User login
Knee pain and injury: When is a surgical consult needed?
Evidence supports what family physicians know to be true: Knee pain is an exceedingly common presenting problem in the primary care office. Estimates of lifetime incidence reach as high as 54%,1 and the prevalence of knee pain in the general population is increasing.2 Knee disability can result from acute or traumatic injuries as well as chronic, degenerative conditions such as osteoarthritis (OA). The decision to pursue orthopedic consultation for a particular injury or painful knee condition can be challenging. To address this, we highlight specific knee diagnoses known to cause pain, with the aim of describing which conditions likely will necessitate surgical consultation—and which won’t.
Acute or nondegenerative knee injuries and pain
Acute knee injuries range in severity from simple contusions and sprains to high-energy, traumatic injuries with resulting joint instability and potential neurovascular compromise. While conservative treatment often is successful for many simple injuries, surgical management—sometimes urgently or emergently—is needed in other cases, as will be detailed shortly.
Neurovascular injury associated with knee dislocations
Acute neurovascular injuries often require emergent surgical intervention. Although rare, tibiofemoral (knee) dislocations pose a significant challenge to the clinician in both diagnosis and management. The reported frequency of popliteal artery injury or rupture following a dislocation varies widely, with rates ranging from 5% to 64%, according to older studies; more recent data, however, suggest the rate is actually as low as 3.3%.3
Immediate immobilization and emergency department transport for monitoring, orthopedics consultation, and vascular studies or vascular surgery consultation is recommended in the case of a suspected knee dislocation. In one cross-sectional cohort study, the surgical management of knee dislocations yielded favorable outcomes in > 75% of cases.5
Tibial plateau fracture
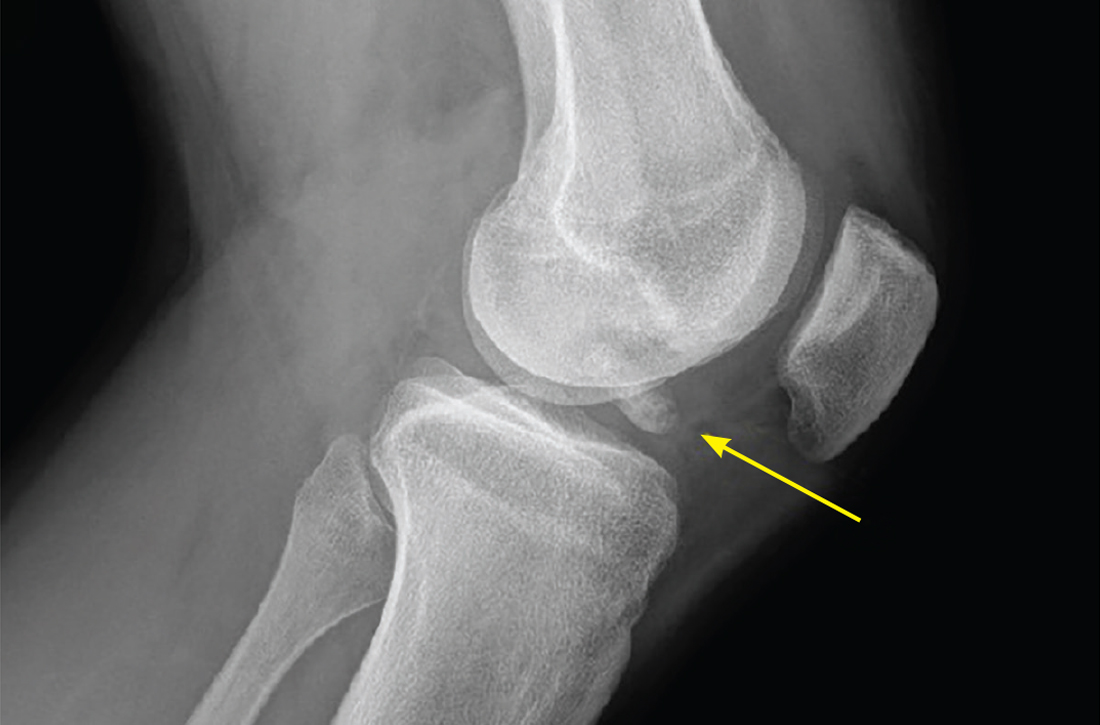
This fracture often occurs as a result of high-energy trauma, such as contact sports or motor vehicle accidents, and is characterized by a proximal tibial fracture line with extension to the articular surface. X-rays often are sufficient for initial diagnosis. Computed tomography can help rule out a fracture line when clinical suspicion is high and x-rays are nondiagnostic. As noted earlier, any suggestion of neurovascular compromise on physical exam requires an emergent orthopedic surgeon consultation for a possible displaced and unstable (or more complex) injury (FIGURE 1).6-8
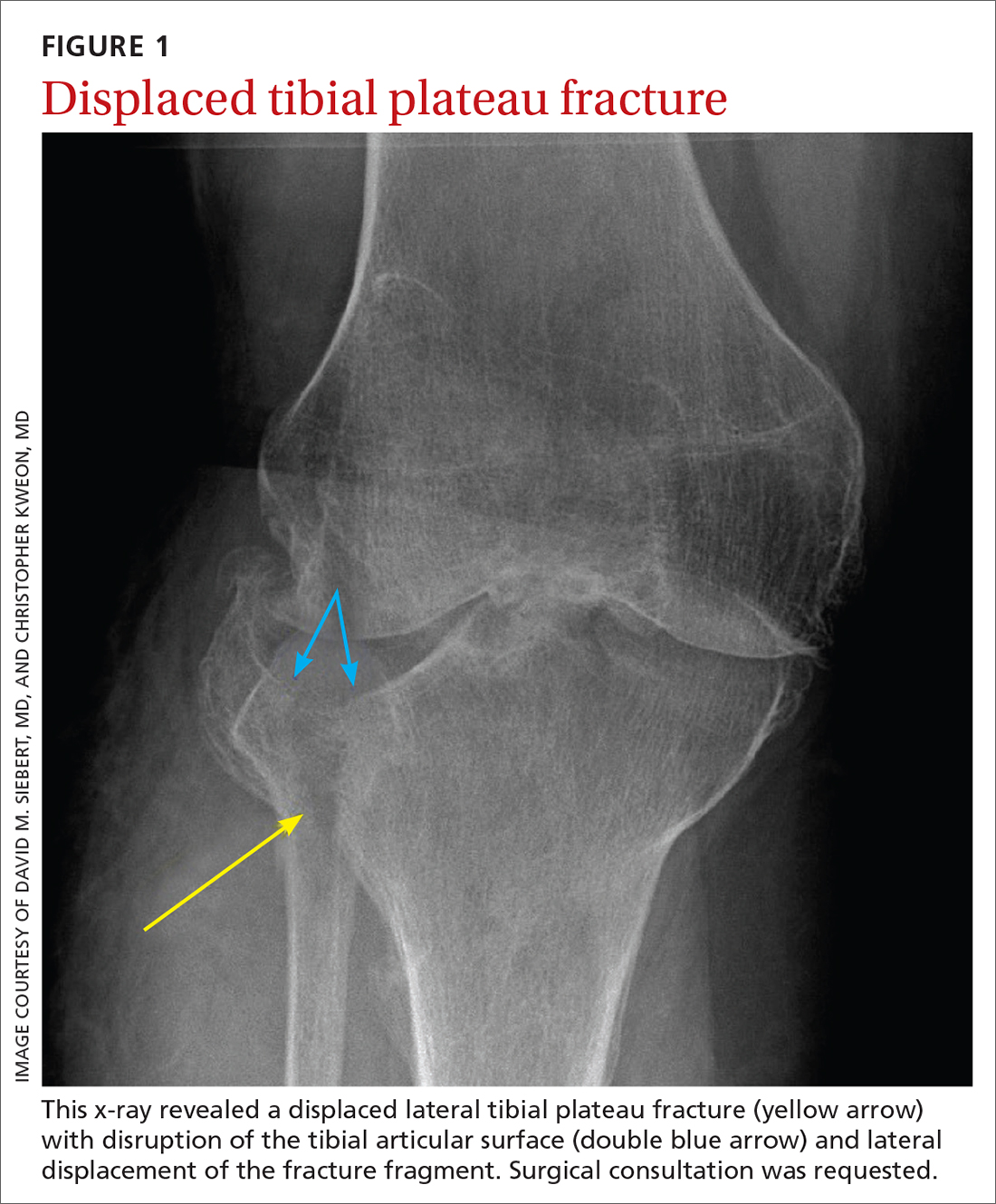
Nondisplaced tibial plateau fractures without supraphysiologic ligamentous laxity on valgus or varus stress testing can be managed safely with protection and early mobilization, gradual progression of weight-bearing, and serial x-rays to ensure fracture healing and stability.
Gross joint instability identified by positive valgus or varus stress testing, positive anterior or posterior drawer testing, or patient inability to tolerate these maneuvers due to pain similarly should raise suspicion for a more significant fracture at risk for concurrent neurovascular injury. Acute compartment syndrome also is a known complication of tibial plateau fractures and similarly requires emergent operative management. Urgent surgical consultation is recommended for fractures with displaced fracture fragments, tibial articular surface step-off or depression, fractures with concurrent joint laxity, or medial plateau fractures.6-8
Continue to: Patella fractures
Patella fractures
These fractures occur as a direct blow to the front of the knee, such as falling forward onto a hard surface, or indirectly due to a sudden extreme eccentric contraction of the quadriceps muscle. Nondisplaced fractures with an intact knee extension mechanism, which is examined via a supine straight-leg raise or seated knee extension, are managed with weight-bearing as tolerated in strict immobilization in full extension for 4 to 6 weeks, with active range-of-motion and isometric quadriceps exercises beginning in 1 to 2 weeks. Serial x-rays also are obtained to ensure fracture displacement does not occur during the rehabilitation process.9
High-quality evidence guiding follow-up care and comparing outcomes of surgical and nonsurgical management of patella fractures is lacking, and studies comparing different surgical techniques are of lower methodological quality.10 Nevertheless, displaced or comminuted patellar fractures are referred urgently to orthopedic surgical care for fixation, as are those with concurrent loose bodies, chondral surface injuries or articular step-off, or osteochondral fractures.9 Inability to perform a straight-leg raise (ie, clinical loss of the knee extension mechanism) suggests a fracture under tension that likely also requires surgical fixation for successful recovery. Neurovascular injuries are unlikely in most patellar fractures but would require emergent surgical consultation.9
Ligamentous injury
Tibiofemoral joint laxity occurs as a result of ligamentous injury, with or without tibial plateau fracture. The anterior cruciate ligament (ACL), posterior cruciate ligament (PCL), medial collateral ligament (MCL), and lateral collateral ligament (LCL) comprise the 4 main ligaments of the knee. The ACL resists anterior tibial translation and rotational forces, while the PCL resists posterior tibial translation. The MCL and LCL resist valgus and varus stress, respectively.
Ligament injuries are classified as Grades 1 to 311:
- Grade 1 sprains. The ligament is stretched, but there is no macroscopic tearing; joint stability is maintained.
- Grade 2 sprains. There are partial macroscopic ligament tears. There is joint laxity due to the partial loss of the ligament’s structural integrity.
- Grade 3 sprains. The ligament is fully avulsed or ruptured with resultant gross joint instability.
Continue to: ACL tears
ACL tears occur most commonly via a noncontact event, as when an individual plants their foot and suddenly changes direction during sport or other physical activity. Treatment hinges on patient activity levels and participation in sports. Patients who do not plan to engage in athletic movements (that require changes in direction or planting and twisting) and who otherwise maintain satisfactory joint stability during activities of daily living may elect to defer or even altogether avoid surgical reconstruction of isolated ACL tears. One pair of studies demonstrated equivalent outcomes in surgical and nonsurgical management in 121 young, nonelite athletes at 2- and 5-year follow-up, although the crossover from the nonsurgical to surgical groups was high.12,13 Athletes who regain satisfactory function and stability nonoperatively can defer surgical intervention. However, the majority of active patients and athletes will require surgical ACL reconstruction to return to pre-injury functional levels.14
PCL sprains occur as a result of sudden posteriorly directed force on the tibia, such as when the knee is hyperextended or a patient falls directly onto a flexed knee. Patients with Grade 1 and 2 isolated sprains generally will recover with conservative care, as will patients with some Grade 3 complete tears that do not fully compromise joint stability. However, high-grade PCL injuries often are comorbid with posterolateral corner or other injuries, leading to a higher likelihood of joint instability and thus the need for surgical intervention for the best chance at an optimal outcome.15
MCL sprain. Surgical management is not required in an isolated Grade 1 or 2 MCL sprain, as the hallmarks of recovery—return of joint stability, knee strength and range of motion, and pain reduction—can be achieved successfully with conservative management. Isolated Grade 3 MCL sprains are also successfully managed nonoperatively16 except in specific cases, such as a concurrent large avulsion fracture.17
LCL sprain. Similarly, isolated Grade 1 and 2 LCL sprains generally do not require surgical intervention. However, Grade 3 LCL injuries usually do, as persistent joint instability and poor functional outcomes are more common with nonsurgical management.18-20 Additionally, high-grade LCL injuries frequently manifest with comorbid meniscus injuries or sprains of the posterolateral corner of the knee, a complex anatomic structure that provides both static and dynamic tibiofemoral joint stability. Surgical repair or reconstruction of the posterolateral corner frequently is necessary for optimal functional outcomes.21
Multiligamentous sprains frequently lead to gross joint instability and necessitate orthopedic surgeon consultation to determine the best treatment plan; this should be done emergently if neurovascular compromise is suspected. A common injury combination is simultaneous ACL and MCL sprains with or without meniscus injury. In these cases, some surgeons will choose to defer ACL reconstruction until after MCL healing is achieved. This allows the patient to regain valgus stability of the joint prior to performing ACL reconstruction to regain rotational and anterior stability.20
Continue to: Patellar dislocations
Patellar dislocations represent a relatively common knee injury in young active patients, often occurring in a noncontact fashion when a valgus force is applied to an externally rotated and planted lower leg.
Major tendon rupture
Patellar tendon ruptures occur when a sudden eccentric force is applied to the knee, such as when landing from a jump with the knee flexed. Patellar tendon ruptures frequently are clinically apparent, with patients demonstrating a high-riding patella and loss of active knee extension. Quadriceps tendon ruptures often result from a similar injury mechanism in older patients, with a similar loss of active knee extension and a palpable gap superior to the patella.24
Partial tears in patients who can maintain full extension of the knee against gravity are treated nonoperatively, but early surgical repair is indicated for complete quadriceps or patellar tendon ruptures to achieve optimal outcomes.
Even with prompt treatment, return to sport is not guaranteed. According to a recent systematic review, athletes returned to play 88.9% and 89.8% of the time following patellar and quadriceps tendon repairs, respectively. However, returning to the same level of play was less common and achieved 80.8% (patellar tendon repair) and 70% (quadriceps tendon repair) of the time. Return-to-work rates were higher, at 96% for both surgical treatments.29
Locked knee and acute meniscus tears in younger patients
In some acute knee injuries, meniscus tears, loose cartilage bodies or osteochondral defects, or other internal structures can become interposed between the femoral and tibial surfaces, preventing both active and passive knee extension. Such injuries are often severely painful and functionally debilitating. While manipulation under anesthesia can acutely restore joint function,30 diagnostic and therapeutic arthroscopy often is pursued for definitive treatment.31 Compared to the gold standard of diagnostic arthroscopy, preoperative magnetic resonance imaging (MRI) carries positive and negative predictive values of 85% and 77%, respectively, in identifying or ruling out the anatomic structure responsible for a locked knee. 32 As such,
Continue to: Depending on the location...
Depending on the location, size, and shape of an acute meniscus tear in younger patients, surgical repair may be an option to preserve long-term joint function. In one case series of patients younger than 20 years, 62% of meniscus repairs yielded good outcomes after a mean follow-up period of 16.8 years.33
Osteochondritis dissecans
Osteochondritis dissecans is characterized by subchondral bone osteonecrosis that most often occurs in pediatric patients, potentially causing the separation of a fragment of articular cartilage and subchondral bone into the joint space (FIGURE 2). In early stages, nonoperative treatment consisting of prolonged rest followed by physical therapy to gradually return to activity is recommended to prevent small, low-grade lesions from progressing to unstable or separated fragments. Arthroscopy, which consists of microfracture or other surgical resurfacing techniques to restore joint integrity, is pursued in more advanced cases of unstable or separated fragments.
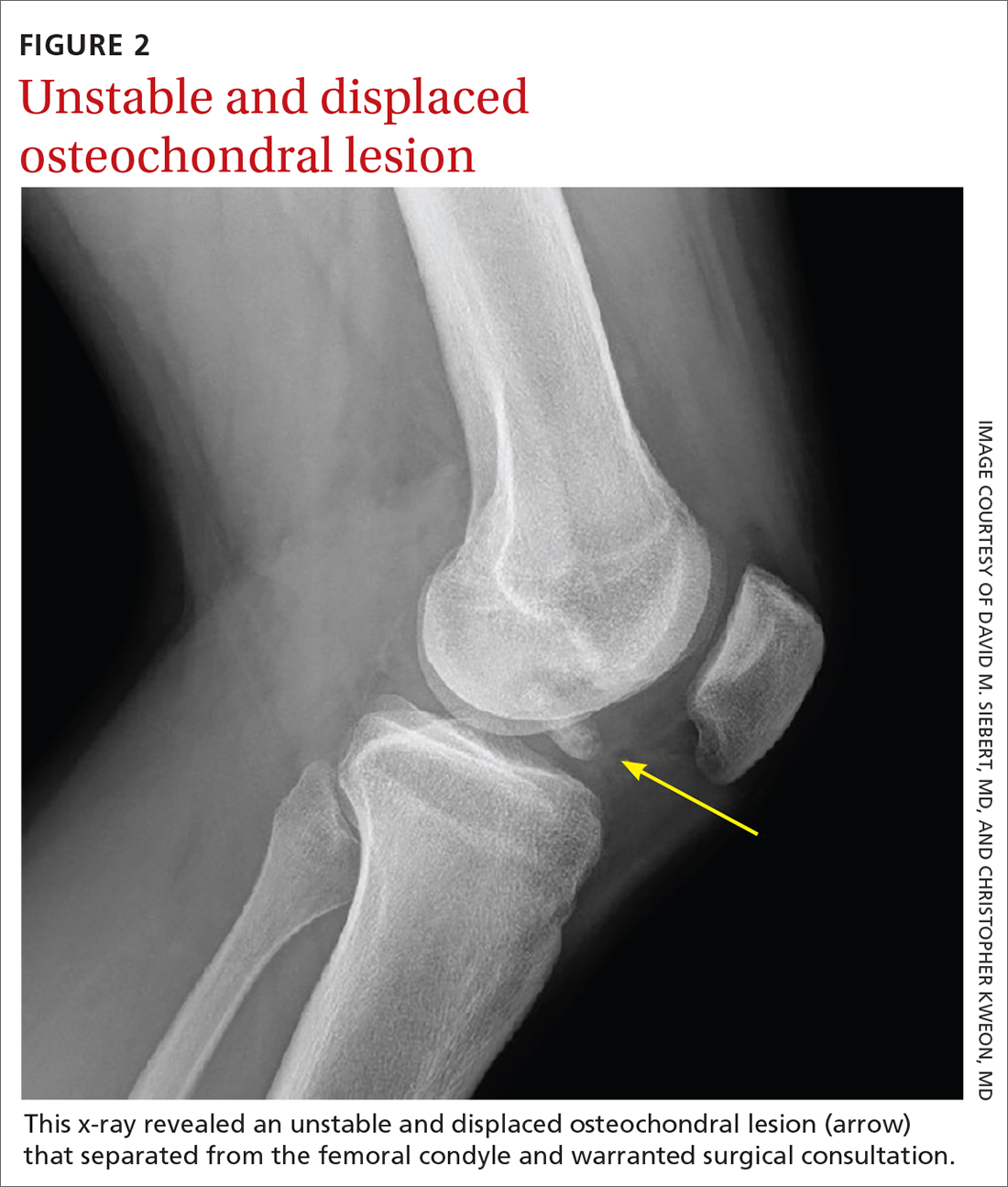
High-quality data guiding the management of osteochondritis dissecans are lacking, and these recommendations are based on consensus guidelines.34
Septic arthritis
Septic arthritis is a medical emergency caused by the hematogenous spread of microorganisms, most often staphylococci and streptococci species. Less commonly, it arises from direct inoculation through an open wound or, rarely, iatrogenically following a joint injection procedure. Clinical signs of septic arthritis include joint pain, joint swelling, and fever. Passive range of motion of the joint is often severely painful. Synovial fluid studies consistent with septic arthritis include an elevated white blood cell count greater than 25,000/mcL with polymorphonuclear cell predominance.35 The knee accounts for more than 50% of septic arthritis cases, and surgical drainage usually is required to achieve infection source control and decrease morbidity and mortality due to destruction of articular cartilage when treatment is delayed.36
Chronic knee injuries and pain
Surgical intervention for chronic knee injuries and pain generally is considered when patients demonstrate significant functional impairment and persistent symptoms despite pursuing numerous nonsurgical treatment options. A significant portion of chronic knee pain is due to degenerative processes such as OA or meniscus injuries, or tears without a history of trauma that do not cause locking of the knee. Treatments for degenerative knee pain include supervised exercise, physical therapy, bracing, offloading with a cane or other equipment, topical or oral anti-inflammatories or analgesics, and injectable therapies such as intra-articular corticosteroids.37
Continue to: Other common causes...
Other common causes of chronic knee pain include chronic tendinopathy or biomechanical syndromes such as patellofemoral pain syndrome or iliotibial band syndrome. Surgical treatment of these conditions is pursued in select cases and only after exhausting nonoperative treatment programs, as recommended by international consensus statements,38 societal guidelines,39 and expert opinion.40 High-quality data on the effectiveness, or ineffectiveness, of surgical intervention for these conditions are lacking.
Despite being one of the most commonly performed surgical procedures in the United States,41 arthroscopic partial meniscectomy treatment of degenerative meniscus tears does not lead to improved outcomes compared to nonsurgical management, according to multiple recent studies.42-45 Evidence does not support routine arthroscopic intervention for degenerative meniscus tears or OA,42 and recent guidelines recommend against it46 or to pursue it only after nonsurgical treatments have failed.37
Surgical management of degenerative knee conditions generally consists of partial or total arthroplasty and is similarly considered after failure of conservative measures. Appropriate use criteria that account for multiple clinical and patient factors are used to enhance patient selection for the procedure.47
Takeaways
Primary care clinicians will treat patients sustaining knee injuries and see many patients with knee pain in the outpatient setting. Treatment options vary considerably depending on the underlying diagnosis and resulting functional losses. Several categories of clinical presentation, including neurovascular injury, unstable or displaced fractures, joint instability, major tendon rupture, significant mechanical symptoms such as a locked knee, certain osteochondral injuries, and septic arthritis, likely or almost always warrant surgical consultation (TABLE3-10,12-36). Occasionally, as in the case of neurovascular injury or septic arthritis, such consultation should be emergent.
CORRESPONDENCE
David M. Siebert, MD, Sports Medicine Center at Husky Stadium, 3800 Montlake Boulevard NE, Seattle, WA 98195; siebert@uw.edu
1. Baker P, Reading I, Cooper C, et al. Knee disorders in the general population and their relation to occupation. Occup Environ Med. 2003;60:794-797. doi: 10.1136/oem.60.10.794
2. Nguyen UD, Zhang Y, Zhu Y, et al. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann Intern Med. 20116;155:725-732. doi: 10.7326/0003-4819-155-11-201112060-00004
3. Natsuhara KM, Yeranosian MG, Cohen JR, et al. What is the frequency of vascular injury after knee dislocation? Clin Orthop Relat Res. 2014;472:2615-2620. doi: 10.1007/s11999-014-3566-1
4. Seroyer ST, Musahl V, Harner CD. Management of the acute knee dislocation: the Pittsburgh experience. Injury. 2008;39:710-718. doi: 10.1016/j.injury.2007.11.022
5. Sinan SM, Elsoe R, Mikkelsen C, et al. Clinical, functional, and patient-reported outcome of traumatic knee dislocations: a retrospective cohort study of 75 patients with 6.5-year follow up. Arch Orthop Trauma Surg. 2023;143:2589-2597. doi: 10.1007/s00402-022-04578-z
6. Schatzker J, Kfuri M. Revisiting the management of tibial plateau fractures. Injury. 2022;53:2207-2218. doi: 10.1016/j.injury.2022.04.006
7. Rudran B, Little C, Wiik A, et al. Tibial plateau fracture: anatomy, diagnosis and management. Br J Hosp Med (Lond). 2020;81:1-9. doi: 10.12968/hmed.2020.0339
8. Tscherne H, Lobenhoffer P. Tibial plateau fractures: management and expected results. Clin Orthop Relat Res. 1993;(292):87-100.
9. Melvin JS, Mehta S. Patellar fractures in adults. J Am Acad Orthop Surg. 2011;19:198-207. doi: 10.5435/00124635-201104000-00004
10. Filho JS, Lenza M, Tamaoki MJ, et al. Interventions for treating fractures of the patella in adults. Cochrane Database Syst Rev. 2021;2:CD009651. doi: 10.1002/14651858.CD009651.pub3
11. Palmer W, Bancroft L, Bonar F, et al. Glossary of terms for musculoskeletal radiology. Skeletal Radiol. 2020;49(suppl 1):1-33. doi: 10.1007/s00256-020-03465-1
12. Frobell RB, Roos EM, Roos HP, et al. A randomized trial of treatment for acute anterior cruciate ligament tears. N Engl J Med. 2010;363:331-342. doi: 10.1056/NEJMoa0907797
13. Frobell RB, Roos HP, Roos EM, et al. Treatment for acute anterior cruciate ligament tear: five year outcome of randomized trial. Br J Sports Med. 2015;49:700. doi: 10.1136/bmj.f232
14. Diermeier TA, Rothrauff BB, Engebretsen L, et al; Panther Symposium ACL Treatment Consensus Group. Treatment after anterior cruciate ligament injury: Panther Symposium ACL Treatment Consensus Group. Br J Sports Med. 2021;55:14-22. doi: 10.1136/bjsports-2020-102200
15. Bedi A, Musahl V, Cowan JB. Management of posterior cruciate ligament injuries: an evidence-based review. J Am Acad Orthop Surg. 2016;24:277-289. doi: 10.5435/JAAOS-D-14-00326
16. Edson CJ. Conservative and postoperative rehabilitation of isolated and combined injuries of the medial collateral ligament. Sports Med Arthrosc Rev. 2006;14:105-110. doi: 10.1097/01.jsa.0000212308.32076.f2
17. Vosoughi F, Dogahe RR, Nuri A, et al. Medial collateral ligament injury of the knee: a review on current concept and management. Arch Bone Jt Surg. 2021;9:255-262. doi: 10.22038/abjs.2021.48458.2401
18. Kannus P. Nonoperative treatment of grade II and III sprains of the lateral ligament compartment of the knee. Am J Sports Med. 1989;17:83-88. doi: 10.1177/036354658901700114
19. Krukhaug Y, Mølster A, Rodt A, et al. Lateral ligament injuries of the knee. Knee Surg Sports Traumatol Arthrosc. 1998;6:21-25. doi: 10.1007/s001670050067
20. Grawe B, Schroeder AJ, Kakazu R, et al. Lateral collateral ligament injury about the knee: anatomy, evaluation, and management. J Am Acad Orthop Surg. 2018 15;26:e120-127. doi: 10.5435/JAAOS-D-16-00028
21. Ranawat A, Baker III CL, Henry S, et al. Posterolateral corner injury of the knee: evaluation and management. J Am Acad Orthop Surg. 2008;16:506-518.
22. Palmu S, Kallio PE, Donell ST, et al. Acute patellar dislocation in children and adolescents: a randomized clinical trial. J Bone Joint Surg Am. 2008;90:463-470. doi: 10.2106/JBJS.G.00072
23. Cohen D, Le N, Zakharia A, et al. MPFL reconstruction results in lower redislocation rates and higher functional outcomes than rehabilitation: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2022;30:3784-3795. doi: 10.1007/s00167-022-07003-5
24. Siwek CW, Rao JP. Ruptures of the extensor mechanism of the knee joint. J Bone Joint Surg Am. 1981;63:932-937.
25. Konrath GA, Chen D, Lock T, et al. Outcomes following repair of quadriceps tendon ruptures. J Orthop Trauma. 1998;12:273-279. doi: 10.1097/00005131-199805000-00010
26. Rasul Jr. AT, Fischer DA. Primary repair of quadriceps tendon ruptures: results of treatment. Clin Orthop Relat Res. 1993;(289):205-207.
27. Rougraff BT, Reeck CC, Essenmacher J. Complete quadriceps tendon ruptures. Orthopedics. 1996;19:509-514.
28. Bui CN, Learned JR, Scolaro JA. Treatment of patellar fractures and injuries to the extensor mechanism of the knee: a critical analysis review. JBJS Rev. 2018;6:e1. doi: 10.2106/JBJS.RVW.17.00172
29. Haskel JD, Fried JW, Hurley ET, et al. High rates of return to play and work follow knee extensor tendon ruptures but low rate of return to pre-injury level of play. Knee Surg Sports Traumatol Arthrosc. 2021;29:2695-2700. doi: 10.1007/s00167-021-06537-4
30. Critchley IJ, Bracey DJ. The acutely locked knee—is a manipulation worth while? Injury. 1985;16:281-283. doi: 10.1016/s0020-1383(85)80020-6
31. Allum RL, Jones JR. The locked knee. Injury. 1986;17:256-258. doi: 10.1016/0020-1383(86)90231-7
32. Helmark IC, Neergaard K, Krogsgaard MR. Traumatic knee extension deficit (the locked knee): can MRI reduce the need for arthroscopy? Knee Surg Sports Traumatol Arthrosc. 2007;15:863-868. doi: 10.1007/s00167-006-0244-1
33. Noyes FR, Chen RC, Barber-Westin SD, et al. Greater than 10-year results of red-white longitudinal meniscal repairs in patients 20 years of age or younger. Am J Sports Med. 2011;39:1008-1017. doi: 10.1177/0363546510392014
34. Chambers HG, Shea KG, Anderson AF, et al; American Academy of Orthopedic Surgeons. Diagnosis and treatment of osteochondritis dissecans. J Am Acad Orthop Surg. 2011;19:297-306. doi: 10.5435/00124635-201105000-00007
35. Margaretten ME, Kohlwes J, Moore D, et al. Does this adult patient have septic arthritis? JAMA. 2007;297:1478-1488. doi: 10.1001/jama.297.13.1478
36. Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology (Oxford). 2001;40:24-30. doi: 10.1093/rheumatology/40.1.24
37. Brophy RH, Fillingham YA. AAOS clinical practice guideline summary: management of osteoarthritis of the knee (nonarthroplasty), 3rd edition. J Am Acad Orthop Surg. 2022;30:e721-729. doi: 10.5435/JAAOS-D-21-01233
38. Collins NJ, Barton CJ, van Middelkoop M, et al. 2018 Consensus statement on exercise therapy and physical interventions (orthoses, taping and manual therapy) to treat patellofemoral pain: recommendations from the 5th International Patellofemoral Pain Research Retreat, Gold Coast, Australia, 2017. Br J Sports Med. 2018;52:1170-1178. doi: 10.1136/bjsports-2018-099397
39. Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19:728-736. doi: 10.5435/00124635-201112000-00003
40. Millar NL, Murrell GAC, Kirwan P. Time to put down the scalpel? The role of surgery in tendinopathy. Br J Sports Med. 2020;54:441-442. doi: 10.1136/bjsports-2019-101084
41. Hall MJ, Schwartzman A, Zhang J, et al. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017;(102):1-15.
42. Kise NJ, Risberg MA, Stensrud S, et al. Exercise therapy versus arthroscopic partial meniscectomy for degenerative meniscal tear in middle aged patients: randomized controlled trial with two year follow-up. BMJ. 2016;354:i3740. doi: 10.1136/bmj.i3740
43. Sihvonen R, Paavola M, Malmivaara A, et al, FIDELITY (Finnish Degenerative Meniscus Lesion Study) Investigators. Arthroscopic partial meniscectomy for a degenerative meniscus tear: a 5 year follow-up of the placebo-surgery controlled FIDELITY (Finnish Degenerative Meniscus Lesion Study) trial. Br J Sports Med. 2020;54:1332-1339. doi: 10.1136/bjsports-2020-102813
44. Pihl K, Ensor J, Peat G, et al. Wild goose chase—no predictable patient subgroups benefit from meniscal surgery: patient-reported outcomes of 641 patients 1 year after surgery. Br J Sports Med. 2020;54:13-22. doi: 10.1136/bjsports-2018-100321
45. O’Connor D, Johnston RV, Brignardello-Petersen R, et al. Athroscopic surgery for degenerative knee disease (osteoarthritis including degenerative meniscal tears). Cochrane Database Syst Rev. 2022;3:CD014328. doi: 10.1002/14651858.CD014328
46. Siemieniuk RAC, Harris IA, Agoritsas T, et al. Arthroscopic surgery for degenerative knee arthritis and meniscal tears: a clinical practice guideline. Br J Sports Med. 2018;52:313. doi: 10.1136/bjsports-2017-j1982rep
47. Manner PA, Tubb CC, Levine BR. AAOS appropriate use criteria: surgical management of osteoarthritis of the knee. J Am Acad Orthop Surg. 2018;26:e194-197. doi: 10.5435/JAAOS-D-17-00425
Evidence supports what family physicians know to be true: Knee pain is an exceedingly common presenting problem in the primary care office. Estimates of lifetime incidence reach as high as 54%,1 and the prevalence of knee pain in the general population is increasing.2 Knee disability can result from acute or traumatic injuries as well as chronic, degenerative conditions such as osteoarthritis (OA). The decision to pursue orthopedic consultation for a particular injury or painful knee condition can be challenging. To address this, we highlight specific knee diagnoses known to cause pain, with the aim of describing which conditions likely will necessitate surgical consultation—and which won’t.
Acute or nondegenerative knee injuries and pain
Acute knee injuries range in severity from simple contusions and sprains to high-energy, traumatic injuries with resulting joint instability and potential neurovascular compromise. While conservative treatment often is successful for many simple injuries, surgical management—sometimes urgently or emergently—is needed in other cases, as will be detailed shortly.
Neurovascular injury associated with knee dislocations
Acute neurovascular injuries often require emergent surgical intervention. Although rare, tibiofemoral (knee) dislocations pose a significant challenge to the clinician in both diagnosis and management. The reported frequency of popliteal artery injury or rupture following a dislocation varies widely, with rates ranging from 5% to 64%, according to older studies; more recent data, however, suggest the rate is actually as low as 3.3%.3
Immediate immobilization and emergency department transport for monitoring, orthopedics consultation, and vascular studies or vascular surgery consultation is recommended in the case of a suspected knee dislocation. In one cross-sectional cohort study, the surgical management of knee dislocations yielded favorable outcomes in > 75% of cases.5
Tibial plateau fracture
This fracture often occurs as a result of high-energy trauma, such as contact sports or motor vehicle accidents, and is characterized by a proximal tibial fracture line with extension to the articular surface. X-rays often are sufficient for initial diagnosis. Computed tomography can help rule out a fracture line when clinical suspicion is high and x-rays are nondiagnostic. As noted earlier, any suggestion of neurovascular compromise on physical exam requires an emergent orthopedic surgeon consultation for a possible displaced and unstable (or more complex) injury (FIGURE 1).6-8

Nondisplaced tibial plateau fractures without supraphysiologic ligamentous laxity on valgus or varus stress testing can be managed safely with protection and early mobilization, gradual progression of weight-bearing, and serial x-rays to ensure fracture healing and stability.
Gross joint instability identified by positive valgus or varus stress testing, positive anterior or posterior drawer testing, or patient inability to tolerate these maneuvers due to pain similarly should raise suspicion for a more significant fracture at risk for concurrent neurovascular injury. Acute compartment syndrome also is a known complication of tibial plateau fractures and similarly requires emergent operative management. Urgent surgical consultation is recommended for fractures with displaced fracture fragments, tibial articular surface step-off or depression, fractures with concurrent joint laxity, or medial plateau fractures.6-8
Continue to: Patella fractures
Patella fractures
These fractures occur as a direct blow to the front of the knee, such as falling forward onto a hard surface, or indirectly due to a sudden extreme eccentric contraction of the quadriceps muscle. Nondisplaced fractures with an intact knee extension mechanism, which is examined via a supine straight-leg raise or seated knee extension, are managed with weight-bearing as tolerated in strict immobilization in full extension for 4 to 6 weeks, with active range-of-motion and isometric quadriceps exercises beginning in 1 to 2 weeks. Serial x-rays also are obtained to ensure fracture displacement does not occur during the rehabilitation process.9
High-quality evidence guiding follow-up care and comparing outcomes of surgical and nonsurgical management of patella fractures is lacking, and studies comparing different surgical techniques are of lower methodological quality.10 Nevertheless, displaced or comminuted patellar fractures are referred urgently to orthopedic surgical care for fixation, as are those with concurrent loose bodies, chondral surface injuries or articular step-off, or osteochondral fractures.9 Inability to perform a straight-leg raise (ie, clinical loss of the knee extension mechanism) suggests a fracture under tension that likely also requires surgical fixation for successful recovery. Neurovascular injuries are unlikely in most patellar fractures but would require emergent surgical consultation.9
Ligamentous injury
Tibiofemoral joint laxity occurs as a result of ligamentous injury, with or without tibial plateau fracture. The anterior cruciate ligament (ACL), posterior cruciate ligament (PCL), medial collateral ligament (MCL), and lateral collateral ligament (LCL) comprise the 4 main ligaments of the knee. The ACL resists anterior tibial translation and rotational forces, while the PCL resists posterior tibial translation. The MCL and LCL resist valgus and varus stress, respectively.
Ligament injuries are classified as Grades 1 to 311:
- Grade 1 sprains. The ligament is stretched, but there is no macroscopic tearing; joint stability is maintained.
- Grade 2 sprains. There are partial macroscopic ligament tears. There is joint laxity due to the partial loss of the ligament’s structural integrity.
- Grade 3 sprains. The ligament is fully avulsed or ruptured with resultant gross joint instability.
Continue to: ACL tears
ACL tears occur most commonly via a noncontact event, as when an individual plants their foot and suddenly changes direction during sport or other physical activity. Treatment hinges on patient activity levels and participation in sports. Patients who do not plan to engage in athletic movements (that require changes in direction or planting and twisting) and who otherwise maintain satisfactory joint stability during activities of daily living may elect to defer or even altogether avoid surgical reconstruction of isolated ACL tears. One pair of studies demonstrated equivalent outcomes in surgical and nonsurgical management in 121 young, nonelite athletes at 2- and 5-year follow-up, although the crossover from the nonsurgical to surgical groups was high.12,13 Athletes who regain satisfactory function and stability nonoperatively can defer surgical intervention. However, the majority of active patients and athletes will require surgical ACL reconstruction to return to pre-injury functional levels.14
PCL sprains occur as a result of sudden posteriorly directed force on the tibia, such as when the knee is hyperextended or a patient falls directly onto a flexed knee. Patients with Grade 1 and 2 isolated sprains generally will recover with conservative care, as will patients with some Grade 3 complete tears that do not fully compromise joint stability. However, high-grade PCL injuries often are comorbid with posterolateral corner or other injuries, leading to a higher likelihood of joint instability and thus the need for surgical intervention for the best chance at an optimal outcome.15
MCL sprain. Surgical management is not required in an isolated Grade 1 or 2 MCL sprain, as the hallmarks of recovery—return of joint stability, knee strength and range of motion, and pain reduction—can be achieved successfully with conservative management. Isolated Grade 3 MCL sprains are also successfully managed nonoperatively16 except in specific cases, such as a concurrent large avulsion fracture.17
LCL sprain. Similarly, isolated Grade 1 and 2 LCL sprains generally do not require surgical intervention. However, Grade 3 LCL injuries usually do, as persistent joint instability and poor functional outcomes are more common with nonsurgical management.18-20 Additionally, high-grade LCL injuries frequently manifest with comorbid meniscus injuries or sprains of the posterolateral corner of the knee, a complex anatomic structure that provides both static and dynamic tibiofemoral joint stability. Surgical repair or reconstruction of the posterolateral corner frequently is necessary for optimal functional outcomes.21
Multiligamentous sprains frequently lead to gross joint instability and necessitate orthopedic surgeon consultation to determine the best treatment plan; this should be done emergently if neurovascular compromise is suspected. A common injury combination is simultaneous ACL and MCL sprains with or without meniscus injury. In these cases, some surgeons will choose to defer ACL reconstruction until after MCL healing is achieved. This allows the patient to regain valgus stability of the joint prior to performing ACL reconstruction to regain rotational and anterior stability.20
Continue to: Patellar dislocations
Patellar dislocations represent a relatively common knee injury in young active patients, often occurring in a noncontact fashion when a valgus force is applied to an externally rotated and planted lower leg.
Major tendon rupture
Patellar tendon ruptures occur when a sudden eccentric force is applied to the knee, such as when landing from a jump with the knee flexed. Patellar tendon ruptures frequently are clinically apparent, with patients demonstrating a high-riding patella and loss of active knee extension. Quadriceps tendon ruptures often result from a similar injury mechanism in older patients, with a similar loss of active knee extension and a palpable gap superior to the patella.24
Partial tears in patients who can maintain full extension of the knee against gravity are treated nonoperatively, but early surgical repair is indicated for complete quadriceps or patellar tendon ruptures to achieve optimal outcomes.
Even with prompt treatment, return to sport is not guaranteed. According to a recent systematic review, athletes returned to play 88.9% and 89.8% of the time following patellar and quadriceps tendon repairs, respectively. However, returning to the same level of play was less common and achieved 80.8% (patellar tendon repair) and 70% (quadriceps tendon repair) of the time. Return-to-work rates were higher, at 96% for both surgical treatments.29
Locked knee and acute meniscus tears in younger patients
In some acute knee injuries, meniscus tears, loose cartilage bodies or osteochondral defects, or other internal structures can become interposed between the femoral and tibial surfaces, preventing both active and passive knee extension. Such injuries are often severely painful and functionally debilitating. While manipulation under anesthesia can acutely restore joint function,30 diagnostic and therapeutic arthroscopy often is pursued for definitive treatment.31 Compared to the gold standard of diagnostic arthroscopy, preoperative magnetic resonance imaging (MRI) carries positive and negative predictive values of 85% and 77%, respectively, in identifying or ruling out the anatomic structure responsible for a locked knee. 32 As such,
Continue to: Depending on the location...
Depending on the location, size, and shape of an acute meniscus tear in younger patients, surgical repair may be an option to preserve long-term joint function. In one case series of patients younger than 20 years, 62% of meniscus repairs yielded good outcomes after a mean follow-up period of 16.8 years.33
Osteochondritis dissecans
Osteochondritis dissecans is characterized by subchondral bone osteonecrosis that most often occurs in pediatric patients, potentially causing the separation of a fragment of articular cartilage and subchondral bone into the joint space (FIGURE 2). In early stages, nonoperative treatment consisting of prolonged rest followed by physical therapy to gradually return to activity is recommended to prevent small, low-grade lesions from progressing to unstable or separated fragments. Arthroscopy, which consists of microfracture or other surgical resurfacing techniques to restore joint integrity, is pursued in more advanced cases of unstable or separated fragments.

