User login
Less Lumens-Less Risk: A Pilot Intervention to Increase the Use of Single-Lumen Peripherally Inserted Central Catheters
Vascular access is a cornerstone of safe and effective medical care. The use of peripherally inserted central catheters (PICCs) to meet vascular access needs has recently increased.1,2 PICCs offer several advantages over other central venous catheters. These advantages include increased reliability over intermediate to long-term use and reductions in complication rates during insertion.3,4
Multiple studies have suggested a strong association between the number of PICC lumens and risk of complications, such as central-line associated bloodstream infection (CLABSI), venous thrombosis, and catheter occlusion.5-8,9,10-12 These complications may lead to device failure, interrupt therapy, prolonged length of stay, and increased healthcare costs.13-15 Thus, available guidelines recommend using PICCs with the least clinically necessary number of lumens.1,16 Quality improvement strategies that have targeted decreasing the number of PICC lumens have reduced complications and healthcare costs.17-19 However, variability exists in the selection of the number of PICC lumens, and many providers request multilumen devices “just in case” additional lumens are needed.20,21 Such variation in device selection may stem from the paucity of information that defines the appropriate indications for the use of single- versus multi-lumen PICCs.
Therefore, to ensure appropriateness of PICC use, we designed an intervention to improve selection of the number of PICC lumens.
METHODS
We conducted this pre–post quasi-experimental study in accordance with SQUIRE guidelines.22 Details regarding clinical parameters associated with the decision to place a PICC, patient characteristics, comorbidities, complications, and laboratory values were collected from the medical records of patients. All PICCs were placed by the Vascular Access Service Team (VAST) during the study period.
Intervention
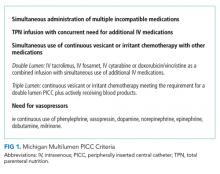
The intervention consisted of three components: first, all hospitalists, pharmacists, and VAST nurses received education in the form of a CME lecture that emphasized use of the Michigan Appropriateness Guide for Intravenous Catheters (MAGIC).1 These criteria define when use of a PICC is appropriate and emphasize how best to select the most appropriate device characteristics such as lumens and catheter gauge. Next, a multidisciplinary task force that consisted of hospitalists, VAST nurses, and pharmacists developed a list of indications specifying when use of a multilumen PICC was appropriate.1 Third, the order for a PICC in our electronic medical record (EMR) system was modified to set single-lumen PICCs as default. If a multilumen PICC was requested, text-based justification from the ordering clinician was required.
As an additional safeguard, a VAST nurse reviewed the number of lumens and clinical scenario for each PICC order prior to insertion. If the number of lumens ordered was considered inappropriate on the basis of the developed list of MAGIC recommendations, the case was referred to a pharmacist for additional review. The pharmacist then reviewed active and anticipated medications, explored options for adjusting the medication delivery plan, and discussed these options with the ordering clinician to determine the most appropriate number of lumens.
Measures and Definitions
In accordance with the criteria set by the Centers for Disease Control National Healthcare Safety Network,23 CLABSI was defined as a confirmed positive blood culture with a PICC in place for 48 hours or longer without another identified infection source or a positive PICC tip culture in the setting of clinically suspected infection. Venous thrombosis was defined as symptomatic upper extremity deep vein thromboembolism or pulmonary embolism that was radiographically confirmed after the placement of a PICC or within one week of device removal. Catheter occlusion was captured when documented or when tPA was administered for problems related to the PICC. The appropriateness of the number of PICC lumens was independently adjudicated by an attending physician and clinical pharmacist by comparing the indications of the device placed against predefined appropriateness criteria.
Outcomes
The primary outcome of interest was the change in the proportion of single-lumen PICCs placed. Secondary outcomes included (1) the placement of PICCs with an appropriate number of lumens, (2) the occurrence of PICC-related complications (CLABSI, venous thrombosis, and catheter occlusion), and (3) the need for a second procedure to place a multilumen device or additional vascular access.
Statistical Analysis
Descriptive statistics were used to tabulate and summarize patient and PICC characteristics. Differences between pre- and postintervention populations were assessed using χ2, Fishers exact, t-, and Wilcoxon rank sum tests. Differences in complications were assessed using the two-sample tests of proportions. Results were reported as medians (IQR) and percentages with corresponding 95% confidence intervals. All statistical tests were two-sided, with P < .05 considered statistically significant. Analyses were conducted with Stata v.14 (stataCorp, College Station, Texas).
Ethical and Regulatory Oversight
This study was approved by the Institutional Review Board at the University of Michigan (IRB#HUM00118168).
RESULTS
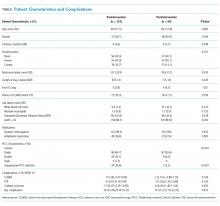
Of the 133 PICCs placed preintervention, 64.7% (n = 86) were single lumen, 33.1% (n = 44) were double lumen, and 2.3% (n = 3) were triple lumen. Compared with the preintervention period, the use of single-lumen PICCs significantly increased following the intervention (64.7% to 93.6%; P < .001; Figure 1). As well, the proportion of PICCs with an inappropriate number of lumens decreased from 25.6% to 2.2% (P < .001; Table 1).
Preintervention, 14.3% (95% CI = 8.34-20.23) of the patients with PICCs experienced at least one complication (n = 19). Following the intervention, 15.1% (95% CI = 7.79-22.32) of the 93 patients with PICCs experienced at least one complication (absolute difference = 0.8%, P = .872). With respect to individual complications, CLABSI decreased from 5.3% (n = 7; 95% CI = 1.47-9.06) to 2.2% (n = 2; 95% CI = −0.80-5.10) (P = .239). Similarly, the incidence of catheter occlusion decreased from 8.3% (n = 11; 95% CI = 3.59-12.95) to 6.5% (n = 6; 95% CI = 1.46-11.44; P = .610; Table). Notably, only 12.1% (n = 21) of patients with a single-lumen PICC experienced any complication, whereas 20.0% (n = 10) of patients with a double lumen, and 66.7% (n = 2) with a triple lumen experienced a PICC-associated complication (P = .022). Patients with triple lumens had a significantly higher incidence of catheter occlusion compared with patients that received double- and single-lumen PICCs (66.7% vs. 12.0% and 5.2%, respectively; P = .003).
No patient who received a single-lumen device required a second procedure for the placement of a device with additional lumens. Similarly, no documentation suggesting an insufficient number of PICC lumens or the need for additional vascular access (eg, placement of additional PICCs) was found in medical records of patients postintervention. Pharmacists supporting the interventions and VAST team members reported no disagreements when discussing number of lumens or appropriateness of catheter choice.
DISCUSSION
In this single center, pre–post quasi-experimental study, a multimodal intervention based on the MAGIC criteria significantly reduced the use of multilumen PICCs. Additionally, a trend toward reductions in complications, including CLABSI and catheter occlusion, was also observed. Notably, these changes in ordering practices did not lead to requests for additional devices or replacement with a multilumen PICC when a single-lumen device was inserted. Collectively, our findings suggest that the use of single-lumen devices in a large direct care service can be feasibly and safely increased through this approach. Larger scale studies that implement MAGIC to inform placement of multilumen PICCs and reduce PICC-related complications now appear necessary.
The presence of a PICC, even for short periods, significantly increases the risk of CLABSI and is one of the strongest predictors of venous thrombosis risk in the hospital setting.19,24,25 Although some factors that lead to this increased risk are patient-related and not modifiable (eg, malignancy or intensive care unit status), increased risk linked to the gauge of PICCs and the number of PICC lumens can be modified by improving device selection.9,18,26 Deliberate use of PICCs with the least numbers of clinically necessary lumens decreases risk of CLABSI, venous thrombosis and overall cost.17,19,26 Additionally, greater rates of occlusion with each additional PICC lumen may result in the interruption of intravenous therapy, the administration of costly medications (eg, tissue plasminogen activator) to salvage the PICC, and premature removal of devices should the occlusion prove irreversible.8
We observed a trend toward decreased PICC complications following implementation of our criteria, especially for the outcomes of CLABSI and catheter occlusion. Given the pilot nature of this study, we were underpowered to detect a statistically significant change in PICC adverse events. However, we did observe a statistically significant increase in the rate of single-lumen PICC use following our intervention. Notably, this increase occurred in the setting of high rates of single-lumen PICC use at baseline (64%). Therefore, an important takeaway from our findings is that room for improving PICC appropriateness exists even among high performers. This finding In turn, high baseline use of single-lumen PICCs may also explain why a robust reduction in PICC complications was not observed in our study, given that other studies showing reduction in the rates of complications began with considerably low rates of single-lumen device use.19 Outcomes may improve, however, if we expand and sustain these changes or expand to larger settings. For example, (based on assumptions from a previously published simulation study and our average hospital medicine daily census of 98 patients) the increased use of single-over multilumen PICCs is expected to decrease CLABSI events and venous thrombosis episodes by 2.4-fold in our hospital medicine service with an associated cost savings of $74,300 each year.17 Additionally, we would also expect the increase in the proportion of single-lumen PICCs to reduce rates of catheter occlusion. This reduction, in turn, would lessen interruptions in intravenous therapy, the need for medications to treat occlusion, and the need for device replacement all leading to reduced costs.27 Overall, then, our intervention (informed by appropriateness criteria) provides substantial benefits to hospital savings and patient safety.
After our intervention, 98% of all PICCs placed were found to comply with appropriate criteria for multilumen PICC use. We unexpectedly found that the most important factor driving our findings was not oversight or order modification by the pharmacy team or VAST nurses, but rather better decisions made by physicians at the outset. Specifically, we did not find a single instance wherein the original PICC order was changed to a device with a different number of lumens after review from the VAST team. We attribute this finding to receptiveness of physicians to change ordering practices following education and the redesign of the default EMR PICC order, both of which provided a scientific rationale for multilumen PICC use. Clarifying the risk and criteria of the use of multilumen devices along with providing an EMR ordering process that supports best practice helped hospitalists “do the right thing”. Additionally, setting single-lumen devices as the preselected EMR order and requiring text-based justification for placement of a multilumen PICC helped provide a nudge to physicians, much as it has done with antibiotic choices.28
Our study has limitations. First, we were only able to identify complications that were captured by our EMR. Given that over 70% of the patients in our study were discharged with a PICC in place, we do not know whether complications may have developed outside the hospital. Second, our intervention was resource intensive and required partnership with pharmacy, VAST, and hospitalists. Thus, the generalizability of our intervention to other institutions without similar support is unclear. Third, despite an increase in the use of single-lumen PICCs and a decrease in multilumen devices, we did not observe a significant reduction in all types of complications. While our high rate of single-lumen PICC use may account for these findings, larger scale studies are needed to better study the impact of MAGIC and appropriateness criteria on PICC complications. Finally, given our approach, we cannot identify the most effective modality within our bundled intervention. Stepped wedge or single-component studies are needed to further address this question.
In conclusion, we piloted a multimodal intervention to promote the use of single-lumen PICCs while lowering the use of multilumen devices. By using MAGIC to create appropriate indications, the use of multilumen PICCs declined and complications trended downwards. Larger, multicenter studies to validate our findings and examine the sustainability of this intervention would be welcomed.
Disclosures
The authors have nothing to disclose.
1. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. doi: 10.7326/M15-0744. PubMed
2. Taylor RW, Palagiri AV. Central venous catheterization. Crit Care Med. 2007;35(5):1390-1396. doi: 10.1097/01.CCM.0000260241.80346.1B. PubMed
3. Pikwer A, Akeson J, Lindgren S. Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65-71. doi: 10.1111/j.1365-2044.2011.06911.x. PubMed
4. Johansson E, Hammarskjold F, Lundberg D, Arnlind MH. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Onco. 2013;52(5):886-892. doi: 10.3109/0284186X.2013.773072. PubMed
5. Pan L, Zhao Q, Yang X. Risk factors for venous thrombosis associated with peripherally inserted central venous catheters. Int J Clin Exp Med. 2014;7(12):5814-5819. PubMed
6. Herc E, Patel P, Washer LL, Conlon A, Flanders SA, Chopra V. A model to predict central-line-associated bloodstream infection among patients with peripherally inserted central catheters: The MPC score. Infect Cont Hosp Ep. 2017;38(10):1155-1166. doi: 10.1017/ice.2017.167. PubMed
7. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–1171. doi: 10.4065/81.9.1159. PubMed
8. Smith SN, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e742. doi: 10.1016/j.jvir.2017.02.005. PubMed
9. Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-325. doi: 10.1016/S0140-6736(13)60592-9. PubMed
10. Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014;12(6):847-854. doi: 10.1111/jth.12549. PubMed
11. Carter JH, Langley JM, Kuhle S, Kirkland S. Risk factors for central venous catheter-associated bloodstream infection in pediatric patients: A cohort study. Infect Control Hosp Epidemiol. 2016;37(8):939-945. doi: 10.1017/ice.2016.83. PubMed
12. Chopra V, Ratz D, Kuhn L, Lopus T, Chenoweth C, Krein S. PICC-associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319-328. doi: 10.1016/j.amjmed.2014.01.001. PubMed
13. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193. doi: 10.1093/cid/cir257. PubMed
14. Parkinson R, Gandhi M, Harper J, Archibald C. Establishing an ultrasound guided peripherally inserted central catheter (PICC) insertion service. Clin Radiol. 1998;53(1):33-36. doi: 10.1016/S0009-9260(98)80031-7. PubMed
15. Shannon RP, Patel B, Cummins D, Shannon AH, Ganguli G, Lu Y. Economics of central line--associated bloodstream infections. Am J Med Qual. 2006;21(6 Suppl):7s–16s. doi: 10.1177/1062860606294631. PubMed
16. Mermis JD, Strom JC, Greenwood JP, et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404-1410. doi: 10.1513/AnnalsATS.201404-175OC. PubMed
17. Ratz D, Hofer T, Flanders SA, Saint S, Chopra V. Limiting the number of lumens in peripherally inserted central catheters to improve outcomes and reduce cost: A simulation study. Infect Control Hosp Epidemiol. 2016;37(7):811-817. doi: 10.1017/ice.2016.55. PubMed
18. Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733-741. doi: 10.1016/j.amjmed.2012.04.010. PubMed
19. O’Brien J, Paquet F, Lindsay R, Valenti D. Insertion of PICCs with minimum number of lumens reduces complications and costs. J Am Coll Radiol. 2013;10(11):864-868. doi: 10.1016/j.jacr.2013.06.003. PubMed
20. Tiwari MM, Hermsen ED, Charlton ME, Anderson JR, Rupp ME. Inappropriate intravascular device use: a prospective study. J Hosp Infect. 2011;78(2):128-132. doi: 10.1016/j.jhin.2011.03.004. PubMed
21. Chopra V, Kuhn L, Flanders SA, Saint S, Krein SL. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: results of a national survey. J Hosp Med. 2013;8(11):635-638. doi: 10.1002/jhm.2095. PubMed
22. Goodman D, Ogrinc G, Davies L, et al. Explanation and elaboration of the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, V.2.0: examples of SQUIRE elements in the healthcare improvement literature. BMJ Qual Saf. 2016;25(12):e7. doi: 10.1136/bmjqs-2015-004480. PubMed
23. CDC Bloodstream Infection/Device Associated Infection Module. https://wwwcdcgov/nhsn/pdfs/pscmanual/4psc_clabscurrentpdf 2017. Accessed April 11, 2017.
24. Woller SC, Stevens SM, Jones JP, et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947-954.e2. doi: 10.1016/j.amjmed.2011.06.004. PubMed
25. Paje D, Conlon A, Kaatz S, et al. Patterns and predictors of short-term peripherally inserted central catheter use: A multicenter prospective cohort study. J Hosp Med. 2018;13(2):76-82. doi: 10.12788/jhm.2847. PubMed
26. Evans RS, Sharp JH, Linford LH, et al. Reduction of peripherally inserted central catheter-associated DVT. Chest. 2013;143(3):627-633. doi: 10.1378/chest.12-0923. PubMed
27. Smith S, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e2. doi: 10.1016/j.jvir.2017.02.005. PubMed
28. Vaughn VM, Linder JA. Thoughtless design of the electronic health record drives overuse, but purposeful design can nudge improved patient care. BMJ Qual Saf. 2018;27(8):583-586. doi: 10.1136/bmjqs-2017-007578. PubMed
Vascular access is a cornerstone of safe and effective medical care. The use of peripherally inserted central catheters (PICCs) to meet vascular access needs has recently increased.1,2 PICCs offer several advantages over other central venous catheters. These advantages include increased reliability over intermediate to long-term use and reductions in complication rates during insertion.3,4
Multiple studies have suggested a strong association between the number of PICC lumens and risk of complications, such as central-line associated bloodstream infection (CLABSI), venous thrombosis, and catheter occlusion.5-8,9,10-12 These complications may lead to device failure, interrupt therapy, prolonged length of stay, and increased healthcare costs.13-15 Thus, available guidelines recommend using PICCs with the least clinically necessary number of lumens.1,16 Quality improvement strategies that have targeted decreasing the number of PICC lumens have reduced complications and healthcare costs.17-19 However, variability exists in the selection of the number of PICC lumens, and many providers request multilumen devices “just in case” additional lumens are needed.20,21 Such variation in device selection may stem from the paucity of information that defines the appropriate indications for the use of single- versus multi-lumen PICCs.
Therefore, to ensure appropriateness of PICC use, we designed an intervention to improve selection of the number of PICC lumens.
METHODS
We conducted this pre–post quasi-experimental study in accordance with SQUIRE guidelines.22 Details regarding clinical parameters associated with the decision to place a PICC, patient characteristics, comorbidities, complications, and laboratory values were collected from the medical records of patients. All PICCs were placed by the Vascular Access Service Team (VAST) during the study period.
Intervention
The intervention consisted of three components: first, all hospitalists, pharmacists, and VAST nurses received education in the form of a CME lecture that emphasized use of the Michigan Appropriateness Guide for Intravenous Catheters (MAGIC).1 These criteria define when use of a PICC is appropriate and emphasize how best to select the most appropriate device characteristics such as lumens and catheter gauge. Next, a multidisciplinary task force that consisted of hospitalists, VAST nurses, and pharmacists developed a list of indications specifying when use of a multilumen PICC was appropriate.1 Third, the order for a PICC in our electronic medical record (EMR) system was modified to set single-lumen PICCs as default. If a multilumen PICC was requested, text-based justification from the ordering clinician was required.
As an additional safeguard, a VAST nurse reviewed the number of lumens and clinical scenario for each PICC order prior to insertion. If the number of lumens ordered was considered inappropriate on the basis of the developed list of MAGIC recommendations, the case was referred to a pharmacist for additional review. The pharmacist then reviewed active and anticipated medications, explored options for adjusting the medication delivery plan, and discussed these options with the ordering clinician to determine the most appropriate number of lumens.
Measures and Definitions
In accordance with the criteria set by the Centers for Disease Control National Healthcare Safety Network,23 CLABSI was defined as a confirmed positive blood culture with a PICC in place for 48 hours or longer without another identified infection source or a positive PICC tip culture in the setting of clinically suspected infection. Venous thrombosis was defined as symptomatic upper extremity deep vein thromboembolism or pulmonary embolism that was radiographically confirmed after the placement of a PICC or within one week of device removal. Catheter occlusion was captured when documented or when tPA was administered for problems related to the PICC. The appropriateness of the number of PICC lumens was independently adjudicated by an attending physician and clinical pharmacist by comparing the indications of the device placed against predefined appropriateness criteria.
Outcomes
The primary outcome of interest was the change in the proportion of single-lumen PICCs placed. Secondary outcomes included (1) the placement of PICCs with an appropriate number of lumens, (2) the occurrence of PICC-related complications (CLABSI, venous thrombosis, and catheter occlusion), and (3) the need for a second procedure to place a multilumen device or additional vascular access.
Statistical Analysis
Descriptive statistics were used to tabulate and summarize patient and PICC characteristics. Differences between pre- and postintervention populations were assessed using χ2, Fishers exact, t-, and Wilcoxon rank sum tests. Differences in complications were assessed using the two-sample tests of proportions. Results were reported as medians (IQR) and percentages with corresponding 95% confidence intervals. All statistical tests were two-sided, with P < .05 considered statistically significant. Analyses were conducted with Stata v.14 (stataCorp, College Station, Texas).
Ethical and Regulatory Oversight
This study was approved by the Institutional Review Board at the University of Michigan (IRB#HUM00118168).
RESULTS
Of the 133 PICCs placed preintervention, 64.7% (n = 86) were single lumen, 33.1% (n = 44) were double lumen, and 2.3% (n = 3) were triple lumen. Compared with the preintervention period, the use of single-lumen PICCs significantly increased following the intervention (64.7% to 93.6%; P < .001; Figure 1). As well, the proportion of PICCs with an inappropriate number of lumens decreased from 25.6% to 2.2% (P < .001; Table 1).
Preintervention, 14.3% (95% CI = 8.34-20.23) of the patients with PICCs experienced at least one complication (n = 19). Following the intervention, 15.1% (95% CI = 7.79-22.32) of the 93 patients with PICCs experienced at least one complication (absolute difference = 0.8%, P = .872). With respect to individual complications, CLABSI decreased from 5.3% (n = 7; 95% CI = 1.47-9.06) to 2.2% (n = 2; 95% CI = −0.80-5.10) (P = .239). Similarly, the incidence of catheter occlusion decreased from 8.3% (n = 11; 95% CI = 3.59-12.95) to 6.5% (n = 6; 95% CI = 1.46-11.44; P = .610; Table). Notably, only 12.1% (n = 21) of patients with a single-lumen PICC experienced any complication, whereas 20.0% (n = 10) of patients with a double lumen, and 66.7% (n = 2) with a triple lumen experienced a PICC-associated complication (P = .022). Patients with triple lumens had a significantly higher incidence of catheter occlusion compared with patients that received double- and single-lumen PICCs (66.7% vs. 12.0% and 5.2%, respectively; P = .003).
No patient who received a single-lumen device required a second procedure for the placement of a device with additional lumens. Similarly, no documentation suggesting an insufficient number of PICC lumens or the need for additional vascular access (eg, placement of additional PICCs) was found in medical records of patients postintervention. Pharmacists supporting the interventions and VAST team members reported no disagreements when discussing number of lumens or appropriateness of catheter choice.
DISCUSSION
In this single center, pre–post quasi-experimental study, a multimodal intervention based on the MAGIC criteria significantly reduced the use of multilumen PICCs. Additionally, a trend toward reductions in complications, including CLABSI and catheter occlusion, was also observed. Notably, these changes in ordering practices did not lead to requests for additional devices or replacement with a multilumen PICC when a single-lumen device was inserted. Collectively, our findings suggest that the use of single-lumen devices in a large direct care service can be feasibly and safely increased through this approach. Larger scale studies that implement MAGIC to inform placement of multilumen PICCs and reduce PICC-related complications now appear necessary.
The presence of a PICC, even for short periods, significantly increases the risk of CLABSI and is one of the strongest predictors of venous thrombosis risk in the hospital setting.19,24,25 Although some factors that lead to this increased risk are patient-related and not modifiable (eg, malignancy or intensive care unit status), increased risk linked to the gauge of PICCs and the number of PICC lumens can be modified by improving device selection.9,18,26 Deliberate use of PICCs with the least numbers of clinically necessary lumens decreases risk of CLABSI, venous thrombosis and overall cost.17,19,26 Additionally, greater rates of occlusion with each additional PICC lumen may result in the interruption of intravenous therapy, the administration of costly medications (eg, tissue plasminogen activator) to salvage the PICC, and premature removal of devices should the occlusion prove irreversible.8
We observed a trend toward decreased PICC complications following implementation of our criteria, especially for the outcomes of CLABSI and catheter occlusion. Given the pilot nature of this study, we were underpowered to detect a statistically significant change in PICC adverse events. However, we did observe a statistically significant increase in the rate of single-lumen PICC use following our intervention. Notably, this increase occurred in the setting of high rates of single-lumen PICC use at baseline (64%). Therefore, an important takeaway from our findings is that room for improving PICC appropriateness exists even among high performers. This finding In turn, high baseline use of single-lumen PICCs may also explain why a robust reduction in PICC complications was not observed in our study, given that other studies showing reduction in the rates of complications began with considerably low rates of single-lumen device use.19 Outcomes may improve, however, if we expand and sustain these changes or expand to larger settings. For example, (based on assumptions from a previously published simulation study and our average hospital medicine daily census of 98 patients) the increased use of single-over multilumen PICCs is expected to decrease CLABSI events and venous thrombosis episodes by 2.4-fold in our hospital medicine service with an associated cost savings of $74,300 each year.17 Additionally, we would also expect the increase in the proportion of single-lumen PICCs to reduce rates of catheter occlusion. This reduction, in turn, would lessen interruptions in intravenous therapy, the need for medications to treat occlusion, and the need for device replacement all leading to reduced costs.27 Overall, then, our intervention (informed by appropriateness criteria) provides substantial benefits to hospital savings and patient safety.
After our intervention, 98% of all PICCs placed were found to comply with appropriate criteria for multilumen PICC use. We unexpectedly found that the most important factor driving our findings was not oversight or order modification by the pharmacy team or VAST nurses, but rather better decisions made by physicians at the outset. Specifically, we did not find a single instance wherein the original PICC order was changed to a device with a different number of lumens after review from the VAST team. We attribute this finding to receptiveness of physicians to change ordering practices following education and the redesign of the default EMR PICC order, both of which provided a scientific rationale for multilumen PICC use. Clarifying the risk and criteria of the use of multilumen devices along with providing an EMR ordering process that supports best practice helped hospitalists “do the right thing”. Additionally, setting single-lumen devices as the preselected EMR order and requiring text-based justification for placement of a multilumen PICC helped provide a nudge to physicians, much as it has done with antibiotic choices.28
Our study has limitations. First, we were only able to identify complications that were captured by our EMR. Given that over 70% of the patients in our study were discharged with a PICC in place, we do not know whether complications may have developed outside the hospital. Second, our intervention was resource intensive and required partnership with pharmacy, VAST, and hospitalists. Thus, the generalizability of our intervention to other institutions without similar support is unclear. Third, despite an increase in the use of single-lumen PICCs and a decrease in multilumen devices, we did not observe a significant reduction in all types of complications. While our high rate of single-lumen PICC use may account for these findings, larger scale studies are needed to better study the impact of MAGIC and appropriateness criteria on PICC complications. Finally, given our approach, we cannot identify the most effective modality within our bundled intervention. Stepped wedge or single-component studies are needed to further address this question.
In conclusion, we piloted a multimodal intervention to promote the use of single-lumen PICCs while lowering the use of multilumen devices. By using MAGIC to create appropriate indications, the use of multilumen PICCs declined and complications trended downwards. Larger, multicenter studies to validate our findings and examine the sustainability of this intervention would be welcomed.
Disclosures
The authors have nothing to disclose.
Vascular access is a cornerstone of safe and effective medical care. The use of peripherally inserted central catheters (PICCs) to meet vascular access needs has recently increased.1,2 PICCs offer several advantages over other central venous catheters. These advantages include increased reliability over intermediate to long-term use and reductions in complication rates during insertion.3,4
Multiple studies have suggested a strong association between the number of PICC lumens and risk of complications, such as central-line associated bloodstream infection (CLABSI), venous thrombosis, and catheter occlusion.5-8,9,10-12 These complications may lead to device failure, interrupt therapy, prolonged length of stay, and increased healthcare costs.13-15 Thus, available guidelines recommend using PICCs with the least clinically necessary number of lumens.1,16 Quality improvement strategies that have targeted decreasing the number of PICC lumens have reduced complications and healthcare costs.17-19 However, variability exists in the selection of the number of PICC lumens, and many providers request multilumen devices “just in case” additional lumens are needed.20,21 Such variation in device selection may stem from the paucity of information that defines the appropriate indications for the use of single- versus multi-lumen PICCs.
Therefore, to ensure appropriateness of PICC use, we designed an intervention to improve selection of the number of PICC lumens.
METHODS
We conducted this pre–post quasi-experimental study in accordance with SQUIRE guidelines.22 Details regarding clinical parameters associated with the decision to place a PICC, patient characteristics, comorbidities, complications, and laboratory values were collected from the medical records of patients. All PICCs were placed by the Vascular Access Service Team (VAST) during the study period.
Intervention
The intervention consisted of three components: first, all hospitalists, pharmacists, and VAST nurses received education in the form of a CME lecture that emphasized use of the Michigan Appropriateness Guide for Intravenous Catheters (MAGIC).1 These criteria define when use of a PICC is appropriate and emphasize how best to select the most appropriate device characteristics such as lumens and catheter gauge. Next, a multidisciplinary task force that consisted of hospitalists, VAST nurses, and pharmacists developed a list of indications specifying when use of a multilumen PICC was appropriate.1 Third, the order for a PICC in our electronic medical record (EMR) system was modified to set single-lumen PICCs as default. If a multilumen PICC was requested, text-based justification from the ordering clinician was required.
As an additional safeguard, a VAST nurse reviewed the number of lumens and clinical scenario for each PICC order prior to insertion. If the number of lumens ordered was considered inappropriate on the basis of the developed list of MAGIC recommendations, the case was referred to a pharmacist for additional review. The pharmacist then reviewed active and anticipated medications, explored options for adjusting the medication delivery plan, and discussed these options with the ordering clinician to determine the most appropriate number of lumens.
Measures and Definitions
In accordance with the criteria set by the Centers for Disease Control National Healthcare Safety Network,23 CLABSI was defined as a confirmed positive blood culture with a PICC in place for 48 hours or longer without another identified infection source or a positive PICC tip culture in the setting of clinically suspected infection. Venous thrombosis was defined as symptomatic upper extremity deep vein thromboembolism or pulmonary embolism that was radiographically confirmed after the placement of a PICC or within one week of device removal. Catheter occlusion was captured when documented or when tPA was administered for problems related to the PICC. The appropriateness of the number of PICC lumens was independently adjudicated by an attending physician and clinical pharmacist by comparing the indications of the device placed against predefined appropriateness criteria.
Outcomes
The primary outcome of interest was the change in the proportion of single-lumen PICCs placed. Secondary outcomes included (1) the placement of PICCs with an appropriate number of lumens, (2) the occurrence of PICC-related complications (CLABSI, venous thrombosis, and catheter occlusion), and (3) the need for a second procedure to place a multilumen device or additional vascular access.
Statistical Analysis
Descriptive statistics were used to tabulate and summarize patient and PICC characteristics. Differences between pre- and postintervention populations were assessed using χ2, Fishers exact, t-, and Wilcoxon rank sum tests. Differences in complications were assessed using the two-sample tests of proportions. Results were reported as medians (IQR) and percentages with corresponding 95% confidence intervals. All statistical tests were two-sided, with P < .05 considered statistically significant. Analyses were conducted with Stata v.14 (stataCorp, College Station, Texas).
Ethical and Regulatory Oversight
This study was approved by the Institutional Review Board at the University of Michigan (IRB#HUM00118168).
RESULTS
Of the 133 PICCs placed preintervention, 64.7% (n = 86) were single lumen, 33.1% (n = 44) were double lumen, and 2.3% (n = 3) were triple lumen. Compared with the preintervention period, the use of single-lumen PICCs significantly increased following the intervention (64.7% to 93.6%; P < .001; Figure 1). As well, the proportion of PICCs with an inappropriate number of lumens decreased from 25.6% to 2.2% (P < .001; Table 1).
Preintervention, 14.3% (95% CI = 8.34-20.23) of the patients with PICCs experienced at least one complication (n = 19). Following the intervention, 15.1% (95% CI = 7.79-22.32) of the 93 patients with PICCs experienced at least one complication (absolute difference = 0.8%, P = .872). With respect to individual complications, CLABSI decreased from 5.3% (n = 7; 95% CI = 1.47-9.06) to 2.2% (n = 2; 95% CI = −0.80-5.10) (P = .239). Similarly, the incidence of catheter occlusion decreased from 8.3% (n = 11; 95% CI = 3.59-12.95) to 6.5% (n = 6; 95% CI = 1.46-11.44; P = .610; Table). Notably, only 12.1% (n = 21) of patients with a single-lumen PICC experienced any complication, whereas 20.0% (n = 10) of patients with a double lumen, and 66.7% (n = 2) with a triple lumen experienced a PICC-associated complication (P = .022). Patients with triple lumens had a significantly higher incidence of catheter occlusion compared with patients that received double- and single-lumen PICCs (66.7% vs. 12.0% and 5.2%, respectively; P = .003).
No patient who received a single-lumen device required a second procedure for the placement of a device with additional lumens. Similarly, no documentation suggesting an insufficient number of PICC lumens or the need for additional vascular access (eg, placement of additional PICCs) was found in medical records of patients postintervention. Pharmacists supporting the interventions and VAST team members reported no disagreements when discussing number of lumens or appropriateness of catheter choice.
DISCUSSION
In this single center, pre–post quasi-experimental study, a multimodal intervention based on the MAGIC criteria significantly reduced the use of multilumen PICCs. Additionally, a trend toward reductions in complications, including CLABSI and catheter occlusion, was also observed. Notably, these changes in ordering practices did not lead to requests for additional devices or replacement with a multilumen PICC when a single-lumen device was inserted. Collectively, our findings suggest that the use of single-lumen devices in a large direct care service can be feasibly and safely increased through this approach. Larger scale studies that implement MAGIC to inform placement of multilumen PICCs and reduce PICC-related complications now appear necessary.
The presence of a PICC, even for short periods, significantly increases the risk of CLABSI and is one of the strongest predictors of venous thrombosis risk in the hospital setting.19,24,25 Although some factors that lead to this increased risk are patient-related and not modifiable (eg, malignancy or intensive care unit status), increased risk linked to the gauge of PICCs and the number of PICC lumens can be modified by improving device selection.9,18,26 Deliberate use of PICCs with the least numbers of clinically necessary lumens decreases risk of CLABSI, venous thrombosis and overall cost.17,19,26 Additionally, greater rates of occlusion with each additional PICC lumen may result in the interruption of intravenous therapy, the administration of costly medications (eg, tissue plasminogen activator) to salvage the PICC, and premature removal of devices should the occlusion prove irreversible.8
We observed a trend toward decreased PICC complications following implementation of our criteria, especially for the outcomes of CLABSI and catheter occlusion. Given the pilot nature of this study, we were underpowered to detect a statistically significant change in PICC adverse events. However, we did observe a statistically significant increase in the rate of single-lumen PICC use following our intervention. Notably, this increase occurred in the setting of high rates of single-lumen PICC use at baseline (64%). Therefore, an important takeaway from our findings is that room for improving PICC appropriateness exists even among high performers. This finding In turn, high baseline use of single-lumen PICCs may also explain why a robust reduction in PICC complications was not observed in our study, given that other studies showing reduction in the rates of complications began with considerably low rates of single-lumen device use.19 Outcomes may improve, however, if we expand and sustain these changes or expand to larger settings. For example, (based on assumptions from a previously published simulation study and our average hospital medicine daily census of 98 patients) the increased use of single-over multilumen PICCs is expected to decrease CLABSI events and venous thrombosis episodes by 2.4-fold in our hospital medicine service with an associated cost savings of $74,300 each year.17 Additionally, we would also expect the increase in the proportion of single-lumen PICCs to reduce rates of catheter occlusion. This reduction, in turn, would lessen interruptions in intravenous therapy, the need for medications to treat occlusion, and the need for device replacement all leading to reduced costs.27 Overall, then, our intervention (informed by appropriateness criteria) provides substantial benefits to hospital savings and patient safety.
After our intervention, 98% of all PICCs placed were found to comply with appropriate criteria for multilumen PICC use. We unexpectedly found that the most important factor driving our findings was not oversight or order modification by the pharmacy team or VAST nurses, but rather better decisions made by physicians at the outset. Specifically, we did not find a single instance wherein the original PICC order was changed to a device with a different number of lumens after review from the VAST team. We attribute this finding to receptiveness of physicians to change ordering practices following education and the redesign of the default EMR PICC order, both of which provided a scientific rationale for multilumen PICC use. Clarifying the risk and criteria of the use of multilumen devices along with providing an EMR ordering process that supports best practice helped hospitalists “do the right thing”. Additionally, setting single-lumen devices as the preselected EMR order and requiring text-based justification for placement of a multilumen PICC helped provide a nudge to physicians, much as it has done with antibiotic choices.28
Our study has limitations. First, we were only able to identify complications that were captured by our EMR. Given that over 70% of the patients in our study were discharged with a PICC in place, we do not know whether complications may have developed outside the hospital. Second, our intervention was resource intensive and required partnership with pharmacy, VAST, and hospitalists. Thus, the generalizability of our intervention to other institutions without similar support is unclear. Third, despite an increase in the use of single-lumen PICCs and a decrease in multilumen devices, we did not observe a significant reduction in all types of complications. While our high rate of single-lumen PICC use may account for these findings, larger scale studies are needed to better study the impact of MAGIC and appropriateness criteria on PICC complications. Finally, given our approach, we cannot identify the most effective modality within our bundled intervention. Stepped wedge or single-component studies are needed to further address this question.
In conclusion, we piloted a multimodal intervention to promote the use of single-lumen PICCs while lowering the use of multilumen devices. By using MAGIC to create appropriate indications, the use of multilumen PICCs declined and complications trended downwards. Larger, multicenter studies to validate our findings and examine the sustainability of this intervention would be welcomed.
Disclosures
The authors have nothing to disclose.
1. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. doi: 10.7326/M15-0744. PubMed
2. Taylor RW, Palagiri AV. Central venous catheterization. Crit Care Med. 2007;35(5):1390-1396. doi: 10.1097/01.CCM.0000260241.80346.1B. PubMed
3. Pikwer A, Akeson J, Lindgren S. Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65-71. doi: 10.1111/j.1365-2044.2011.06911.x. PubMed
4. Johansson E, Hammarskjold F, Lundberg D, Arnlind MH. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Onco. 2013;52(5):886-892. doi: 10.3109/0284186X.2013.773072. PubMed
5. Pan L, Zhao Q, Yang X. Risk factors for venous thrombosis associated with peripherally inserted central venous catheters. Int J Clin Exp Med. 2014;7(12):5814-5819. PubMed
6. Herc E, Patel P, Washer LL, Conlon A, Flanders SA, Chopra V. A model to predict central-line-associated bloodstream infection among patients with peripherally inserted central catheters: The MPC score. Infect Cont Hosp Ep. 2017;38(10):1155-1166. doi: 10.1017/ice.2017.167. PubMed
7. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–1171. doi: 10.4065/81.9.1159. PubMed
8. Smith SN, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e742. doi: 10.1016/j.jvir.2017.02.005. PubMed
9. Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-325. doi: 10.1016/S0140-6736(13)60592-9. PubMed
10. Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014;12(6):847-854. doi: 10.1111/jth.12549. PubMed
11. Carter JH, Langley JM, Kuhle S, Kirkland S. Risk factors for central venous catheter-associated bloodstream infection in pediatric patients: A cohort study. Infect Control Hosp Epidemiol. 2016;37(8):939-945. doi: 10.1017/ice.2016.83. PubMed
12. Chopra V, Ratz D, Kuhn L, Lopus T, Chenoweth C, Krein S. PICC-associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319-328. doi: 10.1016/j.amjmed.2014.01.001. PubMed
13. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193. doi: 10.1093/cid/cir257. PubMed
14. Parkinson R, Gandhi M, Harper J, Archibald C. Establishing an ultrasound guided peripherally inserted central catheter (PICC) insertion service. Clin Radiol. 1998;53(1):33-36. doi: 10.1016/S0009-9260(98)80031-7. PubMed
15. Shannon RP, Patel B, Cummins D, Shannon AH, Ganguli G, Lu Y. Economics of central line--associated bloodstream infections. Am J Med Qual. 2006;21(6 Suppl):7s–16s. doi: 10.1177/1062860606294631. PubMed
16. Mermis JD, Strom JC, Greenwood JP, et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404-1410. doi: 10.1513/AnnalsATS.201404-175OC. PubMed
17. Ratz D, Hofer T, Flanders SA, Saint S, Chopra V. Limiting the number of lumens in peripherally inserted central catheters to improve outcomes and reduce cost: A simulation study. Infect Control Hosp Epidemiol. 2016;37(7):811-817. doi: 10.1017/ice.2016.55. PubMed
18. Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733-741. doi: 10.1016/j.amjmed.2012.04.010. PubMed
19. O’Brien J, Paquet F, Lindsay R, Valenti D. Insertion of PICCs with minimum number of lumens reduces complications and costs. J Am Coll Radiol. 2013;10(11):864-868. doi: 10.1016/j.jacr.2013.06.003. PubMed
20. Tiwari MM, Hermsen ED, Charlton ME, Anderson JR, Rupp ME. Inappropriate intravascular device use: a prospective study. J Hosp Infect. 2011;78(2):128-132. doi: 10.1016/j.jhin.2011.03.004. PubMed
21. Chopra V, Kuhn L, Flanders SA, Saint S, Krein SL. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: results of a national survey. J Hosp Med. 2013;8(11):635-638. doi: 10.1002/jhm.2095. PubMed
22. Goodman D, Ogrinc G, Davies L, et al. Explanation and elaboration of the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, V.2.0: examples of SQUIRE elements in the healthcare improvement literature. BMJ Qual Saf. 2016;25(12):e7. doi: 10.1136/bmjqs-2015-004480. PubMed
23. CDC Bloodstream Infection/Device Associated Infection Module. https://wwwcdcgov/nhsn/pdfs/pscmanual/4psc_clabscurrentpdf 2017. Accessed April 11, 2017.
