with a waiver of patient consent.
Transfer Process Characteristics
Predictors included select characteristics of the transfer process, including (1) Day of week of transfer, dichotomized into Friday through Sunday (“weekend”), versus Monday through Thursday (“weekday”);9 Friday was included with “weekend” given the suggestion of increased volume of transfers in advance of the weekend; (2) Time of arrival of the transferred patient, categorized into “daytime” (7
Outcomes
Outcomes included transfer to the intensive care unit (ICU) within 48 hours of arrival and 30-day mortality from date of index admission.5,6
Patient Characteristics
Covariates for adjustment included: patient age, sex, race, Elixhauser comorbidity score,11 Diagnosis-Related Group (DRG)-weight, insurance status, year of admission, number of preadmission medications, and service of admission.
Statistical Analyses
We used descriptive statistics to display baseline characteristics and performed a series of univariable and multivariable logistic regression models to obtain the adjusted odds of each transfer process characteristic on each outcome, adjusting for all covariates (proc logistic, SAS Statistical Software, Cary, North Carolina). For analyses of ICU transfer within 48 hours of arrival, all patients initially admitted to the ICU at time of transfer were excluded.
In the secondary analyses, we used a combined day-of-week and time-of-day variable (ie, Monday day, Monday evening, Monday night, Tuesday day, and so on, with Monday day as the reference group) to obtain a more detailed evaluation of timing of transfer on patient outcomes. We also performed stratified analyses to evaluate each transfer process characteristic on adjusted odds of 30-day mortality stratified by service of admission (ie, at the time of transfer to BWH), adjusting for all covariates. For all analyses, two-sided P values < .05 were considered significant.
RESULTS
Overall, 24,352 patients met our inclusion criteria and underwent IHT, of whom 2,174 (8.9%) died within 30 days. Of the 22,910 transferred patients originally admitted to a non-ICU service, 5,464 (23.8%) underwent ICU transfer within 48 hours of arrival. Cohort characteristics are shown in Table 1.
Multivariable regression analyses demonstrated no significant association between weekend (versus weekday) transfer or increased time delay between patient acceptance and arrival (>48 hours) and adjusted odds of ICU transfer within 48 hours or 30-day mortality. However, they did demonstrate that nighttime (versus daytime) transfer was associated with greater adjusted odds of both ICU transfer and 30-day mortality. Increased admitting team busyness was associated with lower adjusted odds of ICU transfer but was not significantly associated with adjusted odds of 30-day mortality (Table 2). As expected, decreased time delay between patient acceptance and arrival (0-12 hours) was associated with increased adjusted odds of both ICU transfer (adjusted OR 2.68; 95% CI 2.29, 3.15) and 30-day mortality (adjusted OR 1.25; 95% CI 1.03, 1.53) compared with 12-24 hours (results not shown). Time delay >48 hours was not associated with either outcome.
Regression analyses with the combined day/time variable demonstrated that compared with Monday daytime transfer, Sunday night transfer was significantly associated with increased adjusted odds of 30-day mortality, and Friday night transfer was associated with a trend toward increased 30-day mortality (adjusted OR [aOR] 1.88; 95% CI 1.25, 2.82, and aOR 1.43; 95% CI 0.99, 2.06, respectively). We also found that all nighttime transfers (ie, Monday through Sunday night) were associated with increased adjusted odds of ICU transfer within 48 hours (as compared with Monday daytime transfer). Other days/time analyses were not significant.
Univariable and multivariable analyses stratified by service were performed (Appendix). Multivariable stratified analyses demonstrated that weekend transfer, nighttime transfer, and increased admitting team busyness were associated with increased adjusted odds of 30-day mortality among cardiothoracic (CT) and gastrointestinal (GI) surgical service patients. Increased admitting team busyness was also associated with increased mortality among ICU service patients but was associated with decreased mortality among cardiology service patients. An increased time delay between patient acceptance and arrival was associated with decreased mortality among CT and GI surgical service patients (Figure; Appendix). Other adjusted stratified outcomes were not significant.
DISCUSSION
In this study of 24,352 patients undergoing IHT, we found no significant association between weekend transfer or increased time delay between transfer acceptance and arrival and patient outcomes in the cohort as a whole; but we found that nighttime transfer is associated with increased adjusted odds of both ICU transfer within 48 hours and 30-day mortality. Our analyses combining day-of-week and time-of-day demonstrate that Sunday night transfer is particularly associated with increased adjusted odds of 30-day mortality (as compared with Monday daytime transfer), and show a trend toward increased mortality with Friday night transfers. These detailed analyses otherwise reinforce that nighttime transfer across all nights of the week is associated with increased adjusted odds of ICU transfer within 48 hours. We also found that increased admitting team busyness on the day of patient transfer is associated with decreased odds of ICU transfer, though this may solely be reflective of higher turnover services (ie, cardiology) caring for lower acuity patients, as suggested by secondary analyses stratified by service. In addition, secondary analyses demonstrated differential associations between weekend transfers, nighttime transfers, and increased team busyness on the odds of 30-day mortality based on service of transfer. These analyses showed that patients transferred to higher acuity services requiring procedural care, including CT surgery, GI surgery, and Medical ICU, do worse under all three circumstances as compared with patients transferred to other services. Secondary analyses also demonstrated that increased time delay between patient acceptance and arrival is inversely associated with 30-day mortality among CT and GI surgery service patients, likely reflecting lower acuity patients (ie, less sick patients are less rapidly transferred).
There are several possible explanations for these findings. Patients transferred to surgical services at night may reflect a more urgent need for surgery and include a sicker cohort of patients, possibly explaining these findings. Alternatively, or in addition, both weekend and nighttime hospital admission expose patients to similar potential risks, ie, limited resources available during off-peak hours. Our findings could, therefore, reflect the possibility that patients transferred to higher acuity services in need of procedural care are most vulnerable to off-peak timing of transfer. Similar data looking at patients admitted through the emergency room (ER) find the strongest effect of off-peak admissions on patients in need of procedures, including GI hemorrhage,12 atrial fibrillation13 and acute myocardial infarction (AMI),14 arguably because of the limited availability of necessary interventions. Patients undergoing IHT are a sicker cohort of patients than those admitted through the ER, and, therefore, may be even more vulnerable to these issues.3,5 This is supported by our findings that Sunday night transfers (and trend toward Friday night transfers) are associated with greater mortality compared with Monday daytime transfers, when at-the-ready resources and/or specialty personnel may be less available (Sunday night), and delays until receipt of necessary procedures may be longer (Friday night). Though we did not observe similar results among cardiology service transfers, as may be expected based on existing literature,13,14 this subset of patients includes more heterogeneous diagnoses, (ie, not solely those that require acute intervention) and exhibited a low level of acuity (low Elixhauser score and DRG-weight, data not shown).
We also found that increased admitting team busyness on the day of patient transfer is associated with increased odds of 30-day mortality among CT surgery, GI surgery, and ICU service transfers. As above, there are several possible explanations for this finding. It is possible that among these services, only the sickest/neediest patients are accepted for transfer when teams are busiest, explaining our findings. Though this explanation is possible, the measure of team “busyness” includes patient discharge, thereby increasing, not decreasing, availability for incoming patients, making this explanation less likely. Alternatively, it is possible that this finding is reflective of reverse causation, ie, that teams have less ability to discharge/admit new patients when caring for particularly sick/unstable patient transfers, though this assumes that transferred patients arrive earlier in the day, (eg, in time to influence discharge decisions), which infrequently occurs (Table 1). Lastly, it is possible that this subset of patients will be more vulnerable to the workload of the team that is caring for them at the time of their arrival. With high patient turnover (admissions/discharges), the time allocated to each patient’s care may be diminished (ie, “work compression,” trying to do the same amount of work in less time), and may result in decreased time to care for the transferred patient. This has been shown to influence patient outcomes at the time of patient discharge.10
In trying to understand why we observed an inverse relationship between admitting team busyness and odds of ICU transfer within 48 hours, we believe this finding is largely driven by cardiology service transfers, which comprise the highest volume of transferred patients in our cohort (Table 1), and are low acuity patients. Within this population of patients, admitting team busyness is likely a surrogate variable for high turnover/low acuity. This idea is supported by our findings that admitting team busyness is associated with decreased adjusted odds of 30-day mortality in this group (and only in this group).
Similarly, our observed inverse relationship between increased time delay and 30-day mortality among CT and GI surgical service patients is also likely reflective of lower acuity patients. We anticipated that decreased time delay (0-12 hours) would be reflective of greater patient acuity (supported by our findings that decreased time delay is associated with increased odds of ICU transfer and 30-day mortality). However, our findings also suggest that increased time delay (>48 hours) is similarly representative of lower patient acuity and therefore an imperfect measure of discontinuity and/or harmful delays in care during IHT (see limitations below).
Our study is subject to several limitations. This is a single site study; given known variation in transfer practices between hospitals,3 it is possible that our findings are not generalizable. However, given similar existing data on patients admitted through the ER, it is likely our findings may be reflective of IHT to similar tertiary referral hospitals. Second, although we adjusted for patient characteristics, there remains the possibility of unmeasured confounding and other bias that account for our results, as discussed. Third, although the definition of “busyness” used in this study was chosen based on prior data demonstrating an effect on patient outcomes,10 we did not include other measures of busyness that may influence outcomes of transferred patients such as overall team census or hospital busyness. However, the workload associated with a high volume of patient admissions and discharges is arguably a greater reflection of “work compression” for the admitting team compared with overall team census, which may reflect a more static workload with less impact on the care of a newly transferred patient. Also, although hospital census may influence the ability to transfer (ie, lower volume of transferred patients during times of high hospital census), this likely has less of an impact on the direct care of transferred patients than the admitting team’s workload. It is more likely that it would serve as a confounder (eg, sicker patients are accepted for transfer despite high hospital census, while lower risk patients are not).
Nevertheless, future studies should further evaluate the association with other measures of busyness/workload and outcomes of transferred patients. Lastly, though we anticipated time delay between transfer acceptance and arrival would be correlated with patient acuity, we hypothesized that longer delay might affect patient continuity and communication and impact patient outcomes. However, our results demonstrate that our measurement of this variable was unsuccessful in unraveling patient acuity from our intended evaluation of these vulnerable aspects of IHT. It is likely that a more detailed evaluation is required to explore potential challenges more fully that may occur with greater time delays (eg, suboptimal communication regarding changes in clinical status during this time period, delays in treatment). Similarly, though our study evaluates the association between nighttime and weekend transfer (and the interaction between these) with patient outcomes, we did not evaluate other intermediate outcomes that may be more affected by the timing of transfer, such as diagnostic errors or delays in procedural care, which warrant further investigation. We do not directly examine the underlying reasons that explain our observed associations, and thus more research is needed to identify these as well as design and evaluate solutions.
Collectively, our findings suggest that high acuity patients in need of procedural care experience worse outcomes during off-peak times of transfer, and during times of high care-team workload. Though further research is needed to identify underlying reasons to explain our findings, both the timing of patient transfer (when modifiable) and workload of the team caring for the patient on arrival may serve as potential targets for interventions to improve the quality and safety of IHT for patients at greatest risk.
Disclosures
Dr. Mueller and Dr. Schnipper have nothing to disclose. Ms. Fiskio has nothing to disclose. Dr. Schnipper is the recipient of grant funding from Mallinckrodt Pharmaceuticals to conduct an investigator-initiated study of predictors and impact of opioid-related adverse drug events.
The transfer of patients between acute care hospitals (interhospital transfer [IHT]) occurs regularly among patients with a variety of diagnoses, in theory, to gain access to unique specialty services and/or a higher level of care, among other reasons.1,2
However, the practice of IHT is variable and nonstandardized,3,4 and existing data largely suggests that transferred patients experience worse outcomes, including longer length of stay, higher hospitalization costs, longer ICU time, and greater mortality, even with rigorous adjustment for confounding by indication.5,6 Though there are many possible reasons for these findings, existing literature suggests that there may be aspects of the transfer process itself which contribute to these outcomes.2,6,7
Understanding which aspects of the transfer process contribute to poor patient outcomes is a key first step toward the development of targeted quality improvement initiatives to improve this process of care. In this study, we aim to examine the association between select characteristics of the transfer process, including the timing of transfer and workload of the admitting physician team, and clinical outcomes among patients undergoing IHT.
METHODS
Data and Study Population
We performed a retrospective analysis of patients ≥age 18 years who transferred to Brigham and Women’s Hospital (BWH), a 777-bed tertiary care hospital, from another acute care hospital between January 2005, and September 2013. Dates of inclusion were purposefully chosen prior to BWH implementation of a new electronic health records system to avoid potential information bias. As at most academic medical centers, night coverage at BWH differs by service and includes a combination of long-call admitting teams and night float coverage. On weekends, many services are less well staffed, and some procedures may only be available if needed emergently. Some services have caps on the daily number of admissions or total patient census, but none have caps on the number of discharges per day. Patients were excluded from analysis if they left BWH against medical advice, were transferred from closely affiliated hospitals with shared personnel and electronic health records (Brigham and Women’s Faulkner Hospital, Dana Farber Cancer Institute), transferred from inpatient psychiatric or inpatient hospice facilities, or transferred to obstetrics or nursery services. Data were obtained from administrative sources and the research patient data repository (RPDR), a centralized clinical data repository that gathers data from various hospital legacy systems and stores them in one data warehouse.8 Our study was approved by the Partners Institutional Review Board (IRB) with a waiver of patient consent.
Transfer Process Characteristics
Predictors included select characteristics of the transfer process, including (1) Day of week of transfer, dichotomized into Friday through Sunday (“weekend”), versus Monday through Thursday (“weekday”);9 Friday was included with “weekend” given the suggestion of increased volume of transfers in advance of the weekend; (2) Time of arrival of the transferred patient, categorized into “daytime” (7
Outcomes
Outcomes included transfer to the intensive care unit (ICU) within 48 hours of arrival and 30-day mortality from date of index admission.5,6
Patient Characteristics
Covariates for adjustment included: patient age, sex, race, Elixhauser comorbidity score,11 Diagnosis-Related Group (DRG)-weight, insurance status, year of admission, number of preadmission medications, and service of admission.
Statistical Analyses
We used descriptive statistics to display baseline characteristics and performed a series of univariable and multivariable logistic regression models to obtain the adjusted odds of each transfer process characteristic on each outcome, adjusting for all covariates (proc logistic, SAS Statistical Software, Cary, North Carolina). For analyses of ICU transfer within 48 hours of arrival, all patients initially admitted to the ICU at time of transfer were excluded.
In the secondary analyses, we used a combined day-of-week and time-of-day variable (ie, Monday day, Monday evening, Monday night, Tuesday day, and so on, with Monday day as the reference group) to obtain a more detailed evaluation of timing of transfer on patient outcomes. We also performed stratified analyses to evaluate each transfer process characteristic on adjusted odds of 30-day mortality stratified by service of admission (ie, at the time of transfer to BWH), adjusting for all covariates. For all analyses, two-sided P values < .05 were considered significant.
RESULTS
Overall, 24,352 patients met our inclusion criteria and underwent IHT, of whom 2,174 (8.9%) died within 30 days. Of the 22,910 transferred patients originally admitted to a non-ICU service, 5,464 (23.8%) underwent ICU transfer within 48 hours of arrival. Cohort characteristics are shown in Table 1.
Multivariable regression analyses demonstrated no significant association between weekend (versus weekday) transfer or increased time delay between patient acceptance and arrival (>48 hours) and adjusted odds of ICU transfer within 48 hours or 30-day mortality. However, they did demonstrate that nighttime (versus daytime) transfer was associated with greater adjusted odds of both ICU transfer and 30-day mortality. Increased admitting team busyness was associated with lower adjusted odds of ICU transfer but was not significantly associated with adjusted odds of 30-day mortality (Table 2). As expected, decreased time delay between patient acceptance and arrival (0-12 hours) was associated with increased adjusted odds of both ICU transfer (adjusted OR 2.68; 95% CI 2.29, 3.15) and 30-day mortality (adjusted OR 1.25; 95% CI 1.03, 1.53) compared with 12-24 hours (results not shown). Time delay >48 hours was not associated with either outcome.
Regression analyses with the combined day/time variable demonstrated that compared with Monday daytime transfer, Sunday night transfer was significantly associated with increased adjusted odds of 30-day mortality, and Friday night transfer was associated with a trend toward increased 30-day mortality (adjusted OR [aOR] 1.88; 95% CI 1.25, 2.82, and aOR 1.43; 95% CI 0.99, 2.06, respectively). We also found that all nighttime transfers (ie, Monday through Sunday night) were associated with increased adjusted odds of ICU transfer within 48 hours (as compared with Monday daytime transfer). Other days/time analyses were not significant.
Univariable and multivariable analyses stratified by service were performed (Appendix). Multivariable stratified analyses demonstrated that weekend transfer, nighttime transfer, and increased admitting team busyness were associated with increased adjusted odds of 30-day mortality among cardiothoracic (CT) and gastrointestinal (GI) surgical service patients. Increased admitting team busyness was also associated with increased mortality among ICU service patients but was associated with decreased mortality among cardiology service patients. An increased time delay between patient acceptance and arrival was associated with decreased mortality among CT and GI surgical service patients (Figure; Appendix). Other adjusted stratified outcomes were not significant.
DISCUSSION
In this study of 24,352 patients undergoing IHT, we found no significant association between weekend transfer or increased time delay between transfer acceptance and arrival and patient outcomes in the cohort as a whole; but we found that nighttime transfer is associated with increased adjusted odds of both ICU transfer within 48 hours and 30-day mortality. Our analyses combining day-of-week and time-of-day demonstrate that Sunday night transfer is particularly associated with increased adjusted odds of 30-day mortality (as compared with Monday daytime transfer), and show a trend toward increased mortality with Friday night transfers. These detailed analyses otherwise reinforce that nighttime transfer across all nights of the week is associated with increased adjusted odds of ICU transfer within 48 hours. We also found that increased admitting team busyness on the day of patient transfer is associated with decreased odds of ICU transfer, though this may solely be reflective of higher turnover services (ie, cardiology) caring for lower acuity patients, as suggested by secondary analyses stratified by service. In addition, secondary analyses demonstrated differential associations between weekend transfers, nighttime transfers, and increased team busyness on the odds of 30-day mortality based on service of transfer. These analyses showed that patients transferred to higher acuity services requiring procedural care, including CT surgery, GI surgery, and Medical ICU, do worse under all three circumstances as compared with patients transferred to other services. Secondary analyses also demonstrated that increased time delay between patient acceptance and arrival is inversely associated with 30-day mortality among CT and GI surgery service patients, likely reflecting lower acuity patients (ie, less sick patients are less rapidly transferred).
There are several possible explanations for these findings. Patients transferred to surgical services at night may reflect a more urgent need for surgery and include a sicker cohort of patients, possibly explaining these findings. Alternatively, or in addition, both weekend and nighttime hospital admission expose patients to similar potential risks, ie, limited resources available during off-peak hours. Our findings could, therefore, reflect the possibility that patients transferred to higher acuity services in need of procedural care are most vulnerable to off-peak timing of transfer. Similar data looking at patients admitted through the emergency room (ER) find the strongest effect of off-peak admissions on patients in need of procedures, including GI hemorrhage,12 atrial fibrillation13 and acute myocardial infarction (AMI),14 arguably because of the limited availability of necessary interventions. Patients undergoing IHT are a sicker cohort of patients than those admitted through the ER, and, therefore, may be even more vulnerable to these issues.3,5 This is supported by our findings that Sunday night transfers (and trend toward Friday night transfers) are associated with greater mortality compared with Monday daytime transfers, when at-the-ready resources and/or specialty personnel may be less available (Sunday night), and delays until receipt of necessary procedures may be longer (Friday night). Though we did not observe similar results among cardiology service transfers, as may be expected based on existing literature,13,14 this subset of patients includes more heterogeneous diagnoses, (ie, not solely those that require acute intervention) and exhibited a low level of acuity (low Elixhauser score and DRG-weight, data not shown).
We also found that increased admitting team busyness on the day of patient transfer is associated with increased odds of 30-day mortality among CT surgery, GI surgery, and ICU service transfers. As above, there are several possible explanations for this finding. It is possible that among these services, only the sickest/neediest patients are accepted for transfer when teams are busiest, explaining our findings. Though this explanation is possible, the measure of team “busyness” includes patient discharge, thereby increasing, not decreasing, availability for incoming patients, making this explanation less likely. Alternatively, it is possible that this finding is reflective of reverse causation, ie, that teams have less ability to discharge/admit new patients when caring for particularly sick/unstable patient transfers, though this assumes that transferred patients arrive earlier in the day, (eg, in time to influence discharge decisions), which infrequently occurs (Table 1). Lastly, it is possible that this subset of patients will be more vulnerable to the workload of the team that is caring for them at the time of their arrival. With high patient turnover (admissions/discharges), the time allocated to each patient’s care may be diminished (ie, “work compression,” trying to do the same amount of work in less time), and may result in decreased time to care for the transferred patient. This has been shown to influence patient outcomes at the time of patient discharge.10
In trying to understand why we observed an inverse relationship between admitting team busyness and odds of ICU transfer within 48 hours, we believe this finding is largely driven by cardiology service transfers, which comprise the highest volume of transferred patients in our cohort (Table 1), and are low acuity patients. Within this population of patients, admitting team busyness is likely a surrogate variable for high turnover/low acuity. This idea is supported by our findings that admitting team busyness is associated with decreased adjusted odds of 30-day mortality in this group (and only in this group).
Similarly, our observed inverse relationship between increased time delay and 30-day mortality among CT and GI surgical service patients is also likely reflective of lower acuity patients. We anticipated that decreased time delay (0-12 hours) would be reflective of greater patient acuity (supported by our findings that decreased time delay is associated with increased odds of ICU transfer and 30-day mortality). However, our findings also suggest that increased time delay (>48 hours) is similarly representative of lower patient acuity and therefore an imperfect measure of discontinuity and/or harmful delays in care during IHT (see limitations below).
Our study is subject to several limitations. This is a single site study; given known variation in transfer practices between hospitals,3 it is possible that our findings are not generalizable. However, given similar existing data on patients admitted through the ER, it is likely our findings may be reflective of IHT to similar tertiary referral hospitals. Second, although we adjusted for patient characteristics, there remains the possibility of unmeasured confounding and other bias that account for our results, as discussed. Third, although the definition of “busyness” used in this study was chosen based on prior data demonstrating an effect on patient outcomes,10 we did not include other measures of busyness that may influence outcomes of transferred patients such as overall team census or hospital busyness. However, the workload associated with a high volume of patient admissions and discharges is arguably a greater reflection of “work compression” for the admitting team compared with overall team census, which may reflect a more static workload with less impact on the care of a newly transferred patient. Also, although hospital census may influence the ability to transfer (ie, lower volume of transferred patients during times of high hospital census), this likely has less of an impact on the direct care of transferred patients than the admitting team’s workload. It is more likely that it would serve as a confounder (eg, sicker patients are accepted for transfer despite high hospital census, while lower risk patients are not).
Nevertheless, future studies should further evaluate the association with other measures of busyness/workload and outcomes of transferred patients. Lastly, though we anticipated time delay between transfer acceptance and arrival would be correlated with patient acuity, we hypothesized that longer delay might affect patient continuity and communication and impact patient outcomes. However, our results demonstrate that our measurement of this variable was unsuccessful in unraveling patient acuity from our intended evaluation of these vulnerable aspects of IHT. It is likely that a more detailed evaluation is required to explore potential challenges more fully that may occur with greater time delays (eg, suboptimal communication regarding changes in clinical status during this time period, delays in treatment). Similarly, though our study evaluates the association between nighttime and weekend transfer (and the interaction between these) with patient outcomes, we did not evaluate other intermediate outcomes that may be more affected by the timing of transfer, such as diagnostic errors or delays in procedural care, which warrant further investigation. We do not directly examine the underlying reasons that explain our observed associations, and thus more research is needed to identify these as well as design and evaluate solutions.
Collectively, our findings suggest that high acuity patients in need of procedural care experience worse outcomes during off-peak times of transfer, and during times of high care-team workload. Though further research is needed to identify underlying reasons to explain our findings, both the timing of patient transfer (when modifiable) and workload of the team caring for the patient on arrival may serve as potential targets for interventions to improve the quality and safety of IHT for patients at greatest risk.
Disclosures
Dr. Mueller and Dr. Schnipper have nothing to disclose. Ms. Fiskio has nothing to disclose. Dr. Schnipper is the recipient of grant funding from Mallinckrodt Pharmaceuticals to conduct an investigator-initiated study of predictors and impact of opioid-related adverse drug events.
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2 012;40(8):2470-2478. https://doi.org/10.1097/CCM.0b013e318254516f.
2. Mueller SK, Shannon E, Dalal A, Schnipper JL, Dykes P. Patient and physician experience with interhospital transfer: a qualitative study. J Patient Saf. 2018. https://doi.org/10.1097/PTS.0000000000000501
3. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Rates, predictors and variability of interhospital transfers: a national evaluation. J Hosp Med. 2017;12(6):435-442.https://doi.org/10.12788/jhm.2747.
4. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. https://doi.org/10.1097/MLR.0b013e31820fb71b.
5. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: characteristics and outcomes. J Hosp Med. 2016;11(4):245-50. https://doi.org/10.1002/jhm.2515.
6. Mueller S, Zheng J, Orav EJP, Schnipper JL. Inter-hospital transfer and patient outcomes: a retrospective cohort study. BMJ Qual Saf. 2018. https://doi.org/10.1136/bmjqs-2018-008087.
7. Mueller SK, Schnipper JL. Physician perspectives on interhospital transfers. J Patient Saf. 2016. https://doi.org/10.1097/PTS.0000000000000312.
8. Research Patient Data Registry (RPDR). http://rc.partners.org/rpdr. Accessed April 20, 2018.
9. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. https://doi.org/10.1056/NEJMsa003376
10. Mueller SK, Donze J, Schnipper JL. Intern workload and discontinuity of care on 30-day readmission. Am J Med. 2013;126(1):81-88. https://doi.org/10.1016/j.amjmed.2012.09.003.
11. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. PubMed
12. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol. 2009;7(3):296-302e1. https://doi.org/10.1016/j.cgh.2008.08.013.
13. Deshmukh A, Pant S, Kumar G, Bursac Z, Paydak H, Mehta JL. Comparison of outcomes of weekend versus weekday admissions for atrial fibrillation. Am J Cardiol. 2012;110(2):208-211. https://doi.org/10.1016/j.amjcard.2012.03.011.
14. Clarke MS, Wills RA, Bowman RV, et al. Exploratory study of the ‘weekend effect’ for acute medical admissions to public hospitals in Queensland, Australia. Intern Med J. 2010;40(11):777-783. https://doi.org/-10.1111/j.1445-5994.2009.02067.x.
1. Iwashyna TJ. The incomplete infrastructure for interhospital patient transfer. Crit Care Med. 2 012;40(8):2470-2478. https://doi.org/10.1097/CCM.0b013e318254516f.
2. Mueller SK, Shannon E, Dalal A, Schnipper JL, Dykes P. Patient and physician experience with interhospital transfer: a qualitative study. J Patient Saf. 2018. https://doi.org/10.1097/PTS.0000000000000501
3. Mueller SK, Zheng J, Orav EJ, Schnipper JL. Rates, predictors and variability of interhospital transfers: a national evaluation. J Hosp Med. 2017;12(6):435-442.https://doi.org/10.12788/jhm.2747.