High-quality data guiding the management of osteochondritis dissecans are lacking, and these recommendations are based on consensus guidelines.34
Septic arthritis
Septic arthritis is a medical emergency caused by the hematogenous spread of microorganisms, most often staphylococci and streptococci species. Less commonly, it arises from direct inoculation through an open wound or, rarely, iatrogenically following a joint injection procedure. Clinical signs of septic arthritis include joint pain, joint swelling, and fever. Passive range of motion of the joint is often severely painful. Synovial fluid studies consistent with septic arthritis include an elevated white blood cell count greater than 25,000/mcL with polymorphonuclear cell predominance.35 The knee accounts for more than 50% of septic arthritis cases, and surgical drainage usually is required to achieve infection source control and decrease morbidity and mortality due to destruction of articular cartilage when treatment is delayed.36
Chronic knee injuries and pain
Surgical intervention for chronic knee injuries and pain generally is considered when patients demonstrate significant functional impairment and persistent symptoms despite pursuing numerous nonsurgical treatment options. A significant portion of chronic knee pain is due to degenerative processes such as OA or meniscus injuries, or tears without a history of trauma that do not cause locking of the knee. Treatments for degenerative knee pain include supervised exercise, physical therapy, bracing, offloading with a cane or other equipment, topical or oral anti-inflammatories or analgesics, and injectable therapies such as intra-articular corticosteroids.37
Continue to: Other common causes...
Other common causes of chronic knee pain include chronic tendinopathy or biomechanical syndromes such as patellofemoral pain syndrome or iliotibial band syndrome. Surgical treatment of these conditions is pursued in select cases and only after exhausting nonoperative treatment programs, as recommended by international consensus statements,38 societal guidelines,39 and expert opinion.40 High-quality data on the effectiveness, or ineffectiveness, of surgical intervention for these conditions are lacking.
Despite being one of the most commonly performed surgical procedures in the United States,41 arthroscopic partial meniscectomy treatment of degenerative meniscus tears does not lead to improved outcomes compared to nonsurgical management, according to multiple recent studies.42-45 Evidence does not support routine arthroscopic intervention for degenerative meniscus tears or OA,42 and recent guidelines recommend against it46 or to pursue it only after nonsurgical treatments have failed.37
Surgical management of degenerative knee conditions generally consists of partial or total arthroplasty and is similarly considered after failure of conservative measures. Appropriate use criteria that account for multiple clinical and patient factors are used to enhance patient selection for the procedure.47
Takeaways
Primary care clinicians will treat patients sustaining knee injuries and see many patients with knee pain in the outpatient setting. Treatment options vary considerably depending on the underlying diagnosis and resulting functional losses. Several categories of clinical presentation, including neurovascular injury, unstable or displaced fractures, joint instability, major tendon rupture, significant mechanical symptoms such as a locked knee, certain osteochondral injuries, and septic arthritis, likely or almost always warrant surgical consultation (TABLE3-10,12-36). Occasionally, as in the case of neurovascular injury or septic arthritis, such consultation should be emergent.

CORRESPONDENCE
David M. Siebert, MD, Sports Medicine Center at Husky Stadium, 3800 Montlake Boulevard NE, Seattle, WA 98195; siebert@uw.edu
Evidence supports what family physicians know to be true: Knee pain is an exceedingly common presenting problem in the primary care office. Estimates of lifetime incidence reach as high as 54%,1 and the prevalence of knee pain in the general population is increasing.2 Knee disability can result from acute or traumatic injuries as well as chronic, degenerative conditions such as osteoarthritis (OA). The decision to pursue orthopedic consultation for a particular injury or painful knee condition can be challenging. To address this, we highlight specific knee diagnoses known to cause pain, with the aim of describing which conditions likely will necessitate surgical consultation—and which won’t.
Acute or nondegenerative knee injuries and pain
Acute knee injuries range in severity from simple contusions and sprains to high-energy, traumatic injuries with resulting joint instability and potential neurovascular compromise. While conservative treatment often is successful for many simple injuries, surgical management—sometimes urgently or emergently—is needed in other cases, as will be detailed shortly.
Neurovascular injury associated with knee dislocations
Acute neurovascular injuries often require emergent surgical intervention. Although rare, tibiofemoral (knee) dislocations pose a significant challenge to the clinician in both diagnosis and management. The reported frequency of popliteal artery injury or rupture following a dislocation varies widely, with rates ranging from 5% to 64%, according to older studies; more recent data, however, suggest the rate is actually as low as 3.3%.3
Immediate immobilization and emergency department transport for monitoring, orthopedics consultation, and vascular studies or vascular surgery consultation is recommended in the case of a suspected knee dislocation. In one cross-sectional cohort study, the surgical management of knee dislocations yielded favorable outcomes in > 75% of cases.5
Tibial plateau fracture
This fracture often occurs as a result of high-energy trauma, such as contact sports or motor vehicle accidents, and is characterized by a proximal tibial fracture line with extension to the articular surface. X-rays often are sufficient for initial diagnosis. Computed tomography can help rule out a fracture line when clinical suspicion is high and x-rays are nondiagnostic. As noted earlier, any suggestion of neurovascular compromise on physical exam requires an emergent orthopedic surgeon consultation for a possible displaced and unstable (or more complex) injury (FIGURE 1).6-8

Nondisplaced tibial plateau fractures without supraphysiologic ligamentous laxity on valgus or varus stress testing can be managed safely with protection and early mobilization, gradual progression of weight-bearing, and serial x-rays to ensure fracture healing and stability.
Gross joint instability identified by positive valgus or varus stress testing, positive anterior or posterior drawer testing, or patient inability to tolerate these maneuvers due to pain similarly should raise suspicion for a more significant fracture at risk for concurrent neurovascular injury. Acute compartment syndrome also is a known complication of tibial plateau fractures and similarly requires emergent operative management. Urgent surgical consultation is recommended for fractures with displaced fracture fragments, tibial articular surface step-off or depression, fractures with concurrent joint laxity, or medial plateau fractures.6-8
Continue to: Patella fractures
Patella fractures
These fractures occur as a direct blow to the front of the knee, such as falling forward onto a hard surface, or indirectly due to a sudden extreme eccentric contraction of the quadriceps muscle. Nondisplaced fractures with an intact knee extension mechanism, which is examined via a supine straight-leg raise or seated knee extension, are managed with weight-bearing as tolerated in strict immobilization in full extension for 4 to 6 weeks, with active range-of-motion and isometric quadriceps exercises beginning in 1 to 2 weeks. Serial x-rays also are obtained to ensure fracture displacement does not occur during the rehabilitation process.9
High-quality evidence guiding follow-up care and comparing outcomes of surgical and nonsurgical management of patella fractures is lacking, and studies comparing different surgical techniques are of lower methodological quality.10 Nevertheless, displaced or comminuted patellar fractures are referred urgently to orthopedic surgical care for fixation, as are those with concurrent loose bodies, chondral surface injuries or articular step-off, or osteochondral fractures.9 Inability to perform a straight-leg raise (ie, clinical loss of the knee extension mechanism) suggests a fracture under tension that likely also requires surgical fixation for successful recovery. Neurovascular injuries are unlikely in most patellar fractures but would require emergent surgical consultation.9
Ligamentous injury
Tibiofemoral joint laxity occurs as a result of ligamentous injury, with or without tibial plateau fracture. The anterior cruciate ligament (ACL), posterior cruciate ligament (PCL), medial collateral ligament (MCL), and lateral collateral ligament (LCL) comprise the 4 main ligaments of the knee. The ACL resists anterior tibial translation and rotational forces, while the PCL resists posterior tibial translation. The MCL and LCL resist valgus and varus stress, respectively.
Ligament injuries are classified as Grades 1 to 311:
- Grade 1 sprains. The ligament is stretched, but there is no macroscopic tearing; joint stability is maintained.
- Grade 2 sprains. There are partial macroscopic ligament tears. There is joint laxity due to the partial loss of the ligament’s structural integrity.
- Grade 3 sprains. The ligament is fully avulsed or ruptured with resultant gross joint instability.
Continue to: ACL tears
ACL tears occur most commonly via a noncontact event, as when an individual plants their foot and suddenly changes direction during sport or other physical activity. Treatment hinges on patient activity levels and participation in sports. Patients who do not plan to engage in athletic movements (that require changes in direction or planting and twisting) and who otherwise maintain satisfactory joint stability during activities of daily living may elect to defer or even altogether avoid surgical reconstruction of isolated ACL tears. One pair of studies demonstrated equivalent outcomes in surgical and nonsurgical management in 121 young, nonelite athletes at 2- and 5-year follow-up, although the crossover from the nonsurgical to surgical groups was high.12,13 Athletes who regain satisfactory function and stability nonoperatively can defer surgical intervention. However, the majority of active patients and athletes will require surgical ACL reconstruction to return to pre-injury functional levels.14
PCL sprains occur as a result of sudden posteriorly directed force on the tibia, such as when the knee is hyperextended or a patient falls directly onto a flexed knee. Patients with Grade 1 and 2 isolated sprains generally will recover with conservative care, as will patients with some Grade 3 complete tears that do not fully compromise joint stability. However, high-grade PCL injuries often are comorbid with posterolateral corner or other injuries, leading to a higher likelihood of joint instability and thus the need for surgical intervention for the best chance at an optimal outcome.15
MCL sprain. Surgical management is not required in an isolated Grade 1 or 2 MCL sprain, as the hallmarks of recovery—return of joint stability, knee strength and range of motion, and pain reduction—can be achieved successfully with conservative management. Isolated Grade 3 MCL sprains are also successfully managed nonoperatively16 except in specific cases, such as a concurrent large avulsion fracture.17
LCL sprain. Similarly, isolated Grade 1 and 2 LCL sprains generally do not require surgical intervention. However, Grade 3 LCL injuries usually do, as persistent joint instability and poor functional outcomes are more common with nonsurgical management.18-20 Additionally, high-grade LCL injuries frequently manifest with comorbid meniscus injuries or sprains of the posterolateral corner of the knee, a complex anatomic structure that provides both static and dynamic tibiofemoral joint stability. Surgical repair or reconstruction of the posterolateral corner frequently is necessary for optimal functional outcomes.21
Multiligamentous sprains frequently lead to gross joint instability and necessitate orthopedic surgeon consultation to determine the best treatment plan; this should be done emergently if neurovascular compromise is suspected. A common injury combination is simultaneous ACL and MCL sprains with or without meniscus injury. In these cases, some surgeons will choose to defer ACL reconstruction until after MCL healing is achieved. This allows the patient to regain valgus stability of the joint prior to performing ACL reconstruction to regain rotational and anterior stability.20
Continue to: Patellar dislocations
Patellar dislocations represent a relatively common knee injury in young active patients, often occurring in a noncontact fashion when a valgus force is applied to an externally rotated and planted lower leg.
Major tendon rupture
Patellar tendon ruptures occur when a sudden eccentric force is applied to the knee, such as when landing from a jump with the knee flexed. Patellar tendon ruptures frequently are clinically apparent, with patients demonstrating a high-riding patella and loss of active knee extension. Quadriceps tendon ruptures often result from a similar injury mechanism in older patients, with a similar loss of active knee extension and a palpable gap superior to the patella.24
Partial tears in patients who can maintain full extension of the knee against gravity are treated nonoperatively, but early surgical repair is indicated for complete quadriceps or patellar tendon ruptures to achieve optimal outcomes.
Even with prompt treatment, return to sport is not guaranteed. According to a recent systematic review, athletes returned to play 88.9% and 89.8% of the time following patellar and quadriceps tendon repairs, respectively. However, returning to the same level of play was less common and achieved 80.8% (patellar tendon repair) and 70% (quadriceps tendon repair) of the time. Return-to-work rates were higher, at 96% for both surgical treatments.29
Locked knee and acute meniscus tears in younger patients
In some acute knee injuries, meniscus tears, loose cartilage bodies or osteochondral defects, or other internal structures can become interposed between the femoral and tibial surfaces, preventing both active and passive knee extension. Such injuries are often severely painful and functionally debilitating. While manipulation under anesthesia can acutely restore joint function,30 diagnostic and therapeutic arthroscopy often is pursued for definitive treatment.31 Compared to the gold standard of diagnostic arthroscopy, preoperative magnetic resonance imaging (MRI) carries positive and negative predictive values of 85% and 77%, respectively, in identifying or ruling out the anatomic structure responsible for a locked knee. 32 As such,
Continue to: Depending on the location...
Depending on the location, size, and shape of an acute meniscus tear in younger patients, surgical repair may be an option to preserve long-term joint function. In one case series of patients younger than 20 years, 62% of meniscus repairs yielded good outcomes after a mean follow-up period of 16.8 years.33
Osteochondritis dissecans
Osteochondritis dissecans is characterized by subchondral bone osteonecrosis that most often occurs in pediatric patients, potentially causing the separation of a fragment of articular cartilage and subchondral bone into the joint space (FIGURE 2). In early stages, nonoperative treatment consisting of prolonged rest followed by physical therapy to gradually return to activity is recommended to prevent small, low-grade lesions from progressing to unstable or separated fragments. Arthroscopy, which consists of microfracture or other surgical resurfacing techniques to restore joint integrity, is pursued in more advanced cases of unstable or separated fragments.

High-quality data guiding the management of osteochondritis dissecans are lacking, and these recommendations are based on consensus guidelines.34
Septic arthritis
Septic arthritis is a medical emergency caused by the hematogenous spread of microorganisms, most often staphylococci and streptococci species. Less commonly, it arises from direct inoculation through an open wound or, rarely, iatrogenically following a joint injection procedure. Clinical signs of septic arthritis include joint pain, joint swelling, and fever. Passive range of motion of the joint is often severely painful. Synovial fluid studies consistent with septic arthritis include an elevated white blood cell count greater than 25,000/mcL with polymorphonuclear cell predominance.35 The knee accounts for more than 50% of septic arthritis cases, and surgical drainage usually is required to achieve infection source control and decrease morbidity and mortality due to destruction of articular cartilage when treatment is delayed.36
Chronic knee injuries and pain
Surgical intervention for chronic knee injuries and pain generally is considered when patients demonstrate significant functional impairment and persistent symptoms despite pursuing numerous nonsurgical treatment options. A significant portion of chronic knee pain is due to degenerative processes such as OA or meniscus injuries, or tears without a history of trauma that do not cause locking of the knee. Treatments for degenerative knee pain include supervised exercise, physical therapy, bracing, offloading with a cane or other equipment, topical or oral anti-inflammatories or analgesics, and injectable therapies such as intra-articular corticosteroids.37
Continue to: Other common causes...
Other common causes of chronic knee pain include chronic tendinopathy or biomechanical syndromes such as patellofemoral pain syndrome or iliotibial band syndrome. Surgical treatment of these conditions is pursued in select cases and only after exhausting nonoperative treatment programs, as recommended by international consensus statements,38 societal guidelines,39 and expert opinion.40 High-quality data on the effectiveness, or ineffectiveness, of surgical intervention for these conditions are lacking.
Despite being one of the most commonly performed surgical procedures in the United States,41 arthroscopic partial meniscectomy treatment of degenerative meniscus tears does not lead to improved outcomes compared to nonsurgical management, according to multiple recent studies.42-45 Evidence does not support routine arthroscopic intervention for degenerative meniscus tears or OA,42 and recent guidelines recommend against it46 or to pursue it only after nonsurgical treatments have failed.37
Surgical management of degenerative knee conditions generally consists of partial or total arthroplasty and is similarly considered after failure of conservative measures. Appropriate use criteria that account for multiple clinical and patient factors are used to enhance patient selection for the procedure.47
Takeaways
Primary care clinicians will treat patients sustaining knee injuries and see many patients with knee pain in the outpatient setting. Treatment options vary considerably depending on the underlying diagnosis and resulting functional losses. Several categories of clinical presentation, including neurovascular injury, unstable or displaced fractures, joint instability, major tendon rupture, significant mechanical symptoms such as a locked knee, certain osteochondral injuries, and septic arthritis, likely or almost always warrant surgical consultation (TABLE3-10,12-36). Occasionally, as in the case of neurovascular injury or septic arthritis, such consultation should be emergent.

CORRESPONDENCE
David M. Siebert, MD, Sports Medicine Center at Husky Stadium, 3800 Montlake Boulevard NE, Seattle, WA 98195; siebert@uw.edu
1. Baker P, Reading I, Cooper C, et al. Knee disorders in the general population and their relation to occupation. Occup Environ Med. 2003;60:794-797. doi: 10.1136/oem.60.10.794
2. Nguyen UD, Zhang Y, Zhu Y, et al. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann Intern Med. 20116;155:725-732. doi: 10.7326/0003-4819-155-11-201112060-00004
3. Natsuhara KM, Yeranosian MG, Cohen JR, et al. What is the frequency of vascular injury after knee dislocation? Clin Orthop Relat Res. 2014;472:2615-2620. doi: 10.1007/s11999-014-3566-1
4. Seroyer ST, Musahl V, Harner CD. Management of the acute knee dislocation: the Pittsburgh experience. Injury. 2008;39:710-718. doi: 10.1016/j.injury.2007.11.022
5. Sinan SM, Elsoe R, Mikkelsen C, et al. Clinical, functional, and patient-reported outcome of traumatic knee dislocations: a retrospective cohort study of 75 patients with 6.5-year follow up. Arch Orthop Trauma Surg. 2023;143:2589-2597. doi: 10.1007/s00402-022-04578-z
6. Schatzker J, Kfuri M. Revisiting the management of tibial plateau fractures. Injury. 2022;53:2207-2218. doi: 10.1016/j.injury.2022.04.006
7. Rudran B, Little C, Wiik A, et al. Tibial plateau fracture: anatomy, diagnosis and management. Br J Hosp Med (Lond). 2020;81:1-9. doi: 10.12968/hmed.2020.0339
8. Tscherne H, Lobenhoffer P. Tibial plateau fractures: management and expected results. Clin Orthop Relat Res. 1993;(292):87-100.
9. Melvin JS, Mehta S. Patellar fractures in adults. J Am Acad Orthop Surg. 2011;19:198-207. doi: 10.5435/00124635-201104000-00004
10. Filho JS, Lenza M, Tamaoki MJ, et al. Interventions for treating fractures of the patella in adults. Cochrane Database Syst Rev. 2021;2:CD009651. doi: 10.1002/14651858.CD009651.pub3
11. Palmer W, Bancroft L, Bonar F, et al. Glossary of terms for musculoskeletal radiology. Skeletal Radiol. 2020;49(suppl 1):1-33. doi: 10.1007/s00256-020-03465-1
12. Frobell RB, Roos EM, Roos HP, et al. A randomized trial of treatment for acute anterior cruciate ligament tears. N Engl J Med. 2010;363:331-342. doi: 10.1056/NEJMoa0907797
13. Frobell RB, Roos HP, Roos EM, et al. Treatment for acute anterior cruciate ligament tear: five year outcome of randomized trial. Br J Sports Med. 2015;49:700. doi: 10.1136/bmj.f232
14. Diermeier TA, Rothrauff BB, Engebretsen L, et al; Panther Symposium ACL Treatment Consensus Group. Treatment after anterior cruciate ligament injury: Panther Symposium ACL Treatment Consensus Group. Br J Sports Med. 2021;55:14-22. doi: 10.1136/bjsports-2020-102200
15. Bedi A, Musahl V, Cowan JB. Management of posterior cruciate ligament injuries: an evidence-based review. J Am Acad Orthop Surg. 2016;24:277-289. doi: 10.5435/JAAOS-D-14-00326
16. Edson CJ. Conservative and postoperative rehabilitation of isolated and combined injuries of the medial collateral ligament. Sports Med Arthrosc Rev. 2006;14:105-110. doi: 10.1097/01.jsa.0000212308.32076.f2
17. Vosoughi F, Dogahe RR, Nuri A, et al. Medial collateral ligament injury of the knee: a review on current concept and management. Arch Bone Jt Surg. 2021;9:255-262. doi: 10.22038/abjs.2021.48458.2401
18. Kannus P. Nonoperative treatment of grade II and III sprains of the lateral ligament compartment of the knee. Am J Sports Med. 1989;17:83-88. doi: 10.1177/036354658901700114
19. Krukhaug Y, Mølster A, Rodt A, et al. Lateral ligament injuries of the knee. Knee Surg Sports Traumatol Arthrosc. 1998;6:21-25. doi: 10.1007/s001670050067
20. Grawe B, Schroeder AJ, Kakazu R, et al. Lateral collateral ligament injury about the knee: anatomy, evaluation, and management. J Am Acad Orthop Surg. 2018 15;26:e120-127. doi: 10.5435/JAAOS-D-16-00028
21. Ranawat A, Baker III CL, Henry S, et al. Posterolateral corner injury of the knee: evaluation and management. J Am Acad Orthop Surg. 2008;16:506-518.
22. Palmu S, Kallio PE, Donell ST, et al. Acute patellar dislocation in children and adolescents: a randomized clinical trial. J Bone Joint Surg Am. 2008;90:463-470. doi: 10.2106/JBJS.G.00072
23. Cohen D, Le N, Zakharia A, et al. MPFL reconstruction results in lower redislocation rates and higher functional outcomes than rehabilitation: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2022;30:3784-3795. doi: 10.1007/s00167-022-07003-5
24. Siwek CW, Rao JP. Ruptures of the extensor mechanism of the knee joint. J Bone Joint Surg Am. 1981;63:932-937.
25. Konrath GA, Chen D, Lock T, et al. Outcomes following repair of quadriceps tendon ruptures. J Orthop Trauma. 1998;12:273-279. doi: 10.1097/00005131-199805000-00010
26. Rasul Jr. AT, Fischer DA. Primary repair of quadriceps tendon ruptures: results of treatment. Clin Orthop Relat Res. 1993;(289):205-207.
27. Rougraff BT, Reeck CC, Essenmacher J. Complete quadriceps tendon ruptures. Orthopedics. 1996;19:509-514.
28. Bui CN, Learned JR, Scolaro JA. Treatment of patellar fractures and injuries to the extensor mechanism of the knee: a critical analysis review. JBJS Rev. 2018;6:e1. doi: 10.2106/JBJS.RVW.17.00172
29. Haskel JD, Fried JW, Hurley ET, et al. High rates of return to play and work follow knee extensor tendon ruptures but low rate of return to pre-injury level of play. Knee Surg Sports Traumatol Arthrosc. 2021;29:2695-2700. doi: 10.1007/s00167-021-06537-4
30. Critchley IJ, Bracey DJ. The acutely locked knee—is a manipulation worth while? Injury. 1985;16:281-283. doi: 10.1016/s0020-1383(85)80020-6
31. Allum RL, Jones JR. The locked knee. Injury. 1986;17:256-258. doi: 10.1016/0020-1383(86)90231-7
32. Helmark IC, Neergaard K, Krogsgaard MR. Traumatic knee extension deficit (the locked knee): can MRI reduce the need for arthroscopy? Knee Surg Sports Traumatol Arthrosc. 2007;15:863-868. doi: 10.1007/s00167-006-0244-1
33. Noyes FR, Chen RC, Barber-Westin SD, et al. Greater than 10-year results of red-white longitudinal meniscal repairs in patients 20 years of age or younger. Am J Sports Med. 2011;39:1008-1017. doi: 10.1177/0363546510392014
34. Chambers HG, Shea KG, Anderson AF, et al; American Academy of Orthopedic Surgeons. Diagnosis and treatment of osteochondritis dissecans. J Am Acad Orthop Surg. 2011;19:297-306. doi: 10.5435/00124635-201105000-00007
35. Margaretten ME, Kohlwes J, Moore D, et al. Does this adult patient have septic arthritis? JAMA. 2007;297:1478-1488. doi: 10.1001/jama.297.13.1478
36. Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology (Oxford). 2001;40:24-30. doi: 10.1093/rheumatology/40.1.24
37. Brophy RH, Fillingham YA. AAOS clinical practice guideline summary: management of osteoarthritis of the knee (nonarthroplasty), 3rd edition. J Am Acad Orthop Surg. 2022;30:e721-729. doi: 10.5435/JAAOS-D-21-01233
38. Collins NJ, Barton CJ, van Middelkoop M, et al. 2018 Consensus statement on exercise therapy and physical interventions (orthoses, taping and manual therapy) to treat patellofemoral pain: recommendations from the 5th International Patellofemoral Pain Research Retreat, Gold Coast, Australia, 2017. Br J Sports Med. 2018;52:1170-1178. doi: 10.1136/bjsports-2018-099397
39. Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19:728-736. doi: 10.5435/00124635-201112000-00003
40. Millar NL, Murrell GAC, Kirwan P. Time to put down the scalpel? The role of surgery in tendinopathy. Br J Sports Med. 2020;54:441-442. doi: 10.1136/bjsports-2019-101084
41. Hall MJ, Schwartzman A, Zhang J, et al. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017;(102):1-15.
42. Kise NJ, Risberg MA, Stensrud S, et al. Exercise therapy versus arthroscopic partial meniscectomy for degenerative meniscal tear in middle aged patients: randomized controlled trial with two year follow-up. BMJ. 2016;354:i3740. doi: 10.1136/bmj.i3740
43. Sihvonen R, Paavola M, Malmivaara A, et al, FIDELITY (Finnish Degenerative Meniscus Lesion Study) Investigators. Arthroscopic partial meniscectomy for a degenerative meniscus tear: a 5 year follow-up of the placebo-surgery controlled FIDELITY (Finnish Degenerative Meniscus Lesion Study) trial. Br J Sports Med. 2020;54:1332-1339. doi: 10.1136/bjsports-2020-102813
44. Pihl K, Ensor J, Peat G, et al. Wild goose chase—no predictable patient subgroups benefit from meniscal surgery: patient-reported outcomes of 641 patients 1 year after surgery. Br J Sports Med. 2020;54:13-22. doi: 10.1136/bjsports-2018-100321
45. O’Connor D, Johnston RV, Brignardello-Petersen R, et al. Athroscopic surgery for degenerative knee disease (osteoarthritis including degenerative meniscal tears). Cochrane Database Syst Rev. 2022;3:CD014328. doi: 10.1002/14651858.CD014328
46. Siemieniuk RAC, Harris IA, Agoritsas T, et al. Arthroscopic surgery for degenerative knee arthritis and meniscal tears: a clinical practice guideline. Br J Sports Med. 2018;52:313. doi: 10.1136/bjsports-2017-j1982rep
47. Manner PA, Tubb CC, Levine BR. AAOS appropriate use criteria: surgical management of osteoarthritis of the knee. J Am Acad Orthop Surg. 2018;26:e194-197. doi: 10.5435/JAAOS-D-17-00425
1. Baker P, Reading I, Cooper C, et al. Knee disorders in the general population and their relation to occupation. Occup Environ Med. 2003;60:794-797. doi: 10.1136/oem.60.10.794
2. Nguyen UD, Zhang Y, Zhu Y, et al. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann Intern Med. 20116;155:725-732. doi: 10.7326/0003-4819-155-11-201112060-00004
3. Natsuhara KM, Yeranosian MG, Cohen JR, et al. What is the frequency of vascular injury after knee dislocation? Clin Orthop Relat Res. 2014;472:2615-2620. doi: 10.1007/s11999-014-3566-1
4. Seroyer ST, Musahl V, Harner CD. Management of the acute knee dislocation: the Pittsburgh experience. Injury. 2008;39:710-718. doi: 10.1016/j.injury.2007.11.022
5. Sinan SM, Elsoe R, Mikkelsen C, et al. Clinical, functional, and patient-reported outcome of traumatic knee dislocations: a retrospective cohort study of 75 patients with 6.5-year follow up. Arch Orthop Trauma Surg. 2023;143:2589-2597. doi: 10.1007/s00402-022-04578-z
6. Schatzker J, Kfuri M. Revisiting the management of tibial plateau fractures. Injury. 2022;53:2207-2218. doi: 10.1016/j.injury.2022.04.006
7. Rudran B, Little C, Wiik A, et al. Tibial plateau fracture: anatomy, diagnosis and management. Br J Hosp Med (Lond). 2020;81:1-9. doi: 10.12968/hmed.2020.0339
8. Tscherne H, Lobenhoffer P. Tibial plateau fractures: management and expected results. Clin Orthop Relat Res. 1993;(292):87-100.
9. Melvin JS, Mehta S. Patellar fractures in adults. J Am Acad Orthop Surg. 2011;19:198-207. doi: 10.5435/00124635-201104000-00004
10. Filho JS, Lenza M, Tamaoki MJ, et al. Interventions for treating fractures of the patella in adults. Cochrane Database Syst Rev. 2021;2:CD009651. doi: 10.1002/14651858.CD009651.pub3
11. Palmer W, Bancroft L, Bonar F, et al. Glossary of terms for musculoskeletal radiology. Skeletal Radiol. 2020;49(suppl 1):1-33. doi: 10.1007/s00256-020-03465-1
12. Frobell RB, Roos EM, Roos HP, et al. A randomized trial of treatment for acute anterior cruciate ligament tears. N Engl J Med. 2010;363:331-342. doi: 10.1056/NEJMoa0907797
13. Frobell RB, Roos HP, Roos EM, et al. Treatment for acute anterior cruciate ligament tear: five year outcome of randomized trial. Br J Sports Med. 2015;49:700. doi: 10.1136/bmj.f232
14. Diermeier TA, Rothrauff BB, Engebretsen L, et al; Panther Symposium ACL Treatment Consensus Group. Treatment after anterior cruciate ligament injury: Panther Symposium ACL Treatment Consensus Group. Br J Sports Med. 2021;55:14-22. doi: 10.1136/bjsports-2020-102200
15. Bedi A, Musahl V, Cowan JB. Management of posterior cruciate ligament injuries: an evidence-based review. J Am Acad Orthop Surg. 2016;24:277-289. doi: 10.5435/JAAOS-D-14-00326
16. Edson CJ. Conservative and postoperative rehabilitation of isolated and combined injuries of the medial collateral ligament. Sports Med Arthrosc Rev. 2006;14:105-110. doi: 10.1097/01.jsa.0000212308.32076.f2
17. Vosoughi F, Dogahe RR, Nuri A, et al. Medial collateral ligament injury of the knee: a review on current concept and management. Arch Bone Jt Surg. 2021;9:255-262. doi: 10.22038/abjs.2021.48458.2401
18. Kannus P. Nonoperative treatment of grade II and III sprains of the lateral ligament compartment of the knee. Am J Sports Med. 1989;17:83-88. doi: 10.1177/036354658901700114
19. Krukhaug Y, Mølster A, Rodt A, et al. Lateral ligament injuries of the knee. Knee Surg Sports Traumatol Arthrosc. 1998;6:21-25. doi: 10.1007/s001670050067
20. Grawe B, Schroeder AJ, Kakazu R, et al. Lateral collateral ligament injury about the knee: anatomy, evaluation, and management. J Am Acad Orthop Surg. 2018 15;26:e120-127. doi: 10.5435/JAAOS-D-16-00028
21. Ranawat A, Baker III CL, Henry S, et al. Posterolateral corner injury of the knee: evaluation and management. J Am Acad Orthop Surg. 2008;16:506-518.
22. Palmu S, Kallio PE, Donell ST, et al. Acute patellar dislocation in children and adolescents: a randomized clinical trial. J Bone Joint Surg Am. 2008;90:463-470. doi: 10.2106/JBJS.G.00072
23. Cohen D, Le N, Zakharia A, et al. MPFL reconstruction results in lower redislocation rates and higher functional outcomes than rehabilitation: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2022;30:3784-3795. doi: 10.1007/s00167-022-07003-5
24. Siwek CW, Rao JP. Ruptures of the extensor mechanism of the knee joint. J Bone Joint Surg Am. 1981;63:932-937.
25. Konrath GA, Chen D, Lock T, et al. Outcomes following repair of quadriceps tendon ruptures. J Orthop Trauma. 1998;12:273-279. doi: 10.1097/00005131-199805000-00010
26. Rasul Jr. AT, Fischer DA. Primary repair of quadriceps tendon ruptures: results of treatment. Clin Orthop Relat Res. 1993;(289):205-207.
27. Rougraff BT, Reeck CC, Essenmacher J. Complete quadriceps tendon ruptures. Orthopedics. 1996;19:509-514.
28. Bui CN, Learned JR, Scolaro JA. Treatment of patellar fractures and injuries to the extensor mechanism of the knee: a critical analysis review. JBJS Rev. 2018;6:e1. doi: 10.2106/JBJS.RVW.17.00172
29. Haskel JD, Fried JW, Hurley ET, et al. High rates of return to play and work follow knee extensor tendon ruptures but low rate of return to pre-injury level of play. Knee Surg Sports Traumatol Arthrosc. 2021;29:2695-2700. doi: 10.1007/s00167-021-06537-4
30. Critchley IJ, Bracey DJ. The acutely locked knee—is a manipulation worth while? Injury. 1985;16:281-283. doi: 10.1016/s0020-1383(85)80020-6
31. Allum RL, Jones JR. The locked knee. Injury. 1986;17:256-258. doi: 10.1016/0020-1383(86)90231-7
32. Helmark IC, Neergaard K, Krogsgaard MR. Traumatic knee extension deficit (the locked knee): can MRI reduce the need for arthroscopy? Knee Surg Sports Traumatol Arthrosc. 2007;15:863-868. doi: 10.1007/s00167-006-0244-1
33. Noyes FR, Chen RC, Barber-Westin SD, et al. Greater than 10-year results of red-white longitudinal meniscal repairs in patients 20 years of age or younger. Am J Sports Med. 2011;39:1008-1017. doi: 10.1177/0363546510392014
34. Chambers HG, Shea KG, Anderson AF, et al; American Academy of Orthopedic Surgeons. Diagnosis and treatment of osteochondritis dissecans. J Am Acad Orthop Surg. 2011;19:297-306. doi: 10.5435/00124635-201105000-00007
35. Margaretten ME, Kohlwes J, Moore D, et al. Does this adult patient have septic arthritis? JAMA. 2007;297:1478-1488. doi: 10.1001/jama.297.13.1478
36. Gupta MN, Sturrock RD, Field M. A prospective 2-year study of 75 patients with adult-onset septic arthritis. Rheumatology (Oxford). 2001;40:24-30. doi: 10.1093/rheumatology/40.1.24
37. Brophy RH, Fillingham YA. AAOS clinical practice guideline summary: management of osteoarthritis of the knee (nonarthroplasty), 3rd edition. J Am Acad Orthop Surg. 2022;30:e721-729. doi: 10.5435/JAAOS-D-21-01233
38. Collins NJ, Barton CJ, van Middelkoop M, et al. 2018 Consensus statement on exercise therapy and physical interventions (orthoses, taping and manual therapy) to treat patellofemoral pain: recommendations from the 5th International Patellofemoral Pain Research Retreat, Gold Coast, Australia, 2017. Br J Sports Med. 2018;52:1170-1178. doi: 10.1136/bjsports-2018-099397
39. Strauss EJ, Kim S, Calcei JG, et al. Iliotibial band syndrome: evaluation and management. J Am Acad Orthop Surg. 2011;19:728-736. doi: 10.5435/00124635-201112000-00003
40. Millar NL, Murrell GAC, Kirwan P. Time to put down the scalpel? The role of surgery in tendinopathy. Br J Sports Med. 2020;54:441-442. doi: 10.1136/bjsports-2019-101084
41. Hall MJ, Schwartzman A, Zhang J, et al. Ambulatory surgery data from hospitals and ambulatory surgery centers: United States, 2010. Natl Health Stat Report. 2017;(102):1-15.
42. Kise NJ, Risberg MA, Stensrud S, et al. Exercise therapy versus arthroscopic partial meniscectomy for degenerative meniscal tear in middle aged patients: randomized controlled trial with two year follow-up. BMJ. 2016;354:i3740. doi: 10.1136/bmj.i3740
43. Sihvonen R, Paavola M, Malmivaara A, et al, FIDELITY (Finnish Degenerative Meniscus Lesion Study) Investigators. Arthroscopic partial meniscectomy for a degenerative meniscus tear: a 5 year follow-up of the placebo-surgery controlled FIDELITY (Finnish Degenerative Meniscus Lesion Study) trial. Br J Sports Med. 2020;54:1332-1339. doi: 10.1136/bjsports-2020-102813
44. Pihl K, Ensor J, Peat G, et al. Wild goose chase—no predictable patient subgroups benefit from meniscal surgery: patient-reported outcomes of 641 patients 1 year after surgery. Br J Sports Med. 2020;54:13-22. doi: 10.1136/bjsports-2018-100321
45. O’Connor D, Johnston RV, Brignardello-Petersen R, et al. Athroscopic surgery for degenerative knee disease (osteoarthritis including degenerative meniscal tears). Cochrane Database Syst Rev. 2022;3:CD014328. doi: 10.1002/14651858.CD014328
46. Siemieniuk RAC, Harris IA, Agoritsas T, et al. Arthroscopic surgery for degenerative knee arthritis and meniscal tears: a clinical practice guideline. Br J Sports Med. 2018;52:313. doi: 10.1136/bjsports-2017-j1982rep
47. Manner PA, Tubb CC, Levine BR. AAOS appropriate use criteria: surgical management of osteoarthritis of the knee. J Am Acad Orthop Surg. 2018;26:e194-197. doi: 10.5435/JAAOS-D-17-00425
PRACTICE RECOMMENDATIONS
› Consider surgical management, potentially emergently, for acute knee injuries that result in significant joint instability, unstable fractures, or neurovascular compromise. A
› Avoid arthroscopy for chronic, degenerative sources of knee pain, such as osteoarthritis and degenerative meniscus tears, as it is no longer routinely recommended. A
› Treat osteoarthritis surgically after nonsurgical treatments have failed. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Prescribing DOACs with specific patient populations in mind
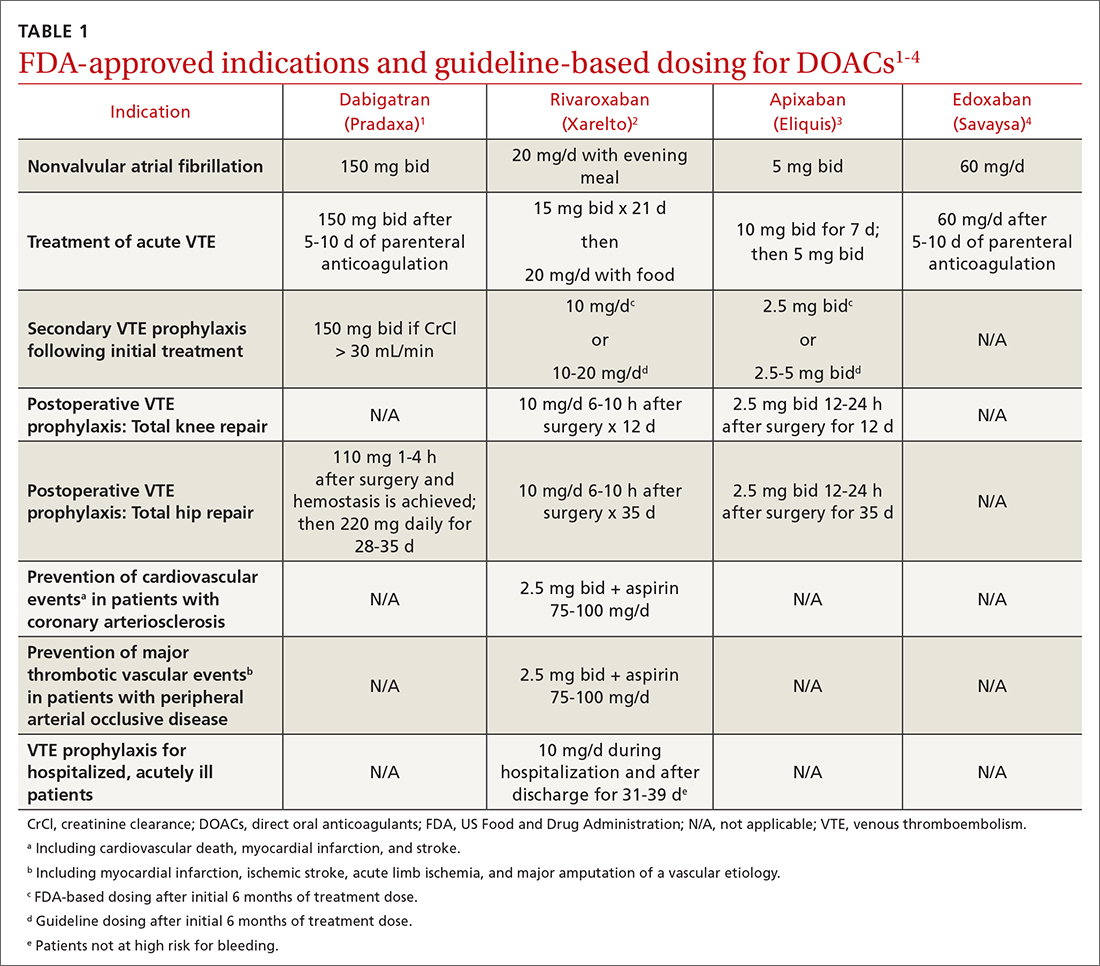
Four medications comprise the drug category known as direct oral anticoagulants (DOACs). Dabigatran (Pradaxa)1 was the first to gain approval. It was approved by the US Food and Drug Administration (FDA) in 2010 for the reduction of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (AF). This was followed by approvals for rivaroxaban (Xarelto)2 in 2011, apixaban (Eliquis)3 in 2012, and edoxaban (Savaysa)4 in 2015. Betrixaban (Bevyxxa)5 was approved in 2017 for venous thromboembolism (VTE) prophylaxis in acutely ill hospitalized patients with restricted mobility, but it was removed from the market in 2020.

