User login
A patient-centered approach to tapering opioids
Many Americans who are treated with prescription opioid analgesics would be better off with less opioid or none at all. To that end, published opioid prescribing guidelines do provide guidance on the mechanics of tapering patients off opioids1-4—but they have a major flaw: They do not adequately account for the fact that people who have a diagnosis of chronic pain are a heterogeneous group and require diagnosis-specific treatment planning. A patient-centered approach to opioid tapers must account for the reality that many people who are given a prescription for an opioid to treat pain have significant mental health conditions—for which opioids act as a psychotropic agent. An opioid taper must therefore address psychological trauma, in particular.5 (See “Tapering and harm-reduction strategies have failed.”6-14)
SIDEBAR
Tapering and harm-reduction strategies have failed
Efforts to address the rising number of overdose events that involve opioids began in earnest in 2010. In a 2011 Government Accountability Office report to Congress, the Drug Enforcement Agency reported that “the number of regulatory investigations (of medical providers who prescribed opioids) tripled between fiscal years 2009- 2010.”6
How has it gone since 2010? High-dosage prescribing of opioids has fallen by 48% since 2011, yet the decline has not reduced overdose events of any kind.7,8 Just the opposite: The 19,000 overdose deaths recorded in 2010 involving any opioid increased to 49,068 by 2017, the National Institute on Drug Abuse reports.9 The increase in opioid overdose deaths is fueled by a recent 9-fold increase in consumption of the synthetic opioid fentanyl: “The rate of drug overdose deaths involving synthetic opioids other than methadone … increased on average by 8% per year from 1999 through 2013 and by 71% per year from 2013 through 2017.”10
These and other statistics document only a modest rise in deaths that involve prescription opioids: from 15,000 in 2010 to 19,000 in 2016.9,10 Since 2010, the crisis of opioid overdose deaths burns hotter, and the pattern of opioid use has shifted from prescription drugs to much deadlier illicit drugs, such as heroin.
Interventions have not been successful overall. Results of research focused on the impact of opioid tapering and harm-reduction strategies implemented this decade are likewise discouraging. In 2018, the US Department of Veterans Affairs reported that opioid discontinuation was not associated with a reduction in overdose but was associated with an increase in suicide.11,12 Von Korff and colleagues, in a 2017 report, concluded that “Long-term implementation of opioid dose and risk reduction initiatives [in Washington state] was not associated with lower rates of prescription opioid use disorder among prevalent [chronic opioid therapy] patients.”13
Evidence suggests that efforts to address the opioid crisis of the past decade have had an effect that is the opposite of what was intended. The federal government recognized this in April 2019 in a Drug Safety Communication: “The US Food and Drug Administration (FDA) has received reports of serious harm in patients who are physically dependent on opioid pain medicines suddenly having these medicines discontinued or the dose rapidly decreased. These include serious withdrawal symptoms, uncontrolled pain, psychological distress, and suicide.”14
In this article, we present an evidence-based consensus approach to opioid tapering for your practice that is informed by a broader understanding of why patients take prescription opioids and why they, occasionally, switch to illicit drugs when their prescription is tapered. This consensus approach is based on the experience of the authors, members of the pain faculty of Project ECHO (Extension for Community Healthcare Outcomes) of the ECHO Institute, a worldwide initiative that uses adult learning techniques and interactive video technology to connect community providers with specialists at centers of excellence in regular real-time collaborative sessions. We are variously experts in pain medicine, primary care, psychology, addiction medicine, pharmacy, behavioral health therapy, occupational medicine, and Chinese medicine.
Why Americans obtain prescription opioids
There are 4 principal reasons why patients obtain prescription opioids, beyond indicated analgesic uses:
1. Patients seek the antianxiety and antidepressant effects of opioids. Multiple converging lines of evidence suggest that antianxiety and antidepressant effects of opioids are a significant reason that patients in the United States persist in requesting prescriptions for opioids:
- In our experience with more than 500 primary care telemedicine case presentations, at least 50% of patients say that the main effect of opioids prescribed for them is “it makes me feel calm” or “more relaxed.”
- In a 2007 survey of 91,823 US residents older than 18 years, nonmedical use of opioids was statistically associated with panic, social anxiety, and depressive symptoms.15
- Ten years later, Von Korff and colleagues found that more than half of opioid prescriptions written in the United States were for the small percentage of patients who have a diagnosis of serious anxiety or depression.13
- In 2016, Yovell and colleagues reported that ultra-low-dosage buprenorphine markedly reduced suicidal ideation over 4 weeks in 62 patients with varied levels of depression.16
There is also mechanistic evidence that the antianxiety and antidepressant effects of opioids are significant reasons Americans persist in requesting prescription opioids. The literature suggests that opioid receptors play a role in mood regulation, including alleviation of depression and anxiety; recent research suggests that oxycodone might be a unique mood-altering drug compared to other common prescription opioids because of its ability to affect mood through the δ opioid receptor.17-20
It should not be a surprise that Americans often turn to opioids to address posttraumatic stress disorder (PTSD), anxiety, and depression. A recent study of the state of the US mental health system concluded that mental health services in the United States are inadequate—despite evidence that > 50% of Americans seek, or consider seeking, treatment for mental health problems for themselves or others.21
2. Patients experience pain unrelated to tissue damage. Rather, they are in pain “for psychological reasons.”22 In 2016, Davis and Vanderah wrote: “We theorize that a functional change in the [central nervous system] can occur in response to certain emotional states or traumatic experiences (eg, child abuse, assault, accidents).” They connect this change to central sensitization and a reduced pain-perception threshold,23 and strongly suspect that many patients with chronic pain have undiagnosed and untreated psychological trauma that has changed the way their central nervous system processes sensory stimuli. The authors call this “trauma-induced hyperalgesia.”
Continue to: Psychological trauma...
Psychological trauma is uniquely capable of producing hyperalgesia, compared to anxiety or depression. In a study of veterans, Defrin and colleagues demonstrated hyperalgesia in patients who had a diagnosis of PTSD but not in controls group who had an anxiety disorder only.24
To support successful opioid tapering, trauma-induced hyperalgesia, when present, must be addressed. Treatment of what the International Association for the Study of Pain calls “pain due to psychological factors”22 requires specific trauma therapy. However, our experience validates what researchers have to say about access to treatment of psychological trauma in the United States: “…[C]linical research has identified certain psychological interventions that effectively ameliorate the symptoms of PTSD. But most people struggling with PTSD don’t receive those treatments.”25
We have no doubt that this is due, in part, to underdiagnosis of psychological trauma, even in mental health clinics. According to Miele and colleagues, “PTSD remains largely undiagnosed and undertreated in mental health outpatients, even in teaching hospitals, with diagnosis rates as low as 4% while published prevalence is between 7% and 50% in this population.”26
3. Patients suffer from opioid use disorder (OUD) and complain of pain to obtain opioids by prescription. For patients with OUD, their use is out of control; they devote increasing mental and physical resources to obtaining, using, and recovering from substances; and they continue to use despite adverse consequences.27 The prevalence of OUD in primary care clinics varies strikingly by the location of clinics. In Washington state, the prevalence of moderate and severe OUD in a large population of patients who had been prescribed opioids through primary care clinics was recently determined to be between 21.5% and 23.9%.13
4. Patients are obtaining opioid prescriptions for people other than themselves. While this is a reason that patients obtain opioid prescriptions, it is not necessarily common. Statistics show that the likelihood of a prescription being diverted intentionally is low: Dart and colleagues found that diversion has become uncommon in the general population.28
Continue to: Why we taper opioid analgesics
Why we taper opioid analgesics
Reasons for an opioid taper include concern that the patient has, or will develop, an OUD; will experience accidental or intentional overdose; might be diverting opioids; is not benefiting from opioid therapy for pain; or is experiencing severe adverse effects. A patient who has nociceptive pain and might have opioid-induced hyperalgesia will require a much different opioid taper plan than a patient with untreated PTSD or a patient with severe OUD.
Misunderstanding can lead to inappropriate tapering
We often encounter primary care providers who believe that a large percentage of patients on chronic opioid therapy inevitably develop OUD. This is a common reason for initiating opioid taper. Most patients on a chronic opioid do become physically dependent, but only a small percentage of patients develop psychological dependence (ie, addiction or OUD).29
Physical dependence is “a state of adaptation that is manifested by a drug class–specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist.”30 Symptoms of opioid withdrawal include muscle aches; abdominal cramping; increased lacrimation, rhinorrhea, and perspiration; diarrhea; agitation and anxiety; insomnia; and piloerection. Opioid withdrawal symptoms are caused by physical dependence, not by addiction. They can be mitigated by tapering slowly and instituting adjuvant medications, such as clonidine, to attenuate symptoms.
Psychological dependence, or addiction (that is, OUD, as described in the Diagnostic and Statistical Manual of Mental Disorders 5th edition27), comprises primarily 3 behavioral criteria:
- Loss of control of the medication, with compulsive use
- Continued use despite adverse consequences of using opioids, such as arrest for driving under the influence and deterioration of social, family, or work performance
- Obsession or preoccupation with obtaining and using the substance. In properly selected chronic opioid therapy patients, there is evidence that new-onset OUD is not as common as has been thought. A recent study of the risk for opioid addiction after use of an opioid for ≥ 90 days for chronic noncancer pain found that the absolute rate of de novo OUD among patients treated for 90 days was 0.72%.29 A systematic review by Fishbain and colleagues of 24 studies of opioid-exposed patients found a risk of 3.27% overall—0.19% for patients who did not have a history of abuse or addiction.31 As Director of the National Institute on Drug Abuse Norma Volkow, MD, wrote in 2016: “Addiction occurs in only a small percentage of people who are exposed to opioids—even among those with preexisting vulnerabilities.”32
Assessment should focus on why the patient is taking an opioid
A strong case can be made that less opioid is better for many of the people for whom these medications are prescribed for chronic noncancer pain. However, a one-size-fits-all dosage reduction and addiction-focused approach to opioid tapering has not worked: The assessment and treatment paradigm must change, in our view.
Continue to: During assessment...
During assessment, we must adopt the means to identify the reason that a patient is using a prescription opioid. It is of particular importance that we identify patients using opioids for their psychotropic properties, particularly when the goal is to cope with the effects of psychological trauma. The subsequent treatment protocol will then need to include time for effective, evidence-based behavioral health treatment of anxiety, PTSD, or depression. If opioids are serving primarily as psychotropic medication, an attempt to taper before establishing effective behavioral health treatment might lead the patient to pursue illegal means of procuring opioid medication.
We acknowledge that primary care physicians are not reimbursed for trauma screening and that evidence-based intensive trauma treatment is generally unavailable in the United States. Both of these shortcomings must be corrected if we want to stem the opioid crisis.
If diversion is suspected and there is evidence that the patient is not currently taking prescribed opioids (eg, a negative urine drug screen), discontinuing the opioid prescription is the immediate next step for the sake of public safety.
SIDEBAR
2 decisions to make before continuing to prescribe an opioid for chronic noncancer pain
#1 Should I provide the patient with a prescription for an opioid for a few days, while I await more information?a
Yes. Writing a prescription is a reasonable decision if all of the following apply:
- You do not have significant suspicion of diversion (based on a clinical interview).
- You do not suspect an active addiction disorder, based on the score of the 10-question Drug Abuse Screening Test (DAST-10) and on a clinical interview. (DAST-10 is available at: https://cde.drugabuse.gov/instrument/e9053390-ee9c-9140-e040-bb89ad433d69.)
- The patient is likely to experience withdrawal symptoms if you don’t provide the medication immediately.
- The patient’s pain and function are likely to be impaired if you do not provide the medication.
- The patient does not display altered mental status during the visit (eg, drowsy, slurred speech).
No. If writing a prescription for an opioid for a few days does not seem to be a reasonable decision because the criteria above are not met, but withdrawal symptoms are likely, you can prescribe medication to mitigate symptoms or refer the patient for treatment of withdrawal.
#2 I’ve decided to provide the patient with a prescription for an opioid. For how many days should I write it?
The usual practice, for a patient whose case is familiar to you, is to prescribe a 1-month supply.
However, if any 1 of the following criteria is met, prescribing a 1-month supply is unsafe under most circumstances:
- An unstable social or living environment places the patient at risk by possessing a supply of opioids (based on a clinical interview).
- You suspect an unstable or severe behavioral health condition or a mental health diagnosis (based on a clinical interview or on the patient record from outside your practice).
- The patient scores as “high risk” on the Opioid Risk Tool (ORT; www.drugabuse.gov/sites/default/files/files/OpioidRiskTool.pdf), Screener and Opioid Assessment for Patients with Pain–Revised (SOAPP-R; www.ncbi.nlm.nih.gov/pmc/articles/PMC4706778/), or a similar opioid risk assessment tool.
When 1 or more of these exclusionary criteria are met, you have 3 options:
- Prescribe an opioid for a brief duration and see the patient often.
- Do not prescribe an opioid; instead, refer the patient as necessary for treatment of withdrawal.
- Refer the patient for treatment of the underlying behavioral health condition.
a Additional information might include findings from consultants you’ve engaged regarding the patient’s diagnosis; a response to your call from a past prescriber; urine drug screen results; and results of a prescription monitoring program check.
Considering a taper? Take this 5-step approach
Once it appears that tapering an opioid is indicated, we propose that you take the following steps:
- Establish whether it is safe to continue prescribing (follow the route provided in “2 decisions to make before continuing to prescribe an opioid for chronic noncancer pain”); if continuing it is not safe, take steps to protect the patient and the community
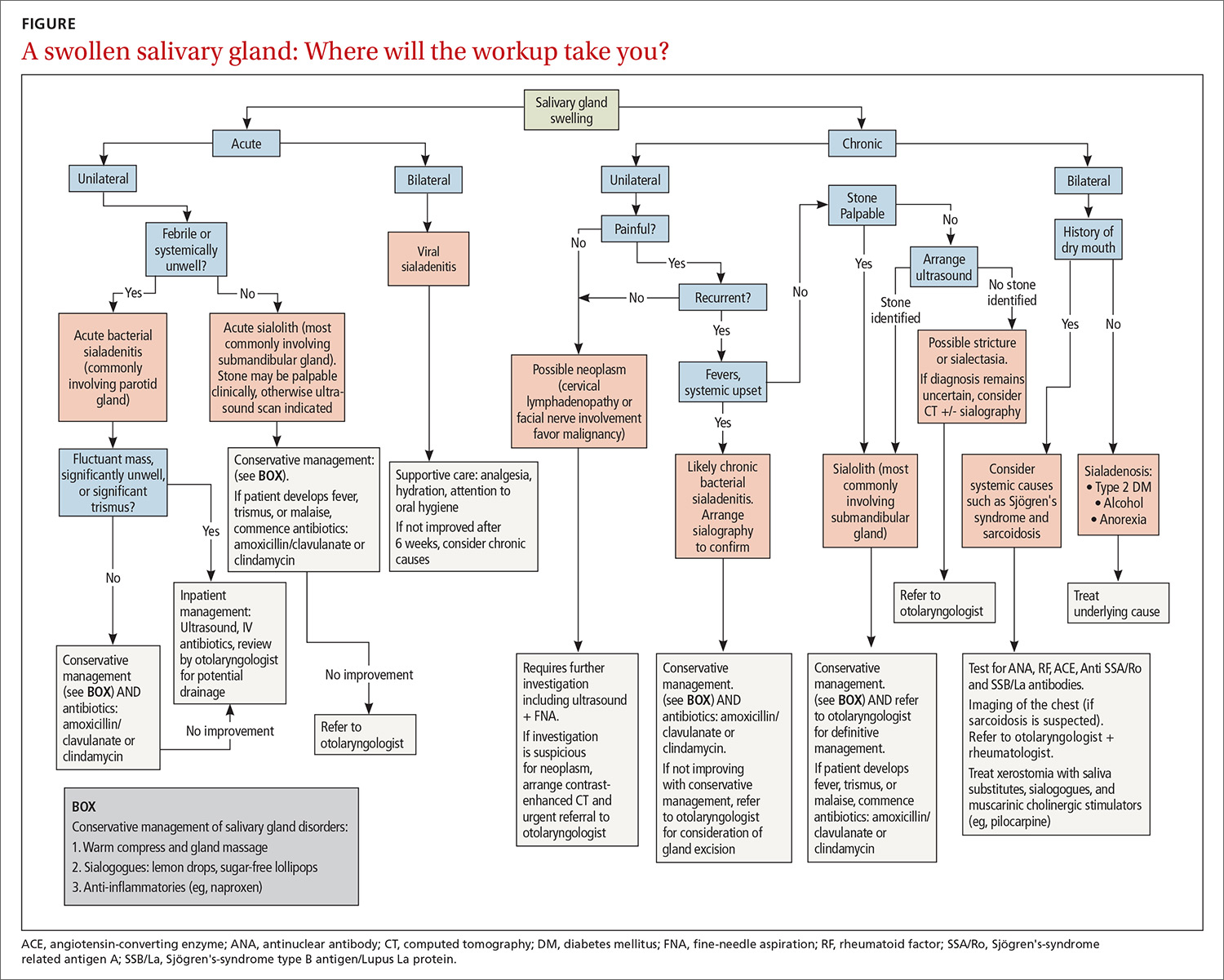
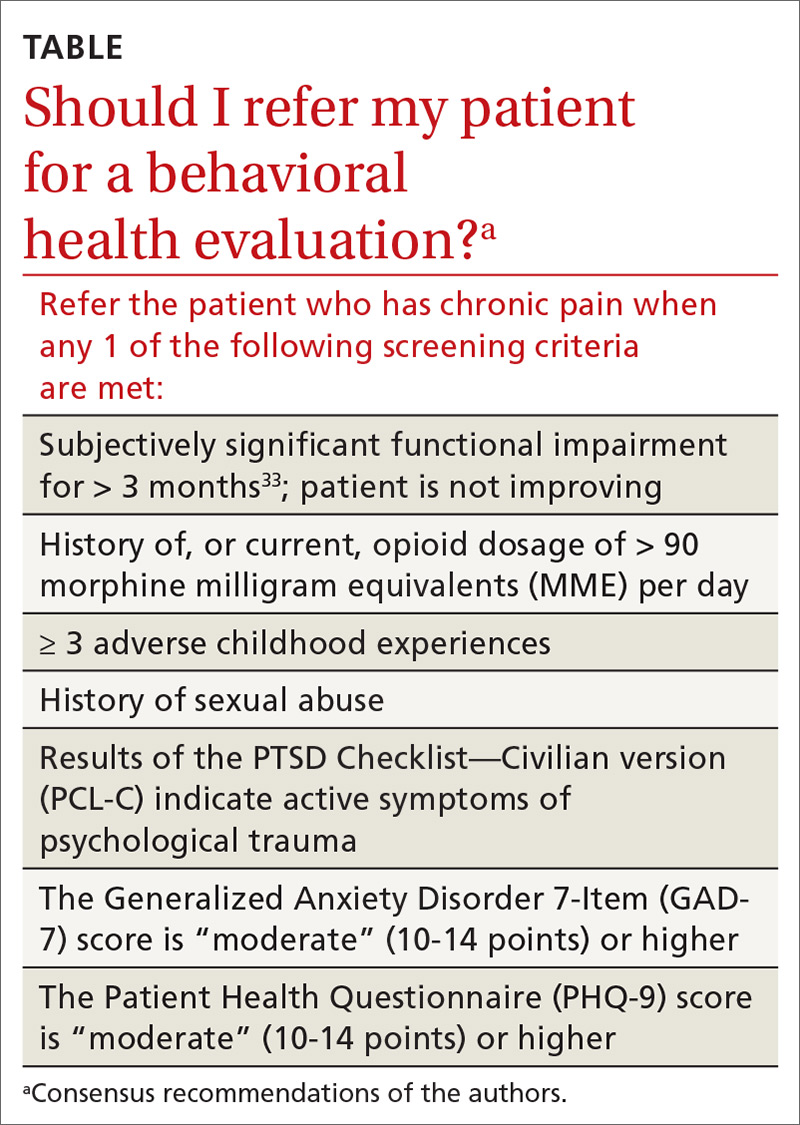
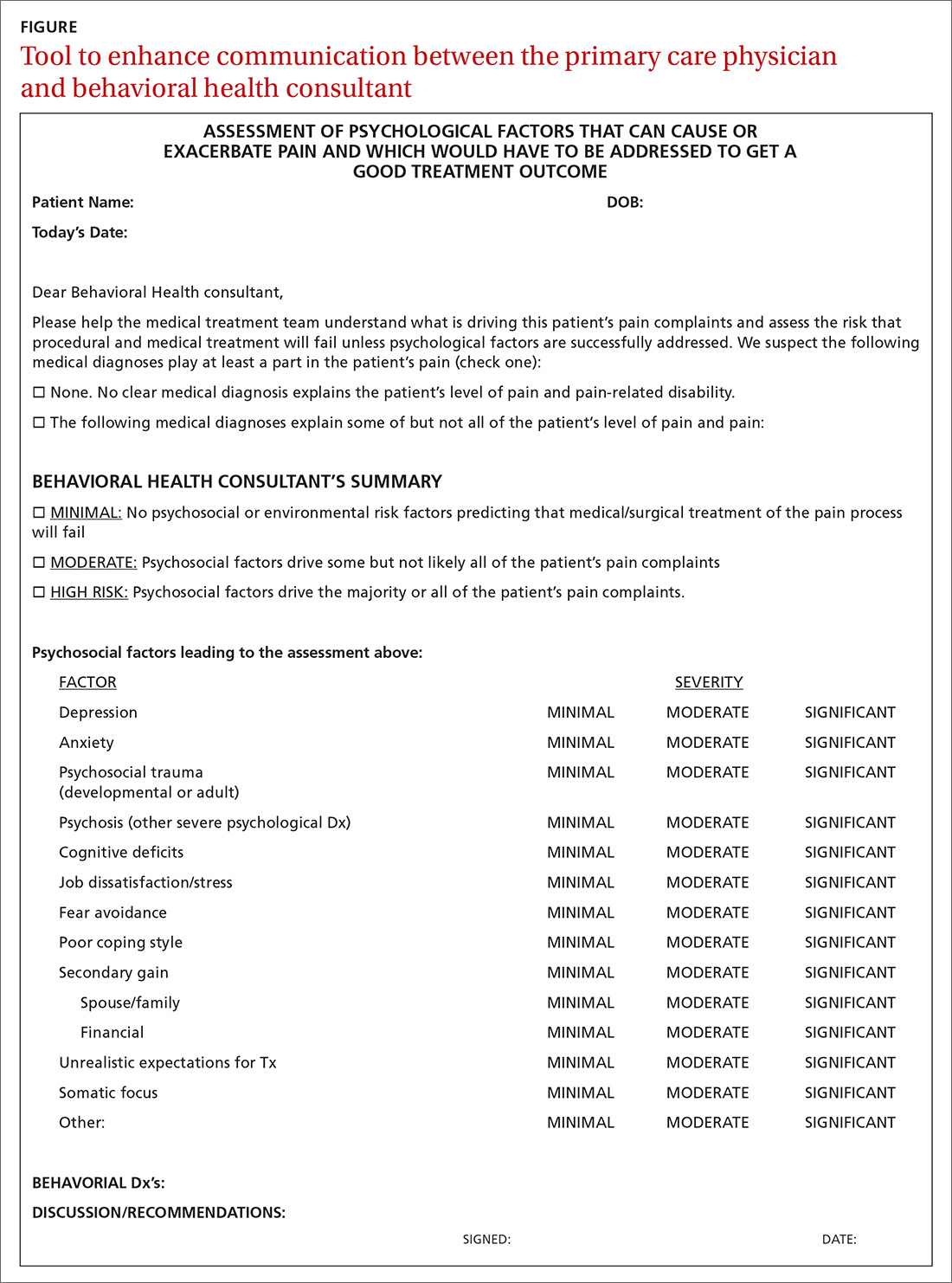
- Determine whether assessment by a trauma-informed behavioral health expert is needed, assuming that, in your judgment, it is safe to continue the opioid (TABLE33). When behavioral health assessment is needed, you need 3 questions answered by that assessment: (1) Are psychological factors present that might put the patient at risk during an opioid taper? (2) What are those factors? (3) What needs to done about them before the taper is started? Recalling that psychological trauma often is not assessed by behavioral health colleagues, it is necessary to provide the behavioral health provider with a specific request to assess trauma burden, and state the physical diagnoses that are causing pain or provide a clear statement that no such diagnoses can be made. (See the FIGURE, which we developed in conjunction with behavioral health colleagues to help the consultant understand what the primary care physician needs from a behavioral health assessment.)
- Obtain consultation from a physical therapist, pain medicine specialist, and, if possible, an alternative or complementary medicine provider to determine what nonpharmacotherapeutic modalities can be instituted to treat pain before tapering the opioid.
- Initiate the Screening, Brief Intervention and Referral to Treatment (SBIRT) approach if OUD is suspected (www.samhsa.gov/sbirt).34 This motivational interviewing tool identifies patients with a substance use disorder, severity of use, and appropriate level of treatment. (If OUD is suspected during assessment, next steps are to stop prescribing and implement harm-reduction strategies, such as primary care level medically assisted treatment [MAT] with buprenorphine, followed by expert behavioral health-centered addiction treatment.)
- Experiment with dosage reduction according to published guidance, if (1) psychological factors are absent or have been adequately addressed, according to the behavioral health consultant, and (2) nonpharmacotherapeutic strategies are in place.8-11
Shifting to a patient-centered approach
The timing and choice of opioid tapers, in relation to harm reduction and intervention targeting the root cause of a patient’s complaint of pain, have not been adequately explored. In our practice, we’ve shifted from an addiction-centered, dosage-centered approach to opioid taper to a patient-centered approach35 that emphasizes behavioral-medical integration—an approach that we broadly endorse. Such an approach (1) is based on a clear understanding of why the patient is taking opioid pain medication, (2) engages medical and complementary or alternative medicine specialists, (3) addresses underdiagnosis of psychological trauma, and (4) requires a quantum leap in access to trauma-specific behavioral health treatment resources. 36
Continue to: To underscore the case...
To underscore the case for shifting to a patient-centered approach35 we present sample cases in “How a patient-centered approach to tapering opioids looks in practice.”
SIDEBAR
How a patient-centered approach to tapering opioids looks in practice
Five hypothetical cases illustrate what might happen when a practice shifts from an addiction-centered, dosage-centered approach to one that places the individual at the center of care.
CASE #1: Brett F
Mr. F appears to use medication responsibly; benefits functionally from an opioid; has tolerable adverse effects; does not have significant psychosocial risk factors (based on the score of the Opioid Risk Tool [ORT] or the Screener and Opioid Assessment for Patients with Pain–Revised [SOAPP-R]); and is engaged in effective self-management. Most of Mr. F’s pain is thought to have a nociceptive or neuropathic source.
Mr F could reasonably contemplate continuing current opioid treatment.
Action: If the daily morphine milligram equivalent (MME) dosage is high, Mr. F should be referred to a pain medicine specialist. We recommend a periodic (at least annually) empiric trial of dosage reduction to see whether he is indeed best served by the current dosage.
CASE #2: Brett F (version 2.0)
Envision Mr. F having the same profile in all respects except that he is not engaged in effective self-management.
Optimal treatment of chronic pain often requires supplemental modalities beyond opioids.
Action: Physical therapy; an individualized, ongoing exercise regimen; interventional procedures; weight loss (if the patient is obese); smoking cessation; and improving coping skills for anxiety and depression without pharmacotherapy might not only temporarily alleviate the pain but, over time, improve Mr. F’s physical condition.
If Mr. F is not willing to do more than take the prescribed opioids, nothing is likely to change: Over time, his condition is likely to deteriorate. A patient like Mr. F can be harmed if opioids continue to be prescribed for him long-term.
Further action: If Mr. F won’t engage in broadening the approach to treating his pain, the opioid medication should be tapered, in his long-term best interest. A carrot-and-stick approach can facilitate Mr. F’s involvement in his care.
CASE #3: Clark S
Mr. S has a significant psychosocial component driving his pain: depression.a
Prescribing opioids without addressing the root cause of trauma is not in the patient’s best interest.
Action: Because of Mr. S’s depression, refer him to a behavioral health provider. If you determine that he is emotionally stable, wait until he is engaged in trauma treatment to begin the taper. If he appears unstable (eg, crying in the office, recent psychological stressors, recent impulsive behaviors, poor insight) consider (1) urgent behavioral health referral and (2) prescribing only enough opioid medication (ie, at close intervals) to prevent withdrawal and panic. Consider whether a psychotropic medication might be of benefit (eg, a serotonin–norepinephrine reuptake inhibitor or selective serotonin reuptake inhibitor).
Further action: Harm-reduction steps, such as close monitoring and, perhaps, a change to a buprenorphine product, is indicated, especially when the patient is overwhelmed by recent psychosocial stressors. Harm-reduction treatment is available through Medication-Assisted Therapy (MAT) programs; however, patients often run into difficulty obtaining access to these programs because regulations and laws restrict MAT to patients who have a diagnosis of opioid use disorder (OUD) and because some health plans and pharmacy benefit managers require prior authorization.
CASE #4: Gloria B
Ms. B isn’t managing her medications responsibly—although you don’t suspect OUD.
When a patient has shown the inability to manage opioid medication responsibly, you should delve into the reason to determine your next step.
Action: Evaluate Ms. B for a cognitive disorder or a thought disorder. Alternatively, as in the case of Mr. S, a psychosocial component might underlie her pain; in that case, the same recommendations can be made for her. In addition, you can propose that she identify a responsible person to dispense her medication.
CASE #5: Nicole L
You suspect that Ms. L, who is taking opioid medication to alleviate pain, also has a substance use disorder.
Action: Implement harm-reduction early for Ms. L: Obtain addiction medicine consultation and implement behavioral health strategies for addiction treatment.
A key characteristic of a substance use disorder is loss of control over use of the substance. A patient like Ms. L—who is in pain and who has an active OUD—cannot be expected to manage her opioid use responsibly.
Further action: We recommend that Ms. L be referred to an addiction specialist for MAT. Evidence of the harmreduction benefit of MAT is sufficient to strongly recommend it. Continue any other treatment modalities for pain that Ms. L has been using, such as non-opioid medication, physical therapy, alternative treatments, and behavioral therapy, or begin such treatments as appropriate.
a Depression is not the only psychosocial component that can underlie pain. Others include anxiety, posttraumatic stress disorder, and grief.
An eye toward the future. To inform future approaches to opioid tapering, more resources need to be deployed to
- support screening and risk stratification for PTSD, anxiety, and related disorders at the primary care level,
- continue the effort to identify and treat OUD,
- develop best-practice responses to screening, and
- make harm-reduction strategies that are now reserved for patients with OUD available to those who don't have OUD.
We urge that research be pursued into best practices for chronic pain interventions that target psychological trauma, anxiety, and depression.
CORRESPONDENCE
Bennet Davis MD, 2092 East Calle de Dulcinea, Tucson, AZ 85718; bdavis@ipcaz.org.
1. Centers for Disease Control and Prevention. Pocket guide: tapering for chronic pain. https://www.cdc.gov/drugoverdose/pdf/clinical_pocket_guide_tapering-a.pdf. Accessed November 25, 2019.
2. Kral LA, Jackson K, Uritsky TJ. A practical guide to tapering opioids. Ment Health Clin. 2015;5:102-108.
3. Murphy L, Babaei-Rad R, Buna D, et al. Guidance on opioid tapering in the context of chronic pain: evidence, practical advice and frequently asked questions. Can Pharm J (Ott). 2018;151:114-120.
4. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic noncancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2005;90:828-842.
5. Davis M. Prescription opioid use among adults with mental health disorders in the United States. J Am Board Fam Med. 2017;30:407-417.
6. US Government Accountability Office. Report to Congressional Requestors. Prescription drug control: DEA has enhanced efforts to combat diversion, but could better assess and report program results. August 2011. www.gao.gov/assets/520/511464.pdf. Accessed November 25, 2019.
7. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. Annual surveillance report of drug-related risks and outcomes. United States, 2017. www.cdc.gov/drugoverdose/pdf/pubs/2017-cdc-drug-surveillance-report.pdf. Accessed November 25, 2019.
8. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016, NCHS Data Brief No. 294. December 21, 2017. Hyattsville, MD: National Center for Health Statistics. www.cdc.gov/nchs/products/databriefs/db294.htm. Accessed November 25, 2019.
9. Overdose death rates. Bethesda, MD: National Institute on Drug Abuse. January 2019. www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Accessed November 25, 2019.
10. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. NCHS Data Brief No. 329. November 2018. Hyattsville, MD: National Center for Health Statistics. www.cdc.gov/nchs/data/databriefs/db329-h.pdf . Accessed November 25, 2019.
11. Manhapra A, Kertesz S, Oliva A, et al. VA data about Rx opioids and overdose and suicide: clinical implications. Presented at the 2018 National Rx Drug Abuse and Heroin Summit, Atlanta Georgia, April 4, 2018.
12. Demidenko M, Dobscha SK, Morasco BJ, et al. Suicidal ideation and suicidal self-directed violence following clinician-initiated prescription opioid discontinuation among long-term opioid users. Gen Hosp Psychiatry. 2017;47:29-35.
13. Von Korff M, Walker RL, Saunders K, et al. Prevalence of prescription opioid use disorder among chronic opioid therapy patients after health plan opioid dose and risk reduction initiatives. Int J Drug Policy. 2017;46:90-98.
14. United States Food and Drug Administration. FDA Drug Safety Communication: FDA identifies harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering. April 9, 2019. www.fda.gov/Drugs/DrugSafety/ucm635038.htm. Accessed November 25, 2019.
15. Becker W, Sullivan LE, Tetrault JM, et al. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: psychiatric, medical and substance use correlates. Drug Alcohol Depend. 2008;94:38-47.
16. Yovell Y, Bar G, Mashiah M, et al. Ultra-low-dose buprenorphine as a time-limited treatment for severe suicidal ideation: a randomized controlled trial. Am J Psychiatry. 2016;173:491-498.
17. Pradhan AA, Befort K, Nozaki C, et al. The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci. 2011;32:581-590.
18. Sugiyama A, Yamada M, Saitoh A, et al. Administration of a delta opioid receptor agonist KNT-127 to the basolateral amygdala has robust anxiolytic-like effects in rats. Psychopharmacology (Berl). 2018;235:2947-2955.
19. Richards EM, Mathews DC, Luckenbaugh DA, et al. A randomized, placebo-controlled pilot trial of the delta opioid receptor agonist AZD2327 in anxious depression. Psychopharmacology (Berl). 2016;233:1119-1130.
20. Yang PP, Yeh GC, Yeh TK, et al. Activation of delta-opioid receptor contributes to the antinociceptive effect of oxycodone in mice. Pharmacol Res. 2016;111:867-876.
21. America’s mental health 2018. Stamford, CT: Cohen Veterans Network. October 10, 2018. https://www.cohenveteransnetwork.org/wp-content/uploads/2018/10/Research-Summary-10-10-2018.pdf. Accessed November 25, 2019.
22. Classification of Chronic Pain, Second Edition (Revised). Washington, DC: International Association for the Study of Pain. Updated 2012. www.iasp-pain.org/PublicationsNews/Content.aspx?ItemNumber=1673. Accessed November 25, 2019.
23. Davis B, Vanderah TW. A new paradigm for pain? J Fam Pract. 2016 65:598-605.
24. Defrin R, Ginzburg K, Solomon Z, et al. Quantitative testing of pain perception in subjects with PTSD—implications for the mechanism of the coexistence between PTSD and chronic pain. Pain. 2008;138:450-459.
25. Foa EB, Gillihan SJ, Bryant RA. Challenges and successes in dissemination of evidence-based treatments for posttraumatic stress: lessons learned from prolonged exposure therapy for PTSD. Psychol Science Public Interest. 2013;14:65-111.
26. Miele D, O’Brien EJ. Underdiagnosis of posttraumatic stress disorder in at risk youth. J Trauma Stress. 2010;23:591-598.
27. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Washington, DC: American Psychiatric Publishing; 2013:541.
28. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241-248.
29. Schuchat A, Houry D, Guy GP Jr. New data on opioid use and prescribing in the United States. JAMA. 2017;318:425-426.
30. American Academy of Pain Medicine, American Pain Society, American Society of Addiction Medicine. Definitions related to the use of opioids for the treatment of pain. 2001. www.naabt.org/documents/APS_consensus_document.pdf. Accessed November 25, 2019.
31. Fishbain DA, Cole B, Lewis J, et al. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 2008;9:444-459.
32. Volkow ND, McClellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374:1253-1263.
33. Treede RD, Rief W, Barke A. A classification of chronic pain for ICD-11. Pain. 2015;156:1003-1007.
34. Screening, brief intervention, and referral to treatment (SBIRT). Rockville, MD: Substance Abuse and Mental Health Services Administration. www.samhsa.gov/sbirt. Accessed November 25, 2019.
35. Schneider JP, Davis B. How well do you know your patient? Pract Pain Manag. 2017;17(2). www.practicalpainmanagement.com/resources/practice-management/how-well-do-you-know-your-patient. Accessed November 25, 2019.
36. Schneider JP. A patient-centered approach to the opioid overdose crisis. J Miss State Med Assoc. 2018;59:232-233.
Many Americans who are treated with prescription opioid analgesics would be better off with less opioid or none at all. To that end, published opioid prescribing guidelines do provide guidance on the mechanics of tapering patients off opioids1-4—but they have a major flaw: They do not adequately account for the fact that people who have a diagnosis of chronic pain are a heterogeneous group and require diagnosis-specific treatment planning. A patient-centered approach to opioid tapers must account for the reality that many people who are given a prescription for an opioid to treat pain have significant mental health conditions—for which opioids act as a psychotropic agent. An opioid taper must therefore address psychological trauma, in particular.5 (See “Tapering and harm-reduction strategies have failed.”6-14)
SIDEBAR
Tapering and harm-reduction strategies have failed
Efforts to address the rising number of overdose events that involve opioids began in earnest in 2010. In a 2011 Government Accountability Office report to Congress, the Drug Enforcement Agency reported that “the number of regulatory investigations (of medical providers who prescribed opioids) tripled between fiscal years 2009- 2010.”6
How has it gone since 2010? High-dosage prescribing of opioids has fallen by 48% since 2011, yet the decline has not reduced overdose events of any kind.7,8 Just the opposite: The 19,000 overdose deaths recorded in 2010 involving any opioid increased to 49,068 by 2017, the National Institute on Drug Abuse reports.9 The increase in opioid overdose deaths is fueled by a recent 9-fold increase in consumption of the synthetic opioid fentanyl: “The rate of drug overdose deaths involving synthetic opioids other than methadone … increased on average by 8% per year from 1999 through 2013 and by 71% per year from 2013 through 2017.”10
These and other statistics document only a modest rise in deaths that involve prescription opioids: from 15,000 in 2010 to 19,000 in 2016.9,10 Since 2010, the crisis of opioid overdose deaths burns hotter, and the pattern of opioid use has shifted from prescription drugs to much deadlier illicit drugs, such as heroin.
Interventions have not been successful overall. Results of research focused on the impact of opioid tapering and harm-reduction strategies implemented this decade are likewise discouraging. In 2018, the US Department of Veterans Affairs reported that opioid discontinuation was not associated with a reduction in overdose but was associated with an increase in suicide.11,12 Von Korff and colleagues, in a 2017 report, concluded that “Long-term implementation of opioid dose and risk reduction initiatives [in Washington state] was not associated with lower rates of prescription opioid use disorder among prevalent [chronic opioid therapy] patients.”13
Evidence suggests that efforts to address the opioid crisis of the past decade have had an effect that is the opposite of what was intended. The federal government recognized this in April 2019 in a Drug Safety Communication: “The US Food and Drug Administration (FDA) has received reports of serious harm in patients who are physically dependent on opioid pain medicines suddenly having these medicines discontinued or the dose rapidly decreased. These include serious withdrawal symptoms, uncontrolled pain, psychological distress, and suicide.”14
In this article, we present an evidence-based consensus approach to opioid tapering for your practice that is informed by a broader understanding of why patients take prescription opioids and why they, occasionally, switch to illicit drugs when their prescription is tapered. This consensus approach is based on the experience of the authors, members of the pain faculty of Project ECHO (Extension for Community Healthcare Outcomes) of the ECHO Institute, a worldwide initiative that uses adult learning techniques and interactive video technology to connect community providers with specialists at centers of excellence in regular real-time collaborative sessions. We are variously experts in pain medicine, primary care, psychology, addiction medicine, pharmacy, behavioral health therapy, occupational medicine, and Chinese medicine.
Why Americans obtain prescription opioids
There are 4 principal reasons why patients obtain prescription opioids, beyond indicated analgesic uses:
1. Patients seek the antianxiety and antidepressant effects of opioids. Multiple converging lines of evidence suggest that antianxiety and antidepressant effects of opioids are a significant reason that patients in the United States persist in requesting prescriptions for opioids:
- In our experience with more than 500 primary care telemedicine case presentations, at least 50% of patients say that the main effect of opioids prescribed for them is “it makes me feel calm” or “more relaxed.”
- In a 2007 survey of 91,823 US residents older than 18 years, nonmedical use of opioids was statistically associated with panic, social anxiety, and depressive symptoms.15
- Ten years later, Von Korff and colleagues found that more than half of opioid prescriptions written in the United States were for the small percentage of patients who have a diagnosis of serious anxiety or depression.13
- In 2016, Yovell and colleagues reported that ultra-low-dosage buprenorphine markedly reduced suicidal ideation over 4 weeks in 62 patients with varied levels of depression.16
There is also mechanistic evidence that the antianxiety and antidepressant effects of opioids are significant reasons Americans persist in requesting prescription opioids. The literature suggests that opioid receptors play a role in mood regulation, including alleviation of depression and anxiety; recent research suggests that oxycodone might be a unique mood-altering drug compared to other common prescription opioids because of its ability to affect mood through the δ opioid receptor.17-20
It should not be a surprise that Americans often turn to opioids to address posttraumatic stress disorder (PTSD), anxiety, and depression. A recent study of the state of the US mental health system concluded that mental health services in the United States are inadequate—despite evidence that > 50% of Americans seek, or consider seeking, treatment for mental health problems for themselves or others.21
2. Patients experience pain unrelated to tissue damage. Rather, they are in pain “for psychological reasons.”22 In 2016, Davis and Vanderah wrote: “We theorize that a functional change in the [central nervous system] can occur in response to certain emotional states or traumatic experiences (eg, child abuse, assault, accidents).” They connect this change to central sensitization and a reduced pain-perception threshold,23 and strongly suspect that many patients with chronic pain have undiagnosed and untreated psychological trauma that has changed the way their central nervous system processes sensory stimuli. The authors call this “trauma-induced hyperalgesia.”
Continue to: Psychological trauma...
Psychological trauma is uniquely capable of producing hyperalgesia, compared to anxiety or depression. In a study of veterans, Defrin and colleagues demonstrated hyperalgesia in patients who had a diagnosis of PTSD but not in controls group who had an anxiety disorder only.24
To support successful opioid tapering, trauma-induced hyperalgesia, when present, must be addressed. Treatment of what the International Association for the Study of Pain calls “pain due to psychological factors”22 requires specific trauma therapy. However, our experience validates what researchers have to say about access to treatment of psychological trauma in the United States: “…[C]linical research has identified certain psychological interventions that effectively ameliorate the symptoms of PTSD. But most people struggling with PTSD don’t receive those treatments.”25
We have no doubt that this is due, in part, to underdiagnosis of psychological trauma, even in mental health clinics. According to Miele and colleagues, “PTSD remains largely undiagnosed and undertreated in mental health outpatients, even in teaching hospitals, with diagnosis rates as low as 4% while published prevalence is between 7% and 50% in this population.”26
3. Patients suffer from opioid use disorder (OUD) and complain of pain to obtain opioids by prescription. For patients with OUD, their use is out of control; they devote increasing mental and physical resources to obtaining, using, and recovering from substances; and they continue to use despite adverse consequences.27 The prevalence of OUD in primary care clinics varies strikingly by the location of clinics. In Washington state, the prevalence of moderate and severe OUD in a large population of patients who had been prescribed opioids through primary care clinics was recently determined to be between 21.5% and 23.9%.13
4. Patients are obtaining opioid prescriptions for people other than themselves. While this is a reason that patients obtain opioid prescriptions, it is not necessarily common. Statistics show that the likelihood of a prescription being diverted intentionally is low: Dart and colleagues found that diversion has become uncommon in the general population.28
Continue to: Why we taper opioid analgesics
Why we taper opioid analgesics
Reasons for an opioid taper include concern that the patient has, or will develop, an OUD; will experience accidental or intentional overdose; might be diverting opioids; is not benefiting from opioid therapy for pain; or is experiencing severe adverse effects. A patient who has nociceptive pain and might have opioid-induced hyperalgesia will require a much different opioid taper plan than a patient with untreated PTSD or a patient with severe OUD.
Misunderstanding can lead to inappropriate tapering
We often encounter primary care providers who believe that a large percentage of patients on chronic opioid therapy inevitably develop OUD. This is a common reason for initiating opioid taper. Most patients on a chronic opioid do become physically dependent, but only a small percentage of patients develop psychological dependence (ie, addiction or OUD).29
Physical dependence is “a state of adaptation that is manifested by a drug class–specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist.”30 Symptoms of opioid withdrawal include muscle aches; abdominal cramping; increased lacrimation, rhinorrhea, and perspiration; diarrhea; agitation and anxiety; insomnia; and piloerection. Opioid withdrawal symptoms are caused by physical dependence, not by addiction. They can be mitigated by tapering slowly and instituting adjuvant medications, such as clonidine, to attenuate symptoms.
Psychological dependence, or addiction (that is, OUD, as described in the Diagnostic and Statistical Manual of Mental Disorders 5th edition27), comprises primarily 3 behavioral criteria:
- Loss of control of the medication, with compulsive use
- Continued use despite adverse consequences of using opioids, such as arrest for driving under the influence and deterioration of social, family, or work performance
- Obsession or preoccupation with obtaining and using the substance. In properly selected chronic opioid therapy patients, there is evidence that new-onset OUD is not as common as has been thought. A recent study of the risk for opioid addiction after use of an opioid for ≥ 90 days for chronic noncancer pain found that the absolute rate of de novo OUD among patients treated for 90 days was 0.72%.29 A systematic review by Fishbain and colleagues of 24 studies of opioid-exposed patients found a risk of 3.27% overall—0.19% for patients who did not have a history of abuse or addiction.31 As Director of the National Institute on Drug Abuse Norma Volkow, MD, wrote in 2016: “Addiction occurs in only a small percentage of people who are exposed to opioids—even among those with preexisting vulnerabilities.”32
Assessment should focus on why the patient is taking an opioid
A strong case can be made that less opioid is better for many of the people for whom these medications are prescribed for chronic noncancer pain. However, a one-size-fits-all dosage reduction and addiction-focused approach to opioid tapering has not worked: The assessment and treatment paradigm must change, in our view.
Continue to: During assessment...
During assessment, we must adopt the means to identify the reason that a patient is using a prescription opioid. It is of particular importance that we identify patients using opioids for their psychotropic properties, particularly when the goal is to cope with the effects of psychological trauma. The subsequent treatment protocol will then need to include time for effective, evidence-based behavioral health treatment of anxiety, PTSD, or depression. If opioids are serving primarily as psychotropic medication, an attempt to taper before establishing effective behavioral health treatment might lead the patient to pursue illegal means of procuring opioid medication.
We acknowledge that primary care physicians are not reimbursed for trauma screening and that evidence-based intensive trauma treatment is generally unavailable in the United States. Both of these shortcomings must be corrected if we want to stem the opioid crisis.
If diversion is suspected and there is evidence that the patient is not currently taking prescribed opioids (eg, a negative urine drug screen), discontinuing the opioid prescription is the immediate next step for the sake of public safety.
SIDEBAR
2 decisions to make before continuing to prescribe an opioid for chronic noncancer pain
#1 Should I provide the patient with a prescription for an opioid for a few days, while I await more information?a
Yes. Writing a prescription is a reasonable decision if all of the following apply:
- You do not have significant suspicion of diversion (based on a clinical interview).
- You do not suspect an active addiction disorder, based on the score of the 10-question Drug Abuse Screening Test (DAST-10) and on a clinical interview. (DAST-10 is available at: https://cde.drugabuse.gov/instrument/e9053390-ee9c-9140-e040-bb89ad433d69.)
- The patient is likely to experience withdrawal symptoms if you don’t provide the medication immediately.
- The patient’s pain and function are likely to be impaired if you do not provide the medication.
- The patient does not display altered mental status during the visit (eg, drowsy, slurred speech).
No. If writing a prescription for an opioid for a few days does not seem to be a reasonable decision because the criteria above are not met, but withdrawal symptoms are likely, you can prescribe medication to mitigate symptoms or refer the patient for treatment of withdrawal.
#2 I’ve decided to provide the patient with a prescription for an opioid. For how many days should I write it?
The usual practice, for a patient whose case is familiar to you, is to prescribe a 1-month supply.
However, if any 1 of the following criteria is met, prescribing a 1-month supply is unsafe under most circumstances:
- An unstable social or living environment places the patient at risk by possessing a supply of opioids (based on a clinical interview).
- You suspect an unstable or severe behavioral health condition or a mental health diagnosis (based on a clinical interview or on the patient record from outside your practice).
- The patient scores as “high risk” on the Opioid Risk Tool (ORT; www.drugabuse.gov/sites/default/files/files/OpioidRiskTool.pdf), Screener and Opioid Assessment for Patients with Pain–Revised (SOAPP-R; www.ncbi.nlm.nih.gov/pmc/articles/PMC4706778/), or a similar opioid risk assessment tool.
When 1 or more of these exclusionary criteria are met, you have 3 options:
- Prescribe an opioid for a brief duration and see the patient often.
- Do not prescribe an opioid; instead, refer the patient as necessary for treatment of withdrawal.
- Refer the patient for treatment of the underlying behavioral health condition.
a Additional information might include findings from consultants you’ve engaged regarding the patient’s diagnosis; a response to your call from a past prescriber; urine drug screen results; and results of a prescription monitoring program check.

Considering a taper? Take this 5-step approach
Once it appears that tapering an opioid is indicated, we propose that you take the following steps:
- Establish whether it is safe to continue prescribing (follow the route provided in “2 decisions to make before continuing to prescribe an opioid for chronic noncancer pain”); if continuing it is not safe, take steps to protect the patient and the community
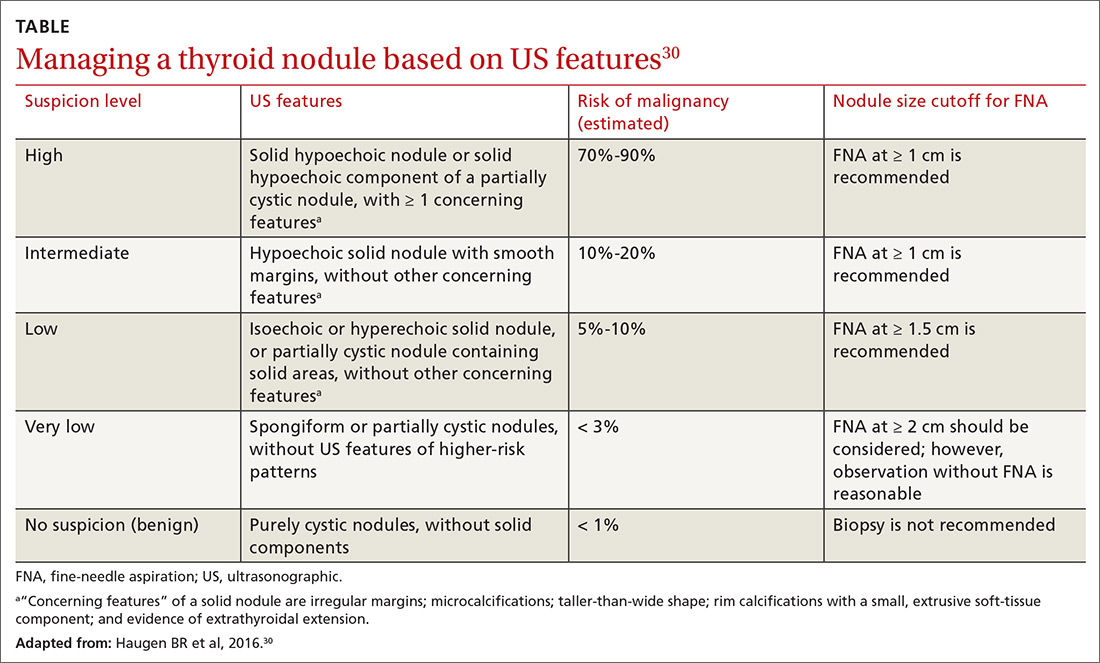
- Determine whether assessment by a trauma-informed behavioral health expert is needed, assuming that, in your judgment, it is safe to continue the opioid (TABLE33). When behavioral health assessment is needed, you need 3 questions answered by that assessment: (1) Are psychological factors present that might put the patient at risk during an opioid taper? (2) What are those factors? (3) What needs to done about them before the taper is started? Recalling that psychological trauma often is not assessed by behavioral health colleagues, it is necessary to provide the behavioral health provider with a specific request to assess trauma burden, and state the physical diagnoses that are causing pain or provide a clear statement that no such diagnoses can be made. (See the FIGURE, which we developed in conjunction with behavioral health colleagues to help the consultant understand what the primary care physician needs from a behavioral health assessment.)
- Obtain consultation from a physical therapist, pain medicine specialist, and, if possible, an alternative or complementary medicine provider to determine what nonpharmacotherapeutic modalities can be instituted to treat pain before tapering the opioid.
- Initiate the Screening, Brief Intervention and Referral to Treatment (SBIRT) approach if OUD is suspected (www.samhsa.gov/sbirt).34 This motivational interviewing tool identifies patients with a substance use disorder, severity of use, and appropriate level of treatment. (If OUD is suspected during assessment, next steps are to stop prescribing and implement harm-reduction strategies, such as primary care level medically assisted treatment [MAT] with buprenorphine, followed by expert behavioral health-centered addiction treatment.)
- Experiment with dosage reduction according to published guidance, if (1) psychological factors are absent or have been adequately addressed, according to the behavioral health consultant, and (2) nonpharmacotherapeutic strategies are in place.8-11

Shifting to a patient-centered approach
The timing and choice of opioid tapers, in relation to harm reduction and intervention targeting the root cause of a patient’s complaint of pain, have not been adequately explored. In our practice, we’ve shifted from an addiction-centered, dosage-centered approach to opioid taper to a patient-centered approach35 that emphasizes behavioral-medical integration—an approach that we broadly endorse. Such an approach (1) is based on a clear understanding of why the patient is taking opioid pain medication, (2) engages medical and complementary or alternative medicine specialists, (3) addresses underdiagnosis of psychological trauma, and (4) requires a quantum leap in access to trauma-specific behavioral health treatment resources. 36
Continue to: To underscore the case...
To underscore the case for shifting to a patient-centered approach35 we present sample cases in “How a patient-centered approach to tapering opioids looks in practice.”
SIDEBAR
How a patient-centered approach to tapering opioids looks in practice
Five hypothetical cases illustrate what might happen when a practice shifts from an addiction-centered, dosage-centered approach to one that places the individual at the center of care.
CASE #1: Brett F
Mr. F appears to use medication responsibly; benefits functionally from an opioid; has tolerable adverse effects; does not have significant psychosocial risk factors (based on the score of the Opioid Risk Tool [ORT] or the Screener and Opioid Assessment for Patients with Pain–Revised [SOAPP-R]); and is engaged in effective self-management. Most of Mr. F’s pain is thought to have a nociceptive or neuropathic source.
Mr F could reasonably contemplate continuing current opioid treatment.
Action: If the daily morphine milligram equivalent (MME) dosage is high, Mr. F should be referred to a pain medicine specialist. We recommend a periodic (at least annually) empiric trial of dosage reduction to see whether he is indeed best served by the current dosage.
CASE #2: Brett F (version 2.0)
Envision Mr. F having the same profile in all respects except that he is not engaged in effective self-management.
Optimal treatment of chronic pain often requires supplemental modalities beyond opioids.
Action: Physical therapy; an individualized, ongoing exercise regimen; interventional procedures; weight loss (if the patient is obese); smoking cessation; and improving coping skills for anxiety and depression without pharmacotherapy might not only temporarily alleviate the pain but, over time, improve Mr. F’s physical condition.
If Mr. F is not willing to do more than take the prescribed opioids, nothing is likely to change: Over time, his condition is likely to deteriorate. A patient like Mr. F can be harmed if opioids continue to be prescribed for him long-term.
Further action: If Mr. F won’t engage in broadening the approach to treating his pain, the opioid medication should be tapered, in his long-term best interest. A carrot-and-stick approach can facilitate Mr. F’s involvement in his care.
CASE #3: Clark S
Mr. S has a significant psychosocial component driving his pain: depression.a
Prescribing opioids without addressing the root cause of trauma is not in the patient’s best interest.
Action: Because of Mr. S’s depression, refer him to a behavioral health provider. If you determine that he is emotionally stable, wait until he is engaged in trauma treatment to begin the taper. If he appears unstable (eg, crying in the office, recent psychological stressors, recent impulsive behaviors, poor insight) consider (1) urgent behavioral health referral and (2) prescribing only enough opioid medication (ie, at close intervals) to prevent withdrawal and panic. Consider whether a psychotropic medication might be of benefit (eg, a serotonin–norepinephrine reuptake inhibitor or selective serotonin reuptake inhibitor).
Further action: Harm-reduction steps, such as close monitoring and, perhaps, a change to a buprenorphine product, is indicated, especially when the patient is overwhelmed by recent psychosocial stressors. Harm-reduction treatment is available through Medication-Assisted Therapy (MAT) programs; however, patients often run into difficulty obtaining access to these programs because regulations and laws restrict MAT to patients who have a diagnosis of opioid use disorder (OUD) and because some health plans and pharmacy benefit managers require prior authorization.
CASE #4: Gloria B
Ms. B isn’t managing her medications responsibly—although you don’t suspect OUD.
When a patient has shown the inability to manage opioid medication responsibly, you should delve into the reason to determine your next step.
Action: Evaluate Ms. B for a cognitive disorder or a thought disorder. Alternatively, as in the case of Mr. S, a psychosocial component might underlie her pain; in that case, the same recommendations can be made for her. In addition, you can propose that she identify a responsible person to dispense her medication.
CASE #5: Nicole L
You suspect that Ms. L, who is taking opioid medication to alleviate pain, also has a substance use disorder.
Action: Implement harm-reduction early for Ms. L: Obtain addiction medicine consultation and implement behavioral health strategies for addiction treatment.
A key characteristic of a substance use disorder is loss of control over use of the substance. A patient like Ms. L—who is in pain and who has an active OUD—cannot be expected to manage her opioid use responsibly.
Further action: We recommend that Ms. L be referred to an addiction specialist for MAT. Evidence of the harmreduction benefit of MAT is sufficient to strongly recommend it. Continue any other treatment modalities for pain that Ms. L has been using, such as non-opioid medication, physical therapy, alternative treatments, and behavioral therapy, or begin such treatments as appropriate.
a Depression is not the only psychosocial component that can underlie pain. Others include anxiety, posttraumatic stress disorder, and grief.
An eye toward the future. To inform future approaches to opioid tapering, more resources need to be deployed to
- support screening and risk stratification for PTSD, anxiety, and related disorders at the primary care level,
- continue the effort to identify and treat OUD,
- develop best-practice responses to screening, and
- make harm-reduction strategies that are now reserved for patients with OUD available to those who don't have OUD.
We urge that research be pursued into best practices for chronic pain interventions that target psychological trauma, anxiety, and depression.
CORRESPONDENCE
Bennet Davis MD, 2092 East Calle de Dulcinea, Tucson, AZ 85718; bdavis@ipcaz.org.
Many Americans who are treated with prescription opioid analgesics would be better off with less opioid or none at all. To that end, published opioid prescribing guidelines do provide guidance on the mechanics of tapering patients off opioids1-4—but they have a major flaw: They do not adequately account for the fact that people who have a diagnosis of chronic pain are a heterogeneous group and require diagnosis-specific treatment planning. A patient-centered approach to opioid tapers must account for the reality that many people who are given a prescription for an opioid to treat pain have significant mental health conditions—for which opioids act as a psychotropic agent. An opioid taper must therefore address psychological trauma, in particular.5 (See “Tapering and harm-reduction strategies have failed.”6-14)
SIDEBAR
Tapering and harm-reduction strategies have failed
Efforts to address the rising number of overdose events that involve opioids began in earnest in 2010. In a 2011 Government Accountability Office report to Congress, the Drug Enforcement Agency reported that “the number of regulatory investigations (of medical providers who prescribed opioids) tripled between fiscal years 2009- 2010.”6
How has it gone since 2010? High-dosage prescribing of opioids has fallen by 48% since 2011, yet the decline has not reduced overdose events of any kind.7,8 Just the opposite: The 19,000 overdose deaths recorded in 2010 involving any opioid increased to 49,068 by 2017, the National Institute on Drug Abuse reports.9 The increase in opioid overdose deaths is fueled by a recent 9-fold increase in consumption of the synthetic opioid fentanyl: “The rate of drug overdose deaths involving synthetic opioids other than methadone … increased on average by 8% per year from 1999 through 2013 and by 71% per year from 2013 through 2017.”10
These and other statistics document only a modest rise in deaths that involve prescription opioids: from 15,000 in 2010 to 19,000 in 2016.9,10 Since 2010, the crisis of opioid overdose deaths burns hotter, and the pattern of opioid use has shifted from prescription drugs to much deadlier illicit drugs, such as heroin.
Interventions have not been successful overall. Results of research focused on the impact of opioid tapering and harm-reduction strategies implemented this decade are likewise discouraging. In 2018, the US Department of Veterans Affairs reported that opioid discontinuation was not associated with a reduction in overdose but was associated with an increase in suicide.11,12 Von Korff and colleagues, in a 2017 report, concluded that “Long-term implementation of opioid dose and risk reduction initiatives [in Washington state] was not associated with lower rates of prescription opioid use disorder among prevalent [chronic opioid therapy] patients.”13
Evidence suggests that efforts to address the opioid crisis of the past decade have had an effect that is the opposite of what was intended. The federal government recognized this in April 2019 in a Drug Safety Communication: “The US Food and Drug Administration (FDA) has received reports of serious harm in patients who are physically dependent on opioid pain medicines suddenly having these medicines discontinued or the dose rapidly decreased. These include serious withdrawal symptoms, uncontrolled pain, psychological distress, and suicide.”14
In this article, we present an evidence-based consensus approach to opioid tapering for your practice that is informed by a broader understanding of why patients take prescription opioids and why they, occasionally, switch to illicit drugs when their prescription is tapered. This consensus approach is based on the experience of the authors, members of the pain faculty of Project ECHO (Extension for Community Healthcare Outcomes) of the ECHO Institute, a worldwide initiative that uses adult learning techniques and interactive video technology to connect community providers with specialists at centers of excellence in regular real-time collaborative sessions. We are variously experts in pain medicine, primary care, psychology, addiction medicine, pharmacy, behavioral health therapy, occupational medicine, and Chinese medicine.
Why Americans obtain prescription opioids
There are 4 principal reasons why patients obtain prescription opioids, beyond indicated analgesic uses:
1. Patients seek the antianxiety and antidepressant effects of opioids. Multiple converging lines of evidence suggest that antianxiety and antidepressant effects of opioids are a significant reason that patients in the United States persist in requesting prescriptions for opioids:
- In our experience with more than 500 primary care telemedicine case presentations, at least 50% of patients say that the main effect of opioids prescribed for them is “it makes me feel calm” or “more relaxed.”
- In a 2007 survey of 91,823 US residents older than 18 years, nonmedical use of opioids was statistically associated with panic, social anxiety, and depressive symptoms.15
- Ten years later, Von Korff and colleagues found that more than half of opioid prescriptions written in the United States were for the small percentage of patients who have a diagnosis of serious anxiety or depression.13
- In 2016, Yovell and colleagues reported that ultra-low-dosage buprenorphine markedly reduced suicidal ideation over 4 weeks in 62 patients with varied levels of depression.16
There is also mechanistic evidence that the antianxiety and antidepressant effects of opioids are significant reasons Americans persist in requesting prescription opioids. The literature suggests that opioid receptors play a role in mood regulation, including alleviation of depression and anxiety; recent research suggests that oxycodone might be a unique mood-altering drug compared to other common prescription opioids because of its ability to affect mood through the δ opioid receptor.17-20
It should not be a surprise that Americans often turn to opioids to address posttraumatic stress disorder (PTSD), anxiety, and depression. A recent study of the state of the US mental health system concluded that mental health services in the United States are inadequate—despite evidence that > 50% of Americans seek, or consider seeking, treatment for mental health problems for themselves or others.21
2. Patients experience pain unrelated to tissue damage. Rather, they are in pain “for psychological reasons.”22 In 2016, Davis and Vanderah wrote: “We theorize that a functional change in the [central nervous system] can occur in response to certain emotional states or traumatic experiences (eg, child abuse, assault, accidents).” They connect this change to central sensitization and a reduced pain-perception threshold,23 and strongly suspect that many patients with chronic pain have undiagnosed and untreated psychological trauma that has changed the way their central nervous system processes sensory stimuli. The authors call this “trauma-induced hyperalgesia.”