24. Woller SC, Stevens SM, Jones JP, et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947-954.e2. doi: 10.1016/j.amjmed.2011.06.004. PubMed
25. Paje D, Conlon A, Kaatz S, et al. Patterns and predictors of short-term peripherally inserted central catheter use: A multicenter prospective cohort study. J Hosp Med. 2018;13(2):76-82. doi: 10.12788/jhm.2847. PubMed
26. Evans RS, Sharp JH, Linford LH, et al. Reduction of peripherally inserted central catheter-associated DVT. Chest. 2013;143(3):627-633. doi: 10.1378/chest.12-0923. PubMed
27. Smith S, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e2. doi: 10.1016/j.jvir.2017.02.005. PubMed
28. Vaughn VM, Linder JA. Thoughtless design of the electronic health record drives overuse, but purposeful design can nudge improved patient care. BMJ Qual Saf. 2018;27(8):583-586. doi: 10.1136/bmjqs-2017-007578. PubMed
1. Chopra V, Flanders SA, Saint S, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results from a multispecialty panel using the RAND/UCLA appropriateness method. Ann Intern Med. 2015;163(6 Suppl):S1-S40. doi: 10.7326/M15-0744. PubMed
2. Taylor RW, Palagiri AV. Central venous catheterization. Crit Care Med. 2007;35(5):1390-1396. doi: 10.1097/01.CCM.0000260241.80346.1B. PubMed
3. Pikwer A, Akeson J, Lindgren S. Complications associated with peripheral or central routes for central venous cannulation. Anaesthesia. 2012;67(1):65-71. doi: 10.1111/j.1365-2044.2011.06911.x. PubMed
4. Johansson E, Hammarskjold F, Lundberg D, Arnlind MH. Advantages and disadvantages of peripherally inserted central venous catheters (PICC) compared to other central venous lines: a systematic review of the literature. Acta Onco. 2013;52(5):886-892. doi: 10.3109/0284186X.2013.773072. PubMed
5. Pan L, Zhao Q, Yang X. Risk factors for venous thrombosis associated with peripherally inserted central venous catheters. Int J Clin Exp Med. 2014;7(12):5814-5819. PubMed
6. Herc E, Patel P, Washer LL, Conlon A, Flanders SA, Chopra V. A model to predict central-line-associated bloodstream infection among patients with peripherally inserted central catheters: The MPC score. Infect Cont Hosp Ep. 2017;38(10):1155-1166. doi: 10.1017/ice.2017.167. PubMed
7. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81(9):1159–1171. doi: 10.4065/81.9.1159. PubMed
8. Smith SN, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e742. doi: 10.1016/j.jvir.2017.02.005. PubMed
9. Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet. 2013;382(9889):311-325. doi: 10.1016/S0140-6736(13)60592-9. PubMed
10. Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost. 2014;12(6):847-854. doi: 10.1111/jth.12549. PubMed
11. Carter JH, Langley JM, Kuhle S, Kirkland S. Risk factors for central venous catheter-associated bloodstream infection in pediatric patients: A cohort study. Infect Control Hosp Epidemiol. 2016;37(8):939-945. doi: 10.1017/ice.2016.83. PubMed
12. Chopra V, Ratz D, Kuhn L, Lopus T, Chenoweth C, Krein S. PICC-associated bloodstream infections: prevalence, patterns, and predictors. Am J Med. 2014;127(4):319-328. doi: 10.1016/j.amjmed.2014.01.001. PubMed
13. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52(9):e162-e193. doi: 10.1093/cid/cir257. PubMed
14. Parkinson R, Gandhi M, Harper J, Archibald C. Establishing an ultrasound guided peripherally inserted central catheter (PICC) insertion service. Clin Radiol. 1998;53(1):33-36. doi: 10.1016/S0009-9260(98)80031-7. PubMed
15. Shannon RP, Patel B, Cummins D, Shannon AH, Ganguli G, Lu Y. Economics of central line--associated bloodstream infections. Am J Med Qual. 2006;21(6 Suppl):7s–16s. doi: 10.1177/1062860606294631. PubMed
16. Mermis JD, Strom JC, Greenwood JP, et al. Quality improvement initiative to reduce deep vein thrombosis associated with peripherally inserted central catheters in adults with cystic fibrosis. Ann Am Thorac Soc. 2014;11(9):1404-1410. doi: 10.1513/AnnalsATS.201404-175OC. PubMed
17. Ratz D, Hofer T, Flanders SA, Saint S, Chopra V. Limiting the number of lumens in peripherally inserted central catheters to improve outcomes and reduce cost: A simulation study. Infect Control Hosp Epidemiol. 2016;37(7):811-817. doi: 10.1017/ice.2016.55. PubMed
18. Chopra V, Anand S, Krein SL, Chenoweth C, Saint S. Bloodstream infection, venous thrombosis, and peripherally inserted central catheters: reappraising the evidence. Am J Med. 2012;125(8):733-741. doi: 10.1016/j.amjmed.2012.04.010. PubMed
19. O’Brien J, Paquet F, Lindsay R, Valenti D. Insertion of PICCs with minimum number of lumens reduces complications and costs. J Am Coll Radiol. 2013;10(11):864-868. doi: 10.1016/j.jacr.2013.06.003. PubMed
20. Tiwari MM, Hermsen ED, Charlton ME, Anderson JR, Rupp ME. Inappropriate intravascular device use: a prospective study. J Hosp Infect. 2011;78(2):128-132. doi: 10.1016/j.jhin.2011.03.004. PubMed
21. Chopra V, Kuhn L, Flanders SA, Saint S, Krein SL. Hospitalist experiences, practice, opinions, and knowledge regarding peripherally inserted central catheters: results of a national survey. J Hosp Med. 2013;8(11):635-638. doi: 10.1002/jhm.2095. PubMed
22. Goodman D, Ogrinc G, Davies L, et al. Explanation and elaboration of the SQUIRE (Standards for Quality Improvement Reporting Excellence) Guidelines, V.2.0: examples of SQUIRE elements in the healthcare improvement literature. BMJ Qual Saf. 2016;25(12):e7. doi: 10.1136/bmjqs-2015-004480. PubMed
23. CDC Bloodstream Infection/Device Associated Infection Module. https://wwwcdcgov/nhsn/pdfs/pscmanual/4psc_clabscurrentpdf 2017. Accessed April 11, 2017.
24. Woller SC, Stevens SM, Jones JP, et al. Derivation and validation of a simple model to identify venous thromboembolism risk in medical patients. Am J Med. 2011;124(10):947-954.e2. doi: 10.1016/j.amjmed.2011.06.004. PubMed
25. Paje D, Conlon A, Kaatz S, et al. Patterns and predictors of short-term peripherally inserted central catheter use: A multicenter prospective cohort study. J Hosp Med. 2018;13(2):76-82. doi: 10.12788/jhm.2847. PubMed
26. Evans RS, Sharp JH, Linford LH, et al. Reduction of peripherally inserted central catheter-associated DVT. Chest. 2013;143(3):627-633. doi: 10.1378/chest.12-0923. PubMed
27. Smith S, Moureau N, Vaughn VM, et al. Patterns and predictors of peripherally inserted central catheter occlusion: The 3P-O study. J Vasc Interv Radiol. 2017;28(5):749-756.e2. doi: 10.1016/j.jvir.2017.02.005. PubMed
28. Vaughn VM, Linder JA. Thoughtless design of the electronic health record drives overuse, but purposeful design can nudge improved patient care. BMJ Qual Saf. 2018;27(8):583-586. doi: 10.1136/bmjqs-2017-007578. PubMed
© 2019 Society of Hospital Medicine
You Can’t Have It All: The Experience of Academic Hospitalists During Pregnancy, Parental Leave, and Return to Work
Despite recent advances made in medicine, gender-based disparities persist.1-3 In particular, women with children have barriers to career advancement and show evidence of slower career advancement.1,2 Multiple challenges for working women experiencing motherhood have been described. In academic medicine in the United States, women have limited access to paid parental leave.4-6 For women who choose to breastfeed, there is limited time, space, and support available for breastfeeding.7 Furthermore, sleep deprivation in the postpartum period significantly impacts the ability to function at work.8
Hospital medicine is a unique specialty as it comprises 47% women, 80% of whom are aged less than 40 years, suggesting that a large portion are women of childbearing age.9 The field poses known challenges to this population, including shift work, atypical schedules, and unpredictable hours. We conducted a descriptive qualitative study to improve our understanding of the experience of female academic hospitalists who have experienced pregnancy, parental leave, and the return to work as faculty. Our goal was to both explore the challenges to undergoing this experience and discover solutions to support female academic hospitalists.
METHODS
Study Design
We conducted a qualitative descriptive study of female hospitalists recruited from academic institutions represented in Society of Hospital Medicine (SHM) committees. Interviews were conducted between November 2017 and February 2018. Participants completed an informed consent and a demographic survey prior to the interview. Each interview lasted approximately 30 minutes; discussions were recorded on digital records and transcribed verbatim. This protocol was reviewed and granted exemption by the Institutional Review Board at the University of Colorado.
Population
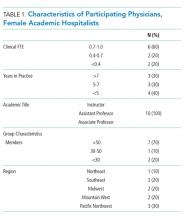
We recruited participants from a selection of hospital medicine groups nationally, chosen from SHM committee representation. A purposeful snowball approach was used to identify hospitalists from representative programs and seek their recommendation for hospitalists from other targeted programs. Ten hospitalists were approached by e-mail to determine their interest in participation, and all of them agreed to participate. Each participant experienced new parenthood within the last seven years.
Framework
We constructed our interview to represent the following timeline associated with having children as it pertains to a hospitalist position: pregnancy, parental leave, and the return to work. The interview guide was structured to invoke the positive aspects, challenges, and solutions within each domain (Appendix 1).
Analysis
Codes were inductively developed from the interview data by a team of three board-certified internal medicine physicians (E.G., A.M., and C.J.), one of whom had prior training and experience with qualitative interviews and analysis (C.J.). Among the coders, two (E.G. and A.M.) conducted the semistructured interviews. Code disparities were reconciled by team consensus, where the primary coder facilitated the discussions. Themes were developed inductively from the codes, and the analysis was completed using a team-based iterative approach that was facilitated using ATLAS.ti.10 Thematic saturation was achieved. This study was approved by the Colorado Multiple Institutional Review Board.
RESULTS
The demographics and the characteristics of the hospital medicine group are shown in Table 1. Although we asked questions about both the positive and challenging aspects of the experience of parenthood, the interviews tended to focus more on the challenges faced and on areas for optimization.
Paid Parental leave
Most of the participants described inadequate paid parental leave, with minimal transparency in the processes for ensuring time off following the birth of their child, resulting in “haggling” with bosses, human resources, and the administrative staff. Rarely was a formal parental leave policy in place. Once a parental leave plan was established, several women reported the financial burden associated with a leave that was partially, or fully, unpaid.
“All of my leave was unpaid. .. managed to finagle short-term disability into paying for it… the system was otherwise set up to screw me financially.”
For the three women who did experience sufficient paid parental leave, they recognized the financial and emotional benefit and suggested that further optimization would include a prebirth schedule to account for the physical challenges and potential complications.
Physical Challenges
All of the women described significant physical challenges when working during pregnancy, resulting in limited bandwidth for additional academic activities outside of direct clinical care responsibilities.
“Exhaustion that hits you in your pregnancy and then you have to round. I used to lie on the floor of my office, take a little nap, wake up, write some notes, go home, take another nap, wake up, write some more notes.”
Upon return to work, women reported additional physical challenges related to sleep deprivation, impacting their productivity with academic work and emotional well-being.
“I came back from maternity leave and I was sleep-deprived and exhausted, I didn’t have the energy. All of these great projects that I had started or dreamed of … dwindled and died on the vine.”
Solutions suggested by the participants included creation of a flexible schedule with a ramp-up and ramp-down period around the birth.
Breastfeeding
The majority of participants in this study encountered several challenges associated with a shared goal of breastfeeding according to evidence-based guidelines.11 Designated pumping areas were often inconveniently located and not conducive to multitasking.
“It’s two chairs that are behind a curtain in a women’s locker room in the basement of the hospital, that are tiny and gross. No computers, so I felt like I was wasting time.”
One hospitalist described carving out time for pumping in her office while multitasking with clinical work.
“I would get to work, set up, and pump while chart reviewing. Then I would go and see people… and come back to my office and pump and write a few notes. And go out and see more patients, and then pump and write a few more notes. And then pump, and then go home. I was like a cow.”
Women highlighted the barriers that could be optimized such as creating time in the clinical schedule for pumping, a physical space to breastfeed or pump, and accessible milk storage facilities.
Career Opportunities
When asked about the impact of parental leave on career opportunities, a few of the women described a phenomenon of no longer being asked to participate or being left out of prior projects.
“People didn’t want to offer you things or give you things because they realize you’re having this transition in your life. Not out of animosity, but out of courtesy that they don’t want to fill up your place even more. Her plate is full; we are not going to ask her to do anything extra.”
However, two women specifically reported a supportive environment without a loss of opportunities, often referenced as a boss who “saved” projects for their return.
Colleague Responses
One participant used the term “microaggressions,” to describe passive aggressions encountered by their colleagues or leadership.
“(A colleague) was diagnosed with pre-eclampsia, and very urgently had to deliver and couldn’t cover a week of shifts…She was asked initially to find her own coverage…Not treating (pregnancy) similar to other serious illnesses is what I would term a microaggression.”
Yet, women in our study also reported positive responses from colleagues and the importance of support networks of physician mothers (Table 2).
Empathy in Patient Care
Finally, the experience of motherhood impacted all of the women as physicians, described as increased empathy, patience, and understanding of difficult family situations.
“I’m just more sensitive to people’s lives outside the hospital, so, you know, when it’s difficult for a family member to get there because they have three other kids they are taking care of or, somebody that says they are leaving AMA, but it’s because they have a sick kid at home. I just have a better context for that.”
DISCUSSION
Gender disparities persist in both internal medicine and hospital medicine.1 Providers in this descriptive qualitative study suggested that the following factors contribute: lack of paid parental leave and the associated financial penalties, loss of career opportunities, the physical challenges associated with pregnancy, decreasing productivity, and the amount of time and effort involved in breastfeeding. However, the participants also shared valuable ideas for future solutions to relieve the challenges imposed on working physician mothers (Table 2).
Breaking the Glass Ceiling
Participants noted the importance of a paid leave policy that encompasses not only maternity leave but also a flexible scheduling period before and after the leave to account for the challenges of pregnancy and new motherhood. Paid parental leave is rare in academic settings, but studies from other industries show that when women take paid leave, they are more likely to remain in the workforce 9-12 months afterward, work more weekly hours, and feel more loyal to their organization.12,13 In the rare instance when negotiations around leave violate local policy or the law, women should be encouraged to seek guidance from their human resources department.
Me Too: Building Solidarity
Women in our study reported the value of a supportive workplace in easing their transition into motherhood. Specifically, they noted that a supportive boss who protected their career opportunities prevented momentum loss in their career trajectory. Access to mutual supports such as the Physicians Mom Group, a well-established Facebook group comprising more than 70,000 women, was referenced as a meaningful way to share joys and tribulations related to balancing a career as a physician and motherhood. Growth of similar support systems within institutions will further support this experience.
Time’s Up: The Promotion Clock
Women in our study described a prolonged period of diminished productivity related to having children, coinciding with a set time to promotion in academics. Flexible promotion schedules may impact women’s ability to successfully undergo promotion.
FUTURE DIRECTION
The aim of this study was to represent a shared set of experiences of female academic hospitalists who participated; therefore, the results may not be generalizable beyond this group. Due to the use of a purposeful snowball approach, there was a potential for selection bias. Future research may include comparing the experience of women at institutions that offer paid leave versus those that do not and the impact on retention, promotion, and well-being.
CONCLUSION
Women in hospital medicine encounter several challenges to having children, but they are also motivated to provide solutions. Efforts to improve the institutional and cultural landscape to better support women physicians with children are critical to prevent attrition of women and ensure equitable academic promotion and achievement of leadership positions.
Disclosures
The authors have no conflicts of interest to report.
Author Contributions
Each author was involved in the creation of the study protocol, data collection and analysis, and creation of the manuscript.
1. Association of American Medical Colleges. The State of Women in Academic Medicine: The pipeline and pathways to leadership, 2013-2014. https://www.hopkinsmedicine.org/women_science_medicine/_pdfs/The%20State%20of%20Women%20in%20Academic%20Medicine%202013-2014%20FINAL.pdf. Accessed February 26, 2018.
2. Carr PL, Ash AS, Friedman RH, et al. Relation of family responsibilities and gender to the productivity and career satisfaction of medical faculty. Ann Int Med. 1998;129(7):532-538. doi: 10.7326/0003-4819-129-7-199810010-00004. PubMed
3. Burden M, Frank MG, Keniston A, et al. Gender disparities for academic hospitalists. J Hosp Med. 2015;10(8):481-485. doi:10.1002/jhm.2340. PubMed
4. Bristol MN, Abbuhl S, Cappola AR, Sonnad SS. Work-life policies for faculty at the top ten medical schools. J Women’s Health. 2008;17(8):1311-1320. doi: 10.1089/jwh.2007.0682. PubMed
5. Welch JL, Wiehe SE, Palmer-Smith V, Dankoski ME. Flexibility in faculty work-life policies at medical schools in the big ten conference. J Women’s Health. 2011;20(5):725-732. doi: 10.1089/jwh.2010.2553. PubMed
6. Riano NS, Linos E, Accurso EC, et al. Paid family and childbearing leave policies at top US medical schools. JAMA. 2018;319(6):611-614. doi: 10.1001/jama.2017.19519. PubMed
7. Arthur CR, Saenz RB, Replogle WH. The employment-related breastfeeding decisions of physician mothers. J Miss State Med Assoc. 2003;44(12):383-387. PubMed
8. Filtness AJ, MacKenzie J, Armstrong K. Longitudinal change in sleep and daytime sleepiness in postpartum women. PLoS ONE. 2014;9(7):e103513. doi: 10.1371/journal.pone.0103513. PubMed
9. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. doi: 10.1007/s11606-011-1892-5. PubMed
10. Jones J, Nowels CT, Sudore R, Ahluwalia S, Bekelman DB. The future as a series of transitions: qualitative study of heart failure patients and their informal caregivers. J Gen Intern Med. 2015;30(2):176-182. doi: 10.1007/s11606-014-3085-5. PubMed
11. American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827-e841. doi: 10.1542/peds.2011-3552. PubMed
12. Houser, L, Vartanian, T. Pay matters: the positive economic impact of paid family Leave for families, businesses and the public. Center for Women and Work at Rutgers. January, 2012. http://go.nationalpartnership.org/site/DocServer/Pay_Matters_Positive_Economic_Impacts_of_Paid_Fam ily_L.pdf?docID=9681. Accessed February 26, 2018.
13. Rossin-Slater M, Ruhm C, Waldfogel J. The effects of California’s paid family leave program on mothers’ leave-taking and subsequent labor market outcomes. J Policy Anal Manage. 2013;32(2):224-2 45. doi: 10.1002/pam.21676. PubMed
Despite recent advances made in medicine, gender-based disparities persist.1-3 In particular, women with children have barriers to career advancement and show evidence of slower career advancement.1,2 Multiple challenges for working women experiencing motherhood have been described. In academic medicine in the United States, women have limited access to paid parental leave.4-6 For women who choose to breastfeed, there is limited time, space, and support available for breastfeeding.7 Furthermore, sleep deprivation in the postpartum period significantly impacts the ability to function at work.8
Hospital medicine is a unique specialty as it comprises 47% women, 80% of whom are aged less than 40 years, suggesting that a large portion are women of childbearing age.9 The field poses known challenges to this population, including shift work, atypical schedules, and unpredictable hours. We conducted a descriptive qualitative study to improve our understanding of the experience of female academic hospitalists who have experienced pregnancy, parental leave, and the return to work as faculty. Our goal was to both explore the challenges to undergoing this experience and discover solutions to support female academic hospitalists.
METHODS
Study Design
We conducted a qualitative descriptive study of female hospitalists recruited from academic institutions represented in Society of Hospital Medicine (SHM) committees. Interviews were conducted between November 2017 and February 2018. Participants completed an informed consent and a demographic survey prior to the interview. Each interview lasted approximately 30 minutes; discussions were recorded on digital records and transcribed verbatim. This protocol was reviewed and granted exemption by the Institutional Review Board at the University of Colorado.
Population
We recruited participants from a selection of hospital medicine groups nationally, chosen from SHM committee representation. A purposeful snowball approach was used to identify hospitalists from representative programs and seek their recommendation for hospitalists from other targeted programs. Ten hospitalists were approached by e-mail to determine their interest in participation, and all of them agreed to participate. Each participant experienced new parenthood within the last seven years.
Framework
We constructed our interview to represent the following timeline associated with having children as it pertains to a hospitalist position: pregnancy, parental leave, and the return to work. The interview guide was structured to invoke the positive aspects, challenges, and solutions within each domain (Appendix 1).
Analysis
Codes were inductively developed from the interview data by a team of three board-certified internal medicine physicians (E.G., A.M., and C.J.), one of whom had prior training and experience with qualitative interviews and analysis (C.J.). Among the coders, two (E.G. and A.M.) conducted the semistructured interviews. Code disparities were reconciled by team consensus, where the primary coder facilitated the discussions. Themes were developed inductively from the codes, and the analysis was completed using a team-based iterative approach that was facilitated using ATLAS.ti.10 Thematic saturation was achieved. This study was approved by the Colorado Multiple Institutional Review Board.
RESULTS
The demographics and the characteristics of the hospital medicine group are shown in Table 1. Although we asked questions about both the positive and challenging aspects of the experience of parenthood, the interviews tended to focus more on the challenges faced and on areas for optimization.
Paid Parental leave
Most of the participants described inadequate paid parental leave, with minimal transparency in the processes for ensuring time off following the birth of their child, resulting in “haggling” with bosses, human resources, and the administrative staff. Rarely was a formal parental leave policy in place. Once a parental leave plan was established, several women reported the financial burden associated with a leave that was partially, or fully, unpaid.
“All of my leave was unpaid. .. managed to finagle short-term disability into paying for it… the system was otherwise set up to screw me financially.”
For the three women who did experience sufficient paid parental leave, they recognized the financial and emotional benefit and suggested that further optimization would include a prebirth schedule to account for the physical challenges and potential complications.
Physical Challenges
All of the women described significant physical challenges when working during pregnancy, resulting in limited bandwidth for additional academic activities outside of direct clinical care responsibilities.
“Exhaustion that hits you in your pregnancy and then you have to round. I used to lie on the floor of my office, take a little nap, wake up, write some notes, go home, take another nap, wake up, write some more notes.”
Upon return to work, women reported additional physical challenges related to sleep deprivation, impacting their productivity with academic work and emotional well-being.
“I came back from maternity leave and I was sleep-deprived and exhausted, I didn’t have the energy. All of these great projects that I had started or dreamed of … dwindled and died on the vine.”
Solutions suggested by the participants included creation of a flexible schedule with a ramp-up and ramp-down period around the birth.
Breastfeeding
The majority of participants in this study encountered several challenges associated with a shared goal of breastfeeding according to evidence-based guidelines.11 Designated pumping areas were often inconveniently located and not conducive to multitasking.
“It’s two chairs that are behind a curtain in a women’s locker room in the basement of the hospital, that are tiny and gross. No computers, so I felt like I was wasting time.”
One hospitalist described carving out time for pumping in her office while multitasking with clinical work.
“I would get to work, set up, and pump while chart reviewing. Then I would go and see people… and come back to my office and pump and write a few notes. And go out and see more patients, and then pump and write a few more notes. And then pump, and then go home. I was like a cow.”
Women highlighted the barriers that could be optimized such as creating time in the clinical schedule for pumping, a physical space to breastfeed or pump, and accessible milk storage facilities.
Career Opportunities
When asked about the impact of parental leave on career opportunities, a few of the women described a phenomenon of no longer being asked to participate or being left out of prior projects.
“People didn’t want to offer you things or give you things because they realize you’re having this transition in your life. Not out of animosity, but out of courtesy that they don’t want to fill up your place even more. Her plate is full; we are not going to ask her to do anything extra.”
However, two women specifically reported a supportive environment without a loss of opportunities, often referenced as a boss who “saved” projects for their return.
Colleague Responses
One participant used the term “microaggressions,” to describe passive aggressions encountered by their colleagues or leadership.
“(A colleague) was diagnosed with pre-eclampsia, and very urgently had to deliver and couldn’t cover a week of shifts…She was asked initially to find her own coverage…Not treating (pregnancy) similar to other serious illnesses is what I would term a microaggression.”
Yet, women in our study also reported positive responses from colleagues and the importance of support networks of physician mothers (Table 2).
Empathy in Patient Care
Finally, the experience of motherhood impacted all of the women as physicians, described as increased empathy, patience, and understanding of difficult family situations.
“I’m just more sensitive to people’s lives outside the hospital, so, you know, when it’s difficult for a family member to get there because they have three other kids they are taking care of or, somebody that says they are leaving AMA, but it’s because they have a sick kid at home. I just have a better context for that.”
DISCUSSION
Gender disparities persist in both internal medicine and hospital medicine.1 Providers in this descriptive qualitative study suggested that the following factors contribute: lack of paid parental leave and the associated financial penalties, loss of career opportunities, the physical challenges associated with pregnancy, decreasing productivity, and the amount of time and effort involved in breastfeeding. However, the participants also shared valuable ideas for future solutions to relieve the challenges imposed on working physician mothers (Table 2).
Breaking the Glass Ceiling
Participants noted the importance of a paid leave policy that encompasses not only maternity leave but also a flexible scheduling period before and after the leave to account for the challenges of pregnancy and new motherhood. Paid parental leave is rare in academic settings, but studies from other industries show that when women take paid leave, they are more likely to remain in the workforce 9-12 months afterward, work more weekly hours, and feel more loyal to their organization.12,13 In the rare instance when negotiations around leave violate local policy or the law, women should be encouraged to seek guidance from their human resources department.
Me Too: Building Solidarity
Women in our study reported the value of a supportive workplace in easing their transition into motherhood. Specifically, they noted that a supportive boss who protected their career opportunities prevented momentum loss in their career trajectory. Access to mutual supports such as the Physicians Mom Group, a well-established Facebook group comprising more than 70,000 women, was referenced as a meaningful way to share joys and tribulations related to balancing a career as a physician and motherhood. Growth of similar support systems within institutions will further support this experience.
Time’s Up: The Promotion Clock
Women in our study described a prolonged period of diminished productivity related to having children, coinciding with a set time to promotion in academics. Flexible promotion schedules may impact women’s ability to successfully undergo promotion.
FUTURE DIRECTION
The aim of this study was to represent a shared set of experiences of female academic hospitalists who participated; therefore, the results may not be generalizable beyond this group. Due to the use of a purposeful snowball approach, there was a potential for selection bias. Future research may include comparing the experience of women at institutions that offer paid leave versus those that do not and the impact on retention, promotion, and well-being.
CONCLUSION
Women in hospital medicine encounter several challenges to having children, but they are also motivated to provide solutions. Efforts to improve the institutional and cultural landscape to better support women physicians with children are critical to prevent attrition of women and ensure equitable academic promotion and achievement of leadership positions.
Disclosures
The authors have no conflicts of interest to report.
Author Contributions
Each author was involved in the creation of the study protocol, data collection and analysis, and creation of the manuscript.
Despite recent advances made in medicine, gender-based disparities persist.1-3 In particular, women with children have barriers to career advancement and show evidence of slower career advancement.1,2 Multiple challenges for working women experiencing motherhood have been described. In academic medicine in the United States, women have limited access to paid parental leave.4-6 For women who choose to breastfeed, there is limited time, space, and support available for breastfeeding.7 Furthermore, sleep deprivation in the postpartum period significantly impacts the ability to function at work.8
Hospital medicine is a unique specialty as it comprises 47% women, 80% of whom are aged less than 40 years, suggesting that a large portion are women of childbearing age.9 The field poses known challenges to this population, including shift work, atypical schedules, and unpredictable hours. We conducted a descriptive qualitative study to improve our understanding of the experience of female academic hospitalists who have experienced pregnancy, parental leave, and the return to work as faculty. Our goal was to both explore the challenges to undergoing this experience and discover solutions to support female academic hospitalists.
METHODS
Study Design
We conducted a qualitative descriptive study of female hospitalists recruited from academic institutions represented in Society of Hospital Medicine (SHM) committees. Interviews were conducted between November 2017 and February 2018. Participants completed an informed consent and a demographic survey prior to the interview. Each interview lasted approximately 30 minutes; discussions were recorded on digital records and transcribed verbatim. This protocol was reviewed and granted exemption by the Institutional Review Board at the University of Colorado.
Population
We recruited participants from a selection of hospital medicine groups nationally, chosen from SHM committee representation. A purposeful snowball approach was used to identify hospitalists from representative programs and seek their recommendation for hospitalists from other targeted programs. Ten hospitalists were approached by e-mail to determine their interest in participation, and all of them agreed to participate. Each participant experienced new parenthood within the last seven years.
Framework
We constructed our interview to represent the following timeline associated with having children as it pertains to a hospitalist position: pregnancy, parental leave, and the return to work. The interview guide was structured to invoke the positive aspects, challenges, and solutions within each domain (Appendix 1).
Analysis
Codes were inductively developed from the interview data by a team of three board-certified internal medicine physicians (E.G., A.M., and C.J.), one of whom had prior training and experience with qualitative interviews and analysis (C.J.). Among the coders, two (E.G. and A.M.) conducted the semistructured interviews. Code disparities were reconciled by team consensus, where the primary coder facilitated the discussions. Themes were developed inductively from the codes, and the analysis was completed using a team-based iterative approach that was facilitated using ATLAS.ti.10 Thematic saturation was achieved. This study was approved by the Colorado Multiple Institutional Review Board.
RESULTS
The demographics and the characteristics of the hospital medicine group are shown in Table 1. Although we asked questions about both the positive and challenging aspects of the experience of parenthood, the interviews tended to focus more on the challenges faced and on areas for optimization.
Paid Parental leave
Most of the participants described inadequate paid parental leave, with minimal transparency in the processes for ensuring time off following the birth of their child, resulting in “haggling” with bosses, human resources, and the administrative staff. Rarely was a formal parental leave policy in place. Once a parental leave plan was established, several women reported the financial burden associated with a leave that was partially, or fully, unpaid.
“All of my leave was unpaid. .. managed to finagle short-term disability into paying for it… the system was otherwise set up to screw me financially.”
For the three women who did experience sufficient paid parental leave, they recognized the financial and emotional benefit and suggested that further optimization would include a prebirth schedule to account for the physical challenges and potential complications.
Physical Challenges
All of the women described significant physical challenges when working during pregnancy, resulting in limited bandwidth for additional academic activities outside of direct clinical care responsibilities.
“Exhaustion that hits you in your pregnancy and then you have to round. I used to lie on the floor of my office, take a little nap, wake up, write some notes, go home, take another nap, wake up, write some more notes.”
Upon return to work, women reported additional physical challenges related to sleep deprivation, impacting their productivity with academic work and emotional well-being.
“I came back from maternity leave and I was sleep-deprived and exhausted, I didn’t have the energy. All of these great projects that I had started or dreamed of … dwindled and died on the vine.”
Solutions suggested by the participants included creation of a flexible schedule with a ramp-up and ramp-down period around the birth.
Breastfeeding
The majority of participants in this study encountered several challenges associated with a shared goal of breastfeeding according to evidence-based guidelines.11 Designated pumping areas were often inconveniently located and not conducive to multitasking.
“It’s two chairs that are behind a curtain in a women’s locker room in the basement of the hospital, that are tiny and gross. No computers, so I felt like I was wasting time.”
One hospitalist described carving out time for pumping in her office while multitasking with clinical work.
“I would get to work, set up, and pump while chart reviewing. Then I would go and see people… and come back to my office and pump and write a few notes. And go out and see more patients, and then pump and write a few more notes. And then pump, and then go home. I was like a cow.”
Women highlighted the barriers that could be optimized such as creating time in the clinical schedule for pumping, a physical space to breastfeed or pump, and accessible milk storage facilities.
Career Opportunities
When asked about the impact of parental leave on career opportunities, a few of the women described a phenomenon of no longer being asked to participate or being left out of prior projects.
“People didn’t want to offer you things or give you things because they realize you’re having this transition in your life. Not out of animosity, but out of courtesy that they don’t want to fill up your place even more. Her plate is full; we are not going to ask her to do anything extra.”
However, two women specifically reported a supportive environment without a loss of opportunities, often referenced as a boss who “saved” projects for their return.
Colleague Responses
One participant used the term “microaggressions,” to describe passive aggressions encountered by their colleagues or leadership.
“(A colleague) was diagnosed with pre-eclampsia, and very urgently had to deliver and couldn’t cover a week of shifts…She was asked initially to find her own coverage…Not treating (pregnancy) similar to other serious illnesses is what I would term a microaggression.”
Yet, women in our study also reported positive responses from colleagues and the importance of support networks of physician mothers (Table 2).
Empathy in Patient Care
Finally, the experience of motherhood impacted all of the women as physicians, described as increased empathy, patience, and understanding of difficult family situations.
“I’m just more sensitive to people’s lives outside the hospital, so, you know, when it’s difficult for a family member to get there because they have three other kids they are taking care of or, somebody that says they are leaving AMA, but it’s because they have a sick kid at home. I just have a better context for that.”
DISCUSSION
Gender disparities persist in both internal medicine and hospital medicine.1 Providers in this descriptive qualitative study suggested that the following factors contribute: lack of paid parental leave and the associated financial penalties, loss of career opportunities, the physical challenges associated with pregnancy, decreasing productivity, and the amount of time and effort involved in breastfeeding. However, the participants also shared valuable ideas for future solutions to relieve the challenges imposed on working physician mothers (Table 2).
Breaking the Glass Ceiling
Participants noted the importance of a paid leave policy that encompasses not only maternity leave but also a flexible scheduling period before and after the leave to account for the challenges of pregnancy and new motherhood. Paid parental leave is rare in academic settings, but studies from other industries show that when women take paid leave, they are more likely to remain in the workforce 9-12 months afterward, work more weekly hours, and feel more loyal to their organization.12,13 In the rare instance when negotiations around leave violate local policy or the law, women should be encouraged to seek guidance from their human resources department.
Me Too: Building Solidarity
Women in our study reported the value of a supportive workplace in easing their transition into motherhood. Specifically, they noted that a supportive boss who protected their career opportunities prevented momentum loss in their career trajectory. Access to mutual supports such as the Physicians Mom Group, a well-established Facebook group comprising more than 70,000 women, was referenced as a meaningful way to share joys and tribulations related to balancing a career as a physician and motherhood. Growth of similar support systems within institutions will further support this experience.
Time’s Up: The Promotion Clock
Women in our study described a prolonged period of diminished productivity related to having children, coinciding with a set time to promotion in academics. Flexible promotion schedules may impact women’s ability to successfully undergo promotion.
FUTURE DIRECTION
The aim of this study was to represent a shared set of experiences of female academic hospitalists who participated; therefore, the results may not be generalizable beyond this group. Due to the use of a purposeful snowball approach, there was a potential for selection bias. Future research may include comparing the experience of women at institutions that offer paid leave versus those that do not and the impact on retention, promotion, and well-being.
CONCLUSION
Women in hospital medicine encounter several challenges to having children, but they are also motivated to provide solutions. Efforts to improve the institutional and cultural landscape to better support women physicians with children are critical to prevent attrition of women and ensure equitable academic promotion and achievement of leadership positions.
Disclosures
The authors have no conflicts of interest to report.
Author Contributions
Each author was involved in the creation of the study protocol, data collection and analysis, and creation of the manuscript.
1. Association of American Medical Colleges. The State of Women in Academic Medicine: The pipeline and pathways to leadership, 2013-2014. https://www.hopkinsmedicine.org/women_science_medicine/_pdfs/The%20State%20of%20Women%20in%20Academic%20Medicine%202013-2014%20FINAL.pdf. Accessed February 26, 2018.
2. Carr PL, Ash AS, Friedman RH, et al. Relation of family responsibilities and gender to the productivity and career satisfaction of medical faculty. Ann Int Med. 1998;129(7):532-538. doi: 10.7326/0003-4819-129-7-199810010-00004. PubMed
3. Burden M, Frank MG, Keniston A, et al. Gender disparities for academic hospitalists. J Hosp Med. 2015;10(8):481-485. doi:10.1002/jhm.2340. PubMed
4. Bristol MN, Abbuhl S, Cappola AR, Sonnad SS. Work-life policies for faculty at the top ten medical schools. J Women’s Health. 2008;17(8):1311-1320. doi: 10.1089/jwh.2007.0682. PubMed
5. Welch JL, Wiehe SE, Palmer-Smith V, Dankoski ME. Flexibility in faculty work-life policies at medical schools in the big ten conference. J Women’s Health. 2011;20(5):725-732. doi: 10.1089/jwh.2010.2553. PubMed
6. Riano NS, Linos E, Accurso EC, et al. Paid family and childbearing leave policies at top US medical schools. JAMA. 2018;319(6):611-614. doi: 10.1001/jama.2017.19519. PubMed
7. Arthur CR, Saenz RB, Replogle WH. The employment-related breastfeeding decisions of physician mothers. J Miss State Med Assoc. 2003;44(12):383-387. PubMed
8. Filtness AJ, MacKenzie J, Armstrong K. Longitudinal change in sleep and daytime sleepiness in postpartum women. PLoS ONE. 2014;9(7):e103513. doi: 10.1371/journal.pone.0103513. PubMed
9. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. doi: 10.1007/s11606-011-1892-5. PubMed
10. Jones J, Nowels CT, Sudore R, Ahluwalia S, Bekelman DB. The future as a series of transitions: qualitative study of heart failure patients and their informal caregivers. J Gen Intern Med. 2015;30(2):176-182. doi: 10.1007/s11606-014-3085-5. PubMed
11. American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827-e841. doi: 10.1542/peds.2011-3552. PubMed
12. Houser, L, Vartanian, T. Pay matters: the positive economic impact of paid family Leave for families, businesses and the public. Center for Women and Work at Rutgers. January, 2012. http://go.nationalpartnership.org/site/DocServer/Pay_Matters_Positive_Economic_Impacts_of_Paid_Fam ily_L.pdf?docID=9681. Accessed February 26, 2018.
13. Rossin-Slater M, Ruhm C, Waldfogel J. The effects of California’s paid family leave program on mothers’ leave-taking and subsequent labor market outcomes. J Policy Anal Manage. 2013;32(2):224-2 45. doi: 10.1002/pam.21676. PubMed
1. Association of American Medical Colleges. The State of Women in Academic Medicine: The pipeline and pathways to leadership, 2013-2014. https://www.hopkinsmedicine.org/women_science_medicine/_pdfs/The%20State%20of%20Women%20in%20Academic%20Medicine%202013-2014%20FINAL.pdf. Accessed February 26, 2018.
2. Carr PL, Ash AS, Friedman RH, et al. Relation of family responsibilities and gender to the productivity and career satisfaction of medical faculty. Ann Int Med. 1998;129(7):532-538. doi: 10.7326/0003-4819-129-7-199810010-00004. PubMed
3. Burden M, Frank MG, Keniston A, et al. Gender disparities for academic hospitalists. J Hosp Med. 2015;10(8):481-485. doi:10.1002/jhm.2340. PubMed
4. Bristol MN, Abbuhl S, Cappola AR, Sonnad SS. Work-life policies for faculty at the top ten medical schools. J Women’s Health. 2008;17(8):1311-1320. doi: 10.1089/jwh.2007.0682. PubMed
5. Welch JL, Wiehe SE, Palmer-Smith V, Dankoski ME. Flexibility in faculty work-life policies at medical schools in the big ten conference. J Women’s Health. 2011;20(5):725-732. doi: 10.1089/jwh.2010.2553. PubMed
6. Riano NS, Linos E, Accurso EC, et al. Paid family and childbearing leave policies at top US medical schools. JAMA. 2018;319(6):611-614. doi: 10.1001/jama.2017.19519. PubMed
7. Arthur CR, Saenz RB, Replogle WH. The employment-related breastfeeding decisions of physician mothers. J Miss State Med Assoc. 2003;44(12):383-387. PubMed
8. Filtness AJ, MacKenzie J, Armstrong K. Longitudinal change in sleep and daytime sleepiness in postpartum women. PLoS ONE. 2014;9(7):e103513. doi: 10.1371/journal.pone.0103513. PubMed
9. Reid MB, Misky GJ, Harrison RA, Sharpe B, Auerbach A, Glasheen JJ. Mentorship, productivity, and promotion among academic hospitalists. J Gen Intern Med. 2012;27(1):23-27. doi: 10.1007/s11606-011-1892-5. PubMed
10. Jones J, Nowels CT, Sudore R, Ahluwalia S, Bekelman DB. The future as a series of transitions: qualitative study of heart failure patients and their informal caregivers. J Gen Intern Med. 2015;30(2):176-182. doi: 10.1007/s11606-014-3085-5. PubMed
11. American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics. 2012;129(3):e827-e841. doi: 10.1542/peds.2011-3552. PubMed
12. Houser, L, Vartanian, T. Pay matters: the positive economic impact of paid family Leave for families, businesses and the public. Center for Women and Work at Rutgers. January, 2012. http://go.nationalpartnership.org/site/DocServer/Pay_Matters_Positive_Economic_Impacts_of_Paid_Fam ily_L.pdf?docID=9681. Accessed February 26, 2018.
13. Rossin-Slater M, Ruhm C, Waldfogel J. The effects of California’s paid family leave program on mothers’ leave-taking and subsequent labor market outcomes. J Policy Anal Manage. 2013;32(2):224-2 45. doi: 10.1002/pam.21676. PubMed
© 2018 Society of Hospital Medicine
Who Consults Us and Why? An Evaluation of Medicine Consult/Comanagement Services at Academic Medical Centers
The role of internists in consultation has considerably expanded over the past half century. Consulting general internists increasingly work across disciplines to coordinate complex care.1,2 Some internists assume a “comanagement” role with surgical specialties. This role requires sharing responsibility and accountability and involvement in admission/discharge processes.3-6 Internal medicine (IM) residents are required to serve as consultants.7 Yet, aside from observations collected 30 to 40 years ago, limited information is available for guiding educators in developing consultative curricula.2,8-10 We sought to assess current consultative practices across a sample of IM training programs. Specifically, we examined which services consult IM and their reasons for consultation (RFCs).
METHODS
We collected data on consultation requests at 11 US academic medical centers (AMCs). We applied a selective sampling approach that leveraged existing relationships and interest in consultative medicine to identify institutions across a variety of geographic locations. We collected data regarding the consult service structure at each site, including data on the presence or absence of comanagement services and consult requests received.