In addition to stroke prevention in nonvalvular AF, each DOAC has been approved for other indications and has been addressed further in guideline-based recommendations outside FDA-approved indications.
Overview of DOACs
Dabigatran is the only direct thrombin inhibitor; the other agents inhibit factor Xa. TABLE 11-4 summarizes FDA-approved indications and dosing and guideline-based dosing. Dabigatran and edoxaban require parenteral anticoagulation for 5 to 10 days prior to initiation for acute VTE, limiting their use.1,4TABLE 21-4 highlights pharmacokinetic differences among the agents. For example, dabigatran is 80% renally cleared, is somewhat dialyzable, and can accumulate in patients with renal dysfunction.1 Edoxaban is contraindicated for nonvalvular AF in patients with a creatinine clearance (CrCl) > 95 mL/min because an increased stroke risk was demonstrated.4 Therefore, rivaroxaban and apixaban are prescribed most often in the United States.6,7
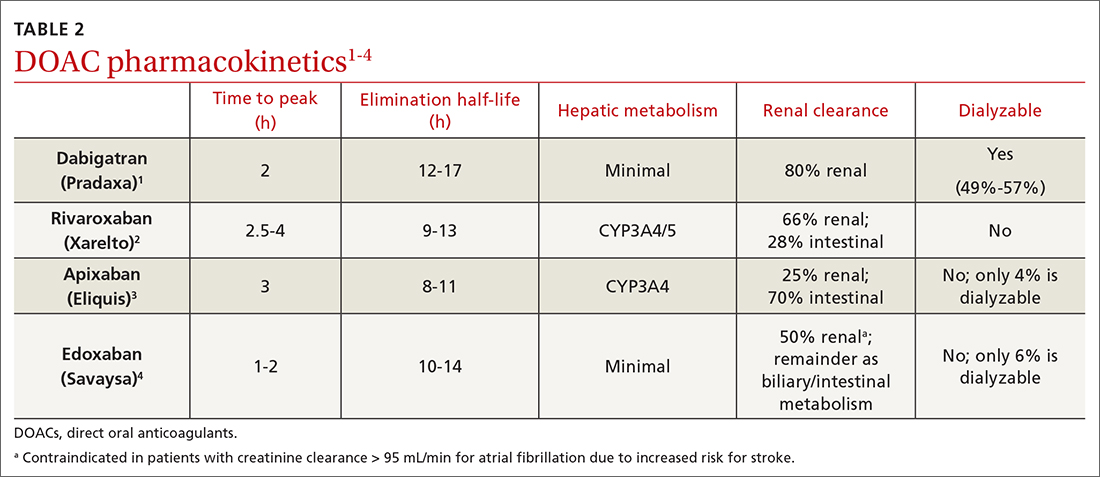
Applications in special patient populations
Obesity
As of 2020, more than 40% of adults in the United States were obese (body mass index [BMI] ≥ 30), with 9% classified as class 3 or severely obese (BMI ≥ 40).8 Altered drug pharmacokinetics in patients with severe obesity raises concern for undertreatment with fixed-dose DOACs. Phase III DOAC approval trials included patients with obesity, but weight cutoffs differed, making extrapolating efficacy and safety data difficult across different obesity stages.9 Although no FDA-labeled dosing adjustments exist for patients with obesity, the International Society on Thrombosis and Haemostasis (ISTH) does provide such recommendations.
ISTH changes position on measuring drug levels. ISTH previously recommended avoiding DOACs in those with a BMI > 40 or body weight > 120 kg. If a DOAC was used, ISTH advised obtaining peak and trough drug levels.10 However, DOAC drug levels have not been associated with clinical outcomes or sufficient degrees of anticoagulation.11
Men and women are affected equally by fibrolipomas. Prevalence does not differ by race or ethnicity.
In April 2021, ISTH updated guidance on DOACs in obesity, indicating standard doses of rivaroxaban or apixaban can be used for the treatment and prevention of VTE in all patients regardless of weight or BMI. Because data in obesity are lacking for dabigatran and edoxaban, avoid using these agents in patients with a BMI > 40 or weight > 120 kg. Additionally, assessing drug levels is no longer recommended, as there is insufficient evidence that these impact clinical outcomes.12
The 2021 American College of Chest Physicians (CHEST) guideline update
Continue to: Effectiveness of DOACs for AF in patients with obesity isn't clear
Effectiveness of DOACs for AF in patients with obesity isn’t clear, as most data are from retrospective cohort analyses. In patients weighing > 120 kg, dabigatran has shown efficacy in thrombosis prevention similar to that achieved in those weighing ≤ 120 kg, but it has increased the risk for gastrointestinal (GI) bleeding.15 Another study indicated a 15-mg dose of rivaroxaban may be associated with increased thromboembolic complications in patients with a BMI ≥ 35.16 Alternatively, another retrospective study of rivaroxaban demonstrated a small absolute risk reduction in ischemic stroke among patients in all stages of obesity and no difference in significant bleeding events.17 One further retrospective cohort showed that, in patients with a BMI ≥ 50 kg, the effectiveness of rivaroxaban and apixaban in thrombosis prevention and bleeding safety outcomes was comparable to that seen in those with a BMI < 30.18
As a result of conflicting data, and a lack of prospective randomized controlled trials (RCTs), ISTH continued recommending international normalized ratio (INR)–based dosing of warfarin for class 3 or severely obese patients with AF. The 2018 CHEST guidelines19 and the 2020 ESC guidelines20 make no mention of DOAC avoidance in patients with obesity and AF.
Advanced and end-stage renal disease
DOACs are renally dosed based on indication, drug-drug interactions, and degree of renal function (TABLE 31-4). For example, patients with AF who are anticoagulated with apixaban are prescribed 2.5 mg twice daily when 2 of the 3 following criteria are met: age ≥ 80 years, body weight ≤ 60 kg, serum creatinine ≥ 1.5 mg/dL. However, no dosage adjustment is necessary for VTE treatment or prophylaxis with apixaban regardless of renal function.3
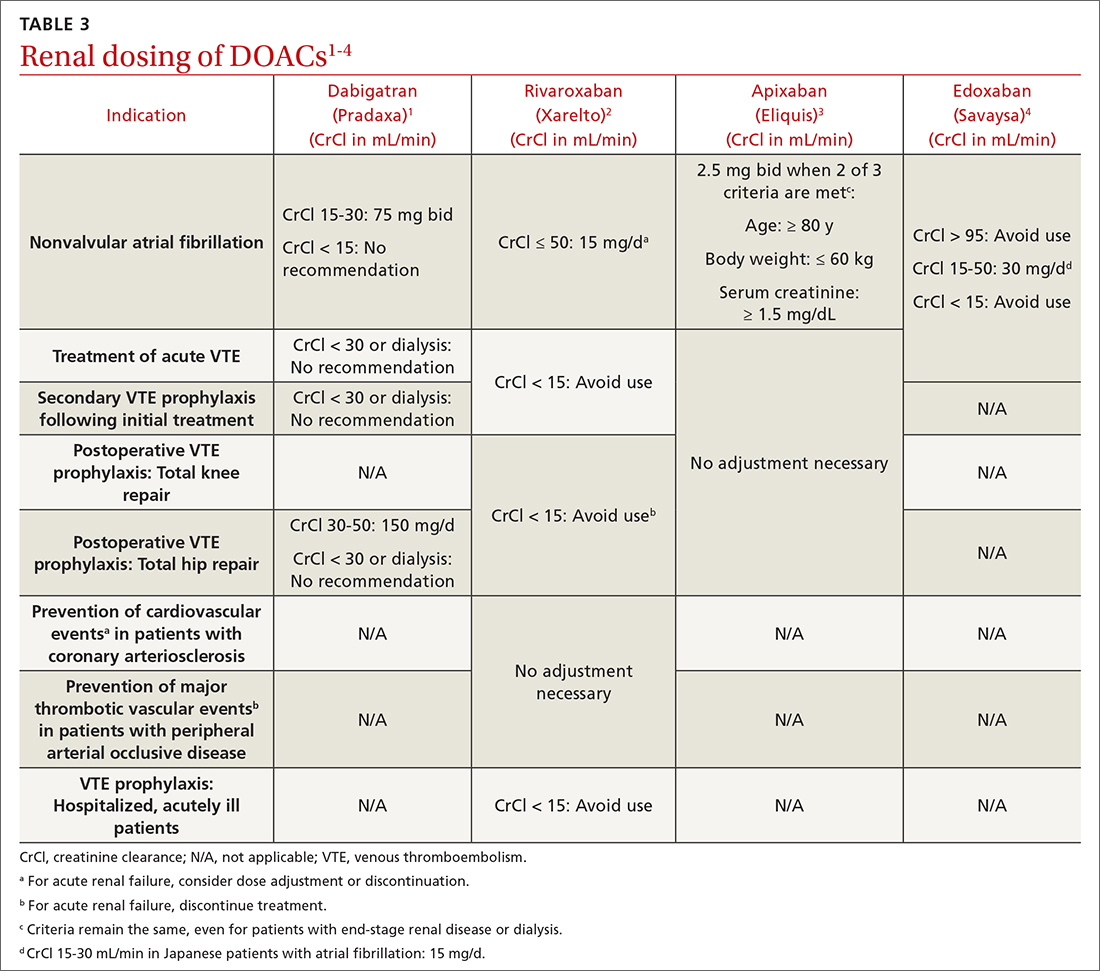
Data supporting the safety and efficacy of DOACs in end-stage renal disease (ESRD) are sparse. All DOACs are renally cleared to varying degrees (TABLE 21-4), theoretically increasing bleeding risk as kidney disease progresses. Apixaban is the least renally cleared of the DOACs and has been evaluated in the greatest number of trials for patients with ESRD for both VTE treatment and prevention and nonvalvular AF.21 As a result, the FDA approved standard-dose apixaban (5 mg twice daily) for VTE treatment and prevention and nonvalvular AF in patients with ESRD, even those requiring dialysis. Use the reduced apixaban dose (2.5 mg twice daily) in patients with ESRD and AF only if they are ≥ 80 years of age or their body weight is ≤ 60 kg.3
Patients with cancer
Cancer-associated acute VTE treatment. Cancer is an established risk factor for acute VTE but it also increases the risk for treatment-associated bleeding compared with patients without cancer.22 Historically, low-molecular-weight heparin (LMWH) was recommended over warfarin and DOACs for cancer-associated thromboses (CAT).23 Compared with warfarin, LMWH reduced the rate of recurrent VTE and had similar or reduced bleeding rates at 6 to 12 months.24-26 However, clinicians and patients often chose warfarin to avoid subcutaneous injections.27
CHEST guidelines recommend oral Xa inhibitors over LMWH for the treatment of CAT.13 The 2020 guidelines of the National Institute for Health and Care Excellence (NICE) recommend DOACs as an option for CAT along with LMWH or LMWH transitioned to warfarin.28 The American Society of Clinical Oncology (ASCO) recommends rivaroxaban for acute VTE treatment in CAT. No head-to-head trials have evaluated comparative efficacy of DOACs for CAT. However, edoxaban and rivaroxaban are associated with a greater risk for GI bleeding; therefore, apixaban is preferred in patients with GI malignancies.29 Standard DOAC VTE treatment dosing is recommended for all 3 agents.2-4
When using DOACs for patients with CAT, consider potential drug-drug interactions with chemotherapy regimens. All DOACs are transported by p-glycoprotein, while rivaroxaban and apixaban are substrates of cytochrome P450, leading to potentially significant drug-drug interactions.30 These interactions could affect the patient’s chemotherapeutic regimen, decrease the efficacy of the DOAC, or increase the risk for bleeding. Therefore, anticoagulation choice should be made in collaboration with the hematology/oncology team.
Continue to: Cancer-associated VTE prophylaxis...
Cancer-associated VTE prophylaxis. VTE prophylaxis for patients with cancer is complex and necessitates a global assessment of cancer location and treatment regimen and setting. Hospitalized patients receiving chemotherapy are at high risk for VTE if mobility is reduced or if other VTE risk factors are present. The International Initiative on Thrombosis and Cancer (ITAC)31 and ISTH32 recommend VTE prophylaxis with unfractionated heparin or LMWH (ISTH recommends LMWH more strongly). The 2020 ASCO Guidelines recommend pharmacologic anticoagulation but make no drug-specific recommendation.29 Parenteral treatment in hospitalized patients is not as burdensome as it is in ambulatory patients; therefore, these recommendations are less likely to elicit inpatient opposition.
In the ambulatory setting, patient avoidance of subcutaneous injections necessitates consideration of DOACs for CAT prophylaxis. The Khorana Risk Score (KRS) is a validated tool (scale, 0-7) to predict VTE risk in ambulatory patients receiving chemotherapy.33 KRS scores ≥ 2 indicate high thrombotic risk and the need for prophylactic anticoagulation. ASCO recommends apixaban, rivaroxaban, or LMWH.29 ISTH and ITAC both recommend apixaban or rivaroxaban over LMWH.31,34 An RCT published in June 2023 confirmed that, for adults with cancer and VTE, DOACs were noninferior to LMWH for preventing recurrent VTE for 6 months.35 The recommended doses for apixaban (2.5 mg twice daily) and rivaroxaban (10 mg daily) for CAT VTE prophylaxis are lower than FDA-approved treatment doses.31
Patients with thrombophilia: VTE prevention
Thrombophilias are broadly categorized as inherited or acquired, with inherited thrombophilia being more prevalent. The Factor V Leiden (FVL) variant affects 2% to 7% of the population, and prothrombin gene mutation (PGM) affects 1% to 2% of the population.36 Other forms of inherited thrombophilia, such as protein C deficiency, protein S deficiency, and antithrombin deficiency, occur less commonly (< 0.7% of the population).36 Antiphospholipid syndrome (APS), the most common acquired thrombophilia, affects approximately 2% of the population.36 APS involves multiple antibodies: anticardiolipin antibodies, lupus anticoagulant, and anti-beta-2 glycoprotein 1 antibodies. Establishing risk for thrombosis across the varying types of thrombophilia has proven difficult, but APS is considered the most thrombogenic thrombophilia apart from extremely rare homozygous inherited thrombophilias.36 Therefore, DOAC recommendations are thrombophilia specific.
A prospective cohort study evaluated DOACs compared with heparin/warfarin for VTE treatment in patients with inherited thrombophilias.37 Although all 4 available DOACs were included, most patients (61.1%) received rivaroxaban. Patients with an array of inherited thrombophilias, including rare homozygous mutations, were enrolled in this trial. While most patients (66.9%) had a “mild thrombophilia” defined as either FVL or PGM, the remainder had more severe thrombophilias.37 VTE recurrence was similar and uncommon in the DOAC and heparin/warfarin groups, consistent with a previous meta-analysis.38 Surprisingly, an increase in the cumulative risk for bleeding was seen in the DOAC group compared with the warfarin group, a finding inconsistent with prior trials.38 There were no major bleeding events in the DOAC group, but 3 such events occurred in the heparin/warfarin group, including 2 intracranial hemorrhages.
Currently NICE, CHEST, and ISTH do not make a recommendation for a preferred agent in patients with an acute VTE and inherited thrombophilia; however, DOACs would not be inappropriate.23,28,32 The American Society of Hematology (ASH) had planned to release recommendations related to the treatment of thrombophilia in 2020, but they were delayed by the COVID-19 pandemic.39
APS presents challenges for acute VTE anticoagulation. First, it causes a strongly thrombogenic state necessitating therapeutic anticoagulation. Second, for patients with positive lupus anticoagulant, INR monitoring and standardized INR goals may be inadequate.40 Therefore, using fixed-dose DOACs without the need for therapeutic monitoring is appealing, but significant concerns exist for using DOACs in patients with APS.41-45 ISTH and CHEST recommend warfarin for the treatment and prevention of acute VTE in patients with APS, especially those with triple-positive (anticardiolipin, lupus anticoagulant, and anti-beta-2 glycoprotein 1) APS.13,46 Package labeling for all DOACs recommends avoidance in triple-positive APS.1-4
ASTRO-APS is the most recent RCT to compare apixaban and warfarin for patients with APS,47 and it was terminated early after 6 of 23 patients in the apixaban group had thrombotic events, while no one in the warfarin group had such an event.48 Subsequently, a meta-analysis49 demonstrated that patients with thrombotic APS appear to have a greater risk for arterial thrombosis when treated with DOACs compared with warfarin. These 2 studies may lead to changes in recommendations to avoid DOACs in all patients with APS or may prompt more focused trials for DOAC use in patients with APS plus an antiplatelet to mitigate arterial thrombotic risk.
Continue to: Expanded clinical indications
Expanded clinical indications
Superficial vein thrombosis
Superficial thrombophlebitis or superficial vein thrombosis (SVT) is estimated to occur 6 times more frequently than VTE.50 Management of patients with isolated, uncomplicated thrombophlebitis who are at low risk for extension of the SVT involves symptomatic treatment with nonsteroidal anti-inflammatory drugs, topical agents, or compression therapy. However, depending on risk for progression, anticoagulation may be recommended.51
Patients at intermediate risk for extension or propagation of SVT are candidates for anticoagulation. The CHEST guidelines recommend
Certain situations should prompt one to consider using a treatment dose of a DOAC for 3 months. These include cases in which the SVT is located within 3 cm of the deep venous system, expands despite an appropriate prophylactic regimen, or recurs after discontinuation of prophylactic anticoagulation.13,50
Acute coronary syndrome
The American College of Cardiology/American Heart Association (ACC/AHA) recommend combination antiplatelet therapy and anticoagulation for management of acute coronary syndrome in hospitalized patients.52 Data are mixed regarding longer-term anticoagulation in addition to dual antiplatelet therapy in outpatient settings to prevent thrombosis recurrence in the absence of AF.
The APPRAISE-2 trial enrolled high-risk patients with ACS within 7 days of the event.53 Apixaban 5 mg twice daily was compared with placebo in patients taking aspirin or aspirin plus clopidogrel. The trial was terminated early because major bleeding events increased with apixaban without reduction in recurrent ischemic events. The ATLAS ACS-TIMI 46 trial evaluated different rivaroxaban doses (5-20 mg daily) in ACS patients.54 The study revealed possible thrombosis benefit but also increased risk for bleeding, particularly at higher doses. As a result, another study—ATLAS ACS 2-TIMI 51—was conducted and compared the use of low-dose rivaroxaban (2.5 mg twice daily or 5 mg twice daily) vs placebo for patients with recent ACS.55 All patients were receiving low-dose aspirin, and approximately 93% of patients in each group also were receiving clopidogrel or ticlopidine. As in the APPRAISE-2 trial, rivaroxaban increased the rate of major bleeding and intracranial hemorrhage; however, it did not increase the incidence of fatal bleeding. Unlike APPRAISE-2, rivaroxaban significantly reduced the primary efficacy end point, a composite of death from cardiovascular causes, myocardial infarction, or stroke (absolute risk reduction = 1.8%; number needed to treat = 56 for combined rivaroxaban doses).55
A secondary subgroup analysis combined data from the ATLAS ACMS-TIMI 46 and ATLAS ACS 2-TIMI 51 trials to evaluate outcomes in patients receiving aspirin monotherapy when combined with rivaroxaban 2.5 mg twice daily or 5 mg twice daily or with placebo.56 The primary efficacy end point was a composite of cardiovascular death, myocardial infarction, or stroke. When the 2 trials were evaluated separately, neither rivaroxaban dose was associated with reduction of the primary efficacy outcomes compared with aspirin alone. However, when the data were pooled, both the combined rivaroxaban doses (particularly the 5-mg dose) were associated with reduced cardiovascular outcomes. From a safety perspective, the 2.5-mg twice-daily dose of rivaroxaban was the only dose not associated with increased major bleeding risk. Thus, the 2.5-mg twice-daily dose of rivaroxaban may not provide sufficient cardiovascular benefit in patients with ACS, while the larger dose may increase the risk for nonfatal major bleeding events.56
The European Medicines Agency57 approved rivaroxaban 2.5 mg twice daily for ACS, and the 2020 ESC guidelines58 consider it an appropriate therapeutic option in addition to aspirin for patients at high ischemic risk and low bleeding risk. ACS is not an FDA-approved indication for DOACs, and the ACC/AHA Guideline for the Management of ACS, last updated in 2014, does not include DOACs for ACS unless patients have AF.52 Ongoing trials are further investigating rivaroxaban for ACS, so the use of DOACs in the post-acute phase of ACS may become clearer in the future.59
Continue to: Heparin-induced thrombocytopenia
Heparin-induced thrombocytopenia
Historically, nonheparin parenteral anticoagulants argatroban, bivalirudin, and fondaparinux were recommended for patients at risk for or who had heparin-induced thrombocytopenia (HIT). Argatroban is the only drug FDA approved for the treatment and prophylaxis of HIT; recommendations for the others are based on guideline recommendations.23,60,61 The nonheparin parenteral anticoagulants cost between $700 and $1500 per day; therefore most patients with HIT are transitioned to warfarin.62 However, protein C and S inhibition and a subsequent prothrombotic state conveyed by warfarin initiation necessitates a minimum 5-day bridge to therapeutic warfarin with a nonheparin parenteral anticoagulant.
In vitro tests show that DOACs do not promote development of HIT antibodies63 or affect platelet activation or aggregation.64 A literature summary of DOACs for HIT determined that in 104 patients, all but 1 achieved platelet recovery (defined as > 150,000/mcL) within a median time of 7 days. Therapeutically, DOACs prevented new or recurrent VTE in 102/104 cases (98%), and only 3% of patients experienced significant bleeding events.62
The 2018 ASH guidelines for VTE management in HIT include (with very low certainty of evidence) dabigatran, rivaroxaban, or apixaban for consideration in addition to previously recommended nonheparin parenteral anticoagulants.61 The dosing of each agent is contingent upon treatment of patients with HIT and an acute thrombosis (HITT) or HIT in the absence of VTE. For patients with HITT, treatment doses for acute VTE should be used for the appropriate duration of therapy (ie, 3 months). Importantly, dabigatran requires a 5-day pretreatment period with a parenteral anticoagulant, so it is not an ideal option. When treating isolated HIT (in the absence of VTE), ASH recommends all agents be dosed twice daily—dabigatran 150 mg twice daily (no 5-day parenteral pretreatment necessary), rivaroxaban 15 mg twice daily, or apixaban 5 mg twice daily—until platelet recovery (≥ 150,000/mcL) is achieved.61
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; kevin-schleich@uiowa.edu
1. Dabigatran. Package Insert. Boehringer Ingelheim Pharmaceuticals, Inc.; 2021.
2. Rivaroxaban. Package insert. Janssen Pharmaceuticals, Inc; 2022.
3. Apixaban. Package insert. Bristol-Myers Squibb; 2021.
4. Edoxaban. Package insert. Daiichi Sankyo, Inc; 2015.
5. Betrixaban. Package insert. Portola Pharmaceuticals, Inc; 2017.
6. Wheelock KM, Ross JS, Murugiah K, et al. Clinician trends in prescribing direct oral anticoagulants for US Medicare beneficiaries. JAMA Netw Open. 2021;4:e2137288. doi: 10.1001/jamanetworkopen.2021.37288
7. Colacci M, Tseng EK, Sacks CA, et al. Oral anticoagulant utilization in the United States and United Kingdom. J Gen Intern Med. 2020;35:2505-2507. doi: 10.1007/s11606-020-05904-0
8. CDC. Adult obesity facts. Accessed May 9, 2023. www.cdc.gov/obesity/data/adult.html
9. Mocini D, Di Fusco SA, Mocini E, et al. Direct oral anticoagulants in patients with obesity and atrial fibrillation: position paper of Italian National Association of Hospital Cardiologists (ANMCO). J Clin Med. 2021;10:4185. doi: 10.3390/jcm10184185
10. Martin K, Beyer-Westendorf J, Davidson BL, et al. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:1308-1313. doi: 10.1111/jth.13323
11. Gu TM, Garcia DA, Sabath DE. Assessment of direct oral anticoagulant assay use in clinical practice. J Thromb Thrombolysis. 2019;47:403-408. doi: 10.1007/s11239-018-1793-0
12. Martin KA, Beyer-Westendorf J, Davidson BL, et al. Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: updated communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J Thromb Haemost. 2021;19:1874-1882. doi: 10.1111/jth.15358
13. Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160:e545-e608. doi: 10.1016/j.chest.2021.07.055
14. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. doi: 10.1093/eurheartj/ehz405
15. Coates J, Bitton E, Hendje A, et al. Clinical outcomes of dabigatran use in patients with non-valvular atrial fibrillation and weight >120 kg. Thromb Res. 2021;208:176-180. doi: 10.1016/j.thromres.2021.11.007
16. Li X, Zuo C, Ji Q, et al. Body mass index influence on the clinical outcomes for nonvalvular atrial fibrillation patients admitted to a hospital treated with direct oral anticoagulants: a retrospective cohort study. Drug Des Devel Ther. 2021;15:1931-1943. doi: 10.2147/dddt.S303219
17. Barakat AF, Jain S, Masri A, et al. Outcomes of direct oral anticoagulants in atrial fibrillation patients across different body mass index categories. JACC Clin Electrophysiol. 2021;7:649-658. doi: 10.1016/j.jacep.2021.02.002
18. O’Kane CP, Avalon JCO, Lacoste JL, et al. Apixaban and rivaroxaban use for atrial fibrillation in patients with obesity and BMI ≥50 kg/m2. Pharmacotherapy. 2022;42:112-118. doi: https://doi.org/10.1002/phar.2651
19. Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST Guideline and Expert Panel Report. Chest. 2018;154:1121-1201. doi: 10.1016/j.chest.2018.07.040
20. Sepehri Shamloo A, Dagres N, Hindricks G. [2020 ESC guidelines on atrial fibrillation: summary of the most relevant recommendations and innovations]. Herz. 2021;46:28-37. doi: 10.1007/s00059-020-05005-y
21. Chokesuwattanaskul R, Thongprayoon C, Tanawuttiwat T, et al. Safety and efficacy of apixaban versus warfarin in patients with end-stage renal disease: meta-analysis. Pacing Clin Electrophysiol. 2018;41:627-634. doi: 10.1111/pace.13331
22. Wang T-F, Li A, Garcia D. Managing thrombosis in cancer patients. Res Pract Thromb Haemost. 2018;2:429-438. doi: https://doi.org/10.1002/rth2.12102
23. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. CHEST. 2016;149:315-352. doi: 10.1016/j.chest.2015.11.026
24. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146-153. doi: 10.1056/NEJMoa025313
25. Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162:1729-1735. doi: 10.1001/archinte.162.15.1729
26. Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119:1062-1072. doi: 10.1016/j.amjmed.2006.02.022
27. Lee AYY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314:677-686. doi: 10.1001/jama.2015.9243
28. NICE Guideline. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. Accessed May 9, 2023. www.ncbi.nlm.nih.gov/books/NBK556698/
29. Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2020;38:496-520. doi: 10.1200/jco.19.01461
30. Galgani A, Palleria C, Iannone LF, et al. Pharmacokinetic interactions of clinical interest between direct oral anticoagulants and antiepileptic drugs. Front Neurol. 2018;9:1067. doi: 10.3389/fneur.2018.01067
31. Farge D, Frere C, Connors JM, et al. 2019 International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20:e566-e581. doi: 10.1016/s1470-2045(19)30336-5
32. Di Nisio M, Carrier M, Lyman GH, et al. Prevention of venous thromboembolism in hospitalized medical cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2014;12:1746-1749. doi: 10.1111/jth.12683
33. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907. doi: 10.1182/blood-2007-10-116327
34. Wang TF, Zwicker JI, Ay C, et al. The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2019;17:1772-1778. doi: 10.1111/jth.14564
35. Schrag D, Uno H, Rosovsky R, et al. Direct oral anticoagulants vs low-molecular-weight heparin and recurrent VTE in patients with cancer: a randomized clinical trial. JAMA. 2023;329:1924-1933. doi: 10.1001/jama.2023.7843
36. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41:154-164. doi: 10.1007/s11239-015-1316-1
37. Campello E, Spiezia L, Simion C, et al. Direct oral anticoagulants in patients with inherited thrombophilia and venous thromboembolism: a prospective cohort study. J Am Heart Assoc. 2020;9:e018917. doi: 10.1161/jaha.120.018917
38. Elsebaie MAT, van Es N, Langston A, et al. Direct oral anticoagulants in patients with venous thromboembolism and thrombophilia: a systematic review and meta-analysis. J Thromb Haemost. 2019;17:645-656. doi: 10.1111/jth.14398
39. ASH. ASH Clinical Practice Guidelines on Venous Thromboembolism. Accessed May 10, 2023. www.hematology.org/education/clinicians/guidelines-and-quality-care/clinical-practice-guidelines/venous-thromboembolism-guidelines
40. Baquero-Salamanca M, Téllez-Arévalo AM, Calderon-Ospina C. Variability in the international normalised ratio (INR) in patients with antiphospholipid syndrome and positive lupus anticoagulant: should the INR targets be higher? BMJ Case Rep. 2015;2015:bcr2014209013. doi: 10.1136/bcr-2014-209013
41. Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018;132:1365-1371. doi: 10.1182/blood-2018-04-848333
42. Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M, et al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome: a randomized noninferiority trial. Ann Intern Med. 2019;171:685-694. doi: 10.7326/m19-0291
43. Sato T, Nakamura H, Fujieda Y, et al. Factor Xa inhibitors for preventing recurrent thrombosis in patients with antiphospholipid syndrome: a longitudinal cohort study. Lupus. 2019;28:1577-1582. doi: 10.1177/0961203319881200
44. Malec K, Broniatowska E, Undas A. Direct oral anticoagulants in patients with antiphospholipid syndrome: a cohort study. Lupus. 2020;29:37-44. doi: 10.1177/0961203319889156
45. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome. Dr. Hannah Cohen about the results of the RAPS trial (Lancet Haematol 2016; 3: e426-36). Rheumatology (Oxford). 2017;56:e23. doi: 10.1093/rheumatology/kex290
46. Zuily S, Cohen H, Isenberg D, et al. Use of direct oral anticoagulants in patients with thrombotic antiphospholipid syndrome: guidance from the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2020;18:2126-2137. doi: https://doi.org/10.1111/jth.14935
47. NIH. ClinicalTrials.gov. Apixaban for the secondary prevention of thromboembolism among patients with antiphospholipid syndrome (ASTRO-APS). Accessed May 10, 2023. https://clinicaltrials.gov/ct2/show/NCT02295475?term=apixaban&cond=Anti+Phospholipid+Syndrome&draw=2&rank=1
48. Woller SC, Stevens SM, Kaplan D, et al. Apixaban compared with warfarin to prevent thrombosis in thrombotic antiphospholipid syndrome: a randomized trial. Blood Adv. 2022;6:1661-1670. doi: 10.1182/bloodadvances.2021005808
49. Khairani CD, Bejjani A, Piazza G, et al. Direct oral anticoagulants vs vitamin K antagonists in patients with antiphospholipid syndromes: meta-analysis of randomized trials. J Am Coll Cardiol. 2023;81:16-30. doi: 10.1016/j.jacc.2022.10.008
50. Superficial thrombophlebitis, superficial vein thrombosis. 2021. Accessed May 10, 2023. thrombosiscanada.ca/wp-content/uploads/2021/07/47.-Superficial-Vein-Thrombosis_16July2021.pdf
51. Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev. 2018;2:CD004982. doi: 10.1002/14651858.CD004982.pub6
52. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139-e228. doi: 10.1016/j.jacc.2014.09.017
53. Alexander JH, Lopes RD, James S, et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365:699-708. doi: 10.1056/NEJMoa1105819
54. Mega JL, Braunwald E, Mohanavelu S, et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet. 2009;374:29-38. doi: 10.1016/s0140-6736(09)60738-8
55. Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9-19. doi: 10.1056/NEJMoa1112277
56. Gibson WJ, Gibson CM, Yee MK, et al. Safety and efficacy of rivaroxaban when added to aspirin monotherapy among stabilized post‐acute coronary syndrome patients: a pooled analysis study of ATLAS ACS‐TIMI 46 and ATLAS ACS 2‐TIMI 51. J Am Heart Assoc. 2019. Accessed May 10, 2023. Doi: 10.1161/JAHA.118.009451
57. European Medicines Agency. Xarelto (rivaroxaban). 2008. Accessed June 23, 2023.
58. Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289-1367. doi: 10.1093/eurheartj/ehaa575
59. NIH. ClinicalTrials.gov. Accessed May 10, 2023. www.clinicaltrials.gov/ct2/results?cond=Acute+Coronary+Syndrome&term=rivaroxaban+&cntry=&state=&city=&dist=#
60. Watson H, Davidson S, Keeling D. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: second edition. Br J Haematol. 2012;159:528-40. doi: 10.1111/bjh.12059
61. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2:3360-3392. doi: 10.1182/bloodadvances.2018024489
62. Momin J, Lee C-S. The role of direct oral anticoagulants in the management of heparin-induced thrombocytopenia US Pharmacist. 2020;45:3-10. Accessed May 10, 2023. www.uspharmacist.com/article/the-role-of-direct-oral-anticoagulants-in-the-management-of-heparininduced-thrombocytopenia
63. Warkentin TE, Pai M, Linkins LA. Direct oral anticoagulants for treatment of HIT: update of Hamilton experience and literature review. Blood. 2017;130:1104-1113. doi: 10.1182/blood-2017-04-778993
64. Krauel K, Hackbarth C, Fürll B, et al. Heparin-induced thrombocytopenia: in vitro studies on the interaction of dabigatran, rivaroxaban, and low-sulfated heparin, with platelet factor 4 and anti-PF4/heparin antibodies. Blood. 2012;119:1248-1255. doi: 10.1182/blood-2011-05-353391
Four medications comprise the drug category known as direct oral anticoagulants (DOACs). Dabigatran (Pradaxa)1 was the first to gain approval. It was approved by the US Food and Drug Administration (FDA) in 2010 for the reduction of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (AF). This was followed by approvals for rivaroxaban (Xarelto)2 in 2011, apixaban (Eliquis)3 in 2012, and edoxaban (Savaysa)4 in 2015. Betrixaban (Bevyxxa)5 was approved in 2017 for venous thromboembolism (VTE) prophylaxis in acutely ill hospitalized patients with restricted mobility, but it was removed from the market in 2020.

In addition to stroke prevention in nonvalvular AF, each DOAC has been approved for other indications and has been addressed further in guideline-based recommendations outside FDA-approved indications.