Continue to: Psychological trauma...
Psychological trauma is uniquely capable of producing hyperalgesia, compared to anxiety or depression. In a study of veterans, Defrin and colleagues demonstrated hyperalgesia in patients who had a diagnosis of PTSD but not in controls group who had an anxiety disorder only.24
To support successful opioid tapering, trauma-induced hyperalgesia, when present, must be addressed. Treatment of what the International Association for the Study of Pain calls “pain due to psychological factors”22 requires specific trauma therapy. However, our experience validates what researchers have to say about access to treatment of psychological trauma in the United States: “…[C]linical research has identified certain psychological interventions that effectively ameliorate the symptoms of PTSD. But most people struggling with PTSD don’t receive those treatments.”25
We have no doubt that this is due, in part, to underdiagnosis of psychological trauma, even in mental health clinics. According to Miele and colleagues, “PTSD remains largely undiagnosed and undertreated in mental health outpatients, even in teaching hospitals, with diagnosis rates as low as 4% while published prevalence is between 7% and 50% in this population.”26
3. Patients suffer from opioid use disorder (OUD) and complain of pain to obtain opioids by prescription. For patients with OUD, their use is out of control; they devote increasing mental and physical resources to obtaining, using, and recovering from substances; and they continue to use despite adverse consequences.27 The prevalence of OUD in primary care clinics varies strikingly by the location of clinics. In Washington state, the prevalence of moderate and severe OUD in a large population of patients who had been prescribed opioids through primary care clinics was recently determined to be between 21.5% and 23.9%.13
4. Patients are obtaining opioid prescriptions for people other than themselves. While this is a reason that patients obtain opioid prescriptions, it is not necessarily common. Statistics show that the likelihood of a prescription being diverted intentionally is low: Dart and colleagues found that diversion has become uncommon in the general population.28
Continue to: Why we taper opioid analgesics
Why we taper opioid analgesics
Reasons for an opioid taper include concern that the patient has, or will develop, an OUD; will experience accidental or intentional overdose; might be diverting opioids; is not benefiting from opioid therapy for pain; or is experiencing severe adverse effects. A patient who has nociceptive pain and might have opioid-induced hyperalgesia will require a much different opioid taper plan than a patient with untreated PTSD or a patient with severe OUD.
Misunderstanding can lead to inappropriate tapering
We often encounter primary care providers who believe that a large percentage of patients on chronic opioid therapy inevitably develop OUD. This is a common reason for initiating opioid taper. Most patients on a chronic opioid do become physically dependent, but only a small percentage of patients develop psychological dependence (ie, addiction or OUD).29
Physical dependence is “a state of adaptation that is manifested by a drug class–specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug, and/or administration of an antagonist.”30 Symptoms of opioid withdrawal include muscle aches; abdominal cramping; increased lacrimation, rhinorrhea, and perspiration; diarrhea; agitation and anxiety; insomnia; and piloerection. Opioid withdrawal symptoms are caused by physical dependence, not by addiction. They can be mitigated by tapering slowly and instituting adjuvant medications, such as clonidine, to attenuate symptoms.
Psychological dependence, or addiction (that is, OUD, as described in the Diagnostic and Statistical Manual of Mental Disorders 5th edition27), comprises primarily 3 behavioral criteria:
- Loss of control of the medication, with compulsive use
- Continued use despite adverse consequences of using opioids, such as arrest for driving under the influence and deterioration of social, family, or work performance
- Obsession or preoccupation with obtaining and using the substance. In properly selected chronic opioid therapy patients, there is evidence that new-onset OUD is not as common as has been thought. A recent study of the risk for opioid addiction after use of an opioid for ≥ 90 days for chronic noncancer pain found that the absolute rate of de novo OUD among patients treated for 90 days was 0.72%.29 A systematic review by Fishbain and colleagues of 24 studies of opioid-exposed patients found a risk of 3.27% overall—0.19% for patients who did not have a history of abuse or addiction.31 As Director of the National Institute on Drug Abuse Norma Volkow, MD, wrote in 2016: “Addiction occurs in only a small percentage of people who are exposed to opioids—even among those with preexisting vulnerabilities.”32
Assessment should focus on why the patient is taking an opioid
A strong case can be made that less opioid is better for many of the people for whom these medications are prescribed for chronic noncancer pain. However, a one-size-fits-all dosage reduction and addiction-focused approach to opioid tapering has not worked: The assessment and treatment paradigm must change, in our view.
Continue to: During assessment...
During assessment, we must adopt the means to identify the reason that a patient is using a prescription opioid. It is of particular importance that we identify patients using opioids for their psychotropic properties, particularly when the goal is to cope with the effects of psychological trauma. The subsequent treatment protocol will then need to include time for effective, evidence-based behavioral health treatment of anxiety, PTSD, or depression. If opioids are serving primarily as psychotropic medication, an attempt to taper before establishing effective behavioral health treatment might lead the patient to pursue illegal means of procuring opioid medication.
We acknowledge that primary care physicians are not reimbursed for trauma screening and that evidence-based intensive trauma treatment is generally unavailable in the United States. Both of these shortcomings must be corrected if we want to stem the opioid crisis.
If diversion is suspected and there is evidence that the patient is not currently taking prescribed opioids (eg, a negative urine drug screen), discontinuing the opioid prescription is the immediate next step for the sake of public safety.
SIDEBAR
2 decisions to make before continuing to prescribe an opioid for chronic noncancer pain
#1 Should I provide the patient with a prescription for an opioid for a few days, while I await more information?a
Yes. Writing a prescription is a reasonable decision if all of the following apply:
- You do not have significant suspicion of diversion (based on a clinical interview).
- You do not suspect an active addiction disorder, based on the score of the 10-question Drug Abuse Screening Test (DAST-10) and on a clinical interview. (DAST-10 is available at: https://cde.drugabuse.gov/instrument/e9053390-ee9c-9140-e040-bb89ad433d69.)
- The patient is likely to experience withdrawal symptoms if you don’t provide the medication immediately.
- The patient’s pain and function are likely to be impaired if you do not provide the medication.
- The patient does not display altered mental status during the visit (eg, drowsy, slurred speech).
No. If writing a prescription for an opioid for a few days does not seem to be a reasonable decision because the criteria above are not met, but withdrawal symptoms are likely, you can prescribe medication to mitigate symptoms or refer the patient for treatment of withdrawal.
#2 I’ve decided to provide the patient with a prescription for an opioid. For how many days should I write it?
The usual practice, for a patient whose case is familiar to you, is to prescribe a 1-month supply.
However, if any 1 of the following criteria is met, prescribing a 1-month supply is unsafe under most circumstances:
- An unstable social or living environment places the patient at risk by possessing a supply of opioids (based on a clinical interview).
- You suspect an unstable or severe behavioral health condition or a mental health diagnosis (based on a clinical interview or on the patient record from outside your practice).
- The patient scores as “high risk” on the Opioid Risk Tool (ORT; www.drugabuse.gov/sites/default/files/files/OpioidRiskTool.pdf), Screener and Opioid Assessment for Patients with Pain–Revised (SOAPP-R; www.ncbi.nlm.nih.gov/pmc/articles/PMC4706778/), or a similar opioid risk assessment tool.
When 1 or more of these exclusionary criteria are met, you have 3 options:
- Prescribe an opioid for a brief duration and see the patient often.
- Do not prescribe an opioid; instead, refer the patient as necessary for treatment of withdrawal.
- Refer the patient for treatment of the underlying behavioral health condition.
a Additional information might include findings from consultants you’ve engaged regarding the patient’s diagnosis; a response to your call from a past prescriber; urine drug screen results; and results of a prescription monitoring program check.

Considering a taper? Take this 5-step approach
Once it appears that tapering an opioid is indicated, we propose that you take the following steps:
- Establish whether it is safe to continue prescribing (follow the route provided in “2 decisions to make before continuing to prescribe an opioid for chronic noncancer pain”); if continuing it is not safe, take steps to protect the patient and the community
- Determine whether assessment by a trauma-informed behavioral health expert is needed, assuming that, in your judgment, it is safe to continue the opioid (TABLE33). When behavioral health assessment is needed, you need 3 questions answered by that assessment: (1) Are psychological factors present that might put the patient at risk during an opioid taper? (2) What are those factors? (3) What needs to done about them before the taper is started? Recalling that psychological trauma often is not assessed by behavioral health colleagues, it is necessary to provide the behavioral health provider with a specific request to assess trauma burden, and state the physical diagnoses that are causing pain or provide a clear statement that no such diagnoses can be made. (See the FIGURE, which we developed in conjunction with behavioral health colleagues to help the consultant understand what the primary care physician needs from a behavioral health assessment.)
- Obtain consultation from a physical therapist, pain medicine specialist, and, if possible, an alternative or complementary medicine provider to determine what nonpharmacotherapeutic modalities can be instituted to treat pain before tapering the opioid.
- Initiate the Screening, Brief Intervention and Referral to Treatment (SBIRT) approach if OUD is suspected (www.samhsa.gov/sbirt).34 This motivational interviewing tool identifies patients with a substance use disorder, severity of use, and appropriate level of treatment. (If OUD is suspected during assessment, next steps are to stop prescribing and implement harm-reduction strategies, such as primary care level medically assisted treatment [MAT] with buprenorphine, followed by expert behavioral health-centered addiction treatment.)
- Experiment with dosage reduction according to published guidance, if (1) psychological factors are absent or have been adequately addressed, according to the behavioral health consultant, and (2) nonpharmacotherapeutic strategies are in place.8-11

Shifting to a patient-centered approach
The timing and choice of opioid tapers, in relation to harm reduction and intervention targeting the root cause of a patient’s complaint of pain, have not been adequately explored. In our practice, we’ve shifted from an addiction-centered, dosage-centered approach to opioid taper to a patient-centered approach35 that emphasizes behavioral-medical integration—an approach that we broadly endorse. Such an approach (1) is based on a clear understanding of why the patient is taking opioid pain medication, (2) engages medical and complementary or alternative medicine specialists, (3) addresses underdiagnosis of psychological trauma, and (4) requires a quantum leap in access to trauma-specific behavioral health treatment resources. 36
Continue to: To underscore the case...
To underscore the case for shifting to a patient-centered approach35 we present sample cases in “How a patient-centered approach to tapering opioids looks in practice.”
SIDEBAR
How a patient-centered approach to tapering opioids looks in practice
Five hypothetical cases illustrate what might happen when a practice shifts from an addiction-centered, dosage-centered approach to one that places the individual at the center of care.
CASE #1: Brett F
Mr. F appears to use medication responsibly; benefits functionally from an opioid; has tolerable adverse effects; does not have significant psychosocial risk factors (based on the score of the Opioid Risk Tool [ORT] or the Screener and Opioid Assessment for Patients with Pain–Revised [SOAPP-R]); and is engaged in effective self-management. Most of Mr. F’s pain is thought to have a nociceptive or neuropathic source.
Mr F could reasonably contemplate continuing current opioid treatment.
Action: If the daily morphine milligram equivalent (MME) dosage is high, Mr. F should be referred to a pain medicine specialist. We recommend a periodic (at least annually) empiric trial of dosage reduction to see whether he is indeed best served by the current dosage.
CASE #2: Brett F (version 2.0)
Envision Mr. F having the same profile in all respects except that he is not engaged in effective self-management.
Optimal treatment of chronic pain often requires supplemental modalities beyond opioids.
Action: Physical therapy; an individualized, ongoing exercise regimen; interventional procedures; weight loss (if the patient is obese); smoking cessation; and improving coping skills for anxiety and depression without pharmacotherapy might not only temporarily alleviate the pain but, over time, improve Mr. F’s physical condition.
If Mr. F is not willing to do more than take the prescribed opioids, nothing is likely to change: Over time, his condition is likely to deteriorate. A patient like Mr. F can be harmed if opioids continue to be prescribed for him long-term.
Further action: If Mr. F won’t engage in broadening the approach to treating his pain, the opioid medication should be tapered, in his long-term best interest. A carrot-and-stick approach can facilitate Mr. F’s involvement in his care.
CASE #3: Clark S
Mr. S has a significant psychosocial component driving his pain: depression.a
Prescribing opioids without addressing the root cause of trauma is not in the patient’s best interest.
Action: Because of Mr. S’s depression, refer him to a behavioral health provider. If you determine that he is emotionally stable, wait until he is engaged in trauma treatment to begin the taper. If he appears unstable (eg, crying in the office, recent psychological stressors, recent impulsive behaviors, poor insight) consider (1) urgent behavioral health referral and (2) prescribing only enough opioid medication (ie, at close intervals) to prevent withdrawal and panic. Consider whether a psychotropic medication might be of benefit (eg, a serotonin–norepinephrine reuptake inhibitor or selective serotonin reuptake inhibitor).
Further action: Harm-reduction steps, such as close monitoring and, perhaps, a change to a buprenorphine product, is indicated, especially when the patient is overwhelmed by recent psychosocial stressors. Harm-reduction treatment is available through Medication-Assisted Therapy (MAT) programs; however, patients often run into difficulty obtaining access to these programs because regulations and laws restrict MAT to patients who have a diagnosis of opioid use disorder (OUD) and because some health plans and pharmacy benefit managers require prior authorization.
CASE #4: Gloria B
Ms. B isn’t managing her medications responsibly—although you don’t suspect OUD.
When a patient has shown the inability to manage opioid medication responsibly, you should delve into the reason to determine your next step.
Action: Evaluate Ms. B for a cognitive disorder or a thought disorder. Alternatively, as in the case of Mr. S, a psychosocial component might underlie her pain; in that case, the same recommendations can be made for her. In addition, you can propose that she identify a responsible person to dispense her medication.
CASE #5: Nicole L
You suspect that Ms. L, who is taking opioid medication to alleviate pain, also has a substance use disorder.
Action: Implement harm-reduction early for Ms. L: Obtain addiction medicine consultation and implement behavioral health strategies for addiction treatment.
A key characteristic of a substance use disorder is loss of control over use of the substance. A patient like Ms. L—who is in pain and who has an active OUD—cannot be expected to manage her opioid use responsibly.
Further action: We recommend that Ms. L be referred to an addiction specialist for MAT. Evidence of the harmreduction benefit of MAT is sufficient to strongly recommend it. Continue any other treatment modalities for pain that Ms. L has been using, such as non-opioid medication, physical therapy, alternative treatments, and behavioral therapy, or begin such treatments as appropriate.
a Depression is not the only psychosocial component that can underlie pain. Others include anxiety, posttraumatic stress disorder, and grief.
An eye toward the future. To inform future approaches to opioid tapering, more resources need to be deployed to
- support screening and risk stratification for PTSD, anxiety, and related disorders at the primary care level,
- continue the effort to identify and treat OUD,
- develop best-practice responses to screening, and
- make harm-reduction strategies that are now reserved for patients with OUD available to those who don't have OUD.
We urge that research be pursued into best practices for chronic pain interventions that target psychological trauma, anxiety, and depression.
CORRESPONDENCE
Bennet Davis MD, 2092 East Calle de Dulcinea, Tucson, AZ 85718; bdavis@ipcaz.org.
1. Centers for Disease Control and Prevention. Pocket guide: tapering for chronic pain. https://www.cdc.gov/drugoverdose/pdf/clinical_pocket_guide_tapering-a.pdf. Accessed November 25, 2019.
2. Kral LA, Jackson K, Uritsky TJ. A practical guide to tapering opioids. Ment Health Clin. 2015;5:102-108.
3. Murphy L, Babaei-Rad R, Buna D, et al. Guidance on opioid tapering in the context of chronic pain: evidence, practical advice and frequently asked questions. Can Pharm J (Ott). 2018;151:114-120.
4. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic noncancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2005;90:828-842.
5. Davis M. Prescription opioid use among adults with mental health disorders in the United States. J Am Board Fam Med. 2017;30:407-417.
6. US Government Accountability Office. Report to Congressional Requestors. Prescription drug control: DEA has enhanced efforts to combat diversion, but could better assess and report program results. August 2011. www.gao.gov/assets/520/511464.pdf. Accessed November 25, 2019.
7. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. Annual surveillance report of drug-related risks and outcomes. United States, 2017. www.cdc.gov/drugoverdose/pdf/pubs/2017-cdc-drug-surveillance-report.pdf. Accessed November 25, 2019.
8. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016, NCHS Data Brief No. 294. December 21, 2017. Hyattsville, MD: National Center for Health Statistics. www.cdc.gov/nchs/products/databriefs/db294.htm. Accessed November 25, 2019.
9. Overdose death rates. Bethesda, MD: National Institute on Drug Abuse. January 2019. www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Accessed November 25, 2019.
10. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. NCHS Data Brief No. 329. November 2018. Hyattsville, MD: National Center for Health Statistics. www.cdc.gov/nchs/data/databriefs/db329-h.pdf . Accessed November 25, 2019.
11. Manhapra A, Kertesz S, Oliva A, et al. VA data about Rx opioids and overdose and suicide: clinical implications. Presented at the 2018 National Rx Drug Abuse and Heroin Summit, Atlanta Georgia, April 4, 2018.
12. Demidenko M, Dobscha SK, Morasco BJ, et al. Suicidal ideation and suicidal self-directed violence following clinician-initiated prescription opioid discontinuation among long-term opioid users. Gen Hosp Psychiatry. 2017;47:29-35.
13. Von Korff M, Walker RL, Saunders K, et al. Prevalence of prescription opioid use disorder among chronic opioid therapy patients after health plan opioid dose and risk reduction initiatives. Int J Drug Policy. 2017;46:90-98.
14. United States Food and Drug Administration. FDA Drug Safety Communication: FDA identifies harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering. April 9, 2019. www.fda.gov/Drugs/DrugSafety/ucm635038.htm. Accessed November 25, 2019.
15. Becker W, Sullivan LE, Tetrault JM, et al. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: psychiatric, medical and substance use correlates. Drug Alcohol Depend. 2008;94:38-47.
16. Yovell Y, Bar G, Mashiah M, et al. Ultra-low-dose buprenorphine as a time-limited treatment for severe suicidal ideation: a randomized controlled trial. Am J Psychiatry. 2016;173:491-498.
17. Pradhan AA, Befort K, Nozaki C, et al. The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci. 2011;32:581-590.
18. Sugiyama A, Yamada M, Saitoh A, et al. Administration of a delta opioid receptor agonist KNT-127 to the basolateral amygdala has robust anxiolytic-like effects in rats. Psychopharmacology (Berl). 2018;235:2947-2955.
19. Richards EM, Mathews DC, Luckenbaugh DA, et al. A randomized, placebo-controlled pilot trial of the delta opioid receptor agonist AZD2327 in anxious depression. Psychopharmacology (Berl). 2016;233:1119-1130.
20. Yang PP, Yeh GC, Yeh TK, et al. Activation of delta-opioid receptor contributes to the antinociceptive effect of oxycodone in mice. Pharmacol Res. 2016;111:867-876.
21. America’s mental health 2018. Stamford, CT: Cohen Veterans Network. October 10, 2018. https://www.cohenveteransnetwork.org/wp-content/uploads/2018/10/Research-Summary-10-10-2018.pdf. Accessed November 25, 2019.
22. Classification of Chronic Pain, Second Edition (Revised). Washington, DC: International Association for the Study of Pain. Updated 2012. www.iasp-pain.org/PublicationsNews/Content.aspx?ItemNumber=1673. Accessed November 25, 2019.
23. Davis B, Vanderah TW. A new paradigm for pain? J Fam Pract. 2016 65:598-605.
24. Defrin R, Ginzburg K, Solomon Z, et al. Quantitative testing of pain perception in subjects with PTSD—implications for the mechanism of the coexistence between PTSD and chronic pain. Pain. 2008;138:450-459.
25. Foa EB, Gillihan SJ, Bryant RA. Challenges and successes in dissemination of evidence-based treatments for posttraumatic stress: lessons learned from prolonged exposure therapy for PTSD. Psychol Science Public Interest. 2013;14:65-111.
26. Miele D, O’Brien EJ. Underdiagnosis of posttraumatic stress disorder in at risk youth. J Trauma Stress. 2010;23:591-598.
27. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Washington, DC: American Psychiatric Publishing; 2013:541.
28. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241-248.
29. Schuchat A, Houry D, Guy GP Jr. New data on opioid use and prescribing in the United States. JAMA. 2017;318:425-426.
30. American Academy of Pain Medicine, American Pain Society, American Society of Addiction Medicine. Definitions related to the use of opioids for the treatment of pain. 2001. www.naabt.org/documents/APS_consensus_document.pdf. Accessed November 25, 2019.
31. Fishbain DA, Cole B, Lewis J, et al. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 2008;9:444-459.
32. Volkow ND, McClellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374:1253-1263.
33. Treede RD, Rief W, Barke A. A classification of chronic pain for ICD-11. Pain. 2015;156:1003-1007.
34. Screening, brief intervention, and referral to treatment (SBIRT). Rockville, MD: Substance Abuse and Mental Health Services Administration. www.samhsa.gov/sbirt. Accessed November 25, 2019.
35. Schneider JP, Davis B. How well do you know your patient? Pract Pain Manag. 2017;17(2). www.practicalpainmanagement.com/resources/practice-management/how-well-do-you-know-your-patient. Accessed November 25, 2019.
36. Schneider JP. A patient-centered approach to the opioid overdose crisis. J Miss State Med Assoc. 2018;59:232-233.
1. Centers for Disease Control and Prevention. Pocket guide: tapering for chronic pain. https://www.cdc.gov/drugoverdose/pdf/clinical_pocket_guide_tapering-a.pdf. Accessed November 25, 2019.
2. Kral LA, Jackson K, Uritsky TJ. A practical guide to tapering opioids. Ment Health Clin. 2015;5:102-108.
3. Murphy L, Babaei-Rad R, Buna D, et al. Guidance on opioid tapering in the context of chronic pain: evidence, practical advice and frequently asked questions. Can Pharm J (Ott). 2018;151:114-120.
4. Berna C, Kulich RJ, Rathmell JP. Tapering long-term opioid therapy in chronic noncancer pain: evidence and recommendations for everyday practice. Mayo Clin Proc. 2005;90:828-842.
5. Davis M. Prescription opioid use among adults with mental health disorders in the United States. J Am Board Fam Med. 2017;30:407-417.
6. US Government Accountability Office. Report to Congressional Requestors. Prescription drug control: DEA has enhanced efforts to combat diversion, but could better assess and report program results. August 2011. www.gao.gov/assets/520/511464.pdf. Accessed November 25, 2019.
7. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. Annual surveillance report of drug-related risks and outcomes. United States, 2017. www.cdc.gov/drugoverdose/pdf/pubs/2017-cdc-drug-surveillance-report.pdf. Accessed November 25, 2019.
8. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016, NCHS Data Brief No. 294. December 21, 2017. Hyattsville, MD: National Center for Health Statistics. www.cdc.gov/nchs/products/databriefs/db294.htm. Accessed November 25, 2019.
9. Overdose death rates. Bethesda, MD: National Institute on Drug Abuse. January 2019. www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates. Accessed November 25, 2019.
10. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999-2017. NCHS Data Brief No. 329. November 2018. Hyattsville, MD: National Center for Health Statistics. www.cdc.gov/nchs/data/databriefs/db329-h.pdf . Accessed November 25, 2019.
11. Manhapra A, Kertesz S, Oliva A, et al. VA data about Rx opioids and overdose and suicide: clinical implications. Presented at the 2018 National Rx Drug Abuse and Heroin Summit, Atlanta Georgia, April 4, 2018.
12. Demidenko M, Dobscha SK, Morasco BJ, et al. Suicidal ideation and suicidal self-directed violence following clinician-initiated prescription opioid discontinuation among long-term opioid users. Gen Hosp Psychiatry. 2017;47:29-35.
13. Von Korff M, Walker RL, Saunders K, et al. Prevalence of prescription opioid use disorder among chronic opioid therapy patients after health plan opioid dose and risk reduction initiatives. Int J Drug Policy. 2017;46:90-98.
14. United States Food and Drug Administration. FDA Drug Safety Communication: FDA identifies harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering. April 9, 2019. www.fda.gov/Drugs/DrugSafety/ucm635038.htm. Accessed November 25, 2019.
15. Becker W, Sullivan LE, Tetrault JM, et al. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: psychiatric, medical and substance use correlates. Drug Alcohol Depend. 2008;94:38-47.
16. Yovell Y, Bar G, Mashiah M, et al. Ultra-low-dose buprenorphine as a time-limited treatment for severe suicidal ideation: a randomized controlled trial. Am J Psychiatry. 2016;173:491-498.
17. Pradhan AA, Befort K, Nozaki C, et al. The delta opioid receptor: an evolving target for the treatment of brain disorders. Trends Pharmacol Sci. 2011;32:581-590.
18. Sugiyama A, Yamada M, Saitoh A, et al. Administration of a delta opioid receptor agonist KNT-127 to the basolateral amygdala has robust anxiolytic-like effects in rats. Psychopharmacology (Berl). 2018;235:2947-2955.
19. Richards EM, Mathews DC, Luckenbaugh DA, et al. A randomized, placebo-controlled pilot trial of the delta opioid receptor agonist AZD2327 in anxious depression. Psychopharmacology (Berl). 2016;233:1119-1130.
20. Yang PP, Yeh GC, Yeh TK, et al. Activation of delta-opioid receptor contributes to the antinociceptive effect of oxycodone in mice. Pharmacol Res. 2016;111:867-876.
21. America’s mental health 2018. Stamford, CT: Cohen Veterans Network. October 10, 2018. https://www.cohenveteransnetwork.org/wp-content/uploads/2018/10/Research-Summary-10-10-2018.pdf. Accessed November 25, 2019.
22. Classification of Chronic Pain, Second Edition (Revised). Washington, DC: International Association for the Study of Pain. Updated 2012. www.iasp-pain.org/PublicationsNews/Content.aspx?ItemNumber=1673. Accessed November 25, 2019.
23. Davis B, Vanderah TW. A new paradigm for pain? J Fam Pract. 2016 65:598-605.
24. Defrin R, Ginzburg K, Solomon Z, et al. Quantitative testing of pain perception in subjects with PTSD—implications for the mechanism of the coexistence between PTSD and chronic pain. Pain. 2008;138:450-459.
25. Foa EB, Gillihan SJ, Bryant RA. Challenges and successes in dissemination of evidence-based treatments for posttraumatic stress: lessons learned from prolonged exposure therapy for PTSD. Psychol Science Public Interest. 2013;14:65-111.
26. Miele D, O’Brien EJ. Underdiagnosis of posttraumatic stress disorder in at risk youth. J Trauma Stress. 2010;23:591-598.
27. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Washington, DC: American Psychiatric Publishing; 2013:541.
28. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372:241-248.
29. Schuchat A, Houry D, Guy GP Jr. New data on opioid use and prescribing in the United States. JAMA. 2017;318:425-426.
30. American Academy of Pain Medicine, American Pain Society, American Society of Addiction Medicine. Definitions related to the use of opioids for the treatment of pain. 2001. www.naabt.org/documents/APS_consensus_document.pdf. Accessed November 25, 2019.
31. Fishbain DA, Cole B, Lewis J, et al. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. 2008;9:444-459.
32. Volkow ND, McClellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med. 2016;374:1253-1263.
33. Treede RD, Rief W, Barke A. A classification of chronic pain for ICD-11. Pain. 2015;156:1003-1007.
34. Screening, brief intervention, and referral to treatment (SBIRT). Rockville, MD: Substance Abuse and Mental Health Services Administration. www.samhsa.gov/sbirt. Accessed November 25, 2019.
35. Schneider JP, Davis B. How well do you know your patient? Pract Pain Manag. 2017;17(2). www.practicalpainmanagement.com/resources/practice-management/how-well-do-you-know-your-patient. Accessed November 25, 2019.
36. Schneider JP. A patient-centered approach to the opioid overdose crisis. J Miss State Med Assoc. 2018;59:232-233.
PRACTICE RECOMMENDATIONS
› Screen for developmental and adult trauma, for current trauma symptoms, and for opioid use disorder before tapering an opioid. B
› Refer the patient for in-depth behavioral health evaluation when screening identifies risk of behavioral problems, to identify psychological, behavioral, emotional, cognitive, and social factors pertinent to the prevention, treatment, or management of physical health problems, such as chronic pain. A
› Refer the patient for addiction medicine treatment, either within your practice or to an outside consultant, when screening for opioid use disorder indicates that the patient is at risk. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
When guideline treatment of asthma fails, consider a macrolide antibiotic
In vitro laboratory and in vivo animal models support the biologic plausibility that chronic infection is a potential cause of asthma.1,2 Arising from that hypothesis, macrolide antibiotics have been the subject of clinical trials and other studies to determine whether these drugs are efficacious in the long-term management of asthma in adults and children. Macrolides might also have immunomodulatory and antiviral properties that can benefit patients with asthma.3
This article looks at the evidence and clinical scenarios for the use of macrolides in asthma, provides proposed dosing schedules, and reviews associated concerns, including adverse effects, risk of bacterial resistance, and cost.
3 cases to consider
CASE 1 Paul D developed severe, refractory asthma at 30 years of age after an acute respiratory illness. At age 40, he was treated with 14 weekly doses of azithromycin. His asthma resolved slowly over 12 months.
Outcome. Mr. D has remained free of symptoms of asthma for more than 20 years.
CASE 2 Casey K developed severe wheezing at 18 months of age after an acute respiratory illness. Refractory asthma symptoms persisted until 6 years of age, at which time he was given 12 weekly doses of azithromycin. Asthma symptoms gradually resolved.
Outcome. Casey was able to resume normal physical activities, including competitive swimming.
CASE 3 Amy S, who had no history of respiratory problems, presented at 30 years of age with a 3-month history of wheezing and dyspnea after an acute respiratory illness. She was treated symptomatically with bronchodilators; wheezing failed to resolve. After 6 months of persistent wheezing that significantly affected her exercise capacity, Ms. S was given a diagnosis of persistent asthma and received 12 weekly doses of azithromycin.
[polldaddy:10475438]
Continue to: Outcome...
Outcome. Ms. S’s symptoms resolved completely within months.
Evidence of benefit of macrolides in asthma
These 3 cases, taken from my practice (but with names changed), demonstrate the therapeutic potential of macrolide antibiotics for patients with asthma under specific clinical circumstances. The cases are referenced again in the following examination of the literature on macrolides for asthma
SIDEBAR
Macrolides for Asthma: Registry of Clinical Experience
More information is needed about the “real world” effectiveness of antibiotic treatment for severe refractory and new-onset asthma. If you are a prescribing clinician who cares for patients with asthma and you are considering prescribing antibiotics for asthma, you are invited to document your outcomes by entering prospective, de-identified patient data into a human subjects committee-approved online registry. To gain access to the registry, and for more information, contact the author at dlhahn@wisc.edu or visit https://www.fammed.wisc.edu/wren/resources/macrolides-for-asthma/ .
Meta-analysis. Reiter et al4 performed a meta-analysis of 12 randomized clinical trials of macrolides for long-term management of asthma in children and adults. Prolonged treatment was defined as > 3 weeks of continuous administration of a macrolide. The pooled effect of macrolides on forced expiratory volume in 1 second (FEV1) was not significant; however, a significant effect on peak expiratory flow, symptom scores, quality of life, and airway hyperreactivity was observed.
Comment: The study’s authors concluded: “Macrolides may therefore be beneficial as adjunct asthma therapy. Future trials, focusing on long-term safety and effectiveness, should use standardized outcomes and procedures.”
Cochrane meta-analysis. Kew et al5 performed a meta-analysis of 23 studies of macrolides for managing chronic asthma for the Cochrane Database of Systematic Reviews. In their review, they reported
- no significant effects of macrolides on asthma exacerbations, asthma control, quality of life, and rescue medication use; and
- significant effects of macrolides for asthma symptoms and FEV1.
Continue to: Two within-study subgroup...
Two within-study subgroup analyses showed a possible benefit of macrolides for non-eosinophilic asthma, defined by a predominance of neutrophils in a bronchoalveolar lavage specimen. Kew et al5 noted that (1) most of the evidence examined in the review was of low quality and (2) inclusion criteria, interventions, and outcomes were highly variable.
Comment: The validity of a meta-analysis depends on the validity and similarity of underlying trials. Both meta-analyses just described were characterized by (1) grouping trials of older and newer macrolides and (2) significant selection bias in the underlying trials.
Selection bias is prevalent in asthma research and is a major contributor to uncertainty: Randomized controlled trials upon which guideline treatments are based have systematically excluded > 90% of people with asthma.6 Exclusions include past or current smoking, the asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome, severe asthma, and acute respiratory illness; these exclusion criteria have also been applied to studies of macrolides. Importantly, patients in the excluded groups are probably those most likely to respond to a macrolide.2 Pragmatic effectiveness studies (broad eligibility criteria, adequate duration of azithromycin treatment, a posttreatment observation period, and pre-specified biomarker subgroup analyses) have been recommended to address the hypothesis of what has been termed infectious asthma.2
Inconsistent evidence, the generally poor quality of underlying studies, and uncertainty about which subgroup(s) of asthma patients might benefit all contribute to a strength of recommendation of “B” for treating asthma with macrolides. Two recent randomized trials7,8 that were not included in the cited meta-analyses, along with other evidence,2 point to 2 groups of patients who are candidates for a trial of azithromycin: those with severe refractory asthma and those with new-onset asthma.
Clinical trial in adults. Gibson et al7 conducted a randomized, double-blind, placebo-controlled trial of azithromycin 500 mg 3 times a week or placebo for 1 year in 420 adults who had uncontrolled persistent asthma despite taking medium-to-high doses of an inhaled corticosteroid (ICS) plus a long-acting β agonist (LABA) (the AMAZES [Asthma and Macrolides: The Azithromycin Efficacy and Safety] trial; Level 1 study). The mean baseline asthma control questionnaire score was 1.5, equivalent to an Asthma Control Test (ACT) score* of 15.9
Continue to: Azithromycin reduced the frequency...
Azithromycin reduced the frequency of asthma exacerbations (to 1.07 per patient–year for azithromycin, compared with 1.86 per patient–year for placebo [incidence rate ratio = 0.59; 95% confidence interval (CI), 0.47-0.74]). The percentage of patients experiencing at least 1 exacerbation was reduced with azithromycin treatment (61% of patients in the placebo group experienced ≥ 1 exacerbation, compared with 44% in the azithromycin group [P < .0001; number needed to treat = 6]). Asthma quality of life was also improved by azithromycin (P = .001).
There was no significant difference between azithromycin and placebo in the overall rate of serious adverse events. Diarrhea that did not require treatment discontinuation was more common in patients treated with azithromycin (34%) than in the placebo group (19%). There was no posttreatment observation period to assess whether these azithromycin benefits waned or persisted after treatment was stopped.
Other evidence10 indicates that at least some patients who respond to azithromycin will experience persistent improvement after antibiotic treatment is completed (see CASE 1).
Pediatric clinical trial. Stokholm et al8 performed a randomized, double-blind, placebo-controlled trial of azithromycin in children 1 to 3 years of age who had been given a diagnosis of recurrent asthma-like symptoms (Level 1 study). Treatment was a 3-day course of azithromycin oral solution, 10 mg/kg/d, or placebo. Random allocation was performed for 158 asthma-like episodes in 72 children.
Azithromycin reduced the wheezing episode to a mean duration of 3.4 days, compared with 7.7 days for placebo (risk reduction = 63.3%; 95% CI, 56%-69.3% [P < .0001]). Effect size increased with early initiation of treatment: ie, an 83% reduction in episode duration was seen when treatment was initiated before Day 6 of the episode, compared with a 36% reduction if treatment was initiated on or after Day 6 (P < .0001).
Continue to: No differences between...
No differences between the randomized groups were observed in clinical adverse effects.
Comment: The brief course of azithromycin provided to patients in this trial did not have a significant impact on time to next episode of troublesome lung symptoms in individual children. Previous clinical observations have suggested that a longer duration of treatment (3-6 months) might be required to achieve lasting improvement or remission in selected patients with asthma (see CASE 2).10,11 The short-term benefit of azithromycin for acute wheezing is limited to children: Two comparable acute dosing trials in adults have shown little12 or no13 short-term benefit; however, these negative findings have been hypothesized to be the result of selection bias.14
Other evidence is worth examining
Other studies not included in the meta-analyses of randomized controlled trials provide additional evidence to support a recommendation of a trial of azithromycin in patients with severe, refractory, or new-onset asthma.
Nonrandomized controlled evidence. AZMATICS (AZithroMycin/Asthma Trial In Community Settings)15 is the sole randomized, double-blind, placebo-controlled trial of long-term azithromycin that included a 9-month posttreatment observation period. Seventy-five participants were randomized to receive a loading dose of 600 mg of azithromycin or placebo once daily for 3 days in Week 1. They then received either azithromycin 600 mg or placebo once weekly for 11 weeks. Posttreatment observation was performed until 48 weeks after randomization.
However, many eligible subjects, whom the principal investigator believed were ideal candidates for randomization, declined randomization because they did not want to risk receiving placebo. To accommodate those patients, the protocol was amended to include an open-label (OL) azithromycin arm, in which each participant’s personal physician prescribed azithromycin 750 mg for 11 weeks after a loading dose16 (OL cohort only, Level 2 study: controlled, nonrandomized, nonblinded). The OL group had (1) a higher baseline prevalence of severe, persistent asthma (32%) than the randomized group (8%) (P = .012); and (2) worse asthma quality of life than the randomized patients (P = .023). The OL group represented selection bias attributable to patient preference.
Continue to: The less severely...
The less severely affected randomized group of the trial did not exhibit significant effects attributable to azithromycin. The more severely affected OL cohort demonstrated significant, and large, azithromycin treatment effects for asthma symptoms, asthma quality of life, and asthma control (P < .05 for both groups; number needed to treat [NNT] = 3) that persisted during the posttreatment observation period.
Comment: The authors concluded: “Pending further randomized trials and given the relative safety of azithromycin and the significant disease burden from severe, refractory asthma, prescribing prolonged azithromycin therapy to patients with uncontrolled asthma may be considered by managing clinicians, particularly for patients who have failed to respond to conventional treatment and as an alternative to instituting immunomodulatory agents.”15
Before-and-after trial. Forty-six patients with moderate or severe chronic, persistent, stable asthma were selected as a cohort unlikely to experience spontaneous remission (ie, patients in exacerbation were excluded) (Level 2 study: prospective cohort).17 Subjects were treated for a median of 4 weeks (range, 3 to 9 weeks) with oral doxycycline, 100 mg bid; azithromycin, 1000 mg, once weekly; or erythromycin, 1000 mg/d in divided doses. Average duration of posttreatment follow-up was 6 months. All subjects were positive for antibodies to Chlamydia pneumoniae.
Four patients with diagnosed acuteC pneumoniae respiratory infection developed chronic asthma, which disappeared in each case after treatment. Of the other 42 seroreactive patients who were treated a mean of 6 years after they developed chronic asthma, 21 had either complete remission of asthma symptoms (n = 3) or major persistent clinical improvement (n = 18). Clinical improvement was more likely to occur in patients with early disease (P = .01) and before development of fixed airway obstruction (P < .01).
These results are consistent with the hypothesis that chronic infection of the lower respiratory tract contributes to the development and progression of asthma.17 Although clinical improvement was more likely in early asthma compared with asthma with fixed airway obstruction, improvement was nevertheless noted in the latter group.
Continue to: Physicians should also note...
Physicians should also note the landmark trial of azithromycin in severe, smoking-associated COPD, which found a clinically significant benefit in reducing exacerbations and improving quality of life (NNT = 3, to prevent 1 exacerbation).18
Case series. In a prospective case series (Level 2 study: prospective cohort), 163 primary care outpatients (adolescents and adults) who had acute wheezing illnesses or chronic asthma were evaluated for C pneumoniae infection by serologic testing.19 A subgroup of this cohort also had nasopharyngeal cultures tested for C pneumoniae.
Twenty patients (12%) were given a diagnosis of C pneumoniae infection defined by serology (n = 15), culture isolation (n = 3), or both (n = 2). Of the 20, 10 wheezed for the first time—6 of whom subsequently developed chronic asthma (n = 5) or chronic bronchitis (n = 1), with a serologic profile suggesting chronic infection. The other 10 patients who had a diagnosis of C pneumoniae infection already had a diagnosis of chronic asthma. In patients with established chronic asthma, initial serologic findings suggested chronic, rather than acute, C pneumoniae infection.
Tx recommendations: When to consider azithromycin
Randomized7 and nonrandomized15 evidence supports treating severely uncontrolled or refractory asthma (strength of recommendation [SOR], B); no comparable randomized trials of azithromycin have been conducted for new-onset asthma (SOR, C). Consider prescribing empiric azithromycin for patients with new-onset asthma in the context of shared decision making about potential benefits, harms, and consequences of chronic asthma (SOR, C).
It is important to note that wheezing is frequently associated with uncomplicated acute bronchitis that resolves spontaneously without antibiotic treatment.11 Azithromycin treatment for new-onset asthma should therefore be reserved for patients in whom apparent uncomplicated acute bronchitis fails to resolve after 3 to 6 months, and whose illness is diagnosable as asthma (see CASE 3).10
Continue to: Do biomarkers predict response?
Do biomarkers predict response?
Confirming C pneumoniae infection by bronchoscopy before beginning treatment has been recommended20 but might be impractical; also, diagnostic testing for C pneumoniae is limited in availability and has potentially low sensitivity for diagnosing chronic deep lung infection.
So should you test for C pneumoniae biomarkers (or for biomarkers of Mycoplasma pneumoniae, another atypical infection implicated in the pathogenesis of asthma21) before initiating treatment? Azithromycin has antimicrobial, immunomodulatory, and potential antiviral properties.3 The body of evidence reviewed here indicates that the effects of macrolides on asthma might be, at least in part, antimicrobial. However, there is no direct evidence that the benefit of azithromycin in asthma is limited to patients who have positive infection biomarkers.22 Therefore, infection biomarker testing as a decision aid cannot be recommended at this time (although future research might alter this recommendation).
Acute bronchitis and asthma-onset associated with an acute lower respiratory tract infection have been statistically associated with biomarkers of C pneumoniae infection.23 However, C pneumoniae biomarkers are also prevalent in patients who have asthma that is not associated with an infectious onset.23 Several other matters are worth noting:
- C pneumoniae-specific IgA23 and IgE24 are promising biomarkers that deserve further investigation.
- M pneumoniae infection has also been associated with asthma and a response to antibiotic therapy.21,25
- Noneosinophilic severe asthma is another potential predictive characteristic.26 The applicability of this biomarker to primary care practice is limited, however, by the invasive nature of bronchoscopy and by the uncertain validity of the diagnostic concept: There is no guarantee that dynamic inflammatory infiltrates remain stable over a lifetime. Furthermore, the AMAZES Trial7 reported that azithromycin benefit was comparable in eosinophilic and noneosinophilic asthma.
Potential for harm withlong-term macrolide use?
Controversies about the role of macrolides in asthma involve uncertainty about who might benefit from treatment and the potential harms of macrolides use (TABLE 127,28 and discussed below).29
Adverse effects. The newer macrolides azithromycin and clarithromycin offer favorable safety and tolerability profiles, compared with those of older agents.30 In clinical trials of azithromycin, gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhea) were usually mild or moderate and rarely (< 2% of subjects) required discontinuation of study medication.31,32Clostridium difficile diarrhea has not been reported in any of the large clinical trials, in which thousands of patients received azithromycin for 3 to 12 months.31,32 The major clinical “side effects” attributable to azithromycin are a significant reduction, compared to placebo, in acute respiratory illness, bronchitis, pneumonia, and sinusitis.31,32
Continue to: Antibiotic resistance
Antibiotic resistance. Exposure of populations to macrolides can increase the percentage of macrolide-resistant bacterial respiratory pathogens33; policies aimed at decreasing inappropriate macrolide prescribing can significantly lower that percentage.34 There is no evidence, however, of any detrimental effects of macrolide resistance in individual patients receiving azithromycin.33
In trials of azithromycin for the treatment of trachoma in Africa, significantly fewer deaths occurred in villages where subjects were treated with azithromycin than in villages where azithromycin therapy was not provided.35 In the United States, weekly azithromycin treatment for 3 to 12 months in adults with heart disease resulted in fewer cases of acute bronchitis and pneumonia, compared with the placebo-treated groups31,32; similar benefit for azithromycin was seen in children who had recurrent lung infection.8,36
Nevertheless, concern over the spread of macrolide-resistant bacteria to the surrounding community is a concern and a possibility—and should be the subject of future research.
Sudden cardiac death. In a Medicaid population, the risk of sudden cardiac death from taking a macrolide among patients at high risk of cardiovascular disease was 1 in every 4000 administrations.27 Compare that level of risk with the 1 in 167 risk of an acute cardiovascular event in patients with COPD who start taking a LABA.37 There is no detectable increase in the risk of sudden cardiac death when taking azithromycin in the general (ie, average cardiovascular risk) population38,39 or when azithromycin is coadministered with a LABA.3
Hearing loss. An excess of 18 (< 1%) patients affected by hearing loss, 7 of whom sought medical attention, was reported among 2004 patients who had stable coronary artery disease and had been treated once weekly with azithromycin for 12 months (P = .02, compared with placebo).32 In another study, hearing test changes leading to discontinuation of azithromycin were detected in an excess of 32
Continue to: Physicians who prescribe...
Physicians who prescribe long-term azithromycin should instruct patients to report any hearing loss.
Drug–drug interactions. Azithromycin is free of the drug–drug interactions characteristic of conventional macrolides, such as clarithromycin.40 Nevertheless:
- Caution is advised when giving azithromycin in conjunction with coumadin or theophylline.
- Giving azithromycin with antacids that contain aluminum or magnesium salts can reduce the rate, although not the extent, of the absorption of azithromycin.
- The serum concentration of azithromycin is markedly increased when it is given with nelfinavir.40
Microbiome effects. The host microbiome can have a significant effect on the risk of asthma.2 A cross-sectional study indicated that lower respiratory bacterial burden is greater in patients with asthma, compared with that of healthy control subjects, and correlates with bronchial hyperresponsiveness.41 Early colonization of the infant nasopharynx, particularly with Streptococcus spp, is a predictor of asthma risk.42,43 Bacterial pathogens in the nasopharyngeal biome at the time of upper respiratory viral infection are significant determinants of risk for the spread of infection to the lower airways, suggesting that these microorganisms contribute to the risk of persistent asthma.41
Investigators have speculated that, rather than increasing the risk of asthma by disrupting the “healthy” microbiome, azithromycin might be helpful in treating an “unhealthy” microbiome.42,43 Recently, it was shown in a randomized trial that azithromycin induced a perturbation in the gut microbiota of children 14 days after randomization, although the drug did not have a long-lasting effect on the composition of gut microbiota.44
What about cost?
Inhaled corticosteroids and combination formulations of an ICS and a LABA are expensive and must be taken for the long term. A 3-month course of generic azithromycin—comparable to what was used in the OL subgroup of AZMATICS15—costs about as much as 1 ICS and LABA combination inhaler. Using published results,15,45 a pilot cost-effectiveness analysis in patients with persistent asthma compared doubling the ICS dosage, adding salmeterol, adding tiotropium, or prescribing 3 months of azithromycin. In the long run, azithromycin was 10 to 20 times as cost-effective as the other 3 therapeutic options for improving asthma quality-of-life outcomes.* However, reliable cost-effectiveness analyses require more, and better, evidence.
Continue to: Recommendations to reflect on for your practice
Recommendations to reflect on for your practice
Table 27,15 outlines selected long-term (≥ 3 months) macrolide dosing schedules in the management of asthma. Consider a trial of azithromycin for your patients
- whose asthma is refractory (poorly controlled persistent asthma), despite treatment with either an ICS and LABA combination or an ICS and long-acting muscarinic antagonist combination; and
- who have new-onset asthma.
Last, there is no evidence for or against prescribing azithromycin for patients who have chronic asthma that is not refractory but is uncontrolled because they are not being treated according to guidelines.
*Data available from the author upon request. See “Correspondence,” at end of article.
CORRESPONDENCE
David L. Hahn, MD, MS, Department of Family Medicine & Community Health, University of Wisconsin School of Medicine & Public Health, 1100 Delaplaine Court, Madison, WI 53715; dlhahn@wisc.edu.
1. Hahn DL. Role of Chlamydia pneumoniae as an inducer of asthma. In: Friedman H, Yamamoto Y, Bendinelli M, eds. Chlamydia Pneumoniae: Infection and Disease. New York: Kluwer Academic/Plenum Publishers; 2004:239-262.
2. Webley WC, Hahn DL. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir Res. 2017;18:98.
3. Wong EH, Porter JD, Edwards MR, et al. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014;2:657-670.
4. Reiter J, Demirel N, Mendy A, et al. Macrolides for the long-term management of asthma—a meta-analysis of randomized clinical trials. Allergy. 2013;68:1040-1049.
5. Kew KM, Undela K, Kotortsi I, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015(9):CD002997.
6. Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219-223.
7. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
8. Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19-26.
9. Korn S, Both J, Jung M, et al. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107:474-479.
10. Hahn DL. A Cure for Asthma? What Your Doctor Isn’t Telling You—and Why. Durham, North Carolina: Peoples Pharmacy Press; 2013.
11. Hahn DL. Acute asthmatic bronchitis: a new twist to an old problem. J Fam Pract. 1994;39:431-435.
12. Johnston SL, Blasi F, Black PN, et al; TELICAST Investigators. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589-1600.
13. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma: the AZALEA Randomized Clinical Trial. JAMA Intern Med. 2016;176:1630-1637.
14. Brusselle GG, Van Braeckel E. AZALEA trial highlights antibiotic overuse in acute asthma attacks. JAMA Intern Med. 2016;176:1637-1638.
15. Hahn DL, Grasmick M, Hetzel S, et al; AZMATICS (AZithroMycinAsthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012;25:442-459.
16. Hahn DL. An unanticipated effect of clinical trial registration. BMJ.com. November 2, 2007. https://www.bmj.com/rapid-response/2011/11/01/unanticipated-effect-clinical-trial-registration. Accessed November 2, 2019.
17. Hahn DL. Treatment of Chlamydia pneumoniae infection in adult asthma: a before-after trial. J Fam Pract. 1995;41:345-351.
18. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
19. Hahn DL, McDonald R. Can acute Chlamydia pneumoniae infection initiate chronic asthma? Ann Allergy Asthma Immunol. 1998;81:339-344.
20. Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Curr Allergy Asthma Rep. 2010;10:67-73.
21. Martin RJ, Kraft M, Chu HW, et al. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595-601.
22. Hahn DL, Plane MB, Mahdi OS, et al. Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma. PLoS Clin Trials. 2006;1:e11.
23. Hahn DL, Peeling RW, Dillon E, et al. Serologic markers for Chlamydia pneumoniae in asthma. Ann Allergy Asthma Immunol. 2000;84: 227-233.
24. Hahn DL, Schure A, Patel K, et al. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One. 2012;7:e35945.
25. Kraft M, Cassell GH, Pak J, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782-1788.
26. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
27. Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
28. Jespersen CM, Als-Nielsen B, Damgaard M, et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ. 2006;332:22-27.
29. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343-373.
30. Jackson LA, Stewart DK, Wang SP, et al. Safety and effect on antiChlamydia pneumoniae antibody titres of a 1 month course of daily azithromycin in adults with coronary artery disease. J Antimicrob Chemother. 1999;44:411-414.
31. O’Connor CM, Dunne MW, Pfeffer MA, et al; Investigators in the WIZARD Study. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459-1466.
32. Grayston JT, Kronmal RA, Jackson LA, et al; ACES Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637-1645.
33. Skalet AH, Cevallos V, Ayele B, et al. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med. 2010;7:e1000377.
34. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441-446.
35. Keenan JD, Emerson PM, Gaynor BD, et al. Adult mortality in a randomized trial of mass azithromycin for trachoma. JAMA Intern Med. 2013;173:821-833.
36. Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034-2044.
37. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178:229-238.
38. Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368:1704-1712.
39. Khosropour CM, Capizzi JD, Schafer SD, et al. Lack of association between azithromycin and death from cardiovascular causes. N Engl J Med. 2014;370:1961-1962.
40. Bakheit AH, Al-Hadiya BM, Abd-Elgalil AA. Azithromycin. Profiles Drug Subst Excip Relat Methodol. 2014;39:1-40.
41. Huang YJ, Nelson CE, Brodie EL, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372-381.e1-3.
42. Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978.
43. Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704-715.
44. Wei S, Mortensen MS, Stokholm J, et al. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: a double-blind, randomized, placebo-controlled trial. EBioMedicine. 2018;38:265-272.
45. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. New Engl J Med. 2010;363:1715-1726.
In vitro laboratory and in vivo animal models support the biologic plausibility that chronic infection is a potential cause of asthma.1,2 Arising from that hypothesis, macrolide antibiotics have been the subject of clinical trials and other studies to determine whether these drugs are efficacious in the long-term management of asthma in adults and children. Macrolides might also have immunomodulatory and antiviral properties that can benefit patients with asthma.3
This article looks at the evidence and clinical scenarios for the use of macrolides in asthma, provides proposed dosing schedules, and reviews associated concerns, including adverse effects, risk of bacterial resistance, and cost.
3 cases to consider
CASE 1 Paul D developed severe, refractory asthma at 30 years of age after an acute respiratory illness. At age 40, he was treated with 14 weekly doses of azithromycin. His asthma resolved slowly over 12 months.
Outcome. Mr. D has remained free of symptoms of asthma for more than 20 years.
CASE 2 Casey K developed severe wheezing at 18 months of age after an acute respiratory illness. Refractory asthma symptoms persisted until 6 years of age, at which time he was given 12 weekly doses of azithromycin. Asthma symptoms gradually resolved.
Outcome. Casey was able to resume normal physical activities, including competitive swimming.
CASE 3 Amy S, who had no history of respiratory problems, presented at 30 years of age with a 3-month history of wheezing and dyspnea after an acute respiratory illness. She was treated symptomatically with bronchodilators; wheezing failed to resolve. After 6 months of persistent wheezing that significantly affected her exercise capacity, Ms. S was given a diagnosis of persistent asthma and received 12 weekly doses of azithromycin.
[polldaddy:10475438]
Continue to: Outcome...
Outcome. Ms. S’s symptoms resolved completely within months.
Evidence of benefit of macrolides in asthma
These 3 cases, taken from my practice (but with names changed), demonstrate the therapeutic potential of macrolide antibiotics for patients with asthma under specific clinical circumstances. The cases are referenced again in the following examination of the literature on macrolides for asthma
SIDEBAR
Macrolides for Asthma: Registry of Clinical Experience
More information is needed about the “real world” effectiveness of antibiotic treatment for severe refractory and new-onset asthma. If you are a prescribing clinician who cares for patients with asthma and you are considering prescribing antibiotics for asthma, you are invited to document your outcomes by entering prospective, de-identified patient data into a human subjects committee-approved online registry. To gain access to the registry, and for more information, contact the author at dlhahn@wisc.edu or visit https://www.fammed.wisc.edu/wren/resources/macrolides-for-asthma/ .
Meta-analysis. Reiter et al4 performed a meta-analysis of 12 randomized clinical trials of macrolides for long-term management of asthma in children and adults. Prolonged treatment was defined as > 3 weeks of continuous administration of a macrolide. The pooled effect of macrolides on forced expiratory volume in 1 second (FEV1) was not significant; however, a significant effect on peak expiratory flow, symptom scores, quality of life, and airway hyperreactivity was observed.
Comment: The study’s authors concluded: “Macrolides may therefore be beneficial as adjunct asthma therapy. Future trials, focusing on long-term safety and effectiveness, should use standardized outcomes and procedures.”
Cochrane meta-analysis. Kew et al5 performed a meta-analysis of 23 studies of macrolides for managing chronic asthma for the Cochrane Database of Systematic Reviews. In their review, they reported
- no significant effects of macrolides on asthma exacerbations, asthma control, quality of life, and rescue medication use; and
- significant effects of macrolides for asthma symptoms and FEV1.
Continue to: Two within-study subgroup...
Two within-study subgroup analyses showed a possible benefit of macrolides for non-eosinophilic asthma, defined by a predominance of neutrophils in a bronchoalveolar lavage specimen. Kew et al5 noted that (1) most of the evidence examined in the review was of low quality and (2) inclusion criteria, interventions, and outcomes were highly variable.
Comment: The validity of a meta-analysis depends on the validity and similarity of underlying trials. Both meta-analyses just described were characterized by (1) grouping trials of older and newer macrolides and (2) significant selection bias in the underlying trials.
Selection bias is prevalent in asthma research and is a major contributor to uncertainty: Randomized controlled trials upon which guideline treatments are based have systematically excluded > 90% of people with asthma.6 Exclusions include past or current smoking, the asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome, severe asthma, and acute respiratory illness; these exclusion criteria have also been applied to studies of macrolides. Importantly, patients in the excluded groups are probably those most likely to respond to a macrolide.2 Pragmatic effectiveness studies (broad eligibility criteria, adequate duration of azithromycin treatment, a posttreatment observation period, and pre-specified biomarker subgroup analyses) have been recommended to address the hypothesis of what has been termed infectious asthma.2
Inconsistent evidence, the generally poor quality of underlying studies, and uncertainty about which subgroup(s) of asthma patients might benefit all contribute to a strength of recommendation of “B” for treating asthma with macrolides. Two recent randomized trials7,8 that were not included in the cited meta-analyses, along with other evidence,2 point to 2 groups of patients who are candidates for a trial of azithromycin: those with severe refractory asthma and those with new-onset asthma.
Clinical trial in adults. Gibson et al7 conducted a randomized, double-blind, placebo-controlled trial of azithromycin 500 mg 3 times a week or placebo for 1 year in 420 adults who had uncontrolled persistent asthma despite taking medium-to-high doses of an inhaled corticosteroid (ICS) plus a long-acting β agonist (LABA) (the AMAZES [Asthma and Macrolides: The Azithromycin Efficacy and Safety] trial; Level 1 study). The mean baseline asthma control questionnaire score was 1.5, equivalent to an Asthma Control Test (ACT) score* of 15.9
Continue to: Azithromycin reduced the frequency...
Azithromycin reduced the frequency of asthma exacerbations (to 1.07 per patient–year for azithromycin, compared with 1.86 per patient–year for placebo [incidence rate ratio = 0.59; 95% confidence interval (CI), 0.47-0.74]). The percentage of patients experiencing at least 1 exacerbation was reduced with azithromycin treatment (61% of patients in the placebo group experienced ≥ 1 exacerbation, compared with 44% in the azithromycin group [P < .0001; number needed to treat = 6]). Asthma quality of life was also improved by azithromycin (P = .001).
There was no significant difference between azithromycin and placebo in the overall rate of serious adverse events. Diarrhea that did not require treatment discontinuation was more common in patients treated with azithromycin (34%) than in the placebo group (19%). There was no posttreatment observation period to assess whether these azithromycin benefits waned or persisted after treatment was stopped.
Other evidence10 indicates that at least some patients who respond to azithromycin will experience persistent improvement after antibiotic treatment is completed (see CASE 1).
Pediatric clinical trial. Stokholm et al8 performed a randomized, double-blind, placebo-controlled trial of azithromycin in children 1 to 3 years of age who had been given a diagnosis of recurrent asthma-like symptoms (Level 1 study). Treatment was a 3-day course of azithromycin oral solution, 10 mg/kg/d, or placebo. Random allocation was performed for 158 asthma-like episodes in 72 children.
Azithromycin reduced the wheezing episode to a mean duration of 3.4 days, compared with 7.7 days for placebo (risk reduction = 63.3%; 95% CI, 56%-69.3% [P < .0001]). Effect size increased with early initiation of treatment: ie, an 83% reduction in episode duration was seen when treatment was initiated before Day 6 of the episode, compared with a 36% reduction if treatment was initiated on or after Day 6 (P < .0001).
Continue to: No differences between...
No differences between the randomized groups were observed in clinical adverse effects.
Comment: The brief course of azithromycin provided to patients in this trial did not have a significant impact on time to next episode of troublesome lung symptoms in individual children. Previous clinical observations have suggested that a longer duration of treatment (3-6 months) might be required to achieve lasting improvement or remission in selected patients with asthma (see CASE 2).10,11 The short-term benefit of azithromycin for acute wheezing is limited to children: Two comparable acute dosing trials in adults have shown little12 or no13 short-term benefit; however, these negative findings have been hypothesized to be the result of selection bias.14
Other evidence is worth examining
Other studies not included in the meta-analyses of randomized controlled trials provide additional evidence to support a recommendation of a trial of azithromycin in patients with severe, refractory, or new-onset asthma.
Nonrandomized controlled evidence. AZMATICS (AZithroMycin/Asthma Trial In Community Settings)15 is the sole randomized, double-blind, placebo-controlled trial of long-term azithromycin that included a 9-month posttreatment observation period. Seventy-five participants were randomized to receive a loading dose of 600 mg of azithromycin or placebo once daily for 3 days in Week 1. They then received either azithromycin 600 mg or placebo once weekly for 11 weeks. Posttreatment observation was performed until 48 weeks after randomization.
However, many eligible subjects, whom the principal investigator believed were ideal candidates for randomization, declined randomization because they did not want to risk receiving placebo. To accommodate those patients, the protocol was amended to include an open-label (OL) azithromycin arm, in which each participant’s personal physician prescribed azithromycin 750 mg for 11 weeks after a loading dose16 (OL cohort only, Level 2 study: controlled, nonrandomized, nonblinded). The OL group had (1) a higher baseline prevalence of severe, persistent asthma (32%) than the randomized group (8%) (P = .012); and (2) worse asthma quality of life than the randomized patients (P = .023). The OL group represented selection bias attributable to patient preference.
Continue to: The less severely...
The less severely affected randomized group of the trial did not exhibit significant effects attributable to azithromycin. The more severely affected OL cohort demonstrated significant, and large, azithromycin treatment effects for asthma symptoms, asthma quality of life, and asthma control (P < .05 for both groups; number needed to treat [NNT] = 3) that persisted during the posttreatment observation period.
Comment: The authors concluded: “Pending further randomized trials and given the relative safety of azithromycin and the significant disease burden from severe, refractory asthma, prescribing prolonged azithromycin therapy to patients with uncontrolled asthma may be considered by managing clinicians, particularly for patients who have failed to respond to conventional treatment and as an alternative to instituting immunomodulatory agents.”15
Before-and-after trial. Forty-six patients with moderate or severe chronic, persistent, stable asthma were selected as a cohort unlikely to experience spontaneous remission (ie, patients in exacerbation were excluded) (Level 2 study: prospective cohort).17 Subjects were treated for a median of 4 weeks (range, 3 to 9 weeks) with oral doxycycline, 100 mg bid; azithromycin, 1000 mg, once weekly; or erythromycin, 1000 mg/d in divided doses. Average duration of posttreatment follow-up was 6 months. All subjects were positive for antibodies to Chlamydia pneumoniae.
Four patients with diagnosed acuteC pneumoniae respiratory infection developed chronic asthma, which disappeared in each case after treatment. Of the other 42 seroreactive patients who were treated a mean of 6 years after they developed chronic asthma, 21 had either complete remission of asthma symptoms (n = 3) or major persistent clinical improvement (n = 18). Clinical improvement was more likely to occur in patients with early disease (P = .01) and before development of fixed airway obstruction (P < .01).
These results are consistent with the hypothesis that chronic infection of the lower respiratory tract contributes to the development and progression of asthma.17 Although clinical improvement was more likely in early asthma compared with asthma with fixed airway obstruction, improvement was nevertheless noted in the latter group.
Continue to: Physicians should also note...
Physicians should also note the landmark trial of azithromycin in severe, smoking-associated COPD, which found a clinically significant benefit in reducing exacerbations and improving quality of life (NNT = 3, to prevent 1 exacerbation).18
Case series. In a prospective case series (Level 2 study: prospective cohort), 163 primary care outpatients (adolescents and adults) who had acute wheezing illnesses or chronic asthma were evaluated for C pneumoniae infection by serologic testing.19 A subgroup of this cohort also had nasopharyngeal cultures tested for C pneumoniae.
Twenty patients (12%) were given a diagnosis of C pneumoniae infection defined by serology (n = 15), culture isolation (n = 3), or both (n = 2). Of the 20, 10 wheezed for the first time—6 of whom subsequently developed chronic asthma (n = 5) or chronic bronchitis (n = 1), with a serologic profile suggesting chronic infection. The other 10 patients who had a diagnosis of C pneumoniae infection already had a diagnosis of chronic asthma. In patients with established chronic asthma, initial serologic findings suggested chronic, rather than acute, C pneumoniae infection.
Tx recommendations: When to consider azithromycin
Randomized7 and nonrandomized15 evidence supports treating severely uncontrolled or refractory asthma (strength of recommendation [SOR], B); no comparable randomized trials of azithromycin have been conducted for new-onset asthma (SOR, C). Consider prescribing empiric azithromycin for patients with new-onset asthma in the context of shared decision making about potential benefits, harms, and consequences of chronic asthma (SOR, C).
It is important to note that wheezing is frequently associated with uncomplicated acute bronchitis that resolves spontaneously without antibiotic treatment.11 Azithromycin treatment for new-onset asthma should therefore be reserved for patients in whom apparent uncomplicated acute bronchitis fails to resolve after 3 to 6 months, and whose illness is diagnosable as asthma (see CASE 3).10
Continue to: Do biomarkers predict response?
Do biomarkers predict response?