Data Collection Tool
Investigators at the University of Texas Health San Antonio (UTHSA) drafted the data collection tool. Iterative feedback on the data collection tool was obtained from the research consortium (final tool, Supplemental Figure). Data collected included service requesting consultation, RFC, time request was made (day/night), who first saw the patient (eg, resident, attending), whether requesting and consulting providers verbally communicated, and whether patients were transferred to medicine. Respondents also estimated how often RFCs were encountered during their general medicine services.
To streamline data collection, we used click boxes and drop-down lists that included diagnoses and symptoms. The use of these predetermined RFCs was based on prior studies and discussion with the research consortium on common RFCs in clinical practice. A write-in field was also included. Respondents could select multiple RFCs in the case of multiple questions. Respondents also provided data regarding clinical issues that were incidentally identified during their initial patient assessments. Incidentally identified issues are hereafter called “additional RFCs” for differentiation from stated RFCs. Prior to data collection, the tool was piloted at UTHSA.
Data Collection, Categorization, and Analysis
Participants submitted data using Survey Monkey (Palo Alto, California). Emails with the survey link were sent daily. Specific participants for each data collection period were chosen by each site. Days with no data entry were confirmed by the study coordinator. Each institution collected data for four 2-week periods from July 2014 to July 2015 for a total of 8 weeks. We did not track follow-up encounters. Repeat consultations for different reasons were considered new consults.
All survey responses and free-text RFC entries were independently reviewed and categorized by 2 authors (E.W. and M.S.). New categories were created if needed. If reviewers disagreed, a third reviewer (C.M.) reviewed the RFC. The research consortium reviewed the final list of categories and entries.
We calculated descriptive statistics using SAS version 9.3 (SAS Institute, Inc., Cary, North Carolina). Each analysis used complete responses for each survey component. We separately analyzed services with and without comanagement components. The study was approved by UTHSA’s Institutional Review Board.
RESULTS
A total of 11 AMCs that represent 9 academic affiliations participated in this study (Table 1). Of the 11 AMCs, 7 were public nonprofit, 3 were private nonprofit, and 1 was a Veterans Health Administration facility. Out of the 11 AMCs, 9 sites included residents on the consult service, and the rotation was required at 6 of the sites. Most sites with residents had a formal curriculum that ranged from curated articles to online modules. Out of the 11 services, 4 were consult and comanagement services. All 4 co-managed orthopedic patients, and 1 also included other patients.
Data for 1,264 patient encounters with 2,778 RFCs were collected. A total of 1,218 of the surveys (96.4%) were fully completed, and only 5 surveys were missing data for multiple questions. A total of 7 sites adhered to the planned protocol. Among the sites, 1 site had 1 incomplete collection period, 1 site missed 1 collection period, and 1 site missed 2 collection periods.
Most consultations (87.1%) were requested during the day. Many patients (55.9%) were initially seen by residents, and 32.4% of the patients were initially seen by an attending. Respondents reported communicating verbally with the requesting team in 93.9% of instances. Among the patients, 7.8% were transferred to medicine following initial consultation. This percentage was higher (10.2%) in services without comanagement.
The average number of new consults per day per site was 2.24. The range for individual sites was 1.36-3.48. The maximum number of new consults in 1 day was 10. All sites had at least 1 day without new consults. The mean number of RFCs per encounter was 2.20 (median 2, range 1-13). In 226 of 360 encounters in which comanagement was an RFC, the respondent enumerated the other specific RFCs addressed. In these encounters, the mean number of RFCs (in addition to comanagement) was 3.02.
Most requests (82.2%) originated from surgical services. Among all surgical services, orthopedic surgery requested the highest number of consultations (67.5% for services with a comanagement component; 28.5% for services without) and 81.2% of the 360 comanagement encounters. Refer to Supplemental Table 1 for detailed information on the services that requested consultation.
The most common RFC was comanagement (13.0% across the entire study; 23.3% for services with a comanagement component; Table 2). For services without comanagement, preoperative evaluation was the most common RFC (16.4%). Other frequent RFCs across the entire study included blood pressure management (8.9%), glycemic management (7.2%), and renal failure (3.9%). Additional (unstated) RFCs were addressed in 944 patients (34.0%), and blood pressure management was the most common additional RFC.
Respondents indicated that 54.9% of RFCs were clinical topics that are “often” or “always” encountered in IM inpatient services. In 11.8% of encounters, the RFC was “rarely” or “never” encountered; the most common RFCs in such encounters were comanagement (53.4%), preoperative evaluation (17.4%), and transfer to medicine (5.4%).
DISCUSSION
Our study provides insights into the consultative landscape of AMCs and identified who consults IMs and their RFCs. Thus, our study has implications for resident consultative education. The consult services included in our study presented varied structures, including those that require medicine consultation as a resident rotation and those with comanagement agreements. Consistent with the results of prior studies, surgical services requested the majority of consults, with orthopedic surgery generating the highest number of requests. Consultation requests from neurosurgery were higher than previously reported.2,8,9
Our study reveals that comanagement and preoperative evaluation are the most common RFCs and are the least commonly encountered RFCs in IM inpatient services. The broad nature of these RFCs speaks to an increasing need for comprehensive consultative care. Consultants addressed a wide range of clinical issues, including rare entities that defy easy categorization (eg, Moyamoya disease). This broad landscape presents challenges in focusing curricular content areas outside of comanagement and preoperative evaluation but does provide evidence “to expect the unexpected” in IM consultation, as has been previously noted.8
In over a third of encounters, consultants addressed an issue that was not stated in the initial RFC. Consultants also addressed more than 2 RFCs per encounter. These observations suggest that medicine consult services may be essentially comanaging some patients even when a comanagement care model is not formally in place. These findings provide rationale for the continued expansion of comanagement services.11
Our study provides further evidence that, in modern consultative practice, “determining your customer” is more important than “determining the question.”12-14 We work in an era in which comanagement services are increasingly prevalent but are not ubiquitous and in which IM consultants routinely address multiple issues. Prior studies indicated that most surgeons do not believe that consults should be limited to specific questions and instead prefer comanagement.13 Understanding the expectations of the requesting physician is therefore important and highlights the importance of verbal communication at the time of initial consultation. Ongoing interprofessional communication is a vital skill that residents should acquire.
Our study has several limitations. Although our sites represented a varied sample, we focused on AMCs. Therefore, our study may not reflect consultative experiences in nonacademic hospitals or sites without dedicated consult services. Trade-offs exist in our data collection approach, which provided predetermined RFCs. We selected our methodology to facilitate data entry and to aid RFC categorization. Nevertheless, it may have lessened the clinical nuance of submitted data. The provision of predetermined RFCs may have influenced issue selection by the respondents. However, in 473 encounters (37.4%), the survey respondents provided free-text entries for the stated RFC, and 944 additional RFCs were written in as responses. These results demonstrated that respondents did not limit themselves to the predetermined list. We did not perform chart reviews to validate data. Finally, our data were a cross-section of initial consultations. We lack information on subsequent diagnoses or additional clinical issues that developed later.
In conclusion, we found varied consultative experiences across AMCs. However, preoperative evaluation and perioperative comanagement – particularly of orthopedic and neurosurgical patients – were common and should be included in curricula. Faculty should recognize the unique nature of IM consultation to prepare residents. Specifically, faculty should prepare residents to expect to identify and address unstated medical issues and to provide comprehensive assessments regardless of whether the consultative structure has a comanagement component. Given the unique nature of consultative IM work and the possibility of discordant expectations between consulting and requesting physicians, perhaps the most valuable skill to impart to residents is effective and regular communication.
Medicine Consult/Comanagement Consortium Members
The Medicine Consult/Comanagement Consortium consists of: Mary Anderson Wallace, MD, Brian Wolfe, MD (University of Colorado), Meridale Baggett, MD, Douglas Wright, MD, PhD (Harvard University), Joyeeta G. Dastidar MD, Maureen Kelly, MD (Columbia University), Leonard S. Feldman, MD (Johns Hopkins University), Cecily J. Gallup, MD, MPH (University of California, San Francisco), Paul J. Grant, MD (University of Michigan), Craig R. Keenan, MD (University of California, Davis), Fletcher Penney, MD (Medical University of South Carolina).
Acknowledgments
The authors thank the clinicians at each site who were involved in data collection for this study, including Barbara Statland, MD. The authors also thank Timothy Niessen, MD for data and physician coordination and Musarrat Nahid, MSc. for statistical analysis.
Disclosures
Paul J. Grant receives royalties from the medical textbook Perioperative Medicine: Medical Consultation and Comanagement, Wiley Publishing 2012. Craig R. Keenan receives medicolegal consultation fees from Weiss-Salinas Law Group and American Psychiatric Association Publishers for book royalties. All other authors declare that they do not have any conflicts of interest.
Funding Information
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration. Investigator salary support is provided through the South Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or our institutions.
1. Hollenberg CH, Langley GR. The Canadian general internist: education and future role. CMAJ. 1978;118(4):397-400. PubMed
2. Charlson ME, Cohen RP, Sears CL. General medicine consultation: lessons from a clinical service. Am J Med. 1983;75(1):121-128. https://doi.org/10.1016/0002-9343(83)91175-0. PubMed
3. Society of Hospital Medicine. The evolution of co-management in hospital medicine. http://www.hospitalmedicine.org/Web/Practice_Management/CoManagement.aspx. Accessed March 8, 2018.
4. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. 10.1001/archinternmed.2010.432. PubMed
5. Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368. 10.1001/archinternmed.2009.553. PubMed
6. Thompson RE, Pfeifer K, Grant PJ, et al. Hospital medicine and perioperative care: a framework for high-quality, high-value collaborative care. J Hosp Med. 2017;12(4):277-282. 10.12788/jhm.2717. PubMed
7. Accreditation Council for Graduate Medical Education. Common Program Requirements. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/140_internal_medicine_2017-07-01.pdf. Accessed March 8, 2018.
8. Moore RA, Kammerer WS, McGlynn TJ, Trautlein JJ, Burnside JW. Consultations in internal medicine: a training program resource. J Med Educ. 1977;52(4):323-327. PubMed
9. Robie PW. The service and educational contributions of a general medicine consultation service. J Gen Intern Med. 1986;1(4):225-227. https://doi.org/10.1007/BF02596187. PubMed
10. Devor M, Renvall M, Ramsdell J. Practice patterns and the adequacy of residency training in consultation medicine. J Gen Intern Med. 1993;8(10):554-560. 10.1007/BF02599639. PubMed
11. Siegal EM. Just because you can, doesn’t mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398-402. 10.1002/jhm.361. PubMed
12. Goldman L, Lee T, Rudd P. Ten commandments for effective consultations. Arch Intern Med. 1983;143(9):1753-1755. 10.1001/archinte.1983.00350090131022. PubMed
13. Salerno SM. Principles of effective consultation: an update for the 21st-century consultant. Arch Intern Med. 2007;167:271-275. 10.1001/archinte.167.3.271. PubMed
14. Merli GJ, Weitz HH. Medical management of the surgical patient E-Book. Elsevier Health Sciences; 2008. PubMed
The role of internists in consultation has considerably expanded over the past half century. Consulting general internists increasingly work across disciplines to coordinate complex care.1,2 Some internists assume a “comanagement” role with surgical specialties. This role requires sharing responsibility and accountability and involvement in admission/discharge processes.3-6 Internal medicine (IM) residents are required to serve as consultants.7 Yet, aside from observations collected 30 to 40 years ago, limited information is available for guiding educators in developing consultative curricula.2,8-10 We sought to assess current consultative practices across a sample of IM training programs. Specifically, we examined which services consult IM and their reasons for consultation (RFCs).
METHODS
We collected data on consultation requests at 11 US academic medical centers (AMCs). We applied a selective sampling approach that leveraged existing relationships and interest in consultative medicine to identify institutions across a variety of geographic locations. We collected data regarding the consult service structure at each site, including data on the presence or absence of comanagement services and consult requests received.
Data Collection Tool
Investigators at the University of Texas Health San Antonio (UTHSA) drafted the data collection tool. Iterative feedback on the data collection tool was obtained from the research consortium (final tool, Supplemental Figure). Data collected included service requesting consultation, RFC, time request was made (day/night), who first saw the patient (eg, resident, attending), whether requesting and consulting providers verbally communicated, and whether patients were transferred to medicine. Respondents also estimated how often RFCs were encountered during their general medicine services.
To streamline data collection, we used click boxes and drop-down lists that included diagnoses and symptoms. The use of these predetermined RFCs was based on prior studies and discussion with the research consortium on common RFCs in clinical practice. A write-in field was also included. Respondents could select multiple RFCs in the case of multiple questions. Respondents also provided data regarding clinical issues that were incidentally identified during their initial patient assessments. Incidentally identified issues are hereafter called “additional RFCs” for differentiation from stated RFCs. Prior to data collection, the tool was piloted at UTHSA.
Data Collection, Categorization, and Analysis
Participants submitted data using Survey Monkey (Palo Alto, California). Emails with the survey link were sent daily. Specific participants for each data collection period were chosen by each site. Days with no data entry were confirmed by the study coordinator. Each institution collected data for four 2-week periods from July 2014 to July 2015 for a total of 8 weeks. We did not track follow-up encounters. Repeat consultations for different reasons were considered new consults.
All survey responses and free-text RFC entries were independently reviewed and categorized by 2 authors (E.W. and M.S.). New categories were created if needed. If reviewers disagreed, a third reviewer (C.M.) reviewed the RFC. The research consortium reviewed the final list of categories and entries.
We calculated descriptive statistics using SAS version 9.3 (SAS Institute, Inc., Cary, North Carolina). Each analysis used complete responses for each survey component. We separately analyzed services with and without comanagement components. The study was approved by UTHSA’s Institutional Review Board.
RESULTS
A total of 11 AMCs that represent 9 academic affiliations participated in this study (Table 1). Of the 11 AMCs, 7 were public nonprofit, 3 were private nonprofit, and 1 was a Veterans Health Administration facility. Out of the 11 AMCs, 9 sites included residents on the consult service, and the rotation was required at 6 of the sites. Most sites with residents had a formal curriculum that ranged from curated articles to online modules. Out of the 11 services, 4 were consult and comanagement services. All 4 co-managed orthopedic patients, and 1 also included other patients.
Data for 1,264 patient encounters with 2,778 RFCs were collected. A total of 1,218 of the surveys (96.4%) were fully completed, and only 5 surveys were missing data for multiple questions. A total of 7 sites adhered to the planned protocol. Among the sites, 1 site had 1 incomplete collection period, 1 site missed 1 collection period, and 1 site missed 2 collection periods.
Most consultations (87.1%) were requested during the day. Many patients (55.9%) were initially seen by residents, and 32.4% of the patients were initially seen by an attending. Respondents reported communicating verbally with the requesting team in 93.9% of instances. Among the patients, 7.8% were transferred to medicine following initial consultation. This percentage was higher (10.2%) in services without comanagement.
The average number of new consults per day per site was 2.24. The range for individual sites was 1.36-3.48. The maximum number of new consults in 1 day was 10. All sites had at least 1 day without new consults. The mean number of RFCs per encounter was 2.20 (median 2, range 1-13). In 226 of 360 encounters in which comanagement was an RFC, the respondent enumerated the other specific RFCs addressed. In these encounters, the mean number of RFCs (in addition to comanagement) was 3.02.
Most requests (82.2%) originated from surgical services. Among all surgical services, orthopedic surgery requested the highest number of consultations (67.5% for services with a comanagement component; 28.5% for services without) and 81.2% of the 360 comanagement encounters. Refer to Supplemental Table 1 for detailed information on the services that requested consultation.
The most common RFC was comanagement (13.0% across the entire study; 23.3% for services with a comanagement component; Table 2). For services without comanagement, preoperative evaluation was the most common RFC (16.4%). Other frequent RFCs across the entire study included blood pressure management (8.9%), glycemic management (7.2%), and renal failure (3.9%). Additional (unstated) RFCs were addressed in 944 patients (34.0%), and blood pressure management was the most common additional RFC.
Respondents indicated that 54.9% of RFCs were clinical topics that are “often” or “always” encountered in IM inpatient services. In 11.8% of encounters, the RFC was “rarely” or “never” encountered; the most common RFCs in such encounters were comanagement (53.4%), preoperative evaluation (17.4%), and transfer to medicine (5.4%).
DISCUSSION
Our study provides insights into the consultative landscape of AMCs and identified who consults IMs and their RFCs. Thus, our study has implications for resident consultative education. The consult services included in our study presented varied structures, including those that require medicine consultation as a resident rotation and those with comanagement agreements. Consistent with the results of prior studies, surgical services requested the majority of consults, with orthopedic surgery generating the highest number of requests. Consultation requests from neurosurgery were higher than previously reported.2,8,9
Our study reveals that comanagement and preoperative evaluation are the most common RFCs and are the least commonly encountered RFCs in IM inpatient services. The broad nature of these RFCs speaks to an increasing need for comprehensive consultative care. Consultants addressed a wide range of clinical issues, including rare entities that defy easy categorization (eg, Moyamoya disease). This broad landscape presents challenges in focusing curricular content areas outside of comanagement and preoperative evaluation but does provide evidence “to expect the unexpected” in IM consultation, as has been previously noted.8
In over a third of encounters, consultants addressed an issue that was not stated in the initial RFC. Consultants also addressed more than 2 RFCs per encounter. These observations suggest that medicine consult services may be essentially comanaging some patients even when a comanagement care model is not formally in place. These findings provide rationale for the continued expansion of comanagement services.11
Our study provides further evidence that, in modern consultative practice, “determining your customer” is more important than “determining the question.”12-14 We work in an era in which comanagement services are increasingly prevalent but are not ubiquitous and in which IM consultants routinely address multiple issues. Prior studies indicated that most surgeons do not believe that consults should be limited to specific questions and instead prefer comanagement.13 Understanding the expectations of the requesting physician is therefore important and highlights the importance of verbal communication at the time of initial consultation. Ongoing interprofessional communication is a vital skill that residents should acquire.
Our study has several limitations. Although our sites represented a varied sample, we focused on AMCs. Therefore, our study may not reflect consultative experiences in nonacademic hospitals or sites without dedicated consult services. Trade-offs exist in our data collection approach, which provided predetermined RFCs. We selected our methodology to facilitate data entry and to aid RFC categorization. Nevertheless, it may have lessened the clinical nuance of submitted data. The provision of predetermined RFCs may have influenced issue selection by the respondents. However, in 473 encounters (37.4%), the survey respondents provided free-text entries for the stated RFC, and 944 additional RFCs were written in as responses. These results demonstrated that respondents did not limit themselves to the predetermined list. We did not perform chart reviews to validate data. Finally, our data were a cross-section of initial consultations. We lack information on subsequent diagnoses or additional clinical issues that developed later.
In conclusion, we found varied consultative experiences across AMCs. However, preoperative evaluation and perioperative comanagement – particularly of orthopedic and neurosurgical patients – were common and should be included in curricula. Faculty should recognize the unique nature of IM consultation to prepare residents. Specifically, faculty should prepare residents to expect to identify and address unstated medical issues and to provide comprehensive assessments regardless of whether the consultative structure has a comanagement component. Given the unique nature of consultative IM work and the possibility of discordant expectations between consulting and requesting physicians, perhaps the most valuable skill to impart to residents is effective and regular communication.
Medicine Consult/Comanagement Consortium Members
The Medicine Consult/Comanagement Consortium consists of: Mary Anderson Wallace, MD, Brian Wolfe, MD (University of Colorado), Meridale Baggett, MD, Douglas Wright, MD, PhD (Harvard University), Joyeeta G. Dastidar MD, Maureen Kelly, MD (Columbia University), Leonard S. Feldman, MD (Johns Hopkins University), Cecily J. Gallup, MD, MPH (University of California, San Francisco), Paul J. Grant, MD (University of Michigan), Craig R. Keenan, MD (University of California, Davis), Fletcher Penney, MD (Medical University of South Carolina).
Acknowledgments
The authors thank the clinicians at each site who were involved in data collection for this study, including Barbara Statland, MD. The authors also thank Timothy Niessen, MD for data and physician coordination and Musarrat Nahid, MSc. for statistical analysis.
Disclosures
Paul J. Grant receives royalties from the medical textbook Perioperative Medicine: Medical Consultation and Comanagement, Wiley Publishing 2012. Craig R. Keenan receives medicolegal consultation fees from Weiss-Salinas Law Group and American Psychiatric Association Publishers for book royalties. All other authors declare that they do not have any conflicts of interest.
Funding Information
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration. Investigator salary support is provided through the South Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or our institutions.
The role of internists in consultation has considerably expanded over the past half century. Consulting general internists increasingly work across disciplines to coordinate complex care.1,2 Some internists assume a “comanagement” role with surgical specialties. This role requires sharing responsibility and accountability and involvement in admission/discharge processes.3-6 Internal medicine (IM) residents are required to serve as consultants.7 Yet, aside from observations collected 30 to 40 years ago, limited information is available for guiding educators in developing consultative curricula.2,8-10 We sought to assess current consultative practices across a sample of IM training programs. Specifically, we examined which services consult IM and their reasons for consultation (RFCs).
METHODS
We collected data on consultation requests at 11 US academic medical centers (AMCs). We applied a selective sampling approach that leveraged existing relationships and interest in consultative medicine to identify institutions across a variety of geographic locations. We collected data regarding the consult service structure at each site, including data on the presence or absence of comanagement services and consult requests received.
Data Collection Tool
Investigators at the University of Texas Health San Antonio (UTHSA) drafted the data collection tool. Iterative feedback on the data collection tool was obtained from the research consortium (final tool, Supplemental Figure). Data collected included service requesting consultation, RFC, time request was made (day/night), who first saw the patient (eg, resident, attending), whether requesting and consulting providers verbally communicated, and whether patients were transferred to medicine. Respondents also estimated how often RFCs were encountered during their general medicine services.
To streamline data collection, we used click boxes and drop-down lists that included diagnoses and symptoms. The use of these predetermined RFCs was based on prior studies and discussion with the research consortium on common RFCs in clinical practice. A write-in field was also included. Respondents could select multiple RFCs in the case of multiple questions. Respondents also provided data regarding clinical issues that were incidentally identified during their initial patient assessments. Incidentally identified issues are hereafter called “additional RFCs” for differentiation from stated RFCs. Prior to data collection, the tool was piloted at UTHSA.
Data Collection, Categorization, and Analysis
Participants submitted data using Survey Monkey (Palo Alto, California). Emails with the survey link were sent daily. Specific participants for each data collection period were chosen by each site. Days with no data entry were confirmed by the study coordinator. Each institution collected data for four 2-week periods from July 2014 to July 2015 for a total of 8 weeks. We did not track follow-up encounters. Repeat consultations for different reasons were considered new consults.
All survey responses and free-text RFC entries were independently reviewed and categorized by 2 authors (E.W. and M.S.). New categories were created if needed. If reviewers disagreed, a third reviewer (C.M.) reviewed the RFC. The research consortium reviewed the final list of categories and entries.
We calculated descriptive statistics using SAS version 9.3 (SAS Institute, Inc., Cary, North Carolina). Each analysis used complete responses for each survey component. We separately analyzed services with and without comanagement components. The study was approved by UTHSA’s Institutional Review Board.
RESULTS
A total of 11 AMCs that represent 9 academic affiliations participated in this study (Table 1). Of the 11 AMCs, 7 were public nonprofit, 3 were private nonprofit, and 1 was a Veterans Health Administration facility. Out of the 11 AMCs, 9 sites included residents on the consult service, and the rotation was required at 6 of the sites. Most sites with residents had a formal curriculum that ranged from curated articles to online modules. Out of the 11 services, 4 were consult and comanagement services. All 4 co-managed orthopedic patients, and 1 also included other patients.
Data for 1,264 patient encounters with 2,778 RFCs were collected. A total of 1,218 of the surveys (96.4%) were fully completed, and only 5 surveys were missing data for multiple questions. A total of 7 sites adhered to the planned protocol. Among the sites, 1 site had 1 incomplete collection period, 1 site missed 1 collection period, and 1 site missed 2 collection periods.
Most consultations (87.1%) were requested during the day. Many patients (55.9%) were initially seen by residents, and 32.4% of the patients were initially seen by an attending. Respondents reported communicating verbally with the requesting team in 93.9% of instances. Among the patients, 7.8% were transferred to medicine following initial consultation. This percentage was higher (10.2%) in services without comanagement.
The average number of new consults per day per site was 2.24. The range for individual sites was 1.36-3.48. The maximum number of new consults in 1 day was 10. All sites had at least 1 day without new consults. The mean number of RFCs per encounter was 2.20 (median 2, range 1-13). In 226 of 360 encounters in which comanagement was an RFC, the respondent enumerated the other specific RFCs addressed. In these encounters, the mean number of RFCs (in addition to comanagement) was 3.02.
Most requests (82.2%) originated from surgical services. Among all surgical services, orthopedic surgery requested the highest number of consultations (67.5% for services with a comanagement component; 28.5% for services without) and 81.2% of the 360 comanagement encounters. Refer to Supplemental Table 1 for detailed information on the services that requested consultation.
The most common RFC was comanagement (13.0% across the entire study; 23.3% for services with a comanagement component; Table 2). For services without comanagement, preoperative evaluation was the most common RFC (16.4%). Other frequent RFCs across the entire study included blood pressure management (8.9%), glycemic management (7.2%), and renal failure (3.9%). Additional (unstated) RFCs were addressed in 944 patients (34.0%), and blood pressure management was the most common additional RFC.
Respondents indicated that 54.9% of RFCs were clinical topics that are “often” or “always” encountered in IM inpatient services. In 11.8% of encounters, the RFC was “rarely” or “never” encountered; the most common RFCs in such encounters were comanagement (53.4%), preoperative evaluation (17.4%), and transfer to medicine (5.4%).
DISCUSSION
Our study provides insights into the consultative landscape of AMCs and identified who consults IMs and their RFCs. Thus, our study has implications for resident consultative education. The consult services included in our study presented varied structures, including those that require medicine consultation as a resident rotation and those with comanagement agreements. Consistent with the results of prior studies, surgical services requested the majority of consults, with orthopedic surgery generating the highest number of requests. Consultation requests from neurosurgery were higher than previously reported.2,8,9
Our study reveals that comanagement and preoperative evaluation are the most common RFCs and are the least commonly encountered RFCs in IM inpatient services. The broad nature of these RFCs speaks to an increasing need for comprehensive consultative care. Consultants addressed a wide range of clinical issues, including rare entities that defy easy categorization (eg, Moyamoya disease). This broad landscape presents challenges in focusing curricular content areas outside of comanagement and preoperative evaluation but does provide evidence “to expect the unexpected” in IM consultation, as has been previously noted.8
In over a third of encounters, consultants addressed an issue that was not stated in the initial RFC. Consultants also addressed more than 2 RFCs per encounter. These observations suggest that medicine consult services may be essentially comanaging some patients even when a comanagement care model is not formally in place. These findings provide rationale for the continued expansion of comanagement services.11
Our study provides further evidence that, in modern consultative practice, “determining your customer” is more important than “determining the question.”12-14 We work in an era in which comanagement services are increasingly prevalent but are not ubiquitous and in which IM consultants routinely address multiple issues. Prior studies indicated that most surgeons do not believe that consults should be limited to specific questions and instead prefer comanagement.13 Understanding the expectations of the requesting physician is therefore important and highlights the importance of verbal communication at the time of initial consultation. Ongoing interprofessional communication is a vital skill that residents should acquire.
Our study has several limitations. Although our sites represented a varied sample, we focused on AMCs. Therefore, our study may not reflect consultative experiences in nonacademic hospitals or sites without dedicated consult services. Trade-offs exist in our data collection approach, which provided predetermined RFCs. We selected our methodology to facilitate data entry and to aid RFC categorization. Nevertheless, it may have lessened the clinical nuance of submitted data. The provision of predetermined RFCs may have influenced issue selection by the respondents. However, in 473 encounters (37.4%), the survey respondents provided free-text entries for the stated RFC, and 944 additional RFCs were written in as responses. These results demonstrated that respondents did not limit themselves to the predetermined list. We did not perform chart reviews to validate data. Finally, our data were a cross-section of initial consultations. We lack information on subsequent diagnoses or additional clinical issues that developed later.
In conclusion, we found varied consultative experiences across AMCs. However, preoperative evaluation and perioperative comanagement – particularly of orthopedic and neurosurgical patients – were common and should be included in curricula. Faculty should recognize the unique nature of IM consultation to prepare residents. Specifically, faculty should prepare residents to expect to identify and address unstated medical issues and to provide comprehensive assessments regardless of whether the consultative structure has a comanagement component. Given the unique nature of consultative IM work and the possibility of discordant expectations between consulting and requesting physicians, perhaps the most valuable skill to impart to residents is effective and regular communication.
Medicine Consult/Comanagement Consortium Members
The Medicine Consult/Comanagement Consortium consists of: Mary Anderson Wallace, MD, Brian Wolfe, MD (University of Colorado), Meridale Baggett, MD, Douglas Wright, MD, PhD (Harvard University), Joyeeta G. Dastidar MD, Maureen Kelly, MD (Columbia University), Leonard S. Feldman, MD (Johns Hopkins University), Cecily J. Gallup, MD, MPH (University of California, San Francisco), Paul J. Grant, MD (University of Michigan), Craig R. Keenan, MD (University of California, Davis), Fletcher Penney, MD (Medical University of South Carolina).
Acknowledgments
The authors thank the clinicians at each site who were involved in data collection for this study, including Barbara Statland, MD. The authors also thank Timothy Niessen, MD for data and physician coordination and Musarrat Nahid, MSc. for statistical analysis.
Disclosures
Paul J. Grant receives royalties from the medical textbook Perioperative Medicine: Medical Consultation and Comanagement, Wiley Publishing 2012. Craig R. Keenan receives medicolegal consultation fees from Weiss-Salinas Law Group and American Psychiatric Association Publishers for book royalties. All other authors declare that they do not have any conflicts of interest.
Funding Information
The research reported here was supported by the Department of Veterans Affairs, Veterans Health Administration. Investigator salary support is provided through the South Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or our institutions.
1. Hollenberg CH, Langley GR. The Canadian general internist: education and future role. CMAJ. 1978;118(4):397-400. PubMed
2. Charlson ME, Cohen RP, Sears CL. General medicine consultation: lessons from a clinical service. Am J Med. 1983;75(1):121-128. https://doi.org/10.1016/0002-9343(83)91175-0. PubMed
3. Society of Hospital Medicine. The evolution of co-management in hospital medicine. http://www.hospitalmedicine.org/Web/Practice_Management/CoManagement.aspx. Accessed March 8, 2018.
4. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. 10.1001/archinternmed.2010.432. PubMed
5. Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368. 10.1001/archinternmed.2009.553. PubMed
6. Thompson RE, Pfeifer K, Grant PJ, et al. Hospital medicine and perioperative care: a framework for high-quality, high-value collaborative care. J Hosp Med. 2017;12(4):277-282. 10.12788/jhm.2717. PubMed
7. Accreditation Council for Graduate Medical Education. Common Program Requirements. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/140_internal_medicine_2017-07-01.pdf. Accessed March 8, 2018.
8. Moore RA, Kammerer WS, McGlynn TJ, Trautlein JJ, Burnside JW. Consultations in internal medicine: a training program resource. J Med Educ. 1977;52(4):323-327. PubMed
9. Robie PW. The service and educational contributions of a general medicine consultation service. J Gen Intern Med. 1986;1(4):225-227. https://doi.org/10.1007/BF02596187. PubMed
10. Devor M, Renvall M, Ramsdell J. Practice patterns and the adequacy of residency training in consultation medicine. J Gen Intern Med. 1993;8(10):554-560. 10.1007/BF02599639. PubMed
11. Siegal EM. Just because you can, doesn’t mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398-402. 10.1002/jhm.361. PubMed
12. Goldman L, Lee T, Rudd P. Ten commandments for effective consultations. Arch Intern Med. 1983;143(9):1753-1755. 10.1001/archinte.1983.00350090131022. PubMed
13. Salerno SM. Principles of effective consultation: an update for the 21st-century consultant. Arch Intern Med. 2007;167:271-275. 10.1001/archinte.167.3.271. PubMed
14. Merli GJ, Weitz HH. Medical management of the surgical patient E-Book. Elsevier Health Sciences; 2008. PubMed
1. Hollenberg CH, Langley GR. The Canadian general internist: education and future role. CMAJ. 1978;118(4):397-400. PubMed
2. Charlson ME, Cohen RP, Sears CL. General medicine consultation: lessons from a clinical service. Am J Med. 1983;75(1):121-128. https://doi.org/10.1016/0002-9343(83)91175-0. PubMed
3. Society of Hospital Medicine. The evolution of co-management in hospital medicine. http://www.hospitalmedicine.org/Web/Practice_Management/CoManagement.aspx. Accessed March 8, 2018.
4. Auerbach AD, Wachter RM, Cheng HQ, et al. Comanagement of surgical patients between neurosurgeons and hospitalists. Arch Intern Med. 2010;170(22):2004-2010. 10.1001/archinternmed.2010.432. PubMed
5. Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363-368. 10.1001/archinternmed.2009.553. PubMed
6. Thompson RE, Pfeifer K, Grant PJ, et al. Hospital medicine and perioperative care: a framework for high-quality, high-value collaborative care. J Hosp Med. 2017;12(4):277-282. 10.12788/jhm.2717. PubMed
7. Accreditation Council for Graduate Medical Education. Common Program Requirements. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/140_internal_medicine_2017-07-01.pdf. Accessed March 8, 2018.
8. Moore RA, Kammerer WS, McGlynn TJ, Trautlein JJ, Burnside JW. Consultations in internal medicine: a training program resource. J Med Educ. 1977;52(4):323-327. PubMed
9. Robie PW. The service and educational contributions of a general medicine consultation service. J Gen Intern Med. 1986;1(4):225-227. https://doi.org/10.1007/BF02596187. PubMed
10. Devor M, Renvall M, Ramsdell J. Practice patterns and the adequacy of residency training in consultation medicine. J Gen Intern Med. 1993;8(10):554-560. 10.1007/BF02599639. PubMed
11. Siegal EM. Just because you can, doesn’t mean that you should: a call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398-402. 10.1002/jhm.361. PubMed
12. Goldman L, Lee T, Rudd P. Ten commandments for effective consultations. Arch Intern Med. 1983;143(9):1753-1755. 10.1001/archinte.1983.00350090131022. PubMed
13. Salerno SM. Principles of effective consultation: an update for the 21st-century consultant. Arch Intern Med. 2007;167:271-275. 10.1001/archinte.167.3.271. PubMed
14. Merli GJ, Weitz HH. Medical management of the surgical patient E-Book. Elsevier Health Sciences; 2008. PubMed
© 2018 Society of Hospital Medicine
Impact of Clinical Specialty on Attitudes Regarding Overuse of Inpatient Laboratory Testing
Routine laboratory testing in hospitalized patients is common, with a high prevalence of unnecessary tests that do not contribute to patient management.1 Excessive laboratory testing of hospitalized patients can contribute to anemia2 and may cause patient discomfort, additional unnecessary testing resulting from false positive results, and higher out-of-pocket patient costs. Excessive testing can impact hospital budgets both directly (though direct costs are often low) and indirectly through costly downstream services and prolonged hospital stay.3 As part of the American Board of Internal Medicine (ABIM) Foundation’s Choosing Wisely initiative, several professional societies have recommended against routine laboratory testing of hospitalized adult patients.4
Excessive inpatient laboratory testing has been documented mostly among adult internal medicine (IM) patients with studies of drivers of unnecessary testing and efforts to reduce it conducted in IM settings.5, 6 Attitudes toward other issues related to testing overuse differ by specialty7 and are likely to similarly vary with regard to unnecessary laboratory testing. Understanding differences in attitudes by clinical specialty is critical for framing tailored approaches to reducing inappropriate care.
We performed a cross-sectional survey of a diverse group of hospital clinicians to describe attitudes and beliefs regarding laboratory testing and its overuse across clinical specialties (eg, medical, surgical, and pediatric). We hypothesized that attitudes toward the need for testing would differ across specialties.
METHODS
Survey Development and Administration
The study was conducted at Memorial Sloan Kettering Cancer Center, a tertiary academic cancer hospital in New York City. The 12-item survey was adopted from a previously administered but not formally validated survey (Online-only Appendix).5,8 The survey was pilot tested with 4 physicians, 3 NPs, 2 PAs, and 3 RNs and edited for content and clarity. All staff providers including NPs, PAs, RNs, and resident, fellow, and attending MDs working in the hospital during the 2-week survey period (November 2-15, 2015) were eligible to participate and were emailed a link to the survey. The email invitation was resent 3 times during the survey period. Participants who completed the survey received a coupon for a free coffee. The study was reviewed by the Institutional Review Board and exempted from ongoing oversight.
Measures
Demographic items included clinical specialty, provider type, and gender (Online-only Appendix). The remaining survey questions included the following categories:
1. Attitudes toward laboratory testing were evaluated by 3 items about accepted norms for lab testing and 2 items about fears (Table 2). Responses to these items used a 4-point Likert scale (strongly agree to strongly disagree).
2. Drivers contributing to unnecessary testing were evaluated by presenting a list of possible contributing factors (Table 2). Responses to these items used a 3-point Likert scale (contributes a lot, contributes a little, or does not contribute).
Analysis
We used univariate statistics to describe demographics and survey responses. We used the chi-square statistic to evaluate differences in attitudes and drivers by clinical specialty. We dichotomized responses regarding attitudes toward lab testing (“strongly agree” and “somewhat agree” vs. “somewhat disagree” and “strongly disagree.”) and beliefs regarding contributing drivers (“contributes a lot” vs all others). We grouped clinical specialty into medical/med-oncology, surgical, pediatric, and other (gynecological, critical care, and other).
We used logistic regression to explore the associations between attitudes/drivers and clinical specialty after adjusting for provider type, and report the overall P-value. We used pediatrics as the reference group to assess direct comparisons with each of the other specialties. We performed analyses with SAS statistical software, version 9.4 (SAS Institute, Cary, North Carolina) and considered P < .05 to be significant.
RESULTS
Among 1580 eligible participants, 837 (53%) completed surveys. Attending MD response rates ranged between 61% (surgical) to 86% (pediatric); rates were 59% for all trainees, 72% for PAs and 46% for RNs and NPs combined. Given privacy concerns, we were unable to collect detailed response rate information or any information about nonrespondents. The demographics are shown in Table 1.
Attitudes toward Laboratory Testing
The majority of respondents agreed that hospitalized patients should get daily labs (59%), testing on the discharge day (52%), and that daily testing generally enhances safety (55%; Table 2). Fewer pediatric and surgical clinicians endorsed that laboratory testing should be done daily (56% and 47% respectively) and enhances patient safety (46% and 47%). These differences were significant after adjusting for provider type. In addition, fewer pediatric providers endorsed the statement that daily laboratory testing helps avoid malpractice litigation. Overall, 68% of respondents agreed they would be comfortable with less testing.
Drivers Contributing to Unnecessary Laboratory Testing
The strongest drivers of unnecessary testing were seen as habit (94% responding “contributes a lot”) and institutional culture (89% responding “contributes a lot”; Table 2). After adjusting for provider type, significant differences were observed based on clinical specialty. In particular, pediatric specialists were less likely to endorse fear of litigation (P < .001) and more likely to endorse pressure from patient/family (P = .0003) compared to all other specialties (Table 2, odd ratios not shown).
DISCUSSION
Overuse of laboratory testing in hospitalized patients is widely recognized in IM and likely to be prevalent in other clinical specialties. Our study elucidated differences in attitudes toward unnecessary testing and self-identified drivers across specialties in a diverse group of clinical providers at an academic cancer center. We found differences based on clinical specialty, with those caring for pediatric and surgical patients less likely than others to believe that testing should be done daily and that daily testing enhances patient safety. Furthermore, comfort with less testing was highest among pediatric specialists. Habit and institutional culture were recognized broadly as the strongest drivers of laboratory testing overuse.
Our findings regarding differences based on clinical specialty are novel. Respondents caring for pediatric patients generally placed lower value on testing, and IM clinicians were the most likely to endorse daily testing and to believe that it enhances patient safety and helps avoid malpractice litigation. The difference between adult and pediatric clinicians is surprising given the fundamental similarities between these specialties.9 Although some resource use studies have described differences across specialties, none has examined differences in laboratory testing or examined the practice patterns of clinicians who are not physicians across specialties.10 Prior studies have documented the impact of training location on practice11,12, suggesting the importance of the local training culture.13 As physician personalities vary across clinical specialties14 it is likely that culture varies as well. Specialty-specific cultures are likely to strongly influence attitudes and practice patterns and warrant further exploration.
Clinicians in our sample identified drivers of unnecessary laboratory testing that were consistent with other studies, most frequently endorsing habit, followed by culture, discomfort with not knowing, and concern that someone will ask for the results.5,15 Previous studies have focused on IM and have not included nonphysicians or compared attitudes across specialties. We found that the largest differences in drivers by specialty were related to malpractice concerns and the perception of pressure from patients or families. The low endorsement of defensive medicine among clinicians serving pediatric populations may imply that interventions to reduce unnecessary care in hospitalized children may not need to address malpractice fear. In contrast, clinicians from pediatrics identified family pressure as a greater driver of unnecessary testing. Efforts to reduce unnecessary laboratory testing in pediatrics will need to address parent expectations.
Our findings have implications for efforts to reduce unnecessary testing. Culture, identified as a key driver of testing, reflects leadership priorities, institutional history, and other factors and is difficult to specifically target. Habit, the other most-endorsed driver, is a more promising target for quality improvement interventions, particularly those addressing care processes (eg, electronic ordering). Discomfort with not knowing and fear of being asked are drivers that might be influenced by better communication about information expectations by supervising physicians and hospital administration. Lastly, education about the potential harms of excessive testing may facilitate more targeted efforts to reduce testing overuse.
Our study has important limitations. The cancer focus of the center may have influenced provider attitudes and practices. Attitudes may differ at community centers, though important differences regarding routine laboratory testing are unlikely. Second, although our sample was large, our response rate was modest at 53% and as low as 46% among RNs and NPs and we have no information regarding nonresponders. This response rate, though, was comparable to response rates seen in other large surveys.5,15 In addition, our results reflect clinician self-report; perceptions of necessity and the true need for testing may vary across specialties and the true subconscious drivers of behavior may differ. However, differences across specialties are likely to be valid even if there are other factors at play. Self assessment of unnecessary testing may also underestimate prevalence of the problem. Finally, our findings related to drivers of unnecessary testing are descriptive rather than quantitative given the lack of validated scales.