4. Bosk EA, Veinot T, Iwashyna TJ. Which patients and where: a qualitative study of patient transfers from community hospitals. Med Care. 2011;49(6):592-598. https://doi.org/10.1097/MLR.0b013e31820fb71b.
5. Sokol-Hessner L, White AA, Davis KF, Herzig SJ, Hohmann SF. Interhospital transfer patients discharged by academic hospitalists and general internists: characteristics and outcomes. J Hosp Med. 2016;11(4):245-50. https://doi.org/10.1002/jhm.2515.
6. Mueller S, Zheng J, Orav EJP, Schnipper JL. Inter-hospital transfer and patient outcomes: a retrospective cohort study. BMJ Qual Saf. 2018. https://doi.org/10.1136/bmjqs-2018-008087.
7. Mueller SK, Schnipper JL. Physician perspectives on interhospital transfers. J Patient Saf. 2016. https://doi.org/10.1097/PTS.0000000000000312.
8. Research Patient Data Registry (RPDR). http://rc.partners.org/rpdr. Accessed April 20, 2018.
9. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. https://doi.org/10.1056/NEJMsa003376
10. Mueller SK, Donze J, Schnipper JL. Intern workload and discontinuity of care on 30-day readmission. Am J Med. 2013;126(1):81-88. https://doi.org/10.1016/j.amjmed.2012.09.003.
11. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. PubMed
12. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol. 2009;7(3):296-302e1. https://doi.org/10.1016/j.cgh.2008.08.013.
13. Deshmukh A, Pant S, Kumar G, Bursac Z, Paydak H, Mehta JL. Comparison of outcomes of weekend versus weekday admissions for atrial fibrillation. Am J Cardiol. 2012;110(2):208-211. https://doi.org/10.1016/j.amjcard.2012.03.011.
14. Clarke MS, Wills RA, Bowman RV, et al. Exploratory study of the ‘weekend effect’ for acute medical admissions to public hospitals in Queensland, Australia. Intern Med J. 2010;40(11):777-783. https://doi.org/-10.1111/j.1445-5994.2009.02067.x.
© 2019 Society of Hospital Medicine
Critical Errors in Inhaler Technique among Children Hospitalized with Asthma
Many studies have shown that improved control can be achieved for most children with asthma if inhaled medications are taken correctly and adequately.1-3 Drug delivery studies have shown that bioavailability of medication with a pressurized metered-dose inhaler (MDI) improves from 34% to 83% with the addition of spacer devices. This difference is largely due to the decrease in oropharyngeal deposition,1,4,5 and therefore, the use of a spacer with proper technique has been recommended in all pediatric patients.1,6
Poor inhaler technique is common among children.1,7 Previous studies of children with asthma have evaluated inhaler technique, primarily in the outpatient and community settings, and reported variable rates of error (from 45% to >90%).8,9 No studies have evaluated children hospitalized with asthma. As these children represent a particularly high-risk group for morbidity and mortality,10,11 the objectives of this study were to assess errors in inhaler technique in hospitalized asthmatic children and identify risk factors for improper use.
METHODS
As part of a larger interventional study, we conducted a prospective cross-sectional study at a tertiary urban children’s hospital. We enrolled a convenience sample of children aged 2-16 years admitted to the inpatient ward with an asthma exacerbation Monday-Friday from 8 AM to 6 PM. Participants were required to have a diagnosis of asthma (an established diagnosis by their primary care provider or meets the National Heart, Lung, and Blood Institute [NHLBI] criteria1), have a consenting adult available, and speak English. Patients were excluded if they had a codiagnosis of an additional respiratory disease (ie, pneumonia), cardiac disease, or sickle cell anemia. The Institutional Review Board approved this study.
We asked caregivers, or children >10 years old if they independently use their inhaler, to demonstrate their typical home inhaler technique using a spacer with mask (SM), spacer with mouthpiece (SMP), or no spacer (per their usual home practice). Inhaler technique was scored using a previously validated asthma checklist (Table 1).12 Certain steps in the checklist were identified as critical: (Step 1) removing the cap, (Step 3) attaching to a spacer, (Step 7) taking six breaths (SM), and (Step 9) holding breath for five seconds (SMP). Caregivers only were also asked to complete questionnaires assessing their literacy (Brief Health Literacy Screen [BHLS]), confidence (Parent Asthma Management Self-Efficacy scale [PAMSE]), and any barriers to managing their child’s asthma (Barriers to Asthma Care). Demographic and medical history information was extracted from the medical chart.
Inhaler technique was evaluated in two ways by comparing: (1) patients who missed more than one critical step with those who missed zero critical steps and (2) patients with an asthma checklist score <7 versus ≥7. While there is a lot of variability in how inhaler technique has been measured in past studies, these two markers (75% of steps and critical errors) were the most common.8
We assessed a number of variables to evaluate their association with improper inhaler technique. For categorical variables, the association with each outcome was evaluated using relative risks (RRs). Bivariate P-values were calculated using chi-square or Fisher’s exact tests, as appropriate. Continuous variables were assessed for associations with each outcome using two-sample t-tests. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using logistic regression analyses. Using a model entry criterion of P < .10 on univariate tests, variables were entered into a multivariable logistic regression model for each outcome. Full models with all eligible covariates and reduced models selected via a manual backward selection process were evaluated. Two-sided P-values <.05 were considered statistically significant.
RESULTS
Participants
From October 2016 to June 2017, 380 participants were assessed for participation; 215 were excluded for not having a parent available (59%), not speaking English (27%), not having an asthma diagnosis (ie, viral wheezing; 14%), and 52 (14%) declined to participate. Therefore, a total of 113 participants were enrolled, with demonstrations provided by 100 caregivers and 13 children. The mean age of the patients overall was 6.6 ± 3.4 years and over half (55%) of the participants had uncontrolled asthma (NHLBI criteria1).
Errors in Inhaler Technique
The mean asthma checklist score was 6.7 (maximum score of 10 for SM and 12 for SMP). A third (35%) scored <7 on the asthma checklist and 42% of participants missed at least one critical step. Overall, children who missed a critical step were significantly older (7.8 [6.7-8.9] vs 5.8 [5.1-6.5] years; P = .002). More participants missed a critical step with the SMP than the SM (75% [51%-90%] vs 36% [27%-46%]; P = .003), and this was the most prominent factor for missing a critical step in the adjusted regression analysis (OR 6.95 [1.71-28.23], P = .007). The most commonly missed steps were breathing normally for 30 seconds for SM, and for SMP, it was breathing out fully and breathing away from the spacer (Table 1). Twenty participants (18%) did not use a spacer device; these patients were older than those who did use a spacer (mean age 8.5 [6.7-10.4] vs 6.2 [5.6-6.9] years; P = .005); however, no other significant differences were identified.
Demographic, Medical History, and Socioeconomic Characteristics
Overall, race, ethnicity, and insurance status did not vary significantly based on asthma checklist score ≥7 or missing a critical step. Patients in the SM group who had received inpatient asthma education during a previous admission, had a history of pediatric intensive care unit (PICU) admission, and had been prescribed a daily controller were less likely to miss a critical step (Table 2). Parental education level varied, with 33% having a high school degree or less, but was not associated with asthma checklist score or missing critical steps. Parental BHLS and parental confidence (PAMSE) were not significantly associated with inhaler proficiency. However, transportation-related barriers were more common in patients with checklist scores <7 and more missed critical steps (OR 1.62 [1.06-2.46]; P = .02).
Nearly half of the participants in this study missed at least one critical step in inhaler use. In addition, 18% did not use a spacer when demonstrating their inhaler technique. Despite robust studies demonstrating how asthma education can improve both asthma skills and clinical outcomes,13 our study demonstrates that a large gap remains in proper inhaler technique among asthmatic patients presenting for inpatient care. Specifically, in the mouthpiece group, steps related to breathing technique were the most commonly missed. Our results also show that inhaler technique errors were most prominent in the adolescent population, possibly coinciding with the process of transitioning to a mouthpiece and more independence in medication administration. Adolescents may be a high-impact population on which to focus inpatient asthma education. Additionally, we found that a previous PICU admission and previous inpatient asthma education were associated with missing fewer critical steps in inhaler technique. This finding is consistent with those of another study that evaluated inhaler technique in the emergency department and found that previous hospitalization for asthma was inversely related to improper inhaler use (RR 0.55, 95% CI 0.36-0.84).14 This supports that when provided, inpatient education can increase inhaler administration skills.
Previous studies conducted in the outpatient setting have demonstrated variable rates of inhaler skill, from 0% to approximately 89% of children performing all steps of inhalation correctly.8 This wide range may be related to variations in the number and definition of critical steps between the different studies. In our study, we highlighted removing the cap, attaching a spacer, and adequate breathing technique as critical steps, because failure to complete them would significantly reduce lung deposition of medication. While past studies did evaluate both MDIs and discuss the devices, our study is the first to report difference in problems with technique between SM and SMP. As asthma educational interventions are developed and/or implemented, it is important to stress that different steps in inhaler technique are being missed in those using a mask versus mouthpiece.
The limitations of this study include that it was at a single center with a primarily urban and English-speaking population; however, this study population reflects the racial diversity of pediatric asthma patients. Further studies may explore the reproducibility of these findings at multiple centers and with non-English-speaking families. This study included younger patients than in some previous publications investigating asthma; however, all patients met the criteria for asthma diagnosis and this age range is reflective of patients presenting for inpatient asthma care. Furthermore, because of our daytime research hours, 59% of patients were excluded because a primary caregiver was not available. It is possible that these families have decreased access to inpatient asthma educators as well and may be another target group for future studies. Finally, a large proportion of parents had a college education or greater in our sample. However, there was no association within our analysis between parental education level and inhaler proficiency.
The findings from this study indicate that continued efforts are needed to establish that inhaler technique is adequate for all families regardless of their educational status or socioeconomic background, especially for adolescents and in the setting of poor asthma control. Furthermore, our findings support that inhaler technique education may be beneficial in the inpatient setting and that acute care settings can provide a valuable “teachable moment.”14,15
CONCLUSION
Errors in inhaler technique are prevalent in pediatric inpatients with asthma, primarily those using a mouthpiece device. Educational efforts in both inpatient and outpatient settings have the potential to improve drug delivery and therefore asthma control. Inpatient hospitalization may serve as a platform for further studies to investigate innovative educational interventions.
Acknowledgments
The authors thank Tina Carter for her assistance in the recruitment and data collection and Ashley Hull and Susannah Butters for training the study staff on the use of the asthma checklist.
Disclosures
Dr. Gupta receives research grant support from the National Institutes of Health and the United Healthcare Group. Dr. Gupta serves as a consultant for DBV Technology, Aimmune Therapeutics, Kaleo & BEFORE Brands. Dr. Gupta has received lecture fees/honorariums from the Allergy Asthma Network & the American College of Asthma, Allergy & Immunology. Dr. Press reports research support from the Chicago Center for Diabetes Translation Research Pilot and Feasibility Grant, the Bucksbaum Institute for Clinical Excellence Pilot Grant Program, the Academy of Distinguished Medical Educators, the Development of Novel Hospital-initiated Care Bundle in Adults Hospitalized for Acute Asthma: the 41st Multicenter Airway Research Collaboration (MARC-41) Study, UCM’s Innovation Grant Program, the University of Chicago-Chapin Hall Join Research Fund, the NIH/NHLBI Loan Repayment Program, 1 K23 HL118151 01, NIH NLBHI R03 (RFA-HL-18-025), the George and Carol Abramson Pilot Awards, the COPD Foundation Green Shoots Grant, the University of Chicago Women’s Board Grant, NIH NHLBI UG1 (RFA-HL-17-009), and the CTSA Pilot Award, outside the submitted work. These disclosures have been reported to Dr. Press’ institutional IRB board. Additionally, a management plan is on file that details how to address conflicts such as these which are sources of research support but do not directly support the work at hand. The remaining authors have no conflicts of interest relevant to the article to disclose.
Funding
This study was funded by internal grants from Ann and Robert H. Lurie Children’s Hospital of Chicago. Dr. Press was funded by a K23HL118151.
1. Expert Panel Report 3: guidelines for the diagnosis and management of asthma: full report. Washington, DC: US Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute; 2007. PubMed
2. Hekking PP, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896-902. doi: 10.1016/j.jaci.2014.08.042. PubMed
3. Peters SP, Ferguson G, Deniz Y, Reisner C. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med. 2006;100(7):1139-1151. doi: 10.1016/j.rmed.2006.03.031. PubMed
4. Dickens GR, Wermeling DP, Matheny CJ, et al. Pharmacokinetics of flunisolide administered via metered dose inhaler with and without a spacer device and following oral administration. Ann Allergy Asthma Immunol. 2000;84(5):528-532. doi: 10.1016/S1081-1206(10)62517-3. PubMed
5. Nikander K, Nicholls C, Denyer J, Pritchard J. The evolution of spacers and valved holding chambers. J Aerosol Med Pulm Drug Deliv. 2014;27(1):S4-S23. doi: 10.1089/jamp.2013.1076. PubMed
6. Rubin BK, Fink JB. The delivery of inhaled medication to the young child. Pediatr Clin North Am. 2003;50(3):717-731. doi:10.1016/S0031-3955(03)00049-X. PubMed
7. Roland NJ, Bhalla RK, Earis J. The local side effects of inhaled corticosteroids: current understanding and review of the literature. Chest. 2004;126(1):213-219. doi: 10.1378/chest.126.1.213. PubMed
8. Gillette C, Rockich-Winston N, Kuhn JA, Flesher S, Shepherd M. Inhaler technique in children with asthma: a systematic review. Acad Pediatr. 2016;16(7):605-615. doi: 10.1016/j.acap.2016.04.006. PubMed
9. Pappalardo AA, Karavolos K, Martin MA. What really happens in the home: the medication environment of urban, minority youth. J Allergy Clin Immunol Pract. 2017;5(3):764-770. doi: 10.1016/j.jaip.2016.09.046. PubMed
10. Crane J, Pearce N, Burgess C, Woodman K, Robson B, Beasley R. Markers of risk of asthma death or readmission in the 12 months following a hospital admission for asthma. Int J Epidemiol. 1992;21(4):737-744. doi: 10.1093/ije/21.4.737. PubMed
11. Turner MO, Noertjojo K, Vedal S, Bai T, Crump S, Fitzgerald JM. Risk factors for near-fatal asthma. A case-control study in hospitalized patients with asthma. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1804-1809. doi: 10.1164/ajrccm.157.6.9708092. PubMed
12. Press VG, Arora VM, Shah LM, et al. Misuse of respiratory inhalers in hospitalized patients with asthma or COPD. J Gen Intern Med. 2011;26(6):635-642. doi: 10.1007/s11606-010-1624-2. PubMed
13. Guevara JP, Wolf FM, Grum CM, Clark NM. Effects of educational interventions for self management of asthma in children and adolescents: systematic review and meta-analysis. BMJ. 2003;326(7402):1308-1309. doi: 10.1136/bmj.326.7402.1308. PubMed
14. Scarfone RJ, Capraro GA, Zorc JJ, Zhao H. Demonstrated use of metered-dose inhalers and peak flow meters by children and adolescents with acute asthma exacerbations. Arch Pediatr Adolesc Med. 2002;156(4):378-383. doi: 10.1001/archpedi.156.4.378. PubMed
15. Sockrider MM, Abramson S, Brooks E, et al. Delivering tailored asthma family education in a pediatric emergency department setting: a pilot study. Pediatrics. 2006;117(4 Pt 2):S135-144. doi: 10.1542/peds.2005-2000K. PubMed
Many studies have shown that improved control can be achieved for most children with asthma if inhaled medications are taken correctly and adequately.1-3 Drug delivery studies have shown that bioavailability of medication with a pressurized metered-dose inhaler (MDI) improves from 34% to 83% with the addition of spacer devices. This difference is largely due to the decrease in oropharyngeal deposition,1,4,5 and therefore, the use of a spacer with proper technique has been recommended in all pediatric patients.1,6
Poor inhaler technique is common among children.1,7 Previous studies of children with asthma have evaluated inhaler technique, primarily in the outpatient and community settings, and reported variable rates of error (from 45% to >90%).8,9 No studies have evaluated children hospitalized with asthma. As these children represent a particularly high-risk group for morbidity and mortality,10,11 the objectives of this study were to assess errors in inhaler technique in hospitalized asthmatic children and identify risk factors for improper use.
METHODS
As part of a larger interventional study, we conducted a prospective cross-sectional study at a tertiary urban children’s hospital. We enrolled a convenience sample of children aged 2-16 years admitted to the inpatient ward with an asthma exacerbation Monday-Friday from 8 AM to 6 PM. Participants were required to have a diagnosis of asthma (an established diagnosis by their primary care provider or meets the National Heart, Lung, and Blood Institute [NHLBI] criteria1), have a consenting adult available, and speak English. Patients were excluded if they had a codiagnosis of an additional respiratory disease (ie, pneumonia), cardiac disease, or sickle cell anemia. The Institutional Review Board approved this study.
We asked caregivers, or children >10 years old if they independently use their inhaler, to demonstrate their typical home inhaler technique using a spacer with mask (SM), spacer with mouthpiece (SMP), or no spacer (per their usual home practice). Inhaler technique was scored using a previously validated asthma checklist (Table 1).12 Certain steps in the checklist were identified as critical: (Step 1) removing the cap, (Step 3) attaching to a spacer, (Step 7) taking six breaths (SM), and (Step 9) holding breath for five seconds (SMP). Caregivers only were also asked to complete questionnaires assessing their literacy (Brief Health Literacy Screen [BHLS]), confidence (Parent Asthma Management Self-Efficacy scale [PAMSE]), and any barriers to managing their child’s asthma (Barriers to Asthma Care). Demographic and medical history information was extracted from the medical chart.
Inhaler technique was evaluated in two ways by comparing: (1) patients who missed more than one critical step with those who missed zero critical steps and (2) patients with an asthma checklist score <7 versus ≥7. While there is a lot of variability in how inhaler technique has been measured in past studies, these two markers (75% of steps and critical errors) were the most common.8
We assessed a number of variables to evaluate their association with improper inhaler technique. For categorical variables, the association with each outcome was evaluated using relative risks (RRs). Bivariate P-values were calculated using chi-square or Fisher’s exact tests, as appropriate. Continuous variables were assessed for associations with each outcome using two-sample t-tests. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using logistic regression analyses. Using a model entry criterion of P < .10 on univariate tests, variables were entered into a multivariable logistic regression model for each outcome. Full models with all eligible covariates and reduced models selected via a manual backward selection process were evaluated. Two-sided P-values <.05 were considered statistically significant.
RESULTS
Participants
From October 2016 to June 2017, 380 participants were assessed for participation; 215 were excluded for not having a parent available (59%), not speaking English (27%), not having an asthma diagnosis (ie, viral wheezing; 14%), and 52 (14%) declined to participate. Therefore, a total of 113 participants were enrolled, with demonstrations provided by 100 caregivers and 13 children. The mean age of the patients overall was 6.6 ± 3.4 years and over half (55%) of the participants had uncontrolled asthma (NHLBI criteria1).
Errors in Inhaler Technique
The mean asthma checklist score was 6.7 (maximum score of 10 for SM and 12 for SMP). A third (35%) scored <7 on the asthma checklist and 42% of participants missed at least one critical step. Overall, children who missed a critical step were significantly older (7.8 [6.7-8.9] vs 5.8 [5.1-6.5] years; P = .002). More participants missed a critical step with the SMP than the SM (75% [51%-90%] vs 36% [27%-46%]; P = .003), and this was the most prominent factor for missing a critical step in the adjusted regression analysis (OR 6.95 [1.71-28.23], P = .007). The most commonly missed steps were breathing normally for 30 seconds for SM, and for SMP, it was breathing out fully and breathing away from the spacer (Table 1). Twenty participants (18%) did not use a spacer device; these patients were older than those who did use a spacer (mean age 8.5 [6.7-10.4] vs 6.2 [5.6-6.9] years; P = .005); however, no other significant differences were identified.
Demographic, Medical History, and Socioeconomic Characteristics
Overall, race, ethnicity, and insurance status did not vary significantly based on asthma checklist score ≥7 or missing a critical step. Patients in the SM group who had received inpatient asthma education during a previous admission, had a history of pediatric intensive care unit (PICU) admission, and had been prescribed a daily controller were less likely to miss a critical step (Table 2). Parental education level varied, with 33% having a high school degree or less, but was not associated with asthma checklist score or missing critical steps. Parental BHLS and parental confidence (PAMSE) were not significantly associated with inhaler proficiency. However, transportation-related barriers were more common in patients with checklist scores <7 and more missed critical steps (OR 1.62 [1.06-2.46]; P = .02).
Nearly half of the participants in this study missed at least one critical step in inhaler use. In addition, 18% did not use a spacer when demonstrating their inhaler technique. Despite robust studies demonstrating how asthma education can improve both asthma skills and clinical outcomes,13 our study demonstrates that a large gap remains in proper inhaler technique among asthmatic patients presenting for inpatient care. Specifically, in the mouthpiece group, steps related to breathing technique were the most commonly missed. Our results also show that inhaler technique errors were most prominent in the adolescent population, possibly coinciding with the process of transitioning to a mouthpiece and more independence in medication administration. Adolescents may be a high-impact population on which to focus inpatient asthma education. Additionally, we found that a previous PICU admission and previous inpatient asthma education were associated with missing fewer critical steps in inhaler technique. This finding is consistent with those of another study that evaluated inhaler technique in the emergency department and found that previous hospitalization for asthma was inversely related to improper inhaler use (RR 0.55, 95% CI 0.36-0.84).14 This supports that when provided, inpatient education can increase inhaler administration skills.
Previous studies conducted in the outpatient setting have demonstrated variable rates of inhaler skill, from 0% to approximately 89% of children performing all steps of inhalation correctly.8 This wide range may be related to variations in the number and definition of critical steps between the different studies. In our study, we highlighted removing the cap, attaching a spacer, and adequate breathing technique as critical steps, because failure to complete them would significantly reduce lung deposition of medication. While past studies did evaluate both MDIs and discuss the devices, our study is the first to report difference in problems with technique between SM and SMP. As asthma educational interventions are developed and/or implemented, it is important to stress that different steps in inhaler technique are being missed in those using a mask versus mouthpiece.
The limitations of this study include that it was at a single center with a primarily urban and English-speaking population; however, this study population reflects the racial diversity of pediatric asthma patients. Further studies may explore the reproducibility of these findings at multiple centers and with non-English-speaking families. This study included younger patients than in some previous publications investigating asthma; however, all patients met the criteria for asthma diagnosis and this age range is reflective of patients presenting for inpatient asthma care. Furthermore, because of our daytime research hours, 59% of patients were excluded because a primary caregiver was not available. It is possible that these families have decreased access to inpatient asthma educators as well and may be another target group for future studies. Finally, a large proportion of parents had a college education or greater in our sample. However, there was no association within our analysis between parental education level and inhaler proficiency.
The findings from this study indicate that continued efforts are needed to establish that inhaler technique is adequate for all families regardless of their educational status or socioeconomic background, especially for adolescents and in the setting of poor asthma control. Furthermore, our findings support that inhaler technique education may be beneficial in the inpatient setting and that acute care settings can provide a valuable “teachable moment.”14,15
CONCLUSION
Errors in inhaler technique are prevalent in pediatric inpatients with asthma, primarily those using a mouthpiece device. Educational efforts in both inpatient and outpatient settings have the potential to improve drug delivery and therefore asthma control. Inpatient hospitalization may serve as a platform for further studies to investigate innovative educational interventions.
Acknowledgments
The authors thank Tina Carter for her assistance in the recruitment and data collection and Ashley Hull and Susannah Butters for training the study staff on the use of the asthma checklist.
Disclosures
Dr. Gupta receives research grant support from the National Institutes of Health and the United Healthcare Group. Dr. Gupta serves as a consultant for DBV Technology, Aimmune Therapeutics, Kaleo & BEFORE Brands. Dr. Gupta has received lecture fees/honorariums from the Allergy Asthma Network & the American College of Asthma, Allergy & Immunology. Dr. Press reports research support from the Chicago Center for Diabetes Translation Research Pilot and Feasibility Grant, the Bucksbaum Institute for Clinical Excellence Pilot Grant Program, the Academy of Distinguished Medical Educators, the Development of Novel Hospital-initiated Care Bundle in Adults Hospitalized for Acute Asthma: the 41st Multicenter Airway Research Collaboration (MARC-41) Study, UCM’s Innovation Grant Program, the University of Chicago-Chapin Hall Join Research Fund, the NIH/NHLBI Loan Repayment Program, 1 K23 HL118151 01, NIH NLBHI R03 (RFA-HL-18-025), the George and Carol Abramson Pilot Awards, the COPD Foundation Green Shoots Grant, the University of Chicago Women’s Board Grant, NIH NHLBI UG1 (RFA-HL-17-009), and the CTSA Pilot Award, outside the submitted work. These disclosures have been reported to Dr. Press’ institutional IRB board. Additionally, a management plan is on file that details how to address conflicts such as these which are sources of research support but do not directly support the work at hand. The remaining authors have no conflicts of interest relevant to the article to disclose.
Funding
This study was funded by internal grants from Ann and Robert H. Lurie Children’s Hospital of Chicago. Dr. Press was funded by a K23HL118151.
Many studies have shown that improved control can be achieved for most children with asthma if inhaled medications are taken correctly and adequately.1-3 Drug delivery studies have shown that bioavailability of medication with a pressurized metered-dose inhaler (MDI) improves from 34% to 83% with the addition of spacer devices. This difference is largely due to the decrease in oropharyngeal deposition,1,4,5 and therefore, the use of a spacer with proper technique has been recommended in all pediatric patients.1,6
Poor inhaler technique is common among children.1,7 Previous studies of children with asthma have evaluated inhaler technique, primarily in the outpatient and community settings, and reported variable rates of error (from 45% to >90%).8,9 No studies have evaluated children hospitalized with asthma. As these children represent a particularly high-risk group for morbidity and mortality,10,11 the objectives of this study were to assess errors in inhaler technique in hospitalized asthmatic children and identify risk factors for improper use.
METHODS
As part of a larger interventional study, we conducted a prospective cross-sectional study at a tertiary urban children’s hospital. We enrolled a convenience sample of children aged 2-16 years admitted to the inpatient ward with an asthma exacerbation Monday-Friday from 8 AM to 6 PM. Participants were required to have a diagnosis of asthma (an established diagnosis by their primary care provider or meets the National Heart, Lung, and Blood Institute [NHLBI] criteria1), have a consenting adult available, and speak English. Patients were excluded if they had a codiagnosis of an additional respiratory disease (ie, pneumonia), cardiac disease, or sickle cell anemia. The Institutional Review Board approved this study.
We asked caregivers, or children >10 years old if they independently use their inhaler, to demonstrate their typical home inhaler technique using a spacer with mask (SM), spacer with mouthpiece (SMP), or no spacer (per their usual home practice). Inhaler technique was scored using a previously validated asthma checklist (Table 1).12 Certain steps in the checklist were identified as critical: (Step 1) removing the cap, (Step 3) attaching to a spacer, (Step 7) taking six breaths (SM), and (Step 9) holding breath for five seconds (SMP). Caregivers only were also asked to complete questionnaires assessing their literacy (Brief Health Literacy Screen [BHLS]), confidence (Parent Asthma Management Self-Efficacy scale [PAMSE]), and any barriers to managing their child’s asthma (Barriers to Asthma Care). Demographic and medical history information was extracted from the medical chart.