Confirming C pneumoniae infection by bronchoscopy before beginning treatment has been recommended20 but might be impractical; also, diagnostic testing for C pneumoniae is limited in availability and has potentially low sensitivity for diagnosing chronic deep lung infection.
So should you test for C pneumoniae biomarkers (or for biomarkers of Mycoplasma pneumoniae, another atypical infection implicated in the pathogenesis of asthma21) before initiating treatment? Azithromycin has antimicrobial, immunomodulatory, and potential antiviral properties.3 The body of evidence reviewed here indicates that the effects of macrolides on asthma might be, at least in part, antimicrobial. However, there is no direct evidence that the benefit of azithromycin in asthma is limited to patients who have positive infection biomarkers.22 Therefore, infection biomarker testing as a decision aid cannot be recommended at this time (although future research might alter this recommendation).
Acute bronchitis and asthma-onset associated with an acute lower respiratory tract infection have been statistically associated with biomarkers of C pneumoniae infection.23 However, C pneumoniae biomarkers are also prevalent in patients who have asthma that is not associated with an infectious onset.23 Several other matters are worth noting:
- C pneumoniae-specific IgA23 and IgE24 are promising biomarkers that deserve further investigation.
- M pneumoniae infection has also been associated with asthma and a response to antibiotic therapy.21,25
- Noneosinophilic severe asthma is another potential predictive characteristic.26 The applicability of this biomarker to primary care practice is limited, however, by the invasive nature of bronchoscopy and by the uncertain validity of the diagnostic concept: There is no guarantee that dynamic inflammatory infiltrates remain stable over a lifetime. Furthermore, the AMAZES Trial7 reported that azithromycin benefit was comparable in eosinophilic and noneosinophilic asthma.
Potential for harm withlong-term macrolide use?
Controversies about the role of macrolides in asthma involve uncertainty about who might benefit from treatment and the potential harms of macrolides use (TABLE 127,28 and discussed below).29
Adverse effects. The newer macrolides azithromycin and clarithromycin offer favorable safety and tolerability profiles, compared with those of older agents.30 In clinical trials of azithromycin, gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhea) were usually mild or moderate and rarely (< 2% of subjects) required discontinuation of study medication.31,32Clostridium difficile diarrhea has not been reported in any of the large clinical trials, in which thousands of patients received azithromycin for 3 to 12 months.31,32 The major clinical “side effects” attributable to azithromycin are a significant reduction, compared to placebo, in acute respiratory illness, bronchitis, pneumonia, and sinusitis.31,32
Continue to: Antibiotic resistance
Antibiotic resistance. Exposure of populations to macrolides can increase the percentage of macrolide-resistant bacterial respiratory pathogens33; policies aimed at decreasing inappropriate macrolide prescribing can significantly lower that percentage.34 There is no evidence, however, of any detrimental effects of macrolide resistance in individual patients receiving azithromycin.33
In trials of azithromycin for the treatment of trachoma in Africa, significantly fewer deaths occurred in villages where subjects were treated with azithromycin than in villages where azithromycin therapy was not provided.35 In the United States, weekly azithromycin treatment for 3 to 12 months in adults with heart disease resulted in fewer cases of acute bronchitis and pneumonia, compared with the placebo-treated groups31,32; similar benefit for azithromycin was seen in children who had recurrent lung infection.8,36
Nevertheless, concern over the spread of macrolide-resistant bacteria to the surrounding community is a concern and a possibility—and should be the subject of future research.
Sudden cardiac death. In a Medicaid population, the risk of sudden cardiac death from taking a macrolide among patients at high risk of cardiovascular disease was 1 in every 4000 administrations.27 Compare that level of risk with the 1 in 167 risk of an acute cardiovascular event in patients with COPD who start taking a LABA.37 There is no detectable increase in the risk of sudden cardiac death when taking azithromycin in the general (ie, average cardiovascular risk) population38,39 or when azithromycin is coadministered with a LABA.3
Hearing loss. An excess of 18 (< 1%) patients affected by hearing loss, 7 of whom sought medical attention, was reported among 2004 patients who had stable coronary artery disease and had been treated once weekly with azithromycin for 12 months (P = .02, compared with placebo).32 In another study, hearing test changes leading to discontinuation of azithromycin were detected in an excess of 32
Continue to: Physicians who prescribe...
Physicians who prescribe long-term azithromycin should instruct patients to report any hearing loss.
Drug–drug interactions. Azithromycin is free of the drug–drug interactions characteristic of conventional macrolides, such as clarithromycin.40 Nevertheless:
- Caution is advised when giving azithromycin in conjunction with coumadin or theophylline.
- Giving azithromycin with antacids that contain aluminum or magnesium salts can reduce the rate, although not the extent, of the absorption of azithromycin.
- The serum concentration of azithromycin is markedly increased when it is given with nelfinavir.40
Microbiome effects. The host microbiome can have a significant effect on the risk of asthma.2 A cross-sectional study indicated that lower respiratory bacterial burden is greater in patients with asthma, compared with that of healthy control subjects, and correlates with bronchial hyperresponsiveness.41 Early colonization of the infant nasopharynx, particularly with Streptococcus spp, is a predictor of asthma risk.42,43 Bacterial pathogens in the nasopharyngeal biome at the time of upper respiratory viral infection are significant determinants of risk for the spread of infection to the lower airways, suggesting that these microorganisms contribute to the risk of persistent asthma.41
Investigators have speculated that, rather than increasing the risk of asthma by disrupting the “healthy” microbiome, azithromycin might be helpful in treating an “unhealthy” microbiome.42,43 Recently, it was shown in a randomized trial that azithromycin induced a perturbation in the gut microbiota of children 14 days after randomization, although the drug did not have a long-lasting effect on the composition of gut microbiota.44
What about cost?
Inhaled corticosteroids and combination formulations of an ICS and a LABA are expensive and must be taken for the long term. A 3-month course of generic azithromycin—comparable to what was used in the OL subgroup of AZMATICS15—costs about as much as 1 ICS and LABA combination inhaler. Using published results,15,45 a pilot cost-effectiveness analysis in patients with persistent asthma compared doubling the ICS dosage, adding salmeterol, adding tiotropium, or prescribing 3 months of azithromycin. In the long run, azithromycin was 10 to 20 times as cost-effective as the other 3 therapeutic options for improving asthma quality-of-life outcomes.* However, reliable cost-effectiveness analyses require more, and better, evidence.
Continue to: Recommendations to reflect on for your practice
Recommendations to reflect on for your practice
Table 27,15 outlines selected long-term (≥ 3 months) macrolide dosing schedules in the management of asthma. Consider a trial of azithromycin for your patients
- whose asthma is refractory (poorly controlled persistent asthma), despite treatment with either an ICS and LABA combination or an ICS and long-acting muscarinic antagonist combination; and
- who have new-onset asthma.
Last, there is no evidence for or against prescribing azithromycin for patients who have chronic asthma that is not refractory but is uncontrolled because they are not being treated according to guidelines.
*Data available from the author upon request. See “Correspondence,” at end of article.
CORRESPONDENCE
David L. Hahn, MD, MS, Department of Family Medicine & Community Health, University of Wisconsin School of Medicine & Public Health, 1100 Delaplaine Court, Madison, WI 53715; dlhahn@wisc.edu.
In vitro laboratory and in vivo animal models support the biologic plausibility that chronic infection is a potential cause of asthma.1,2 Arising from that hypothesis, macrolide antibiotics have been the subject of clinical trials and other studies to determine whether these drugs are efficacious in the long-term management of asthma in adults and children. Macrolides might also have immunomodulatory and antiviral properties that can benefit patients with asthma.3
This article looks at the evidence and clinical scenarios for the use of macrolides in asthma, provides proposed dosing schedules, and reviews associated concerns, including adverse effects, risk of bacterial resistance, and cost.
3 cases to consider
CASE 1 Paul D developed severe, refractory asthma at 30 years of age after an acute respiratory illness. At age 40, he was treated with 14 weekly doses of azithromycin. His asthma resolved slowly over 12 months.
Outcome. Mr. D has remained free of symptoms of asthma for more than 20 years.
CASE 2 Casey K developed severe wheezing at 18 months of age after an acute respiratory illness. Refractory asthma symptoms persisted until 6 years of age, at which time he was given 12 weekly doses of azithromycin. Asthma symptoms gradually resolved.
Outcome. Casey was able to resume normal physical activities, including competitive swimming.
CASE 3 Amy S, who had no history of respiratory problems, presented at 30 years of age with a 3-month history of wheezing and dyspnea after an acute respiratory illness. She was treated symptomatically with bronchodilators; wheezing failed to resolve. After 6 months of persistent wheezing that significantly affected her exercise capacity, Ms. S was given a diagnosis of persistent asthma and received 12 weekly doses of azithromycin.
[polldaddy:10475438]
Continue to: Outcome...
Outcome. Ms. S’s symptoms resolved completely within months.
Evidence of benefit of macrolides in asthma
These 3 cases, taken from my practice (but with names changed), demonstrate the therapeutic potential of macrolide antibiotics for patients with asthma under specific clinical circumstances. The cases are referenced again in the following examination of the literature on macrolides for asthma
SIDEBAR
Macrolides for Asthma: Registry of Clinical Experience
More information is needed about the “real world” effectiveness of antibiotic treatment for severe refractory and new-onset asthma. If you are a prescribing clinician who cares for patients with asthma and you are considering prescribing antibiotics for asthma, you are invited to document your outcomes by entering prospective, de-identified patient data into a human subjects committee-approved online registry. To gain access to the registry, and for more information, contact the author at dlhahn@wisc.edu or visit https://www.fammed.wisc.edu/wren/resources/macrolides-for-asthma/ .
Meta-analysis. Reiter et al4 performed a meta-analysis of 12 randomized clinical trials of macrolides for long-term management of asthma in children and adults. Prolonged treatment was defined as > 3 weeks of continuous administration of a macrolide. The pooled effect of macrolides on forced expiratory volume in 1 second (FEV1) was not significant; however, a significant effect on peak expiratory flow, symptom scores, quality of life, and airway hyperreactivity was observed.
Comment: The study’s authors concluded: “Macrolides may therefore be beneficial as adjunct asthma therapy. Future trials, focusing on long-term safety and effectiveness, should use standardized outcomes and procedures.”
Cochrane meta-analysis. Kew et al5 performed a meta-analysis of 23 studies of macrolides for managing chronic asthma for the Cochrane Database of Systematic Reviews. In their review, they reported
- no significant effects of macrolides on asthma exacerbations, asthma control, quality of life, and rescue medication use; and
- significant effects of macrolides for asthma symptoms and FEV1.
Continue to: Two within-study subgroup...
Two within-study subgroup analyses showed a possible benefit of macrolides for non-eosinophilic asthma, defined by a predominance of neutrophils in a bronchoalveolar lavage specimen. Kew et al5 noted that (1) most of the evidence examined in the review was of low quality and (2) inclusion criteria, interventions, and outcomes were highly variable.
Comment: The validity of a meta-analysis depends on the validity and similarity of underlying trials. Both meta-analyses just described were characterized by (1) grouping trials of older and newer macrolides and (2) significant selection bias in the underlying trials.
Selection bias is prevalent in asthma research and is a major contributor to uncertainty: Randomized controlled trials upon which guideline treatments are based have systematically excluded > 90% of people with asthma.6 Exclusions include past or current smoking, the asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome, severe asthma, and acute respiratory illness; these exclusion criteria have also been applied to studies of macrolides. Importantly, patients in the excluded groups are probably those most likely to respond to a macrolide.2 Pragmatic effectiveness studies (broad eligibility criteria, adequate duration of azithromycin treatment, a posttreatment observation period, and pre-specified biomarker subgroup analyses) have been recommended to address the hypothesis of what has been termed infectious asthma.2
Inconsistent evidence, the generally poor quality of underlying studies, and uncertainty about which subgroup(s) of asthma patients might benefit all contribute to a strength of recommendation of “B” for treating asthma with macrolides. Two recent randomized trials7,8 that were not included in the cited meta-analyses, along with other evidence,2 point to 2 groups of patients who are candidates for a trial of azithromycin: those with severe refractory asthma and those with new-onset asthma.
Clinical trial in adults. Gibson et al7 conducted a randomized, double-blind, placebo-controlled trial of azithromycin 500 mg 3 times a week or placebo for 1 year in 420 adults who had uncontrolled persistent asthma despite taking medium-to-high doses of an inhaled corticosteroid (ICS) plus a long-acting β agonist (LABA) (the AMAZES [Asthma and Macrolides: The Azithromycin Efficacy and Safety] trial; Level 1 study). The mean baseline asthma control questionnaire score was 1.5, equivalent to an Asthma Control Test (ACT) score* of 15.9
Continue to: Azithromycin reduced the frequency...
Azithromycin reduced the frequency of asthma exacerbations (to 1.07 per patient–year for azithromycin, compared with 1.86 per patient–year for placebo [incidence rate ratio = 0.59; 95% confidence interval (CI), 0.47-0.74]). The percentage of patients experiencing at least 1 exacerbation was reduced with azithromycin treatment (61% of patients in the placebo group experienced ≥ 1 exacerbation, compared with 44% in the azithromycin group [P < .0001; number needed to treat = 6]). Asthma quality of life was also improved by azithromycin (P = .001).
There was no significant difference between azithromycin and placebo in the overall rate of serious adverse events. Diarrhea that did not require treatment discontinuation was more common in patients treated with azithromycin (34%) than in the placebo group (19%). There was no posttreatment observation period to assess whether these azithromycin benefits waned or persisted after treatment was stopped.
Other evidence10 indicates that at least some patients who respond to azithromycin will experience persistent improvement after antibiotic treatment is completed (see CASE 1).
Pediatric clinical trial. Stokholm et al8 performed a randomized, double-blind, placebo-controlled trial of azithromycin in children 1 to 3 years of age who had been given a diagnosis of recurrent asthma-like symptoms (Level 1 study). Treatment was a 3-day course of azithromycin oral solution, 10 mg/kg/d, or placebo. Random allocation was performed for 158 asthma-like episodes in 72 children.
Azithromycin reduced the wheezing episode to a mean duration of 3.4 days, compared with 7.7 days for placebo (risk reduction = 63.3%; 95% CI, 56%-69.3% [P < .0001]). Effect size increased with early initiation of treatment: ie, an 83% reduction in episode duration was seen when treatment was initiated before Day 6 of the episode, compared with a 36% reduction if treatment was initiated on or after Day 6 (P < .0001).
Continue to: No differences between...
No differences between the randomized groups were observed in clinical adverse effects.
Comment: The brief course of azithromycin provided to patients in this trial did not have a significant impact on time to next episode of troublesome lung symptoms in individual children. Previous clinical observations have suggested that a longer duration of treatment (3-6 months) might be required to achieve lasting improvement or remission in selected patients with asthma (see CASE 2).10,11 The short-term benefit of azithromycin for acute wheezing is limited to children: Two comparable acute dosing trials in adults have shown little12 or no13 short-term benefit; however, these negative findings have been hypothesized to be the result of selection bias.14
Other evidence is worth examining
Other studies not included in the meta-analyses of randomized controlled trials provide additional evidence to support a recommendation of a trial of azithromycin in patients with severe, refractory, or new-onset asthma.
Nonrandomized controlled evidence. AZMATICS (AZithroMycin/Asthma Trial In Community Settings)15 is the sole randomized, double-blind, placebo-controlled trial of long-term azithromycin that included a 9-month posttreatment observation period. Seventy-five participants were randomized to receive a loading dose of 600 mg of azithromycin or placebo once daily for 3 days in Week 1. They then received either azithromycin 600 mg or placebo once weekly for 11 weeks. Posttreatment observation was performed until 48 weeks after randomization.
However, many eligible subjects, whom the principal investigator believed were ideal candidates for randomization, declined randomization because they did not want to risk receiving placebo. To accommodate those patients, the protocol was amended to include an open-label (OL) azithromycin arm, in which each participant’s personal physician prescribed azithromycin 750 mg for 11 weeks after a loading dose16 (OL cohort only, Level 2 study: controlled, nonrandomized, nonblinded). The OL group had (1) a higher baseline prevalence of severe, persistent asthma (32%) than the randomized group (8%) (P = .012); and (2) worse asthma quality of life than the randomized patients (P = .023). The OL group represented selection bias attributable to patient preference.
Continue to: The less severely...
The less severely affected randomized group of the trial did not exhibit significant effects attributable to azithromycin. The more severely affected OL cohort demonstrated significant, and large, azithromycin treatment effects for asthma symptoms, asthma quality of life, and asthma control (P < .05 for both groups; number needed to treat [NNT] = 3) that persisted during the posttreatment observation period.
Comment: The authors concluded: “Pending further randomized trials and given the relative safety of azithromycin and the significant disease burden from severe, refractory asthma, prescribing prolonged azithromycin therapy to patients with uncontrolled asthma may be considered by managing clinicians, particularly for patients who have failed to respond to conventional treatment and as an alternative to instituting immunomodulatory agents.”15
Before-and-after trial. Forty-six patients with moderate or severe chronic, persistent, stable asthma were selected as a cohort unlikely to experience spontaneous remission (ie, patients in exacerbation were excluded) (Level 2 study: prospective cohort).17 Subjects were treated for a median of 4 weeks (range, 3 to 9 weeks) with oral doxycycline, 100 mg bid; azithromycin, 1000 mg, once weekly; or erythromycin, 1000 mg/d in divided doses. Average duration of posttreatment follow-up was 6 months. All subjects were positive for antibodies to Chlamydia pneumoniae.
Four patients with diagnosed acuteC pneumoniae respiratory infection developed chronic asthma, which disappeared in each case after treatment. Of the other 42 seroreactive patients who were treated a mean of 6 years after they developed chronic asthma, 21 had either complete remission of asthma symptoms (n = 3) or major persistent clinical improvement (n = 18). Clinical improvement was more likely to occur in patients with early disease (P = .01) and before development of fixed airway obstruction (P < .01).
These results are consistent with the hypothesis that chronic infection of the lower respiratory tract contributes to the development and progression of asthma.17 Although clinical improvement was more likely in early asthma compared with asthma with fixed airway obstruction, improvement was nevertheless noted in the latter group.
Continue to: Physicians should also note...
Physicians should also note the landmark trial of azithromycin in severe, smoking-associated COPD, which found a clinically significant benefit in reducing exacerbations and improving quality of life (NNT = 3, to prevent 1 exacerbation).18
Case series. In a prospective case series (Level 2 study: prospective cohort), 163 primary care outpatients (adolescents and adults) who had acute wheezing illnesses or chronic asthma were evaluated for C pneumoniae infection by serologic testing.19 A subgroup of this cohort also had nasopharyngeal cultures tested for C pneumoniae.
Twenty patients (12%) were given a diagnosis of C pneumoniae infection defined by serology (n = 15), culture isolation (n = 3), or both (n = 2). Of the 20, 10 wheezed for the first time—6 of whom subsequently developed chronic asthma (n = 5) or chronic bronchitis (n = 1), with a serologic profile suggesting chronic infection. The other 10 patients who had a diagnosis of C pneumoniae infection already had a diagnosis of chronic asthma. In patients with established chronic asthma, initial serologic findings suggested chronic, rather than acute, C pneumoniae infection.
Tx recommendations: When to consider azithromycin
Randomized7 and nonrandomized15 evidence supports treating severely uncontrolled or refractory asthma (strength of recommendation [SOR], B); no comparable randomized trials of azithromycin have been conducted for new-onset asthma (SOR, C). Consider prescribing empiric azithromycin for patients with new-onset asthma in the context of shared decision making about potential benefits, harms, and consequences of chronic asthma (SOR, C).
It is important to note that wheezing is frequently associated with uncomplicated acute bronchitis that resolves spontaneously without antibiotic treatment.11 Azithromycin treatment for new-onset asthma should therefore be reserved for patients in whom apparent uncomplicated acute bronchitis fails to resolve after 3 to 6 months, and whose illness is diagnosable as asthma (see CASE 3).10
Continue to: Do biomarkers predict response?
Do biomarkers predict response?
Confirming C pneumoniae infection by bronchoscopy before beginning treatment has been recommended20 but might be impractical; also, diagnostic testing for C pneumoniae is limited in availability and has potentially low sensitivity for diagnosing chronic deep lung infection.
So should you test for C pneumoniae biomarkers (or for biomarkers of Mycoplasma pneumoniae, another atypical infection implicated in the pathogenesis of asthma21) before initiating treatment? Azithromycin has antimicrobial, immunomodulatory, and potential antiviral properties.3 The body of evidence reviewed here indicates that the effects of macrolides on asthma might be, at least in part, antimicrobial. However, there is no direct evidence that the benefit of azithromycin in asthma is limited to patients who have positive infection biomarkers.22 Therefore, infection biomarker testing as a decision aid cannot be recommended at this time (although future research might alter this recommendation).
Acute bronchitis and asthma-onset associated with an acute lower respiratory tract infection have been statistically associated with biomarkers of C pneumoniae infection.23 However, C pneumoniae biomarkers are also prevalent in patients who have asthma that is not associated with an infectious onset.23 Several other matters are worth noting:
- C pneumoniae-specific IgA23 and IgE24 are promising biomarkers that deserve further investigation.
- M pneumoniae infection has also been associated with asthma and a response to antibiotic therapy.21,25
- Noneosinophilic severe asthma is another potential predictive characteristic.26 The applicability of this biomarker to primary care practice is limited, however, by the invasive nature of bronchoscopy and by the uncertain validity of the diagnostic concept: There is no guarantee that dynamic inflammatory infiltrates remain stable over a lifetime. Furthermore, the AMAZES Trial7 reported that azithromycin benefit was comparable in eosinophilic and noneosinophilic asthma.
Potential for harm withlong-term macrolide use?
Controversies about the role of macrolides in asthma involve uncertainty about who might benefit from treatment and the potential harms of macrolides use (TABLE 127,28 and discussed below).29
Adverse effects. The newer macrolides azithromycin and clarithromycin offer favorable safety and tolerability profiles, compared with those of older agents.30 In clinical trials of azithromycin, gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhea) were usually mild or moderate and rarely (< 2% of subjects) required discontinuation of study medication.31,32Clostridium difficile diarrhea has not been reported in any of the large clinical trials, in which thousands of patients received azithromycin for 3 to 12 months.31,32 The major clinical “side effects” attributable to azithromycin are a significant reduction, compared to placebo, in acute respiratory illness, bronchitis, pneumonia, and sinusitis.31,32
Continue to: Antibiotic resistance
Antibiotic resistance. Exposure of populations to macrolides can increase the percentage of macrolide-resistant bacterial respiratory pathogens33; policies aimed at decreasing inappropriate macrolide prescribing can significantly lower that percentage.34 There is no evidence, however, of any detrimental effects of macrolide resistance in individual patients receiving azithromycin.33
In trials of azithromycin for the treatment of trachoma in Africa, significantly fewer deaths occurred in villages where subjects were treated with azithromycin than in villages where azithromycin therapy was not provided.35 In the United States, weekly azithromycin treatment for 3 to 12 months in adults with heart disease resulted in fewer cases of acute bronchitis and pneumonia, compared with the placebo-treated groups31,32; similar benefit for azithromycin was seen in children who had recurrent lung infection.8,36
Nevertheless, concern over the spread of macrolide-resistant bacteria to the surrounding community is a concern and a possibility—and should be the subject of future research.
Sudden cardiac death. In a Medicaid population, the risk of sudden cardiac death from taking a macrolide among patients at high risk of cardiovascular disease was 1 in every 4000 administrations.27 Compare that level of risk with the 1 in 167 risk of an acute cardiovascular event in patients with COPD who start taking a LABA.37 There is no detectable increase in the risk of sudden cardiac death when taking azithromycin in the general (ie, average cardiovascular risk) population38,39 or when azithromycin is coadministered with a LABA.3
Hearing loss. An excess of 18 (< 1%) patients affected by hearing loss, 7 of whom sought medical attention, was reported among 2004 patients who had stable coronary artery disease and had been treated once weekly with azithromycin for 12 months (P = .02, compared with placebo).32 In another study, hearing test changes leading to discontinuation of azithromycin were detected in an excess of 32
Continue to: Physicians who prescribe...
Physicians who prescribe long-term azithromycin should instruct patients to report any hearing loss.
Drug–drug interactions. Azithromycin is free of the drug–drug interactions characteristic of conventional macrolides, such as clarithromycin.40 Nevertheless:
- Caution is advised when giving azithromycin in conjunction with coumadin or theophylline.
- Giving azithromycin with antacids that contain aluminum or magnesium salts can reduce the rate, although not the extent, of the absorption of azithromycin.
- The serum concentration of azithromycin is markedly increased when it is given with nelfinavir.40
Microbiome effects. The host microbiome can have a significant effect on the risk of asthma.2 A cross-sectional study indicated that lower respiratory bacterial burden is greater in patients with asthma, compared with that of healthy control subjects, and correlates with bronchial hyperresponsiveness.41 Early colonization of the infant nasopharynx, particularly with Streptococcus spp, is a predictor of asthma risk.42,43 Bacterial pathogens in the nasopharyngeal biome at the time of upper respiratory viral infection are significant determinants of risk for the spread of infection to the lower airways, suggesting that these microorganisms contribute to the risk of persistent asthma.41
Investigators have speculated that, rather than increasing the risk of asthma by disrupting the “healthy” microbiome, azithromycin might be helpful in treating an “unhealthy” microbiome.42,43 Recently, it was shown in a randomized trial that azithromycin induced a perturbation in the gut microbiota of children 14 days after randomization, although the drug did not have a long-lasting effect on the composition of gut microbiota.44
What about cost?
Inhaled corticosteroids and combination formulations of an ICS and a LABA are expensive and must be taken for the long term. A 3-month course of generic azithromycin—comparable to what was used in the OL subgroup of AZMATICS15—costs about as much as 1 ICS and LABA combination inhaler. Using published results,15,45 a pilot cost-effectiveness analysis in patients with persistent asthma compared doubling the ICS dosage, adding salmeterol, adding tiotropium, or prescribing 3 months of azithromycin. In the long run, azithromycin was 10 to 20 times as cost-effective as the other 3 therapeutic options for improving asthma quality-of-life outcomes.* However, reliable cost-effectiveness analyses require more, and better, evidence.
Continue to: Recommendations to reflect on for your practice
Recommendations to reflect on for your practice
Table 27,15 outlines selected long-term (≥ 3 months) macrolide dosing schedules in the management of asthma. Consider a trial of azithromycin for your patients
- whose asthma is refractory (poorly controlled persistent asthma), despite treatment with either an ICS and LABA combination or an ICS and long-acting muscarinic antagonist combination; and
- who have new-onset asthma.
Last, there is no evidence for or against prescribing azithromycin for patients who have chronic asthma that is not refractory but is uncontrolled because they are not being treated according to guidelines.
*Data available from the author upon request. See “Correspondence,” at end of article.
CORRESPONDENCE
David L. Hahn, MD, MS, Department of Family Medicine & Community Health, University of Wisconsin School of Medicine & Public Health, 1100 Delaplaine Court, Madison, WI 53715; dlhahn@wisc.edu.
1. Hahn DL. Role of Chlamydia pneumoniae as an inducer of asthma. In: Friedman H, Yamamoto Y, Bendinelli M, eds. Chlamydia Pneumoniae: Infection and Disease. New York: Kluwer Academic/Plenum Publishers; 2004:239-262.
2. Webley WC, Hahn DL. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir Res. 2017;18:98.
3. Wong EH, Porter JD, Edwards MR, et al. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014;2:657-670.
4. Reiter J, Demirel N, Mendy A, et al. Macrolides for the long-term management of asthma—a meta-analysis of randomized clinical trials. Allergy. 2013;68:1040-1049.
5. Kew KM, Undela K, Kotortsi I, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015(9):CD002997.
6. Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219-223.
7. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
8. Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19-26.
9. Korn S, Both J, Jung M, et al. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107:474-479.
10. Hahn DL. A Cure for Asthma? What Your Doctor Isn’t Telling You—and Why. Durham, North Carolina: Peoples Pharmacy Press; 2013.
11. Hahn DL. Acute asthmatic bronchitis: a new twist to an old problem. J Fam Pract. 1994;39:431-435.
12. Johnston SL, Blasi F, Black PN, et al; TELICAST Investigators. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589-1600.
13. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma: the AZALEA Randomized Clinical Trial. JAMA Intern Med. 2016;176:1630-1637.
14. Brusselle GG, Van Braeckel E. AZALEA trial highlights antibiotic overuse in acute asthma attacks. JAMA Intern Med. 2016;176:1637-1638.
15. Hahn DL, Grasmick M, Hetzel S, et al; AZMATICS (AZithroMycinAsthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012;25:442-459.
16. Hahn DL. An unanticipated effect of clinical trial registration. BMJ.com. November 2, 2007. https://www.bmj.com/rapid-response/2011/11/01/unanticipated-effect-clinical-trial-registration. Accessed November 2, 2019.
17. Hahn DL. Treatment of Chlamydia pneumoniae infection in adult asthma: a before-after trial. J Fam Pract. 1995;41:345-351.
18. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
19. Hahn DL, McDonald R. Can acute Chlamydia pneumoniae infection initiate chronic asthma? Ann Allergy Asthma Immunol. 1998;81:339-344.
20. Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Curr Allergy Asthma Rep. 2010;10:67-73.
21. Martin RJ, Kraft M, Chu HW, et al. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595-601.
22. Hahn DL, Plane MB, Mahdi OS, et al. Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma. PLoS Clin Trials. 2006;1:e11.
23. Hahn DL, Peeling RW, Dillon E, et al. Serologic markers for Chlamydia pneumoniae in asthma. Ann Allergy Asthma Immunol. 2000;84: 227-233.
24. Hahn DL, Schure A, Patel K, et al. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One. 2012;7:e35945.
25. Kraft M, Cassell GH, Pak J, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782-1788.
26. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
27. Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
28. Jespersen CM, Als-Nielsen B, Damgaard M, et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ. 2006;332:22-27.
29. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343-373.
30. Jackson LA, Stewart DK, Wang SP, et al. Safety and effect on antiChlamydia pneumoniae antibody titres of a 1 month course of daily azithromycin in adults with coronary artery disease. J Antimicrob Chemother. 1999;44:411-414.
31. O’Connor CM, Dunne MW, Pfeffer MA, et al; Investigators in the WIZARD Study. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459-1466.
32. Grayston JT, Kronmal RA, Jackson LA, et al; ACES Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637-1645.
33. Skalet AH, Cevallos V, Ayele B, et al. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med. 2010;7:e1000377.
34. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441-446.
35. Keenan JD, Emerson PM, Gaynor BD, et al. Adult mortality in a randomized trial of mass azithromycin for trachoma. JAMA Intern Med. 2013;173:821-833.
36. Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034-2044.
37. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178:229-238.
38. Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368:1704-1712.
39. Khosropour CM, Capizzi JD, Schafer SD, et al. Lack of association between azithromycin and death from cardiovascular causes. N Engl J Med. 2014;370:1961-1962.
40. Bakheit AH, Al-Hadiya BM, Abd-Elgalil AA. Azithromycin. Profiles Drug Subst Excip Relat Methodol. 2014;39:1-40.
41. Huang YJ, Nelson CE, Brodie EL, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372-381.e1-3.
42. Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978.
43. Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704-715.
44. Wei S, Mortensen MS, Stokholm J, et al. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: a double-blind, randomized, placebo-controlled trial. EBioMedicine. 2018;38:265-272.
45. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. New Engl J Med. 2010;363:1715-1726.
1. Hahn DL. Role of Chlamydia pneumoniae as an inducer of asthma. In: Friedman H, Yamamoto Y, Bendinelli M, eds. Chlamydia Pneumoniae: Infection and Disease. New York: Kluwer Academic/Plenum Publishers; 2004:239-262.
2. Webley WC, Hahn DL. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir Res. 2017;18:98.
3. Wong EH, Porter JD, Edwards MR, et al. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014;2:657-670.
4. Reiter J, Demirel N, Mendy A, et al. Macrolides for the long-term management of asthma—a meta-analysis of randomized clinical trials. Allergy. 2013;68:1040-1049.
5. Kew KM, Undela K, Kotortsi I, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015(9):CD002997.
6. Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219-223.
7. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
8. Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19-26.
9. Korn S, Both J, Jung M, et al. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107:474-479.
10. Hahn DL. A Cure for Asthma? What Your Doctor Isn’t Telling You—and Why. Durham, North Carolina: Peoples Pharmacy Press; 2013.
11. Hahn DL. Acute asthmatic bronchitis: a new twist to an old problem. J Fam Pract. 1994;39:431-435.
12. Johnston SL, Blasi F, Black PN, et al; TELICAST Investigators. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589-1600.
13. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma: the AZALEA Randomized Clinical Trial. JAMA Intern Med. 2016;176:1630-1637.
14. Brusselle GG, Van Braeckel E. AZALEA trial highlights antibiotic overuse in acute asthma attacks. JAMA Intern Med. 2016;176:1637-1638.
15. Hahn DL, Grasmick M, Hetzel S, et al; AZMATICS (AZithroMycinAsthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012;25:442-459.
16. Hahn DL. An unanticipated effect of clinical trial registration. BMJ.com. November 2, 2007. https://www.bmj.com/rapid-response/2011/11/01/unanticipated-effect-clinical-trial-registration. Accessed November 2, 2019.
17. Hahn DL. Treatment of Chlamydia pneumoniae infection in adult asthma: a before-after trial. J Fam Pract. 1995;41:345-351.
18. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
19. Hahn DL, McDonald R. Can acute Chlamydia pneumoniae infection initiate chronic asthma? Ann Allergy Asthma Immunol. 1998;81:339-344.
20. Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Curr Allergy Asthma Rep. 2010;10:67-73.
21. Martin RJ, Kraft M, Chu HW, et al. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595-601.
22. Hahn DL, Plane MB, Mahdi OS, et al. Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma. PLoS Clin Trials. 2006;1:e11.
23. Hahn DL, Peeling RW, Dillon E, et al. Serologic markers for Chlamydia pneumoniae in asthma. Ann Allergy Asthma Immunol. 2000;84: 227-233.
24. Hahn DL, Schure A, Patel K, et al. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One. 2012;7:e35945.
25. Kraft M, Cassell GH, Pak J, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782-1788.
26. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
27. Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
28. Jespersen CM, Als-Nielsen B, Damgaard M, et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ. 2006;332:22-27.
29. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343-373.
30. Jackson LA, Stewart DK, Wang SP, et al. Safety and effect on antiChlamydia pneumoniae antibody titres of a 1 month course of daily azithromycin in adults with coronary artery disease. J Antimicrob Chemother. 1999;44:411-414.
31. O’Connor CM, Dunne MW, Pfeffer MA, et al; Investigators in the WIZARD Study. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459-1466.
32. Grayston JT, Kronmal RA, Jackson LA, et al; ACES Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637-1645.
33. Skalet AH, Cevallos V, Ayele B, et al. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med. 2010;7:e1000377.
34. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441-446.
35. Keenan JD, Emerson PM, Gaynor BD, et al. Adult mortality in a randomized trial of mass azithromycin for trachoma. JAMA Intern Med. 2013;173:821-833.
36. Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034-2044.
37. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178:229-238.
38. Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368:1704-1712.
39. Khosropour CM, Capizzi JD, Schafer SD, et al. Lack of association between azithromycin and death from cardiovascular causes. N Engl J Med. 2014;370:1961-1962.
40. Bakheit AH, Al-Hadiya BM, Abd-Elgalil AA. Azithromycin. Profiles Drug Subst Excip Relat Methodol. 2014;39:1-40.
41. Huang YJ, Nelson CE, Brodie EL, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372-381.e1-3.
42. Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978.
43. Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704-715.
44. Wei S, Mortensen MS, Stokholm J, et al. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: a double-blind, randomized, placebo-controlled trial. EBioMedicine. 2018;38:265-272.
45. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. New Engl J Med. 2010;363:1715-1726.
PRACTICE RECOMMENDATIONS
› Consider a trial of azithromycin for patients who have poorly controlled persistent asthma and are not responding to guideline treatment with the combination of an inhaled corticosteroid and either a long-acting bronchodilator or long-acting muscarinic antagonist. B
› Consider a trial of azithromycin in addition to first-line guideline therapy for patients who have new-onset asthma. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Head & neck cancers: What you’ll see, how to proceed
The statistics reveal a serious problem: This year, an estimated 63,030 Americans will be given a diagnosis of head and neck cancer (which includes laryngeal, oropharyngeal, sinonasal, nasopharyngeal, and salivary gland cancer1); approximately 13,360 of them will die. Furthermore, thyroid cancer is the most rapidly increasing cancer diagnosis in the United States, with an estimated 56,870 cases in 2017.1,2 Major risk factors for head and neck cancer are tobacco and alcohol exposure and infection with Epstein-Barr virus and human papillomavirus (HPV).3
In this article, we review the background for each of the principal types of head and neck cancer with which you should be familiar. We also discuss how to evaluate signs and symptoms that raise suspicion of these neoplasms; outline the diagnostic strategy in the face of such suspicion; and summarize accepted therapeutic approaches. Last, we describe the important role that you, the family physician, play in providing posttreatment care for these patients, especially prevention and management of late adverse effects of radiation therapy.
General characterizationsof these cancers
Approximately one-half of patients with head and neck cancer present initially with a nonspecific, persistent neck mass that should be deemed malignant until proven otherwise, because a delay in diagnosis is associated with a worse outcome.4 In a series of 100 patients with head and neck cancer, for example, delay in diagnosis occurred in nearly 25%—most often because of time spent providing inappropriate antibiotic treatment.5 Guidelines for management of neck masses recommend against the use of antibiotics in patients who do not have evidence of infection.6
Patients with a neck mass that has been present for longer than 2 weeks or that is ulcerated, fixed to underlying tissues, of firm consistency, or > 1.5 cm should have a physical examination that includes visualization of the base of tongue, pharynx, and larynx. The mass should be evaluated with fine-needle aspiration (FNA) biopsy, which has a positive predictive value of 96% and negative predictive value of 90% for the diagnosis of a head and neck mass. (Note: Anticoagulation therapy is not an absolute contraindication to FNA, which is not associated with an increased risk of bleeding.6)
Laryngeal cancer
What you need to know. More than 90% of laryngeal cancers are squamous cell carcinoma (SCC). Smoking or heavy drinking (> 8 drinks/d), compared to neither behavior, is associated with an increased risk of laryngeal cancer (odds ratio, 9.4 and 2.5, respectively).7 The risk of cancer is directly proportional to the degree of tobacco exposure.
Laryngeal cancer occurs in the supraglottic region in one-third of patients; in the glottic region in one-half; and in the subglottic region in a very few.8 Glottic cancer presents earlier than supraglottic cancer with hoarseness, whereas supraglottic cancer presents with more advanced disease, causing stridor, dysphagia, and throat pain. (Note: Guidelines recommend against prescribing acid suppressants in patients with hoarseness who do not have symptoms of reflux.9)
Stage 1 and Stage 2 laryngeal cancers are localized; Stages 3-4B are locally advanced or involve lymph nodes, or both; Stage 4C is metastatic disease. Overall, 60% of patients have Stage 3 or Stage 4 disease at diagnosis.10
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Laryngoscopy should be performed before computed tomography (CT) or magnetic resonance imaging is considered in a patient with hoarseness that does not resolve after 3 months—or sooner, if there is suspicion of malignancy.
How is it treated? Most patients presenting with Stage 1 or Stage 2 cancer can be treated with local radiation or, less commonly, larynx-preserving surgery. Patients with Stage 3 or Stage 4 disease can be treated with a combination of radiation and chemotherapy, which, compared to radiation alone, confers a decreased risk of local recurrence and increased laryngectomy-free survival.11 Patients whose vocal cords are destroyed or who have recurrence following radiation and chemotherapy might need total laryngectomy and formation of a tracheostomy and prosthetic for voice creation.
Five-year overall survival for Stage 1 and Stage 2 supraglottic and glottic cancers is 80%—lower, however, for later-presenting subglottic cancers.12
Oropharyngeal cancer
What you need to know. The lifetime risk for cancer of the oropharynx is approximately 1%.13 SCC is responsible for approximately 90% of these cancers. Early detection is important: The 5-year survival rate is more than twice as high for localized disease (83%) than it is for metastatic disease (39%) at detection.13
At any given time, 7% of the US population has HPV infection of the oropharynx. Most of these cases clear spontaneously, but persistent high-risk HPV infection led to a 225% increase in HPV-positive oropharyngeal SCC from 1988 to 2004.14 The representative case of HPV-positive oropharyngeal SCC is a middle-aged (40- to 59-year-old) white male with a history of multiple sexual partners and with little or no tobacco exposure and low alcohol consumption.
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Oral cancers present with a lesion, often ulcerative, that should be examined by palpation with a gloved finger to describe the presence, color, and number of lesions; any tenderness; tissue consistency (soft, firm, hard); and fixation to underlying structures.15 The oropharynx should be examined without protrusion of the tongue, which obscures the oropharynx and can make it harder to depress the posterior part of the tongue.
A finding of leukoplakia (white plaques) and erythroplakia (red plaques) of the oropharynx might reflect benign hyperkeratosis or premalignant lesions; the plaques do not wipe off on examination. Referral to a dentist or otorhinolaryngologist for biopsy is indicated for all erythroplakia and leukoplakia, and for ulcers that persist longer than 2 weeks.16
(Note: Evidence is insufficient to support screening asymptomatic patients for oral and oropharyngeal cancers by physical examination. There is no US Food and Drug Administration-approved screening test for oral HPV infection.17)
How is it treated? A diagnosis of moderate dysplasia or carcinoma in situ should be treated with surgical excision to clear margins followed by routine monitoring every 3 to 6 months, for life.18 Topical medication, electrocautery, laser ablation, and cryosurgery are management options for less severe dysplasia.
Sinonasal cancer
What you need to know. Worldwide, sinonasal cancer accounts for approximately 0.7% of all new cancers but demonstrates strong genetic and regional associations, particularly among the Cantonese population of southern China.19 One-half of new sinonasal malignancies are SCC; the rest are adenocarcinoma, lymphoepithelial carcinoma, and rare subtypes.20
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Presentation tends to mimic common, nonmalignant conditions, such as sinusitis, until invasion into adjacent structures. When sinonasal passages are involved, the history might include epistaxis or nasal discharge; facial or dental pain; unilateral nasal obstruction with unexplained onset later in life; and failure to respond to treatment of presumed rhinosinusitis. Physical examination should include assessment of cranial nerves, palpation of the sinuses, and anterior rhinoscopy.
Thin-cut CT of the paranasal sinuses is the first-line imaging study. Sinonasal endoscopy, with targeted biopsy of suspicious lesions, is the evaluation of choice when malignancy is suspected.
How is it treated? Surgery is the treatment of choice, with postoperative radiation for patients at higher risk of recurrence because of more extensive disase.12 Five-year survival for advanced disease is poor (35%); only 15% of cases are diagnosed at a localized stage because presenting symptoms are nonspecific.21
Nasopharyngeal cancer
What you need to know. Nasopharyngeal cancer is rare in the United States and Europe, compared with China, where it is endemic (and where a variety of risk factors, including intake of salt-preserved fish, have been proposed22). Epstein-Barr virus infection and a history of smoking increase the risk.
Patients with nasopharyngeal cancer can present with epistaxis, nasal obstruction, and auditory symptoms, such as serous otitis media. Direct extension of the tumor can lead to cranial-nerve palsy, most commonly III, V, VI, and XII.23
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Three-quarters of patients present with a neck mass from lymph-node metastases. Patients with the risk factors for nasopharyngeal cancer noted above who present with concerning symptoms should have nasoendoscopy with biopsy.
How is it treated? Radiation is the primary treatment, which is combined with chemotherapy for more advanced disease.23 Screening high-risk populations for antibodies to Epstein-Barr virus and performing nasopharyngeal endoscopy on patients who screen positive increases the detection rate of nasopharyngeal cancer; however, this strategy has not been shown to improve survival.9
Salivary gland tumors
What you need to know. Salivary gland neoplasms are a rare and heterogeneous entity, comprising 6% to 8% of head and neck cancers.24 More than 70% of these tumors are located in the parotid gland; 8%, in the submandibular glands; 1%, in the sublingual glands; and the rest, in the minor salivary glands. Most salivary gland tumors are benign; the most prevalent malignant tumors are mucoepidermoid carcinoma (30%) and adenoid cystic carcinoma (10%).25 Additional identified risk factors for a salivary gland tumor include irradiation, prior head and neck cancer, and environmental exposures, including hairdressing, rubber manufacturing, and exposure to nickel compounds.26
What is the diagnostic strategy? The history and physical exam are essential to distinguish a salivary gland tumor from an infectious cause and sialolithiasis. Parotid tumors most commonly present as asymptomatic parotid swelling, although pain can be present in as many as 40% of malignant parotid tumors.25 Facial nerve weakness is found in 25% of parotid tumors; although the differential diagnosis of facial nerve palsy is broad, suspicion of malignancy should be raised in the presence of a parotid mass, progressive unilateral symptoms, hemifacial spasm progressing to weakness, and a history of skin cancer on the face or scalp. Additional characteristics that favor a neoplastic cause are trismus and nontender lymphadenopathy.25
In contrast, sialolithiasis is associated with intermittent pain caused by eating and is more common in the settings of dehydration and poor dental hygiene. Sialadenitis should be suspected when the presentation is fever, increased pain and swelling, erythema, and expression of pus from the salivary gland.
Continue to: If malignancy is suspected...
If malignancy is suspected, the initial diagnostic evaluation should include ultrasonography (US); concurrent FNA biopsy should be performed if a mass is detected.27 US-guided FNA has a sensitivity of 73% to 86% for salivary neoplasm.7 CT and magnetic resonance imaging are useful for further characterization of tumors and can be advantageous for surgical planning.
How is it treated? Treatment of a salivary gland tumor involves surgical resection, followed by radiotherapy for patients in whom disease is more extensive or who exhibit high-risk pathology. Primary radiotherapy can be used in patients with an unresectable tumor. Typically, chemotherapy is used only for palliative purposes in relapsing disease, when a tumor is not amenable to radiotherapy, and in metastatic disease.25
Prognosis varies by histotype but is generally favorable. The survival rates for a malignant salivary gland tumor are 83% at 1 year, 69% at 3 years, and 65% at 5 years.28 Distant metastases are the most common cause of death, occurring primarily in the lungs (80%), bone (15%), and liver.27 Factors that indicate poor prognosis include facial nerve involvement, trismus, a tumor > 4 cm, bone involvement, nodal spread, and recurrence.25
Thyroid cancer
What you need to know. Thyroid cancer is the most rapidly increasing cancer diagnosis in the United States, with an annual incidence of 4.5%.1 In the United States, most thyroid cancers are differentiated thyroid cancer (DTC), which includes papillary and follicular cancers. Less-differentiated medullary thyroid cancer (MTC), typically associated with multiple endocrine neoplasia (MEN) 2A or 2B, and undifferentiated or anaplastic thyroid cancer are less common. The increasing incidence of thyroid cancer is primarily the result of an increase in nonclinically relevant DTC.
What is the diagnostic strategy? Thyroid cancer usually presents as a thyroid nodule found by the patient or incidentally on physical examination or imaging. Other presenting signs and symptoms include hoarseness, voice changes, and dysphagia.
Continue to: Thyroid US is the study of...
Thyroid US is the study of choice for initial evaluation of the size and features of a nodule; findings are used to make recommendations for further workup. If further evaluation is indicated, FNA biopsy is the test of choice.29
In 2016, the American Thyroid Association released updated guidelines for evaluating thyroid nodules (TABLE).30 The US Preventive Services Task Force recommends against screening for thyroid cancer by neck palpation or US in asymptomatic patients because evidence of significant mortality benefit is lacking.31
How is it treated? Treatment of thyroid cancer focuses on local excision of the nodule by partial or total thyroidectomy (depending on the size and type of cancer) and surgical removal of involved lymph nodes. Differentiated thyroid cancer is categorized as high-, medium-, or low-risk, depending on tumor extension, incomplete tumor resection, size of lymph nodes > 3 cm, and distant metastases. Adjuvant treatment with radioactive iodine can be considered for intermediate-risk DTC and is recommended for high-risk DTC.32
Following surgical treatment, thyroid-stimulating hormone suppression is recommended using levothyroxine.33 Patients at higher risk of recurrence should have longer and more intense suppression of thyroid-stimulating hormone.30 Levels of serum thyroglobulin and anti-thyroglobulin antibody should be followed postoperatively; rising values can indicate recurrent disease. The calcitonin level should be followed in patients with a history of MTC. Thyroid US should be performed 6 to 12 months postoperatively, then periodically, depending on determination of recurrence risk and any change in the thyroglobulin level.30
(Note: Glucagon-like peptide-1 [GLP-1] receptor agonists, used to treat type 2 diabetes mellitus, carry a black-box warning for their risk of MTC and are contraindicated in patients who have a personal or family history of MTC, MEN2A, or MEN2B.34)
Continue to: Anaplastic thyroid cancer...
Anaplastic thyroid cancer, a rare form of thyroid cancer, carries a high mortality rate, with a median survival of 5 months from diagnosis and 1-year survival of 20%. Patients require expeditious total thyroidectomy and neck dissection, followed by external-beam radiation with or without chemotherapy. If this strategy is not feasible, tracheostomy might be necessary to maintain a patent airway.2 Family physicians treating a patient who has anaplastic thyroid cancer can fulfill a crucial role by ensuring that an advance directive is established, a surrogate decision-maker is appointed, and goals of care are well defined.
Follow-up care for head and neck Ca
The risk of adverse effects after radiation therapy for head and neck cancer calls for close monitoring, appropriate treatment, and referral and counseling as needed. See “Follow-up care after treatment of head and neck cancer.” 35-39
SIDEBAR
Follow-up care after treatment of head and neck cancer35-39
Challenge: After radiation to the head and neck, as many as 53% of patients develop subclinical hypothyroidism and 33% develop clinical hypothyroidism.35Strategy: Measure the thyroid-stimulating hormone level within 1 year of the completion of radiotherapy and every 6 to 12 months thereafter.36
Challenge: Radiation to the head and neck can decrease the function of salivary glands, causing xerostomia in as many as 40% of patients. This condition can lead to problems with oral hygiene and difficulty with speech, eating, and swallowing.37Strategy:
- Treat xerostomia with artificial saliva, sugar-free candy and gum, or muscarinic cholinergic agonists, such as pilocarpine and cevimeline.
- Consider treatment with pilocarpine or cevimeline. Pilocarpine alleviates xerostomia in approximately 50% of patients who develop the condition, although its use can be limited by adverse cholinergic effects.3,7 Cevimeline causes fewer and less pronounced adverse effects than pilocarpine because it acts more specifically on receptors in the salivary glands.38
- Mention the possibility of acupuncture to your patients. There is evidence that it can stimulate salivary flow.39
Challenge: Patients who have had radiation to the head and neck have an increased risk of dental caries from xerostomia and the direct effect of radiation, which causes demineralization of teeth.
Strategy: Following radiation, instruct the patient about appropriate oral hygiene:
- regular flossing
- brushing and application of daily fluoride
- regular visits for dental care.39
Challenge: Trismus occurs in 5% to 25% of patients, depending on the type of radiation.36Strategy: Recommend exercise-based treatment, the treatment of choice. Surgery is indicated for severe cases.
Challenge: Dysphagia occurs in approximately 25% of patients treated with radiation.36Strategy: Provide a referral for swallowing exercises, which might be helpful. Some cases are severe enough to warrant placement of a feeding tube.37
Last, counsel all patients who have been treated for cancer of the head or neck, with any modality, about cessation of smoking and alcohol.
CORRESPONDENCE
Anne Mounsey, MD, Family Medicine Residency, The University of North Carolina at Chapel Hill, 590 Manning Dr., Chapel Hill, NC 27599; Anne_mounsey@med.unc.edu
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Smallridge RC, Ain KB, Asa SL, et al; American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104-1139.
3. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83:489-501.
4. Seoane J, Alvarez-Novoa P, Gomez I, et al. Early oral cancer diagnosis: The Aarhus statement perspective. A systematic review and meta-analysis. Head Neck. 2016;38(suppl 1):E2182-E2189.
5. Franco J, Elghouche AN, Harris MS, et al Diagnostic delays and errors in head and neck cancer patients: opportunities for improvement. Am J Med Qual. 2017;32:330-335.
6. Pynnonen MA, Gillespie MB, Roman B, et al. Clinical practice guideline: evaluation of the neck mass in adults. Otolaryngol Head Neck Surg. 2017;157(suppl 2):S1-S30.
7. Bosetti C, Gallus S, Franceschi S, et al. Cancer of the larynx in non-smoking alcohol drinkers and in non-drinking tobacco smokers. Br J Cancer. 2002;87:516-518.
8. Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116(9 pt 2 suppl 111):1-13.
9. Schwartz SR, Cohen SM, Dailey SH, et al. Clinical practice guideline: hoarseness (dysphonia). Otolaryngol Head Neck Surg. 2009;141(3 suppl 2):S1-S31.
10. Steuer CE, El-Deiry M, Parks JR, et al. An update on larynx cancer. CA Cancer J Clin. 2017;67:31-50.
11. Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091-2098.
12. Mendenhall WM, Werning JW, Hinerman RW, et al. Management of T1-T2 glottic carcinomas. Cancer. 2004;100:1786-1792.
13. Surveillance, Epidemiology, and End Results Unit. National Cancer Institute. Cancer stat facts: oral cavity and pharynx. https://seer.cancer.gov/statfacts/html/oralcav.html. Accessed October 18, 2019.
14. Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014;50:380-386.
15. Tarakji B, Gazal G, Al-Maweri SA, et al. Guideline for the diagnosis and treatment of recurrent aphthous stomatitis for dental practitioners. J Int Oral Health. 2015;7:74-80.
16. Siu A, Landon K, Ramos DM. Differential diagnosis and management of oral ulcers. Semin Cutan Med Surg. 2015;34:171-177.
17. US Preventive Services Task Force. Final recommendation statement: oral cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/oral-cancer-screening1. Updated November 2013. Accessed October 18, 2019.
18. Villa A, Woo SB. Leukoplakia—a diagnostic and management algorithm. J Oral Maxillofac Surg. 2017;75:723-734.
19. Yang S, Wu S, Zhou J, et al. Screening for nasopharyngeal cancer. Cochrane Database Syst Rev. 2015;(11):CD008423.
20. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877-885.
21. Ou SH, Zell JA, Ziogas A, et al. Epidemiology of nasopharyngeal carcinoma in the United States: improved survival of Chinese patients within the keratinizing squamous cell carcinoma histology. Ann Oncol. 2007;18:29-35.
22. Chang ET, Adami H-O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765-1777.
23. Chua MLK, Wee JTS, Hui EP, et al. Nasopharyngeal carcinoma. Lancet. 2016;387:1012-1024.
24. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177-184.
25. Lewis JS. Sinonasal squamous cell carcinoma: a review with emphasis on emerging histologic subtypes and the role of human papillomavirus. Head Neck Pathol. 2016;10:60-67.
26. Horn-Ross PL, Ljung BM, Morrow M. Environmental factors and the risk of salivary gland cancer. Epidemiology. 1997;8:414-419.
27. Colella G, Cannavale R, Flamminio F, et al. Fine-needle aspiration cytology of salivary gland lesions: a systematic review. J Oral Maxillofac Surg. 2010;68:2146-2153.
28. Berrino F, De Angelis R, Sant M, et al; EUROCARE Working Group. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995-99: results of the EUROCARE-4 study. Lancet Oncol. 2007;8:773-783.
29. Baloch ZW, LiVolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425-437.
30. Haugen BR, Alexander EK, Bible KC, et al; The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133.
31. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for thyroid Cancer: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:1882-1887.
32. Jonklaas J, Cooper DS, Ain KB, et al; National Thyroid Cancer Treatment Cooperative Study Group. Radioiodine therapy in patients with stage I differentiated thyroid cancer. Thyroid. 2010;20:1423-1424.
33. Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid. 1998;8:737-744.
34. US Food and Drug Administration. Highlight of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125431s020lbl.pdf. Updated December 2017. Accessed October 30, 1019.
35. Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol. 2011;99:1-5.
36. The development of quality of care measures for oral cavity cancer. Arch Otolaryngol Head Neck Surg. 2008;134:672.
37. Strojan P, Hutcheson KA, Eisbruch A, et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat Rev. 2017;59:79-92.
38. Chambers MS, Posner M, Jones CU, et al. Cevimeline for the treatment of postirradiation xerostomia in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:1102-1109.
39. Gupta N, Pal M, Rawat S, et al. Radiation-induced dental caries, prevention and treatment - a systematic review. Natl J Maxillofac Surg. 2015;6:160-166.
The statistics reveal a serious problem: This year, an estimated 63,030 Americans will be given a diagnosis of head and neck cancer (which includes laryngeal, oropharyngeal, sinonasal, nasopharyngeal, and salivary gland cancer1); approximately 13,360 of them will die. Furthermore, thyroid cancer is the most rapidly increasing cancer diagnosis in the United States, with an estimated 56,870 cases in 2017.1,2 Major risk factors for head and neck cancer are tobacco and alcohol exposure and infection with Epstein-Barr virus and human papillomavirus (HPV).3
In this article, we review the background for each of the principal types of head and neck cancer with which you should be familiar. We also discuss how to evaluate signs and symptoms that raise suspicion of these neoplasms; outline the diagnostic strategy in the face of such suspicion; and summarize accepted therapeutic approaches. Last, we describe the important role that you, the family physician, play in providing posttreatment care for these patients, especially prevention and management of late adverse effects of radiation therapy.
General characterizationsof these cancers
Approximately one-half of patients with head and neck cancer present initially with a nonspecific, persistent neck mass that should be deemed malignant until proven otherwise, because a delay in diagnosis is associated with a worse outcome.4 In a series of 100 patients with head and neck cancer, for example, delay in diagnosis occurred in nearly 25%—most often because of time spent providing inappropriate antibiotic treatment.5 Guidelines for management of neck masses recommend against the use of antibiotics in patients who do not have evidence of infection.6
Patients with a neck mass that has been present for longer than 2 weeks or that is ulcerated, fixed to underlying tissues, of firm consistency, or > 1.5 cm should have a physical examination that includes visualization of the base of tongue, pharynx, and larynx. The mass should be evaluated with fine-needle aspiration (FNA) biopsy, which has a positive predictive value of 96% and negative predictive value of 90% for the diagnosis of a head and neck mass. (Note: Anticoagulation therapy is not an absolute contraindication to FNA, which is not associated with an increased risk of bleeding.6)
Laryngeal cancer
What you need to know. More than 90% of laryngeal cancers are squamous cell carcinoma (SCC). Smoking or heavy drinking (> 8 drinks/d), compared to neither behavior, is associated with an increased risk of laryngeal cancer (odds ratio, 9.4 and 2.5, respectively).7 The risk of cancer is directly proportional to the degree of tobacco exposure.
Laryngeal cancer occurs in the supraglottic region in one-third of patients; in the glottic region in one-half; and in the subglottic region in a very few.8 Glottic cancer presents earlier than supraglottic cancer with hoarseness, whereas supraglottic cancer presents with more advanced disease, causing stridor, dysphagia, and throat pain. (Note: Guidelines recommend against prescribing acid suppressants in patients with hoarseness who do not have symptoms of reflux.9)
Stage 1 and Stage 2 laryngeal cancers are localized; Stages 3-4B are locally advanced or involve lymph nodes, or both; Stage 4C is metastatic disease. Overall, 60% of patients have Stage 3 or Stage 4 disease at diagnosis.10
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Laryngoscopy should be performed before computed tomography (CT) or magnetic resonance imaging is considered in a patient with hoarseness that does not resolve after 3 months—or sooner, if there is suspicion of malignancy.
How is it treated? Most patients presenting with Stage 1 or Stage 2 cancer can be treated with local radiation or, less commonly, larynx-preserving surgery. Patients with Stage 3 or Stage 4 disease can be treated with a combination of radiation and chemotherapy, which, compared to radiation alone, confers a decreased risk of local recurrence and increased laryngectomy-free survival.11 Patients whose vocal cords are destroyed or who have recurrence following radiation and chemotherapy might need total laryngectomy and formation of a tracheostomy and prosthetic for voice creation.
Five-year overall survival for Stage 1 and Stage 2 supraglottic and glottic cancers is 80%—lower, however, for later-presenting subglottic cancers.12
Oropharyngeal cancer
What you need to know. The lifetime risk for cancer of the oropharynx is approximately 1%.13 SCC is responsible for approximately 90% of these cancers. Early detection is important: The 5-year survival rate is more than twice as high for localized disease (83%) than it is for metastatic disease (39%) at detection.13
At any given time, 7% of the US population has HPV infection of the oropharynx. Most of these cases clear spontaneously, but persistent high-risk HPV infection led to a 225% increase in HPV-positive oropharyngeal SCC from 1988 to 2004.14 The representative case of HPV-positive oropharyngeal SCC is a middle-aged (40- to 59-year-old) white male with a history of multiple sexual partners and with little or no tobacco exposure and low alcohol consumption.
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Oral cancers present with a lesion, often ulcerative, that should be examined by palpation with a gloved finger to describe the presence, color, and number of lesions; any tenderness; tissue consistency (soft, firm, hard); and fixation to underlying structures.15 The oropharynx should be examined without protrusion of the tongue, which obscures the oropharynx and can make it harder to depress the posterior part of the tongue.
A finding of leukoplakia (white plaques) and erythroplakia (red plaques) of the oropharynx might reflect benign hyperkeratosis or premalignant lesions; the plaques do not wipe off on examination. Referral to a dentist or otorhinolaryngologist for biopsy is indicated for all erythroplakia and leukoplakia, and for ulcers that persist longer than 2 weeks.16
(Note: Evidence is insufficient to support screening asymptomatic patients for oral and oropharyngeal cancers by physical examination. There is no US Food and Drug Administration-approved screening test for oral HPV infection.17)
How is it treated? A diagnosis of moderate dysplasia or carcinoma in situ should be treated with surgical excision to clear margins followed by routine monitoring every 3 to 6 months, for life.18 Topical medication, electrocautery, laser ablation, and cryosurgery are management options for less severe dysplasia.
Sinonasal cancer
What you need to know. Worldwide, sinonasal cancer accounts for approximately 0.7% of all new cancers but demonstrates strong genetic and regional associations, particularly among the Cantonese population of southern China.19 One-half of new sinonasal malignancies are SCC; the rest are adenocarcinoma, lymphoepithelial carcinoma, and rare subtypes.20
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Presentation tends to mimic common, nonmalignant conditions, such as sinusitis, until invasion into adjacent structures. When sinonasal passages are involved, the history might include epistaxis or nasal discharge; facial or dental pain; unilateral nasal obstruction with unexplained onset later in life; and failure to respond to treatment of presumed rhinosinusitis. Physical examination should include assessment of cranial nerves, palpation of the sinuses, and anterior rhinoscopy.
Thin-cut CT of the paranasal sinuses is the first-line imaging study. Sinonasal endoscopy, with targeted biopsy of suspicious lesions, is the evaluation of choice when malignancy is suspected.
How is it treated? Surgery is the treatment of choice, with postoperative radiation for patients at higher risk of recurrence because of more extensive disase.12 Five-year survival for advanced disease is poor (35%); only 15% of cases are diagnosed at a localized stage because presenting symptoms are nonspecific.21
Nasopharyngeal cancer
What you need to know. Nasopharyngeal cancer is rare in the United States and Europe, compared with China, where it is endemic (and where a variety of risk factors, including intake of salt-preserved fish, have been proposed22). Epstein-Barr virus infection and a history of smoking increase the risk.
Patients with nasopharyngeal cancer can present with epistaxis, nasal obstruction, and auditory symptoms, such as serous otitis media. Direct extension of the tumor can lead to cranial-nerve palsy, most commonly III, V, VI, and XII.23
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Three-quarters of patients present with a neck mass from lymph-node metastases. Patients with the risk factors for nasopharyngeal cancer noted above who present with concerning symptoms should have nasoendoscopy with biopsy.
How is it treated? Radiation is the primary treatment, which is combined with chemotherapy for more advanced disease.23 Screening high-risk populations for antibodies to Epstein-Barr virus and performing nasopharyngeal endoscopy on patients who screen positive increases the detection rate of nasopharyngeal cancer; however, this strategy has not been shown to improve survival.9
Salivary gland tumors
What you need to know. Salivary gland neoplasms are a rare and heterogeneous entity, comprising 6% to 8% of head and neck cancers.24 More than 70% of these tumors are located in the parotid gland; 8%, in the submandibular glands; 1%, in the sublingual glands; and the rest, in the minor salivary glands. Most salivary gland tumors are benign; the most prevalent malignant tumors are mucoepidermoid carcinoma (30%) and adenoid cystic carcinoma (10%).25 Additional identified risk factors for a salivary gland tumor include irradiation, prior head and neck cancer, and environmental exposures, including hairdressing, rubber manufacturing, and exposure to nickel compounds.26
What is the diagnostic strategy? The history and physical exam are essential to distinguish a salivary gland tumor from an infectious cause and sialolithiasis. Parotid tumors most commonly present as asymptomatic parotid swelling, although pain can be present in as many as 40% of malignant parotid tumors.25 Facial nerve weakness is found in 25% of parotid tumors; although the differential diagnosis of facial nerve palsy is broad, suspicion of malignancy should be raised in the presence of a parotid mass, progressive unilateral symptoms, hemifacial spasm progressing to weakness, and a history of skin cancer on the face or scalp. Additional characteristics that favor a neoplastic cause are trismus and nontender lymphadenopathy.25
In contrast, sialolithiasis is associated with intermittent pain caused by eating and is more common in the settings of dehydration and poor dental hygiene. Sialadenitis should be suspected when the presentation is fever, increased pain and swelling, erythema, and expression of pus from the salivary gland.