In conclusion, we evaluated attitudes toward routine laboratory testing in hospitalized patients in clinicians across specialties and found important differences. These findings speak to the diversity of cultures of medical care even within a single institution and point to the importance of studying attitudes about overused services across clinical specialties. In particular, as medical fields beyond IM increasingly recognize the importance of reducing medical overuse both in and out of the hospital, our findings highlight the importance of elucidating specialty-specific attitudes to optimize interventions to address unnecessary testing.
Disclosures
Mr. Husain, Ms. Gennarelli, Ms. White4, Mr. Masciale, MA5, and Dr. Roman, MD, have nothing to disclose. The work of Dr. Roman and Dr. Korenstein on this project was supported, in part, by a Cancer Center Support Grant from the National Cancer Institute to Memorial Sloan Kettering Cancer Center (P30 CA008748)
1. Zhi M, Ding EL, Theisen-Toupal J, Whelan J, Arnaout R. The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PloS One. 2013;8(11):e78962. DOI: 10.1371/journal.pone.0078962. PubMed
2. Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20(6):520-524. DOI: 10.1111/j.1525-1497.2005.0094.x. PubMed
3. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-1839. DOI: 10.1001/jamainternmed.2017.5152 PubMed
4. Choosing wisely. http://www.choosingwisely.org/resources/. Accessed November 21, 2017.
5. Sedrak MS, Patel MS, Ziemba JB, et al. Residents’ self-report on why they order perceived unnecessary inpatient laboratory tests. J Hosp Med. 2016;11(12):869-872. DOI: 10.1002/jhm.2645. PubMed
6. Thakkar RN, Kim D, Knight AM, Riedel S, Vaidya D, Wright SM. Impact of an educational intervention on the frequency of daily blood test orders for hospitalized patients. Am J Clin Pathol. 2015;143(3):393-397. DOI: 10.1309/AJCPJS4EEM7UAUBV. PubMed
7. Sheeler RD, Mundell T, Hurst SA, et al. Self-reported rationing behavior among US physicians: a national survey. J Gen Intern Med. 2016;31(12):1444-1451. DOI: 10.1007/s11606-016-3756-5. PubMed
8. Roman BR, Yang A, Masciale J, Korenstein D. Association of attitudes regarding overuse of inpatient laboratory testing with health care provider type. JAMA Intern Med. 2017;177(8):1205-1207. DOI: 10.1001/jamainternmed.2017.1634. PubMed
9. Schatz IJ, Realini JP, Charney E. Family practice, internal medicine, and pediatrics as partners in the education of generalists. Acad Med. 1996;71(1):35-39. PubMed
10. Johnson RE, Freeborn DK, Mullooly JP. Physicians’ use of laboratory, radiology, and drugs in a prepaid group practice HMO. Health Serv Res. 1985;20(5):525-547. PubMed
11. Chen C, Petterson S, Phillips R, Bazemore A, Mullan F. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. Dec 10, 2014;312(22):2385-2393. DOI: 10.1001/jama.2014.15973. PubMed
12. Sirovich BE, Lipner RS, Johnston M, Holmboe ES. The association between residency training and internists’ ability to practice conservatively. JAMA Intern Med. 2014;174(10):1640-1648. DOI: 10.1001/jamainternmed.2014.3337. PubMed
13. Smith CD, Korenstein D. Harnessing the power of peer pressure to reduce health care waste and improve clinical outcomes. Mayo Clin Proc. 2015;90(3):311-312. DOI: https://doi.org/10.1017/ice.2015.136 PubMed
14. Vaidya NA, Sierles FS, Raida MD, Fakhoury FJ, Przybeck TR, Cloninger CR. Relationship between specialty choice and medical student temperament and character assessed with Cloninger Inventory. Teach Learn Med. 2004;16(2):150-156. DOI: 10.1207/s15328015tlm1602_6 PubMed
15. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617. DOI: 10.1001/jama.293.21.2609 PubMed
Routine laboratory testing in hospitalized patients is common, with a high prevalence of unnecessary tests that do not contribute to patient management.1 Excessive laboratory testing of hospitalized patients can contribute to anemia2 and may cause patient discomfort, additional unnecessary testing resulting from false positive results, and higher out-of-pocket patient costs. Excessive testing can impact hospital budgets both directly (though direct costs are often low) and indirectly through costly downstream services and prolonged hospital stay.3 As part of the American Board of Internal Medicine (ABIM) Foundation’s Choosing Wisely initiative, several professional societies have recommended against routine laboratory testing of hospitalized adult patients.4
Excessive inpatient laboratory testing has been documented mostly among adult internal medicine (IM) patients with studies of drivers of unnecessary testing and efforts to reduce it conducted in IM settings.5, 6 Attitudes toward other issues related to testing overuse differ by specialty7 and are likely to similarly vary with regard to unnecessary laboratory testing. Understanding differences in attitudes by clinical specialty is critical for framing tailored approaches to reducing inappropriate care.
We performed a cross-sectional survey of a diverse group of hospital clinicians to describe attitudes and beliefs regarding laboratory testing and its overuse across clinical specialties (eg, medical, surgical, and pediatric). We hypothesized that attitudes toward the need for testing would differ across specialties.
METHODS
Survey Development and Administration
The study was conducted at Memorial Sloan Kettering Cancer Center, a tertiary academic cancer hospital in New York City. The 12-item survey was adopted from a previously administered but not formally validated survey (Online-only Appendix).5,8 The survey was pilot tested with 4 physicians, 3 NPs, 2 PAs, and 3 RNs and edited for content and clarity. All staff providers including NPs, PAs, RNs, and resident, fellow, and attending MDs working in the hospital during the 2-week survey period (November 2-15, 2015) were eligible to participate and were emailed a link to the survey. The email invitation was resent 3 times during the survey period. Participants who completed the survey received a coupon for a free coffee. The study was reviewed by the Institutional Review Board and exempted from ongoing oversight.
Measures
Demographic items included clinical specialty, provider type, and gender (Online-only Appendix). The remaining survey questions included the following categories:
1. Attitudes toward laboratory testing were evaluated by 3 items about accepted norms for lab testing and 2 items about fears (Table 2). Responses to these items used a 4-point Likert scale (strongly agree to strongly disagree).
2. Drivers contributing to unnecessary testing were evaluated by presenting a list of possible contributing factors (Table 2). Responses to these items used a 3-point Likert scale (contributes a lot, contributes a little, or does not contribute).
Analysis
We used univariate statistics to describe demographics and survey responses. We used the chi-square statistic to evaluate differences in attitudes and drivers by clinical specialty. We dichotomized responses regarding attitudes toward lab testing (“strongly agree” and “somewhat agree” vs. “somewhat disagree” and “strongly disagree.”) and beliefs regarding contributing drivers (“contributes a lot” vs all others). We grouped clinical specialty into medical/med-oncology, surgical, pediatric, and other (gynecological, critical care, and other).
We used logistic regression to explore the associations between attitudes/drivers and clinical specialty after adjusting for provider type, and report the overall P-value. We used pediatrics as the reference group to assess direct comparisons with each of the other specialties. We performed analyses with SAS statistical software, version 9.4 (SAS Institute, Cary, North Carolina) and considered P < .05 to be significant.
RESULTS
Among 1580 eligible participants, 837 (53%) completed surveys. Attending MD response rates ranged between 61% (surgical) to 86% (pediatric); rates were 59% for all trainees, 72% for PAs and 46% for RNs and NPs combined. Given privacy concerns, we were unable to collect detailed response rate information or any information about nonrespondents. The demographics are shown in Table 1.
Attitudes toward Laboratory Testing
The majority of respondents agreed that hospitalized patients should get daily labs (59%), testing on the discharge day (52%), and that daily testing generally enhances safety (55%; Table 2). Fewer pediatric and surgical clinicians endorsed that laboratory testing should be done daily (56% and 47% respectively) and enhances patient safety (46% and 47%). These differences were significant after adjusting for provider type. In addition, fewer pediatric providers endorsed the statement that daily laboratory testing helps avoid malpractice litigation. Overall, 68% of respondents agreed they would be comfortable with less testing.
Drivers Contributing to Unnecessary Laboratory Testing
The strongest drivers of unnecessary testing were seen as habit (94% responding “contributes a lot”) and institutional culture (89% responding “contributes a lot”; Table 2). After adjusting for provider type, significant differences were observed based on clinical specialty. In particular, pediatric specialists were less likely to endorse fear of litigation (P < .001) and more likely to endorse pressure from patient/family (P = .0003) compared to all other specialties (Table 2, odd ratios not shown).
DISCUSSION
Overuse of laboratory testing in hospitalized patients is widely recognized in IM and likely to be prevalent in other clinical specialties. Our study elucidated differences in attitudes toward unnecessary testing and self-identified drivers across specialties in a diverse group of clinical providers at an academic cancer center. We found differences based on clinical specialty, with those caring for pediatric and surgical patients less likely than others to believe that testing should be done daily and that daily testing enhances patient safety. Furthermore, comfort with less testing was highest among pediatric specialists. Habit and institutional culture were recognized broadly as the strongest drivers of laboratory testing overuse.
Our findings regarding differences based on clinical specialty are novel. Respondents caring for pediatric patients generally placed lower value on testing, and IM clinicians were the most likely to endorse daily testing and to believe that it enhances patient safety and helps avoid malpractice litigation. The difference between adult and pediatric clinicians is surprising given the fundamental similarities between these specialties.9 Although some resource use studies have described differences across specialties, none has examined differences in laboratory testing or examined the practice patterns of clinicians who are not physicians across specialties.10 Prior studies have documented the impact of training location on practice11,12, suggesting the importance of the local training culture.13 As physician personalities vary across clinical specialties14 it is likely that culture varies as well. Specialty-specific cultures are likely to strongly influence attitudes and practice patterns and warrant further exploration.
Clinicians in our sample identified drivers of unnecessary laboratory testing that were consistent with other studies, most frequently endorsing habit, followed by culture, discomfort with not knowing, and concern that someone will ask for the results.5,15 Previous studies have focused on IM and have not included nonphysicians or compared attitudes across specialties. We found that the largest differences in drivers by specialty were related to malpractice concerns and the perception of pressure from patients or families. The low endorsement of defensive medicine among clinicians serving pediatric populations may imply that interventions to reduce unnecessary care in hospitalized children may not need to address malpractice fear. In contrast, clinicians from pediatrics identified family pressure as a greater driver of unnecessary testing. Efforts to reduce unnecessary laboratory testing in pediatrics will need to address parent expectations.
Our findings have implications for efforts to reduce unnecessary testing. Culture, identified as a key driver of testing, reflects leadership priorities, institutional history, and other factors and is difficult to specifically target. Habit, the other most-endorsed driver, is a more promising target for quality improvement interventions, particularly those addressing care processes (eg, electronic ordering). Discomfort with not knowing and fear of being asked are drivers that might be influenced by better communication about information expectations by supervising physicians and hospital administration. Lastly, education about the potential harms of excessive testing may facilitate more targeted efforts to reduce testing overuse.
Our study has important limitations. The cancer focus of the center may have influenced provider attitudes and practices. Attitudes may differ at community centers, though important differences regarding routine laboratory testing are unlikely. Second, although our sample was large, our response rate was modest at 53% and as low as 46% among RNs and NPs and we have no information regarding nonresponders. This response rate, though, was comparable to response rates seen in other large surveys.5,15 In addition, our results reflect clinician self-report; perceptions of necessity and the true need for testing may vary across specialties and the true subconscious drivers of behavior may differ. However, differences across specialties are likely to be valid even if there are other factors at play. Self assessment of unnecessary testing may also underestimate prevalence of the problem. Finally, our findings related to drivers of unnecessary testing are descriptive rather than quantitative given the lack of validated scales.
In conclusion, we evaluated attitudes toward routine laboratory testing in hospitalized patients in clinicians across specialties and found important differences. These findings speak to the diversity of cultures of medical care even within a single institution and point to the importance of studying attitudes about overused services across clinical specialties. In particular, as medical fields beyond IM increasingly recognize the importance of reducing medical overuse both in and out of the hospital, our findings highlight the importance of elucidating specialty-specific attitudes to optimize interventions to address unnecessary testing.
Disclosures
Mr. Husain, Ms. Gennarelli, Ms. White4, Mr. Masciale, MA5, and Dr. Roman, MD, have nothing to disclose. The work of Dr. Roman and Dr. Korenstein on this project was supported, in part, by a Cancer Center Support Grant from the National Cancer Institute to Memorial Sloan Kettering Cancer Center (P30 CA008748)
Routine laboratory testing in hospitalized patients is common, with a high prevalence of unnecessary tests that do not contribute to patient management.1 Excessive laboratory testing of hospitalized patients can contribute to anemia2 and may cause patient discomfort, additional unnecessary testing resulting from false positive results, and higher out-of-pocket patient costs. Excessive testing can impact hospital budgets both directly (though direct costs are often low) and indirectly through costly downstream services and prolonged hospital stay.3 As part of the American Board of Internal Medicine (ABIM) Foundation’s Choosing Wisely initiative, several professional societies have recommended against routine laboratory testing of hospitalized adult patients.4
Excessive inpatient laboratory testing has been documented mostly among adult internal medicine (IM) patients with studies of drivers of unnecessary testing and efforts to reduce it conducted in IM settings.5, 6 Attitudes toward other issues related to testing overuse differ by specialty7 and are likely to similarly vary with regard to unnecessary laboratory testing. Understanding differences in attitudes by clinical specialty is critical for framing tailored approaches to reducing inappropriate care.
We performed a cross-sectional survey of a diverse group of hospital clinicians to describe attitudes and beliefs regarding laboratory testing and its overuse across clinical specialties (eg, medical, surgical, and pediatric). We hypothesized that attitudes toward the need for testing would differ across specialties.
METHODS
Survey Development and Administration
The study was conducted at Memorial Sloan Kettering Cancer Center, a tertiary academic cancer hospital in New York City. The 12-item survey was adopted from a previously administered but not formally validated survey (Online-only Appendix).5,8 The survey was pilot tested with 4 physicians, 3 NPs, 2 PAs, and 3 RNs and edited for content and clarity. All staff providers including NPs, PAs, RNs, and resident, fellow, and attending MDs working in the hospital during the 2-week survey period (November 2-15, 2015) were eligible to participate and were emailed a link to the survey. The email invitation was resent 3 times during the survey period. Participants who completed the survey received a coupon for a free coffee. The study was reviewed by the Institutional Review Board and exempted from ongoing oversight.
Measures
Demographic items included clinical specialty, provider type, and gender (Online-only Appendix). The remaining survey questions included the following categories:
1. Attitudes toward laboratory testing were evaluated by 3 items about accepted norms for lab testing and 2 items about fears (Table 2). Responses to these items used a 4-point Likert scale (strongly agree to strongly disagree).
2. Drivers contributing to unnecessary testing were evaluated by presenting a list of possible contributing factors (Table 2). Responses to these items used a 3-point Likert scale (contributes a lot, contributes a little, or does not contribute).
Analysis
We used univariate statistics to describe demographics and survey responses. We used the chi-square statistic to evaluate differences in attitudes and drivers by clinical specialty. We dichotomized responses regarding attitudes toward lab testing (“strongly agree” and “somewhat agree” vs. “somewhat disagree” and “strongly disagree.”) and beliefs regarding contributing drivers (“contributes a lot” vs all others). We grouped clinical specialty into medical/med-oncology, surgical, pediatric, and other (gynecological, critical care, and other).
We used logistic regression to explore the associations between attitudes/drivers and clinical specialty after adjusting for provider type, and report the overall P-value. We used pediatrics as the reference group to assess direct comparisons with each of the other specialties. We performed analyses with SAS statistical software, version 9.4 (SAS Institute, Cary, North Carolina) and considered P < .05 to be significant.
RESULTS
Among 1580 eligible participants, 837 (53%) completed surveys. Attending MD response rates ranged between 61% (surgical) to 86% (pediatric); rates were 59% for all trainees, 72% for PAs and 46% for RNs and NPs combined. Given privacy concerns, we were unable to collect detailed response rate information or any information about nonrespondents. The demographics are shown in Table 1.
Attitudes toward Laboratory Testing
The majority of respondents agreed that hospitalized patients should get daily labs (59%), testing on the discharge day (52%), and that daily testing generally enhances safety (55%; Table 2). Fewer pediatric and surgical clinicians endorsed that laboratory testing should be done daily (56% and 47% respectively) and enhances patient safety (46% and 47%). These differences were significant after adjusting for provider type. In addition, fewer pediatric providers endorsed the statement that daily laboratory testing helps avoid malpractice litigation. Overall, 68% of respondents agreed they would be comfortable with less testing.
Drivers Contributing to Unnecessary Laboratory Testing
The strongest drivers of unnecessary testing were seen as habit (94% responding “contributes a lot”) and institutional culture (89% responding “contributes a lot”; Table 2). After adjusting for provider type, significant differences were observed based on clinical specialty. In particular, pediatric specialists were less likely to endorse fear of litigation (P < .001) and more likely to endorse pressure from patient/family (P = .0003) compared to all other specialties (Table 2, odd ratios not shown).
DISCUSSION
Overuse of laboratory testing in hospitalized patients is widely recognized in IM and likely to be prevalent in other clinical specialties. Our study elucidated differences in attitudes toward unnecessary testing and self-identified drivers across specialties in a diverse group of clinical providers at an academic cancer center. We found differences based on clinical specialty, with those caring for pediatric and surgical patients less likely than others to believe that testing should be done daily and that daily testing enhances patient safety. Furthermore, comfort with less testing was highest among pediatric specialists. Habit and institutional culture were recognized broadly as the strongest drivers of laboratory testing overuse.
Our findings regarding differences based on clinical specialty are novel. Respondents caring for pediatric patients generally placed lower value on testing, and IM clinicians were the most likely to endorse daily testing and to believe that it enhances patient safety and helps avoid malpractice litigation. The difference between adult and pediatric clinicians is surprising given the fundamental similarities between these specialties.9 Although some resource use studies have described differences across specialties, none has examined differences in laboratory testing or examined the practice patterns of clinicians who are not physicians across specialties.10 Prior studies have documented the impact of training location on practice11,12, suggesting the importance of the local training culture.13 As physician personalities vary across clinical specialties14 it is likely that culture varies as well. Specialty-specific cultures are likely to strongly influence attitudes and practice patterns and warrant further exploration.
Clinicians in our sample identified drivers of unnecessary laboratory testing that were consistent with other studies, most frequently endorsing habit, followed by culture, discomfort with not knowing, and concern that someone will ask for the results.5,15 Previous studies have focused on IM and have not included nonphysicians or compared attitudes across specialties. We found that the largest differences in drivers by specialty were related to malpractice concerns and the perception of pressure from patients or families. The low endorsement of defensive medicine among clinicians serving pediatric populations may imply that interventions to reduce unnecessary care in hospitalized children may not need to address malpractice fear. In contrast, clinicians from pediatrics identified family pressure as a greater driver of unnecessary testing. Efforts to reduce unnecessary laboratory testing in pediatrics will need to address parent expectations.
Our findings have implications for efforts to reduce unnecessary testing. Culture, identified as a key driver of testing, reflects leadership priorities, institutional history, and other factors and is difficult to specifically target. Habit, the other most-endorsed driver, is a more promising target for quality improvement interventions, particularly those addressing care processes (eg, electronic ordering). Discomfort with not knowing and fear of being asked are drivers that might be influenced by better communication about information expectations by supervising physicians and hospital administration. Lastly, education about the potential harms of excessive testing may facilitate more targeted efforts to reduce testing overuse.
Our study has important limitations. The cancer focus of the center may have influenced provider attitudes and practices. Attitudes may differ at community centers, though important differences regarding routine laboratory testing are unlikely. Second, although our sample was large, our response rate was modest at 53% and as low as 46% among RNs and NPs and we have no information regarding nonresponders. This response rate, though, was comparable to response rates seen in other large surveys.5,15 In addition, our results reflect clinician self-report; perceptions of necessity and the true need for testing may vary across specialties and the true subconscious drivers of behavior may differ. However, differences across specialties are likely to be valid even if there are other factors at play. Self assessment of unnecessary testing may also underestimate prevalence of the problem. Finally, our findings related to drivers of unnecessary testing are descriptive rather than quantitative given the lack of validated scales.
In conclusion, we evaluated attitudes toward routine laboratory testing in hospitalized patients in clinicians across specialties and found important differences. These findings speak to the diversity of cultures of medical care even within a single institution and point to the importance of studying attitudes about overused services across clinical specialties. In particular, as medical fields beyond IM increasingly recognize the importance of reducing medical overuse both in and out of the hospital, our findings highlight the importance of elucidating specialty-specific attitudes to optimize interventions to address unnecessary testing.
Disclosures
Mr. Husain, Ms. Gennarelli, Ms. White4, Mr. Masciale, MA5, and Dr. Roman, MD, have nothing to disclose. The work of Dr. Roman and Dr. Korenstein on this project was supported, in part, by a Cancer Center Support Grant from the National Cancer Institute to Memorial Sloan Kettering Cancer Center (P30 CA008748)
1. Zhi M, Ding EL, Theisen-Toupal J, Whelan J, Arnaout R. The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PloS One. 2013;8(11):e78962. DOI: 10.1371/journal.pone.0078962. PubMed
2. Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20(6):520-524. DOI: 10.1111/j.1525-1497.2005.0094.x. PubMed
3. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-1839. DOI: 10.1001/jamainternmed.2017.5152 PubMed
4. Choosing wisely. http://www.choosingwisely.org/resources/. Accessed November 21, 2017.
5. Sedrak MS, Patel MS, Ziemba JB, et al. Residents’ self-report on why they order perceived unnecessary inpatient laboratory tests. J Hosp Med. 2016;11(12):869-872. DOI: 10.1002/jhm.2645. PubMed
6. Thakkar RN, Kim D, Knight AM, Riedel S, Vaidya D, Wright SM. Impact of an educational intervention on the frequency of daily blood test orders for hospitalized patients. Am J Clin Pathol. 2015;143(3):393-397. DOI: 10.1309/AJCPJS4EEM7UAUBV. PubMed
7. Sheeler RD, Mundell T, Hurst SA, et al. Self-reported rationing behavior among US physicians: a national survey. J Gen Intern Med. 2016;31(12):1444-1451. DOI: 10.1007/s11606-016-3756-5. PubMed
8. Roman BR, Yang A, Masciale J, Korenstein D. Association of attitudes regarding overuse of inpatient laboratory testing with health care provider type. JAMA Intern Med. 2017;177(8):1205-1207. DOI: 10.1001/jamainternmed.2017.1634. PubMed
9. Schatz IJ, Realini JP, Charney E. Family practice, internal medicine, and pediatrics as partners in the education of generalists. Acad Med. 1996;71(1):35-39. PubMed
10. Johnson RE, Freeborn DK, Mullooly JP. Physicians’ use of laboratory, radiology, and drugs in a prepaid group practice HMO. Health Serv Res. 1985;20(5):525-547. PubMed
11. Chen C, Petterson S, Phillips R, Bazemore A, Mullan F. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. Dec 10, 2014;312(22):2385-2393. DOI: 10.1001/jama.2014.15973. PubMed
12. Sirovich BE, Lipner RS, Johnston M, Holmboe ES. The association between residency training and internists’ ability to practice conservatively. JAMA Intern Med. 2014;174(10):1640-1648. DOI: 10.1001/jamainternmed.2014.3337. PubMed
13. Smith CD, Korenstein D. Harnessing the power of peer pressure to reduce health care waste and improve clinical outcomes. Mayo Clin Proc. 2015;90(3):311-312. DOI: https://doi.org/10.1017/ice.2015.136 PubMed
14. Vaidya NA, Sierles FS, Raida MD, Fakhoury FJ, Przybeck TR, Cloninger CR. Relationship between specialty choice and medical student temperament and character assessed with Cloninger Inventory. Teach Learn Med. 2004;16(2):150-156. DOI: 10.1207/s15328015tlm1602_6 PubMed
15. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617. DOI: 10.1001/jama.293.21.2609 PubMed
1. Zhi M, Ding EL, Theisen-Toupal J, Whelan J, Arnaout R. The landscape of inappropriate laboratory testing: a 15-year meta-analysis. PloS One. 2013;8(11):e78962. DOI: 10.1371/journal.pone.0078962. PubMed
2. Thavendiranathan P, Bagai A, Ebidia A, Detsky AS, Choudhry NK. Do blood tests cause anemia in hospitalized patients? The effect of diagnostic phlebotomy on hemoglobin and hematocrit levels. J Gen Intern Med. 2005;20(6):520-524. DOI: 10.1111/j.1525-1497.2005.0094.x. PubMed
3. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-1839. DOI: 10.1001/jamainternmed.2017.5152 PubMed
4. Choosing wisely. http://www.choosingwisely.org/resources/. Accessed November 21, 2017.
5. Sedrak MS, Patel MS, Ziemba JB, et al. Residents’ self-report on why they order perceived unnecessary inpatient laboratory tests. J Hosp Med. 2016;11(12):869-872. DOI: 10.1002/jhm.2645. PubMed
6. Thakkar RN, Kim D, Knight AM, Riedel S, Vaidya D, Wright SM. Impact of an educational intervention on the frequency of daily blood test orders for hospitalized patients. Am J Clin Pathol. 2015;143(3):393-397. DOI: 10.1309/AJCPJS4EEM7UAUBV. PubMed
7. Sheeler RD, Mundell T, Hurst SA, et al. Self-reported rationing behavior among US physicians: a national survey. J Gen Intern Med. 2016;31(12):1444-1451. DOI: 10.1007/s11606-016-3756-5. PubMed
8. Roman BR, Yang A, Masciale J, Korenstein D. Association of attitudes regarding overuse of inpatient laboratory testing with health care provider type. JAMA Intern Med. 2017;177(8):1205-1207. DOI: 10.1001/jamainternmed.2017.1634. PubMed
9. Schatz IJ, Realini JP, Charney E. Family practice, internal medicine, and pediatrics as partners in the education of generalists. Acad Med. 1996;71(1):35-39. PubMed
10. Johnson RE, Freeborn DK, Mullooly JP. Physicians’ use of laboratory, radiology, and drugs in a prepaid group practice HMO. Health Serv Res. 1985;20(5):525-547. PubMed
11. Chen C, Petterson S, Phillips R, Bazemore A, Mullan F. Spending patterns in region of residency training and subsequent expenditures for care provided by practicing physicians for Medicare beneficiaries. JAMA. Dec 10, 2014;312(22):2385-2393. DOI: 10.1001/jama.2014.15973. PubMed
12. Sirovich BE, Lipner RS, Johnston M, Holmboe ES. The association between residency training and internists’ ability to practice conservatively. JAMA Intern Med. 2014;174(10):1640-1648. DOI: 10.1001/jamainternmed.2014.3337. PubMed
13. Smith CD, Korenstein D. Harnessing the power of peer pressure to reduce health care waste and improve clinical outcomes. Mayo Clin Proc. 2015;90(3):311-312. DOI: https://doi.org/10.1017/ice.2015.136 PubMed
14. Vaidya NA, Sierles FS, Raida MD, Fakhoury FJ, Przybeck TR, Cloninger CR. Relationship between specialty choice and medical student temperament and character assessed with Cloninger Inventory. Teach Learn Med. 2004;16(2):150-156. DOI: 10.1207/s15328015tlm1602_6 PubMed
15. Studdert DM, Mello MM, Sage WM, et al. Defensive medicine among high-risk specialist physicians in a volatile malpractice environment. JAMA. 2005;293(21):2609-2617. DOI: 10.1001/jama.293.21.2609 PubMed
© 2018 Society of Hospital Medicine
Prevalence of Staphylococcus aureus and Use of Antistaphylococcal Therapy in Children Hospitalized with Pneumonia
Although Staphylococcus aureus pneumonia is common in children with cystic fibrosis and those with healthcare-associated infections (eg, ventilator-associated pneumonia),1,2 S. aureus is an uncommon cause of community-acquired pneumonia in children. In recent years, concerns have arisen about the increasing frequency and severity of staphylococcal pneumonia, largely fueled by the emergence of community-associated methicillin-resistant S. aureus (MRSA).3,4 Thus, therapy with clindamycin or vancomycin, both active against MRSA, has been recommended when S. aureus is suspected.5 Given the lack of rapid and sensitive approaches to the detection of the etiologies of pneumonia, antibiotic selection is most often empirical, contributing to overuse of anti-MRSA antibiotics. In addition, resistance against these antibiotics, especially clindamycin, has been increasing.6,7
A better understanding of the likelihood of staphylococcal pneumonia would help to optimize empirical antibiotic selection, allowing for judicious use of antistaphylococcal antibiotics, while also avoiding poor outcomes due to delays in effective treatment when S. aureus is present.8 Using data from a multicenter, population-based study of pneumonia hospitalizations in children, we sought to describe the prevalence, clinical characteristics, and in-hospital outcomes of staphylococcal pneumonia and the prevalence of antistaphylococcal antibiotic use.
METHODS
The Etiology of Pneumonia in the Community (EPIC) study was a prospective, active, population-based surveillance study of pneumonia hospitalizations among children (age <18 years) conducted between 2010 and 2012 at three children’s hospitals, including two in Tennessee and one in Utah.9 Children hospitalized with clinical evidence of pneumonia and radiographic evidence confirmed by a blinded review by study radiologists were enrolled. Etiologic assessments included blood analysis for bacterial culture, serology for eight respiratory viruses, pneumococcal and group A streptococcal polymerase chain reaction (PCR), and naso/oro-pharyngeal swabs for PCR for 13 respiratory viruses, Mycoplasma pneumoniae, and Chlamydophila pneumoniae. Data from other clinical specimens (pleural fluid, high-quality endotracheal aspirate, or quantified bronchoalveolar lavage fluid) were also recorded. For this study, we included only children with at least one bacterial culture and complete information about antibiotic use. Those with confirmed fungal pneumonia were excluded. Additional details regarding the study population and methods have been published previously.9
Staphylococcal pneumonia was defined based on the detection of S. aureus by culture (any site) or PCR (pleural fluid only), regardless of codetection of other pathogens. Antibiotic susceptibility profiles were used to classify S. aureus isolates as MRSA or methicillin-sensitive S. aureus (MSSA). The remaining children were classified as nonstaphylococcal pneumonia including children with other bacterial pathogens detected (classified as other bacterial pneumonia, excludes atypical bacteria), atypical bacteria, viruses, and no pathogens detected.
Use of anti-MRSA antibiotics (vancomycin, clindamycin, linezolid, doxycycline, and trimethoprim-sulfamethoxazole) and any antistaphylococcal antibiotics (anti-MRSA agents plus oxacillin, nafcillin, and cefazolin) during and after the first two calendar days of admission was identified by medical record review.
Descriptive statistics included number (%) and median (interquartile range, [IQR]) for categorical and continuous variables, respectively. Baseline clinical characteristics and outcomes were compared between children with staphylococcal versus nonstaphylococcal pneumonia, those with staphylococcal versus other bacterial pneumonia, and those with MRSA versus MSSA pneumonia using Wilcoxon rank-sum and Pearson’s chi-square tests where appropriate. To account for multiple comparisons, we used a Bonferroni corrected P value threshold of <.001 to determine statistical significance.
RESULTS
Of the 2,358 children enrolled in the EPIC study hospitalized with radiographically confirmed pneumonia, 2,146 (91.0%) had ≥1 bacterial culture obtained. Two children with Histoplasma capsulatum fungal infection and six children with incomplete antibiotic utilization data were excluded, yielding a final study population of 2,138 children. Among these, blood samples were obtained from 2,134 (>99%) children for culture, pleural fluid from 87 (4%) children, bronchoalveolar lavage fluid from 31 (1%) children, and endotracheal aspirate from 80 (4%) children. Across all culture types, there were 2,332 initial cultures; 2,150 (92%) were collected within the first 24 hours.
Staphylococcal pneumonia was detected in 23 of the 2,138 children (1% [95% CI 0.7, 1.6]; 17 MRSA, 6 MSSA). Of these, 6/23 (26%) had bacteremia, 12/23 (52%) had a positive pleural fluid, and 9/23 (39%) had a positive culture from bronchoalveolar lavage fluid or endotracheal aspirate; 4/23 (17%) children had S. aureus detected from more than one site. Three children (13%) with S. aureus had a viral codetection, including two with influenza.
Compared with children with nonstaphylococcal pneumonia, those with staphylococcal pneumonia were more likely to have a parapneumonic effusion (78% vs 12%, P < .001), but less likely to have cough (78% vs 95%, P < .001). Other baseline characteristics were similar between the two groups. Children with staphylococcal pneumonia had more adverse outcomes than those without (Table), including longer median length of stay (10 vs 3 days, P < .001), more frequent admission to intensive care (83% vs 21%, P < .001), and more frequent invasive mechanical ventilation (65% vs 7%, P < .001). Similar findings were noted when staphylococcal pneumonia was compared with pneumonia caused due to other bacterial pathogens (n = 124). There were no significant differences in baseline characteristics or clinical course between children with MRSA and MSSA pneumonia, although the numbers were small. Overall, S. aureus was detected in 18/267 (7%) children with parapneumonic effusion and 19/462 (4%) children admitted to intensive care. Importantly, there were no confirmed S. aureus cases among children with less severe pneumonia, defined as lacking both parapneumonic effusion and intensive care admission (n = 1,488).
Overall, 519 children (24%) received antistaphylococcal therapy during their hospitalization (512/519, 99% received anti-MRSA therapy), including 22 of the 23 children with S. aureus detected (the only child without antistaphylococcal therapy had S. aureus detected from a high-quality endotracheal tube aspirate only and also had respiratory syncytial virus detected). Clindamycin was most often used (n = 266, 51%), followed by vancomycin (n = 128, 24%), clindamycin plus vancomycin (n = 83, 16%), and others (n = 42, 8%). During the first two days of hospitalization, 479 children (22%) received antistaphylococcal therapy (477 received anti-MRSA therapy). After the first two days, 351 children (16%) received antistaphylococcal therapy (346/351, 99% received anti-MRSA therapy). Use of antistaphylococcal therapy was very common in those admitted to intensive care (182/462, 39%; all but two received anti-MRSA therapy) and in those requiring invasive mechanical ventilation (103/159, 65%). Among those lacking both parapneumonic effusion and intensive care admission (n = 1488), 232 (16%) received antistaphylococcal therapy.
DISCUSSION
In our large, population-based study of >2,000 children hospitalized with community-acquired pneumonia, S. aureus was identified in only 1% of children. Compared with children with other pneumonia etiologies, staphylococcal pneumonia was associated with increased disease severity. Among the small numbers studied, no differences in outcomes were found between children with MRSA and MSSA disease. Despite the low prevalence of staphylococcal pneumonia, almost 1 in 4 children received antistaphylococcal antibiotic therapy; anti-MRSA therapy was used almost exclusively.
The severity of staphylococcal pneumonia was striking, with >80% of children with S. aureus detected being admitted to intensive care, about 65% requiring invasive mechanical ventilation, and >75% with parapneumonic effusion. These findings are similar to those of prior retrospective studies.4,10 The association between staphylococcal pneumonia and adverse outcomes underscores the importance of prompt institution of antimicrobial therapy targeting S. aureus in high-risk patients. This is noteworthy given recent epidemiological data demonstrating increases in MSSA relative to MRSA infections in children,6 and the known superiority of beta-lactam versus vancomycin for MSSA infections, including pneumonia.11
Although detection of staphylococcal infection was rare, almost a quarter of children received antistaphylococcal therapy; nearly all of these children received anti-MRSA therapy. Confirming a bacterial etiology of pneumonia, however, is challenging. Given the severity associated with staphylococcal pneumonia, it is not surprising that use of antistaphylococcal therapy outpaced staphylococcal detections. Antistaphylococcal therapy was especially common in those with severe pneumonia, suggesting that disease severity is an important factor that influences initial antibiotic treatment decisions. Even so, two children with MRSA detected did not initially receive anti-MRSA therapy, highlighting the challenge of balancing judicious antibiotic selection along with ensuring effective treatment. Perhaps more striking is the finding that 16% of children received antistaphylococcal therapy beyond the first two days of hospitalization, presumably after the initial culture results were available. This suggests that clinicians are reluctant to stop antistaphylococcal therapy when the etiology is unknown, although certain features, such as negative cultures, rapid clinical improvement, and lack of risk factors for staphylococcal disease, may provide important clues to support de-escalation of empiric antibiotic therapy. It is also possible that some antibiotics with antistaphylococcal activity were used for alternative indications (eg, clindamycin for penicillin allergy or concern for aspiration pneumonia).
A simple strategy for tailoring antibiotic treatment is maximizing opportunities to identify a causative pathogen. Despite the very low yield of blood cultures in children with pneumonia overall, bacteremia is more common in children with severe pneumonia and those with parapneumonic effusion, especially when cultures are obtained prior to antibiotic use.12,13 Similarly, obtaining pleural fluid is often therapeutic and significantly improves the chances of identifying a bacterial pathogen.14 Moreover, at least one study suggests that S. aureus is much less likely in cases of culture-negative parapneumonic effusions.15 Institutional guidelines, order sets, and antimicrobial stewardship teams are also effective strategies that can facilitate judicious antibiotic use. In particular, stewardship experts can be very useful in assisting clinicians around de-escalation of therapy.16 Use of procalcitonin, a biomarker associated with bacterial infections,17 and prognostic tools to identify risk for adverse outcomes,18 may also inform treatment decisions and are deserving of further study.
Our study must be considered in
Our study demonstrates a very low prevalence of S. aureus detection among children hospitalized with pneumonia and highlights the association between staphylococcal disease and adverse in-hospital outcomes. We also document important discrepancies between disease prevalence and utilization of antistaphylococcal therapy, especially anti-MRSA therapy. Improved approaches are needed to minimize overuse of antistaphylococcal antibiotics while also ensuring adequate therapy for those who need it.
Disclosures
Drs. Zhu, Edwards, Self, Ampofo, Arnold, McCullers, and Williams report grants from the Centers for Disease Control and Prevention during the conduct of the study. Ms. Frush has nothing to disclose. Dr. Jain has nothing to disclose. Dr. Grijalva reports other from Merck, grants and other from Sanofi, other from Pfizer, grants from CDC, grants from AHRQ, grants from NIH, and grants from Campbell Alliance, outside the submitted work. Dr. Self reports grants from CDC, during the conduct of the study; personal fees from Cempra Pharmaceuticals, grants and personal fees from Ferring Pharmaceuticals, personal fees from BioTest AG, personal fees from Abbott Point of Care, personal fees from Gilead Pharmaceuticals, personal fees from Pfizer, grants from Merck, outside the submitted work. Dr. Thomsen has nothing to disclose. Dr. Ampofo reports grants from CDC, during the conduct of the study; other from GlaxoSmithKline, other from Cubist Pharmaceuticals outside the submitted work; and KA collaborate with BioFire Diagnostics, Inc. (formerly Idaho Technology, Inc.) on several NIH grants. Dr. Pavia reports grants from NAID/NIH, grants from NAID/NIH, grants from CDC, personal fees from WebMD, personal fees from Antimicrobial Therapy Inc., outside the submitted work.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number K23AI104779 to D.J.W. and Award 1K23AI113150 to I.P.T., the National Institute of General Medical Sciences under Award K23GM110469 to W.H.S., and the Agency for Healthcare Research and Quality under Award R03HS022342 to C.G.G. The EPIC study was supported by the Influenza Division in the National Center for Immunizations and Respiratory Diseases at the Centers for Disease Control and Prevention through cooperative agreements with each study site and was based on a competitive research funding opportunity. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute of Allergy and Infectious Diseases, the National Institute of General Medical Sciences, the Agency for Healthcare Research and Quality, or the Centers for Disease Control and Prevention.
1. Akil N, Muhlebach MS. Biology and management of methicillin resistant Staphylococcus aureus in cystic fibrosis. Pediatr Pulmonol. 2018. doi: 10.1002/ppul.24139. PubMed
2. Srinivasan R, Asselin J, Gildengorin G, Wiener-Kronish J, Flori HR. A prospective study of ventilator-associated pneumonia in children. Pediatrics.
