User login
Combine these screening tools to detect bipolar depression
THE CASE
A 35-year-old police officer visited his family physician (FP) with complaints of low energy, trouble sleeping, a lack of enjoyment in life, and feelings of hopelessness that have persisted for several months. He was worried about the impact they were having on his marriage and work. He had not experienced suicidal thoughts. His Patient Health Questionnaire (PHQ9) score was 18 (moderately severe depression). He had been seen intermittently for similar complaints and had tried several medications (fluoxetine, bupropion, and citalopram) without much effect. He was taking no medications now other than an over-the-counter multivitamin. He had one brother with anxiety and depression. He said his marriage counselor expressed concerns that he might have bipolar disorder or borderline personality disorder.
How would you proceed with this patient?
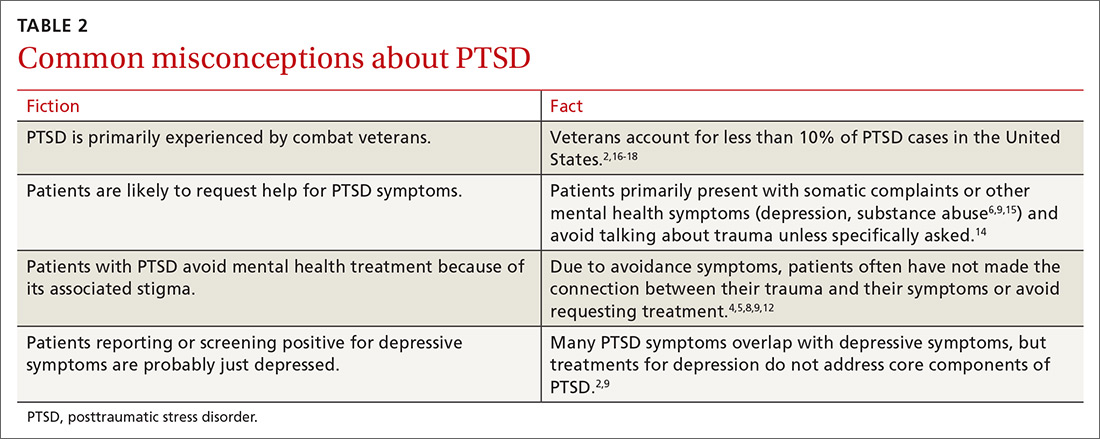
The prevalence of a spectrum of bipolarity in the community has been shown to be 6.4%.1 Depressive episodes predominate in bipolar disorder (BPD),2 with patients spending less time in manic or hypomanic states.3 Not surprisingly, then, depressive episodes are the most common presentation of BPD.
The depressive symptoms of BPD and unipolar depression, or major depressive disorder (MDD), are similar, making it difficult to distinguish between the disorders.3 As a result, BPD is often misdiagnosed as MDD.4,5 Zimmerman et al point out that “bipolar disorder is prone to being overlooked because its diagnosis is more often based on retrospective report rather than presenting symptoms of mania or hypomania assessment.”6
Accurately recognizing BPD is essential in selecting effective treatment. It’s estimated that approximately one-third of patients given antidepressants for major depression show no treatment response,7 possibly due in part to undiagnosed BPD being more prevalent than previously thought.4,8 Failure to distinguish between depressive episodes of BPD and MDD before prescribing medication introduces the risk of ineffective or suboptimal treatment. Inappropriate treatment can worsen or destabilize the course of bipolar illness by, for instance, inducing rapid cycling or, less commonly, manic symptoms.
Screen for BPD when depressive symptoms are present
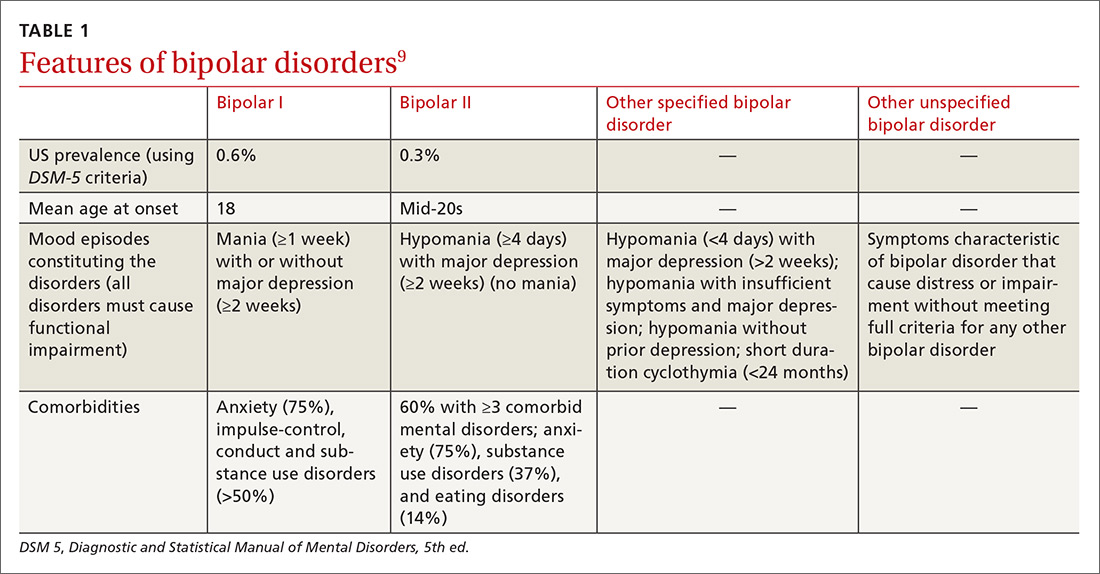
Identifying BPD in a patient with current or past depressive symptoms requires screening for manic, hypomanic, and mixed episodes (TABLE 19). Two brief, complementary screening tools — the Mood Disorder Questionnaire (MDQ) and the 9-item PHQ9—are helpful in this assessment. Both questionnaires (TABLE 28,10-14) can be conveniently completed by the patient in the waiting room or with staff assistance before the physician encounter.
The MDQ screen is for past/lifetime or current manic/hypomanic symptoms (https://www.integration.samhsa.gov/images/res/MDQ.pdf). A positive screen requires answering “Yes” to at least 7 of the 13 items on question 1, answering “yes” on question 2, and answering “moderate problems” or “serious problems” on question 3.
Continue to: The PHQ9 screens for...
The PHQ9 screens for current depressive symptoms/episodes (https://www.uspreventiveservicestaskforce.org/Home/GetFileByID/218).
The value of combining the MDQ and PHQ9. The PHQ9 screens for and assesses the severity of depressive episodes along with clinician assessment, but it cannot distinguish between depressive episodes of MDD or BPD. A brief instrument, such as MDQ, screens for current or past manic or hypomanic symptoms, which, when combined with the clinical interview and patient history, enables detection of BPD if present and avoids erroneously assigning depressive symptoms to MDD.
One cross-sectional study found that the combined MDQ and PHQ9 questionnaires have a higher sensitivity in detecting mood disorder than does routine assessment by general practitioners (0.8 [95% confidence interval (CI), 0.71-0.81] vs 0.2 [95% CI, 0.12- 0.25]) and without loss of specificity (0.9 [95% CI, 0.86-0.96] vs 0.9 [95% CI, 0.88-0.97]).15 In this same study, using a structured clinical interview for DSM-III-R Axis I Disorders (SCID-I) as the gold standard, researchers also found the screening tools to be more accurate (Cohen’s Kappa 0.7 [SE=0.05; 95% CI, 0.5-0.7]) than the general practitioner assessment (Cohen’s Kappa 0.2 [SE=0.07 (95% CI, 0.12-0.27]).15
Delve deeper with a patient interview
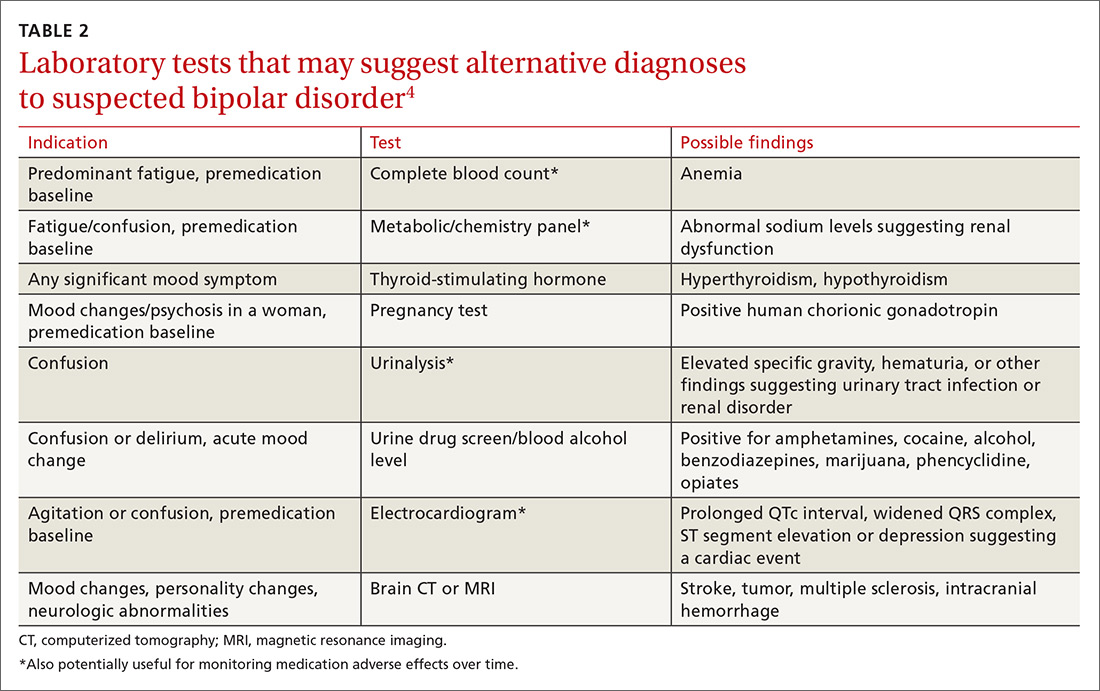
Use targeted questions and laboratory tests to rule out other possible causes of depressed mood, such as substance abuse or medical conditions (eg, hypothyroidism). Keep in mind that even when MDD or BPD is present, other medical disorders or substance abuse could be coexistent. Also ask about a personal or family psychiatric history and assess for suicidality. If family members are available, they may be able to help in identifying the patient’s age when symptoms first appeared or in adding information about the affective episode or behavior that the patient may not recollect.
Beyond a history of manic, hypomanic, or mixed episodes, other symptoms and features may assist in distinguishing between bipolar and unipolar depression or in helping the clinician identify depressed patients who may be at higher risk for, or have, BPD. One meta-analysis of 3 multicenter clinical trials assessed sociodemographic factors and clinical features of BPD compared with unipolar depression. The average age of onset of mood symptoms in individuals with BPD was significantly younger (21.2 years) than that of patients with MDD (29.7 years).16 Another study found that patients with either bipolar I or bipolar II similarly experienced their first mood disorder episode 10 years earlier than those with MDD.17
Continue to: BPD is often associated with...
BPD is often associated with more frequent depressive episodes and a higher number of depressive symptoms per episode than is MDD, as well as more frequent family psychiatric histories (especially of mood disorders), anxiety disorders, alcohol and drug use disorders, and personality disorders.17 Other factors more closely associated with BPD than MDD include atypical features such as hypersomnia and psychomotor retardation, psychotic symptoms during the depressive episode, and more frequent recurrences of depressive episodes.18-22 Also, depressive episodes during the postpartum period indicate a higher risk of BPD than do episodes in women outside the postpartum period, with a hazard ratio (HR) of 1.66 (95% CI, 1.12-2.48).23 The risk is much greater when postpartum depressive episodes are associated with anxiety symptoms (HR=10.15; 95% CI, 7.13-14.46).23
Final thoughts
Increased awareness and screening for BPD in primary care—where most individuals with depressive symptoms are first encountered—should lead to more accurate diagnoses and decrease the years-long gaps between symptom onset and detection of BPD,4,5 thereby improving treatment and patient outcomes. Still, some cases of BPD may be difficult to recognize—particularly patients who present predominantly with depression with past irritability and other hypomanic symptoms (but not euphoria).
A positive MDQ screen should also prompt, if possible, a more detailed clinical interview by a mental health care professional, particularly if there is uncertainty about the diagnosis. Complex cases of BPD may require the expertise of a psychiatrist.
THE CASE
The patient’s FP referred him to a psychiatrist colleague, whose inquiry also revealed low mood, anhedonia, hopelessness, difficulty sleeping, low energy, poor appetite, guilt, poor concentration, and psychomotor retardation. The patient had experienced multiple depressive episodes over the past 20 years. Significant interpersonal conflicts frequently triggered his depressive episodes, which were accompanied by mood irritability, racing thoughts, distractibility, increased libido, excessive spending, increased energy, and engagement in risky behaviors.
The patient’s score on the MDQ administered by the psychiatrist was positive, with 7 points on question 1. He also had posttraumatic symptoms related to his police work, which were not the main reason for the visit. He had been divorced 3 times. In prior manic episodes, he had not displayed euphoria, grandiosity, psychotic symptoms, or anxiety, but rather irritability with other manic symptoms.
Continue to: Based on his MDQ results...
Based on his MDQ results, the clinical interview, and current episode with mixed features, the patient was given a diagnosis of bipolar II disorder. The psychiatrist prescribed divalproex 500 mg at bedtime and scheduled a return visit with a plan for further laboratory monitoring and up-titration if needed. He was also encouraged to follow up with his FP.
CORRESPONDENCE
Nagy A. Youssef, MD, Medical College of Georgia at Augusta University, 997 St. Sebastian Way, Augusta, GA 30912; nyoussef@augusta.edu.
SUPPORT AND ACKNOWLEDGMENT
Dr. Youssef’s work on this paper was supported by the Office of Academic Affairs, Medical College of Georgia at Augusta University. We thank Mark Yassa, BS, for his assistance in editing.
1. Judd LL, Akiskal HS. The prevalence and disability of bipolar spectrum disorders in the US population: re-analysis of the ECA database taking into account subthreshold cases. J Affect Disord. 2003;73:123-131.
2. Yatham LN, Lecrubier Y, Fieve RR, et al. Quality of life in patients with bipolar I depression: data from 920 patients. Bipolar Disord. 2004;6:379-385.
3. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530-537.
4. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord. 1999;52:135-144.
5. Cha B, Kim JH, Ha TH, et al. Polarity of the first episode and time to diagnosis of bipolar I disorder. Psychiatry Investig. 2009;6:96-101. Available at: http://psychiatryinvestigation.org/journal/view.php?doi=10.4306/pi.2009.6.2.96. Accessed June 25, 2018.
6. Zimmerman M, Galione JN, Chelminski I, et al. Psychiatric diagnoses in patients who screen positive on the Mood Disorder Questionnaire: implications for using the scale as a case-finding instrument for bipolar disorder. Psychiatry Res. 2011;185:444-449.
7. Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369-388.
8. Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Pract. 2005;18:233-239.
9. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, D.C.: American Psychiatric Publishing. 2013.
10. Poon Y, Chung KF, Tso KC, et al. The use of Mood Disorder Questionnaire, Hypomania Checklist-32 and clinical predictors for screening previously unrecognised bipolar disorder in a general psychiatric setting. Psychiatry Res. 2012;195:111-117.
11. Gilbody S, Richards D, Brealey S, et al. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med. 2007;22:1596-1602.
12. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
13. Miller CJ, Klugman J, Berv DA, et al. Sensitivity and specificity of the Mood Disorder Questionnaire for detecting bipolar disorder. J Affect Disord. 2004;81:167-171.
14. Sasdelli A, Lia L, Luciano CC, et al. Screening for bipolar disorder symptoms in depressed primary care attenders: comparison between Mood Disorder Questionnaire and Hypomania Checklist (HCL-32). Psychiatry J. 2013;2013:548349.
15. Vohringer PA, Jimenez MI, Igor MA, et al. Detecting mood disorder in resource-limited primary care settings: comparison of a self-administered screening tool to general practitioner assessment. J Med Screen. 2013;20:118-124.
16. Perlis RH, Brown E, Baker RW, et al. Clinical features of bipolar depression versus major depressive disorder in large multicenter trials. Am J Psychiatry. 2006;163:225-231.
17. Moreno C, Hasin DS, Arango C, et al. Depression in bipolar disorder versus major depressive disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Bipolar Disord. 2012;14:271-282.
18. Mitchell PB, Malhi GS. Bipolar depression: phenomenological overview and clinical characteristics. Bipolar Disord. 2004;6:530-539.
19. Solomon DA, Leon AC, Maser JD, et al. Distinguishing bipolar major depression from unipolar major depression with the screening assessment of depression-polarity (SAD-P). J Clin Psychiatry. 2006;67:434-442.
20. Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord. 2005;84:117-125.
21. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9:901-906.
22. Buzuk G, Lojko D, Owecki M, et al. Depression with atypical features in various kinds of affective disorders. Psychiatr Pol. 2016;50:827-838.
23. Liu X, Agerbo E, Li J, et al. Depression and anxiety in the postpartum period and risk of bipolar disorder: a Danish Nationwide Register-Based Cohort Study. J Clin Psychiatry. 2017;78:e469-e476.
THE CASE
A 35-year-old police officer visited his family physician (FP) with complaints of low energy, trouble sleeping, a lack of enjoyment in life, and feelings of hopelessness that have persisted for several months. He was worried about the impact they were having on his marriage and work. He had not experienced suicidal thoughts. His Patient Health Questionnaire (PHQ9) score was 18 (moderately severe depression). He had been seen intermittently for similar complaints and had tried several medications (fluoxetine, bupropion, and citalopram) without much effect. He was taking no medications now other than an over-the-counter multivitamin. He had one brother with anxiety and depression. He said his marriage counselor expressed concerns that he might have bipolar disorder or borderline personality disorder.
How would you proceed with this patient?
The prevalence of a spectrum of bipolarity in the community has been shown to be 6.4%.1 Depressive episodes predominate in bipolar disorder (BPD),2 with patients spending less time in manic or hypomanic states.3 Not surprisingly, then, depressive episodes are the most common presentation of BPD.
The depressive symptoms of BPD and unipolar depression, or major depressive disorder (MDD), are similar, making it difficult to distinguish between the disorders.3 As a result, BPD is often misdiagnosed as MDD.4,5 Zimmerman et al point out that “bipolar disorder is prone to being overlooked because its diagnosis is more often based on retrospective report rather than presenting symptoms of mania or hypomania assessment.”6
Accurately recognizing BPD is essential in selecting effective treatment. It’s estimated that approximately one-third of patients given antidepressants for major depression show no treatment response,7 possibly due in part to undiagnosed BPD being more prevalent than previously thought.4,8 Failure to distinguish between depressive episodes of BPD and MDD before prescribing medication introduces the risk of ineffective or suboptimal treatment. Inappropriate treatment can worsen or destabilize the course of bipolar illness by, for instance, inducing rapid cycling or, less commonly, manic symptoms.
Screen for BPD when depressive symptoms are present
Identifying BPD in a patient with current or past depressive symptoms requires screening for manic, hypomanic, and mixed episodes (TABLE 19). Two brief, complementary screening tools — the Mood Disorder Questionnaire (MDQ) and the 9-item PHQ9—are helpful in this assessment. Both questionnaires (TABLE 28,10-14) can be conveniently completed by the patient in the waiting room or with staff assistance before the physician encounter.
The MDQ screen is for past/lifetime or current manic/hypomanic symptoms (https://www.integration.samhsa.gov/images/res/MDQ.pdf). A positive screen requires answering “Yes” to at least 7 of the 13 items on question 1, answering “yes” on question 2, and answering “moderate problems” or “serious problems” on question 3.
Continue to: The PHQ9 screens for...
The PHQ9 screens for current depressive symptoms/episodes (https://www.uspreventiveservicestaskforce.org/Home/GetFileByID/218).
The value of combining the MDQ and PHQ9. The PHQ9 screens for and assesses the severity of depressive episodes along with clinician assessment, but it cannot distinguish between depressive episodes of MDD or BPD. A brief instrument, such as MDQ, screens for current or past manic or hypomanic symptoms, which, when combined with the clinical interview and patient history, enables detection of BPD if present and avoids erroneously assigning depressive symptoms to MDD.
One cross-sectional study found that the combined MDQ and PHQ9 questionnaires have a higher sensitivity in detecting mood disorder than does routine assessment by general practitioners (0.8 [95% confidence interval (CI), 0.71-0.81] vs 0.2 [95% CI, 0.12- 0.25]) and without loss of specificity (0.9 [95% CI, 0.86-0.96] vs 0.9 [95% CI, 0.88-0.97]).15 In this same study, using a structured clinical interview for DSM-III-R Axis I Disorders (SCID-I) as the gold standard, researchers also found the screening tools to be more accurate (Cohen’s Kappa 0.7 [SE=0.05; 95% CI, 0.5-0.7]) than the general practitioner assessment (Cohen’s Kappa 0.2 [SE=0.07 (95% CI, 0.12-0.27]).15
Delve deeper with a patient interview
Use targeted questions and laboratory tests to rule out other possible causes of depressed mood, such as substance abuse or medical conditions (eg, hypothyroidism). Keep in mind that even when MDD or BPD is present, other medical disorders or substance abuse could be coexistent. Also ask about a personal or family psychiatric history and assess for suicidality. If family members are available, they may be able to help in identifying the patient’s age when symptoms first appeared or in adding information about the affective episode or behavior that the patient may not recollect.
Beyond a history of manic, hypomanic, or mixed episodes, other symptoms and features may assist in distinguishing between bipolar and unipolar depression or in helping the clinician identify depressed patients who may be at higher risk for, or have, BPD. One meta-analysis of 3 multicenter clinical trials assessed sociodemographic factors and clinical features of BPD compared with unipolar depression. The average age of onset of mood symptoms in individuals with BPD was significantly younger (21.2 years) than that of patients with MDD (29.7 years).16 Another study found that patients with either bipolar I or bipolar II similarly experienced their first mood disorder episode 10 years earlier than those with MDD.17
Continue to: BPD is often associated with...
BPD is often associated with more frequent depressive episodes and a higher number of depressive symptoms per episode than is MDD, as well as more frequent family psychiatric histories (especially of mood disorders), anxiety disorders, alcohol and drug use disorders, and personality disorders.17 Other factors more closely associated with BPD than MDD include atypical features such as hypersomnia and psychomotor retardation, psychotic symptoms during the depressive episode, and more frequent recurrences of depressive episodes.18-22 Also, depressive episodes during the postpartum period indicate a higher risk of BPD than do episodes in women outside the postpartum period, with a hazard ratio (HR) of 1.66 (95% CI, 1.12-2.48).23 The risk is much greater when postpartum depressive episodes are associated with anxiety symptoms (HR=10.15; 95% CI, 7.13-14.46).23
Final thoughts
Increased awareness and screening for BPD in primary care—where most individuals with depressive symptoms are first encountered—should lead to more accurate diagnoses and decrease the years-long gaps between symptom onset and detection of BPD,4,5 thereby improving treatment and patient outcomes. Still, some cases of BPD may be difficult to recognize—particularly patients who present predominantly with depression with past irritability and other hypomanic symptoms (but not euphoria).
A positive MDQ screen should also prompt, if possible, a more detailed clinical interview by a mental health care professional, particularly if there is uncertainty about the diagnosis. Complex cases of BPD may require the expertise of a psychiatrist.
THE CASE
The patient’s FP referred him to a psychiatrist colleague, whose inquiry also revealed low mood, anhedonia, hopelessness, difficulty sleeping, low energy, poor appetite, guilt, poor concentration, and psychomotor retardation. The patient had experienced multiple depressive episodes over the past 20 years. Significant interpersonal conflicts frequently triggered his depressive episodes, which were accompanied by mood irritability, racing thoughts, distractibility, increased libido, excessive spending, increased energy, and engagement in risky behaviors.
The patient’s score on the MDQ administered by the psychiatrist was positive, with 7 points on question 1. He also had posttraumatic symptoms related to his police work, which were not the main reason for the visit. He had been divorced 3 times. In prior manic episodes, he had not displayed euphoria, grandiosity, psychotic symptoms, or anxiety, but rather irritability with other manic symptoms.
Continue to: Based on his MDQ results...
Based on his MDQ results, the clinical interview, and current episode with mixed features, the patient was given a diagnosis of bipolar II disorder. The psychiatrist prescribed divalproex 500 mg at bedtime and scheduled a return visit with a plan for further laboratory monitoring and up-titration if needed. He was also encouraged to follow up with his FP.
CORRESPONDENCE
Nagy A. Youssef, MD, Medical College of Georgia at Augusta University, 997 St. Sebastian Way, Augusta, GA 30912; nyoussef@augusta.edu.
SUPPORT AND ACKNOWLEDGMENT
Dr. Youssef’s work on this paper was supported by the Office of Academic Affairs, Medical College of Georgia at Augusta University. We thank Mark Yassa, BS, for his assistance in editing.
THE CASE
A 35-year-old police officer visited his family physician (FP) with complaints of low energy, trouble sleeping, a lack of enjoyment in life, and feelings of hopelessness that have persisted for several months. He was worried about the impact they were having on his marriage and work. He had not experienced suicidal thoughts. His Patient Health Questionnaire (PHQ9) score was 18 (moderately severe depression). He had been seen intermittently for similar complaints and had tried several medications (fluoxetine, bupropion, and citalopram) without much effect. He was taking no medications now other than an over-the-counter multivitamin. He had one brother with anxiety and depression. He said his marriage counselor expressed concerns that he might have bipolar disorder or borderline personality disorder.
How would you proceed with this patient?
The prevalence of a spectrum of bipolarity in the community has been shown to be 6.4%.1 Depressive episodes predominate in bipolar disorder (BPD),2 with patients spending less time in manic or hypomanic states.3 Not surprisingly, then, depressive episodes are the most common presentation of BPD.
The depressive symptoms of BPD and unipolar depression, or major depressive disorder (MDD), are similar, making it difficult to distinguish between the disorders.3 As a result, BPD is often misdiagnosed as MDD.4,5 Zimmerman et al point out that “bipolar disorder is prone to being overlooked because its diagnosis is more often based on retrospective report rather than presenting symptoms of mania or hypomania assessment.”6
Accurately recognizing BPD is essential in selecting effective treatment. It’s estimated that approximately one-third of patients given antidepressants for major depression show no treatment response,7 possibly due in part to undiagnosed BPD being more prevalent than previously thought.4,8 Failure to distinguish between depressive episodes of BPD and MDD before prescribing medication introduces the risk of ineffective or suboptimal treatment. Inappropriate treatment can worsen or destabilize the course of bipolar illness by, for instance, inducing rapid cycling or, less commonly, manic symptoms.
Screen for BPD when depressive symptoms are present
Identifying BPD in a patient with current or past depressive symptoms requires screening for manic, hypomanic, and mixed episodes (TABLE 19). Two brief, complementary screening tools — the Mood Disorder Questionnaire (MDQ) and the 9-item PHQ9—are helpful in this assessment. Both questionnaires (TABLE 28,10-14) can be conveniently completed by the patient in the waiting room or with staff assistance before the physician encounter.
The MDQ screen is for past/lifetime or current manic/hypomanic symptoms (https://www.integration.samhsa.gov/images/res/MDQ.pdf). A positive screen requires answering “Yes” to at least 7 of the 13 items on question 1, answering “yes” on question 2, and answering “moderate problems” or “serious problems” on question 3.
Continue to: The PHQ9 screens for...
The PHQ9 screens for current depressive symptoms/episodes (https://www.uspreventiveservicestaskforce.org/Home/GetFileByID/218).
The value of combining the MDQ and PHQ9. The PHQ9 screens for and assesses the severity of depressive episodes along with clinician assessment, but it cannot distinguish between depressive episodes of MDD or BPD. A brief instrument, such as MDQ, screens for current or past manic or hypomanic symptoms, which, when combined with the clinical interview and patient history, enables detection of BPD if present and avoids erroneously assigning depressive symptoms to MDD.
One cross-sectional study found that the combined MDQ and PHQ9 questionnaires have a higher sensitivity in detecting mood disorder than does routine assessment by general practitioners (0.8 [95% confidence interval (CI), 0.71-0.81] vs 0.2 [95% CI, 0.12- 0.25]) and without loss of specificity (0.9 [95% CI, 0.86-0.96] vs 0.9 [95% CI, 0.88-0.97]).15 In this same study, using a structured clinical interview for DSM-III-R Axis I Disorders (SCID-I) as the gold standard, researchers also found the screening tools to be more accurate (Cohen’s Kappa 0.7 [SE=0.05; 95% CI, 0.5-0.7]) than the general practitioner assessment (Cohen’s Kappa 0.2 [SE=0.07 (95% CI, 0.12-0.27]).15
Delve deeper with a patient interview
Use targeted questions and laboratory tests to rule out other possible causes of depressed mood, such as substance abuse or medical conditions (eg, hypothyroidism). Keep in mind that even when MDD or BPD is present, other medical disorders or substance abuse could be coexistent. Also ask about a personal or family psychiatric history and assess for suicidality. If family members are available, they may be able to help in identifying the patient’s age when symptoms first appeared or in adding information about the affective episode or behavior that the patient may not recollect.
Beyond a history of manic, hypomanic, or mixed episodes, other symptoms and features may assist in distinguishing between bipolar and unipolar depression or in helping the clinician identify depressed patients who may be at higher risk for, or have, BPD. One meta-analysis of 3 multicenter clinical trials assessed sociodemographic factors and clinical features of BPD compared with unipolar depression. The average age of onset of mood symptoms in individuals with BPD was significantly younger (21.2 years) than that of patients with MDD (29.7 years).16 Another study found that patients with either bipolar I or bipolar II similarly experienced their first mood disorder episode 10 years earlier than those with MDD.17
Continue to: BPD is often associated with...