Overview of DOACs
Dabigatran is the only direct thrombin inhibitor; the other agents inhibit factor Xa. TABLE 11-4 summarizes FDA-approved indications and dosing and guideline-based dosing. Dabigatran and edoxaban require parenteral anticoagulation for 5 to 10 days prior to initiation for acute VTE, limiting their use.1,4TABLE 21-4 highlights pharmacokinetic differences among the agents. For example, dabigatran is 80% renally cleared, is somewhat dialyzable, and can accumulate in patients with renal dysfunction.1 Edoxaban is contraindicated for nonvalvular AF in patients with a creatinine clearance (CrCl) > 95 mL/min because an increased stroke risk was demonstrated.4 Therefore, rivaroxaban and apixaban are prescribed most often in the United States.6,7

Applications in special patient populations
Obesity
As of 2020, more than 40% of adults in the United States were obese (body mass index [BMI] ≥ 30), with 9% classified as class 3 or severely obese (BMI ≥ 40).8 Altered drug pharmacokinetics in patients with severe obesity raises concern for undertreatment with fixed-dose DOACs. Phase III DOAC approval trials included patients with obesity, but weight cutoffs differed, making extrapolating efficacy and safety data difficult across different obesity stages.9 Although no FDA-labeled dosing adjustments exist for patients with obesity, the International Society on Thrombosis and Haemostasis (ISTH) does provide such recommendations.
ISTH changes position on measuring drug levels. ISTH previously recommended avoiding DOACs in those with a BMI > 40 or body weight > 120 kg. If a DOAC was used, ISTH advised obtaining peak and trough drug levels.10 However, DOAC drug levels have not been associated with clinical outcomes or sufficient degrees of anticoagulation.11
Men and women are affected equally by fibrolipomas. Prevalence does not differ by race or ethnicity.
In April 2021, ISTH updated guidance on DOACs in obesity, indicating standard doses of rivaroxaban or apixaban can be used for the treatment and prevention of VTE in all patients regardless of weight or BMI. Because data in obesity are lacking for dabigatran and edoxaban, avoid using these agents in patients with a BMI > 40 or weight > 120 kg. Additionally, assessing drug levels is no longer recommended, as there is insufficient evidence that these impact clinical outcomes.12
The 2021 American College of Chest Physicians (CHEST) guideline update
Continue to: Effectiveness of DOACs for AF in patients with obesity isn't clear
Effectiveness of DOACs for AF in patients with obesity isn’t clear, as most data are from retrospective cohort analyses. In patients weighing > 120 kg, dabigatran has shown efficacy in thrombosis prevention similar to that achieved in those weighing ≤ 120 kg, but it has increased the risk for gastrointestinal (GI) bleeding.15 Another study indicated a 15-mg dose of rivaroxaban may be associated with increased thromboembolic complications in patients with a BMI ≥ 35.16 Alternatively, another retrospective study of rivaroxaban demonstrated a small absolute risk reduction in ischemic stroke among patients in all stages of obesity and no difference in significant bleeding events.17 One further retrospective cohort showed that, in patients with a BMI ≥ 50 kg, the effectiveness of rivaroxaban and apixaban in thrombosis prevention and bleeding safety outcomes was comparable to that seen in those with a BMI < 30.18
As a result of conflicting data, and a lack of prospective randomized controlled trials (RCTs), ISTH continued recommending international normalized ratio (INR)–based dosing of warfarin for class 3 or severely obese patients with AF. The 2018 CHEST guidelines19 and the 2020 ESC guidelines20 make no mention of DOAC avoidance in patients with obesity and AF.
Advanced and end-stage renal disease
DOACs are renally dosed based on indication, drug-drug interactions, and degree of renal function (TABLE 31-4). For example, patients with AF who are anticoagulated with apixaban are prescribed 2.5 mg twice daily when 2 of the 3 following criteria are met: age ≥ 80 years, body weight ≤ 60 kg, serum creatinine ≥ 1.5 mg/dL. However, no dosage adjustment is necessary for VTE treatment or prophylaxis with apixaban regardless of renal function.3

Data supporting the safety and efficacy of DOACs in end-stage renal disease (ESRD) are sparse. All DOACs are renally cleared to varying degrees (TABLE 21-4), theoretically increasing bleeding risk as kidney disease progresses. Apixaban is the least renally cleared of the DOACs and has been evaluated in the greatest number of trials for patients with ESRD for both VTE treatment and prevention and nonvalvular AF.21 As a result, the FDA approved standard-dose apixaban (5 mg twice daily) for VTE treatment and prevention and nonvalvular AF in patients with ESRD, even those requiring dialysis. Use the reduced apixaban dose (2.5 mg twice daily) in patients with ESRD and AF only if they are ≥ 80 years of age or their body weight is ≤ 60 kg.3
Patients with cancer
Cancer-associated acute VTE treatment. Cancer is an established risk factor for acute VTE but it also increases the risk for treatment-associated bleeding compared with patients without cancer.22 Historically, low-molecular-weight heparin (LMWH) was recommended over warfarin and DOACs for cancer-associated thromboses (CAT).23 Compared with warfarin, LMWH reduced the rate of recurrent VTE and had similar or reduced bleeding rates at 6 to 12 months.24-26 However, clinicians and patients often chose warfarin to avoid subcutaneous injections.27
CHEST guidelines recommend oral Xa inhibitors over LMWH for the treatment of CAT.13 The 2020 guidelines of the National Institute for Health and Care Excellence (NICE) recommend DOACs as an option for CAT along with LMWH or LMWH transitioned to warfarin.28 The American Society of Clinical Oncology (ASCO) recommends rivaroxaban for acute VTE treatment in CAT. No head-to-head trials have evaluated comparative efficacy of DOACs for CAT. However, edoxaban and rivaroxaban are associated with a greater risk for GI bleeding; therefore, apixaban is preferred in patients with GI malignancies.29 Standard DOAC VTE treatment dosing is recommended for all 3 agents.2-4
When using DOACs for patients with CAT, consider potential drug-drug interactions with chemotherapy regimens. All DOACs are transported by p-glycoprotein, while rivaroxaban and apixaban are substrates of cytochrome P450, leading to potentially significant drug-drug interactions.30 These interactions could affect the patient’s chemotherapeutic regimen, decrease the efficacy of the DOAC, or increase the risk for bleeding. Therefore, anticoagulation choice should be made in collaboration with the hematology/oncology team.
Continue to: Cancer-associated VTE prophylaxis...
Cancer-associated VTE prophylaxis. VTE prophylaxis for patients with cancer is complex and necessitates a global assessment of cancer location and treatment regimen and setting. Hospitalized patients receiving chemotherapy are at high risk for VTE if mobility is reduced or if other VTE risk factors are present. The International Initiative on Thrombosis and Cancer (ITAC)31 and ISTH32 recommend VTE prophylaxis with unfractionated heparin or LMWH (ISTH recommends LMWH more strongly). The 2020 ASCO Guidelines recommend pharmacologic anticoagulation but make no drug-specific recommendation.29 Parenteral treatment in hospitalized patients is not as burdensome as it is in ambulatory patients; therefore, these recommendations are less likely to elicit inpatient opposition.
In the ambulatory setting, patient avoidance of subcutaneous injections necessitates consideration of DOACs for CAT prophylaxis. The Khorana Risk Score (KRS) is a validated tool (scale, 0-7) to predict VTE risk in ambulatory patients receiving chemotherapy.33 KRS scores ≥ 2 indicate high thrombotic risk and the need for prophylactic anticoagulation. ASCO recommends apixaban, rivaroxaban, or LMWH.29 ISTH and ITAC both recommend apixaban or rivaroxaban over LMWH.31,34 An RCT published in June 2023 confirmed that, for adults with cancer and VTE, DOACs were noninferior to LMWH for preventing recurrent VTE for 6 months.35 The recommended doses for apixaban (2.5 mg twice daily) and rivaroxaban (10 mg daily) for CAT VTE prophylaxis are lower than FDA-approved treatment doses.31
Patients with thrombophilia: VTE prevention
Thrombophilias are broadly categorized as inherited or acquired, with inherited thrombophilia being more prevalent. The Factor V Leiden (FVL) variant affects 2% to 7% of the population, and prothrombin gene mutation (PGM) affects 1% to 2% of the population.36 Other forms of inherited thrombophilia, such as protein C deficiency, protein S deficiency, and antithrombin deficiency, occur less commonly (< 0.7% of the population).36 Antiphospholipid syndrome (APS), the most common acquired thrombophilia, affects approximately 2% of the population.36 APS involves multiple antibodies: anticardiolipin antibodies, lupus anticoagulant, and anti-beta-2 glycoprotein 1 antibodies. Establishing risk for thrombosis across the varying types of thrombophilia has proven difficult, but APS is considered the most thrombogenic thrombophilia apart from extremely rare homozygous inherited thrombophilias.36 Therefore, DOAC recommendations are thrombophilia specific.
A prospective cohort study evaluated DOACs compared with heparin/warfarin for VTE treatment in patients with inherited thrombophilias.37 Although all 4 available DOACs were included, most patients (61.1%) received rivaroxaban. Patients with an array of inherited thrombophilias, including rare homozygous mutations, were enrolled in this trial. While most patients (66.9%) had a “mild thrombophilia” defined as either FVL or PGM, the remainder had more severe thrombophilias.37 VTE recurrence was similar and uncommon in the DOAC and heparin/warfarin groups, consistent with a previous meta-analysis.38 Surprisingly, an increase in the cumulative risk for bleeding was seen in the DOAC group compared with the warfarin group, a finding inconsistent with prior trials.38 There were no major bleeding events in the DOAC group, but 3 such events occurred in the heparin/warfarin group, including 2 intracranial hemorrhages.
Currently NICE, CHEST, and ISTH do not make a recommendation for a preferred agent in patients with an acute VTE and inherited thrombophilia; however, DOACs would not be inappropriate.23,28,32 The American Society of Hematology (ASH) had planned to release recommendations related to the treatment of thrombophilia in 2020, but they were delayed by the COVID-19 pandemic.39
APS presents challenges for acute VTE anticoagulation. First, it causes a strongly thrombogenic state necessitating therapeutic anticoagulation. Second, for patients with positive lupus anticoagulant, INR monitoring and standardized INR goals may be inadequate.40 Therefore, using fixed-dose DOACs without the need for therapeutic monitoring is appealing, but significant concerns exist for using DOACs in patients with APS.41-45 ISTH and CHEST recommend warfarin for the treatment and prevention of acute VTE in patients with APS, especially those with triple-positive (anticardiolipin, lupus anticoagulant, and anti-beta-2 glycoprotein 1) APS.13,46 Package labeling for all DOACs recommends avoidance in triple-positive APS.1-4
ASTRO-APS is the most recent RCT to compare apixaban and warfarin for patients with APS,47 and it was terminated early after 6 of 23 patients in the apixaban group had thrombotic events, while no one in the warfarin group had such an event.48 Subsequently, a meta-analysis49 demonstrated that patients with thrombotic APS appear to have a greater risk for arterial thrombosis when treated with DOACs compared with warfarin. These 2 studies may lead to changes in recommendations to avoid DOACs in all patients with APS or may prompt more focused trials for DOAC use in patients with APS plus an antiplatelet to mitigate arterial thrombotic risk.
Continue to: Expanded clinical indications
Expanded clinical indications
Superficial vein thrombosis
Superficial thrombophlebitis or superficial vein thrombosis (SVT) is estimated to occur 6 times more frequently than VTE.50 Management of patients with isolated, uncomplicated thrombophlebitis who are at low risk for extension of the SVT involves symptomatic treatment with nonsteroidal anti-inflammatory drugs, topical agents, or compression therapy. However, depending on risk for progression, anticoagulation may be recommended.51
Patients at intermediate risk for extension or propagation of SVT are candidates for anticoagulation. The CHEST guidelines recommend
Certain situations should prompt one to consider using a treatment dose of a DOAC for 3 months. These include cases in which the SVT is located within 3 cm of the deep venous system, expands despite an appropriate prophylactic regimen, or recurs after discontinuation of prophylactic anticoagulation.13,50
Acute coronary syndrome
The American College of Cardiology/American Heart Association (ACC/AHA) recommend combination antiplatelet therapy and anticoagulation for management of acute coronary syndrome in hospitalized patients.52 Data are mixed regarding longer-term anticoagulation in addition to dual antiplatelet therapy in outpatient settings to prevent thrombosis recurrence in the absence of AF.
The APPRAISE-2 trial enrolled high-risk patients with ACS within 7 days of the event.53 Apixaban 5 mg twice daily was compared with placebo in patients taking aspirin or aspirin plus clopidogrel. The trial was terminated early because major bleeding events increased with apixaban without reduction in recurrent ischemic events. The ATLAS ACS-TIMI 46 trial evaluated different rivaroxaban doses (5-20 mg daily) in ACS patients.54 The study revealed possible thrombosis benefit but also increased risk for bleeding, particularly at higher doses. As a result, another study—ATLAS ACS 2-TIMI 51—was conducted and compared the use of low-dose rivaroxaban (2.5 mg twice daily or 5 mg twice daily) vs placebo for patients with recent ACS.55 All patients were receiving low-dose aspirin, and approximately 93% of patients in each group also were receiving clopidogrel or ticlopidine. As in the APPRAISE-2 trial, rivaroxaban increased the rate of major bleeding and intracranial hemorrhage; however, it did not increase the incidence of fatal bleeding. Unlike APPRAISE-2, rivaroxaban significantly reduced the primary efficacy end point, a composite of death from cardiovascular causes, myocardial infarction, or stroke (absolute risk reduction = 1.8%; number needed to treat = 56 for combined rivaroxaban doses).55
A secondary subgroup analysis combined data from the ATLAS ACMS-TIMI 46 and ATLAS ACS 2-TIMI 51 trials to evaluate outcomes in patients receiving aspirin monotherapy when combined with rivaroxaban 2.5 mg twice daily or 5 mg twice daily or with placebo.56 The primary efficacy end point was a composite of cardiovascular death, myocardial infarction, or stroke. When the 2 trials were evaluated separately, neither rivaroxaban dose was associated with reduction of the primary efficacy outcomes compared with aspirin alone. However, when the data were pooled, both the combined rivaroxaban doses (particularly the 5-mg dose) were associated with reduced cardiovascular outcomes. From a safety perspective, the 2.5-mg twice-daily dose of rivaroxaban was the only dose not associated with increased major bleeding risk. Thus, the 2.5-mg twice-daily dose of rivaroxaban may not provide sufficient cardiovascular benefit in patients with ACS, while the larger dose may increase the risk for nonfatal major bleeding events.56
The European Medicines Agency57 approved rivaroxaban 2.5 mg twice daily for ACS, and the 2020 ESC guidelines58 consider it an appropriate therapeutic option in addition to aspirin for patients at high ischemic risk and low bleeding risk. ACS is not an FDA-approved indication for DOACs, and the ACC/AHA Guideline for the Management of ACS, last updated in 2014, does not include DOACs for ACS unless patients have AF.52 Ongoing trials are further investigating rivaroxaban for ACS, so the use of DOACs in the post-acute phase of ACS may become clearer in the future.59
Continue to: Heparin-induced thrombocytopenia
Heparin-induced thrombocytopenia
Historically, nonheparin parenteral anticoagulants argatroban, bivalirudin, and fondaparinux were recommended for patients at risk for or who had heparin-induced thrombocytopenia (HIT). Argatroban is the only drug FDA approved for the treatment and prophylaxis of HIT; recommendations for the others are based on guideline recommendations.23,60,61 The nonheparin parenteral anticoagulants cost between $700 and $1500 per day; therefore most patients with HIT are transitioned to warfarin.62 However, protein C and S inhibition and a subsequent prothrombotic state conveyed by warfarin initiation necessitates a minimum 5-day bridge to therapeutic warfarin with a nonheparin parenteral anticoagulant.
In vitro tests show that DOACs do not promote development of HIT antibodies63 or affect platelet activation or aggregation.64 A literature summary of DOACs for HIT determined that in 104 patients, all but 1 achieved platelet recovery (defined as > 150,000/mcL) within a median time of 7 days. Therapeutically, DOACs prevented new or recurrent VTE in 102/104 cases (98%), and only 3% of patients experienced significant bleeding events.62
The 2018 ASH guidelines for VTE management in HIT include (with very low certainty of evidence) dabigatran, rivaroxaban, or apixaban for consideration in addition to previously recommended nonheparin parenteral anticoagulants.61 The dosing of each agent is contingent upon treatment of patients with HIT and an acute thrombosis (HITT) or HIT in the absence of VTE. For patients with HITT, treatment doses for acute VTE should be used for the appropriate duration of therapy (ie, 3 months). Importantly, dabigatran requires a 5-day pretreatment period with a parenteral anticoagulant, so it is not an ideal option. When treating isolated HIT (in the absence of VTE), ASH recommends all agents be dosed twice daily—dabigatran 150 mg twice daily (no 5-day parenteral pretreatment necessary), rivaroxaban 15 mg twice daily, or apixaban 5 mg twice daily—until platelet recovery (≥ 150,000/mcL) is achieved.61
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; kevin-schleich@uiowa.edu
Four medications comprise the drug category known as direct oral anticoagulants (DOACs). Dabigatran (Pradaxa)1 was the first to gain approval. It was approved by the US Food and Drug Administration (FDA) in 2010 for the reduction of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (AF). This was followed by approvals for rivaroxaban (Xarelto)2 in 2011, apixaban (Eliquis)3 in 2012, and edoxaban (Savaysa)4 in 2015. Betrixaban (Bevyxxa)5 was approved in 2017 for venous thromboembolism (VTE) prophylaxis in acutely ill hospitalized patients with restricted mobility, but it was removed from the market in 2020.

In addition to stroke prevention in nonvalvular AF, each DOAC has been approved for other indications and has been addressed further in guideline-based recommendations outside FDA-approved indications.

Overview of DOACs
Dabigatran is the only direct thrombin inhibitor; the other agents inhibit factor Xa. TABLE 11-4 summarizes FDA-approved indications and dosing and guideline-based dosing. Dabigatran and edoxaban require parenteral anticoagulation for 5 to 10 days prior to initiation for acute VTE, limiting their use.1,4TABLE 21-4 highlights pharmacokinetic differences among the agents. For example, dabigatran is 80% renally cleared, is somewhat dialyzable, and can accumulate in patients with renal dysfunction.1 Edoxaban is contraindicated for nonvalvular AF in patients with a creatinine clearance (CrCl) > 95 mL/min because an increased stroke risk was demonstrated.4 Therefore, rivaroxaban and apixaban are prescribed most often in the United States.6,7

Applications in special patient populations
Obesity
As of 2020, more than 40% of adults in the United States were obese (body mass index [BMI] ≥ 30), with 9% classified as class 3 or severely obese (BMI ≥ 40).8 Altered drug pharmacokinetics in patients with severe obesity raises concern for undertreatment with fixed-dose DOACs. Phase III DOAC approval trials included patients with obesity, but weight cutoffs differed, making extrapolating efficacy and safety data difficult across different obesity stages.9 Although no FDA-labeled dosing adjustments exist for patients with obesity, the International Society on Thrombosis and Haemostasis (ISTH) does provide such recommendations.
ISTH changes position on measuring drug levels. ISTH previously recommended avoiding DOACs in those with a BMI > 40 or body weight > 120 kg. If a DOAC was used, ISTH advised obtaining peak and trough drug levels.10 However, DOAC drug levels have not been associated with clinical outcomes or sufficient degrees of anticoagulation.11
Men and women are affected equally by fibrolipomas. Prevalence does not differ by race or ethnicity.
In April 2021, ISTH updated guidance on DOACs in obesity, indicating standard doses of rivaroxaban or apixaban can be used for the treatment and prevention of VTE in all patients regardless of weight or BMI. Because data in obesity are lacking for dabigatran and edoxaban, avoid using these agents in patients with a BMI > 40 or weight > 120 kg. Additionally, assessing drug levels is no longer recommended, as there is insufficient evidence that these impact clinical outcomes.12
The 2021 American College of Chest Physicians (CHEST) guideline update
Continue to: Effectiveness of DOACs for AF in patients with obesity isn't clear
Effectiveness of DOACs for AF in patients with obesity isn’t clear, as most data are from retrospective cohort analyses. In patients weighing > 120 kg, dabigatran has shown efficacy in thrombosis prevention similar to that achieved in those weighing ≤ 120 kg, but it has increased the risk for gastrointestinal (GI) bleeding.15 Another study indicated a 15-mg dose of rivaroxaban may be associated with increased thromboembolic complications in patients with a BMI ≥ 35.16 Alternatively, another retrospective study of rivaroxaban demonstrated a small absolute risk reduction in ischemic stroke among patients in all stages of obesity and no difference in significant bleeding events.17 One further retrospective cohort showed that, in patients with a BMI ≥ 50 kg, the effectiveness of rivaroxaban and apixaban in thrombosis prevention and bleeding safety outcomes was comparable to that seen in those with a BMI < 30.18
As a result of conflicting data, and a lack of prospective randomized controlled trials (RCTs), ISTH continued recommending international normalized ratio (INR)–based dosing of warfarin for class 3 or severely obese patients with AF. The 2018 CHEST guidelines19 and the 2020 ESC guidelines20 make no mention of DOAC avoidance in patients with obesity and AF.
Advanced and end-stage renal disease
DOACs are renally dosed based on indication, drug-drug interactions, and degree of renal function (TABLE 31-4). For example, patients with AF who are anticoagulated with apixaban are prescribed 2.5 mg twice daily when 2 of the 3 following criteria are met: age ≥ 80 years, body weight ≤ 60 kg, serum creatinine ≥ 1.5 mg/dL. However, no dosage adjustment is necessary for VTE treatment or prophylaxis with apixaban regardless of renal function.3