Continue to: If malignancy is suspected...
If malignancy is suspected, the initial diagnostic evaluation should include ultrasonography (US); concurrent FNA biopsy should be performed if a mass is detected.27 US-guided FNA has a sensitivity of 73% to 86% for salivary neoplasm.7 CT and magnetic resonance imaging are useful for further characterization of tumors and can be advantageous for surgical planning.
How is it treated? Treatment of a salivary gland tumor involves surgical resection, followed by radiotherapy for patients in whom disease is more extensive or who exhibit high-risk pathology. Primary radiotherapy can be used in patients with an unresectable tumor. Typically, chemotherapy is used only for palliative purposes in relapsing disease, when a tumor is not amenable to radiotherapy, and in metastatic disease.25
Prognosis varies by histotype but is generally favorable. The survival rates for a malignant salivary gland tumor are 83% at 1 year, 69% at 3 years, and 65% at 5 years.28 Distant metastases are the most common cause of death, occurring primarily in the lungs (80%), bone (15%), and liver.27 Factors that indicate poor prognosis include facial nerve involvement, trismus, a tumor > 4 cm, bone involvement, nodal spread, and recurrence.25
Thyroid cancer
What you need to know. Thyroid cancer is the most rapidly increasing cancer diagnosis in the United States, with an annual incidence of 4.5%.1 In the United States, most thyroid cancers are differentiated thyroid cancer (DTC), which includes papillary and follicular cancers. Less-differentiated medullary thyroid cancer (MTC), typically associated with multiple endocrine neoplasia (MEN) 2A or 2B, and undifferentiated or anaplastic thyroid cancer are less common. The increasing incidence of thyroid cancer is primarily the result of an increase in nonclinically relevant DTC.
What is the diagnostic strategy? Thyroid cancer usually presents as a thyroid nodule found by the patient or incidentally on physical examination or imaging. Other presenting signs and symptoms include hoarseness, voice changes, and dysphagia.
Continue to: Thyroid US is the study of...
Thyroid US is the study of choice for initial evaluation of the size and features of a nodule; findings are used to make recommendations for further workup. If further evaluation is indicated, FNA biopsy is the test of choice.29
In 2016, the American Thyroid Association released updated guidelines for evaluating thyroid nodules (TABLE).30 The US Preventive Services Task Force recommends against screening for thyroid cancer by neck palpation or US in asymptomatic patients because evidence of significant mortality benefit is lacking.31
How is it treated? Treatment of thyroid cancer focuses on local excision of the nodule by partial or total thyroidectomy (depending on the size and type of cancer) and surgical removal of involved lymph nodes. Differentiated thyroid cancer is categorized as high-, medium-, or low-risk, depending on tumor extension, incomplete tumor resection, size of lymph nodes > 3 cm, and distant metastases. Adjuvant treatment with radioactive iodine can be considered for intermediate-risk DTC and is recommended for high-risk DTC.32
Following surgical treatment, thyroid-stimulating hormone suppression is recommended using levothyroxine.33 Patients at higher risk of recurrence should have longer and more intense suppression of thyroid-stimulating hormone.30 Levels of serum thyroglobulin and anti-thyroglobulin antibody should be followed postoperatively; rising values can indicate recurrent disease. The calcitonin level should be followed in patients with a history of MTC. Thyroid US should be performed 6 to 12 months postoperatively, then periodically, depending on determination of recurrence risk and any change in the thyroglobulin level.30
(Note: Glucagon-like peptide-1 [GLP-1] receptor agonists, used to treat type 2 diabetes mellitus, carry a black-box warning for their risk of MTC and are contraindicated in patients who have a personal or family history of MTC, MEN2A, or MEN2B.34)
Continue to: Anaplastic thyroid cancer...
Anaplastic thyroid cancer, a rare form of thyroid cancer, carries a high mortality rate, with a median survival of 5 months from diagnosis and 1-year survival of 20%. Patients require expeditious total thyroidectomy and neck dissection, followed by external-beam radiation with or without chemotherapy. If this strategy is not feasible, tracheostomy might be necessary to maintain a patent airway.2 Family physicians treating a patient who has anaplastic thyroid cancer can fulfill a crucial role by ensuring that an advance directive is established, a surrogate decision-maker is appointed, and goals of care are well defined.
Follow-up care for head and neck Ca
The risk of adverse effects after radiation therapy for head and neck cancer calls for close monitoring, appropriate treatment, and referral and counseling as needed. See “Follow-up care after treatment of head and neck cancer.” 35-39
SIDEBAR
Follow-up care after treatment of head and neck cancer35-39
Challenge: After radiation to the head and neck, as many as 53% of patients develop subclinical hypothyroidism and 33% develop clinical hypothyroidism.35Strategy: Measure the thyroid-stimulating hormone level within 1 year of the completion of radiotherapy and every 6 to 12 months thereafter.36
Challenge: Radiation to the head and neck can decrease the function of salivary glands, causing xerostomia in as many as 40% of patients. This condition can lead to problems with oral hygiene and difficulty with speech, eating, and swallowing.37Strategy:
- Treat xerostomia with artificial saliva, sugar-free candy and gum, or muscarinic cholinergic agonists, such as pilocarpine and cevimeline.
- Consider treatment with pilocarpine or cevimeline. Pilocarpine alleviates xerostomia in approximately 50% of patients who develop the condition, although its use can be limited by adverse cholinergic effects.3,7 Cevimeline causes fewer and less pronounced adverse effects than pilocarpine because it acts more specifically on receptors in the salivary glands.38
- Mention the possibility of acupuncture to your patients. There is evidence that it can stimulate salivary flow.39
Challenge: Patients who have had radiation to the head and neck have an increased risk of dental caries from xerostomia and the direct effect of radiation, which causes demineralization of teeth.
Strategy: Following radiation, instruct the patient about appropriate oral hygiene:
- regular flossing
- brushing and application of daily fluoride
- regular visits for dental care.39
Challenge: Trismus occurs in 5% to 25% of patients, depending on the type of radiation.36Strategy: Recommend exercise-based treatment, the treatment of choice. Surgery is indicated for severe cases.
Challenge: Dysphagia occurs in approximately 25% of patients treated with radiation.36Strategy: Provide a referral for swallowing exercises, which might be helpful. Some cases are severe enough to warrant placement of a feeding tube.37
Last, counsel all patients who have been treated for cancer of the head or neck, with any modality, about cessation of smoking and alcohol.
CORRESPONDENCE
Anne Mounsey, MD, Family Medicine Residency, The University of North Carolina at Chapel Hill, 590 Manning Dr., Chapel Hill, NC 27599; Anne_mounsey@med.unc.edu
The statistics reveal a serious problem: This year, an estimated 63,030 Americans will be given a diagnosis of head and neck cancer (which includes laryngeal, oropharyngeal, sinonasal, nasopharyngeal, and salivary gland cancer1); approximately 13,360 of them will die. Furthermore, thyroid cancer is the most rapidly increasing cancer diagnosis in the United States, with an estimated 56,870 cases in 2017.1,2 Major risk factors for head and neck cancer are tobacco and alcohol exposure and infection with Epstein-Barr virus and human papillomavirus (HPV).3
In this article, we review the background for each of the principal types of head and neck cancer with which you should be familiar. We also discuss how to evaluate signs and symptoms that raise suspicion of these neoplasms; outline the diagnostic strategy in the face of such suspicion; and summarize accepted therapeutic approaches. Last, we describe the important role that you, the family physician, play in providing posttreatment care for these patients, especially prevention and management of late adverse effects of radiation therapy.
General characterizationsof these cancers
Approximately one-half of patients with head and neck cancer present initially with a nonspecific, persistent neck mass that should be deemed malignant until proven otherwise, because a delay in diagnosis is associated with a worse outcome.4 In a series of 100 patients with head and neck cancer, for example, delay in diagnosis occurred in nearly 25%—most often because of time spent providing inappropriate antibiotic treatment.5 Guidelines for management of neck masses recommend against the use of antibiotics in patients who do not have evidence of infection.6
Patients with a neck mass that has been present for longer than 2 weeks or that is ulcerated, fixed to underlying tissues, of firm consistency, or > 1.5 cm should have a physical examination that includes visualization of the base of tongue, pharynx, and larynx. The mass should be evaluated with fine-needle aspiration (FNA) biopsy, which has a positive predictive value of 96% and negative predictive value of 90% for the diagnosis of a head and neck mass. (Note: Anticoagulation therapy is not an absolute contraindication to FNA, which is not associated with an increased risk of bleeding.6)
Laryngeal cancer
What you need to know. More than 90% of laryngeal cancers are squamous cell carcinoma (SCC). Smoking or heavy drinking (> 8 drinks/d), compared to neither behavior, is associated with an increased risk of laryngeal cancer (odds ratio, 9.4 and 2.5, respectively).7 The risk of cancer is directly proportional to the degree of tobacco exposure.
Laryngeal cancer occurs in the supraglottic region in one-third of patients; in the glottic region in one-half; and in the subglottic region in a very few.8 Glottic cancer presents earlier than supraglottic cancer with hoarseness, whereas supraglottic cancer presents with more advanced disease, causing stridor, dysphagia, and throat pain. (Note: Guidelines recommend against prescribing acid suppressants in patients with hoarseness who do not have symptoms of reflux.9)
Stage 1 and Stage 2 laryngeal cancers are localized; Stages 3-4B are locally advanced or involve lymph nodes, or both; Stage 4C is metastatic disease. Overall, 60% of patients have Stage 3 or Stage 4 disease at diagnosis.10
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Laryngoscopy should be performed before computed tomography (CT) or magnetic resonance imaging is considered in a patient with hoarseness that does not resolve after 3 months—or sooner, if there is suspicion of malignancy.
How is it treated? Most patients presenting with Stage 1 or Stage 2 cancer can be treated with local radiation or, less commonly, larynx-preserving surgery. Patients with Stage 3 or Stage 4 disease can be treated with a combination of radiation and chemotherapy, which, compared to radiation alone, confers a decreased risk of local recurrence and increased laryngectomy-free survival.11 Patients whose vocal cords are destroyed or who have recurrence following radiation and chemotherapy might need total laryngectomy and formation of a tracheostomy and prosthetic for voice creation.
Five-year overall survival for Stage 1 and Stage 2 supraglottic and glottic cancers is 80%—lower, however, for later-presenting subglottic cancers.12
Oropharyngeal cancer
What you need to know. The lifetime risk for cancer of the oropharynx is approximately 1%.13 SCC is responsible for approximately 90% of these cancers. Early detection is important: The 5-year survival rate is more than twice as high for localized disease (83%) than it is for metastatic disease (39%) at detection.13
At any given time, 7% of the US population has HPV infection of the oropharynx. Most of these cases clear spontaneously, but persistent high-risk HPV infection led to a 225% increase in HPV-positive oropharyngeal SCC from 1988 to 2004.14 The representative case of HPV-positive oropharyngeal SCC is a middle-aged (40- to 59-year-old) white male with a history of multiple sexual partners and with little or no tobacco exposure and low alcohol consumption.
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Oral cancers present with a lesion, often ulcerative, that should be examined by palpation with a gloved finger to describe the presence, color, and number of lesions; any tenderness; tissue consistency (soft, firm, hard); and fixation to underlying structures.15 The oropharynx should be examined without protrusion of the tongue, which obscures the oropharynx and can make it harder to depress the posterior part of the tongue.
A finding of leukoplakia (white plaques) and erythroplakia (red plaques) of the oropharynx might reflect benign hyperkeratosis or premalignant lesions; the plaques do not wipe off on examination. Referral to a dentist or otorhinolaryngologist for biopsy is indicated for all erythroplakia and leukoplakia, and for ulcers that persist longer than 2 weeks.16
(Note: Evidence is insufficient to support screening asymptomatic patients for oral and oropharyngeal cancers by physical examination. There is no US Food and Drug Administration-approved screening test for oral HPV infection.17)
How is it treated? A diagnosis of moderate dysplasia or carcinoma in situ should be treated with surgical excision to clear margins followed by routine monitoring every 3 to 6 months, for life.18 Topical medication, electrocautery, laser ablation, and cryosurgery are management options for less severe dysplasia.
Sinonasal cancer
What you need to know. Worldwide, sinonasal cancer accounts for approximately 0.7% of all new cancers but demonstrates strong genetic and regional associations, particularly among the Cantonese population of southern China.19 One-half of new sinonasal malignancies are SCC; the rest are adenocarcinoma, lymphoepithelial carcinoma, and rare subtypes.20
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Presentation tends to mimic common, nonmalignant conditions, such as sinusitis, until invasion into adjacent structures. When sinonasal passages are involved, the history might include epistaxis or nasal discharge; facial or dental pain; unilateral nasal obstruction with unexplained onset later in life; and failure to respond to treatment of presumed rhinosinusitis. Physical examination should include assessment of cranial nerves, palpation of the sinuses, and anterior rhinoscopy.
Thin-cut CT of the paranasal sinuses is the first-line imaging study. Sinonasal endoscopy, with targeted biopsy of suspicious lesions, is the evaluation of choice when malignancy is suspected.
How is it treated? Surgery is the treatment of choice, with postoperative radiation for patients at higher risk of recurrence because of more extensive disase.12 Five-year survival for advanced disease is poor (35%); only 15% of cases are diagnosed at a localized stage because presenting symptoms are nonspecific.21
Nasopharyngeal cancer
What you need to know. Nasopharyngeal cancer is rare in the United States and Europe, compared with China, where it is endemic (and where a variety of risk factors, including intake of salt-preserved fish, have been proposed22). Epstein-Barr virus infection and a history of smoking increase the risk.
Patients with nasopharyngeal cancer can present with epistaxis, nasal obstruction, and auditory symptoms, such as serous otitis media. Direct extension of the tumor can lead to cranial-nerve palsy, most commonly III, V, VI, and XII.23
Continue to: What is the diagnostic strategy?
What is the diagnostic strategy? Three-quarters of patients present with a neck mass from lymph-node metastases. Patients with the risk factors for nasopharyngeal cancer noted above who present with concerning symptoms should have nasoendoscopy with biopsy.
How is it treated? Radiation is the primary treatment, which is combined with chemotherapy for more advanced disease.23 Screening high-risk populations for antibodies to Epstein-Barr virus and performing nasopharyngeal endoscopy on patients who screen positive increases the detection rate of nasopharyngeal cancer; however, this strategy has not been shown to improve survival.9
Salivary gland tumors
What you need to know. Salivary gland neoplasms are a rare and heterogeneous entity, comprising 6% to 8% of head and neck cancers.24 More than 70% of these tumors are located in the parotid gland; 8%, in the submandibular glands; 1%, in the sublingual glands; and the rest, in the minor salivary glands. Most salivary gland tumors are benign; the most prevalent malignant tumors are mucoepidermoid carcinoma (30%) and adenoid cystic carcinoma (10%).25 Additional identified risk factors for a salivary gland tumor include irradiation, prior head and neck cancer, and environmental exposures, including hairdressing, rubber manufacturing, and exposure to nickel compounds.26
What is the diagnostic strategy? The history and physical exam are essential to distinguish a salivary gland tumor from an infectious cause and sialolithiasis. Parotid tumors most commonly present as asymptomatic parotid swelling, although pain can be present in as many as 40% of malignant parotid tumors.25 Facial nerve weakness is found in 25% of parotid tumors; although the differential diagnosis of facial nerve palsy is broad, suspicion of malignancy should be raised in the presence of a parotid mass, progressive unilateral symptoms, hemifacial spasm progressing to weakness, and a history of skin cancer on the face or scalp. Additional characteristics that favor a neoplastic cause are trismus and nontender lymphadenopathy.25
In contrast, sialolithiasis is associated with intermittent pain caused by eating and is more common in the settings of dehydration and poor dental hygiene. Sialadenitis should be suspected when the presentation is fever, increased pain and swelling, erythema, and expression of pus from the salivary gland.
Continue to: If malignancy is suspected...
If malignancy is suspected, the initial diagnostic evaluation should include ultrasonography (US); concurrent FNA biopsy should be performed if a mass is detected.27 US-guided FNA has a sensitivity of 73% to 86% for salivary neoplasm.7 CT and magnetic resonance imaging are useful for further characterization of tumors and can be advantageous for surgical planning.
How is it treated? Treatment of a salivary gland tumor involves surgical resection, followed by radiotherapy for patients in whom disease is more extensive or who exhibit high-risk pathology. Primary radiotherapy can be used in patients with an unresectable tumor. Typically, chemotherapy is used only for palliative purposes in relapsing disease, when a tumor is not amenable to radiotherapy, and in metastatic disease.25
Prognosis varies by histotype but is generally favorable. The survival rates for a malignant salivary gland tumor are 83% at 1 year, 69% at 3 years, and 65% at 5 years.28 Distant metastases are the most common cause of death, occurring primarily in the lungs (80%), bone (15%), and liver.27 Factors that indicate poor prognosis include facial nerve involvement, trismus, a tumor > 4 cm, bone involvement, nodal spread, and recurrence.25
Thyroid cancer
What you need to know. Thyroid cancer is the most rapidly increasing cancer diagnosis in the United States, with an annual incidence of 4.5%.1 In the United States, most thyroid cancers are differentiated thyroid cancer (DTC), which includes papillary and follicular cancers. Less-differentiated medullary thyroid cancer (MTC), typically associated with multiple endocrine neoplasia (MEN) 2A or 2B, and undifferentiated or anaplastic thyroid cancer are less common. The increasing incidence of thyroid cancer is primarily the result of an increase in nonclinically relevant DTC.
What is the diagnostic strategy? Thyroid cancer usually presents as a thyroid nodule found by the patient or incidentally on physical examination or imaging. Other presenting signs and symptoms include hoarseness, voice changes, and dysphagia.
Continue to: Thyroid US is the study of...
Thyroid US is the study of choice for initial evaluation of the size and features of a nodule; findings are used to make recommendations for further workup. If further evaluation is indicated, FNA biopsy is the test of choice.29
In 2016, the American Thyroid Association released updated guidelines for evaluating thyroid nodules (TABLE).30 The US Preventive Services Task Force recommends against screening for thyroid cancer by neck palpation or US in asymptomatic patients because evidence of significant mortality benefit is lacking.31
How is it treated? Treatment of thyroid cancer focuses on local excision of the nodule by partial or total thyroidectomy (depending on the size and type of cancer) and surgical removal of involved lymph nodes. Differentiated thyroid cancer is categorized as high-, medium-, or low-risk, depending on tumor extension, incomplete tumor resection, size of lymph nodes > 3 cm, and distant metastases. Adjuvant treatment with radioactive iodine can be considered for intermediate-risk DTC and is recommended for high-risk DTC.32
Following surgical treatment, thyroid-stimulating hormone suppression is recommended using levothyroxine.33 Patients at higher risk of recurrence should have longer and more intense suppression of thyroid-stimulating hormone.30 Levels of serum thyroglobulin and anti-thyroglobulin antibody should be followed postoperatively; rising values can indicate recurrent disease. The calcitonin level should be followed in patients with a history of MTC. Thyroid US should be performed 6 to 12 months postoperatively, then periodically, depending on determination of recurrence risk and any change in the thyroglobulin level.30
(Note: Glucagon-like peptide-1 [GLP-1] receptor agonists, used to treat type 2 diabetes mellitus, carry a black-box warning for their risk of MTC and are contraindicated in patients who have a personal or family history of MTC, MEN2A, or MEN2B.34)
Continue to: Anaplastic thyroid cancer...
Anaplastic thyroid cancer, a rare form of thyroid cancer, carries a high mortality rate, with a median survival of 5 months from diagnosis and 1-year survival of 20%. Patients require expeditious total thyroidectomy and neck dissection, followed by external-beam radiation with or without chemotherapy. If this strategy is not feasible, tracheostomy might be necessary to maintain a patent airway.2 Family physicians treating a patient who has anaplastic thyroid cancer can fulfill a crucial role by ensuring that an advance directive is established, a surrogate decision-maker is appointed, and goals of care are well defined.
Follow-up care for head and neck Ca
The risk of adverse effects after radiation therapy for head and neck cancer calls for close monitoring, appropriate treatment, and referral and counseling as needed. See “Follow-up care after treatment of head and neck cancer.” 35-39
SIDEBAR
Follow-up care after treatment of head and neck cancer35-39
Challenge: After radiation to the head and neck, as many as 53% of patients develop subclinical hypothyroidism and 33% develop clinical hypothyroidism.35Strategy: Measure the thyroid-stimulating hormone level within 1 year of the completion of radiotherapy and every 6 to 12 months thereafter.36
Challenge: Radiation to the head and neck can decrease the function of salivary glands, causing xerostomia in as many as 40% of patients. This condition can lead to problems with oral hygiene and difficulty with speech, eating, and swallowing.37Strategy:
- Treat xerostomia with artificial saliva, sugar-free candy and gum, or muscarinic cholinergic agonists, such as pilocarpine and cevimeline.
- Consider treatment with pilocarpine or cevimeline. Pilocarpine alleviates xerostomia in approximately 50% of patients who develop the condition, although its use can be limited by adverse cholinergic effects.3,7 Cevimeline causes fewer and less pronounced adverse effects than pilocarpine because it acts more specifically on receptors in the salivary glands.38
- Mention the possibility of acupuncture to your patients. There is evidence that it can stimulate salivary flow.39
Challenge: Patients who have had radiation to the head and neck have an increased risk of dental caries from xerostomia and the direct effect of radiation, which causes demineralization of teeth.
Strategy: Following radiation, instruct the patient about appropriate oral hygiene:
- regular flossing
- brushing and application of daily fluoride
- regular visits for dental care.39
Challenge: Trismus occurs in 5% to 25% of patients, depending on the type of radiation.36Strategy: Recommend exercise-based treatment, the treatment of choice. Surgery is indicated for severe cases.
Challenge: Dysphagia occurs in approximately 25% of patients treated with radiation.36Strategy: Provide a referral for swallowing exercises, which might be helpful. Some cases are severe enough to warrant placement of a feeding tube.37
Last, counsel all patients who have been treated for cancer of the head or neck, with any modality, about cessation of smoking and alcohol.
CORRESPONDENCE
Anne Mounsey, MD, Family Medicine Residency, The University of North Carolina at Chapel Hill, 590 Manning Dr., Chapel Hill, NC 27599; Anne_mounsey@med.unc.edu
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Smallridge RC, Ain KB, Asa SL, et al; American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104-1139.
3. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83:489-501.
4. Seoane J, Alvarez-Novoa P, Gomez I, et al. Early oral cancer diagnosis: The Aarhus statement perspective. A systematic review and meta-analysis. Head Neck. 2016;38(suppl 1):E2182-E2189.
5. Franco J, Elghouche AN, Harris MS, et al Diagnostic delays and errors in head and neck cancer patients: opportunities for improvement. Am J Med Qual. 2017;32:330-335.
6. Pynnonen MA, Gillespie MB, Roman B, et al. Clinical practice guideline: evaluation of the neck mass in adults. Otolaryngol Head Neck Surg. 2017;157(suppl 2):S1-S30.
7. Bosetti C, Gallus S, Franceschi S, et al. Cancer of the larynx in non-smoking alcohol drinkers and in non-drinking tobacco smokers. Br J Cancer. 2002;87:516-518.
8. Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116(9 pt 2 suppl 111):1-13.
9. Schwartz SR, Cohen SM, Dailey SH, et al. Clinical practice guideline: hoarseness (dysphonia). Otolaryngol Head Neck Surg. 2009;141(3 suppl 2):S1-S31.
10. Steuer CE, El-Deiry M, Parks JR, et al. An update on larynx cancer. CA Cancer J Clin. 2017;67:31-50.
11. Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091-2098.
12. Mendenhall WM, Werning JW, Hinerman RW, et al. Management of T1-T2 glottic carcinomas. Cancer. 2004;100:1786-1792.
13. Surveillance, Epidemiology, and End Results Unit. National Cancer Institute. Cancer stat facts: oral cavity and pharynx. https://seer.cancer.gov/statfacts/html/oralcav.html. Accessed October 18, 2019.
14. Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014;50:380-386.
15. Tarakji B, Gazal G, Al-Maweri SA, et al. Guideline for the diagnosis and treatment of recurrent aphthous stomatitis for dental practitioners. J Int Oral Health. 2015;7:74-80.
16. Siu A, Landon K, Ramos DM. Differential diagnosis and management of oral ulcers. Semin Cutan Med Surg. 2015;34:171-177.
17. US Preventive Services Task Force. Final recommendation statement: oral cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/oral-cancer-screening1. Updated November 2013. Accessed October 18, 2019.
18. Villa A, Woo SB. Leukoplakia—a diagnostic and management algorithm. J Oral Maxillofac Surg. 2017;75:723-734.
19. Yang S, Wu S, Zhou J, et al. Screening for nasopharyngeal cancer. Cochrane Database Syst Rev. 2015;(11):CD008423.
20. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877-885.
21. Ou SH, Zell JA, Ziogas A, et al. Epidemiology of nasopharyngeal carcinoma in the United States: improved survival of Chinese patients within the keratinizing squamous cell carcinoma histology. Ann Oncol. 2007;18:29-35.
22. Chang ET, Adami H-O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765-1777.
23. Chua MLK, Wee JTS, Hui EP, et al. Nasopharyngeal carcinoma. Lancet. 2016;387:1012-1024.
24. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177-184.
25. Lewis JS. Sinonasal squamous cell carcinoma: a review with emphasis on emerging histologic subtypes and the role of human papillomavirus. Head Neck Pathol. 2016;10:60-67.
26. Horn-Ross PL, Ljung BM, Morrow M. Environmental factors and the risk of salivary gland cancer. Epidemiology. 1997;8:414-419.
27. Colella G, Cannavale R, Flamminio F, et al. Fine-needle aspiration cytology of salivary gland lesions: a systematic review. J Oral Maxillofac Surg. 2010;68:2146-2153.
28. Berrino F, De Angelis R, Sant M, et al; EUROCARE Working Group. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995-99: results of the EUROCARE-4 study. Lancet Oncol. 2007;8:773-783.
29. Baloch ZW, LiVolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425-437.
30. Haugen BR, Alexander EK, Bible KC, et al; The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133.
31. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for thyroid Cancer: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:1882-1887.
32. Jonklaas J, Cooper DS, Ain KB, et al; National Thyroid Cancer Treatment Cooperative Study Group. Radioiodine therapy in patients with stage I differentiated thyroid cancer. Thyroid. 2010;20:1423-1424.
33. Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid. 1998;8:737-744.
34. US Food and Drug Administration. Highlight of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125431s020lbl.pdf. Updated December 2017. Accessed October 30, 1019.
35. Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol. 2011;99:1-5.
36. The development of quality of care measures for oral cavity cancer. Arch Otolaryngol Head Neck Surg. 2008;134:672.
37. Strojan P, Hutcheson KA, Eisbruch A, et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat Rev. 2017;59:79-92.
38. Chambers MS, Posner M, Jones CU, et al. Cevimeline for the treatment of postirradiation xerostomia in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:1102-1109.
39. Gupta N, Pal M, Rawat S, et al. Radiation-induced dental caries, prevention and treatment - a systematic review. Natl J Maxillofac Surg. 2015;6:160-166.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
2. Smallridge RC, Ain KB, Asa SL, et al; American Thyroid Association Anaplastic Thyroid Cancer Guidelines Taskforce. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104-1139.
3. Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2008;83:489-501.
4. Seoane J, Alvarez-Novoa P, Gomez I, et al. Early oral cancer diagnosis: The Aarhus statement perspective. A systematic review and meta-analysis. Head Neck. 2016;38(suppl 1):E2182-E2189.
5. Franco J, Elghouche AN, Harris MS, et al Diagnostic delays and errors in head and neck cancer patients: opportunities for improvement. Am J Med Qual. 2017;32:330-335.
6. Pynnonen MA, Gillespie MB, Roman B, et al. Clinical practice guideline: evaluation of the neck mass in adults. Otolaryngol Head Neck Surg. 2017;157(suppl 2):S1-S30.
7. Bosetti C, Gallus S, Franceschi S, et al. Cancer of the larynx in non-smoking alcohol drinkers and in non-drinking tobacco smokers. Br J Cancer. 2002;87:516-518.
8. Hoffman HT, Porter K, Karnell LH, et al. Laryngeal cancer in the United States: changes in demographics, patterns of care, and survival. Laryngoscope. 2006;116(9 pt 2 suppl 111):1-13.
9. Schwartz SR, Cohen SM, Dailey SH, et al. Clinical practice guideline: hoarseness (dysphonia). Otolaryngol Head Neck Surg. 2009;141(3 suppl 2):S1-S31.
10. Steuer CE, El-Deiry M, Parks JR, et al. An update on larynx cancer. CA Cancer J Clin. 2017;67:31-50.
11. Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091-2098.
12. Mendenhall WM, Werning JW, Hinerman RW, et al. Management of T1-T2 glottic carcinomas. Cancer. 2004;100:1786-1792.
13. Surveillance, Epidemiology, and End Results Unit. National Cancer Institute. Cancer stat facts: oral cavity and pharynx. https://seer.cancer.gov/statfacts/html/oralcav.html. Accessed October 18, 2019.
14. Pytynia KB, Dahlstrom KR, Sturgis EM. Epidemiology of HPV-associated oropharyngeal cancer. Oral Oncol. 2014;50:380-386.
15. Tarakji B, Gazal G, Al-Maweri SA, et al. Guideline for the diagnosis and treatment of recurrent aphthous stomatitis for dental practitioners. J Int Oral Health. 2015;7:74-80.
16. Siu A, Landon K, Ramos DM. Differential diagnosis and management of oral ulcers. Semin Cutan Med Surg. 2015;34:171-177.
17. US Preventive Services Task Force. Final recommendation statement: oral cancer: screening. https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/oral-cancer-screening1. Updated November 2013. Accessed October 18, 2019.
18. Villa A, Woo SB. Leukoplakia—a diagnostic and management algorithm. J Oral Maxillofac Surg. 2017;75:723-734.
19. Yang S, Wu S, Zhou J, et al. Screening for nasopharyngeal cancer. Cochrane Database Syst Rev. 2015;(11):CD008423.
20. Turner JH, Reh DD. Incidence and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck. 2012;34:877-885.
21. Ou SH, Zell JA, Ziogas A, et al. Epidemiology of nasopharyngeal carcinoma in the United States: improved survival of Chinese patients within the keratinizing squamous cell carcinoma histology. Ann Oncol. 2007;18:29-35.
22. Chang ET, Adami H-O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765-1777.
23. Chua MLK, Wee JTS, Hui EP, et al. Nasopharyngeal carcinoma. Lancet. 2016;387:1012-1024.
24. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177-184.
25. Lewis JS. Sinonasal squamous cell carcinoma: a review with emphasis on emerging histologic subtypes and the role of human papillomavirus. Head Neck Pathol. 2016;10:60-67.
26. Horn-Ross PL, Ljung BM, Morrow M. Environmental factors and the risk of salivary gland cancer. Epidemiology. 1997;8:414-419.
27. Colella G, Cannavale R, Flamminio F, et al. Fine-needle aspiration cytology of salivary gland lesions: a systematic review. J Oral Maxillofac Surg. 2010;68:2146-2153.
28. Berrino F, De Angelis R, Sant M, et al; EUROCARE Working Group. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995-99: results of the EUROCARE-4 study. Lancet Oncol. 2007;8:773-783.
29. Baloch ZW, LiVolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425-437.
30. Haugen BR, Alexander EK, Bible KC, et al; The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133.
31. US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for thyroid Cancer: US Preventive Services Task Force recommendation statement. JAMA. 2017;317:1882-1887.
32. Jonklaas J, Cooper DS, Ain KB, et al; National Thyroid Cancer Treatment Cooperative Study Group. Radioiodine therapy in patients with stage I differentiated thyroid cancer. Thyroid. 2010;20:1423-1424.
33. Cooper DS, Specker B, Ho M, et al. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid. 1998;8:737-744.
34. US Food and Drug Administration. Highlight of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125431s020lbl.pdf. Updated December 2017. Accessed October 30, 1019.
35. Boomsma MJ, Bijl HP, Langendijk JA. Radiation-induced hypothyroidism in head and neck cancer patients: a systematic review. Radiother Oncol. 2011;99:1-5.
36. The development of quality of care measures for oral cavity cancer. Arch Otolaryngol Head Neck Surg. 2008;134:672.
37. Strojan P, Hutcheson KA, Eisbruch A, et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat Rev. 2017;59:79-92.
38. Chambers MS, Posner M, Jones CU, et al. Cevimeline for the treatment of postirradiation xerostomia in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:1102-1109.
39. Gupta N, Pal M, Rawat S, et al. Radiation-induced dental caries, prevention and treatment - a systematic review. Natl J Maxillofac Surg. 2015;6:160-166.
PRACTICE RECOMMENDATIONS
› Do not treat a neck mass with antibiotics unless it has features consistent with infection. C
› Order laryngoscopy for all patients with hoarseness that does not resolve after 3 months—or sooner, if malignancy is suspected. C
› Order ultrasonography-guided fine-needle aspiration for diagnostic evaluation of salivary gland masses. B
› Manage a thyroid nodule based on its sonographic features, including size, consistency, and the presence of concerning features. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
An FP’s guide to AI-enabled clinical decision support
Computer technology and artificial intelligence (AI) have come a long way in several decades:
- Between 1971 and 1996, access to the Medline database was primarily limited to university libraries and other institutions; in 1997, the database became universally available online as PubMed.1
- In 2004, the President of the United States issued an executive order that launched a 10-year plan to put electronic health records (EHRs) in place nationwide; EHRs are now employed in nearly 9 of 10 (85.9%) medical offices.2
Over time, numerous online resources sprouted as well, including DxPlain, UpToDate, and Clinical Key, to name a few. These digital tools were impressive for their time, but many of them are now considered “old-school” AI-enabled clinical decision support.
In the past 2 to 3 years, innovative clinicians and technologists have pushed medicine into a new era that takes advantage of machine learning (ML)-enhanced diagnostic aids, software systems that predict disease progression, and advanced clinical pathways to help individualize treatment. Enthusiastic early adopters believe these resources are transforming patient care—although skeptics remain unconvinced, cautioning that they have yet to prove their worth in everyday clinical practice.
In this review, we first analyze the strengths and weaknesses of evidence supporting these tools, then propose a potential role for them in family medicine.
Machine learning takes on retinopathy
The term “artificial intelligence” has been with us for longer than a half century.3 In the broadest sense, AI refers to any computer system capable of automating a process usually performed manually by humans. But the latest innovations in AI take advantage of a subset of AI called “machine learning”: the ability of software systems to learn new functionality or insights on their own, without additional programming from human data engineers. Case in point: A software platform has been developed that is capable of diagnosing or screening for diabetic retinopathy without the involvement of an experienced ophthalmologist.
The landmark study that started clinicians and health care executives thinking seriously about the potential role of ML in medical practice was spearheaded by Varun Gulshan, PhD, at Google, and associates from several medical schools.4 Gulshan used an artificial neural network designed to mimic the functions of the human nervous system to analyze more than 128,000 retinal images, looking for evidence of diabetic retinopathy. (See “Deciphering artificial neural networks,” for an explanation of how such networks function.5) The algorithm they employed was compared with the diagnostic skills of several board-certified ophthalmologists.
[polldaddy:10453606]
Continue to: Deciperhing artificial neural networks
Deciphering artificial neural networks
The promise of health care information technology relies heavily on statistical methods and software constructs, including logistic regression, random forest modeling, clustering, and neural networks. The machine learning-enabled image analysis used to detect diabetic retinopathy and to differentiate a malignant melanoma and a normal mole is based on neural networking.
As we discussed in the body of this article, these networks mimic the nervous system, in that they comprise computer-generated “neurons,” or nodes, and are connected by “synapses” (FIGURE5). When a node in Layer 1 is excited by pixels coming from a scanned image, it sends on that excitement, represented by a numerical value, to a second set of nodes in Layer 2, which, in turns, sends signals to the next layer— and so on.
Eventually, the software’s interpretation of the pixels of the image reaches the output layer of the network, generating a negative or positive diagnosis. The initial process results in many interpretations, which are corrected by a backward analytic process called backpropagation. The video tutorials mentioned in the main text provide a more detailed explanation of neural networking.
Using an area-under-the-receiver operating curve (AUROC) as a metric, and choosing an operating point for high specificity, the algorithm generated sensitivity of 87% and 90.3% and specificity of 98.1% and 98.5% for 2 validation data sets for detecting referable retinopathy, as defined by a panel of at least 7 ophthalmologists. When AUROC was set for high sensitivity, the algorithm generated sensitivity of 97.5% and 96.1% and specificity of 93.4% and 93.9% for the 2 data sets.
These results are impressive, but the researchers used a retrospective approach in their analysis. A prospective analysis would provide stronger evidence.
That shortcoming was addressed by a pivotal clinical trial that convinced the US Food and Drug Administration (FDA) to approve the technology. Michael Abramoff, MD, PhD, at the University of Iowa Department of Ophthalmology and Visual Sciences and his associates6 conducted a prospective study that compared the gold standard for detecting retinopathy, the Fundus Photograph Reading Center (of the University of Wisconsin School of Medicine and Public Health), to an ML-based algorithm, the commercialized IDx-DR. The IDx-DR is a software system that is used in combination with a fundal camera to capture retinal images. The researchers found that “the AI system exceeded all pre-specified superiority endpoints at sensitivity of 87.2% ... [and] specificity of 90.7% ....”
Continue to: The FDA clearance statement...
The FDA clearance statement for this technology7 limits its use, emphasizing that it is intended only as a screening tool, not a stand-alone diagnostic system. Because IDx-DR is being used in primary care, the FDA states that patients who have a positive result should be referred to an eye care professional. The technology is contraindicated in patients who have a history of laser treatment, surgery, or injection in the eye or who have any of the following: persistent vision loss, blurred vision, floaters, previously diagnosed macular edema, severe nonproliferative retinopathy, proliferative retinopathy, radiation retinopathy, and retinal vein occlusion. It is also not intended for pregnant patients because their eye disease often progresses rapidly.
Additional caveats to keep in mind when evaluating this new technology include that, although the software can help detect retinopathy, it does not address other key issues for this patient population, including cataracts and glaucoma. The cost of the new technology also requires attention: Software must be used in conjunction with a specific retinal camera, the Topcon TRC-NW400, which is expensive (new, as much as $20,000).
Speaking of cost: Health care providers and insurers still question whether implementing AI-enabled systems is cost-effective. It is too early to say definitively how AI and machine learning will have an impact on health care expenditures, because the most promising technological systems have yet to be fully implemented in hospitals and medical practices nationwide. Projections by Forbes suggest that private investment in health care AI will reach $6.6 billion by 2021; on a more confident note, an Accenture analysis predicts that the best possible application of AI might save the health care sector $150 billion annually by 2026.8
What role might this diabetic retinopathy technology play in family medicine? Physicians are constantly advising patients who have diabetes about the need to have a regular ophthalmic examination to check for early signs of retinopathy—advice that is often ignored. The American Academy of Ophthalmology points out that “6 out of 10 people with diabetes skip a sight-saving exam.”9 When a patient is screened with this type of device and found to be at high risk of eye disease, however, the advice to see an eye-care specialist might carry more weight.
Screening colonoscopy: Improving patient incentives
No responsible physician doubts the value of screening colonoscopy in patients 50 years and older, but many patients have yet to realize that the procedure just might save their life. Is there a way to incentivize resistant patients to have a colonoscopy performed? An ML-based software system that only requires access to a few readily available parameters might be the needed impetus for many patients.
Continue to: A large-scale validation...
A large-scale validation study performed on data from Kaiser Permanente Northwest found that it is possible to estimate a person’s risk of colorectal cancer by using age, gender, and complete blood count.10 This retrospective investigation analyzed more than 17,000 Kaiser Permanente patients, including 900 who already had colorectal cancer. The analysis generated a risk score for patients who did not have the malignancy to gauge their likelihood of developing it. The algorithms were more sensitive for detecting tumors of the cecum and ascending colon, and less sensitive for detection of tumors of the transverse and sigmoid colon and rectum.
To provide more definitive evidence to support the value of the software platform, a prospective study was subsequently conducted on more than 79,000 patients who had initially declined to undergo colorectal screening. The platform, called ColonFlag, was used to detect 688 patients at highest risk, who were then offered screening colonoscopy. In this subgroup, 254 agreed to the procedure; ColonFlag identified 19 malignancies (7.5%) among patients within the Maccabi Health System (Israel), and 15 more in patients outside that health system.11 (In the United States, the same program is known as LGI Flag and has been cleared by the FDA.)
Although ColonFlag has the potential to reduce the incidence of colorectal cancer, other evidence-based screening modalities are highlighted in US Preventive Services Task Force guidelines, including the guaiac-based fecal occult blood test and the fecal immunochemical test.12
Beyond screening to applications in managing disease
The complex etiology of sepsis makes the condition difficult to treat. That complexity has also led to disagreement on the best course of management. Using an ML algorithm called an “Artificial Intelligence Clinician,” Komorowski and associates13 extracted data from a large data set from 2 nonoverlapping intensive care unit databases collected from US adults.The researchers’ analysis suggested a list of 48 variables that likely influence sepsis outcomes, including:
- demographics,
- Elixhauser premorbid status,
- vital signs,
- clinical laboratory data,
- intravenous fluids given, and
- vasopressors administered.
Komorowski and co-workers concluded that “… mortality was lowest in patients for whom clinicians’ actual doses matched the AI decisions. Our model provides individualized and clinically interpretable treatment decisions for sepsis that could improve patient outcomes.”
A randomized clinical trial has found that an ML program that uses only 6 common clinical markers—blood pressure, heart rate, temperature, respiratory rate, peripheral capillary oxygen saturation (SpO2), and age—can improve clinical outcomes in patients with severe sepsis.14 The alerts generated by the algorithm were used to guide treatment. Average length of stay was 13 days in controls, compared with 10.3 days in those evaluated with the ML algorithm. The algorithm was also associated with a 12.4% drop in in-hospital mortality.
Continue to: Addressing challenges, tapping resources
Addressing challenges, tapping resources
Advances in the management of diabetic retinopathy, colorectal cancer, and sepsis are the tip of the AI iceberg. There are now ML programs to distinguish melanoma from benign nevi; to improve insulin dosing for patients with type 1 diabetes; to predict which hospital patients are most likely to end up in the intensive care unit; and to mitigate the opioid epidemic.
An ML Web page on the JAMA Network (https://sites.jamanetwork.com/machine-learning/) features a long list of published research studies, reviews, and opinion papers suggesting that the future of medicine is closely tied to innovative developments in this area. This Web page also addresses the potential use of ML in detecting lymph node metastases in breast cancer, the need to temper AI with human intelligence, the role of AI in clinical decision support, and more.
The JAMA Network also discusses a few of the challenges that still need to be overcome in developing ML tools for clinical medicine—challenges that you will want to be cognizant of as you evaluate new research in the field.
Black-box dilemma. A challenge that technologists face as they introduce new programs that have the potential to improve diagnosis, treatment, and prognosis is a phenomenon called the “black-box dilemma,” which refers to the complex data science, advanced statistics, and mathematical equations that underpin ML algorithms. These complexities make it difficult to explain the mechanism of action upon which software is based, which, in turn, makes many clinicians skeptical about its worth.
For example, the neural networks that are the backbone of the retinopathy algorithm discussed earlier might seem like voodoo science to those unfamiliar with the technology. It’s fortunate that several technology-savvy physicians have mastered these digital tools and have the teaching skills to explain them in plain-English tutorials. One such tutorial, “Understanding How Machine Learning Works,” is posted on the JAMA Network (https://sites.jamanetwork.com/machine-learning/#multimedia). A more basic explanation was included in a recent Public Broadcasting System “Nova” episode, viewable at www.youtube.com/watch?v=xS2G0oolHpo.
Continue to: Limited analysis
Limited analysis. Another problem that plagues many ML-based algorithms is that they have been tested on only a single data set. (Typically, a data set refers to a collection of clinical parameters from a patient population.) For example, researchers developing an algorithm might collect their data from a single health care system.
Several investigators have addressed this shortcoming by testing their software on 2 completely independent patient populations. Banda and colleagues15 recently developed a software platform to improve the detection rate in familial hypercholesterolemia, a significant cause of premature cardiovascular disease and death that affects approximately 1 of every 250 people. Despite the urgency of identifying the disorder and providing potentially lifesaving treatment, only 10% of patients receive an accurate diagnosis.16 Banda and colleagues developed a deep-learning algorithm that is far more effective than the traditional screening approach now in use.
To address the generalizability of the algorithm, it was tested on EHR data from 2 independent health care systems: Stanford Health Care and Geisinger Health System. In Stanford patients, the positive predictive value of the algorithm was 88%, with a sensitivity of 75%; it identified 84% of affected patients at the highest probability threshold. In Geisinger patients, the classifier generated a positive predictive value of 85%.
The future of these technologies
AI and ML are not panaceas that will revolutionize medicine in the near future. Likewise, the digital tools discussed in this article are not going to solve multiple complex medical problems addressed during a single office visit. But physicians who ignore mounting evidence that supports these emerging technologies will be left behind by more forward-thinking colleagues.
A recent commentary in Gastroenterology17 sums up the situation best: “It is now too conservative to suggest that CADe [computer-assisted detection] and CADx [computer-assisted diagnosis] carry the potential to revolutionize colonoscopy. The artificial intelligence revolution has already begun.”
CORRESPONDENCE
Paul Cerrato, MA, cerrato@aol.com, pcerrato@optonline.net. John Halamka, MD, MS, john.halamka@bilh.org.
1. Lindberg DA. Internet access to National Library of Medicine. Eff Clin Pract. 2000;3:256-260.
2. National Center for Health Statistics, Centers for Disease Control and Prevention. Electronic medical records/electronic health records (EMRs/EHRs). www.cdc.gov/nchs/fastats/electronic-medical-records.htm. Updated March 31, 2017. Accessed October 1, 2019.
3. Smith C, McGuire B, Huang T, et al. The history of artificial intelligence. University of Washington. https://courses.cs.washington.edu/courses/csep590/06au/projects/history-ai.pdf. Published December 2006. Accessed October 1, 2019.
4. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA; 2016;316:2402-2410.
5. Cerrato P, Halamka J. The Transformative Power of Mobile Medicine. Cambridge, MA: Academic Press; 2019.
6. Abràmoff MD, Lavin PT, Birch M, et al. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39.
7. US Food and Drug Administration. FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems. Press release. www.fda.gov/news-events/press-announcements/fda-permits-marketing-artificial-intelligence-based-device-detect-certain-diabetes-related-eye. Published April 11, 2018. Accessed October 1, 2019.
8. AI and healthcare: a giant opportunity. Forbes Web site. www.forbes.com/sites/insights-intelai/2019/02/11/ai-and-healthcare-a-giant-opportunity/#5906c4014c68. Published February 11, 2019. Accessed October 25, 2019.
9. Boyd K. Six out of 10 people with diabetes skip a sight-saving exam. American Academy of Ophthalmology Website. https://www.aao.org/eye-health/news/sixty-percent-skip-diabetic-eye-exams. Published November 1, 2016. Accessed October 25, 2019.
10. Hornbrook MC, Goshen R, Choman E, et al. Early colorectal cancer detected by machine learning model using gender, age, and complete blood count data. Dig Dis Sci. 2017;62:2719-2727.
11. Goshen R, Choman E, Ran A, et al. Computer-assisted flagging of individuals at high risk of colorectal cancer in a large health maintenance organization using the ColonFlag test. JCO Clin Cancer Inform. 2018;2:1-8.
12. US Preventive Services Task Force. Final recommendation statement: colorectal cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/colorectal-cancer-screening2#tab. Published May 2019. Accessed October 1, 2019.
13. Komorowski M, Celi LA, Badawi O, et al. The artificial intelligence clinician learns optimal treatment strategies for sepsis in intensive care. Nat Med. 2018;24:1716-1720.
14. Shimabukuro DW, Barton CW, Feldman MD, et al. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Respir Res. 2017;4:e000234.
15. Banda J, Sarraju A, Abbasi F, et al. Finding missed cases of familial hypercholesterolemia in health systems using machine learning. NPJ Digit Med. 2019;2:23.
16. What is familial hypercholesterolemia? FH Foundation Web site. https://thefhfoundation.org/familial-hypercholesterolemia/what-is-familial-hypercholesterolemia. Accessed November 1, 2019.
17. Byrne MF, Shahidi N, Rex DK. Will computer-aided detection and diagnosis revolutionize colonoscopy? Gastroenterology. 2017;153:1460-1464.E1.
Computer technology and artificial intelligence (AI) have come a long way in several decades:
- Between 1971 and 1996, access to the Medline database was primarily limited to university libraries and other institutions; in 1997, the database became universally available online as PubMed.1
- In 2004, the President of the United States issued an executive order that launched a 10-year plan to put electronic health records (EHRs) in place nationwide; EHRs are now employed in nearly 9 of 10 (85.9%) medical offices.2
Over time, numerous online resources sprouted as well, including DxPlain, UpToDate, and Clinical Key, to name a few. These digital tools were impressive for their time, but many of them are now considered “old-school” AI-enabled clinical decision support.
In the past 2 to 3 years, innovative clinicians and technologists have pushed medicine into a new era that takes advantage of machine learning (ML)-enhanced diagnostic aids, software systems that predict disease progression, and advanced clinical pathways to help individualize treatment. Enthusiastic early adopters believe these resources are transforming patient care—although skeptics remain unconvinced, cautioning that they have yet to prove their worth in everyday clinical practice.
In this review, we first analyze the strengths and weaknesses of evidence supporting these tools, then propose a potential role for them in family medicine.
Machine learning takes on retinopathy
The term “artificial intelligence” has been with us for longer than a half century.3 In the broadest sense, AI refers to any computer system capable of automating a process usually performed manually by humans. But the latest innovations in AI take advantage of a subset of AI called “machine learning”: the ability of software systems to learn new functionality or insights on their own, without additional programming from human data engineers. Case in point: A software platform has been developed that is capable of diagnosing or screening for diabetic retinopathy without the involvement of an experienced ophthalmologist.
The landmark study that started clinicians and health care executives thinking seriously about the potential role of ML in medical practice was spearheaded by Varun Gulshan, PhD, at Google, and associates from several medical schools.4 Gulshan used an artificial neural network designed to mimic the functions of the human nervous system to analyze more than 128,000 retinal images, looking for evidence of diabetic retinopathy. (See “Deciphering artificial neural networks,” for an explanation of how such networks function.5) The algorithm they employed was compared with the diagnostic skills of several board-certified ophthalmologists.
[polldaddy:10453606]
Continue to: Deciperhing artificial neural networks
Deciphering artificial neural networks
The promise of health care information technology relies heavily on statistical methods and software constructs, including logistic regression, random forest modeling, clustering, and neural networks. The machine learning-enabled image analysis used to detect diabetic retinopathy and to differentiate a malignant melanoma and a normal mole is based on neural networking.
As we discussed in the body of this article, these networks mimic the nervous system, in that they comprise computer-generated “neurons,” or nodes, and are connected by “synapses” (FIGURE5). When a node in Layer 1 is excited by pixels coming from a scanned image, it sends on that excitement, represented by a numerical value, to a second set of nodes in Layer 2, which, in turns, sends signals to the next layer— and so on.
Eventually, the software’s interpretation of the pixels of the image reaches the output layer of the network, generating a negative or positive diagnosis. The initial process results in many interpretations, which are corrected by a backward analytic process called backpropagation. The video tutorials mentioned in the main text provide a more detailed explanation of neural networking.
Using an area-under-the-receiver operating curve (AUROC) as a metric, and choosing an operating point for high specificity, the algorithm generated sensitivity of 87% and 90.3% and specificity of 98.1% and 98.5% for 2 validation data sets for detecting referable retinopathy, as defined by a panel of at least 7 ophthalmologists. When AUROC was set for high sensitivity, the algorithm generated sensitivity of 97.5% and 96.1% and specificity of 93.4% and 93.9% for the 2 data sets.
These results are impressive, but the researchers used a retrospective approach in their analysis. A prospective analysis would provide stronger evidence.
That shortcoming was addressed by a pivotal clinical trial that convinced the US Food and Drug Administration (FDA) to approve the technology. Michael Abramoff, MD, PhD, at the University of Iowa Department of Ophthalmology and Visual Sciences and his associates6 conducted a prospective study that compared the gold standard for detecting retinopathy, the Fundus Photograph Reading Center (of the University of Wisconsin School of Medicine and Public Health), to an ML-based algorithm, the commercialized IDx-DR. The IDx-DR is a software system that is used in combination with a fundal camera to capture retinal images. The researchers found that “the AI system exceeded all pre-specified superiority endpoints at sensitivity of 87.2% ... [and] specificity of 90.7% ....”
Continue to: The FDA clearance statement...
The FDA clearance statement for this technology7 limits its use, emphasizing that it is intended only as a screening tool, not a stand-alone diagnostic system. Because IDx-DR is being used in primary care, the FDA states that patients who have a positive result should be referred to an eye care professional. The technology is contraindicated in patients who have a history of laser treatment, surgery, or injection in the eye or who have any of the following: persistent vision loss, blurred vision, floaters, previously diagnosed macular edema, severe nonproliferative retinopathy, proliferative retinopathy, radiation retinopathy, and retinal vein occlusion. It is also not intended for pregnant patients because their eye disease often progresses rapidly.
Additional caveats to keep in mind when evaluating this new technology include that, although the software can help detect retinopathy, it does not address other key issues for this patient population, including cataracts and glaucoma. The cost of the new technology also requires attention: Software must be used in conjunction with a specific retinal camera, the Topcon TRC-NW400, which is expensive (new, as much as $20,000).
Speaking of cost: Health care providers and insurers still question whether implementing AI-enabled systems is cost-effective. It is too early to say definitively how AI and machine learning will have an impact on health care expenditures, because the most promising technological systems have yet to be fully implemented in hospitals and medical practices nationwide. Projections by Forbes suggest that private investment in health care AI will reach $6.6 billion by 2021; on a more confident note, an Accenture analysis predicts that the best possible application of AI might save the health care sector $150 billion annually by 2026.8
What role might this diabetic retinopathy technology play in family medicine? Physicians are constantly advising patients who have diabetes about the need to have a regular ophthalmic examination to check for early signs of retinopathy—advice that is often ignored. The American Academy of Ophthalmology points out that “6 out of 10 people with diabetes skip a sight-saving exam.”9 When a patient is screened with this type of device and found to be at high risk of eye disease, however, the advice to see an eye-care specialist might carry more weight.
Screening colonoscopy: Improving patient incentives
No responsible physician doubts the value of screening colonoscopy in patients 50 years and older, but many patients have yet to realize that the procedure just might save their life. Is there a way to incentivize resistant patients to have a colonoscopy performed? An ML-based software system that only requires access to a few readily available parameters might be the needed impetus for many patients.
Continue to: A large-scale validation...
A large-scale validation study performed on data from Kaiser Permanente Northwest found that it is possible to estimate a person’s risk of colorectal cancer by using age, gender, and complete blood count.10 This retrospective investigation analyzed more than 17,000 Kaiser Permanente patients, including 900 who already had colorectal cancer. The analysis generated a risk score for patients who did not have the malignancy to gauge their likelihood of developing it. The algorithms were more sensitive for detecting tumors of the cecum and ascending colon, and less sensitive for detection of tumors of the transverse and sigmoid colon and rectum.
To provide more definitive evidence to support the value of the software platform, a prospective study was subsequently conducted on more than 79,000 patients who had initially declined to undergo colorectal screening. The platform, called ColonFlag, was used to detect 688 patients at highest risk, who were then offered screening colonoscopy. In this subgroup, 254 agreed to the procedure; ColonFlag identified 19 malignancies (7.5%) among patients within the Maccabi Health System (Israel), and 15 more in patients outside that health system.11 (In the United States, the same program is known as LGI Flag and has been cleared by the FDA.)
Although ColonFlag has the potential to reduce the incidence of colorectal cancer, other evidence-based screening modalities are highlighted in US Preventive Services Task Force guidelines, including the guaiac-based fecal occult blood test and the fecal immunochemical test.12
Beyond screening to applications in managing disease
The complex etiology of sepsis makes the condition difficult to treat. That complexity has also led to disagreement on the best course of management. Using an ML algorithm called an “Artificial Intelligence Clinician,” Komorowski and associates13 extracted data from a large data set from 2 nonoverlapping intensive care unit databases collected from US adults.The researchers’ analysis suggested a list of 48 variables that likely influence sepsis outcomes, including:
- demographics,
- Elixhauser premorbid status,
- vital signs,
- clinical laboratory data,
- intravenous fluids given, and
- vasopressors administered.
Komorowski and co-workers concluded that “… mortality was lowest in patients for whom clinicians’ actual doses matched the AI decisions. Our model provides individualized and clinically interpretable treatment decisions for sepsis that could improve patient outcomes.”
A randomized clinical trial has found that an ML program that uses only 6 common clinical markers—blood pressure, heart rate, temperature, respiratory rate, peripheral capillary oxygen saturation (SpO2), and age—can improve clinical outcomes in patients with severe sepsis.14 The alerts generated by the algorithm were used to guide treatment. Average length of stay was 13 days in controls, compared with 10.3 days in those evaluated with the ML algorithm. The algorithm was also associated with a 12.4% drop in in-hospital mortality.
Continue to: Addressing challenges, tapping resources
Addressing challenges, tapping resources
Advances in the management of diabetic retinopathy, colorectal cancer, and sepsis are the tip of the AI iceberg. There are now ML programs to distinguish melanoma from benign nevi; to improve insulin dosing for patients with type 1 diabetes; to predict which hospital patients are most likely to end up in the intensive care unit; and to mitigate the opioid epidemic.
An ML Web page on the JAMA Network (https://sites.jamanetwork.com/machine-learning/) features a long list of published research studies, reviews, and opinion papers suggesting that the future of medicine is closely tied to innovative developments in this area. This Web page also addresses the potential use of ML in detecting lymph node metastases in breast cancer, the need to temper AI with human intelligence, the role of AI in clinical decision support, and more.
The JAMA Network also discusses a few of the challenges that still need to be overcome in developing ML tools for clinical medicine—challenges that you will want to be cognizant of as you evaluate new research in the field.
Black-box dilemma. A challenge that technologists face as they introduce new programs that have the potential to improve diagnosis, treatment, and prognosis is a phenomenon called the “black-box dilemma,” which refers to the complex data science, advanced statistics, and mathematical equations that underpin ML algorithms. These complexities make it difficult to explain the mechanism of action upon which software is based, which, in turn, makes many clinicians skeptical about its worth.
For example, the neural networks that are the backbone of the retinopathy algorithm discussed earlier might seem like voodoo science to those unfamiliar with the technology. It’s fortunate that several technology-savvy physicians have mastered these digital tools and have the teaching skills to explain them in plain-English tutorials. One such tutorial, “Understanding How Machine Learning Works,” is posted on the JAMA Network (https://sites.jamanetwork.com/machine-learning/#multimedia). A more basic explanation was included in a recent Public Broadcasting System “Nova” episode, viewable at www.youtube.com/watch?v=xS2G0oolHpo.
Continue to: Limited analysis
Limited analysis. Another problem that plagues many ML-based algorithms is that they have been tested on only a single data set. (Typically, a data set refers to a collection of clinical parameters from a patient population.) For example, researchers developing an algorithm might collect their data from a single health care system.
Several investigators have addressed this shortcoming by testing their software on 2 completely independent patient populations. Banda and colleagues15 recently developed a software platform to improve the detection rate in familial hypercholesterolemia, a significant cause of premature cardiovascular disease and death that affects approximately 1 of every 250 people. Despite the urgency of identifying the disorder and providing potentially lifesaving treatment, only 10% of patients receive an accurate diagnosis.16 Banda and colleagues developed a deep-learning algorithm that is far more effective than the traditional screening approach now in use.
To address the generalizability of the algorithm, it was tested on EHR data from 2 independent health care systems: Stanford Health Care and Geisinger Health System. In Stanford patients, the positive predictive value of the algorithm was 88%, with a sensitivity of 75%; it identified 84% of affected patients at the highest probability threshold. In Geisinger patients, the classifier generated a positive predictive value of 85%.
The future of these technologies
AI and ML are not panaceas that will revolutionize medicine in the near future. Likewise, the digital tools discussed in this article are not going to solve multiple complex medical problems addressed during a single office visit. But physicians who ignore mounting evidence that supports these emerging technologies will be left behind by more forward-thinking colleagues.
A recent commentary in Gastroenterology17 sums up the situation best: “It is now too conservative to suggest that CADe [computer-assisted detection] and CADx [computer-assisted diagnosis] carry the potential to revolutionize colonoscopy. The artificial intelligence revolution has already begun.”
CORRESPONDENCE
Paul Cerrato, MA, cerrato@aol.com, pcerrato@optonline.net. John Halamka, MD, MS, john.halamka@bilh.org.
Computer technology and artificial intelligence (AI) have come a long way in several decades:
- Between 1971 and 1996, access to the Medline database was primarily limited to university libraries and other institutions; in 1997, the database became universally available online as PubMed.1
- In 2004, the President of the United States issued an executive order that launched a 10-year plan to put electronic health records (EHRs) in place nationwide; EHRs are now employed in nearly 9 of 10 (85.9%) medical offices.2
Over time, numerous online resources sprouted as well, including DxPlain, UpToDate, and Clinical Key, to name a few. These digital tools were impressive for their time, but many of them are now considered “old-school” AI-enabled clinical decision support.
In the past 2 to 3 years, innovative clinicians and technologists have pushed medicine into a new era that takes advantage of machine learning (ML)-enhanced diagnostic aids, software systems that predict disease progression, and advanced clinical pathways to help individualize treatment. Enthusiastic early adopters believe these resources are transforming patient care—although skeptics remain unconvinced, cautioning that they have yet to prove their worth in everyday clinical practice.
In this review, we first analyze the strengths and weaknesses of evidence supporting these tools, then propose a potential role for them in family medicine.
Machine learning takes on retinopathy
The term “artificial intelligence” has been with us for longer than a half century.3 In the broadest sense, AI refers to any computer system capable of automating a process usually performed manually by humans. But the latest innovations in AI take advantage of a subset of AI called “machine learning”: the ability of software systems to learn new functionality or insights on their own, without additional programming from human data engineers. Case in point: A software platform has been developed that is capable of diagnosing or screening for diabetic retinopathy without the involvement of an experienced ophthalmologist.
The landmark study that started clinicians and health care executives thinking seriously about the potential role of ML in medical practice was spearheaded by Varun Gulshan, PhD, at Google, and associates from several medical schools.4 Gulshan used an artificial neural network designed to mimic the functions of the human nervous system to analyze more than 128,000 retinal images, looking for evidence of diabetic retinopathy. (See “Deciphering artificial neural networks,” for an explanation of how such networks function.5) The algorithm they employed was compared with the diagnostic skills of several board-certified ophthalmologists.
[polldaddy:10453606]
Continue to: Deciperhing artificial neural networks
Deciphering artificial neural networks
The promise of health care information technology relies heavily on statistical methods and software constructs, including logistic regression, random forest modeling, clustering, and neural networks. The machine learning-enabled image analysis used to detect diabetic retinopathy and to differentiate a malignant melanoma and a normal mole is based on neural networking.
As we discussed in the body of this article, these networks mimic the nervous system, in that they comprise computer-generated “neurons,” or nodes, and are connected by “synapses” (FIGURE5). When a node in Layer 1 is excited by pixels coming from a scanned image, it sends on that excitement, represented by a numerical value, to a second set of nodes in Layer 2, which, in turns, sends signals to the next layer— and so on.
Eventually, the software’s interpretation of the pixels of the image reaches the output layer of the network, generating a negative or positive diagnosis. The initial process results in many interpretations, which are corrected by a backward analytic process called backpropagation. The video tutorials mentioned in the main text provide a more detailed explanation of neural networking.
Using an area-under-the-receiver operating curve (AUROC) as a metric, and choosing an operating point for high specificity, the algorithm generated sensitivity of 87% and 90.3% and specificity of 98.1% and 98.5% for 2 validation data sets for detecting referable retinopathy, as defined by a panel of at least 7 ophthalmologists. When AUROC was set for high sensitivity, the algorithm generated sensitivity of 97.5% and 96.1% and specificity of 93.4% and 93.9% for the 2 data sets.
These results are impressive, but the researchers used a retrospective approach in their analysis. A prospective analysis would provide stronger evidence.
That shortcoming was addressed by a pivotal clinical trial that convinced the US Food and Drug Administration (FDA) to approve the technology. Michael Abramoff, MD, PhD, at the University of Iowa Department of Ophthalmology and Visual Sciences and his associates6 conducted a prospective study that compared the gold standard for detecting retinopathy, the Fundus Photograph Reading Center (of the University of Wisconsin School of Medicine and Public Health), to an ML-based algorithm, the commercialized IDx-DR. The IDx-DR is a software system that is used in combination with a fundal camera to capture retinal images. The researchers found that “the AI system exceeded all pre-specified superiority endpoints at sensitivity of 87.2% ... [and] specificity of 90.7% ....”