Inhaler technique was evaluated in two ways by comparing: (1) patients who missed more than one critical step with those who missed zero critical steps and (2) patients with an asthma checklist score <7 versus ≥7. While there is a lot of variability in how inhaler technique has been measured in past studies, these two markers (75% of steps and critical errors) were the most common.8
We assessed a number of variables to evaluate their association with improper inhaler technique. For categorical variables, the association with each outcome was evaluated using relative risks (RRs). Bivariate P-values were calculated using chi-square or Fisher’s exact tests, as appropriate. Continuous variables were assessed for associations with each outcome using two-sample t-tests. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using logistic regression analyses. Using a model entry criterion of P < .10 on univariate tests, variables were entered into a multivariable logistic regression model for each outcome. Full models with all eligible covariates and reduced models selected via a manual backward selection process were evaluated. Two-sided P-values <.05 were considered statistically significant.
RESULTS
Participants
From October 2016 to June 2017, 380 participants were assessed for participation; 215 were excluded for not having a parent available (59%), not speaking English (27%), not having an asthma diagnosis (ie, viral wheezing; 14%), and 52 (14%) declined to participate. Therefore, a total of 113 participants were enrolled, with demonstrations provided by 100 caregivers and 13 children. The mean age of the patients overall was 6.6 ± 3.4 years and over half (55%) of the participants had uncontrolled asthma (NHLBI criteria1).
Errors in Inhaler Technique
The mean asthma checklist score was 6.7 (maximum score of 10 for SM and 12 for SMP). A third (35%) scored <7 on the asthma checklist and 42% of participants missed at least one critical step. Overall, children who missed a critical step were significantly older (7.8 [6.7-8.9] vs 5.8 [5.1-6.5] years; P = .002). More participants missed a critical step with the SMP than the SM (75% [51%-90%] vs 36% [27%-46%]; P = .003), and this was the most prominent factor for missing a critical step in the adjusted regression analysis (OR 6.95 [1.71-28.23], P = .007). The most commonly missed steps were breathing normally for 30 seconds for SM, and for SMP, it was breathing out fully and breathing away from the spacer (Table 1). Twenty participants (18%) did not use a spacer device; these patients were older than those who did use a spacer (mean age 8.5 [6.7-10.4] vs 6.2 [5.6-6.9] years; P = .005); however, no other significant differences were identified.
Demographic, Medical History, and Socioeconomic Characteristics
Overall, race, ethnicity, and insurance status did not vary significantly based on asthma checklist score ≥7 or missing a critical step. Patients in the SM group who had received inpatient asthma education during a previous admission, had a history of pediatric intensive care unit (PICU) admission, and had been prescribed a daily controller were less likely to miss a critical step (Table 2). Parental education level varied, with 33% having a high school degree or less, but was not associated with asthma checklist score or missing critical steps. Parental BHLS and parental confidence (PAMSE) were not significantly associated with inhaler proficiency. However, transportation-related barriers were more common in patients with checklist scores <7 and more missed critical steps (OR 1.62 [1.06-2.46]; P = .02).
Nearly half of the participants in this study missed at least one critical step in inhaler use. In addition, 18% did not use a spacer when demonstrating their inhaler technique. Despite robust studies demonstrating how asthma education can improve both asthma skills and clinical outcomes,13 our study demonstrates that a large gap remains in proper inhaler technique among asthmatic patients presenting for inpatient care. Specifically, in the mouthpiece group, steps related to breathing technique were the most commonly missed. Our results also show that inhaler technique errors were most prominent in the adolescent population, possibly coinciding with the process of transitioning to a mouthpiece and more independence in medication administration. Adolescents may be a high-impact population on which to focus inpatient asthma education. Additionally, we found that a previous PICU admission and previous inpatient asthma education were associated with missing fewer critical steps in inhaler technique. This finding is consistent with those of another study that evaluated inhaler technique in the emergency department and found that previous hospitalization for asthma was inversely related to improper inhaler use (RR 0.55, 95% CI 0.36-0.84).14 This supports that when provided, inpatient education can increase inhaler administration skills.
Previous studies conducted in the outpatient setting have demonstrated variable rates of inhaler skill, from 0% to approximately 89% of children performing all steps of inhalation correctly.8 This wide range may be related to variations in the number and definition of critical steps between the different studies. In our study, we highlighted removing the cap, attaching a spacer, and adequate breathing technique as critical steps, because failure to complete them would significantly reduce lung deposition of medication. While past studies did evaluate both MDIs and discuss the devices, our study is the first to report difference in problems with technique between SM and SMP. As asthma educational interventions are developed and/or implemented, it is important to stress that different steps in inhaler technique are being missed in those using a mask versus mouthpiece.
The limitations of this study include that it was at a single center with a primarily urban and English-speaking population; however, this study population reflects the racial diversity of pediatric asthma patients. Further studies may explore the reproducibility of these findings at multiple centers and with non-English-speaking families. This study included younger patients than in some previous publications investigating asthma; however, all patients met the criteria for asthma diagnosis and this age range is reflective of patients presenting for inpatient asthma care. Furthermore, because of our daytime research hours, 59% of patients were excluded because a primary caregiver was not available. It is possible that these families have decreased access to inpatient asthma educators as well and may be another target group for future studies. Finally, a large proportion of parents had a college education or greater in our sample. However, there was no association within our analysis between parental education level and inhaler proficiency.
The findings from this study indicate that continued efforts are needed to establish that inhaler technique is adequate for all families regardless of their educational status or socioeconomic background, especially for adolescents and in the setting of poor asthma control. Furthermore, our findings support that inhaler technique education may be beneficial in the inpatient setting and that acute care settings can provide a valuable “teachable moment.”14,15
CONCLUSION
Errors in inhaler technique are prevalent in pediatric inpatients with asthma, primarily those using a mouthpiece device. Educational efforts in both inpatient and outpatient settings have the potential to improve drug delivery and therefore asthma control. Inpatient hospitalization may serve as a platform for further studies to investigate innovative educational interventions.
Acknowledgments
The authors thank Tina Carter for her assistance in the recruitment and data collection and Ashley Hull and Susannah Butters for training the study staff on the use of the asthma checklist.
Disclosures
Dr. Gupta receives research grant support from the National Institutes of Health and the United Healthcare Group. Dr. Gupta serves as a consultant for DBV Technology, Aimmune Therapeutics, Kaleo & BEFORE Brands. Dr. Gupta has received lecture fees/honorariums from the Allergy Asthma Network & the American College of Asthma, Allergy & Immunology. Dr. Press reports research support from the Chicago Center for Diabetes Translation Research Pilot and Feasibility Grant, the Bucksbaum Institute for Clinical Excellence Pilot Grant Program, the Academy of Distinguished Medical Educators, the Development of Novel Hospital-initiated Care Bundle in Adults Hospitalized for Acute Asthma: the 41st Multicenter Airway Research Collaboration (MARC-41) Study, UCM’s Innovation Grant Program, the University of Chicago-Chapin Hall Join Research Fund, the NIH/NHLBI Loan Repayment Program, 1 K23 HL118151 01, NIH NLBHI R03 (RFA-HL-18-025), the George and Carol Abramson Pilot Awards, the COPD Foundation Green Shoots Grant, the University of Chicago Women’s Board Grant, NIH NHLBI UG1 (RFA-HL-17-009), and the CTSA Pilot Award, outside the submitted work. These disclosures have been reported to Dr. Press’ institutional IRB board. Additionally, a management plan is on file that details how to address conflicts such as these which are sources of research support but do not directly support the work at hand. The remaining authors have no conflicts of interest relevant to the article to disclose.
Funding
This study was funded by internal grants from Ann and Robert H. Lurie Children’s Hospital of Chicago. Dr. Press was funded by a K23HL118151.
1. Expert Panel Report 3: guidelines for the diagnosis and management of asthma: full report. Washington, DC: US Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute; 2007. PubMed
2. Hekking PP, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896-902. doi: 10.1016/j.jaci.2014.08.042. PubMed
3. Peters SP, Ferguson G, Deniz Y, Reisner C. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med. 2006;100(7):1139-1151. doi: 10.1016/j.rmed.2006.03.031. PubMed
4. Dickens GR, Wermeling DP, Matheny CJ, et al. Pharmacokinetics of flunisolide administered via metered dose inhaler with and without a spacer device and following oral administration. Ann Allergy Asthma Immunol. 2000;84(5):528-532. doi: 10.1016/S1081-1206(10)62517-3. PubMed
5. Nikander K, Nicholls C, Denyer J, Pritchard J. The evolution of spacers and valved holding chambers. J Aerosol Med Pulm Drug Deliv. 2014;27(1):S4-S23. doi: 10.1089/jamp.2013.1076. PubMed
6. Rubin BK, Fink JB. The delivery of inhaled medication to the young child. Pediatr Clin North Am. 2003;50(3):717-731. doi:10.1016/S0031-3955(03)00049-X. PubMed
7. Roland NJ, Bhalla RK, Earis J. The local side effects of inhaled corticosteroids: current understanding and review of the literature. Chest. 2004;126(1):213-219. doi: 10.1378/chest.126.1.213. PubMed
8. Gillette C, Rockich-Winston N, Kuhn JA, Flesher S, Shepherd M. Inhaler technique in children with asthma: a systematic review. Acad Pediatr. 2016;16(7):605-615. doi: 10.1016/j.acap.2016.04.006. PubMed
9. Pappalardo AA, Karavolos K, Martin MA. What really happens in the home: the medication environment of urban, minority youth. J Allergy Clin Immunol Pract. 2017;5(3):764-770. doi: 10.1016/j.jaip.2016.09.046. PubMed
10. Crane J, Pearce N, Burgess C, Woodman K, Robson B, Beasley R. Markers of risk of asthma death or readmission in the 12 months following a hospital admission for asthma. Int J Epidemiol. 1992;21(4):737-744. doi: 10.1093/ije/21.4.737. PubMed
11. Turner MO, Noertjojo K, Vedal S, Bai T, Crump S, Fitzgerald JM. Risk factors for near-fatal asthma. A case-control study in hospitalized patients with asthma. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1804-1809. doi: 10.1164/ajrccm.157.6.9708092. PubMed
12. Press VG, Arora VM, Shah LM, et al. Misuse of respiratory inhalers in hospitalized patients with asthma or COPD. J Gen Intern Med. 2011;26(6):635-642. doi: 10.1007/s11606-010-1624-2. PubMed
13. Guevara JP, Wolf FM, Grum CM, Clark NM. Effects of educational interventions for self management of asthma in children and adolescents: systematic review and meta-analysis. BMJ. 2003;326(7402):1308-1309. doi: 10.1136/bmj.326.7402.1308. PubMed
14. Scarfone RJ, Capraro GA, Zorc JJ, Zhao H. Demonstrated use of metered-dose inhalers and peak flow meters by children and adolescents with acute asthma exacerbations. Arch Pediatr Adolesc Med. 2002;156(4):378-383. doi: 10.1001/archpedi.156.4.378. PubMed
15. Sockrider MM, Abramson S, Brooks E, et al. Delivering tailored asthma family education in a pediatric emergency department setting: a pilot study. Pediatrics. 2006;117(4 Pt 2):S135-144. doi: 10.1542/peds.2005-2000K. PubMed
1. Expert Panel Report 3: guidelines for the diagnosis and management of asthma: full report. Washington, DC: US Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute; 2007. PubMed
2. Hekking PP, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896-902. doi: 10.1016/j.jaci.2014.08.042. PubMed
3. Peters SP, Ferguson G, Deniz Y, Reisner C. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med. 2006;100(7):1139-1151. doi: 10.1016/j.rmed.2006.03.031. PubMed
4. Dickens GR, Wermeling DP, Matheny CJ, et al. Pharmacokinetics of flunisolide administered via metered dose inhaler with and without a spacer device and following oral administration. Ann Allergy Asthma Immunol. 2000;84(5):528-532. doi: 10.1016/S1081-1206(10)62517-3. PubMed
5. Nikander K, Nicholls C, Denyer J, Pritchard J. The evolution of spacers and valved holding chambers. J Aerosol Med Pulm Drug Deliv. 2014;27(1):S4-S23. doi: 10.1089/jamp.2013.1076. PubMed
6. Rubin BK, Fink JB. The delivery of inhaled medication to the young child. Pediatr Clin North Am. 2003;50(3):717-731. doi:10.1016/S0031-3955(03)00049-X. PubMed
7. Roland NJ, Bhalla RK, Earis J. The local side effects of inhaled corticosteroids: current understanding and review of the literature. Chest. 2004;126(1):213-219. doi: 10.1378/chest.126.1.213. PubMed
8. Gillette C, Rockich-Winston N, Kuhn JA, Flesher S, Shepherd M. Inhaler technique in children with asthma: a systematic review. Acad Pediatr. 2016;16(7):605-615. doi: 10.1016/j.acap.2016.04.006. PubMed
9. Pappalardo AA, Karavolos K, Martin MA. What really happens in the home: the medication environment of urban, minority youth. J Allergy Clin Immunol Pract. 2017;5(3):764-770. doi: 10.1016/j.jaip.2016.09.046. PubMed
10. Crane J, Pearce N, Burgess C, Woodman K, Robson B, Beasley R. Markers of risk of asthma death or readmission in the 12 months following a hospital admission for asthma. Int J Epidemiol. 1992;21(4):737-744. doi: 10.1093/ije/21.4.737. PubMed
11. Turner MO, Noertjojo K, Vedal S, Bai T, Crump S, Fitzgerald JM. Risk factors for near-fatal asthma. A case-control study in hospitalized patients with asthma. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1804-1809. doi: 10.1164/ajrccm.157.6.9708092. PubMed
12. Press VG, Arora VM, Shah LM, et al. Misuse of respiratory inhalers in hospitalized patients with asthma or COPD. J Gen Intern Med. 2011;26(6):635-642. doi: 10.1007/s11606-010-1624-2. PubMed
13. Guevara JP, Wolf FM, Grum CM, Clark NM. Effects of educational interventions for self management of asthma in children and adolescents: systematic review and meta-analysis. BMJ. 2003;326(7402):1308-1309. doi: 10.1136/bmj.326.7402.1308. PubMed
14. Scarfone RJ, Capraro GA, Zorc JJ, Zhao H. Demonstrated use of metered-dose inhalers and peak flow meters by children and adolescents with acute asthma exacerbations. Arch Pediatr Adolesc Med. 2002;156(4):378-383. doi: 10.1001/archpedi.156.4.378. PubMed
15. Sockrider MM, Abramson S, Brooks E, et al. Delivering tailored asthma family education in a pediatric emergency department setting: a pilot study. Pediatrics. 2006;117(4 Pt 2):S135-144. doi: 10.1542/peds.2005-2000K. PubMed
© 2019 Society of Hospital Medicine
Contemporary Rates of Preoperative Cardiac Testing Prior to Inpatient Hip Fracture Surgery
Hip fracture is a common reason for unexpected, urgent inpatient surgery in older patients. In 2005, the incidence of hip fracture was 369.0 and 793.5 per 100,000 in men and women respectively.1 These numbers declined over the preceding decade, potentially as a result of bisphosphonate use. Age- and risk-adjusted 30-day mortality rates for men and women in 2005 were approximately 10% and 5%, respectively.
Evidence suggests that timely surgical repair of hip fractures improves outcomes, although the optimal timing is controversial. Guidelines from the American College of Surgeons Committee on Trauma from 2015 recommend surgical intervention within 48 hours for geriatric hip fracures.2 A 2008 systematic review found that operative delay beyond 48 hours was associated with a 41% increase in 30-day all-cause mortality and a 32% increase in one-year all-cause mortality.3 Recent evidence suggests that the rate of complications begins to increase with delays beyond 24 hours.4
There has been a focus over the past decade on overuse of preoperative testing for low- and intermediate-risk surgeries.5-7 Beginning in 2012, the American Board of Internal Medicine initiated the Choosing Wisely® campaign in which numerous societies issued recommendations on reducing utilization of various diagnostic tests, a number of which have focused on preoperative tests. Two groups—the American Society of Anesthesiologists (ASA) and the American Society of Echocardiography (ASE)— issued specific recommendations on preoperative cardiac testing.8 In February 2013, the ASE recommended avoiding preoperative echocardiograms in patients without a history or symptoms of heart disease. In October 2013, the ASA recommended against transthoracic echocardiogram (TTE), transesophageal echocardiogram (TEE), or stress testing for low- or intermediate-risk noncardiac surgery for patients with stable cardiac disease.
Finally, in 2014, the American College of Cardiology (ACC)/American Heart Association (AHA) issued updated perioperative guidelines for patients undergoing noncardiac surgeries.9 They recommended preoperative stress testing only in a small subset of cases (patients with an elevated perioperative risk of major adverse cardiac event, a poor or unknown functional capacity, or those in whom stress testing would impact perioperative care).
Given the high cost of preoperative cardiac testing, the potential for delays in care that can adversely impact outcomes, and the recent recommendations, we sought to characterize the rates of inpatient preoperative cardiac testing prior to hip fracture surgery in recent years and to see whether recent recommendations to curb use of these tests were temporally associated with changing rates.
METHODS
Overview
We utilized two datasets—the Healthcare Cost and Utilization Project (HCUP) State Inpatient Databases (SID) and the American Hospital Association (AHA) Annual Survey—to characterize preoperative cardiac testing. SID data from Maryland, New Jersey, and Washington State from 2011 through September 2015 were used (the ICD coding system changed from ICD9 to ICD10 on October 1). This was combined with AHA data for these years. We included all hospitalizations with a primary ICD9 procedure code for hip fracture repair—78.55, 78.65, 79.05, 79.15, 79.25, 79.35, 79.45, 79.55, 79.65, 79.75, 79.85, and 79.95. We excluded all observations that involved an interhospital transfer. This study was exempt from institutional review board approval.
Measurement and Outcomes
We summarized demographic data for the hospitalizations that met the inclusion criteria as well as the associated hospitals. The primary outcome was the percentage of patients undergoing TTE, stress test, and cardiac catheterization during a hospitalization with a primary procedure code of hip fracture repair. Random effects logistic regression models for each type of diagnostic test were developed to determine the factors that might impact test utilization. In addition to running each test as a separate model, we also performed an analysis in which the outcome was performance of any of these three cardiac tests. Random effects were used to account for clustering of testing within hospitals. Variables included time (3-month intervals), state, age (continuous variable), gender, length of stay, payer (Medicare/Medicaid/private insurance/self-pay/other), hospital teaching status (major teaching/minor teaching/nonteaching), hospital size according to number of beds (continuous variable), and mortality score. Major teaching hospitals are defined as members of the Council of Teaching Hospitals. Minor teaching hospitals are defined as (1) those with one or more postgraduate training programs recognized by the American Council on Graduate Medical Education, (2) those with a medical school affiliation reported to the American Medical Association, or (3) those with an internship or residency approved by the American Osteopathic Association.
The SID has a specific binary indicator variable for each of the three diagnostic tests we evaluated. The use of the diagnostic test is evaluated through both UB-92 revenue codes and ICD9 procedure codes, with the presence of either leading to the indicator variable being positive.10 Finally, we performed a sensitivity analysis to evaluate the significance of changing utilization trends by interrupted time series analysis. A level of 0.05 was used to determine statistical significance. Analyses were done in STATA 15 (College Station, Texas).
RESULTS
The dataset included 75,144 hospitalizations with a primary procedure code of hip fracture over the study period (Table). The number of hospitalizations per year was fairly consistent over the study period in each state, although there were fewer hospitalizations for 2015 as this included only January through September. The mean age was 72.8 years, and 67% were female. The primary payer was Medicare for 71.7% of hospitalizations. Hospitalizations occurred at 181 hospitals, the plurality of which (42.9%) were minor teaching hospitals. The proportions of hospitalizations that included a TTE, stress test, and cardiac catheterization were 12.6%, 1.1%, and 0.5%, respectively. Overall, 13.5% of patients underwent any cardiac testing.
There was a statistically significantly lower rate of stress tests (odds ratio [OR], 0.32; 95% CI, 0.19-0.54) and cardiac catheterizations (OR, 0.46; 95% CI, 0.27-0.79) in Washington than in Maryland and New Jersey. Female gender was associated with significantly lower adjusted ORs for stress tests (OR, 0.74; 95% CI, 0.63-0.86) and cardiac catheterizations (OR, 0.73; 95% CI, 0.59-0.91), and increasing age was associated with higher adjusted ORs for each test (TTE, OR, 1.033; 95% CI, 1.031-1.035; stress tests, OR, 1.007; 95% CI, 1.001-1.013; cardiac catheterizations, OR, 1.011; 95% CI, 1.003-1.019). Private insurance was associated with a lower likelihood of stress tests (OR, 0.65; 95% CI, 0.50-0.85) and cardiac catheterizations (OR, 0.67; 95% CI,0.46-0.98), and self-pay was associated with a lower likelihood of TTE (OR, 0.76; 95% CI, 0.61-0.95) and stress test (OR, 0.43; 95% CI, 0.21-0.90), all compared with Medicare.
Larger hospitals were associated with a greater likelihood of cardiac catheterizations (OR, 1.18; 95% CI, 1.03-1.36) and a lower likelihood of TTE (OR, 0.89; 95% CI, 0.82-0.96). An unweighted average of these tests between 2011 and October 2015 showed a modest increase in TTEs and a modest decrease in stress tests and cardiac catheterizations (Figure). A multivariable random effects regression for use of TTEs revealed a significantly increasing trend from 2011 to 2014 (OR, 1.04, P < .0001), but the decreasing trend for 2015 was not statistically significant when analyzed according to quarters or months (for which data from only New Jersey and Washington are available).
In the combined model with any cardiac testing as the outcome, the likelihood of testing was lower in Washington (OR, 0.56; 95% CI, 0.31-0.995). Primary payer status of self-pay was associated with a lower likelihood of cardiac testing (OR, 0.73; 95% CI, 0.58-0.90). Female gender was associated with a lower likelihood of testing (OR, 0.93; 95% CI, 0.88-0.98), and high mortality score was associated with a higher likelihood of testing (OR, 1.030; 95% CI, 1.027-1.033). TTEs were the major driver of this model as these were the most heavily utilized test.
DISCUSSION
There has been limited research into how often preoperative cardiac testing occurs in the inpatient setting. Our aim was to study its prevalence prior to hip fracture surgery during a time period when multiple recommendations had been issued to limit its use. We found rates of ischemic testing (stress tests and cardiac catheterizations) to be appropriately, and perhaps surprisingly, low. Our results on ischemic testing rates are consistent with previous studies, which have focused on the outpatient setting where much of the preoperative workup for nonurgent surgeries occurs. The rate of TTEs was higher than in previous studies of the outpatient preoperative setting, although it is unclear what an optimal rate of TTEs is.
A recent study examining outpatient preoperative stress tests within the 30 days before cataract surgeries, knee arthroscopies, or shoulder arthroscopies found a rate of 2.1% for Medicare fee-for-service patients in 2009 with little regional variation.11 Another evaluation using 2009 Medicare claims data found rates of preoperative TTEs and stress tests to be 0.8% and 0.7%, respectively.12 They included TTEs and stress tests performed within 30 days of a low- or intermediate-risk surgery. A study analyzing the rate of preoperative TTEs between 2009 and 2014 found that rates varied from 2.0% to 3.4% for commercially insured patients aged 50-64 years and Medicare-advantage patients, respectively, in 2009.13 These rates decreased by 7.0% and 12.6% from 2009 to 2014. These studies, like ours, suggest that preoperative cardiac testing has not been a major source of wasteful spending. One explanation for the higher rate of TTEs we observed in the inpatient setting might be that primary care physicians in the outpatient setting are more likely to have historical cardiac testing results compared with physicians in a hospital.
We found that the rate of stress testing and cardiac catheterization in Washington was significantly lower than that in Maryland and New Jersey. This is consistent with a number of measures of healthcare utilization – total Medicare reimbursement in the last six months of life, mean number of hospital days in the last six months of life, and healthcare intensity index—for all of which Washington was below the national mean and Maryland and New Jersey were above it.14
Finally, we found evidence of a lower rate of preoperative stress tests and cardiac catheterizations for women despite controlling for age and mortality score. Of course, we did not control directly for cardiovascular comorbidities; as a result, there could be residual confounding. However, these results are consistent with previous findings of gender bias in both pharmacologic management of coronary artery disease (CAD)15 and diagnostic testing for suspected CAD.16
We focused on hospitalizations with a primary procedure code to surgically treat hip fracture. We are unable to tell if the cardiac testing of these patients had occurred before or after the procedure. However, we suspect that the vast majority were completed for preoperative evaluation. It is likely that a small subset were done to diagnose and manage cardiac complications that either accompanied the hip fracture or occurred postoperatively. Another limitation is that we cannot determine if a patient had one of these tests recently in the emergency department or as an outpatient.
We also chose to include only patients who actually had hip fracture surgery. It is possible that the testing rate is higher for all patients admitted for hip fracture and that some of these patients did not have surgery because of abnormal cardiac testing. However, we suspect that this is a very small fraction given the high degree of morbidity and mortality associated with untreated hip fracture.
CONCLUSION
We found a low rate of preoperative cardiac testing in patients hospitalized for hip fracture surgery both in the years before and after the issuance of recommendations intended to curb its use. Although it is reassuring that the volume of low-value testing is lower than we expected, these findings highlight the importance of targeting utilization improvement efforts toward low-value tests and procedures that are more heavily used, since further curbing the use of infrequently utilized tests and procedures will have only a modest impact on overall healthcare expenditure. Our findings highlight the necessity that professional organizations ensure that they focus on true areas of inappropriate utilization. These are the areas in which improvements will have a major impact on healthcare spending. Further research should aim to quantify unwarranted cardiac testing for other inpatient surgeries that are less urgent, as the urgency of hip fracture repair may be driving the relatively low utilization of inpatient cardiac testing.
Disclosures
The authors have nothing to disclose.
Funding
This project was supported by the Johns Hopkins Hospitalist Scholars Fund and the Johns Hopkins School of Medicine Biostatistics, Epidemiology and Data Management (BEAD) Core.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen A. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573-1579. PubMed
2. ACS TQIP - Best Practices in the Management of Orthopaedic Trauma. https://www.facs.org/~/media/files/quality programs/trauma/tqip/tqip bpgs in the management of orthopaedic traumafinal.ashx. Published 2015. Accessed July 13, 2018.
3. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis, and meta-regression. Can J Anesth. 2008;55(3):146-154. PubMed
4. Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017;318(20):1994. PubMed
5. Clair CM, Shah M, Diver EJ, et al. Adherence to evidence-based guidelines for preoperative testing in women undergoing gynecologic surgery. Obstet Gynecol. 2010;116(3):694-700. PubMed
6. Chen CL, Lin GA, Bardach NS, et al. Preoperative medical testing in Medicare patients undergoing cataract surgery. N Engl J Med. 2015;372(16):1530-1538. PubMed
7. Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012; 256(3):518-528. PubMed
8. Choosing Wisely - An Initiative of the ABIM Foundation. http://www.choosingwisely.org/clinician-lists. Accessed July 16, 2018.
9. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA Guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. JACC. 2014;64(22):e278 LP-e333. PubMed
10. HCUP Methods Series - Development of Utilization Flags for Use with UB-92 Administrative Data; Report # 2006-04. https://www.hcup-us.ahrq.gov/reports/methods/2006_4.pdf.
11. Kerr EA, Chen J, Sussman JB, Klamerus ML, Nallamothu BK. Stress testing before low-risk surgery - so many recommendations, so little overuse. JAMA Intern Med. 2015;175(4):645-647. PubMed
12. Schwartz AL, Landon BE, Elshaug AG, Chernew ME, McWilliams JM. Measuring low-value care in medicare. JAMA Intern Med. 2014;174(7):1067-1076. PubMed
13. Carter EA, Morin PE, Lind KD. Costs and trends in utilization of low-value services among older adults with commercial insurance or Medicare advantage. Med Care. 2017;55(11):931-939. PubMed
14. The Dartmouth Atlas of Health Care. http://www.dartmouthatlas.org. Accessed December 7, 2017.
15. Williams D, Bennett K, Feely J. Evidence for an age and gender bias in the secondary prevention of ischaemic heart disease in primary care. Br J Clin Pharmacol. 2003;55(6):604-608. PubMed
16. Chang AM, Mumma B, Sease KL, Robey JL, Shofer FS, Hollander JE. Gender bias in cardiovascular testing persists after adjustment for presenting characteristics and cardiac risk. Acad Emerg Med. 2007;14(7):599-605. PubMed
Hip fracture is a common reason for unexpected, urgent inpatient surgery in older patients. In 2005, the incidence of hip fracture was 369.0 and 793.5 per 100,000 in men and women respectively.1 These numbers declined over the preceding decade, potentially as a result of bisphosphonate use. Age- and risk-adjusted 30-day mortality rates for men and women in 2005 were approximately 10% and 5%, respectively.
Evidence suggests that timely surgical repair of hip fractures improves outcomes, although the optimal timing is controversial. Guidelines from the American College of Surgeons Committee on Trauma from 2015 recommend surgical intervention within 48 hours for geriatric hip fracures.2 A 2008 systematic review found that operative delay beyond 48 hours was associated with a 41% increase in 30-day all-cause mortality and a 32% increase in one-year all-cause mortality.3 Recent evidence suggests that the rate of complications begins to increase with delays beyond 24 hours.4
There has been a focus over the past decade on overuse of preoperative testing for low- and intermediate-risk surgeries.5-7 Beginning in 2012, the American Board of Internal Medicine initiated the Choosing Wisely® campaign in which numerous societies issued recommendations on reducing utilization of various diagnostic tests, a number of which have focused on preoperative tests. Two groups—the American Society of Anesthesiologists (ASA) and the American Society of Echocardiography (ASE)— issued specific recommendations on preoperative cardiac testing.8 In February 2013, the ASE recommended avoiding preoperative echocardiograms in patients without a history or symptoms of heart disease. In October 2013, the ASA recommended against transthoracic echocardiogram (TTE), transesophageal echocardiogram (TEE), or stress testing for low- or intermediate-risk noncardiac surgery for patients with stable cardiac disease.
Finally, in 2014, the American College of Cardiology (ACC)/American Heart Association (AHA) issued updated perioperative guidelines for patients undergoing noncardiac surgeries.9 They recommended preoperative stress testing only in a small subset of cases (patients with an elevated perioperative risk of major adverse cardiac event, a poor or unknown functional capacity, or those in whom stress testing would impact perioperative care).
Given the high cost of preoperative cardiac testing, the potential for delays in care that can adversely impact outcomes, and the recent recommendations, we sought to characterize the rates of inpatient preoperative cardiac testing prior to hip fracture surgery in recent years and to see whether recent recommendations to curb use of these tests were temporally associated with changing rates.
METHODS
Overview
We utilized two datasets—the Healthcare Cost and Utilization Project (HCUP) State Inpatient Databases (SID) and the American Hospital Association (AHA) Annual Survey—to characterize preoperative cardiac testing. SID data from Maryland, New Jersey, and Washington State from 2011 through September 2015 were used (the ICD coding system changed from ICD9 to ICD10 on October 1). This was combined with AHA data for these years. We included all hospitalizations with a primary ICD9 procedure code for hip fracture repair—78.55, 78.65, 79.05, 79.15, 79.25, 79.35, 79.45, 79.55, 79.65, 79.75, 79.85, and 79.95. We excluded all observations that involved an interhospital transfer. This study was exempt from institutional review board approval.
Measurement and Outcomes
We summarized demographic data for the hospitalizations that met the inclusion criteria as well as the associated hospitals. The primary outcome was the percentage of patients undergoing TTE, stress test, and cardiac catheterization during a hospitalization with a primary procedure code of hip fracture repair. Random effects logistic regression models for each type of diagnostic test were developed to determine the factors that might impact test utilization. In addition to running each test as a separate model, we also performed an analysis in which the outcome was performance of any of these three cardiac tests. Random effects were used to account for clustering of testing within hospitals. Variables included time (3-month intervals), state, age (continuous variable), gender, length of stay, payer (Medicare/Medicaid/private insurance/self-pay/other), hospital teaching status (major teaching/minor teaching/nonteaching), hospital size according to number of beds (continuous variable), and mortality score. Major teaching hospitals are defined as members of the Council of Teaching Hospitals. Minor teaching hospitals are defined as (1) those with one or more postgraduate training programs recognized by the American Council on Graduate Medical Education, (2) those with a medical school affiliation reported to the American Medical Association, or (3) those with an internship or residency approved by the American Osteopathic Association.
The SID has a specific binary indicator variable for each of the three diagnostic tests we evaluated. The use of the diagnostic test is evaluated through both UB-92 revenue codes and ICD9 procedure codes, with the presence of either leading to the indicator variable being positive.10 Finally, we performed a sensitivity analysis to evaluate the significance of changing utilization trends by interrupted time series analysis. A level of 0.05 was used to determine statistical significance. Analyses were done in STATA 15 (College Station, Texas).
RESULTS
The dataset included 75,144 hospitalizations with a primary procedure code of hip fracture over the study period (Table). The number of hospitalizations per year was fairly consistent over the study period in each state, although there were fewer hospitalizations for 2015 as this included only January through September. The mean age was 72.8 years, and 67% were female. The primary payer was Medicare for 71.7% of hospitalizations. Hospitalizations occurred at 181 hospitals, the plurality of which (42.9%) were minor teaching hospitals. The proportions of hospitalizations that included a TTE, stress test, and cardiac catheterization were 12.6%, 1.1%, and 0.5%, respectively. Overall, 13.5% of patients underwent any cardiac testing.
There was a statistically significantly lower rate of stress tests (odds ratio [OR], 0.32; 95% CI, 0.19-0.54) and cardiac catheterizations (OR, 0.46; 95% CI, 0.27-0.79) in Washington than in Maryland and New Jersey. Female gender was associated with significantly lower adjusted ORs for stress tests (OR, 0.74; 95% CI, 0.63-0.86) and cardiac catheterizations (OR, 0.73; 95% CI, 0.59-0.91), and increasing age was associated with higher adjusted ORs for each test (TTE, OR, 1.033; 95% CI, 1.031-1.035; stress tests, OR, 1.007; 95% CI, 1.001-1.013; cardiac catheterizations, OR, 1.011; 95% CI, 1.003-1.019). Private insurance was associated with a lower likelihood of stress tests (OR, 0.65; 95% CI, 0.50-0.85) and cardiac catheterizations (OR, 0.67; 95% CI,0.46-0.98), and self-pay was associated with a lower likelihood of TTE (OR, 0.76; 95% CI, 0.61-0.95) and stress test (OR, 0.43; 95% CI, 0.21-0.90), all compared with Medicare.
Larger hospitals were associated with a greater likelihood of cardiac catheterizations (OR, 1.18; 95% CI, 1.03-1.36) and a lower likelihood of TTE (OR, 0.89; 95% CI, 0.82-0.96). An unweighted average of these tests between 2011 and October 2015 showed a modest increase in TTEs and a modest decrease in stress tests and cardiac catheterizations (Figure). A multivariable random effects regression for use of TTEs revealed a significantly increasing trend from 2011 to 2014 (OR, 1.04, P < .0001), but the decreasing trend for 2015 was not statistically significant when analyzed according to quarters or months (for which data from only New Jersey and Washington are available).
In the combined model with any cardiac testing as the outcome, the likelihood of testing was lower in Washington (OR, 0.56; 95% CI, 0.31-0.995). Primary payer status of self-pay was associated with a lower likelihood of cardiac testing (OR, 0.73; 95% CI, 0.58-0.90). Female gender was associated with a lower likelihood of testing (OR, 0.93; 95% CI, 0.88-0.98), and high mortality score was associated with a higher likelihood of testing (OR, 1.030; 95% CI, 1.027-1.033). TTEs were the major driver of this model as these were the most heavily utilized test.
DISCUSSION
There has been limited research into how often preoperative cardiac testing occurs in the inpatient setting. Our aim was to study its prevalence prior to hip fracture surgery during a time period when multiple recommendations had been issued to limit its use. We found rates of ischemic testing (stress tests and cardiac catheterizations) to be appropriately, and perhaps surprisingly, low. Our results on ischemic testing rates are consistent with previous studies, which have focused on the outpatient setting where much of the preoperative workup for nonurgent surgeries occurs. The rate of TTEs was higher than in previous studies of the outpatient preoperative setting, although it is unclear what an optimal rate of TTEs is.
A recent study examining outpatient preoperative stress tests within the 30 days before cataract surgeries, knee arthroscopies, or shoulder arthroscopies found a rate of 2.1% for Medicare fee-for-service patients in 2009 with little regional variation.11 Another evaluation using 2009 Medicare claims data found rates of preoperative TTEs and stress tests to be 0.8% and 0.7%, respectively.12 They included TTEs and stress tests performed within 30 days of a low- or intermediate-risk surgery. A study analyzing the rate of preoperative TTEs between 2009 and 2014 found that rates varied from 2.0% to 3.4% for commercially insured patients aged 50-64 years and Medicare-advantage patients, respectively, in 2009.13 These rates decreased by 7.0% and 12.6% from 2009 to 2014. These studies, like ours, suggest that preoperative cardiac testing has not been a major source of wasteful spending. One explanation for the higher rate of TTEs we observed in the inpatient setting might be that primary care physicians in the outpatient setting are more likely to have historical cardiac testing results compared with physicians in a hospital.
We found that the rate of stress testing and cardiac catheterization in Washington was significantly lower than that in Maryland and New Jersey. This is consistent with a number of measures of healthcare utilization – total Medicare reimbursement in the last six months of life, mean number of hospital days in the last six months of life, and healthcare intensity index—for all of which Washington was below the national mean and Maryland and New Jersey were above it.14
Finally, we found evidence of a lower rate of preoperative stress tests and cardiac catheterizations for women despite controlling for age and mortality score. Of course, we did not control directly for cardiovascular comorbidities; as a result, there could be residual confounding. However, these results are consistent with previous findings of gender bias in both pharmacologic management of coronary artery disease (CAD)15 and diagnostic testing for suspected CAD.16
We focused on hospitalizations with a primary procedure code to surgically treat hip fracture. We are unable to tell if the cardiac testing of these patients had occurred before or after the procedure. However, we suspect that the vast majority were completed for preoperative evaluation. It is likely that a small subset were done to diagnose and manage cardiac complications that either accompanied the hip fracture or occurred postoperatively. Another limitation is that we cannot determine if a patient had one of these tests recently in the emergency department or as an outpatient.
We also chose to include only patients who actually had hip fracture surgery. It is possible that the testing rate is higher for all patients admitted for hip fracture and that some of these patients did not have surgery because of abnormal cardiac testing. However, we suspect that this is a very small fraction given the high degree of morbidity and mortality associated with untreated hip fracture.
CONCLUSION
We found a low rate of preoperative cardiac testing in patients hospitalized for hip fracture surgery both in the years before and after the issuance of recommendations intended to curb its use. Although it is reassuring that the volume of low-value testing is lower than we expected, these findings highlight the importance of targeting utilization improvement efforts toward low-value tests and procedures that are more heavily used, since further curbing the use of infrequently utilized tests and procedures will have only a modest impact on overall healthcare expenditure. Our findings highlight the necessity that professional organizations ensure that they focus on true areas of inappropriate utilization. These are the areas in which improvements will have a major impact on healthcare spending. Further research should aim to quantify unwarranted cardiac testing for other inpatient surgeries that are less urgent, as the urgency of hip fracture repair may be driving the relatively low utilization of inpatient cardiac testing.
Disclosures
The authors have nothing to disclose.
Funding
This project was supported by the Johns Hopkins Hospitalist Scholars Fund and the Johns Hopkins School of Medicine Biostatistics, Epidemiology and Data Management (BEAD) Core.
Hip fracture is a common reason for unexpected, urgent inpatient surgery in older patients. In 2005, the incidence of hip fracture was 369.0 and 793.5 per 100,000 in men and women respectively.1 These numbers declined over the preceding decade, potentially as a result of bisphosphonate use. Age- and risk-adjusted 30-day mortality rates for men and women in 2005 were approximately 10% and 5%, respectively.
Evidence suggests that timely surgical repair of hip fractures improves outcomes, although the optimal timing is controversial. Guidelines from the American College of Surgeons Committee on Trauma from 2015 recommend surgical intervention within 48 hours for geriatric hip fracures.2 A 2008 systematic review found that operative delay beyond 48 hours was associated with a 41% increase in 30-day all-cause mortality and a 32% increase in one-year all-cause mortality.3 Recent evidence suggests that the rate of complications begins to increase with delays beyond 24 hours.4
There has been a focus over the past decade on overuse of preoperative testing for low- and intermediate-risk surgeries.5-7 Beginning in 2012, the American Board of Internal Medicine initiated the Choosing Wisely® campaign in which numerous societies issued recommendations on reducing utilization of various diagnostic tests, a number of which have focused on preoperative tests. Two groups—the American Society of Anesthesiologists (ASA) and the American Society of Echocardiography (ASE)— issued specific recommendations on preoperative cardiac testing.8 In February 2013, the ASE recommended avoiding preoperative echocardiograms in patients without a history or symptoms of heart disease. In October 2013, the ASA recommended against transthoracic echocardiogram (TTE), transesophageal echocardiogram (TEE), or stress testing for low- or intermediate-risk noncardiac surgery for patients with stable cardiac disease.
Finally, in 2014, the American College of Cardiology (ACC)/American Heart Association (AHA) issued updated perioperative guidelines for patients undergoing noncardiac surgeries.9 They recommended preoperative stress testing only in a small subset of cases (patients with an elevated perioperative risk of major adverse cardiac event, a poor or unknown functional capacity, or those in whom stress testing would impact perioperative care).
Given the high cost of preoperative cardiac testing, the potential for delays in care that can adversely impact outcomes, and the recent recommendations, we sought to characterize the rates of inpatient preoperative cardiac testing prior to hip fracture surgery in recent years and to see whether recent recommendations to curb use of these tests were temporally associated with changing rates.
METHODS
Overview
We utilized two datasets—the Healthcare Cost and Utilization Project (HCUP) State Inpatient Databases (SID) and the American Hospital Association (AHA) Annual Survey—to characterize preoperative cardiac testing. SID data from Maryland, New Jersey, and Washington State from 2011 through September 2015 were used (the ICD coding system changed from ICD9 to ICD10 on October 1). This was combined with AHA data for these years. We included all hospitalizations with a primary ICD9 procedure code for hip fracture repair—78.55, 78.65, 79.05, 79.15, 79.25, 79.35, 79.45, 79.55, 79.65, 79.75, 79.85, and 79.95. We excluded all observations that involved an interhospital transfer. This study was exempt from institutional review board approval.
Measurement and Outcomes
We summarized demographic data for the hospitalizations that met the inclusion criteria as well as the associated hospitals. The primary outcome was the percentage of patients undergoing TTE, stress test, and cardiac catheterization during a hospitalization with a primary procedure code of hip fracture repair. Random effects logistic regression models for each type of diagnostic test were developed to determine the factors that might impact test utilization. In addition to running each test as a separate model, we also performed an analysis in which the outcome was performance of any of these three cardiac tests. Random effects were used to account for clustering of testing within hospitals. Variables included time (3-month intervals), state, age (continuous variable), gender, length of stay, payer (Medicare/Medicaid/private insurance/self-pay/other), hospital teaching status (major teaching/minor teaching/nonteaching), hospital size according to number of beds (continuous variable), and mortality score. Major teaching hospitals are defined as members of the Council of Teaching Hospitals. Minor teaching hospitals are defined as (1) those with one or more postgraduate training programs recognized by the American Council on Graduate Medical Education, (2) those with a medical school affiliation reported to the American Medical Association, or (3) those with an internship or residency approved by the American Osteopathic Association.
The SID has a specific binary indicator variable for each of the three diagnostic tests we evaluated. The use of the diagnostic test is evaluated through both UB-92 revenue codes and ICD9 procedure codes, with the presence of either leading to the indicator variable being positive.10 Finally, we performed a sensitivity analysis to evaluate the significance of changing utilization trends by interrupted time series analysis. A level of 0.05 was used to determine statistical significance. Analyses were done in STATA 15 (College Station, Texas).
RESULTS
The dataset included 75,144 hospitalizations with a primary procedure code of hip fracture over the study period (Table). The number of hospitalizations per year was fairly consistent over the study period in each state, although there were fewer hospitalizations for 2015 as this included only January through September. The mean age was 72.8 years, and 67% were female. The primary payer was Medicare for 71.7% of hospitalizations. Hospitalizations occurred at 181 hospitals, the plurality of which (42.9%) were minor teaching hospitals. The proportions of hospitalizations that included a TTE, stress test, and cardiac catheterization were 12.6%, 1.1%, and 0.5%, respectively. Overall, 13.5% of patients underwent any cardiac testing.
There was a statistically significantly lower rate of stress tests (odds ratio [OR], 0.32; 95% CI, 0.19-0.54) and cardiac catheterizations (OR, 0.46; 95% CI, 0.27-0.79) in Washington than in Maryland and New Jersey. Female gender was associated with significantly lower adjusted ORs for stress tests (OR, 0.74; 95% CI, 0.63-0.86) and cardiac catheterizations (OR, 0.73; 95% CI, 0.59-0.91), and increasing age was associated with higher adjusted ORs for each test (TTE, OR, 1.033; 95% CI, 1.031-1.035; stress tests, OR, 1.007; 95% CI, 1.001-1.013; cardiac catheterizations, OR, 1.011; 95% CI, 1.003-1.019). Private insurance was associated with a lower likelihood of stress tests (OR, 0.65; 95% CI, 0.50-0.85) and cardiac catheterizations (OR, 0.67; 95% CI,0.46-0.98), and self-pay was associated with a lower likelihood of TTE (OR, 0.76; 95% CI, 0.61-0.95) and stress test (OR, 0.43; 95% CI, 0.21-0.90), all compared with Medicare.
Larger hospitals were associated with a greater likelihood of cardiac catheterizations (OR, 1.18; 95% CI, 1.03-1.36) and a lower likelihood of TTE (OR, 0.89; 95% CI, 0.82-0.96). An unweighted average of these tests between 2011 and October 2015 showed a modest increase in TTEs and a modest decrease in stress tests and cardiac catheterizations (Figure). A multivariable random effects regression for use of TTEs revealed a significantly increasing trend from 2011 to 2014 (OR, 1.04, P < .0001), but the decreasing trend for 2015 was not statistically significant when analyzed according to quarters or months (for which data from only New Jersey and Washington are available).
In the combined model with any cardiac testing as the outcome, the likelihood of testing was lower in Washington (OR, 0.56; 95% CI, 0.31-0.995). Primary payer status of self-pay was associated with a lower likelihood of cardiac testing (OR, 0.73; 95% CI, 0.58-0.90). Female gender was associated with a lower likelihood of testing (OR, 0.93; 95% CI, 0.88-0.98), and high mortality score was associated with a higher likelihood of testing (OR, 1.030; 95% CI, 1.027-1.033). TTEs were the major driver of this model as these were the most heavily utilized test.
DISCUSSION
There has been limited research into how often preoperative cardiac testing occurs in the inpatient setting. Our aim was to study its prevalence prior to hip fracture surgery during a time period when multiple recommendations had been issued to limit its use. We found rates of ischemic testing (stress tests and cardiac catheterizations) to be appropriately, and perhaps surprisingly, low. Our results on ischemic testing rates are consistent with previous studies, which have focused on the outpatient setting where much of the preoperative workup for nonurgent surgeries occurs. The rate of TTEs was higher than in previous studies of the outpatient preoperative setting, although it is unclear what an optimal rate of TTEs is.
A recent study examining outpatient preoperative stress tests within the 30 days before cataract surgeries, knee arthroscopies, or shoulder arthroscopies found a rate of 2.1% for Medicare fee-for-service patients in 2009 with little regional variation.11 Another evaluation using 2009 Medicare claims data found rates of preoperative TTEs and stress tests to be 0.8% and 0.7%, respectively.12 They included TTEs and stress tests performed within 30 days of a low- or intermediate-risk surgery. A study analyzing the rate of preoperative TTEs between 2009 and 2014 found that rates varied from 2.0% to 3.4% for commercially insured patients aged 50-64 years and Medicare-advantage patients, respectively, in 2009.13 These rates decreased by 7.0% and 12.6% from 2009 to 2014. These studies, like ours, suggest that preoperative cardiac testing has not been a major source of wasteful spending. One explanation for the higher rate of TTEs we observed in the inpatient setting might be that primary care physicians in the outpatient setting are more likely to have historical cardiac testing results compared with physicians in a hospital.
We found that the rate of stress testing and cardiac catheterization in Washington was significantly lower than that in Maryland and New Jersey. This is consistent with a number of measures of healthcare utilization – total Medicare reimbursement in the last six months of life, mean number of hospital days in the last six months of life, and healthcare intensity index—for all of which Washington was below the national mean and Maryland and New Jersey were above it.14
Finally, we found evidence of a lower rate of preoperative stress tests and cardiac catheterizations for women despite controlling for age and mortality score. Of course, we did not control directly for cardiovascular comorbidities; as a result, there could be residual confounding. However, these results are consistent with previous findings of gender bias in both pharmacologic management of coronary artery disease (CAD)15 and diagnostic testing for suspected CAD.16
We focused on hospitalizations with a primary procedure code to surgically treat hip fracture. We are unable to tell if the cardiac testing of these patients had occurred before or after the procedure. However, we suspect that the vast majority were completed for preoperative evaluation. It is likely that a small subset were done to diagnose and manage cardiac complications that either accompanied the hip fracture or occurred postoperatively. Another limitation is that we cannot determine if a patient had one of these tests recently in the emergency department or as an outpatient.
We also chose to include only patients who actually had hip fracture surgery. It is possible that the testing rate is higher for all patients admitted for hip fracture and that some of these patients did not have surgery because of abnormal cardiac testing. However, we suspect that this is a very small fraction given the high degree of morbidity and mortality associated with untreated hip fracture.
CONCLUSION
We found a low rate of preoperative cardiac testing in patients hospitalized for hip fracture surgery both in the years before and after the issuance of recommendations intended to curb its use. Although it is reassuring that the volume of low-value testing is lower than we expected, these findings highlight the importance of targeting utilization improvement efforts toward low-value tests and procedures that are more heavily used, since further curbing the use of infrequently utilized tests and procedures will have only a modest impact on overall healthcare expenditure. Our findings highlight the necessity that professional organizations ensure that they focus on true areas of inappropriate utilization. These are the areas in which improvements will have a major impact on healthcare spending. Further research should aim to quantify unwarranted cardiac testing for other inpatient surgeries that are less urgent, as the urgency of hip fracture repair may be driving the relatively low utilization of inpatient cardiac testing.
Disclosures
The authors have nothing to disclose.
Funding
This project was supported by the Johns Hopkins Hospitalist Scholars Fund and the Johns Hopkins School of Medicine Biostatistics, Epidemiology and Data Management (BEAD) Core.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen A. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573-1579. PubMed
2. ACS TQIP - Best Practices in the Management of Orthopaedic Trauma. https://www.facs.org/~/media/files/quality programs/trauma/tqip/tqip bpgs in the management of orthopaedic traumafinal.ashx. Published 2015. Accessed July 13, 2018.
3. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis, and meta-regression. Can J Anesth. 2008;55(3):146-154. PubMed
4. Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017;318(20):1994. PubMed
5. Clair CM, Shah M, Diver EJ, et al. Adherence to evidence-based guidelines for preoperative testing in women undergoing gynecologic surgery. Obstet Gynecol. 2010;116(3):694-700. PubMed
6. Chen CL, Lin GA, Bardach NS, et al. Preoperative medical testing in Medicare patients undergoing cataract surgery. N Engl J Med. 2015;372(16):1530-1538. PubMed
7. Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012; 256(3):518-528. PubMed
8. Choosing Wisely - An Initiative of the ABIM Foundation. http://www.choosingwisely.org/clinician-lists. Accessed July 16, 2018.
9. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA Guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. JACC. 2014;64(22):e278 LP-e333. PubMed
10. HCUP Methods Series - Development of Utilization Flags for Use with UB-92 Administrative Data; Report # 2006-04. https://www.hcup-us.ahrq.gov/reports/methods/2006_4.pdf.
11. Kerr EA, Chen J, Sussman JB, Klamerus ML, Nallamothu BK. Stress testing before low-risk surgery - so many recommendations, so little overuse. JAMA Intern Med. 2015;175(4):645-647. PubMed
12. Schwartz AL, Landon BE, Elshaug AG, Chernew ME, McWilliams JM. Measuring low-value care in medicare. JAMA Intern Med. 2014;174(7):1067-1076. PubMed
13. Carter EA, Morin PE, Lind KD. Costs and trends in utilization of low-value services among older adults with commercial insurance or Medicare advantage. Med Care. 2017;55(11):931-939. PubMed
14. The Dartmouth Atlas of Health Care. http://www.dartmouthatlas.org. Accessed December 7, 2017.
15. Williams D, Bennett K, Feely J. Evidence for an age and gender bias in the secondary prevention of ischaemic heart disease in primary care. Br J Clin Pharmacol. 2003;55(6):604-608. PubMed
16. Chang AM, Mumma B, Sease KL, Robey JL, Shofer FS, Hollander JE. Gender bias in cardiovascular testing persists after adjustment for presenting characteristics and cardiac risk. Acad Emerg Med. 2007;14(7):599-605. PubMed
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen A. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573-1579. PubMed
2. ACS TQIP - Best Practices in the Management of Orthopaedic Trauma. https://www.facs.org/~/media/files/quality programs/trauma/tqip/tqip bpgs in the management of orthopaedic traumafinal.ashx. Published 2015. Accessed July 13, 2018.
3. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis, and meta-regression. Can J Anesth. 2008;55(3):146-154. PubMed
4. Pincus D, Ravi B, Wasserstein D, et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017;318(20):1994. PubMed
5. Clair CM, Shah M, Diver EJ, et al. Adherence to evidence-based guidelines for preoperative testing in women undergoing gynecologic surgery. Obstet Gynecol. 2010;116(3):694-700. PubMed
6. Chen CL, Lin GA, Bardach NS, et al. Preoperative medical testing in Medicare patients undergoing cataract surgery. N Engl J Med. 2015;372(16):1530-1538. PubMed
7. Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012; 256(3):518-528. PubMed
8. Choosing Wisely - An Initiative of the ABIM Foundation. http://www.choosingwisely.org/clinician-lists. Accessed July 16, 2018.
9. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA Guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. JACC. 2014;64(22):e278 LP-e333. PubMed
10. HCUP Methods Series - Development of Utilization Flags for Use with UB-92 Administrative Data; Report # 2006-04. https://www.hcup-us.ahrq.gov/reports/methods/2006_4.pdf.
11. Kerr EA, Chen J, Sussman JB, Klamerus ML, Nallamothu BK. Stress testing before low-risk surgery - so many recommendations, so little overuse. JAMA Intern Med. 2015;175(4):645-647. PubMed
12. Schwartz AL, Landon BE, Elshaug AG, Chernew ME, McWilliams JM. Measuring low-value care in medicare. JAMA Intern Med. 2014;174(7):1067-1076. PubMed
13. Carter EA, Morin PE, Lind KD. Costs and trends in utilization of low-value services among older adults with commercial insurance or Medicare advantage. Med Care. 2017;55(11):931-939. PubMed
14. The Dartmouth Atlas of Health Care. http://www.dartmouthatlas.org. Accessed December 7, 2017.
15. Williams D, Bennett K, Feely J. Evidence for an age and gender bias in the secondary prevention of ischaemic heart disease in primary care. Br J Clin Pharmacol. 2003;55(6):604-608. PubMed
16. Chang AM, Mumma B, Sease KL, Robey JL, Shofer FS, Hollander JE. Gender bias in cardiovascular testing persists after adjustment for presenting characteristics and cardiac risk. Acad Emerg Med. 2007;14(7):599-605. PubMed
© 2019 Society of Hospital Medicine
Use of Advance Care Planning Billing Codes for Hospitalized Older Adults at High Risk of Dying: A National Observational Study
Advance care planning (ACP) is the process wherein patients, in discussions with their healthcare providers, family members, and other loved ones, make individual decisions about their future healthcare or prepare proxies to guide future medical treatment decisions.1,2 In 2016, the Centers for Medicare and Medicaid Services (CMS) began paying providers for ACP by using billing codes 99497 (first 30 min of ACP) and 99498 (additional 30 min of ACP). According to the CMS, during the first year after the billing codes were introduced, 22,864 providers billed for ACP conversations with 574,621 patients.3 While all adults are eligible, common triggers for ACP include advanced age, serious illness, and functional status changes that confer an increased risk of dying. We explored the early uptake of the ACP billing code in a large national physician practice that provided mandatory education in use of the ACP billing code, offered a small financial incentive for ACP documentation, and primed physicians to reflect on the patient’s risk of dying in the next year at the time of hospital admission.
METHODS
We analyzed ACP billing for hospitalized adults aged 65 years or above and who were managed by a large national physician practice that employs acute care providers in hospital medicine, emergency medicine and critical care between January 1, 2017 and March 31, 2017. This practice employs approximately 2,500 hospital-based physicians in 250 community hospitals in 38 states. They collect data through handheld and desktop information technology (IT) tools to facilitate coding, billing, and compliance by hospitalists. Hospitalists receive mandatory web-based training in compliance with CMS ACP billing and templated ACP documentation. Additionally, they receive web-based training in serious illness communication skills during the first two years of employment. The training includes didactic content regarding steps for collaborative decision making, words to use during the encounter, and videos of simulated patient encounters demonstrating best practices. Hospitalists also receive a small financial incentive ($20) for each properly documented ACP conversation that meets CMS criteria for ACP code payment.
Beginning in 2017, hospitalists were required to answer the validated Surprise Question4 (SQ; “Would you be surprised if the patient died in the next year?”) for all admitted patients aged 65 years and older. The SQ is useful because it is intuitive and not burdensome for physicians to answer. Moreover, it is predictive of mortality. The pooled prognostic characteristics of the SQ across multiple populations for predicting the outcome of death at 6 months to 18 months include a sensitivity of 67.0% (95% confidence interval [CI] 55.7%-76.7%), a specificity of 80.2% (95% CI 73.3%-85.6%), a positive likelihood ratio of 3.4 (95% CI 2.8–4.1), a negative likelihood ratio of 0.41 (95% CI 0.32-0.54), a positive predictive value of 37.1% (95% CI 30.2%-44.6%), and a negative predictive value of 93.1% (95% CI 91.0%-94.8%).5 The SQ primed the admitting physician and triggered an “EoL” (end-of-life) icon next to the patient’s name on the hospitalists’ handheld electronic patient census.
We summarized ACP billing rates and used mixed-effects regression to estimate adjusted ACP rates accounting for patient covariates and clustering at the provider and hospital level. Patient covariates included age; answer to the SQ [“yes,” “no,” or “missing”]); and the presence or absence of seven comorbidities: dementia, heart failure, chronic obstructive pulmonary disease, renal failure, liver failure, metastatic cancer, and nonmetastatic cancer. We quantified the magnitude of provider and hospital variation in ACP rates by using the intraclass correlation coefficient (ICC).
RESULTS
In the first quarter of 2017, hospitalists admitted 113,612 patients aged 65 years and older. Hospitalists were prompted to answer the SQ for 73,731 (65%) of the patients. They were not prompted to answer the SQ for 39,881 (35%) of the patients (ie, missing data for the SQ). Reasons for not prompting include delayed implementation at a site and the patient not being admitted to the hospital (eg, managed on observation status). When prompted, hospitalists answered “no” to the SQ for 41,276/73,731 (56%) of admissions.
Only 6,146/113,612 (5.4%) of all admissions involved a billed ACP conversation. Rates were highest among SQ-prompted/answer “no” cases (8.3%) compared with SQ-prompted/answer “yes” cases (4.1%) and non-SQ-prompted cases (3.5%), with all pairwise differences being statistically significant (P values “yes” vs “no” = .0079, “yes” vs not prompted = .0043, “no” vs not prompted < .0001; see Table 1).
In addition to being more likely to have a “no” response to the SQ, those with a billed ACP conversations were older (80 vs 78, P < .001); more likely to be diagnosed with dementia (5.9% vs 3.5%, P < .001), congestive heart failure (12.3% vs 9.9%, P < .001), and cancer (6.1% vs 3.3%, P < .001); more likely to die during the admission (16.5% vs 10.9%, P < .001); and, conditional on survival to discharge, more likely to be discharged with hospice (17% vs 3%, P < .001) than those without (Table 2).
At the hospital level, ACP rates varied from 0% to 35% (mean 5.2%) of all admissions. In analyses restricted to physicians seeing at least 30 patients 65 years of age and older during the quarter, physician-level ACP rates varied from 0% to 93% (mean 5.4%). The majority of all ACP discussions were attributable to one-quarter of physicians. One-third of physicians never billed for ACP.
In a hierarchical logistic regression model accounting for observable patient characteristics and clustering at the physician and hospital level, the adjusted ACP rate for an “average” patient (age 77.85 with the most common clinical conditions) was 13.6% if the hospitalist answered “no” to the SQ, 9.6% if the hospitalist answered “yes,” and 10.1% if the hospitalist was not asked the SQ (P value of difference < .0001). From this model, we also calculated an ICC at the physician level of 0.044 and at the hospital level of 0.079. The physician level ICC corresponds to a 4.5% absolute increase in ACP when one moves from a physician at the mean to a physician 1 SD above the mean (ie, moving 1 SD up the scale of the latent variable underlying the random effect). The hospital level ICC corresponds to a 6.3% absolute increase in ACP when one moves from a hospital at the mean to a hospital 1 SD above the mean. The 4.5% absolute increase in ACP due to physician practice patterns and 6.3% absolute increase in ACP due to hospital practice patterns are both greater than the estimated increase in ACP from the hospitalist answering “no” instead of “yes” to the SQ (3.6%).
DISCUSSION
In this large national hospital-based physician practice group, the rates of ACP among acute care patients 65 years of age and older were very low despite the use of education and IT- and incentive-based strategies to encourage ACP conversations among seriously ill older adults. Priming physicians to reflect on the patient’s risk of dying at the time of admission was associated with the doubling of ACP rates.
Despite some lawmakers’ concerns that the ACP billing code may be overused and therefore become a financial burden to the Medicare program6, we find the very low use of ACP billing in a population for whom having goals of care conversations is critical—seriously ill older adults who the physician would not be surprised if they died in the next year. This gap is significant because these ACP conversations, when they did occur, were associated with a comfort-focused trajectory, including a more than four-fold increase in hospice referral at discharge.
Causal inference is limited because of the observational nature of the study. While we hypothesize that priming the physicians to reflect on prognosis activated them to prioritize ACP, based on a prior scenario-based randomized trial,7 illness severity likely drives ACP conversations. Specifically, patients on observation status (who had missing SQ data) and those for whom the physician answered “yes” to the SQ are less sick than other patients. Additional decision-making heuristics in addition to mortality risk may influence ACP conversations, as suggested by the independent influence of diagnoses, such as dementia or cancer, on ACP. Notably, however, the large amounts of unexplained variation at the physician and the hospital levels exceed the amounts explained by any individual observed patient factor.
Other key limitations of this study include the use of ACP billing as a primary outcome rather than observed and documented ACP conversations and the lack of information on the quality of ACP conversations. These findings reflect the uptake of ACP billing rates soon after the code was introduced. ACP billing rates have likely increased since the first quarter of 2017. Future work should explore diffusion and variation in physician-specific use over time. Finally, despite the nationwide sample, findings may not be generalizable to hospitalists who have not received training and financial incentives for ACP billing.
This study reinforces the possibility that variation in ACP conversations may contribute to variation in end-of-life treatment intensity between providers.8-10 Low ACP rates among even those with high hospitalist-predicted mortality risk and considerable between-provider variation underscore the need for quality improvement interventions to increase hospital-based ACP.
Acknowledgments
The authors thank Jared Wasserman, Maxwell Bessler, Devon Zoller MD, Mark Rudolph MD, Kristi Franz, and Weiping Zhou for their research assistance.
Disclosures
The authors have nothing to disclose.
Funding
National Institute on Aging award P01 AG019783
1. Mullick A, Martin J, Sallnow L. An introduction to advance care planning in practice. BMJ. 2013;347:f6064. PubMed
2. Sudore RL, Lum HD, You JJ, et al. Defining advance care planning for adults: a consensus definition from a multidisciplinary Delphi panel. J Pain Symptom Manage. 2017;53(5):821-832. PubMed
3. Medicare spending and utilization for advance care planning (ACP) services in 2016. Analysis of CMS data posted by the Coalition to Transform Advanced Care https://www.thectac.org/2017/08/use-billing-codes-advance-care-planning-exceeds-projections/. Accessed February 2018.
4. Moss AH, Ganjoo J, Sharma S, et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3(5):1379-1384. PubMed
5. Downar J, Goldman R, Pinto R, Englesakis M, Adhikari NK. The “surprise question” for predicting death in seriously ill patients: a systematic review and meta-analysis. CMAJ. 2017;189(13):E484-E493. PubMed
6. Aleccia J. Docs bill Medicare for end-of-life advice as ‘death panel’ fears reemerge. Kaiser Health News, February 2017.
7. Turnbull AE, Krall JR, Ruhl AP, et al. A scenario-based, randomized trial of patient values and functional prognosis on intensivist intent to discuss withdrawing life support. Crit Care Med. 2014;42(6):1455-1462. PubMed
8. Barnato AE, Mohan D, Lane RK, et al. Advance care planning norms may contribute to hospital variation in end-of-life ICU use: a simulation study. Med Decis Making. 2014;34(4):473-484. PubMed
9. Barnato AE, Tate JA, Rodriguez KL, Zickmund SL, Arnold RM. Norms of decision making in the ICU: a case study of two academic medical centers at the extremes of end-of-life treatment intensity. Intensive Care Med. 2012;38(11):1886-1896. PubMed
10. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673. PubMed
Advance care planning (ACP) is the process wherein patients, in discussions with their healthcare providers, family members, and other loved ones, make individual decisions about their future healthcare or prepare proxies to guide future medical treatment decisions.1,2 In 2016, the Centers for Medicare and Medicaid Services (CMS) began paying providers for ACP by using billing codes 99497 (first 30 min of ACP) and 99498 (additional 30 min of ACP). According to the CMS, during the first year after the billing codes were introduced, 22,864 providers billed for ACP conversations with 574,621 patients.3 While all adults are eligible, common triggers for ACP include advanced age, serious illness, and functional status changes that confer an increased risk of dying. We explored the early uptake of the ACP billing code in a large national physician practice that provided mandatory education in use of the ACP billing code, offered a small financial incentive for ACP documentation, and primed physicians to reflect on the patient’s risk of dying in the next year at the time of hospital admission.
METHODS
We analyzed ACP billing for hospitalized adults aged 65 years or above and who were managed by a large national physician practice that employs acute care providers in hospital medicine, emergency medicine and critical care between January 1, 2017 and March 31, 2017. This practice employs approximately 2,500 hospital-based physicians in 250 community hospitals in 38 states. They collect data through handheld and desktop information technology (IT) tools to facilitate coding, billing, and compliance by hospitalists. Hospitalists receive mandatory web-based training in compliance with CMS ACP billing and templated ACP documentation. Additionally, they receive web-based training in serious illness communication skills during the first two years of employment. The training includes didactic content regarding steps for collaborative decision making, words to use during the encounter, and videos of simulated patient encounters demonstrating best practices. Hospitalists also receive a small financial incentive ($20) for each properly documented ACP conversation that meets CMS criteria for ACP code payment.
Beginning in 2017, hospitalists were required to answer the validated Surprise Question4 (SQ; “Would you be surprised if the patient died in the next year?”) for all admitted patients aged 65 years and older. The SQ is useful because it is intuitive and not burdensome for physicians to answer. Moreover, it is predictive of mortality. The pooled prognostic characteristics of the SQ across multiple populations for predicting the outcome of death at 6 months to 18 months include a sensitivity of 67.0% (95% confidence interval [CI] 55.7%-76.7%), a specificity of 80.2% (95% CI 73.3%-85.6%), a positive likelihood ratio of 3.4 (95% CI 2.8–4.1), a negative likelihood ratio of 0.41 (95% CI 0.32-0.54), a positive predictive value of 37.1% (95% CI 30.2%-44.6%), and a negative predictive value of 93.1% (95% CI 91.0%-94.8%).5 The SQ primed the admitting physician and triggered an “EoL” (end-of-life) icon next to the patient’s name on the hospitalists’ handheld electronic patient census.
We summarized ACP billing rates and used mixed-effects regression to estimate adjusted ACP rates accounting for patient covariates and clustering at the provider and hospital level. Patient covariates included age; answer to the SQ [“yes,” “no,” or “missing”]); and the presence or absence of seven comorbidities: dementia, heart failure, chronic obstructive pulmonary disease, renal failure, liver failure, metastatic cancer, and nonmetastatic cancer. We quantified the magnitude of provider and hospital variation in ACP rates by using the intraclass correlation coefficient (ICC).
RESULTS
In the first quarter of 2017, hospitalists admitted 113,612 patients aged 65 years and older. Hospitalists were prompted to answer the SQ for 73,731 (65%) of the patients. They were not prompted to answer the SQ for 39,881 (35%) of the patients (ie, missing data for the SQ). Reasons for not prompting include delayed implementation at a site and the patient not being admitted to the hospital (eg, managed on observation status). When prompted, hospitalists answered “no” to the SQ for 41,276/73,731 (56%) of admissions.
Only 6,146/113,612 (5.4%) of all admissions involved a billed ACP conversation. Rates were highest among SQ-prompted/answer “no” cases (8.3%) compared with SQ-prompted/answer “yes” cases (4.1%) and non-SQ-prompted cases (3.5%), with all pairwise differences being statistically significant (P values “yes” vs “no” = .0079, “yes” vs not prompted = .0043, “no” vs not prompted < .0001; see Table 1).
In addition to being more likely to have a “no” response to the SQ, those with a billed ACP conversations were older (80 vs 78, P < .001); more likely to be diagnosed with dementia (5.9% vs 3.5%, P < .001), congestive heart failure (12.3% vs 9.9%, P < .001), and cancer (6.1% vs 3.3%, P < .001); more likely to die during the admission (16.5% vs 10.9%, P < .001); and, conditional on survival to discharge, more likely to be discharged with hospice (17% vs 3%, P < .001) than those without (Table 2).
At the hospital level, ACP rates varied from 0% to 35% (mean 5.2%) of all admissions. In analyses restricted to physicians seeing at least 30 patients 65 years of age and older during the quarter, physician-level ACP rates varied from 0% to 93% (mean 5.4%). The majority of all ACP discussions were attributable to one-quarter of physicians. One-third of physicians never billed for ACP.
In a hierarchical logistic regression model accounting for observable patient characteristics and clustering at the physician and hospital level, the adjusted ACP rate for an “average” patient (age 77.85 with the most common clinical conditions) was 13.6% if the hospitalist answered “no” to the SQ, 9.6% if the hospitalist answered “yes,” and 10.1% if the hospitalist was not asked the SQ (P value of difference < .0001). From this model, we also calculated an ICC at the physician level of 0.044 and at the hospital level of 0.079. The physician level ICC corresponds to a 4.5% absolute increase in ACP when one moves from a physician at the mean to a physician 1 SD above the mean (ie, moving 1 SD up the scale of the latent variable underlying the random effect). The hospital level ICC corresponds to a 6.3% absolute increase in ACP when one moves from a hospital at the mean to a hospital 1 SD above the mean. The 4.5% absolute increase in ACP due to physician practice patterns and 6.3% absolute increase in ACP due to hospital practice patterns are both greater than the estimated increase in ACP from the hospitalist answering “no” instead of “yes” to the SQ (3.6%).
DISCUSSION
In this large national hospital-based physician practice group, the rates of ACP among acute care patients 65 years of age and older were very low despite the use of education and IT- and incentive-based strategies to encourage ACP conversations among seriously ill older adults. Priming physicians to reflect on the patient’s risk of dying at the time of admission was associated with the doubling of ACP rates.
Despite some lawmakers’ concerns that the ACP billing code may be overused and therefore become a financial burden to the Medicare program6, we find the very low use of ACP billing in a population for whom having goals of care conversations is critical—seriously ill older adults who the physician would not be surprised if they died in the next year. This gap is significant because these ACP conversations, when they did occur, were associated with a comfort-focused trajectory, including a more than four-fold increase in hospice referral at discharge.
Causal inference is limited because of the observational nature of the study. While we hypothesize that priming the physicians to reflect on prognosis activated them to prioritize ACP, based on a prior scenario-based randomized trial,7 illness severity likely drives ACP conversations. Specifically, patients on observation status (who had missing SQ data) and those for whom the physician answered “yes” to the SQ are less sick than other patients. Additional decision-making heuristics in addition to mortality risk may influence ACP conversations, as suggested by the independent influence of diagnoses, such as dementia or cancer, on ACP. Notably, however, the large amounts of unexplained variation at the physician and the hospital levels exceed the amounts explained by any individual observed patient factor.
Other key limitations of this study include the use of ACP billing as a primary outcome rather than observed and documented ACP conversations and the lack of information on the quality of ACP conversations. These findings reflect the uptake of ACP billing rates soon after the code was introduced. ACP billing rates have likely increased since the first quarter of 2017. Future work should explore diffusion and variation in physician-specific use over time. Finally, despite the nationwide sample, findings may not be generalizable to hospitalists who have not received training and financial incentives for ACP billing.
This study reinforces the possibility that variation in ACP conversations may contribute to variation in end-of-life treatment intensity between providers.8-10 Low ACP rates among even those with high hospitalist-predicted mortality risk and considerable between-provider variation underscore the need for quality improvement interventions to increase hospital-based ACP.
Acknowledgments
The authors thank Jared Wasserman, Maxwell Bessler, Devon Zoller MD, Mark Rudolph MD, Kristi Franz, and Weiping Zhou for their research assistance.
Disclosures
The authors have nothing to disclose.
Funding
National Institute on Aging award P01 AG019783
Advance care planning (ACP) is the process wherein patients, in discussions with their healthcare providers, family members, and other loved ones, make individual decisions about their future healthcare or prepare proxies to guide future medical treatment decisions.1,2 In 2016, the Centers for Medicare and Medicaid Services (CMS) began paying providers for ACP by using billing codes 99497 (first 30 min of ACP) and 99498 (additional 30 min of ACP). According to the CMS, during the first year after the billing codes were introduced, 22,864 providers billed for ACP conversations with 574,621 patients.3 While all adults are eligible, common triggers for ACP include advanced age, serious illness, and functional status changes that confer an increased risk of dying. We explored the early uptake of the ACP billing code in a large national physician practice that provided mandatory education in use of the ACP billing code, offered a small financial incentive for ACP documentation, and primed physicians to reflect on the patient’s risk of dying in the next year at the time of hospital admission.
METHODS
We analyzed ACP billing for hospitalized adults aged 65 years or above and who were managed by a large national physician practice that employs acute care providers in hospital medicine, emergency medicine and critical care between January 1, 2017 and March 31, 2017. This practice employs approximately 2,500 hospital-based physicians in 250 community hospitals in 38 states. They collect data through handheld and desktop information technology (IT) tools to facilitate coding, billing, and compliance by hospitalists. Hospitalists receive mandatory web-based training in compliance with CMS ACP billing and templated ACP documentation. Additionally, they receive web-based training in serious illness communication skills during the first two years of employment. The training includes didactic content regarding steps for collaborative decision making, words to use during the encounter, and videos of simulated patient encounters demonstrating best practices. Hospitalists also receive a small financial incentive ($20) for each properly documented ACP conversation that meets CMS criteria for ACP code payment.
Beginning in 2017, hospitalists were required to answer the validated Surprise Question4 (SQ; “Would you be surprised if the patient died in the next year?”) for all admitted patients aged 65 years and older. The SQ is useful because it is intuitive and not burdensome for physicians to answer. Moreover, it is predictive of mortality. The pooled prognostic characteristics of the SQ across multiple populations for predicting the outcome of death at 6 months to 18 months include a sensitivity of 67.0% (95% confidence interval [CI] 55.7%-76.7%), a specificity of 80.2% (95% CI 73.3%-85.6%), a positive likelihood ratio of 3.4 (95% CI 2.8–4.1), a negative likelihood ratio of 0.41 (95% CI 0.32-0.54), a positive predictive value of 37.1% (95% CI 30.2%-44.6%), and a negative predictive value of 93.1% (95% CI 91.0%-94.8%).5 The SQ primed the admitting physician and triggered an “EoL” (end-of-life) icon next to the patient’s name on the hospitalists’ handheld electronic patient census.
We summarized ACP billing rates and used mixed-effects regression to estimate adjusted ACP rates accounting for patient covariates and clustering at the provider and hospital level. Patient covariates included age; answer to the SQ [“yes,” “no,” or “missing”]); and the presence or absence of seven comorbidities: dementia, heart failure, chronic obstructive pulmonary disease, renal failure, liver failure, metastatic cancer, and nonmetastatic cancer. We quantified the magnitude of provider and hospital variation in ACP rates by using the intraclass correlation coefficient (ICC).
RESULTS
In the first quarter of 2017, hospitalists admitted 113,612 patients aged 65 years and older. Hospitalists were prompted to answer the SQ for 73,731 (65%) of the patients. They were not prompted to answer the SQ for 39,881 (35%) of the patients (ie, missing data for the SQ). Reasons for not prompting include delayed implementation at a site and the patient not being admitted to the hospital (eg, managed on observation status). When prompted, hospitalists answered “no” to the SQ for 41,276/73,731 (56%) of admissions.
Only 6,146/113,612 (5.4%) of all admissions involved a billed ACP conversation. Rates were highest among SQ-prompted/answer “no” cases (8.3%) compared with SQ-prompted/answer “yes” cases (4.1%) and non-SQ-prompted cases (3.5%), with all pairwise differences being statistically significant (P values “yes” vs “no” = .0079, “yes” vs not prompted = .0043, “no” vs not prompted < .0001; see Table 1).
In addition to being more likely to have a “no” response to the SQ, those with a billed ACP conversations were older (80 vs 78, P < .001); more likely to be diagnosed with dementia (5.9% vs 3.5%, P < .001), congestive heart failure (12.3% vs 9.9%, P < .001), and cancer (6.1% vs 3.3%, P < .001); more likely to die during the admission (16.5% vs 10.9%, P < .001); and, conditional on survival to discharge, more likely to be discharged with hospice (17% vs 3%, P < .001) than those without (Table 2).
At the hospital level, ACP rates varied from 0% to 35% (mean 5.2%) of all admissions. In analyses restricted to physicians seeing at least 30 patients 65 years of age and older during the quarter, physician-level ACP rates varied from 0% to 93% (mean 5.4%). The majority of all ACP discussions were attributable to one-quarter of physicians. One-third of physicians never billed for ACP.
In a hierarchical logistic regression model accounting for observable patient characteristics and clustering at the physician and hospital level, the adjusted ACP rate for an “average” patient (age 77.85 with the most common clinical conditions) was 13.6% if the hospitalist answered “no” to the SQ, 9.6% if the hospitalist answered “yes,” and 10.1% if the hospitalist was not asked the SQ (P value of difference < .0001). From this model, we also calculated an ICC at the physician level of 0.044 and at the hospital level of 0.079. The physician level ICC corresponds to a 4.5% absolute increase in ACP when one moves from a physician at the mean to a physician 1 SD above the mean (ie, moving 1 SD up the scale of the latent variable underlying the random effect). The hospital level ICC corresponds to a 6.3% absolute increase in ACP when one moves from a hospital at the mean to a hospital 1 SD above the mean. The 4.5% absolute increase in ACP due to physician practice patterns and 6.3% absolute increase in ACP due to hospital practice patterns are both greater than the estimated increase in ACP from the hospitalist answering “no” instead of “yes” to the SQ (3.6%).
DISCUSSION
In this large national hospital-based physician practice group, the rates of ACP among acute care patients 65 years of age and older were very low despite the use of education and IT- and incentive-based strategies to encourage ACP conversations among seriously ill older adults. Priming physicians to reflect on the patient’s risk of dying at the time of admission was associated with the doubling of ACP rates.
Despite some lawmakers’ concerns that the ACP billing code may be overused and therefore become a financial burden to the Medicare program6, we find the very low use of ACP billing in a population for whom having goals of care conversations is critical—seriously ill older adults who the physician would not be surprised if they died in the next year. This gap is significant because these ACP conversations, when they did occur, were associated with a comfort-focused trajectory, including a more than four-fold increase in hospice referral at discharge.
Causal inference is limited because of the observational nature of the study. While we hypothesize that priming the physicians to reflect on prognosis activated them to prioritize ACP, based on a prior scenario-based randomized trial,7 illness severity likely drives ACP conversations. Specifically, patients on observation status (who had missing SQ data) and those for whom the physician answered “yes” to the SQ are less sick than other patients. Additional decision-making heuristics in addition to mortality risk may influence ACP conversations, as suggested by the independent influence of diagnoses, such as dementia or cancer, on ACP. Notably, however, the large amounts of unexplained variation at the physician and the hospital levels exceed the amounts explained by any individual observed patient factor.