Data supporting the safety and efficacy of DOACs in end-stage renal disease (ESRD) are sparse. All DOACs are renally cleared to varying degrees (TABLE 21-4), theoretically increasing bleeding risk as kidney disease progresses. Apixaban is the least renally cleared of the DOACs and has been evaluated in the greatest number of trials for patients with ESRD for both VTE treatment and prevention and nonvalvular AF.21 As a result, the FDA approved standard-dose apixaban (5 mg twice daily) for VTE treatment and prevention and nonvalvular AF in patients with ESRD, even those requiring dialysis. Use the reduced apixaban dose (2.5 mg twice daily) in patients with ESRD and AF only if they are ≥ 80 years of age or their body weight is ≤ 60 kg.3
Patients with cancer
Cancer-associated acute VTE treatment. Cancer is an established risk factor for acute VTE but it also increases the risk for treatment-associated bleeding compared with patients without cancer.22 Historically, low-molecular-weight heparin (LMWH) was recommended over warfarin and DOACs for cancer-associated thromboses (CAT).23 Compared with warfarin, LMWH reduced the rate of recurrent VTE and had similar or reduced bleeding rates at 6 to 12 months.24-26 However, clinicians and patients often chose warfarin to avoid subcutaneous injections.27
CHEST guidelines recommend oral Xa inhibitors over LMWH for the treatment of CAT.13 The 2020 guidelines of the National Institute for Health and Care Excellence (NICE) recommend DOACs as an option for CAT along with LMWH or LMWH transitioned to warfarin.28 The American Society of Clinical Oncology (ASCO) recommends rivaroxaban for acute VTE treatment in CAT. No head-to-head trials have evaluated comparative efficacy of DOACs for CAT. However, edoxaban and rivaroxaban are associated with a greater risk for GI bleeding; therefore, apixaban is preferred in patients with GI malignancies.29 Standard DOAC VTE treatment dosing is recommended for all 3 agents.2-4
When using DOACs for patients with CAT, consider potential drug-drug interactions with chemotherapy regimens. All DOACs are transported by p-glycoprotein, while rivaroxaban and apixaban are substrates of cytochrome P450, leading to potentially significant drug-drug interactions.30 These interactions could affect the patient’s chemotherapeutic regimen, decrease the efficacy of the DOAC, or increase the risk for bleeding. Therefore, anticoagulation choice should be made in collaboration with the hematology/oncology team.
Continue to: Cancer-associated VTE prophylaxis...
Cancer-associated VTE prophylaxis. VTE prophylaxis for patients with cancer is complex and necessitates a global assessment of cancer location and treatment regimen and setting. Hospitalized patients receiving chemotherapy are at high risk for VTE if mobility is reduced or if other VTE risk factors are present. The International Initiative on Thrombosis and Cancer (ITAC)31 and ISTH32 recommend VTE prophylaxis with unfractionated heparin or LMWH (ISTH recommends LMWH more strongly). The 2020 ASCO Guidelines recommend pharmacologic anticoagulation but make no drug-specific recommendation.29 Parenteral treatment in hospitalized patients is not as burdensome as it is in ambulatory patients; therefore, these recommendations are less likely to elicit inpatient opposition.
In the ambulatory setting, patient avoidance of subcutaneous injections necessitates consideration of DOACs for CAT prophylaxis. The Khorana Risk Score (KRS) is a validated tool (scale, 0-7) to predict VTE risk in ambulatory patients receiving chemotherapy.33 KRS scores ≥ 2 indicate high thrombotic risk and the need for prophylactic anticoagulation. ASCO recommends apixaban, rivaroxaban, or LMWH.29 ISTH and ITAC both recommend apixaban or rivaroxaban over LMWH.31,34 An RCT published in June 2023 confirmed that, for adults with cancer and VTE, DOACs were noninferior to LMWH for preventing recurrent VTE for 6 months.35 The recommended doses for apixaban (2.5 mg twice daily) and rivaroxaban (10 mg daily) for CAT VTE prophylaxis are lower than FDA-approved treatment doses.31
Patients with thrombophilia: VTE prevention
Thrombophilias are broadly categorized as inherited or acquired, with inherited thrombophilia being more prevalent. The Factor V Leiden (FVL) variant affects 2% to 7% of the population, and prothrombin gene mutation (PGM) affects 1% to 2% of the population.36 Other forms of inherited thrombophilia, such as protein C deficiency, protein S deficiency, and antithrombin deficiency, occur less commonly (< 0.7% of the population).36 Antiphospholipid syndrome (APS), the most common acquired thrombophilia, affects approximately 2% of the population.36 APS involves multiple antibodies: anticardiolipin antibodies, lupus anticoagulant, and anti-beta-2 glycoprotein 1 antibodies. Establishing risk for thrombosis across the varying types of thrombophilia has proven difficult, but APS is considered the most thrombogenic thrombophilia apart from extremely rare homozygous inherited thrombophilias.36 Therefore, DOAC recommendations are thrombophilia specific.
A prospective cohort study evaluated DOACs compared with heparin/warfarin for VTE treatment in patients with inherited thrombophilias.37 Although all 4 available DOACs were included, most patients (61.1%) received rivaroxaban. Patients with an array of inherited thrombophilias, including rare homozygous mutations, were enrolled in this trial. While most patients (66.9%) had a “mild thrombophilia” defined as either FVL or PGM, the remainder had more severe thrombophilias.37 VTE recurrence was similar and uncommon in the DOAC and heparin/warfarin groups, consistent with a previous meta-analysis.38 Surprisingly, an increase in the cumulative risk for bleeding was seen in the DOAC group compared with the warfarin group, a finding inconsistent with prior trials.38 There were no major bleeding events in the DOAC group, but 3 such events occurred in the heparin/warfarin group, including 2 intracranial hemorrhages.
Currently NICE, CHEST, and ISTH do not make a recommendation for a preferred agent in patients with an acute VTE and inherited thrombophilia; however, DOACs would not be inappropriate.23,28,32 The American Society of Hematology (ASH) had planned to release recommendations related to the treatment of thrombophilia in 2020, but they were delayed by the COVID-19 pandemic.39
APS presents challenges for acute VTE anticoagulation. First, it causes a strongly thrombogenic state necessitating therapeutic anticoagulation. Second, for patients with positive lupus anticoagulant, INR monitoring and standardized INR goals may be inadequate.40 Therefore, using fixed-dose DOACs without the need for therapeutic monitoring is appealing, but significant concerns exist for using DOACs in patients with APS.41-45 ISTH and CHEST recommend warfarin for the treatment and prevention of acute VTE in patients with APS, especially those with triple-positive (anticardiolipin, lupus anticoagulant, and anti-beta-2 glycoprotein 1) APS.13,46 Package labeling for all DOACs recommends avoidance in triple-positive APS.1-4
ASTRO-APS is the most recent RCT to compare apixaban and warfarin for patients with APS,47 and it was terminated early after 6 of 23 patients in the apixaban group had thrombotic events, while no one in the warfarin group had such an event.48 Subsequently, a meta-analysis49 demonstrated that patients with thrombotic APS appear to have a greater risk for arterial thrombosis when treated with DOACs compared with warfarin. These 2 studies may lead to changes in recommendations to avoid DOACs in all patients with APS or may prompt more focused trials for DOAC use in patients with APS plus an antiplatelet to mitigate arterial thrombotic risk.
Continue to: Expanded clinical indications
Expanded clinical indications
Superficial vein thrombosis
Superficial thrombophlebitis or superficial vein thrombosis (SVT) is estimated to occur 6 times more frequently than VTE.50 Management of patients with isolated, uncomplicated thrombophlebitis who are at low risk for extension of the SVT involves symptomatic treatment with nonsteroidal anti-inflammatory drugs, topical agents, or compression therapy. However, depending on risk for progression, anticoagulation may be recommended.51
Patients at intermediate risk for extension or propagation of SVT are candidates for anticoagulation. The CHEST guidelines recommend
Certain situations should prompt one to consider using a treatment dose of a DOAC for 3 months. These include cases in which the SVT is located within 3 cm of the deep venous system, expands despite an appropriate prophylactic regimen, or recurs after discontinuation of prophylactic anticoagulation.13,50
Acute coronary syndrome
The American College of Cardiology/American Heart Association (ACC/AHA) recommend combination antiplatelet therapy and anticoagulation for management of acute coronary syndrome in hospitalized patients.52 Data are mixed regarding longer-term anticoagulation in addition to dual antiplatelet therapy in outpatient settings to prevent thrombosis recurrence in the absence of AF.
The APPRAISE-2 trial enrolled high-risk patients with ACS within 7 days of the event.53 Apixaban 5 mg twice daily was compared with placebo in patients taking aspirin or aspirin plus clopidogrel. The trial was terminated early because major bleeding events increased with apixaban without reduction in recurrent ischemic events. The ATLAS ACS-TIMI 46 trial evaluated different rivaroxaban doses (5-20 mg daily) in ACS patients.54 The study revealed possible thrombosis benefit but also increased risk for bleeding, particularly at higher doses. As a result, another study—ATLAS ACS 2-TIMI 51—was conducted and compared the use of low-dose rivaroxaban (2.5 mg twice daily or 5 mg twice daily) vs placebo for patients with recent ACS.55 All patients were receiving low-dose aspirin, and approximately 93% of patients in each group also were receiving clopidogrel or ticlopidine. As in the APPRAISE-2 trial, rivaroxaban increased the rate of major bleeding and intracranial hemorrhage; however, it did not increase the incidence of fatal bleeding. Unlike APPRAISE-2, rivaroxaban significantly reduced the primary efficacy end point, a composite of death from cardiovascular causes, myocardial infarction, or stroke (absolute risk reduction = 1.8%; number needed to treat = 56 for combined rivaroxaban doses).55
A secondary subgroup analysis combined data from the ATLAS ACMS-TIMI 46 and ATLAS ACS 2-TIMI 51 trials to evaluate outcomes in patients receiving aspirin monotherapy when combined with rivaroxaban 2.5 mg twice daily or 5 mg twice daily or with placebo.56 The primary efficacy end point was a composite of cardiovascular death, myocardial infarction, or stroke. When the 2 trials were evaluated separately, neither rivaroxaban dose was associated with reduction of the primary efficacy outcomes compared with aspirin alone. However, when the data were pooled, both the combined rivaroxaban doses (particularly the 5-mg dose) were associated with reduced cardiovascular outcomes. From a safety perspective, the 2.5-mg twice-daily dose of rivaroxaban was the only dose not associated with increased major bleeding risk. Thus, the 2.5-mg twice-daily dose of rivaroxaban may not provide sufficient cardiovascular benefit in patients with ACS, while the larger dose may increase the risk for nonfatal major bleeding events.56
The European Medicines Agency57 approved rivaroxaban 2.5 mg twice daily for ACS, and the 2020 ESC guidelines58 consider it an appropriate therapeutic option in addition to aspirin for patients at high ischemic risk and low bleeding risk. ACS is not an FDA-approved indication for DOACs, and the ACC/AHA Guideline for the Management of ACS, last updated in 2014, does not include DOACs for ACS unless patients have AF.52 Ongoing trials are further investigating rivaroxaban for ACS, so the use of DOACs in the post-acute phase of ACS may become clearer in the future.59
Continue to: Heparin-induced thrombocytopenia
Heparin-induced thrombocytopenia
Historically, nonheparin parenteral anticoagulants argatroban, bivalirudin, and fondaparinux were recommended for patients at risk for or who had heparin-induced thrombocytopenia (HIT). Argatroban is the only drug FDA approved for the treatment and prophylaxis of HIT; recommendations for the others are based on guideline recommendations.23,60,61 The nonheparin parenteral anticoagulants cost between $700 and $1500 per day; therefore most patients with HIT are transitioned to warfarin.62 However, protein C and S inhibition and a subsequent prothrombotic state conveyed by warfarin initiation necessitates a minimum 5-day bridge to therapeutic warfarin with a nonheparin parenteral anticoagulant.
In vitro tests show that DOACs do not promote development of HIT antibodies63 or affect platelet activation or aggregation.64 A literature summary of DOACs for HIT determined that in 104 patients, all but 1 achieved platelet recovery (defined as > 150,000/mcL) within a median time of 7 days. Therapeutically, DOACs prevented new or recurrent VTE in 102/104 cases (98%), and only 3% of patients experienced significant bleeding events.62
The 2018 ASH guidelines for VTE management in HIT include (with very low certainty of evidence) dabigatran, rivaroxaban, or apixaban for consideration in addition to previously recommended nonheparin parenteral anticoagulants.61 The dosing of each agent is contingent upon treatment of patients with HIT and an acute thrombosis (HITT) or HIT in the absence of VTE. For patients with HITT, treatment doses for acute VTE should be used for the appropriate duration of therapy (ie, 3 months). Importantly, dabigatran requires a 5-day pretreatment period with a parenteral anticoagulant, so it is not an ideal option. When treating isolated HIT (in the absence of VTE), ASH recommends all agents be dosed twice daily—dabigatran 150 mg twice daily (no 5-day parenteral pretreatment necessary), rivaroxaban 15 mg twice daily, or apixaban 5 mg twice daily—until platelet recovery (≥ 150,000/mcL) is achieved.61
CORRESPONDENCE
Kevin Schleich, PharmD, BCACP, Departments of Pharmaceutical Care and Family Medicine, University of Iowa, 200 Hawkins Drive, 01102-D PFP, Iowa City, IA, 52242; kevin-schleich@uiowa.edu
1. Dabigatran. Package Insert. Boehringer Ingelheim Pharmaceuticals, Inc.; 2021.
2. Rivaroxaban. Package insert. Janssen Pharmaceuticals, Inc; 2022.
3. Apixaban. Package insert. Bristol-Myers Squibb; 2021.
4. Edoxaban. Package insert. Daiichi Sankyo, Inc; 2015.
5. Betrixaban. Package insert. Portola Pharmaceuticals, Inc; 2017.
6. Wheelock KM, Ross JS, Murugiah K, et al. Clinician trends in prescribing direct oral anticoagulants for US Medicare beneficiaries. JAMA Netw Open. 2021;4:e2137288. doi: 10.1001/jamanetworkopen.2021.37288
7. Colacci M, Tseng EK, Sacks CA, et al. Oral anticoagulant utilization in the United States and United Kingdom. J Gen Intern Med. 2020;35:2505-2507. doi: 10.1007/s11606-020-05904-0
8. CDC. Adult obesity facts. Accessed May 9, 2023. www.cdc.gov/obesity/data/adult.html
9. Mocini D, Di Fusco SA, Mocini E, et al. Direct oral anticoagulants in patients with obesity and atrial fibrillation: position paper of Italian National Association of Hospital Cardiologists (ANMCO). J Clin Med. 2021;10:4185. doi: 10.3390/jcm10184185
10. Martin K, Beyer-Westendorf J, Davidson BL, et al. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:1308-1313. doi: 10.1111/jth.13323
11. Gu TM, Garcia DA, Sabath DE. Assessment of direct oral anticoagulant assay use in clinical practice. J Thromb Thrombolysis. 2019;47:403-408. doi: 10.1007/s11239-018-1793-0
12. Martin KA, Beyer-Westendorf J, Davidson BL, et al. Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: updated communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J Thromb Haemost. 2021;19:1874-1882. doi: 10.1111/jth.15358
13. Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160:e545-e608. doi: 10.1016/j.chest.2021.07.055
14. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. doi: 10.1093/eurheartj/ehz405
15. Coates J, Bitton E, Hendje A, et al. Clinical outcomes of dabigatran use in patients with non-valvular atrial fibrillation and weight >120 kg. Thromb Res. 2021;208:176-180. doi: 10.1016/j.thromres.2021.11.007
16. Li X, Zuo C, Ji Q, et al. Body mass index influence on the clinical outcomes for nonvalvular atrial fibrillation patients admitted to a hospital treated with direct oral anticoagulants: a retrospective cohort study. Drug Des Devel Ther. 2021;15:1931-1943. doi: 10.2147/dddt.S303219
17. Barakat AF, Jain S, Masri A, et al. Outcomes of direct oral anticoagulants in atrial fibrillation patients across different body mass index categories. JACC Clin Electrophysiol. 2021;7:649-658. doi: 10.1016/j.jacep.2021.02.002
18. O’Kane CP, Avalon JCO, Lacoste JL, et al. Apixaban and rivaroxaban use for atrial fibrillation in patients with obesity and BMI ≥50 kg/m2. Pharmacotherapy. 2022;42:112-118. doi: https://doi.org/10.1002/phar.2651
19. Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST Guideline and Expert Panel Report. Chest. 2018;154:1121-1201. doi: 10.1016/j.chest.2018.07.040
20. Sepehri Shamloo A, Dagres N, Hindricks G. [2020 ESC guidelines on atrial fibrillation: summary of the most relevant recommendations and innovations]. Herz. 2021;46:28-37. doi: 10.1007/s00059-020-05005-y
21. Chokesuwattanaskul R, Thongprayoon C, Tanawuttiwat T, et al. Safety and efficacy of apixaban versus warfarin in patients with end-stage renal disease: meta-analysis. Pacing Clin Electrophysiol. 2018;41:627-634. doi: 10.1111/pace.13331
22. Wang T-F, Li A, Garcia D. Managing thrombosis in cancer patients. Res Pract Thromb Haemost. 2018;2:429-438. doi: https://doi.org/10.1002/rth2.12102
23. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. CHEST. 2016;149:315-352. doi: 10.1016/j.chest.2015.11.026
24. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146-153. doi: 10.1056/NEJMoa025313
25. Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162:1729-1735. doi: 10.1001/archinte.162.15.1729
26. Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119:1062-1072. doi: 10.1016/j.amjmed.2006.02.022
27. Lee AYY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314:677-686. doi: 10.1001/jama.2015.9243
28. NICE Guideline. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. Accessed May 9, 2023. www.ncbi.nlm.nih.gov/books/NBK556698/
29. Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2020;38:496-520. doi: 10.1200/jco.19.01461
30. Galgani A, Palleria C, Iannone LF, et al. Pharmacokinetic interactions of clinical interest between direct oral anticoagulants and antiepileptic drugs. Front Neurol. 2018;9:1067. doi: 10.3389/fneur.2018.01067
31. Farge D, Frere C, Connors JM, et al. 2019 International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20:e566-e581. doi: 10.1016/s1470-2045(19)30336-5
32. Di Nisio M, Carrier M, Lyman GH, et al. Prevention of venous thromboembolism in hospitalized medical cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2014;12:1746-1749. doi: 10.1111/jth.12683
33. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907. doi: 10.1182/blood-2007-10-116327
34. Wang TF, Zwicker JI, Ay C, et al. The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2019;17:1772-1778. doi: 10.1111/jth.14564
35. Schrag D, Uno H, Rosovsky R, et al. Direct oral anticoagulants vs low-molecular-weight heparin and recurrent VTE in patients with cancer: a randomized clinical trial. JAMA. 2023;329:1924-1933. doi: 10.1001/jama.2023.7843
36. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41:154-164. doi: 10.1007/s11239-015-1316-1
37. Campello E, Spiezia L, Simion C, et al. Direct oral anticoagulants in patients with inherited thrombophilia and venous thromboembolism: a prospective cohort study. J Am Heart Assoc. 2020;9:e018917. doi: 10.1161/jaha.120.018917
38. Elsebaie MAT, van Es N, Langston A, et al. Direct oral anticoagulants in patients with venous thromboembolism and thrombophilia: a systematic review and meta-analysis. J Thromb Haemost. 2019;17:645-656. doi: 10.1111/jth.14398
39. ASH. ASH Clinical Practice Guidelines on Venous Thromboembolism. Accessed May 10, 2023. www.hematology.org/education/clinicians/guidelines-and-quality-care/clinical-practice-guidelines/venous-thromboembolism-guidelines
40. Baquero-Salamanca M, Téllez-Arévalo AM, Calderon-Ospina C. Variability in the international normalised ratio (INR) in patients with antiphospholipid syndrome and positive lupus anticoagulant: should the INR targets be higher? BMJ Case Rep. 2015;2015:bcr2014209013. doi: 10.1136/bcr-2014-209013
41. Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018;132:1365-1371. doi: 10.1182/blood-2018-04-848333
42. Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M, et al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome: a randomized noninferiority trial. Ann Intern Med. 2019;171:685-694. doi: 10.7326/m19-0291
43. Sato T, Nakamura H, Fujieda Y, et al. Factor Xa inhibitors for preventing recurrent thrombosis in patients with antiphospholipid syndrome: a longitudinal cohort study. Lupus. 2019;28:1577-1582. doi: 10.1177/0961203319881200
44. Malec K, Broniatowska E, Undas A. Direct oral anticoagulants in patients with antiphospholipid syndrome: a cohort study. Lupus. 2020;29:37-44. doi: 10.1177/0961203319889156
45. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome. Dr. Hannah Cohen about the results of the RAPS trial (Lancet Haematol 2016; 3: e426-36). Rheumatology (Oxford). 2017;56:e23. doi: 10.1093/rheumatology/kex290
46. Zuily S, Cohen H, Isenberg D, et al. Use of direct oral anticoagulants in patients with thrombotic antiphospholipid syndrome: guidance from the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2020;18:2126-2137. doi: https://doi.org/10.1111/jth.14935
47. NIH. ClinicalTrials.gov. Apixaban for the secondary prevention of thromboembolism among patients with antiphospholipid syndrome (ASTRO-APS). Accessed May 10, 2023. https://clinicaltrials.gov/ct2/show/NCT02295475?term=apixaban&cond=Anti+Phospholipid+Syndrome&draw=2&rank=1
48. Woller SC, Stevens SM, Kaplan D, et al. Apixaban compared with warfarin to prevent thrombosis in thrombotic antiphospholipid syndrome: a randomized trial. Blood Adv. 2022;6:1661-1670. doi: 10.1182/bloodadvances.2021005808
49. Khairani CD, Bejjani A, Piazza G, et al. Direct oral anticoagulants vs vitamin K antagonists in patients with antiphospholipid syndromes: meta-analysis of randomized trials. J Am Coll Cardiol. 2023;81:16-30. doi: 10.1016/j.jacc.2022.10.008
50. Superficial thrombophlebitis, superficial vein thrombosis. 2021. Accessed May 10, 2023. thrombosiscanada.ca/wp-content/uploads/2021/07/47.-Superficial-Vein-Thrombosis_16July2021.pdf
51. Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev. 2018;2:CD004982. doi: 10.1002/14651858.CD004982.pub6
52. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139-e228. doi: 10.1016/j.jacc.2014.09.017
53. Alexander JH, Lopes RD, James S, et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365:699-708. doi: 10.1056/NEJMoa1105819
54. Mega JL, Braunwald E, Mohanavelu S, et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet. 2009;374:29-38. doi: 10.1016/s0140-6736(09)60738-8
55. Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9-19. doi: 10.1056/NEJMoa1112277
56. Gibson WJ, Gibson CM, Yee MK, et al. Safety and efficacy of rivaroxaban when added to aspirin monotherapy among stabilized post‐acute coronary syndrome patients: a pooled analysis study of ATLAS ACS‐TIMI 46 and ATLAS ACS 2‐TIMI 51. J Am Heart Assoc. 2019. Accessed May 10, 2023. Doi: 10.1161/JAHA.118.009451
57. European Medicines Agency. Xarelto (rivaroxaban). 2008. Accessed June 23, 2023.
58. Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289-1367. doi: 10.1093/eurheartj/ehaa575
59. NIH. ClinicalTrials.gov. Accessed May 10, 2023. www.clinicaltrials.gov/ct2/results?cond=Acute+Coronary+Syndrome&term=rivaroxaban+&cntry=&state=&city=&dist=#
60. Watson H, Davidson S, Keeling D. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: second edition. Br J Haematol. 2012;159:528-40. doi: 10.1111/bjh.12059
61. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2:3360-3392. doi: 10.1182/bloodadvances.2018024489
62. Momin J, Lee C-S. The role of direct oral anticoagulants in the management of heparin-induced thrombocytopenia US Pharmacist. 2020;45:3-10. Accessed May 10, 2023. www.uspharmacist.com/article/the-role-of-direct-oral-anticoagulants-in-the-management-of-heparininduced-thrombocytopenia
63. Warkentin TE, Pai M, Linkins LA. Direct oral anticoagulants for treatment of HIT: update of Hamilton experience and literature review. Blood. 2017;130:1104-1113. doi: 10.1182/blood-2017-04-778993
64. Krauel K, Hackbarth C, Fürll B, et al. Heparin-induced thrombocytopenia: in vitro studies on the interaction of dabigatran, rivaroxaban, and low-sulfated heparin, with platelet factor 4 and anti-PF4/heparin antibodies. Blood. 2012;119:1248-1255. doi: 10.1182/blood-2011-05-353391
1. Dabigatran. Package Insert. Boehringer Ingelheim Pharmaceuticals, Inc.; 2021.
2. Rivaroxaban. Package insert. Janssen Pharmaceuticals, Inc; 2022.
3. Apixaban. Package insert. Bristol-Myers Squibb; 2021.
4. Edoxaban. Package insert. Daiichi Sankyo, Inc; 2015.
5. Betrixaban. Package insert. Portola Pharmaceuticals, Inc; 2017.
6. Wheelock KM, Ross JS, Murugiah K, et al. Clinician trends in prescribing direct oral anticoagulants for US Medicare beneficiaries. JAMA Netw Open. 2021;4:e2137288. doi: 10.1001/jamanetworkopen.2021.37288
7. Colacci M, Tseng EK, Sacks CA, et al. Oral anticoagulant utilization in the United States and United Kingdom. J Gen Intern Med. 2020;35:2505-2507. doi: 10.1007/s11606-020-05904-0
8. CDC. Adult obesity facts. Accessed May 9, 2023. www.cdc.gov/obesity/data/adult.html
9. Mocini D, Di Fusco SA, Mocini E, et al. Direct oral anticoagulants in patients with obesity and atrial fibrillation: position paper of Italian National Association of Hospital Cardiologists (ANMCO). J Clin Med. 2021;10:4185. doi: 10.3390/jcm10184185
10. Martin K, Beyer-Westendorf J, Davidson BL, et al. Use of the direct oral anticoagulants in obese patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2016;14:1308-1313. doi: 10.1111/jth.13323
11. Gu TM, Garcia DA, Sabath DE. Assessment of direct oral anticoagulant assay use in clinical practice. J Thromb Thrombolysis. 2019;47:403-408. doi: 10.1007/s11239-018-1793-0
12. Martin KA, Beyer-Westendorf J, Davidson BL, et al. Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: updated communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J Thromb Haemost. 2021;19:1874-1882. doi: 10.1111/jth.15358
13. Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160:e545-e608. doi: 10.1016/j.chest.2021.07.055
14. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41:543-603. doi: 10.1093/eurheartj/ehz405
15. Coates J, Bitton E, Hendje A, et al. Clinical outcomes of dabigatran use in patients with non-valvular atrial fibrillation and weight >120 kg. Thromb Res. 2021;208:176-180. doi: 10.1016/j.thromres.2021.11.007
16. Li X, Zuo C, Ji Q, et al. Body mass index influence on the clinical outcomes for nonvalvular atrial fibrillation patients admitted to a hospital treated with direct oral anticoagulants: a retrospective cohort study. Drug Des Devel Ther. 2021;15:1931-1943. doi: 10.2147/dddt.S303219
17. Barakat AF, Jain S, Masri A, et al. Outcomes of direct oral anticoagulants in atrial fibrillation patients across different body mass index categories. JACC Clin Electrophysiol. 2021;7:649-658. doi: 10.1016/j.jacep.2021.02.002
18. O’Kane CP, Avalon JCO, Lacoste JL, et al. Apixaban and rivaroxaban use for atrial fibrillation in patients with obesity and BMI ≥50 kg/m2. Pharmacotherapy. 2022;42:112-118. doi: https://doi.org/10.1002/phar.2651
19. Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST Guideline and Expert Panel Report. Chest. 2018;154:1121-1201. doi: 10.1016/j.chest.2018.07.040
20. Sepehri Shamloo A, Dagres N, Hindricks G. [2020 ESC guidelines on atrial fibrillation: summary of the most relevant recommendations and innovations]. Herz. 2021;46:28-37. doi: 10.1007/s00059-020-05005-y
21. Chokesuwattanaskul R, Thongprayoon C, Tanawuttiwat T, et al. Safety and efficacy of apixaban versus warfarin in patients with end-stage renal disease: meta-analysis. Pacing Clin Electrophysiol. 2018;41:627-634. doi: 10.1111/pace.13331
22. Wang T-F, Li A, Garcia D. Managing thrombosis in cancer patients. Res Pract Thromb Haemost. 2018;2:429-438. doi: https://doi.org/10.1002/rth2.12102
23. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. CHEST. 2016;149:315-352. doi: 10.1016/j.chest.2015.11.026
24. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146-153. doi: 10.1056/NEJMoa025313
25. Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162:1729-1735. doi: 10.1001/archinte.162.15.1729
26. Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119:1062-1072. doi: 10.1016/j.amjmed.2006.02.022
27. Lee AYY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314:677-686. doi: 10.1001/jama.2015.9243
28. NICE Guideline. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. Accessed May 9, 2023. www.ncbi.nlm.nih.gov/books/NBK556698/
29. Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2020;38:496-520. doi: 10.1200/jco.19.01461
30. Galgani A, Palleria C, Iannone LF, et al. Pharmacokinetic interactions of clinical interest between direct oral anticoagulants and antiepileptic drugs. Front Neurol. 2018;9:1067. doi: 10.3389/fneur.2018.01067
31. Farge D, Frere C, Connors JM, et al. 2019 International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20:e566-e581. doi: 10.1016/s1470-2045(19)30336-5
32. Di Nisio M, Carrier M, Lyman GH, et al. Prevention of venous thromboembolism in hospitalized medical cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2014;12:1746-1749. doi: 10.1111/jth.12683
33. Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907. doi: 10.1182/blood-2007-10-116327
34. Wang TF, Zwicker JI, Ay C, et al. The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2019;17:1772-1778. doi: 10.1111/jth.14564
35. Schrag D, Uno H, Rosovsky R, et al. Direct oral anticoagulants vs low-molecular-weight heparin and recurrent VTE in patients with cancer: a randomized clinical trial. JAMA. 2023;329:1924-1933. doi: 10.1001/jama.2023.7843
36. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41:154-164. doi: 10.1007/s11239-015-1316-1
37. Campello E, Spiezia L, Simion C, et al. Direct oral anticoagulants in patients with inherited thrombophilia and venous thromboembolism: a prospective cohort study. J Am Heart Assoc. 2020;9:e018917. doi: 10.1161/jaha.120.018917
38. Elsebaie MAT, van Es N, Langston A, et al. Direct oral anticoagulants in patients with venous thromboembolism and thrombophilia: a systematic review and meta-analysis. J Thromb Haemost. 2019;17:645-656. doi: 10.1111/jth.14398
39. ASH. ASH Clinical Practice Guidelines on Venous Thromboembolism. Accessed May 10, 2023. www.hematology.org/education/clinicians/guidelines-and-quality-care/clinical-practice-guidelines/venous-thromboembolism-guidelines
40. Baquero-Salamanca M, Téllez-Arévalo AM, Calderon-Ospina C. Variability in the international normalised ratio (INR) in patients with antiphospholipid syndrome and positive lupus anticoagulant: should the INR targets be higher? BMJ Case Rep. 2015;2015:bcr2014209013. doi: 10.1136/bcr-2014-209013
41. Pengo V, Denas G, Zoppellaro G, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018;132:1365-1371. doi: 10.1182/blood-2018-04-848333
42. Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M, et al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome: a randomized noninferiority trial. Ann Intern Med. 2019;171:685-694. doi: 10.7326/m19-0291
43. Sato T, Nakamura H, Fujieda Y, et al. Factor Xa inhibitors for preventing recurrent thrombosis in patients with antiphospholipid syndrome: a longitudinal cohort study. Lupus. 2019;28:1577-1582. doi: 10.1177/0961203319881200
44. Malec K, Broniatowska E, Undas A. Direct oral anticoagulants in patients with antiphospholipid syndrome: a cohort study. Lupus. 2020;29:37-44. doi: 10.1177/0961203319889156
45. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome. Dr. Hannah Cohen about the results of the RAPS trial (Lancet Haematol 2016; 3: e426-36). Rheumatology (Oxford). 2017;56:e23. doi: 10.1093/rheumatology/kex290
46. Zuily S, Cohen H, Isenberg D, et al. Use of direct oral anticoagulants in patients with thrombotic antiphospholipid syndrome: guidance from the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2020;18:2126-2137. doi: https://doi.org/10.1111/jth.14935
47. NIH. ClinicalTrials.gov. Apixaban for the secondary prevention of thromboembolism among patients with antiphospholipid syndrome (ASTRO-APS). Accessed May 10, 2023. https://clinicaltrials.gov/ct2/show/NCT02295475?term=apixaban&cond=Anti+Phospholipid+Syndrome&draw=2&rank=1
48. Woller SC, Stevens SM, Kaplan D, et al. Apixaban compared with warfarin to prevent thrombosis in thrombotic antiphospholipid syndrome: a randomized trial. Blood Adv. 2022;6:1661-1670. doi: 10.1182/bloodadvances.2021005808
49. Khairani CD, Bejjani A, Piazza G, et al. Direct oral anticoagulants vs vitamin K antagonists in patients with antiphospholipid syndromes: meta-analysis of randomized trials. J Am Coll Cardiol. 2023;81:16-30. doi: 10.1016/j.jacc.2022.10.008
50. Superficial thrombophlebitis, superficial vein thrombosis. 2021. Accessed May 10, 2023. thrombosiscanada.ca/wp-content/uploads/2021/07/47.-Superficial-Vein-Thrombosis_16July2021.pdf
51. Di Nisio M, Wichers IM, Middeldorp S. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev. 2018;2:CD004982. doi: 10.1002/14651858.CD004982.pub6
52. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139-e228. doi: 10.1016/j.jacc.2014.09.017
53. Alexander JH, Lopes RD, James S, et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365:699-708. doi: 10.1056/NEJMoa1105819
54. Mega JL, Braunwald E, Mohanavelu S, et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomised, double-blind, phase II trial. Lancet. 2009;374:29-38. doi: 10.1016/s0140-6736(09)60738-8
55. Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9-19. doi: 10.1056/NEJMoa1112277
56. Gibson WJ, Gibson CM, Yee MK, et al. Safety and efficacy of rivaroxaban when added to aspirin monotherapy among stabilized post‐acute coronary syndrome patients: a pooled analysis study of ATLAS ACS‐TIMI 46 and ATLAS ACS 2‐TIMI 51. J Am Heart Assoc. 2019. Accessed May 10, 2023. Doi: 10.1161/JAHA.118.009451
57. European Medicines Agency. Xarelto (rivaroxaban). 2008. Accessed June 23, 2023.
58. Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289-1367. doi: 10.1093/eurheartj/ehaa575
59. NIH. ClinicalTrials.gov. Accessed May 10, 2023. www.clinicaltrials.gov/ct2/results?cond=Acute+Coronary+Syndrome&term=rivaroxaban+&cntry=&state=&city=&dist=#
60. Watson H, Davidson S, Keeling D. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: second edition. Br J Haematol. 2012;159:528-40. doi: 10.1111/bjh.12059
61. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2:3360-3392. doi: 10.1182/bloodadvances.2018024489
62. Momin J, Lee C-S. The role of direct oral anticoagulants in the management of heparin-induced thrombocytopenia US Pharmacist. 2020;45:3-10. Accessed May 10, 2023. www.uspharmacist.com/article/the-role-of-direct-oral-anticoagulants-in-the-management-of-heparininduced-thrombocytopenia
63. Warkentin TE, Pai M, Linkins LA. Direct oral anticoagulants for treatment of HIT: update of Hamilton experience and literature review. Blood. 2017;130:1104-1113. doi: 10.1182/blood-2017-04-778993
64. Krauel K, Hackbarth C, Fürll B, et al. Heparin-induced thrombocytopenia: in vitro studies on the interaction of dabigatran, rivaroxaban, and low-sulfated heparin, with platelet factor 4 and anti-PF4/heparin antibodies. Blood. 2012;119:1248-1255. doi: 10.1182/blood-2011-05-353391
PRACTICE RECOMMENDATIONS
› Consider a direct oral anticoagulant (DOAC) when treating venous thromboembolism (VTE) in patients with advanced chronic kidney disease or obesity. C
› Select apixaban for treatment of VTE or nonvalvular atrial fibrillation in patients with end-stage renal disease, due to its minimal renal clearance compared with other DOACs. B
› Consider DOACs such as dabigatran, rivaroxaban, or apixaban for treatment of VTE in the context of heparin-induced thrombocytopenia. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
How telehealth can work best for our patients
Social distancing measures instituted during the COVID-19 pandemic challenged the usual way of operating in primary care. To continue delivering medical services, physicians had to transition quickly to forms of remote interaction with patients. Use of technology appeared to be the answer. And it gave clinicians the ability to do what many had long hoped for: offer patients the option of telehealth.
The terms telemedicine and telehealth have similar definitions and are commonly used interchangeably. We think most practices probably would have adopted telehealth earlier were it not for reimbursement barriers. In this article, we adopt the World Health Organization’s definition of telemedicine as: “The delivery of healthcare services, where distance is a critical factor, by all healthcare professionals using information and communication technologies for the exchange of valid information for the diagnosis, treatment, and prevention of disease and injuries, research and evaluation, and for the continuing education of healthcare providers, all in the interests of advancing the health of individuals and their communities.”1
To provide family medicine clinicians with evidence-based recommendations about telehealth, we conducted a critical review of the literature published through April 30, 2021. The scope of this review includes studies found using the PubMed and Google Scholar databases. In addition, we used the keywords “telehealth,” “telemedicine,” “family medicine,” and “primary care.” We divided this review into 6 sections, including focus areas on implementation in primary care, remote diagnostic accuracy, conditions lending themselves to telehealth, physician and patient perceptions, disparities in telehealth, and finally, the conclusions.
Telehealth implementation in primary care
Telehealth in various forms had been around for years before the pandemic, mainly in the form of commercial telehealth businesses. Telehealth was being used in rural and remote areas where it could be difficult to see a primary care provider—let alone a specialist. The family medicine department of the University of Colorado was an early adopter of telehealth and had navigated this transition since 2017, with clinical champions guiding the process. By 2019, 54% of their clinicians were conducting telehealth encounters.2
However, telehealth implementation elsewhere was not accepted so readily. Before the pandemic, a cross-sectional study of more than 1.1 million patients in Northern California showed that 86% preferred in-person care over video.3 Even as the pandemic began and social distancing measures were implemented, a quality improvement project at a family medicine residency clinic in Florida documented that clinicians still preferred telephone interviews despite the capacity for video visits.4 And many primary care systems were simply unprepared to adopt telehealth technologies.
With time, however, family physicians began to improvise using popular videoconferencing technologies (eg, Zoom) that were readily available and familiar to patients, and medical centers began to repurpose their existing videoconferencing systems.5 The Ohio State University Wexner Medical Center launched a virtual health initiative just before the pandemic struck, at which time fewer than 5% of patient visits were conducted through telehealth. Weeks later, nearly 93% of patient visits were offered through telehealth.6
Reimbursement. Another significant impediment to early telehealth uptake was the late reaction by the Centers for Medicare and Medicaid Services (CMS) in changing the payment system. Hectic expansion of telehealth in response to the crisis pointed to the lack of policies that supported primary care with payments based on outcomes rather than fee-for-service models.7 By the end of April 2020, CMS finally announced that video visits would be reimbursed at the same rate as in-person visits. However, telephone-only visits are still very limited in coverage, and appropriate codes should be verified with payers.
Continue to: Remote diagnosis comes with a caveat
Remote diagnosis comes with a caveat
Some primary care practices have found that images of skin lesions submitted by patients (usually by cell phone) suffice for accurate diagnosis in lieu of office visits.8 With chronic conditions, home-based remote monitoring of vital signs may assist in diagnosing and managing acute issues. More efficient triage of patients is increasingly possible with the receipt of still images or video files of concerning lesions (eg, burns, rash, chronic wounds) sent from smartphones alone9,10 or with devices attached to smartphones (eg, parent-managed otoscopes).11,12
Family physicians historically have relied on in-person visits for holistic assessment and diagnosis. Telehealth video visits have the potential to assist with this goal, but there are risks. For example, one patient cut her foot while swimming and the wound became infected.
Specific conditions usually suitable for telehealth evaluation
The pandemic helped us understand that some situations and conditions are better suited than others to coverage by telehealth. The National Ambulatory Medical Care Survey examined 850 million patient–physician encounters and found that 66% of all ambulatory primary care visits required in-office care,15 suggesting that about one-third of patient encounters could be treated via telehealth.
As an example, our southeastern Wisconsin urban clinic has about 20,000 office visits per year. We launched telehealth in March 2020 in direct response to the pandemic. Telehealth usage peaked at the beginning of the pandemic (FIGURE), fell gradually, hit a lower peak in November and December as COVID case counts increased, and then decreased again as our community changed from a “quarantine/lockdown” mentality to “opening up/back to new normal.
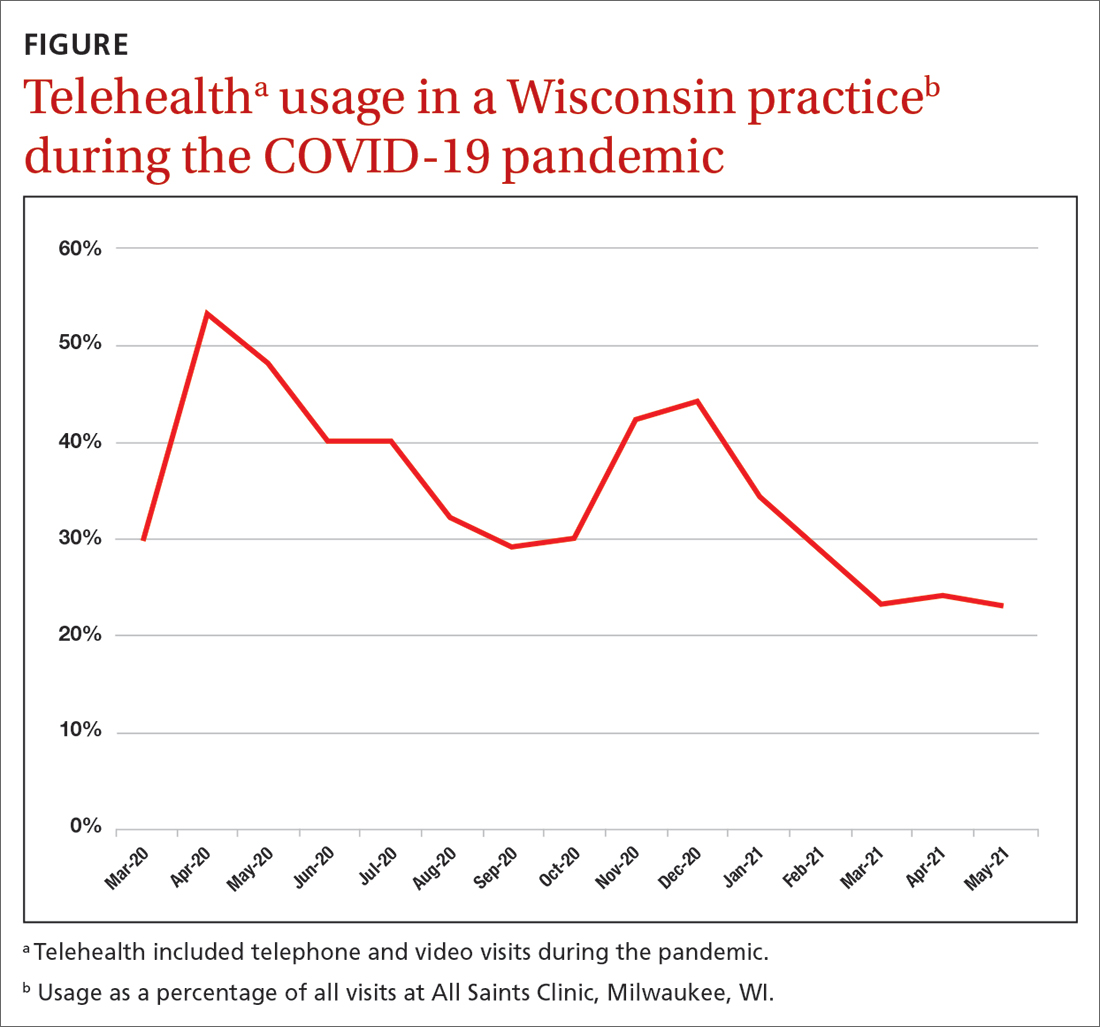
Some conditions can be managed favorably with the telehealth format:
Infectious diseases may be treatable remotely.16,17 Following an initial telehealth visit, the physician can evaluate and recommend further care.
Stable, chronic conditions. Telehealth can be used for stable, chronic conditions such as diabetes, chronic obstructive pulmonary disease, and heart failure when lab or imaging studies are not needed.18
Mental health. Telehealth can be useful in counseling and providing mental health and social support.18 Safeguards can be put in place to protect patient privacy in this setting.19
Behavioral change. Telehealth can be effective in providing support for patients actively trying to quit smoking or lose weight, and for caregivers. A physician who “checks in” can be a positive motivator and can promote a patient’s continued success.20
Continue to: Telehealth is less beneficial...
Telehealth is less beneficial when a physical exam is needed to assess pain, tenderness, strength, or other sensations. Office visits also are required for lab assays and imaging, as in periodic checks of A1C levels in patients with diabetes. As technology advances, home-based laboratory kits and sensors likely will change this picture. New patients may be better served through an initial office visit to develop the patient–physician relationship.
Visual assessment of conditions may be limited by telehealth depending on the quality of the devices used. For example, rashes may be difficult to assess given the clarity of the picture on the device and the ability to see only in 2D. There is still a need for more controlled trials to clarify which conditions can be evaluated and managed by telehealth and which ones need in-person care.21
Physician and patient perceptions of telehealth encounters
Research into family physicians’ perceptions of telehealth is scant. However, 3 studies published in 2021 reveal some advantages and challenges for telehealth adoption.
- A qualitative study found that physicians valued the increased access to care for some patients, changes to reimbursement practices not covered before, and the opportunity to see patients’ home environments.22 Disadvantages included an inability to examine the patient, problems with diagnostic accuracy, hindrances to developing personal connections, and the potential for burnout with on-demand care.22 The researchers suggested that telehealth might better serve to augment in-person care.
- A second study found that clinicians are satisfied with the use of telehealth in general. However, it also noted that the lack of physical examination could hinder accurate diagnosis and treatment.23
- A third study surveyed 109 family physicians, reinforcing the importance of physical exams and highlighting the lack of body language as another barrier.24
In addition, all 3 studies noted that video visits are typically briefer than in-person visits. Previous research predominantly done in specialty and mental health care showed that the benefits of telehealth for physicians include an increase in efficiency, reduced commute time, and improved work-life balance.25
Patient perspectives. Many patients have reported that they prefer telehealth because of lower costs, decreased travel time, and faster health care access.26,27 However, patients also have expressed concerns that the telehealth environment may reduce physician attention, can limit personal interaction (and impart a sense of being rushed), and lacks the physical examination that may be key to an adequate diagnosis.28
Continue to: A survey of 223 patients showed...
A survey of 223 patients showed that sicker patients choose in-person care because they want more in-depth visits with more attention to detail than healthier patients do.29 In a Veterans Affairs health care system qualitative study, patients voiced concerns about communicating with physicians via telehealth, including the potential for errors, less attention paid to their needs, audio difficulties, and challenges to establishing a physician–patient relationship.30 Some patients thought telehealth inhibited their personal expression or that the clinician was not attentive enough. These patient reports underscore the importance of patient–clinician relationships developed in person.31 The perceived level of complexity involved in a visit appears to be an essential factor in a patient opting for telehealth—or not.
In light of these known physician and patient perspectives, it seems wise to develop a hybrid model approach in which visits alternate between telehealth and office.
Patient disparities that may limit the use of telehealth
Race and ethnicity is a major factor in telehealth use. Patients who are Black or Hispanic use telehealth services less often than patients who are White.32,33 A study that looked at patients with chronic conditions—hypertension and diabetes—that disproportionately affect Black and Hispanic patients found that patients in these populations with either of these conditions had a lower prevalence of Internet use when compared with White patients.34 However, subpopulations can vary in their usage. For example, a study in East Harlem, New York, found that Hispanic pregnant women used telehealth frequently for prenatal care and perceived the care as satisfactory.35
Age is also a significant variable in the adoption of telehealth, with pre-COVID-19 studies finding lower use of technology among older adults. However, a study performed at the University of Missouri during the first months of the pandemic found an increase in telehealth use in seniors,32 although the increase was in telephone use and not full video sessions.
Many patients in need of health care services may have older devices and/or low-speed or no Internet access; they also may lack the technical know-how to conduct a telehealth visit.4,36 For example, regardless of race or ethnicity, patients on government insurance (Medicaid and Medicare) have been shown to complete more telephone than video visits,37 underscoring the importance of telehealth practice flexibility and the need for increased technology support to decrease the digital divide. Even with adequate technological support and patient training, telehealth may be more complicated if patients have such comorbidities as hearing, visual, or cognitive impairment.31 Patients from a lower socioeconomic status may feel uncomfortable with providers seeing their home environment on video.38
Overall, incorporating telehealth for the care of older and/or vulnerable patients will present a unique set of challenges that organizations must address. Efforts must be made to understand the available technologies and patients’ comfort in using them. A hybrid model offering telehealth and in-office encounters may be the best solution.
Hernan Barenboim, PhD, KPC Health Group, 301 North San Jacinto Street, Hemet, CA 92543; hbarenboim@gmail.com
1. WHO. A health telematics policy: in support of WHO’s Health-for-All strategy for global health development. 1997. Accessed February 8, 2023. https://apps.who.int/iris/bitstream/handle/10665/63857/WHO_DGO_98.1.pdf?sequence=1&isAllowed=y
2. Knierim K, Palmer C, Kramer ES, et al. Lessons learned during COVID-19 that can move telehealth in primary care forward. J Am Board Fam Med. Supplement 2021;34(suppl):S196-S202. doi: 10.3122/jabfm.2021.S1.200419
3. Reed ME, Huang J, Graetz I, et al. Patient characteristics associated with choosing a telemedicine visit vs office visit with the same primary care clinicians. JAMA Netw Open. 2020;3:e205873. doi: 10.1001/jamanetworkopen.2020.5873
4. Silver SL, Lewis MN, Ledford CJ. A stepwise transition to telemedicine in response to COVID-19. J Am Board Fam Med. 2021;34(suppl):S152-S161. doi: 10.3122/jabfm.2021.S1.200358
5. Hron JD, Parsons CR, Williams LA, et al. Rapid implementation of an inpatient telehealth program during the COVID-19 pandemic. Appl Clin Inform. 2020;3:452-459. doi: 10.1055/s-0040-1713635
6. Olayiwola JN, Magaña C, Harmon A, et al. Telehealth as a bright spot of the COVID-19 pandemic: recommendations from the virtual frontlines (“Frontweb”). JMIR Public Health Surveill. 2020;6:e19045. doi: 10.2196/19045
7. Gausvik C, Jabbarpour Y. COVID-19 timeline: Centers for Medicare and Medicaid Services (CMS) changes and primary care support were not enough to prevent practice losses. J Am Board Fam Med. 2021;34(suppl):S7-S9. doi: 10.3122/jabfm.2021.S1.200305
8. Marin-Gomez FX, Vidal-Alaball J, Poch PR, et al. Diagnosis of skin lesions using photographs taken with a mobile phone: an online survey of primary care physicians. J Prim Care Community Health. 2020;11:2150132720937831. doi: 10.1177/2150132720937831
9. Garber RN, Garcia E, Goodwin CW, et al. (2020). Pictures do influence the decision to transfer: outcomes of a telemedicine program serving an eight-state rural population. J Burn Care Res. 2020;41:690-694. doi: 10.1093/jbcr/iraa017
10. Felix F, Greenblatt M, Shin L. Saving limbs in the time of COVID. 2020. Accessed February 8, 2023. https://podiatrym.com/pdf/2020/7/FelixGreenblattShin820web.pdf
11. Erkkola-Anttinen N, Irjala H, Laine MK, et al. Smartphone otoscopy performed by parents. Telemed J E Health. 2019;25:477-484. doi: 10.1089/tmj.2018.0062
12. Verzantvoort NC, Teunis T, Verheij TJ, et al. Self-triage for acute primary care via a smartphone application: practical, safe and efficient? PLoS One. 2018;13:e0199284. doi: 10.1371/journal.pone.0199284
13. Hickner J. When patients don’t get the care they should. J Fam Pract. 2020;69:427.
14. Pappan N, Benkhadra R, Papincak D, et al. Values and limits of telemedicine: a case report. SN Compr Clin Med. 2021;3:317-319. doi: 10.1007/s42399-020-00725-y
15. Jabbarpour Y, Jetty A, Westfall M, et al. Not telehealth: which primary care visits need in-person care? J Am Board Fam Med. 2021;34(suppl):S162-S169. doi: 10.3122/jabfm.2021.S1.200247
16. Parmar P, Mackie D, Varghese S, et al. Use of telemedicine technologies in the management of infectious diseases: a review. Clin Infect Dis. 2015;60:1084-1094. doi: 10.1093/cid/ciu1143
17. Young JD, Abdel-Massih R, Herchline T, et al. Infectious Diseases Society of America position statement on Telehealth and Telemedicine as Applied to the Practice of Infectious Diseases. Clin Infect Dis. 2019;68:1437-1443. doi: 10.1093/cid/ciy907
18. ARHQ. Telehealth: mapping the evidence for patient outcomes from systematic reviews. 2016. Accessed March 27, 2023. https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/telehealth_technical-brief.pdf
19. Lustgarten SD, Garrison YL, Sinnard MT, et al. Digital privacy in mental healthcare: current issues and recommendations for technology use. Curr Opin Psychol. 2020;36:25-31. doi: 10.1016/j.copsyc.2020.03.012
20. Baird A, Xia Y, Cheng Y. Consumer perceptions of telehealth for mental health or substance abuse: a Twitter-based topic modeling analysis. JAMIA Open. 2022;5:ooac028. doi: 10.1093/jamiaopen/ooac028
21. Flumignan CD, da Rocha AP, Pinto AC, et al. What do Cochrane systematic reviews say about telemedicine for healthcare? Sao Paulo Med J. 2019;137:184-192. doi: 10.1590/1516-3180.0177240419
22. Gomez T, Anaya YB, Shih KJ, et al. A qualitative study of primary care physicians’ experiences with telemedicine during COVID-19. J Am Board Fam Med. 2021;34(suppl):S61-S70. doi: 10.3122/jabfm.2021.S1.200517
23. Malliaras P, Merolli M, Williams CM, et al. ‘It’s not hands-on therapy, so it’s very limited’: telehealth use and views among allied health clinicians during the coronavirus pandemic. Musculoskelet Sci Pract. 2021;52:102340. doi: 10.1016/j.msksp.2021.102340
24. Gold KJ, Laurie AR, Kinney DR, et al. Video visits: family physician experiences with uptake during the COVID-19 pandemic. Fam Med. 53:207-210. doi: 10.22454/FamMed.2021.613099
25. Björndell C, Premberg A. Physicians’ experiences of video consultation with patients at a public virtual primary care clinic: a qualitative interview study. Scand J Prim Health Care. 2021;39:67-76. doi: 10.1080/02813432.2021.1882082
26. Powell RE, Henstenburg JM, Cooper G, et al. Patient perceptions of telehealth primary care video visits. Ann Fam Med. 2017;15:225-229. doi: 10.1370/afm.2095
27. Imlach F, McKinlay E, Middleton L, et al. Telehealth consultations in general practice during a pandemic lockdown: survey and interviews on patient experiences and preferences. BMC Fam Pract. 2020;21:1-14. doi: 10.1186/s12875-020-01336-1
28. Gordon HS, Solanki P, Bokhour BG, et al. “I’m not feeling like I’m part of the conversation” patients’ perspectives on communicating in clinical video telehealth visits. J Gen Intern Med. 2020;35:1751-1758. doi: 10.1007/s11606-020-05673-w
29. Volcy J, Smith W, Mills K, et al. Assessment of patient and provider satisfaction with the change to telehealth from in-person visits at an academic safety net institution during the COVID-19 pandemic. J Am Board Fam Med. 2021;34(suppl):S71-S76. doi: 10.3122/jabfm.2021.S1.200393
30. Gopal RK, Solanki P, Bokhour BG, et al. Provider, staff, and patient perspectives on medical visits using clinical video telehealth: a foundation for educational initiatives to improve medical care in telehealth. J Nurse Pract. 2021;17:582-587. doi: 10.1016/j.nurpra.2021.02.020
31. Edgoose JY. Exploring the face-to-face: revisiting patient-doctor relationships in a time of expanding telemedicine. J Am Board Fam Med. 2021;34(suppl):S252-S254. doi: 10.3122/jabfm.2021.S1.200398
32. Pierce RP, Stevermer JJ. Disparities in use of telehealth at the onset of the COVID-19 public health emergency. J Telemed Telecare. 2023;29:3-9. doi: 10.1177/1357633X20963893
33. Lame M, Leyden D, Platt SL. Geocode maps spotlight disparities in telehealth utilization during the COVID-19 pandemic in New York City. Telemed J E Health. 2021;27:251-253. doi: 10.1089/tmj.2020.0297
34. Jain V, Al Rifai M, Lee MT, et al. Racial and geographic disparities in internet use in the US among patients with hypertension or diabetes: implications for telehealth in the era of COVID-19. Diabetes Care. 2021;44:e15-e17. doi: 10.2337/dc20-2016
35. Futterman I, Rosenfeld E, Toaff M, et al. Addressing disparities in prenatal care via telehealth during COVID-19: prenatal satisfaction survey in East Harlem. Am J Perinatol. 2021;38:88-92. doi: 10.1055/s-0040-1718695
36. Wegermann K, Wilder JM, Parish A, et al. Racial and socioeconomic disparities in utilization of telehealth in patients with liver disease during COVID-19. Dig Dis Sci. 2022;67:93-99. doi: 10.1007/s10620-021-06842-5.
37. ASPE. National survey trends in telehealth use in 2021: disparities in utilization and audio vs. video services. Issue brief: February 21, 2022. Accessed March 27, 2023. https://aspe.hhs.gov/sites/default/files/documents/4e1853c0b4885112b2994680a58af9ed/telehealth-hps-ib.pdf
38. Ukoha EP, Davis K, Yinger M, et al. Ensuring equitable implementation of telemedicine in perinatal care. Obstet Gynecol. 2021;137:487-492. doi: 10.1097/AOG.0000000000004276
Social distancing measures instituted during the COVID-19 pandemic challenged the usual way of operating in primary care. To continue delivering medical services, physicians had to transition quickly to forms of remote interaction with patients. Use of technology appeared to be the answer. And it gave clinicians the ability to do what many had long hoped for: offer patients the option of telehealth.
The terms telemedicine and telehealth have similar definitions and are commonly used interchangeably. We think most practices probably would have adopted telehealth earlier were it not for reimbursement barriers. In this article, we adopt the World Health Organization’s definition of telemedicine as: “The delivery of healthcare services, where distance is a critical factor, by all healthcare professionals using information and communication technologies for the exchange of valid information for the diagnosis, treatment, and prevention of disease and injuries, research and evaluation, and for the continuing education of healthcare providers, all in the interests of advancing the health of individuals and their communities.”1
To provide family medicine clinicians with evidence-based recommendations about telehealth, we conducted a critical review of the literature published through April 30, 2021. The scope of this review includes studies found using the PubMed and Google Scholar databases. In addition, we used the keywords “telehealth,” “telemedicine,” “family medicine,” and “primary care.” We divided this review into 6 sections, including focus areas on implementation in primary care, remote diagnostic accuracy, conditions lending themselves to telehealth, physician and patient perceptions, disparities in telehealth, and finally, the conclusions.
Telehealth implementation in primary care
Telehealth in various forms had been around for years before the pandemic, mainly in the form of commercial telehealth businesses. Telehealth was being used in rural and remote areas where it could be difficult to see a primary care provider—let alone a specialist. The family medicine department of the University of Colorado was an early adopter of telehealth and had navigated this transition since 2017, with clinical champions guiding the process. By 2019, 54% of their clinicians were conducting telehealth encounters.2
However, telehealth implementation elsewhere was not accepted so readily. Before the pandemic, a cross-sectional study of more than 1.1 million patients in Northern California showed that 86% preferred in-person care over video.3 Even as the pandemic began and social distancing measures were implemented, a quality improvement project at a family medicine residency clinic in Florida documented that clinicians still preferred telephone interviews despite the capacity for video visits.4 And many primary care systems were simply unprepared to adopt telehealth technologies.
With time, however, family physicians began to improvise using popular videoconferencing technologies (eg, Zoom) that were readily available and familiar to patients, and medical centers began to repurpose their existing videoconferencing systems.5 The Ohio State University Wexner Medical Center launched a virtual health initiative just before the pandemic struck, at which time fewer than 5% of patient visits were conducted through telehealth. Weeks later, nearly 93% of patient visits were offered through telehealth.6
Reimbursement. Another significant impediment to early telehealth uptake was the late reaction by the Centers for Medicare and Medicaid Services (CMS) in changing the payment system. Hectic expansion of telehealth in response to the crisis pointed to the lack of policies that supported primary care with payments based on outcomes rather than fee-for-service models.7 By the end of April 2020, CMS finally announced that video visits would be reimbursed at the same rate as in-person visits. However, telephone-only visits are still very limited in coverage, and appropriate codes should be verified with payers.
Continue to: Remote diagnosis comes with a caveat
Remote diagnosis comes with a caveat
Some primary care practices have found that images of skin lesions submitted by patients (usually by cell phone) suffice for accurate diagnosis in lieu of office visits.8 With chronic conditions, home-based remote monitoring of vital signs may assist in diagnosing and managing acute issues. More efficient triage of patients is increasingly possible with the receipt of still images or video files of concerning lesions (eg, burns, rash, chronic wounds) sent from smartphones alone9,10 or with devices attached to smartphones (eg, parent-managed otoscopes).11,12
Family physicians historically have relied on in-person visits for holistic assessment and diagnosis. Telehealth video visits have the potential to assist with this goal, but there are risks. For example, one patient cut her foot while swimming and the wound became infected.
Specific conditions usually suitable for telehealth evaluation
The pandemic helped us understand that some situations and conditions are better suited than others to coverage by telehealth. The National Ambulatory Medical Care Survey examined 850 million patient–physician encounters and found that 66% of all ambulatory primary care visits required in-office care,15 suggesting that about one-third of patient encounters could be treated via telehealth.
As an example, our southeastern Wisconsin urban clinic has about 20,000 office visits per year. We launched telehealth in March 2020 in direct response to the pandemic. Telehealth usage peaked at the beginning of the pandemic (FIGURE), fell gradually, hit a lower peak in November and December as COVID case counts increased, and then decreased again as our community changed from a “quarantine/lockdown” mentality to “opening up/back to new normal.