BPD is often associated with more frequent depressive episodes and a higher number of depressive symptoms per episode than is MDD, as well as more frequent family psychiatric histories (especially of mood disorders), anxiety disorders, alcohol and drug use disorders, and personality disorders.17 Other factors more closely associated with BPD than MDD include atypical features such as hypersomnia and psychomotor retardation, psychotic symptoms during the depressive episode, and more frequent recurrences of depressive episodes.18-22 Also, depressive episodes during the postpartum period indicate a higher risk of BPD than do episodes in women outside the postpartum period, with a hazard ratio (HR) of 1.66 (95% CI, 1.12-2.48).23 The risk is much greater when postpartum depressive episodes are associated with anxiety symptoms (HR=10.15; 95% CI, 7.13-14.46).23
Final thoughts
Increased awareness and screening for BPD in primary care—where most individuals with depressive symptoms are first encountered—should lead to more accurate diagnoses and decrease the years-long gaps between symptom onset and detection of BPD,4,5 thereby improving treatment and patient outcomes. Still, some cases of BPD may be difficult to recognize—particularly patients who present predominantly with depression with past irritability and other hypomanic symptoms (but not euphoria).
A positive MDQ screen should also prompt, if possible, a more detailed clinical interview by a mental health care professional, particularly if there is uncertainty about the diagnosis. Complex cases of BPD may require the expertise of a psychiatrist.
THE CASE
The patient’s FP referred him to a psychiatrist colleague, whose inquiry also revealed low mood, anhedonia, hopelessness, difficulty sleeping, low energy, poor appetite, guilt, poor concentration, and psychomotor retardation. The patient had experienced multiple depressive episodes over the past 20 years. Significant interpersonal conflicts frequently triggered his depressive episodes, which were accompanied by mood irritability, racing thoughts, distractibility, increased libido, excessive spending, increased energy, and engagement in risky behaviors.
The patient’s score on the MDQ administered by the psychiatrist was positive, with 7 points on question 1. He also had posttraumatic symptoms related to his police work, which were not the main reason for the visit. He had been divorced 3 times. In prior manic episodes, he had not displayed euphoria, grandiosity, psychotic symptoms, or anxiety, but rather irritability with other manic symptoms.
Continue to: Based on his MDQ results...
Based on his MDQ results, the clinical interview, and current episode with mixed features, the patient was given a diagnosis of bipolar II disorder. The psychiatrist prescribed divalproex 500 mg at bedtime and scheduled a return visit with a plan for further laboratory monitoring and up-titration if needed. He was also encouraged to follow up with his FP.
CORRESPONDENCE
Nagy A. Youssef, MD, Medical College of Georgia at Augusta University, 997 St. Sebastian Way, Augusta, GA 30912; nyoussef@augusta.edu.
SUPPORT AND ACKNOWLEDGMENT
Dr. Youssef’s work on this paper was supported by the Office of Academic Affairs, Medical College of Georgia at Augusta University. We thank Mark Yassa, BS, for his assistance in editing.
1. Judd LL, Akiskal HS. The prevalence and disability of bipolar spectrum disorders in the US population: re-analysis of the ECA database taking into account subthreshold cases. J Affect Disord. 2003;73:123-131.
2. Yatham LN, Lecrubier Y, Fieve RR, et al. Quality of life in patients with bipolar I depression: data from 920 patients. Bipolar Disord. 2004;6:379-385.
3. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530-537.
4. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord. 1999;52:135-144.
5. Cha B, Kim JH, Ha TH, et al. Polarity of the first episode and time to diagnosis of bipolar I disorder. Psychiatry Investig. 2009;6:96-101. Available at: http://psychiatryinvestigation.org/journal/view.php?doi=10.4306/pi.2009.6.2.96. Accessed June 25, 2018.
6. Zimmerman M, Galione JN, Chelminski I, et al. Psychiatric diagnoses in patients who screen positive on the Mood Disorder Questionnaire: implications for using the scale as a case-finding instrument for bipolar disorder. Psychiatry Res. 2011;185:444-449.
7. Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369-388.
8. Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Pract. 2005;18:233-239.
9. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, D.C.: American Psychiatric Publishing. 2013.
10. Poon Y, Chung KF, Tso KC, et al. The use of Mood Disorder Questionnaire, Hypomania Checklist-32 and clinical predictors for screening previously unrecognised bipolar disorder in a general psychiatric setting. Psychiatry Res. 2012;195:111-117.
11. Gilbody S, Richards D, Brealey S, et al. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med. 2007;22:1596-1602.
12. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
13. Miller CJ, Klugman J, Berv DA, et al. Sensitivity and specificity of the Mood Disorder Questionnaire for detecting bipolar disorder. J Affect Disord. 2004;81:167-171.
14. Sasdelli A, Lia L, Luciano CC, et al. Screening for bipolar disorder symptoms in depressed primary care attenders: comparison between Mood Disorder Questionnaire and Hypomania Checklist (HCL-32). Psychiatry J. 2013;2013:548349.
15. Vohringer PA, Jimenez MI, Igor MA, et al. Detecting mood disorder in resource-limited primary care settings: comparison of a self-administered screening tool to general practitioner assessment. J Med Screen. 2013;20:118-124.
16. Perlis RH, Brown E, Baker RW, et al. Clinical features of bipolar depression versus major depressive disorder in large multicenter trials. Am J Psychiatry. 2006;163:225-231.
17. Moreno C, Hasin DS, Arango C, et al. Depression in bipolar disorder versus major depressive disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Bipolar Disord. 2012;14:271-282.
18. Mitchell PB, Malhi GS. Bipolar depression: phenomenological overview and clinical characteristics. Bipolar Disord. 2004;6:530-539.
19. Solomon DA, Leon AC, Maser JD, et al. Distinguishing bipolar major depression from unipolar major depression with the screening assessment of depression-polarity (SAD-P). J Clin Psychiatry. 2006;67:434-442.
20. Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord. 2005;84:117-125.
21. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9:901-906.
22. Buzuk G, Lojko D, Owecki M, et al. Depression with atypical features in various kinds of affective disorders. Psychiatr Pol. 2016;50:827-838.
23. Liu X, Agerbo E, Li J, et al. Depression and anxiety in the postpartum period and risk of bipolar disorder: a Danish Nationwide Register-Based Cohort Study. J Clin Psychiatry. 2017;78:e469-e476.
1. Judd LL, Akiskal HS. The prevalence and disability of bipolar spectrum disorders in the US population: re-analysis of the ECA database taking into account subthreshold cases. J Affect Disord. 2003;73:123-131.
2. Yatham LN, Lecrubier Y, Fieve RR, et al. Quality of life in patients with bipolar I depression: data from 920 patients. Bipolar Disord. 2004;6:379-385.
3. Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530-537.
4. Ghaemi SN, Sachs GS, Chiou AM, et al. Is bipolar disorder still underdiagnosed? Are antidepressants overutilized? J Affect Disord. 1999;52:135-144.
5. Cha B, Kim JH, Ha TH, et al. Polarity of the first episode and time to diagnosis of bipolar I disorder. Psychiatry Investig. 2009;6:96-101. Available at: http://psychiatryinvestigation.org/journal/view.php?doi=10.4306/pi.2009.6.2.96. Accessed June 25, 2018.
6. Zimmerman M, Galione JN, Chelminski I, et al. Psychiatric diagnoses in patients who screen positive on the Mood Disorder Questionnaire: implications for using the scale as a case-finding instrument for bipolar disorder. Psychiatry Res. 2011;185:444-449.
7. Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369-388.
8. Hirschfeld RM, Cass AR, Holt DC, et al. Screening for bipolar disorder in patients treated for depression in a family medicine clinic. J Am Board Fam Pract. 2005;18:233-239.
9. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, D.C.: American Psychiatric Publishing. 2013.
10. Poon Y, Chung KF, Tso KC, et al. The use of Mood Disorder Questionnaire, Hypomania Checklist-32 and clinical predictors for screening previously unrecognised bipolar disorder in a general psychiatric setting. Psychiatry Res. 2012;195:111-117.
11. Gilbody S, Richards D, Brealey S, et al. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med. 2007;22:1596-1602.
12. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
13. Miller CJ, Klugman J, Berv DA, et al. Sensitivity and specificity of the Mood Disorder Questionnaire for detecting bipolar disorder. J Affect Disord. 2004;81:167-171.
14. Sasdelli A, Lia L, Luciano CC, et al. Screening for bipolar disorder symptoms in depressed primary care attenders: comparison between Mood Disorder Questionnaire and Hypomania Checklist (HCL-32). Psychiatry J. 2013;2013:548349.
15. Vohringer PA, Jimenez MI, Igor MA, et al. Detecting mood disorder in resource-limited primary care settings: comparison of a self-administered screening tool to general practitioner assessment. J Med Screen. 2013;20:118-124.
16. Perlis RH, Brown E, Baker RW, et al. Clinical features of bipolar depression versus major depressive disorder in large multicenter trials. Am J Psychiatry. 2006;163:225-231.
17. Moreno C, Hasin DS, Arango C, et al. Depression in bipolar disorder versus major depressive disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Bipolar Disord. 2012;14:271-282.
18. Mitchell PB, Malhi GS. Bipolar depression: phenomenological overview and clinical characteristics. Bipolar Disord. 2004;6:530-539.
19. Solomon DA, Leon AC, Maser JD, et al. Distinguishing bipolar major depression from unipolar major depression with the screening assessment of depression-polarity (SAD-P). J Clin Psychiatry. 2006;67:434-442.
20. Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord. 2005;84:117-125.
21. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9:901-906.
22. Buzuk G, Lojko D, Owecki M, et al. Depression with atypical features in various kinds of affective disorders. Psychiatr Pol. 2016;50:827-838.
23. Liu X, Agerbo E, Li J, et al. Depression and anxiety in the postpartum period and risk of bipolar disorder: a Danish Nationwide Register-Based Cohort Study. J Clin Psychiatry. 2017;78:e469-e476.
Schizophrenia: Ensuring an accurate Dx, optimizing treatment
THE CASE
Steven R,* a 21-year-old man, visited the clinic accompanied by his mother. He did not speak much, and his mother provided his history. Over the previous 2 months, she had overheard him whispering in an agitated voice, even though no one else was nearby. And, lately, he refused to answer or make calls on his cell phone, claiming that if he did it would activate a deadly chip that had been implanted in his brain by evil aliens. He also stopped attending classes at the community college. He occasionally had a few beers with his friends, but he had never been known to abuse alcohol or use other recreational drugs.
How would you proceed with this patient?
* The patient’s name has been changed to protect his identity.
CHARACTERISTICS AND SCOPE OF SCHIZOPHRENIA
Schizophrenia is a psychotic illness in which the individual loses contact with reality and often experiences hallucinations, delusions, or thought disorders. Criteria for schizophrenia described in the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5) include signs and symptoms of at least 6 months’ duration, as well as at least one month of active-phase positive and negative symptoms.1
Delusions, hallucinations, disorganized speech, and disorganized behavior are examples of positive symptoms. Negative symptoms include a decrease in the range and intensity of expressed emotions (ie, affective flattening) and a diminished initiation of goal-directed activities (ie, avolition).
Approximately 7 in 1000 people will develop the disorder in their lifetime.2 Schizophrenia is considered a “serious mental illness” because of its chronic course and often poor long-term social and vocational outcomes.3,4 Symptom onset is generally between late adolescence and the mid-30s.5
Getting closer to understanding its origin
Both genetic susceptibility and environmental factors influence the incidence of schizophrenia.4 Newer models of the disease have identified genes (ZDHHC8 and DTNBP1) whose mutations may increase the risk of schizophrenia.6 Physiologic insults during fetal life—hypoxia, maternal infection, maternal stress, and maternal malnutrition—account for a small portion of schizophrenia cases.6
Abnormalities in neurotransmission are the basis for theories on the pathophysiology of schizophrenia. Most of these theories center on either an excess or a deficiency of neurotransmitters, including dopamine, serotonin, and glutamate. Other theories implicate aspartate, glycine, and gamma-aminobutyric acid as part of the neurochemical imbalance of schizophrenia.7
ESTABLISHING A DIAGNOSIS
Although psychotic symptoms may be a prominent part of schizophrenia, not all psychoses indicate a primary psychiatric disorder such as schizophrenia. Broadly, psychoses can be categorized as primary or secondary.
Primary psychoses include schizophrenia, schizoaffective disorder, schizophreniform disorder, brief psychotic disorder, delusional disorder, and mood disorders (major depressive disorder and borderline personality disorder) with psychotic features.1 Difficulty in distinguishing between these entities can necessitate referral to a psychiatrist.
Secondary psychoses arise from a precursor such as delirium, dementia, medical illness, or adverse effects of medications or illicit substances. Medical illnesses that cause psychotic symptoms include: 5,8
- seizures (especially temporal lobe epilepsy),
- cerebrovascular accidents,
- intracranial space-occupying lesions,
- neuropsychiatric disorders (eg, Wilson’s or Parkinson’s disease),
- endocrine disorders (eg, thyroid or adrenal disease),
- autoimmune disease (eg, systemic lupus erythematosus, Hashimoto encephalopathy),
- deficiencies of vitamins A, B1, B12, or niacin,
- infections (eg, human immunodeficiency virus [HIV], encephalitis, parasites, and prion disease),
- narcolepsy, and
- metabolic disease (eg, acute intermittent porphyria, Tay-Sach’s disease, Niemann-Pick disease).
Several recreational drugs can cause psychotic symptoms: cocaine, amphetamines, cannabis, synthetic cannabinoids, inhalants, opioids, and hallucinogens. Psychotic symptoms can also appear during withdrawal from alcohol (delirium tremens) and from sedative hypnotics such as benzodiazepines. Prescribed medications such as anticholinergics, corticosteroids, dopaminergic agents (L-dopa), stimulants (amphetamines), and interferons can also induce psychotic symptoms.
First rule out causes of secondary psychosis
Rule out causes of secondary psychosis by conducting a detailed history and physical examination and ordering appropriate lab tests and imaging studies. If the patient’s psychosis is of recent onset, make sure the laboratory work-up includes
Consider cranial computed tomography or magnetic resonance imaging if there are focal neurologic deficits or if the patient’s presentation is atypical (eg, new onset psychosis in old age).9 Clinical presentation may also indicate a need for electroencephalography, ceruloplasmin measurement, a dexamethasone suppression test, a corticotropin stimulation test, 24-hour urine porphyrin and copper assays, chest radiography, or cerebrospinal fluid analysis.9
FACTORS TO CONSIDER IN TREATMENT DECISIONS
Although primary care physicians may encounter individuals experiencing their first episode of psychosis, it’s more likely that patients presenting with signs and symptoms of the disorder have been experiencing them for some time and have received no psychiatric care. In both instances, schizophrenia is best managed in conjunction with a psychiatrist until symptoms are stabilized.5 Psychosis does not always require hospitalization. But urgent psychiatry referral is recommended, if possible. Consider admission to a psychiatric inpatient unit for anyone who poses a danger to self or others.8,10
Treatment for schizophrenia is most effective with an interprofessional and collaborative approach that includes medication, psychological treatment, social supports, and primary care clinical management.11,12 The last aspect takes on particular importance given that people with schizophrenia, compared with the general population, have a higher incidence of medical illness, particularly cardiovascular disease.13
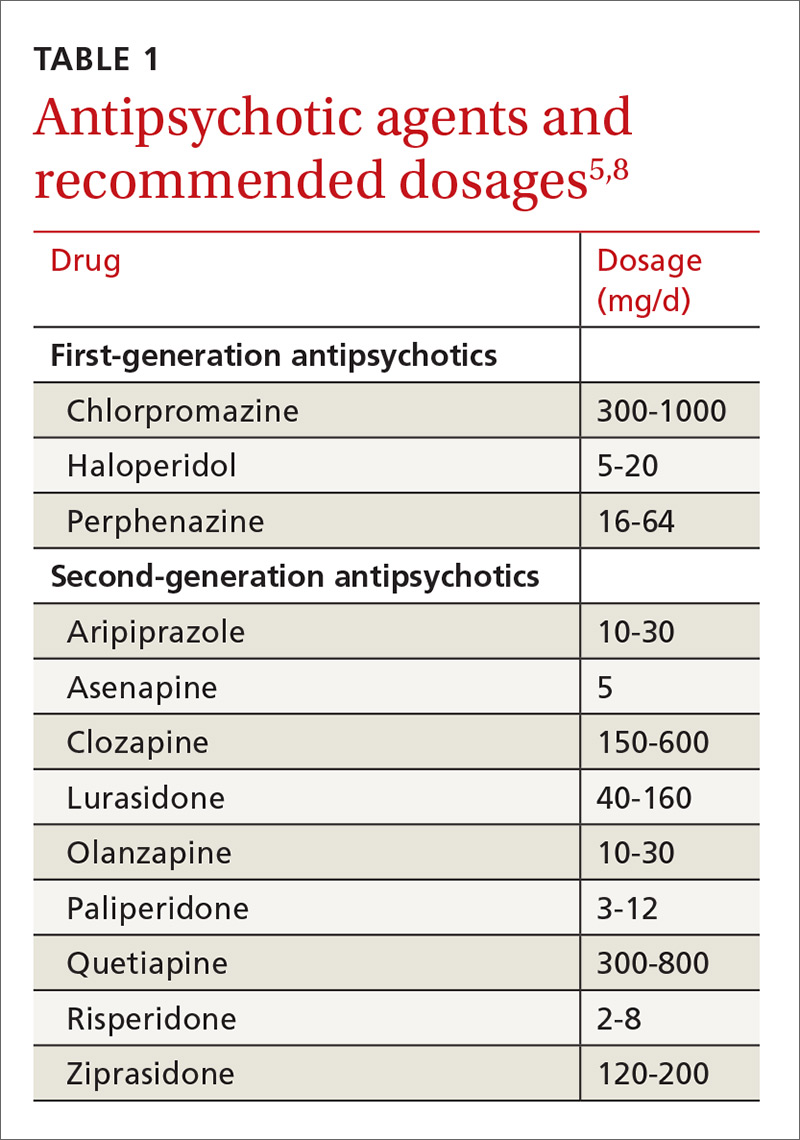
Medications (TABLE 15,8) are grouped into first-generation antipsychotics (FGAs) and second-generation, or atypical, antipsychotics (SGAs), with the 2 classes being equally effective.14-16 Quality of life is also similar at one year for patients treated with either drug class.14
Adverse effects can differ. The main difference between these medications is their adverse effect profiles. FGAs cause extrapyramidal symptoms (dystonia, akathisia, and tardive dyskinesia) more often than SGAs. Among the SGAs, olanzapine, asenapine, paliperidone, clozapine, and quetiapine cause significant weight gain, glucose dysregulation, and lipid abnormalities.5,8,12,17 Clozapine is associated with agranulocytosis, as well. Risperidone causes mild to moderate weight gain.5,8,12,17 Aripiprazole, lurasidone, and ziprasidone are considered weight neutral and cause no significant glucose dysregulation or lipid abnormalities.5,8,12,17 All antipsychotics can cause QT prolongation and neuroleptic malignant syndrome.5,8,12,17
Keys to successful treatment. Antipsychotics are most effective in treating positive symptoms of schizophrenia and show limited, if any, effect on negative or cognitive symptoms.18,19 Give patients an adequate trial of therapy (at least 4 weeks at a therapeutic dose) before discontinuing the drug or offering a different medication.20 All patients who report symptom relief while receiving antipsychotics should receive maintenance therapy.12
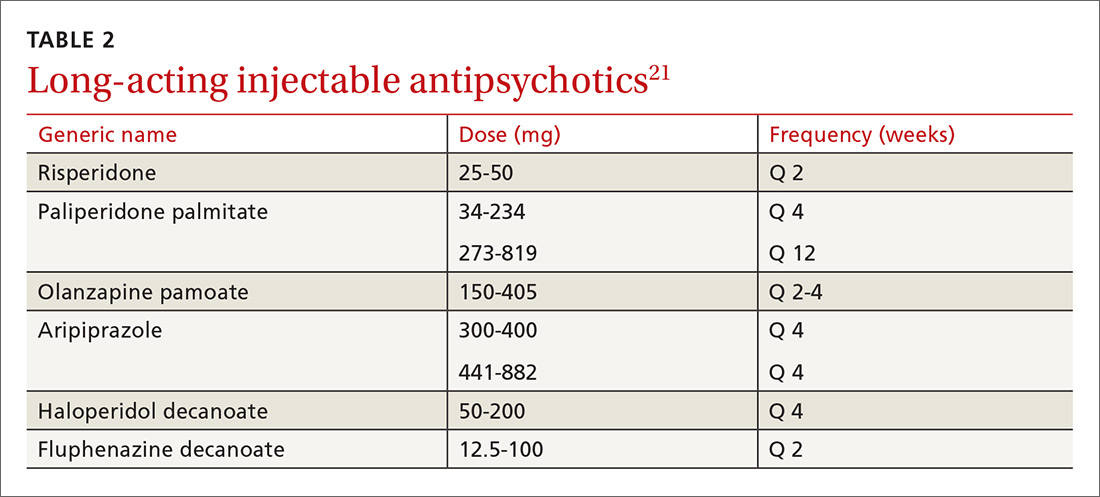
As with all chronic illnesses, success in managing schizophrenia requires patient adherence to the medication regimen. Discontinuation of antipsychotics is a common problem in schizophrenia, resulting in relapse. Long-acting injectable agents (LAIs) were developed to address this problem (TABLE 2).21 Although LAIs are typically used to ensure adherence during maintenance treatment, recent research has suggested they may also be effective for patients with early-phase or first-episode disease.22
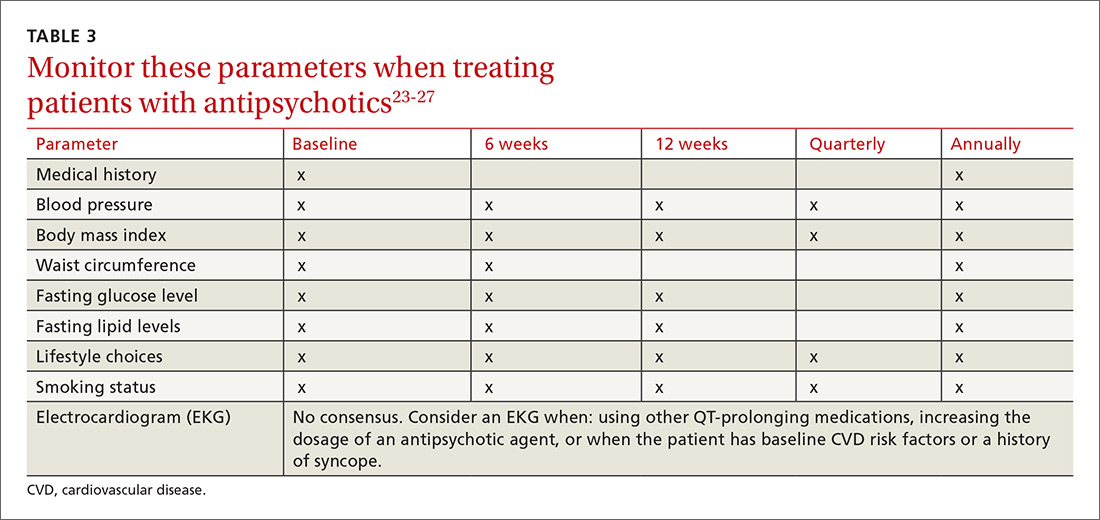
What to watch for. Patients on SGAs may develop metabolic abnormalities, and ongoing monitoring of relevant parameters is key (TABLE 323-27). More frequent monitoring may be necessary in patients with cardiovascular risk factors. Continue antipsychotics for at least 6 months to prevent relapse.12 Also keep in mind the “Choosing Wisely” recommendation from the American Psychiatric Association of not prescribing 2 or more antipsychotics concurrently.28
Adjunctive treatment should also be offered
In addition to receiving medication, patients with schizophrenia should be offered adjunctive therapies such as cognitive behavioral therapy, family intervention, and social skills training.10-12 Among patients with schizophrenia, the incidences of anxiety disorder, panic symptoms, posttraumatic stress disorder, and obsessive compulsive disorder are higher than in the general population.29 To address these conditions, medications such as selective serotonin reuptake inhibitors and anxiolytics can be used simultaneously with antipsychotic agents.
CLINICAL COURSE AND PROGNOSIS CAN VARY
Schizophrenia can have a variable clinical course that includes remissions and exacerbations, or it can follow a more persistently chronic course.
Mortality for patients with schizophrenia is 2 to 3 times higher than that of the general population.30 Most deaths are due to an increased incidence of cardiovascular disease, respiratory illness, cancer, stroke, and other thromboembolic events.30
The lifetime prevalence of suicide attempts among individuals with schizophrenia is 20% to 40%,31 and approximately 5% complete suicide.32 Risk factors include command hallucinations, a history of suicide attempts, intoxication with substances, anxiety, and physical pain.32 Clozapine has been shown to reduce suicide risk and may be considered for patients who are at high risk for suicide.32
Therapeutic response varies among patients with schizophrenia, with one-third remaining symptomatic despite adequate treatment regimens.4
CARE MANAGERS CAN HELP ADDRESS BARRIERS TO CARE
A review of the literature suggests that up to one-third of individuals with serious mental illnesses who have had some contact with the mental health system disengage from care.12 Poor engagement may lead to worse clinical outcomes, with symptom relapse and re-hospitalizations. Disengagement from treatment may indicate a patient’s belief that treatment is not necessary, is not meeting his or her needs, or is not being provided in a collaborative manner.
Although shared decision-making is difficult with patients who have schizophrenia, emerging evidence suggests that this approach coupled with patient-centered care will improve engagement with mental health treatment.12 Models of integrated care are being developed and have shown promise in ensuring access to behavioral health for these patients.34
CASE
The primary care physician talked with Mr. R and his mother about the diagnosis of schizophrenia. He screened for suicide risk, and the patient denied having suicidal thoughts. Both the patient and his mother agreed to his starting medication.
Blood and urine samples were collected for a CBC and ESR, as well as to evaluate renal function, electrolytes, glucose, TSH, vitamin B12, folic acid, ANAs, and HIV antibodies. A serum FTA-ABS test was done, as was a urine culture and sensitivity test and a toxicology screen. Because of the patient’s obesity, the physician decided to prescribe a weight-neutral SGA, aripiprazole 10 mg/d. The physician spoke with the clinic’s care coordinator to schedule an appointment with the psychiatry intake department and to follow up on the phone with the patient and his mother. He also scheduled a follow-up appointment for 2 weeks later.
At the follow-up visit, the patient showed no improvement. His blood and urine test results revealed no metabolic abnormalities or infectious or inflammatory illnesses. His urine toxicology result showed no illicit substances. The physician increased the dosage of aripiprazole to 15 mg/d and asked the patient to return in 2 weeks.
At the next follow-up visit, the patient was more verbal and said he was not hearing voices. His mother also acknowledged an improvement. He had already been scheduled for a psychiatry intake appointment, and he and his mother were reminded about this. Mr. R was also asked to make a follow-up primary care appointment for one month from the current visit.
CORRESPONDENCE
Rajesh (Fnu) Rajesh, MD, MetroHealth Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; frajesh@metrohealth.org.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5). Washington, DC: American Psychiatric Publishing; 2013.
2. McGrath J, Saha S, Chant D, et al. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67-76.
3. Henry LP, Amminger GP, Harris MG, et al. The EPPIC follow-up study of first-episode psychosis: longer-term and clinical and functional outcome 7 years after index admission. J Clin Psychiatry. 2010;71:716-728.
4. van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635-645.
5. Holder SD, Wayhs A. Schizophrenia. Am Fam Phys. 2014;90:775-82.
6. Lakhan SE, Vieira KF. Schizophrenia pathophysiology: are we any closer to a complete model? Ann Gen Psychiatry. 2009;8:12.
7. Crismon L, Argo TR, Buckley PF. Schizophrenia. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, New York: McGraw-Hill; 2014:1019-1046.
8. Viron M, Baggett T, Hill M, et al. Schizophrenia for primary care providers: how to contribute to the care of a vulnerable patient population. Am J Med. 2012;125:223-230.
9. Freudenreich O, Charles Schulz SC, Goff DC. Initial medical work-up of first-episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3:10-18.
10. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: Prevention and management. 2014. Available at: http://www.nice.org.uk/Guidance/CG178. Accessed January 3, 2017.
11. Guo X, Zhai J, Liu Z, et al. Effect of antipsychotic medication alone vs combined with psychosocial intervention on outcomes of early-stage schizophrenia: a randomized 1-year study. Arch Gen Psychiatry. 2010;67:895-904.
12. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2009;36:94-103.
13. Viron MJ, Stern TA. The impact of serious mental illness on health and healthcare. Psychosomatics. 2010;51:458-465.
14. Jones PB, Barnes TRE, Davies L, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry. 2006;63:1079-1087.
15. Hartling L, Abou-Setta AM, Dursun S, et al. Antipsychotics in adults with schizophrenia: comparative effectiveness of first-generation versus second-generation medications: a systematic review and meta-analysis. Ann Intern Med. 2012;157:498-511.
16. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209-1223.
17. Tandon R. Antipsychotics in the treatment of schizophrenia: an overview. J Clin Psychiatry. 2011;72(suppl 1):4-8.
18. Salimi K, Jarskog LF, Lieberman JA. Antipsychotic drugs for first-episode schizophrenia: a comparative review. CNS Drugs. 2009;23:837-855.
19. Fusar-Poli P, Papanastasiou E, Stahl D, et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41:892-899.
20. Moore TA, Buchanan RW, Buckley PF, et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J Clin Psychiatry. 2007;68:1751-1762.
21. Bera R. Patient outcomes within schizophrenia treatment: a look at the role of long-acting injectable antipsychotics. J Clin Psychiatry. 2014;75(suppl 2):30-33.
22. Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
23. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123:225-233.
24. De Hert M, Vancampfort D, Correll CU, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. 2011;199:99-105.
25. Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—a systematic review and meta-analysis. Schizophr Bull. 2013;39:306-318.
26. Lieberman JA, Merrill D, Parameswaran S. APA guidance on the use of antipsychotic drugs and cardiac sudden death.
27. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161:1334-1349.
28. American Psychiatric Association. Five things physicians and patients should question. Available at: http://www.choosingwisely.org/societies/american-psychiatric-association/. Accessed February 28, 2017.
29. Buckley PF, Miller BJ, Lehrer DS, et al. Psychiatric comorbidities and schizophrenia. Schizophr Bull. 2009;35:383-402.
30. Lwin AM, Symeon C, Jan F, et al. Morbidity and mortality in schizophrenia. Br J Hosp Med (Lond). 2011;72:628-630.
31. Pompili M, Amador XF, Girardi P, et al. Suicide risk in schizophrenia: learning from the past to change the future. Ann Gen Psychiatry. 2007;6:10.
32. Hor K, Taylor M. Suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol. 2010;24(4 suppl):81-90.
33. Druss BG, von Esenwein SA, Compton MT, et al. A randomized trial of medical care management for community mental health settings: the Primary Care Access, Referral, and Evaluation (PCARE) study. Am J Psychiatry. 2010;167:151-159.
34. Gerrity M. Evolving models of behavioral health integration: Evidence update 2010-2015. Milbank Memorial Fund. Available at: https://www.milbank.org/wp-content/uploads/2016/05/Evolv ing-Models-of-BHI.pdf. Accessed January 11, 2018.
THE CASE
Steven R,* a 21-year-old man, visited the clinic accompanied by his mother. He did not speak much, and his mother provided his history. Over the previous 2 months, she had overheard him whispering in an agitated voice, even though no one else was nearby. And, lately, he refused to answer or make calls on his cell phone, claiming that if he did it would activate a deadly chip that had been implanted in his brain by evil aliens. He also stopped attending classes at the community college. He occasionally had a few beers with his friends, but he had never been known to abuse alcohol or use other recreational drugs.
How would you proceed with this patient?
* The patient’s name has been changed to protect his identity.
CHARACTERISTICS AND SCOPE OF SCHIZOPHRENIA
Schizophrenia is a psychotic illness in which the individual loses contact with reality and often experiences hallucinations, delusions, or thought disorders. Criteria for schizophrenia described in the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5) include signs and symptoms of at least 6 months’ duration, as well as at least one month of active-phase positive and negative symptoms.1
Delusions, hallucinations, disorganized speech, and disorganized behavior are examples of positive symptoms. Negative symptoms include a decrease in the range and intensity of expressed emotions (ie, affective flattening) and a diminished initiation of goal-directed activities (ie, avolition).
Approximately 7 in 1000 people will develop the disorder in their lifetime.2 Schizophrenia is considered a “serious mental illness” because of its chronic course and often poor long-term social and vocational outcomes.3,4 Symptom onset is generally between late adolescence and the mid-30s.5
Getting closer to understanding its origin
Both genetic susceptibility and environmental factors influence the incidence of schizophrenia.4 Newer models of the disease have identified genes (ZDHHC8 and DTNBP1) whose mutations may increase the risk of schizophrenia.6 Physiologic insults during fetal life—hypoxia, maternal infection, maternal stress, and maternal malnutrition—account for a small portion of schizophrenia cases.6
Abnormalities in neurotransmission are the basis for theories on the pathophysiology of schizophrenia. Most of these theories center on either an excess or a deficiency of neurotransmitters, including dopamine, serotonin, and glutamate. Other theories implicate aspartate, glycine, and gamma-aminobutyric acid as part of the neurochemical imbalance of schizophrenia.7
ESTABLISHING A DIAGNOSIS
Although psychotic symptoms may be a prominent part of schizophrenia, not all psychoses indicate a primary psychiatric disorder such as schizophrenia. Broadly, psychoses can be categorized as primary or secondary.
Primary psychoses include schizophrenia, schizoaffective disorder, schizophreniform disorder, brief psychotic disorder, delusional disorder, and mood disorders (major depressive disorder and borderline personality disorder) with psychotic features.1 Difficulty in distinguishing between these entities can necessitate referral to a psychiatrist.
Secondary psychoses arise from a precursor such as delirium, dementia, medical illness, or adverse effects of medications or illicit substances. Medical illnesses that cause psychotic symptoms include: 5,8
- seizures (especially temporal lobe epilepsy),
- cerebrovascular accidents,
- intracranial space-occupying lesions,
- neuropsychiatric disorders (eg, Wilson’s or Parkinson’s disease),
- endocrine disorders (eg, thyroid or adrenal disease),
- autoimmune disease (eg, systemic lupus erythematosus, Hashimoto encephalopathy),
- deficiencies of vitamins A, B1, B12, or niacin,
- infections (eg, human immunodeficiency virus [HIV], encephalitis, parasites, and prion disease),
- narcolepsy, and
- metabolic disease (eg, acute intermittent porphyria, Tay-Sach’s disease, Niemann-Pick disease).
Several recreational drugs can cause psychotic symptoms: cocaine, amphetamines, cannabis, synthetic cannabinoids, inhalants, opioids, and hallucinogens. Psychotic symptoms can also appear during withdrawal from alcohol (delirium tremens) and from sedative hypnotics such as benzodiazepines. Prescribed medications such as anticholinergics, corticosteroids, dopaminergic agents (L-dopa), stimulants (amphetamines), and interferons can also induce psychotic symptoms.
First rule out causes of secondary psychosis
Rule out causes of secondary psychosis by conducting a detailed history and physical examination and ordering appropriate lab tests and imaging studies. If the patient’s psychosis is of recent onset, make sure the laboratory work-up includes
Consider cranial computed tomography or magnetic resonance imaging if there are focal neurologic deficits or if the patient’s presentation is atypical (eg, new onset psychosis in old age).9 Clinical presentation may also indicate a need for electroencephalography, ceruloplasmin measurement, a dexamethasone suppression test, a corticotropin stimulation test, 24-hour urine porphyrin and copper assays, chest radiography, or cerebrospinal fluid analysis.9
FACTORS TO CONSIDER IN TREATMENT DECISIONS
Although primary care physicians may encounter individuals experiencing their first episode of psychosis, it’s more likely that patients presenting with signs and symptoms of the disorder have been experiencing them for some time and have received no psychiatric care. In both instances, schizophrenia is best managed in conjunction with a psychiatrist until symptoms are stabilized.5 Psychosis does not always require hospitalization. But urgent psychiatry referral is recommended, if possible. Consider admission to a psychiatric inpatient unit for anyone who poses a danger to self or others.8,10
Treatment for schizophrenia is most effective with an interprofessional and collaborative approach that includes medication, psychological treatment, social supports, and primary care clinical management.11,12 The last aspect takes on particular importance given that people with schizophrenia, compared with the general population, have a higher incidence of medical illness, particularly cardiovascular disease.13
Medications (TABLE 15,8) are grouped into first-generation antipsychotics (FGAs) and second-generation, or atypical, antipsychotics (SGAs), with the 2 classes being equally effective.14-16 Quality of life is also similar at one year for patients treated with either drug class.14
Adverse effects can differ. The main difference between these medications is their adverse effect profiles. FGAs cause extrapyramidal symptoms (dystonia, akathisia, and tardive dyskinesia) more often than SGAs. Among the SGAs, olanzapine, asenapine, paliperidone, clozapine, and quetiapine cause significant weight gain, glucose dysregulation, and lipid abnormalities.5,8,12,17 Clozapine is associated with agranulocytosis, as well. Risperidone causes mild to moderate weight gain.5,8,12,17 Aripiprazole, lurasidone, and ziprasidone are considered weight neutral and cause no significant glucose dysregulation or lipid abnormalities.5,8,12,17 All antipsychotics can cause QT prolongation and neuroleptic malignant syndrome.5,8,12,17
Keys to successful treatment. Antipsychotics are most effective in treating positive symptoms of schizophrenia and show limited, if any, effect on negative or cognitive symptoms.18,19 Give patients an adequate trial of therapy (at least 4 weeks at a therapeutic dose) before discontinuing the drug or offering a different medication.20 All patients who report symptom relief while receiving antipsychotics should receive maintenance therapy.12
As with all chronic illnesses, success in managing schizophrenia requires patient adherence to the medication regimen. Discontinuation of antipsychotics is a common problem in schizophrenia, resulting in relapse. Long-acting injectable agents (LAIs) were developed to address this problem (TABLE 2).21 Although LAIs are typically used to ensure adherence during maintenance treatment, recent research has suggested they may also be effective for patients with early-phase or first-episode disease.22
What to watch for. Patients on SGAs may develop metabolic abnormalities, and ongoing monitoring of relevant parameters is key (TABLE 323-27). More frequent monitoring may be necessary in patients with cardiovascular risk factors. Continue antipsychotics for at least 6 months to prevent relapse.12 Also keep in mind the “Choosing Wisely” recommendation from the American Psychiatric Association of not prescribing 2 or more antipsychotics concurrently.28
Adjunctive treatment should also be offered
In addition to receiving medication, patients with schizophrenia should be offered adjunctive therapies such as cognitive behavioral therapy, family intervention, and social skills training.10-12 Among patients with schizophrenia, the incidences of anxiety disorder, panic symptoms, posttraumatic stress disorder, and obsessive compulsive disorder are higher than in the general population.29 To address these conditions, medications such as selective serotonin reuptake inhibitors and anxiolytics can be used simultaneously with antipsychotic agents.
CLINICAL COURSE AND PROGNOSIS CAN VARY
Schizophrenia can have a variable clinical course that includes remissions and exacerbations, or it can follow a more persistently chronic course.
Mortality for patients with schizophrenia is 2 to 3 times higher than that of the general population.30 Most deaths are due to an increased incidence of cardiovascular disease, respiratory illness, cancer, stroke, and other thromboembolic events.30
The lifetime prevalence of suicide attempts among individuals with schizophrenia is 20% to 40%,31 and approximately 5% complete suicide.32 Risk factors include command hallucinations, a history of suicide attempts, intoxication with substances, anxiety, and physical pain.32 Clozapine has been shown to reduce suicide risk and may be considered for patients who are at high risk for suicide.32
Therapeutic response varies among patients with schizophrenia, with one-third remaining symptomatic despite adequate treatment regimens.4
CARE MANAGERS CAN HELP ADDRESS BARRIERS TO CARE
A review of the literature suggests that up to one-third of individuals with serious mental illnesses who have had some contact with the mental health system disengage from care.12 Poor engagement may lead to worse clinical outcomes, with symptom relapse and re-hospitalizations. Disengagement from treatment may indicate a patient’s belief that treatment is not necessary, is not meeting his or her needs, or is not being provided in a collaborative manner.
Although shared decision-making is difficult with patients who have schizophrenia, emerging evidence suggests that this approach coupled with patient-centered care will improve engagement with mental health treatment.12 Models of integrated care are being developed and have shown promise in ensuring access to behavioral health for these patients.34
CASE
The primary care physician talked with Mr. R and his mother about the diagnosis of schizophrenia. He screened for suicide risk, and the patient denied having suicidal thoughts. Both the patient and his mother agreed to his starting medication.
Blood and urine samples were collected for a CBC and ESR, as well as to evaluate renal function, electrolytes, glucose, TSH, vitamin B12, folic acid, ANAs, and HIV antibodies. A serum FTA-ABS test was done, as was a urine culture and sensitivity test and a toxicology screen. Because of the patient’s obesity, the physician decided to prescribe a weight-neutral SGA, aripiprazole 10 mg/d. The physician spoke with the clinic’s care coordinator to schedule an appointment with the psychiatry intake department and to follow up on the phone with the patient and his mother. He also scheduled a follow-up appointment for 2 weeks later.
At the follow-up visit, the patient showed no improvement. His blood and urine test results revealed no metabolic abnormalities or infectious or inflammatory illnesses. His urine toxicology result showed no illicit substances. The physician increased the dosage of aripiprazole to 15 mg/d and asked the patient to return in 2 weeks.
At the next follow-up visit, the patient was more verbal and said he was not hearing voices. His mother also acknowledged an improvement. He had already been scheduled for a psychiatry intake appointment, and he and his mother were reminded about this. Mr. R was also asked to make a follow-up primary care appointment for one month from the current visit.
CORRESPONDENCE
Rajesh (Fnu) Rajesh, MD, MetroHealth Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; frajesh@metrohealth.org.
THE CASE
Steven R,* a 21-year-old man, visited the clinic accompanied by his mother. He did not speak much, and his mother provided his history. Over the previous 2 months, she had overheard him whispering in an agitated voice, even though no one else was nearby. And, lately, he refused to answer or make calls on his cell phone, claiming that if he did it would activate a deadly chip that had been implanted in his brain by evil aliens. He also stopped attending classes at the community college. He occasionally had a few beers with his friends, but he had never been known to abuse alcohol or use other recreational drugs.
How would you proceed with this patient?
* The patient’s name has been changed to protect his identity.
CHARACTERISTICS AND SCOPE OF SCHIZOPHRENIA
Schizophrenia is a psychotic illness in which the individual loses contact with reality and often experiences hallucinations, delusions, or thought disorders. Criteria for schizophrenia described in the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-5) include signs and symptoms of at least 6 months’ duration, as well as at least one month of active-phase positive and negative symptoms.1
Delusions, hallucinations, disorganized speech, and disorganized behavior are examples of positive symptoms. Negative symptoms include a decrease in the range and intensity of expressed emotions (ie, affective flattening) and a diminished initiation of goal-directed activities (ie, avolition).
Approximately 7 in 1000 people will develop the disorder in their lifetime.2 Schizophrenia is considered a “serious mental illness” because of its chronic course and often poor long-term social and vocational outcomes.3,4 Symptom onset is generally between late adolescence and the mid-30s.5
Getting closer to understanding its origin
Both genetic susceptibility and environmental factors influence the incidence of schizophrenia.4 Newer models of the disease have identified genes (ZDHHC8 and DTNBP1) whose mutations may increase the risk of schizophrenia.6 Physiologic insults during fetal life—hypoxia, maternal infection, maternal stress, and maternal malnutrition—account for a small portion of schizophrenia cases.6
Abnormalities in neurotransmission are the basis for theories on the pathophysiology of schizophrenia. Most of these theories center on either an excess or a deficiency of neurotransmitters, including dopamine, serotonin, and glutamate. Other theories implicate aspartate, glycine, and gamma-aminobutyric acid as part of the neurochemical imbalance of schizophrenia.7
ESTABLISHING A DIAGNOSIS
Although psychotic symptoms may be a prominent part of schizophrenia, not all psychoses indicate a primary psychiatric disorder such as schizophrenia. Broadly, psychoses can be categorized as primary or secondary.
Primary psychoses include schizophrenia, schizoaffective disorder, schizophreniform disorder, brief psychotic disorder, delusional disorder, and mood disorders (major depressive disorder and borderline personality disorder) with psychotic features.1 Difficulty in distinguishing between these entities can necessitate referral to a psychiatrist.
Secondary psychoses arise from a precursor such as delirium, dementia, medical illness, or adverse effects of medications or illicit substances. Medical illnesses that cause psychotic symptoms include: 5,8
- seizures (especially temporal lobe epilepsy),
- cerebrovascular accidents,
- intracranial space-occupying lesions,
- neuropsychiatric disorders (eg, Wilson’s or Parkinson’s disease),
- endocrine disorders (eg, thyroid or adrenal disease),
- autoimmune disease (eg, systemic lupus erythematosus, Hashimoto encephalopathy),
- deficiencies of vitamins A, B1, B12, or niacin,
- infections (eg, human immunodeficiency virus [HIV], encephalitis, parasites, and prion disease),
- narcolepsy, and
- metabolic disease (eg, acute intermittent porphyria, Tay-Sach’s disease, Niemann-Pick disease).
Several recreational drugs can cause psychotic symptoms: cocaine, amphetamines, cannabis, synthetic cannabinoids, inhalants, opioids, and hallucinogens. Psychotic symptoms can also appear during withdrawal from alcohol (delirium tremens) and from sedative hypnotics such as benzodiazepines. Prescribed medications such as anticholinergics, corticosteroids, dopaminergic agents (L-dopa), stimulants (amphetamines), and interferons can also induce psychotic symptoms.
First rule out causes of secondary psychosis
Rule out causes of secondary psychosis by conducting a detailed history and physical examination and ordering appropriate lab tests and imaging studies. If the patient’s psychosis is of recent onset, make sure the laboratory work-up includes
Consider cranial computed tomography or magnetic resonance imaging if there are focal neurologic deficits or if the patient’s presentation is atypical (eg, new onset psychosis in old age).9 Clinical presentation may also indicate a need for electroencephalography, ceruloplasmin measurement, a dexamethasone suppression test, a corticotropin stimulation test, 24-hour urine porphyrin and copper assays, chest radiography, or cerebrospinal fluid analysis.9
FACTORS TO CONSIDER IN TREATMENT DECISIONS
Although primary care physicians may encounter individuals experiencing their first episode of psychosis, it’s more likely that patients presenting with signs and symptoms of the disorder have been experiencing them for some time and have received no psychiatric care. In both instances, schizophrenia is best managed in conjunction with a psychiatrist until symptoms are stabilized.5 Psychosis does not always require hospitalization. But urgent psychiatry referral is recommended, if possible. Consider admission to a psychiatric inpatient unit for anyone who poses a danger to self or others.8,10
Treatment for schizophrenia is most effective with an interprofessional and collaborative approach that includes medication, psychological treatment, social supports, and primary care clinical management.11,12 The last aspect takes on particular importance given that people with schizophrenia, compared with the general population, have a higher incidence of medical illness, particularly cardiovascular disease.13
Medications (TABLE 15,8) are grouped into first-generation antipsychotics (FGAs) and second-generation, or atypical, antipsychotics (SGAs), with the 2 classes being equally effective.14-16 Quality of life is also similar at one year for patients treated with either drug class.14
Adverse effects can differ. The main difference between these medications is their adverse effect profiles. FGAs cause extrapyramidal symptoms (dystonia, akathisia, and tardive dyskinesia) more often than SGAs. Among the SGAs, olanzapine, asenapine, paliperidone, clozapine, and quetiapine cause significant weight gain, glucose dysregulation, and lipid abnormalities.5,8,12,17 Clozapine is associated with agranulocytosis, as well. Risperidone causes mild to moderate weight gain.5,8,12,17 Aripiprazole, lurasidone, and ziprasidone are considered weight neutral and cause no significant glucose dysregulation or lipid abnormalities.5,8,12,17 All antipsychotics can cause QT prolongation and neuroleptic malignant syndrome.5,8,12,17
Keys to successful treatment. Antipsychotics are most effective in treating positive symptoms of schizophrenia and show limited, if any, effect on negative or cognitive symptoms.18,19 Give patients an adequate trial of therapy (at least 4 weeks at a therapeutic dose) before discontinuing the drug or offering a different medication.20 All patients who report symptom relief while receiving antipsychotics should receive maintenance therapy.12
As with all chronic illnesses, success in managing schizophrenia requires patient adherence to the medication regimen. Discontinuation of antipsychotics is a common problem in schizophrenia, resulting in relapse. Long-acting injectable agents (LAIs) were developed to address this problem (TABLE 2).21 Although LAIs are typically used to ensure adherence during maintenance treatment, recent research has suggested they may also be effective for patients with early-phase or first-episode disease.22
What to watch for. Patients on SGAs may develop metabolic abnormalities, and ongoing monitoring of relevant parameters is key (TABLE 323-27). More frequent monitoring may be necessary in patients with cardiovascular risk factors. Continue antipsychotics for at least 6 months to prevent relapse.12 Also keep in mind the “Choosing Wisely” recommendation from the American Psychiatric Association of not prescribing 2 or more antipsychotics concurrently.28
Adjunctive treatment should also be offered
In addition to receiving medication, patients with schizophrenia should be offered adjunctive therapies such as cognitive behavioral therapy, family intervention, and social skills training.10-12 Among patients with schizophrenia, the incidences of anxiety disorder, panic symptoms, posttraumatic stress disorder, and obsessive compulsive disorder are higher than in the general population.29 To address these conditions, medications such as selective serotonin reuptake inhibitors and anxiolytics can be used simultaneously with antipsychotic agents.
CLINICAL COURSE AND PROGNOSIS CAN VARY
Schizophrenia can have a variable clinical course that includes remissions and exacerbations, or it can follow a more persistently chronic course.
Mortality for patients with schizophrenia is 2 to 3 times higher than that of the general population.30 Most deaths are due to an increased incidence of cardiovascular disease, respiratory illness, cancer, stroke, and other thromboembolic events.30
The lifetime prevalence of suicide attempts among individuals with schizophrenia is 20% to 40%,31 and approximately 5% complete suicide.32 Risk factors include command hallucinations, a history of suicide attempts, intoxication with substances, anxiety, and physical pain.32 Clozapine has been shown to reduce suicide risk and may be considered for patients who are at high risk for suicide.32
Therapeutic response varies among patients with schizophrenia, with one-third remaining symptomatic despite adequate treatment regimens.4
CARE MANAGERS CAN HELP ADDRESS BARRIERS TO CARE
A review of the literature suggests that up to one-third of individuals with serious mental illnesses who have had some contact with the mental health system disengage from care.12 Poor engagement may lead to worse clinical outcomes, with symptom relapse and re-hospitalizations. Disengagement from treatment may indicate a patient’s belief that treatment is not necessary, is not meeting his or her needs, or is not being provided in a collaborative manner.
Although shared decision-making is difficult with patients who have schizophrenia, emerging evidence suggests that this approach coupled with patient-centered care will improve engagement with mental health treatment.12 Models of integrated care are being developed and have shown promise in ensuring access to behavioral health for these patients.34
CASE
The primary care physician talked with Mr. R and his mother about the diagnosis of schizophrenia. He screened for suicide risk, and the patient denied having suicidal thoughts. Both the patient and his mother agreed to his starting medication.
Blood and urine samples were collected for a CBC and ESR, as well as to evaluate renal function, electrolytes, glucose, TSH, vitamin B12, folic acid, ANAs, and HIV antibodies. A serum FTA-ABS test was done, as was a urine culture and sensitivity test and a toxicology screen. Because of the patient’s obesity, the physician decided to prescribe a weight-neutral SGA, aripiprazole 10 mg/d. The physician spoke with the clinic’s care coordinator to schedule an appointment with the psychiatry intake department and to follow up on the phone with the patient and his mother. He also scheduled a follow-up appointment for 2 weeks later.
At the follow-up visit, the patient showed no improvement. His blood and urine test results revealed no metabolic abnormalities or infectious or inflammatory illnesses. His urine toxicology result showed no illicit substances. The physician increased the dosage of aripiprazole to 15 mg/d and asked the patient to return in 2 weeks.
At the next follow-up visit, the patient was more verbal and said he was not hearing voices. His mother also acknowledged an improvement. He had already been scheduled for a psychiatry intake appointment, and he and his mother were reminded about this. Mr. R was also asked to make a follow-up primary care appointment for one month from the current visit.
CORRESPONDENCE
Rajesh (Fnu) Rajesh, MD, MetroHealth Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109; frajesh@metrohealth.org.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5). Washington, DC: American Psychiatric Publishing; 2013.
2. McGrath J, Saha S, Chant D, et al. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67-76.
3. Henry LP, Amminger GP, Harris MG, et al. The EPPIC follow-up study of first-episode psychosis: longer-term and clinical and functional outcome 7 years after index admission. J Clin Psychiatry. 2010;71:716-728.
4. van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635-645.
5. Holder SD, Wayhs A. Schizophrenia. Am Fam Phys. 2014;90:775-82.
6. Lakhan SE, Vieira KF. Schizophrenia pathophysiology: are we any closer to a complete model? Ann Gen Psychiatry. 2009;8:12.
7. Crismon L, Argo TR, Buckley PF. Schizophrenia. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, New York: McGraw-Hill; 2014:1019-1046.
8. Viron M, Baggett T, Hill M, et al. Schizophrenia for primary care providers: how to contribute to the care of a vulnerable patient population. Am J Med. 2012;125:223-230.
9. Freudenreich O, Charles Schulz SC, Goff DC. Initial medical work-up of first-episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3:10-18.
10. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: Prevention and management. 2014. Available at: http://www.nice.org.uk/Guidance/CG178. Accessed January 3, 2017.
11. Guo X, Zhai J, Liu Z, et al. Effect of antipsychotic medication alone vs combined with psychosocial intervention on outcomes of early-stage schizophrenia: a randomized 1-year study. Arch Gen Psychiatry. 2010;67:895-904.
12. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2009;36:94-103.
13. Viron MJ, Stern TA. The impact of serious mental illness on health and healthcare. Psychosomatics. 2010;51:458-465.
14. Jones PB, Barnes TRE, Davies L, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry. 2006;63:1079-1087.
15. Hartling L, Abou-Setta AM, Dursun S, et al. Antipsychotics in adults with schizophrenia: comparative effectiveness of first-generation versus second-generation medications: a systematic review and meta-analysis. Ann Intern Med. 2012;157:498-511.
16. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209-1223.
17. Tandon R. Antipsychotics in the treatment of schizophrenia: an overview. J Clin Psychiatry. 2011;72(suppl 1):4-8.
18. Salimi K, Jarskog LF, Lieberman JA. Antipsychotic drugs for first-episode schizophrenia: a comparative review. CNS Drugs. 2009;23:837-855.
19. Fusar-Poli P, Papanastasiou E, Stahl D, et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41:892-899.
20. Moore TA, Buchanan RW, Buckley PF, et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J Clin Psychiatry. 2007;68:1751-1762.
21. Bera R. Patient outcomes within schizophrenia treatment: a look at the role of long-acting injectable antipsychotics. J Clin Psychiatry. 2014;75(suppl 2):30-33.
22. Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
23. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123:225-233.
24. De Hert M, Vancampfort D, Correll CU, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. 2011;199:99-105.
25. Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—a systematic review and meta-analysis. Schizophr Bull. 2013;39:306-318.
26. Lieberman JA, Merrill D, Parameswaran S. APA guidance on the use of antipsychotic drugs and cardiac sudden death.
27. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161:1334-1349.
28. American Psychiatric Association. Five things physicians and patients should question. Available at: http://www.choosingwisely.org/societies/american-psychiatric-association/. Accessed February 28, 2017.
29. Buckley PF, Miller BJ, Lehrer DS, et al. Psychiatric comorbidities and schizophrenia. Schizophr Bull. 2009;35:383-402.
30. Lwin AM, Symeon C, Jan F, et al. Morbidity and mortality in schizophrenia. Br J Hosp Med (Lond). 2011;72:628-630.
31. Pompili M, Amador XF, Girardi P, et al. Suicide risk in schizophrenia: learning from the past to change the future. Ann Gen Psychiatry. 2007;6:10.
32. Hor K, Taylor M. Suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol. 2010;24(4 suppl):81-90.
33. Druss BG, von Esenwein SA, Compton MT, et al. A randomized trial of medical care management for community mental health settings: the Primary Care Access, Referral, and Evaluation (PCARE) study. Am J Psychiatry. 2010;167:151-159.
34. Gerrity M. Evolving models of behavioral health integration: Evidence update 2010-2015. Milbank Memorial Fund. Available at: https://www.milbank.org/wp-content/uploads/2016/05/Evolv ing-Models-of-BHI.pdf. Accessed January 11, 2018.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5). Washington, DC: American Psychiatric Publishing; 2013.
2. McGrath J, Saha S, Chant D, et al. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. 2008;30:67-76.
3. Henry LP, Amminger GP, Harris MG, et al. The EPPIC follow-up study of first-episode psychosis: longer-term and clinical and functional outcome 7 years after index admission. J Clin Psychiatry. 2010;71:716-728.
4. van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635-645.
5. Holder SD, Wayhs A. Schizophrenia. Am Fam Phys. 2014;90:775-82.
6. Lakhan SE, Vieira KF. Schizophrenia pathophysiology: are we any closer to a complete model? Ann Gen Psychiatry. 2009;8:12.
7. Crismon L, Argo TR, Buckley PF. Schizophrenia. In: DiPiro JT, Talbert RL, Yee GC, et al, eds. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, New York: McGraw-Hill; 2014:1019-1046.
8. Viron M, Baggett T, Hill M, et al. Schizophrenia for primary care providers: how to contribute to the care of a vulnerable patient population. Am J Med. 2012;125:223-230.
9. Freudenreich O, Charles Schulz SC, Goff DC. Initial medical work-up of first-episode psychosis: a conceptual review. Early Interv Psychiatry. 2009;3:10-18.
10. National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: Prevention and management. 2014. Available at: http://www.nice.org.uk/Guidance/CG178. Accessed January 3, 2017.
11. Guo X, Zhai J, Liu Z, et al. Effect of antipsychotic medication alone vs combined with psychosocial intervention on outcomes of early-stage schizophrenia: a randomized 1-year study. Arch Gen Psychiatry. 2010;67:895-904.
12. Kreyenbuhl J, Buchanan RW, Dickerson FB, et al. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2009. Schizophr Bull. 2009;36:94-103.
13. Viron MJ, Stern TA. The impact of serious mental illness on health and healthcare. Psychosomatics. 2010;51:458-465.
14. Jones PB, Barnes TRE, Davies L, et al. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch Gen Psychiatry. 2006;63:1079-1087.
15. Hartling L, Abou-Setta AM, Dursun S, et al. Antipsychotics in adults with schizophrenia: comparative effectiveness of first-generation versus second-generation medications: a systematic review and meta-analysis. Ann Intern Med. 2012;157:498-511.
16. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209-1223.