Other key limitations of this study include the use of ACP billing as a primary outcome rather than observed and documented ACP conversations and the lack of information on the quality of ACP conversations. These findings reflect the uptake of ACP billing rates soon after the code was introduced. ACP billing rates have likely increased since the first quarter of 2017. Future work should explore diffusion and variation in physician-specific use over time. Finally, despite the nationwide sample, findings may not be generalizable to hospitalists who have not received training and financial incentives for ACP billing.
This study reinforces the possibility that variation in ACP conversations may contribute to variation in end-of-life treatment intensity between providers.8-10 Low ACP rates among even those with high hospitalist-predicted mortality risk and considerable between-provider variation underscore the need for quality improvement interventions to increase hospital-based ACP.
Acknowledgments
The authors thank Jared Wasserman, Maxwell Bessler, Devon Zoller MD, Mark Rudolph MD, Kristi Franz, and Weiping Zhou for their research assistance.
Disclosures
The authors have nothing to disclose.
Funding
National Institute on Aging award P01 AG019783
1. Mullick A, Martin J, Sallnow L. An introduction to advance care planning in practice. BMJ. 2013;347:f6064. PubMed
2. Sudore RL, Lum HD, You JJ, et al. Defining advance care planning for adults: a consensus definition from a multidisciplinary Delphi panel. J Pain Symptom Manage. 2017;53(5):821-832. PubMed
3. Medicare spending and utilization for advance care planning (ACP) services in 2016. Analysis of CMS data posted by the Coalition to Transform Advanced Care https://www.thectac.org/2017/08/use-billing-codes-advance-care-planning-exceeds-projections/. Accessed February 2018.
4. Moss AH, Ganjoo J, Sharma S, et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3(5):1379-1384. PubMed
5. Downar J, Goldman R, Pinto R, Englesakis M, Adhikari NK. The “surprise question” for predicting death in seriously ill patients: a systematic review and meta-analysis. CMAJ. 2017;189(13):E484-E493. PubMed
6. Aleccia J. Docs bill Medicare for end-of-life advice as ‘death panel’ fears reemerge. Kaiser Health News, February 2017.
7. Turnbull AE, Krall JR, Ruhl AP, et al. A scenario-based, randomized trial of patient values and functional prognosis on intensivist intent to discuss withdrawing life support. Crit Care Med. 2014;42(6):1455-1462. PubMed
8. Barnato AE, Mohan D, Lane RK, et al. Advance care planning norms may contribute to hospital variation in end-of-life ICU use: a simulation study. Med Decis Making. 2014;34(4):473-484. PubMed
9. Barnato AE, Tate JA, Rodriguez KL, Zickmund SL, Arnold RM. Norms of decision making in the ICU: a case study of two academic medical centers at the extremes of end-of-life treatment intensity. Intensive Care Med. 2012;38(11):1886-1896. PubMed
10. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673. PubMed
1. Mullick A, Martin J, Sallnow L. An introduction to advance care planning in practice. BMJ. 2013;347:f6064. PubMed
2. Sudore RL, Lum HD, You JJ, et al. Defining advance care planning for adults: a consensus definition from a multidisciplinary Delphi panel. J Pain Symptom Manage. 2017;53(5):821-832. PubMed
3. Medicare spending and utilization for advance care planning (ACP) services in 2016. Analysis of CMS data posted by the Coalition to Transform Advanced Care https://www.thectac.org/2017/08/use-billing-codes-advance-care-planning-exceeds-projections/. Accessed February 2018.
4. Moss AH, Ganjoo J, Sharma S, et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3(5):1379-1384. PubMed
5. Downar J, Goldman R, Pinto R, Englesakis M, Adhikari NK. The “surprise question” for predicting death in seriously ill patients: a systematic review and meta-analysis. CMAJ. 2017;189(13):E484-E493. PubMed
6. Aleccia J. Docs bill Medicare for end-of-life advice as ‘death panel’ fears reemerge. Kaiser Health News, February 2017.
7. Turnbull AE, Krall JR, Ruhl AP, et al. A scenario-based, randomized trial of patient values and functional prognosis on intensivist intent to discuss withdrawing life support. Crit Care Med. 2014;42(6):1455-1462. PubMed
8. Barnato AE, Mohan D, Lane RK, et al. Advance care planning norms may contribute to hospital variation in end-of-life ICU use: a simulation study. Med Decis Making. 2014;34(4):473-484. PubMed
9. Barnato AE, Tate JA, Rodriguez KL, Zickmund SL, Arnold RM. Norms of decision making in the ICU: a case study of two academic medical centers at the extremes of end-of-life treatment intensity. Intensive Care Med. 2012;38(11):1886-1896. PubMed
10. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673. PubMed
© 2019 Society of Hospital Medicine
Frequency of Ethical Issues on a Hospitalist Teaching Service at an Urban, Tertiary Care Center
Much has been written about the sources of the hidden curriculum in clerkships and postgraduate medical education.1-3 However, these descriptions do not adequately account for the critical role that hospitalists play in the development of trainees when they encounter ethical challenges on teaching services.4 As a role model, teacher, and the attending of record, a hospitalist’s response to ethical issues in practice can have a pivotal influence on the life and work of trainees, either instilling positive virtues or perpetuating the negative impact of the hidden curriculum.5-8 Understanding the epidemiology of ethical issues arising on academic hospitalist services has important implications for medical education, clinical ethics, and professionalism, as well as for patient care.
METHODS
Study Setting and Design
We conducted a mixed-method observational study at NewYork–Presbyterian–Weill Cornell Medical Center, an 862-bed, tertiary-care, academic institution located in New York, New York. We performed a prospective description of the frequency of all consecutively identified ethical and contextual issues pertinent to clinical decision-making by observing morning rounds with housestaff hospitalist services. Ethical issues were categorized using a comprehensive standardized instrument previously developed and published by the Division of Medical Ethics.9
The Division of Hospital Medicine employs 79 physicians, 30 of whom are dedicated full-time to daytime care on house-staff (or teaching) or physician assistant services. Of these 30 physicians, two (7%) were coinvestigators in this project and were excluded from participation to avoid bias. Between September 2017 and May 2018, the attending physicians of record of all available housestaff services were invited to participate with their teams in our research study on a weekly basis. We observed 10 different Hospital Medicine attending physicians (10/28, 36% of the available physician sample) over 19 sessions. Before rounds, a brief introduction to the nature of the study was provided to each team. It was explicitly stated that the observers were present to identify and document possible ethical issues that may arise while discussing the patients on rounds, and that the purpose of the study was neither an evaluation of the team members or their decisions nor a critique or quality improvement exercise. Observing researchers were not allowed to participate in the discussion of any case.
To avoid potential case duplication, we allowed for a minimum two-week interval before rounding twice on any particular team. To control for interobserver variability, we observed in pairs during these sessions. Discrepancies between observers were resolved by post hoc discussion and application of the definitions of the standardized instrument used to identify and catalog ethical and contextual issues.
Study Variables and Definitions
The following variables were collected in all cases: observation date, name of reviewers, demographic characteristics of the patient (age, gender, race, ethnicity, marital status, religion, preferred language, insurance type, and living situation before the admission), patient’s location during the admission (emergency room, regular nursing floor, step-down unit, or other), and ethical and contextual issues. “Ethical issues” were defined as those situations involving a conflict of values or preferences among different stakeholders, including, but not limited to, providers, patients, and/or families. Explicit definitions of each issue were generated, and additional standard rules for completion were provided.
Statistical Analysis
Results are presented as n (%) or mean ± standard deviation. Percentages were rounded to the closest integer. Interobserver variability between the observers in relation to evaluating the presence or absence of ethical or contextual issues was assessed by the kappa statistic. All P values are two-sided, with statistical significance evaluated at the 0.05 alpha level. A 95% confidence interval (95% CI) for the kappa statistic (ie, for assessing interobserver variability) was calculated to assess the precision of the obtained kappa estimate. All analyses were performed in SAS Version 9.4 (SAS Institute, Inc., Cary, NC) and Stata Version 14.0 (StataCorp, College Station, TX).
RESULTS
General Characteristics of the Study Sample
In total, 270 patients were evaluated from the teaching hospitalist services during the observation period. Ethical issues were identified in 86 of these patients (31.8%). Observer ethicists disagreed in their initial evaluation of 17 cases (6.3%). After review of and adjudication, both observers agreed that nine of these 17 cases (3.3%) should be excluded from the final analysis, as none reached the necessary threshold to be considered as a true ethical issue. Hence, we report the results of 77 patients (28.5%). These cases comprised the Hospitalist group and involved 113 ethical issues (1.48 ± 0.5 ethical issues/case). Only five patients in the Hospitalist group had a formal clinical ethics consult before our observation (5/270 patients [1.9%] vs 77/270 patients [28.5%] with an ethical issue, respectively, P < .001). Although the majority of ethical issues were noted by members of the primary team (84%), 12 of the 77 cases in the Hospitalist group (16%) were identified only by the observing ethicists. The kappa statistic for interobserver variability between the observing ethicists was 0.85 (95% CI = 0.76-0.92). The major demographic characteristics are summarized in Table 1.
Ethical Challenges
The most common ethical issues hospitalists encountered involved discussions about goals of care (including decisions to pursue aggressive treatment versus hospice care, or debates about the team’s ambivalence about the benefits and risks of pursuing investigational chemotherapy), treatment refusals (including the decision to forgo biopsy of a suspected malignancy), or decision-making capacity (Table 2). Less common were issues pertaining to resource allocation (specially related to pressures to discharge patients), pain management (some patients were suspected of drug-seeking behavior), or surrogate decision-making (when alternative decision-makers were suspected to lack decision-making capacity). Discussions about forgoing life-sustaining treatments occurred only in four cases (5%). These involved considerations of withdrawing Bilevel Positive Airway Pressure (BiPAP), artificial nutrition and hydration, and/or stopping antibiotic treatment.
DISCUSSION
Our data are the first prospective description of ethical issues arising on an academic hospitalist teaching service. These results indicate that there is an ethics epidemiology in the routine practice of Hospital Medicine that has heretofore not been characterized. By this, we mean a discreet incidence and prevalence of ethical challenges in Hospital Medicine that is distinct from that which is encountered by clinical ethics consultation (CEC) services. Although most practitioners recognize the utility of a traditional ethics consultation, there is a surprising paucity of data about the sources of ethical conflict encountered by academic hospitalists at the bedside, particularly those addressed without CEC. This suggests that the criteria for requesting a formal ethics consult could be limited and restrictive, which is both undersensitive and overspecific.10 Because of these limitations, viewing traditional ethics consultation as a proxy for ethical issues arising in daily hospitalist practice would lead to an underestimation of the true prevalence, as our data indicate.
More than one-fourth of the patients admitted to hospitalist teaching services pose ethical conflicts. Some of these are addressed on rounds, some are not, and only a handful of these cases will ever be referred to an ethicist. CEC services are made aware of the “tip of the iceberg,” which accounts for a vanishingly small percentage of ethical issues that arise on daily rounds. Some hospitalists may not involve CEC simply because they believe that the services are not helpful. However, the failure to obtain consultation may also reflect an inability to recognize a “problematic situation” and formulate a referral that might benefit from the assistance of an ethics consultation.11
Our study faces several potential limitations. We are presenting a single-center experience that focuses on the perspective of physicians and trainees. Some ethical issues might have been underestimated because the perspectives of patients, families, nurses, social workers, or other ancillary staff were not directly included. Furthermore, since any ethical challenge could have been discussed on any moment other than on morning rounds, our results may underestimate the prevalence of ethical issues arising from the hospital floors. Moreover, medical teams participating in the study could have been subject to the Hawthorne effect and could have tried to identify a greater number of ethical issues on rounds, which would not reflect actual practice.
CONCLUSION
Almost two decades ago, Coulehan and Williams wrote about the positive impact that ethics and humanities could have if these disciplines could be embedded in the daily practice of medicine, which is as follows:
…ethics and humanities curricula are irrelevant unless they can produce a substantive and continuing impact on hospital culture (…) The idea, of course, is to infiltrate the culture by coopting residents and attending physicians(…) If an ethics program can somehow achieve a critical mass of ‘‘value-sensitive’’ clinical faculty, it may begin to influence the institution’s ethos.12
Coulehan and Williams wrote of a need to bring ethics to the bedside. Our data suggest that an ethics epidemiology is deeply embedded in hospitalist services and is waiting to be fully characterized to better inform the care of patients and guide the professional formation and education of students and trainees. Hospitalists frequently confront ethical problems in daily practice that do not come to the attention of the CEC services or the institutional ethics committee. Understanding this emerging epidemiology presents an unrealized opportunity to improve bedside teaching, reinforce normative reasoning, and enhance patient care.
Acknowledgments
The authors want to acknowledge Drs. Augustine I. Choi, Michael G. Stewart, Laura L. Forese, and Anthony Hollenberg for their support of the fellowship in medical ethics and thank Drs. Arthur T. Evans and Monika M. Safford for their guidance.
Disclosures
The authors report no conflicts of interest.
Funding
This work was supported by a Weill Cornell General Internal Medicine Primary Care Innovations Initiative seed grant. Dr. Paul Christos was partially supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (1-UL1-TR002384-01).
1. Doja A, Bould MD, Clarkin C, Eady K, Sutherland S, Writer H. The hidden and informal curriculum across the continuum of training: a cross-sectional qualitative study. Med Teach. 2016;38(4):410-418. doi: 10.3109/0142159X.2015.1073241. PubMed
2. Martimianakis MA, Hafferty FW. Exploring the interstitial space between the ideal and the practised: humanism and the hidden curriculum of system reform. Med Educ. 2016;50(3):278-280. doi: 10.1111/medu.12982. PubMed
3. Lawrence C, Mhlaba T, Stewart KA, Moletsane R, Gaede B, Moshabela M. The hidden curricula of medical education: a scoping review. Acad Med. 2017;93(4):648-656. doi: 10.1097/ACM.0000000000002004. PubMed
4. McCarthy MW, Real de Asua D, Fins JJ. The rise of hospitalists: an opportunity for clinical ethics. J Clin Ethics. 2017;28(4):325-332. PubMed
5. McCarthy M, Fins J. Teaching clinical ethics at the bedside: William Osler and the essential role of the hospitalist. AMA J Ethics. 2017;19(6):528-532. doi: 10.1001/journalofethics.2017.19.6.peer2-1706. PubMed
6. Gabbay E, McCarthy MW, Fins JJ. The care of the ultra-orthodox Jewish patient. J Relig Health. 2017;56(2):545-560. doi: 10.1007/s10943-017-0356-6. PubMed
7. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. doi: 10.1056/NEJM199608153350713. PubMed
8. Hauer KE, Wachter RM, McCulloch CE, Woo GA, Auerbach AD. Effects of hospitalist attending physicians on trainee satisfaction with teaching and with internal medicine rotations. Arch Intern Med. 2004;164(17):1866-1871. doi: 10.1001/archinte.164.17.1866. PubMed
9. Nilson EG, Acres CA, Tamerin NG, Fins JJ. Clinical ethics and the quality initiative: a pilot study for the empirical evaluation of ethics case consultation. Am J Med Qual. 2008;23(5):356-364. doi: 10.1177/1062860608316729. PubMed
10. Hurst SA, Reiter-Theil S, Perrier A, et al. Physicians’ access to ethics support services in four European countries. Health Care Anal. 2007;15(4):321-335. doi: 10.1007/s10728-007-0072-6. PubMed
11. Fins JJ, Bacchetta MD, Miller FG. Clinical pragmatism: a method of moral problem solving. Kennedy Inst Ethics J. 1997;7(2):129-145. doi: 10.1353/ken.1997.0013. PubMed
12. Coulehan J, Williams PC. Vanquishing virtue: the impact of medical education. Acad Med. 2001;76(6):598-605. PubMed
Much has been written about the sources of the hidden curriculum in clerkships and postgraduate medical education.1-3 However, these descriptions do not adequately account for the critical role that hospitalists play in the development of trainees when they encounter ethical challenges on teaching services.4 As a role model, teacher, and the attending of record, a hospitalist’s response to ethical issues in practice can have a pivotal influence on the life and work of trainees, either instilling positive virtues or perpetuating the negative impact of the hidden curriculum.5-8 Understanding the epidemiology of ethical issues arising on academic hospitalist services has important implications for medical education, clinical ethics, and professionalism, as well as for patient care.
METHODS
Study Setting and Design
We conducted a mixed-method observational study at NewYork–Presbyterian–Weill Cornell Medical Center, an 862-bed, tertiary-care, academic institution located in New York, New York. We performed a prospective description of the frequency of all consecutively identified ethical and contextual issues pertinent to clinical decision-making by observing morning rounds with housestaff hospitalist services. Ethical issues were categorized using a comprehensive standardized instrument previously developed and published by the Division of Medical Ethics.9
The Division of Hospital Medicine employs 79 physicians, 30 of whom are dedicated full-time to daytime care on house-staff (or teaching) or physician assistant services. Of these 30 physicians, two (7%) were coinvestigators in this project and were excluded from participation to avoid bias. Between September 2017 and May 2018, the attending physicians of record of all available housestaff services were invited to participate with their teams in our research study on a weekly basis. We observed 10 different Hospital Medicine attending physicians (10/28, 36% of the available physician sample) over 19 sessions. Before rounds, a brief introduction to the nature of the study was provided to each team. It was explicitly stated that the observers were present to identify and document possible ethical issues that may arise while discussing the patients on rounds, and that the purpose of the study was neither an evaluation of the team members or their decisions nor a critique or quality improvement exercise. Observing researchers were not allowed to participate in the discussion of any case.
To avoid potential case duplication, we allowed for a minimum two-week interval before rounding twice on any particular team. To control for interobserver variability, we observed in pairs during these sessions. Discrepancies between observers were resolved by post hoc discussion and application of the definitions of the standardized instrument used to identify and catalog ethical and contextual issues.
Study Variables and Definitions
The following variables were collected in all cases: observation date, name of reviewers, demographic characteristics of the patient (age, gender, race, ethnicity, marital status, religion, preferred language, insurance type, and living situation before the admission), patient’s location during the admission (emergency room, regular nursing floor, step-down unit, or other), and ethical and contextual issues. “Ethical issues” were defined as those situations involving a conflict of values or preferences among different stakeholders, including, but not limited to, providers, patients, and/or families. Explicit definitions of each issue were generated, and additional standard rules for completion were provided.
Statistical Analysis
Results are presented as n (%) or mean ± standard deviation. Percentages were rounded to the closest integer. Interobserver variability between the observers in relation to evaluating the presence or absence of ethical or contextual issues was assessed by the kappa statistic. All P values are two-sided, with statistical significance evaluated at the 0.05 alpha level. A 95% confidence interval (95% CI) for the kappa statistic (ie, for assessing interobserver variability) was calculated to assess the precision of the obtained kappa estimate. All analyses were performed in SAS Version 9.4 (SAS Institute, Inc., Cary, NC) and Stata Version 14.0 (StataCorp, College Station, TX).
RESULTS
General Characteristics of the Study Sample
In total, 270 patients were evaluated from the teaching hospitalist services during the observation period. Ethical issues were identified in 86 of these patients (31.8%). Observer ethicists disagreed in their initial evaluation of 17 cases (6.3%). After review of and adjudication, both observers agreed that nine of these 17 cases (3.3%) should be excluded from the final analysis, as none reached the necessary threshold to be considered as a true ethical issue. Hence, we report the results of 77 patients (28.5%). These cases comprised the Hospitalist group and involved 113 ethical issues (1.48 ± 0.5 ethical issues/case). Only five patients in the Hospitalist group had a formal clinical ethics consult before our observation (5/270 patients [1.9%] vs 77/270 patients [28.5%] with an ethical issue, respectively, P < .001). Although the majority of ethical issues were noted by members of the primary team (84%), 12 of the 77 cases in the Hospitalist group (16%) were identified only by the observing ethicists. The kappa statistic for interobserver variability between the observing ethicists was 0.85 (95% CI = 0.76-0.92). The major demographic characteristics are summarized in Table 1.
Ethical Challenges
The most common ethical issues hospitalists encountered involved discussions about goals of care (including decisions to pursue aggressive treatment versus hospice care, or debates about the team’s ambivalence about the benefits and risks of pursuing investigational chemotherapy), treatment refusals (including the decision to forgo biopsy of a suspected malignancy), or decision-making capacity (Table 2). Less common were issues pertaining to resource allocation (specially related to pressures to discharge patients), pain management (some patients were suspected of drug-seeking behavior), or surrogate decision-making (when alternative decision-makers were suspected to lack decision-making capacity). Discussions about forgoing life-sustaining treatments occurred only in four cases (5%). These involved considerations of withdrawing Bilevel Positive Airway Pressure (BiPAP), artificial nutrition and hydration, and/or stopping antibiotic treatment.
DISCUSSION
Our data are the first prospective description of ethical issues arising on an academic hospitalist teaching service. These results indicate that there is an ethics epidemiology in the routine practice of Hospital Medicine that has heretofore not been characterized. By this, we mean a discreet incidence and prevalence of ethical challenges in Hospital Medicine that is distinct from that which is encountered by clinical ethics consultation (CEC) services. Although most practitioners recognize the utility of a traditional ethics consultation, there is a surprising paucity of data about the sources of ethical conflict encountered by academic hospitalists at the bedside, particularly those addressed without CEC. This suggests that the criteria for requesting a formal ethics consult could be limited and restrictive, which is both undersensitive and overspecific.10 Because of these limitations, viewing traditional ethics consultation as a proxy for ethical issues arising in daily hospitalist practice would lead to an underestimation of the true prevalence, as our data indicate.
More than one-fourth of the patients admitted to hospitalist teaching services pose ethical conflicts. Some of these are addressed on rounds, some are not, and only a handful of these cases will ever be referred to an ethicist. CEC services are made aware of the “tip of the iceberg,” which accounts for a vanishingly small percentage of ethical issues that arise on daily rounds. Some hospitalists may not involve CEC simply because they believe that the services are not helpful. However, the failure to obtain consultation may also reflect an inability to recognize a “problematic situation” and formulate a referral that might benefit from the assistance of an ethics consultation.11
Our study faces several potential limitations. We are presenting a single-center experience that focuses on the perspective of physicians and trainees. Some ethical issues might have been underestimated because the perspectives of patients, families, nurses, social workers, or other ancillary staff were not directly included. Furthermore, since any ethical challenge could have been discussed on any moment other than on morning rounds, our results may underestimate the prevalence of ethical issues arising from the hospital floors. Moreover, medical teams participating in the study could have been subject to the Hawthorne effect and could have tried to identify a greater number of ethical issues on rounds, which would not reflect actual practice.
CONCLUSION
Almost two decades ago, Coulehan and Williams wrote about the positive impact that ethics and humanities could have if these disciplines could be embedded in the daily practice of medicine, which is as follows:
…ethics and humanities curricula are irrelevant unless they can produce a substantive and continuing impact on hospital culture (…) The idea, of course, is to infiltrate the culture by coopting residents and attending physicians(…) If an ethics program can somehow achieve a critical mass of ‘‘value-sensitive’’ clinical faculty, it may begin to influence the institution’s ethos.12
Coulehan and Williams wrote of a need to bring ethics to the bedside. Our data suggest that an ethics epidemiology is deeply embedded in hospitalist services and is waiting to be fully characterized to better inform the care of patients and guide the professional formation and education of students and trainees. Hospitalists frequently confront ethical problems in daily practice that do not come to the attention of the CEC services or the institutional ethics committee. Understanding this emerging epidemiology presents an unrealized opportunity to improve bedside teaching, reinforce normative reasoning, and enhance patient care.
Acknowledgments
The authors want to acknowledge Drs. Augustine I. Choi, Michael G. Stewart, Laura L. Forese, and Anthony Hollenberg for their support of the fellowship in medical ethics and thank Drs. Arthur T. Evans and Monika M. Safford for their guidance.
Disclosures
The authors report no conflicts of interest.
Funding
This work was supported by a Weill Cornell General Internal Medicine Primary Care Innovations Initiative seed grant. Dr. Paul Christos was partially supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (1-UL1-TR002384-01).
Much has been written about the sources of the hidden curriculum in clerkships and postgraduate medical education.1-3 However, these descriptions do not adequately account for the critical role that hospitalists play in the development of trainees when they encounter ethical challenges on teaching services.4 As a role model, teacher, and the attending of record, a hospitalist’s response to ethical issues in practice can have a pivotal influence on the life and work of trainees, either instilling positive virtues or perpetuating the negative impact of the hidden curriculum.5-8 Understanding the epidemiology of ethical issues arising on academic hospitalist services has important implications for medical education, clinical ethics, and professionalism, as well as for patient care.
METHODS
Study Setting and Design
We conducted a mixed-method observational study at NewYork–Presbyterian–Weill Cornell Medical Center, an 862-bed, tertiary-care, academic institution located in New York, New York. We performed a prospective description of the frequency of all consecutively identified ethical and contextual issues pertinent to clinical decision-making by observing morning rounds with housestaff hospitalist services. Ethical issues were categorized using a comprehensive standardized instrument previously developed and published by the Division of Medical Ethics.9
The Division of Hospital Medicine employs 79 physicians, 30 of whom are dedicated full-time to daytime care on house-staff (or teaching) or physician assistant services. Of these 30 physicians, two (7%) were coinvestigators in this project and were excluded from participation to avoid bias. Between September 2017 and May 2018, the attending physicians of record of all available housestaff services were invited to participate with their teams in our research study on a weekly basis. We observed 10 different Hospital Medicine attending physicians (10/28, 36% of the available physician sample) over 19 sessions. Before rounds, a brief introduction to the nature of the study was provided to each team. It was explicitly stated that the observers were present to identify and document possible ethical issues that may arise while discussing the patients on rounds, and that the purpose of the study was neither an evaluation of the team members or their decisions nor a critique or quality improvement exercise. Observing researchers were not allowed to participate in the discussion of any case.
To avoid potential case duplication, we allowed for a minimum two-week interval before rounding twice on any particular team. To control for interobserver variability, we observed in pairs during these sessions. Discrepancies between observers were resolved by post hoc discussion and application of the definitions of the standardized instrument used to identify and catalog ethical and contextual issues.
Study Variables and Definitions
The following variables were collected in all cases: observation date, name of reviewers, demographic characteristics of the patient (age, gender, race, ethnicity, marital status, religion, preferred language, insurance type, and living situation before the admission), patient’s location during the admission (emergency room, regular nursing floor, step-down unit, or other), and ethical and contextual issues. “Ethical issues” were defined as those situations involving a conflict of values or preferences among different stakeholders, including, but not limited to, providers, patients, and/or families. Explicit definitions of each issue were generated, and additional standard rules for completion were provided.
Statistical Analysis
Results are presented as n (%) or mean ± standard deviation. Percentages were rounded to the closest integer. Interobserver variability between the observers in relation to evaluating the presence or absence of ethical or contextual issues was assessed by the kappa statistic. All P values are two-sided, with statistical significance evaluated at the 0.05 alpha level. A 95% confidence interval (95% CI) for the kappa statistic (ie, for assessing interobserver variability) was calculated to assess the precision of the obtained kappa estimate. All analyses were performed in SAS Version 9.4 (SAS Institute, Inc., Cary, NC) and Stata Version 14.0 (StataCorp, College Station, TX).