Continue to: The FDA clearance statement...
The FDA clearance statement for this technology7 limits its use, emphasizing that it is intended only as a screening tool, not a stand-alone diagnostic system. Because IDx-DR is being used in primary care, the FDA states that patients who have a positive result should be referred to an eye care professional. The technology is contraindicated in patients who have a history of laser treatment, surgery, or injection in the eye or who have any of the following: persistent vision loss, blurred vision, floaters, previously diagnosed macular edema, severe nonproliferative retinopathy, proliferative retinopathy, radiation retinopathy, and retinal vein occlusion. It is also not intended for pregnant patients because their eye disease often progresses rapidly.
Additional caveats to keep in mind when evaluating this new technology include that, although the software can help detect retinopathy, it does not address other key issues for this patient population, including cataracts and glaucoma. The cost of the new technology also requires attention: Software must be used in conjunction with a specific retinal camera, the Topcon TRC-NW400, which is expensive (new, as much as $20,000).
Speaking of cost: Health care providers and insurers still question whether implementing AI-enabled systems is cost-effective. It is too early to say definitively how AI and machine learning will have an impact on health care expenditures, because the most promising technological systems have yet to be fully implemented in hospitals and medical practices nationwide. Projections by Forbes suggest that private investment in health care AI will reach $6.6 billion by 2021; on a more confident note, an Accenture analysis predicts that the best possible application of AI might save the health care sector $150 billion annually by 2026.8
What role might this diabetic retinopathy technology play in family medicine? Physicians are constantly advising patients who have diabetes about the need to have a regular ophthalmic examination to check for early signs of retinopathy—advice that is often ignored. The American Academy of Ophthalmology points out that “6 out of 10 people with diabetes skip a sight-saving exam.”9 When a patient is screened with this type of device and found to be at high risk of eye disease, however, the advice to see an eye-care specialist might carry more weight.
Screening colonoscopy: Improving patient incentives
No responsible physician doubts the value of screening colonoscopy in patients 50 years and older, but many patients have yet to realize that the procedure just might save their life. Is there a way to incentivize resistant patients to have a colonoscopy performed? An ML-based software system that only requires access to a few readily available parameters might be the needed impetus for many patients.
Continue to: A large-scale validation...
A large-scale validation study performed on data from Kaiser Permanente Northwest found that it is possible to estimate a person’s risk of colorectal cancer by using age, gender, and complete blood count.10 This retrospective investigation analyzed more than 17,000 Kaiser Permanente patients, including 900 who already had colorectal cancer. The analysis generated a risk score for patients who did not have the malignancy to gauge their likelihood of developing it. The algorithms were more sensitive for detecting tumors of the cecum and ascending colon, and less sensitive for detection of tumors of the transverse and sigmoid colon and rectum.
To provide more definitive evidence to support the value of the software platform, a prospective study was subsequently conducted on more than 79,000 patients who had initially declined to undergo colorectal screening. The platform, called ColonFlag, was used to detect 688 patients at highest risk, who were then offered screening colonoscopy. In this subgroup, 254 agreed to the procedure; ColonFlag identified 19 malignancies (7.5%) among patients within the Maccabi Health System (Israel), and 15 more in patients outside that health system.11 (In the United States, the same program is known as LGI Flag and has been cleared by the FDA.)
Although ColonFlag has the potential to reduce the incidence of colorectal cancer, other evidence-based screening modalities are highlighted in US Preventive Services Task Force guidelines, including the guaiac-based fecal occult blood test and the fecal immunochemical test.12
Beyond screening to applications in managing disease
The complex etiology of sepsis makes the condition difficult to treat. That complexity has also led to disagreement on the best course of management. Using an ML algorithm called an “Artificial Intelligence Clinician,” Komorowski and associates13 extracted data from a large data set from 2 nonoverlapping intensive care unit databases collected from US adults.The researchers’ analysis suggested a list of 48 variables that likely influence sepsis outcomes, including:
- demographics,
- Elixhauser premorbid status,
- vital signs,
- clinical laboratory data,
- intravenous fluids given, and
- vasopressors administered.
Komorowski and co-workers concluded that “… mortality was lowest in patients for whom clinicians’ actual doses matched the AI decisions. Our model provides individualized and clinically interpretable treatment decisions for sepsis that could improve patient outcomes.”
A randomized clinical trial has found that an ML program that uses only 6 common clinical markers—blood pressure, heart rate, temperature, respiratory rate, peripheral capillary oxygen saturation (SpO2), and age—can improve clinical outcomes in patients with severe sepsis.14 The alerts generated by the algorithm were used to guide treatment. Average length of stay was 13 days in controls, compared with 10.3 days in those evaluated with the ML algorithm. The algorithm was also associated with a 12.4% drop in in-hospital mortality.
Continue to: Addressing challenges, tapping resources
Addressing challenges, tapping resources
Advances in the management of diabetic retinopathy, colorectal cancer, and sepsis are the tip of the AI iceberg. There are now ML programs to distinguish melanoma from benign nevi; to improve insulin dosing for patients with type 1 diabetes; to predict which hospital patients are most likely to end up in the intensive care unit; and to mitigate the opioid epidemic.
An ML Web page on the JAMA Network (https://sites.jamanetwork.com/machine-learning/) features a long list of published research studies, reviews, and opinion papers suggesting that the future of medicine is closely tied to innovative developments in this area. This Web page also addresses the potential use of ML in detecting lymph node metastases in breast cancer, the need to temper AI with human intelligence, the role of AI in clinical decision support, and more.
The JAMA Network also discusses a few of the challenges that still need to be overcome in developing ML tools for clinical medicine—challenges that you will want to be cognizant of as you evaluate new research in the field.
Black-box dilemma. A challenge that technologists face as they introduce new programs that have the potential to improve diagnosis, treatment, and prognosis is a phenomenon called the “black-box dilemma,” which refers to the complex data science, advanced statistics, and mathematical equations that underpin ML algorithms. These complexities make it difficult to explain the mechanism of action upon which software is based, which, in turn, makes many clinicians skeptical about its worth.
For example, the neural networks that are the backbone of the retinopathy algorithm discussed earlier might seem like voodoo science to those unfamiliar with the technology. It’s fortunate that several technology-savvy physicians have mastered these digital tools and have the teaching skills to explain them in plain-English tutorials. One such tutorial, “Understanding How Machine Learning Works,” is posted on the JAMA Network (https://sites.jamanetwork.com/machine-learning/#multimedia). A more basic explanation was included in a recent Public Broadcasting System “Nova” episode, viewable at www.youtube.com/watch?v=xS2G0oolHpo.
Continue to: Limited analysis
Limited analysis. Another problem that plagues many ML-based algorithms is that they have been tested on only a single data set. (Typically, a data set refers to a collection of clinical parameters from a patient population.) For example, researchers developing an algorithm might collect their data from a single health care system.
Several investigators have addressed this shortcoming by testing their software on 2 completely independent patient populations. Banda and colleagues15 recently developed a software platform to improve the detection rate in familial hypercholesterolemia, a significant cause of premature cardiovascular disease and death that affects approximately 1 of every 250 people. Despite the urgency of identifying the disorder and providing potentially lifesaving treatment, only 10% of patients receive an accurate diagnosis.16 Banda and colleagues developed a deep-learning algorithm that is far more effective than the traditional screening approach now in use.
To address the generalizability of the algorithm, it was tested on EHR data from 2 independent health care systems: Stanford Health Care and Geisinger Health System. In Stanford patients, the positive predictive value of the algorithm was 88%, with a sensitivity of 75%; it identified 84% of affected patients at the highest probability threshold. In Geisinger patients, the classifier generated a positive predictive value of 85%.
The future of these technologies
AI and ML are not panaceas that will revolutionize medicine in the near future. Likewise, the digital tools discussed in this article are not going to solve multiple complex medical problems addressed during a single office visit. But physicians who ignore mounting evidence that supports these emerging technologies will be left behind by more forward-thinking colleagues.
A recent commentary in Gastroenterology17 sums up the situation best: “It is now too conservative to suggest that CADe [computer-assisted detection] and CADx [computer-assisted diagnosis] carry the potential to revolutionize colonoscopy. The artificial intelligence revolution has already begun.”
CORRESPONDENCE
Paul Cerrato, MA, cerrato@aol.com, pcerrato@optonline.net. John Halamka, MD, MS, john.halamka@bilh.org.
1. Lindberg DA. Internet access to National Library of Medicine. Eff Clin Pract. 2000;3:256-260.
2. National Center for Health Statistics, Centers for Disease Control and Prevention. Electronic medical records/electronic health records (EMRs/EHRs). www.cdc.gov/nchs/fastats/electronic-medical-records.htm. Updated March 31, 2017. Accessed October 1, 2019.
3. Smith C, McGuire B, Huang T, et al. The history of artificial intelligence. University of Washington. https://courses.cs.washington.edu/courses/csep590/06au/projects/history-ai.pdf. Published December 2006. Accessed October 1, 2019.
4. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA; 2016;316:2402-2410.
5. Cerrato P, Halamka J. The Transformative Power of Mobile Medicine. Cambridge, MA: Academic Press; 2019.
6. Abràmoff MD, Lavin PT, Birch M, et al. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39.
7. US Food and Drug Administration. FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems. Press release. www.fda.gov/news-events/press-announcements/fda-permits-marketing-artificial-intelligence-based-device-detect-certain-diabetes-related-eye. Published April 11, 2018. Accessed October 1, 2019.
8. AI and healthcare: a giant opportunity. Forbes Web site. www.forbes.com/sites/insights-intelai/2019/02/11/ai-and-healthcare-a-giant-opportunity/#5906c4014c68. Published February 11, 2019. Accessed October 25, 2019.
9. Boyd K. Six out of 10 people with diabetes skip a sight-saving exam. American Academy of Ophthalmology Website. https://www.aao.org/eye-health/news/sixty-percent-skip-diabetic-eye-exams. Published November 1, 2016. Accessed October 25, 2019.
10. Hornbrook MC, Goshen R, Choman E, et al. Early colorectal cancer detected by machine learning model using gender, age, and complete blood count data. Dig Dis Sci. 2017;62:2719-2727.
11. Goshen R, Choman E, Ran A, et al. Computer-assisted flagging of individuals at high risk of colorectal cancer in a large health maintenance organization using the ColonFlag test. JCO Clin Cancer Inform. 2018;2:1-8.
12. US Preventive Services Task Force. Final recommendation statement: colorectal cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/colorectal-cancer-screening2#tab. Published May 2019. Accessed October 1, 2019.
13. Komorowski M, Celi LA, Badawi O, et al. The artificial intelligence clinician learns optimal treatment strategies for sepsis in intensive care. Nat Med. 2018;24:1716-1720.
14. Shimabukuro DW, Barton CW, Feldman MD, et al. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Respir Res. 2017;4:e000234.
15. Banda J, Sarraju A, Abbasi F, et al. Finding missed cases of familial hypercholesterolemia in health systems using machine learning. NPJ Digit Med. 2019;2:23.
16. What is familial hypercholesterolemia? FH Foundation Web site. https://thefhfoundation.org/familial-hypercholesterolemia/what-is-familial-hypercholesterolemia. Accessed November 1, 2019.
17. Byrne MF, Shahidi N, Rex DK. Will computer-aided detection and diagnosis revolutionize colonoscopy? Gastroenterology. 2017;153:1460-1464.E1.
1. Lindberg DA. Internet access to National Library of Medicine. Eff Clin Pract. 2000;3:256-260.
2. National Center for Health Statistics, Centers for Disease Control and Prevention. Electronic medical records/electronic health records (EMRs/EHRs). www.cdc.gov/nchs/fastats/electronic-medical-records.htm. Updated March 31, 2017. Accessed October 1, 2019.
3. Smith C, McGuire B, Huang T, et al. The history of artificial intelligence. University of Washington. https://courses.cs.washington.edu/courses/csep590/06au/projects/history-ai.pdf. Published December 2006. Accessed October 1, 2019.
4. Gulshan V, Peng L, Coram M, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA; 2016;316:2402-2410.
5. Cerrato P, Halamka J. The Transformative Power of Mobile Medicine. Cambridge, MA: Academic Press; 2019.
6. Abràmoff MD, Lavin PT, Birch M, et al. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018;1:39.
7. US Food and Drug Administration. FDA permits marketing of artificial intelligence-based device to detect certain diabetes-related eye problems. Press release. www.fda.gov/news-events/press-announcements/fda-permits-marketing-artificial-intelligence-based-device-detect-certain-diabetes-related-eye. Published April 11, 2018. Accessed October 1, 2019.
8. AI and healthcare: a giant opportunity. Forbes Web site. www.forbes.com/sites/insights-intelai/2019/02/11/ai-and-healthcare-a-giant-opportunity/#5906c4014c68. Published February 11, 2019. Accessed October 25, 2019.
9. Boyd K. Six out of 10 people with diabetes skip a sight-saving exam. American Academy of Ophthalmology Website. https://www.aao.org/eye-health/news/sixty-percent-skip-diabetic-eye-exams. Published November 1, 2016. Accessed October 25, 2019.
10. Hornbrook MC, Goshen R, Choman E, et al. Early colorectal cancer detected by machine learning model using gender, age, and complete blood count data. Dig Dis Sci. 2017;62:2719-2727.
11. Goshen R, Choman E, Ran A, et al. Computer-assisted flagging of individuals at high risk of colorectal cancer in a large health maintenance organization using the ColonFlag test. JCO Clin Cancer Inform. 2018;2:1-8.
12. US Preventive Services Task Force. Final recommendation statement: colorectal cancer: screening. www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/colorectal-cancer-screening2#tab. Published May 2019. Accessed October 1, 2019.
13. Komorowski M, Celi LA, Badawi O, et al. The artificial intelligence clinician learns optimal treatment strategies for sepsis in intensive care. Nat Med. 2018;24:1716-1720.
14. Shimabukuro DW, Barton CW, Feldman MD, et al. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Respir Res. 2017;4:e000234.
15. Banda J, Sarraju A, Abbasi F, et al. Finding missed cases of familial hypercholesterolemia in health systems using machine learning. NPJ Digit Med. 2019;2:23.
16. What is familial hypercholesterolemia? FH Foundation Web site. https://thefhfoundation.org/familial-hypercholesterolemia/what-is-familial-hypercholesterolemia. Accessed November 1, 2019.
17. Byrne MF, Shahidi N, Rex DK. Will computer-aided detection and diagnosis revolutionize colonoscopy? Gastroenterology. 2017;153:1460-1464.E1.
PRACTICE RECOMMENDATIONS
› Encourage patients with diabetes who are unwilling to have a regular eye exam to have an artificial intelligence-based retinal scan that can detect retinopathy. B
› Consider using a machine learning-based algorithm to help evaluate the risk of colorectal cancer in patients who are resistant to screening colonoscopy. B
› Question the effectiveness of any artificial intelligence-based software algorithm that has not been validated by at least 2 independent data sets derived from clinical parameters. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
How to use type 2 diabetes meds to lower CV disease risk
The association between type 2 diabetes (T2D) and cardiovascular (CV) disease is well-established:
- Type 2 diabetes approximately doubles the risk of coronary artery disease, stroke, and peripheral arterial disease, independent of conventional risk factors1
- CV disease is the leading cause of morbidity and mortality in patients with T2D
- CV disease is the largest contributor to direct and indirect costs of the health care of patients who have T2D.2
In recent years, new classes of agents for treating T2D have been introduced (TABLE 1). Prior to 2008, the US Food and Drug Administration (FDA) approved drugs in those new classes based simply on their effectiveness in reducing the blood glucose level. Concerns about the CV safety of specific drugs (eg, rosiglitazone, muraglitazar) emerged from a number of trials, suggesting that these agents might increase the risk of CV events.3,4
Consequently, in 2008, the FDA issued guidance to the pharmaceutical industry: Preapproval and postapproval trials of all new antidiabetic drugs must now assess potential excess CV risk.5 CV outcomes trials (CVOTs), performed in accordance with FDA guidelines, have therefore become the focus of evaluating novel treatment options. In most CVOTs, combined primary CV endpoints have included CV mortality, nonfatal myocardial infarction (MI), and nonfatal stroke—taken together, what is known as the composite of these 3 major adverse CV events, or MACE-3.
To date, 15 CVOTs have been completed, assessing 3 novel classes of antihyperglycemic agents:
- dipeptidyl peptidase-4 (DPP-4) inhibitors
- glucagon-like peptide-1 (GLP-1) receptor agonists
- sodium–glucose cotransporter-2 (SGLT-2) inhibitors.
None of these trials identified any increased incidence of MACE; 7 found CV benefit. This review summarizes what the CVOTs revealed about these antihyperglycemic agents and their ability to yield a reduction in MACE and a decrease in all-cause mortality in patients with T2D and elevated CV disease risk. Armed with this information, you will have the tools you need to offer patients with T2D CV benefit while managing their primary disease.
Cardiovascular outcomes trials: DPP-4 inhibitors
Four trials. Trials of DPP-4 inhibitors that have been completed and reported are of saxagliptin (SAVOR-TIMI 536), alogliptin (EXAMINE7), sitagliptin (TECOS8), and linagliptin (CARMELINA9); others are in progress. In general, researchers enrolled patients at high risk of CV events, although inclusion criteria varied substantially. Consistently, these studies demonstrated that DPP-4 inhibition neither increased nor decreased (ie, were noninferior) the 3-point MACE (SAVOR-TIMI 53 noninferiority, P < .001; EXAMINE, P < .001; TECOS, P < .001).
Continue to: Rather than improve...
Rather than improve CV outcomes, there was some evidence that DPP-4 inhibitors might be associated with an increased risk of hospitalization for heart failure (HHF). In the SAVOR-TIMI 53 trial, patients randomized to saxagliptin had a 0.7% absolute increase in risk of HHF (P = .98).6 In the EXAMINE trial, patients treated with alogliptin showed a nonsignificant trend for HHF.10 In both the TECOS and CARMELINA trials, no difference was recorded in the rate of HHF.8,9,11 Subsequent meta-analysis that summarized the risk of HHF in CVOTs with DPP-4 inhibitors indicated a nonsignificant trend to increased risk.12
From these trials alone, it appears that DPP-4 inhibitors are unlikely to provide CV benefit. Data from additional trials are needed to evaluate the possible association between these medications and heart failure (HF). However, largely as a result of the findings from SAVOR-TIMI 53 and EXAMINE, the FDA issued a Drug Safety Communication in April 2016, adding warnings about HF to the labeling of saxagliptin and alogliptin.13
CARMELINA was designed to also evaluate kidney outcomes in patients with T2D. As with other DPP-4 inhibitor trials, the primary aim was to establish noninferiority, compared with placebo, for time to MACE-3 (P < .001). Secondary outcomes were defined as time to first occurrence of end-stage renal disease, death due to renal failure, and sustained decrease from baseline of ≥ 40% in the estimated glomerular filtration rate. The incidence of the secondary kidney composite results was not significantly different between groups randomized to linagliptin or placebo.9
Cardiovascular outcomes trials: GLP-1 receptor agonists
ELIXA. The CV safety of GLP-1 receptor agonists has been evaluated in several randomized clinical trials. The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial was the first14: Lixisenatide was studied in 6068 patients with recent hospitalization for acute coronary syndrome. Lixisenatide therapy was neutral with regard to CV outcomes, which met the primary endpoint: noninferiority to placebo (P < .001). There was no increase in either HF or HHF.
Continue to: LEADER
LEADER. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial (LEADER) evaluated long-term effects of liraglutide, compared to placebo, on CV events in patients with T2D.15 It was a multicenter, double-blind, placebocontrolled study that followed 9340 participants, most (81%) of whom had established CV disease, over 5 years. LEADER is considered a landmark study because it was the first large CVOT to show significant benefit for a GLP-1 receptor agonist.
Liraglutide demonstrated reductions in first occurrence of death from CV causes, nonfatal MI or nonfatal stroke, overall CV mortality, and all-cause mortality. The composite MACE-3 showed a relative risk reduction (RRR) of 13%, equivalent to an absolute risk reduction (ARR) of 1.9% (noninferiority, P < .001; superiority, P < .01). The RRR was 22% for death from CV causes, with an ARR of 1.3% (P = .007); the RRR for death from any cause was 15%, with an ARR of 1.4% (P = .02).
In addition, there was a lower rate of nephropathy (1.5 events for every 100 patient–years in the liraglutide group [P = .003], compared with 1.9 events every 100 patient–years in the placebo group).15
Results clearly demonstrated benefit. No significant difference was seen in the liraglutide rate of HHF, compared to the rate in the placebo group.
SUSTAIN-6. Evidence for the CV benefit of GLP-1 receptor agonists was also demonstrated in the phase 3 Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6).16 This was a study of 3297 patients with T2D at high risk of CV disease and with a mean hemoglobin A1c (HbA1c) value of 8.7%, 83% of whom had established CV disease. Patients were randomized to semaglutide or placebo. Note: SUSTAIN-6 was a noninferiority safety study; as such, it was not actually designed to assess or establish superiority.
Continue to: The incidence of MACE-3...
The incidence of MACE-3 was significantly reduced among patients treated with semaglutide (P = .02) after median followup of 2.1 years. The expanded composite outcome (death from CV causes, nonfatal MI, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina or HF), also showed a significant reduction with semaglutide (P = .002), compared with placebo. There was no difference in the overall hospitalization rate or rate of death from any cause.
EXSCEL. The Exenatide Study of Cardiovascular Event Lowering trial (EXSCEL)17,18 was a phase III/IV, double-blind, pragmatic placebo-controlled study of 14,752 patients at any level of CV risk, for a median 3.2 years. The study population was intentionally more diverse than in earlier GLP-1 receptor agonist studies. The researchers hypothesized that patients at increased risk of MACE would experience a comparatively greater relative treatment benefit with exenatide than those at lower risk. That did not prove to be the case.
EXSCEL did confirm noninferiority compared with placebo (P < .001), but once-weekly exenatide resulted in a nonsignificant reduction in major adverse CV events, and a trend for RRR in all-cause mortality (RRR = 14%; ARR = 1% [P = .06]).
HARMONY OUTCOMES. The Albiglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes and Cardiovascular Disease study (HARMONY OUTCOMES)19 was a double-blind, randomized, placebocontrolled trial conducted at 610 sites across 28 countries. The study investigated albiglutide, 30 to 50 mg once weekly, compared with placebo. It included 9463 patients ages ≥ 40 years with T2D who had an HbA1c > 7% (median value, 8.7%) and established CV disease. Patients were evaluated for a median 1.6 years.
Albiglutide reduced the risk of CV causes of death, nonfatal MI, and nonfatal stroke by an RRR of 22%, (ARR, 2%) (noninferiority, P < .0001; superiority, P < .0006).
Continue to: REWIND
REWIND. The Researching Cardiovascular Events with a Weekly INcretin in Diabetes trial (REWIND),20 the most recently completed GLP-1 receptor agonist CVOT (presented at the 2019 American Diabetes Association [ADA] Conference in June and published simultaneously in The Lancet), was a multicenter, randomized, double-blind placebo-controlled trial designed to assess the effect of weekly dulaglutide, 1.5 mg, compared with placebo, in 9901 participants enrolled at 371 sites in 24 countries. Mean patient age was 66.2 years, with women constituting 4589 (46.3%) of participants.
REWIND was distinct from other CVOTs in several ways:
- Other CVOTs were designed to show noninferiority compared with placebo regarding CV events; REWIND was designed to establish superiority
- In contrast to trials of other GLP-1 receptor agonists, in which most patients had established CV disease, only 31% of REWIND participants had a history of CV disease or a prior CV event (although 69% did have CV risk factors without underlying disease)
- REWIND was much longer (median follow-up, 5.4 years) than other GLP-1 receptor agonist trials (median follow-up, 1.5 to 3.8 years).
In REWIND, the primary composite outcome of MACE-3 occurred in 12% of participants assigned to dulaglutide, compared with 13.1% assigned to placebo (P = .026). This equated to 2.4 events for every 100 person– years on dulaglutide, compared with 2.7 events for every 100 person–years on placebo. There was a consistent effect on all MACE-3 components, although the greatest reductions were observed in nonfatal stroke (P = .017). Overall risk reduction was the same for primary and secondary prevention cohorts (P = .97), as well as in patients with either an HbA1c value < 7.2% or ≥ 7.2% (P = .75). Risk reduction was consistent across age, sex, duration of T2D, and body mass index.
Dulaglutide did not significantly affect the incidence of all-cause mortality, heart failure, revascularization, or hospital admission. Forty-seven percent of patients taking dulaglutide reported gastrointestinal adverse effects (P = .0001).
In a separate analysis of secondary outcomes, 21 dulaglutide reduced the composite renal outcomes of new-onset macroalbuminuria (P = .0001); decline of ≥ 30% in the estimated glomerular filtration rate (P = .066); and chronic renal replacement therapy (P = .39). Investigators estimated that 1 composite renal outcome event would be prevented for every 31 patients treated with dulaglutide for a median 5.4 years.
Continue to: Cardiovascular outcomes trials...
Cardiovascular outcomes trials: SGLT-2 inhibitors
EMPA-REG OUTCOME. The Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes trial (EMPA-REG OUTCOME) was also a landmark study because it was the first dedicated CVOT to show that an antihyperglycemic agent 1) decreased CV mortality and all-cause mortality, and 2) reduced HHF in patients with T2D and established CV disease.22 In this trial, 7020 patients with T2D who were at high risk of CV events were randomized and treated with empagliflozin, 10 or 25 mg, or placebo, in addition to standard care, and were followed for a median 2.6 years.
Compared with placebo, empagliflozin resulted in an RRR of 14% (ARR, 1.6%) in the primary endpoint of CV death, nonfatal MI, and stroke, confirming study drug superiority (P = .04). When compared with placebo, the empagliflozin group had an RRR of 38% in CV mortality, (ARR < 2.2%) (P < .001); an RRR of 35% in HHF (ARR, 1.4%) (P = .002); and an RRR of 32% (ARR, 2.6%) in death from any cause (P < .001).
CANVAS. The Canagliflozin Cardiovascular Assessment Study (CANVAS) integrated 2 multicenter, placebo-controlled, randomized trials with 10,142 participants and a mean follow-up of 3.6 years.23 Patients were randomized to receive canagliflozin (100-300 mg/d) or placebo. Approximately two-thirds of patients had a history of CV disease (therefore representing secondary prevention); one-third had CV risk factors only (primary prevention).
In CANVAS, patients receiving canagliflozin had a risk reduction in MACE-3, establishing superiority compared with placebo (P < .001). There was also a significant reduction in progression of albuminuria (P < .05). Superiority was not shown for the secondary outcome of death from any cause. Canagliflozin had no effect on the primary endpoint (MACE-3) in the subgroup of participants who did not have a history of CV disease. Similar to what was found with empagliflozin in EMPA-REG OUTCOME, CANVAS participants had a reduced risk of HHF.
Continue to: Patients on canagliflozin...
Patients on canagliflozin unexpectedly had an increased incidence of amputations (6.3 participants, compared with 3.4 participants, for every 1000 patient–years). This finding led to a black box warning for canagliflozin about the risk of lower-limb amputation.
DECLARE-TIMI 58. The Dapagliflozin Effect of Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 trial (DECLARETIMI 58) was the largest SGLT-2 inhibitor outcomes trial to date, enrolling 17,160 patients with T2D who also had established CV disease or multiple risk factors for atherosclerotic CV disease. The trial compared dapagliflozin, 10 mg/d, and placebo, following patients for a median 4.2 years.24 Unlike CANVAS and EMPA-REG OUTCOME, DECLARE-TIMI 58 included CV death and HHF as primary outcomes, in addition to MACE-3.
Dapagliflozin was noninferior to placebo with regard to MACE-3. However, its use did result in a lower rate of CV death and HHF by an RRR of 17% (ARR, 1.9%). Risk reduction was greatest in patients with HF who had a reduced ejection fraction (ARR = 9.2%).25
In October, the FDA approved dapagliflozin to reduce the risk of HHF in adults with T2D and established CV disease or multiple CV risk factors. Before initiating the drug, physicians should evaluate the patient's renal function and monitor periodically.
Meta-analyses of SGLT-2 inhibitors
Systematic review. Usman et al released a meta-analysis in 2018 that included 35 randomized, placebo-controlled trials (including EMPA-REG OUTCOME, CANVAS, and DECLARE-TIMI 58) that had assessed the use of SGLT-2 inhibitors in nearly 35,000 patients with T2D.26 This review concluded that, as a class, SGLT-2 inhibitors reduce all-cause mortality, major adverse cardiac events, nonfatal MI, and HF and HHF, compared with placebo.
Continue to: CVD-REAL
CVD-REAL. A separate study, Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors (CVD-REAL), of 154,528 patients who were treated with canagliflozin, dapagliflozin, or empagliflozin, showed that initiation of SGLT-2 inhibitors, compared with other glucose- lowering therapies, was associated with a 39% reduction in HHF; a 51% reduction in death from any cause; and a 46% reduction in the composite of HHF or death (P < .001).27
CVD-REAL was unique because it was the largest real-world study to assess the effectiveness of SGLT-2 inhibitors on HHF and mortality. The study utilized data from patients in the United States, Norway, Denmark, Sweden, Germany, and the United Kingdom, based on information obtained from medical claims, primary care and hospital records, and national registries that compared patients who were either newly started on an SGLT-2 inhibitor or another glucose-lowering drug. The drug used by most patients in the trial was canagliflozin (53%), followed by dapagliflozin (42%), and empagliflozin (5%).
In this meta-analysis, similar therapeutic effects were seen across countries, regardless of geographic differences, in the use of specific SGLT-2 inhibitors, suggesting a class effect. Of particular significance was that most (87%) patients enrolled in CVD-REAL did not have prior CV disease. Despite this, results for examined outcomes in CVD-REAL were similar to what was seen in other SGLT-2 inhibitor trials that were designed to study patients with established CV disease.
Risk of adverse effects of newer antidiabetic agents
DPP-4 inhibitors. Alogliptin and sitagliptin carry a black-box warning about potential risk of HF. In SAVOR-TIMI, a 27% increase was detected in the rate of HHF after approximately 2 years of saxagliptin therapy.6 Although HF should not be considered a class effect for DPP-4 inhibitors, patients who have risk factors for HF should be monitored for signs and symptoms of HF.
Continue to: Cases of acute pancreatitis...
Cases of acute pancreatitis have been reported in association with all DPP-4 inhibitors available in the United States. A combined analysis of DDP-4 inhibitor trials suggested an increased relative risk of 79% and an absolute risk of 0.13%, which translates to 1 or 2 additional cases of acute pancreatitis for every 1000 patients treated for 2 years.28
There have been numerous postmarketing reports of severe joint pain in patients taking a DPP-4 inhibitor. Most recently, cases of bullous pemphigoid have been reported after initiation of DPP-4 inhibitor therapy.29
GLP-1 receptor agonists carry a black box warning for medullary thyroid (C-cell) tumor risk. GLP-1 receptor agonists are contraindicated in patients with a personal or family history of this cancer, although this FDA warning is based solely on observations from animal models.
In addition, GLP-1 receptor agonists can increase the risk of cholecystitis and pancreatitis. Not uncommonly, they cause gastrointestinal symptoms when first started and when the dosage is titrated upward. Most GLP-1 receptor agonists can be used in patients with renal impairment, although data regarding their use in Stages 4 and 5 chronic kidney disease are limited.30 Semaglutide was found, in the SUSTAIN-6 trial, to be associated with an increased rate of complications of retinopathy, including vitreous hemorrhage and blindness (P = .02)31
SGLT-2 inhibitors are associated with an increased incidence of genitourinary infection, bone fracture (canagliflozin), amputation (canagliflozin), and euglycemic diabetic ketoacidosis. Agents in this class should be avoided in patients with moderate or severe renal impairment, primarily due to a lack of efficacy. They are contraindicated in patients with an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2. (Dapagliflozin is not recommended when eGFR is < 45 mL/min/ 1.73 m2.) These agents carry an FDA warning about the risk of acute kidney injury.30
Continue to: Summing up
Summing up
All glucose-lowering medications used to treat T2D are not equally effective in reducing CV complications. Recent CVOTs have uncovered evidence that certain antidiabetic agents might confer CV and all-cause mortality benefits (TABLE 26,7,9,11,14-17,19-24).
Discussion of proposed mechanisms for CV outcome superiority of these agents is beyond the scope of this review. It is generally believed that benefits result from mechanisms other than a reduction in the serum glucose level, given the relatively short time frame of the studies and the magnitude of the CV benefit. It is almost certain that mechanisms of CV benefit in the 2 landmark studies—LEADER and EMPA-REG OUTCOME—are distinct from each other.32
See “When planning T2D pharmacotherapy, include newer agents that offer CV benefit,” 33-38 for a stepwise approach to treating T2D, including the role of agents that have efficacy in modifying the risk of CV disease.
SIDEBAR
When planning T2D pharmacotherapy, include newer agents that offer CV benefit33-38
First-line management. The 2019 Standards of Medical Care in Diabetes Guidelines established by the American Diabetes Association (ADA) recommend metformin as first-line pharmacotherapy for type 2 diabetes (T2D).33 This recommendation is based on metformin’s efficacy in reducing the blood glucose level and hemoglobin A1C (HbA1C); safety; tolerability; extensive clinical experience; and findings from the UK Prospective Diabetes Study demonstrating a substantial beneficial effect of metformin on cardiovascular (CV) disease.34 Additional benefits of metformin include a decrease in body weight, low-density lipoprotein level, and the need for insulin.
Second-line additive benefit. In addition, ADA guidelines make a highest level (Level-A) recommendation that patients with T2D and established atherosclerotic CV disease be treated with one of the sodium–glucose cotransporter-2 (SGLT-2) inhibitors or glucagon-like peptide-1 (GLP-1) receptor agonists that have demonstrated efficacy in CV disease risk reduction as part of an antihyperglycemic regimen.35 Seven agents described in this article from these 2 unique classes of medications meet the CV disease benefit criterion: liraglutide, semaglutide, albiglutide, dulaglutide, empagliflozin, canagliflozin, and dapagliflozin. Only empagliflozin and liraglutide have received a US Food and Drug Administration indication for risk reduction in major CV events in adults with T2D and established CV disease.
Regarding dulaglutide, although the findings of REWIND are encouraging, results were not robust; further analysis is necessary to make a recommendation for treating patients who do not have a history of established CV disease with this medication.
Individualized decision-making. From a clinical perspective, patient-specific considerations and shared decision-making should be incorporated into T2D treatment decisions:
- For patients with T2D and established atherosclerotic CV disease, SGLT-2 inhibitors and GLP-1 receptor agonists are recommended agents after metformin.
- SGLT-2 inhibitors are preferred in T2D patients with established CV disease and a history of heart failure.
- GLP-1 receptor agonists with proven CV disease benefit are preferred in patients with established CV disease and chronic kidney disease.
Add-on Tx. In ADA guidelines, dipeptidyl peptidase-4 (DDP-4) inhibitors are recommended as an optional add-on for patients without clinical atherosclerotic CV disease who are unable to reach their HbA1C goal after taking metformin for 3 months.33 Furthermore, the American Association of Clinical Endocrinologists lists DPP-4 inhibitors as alternatives for patients with an HbA1C < 7.5% in whom metformin is contraindicated.36 DPP-4 inhibitors are not an ideal choice as a second agent when the patient has a history of heart failure, and should not be recommended over GLP-1 receptor agonists or SGLT-2 inhibitors as second-line agents in patients with T2D and CV disease.
Individualizing management. The current algorithm for T2D management,37 based primarily on HbA1C reduction, is shifting toward concurrent attention to reduction of CV risk (FIGURE38). Our challenge, as physicians, is to translate the results of recent CV outcomes trials into a more targeted management strategy that focuses on eligible populations.
ACKNOWLEDGMENTS
Linda Speer, MD, Kevin Phelps, DO, and Jay Shubrook, DO, provided support and editorial assistance.
CORRESPONDENCE
Robert Gotfried, DO, FAAFP, Department of Family Medicine, University of Toledo College of Medicine, 3333 Glendale Avenue, Toledo, OH 43614; Robert.gotfried@utoledo.edu.
1. Emerging Risk Factors Collaboration; Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215-2222.
2. Chamberlain JJ, Johnson EL, Leal S, et al. Cardiovascular disease and risk management: review of the American Diabetes Association Standards of Medical Care in Diabetes 2018. Ann Intern Med. 2018;168:640-650.
3. Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294:2581-2586.
4. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457-2471.
5. Center for Drug Evaluation and Research, US Food and Drug Administration. Guidance document: Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. www.fda.gov/downloads/drugs/guidance
complianceregulatoryinformation/guidances/ucm071627.pdf. Published December 2008. Accessed October 4, 2019.
6. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patient with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
7. White WB, Canon CP, Heller SR, et al; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327-1335.
8. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
9. Rosenstock J, Perkovic V, Johansen OE, et al; CARMELINA Investigators. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69-79.
10. Zannad F, Cannon CP, Cushman WC, et al. EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
11. McGuire DK, Van de Werf F, Armstrong PW, et al; Trial Evaluating Cardiovascular Outcomes with Sitagliptin Study Group. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:126-135.
12. Toh S, Hampp C, Reichman ME, et al. Risk for hospitalized heart failure among new users of saxagliptin, sitagliptin, and other antihyperglycemic drugs: a retrospective cohort study. Ann Intern Med. 2016;164:705-714.
13. US Food and Drug Administration. FDA drug safety communication: FDA adds warning about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. www.fda.gov/Drugs/DrugSafety/ucm486096.htm. Updated April 5, 2016. Accessed October 4, 2019.
14. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patient with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247-2257.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
17. Mentz RJ, Bethel MA, Merrill P, et al; EXSCEL Study Group. Effect of once-weekly exenatide on clinical outcomes according to baseline risk in patients with type 2 diabetes mellitus: insights from the EXSCEL Trial. J Am Heart Assoc. 2018;7:e009304.
18. Holman RR, Bethel MA, George J, et al. Rationale and design of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial. Am Heart J. 2016;174:103-110.
19. Hernandez AF, Green JB, Janmohamed S, et al; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519-1529.
20. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121-130.
21. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomized, placebo-controlled trial. Lancet. 2019;394:131-138.
22. Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
23. Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
24. Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
25. Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528-2536.
26. Usman MS, Siddiqi TJ, Memon MM, et al. Sodium-glucose cotransporter 2 inhibitors and cardiovascular outcomes: a systematic review and meta-analysis. Eur J Prev Cardiol. 2018;25:495-502.
27. Kosiborod M, Cavender MA, Fu AZ, et al; CVD-REAL Investigators and Study Group. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation. 2017;136:249-259.
28. Tkáč I, Raz I. Combined analysis of three large interventional trials with gliptins indicates increased incidence of acute pancreatitis in patients with type 2 diabetes. Diabetes Care. 2017;40:284-286.
29. Schaffer C, Buclin T, Jornayvaz FR, et al. Use of dipeptidyl-peptidase IV inhibitors and bullous pemphigoid. Dermatology. 2017;233:401-403.
30. Madievsky R. Spotlight on antidiabetic agents with cardiovascular or renoprotective benefits. Perm J. 2018;22:18-034.
31. Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated hemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20:889-897.
32. Kosiborod M. Following the LEADER–why this and other recent trials signal a major paradigm shift in the management of type 2 diabetes. J Diabetes Complications. 2017;31:517-519.
33. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S90-S102.
34. Holman R. Metformin as first choice in oral diabetes treatment: the UKPDS experience. Journ Annu Diabetol Hotel Dieu. 2007:13-20.
35. American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S103-S123.
36. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2018 executive summary. Endocr Pract. 2018;24:91-120.
37. Inzucci SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140-149.
38. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669-2701.
The association between type 2 diabetes (T2D) and cardiovascular (CV) disease is well-established:
- Type 2 diabetes approximately doubles the risk of coronary artery disease, stroke, and peripheral arterial disease, independent of conventional risk factors1
- CV disease is the leading cause of morbidity and mortality in patients with T2D
- CV disease is the largest contributor to direct and indirect costs of the health care of patients who have T2D.2
In recent years, new classes of agents for treating T2D have been introduced (TABLE 1). Prior to 2008, the US Food and Drug Administration (FDA) approved drugs in those new classes based simply on their effectiveness in reducing the blood glucose level. Concerns about the CV safety of specific drugs (eg, rosiglitazone, muraglitazar) emerged from a number of trials, suggesting that these agents might increase the risk of CV events.3,4
Consequently, in 2008, the FDA issued guidance to the pharmaceutical industry: Preapproval and postapproval trials of all new antidiabetic drugs must now assess potential excess CV risk.5 CV outcomes trials (CVOTs), performed in accordance with FDA guidelines, have therefore become the focus of evaluating novel treatment options. In most CVOTs, combined primary CV endpoints have included CV mortality, nonfatal myocardial infarction (MI), and nonfatal stroke—taken together, what is known as the composite of these 3 major adverse CV events, or MACE-3.
To date, 15 CVOTs have been completed, assessing 3 novel classes of antihyperglycemic agents:
- dipeptidyl peptidase-4 (DPP-4) inhibitors
- glucagon-like peptide-1 (GLP-1) receptor agonists
- sodium–glucose cotransporter-2 (SGLT-2) inhibitors.
None of these trials identified any increased incidence of MACE; 7 found CV benefit. This review summarizes what the CVOTs revealed about these antihyperglycemic agents and their ability to yield a reduction in MACE and a decrease in all-cause mortality in patients with T2D and elevated CV disease risk. Armed with this information, you will have the tools you need to offer patients with T2D CV benefit while managing their primary disease.
Cardiovascular outcomes trials: DPP-4 inhibitors
Four trials. Trials of DPP-4 inhibitors that have been completed and reported are of saxagliptin (SAVOR-TIMI 536), alogliptin (EXAMINE7), sitagliptin (TECOS8), and linagliptin (CARMELINA9); others are in progress. In general, researchers enrolled patients at high risk of CV events, although inclusion criteria varied substantially. Consistently, these studies demonstrated that DPP-4 inhibition neither increased nor decreased (ie, were noninferior) the 3-point MACE (SAVOR-TIMI 53 noninferiority, P < .001; EXAMINE, P < .001; TECOS, P < .001).
Continue to: Rather than improve...
Rather than improve CV outcomes, there was some evidence that DPP-4 inhibitors might be associated with an increased risk of hospitalization for heart failure (HHF). In the SAVOR-TIMI 53 trial, patients randomized to saxagliptin had a 0.7% absolute increase in risk of HHF (P = .98).6 In the EXAMINE trial, patients treated with alogliptin showed a nonsignificant trend for HHF.10 In both the TECOS and CARMELINA trials, no difference was recorded in the rate of HHF.8,9,11 Subsequent meta-analysis that summarized the risk of HHF in CVOTs with DPP-4 inhibitors indicated a nonsignificant trend to increased risk.12
From these trials alone, it appears that DPP-4 inhibitors are unlikely to provide CV benefit. Data from additional trials are needed to evaluate the possible association between these medications and heart failure (HF). However, largely as a result of the findings from SAVOR-TIMI 53 and EXAMINE, the FDA issued a Drug Safety Communication in April 2016, adding warnings about HF to the labeling of saxagliptin and alogliptin.13
CARMELINA was designed to also evaluate kidney outcomes in patients with T2D. As with other DPP-4 inhibitor trials, the primary aim was to establish noninferiority, compared with placebo, for time to MACE-3 (P < .001). Secondary outcomes were defined as time to first occurrence of end-stage renal disease, death due to renal failure, and sustained decrease from baseline of ≥ 40% in the estimated glomerular filtration rate. The incidence of the secondary kidney composite results was not significantly different between groups randomized to linagliptin or placebo.9
Cardiovascular outcomes trials: GLP-1 receptor agonists
ELIXA. The CV safety of GLP-1 receptor agonists has been evaluated in several randomized clinical trials. The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial was the first14: Lixisenatide was studied in 6068 patients with recent hospitalization for acute coronary syndrome. Lixisenatide therapy was neutral with regard to CV outcomes, which met the primary endpoint: noninferiority to placebo (P < .001). There was no increase in either HF or HHF.
Continue to: LEADER
LEADER. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial (LEADER) evaluated long-term effects of liraglutide, compared to placebo, on CV events in patients with T2D.15 It was a multicenter, double-blind, placebocontrolled study that followed 9340 participants, most (81%) of whom had established CV disease, over 5 years. LEADER is considered a landmark study because it was the first large CVOT to show significant benefit for a GLP-1 receptor agonist.
Liraglutide demonstrated reductions in first occurrence of death from CV causes, nonfatal MI or nonfatal stroke, overall CV mortality, and all-cause mortality. The composite MACE-3 showed a relative risk reduction (RRR) of 13%, equivalent to an absolute risk reduction (ARR) of 1.9% (noninferiority, P < .001; superiority, P < .01). The RRR was 22% for death from CV causes, with an ARR of 1.3% (P = .007); the RRR for death from any cause was 15%, with an ARR of 1.4% (P = .02).
In addition, there was a lower rate of nephropathy (1.5 events for every 100 patient–years in the liraglutide group [P = .003], compared with 1.9 events every 100 patient–years in the placebo group).15
Results clearly demonstrated benefit. No significant difference was seen in the liraglutide rate of HHF, compared to the rate in the placebo group.
SUSTAIN-6. Evidence for the CV benefit of GLP-1 receptor agonists was also demonstrated in the phase 3 Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6).16 This was a study of 3297 patients with T2D at high risk of CV disease and with a mean hemoglobin A1c (HbA1c) value of 8.7%, 83% of whom had established CV disease. Patients were randomized to semaglutide or placebo. Note: SUSTAIN-6 was a noninferiority safety study; as such, it was not actually designed to assess or establish superiority.
Continue to: The incidence of MACE-3...
The incidence of MACE-3 was significantly reduced among patients treated with semaglutide (P = .02) after median followup of 2.1 years. The expanded composite outcome (death from CV causes, nonfatal MI, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina or HF), also showed a significant reduction with semaglutide (P = .002), compared with placebo. There was no difference in the overall hospitalization rate or rate of death from any cause.
EXSCEL. The Exenatide Study of Cardiovascular Event Lowering trial (EXSCEL)17,18 was a phase III/IV, double-blind, pragmatic placebo-controlled study of 14,752 patients at any level of CV risk, for a median 3.2 years. The study population was intentionally more diverse than in earlier GLP-1 receptor agonist studies. The researchers hypothesized that patients at increased risk of MACE would experience a comparatively greater relative treatment benefit with exenatide than those at lower risk. That did not prove to be the case.
EXSCEL did confirm noninferiority compared with placebo (P < .001), but once-weekly exenatide resulted in a nonsignificant reduction in major adverse CV events, and a trend for RRR in all-cause mortality (RRR = 14%; ARR = 1% [P = .06]).
HARMONY OUTCOMES. The Albiglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes and Cardiovascular Disease study (HARMONY OUTCOMES)19 was a double-blind, randomized, placebocontrolled trial conducted at 610 sites across 28 countries. The study investigated albiglutide, 30 to 50 mg once weekly, compared with placebo. It included 9463 patients ages ≥ 40 years with T2D who had an HbA1c > 7% (median value, 8.7%) and established CV disease. Patients were evaluated for a median 1.6 years.
Albiglutide reduced the risk of CV causes of death, nonfatal MI, and nonfatal stroke by an RRR of 22%, (ARR, 2%) (noninferiority, P < .0001; superiority, P < .0006).
Continue to: REWIND
REWIND. The Researching Cardiovascular Events with a Weekly INcretin in Diabetes trial (REWIND),20 the most recently completed GLP-1 receptor agonist CVOT (presented at the 2019 American Diabetes Association [ADA] Conference in June and published simultaneously in The Lancet), was a multicenter, randomized, double-blind placebo-controlled trial designed to assess the effect of weekly dulaglutide, 1.5 mg, compared with placebo, in 9901 participants enrolled at 371 sites in 24 countries. Mean patient age was 66.2 years, with women constituting 4589 (46.3%) of participants.
REWIND was distinct from other CVOTs in several ways:
- Other CVOTs were designed to show noninferiority compared with placebo regarding CV events; REWIND was designed to establish superiority
- In contrast to trials of other GLP-1 receptor agonists, in which most patients had established CV disease, only 31% of REWIND participants had a history of CV disease or a prior CV event (although 69% did have CV risk factors without underlying disease)
- REWIND was much longer (median follow-up, 5.4 years) than other GLP-1 receptor agonist trials (median follow-up, 1.5 to 3.8 years).
In REWIND, the primary composite outcome of MACE-3 occurred in 12% of participants assigned to dulaglutide, compared with 13.1% assigned to placebo (P = .026). This equated to 2.4 events for every 100 person– years on dulaglutide, compared with 2.7 events for every 100 person–years on placebo. There was a consistent effect on all MACE-3 components, although the greatest reductions were observed in nonfatal stroke (P = .017). Overall risk reduction was the same for primary and secondary prevention cohorts (P = .97), as well as in patients with either an HbA1c value < 7.2% or ≥ 7.2% (P = .75). Risk reduction was consistent across age, sex, duration of T2D, and body mass index.
Dulaglutide did not significantly affect the incidence of all-cause mortality, heart failure, revascularization, or hospital admission. Forty-seven percent of patients taking dulaglutide reported gastrointestinal adverse effects (P = .0001).
In a separate analysis of secondary outcomes, 21 dulaglutide reduced the composite renal outcomes of new-onset macroalbuminuria (P = .0001); decline of ≥ 30% in the estimated glomerular filtration rate (P = .066); and chronic renal replacement therapy (P = .39). Investigators estimated that 1 composite renal outcome event would be prevented for every 31 patients treated with dulaglutide for a median 5.4 years.
Continue to: Cardiovascular outcomes trials...
Cardiovascular outcomes trials: SGLT-2 inhibitors
EMPA-REG OUTCOME. The Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes trial (EMPA-REG OUTCOME) was also a landmark study because it was the first dedicated CVOT to show that an antihyperglycemic agent 1) decreased CV mortality and all-cause mortality, and 2) reduced HHF in patients with T2D and established CV disease.22 In this trial, 7020 patients with T2D who were at high risk of CV events were randomized and treated with empagliflozin, 10 or 25 mg, or placebo, in addition to standard care, and were followed for a median 2.6 years.
Compared with placebo, empagliflozin resulted in an RRR of 14% (ARR, 1.6%) in the primary endpoint of CV death, nonfatal MI, and stroke, confirming study drug superiority (P = .04). When compared with placebo, the empagliflozin group had an RRR of 38% in CV mortality, (ARR < 2.2%) (P < .001); an RRR of 35% in HHF (ARR, 1.4%) (P = .002); and an RRR of 32% (ARR, 2.6%) in death from any cause (P < .001).
CANVAS. The Canagliflozin Cardiovascular Assessment Study (CANVAS) integrated 2 multicenter, placebo-controlled, randomized trials with 10,142 participants and a mean follow-up of 3.6 years.23 Patients were randomized to receive canagliflozin (100-300 mg/d) or placebo. Approximately two-thirds of patients had a history of CV disease (therefore representing secondary prevention); one-third had CV risk factors only (primary prevention).
In CANVAS, patients receiving canagliflozin had a risk reduction in MACE-3, establishing superiority compared with placebo (P < .001). There was also a significant reduction in progression of albuminuria (P < .05). Superiority was not shown for the secondary outcome of death from any cause. Canagliflozin had no effect on the primary endpoint (MACE-3) in the subgroup of participants who did not have a history of CV disease. Similar to what was found with empagliflozin in EMPA-REG OUTCOME, CANVAS participants had a reduced risk of HHF.
Continue to: Patients on canagliflozin...
Patients on canagliflozin unexpectedly had an increased incidence of amputations (6.3 participants, compared with 3.4 participants, for every 1000 patient–years). This finding led to a black box warning for canagliflozin about the risk of lower-limb amputation.
DECLARE-TIMI 58. The Dapagliflozin Effect of Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 trial (DECLARETIMI 58) was the largest SGLT-2 inhibitor outcomes trial to date, enrolling 17,160 patients with T2D who also had established CV disease or multiple risk factors for atherosclerotic CV disease. The trial compared dapagliflozin, 10 mg/d, and placebo, following patients for a median 4.2 years.24 Unlike CANVAS and EMPA-REG OUTCOME, DECLARE-TIMI 58 included CV death and HHF as primary outcomes, in addition to MACE-3.
Dapagliflozin was noninferior to placebo with regard to MACE-3. However, its use did result in a lower rate of CV death and HHF by an RRR of 17% (ARR, 1.9%). Risk reduction was greatest in patients with HF who had a reduced ejection fraction (ARR = 9.2%).25
In October, the FDA approved dapagliflozin to reduce the risk of HHF in adults with T2D and established CV disease or multiple CV risk factors. Before initiating the drug, physicians should evaluate the patient's renal function and monitor periodically.
Meta-analyses of SGLT-2 inhibitors
Systematic review. Usman et al released a meta-analysis in 2018 that included 35 randomized, placebo-controlled trials (including EMPA-REG OUTCOME, CANVAS, and DECLARE-TIMI 58) that had assessed the use of SGLT-2 inhibitors in nearly 35,000 patients with T2D.26 This review concluded that, as a class, SGLT-2 inhibitors reduce all-cause mortality, major adverse cardiac events, nonfatal MI, and HF and HHF, compared with placebo.
Continue to: CVD-REAL
CVD-REAL. A separate study, Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors (CVD-REAL), of 154,528 patients who were treated with canagliflozin, dapagliflozin, or empagliflozin, showed that initiation of SGLT-2 inhibitors, compared with other glucose- lowering therapies, was associated with a 39% reduction in HHF; a 51% reduction in death from any cause; and a 46% reduction in the composite of HHF or death (P < .001).27
CVD-REAL was unique because it was the largest real-world study to assess the effectiveness of SGLT-2 inhibitors on HHF and mortality. The study utilized data from patients in the United States, Norway, Denmark, Sweden, Germany, and the United Kingdom, based on information obtained from medical claims, primary care and hospital records, and national registries that compared patients who were either newly started on an SGLT-2 inhibitor or another glucose-lowering drug. The drug used by most patients in the trial was canagliflozin (53%), followed by dapagliflozin (42%), and empagliflozin (5%).
In this meta-analysis, similar therapeutic effects were seen across countries, regardless of geographic differences, in the use of specific SGLT-2 inhibitors, suggesting a class effect. Of particular significance was that most (87%) patients enrolled in CVD-REAL did not have prior CV disease. Despite this, results for examined outcomes in CVD-REAL were similar to what was seen in other SGLT-2 inhibitor trials that were designed to study patients with established CV disease.
Risk of adverse effects of newer antidiabetic agents
DPP-4 inhibitors. Alogliptin and sitagliptin carry a black-box warning about potential risk of HF. In SAVOR-TIMI, a 27% increase was detected in the rate of HHF after approximately 2 years of saxagliptin therapy.6 Although HF should not be considered a class effect for DPP-4 inhibitors, patients who have risk factors for HF should be monitored for signs and symptoms of HF.
Continue to: Cases of acute pancreatitis...
Cases of acute pancreatitis have been reported in association with all DPP-4 inhibitors available in the United States. A combined analysis of DDP-4 inhibitor trials suggested an increased relative risk of 79% and an absolute risk of 0.13%, which translates to 1 or 2 additional cases of acute pancreatitis for every 1000 patients treated for 2 years.28
There have been numerous postmarketing reports of severe joint pain in patients taking a DPP-4 inhibitor. Most recently, cases of bullous pemphigoid have been reported after initiation of DPP-4 inhibitor therapy.29
GLP-1 receptor agonists carry a black box warning for medullary thyroid (C-cell) tumor risk. GLP-1 receptor agonists are contraindicated in patients with a personal or family history of this cancer, although this FDA warning is based solely on observations from animal models.
In addition, GLP-1 receptor agonists can increase the risk of cholecystitis and pancreatitis. Not uncommonly, they cause gastrointestinal symptoms when first started and when the dosage is titrated upward. Most GLP-1 receptor agonists can be used in patients with renal impairment, although data regarding their use in Stages 4 and 5 chronic kidney disease are limited.30 Semaglutide was found, in the SUSTAIN-6 trial, to be associated with an increased rate of complications of retinopathy, including vitreous hemorrhage and blindness (P = .02)31
SGLT-2 inhibitors are associated with an increased incidence of genitourinary infection, bone fracture (canagliflozin), amputation (canagliflozin), and euglycemic diabetic ketoacidosis. Agents in this class should be avoided in patients with moderate or severe renal impairment, primarily due to a lack of efficacy. They are contraindicated in patients with an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2. (Dapagliflozin is not recommended when eGFR is < 45 mL/min/ 1.73 m2.) These agents carry an FDA warning about the risk of acute kidney injury.30
Continue to: Summing up
Summing up
All glucose-lowering medications used to treat T2D are not equally effective in reducing CV complications. Recent CVOTs have uncovered evidence that certain antidiabetic agents might confer CV and all-cause mortality benefits (TABLE 26,7,9,11,14-17,19-24).
Discussion of proposed mechanisms for CV outcome superiority of these agents is beyond the scope of this review. It is generally believed that benefits result from mechanisms other than a reduction in the serum glucose level, given the relatively short time frame of the studies and the magnitude of the CV benefit. It is almost certain that mechanisms of CV benefit in the 2 landmark studies—LEADER and EMPA-REG OUTCOME—are distinct from each other.32
See “When planning T2D pharmacotherapy, include newer agents that offer CV benefit,” 33-38 for a stepwise approach to treating T2D, including the role of agents that have efficacy in modifying the risk of CV disease.
SIDEBAR
When planning T2D pharmacotherapy, include newer agents that offer CV benefit33-38
First-line management. The 2019 Standards of Medical Care in Diabetes Guidelines established by the American Diabetes Association (ADA) recommend metformin as first-line pharmacotherapy for type 2 diabetes (T2D).33 This recommendation is based on metformin’s efficacy in reducing the blood glucose level and hemoglobin A1C (HbA1C); safety; tolerability; extensive clinical experience; and findings from the UK Prospective Diabetes Study demonstrating a substantial beneficial effect of metformin on cardiovascular (CV) disease.34 Additional benefits of metformin include a decrease in body weight, low-density lipoprotein level, and the need for insulin.
Second-line additive benefit. In addition, ADA guidelines make a highest level (Level-A) recommendation that patients with T2D and established atherosclerotic CV disease be treated with one of the sodium–glucose cotransporter-2 (SGLT-2) inhibitors or glucagon-like peptide-1 (GLP-1) receptor agonists that have demonstrated efficacy in CV disease risk reduction as part of an antihyperglycemic regimen.35 Seven agents described in this article from these 2 unique classes of medications meet the CV disease benefit criterion: liraglutide, semaglutide, albiglutide, dulaglutide, empagliflozin, canagliflozin, and dapagliflozin. Only empagliflozin and liraglutide have received a US Food and Drug Administration indication for risk reduction in major CV events in adults with T2D and established CV disease.
Regarding dulaglutide, although the findings of REWIND are encouraging, results were not robust; further analysis is necessary to make a recommendation for treating patients who do not have a history of established CV disease with this medication.
Individualized decision-making. From a clinical perspective, patient-specific considerations and shared decision-making should be incorporated into T2D treatment decisions:
- For patients with T2D and established atherosclerotic CV disease, SGLT-2 inhibitors and GLP-1 receptor agonists are recommended agents after metformin.
- SGLT-2 inhibitors are preferred in T2D patients with established CV disease and a history of heart failure.
- GLP-1 receptor agonists with proven CV disease benefit are preferred in patients with established CV disease and chronic kidney disease.
Add-on Tx. In ADA guidelines, dipeptidyl peptidase-4 (DDP-4) inhibitors are recommended as an optional add-on for patients without clinical atherosclerotic CV disease who are unable to reach their HbA1C goal after taking metformin for 3 months.33 Furthermore, the American Association of Clinical Endocrinologists lists DPP-4 inhibitors as alternatives for patients with an HbA1C < 7.5% in whom metformin is contraindicated.36 DPP-4 inhibitors are not an ideal choice as a second agent when the patient has a history of heart failure, and should not be recommended over GLP-1 receptor agonists or SGLT-2 inhibitors as second-line agents in patients with T2D and CV disease.
Individualizing management. The current algorithm for T2D management,37 based primarily on HbA1C reduction, is shifting toward concurrent attention to reduction of CV risk (FIGURE38). Our challenge, as physicians, is to translate the results of recent CV outcomes trials into a more targeted management strategy that focuses on eligible populations.
ACKNOWLEDGMENTS
Linda Speer, MD, Kevin Phelps, DO, and Jay Shubrook, DO, provided support and editorial assistance.
CORRESPONDENCE
Robert Gotfried, DO, FAAFP, Department of Family Medicine, University of Toledo College of Medicine, 3333 Glendale Avenue, Toledo, OH 43614; Robert.gotfried@utoledo.edu.
The association between type 2 diabetes (T2D) and cardiovascular (CV) disease is well-established:
- Type 2 diabetes approximately doubles the risk of coronary artery disease, stroke, and peripheral arterial disease, independent of conventional risk factors1
- CV disease is the leading cause of morbidity and mortality in patients with T2D
- CV disease is the largest contributor to direct and indirect costs of the health care of patients who have T2D.2
In recent years, new classes of agents for treating T2D have been introduced (TABLE 1). Prior to 2008, the US Food and Drug Administration (FDA) approved drugs in those new classes based simply on their effectiveness in reducing the blood glucose level. Concerns about the CV safety of specific drugs (eg, rosiglitazone, muraglitazar) emerged from a number of trials, suggesting that these agents might increase the risk of CV events.3,4
Consequently, in 2008, the FDA issued guidance to the pharmaceutical industry: Preapproval and postapproval trials of all new antidiabetic drugs must now assess potential excess CV risk.5 CV outcomes trials (CVOTs), performed in accordance with FDA guidelines, have therefore become the focus of evaluating novel treatment options. In most CVOTs, combined primary CV endpoints have included CV mortality, nonfatal myocardial infarction (MI), and nonfatal stroke—taken together, what is known as the composite of these 3 major adverse CV events, or MACE-3.
To date, 15 CVOTs have been completed, assessing 3 novel classes of antihyperglycemic agents:
- dipeptidyl peptidase-4 (DPP-4) inhibitors
- glucagon-like peptide-1 (GLP-1) receptor agonists
- sodium–glucose cotransporter-2 (SGLT-2) inhibitors.
None of these trials identified any increased incidence of MACE; 7 found CV benefit. This review summarizes what the CVOTs revealed about these antihyperglycemic agents and their ability to yield a reduction in MACE and a decrease in all-cause mortality in patients with T2D and elevated CV disease risk. Armed with this information, you will have the tools you need to offer patients with T2D CV benefit while managing their primary disease.
Cardiovascular outcomes trials: DPP-4 inhibitors
Four trials. Trials of DPP-4 inhibitors that have been completed and reported are of saxagliptin (SAVOR-TIMI 536), alogliptin (EXAMINE7), sitagliptin (TECOS8), and linagliptin (CARMELINA9); others are in progress. In general, researchers enrolled patients at high risk of CV events, although inclusion criteria varied substantially. Consistently, these studies demonstrated that DPP-4 inhibition neither increased nor decreased (ie, were noninferior) the 3-point MACE (SAVOR-TIMI 53 noninferiority, P < .001; EXAMINE, P < .001; TECOS, P < .001).
Continue to: Rather than improve...
Rather than improve CV outcomes, there was some evidence that DPP-4 inhibitors might be associated with an increased risk of hospitalization for heart failure (HHF). In the SAVOR-TIMI 53 trial, patients randomized to saxagliptin had a 0.7% absolute increase in risk of HHF (P = .98).6 In the EXAMINE trial, patients treated with alogliptin showed a nonsignificant trend for HHF.10 In both the TECOS and CARMELINA trials, no difference was recorded in the rate of HHF.8,9,11 Subsequent meta-analysis that summarized the risk of HHF in CVOTs with DPP-4 inhibitors indicated a nonsignificant trend to increased risk.12
From these trials alone, it appears that DPP-4 inhibitors are unlikely to provide CV benefit. Data from additional trials are needed to evaluate the possible association between these medications and heart failure (HF). However, largely as a result of the findings from SAVOR-TIMI 53 and EXAMINE, the FDA issued a Drug Safety Communication in April 2016, adding warnings about HF to the labeling of saxagliptin and alogliptin.13
CARMELINA was designed to also evaluate kidney outcomes in patients with T2D. As with other DPP-4 inhibitor trials, the primary aim was to establish noninferiority, compared with placebo, for time to MACE-3 (P < .001). Secondary outcomes were defined as time to first occurrence of end-stage renal disease, death due to renal failure, and sustained decrease from baseline of ≥ 40% in the estimated glomerular filtration rate. The incidence of the secondary kidney composite results was not significantly different between groups randomized to linagliptin or placebo.9
Cardiovascular outcomes trials: GLP-1 receptor agonists
ELIXA. The CV safety of GLP-1 receptor agonists has been evaluated in several randomized clinical trials. The Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) trial was the first14: Lixisenatide was studied in 6068 patients with recent hospitalization for acute coronary syndrome. Lixisenatide therapy was neutral with regard to CV outcomes, which met the primary endpoint: noninferiority to placebo (P < .001). There was no increase in either HF or HHF.