17. Tandon R. Antipsychotics in the treatment of schizophrenia: an overview. J Clin Psychiatry. 2011;72(suppl 1):4-8.
18. Salimi K, Jarskog LF, Lieberman JA. Antipsychotic drugs for first-episode schizophrenia: a comparative review. CNS Drugs. 2009;23:837-855.
19. Fusar-Poli P, Papanastasiou E, Stahl D, et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. 2015;41:892-899.
20. Moore TA, Buchanan RW, Buckley PF, et al. The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J Clin Psychiatry. 2007;68:1751-1762.
21. Bera R. Patient outcomes within schizophrenia treatment: a look at the role of long-acting injectable antipsychotics. J Clin Psychiatry. 2014;75(suppl 2):30-33.
22. Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
23. Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2010;123:225-233.
24. De Hert M, Vancampfort D, Correll CU, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. 2011;199:99-105.
25. Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—a systematic review and meta-analysis. Schizophr Bull. 2013;39:306-318.
26. Lieberman JA, Merrill D, Parameswaran S. APA guidance on the use of antipsychotic drugs and cardiac sudden death.
27. Marder SR, Essock SM, Miller AL, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161:1334-1349.
28. American Psychiatric Association. Five things physicians and patients should question. Available at: http://www.choosingwisely.org/societies/american-psychiatric-association/. Accessed February 28, 2017.
29. Buckley PF, Miller BJ, Lehrer DS, et al. Psychiatric comorbidities and schizophrenia. Schizophr Bull. 2009;35:383-402.
30. Lwin AM, Symeon C, Jan F, et al. Morbidity and mortality in schizophrenia. Br J Hosp Med (Lond). 2011;72:628-630.
31. Pompili M, Amador XF, Girardi P, et al. Suicide risk in schizophrenia: learning from the past to change the future. Ann Gen Psychiatry. 2007;6:10.
32. Hor K, Taylor M. Suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol. 2010;24(4 suppl):81-90.
33. Druss BG, von Esenwein SA, Compton MT, et al. A randomized trial of medical care management for community mental health settings: the Primary Care Access, Referral, and Evaluation (PCARE) study. Am J Psychiatry. 2010;167:151-159.
34. Gerrity M. Evolving models of behavioral health integration: Evidence update 2010-2015. Milbank Memorial Fund. Available at: https://www.milbank.org/wp-content/uploads/2016/05/Evolv ing-Models-of-BHI.pdf. Accessed January 11, 2018.
Ensuring prompt recognition and treatment of panic disorder
THE CASE
Lorna D* was seen by her primary care physician (PCP) as follow-up to a visit she made to the emergency department (ED). The 37 year old had gone to the ED 4 times in the previous year. Each time she presented with tachycardia, dyspnea, nausea, numbness in her extremities, and a fear that she was having a heart attack. In spite of negative work-ups at each visit (electrocardiogram, cardiac enzymes, complete blood count, toxicology screen, Holter monitoring), Ms. D was terrified that the ED doctors were missing something. She was still “rattled” by the chest pain and shortness of breath she had experienced. Mild symptoms were persisting and she was worried that she would have a heart attack and die without the treatment she believed she needed.
How would you proceed with this patient?
*The patient’s name has been changed to protect her privacy.
MANY PANIC ATTACKS PROMPT AN ED VISIT
Panic disorder (PD) is characterized by the spontaneous and unexpected occurrence of panic attacks, and by at least one month of persistent worry about having another attack or significant maladaptive behaviors related to the attack. Frequency of such attacks can vary from several a day to only a few per year. In a panic attack, an intense fear develops abruptly and peaks within 10 minutes of onset. At least 4 of the following 13 symptoms must accompany the attack, according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5):1
- palpitations, pounding heart, or accelerated heart rate
- sweating
- trembling or shaking
- sensations of shortness of breath or smothering
- feeling of choking
- chest pain or discomfort
- nausea or abdominal distress
- feeling dizzy, unsteady, lightheaded, or faint
- de-realization (feelings of unreality) or depersonalization (being detached from oneself)
- fear of losing control or going crazy
- fear of dying
- paresthesia (numbness or tingling sensations)
- chills or hot flushes.
Lifetime incidence rates of panic disorder are 1% to 3% for the general population.2 A closer look at patients presenting to the ED with chest pain reveals that 17% to 25% meet criteria for panic disorder.3,4 And an estimated 6% of individuals experiencing a panic attack present to their primary physician.5 Patients with panic disorder tend to use health care resources at a disproportionately high rate.6
An international review of panic disorder research suggests the average age of onset for PD is 32 years.7 Triggers can vary widely and no single
Differential goes far beyond myocardial infarction
Many medical conditions can mimic panic disorder symptoms: cardiovascular, pulmonary, and neurologic diseases; endocrine diseases (eg, hyperthyroidism); drug intoxication (eg, stimulants such as cocaine, amphetamines); drug withdrawal (eg, benzodiazepines, alcohol, sedative-hypnotics); and ingestion of excessive quantities of caffeine. Common comorbid medical disorders include asthma, coronary artery disease, cancer, thyroid disease, hypertension, ulcer, and migraine headaches.8
When patients present with panic-like symptoms, suspect a possible medical condition when those symptoms include ataxia, altered mental status, or loss of bladder control, or when onset of panic symptoms occur later in life for a patient with no significant psychiatric history.
RULE OUT ORGANIC CAUSES
In addition to obtaining a complete history and doing a physical exam on patients with panic-like symptoms, you’ll also need to ensure that the following are done: a neurologic examination, standard laboratory testing (thyroid function, complete blood cell count, chemistry panel), and possible additional testing (eg, urine toxicology screen and D-dimer assay to exclude pulmonary embolism).
If organic causes are ruled out, focus on a psychiatric assessment:9
- history of the present illness (onset, symptoms, frequency, predisposing/precipitating factors)
- psychiatric history
- history of substance use
- family history of psychiatric disorders (especially anxiety disorders)
- social history (life events, including those preceding onset of panic; history of child abuse)
- medications
- mental status examination
- safety (panic disorder is associated with higher risk of suicidal ideation).
TREATMENT INCLUDES CBT AND MEDICATION
PD is a chronic disease with a variable course, but the long-term prognosis is good. PD is usually treated in an outpatient setting. Consider hospitalization if the patient is suicidal, if the potential for life-threatening withdrawal symptoms is high (as with alcohol or benzodiazepines), or if the symptoms are severely debilitating or attempted outpatient treatment is unsuccessful. Pharmacologic and psychotherapeutic interventions are used for PD (FIGURE9), although there is not enough evidence to recommend one vs the other or combination therapy vs monotherapy.9
CASE
For Ms. D, all medical test results came back negative, and the psychiatric assessment revealed that she met the DSM-5 criteria for panic disorder. Counting on the strength of their relationship, her physician talked to her about PD and discussed treatment options, which included counseling, medication, or both. Ms. D agreed to a referral for cognitive behavioral therapy (CBT) with a psychologist embedded at her physician’s primary care clinic and to begin taking medication. Her PCP started her on sertraline 25 mg/d.
In CBT, Ms. D’s psychologist taught her about “fight or flight” and explained that it was a normal physiologic response that could lead to panic. Ms. D. learned to approach her physical symptoms in a different way, and how to breathe in a way that slowed her panic reaction.
Consider SSRIs and SNRIs
First-line medication is a selective serotonin reuptake inhibitor (SSRI) or a serotonin-norepinephrine reuptake inhibitor (SNRI) due to the better tolerability and lower adverse effect profile of these classes compared with the tricyclic antidepressants or monoamine oxidase inhibitors. MAOIs are usually reserved for patients in whom multiple medication trials have failed.
Special considerations. American Psychiatric Association guidelines advise starting with a very low dose of an SSRI or SNRI, such as paroxetine 10 mg/d (although many clinicians start lower, at 5 mg/d), to avoid hypersensitivity reactions. Gradually titrate the dose upward within 3 to 7 days after initiation until a therapeutic dose is reached over 2 to 6 weeks. Schedule follow-up visits for every one to 2 weeks at the beginning of treatment and every 2 to 4 weeks until the therapeutic dose is reached. Assess safety/suicidality at each visit.
Keep in mind that the onset of therapeutic effect is between 2 and 4 weeks, but that clinical response can take up to 8 to 12 weeks. Continue pharmacotherapy for at least one year. When discontinuing the medication, taper it slowly, and monitor the patient for withdrawal symptoms and recurrence of PD.9
Consider adding a benzodiazepine if symptoms are debilitating.9 Keep in mind, though, that the potential for addiction with these medications is high and they are intended to be used for only 4 to 12 weeks.8 Onset of action is within the first week, and a scheduled dosing regimen is preferred to giving the medication as needed. The starting dose (eg, clonazepam 0.25 mg bid)9 may be increased 3 to 5 days following initiation.
The evidence supports the use of CBT for panic disorder
CBT is an evidenced-based treatment for panic disorder.10-13 Up to 75% of patients treated with CBT are panic free within 4 months.10 Other techniques proven effective are progressive muscle relaxation training, breathing retraining, psycho-education, exposure, and imagery.14
Treatment with medications and CBT either combined or used individually is effective in 80% to 90% of cases.15 CBT has been shown to decrease the likelihood of relapse in the year following treatment.15 Good premorbid functioning and a brief duration of symptoms increase the likelihood of a good prognosis.15
WHEN TO REFER TO A PSYCHIATRIST
Consider referral to a psychiatrist when patients have a comorbid psychiatric condition that complicates the clinical picture (eg, substance abuse disorder), if the diagnosis is uncertain, or if the patient does not respond to one or 2 adequate trials of medication and psychotherapy. Although psychiatric follow-up is sometimes difficult due to a lack of psychiatrist availability locally, it is a best-practice recommendation.
CASE
Ten days after Ms. D started the sertraline 25 mg/d, she called the PCP to report daily diarrhea. She stopped the sertraline on her own and asked for another medication. She also expressed her frustration with the severity of the symptoms. She was having 3 to 5 panic attacks daily and had been missing many days from work.
On the day of her follow-up PCP appointment, Ms. D also saw the psychologist. She reported that she’d been practicing relaxation breathing, tracking her panic attacks, limiting caffeine intake, and exercising regularly. But the attacks were still occurring.
The PCP switched her to paroxetine 10 mg/d and, due to the severity of the symptoms, prescribed clonazepam 0.5 mg bid. Two weeks later, Ms. D reported that she was feeling a little better, had returned to work, and was hopeful that she would be her “normal self again.” The PCP planned to encourage continuation of CBT, titrate the paroxetine to 20 to 40 mg/d based on symptoms, and to slowly taper the clonazepam toward discontinuation in the near future.
CORRESPONDENCE
Eric H. Berko, PhD, Case Western Reserve University School of Medicine, Department of Family Medicine, MetroHealth Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109-7878; eberko@metrohealth.org.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing: Arlington, VA; 2013.
2. Kumar S, Oakley-Browne M. Panic disorder. Clin Evid. 2006;15:1438-1452.
3. Yingling KW, Wulsin LR, Arnold LM, et al. Estimated prevalences of panic disorder and depression among consecutive patients seen in an emergency department with acute chest pain. J Gen Intern Med. 1993;8:231-235.
4. Fleet RP, Dupuis G, Marchand A, et al. Panic disorder in emergency department chest pain patients: prevalence, comorbidity, suicidal ideation, and physician recognition. Am J Med. 1996;101:371-380.
5. Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272:1749-1756.
6. Taylor CB. Panic disorder. BMJ. 2006;332:951-955.
7. de Jonge P, Roest AM, Lim CC, et al. Cross-national epidemiology of panic disorder and panic attacks in the world mental health surveys. Depress Anxiety. 2016;33:1155-1177.
8. Sadock BJ, Sadock VA, Ruiz P. Panic disorder. In: Kaplan & Sadock’s Synopsis of Psychiatry. 11th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015:392-397.
9. Stein MB, Goin MK, Pollack MH, et al. Practice guideline for the treatment of patients with panic disorder, 2nd ed. 2010. American Psychiatric Association; Washington DC. Available at: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Accessed October 26, 2017.
10. Westen D, Morrison K. A multidimensional meta-analysis of treatments for depression, panic, and generalized anxiety disorder: an empirical examination of the status of empirically supported therapies. J Consult Clin Psychol. 2001;69:875-899.
11. Gould RA, Otto MW, Pollack MH. A meta-analysis of treatment outcome for panic disorder. Available at: https://www.ncbi.nlm.nih.gov/books/NBK66380/. Accessed October 26, 2017.
12. Clum GA, Clum GA, Surls R. A meta-analysis of treatments for panic disorder. J Consult Clin Psychol. 1993;61:317-326.
13. Shear MK, Houck P, Greeno C, et al. Emotion-focused psychotherapy for patients with panic disorder. Am J Psychiatry. 2001;158:1993-1998.
14. Stewart RE., Chambless DL. Cognitive-behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77:595–606
15. Craske M. Psychotherapy for panic disorder in adults. Up to Date. 2017. Available at: https://www.uptodate.com/contents/psychotherapy-for-panic-disorder-with-or-without-agoraphobia-in-adults. Accessed October 26, 2017.
THE CASE
Lorna D* was seen by her primary care physician (PCP) as follow-up to a visit she made to the emergency department (ED). The 37 year old had gone to the ED 4 times in the previous year. Each time she presented with tachycardia, dyspnea, nausea, numbness in her extremities, and a fear that she was having a heart attack. In spite of negative work-ups at each visit (electrocardiogram, cardiac enzymes, complete blood count, toxicology screen, Holter monitoring), Ms. D was terrified that the ED doctors were missing something. She was still “rattled” by the chest pain and shortness of breath she had experienced. Mild symptoms were persisting and she was worried that she would have a heart attack and die without the treatment she believed she needed.
How would you proceed with this patient?
*The patient’s name has been changed to protect her privacy.
MANY PANIC ATTACKS PROMPT AN ED VISIT
Panic disorder (PD) is characterized by the spontaneous and unexpected occurrence of panic attacks, and by at least one month of persistent worry about having another attack or significant maladaptive behaviors related to the attack. Frequency of such attacks can vary from several a day to only a few per year. In a panic attack, an intense fear develops abruptly and peaks within 10 minutes of onset. At least 4 of the following 13 symptoms must accompany the attack, according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5):1
- palpitations, pounding heart, or accelerated heart rate
- sweating
- trembling or shaking
- sensations of shortness of breath or smothering
- feeling of choking
- chest pain or discomfort
- nausea or abdominal distress
- feeling dizzy, unsteady, lightheaded, or faint
- de-realization (feelings of unreality) or depersonalization (being detached from oneself)
- fear of losing control or going crazy
- fear of dying
- paresthesia (numbness or tingling sensations)
- chills or hot flushes.
Lifetime incidence rates of panic disorder are 1% to 3% for the general population.2 A closer look at patients presenting to the ED with chest pain reveals that 17% to 25% meet criteria for panic disorder.3,4 And an estimated 6% of individuals experiencing a panic attack present to their primary physician.5 Patients with panic disorder tend to use health care resources at a disproportionately high rate.6
An international review of panic disorder research suggests the average age of onset for PD is 32 years.7 Triggers can vary widely and no single
Differential goes far beyond myocardial infarction
Many medical conditions can mimic panic disorder symptoms: cardiovascular, pulmonary, and neurologic diseases; endocrine diseases (eg, hyperthyroidism); drug intoxication (eg, stimulants such as cocaine, amphetamines); drug withdrawal (eg, benzodiazepines, alcohol, sedative-hypnotics); and ingestion of excessive quantities of caffeine. Common comorbid medical disorders include asthma, coronary artery disease, cancer, thyroid disease, hypertension, ulcer, and migraine headaches.8
When patients present with panic-like symptoms, suspect a possible medical condition when those symptoms include ataxia, altered mental status, or loss of bladder control, or when onset of panic symptoms occur later in life for a patient with no significant psychiatric history.
RULE OUT ORGANIC CAUSES
In addition to obtaining a complete history and doing a physical exam on patients with panic-like symptoms, you’ll also need to ensure that the following are done: a neurologic examination, standard laboratory testing (thyroid function, complete blood cell count, chemistry panel), and possible additional testing (eg, urine toxicology screen and D-dimer assay to exclude pulmonary embolism).
If organic causes are ruled out, focus on a psychiatric assessment:9
- history of the present illness (onset, symptoms, frequency, predisposing/precipitating factors)
- psychiatric history
- history of substance use
- family history of psychiatric disorders (especially anxiety disorders)
- social history (life events, including those preceding onset of panic; history of child abuse)
- medications
- mental status examination
- safety (panic disorder is associated with higher risk of suicidal ideation).
TREATMENT INCLUDES CBT AND MEDICATION
PD is a chronic disease with a variable course, but the long-term prognosis is good. PD is usually treated in an outpatient setting. Consider hospitalization if the patient is suicidal, if the potential for life-threatening withdrawal symptoms is high (as with alcohol or benzodiazepines), or if the symptoms are severely debilitating or attempted outpatient treatment is unsuccessful. Pharmacologic and psychotherapeutic interventions are used for PD (FIGURE9), although there is not enough evidence to recommend one vs the other or combination therapy vs monotherapy.9
CASE
For Ms. D, all medical test results came back negative, and the psychiatric assessment revealed that she met the DSM-5 criteria for panic disorder. Counting on the strength of their relationship, her physician talked to her about PD and discussed treatment options, which included counseling, medication, or both. Ms. D agreed to a referral for cognitive behavioral therapy (CBT) with a psychologist embedded at her physician’s primary care clinic and to begin taking medication. Her PCP started her on sertraline 25 mg/d.
In CBT, Ms. D’s psychologist taught her about “fight or flight” and explained that it was a normal physiologic response that could lead to panic. Ms. D. learned to approach her physical symptoms in a different way, and how to breathe in a way that slowed her panic reaction.
Consider SSRIs and SNRIs
First-line medication is a selective serotonin reuptake inhibitor (SSRI) or a serotonin-norepinephrine reuptake inhibitor (SNRI) due to the better tolerability and lower adverse effect profile of these classes compared with the tricyclic antidepressants or monoamine oxidase inhibitors. MAOIs are usually reserved for patients in whom multiple medication trials have failed.
Special considerations. American Psychiatric Association guidelines advise starting with a very low dose of an SSRI or SNRI, such as paroxetine 10 mg/d (although many clinicians start lower, at 5 mg/d), to avoid hypersensitivity reactions. Gradually titrate the dose upward within 3 to 7 days after initiation until a therapeutic dose is reached over 2 to 6 weeks. Schedule follow-up visits for every one to 2 weeks at the beginning of treatment and every 2 to 4 weeks until the therapeutic dose is reached. Assess safety/suicidality at each visit.
Keep in mind that the onset of therapeutic effect is between 2 and 4 weeks, but that clinical response can take up to 8 to 12 weeks. Continue pharmacotherapy for at least one year. When discontinuing the medication, taper it slowly, and monitor the patient for withdrawal symptoms and recurrence of PD.9
Consider adding a benzodiazepine if symptoms are debilitating.9 Keep in mind, though, that the potential for addiction with these medications is high and they are intended to be used for only 4 to 12 weeks.8 Onset of action is within the first week, and a scheduled dosing regimen is preferred to giving the medication as needed. The starting dose (eg, clonazepam 0.25 mg bid)9 may be increased 3 to 5 days following initiation.
The evidence supports the use of CBT for panic disorder
CBT is an evidenced-based treatment for panic disorder.10-13 Up to 75% of patients treated with CBT are panic free within 4 months.10 Other techniques proven effective are progressive muscle relaxation training, breathing retraining, psycho-education, exposure, and imagery.14
Treatment with medications and CBT either combined or used individually is effective in 80% to 90% of cases.15 CBT has been shown to decrease the likelihood of relapse in the year following treatment.15 Good premorbid functioning and a brief duration of symptoms increase the likelihood of a good prognosis.15
WHEN TO REFER TO A PSYCHIATRIST
Consider referral to a psychiatrist when patients have a comorbid psychiatric condition that complicates the clinical picture (eg, substance abuse disorder), if the diagnosis is uncertain, or if the patient does not respond to one or 2 adequate trials of medication and psychotherapy. Although psychiatric follow-up is sometimes difficult due to a lack of psychiatrist availability locally, it is a best-practice recommendation.
CASE
Ten days after Ms. D started the sertraline 25 mg/d, she called the PCP to report daily diarrhea. She stopped the sertraline on her own and asked for another medication. She also expressed her frustration with the severity of the symptoms. She was having 3 to 5 panic attacks daily and had been missing many days from work.
On the day of her follow-up PCP appointment, Ms. D also saw the psychologist. She reported that she’d been practicing relaxation breathing, tracking her panic attacks, limiting caffeine intake, and exercising regularly. But the attacks were still occurring.
The PCP switched her to paroxetine 10 mg/d and, due to the severity of the symptoms, prescribed clonazepam 0.5 mg bid. Two weeks later, Ms. D reported that she was feeling a little better, had returned to work, and was hopeful that she would be her “normal self again.” The PCP planned to encourage continuation of CBT, titrate the paroxetine to 20 to 40 mg/d based on symptoms, and to slowly taper the clonazepam toward discontinuation in the near future.
CORRESPONDENCE
Eric H. Berko, PhD, Case Western Reserve University School of Medicine, Department of Family Medicine, MetroHealth Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109-7878; eberko@metrohealth.org.
THE CASE
Lorna D* was seen by her primary care physician (PCP) as follow-up to a visit she made to the emergency department (ED). The 37 year old had gone to the ED 4 times in the previous year. Each time she presented with tachycardia, dyspnea, nausea, numbness in her extremities, and a fear that she was having a heart attack. In spite of negative work-ups at each visit (electrocardiogram, cardiac enzymes, complete blood count, toxicology screen, Holter monitoring), Ms. D was terrified that the ED doctors were missing something. She was still “rattled” by the chest pain and shortness of breath she had experienced. Mild symptoms were persisting and she was worried that she would have a heart attack and die without the treatment she believed she needed.
How would you proceed with this patient?
*The patient’s name has been changed to protect her privacy.
MANY PANIC ATTACKS PROMPT AN ED VISIT
Panic disorder (PD) is characterized by the spontaneous and unexpected occurrence of panic attacks, and by at least one month of persistent worry about having another attack or significant maladaptive behaviors related to the attack. Frequency of such attacks can vary from several a day to only a few per year. In a panic attack, an intense fear develops abruptly and peaks within 10 minutes of onset. At least 4 of the following 13 symptoms must accompany the attack, according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5):1
- palpitations, pounding heart, or accelerated heart rate
- sweating
- trembling or shaking
- sensations of shortness of breath or smothering
- feeling of choking
- chest pain or discomfort
- nausea or abdominal distress
- feeling dizzy, unsteady, lightheaded, or faint
- de-realization (feelings of unreality) or depersonalization (being detached from oneself)
- fear of losing control or going crazy
- fear of dying
- paresthesia (numbness or tingling sensations)
- chills or hot flushes.
Lifetime incidence rates of panic disorder are 1% to 3% for the general population.2 A closer look at patients presenting to the ED with chest pain reveals that 17% to 25% meet criteria for panic disorder.3,4 And an estimated 6% of individuals experiencing a panic attack present to their primary physician.5 Patients with panic disorder tend to use health care resources at a disproportionately high rate.6
An international review of panic disorder research suggests the average age of onset for PD is 32 years.7 Triggers can vary widely and no single
Differential goes far beyond myocardial infarction
Many medical conditions can mimic panic disorder symptoms: cardiovascular, pulmonary, and neurologic diseases; endocrine diseases (eg, hyperthyroidism); drug intoxication (eg, stimulants such as cocaine, amphetamines); drug withdrawal (eg, benzodiazepines, alcohol, sedative-hypnotics); and ingestion of excessive quantities of caffeine. Common comorbid medical disorders include asthma, coronary artery disease, cancer, thyroid disease, hypertension, ulcer, and migraine headaches.8
When patients present with panic-like symptoms, suspect a possible medical condition when those symptoms include ataxia, altered mental status, or loss of bladder control, or when onset of panic symptoms occur later in life for a patient with no significant psychiatric history.
RULE OUT ORGANIC CAUSES
In addition to obtaining a complete history and doing a physical exam on patients with panic-like symptoms, you’ll also need to ensure that the following are done: a neurologic examination, standard laboratory testing (thyroid function, complete blood cell count, chemistry panel), and possible additional testing (eg, urine toxicology screen and D-dimer assay to exclude pulmonary embolism).
If organic causes are ruled out, focus on a psychiatric assessment:9
- history of the present illness (onset, symptoms, frequency, predisposing/precipitating factors)
- psychiatric history
- history of substance use
- family history of psychiatric disorders (especially anxiety disorders)
- social history (life events, including those preceding onset of panic; history of child abuse)
- medications
- mental status examination
- safety (panic disorder is associated with higher risk of suicidal ideation).
TREATMENT INCLUDES CBT AND MEDICATION
PD is a chronic disease with a variable course, but the long-term prognosis is good. PD is usually treated in an outpatient setting. Consider hospitalization if the patient is suicidal, if the potential for life-threatening withdrawal symptoms is high (as with alcohol or benzodiazepines), or if the symptoms are severely debilitating or attempted outpatient treatment is unsuccessful. Pharmacologic and psychotherapeutic interventions are used for PD (FIGURE9), although there is not enough evidence to recommend one vs the other or combination therapy vs monotherapy.9
CASE
For Ms. D, all medical test results came back negative, and the psychiatric assessment revealed that she met the DSM-5 criteria for panic disorder. Counting on the strength of their relationship, her physician talked to her about PD and discussed treatment options, which included counseling, medication, or both. Ms. D agreed to a referral for cognitive behavioral therapy (CBT) with a psychologist embedded at her physician’s primary care clinic and to begin taking medication. Her PCP started her on sertraline 25 mg/d.
In CBT, Ms. D’s psychologist taught her about “fight or flight” and explained that it was a normal physiologic response that could lead to panic. Ms. D. learned to approach her physical symptoms in a different way, and how to breathe in a way that slowed her panic reaction.
Consider SSRIs and SNRIs
First-line medication is a selective serotonin reuptake inhibitor (SSRI) or a serotonin-norepinephrine reuptake inhibitor (SNRI) due to the better tolerability and lower adverse effect profile of these classes compared with the tricyclic antidepressants or monoamine oxidase inhibitors. MAOIs are usually reserved for patients in whom multiple medication trials have failed.
Special considerations. American Psychiatric Association guidelines advise starting with a very low dose of an SSRI or SNRI, such as paroxetine 10 mg/d (although many clinicians start lower, at 5 mg/d), to avoid hypersensitivity reactions. Gradually titrate the dose upward within 3 to 7 days after initiation until a therapeutic dose is reached over 2 to 6 weeks. Schedule follow-up visits for every one to 2 weeks at the beginning of treatment and every 2 to 4 weeks until the therapeutic dose is reached. Assess safety/suicidality at each visit.
Keep in mind that the onset of therapeutic effect is between 2 and 4 weeks, but that clinical response can take up to 8 to 12 weeks. Continue pharmacotherapy for at least one year. When discontinuing the medication, taper it slowly, and monitor the patient for withdrawal symptoms and recurrence of PD.9
Consider adding a benzodiazepine if symptoms are debilitating.9 Keep in mind, though, that the potential for addiction with these medications is high and they are intended to be used for only 4 to 12 weeks.8 Onset of action is within the first week, and a scheduled dosing regimen is preferred to giving the medication as needed. The starting dose (eg, clonazepam 0.25 mg bid)9 may be increased 3 to 5 days following initiation.
The evidence supports the use of CBT for panic disorder
CBT is an evidenced-based treatment for panic disorder.10-13 Up to 75% of patients treated with CBT are panic free within 4 months.10 Other techniques proven effective are progressive muscle relaxation training, breathing retraining, psycho-education, exposure, and imagery.14
Treatment with medications and CBT either combined or used individually is effective in 80% to 90% of cases.15 CBT has been shown to decrease the likelihood of relapse in the year following treatment.15 Good premorbid functioning and a brief duration of symptoms increase the likelihood of a good prognosis.15
WHEN TO REFER TO A PSYCHIATRIST
Consider referral to a psychiatrist when patients have a comorbid psychiatric condition that complicates the clinical picture (eg, substance abuse disorder), if the diagnosis is uncertain, or if the patient does not respond to one or 2 adequate trials of medication and psychotherapy. Although psychiatric follow-up is sometimes difficult due to a lack of psychiatrist availability locally, it is a best-practice recommendation.
CASE
Ten days after Ms. D started the sertraline 25 mg/d, she called the PCP to report daily diarrhea. She stopped the sertraline on her own and asked for another medication. She also expressed her frustration with the severity of the symptoms. She was having 3 to 5 panic attacks daily and had been missing many days from work.
On the day of her follow-up PCP appointment, Ms. D also saw the psychologist. She reported that she’d been practicing relaxation breathing, tracking her panic attacks, limiting caffeine intake, and exercising regularly. But the attacks were still occurring.
The PCP switched her to paroxetine 10 mg/d and, due to the severity of the symptoms, prescribed clonazepam 0.5 mg bid. Two weeks later, Ms. D reported that she was feeling a little better, had returned to work, and was hopeful that she would be her “normal self again.” The PCP planned to encourage continuation of CBT, titrate the paroxetine to 20 to 40 mg/d based on symptoms, and to slowly taper the clonazepam toward discontinuation in the near future.
CORRESPONDENCE
Eric H. Berko, PhD, Case Western Reserve University School of Medicine, Department of Family Medicine, MetroHealth Medical Center, 2500 MetroHealth Drive, Cleveland, OH 44109-7878; eberko@metrohealth.org.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing: Arlington, VA; 2013.
2. Kumar S, Oakley-Browne M. Panic disorder. Clin Evid. 2006;15:1438-1452.
3. Yingling KW, Wulsin LR, Arnold LM, et al. Estimated prevalences of panic disorder and depression among consecutive patients seen in an emergency department with acute chest pain. J Gen Intern Med. 1993;8:231-235.