Some conditions can be managed favorably with the telehealth format:
Infectious diseases may be treatable remotely.16,17 Following an initial telehealth visit, the physician can evaluate and recommend further care.
Stable, chronic conditions. Telehealth can be used for stable, chronic conditions such as diabetes, chronic obstructive pulmonary disease, and heart failure when lab or imaging studies are not needed.18
Mental health. Telehealth can be useful in counseling and providing mental health and social support.18 Safeguards can be put in place to protect patient privacy in this setting.19
Behavioral change. Telehealth can be effective in providing support for patients actively trying to quit smoking or lose weight, and for caregivers. A physician who “checks in” can be a positive motivator and can promote a patient’s continued success.20
Continue to: Telehealth is less beneficial...
Telehealth is less beneficial when a physical exam is needed to assess pain, tenderness, strength, or other sensations. Office visits also are required for lab assays and imaging, as in periodic checks of A1C levels in patients with diabetes. As technology advances, home-based laboratory kits and sensors likely will change this picture. New patients may be better served through an initial office visit to develop the patient–physician relationship.
Visual assessment of conditions may be limited by telehealth depending on the quality of the devices used. For example, rashes may be difficult to assess given the clarity of the picture on the device and the ability to see only in 2D. There is still a need for more controlled trials to clarify which conditions can be evaluated and managed by telehealth and which ones need in-person care.21
Physician and patient perceptions of telehealth encounters
Research into family physicians’ perceptions of telehealth is scant. However, 3 studies published in 2021 reveal some advantages and challenges for telehealth adoption.
- A qualitative study found that physicians valued the increased access to care for some patients, changes to reimbursement practices not covered before, and the opportunity to see patients’ home environments.22 Disadvantages included an inability to examine the patient, problems with diagnostic accuracy, hindrances to developing personal connections, and the potential for burnout with on-demand care.22 The researchers suggested that telehealth might better serve to augment in-person care.
- A second study found that clinicians are satisfied with the use of telehealth in general. However, it also noted that the lack of physical examination could hinder accurate diagnosis and treatment.23
- A third study surveyed 109 family physicians, reinforcing the importance of physical exams and highlighting the lack of body language as another barrier.24
In addition, all 3 studies noted that video visits are typically briefer than in-person visits. Previous research predominantly done in specialty and mental health care showed that the benefits of telehealth for physicians include an increase in efficiency, reduced commute time, and improved work-life balance.25
Patient perspectives. Many patients have reported that they prefer telehealth because of lower costs, decreased travel time, and faster health care access.26,27 However, patients also have expressed concerns that the telehealth environment may reduce physician attention, can limit personal interaction (and impart a sense of being rushed), and lacks the physical examination that may be key to an adequate diagnosis.28
Continue to: A survey of 223 patients showed...
A survey of 223 patients showed that sicker patients choose in-person care because they want more in-depth visits with more attention to detail than healthier patients do.29 In a Veterans Affairs health care system qualitative study, patients voiced concerns about communicating with physicians via telehealth, including the potential for errors, less attention paid to their needs, audio difficulties, and challenges to establishing a physician–patient relationship.30 Some patients thought telehealth inhibited their personal expression or that the clinician was not attentive enough. These patient reports underscore the importance of patient–clinician relationships developed in person.31 The perceived level of complexity involved in a visit appears to be an essential factor in a patient opting for telehealth—or not.
In light of these known physician and patient perspectives, it seems wise to develop a hybrid model approach in which visits alternate between telehealth and office.
Patient disparities that may limit the use of telehealth
Race and ethnicity is a major factor in telehealth use. Patients who are Black or Hispanic use telehealth services less often than patients who are White.32,33 A study that looked at patients with chronic conditions—hypertension and diabetes—that disproportionately affect Black and Hispanic patients found that patients in these populations with either of these conditions had a lower prevalence of Internet use when compared with White patients.34 However, subpopulations can vary in their usage. For example, a study in East Harlem, New York, found that Hispanic pregnant women used telehealth frequently for prenatal care and perceived the care as satisfactory.35
Age is also a significant variable in the adoption of telehealth, with pre-COVID-19 studies finding lower use of technology among older adults. However, a study performed at the University of Missouri during the first months of the pandemic found an increase in telehealth use in seniors,32 although the increase was in telephone use and not full video sessions.
Many patients in need of health care services may have older devices and/or low-speed or no Internet access; they also may lack the technical know-how to conduct a telehealth visit.4,36 For example, regardless of race or ethnicity, patients on government insurance (Medicaid and Medicare) have been shown to complete more telephone than video visits,37 underscoring the importance of telehealth practice flexibility and the need for increased technology support to decrease the digital divide. Even with adequate technological support and patient training, telehealth may be more complicated if patients have such comorbidities as hearing, visual, or cognitive impairment.31 Patients from a lower socioeconomic status may feel uncomfortable with providers seeing their home environment on video.38
Overall, incorporating telehealth for the care of older and/or vulnerable patients will present a unique set of challenges that organizations must address. Efforts must be made to understand the available technologies and patients’ comfort in using them. A hybrid model offering telehealth and in-office encounters may be the best solution.
Hernan Barenboim, PhD, KPC Health Group, 301 North San Jacinto Street, Hemet, CA 92543; hbarenboim@gmail.com
Social distancing measures instituted during the COVID-19 pandemic challenged the usual way of operating in primary care. To continue delivering medical services, physicians had to transition quickly to forms of remote interaction with patients. Use of technology appeared to be the answer. And it gave clinicians the ability to do what many had long hoped for: offer patients the option of telehealth.
The terms telemedicine and telehealth have similar definitions and are commonly used interchangeably. We think most practices probably would have adopted telehealth earlier were it not for reimbursement barriers. In this article, we adopt the World Health Organization’s definition of telemedicine as: “The delivery of healthcare services, where distance is a critical factor, by all healthcare professionals using information and communication technologies for the exchange of valid information for the diagnosis, treatment, and prevention of disease and injuries, research and evaluation, and for the continuing education of healthcare providers, all in the interests of advancing the health of individuals and their communities.”1
To provide family medicine clinicians with evidence-based recommendations about telehealth, we conducted a critical review of the literature published through April 30, 2021. The scope of this review includes studies found using the PubMed and Google Scholar databases. In addition, we used the keywords “telehealth,” “telemedicine,” “family medicine,” and “primary care.” We divided this review into 6 sections, including focus areas on implementation in primary care, remote diagnostic accuracy, conditions lending themselves to telehealth, physician and patient perceptions, disparities in telehealth, and finally, the conclusions.
Telehealth implementation in primary care
Telehealth in various forms had been around for years before the pandemic, mainly in the form of commercial telehealth businesses. Telehealth was being used in rural and remote areas where it could be difficult to see a primary care provider—let alone a specialist. The family medicine department of the University of Colorado was an early adopter of telehealth and had navigated this transition since 2017, with clinical champions guiding the process. By 2019, 54% of their clinicians were conducting telehealth encounters.2
However, telehealth implementation elsewhere was not accepted so readily. Before the pandemic, a cross-sectional study of more than 1.1 million patients in Northern California showed that 86% preferred in-person care over video.3 Even as the pandemic began and social distancing measures were implemented, a quality improvement project at a family medicine residency clinic in Florida documented that clinicians still preferred telephone interviews despite the capacity for video visits.4 And many primary care systems were simply unprepared to adopt telehealth technologies.
With time, however, family physicians began to improvise using popular videoconferencing technologies (eg, Zoom) that were readily available and familiar to patients, and medical centers began to repurpose their existing videoconferencing systems.5 The Ohio State University Wexner Medical Center launched a virtual health initiative just before the pandemic struck, at which time fewer than 5% of patient visits were conducted through telehealth. Weeks later, nearly 93% of patient visits were offered through telehealth.6
Reimbursement. Another significant impediment to early telehealth uptake was the late reaction by the Centers for Medicare and Medicaid Services (CMS) in changing the payment system. Hectic expansion of telehealth in response to the crisis pointed to the lack of policies that supported primary care with payments based on outcomes rather than fee-for-service models.7 By the end of April 2020, CMS finally announced that video visits would be reimbursed at the same rate as in-person visits. However, telephone-only visits are still very limited in coverage, and appropriate codes should be verified with payers.
Continue to: Remote diagnosis comes with a caveat
Remote diagnosis comes with a caveat
Some primary care practices have found that images of skin lesions submitted by patients (usually by cell phone) suffice for accurate diagnosis in lieu of office visits.8 With chronic conditions, home-based remote monitoring of vital signs may assist in diagnosing and managing acute issues. More efficient triage of patients is increasingly possible with the receipt of still images or video files of concerning lesions (eg, burns, rash, chronic wounds) sent from smartphones alone9,10 or with devices attached to smartphones (eg, parent-managed otoscopes).11,12
Family physicians historically have relied on in-person visits for holistic assessment and diagnosis. Telehealth video visits have the potential to assist with this goal, but there are risks. For example, one patient cut her foot while swimming and the wound became infected.
Specific conditions usually suitable for telehealth evaluation
The pandemic helped us understand that some situations and conditions are better suited than others to coverage by telehealth. The National Ambulatory Medical Care Survey examined 850 million patient–physician encounters and found that 66% of all ambulatory primary care visits required in-office care,15 suggesting that about one-third of patient encounters could be treated via telehealth.
As an example, our southeastern Wisconsin urban clinic has about 20,000 office visits per year. We launched telehealth in March 2020 in direct response to the pandemic. Telehealth usage peaked at the beginning of the pandemic (FIGURE), fell gradually, hit a lower peak in November and December as COVID case counts increased, and then decreased again as our community changed from a “quarantine/lockdown” mentality to “opening up/back to new normal.