Continue to: LEADER
LEADER. The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results trial (LEADER) evaluated long-term effects of liraglutide, compared to placebo, on CV events in patients with T2D.15 It was a multicenter, double-blind, placebocontrolled study that followed 9340 participants, most (81%) of whom had established CV disease, over 5 years. LEADER is considered a landmark study because it was the first large CVOT to show significant benefit for a GLP-1 receptor agonist.
Liraglutide demonstrated reductions in first occurrence of death from CV causes, nonfatal MI or nonfatal stroke, overall CV mortality, and all-cause mortality. The composite MACE-3 showed a relative risk reduction (RRR) of 13%, equivalent to an absolute risk reduction (ARR) of 1.9% (noninferiority, P < .001; superiority, P < .01). The RRR was 22% for death from CV causes, with an ARR of 1.3% (P = .007); the RRR for death from any cause was 15%, with an ARR of 1.4% (P = .02).
In addition, there was a lower rate of nephropathy (1.5 events for every 100 patient–years in the liraglutide group [P = .003], compared with 1.9 events every 100 patient–years in the placebo group).15
Results clearly demonstrated benefit. No significant difference was seen in the liraglutide rate of HHF, compared to the rate in the placebo group.
SUSTAIN-6. Evidence for the CV benefit of GLP-1 receptor agonists was also demonstrated in the phase 3 Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6).16 This was a study of 3297 patients with T2D at high risk of CV disease and with a mean hemoglobin A1c (HbA1c) value of 8.7%, 83% of whom had established CV disease. Patients were randomized to semaglutide or placebo. Note: SUSTAIN-6 was a noninferiority safety study; as such, it was not actually designed to assess or establish superiority.
Continue to: The incidence of MACE-3...
The incidence of MACE-3 was significantly reduced among patients treated with semaglutide (P = .02) after median followup of 2.1 years. The expanded composite outcome (death from CV causes, nonfatal MI, nonfatal stroke, coronary revascularization, or hospitalization for unstable angina or HF), also showed a significant reduction with semaglutide (P = .002), compared with placebo. There was no difference in the overall hospitalization rate or rate of death from any cause.
EXSCEL. The Exenatide Study of Cardiovascular Event Lowering trial (EXSCEL)17,18 was a phase III/IV, double-blind, pragmatic placebo-controlled study of 14,752 patients at any level of CV risk, for a median 3.2 years. The study population was intentionally more diverse than in earlier GLP-1 receptor agonist studies. The researchers hypothesized that patients at increased risk of MACE would experience a comparatively greater relative treatment benefit with exenatide than those at lower risk. That did not prove to be the case.
EXSCEL did confirm noninferiority compared with placebo (P < .001), but once-weekly exenatide resulted in a nonsignificant reduction in major adverse CV events, and a trend for RRR in all-cause mortality (RRR = 14%; ARR = 1% [P = .06]).
HARMONY OUTCOMES. The Albiglutide and Cardiovascular Outcomes in Patients With Type 2 Diabetes and Cardiovascular Disease study (HARMONY OUTCOMES)19 was a double-blind, randomized, placebocontrolled trial conducted at 610 sites across 28 countries. The study investigated albiglutide, 30 to 50 mg once weekly, compared with placebo. It included 9463 patients ages ≥ 40 years with T2D who had an HbA1c > 7% (median value, 8.7%) and established CV disease. Patients were evaluated for a median 1.6 years.
Albiglutide reduced the risk of CV causes of death, nonfatal MI, and nonfatal stroke by an RRR of 22%, (ARR, 2%) (noninferiority, P < .0001; superiority, P < .0006).
Continue to: REWIND
REWIND. The Researching Cardiovascular Events with a Weekly INcretin in Diabetes trial (REWIND),20 the most recently completed GLP-1 receptor agonist CVOT (presented at the 2019 American Diabetes Association [ADA] Conference in June and published simultaneously in The Lancet), was a multicenter, randomized, double-blind placebo-controlled trial designed to assess the effect of weekly dulaglutide, 1.5 mg, compared with placebo, in 9901 participants enrolled at 371 sites in 24 countries. Mean patient age was 66.2 years, with women constituting 4589 (46.3%) of participants.
REWIND was distinct from other CVOTs in several ways:
- Other CVOTs were designed to show noninferiority compared with placebo regarding CV events; REWIND was designed to establish superiority
- In contrast to trials of other GLP-1 receptor agonists, in which most patients had established CV disease, only 31% of REWIND participants had a history of CV disease or a prior CV event (although 69% did have CV risk factors without underlying disease)
- REWIND was much longer (median follow-up, 5.4 years) than other GLP-1 receptor agonist trials (median follow-up, 1.5 to 3.8 years).
In REWIND, the primary composite outcome of MACE-3 occurred in 12% of participants assigned to dulaglutide, compared with 13.1% assigned to placebo (P = .026). This equated to 2.4 events for every 100 person– years on dulaglutide, compared with 2.7 events for every 100 person–years on placebo. There was a consistent effect on all MACE-3 components, although the greatest reductions were observed in nonfatal stroke (P = .017). Overall risk reduction was the same for primary and secondary prevention cohorts (P = .97), as well as in patients with either an HbA1c value < 7.2% or ≥ 7.2% (P = .75). Risk reduction was consistent across age, sex, duration of T2D, and body mass index.
Dulaglutide did not significantly affect the incidence of all-cause mortality, heart failure, revascularization, or hospital admission. Forty-seven percent of patients taking dulaglutide reported gastrointestinal adverse effects (P = .0001).
In a separate analysis of secondary outcomes, 21 dulaglutide reduced the composite renal outcomes of new-onset macroalbuminuria (P = .0001); decline of ≥ 30% in the estimated glomerular filtration rate (P = .066); and chronic renal replacement therapy (P = .39). Investigators estimated that 1 composite renal outcome event would be prevented for every 31 patients treated with dulaglutide for a median 5.4 years.
Continue to: Cardiovascular outcomes trials...
Cardiovascular outcomes trials: SGLT-2 inhibitors
EMPA-REG OUTCOME. The Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes trial (EMPA-REG OUTCOME) was also a landmark study because it was the first dedicated CVOT to show that an antihyperglycemic agent 1) decreased CV mortality and all-cause mortality, and 2) reduced HHF in patients with T2D and established CV disease.22 In this trial, 7020 patients with T2D who were at high risk of CV events were randomized and treated with empagliflozin, 10 or 25 mg, or placebo, in addition to standard care, and were followed for a median 2.6 years.
Compared with placebo, empagliflozin resulted in an RRR of 14% (ARR, 1.6%) in the primary endpoint of CV death, nonfatal MI, and stroke, confirming study drug superiority (P = .04). When compared with placebo, the empagliflozin group had an RRR of 38% in CV mortality, (ARR < 2.2%) (P < .001); an RRR of 35% in HHF (ARR, 1.4%) (P = .002); and an RRR of 32% (ARR, 2.6%) in death from any cause (P < .001).
CANVAS. The Canagliflozin Cardiovascular Assessment Study (CANVAS) integrated 2 multicenter, placebo-controlled, randomized trials with 10,142 participants and a mean follow-up of 3.6 years.23 Patients were randomized to receive canagliflozin (100-300 mg/d) or placebo. Approximately two-thirds of patients had a history of CV disease (therefore representing secondary prevention); one-third had CV risk factors only (primary prevention).
In CANVAS, patients receiving canagliflozin had a risk reduction in MACE-3, establishing superiority compared with placebo (P < .001). There was also a significant reduction in progression of albuminuria (P < .05). Superiority was not shown for the secondary outcome of death from any cause. Canagliflozin had no effect on the primary endpoint (MACE-3) in the subgroup of participants who did not have a history of CV disease. Similar to what was found with empagliflozin in EMPA-REG OUTCOME, CANVAS participants had a reduced risk of HHF.
Continue to: Patients on canagliflozin...
Patients on canagliflozin unexpectedly had an increased incidence of amputations (6.3 participants, compared with 3.4 participants, for every 1000 patient–years). This finding led to a black box warning for canagliflozin about the risk of lower-limb amputation.
DECLARE-TIMI 58. The Dapagliflozin Effect of Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 trial (DECLARETIMI 58) was the largest SGLT-2 inhibitor outcomes trial to date, enrolling 17,160 patients with T2D who also had established CV disease or multiple risk factors for atherosclerotic CV disease. The trial compared dapagliflozin, 10 mg/d, and placebo, following patients for a median 4.2 years.24 Unlike CANVAS and EMPA-REG OUTCOME, DECLARE-TIMI 58 included CV death and HHF as primary outcomes, in addition to MACE-3.
Dapagliflozin was noninferior to placebo with regard to MACE-3. However, its use did result in a lower rate of CV death and HHF by an RRR of 17% (ARR, 1.9%). Risk reduction was greatest in patients with HF who had a reduced ejection fraction (ARR = 9.2%).25
In October, the FDA approved dapagliflozin to reduce the risk of HHF in adults with T2D and established CV disease or multiple CV risk factors. Before initiating the drug, physicians should evaluate the patient's renal function and monitor periodically.
Meta-analyses of SGLT-2 inhibitors
Systematic review. Usman et al released a meta-analysis in 2018 that included 35 randomized, placebo-controlled trials (including EMPA-REG OUTCOME, CANVAS, and DECLARE-TIMI 58) that had assessed the use of SGLT-2 inhibitors in nearly 35,000 patients with T2D.26 This review concluded that, as a class, SGLT-2 inhibitors reduce all-cause mortality, major adverse cardiac events, nonfatal MI, and HF and HHF, compared with placebo.
Continue to: CVD-REAL
CVD-REAL. A separate study, Comparative Effectiveness of Cardiovascular Outcomes in New Users of SGLT-2 Inhibitors (CVD-REAL), of 154,528 patients who were treated with canagliflozin, dapagliflozin, or empagliflozin, showed that initiation of SGLT-2 inhibitors, compared with other glucose- lowering therapies, was associated with a 39% reduction in HHF; a 51% reduction in death from any cause; and a 46% reduction in the composite of HHF or death (P < .001).27
CVD-REAL was unique because it was the largest real-world study to assess the effectiveness of SGLT-2 inhibitors on HHF and mortality. The study utilized data from patients in the United States, Norway, Denmark, Sweden, Germany, and the United Kingdom, based on information obtained from medical claims, primary care and hospital records, and national registries that compared patients who were either newly started on an SGLT-2 inhibitor or another glucose-lowering drug. The drug used by most patients in the trial was canagliflozin (53%), followed by dapagliflozin (42%), and empagliflozin (5%).
In this meta-analysis, similar therapeutic effects were seen across countries, regardless of geographic differences, in the use of specific SGLT-2 inhibitors, suggesting a class effect. Of particular significance was that most (87%) patients enrolled in CVD-REAL did not have prior CV disease. Despite this, results for examined outcomes in CVD-REAL were similar to what was seen in other SGLT-2 inhibitor trials that were designed to study patients with established CV disease.
Risk of adverse effects of newer antidiabetic agents
DPP-4 inhibitors. Alogliptin and sitagliptin carry a black-box warning about potential risk of HF. In SAVOR-TIMI, a 27% increase was detected in the rate of HHF after approximately 2 years of saxagliptin therapy.6 Although HF should not be considered a class effect for DPP-4 inhibitors, patients who have risk factors for HF should be monitored for signs and symptoms of HF.
Continue to: Cases of acute pancreatitis...
Cases of acute pancreatitis have been reported in association with all DPP-4 inhibitors available in the United States. A combined analysis of DDP-4 inhibitor trials suggested an increased relative risk of 79% and an absolute risk of 0.13%, which translates to 1 or 2 additional cases of acute pancreatitis for every 1000 patients treated for 2 years.28
There have been numerous postmarketing reports of severe joint pain in patients taking a DPP-4 inhibitor. Most recently, cases of bullous pemphigoid have been reported after initiation of DPP-4 inhibitor therapy.29
GLP-1 receptor agonists carry a black box warning for medullary thyroid (C-cell) tumor risk. GLP-1 receptor agonists are contraindicated in patients with a personal or family history of this cancer, although this FDA warning is based solely on observations from animal models.
In addition, GLP-1 receptor agonists can increase the risk of cholecystitis and pancreatitis. Not uncommonly, they cause gastrointestinal symptoms when first started and when the dosage is titrated upward. Most GLP-1 receptor agonists can be used in patients with renal impairment, although data regarding their use in Stages 4 and 5 chronic kidney disease are limited.30 Semaglutide was found, in the SUSTAIN-6 trial, to be associated with an increased rate of complications of retinopathy, including vitreous hemorrhage and blindness (P = .02)31
SGLT-2 inhibitors are associated with an increased incidence of genitourinary infection, bone fracture (canagliflozin), amputation (canagliflozin), and euglycemic diabetic ketoacidosis. Agents in this class should be avoided in patients with moderate or severe renal impairment, primarily due to a lack of efficacy. They are contraindicated in patients with an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2. (Dapagliflozin is not recommended when eGFR is < 45 mL/min/ 1.73 m2.) These agents carry an FDA warning about the risk of acute kidney injury.30
Continue to: Summing up
Summing up
All glucose-lowering medications used to treat T2D are not equally effective in reducing CV complications. Recent CVOTs have uncovered evidence that certain antidiabetic agents might confer CV and all-cause mortality benefits (TABLE 26,7,9,11,14-17,19-24).
Discussion of proposed mechanisms for CV outcome superiority of these agents is beyond the scope of this review. It is generally believed that benefits result from mechanisms other than a reduction in the serum glucose level, given the relatively short time frame of the studies and the magnitude of the CV benefit. It is almost certain that mechanisms of CV benefit in the 2 landmark studies—LEADER and EMPA-REG OUTCOME—are distinct from each other.32
See “When planning T2D pharmacotherapy, include newer agents that offer CV benefit,” 33-38 for a stepwise approach to treating T2D, including the role of agents that have efficacy in modifying the risk of CV disease.
SIDEBAR
When planning T2D pharmacotherapy, include newer agents that offer CV benefit33-38
First-line management. The 2019 Standards of Medical Care in Diabetes Guidelines established by the American Diabetes Association (ADA) recommend metformin as first-line pharmacotherapy for type 2 diabetes (T2D).33 This recommendation is based on metformin’s efficacy in reducing the blood glucose level and hemoglobin A1C (HbA1C); safety; tolerability; extensive clinical experience; and findings from the UK Prospective Diabetes Study demonstrating a substantial beneficial effect of metformin on cardiovascular (CV) disease.34 Additional benefits of metformin include a decrease in body weight, low-density lipoprotein level, and the need for insulin.
Second-line additive benefit. In addition, ADA guidelines make a highest level (Level-A) recommendation that patients with T2D and established atherosclerotic CV disease be treated with one of the sodium–glucose cotransporter-2 (SGLT-2) inhibitors or glucagon-like peptide-1 (GLP-1) receptor agonists that have demonstrated efficacy in CV disease risk reduction as part of an antihyperglycemic regimen.35 Seven agents described in this article from these 2 unique classes of medications meet the CV disease benefit criterion: liraglutide, semaglutide, albiglutide, dulaglutide, empagliflozin, canagliflozin, and dapagliflozin. Only empagliflozin and liraglutide have received a US Food and Drug Administration indication for risk reduction in major CV events in adults with T2D and established CV disease.
Regarding dulaglutide, although the findings of REWIND are encouraging, results were not robust; further analysis is necessary to make a recommendation for treating patients who do not have a history of established CV disease with this medication.
Individualized decision-making. From a clinical perspective, patient-specific considerations and shared decision-making should be incorporated into T2D treatment decisions:
- For patients with T2D and established atherosclerotic CV disease, SGLT-2 inhibitors and GLP-1 receptor agonists are recommended agents after metformin.
- SGLT-2 inhibitors are preferred in T2D patients with established CV disease and a history of heart failure.
- GLP-1 receptor agonists with proven CV disease benefit are preferred in patients with established CV disease and chronic kidney disease.
Add-on Tx. In ADA guidelines, dipeptidyl peptidase-4 (DDP-4) inhibitors are recommended as an optional add-on for patients without clinical atherosclerotic CV disease who are unable to reach their HbA1C goal after taking metformin for 3 months.33 Furthermore, the American Association of Clinical Endocrinologists lists DPP-4 inhibitors as alternatives for patients with an HbA1C < 7.5% in whom metformin is contraindicated.36 DPP-4 inhibitors are not an ideal choice as a second agent when the patient has a history of heart failure, and should not be recommended over GLP-1 receptor agonists or SGLT-2 inhibitors as second-line agents in patients with T2D and CV disease.
Individualizing management. The current algorithm for T2D management,37 based primarily on HbA1C reduction, is shifting toward concurrent attention to reduction of CV risk (FIGURE38). Our challenge, as physicians, is to translate the results of recent CV outcomes trials into a more targeted management strategy that focuses on eligible populations.
ACKNOWLEDGMENTS
Linda Speer, MD, Kevin Phelps, DO, and Jay Shubrook, DO, provided support and editorial assistance.
CORRESPONDENCE
Robert Gotfried, DO, FAAFP, Department of Family Medicine, University of Toledo College of Medicine, 3333 Glendale Avenue, Toledo, OH 43614; Robert.gotfried@utoledo.edu.
1. Emerging Risk Factors Collaboration; Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215-2222.
2. Chamberlain JJ, Johnson EL, Leal S, et al. Cardiovascular disease and risk management: review of the American Diabetes Association Standards of Medical Care in Diabetes 2018. Ann Intern Med. 2018;168:640-650.
3. Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294:2581-2586.
4. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457-2471.
5. Center for Drug Evaluation and Research, US Food and Drug Administration. Guidance document: Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. www.fda.gov/downloads/drugs/guidance
complianceregulatoryinformation/guidances/ucm071627.pdf. Published December 2008. Accessed October 4, 2019.
6. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patient with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
7. White WB, Canon CP, Heller SR, et al; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327-1335.
8. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
9. Rosenstock J, Perkovic V, Johansen OE, et al; CARMELINA Investigators. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69-79.
10. Zannad F, Cannon CP, Cushman WC, et al. EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
11. McGuire DK, Van de Werf F, Armstrong PW, et al; Trial Evaluating Cardiovascular Outcomes with Sitagliptin Study Group. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:126-135.
12. Toh S, Hampp C, Reichman ME, et al. Risk for hospitalized heart failure among new users of saxagliptin, sitagliptin, and other antihyperglycemic drugs: a retrospective cohort study. Ann Intern Med. 2016;164:705-714.
13. US Food and Drug Administration. FDA drug safety communication: FDA adds warning about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. www.fda.gov/Drugs/DrugSafety/ucm486096.htm. Updated April 5, 2016. Accessed October 4, 2019.
14. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patient with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247-2257.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
17. Mentz RJ, Bethel MA, Merrill P, et al; EXSCEL Study Group. Effect of once-weekly exenatide on clinical outcomes according to baseline risk in patients with type 2 diabetes mellitus: insights from the EXSCEL Trial. J Am Heart Assoc. 2018;7:e009304.
18. Holman RR, Bethel MA, George J, et al. Rationale and design of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial. Am Heart J. 2016;174:103-110.
19. Hernandez AF, Green JB, Janmohamed S, et al; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519-1529.
20. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121-130.
21. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomized, placebo-controlled trial. Lancet. 2019;394:131-138.
22. Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
23. Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
24. Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
25. Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528-2536.
26. Usman MS, Siddiqi TJ, Memon MM, et al. Sodium-glucose cotransporter 2 inhibitors and cardiovascular outcomes: a systematic review and meta-analysis. Eur J Prev Cardiol. 2018;25:495-502.
27. Kosiborod M, Cavender MA, Fu AZ, et al; CVD-REAL Investigators and Study Group. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation. 2017;136:249-259.
28. Tkáč I, Raz I. Combined analysis of three large interventional trials with gliptins indicates increased incidence of acute pancreatitis in patients with type 2 diabetes. Diabetes Care. 2017;40:284-286.
29. Schaffer C, Buclin T, Jornayvaz FR, et al. Use of dipeptidyl-peptidase IV inhibitors and bullous pemphigoid. Dermatology. 2017;233:401-403.
30. Madievsky R. Spotlight on antidiabetic agents with cardiovascular or renoprotective benefits. Perm J. 2018;22:18-034.
31. Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated hemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20:889-897.
32. Kosiborod M. Following the LEADER–why this and other recent trials signal a major paradigm shift in the management of type 2 diabetes. J Diabetes Complications. 2017;31:517-519.
33. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S90-S102.
34. Holman R. Metformin as first choice in oral diabetes treatment: the UKPDS experience. Journ Annu Diabetol Hotel Dieu. 2007:13-20.
35. American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S103-S123.
36. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2018 executive summary. Endocr Pract. 2018;24:91-120.
37. Inzucci SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140-149.
38. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669-2701.
1. Emerging Risk Factors Collaboration; Sarwar N, Gao P, Seshasai SR, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215-2222.
2. Chamberlain JJ, Johnson EL, Leal S, et al. Cardiovascular disease and risk management: review of the American Diabetes Association Standards of Medical Care in Diabetes 2018. Ann Intern Med. 2018;168:640-650.
3. Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA. 2005;294:2581-2586.
4. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457-2471.
5. Center for Drug Evaluation and Research, US Food and Drug Administration. Guidance document: Diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. www.fda.gov/downloads/drugs/guidance
complianceregulatoryinformation/guidances/ucm071627.pdf. Published December 2008. Accessed October 4, 2019.
6. Scirica BM, Bhatt DL, Braunwald E, et al; SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patient with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317-1326.
7. White WB, Canon CP, Heller SR, et al; EXAMINE Investigators. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327-1335.
8. Green JB, Bethel MA, Armstrong PW, et al; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232-242.
9. Rosenstock J, Perkovic V, Johansen OE, et al; CARMELINA Investigators. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69-79.
10. Zannad F, Cannon CP, Cushman WC, et al. EXAMINE Investigators. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet. 2015;385:2067-2076.
11. McGuire DK, Van de Werf F, Armstrong PW, et al; Trial Evaluating Cardiovascular Outcomes with Sitagliptin Study Group. Association between sitagliptin use and heart failure hospitalization and related outcomes in type 2 diabetes mellitus: secondary analysis of a randomized clinical trial. JAMA Cardiol. 2016;1:126-135.
12. Toh S, Hampp C, Reichman ME, et al. Risk for hospitalized heart failure among new users of saxagliptin, sitagliptin, and other antihyperglycemic drugs: a retrospective cohort study. Ann Intern Med. 2016;164:705-714.
13. US Food and Drug Administration. FDA drug safety communication: FDA adds warning about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. www.fda.gov/Drugs/DrugSafety/ucm486096.htm. Updated April 5, 2016. Accessed October 4, 2019.
14. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patient with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247-2257.
15. Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
16. Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834-1844.
17. Mentz RJ, Bethel MA, Merrill P, et al; EXSCEL Study Group. Effect of once-weekly exenatide on clinical outcomes according to baseline risk in patients with type 2 diabetes mellitus: insights from the EXSCEL Trial. J Am Heart Assoc. 2018;7:e009304.
18. Holman RR, Bethel MA, George J, et al. Rationale and design of the EXenatide Study of Cardiovascular Event Lowering (EXSCEL) trial. Am Heart J. 2016;174:103-110.
19. Hernandez AF, Green JB, Janmohamed S, et al; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519-1529.
20. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121-130.
21. Gerstein HC, Colhoun HM, Dagenais GR, et al; REWIND Investigators. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomized, placebo-controlled trial. Lancet. 2019;394:131-138.
22. Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
23. Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657.
24. Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357.
25. Kato ET, Silverman MG, Mosenzon O, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019;139:2528-2536.
26. Usman MS, Siddiqi TJ, Memon MM, et al. Sodium-glucose cotransporter 2 inhibitors and cardiovascular outcomes: a systematic review and meta-analysis. Eur J Prev Cardiol. 2018;25:495-502.
27. Kosiborod M, Cavender MA, Fu AZ, et al; CVD-REAL Investigators and Study Group. Lower risk of heart failure and death in patients initiated on sodium-glucose cotransporter-2 inhibitors versus other glucose-lowering drugs: the CVD-REAL study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation. 2017;136:249-259.
28. Tkáč I, Raz I. Combined analysis of three large interventional trials with gliptins indicates increased incidence of acute pancreatitis in patients with type 2 diabetes. Diabetes Care. 2017;40:284-286.
29. Schaffer C, Buclin T, Jornayvaz FR, et al. Use of dipeptidyl-peptidase IV inhibitors and bullous pemphigoid. Dermatology. 2017;233:401-403.
30. Madievsky R. Spotlight on antidiabetic agents with cardiovascular or renoprotective benefits. Perm J. 2018;22:18-034.
31. Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated hemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20:889-897.
32. Kosiborod M. Following the LEADER–why this and other recent trials signal a major paradigm shift in the management of type 2 diabetes. J Diabetes Complications. 2017;31:517-519.
33. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S90-S102.
34. Holman R. Metformin as first choice in oral diabetes treatment: the UKPDS experience. Journ Annu Diabetol Hotel Dieu. 2007:13-20.
35. American Diabetes Association. 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S103-S123.
36. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2018 executive summary. Endocr Pract. 2018;24:91-120.
37. Inzucci SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140-149.
38. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669-2701.
PRACTICE RECOMMENDATIONS
› Consider American Diabetes Association (ADA) guidance and prescribe a sodium–glucose cotransporter-2 (SGLT-2) inhibitor or glucagon-like peptide- 1 (GLP-1) receptor agonist that has demonstrated cardiovascular (CV) disease benefit for your patients who have type 2 diabetes (T2D) and established atherosclerotic CV disease. A
› Consider ADA’s recommendation for preferred therapy and prescribe an SGLT-2 inhibitor for your patients with T2D who have atherosclerotic CV disease and are at high risk of heart failure or in whom heart failure coexists. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Presentation is key to diagnosing salivary gland disorders
Making a diagnosis of a salivary gland disorder can be difficult. Common presentations, such as a painful or swollen gland, can be caused by numerous disorders of strikingly variable severity and consequences, including inflammatory, infectious, and neoplastic conditions, for which treatment can differ significantly, and referral for specialty care is sometimes necessary.
Yet it is the patient’s presentation that can aid you in making the diagnosis that will guide management. Consider that acute symptoms often result from infection, for example, and chronic or recurrent symptoms are caused more often by obstructive or nonobstructive inflammatory conditions and neoplasms. Diagnosis of an apparent neoplasm, prompted by clinical findings, is made using imaging and fine-needle aspiration (FNA) biopsy. Acute infection usually resolves with antibiotics and supportive management; calculi that cause persistent symptoms warrant referral for consideration of stone or gland removal; and malignant neoplasms usually require excision as well as neck dissection and chemotherapy or radiotherapy, or both—calling for multidisciplinary care.
In this article, we clarify what can be an imprecise and perplexing path from the presentation to diagnosis to treatment of disorders of the salivary glands. To begin, see “Geography of the salivary glands,” for an overview of the location, structure, and corresponding ducts of the component salivary glands (parotid, submandibular, sublingual, and minor glands).
SIDEBAR
Geography of the salivary glands
The salivary glands comprise the major paired parotid, submandibular, and sublingual glands, as well as minor salivary glands that line the oropharyngeal mucosa. Secretion of saliva is modulated by both autonomic and humoral factors.
The parotid gland sits between the mastoid process, the ramus of the mandible, and the styloid process, extend- ing from the external auditory meatus superiorly to below the angle of the mandible and into the neck inferiorly. The gland is surrounded by a tough capsule. Embedded within the gland is the facial nerve, which divides into its 5 branches within the substance of the gland. The parotid (Stensen’s) duct passes anteriorly before turning medially to pierce the buccinator muscle, opening onto the mucous membrane of the cheek opposite the second upper molar.
The submandibular gland comprises (1) a large superficial part that fills the space between the mandible and the floor of the mouth and (2) a small deep part that wraps around the posterior border of the mylohyoid muscle. The submandibular (Wharton’s) duct runs anteriorly to open onto the floor of the mouth, alongside the frenulum.
The sublingual gland, the smallest of the major salivary glands, lies anteriorly in the floor of the mouth, with many small ducts opening either into the submandibular duct or directly into the mouth.
Basic secretory units of salivary glands are clusters of cells, each called an acinus. These cells secrete a fluid that contains water, electrolytes, mucous, and enzymes, all of which flow out of the acini into collecting ducts. The saliva produced by the parotid is mainly serous; by the submandibular gland, mixed; and by the sublingual and minor salivary glands, mucoid.
Presentation helps establish the differential Dx
Ask: Are the glands swollen?
Painless salivary gland swelling has a variety of causes, including neoplasm, sialadenosis, and the eating disorders bulimia and anorexia nervosa. There is significant overlap of presentations among those causes (FIGURE). Pain accompanying swelling is uncommon but not unheard of.
Neoplasms. Tumors of the salivary gland are relatively uncommon, constituting approximately 2% of head and neck neoplasms; most (80%) occur in the parotid gland, and most of those are benign.1 Although benign and malignant salivary gland neoplasms do not usually present with pain, pain can be associated with a neoplasm secondary to suppuration, hemorrhage into a mass, or infiltration of a malignancy into adjacent tissue.
Benign tumors. The majority of benign tumors are pleomorphic adenomas of the parotid, accounting for approximately 60% of salivary gland neoplasms.1,2 Tumors localized to the submandibular gland are often (in 50% of cases) malignant, however.3
Benign tumors are typically slow-growing and, generally, painless. On examination, they are well-circumscribed, mobile, and nontender. Patients presenting late with a large tumor might, however, experience pain secondary to stretching of the parotid capsule or compression of local structures.
Continue to: Ultrasonograhpy (US) is an excellent...
Ultrasonography (US) is an excellent initial imaging choice for investigating a possible salivary gland tumor; US is combined with FNA, which is safe and highly reliable for differentiating neoplastic and non-neoplastic disorders.4 (Avoid open biopsy of a neoplasm because of the risk of tumor spillage.) In patients with suspected neoplasm, contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) should also be performed, because both modalities allow delineation of the tumor mass and demonstration of any infiltration of surrounding structures.
Treatment of benign neoplasms involves complete excision because, with some tumors, particularly pleomorphic adenomas, there is risk of malignant transformation over time. Superficial parotidectomy is the most common procedure, because most benign tumors occur in the superficial lobe of the parotid gland. Delicate dissection of the facial nerve is integral to the operation, although temporary facial nerve palsy will still occur in 5% to 10% of patients undergoing superficial parotidectomy for a benign tumor, with permanent injury occurring in fewer than 1%.5
Malignancy. Features of a tumor that raise concern of malignancy include6:
- rapid growth
- pain
- tethering to underlying structures or overlying skin
- firm mass
- associated cervical lymphadenopathy
- facial-nerve palsy.
The workup of a malignant tumor is the same as it is for a benign neoplasm: US-guided FNA, essential for diagnosis, and contrast-enhanced CT or MRI to delineate the tumor.
Malignant salivary gland neoplasms usually require excision as well as neck dissection and chemotherapy or radiotherapy, or both, necessitating a multidisciplinary approach. Also, there is potential for squamous-cell carcinoma and melanoma of the head to metastasize to salivary gland lymph nodes; it is important, therefore, to examine for, and elicit any history of, cutaneous malignancy of the scalp or face.
Continue to: Sialadenosis...
Sialadenosis presents with asymptomatic bilateral hypertrophy of the salivary glands—more commonly the parotids and rarely the submandibular glands. Swelling is persistent, symmetrical, painless, and of normal tone on palpation.
Causes of sialadenosis include alcoholism and, less commonly, diabetes mellitus and malnutrition; some cases are idiopathic. An autonomic neuropathy, causing excessive salivary acinar protein synthesis or failure of adequate secretion, or both, is common to alcoholism, diabetes, and malnutrition.7 Subsequent engorgement of acinar cells leads to clinical parotid hypertrophy.
Diagnosis is based on history and examination, as well as on the findings of US or CT, which will reveal bilateral gland enlargement and increased density. The glands appear dense because adipose cells are displaced by acinar cell hypertrophy; however, end-stage changes can result in the opposite appearance: a lucent enlargement caused by fatty infiltration.2 FNA is unnecessary, unless there is suspicion of neoplasm, as there would be in patients with asymmetrical parotid enlargement, pain, lymph node enlargement, or facial-nerve involvement. In patients with sialadenosis, in contrast, acinar cell hypertrophy alone will be present.
Treatment of sialadenosis is best aimed at rectifying the underlying medical condition, which might, over time, lead to some reduction in the size of the gland. There is no specific effective therapy for elimination of glandular swelling.
Bulimia and anorexia nervosa. Bulimia nervosa, the induction of vomiting after binge eating, can be associated with bilateral or occasionally unilateral parotid swelling. Anorexia, a form of self-starvation, can occur in association with bulimia, with patients also presenting with parotid swelling. Associated parotid swelling is similar to what is seen in sialadenosis: painless, persistent, and of nonpathologic consistency.
The pathophysiology of bulimia- and anorexia-associated parotid-gland swelling is identical to what is seen in sialadenosis: dysregulation of acinar cell sympathetic nerve supply that leads to enlargement of individual parenchymal cells.8 Contrast-enhanced CT can reveal increased vascularity associated with active bulimia. FNA and CT, however, are required only in patients in whom the diagnosis is not clear and when neoplasm is suspected.
Continue to: Treatment includes...
Treatment includes correcting electrolyte abnormalities and, more importantly, addressing underlying emotional issues to stop purging episodes. Psychiatric input and social support are invaluable. Parotid gland swelling generally improves with cessation of vomiting episodes.
Ask: Is the patient in pain?
Causes of salivary gland pain include sialolithiasis, sialadenitis, and recurrent parotitis of childhood. Pain occurs secondary to stretching of the fibrous capsule in which the parotid or submandibular gland is surrounded, compression of pain fibers by an expanding mass, or infiltration of nerves by neoplasia.
Sialolithiasis. Sialolithiasis, or salivary stones, are primarily calcium carbonate concentrations within the salivary ductal system. More than 80% occur in the submandibular gland or duct9 as a result of production of mixed mucoid and serous saliva and a tortuous duct path.
Patients usually present with a history of intermittent swelling and pain of the involved gland associated with eating. Increased production of saliva during meals, which then passes through a partially obstructed salivary duct, leads to salivary retention and glandular swelling. Thus, a recurring pattern can develop, with varying periods of remission,7 eventually leading to an acute suppurative process or sialadenitis (described below). Chronic salivary disease can also be caused by stricture of a duct or, rarely, external compression by a tumor mass.
Examination often reveals an enlarged and often tender gland; conversely, chronic disease can lead to gland atrophy. Usually, only minimal saliva is able to be expressed from an obstructed duct. For a submandibular duct stone, bimanual palpation might reveal its position if it is located distally in the floor of the mouth; a proximal stone might not be palpable.
Continue to: Although US is operator-dependent...
Although US is operator-dependent, it is the imaging modality of choice for identifying sialolithiasis10 because it can identify gland architecture, duct dilation, and both radiolucent and radiopaque stones. For patients in whom US findings are normal despite a convincing clinical presentation of sialolithiasis, CT should be performed because small stones can be missed on US.11
Supportive measures for sialolithiasis are listed in the TABLE. Reserve antibiotics for patients who have signs or symptoms of infection, including pyrexia, trismus, and malaise. A beta-lactam antibiotic, such as amoxicillin–clavulanate, 875 mg orally bid, or a cephalosporin, such as cephalexin, 500 mg orally qid, are appropriate first-line options. Clindamycin, 300 mg orally tid, or metronidazole, 500 mg orally tid, are acceptable alternatives. When signs or symptoms are persistent or recurrent, refer the patient for a surgical opinion.
Stones located in the floor of the mouth are usually excised through an intraoral approach. In the past, gland excision was advocated when a sialolith was found more proximally within the gland parenchyma. More recently, however, sialendoscopy, involving insertion of a small, semirigid endoscope into the salivary duct, has been shown safe and effective for removing a stone; successful removal, in as many as 80% of cases, increases to 90% when performed using a minimally invasive surgical technique.12 Although sialendoscopy is effective, the technique cannot always treat the underlying abnormality of the salivary gland; gland excision is therefore warranted in some cases.
Last, extracorporeal shock wave therapy is aimed at fragmenting salivary stones before retrieval. Results are variable, however, and treatment should be guided by an otolaryngologist.13,14
Sialadenitis (bacterial and viral infection). Acute suppurative sialadenitis occurs secondary to retrograde ductal bacterial infection. The parotid gland is most frequently involved,15 although submandibular sialadenitis is not uncommon. Patients usually present with sudden-onset unilateral, painful swelling.
Continue to: Pathophysiology involves...
Pathophysiology involves dehydration or decreased oral intake leading to salivary stasis and subsequent bacterial migration into the gland. Medically debilitated and postoperative patients are therefore at greater risk; so are patients with diabetes mellitus, poor oral hygiene, Sjögren’s syndrome, hypothyroidism, or renal failure.16 Certain medications, including anticholinergics, can also predispose to hyposalivation.17
(As discussed, sialolithiasis and stricture of salivary ducts can also cause acute bacterial infection; in such cases, however, the typical presentation is one of chronic or recurrent infection.)
Examination might reveal an exquisitely tender, indurated, and inflamed gland; pus can often be expressed from the respective intraoral orifice. Any expressed pus should be sent for culture to guide antibiotic therapy.
Treatment should focus on hydration, oral hygiene, and antibiotics, while reversing or minimizing any underlying contributing medical condition. Warm compresses applied to the involved gland, massage, and sialagogues, such as lemon drops or sugar-free lollipops, can stimulate salivary flow and prevent stasis.
More than 80% of infections are caused by Staphylococcus aureus17; anaerobic and mixed infections have also been recognized.A beta-lactam penicillin, such as amoxicillin-clavulanate, is the antibiotic of choice. A patient who is systemically unwell should be treated as an inpatient with nafcillin and metronidazole. Methicillin-resistant S aureus must also be considered in patients with comorbid disease, such as diabetes mellitus or intravenous drug use, or in patients residing in an area of substantial incidence of methicillin-resistant S aureus. In those cases, substitute vancomycin or linezolid for nafcillin.18
Continue to: Less commonly...
Less commonly, abscess can form, with the patient presenting as systemically unwell with a fluctuant mass. If the diagnosis is unclear or the patient does not improve, abscess can be confirmed by US. Expedient surgical review and inpatient admission can then be arranged.
Unlike bacterial sialadenitis, causes of viral sialadenitis are often bilateral. Mumps (a paramyxovirus) is the most common viral cause, affecting primarily children < 15 years.19 The parotid glands are most often involved, with inflammation and edema causing significant pain because of increasing intraparotid pressure as expansion of the gland is limited by its tense fibrous capsule. Complications of mumps include orchitis, meningitis, pancreatitis, and oophoritis.
Mumps is highly contagious; it is spread through contact with airborne saliva droplets, with viral entry through the nose or mouth, followed by proliferation in the salivary glands or on surface epithelium of the respiratory tract.7 Diagnosis is confirmed by viral serology. A positive test of serum immunoglobulin M confirms the diagnosis, but this test should not be performed until 3 days after onset of symptoms because a false-negative result is otherwise possible.20 Immunoglobulin G serologic testing can further aid diagnosis; the titer is measured approximately 4 days after onset of symptoms and again 2 to 3 weeks later. A 4-fold rise in titer confirms mumps.
Other viral infections that can cause sialadenitis include Epstein-Barr virus, cytomegalovirus, human immunodeficiency virus, coxsackievirus, and influenza. Treatment is supportive: analgesia, hydration, oral hygiene, and rest. Inflammation might take weeks to resolve, but expect complete resolution. For a patient who has significant trismus, poor oral intake, or a potentially threatened airway, inpatient care should be provided.
Recurrent parotitis of childhood is an inflammatory condition that usually affects one, but at times both, parotid glands. It is characterized by episodes of painful swelling. Incidence peaks at 3 to 6 years of age.7 Episodes can be frequent, occurring 1 to 5 times a year and lasting 3 to 7 days—sometimes longer—and usually resolving without treatment.
Continue to: The precise etiology...
The precise etiology of recurrent parotitis of childhood is unclear; possibly, saliva aggregates to form obstructive mucous plugs, thus causing stasis and swelling of the gland. As pressure builds, spontaneous plug extrusion occurs and symptoms resolve, provided infection is not a factor. US demonstrates multiple round, hypoechoic areas consistent with duct dilation, and surrounding infiltration by lymphocytes.1
Supportive care—adequate hydration, gland massage, warm compresses, and sialogogues—are mainstays of treatment. Fever and malaise warrant treatment with oral antibiotics. Sialadenoscopy, which can be considered in children with frequent episodes, can decrease the frequency and severity of episodes.21 The condition usually resolves spontaneously at puberty.
Ask: Does the patient have dry mouth?
In-depth review of xerostomia is beyond the scope of this article. Causes include Sjögren's syndrome, immunoglobulin G4-related sialadenitis, sarcoidosis, radiation therapy, diabetes, chronic infection, and medications—in particular those with anticholinergic effects.
Treatment of xerostomia includes saliva substitutes, sialagogues, and, for oral candidiasis, antifungals. Muscarinic cholinergic stimulators, such as pilocarpine, 5 mg qid have been used with some success22; patients should be advised of potential adverse effects with these agents, including sweating, urinary frequency, flushing, and chills.
CORRESPONDENCE
Shankar Haran, MBBS, ENT Department, Townsville Hospital, 100 Angus Smith Dr, Douglas, Queensland, Australia 4814; Shankar.haran01@gmail.com.
1. de Oliveira FA, Duarte EC, Taveira CT, et al. Salivary gland tumor: a review of 599 cases in a Brazilian population. Head Neck Pathol. 2009;3:271-275.
2. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177-184.
3. Bova R. A guide to salivary gland disorders. Medicine Today. 2006;7:44-48.
4. Zhang S, Bao R, Bagby J, et al. Fine needle aspiration of salivary glands: 5-year experience from a single academic center. Acta Cytol. 2009;53:375-382.
5. Bova R, Saylor A, Coman WB. Parotidectomy: review of treatment and outcomes. ANZ J Surg. 2004;74:563-568.
6. Sood S, McGurk M, Vaz F. Management of salivary gland tumours: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130:S142-S149.
7. Mandel L. Salivary gland disorders. Med Clin North Am. 2014;98:1407-1449.
8. Mandel L, Abai S. Diagnosing bulimia nervosa with parotid gland swelling. J Am Dent Assoc. 2004;135:613–616.
9. Lustmann J, Regev E, Melamed Y. Sialolithiasis. A survey on 245 patients and a review of literature. Int J Oral Maxillofac Surg. 1990;19:135–138.
10. Vogl TJ, Al-Nawas B, Beutner D, et al. Updated S2K AWMF guideline for the diagnosis and follow-up of obstructive sialadenitis—relevance for radiologic imaging. Rofo. 2014;186:843-846.
11. Schwarz D, Kabbasch C, Scheer M, et al. Comparative analysis of sialendoscopy, sonography, and CBCT in the detection of sialolithiasis. Laryngoscope. 2015;125:1098–1101.
12. Atienza G, López-Cedrún JL. Management of obstructive salivary disorders by sialendoscopy: a systematic review. Br J Oral Maxillofac Surg. 2015;53:507-519.
13. Escudier MP, Brown JE, Putcha V, et al. Factors influencing the outcome of extracorporeal shock wave lithotripsy in the management of salivary calculi. Laryngoscope. 2010;120:1545-1549.
14. Koch M, Schapher M, Mantsopoulos K, et al. Multimodal treatment in difficult sialolithiasis: Role of extracorporeal shock-wave lithotripsy and intraductal pneumatic lithotripsy. Laryngoscope. 2018;128:E332-E338.
15. McQuone SJ. Acute viral and bacterial infections of the salivary glands. Otolaryngol Clin North Am. 1999;32:793-811.
16. O’Neil C, Sidhu S. Salivary gland disorders. Australian Doctor. 2011;28:19-25.
17. Mandel L. Differentiating acute suppurative parotitis from acute exacerbation of a chronic parotitis: case reports. J Oral Maxillofac Surg. 2008;66:1964-1968.
18. Chow AW. Suppurative parotitis in adults. UpToDate.com. www.uptodate.com/contents/suppurative-parotitis-in-adults. Accessed September 25, 2019.
19. Katz SL, Gershon AA, Hotez PJ. Infectious Diseases of Children. New York, NY: Mosby Year Book; 1998:280-289.
20. Krause CH, Molyneaux PJ, Ho-Yen DO, et al. Comparison of mumps-IgM ELISAs in acute infection. J Clin Virol. 2007;38:153-156.
21. Quenin S, Plouin-Gaudon I, Marchal F, et al. Juvenile recurrent parotitis: sialendoscopic approach. Arch Otolaryngol Head Neck Surg. 2008;134:715-719.
22. Papas AS, Sherrer YS, Charney M, et al. Successful treatment of dry mouth and dry eye symptoms in Sjögren’s syndrome patients with oral pilocarpine: a randomized, placebo-controlled, dose-adjustment study. J Clin Rheumatol. 2004;10:169-177.
Making a diagnosis of a salivary gland disorder can be difficult. Common presentations, such as a painful or swollen gland, can be caused by numerous disorders of strikingly variable severity and consequences, including inflammatory, infectious, and neoplastic conditions, for which treatment can differ significantly, and referral for specialty care is sometimes necessary.
Yet it is the patient’s presentation that can aid you in making the diagnosis that will guide management. Consider that acute symptoms often result from infection, for example, and chronic or recurrent symptoms are caused more often by obstructive or nonobstructive inflammatory conditions and neoplasms. Diagnosis of an apparent neoplasm, prompted by clinical findings, is made using imaging and fine-needle aspiration (FNA) biopsy. Acute infection usually resolves with antibiotics and supportive management; calculi that cause persistent symptoms warrant referral for consideration of stone or gland removal; and malignant neoplasms usually require excision as well as neck dissection and chemotherapy or radiotherapy, or both—calling for multidisciplinary care.
In this article, we clarify what can be an imprecise and perplexing path from the presentation to diagnosis to treatment of disorders of the salivary glands. To begin, see “Geography of the salivary glands,” for an overview of the location, structure, and corresponding ducts of the component salivary glands (parotid, submandibular, sublingual, and minor glands).
SIDEBAR
Geography of the salivary glands
The salivary glands comprise the major paired parotid, submandibular, and sublingual glands, as well as minor salivary glands that line the oropharyngeal mucosa. Secretion of saliva is modulated by both autonomic and humoral factors.
The parotid gland sits between the mastoid process, the ramus of the mandible, and the styloid process, extend- ing from the external auditory meatus superiorly to below the angle of the mandible and into the neck inferiorly. The gland is surrounded by a tough capsule. Embedded within the gland is the facial nerve, which divides into its 5 branches within the substance of the gland. The parotid (Stensen’s) duct passes anteriorly before turning medially to pierce the buccinator muscle, opening onto the mucous membrane of the cheek opposite the second upper molar.
The submandibular gland comprises (1) a large superficial part that fills the space between the mandible and the floor of the mouth and (2) a small deep part that wraps around the posterior border of the mylohyoid muscle. The submandibular (Wharton’s) duct runs anteriorly to open onto the floor of the mouth, alongside the frenulum.
The sublingual gland, the smallest of the major salivary glands, lies anteriorly in the floor of the mouth, with many small ducts opening either into the submandibular duct or directly into the mouth.
Basic secretory units of salivary glands are clusters of cells, each called an acinus. These cells secrete a fluid that contains water, electrolytes, mucous, and enzymes, all of which flow out of the acini into collecting ducts. The saliva produced by the parotid is mainly serous; by the submandibular gland, mixed; and by the sublingual and minor salivary glands, mucoid.
Presentation helps establish the differential Dx
Ask: Are the glands swollen?
Painless salivary gland swelling has a variety of causes, including neoplasm, sialadenosis, and the eating disorders bulimia and anorexia nervosa. There is significant overlap of presentations among those causes (FIGURE). Pain accompanying swelling is uncommon but not unheard of.
Neoplasms. Tumors of the salivary gland are relatively uncommon, constituting approximately 2% of head and neck neoplasms; most (80%) occur in the parotid gland, and most of those are benign.1 Although benign and malignant salivary gland neoplasms do not usually present with pain, pain can be associated with a neoplasm secondary to suppuration, hemorrhage into a mass, or infiltration of a malignancy into adjacent tissue.
Benign tumors. The majority of benign tumors are pleomorphic adenomas of the parotid, accounting for approximately 60% of salivary gland neoplasms.1,2 Tumors localized to the submandibular gland are often (in 50% of cases) malignant, however.3
Benign tumors are typically slow-growing and, generally, painless. On examination, they are well-circumscribed, mobile, and nontender. Patients presenting late with a large tumor might, however, experience pain secondary to stretching of the parotid capsule or compression of local structures.
Continue to: Ultrasonograhpy (US) is an excellent...
Ultrasonography (US) is an excellent initial imaging choice for investigating a possible salivary gland tumor; US is combined with FNA, which is safe and highly reliable for differentiating neoplastic and non-neoplastic disorders.4 (Avoid open biopsy of a neoplasm because of the risk of tumor spillage.) In patients with suspected neoplasm, contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) should also be performed, because both modalities allow delineation of the tumor mass and demonstration of any infiltration of surrounding structures.
Treatment of benign neoplasms involves complete excision because, with some tumors, particularly pleomorphic adenomas, there is risk of malignant transformation over time. Superficial parotidectomy is the most common procedure, because most benign tumors occur in the superficial lobe of the parotid gland. Delicate dissection of the facial nerve is integral to the operation, although temporary facial nerve palsy will still occur in 5% to 10% of patients undergoing superficial parotidectomy for a benign tumor, with permanent injury occurring in fewer than 1%.5
Malignancy. Features of a tumor that raise concern of malignancy include6:
- rapid growth
- pain
- tethering to underlying structures or overlying skin
- firm mass
- associated cervical lymphadenopathy
- facial-nerve palsy.
The workup of a malignant tumor is the same as it is for a benign neoplasm: US-guided FNA, essential for diagnosis, and contrast-enhanced CT or MRI to delineate the tumor.
Malignant salivary gland neoplasms usually require excision as well as neck dissection and chemotherapy or radiotherapy, or both, necessitating a multidisciplinary approach. Also, there is potential for squamous-cell carcinoma and melanoma of the head to metastasize to salivary gland lymph nodes; it is important, therefore, to examine for, and elicit any history of, cutaneous malignancy of the scalp or face.
Continue to: Sialadenosis...
Sialadenosis presents with asymptomatic bilateral hypertrophy of the salivary glands—more commonly the parotids and rarely the submandibular glands. Swelling is persistent, symmetrical, painless, and of normal tone on palpation.
Causes of sialadenosis include alcoholism and, less commonly, diabetes mellitus and malnutrition; some cases are idiopathic. An autonomic neuropathy, causing excessive salivary acinar protein synthesis or failure of adequate secretion, or both, is common to alcoholism, diabetes, and malnutrition.7 Subsequent engorgement of acinar cells leads to clinical parotid hypertrophy.
Diagnosis is based on history and examination, as well as on the findings of US or CT, which will reveal bilateral gland enlargement and increased density. The glands appear dense because adipose cells are displaced by acinar cell hypertrophy; however, end-stage changes can result in the opposite appearance: a lucent enlargement caused by fatty infiltration.2 FNA is unnecessary, unless there is suspicion of neoplasm, as there would be in patients with asymmetrical parotid enlargement, pain, lymph node enlargement, or facial-nerve involvement. In patients with sialadenosis, in contrast, acinar cell hypertrophy alone will be present.
Treatment of sialadenosis is best aimed at rectifying the underlying medical condition, which might, over time, lead to some reduction in the size of the gland. There is no specific effective therapy for elimination of glandular swelling.
Bulimia and anorexia nervosa. Bulimia nervosa, the induction of vomiting after binge eating, can be associated with bilateral or occasionally unilateral parotid swelling. Anorexia, a form of self-starvation, can occur in association with bulimia, with patients also presenting with parotid swelling. Associated parotid swelling is similar to what is seen in sialadenosis: painless, persistent, and of nonpathologic consistency.
The pathophysiology of bulimia- and anorexia-associated parotid-gland swelling is identical to what is seen in sialadenosis: dysregulation of acinar cell sympathetic nerve supply that leads to enlargement of individual parenchymal cells.8 Contrast-enhanced CT can reveal increased vascularity associated with active bulimia. FNA and CT, however, are required only in patients in whom the diagnosis is not clear and when neoplasm is suspected.
Continue to: Treatment includes...
Treatment includes correcting electrolyte abnormalities and, more importantly, addressing underlying emotional issues to stop purging episodes. Psychiatric input and social support are invaluable. Parotid gland swelling generally improves with cessation of vomiting episodes.
Ask: Is the patient in pain?
Causes of salivary gland pain include sialolithiasis, sialadenitis, and recurrent parotitis of childhood. Pain occurs secondary to stretching of the fibrous capsule in which the parotid or submandibular gland is surrounded, compression of pain fibers by an expanding mass, or infiltration of nerves by neoplasia.
Sialolithiasis. Sialolithiasis, or salivary stones, are primarily calcium carbonate concentrations within the salivary ductal system. More than 80% occur in the submandibular gland or duct9 as a result of production of mixed mucoid and serous saliva and a tortuous duct path.
Patients usually present with a history of intermittent swelling and pain of the involved gland associated with eating. Increased production of saliva during meals, which then passes through a partially obstructed salivary duct, leads to salivary retention and glandular swelling. Thus, a recurring pattern can develop, with varying periods of remission,7 eventually leading to an acute suppurative process or sialadenitis (described below). Chronic salivary disease can also be caused by stricture of a duct or, rarely, external compression by a tumor mass.
Examination often reveals an enlarged and often tender gland; conversely, chronic disease can lead to gland atrophy. Usually, only minimal saliva is able to be expressed from an obstructed duct. For a submandibular duct stone, bimanual palpation might reveal its position if it is located distally in the floor of the mouth; a proximal stone might not be palpable.
Continue to: Although US is operator-dependent...
Although US is operator-dependent, it is the imaging modality of choice for identifying sialolithiasis10 because it can identify gland architecture, duct dilation, and both radiolucent and radiopaque stones. For patients in whom US findings are normal despite a convincing clinical presentation of sialolithiasis, CT should be performed because small stones can be missed on US.11
Supportive measures for sialolithiasis are listed in the TABLE. Reserve antibiotics for patients who have signs or symptoms of infection, including pyrexia, trismus, and malaise. A beta-lactam antibiotic, such as amoxicillin–clavulanate, 875 mg orally bid, or a cephalosporin, such as cephalexin, 500 mg orally qid, are appropriate first-line options. Clindamycin, 300 mg orally tid, or metronidazole, 500 mg orally tid, are acceptable alternatives. When signs or symptoms are persistent or recurrent, refer the patient for a surgical opinion.
Stones located in the floor of the mouth are usually excised through an intraoral approach. In the past, gland excision was advocated when a sialolith was found more proximally within the gland parenchyma. More recently, however, sialendoscopy, involving insertion of a small, semirigid endoscope into the salivary duct, has been shown safe and effective for removing a stone; successful removal, in as many as 80% of cases, increases to 90% when performed using a minimally invasive surgical technique.12 Although sialendoscopy is effective, the technique cannot always treat the underlying abnormality of the salivary gland; gland excision is therefore warranted in some cases.
Last, extracorporeal shock wave therapy is aimed at fragmenting salivary stones before retrieval. Results are variable, however, and treatment should be guided by an otolaryngologist.13,14
Sialadenitis (bacterial and viral infection). Acute suppurative sialadenitis occurs secondary to retrograde ductal bacterial infection. The parotid gland is most frequently involved,15 although submandibular sialadenitis is not uncommon. Patients usually present with sudden-onset unilateral, painful swelling.
Continue to: Pathophysiology involves...
Pathophysiology involves dehydration or decreased oral intake leading to salivary stasis and subsequent bacterial migration into the gland. Medically debilitated and postoperative patients are therefore at greater risk; so are patients with diabetes mellitus, poor oral hygiene, Sjögren’s syndrome, hypothyroidism, or renal failure.16 Certain medications, including anticholinergics, can also predispose to hyposalivation.17
(As discussed, sialolithiasis and stricture of salivary ducts can also cause acute bacterial infection; in such cases, however, the typical presentation is one of chronic or recurrent infection.)
Examination might reveal an exquisitely tender, indurated, and inflamed gland; pus can often be expressed from the respective intraoral orifice. Any expressed pus should be sent for culture to guide antibiotic therapy.
Treatment should focus on hydration, oral hygiene, and antibiotics, while reversing or minimizing any underlying contributing medical condition. Warm compresses applied to the involved gland, massage, and sialagogues, such as lemon drops or sugar-free lollipops, can stimulate salivary flow and prevent stasis.
More than 80% of infections are caused by Staphylococcus aureus17; anaerobic and mixed infections have also been recognized.A beta-lactam penicillin, such as amoxicillin-clavulanate, is the antibiotic of choice. A patient who is systemically unwell should be treated as an inpatient with nafcillin and metronidazole. Methicillin-resistant S aureus must also be considered in patients with comorbid disease, such as diabetes mellitus or intravenous drug use, or in patients residing in an area of substantial incidence of methicillin-resistant S aureus. In those cases, substitute vancomycin or linezolid for nafcillin.18
Continue to: Less commonly...
Less commonly, abscess can form, with the patient presenting as systemically unwell with a fluctuant mass. If the diagnosis is unclear or the patient does not improve, abscess can be confirmed by US. Expedient surgical review and inpatient admission can then be arranged.
Unlike bacterial sialadenitis, causes of viral sialadenitis are often bilateral. Mumps (a paramyxovirus) is the most common viral cause, affecting primarily children < 15 years.19 The parotid glands are most often involved, with inflammation and edema causing significant pain because of increasing intraparotid pressure as expansion of the gland is limited by its tense fibrous capsule. Complications of mumps include orchitis, meningitis, pancreatitis, and oophoritis.
Mumps is highly contagious; it is spread through contact with airborne saliva droplets, with viral entry through the nose or mouth, followed by proliferation in the salivary glands or on surface epithelium of the respiratory tract.7 Diagnosis is confirmed by viral serology. A positive test of serum immunoglobulin M confirms the diagnosis, but this test should not be performed until 3 days after onset of symptoms because a false-negative result is otherwise possible.20 Immunoglobulin G serologic testing can further aid diagnosis; the titer is measured approximately 4 days after onset of symptoms and again 2 to 3 weeks later. A 4-fold rise in titer confirms mumps.
Other viral infections that can cause sialadenitis include Epstein-Barr virus, cytomegalovirus, human immunodeficiency virus, coxsackievirus, and influenza. Treatment is supportive: analgesia, hydration, oral hygiene, and rest. Inflammation might take weeks to resolve, but expect complete resolution. For a patient who has significant trismus, poor oral intake, or a potentially threatened airway, inpatient care should be provided.
Recurrent parotitis of childhood is an inflammatory condition that usually affects one, but at times both, parotid glands. It is characterized by episodes of painful swelling. Incidence peaks at 3 to 6 years of age.7 Episodes can be frequent, occurring 1 to 5 times a year and lasting 3 to 7 days—sometimes longer—and usually resolving without treatment.
Continue to: The precise etiology...
The precise etiology of recurrent parotitis of childhood is unclear; possibly, saliva aggregates to form obstructive mucous plugs, thus causing stasis and swelling of the gland. As pressure builds, spontaneous plug extrusion occurs and symptoms resolve, provided infection is not a factor. US demonstrates multiple round, hypoechoic areas consistent with duct dilation, and surrounding infiltration by lymphocytes.1
Supportive care—adequate hydration, gland massage, warm compresses, and sialogogues—are mainstays of treatment. Fever and malaise warrant treatment with oral antibiotics. Sialadenoscopy, which can be considered in children with frequent episodes, can decrease the frequency and severity of episodes.21 The condition usually resolves spontaneously at puberty.
Ask: Does the patient have dry mouth?
In-depth review of xerostomia is beyond the scope of this article. Causes include Sjögren's syndrome, immunoglobulin G4-related sialadenitis, sarcoidosis, radiation therapy, diabetes, chronic infection, and medications—in particular those with anticholinergic effects.
Treatment of xerostomia includes saliva substitutes, sialagogues, and, for oral candidiasis, antifungals. Muscarinic cholinergic stimulators, such as pilocarpine, 5 mg qid have been used with some success22; patients should be advised of potential adverse effects with these agents, including sweating, urinary frequency, flushing, and chills.
CORRESPONDENCE
Shankar Haran, MBBS, ENT Department, Townsville Hospital, 100 Angus Smith Dr, Douglas, Queensland, Australia 4814; Shankar.haran01@gmail.com.
Making a diagnosis of a salivary gland disorder can be difficult. Common presentations, such as a painful or swollen gland, can be caused by numerous disorders of strikingly variable severity and consequences, including inflammatory, infectious, and neoplastic conditions, for which treatment can differ significantly, and referral for specialty care is sometimes necessary.
Yet it is the patient’s presentation that can aid you in making the diagnosis that will guide management. Consider that acute symptoms often result from infection, for example, and chronic or recurrent symptoms are caused more often by obstructive or nonobstructive inflammatory conditions and neoplasms. Diagnosis of an apparent neoplasm, prompted by clinical findings, is made using imaging and fine-needle aspiration (FNA) biopsy. Acute infection usually resolves with antibiotics and supportive management; calculi that cause persistent symptoms warrant referral for consideration of stone or gland removal; and malignant neoplasms usually require excision as well as neck dissection and chemotherapy or radiotherapy, or both—calling for multidisciplinary care.
In this article, we clarify what can be an imprecise and perplexing path from the presentation to diagnosis to treatment of disorders of the salivary glands. To begin, see “Geography of the salivary glands,” for an overview of the location, structure, and corresponding ducts of the component salivary glands (parotid, submandibular, sublingual, and minor glands).
SIDEBAR
Geography of the salivary glands
The salivary glands comprise the major paired parotid, submandibular, and sublingual glands, as well as minor salivary glands that line the oropharyngeal mucosa. Secretion of saliva is modulated by both autonomic and humoral factors.
The parotid gland sits between the mastoid process, the ramus of the mandible, and the styloid process, extend- ing from the external auditory meatus superiorly to below the angle of the mandible and into the neck inferiorly. The gland is surrounded by a tough capsule. Embedded within the gland is the facial nerve, which divides into its 5 branches within the substance of the gland. The parotid (Stensen’s) duct passes anteriorly before turning medially to pierce the buccinator muscle, opening onto the mucous membrane of the cheek opposite the second upper molar.
The submandibular gland comprises (1) a large superficial part that fills the space between the mandible and the floor of the mouth and (2) a small deep part that wraps around the posterior border of the mylohyoid muscle. The submandibular (Wharton’s) duct runs anteriorly to open onto the floor of the mouth, alongside the frenulum.
The sublingual gland, the smallest of the major salivary glands, lies anteriorly in the floor of the mouth, with many small ducts opening either into the submandibular duct or directly into the mouth.
Basic secretory units of salivary glands are clusters of cells, each called an acinus. These cells secrete a fluid that contains water, electrolytes, mucous, and enzymes, all of which flow out of the acini into collecting ducts. The saliva produced by the parotid is mainly serous; by the submandibular gland, mixed; and by the sublingual and minor salivary glands, mucoid.
Presentation helps establish the differential Dx
Ask: Are the glands swollen?
Painless salivary gland swelling has a variety of causes, including neoplasm, sialadenosis, and the eating disorders bulimia and anorexia nervosa. There is significant overlap of presentations among those causes (FIGURE). Pain accompanying swelling is uncommon but not unheard of.
Neoplasms. Tumors of the salivary gland are relatively uncommon, constituting approximately 2% of head and neck neoplasms; most (80%) occur in the parotid gland, and most of those are benign.1 Although benign and malignant salivary gland neoplasms do not usually present with pain, pain can be associated with a neoplasm secondary to suppuration, hemorrhage into a mass, or infiltration of a malignancy into adjacent tissue.
Benign tumors. The majority of benign tumors are pleomorphic adenomas of the parotid, accounting for approximately 60% of salivary gland neoplasms.1,2 Tumors localized to the submandibular gland are often (in 50% of cases) malignant, however.3
Benign tumors are typically slow-growing and, generally, painless. On examination, they are well-circumscribed, mobile, and nontender. Patients presenting late with a large tumor might, however, experience pain secondary to stretching of the parotid capsule or compression of local structures.
Continue to: Ultrasonograhpy (US) is an excellent...
Ultrasonography (US) is an excellent initial imaging choice for investigating a possible salivary gland tumor; US is combined with FNA, which is safe and highly reliable for differentiating neoplastic and non-neoplastic disorders.4 (Avoid open biopsy of a neoplasm because of the risk of tumor spillage.) In patients with suspected neoplasm, contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) should also be performed, because both modalities allow delineation of the tumor mass and demonstration of any infiltration of surrounding structures.
Treatment of benign neoplasms involves complete excision because, with some tumors, particularly pleomorphic adenomas, there is risk of malignant transformation over time. Superficial parotidectomy is the most common procedure, because most benign tumors occur in the superficial lobe of the parotid gland. Delicate dissection of the facial nerve is integral to the operation, although temporary facial nerve palsy will still occur in 5% to 10% of patients undergoing superficial parotidectomy for a benign tumor, with permanent injury occurring in fewer than 1%.5
Malignancy. Features of a tumor that raise concern of malignancy include6:
- rapid growth
- pain
- tethering to underlying structures or overlying skin
- firm mass
- associated cervical lymphadenopathy
- facial-nerve palsy.