4. Fleet RP, Dupuis G, Marchand A, et al. Panic disorder in emergency department chest pain patients: prevalence, comorbidity, suicidal ideation, and physician recognition. Am J Med. 1996;101:371-380.
5. Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272:1749-1756.
6. Taylor CB. Panic disorder. BMJ. 2006;332:951-955.
7. de Jonge P, Roest AM, Lim CC, et al. Cross-national epidemiology of panic disorder and panic attacks in the world mental health surveys. Depress Anxiety. 2016;33:1155-1177.
8. Sadock BJ, Sadock VA, Ruiz P. Panic disorder. In: Kaplan & Sadock’s Synopsis of Psychiatry. 11th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015:392-397.
9. Stein MB, Goin MK, Pollack MH, et al. Practice guideline for the treatment of patients with panic disorder, 2nd ed. 2010. American Psychiatric Association; Washington DC. Available at: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Accessed October 26, 2017.
10. Westen D, Morrison K. A multidimensional meta-analysis of treatments for depression, panic, and generalized anxiety disorder: an empirical examination of the status of empirically supported therapies. J Consult Clin Psychol. 2001;69:875-899.
11. Gould RA, Otto MW, Pollack MH. A meta-analysis of treatment outcome for panic disorder. Available at: https://www.ncbi.nlm.nih.gov/books/NBK66380/. Accessed October 26, 2017.
12. Clum GA, Clum GA, Surls R. A meta-analysis of treatments for panic disorder. J Consult Clin Psychol. 1993;61:317-326.
13. Shear MK, Houck P, Greeno C, et al. Emotion-focused psychotherapy for patients with panic disorder. Am J Psychiatry. 2001;158:1993-1998.
14. Stewart RE., Chambless DL. Cognitive-behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77:595–606
15. Craske M. Psychotherapy for panic disorder in adults. Up to Date. 2017. Available at: https://www.uptodate.com/contents/psychotherapy-for-panic-disorder-with-or-without-agoraphobia-in-adults. Accessed October 26, 2017.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Publishing: Arlington, VA; 2013.
2. Kumar S, Oakley-Browne M. Panic disorder. Clin Evid. 2006;15:1438-1452.
3. Yingling KW, Wulsin LR, Arnold LM, et al. Estimated prevalences of panic disorder and depression among consecutive patients seen in an emergency department with acute chest pain. J Gen Intern Med. 1993;8:231-235.
4. Fleet RP, Dupuis G, Marchand A, et al. Panic disorder in emergency department chest pain patients: prevalence, comorbidity, suicidal ideation, and physician recognition. Am J Med. 1996;101:371-380.
5. Spitzer RL, Williams JB, Kroenke K, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272:1749-1756.
6. Taylor CB. Panic disorder. BMJ. 2006;332:951-955.
7. de Jonge P, Roest AM, Lim CC, et al. Cross-national epidemiology of panic disorder and panic attacks in the world mental health surveys. Depress Anxiety. 2016;33:1155-1177.
8. Sadock BJ, Sadock VA, Ruiz P. Panic disorder. In: Kaplan & Sadock’s Synopsis of Psychiatry. 11th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2015:392-397.
9. Stein MB, Goin MK, Pollack MH, et al. Practice guideline for the treatment of patients with panic disorder, 2nd ed. 2010. American Psychiatric Association; Washington DC. Available at: http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Accessed October 26, 2017.
10. Westen D, Morrison K. A multidimensional meta-analysis of treatments for depression, panic, and generalized anxiety disorder: an empirical examination of the status of empirically supported therapies. J Consult Clin Psychol. 2001;69:875-899.
11. Gould RA, Otto MW, Pollack MH. A meta-analysis of treatment outcome for panic disorder. Available at: https://www.ncbi.nlm.nih.gov/books/NBK66380/. Accessed October 26, 2017.
12. Clum GA, Clum GA, Surls R. A meta-analysis of treatments for panic disorder. J Consult Clin Psychol. 1993;61:317-326.
13. Shear MK, Houck P, Greeno C, et al. Emotion-focused psychotherapy for patients with panic disorder. Am J Psychiatry. 2001;158:1993-1998.
14. Stewart RE., Chambless DL. Cognitive-behavioral therapy for adult anxiety disorders in clinical practice: a meta-analysis of effectiveness studies. J Consult Clin Psychol. 2009;77:595–606
15. Craske M. Psychotherapy for panic disorder in adults. Up to Date. 2017. Available at: https://www.uptodate.com/contents/psychotherapy-for-panic-disorder-with-or-without-agoraphobia-in-adults. Accessed October 26, 2017.
Posttraumatic stress disorder: Often missed in primary care
THE CASE
DeSean W,* a 47-year-old man, returned to his primary care clinic with a new complaint of epigastric burning that had been bothering him for the past 4 months. He had tried several over-the-counter remedies, which provided no relief. He also remained concerned—despite assurances to the contrary at previous clinic visits—that he had contracted a sexually-transmitted disease (STD) after going to a bar one night 4 to 5 months ago. At 2 other clinic visits since that time, STD test results were negative. At this current visit, symptoms and details of sexual history were unchanged since the last visit, with the exception of the epigastric pain.
When asked if he thought he had contracted an STD through a sexual encounter the night he went to the bar, he emphatically said he would not cheat on his wife. Surprisingly, given his concern, he avoided further discussion on modes of contracting an STD.
The physician prescribed ranitidine 150 mg bid for the epigastric burning and explained, once more, the significance of the STD test results. However, he also decided to further examine Mr. W’s concern about STDs and the night he may have contracted one.
HOW WOULD YOU PROCEED WITH THIS PATIENT?
*The patient’s name has been changed to protect his privacy.
SCOPE OF THE PROBLEM
Despite being as common as asthma, posttraumatic stress disorder (PTSD) often remains undiagnosed and untreated in primary care.1 In brief, the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) defines PTSD as persistent and long-term changes in thoughts or mood following actual or threatened exposure to death, serious injury, or sexual assault that leads to re-experiencing, functional impairment, physiologic stress reactions, and avoidance of thoughts or situations associated with the original trauma.2 More than one in 10 women and one in 20 men experience PTSD in their lifetime.2,3 Population-based studies have not yet determined the prevalence among children.3 Almost 40% of US adults report having experienced a trauma before age 13, and about one-third of these go on to develop PTSD.4
Individuals with PTSD have higher rates of somatic complaints, overall medical utilization, prescription use, physical and social disability, attempted suicide, and all-cause mortality.3,5-7 PTSD is associated with increased risk for cardiac, gastrointestinal, metabolic, and immunologic illnesses, other psychiatric illnesses, risky health behaviors, and decreased medical adherence.4,6 Additionally, prevention and treatment efforts for STDs and obesity are less effective among those with trauma histories.4 Thus, detection and treatment of PTSD improves the likelihood of successfully treating other health concerns.
THE ESSENTIALS OF A PTSD DIAGNOSIS
DSM-5 diagnosis of PTSD requires the experience of a trauma and resultant symptoms from each of 4 symptom-clusters:2
- one or more re-experiencing symptoms (eg, intrusive memories or recurrent distressing dreams, psychological distress or physiologic reactions to reminders of the trauma)
- one or more avoidance symptoms (eg, avoidance of trauma memories or of people and places that trigger a reminder of the trauma)
- two or more changes in thoughts or mood (eg, negative beliefs about self or others, social detachment, anhedonia)
- two or more changes in arousal activity (eg, sleep problems, hypervigilance, inability to concentrate).
Since many people experiencing traumas do not develop PTSD,5,8 symptoms must last at least one month to meet the criteria for diagnosis.2 Sexual trauma, experiencing multiple traumas, and lack of social support increase the risk that an individual will develop PTSD.9-11 Notably, symptom onset will be delayed 6 months or more in some individuals,2,8 making it more difficult for those patients and clinicians to connect symptoms to the trauma.12
Differential diagnosis
PTSD must be differentiated from other mental health conditions with overlapping symptoms (TABLE 12,8,13), but it may also be comorbid with one or more of these other conditions. When patients with PTSD do report mental health symptoms, providers often focus on the depressive symptoms that overlap with PTSD, and on substance use, which often accompanies PTSD, leaving PTSD undetected.9
Given that depressed/irritable mood, decreased participation in pleasurable activities, negative views of the world, attention difficulties, sleep difficulties, feelings of guilt, and agitation/restlessness are symptoms of both depression and PTSD,2 it is particularly important to screen patients with depressive symptoms for trauma history.
Why PTSD is often missed
Due to the impact of PTSD on overall health, the rates of PTSD in primary care clinics may be higher than in the general population.14 Thus, primary care clinicians are likely seeing PTSD more often than they realize. In fact, a systematic review showed that clinicians detected 0% to 52% of their patients with PTSD, missing at least half of all PTSD diagnoses.9
Detecting PTSD can be challenging for several reasons. Symptoms can span the emotional, social, physical, and behavioral aspects of an individual’s life, so patients and clinicians alike may regard symptoms as unrelated to PTSD.8 Primary symptom presentation may vary, with some people reporting anxiety symptoms, others mostly depressive symptoms, and others arousal, dissociative, or—as in our patient’s case—somatic symptoms.2 In affected children, parents may report emotional or behavioral problems without mentioning the trauma.2 Additionally, for traumas that were not a single event, such as long-term child abuse, patients may have difficulty identifying symptom onset.2
CASE
The physician screened Mr. W for trauma exposure as part of the differential. Mr. W revealed that he had blacked out at the bar, despite drinking only moderately, and that he awoke with anal pain. He believed he had been drugged and sexually assaulted. Further screening for PTSD symptoms related to this event confirmed multiple associated symptoms. He acknowledged that his epigastric pain had started soon after the trauma and, after further discussion, began to link his stomach pain and other new symptoms revealed by the PTSD screen (hypervigilance, avoidance, change in mood) to the trauma.
As happened in this case, most PTSD patients present with somatic complaints rather than reporting a traumatic experience and having associated effects. This in turn usually leads clinicians to consider only non-PTSD diagnoses.6,9,15 Core avoidance symptoms are a major reason for such a presentation in PTSD patients.14 Patients avoid thoughts, feelings, and conversations that remind them of the trauma.13 As a result, they are less likely to voluntarily report trauma. They avoid thinking about how their current symptoms may be associated with their trauma and are reluctant to talk about their trauma with clinicians.5,9,8,12
Another barrier to diagnosis is a belief that PTSD is primarily experienced by combat veterans1 (TABLE 22,4-6,8,9,12,14-18). While PTSD is detected more often among veterans due to regular screening through the Department of Veterans Affairs,14 the vast majority of PTSD cases are related to civilian traumas such as sexual assault, child abuse, and car accidents.5,9 In fact, the estimated
SCREENING: WHAT TO LOOK FOR
Since individuals with PTSD mainly seek treatment for associated physical symptoms,14 primary care is particularly important for identification of PTSD and treatment access. The US Preventative Services Task Force does not yet have any recommendations for screening for PTSD. The American Psychiatric Association recommends that a trauma history be included in all initial psychiatric evaluations of adults.19 Screens can target high-risk populations and can be repeated across the lifespan,9 as traumas can occur at any age and symptoms may not emerge until years after the trauma.2,4 Factors in a patient’s history associated with high risk of PTSD include the following:
- known trauma exposure (eg, treatment at the emergency department following motor vehicle collision, natural disaster, assault),6
- frequent medical visits or unexplained physical symptoms,5,8
- family members who are trauma victims,8
- involvement in juvenile justice system,4,12
- sensitive or invasive exams (eg, pelvic exams) that trigger symptoms or contribute to retraumatization,12,20 and
- any medical condition (eg, hypertension, chronic pain, sleep disorder), self-destructive behavior (eg, drug or alcohol abuse, low impulse control), or social/occupational issues (eg, unemployment, social isolation, fighting) with a known link to PTSD.2,4,6,8
The first step in screening. Given a patient’s reported symptoms, assess for trauma exposure to determine whether PTSD should be included in the differential diagnosis. Overlooking PTSD as a possible source of symptoms can result in misattributing them to other causes.4,8 Listing common traumas, or using a standardized list such as the Life Events Checklist, can help identify patients with trauma exposure.8,21 However, do not make the patient provide details of the traumatic event(s), as that can exacerbate symptoms if PTSD is present.6 It is sufficient to know the category of the trauma (eg, sexual assault) without making the patient describe who was involved and what happened.6
The second step in screening. If a patient reveals trauma exposure, consider using an instrument such as the Primary Care PTSD Screen (PC-PTSD) or the PTSD Checklist, both available online, to screen for PTSD symptoms related to the identified trauma.6,9,21-23 Since these measures screen for symptoms but do not ask about trauma exposure, false positives can occur if a trauma is not first identified (such as through the Life Events Checklist) due to symptom overlap with other conditions (TABLE 12,8,13).21
Treatment is effective, even decades after a traumatic event
Provide anyone who has been traumatized with information about common after effects, symptoms of PTSD, and available treatments.8 Keep in mind that initial symptom severity after trauma exposure does not correlate with long-term symptoms,8 and about half of adults will recover without treatment within 3 months.1,2,5 The first month of symptoms may be addressed with patient education and watchful waiting. But if symptoms don’t subside after a month, consider offering treatment1 with the understanding that, for some individuals, symptoms may yet resolve on their own.
Detecting and treating PTSD early can decrease its deleterious effects on health and cut down on years of functional impairment.1 Even decades after an initial traumatic event, PTSD treatments can be effective.8 Children may experience functional impairment without meeting full criteria for PTSD, and can also benefit from treatment.7
INTEGRATING EXPOSURE AND COGNITIVE THERAPIES IS KEY
Offer any patient who meets criteria for PTSD a referral for exposure therapy and trauma-focused cognitive behavioral therapy (TF-CBT), the first-line treatments for PTSD.1,4,8,24,25
Exposure therapies for PTSD are supported by strong evidence and help patients to become desensitized to distressful memories through gradual, repeated exposures in a relaxed or safe space.8,26
Cognitive methods, such as cognitive processing therapy, cognitive behavioral therapy, and cognitive reprocessing have moderate strength of evidence, and may be combined with exposure therapy.26 Cognitive therapies help patients change thoughts, beliefs, and behaviors that contribute to PTSD symptoms.8,26
Exposure and TF-CBT have the most empirical evidence for child, adolescent, and adult PTSD, and are effective for the range of PTSD symptoms,4,8,25 including avoidance—a fundamental component of PTSD that drives other PTSD symptoms27—comorbid depression, and other emotions associated with trauma (eg, shame, guilt, and anger).8,25 Family involvement is recommended for children and adolescents.4
For patients with comorbid substance abuse, offer integrated PTSD/substance abuse treatment, which is more effective than isolated treatment of each.4 Relaxation training can be helpful as an adjunct to TF-CBT, but is not sufficient as a stand-alone treatment.13 Other psychotherapies, such as supportive, psychodynamic, systemic, and hypnotherapy, have not proved effective.14
Eye Movement Desensitization and Reprocessing (EMDR), a much publicized but controversial treatment, integrates components of exposure and cognitive therapies with therapist-directed eye movements.28-30 Patients imagine their trauma while the therapist directs their eye movements, which is thought to provide exposure to trauma images and memories, thereby reducing symptoms. EMDR has been found to reduce PTSD symptoms with a low to moderate strength-of-evidence rating.26 However, it has not proved more effective than other exposure and cognitive therapies, and its unique component (eg, eye movements) has not added any effect to outcomes.28-31
Other newer therapies, such as Acceptance and Commitment Therapy7,24,27 and online and computer-assisted treatments, are being evaluated.14
Medications take on an adjunct role to therapy
Drug treatment of PTSD has not been effective in children or adolescents.4,8 In adults, medications have helped relieve some symptoms of PTSD. However, given their low effect sizes, medications are not recommended as first-line treatments over exposure and TF-CBT. Their usefulness lies chiefly in an adjunct role to exposure and cognitive therapies or for patients who refuse psychotherapy.4,8,25
Selective serotonin reuptake inhibitors such as fluoxetine, paroxetine, and sertraline, have been effective for such PTSD symptoms as intrusive thoughts, negative or irritable mood, anxiety, restlessness, attention difficulties, and hyperarousal.1,8
While benzodiazepines have been used to control anxiety, hyperarousal, and insomnia, they have not been effective for most other PTSD symptoms, including avoidance, re-experiencing, and cognitive symptoms. Furthermore, they are not recommended given their augmentative effect on other related symptoms and associated conditions (eg, dissociation, disinhibition, substance abuse) and possible interference with desensitization that occurs in exposure therapy.1,5
If a patient has significant insomnia and PTSD-related nightmares, consider starting prazosin at 1 mg/d and titrating up to an effective dose, which typically ranges from 5 to 20 mg per day.1,5 Additionally, trazodone or antihistamines may be used to enhance sleep.1
COORDINATION OF CARE
Upon identifying PTSD and offering treatment, introduce the patient to a mental health provider as part of the referral process, which strongly encourages patient engagement in treatment.14 Collaborate with the psychotherapist throughout treatment to facilitate a biopsychosocial approach to the patient’s care, and coordinate the monitoring and treatment of any comorbid physical conditions.
The Substance Abuse and Mental Health Services Administration has proposed a framework for multisystem Trauma-Informed Care (TIC), in which the primary care physician has many roles, including:12,20
- recording or communicating sensitive private information to other providers through the electronic medical record in a manner that does not interfere with a patient’s development of trust or lead to exposure and retraumatization,
- performing invasive physical exams in a sensitive and patient-centered manner, and
- using support and shared decision-making in clinical encounters.
Physicians can also connect patients with PTSD to programs or groups that aid in developing resilience, such as physical exercise classes, social support networks, and community involvement opportunities.4
CASE
The physician referred Mr. W to an onsite psychologist. At a subsequent clinic visit in which he was seen by a different primary care physician, Mr. W expressed new concerns about shoulder pain and changes in a mole. During this visit, Mr. W was asked whether he had followed up on the earlier referral for counseling. He replied that he had attended an intake appointment with the psychologist, but that he had not wanted to talk about what had happened to him and therefore avoided future appointments.*
He remained concerned that he might have an STD, but declined medication for PTSD because he felt he was “moving on” with his life.
*Author’s note: Getting patients to open up about their trauma exposure can be difficult. If the patient isn’t ready, simply bringing up the experience can trigger avoidance. It’s often helpful to encourage patients to first develop a relationship with their therapist, then later discuss the details of their trauma when they are ready. This encourages patients to engage in the counseling process.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC663, Chicago, IL 60612; awms@uic.edu.
1. Bobo WV, Warner CH, Warner CM. The management of post traumatic stress disorder (PTSD) in the primary care setting. South Med J. 2007;100:797-802.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
3. Gradus JL. Epidemiology of PTSD. National Center for PTSD. Available at: http://www.ptsd.va.gov/professional/PTSD-overview/epidemiological-facts-ptsd.asp. Updated 2017. Accessed August 16, 2017.
4. Gerson R, Rappaport N. Traumatic stress and posttraumatic stress disorder in youth: recent research findings on clinical impact, assessment, and treatment. J Adolesc Health. 2013;52:137-143.
5. Zohar J, Juven-Wetzler A, Myers V, et al. Post-traumatic stress disorder: facts and fiction. Curr Opin Psychiatry. 2008;21:74-77.
6. Spoont MR, Williams JW Jr, Kehle-Forbes S, et al. Does this patient have posttraumatic stress disorder? Rational clinical examination systematic review. JAMA. 2015;314:501-510.
7. Woidneck MR, Morrison KL, Twohig MP. Acceptance and commitment therapy for the treatment of posttraumatic stress among adolescents. Behav Modif. 2014;38:451-476.
8. National Collaborating Centre for Mental Health (UK). Post-traumatic stress disorder: the management of PTSD in adults and children in primary and secondary care. Available at: https://www.ncbi.nlm.nih.gov/books/NBK56494. Accessed August 16, 2017.
9. Greene T, Neria Y, Gross R. Prevalence, detection and correlates of PTSD in the primary care setting: a systematic review. J Clin Psychol Med Settings. 2016;23:160-180.
10. Gavranidou M, Rosner R. The weaker sex? Gender and post-traumatic stress disorder. Depress Anxiety. 2003;17:130-139.
11. Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748-766.
12. SAMHSA’s Trauma and Justice Strategic Initiative. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. Available at: http://store.samhsa.gov/shin/content/SMA14-4884/SMA14-4884.pdf. Accessed September 13, 2017.
13. Mulick PS, Landes SJ, Kanter JW. Contextual behavior therapies in the treatment of PTSD: a review. Int J Behav Consult Ther. 2005;1:223-238.
14. Possemato K. The current state of intervention research for posttraumatic stress disorder within the primary care setting. J Clin Psychol Med Settings. 2011;18:268-280.
15. Forneris CA, Gartlehner G, Brownley KA, et al. Interventions to prevent post-traumatic stress disorder: a systematic review. Am J Prev Med. 2013;44:635-650.
16. Trivedi RB, Post EP, Sun H, et al. Prevalence, comorbidity, and prognosis of mental health among US veterans. Am J Public Health. 2015;105:2564-2569.
17. United States Census Bureau. Facts for features: Veteran’s day 2016: Nov. 11. Available at: https://www.census.gov/newsroom/facts-for-features/2016/cb16-ff21.html. Accessed August 16, 2017.
18. United States Census Bureau. U.S. and World Population Clock. Available at: https://www.census.gov/popclock/. Accessed August 16, 2017.
19. American Psychiatric Association. Guidelines and implementation. In: Practice Guidelines for the Psychiatric Evaluation of Adults. 3rd ed. Arlington, Va: American Psychiatric Association; 2015:9-45.
20. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
21. U.S. Department of Veterans Affairs. Life events checklist for DSM-5 (LEC-5). Available at: http://www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp. Accessed September 13, 2017.
22. U.S. Department of Veterans Affairs. Primary care PTSD screen for DSM-5 (PC-PTSD). Available at: http://www.ptsd.va.gov/professional/assessment/screens/pc-ptsd.asp. Accessed September 13, 2017.
23. Spoont M, Arbisi P, Fu S, et al. Screening for Post-Traumatic Stress Disorder (PTSD) in Primary Care: A Systematic Review. Available at: https://www.ncbi.nlm.nih.gov/books/NBK126691/. Accessed Sept 13, 2017
24. Gallagher MW, Thompson-Hollands J, Bourgeois ML, et al. Cognitive behavioral treatments for adult posttraumatic stress disorder: current status and future directions. J Contemp Psychother. 2015;45:235-243.
25. Kar N. Cognitive behavioral therapy for the treatment of post-traumatic stress disorder: a review. Neuropsychiatr Dis Treat. 2011;7:167-181.
26. Cusack K, Jonas DE, Forneris CA, et al. Psychological treatments for adults with posttraumatic stress disorder: a systematic review and meta-analysis. Clin Psychol Rev. 2016;43:128-141.
27. Thompson BL, Luoma JB, LeJeune JT. Using acceptance and commitment therapy to guide exposure-based interventions for posttraumatic stress disorder. J Contemp Psychother. 2013;43:133-140.
28. Lohr JM, Hooke W, Gist R, et al. Novel and controversial treatments for trauma-related stress disorders. In: Lilienfeld SO, Lynn SJ, Lohr JM, eds. Science and Pseudoscience in Clinical Psychology. New York, NY: Guilford Press; 2003:243-272.
29. Sikes C, Sikes V. EMDR: Why the controversy? Traumatol. 2003;9:169-182.
30. Davidson PR, Parker KCH. Eye movement desensitization and reprocessing (EMDR): a meta-analysis. J Consult Clin Psychol. 2001;69:305-316.
31. Devilly GJ. Power therapies and possible threats to the science of psychology and psychiatry. Aust N Z J Psychiatry. 2005;39:437-445.
THE CASE
DeSean W,* a 47-year-old man, returned to his primary care clinic with a new complaint of epigastric burning that had been bothering him for the past 4 months. He had tried several over-the-counter remedies, which provided no relief. He also remained concerned—despite assurances to the contrary at previous clinic visits—that he had contracted a sexually-transmitted disease (STD) after going to a bar one night 4 to 5 months ago. At 2 other clinic visits since that time, STD test results were negative. At this current visit, symptoms and details of sexual history were unchanged since the last visit, with the exception of the epigastric pain.
When asked if he thought he had contracted an STD through a sexual encounter the night he went to the bar, he emphatically said he would not cheat on his wife. Surprisingly, given his concern, he avoided further discussion on modes of contracting an STD.
The physician prescribed ranitidine 150 mg bid for the epigastric burning and explained, once more, the significance of the STD test results. However, he also decided to further examine Mr. W’s concern about STDs and the night he may have contracted one.
HOW WOULD YOU PROCEED WITH THIS PATIENT?
*The patient’s name has been changed to protect his privacy.
SCOPE OF THE PROBLEM
Despite being as common as asthma, posttraumatic stress disorder (PTSD) often remains undiagnosed and untreated in primary care.1 In brief, the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) defines PTSD as persistent and long-term changes in thoughts or mood following actual or threatened exposure to death, serious injury, or sexual assault that leads to re-experiencing, functional impairment, physiologic stress reactions, and avoidance of thoughts or situations associated with the original trauma.2 More than one in 10 women and one in 20 men experience PTSD in their lifetime.2,3 Population-based studies have not yet determined the prevalence among children.3 Almost 40% of US adults report having experienced a trauma before age 13, and about one-third of these go on to develop PTSD.4
Individuals with PTSD have higher rates of somatic complaints, overall medical utilization, prescription use, physical and social disability, attempted suicide, and all-cause mortality.3,5-7 PTSD is associated with increased risk for cardiac, gastrointestinal, metabolic, and immunologic illnesses, other psychiatric illnesses, risky health behaviors, and decreased medical adherence.4,6 Additionally, prevention and treatment efforts for STDs and obesity are less effective among those with trauma histories.4 Thus, detection and treatment of PTSD improves the likelihood of successfully treating other health concerns.
THE ESSENTIALS OF A PTSD DIAGNOSIS
DSM-5 diagnosis of PTSD requires the experience of a trauma and resultant symptoms from each of 4 symptom-clusters:2
- one or more re-experiencing symptoms (eg, intrusive memories or recurrent distressing dreams, psychological distress or physiologic reactions to reminders of the trauma)
- one or more avoidance symptoms (eg, avoidance of trauma memories or of people and places that trigger a reminder of the trauma)
- two or more changes in thoughts or mood (eg, negative beliefs about self or others, social detachment, anhedonia)
- two or more changes in arousal activity (eg, sleep problems, hypervigilance, inability to concentrate).
Since many people experiencing traumas do not develop PTSD,5,8 symptoms must last at least one month to meet the criteria for diagnosis.2 Sexual trauma, experiencing multiple traumas, and lack of social support increase the risk that an individual will develop PTSD.9-11 Notably, symptom onset will be delayed 6 months or more in some individuals,2,8 making it more difficult for those patients and clinicians to connect symptoms to the trauma.12
Differential diagnosis
PTSD must be differentiated from other mental health conditions with overlapping symptoms (TABLE 12,8,13), but it may also be comorbid with one or more of these other conditions. When patients with PTSD do report mental health symptoms, providers often focus on the depressive symptoms that overlap with PTSD, and on substance use, which often accompanies PTSD, leaving PTSD undetected.9
Given that depressed/irritable mood, decreased participation in pleasurable activities, negative views of the world, attention difficulties, sleep difficulties, feelings of guilt, and agitation/restlessness are symptoms of both depression and PTSD,2 it is particularly important to screen patients with depressive symptoms for trauma history.
Why PTSD is often missed
Due to the impact of PTSD on overall health, the rates of PTSD in primary care clinics may be higher than in the general population.14 Thus, primary care clinicians are likely seeing PTSD more often than they realize. In fact, a systematic review showed that clinicians detected 0% to 52% of their patients with PTSD, missing at least half of all PTSD diagnoses.9
Detecting PTSD can be challenging for several reasons. Symptoms can span the emotional, social, physical, and behavioral aspects of an individual’s life, so patients and clinicians alike may regard symptoms as unrelated to PTSD.8 Primary symptom presentation may vary, with some people reporting anxiety symptoms, others mostly depressive symptoms, and others arousal, dissociative, or—as in our patient’s case—somatic symptoms.2 In affected children, parents may report emotional or behavioral problems without mentioning the trauma.2 Additionally, for traumas that were not a single event, such as long-term child abuse, patients may have difficulty identifying symptom onset.2
CASE
The physician screened Mr. W for trauma exposure as part of the differential. Mr. W revealed that he had blacked out at the bar, despite drinking only moderately, and that he awoke with anal pain. He believed he had been drugged and sexually assaulted. Further screening for PTSD symptoms related to this event confirmed multiple associated symptoms. He acknowledged that his epigastric pain had started soon after the trauma and, after further discussion, began to link his stomach pain and other new symptoms revealed by the PTSD screen (hypervigilance, avoidance, change in mood) to the trauma.