2009;123(4):1108-1115. doi: 10.1542/peds.2008-1211. PubMed
3. Gonzalez BE, Martinez-Aguilar G, Hulten KG, et al. Severe Staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant Staphylococcus aureus. Pediatrics. 2005;115(3):642-648. doi: 10.1542/peds.2004-2300. PubMed
4. Carrillo-Marquez MA, Hulten KG, Hammerman W, Lamberth L, Mason EO, Kaplan SL. Staphylococcus aureus pneumonia in children in the era of community-acquired methicillin-resistance at Texas Children’s Hospital. Pediatr Infect Dis J. 2011;30(7):545-550. doi: 10.1097/INF.0b013e31821618be. PubMed
5. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the pediatric infectious diseases society and the infectious diseases society of America. Clin Infect Dis. 2011;53(7):e25-e76. doi: 10.1093/cid/cir531. PubMed
6. Sutter DE, Milburn E, Chukwuma U, Dzialowy N, Maranich AM, Hospenthal DR. Changing susceptibility of Staphylococcus aureus in a US pediatric population. Pediatrics. 2016;137(4):e20153099–e20153099. doi: 10.1542/peds.2015-3099. PubMed
7. Sakoulas G, Moellering RC, Jr. Increasing antibiotic resistance among methicillin-resistant Staphylococcus aureus strains. Clin Infect Dis. 2008;46(Suppl 5):S360-S367. doi: 10.1086/533592. PubMed
8. Rubinstein E, Kollef MH, Nathwani D. Pneumonia caused by methicillin-resistant
Staphylococcus aureus. Clin Infect Dis. 2008;46(Suppl 5):S378-S385. doi: 10.1086/533594. PubMed
9. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. doi: 10.1056/NEJMoa1405870. PubMed
10. Kallen AJ, Reed C, Patton M, Arnold KE, Finelli L, Hageman J. Staphylococcus aureus community-onset pneumonia in patients admitted to children’s hospitals during autumn and winter of 2006-2007. Epidemiol Infect. 2010;138(5):666-672. doi: 10.1017/S095026880999135X. PubMed
11. González C, Rubio M, Romero-Vivas J, González M, Picazo JJ. Bacteremic pneumonia due to Staphylococcus aureus: A comparison of disease caused by methicillin-resistant and methicillin-susceptible organisms. Clin Infect Dis. 1999;29(5):1171-1177. doi: 10.1086/313440. PubMed
12. Myers AL, Hall M, Williams DJ, et al. Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J. 2013;32(7):736-740. doi: 10.1097/INF.0b013e318290bf63. PubMed
13. Iroh Tam PY, Bernstein E, Ma X, Ferrieri P. Blood culture in evaluation of pediatric community-acquired pneumonia: A systematic review and meta-analysis. Hosp Pediatr. 2015;5(6):324-336. doi: 10.1542/hpeds.2014-0138. PubMed
14. Byington CL, Spencer LY, Johnson TA, et al. An epidemiological investigation of a sustained high rate of pediatric parapneumonic empyema: risk factors and microbiological associations. Clin Infect Dis. 2002;34(4):434-440. doi: 10.1086/338460. PubMed
15. Blaschke AJ, Heyrend C, Byington CL, et al. Molecular analysis improves pathogen identifi cation and epidemiologic study of pediatric parapneumonic empyema. Pediatr Infect Dis J. 2011;30(4):289-294. doi: 10.1097/INF.0b013e3182002d14. PubMed
16. Banerjee R, Teng CB, Cunningham SA, et al. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identifi cation and susceptibility testing. Clin Infect Dis. 2015;61(7):1071-1080. doi: 10.1093/cid/civ447. PubMed
17. Stockmann C, Ampofo K, Killpack J, et al. Procalcitonin accurately identifies hospitalized children with low risk of bacterial community-acquired pneumonia. J Pediatr Infect Dis Soc. 2018;7(1):46–53. doi: 10.1093/jpids/piw091. PubMed
18. Williams DJ, Zhu Y, Grijalva CG, et al. Predicting severe pneumonia outcomes in children. Pediatrics. 2016;138(4). doi: 10.1542/peds.2016-1019. PubMed
19. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. doi: 10.1097/INF.0b013e-31825f2b10. PubMed
Although Staphylococcus aureus pneumonia is common in children with cystic fibrosis and those with healthcare-associated infections (eg, ventilator-associated pneumonia),1,2 S. aureus is an uncommon cause of community-acquired pneumonia in children. In recent years, concerns have arisen about the increasing frequency and severity of staphylococcal pneumonia, largely fueled by the emergence of community-associated methicillin-resistant S. aureus (MRSA).3,4 Thus, therapy with clindamycin or vancomycin, both active against MRSA, has been recommended when S. aureus is suspected.5 Given the lack of rapid and sensitive approaches to the detection of the etiologies of pneumonia, antibiotic selection is most often empirical, contributing to overuse of anti-MRSA antibiotics. In addition, resistance against these antibiotics, especially clindamycin, has been increasing.6,7
A better understanding of the likelihood of staphylococcal pneumonia would help to optimize empirical antibiotic selection, allowing for judicious use of antistaphylococcal antibiotics, while also avoiding poor outcomes due to delays in effective treatment when S. aureus is present.8 Using data from a multicenter, population-based study of pneumonia hospitalizations in children, we sought to describe the prevalence, clinical characteristics, and in-hospital outcomes of staphylococcal pneumonia and the prevalence of antistaphylococcal antibiotic use.
METHODS
The Etiology of Pneumonia in the Community (EPIC) study was a prospective, active, population-based surveillance study of pneumonia hospitalizations among children (age <18 years) conducted between 2010 and 2012 at three children’s hospitals, including two in Tennessee and one in Utah.9 Children hospitalized with clinical evidence of pneumonia and radiographic evidence confirmed by a blinded review by study radiologists were enrolled. Etiologic assessments included blood analysis for bacterial culture, serology for eight respiratory viruses, pneumococcal and group A streptococcal polymerase chain reaction (PCR), and naso/oro-pharyngeal swabs for PCR for 13 respiratory viruses, Mycoplasma pneumoniae, and Chlamydophila pneumoniae. Data from other clinical specimens (pleural fluid, high-quality endotracheal aspirate, or quantified bronchoalveolar lavage fluid) were also recorded. For this study, we included only children with at least one bacterial culture and complete information about antibiotic use. Those with confirmed fungal pneumonia were excluded. Additional details regarding the study population and methods have been published previously.9
Staphylococcal pneumonia was defined based on the detection of S. aureus by culture (any site) or PCR (pleural fluid only), regardless of codetection of other pathogens. Antibiotic susceptibility profiles were used to classify S. aureus isolates as MRSA or methicillin-sensitive S. aureus (MSSA). The remaining children were classified as nonstaphylococcal pneumonia including children with other bacterial pathogens detected (classified as other bacterial pneumonia, excludes atypical bacteria), atypical bacteria, viruses, and no pathogens detected.
Use of anti-MRSA antibiotics (vancomycin, clindamycin, linezolid, doxycycline, and trimethoprim-sulfamethoxazole) and any antistaphylococcal antibiotics (anti-MRSA agents plus oxacillin, nafcillin, and cefazolin) during and after the first two calendar days of admission was identified by medical record review.
Descriptive statistics included number (%) and median (interquartile range, [IQR]) for categorical and continuous variables, respectively. Baseline clinical characteristics and outcomes were compared between children with staphylococcal versus nonstaphylococcal pneumonia, those with staphylococcal versus other bacterial pneumonia, and those with MRSA versus MSSA pneumonia using Wilcoxon rank-sum and Pearson’s chi-square tests where appropriate. To account for multiple comparisons, we used a Bonferroni corrected P value threshold of <.001 to determine statistical significance.
RESULTS
Of the 2,358 children enrolled in the EPIC study hospitalized with radiographically confirmed pneumonia, 2,146 (91.0%) had ≥1 bacterial culture obtained. Two children with Histoplasma capsulatum fungal infection and six children with incomplete antibiotic utilization data were excluded, yielding a final study population of 2,138 children. Among these, blood samples were obtained from 2,134 (>99%) children for culture, pleural fluid from 87 (4%) children, bronchoalveolar lavage fluid from 31 (1%) children, and endotracheal aspirate from 80 (4%) children. Across all culture types, there were 2,332 initial cultures; 2,150 (92%) were collected within the first 24 hours.
Staphylococcal pneumonia was detected in 23 of the 2,138 children (1% [95% CI 0.7, 1.6]; 17 MRSA, 6 MSSA). Of these, 6/23 (26%) had bacteremia, 12/23 (52%) had a positive pleural fluid, and 9/23 (39%) had a positive culture from bronchoalveolar lavage fluid or endotracheal aspirate; 4/23 (17%) children had S. aureus detected from more than one site. Three children (13%) with S. aureus had a viral codetection, including two with influenza.
Compared with children with nonstaphylococcal pneumonia, those with staphylococcal pneumonia were more likely to have a parapneumonic effusion (78% vs 12%, P < .001), but less likely to have cough (78% vs 95%, P < .001). Other baseline characteristics were similar between the two groups. Children with staphylococcal pneumonia had more adverse outcomes than those without (Table), including longer median length of stay (10 vs 3 days, P < .001), more frequent admission to intensive care (83% vs 21%, P < .001), and more frequent invasive mechanical ventilation (65% vs 7%, P < .001). Similar findings were noted when staphylococcal pneumonia was compared with pneumonia caused due to other bacterial pathogens (n = 124). There were no significant differences in baseline characteristics or clinical course between children with MRSA and MSSA pneumonia, although the numbers were small. Overall, S. aureus was detected in 18/267 (7%) children with parapneumonic effusion and 19/462 (4%) children admitted to intensive care. Importantly, there were no confirmed S. aureus cases among children with less severe pneumonia, defined as lacking both parapneumonic effusion and intensive care admission (n = 1,488).
Overall, 519 children (24%) received antistaphylococcal therapy during their hospitalization (512/519, 99% received anti-MRSA therapy), including 22 of the 23 children with S. aureus detected (the only child without antistaphylococcal therapy had S. aureus detected from a high-quality endotracheal tube aspirate only and also had respiratory syncytial virus detected). Clindamycin was most often used (n = 266, 51%), followed by vancomycin (n = 128, 24%), clindamycin plus vancomycin (n = 83, 16%), and others (n = 42, 8%). During the first two days of hospitalization, 479 children (22%) received antistaphylococcal therapy (477 received anti-MRSA therapy). After the first two days, 351 children (16%) received antistaphylococcal therapy (346/351, 99% received anti-MRSA therapy). Use of antistaphylococcal therapy was very common in those admitted to intensive care (182/462, 39%; all but two received anti-MRSA therapy) and in those requiring invasive mechanical ventilation (103/159, 65%). Among those lacking both parapneumonic effusion and intensive care admission (n = 1488), 232 (16%) received antistaphylococcal therapy.
DISCUSSION
In our large, population-based study of >2,000 children hospitalized with community-acquired pneumonia, S. aureus was identified in only 1% of children. Compared with children with other pneumonia etiologies, staphylococcal pneumonia was associated with increased disease severity. Among the small numbers studied, no differences in outcomes were found between children with MRSA and MSSA disease. Despite the low prevalence of staphylococcal pneumonia, almost 1 in 4 children received antistaphylococcal antibiotic therapy; anti-MRSA therapy was used almost exclusively.
The severity of staphylococcal pneumonia was striking, with >80% of children with S. aureus detected being admitted to intensive care, about 65% requiring invasive mechanical ventilation, and >75% with parapneumonic effusion. These findings are similar to those of prior retrospective studies.4,10 The association between staphylococcal pneumonia and adverse outcomes underscores the importance of prompt institution of antimicrobial therapy targeting S. aureus in high-risk patients. This is noteworthy given recent epidemiological data demonstrating increases in MSSA relative to MRSA infections in children,6 and the known superiority of beta-lactam versus vancomycin for MSSA infections, including pneumonia.11
Although detection of staphylococcal infection was rare, almost a quarter of children received antistaphylococcal therapy; nearly all of these children received anti-MRSA therapy. Confirming a bacterial etiology of pneumonia, however, is challenging. Given the severity associated with staphylococcal pneumonia, it is not surprising that use of antistaphylococcal therapy outpaced staphylococcal detections. Antistaphylococcal therapy was especially common in those with severe pneumonia, suggesting that disease severity is an important factor that influences initial antibiotic treatment decisions. Even so, two children with MRSA detected did not initially receive anti-MRSA therapy, highlighting the challenge of balancing judicious antibiotic selection along with ensuring effective treatment. Perhaps more striking is the finding that 16% of children received antistaphylococcal therapy beyond the first two days of hospitalization, presumably after the initial culture results were available. This suggests that clinicians are reluctant to stop antistaphylococcal therapy when the etiology is unknown, although certain features, such as negative cultures, rapid clinical improvement, and lack of risk factors for staphylococcal disease, may provide important clues to support de-escalation of empiric antibiotic therapy. It is also possible that some antibiotics with antistaphylococcal activity were used for alternative indications (eg, clindamycin for penicillin allergy or concern for aspiration pneumonia).
A simple strategy for tailoring antibiotic treatment is maximizing opportunities to identify a causative pathogen. Despite the very low yield of blood cultures in children with pneumonia overall, bacteremia is more common in children with severe pneumonia and those with parapneumonic effusion, especially when cultures are obtained prior to antibiotic use.12,13 Similarly, obtaining pleural fluid is often therapeutic and significantly improves the chances of identifying a bacterial pathogen.14 Moreover, at least one study suggests that S. aureus is much less likely in cases of culture-negative parapneumonic effusions.15 Institutional guidelines, order sets, and antimicrobial stewardship teams are also effective strategies that can facilitate judicious antibiotic use. In particular, stewardship experts can be very useful in assisting clinicians around de-escalation of therapy.16 Use of procalcitonin, a biomarker associated with bacterial infections,17 and prognostic tools to identify risk for adverse outcomes,18 may also inform treatment decisions and are deserving of further study.
Our study must be considered in
Our study demonstrates a very low prevalence of S. aureus detection among children hospitalized with pneumonia and highlights the association between staphylococcal disease and adverse in-hospital outcomes. We also document important discrepancies between disease prevalence and utilization of antistaphylococcal therapy, especially anti-MRSA therapy. Improved approaches are needed to minimize overuse of antistaphylococcal antibiotics while also ensuring adequate therapy for those who need it.
Disclosures
Drs. Zhu, Edwards, Self, Ampofo, Arnold, McCullers, and Williams report grants from the Centers for Disease Control and Prevention during the conduct of the study. Ms. Frush has nothing to disclose. Dr. Jain has nothing to disclose. Dr. Grijalva reports other from Merck, grants and other from Sanofi, other from Pfizer, grants from CDC, grants from AHRQ, grants from NIH, and grants from Campbell Alliance, outside the submitted work. Dr. Self reports grants from CDC, during the conduct of the study; personal fees from Cempra Pharmaceuticals, grants and personal fees from Ferring Pharmaceuticals, personal fees from BioTest AG, personal fees from Abbott Point of Care, personal fees from Gilead Pharmaceuticals, personal fees from Pfizer, grants from Merck, outside the submitted work. Dr. Thomsen has nothing to disclose. Dr. Ampofo reports grants from CDC, during the conduct of the study; other from GlaxoSmithKline, other from Cubist Pharmaceuticals outside the submitted work; and KA collaborate with BioFire Diagnostics, Inc. (formerly Idaho Technology, Inc.) on several NIH grants. Dr. Pavia reports grants from NAID/NIH, grants from NAID/NIH, grants from CDC, personal fees from WebMD, personal fees from Antimicrobial Therapy Inc., outside the submitted work.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number K23AI104779 to D.J.W. and Award 1K23AI113150 to I.P.T., the National Institute of General Medical Sciences under Award K23GM110469 to W.H.S., and the Agency for Healthcare Research and Quality under Award R03HS022342 to C.G.G. The EPIC study was supported by the Influenza Division in the National Center for Immunizations and Respiratory Diseases at the Centers for Disease Control and Prevention through cooperative agreements with each study site and was based on a competitive research funding opportunity. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute of Allergy and Infectious Diseases, the National Institute of General Medical Sciences, the Agency for Healthcare Research and Quality, or the Centers for Disease Control and Prevention.
Although Staphylococcus aureus pneumonia is common in children with cystic fibrosis and those with healthcare-associated infections (eg, ventilator-associated pneumonia),1,2 S. aureus is an uncommon cause of community-acquired pneumonia in children. In recent years, concerns have arisen about the increasing frequency and severity of staphylococcal pneumonia, largely fueled by the emergence of community-associated methicillin-resistant S. aureus (MRSA).3,4 Thus, therapy with clindamycin or vancomycin, both active against MRSA, has been recommended when S. aureus is suspected.5 Given the lack of rapid and sensitive approaches to the detection of the etiologies of pneumonia, antibiotic selection is most often empirical, contributing to overuse of anti-MRSA antibiotics. In addition, resistance against these antibiotics, especially clindamycin, has been increasing.6,7
A better understanding of the likelihood of staphylococcal pneumonia would help to optimize empirical antibiotic selection, allowing for judicious use of antistaphylococcal antibiotics, while also avoiding poor outcomes due to delays in effective treatment when S. aureus is present.8 Using data from a multicenter, population-based study of pneumonia hospitalizations in children, we sought to describe the prevalence, clinical characteristics, and in-hospital outcomes of staphylococcal pneumonia and the prevalence of antistaphylococcal antibiotic use.
METHODS
The Etiology of Pneumonia in the Community (EPIC) study was a prospective, active, population-based surveillance study of pneumonia hospitalizations among children (age <18 years) conducted between 2010 and 2012 at three children’s hospitals, including two in Tennessee and one in Utah.9 Children hospitalized with clinical evidence of pneumonia and radiographic evidence confirmed by a blinded review by study radiologists were enrolled. Etiologic assessments included blood analysis for bacterial culture, serology for eight respiratory viruses, pneumococcal and group A streptococcal polymerase chain reaction (PCR), and naso/oro-pharyngeal swabs for PCR for 13 respiratory viruses, Mycoplasma pneumoniae, and Chlamydophila pneumoniae. Data from other clinical specimens (pleural fluid, high-quality endotracheal aspirate, or quantified bronchoalveolar lavage fluid) were also recorded. For this study, we included only children with at least one bacterial culture and complete information about antibiotic use. Those with confirmed fungal pneumonia were excluded. Additional details regarding the study population and methods have been published previously.9
Staphylococcal pneumonia was defined based on the detection of S. aureus by culture (any site) or PCR (pleural fluid only), regardless of codetection of other pathogens. Antibiotic susceptibility profiles were used to classify S. aureus isolates as MRSA or methicillin-sensitive S. aureus (MSSA). The remaining children were classified as nonstaphylococcal pneumonia including children with other bacterial pathogens detected (classified as other bacterial pneumonia, excludes atypical bacteria), atypical bacteria, viruses, and no pathogens detected.
Use of anti-MRSA antibiotics (vancomycin, clindamycin, linezolid, doxycycline, and trimethoprim-sulfamethoxazole) and any antistaphylococcal antibiotics (anti-MRSA agents plus oxacillin, nafcillin, and cefazolin) during and after the first two calendar days of admission was identified by medical record review.
Descriptive statistics included number (%) and median (interquartile range, [IQR]) for categorical and continuous variables, respectively. Baseline clinical characteristics and outcomes were compared between children with staphylococcal versus nonstaphylococcal pneumonia, those with staphylococcal versus other bacterial pneumonia, and those with MRSA versus MSSA pneumonia using Wilcoxon rank-sum and Pearson’s chi-square tests where appropriate. To account for multiple comparisons, we used a Bonferroni corrected P value threshold of <.001 to determine statistical significance.
RESULTS
Of the 2,358 children enrolled in the EPIC study hospitalized with radiographically confirmed pneumonia, 2,146 (91.0%) had ≥1 bacterial culture obtained. Two children with Histoplasma capsulatum fungal infection and six children with incomplete antibiotic utilization data were excluded, yielding a final study population of 2,138 children. Among these, blood samples were obtained from 2,134 (>99%) children for culture, pleural fluid from 87 (4%) children, bronchoalveolar lavage fluid from 31 (1%) children, and endotracheal aspirate from 80 (4%) children. Across all culture types, there were 2,332 initial cultures; 2,150 (92%) were collected within the first 24 hours.
Staphylococcal pneumonia was detected in 23 of the 2,138 children (1% [95% CI 0.7, 1.6]; 17 MRSA, 6 MSSA). Of these, 6/23 (26%) had bacteremia, 12/23 (52%) had a positive pleural fluid, and 9/23 (39%) had a positive culture from bronchoalveolar lavage fluid or endotracheal aspirate; 4/23 (17%) children had S. aureus detected from more than one site. Three children (13%) with S. aureus had a viral codetection, including two with influenza.
Compared with children with nonstaphylococcal pneumonia, those with staphylococcal pneumonia were more likely to have a parapneumonic effusion (78% vs 12%, P < .001), but less likely to have cough (78% vs 95%, P < .001). Other baseline characteristics were similar between the two groups. Children with staphylococcal pneumonia had more adverse outcomes than those without (Table), including longer median length of stay (10 vs 3 days, P < .001), more frequent admission to intensive care (83% vs 21%, P < .001), and more frequent invasive mechanical ventilation (65% vs 7%, P < .001). Similar findings were noted when staphylococcal pneumonia was compared with pneumonia caused due to other bacterial pathogens (n = 124). There were no significant differences in baseline characteristics or clinical course between children with MRSA and MSSA pneumonia, although the numbers were small. Overall, S. aureus was detected in 18/267 (7%) children with parapneumonic effusion and 19/462 (4%) children admitted to intensive care. Importantly, there were no confirmed S. aureus cases among children with less severe pneumonia, defined as lacking both parapneumonic effusion and intensive care admission (n = 1,488).
Overall, 519 children (24%) received antistaphylococcal therapy during their hospitalization (512/519, 99% received anti-MRSA therapy), including 22 of the 23 children with S. aureus detected (the only child without antistaphylococcal therapy had S. aureus detected from a high-quality endotracheal tube aspirate only and also had respiratory syncytial virus detected). Clindamycin was most often used (n = 266, 51%), followed by vancomycin (n = 128, 24%), clindamycin plus vancomycin (n = 83, 16%), and others (n = 42, 8%). During the first two days of hospitalization, 479 children (22%) received antistaphylococcal therapy (477 received anti-MRSA therapy). After the first two days, 351 children (16%) received antistaphylococcal therapy (346/351, 99% received anti-MRSA therapy). Use of antistaphylococcal therapy was very common in those admitted to intensive care (182/462, 39%; all but two received anti-MRSA therapy) and in those requiring invasive mechanical ventilation (103/159, 65%). Among those lacking both parapneumonic effusion and intensive care admission (n = 1488), 232 (16%) received antistaphylococcal therapy.
DISCUSSION
In our large, population-based study of >2,000 children hospitalized with community-acquired pneumonia, S. aureus was identified in only 1% of children. Compared with children with other pneumonia etiologies, staphylococcal pneumonia was associated with increased disease severity. Among the small numbers studied, no differences in outcomes were found between children with MRSA and MSSA disease. Despite the low prevalence of staphylococcal pneumonia, almost 1 in 4 children received antistaphylococcal antibiotic therapy; anti-MRSA therapy was used almost exclusively.
The severity of staphylococcal pneumonia was striking, with >80% of children with S. aureus detected being admitted to intensive care, about 65% requiring invasive mechanical ventilation, and >75% with parapneumonic effusion. These findings are similar to those of prior retrospective studies.4,10 The association between staphylococcal pneumonia and adverse outcomes underscores the importance of prompt institution of antimicrobial therapy targeting S. aureus in high-risk patients. This is noteworthy given recent epidemiological data demonstrating increases in MSSA relative to MRSA infections in children,6 and the known superiority of beta-lactam versus vancomycin for MSSA infections, including pneumonia.11
Although detection of staphylococcal infection was rare, almost a quarter of children received antistaphylococcal therapy; nearly all of these children received anti-MRSA therapy. Confirming a bacterial etiology of pneumonia, however, is challenging. Given the severity associated with staphylococcal pneumonia, it is not surprising that use of antistaphylococcal therapy outpaced staphylococcal detections. Antistaphylococcal therapy was especially common in those with severe pneumonia, suggesting that disease severity is an important factor that influences initial antibiotic treatment decisions. Even so, two children with MRSA detected did not initially receive anti-MRSA therapy, highlighting the challenge of balancing judicious antibiotic selection along with ensuring effective treatment. Perhaps more striking is the finding that 16% of children received antistaphylococcal therapy beyond the first two days of hospitalization, presumably after the initial culture results were available. This suggests that clinicians are reluctant to stop antistaphylococcal therapy when the etiology is unknown, although certain features, such as negative cultures, rapid clinical improvement, and lack of risk factors for staphylococcal disease, may provide important clues to support de-escalation of empiric antibiotic therapy. It is also possible that some antibiotics with antistaphylococcal activity were used for alternative indications (eg, clindamycin for penicillin allergy or concern for aspiration pneumonia).
A simple strategy for tailoring antibiotic treatment is maximizing opportunities to identify a causative pathogen. Despite the very low yield of blood cultures in children with pneumonia overall, bacteremia is more common in children with severe pneumonia and those with parapneumonic effusion, especially when cultures are obtained prior to antibiotic use.12,13 Similarly, obtaining pleural fluid is often therapeutic and significantly improves the chances of identifying a bacterial pathogen.14 Moreover, at least one study suggests that S. aureus is much less likely in cases of culture-negative parapneumonic effusions.15 Institutional guidelines, order sets, and antimicrobial stewardship teams are also effective strategies that can facilitate judicious antibiotic use. In particular, stewardship experts can be very useful in assisting clinicians around de-escalation of therapy.16 Use of procalcitonin, a biomarker associated with bacterial infections,17 and prognostic tools to identify risk for adverse outcomes,18 may also inform treatment decisions and are deserving of further study.
Our study must be considered in
Our study demonstrates a very low prevalence of S. aureus detection among children hospitalized with pneumonia and highlights the association between staphylococcal disease and adverse in-hospital outcomes. We also document important discrepancies between disease prevalence and utilization of antistaphylococcal therapy, especially anti-MRSA therapy. Improved approaches are needed to minimize overuse of antistaphylococcal antibiotics while also ensuring adequate therapy for those who need it.
Disclosures
Drs. Zhu, Edwards, Self, Ampofo, Arnold, McCullers, and Williams report grants from the Centers for Disease Control and Prevention during the conduct of the study. Ms. Frush has nothing to disclose. Dr. Jain has nothing to disclose. Dr. Grijalva reports other from Merck, grants and other from Sanofi, other from Pfizer, grants from CDC, grants from AHRQ, grants from NIH, and grants from Campbell Alliance, outside the submitted work. Dr. Self reports grants from CDC, during the conduct of the study; personal fees from Cempra Pharmaceuticals, grants and personal fees from Ferring Pharmaceuticals, personal fees from BioTest AG, personal fees from Abbott Point of Care, personal fees from Gilead Pharmaceuticals, personal fees from Pfizer, grants from Merck, outside the submitted work. Dr. Thomsen has nothing to disclose. Dr. Ampofo reports grants from CDC, during the conduct of the study; other from GlaxoSmithKline, other from Cubist Pharmaceuticals outside the submitted work; and KA collaborate with BioFire Diagnostics, Inc. (formerly Idaho Technology, Inc.) on several NIH grants. Dr. Pavia reports grants from NAID/NIH, grants from NAID/NIH, grants from CDC, personal fees from WebMD, personal fees from Antimicrobial Therapy Inc., outside the submitted work.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number K23AI104779 to D.J.W. and Award 1K23AI113150 to I.P.T., the National Institute of General Medical Sciences under Award K23GM110469 to W.H.S., and the Agency for Healthcare Research and Quality under Award R03HS022342 to C.G.G. The EPIC study was supported by the Influenza Division in the National Center for Immunizations and Respiratory Diseases at the Centers for Disease Control and Prevention through cooperative agreements with each study site and was based on a competitive research funding opportunity. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute of Allergy and Infectious Diseases, the National Institute of General Medical Sciences, the Agency for Healthcare Research and Quality, or the Centers for Disease Control and Prevention.
1. Akil N, Muhlebach MS. Biology and management of methicillin resistant Staphylococcus aureus in cystic fibrosis. Pediatr Pulmonol. 2018. doi: 10.1002/ppul.24139. PubMed
2. Srinivasan R, Asselin J, Gildengorin G, Wiener-Kronish J, Flori HR. A prospective study of ventilator-associated pneumonia in children. Pediatrics.
2009;123(4):1108-1115. doi: 10.1542/peds.2008-1211. PubMed
3. Gonzalez BE, Martinez-Aguilar G, Hulten KG, et al. Severe Staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant Staphylococcus aureus. Pediatrics. 2005;115(3):642-648. doi: 10.1542/peds.2004-2300. PubMed
4. Carrillo-Marquez MA, Hulten KG, Hammerman W, Lamberth L, Mason EO, Kaplan SL. Staphylococcus aureus pneumonia in children in the era of community-acquired methicillin-resistance at Texas Children’s Hospital. Pediatr Infect Dis J. 2011;30(7):545-550. doi: 10.1097/INF.0b013e31821618be. PubMed
5. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the pediatric infectious diseases society and the infectious diseases society of America. Clin Infect Dis. 2011;53(7):e25-e76. doi: 10.1093/cid/cir531. PubMed
6. Sutter DE, Milburn E, Chukwuma U, Dzialowy N, Maranich AM, Hospenthal DR. Changing susceptibility of Staphylococcus aureus in a US pediatric population. Pediatrics. 2016;137(4):e20153099–e20153099. doi: 10.1542/peds.2015-3099. PubMed
7. Sakoulas G, Moellering RC, Jr. Increasing antibiotic resistance among methicillin-resistant Staphylococcus aureus strains. Clin Infect Dis. 2008;46(Suppl 5):S360-S367. doi: 10.1086/533592. PubMed
8. Rubinstein E, Kollef MH, Nathwani D. Pneumonia caused by methicillin-resistant
Staphylococcus aureus. Clin Infect Dis. 2008;46(Suppl 5):S378-S385. doi: 10.1086/533594. PubMed
9. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. doi: 10.1056/NEJMoa1405870. PubMed
10. Kallen AJ, Reed C, Patton M, Arnold KE, Finelli L, Hageman J. Staphylococcus aureus community-onset pneumonia in patients admitted to children’s hospitals during autumn and winter of 2006-2007. Epidemiol Infect. 2010;138(5):666-672. doi: 10.1017/S095026880999135X. PubMed
11. González C, Rubio M, Romero-Vivas J, González M, Picazo JJ. Bacteremic pneumonia due to Staphylococcus aureus: A comparison of disease caused by methicillin-resistant and methicillin-susceptible organisms. Clin Infect Dis. 1999;29(5):1171-1177. doi: 10.1086/313440. PubMed
12. Myers AL, Hall M, Williams DJ, et al. Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J. 2013;32(7):736-740. doi: 10.1097/INF.0b013e318290bf63. PubMed
13. Iroh Tam PY, Bernstein E, Ma X, Ferrieri P. Blood culture in evaluation of pediatric community-acquired pneumonia: A systematic review and meta-analysis. Hosp Pediatr. 2015;5(6):324-336. doi: 10.1542/hpeds.2014-0138. PubMed
14. Byington CL, Spencer LY, Johnson TA, et al. An epidemiological investigation of a sustained high rate of pediatric parapneumonic empyema: risk factors and microbiological associations. Clin Infect Dis. 2002;34(4):434-440. doi: 10.1086/338460. PubMed
15. Blaschke AJ, Heyrend C, Byington CL, et al. Molecular analysis improves pathogen identifi cation and epidemiologic study of pediatric parapneumonic empyema. Pediatr Infect Dis J. 2011;30(4):289-294. doi: 10.1097/INF.0b013e3182002d14. PubMed
16. Banerjee R, Teng CB, Cunningham SA, et al. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identifi cation and susceptibility testing. Clin Infect Dis. 2015;61(7):1071-1080. doi: 10.1093/cid/civ447. PubMed
17. Stockmann C, Ampofo K, Killpack J, et al. Procalcitonin accurately identifies hospitalized children with low risk of bacterial community-acquired pneumonia. J Pediatr Infect Dis Soc. 2018;7(1):46–53. doi: 10.1093/jpids/piw091. PubMed
18. Williams DJ, Zhu Y, Grijalva CG, et al. Predicting severe pneumonia outcomes in children. Pediatrics. 2016;138(4). doi: 10.1542/peds.2016-1019. PubMed
19. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. doi: 10.1097/INF.0b013e-31825f2b10. PubMed
1. Akil N, Muhlebach MS. Biology and management of methicillin resistant Staphylococcus aureus in cystic fibrosis. Pediatr Pulmonol. 2018. doi: 10.1002/ppul.24139. PubMed
2. Srinivasan R, Asselin J, Gildengorin G, Wiener-Kronish J, Flori HR. A prospective study of ventilator-associated pneumonia in children. Pediatrics.
2009;123(4):1108-1115. doi: 10.1542/peds.2008-1211. PubMed
3. Gonzalez BE, Martinez-Aguilar G, Hulten KG, et al. Severe Staphylococcal sepsis in adolescents in the era of community-acquired methicillin-resistant Staphylococcus aureus. Pediatrics. 2005;115(3):642-648. doi: 10.1542/peds.2004-2300. PubMed
4. Carrillo-Marquez MA, Hulten KG, Hammerman W, Lamberth L, Mason EO, Kaplan SL. Staphylococcus aureus pneumonia in children in the era of community-acquired methicillin-resistance at Texas Children’s Hospital. Pediatr Infect Dis J. 2011;30(7):545-550. doi: 10.1097/INF.0b013e31821618be. PubMed
5. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the pediatric infectious diseases society and the infectious diseases society of America. Clin Infect Dis. 2011;53(7):e25-e76. doi: 10.1093/cid/cir531. PubMed
6. Sutter DE, Milburn E, Chukwuma U, Dzialowy N, Maranich AM, Hospenthal DR. Changing susceptibility of Staphylococcus aureus in a US pediatric population. Pediatrics. 2016;137(4):e20153099–e20153099. doi: 10.1542/peds.2015-3099. PubMed
7. Sakoulas G, Moellering RC, Jr. Increasing antibiotic resistance among methicillin-resistant Staphylococcus aureus strains. Clin Infect Dis. 2008;46(Suppl 5):S360-S367. doi: 10.1086/533592. PubMed
8. Rubinstein E, Kollef MH, Nathwani D. Pneumonia caused by methicillin-resistant
Staphylococcus aureus. Clin Infect Dis. 2008;46(Suppl 5):S378-S385. doi: 10.1086/533594. PubMed
9. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845. doi: 10.1056/NEJMoa1405870. PubMed
10. Kallen AJ, Reed C, Patton M, Arnold KE, Finelli L, Hageman J. Staphylococcus aureus community-onset pneumonia in patients admitted to children’s hospitals during autumn and winter of 2006-2007. Epidemiol Infect. 2010;138(5):666-672. doi: 10.1017/S095026880999135X. PubMed
11. González C, Rubio M, Romero-Vivas J, González M, Picazo JJ. Bacteremic pneumonia due to Staphylococcus aureus: A comparison of disease caused by methicillin-resistant and methicillin-susceptible organisms. Clin Infect Dis. 1999;29(5):1171-1177. doi: 10.1086/313440. PubMed
12. Myers AL, Hall M, Williams DJ, et al. Prevalence of bacteremia in hospitalized pediatric patients with community-acquired pneumonia. Pediatr Infect Dis J. 2013;32(7):736-740. doi: 10.1097/INF.0b013e318290bf63. PubMed
13. Iroh Tam PY, Bernstein E, Ma X, Ferrieri P. Blood culture in evaluation of pediatric community-acquired pneumonia: A systematic review and meta-analysis. Hosp Pediatr. 2015;5(6):324-336. doi: 10.1542/hpeds.2014-0138. PubMed
14. Byington CL, Spencer LY, Johnson TA, et al. An epidemiological investigation of a sustained high rate of pediatric parapneumonic empyema: risk factors and microbiological associations. Clin Infect Dis. 2002;34(4):434-440. doi: 10.1086/338460. PubMed
15. Blaschke AJ, Heyrend C, Byington CL, et al. Molecular analysis improves pathogen identifi cation and epidemiologic study of pediatric parapneumonic empyema. Pediatr Infect Dis J. 2011;30(4):289-294. doi: 10.1097/INF.0b013e3182002d14. PubMed
16. Banerjee R, Teng CB, Cunningham SA, et al. Randomized trial of rapid multiplex polymerase chain reaction-based blood culture identifi cation and susceptibility testing. Clin Infect Dis. 2015;61(7):1071-1080. doi: 10.1093/cid/civ447. PubMed
17. Stockmann C, Ampofo K, Killpack J, et al. Procalcitonin accurately identifies hospitalized children with low risk of bacterial community-acquired pneumonia. J Pediatr Infect Dis Soc. 2018;7(1):46–53. doi: 10.1093/jpids/piw091. PubMed
18. Williams DJ, Zhu Y, Grijalva CG, et al. Predicting severe pneumonia outcomes in children. Pediatrics. 2016;138(4). doi: 10.1542/peds.2016-1019. PubMed
19. Brogan TV, Hall M, Williams DJ, et al. Variability in processes of care and outcomes among children hospitalized with community-acquired pneumonia. Pediatr Infect Dis J. 2012;31(10):1036-1041. doi: 10.1097/INF.0b013e-31825f2b10. PubMed
©2018 Society of Hospital Medicine
Estimating the Accuracy of Dobutamine Stress Echocardiography and Single-Photon Emission Computed Tomography among Patients Undergoing Noncardiac Surgery
Cardiac complications account for at least one-third of perioperative deaths, and lead to substantial morbidity and cost.1-4 Current guidelines recommend that patients undergo assessment of cardiac risk and functional status prior to noncardiac surgery.5 Preoperative cardiac stress testing is recommended for patients whose predicted cardiac risk exceeds 1%, whose functional status is limited, and for whom testing may change management.5
However, patients are not specifically selected according to risk of coronary artery disease (CAD) in current guidelines. The pretest probability of CAD may vary widely in this patient population, and the resultant accuracy of cardiac stress testing in making the diagnosis of CAD may vary as well.5 Meanwhile, CAD is a clear risk factor for perioperative cardiac events.6-8
Because the pretest probability of CAD is heterogeneous, the optimal modality of cardiac stress testing in this population is unclear. False-positive results would likely lead to inflated estimates of operative risk, expensive and high-risk downstream testing, and potentially cancellation of otherwise beneficial surgeries. Meanwhile, false-negative results would lead to overly optimistic estimates of surgical risk and potentially to surgical intervention at higher levels of risk than would be desirable. Current guidelines leave the selection of either dobutamine stress echocardiography (DSE) or pharmacological stress myocardial perfusion imaging to the clinician.5 To inform decisions regarding the selection of cardiac stress testing modality prior to noncardiac surgery, we conducted this study to estimate the diagnostic accuracy of DSE and single-photon emission computed tomography (SPECT) among this patient population.
METHODS
Surgical Cohort
The American College of Surgeons’ National Surgical Quality Improvement Program (NSQIP) samples patients undergoing surgery at participating hospitals and collects standardized clinical data on preoperative risk factors and postoperative complications.9 We acquired public use data from the 2009 NSQIP cohort, which included more than 336,000 surgical cases from 237 hospitals (principally in the United States). We excluded from our analysis patients undergoing cardiac surgery, patients with a prior diagnosis of CAD, and patients undergoing experimental surgeries. This left a sample of 300,462 for analysis.
Prediction of Dyslipidemia
The model we used to predict the presence of obstructive CAD required the presence or absence of dyslipidemia. A number of variables are common to both NSQIP and the National Health and Nutrition Examination Survey (NHANES), including age, weight, sex, tobacco use, diabetes, and prior stroke.10 Using those common variables, we developed a logistic regression to predict a diagnosis of dyslipidemia, applied that regression to the NSQIP cohort, and dichotomized. To assess the potential impact of misclassification, we performed separate sensitivity analyses in which either no patients or all patients had dyslipidemia.
Prediction of Obstructive CAD
To estimate the probability of obstructive CAD, we applied the risk prediction tool currently recommended by the European Society of Cardiology.11 The clinical version of this tool relies on age; sex; diagnoses of diabetes, hypertension, and dyslipidemia; active tobacco use; and chest pain characteristics to predict the probability of obstructive CAD on coronary angiography. We assumed that all patients in our cohort had nonspecific chest pain, the referent in the calculator.
Prediction of Perioperative Event Risk
To predict the probability of a perioperative cardiac event, we used the Myocardial Infarction or Cardiac Arrest (MICA) calculator, which was derived from an earlier cohort of NSQIP.12 All variables required for this prediction tool were included in the 2009 NSQIP cohort; our categorization of surgeries is included as an online appendix. MICA is one of three prediction tools included in the current American College of Cardiology/American Heart Association (ACC/AHA) guidelines.5
Prediction of Test Accuracy
We searched the MEDLINE database for estimates of the test characteristics of DSE and SPECT that adjusted for workup bias.13 (Also known as sequential-ordering bias, here we refer to the phenomenon whereby further workup is based on the results of diagnostic testing, resulting in underdiagnosis among patients with negative tests and falsely high estimates of sensitivity.14) Although other modalities of myocardial perfusion imaging exist, SPECT appears to be the most widely available, utilized, and studied modality of MPI.15 Our search strategy paired (“Sensitivity and Specificity” [MeSH Terms] AND “Coronary Disease/diagnostic imaging” [MAJR] AND “bias” [TIAB]) with (“Tomography, Emission-Computed, Single-Photon” [MAJR] OR “Echocardiography, Stress” [MAJR]). We reviewed the results for sensitivity and specificity estimates that corrected for workup bias. For each of SPECT and DSE, we drew the sensitivity and specificity from normal distributions based on literature estimates (see Table). We then calculated the expected accuracy of each modality for each patient in our dataset. All analyses were performed in Stata (version 14, College Station, Texas).
RESULTS
The median predicted probability of obstructive CAD was
Both accuracy and PPV were higher for DSE than for SPECT. The predicted accuracy of DSE was greater than that of SPECT in 73.5% of cases overall and in 60.5% of cases with a predicted operative cardiac risk greater than 1%. The mean PPV of DSE was 32.9% (median: 26.7%), while the equivalent PPVs for SPECT were 14.1% and 8.2%, respectively. Among cases with a predicted operative cardiac risk greater than 1%, the mean PPV of DSE was 57.5% (median: 60.2%), while the equivalent PPVs for SPECT were 29.8% and 26.7%, respectively.
DSE had a mean predicted accuracy of 93.0% (median: 96.2%), while SPECT had a mean accuracy of 92.6% (median: 95.6%). The predicted accuracies of DSE and SPECT are shown in the Figure, stratified by predicted perioperative risk across the 1% risk threshold currently used by ACC/AHA guidelines.
In our sensitivity analyses, dyslipidemia had little effect on the comparative accuracy. If no patients had dyslipidemia, DSE would have a higher accuracy than SPECT in 75.7% of cases. If all patients had dyslipidemia, DSE would have a higher predicted accuracy than SPECT in 72.8% of cases. For patients with an operative cardiac risk greater than 1%, the predicted accuracies were 65.0% and 59.4%, respectively.
DISCUSSION
In this study, we demonstrated that the expected accuracy of DSE in the diagnosis of obstructive CAD among patients undergoing noncardiac surgery is higher than that of SPECT. This finding was true in both unselected patients and those selected by a perioperative risk of greater than 1%. The use of SPECT, compared with DSE, would likely result in greater numbers of false-positive tests in this patient population and less accurate results overall.