The workup of a malignant tumor is the same as it is for a benign neoplasm: US-guided FNA, essential for diagnosis, and contrast-enhanced CT or MRI to delineate the tumor.
Malignant salivary gland neoplasms usually require excision as well as neck dissection and chemotherapy or radiotherapy, or both, necessitating a multidisciplinary approach. Also, there is potential for squamous-cell carcinoma and melanoma of the head to metastasize to salivary gland lymph nodes; it is important, therefore, to examine for, and elicit any history of, cutaneous malignancy of the scalp or face.
Continue to: Sialadenosis...
Sialadenosis presents with asymptomatic bilateral hypertrophy of the salivary glands—more commonly the parotids and rarely the submandibular glands. Swelling is persistent, symmetrical, painless, and of normal tone on palpation.
Causes of sialadenosis include alcoholism and, less commonly, diabetes mellitus and malnutrition; some cases are idiopathic. An autonomic neuropathy, causing excessive salivary acinar protein synthesis or failure of adequate secretion, or both, is common to alcoholism, diabetes, and malnutrition.7 Subsequent engorgement of acinar cells leads to clinical parotid hypertrophy.
Diagnosis is based on history and examination, as well as on the findings of US or CT, which will reveal bilateral gland enlargement and increased density. The glands appear dense because adipose cells are displaced by acinar cell hypertrophy; however, end-stage changes can result in the opposite appearance: a lucent enlargement caused by fatty infiltration.2 FNA is unnecessary, unless there is suspicion of neoplasm, as there would be in patients with asymmetrical parotid enlargement, pain, lymph node enlargement, or facial-nerve involvement. In patients with sialadenosis, in contrast, acinar cell hypertrophy alone will be present.
Treatment of sialadenosis is best aimed at rectifying the underlying medical condition, which might, over time, lead to some reduction in the size of the gland. There is no specific effective therapy for elimination of glandular swelling.
Bulimia and anorexia nervosa. Bulimia nervosa, the induction of vomiting after binge eating, can be associated with bilateral or occasionally unilateral parotid swelling. Anorexia, a form of self-starvation, can occur in association with bulimia, with patients also presenting with parotid swelling. Associated parotid swelling is similar to what is seen in sialadenosis: painless, persistent, and of nonpathologic consistency.
The pathophysiology of bulimia- and anorexia-associated parotid-gland swelling is identical to what is seen in sialadenosis: dysregulation of acinar cell sympathetic nerve supply that leads to enlargement of individual parenchymal cells.8 Contrast-enhanced CT can reveal increased vascularity associated with active bulimia. FNA and CT, however, are required only in patients in whom the diagnosis is not clear and when neoplasm is suspected.
Continue to: Treatment includes...
Treatment includes correcting electrolyte abnormalities and, more importantly, addressing underlying emotional issues to stop purging episodes. Psychiatric input and social support are invaluable. Parotid gland swelling generally improves with cessation of vomiting episodes.
Ask: Is the patient in pain?
Causes of salivary gland pain include sialolithiasis, sialadenitis, and recurrent parotitis of childhood. Pain occurs secondary to stretching of the fibrous capsule in which the parotid or submandibular gland is surrounded, compression of pain fibers by an expanding mass, or infiltration of nerves by neoplasia.
Sialolithiasis. Sialolithiasis, or salivary stones, are primarily calcium carbonate concentrations within the salivary ductal system. More than 80% occur in the submandibular gland or duct9 as a result of production of mixed mucoid and serous saliva and a tortuous duct path.
Patients usually present with a history of intermittent swelling and pain of the involved gland associated with eating. Increased production of saliva during meals, which then passes through a partially obstructed salivary duct, leads to salivary retention and glandular swelling. Thus, a recurring pattern can develop, with varying periods of remission,7 eventually leading to an acute suppurative process or sialadenitis (described below). Chronic salivary disease can also be caused by stricture of a duct or, rarely, external compression by a tumor mass.
Examination often reveals an enlarged and often tender gland; conversely, chronic disease can lead to gland atrophy. Usually, only minimal saliva is able to be expressed from an obstructed duct. For a submandibular duct stone, bimanual palpation might reveal its position if it is located distally in the floor of the mouth; a proximal stone might not be palpable.
Continue to: Although US is operator-dependent...
Although US is operator-dependent, it is the imaging modality of choice for identifying sialolithiasis10 because it can identify gland architecture, duct dilation, and both radiolucent and radiopaque stones. For patients in whom US findings are normal despite a convincing clinical presentation of sialolithiasis, CT should be performed because small stones can be missed on US.11
Supportive measures for sialolithiasis are listed in the TABLE. Reserve antibiotics for patients who have signs or symptoms of infection, including pyrexia, trismus, and malaise. A beta-lactam antibiotic, such as amoxicillin–clavulanate, 875 mg orally bid, or a cephalosporin, such as cephalexin, 500 mg orally qid, are appropriate first-line options. Clindamycin, 300 mg orally tid, or metronidazole, 500 mg orally tid, are acceptable alternatives. When signs or symptoms are persistent or recurrent, refer the patient for a surgical opinion.
Stones located in the floor of the mouth are usually excised through an intraoral approach. In the past, gland excision was advocated when a sialolith was found more proximally within the gland parenchyma. More recently, however, sialendoscopy, involving insertion of a small, semirigid endoscope into the salivary duct, has been shown safe and effective for removing a stone; successful removal, in as many as 80% of cases, increases to 90% when performed using a minimally invasive surgical technique.12 Although sialendoscopy is effective, the technique cannot always treat the underlying abnormality of the salivary gland; gland excision is therefore warranted in some cases.
Last, extracorporeal shock wave therapy is aimed at fragmenting salivary stones before retrieval. Results are variable, however, and treatment should be guided by an otolaryngologist.13,14
Sialadenitis (bacterial and viral infection). Acute suppurative sialadenitis occurs secondary to retrograde ductal bacterial infection. The parotid gland is most frequently involved,15 although submandibular sialadenitis is not uncommon. Patients usually present with sudden-onset unilateral, painful swelling.
Continue to: Pathophysiology involves...
Pathophysiology involves dehydration or decreased oral intake leading to salivary stasis and subsequent bacterial migration into the gland. Medically debilitated and postoperative patients are therefore at greater risk; so are patients with diabetes mellitus, poor oral hygiene, Sjögren’s syndrome, hypothyroidism, or renal failure.16 Certain medications, including anticholinergics, can also predispose to hyposalivation.17
(As discussed, sialolithiasis and stricture of salivary ducts can also cause acute bacterial infection; in such cases, however, the typical presentation is one of chronic or recurrent infection.)
Examination might reveal an exquisitely tender, indurated, and inflamed gland; pus can often be expressed from the respective intraoral orifice. Any expressed pus should be sent for culture to guide antibiotic therapy.
Treatment should focus on hydration, oral hygiene, and antibiotics, while reversing or minimizing any underlying contributing medical condition. Warm compresses applied to the involved gland, massage, and sialagogues, such as lemon drops or sugar-free lollipops, can stimulate salivary flow and prevent stasis.
More than 80% of infections are caused by Staphylococcus aureus17; anaerobic and mixed infections have also been recognized.A beta-lactam penicillin, such as amoxicillin-clavulanate, is the antibiotic of choice. A patient who is systemically unwell should be treated as an inpatient with nafcillin and metronidazole. Methicillin-resistant S aureus must also be considered in patients with comorbid disease, such as diabetes mellitus or intravenous drug use, or in patients residing in an area of substantial incidence of methicillin-resistant S aureus. In those cases, substitute vancomycin or linezolid for nafcillin.18
Continue to: Less commonly...
Less commonly, abscess can form, with the patient presenting as systemically unwell with a fluctuant mass. If the diagnosis is unclear or the patient does not improve, abscess can be confirmed by US. Expedient surgical review and inpatient admission can then be arranged.
Unlike bacterial sialadenitis, causes of viral sialadenitis are often bilateral. Mumps (a paramyxovirus) is the most common viral cause, affecting primarily children < 15 years.19 The parotid glands are most often involved, with inflammation and edema causing significant pain because of increasing intraparotid pressure as expansion of the gland is limited by its tense fibrous capsule. Complications of mumps include orchitis, meningitis, pancreatitis, and oophoritis.
Mumps is highly contagious; it is spread through contact with airborne saliva droplets, with viral entry through the nose or mouth, followed by proliferation in the salivary glands or on surface epithelium of the respiratory tract.7 Diagnosis is confirmed by viral serology. A positive test of serum immunoglobulin M confirms the diagnosis, but this test should not be performed until 3 days after onset of symptoms because a false-negative result is otherwise possible.20 Immunoglobulin G serologic testing can further aid diagnosis; the titer is measured approximately 4 days after onset of symptoms and again 2 to 3 weeks later. A 4-fold rise in titer confirms mumps.
Other viral infections that can cause sialadenitis include Epstein-Barr virus, cytomegalovirus, human immunodeficiency virus, coxsackievirus, and influenza. Treatment is supportive: analgesia, hydration, oral hygiene, and rest. Inflammation might take weeks to resolve, but expect complete resolution. For a patient who has significant trismus, poor oral intake, or a potentially threatened airway, inpatient care should be provided.
Recurrent parotitis of childhood is an inflammatory condition that usually affects one, but at times both, parotid glands. It is characterized by episodes of painful swelling. Incidence peaks at 3 to 6 years of age.7 Episodes can be frequent, occurring 1 to 5 times a year and lasting 3 to 7 days—sometimes longer—and usually resolving without treatment.
Continue to: The precise etiology...
The precise etiology of recurrent parotitis of childhood is unclear; possibly, saliva aggregates to form obstructive mucous plugs, thus causing stasis and swelling of the gland. As pressure builds, spontaneous plug extrusion occurs and symptoms resolve, provided infection is not a factor. US demonstrates multiple round, hypoechoic areas consistent with duct dilation, and surrounding infiltration by lymphocytes.1
Supportive care—adequate hydration, gland massage, warm compresses, and sialogogues—are mainstays of treatment. Fever and malaise warrant treatment with oral antibiotics. Sialadenoscopy, which can be considered in children with frequent episodes, can decrease the frequency and severity of episodes.21 The condition usually resolves spontaneously at puberty.
Ask: Does the patient have dry mouth?
In-depth review of xerostomia is beyond the scope of this article. Causes include Sjögren's syndrome, immunoglobulin G4-related sialadenitis, sarcoidosis, radiation therapy, diabetes, chronic infection, and medications—in particular those with anticholinergic effects.
Treatment of xerostomia includes saliva substitutes, sialagogues, and, for oral candidiasis, antifungals. Muscarinic cholinergic stimulators, such as pilocarpine, 5 mg qid have been used with some success22; patients should be advised of potential adverse effects with these agents, including sweating, urinary frequency, flushing, and chills.
CORRESPONDENCE
Shankar Haran, MBBS, ENT Department, Townsville Hospital, 100 Angus Smith Dr, Douglas, Queensland, Australia 4814; Shankar.haran01@gmail.com.
1. de Oliveira FA, Duarte EC, Taveira CT, et al. Salivary gland tumor: a review of 599 cases in a Brazilian population. Head Neck Pathol. 2009;3:271-275.
2. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177-184.
3. Bova R. A guide to salivary gland disorders. Medicine Today. 2006;7:44-48.
4. Zhang S, Bao R, Bagby J, et al. Fine needle aspiration of salivary glands: 5-year experience from a single academic center. Acta Cytol. 2009;53:375-382.
5. Bova R, Saylor A, Coman WB. Parotidectomy: review of treatment and outcomes. ANZ J Surg. 2004;74:563-568.
6. Sood S, McGurk M, Vaz F. Management of salivary gland tumours: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130:S142-S149.
7. Mandel L. Salivary gland disorders. Med Clin North Am. 2014;98:1407-1449.
8. Mandel L, Abai S. Diagnosing bulimia nervosa with parotid gland swelling. J Am Dent Assoc. 2004;135:613–616.
9. Lustmann J, Regev E, Melamed Y. Sialolithiasis. A survey on 245 patients and a review of literature. Int J Oral Maxillofac Surg. 1990;19:135–138.
10. Vogl TJ, Al-Nawas B, Beutner D, et al. Updated S2K AWMF guideline for the diagnosis and follow-up of obstructive sialadenitis—relevance for radiologic imaging. Rofo. 2014;186:843-846.
11. Schwarz D, Kabbasch C, Scheer M, et al. Comparative analysis of sialendoscopy, sonography, and CBCT in the detection of sialolithiasis. Laryngoscope. 2015;125:1098–1101.
12. Atienza G, López-Cedrún JL. Management of obstructive salivary disorders by sialendoscopy: a systematic review. Br J Oral Maxillofac Surg. 2015;53:507-519.
13. Escudier MP, Brown JE, Putcha V, et al. Factors influencing the outcome of extracorporeal shock wave lithotripsy in the management of salivary calculi. Laryngoscope. 2010;120:1545-1549.
14. Koch M, Schapher M, Mantsopoulos K, et al. Multimodal treatment in difficult sialolithiasis: Role of extracorporeal shock-wave lithotripsy and intraductal pneumatic lithotripsy. Laryngoscope. 2018;128:E332-E338.
15. McQuone SJ. Acute viral and bacterial infections of the salivary glands. Otolaryngol Clin North Am. 1999;32:793-811.
16. O’Neil C, Sidhu S. Salivary gland disorders. Australian Doctor. 2011;28:19-25.
17. Mandel L. Differentiating acute suppurative parotitis from acute exacerbation of a chronic parotitis: case reports. J Oral Maxillofac Surg. 2008;66:1964-1968.
18. Chow AW. Suppurative parotitis in adults. UpToDate.com. www.uptodate.com/contents/suppurative-parotitis-in-adults. Accessed September 25, 2019.
19. Katz SL, Gershon AA, Hotez PJ. Infectious Diseases of Children. New York, NY: Mosby Year Book; 1998:280-289.
20. Krause CH, Molyneaux PJ, Ho-Yen DO, et al. Comparison of mumps-IgM ELISAs in acute infection. J Clin Virol. 2007;38:153-156.
21. Quenin S, Plouin-Gaudon I, Marchal F, et al. Juvenile recurrent parotitis: sialendoscopic approach. Arch Otolaryngol Head Neck Surg. 2008;134:715-719.
22. Papas AS, Sherrer YS, Charney M, et al. Successful treatment of dry mouth and dry eye symptoms in Sjögren’s syndrome patients with oral pilocarpine: a randomized, placebo-controlled, dose-adjustment study. J Clin Rheumatol. 2004;10:169-177.
1. de Oliveira FA, Duarte EC, Taveira CT, et al. Salivary gland tumor: a review of 599 cases in a Brazilian population. Head Neck Pathol. 2009;3:271-275.
2. Spiro RH. Salivary neoplasms: overview of a 35-year experience with 2,807 patients. Head Neck Surg. 1986;8:177-184.
3. Bova R. A guide to salivary gland disorders. Medicine Today. 2006;7:44-48.
4. Zhang S, Bao R, Bagby J, et al. Fine needle aspiration of salivary glands: 5-year experience from a single academic center. Acta Cytol. 2009;53:375-382.
5. Bova R, Saylor A, Coman WB. Parotidectomy: review of treatment and outcomes. ANZ J Surg. 2004;74:563-568.
6. Sood S, McGurk M, Vaz F. Management of salivary gland tumours: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol. 2016;130:S142-S149.
7. Mandel L. Salivary gland disorders. Med Clin North Am. 2014;98:1407-1449.
8. Mandel L, Abai S. Diagnosing bulimia nervosa with parotid gland swelling. J Am Dent Assoc. 2004;135:613–616.
9. Lustmann J, Regev E, Melamed Y. Sialolithiasis. A survey on 245 patients and a review of literature. Int J Oral Maxillofac Surg. 1990;19:135–138.
10. Vogl TJ, Al-Nawas B, Beutner D, et al. Updated S2K AWMF guideline for the diagnosis and follow-up of obstructive sialadenitis—relevance for radiologic imaging. Rofo. 2014;186:843-846.
11. Schwarz D, Kabbasch C, Scheer M, et al. Comparative analysis of sialendoscopy, sonography, and CBCT in the detection of sialolithiasis. Laryngoscope. 2015;125:1098–1101.
12. Atienza G, López-Cedrún JL. Management of obstructive salivary disorders by sialendoscopy: a systematic review. Br J Oral Maxillofac Surg. 2015;53:507-519.
13. Escudier MP, Brown JE, Putcha V, et al. Factors influencing the outcome of extracorporeal shock wave lithotripsy in the management of salivary calculi. Laryngoscope. 2010;120:1545-1549.
14. Koch M, Schapher M, Mantsopoulos K, et al. Multimodal treatment in difficult sialolithiasis: Role of extracorporeal shock-wave lithotripsy and intraductal pneumatic lithotripsy. Laryngoscope. 2018;128:E332-E338.
15. McQuone SJ. Acute viral and bacterial infections of the salivary glands. Otolaryngol Clin North Am. 1999;32:793-811.
16. O’Neil C, Sidhu S. Salivary gland disorders. Australian Doctor. 2011;28:19-25.
17. Mandel L. Differentiating acute suppurative parotitis from acute exacerbation of a chronic parotitis: case reports. J Oral Maxillofac Surg. 2008;66:1964-1968.
18. Chow AW. Suppurative parotitis in adults. UpToDate.com. www.uptodate.com/contents/suppurative-parotitis-in-adults. Accessed September 25, 2019.
19. Katz SL, Gershon AA, Hotez PJ. Infectious Diseases of Children. New York, NY: Mosby Year Book; 1998:280-289.
20. Krause CH, Molyneaux PJ, Ho-Yen DO, et al. Comparison of mumps-IgM ELISAs in acute infection. J Clin Virol. 2007;38:153-156.
21. Quenin S, Plouin-Gaudon I, Marchal F, et al. Juvenile recurrent parotitis: sialendoscopic approach. Arch Otolaryngol Head Neck Surg. 2008;134:715-719.
22. Papas AS, Sherrer YS, Charney M, et al. Successful treatment of dry mouth and dry eye symptoms in Sjögren’s syndrome patients with oral pilocarpine: a randomized, placebo-controlled, dose-adjustment study. J Clin Rheumatol. 2004;10:169-177.
PRACTICE RECOMMENDATIONS
› Use ultrasonography for initial imaging of a salivary gland. A
› Refer patients with the following findings for further specialty evaluation: abscess, inflammation unresponsive to medical care, recurrent or chronic symptoms, suspected neoplasm (for excision), and suspected sialolithiasis. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Primary care for the declining cancer survivor
As a family physician (FP), you are well positioned to optimize the quality of life of advanced cancer patients as they decline and approach death. You can help them understand their evolving prognosis so that treatment goals can be adjusted, and you can ensure that hospice is implemented early to improve the end-of-life experience. This practical review will help you to provide the best care possible for these patients.
Family physicians can fill a care gap
The term cancer survivor describes a patient who has completed initial cancer treatment. Within this population, many have declining health and ultimately succumb to their disease. There were 16.9 million cancer survivors in the United States as of January 1, 2019,1 with 53% likely to experience significant symptoms and disability.2 More than 600,000 American cancer survivors will die in 2019.3
In 2011, the Commission on Cancer mandated available outpatient palliative care services at certified cancer centers.4 Unfortunately, current palliative care resources fall far short of expected needs. A 2010 estimate of required hospice and palliative care physicians demonstrated a staffing gap of more than 50% among those providing outpatient services.5 The shortage continues,6 and many cancer patients will look to their FP for supportive care.
FPs, in addition to easing symptoms and adverse effects of medication, can educate patients and families about their disease and prognosis. By providing longitudinal care, FPs can identify critical health declines that oncologists, patients, and families often overlook. FPs can also readily appreciate decline, guide patients toward their care goals, and facilitate comfort care—including at the end of life.
Early outpatient palliative care improves quality of life and patient satisfaction. It also may improve survival time and ward off depression.7,8 Some patients and providers resist palliative care due to a misconception that it requires abandoning treatment.9 Actually, palliative care can be given in concert with all active treatments. Many experts recommend a name change from “palliative care” to “supportive care” to dispel this misconception.10
Estimate prognosis using the “surprise question”
Several algorithms are available—using between 2 and 13 patient parameters—to estimate advanced cancer survival. Most of these algorithms are designed to identify the last months or weeks of life, but their utility to predict death within these periods is limited.11
The “surprise question” may be the most valuable prognostic test for primary care. In this test, the physician asks him- or herself: Would I be surprised if this patient died in 1 year? Researchers found that when primary care physicians answered No, their patient was 4 times more likely to die within the year than when they answered Yes.12 This test has a positive predictive value of 20% and a negative predictive value of 95%, making it valuable in distinguishing patients with longer life expectancy.12 Although it overidentifies at-risk patients, the "surprise question" is a simple and sensitive tool for defining prognosis.
Continue to: Priorities for patients likely to live more than a year
Priorities for patients likely to live more than a year
For patients who likely have more than a year to live, the focus is on symptom management and preparation for future decline. Initiate and facilitate discussions about end-of-life topics. Cancer survivors are often open to discussions on these topics, which include advanced directives, home health aides, and hospice.13 Patients can set specific goals for their remaining time, such as engaging in travel, personal projects, or special events. Cancer patients have better end-of-life experiences and families have improved mental health after these discussions.14 Although cancer patients are more likely than other terminal patients to have end-of-life discussions, fewer than 40% ever do.15
Address distressing symptoms with a focus on maintaining function. More than 50% of advanced cancer patients experience fatigue, weakness, pain, weight loss, and anorexia,16 and up to 60% experience psychological distress.17 Deprescribing most preventive medications is recommended with transition to symptomatic treatment.18
Priorities for patients with less than a year to live
For patients who may have less than a year to live, focus shifts to their wishes for the time remaining and priorities for the dying process. Most patients start out with prognostic views more optimistic than those of their physicians, but this gap narrows after end-of-life discussions.19,20 Patients with incurable cancer are less likely to choose aggressive therapy if they believe their 6-month survival probability is less than 90%.21 Honest conversations, with best- and worst-case scenarios, are important to patients and families, and should occur while the patient is well enough to participate and set goals.22
In the last months of life, opioids become the primary treatment for pain and air hunger. As function declines, concerns about such adverse effects as falls and confusion decrease. Opioids have been shown to be most effective over the course of 4 weeks, and avoiding their use in earlier stages may increase their efficacy at the end of life.23
Hospice benefit—more comfort, with limitations
Hospice care consists of services administered by nonprofit and for-profit entities covered by Medicare, Medicaid, and many private insurers.24 Hospice strives to allow patients to approach death in comfort, meeting their goal of a “good death.” A recent literature review identified 4 aspects of a good death that terminally ill patients and their families considered most important: control of the dying process, relief of pain, spirituality, and emotional well-being (TABLE 1).25
Continue to: Hospice use is increasing...
Hospice use is increasing, yet many enroll too late to fully benefit. While cancer patients alone are not currently tracked, the use of hospice by Medicare beneficiaries increased from 44% in 2012 to 48% in 2019.24 In 2017, the median hospice stay was 19 days.24 Unfortunately, though, just 28% of hospice-eligible patients enrolled in hospice in their last week of life.24 Without hospice, patients often receive excessive care near death. More than 6% receive aggressive chemotherapy in their last 2 weeks of life, and nearly 10% receive a life-prolonging procedure in their last month.26
Hospice care replaces standard hospital care, although patients can elect to be followed by their primary care physician.9 Most hospice services are provided as needed or continuously at the patient’s home, including assisted living facilities. And it is also offered as part of hospital care. Hospice services are interdisciplinary, provided by physicians, nurses, social workers, chaplains, and health aides. Hospices have on-call staff to assess and treat complications, avoiding emergency hospital visits.9 And hospice includes up to 5 days respite care for family caregivers, although with a 5% copay.9 Most hospice entities run inpatient facilities for care that cannot be effectively provided at home.
Hospice care has limitations—many set by insurance. Medicare, for example, stipulates that a primary care or hospice physician must certify the patient has a reasonable prognosis of 6 months or less and is expected to have a declining course.27 Patients who survive longer than 6 months are recertified by the same criteria every 60 days.27
Hospice patients forgo treatments aimed at curing their terminal diagnosis.28 Some hospice entities allow noncurative therapies while others do not. Hospice covers prescription medications for symptom control only, although patients can receive care unrelated to the terminal diagnosis under regular benefits.28 Hospice care practices differ from standard care in ways that may surprise patients and families (TABLE 227,28). Patients can disenroll and re-enroll in hospice as they wish.28
Symptom control in advanced cancer
General symptoms
Pain affects 64% of patients with advanced cancer.29 Evidence shows that cancer pain is often undertreated, with a recent systematic review reporting undertreated pain in 32% of patients.30 State and national chronic opioid guidelines do not restrict use for cancer pain.31 Opioids are effective in 75% of cancer patients over 1 month, but there is no evidence of benefit after this period.23 In fact, increasing evidence demonstrates that pain is likely negatively responsive to opioids over longer periods.32 Opioid adverse effects can worsen other cancer symptoms, including depression, anxiety, fatigue, constipation, hypogonadism, and cognitive dysfunction.32 Delaying opioid therapy to end of life can limit adverse effects and may preserve pain-control efficacy for the dying process.
Continue to: Most cancer pain...
Most cancer pain is partially neuropathic, so anticonvulsant and antidepressant medications can help.33 Gabapentin, pregabalin, and duloxetine are recommended based on evidence not restricted to cancer.34 Cannabinoids have been evaluated in 2 trials of cancer pain with 440 patients and showed a borderline significant reduction of pain.35
Palliative radiation therapy can sometimes reduce pain. Bone metastases pain has been studied the most, and the literature suggests that palliative radiation provides improvement for 60% of patients and complete relief to 25% of patients.36 Palliative thoracic radiotherapy for primary or metastatic lung masses reduces pain by more than 70% while improving dyspnea, hemoptysis, and cough in a majority of patients.36
Other uses of palliative radiation have varied evidence. Palliative chemotherapy has less evidence of benefit. In a recent multicenter cohort trial, chemotherapy in end-stage cancer reduced quality of life in patients with good functional status, without affecting quality of life when function was limited.37 Palliative chemotherapy may be beneficial if combined with corticosteroids or radiation therapy.38
Treatment in the last weeks of life centers on opioids; dose increases do not shorten survival.39 Cancer patients are 4 times as likely as noncancer patients to have severe or excruciating pain during the last 3 days of life.40 Narcotics can be titrated aggressively near end of life with less concern for hypotension, respiratory depression, or level of consciousness. Palliative sedation remains an option for uncontrolled pain.41
Anorexia is only a problem if quality of life is affected. Cachexia is caused by increases in cytokines more than reduced calorie intake.42 Reversible causes of reduced eating may be found, including candidiasis, dental problems, depression, or constipation. Megestrol acetate improves weight (number needed to treat = 12), although it significantly increases mortality (number needed to harm = 23), making its use controversial.43 Limited study of cannabinoids has not shown effectiveness in treating anorexia.35
Continue to: Constipation...
Constipation in advanced cancer is often related to opioid therapy, although bowel obstruction must be considered. Opioid-induced constipation affects 40% to 90% of patients on long-term treatment,44 and 5 days of opioid treatment nearly doubles gastrointestinal transit time.45 Opioid-induced constipation can be treated by adding a stimulating laxative followed by a peripheral acting μ-opioid receptor antagonist, such as subcutaneous methylnaltrexone or oral naloxegol.46 These medications are contraindicated if ileus or bowel obstruction is suspected.46
Nausea and vomiting are common in advanced cancer and have numerous causes. Approximately half of reversible causes are medication adverse effects from either chemotherapy or pain medication.47 Opioid rotation may improve symptoms.47 A suspected bowel obstruction should be evaluated by specialists; surgery, palliative chemotherapy, radiation therapy, or stenting may be required. Oncologists can best manage adverse effects of chemotherapy. For nausea and vomiting unrelated to chemotherapy, consider treating constipation and pain. Medication can also be helpful; a systemic review suggests metoclopramide works best, with some evidence supporting other dopaminergic agonists, including haloperidol.47
Fatigue. Both methylphenidate and modafinil have been studied to treat cancer-related fatigue.48 A majority of patients treated with methylphenidate reported less cancer-related fatigue at 4 weeks and wished to continue treatment.49 Modafinil demonstrated minimal improvement in fatigue.50 Sleep disorders, often due to anxiety or sleep apnea, may be a correctable cause.
Later symptoms
Delirium occurs in up to 90% of cancer patients near the end of life, and can signal death.51 Up to half of the delirium seen in palliative care is reversible.51 Reversible causes include uncontrolled pain, medication adverse effects, and urinary and fecal retention (TABLE 348,51). Addressing these factors reduces delirium, based on studies in postoperative patients.52 Consider opioid rotation if neurotoxicity is suspected.51
Delirium can be accompanied by agitation or decreased responsiveness.53 Agitated delirium commonly presents with moaning, facial grimacing, and purposeless repetitive movements, such as plucking bedsheets or removing clothes.51 Delirious patients without agitation have reported, following recovery, distress similar to that experienced by agitated patients.54 Caregivers are most likely to recognize delirium and often become upset. Educating family members about the frequency of delirium can lessen this distress.54
Continue to: Delirium can be treated with...
Delirium can be treated with antipsychotics; haloperidol has been most frequently studied.54 Antipsychotics are effective at reducing agitation but not at restoring cognition.55 Case reports suggest that use of atypical antipsychotics can be beneficial if adverse effects limit haloperidol dosing.56 Agitated delirium is the most frequent indication for palliative sedation.57
Dyspnea. In the last weeks, days, or hours of life, dyspnea is common and often distressing. Dyspnea appears to be multifactorial, worsened by poor control of secretions, airway hyperactivity, and lung pathologies.58 Intravenous hydration may unintentionally exacerbate dyspnea. Hospice providers generally discourage intravenous hydration because relative dehydration reduces terminal respiratory secretions (“death rattle”) and increases patient comfort.59
Some simple nonpharmacologic interventions have benefit. Oxygen is commonly employed, although multiple studies show no benefit over room air.59 Directing a handheld fan at the face does reduce dyspnea, likely by activation of the maxillary branch of the trigeminal nerve.60
Opioids effectively treat dyspnea near the end of life with oral and parenteral dosing, but the evidence does not support nebulized opioids.61 Opioid doses required to treat dyspnea are less than those for pain and do not cause significant respiratory depression.62 If a patient taking opioids experiences dyspnea, a 25% dose increase is recommended.63
Anticholinergic medications can improve excessive airway secretions associated with dyspnea. Glycopyrrolate causes less delirium because it does not cross the blood-brain barrier, while scopolamine patches have reduced anticholinergic adverse effects, but effects are delayed until 12 hours after patch placement.64 Atropine eye drops given sublingually were effective in a small study.65
Continue to: Palliative sedation
Palliative sedation
Palliative sedation can manage intractable symptoms near the end of life. A recent systematic review suggests that palliative sedation does not shorten life.57 Sedation is most often initiated by gradual increases in medication doses.57 Midazolam is most often employed, but antipsychotics are also used.57
CORRESPONDENCE
CDR Michael J. Arnold, MD, Uniformed Services University of the Health Sciences, 4501 Jones Bridge Road, Bethesda, MD 20814; michael.arnold@usuhs.edu.
ACKNOWLEDGEMENT
Kristian Sanchack, MD, and James Higgins, DO, assisted in the preparation of this manuscript.
1. American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2019-2021. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2019-2021.pdf. Accessed September 4, 2019.
2. Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112(11 suppl):2577-2592.
3. National Comprehensive Cancer Network. NCCN Guidelines Version 2. 2019. Palliative Care. www.nccn.org/professionals/physician_gls/pdf/palliative.pdf. (Must register an account for access.) Accessed September 4, 2019.
4. American Cancer Society. New CoC accreditation standards gain strong support. www.facs.org/media/press-releases/2011/coc-standards0811. Accessed September 11, 2019.
5. Lupu D; American Academy of Hospice and Palliative Medicine Workforce Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage. 2010;40:899-911.
6. Lupu D, Quigley L, Mehfoud N, et al. The growing demand for hospice and palliative medicine physicians: will the supply keep up? J Pain Symptom Manage. 2018;55:1216-1223.
7. Rabow MW, Dahlin C, Calton B, et al. New frontiers in outpatient palliative care for patients with cancer. Cancer Control. 2015;22:465-474.
8. Haun MW, Estel S, Rücker G, et al. Early palliative care for adults with advanced cancer. Cochrane Database of Syst Rev. 2017:CD01129.
9. Buss MK, Rock LK, McCarthy EP. Understanding palliative care and hospice: a review for primary care providers. Mayo Clin Proc. 2017;92:280-286.
10. Hui D. Definition of supportive care: does the semantic matter? Curr Opin Oncol. 2014;26:372-379.
11. Simmons CPL, McMillan DC, McWilliams K, et al. Prognostic tools in patients with advanced cancer: a systematic review. J Pain Symptom Manage. 2017;53:962-970.
12. Lakin JR, Robinson MG, Bernacki RE, et al. Estimating 1-year mortality for high-risk primary care patients using the “surprise” question. JAMA Int Med. 2016;176:1863-1865.
13. Walczak A, Henselmans I, Tattersall MH, et al. A qualitative analysis of responses to a question prompt list and prognosis and end-of-life care discussion prompts delivered in a communication support program. Psychoonchology. 2015;24:287-293.
14. Yamaguchi T, Maeda I, Hatano Y, et al. Effects of end-of-life discussions on the mental health of bereaved family members and quality of patient death and care. J Pain Symptom Manage. 2017;54:17-26.
15. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, caregiver bereavement adjustment. JAMA. 2008;300:1665-1673.
16. Teunissen SC, Wesker W, Kruitwagen C, et al. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34:94-104.
17. Gao W, Bennett MI, Stark D, et al. Psychological distress in cancer from survivorship to end of life: prevalence, associated factors and clinical implications. Eur J Cancer. 2010;46:2036-2044.
18. Scott IA, Gray LC, Martin JH, et al. Deciding when to stop: towards evidence-based deprescribing of drugs in older populations. Evid Based Med. 2013;18:121-124.
19. Gramling R, Fiscella K, Xing G, et al. Determinants of patient-oncologist prognostic discordance in advanced cancer. JAMA Oncol. 2016;2:1421-1426.
20. Epstein AS, Prigerson HG, O’Reilly EM, et al. Discussions of life expectancy and changes in illness understanding in patients with advanced cancer. J Clin Oncol. 2016;34:2398-2403.
21. Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709-1714.
22. Myers J. Improving the quality of end-of-life discussions. Curr Opin Support Palliat Care. 2015;9:72-76.
23. Corli O, Floriani I, Roberto A, et al. Are strong opioids equally effective and safe in the treatment of chronic cancer pain? A multicenter randomized phase IV ‘real life’ trial on the variability of response to opioids. Ann Oncolog. 2016;27:1107-1115.
24. National Hospice and Palliative Care Organization. NHPCO Facts and Figures. 2018. www.nhpco.org/wp-content/uploads/2019/07/2018_NHPCO_Facts_Figures.pdf. Accessed September 24, 2019.
25. Meier EA, Gallegos JV, Thomas LP, et al. Defining a good death (successful dying): literature review and a call for research and public dialogue. Am J Geriatr Psychiatry. 2016;24:261-271.
26. Morden NE, Chang CH, Jacobson JO, et al. End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff (Millwood). 2012;31:786-796.
27. Centers for Medicare & Medicaid Services. Medicare Hospice Benefit Facts. www.cgsmedicare.com/hhh/education/materials/pdf/Medicare_Hospice_Benefit_Facts.pdf. Accessed September 11, 2019.
28. Centers for Medicare & Medicaid Services. Medicare Hospice Benefits. www.medicare.gov/pubs/pdf/02154-medicare-hospice-benefits.pdf. Accessed September 11, 2019.
29. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18:1437-1449.
30. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32:4149-4154.
31. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
32. Davis MP, Mehta Z. Opioids and chronic pain: where is the balance? Curr Oncol Rep. 2016;18:71.
33. Leppert W, Zajaczkowska R, Wordliczek J, et al. Pathophysiology and clinical characteristics of pain in most common locations in cancer patients. J Physiol Pharmacol. 2016;67:787-799.
34. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162-173.
35. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
36. Jones JA, Lutz ST, Chow E. et al. Palliative radiotherapy at the end of life: a critical review. CA Cancer J Clin. 2014;64:296-310.
37. Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 2015;1:778-784.
38. Kongsgaard U, Kaasa S, Dale O, et al. Palliative treatment of cancer-related pain. 2005. www.ncbi.nlm.nih.gov/books/NBK464794/. Accessed September 24, 2019.
39. Sathornviriyapong A, Nagaviroj K, Anothaisintawee T. The association between different opioid doses and the survival of advanced cancer patients receiving palliative care. BMC Palliat Care. 2016;15:95.
40. Steindal SA, Bredal IS. Sørbye LW, et al. Pain control at the end of life: a comparative study of hospitalized cancer and noncancer patients. Scand J Caring Sci. 2011;25:771-779.
41. Maltoni M, Setola E. Palliative sedation in patients with cancer. Cancer Control. 2015;22:433-441.
42. Cooper C, Burden ST, Cheng H, et al. Understanding and managing cancer-related weight loss and anorexia: insights from a systematic review of qualitative research. J Cachexia Sarcopenia Muscle. 2015;6:99-111.
43. Ruiz Garcia V, LÓpez-Briz E, Carbonell Sanchis R, et al. Megesterol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2013;28:CD004310.
44. Chey WD, Webster L, Sostek M, et al. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014;370:2387-2396.
45. Poulsen JL, Nilsson M, Brock C, et al. The impact of opioid treatment on regional gastrointestinal transit. J Neurogastroenterol Motil. 2016;22:282-291.
46. Pergolizzi JV, Raffa RB, Pappagallo M, et al. Peripherally acting μ-opioid receptor antagonists as treatment options for constipation in noncancer pain patients on chronic opioid therapy. Patient Prefer Adherence. 2017;11:107-119.
47. Walsh D, Davis M, Ripamonti C, et al. 2016 updated MASCC/ESMO consensus recommendations: management of nausea and vomiting in advanced cancer. Support Care Cancer. 2017;25:333-340.
48. Mücke M, Mochamat, Cuhls H, et al. Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst Rev. 2015(5):CD006788.
49. Escalante CP, Meyers C, Reuben JM, et al. A randomized, double-blind, 2-period, placebo-controlled crossover trial of a sustained-release methylphenidate in the treatment of fatigue in cancer patients. Cancer J. 2014;20:8-14.
50. Hovey E, de Souza P, Marx G, et al. Phase III, randomized, double-blind, placebo-controlled study of modafinil for fatigue in patients treated with docetaxel-based chemotherapy. Support Care Cancer. 2014;22:1233-1242.
51. Hosker CM, Bennett MI. Delirium and agitation at the end of life. BMJ. 2016;353:i3085.
52. Mercantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516-522.
53. Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Int Med. 2001;135:32-40.
54. Breitbart W, Alici Y. Agitation and delirium at the end of life: “We couldn’t manage him." JAMA. 2008;300:2898-2910.
55. Candy B, Jackson KC, Jones L, et al. Drug therapy for delirium in terminally ill patients. Cochrane Database Syst Rev. 2012;11:CD004770.
56. Bascom PB, Bordley JL, Lawton AJ. High-dose neuroleptics and neuroleptic rotation for agitated delirium near the end of life. Am J Hosp Palliat Med. 2014;31:808-811.
57. Maltoni M, Scarpi E, Rosati M, et al. Palliative sedation in end-of-life care and survival: a systematic review. J Clin Oncol. 2012;30:1378-1383.
58. Albert RH. End-of-life care: managing common symptoms. Am Fam Physician. 2017;95:356-361.
59. Arenella C. Artificial nutrition and hydration at the end of life: beneficial or harmful? https://americanhospice.org/caregiving/artificial-nutrition-and-hydration-at-the-end-of-life-beneficial-or-harmful/ Accessed September 11, 2019.
60. Booth S, Moffat C, Burkin J, et al. Nonpharmacological interventions for breathlessness. Curr Opinion Support Pall Care. 2011;5:77-86.
61. Barnes H, McDonald J, Smallwood N, et al. Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Database Syst Rev. 2016(3)CD011008.
62. Lim RB. End-of-life care in patients with advanced lung cancer. Ther Adv Resp Dis. 2016;10:455-467.
63. Kreher M. Symptom control at the end of life. Med Clin North Am. 2016;100:1111-1122.
64. Baralatei FT, Ackerman RJ. Care of patients at the end of life: management of nonpain symptoms. FP Essent. 2016;447:18-24.
65. Protus BM, Grauer PA, Kimbrel JM. Evaluation of atropine 1% ophthalmic solution administered sublingual for the management of terminal respiratory secretions. Am J Hosp Palliat Med. 2013;30:388-392.
As a family physician (FP), you are well positioned to optimize the quality of life of advanced cancer patients as they decline and approach death. You can help them understand their evolving prognosis so that treatment goals can be adjusted, and you can ensure that hospice is implemented early to improve the end-of-life experience. This practical review will help you to provide the best care possible for these patients.
Family physicians can fill a care gap
The term cancer survivor describes a patient who has completed initial cancer treatment. Within this population, many have declining health and ultimately succumb to their disease. There were 16.9 million cancer survivors in the United States as of January 1, 2019,1 with 53% likely to experience significant symptoms and disability.2 More than 600,000 American cancer survivors will die in 2019.3
In 2011, the Commission on Cancer mandated available outpatient palliative care services at certified cancer centers.4 Unfortunately, current palliative care resources fall far short of expected needs. A 2010 estimate of required hospice and palliative care physicians demonstrated a staffing gap of more than 50% among those providing outpatient services.5 The shortage continues,6 and many cancer patients will look to their FP for supportive care.
FPs, in addition to easing symptoms and adverse effects of medication, can educate patients and families about their disease and prognosis. By providing longitudinal care, FPs can identify critical health declines that oncologists, patients, and families often overlook. FPs can also readily appreciate decline, guide patients toward their care goals, and facilitate comfort care—including at the end of life.
Early outpatient palliative care improves quality of life and patient satisfaction. It also may improve survival time and ward off depression.7,8 Some patients and providers resist palliative care due to a misconception that it requires abandoning treatment.9 Actually, palliative care can be given in concert with all active treatments. Many experts recommend a name change from “palliative care” to “supportive care” to dispel this misconception.10
Estimate prognosis using the “surprise question”
Several algorithms are available—using between 2 and 13 patient parameters—to estimate advanced cancer survival. Most of these algorithms are designed to identify the last months or weeks of life, but their utility to predict death within these periods is limited.11
The “surprise question” may be the most valuable prognostic test for primary care. In this test, the physician asks him- or herself: Would I be surprised if this patient died in 1 year? Researchers found that when primary care physicians answered No, their patient was 4 times more likely to die within the year than when they answered Yes.12 This test has a positive predictive value of 20% and a negative predictive value of 95%, making it valuable in distinguishing patients with longer life expectancy.12 Although it overidentifies at-risk patients, the "surprise question" is a simple and sensitive tool for defining prognosis.
Continue to: Priorities for patients likely to live more than a year
Priorities for patients likely to live more than a year
For patients who likely have more than a year to live, the focus is on symptom management and preparation for future decline. Initiate and facilitate discussions about end-of-life topics. Cancer survivors are often open to discussions on these topics, which include advanced directives, home health aides, and hospice.13 Patients can set specific goals for their remaining time, such as engaging in travel, personal projects, or special events. Cancer patients have better end-of-life experiences and families have improved mental health after these discussions.14 Although cancer patients are more likely than other terminal patients to have end-of-life discussions, fewer than 40% ever do.15
Address distressing symptoms with a focus on maintaining function. More than 50% of advanced cancer patients experience fatigue, weakness, pain, weight loss, and anorexia,16 and up to 60% experience psychological distress.17 Deprescribing most preventive medications is recommended with transition to symptomatic treatment.18
Priorities for patients with less than a year to live
For patients who may have less than a year to live, focus shifts to their wishes for the time remaining and priorities for the dying process. Most patients start out with prognostic views more optimistic than those of their physicians, but this gap narrows after end-of-life discussions.19,20 Patients with incurable cancer are less likely to choose aggressive therapy if they believe their 6-month survival probability is less than 90%.21 Honest conversations, with best- and worst-case scenarios, are important to patients and families, and should occur while the patient is well enough to participate and set goals.22
In the last months of life, opioids become the primary treatment for pain and air hunger. As function declines, concerns about such adverse effects as falls and confusion decrease. Opioids have been shown to be most effective over the course of 4 weeks, and avoiding their use in earlier stages may increase their efficacy at the end of life.23
Hospice benefit—more comfort, with limitations
Hospice care consists of services administered by nonprofit and for-profit entities covered by Medicare, Medicaid, and many private insurers.24 Hospice strives to allow patients to approach death in comfort, meeting their goal of a “good death.” A recent literature review identified 4 aspects of a good death that terminally ill patients and their families considered most important: control of the dying process, relief of pain, spirituality, and emotional well-being (TABLE 1).25
Continue to: Hospice use is increasing...
Hospice use is increasing, yet many enroll too late to fully benefit. While cancer patients alone are not currently tracked, the use of hospice by Medicare beneficiaries increased from 44% in 2012 to 48% in 2019.24 In 2017, the median hospice stay was 19 days.24 Unfortunately, though, just 28% of hospice-eligible patients enrolled in hospice in their last week of life.24 Without hospice, patients often receive excessive care near death. More than 6% receive aggressive chemotherapy in their last 2 weeks of life, and nearly 10% receive a life-prolonging procedure in their last month.26
Hospice care replaces standard hospital care, although patients can elect to be followed by their primary care physician.9 Most hospice services are provided as needed or continuously at the patient’s home, including assisted living facilities. And it is also offered as part of hospital care. Hospice services are interdisciplinary, provided by physicians, nurses, social workers, chaplains, and health aides. Hospices have on-call staff to assess and treat complications, avoiding emergency hospital visits.9 And hospice includes up to 5 days respite care for family caregivers, although with a 5% copay.9 Most hospice entities run inpatient facilities for care that cannot be effectively provided at home.
Hospice care has limitations—many set by insurance. Medicare, for example, stipulates that a primary care or hospice physician must certify the patient has a reasonable prognosis of 6 months or less and is expected to have a declining course.27 Patients who survive longer than 6 months are recertified by the same criteria every 60 days.27
Hospice patients forgo treatments aimed at curing their terminal diagnosis.28 Some hospice entities allow noncurative therapies while others do not. Hospice covers prescription medications for symptom control only, although patients can receive care unrelated to the terminal diagnosis under regular benefits.28 Hospice care practices differ from standard care in ways that may surprise patients and families (TABLE 227,28). Patients can disenroll and re-enroll in hospice as they wish.28
Symptom control in advanced cancer
General symptoms
Pain affects 64% of patients with advanced cancer.29 Evidence shows that cancer pain is often undertreated, with a recent systematic review reporting undertreated pain in 32% of patients.30 State and national chronic opioid guidelines do not restrict use for cancer pain.31 Opioids are effective in 75% of cancer patients over 1 month, but there is no evidence of benefit after this period.23 In fact, increasing evidence demonstrates that pain is likely negatively responsive to opioids over longer periods.32 Opioid adverse effects can worsen other cancer symptoms, including depression, anxiety, fatigue, constipation, hypogonadism, and cognitive dysfunction.32 Delaying opioid therapy to end of life can limit adverse effects and may preserve pain-control efficacy for the dying process.
Continue to: Most cancer pain...
Most cancer pain is partially neuropathic, so anticonvulsant and antidepressant medications can help.33 Gabapentin, pregabalin, and duloxetine are recommended based on evidence not restricted to cancer.34 Cannabinoids have been evaluated in 2 trials of cancer pain with 440 patients and showed a borderline significant reduction of pain.35
Palliative radiation therapy can sometimes reduce pain. Bone metastases pain has been studied the most, and the literature suggests that palliative radiation provides improvement for 60% of patients and complete relief to 25% of patients.36 Palliative thoracic radiotherapy for primary or metastatic lung masses reduces pain by more than 70% while improving dyspnea, hemoptysis, and cough in a majority of patients.36
Other uses of palliative radiation have varied evidence. Palliative chemotherapy has less evidence of benefit. In a recent multicenter cohort trial, chemotherapy in end-stage cancer reduced quality of life in patients with good functional status, without affecting quality of life when function was limited.37 Palliative chemotherapy may be beneficial if combined with corticosteroids or radiation therapy.38
Treatment in the last weeks of life centers on opioids; dose increases do not shorten survival.39 Cancer patients are 4 times as likely as noncancer patients to have severe or excruciating pain during the last 3 days of life.40 Narcotics can be titrated aggressively near end of life with less concern for hypotension, respiratory depression, or level of consciousness. Palliative sedation remains an option for uncontrolled pain.41
Anorexia is only a problem if quality of life is affected. Cachexia is caused by increases in cytokines more than reduced calorie intake.42 Reversible causes of reduced eating may be found, including candidiasis, dental problems, depression, or constipation. Megestrol acetate improves weight (number needed to treat = 12), although it significantly increases mortality (number needed to harm = 23), making its use controversial.43 Limited study of cannabinoids has not shown effectiveness in treating anorexia.35
Continue to: Constipation...
Constipation in advanced cancer is often related to opioid therapy, although bowel obstruction must be considered. Opioid-induced constipation affects 40% to 90% of patients on long-term treatment,44 and 5 days of opioid treatment nearly doubles gastrointestinal transit time.45 Opioid-induced constipation can be treated by adding a stimulating laxative followed by a peripheral acting μ-opioid receptor antagonist, such as subcutaneous methylnaltrexone or oral naloxegol.46 These medications are contraindicated if ileus or bowel obstruction is suspected.46
Nausea and vomiting are common in advanced cancer and have numerous causes. Approximately half of reversible causes are medication adverse effects from either chemotherapy or pain medication.47 Opioid rotation may improve symptoms.47 A suspected bowel obstruction should be evaluated by specialists; surgery, palliative chemotherapy, radiation therapy, or stenting may be required. Oncologists can best manage adverse effects of chemotherapy. For nausea and vomiting unrelated to chemotherapy, consider treating constipation and pain. Medication can also be helpful; a systemic review suggests metoclopramide works best, with some evidence supporting other dopaminergic agonists, including haloperidol.47
Fatigue. Both methylphenidate and modafinil have been studied to treat cancer-related fatigue.48 A majority of patients treated with methylphenidate reported less cancer-related fatigue at 4 weeks and wished to continue treatment.49 Modafinil demonstrated minimal improvement in fatigue.50 Sleep disorders, often due to anxiety or sleep apnea, may be a correctable cause.
Later symptoms
Delirium occurs in up to 90% of cancer patients near the end of life, and can signal death.51 Up to half of the delirium seen in palliative care is reversible.51 Reversible causes include uncontrolled pain, medication adverse effects, and urinary and fecal retention (TABLE 348,51). Addressing these factors reduces delirium, based on studies in postoperative patients.52 Consider opioid rotation if neurotoxicity is suspected.51
Delirium can be accompanied by agitation or decreased responsiveness.53 Agitated delirium commonly presents with moaning, facial grimacing, and purposeless repetitive movements, such as plucking bedsheets or removing clothes.51 Delirious patients without agitation have reported, following recovery, distress similar to that experienced by agitated patients.54 Caregivers are most likely to recognize delirium and often become upset. Educating family members about the frequency of delirium can lessen this distress.54
Continue to: Delirium can be treated with...
Delirium can be treated with antipsychotics; haloperidol has been most frequently studied.54 Antipsychotics are effective at reducing agitation but not at restoring cognition.55 Case reports suggest that use of atypical antipsychotics can be beneficial if adverse effects limit haloperidol dosing.56 Agitated delirium is the most frequent indication for palliative sedation.57
Dyspnea. In the last weeks, days, or hours of life, dyspnea is common and often distressing. Dyspnea appears to be multifactorial, worsened by poor control of secretions, airway hyperactivity, and lung pathologies.58 Intravenous hydration may unintentionally exacerbate dyspnea. Hospice providers generally discourage intravenous hydration because relative dehydration reduces terminal respiratory secretions (“death rattle”) and increases patient comfort.59
Some simple nonpharmacologic interventions have benefit. Oxygen is commonly employed, although multiple studies show no benefit over room air.59 Directing a handheld fan at the face does reduce dyspnea, likely by activation of the maxillary branch of the trigeminal nerve.60
Opioids effectively treat dyspnea near the end of life with oral and parenteral dosing, but the evidence does not support nebulized opioids.61 Opioid doses required to treat dyspnea are less than those for pain and do not cause significant respiratory depression.62 If a patient taking opioids experiences dyspnea, a 25% dose increase is recommended.63
Anticholinergic medications can improve excessive airway secretions associated with dyspnea. Glycopyrrolate causes less delirium because it does not cross the blood-brain barrier, while scopolamine patches have reduced anticholinergic adverse effects, but effects are delayed until 12 hours after patch placement.64 Atropine eye drops given sublingually were effective in a small study.65
Continue to: Palliative sedation
Palliative sedation
Palliative sedation can manage intractable symptoms near the end of life. A recent systematic review suggests that palliative sedation does not shorten life.57 Sedation is most often initiated by gradual increases in medication doses.57 Midazolam is most often employed, but antipsychotics are also used.57
CORRESPONDENCE
CDR Michael J. Arnold, MD, Uniformed Services University of the Health Sciences, 4501 Jones Bridge Road, Bethesda, MD 20814; michael.arnold@usuhs.edu.
ACKNOWLEDGEMENT
Kristian Sanchack, MD, and James Higgins, DO, assisted in the preparation of this manuscript.
As a family physician (FP), you are well positioned to optimize the quality of life of advanced cancer patients as they decline and approach death. You can help them understand their evolving prognosis so that treatment goals can be adjusted, and you can ensure that hospice is implemented early to improve the end-of-life experience. This practical review will help you to provide the best care possible for these patients.
Family physicians can fill a care gap
The term cancer survivor describes a patient who has completed initial cancer treatment. Within this population, many have declining health and ultimately succumb to their disease. There were 16.9 million cancer survivors in the United States as of January 1, 2019,1 with 53% likely to experience significant symptoms and disability.2 More than 600,000 American cancer survivors will die in 2019.3
In 2011, the Commission on Cancer mandated available outpatient palliative care services at certified cancer centers.4 Unfortunately, current palliative care resources fall far short of expected needs. A 2010 estimate of required hospice and palliative care physicians demonstrated a staffing gap of more than 50% among those providing outpatient services.5 The shortage continues,6 and many cancer patients will look to their FP for supportive care.
FPs, in addition to easing symptoms and adverse effects of medication, can educate patients and families about their disease and prognosis. By providing longitudinal care, FPs can identify critical health declines that oncologists, patients, and families often overlook. FPs can also readily appreciate decline, guide patients toward their care goals, and facilitate comfort care—including at the end of life.
Early outpatient palliative care improves quality of life and patient satisfaction. It also may improve survival time and ward off depression.7,8 Some patients and providers resist palliative care due to a misconception that it requires abandoning treatment.9 Actually, palliative care can be given in concert with all active treatments. Many experts recommend a name change from “palliative care” to “supportive care” to dispel this misconception.10
Estimate prognosis using the “surprise question”
Several algorithms are available—using between 2 and 13 patient parameters—to estimate advanced cancer survival. Most of these algorithms are designed to identify the last months or weeks of life, but their utility to predict death within these periods is limited.11
The “surprise question” may be the most valuable prognostic test for primary care. In this test, the physician asks him- or herself: Would I be surprised if this patient died in 1 year? Researchers found that when primary care physicians answered No, their patient was 4 times more likely to die within the year than when they answered Yes.12 This test has a positive predictive value of 20% and a negative predictive value of 95%, making it valuable in distinguishing patients with longer life expectancy.12 Although it overidentifies at-risk patients, the "surprise question" is a simple and sensitive tool for defining prognosis.
Continue to: Priorities for patients likely to live more than a year
Priorities for patients likely to live more than a year
For patients who likely have more than a year to live, the focus is on symptom management and preparation for future decline. Initiate and facilitate discussions about end-of-life topics. Cancer survivors are often open to discussions on these topics, which include advanced directives, home health aides, and hospice.13 Patients can set specific goals for their remaining time, such as engaging in travel, personal projects, or special events. Cancer patients have better end-of-life experiences and families have improved mental health after these discussions.14 Although cancer patients are more likely than other terminal patients to have end-of-life discussions, fewer than 40% ever do.15
Address distressing symptoms with a focus on maintaining function. More than 50% of advanced cancer patients experience fatigue, weakness, pain, weight loss, and anorexia,16 and up to 60% experience psychological distress.17 Deprescribing most preventive medications is recommended with transition to symptomatic treatment.18
Priorities for patients with less than a year to live
For patients who may have less than a year to live, focus shifts to their wishes for the time remaining and priorities for the dying process. Most patients start out with prognostic views more optimistic than those of their physicians, but this gap narrows after end-of-life discussions.19,20 Patients with incurable cancer are less likely to choose aggressive therapy if they believe their 6-month survival probability is less than 90%.21 Honest conversations, with best- and worst-case scenarios, are important to patients and families, and should occur while the patient is well enough to participate and set goals.22
In the last months of life, opioids become the primary treatment for pain and air hunger. As function declines, concerns about such adverse effects as falls and confusion decrease. Opioids have been shown to be most effective over the course of 4 weeks, and avoiding their use in earlier stages may increase their efficacy at the end of life.23
Hospice benefit—more comfort, with limitations
Hospice care consists of services administered by nonprofit and for-profit entities covered by Medicare, Medicaid, and many private insurers.24 Hospice strives to allow patients to approach death in comfort, meeting their goal of a “good death.” A recent literature review identified 4 aspects of a good death that terminally ill patients and their families considered most important: control of the dying process, relief of pain, spirituality, and emotional well-being (TABLE 1).25
Continue to: Hospice use is increasing...
Hospice use is increasing, yet many enroll too late to fully benefit. While cancer patients alone are not currently tracked, the use of hospice by Medicare beneficiaries increased from 44% in 2012 to 48% in 2019.24 In 2017, the median hospice stay was 19 days.24 Unfortunately, though, just 28% of hospice-eligible patients enrolled in hospice in their last week of life.24 Without hospice, patients often receive excessive care near death. More than 6% receive aggressive chemotherapy in their last 2 weeks of life, and nearly 10% receive a life-prolonging procedure in their last month.26
Hospice care replaces standard hospital care, although patients can elect to be followed by their primary care physician.9 Most hospice services are provided as needed or continuously at the patient’s home, including assisted living facilities. And it is also offered as part of hospital care. Hospice services are interdisciplinary, provided by physicians, nurses, social workers, chaplains, and health aides. Hospices have on-call staff to assess and treat complications, avoiding emergency hospital visits.9 And hospice includes up to 5 days respite care for family caregivers, although with a 5% copay.9 Most hospice entities run inpatient facilities for care that cannot be effectively provided at home.
Hospice care has limitations—many set by insurance. Medicare, for example, stipulates that a primary care or hospice physician must certify the patient has a reasonable prognosis of 6 months or less and is expected to have a declining course.27 Patients who survive longer than 6 months are recertified by the same criteria every 60 days.27
Hospice patients forgo treatments aimed at curing their terminal diagnosis.28 Some hospice entities allow noncurative therapies while others do not. Hospice covers prescription medications for symptom control only, although patients can receive care unrelated to the terminal diagnosis under regular benefits.28 Hospice care practices differ from standard care in ways that may surprise patients and families (TABLE 227,28). Patients can disenroll and re-enroll in hospice as they wish.28
Symptom control in advanced cancer
General symptoms
Pain affects 64% of patients with advanced cancer.29 Evidence shows that cancer pain is often undertreated, with a recent systematic review reporting undertreated pain in 32% of patients.30 State and national chronic opioid guidelines do not restrict use for cancer pain.31 Opioids are effective in 75% of cancer patients over 1 month, but there is no evidence of benefit after this period.23 In fact, increasing evidence demonstrates that pain is likely negatively responsive to opioids over longer periods.32 Opioid adverse effects can worsen other cancer symptoms, including depression, anxiety, fatigue, constipation, hypogonadism, and cognitive dysfunction.32 Delaying opioid therapy to end of life can limit adverse effects and may preserve pain-control efficacy for the dying process.
Continue to: Most cancer pain...
Most cancer pain is partially neuropathic, so anticonvulsant and antidepressant medications can help.33 Gabapentin, pregabalin, and duloxetine are recommended based on evidence not restricted to cancer.34 Cannabinoids have been evaluated in 2 trials of cancer pain with 440 patients and showed a borderline significant reduction of pain.35
Palliative radiation therapy can sometimes reduce pain. Bone metastases pain has been studied the most, and the literature suggests that palliative radiation provides improvement for 60% of patients and complete relief to 25% of patients.36 Palliative thoracic radiotherapy for primary or metastatic lung masses reduces pain by more than 70% while improving dyspnea, hemoptysis, and cough in a majority of patients.36
Other uses of palliative radiation have varied evidence. Palliative chemotherapy has less evidence of benefit. In a recent multicenter cohort trial, chemotherapy in end-stage cancer reduced quality of life in patients with good functional status, without affecting quality of life when function was limited.37 Palliative chemotherapy may be beneficial if combined with corticosteroids or radiation therapy.38
Treatment in the last weeks of life centers on opioids; dose increases do not shorten survival.39 Cancer patients are 4 times as likely as noncancer patients to have severe or excruciating pain during the last 3 days of life.40 Narcotics can be titrated aggressively near end of life with less concern for hypotension, respiratory depression, or level of consciousness. Palliative sedation remains an option for uncontrolled pain.41
Anorexia is only a problem if quality of life is affected. Cachexia is caused by increases in cytokines more than reduced calorie intake.42 Reversible causes of reduced eating may be found, including candidiasis, dental problems, depression, or constipation. Megestrol acetate improves weight (number needed to treat = 12), although it significantly increases mortality (number needed to harm = 23), making its use controversial.43 Limited study of cannabinoids has not shown effectiveness in treating anorexia.35
Continue to: Constipation...
Constipation in advanced cancer is often related to opioid therapy, although bowel obstruction must be considered. Opioid-induced constipation affects 40% to 90% of patients on long-term treatment,44 and 5 days of opioid treatment nearly doubles gastrointestinal transit time.45 Opioid-induced constipation can be treated by adding a stimulating laxative followed by a peripheral acting μ-opioid receptor antagonist, such as subcutaneous methylnaltrexone or oral naloxegol.46 These medications are contraindicated if ileus or bowel obstruction is suspected.46
Nausea and vomiting are common in advanced cancer and have numerous causes. Approximately half of reversible causes are medication adverse effects from either chemotherapy or pain medication.47 Opioid rotation may improve symptoms.47 A suspected bowel obstruction should be evaluated by specialists; surgery, palliative chemotherapy, radiation therapy, or stenting may be required. Oncologists can best manage adverse effects of chemotherapy. For nausea and vomiting unrelated to chemotherapy, consider treating constipation and pain. Medication can also be helpful; a systemic review suggests metoclopramide works best, with some evidence supporting other dopaminergic agonists, including haloperidol.47
Fatigue. Both methylphenidate and modafinil have been studied to treat cancer-related fatigue.48 A majority of patients treated with methylphenidate reported less cancer-related fatigue at 4 weeks and wished to continue treatment.49 Modafinil demonstrated minimal improvement in fatigue.50 Sleep disorders, often due to anxiety or sleep apnea, may be a correctable cause.
Later symptoms
Delirium occurs in up to 90% of cancer patients near the end of life, and can signal death.51 Up to half of the delirium seen in palliative care is reversible.51 Reversible causes include uncontrolled pain, medication adverse effects, and urinary and fecal retention (TABLE 348,51). Addressing these factors reduces delirium, based on studies in postoperative patients.52 Consider opioid rotation if neurotoxicity is suspected.51
Delirium can be accompanied by agitation or decreased responsiveness.53 Agitated delirium commonly presents with moaning, facial grimacing, and purposeless repetitive movements, such as plucking bedsheets or removing clothes.51 Delirious patients without agitation have reported, following recovery, distress similar to that experienced by agitated patients.54 Caregivers are most likely to recognize delirium and often become upset. Educating family members about the frequency of delirium can lessen this distress.54
Continue to: Delirium can be treated with...
Delirium can be treated with antipsychotics; haloperidol has been most frequently studied.54 Antipsychotics are effective at reducing agitation but not at restoring cognition.55 Case reports suggest that use of atypical antipsychotics can be beneficial if adverse effects limit haloperidol dosing.56 Agitated delirium is the most frequent indication for palliative sedation.57
Dyspnea. In the last weeks, days, or hours of life, dyspnea is common and often distressing. Dyspnea appears to be multifactorial, worsened by poor control of secretions, airway hyperactivity, and lung pathologies.58 Intravenous hydration may unintentionally exacerbate dyspnea. Hospice providers generally discourage intravenous hydration because relative dehydration reduces terminal respiratory secretions (“death rattle”) and increases patient comfort.59
Some simple nonpharmacologic interventions have benefit. Oxygen is commonly employed, although multiple studies show no benefit over room air.59 Directing a handheld fan at the face does reduce dyspnea, likely by activation of the maxillary branch of the trigeminal nerve.60
Opioids effectively treat dyspnea near the end of life with oral and parenteral dosing, but the evidence does not support nebulized opioids.61 Opioid doses required to treat dyspnea are less than those for pain and do not cause significant respiratory depression.62 If a patient taking opioids experiences dyspnea, a 25% dose increase is recommended.63
Anticholinergic medications can improve excessive airway secretions associated with dyspnea. Glycopyrrolate causes less delirium because it does not cross the blood-brain barrier, while scopolamine patches have reduced anticholinergic adverse effects, but effects are delayed until 12 hours after patch placement.64 Atropine eye drops given sublingually were effective in a small study.65
Continue to: Palliative sedation
Palliative sedation
Palliative sedation can manage intractable symptoms near the end of life. A recent systematic review suggests that palliative sedation does not shorten life.57 Sedation is most often initiated by gradual increases in medication doses.57 Midazolam is most often employed, but antipsychotics are also used.57
CORRESPONDENCE
CDR Michael J. Arnold, MD, Uniformed Services University of the Health Sciences, 4501 Jones Bridge Road, Bethesda, MD 20814; michael.arnold@usuhs.edu.