As happened in this case, most PTSD patients present with somatic complaints rather than reporting a traumatic experience and having associated effects. This in turn usually leads clinicians to consider only non-PTSD diagnoses.6,9,15 Core avoidance symptoms are a major reason for such a presentation in PTSD patients.14 Patients avoid thoughts, feelings, and conversations that remind them of the trauma.13 As a result, they are less likely to voluntarily report trauma. They avoid thinking about how their current symptoms may be associated with their trauma and are reluctant to talk about their trauma with clinicians.5,9,8,12
Another barrier to diagnosis is a belief that PTSD is primarily experienced by combat veterans1 (TABLE 22,4-6,8,9,12,14-18). While PTSD is detected more often among veterans due to regular screening through the Department of Veterans Affairs,14 the vast majority of PTSD cases are related to civilian traumas such as sexual assault, child abuse, and car accidents.5,9 In fact, the estimated
SCREENING: WHAT TO LOOK FOR
Since individuals with PTSD mainly seek treatment for associated physical symptoms,14 primary care is particularly important for identification of PTSD and treatment access. The US Preventative Services Task Force does not yet have any recommendations for screening for PTSD. The American Psychiatric Association recommends that a trauma history be included in all initial psychiatric evaluations of adults.19 Screens can target high-risk populations and can be repeated across the lifespan,9 as traumas can occur at any age and symptoms may not emerge until years after the trauma.2,4 Factors in a patient’s history associated with high risk of PTSD include the following:
- known trauma exposure (eg, treatment at the emergency department following motor vehicle collision, natural disaster, assault),6
- frequent medical visits or unexplained physical symptoms,5,8
- family members who are trauma victims,8
- involvement in juvenile justice system,4,12
- sensitive or invasive exams (eg, pelvic exams) that trigger symptoms or contribute to retraumatization,12,20 and
- any medical condition (eg, hypertension, chronic pain, sleep disorder), self-destructive behavior (eg, drug or alcohol abuse, low impulse control), or social/occupational issues (eg, unemployment, social isolation, fighting) with a known link to PTSD.2,4,6,8
The first step in screening. Given a patient’s reported symptoms, assess for trauma exposure to determine whether PTSD should be included in the differential diagnosis. Overlooking PTSD as a possible source of symptoms can result in misattributing them to other causes.4,8 Listing common traumas, or using a standardized list such as the Life Events Checklist, can help identify patients with trauma exposure.8,21 However, do not make the patient provide details of the traumatic event(s), as that can exacerbate symptoms if PTSD is present.6 It is sufficient to know the category of the trauma (eg, sexual assault) without making the patient describe who was involved and what happened.6
The second step in screening. If a patient reveals trauma exposure, consider using an instrument such as the Primary Care PTSD Screen (PC-PTSD) or the PTSD Checklist, both available online, to screen for PTSD symptoms related to the identified trauma.6,9,21-23 Since these measures screen for symptoms but do not ask about trauma exposure, false positives can occur if a trauma is not first identified (such as through the Life Events Checklist) due to symptom overlap with other conditions (TABLE 12,8,13).21
Treatment is effective, even decades after a traumatic event
Provide anyone who has been traumatized with information about common after effects, symptoms of PTSD, and available treatments.8 Keep in mind that initial symptom severity after trauma exposure does not correlate with long-term symptoms,8 and about half of adults will recover without treatment within 3 months.1,2,5 The first month of symptoms may be addressed with patient education and watchful waiting. But if symptoms don’t subside after a month, consider offering treatment1 with the understanding that, for some individuals, symptoms may yet resolve on their own.
Detecting and treating PTSD early can decrease its deleterious effects on health and cut down on years of functional impairment.1 Even decades after an initial traumatic event, PTSD treatments can be effective.8 Children may experience functional impairment without meeting full criteria for PTSD, and can also benefit from treatment.7
INTEGRATING EXPOSURE AND COGNITIVE THERAPIES IS KEY
Offer any patient who meets criteria for PTSD a referral for exposure therapy and trauma-focused cognitive behavioral therapy (TF-CBT), the first-line treatments for PTSD.1,4,8,24,25
Exposure therapies for PTSD are supported by strong evidence and help patients to become desensitized to distressful memories through gradual, repeated exposures in a relaxed or safe space.8,26
Cognitive methods, such as cognitive processing therapy, cognitive behavioral therapy, and cognitive reprocessing have moderate strength of evidence, and may be combined with exposure therapy.26 Cognitive therapies help patients change thoughts, beliefs, and behaviors that contribute to PTSD symptoms.8,26
Exposure and TF-CBT have the most empirical evidence for child, adolescent, and adult PTSD, and are effective for the range of PTSD symptoms,4,8,25 including avoidance—a fundamental component of PTSD that drives other PTSD symptoms27—comorbid depression, and other emotions associated with trauma (eg, shame, guilt, and anger).8,25 Family involvement is recommended for children and adolescents.4
For patients with comorbid substance abuse, offer integrated PTSD/substance abuse treatment, which is more effective than isolated treatment of each.4 Relaxation training can be helpful as an adjunct to TF-CBT, but is not sufficient as a stand-alone treatment.13 Other psychotherapies, such as supportive, psychodynamic, systemic, and hypnotherapy, have not proved effective.14
Eye Movement Desensitization and Reprocessing (EMDR), a much publicized but controversial treatment, integrates components of exposure and cognitive therapies with therapist-directed eye movements.28-30 Patients imagine their trauma while the therapist directs their eye movements, which is thought to provide exposure to trauma images and memories, thereby reducing symptoms. EMDR has been found to reduce PTSD symptoms with a low to moderate strength-of-evidence rating.26 However, it has not proved more effective than other exposure and cognitive therapies, and its unique component (eg, eye movements) has not added any effect to outcomes.28-31
Other newer therapies, such as Acceptance and Commitment Therapy7,24,27 and online and computer-assisted treatments, are being evaluated.14
Medications take on an adjunct role to therapy
Drug treatment of PTSD has not been effective in children or adolescents.4,8 In adults, medications have helped relieve some symptoms of PTSD. However, given their low effect sizes, medications are not recommended as first-line treatments over exposure and TF-CBT. Their usefulness lies chiefly in an adjunct role to exposure and cognitive therapies or for patients who refuse psychotherapy.4,8,25
Selective serotonin reuptake inhibitors such as fluoxetine, paroxetine, and sertraline, have been effective for such PTSD symptoms as intrusive thoughts, negative or irritable mood, anxiety, restlessness, attention difficulties, and hyperarousal.1,8
While benzodiazepines have been used to control anxiety, hyperarousal, and insomnia, they have not been effective for most other PTSD symptoms, including avoidance, re-experiencing, and cognitive symptoms. Furthermore, they are not recommended given their augmentative effect on other related symptoms and associated conditions (eg, dissociation, disinhibition, substance abuse) and possible interference with desensitization that occurs in exposure therapy.1,5
If a patient has significant insomnia and PTSD-related nightmares, consider starting prazosin at 1 mg/d and titrating up to an effective dose, which typically ranges from 5 to 20 mg per day.1,5 Additionally, trazodone or antihistamines may be used to enhance sleep.1
COORDINATION OF CARE
Upon identifying PTSD and offering treatment, introduce the patient to a mental health provider as part of the referral process, which strongly encourages patient engagement in treatment.14 Collaborate with the psychotherapist throughout treatment to facilitate a biopsychosocial approach to the patient’s care, and coordinate the monitoring and treatment of any comorbid physical conditions.
The Substance Abuse and Mental Health Services Administration has proposed a framework for multisystem Trauma-Informed Care (TIC), in which the primary care physician has many roles, including:12,20
- recording or communicating sensitive private information to other providers through the electronic medical record in a manner that does not interfere with a patient’s development of trust or lead to exposure and retraumatization,
- performing invasive physical exams in a sensitive and patient-centered manner, and
- using support and shared decision-making in clinical encounters.
Physicians can also connect patients with PTSD to programs or groups that aid in developing resilience, such as physical exercise classes, social support networks, and community involvement opportunities.4
CASE
The physician referred Mr. W to an onsite psychologist. At a subsequent clinic visit in which he was seen by a different primary care physician, Mr. W expressed new concerns about shoulder pain and changes in a mole. During this visit, Mr. W was asked whether he had followed up on the earlier referral for counseling. He replied that he had attended an intake appointment with the psychologist, but that he had not wanted to talk about what had happened to him and therefore avoided future appointments.*
He remained concerned that he might have an STD, but declined medication for PTSD because he felt he was “moving on” with his life.
*Author’s note: Getting patients to open up about their trauma exposure can be difficult. If the patient isn’t ready, simply bringing up the experience can trigger avoidance. It’s often helpful to encourage patients to first develop a relationship with their therapist, then later discuss the details of their trauma when they are ready. This encourages patients to engage in the counseling process.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC663, Chicago, IL 60612; awms@uic.edu.
THE CASE
DeSean W,* a 47-year-old man, returned to his primary care clinic with a new complaint of epigastric burning that had been bothering him for the past 4 months. He had tried several over-the-counter remedies, which provided no relief. He also remained concerned—despite assurances to the contrary at previous clinic visits—that he had contracted a sexually-transmitted disease (STD) after going to a bar one night 4 to 5 months ago. At 2 other clinic visits since that time, STD test results were negative. At this current visit, symptoms and details of sexual history were unchanged since the last visit, with the exception of the epigastric pain.
When asked if he thought he had contracted an STD through a sexual encounter the night he went to the bar, he emphatically said he would not cheat on his wife. Surprisingly, given his concern, he avoided further discussion on modes of contracting an STD.
The physician prescribed ranitidine 150 mg bid for the epigastric burning and explained, once more, the significance of the STD test results. However, he also decided to further examine Mr. W’s concern about STDs and the night he may have contracted one.
HOW WOULD YOU PROCEED WITH THIS PATIENT?
*The patient’s name has been changed to protect his privacy.
SCOPE OF THE PROBLEM
Despite being as common as asthma, posttraumatic stress disorder (PTSD) often remains undiagnosed and untreated in primary care.1 In brief, the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) defines PTSD as persistent and long-term changes in thoughts or mood following actual or threatened exposure to death, serious injury, or sexual assault that leads to re-experiencing, functional impairment, physiologic stress reactions, and avoidance of thoughts or situations associated with the original trauma.2 More than one in 10 women and one in 20 men experience PTSD in their lifetime.2,3 Population-based studies have not yet determined the prevalence among children.3 Almost 40% of US adults report having experienced a trauma before age 13, and about one-third of these go on to develop PTSD.4
Individuals with PTSD have higher rates of somatic complaints, overall medical utilization, prescription use, physical and social disability, attempted suicide, and all-cause mortality.3,5-7 PTSD is associated with increased risk for cardiac, gastrointestinal, metabolic, and immunologic illnesses, other psychiatric illnesses, risky health behaviors, and decreased medical adherence.4,6 Additionally, prevention and treatment efforts for STDs and obesity are less effective among those with trauma histories.4 Thus, detection and treatment of PTSD improves the likelihood of successfully treating other health concerns.
THE ESSENTIALS OF A PTSD DIAGNOSIS
DSM-5 diagnosis of PTSD requires the experience of a trauma and resultant symptoms from each of 4 symptom-clusters:2
- one or more re-experiencing symptoms (eg, intrusive memories or recurrent distressing dreams, psychological distress or physiologic reactions to reminders of the trauma)
- one or more avoidance symptoms (eg, avoidance of trauma memories or of people and places that trigger a reminder of the trauma)
- two or more changes in thoughts or mood (eg, negative beliefs about self or others, social detachment, anhedonia)
- two or more changes in arousal activity (eg, sleep problems, hypervigilance, inability to concentrate).
Since many people experiencing traumas do not develop PTSD,5,8 symptoms must last at least one month to meet the criteria for diagnosis.2 Sexual trauma, experiencing multiple traumas, and lack of social support increase the risk that an individual will develop PTSD.9-11 Notably, symptom onset will be delayed 6 months or more in some individuals,2,8 making it more difficult for those patients and clinicians to connect symptoms to the trauma.12
Differential diagnosis
PTSD must be differentiated from other mental health conditions with overlapping symptoms (TABLE 12,8,13), but it may also be comorbid with one or more of these other conditions. When patients with PTSD do report mental health symptoms, providers often focus on the depressive symptoms that overlap with PTSD, and on substance use, which often accompanies PTSD, leaving PTSD undetected.9
Given that depressed/irritable mood, decreased participation in pleasurable activities, negative views of the world, attention difficulties, sleep difficulties, feelings of guilt, and agitation/restlessness are symptoms of both depression and PTSD,2 it is particularly important to screen patients with depressive symptoms for trauma history.
Why PTSD is often missed
Due to the impact of PTSD on overall health, the rates of PTSD in primary care clinics may be higher than in the general population.14 Thus, primary care clinicians are likely seeing PTSD more often than they realize. In fact, a systematic review showed that clinicians detected 0% to 52% of their patients with PTSD, missing at least half of all PTSD diagnoses.9
Detecting PTSD can be challenging for several reasons. Symptoms can span the emotional, social, physical, and behavioral aspects of an individual’s life, so patients and clinicians alike may regard symptoms as unrelated to PTSD.8 Primary symptom presentation may vary, with some people reporting anxiety symptoms, others mostly depressive symptoms, and others arousal, dissociative, or—as in our patient’s case—somatic symptoms.2 In affected children, parents may report emotional or behavioral problems without mentioning the trauma.2 Additionally, for traumas that were not a single event, such as long-term child abuse, patients may have difficulty identifying symptom onset.2
CASE
The physician screened Mr. W for trauma exposure as part of the differential. Mr. W revealed that he had blacked out at the bar, despite drinking only moderately, and that he awoke with anal pain. He believed he had been drugged and sexually assaulted. Further screening for PTSD symptoms related to this event confirmed multiple associated symptoms. He acknowledged that his epigastric pain had started soon after the trauma and, after further discussion, began to link his stomach pain and other new symptoms revealed by the PTSD screen (hypervigilance, avoidance, change in mood) to the trauma.
As happened in this case, most PTSD patients present with somatic complaints rather than reporting a traumatic experience and having associated effects. This in turn usually leads clinicians to consider only non-PTSD diagnoses.6,9,15 Core avoidance symptoms are a major reason for such a presentation in PTSD patients.14 Patients avoid thoughts, feelings, and conversations that remind them of the trauma.13 As a result, they are less likely to voluntarily report trauma. They avoid thinking about how their current symptoms may be associated with their trauma and are reluctant to talk about their trauma with clinicians.5,9,8,12
Another barrier to diagnosis is a belief that PTSD is primarily experienced by combat veterans1 (TABLE 22,4-6,8,9,12,14-18). While PTSD is detected more often among veterans due to regular screening through the Department of Veterans Affairs,14 the vast majority of PTSD cases are related to civilian traumas such as sexual assault, child abuse, and car accidents.5,9 In fact, the estimated
SCREENING: WHAT TO LOOK FOR
Since individuals with PTSD mainly seek treatment for associated physical symptoms,14 primary care is particularly important for identification of PTSD and treatment access. The US Preventative Services Task Force does not yet have any recommendations for screening for PTSD. The American Psychiatric Association recommends that a trauma history be included in all initial psychiatric evaluations of adults.19 Screens can target high-risk populations and can be repeated across the lifespan,9 as traumas can occur at any age and symptoms may not emerge until years after the trauma.2,4 Factors in a patient’s history associated with high risk of PTSD include the following:
- known trauma exposure (eg, treatment at the emergency department following motor vehicle collision, natural disaster, assault),6
- frequent medical visits or unexplained physical symptoms,5,8
- family members who are trauma victims,8
- involvement in juvenile justice system,4,12
- sensitive or invasive exams (eg, pelvic exams) that trigger symptoms or contribute to retraumatization,12,20 and
- any medical condition (eg, hypertension, chronic pain, sleep disorder), self-destructive behavior (eg, drug or alcohol abuse, low impulse control), or social/occupational issues (eg, unemployment, social isolation, fighting) with a known link to PTSD.2,4,6,8
The first step in screening. Given a patient’s reported symptoms, assess for trauma exposure to determine whether PTSD should be included in the differential diagnosis. Overlooking PTSD as a possible source of symptoms can result in misattributing them to other causes.4,8 Listing common traumas, or using a standardized list such as the Life Events Checklist, can help identify patients with trauma exposure.8,21 However, do not make the patient provide details of the traumatic event(s), as that can exacerbate symptoms if PTSD is present.6 It is sufficient to know the category of the trauma (eg, sexual assault) without making the patient describe who was involved and what happened.6
The second step in screening. If a patient reveals trauma exposure, consider using an instrument such as the Primary Care PTSD Screen (PC-PTSD) or the PTSD Checklist, both available online, to screen for PTSD symptoms related to the identified trauma.6,9,21-23 Since these measures screen for symptoms but do not ask about trauma exposure, false positives can occur if a trauma is not first identified (such as through the Life Events Checklist) due to symptom overlap with other conditions (TABLE 12,8,13).21
Treatment is effective, even decades after a traumatic event
Provide anyone who has been traumatized with information about common after effects, symptoms of PTSD, and available treatments.8 Keep in mind that initial symptom severity after trauma exposure does not correlate with long-term symptoms,8 and about half of adults will recover without treatment within 3 months.1,2,5 The first month of symptoms may be addressed with patient education and watchful waiting. But if symptoms don’t subside after a month, consider offering treatment1 with the understanding that, for some individuals, symptoms may yet resolve on their own.
Detecting and treating PTSD early can decrease its deleterious effects on health and cut down on years of functional impairment.1 Even decades after an initial traumatic event, PTSD treatments can be effective.8 Children may experience functional impairment without meeting full criteria for PTSD, and can also benefit from treatment.7
INTEGRATING EXPOSURE AND COGNITIVE THERAPIES IS KEY
Offer any patient who meets criteria for PTSD a referral for exposure therapy and trauma-focused cognitive behavioral therapy (TF-CBT), the first-line treatments for PTSD.1,4,8,24,25
Exposure therapies for PTSD are supported by strong evidence and help patients to become desensitized to distressful memories through gradual, repeated exposures in a relaxed or safe space.8,26
Cognitive methods, such as cognitive processing therapy, cognitive behavioral therapy, and cognitive reprocessing have moderate strength of evidence, and may be combined with exposure therapy.26 Cognitive therapies help patients change thoughts, beliefs, and behaviors that contribute to PTSD symptoms.8,26
Exposure and TF-CBT have the most empirical evidence for child, adolescent, and adult PTSD, and are effective for the range of PTSD symptoms,4,8,25 including avoidance—a fundamental component of PTSD that drives other PTSD symptoms27—comorbid depression, and other emotions associated with trauma (eg, shame, guilt, and anger).8,25 Family involvement is recommended for children and adolescents.4
For patients with comorbid substance abuse, offer integrated PTSD/substance abuse treatment, which is more effective than isolated treatment of each.4 Relaxation training can be helpful as an adjunct to TF-CBT, but is not sufficient as a stand-alone treatment.13 Other psychotherapies, such as supportive, psychodynamic, systemic, and hypnotherapy, have not proved effective.14
Eye Movement Desensitization and Reprocessing (EMDR), a much publicized but controversial treatment, integrates components of exposure and cognitive therapies with therapist-directed eye movements.28-30 Patients imagine their trauma while the therapist directs their eye movements, which is thought to provide exposure to trauma images and memories, thereby reducing symptoms. EMDR has been found to reduce PTSD symptoms with a low to moderate strength-of-evidence rating.26 However, it has not proved more effective than other exposure and cognitive therapies, and its unique component (eg, eye movements) has not added any effect to outcomes.28-31
Other newer therapies, such as Acceptance and Commitment Therapy7,24,27 and online and computer-assisted treatments, are being evaluated.14
Medications take on an adjunct role to therapy
Drug treatment of PTSD has not been effective in children or adolescents.4,8 In adults, medications have helped relieve some symptoms of PTSD. However, given their low effect sizes, medications are not recommended as first-line treatments over exposure and TF-CBT. Their usefulness lies chiefly in an adjunct role to exposure and cognitive therapies or for patients who refuse psychotherapy.4,8,25
Selective serotonin reuptake inhibitors such as fluoxetine, paroxetine, and sertraline, have been effective for such PTSD symptoms as intrusive thoughts, negative or irritable mood, anxiety, restlessness, attention difficulties, and hyperarousal.1,8
While benzodiazepines have been used to control anxiety, hyperarousal, and insomnia, they have not been effective for most other PTSD symptoms, including avoidance, re-experiencing, and cognitive symptoms. Furthermore, they are not recommended given their augmentative effect on other related symptoms and associated conditions (eg, dissociation, disinhibition, substance abuse) and possible interference with desensitization that occurs in exposure therapy.1,5
If a patient has significant insomnia and PTSD-related nightmares, consider starting prazosin at 1 mg/d and titrating up to an effective dose, which typically ranges from 5 to 20 mg per day.1,5 Additionally, trazodone or antihistamines may be used to enhance sleep.1
COORDINATION OF CARE
Upon identifying PTSD and offering treatment, introduce the patient to a mental health provider as part of the referral process, which strongly encourages patient engagement in treatment.14 Collaborate with the psychotherapist throughout treatment to facilitate a biopsychosocial approach to the patient’s care, and coordinate the monitoring and treatment of any comorbid physical conditions.
The Substance Abuse and Mental Health Services Administration has proposed a framework for multisystem Trauma-Informed Care (TIC), in which the primary care physician has many roles, including:12,20
- recording or communicating sensitive private information to other providers through the electronic medical record in a manner that does not interfere with a patient’s development of trust or lead to exposure and retraumatization,
- performing invasive physical exams in a sensitive and patient-centered manner, and
- using support and shared decision-making in clinical encounters.
Physicians can also connect patients with PTSD to programs or groups that aid in developing resilience, such as physical exercise classes, social support networks, and community involvement opportunities.4
CASE
The physician referred Mr. W to an onsite psychologist. At a subsequent clinic visit in which he was seen by a different primary care physician, Mr. W expressed new concerns about shoulder pain and changes in a mole. During this visit, Mr. W was asked whether he had followed up on the earlier referral for counseling. He replied that he had attended an intake appointment with the psychologist, but that he had not wanted to talk about what had happened to him and therefore avoided future appointments.*
He remained concerned that he might have an STD, but declined medication for PTSD because he felt he was “moving on” with his life.
*Author’s note: Getting patients to open up about their trauma exposure can be difficult. If the patient isn’t ready, simply bringing up the experience can trigger avoidance. It’s often helpful to encourage patients to first develop a relationship with their therapist, then later discuss the details of their trauma when they are ready. This encourages patients to engage in the counseling process.
CORRESPONDENCE
Adrienne A. Williams, PhD, Department of Family Medicine, University of Illinois at Chicago College of Medicine, 1919 W Taylor Street, MC663, Chicago, IL 60612; awms@uic.edu.
1. Bobo WV, Warner CH, Warner CM. The management of post traumatic stress disorder (PTSD) in the primary care setting. South Med J. 2007;100:797-802.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
3. Gradus JL. Epidemiology of PTSD. National Center for PTSD. Available at: http://www.ptsd.va.gov/professional/PTSD-overview/epidemiological-facts-ptsd.asp. Updated 2017. Accessed August 16, 2017.
4. Gerson R, Rappaport N. Traumatic stress and posttraumatic stress disorder in youth: recent research findings on clinical impact, assessment, and treatment. J Adolesc Health. 2013;52:137-143.
5. Zohar J, Juven-Wetzler A, Myers V, et al. Post-traumatic stress disorder: facts and fiction. Curr Opin Psychiatry. 2008;21:74-77.
6. Spoont MR, Williams JW Jr, Kehle-Forbes S, et al. Does this patient have posttraumatic stress disorder? Rational clinical examination systematic review. JAMA. 2015;314:501-510.
7. Woidneck MR, Morrison KL, Twohig MP. Acceptance and commitment therapy for the treatment of posttraumatic stress among adolescents. Behav Modif. 2014;38:451-476.
8. National Collaborating Centre for Mental Health (UK). Post-traumatic stress disorder: the management of PTSD in adults and children in primary and secondary care. Available at: https://www.ncbi.nlm.nih.gov/books/NBK56494. Accessed August 16, 2017.
9. Greene T, Neria Y, Gross R. Prevalence, detection and correlates of PTSD in the primary care setting: a systematic review. J Clin Psychol Med Settings. 2016;23:160-180.
10. Gavranidou M, Rosner R. The weaker sex? Gender and post-traumatic stress disorder. Depress Anxiety. 2003;17:130-139.
11. Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748-766.
12. SAMHSA’s Trauma and Justice Strategic Initiative. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. Available at: http://store.samhsa.gov/shin/content/SMA14-4884/SMA14-4884.pdf. Accessed September 13, 2017.
13. Mulick PS, Landes SJ, Kanter JW. Contextual behavior therapies in the treatment of PTSD: a review. Int J Behav Consult Ther. 2005;1:223-238.
14. Possemato K. The current state of intervention research for posttraumatic stress disorder within the primary care setting. J Clin Psychol Med Settings. 2011;18:268-280.
15. Forneris CA, Gartlehner G, Brownley KA, et al. Interventions to prevent post-traumatic stress disorder: a systematic review. Am J Prev Med. 2013;44:635-650.
16. Trivedi RB, Post EP, Sun H, et al. Prevalence, comorbidity, and prognosis of mental health among US veterans. Am J Public Health. 2015;105:2564-2569.
17. United States Census Bureau. Facts for features: Veteran’s day 2016: Nov. 11. Available at: https://www.census.gov/newsroom/facts-for-features/2016/cb16-ff21.html. Accessed August 16, 2017.
18. United States Census Bureau. U.S. and World Population Clock. Available at: https://www.census.gov/popclock/. Accessed August 16, 2017.
19. American Psychiatric Association. Guidelines and implementation. In: Practice Guidelines for the Psychiatric Evaluation of Adults. 3rd ed. Arlington, Va: American Psychiatric Association; 2015:9-45.
20. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
21. U.S. Department of Veterans Affairs. Life events checklist for DSM-5 (LEC-5). Available at: http://www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp. Accessed September 13, 2017.
22. U.S. Department of Veterans Affairs. Primary care PTSD screen for DSM-5 (PC-PTSD). Available at: http://www.ptsd.va.gov/professional/assessment/screens/pc-ptsd.asp. Accessed September 13, 2017.
23. Spoont M, Arbisi P, Fu S, et al. Screening for Post-Traumatic Stress Disorder (PTSD) in Primary Care: A Systematic Review. Available at: https://www.ncbi.nlm.nih.gov/books/NBK126691/. Accessed Sept 13, 2017
24. Gallagher MW, Thompson-Hollands J, Bourgeois ML, et al. Cognitive behavioral treatments for adult posttraumatic stress disorder: current status and future directions. J Contemp Psychother. 2015;45:235-243.
25. Kar N. Cognitive behavioral therapy for the treatment of post-traumatic stress disorder: a review. Neuropsychiatr Dis Treat. 2011;7:167-181.
26. Cusack K, Jonas DE, Forneris CA, et al. Psychological treatments for adults with posttraumatic stress disorder: a systematic review and meta-analysis. Clin Psychol Rev. 2016;43:128-141.
27. Thompson BL, Luoma JB, LeJeune JT. Using acceptance and commitment therapy to guide exposure-based interventions for posttraumatic stress disorder. J Contemp Psychother. 2013;43:133-140.
28. Lohr JM, Hooke W, Gist R, et al. Novel and controversial treatments for trauma-related stress disorders. In: Lilienfeld SO, Lynn SJ, Lohr JM, eds. Science and Pseudoscience in Clinical Psychology. New York, NY: Guilford Press; 2003:243-272.
29. Sikes C, Sikes V. EMDR: Why the controversy? Traumatol. 2003;9:169-182.
30. Davidson PR, Parker KCH. Eye movement desensitization and reprocessing (EMDR): a meta-analysis. J Consult Clin Psychol. 2001;69:305-316.
31. Devilly GJ. Power therapies and possible threats to the science of psychology and psychiatry. Aust N Z J Psychiatry. 2005;39:437-445.
1. Bobo WV, Warner CH, Warner CM. The management of post traumatic stress disorder (PTSD) in the primary care setting. South Med J. 2007;100:797-802.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
3. Gradus JL. Epidemiology of PTSD. National Center for PTSD. Available at: http://www.ptsd.va.gov/professional/PTSD-overview/epidemiological-facts-ptsd.asp. Updated 2017. Accessed August 16, 2017.
4. Gerson R, Rappaport N. Traumatic stress and posttraumatic stress disorder in youth: recent research findings on clinical impact, assessment, and treatment. J Adolesc Health. 2013;52:137-143.
5. Zohar J, Juven-Wetzler A, Myers V, et al. Post-traumatic stress disorder: facts and fiction. Curr Opin Psychiatry. 2008;21:74-77.
6. Spoont MR, Williams JW Jr, Kehle-Forbes S, et al. Does this patient have posttraumatic stress disorder? Rational clinical examination systematic review. JAMA. 2015;314:501-510.
7. Woidneck MR, Morrison KL, Twohig MP. Acceptance and commitment therapy for the treatment of posttraumatic stress among adolescents. Behav Modif. 2014;38:451-476.
8. National Collaborating Centre for Mental Health (UK). Post-traumatic stress disorder: the management of PTSD in adults and children in primary and secondary care. Available at: https://www.ncbi.nlm.nih.gov/books/NBK56494. Accessed August 16, 2017.
9. Greene T, Neria Y, Gross R. Prevalence, detection and correlates of PTSD in the primary care setting: a systematic review. J Clin Psychol Med Settings. 2016;23:160-180.
10. Gavranidou M, Rosner R. The weaker sex? Gender and post-traumatic stress disorder. Depress Anxiety. 2003;17:130-139.
11. Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:748-766.
12. SAMHSA’s Trauma and Justice Strategic Initiative. SAMHSA’s concept of trauma and guidance for a trauma-informed approach. Available at: http://store.samhsa.gov/shin/content/SMA14-4884/SMA14-4884.pdf. Accessed September 13, 2017.
13. Mulick PS, Landes SJ, Kanter JW. Contextual behavior therapies in the treatment of PTSD: a review. Int J Behav Consult Ther. 2005;1:223-238.
14. Possemato K. The current state of intervention research for posttraumatic stress disorder within the primary care setting. J Clin Psychol Med Settings. 2011;18:268-280.
15. Forneris CA, Gartlehner G, Brownley KA, et al. Interventions to prevent post-traumatic stress disorder: a systematic review. Am J Prev Med. 2013;44:635-650.
16. Trivedi RB, Post EP, Sun H, et al. Prevalence, comorbidity, and prognosis of mental health among US veterans. Am J Public Health. 2015;105:2564-2569.
17. United States Census Bureau. Facts for features: Veteran’s day 2016: Nov. 11. Available at: https://www.census.gov/newsroom/facts-for-features/2016/cb16-ff21.html. Accessed August 16, 2017.