Cardiac stress testing, as with any diagnostic test, is most useful at intermediate probabilities. Insofar as stress testing offers diagnostic value, our analysis suggests that, in the range of the predicted risk of CAD found in patients undergoing noncardiac surgery, DSE is a more efficient testing strategy. To the extent that making a diagnosis of CAD informs the decision to proceed to surgery, a more accurate test would be preferable. The lower cost of DSE, the lack of ionizing radiation, and the information provided by echocardiography regarding diagnoses other than CAD, if considered, would further amplify that preference.
However, it is important to note that both modalities have limited positive predictive value. In the median patient who meets the currently recommended 1% perioperative event risk threshold, SPECT would lead to 2.74 false positive results for every true positive result. DSE would produce approximately two false positive results for every three true positive results. If lower rates of false-positive testing are desired, different patient selection criteria are required.
A few key limitations of this work warrant discussion. First, our results likely overestimate the probability of obstructive CAD in this population. We assumed that all patients have nonspecific chest pain at the time of the preoperative evaluation, though many patients do not, in fact, have chest pain. Tools to estimate the pretest probability of CAD (such as the ESC tool that we used or the older Diamond-Forrester prediction) are intended to stratify higher-risk patients than are seen in a preoperative setting. If asymptomatic patients seen in preoperative risk assessment clinics have lower risk of CAD than what we have predicted, we will have understated the case for DSE. Moreover, cases sampled from hospitals participating in NSQIP are not representative of the national surgical population. This likely further inflates our estimates of CAD risk compared with the “true” surgical population. Finally, we cannot comment on current practice from these data. Current guidelines recommend preoperative cardiac stress testing for patients whose risk of a perioperative cardiac event exceeds 1%, whose functional status is poor or unknown, and only if said testing will change management.5 Using these data, we cannot determine the pretest probability of patients referred for stress testing before noncardiac surgery.
Still, this analysis suggests that, among patients undergoing noncardiac surgery, selecting patients according to the risk of perioperative events would result in a population at an overall comparatively low risk of CAD, and that in this population, DSE would be more accurate than SPECT for making the diagnosis of CAD. If a diagnosis of CAD would change the decision to proceed to surgery, DSE is likely to be a more efficient test than SPECT.
Acknowledgements
The authors would like to thank Wael Jaber, MD, for his thoughtful comments on a draft of this manuscript.
Disclosures
The authors have nothing to disclose.
Funding
The authors received no specific funding for this work.
1. Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med. 2011;154(8):523-528. doi: 10.7326/0003-4819-154-8-201104190-00003. PubMed
2. Udeh BL, Dalton JE, Hata JS, Udeh CI, Sessler DI. Economic trends from 2003 to 2010 for perioperative myocardial infarction: a retrospective, cohort study. Anesthesiology. 2014;121(1):36-45. doi: 10.1097/ALN.0000000000000233. PubMed
3. van Waes JAR, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013;127(23):2264-2271. doi: 10.1161/CIRCULATIONAHA.113.002128. PubMed
4. Wijeysundera DN, Beattie WS, Austin PC, Hux JE, Laupacis A. Non-invasive cardiac stress testing before elective major non-cardiac surgery: population based cohort study. BMJ. 2010;340(jan28 3):b5526-b5526. doi: 10.1136/bmj.b5526. PubMed
5. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. J Am Coll Cardiol. 2014;64(22):e77-e137. doi: 10.1016/j.jacc.2014.07.944. PubMed
6. Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297(16):845-850. doi:10.1056/NEJM197710202971601. PubMed
7. Devereaux PJ, Sessler DI. Cardiac Complications in patients undergoing major noncardiac surgery. N Engl J Med. 2015;373(23):2258-2269. doi:10.1056/NEJMra1502824. PubMed
8. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049. doi: 10.1161/01.CIR.100.10.1043. PubMed
9. Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217(2):336-46.e1. doi: 10.1016/j.jamcollsurg.2013.02.027. PubMed
10. National Health and Nutrition Examination Survey Data. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2011. Accessed April 20, 2018.
11. Genders TSS, Steyerberg EW, Hunink MGM, et al. Prediction model to estimate presence of coronary artery disease: retrospective pooled analysis of existing cohorts. BMJ. 2012;344:e3485. doi: 10.1136/bmj.e3485. PubMed
12. Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124(4):381-387. doi: 10.1161/CIRCULATIONAHA.110.015701. PubMed
13. Blackstone EH, Lauer MS. Caveat emptor: the treachery of work-up bias. J Thorac Cardiovasc Surg. 2004;128(3):341-344. doi: 10.1016/j.jtcvs.2004.03.039. PubMed
14. Ransohoff DF, Feinstein AR. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med. 1978;299(17):926-930. doi: 10.1056/NEJM197810262991705. PubMed
15. Jaarsma C, Leiner T, Bekkers SC, et al. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2012;59(19):1719-1728. doi: 10.1016/j.jacc.2011.12.040. PubMed
16. Geleijnse ML, Krenning BJ, Soliman OII, Nemes A, Galema TW, Cate ten FJ. Dobutamine stress echocardiography for the detection of coronary artery disease in women. Am J Cardiol. 2007;99(5):714-717. doi: 10.1016/j.amjcard.2006.09.124. PubMed
17. Miller TD, Hodge DO, Christian TF, Milavetz JJ, Bailey KR, Gibbons RJ. Effects of adjustment for referral bias on the sensitivity and specificity of single photon emission computed tomography for the diagnosis of coronary artery disease. Am J Med. 2002;112(4):290-297. doi: 10.1016/S0002-9343(01)01111-1. PubMed
Cardiac complications account for at least one-third of perioperative deaths, and lead to substantial morbidity and cost.1-4 Current guidelines recommend that patients undergo assessment of cardiac risk and functional status prior to noncardiac surgery.5 Preoperative cardiac stress testing is recommended for patients whose predicted cardiac risk exceeds 1%, whose functional status is limited, and for whom testing may change management.5
However, patients are not specifically selected according to risk of coronary artery disease (CAD) in current guidelines. The pretest probability of CAD may vary widely in this patient population, and the resultant accuracy of cardiac stress testing in making the diagnosis of CAD may vary as well.5 Meanwhile, CAD is a clear risk factor for perioperative cardiac events.6-8
Because the pretest probability of CAD is heterogeneous, the optimal modality of cardiac stress testing in this population is unclear. False-positive results would likely lead to inflated estimates of operative risk, expensive and high-risk downstream testing, and potentially cancellation of otherwise beneficial surgeries. Meanwhile, false-negative results would lead to overly optimistic estimates of surgical risk and potentially to surgical intervention at higher levels of risk than would be desirable. Current guidelines leave the selection of either dobutamine stress echocardiography (DSE) or pharmacological stress myocardial perfusion imaging to the clinician.5 To inform decisions regarding the selection of cardiac stress testing modality prior to noncardiac surgery, we conducted this study to estimate the diagnostic accuracy of DSE and single-photon emission computed tomography (SPECT) among this patient population.
METHODS
Surgical Cohort
The American College of Surgeons’ National Surgical Quality Improvement Program (NSQIP) samples patients undergoing surgery at participating hospitals and collects standardized clinical data on preoperative risk factors and postoperative complications.9 We acquired public use data from the 2009 NSQIP cohort, which included more than 336,000 surgical cases from 237 hospitals (principally in the United States). We excluded from our analysis patients undergoing cardiac surgery, patients with a prior diagnosis of CAD, and patients undergoing experimental surgeries. This left a sample of 300,462 for analysis.
Prediction of Dyslipidemia
The model we used to predict the presence of obstructive CAD required the presence or absence of dyslipidemia. A number of variables are common to both NSQIP and the National Health and Nutrition Examination Survey (NHANES), including age, weight, sex, tobacco use, diabetes, and prior stroke.10 Using those common variables, we developed a logistic regression to predict a diagnosis of dyslipidemia, applied that regression to the NSQIP cohort, and dichotomized. To assess the potential impact of misclassification, we performed separate sensitivity analyses in which either no patients or all patients had dyslipidemia.
Prediction of Obstructive CAD
To estimate the probability of obstructive CAD, we applied the risk prediction tool currently recommended by the European Society of Cardiology.11 The clinical version of this tool relies on age; sex; diagnoses of diabetes, hypertension, and dyslipidemia; active tobacco use; and chest pain characteristics to predict the probability of obstructive CAD on coronary angiography. We assumed that all patients in our cohort had nonspecific chest pain, the referent in the calculator.
Prediction of Perioperative Event Risk
To predict the probability of a perioperative cardiac event, we used the Myocardial Infarction or Cardiac Arrest (MICA) calculator, which was derived from an earlier cohort of NSQIP.12 All variables required for this prediction tool were included in the 2009 NSQIP cohort; our categorization of surgeries is included as an online appendix. MICA is one of three prediction tools included in the current American College of Cardiology/American Heart Association (ACC/AHA) guidelines.5
Prediction of Test Accuracy
We searched the MEDLINE database for estimates of the test characteristics of DSE and SPECT that adjusted for workup bias.13 (Also known as sequential-ordering bias, here we refer to the phenomenon whereby further workup is based on the results of diagnostic testing, resulting in underdiagnosis among patients with negative tests and falsely high estimates of sensitivity.14) Although other modalities of myocardial perfusion imaging exist, SPECT appears to be the most widely available, utilized, and studied modality of MPI.15 Our search strategy paired (“Sensitivity and Specificity” [MeSH Terms] AND “Coronary Disease/diagnostic imaging” [MAJR] AND “bias” [TIAB]) with (“Tomography, Emission-Computed, Single-Photon” [MAJR] OR “Echocardiography, Stress” [MAJR]). We reviewed the results for sensitivity and specificity estimates that corrected for workup bias. For each of SPECT and DSE, we drew the sensitivity and specificity from normal distributions based on literature estimates (see Table). We then calculated the expected accuracy of each modality for each patient in our dataset. All analyses were performed in Stata (version 14, College Station, Texas).
RESULTS
The median predicted probability of obstructive CAD was
Both accuracy and PPV were higher for DSE than for SPECT. The predicted accuracy of DSE was greater than that of SPECT in 73.5% of cases overall and in 60.5% of cases with a predicted operative cardiac risk greater than 1%. The mean PPV of DSE was 32.9% (median: 26.7%), while the equivalent PPVs for SPECT were 14.1% and 8.2%, respectively. Among cases with a predicted operative cardiac risk greater than 1%, the mean PPV of DSE was 57.5% (median: 60.2%), while the equivalent PPVs for SPECT were 29.8% and 26.7%, respectively.
DSE had a mean predicted accuracy of 93.0% (median: 96.2%), while SPECT had a mean accuracy of 92.6% (median: 95.6%). The predicted accuracies of DSE and SPECT are shown in the Figure, stratified by predicted perioperative risk across the 1% risk threshold currently used by ACC/AHA guidelines.
In our sensitivity analyses, dyslipidemia had little effect on the comparative accuracy. If no patients had dyslipidemia, DSE would have a higher accuracy than SPECT in 75.7% of cases. If all patients had dyslipidemia, DSE would have a higher predicted accuracy than SPECT in 72.8% of cases. For patients with an operative cardiac risk greater than 1%, the predicted accuracies were 65.0% and 59.4%, respectively.
DISCUSSION
In this study, we demonstrated that the expected accuracy of DSE in the diagnosis of obstructive CAD among patients undergoing noncardiac surgery is higher than that of SPECT. This finding was true in both unselected patients and those selected by a perioperative risk of greater than 1%. The use of SPECT, compared with DSE, would likely result in greater numbers of false-positive tests in this patient population and less accurate results overall.
Cardiac stress testing, as with any diagnostic test, is most useful at intermediate probabilities. Insofar as stress testing offers diagnostic value, our analysis suggests that, in the range of the predicted risk of CAD found in patients undergoing noncardiac surgery, DSE is a more efficient testing strategy. To the extent that making a diagnosis of CAD informs the decision to proceed to surgery, a more accurate test would be preferable. The lower cost of DSE, the lack of ionizing radiation, and the information provided by echocardiography regarding diagnoses other than CAD, if considered, would further amplify that preference.
However, it is important to note that both modalities have limited positive predictive value. In the median patient who meets the currently recommended 1% perioperative event risk threshold, SPECT would lead to 2.74 false positive results for every true positive result. DSE would produce approximately two false positive results for every three true positive results. If lower rates of false-positive testing are desired, different patient selection criteria are required.
A few key limitations of this work warrant discussion. First, our results likely overestimate the probability of obstructive CAD in this population. We assumed that all patients have nonspecific chest pain at the time of the preoperative evaluation, though many patients do not, in fact, have chest pain. Tools to estimate the pretest probability of CAD (such as the ESC tool that we used or the older Diamond-Forrester prediction) are intended to stratify higher-risk patients than are seen in a preoperative setting. If asymptomatic patients seen in preoperative risk assessment clinics have lower risk of CAD than what we have predicted, we will have understated the case for DSE. Moreover, cases sampled from hospitals participating in NSQIP are not representative of the national surgical population. This likely further inflates our estimates of CAD risk compared with the “true” surgical population. Finally, we cannot comment on current practice from these data. Current guidelines recommend preoperative cardiac stress testing for patients whose risk of a perioperative cardiac event exceeds 1%, whose functional status is poor or unknown, and only if said testing will change management.5 Using these data, we cannot determine the pretest probability of patients referred for stress testing before noncardiac surgery.
Still, this analysis suggests that, among patients undergoing noncardiac surgery, selecting patients according to the risk of perioperative events would result in a population at an overall comparatively low risk of CAD, and that in this population, DSE would be more accurate than SPECT for making the diagnosis of CAD. If a diagnosis of CAD would change the decision to proceed to surgery, DSE is likely to be a more efficient test than SPECT.
Acknowledgements
The authors would like to thank Wael Jaber, MD, for his thoughtful comments on a draft of this manuscript.
Disclosures
The authors have nothing to disclose.
Funding
The authors received no specific funding for this work.
Cardiac complications account for at least one-third of perioperative deaths, and lead to substantial morbidity and cost.1-4 Current guidelines recommend that patients undergo assessment of cardiac risk and functional status prior to noncardiac surgery.5 Preoperative cardiac stress testing is recommended for patients whose predicted cardiac risk exceeds 1%, whose functional status is limited, and for whom testing may change management.5
However, patients are not specifically selected according to risk of coronary artery disease (CAD) in current guidelines. The pretest probability of CAD may vary widely in this patient population, and the resultant accuracy of cardiac stress testing in making the diagnosis of CAD may vary as well.5 Meanwhile, CAD is a clear risk factor for perioperative cardiac events.6-8
Because the pretest probability of CAD is heterogeneous, the optimal modality of cardiac stress testing in this population is unclear. False-positive results would likely lead to inflated estimates of operative risk, expensive and high-risk downstream testing, and potentially cancellation of otherwise beneficial surgeries. Meanwhile, false-negative results would lead to overly optimistic estimates of surgical risk and potentially to surgical intervention at higher levels of risk than would be desirable. Current guidelines leave the selection of either dobutamine stress echocardiography (DSE) or pharmacological stress myocardial perfusion imaging to the clinician.5 To inform decisions regarding the selection of cardiac stress testing modality prior to noncardiac surgery, we conducted this study to estimate the diagnostic accuracy of DSE and single-photon emission computed tomography (SPECT) among this patient population.
METHODS
Surgical Cohort
The American College of Surgeons’ National Surgical Quality Improvement Program (NSQIP) samples patients undergoing surgery at participating hospitals and collects standardized clinical data on preoperative risk factors and postoperative complications.9 We acquired public use data from the 2009 NSQIP cohort, which included more than 336,000 surgical cases from 237 hospitals (principally in the United States). We excluded from our analysis patients undergoing cardiac surgery, patients with a prior diagnosis of CAD, and patients undergoing experimental surgeries. This left a sample of 300,462 for analysis.
Prediction of Dyslipidemia
The model we used to predict the presence of obstructive CAD required the presence or absence of dyslipidemia. A number of variables are common to both NSQIP and the National Health and Nutrition Examination Survey (NHANES), including age, weight, sex, tobacco use, diabetes, and prior stroke.10 Using those common variables, we developed a logistic regression to predict a diagnosis of dyslipidemia, applied that regression to the NSQIP cohort, and dichotomized. To assess the potential impact of misclassification, we performed separate sensitivity analyses in which either no patients or all patients had dyslipidemia.
Prediction of Obstructive CAD
To estimate the probability of obstructive CAD, we applied the risk prediction tool currently recommended by the European Society of Cardiology.11 The clinical version of this tool relies on age; sex; diagnoses of diabetes, hypertension, and dyslipidemia; active tobacco use; and chest pain characteristics to predict the probability of obstructive CAD on coronary angiography. We assumed that all patients in our cohort had nonspecific chest pain, the referent in the calculator.
Prediction of Perioperative Event Risk
To predict the probability of a perioperative cardiac event, we used the Myocardial Infarction or Cardiac Arrest (MICA) calculator, which was derived from an earlier cohort of NSQIP.12 All variables required for this prediction tool were included in the 2009 NSQIP cohort; our categorization of surgeries is included as an online appendix. MICA is one of three prediction tools included in the current American College of Cardiology/American Heart Association (ACC/AHA) guidelines.5
Prediction of Test Accuracy
We searched the MEDLINE database for estimates of the test characteristics of DSE and SPECT that adjusted for workup bias.13 (Also known as sequential-ordering bias, here we refer to the phenomenon whereby further workup is based on the results of diagnostic testing, resulting in underdiagnosis among patients with negative tests and falsely high estimates of sensitivity.14) Although other modalities of myocardial perfusion imaging exist, SPECT appears to be the most widely available, utilized, and studied modality of MPI.15 Our search strategy paired (“Sensitivity and Specificity” [MeSH Terms] AND “Coronary Disease/diagnostic imaging” [MAJR] AND “bias” [TIAB]) with (“Tomography, Emission-Computed, Single-Photon” [MAJR] OR “Echocardiography, Stress” [MAJR]). We reviewed the results for sensitivity and specificity estimates that corrected for workup bias. For each of SPECT and DSE, we drew the sensitivity and specificity from normal distributions based on literature estimates (see Table). We then calculated the expected accuracy of each modality for each patient in our dataset. All analyses were performed in Stata (version 14, College Station, Texas).
RESULTS
The median predicted probability of obstructive CAD was
Both accuracy and PPV were higher for DSE than for SPECT. The predicted accuracy of DSE was greater than that of SPECT in 73.5% of cases overall and in 60.5% of cases with a predicted operative cardiac risk greater than 1%. The mean PPV of DSE was 32.9% (median: 26.7%), while the equivalent PPVs for SPECT were 14.1% and 8.2%, respectively. Among cases with a predicted operative cardiac risk greater than 1%, the mean PPV of DSE was 57.5% (median: 60.2%), while the equivalent PPVs for SPECT were 29.8% and 26.7%, respectively.
DSE had a mean predicted accuracy of 93.0% (median: 96.2%), while SPECT had a mean accuracy of 92.6% (median: 95.6%). The predicted accuracies of DSE and SPECT are shown in the Figure, stratified by predicted perioperative risk across the 1% risk threshold currently used by ACC/AHA guidelines.
In our sensitivity analyses, dyslipidemia had little effect on the comparative accuracy. If no patients had dyslipidemia, DSE would have a higher accuracy than SPECT in 75.7% of cases. If all patients had dyslipidemia, DSE would have a higher predicted accuracy than SPECT in 72.8% of cases. For patients with an operative cardiac risk greater than 1%, the predicted accuracies were 65.0% and 59.4%, respectively.
DISCUSSION
In this study, we demonstrated that the expected accuracy of DSE in the diagnosis of obstructive CAD among patients undergoing noncardiac surgery is higher than that of SPECT. This finding was true in both unselected patients and those selected by a perioperative risk of greater than 1%. The use of SPECT, compared with DSE, would likely result in greater numbers of false-positive tests in this patient population and less accurate results overall.
Cardiac stress testing, as with any diagnostic test, is most useful at intermediate probabilities. Insofar as stress testing offers diagnostic value, our analysis suggests that, in the range of the predicted risk of CAD found in patients undergoing noncardiac surgery, DSE is a more efficient testing strategy. To the extent that making a diagnosis of CAD informs the decision to proceed to surgery, a more accurate test would be preferable. The lower cost of DSE, the lack of ionizing radiation, and the information provided by echocardiography regarding diagnoses other than CAD, if considered, would further amplify that preference.
However, it is important to note that both modalities have limited positive predictive value. In the median patient who meets the currently recommended 1% perioperative event risk threshold, SPECT would lead to 2.74 false positive results for every true positive result. DSE would produce approximately two false positive results for every three true positive results. If lower rates of false-positive testing are desired, different patient selection criteria are required.
A few key limitations of this work warrant discussion. First, our results likely overestimate the probability of obstructive CAD in this population. We assumed that all patients have nonspecific chest pain at the time of the preoperative evaluation, though many patients do not, in fact, have chest pain. Tools to estimate the pretest probability of CAD (such as the ESC tool that we used or the older Diamond-Forrester prediction) are intended to stratify higher-risk patients than are seen in a preoperative setting. If asymptomatic patients seen in preoperative risk assessment clinics have lower risk of CAD than what we have predicted, we will have understated the case for DSE. Moreover, cases sampled from hospitals participating in NSQIP are not representative of the national surgical population. This likely further inflates our estimates of CAD risk compared with the “true” surgical population. Finally, we cannot comment on current practice from these data. Current guidelines recommend preoperative cardiac stress testing for patients whose risk of a perioperative cardiac event exceeds 1%, whose functional status is poor or unknown, and only if said testing will change management.5 Using these data, we cannot determine the pretest probability of patients referred for stress testing before noncardiac surgery.
Still, this analysis suggests that, among patients undergoing noncardiac surgery, selecting patients according to the risk of perioperative events would result in a population at an overall comparatively low risk of CAD, and that in this population, DSE would be more accurate than SPECT for making the diagnosis of CAD. If a diagnosis of CAD would change the decision to proceed to surgery, DSE is likely to be a more efficient test than SPECT.
Acknowledgements
The authors would like to thank Wael Jaber, MD, for his thoughtful comments on a draft of this manuscript.
Disclosures
The authors have nothing to disclose.
Funding
The authors received no specific funding for this work.
1. Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med. 2011;154(8):523-528. doi: 10.7326/0003-4819-154-8-201104190-00003. PubMed
2. Udeh BL, Dalton JE, Hata JS, Udeh CI, Sessler DI. Economic trends from 2003 to 2010 for perioperative myocardial infarction: a retrospective, cohort study. Anesthesiology. 2014;121(1):36-45. doi: 10.1097/ALN.0000000000000233. PubMed
3. van Waes JAR, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013;127(23):2264-2271. doi: 10.1161/CIRCULATIONAHA.113.002128. PubMed
4. Wijeysundera DN, Beattie WS, Austin PC, Hux JE, Laupacis A. Non-invasive cardiac stress testing before elective major non-cardiac surgery: population based cohort study. BMJ. 2010;340(jan28 3):b5526-b5526. doi: 10.1136/bmj.b5526. PubMed
5. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. J Am Coll Cardiol. 2014;64(22):e77-e137. doi: 10.1016/j.jacc.2014.07.944. PubMed
6. Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297(16):845-850. doi:10.1056/NEJM197710202971601. PubMed
7. Devereaux PJ, Sessler DI. Cardiac Complications in patients undergoing major noncardiac surgery. N Engl J Med. 2015;373(23):2258-2269. doi:10.1056/NEJMra1502824. PubMed
8. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049. doi: 10.1161/01.CIR.100.10.1043. PubMed
9. Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217(2):336-46.e1. doi: 10.1016/j.jamcollsurg.2013.02.027. PubMed
10. National Health and Nutrition Examination Survey Data. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2011. Accessed April 20, 2018.
11. Genders TSS, Steyerberg EW, Hunink MGM, et al. Prediction model to estimate presence of coronary artery disease: retrospective pooled analysis of existing cohorts. BMJ. 2012;344:e3485. doi: 10.1136/bmj.e3485. PubMed
12. Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124(4):381-387. doi: 10.1161/CIRCULATIONAHA.110.015701. PubMed
13. Blackstone EH, Lauer MS. Caveat emptor: the treachery of work-up bias. J Thorac Cardiovasc Surg. 2004;128(3):341-344. doi: 10.1016/j.jtcvs.2004.03.039. PubMed
14. Ransohoff DF, Feinstein AR. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med. 1978;299(17):926-930. doi: 10.1056/NEJM197810262991705. PubMed
15. Jaarsma C, Leiner T, Bekkers SC, et al. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2012;59(19):1719-1728. doi: 10.1016/j.jacc.2011.12.040. PubMed
16. Geleijnse ML, Krenning BJ, Soliman OII, Nemes A, Galema TW, Cate ten FJ. Dobutamine stress echocardiography for the detection of coronary artery disease in women. Am J Cardiol. 2007;99(5):714-717. doi: 10.1016/j.amjcard.2006.09.124. PubMed
17. Miller TD, Hodge DO, Christian TF, Milavetz JJ, Bailey KR, Gibbons RJ. Effects of adjustment for referral bias on the sensitivity and specificity of single photon emission computed tomography for the diagnosis of coronary artery disease. Am J Med. 2002;112(4):290-297. doi: 10.1016/S0002-9343(01)01111-1. PubMed
1. Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med. 2011;154(8):523-528. doi: 10.7326/0003-4819-154-8-201104190-00003. PubMed
2. Udeh BL, Dalton JE, Hata JS, Udeh CI, Sessler DI. Economic trends from 2003 to 2010 for perioperative myocardial infarction: a retrospective, cohort study. Anesthesiology. 2014;121(1):36-45. doi: 10.1097/ALN.0000000000000233. PubMed
3. van Waes JAR, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013;127(23):2264-2271. doi: 10.1161/CIRCULATIONAHA.113.002128. PubMed
4. Wijeysundera DN, Beattie WS, Austin PC, Hux JE, Laupacis A. Non-invasive cardiac stress testing before elective major non-cardiac surgery: population based cohort study. BMJ. 2010;340(jan28 3):b5526-b5526. doi: 10.1136/bmj.b5526. PubMed
5. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. J Am Coll Cardiol. 2014;64(22):e77-e137. doi: 10.1016/j.jacc.2014.07.944. PubMed
6. Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297(16):845-850. doi:10.1056/NEJM197710202971601. PubMed
7. Devereaux PJ, Sessler DI. Cardiac Complications in patients undergoing major noncardiac surgery. N Engl J Med. 2015;373(23):2258-2269. doi:10.1056/NEJMra1502824. PubMed
8. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049. doi: 10.1161/01.CIR.100.10.1043. PubMed
9. Cohen ME, Ko CY, Bilimoria KY, et al. Optimizing ACS NSQIP modeling for evaluation of surgical quality and risk: patient risk adjustment, procedure mix adjustment, shrinkage adjustment, and surgical focus. J Am Coll Surg. 2013;217(2):336-46.e1. doi: 10.1016/j.jamcollsurg.2013.02.027. PubMed
10. National Health and Nutrition Examination Survey Data. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?BeginYear=2011. Accessed April 20, 2018.
11. Genders TSS, Steyerberg EW, Hunink MGM, et al. Prediction model to estimate presence of coronary artery disease: retrospective pooled analysis of existing cohorts. BMJ. 2012;344:e3485. doi: 10.1136/bmj.e3485. PubMed
12. Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124(4):381-387. doi: 10.1161/CIRCULATIONAHA.110.015701. PubMed
13. Blackstone EH, Lauer MS. Caveat emptor: the treachery of work-up bias. J Thorac Cardiovasc Surg. 2004;128(3):341-344. doi: 10.1016/j.jtcvs.2004.03.039. PubMed
14. Ransohoff DF, Feinstein AR. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. N Engl J Med. 1978;299(17):926-930. doi: 10.1056/NEJM197810262991705. PubMed
15. Jaarsma C, Leiner T, Bekkers SC, et al. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease: a meta-analysis. J Am Coll Cardiol. 2012;59(19):1719-1728. doi: 10.1016/j.jacc.2011.12.040. PubMed
16. Geleijnse ML, Krenning BJ, Soliman OII, Nemes A, Galema TW, Cate ten FJ. Dobutamine stress echocardiography for the detection of coronary artery disease in women. Am J Cardiol. 2007;99(5):714-717. doi: 10.1016/j.amjcard.2006.09.124. PubMed
17. Miller TD, Hodge DO, Christian TF, Milavetz JJ, Bailey KR, Gibbons RJ. Effects of adjustment for referral bias on the sensitivity and specificity of single photon emission computed tomography for the diagnosis of coronary artery disease. Am J Med. 2002;112(4):290-297. doi: 10.1016/S0002-9343(01)01111-1. PubMed
© 2018 Society of Hospital Medicine
An Electronic Health Record Tool Designed to Improve Pediatric Hospital Discharge has Low Predictive Utility for Readmissions
As hospitalized children become more medically complex, hospital-to-home care transitions will become increasingly challenging. During a quality improvement (QI) initiative, we developed an electronic tool to improve the quality of our hospital discharge process.
METHODS
Setting
This work was conducted at the Children’s Hospital Colorado as part of a national QI collaborative. The hospital’s EHR is Epic (Verona, Wisconsin). The project was approved as QI by the Children’s Hospital Organizational Research Risk and Quality Improvement Review Panel, precluding review from the Colorado Multiple Institutional Review Board.
Tool Design, Implementation, and Use
A team of clinicians, nurse–family educators, case managers, social workers, and informatics experts helped design the instrument between 2014 and 2015. In addition to the selected features (number of discharge medications, presence of home health, and language preference), we considered adding the number of consulting specialists but had previously improved our process for scheduling follow-up appointments. Diagnoses were not systematically or discretely documented to be reliably extracted in real time. We excluded known readmission predictor variables (such as length of stay [LOS] and prior hospitalizations) from the initial model to maintain emphasis on the modifiable discharge processes. Additional considerations, such as health literacy and social determinants, were not systematically measured to be operationally usable.
To generate the score, the clinical documentation of home-health orders categorizes children with home care. Each home-care equipment or service category is documented in separate flowsheet rows, allowing for identification of distinct categories (Table). Total parenteral nutrition, intravenous medications, and durable medical equipment and supplies are counted as home care. The number of discharge medications is approximated by inpatient medication orders and finalized as the number of discharge medication orders. The medications include new, historic, and as-needed medications (if included among discharge medication orders).
The electronic score is displayed within the EHR’s Discharge Readiness Report8 and updates automatically as relevant data are entered. The tool displays the individual components and as a composite of 0-3 points. To register a point in each category, a patient needs to exceed (1) the dichotomous discharge medications criterion (ie, ≥6 medications), (2) the dichotomous home-health order criterion (ie, ≥1 home-care order), and (3) to possess documentation of a non-English speaking caregiver. The tool serves as a visual reminder of discharge planning needs during daily coordination rounds attended by clinicians, nursing managers, case managers, and social workers. Case managers use the home-care alert to verify the accuracy of home-care orders.
Evaluation of Predictive Utility for Readmission
We performed a retrospective cohort study on patients aged 0-21 years who were discharged between January 1, 2014 and December 30, 2015. This study was performed to determine the optimal cut points for the continuous variables (discharge medications and home-care orders) and to evaluate the predictive value of the composite score.
Unplanned readmission within 30 days was used as the primary outcome. The index hospitalization was randomly selected for patients with >1 admissions to avoid biasing the results with multiple hospitalizations from individual patients.
Patient characteristics were summarized using percentages for categorical variables and the median and interquartile range (IQR) for continuous variables. We examined bivariate associations for each of the tool’s predictor elements with readmission using Chi-square and Wilcoxon tests
The area under the ROC curve (AUC) was estimated to evaluate the performance of the composite score
RESULTS
Cohort Characteristics
Analysis was restricted to
ROC analysis indicated that dichotomizing number of medications at ≥6 vs. <6 and home health at 0 vs. ≥1 categories maximized the sensitivity and specificity for predicting 30-day unplanned readmissions. In predictive logistic regression analysis, the odds of readmission was significantly higher in children with a composite score of 1 vs. 0 (odds ratio [OR], 1.7; 95% CI 1.5-2) and a score of ≥2 vs. 0 (OR, 4.2; 95% CI, 3.6-4.9). The c statistic for this model was 0.62, and the Brier score was 0.037. Internal validation of the predictive logistic regression model yielded identical results.
DISCUSSION
The instrument’s framework is relatively simple and should reduce barriers to implementation elsewhere. However, this tool was developed for one setting, and the design may require adjustment for other environments. Regional or institutional variation in home-health eligibility or clinical documentation may impact home-care and medication scores. The score may change at discharge if home-health or medication orders are modified late. The tool performs none of the following: measurement of regimen complexity, identification of high-risk medications, distinguishing of new versus preexisting medications/home care, nor measurement of health literacy, parent education, or psychosocial risk. Adding these features might enhance the model. Finally, readmission rates did not rise linearly with each added point. A more sophisticated scoring system (eg, differentially weighting each risk factor) may also improve the performance of the tool.
Despite these limitations, we have implemented a real-time electronic tool with practical potential to improve the discharge process but with low utility for distinguishing readmissions. Additional validation and research is needed to evaluate its impact on hospital discharge quality metrics and family reported outcome measures.
Disclosures
The authors have no relevant financial relationships to disclose.
Funding
This study was supported by an institutional Clinical and Operational Effectiveness and Patient Safety Small Grants Program
1. Holland DE, Conlon PM, Rohlik GM, Gillard KL, Messner PK, Mundy LM. Identifying hospitalized pediatric patients for early discharge planning: a feasibility study. J Pediatr Nurs. 2015;30(3):454-462. doi: 10.1016/j.pedn.2014.12.011. PubMed
2. Brittan M, Albright K, Cifuentes M, Jimenez-Zambrano A, Kempe A. Parent and provider perspectives on pediatric readmissions: what can we learn about readiness for discharge? Hosp Pediatr. 2015;5(11):559-565. doi: 10.1542/hpeds.2015-0034. PubMed
3. Brittan M FV, Martin S, Moss A, Keller D. Provider feedback: a potential method to reduce readmissions. Hosp Pediatr. 2016;6(11):684-688. PubMed
4. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA 2011;305(7):682-690. doi: 10.1001/jama.2011.122. PubMed
5. Feudtner C, Levin JE, Srivastava R, et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics 2009;123(1):286-293. doi: 10.1542/peds.2007-3395. PubMed
6. Jurgens V, Spaeder MC, Pavuluri P, Waldman Z. Hospital readmission in children with complex chronic conditions discharged from subacute care. Hosp Pediatr. 2014;4(3):153-158. doi: 10.1542/hpeds.2013-0094. PubMed
7. Karliner LS, Kim SE, Meltzer DO, Auerbach AD. Influence of language barriers on outcomes of hospital care for general medicine inpatients. J Hosp Med. 2010;5(5):276-282. doi: 10.1002/jhm.658. PubMed
8. Tyler A, Boyer A, Martin S, Neiman J, Bakel LA, Brittan M. Development of a discharge readiness report within the electronic health record-A discharge planning tool. J Hosp Med. 2014;9(8):533-539. doi: 10.1002/jhm.2212. PubMed
9. Youden WJ. Index for rating diagnostic tests. Cancer 1950;3(1):32-35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. PubMed
10. Steyerberg EW, Harrell FE, Jr., Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774-781. doi: 10.1016/S0895-4356(01)00341-9. PubMed
11. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA 2011;306(15):1688-1698. doi: 10.1001/jama.2011.1515. PubMed
12. Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159(9):882-890. doi: 10.1093/aje/kwh101. PubMed
13. Coller RJ, Klitzner TS, Lerner CF, Chung PJ. Predictors of 30-day readmission and association with primary care follow-up plans. J Pediatr. 2013;163(4):1027-1033. doi: 10.1016/j.jpeds.2013.04.013. PubMed
14. Jovanovic M, Radovanovic S, Vukicevic M, Van Poucke S, Delibasic B. Building interpretable predictive models for pediatric hospital readmission using Tree-Lasso logistic regression. Artif Intell Med. 2016;72:12-21. doi: 10.1016/j.artmed.2016.07.003. PubMed
15. Naessens JM, Knoebel E, Johnson M, Branda M. ISQUA16-1722 predicting pediatric readmissions. Int J Qual Health Care. 2016;28(suppl_1):24-25. doi: 10.1093/intqhc/mzw104.34.
As hospitalized children become more medically complex, hospital-to-home care transitions will become increasingly challenging. During a quality improvement (QI) initiative, we developed an electronic tool to improve the quality of our hospital discharge process.
METHODS
Setting
This work was conducted at the Children’s Hospital Colorado as part of a national QI collaborative. The hospital’s EHR is Epic (Verona, Wisconsin). The project was approved as QI by the Children’s Hospital Organizational Research Risk and Quality Improvement Review Panel, precluding review from the Colorado Multiple Institutional Review Board.
Tool Design, Implementation, and Use
A team of clinicians, nurse–family educators, case managers, social workers, and informatics experts helped design the instrument between 2014 and 2015. In addition to the selected features (number of discharge medications, presence of home health, and language preference), we considered adding the number of consulting specialists but had previously improved our process for scheduling follow-up appointments. Diagnoses were not systematically or discretely documented to be reliably extracted in real time. We excluded known readmission predictor variables (such as length of stay [LOS] and prior hospitalizations) from the initial model to maintain emphasis on the modifiable discharge processes. Additional considerations, such as health literacy and social determinants, were not systematically measured to be operationally usable.
To generate the score, the clinical documentation of home-health orders categorizes children with home care. Each home-care equipment or service category is documented in separate flowsheet rows, allowing for identification of distinct categories (Table). Total parenteral nutrition, intravenous medications, and durable medical equipment and supplies are counted as home care. The number of discharge medications is approximated by inpatient medication orders and finalized as the number of discharge medication orders. The medications include new, historic, and as-needed medications (if included among discharge medication orders).
The electronic score is displayed within the EHR’s Discharge Readiness Report8 and updates automatically as relevant data are entered. The tool displays the individual components and as a composite of 0-3 points. To register a point in each category, a patient needs to exceed (1) the dichotomous discharge medications criterion (ie, ≥6 medications), (2) the dichotomous home-health order criterion (ie, ≥1 home-care order), and (3) to possess documentation of a non-English speaking caregiver. The tool serves as a visual reminder of discharge planning needs during daily coordination rounds attended by clinicians, nursing managers, case managers, and social workers. Case managers use the home-care alert to verify the accuracy of home-care orders.
Evaluation of Predictive Utility for Readmission
We performed a retrospective cohort study on patients aged 0-21 years who were discharged between January 1, 2014 and December 30, 2015. This study was performed to determine the optimal cut points for the continuous variables (discharge medications and home-care orders) and to evaluate the predictive value of the composite score.
Unplanned readmission within 30 days was used as the primary outcome. The index hospitalization was randomly selected for patients with >1 admissions to avoid biasing the results with multiple hospitalizations from individual patients.
Patient characteristics were summarized using percentages for categorical variables and the median and interquartile range (IQR) for continuous variables. We examined bivariate associations for each of the tool’s predictor elements with readmission using Chi-square and Wilcoxon tests
The area under the ROC curve (AUC) was estimated to evaluate the performance of the composite score
RESULTS
Cohort Characteristics
Analysis was restricted to
ROC analysis indicated that dichotomizing number of medications at ≥6 vs. <6 and home health at 0 vs. ≥1 categories maximized the sensitivity and specificity for predicting 30-day unplanned readmissions. In predictive logistic regression analysis, the odds of readmission was significantly higher in children with a composite score of 1 vs. 0 (odds ratio [OR], 1.7; 95% CI 1.5-2) and a score of ≥2 vs. 0 (OR, 4.2; 95% CI, 3.6-4.9). The c statistic for this model was 0.62, and the Brier score was 0.037. Internal validation of the predictive logistic regression model yielded identical results.
DISCUSSION
The instrument’s framework is relatively simple and should reduce barriers to implementation elsewhere. However, this tool was developed for one setting, and the design may require adjustment for other environments. Regional or institutional variation in home-health eligibility or clinical documentation may impact home-care and medication scores. The score may change at discharge if home-health or medication orders are modified late. The tool performs none of the following: measurement of regimen complexity, identification of high-risk medications, distinguishing of new versus preexisting medications/home care, nor measurement of health literacy, parent education, or psychosocial risk. Adding these features might enhance the model. Finally, readmission rates did not rise linearly with each added point. A more sophisticated scoring system (eg, differentially weighting each risk factor) may also improve the performance of the tool.
Despite these limitations, we have implemented a real-time electronic tool with practical potential to improve the discharge process but with low utility for distinguishing readmissions. Additional validation and research is needed to evaluate its impact on hospital discharge quality metrics and family reported outcome measures.
Disclosures
The authors have no relevant financial relationships to disclose.
Funding
This study was supported by an institutional Clinical and Operational Effectiveness and Patient Safety Small Grants Program
As hospitalized children become more medically complex, hospital-to-home care transitions will become increasingly challenging. During a quality improvement (QI) initiative, we developed an electronic tool to improve the quality of our hospital discharge process.
METHODS
Setting
This work was conducted at the Children’s Hospital Colorado as part of a national QI collaborative. The hospital’s EHR is Epic (Verona, Wisconsin). The project was approved as QI by the Children’s Hospital Organizational Research Risk and Quality Improvement Review Panel, precluding review from the Colorado Multiple Institutional Review Board.
Tool Design, Implementation, and Use
A team of clinicians, nurse–family educators, case managers, social workers, and informatics experts helped design the instrument between 2014 and 2015. In addition to the selected features (number of discharge medications, presence of home health, and language preference), we considered adding the number of consulting specialists but had previously improved our process for scheduling follow-up appointments. Diagnoses were not systematically or discretely documented to be reliably extracted in real time. We excluded known readmission predictor variables (such as length of stay [LOS] and prior hospitalizations) from the initial model to maintain emphasis on the modifiable discharge processes. Additional considerations, such as health literacy and social determinants, were not systematically measured to be operationally usable.
To generate the score, the clinical documentation of home-health orders categorizes children with home care. Each home-care equipment or service category is documented in separate flowsheet rows, allowing for identification of distinct categories (Table). Total parenteral nutrition, intravenous medications, and durable medical equipment and supplies are counted as home care. The number of discharge medications is approximated by inpatient medication orders and finalized as the number of discharge medication orders. The medications include new, historic, and as-needed medications (if included among discharge medication orders).