ACKNOWLEDGEMENT
Kristian Sanchack, MD, and James Higgins, DO, assisted in the preparation of this manuscript.
1. American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2019-2021. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2019-2021.pdf. Accessed September 4, 2019.
2. Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112(11 suppl):2577-2592.
3. National Comprehensive Cancer Network. NCCN Guidelines Version 2. 2019. Palliative Care. www.nccn.org/professionals/physician_gls/pdf/palliative.pdf. (Must register an account for access.) Accessed September 4, 2019.
4. American Cancer Society. New CoC accreditation standards gain strong support. www.facs.org/media/press-releases/2011/coc-standards0811. Accessed September 11, 2019.
5. Lupu D; American Academy of Hospice and Palliative Medicine Workforce Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage. 2010;40:899-911.
6. Lupu D, Quigley L, Mehfoud N, et al. The growing demand for hospice and palliative medicine physicians: will the supply keep up? J Pain Symptom Manage. 2018;55:1216-1223.
7. Rabow MW, Dahlin C, Calton B, et al. New frontiers in outpatient palliative care for patients with cancer. Cancer Control. 2015;22:465-474.
8. Haun MW, Estel S, Rücker G, et al. Early palliative care for adults with advanced cancer. Cochrane Database of Syst Rev. 2017:CD01129.
9. Buss MK, Rock LK, McCarthy EP. Understanding palliative care and hospice: a review for primary care providers. Mayo Clin Proc. 2017;92:280-286.
10. Hui D. Definition of supportive care: does the semantic matter? Curr Opin Oncol. 2014;26:372-379.
11. Simmons CPL, McMillan DC, McWilliams K, et al. Prognostic tools in patients with advanced cancer: a systematic review. J Pain Symptom Manage. 2017;53:962-970.
12. Lakin JR, Robinson MG, Bernacki RE, et al. Estimating 1-year mortality for high-risk primary care patients using the “surprise” question. JAMA Int Med. 2016;176:1863-1865.
13. Walczak A, Henselmans I, Tattersall MH, et al. A qualitative analysis of responses to a question prompt list and prognosis and end-of-life care discussion prompts delivered in a communication support program. Psychoonchology. 2015;24:287-293.
14. Yamaguchi T, Maeda I, Hatano Y, et al. Effects of end-of-life discussions on the mental health of bereaved family members and quality of patient death and care. J Pain Symptom Manage. 2017;54:17-26.
15. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, caregiver bereavement adjustment. JAMA. 2008;300:1665-1673.
16. Teunissen SC, Wesker W, Kruitwagen C, et al. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34:94-104.
17. Gao W, Bennett MI, Stark D, et al. Psychological distress in cancer from survivorship to end of life: prevalence, associated factors and clinical implications. Eur J Cancer. 2010;46:2036-2044.
18. Scott IA, Gray LC, Martin JH, et al. Deciding when to stop: towards evidence-based deprescribing of drugs in older populations. Evid Based Med. 2013;18:121-124.
19. Gramling R, Fiscella K, Xing G, et al. Determinants of patient-oncologist prognostic discordance in advanced cancer. JAMA Oncol. 2016;2:1421-1426.
20. Epstein AS, Prigerson HG, O’Reilly EM, et al. Discussions of life expectancy and changes in illness understanding in patients with advanced cancer. J Clin Oncol. 2016;34:2398-2403.
21. Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709-1714.
22. Myers J. Improving the quality of end-of-life discussions. Curr Opin Support Palliat Care. 2015;9:72-76.
23. Corli O, Floriani I, Roberto A, et al. Are strong opioids equally effective and safe in the treatment of chronic cancer pain? A multicenter randomized phase IV ‘real life’ trial on the variability of response to opioids. Ann Oncolog. 2016;27:1107-1115.
24. National Hospice and Palliative Care Organization. NHPCO Facts and Figures. 2018. www.nhpco.org/wp-content/uploads/2019/07/2018_NHPCO_Facts_Figures.pdf. Accessed September 24, 2019.
25. Meier EA, Gallegos JV, Thomas LP, et al. Defining a good death (successful dying): literature review and a call for research and public dialogue. Am J Geriatr Psychiatry. 2016;24:261-271.
26. Morden NE, Chang CH, Jacobson JO, et al. End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff (Millwood). 2012;31:786-796.
27. Centers for Medicare & Medicaid Services. Medicare Hospice Benefit Facts. www.cgsmedicare.com/hhh/education/materials/pdf/Medicare_Hospice_Benefit_Facts.pdf. Accessed September 11, 2019.
28. Centers for Medicare & Medicaid Services. Medicare Hospice Benefits. www.medicare.gov/pubs/pdf/02154-medicare-hospice-benefits.pdf. Accessed September 11, 2019.
29. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18:1437-1449.
30. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32:4149-4154.
31. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
32. Davis MP, Mehta Z. Opioids and chronic pain: where is the balance? Curr Oncol Rep. 2016;18:71.
33. Leppert W, Zajaczkowska R, Wordliczek J, et al. Pathophysiology and clinical characteristics of pain in most common locations in cancer patients. J Physiol Pharmacol. 2016;67:787-799.
34. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162-173.
35. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
36. Jones JA, Lutz ST, Chow E. et al. Palliative radiotherapy at the end of life: a critical review. CA Cancer J Clin. 2014;64:296-310.
37. Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 2015;1:778-784.
38. Kongsgaard U, Kaasa S, Dale O, et al. Palliative treatment of cancer-related pain. 2005. www.ncbi.nlm.nih.gov/books/NBK464794/. Accessed September 24, 2019.
39. Sathornviriyapong A, Nagaviroj K, Anothaisintawee T. The association between different opioid doses and the survival of advanced cancer patients receiving palliative care. BMC Palliat Care. 2016;15:95.
40. Steindal SA, Bredal IS. Sørbye LW, et al. Pain control at the end of life: a comparative study of hospitalized cancer and noncancer patients. Scand J Caring Sci. 2011;25:771-779.
41. Maltoni M, Setola E. Palliative sedation in patients with cancer. Cancer Control. 2015;22:433-441.
42. Cooper C, Burden ST, Cheng H, et al. Understanding and managing cancer-related weight loss and anorexia: insights from a systematic review of qualitative research. J Cachexia Sarcopenia Muscle. 2015;6:99-111.
43. Ruiz Garcia V, LÓpez-Briz E, Carbonell Sanchis R, et al. Megesterol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2013;28:CD004310.
44. Chey WD, Webster L, Sostek M, et al. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014;370:2387-2396.
45. Poulsen JL, Nilsson M, Brock C, et al. The impact of opioid treatment on regional gastrointestinal transit. J Neurogastroenterol Motil. 2016;22:282-291.
46. Pergolizzi JV, Raffa RB, Pappagallo M, et al. Peripherally acting μ-opioid receptor antagonists as treatment options for constipation in noncancer pain patients on chronic opioid therapy. Patient Prefer Adherence. 2017;11:107-119.
47. Walsh D, Davis M, Ripamonti C, et al. 2016 updated MASCC/ESMO consensus recommendations: management of nausea and vomiting in advanced cancer. Support Care Cancer. 2017;25:333-340.
48. Mücke M, Mochamat, Cuhls H, et al. Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst Rev. 2015(5):CD006788.
49. Escalante CP, Meyers C, Reuben JM, et al. A randomized, double-blind, 2-period, placebo-controlled crossover trial of a sustained-release methylphenidate in the treatment of fatigue in cancer patients. Cancer J. 2014;20:8-14.
50. Hovey E, de Souza P, Marx G, et al. Phase III, randomized, double-blind, placebo-controlled study of modafinil for fatigue in patients treated with docetaxel-based chemotherapy. Support Care Cancer. 2014;22:1233-1242.
51. Hosker CM, Bennett MI. Delirium and agitation at the end of life. BMJ. 2016;353:i3085.
52. Mercantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516-522.
53. Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Int Med. 2001;135:32-40.
54. Breitbart W, Alici Y. Agitation and delirium at the end of life: “We couldn’t manage him." JAMA. 2008;300:2898-2910.
55. Candy B, Jackson KC, Jones L, et al. Drug therapy for delirium in terminally ill patients. Cochrane Database Syst Rev. 2012;11:CD004770.
56. Bascom PB, Bordley JL, Lawton AJ. High-dose neuroleptics and neuroleptic rotation for agitated delirium near the end of life. Am J Hosp Palliat Med. 2014;31:808-811.
57. Maltoni M, Scarpi E, Rosati M, et al. Palliative sedation in end-of-life care and survival: a systematic review. J Clin Oncol. 2012;30:1378-1383.
58. Albert RH. End-of-life care: managing common symptoms. Am Fam Physician. 2017;95:356-361.
59. Arenella C. Artificial nutrition and hydration at the end of life: beneficial or harmful? https://americanhospice.org/caregiving/artificial-nutrition-and-hydration-at-the-end-of-life-beneficial-or-harmful/ Accessed September 11, 2019.
60. Booth S, Moffat C, Burkin J, et al. Nonpharmacological interventions for breathlessness. Curr Opinion Support Pall Care. 2011;5:77-86.
61. Barnes H, McDonald J, Smallwood N, et al. Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Database Syst Rev. 2016(3)CD011008.
62. Lim RB. End-of-life care in patients with advanced lung cancer. Ther Adv Resp Dis. 2016;10:455-467.
63. Kreher M. Symptom control at the end of life. Med Clin North Am. 2016;100:1111-1122.
64. Baralatei FT, Ackerman RJ. Care of patients at the end of life: management of nonpain symptoms. FP Essent. 2016;447:18-24.
65. Protus BM, Grauer PA, Kimbrel JM. Evaluation of atropine 1% ophthalmic solution administered sublingual for the management of terminal respiratory secretions. Am J Hosp Palliat Med. 2013;30:388-392.
1. American Cancer Society. Cancer Treatment & Survivorship Facts & Figures 2019-2021. www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-treatment-and-survivorship-facts-and-figures/cancer-treatment-and-survivorship-facts-and-figures-2019-2021.pdf. Accessed September 4, 2019.
2. Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112(11 suppl):2577-2592.
3. National Comprehensive Cancer Network. NCCN Guidelines Version 2. 2019. Palliative Care. www.nccn.org/professionals/physician_gls/pdf/palliative.pdf. (Must register an account for access.) Accessed September 4, 2019.
4. American Cancer Society. New CoC accreditation standards gain strong support. www.facs.org/media/press-releases/2011/coc-standards0811. Accessed September 11, 2019.
5. Lupu D; American Academy of Hospice and Palliative Medicine Workforce Task Force. Estimate of current hospice and palliative medicine physician workforce shortage. J Pain Symptom Manage. 2010;40:899-911.
6. Lupu D, Quigley L, Mehfoud N, et al. The growing demand for hospice and palliative medicine physicians: will the supply keep up? J Pain Symptom Manage. 2018;55:1216-1223.
7. Rabow MW, Dahlin C, Calton B, et al. New frontiers in outpatient palliative care for patients with cancer. Cancer Control. 2015;22:465-474.
8. Haun MW, Estel S, Rücker G, et al. Early palliative care for adults with advanced cancer. Cochrane Database of Syst Rev. 2017:CD01129.
9. Buss MK, Rock LK, McCarthy EP. Understanding palliative care and hospice: a review for primary care providers. Mayo Clin Proc. 2017;92:280-286.
10. Hui D. Definition of supportive care: does the semantic matter? Curr Opin Oncol. 2014;26:372-379.
11. Simmons CPL, McMillan DC, McWilliams K, et al. Prognostic tools in patients with advanced cancer: a systematic review. J Pain Symptom Manage. 2017;53:962-970.
12. Lakin JR, Robinson MG, Bernacki RE, et al. Estimating 1-year mortality for high-risk primary care patients using the “surprise” question. JAMA Int Med. 2016;176:1863-1865.
13. Walczak A, Henselmans I, Tattersall MH, et al. A qualitative analysis of responses to a question prompt list and prognosis and end-of-life care discussion prompts delivered in a communication support program. Psychoonchology. 2015;24:287-293.
14. Yamaguchi T, Maeda I, Hatano Y, et al. Effects of end-of-life discussions on the mental health of bereaved family members and quality of patient death and care. J Pain Symptom Manage. 2017;54:17-26.
15. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, caregiver bereavement adjustment. JAMA. 2008;300:1665-1673.
16. Teunissen SC, Wesker W, Kruitwagen C, et al. Symptom prevalence in patients with incurable cancer: a systematic review. J Pain Symptom Manage. 2007;34:94-104.
17. Gao W, Bennett MI, Stark D, et al. Psychological distress in cancer from survivorship to end of life: prevalence, associated factors and clinical implications. Eur J Cancer. 2010;46:2036-2044.
18. Scott IA, Gray LC, Martin JH, et al. Deciding when to stop: towards evidence-based deprescribing of drugs in older populations. Evid Based Med. 2013;18:121-124.
19. Gramling R, Fiscella K, Xing G, et al. Determinants of patient-oncologist prognostic discordance in advanced cancer. JAMA Oncol. 2016;2:1421-1426.
20. Epstein AS, Prigerson HG, O’Reilly EM, et al. Discussions of life expectancy and changes in illness understanding in patients with advanced cancer. J Clin Oncol. 2016;34:2398-2403.
21. Weeks JC, Cook EF, O’Day SJ, et al. Relationship between cancer patients’ predictions of prognosis and their treatment preferences. JAMA. 1998;279:1709-1714.
22. Myers J. Improving the quality of end-of-life discussions. Curr Opin Support Palliat Care. 2015;9:72-76.
23. Corli O, Floriani I, Roberto A, et al. Are strong opioids equally effective and safe in the treatment of chronic cancer pain? A multicenter randomized phase IV ‘real life’ trial on the variability of response to opioids. Ann Oncolog. 2016;27:1107-1115.
24. National Hospice and Palliative Care Organization. NHPCO Facts and Figures. 2018. www.nhpco.org/wp-content/uploads/2019/07/2018_NHPCO_Facts_Figures.pdf. Accessed September 24, 2019.
25. Meier EA, Gallegos JV, Thomas LP, et al. Defining a good death (successful dying): literature review and a call for research and public dialogue. Am J Geriatr Psychiatry. 2016;24:261-271.
26. Morden NE, Chang CH, Jacobson JO, et al. End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff (Millwood). 2012;31:786-796.
27. Centers for Medicare & Medicaid Services. Medicare Hospice Benefit Facts. www.cgsmedicare.com/hhh/education/materials/pdf/Medicare_Hospice_Benefit_Facts.pdf. Accessed September 11, 2019.
28. Centers for Medicare & Medicaid Services. Medicare Hospice Benefits. www.medicare.gov/pubs/pdf/02154-medicare-hospice-benefits.pdf. Accessed September 11, 2019.
29. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, et al. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18:1437-1449.
30. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32:4149-4154.
31. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain — United States, 2016. MMWR Recomm Rep. 2016;65:1-49.
32. Davis MP, Mehta Z. Opioids and chronic pain: where is the balance? Curr Oncol Rep. 2016;18:71.
33. Leppert W, Zajaczkowska R, Wordliczek J, et al. Pathophysiology and clinical characteristics of pain in most common locations in cancer patients. J Physiol Pharmacol. 2016;67:787-799.
34. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162-173.
35. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA. 2015;313:2456-2473.
36. Jones JA, Lutz ST, Chow E. et al. Palliative radiotherapy at the end of life: a critical review. CA Cancer J Clin. 2014;64:296-310.
37. Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 2015;1:778-784.
38. Kongsgaard U, Kaasa S, Dale O, et al. Palliative treatment of cancer-related pain. 2005. www.ncbi.nlm.nih.gov/books/NBK464794/. Accessed September 24, 2019.
39. Sathornviriyapong A, Nagaviroj K, Anothaisintawee T. The association between different opioid doses and the survival of advanced cancer patients receiving palliative care. BMC Palliat Care. 2016;15:95.
40. Steindal SA, Bredal IS. Sørbye LW, et al. Pain control at the end of life: a comparative study of hospitalized cancer and noncancer patients. Scand J Caring Sci. 2011;25:771-779.
41. Maltoni M, Setola E. Palliative sedation in patients with cancer. Cancer Control. 2015;22:433-441.
42. Cooper C, Burden ST, Cheng H, et al. Understanding and managing cancer-related weight loss and anorexia: insights from a systematic review of qualitative research. J Cachexia Sarcopenia Muscle. 2015;6:99-111.
43. Ruiz Garcia V, LÓpez-Briz E, Carbonell Sanchis R, et al. Megesterol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2013;28:CD004310.
44. Chey WD, Webster L, Sostek M, et al. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014;370:2387-2396.
45. Poulsen JL, Nilsson M, Brock C, et al. The impact of opioid treatment on regional gastrointestinal transit. J Neurogastroenterol Motil. 2016;22:282-291.
46. Pergolizzi JV, Raffa RB, Pappagallo M, et al. Peripherally acting μ-opioid receptor antagonists as treatment options for constipation in noncancer pain patients on chronic opioid therapy. Patient Prefer Adherence. 2017;11:107-119.
47. Walsh D, Davis M, Ripamonti C, et al. 2016 updated MASCC/ESMO consensus recommendations: management of nausea and vomiting in advanced cancer. Support Care Cancer. 2017;25:333-340.
48. Mücke M, Mochamat, Cuhls H, et al. Pharmacological treatments for fatigue associated with palliative care. Cochrane Database Syst Rev. 2015(5):CD006788.
49. Escalante CP, Meyers C, Reuben JM, et al. A randomized, double-blind, 2-period, placebo-controlled crossover trial of a sustained-release methylphenidate in the treatment of fatigue in cancer patients. Cancer J. 2014;20:8-14.
50. Hovey E, de Souza P, Marx G, et al. Phase III, randomized, double-blind, placebo-controlled study of modafinil for fatigue in patients treated with docetaxel-based chemotherapy. Support Care Cancer. 2014;22:1233-1242.
51. Hosker CM, Bennett MI. Delirium and agitation at the end of life. BMJ. 2016;353:i3085.
52. Mercantonio ER, Flacker JM, Wright RJ, et al. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. 2001;49:516-522.
53. Casarett DJ, Inouye SK. Diagnosis and management of delirium near the end of life. Ann Int Med. 2001;135:32-40.
54. Breitbart W, Alici Y. Agitation and delirium at the end of life: “We couldn’t manage him." JAMA. 2008;300:2898-2910.
55. Candy B, Jackson KC, Jones L, et al. Drug therapy for delirium in terminally ill patients. Cochrane Database Syst Rev. 2012;11:CD004770.
56. Bascom PB, Bordley JL, Lawton AJ. High-dose neuroleptics and neuroleptic rotation for agitated delirium near the end of life. Am J Hosp Palliat Med. 2014;31:808-811.
57. Maltoni M, Scarpi E, Rosati M, et al. Palliative sedation in end-of-life care and survival: a systematic review. J Clin Oncol. 2012;30:1378-1383.
58. Albert RH. End-of-life care: managing common symptoms. Am Fam Physician. 2017;95:356-361.
59. Arenella C. Artificial nutrition and hydration at the end of life: beneficial or harmful? https://americanhospice.org/caregiving/artificial-nutrition-and-hydration-at-the-end-of-life-beneficial-or-harmful/ Accessed September 11, 2019.
60. Booth S, Moffat C, Burkin J, et al. Nonpharmacological interventions for breathlessness. Curr Opinion Support Pall Care. 2011;5:77-86.
61. Barnes H, McDonald J, Smallwood N, et al. Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Database Syst Rev. 2016(3)CD011008.
62. Lim RB. End-of-life care in patients with advanced lung cancer. Ther Adv Resp Dis. 2016;10:455-467.
63. Kreher M. Symptom control at the end of life. Med Clin North Am. 2016;100:1111-1122.
64. Baralatei FT, Ackerman RJ. Care of patients at the end of life: management of nonpain symptoms. FP Essent. 2016;447:18-24.
65. Protus BM, Grauer PA, Kimbrel JM. Evaluation of atropine 1% ophthalmic solution administered sublingual for the management of terminal respiratory secretions. Am J Hosp Palliat Med. 2013;30:388-392.
PRACTICE RECOMMENDATIONS
› Implement palliative/ supportive care shortly after the diagnosis of an incurable cancer. A
› Candidly communicate prognoses to patients and help them adjust their goals of care. B
› Recommend hospice care for patients who likely have less than 6 months to live, especially with treatmentrelated complications or significant caregiver stress. B
› Delay opioid therapy— if possible—to better control symptoms near the end of life. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Strategies to reduce and prevent polypharmacy in older patients
CASE
Ronald Wa is a 74-year old man with an extensive medical history: diabetes, hypertension, heart failure, atrial fibrillation, pancreatitis, hyperlipidemia, gout, depression, generalized anxiety, obstructive sleep apnea, and benign prostatic hypertrophy. He arrives at the emergency department (ED) of the hospital by nonemergent ambulance from home for evaluation of lethargy and confusion over the past week.
In the ED, Mr. W is afebrile, normotensive, and oxygenating on room air. Mucous membranes are dry. On physical examination, he appears pale, fatigued, and modestly confused but is able to state his name and birthday, although not the location or date.
Laboratory testing reveals: blood glucose, 107 mg/dL; serum creatinine, 2.3 mg/dL; sodium, 127 mEq/L; and hemoglobin level and hematocrit, within normal limits. Urinalysis is negative. Renal ultrasonography is unremarkable, without evidence of urinary tract obstruction.
Mr. W is admitted to the general medical unit with hyponatremia. The pharmacy admission specialist begins reconciliation of the long list of the patient’s home medications.
Overprescribing: Often, more is not better
Some experts consider prescribing medication to be the most common form of medical intervention; beyond that, polypharmacy—often defined as the use of more medications than are medically necessary (see the next section on terminology)—is recognized as an increasingly serious problem in many medical specialties.1 Here are specifics about the extent of, and harm caused by, the problem2,3:
- The US General Accounting Office reports that inappropriate polypharmacy is associated with significant morbidity and mortality.2 Research has established a strong relationship between polypharmacy and harmful clinical consequences,3 to which the older patient population is most susceptible.
- Polypharmacy is also recognized as an expensive practice; the US Center for Medicare and Medicaid Services estimates that polypharmacy cost US health insurers more than $50 billion annually.2
- Worldwide, with more and more people older than 65 years, polypharmacy is becoming more prevalent, and a growing concern, in older adults; approximately 50% of them take ≥ 1 medications that are medically unnecessary.3
Despite many programs to help with deprescribing, drug–drug interactions and the so-called prescribing cascade (ie, when signs and symptoms of an adverse drug effect are misdiagnosed as a new medical condition) continue to affect patients, leading to comorbidities. It is important, therefore, for physicians to be aware of commonly used tools to prevent polypharmacy and its consequences.
What is “polypharmacy” understood to mean?
Despite the compelling association of polypharmacy with the presence of multiple morbidities in the older patient population, there is no consensus on its definition:
- Starting with the dictionary, “polypharmacy” derives from 2 words in Ancient Greek: poly, “more than one,” and “pharmakon, “drug.”3
- The definition can vary based on the number of drugs a patient has been prescribed, their safety, and the appropriateness of their use.1
- Another definition is the use of more medications than are medically necessary; such a grouping includes agents that are not indicated, are ineffective, or constitute a therapeutic duplication. Although this definition is more clinically relevant than the others, it is premised on undertaking a clinical review of a medication regimen.3
- A numerical definition is the most commonly reported category, a number that varies from study to study—from ≥ 2 to ≥ 11 medications. When applied to health care settings, accepted definitions are ≥ 5 medications at hospital discharge and ≥ 10 during a hospital stay.4 Numerical definitions of polypharmacy do not ascertain the clinical appropriateness of therapy nor the process of rationalizing those medications.1
aA composite, hypothetical patient, based on the authors' clinical experience.
Continue to: Appropriateness
Appropriateness
Polypharmacy is classified as appropriate or inappropriate:
- Appropriate polypharmacy is the optimization of medications for patients with complex or multiple conditions, when the use of medicine is in agreement with best evidence.
- Inappropriate polypharmacy can increase the risk of adverse drug effects and drug–drug interactions and can be characterized by medication underuse and duplication.4
There are subdefinitions of “appropriateness,” but these are beyond the scope of this article.
What variables contribute to polypharmacy?
Multimorbidity is common in the older population. The presence of multiple chronic conditions increases the complexity of therapeutic management for health professionals and patients; such complexity can have a harmful impact on health outcomes. Combinations of medications to treat chronic diseases automatically push many patients into polypharmacy. Few treatment guidelines provide recommendations on when to stop medications.
Consequences of polypharmacy, some of which are masked as syndromes in the older patient, include delirium and dementia, urinary incontinence, dizziness, falls, adverse drug reactions, increased length of hospital stay, readmission soon after discharge, and death.3-5 Relatively high rates of drug consumption and other variables (eg, decreased renal and hepatic function, decreased total body water and lean body mass, cognitive impairment, age-related decline in vision and hearing, frequency of chronic diseases and medical comorbidities, communication barriers, prescribing cascades, and health care delivery involving multiple prescribers) can contribute to an increased prevalence of medication-associated morbidity and mortality as the result of polypharmacy.
In a descriptive study6 that examined these variables, researchers explored whether general practitioners experience barriers to medication review in multimorbid patients with polypharmacy. They concluded that the primary barriers were (1) lack of communication and teamwork with specialists and (2) the challenge of handling polypharmacy in a culture that encourages adding medications and inhibits conversations about medication withdrawal.6
Continue to: Reducing consequences of polypharmacy
Reducing consequences of polypharmacy
Collaborative medication review
Interventions to help physicians reduce polypharmacy include reviewing medications with older patients at every office visit and during transitions of care into and out of the hospital or other care facility. A 2016 Cochrane review of 5 randomized trials of inpatient medication reviews led by pharmacists, physicians, and other health care professionals showed a 36% reduction in ED visits 30 days to 1 year after discharge.7
Patients can collaborate in this effort by bringing all medications to each appointment or upon hospital admission—not just a list but the actual supply, to ensure that a correct medication list is compiled and a thorough review conducted.8 Explicitly ask open-ended questions of the patient about over-the-counter medications, herbal products, and other home remedies that have not been prescribed; many patients may have trouble with recall or are uncertain what fits the definition of a nonprescription medication.8,9
Compare the medication list with the patient’s current problem list; consider removing medications that do not have a pertinent indication. (Physicians can help in this regard when prescribing by making note in the medical record of the indication for each medication they prescribe.)
Evaluate the patient’s signs and symptoms as a possible drug-related adverse effect, thus making an effort to minimize the chance of a prescribing cascade.9
Use Beers criteria,10 which list potentially inappropriate medications to be avoided in older adults. The criteria serve as a filter when considering starting a new medication and aiding in the review process.8
Continue to: The NO TEARS tool...
The NO TEARS tool11 can be useful for simplifying the medication review process. Components of this tool are:
- Need and indication: Does the patient still require each of his medications? Was long-term treatment anticipated?
- Open questions: Ask the patient for his views about his medications; for example, “Do you think the drugs you take work?”
- Tests and monitoring: Are any of the patient’s conditions undertreated, based on laboratory and clinical findings?
- Evidence and guidelines: Has the base of evidence been updated for each of the patient’s medications since they were started?
- Adverse events: Is the patient experiencing adverse effects of medication? Have possible adverse drug interactions been noted?
- Risk reduction or prevention: Does the patient face risks of treatment (eg, loss of appetite, urinary incontinence) that can be reduced by optimizing the medication plan?
- Simplification and switches: Can treatment be simplified while maintaining effectiveness?
There are strategies to promote patient advocacy, as well. Encourage patients to use a holistic approach by asking you, their other physicians, and their pharmacist about how their condition is being treated:
- What other treatment options exist, including nonpharmacotherapeutic options?
- What are the possible benefits and harms of medical therapy?
- Under what circumstances would discontinuing a medication be appropriate?12
CASE
Medication reconciliation identifies > 20 medications that had been prescribed for the patient to take at home (TABLE 1). A clinical pharmacist then performs a home medication review as part of routine patient care upon transition of care into the hospital.
Identifying polypharmacy
Implementing polypharmacy identification tools is a necessary first step in the process of mitigating the risk of multiple concurrent medications (TABLE 22,10,12-18). In addition to tools that are used to identify polypharmacy, there are steps that physicians and pharmacists can take to decrease the risk of polypharmacy.
For example, in a longitudinal, time-series cohort study measuring polypharmacy events, a pharmacist intervention was used as the means to decrease polypharmacy.19 Pharmacists intervened twice (each intervention separated by 1 year) to identify and manage 5 categories of high-risk drugs in patients whose care was provided by a managed care plan.19 During that time, pharmacists provided drug therapy reviews, education to physicians and patients about drug safety, and information for physicians on ways to correct problems with polypharmacy.19
Continue to: Over the course of the 2 interventions...
Over the course of the 2 interventions, the overall rate of polypharmacy events decreased 67% after the first intervention and 39% after the second. The practice of having pharmacists spearhead this task was shown to reduce the cost and number of prescriptions in patients at risk for polypharmacy. (In fact, some general practitioners report that they deem multidisciplinary decision-making with pharmacists a necessary component of managing polypharmacy effectively.6)
Screening for medications as a cause of signs and symptoms
As noted earlier, a prescribing cascade arises when a drug administered to a patient causes an adverse event that is then mistakenly identified as a new condition, resulting in a new medication being prescribed.9 The pattern of a cascade then repeats itself, resulting in inappropriate polypharmacy.
Erroneous treatment of an adverse drug event as a medical condition is often the result of a lack of pharmacologic knowledge—which is why it is necessary to evaluate each new symptom with the mindset that a medication might, in fact, be causing the sign or symptom and with the aim of reducing the risk of a prescribing cascade.8,9 Routinely update a patient’s medication list in the event that a medication no longer has an indication aligned with the patient’s problem list; then, ideally, the initial therapy can be adjusted instead of starting additional medications.9
CASE
A review of Mr. W’s home medications reveals 1 therapeutic duplication and 2 drugs that lacked an indication. Application of the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP)15 and Beers criteria10 helped the pharmacist identify additional elements of inappropriate polypharmacy, including inappropriate medication use, drug–disease interactions, contraindications, and recommendations for dosage adjustment based on kidney function. Specifically:
- Aripiprazole and quetiapine: Present an increased risk of falls. (General recommendation: Avoid using Frutiger LT Std≥ 3 drugs that act on the central nervous system [CNS], due to an increased risk of falls.)
- Fluoxetine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Gabapentin: Presents an increased risk of CNS adverse effects. Reduce the dosage when the estimated creatinine clearance is < 60 mL/min.
- Hydrocodone–acetaminophen: Presents an increased risk of falls. (Again, avoid or minimize the number of drugs that act on the CNS.)
- Lorazepam: Indication is missing. Avoid use of this drug due to an increased risk of cognitive impairment and decreased metabolism of medication.
- Mirtazapine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Pantoprazole: Avoid scheduled use for > 8 weeks, except in high-risk patients, due to the risk of Clostridium difficile infection and bone loss and fractures.
- Prazosin: Indication is missing. Avoid use of this drug as an antihypertensive due to the high risk of orthostatic hypotension.
- Ranitidine: Duplicates concurrent treatment with pantoprazole. Reduce the dosage when the estimated creatinine clearance is < 50 mL/min.
The value of deprescribing
Direct evidence of the efficacy and safety of deprescribing, and strategies for deprescribing, have been documented in the literature:
Observational study. Cessation of inappropriate antihypertensive agents was associated with fewer cardiovascular events and deaths over a 5-year follow-up period.20
Continue to: Deprescribing protocol
Deprescribing protocol. A method developed by Scott and co-workers21 is an additional resource to consider. Appropriate times to consider deprescribing are (1) when new symptoms suggest an adverse drug effect; (2) in the presence of end-stage disease, terminal illness, dementia, extreme frailty, or full dependence on others for all care; (3) upon receipt of high-risk medications or combinations; and (4) upon receipt of preventive medications for which risk outweighs benefit.21
This suggested method of deprescribing comprises several steps: (1) collecting all medications that the patient is taking and identifying the indication for each; (2) considering the overall risk of drug-induced harm to determine necessary intensity of deprescribing; (3) assessing each drug for its eligibility to be discontinued, such as no indication, part of a prescribing cascade, or lack of benefit; (4) prioritizing drugs for discontinuation; and (5) implementing and monitoring the drug discontinuation regimen.21
Drug-by-drug elimination trial. Reducing the dosage of, or stopping, only 1 medication at a time has been shown to be paramount to assessing development of medication-associated problems and then identifying a likely cause.14
Good Palliative-Geriatric Practice algorithm. This algorithm22 can be used to guide discontinuation of inappropriate medications and improve drug therapy in community-dwelling older adults. The algorithm has been shown to improve the overall well-being of patients studied; however, it has been tested only in patients in long-term care settings and community-dwelling palliative care patients, limiting its generalizability to a larger population. The algorithm is also difficult to apply to patients who have multiple comorbidities.
Risk vs. benefit of discontinuing chronic medical therapy. A systematic review of the effects of discontinuing chronic medication reveals that the risk of doing so might outweigh benefit14; this finding is thought to be due to potential relapse in the disease state being treated.11 The risks of discontinuation should be contemplated before removing the medication or reducing the dosage. Medications that can be considered to present a risk when discontinued include, but are not limited to, benzodiazepines, oral corticosteroids, antidepressants, acid suppressants, bisphosphonates, statins, and transdermal opioids.1
Continue to: CASE
CASE
After applying Beers criteria10 and STOPP15, the pharmacist makes several recommendations:
- Use aripiprazole and quetiapine with caution.
- Consider discontinuing fluoxetine, hydrocodone–acetaminophen, lorazepam, pantoprazole, and ranitidine.
- Reduce the dosage of gabapentin.
- Clarify the indication for prazosin. Consider discontinuing if being used as an antihypertensive.
In addition, the pharmacist recommends holding metformin because lactic acidosis can develop (however rarely) when a person taking metformin experiences acute kidney injury.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; tbaher@uwyo.edu
1. All Wales Medicines Strategy Group. Polypharmacy: Guidance for Prescribing. July 2014. http://awmsg.org/docs/awmsg/medman/Polypharmacy%20-%20Guidance%20for%20Prescribing.pdf. Accessed October 3, 2019.
2. Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3:383-389.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
4. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
5. Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606-609.
6. Laursen J, Kornholt J, Betzer C, et al. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:2333392818792169.
7. Christensen M, Lundh A. Medication review in hospitalized patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
8. Zurakowski T. The practicalities and pitfalls of polypharmacy. Nurse Pract. 2009;34:36-41.
9. Ponte ML, Wachs L, Wachs A, et al. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77:13-16.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434.
12. Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse events in older people. BMC Geriatr. 2009;9:5.
13. Skinner M. A literature review: polypharmacy protocol for primary care. Geriatr Nurs. 2015;36:367-371.
14. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
15. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37:673-679.
16. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045-1051.
17. Samsa G, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891-896.
18. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481-1486.
19. Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636-1645.
20. Thio SL, Nam J, van Driel ML, et al. Effects of discontinuation of chronic medication in primary care: a systematic review of deprescribing trials. Br J Gen Pract. 2018;68:e663-e672.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
22. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
CASE
Ronald Wa is a 74-year old man with an extensive medical history: diabetes, hypertension, heart failure, atrial fibrillation, pancreatitis, hyperlipidemia, gout, depression, generalized anxiety, obstructive sleep apnea, and benign prostatic hypertrophy. He arrives at the emergency department (ED) of the hospital by nonemergent ambulance from home for evaluation of lethargy and confusion over the past week.
In the ED, Mr. W is afebrile, normotensive, and oxygenating on room air. Mucous membranes are dry. On physical examination, he appears pale, fatigued, and modestly confused but is able to state his name and birthday, although not the location or date.
Laboratory testing reveals: blood glucose, 107 mg/dL; serum creatinine, 2.3 mg/dL; sodium, 127 mEq/L; and hemoglobin level and hematocrit, within normal limits. Urinalysis is negative. Renal ultrasonography is unremarkable, without evidence of urinary tract obstruction.
Mr. W is admitted to the general medical unit with hyponatremia. The pharmacy admission specialist begins reconciliation of the long list of the patient’s home medications.
Overprescribing: Often, more is not better
Some experts consider prescribing medication to be the most common form of medical intervention; beyond that, polypharmacy—often defined as the use of more medications than are medically necessary (see the next section on terminology)—is recognized as an increasingly serious problem in many medical specialties.1 Here are specifics about the extent of, and harm caused by, the problem2,3:
- The US General Accounting Office reports that inappropriate polypharmacy is associated with significant morbidity and mortality.2 Research has established a strong relationship between polypharmacy and harmful clinical consequences,3 to which the older patient population is most susceptible.
- Polypharmacy is also recognized as an expensive practice; the US Center for Medicare and Medicaid Services estimates that polypharmacy cost US health insurers more than $50 billion annually.2
- Worldwide, with more and more people older than 65 years, polypharmacy is becoming more prevalent, and a growing concern, in older adults; approximately 50% of them take ≥ 1 medications that are medically unnecessary.3
Despite many programs to help with deprescribing, drug–drug interactions and the so-called prescribing cascade (ie, when signs and symptoms of an adverse drug effect are misdiagnosed as a new medical condition) continue to affect patients, leading to comorbidities. It is important, therefore, for physicians to be aware of commonly used tools to prevent polypharmacy and its consequences.
What is “polypharmacy” understood to mean?
Despite the compelling association of polypharmacy with the presence of multiple morbidities in the older patient population, there is no consensus on its definition:
- Starting with the dictionary, “polypharmacy” derives from 2 words in Ancient Greek: poly, “more than one,” and “pharmakon, “drug.”3
- The definition can vary based on the number of drugs a patient has been prescribed, their safety, and the appropriateness of their use.1
- Another definition is the use of more medications than are medically necessary; such a grouping includes agents that are not indicated, are ineffective, or constitute a therapeutic duplication. Although this definition is more clinically relevant than the others, it is premised on undertaking a clinical review of a medication regimen.3
- A numerical definition is the most commonly reported category, a number that varies from study to study—from ≥ 2 to ≥ 11 medications. When applied to health care settings, accepted definitions are ≥ 5 medications at hospital discharge and ≥ 10 during a hospital stay.4 Numerical definitions of polypharmacy do not ascertain the clinical appropriateness of therapy nor the process of rationalizing those medications.1
aA composite, hypothetical patient, based on the authors' clinical experience.
Continue to: Appropriateness
Appropriateness
Polypharmacy is classified as appropriate or inappropriate:
- Appropriate polypharmacy is the optimization of medications for patients with complex or multiple conditions, when the use of medicine is in agreement with best evidence.
- Inappropriate polypharmacy can increase the risk of adverse drug effects and drug–drug interactions and can be characterized by medication underuse and duplication.4
There are subdefinitions of “appropriateness,” but these are beyond the scope of this article.
What variables contribute to polypharmacy?
Multimorbidity is common in the older population. The presence of multiple chronic conditions increases the complexity of therapeutic management for health professionals and patients; such complexity can have a harmful impact on health outcomes. Combinations of medications to treat chronic diseases automatically push many patients into polypharmacy. Few treatment guidelines provide recommendations on when to stop medications.
Consequences of polypharmacy, some of which are masked as syndromes in the older patient, include delirium and dementia, urinary incontinence, dizziness, falls, adverse drug reactions, increased length of hospital stay, readmission soon after discharge, and death.3-5 Relatively high rates of drug consumption and other variables (eg, decreased renal and hepatic function, decreased total body water and lean body mass, cognitive impairment, age-related decline in vision and hearing, frequency of chronic diseases and medical comorbidities, communication barriers, prescribing cascades, and health care delivery involving multiple prescribers) can contribute to an increased prevalence of medication-associated morbidity and mortality as the result of polypharmacy.
In a descriptive study6 that examined these variables, researchers explored whether general practitioners experience barriers to medication review in multimorbid patients with polypharmacy. They concluded that the primary barriers were (1) lack of communication and teamwork with specialists and (2) the challenge of handling polypharmacy in a culture that encourages adding medications and inhibits conversations about medication withdrawal.6
Continue to: Reducing consequences of polypharmacy
Reducing consequences of polypharmacy
Collaborative medication review
Interventions to help physicians reduce polypharmacy include reviewing medications with older patients at every office visit and during transitions of care into and out of the hospital or other care facility. A 2016 Cochrane review of 5 randomized trials of inpatient medication reviews led by pharmacists, physicians, and other health care professionals showed a 36% reduction in ED visits 30 days to 1 year after discharge.7
Patients can collaborate in this effort by bringing all medications to each appointment or upon hospital admission—not just a list but the actual supply, to ensure that a correct medication list is compiled and a thorough review conducted.8 Explicitly ask open-ended questions of the patient about over-the-counter medications, herbal products, and other home remedies that have not been prescribed; many patients may have trouble with recall or are uncertain what fits the definition of a nonprescription medication.8,9
Compare the medication list with the patient’s current problem list; consider removing medications that do not have a pertinent indication. (Physicians can help in this regard when prescribing by making note in the medical record of the indication for each medication they prescribe.)
Evaluate the patient’s signs and symptoms as a possible drug-related adverse effect, thus making an effort to minimize the chance of a prescribing cascade.9
Use Beers criteria,10 which list potentially inappropriate medications to be avoided in older adults. The criteria serve as a filter when considering starting a new medication and aiding in the review process.8
Continue to: The NO TEARS tool...
The NO TEARS tool11 can be useful for simplifying the medication review process. Components of this tool are:
- Need and indication: Does the patient still require each of his medications? Was long-term treatment anticipated?
- Open questions: Ask the patient for his views about his medications; for example, “Do you think the drugs you take work?”
- Tests and monitoring: Are any of the patient’s conditions undertreated, based on laboratory and clinical findings?
- Evidence and guidelines: Has the base of evidence been updated for each of the patient’s medications since they were started?
- Adverse events: Is the patient experiencing adverse effects of medication? Have possible adverse drug interactions been noted?
- Risk reduction or prevention: Does the patient face risks of treatment (eg, loss of appetite, urinary incontinence) that can be reduced by optimizing the medication plan?
- Simplification and switches: Can treatment be simplified while maintaining effectiveness?
There are strategies to promote patient advocacy, as well. Encourage patients to use a holistic approach by asking you, their other physicians, and their pharmacist about how their condition is being treated:
- What other treatment options exist, including nonpharmacotherapeutic options?
- What are the possible benefits and harms of medical therapy?
- Under what circumstances would discontinuing a medication be appropriate?12
CASE
Medication reconciliation identifies > 20 medications that had been prescribed for the patient to take at home (TABLE 1). A clinical pharmacist then performs a home medication review as part of routine patient care upon transition of care into the hospital.
Identifying polypharmacy
Implementing polypharmacy identification tools is a necessary first step in the process of mitigating the risk of multiple concurrent medications (TABLE 22,10,12-18). In addition to tools that are used to identify polypharmacy, there are steps that physicians and pharmacists can take to decrease the risk of polypharmacy.
For example, in a longitudinal, time-series cohort study measuring polypharmacy events, a pharmacist intervention was used as the means to decrease polypharmacy.19 Pharmacists intervened twice (each intervention separated by 1 year) to identify and manage 5 categories of high-risk drugs in patients whose care was provided by a managed care plan.19 During that time, pharmacists provided drug therapy reviews, education to physicians and patients about drug safety, and information for physicians on ways to correct problems with polypharmacy.19
Continue to: Over the course of the 2 interventions...
Over the course of the 2 interventions, the overall rate of polypharmacy events decreased 67% after the first intervention and 39% after the second. The practice of having pharmacists spearhead this task was shown to reduce the cost and number of prescriptions in patients at risk for polypharmacy. (In fact, some general practitioners report that they deem multidisciplinary decision-making with pharmacists a necessary component of managing polypharmacy effectively.6)
Screening for medications as a cause of signs and symptoms
As noted earlier, a prescribing cascade arises when a drug administered to a patient causes an adverse event that is then mistakenly identified as a new condition, resulting in a new medication being prescribed.9 The pattern of a cascade then repeats itself, resulting in inappropriate polypharmacy.
Erroneous treatment of an adverse drug event as a medical condition is often the result of a lack of pharmacologic knowledge—which is why it is necessary to evaluate each new symptom with the mindset that a medication might, in fact, be causing the sign or symptom and with the aim of reducing the risk of a prescribing cascade.8,9 Routinely update a patient’s medication list in the event that a medication no longer has an indication aligned with the patient’s problem list; then, ideally, the initial therapy can be adjusted instead of starting additional medications.9
CASE
A review of Mr. W’s home medications reveals 1 therapeutic duplication and 2 drugs that lacked an indication. Application of the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP)15 and Beers criteria10 helped the pharmacist identify additional elements of inappropriate polypharmacy, including inappropriate medication use, drug–disease interactions, contraindications, and recommendations for dosage adjustment based on kidney function. Specifically:
- Aripiprazole and quetiapine: Present an increased risk of falls. (General recommendation: Avoid using Frutiger LT Std≥ 3 drugs that act on the central nervous system [CNS], due to an increased risk of falls.)
- Fluoxetine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Gabapentin: Presents an increased risk of CNS adverse effects. Reduce the dosage when the estimated creatinine clearance is < 60 mL/min.
- Hydrocodone–acetaminophen: Presents an increased risk of falls. (Again, avoid or minimize the number of drugs that act on the CNS.)
- Lorazepam: Indication is missing. Avoid use of this drug due to an increased risk of cognitive impairment and decreased metabolism of medication.
- Mirtazapine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Pantoprazole: Avoid scheduled use for > 8 weeks, except in high-risk patients, due to the risk of Clostridium difficile infection and bone loss and fractures.
- Prazosin: Indication is missing. Avoid use of this drug as an antihypertensive due to the high risk of orthostatic hypotension.
- Ranitidine: Duplicates concurrent treatment with pantoprazole. Reduce the dosage when the estimated creatinine clearance is < 50 mL/min.
The value of deprescribing
Direct evidence of the efficacy and safety of deprescribing, and strategies for deprescribing, have been documented in the literature:
Observational study. Cessation of inappropriate antihypertensive agents was associated with fewer cardiovascular events and deaths over a 5-year follow-up period.20
Continue to: Deprescribing protocol
Deprescribing protocol. A method developed by Scott and co-workers21 is an additional resource to consider. Appropriate times to consider deprescribing are (1) when new symptoms suggest an adverse drug effect; (2) in the presence of end-stage disease, terminal illness, dementia, extreme frailty, or full dependence on others for all care; (3) upon receipt of high-risk medications or combinations; and (4) upon receipt of preventive medications for which risk outweighs benefit.21
This suggested method of deprescribing comprises several steps: (1) collecting all medications that the patient is taking and identifying the indication for each; (2) considering the overall risk of drug-induced harm to determine necessary intensity of deprescribing; (3) assessing each drug for its eligibility to be discontinued, such as no indication, part of a prescribing cascade, or lack of benefit; (4) prioritizing drugs for discontinuation; and (5) implementing and monitoring the drug discontinuation regimen.21
Drug-by-drug elimination trial. Reducing the dosage of, or stopping, only 1 medication at a time has been shown to be paramount to assessing development of medication-associated problems and then identifying a likely cause.14
Good Palliative-Geriatric Practice algorithm. This algorithm22 can be used to guide discontinuation of inappropriate medications and improve drug therapy in community-dwelling older adults. The algorithm has been shown to improve the overall well-being of patients studied; however, it has been tested only in patients in long-term care settings and community-dwelling palliative care patients, limiting its generalizability to a larger population. The algorithm is also difficult to apply to patients who have multiple comorbidities.
Risk vs. benefit of discontinuing chronic medical therapy. A systematic review of the effects of discontinuing chronic medication reveals that the risk of doing so might outweigh benefit14; this finding is thought to be due to potential relapse in the disease state being treated.11 The risks of discontinuation should be contemplated before removing the medication or reducing the dosage. Medications that can be considered to present a risk when discontinued include, but are not limited to, benzodiazepines, oral corticosteroids, antidepressants, acid suppressants, bisphosphonates, statins, and transdermal opioids.1
Continue to: CASE
CASE
After applying Beers criteria10 and STOPP15, the pharmacist makes several recommendations:
- Use aripiprazole and quetiapine with caution.
- Consider discontinuing fluoxetine, hydrocodone–acetaminophen, lorazepam, pantoprazole, and ranitidine.
- Reduce the dosage of gabapentin.
- Clarify the indication for prazosin. Consider discontinuing if being used as an antihypertensive.
In addition, the pharmacist recommends holding metformin because lactic acidosis can develop (however rarely) when a person taking metformin experiences acute kidney injury.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; tbaher@uwyo.edu
CASE
Ronald Wa is a 74-year old man with an extensive medical history: diabetes, hypertension, heart failure, atrial fibrillation, pancreatitis, hyperlipidemia, gout, depression, generalized anxiety, obstructive sleep apnea, and benign prostatic hypertrophy. He arrives at the emergency department (ED) of the hospital by nonemergent ambulance from home for evaluation of lethargy and confusion over the past week.
In the ED, Mr. W is afebrile, normotensive, and oxygenating on room air. Mucous membranes are dry. On physical examination, he appears pale, fatigued, and modestly confused but is able to state his name and birthday, although not the location or date.
Laboratory testing reveals: blood glucose, 107 mg/dL; serum creatinine, 2.3 mg/dL; sodium, 127 mEq/L; and hemoglobin level and hematocrit, within normal limits. Urinalysis is negative. Renal ultrasonography is unremarkable, without evidence of urinary tract obstruction.
Mr. W is admitted to the general medical unit with hyponatremia. The pharmacy admission specialist begins reconciliation of the long list of the patient’s home medications.
Overprescribing: Often, more is not better
Some experts consider prescribing medication to be the most common form of medical intervention; beyond that, polypharmacy—often defined as the use of more medications than are medically necessary (see the next section on terminology)—is recognized as an increasingly serious problem in many medical specialties.1 Here are specifics about the extent of, and harm caused by, the problem2,3:
- The US General Accounting Office reports that inappropriate polypharmacy is associated with significant morbidity and mortality.2 Research has established a strong relationship between polypharmacy and harmful clinical consequences,3 to which the older patient population is most susceptible.
- Polypharmacy is also recognized as an expensive practice; the US Center for Medicare and Medicaid Services estimates that polypharmacy cost US health insurers more than $50 billion annually.2
- Worldwide, with more and more people older than 65 years, polypharmacy is becoming more prevalent, and a growing concern, in older adults; approximately 50% of them take ≥ 1 medications that are medically unnecessary.3
Despite many programs to help with deprescribing, drug–drug interactions and the so-called prescribing cascade (ie, when signs and symptoms of an adverse drug effect are misdiagnosed as a new medical condition) continue to affect patients, leading to comorbidities. It is important, therefore, for physicians to be aware of commonly used tools to prevent polypharmacy and its consequences.
What is “polypharmacy” understood to mean?
Despite the compelling association of polypharmacy with the presence of multiple morbidities in the older patient population, there is no consensus on its definition:
- Starting with the dictionary, “polypharmacy” derives from 2 words in Ancient Greek: poly, “more than one,” and “pharmakon, “drug.”3
- The definition can vary based on the number of drugs a patient has been prescribed, their safety, and the appropriateness of their use.1
- Another definition is the use of more medications than are medically necessary; such a grouping includes agents that are not indicated, are ineffective, or constitute a therapeutic duplication. Although this definition is more clinically relevant than the others, it is premised on undertaking a clinical review of a medication regimen.3
- A numerical definition is the most commonly reported category, a number that varies from study to study—from ≥ 2 to ≥ 11 medications. When applied to health care settings, accepted definitions are ≥ 5 medications at hospital discharge and ≥ 10 during a hospital stay.4 Numerical definitions of polypharmacy do not ascertain the clinical appropriateness of therapy nor the process of rationalizing those medications.1
aA composite, hypothetical patient, based on the authors' clinical experience.
Continue to: Appropriateness
Appropriateness
Polypharmacy is classified as appropriate or inappropriate:
- Appropriate polypharmacy is the optimization of medications for patients with complex or multiple conditions, when the use of medicine is in agreement with best evidence.
- Inappropriate polypharmacy can increase the risk of adverse drug effects and drug–drug interactions and can be characterized by medication underuse and duplication.4
There are subdefinitions of “appropriateness,” but these are beyond the scope of this article.
What variables contribute to polypharmacy?
Multimorbidity is common in the older population. The presence of multiple chronic conditions increases the complexity of therapeutic management for health professionals and patients; such complexity can have a harmful impact on health outcomes. Combinations of medications to treat chronic diseases automatically push many patients into polypharmacy. Few treatment guidelines provide recommendations on when to stop medications.
Consequences of polypharmacy, some of which are masked as syndromes in the older patient, include delirium and dementia, urinary incontinence, dizziness, falls, adverse drug reactions, increased length of hospital stay, readmission soon after discharge, and death.3-5 Relatively high rates of drug consumption and other variables (eg, decreased renal and hepatic function, decreased total body water and lean body mass, cognitive impairment, age-related decline in vision and hearing, frequency of chronic diseases and medical comorbidities, communication barriers, prescribing cascades, and health care delivery involving multiple prescribers) can contribute to an increased prevalence of medication-associated morbidity and mortality as the result of polypharmacy.
In a descriptive study6 that examined these variables, researchers explored whether general practitioners experience barriers to medication review in multimorbid patients with polypharmacy. They concluded that the primary barriers were (1) lack of communication and teamwork with specialists and (2) the challenge of handling polypharmacy in a culture that encourages adding medications and inhibits conversations about medication withdrawal.6
Continue to: Reducing consequences of polypharmacy
Reducing consequences of polypharmacy
Collaborative medication review
Interventions to help physicians reduce polypharmacy include reviewing medications with older patients at every office visit and during transitions of care into and out of the hospital or other care facility. A 2016 Cochrane review of 5 randomized trials of inpatient medication reviews led by pharmacists, physicians, and other health care professionals showed a 36% reduction in ED visits 30 days to 1 year after discharge.7
Patients can collaborate in this effort by bringing all medications to each appointment or upon hospital admission—not just a list but the actual supply, to ensure that a correct medication list is compiled and a thorough review conducted.8 Explicitly ask open-ended questions of the patient about over-the-counter medications, herbal products, and other home remedies that have not been prescribed; many patients may have trouble with recall or are uncertain what fits the definition of a nonprescription medication.8,9
Compare the medication list with the patient’s current problem list; consider removing medications that do not have a pertinent indication. (Physicians can help in this regard when prescribing by making note in the medical record of the indication for each medication they prescribe.)
Evaluate the patient’s signs and symptoms as a possible drug-related adverse effect, thus making an effort to minimize the chance of a prescribing cascade.9
Use Beers criteria,10 which list potentially inappropriate medications to be avoided in older adults. The criteria serve as a filter when considering starting a new medication and aiding in the review process.8
Continue to: The NO TEARS tool...
The NO TEARS tool11 can be useful for simplifying the medication review process. Components of this tool are:
- Need and indication: Does the patient still require each of his medications? Was long-term treatment anticipated?
- Open questions: Ask the patient for his views about his medications; for example, “Do you think the drugs you take work?”
- Tests and monitoring: Are any of the patient’s conditions undertreated, based on laboratory and clinical findings?
- Evidence and guidelines: Has the base of evidence been updated for each of the patient’s medications since they were started?
- Adverse events: Is the patient experiencing adverse effects of medication? Have possible adverse drug interactions been noted?
- Risk reduction or prevention: Does the patient face risks of treatment (eg, loss of appetite, urinary incontinence) that can be reduced by optimizing the medication plan?
- Simplification and switches: Can treatment be simplified while maintaining effectiveness?
There are strategies to promote patient advocacy, as well. Encourage patients to use a holistic approach by asking you, their other physicians, and their pharmacist about how their condition is being treated:
- What other treatment options exist, including nonpharmacotherapeutic options?
- What are the possible benefits and harms of medical therapy?
- Under what circumstances would discontinuing a medication be appropriate?12
CASE
Medication reconciliation identifies > 20 medications that had been prescribed for the patient to take at home (TABLE 1). A clinical pharmacist then performs a home medication review as part of routine patient care upon transition of care into the hospital.
Identifying polypharmacy
Implementing polypharmacy identification tools is a necessary first step in the process of mitigating the risk of multiple concurrent medications (TABLE 22,10,12-18). In addition to tools that are used to identify polypharmacy, there are steps that physicians and pharmacists can take to decrease the risk of polypharmacy.
For example, in a longitudinal, time-series cohort study measuring polypharmacy events, a pharmacist intervention was used as the means to decrease polypharmacy.19 Pharmacists intervened twice (each intervention separated by 1 year) to identify and manage 5 categories of high-risk drugs in patients whose care was provided by a managed care plan.19 During that time, pharmacists provided drug therapy reviews, education to physicians and patients about drug safety, and information for physicians on ways to correct problems with polypharmacy.19
Continue to: Over the course of the 2 interventions...
Over the course of the 2 interventions, the overall rate of polypharmacy events decreased 67% after the first intervention and 39% after the second. The practice of having pharmacists spearhead this task was shown to reduce the cost and number of prescriptions in patients at risk for polypharmacy. (In fact, some general practitioners report that they deem multidisciplinary decision-making with pharmacists a necessary component of managing polypharmacy effectively.6)
Screening for medications as a cause of signs and symptoms
As noted earlier, a prescribing cascade arises when a drug administered to a patient causes an adverse event that is then mistakenly identified as a new condition, resulting in a new medication being prescribed.9 The pattern of a cascade then repeats itself, resulting in inappropriate polypharmacy.
Erroneous treatment of an adverse drug event as a medical condition is often the result of a lack of pharmacologic knowledge—which is why it is necessary to evaluate each new symptom with the mindset that a medication might, in fact, be causing the sign or symptom and with the aim of reducing the risk of a prescribing cascade.8,9 Routinely update a patient’s medication list in the event that a medication no longer has an indication aligned with the patient’s problem list; then, ideally, the initial therapy can be adjusted instead of starting additional medications.9
CASE
A review of Mr. W’s home medications reveals 1 therapeutic duplication and 2 drugs that lacked an indication. Application of the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP)15 and Beers criteria10 helped the pharmacist identify additional elements of inappropriate polypharmacy, including inappropriate medication use, drug–disease interactions, contraindications, and recommendations for dosage adjustment based on kidney function. Specifically:
- Aripiprazole and quetiapine: Present an increased risk of falls. (General recommendation: Avoid using Frutiger LT Std≥ 3 drugs that act on the central nervous system [CNS], due to an increased risk of falls.)
- Fluoxetine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Gabapentin: Presents an increased risk of CNS adverse effects. Reduce the dosage when the estimated creatinine clearance is < 60 mL/min.
- Hydrocodone–acetaminophen: Presents an increased risk of falls. (Again, avoid or minimize the number of drugs that act on the CNS.)
- Lorazepam: Indication is missing. Avoid use of this drug due to an increased risk of cognitive impairment and decreased metabolism of medication.
- Mirtazapine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Pantoprazole: Avoid scheduled use for > 8 weeks, except in high-risk patients, due to the risk of Clostridium difficile infection and bone loss and fractures.
- Prazosin: Indication is missing. Avoid use of this drug as an antihypertensive due to the high risk of orthostatic hypotension.
- Ranitidine: Duplicates concurrent treatment with pantoprazole. Reduce the dosage when the estimated creatinine clearance is < 50 mL/min.
The value of deprescribing
Direct evidence of the efficacy and safety of deprescribing, and strategies for deprescribing, have been documented in the literature:
Observational study. Cessation of inappropriate antihypertensive agents was associated with fewer cardiovascular events and deaths over a 5-year follow-up period.20
Continue to: Deprescribing protocol
Deprescribing protocol. A method developed by Scott and co-workers21 is an additional resource to consider. Appropriate times to consider deprescribing are (1) when new symptoms suggest an adverse drug effect; (2) in the presence of end-stage disease, terminal illness, dementia, extreme frailty, or full dependence on others for all care; (3) upon receipt of high-risk medications or combinations; and (4) upon receipt of preventive medications for which risk outweighs benefit.21
This suggested method of deprescribing comprises several steps: (1) collecting all medications that the patient is taking and identifying the indication for each; (2) considering the overall risk of drug-induced harm to determine necessary intensity of deprescribing; (3) assessing each drug for its eligibility to be discontinued, such as no indication, part of a prescribing cascade, or lack of benefit; (4) prioritizing drugs for discontinuation; and (5) implementing and monitoring the drug discontinuation regimen.21
Drug-by-drug elimination trial. Reducing the dosage of, or stopping, only 1 medication at a time has been shown to be paramount to assessing development of medication-associated problems and then identifying a likely cause.14
Good Palliative-Geriatric Practice algorithm. This algorithm22 can be used to guide discontinuation of inappropriate medications and improve drug therapy in community-dwelling older adults. The algorithm has been shown to improve the overall well-being of patients studied; however, it has been tested only in patients in long-term care settings and community-dwelling palliative care patients, limiting its generalizability to a larger population. The algorithm is also difficult to apply to patients who have multiple comorbidities.
Risk vs. benefit of discontinuing chronic medical therapy. A systematic review of the effects of discontinuing chronic medication reveals that the risk of doing so might outweigh benefit14; this finding is thought to be due to potential relapse in the disease state being treated.11 The risks of discontinuation should be contemplated before removing the medication or reducing the dosage. Medications that can be considered to present a risk when discontinued include, but are not limited to, benzodiazepines, oral corticosteroids, antidepressants, acid suppressants, bisphosphonates, statins, and transdermal opioids.1
Continue to: CASE
CASE
After applying Beers criteria10 and STOPP15, the pharmacist makes several recommendations:
- Use aripiprazole and quetiapine with caution.
- Consider discontinuing fluoxetine, hydrocodone–acetaminophen, lorazepam, pantoprazole, and ranitidine.
- Reduce the dosage of gabapentin.
- Clarify the indication for prazosin. Consider discontinuing if being used as an antihypertensive.
In addition, the pharmacist recommends holding metformin because lactic acidosis can develop (however rarely) when a person taking metformin experiences acute kidney injury.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; tbaher@uwyo.edu
1. All Wales Medicines Strategy Group. Polypharmacy: Guidance for Prescribing. July 2014. http://awmsg.org/docs/awmsg/medman/Polypharmacy%20-%20Guidance%20for%20Prescribing.pdf. Accessed October 3, 2019.
2. Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3:383-389.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
4. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
5. Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606-609.
6. Laursen J, Kornholt J, Betzer C, et al. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:2333392818792169.
7. Christensen M, Lundh A. Medication review in hospitalized patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
8. Zurakowski T. The practicalities and pitfalls of polypharmacy. Nurse Pract. 2009;34:36-41.
9. Ponte ML, Wachs L, Wachs A, et al. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77:13-16.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434.
12. Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse events in older people. BMC Geriatr. 2009;9:5.
13. Skinner M. A literature review: polypharmacy protocol for primary care. Geriatr Nurs. 2015;36:367-371.
14. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
15. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37:673-679.
16. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045-1051.
17. Samsa G, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891-896.
18. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481-1486.
19. Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636-1645.
20. Thio SL, Nam J, van Driel ML, et al. Effects of discontinuation of chronic medication in primary care: a systematic review of deprescribing trials. Br J Gen Pract. 2018;68:e663-e672.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
22. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
1. All Wales Medicines Strategy Group. Polypharmacy: Guidance for Prescribing. July 2014. http://awmsg.org/docs/awmsg/medman/Polypharmacy%20-%20Guidance%20for%20Prescribing.pdf. Accessed October 3, 2019.
2. Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3:383-389.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
4. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
5. Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606-609.
6. Laursen J, Kornholt J, Betzer C, et al. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:2333392818792169.
7. Christensen M, Lundh A. Medication review in hospitalized patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
8. Zurakowski T. The practicalities and pitfalls of polypharmacy. Nurse Pract. 2009;34:36-41.
9. Ponte ML, Wachs L, Wachs A, et al. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77:13-16.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434.
12. Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse events in older people. BMC Geriatr. 2009;9:5.
13. Skinner M. A literature review: polypharmacy protocol for primary care. Geriatr Nurs. 2015;36:367-371.
14. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
15. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37:673-679.
16. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045-1051.
17. Samsa G, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891-896.
18. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481-1486.
19. Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636-1645.
20. Thio SL, Nam J, van Driel ML, et al. Effects of discontinuation of chronic medication in primary care: a systematic review of deprescribing trials. Br J Gen Pract. 2018;68:e663-e672.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
22. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
PRACTICE RECOMMENDATIONS
› Use one of the available tested and recommended screening tools to identify polypharmacy. C
› Engage in collaborative medication review to reduce the incidence of polypharmacy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series