18. United States Census Bureau. U.S. and World Population Clock. Available at: https://www.census.gov/popclock/. Accessed August 16, 2017.
19. American Psychiatric Association. Guidelines and implementation. In: Practice Guidelines for the Psychiatric Evaluation of Adults. 3rd ed. Arlington, Va: American Psychiatric Association; 2015:9-45.
20. Williams AA, Williams M. A guide to performing pelvic speculum exams: a patient-centered approach to reducing iatrogenic effects. Teach Learn Med. 2013;25:383-391.
21. U.S. Department of Veterans Affairs. Life events checklist for DSM-5 (LEC-5). Available at: http://www.ptsd.va.gov/professional/assessment/te-measures/life_events_checklist.asp. Accessed September 13, 2017.
22. U.S. Department of Veterans Affairs. Primary care PTSD screen for DSM-5 (PC-PTSD). Available at: http://www.ptsd.va.gov/professional/assessment/screens/pc-ptsd.asp. Accessed September 13, 2017.
23. Spoont M, Arbisi P, Fu S, et al. Screening for Post-Traumatic Stress Disorder (PTSD) in Primary Care: A Systematic Review. Available at: https://www.ncbi.nlm.nih.gov/books/NBK126691/. Accessed Sept 13, 2017
24. Gallagher MW, Thompson-Hollands J, Bourgeois ML, et al. Cognitive behavioral treatments for adult posttraumatic stress disorder: current status and future directions. J Contemp Psychother. 2015;45:235-243.
25. Kar N. Cognitive behavioral therapy for the treatment of post-traumatic stress disorder: a review. Neuropsychiatr Dis Treat. 2011;7:167-181.
26. Cusack K, Jonas DE, Forneris CA, et al. Psychological treatments for adults with posttraumatic stress disorder: a systematic review and meta-analysis. Clin Psychol Rev. 2016;43:128-141.
27. Thompson BL, Luoma JB, LeJeune JT. Using acceptance and commitment therapy to guide exposure-based interventions for posttraumatic stress disorder. J Contemp Psychother. 2013;43:133-140.
28. Lohr JM, Hooke W, Gist R, et al. Novel and controversial treatments for trauma-related stress disorders. In: Lilienfeld SO, Lynn SJ, Lohr JM, eds. Science and Pseudoscience in Clinical Psychology. New York, NY: Guilford Press; 2003:243-272.
29. Sikes C, Sikes V. EMDR: Why the controversy? Traumatol. 2003;9:169-182.
30. Davidson PR, Parker KCH. Eye movement desensitization and reprocessing (EMDR): a meta-analysis. J Consult Clin Psychol. 2001;69:305-316.
31. Devilly GJ. Power therapies and possible threats to the science of psychology and psychiatry. Aust N Z J Psychiatry. 2005;39:437-445.
Obsessive-compulsive disorder: Under-recognized and responsive to treatment
THE CASE
Ms. L is a 26-year-old woman in acute distress because of a recurrent thought. She worries: “What if I sexually molest my son?” She says she has no desire to act on this recurring thought and recognizes that it is unlikely to be true. The thought is so upsetting, however, that she has begun having panic attacks and avoids being left alone with her child.
She also reports past episodes of thoughts that she may be homosexual and thoughts that something catastrophic happened without her awareness (eg, that she unknowingly ran over someone while driving). She has attempted self-management, including trying to reason with herself, trying to stop thinking the thoughts, and seeking reassurance from her boyfriend and medical providers.
These compulsive behaviors lowered her distress temporarily, thereby reinforcing her need to check and seek reassurance.
HOW WOULD YOU PROCEED WITH THIS PATIENT?
Obsessive-compulsive disorder (OCD) is a common psychiatric disorder with a 12-month prevalence of 1.2% in the United States and internationally.1 Like other psychiatric disorders, patients with OCD present more often to primary care than specialty settings.2 Despite high distress and impairment levels, individuals with OCD are often undiagnosed and do not receive evidence-based care.3,4 This can be particularly problematic in fast-paced primary care settings due to high medical utilization and increased costs associated with OCD.5
A time-consuming disorder associated with distress
OCD is characterized by obsessive thoughts and/or compulsions.1
Obsessions are repeated, unwanted, distressing thoughts or images (eg, of being contaminated by dirt/germs, fears of causing harm to others without wanting to). Individuals with OCD attempt to avoid these thoughts by suppressing or neutralizing them.
Compulsions are mental or behavioral rituals that the individual feels compelled to perform to reduce distress or prevent a feared consequence (eg, hand-washing, checking locks, counting). Compulsions are not reasonable safety efforts, but are instead out-of-proportion reactions to the situation.
Onset of OCD usually occurs by young adulthood, but may be present in children. Pregnancy and postpartum periods may be associated with increased risk for symptoms.6 The course is typically chronic if left untreated, although symptoms can occur episodically.1
Intrusive thoughts and compulsive behaviors are surprisingly common in the general population. One study found that most individuals in a non-clinical sample reported having occasional intrusive thoughts such as whether they may have accidentally left the stove on, running their car off the road, or engaging in a “disgusting” sex act.7 With OCD, however, obsessions and compulsions are time-consuming and associated with distress and/or impairment.1
For example, an individual with OCD may restrict their diet due to fears of handling foods that other people may have touched or may limit contact with people for fear they will lose control and act violently. This is partly the result of overestimating the significance of the thoughts.8 Individuals with OCD may believe that the thoughts mean something negative about them (eg, that they are immoral) or could lead to serious consequences (eg, thinking about a car accident makes it more likely to occur).
Distinguishing features of OCD
OCD is commonly misdiagnosed,9 which may contribute to the long duration of untreated illness (average 17 years).10 In one study, primary care providers were given vignettes describing OCD symptoms; half of these cases were misidentified.9 Certain types of obsessions (eg, aggression, fear of saying certain things, homosexuality, pedophilia) were misdiagnosed 70% to 85% of the time.9
Although OCD shares characteristics with other disorders, several features can help family physicians correctly identify OCD. Fears associated with OCD are usually not about everyday concerns or worries. For example, a patient with social anxiety disorder may report fear of embarrassing themselves in public, whereas a patient with OCD may report fear that they will lose control and do something outlandish such as start swearing loudly.
Additionally, obsessions and compulsions in OCD are not exclusively tied to a traumatic experience as in posttraumatic stress disorder. Someone with OCD who has harm-related or sexual obsessions (eg, homosexuality) will report that this is not consistent with their interests and desires. Furthermore, a small subset of people with OCD may have poor insight, meaning they have low self-awareness of the nature of their obsessions or compulsions, but they do not experience psychotic symptoms.
How to make the diagnosis
A clinical interview is an essential component of assessing OCD in primary care. Ask patients who have mood or anxiety concerns about OCD symptoms, due to the high comorbidity rates of these entities.1 If a family history of OCD is known, assess the patient for this disorder, as higher rates exist among family members.10 Although primary care providers should indeed screen for OCD and provide provisional diagnoses as warranted, additional assessment by a behavioral health practitioner is recommended, given their specialty training in this area.
Patients with OCD are often reluctant to disclose intrusive thoughts due to perceiving them as shameful or unacceptable. Consider asking direct questions to facilitate the evaluation:
- “Do you ever feel bothered by unwanted or unusual thoughts that you cannot get out of your mind even though you try to?”
- “Do you feel that you have to do anything to get rid of these thoughts or prevent something bad from happening?”
- “Will you feel very uncomfortable if you don’t do something a specific way?”
Evidence-based self-report measures are also available. The Yale-Brown Obsessive Compulsive Scale (Y-BOCS)11 has become the gold standard psychometric measure for OCD. The updated version (Y-BOCS-II)12 and child/adolescent version (CY-BOCS)11 are also available.
Treatment: A tandem approach is most effective
Patient characteristics (eg, comorbid depression, level of adherence to treatment) may also help guide prescribing choices.10 For patients not responding to pharmacologic treatment in 8 to 12 weeks, consider referral to a psychiatrist.14
For OCD, CBT provided by a trained specialist typically involves exposure with response prevention (ERP). This entails confronting difficult thoughts and feared situations through exposure therapy and learning to reduce compulsive and excessive safety behaviors (eg, thinking about being contaminated by germs and then refraining from washing hands). Research suggests that CBT with ERP produces outcomes equivalent or superior to those achieved with pharmacotherapy.13 In addition to finding large effect sizes, clinical trials have demonstrated a treatment response of 86% in those completing CBT with ERP, compared with 48% of those receiving clomipramine.15 And Y-BOCS symptom scores have been reduced by 50% to 60% with CBT and ERP.16
How best to navigate coordination-of-care issues
When selecting a psychotherapy treatment provider for a patient with OCD, ask whether they are trained in ERP. If a trained psychotherapist is not available in the local health care system, you may refer to the International OCD Foundation (iocdf.org), which maintains an online directory of psychotherapists specializing in OCD.
Primary care physicians can also work with psychiatrists or psychotherapy providers to develop shared treatment plans. Part of this plan may involve reducing excess medical utilization and checking/reassurance (eg, requesting repeat medical tests). When there is concern about safety issues (eg, intrusive homicidal or suicidal thoughts), a risk assessment is strongly recommended.
CASE
An on-site psychologist evaluated Ms. L and diagnosed OCD. Ms. L had talked to the doctor about the possibility of medication, but she preferred to try behavioral treatment first. Ms. L agreed to participate in CBT with ERP. Treatment included imaginal and situational exposure exercises to decrease emotional reactivity associated with the thoughts, and to challenge beliefs that the thoughts are meaningful (eg, that having the thought means that she may act on it or that she is an unfit mother).
For example, Ms. L practiced repeating the thoughts aloud and going into feared situations (eg, being alone with her child). This was paired with response prevention, meaning that Ms. L was instructed to avoid checking with, or seeking, reassurance from others. She engaged in 4 ERP sessions in the primary care setting, and treatment led to significant symptom improvement that was maintained at follow-up with her primary care provider 6 and 12 months later.
CORRESPONDENCE
Jared L. Skillings, PhD, ABPP, Division of Psychiatry and Behavioral Medicine, Spectrum Health System, 2750 East Beltline NE, Grand Rapids, MI 49525; jared.skillings@spectrumhealth.org.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
2. Deacon B, Lickel J, Abramowitz JS. Medical utilization across the anxiety disorders. J Anxiety Disord. 2008;22:344-350.
3. Glazier K, Calizte RM, Rothschild R, et al. High rates of OCD symptom misidentification by mental health professionals. Ann Clin Psychiatry. 2013;25:201-209.
4. Schwartz C, Schlegl S, Kuelz AK, et al. Treatment-seeking in OCD community cases and psychological treatment actually provided to treatment-seeking patients: a systematic review. J Obsessive Compuls Relat Disord. 2013;2:448-456.
5. Marciniak M, Lage MJ, Landbloom RP, et al. Medical and productivity costs of anxiety disorders: case control study. Depress Anxiety. 2004;19:112-120.
6. Forray A, Focseneanu M, Pittman B, et al. Onset and exacerbation of obsessive-compulsive disorder in pregnancy and the postpartum period. J Clin Psychiatry. 2010;71:1061-1068.
7. Purdon C, Clark DA. Obsessive intrusive thoughts in nonclinical subjects. Part I. Content and relation with depressive, anxious and obsessional symptoms. Behav Res Ther. 1993;31:713-720.
8. Clark DA. Cognitive-Behavioral Therapy for OCD. New York: The Guilford Press; 2002.
9. Glazier K, Swing M, McGinn LK. Half of obsessive-compulsive disorder cases misdiagnosed: vignette-based survey of primary care physicians. J Clin Psychiatry. 2015;26:e761-e767.
10. Fineberg NA, Reghunandanan S, Simpson HB, et al. Obsessive-compulsive disorder (OCD): practical strategies for pharmacological and somatic treatment in adults. Psych Res. 2015;227:114-125.
11. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006-1011.
12. Storch EA, Larson MJ, Goodman WK, et al. Development and psychometric evaluation of the Yale-Brown Obsessive-Compulsive Scale—second edition. Psychol Assess. 2010;22:223-232.
13. Katzman MA, Bleau P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14(Suppl 1):S1.
14. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder, and posttraumatic stress disorder in primary care. Int J Psychiatry Clin. 2012;16:77-84.
15. Foa FB, Liebowitz MR, Kozak MJ, et al. Randomized, placebo-controlled trial of exposure and response prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 2005;162:151-161.
16. Abramowitz JS. The psychological treatment of obsessive compulsive disorder. Can J Psychiatry. 2006;51:407-416.
THE CASE
Ms. L is a 26-year-old woman in acute distress because of a recurrent thought. She worries: “What if I sexually molest my son?” She says she has no desire to act on this recurring thought and recognizes that it is unlikely to be true. The thought is so upsetting, however, that she has begun having panic attacks and avoids being left alone with her child.
She also reports past episodes of thoughts that she may be homosexual and thoughts that something catastrophic happened without her awareness (eg, that she unknowingly ran over someone while driving). She has attempted self-management, including trying to reason with herself, trying to stop thinking the thoughts, and seeking reassurance from her boyfriend and medical providers.
These compulsive behaviors lowered her distress temporarily, thereby reinforcing her need to check and seek reassurance.
HOW WOULD YOU PROCEED WITH THIS PATIENT?
Obsessive-compulsive disorder (OCD) is a common psychiatric disorder with a 12-month prevalence of 1.2% in the United States and internationally.1 Like other psychiatric disorders, patients with OCD present more often to primary care than specialty settings.2 Despite high distress and impairment levels, individuals with OCD are often undiagnosed and do not receive evidence-based care.3,4 This can be particularly problematic in fast-paced primary care settings due to high medical utilization and increased costs associated with OCD.5
A time-consuming disorder associated with distress
OCD is characterized by obsessive thoughts and/or compulsions.1
Obsessions are repeated, unwanted, distressing thoughts or images (eg, of being contaminated by dirt/germs, fears of causing harm to others without wanting to). Individuals with OCD attempt to avoid these thoughts by suppressing or neutralizing them.
Compulsions are mental or behavioral rituals that the individual feels compelled to perform to reduce distress or prevent a feared consequence (eg, hand-washing, checking locks, counting). Compulsions are not reasonable safety efforts, but are instead out-of-proportion reactions to the situation.
Onset of OCD usually occurs by young adulthood, but may be present in children. Pregnancy and postpartum periods may be associated with increased risk for symptoms.6 The course is typically chronic if left untreated, although symptoms can occur episodically.1
Intrusive thoughts and compulsive behaviors are surprisingly common in the general population. One study found that most individuals in a non-clinical sample reported having occasional intrusive thoughts such as whether they may have accidentally left the stove on, running their car off the road, or engaging in a “disgusting” sex act.7 With OCD, however, obsessions and compulsions are time-consuming and associated with distress and/or impairment.1
For example, an individual with OCD may restrict their diet due to fears of handling foods that other people may have touched or may limit contact with people for fear they will lose control and act violently. This is partly the result of overestimating the significance of the thoughts.8 Individuals with OCD may believe that the thoughts mean something negative about them (eg, that they are immoral) or could lead to serious consequences (eg, thinking about a car accident makes it more likely to occur).
Distinguishing features of OCD
OCD is commonly misdiagnosed,9 which may contribute to the long duration of untreated illness (average 17 years).10 In one study, primary care providers were given vignettes describing OCD symptoms; half of these cases were misidentified.9 Certain types of obsessions (eg, aggression, fear of saying certain things, homosexuality, pedophilia) were misdiagnosed 70% to 85% of the time.9
Although OCD shares characteristics with other disorders, several features can help family physicians correctly identify OCD. Fears associated with OCD are usually not about everyday concerns or worries. For example, a patient with social anxiety disorder may report fear of embarrassing themselves in public, whereas a patient with OCD may report fear that they will lose control and do something outlandish such as start swearing loudly.
Additionally, obsessions and compulsions in OCD are not exclusively tied to a traumatic experience as in posttraumatic stress disorder. Someone with OCD who has harm-related or sexual obsessions (eg, homosexuality) will report that this is not consistent with their interests and desires. Furthermore, a small subset of people with OCD may have poor insight, meaning they have low self-awareness of the nature of their obsessions or compulsions, but they do not experience psychotic symptoms.
How to make the diagnosis
A clinical interview is an essential component of assessing OCD in primary care. Ask patients who have mood or anxiety concerns about OCD symptoms, due to the high comorbidity rates of these entities.1 If a family history of OCD is known, assess the patient for this disorder, as higher rates exist among family members.10 Although primary care providers should indeed screen for OCD and provide provisional diagnoses as warranted, additional assessment by a behavioral health practitioner is recommended, given their specialty training in this area.
Patients with OCD are often reluctant to disclose intrusive thoughts due to perceiving them as shameful or unacceptable. Consider asking direct questions to facilitate the evaluation:
- “Do you ever feel bothered by unwanted or unusual thoughts that you cannot get out of your mind even though you try to?”
- “Do you feel that you have to do anything to get rid of these thoughts or prevent something bad from happening?”
- “Will you feel very uncomfortable if you don’t do something a specific way?”
Evidence-based self-report measures are also available. The Yale-Brown Obsessive Compulsive Scale (Y-BOCS)11 has become the gold standard psychometric measure for OCD. The updated version (Y-BOCS-II)12 and child/adolescent version (CY-BOCS)11 are also available.
Treatment: A tandem approach is most effective
Patient characteristics (eg, comorbid depression, level of adherence to treatment) may also help guide prescribing choices.10 For patients not responding to pharmacologic treatment in 8 to 12 weeks, consider referral to a psychiatrist.14
For OCD, CBT provided by a trained specialist typically involves exposure with response prevention (ERP). This entails confronting difficult thoughts and feared situations through exposure therapy and learning to reduce compulsive and excessive safety behaviors (eg, thinking about being contaminated by germs and then refraining from washing hands). Research suggests that CBT with ERP produces outcomes equivalent or superior to those achieved with pharmacotherapy.13 In addition to finding large effect sizes, clinical trials have demonstrated a treatment response of 86% in those completing CBT with ERP, compared with 48% of those receiving clomipramine.15 And Y-BOCS symptom scores have been reduced by 50% to 60% with CBT and ERP.16
How best to navigate coordination-of-care issues
When selecting a psychotherapy treatment provider for a patient with OCD, ask whether they are trained in ERP. If a trained psychotherapist is not available in the local health care system, you may refer to the International OCD Foundation (iocdf.org), which maintains an online directory of psychotherapists specializing in OCD.
Primary care physicians can also work with psychiatrists or psychotherapy providers to develop shared treatment plans. Part of this plan may involve reducing excess medical utilization and checking/reassurance (eg, requesting repeat medical tests). When there is concern about safety issues (eg, intrusive homicidal or suicidal thoughts), a risk assessment is strongly recommended.
CASE
An on-site psychologist evaluated Ms. L and diagnosed OCD. Ms. L had talked to the doctor about the possibility of medication, but she preferred to try behavioral treatment first. Ms. L agreed to participate in CBT with ERP. Treatment included imaginal and situational exposure exercises to decrease emotional reactivity associated with the thoughts, and to challenge beliefs that the thoughts are meaningful (eg, that having the thought means that she may act on it or that she is an unfit mother).
For example, Ms. L practiced repeating the thoughts aloud and going into feared situations (eg, being alone with her child). This was paired with response prevention, meaning that Ms. L was instructed to avoid checking with, or seeking, reassurance from others. She engaged in 4 ERP sessions in the primary care setting, and treatment led to significant symptom improvement that was maintained at follow-up with her primary care provider 6 and 12 months later.
CORRESPONDENCE
Jared L. Skillings, PhD, ABPP, Division of Psychiatry and Behavioral Medicine, Spectrum Health System, 2750 East Beltline NE, Grand Rapids, MI 49525; jared.skillings@spectrumhealth.org.
THE CASE
Ms. L is a 26-year-old woman in acute distress because of a recurrent thought. She worries: “What if I sexually molest my son?” She says she has no desire to act on this recurring thought and recognizes that it is unlikely to be true. The thought is so upsetting, however, that she has begun having panic attacks and avoids being left alone with her child.
She also reports past episodes of thoughts that she may be homosexual and thoughts that something catastrophic happened without her awareness (eg, that she unknowingly ran over someone while driving). She has attempted self-management, including trying to reason with herself, trying to stop thinking the thoughts, and seeking reassurance from her boyfriend and medical providers.
These compulsive behaviors lowered her distress temporarily, thereby reinforcing her need to check and seek reassurance.
HOW WOULD YOU PROCEED WITH THIS PATIENT?
Obsessive-compulsive disorder (OCD) is a common psychiatric disorder with a 12-month prevalence of 1.2% in the United States and internationally.1 Like other psychiatric disorders, patients with OCD present more often to primary care than specialty settings.2 Despite high distress and impairment levels, individuals with OCD are often undiagnosed and do not receive evidence-based care.3,4 This can be particularly problematic in fast-paced primary care settings due to high medical utilization and increased costs associated with OCD.5
A time-consuming disorder associated with distress
OCD is characterized by obsessive thoughts and/or compulsions.1
Obsessions are repeated, unwanted, distressing thoughts or images (eg, of being contaminated by dirt/germs, fears of causing harm to others without wanting to). Individuals with OCD attempt to avoid these thoughts by suppressing or neutralizing them.
Compulsions are mental or behavioral rituals that the individual feels compelled to perform to reduce distress or prevent a feared consequence (eg, hand-washing, checking locks, counting). Compulsions are not reasonable safety efforts, but are instead out-of-proportion reactions to the situation.
Onset of OCD usually occurs by young adulthood, but may be present in children. Pregnancy and postpartum periods may be associated with increased risk for symptoms.6 The course is typically chronic if left untreated, although symptoms can occur episodically.1
Intrusive thoughts and compulsive behaviors are surprisingly common in the general population. One study found that most individuals in a non-clinical sample reported having occasional intrusive thoughts such as whether they may have accidentally left the stove on, running their car off the road, or engaging in a “disgusting” sex act.7 With OCD, however, obsessions and compulsions are time-consuming and associated with distress and/or impairment.1
For example, an individual with OCD may restrict their diet due to fears of handling foods that other people may have touched or may limit contact with people for fear they will lose control and act violently. This is partly the result of overestimating the significance of the thoughts.8 Individuals with OCD may believe that the thoughts mean something negative about them (eg, that they are immoral) or could lead to serious consequences (eg, thinking about a car accident makes it more likely to occur).
Distinguishing features of OCD
OCD is commonly misdiagnosed,9 which may contribute to the long duration of untreated illness (average 17 years).10 In one study, primary care providers were given vignettes describing OCD symptoms; half of these cases were misidentified.9 Certain types of obsessions (eg, aggression, fear of saying certain things, homosexuality, pedophilia) were misdiagnosed 70% to 85% of the time.9
Although OCD shares characteristics with other disorders, several features can help family physicians correctly identify OCD. Fears associated with OCD are usually not about everyday concerns or worries. For example, a patient with social anxiety disorder may report fear of embarrassing themselves in public, whereas a patient with OCD may report fear that they will lose control and do something outlandish such as start swearing loudly.
Additionally, obsessions and compulsions in OCD are not exclusively tied to a traumatic experience as in posttraumatic stress disorder. Someone with OCD who has harm-related or sexual obsessions (eg, homosexuality) will report that this is not consistent with their interests and desires. Furthermore, a small subset of people with OCD may have poor insight, meaning they have low self-awareness of the nature of their obsessions or compulsions, but they do not experience psychotic symptoms.
How to make the diagnosis
A clinical interview is an essential component of assessing OCD in primary care. Ask patients who have mood or anxiety concerns about OCD symptoms, due to the high comorbidity rates of these entities.1 If a family history of OCD is known, assess the patient for this disorder, as higher rates exist among family members.10 Although primary care providers should indeed screen for OCD and provide provisional diagnoses as warranted, additional assessment by a behavioral health practitioner is recommended, given their specialty training in this area.
Patients with OCD are often reluctant to disclose intrusive thoughts due to perceiving them as shameful or unacceptable. Consider asking direct questions to facilitate the evaluation:
- “Do you ever feel bothered by unwanted or unusual thoughts that you cannot get out of your mind even though you try to?”
- “Do you feel that you have to do anything to get rid of these thoughts or prevent something bad from happening?”
- “Will you feel very uncomfortable if you don’t do something a specific way?”
Evidence-based self-report measures are also available. The Yale-Brown Obsessive Compulsive Scale (Y-BOCS)11 has become the gold standard psychometric measure for OCD. The updated version (Y-BOCS-II)12 and child/adolescent version (CY-BOCS)11 are also available.
Treatment: A tandem approach is most effective
Patient characteristics (eg, comorbid depression, level of adherence to treatment) may also help guide prescribing choices.10 For patients not responding to pharmacologic treatment in 8 to 12 weeks, consider referral to a psychiatrist.14
For OCD, CBT provided by a trained specialist typically involves exposure with response prevention (ERP). This entails confronting difficult thoughts and feared situations through exposure therapy and learning to reduce compulsive and excessive safety behaviors (eg, thinking about being contaminated by germs and then refraining from washing hands). Research suggests that CBT with ERP produces outcomes equivalent or superior to those achieved with pharmacotherapy.13 In addition to finding large effect sizes, clinical trials have demonstrated a treatment response of 86% in those completing CBT with ERP, compared with 48% of those receiving clomipramine.15 And Y-BOCS symptom scores have been reduced by 50% to 60% with CBT and ERP.16
How best to navigate coordination-of-care issues
When selecting a psychotherapy treatment provider for a patient with OCD, ask whether they are trained in ERP. If a trained psychotherapist is not available in the local health care system, you may refer to the International OCD Foundation (iocdf.org), which maintains an online directory of psychotherapists specializing in OCD.
Primary care physicians can also work with psychiatrists or psychotherapy providers to develop shared treatment plans. Part of this plan may involve reducing excess medical utilization and checking/reassurance (eg, requesting repeat medical tests). When there is concern about safety issues (eg, intrusive homicidal or suicidal thoughts), a risk assessment is strongly recommended.
CASE
An on-site psychologist evaluated Ms. L and diagnosed OCD. Ms. L had talked to the doctor about the possibility of medication, but she preferred to try behavioral treatment first. Ms. L agreed to participate in CBT with ERP. Treatment included imaginal and situational exposure exercises to decrease emotional reactivity associated with the thoughts, and to challenge beliefs that the thoughts are meaningful (eg, that having the thought means that she may act on it or that she is an unfit mother).
For example, Ms. L practiced repeating the thoughts aloud and going into feared situations (eg, being alone with her child). This was paired with response prevention, meaning that Ms. L was instructed to avoid checking with, or seeking, reassurance from others. She engaged in 4 ERP sessions in the primary care setting, and treatment led to significant symptom improvement that was maintained at follow-up with her primary care provider 6 and 12 months later.
CORRESPONDENCE
Jared L. Skillings, PhD, ABPP, Division of Psychiatry and Behavioral Medicine, Spectrum Health System, 2750 East Beltline NE, Grand Rapids, MI 49525; jared.skillings@spectrumhealth.org.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
2. Deacon B, Lickel J, Abramowitz JS. Medical utilization across the anxiety disorders. J Anxiety Disord. 2008;22:344-350.
3. Glazier K, Calizte RM, Rothschild R, et al. High rates of OCD symptom misidentification by mental health professionals. Ann Clin Psychiatry. 2013;25:201-209.
4. Schwartz C, Schlegl S, Kuelz AK, et al. Treatment-seeking in OCD community cases and psychological treatment actually provided to treatment-seeking patients: a systematic review. J Obsessive Compuls Relat Disord. 2013;2:448-456.
5. Marciniak M, Lage MJ, Landbloom RP, et al. Medical and productivity costs of anxiety disorders: case control study. Depress Anxiety. 2004;19:112-120.
6. Forray A, Focseneanu M, Pittman B, et al. Onset and exacerbation of obsessive-compulsive disorder in pregnancy and the postpartum period. J Clin Psychiatry. 2010;71:1061-1068.
7. Purdon C, Clark DA. Obsessive intrusive thoughts in nonclinical subjects. Part I. Content and relation with depressive, anxious and obsessional symptoms. Behav Res Ther. 1993;31:713-720.
8. Clark DA. Cognitive-Behavioral Therapy for OCD. New York: The Guilford Press; 2002.
9. Glazier K, Swing M, McGinn LK. Half of obsessive-compulsive disorder cases misdiagnosed: vignette-based survey of primary care physicians. J Clin Psychiatry. 2015;26:e761-e767.
10. Fineberg NA, Reghunandanan S, Simpson HB, et al. Obsessive-compulsive disorder (OCD): practical strategies for pharmacological and somatic treatment in adults. Psych Res. 2015;227:114-125.
11. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006-1011.
12. Storch EA, Larson MJ, Goodman WK, et al. Development and psychometric evaluation of the Yale-Brown Obsessive-Compulsive Scale—second edition. Psychol Assess. 2010;22:223-232.
13. Katzman MA, Bleau P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14(Suppl 1):S1.
14. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder, and posttraumatic stress disorder in primary care. Int J Psychiatry Clin. 2012;16:77-84.
15. Foa FB, Liebowitz MR, Kozak MJ, et al. Randomized, placebo-controlled trial of exposure and response prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 2005;162:151-161.
16. Abramowitz JS. The psychological treatment of obsessive compulsive disorder. Can J Psychiatry. 2006;51:407-416.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
2. Deacon B, Lickel J, Abramowitz JS. Medical utilization across the anxiety disorders. J Anxiety Disord. 2008;22:344-350.
3. Glazier K, Calizte RM, Rothschild R, et al. High rates of OCD symptom misidentification by mental health professionals. Ann Clin Psychiatry. 2013;25:201-209.
4. Schwartz C, Schlegl S, Kuelz AK, et al. Treatment-seeking in OCD community cases and psychological treatment actually provided to treatment-seeking patients: a systematic review. J Obsessive Compuls Relat Disord. 2013;2:448-456.