Some conditions can be managed favorably with the telehealth format:
Infectious diseases may be treatable remotely.16,17 Following an initial telehealth visit, the physician can evaluate and recommend further care.
Stable, chronic conditions. Telehealth can be used for stable, chronic conditions such as diabetes, chronic obstructive pulmonary disease, and heart failure when lab or imaging studies are not needed.18
Mental health. Telehealth can be useful in counseling and providing mental health and social support.18 Safeguards can be put in place to protect patient privacy in this setting.19
Behavioral change. Telehealth can be effective in providing support for patients actively trying to quit smoking or lose weight, and for caregivers. A physician who “checks in” can be a positive motivator and can promote a patient’s continued success.20
Continue to: Telehealth is less beneficial...
Telehealth is less beneficial when a physical exam is needed to assess pain, tenderness, strength, or other sensations. Office visits also are required for lab assays and imaging, as in periodic checks of A1C levels in patients with diabetes. As technology advances, home-based laboratory kits and sensors likely will change this picture. New patients may be better served through an initial office visit to develop the patient–physician relationship.
Visual assessment of conditions may be limited by telehealth depending on the quality of the devices used. For example, rashes may be difficult to assess given the clarity of the picture on the device and the ability to see only in 2D. There is still a need for more controlled trials to clarify which conditions can be evaluated and managed by telehealth and which ones need in-person care.21
Physician and patient perceptions of telehealth encounters
Research into family physicians’ perceptions of telehealth is scant. However, 3 studies published in 2021 reveal some advantages and challenges for telehealth adoption.
- A qualitative study found that physicians valued the increased access to care for some patients, changes to reimbursement practices not covered before, and the opportunity to see patients’ home environments.22 Disadvantages included an inability to examine the patient, problems with diagnostic accuracy, hindrances to developing personal connections, and the potential for burnout with on-demand care.22 The researchers suggested that telehealth might better serve to augment in-person care.
- A second study found that clinicians are satisfied with the use of telehealth in general. However, it also noted that the lack of physical examination could hinder accurate diagnosis and treatment.23
- A third study surveyed 109 family physicians, reinforcing the importance of physical exams and highlighting the lack of body language as another barrier.24
In addition, all 3 studies noted that video visits are typically briefer than in-person visits. Previous research predominantly done in specialty and mental health care showed that the benefits of telehealth for physicians include an increase in efficiency, reduced commute time, and improved work-life balance.25
Patient perspectives. Many patients have reported that they prefer telehealth because of lower costs, decreased travel time, and faster health care access.26,27 However, patients also have expressed concerns that the telehealth environment may reduce physician attention, can limit personal interaction (and impart a sense of being rushed), and lacks the physical examination that may be key to an adequate diagnosis.28
Continue to: A survey of 223 patients showed...
A survey of 223 patients showed that sicker patients choose in-person care because they want more in-depth visits with more attention to detail than healthier patients do.29 In a Veterans Affairs health care system qualitative study, patients voiced concerns about communicating with physicians via telehealth, including the potential for errors, less attention paid to their needs, audio difficulties, and challenges to establishing a physician–patient relationship.30 Some patients thought telehealth inhibited their personal expression or that the clinician was not attentive enough. These patient reports underscore the importance of patient–clinician relationships developed in person.31 The perceived level of complexity involved in a visit appears to be an essential factor in a patient opting for telehealth—or not.
In light of these known physician and patient perspectives, it seems wise to develop a hybrid model approach in which visits alternate between telehealth and office.
Patient disparities that may limit the use of telehealth
Race and ethnicity is a major factor in telehealth use. Patients who are Black or Hispanic use telehealth services less often than patients who are White.32,33 A study that looked at patients with chronic conditions—hypertension and diabetes—that disproportionately affect Black and Hispanic patients found that patients in these populations with either of these conditions had a lower prevalence of Internet use when compared with White patients.34 However, subpopulations can vary in their usage. For example, a study in East Harlem, New York, found that Hispanic pregnant women used telehealth frequently for prenatal care and perceived the care as satisfactory.35
Age is also a significant variable in the adoption of telehealth, with pre-COVID-19 studies finding lower use of technology among older adults. However, a study performed at the University of Missouri during the first months of the pandemic found an increase in telehealth use in seniors,32 although the increase was in telephone use and not full video sessions.
Many patients in need of health care services may have older devices and/or low-speed or no Internet access; they also may lack the technical know-how to conduct a telehealth visit.4,36 For example, regardless of race or ethnicity, patients on government insurance (Medicaid and Medicare) have been shown to complete more telephone than video visits,37 underscoring the importance of telehealth practice flexibility and the need for increased technology support to decrease the digital divide. Even with adequate technological support and patient training, telehealth may be more complicated if patients have such comorbidities as hearing, visual, or cognitive impairment.31 Patients from a lower socioeconomic status may feel uncomfortable with providers seeing their home environment on video.38
Overall, incorporating telehealth for the care of older and/or vulnerable patients will present a unique set of challenges that organizations must address. Efforts must be made to understand the available technologies and patients’ comfort in using them. A hybrid model offering telehealth and in-office encounters may be the best solution.
Hernan Barenboim, PhD, KPC Health Group, 301 North San Jacinto Street, Hemet, CA 92543; hbarenboim@gmail.com
1. WHO. A health telematics policy: in support of WHO’s Health-for-All strategy for global health development. 1997. Accessed February 8, 2023. https://apps.who.int/iris/bitstream/handle/10665/63857/WHO_DGO_98.1.pdf?sequence=1&isAllowed=y
2. Knierim K, Palmer C, Kramer ES, et al. Lessons learned during COVID-19 that can move telehealth in primary care forward. J Am Board Fam Med. Supplement 2021;34(suppl):S196-S202. doi: 10.3122/jabfm.2021.S1.200419
3. Reed ME, Huang J, Graetz I, et al. Patient characteristics associated with choosing a telemedicine visit vs office visit with the same primary care clinicians. JAMA Netw Open. 2020;3:e205873. doi: 10.1001/jamanetworkopen.2020.5873
4. Silver SL, Lewis MN, Ledford CJ. A stepwise transition to telemedicine in response to COVID-19. J Am Board Fam Med. 2021;34(suppl):S152-S161. doi: 10.3122/jabfm.2021.S1.200358
5. Hron JD, Parsons CR, Williams LA, et al. Rapid implementation of an inpatient telehealth program during the COVID-19 pandemic. Appl Clin Inform. 2020;3:452-459. doi: 10.1055/s-0040-1713635
6. Olayiwola JN, Magaña C, Harmon A, et al. Telehealth as a bright spot of the COVID-19 pandemic: recommendations from the virtual frontlines (“Frontweb”). JMIR Public Health Surveill. 2020;6:e19045. doi: 10.2196/19045
7. Gausvik C, Jabbarpour Y. COVID-19 timeline: Centers for Medicare and Medicaid Services (CMS) changes and primary care support were not enough to prevent practice losses. J Am Board Fam Med. 2021;34(suppl):S7-S9. doi: 10.3122/jabfm.2021.S1.200305
8. Marin-Gomez FX, Vidal-Alaball J, Poch PR, et al. Diagnosis of skin lesions using photographs taken with a mobile phone: an online survey of primary care physicians. J Prim Care Community Health. 2020;11:2150132720937831. doi: 10.1177/2150132720937831
9. Garber RN, Garcia E, Goodwin CW, et al. (2020). Pictures do influence the decision to transfer: outcomes of a telemedicine program serving an eight-state rural population. J Burn Care Res. 2020;41:690-694. doi: 10.1093/jbcr/iraa017
10. Felix F, Greenblatt M, Shin L. Saving limbs in the time of COVID. 2020. Accessed February 8, 2023. https://podiatrym.com/pdf/2020/7/FelixGreenblattShin820web.pdf
11. Erkkola-Anttinen N, Irjala H, Laine MK, et al. Smartphone otoscopy performed by parents. Telemed J E Health. 2019;25:477-484. doi: 10.1089/tmj.2018.0062
12. Verzantvoort NC, Teunis T, Verheij TJ, et al. Self-triage for acute primary care via a smartphone application: practical, safe and efficient? PLoS One. 2018;13:e0199284. doi: 10.1371/journal.pone.0199284
13. Hickner J. When patients don’t get the care they should. J Fam Pract. 2020;69:427.
14. Pappan N, Benkhadra R, Papincak D, et al. Values and limits of telemedicine: a case report. SN Compr Clin Med. 2021;3:317-319. doi: 10.1007/s42399-020-00725-y
15. Jabbarpour Y, Jetty A, Westfall M, et al. Not telehealth: which primary care visits need in-person care? J Am Board Fam Med. 2021;34(suppl):S162-S169. doi: 10.3122/jabfm.2021.S1.200247
16. Parmar P, Mackie D, Varghese S, et al. Use of telemedicine technologies in the management of infectious diseases: a review. Clin Infect Dis. 2015;60:1084-1094. doi: 10.1093/cid/ciu1143
17. Young JD, Abdel-Massih R, Herchline T, et al. Infectious Diseases Society of America position statement on Telehealth and Telemedicine as Applied to the Practice of Infectious Diseases. Clin Infect Dis. 2019;68:1437-1443. doi: 10.1093/cid/ciy907
18. ARHQ. Telehealth: mapping the evidence for patient outcomes from systematic reviews. 2016. Accessed March 27, 2023. https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/telehealth_technical-brief.pdf
19. Lustgarten SD, Garrison YL, Sinnard MT, et al. Digital privacy in mental healthcare: current issues and recommendations for technology use. Curr Opin Psychol. 2020;36:25-31. doi: 10.1016/j.copsyc.2020.03.012
20. Baird A, Xia Y, Cheng Y. Consumer perceptions of telehealth for mental health or substance abuse: a Twitter-based topic modeling analysis. JAMIA Open. 2022;5:ooac028. doi: 10.1093/jamiaopen/ooac028
21. Flumignan CD, da Rocha AP, Pinto AC, et al. What do Cochrane systematic reviews say about telemedicine for healthcare? Sao Paulo Med J. 2019;137:184-192. doi: 10.1590/1516-3180.0177240419
22. Gomez T, Anaya YB, Shih KJ, et al. A qualitative study of primary care physicians’ experiences with telemedicine during COVID-19. J Am Board Fam Med. 2021;34(suppl):S61-S70. doi: 10.3122/jabfm.2021.S1.200517
23. Malliaras P, Merolli M, Williams CM, et al. ‘It’s not hands-on therapy, so it’s very limited’: telehealth use and views among allied health clinicians during the coronavirus pandemic. Musculoskelet Sci Pract. 2021;52:102340. doi: 10.1016/j.msksp.2021.102340
24. Gold KJ, Laurie AR, Kinney DR, et al. Video visits: family physician experiences with uptake during the COVID-19 pandemic. Fam Med. 53:207-210. doi: 10.22454/FamMed.2021.613099
25. Björndell C, Premberg A. Physicians’ experiences of video consultation with patients at a public virtual primary care clinic: a qualitative interview study. Scand J Prim Health Care. 2021;39:67-76. doi: 10.1080/02813432.2021.1882082
26. Powell RE, Henstenburg JM, Cooper G, et al. Patient perceptions of telehealth primary care video visits. Ann Fam Med. 2017;15:225-229. doi: 10.1370/afm.2095
27. Imlach F, McKinlay E, Middleton L, et al. Telehealth consultations in general practice during a pandemic lockdown: survey and interviews on patient experiences and preferences. BMC Fam Pract. 2020;21:1-14. doi: 10.1186/s12875-020-01336-1
28. Gordon HS, Solanki P, Bokhour BG, et al. “I’m not feeling like I’m part of the conversation” patients’ perspectives on communicating in clinical video telehealth visits. J Gen Intern Med. 2020;35:1751-1758. doi: 10.1007/s11606-020-05673-w
29. Volcy J, Smith W, Mills K, et al. Assessment of patient and provider satisfaction with the change to telehealth from in-person visits at an academic safety net institution during the COVID-19 pandemic. J Am Board Fam Med. 2021;34(suppl):S71-S76. doi: 10.3122/jabfm.2021.S1.200393
30. Gopal RK, Solanki P, Bokhour BG, et al. Provider, staff, and patient perspectives on medical visits using clinical video telehealth: a foundation for educational initiatives to improve medical care in telehealth. J Nurse Pract. 2021;17:582-587. doi: 10.1016/j.nurpra.2021.02.020
31. Edgoose JY. Exploring the face-to-face: revisiting patient-doctor relationships in a time of expanding telemedicine. J Am Board Fam Med. 2021;34(suppl):S252-S254. doi: 10.3122/jabfm.2021.S1.200398
32. Pierce RP, Stevermer JJ. Disparities in use of telehealth at the onset of the COVID-19 public health emergency. J Telemed Telecare. 2023;29:3-9. doi: 10.1177/1357633X20963893
33. Lame M, Leyden D, Platt SL. Geocode maps spotlight disparities in telehealth utilization during the COVID-19 pandemic in New York City. Telemed J E Health. 2021;27:251-253. doi: 10.1089/tmj.2020.0297
34. Jain V, Al Rifai M, Lee MT, et al. Racial and geographic disparities in internet use in the US among patients with hypertension or diabetes: implications for telehealth in the era of COVID-19. Diabetes Care. 2021;44:e15-e17. doi: 10.2337/dc20-2016
35. Futterman I, Rosenfeld E, Toaff M, et al. Addressing disparities in prenatal care via telehealth during COVID-19: prenatal satisfaction survey in East Harlem. Am J Perinatol. 2021;38:88-92. doi: 10.1055/s-0040-1718695
36. Wegermann K, Wilder JM, Parish A, et al. Racial and socioeconomic disparities in utilization of telehealth in patients with liver disease during COVID-19. Dig Dis Sci. 2022;67:93-99. doi: 10.1007/s10620-021-06842-5.
37. ASPE. National survey trends in telehealth use in 2021: disparities in utilization and audio vs. video services. Issue brief: February 21, 2022. Accessed March 27, 2023. https://aspe.hhs.gov/sites/default/files/documents/4e1853c0b4885112b2994680a58af9ed/telehealth-hps-ib.pdf
38. Ukoha EP, Davis K, Yinger M, et al. Ensuring equitable implementation of telemedicine in perinatal care. Obstet Gynecol. 2021;137:487-492. doi: 10.1097/AOG.0000000000004276
1. WHO. A health telematics policy: in support of WHO’s Health-for-All strategy for global health development. 1997. Accessed February 8, 2023. https://apps.who.int/iris/bitstream/handle/10665/63857/WHO_DGO_98.1.pdf?sequence=1&isAllowed=y
2. Knierim K, Palmer C, Kramer ES, et al. Lessons learned during COVID-19 that can move telehealth in primary care forward. J Am Board Fam Med. Supplement 2021;34(suppl):S196-S202. doi: 10.3122/jabfm.2021.S1.200419
3. Reed ME, Huang J, Graetz I, et al. Patient characteristics associated with choosing a telemedicine visit vs office visit with the same primary care clinicians. JAMA Netw Open. 2020;3:e205873. doi: 10.1001/jamanetworkopen.2020.5873
4. Silver SL, Lewis MN, Ledford CJ. A stepwise transition to telemedicine in response to COVID-19. J Am Board Fam Med. 2021;34(suppl):S152-S161. doi: 10.3122/jabfm.2021.S1.200358
5. Hron JD, Parsons CR, Williams LA, et al. Rapid implementation of an inpatient telehealth program during the COVID-19 pandemic. Appl Clin Inform. 2020;3:452-459. doi: 10.1055/s-0040-1713635
6. Olayiwola JN, Magaña C, Harmon A, et al. Telehealth as a bright spot of the COVID-19 pandemic: recommendations from the virtual frontlines (“Frontweb”). JMIR Public Health Surveill. 2020;6:e19045. doi: 10.2196/19045
7. Gausvik C, Jabbarpour Y. COVID-19 timeline: Centers for Medicare and Medicaid Services (CMS) changes and primary care support were not enough to prevent practice losses. J Am Board Fam Med. 2021;34(suppl):S7-S9. doi: 10.3122/jabfm.2021.S1.200305
8. Marin-Gomez FX, Vidal-Alaball J, Poch PR, et al. Diagnosis of skin lesions using photographs taken with a mobile phone: an online survey of primary care physicians. J Prim Care Community Health. 2020;11:2150132720937831. doi: 10.1177/2150132720937831
9. Garber RN, Garcia E, Goodwin CW, et al. (2020). Pictures do influence the decision to transfer: outcomes of a telemedicine program serving an eight-state rural population. J Burn Care Res. 2020;41:690-694. doi: 10.1093/jbcr/iraa017
10. Felix F, Greenblatt M, Shin L. Saving limbs in the time of COVID. 2020. Accessed February 8, 2023. https://podiatrym.com/pdf/2020/7/FelixGreenblattShin820web.pdf
11. Erkkola-Anttinen N, Irjala H, Laine MK, et al. Smartphone otoscopy performed by parents. Telemed J E Health. 2019;25:477-484. doi: 10.1089/tmj.2018.0062
12. Verzantvoort NC, Teunis T, Verheij TJ, et al. Self-triage for acute primary care via a smartphone application: practical, safe and efficient? PLoS One. 2018;13:e0199284. doi: 10.1371/journal.pone.0199284
13. Hickner J. When patients don’t get the care they should. J Fam Pract. 2020;69:427.
14. Pappan N, Benkhadra R, Papincak D, et al. Values and limits of telemedicine: a case report. SN Compr Clin Med. 2021;3:317-319. doi: 10.1007/s42399-020-00725-y
15. Jabbarpour Y, Jetty A, Westfall M, et al. Not telehealth: which primary care visits need in-person care? J Am Board Fam Med. 2021;34(suppl):S162-S169. doi: 10.3122/jabfm.2021.S1.200247
16. Parmar P, Mackie D, Varghese S, et al. Use of telemedicine technologies in the management of infectious diseases: a review. Clin Infect Dis. 2015;60:1084-1094. doi: 10.1093/cid/ciu1143
17. Young JD, Abdel-Massih R, Herchline T, et al. Infectious Diseases Society of America position statement on Telehealth and Telemedicine as Applied to the Practice of Infectious Diseases. Clin Infect Dis. 2019;68:1437-1443. doi: 10.1093/cid/ciy907
18. ARHQ. Telehealth: mapping the evidence for patient outcomes from systematic reviews. 2016. Accessed March 27, 2023. https://effectivehealthcare.ahrq.gov/sites/default/files/pdf/telehealth_technical-brief.pdf
19. Lustgarten SD, Garrison YL, Sinnard MT, et al. Digital privacy in mental healthcare: current issues and recommendations for technology use. Curr Opin Psychol. 2020;36:25-31. doi: 10.1016/j.copsyc.2020.03.012
20. Baird A, Xia Y, Cheng Y. Consumer perceptions of telehealth for mental health or substance abuse: a Twitter-based topic modeling analysis. JAMIA Open. 2022;5:ooac028. doi: 10.1093/jamiaopen/ooac028
21. Flumignan CD, da Rocha AP, Pinto AC, et al. What do Cochrane systematic reviews say about telemedicine for healthcare? Sao Paulo Med J. 2019;137:184-192. doi: 10.1590/1516-3180.0177240419
22. Gomez T, Anaya YB, Shih KJ, et al. A qualitative study of primary care physicians’ experiences with telemedicine during COVID-19. J Am Board Fam Med. 2021;34(suppl):S61-S70. doi: 10.3122/jabfm.2021.S1.200517
23. Malliaras P, Merolli M, Williams CM, et al. ‘It’s not hands-on therapy, so it’s very limited’: telehealth use and views among allied health clinicians during the coronavirus pandemic. Musculoskelet Sci Pract. 2021;52:102340. doi: 10.1016/j.msksp.2021.102340
24. Gold KJ, Laurie AR, Kinney DR, et al. Video visits: family physician experiences with uptake during the COVID-19 pandemic. Fam Med. 53:207-210. doi: 10.22454/FamMed.2021.613099
25. Björndell C, Premberg A. Physicians’ experiences of video consultation with patients at a public virtual primary care clinic: a qualitative interview study. Scand J Prim Health Care. 2021;39:67-76. doi: 10.1080/02813432.2021.1882082
26. Powell RE, Henstenburg JM, Cooper G, et al. Patient perceptions of telehealth primary care video visits. Ann Fam Med. 2017;15:225-229. doi: 10.1370/afm.2095
27. Imlach F, McKinlay E, Middleton L, et al. Telehealth consultations in general practice during a pandemic lockdown: survey and interviews on patient experiences and preferences. BMC Fam Pract. 2020;21:1-14. doi: 10.1186/s12875-020-01336-1
28. Gordon HS, Solanki P, Bokhour BG, et al. “I’m not feeling like I’m part of the conversation” patients’ perspectives on communicating in clinical video telehealth visits. J Gen Intern Med. 2020;35:1751-1758. doi: 10.1007/s11606-020-05673-w
29. Volcy J, Smith W, Mills K, et al. Assessment of patient and provider satisfaction with the change to telehealth from in-person visits at an academic safety net institution during the COVID-19 pandemic. J Am Board Fam Med. 2021;34(suppl):S71-S76. doi: 10.3122/jabfm.2021.S1.200393
30. Gopal RK, Solanki P, Bokhour BG, et al. Provider, staff, and patient perspectives on medical visits using clinical video telehealth: a foundation for educational initiatives to improve medical care in telehealth. J Nurse Pract. 2021;17:582-587. doi: 10.1016/j.nurpra.2021.02.020
31. Edgoose JY. Exploring the face-to-face: revisiting patient-doctor relationships in a time of expanding telemedicine. J Am Board Fam Med. 2021;34(suppl):S252-S254. doi: 10.3122/jabfm.2021.S1.200398
32. Pierce RP, Stevermer JJ. Disparities in use of telehealth at the onset of the COVID-19 public health emergency. J Telemed Telecare. 2023;29:3-9. doi: 10.1177/1357633X20963893
33. Lame M, Leyden D, Platt SL. Geocode maps spotlight disparities in telehealth utilization during the COVID-19 pandemic in New York City. Telemed J E Health. 2021;27:251-253. doi: 10.1089/tmj.2020.0297
34. Jain V, Al Rifai M, Lee MT, et al. Racial and geographic disparities in internet use in the US among patients with hypertension or diabetes: implications for telehealth in the era of COVID-19. Diabetes Care. 2021;44:e15-e17. doi: 10.2337/dc20-2016
35. Futterman I, Rosenfeld E, Toaff M, et al. Addressing disparities in prenatal care via telehealth during COVID-19: prenatal satisfaction survey in East Harlem. Am J Perinatol. 2021;38:88-92. doi: 10.1055/s-0040-1718695
36. Wegermann K, Wilder JM, Parish A, et al. Racial and socioeconomic disparities in utilization of telehealth in patients with liver disease during COVID-19. Dig Dis Sci. 2022;67:93-99. doi: 10.1007/s10620-021-06842-5.
37. ASPE. National survey trends in telehealth use in 2021: disparities in utilization and audio vs. video services. Issue brief: February 21, 2022. Accessed March 27, 2023. https://aspe.hhs.gov/sites/default/files/documents/4e1853c0b4885112b2994680a58af9ed/telehealth-hps-ib.pdf
38. Ukoha EP, Davis K, Yinger M, et al. Ensuring equitable implementation of telemedicine in perinatal care. Obstet Gynecol. 2021;137:487-492. doi: 10.1097/AOG.0000000000004276
PRACTICE RECOMMENDATIONS
› Consider using telehealth encounters for diagnosing and treating infectious diseases and for monitoring stable chronic conditions. C
› Consider telehealth “check-ins” to encourage patients working on behavioral change, such as smoking cessation. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Patient with newly diagnosed type 2 diabetes? Remember these steps
Nearly 40 antihyperglycemic agents have been approved by the US Food and Drug Administration (FDA) since the approval of human insulin in 1982.1 In addition, existing antihyperglycemic medications are constantly gaining FDA approval for new indications for common type 2 diabetes (T2D) comorbidities. For example, in addition to their glycemic benefits, the sodium-glucose cotransporter-2 (SGLT2) inhibitors have been approved for use in patients with T2D and established atherosclerotic cardiovascular disease (ASCVD) to reduce the risk for major adverse cardiovascular events (MACE; canagliflozin), risk for hospitalization for heart failure (dapagliflozin), and cardiovascular death (empagliflozin).2-4
The plethora of new agents and new data for existing agents, coupled with the annual release of guidelines from the American Diabetes Association (ADA) and practice recommendations from several other professional organizations,5-7 make it challenging for family physicians to stay current and provide the most up-to-date, evidence-based care. In this article, we provide advice on how to approach the screening, diagnosis, and evaluation of T2D, and on how to manage newly diagnosed T2D.
Screening, Dx, and evaluation: A quick review
Screening
Screening recommendations vary among professional organizations (TABLE 15,6,8). The US Preventive Services Task Force (USPSTF) recommends screening adults ages 35 to 70 years who are overweight or obese. Clinicians also can consider screening patients with a higher risk for diabetes.5 The ADA suggests screening all adults starting at 35 years, regardless of risk factors.8 Asymptomatic adults of any age with overweight or obesity and 1 or more risk factors should be screened.8
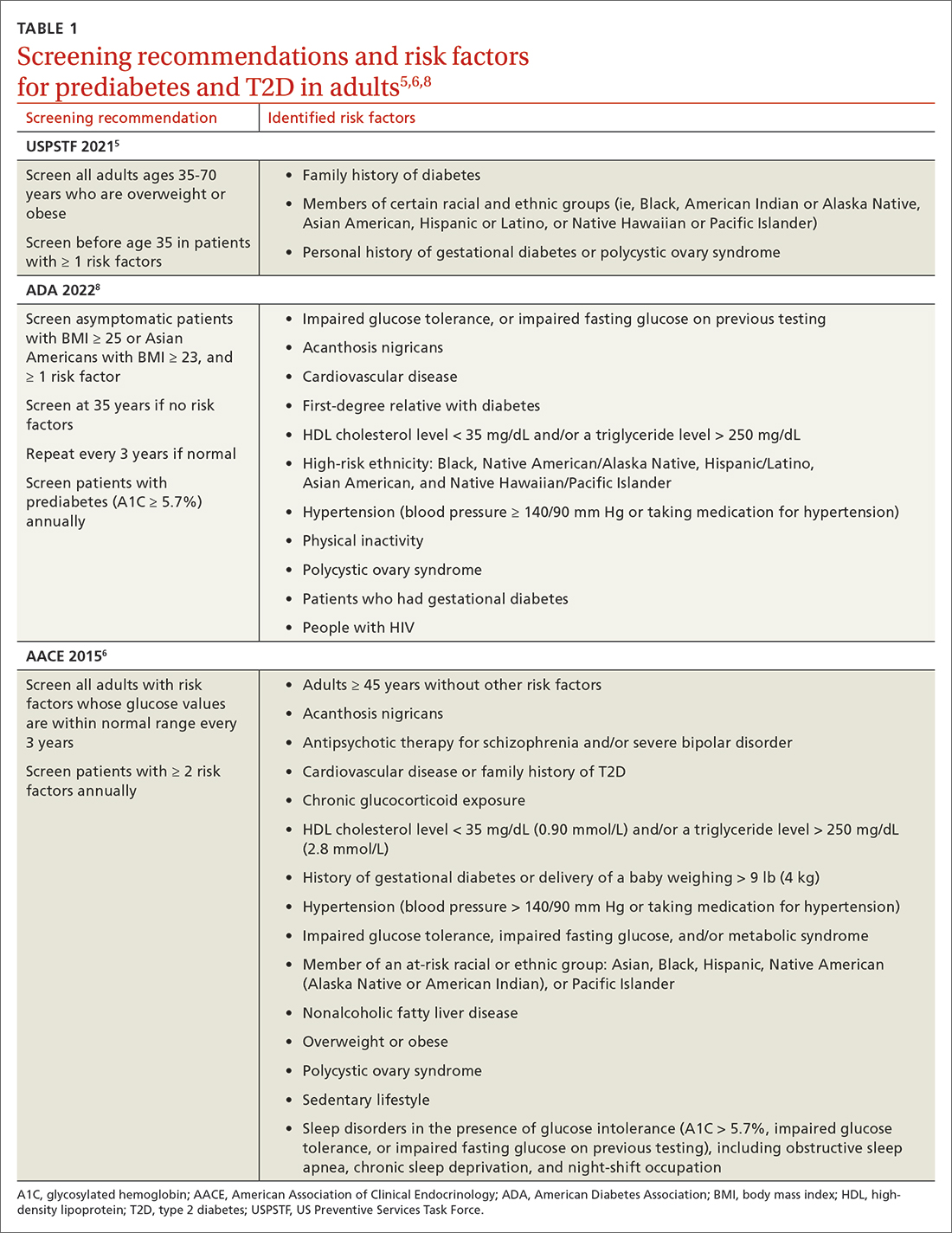
Making the diagnosis
The initial diagnosis of diabetes can be made by a fasting plasma glucose level ≥ 126 mg/dL (7.0 mmol/L); a 2-hour plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) following an oral glucose tolerance test; or an A1C level ≥ 6.5%. Prioritize lab-drawn A1C measurements over point-of-care tests to diagnose T2D. In patients with classic symptoms of hyperglycemia, a random plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) is also diagnostic. Generally, these tests are considered equally appropriate in screening for diabetes and may be used to detect prediabetes. In the absence of clear symptoms of hyperglycemia, the diagnosis of diabetes requires 2 abnormal screening test results, either via 1 blood sample (such as an abnormal A1C and glucose) or 2 separate blood samples of the same test. Further evaluation is advised if there is discordance between the 2 samples.8
Extended evaluations
Patients with newly diagnosed T2D require a thorough evaluation for comorbidities and complications of diabetes. Refer patients to an ophthalmologist for a dilated eye examination, with subsequent exams occurring every 1 to 2 years.6,9 Additional referrals for diabetes education, family planning for women of reproductive age, and dental, social, or mental health services may be clinically appropriate.9
Setting goals for glycemic control
Glycemic control is commonly monitored by the A1C level and by blood glucose monitoring either through traditional point-of-care glucometers or continuous glucose monitors (CGMs).10 Generally, CGMs provide more glycemic data than traditional glucometers and may cue patients to choose healthier dietary options and engage in physical exercise.11 Patients with T2D who use CGMs exhibit lower A1Cs, greater time in glycemic range, and reduced hypoglycemic episodes.11 Generally, CGMs are reserved for patients with type 1 diabetes and patients with T2D who use multiple daily injections, subcutaneous insulin infusions, or basal insulin only.12 Most professional organizations recommend that clinicians consider patient-specific factors to set individualized glycemic goals.6,10,13,14 For example, more stringent glycemic goals could be pursued for patients with longer life expectancy, shorter disease duration, absence of complications (eg, nephropathy, neuropathy, or cardiovascular disease), fewer comorbid conditions, lower hypoglycemia risk, or higher cognitive function.6
More specific A1C goals vary by professional organization. For nonpregnant adults, the ADA recommends an A1C goal of < 7% and a preprandial blood glucose level of 80 to 130 mg/dL (4.4-7.2 mmol/L).10 However, a lower A1C goal may be appropriate if it can be attained safely without causing hypoglycemia or other adverse effects.10 The AACE suggests an A1C goal of ≤ 6.5% and a fasting blood glucose level of < 110 mg/dL when it can be achieved safely.6 More stringent A1C goals may reduce long-term micro- and macrovascular complications—especially in patients with newly diagnosed T2D.10 While older studies such as the ACCORD trial found increased mortality in groups with more stringent glycemic targets, they did not include newer agents (SGLT2 inhibitors or glucagon-like peptide-1 [GLP-1] receptor agonists) that reduce cardiovascular events by mechanisms outside their glycemic-lowering effect. With these newer agents, more aggressive A1C goals can be targeted safely in select patients, particularly those with long life expectancy.10 Both the ADA and AACE recommend a less stringent A1C goal of 7% to 8% for patients with limited life expectancy or risks (eg, a history of hypoglycemia) that outweigh expected benefits.6,10
Continue to: Lifestyle modifications
Lifestyle modifications: As important as medication
Nutrition
The energy-dense Western diet, combined with sedentary behavior, are thought to be a primary cause of T2D.15 Therefore, include lifestyle modifications in the initial management of newly diagnosed T2D. Diets that replace carbohydrates with saturated and trans fats are related to increased mortality in patients with T2D.16 Increased consumption of vegetables, fruits, legumes, nuts, fish, cereal, and oils reduces concentrations of saturated and trans fats and increases dietary intake of monounsaturated fatty acids, fiber, antioxidants, and polyphenols.17
Increasing the intake of fiber, an undigestible carbohydrate, offers numerous benefits in T2D management. High-fiber diets can help regulate blood sugar and lipid levels, increase satiety, reduce inflammation, aid in weight management, and reduce premature mortality.18 Insoluble fiber, found in foods such as whole wheat flour, nuts, and cauliflower, helps food pass more quickly through the stomach and intestines and adds bulk to stool. Soluble fiber, found in foods such as chickpeas, lentils, and Brussels sprouts, absorbs water and forms a gel-like substance that protects nutrients from digestive enzymes and slows down digestion. The result is a more gradual rise in postprandial glucose levels and improved insulin sensitivity.19 Dietary fiber may produce short-chain fatty acids which in turn activate incretin secretion and stimulate a glucose-dependent release of insulin from the pancreas.20
Simple dietary substitutions, such as whole grains and legumes for white rice, can reduce fasting blood glucose and A1C levels.21 In a randomized controlled trial (RCT), increasing whole grain oat intake improved measures of glycemic control, reducing A1C by 1% at 1-year follow-up.19 Encourage patients with T2D to increase consumption of high-fiber foods and replace animal fats and refined grains with vegetable fats (eg, nuts, avocados, olives). Nutritional therapies should be individualized, taking into account personal preferences and cultural customs.22 Nutritional habits may be based on race/ethnicity, religion/spirituality, or even the city in which an individual resides. Nutrition recommendations should account for these differences as well as access to healthy foods. For instance, ethnic groups whose dietary patterns include tortillas could be counseled to choose high-fiber options such as corn instead of flour tortillas and to incorporate vegetables in place of high-fat foods. Additionally, ethnic groups who favor using animal fats in foods such as greens could be advised on ways to add flavor to vegetables without adding saturated fats. Taking this approach may lessen barriers to change and increase ability to make dietary modifications.23
Exercise
Encourage all patients with T2D to exercise regularly. The atherosclerotic plaques found in patients with T2D have increased inflammatory properties and result in worse cardiovascular outcomes compared with plaques in individuals without T2D.24 Regular exercise reduces levels of pro-inflammatory markers—C-reactive protein, interleukin (IL)-6, and tumor necrosis factor alpha—and increases levels of anti-inflammatory markers (IL-4 and IL-10).24 Regular exercise can improve body composition, physical fitness, lipid and glucose metabolism, and insulin sensitivity.25,26
A meta-analysis of RCTs demonstrated that structured exercise > 150 minutes per week resulted in A1C reductions of 0.89%,27 which is comparable to the effect of many oral antihyperglycemic medications.26 The Health Benefits of Aerobic and Resistance Training in individuals with T2D (HART-D) and Diabetes Aerobic and Resistance Exercise (DARE) studies demonstrated that combining endurance and resistance training was superior for improving glycemic control, cardiorespiratory fitness, and body composition, than using either type of training alone.25 Both the American College of Sports Medicine (ACSM) and the ADA recommend that adults engage in at least 150 total minutes of moderate-intensity aerobic activity per week and resistance training 2 to 3 times weekly.26 ACSM defines moderate-intensity exercise as 65% to 75% of maximal heart rate, a rating of perceived exertion of 3 to 4, or a step rate of 100 steps per minute.28
Continue to: Because of their longitudinal relationships...
Because of their longitudinal relationships with patients, family physicians are in an optimal position to assess a patient’s physical capacity level and provide individualized counseling. Several systematic reviews have demonstrated that counseling on exercise increases patients’ participation in physical activity.29 Encourage your patients with T2D to exercise regularly, considering each individual’s ability to engage in physical activity.
Weight loss
Include weight management in the initial treatment of patients with newly diagnosed T2D. Weight loss decreases hepatic glucose production and increases peripheral insulin sensitivity and insulin secretion.30 Moderate decreases in weight (5%-10%) can reduce complications related to diabetes, and sustained significant weight loss (> 10%) can potentially cause T2D remission (A1C < 6.5% after stopping diabetes medications).31,32
Diabetes self-management education supports patients by giving them tools for making and maintaining lifestyle changes. Understanding individual barriers to change and addressing these during motivational interviews is important. Through a qualitative interview study, participants in a diabetes self-management program revealed 4 factors that motivated them to maintain lifestyle changes: support from others, experiencing the impact of the changes they made, fear of T2D complications, and forming new habits.33 Family physicians are key in helping patients acquire knowledge and support to make the lifestyle modifications needed to manage newly diagnosed T2D.
Individualized pharmacotherapy considerations
For decades, the initial pharmacotherapeutic regimen for patients with newly diagnosed T2D considered the patient’s baseline A1C as a major driver for therapy. Metformin has been the mainstay in T2D treatment due to its clinical efficacy, minimal risk for hypoglycemia, and low cost. Regardless of the regimen, pharmacotherapy should be initiated at the time of T2D diagnosis in conjunction with the aforementioned lifestyle modifications.34
When selecting pharmacotherapy, practice guidelines recommend considering the efficacy and adverse effects of medications, patient-specific comorbidities, adherence, cost, and a patient’s lifestyle factors.34 Drug classes with pertinent information are listed in TABLE 2.34-54 After starting medication, monitor the A1C level every 3 months to determine whether therapy should be intensified. Patients should have their labs drawn ahead of the quarterly visit, or point-of-care measurements may be used to facilitate in-person patient–provider discussions.
Continue to: Consider patient-specific factors when starting pharmacotherapy
Consider patient-specific factors when starting pharmacotherapy
ASCVD. Regardless of baseline glycemic control, offer patients who have ASCVD, or who are at high risk for it, an SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin) or a long-acting GLP-1 receptor agonist (dulaglutide, liraglutide, or semaglutide).34,35 SGLT2 inhibitors reduced the risk for MACE by 11% in patients with established ASCVD.55 They also reduced a composite outcome of cardiovascular death or hospitalization for heart failure by 23% in patients with or without ASCVD or heart failure at baseline.55 GLP-1 receptor agonists offer a similar reduction in MACE to SGLT2 inhibitors, but they do not have significant effects in heart failure.56 Thiazolidinediones (TZDs), saxagliptin, and alogliptin should be avoided in patients with heart failure.57 TZDs may reduce the risk for recurrent stroke in patients with T2D.58
Chronic kidney disease (CKD). As with ASCVD, prioritize SGLT2 inhibitors and GLP-1 receptor agonists in patients with CKD. While both classes reduced the risk for progression of kidney disease such as macroalbuminuria, SGLT2 inhibitors offer additional benefits in their reduction of the worsening of estimated glomerular filtration rate, end-stage kidney disease, and renal death.56
Obesity. Consider the effect of each drug class on weight when making initial treatment choices, taking special care to minimize weight gain and potentially promote weight loss.34 The ADA prefers GLP-1 receptor agonists, but also suggests SGLT2 inhibitors in these patients. While all GLP-1 receptor agonists have an impact on weight, weekly subcutaneous semaglutide offers the most pronounced weight loss of 2 to 7 kg over 56 weeks.59 SGLT2 inhibitors promote sustainable weight loss to a lesser degree, contributing to an average loss of 3 kg at 2 years.60 Weight gain is common in patients taking sulfonylureas (2.01-2.3 kg)31 and insulin (3-9 kg weight gain in the first year)61 and should be avoided in patients with T2D and obesity.34
Hypoglycemia risk. In addition to counseling patients on hypoglycemia management and prescribing glucagon rescue kits, offer medications with no or very low risk for hypoglycemia (eg, GLP-1 receptor agonists, SGLT2 inhibitors, dipeptidyl peptidase-4 inhibitors, and TZDs). Generally, avoid insulin and sulfonylureas in patients in whom hypoglycemia is a major concern (eg, older adults, individuals with labile blood glucose levels).34 Patients with reduced renal function are at higher risk for hypoglycemia with insulin or sulfonylureas due to reduced drug clearance. However, insulin is often the only treatment for patients with advanced renal disease. Pay close attention to insulin dosing in patients with advanced renal disease, which may necessitate lower doses and smaller dose adjustments due to this risk.
Social determinants of health. Medication access and cost is a major burden in T2D management and should be considered for every patient. Compared with the period of 2005 to 2007, the annual cost of diabetes medications for an individual in 2015 to 2017 increased by 147%, rising from $1106 to $2727 per year.62 This increase is driven by the cost of insulin and newer medications without generic options.62 Identify local resources in your community, such as patient assistance programs and pharmacies with reduced-price generic prescription programs, which may be useful for patients who are underinsured or uninsured.
Continue to: Even if cost weren't an issue...
Even if cost weren’t an issue, many medications such as insulin and GLP-1 receptor agonists should be kept refrigerated and are only stable at room temperature for a limited time. Medications that are stable at room temperature should be prioritized in patients with limited or inconsistent access to refrigeration or unstable housing who may find it difficult to store their medications appropriately.
Do not delay insulin initiation in patients with high baseline A1C
Whenever possible, a GLP-1 receptor agonist is the preferred injectable medication to insulin. Starting insulin introduces numerous risks, including hypoglycemia, weight gain, and stigma. However, in the patient with newly diagnosed T2D, choose basal insulin when the baseline hyperglycemia is severe,34 as indicated by:
- blood glucose > 300 mg/dL (16.7 mmol/L),
- A1C > 10% (86 mmol/mol),
- symptoms of hyperglycemia (polyuria or polydipsia), or
- evidence of catabolism (weight loss, hypertriglyceridemia, ketosis).
Basal insulin analogs are preferred over NPH given their reduced variability, dosing, and hypoglycemic risk.35 Mixed insulins may be used if a patient is unable to afford an insulin analog, which can be quite costly. However, extensive counseling on dosing and management of hypoglycemia is crucial to patient safety with these agents. The ADA recommends initiating 0.1 to 0.2 units/kg of basal insulin daily or 10 units daily.34 The AACE follows this recommendation for patients with baseline A1C < 8%, but it proposes a more aggressive initiation of 0.2 to 0.3 units/kg/d for patients with baseline A1C > 8%.35 Titrate the dose by 2 units every 3 days to reach the target fasting blood glucose level. As hyperglycemia resolves, simplify the regimen and transition to noninsulin options per the previously discussed considerations.
It’s not just about glycemic control
In addition to the direct effects of hyperglycemia, a T2D diagnosis introduces an increased risk for ASCVD, a reduced ability to fight infection, and heightened risk for depression. Order a lipid panel at the time of T2D diagnosis and initiate lipid management as needed (TABLE 335,63,64). Both the ADA and the American Heart Association recommend starting a moderate-intensity statin as primary prevention for all patients with T2D between 40 and 75 years of age regardless of the 10-year ASCVD risk.63 The AACE uses specific lipid targets and recommends moderate- to high-intensity statin therapy for patients with T2D.35 All recommendations by professional organizations list high-intensity statins for patients with established ASCVD.
It is also vital to recommend that patients with newly diagnosed T2D remain up to date on all indicated vaccinations. They should promptly receive the hepatitis B and pneumococcal vaccines if they have not already done so for a previous indication. COVID-19 and annual influenza vaccines also should be prioritized for these patients.65
Finally, patients with diabetes are twice as likely to develop depression than patients without diabetes.66 Individuals with T2D and depression exhibit poorer medication adherence, lifestyle choices, and glycemic control.66 Screen for and treat these issues in all patients with T2D across the course of the disease.
Overall, work closely with patients to support them in managing their new diagnosis with evidence-based pharmacologic and nonpharmacologic approaches. The importance of lifestyle changes including high-fiber diets, regular exercise, and weight loss should not be overlooked. Do not delay starting pharmacotherapy after diagnosing T2D and consider medication-specific and patient-specific factors to individualize therapy, improve adherence, and prevent complications.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, 833 South Wood Street (MC 886), Chicago, IL 60612; jarrett8@uic.edu
1. Dahlén AD, Dashi G, Maslov I, et al. Trends in antidiabetic drug discovery: FDA approved drugs, new drugs in clinical trials and global sales. Front Pharmacol. 2022;12. Accessed April 19, 2023. www.frontiersin.org/article/10.3389/fphar.2021.807548
2. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128. doi: 10.1056/NEJMoa1504720
3. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657. doi: 10.1056/NEJMoa1611925
4. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357. doi: 10.1056/NEJMoa1812389
5. Davidson KW, Barry MJ, et al. Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:736-743. doi: 10.1001/jama. 2021.12531
6. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract. 2015;21(suppl 1):1-87. doi: 10.4158/EP15672.GL
7. ADA. Introduction: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
8. ADA Professional Practice Committee. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
9. ADA Professional Practice Committee. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S46-S59. doi: 10.2337/dc22-S004
10. ADA Professional Practice Committee. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S83-S96. doi: 10.2337/dc22-S006
11. Janapala RN, Jayaraj JS, Fathima N, et al. Continuous glucose monitoring versus self-monitoring of blood glucose in type 2 diabetes mellitus: a systematic review with meta-analysis. Cureus. 2019;11:e5634. doi: 10.7759/cureus.5634
12. ADA Professional Practice Committee. Diabetes technology: standards of medical care in diabetes - 2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
13. Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168:569-576. doi: 10.7326/M17-0939
14. Moran GM, Bakhai C, Song SH, et al, Guideline Committee. Type 2 diabetes: summary of updated NICE guidance. BMJ. 2022;377:o775. doi: 10.1136/bmj.o775
15. Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. doi: 10.1186/s12916-017-0901-x
16. McMacken M, Shah S. A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol. 2017;14:342-354. doi: 10.11909/j.issn.1671-5411.2017.05.009
17. Asif M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J Educ Health Promot. 2014;3:1. doi: 10.4103/2277-9531.127541
18. Reynolds AN, Akerman AP, Mann J. Dietary fibre and whole grains in diabetes management: systematic review and meta-analyses. PLoS Med. 2020;17(3):e1003053. doi: 10.1371/journal.pmed.1003053
19. Li X, Cai X, Ma X, et al. Short- and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: a randomized control trial. Nutrients. 2016;8:549. doi: 10.3390/nu8090549
20. Fujii H, Iwase M, Ohkuma T, et al. Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. Nutr J. 2013;12:159. doi: 10.1186/1475-2891-12-159
21. Kim M, Jeung SR, Jeong TS, et al. Replacing with whole grains and legumes reduces Lp-PLA2 activities in plasma and PBMCs in patients with prediabetes or T2D. J Lipid Res. 2014;55:1762-1771. doi: 10.1194/jlr.M044834
22. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
23. Caballero AE. The “a to z” of managing type 2 diabetes in culturally diverse populations. Front Endocrinol. 2018;9:479. doi: 10.3389/fendo.2018.00479
24. Golbidi S, Badran M, Laher I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Exp Diabetes Res. 2012; 2012:941868. doi: 10.1155/2012/941868
25. Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol. 2016;94:146-150. doi: 10.1038/icb.2015.101
26. Dugan JA. Exercise recommendations for patients with type 2 diabetes. JAAPA. 2016;29:13-18. doi: 10.1097/01.JAA. 0000475460.77476.f6
27. Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–1799. doi: 10.1001/jama.2011.576
28. Zuhl M. Tips for monitoring aerobic exercise intensity. 2020. Accessed April 19, 2023. www.acsm.org/docs/default-source/files-for-resource-library/exercise-intensity-infographic.pdf? sfvrsn=f467c793_2
29. Williams A, Radford J, O’Brien J, Davison K. Type 2 diabetes and the medicine of exercise: the role of general practice in ensuring exercise is part of every patient’s plan. Aust J Gen Pract. 2020;49:189-193. doi: 10.31128/AJGP-09-19-5091
30. Grams J, Garvey WT. Weight loss and the prevention and treatment of type 2 diabetes using lifestyle therapy, pharmacotherapy, and bariatric surgery: mechanisms of action. Curr Obes Rep. 2015;4:287-302. doi: 10.1007/s13679-015-0155-x
31. Apovian CM, Okemah J, O’Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36:44-58. doi: 10.1007/s12325-018-0824-8
32. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7:344-355. doi: 10.1016/S2213-8587(19)30068-3
33. Rise MB, Pellerud A, Rygg LØ, et al. Making and maintaining lifestyle changes after participating in group based type 2 diabetes self-management educations: a qualitative study. PLoS One. 2013;8:e64009. doi: 10.1371/journal.pone.0064009
34. ADA Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S125-S143. doi: 10.2337/dc22-S009
35. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract. 2020;26:107-139. doi: 10.4158/CS-2019-0472
36. Metformin. Package insert. Bristol-Myers Squibb Company; 2017.
37. Invokana (canagliflozin). Package insert. Janssen Pharmaceuticals, Inc; 2020.
38. Farxiga (dapagliflozin). Package insert. AstraZeneca Pharmaceuticals LP; 2021.
39. Jardiance (empagliflozin). Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
40. Steglatro (ertugliflozin). Package insert. Merck & Co, Inc; 2021.
41. Trulicity (dulaglutide). Package insert. Lilly USA, LLC; 2022.
42. Byetta (exenatide). Package insert. AstraZeneca Canada Inc; 2022.
43. Bydureon (exenatide ER). Package insert. AstraZeneca Pharmaceuticals LP; 2022.
44. Victoza (liraglutide). Package insert. Novo Nordisk; 2022.
45. Adlyxin (lixisenatide). Package insert. Sanofi-Aventis US LLC; 2022.
46. Ozempic (semaglutide). Package insert. Novo Nordisk; 2022.
47. Alogliptin. Package insert. Takeda Pharmaceuticals USA, Inc; 2022.
48. Linagliptin. Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
49. Saxagliptin. Package insert. AstraZeneca Pharmaceuticals LP; 2019.
50. Januvia (sitagliptin). Package insert. Merck Sharp & Dohme LLC; 2022.
51. Glimepiride. Package insert. Sanofi-Aventis US LLC; 2009.
52. Glipizide. Package insert. Roerig; 2023.
53. Glyburide. Package insert. Sanofi-Aventis US LLC; 2009.
54. Pioglitazone. Package insert. Northstar Rx LLC; 2022.
55. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. doi: 10.1016/S0140-6736(18)32590-X
56. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022-2031. doi: 10.1161/CIRCULATIONAHA.118.038868
57. FDA. FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. Accessed April 19, 2023. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-adds-warnings-about-heart-failure-risk-labels-type-2-diabetes
58. Wilcox R, Bousser MG, Betteridge DJ, et al. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04). Stroke. 2007;38:865-873. doi: 10.1161/01.STR.0000257974.06317.49
59. Lingvay I, Hansen T, Macura S, et al. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diabetes Res Care. 2020;8:e001706. doi: 10.1136/bmjdrc-2020-001706
60. Liu XY, Zhang N, Chen R, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years. J Diabetes Complications. 2015;29:1295-1303. doi: 10.1016/j.jdiacomp.2015.07.011
61. Brown A, Guess N, Dornhorst A, et al. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: what can be done? Diabetes Obes Metab. 2017;19:1655-1668. doi: 10.1111/dom.13009
62. Zhou X, Shrestha SS, Shao H, et al. Factors contributing to the rising national cost of glucose-lowering medicines for diabetes during 2005-2007 and 2015-2017. Diabetes Care. 2020;43:2396-2402. doi: 10.2337/dc19-2273
63. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi: 10.1161/CIR.0000000000000625
64. ADA Professional Practice Committee. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S144-S174. doi: 10.2337/dc22-S010
65. CDC. Adult immunization schedule by medical condition and other indication. 2022. Accessed April 19, 2023. www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.htm
66. Semenkovich K, Brown ME, Svrakic DM, et al. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75:577-587. doi: 10.1007/s40265-015-0347-4
Nearly 40 antihyperglycemic agents have been approved by the US Food and Drug Administration (FDA) since the approval of human insulin in 1982.1 In addition, existing antihyperglycemic medications are constantly gaining FDA approval for new indications for common type 2 diabetes (T2D) comorbidities. For example, in addition to their glycemic benefits, the sodium-glucose cotransporter-2 (SGLT2) inhibitors have been approved for use in patients with T2D and established atherosclerotic cardiovascular disease (ASCVD) to reduce the risk for major adverse cardiovascular events (MACE; canagliflozin), risk for hospitalization for heart failure (dapagliflozin), and cardiovascular death (empagliflozin).2-4
The plethora of new agents and new data for existing agents, coupled with the annual release of guidelines from the American Diabetes Association (ADA) and practice recommendations from several other professional organizations,5-7 make it challenging for family physicians to stay current and provide the most up-to-date, evidence-based care. In this article, we provide advice on how to approach the screening, diagnosis, and evaluation of T2D, and on how to manage newly diagnosed T2D.
Screening, Dx, and evaluation: A quick review
Screening
Screening recommendations vary among professional organizations (TABLE 15,6,8). The US Preventive Services Task Force (USPSTF) recommends screening adults ages 35 to 70 years who are overweight or obese. Clinicians also can consider screening patients with a higher risk for diabetes.5 The ADA suggests screening all adults starting at 35 years, regardless of risk factors.8 Asymptomatic adults of any age with overweight or obesity and 1 or more risk factors should be screened.8

Making the diagnosis
The initial diagnosis of diabetes can be made by a fasting plasma glucose level ≥ 126 mg/dL (7.0 mmol/L); a 2-hour plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) following an oral glucose tolerance test; or an A1C level ≥ 6.5%. Prioritize lab-drawn A1C measurements over point-of-care tests to diagnose T2D. In patients with classic symptoms of hyperglycemia, a random plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) is also diagnostic. Generally, these tests are considered equally appropriate in screening for diabetes and may be used to detect prediabetes. In the absence of clear symptoms of hyperglycemia, the diagnosis of diabetes requires 2 abnormal screening test results, either via 1 blood sample (such as an abnormal A1C and glucose) or 2 separate blood samples of the same test. Further evaluation is advised if there is discordance between the 2 samples.8
Extended evaluations
Patients with newly diagnosed T2D require a thorough evaluation for comorbidities and complications of diabetes. Refer patients to an ophthalmologist for a dilated eye examination, with subsequent exams occurring every 1 to 2 years.6,9 Additional referrals for diabetes education, family planning for women of reproductive age, and dental, social, or mental health services may be clinically appropriate.9
Setting goals for glycemic control
Glycemic control is commonly monitored by the A1C level and by blood glucose monitoring either through traditional point-of-care glucometers or continuous glucose monitors (CGMs).10 Generally, CGMs provide more glycemic data than traditional glucometers and may cue patients to choose healthier dietary options and engage in physical exercise.11 Patients with T2D who use CGMs exhibit lower A1Cs, greater time in glycemic range, and reduced hypoglycemic episodes.11 Generally, CGMs are reserved for patients with type 1 diabetes and patients with T2D who use multiple daily injections, subcutaneous insulin infusions, or basal insulin only.12 Most professional organizations recommend that clinicians consider patient-specific factors to set individualized glycemic goals.6,10,13,14 For example, more stringent glycemic goals could be pursued for patients with longer life expectancy, shorter disease duration, absence of complications (eg, nephropathy, neuropathy, or cardiovascular disease), fewer comorbid conditions, lower hypoglycemia risk, or higher cognitive function.6
More specific A1C goals vary by professional organization. For nonpregnant adults, the ADA recommends an A1C goal of < 7% and a preprandial blood glucose level of 80 to 130 mg/dL (4.4-7.2 mmol/L).10 However, a lower A1C goal may be appropriate if it can be attained safely without causing hypoglycemia or other adverse effects.10 The AACE suggests an A1C goal of ≤ 6.5% and a fasting blood glucose level of < 110 mg/dL when it can be achieved safely.6 More stringent A1C goals may reduce long-term micro- and macrovascular complications—especially in patients with newly diagnosed T2D.10 While older studies such as the ACCORD trial found increased mortality in groups with more stringent glycemic targets, they did not include newer agents (SGLT2 inhibitors or glucagon-like peptide-1 [GLP-1] receptor agonists) that reduce cardiovascular events by mechanisms outside their glycemic-lowering effect. With these newer agents, more aggressive A1C goals can be targeted safely in select patients, particularly those with long life expectancy.10 Both the ADA and AACE recommend a less stringent A1C goal of 7% to 8% for patients with limited life expectancy or risks (eg, a history of hypoglycemia) that outweigh expected benefits.6,10
Continue to: Lifestyle modifications
Lifestyle modifications: As important as medication
Nutrition
The energy-dense Western diet, combined with sedentary behavior, are thought to be a primary cause of T2D.15 Therefore, include lifestyle modifications in the initial management of newly diagnosed T2D. Diets that replace carbohydrates with saturated and trans fats are related to increased mortality in patients with T2D.16 Increased consumption of vegetables, fruits, legumes, nuts, fish, cereal, and oils reduces concentrations of saturated and trans fats and increases dietary intake of monounsaturated fatty acids, fiber, antioxidants, and polyphenols.17
Increasing the intake of fiber, an undigestible carbohydrate, offers numerous benefits in T2D management. High-fiber diets can help regulate blood sugar and lipid levels, increase satiety, reduce inflammation, aid in weight management, and reduce premature mortality.18 Insoluble fiber, found in foods such as whole wheat flour, nuts, and cauliflower, helps food pass more quickly through the stomach and intestines and adds bulk to stool. Soluble fiber, found in foods such as chickpeas, lentils, and Brussels sprouts, absorbs water and forms a gel-like substance that protects nutrients from digestive enzymes and slows down digestion. The result is a more gradual rise in postprandial glucose levels and improved insulin sensitivity.19 Dietary fiber may produce short-chain fatty acids which in turn activate incretin secretion and stimulate a glucose-dependent release of insulin from the pancreas.20
Simple dietary substitutions, such as whole grains and legumes for white rice, can reduce fasting blood glucose and A1C levels.21 In a randomized controlled trial (RCT), increasing whole grain oat intake improved measures of glycemic control, reducing A1C by 1% at 1-year follow-up.19 Encourage patients with T2D to increase consumption of high-fiber foods and replace animal fats and refined grains with vegetable fats (eg, nuts, avocados, olives). Nutritional therapies should be individualized, taking into account personal preferences and cultural customs.22 Nutritional habits may be based on race/ethnicity, religion/spirituality, or even the city in which an individual resides. Nutrition recommendations should account for these differences as well as access to healthy foods. For instance, ethnic groups whose dietary patterns include tortillas could be counseled to choose high-fiber options such as corn instead of flour tortillas and to incorporate vegetables in place of high-fat foods. Additionally, ethnic groups who favor using animal fats in foods such as greens could be advised on ways to add flavor to vegetables without adding saturated fats. Taking this approach may lessen barriers to change and increase ability to make dietary modifications.23
Exercise
Encourage all patients with T2D to exercise regularly. The atherosclerotic plaques found in patients with T2D have increased inflammatory properties and result in worse cardiovascular outcomes compared with plaques in individuals without T2D.24 Regular exercise reduces levels of pro-inflammatory markers—C-reactive protein, interleukin (IL)-6, and tumor necrosis factor alpha—and increases levels of anti-inflammatory markers (IL-4 and IL-10).24 Regular exercise can improve body composition, physical fitness, lipid and glucose metabolism, and insulin sensitivity.25,26
A meta-analysis of RCTs demonstrated that structured exercise > 150 minutes per week resulted in A1C reductions of 0.89%,27 which is comparable to the effect of many oral antihyperglycemic medications.26 The Health Benefits of Aerobic and Resistance Training in individuals with T2D (HART-D) and Diabetes Aerobic and Resistance Exercise (DARE) studies demonstrated that combining endurance and resistance training was superior for improving glycemic control, cardiorespiratory fitness, and body composition, than using either type of training alone.25 Both the American College of Sports Medicine (ACSM) and the ADA recommend that adults engage in at least 150 total minutes of moderate-intensity aerobic activity per week and resistance training 2 to 3 times weekly.26 ACSM defines moderate-intensity exercise as 65% to 75% of maximal heart rate, a rating of perceived exertion of 3 to 4, or a step rate of 100 steps per minute.28
Continue to: Because of their longitudinal relationships...
Because of their longitudinal relationships with patients, family physicians are in an optimal position to assess a patient’s physical capacity level and provide individualized counseling. Several systematic reviews have demonstrated that counseling on exercise increases patients’ participation in physical activity.29 Encourage your patients with T2D to exercise regularly, considering each individual’s ability to engage in physical activity.
Weight loss
Include weight management in the initial treatment of patients with newly diagnosed T2D. Weight loss decreases hepatic glucose production and increases peripheral insulin sensitivity and insulin secretion.30 Moderate decreases in weight (5%-10%) can reduce complications related to diabetes, and sustained significant weight loss (> 10%) can potentially cause T2D remission (A1C < 6.5% after stopping diabetes medications).31,32
Diabetes self-management education supports patients by giving them tools for making and maintaining lifestyle changes. Understanding individual barriers to change and addressing these during motivational interviews is important. Through a qualitative interview study, participants in a diabetes self-management program revealed 4 factors that motivated them to maintain lifestyle changes: support from others, experiencing the impact of the changes they made, fear of T2D complications, and forming new habits.33 Family physicians are key in helping patients acquire knowledge and support to make the lifestyle modifications needed to manage newly diagnosed T2D.
Individualized pharmacotherapy considerations
For decades, the initial pharmacotherapeutic regimen for patients with newly diagnosed T2D considered the patient’s baseline A1C as a major driver for therapy. Metformin has been the mainstay in T2D treatment due to its clinical efficacy, minimal risk for hypoglycemia, and low cost. Regardless of the regimen, pharmacotherapy should be initiated at the time of T2D diagnosis in conjunction with the aforementioned lifestyle modifications.34
When selecting pharmacotherapy, practice guidelines recommend considering the efficacy and adverse effects of medications, patient-specific comorbidities, adherence, cost, and a patient’s lifestyle factors.34 Drug classes with pertinent information are listed in TABLE 2.34-54 After starting medication, monitor the A1C level every 3 months to determine whether therapy should be intensified. Patients should have their labs drawn ahead of the quarterly visit, or point-of-care measurements may be used to facilitate in-person patient–provider discussions.


Continue to: Consider patient-specific factors when starting pharmacotherapy
Consider patient-specific factors when starting pharmacotherapy
ASCVD. Regardless of baseline glycemic control, offer patients who have ASCVD, or who are at high risk for it, an SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin) or a long-acting GLP-1 receptor agonist (dulaglutide, liraglutide, or semaglutide).34,35 SGLT2 inhibitors reduced the risk for MACE by 11% in patients with established ASCVD.55 They also reduced a composite outcome of cardiovascular death or hospitalization for heart failure by 23% in patients with or without ASCVD or heart failure at baseline.55 GLP-1 receptor agonists offer a similar reduction in MACE to SGLT2 inhibitors, but they do not have significant effects in heart failure.56 Thiazolidinediones (TZDs), saxagliptin, and alogliptin should be avoided in patients with heart failure.57 TZDs may reduce the risk for recurrent stroke in patients with T2D.58
Chronic kidney disease (CKD). As with ASCVD, prioritize SGLT2 inhibitors and GLP-1 receptor agonists in patients with CKD. While both classes reduced the risk for progression of kidney disease such as macroalbuminuria, SGLT2 inhibitors offer additional benefits in their reduction of the worsening of estimated glomerular filtration rate, end-stage kidney disease, and renal death.56
Obesity. Consider the effect of each drug class on weight when making initial treatment choices, taking special care to minimize weight gain and potentially promote weight loss.34 The ADA prefers GLP-1 receptor agonists, but also suggests SGLT2 inhibitors in these patients. While all GLP-1 receptor agonists have an impact on weight, weekly subcutaneous semaglutide offers the most pronounced weight loss of 2 to 7 kg over 56 weeks.59 SGLT2 inhibitors promote sustainable weight loss to a lesser degree, contributing to an average loss of 3 kg at 2 years.60 Weight gain is common in patients taking sulfonylureas (2.01-2.3 kg)31 and insulin (3-9 kg weight gain in the first year)61 and should be avoided in patients with T2D and obesity.34
Hypoglycemia risk. In addition to counseling patients on hypoglycemia management and prescribing glucagon rescue kits, offer medications with no or very low risk for hypoglycemia (eg, GLP-1 receptor agonists, SGLT2 inhibitors, dipeptidyl peptidase-4 inhibitors, and TZDs). Generally, avoid insulin and sulfonylureas in patients in whom hypoglycemia is a major concern (eg, older adults, individuals with labile blood glucose levels).34 Patients with reduced renal function are at higher risk for hypoglycemia with insulin or sulfonylureas due to reduced drug clearance. However, insulin is often the only treatment for patients with advanced renal disease. Pay close attention to insulin dosing in patients with advanced renal disease, which may necessitate lower doses and smaller dose adjustments due to this risk.
Social determinants of health. Medication access and cost is a major burden in T2D management and should be considered for every patient. Compared with the period of 2005 to 2007, the annual cost of diabetes medications for an individual in 2015 to 2017 increased by 147%, rising from $1106 to $2727 per year.62 This increase is driven by the cost of insulin and newer medications without generic options.62 Identify local resources in your community, such as patient assistance programs and pharmacies with reduced-price generic prescription programs, which may be useful for patients who are underinsured or uninsured.
Continue to: Even if cost weren't an issue...
Even if cost weren’t an issue, many medications such as insulin and GLP-1 receptor agonists should be kept refrigerated and are only stable at room temperature for a limited time. Medications that are stable at room temperature should be prioritized in patients with limited or inconsistent access to refrigeration or unstable housing who may find it difficult to store their medications appropriately.
Do not delay insulin initiation in patients with high baseline A1C
Whenever possible, a GLP-1 receptor agonist is the preferred injectable medication to insulin. Starting insulin introduces numerous risks, including hypoglycemia, weight gain, and stigma. However, in the patient with newly diagnosed T2D, choose basal insulin when the baseline hyperglycemia is severe,34 as indicated by:
- blood glucose > 300 mg/dL (16.7 mmol/L),
- A1C > 10% (86 mmol/mol),
- symptoms of hyperglycemia (polyuria or polydipsia), or
- evidence of catabolism (weight loss, hypertriglyceridemia, ketosis).
Basal insulin analogs are preferred over NPH given their reduced variability, dosing, and hypoglycemic risk.35 Mixed insulins may be used if a patient is unable to afford an insulin analog, which can be quite costly. However, extensive counseling on dosing and management of hypoglycemia is crucial to patient safety with these agents. The ADA recommends initiating 0.1 to 0.2 units/kg of basal insulin daily or 10 units daily.34 The AACE follows this recommendation for patients with baseline A1C < 8%, but it proposes a more aggressive initiation of 0.2 to 0.3 units/kg/d for patients with baseline A1C > 8%.35 Titrate the dose by 2 units every 3 days to reach the target fasting blood glucose level. As hyperglycemia resolves, simplify the regimen and transition to noninsulin options per the previously discussed considerations.
It’s not just about glycemic control
In addition to the direct effects of hyperglycemia, a T2D diagnosis introduces an increased risk for ASCVD, a reduced ability to fight infection, and heightened risk for depression. Order a lipid panel at the time of T2D diagnosis and initiate lipid management as needed (TABLE 335,63,64). Both the ADA and the American Heart Association recommend starting a moderate-intensity statin as primary prevention for all patients with T2D between 40 and 75 years of age regardless of the 10-year ASCVD risk.63 The AACE uses specific lipid targets and recommends moderate- to high-intensity statin therapy for patients with T2D.35 All recommendations by professional organizations list high-intensity statins for patients with established ASCVD.