RESULTS
General Characteristics of the Study Sample
In total, 270 patients were evaluated from the teaching hospitalist services during the observation period. Ethical issues were identified in 86 of these patients (31.8%). Observer ethicists disagreed in their initial evaluation of 17 cases (6.3%). After review of and adjudication, both observers agreed that nine of these 17 cases (3.3%) should be excluded from the final analysis, as none reached the necessary threshold to be considered as a true ethical issue. Hence, we report the results of 77 patients (28.5%). These cases comprised the Hospitalist group and involved 113 ethical issues (1.48 ± 0.5 ethical issues/case). Only five patients in the Hospitalist group had a formal clinical ethics consult before our observation (5/270 patients [1.9%] vs 77/270 patients [28.5%] with an ethical issue, respectively, P < .001). Although the majority of ethical issues were noted by members of the primary team (84%), 12 of the 77 cases in the Hospitalist group (16%) were identified only by the observing ethicists. The kappa statistic for interobserver variability between the observing ethicists was 0.85 (95% CI = 0.76-0.92). The major demographic characteristics are summarized in Table 1.
Ethical Challenges
The most common ethical issues hospitalists encountered involved discussions about goals of care (including decisions to pursue aggressive treatment versus hospice care, or debates about the team’s ambivalence about the benefits and risks of pursuing investigational chemotherapy), treatment refusals (including the decision to forgo biopsy of a suspected malignancy), or decision-making capacity (Table 2). Less common were issues pertaining to resource allocation (specially related to pressures to discharge patients), pain management (some patients were suspected of drug-seeking behavior), or surrogate decision-making (when alternative decision-makers were suspected to lack decision-making capacity). Discussions about forgoing life-sustaining treatments occurred only in four cases (5%). These involved considerations of withdrawing Bilevel Positive Airway Pressure (BiPAP), artificial nutrition and hydration, and/or stopping antibiotic treatment.
DISCUSSION
Our data are the first prospective description of ethical issues arising on an academic hospitalist teaching service. These results indicate that there is an ethics epidemiology in the routine practice of Hospital Medicine that has heretofore not been characterized. By this, we mean a discreet incidence and prevalence of ethical challenges in Hospital Medicine that is distinct from that which is encountered by clinical ethics consultation (CEC) services. Although most practitioners recognize the utility of a traditional ethics consultation, there is a surprising paucity of data about the sources of ethical conflict encountered by academic hospitalists at the bedside, particularly those addressed without CEC. This suggests that the criteria for requesting a formal ethics consult could be limited and restrictive, which is both undersensitive and overspecific.10 Because of these limitations, viewing traditional ethics consultation as a proxy for ethical issues arising in daily hospitalist practice would lead to an underestimation of the true prevalence, as our data indicate.
More than one-fourth of the patients admitted to hospitalist teaching services pose ethical conflicts. Some of these are addressed on rounds, some are not, and only a handful of these cases will ever be referred to an ethicist. CEC services are made aware of the “tip of the iceberg,” which accounts for a vanishingly small percentage of ethical issues that arise on daily rounds. Some hospitalists may not involve CEC simply because they believe that the services are not helpful. However, the failure to obtain consultation may also reflect an inability to recognize a “problematic situation” and formulate a referral that might benefit from the assistance of an ethics consultation.11
Our study faces several potential limitations. We are presenting a single-center experience that focuses on the perspective of physicians and trainees. Some ethical issues might have been underestimated because the perspectives of patients, families, nurses, social workers, or other ancillary staff were not directly included. Furthermore, since any ethical challenge could have been discussed on any moment other than on morning rounds, our results may underestimate the prevalence of ethical issues arising from the hospital floors. Moreover, medical teams participating in the study could have been subject to the Hawthorne effect and could have tried to identify a greater number of ethical issues on rounds, which would not reflect actual practice.
CONCLUSION
Almost two decades ago, Coulehan and Williams wrote about the positive impact that ethics and humanities could have if these disciplines could be embedded in the daily practice of medicine, which is as follows:
…ethics and humanities curricula are irrelevant unless they can produce a substantive and continuing impact on hospital culture (…) The idea, of course, is to infiltrate the culture by coopting residents and attending physicians(…) If an ethics program can somehow achieve a critical mass of ‘‘value-sensitive’’ clinical faculty, it may begin to influence the institution’s ethos.12
Coulehan and Williams wrote of a need to bring ethics to the bedside. Our data suggest that an ethics epidemiology is deeply embedded in hospitalist services and is waiting to be fully characterized to better inform the care of patients and guide the professional formation and education of students and trainees. Hospitalists frequently confront ethical problems in daily practice that do not come to the attention of the CEC services or the institutional ethics committee. Understanding this emerging epidemiology presents an unrealized opportunity to improve bedside teaching, reinforce normative reasoning, and enhance patient care.
Acknowledgments
The authors want to acknowledge Drs. Augustine I. Choi, Michael G. Stewart, Laura L. Forese, and Anthony Hollenberg for their support of the fellowship in medical ethics and thank Drs. Arthur T. Evans and Monika M. Safford for their guidance.
Disclosures
The authors report no conflicts of interest.
Funding
This work was supported by a Weill Cornell General Internal Medicine Primary Care Innovations Initiative seed grant. Dr. Paul Christos was partially supported by the following grant: Clinical and Translational Science Center at Weill Cornell Medical College (1-UL1-TR002384-01).
1. Doja A, Bould MD, Clarkin C, Eady K, Sutherland S, Writer H. The hidden and informal curriculum across the continuum of training: a cross-sectional qualitative study. Med Teach. 2016;38(4):410-418. doi: 10.3109/0142159X.2015.1073241. PubMed
2. Martimianakis MA, Hafferty FW. Exploring the interstitial space between the ideal and the practised: humanism and the hidden curriculum of system reform. Med Educ. 2016;50(3):278-280. doi: 10.1111/medu.12982. PubMed
3. Lawrence C, Mhlaba T, Stewart KA, Moletsane R, Gaede B, Moshabela M. The hidden curricula of medical education: a scoping review. Acad Med. 2017;93(4):648-656. doi: 10.1097/ACM.0000000000002004. PubMed
4. McCarthy MW, Real de Asua D, Fins JJ. The rise of hospitalists: an opportunity for clinical ethics. J Clin Ethics. 2017;28(4):325-332. PubMed
5. McCarthy M, Fins J. Teaching clinical ethics at the bedside: William Osler and the essential role of the hospitalist. AMA J Ethics. 2017;19(6):528-532. doi: 10.1001/journalofethics.2017.19.6.peer2-1706. PubMed
6. Gabbay E, McCarthy MW, Fins JJ. The care of the ultra-orthodox Jewish patient. J Relig Health. 2017;56(2):545-560. doi: 10.1007/s10943-017-0356-6. PubMed
7. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. doi: 10.1056/NEJM199608153350713. PubMed
8. Hauer KE, Wachter RM, McCulloch CE, Woo GA, Auerbach AD. Effects of hospitalist attending physicians on trainee satisfaction with teaching and with internal medicine rotations. Arch Intern Med. 2004;164(17):1866-1871. doi: 10.1001/archinte.164.17.1866. PubMed
9. Nilson EG, Acres CA, Tamerin NG, Fins JJ. Clinical ethics and the quality initiative: a pilot study for the empirical evaluation of ethics case consultation. Am J Med Qual. 2008;23(5):356-364. doi: 10.1177/1062860608316729. PubMed
10. Hurst SA, Reiter-Theil S, Perrier A, et al. Physicians’ access to ethics support services in four European countries. Health Care Anal. 2007;15(4):321-335. doi: 10.1007/s10728-007-0072-6. PubMed
11. Fins JJ, Bacchetta MD, Miller FG. Clinical pragmatism: a method of moral problem solving. Kennedy Inst Ethics J. 1997;7(2):129-145. doi: 10.1353/ken.1997.0013. PubMed
12. Coulehan J, Williams PC. Vanquishing virtue: the impact of medical education. Acad Med. 2001;76(6):598-605. PubMed
1. Doja A, Bould MD, Clarkin C, Eady K, Sutherland S, Writer H. The hidden and informal curriculum across the continuum of training: a cross-sectional qualitative study. Med Teach. 2016;38(4):410-418. doi: 10.3109/0142159X.2015.1073241. PubMed
2. Martimianakis MA, Hafferty FW. Exploring the interstitial space between the ideal and the practised: humanism and the hidden curriculum of system reform. Med Educ. 2016;50(3):278-280. doi: 10.1111/medu.12982. PubMed
3. Lawrence C, Mhlaba T, Stewart KA, Moletsane R, Gaede B, Moshabela M. The hidden curricula of medical education: a scoping review. Acad Med. 2017;93(4):648-656. doi: 10.1097/ACM.0000000000002004. PubMed
4. McCarthy MW, Real de Asua D, Fins JJ. The rise of hospitalists: an opportunity for clinical ethics. J Clin Ethics. 2017;28(4):325-332. PubMed
5. McCarthy M, Fins J. Teaching clinical ethics at the bedside: William Osler and the essential role of the hospitalist. AMA J Ethics. 2017;19(6):528-532. doi: 10.1001/journalofethics.2017.19.6.peer2-1706. PubMed
6. Gabbay E, McCarthy MW, Fins JJ. The care of the ultra-orthodox Jewish patient. J Relig Health. 2017;56(2):545-560. doi: 10.1007/s10943-017-0356-6. PubMed
7. Wachter RM, Goldman L. The emerging role of “hospitalists” in the American health care system. N Engl J Med. 1996;335(7):514-517. doi: 10.1056/NEJM199608153350713. PubMed
8. Hauer KE, Wachter RM, McCulloch CE, Woo GA, Auerbach AD. Effects of hospitalist attending physicians on trainee satisfaction with teaching and with internal medicine rotations. Arch Intern Med. 2004;164(17):1866-1871. doi: 10.1001/archinte.164.17.1866. PubMed
9. Nilson EG, Acres CA, Tamerin NG, Fins JJ. Clinical ethics and the quality initiative: a pilot study for the empirical evaluation of ethics case consultation. Am J Med Qual. 2008;23(5):356-364. doi: 10.1177/1062860608316729. PubMed
10. Hurst SA, Reiter-Theil S, Perrier A, et al. Physicians’ access to ethics support services in four European countries. Health Care Anal. 2007;15(4):321-335. doi: 10.1007/s10728-007-0072-6. PubMed
11. Fins JJ, Bacchetta MD, Miller FG. Clinical pragmatism: a method of moral problem solving. Kennedy Inst Ethics J. 1997;7(2):129-145. doi: 10.1353/ken.1997.0013. PubMed
12. Coulehan J, Williams PC. Vanquishing virtue: the impact of medical education. Acad Med. 2001;76(6):598-605. PubMed
© 2019 Society of Hospital Medicine
Do Hospitals Participating in Accountable Care Organizations Discharge Patients to Higher Quality Nursing Homes?
Accountable care organizations (ACOs) create incentives for more efficient healthcare utilization. For patients being discharged from the hospital, this may mean more efficient use of postacute care (PAC), including discharging patients to higher quality skilled nursing facilities (SNFs) in an effort to limit readmissions and other costly complications. Public reporting of nursing home quality has been associated with improved performance measures, although improvements in preventable hospitalizations have lagged.1 Evidence to date suggests that patients attributed to an ACO are not going to higher quality SNFs,2,3 but these effects may be concentrated in hospitals that participate in ACOs and face stronger incentives to alter their discharge patterns compared with non-ACO hospitals. Therefore, we examined whether hospitals participating in Medicare’s Shared Saving Program (MSSP) increased the use of highly rated SNFs or decreased the use of low-rated SNFs hospital-wide after initiation of their ACO contracts compared with non-ACO hospitals.
METHODS
We used discharge-level data from the 100% MedPAR file for all fee-for-service Medicare beneficiaries discharged from an acute care hospital to an SNF between 2010 and 2013. We measured the SNF quality using Medicare’s Nursing Home Compare star ratings. Our primary outcome was probability of discharge to high-rated (five star) and low-rated (one star) SNFs.
We utilized a difference-in-differences design. Using a linear probability model, we first estimated the change in the probability of discharge to five-star SNFs (compared to all other SNFs) among all beneficiaries discharged from one of the 233 ACO-participating hospitals after the hospital became an ACO provider compared with before and compared withall beneficiaries discharged from one of the 3,081 non-ACO hospitals over the same time period. Individual hospitals were determined to be “ACO-participating” if they were listed on Medicare’s website as being part of an ACO-participating hospital in the MSSP. ACOs joined the MSSP in three waves: April 1, 2012; July 1, 2012; and January 1, 2013, which were also determined based on information on Medicare’s website. We separately estimated the change in probability of discharge to a one-star SNF (compared to all other SNFs) using the same approach. Models were adjusted for beneficiary demographic and clinical characteristics (age, sex, race, dual eligibility, urban ZIP code, diagnosis-related group code, and Elixhauser comorbidities) and market characteristics (the concentration of hospital discharges, SNF discharges, and the number of five-star SNFs, all measured in each hospital referral region).
RESULTS
We examined a total of 12,736,287 discharges, 11.8% from ACO-participating hospitals and 88.2% from non-ACO-participating hospitals. ACO-participating hospitals cared for fewer black patients and fewer patients who were dually enrolled in Medicare and Medicaid (Table 1), but these characteristics did not change differentially between the two groups of hospitals over our study period. ACO-participating hospitals were also more likely to discharge patients to five-star SNFs prior to joining an ACO (in 2010-2011). After joining an ACO, the percentage of hospital discharges going to a 5-star SNF increased by 3.4 percentage points on a base of 15.4% (95% confidence interval [CI] 1.3-5.5, P = .002; Table 2) compared with non-ACO-participating hospitals over the same time period. The differential changes did not extend to SNFs rated as three stars and above (change of 0.5 percentage points, 95% CI, 1.3-2.8, P = .600).
The probability of discharge from an ACO hospital to low-quality (one-star) SNFs did not change significantly from its baseline level of 13.5% after joining an ACO compared with non-ACO-participating hospitals (change of 0.4 percentage points, 95% CI, 0.7-1.5, P = .494).
DISCUSSION
Our findings indicate that ACO-participating hospitals were more likely to discharge patients to the highest rated SNFs after they began their ACO contract but did not change the likelihood of discharge to lower rated SNFs in comparison with non-ACO hospitals. Previous research has suggested that patients attributed to a Medicare ACO were not more likely to use high-quality SNFs. However, we examined the effect of hospital participation in an ACO, not individual beneficiaries attributed to an ACO. These contrasting results suggest that hospitals could be instituting hospital-wide changes in discharge patterns once they join an ACO and that hospital-led ACOs could be particularly well positioned to manage postdischarge care relative to physician-led ACOs. One potential limitation of this study is that ACO-participating hospitals may differ in unobservable ways from non-ACO-participating hospitals. However, using hospital fixed effects, w
Disclosures
Dr. Werner reports receiving personal fees from CarePort Health. Dr. Bain reports no conflicts. Mr. Yuan reports no conflicts. Dr. Navathe reports receiving personal fees from Navvis and Company, Navigant Inc., Lynx Medical, Indegene Inc., Sutherland Global Services, and Agathos, Inc.; personal fees and equity from NavaHealth; an honorarium from Elsevier Press, serving on the board of Integrated Services, Inc. without compensation, and grants from Hawaii Medical Service Association, Anthem Public Policy Institute, and Oscar Health, none of which are related to this manuscript.
Funding
This research was funded by R01-HS024266 by the Agency for Healthcare Research and Quality. Rachel Werner was supported in part by K24-AG047908 from the National Institute on Aging.
1. Ryskina KL, Konetzka RT, Werner RM. Association between 5-star nursing home report card ratings and potentially preventable hospitalizations. Inquiry. 2018;55:46958018787323. doi: 10.1177/0046958018787323. PubMed
2. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the medicare shared savings program. JAMA Intern Med. 2017;177(4):518-526. doi: 10.1001/jamainternmed.2016.9115. PubMed
3. McWilliams JM, Hatfield LA, Chernew ME, Landon BE, Schwartz AL. Early performance of accountable care organizations in medicare. N Engl J Med. 2016;374(24):2357-2366. doi: 10.1056/NEJMsa1600142. PubMed
Accountable care organizations (ACOs) create incentives for more efficient healthcare utilization. For patients being discharged from the hospital, this may mean more efficient use of postacute care (PAC), including discharging patients to higher quality skilled nursing facilities (SNFs) in an effort to limit readmissions and other costly complications. Public reporting of nursing home quality has been associated with improved performance measures, although improvements in preventable hospitalizations have lagged.1 Evidence to date suggests that patients attributed to an ACO are not going to higher quality SNFs,2,3 but these effects may be concentrated in hospitals that participate in ACOs and face stronger incentives to alter their discharge patterns compared with non-ACO hospitals. Therefore, we examined whether hospitals participating in Medicare’s Shared Saving Program (MSSP) increased the use of highly rated SNFs or decreased the use of low-rated SNFs hospital-wide after initiation of their ACO contracts compared with non-ACO hospitals.
METHODS
We used discharge-level data from the 100% MedPAR file for all fee-for-service Medicare beneficiaries discharged from an acute care hospital to an SNF between 2010 and 2013. We measured the SNF quality using Medicare’s Nursing Home Compare star ratings. Our primary outcome was probability of discharge to high-rated (five star) and low-rated (one star) SNFs.
We utilized a difference-in-differences design. Using a linear probability model, we first estimated the change in the probability of discharge to five-star SNFs (compared to all other SNFs) among all beneficiaries discharged from one of the 233 ACO-participating hospitals after the hospital became an ACO provider compared with before and compared withall beneficiaries discharged from one of the 3,081 non-ACO hospitals over the same time period. Individual hospitals were determined to be “ACO-participating” if they were listed on Medicare’s website as being part of an ACO-participating hospital in the MSSP. ACOs joined the MSSP in three waves: April 1, 2012; July 1, 2012; and January 1, 2013, which were also determined based on information on Medicare’s website. We separately estimated the change in probability of discharge to a one-star SNF (compared to all other SNFs) using the same approach. Models were adjusted for beneficiary demographic and clinical characteristics (age, sex, race, dual eligibility, urban ZIP code, diagnosis-related group code, and Elixhauser comorbidities) and market characteristics (the concentration of hospital discharges, SNF discharges, and the number of five-star SNFs, all measured in each hospital referral region).
RESULTS
We examined a total of 12,736,287 discharges, 11.8% from ACO-participating hospitals and 88.2% from non-ACO-participating hospitals. ACO-participating hospitals cared for fewer black patients and fewer patients who were dually enrolled in Medicare and Medicaid (Table 1), but these characteristics did not change differentially between the two groups of hospitals over our study period. ACO-participating hospitals were also more likely to discharge patients to five-star SNFs prior to joining an ACO (in 2010-2011). After joining an ACO, the percentage of hospital discharges going to a 5-star SNF increased by 3.4 percentage points on a base of 15.4% (95% confidence interval [CI] 1.3-5.5, P = .002; Table 2) compared with non-ACO-participating hospitals over the same time period. The differential changes did not extend to SNFs rated as three stars and above (change of 0.5 percentage points, 95% CI, 1.3-2.8, P = .600).
The probability of discharge from an ACO hospital to low-quality (one-star) SNFs did not change significantly from its baseline level of 13.5% after joining an ACO compared with non-ACO-participating hospitals (change of 0.4 percentage points, 95% CI, 0.7-1.5, P = .494).
DISCUSSION
Our findings indicate that ACO-participating hospitals were more likely to discharge patients to the highest rated SNFs after they began their ACO contract but did not change the likelihood of discharge to lower rated SNFs in comparison with non-ACO hospitals. Previous research has suggested that patients attributed to a Medicare ACO were not more likely to use high-quality SNFs. However, we examined the effect of hospital participation in an ACO, not individual beneficiaries attributed to an ACO. These contrasting results suggest that hospitals could be instituting hospital-wide changes in discharge patterns once they join an ACO and that hospital-led ACOs could be particularly well positioned to manage postdischarge care relative to physician-led ACOs. One potential limitation of this study is that ACO-participating hospitals may differ in unobservable ways from non-ACO-participating hospitals. However, using hospital fixed effects, w
Disclosures
Dr. Werner reports receiving personal fees from CarePort Health. Dr. Bain reports no conflicts. Mr. Yuan reports no conflicts. Dr. Navathe reports receiving personal fees from Navvis and Company, Navigant Inc., Lynx Medical, Indegene Inc., Sutherland Global Services, and Agathos, Inc.; personal fees and equity from NavaHealth; an honorarium from Elsevier Press, serving on the board of Integrated Services, Inc. without compensation, and grants from Hawaii Medical Service Association, Anthem Public Policy Institute, and Oscar Health, none of which are related to this manuscript.
Funding
This research was funded by R01-HS024266 by the Agency for Healthcare Research and Quality. Rachel Werner was supported in part by K24-AG047908 from the National Institute on Aging.
Accountable care organizations (ACOs) create incentives for more efficient healthcare utilization. For patients being discharged from the hospital, this may mean more efficient use of postacute care (PAC), including discharging patients to higher quality skilled nursing facilities (SNFs) in an effort to limit readmissions and other costly complications. Public reporting of nursing home quality has been associated with improved performance measures, although improvements in preventable hospitalizations have lagged.1 Evidence to date suggests that patients attributed to an ACO are not going to higher quality SNFs,2,3 but these effects may be concentrated in hospitals that participate in ACOs and face stronger incentives to alter their discharge patterns compared with non-ACO hospitals. Therefore, we examined whether hospitals participating in Medicare’s Shared Saving Program (MSSP) increased the use of highly rated SNFs or decreased the use of low-rated SNFs hospital-wide after initiation of their ACO contracts compared with non-ACO hospitals.
METHODS
We used discharge-level data from the 100% MedPAR file for all fee-for-service Medicare beneficiaries discharged from an acute care hospital to an SNF between 2010 and 2013. We measured the SNF quality using Medicare’s Nursing Home Compare star ratings. Our primary outcome was probability of discharge to high-rated (five star) and low-rated (one star) SNFs.
We utilized a difference-in-differences design. Using a linear probability model, we first estimated the change in the probability of discharge to five-star SNFs (compared to all other SNFs) among all beneficiaries discharged from one of the 233 ACO-participating hospitals after the hospital became an ACO provider compared with before and compared withall beneficiaries discharged from one of the 3,081 non-ACO hospitals over the same time period. Individual hospitals were determined to be “ACO-participating” if they were listed on Medicare’s website as being part of an ACO-participating hospital in the MSSP. ACOs joined the MSSP in three waves: April 1, 2012; July 1, 2012; and January 1, 2013, which were also determined based on information on Medicare’s website. We separately estimated the change in probability of discharge to a one-star SNF (compared to all other SNFs) using the same approach. Models were adjusted for beneficiary demographic and clinical characteristics (age, sex, race, dual eligibility, urban ZIP code, diagnosis-related group code, and Elixhauser comorbidities) and market characteristics (the concentration of hospital discharges, SNF discharges, and the number of five-star SNFs, all measured in each hospital referral region).
RESULTS
We examined a total of 12,736,287 discharges, 11.8% from ACO-participating hospitals and 88.2% from non-ACO-participating hospitals. ACO-participating hospitals cared for fewer black patients and fewer patients who were dually enrolled in Medicare and Medicaid (Table 1), but these characteristics did not change differentially between the two groups of hospitals over our study period. ACO-participating hospitals were also more likely to discharge patients to five-star SNFs prior to joining an ACO (in 2010-2011). After joining an ACO, the percentage of hospital discharges going to a 5-star SNF increased by 3.4 percentage points on a base of 15.4% (95% confidence interval [CI] 1.3-5.5, P = .002; Table 2) compared with non-ACO-participating hospitals over the same time period. The differential changes did not extend to SNFs rated as three stars and above (change of 0.5 percentage points, 95% CI, 1.3-2.8, P = .600).
The probability of discharge from an ACO hospital to low-quality (one-star) SNFs did not change significantly from its baseline level of 13.5% after joining an ACO compared with non-ACO-participating hospitals (change of 0.4 percentage points, 95% CI, 0.7-1.5, P = .494).
DISCUSSION
Our findings indicate that ACO-participating hospitals were more likely to discharge patients to the highest rated SNFs after they began their ACO contract but did not change the likelihood of discharge to lower rated SNFs in comparison with non-ACO hospitals. Previous research has suggested that patients attributed to a Medicare ACO were not more likely to use high-quality SNFs. However, we examined the effect of hospital participation in an ACO, not individual beneficiaries attributed to an ACO. These contrasting results suggest that hospitals could be instituting hospital-wide changes in discharge patterns once they join an ACO and that hospital-led ACOs could be particularly well positioned to manage postdischarge care relative to physician-led ACOs. One potential limitation of this study is that ACO-participating hospitals may differ in unobservable ways from non-ACO-participating hospitals. However, using hospital fixed effects, w
Disclosures
Dr. Werner reports receiving personal fees from CarePort Health. Dr. Bain reports no conflicts. Mr. Yuan reports no conflicts. Dr. Navathe reports receiving personal fees from Navvis and Company, Navigant Inc., Lynx Medical, Indegene Inc., Sutherland Global Services, and Agathos, Inc.; personal fees and equity from NavaHealth; an honorarium from Elsevier Press, serving on the board of Integrated Services, Inc. without compensation, and grants from Hawaii Medical Service Association, Anthem Public Policy Institute, and Oscar Health, none of which are related to this manuscript.
Funding
This research was funded by R01-HS024266 by the Agency for Healthcare Research and Quality. Rachel Werner was supported in part by K24-AG047908 from the National Institute on Aging.
1. Ryskina KL, Konetzka RT, Werner RM. Association between 5-star nursing home report card ratings and potentially preventable hospitalizations. Inquiry. 2018;55:46958018787323. doi: 10.1177/0046958018787323. PubMed
2. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the medicare shared savings program. JAMA Intern Med. 2017;177(4):518-526. doi: 10.1001/jamainternmed.2016.9115. PubMed
3. McWilliams JM, Hatfield LA, Chernew ME, Landon BE, Schwartz AL. Early performance of accountable care organizations in medicare. N Engl J Med. 2016;374(24):2357-2366. doi: 10.1056/NEJMsa1600142. PubMed
1. Ryskina KL, Konetzka RT, Werner RM. Association between 5-star nursing home report card ratings and potentially preventable hospitalizations. Inquiry. 2018;55:46958018787323. doi: 10.1177/0046958018787323. PubMed
2. McWilliams JM, Gilstrap LG, Stevenson DG, Chernew ME, Huskamp HA, Grabowski DC. Changes in postacute care in the medicare shared savings program. JAMA Intern Med. 2017;177(4):518-526. doi: 10.1001/jamainternmed.2016.9115. PubMed
3. McWilliams JM, Hatfield LA, Chernew ME, Landon BE, Schwartz AL. Early performance of accountable care organizations in medicare. N Engl J Med. 2016;374(24):2357-2366. doi: 10.1056/NEJMsa1600142. PubMed
© 2019 Society of Hospital Medicine
Condom Catheters versus Indwelling Urethral Catheters in Men: A Prospective, Observational Study
Millions of patients use urinary collection devices. For men, both indwelling and condom-style urinary catheters (known as “external catheters”) are commonly used. National infection prevention guidelines recommend condom catheters as a preferred alternative to indwelling catheters for patients without urinary retention1,2 to reduce the risk of catheter-associated urinary tract infection (UTI). Unfortunately, little outcome data comparing condom catheters with indwelling urethral catheters exists. We therefore assessed the incidence of infectious and noninfectious complications in condom catheter and indwelling urethral catheter users.