5. Marciniak M, Lage MJ, Landbloom RP, et al. Medical and productivity costs of anxiety disorders: case control study. Depress Anxiety. 2004;19:112-120.
6. Forray A, Focseneanu M, Pittman B, et al. Onset and exacerbation of obsessive-compulsive disorder in pregnancy and the postpartum period. J Clin Psychiatry. 2010;71:1061-1068.
7. Purdon C, Clark DA. Obsessive intrusive thoughts in nonclinical subjects. Part I. Content and relation with depressive, anxious and obsessional symptoms. Behav Res Ther. 1993;31:713-720.
8. Clark DA. Cognitive-Behavioral Therapy for OCD. New York: The Guilford Press; 2002.
9. Glazier K, Swing M, McGinn LK. Half of obsessive-compulsive disorder cases misdiagnosed: vignette-based survey of primary care physicians. J Clin Psychiatry. 2015;26:e761-e767.
10. Fineberg NA, Reghunandanan S, Simpson HB, et al. Obsessive-compulsive disorder (OCD): practical strategies for pharmacological and somatic treatment in adults. Psych Res. 2015;227:114-125.
11. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006-1011.
12. Storch EA, Larson MJ, Goodman WK, et al. Development and psychometric evaluation of the Yale-Brown Obsessive-Compulsive Scale—second edition. Psychol Assess. 2010;22:223-232.
13. Katzman MA, Bleau P, et al. Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry. 2014;14(Suppl 1):S1.
14. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder, and posttraumatic stress disorder in primary care. Int J Psychiatry Clin. 2012;16:77-84.
15. Foa FB, Liebowitz MR, Kozak MJ, et al. Randomized, placebo-controlled trial of exposure and response prevention, clomipramine, and their combination in the treatment of obsessive-compulsive disorder. Am J Psychiatry. 2005;162:151-161.
16. Abramowitz JS. The psychological treatment of obsessive compulsive disorder. Can J Psychiatry. 2006;51:407-416.
Bipolar disorder: Making the Dx, selecting the right Rx
THE CASE
A 23-year-old woman seeks medical attention at the request of her boyfriend because she’s been “miserable” for 3 weeks. In the examination room, she slouches in the chair and says her mood is low, her grades have dropped, and she no longer enjoys social gatherings or her other usual activities. She has no thoughts of suicide, no weight loss, and no somatic symptoms.
She says she is generally healthy, does not take any regular medications, and has never been pregnant. When asked about previous similar episodes, she admits to feeling this way about 3 times a year for one to 2 months at a time. She has tried different antidepressants, which haven’t helped much and have made her irritable and interfered with sleep.
When asked about mania or hypomania, she says there are short periods, roughly a couple of weeks 2 or 3 times a year, when she will get a lot of work done and can get by with little sleep. She has never gone on “spending sprees,” though, or indulged in any other unusual or dangerous behavior. And she has never been hospitalized for symptoms.
Bipolar disorders, over time, typically cause fluctuations in mood, activity, and energy level. If disorders go untreated, a patient’s behavior may cause considerable damage to relationships, finances, and reputations. And for some patients, the disorder can take the ultimate toll, resulting in death by suicide or accident.
Subtypes of bipolar disorder differ in the timing and severity of manic (or hypomanic) and depressive symptoms or episodes. Type I is the classic manic-depressive illness; type II is characterized by chronic treatment-resistant depression punctuated by hypomanic episodes; and cyclothymia leads to chronic fluctuations in mood. The diagnostic category “bipolar disorder not otherwise specified” applies to patients who meet some, but not all, of the criteria for other bipolar disorder subtypes.1
Prevalence. As with other mood symptoms or disorders, patients with bipolar disorder are often seen first in primary care due, in part, to barriers to obtaining psychiatric care or to avoidance of the perceived stigma in seeking such care.2 In a systematic review of patients who were interviewed randomly in primary care settings, 0.5% to 4.3% met criteria for bipolar disorder.3 The average age of onset for bipolar disorder is 15 to 19 years.4 In the United States, the prevalence of bipolar disorder type I is 1%; type II is 1.1%.3
The cause of bipolar disorder is unknown, but familial predisposition, biopsychosocial factors, and environment all seem to play a role. Children of parents with bipolar disorder have a 4% to 15% chance of receiving the same diagnosis, compared with children of parents without bipolar disorder, whose risk is only as high as 2%.5,6
Clinical presentation varies
When patients with bipolar disorder are first seen in the office, their state may be depression, mania, hypomania, or even euthymia. Keep in mind that the first 3 aberrations may indicate other disorders, either
Verify a true depressive episode
Symptoms must last for 2 weeks and include anhedonia or depressed mood, as well as some combination of changes in sleep, increased feelings of guilt, poor concentration, changes in appetite, loss of energy, psychomotor agitation or retardation, or suicidal thoughts.1
Know the criteria for mania
True mania is a distinct period of abnormally and persistently elevated, expansive, or irritable mood, accompanied by abnormally and persistently increased activity or energy, and lasting at least one week for most of the day, nearly every day (or any duration if hospitalization is necessary).
During that time, the patient must also exhibit at least 3 or more of the following symptoms (not counting irritability, if present): 1
- distractibility,
- insomnia,
- grandiosity,
- flights of ideas,
- increased goal-directed activity or agitation,
- rapid/pressured speech, or
- reckless behaviors.
How hypomania differs from mania. The symptoms of hypomania are less severe than those of mania—eg, social functioning is less impaired or is even normal, and there is no need for hospitalization. Patients may feel they have been much more productive than usual or have needed less sleep to engage in daily activities. Hypomania may be present but not reported by patients who perceive nothing wrong.1,4
Rule out alternate diagnoses and apply DSM-5 criteria
There are no objective tests to confirm a diagnosis of bipolar disorder. If you suspect bipolar disorder, focus your clinical evaluation on ruling out competing mental health or medical diagnoses, and on determining whether the patient’s history meets criteria for a bipolar disorder as described in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5).1
Explore the patient’s psychiatric history (including hospitalizations, medications, and electroconvulsive therapy), general medical history, family history of psychiatric disorders (including suicide), and social history (including substance use and abuse). And carefully observe mental status. Confirming a diagnosis of bipolar disorder may take multiple visits, but strongly suggestive symptoms could warrant empirical treatment.
Helpful scales. The Patient Health Questionnaire (PHQ-9; https://www.uspreventiveservicestaskforce.org/Home/GetFileByID/218) and the Beck Depression Inventory (http://www.hr.ucdavis.edu/asap/pdf_files/Beck_Depression_Inventory.pdf) are useful for ruling out depressive disorders. Other scales are available, but they cannot confirm bipolar disorder. Laboratory testing selected according to patient symptoms (TABLE 24) can help rule out alternative diagnoses, but are also useful for establishing a baseline for medications.
Pharmacologic treatment: Match agents to symptoms
When treating bipolar disorder, choose a drug that targets a patient’s specific symptoms (TABLE 3).7-10 In primary care, the most commonly-used treatments for bipolar disorder type II are lamotrigine, valproic acid, and lithium.11
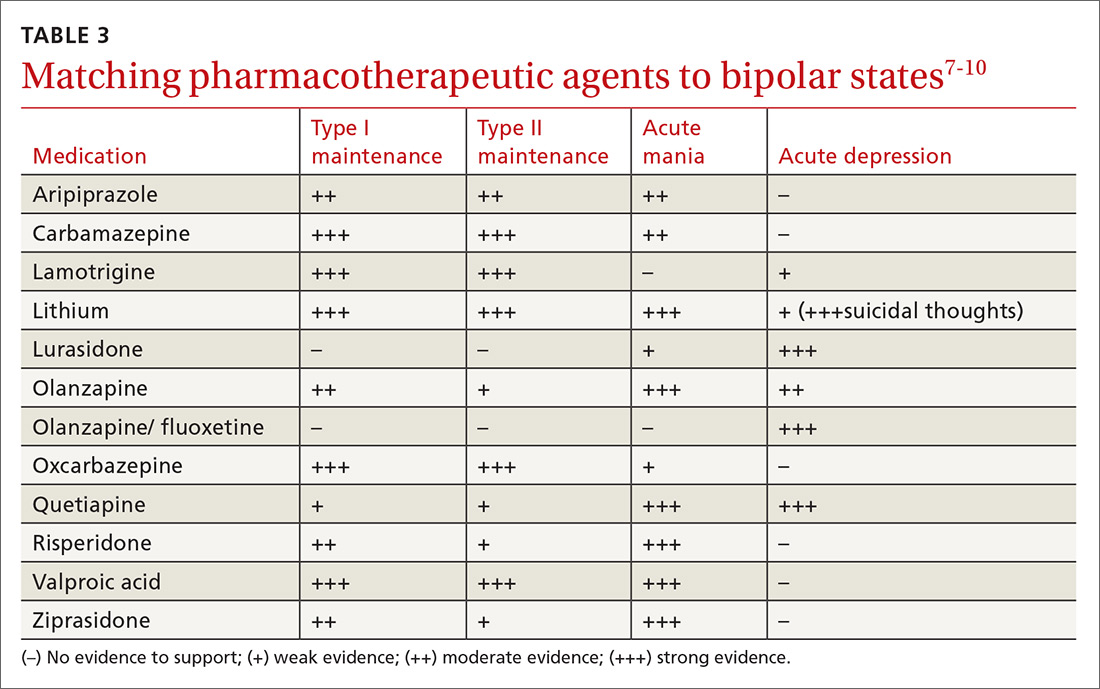
When to refer
Many cases of bipolar disorder type II can be managed successfully in a primary care practice, as can some cases of stable bipolar disorder type I. Psychiatric consultation may be most beneficial if the patient has recently attempted suicide or has suicidal ideation, has symptoms refractory to treatment, has poor medication adherence, or is misusing medications.
Even when patients are co-managed with psychiatric consultation, family physicians often ensure patients’ medication adherence, help patients understand their illness, manage overall health-related behaviors (including getting sufficient sleep), and make sure patients follow up as needed with their psychiatrist. Often, once patients have achieved equilibrium on mood-stabilizing (or other) medications, you can manage them and monitor medications with further consultation only as needed for clinical deterioration or other issues. Cognitive behavioral therapy may be useful as adjunctive treatment, particularly when patients are in active treatment.12
› CASE
This case is typical for many patients with depressed mood. A few key features in the patient’s history suggest bipolar disorder type II:
- depression that has been refractory to treatment
- multiple failed drug treatments, with mood-related adverse effects
- hypomania perceived as a “productive time,” and not as a problem
- absence of overt manic symptoms.
The patient was given a diagnosis of bipolar disorder type II with current depressed mood and no evidence of acute mania. She was started on valproic acid 250 mg po tid. She reported an initial improvement in mood but stopped the medication after one month because it caused intolerable drowsiness. She was then prescribed lamotrigine progressing gradually in 2-week intervals from 25 mg to 100 mg daily. She tolerated the medication well, and after 3 months of treatment, her mood symptoms improved and she had no further episodes of depressed mood.
CORRESPONDENCE
Michael Jason Wells, MD, Department of Family and Geriatric Medicine, University of Louisville School of Medicine, 201 Abraham Flexner Way, Suite 690, Louisville, KY 40202; mjwell04@louisville.edu.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed (DSM-5). Arlington, VA: American Psychiatric Association; 2013.
2. Kilbourne AM, Goodrich DE, O’Donnell AN, et al. Integrated bipolar disorder management in primary care. Curr Psychiatry Rep. 2012;14:687-695.
3. Cerimele JM, Chwastiak LA, Dodson S, et al. The prevalence of bipolar disorder in primary care samples: a systematic review. Gen Hosp Psychiatry. 2014;36:19-25.
4. Malhi GS, Adams D, Lampe L, et al. Clinical practice recommendations for bipolar disorder. Acta Pscyhiatr Scand. 2009:119(Suppl 439):27-46.
5. Abell S, Ey J. Bipolar Disorder. Clin Pediatr. 2009;48:693-694.
6. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders of school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study. Arch Gen Psychiatry. 2009;66:287-296.
7. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646.
8. De Fruyt J, Deschepper E, Audenaert K, et al. Second generation antipsychotics in the treatment of bipolar depression: a systematic review and meta-analysis. J Psychopharmacol. 2012;26:603-617.
9. Gitlin M, Frye MA. Maintenance therapies in bipolar disorders. Bipolar Disord. 2012:14(Suppl 2):51-65.
10. Labbate LA, Fava M, Rosenbaum JF, et al. Handbook of Psychiatric Drug Therapy. 6th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2010.
11. Ostacher M, Tandon R, Suppes T. Florida Best Practice Psychotherapeutic Medication Guidelines for Adults with Bipolar Disorder: a novel, practical, patient-centered guide for clinicians. J Clin Psychiatry. 2016;77:920-926.
12. Szentagotai A, David D. The efficacy of cognitive behavioral therapy in bipolar disorder: a quantitative meta-analysis. J Clin Psychiatry. 2010;71:66-72.
THE CASE
A 23-year-old woman seeks medical attention at the request of her boyfriend because she’s been “miserable” for 3 weeks. In the examination room, she slouches in the chair and says her mood is low, her grades have dropped, and she no longer enjoys social gatherings or her other usual activities. She has no thoughts of suicide, no weight loss, and no somatic symptoms.
She says she is generally healthy, does not take any regular medications, and has never been pregnant. When asked about previous similar episodes, she admits to feeling this way about 3 times a year for one to 2 months at a time. She has tried different antidepressants, which haven’t helped much and have made her irritable and interfered with sleep.
When asked about mania or hypomania, she says there are short periods, roughly a couple of weeks 2 or 3 times a year, when she will get a lot of work done and can get by with little sleep. She has never gone on “spending sprees,” though, or indulged in any other unusual or dangerous behavior. And she has never been hospitalized for symptoms.
Bipolar disorders, over time, typically cause fluctuations in mood, activity, and energy level. If disorders go untreated, a patient’s behavior may cause considerable damage to relationships, finances, and reputations. And for some patients, the disorder can take the ultimate toll, resulting in death by suicide or accident.
Subtypes of bipolar disorder differ in the timing and severity of manic (or hypomanic) and depressive symptoms or episodes. Type I is the classic manic-depressive illness; type II is characterized by chronic treatment-resistant depression punctuated by hypomanic episodes; and cyclothymia leads to chronic fluctuations in mood. The diagnostic category “bipolar disorder not otherwise specified” applies to patients who meet some, but not all, of the criteria for other bipolar disorder subtypes.1
Prevalence. As with other mood symptoms or disorders, patients with bipolar disorder are often seen first in primary care due, in part, to barriers to obtaining psychiatric care or to avoidance of the perceived stigma in seeking such care.2 In a systematic review of patients who were interviewed randomly in primary care settings, 0.5% to 4.3% met criteria for bipolar disorder.3 The average age of onset for bipolar disorder is 15 to 19 years.4 In the United States, the prevalence of bipolar disorder type I is 1%; type II is 1.1%.3
The cause of bipolar disorder is unknown, but familial predisposition, biopsychosocial factors, and environment all seem to play a role. Children of parents with bipolar disorder have a 4% to 15% chance of receiving the same diagnosis, compared with children of parents without bipolar disorder, whose risk is only as high as 2%.5,6
Clinical presentation varies
When patients with bipolar disorder are first seen in the office, their state may be depression, mania, hypomania, or even euthymia. Keep in mind that the first 3 aberrations may indicate other disorders, either
Verify a true depressive episode
Symptoms must last for 2 weeks and include anhedonia or depressed mood, as well as some combination of changes in sleep, increased feelings of guilt, poor concentration, changes in appetite, loss of energy, psychomotor agitation or retardation, or suicidal thoughts.1
Know the criteria for mania
True mania is a distinct period of abnormally and persistently elevated, expansive, or irritable mood, accompanied by abnormally and persistently increased activity or energy, and lasting at least one week for most of the day, nearly every day (or any duration if hospitalization is necessary).
During that time, the patient must also exhibit at least 3 or more of the following symptoms (not counting irritability, if present): 1
- distractibility,
- insomnia,
- grandiosity,
- flights of ideas,
- increased goal-directed activity or agitation,
- rapid/pressured speech, or
- reckless behaviors.
How hypomania differs from mania. The symptoms of hypomania are less severe than those of mania—eg, social functioning is less impaired or is even normal, and there is no need for hospitalization. Patients may feel they have been much more productive than usual or have needed less sleep to engage in daily activities. Hypomania may be present but not reported by patients who perceive nothing wrong.1,4
Rule out alternate diagnoses and apply DSM-5 criteria
There are no objective tests to confirm a diagnosis of bipolar disorder. If you suspect bipolar disorder, focus your clinical evaluation on ruling out competing mental health or medical diagnoses, and on determining whether the patient’s history meets criteria for a bipolar disorder as described in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5).1
Explore the patient’s psychiatric history (including hospitalizations, medications, and electroconvulsive therapy), general medical history, family history of psychiatric disorders (including suicide), and social history (including substance use and abuse). And carefully observe mental status. Confirming a diagnosis of bipolar disorder may take multiple visits, but strongly suggestive symptoms could warrant empirical treatment.
Helpful scales. The Patient Health Questionnaire (PHQ-9; https://www.uspreventiveservicestaskforce.org/Home/GetFileByID/218) and the Beck Depression Inventory (http://www.hr.ucdavis.edu/asap/pdf_files/Beck_Depression_Inventory.pdf) are useful for ruling out depressive disorders. Other scales are available, but they cannot confirm bipolar disorder. Laboratory testing selected according to patient symptoms (TABLE 24) can help rule out alternative diagnoses, but are also useful for establishing a baseline for medications.
Pharmacologic treatment: Match agents to symptoms
When treating bipolar disorder, choose a drug that targets a patient’s specific symptoms (TABLE 3).7-10 In primary care, the most commonly-used treatments for bipolar disorder type II are lamotrigine, valproic acid, and lithium.11

When to refer
Many cases of bipolar disorder type II can be managed successfully in a primary care practice, as can some cases of stable bipolar disorder type I. Psychiatric consultation may be most beneficial if the patient has recently attempted suicide or has suicidal ideation, has symptoms refractory to treatment, has poor medication adherence, or is misusing medications.
Even when patients are co-managed with psychiatric consultation, family physicians often ensure patients’ medication adherence, help patients understand their illness, manage overall health-related behaviors (including getting sufficient sleep), and make sure patients follow up as needed with their psychiatrist. Often, once patients have achieved equilibrium on mood-stabilizing (or other) medications, you can manage them and monitor medications with further consultation only as needed for clinical deterioration or other issues. Cognitive behavioral therapy may be useful as adjunctive treatment, particularly when patients are in active treatment.12
› CASE
This case is typical for many patients with depressed mood. A few key features in the patient’s history suggest bipolar disorder type II:
- depression that has been refractory to treatment
- multiple failed drug treatments, with mood-related adverse effects
- hypomania perceived as a “productive time,” and not as a problem
- absence of overt manic symptoms.
The patient was given a diagnosis of bipolar disorder type II with current depressed mood and no evidence of acute mania. She was started on valproic acid 250 mg po tid. She reported an initial improvement in mood but stopped the medication after one month because it caused intolerable drowsiness. She was then prescribed lamotrigine progressing gradually in 2-week intervals from 25 mg to 100 mg daily. She tolerated the medication well, and after 3 months of treatment, her mood symptoms improved and she had no further episodes of depressed mood.
CORRESPONDENCE
Michael Jason Wells, MD, Department of Family and Geriatric Medicine, University of Louisville School of Medicine, 201 Abraham Flexner Way, Suite 690, Louisville, KY 40202; mjwell04@louisville.edu.
THE CASE
A 23-year-old woman seeks medical attention at the request of her boyfriend because she’s been “miserable” for 3 weeks. In the examination room, she slouches in the chair and says her mood is low, her grades have dropped, and she no longer enjoys social gatherings or her other usual activities. She has no thoughts of suicide, no weight loss, and no somatic symptoms.
She says she is generally healthy, does not take any regular medications, and has never been pregnant. When asked about previous similar episodes, she admits to feeling this way about 3 times a year for one to 2 months at a time. She has tried different antidepressants, which haven’t helped much and have made her irritable and interfered with sleep.
When asked about mania or hypomania, she says there are short periods, roughly a couple of weeks 2 or 3 times a year, when she will get a lot of work done and can get by with little sleep. She has never gone on “spending sprees,” though, or indulged in any other unusual or dangerous behavior. And she has never been hospitalized for symptoms.
Bipolar disorders, over time, typically cause fluctuations in mood, activity, and energy level. If disorders go untreated, a patient’s behavior may cause considerable damage to relationships, finances, and reputations. And for some patients, the disorder can take the ultimate toll, resulting in death by suicide or accident.
Subtypes of bipolar disorder differ in the timing and severity of manic (or hypomanic) and depressive symptoms or episodes. Type I is the classic manic-depressive illness; type II is characterized by chronic treatment-resistant depression punctuated by hypomanic episodes; and cyclothymia leads to chronic fluctuations in mood. The diagnostic category “bipolar disorder not otherwise specified” applies to patients who meet some, but not all, of the criteria for other bipolar disorder subtypes.1
Prevalence. As with other mood symptoms or disorders, patients with bipolar disorder are often seen first in primary care due, in part, to barriers to obtaining psychiatric care or to avoidance of the perceived stigma in seeking such care.2 In a systematic review of patients who were interviewed randomly in primary care settings, 0.5% to 4.3% met criteria for bipolar disorder.3 The average age of onset for bipolar disorder is 15 to 19 years.4 In the United States, the prevalence of bipolar disorder type I is 1%; type II is 1.1%.3
The cause of bipolar disorder is unknown, but familial predisposition, biopsychosocial factors, and environment all seem to play a role. Children of parents with bipolar disorder have a 4% to 15% chance of receiving the same diagnosis, compared with children of parents without bipolar disorder, whose risk is only as high as 2%.5,6
Clinical presentation varies
When patients with bipolar disorder are first seen in the office, their state may be depression, mania, hypomania, or even euthymia. Keep in mind that the first 3 aberrations may indicate other disorders, either
Verify a true depressive episode
Symptoms must last for 2 weeks and include anhedonia or depressed mood, as well as some combination of changes in sleep, increased feelings of guilt, poor concentration, changes in appetite, loss of energy, psychomotor agitation or retardation, or suicidal thoughts.1
Know the criteria for mania
True mania is a distinct period of abnormally and persistently elevated, expansive, or irritable mood, accompanied by abnormally and persistently increased activity or energy, and lasting at least one week for most of the day, nearly every day (or any duration if hospitalization is necessary).
During that time, the patient must also exhibit at least 3 or more of the following symptoms (not counting irritability, if present): 1
- distractibility,
- insomnia,
- grandiosity,
- flights of ideas,
- increased goal-directed activity or agitation,
- rapid/pressured speech, or
- reckless behaviors.
How hypomania differs from mania. The symptoms of hypomania are less severe than those of mania—eg, social functioning is less impaired or is even normal, and there is no need for hospitalization. Patients may feel they have been much more productive than usual or have needed less sleep to engage in daily activities. Hypomania may be present but not reported by patients who perceive nothing wrong.1,4
Rule out alternate diagnoses and apply DSM-5 criteria
There are no objective tests to confirm a diagnosis of bipolar disorder. If you suspect bipolar disorder, focus your clinical evaluation on ruling out competing mental health or medical diagnoses, and on determining whether the patient’s history meets criteria for a bipolar disorder as described in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5).1
Explore the patient’s psychiatric history (including hospitalizations, medications, and electroconvulsive therapy), general medical history, family history of psychiatric disorders (including suicide), and social history (including substance use and abuse). And carefully observe mental status. Confirming a diagnosis of bipolar disorder may take multiple visits, but strongly suggestive symptoms could warrant empirical treatment.
Helpful scales. The Patient Health Questionnaire (PHQ-9; https://www.uspreventiveservicestaskforce.org/Home/GetFileByID/218) and the Beck Depression Inventory (http://www.hr.ucdavis.edu/asap/pdf_files/Beck_Depression_Inventory.pdf) are useful for ruling out depressive disorders. Other scales are available, but they cannot confirm bipolar disorder. Laboratory testing selected according to patient symptoms (TABLE 24) can help rule out alternative diagnoses, but are also useful for establishing a baseline for medications.
Pharmacologic treatment: Match agents to symptoms
When treating bipolar disorder, choose a drug that targets a patient’s specific symptoms (TABLE 3).7-10 In primary care, the most commonly-used treatments for bipolar disorder type II are lamotrigine, valproic acid, and lithium.11

When to refer
Many cases of bipolar disorder type II can be managed successfully in a primary care practice, as can some cases of stable bipolar disorder type I. Psychiatric consultation may be most beneficial if the patient has recently attempted suicide or has suicidal ideation, has symptoms refractory to treatment, has poor medication adherence, or is misusing medications.
Even when patients are co-managed with psychiatric consultation, family physicians often ensure patients’ medication adherence, help patients understand their illness, manage overall health-related behaviors (including getting sufficient sleep), and make sure patients follow up as needed with their psychiatrist. Often, once patients have achieved equilibrium on mood-stabilizing (or other) medications, you can manage them and monitor medications with further consultation only as needed for clinical deterioration or other issues. Cognitive behavioral therapy may be useful as adjunctive treatment, particularly when patients are in active treatment.12
› CASE
This case is typical for many patients with depressed mood. A few key features in the patient’s history suggest bipolar disorder type II:
- depression that has been refractory to treatment
- multiple failed drug treatments, with mood-related adverse effects
- hypomania perceived as a “productive time,” and not as a problem
- absence of overt manic symptoms.
The patient was given a diagnosis of bipolar disorder type II with current depressed mood and no evidence of acute mania. She was started on valproic acid 250 mg po tid. She reported an initial improvement in mood but stopped the medication after one month because it caused intolerable drowsiness. She was then prescribed lamotrigine progressing gradually in 2-week intervals from 25 mg to 100 mg daily. She tolerated the medication well, and after 3 months of treatment, her mood symptoms improved and she had no further episodes of depressed mood.
CORRESPONDENCE
Michael Jason Wells, MD, Department of Family and Geriatric Medicine, University of Louisville School of Medicine, 201 Abraham Flexner Way, Suite 690, Louisville, KY 40202; mjwell04@louisville.edu.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed (DSM-5). Arlington, VA: American Psychiatric Association; 2013.
2. Kilbourne AM, Goodrich DE, O’Donnell AN, et al. Integrated bipolar disorder management in primary care. Curr Psychiatry Rep. 2012;14:687-695.
3. Cerimele JM, Chwastiak LA, Dodson S, et al. The prevalence of bipolar disorder in primary care samples: a systematic review. Gen Hosp Psychiatry. 2014;36:19-25.
4. Malhi GS, Adams D, Lampe L, et al. Clinical practice recommendations for bipolar disorder. Acta Pscyhiatr Scand. 2009:119(Suppl 439):27-46.
5. Abell S, Ey J. Bipolar Disorder. Clin Pediatr. 2009;48:693-694.
6. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders of school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study. Arch Gen Psychiatry. 2009;66:287-296.
7. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646.
8. De Fruyt J, Deschepper E, Audenaert K, et al. Second generation antipsychotics in the treatment of bipolar depression: a systematic review and meta-analysis. J Psychopharmacol. 2012;26:603-617.
9. Gitlin M, Frye MA. Maintenance therapies in bipolar disorders. Bipolar Disord. 2012:14(Suppl 2):51-65.
10. Labbate LA, Fava M, Rosenbaum JF, et al. Handbook of Psychiatric Drug Therapy. 6th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2010.
11. Ostacher M, Tandon R, Suppes T. Florida Best Practice Psychotherapeutic Medication Guidelines for Adults with Bipolar Disorder: a novel, practical, patient-centered guide for clinicians. J Clin Psychiatry. 2016;77:920-926.
12. Szentagotai A, David D. The efficacy of cognitive behavioral therapy in bipolar disorder: a quantitative meta-analysis. J Clin Psychiatry. 2010;71:66-72.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed (DSM-5). Arlington, VA: American Psychiatric Association; 2013.
2. Kilbourne AM, Goodrich DE, O’Donnell AN, et al. Integrated bipolar disorder management in primary care. Curr Psychiatry Rep. 2012;14:687-695.
3. Cerimele JM, Chwastiak LA, Dodson S, et al. The prevalence of bipolar disorder in primary care samples: a systematic review. Gen Hosp Psychiatry. 2014;36:19-25.
4. Malhi GS, Adams D, Lampe L, et al. Clinical practice recommendations for bipolar disorder. Acta Pscyhiatr Scand. 2009:119(Suppl 439):27-46.
5. Abell S, Ey J. Bipolar Disorder. Clin Pediatr. 2009;48:693-694.
6. Birmaher B, Axelson D, Monk K, et al. Lifetime psychiatric disorders of school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study. Arch Gen Psychiatry. 2009;66:287-296.
7. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346:f3646.
8. De Fruyt J, Deschepper E, Audenaert K, et al. Second generation antipsychotics in the treatment of bipolar depression: a systematic review and meta-analysis. J Psychopharmacol. 2012;26:603-617.
9. Gitlin M, Frye MA. Maintenance therapies in bipolar disorders. Bipolar Disord. 2012:14(Suppl 2):51-65.
10. Labbate LA, Fava M, Rosenbaum JF, et al. Handbook of Psychiatric Drug Therapy. 6th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2010.
11. Ostacher M, Tandon R, Suppes T. Florida Best Practice Psychotherapeutic Medication Guidelines for Adults with Bipolar Disorder: a novel, practical, patient-centered guide for clinicians. J Clin Psychiatry. 2016;77:920-926.
12. Szentagotai A, David D. The efficacy of cognitive behavioral therapy in bipolar disorder: a quantitative meta-analysis. J Clin Psychiatry. 2010;71:66-72.