The electronic score is displayed within the EHR’s Discharge Readiness Report8 and updates automatically as relevant data are entered. The tool displays the individual components and as a composite of 0-3 points. To register a point in each category, a patient needs to exceed (1) the dichotomous discharge medications criterion (ie, ≥6 medications), (2) the dichotomous home-health order criterion (ie, ≥1 home-care order), and (3) to possess documentation of a non-English speaking caregiver. The tool serves as a visual reminder of discharge planning needs during daily coordination rounds attended by clinicians, nursing managers, case managers, and social workers. Case managers use the home-care alert to verify the accuracy of home-care orders.
Evaluation of Predictive Utility for Readmission
We performed a retrospective cohort study on patients aged 0-21 years who were discharged between January 1, 2014 and December 30, 2015. This study was performed to determine the optimal cut points for the continuous variables (discharge medications and home-care orders) and to evaluate the predictive value of the composite score.
Unplanned readmission within 30 days was used as the primary outcome. The index hospitalization was randomly selected for patients with >1 admissions to avoid biasing the results with multiple hospitalizations from individual patients.
Patient characteristics were summarized using percentages for categorical variables and the median and interquartile range (IQR) for continuous variables. We examined bivariate associations for each of the tool’s predictor elements with readmission using Chi-square and Wilcoxon tests
The area under the ROC curve (AUC) was estimated to evaluate the performance of the composite score
RESULTS
Cohort Characteristics
Analysis was restricted to
ROC analysis indicated that dichotomizing number of medications at ≥6 vs. <6 and home health at 0 vs. ≥1 categories maximized the sensitivity and specificity for predicting 30-day unplanned readmissions. In predictive logistic regression analysis, the odds of readmission was significantly higher in children with a composite score of 1 vs. 0 (odds ratio [OR], 1.7; 95% CI 1.5-2) and a score of ≥2 vs. 0 (OR, 4.2; 95% CI, 3.6-4.9). The c statistic for this model was 0.62, and the Brier score was 0.037. Internal validation of the predictive logistic regression model yielded identical results.
DISCUSSION
The instrument’s framework is relatively simple and should reduce barriers to implementation elsewhere. However, this tool was developed for one setting, and the design may require adjustment for other environments. Regional or institutional variation in home-health eligibility or clinical documentation may impact home-care and medication scores. The score may change at discharge if home-health or medication orders are modified late. The tool performs none of the following: measurement of regimen complexity, identification of high-risk medications, distinguishing of new versus preexisting medications/home care, nor measurement of health literacy, parent education, or psychosocial risk. Adding these features might enhance the model. Finally, readmission rates did not rise linearly with each added point. A more sophisticated scoring system (eg, differentially weighting each risk factor) may also improve the performance of the tool.
Despite these limitations, we have implemented a real-time electronic tool with practical potential to improve the discharge process but with low utility for distinguishing readmissions. Additional validation and research is needed to evaluate its impact on hospital discharge quality metrics and family reported outcome measures.
Disclosures
The authors have no relevant financial relationships to disclose.
Funding
This study was supported by an institutional Clinical and Operational Effectiveness and Patient Safety Small Grants Program
1. Holland DE, Conlon PM, Rohlik GM, Gillard KL, Messner PK, Mundy LM. Identifying hospitalized pediatric patients for early discharge planning: a feasibility study. J Pediatr Nurs. 2015;30(3):454-462. doi: 10.1016/j.pedn.2014.12.011. PubMed
2. Brittan M, Albright K, Cifuentes M, Jimenez-Zambrano A, Kempe A. Parent and provider perspectives on pediatric readmissions: what can we learn about readiness for discharge? Hosp Pediatr. 2015;5(11):559-565. doi: 10.1542/hpeds.2015-0034. PubMed
3. Brittan M FV, Martin S, Moss A, Keller D. Provider feedback: a potential method to reduce readmissions. Hosp Pediatr. 2016;6(11):684-688. PubMed
4. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA 2011;305(7):682-690. doi: 10.1001/jama.2011.122. PubMed
5. Feudtner C, Levin JE, Srivastava R, et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics 2009;123(1):286-293. doi: 10.1542/peds.2007-3395. PubMed
6. Jurgens V, Spaeder MC, Pavuluri P, Waldman Z. Hospital readmission in children with complex chronic conditions discharged from subacute care. Hosp Pediatr. 2014;4(3):153-158. doi: 10.1542/hpeds.2013-0094. PubMed
7. Karliner LS, Kim SE, Meltzer DO, Auerbach AD. Influence of language barriers on outcomes of hospital care for general medicine inpatients. J Hosp Med. 2010;5(5):276-282. doi: 10.1002/jhm.658. PubMed
8. Tyler A, Boyer A, Martin S, Neiman J, Bakel LA, Brittan M. Development of a discharge readiness report within the electronic health record-A discharge planning tool. J Hosp Med. 2014;9(8):533-539. doi: 10.1002/jhm.2212. PubMed
9. Youden WJ. Index for rating diagnostic tests. Cancer 1950;3(1):32-35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. PubMed
10. Steyerberg EW, Harrell FE, Jr., Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774-781. doi: 10.1016/S0895-4356(01)00341-9. PubMed
11. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA 2011;306(15):1688-1698. doi: 10.1001/jama.2011.1515. PubMed
12. Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159(9):882-890. doi: 10.1093/aje/kwh101. PubMed
13. Coller RJ, Klitzner TS, Lerner CF, Chung PJ. Predictors of 30-day readmission and association with primary care follow-up plans. J Pediatr. 2013;163(4):1027-1033. doi: 10.1016/j.jpeds.2013.04.013. PubMed
14. Jovanovic M, Radovanovic S, Vukicevic M, Van Poucke S, Delibasic B. Building interpretable predictive models for pediatric hospital readmission using Tree-Lasso logistic regression. Artif Intell Med. 2016;72:12-21. doi: 10.1016/j.artmed.2016.07.003. PubMed
15. Naessens JM, Knoebel E, Johnson M, Branda M. ISQUA16-1722 predicting pediatric readmissions. Int J Qual Health Care. 2016;28(suppl_1):24-25. doi: 10.1093/intqhc/mzw104.34.
1. Holland DE, Conlon PM, Rohlik GM, Gillard KL, Messner PK, Mundy LM. Identifying hospitalized pediatric patients for early discharge planning: a feasibility study. J Pediatr Nurs. 2015;30(3):454-462. doi: 10.1016/j.pedn.2014.12.011. PubMed
2. Brittan M, Albright K, Cifuentes M, Jimenez-Zambrano A, Kempe A. Parent and provider perspectives on pediatric readmissions: what can we learn about readiness for discharge? Hosp Pediatr. 2015;5(11):559-565. doi: 10.1542/hpeds.2015-0034. PubMed
3. Brittan M FV, Martin S, Moss A, Keller D. Provider feedback: a potential method to reduce readmissions. Hosp Pediatr. 2016;6(11):684-688. PubMed
4. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA 2011;305(7):682-690. doi: 10.1001/jama.2011.122. PubMed
5. Feudtner C, Levin JE, Srivastava R, et al. How well can hospital readmission be predicted in a cohort of hospitalized children? A retrospective, multicenter study. Pediatrics 2009;123(1):286-293. doi: 10.1542/peds.2007-3395. PubMed
6. Jurgens V, Spaeder MC, Pavuluri P, Waldman Z. Hospital readmission in children with complex chronic conditions discharged from subacute care. Hosp Pediatr. 2014;4(3):153-158. doi: 10.1542/hpeds.2013-0094. PubMed
7. Karliner LS, Kim SE, Meltzer DO, Auerbach AD. Influence of language barriers on outcomes of hospital care for general medicine inpatients. J Hosp Med. 2010;5(5):276-282. doi: 10.1002/jhm.658. PubMed
8. Tyler A, Boyer A, Martin S, Neiman J, Bakel LA, Brittan M. Development of a discharge readiness report within the electronic health record-A discharge planning tool. J Hosp Med. 2014;9(8):533-539. doi: 10.1002/jhm.2212. PubMed
9. Youden WJ. Index for rating diagnostic tests. Cancer 1950;3(1):32-35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. PubMed
10. Steyerberg EW, Harrell FE, Jr., Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54(8):774-781. doi: 10.1016/S0895-4356(01)00341-9. PubMed
11. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA 2011;306(15):1688-1698. doi: 10.1001/jama.2011.1515. PubMed
12. Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159(9):882-890. doi: 10.1093/aje/kwh101. PubMed
13. Coller RJ, Klitzner TS, Lerner CF, Chung PJ. Predictors of 30-day readmission and association with primary care follow-up plans. J Pediatr. 2013;163(4):1027-1033. doi: 10.1016/j.jpeds.2013.04.013. PubMed
14. Jovanovic M, Radovanovic S, Vukicevic M, Van Poucke S, Delibasic B. Building interpretable predictive models for pediatric hospital readmission using Tree-Lasso logistic regression. Artif Intell Med. 2016;72:12-21. doi: 10.1016/j.artmed.2016.07.003. PubMed
15. Naessens JM, Knoebel E, Johnson M, Branda M. ISQUA16-1722 predicting pediatric readmissions. Int J Qual Health Care. 2016;28(suppl_1):24-25. doi: 10.1093/intqhc/mzw104.34.
© 2018 Society of Hospital Medicine
Smoking Cessation after Hospital Discharge: Factors Associated with Abstinence
Cigarette smoking is the leading cause of preventable deaths in the United States.1 Smoking contributes to several health problems that require hospitalization. Hospitalization also offers smokers an opportunity to quit because hospital policies prohibit smoking indoors while a health threat increases the motivation to quit.2 Brief bedside smoking cessation counseling with follow-up contact after discharge increases postdischarge tobacco abstinence rates by 37%.2 Identifying the characteristics of patients who are most likely to stop smoking after hospital discharge could identify strategies for interventions to help more smokers to succeed. It could also guide hospital clinicians’ efforts to provide effective brief messages to promote cessation by inpatients under their care during this teachable moment.
Sociodemographic factors, tobacco use, and psychological and medical factors have been associated with successful quit attempts by smokers in the general population.3,4 Far less is known about the predictors of success in quitting smoking and maintaining abstinence after hospitalization. The characteristics associated with abstinence at postdischarge follow-up in prior studies of hospitalized smokers were male gender, greater confidence in quitting, greater readiness to quit, less nicotine dependence, and having a smoking-related illness.5-8 However, most of the prior studies were limited to 1 geographic region,5,6 focused only on a specific subgroup (eg, coronary patients9), or did not biochemically verify tobacco abstinence.8 In fact, to our knowledge, only one prior study has examined the predictors of quitting among a broad sample of hospital patients enrolled across multiple hospitals and biochemically verified abstinence.6 That study was conducted nearly two decades ago in one Midwestern state.
Thus, the present study aimed to identify factors independently associated with sustained postdischarge tobacco abstinence among hospitalized smokers who planned to quit smoking.10 Building on previous work, this study includes a large number of smokers with varied diagnoses admitted to one of three hospitals in two states, uses biochemically verified abstinence as the outcome measure, and examines multiple variables that were identified during the inpatient stay. We hypothesize that consistent with prior literature on this topic, factors independently associated with cessation in the present study will include confidence and intention to quit, degree of nicotine dependence, and a discharge diagnosis of a smoking-related disease.
METHODS
We analyzed data from the Helping HAND2 Trial (HH2; NCT01714323), a randomized clinical trial conducted at the following three hospitals: Massachusetts General Hospital (MGH) in Boston, MA; University of Pittsburgh Medical Center (UPMC) in Pittsburgh, PA; and North Shore Medical Center (NSMC) in Salem, MA. Enrollment occurred from December 2012 to July 2014. The study methodology has been reported elsewhere.11 This study was approved by the Institutional Review Boards of Partners HealthCare and University of Pittsburgh.
PARTICIPANTS
Hospital inpatients were eligible for enrollment if they were
- >18 years old, daily smokers, received smoking cessation counseling in the hospital (ie, standard of care for inpatient smokers), and planned to quit or try to quit smoking after discharge. Exclusion criteria included no access to a telephone, not speaking English, psychiatric or cognitive impairment, medical instability, or admission to obstetric or psychiatric units. All participants were offered nicotine replacement and one counseling session by a tobacco treatment specialist during hospitalization.
STUDY CONDITIONS
Participants were enrolled before discharge and randomly assigned to Sustained Care (Intervention) or Standard Care (Control) conditions.10,11 In the Standard Care condition, participants received advice to call a free telephone quit line and a tailored recommendation for postdischarge pharmacotherapy. Participants randomized to Sustained Care received a free 30-day supply of their choice of FDA-approved tobacco cessation pharmacotherapy at hospital discharge (refillable twice) and five automated interactive voice response calls over three months postdischarge to allow them to access counseling or refill medications.
MEASURES
Baseline Demographic and Smoking Characteristics
A baseline survey assessed demographic variables (age, gender, race/ethnicity, education), tobacco use (cigarettes smoked per day, time to first morning cigarette,12 other tobacco use, and prior quit attempts), intention to quit after discharge (ie,“What is your plan about smoking after you leave the hospital,” with the intent measured across four categorical response options), perceived importance of and confidence in quitting after discharge (five-point Likert scales ranging from “not at all” to “very”), and the presence of another smoker at home. Depression and anxiety symptoms were assessed using the Patient Health Questionnaire (PHQ-413). Alcohol use (AUDIT-C14) and past-year use of cocaine, stimulants, opioids, and marijuana were also measured. Health insurance, length of stay, and primary discharge diagnoses were abstracted from the medical record. Smoking-related disease categories were derived from the 2014 U.S. Surgeon General’s Report.1
Follow-up Assessment
Telephone surveys were administered by the research staff sixmonths after hospital discharge. Participants who reported past seven-day tobacco abstinence (ie, abstinence from tobacco for the past seven days reported at the 6-month call) were asked to provide a mailed saliva sample to assay for cotinine, a nicotine metabolite, to verify self-reported abstinence. Participants who reported nicotine replacement therapy use were asked to provide an in-person measurement of expired air carbon monoxide (CO) instead. Self-reported abstinence was biochemically verified if saliva cotinine was <10 ng/ml or if CO was <9 ppm.11
Outcomes
The dependent variable, consistent with the parent trial, was biochemically confirmed past seven-day tobacco abstinence at six-month follow-up. Nonrespondents and those failing to provide a sample for confirmation were considered as smokers. In addition, a sensitivity analysis used complete cases only, excluding cases with missing smoking status outcomes.
Analysis
Bivariate associations of baseline predictor variables and biochemically confirmed abstinence were examined using chi-square tests for categorical variables and t tests or Wilcoxon rank sum tests for continuous variables. Using multiple logistic regression analyses, we identified variables that were independently associated with confirmed abstinence. The final models included all factors that were associated with cessation in the bivariate analysis (P < .10), factors associated with abstinence in the literature regardless of statistical significance (gender, AUDIT-C score),4 study site, and study condition. A two-sided p value of <.05 was considered to be statistically significant. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Baseline characteristics of the 1,357 smokers enrolled in the trial are reported in Table 1. One-third of participants had a smoking-related discharge diagnosis. The median self-reported confidence in quitting was three on a five-point scale, and nearly half of the participants reported planning to stay abstinent after discharge. At six-month follow-up, 75% of participants completed the assessment, and seven-day tobacco abstinence was reported by 389 participants (29%) and biochemically confirmed in 218 participants (16%).
Results of the multiple logistic regression analysis predicting biochemically confirmed abstinence at six months are presented in Table 2. Factors independently associated with confirmed abstinence were a smoking-related primary discharge diagnosis (AOR = 1.98, 95% CI: 1.41-2.77), greater confidence in the ability to quit smoking (AOR = 1.31, 95% CI: 1.07-1.60), and stronger intention to quit (plan to stay abstinent after discharge vs. try to stay abstinent; AOR = 1.68, 95% CI: 1.19-2.38). Similar variables emerged as independent predictors of abstinence when the analysis was limited to complete cases, with an exception that one additional predictor, time to first cigarette after 30 minutes of waking, had statistical significance at the 0.05 level (Table 2).
DISCUSSION
We examined the associations between factors that were identifiable in the hospital and postdischarge tobacco abstinence among a general sample of hospitalized patients enrolled in a smoking cessation trial. The odds of biochemically confirmed abstinence at six months were higher among participants who reported higher levels of confidence in quitting smoking, those reporting having a definite plan to quit (vs. try to) after discharge, and those with a smoking-related primary discharge diagnosis.
Our findings are largely consistent with the prior literature on this topic, which has demonstrated that increased confidence in quitting, having a plan to quit smoking, and the presence of a smoking-related disease are associated with quit success at follow-up among hospital patients as well as in the general adult population.3-7 Our finding that nicotine dependence predicted quit success in the complete case analysis, but not when imputing smoking status, aligns with prior studies of hospitalized smokers, which have shown an inconclusive relationship between nicotine dependence and quit success.6,8 Despite a clear relationship of dependence to quit success among adult smokers, evidence in the hospital literature has been inconsistent. This inconsistency is likely due to the differing interventions across studies (eg, counseling vs. pharmacotherapy), the differing outcome variables (eg, self-report vs. biochemically verified), as well as the different patient populations selected to participate.
Unfortunately, smoking cessation is infrequently addressed in routine health care settings,15,16 highlighting a gap in care. For example, one survey study16 found that while many health care professionals report asking about smoking status and advising smokers to quit, fewer clinicians assess smokers’ interest or intention to stop smoking, assist with cessation, or arrange follow-up. Our results indicate that assessing an inpatient smoker’s intentions, motivation, and confidence for cessation and attempting to improve low levels of these factors could enhance cessation success. Because motivation is a malleable construct, repeated assessment by hospital clinicians of a patient’s motivation and confidence to quit is needed.
Our results also confirm that inpatient efforts to improve smoking cessation postdischarge should target smokers’ resolve to quit and confidence in the ability to succeed. Motivational interventions and cognitive-behavioral therapy are effective strategies that can resolve ambivalence and increase confidence to quit and should be components of brief interventions delivered in inpatient settings.17,18 Although individuals with a smoking-related illness may already possess some resolve to quit based on their illness, they may be candidates for interventions focused primarily on developing self-efficacy. Indeed, supporting self-efficacy is a major goal of effective bedside counseling and can be bolstered via problem-solving, motivational techniques, and education about pharmacotherapy during a tobacco-specific consult such as the one that these participants experienced. Armed with these resources, smokers with and without a smoking-related disease may be more likely to execute a plan to quit after discharge.
A study limitation is that our results can be generalized only to hospital inpatients who were willing to try to quit smoking after discharge, because the parent trial excluded smokers with lower levels of motivation. Similarly, these results may not be generalizable to obstetric or psychiatric inpatients, who were excluded from this trial.
In conclusion, our results underscore the importance of assessing motivation and self-efficacy in hospitalized smokers and targeting these factors in intervention efforts. Although future research should aim to identify better methods to alter these factors, in the short run, hospital clinicians could target these factors when discussing tobacco use with inpatient smokers.
Acknowledgments
The authors are grateful for the hard work of MGH, NSMC, and UPMC’s tobacco treatment services, the hospital providers, and study research staff.
Disclosures
Drs. Rigotti and Park received royalties from UpToDate and have received a research grant from Pfizer regarding smoking cessation. Dr. Rigotti has consulted (without pay) for Pfizer. Dr. Singer has served as a consultant to Pfizer but on a topic separate from smoking cessation. No other authors have conflicts of interest to disclose.
Role of Funding Source: The study was funded by NIH/NHLBI [grant #R01-HL11821]. The funding organization had no role in the study design, collection, analysis, and interpretation of the data, preparation of the manuscript, or decision to submit the manuscript for publication.
Clinical Trial Registration: NCT01714323
1. U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014 | SurgeonGeneral.Gov. Office on Smoking and Health: Centers for Disease Control and Prevention; 2014:944. http://www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html. Accessed May 22, 2016.
2. Rigotti NA, Clair C, Munafò MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012;(5):CD001837. 10.1002/14651858.CD001837.pub3 PubMed
3. Vangeli E, Stapleton J, Smit ES, Borland R, West R. Predictors of attempts to stop smoking and their success in adult general population samples: a systematic review. Addict Abingdon Engl. 2011;106(12):2110-2121. 10.1111/j.1360-0443.2011.03565.x PubMed
4. Ockene JK, Emmons KM, Mermelstein RJ, et al. Relapse and maintenance issues for smoking cessation. Health Psychol. 2000;19(1S):17-31. 10.1037/0278-6133.19.Suppl1.17 PubMed
5. Harrington K, Young-Il K, Meifang C, et al. Web-based intervention for transitioning smokers from inpatient to outpatient care: an RCT. Am J Prev Med. 2016;51(4):620-629. 10.1016/j.amepre.2016.04.008 PubMed
6. Lando H, Hennrikus D, McCarty M, Vessey J. Predictors of quitting in hospitalized smokers. Nicotine Tob Res. 2003;5(2):215-222. 10.1080/0955300031000083436 PubMed
7. Hennrikus DJ, Lando HA, McCarty MC, et al. The TEAM project: the effectiveness of smoking cessation intervention with hospital patients. Prev Med. 2005;40(3):249-258. 10.1016/j.ypmed.2004.05.030 PubMed
8. MacKenzie TD, Pereira RI, Mehler PS. Smoking abstinence after hospitalization: predictors of success. Prev Med. 2004;39(6):1087-1092. 10.1016/j.ypmed.2004.04.054 PubMed
9. Holtrop JS, Stommel M, Corser W, Holmes-Rovner M. Predictors of smoking cessation and relapse after hospitalization for acute coronary syndrome. J Hosp Med. 2009;4(3):E3-E9. 10.1002/jhm.415 PubMed
10. Rigotti NA, Tindle HA, Regan S, et al. A post-discharge smoking-cessation intervention for hospital patients: helping Hand 2 randomized clinical trial. Am J Prev Med. 2016;51(4):597-608. 10.1016/j.amepre.2016.04.005 PubMed
11. Reid ZZ, Regan S, Kelley JHK, et al. Comparative effectiveness of post-discharge strategies for hospitalized smokers: study protocol for the helping HAND 2 randomized controlled trial. BMC Public Health. 2015;15:109. 10.1186/s12889-015-1484-0 PubMed
12. Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989;84(7):791-799. http://dx.doi.org/10.1111/j.1360-0443.1989.tb03059.x PubMed
13. Melchior LA, Huba GJ, Brown VB, Reback CJ. A short depression index for women. Educ Psychol Meas. 1993;53(4):1117-1125. 10.1177/0013164493053004024
14. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789-1795. 10.1001/archinte.158.16.1789 PubMed
15. Kruger J, Shaw L, Kahende J, Frank E. Health care providers’ advice to quit smoking, National Health Interview Survey, 2000, 2005, and 2010. Prev Chronic Dis. 2012;9:E130. 10.5888/pcd9.110340 PubMed
16. Tong EK, Strouse R, Hall J, Kovac M, Schroeder SA. National survey of U.S. health professionals’ smoking prevalence, cessation practices, and beliefs. Nicotine Tob Res. 2010;12(7):724-733. 10.1093/ntr/ntq071 PubMed
17. Lindson-Hawley N, Thompson TP, Begh R. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev. 2015;(3):CD006936. 10.1002/14651858.CD006936.pub3 PubMed
18. Hendricks PS, Delucchi KL, Hall SM. Mechanisms of change in extended cognitive behavioral treatment for tobacco dependence. Drug Alcohol Depend. 2010;109(1-3):114-119. 10.1016/j.drugalcdep.2009.12.021 PubMed
Cigarette smoking is the leading cause of preventable deaths in the United States.1 Smoking contributes to several health problems that require hospitalization. Hospitalization also offers smokers an opportunity to quit because hospital policies prohibit smoking indoors while a health threat increases the motivation to quit.2 Brief bedside smoking cessation counseling with follow-up contact after discharge increases postdischarge tobacco abstinence rates by 37%.2 Identifying the characteristics of patients who are most likely to stop smoking after hospital discharge could identify strategies for interventions to help more smokers to succeed. It could also guide hospital clinicians’ efforts to provide effective brief messages to promote cessation by inpatients under their care during this teachable moment.
Sociodemographic factors, tobacco use, and psychological and medical factors have been associated with successful quit attempts by smokers in the general population.3,4 Far less is known about the predictors of success in quitting smoking and maintaining abstinence after hospitalization. The characteristics associated with abstinence at postdischarge follow-up in prior studies of hospitalized smokers were male gender, greater confidence in quitting, greater readiness to quit, less nicotine dependence, and having a smoking-related illness.5-8 However, most of the prior studies were limited to 1 geographic region,5,6 focused only on a specific subgroup (eg, coronary patients9), or did not biochemically verify tobacco abstinence.8 In fact, to our knowledge, only one prior study has examined the predictors of quitting among a broad sample of hospital patients enrolled across multiple hospitals and biochemically verified abstinence.6 That study was conducted nearly two decades ago in one Midwestern state.
Thus, the present study aimed to identify factors independently associated with sustained postdischarge tobacco abstinence among hospitalized smokers who planned to quit smoking.10 Building on previous work, this study includes a large number of smokers with varied diagnoses admitted to one of three hospitals in two states, uses biochemically verified abstinence as the outcome measure, and examines multiple variables that were identified during the inpatient stay. We hypothesize that consistent with prior literature on this topic, factors independently associated with cessation in the present study will include confidence and intention to quit, degree of nicotine dependence, and a discharge diagnosis of a smoking-related disease.
METHODS
We analyzed data from the Helping HAND2 Trial (HH2; NCT01714323), a randomized clinical trial conducted at the following three hospitals: Massachusetts General Hospital (MGH) in Boston, MA; University of Pittsburgh Medical Center (UPMC) in Pittsburgh, PA; and North Shore Medical Center (NSMC) in Salem, MA. Enrollment occurred from December 2012 to July 2014. The study methodology has been reported elsewhere.11 This study was approved by the Institutional Review Boards of Partners HealthCare and University of Pittsburgh.
PARTICIPANTS
Hospital inpatients were eligible for enrollment if they were
- >18 years old, daily smokers, received smoking cessation counseling in the hospital (ie, standard of care for inpatient smokers), and planned to quit or try to quit smoking after discharge. Exclusion criteria included no access to a telephone, not speaking English, psychiatric or cognitive impairment, medical instability, or admission to obstetric or psychiatric units. All participants were offered nicotine replacement and one counseling session by a tobacco treatment specialist during hospitalization.
STUDY CONDITIONS
Participants were enrolled before discharge and randomly assigned to Sustained Care (Intervention) or Standard Care (Control) conditions.10,11 In the Standard Care condition, participants received advice to call a free telephone quit line and a tailored recommendation for postdischarge pharmacotherapy. Participants randomized to Sustained Care received a free 30-day supply of their choice of FDA-approved tobacco cessation pharmacotherapy at hospital discharge (refillable twice) and five automated interactive voice response calls over three months postdischarge to allow them to access counseling or refill medications.
MEASURES
Baseline Demographic and Smoking Characteristics
A baseline survey assessed demographic variables (age, gender, race/ethnicity, education), tobacco use (cigarettes smoked per day, time to first morning cigarette,12 other tobacco use, and prior quit attempts), intention to quit after discharge (ie,“What is your plan about smoking after you leave the hospital,” with the intent measured across four categorical response options), perceived importance of and confidence in quitting after discharge (five-point Likert scales ranging from “not at all” to “very”), and the presence of another smoker at home. Depression and anxiety symptoms were assessed using the Patient Health Questionnaire (PHQ-413). Alcohol use (AUDIT-C14) and past-year use of cocaine, stimulants, opioids, and marijuana were also measured. Health insurance, length of stay, and primary discharge diagnoses were abstracted from the medical record. Smoking-related disease categories were derived from the 2014 U.S. Surgeon General’s Report.1
Follow-up Assessment
Telephone surveys were administered by the research staff sixmonths after hospital discharge. Participants who reported past seven-day tobacco abstinence (ie, abstinence from tobacco for the past seven days reported at the 6-month call) were asked to provide a mailed saliva sample to assay for cotinine, a nicotine metabolite, to verify self-reported abstinence. Participants who reported nicotine replacement therapy use were asked to provide an in-person measurement of expired air carbon monoxide (CO) instead. Self-reported abstinence was biochemically verified if saliva cotinine was <10 ng/ml or if CO was <9 ppm.11
Outcomes
The dependent variable, consistent with the parent trial, was biochemically confirmed past seven-day tobacco abstinence at six-month follow-up. Nonrespondents and those failing to provide a sample for confirmation were considered as smokers. In addition, a sensitivity analysis used complete cases only, excluding cases with missing smoking status outcomes.
Analysis
Bivariate associations of baseline predictor variables and biochemically confirmed abstinence were examined using chi-square tests for categorical variables and t tests or Wilcoxon rank sum tests for continuous variables. Using multiple logistic regression analyses, we identified variables that were independently associated with confirmed abstinence. The final models included all factors that were associated with cessation in the bivariate analysis (P < .10), factors associated with abstinence in the literature regardless of statistical significance (gender, AUDIT-C score),4 study site, and study condition. A two-sided p value of <.05 was considered to be statistically significant. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Baseline characteristics of the 1,357 smokers enrolled in the trial are reported in Table 1. One-third of participants had a smoking-related discharge diagnosis. The median self-reported confidence in quitting was three on a five-point scale, and nearly half of the participants reported planning to stay abstinent after discharge. At six-month follow-up, 75% of participants completed the assessment, and seven-day tobacco abstinence was reported by 389 participants (29%) and biochemically confirmed in 218 participants (16%).
Results of the multiple logistic regression analysis predicting biochemically confirmed abstinence at six months are presented in Table 2. Factors independently associated with confirmed abstinence were a smoking-related primary discharge diagnosis (AOR = 1.98, 95% CI: 1.41-2.77), greater confidence in the ability to quit smoking (AOR = 1.31, 95% CI: 1.07-1.60), and stronger intention to quit (plan to stay abstinent after discharge vs. try to stay abstinent; AOR = 1.68, 95% CI: 1.19-2.38). Similar variables emerged as independent predictors of abstinence when the analysis was limited to complete cases, with an exception that one additional predictor, time to first cigarette after 30 minutes of waking, had statistical significance at the 0.05 level (Table 2).
DISCUSSION
We examined the associations between factors that were identifiable in the hospital and postdischarge tobacco abstinence among a general sample of hospitalized patients enrolled in a smoking cessation trial. The odds of biochemically confirmed abstinence at six months were higher among participants who reported higher levels of confidence in quitting smoking, those reporting having a definite plan to quit (vs. try to) after discharge, and those with a smoking-related primary discharge diagnosis.
Our findings are largely consistent with the prior literature on this topic, which has demonstrated that increased confidence in quitting, having a plan to quit smoking, and the presence of a smoking-related disease are associated with quit success at follow-up among hospital patients as well as in the general adult population.3-7 Our finding that nicotine dependence predicted quit success in the complete case analysis, but not when imputing smoking status, aligns with prior studies of hospitalized smokers, which have shown an inconclusive relationship between nicotine dependence and quit success.6,8 Despite a clear relationship of dependence to quit success among adult smokers, evidence in the hospital literature has been inconsistent. This inconsistency is likely due to the differing interventions across studies (eg, counseling vs. pharmacotherapy), the differing outcome variables (eg, self-report vs. biochemically verified), as well as the different patient populations selected to participate.
Unfortunately, smoking cessation is infrequently addressed in routine health care settings,15,16 highlighting a gap in care. For example, one survey study16 found that while many health care professionals report asking about smoking status and advising smokers to quit, fewer clinicians assess smokers’ interest or intention to stop smoking, assist with cessation, or arrange follow-up. Our results indicate that assessing an inpatient smoker’s intentions, motivation, and confidence for cessation and attempting to improve low levels of these factors could enhance cessation success. Because motivation is a malleable construct, repeated assessment by hospital clinicians of a patient’s motivation and confidence to quit is needed.
Our results also confirm that inpatient efforts to improve smoking cessation postdischarge should target smokers’ resolve to quit and confidence in the ability to succeed. Motivational interventions and cognitive-behavioral therapy are effective strategies that can resolve ambivalence and increase confidence to quit and should be components of brief interventions delivered in inpatient settings.17,18 Although individuals with a smoking-related illness may already possess some resolve to quit based on their illness, they may be candidates for interventions focused primarily on developing self-efficacy. Indeed, supporting self-efficacy is a major goal of effective bedside counseling and can be bolstered via problem-solving, motivational techniques, and education about pharmacotherapy during a tobacco-specific consult such as the one that these participants experienced. Armed with these resources, smokers with and without a smoking-related disease may be more likely to execute a plan to quit after discharge.
A study limitation is that our results can be generalized only to hospital inpatients who were willing to try to quit smoking after discharge, because the parent trial excluded smokers with lower levels of motivation. Similarly, these results may not be generalizable to obstetric or psychiatric inpatients, who were excluded from this trial.
In conclusion, our results underscore the importance of assessing motivation and self-efficacy in hospitalized smokers and targeting these factors in intervention efforts. Although future research should aim to identify better methods to alter these factors, in the short run, hospital clinicians could target these factors when discussing tobacco use with inpatient smokers.
Acknowledgments
The authors are grateful for the hard work of MGH, NSMC, and UPMC’s tobacco treatment services, the hospital providers, and study research staff.
Disclosures
Drs. Rigotti and Park received royalties from UpToDate and have received a research grant from Pfizer regarding smoking cessation. Dr. Rigotti has consulted (without pay) for Pfizer. Dr. Singer has served as a consultant to Pfizer but on a topic separate from smoking cessation. No other authors have conflicts of interest to disclose.
Role of Funding Source: The study was funded by NIH/NHLBI [grant #R01-HL11821]. The funding organization had no role in the study design, collection, analysis, and interpretation of the data, preparation of the manuscript, or decision to submit the manuscript for publication.
Clinical Trial Registration: NCT01714323
Cigarette smoking is the leading cause of preventable deaths in the United States.1 Smoking contributes to several health problems that require hospitalization. Hospitalization also offers smokers an opportunity to quit because hospital policies prohibit smoking indoors while a health threat increases the motivation to quit.2 Brief bedside smoking cessation counseling with follow-up contact after discharge increases postdischarge tobacco abstinence rates by 37%.2 Identifying the characteristics of patients who are most likely to stop smoking after hospital discharge could identify strategies for interventions to help more smokers to succeed. It could also guide hospital clinicians’ efforts to provide effective brief messages to promote cessation by inpatients under their care during this teachable moment.
Sociodemographic factors, tobacco use, and psychological and medical factors have been associated with successful quit attempts by smokers in the general population.3,4 Far less is known about the predictors of success in quitting smoking and maintaining abstinence after hospitalization. The characteristics associated with abstinence at postdischarge follow-up in prior studies of hospitalized smokers were male gender, greater confidence in quitting, greater readiness to quit, less nicotine dependence, and having a smoking-related illness.5-8 However, most of the prior studies were limited to 1 geographic region,5,6 focused only on a specific subgroup (eg, coronary patients9), or did not biochemically verify tobacco abstinence.8 In fact, to our knowledge, only one prior study has examined the predictors of quitting among a broad sample of hospital patients enrolled across multiple hospitals and biochemically verified abstinence.6 That study was conducted nearly two decades ago in one Midwestern state.
Thus, the present study aimed to identify factors independently associated with sustained postdischarge tobacco abstinence among hospitalized smokers who planned to quit smoking.10 Building on previous work, this study includes a large number of smokers with varied diagnoses admitted to one of three hospitals in two states, uses biochemically verified abstinence as the outcome measure, and examines multiple variables that were identified during the inpatient stay. We hypothesize that consistent with prior literature on this topic, factors independently associated with cessation in the present study will include confidence and intention to quit, degree of nicotine dependence, and a discharge diagnosis of a smoking-related disease.
METHODS
We analyzed data from the Helping HAND2 Trial (HH2; NCT01714323), a randomized clinical trial conducted at the following three hospitals: Massachusetts General Hospital (MGH) in Boston, MA; University of Pittsburgh Medical Center (UPMC) in Pittsburgh, PA; and North Shore Medical Center (NSMC) in Salem, MA. Enrollment occurred from December 2012 to July 2014. The study methodology has been reported elsewhere.11 This study was approved by the Institutional Review Boards of Partners HealthCare and University of Pittsburgh.
PARTICIPANTS
Hospital inpatients were eligible for enrollment if they were
- >18 years old, daily smokers, received smoking cessation counseling in the hospital (ie, standard of care for inpatient smokers), and planned to quit or try to quit smoking after discharge. Exclusion criteria included no access to a telephone, not speaking English, psychiatric or cognitive impairment, medical instability, or admission to obstetric or psychiatric units. All participants were offered nicotine replacement and one counseling session by a tobacco treatment specialist during hospitalization.
STUDY CONDITIONS
Participants were enrolled before discharge and randomly assigned to Sustained Care (Intervention) or Standard Care (Control) conditions.10,11 In the Standard Care condition, participants received advice to call a free telephone quit line and a tailored recommendation for postdischarge pharmacotherapy. Participants randomized to Sustained Care received a free 30-day supply of their choice of FDA-approved tobacco cessation pharmacotherapy at hospital discharge (refillable twice) and five automated interactive voice response calls over three months postdischarge to allow them to access counseling or refill medications.
MEASURES
Baseline Demographic and Smoking Characteristics
A baseline survey assessed demographic variables (age, gender, race/ethnicity, education), tobacco use (cigarettes smoked per day, time to first morning cigarette,12 other tobacco use, and prior quit attempts), intention to quit after discharge (ie,“What is your plan about smoking after you leave the hospital,” with the intent measured across four categorical response options), perceived importance of and confidence in quitting after discharge (five-point Likert scales ranging from “not at all” to “very”), and the presence of another smoker at home. Depression and anxiety symptoms were assessed using the Patient Health Questionnaire (PHQ-413). Alcohol use (AUDIT-C14) and past-year use of cocaine, stimulants, opioids, and marijuana were also measured. Health insurance, length of stay, and primary discharge diagnoses were abstracted from the medical record. Smoking-related disease categories were derived from the 2014 U.S. Surgeon General’s Report.1
Follow-up Assessment
Telephone surveys were administered by the research staff sixmonths after hospital discharge. Participants who reported past seven-day tobacco abstinence (ie, abstinence from tobacco for the past seven days reported at the 6-month call) were asked to provide a mailed saliva sample to assay for cotinine, a nicotine metabolite, to verify self-reported abstinence. Participants who reported nicotine replacement therapy use were asked to provide an in-person measurement of expired air carbon monoxide (CO) instead. Self-reported abstinence was biochemically verified if saliva cotinine was <10 ng/ml or if CO was <9 ppm.11
Outcomes
The dependent variable, consistent with the parent trial, was biochemically confirmed past seven-day tobacco abstinence at six-month follow-up. Nonrespondents and those failing to provide a sample for confirmation were considered as smokers. In addition, a sensitivity analysis used complete cases only, excluding cases with missing smoking status outcomes.
Analysis
Bivariate associations of baseline predictor variables and biochemically confirmed abstinence were examined using chi-square tests for categorical variables and t tests or Wilcoxon rank sum tests for continuous variables. Using multiple logistic regression analyses, we identified variables that were independently associated with confirmed abstinence. The final models included all factors that were associated with cessation in the bivariate analysis (P < .10), factors associated with abstinence in the literature regardless of statistical significance (gender, AUDIT-C score),4 study site, and study condition. A two-sided p value of <.05 was considered to be statistically significant. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Baseline characteristics of the 1,357 smokers enrolled in the trial are reported in Table 1. One-third of participants had a smoking-related discharge diagnosis. The median self-reported confidence in quitting was three on a five-point scale, and nearly half of the participants reported planning to stay abstinent after discharge. At six-month follow-up, 75% of participants completed the assessment, and seven-day tobacco abstinence was reported by 389 participants (29%) and biochemically confirmed in 218 participants (16%).
Results of the multiple logistic regression analysis predicting biochemically confirmed abstinence at six months are presented in Table 2. Factors independently associated with confirmed abstinence were a smoking-related primary discharge diagnosis (AOR = 1.98, 95% CI: 1.41-2.77), greater confidence in the ability to quit smoking (AOR = 1.31, 95% CI: 1.07-1.60), and stronger intention to quit (plan to stay abstinent after discharge vs. try to stay abstinent; AOR = 1.68, 95% CI: 1.19-2.38). Similar variables emerged as independent predictors of abstinence when the analysis was limited to complete cases, with an exception that one additional predictor, time to first cigarette after 30 minutes of waking, had statistical significance at the 0.05 level (Table 2).
DISCUSSION
We examined the associations between factors that were identifiable in the hospital and postdischarge tobacco abstinence among a general sample of hospitalized patients enrolled in a smoking cessation trial. The odds of biochemically confirmed abstinence at six months were higher among participants who reported higher levels of confidence in quitting smoking, those reporting having a definite plan to quit (vs. try to) after discharge, and those with a smoking-related primary discharge diagnosis.
Our findings are largely consistent with the prior literature on this topic, which has demonstrated that increased confidence in quitting, having a plan to quit smoking, and the presence of a smoking-related disease are associated with quit success at follow-up among hospital patients as well as in the general adult population.3-7 Our finding that nicotine dependence predicted quit success in the complete case analysis, but not when imputing smoking status, aligns with prior studies of hospitalized smokers, which have shown an inconclusive relationship between nicotine dependence and quit success.6,8 Despite a clear relationship of dependence to quit success among adult smokers, evidence in the hospital literature has been inconsistent. This inconsistency is likely due to the differing interventions across studies (eg, counseling vs. pharmacotherapy), the differing outcome variables (eg, self-report vs. biochemically verified), as well as the different patient populations selected to participate.
Unfortunately, smoking cessation is infrequently addressed in routine health care settings,15,16 highlighting a gap in care. For example, one survey study16 found that while many health care professionals report asking about smoking status and advising smokers to quit, fewer clinicians assess smokers’ interest or intention to stop smoking, assist with cessation, or arrange follow-up. Our results indicate that assessing an inpatient smoker’s intentions, motivation, and confidence for cessation and attempting to improve low levels of these factors could enhance cessation success. Because motivation is a malleable construct, repeated assessment by hospital clinicians of a patient’s motivation and confidence to quit is needed.