PATIENTS AND METHODS
Study Overview
As part of a larger prospective, observational study,3 we compared complications in patients who received a condom catheter during hospitalization with those in patients who received an indwelling urethral catheter. Hospitalized patients with either a condom catheter or indwelling urethral catheter were identified at two Veterans Affairs (VA) medical centers and followed for 30 days after initial catheter placement. Patient-reported data were collected during in-person patient interviews at baseline (within three days of catheter placement), and by in-person or phone interviews at 14 days and 30 days postplacement (Supplementary Appendix A and B). Questions were primarily closed-ended, except for a final question inviting open comments. Information about the catheter and any reported complications was also collected from electronic medical record documentation for each patient. Institutional review board approval was received from both participating study sites.
Data Collection and Inclusion Criteria
Hospitalized patients who had a condom or indwelling urethral catheter placed were eligible to participate if they met the following criteria: (1) were hospitalized on an acute care unit; (2) had a new condom catheter or indwelling urethral catheter placed during this hospital stay that was not present on admission; (3) had a device in place for three days or less; (4) were at least 18 years old; and (5) were able to speak English. Patients were excluded if they: (1) did not have the capacity to give consent or participate in the interview/assessment process; (2) refused to provide written informed consent to participate; or (3) had previously participated in this project.
As the larger study was focused on indwelling urethral catheter users, participants with a condom catheter were recruited from only one facility, while those with an indwelling urethral catheter were recruited from both hospitals. Indwelling catheter patients that had a possible contraindication to condom catheter use (such as urinary retention or perioperative use for a surgical procedure) were excluded to make the groups comparable. Any indication for condom catheterization was permitted.
Information about catheter-related complications was collected from two sources: directly from patients and through medical record review. Patients were interviewed at baseline and approximately 14 days and 30 days after catheter placement. The follow-up assessments asked patients about their symptoms and experience over the previous two weeks. We also conducted a medical record review covering the 30 days after initial catheter placement.
Study Measures
Data Analysis
The primary outcome was the percentage of patients who experienced a complication related to a urinary catheter during the 30 days after the catheter was initially placed. Comparisons by group—condom versus indwelling catheter—were conducted using chi-square tests (Fisher’s exact test when necessary) for categorical variables and the Student’s t-test for continuous variables. All analyses were performed using SAS (Cary, North Carolina). All statistical tests were two-sided with alpha set to .05.
RESULTS
Of the 76 patients invited to participate after having a condom catheter placed, 49 consented (64.5%). Of those, 36 had sufficient data for inclusion in this analysis. The comparison group consisted of 44 patients with an indwelling urethral catheter. There were no statistically significant differences between the two groups in terms of age, race, or ethnicity (Table 1). There were statistically significant differences in patient-reported reasons for catheter placement, but these were due to the exclusion criteria used for indwelling urethral catheter patients.
Both patient-reported and clinician-reported (ie, recorded in the patient’s medical record) outcomes are described in Table 2. In total, 80.6% of condom catheter users reported experiencing at least one catheter-related complication during the month after initial catheter placement compared with 88.6% of indwelling catheter users (P = .32). A similar number of condom catheter patients and indwelling urethral catheter patients experienced an infectious complication according to both self-report data (8.3% condom, 6.8% indwelling; P = .99) and medical record review (11.1% condom, 6.8% indwelling; P = .69).
At least one noninfectious complication was identified in 77.8% of condom catheter patients (28 of 36) and 88.6% of indwelling urethral catheter patients (39 of 44) using combined self-report and medical record review data (P = .19); most of these were based on self-reported data. Significantly fewer condom catheter patients reported complications during placement (eg, pain, discomfort, bleeding, or other trauma) compared with those with indwelling catheters (13.9% vs 43.2%, P < .001). Pain, discomfort, bleeding, or other trauma during catheter removal were commonly reported by both condom catheter and indwelling urethral catheter patients (40.9% vs 42.1%, respectively; P = .99).
Patient-reported noninfectious complications were often not documented in the medical record: 75.0% of condom catheter patients and 86.4% of indwelling catheter patients reported complications, in comparison with the 25.0% of condom catheter patients and 27.3% of indwelling urethral catheter patients with noninfectious complications identified during medical record review.
DISCUSSION
Our study revealed three important findings. First, noninfectious complications greatly outnumbered infectious complications, regardless of the device type. Second, condom catheter users reported significantly less pain related to placement of their device compared with the indwelling urethral catheter group. Finally, many patients reported complications that were not documented in the medical record.
The only randomized trial comparing these devices enrolled 75 men hospitalized at a single VA medical center and found that using a condom catheter rather than an indwelling catheter in patients without urinary retention lowered the composite endpoint of bacteriuria, symptomatic UTI, or death.4 Additionally, patients in this trial reported that the condom catheter was significantly more comfortable (90% vs 58%; P = .02) and less painful (5% vs 36%; P = .02) than the indwelling catheter,4 supporting a previous study in hospitalized male Veterans.5
Importantly, we included patient-reported complications that may be of concern to patients but inconsistently documented in the medical record. Pain associated with removal of both condom catheters and indwelling urethral catheters was reported in over 40% in both groups but was not documented in the medical record. One patient with a condom catheter described removal this way: “It got stuck on my hair, so was hard to get off…” Condom catheters also posed some issues with staying in place as has been previously described.6 As one condom catheter user said: “When I was laying down it was okay, but every time I moved around…it would slide off.”
Recent efforts to reduce catheter-associated UTI,7-9 which have focused on reducing the use of indwelling urethral catheters,10,11 have been relatively successful. Clinical policy makers should consider similar efforts to address the noninfectious harms of both catheter types. Such efforts could include further decreasing any type of catheter use along with improved training of those placing such devices.12 Substantial improvement will require a systematic approach to surveilling noninfectious complications of both types of urinary catheters.
Our study has several limitations. First, we conducted the study at two VA hospitals; therefore, the results may not be generalizable to a non-VA population. Second, we only included 80 patients because we recruited a limited number of condom catheter users.
Limitations notwithstanding, we provide comparison data between condom and indwelling urethral catheters. Condom catheter users reported significantly less pain related to initial placement of their device compared with those using an indwelling urethral catheter. For both devices, patients experienced noninfectious complications much more commonly than infectious ones, underscoring the need to systematically address such complications, perhaps through a surveillance system that includes the patient’s perspective. The patient’s voice is important and necessary in view of the apparent underreporting of noninfectious harms in the medical record.
A cknowledgments
Disclaimer
The funding sources played no role in the design, conducting, or evaluation of this study. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Department of Veterans Affairs.
1. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. doi: 10.1086/651091.
2. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. doi: 10.1086/675718.
3. Saint S, Trautner BW, Fowler KE, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.2417.
4. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. doi: 10.1111/j.1532-5415.2006.00785.x.
5. Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453-1457. doi: 10.1111/j.1532-5415.1999.tb01567.x.
6. Smart C. Male urinary incontinence and the urinary sheath. Br J Nurs. 2014;23(9):S20, S22-S25. doi: 10.12968/bjon.2014.23.Sup9.S20.
7. Saint S, Greene MT, Kowalski CP, Watson SR, Hofer TP, Krein SL. Preventing catheter-associated urinary tract infection in the United States: a national comparative study. JAMA Intern Med. 2013;173(10):874-879. doi: 10.1001/jamainternmed.2013.101.
8. Saint S, Greene MT, Krein SL, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111-2119. doi: 10.1056/NEJMoa1504906.
9. Saint S, Fowler KE, Sermak K, et al. Introducing the No preventable harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. doi: 10.1016/j.ajic.2014.11.016.
10. Fakih MG, Watson SR, Greene MT, et al. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172(3):255-260. doi: 10.1001/archinternmed.2011.627.
11. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. doi: 10.1001/jamainternmed.2013.105.
12. Manojlovich M, Saint S, Meddings J, et al. Indwelling urinary catheter insertion practices in the emergency department: an observational study. Infect Control Hosp Epidemiol. 2016;37(1):117-119. doi: 10.1017/ice.2015.238.
13. Meddings JA, Reichert H, Rogers MA, Saint S, Stephansky J, McMahon LF. Effect of nonpayment for hospital-acquired, catheter-associated urinary tract infection: a statewide analysis. Ann Intern Med. 2012;157(5):305-312. doi: 10.7326/0003-4819-157-5-201209040-00003.
Millions of patients use urinary collection devices. For men, both indwelling and condom-style urinary catheters (known as “external catheters”) are commonly used. National infection prevention guidelines recommend condom catheters as a preferred alternative to indwelling catheters for patients without urinary retention1,2 to reduce the risk of catheter-associated urinary tract infection (UTI). Unfortunately, little outcome data comparing condom catheters with indwelling urethral catheters exists. We therefore assessed the incidence of infectious and noninfectious complications in condom catheter and indwelling urethral catheter users.
PATIENTS AND METHODS
Study Overview
As part of a larger prospective, observational study,3 we compared complications in patients who received a condom catheter during hospitalization with those in patients who received an indwelling urethral catheter. Hospitalized patients with either a condom catheter or indwelling urethral catheter were identified at two Veterans Affairs (VA) medical centers and followed for 30 days after initial catheter placement. Patient-reported data were collected during in-person patient interviews at baseline (within three days of catheter placement), and by in-person or phone interviews at 14 days and 30 days postplacement (Supplementary Appendix A and B). Questions were primarily closed-ended, except for a final question inviting open comments. Information about the catheter and any reported complications was also collected from electronic medical record documentation for each patient. Institutional review board approval was received from both participating study sites.
Data Collection and Inclusion Criteria
Hospitalized patients who had a condom or indwelling urethral catheter placed were eligible to participate if they met the following criteria: (1) were hospitalized on an acute care unit; (2) had a new condom catheter or indwelling urethral catheter placed during this hospital stay that was not present on admission; (3) had a device in place for three days or less; (4) were at least 18 years old; and (5) were able to speak English. Patients were excluded if they: (1) did not have the capacity to give consent or participate in the interview/assessment process; (2) refused to provide written informed consent to participate; or (3) had previously participated in this project.
As the larger study was focused on indwelling urethral catheter users, participants with a condom catheter were recruited from only one facility, while those with an indwelling urethral catheter were recruited from both hospitals. Indwelling catheter patients that had a possible contraindication to condom catheter use (such as urinary retention or perioperative use for a surgical procedure) were excluded to make the groups comparable. Any indication for condom catheterization was permitted.
Information about catheter-related complications was collected from two sources: directly from patients and through medical record review. Patients were interviewed at baseline and approximately 14 days and 30 days after catheter placement. The follow-up assessments asked patients about their symptoms and experience over the previous two weeks. We also conducted a medical record review covering the 30 days after initial catheter placement.
Study Measures
Data Analysis
The primary outcome was the percentage of patients who experienced a complication related to a urinary catheter during the 30 days after the catheter was initially placed. Comparisons by group—condom versus indwelling catheter—were conducted using chi-square tests (Fisher’s exact test when necessary) for categorical variables and the Student’s t-test for continuous variables. All analyses were performed using SAS (Cary, North Carolina). All statistical tests were two-sided with alpha set to .05.
RESULTS
Of the 76 patients invited to participate after having a condom catheter placed, 49 consented (64.5%). Of those, 36 had sufficient data for inclusion in this analysis. The comparison group consisted of 44 patients with an indwelling urethral catheter. There were no statistically significant differences between the two groups in terms of age, race, or ethnicity (Table 1). There were statistically significant differences in patient-reported reasons for catheter placement, but these were due to the exclusion criteria used for indwelling urethral catheter patients.
Both patient-reported and clinician-reported (ie, recorded in the patient’s medical record) outcomes are described in Table 2. In total, 80.6% of condom catheter users reported experiencing at least one catheter-related complication during the month after initial catheter placement compared with 88.6% of indwelling catheter users (P = .32). A similar number of condom catheter patients and indwelling urethral catheter patients experienced an infectious complication according to both self-report data (8.3% condom, 6.8% indwelling; P = .99) and medical record review (11.1% condom, 6.8% indwelling; P = .69).
At least one noninfectious complication was identified in 77.8% of condom catheter patients (28 of 36) and 88.6% of indwelling urethral catheter patients (39 of 44) using combined self-report and medical record review data (P = .19); most of these were based on self-reported data. Significantly fewer condom catheter patients reported complications during placement (eg, pain, discomfort, bleeding, or other trauma) compared with those with indwelling catheters (13.9% vs 43.2%, P < .001). Pain, discomfort, bleeding, or other trauma during catheter removal were commonly reported by both condom catheter and indwelling urethral catheter patients (40.9% vs 42.1%, respectively; P = .99).
Patient-reported noninfectious complications were often not documented in the medical record: 75.0% of condom catheter patients and 86.4% of indwelling catheter patients reported complications, in comparison with the 25.0% of condom catheter patients and 27.3% of indwelling urethral catheter patients with noninfectious complications identified during medical record review.
DISCUSSION
Our study revealed three important findings. First, noninfectious complications greatly outnumbered infectious complications, regardless of the device type. Second, condom catheter users reported significantly less pain related to placement of their device compared with the indwelling urethral catheter group. Finally, many patients reported complications that were not documented in the medical record.
The only randomized trial comparing these devices enrolled 75 men hospitalized at a single VA medical center and found that using a condom catheter rather than an indwelling catheter in patients without urinary retention lowered the composite endpoint of bacteriuria, symptomatic UTI, or death.4 Additionally, patients in this trial reported that the condom catheter was significantly more comfortable (90% vs 58%; P = .02) and less painful (5% vs 36%; P = .02) than the indwelling catheter,4 supporting a previous study in hospitalized male Veterans.5
Importantly, we included patient-reported complications that may be of concern to patients but inconsistently documented in the medical record. Pain associated with removal of both condom catheters and indwelling urethral catheters was reported in over 40% in both groups but was not documented in the medical record. One patient with a condom catheter described removal this way: “It got stuck on my hair, so was hard to get off…” Condom catheters also posed some issues with staying in place as has been previously described.6 As one condom catheter user said: “When I was laying down it was okay, but every time I moved around…it would slide off.”
Recent efforts to reduce catheter-associated UTI,7-9 which have focused on reducing the use of indwelling urethral catheters,10,11 have been relatively successful. Clinical policy makers should consider similar efforts to address the noninfectious harms of both catheter types. Such efforts could include further decreasing any type of catheter use along with improved training of those placing such devices.12 Substantial improvement will require a systematic approach to surveilling noninfectious complications of both types of urinary catheters.
Our study has several limitations. First, we conducted the study at two VA hospitals; therefore, the results may not be generalizable to a non-VA population. Second, we only included 80 patients because we recruited a limited number of condom catheter users.
Limitations notwithstanding, we provide comparison data between condom and indwelling urethral catheters. Condom catheter users reported significantly less pain related to initial placement of their device compared with those using an indwelling urethral catheter. For both devices, patients experienced noninfectious complications much more commonly than infectious ones, underscoring the need to systematically address such complications, perhaps through a surveillance system that includes the patient’s perspective. The patient’s voice is important and necessary in view of the apparent underreporting of noninfectious harms in the medical record.
A cknowledgments
Disclaimer
The funding sources played no role in the design, conducting, or evaluation of this study. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Department of Veterans Affairs.
Millions of patients use urinary collection devices. For men, both indwelling and condom-style urinary catheters (known as “external catheters”) are commonly used. National infection prevention guidelines recommend condom catheters as a preferred alternative to indwelling catheters for patients without urinary retention1,2 to reduce the risk of catheter-associated urinary tract infection (UTI). Unfortunately, little outcome data comparing condom catheters with indwelling urethral catheters exists. We therefore assessed the incidence of infectious and noninfectious complications in condom catheter and indwelling urethral catheter users.
PATIENTS AND METHODS
Study Overview
As part of a larger prospective, observational study,3 we compared complications in patients who received a condom catheter during hospitalization with those in patients who received an indwelling urethral catheter. Hospitalized patients with either a condom catheter or indwelling urethral catheter were identified at two Veterans Affairs (VA) medical centers and followed for 30 days after initial catheter placement. Patient-reported data were collected during in-person patient interviews at baseline (within three days of catheter placement), and by in-person or phone interviews at 14 days and 30 days postplacement (Supplementary Appendix A and B). Questions were primarily closed-ended, except for a final question inviting open comments. Information about the catheter and any reported complications was also collected from electronic medical record documentation for each patient. Institutional review board approval was received from both participating study sites.
Data Collection and Inclusion Criteria
Hospitalized patients who had a condom or indwelling urethral catheter placed were eligible to participate if they met the following criteria: (1) were hospitalized on an acute care unit; (2) had a new condom catheter or indwelling urethral catheter placed during this hospital stay that was not present on admission; (3) had a device in place for three days or less; (4) were at least 18 years old; and (5) were able to speak English. Patients were excluded if they: (1) did not have the capacity to give consent or participate in the interview/assessment process; (2) refused to provide written informed consent to participate; or (3) had previously participated in this project.
As the larger study was focused on indwelling urethral catheter users, participants with a condom catheter were recruited from only one facility, while those with an indwelling urethral catheter were recruited from both hospitals. Indwelling catheter patients that had a possible contraindication to condom catheter use (such as urinary retention or perioperative use for a surgical procedure) were excluded to make the groups comparable. Any indication for condom catheterization was permitted.
Information about catheter-related complications was collected from two sources: directly from patients and through medical record review. Patients were interviewed at baseline and approximately 14 days and 30 days after catheter placement. The follow-up assessments asked patients about their symptoms and experience over the previous two weeks. We also conducted a medical record review covering the 30 days after initial catheter placement.
Study Measures
Data Analysis
The primary outcome was the percentage of patients who experienced a complication related to a urinary catheter during the 30 days after the catheter was initially placed. Comparisons by group—condom versus indwelling catheter—were conducted using chi-square tests (Fisher’s exact test when necessary) for categorical variables and the Student’s t-test for continuous variables. All analyses were performed using SAS (Cary, North Carolina). All statistical tests were two-sided with alpha set to .05.
RESULTS
Of the 76 patients invited to participate after having a condom catheter placed, 49 consented (64.5%). Of those, 36 had sufficient data for inclusion in this analysis. The comparison group consisted of 44 patients with an indwelling urethral catheter. There were no statistically significant differences between the two groups in terms of age, race, or ethnicity (Table 1). There were statistically significant differences in patient-reported reasons for catheter placement, but these were due to the exclusion criteria used for indwelling urethral catheter patients.
Both patient-reported and clinician-reported (ie, recorded in the patient’s medical record) outcomes are described in Table 2. In total, 80.6% of condom catheter users reported experiencing at least one catheter-related complication during the month after initial catheter placement compared with 88.6% of indwelling catheter users (P = .32). A similar number of condom catheter patients and indwelling urethral catheter patients experienced an infectious complication according to both self-report data (8.3% condom, 6.8% indwelling; P = .99) and medical record review (11.1% condom, 6.8% indwelling; P = .69).
At least one noninfectious complication was identified in 77.8% of condom catheter patients (28 of 36) and 88.6% of indwelling urethral catheter patients (39 of 44) using combined self-report and medical record review data (P = .19); most of these were based on self-reported data. Significantly fewer condom catheter patients reported complications during placement (eg, pain, discomfort, bleeding, or other trauma) compared with those with indwelling catheters (13.9% vs 43.2%, P < .001). Pain, discomfort, bleeding, or other trauma during catheter removal were commonly reported by both condom catheter and indwelling urethral catheter patients (40.9% vs 42.1%, respectively; P = .99).
Patient-reported noninfectious complications were often not documented in the medical record: 75.0% of condom catheter patients and 86.4% of indwelling catheter patients reported complications, in comparison with the 25.0% of condom catheter patients and 27.3% of indwelling urethral catheter patients with noninfectious complications identified during medical record review.
DISCUSSION
Our study revealed three important findings. First, noninfectious complications greatly outnumbered infectious complications, regardless of the device type. Second, condom catheter users reported significantly less pain related to placement of their device compared with the indwelling urethral catheter group. Finally, many patients reported complications that were not documented in the medical record.
The only randomized trial comparing these devices enrolled 75 men hospitalized at a single VA medical center and found that using a condom catheter rather than an indwelling catheter in patients without urinary retention lowered the composite endpoint of bacteriuria, symptomatic UTI, or death.4 Additionally, patients in this trial reported that the condom catheter was significantly more comfortable (90% vs 58%; P = .02) and less painful (5% vs 36%; P = .02) than the indwelling catheter,4 supporting a previous study in hospitalized male Veterans.5
Importantly, we included patient-reported complications that may be of concern to patients but inconsistently documented in the medical record. Pain associated with removal of both condom catheters and indwelling urethral catheters was reported in over 40% in both groups but was not documented in the medical record. One patient with a condom catheter described removal this way: “It got stuck on my hair, so was hard to get off…” Condom catheters also posed some issues with staying in place as has been previously described.6 As one condom catheter user said: “When I was laying down it was okay, but every time I moved around…it would slide off.”
Recent efforts to reduce catheter-associated UTI,7-9 which have focused on reducing the use of indwelling urethral catheters,10,11 have been relatively successful. Clinical policy makers should consider similar efforts to address the noninfectious harms of both catheter types. Such efforts could include further decreasing any type of catheter use along with improved training of those placing such devices.12 Substantial improvement will require a systematic approach to surveilling noninfectious complications of both types of urinary catheters.
Our study has several limitations. First, we conducted the study at two VA hospitals; therefore, the results may not be generalizable to a non-VA population. Second, we only included 80 patients because we recruited a limited number of condom catheter users.
Limitations notwithstanding, we provide comparison data between condom and indwelling urethral catheters. Condom catheter users reported significantly less pain related to initial placement of their device compared with those using an indwelling urethral catheter. For both devices, patients experienced noninfectious complications much more commonly than infectious ones, underscoring the need to systematically address such complications, perhaps through a surveillance system that includes the patient’s perspective. The patient’s voice is important and necessary in view of the apparent underreporting of noninfectious harms in the medical record.
A cknowledgments
Disclaimer
The funding sources played no role in the design, conducting, or evaluation of this study. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Department of Veterans Affairs.
1. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. doi: 10.1086/651091.
2. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. doi: 10.1086/675718.
3. Saint S, Trautner BW, Fowler KE, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.2417.
4. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. doi: 10.1111/j.1532-5415.2006.00785.x.
5. Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453-1457. doi: 10.1111/j.1532-5415.1999.tb01567.x.
6. Smart C. Male urinary incontinence and the urinary sheath. Br J Nurs. 2014;23(9):S20, S22-S25. doi: 10.12968/bjon.2014.23.Sup9.S20.
7. Saint S, Greene MT, Kowalski CP, Watson SR, Hofer TP, Krein SL. Preventing catheter-associated urinary tract infection in the United States: a national comparative study. JAMA Intern Med. 2013;173(10):874-879. doi: 10.1001/jamainternmed.2013.101.
8. Saint S, Greene MT, Krein SL, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111-2119. doi: 10.1056/NEJMoa1504906.
9. Saint S, Fowler KE, Sermak K, et al. Introducing the No preventable harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. doi: 10.1016/j.ajic.2014.11.016.
10. Fakih MG, Watson SR, Greene MT, et al. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172(3):255-260. doi: 10.1001/archinternmed.2011.627.
11. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. doi: 10.1001/jamainternmed.2013.105.
12. Manojlovich M, Saint S, Meddings J, et al. Indwelling urinary catheter insertion practices in the emergency department: an observational study. Infect Control Hosp Epidemiol. 2016;37(1):117-119. doi: 10.1017/ice.2015.238.
13. Meddings JA, Reichert H, Rogers MA, Saint S, Stephansky J, McMahon LF. Effect of nonpayment for hospital-acquired, catheter-associated urinary tract infection: a statewide analysis. Ann Intern Med. 2012;157(5):305-312. doi: 10.7326/0003-4819-157-5-201209040-00003.
1. Gould CV, Umscheid CA, Agarwal RK, Kuntz G, Pegues DA, Healthcare Infection Control Practices Advisory Committee. Guideline for prevention of catheter-associated urinary tract infections 2009. Infect Control Hosp Epidemiol. 2010;31(4):319-326. doi: 10.1086/651091.
2. Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(5):464-479. doi: 10.1086/675718.
3. Saint S, Trautner BW, Fowler KE, et al. A multicenter study of patient-reported infectious and noninfectious complications associated with indwelling urethral catheters. JAMA Intern Med. 2018. doi:10.1001/jamainternmed.2018.2417.
4. Saint S, Kaufman SR, Rogers MA, Baker PD, Ossenkop K, Lipsky BA. Condom versus indwelling urinary catheters: a randomized trial. J Am Geriatr Soc. 2006;54(7):1055-1061. doi: 10.1111/j.1532-5415.2006.00785.x.
5. Saint S, Lipsky BA, Baker PD, McDonald LL, Ossenkop K. Urinary catheters: what type do men and their nurses prefer? J Am Geriatr Soc. 1999;47(12):1453-1457. doi: 10.1111/j.1532-5415.1999.tb01567.x.
6. Smart C. Male urinary incontinence and the urinary sheath. Br J Nurs. 2014;23(9):S20, S22-S25. doi: 10.12968/bjon.2014.23.Sup9.S20.
7. Saint S, Greene MT, Kowalski CP, Watson SR, Hofer TP, Krein SL. Preventing catheter-associated urinary tract infection in the United States: a national comparative study. JAMA Intern Med. 2013;173(10):874-879. doi: 10.1001/jamainternmed.2013.101.
8. Saint S, Greene MT, Krein SL, et al. A program to prevent catheter-associated urinary tract infection in acute care. N Engl J Med. 2016;374(22):2111-2119. doi: 10.1056/NEJMoa1504906.
9. Saint S, Fowler KE, Sermak K, et al. Introducing the No preventable harms campaign: creating the safest health care system in the world, starting with catheter-associated urinary tract infection prevention. Am J Infect Control. 2015;43(3):254-259. doi: 10.1016/j.ajic.2014.11.016.
10. Fakih MG, Watson SR, Greene MT, et al. Reducing inappropriate urinary catheter use: a statewide effort. Arch Intern Med. 2012;172(3):255-260. doi: 10.1001/archinternmed.2011.627.
11. Krein SL, Kowalski CP, Harrod M, Forman J, Saint S. Barriers to reducing urinary catheter use: a qualitative assessment of a statewide initiative. JAMA Intern Med. 2013;173(10):881-886. doi: 10.1001/jamainternmed.2013.105.
12. Manojlovich M, Saint S, Meddings J, et al. Indwelling urinary catheter insertion practices in the emergency department: an observational study. Infect Control Hosp Epidemiol. 2016;37(1):117-119. doi: 10.1017/ice.2015.238.
13. Meddings JA, Reichert H, Rogers MA, Saint S, Stephansky J, McMahon LF. Effect of nonpayment for hospital-acquired, catheter-associated urinary tract infection: a statewide analysis. Ann Intern Med. 2012;157(5):305-312. doi: 10.7326/0003-4819-157-5-201209040-00003.
© 2019 Society of Hospital Medicine