Our results also confirm that inpatient efforts to improve smoking cessation postdischarge should target smokers’ resolve to quit and confidence in the ability to succeed. Motivational interventions and cognitive-behavioral therapy are effective strategies that can resolve ambivalence and increase confidence to quit and should be components of brief interventions delivered in inpatient settings.17,18 Although individuals with a smoking-related illness may already possess some resolve to quit based on their illness, they may be candidates for interventions focused primarily on developing self-efficacy. Indeed, supporting self-efficacy is a major goal of effective bedside counseling and can be bolstered via problem-solving, motivational techniques, and education about pharmacotherapy during a tobacco-specific consult such as the one that these participants experienced. Armed with these resources, smokers with and without a smoking-related disease may be more likely to execute a plan to quit after discharge.
A study limitation is that our results can be generalized only to hospital inpatients who were willing to try to quit smoking after discharge, because the parent trial excluded smokers with lower levels of motivation. Similarly, these results may not be generalizable to obstetric or psychiatric inpatients, who were excluded from this trial.
In conclusion, our results underscore the importance of assessing motivation and self-efficacy in hospitalized smokers and targeting these factors in intervention efforts. Although future research should aim to identify better methods to alter these factors, in the short run, hospital clinicians could target these factors when discussing tobacco use with inpatient smokers.
Acknowledgments
The authors are grateful for the hard work of MGH, NSMC, and UPMC’s tobacco treatment services, the hospital providers, and study research staff.
Disclosures
Drs. Rigotti and Park received royalties from UpToDate and have received a research grant from Pfizer regarding smoking cessation. Dr. Rigotti has consulted (without pay) for Pfizer. Dr. Singer has served as a consultant to Pfizer but on a topic separate from smoking cessation. No other authors have conflicts of interest to disclose.
Role of Funding Source: The study was funded by NIH/NHLBI [grant #R01-HL11821]. The funding organization had no role in the study design, collection, analysis, and interpretation of the data, preparation of the manuscript, or decision to submit the manuscript for publication.
Clinical Trial Registration: NCT01714323
1. U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014 | SurgeonGeneral.Gov. Office on Smoking and Health: Centers for Disease Control and Prevention; 2014:944. http://www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html. Accessed May 22, 2016.
2. Rigotti NA, Clair C, Munafò MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012;(5):CD001837. 10.1002/14651858.CD001837.pub3 PubMed
3. Vangeli E, Stapleton J, Smit ES, Borland R, West R. Predictors of attempts to stop smoking and their success in adult general population samples: a systematic review. Addict Abingdon Engl. 2011;106(12):2110-2121. 10.1111/j.1360-0443.2011.03565.x PubMed
4. Ockene JK, Emmons KM, Mermelstein RJ, et al. Relapse and maintenance issues for smoking cessation. Health Psychol. 2000;19(1S):17-31. 10.1037/0278-6133.19.Suppl1.17 PubMed
5. Harrington K, Young-Il K, Meifang C, et al. Web-based intervention for transitioning smokers from inpatient to outpatient care: an RCT. Am J Prev Med. 2016;51(4):620-629. 10.1016/j.amepre.2016.04.008 PubMed
6. Lando H, Hennrikus D, McCarty M, Vessey J. Predictors of quitting in hospitalized smokers. Nicotine Tob Res. 2003;5(2):215-222. 10.1080/0955300031000083436 PubMed
7. Hennrikus DJ, Lando HA, McCarty MC, et al. The TEAM project: the effectiveness of smoking cessation intervention with hospital patients. Prev Med. 2005;40(3):249-258. 10.1016/j.ypmed.2004.05.030 PubMed
8. MacKenzie TD, Pereira RI, Mehler PS. Smoking abstinence after hospitalization: predictors of success. Prev Med. 2004;39(6):1087-1092. 10.1016/j.ypmed.2004.04.054 PubMed
9. Holtrop JS, Stommel M, Corser W, Holmes-Rovner M. Predictors of smoking cessation and relapse after hospitalization for acute coronary syndrome. J Hosp Med. 2009;4(3):E3-E9. 10.1002/jhm.415 PubMed
10. Rigotti NA, Tindle HA, Regan S, et al. A post-discharge smoking-cessation intervention for hospital patients: helping Hand 2 randomized clinical trial. Am J Prev Med. 2016;51(4):597-608. 10.1016/j.amepre.2016.04.005 PubMed
11. Reid ZZ, Regan S, Kelley JHK, et al. Comparative effectiveness of post-discharge strategies for hospitalized smokers: study protocol for the helping HAND 2 randomized controlled trial. BMC Public Health. 2015;15:109. 10.1186/s12889-015-1484-0 PubMed
12. Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989;84(7):791-799. http://dx.doi.org/10.1111/j.1360-0443.1989.tb03059.x PubMed
13. Melchior LA, Huba GJ, Brown VB, Reback CJ. A short depression index for women. Educ Psychol Meas. 1993;53(4):1117-1125. 10.1177/0013164493053004024
14. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789-1795. 10.1001/archinte.158.16.1789 PubMed
15. Kruger J, Shaw L, Kahende J, Frank E. Health care providers’ advice to quit smoking, National Health Interview Survey, 2000, 2005, and 2010. Prev Chronic Dis. 2012;9:E130. 10.5888/pcd9.110340 PubMed
16. Tong EK, Strouse R, Hall J, Kovac M, Schroeder SA. National survey of U.S. health professionals’ smoking prevalence, cessation practices, and beliefs. Nicotine Tob Res. 2010;12(7):724-733. 10.1093/ntr/ntq071 PubMed
17. Lindson-Hawley N, Thompson TP, Begh R. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev. 2015;(3):CD006936. 10.1002/14651858.CD006936.pub3 PubMed
18. Hendricks PS, Delucchi KL, Hall SM. Mechanisms of change in extended cognitive behavioral treatment for tobacco dependence. Drug Alcohol Depend. 2010;109(1-3):114-119. 10.1016/j.drugalcdep.2009.12.021 PubMed
1. U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014 | SurgeonGeneral.Gov. Office on Smoking and Health: Centers for Disease Control and Prevention; 2014:944. http://www.surgeongeneral.gov/library/reports/50-years-of-progress/index.html. Accessed May 22, 2016.
2. Rigotti NA, Clair C, Munafò MR, Stead LF. Interventions for smoking cessation in hospitalised patients. Cochrane Database Syst Rev. 2012;(5):CD001837. 10.1002/14651858.CD001837.pub3 PubMed
3. Vangeli E, Stapleton J, Smit ES, Borland R, West R. Predictors of attempts to stop smoking and their success in adult general population samples: a systematic review. Addict Abingdon Engl. 2011;106(12):2110-2121. 10.1111/j.1360-0443.2011.03565.x PubMed
4. Ockene JK, Emmons KM, Mermelstein RJ, et al. Relapse and maintenance issues for smoking cessation. Health Psychol. 2000;19(1S):17-31. 10.1037/0278-6133.19.Suppl1.17 PubMed
5. Harrington K, Young-Il K, Meifang C, et al. Web-based intervention for transitioning smokers from inpatient to outpatient care: an RCT. Am J Prev Med. 2016;51(4):620-629. 10.1016/j.amepre.2016.04.008 PubMed
6. Lando H, Hennrikus D, McCarty M, Vessey J. Predictors of quitting in hospitalized smokers. Nicotine Tob Res. 2003;5(2):215-222. 10.1080/0955300031000083436 PubMed
7. Hennrikus DJ, Lando HA, McCarty MC, et al. The TEAM project: the effectiveness of smoking cessation intervention with hospital patients. Prev Med. 2005;40(3):249-258. 10.1016/j.ypmed.2004.05.030 PubMed
8. MacKenzie TD, Pereira RI, Mehler PS. Smoking abstinence after hospitalization: predictors of success. Prev Med. 2004;39(6):1087-1092. 10.1016/j.ypmed.2004.04.054 PubMed
9. Holtrop JS, Stommel M, Corser W, Holmes-Rovner M. Predictors of smoking cessation and relapse after hospitalization for acute coronary syndrome. J Hosp Med. 2009;4(3):E3-E9. 10.1002/jhm.415 PubMed
10. Rigotti NA, Tindle HA, Regan S, et al. A post-discharge smoking-cessation intervention for hospital patients: helping Hand 2 randomized clinical trial. Am J Prev Med. 2016;51(4):597-608. 10.1016/j.amepre.2016.04.005 PubMed
11. Reid ZZ, Regan S, Kelley JHK, et al. Comparative effectiveness of post-discharge strategies for hospitalized smokers: study protocol for the helping HAND 2 randomized controlled trial. BMC Public Health. 2015;15:109. 10.1186/s12889-015-1484-0 PubMed
12. Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989;84(7):791-799. http://dx.doi.org/10.1111/j.1360-0443.1989.tb03059.x PubMed
13. Melchior LA, Huba GJ, Brown VB, Reback CJ. A short depression index for women. Educ Psychol Meas. 1993;53(4):1117-1125. 10.1177/0013164493053004024
14. Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789-1795. 10.1001/archinte.158.16.1789 PubMed
15. Kruger J, Shaw L, Kahende J, Frank E. Health care providers’ advice to quit smoking, National Health Interview Survey, 2000, 2005, and 2010. Prev Chronic Dis. 2012;9:E130. 10.5888/pcd9.110340 PubMed
16. Tong EK, Strouse R, Hall J, Kovac M, Schroeder SA. National survey of U.S. health professionals’ smoking prevalence, cessation practices, and beliefs. Nicotine Tob Res. 2010;12(7):724-733. 10.1093/ntr/ntq071 PubMed
17. Lindson-Hawley N, Thompson TP, Begh R. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev. 2015;(3):CD006936. 10.1002/14651858.CD006936.pub3 PubMed
18. Hendricks PS, Delucchi KL, Hall SM. Mechanisms of change in extended cognitive behavioral treatment for tobacco dependence. Drug Alcohol Depend. 2010;109(1-3):114-119. 10.1016/j.drugalcdep.2009.12.021 PubMed
© 2018 Society of Hospital Medicine
Current Perspectives on Transport Medicine in Pediatric Hospital Medicine Fellowships
Transport medicine (TM) involves the provision of care to patients who require transfer to a healthcare facility that can deliver definitive treatment.1 Pediatric interfacility transport occurs in approximately 10% of nonneonatal, nonpregnancy pediatric hospitalizations in the United States.2 Studies document a decline in resident participation in pediatric transports and variability in curricular content.3,4
The Pediatric Hospital Medicine (PHM) Core Competencies include “Transport of the Critically Ill Child.”7 Additionally, the Curriculum Committee of the PHM Fellowship Directors Council proposed a curricular framework that includes a required clinical experience in “Care and Stabilization of the Critically Ill Child,”8 which can occur in a variety of practice settings, including TM. TM is also listed as a potential elective rotation.
In 2014, 60% of PHM fellowships included a required or optional TM rotation.9 A recent study of pediatric emergency, critical care, and neonatal medicine fellowships revealed a paucity of formal or published TM curricula in these programs.10 Furthermore, no standard or published TM curricula have been established for PHM fellowships. The primary objective of our study is to determine attitudes regarding TM training among PHM fellows, recent PHM fellowship graduates, and PHM fellowship program directors (PDs). The secondary objective is to identify how the perspectives of these fellowship stakeholders could influence the design of a TM curriculum.
METHODS
This cross-sectional study focused on 3 stakeholder groups related to PHM fellowships. The subjects included in the study were physicians enrolled in a PHM fellowship (fellow) during the 2015-2016 academic year, graduates of fellowship (graduate) between 2010 and 2015, and fellowship program directors (PD). Unique web-based, anonymous surveys for each group were developed, reviewed by content and methodology experts, and piloted with local pediatric hospitalists. Surveys consisted of unfolding multiple-choice questions and ranking items along Likert scales and the Dreyfus model.
Questions were designed to elicit demographic data, perspectives, and experience related to TM education in PHM fellowships across all respondent groups. Depending on the context, identical or similar questions were asked among the groups. For example, all groups were asked to prioritize learning objectives for a TM rotation. Graduates and PDs reported the most effective teaching methods for use during a TM rotation. Fellows rated their own interest in a TM elective, and PDs were asked to rate the level of interest among their fellows.
Participant contact information was obtained from a website (phmfellows.org) and databases of fellows and graduates, which are maintained by the PHM Fellowship Directors Council (personal communication, Jayne Truckenbrod, DO; February 2, 2017). Between February and April 2016, the participants were individually emailed a link to their respective surveys, and 3 reminder e-mails were sent to nonresponders. The survey was administered through SurveyMonkey (www.surveymonkey.com).
SPSS (IBM SPSS Statistics, IBM Corporation, Armonk, New York) was used for statistical analysis. Descriptive data were presented using mean and standard deviation. Comparisons among fellows, graduates, and PDs were conducted using one-way analyses of variance or Mann-Whitney U test. Frequency of application and self-evaluation of core competency skills before and after the rotation were evaluated using paired sample t-tests. The study protocol was deemed exempt from review by our local Institutional Review Board.
RESULTS
Forty of 70 (57%) fellows, 32 of 87 graduates (37%), and 14 of 32 PDs (44%) responded to the survey. The majority of the participants described their respective programs as 2 years in duration (59% for fellows, 56% for graduates, and 85% for PDs). Most programs (85%) were based at children’s hospitals. Most graduates (84%) practiced in a children’s hospital, and 12% of them practiced in a community site or a combination of sites.
Both fellows and graduates reported limited involvement in several aspects of TM prior to fellowship. Fellows’ interest in completing a TM rotation during fellowship is greater than the interest as perceived by PDs (3.03+1.00 vs. 2.38+1.19, P = .061). Prior TM exposure in residency or perceived proficiency in TM was not associated with lack of interest. Twenty-five percent of graduates completed a TM rotation during PHM fellowship. Many graduates agreed (41%) or strongly agreed (16%) with the statement “I recommend participating in a TM rotation during PHM fellowship.” Graduates who had completed a TM rotation were more likely to agree with this statement (P = .001).
There were similarities between reservations about participating in a TM rotation among fellows and barriers identified by graduates and PDs (Table). However, no graduates cited lack of relevance to a career in PHM as a barrier to participation in a TM rotation. Fellows, graduates, and PDs reported concordant responses regarding the prioritization of learning objectives for a TM rotation (Table). Both graduates and PDs ranked active learning strategies, such as direct patient care and simulation, as the most effective methods for teaching TM.
Discordance was noted between how frequently fellows participated in aspects of TM during fellowship and graduates’ current practice of PHM (Figure). With regard to select TM-related PHM core competencies, such as respiratory failure, shock, and leading a healthcare team, most (63%–90%, depending on the competency) fellows perceived themselves as “competent” prior to the start of the fellowship. Nevertheless, more than 70% of fellows remained very or extremely interested in gaining additional experience in each competency during fellowship.
DISCUSSION
Survey respondents demonstrate variable levels of interest and engagement in TM training; in particular, fellows and graduates often reported greater interest and value in a TM rotation than PDs. Similar to fellows in related fields,10 PHM fellows and graduates selected clinical topics as the most essential elements of TM training. In accordance with the literature, our findings suggest that direct patient care, one-on-one instruction, and simulation would be appropriate and popular methods for delivering this type of educational content.10,11
Curriculum design for a TM rotation should reinforce clinical PHM competencies related to TM while focusing on topics that are specific to the transport environment, such as methods of interfacility transport, handoffs, transitions of care, and team leadership.2,7,12 Trainee comfort level with different forms of transport (eg, fear of flying, motion sickness) and local and state policies regarding interfacility transfer should also be considered. In addition, fellows could engage in clinical research and quality improvement projects related to TM given the overall paucity of literature in the field.13
Several reasons can explain why fellows and graduates place a greater value on a TM rotation than PDs. Fellows and graduates may perceive inherent value in gaining particular knowledge and skills, such as greater understanding of the logistics and personnel involved in transferring patients and experience working with a healthcare team in a unique and dynamic setting.3,10,14
PDs may not be aware of the extent of participation in elements of transport among graduates. A recent workforce survey of pediatric interfacility transport systems indicated that although medical directors are from the fields of emergency, critical care, and neonatal medicine, 20% of medical control physicians are pediatric hospitalists.4 Given that the majority of PHM fellowships are based at children’s hospitals and transport teams are often associated with intensive care or emergency medicine units, PDs may have limited exposure to transport systems that incorporate hospitalists.
Pediatric hospitalists at all practice sites must have clinical and systems skills related to TM. However, the scope of practice for those working at community sites may be more likely to include distinct elements of TM.6 Currently, most fellowship graduates work at free-standing children’s or university-affiliated hospitals and have pursued careers in academic medicine.15 As the field evolves, the number of fellowship-trained pediatric hospitalists working at community sites may increase, making the acquisition of skills relevant to TM during fellowship training more crucial.
This study has several limitations. We attempted to identify all recent PHM fellowship graduates, but sampling bias may exist. Response bias may have been introduced by the self-reporting of skill and proficiency as well as by the small sample size and response rate for some stakeholder groups. The latter may be exacerbated by the fact that we do not have data on the degree or distribution of program representation among the fellow and graduate groups, given the lack of identifying information collected. Finally, we did not collect specific information about existing TM curricula in PHM fellowships.
We report a variable level of interest and engagement in TM among fellowship stakeholders, even though “Transport of the Critically Ill Child” is a PHM Core Competency. Fellows are interested in TM but unsure of its relevance to a PHM career. Graduates support the acquisition of transport skills during fellowship training.
ACKNOWLEDGMENTS
The authors would like to thank Tony Woodward, MD for reviewing the survey tools; Sheree Schrager, PhD and Margaret Trost, MD for their valuable insights into the results; and Grant Christman, MD for reviewing the manuscript.
Disclosures
The authors declare no potential conflicts of interest.
Funding
No funding was secured for this study.
1. Insoft RM, Schwartz HP, Romito J. Guidelines for Air and Ground Transport of Neonatal and Pediatric Patients., 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2016.
2. Rosenthal JL, Romano PS, Kokroko J, Gu W, Okumura MJ. Profiling interfacility transfers for hospitalized pediatric patients. Hosp Pediatr. 2017;7(6):335-343. PubMed
3. Kline-Krammes S, Wheeler DS, Schwartz HP, Forbes M, Bigham MT. Missed opportunities during pediatric residency training. Report of a 10-year follow-up survey in critical care transport medicine. Pediatr Emerg Care. 2012;28(1):1-5. PubMed
4. Tanem J, Triscari D, Chan M, Meyer MT. Workforce survey of pediatric interfacility transport systems in the United States. Pediatr Emer Care. 2016;32(6):364-370. PubMed
5. Freed GL, Dunham KM. Pediatric hospitalists: training, current practice, and career goals. J Hosp Med. 2009;4(3):179-186. PubMed
6. Roberts KB. Pediatric hospitalists in community hospitals: hospital-based generalists with expanded roles. Hosp Pediatr. 2015;5(5):290-292. PubMed
7. Stucky ER, Maniscalco J, Ottolini MC, et al. The Pediatric Hospital Medicine Core Competencies Supplement: a Framework for Curriculum Development by the Society of Hospital Medicine with acknowledgement to pediatric hospitalists from the American Academy of Pediatrics and the Academic Pediatric Association. J Hosp Med. 2010;5(suppl 2):i-xv, 1-114. PubMed
8. Jerardi KE, Fisher E, Rassbach C, et al. Development of a Curricular Framework for Pediatric Hospital Medicine Fellowships. Pediatrics. 2017;140(1):1-8. PubMed
9. Shah NH, Rhim HJH, Maniscalco J, Wilson K, Rassbach C. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11(5):324-328. PubMed
10. Mickells GE, Goodman DM, Rozenfeld RA. Education of pediatric subspecialty fellows in transport medicine: a national survey. BMC Pediatrics. 2017;17(1):13. PubMed
11. Cross B, Wilson D. High-fidelity simulation for transport team training and competency evaluation. Newborn Inf Nurs Rev. 2009;9(4):200-206.
12. Weingart C, Herstich T, Baker P, et al. Making good better: implementing a standardized handoff in pediatric transport. Air Med J. 2013;32(1):40-46. PubMed
13. Kandil SB, Sanford HA, Northrup V, Bigham MT, Giuliano Jr. JS. Transport disposition using transport risk assessment in pediatrics (TRAP) score. Prehosp Emerg Care. 2012;16(3):366-373. PubMed
14. Giardino AP, Tran XG, King J, Giardino ER, Woodward GA, Durbin DR. A longitudinal view of resident education in pediatric emergency interhospital transport. Pediatr Emerg Care. 2010;26(9):653-658. PubMed
15. Oshimurua JM, Bauer BD, Shah N, Nguyen N, Maniscalco J. Current roles and perceived needs of pediatric hospital medicine fellowship graduates. Hosp Pediatr. 2016;6(10):633-637 PubMed
Transport medicine (TM) involves the provision of care to patients who require transfer to a healthcare facility that can deliver definitive treatment.1 Pediatric interfacility transport occurs in approximately 10% of nonneonatal, nonpregnancy pediatric hospitalizations in the United States.2 Studies document a decline in resident participation in pediatric transports and variability in curricular content.3,4
The Pediatric Hospital Medicine (PHM) Core Competencies include “Transport of the Critically Ill Child.”7 Additionally, the Curriculum Committee of the PHM Fellowship Directors Council proposed a curricular framework that includes a required clinical experience in “Care and Stabilization of the Critically Ill Child,”8 which can occur in a variety of practice settings, including TM. TM is also listed as a potential elective rotation.
In 2014, 60% of PHM fellowships included a required or optional TM rotation.9 A recent study of pediatric emergency, critical care, and neonatal medicine fellowships revealed a paucity of formal or published TM curricula in these programs.10 Furthermore, no standard or published TM curricula have been established for PHM fellowships. The primary objective of our study is to determine attitudes regarding TM training among PHM fellows, recent PHM fellowship graduates, and PHM fellowship program directors (PDs). The secondary objective is to identify how the perspectives of these fellowship stakeholders could influence the design of a TM curriculum.
METHODS
This cross-sectional study focused on 3 stakeholder groups related to PHM fellowships. The subjects included in the study were physicians enrolled in a PHM fellowship (fellow) during the 2015-2016 academic year, graduates of fellowship (graduate) between 2010 and 2015, and fellowship program directors (PD). Unique web-based, anonymous surveys for each group were developed, reviewed by content and methodology experts, and piloted with local pediatric hospitalists. Surveys consisted of unfolding multiple-choice questions and ranking items along Likert scales and the Dreyfus model.
Questions were designed to elicit demographic data, perspectives, and experience related to TM education in PHM fellowships across all respondent groups. Depending on the context, identical or similar questions were asked among the groups. For example, all groups were asked to prioritize learning objectives for a TM rotation. Graduates and PDs reported the most effective teaching methods for use during a TM rotation. Fellows rated their own interest in a TM elective, and PDs were asked to rate the level of interest among their fellows.
Participant contact information was obtained from a website (phmfellows.org) and databases of fellows and graduates, which are maintained by the PHM Fellowship Directors Council (personal communication, Jayne Truckenbrod, DO; February 2, 2017). Between February and April 2016, the participants were individually emailed a link to their respective surveys, and 3 reminder e-mails were sent to nonresponders. The survey was administered through SurveyMonkey (www.surveymonkey.com).
SPSS (IBM SPSS Statistics, IBM Corporation, Armonk, New York) was used for statistical analysis. Descriptive data were presented using mean and standard deviation. Comparisons among fellows, graduates, and PDs were conducted using one-way analyses of variance or Mann-Whitney U test. Frequency of application and self-evaluation of core competency skills before and after the rotation were evaluated using paired sample t-tests. The study protocol was deemed exempt from review by our local Institutional Review Board.
RESULTS
Forty of 70 (57%) fellows, 32 of 87 graduates (37%), and 14 of 32 PDs (44%) responded to the survey. The majority of the participants described their respective programs as 2 years in duration (59% for fellows, 56% for graduates, and 85% for PDs). Most programs (85%) were based at children’s hospitals. Most graduates (84%) practiced in a children’s hospital, and 12% of them practiced in a community site or a combination of sites.
Both fellows and graduates reported limited involvement in several aspects of TM prior to fellowship. Fellows’ interest in completing a TM rotation during fellowship is greater than the interest as perceived by PDs (3.03+1.00 vs. 2.38+1.19, P = .061). Prior TM exposure in residency or perceived proficiency in TM was not associated with lack of interest. Twenty-five percent of graduates completed a TM rotation during PHM fellowship. Many graduates agreed (41%) or strongly agreed (16%) with the statement “I recommend participating in a TM rotation during PHM fellowship.” Graduates who had completed a TM rotation were more likely to agree with this statement (P = .001).
There were similarities between reservations about participating in a TM rotation among fellows and barriers identified by graduates and PDs (Table). However, no graduates cited lack of relevance to a career in PHM as a barrier to participation in a TM rotation. Fellows, graduates, and PDs reported concordant responses regarding the prioritization of learning objectives for a TM rotation (Table). Both graduates and PDs ranked active learning strategies, such as direct patient care and simulation, as the most effective methods for teaching TM.
Discordance was noted between how frequently fellows participated in aspects of TM during fellowship and graduates’ current practice of PHM (Figure). With regard to select TM-related PHM core competencies, such as respiratory failure, shock, and leading a healthcare team, most (63%–90%, depending on the competency) fellows perceived themselves as “competent” prior to the start of the fellowship. Nevertheless, more than 70% of fellows remained very or extremely interested in gaining additional experience in each competency during fellowship.
DISCUSSION
Survey respondents demonstrate variable levels of interest and engagement in TM training; in particular, fellows and graduates often reported greater interest and value in a TM rotation than PDs. Similar to fellows in related fields,10 PHM fellows and graduates selected clinical topics as the most essential elements of TM training. In accordance with the literature, our findings suggest that direct patient care, one-on-one instruction, and simulation would be appropriate and popular methods for delivering this type of educational content.10,11
Curriculum design for a TM rotation should reinforce clinical PHM competencies related to TM while focusing on topics that are specific to the transport environment, such as methods of interfacility transport, handoffs, transitions of care, and team leadership.2,7,12 Trainee comfort level with different forms of transport (eg, fear of flying, motion sickness) and local and state policies regarding interfacility transfer should also be considered. In addition, fellows could engage in clinical research and quality improvement projects related to TM given the overall paucity of literature in the field.13
Several reasons can explain why fellows and graduates place a greater value on a TM rotation than PDs. Fellows and graduates may perceive inherent value in gaining particular knowledge and skills, such as greater understanding of the logistics and personnel involved in transferring patients and experience working with a healthcare team in a unique and dynamic setting.3,10,14
PDs may not be aware of the extent of participation in elements of transport among graduates. A recent workforce survey of pediatric interfacility transport systems indicated that although medical directors are from the fields of emergency, critical care, and neonatal medicine, 20% of medical control physicians are pediatric hospitalists.4 Given that the majority of PHM fellowships are based at children’s hospitals and transport teams are often associated with intensive care or emergency medicine units, PDs may have limited exposure to transport systems that incorporate hospitalists.
Pediatric hospitalists at all practice sites must have clinical and systems skills related to TM. However, the scope of practice for those working at community sites may be more likely to include distinct elements of TM.6 Currently, most fellowship graduates work at free-standing children’s or university-affiliated hospitals and have pursued careers in academic medicine.15 As the field evolves, the number of fellowship-trained pediatric hospitalists working at community sites may increase, making the acquisition of skills relevant to TM during fellowship training more crucial.
This study has several limitations. We attempted to identify all recent PHM fellowship graduates, but sampling bias may exist. Response bias may have been introduced by the self-reporting of skill and proficiency as well as by the small sample size and response rate for some stakeholder groups. The latter may be exacerbated by the fact that we do not have data on the degree or distribution of program representation among the fellow and graduate groups, given the lack of identifying information collected. Finally, we did not collect specific information about existing TM curricula in PHM fellowships.
We report a variable level of interest and engagement in TM among fellowship stakeholders, even though “Transport of the Critically Ill Child” is a PHM Core Competency. Fellows are interested in TM but unsure of its relevance to a PHM career. Graduates support the acquisition of transport skills during fellowship training.
ACKNOWLEDGMENTS
The authors would like to thank Tony Woodward, MD for reviewing the survey tools; Sheree Schrager, PhD and Margaret Trost, MD for their valuable insights into the results; and Grant Christman, MD for reviewing the manuscript.
Disclosures
The authors declare no potential conflicts of interest.
Funding
No funding was secured for this study.
Transport medicine (TM) involves the provision of care to patients who require transfer to a healthcare facility that can deliver definitive treatment.1 Pediatric interfacility transport occurs in approximately 10% of nonneonatal, nonpregnancy pediatric hospitalizations in the United States.2 Studies document a decline in resident participation in pediatric transports and variability in curricular content.3,4
The Pediatric Hospital Medicine (PHM) Core Competencies include “Transport of the Critically Ill Child.”7 Additionally, the Curriculum Committee of the PHM Fellowship Directors Council proposed a curricular framework that includes a required clinical experience in “Care and Stabilization of the Critically Ill Child,”8 which can occur in a variety of practice settings, including TM. TM is also listed as a potential elective rotation.
In 2014, 60% of PHM fellowships included a required or optional TM rotation.9 A recent study of pediatric emergency, critical care, and neonatal medicine fellowships revealed a paucity of formal or published TM curricula in these programs.10 Furthermore, no standard or published TM curricula have been established for PHM fellowships. The primary objective of our study is to determine attitudes regarding TM training among PHM fellows, recent PHM fellowship graduates, and PHM fellowship program directors (PDs). The secondary objective is to identify how the perspectives of these fellowship stakeholders could influence the design of a TM curriculum.
METHODS
This cross-sectional study focused on 3 stakeholder groups related to PHM fellowships. The subjects included in the study were physicians enrolled in a PHM fellowship (fellow) during the 2015-2016 academic year, graduates of fellowship (graduate) between 2010 and 2015, and fellowship program directors (PD). Unique web-based, anonymous surveys for each group were developed, reviewed by content and methodology experts, and piloted with local pediatric hospitalists. Surveys consisted of unfolding multiple-choice questions and ranking items along Likert scales and the Dreyfus model.
Questions were designed to elicit demographic data, perspectives, and experience related to TM education in PHM fellowships across all respondent groups. Depending on the context, identical or similar questions were asked among the groups. For example, all groups were asked to prioritize learning objectives for a TM rotation. Graduates and PDs reported the most effective teaching methods for use during a TM rotation. Fellows rated their own interest in a TM elective, and PDs were asked to rate the level of interest among their fellows.
Participant contact information was obtained from a website (phmfellows.org) and databases of fellows and graduates, which are maintained by the PHM Fellowship Directors Council (personal communication, Jayne Truckenbrod, DO; February 2, 2017). Between February and April 2016, the participants were individually emailed a link to their respective surveys, and 3 reminder e-mails were sent to nonresponders. The survey was administered through SurveyMonkey (www.surveymonkey.com).
SPSS (IBM SPSS Statistics, IBM Corporation, Armonk, New York) was used for statistical analysis. Descriptive data were presented using mean and standard deviation. Comparisons among fellows, graduates, and PDs were conducted using one-way analyses of variance or Mann-Whitney U test. Frequency of application and self-evaluation of core competency skills before and after the rotation were evaluated using paired sample t-tests. The study protocol was deemed exempt from review by our local Institutional Review Board.
RESULTS
Forty of 70 (57%) fellows, 32 of 87 graduates (37%), and 14 of 32 PDs (44%) responded to the survey. The majority of the participants described their respective programs as 2 years in duration (59% for fellows, 56% for graduates, and 85% for PDs). Most programs (85%) were based at children’s hospitals. Most graduates (84%) practiced in a children’s hospital, and 12% of them practiced in a community site or a combination of sites.
Both fellows and graduates reported limited involvement in several aspects of TM prior to fellowship. Fellows’ interest in completing a TM rotation during fellowship is greater than the interest as perceived by PDs (3.03+1.00 vs. 2.38+1.19, P = .061). Prior TM exposure in residency or perceived proficiency in TM was not associated with lack of interest. Twenty-five percent of graduates completed a TM rotation during PHM fellowship. Many graduates agreed (41%) or strongly agreed (16%) with the statement “I recommend participating in a TM rotation during PHM fellowship.” Graduates who had completed a TM rotation were more likely to agree with this statement (P = .001).
There were similarities between reservations about participating in a TM rotation among fellows and barriers identified by graduates and PDs (Table). However, no graduates cited lack of relevance to a career in PHM as a barrier to participation in a TM rotation. Fellows, graduates, and PDs reported concordant responses regarding the prioritization of learning objectives for a TM rotation (Table). Both graduates and PDs ranked active learning strategies, such as direct patient care and simulation, as the most effective methods for teaching TM.
Discordance was noted between how frequently fellows participated in aspects of TM during fellowship and graduates’ current practice of PHM (Figure). With regard to select TM-related PHM core competencies, such as respiratory failure, shock, and leading a healthcare team, most (63%–90%, depending on the competency) fellows perceived themselves as “competent” prior to the start of the fellowship. Nevertheless, more than 70% of fellows remained very or extremely interested in gaining additional experience in each competency during fellowship.
DISCUSSION
Survey respondents demonstrate variable levels of interest and engagement in TM training; in particular, fellows and graduates often reported greater interest and value in a TM rotation than PDs. Similar to fellows in related fields,10 PHM fellows and graduates selected clinical topics as the most essential elements of TM training. In accordance with the literature, our findings suggest that direct patient care, one-on-one instruction, and simulation would be appropriate and popular methods for delivering this type of educational content.10,11
Curriculum design for a TM rotation should reinforce clinical PHM competencies related to TM while focusing on topics that are specific to the transport environment, such as methods of interfacility transport, handoffs, transitions of care, and team leadership.2,7,12 Trainee comfort level with different forms of transport (eg, fear of flying, motion sickness) and local and state policies regarding interfacility transfer should also be considered. In addition, fellows could engage in clinical research and quality improvement projects related to TM given the overall paucity of literature in the field.13
Several reasons can explain why fellows and graduates place a greater value on a TM rotation than PDs. Fellows and graduates may perceive inherent value in gaining particular knowledge and skills, such as greater understanding of the logistics and personnel involved in transferring patients and experience working with a healthcare team in a unique and dynamic setting.3,10,14
PDs may not be aware of the extent of participation in elements of transport among graduates. A recent workforce survey of pediatric interfacility transport systems indicated that although medical directors are from the fields of emergency, critical care, and neonatal medicine, 20% of medical control physicians are pediatric hospitalists.4 Given that the majority of PHM fellowships are based at children’s hospitals and transport teams are often associated with intensive care or emergency medicine units, PDs may have limited exposure to transport systems that incorporate hospitalists.
Pediatric hospitalists at all practice sites must have clinical and systems skills related to TM. However, the scope of practice for those working at community sites may be more likely to include distinct elements of TM.6 Currently, most fellowship graduates work at free-standing children’s or university-affiliated hospitals and have pursued careers in academic medicine.15 As the field evolves, the number of fellowship-trained pediatric hospitalists working at community sites may increase, making the acquisition of skills relevant to TM during fellowship training more crucial.
This study has several limitations. We attempted to identify all recent PHM fellowship graduates, but sampling bias may exist. Response bias may have been introduced by the self-reporting of skill and proficiency as well as by the small sample size and response rate for some stakeholder groups. The latter may be exacerbated by the fact that we do not have data on the degree or distribution of program representation among the fellow and graduate groups, given the lack of identifying information collected. Finally, we did not collect specific information about existing TM curricula in PHM fellowships.
We report a variable level of interest and engagement in TM among fellowship stakeholders, even though “Transport of the Critically Ill Child” is a PHM Core Competency. Fellows are interested in TM but unsure of its relevance to a PHM career. Graduates support the acquisition of transport skills during fellowship training.
ACKNOWLEDGMENTS
The authors would like to thank Tony Woodward, MD for reviewing the survey tools; Sheree Schrager, PhD and Margaret Trost, MD for their valuable insights into the results; and Grant Christman, MD for reviewing the manuscript.
Disclosures
The authors declare no potential conflicts of interest.
Funding
No funding was secured for this study.
1. Insoft RM, Schwartz HP, Romito J. Guidelines for Air and Ground Transport of Neonatal and Pediatric Patients., 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2016.
2. Rosenthal JL, Romano PS, Kokroko J, Gu W, Okumura MJ. Profiling interfacility transfers for hospitalized pediatric patients. Hosp Pediatr. 2017;7(6):335-343. PubMed
3. Kline-Krammes S, Wheeler DS, Schwartz HP, Forbes M, Bigham MT. Missed opportunities during pediatric residency training. Report of a 10-year follow-up survey in critical care transport medicine. Pediatr Emerg Care. 2012;28(1):1-5. PubMed
4. Tanem J, Triscari D, Chan M, Meyer MT. Workforce survey of pediatric interfacility transport systems in the United States. Pediatr Emer Care. 2016;32(6):364-370. PubMed
5. Freed GL, Dunham KM. Pediatric hospitalists: training, current practice, and career goals. J Hosp Med. 2009;4(3):179-186. PubMed
6. Roberts KB. Pediatric hospitalists in community hospitals: hospital-based generalists with expanded roles. Hosp Pediatr. 2015;5(5):290-292. PubMed
7. Stucky ER, Maniscalco J, Ottolini MC, et al. The Pediatric Hospital Medicine Core Competencies Supplement: a Framework for Curriculum Development by the Society of Hospital Medicine with acknowledgement to pediatric hospitalists from the American Academy of Pediatrics and the Academic Pediatric Association. J Hosp Med. 2010;5(suppl 2):i-xv, 1-114. PubMed
8. Jerardi KE, Fisher E, Rassbach C, et al. Development of a Curricular Framework for Pediatric Hospital Medicine Fellowships. Pediatrics. 2017;140(1):1-8. PubMed
9. Shah NH, Rhim HJH, Maniscalco J, Wilson K, Rassbach C. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11(5):324-328. PubMed
10. Mickells GE, Goodman DM, Rozenfeld RA. Education of pediatric subspecialty fellows in transport medicine: a national survey. BMC Pediatrics. 2017;17(1):13. PubMed
11. Cross B, Wilson D. High-fidelity simulation for transport team training and competency evaluation. Newborn Inf Nurs Rev. 2009;9(4):200-206.
12. Weingart C, Herstich T, Baker P, et al. Making good better: implementing a standardized handoff in pediatric transport. Air Med J. 2013;32(1):40-46. PubMed
13. Kandil SB, Sanford HA, Northrup V, Bigham MT, Giuliano Jr. JS. Transport disposition using transport risk assessment in pediatrics (TRAP) score. Prehosp Emerg Care. 2012;16(3):366-373. PubMed
14. Giardino AP, Tran XG, King J, Giardino ER, Woodward GA, Durbin DR. A longitudinal view of resident education in pediatric emergency interhospital transport. Pediatr Emerg Care. 2010;26(9):653-658. PubMed
15. Oshimurua JM, Bauer BD, Shah N, Nguyen N, Maniscalco J. Current roles and perceived needs of pediatric hospital medicine fellowship graduates. Hosp Pediatr. 2016;6(10):633-637 PubMed
1. Insoft RM, Schwartz HP, Romito J. Guidelines for Air and Ground Transport of Neonatal and Pediatric Patients., 4th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2016.
2. Rosenthal JL, Romano PS, Kokroko J, Gu W, Okumura MJ. Profiling interfacility transfers for hospitalized pediatric patients. Hosp Pediatr. 2017;7(6):335-343. PubMed
3. Kline-Krammes S, Wheeler DS, Schwartz HP, Forbes M, Bigham MT. Missed opportunities during pediatric residency training. Report of a 10-year follow-up survey in critical care transport medicine. Pediatr Emerg Care. 2012;28(1):1-5. PubMed
4. Tanem J, Triscari D, Chan M, Meyer MT. Workforce survey of pediatric interfacility transport systems in the United States. Pediatr Emer Care. 2016;32(6):364-370. PubMed
5. Freed GL, Dunham KM. Pediatric hospitalists: training, current practice, and career goals. J Hosp Med. 2009;4(3):179-186. PubMed
6. Roberts KB. Pediatric hospitalists in community hospitals: hospital-based generalists with expanded roles. Hosp Pediatr. 2015;5(5):290-292. PubMed
7. Stucky ER, Maniscalco J, Ottolini MC, et al. The Pediatric Hospital Medicine Core Competencies Supplement: a Framework for Curriculum Development by the Society of Hospital Medicine with acknowledgement to pediatric hospitalists from the American Academy of Pediatrics and the Academic Pediatric Association. J Hosp Med. 2010;5(suppl 2):i-xv, 1-114. PubMed
8. Jerardi KE, Fisher E, Rassbach C, et al. Development of a Curricular Framework for Pediatric Hospital Medicine Fellowships. Pediatrics. 2017;140(1):1-8. PubMed
9. Shah NH, Rhim HJH, Maniscalco J, Wilson K, Rassbach C. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11(5):324-328. PubMed
10. Mickells GE, Goodman DM, Rozenfeld RA. Education of pediatric subspecialty fellows in transport medicine: a national survey. BMC Pediatrics. 2017;17(1):13. PubMed
11. Cross B, Wilson D. High-fidelity simulation for transport team training and competency evaluation. Newborn Inf Nurs Rev. 2009;9(4):200-206.
12. Weingart C, Herstich T, Baker P, et al. Making good better: implementing a standardized handoff in pediatric transport. Air Med J. 2013;32(1):40-46. PubMed
13. Kandil SB, Sanford HA, Northrup V, Bigham MT, Giuliano Jr. JS. Transport disposition using transport risk assessment in pediatrics (TRAP) score. Prehosp Emerg Care. 2012;16(3):366-373. PubMed
14. Giardino AP, Tran XG, King J, Giardino ER, Woodward GA, Durbin DR. A longitudinal view of resident education in pediatric emergency interhospital transport. Pediatr Emerg Care. 2010;26(9):653-658. PubMed
15. Oshimurua JM, Bauer BD, Shah N, Nguyen N, Maniscalco J. Current roles and perceived needs of pediatric hospital medicine fellowship graduates. Hosp Pediatr. 2016;6(10):633-637 PubMed
© 2018 Society of Hospital Medicine