It is also vital to recommend that patients with newly diagnosed T2D remain up to date on all indicated vaccinations. They should promptly receive the hepatitis B and pneumococcal vaccines if they have not already done so for a previous indication. COVID-19 and annual influenza vaccines also should be prioritized for these patients.65
Finally, patients with diabetes are twice as likely to develop depression than patients without diabetes.66 Individuals with T2D and depression exhibit poorer medication adherence, lifestyle choices, and glycemic control.66 Screen for and treat these issues in all patients with T2D across the course of the disease.
Overall, work closely with patients to support them in managing their new diagnosis with evidence-based pharmacologic and nonpharmacologic approaches. The importance of lifestyle changes including high-fiber diets, regular exercise, and weight loss should not be overlooked. Do not delay starting pharmacotherapy after diagnosing T2D and consider medication-specific and patient-specific factors to individualize therapy, improve adherence, and prevent complications.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, 833 South Wood Street (MC 886), Chicago, IL 60612; jarrett8@uic.edu
Nearly 40 antihyperglycemic agents have been approved by the US Food and Drug Administration (FDA) since the approval of human insulin in 1982.1 In addition, existing antihyperglycemic medications are constantly gaining FDA approval for new indications for common type 2 diabetes (T2D) comorbidities. For example, in addition to their glycemic benefits, the sodium-glucose cotransporter-2 (SGLT2) inhibitors have been approved for use in patients with T2D and established atherosclerotic cardiovascular disease (ASCVD) to reduce the risk for major adverse cardiovascular events (MACE; canagliflozin), risk for hospitalization for heart failure (dapagliflozin), and cardiovascular death (empagliflozin).2-4
The plethora of new agents and new data for existing agents, coupled with the annual release of guidelines from the American Diabetes Association (ADA) and practice recommendations from several other professional organizations,5-7 make it challenging for family physicians to stay current and provide the most up-to-date, evidence-based care. In this article, we provide advice on how to approach the screening, diagnosis, and evaluation of T2D, and on how to manage newly diagnosed T2D.
Screening, Dx, and evaluation: A quick review
Screening
Screening recommendations vary among professional organizations (TABLE 15,6,8). The US Preventive Services Task Force (USPSTF) recommends screening adults ages 35 to 70 years who are overweight or obese. Clinicians also can consider screening patients with a higher risk for diabetes.5 The ADA suggests screening all adults starting at 35 years, regardless of risk factors.8 Asymptomatic adults of any age with overweight or obesity and 1 or more risk factors should be screened.8

Making the diagnosis
The initial diagnosis of diabetes can be made by a fasting plasma glucose level ≥ 126 mg/dL (7.0 mmol/L); a 2-hour plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) following an oral glucose tolerance test; or an A1C level ≥ 6.5%. Prioritize lab-drawn A1C measurements over point-of-care tests to diagnose T2D. In patients with classic symptoms of hyperglycemia, a random plasma glucose level ≥ 200 mg/dL (11.0 mmol/L) is also diagnostic. Generally, these tests are considered equally appropriate in screening for diabetes and may be used to detect prediabetes. In the absence of clear symptoms of hyperglycemia, the diagnosis of diabetes requires 2 abnormal screening test results, either via 1 blood sample (such as an abnormal A1C and glucose) or 2 separate blood samples of the same test. Further evaluation is advised if there is discordance between the 2 samples.8
Extended evaluations
Patients with newly diagnosed T2D require a thorough evaluation for comorbidities and complications of diabetes. Refer patients to an ophthalmologist for a dilated eye examination, with subsequent exams occurring every 1 to 2 years.6,9 Additional referrals for diabetes education, family planning for women of reproductive age, and dental, social, or mental health services may be clinically appropriate.9
Setting goals for glycemic control
Glycemic control is commonly monitored by the A1C level and by blood glucose monitoring either through traditional point-of-care glucometers or continuous glucose monitors (CGMs).10 Generally, CGMs provide more glycemic data than traditional glucometers and may cue patients to choose healthier dietary options and engage in physical exercise.11 Patients with T2D who use CGMs exhibit lower A1Cs, greater time in glycemic range, and reduced hypoglycemic episodes.11 Generally, CGMs are reserved for patients with type 1 diabetes and patients with T2D who use multiple daily injections, subcutaneous insulin infusions, or basal insulin only.12 Most professional organizations recommend that clinicians consider patient-specific factors to set individualized glycemic goals.6,10,13,14 For example, more stringent glycemic goals could be pursued for patients with longer life expectancy, shorter disease duration, absence of complications (eg, nephropathy, neuropathy, or cardiovascular disease), fewer comorbid conditions, lower hypoglycemia risk, or higher cognitive function.6
More specific A1C goals vary by professional organization. For nonpregnant adults, the ADA recommends an A1C goal of < 7% and a preprandial blood glucose level of 80 to 130 mg/dL (4.4-7.2 mmol/L).10 However, a lower A1C goal may be appropriate if it can be attained safely without causing hypoglycemia or other adverse effects.10 The AACE suggests an A1C goal of ≤ 6.5% and a fasting blood glucose level of < 110 mg/dL when it can be achieved safely.6 More stringent A1C goals may reduce long-term micro- and macrovascular complications—especially in patients with newly diagnosed T2D.10 While older studies such as the ACCORD trial found increased mortality in groups with more stringent glycemic targets, they did not include newer agents (SGLT2 inhibitors or glucagon-like peptide-1 [GLP-1] receptor agonists) that reduce cardiovascular events by mechanisms outside their glycemic-lowering effect. With these newer agents, more aggressive A1C goals can be targeted safely in select patients, particularly those with long life expectancy.10 Both the ADA and AACE recommend a less stringent A1C goal of 7% to 8% for patients with limited life expectancy or risks (eg, a history of hypoglycemia) that outweigh expected benefits.6,10
Continue to: Lifestyle modifications
Lifestyle modifications: As important as medication
Nutrition
The energy-dense Western diet, combined with sedentary behavior, are thought to be a primary cause of T2D.15 Therefore, include lifestyle modifications in the initial management of newly diagnosed T2D. Diets that replace carbohydrates with saturated and trans fats are related to increased mortality in patients with T2D.16 Increased consumption of vegetables, fruits, legumes, nuts, fish, cereal, and oils reduces concentrations of saturated and trans fats and increases dietary intake of monounsaturated fatty acids, fiber, antioxidants, and polyphenols.17
Increasing the intake of fiber, an undigestible carbohydrate, offers numerous benefits in T2D management. High-fiber diets can help regulate blood sugar and lipid levels, increase satiety, reduce inflammation, aid in weight management, and reduce premature mortality.18 Insoluble fiber, found in foods such as whole wheat flour, nuts, and cauliflower, helps food pass more quickly through the stomach and intestines and adds bulk to stool. Soluble fiber, found in foods such as chickpeas, lentils, and Brussels sprouts, absorbs water and forms a gel-like substance that protects nutrients from digestive enzymes and slows down digestion. The result is a more gradual rise in postprandial glucose levels and improved insulin sensitivity.19 Dietary fiber may produce short-chain fatty acids which in turn activate incretin secretion and stimulate a glucose-dependent release of insulin from the pancreas.20
Simple dietary substitutions, such as whole grains and legumes for white rice, can reduce fasting blood glucose and A1C levels.21 In a randomized controlled trial (RCT), increasing whole grain oat intake improved measures of glycemic control, reducing A1C by 1% at 1-year follow-up.19 Encourage patients with T2D to increase consumption of high-fiber foods and replace animal fats and refined grains with vegetable fats (eg, nuts, avocados, olives). Nutritional therapies should be individualized, taking into account personal preferences and cultural customs.22 Nutritional habits may be based on race/ethnicity, religion/spirituality, or even the city in which an individual resides. Nutrition recommendations should account for these differences as well as access to healthy foods. For instance, ethnic groups whose dietary patterns include tortillas could be counseled to choose high-fiber options such as corn instead of flour tortillas and to incorporate vegetables in place of high-fat foods. Additionally, ethnic groups who favor using animal fats in foods such as greens could be advised on ways to add flavor to vegetables without adding saturated fats. Taking this approach may lessen barriers to change and increase ability to make dietary modifications.23
Exercise
Encourage all patients with T2D to exercise regularly. The atherosclerotic plaques found in patients with T2D have increased inflammatory properties and result in worse cardiovascular outcomes compared with plaques in individuals without T2D.24 Regular exercise reduces levels of pro-inflammatory markers—C-reactive protein, interleukin (IL)-6, and tumor necrosis factor alpha—and increases levels of anti-inflammatory markers (IL-4 and IL-10).24 Regular exercise can improve body composition, physical fitness, lipid and glucose metabolism, and insulin sensitivity.25,26
A meta-analysis of RCTs demonstrated that structured exercise > 150 minutes per week resulted in A1C reductions of 0.89%,27 which is comparable to the effect of many oral antihyperglycemic medications.26 The Health Benefits of Aerobic and Resistance Training in individuals with T2D (HART-D) and Diabetes Aerobic and Resistance Exercise (DARE) studies demonstrated that combining endurance and resistance training was superior for improving glycemic control, cardiorespiratory fitness, and body composition, than using either type of training alone.25 Both the American College of Sports Medicine (ACSM) and the ADA recommend that adults engage in at least 150 total minutes of moderate-intensity aerobic activity per week and resistance training 2 to 3 times weekly.26 ACSM defines moderate-intensity exercise as 65% to 75% of maximal heart rate, a rating of perceived exertion of 3 to 4, or a step rate of 100 steps per minute.28
Continue to: Because of their longitudinal relationships...
Because of their longitudinal relationships with patients, family physicians are in an optimal position to assess a patient’s physical capacity level and provide individualized counseling. Several systematic reviews have demonstrated that counseling on exercise increases patients’ participation in physical activity.29 Encourage your patients with T2D to exercise regularly, considering each individual’s ability to engage in physical activity.
Weight loss
Include weight management in the initial treatment of patients with newly diagnosed T2D. Weight loss decreases hepatic glucose production and increases peripheral insulin sensitivity and insulin secretion.30 Moderate decreases in weight (5%-10%) can reduce complications related to diabetes, and sustained significant weight loss (> 10%) can potentially cause T2D remission (A1C < 6.5% after stopping diabetes medications).31,32
Diabetes self-management education supports patients by giving them tools for making and maintaining lifestyle changes. Understanding individual barriers to change and addressing these during motivational interviews is important. Through a qualitative interview study, participants in a diabetes self-management program revealed 4 factors that motivated them to maintain lifestyle changes: support from others, experiencing the impact of the changes they made, fear of T2D complications, and forming new habits.33 Family physicians are key in helping patients acquire knowledge and support to make the lifestyle modifications needed to manage newly diagnosed T2D.
Individualized pharmacotherapy considerations
For decades, the initial pharmacotherapeutic regimen for patients with newly diagnosed T2D considered the patient’s baseline A1C as a major driver for therapy. Metformin has been the mainstay in T2D treatment due to its clinical efficacy, minimal risk for hypoglycemia, and low cost. Regardless of the regimen, pharmacotherapy should be initiated at the time of T2D diagnosis in conjunction with the aforementioned lifestyle modifications.34
When selecting pharmacotherapy, practice guidelines recommend considering the efficacy and adverse effects of medications, patient-specific comorbidities, adherence, cost, and a patient’s lifestyle factors.34 Drug classes with pertinent information are listed in TABLE 2.34-54 After starting medication, monitor the A1C level every 3 months to determine whether therapy should be intensified. Patients should have their labs drawn ahead of the quarterly visit, or point-of-care measurements may be used to facilitate in-person patient–provider discussions.


Continue to: Consider patient-specific factors when starting pharmacotherapy
Consider patient-specific factors when starting pharmacotherapy
ASCVD. Regardless of baseline glycemic control, offer patients who have ASCVD, or who are at high risk for it, an SGLT2 inhibitor (canagliflozin, dapagliflozin, or empagliflozin) or a long-acting GLP-1 receptor agonist (dulaglutide, liraglutide, or semaglutide).34,35 SGLT2 inhibitors reduced the risk for MACE by 11% in patients with established ASCVD.55 They also reduced a composite outcome of cardiovascular death or hospitalization for heart failure by 23% in patients with or without ASCVD or heart failure at baseline.55 GLP-1 receptor agonists offer a similar reduction in MACE to SGLT2 inhibitors, but they do not have significant effects in heart failure.56 Thiazolidinediones (TZDs), saxagliptin, and alogliptin should be avoided in patients with heart failure.57 TZDs may reduce the risk for recurrent stroke in patients with T2D.58
Chronic kidney disease (CKD). As with ASCVD, prioritize SGLT2 inhibitors and GLP-1 receptor agonists in patients with CKD. While both classes reduced the risk for progression of kidney disease such as macroalbuminuria, SGLT2 inhibitors offer additional benefits in their reduction of the worsening of estimated glomerular filtration rate, end-stage kidney disease, and renal death.56
Obesity. Consider the effect of each drug class on weight when making initial treatment choices, taking special care to minimize weight gain and potentially promote weight loss.34 The ADA prefers GLP-1 receptor agonists, but also suggests SGLT2 inhibitors in these patients. While all GLP-1 receptor agonists have an impact on weight, weekly subcutaneous semaglutide offers the most pronounced weight loss of 2 to 7 kg over 56 weeks.59 SGLT2 inhibitors promote sustainable weight loss to a lesser degree, contributing to an average loss of 3 kg at 2 years.60 Weight gain is common in patients taking sulfonylureas (2.01-2.3 kg)31 and insulin (3-9 kg weight gain in the first year)61 and should be avoided in patients with T2D and obesity.34
Hypoglycemia risk. In addition to counseling patients on hypoglycemia management and prescribing glucagon rescue kits, offer medications with no or very low risk for hypoglycemia (eg, GLP-1 receptor agonists, SGLT2 inhibitors, dipeptidyl peptidase-4 inhibitors, and TZDs). Generally, avoid insulin and sulfonylureas in patients in whom hypoglycemia is a major concern (eg, older adults, individuals with labile blood glucose levels).34 Patients with reduced renal function are at higher risk for hypoglycemia with insulin or sulfonylureas due to reduced drug clearance. However, insulin is often the only treatment for patients with advanced renal disease. Pay close attention to insulin dosing in patients with advanced renal disease, which may necessitate lower doses and smaller dose adjustments due to this risk.
Social determinants of health. Medication access and cost is a major burden in T2D management and should be considered for every patient. Compared with the period of 2005 to 2007, the annual cost of diabetes medications for an individual in 2015 to 2017 increased by 147%, rising from $1106 to $2727 per year.62 This increase is driven by the cost of insulin and newer medications without generic options.62 Identify local resources in your community, such as patient assistance programs and pharmacies with reduced-price generic prescription programs, which may be useful for patients who are underinsured or uninsured.
Continue to: Even if cost weren't an issue...
Even if cost weren’t an issue, many medications such as insulin and GLP-1 receptor agonists should be kept refrigerated and are only stable at room temperature for a limited time. Medications that are stable at room temperature should be prioritized in patients with limited or inconsistent access to refrigeration or unstable housing who may find it difficult to store their medications appropriately.
Do not delay insulin initiation in patients with high baseline A1C
Whenever possible, a GLP-1 receptor agonist is the preferred injectable medication to insulin. Starting insulin introduces numerous risks, including hypoglycemia, weight gain, and stigma. However, in the patient with newly diagnosed T2D, choose basal insulin when the baseline hyperglycemia is severe,34 as indicated by:
- blood glucose > 300 mg/dL (16.7 mmol/L),
- A1C > 10% (86 mmol/mol),
- symptoms of hyperglycemia (polyuria or polydipsia), or
- evidence of catabolism (weight loss, hypertriglyceridemia, ketosis).
Basal insulin analogs are preferred over NPH given their reduced variability, dosing, and hypoglycemic risk.35 Mixed insulins may be used if a patient is unable to afford an insulin analog, which can be quite costly. However, extensive counseling on dosing and management of hypoglycemia is crucial to patient safety with these agents. The ADA recommends initiating 0.1 to 0.2 units/kg of basal insulin daily or 10 units daily.34 The AACE follows this recommendation for patients with baseline A1C < 8%, but it proposes a more aggressive initiation of 0.2 to 0.3 units/kg/d for patients with baseline A1C > 8%.35 Titrate the dose by 2 units every 3 days to reach the target fasting blood glucose level. As hyperglycemia resolves, simplify the regimen and transition to noninsulin options per the previously discussed considerations.
It’s not just about glycemic control
In addition to the direct effects of hyperglycemia, a T2D diagnosis introduces an increased risk for ASCVD, a reduced ability to fight infection, and heightened risk for depression. Order a lipid panel at the time of T2D diagnosis and initiate lipid management as needed (TABLE 335,63,64). Both the ADA and the American Heart Association recommend starting a moderate-intensity statin as primary prevention for all patients with T2D between 40 and 75 years of age regardless of the 10-year ASCVD risk.63 The AACE uses specific lipid targets and recommends moderate- to high-intensity statin therapy for patients with T2D.35 All recommendations by professional organizations list high-intensity statins for patients with established ASCVD.

It is also vital to recommend that patients with newly diagnosed T2D remain up to date on all indicated vaccinations. They should promptly receive the hepatitis B and pneumococcal vaccines if they have not already done so for a previous indication. COVID-19 and annual influenza vaccines also should be prioritized for these patients.65
Finally, patients with diabetes are twice as likely to develop depression than patients without diabetes.66 Individuals with T2D and depression exhibit poorer medication adherence, lifestyle choices, and glycemic control.66 Screen for and treat these issues in all patients with T2D across the course of the disease.
Overall, work closely with patients to support them in managing their new diagnosis with evidence-based pharmacologic and nonpharmacologic approaches. The importance of lifestyle changes including high-fiber diets, regular exercise, and weight loss should not be overlooked. Do not delay starting pharmacotherapy after diagnosing T2D and consider medication-specific and patient-specific factors to individualize therapy, improve adherence, and prevent complications.
CORRESPONDENCE
Jennie B. Jarrett, PharmD, MMedEd, 833 South Wood Street (MC 886), Chicago, IL 60612; jarrett8@uic.edu
1. Dahlén AD, Dashi G, Maslov I, et al. Trends in antidiabetic drug discovery: FDA approved drugs, new drugs in clinical trials and global sales. Front Pharmacol. 2022;12. Accessed April 19, 2023. www.frontiersin.org/article/10.3389/fphar.2021.807548
2. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128. doi: 10.1056/NEJMoa1504720
3. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657. doi: 10.1056/NEJMoa1611925
4. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357. doi: 10.1056/NEJMoa1812389
5. Davidson KW, Barry MJ, et al. Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:736-743. doi: 10.1001/jama. 2021.12531
6. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract. 2015;21(suppl 1):1-87. doi: 10.4158/EP15672.GL
7. ADA. Introduction: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
8. ADA Professional Practice Committee. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
9. ADA Professional Practice Committee. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S46-S59. doi: 10.2337/dc22-S004
10. ADA Professional Practice Committee. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S83-S96. doi: 10.2337/dc22-S006
11. Janapala RN, Jayaraj JS, Fathima N, et al. Continuous glucose monitoring versus self-monitoring of blood glucose in type 2 diabetes mellitus: a systematic review with meta-analysis. Cureus. 2019;11:e5634. doi: 10.7759/cureus.5634
12. ADA Professional Practice Committee. Diabetes technology: standards of medical care in diabetes - 2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
13. Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168:569-576. doi: 10.7326/M17-0939
14. Moran GM, Bakhai C, Song SH, et al, Guideline Committee. Type 2 diabetes: summary of updated NICE guidance. BMJ. 2022;377:o775. doi: 10.1136/bmj.o775
15. Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. doi: 10.1186/s12916-017-0901-x
16. McMacken M, Shah S. A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol. 2017;14:342-354. doi: 10.11909/j.issn.1671-5411.2017.05.009
17. Asif M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J Educ Health Promot. 2014;3:1. doi: 10.4103/2277-9531.127541
18. Reynolds AN, Akerman AP, Mann J. Dietary fibre and whole grains in diabetes management: systematic review and meta-analyses. PLoS Med. 2020;17(3):e1003053. doi: 10.1371/journal.pmed.1003053
19. Li X, Cai X, Ma X, et al. Short- and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: a randomized control trial. Nutrients. 2016;8:549. doi: 10.3390/nu8090549
20. Fujii H, Iwase M, Ohkuma T, et al. Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. Nutr J. 2013;12:159. doi: 10.1186/1475-2891-12-159
21. Kim M, Jeung SR, Jeong TS, et al. Replacing with whole grains and legumes reduces Lp-PLA2 activities in plasma and PBMCs in patients with prediabetes or T2D. J Lipid Res. 2014;55:1762-1771. doi: 10.1194/jlr.M044834
22. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
23. Caballero AE. The “a to z” of managing type 2 diabetes in culturally diverse populations. Front Endocrinol. 2018;9:479. doi: 10.3389/fendo.2018.00479
24. Golbidi S, Badran M, Laher I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Exp Diabetes Res. 2012; 2012:941868. doi: 10.1155/2012/941868
25. Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol. 2016;94:146-150. doi: 10.1038/icb.2015.101
26. Dugan JA. Exercise recommendations for patients with type 2 diabetes. JAAPA. 2016;29:13-18. doi: 10.1097/01.JAA. 0000475460.77476.f6
27. Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–1799. doi: 10.1001/jama.2011.576
28. Zuhl M. Tips for monitoring aerobic exercise intensity. 2020. Accessed April 19, 2023. www.acsm.org/docs/default-source/files-for-resource-library/exercise-intensity-infographic.pdf? sfvrsn=f467c793_2
29. Williams A, Radford J, O’Brien J, Davison K. Type 2 diabetes and the medicine of exercise: the role of general practice in ensuring exercise is part of every patient’s plan. Aust J Gen Pract. 2020;49:189-193. doi: 10.31128/AJGP-09-19-5091
30. Grams J, Garvey WT. Weight loss and the prevention and treatment of type 2 diabetes using lifestyle therapy, pharmacotherapy, and bariatric surgery: mechanisms of action. Curr Obes Rep. 2015;4:287-302. doi: 10.1007/s13679-015-0155-x
31. Apovian CM, Okemah J, O’Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36:44-58. doi: 10.1007/s12325-018-0824-8
32. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7:344-355. doi: 10.1016/S2213-8587(19)30068-3
33. Rise MB, Pellerud A, Rygg LØ, et al. Making and maintaining lifestyle changes after participating in group based type 2 diabetes self-management educations: a qualitative study. PLoS One. 2013;8:e64009. doi: 10.1371/journal.pone.0064009
34. ADA Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S125-S143. doi: 10.2337/dc22-S009
35. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract. 2020;26:107-139. doi: 10.4158/CS-2019-0472
36. Metformin. Package insert. Bristol-Myers Squibb Company; 2017.
37. Invokana (canagliflozin). Package insert. Janssen Pharmaceuticals, Inc; 2020.
38. Farxiga (dapagliflozin). Package insert. AstraZeneca Pharmaceuticals LP; 2021.
39. Jardiance (empagliflozin). Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
40. Steglatro (ertugliflozin). Package insert. Merck & Co, Inc; 2021.
41. Trulicity (dulaglutide). Package insert. Lilly USA, LLC; 2022.
42. Byetta (exenatide). Package insert. AstraZeneca Canada Inc; 2022.
43. Bydureon (exenatide ER). Package insert. AstraZeneca Pharmaceuticals LP; 2022.
44. Victoza (liraglutide). Package insert. Novo Nordisk; 2022.
45. Adlyxin (lixisenatide). Package insert. Sanofi-Aventis US LLC; 2022.
46. Ozempic (semaglutide). Package insert. Novo Nordisk; 2022.
47. Alogliptin. Package insert. Takeda Pharmaceuticals USA, Inc; 2022.
48. Linagliptin. Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
49. Saxagliptin. Package insert. AstraZeneca Pharmaceuticals LP; 2019.
50. Januvia (sitagliptin). Package insert. Merck Sharp & Dohme LLC; 2022.
51. Glimepiride. Package insert. Sanofi-Aventis US LLC; 2009.
52. Glipizide. Package insert. Roerig; 2023.
53. Glyburide. Package insert. Sanofi-Aventis US LLC; 2009.
54. Pioglitazone. Package insert. Northstar Rx LLC; 2022.
55. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. doi: 10.1016/S0140-6736(18)32590-X
56. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022-2031. doi: 10.1161/CIRCULATIONAHA.118.038868
57. FDA. FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. Accessed April 19, 2023. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-adds-warnings-about-heart-failure-risk-labels-type-2-diabetes
58. Wilcox R, Bousser MG, Betteridge DJ, et al. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04). Stroke. 2007;38:865-873. doi: 10.1161/01.STR.0000257974.06317.49
59. Lingvay I, Hansen T, Macura S, et al. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diabetes Res Care. 2020;8:e001706. doi: 10.1136/bmjdrc-2020-001706
60. Liu XY, Zhang N, Chen R, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years. J Diabetes Complications. 2015;29:1295-1303. doi: 10.1016/j.jdiacomp.2015.07.011
61. Brown A, Guess N, Dornhorst A, et al. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: what can be done? Diabetes Obes Metab. 2017;19:1655-1668. doi: 10.1111/dom.13009
62. Zhou X, Shrestha SS, Shao H, et al. Factors contributing to the rising national cost of glucose-lowering medicines for diabetes during 2005-2007 and 2015-2017. Diabetes Care. 2020;43:2396-2402. doi: 10.2337/dc19-2273
63. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi: 10.1161/CIR.0000000000000625
64. ADA Professional Practice Committee. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S144-S174. doi: 10.2337/dc22-S010
65. CDC. Adult immunization schedule by medical condition and other indication. 2022. Accessed April 19, 2023. www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.htm
66. Semenkovich K, Brown ME, Svrakic DM, et al. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75:577-587. doi: 10.1007/s40265-015-0347-4
1. Dahlén AD, Dashi G, Maslov I, et al. Trends in antidiabetic drug discovery: FDA approved drugs, new drugs in clinical trials and global sales. Front Pharmacol. 2022;12. Accessed April 19, 2023. www.frontiersin.org/article/10.3389/fphar.2021.807548
2. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128. doi: 10.1056/NEJMoa1504720
3. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644-657. doi: 10.1056/NEJMoa1611925
4. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357. doi: 10.1056/NEJMoa1812389
5. Davidson KW, Barry MJ, et al. Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA. 2021;326:736-743. doi: 10.1001/jama. 2021.12531
6. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract. 2015;21(suppl 1):1-87. doi: 10.4158/EP15672.GL
7. ADA. Introduction: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S1-S2. doi: 10.2337/dc22-Sint
8. ADA Professional Practice Committee. Classification and diagnosis of diabetes: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S17-S38. doi: 10.2337/dc22-S002
9. ADA Professional Practice Committee. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S46-S59. doi: 10.2337/dc22-S004
10. ADA Professional Practice Committee. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S83-S96. doi: 10.2337/dc22-S006
11. Janapala RN, Jayaraj JS, Fathima N, et al. Continuous glucose monitoring versus self-monitoring of blood glucose in type 2 diabetes mellitus: a systematic review with meta-analysis. Cureus. 2019;11:e5634. doi: 10.7759/cureus.5634
12. ADA Professional Practice Committee. Diabetes technology: standards of medical care in diabetes - 2022. Diabetes Care. 2021;45(suppl 1):S97-S112. doi: 10.2337/dc22-S007
13. Qaseem A, Wilt TJ, Kansagara D, et al. Hemoglobin A1c targets for glycemic control with pharmacologic therapy for nonpregnant adults with type 2 diabetes mellitus: a guidance statement update from the American College of Physicians. Ann Intern Med. 2018;168:569-576. doi: 10.7326/M17-0939
14. Moran GM, Bakhai C, Song SH, et al, Guideline Committee. Type 2 diabetes: summary of updated NICE guidance. BMJ. 2022;377:o775. doi: 10.1136/bmj.o775
15. Kolb H, Martin S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017;15:131. doi: 10.1186/s12916-017-0901-x
16. McMacken M, Shah S. A plant-based diet for the prevention and treatment of type 2 diabetes. J Geriatr Cardiol. 2017;14:342-354. doi: 10.11909/j.issn.1671-5411.2017.05.009
17. Asif M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J Educ Health Promot. 2014;3:1. doi: 10.4103/2277-9531.127541
18. Reynolds AN, Akerman AP, Mann J. Dietary fibre and whole grains in diabetes management: systematic review and meta-analyses. PLoS Med. 2020;17(3):e1003053. doi: 10.1371/journal.pmed.1003053
19. Li X, Cai X, Ma X, et al. Short- and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: a randomized control trial. Nutrients. 2016;8:549. doi: 10.3390/nu8090549
20. Fujii H, Iwase M, Ohkuma T, et al. Impact of dietary fiber intake on glycemic control, cardiovascular risk factors and chronic kidney disease in Japanese patients with type 2 diabetes mellitus: the Fukuoka Diabetes Registry. Nutr J. 2013;12:159. doi: 10.1186/1475-2891-12-159
21. Kim M, Jeung SR, Jeong TS, et al. Replacing with whole grains and legumes reduces Lp-PLA2 activities in plasma and PBMCs in patients with prediabetes or T2D. J Lipid Res. 2014;55:1762-1771. doi: 10.1194/jlr.M044834
22. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
23. Caballero AE. The “a to z” of managing type 2 diabetes in culturally diverse populations. Front Endocrinol. 2018;9:479. doi: 10.3389/fendo.2018.00479
24. Golbidi S, Badran M, Laher I. Antioxidant and anti-inflammatory effects of exercise in diabetic patients. Exp Diabetes Res. 2012; 2012:941868. doi: 10.1155/2012/941868
25. Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol. 2016;94:146-150. doi: 10.1038/icb.2015.101
26. Dugan JA. Exercise recommendations for patients with type 2 diabetes. JAAPA. 2016;29:13-18. doi: 10.1097/01.JAA. 0000475460.77476.f6
27. Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–1799. doi: 10.1001/jama.2011.576
28. Zuhl M. Tips for monitoring aerobic exercise intensity. 2020. Accessed April 19, 2023. www.acsm.org/docs/default-source/files-for-resource-library/exercise-intensity-infographic.pdf? sfvrsn=f467c793_2
29. Williams A, Radford J, O’Brien J, Davison K. Type 2 diabetes and the medicine of exercise: the role of general practice in ensuring exercise is part of every patient’s plan. Aust J Gen Pract. 2020;49:189-193. doi: 10.31128/AJGP-09-19-5091
30. Grams J, Garvey WT. Weight loss and the prevention and treatment of type 2 diabetes using lifestyle therapy, pharmacotherapy, and bariatric surgery: mechanisms of action. Curr Obes Rep. 2015;4:287-302. doi: 10.1007/s13679-015-0155-x
31. Apovian CM, Okemah J, O’Neil PM. Body weight considerations in the management of type 2 diabetes. Adv Ther. 2019;36:44-58. doi: 10.1007/s12325-018-0824-8
32. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7:344-355. doi: 10.1016/S2213-8587(19)30068-3
33. Rise MB, Pellerud A, Rygg LØ, et al. Making and maintaining lifestyle changes after participating in group based type 2 diabetes self-management educations: a qualitative study. PLoS One. 2013;8:e64009. doi: 10.1371/journal.pone.0064009
34. ADA Professional Practice Committee. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S125-S143. doi: 10.2337/dc22-S009
35. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive type 2 diabetes management algorithm—2020 executive summary. Endocr Pract. 2020;26:107-139. doi: 10.4158/CS-2019-0472
36. Metformin. Package insert. Bristol-Myers Squibb Company; 2017.
37. Invokana (canagliflozin). Package insert. Janssen Pharmaceuticals, Inc; 2020.
38. Farxiga (dapagliflozin). Package insert. AstraZeneca Pharmaceuticals LP; 2021.
39. Jardiance (empagliflozin). Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
40. Steglatro (ertugliflozin). Package insert. Merck & Co, Inc; 2021.
41. Trulicity (dulaglutide). Package insert. Lilly USA, LLC; 2022.
42. Byetta (exenatide). Package insert. AstraZeneca Canada Inc; 2022.
43. Bydureon (exenatide ER). Package insert. AstraZeneca Pharmaceuticals LP; 2022.
44. Victoza (liraglutide). Package insert. Novo Nordisk; 2022.
45. Adlyxin (lixisenatide). Package insert. Sanofi-Aventis US LLC; 2022.
46. Ozempic (semaglutide). Package insert. Novo Nordisk; 2022.
47. Alogliptin. Package insert. Takeda Pharmaceuticals USA, Inc; 2022.
48. Linagliptin. Package insert. Boehringer Ingelheim Pharmaceuticals, Inc; 2022.
49. Saxagliptin. Package insert. AstraZeneca Pharmaceuticals LP; 2019.
50. Januvia (sitagliptin). Package insert. Merck Sharp & Dohme LLC; 2022.
51. Glimepiride. Package insert. Sanofi-Aventis US LLC; 2009.
52. Glipizide. Package insert. Roerig; 2023.
53. Glyburide. Package insert. Sanofi-Aventis US LLC; 2009.
54. Pioglitazone. Package insert. Northstar Rx LLC; 2022.
55. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. doi: 10.1016/S0140-6736(18)32590-X
56. Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation. 2019;139:2022-2031. doi: 10.1161/CIRCULATIONAHA.118.038868
57. FDA. FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. Accessed April 19, 2023. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-adds-warnings-about-heart-failure-risk-labels-type-2-diabetes
58. Wilcox R, Bousser MG, Betteridge DJ, et al. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04). Stroke. 2007;38:865-873. doi: 10.1161/01.STR.0000257974.06317.49
59. Lingvay I, Hansen T, Macura S, et al. Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ Open Diabetes Res Care. 2020;8:e001706. doi: 10.1136/bmjdrc-2020-001706
60. Liu XY, Zhang N, Chen R, et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes: a meta-analysis of randomized controlled trials for 1 to 2 years. J Diabetes Complications. 2015;29:1295-1303. doi: 10.1016/j.jdiacomp.2015.07.011
61. Brown A, Guess N, Dornhorst A, et al. Insulin-associated weight gain in obese type 2 diabetes mellitus patients: what can be done? Diabetes Obes Metab. 2017;19:1655-1668. doi: 10.1111/dom.13009
62. Zhou X, Shrestha SS, Shao H, et al. Factors contributing to the rising national cost of glucose-lowering medicines for diabetes during 2005-2007 and 2015-2017. Diabetes Care. 2020;43:2396-2402. doi: 10.2337/dc19-2273
63. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082-e1143. doi: 10.1161/CIR.0000000000000625
64. ADA Professional Practice Committee. Cardiovascular disease and risk management: standards of medical care in diabetes—2022. Diabetes Care. 2021;45(suppl 1):S144-S174. doi: 10.2337/dc22-S010
65. CDC. Adult immunization schedule by medical condition and other indication. 2022. Accessed April 19, 2023. www.cdc.gov/vaccines/schedules/hcp/imz/adult-conditions.htm
66. Semenkovich K, Brown ME, Svrakic DM, et al. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75:577-587. doi: 10.1007/s40265-015-0347-4
PRACTICE RECOMMENDATIONS
› Individualize lifestyle modifications, considering personal and cultural experiences, health literacy, access to healthy foods, willingness and ability to make behavior changes, and barriers to change. C
› Initiate medication therapy at diagnosis, considering medication efficacy and cost, hypoglycemia risk, weight effects, benefits in cardiovascular and kidney disease, and patient-specific comorbidities. C
› Start basal insulin as first-line therapy in patients with severe baseline hyperglycemia, symptoms of hyperglycemia, or evidence of catabolism. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Which patients might benefit from platelet-rich plasma?
Platelet-rich plasma (PRP) injections have become a popular treatment option in a variety of specialties including sports medicine, maxillofacial surgery, dermatology, cosmetology, and reproductive medicine.1 PRP is an autologous blood product derived from whole blood, using a centrifuge to isolate a concentrated layer of platelets. The a-granules in platelets release transforming growth factor b 1, vascular endothelial growth factor, platelet-derived growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor 1, and other mediatorsthat enhance the natural healing process.2
When patients ask. Familiarity with the use of PRP to treat specific musculoskeletal (MSK) conditions is essential for family physicians who frequently are asked by patients about whether PRP is right for them. These patients may have experienced failure of medication therapy or declined surgical intervention, or may not be surgical candidates. This review details the evidence surrounding common intra-articular and extra-articular applications of PRP. But first, a word about how PRP is prepared, its contraindications, and costs.
Preparation and types of PRP
Although there are many commercial systems for preparing PRP, there is no consensus on the optimal formulation.2 Other terms for PRP, such as autologous concentrated platelets and super-concentrated platelets, are based on concentration of red blood cells, leukocytes, and fibrin.3 PRP therapies usually are categorized as leukocyte-rich PRP (LR-PRP) or leukocyte-poor PRP (LP-PRP), based on neutrophil concentrations that are above and below baseline.2 Leukocyte concentration is one of the most debated topics in PRP therapy.4
Common commercially available preparation systems produce platelet concentrations between 3 to 6 times the baseline platelet count.5 Although there is no universally agreed upon PRP formulation, studies have shown 2 centrifugation cycles (“double-spun” or “dual centrifugation”) that yield platelet concentrations between 1.8 to 1.9 times the baseline values significantly improve MSK conditions.6-8
For MSK purposes, PRP may be injected into intratendinous, peritendinous, and intra-articular spaces. Currently, there is no consensus regarding injection frequency. Many studies have incorporated single-injection protocols, while some have used 2 to 3 injections repeated over several weeks to months. PRP commonly is injected at point-of-care without requiring storage.
Contraindications. PRP has been shown to be safe, with most adverse effects attributed to local injection site pain, bleeding, swelling, and bruising.9
Contraindications to PRP include active malignancy or recent remission from malignancy with the exception of nonmetastatic skin tumors.10 PRP is not recommended for patients with an allergy to manufacturing components (eg, dimethyl sulfoxide), thrombocytopenia, nonsteroidal anti-inflammatory drug use within 2 weeks, active infection causing fever, and local infection at the injection site.10 Since local anesthetics may impair platelet function, they should not be given at the same injection site as PRP.10
Continue to: Cost
Cost. PRP is not covered by most insurance plans.11,12 The cost for PRP may range from $500 to $2500 for a single injection.12
Evidence-based summary by condition
Knee osteoarthritis
❯❯❯ Consider using PRP
Knee osteoarthritis (OA) is a common cause of pain and disability. Treatment options include physical therapy, pharmacotherapy, and surgery. PRP has gained popularity as a nonsurgical option. A recent meta-analysis by Costa et al13 of 40 studies with 3035 participants comparing intra-articular PRP with hyaluronic acid (HA), corticosteroid, and saline injections, found that PRP appears to be more effective or as effective as other nonsurgical modalities. However, due to study heterogeneity and high risk for bias, the authors could not recommend PRP for knee OA in clinical practice.13
Despite Costa et al’s findings, reproducible data have demonstrated the superiority of PRP over other nonsurgical treatment options for knee OA. A 2021 systematic review and meta-analysis of 18 randomized controlled trials (RCTs; N = 811) by Belk et al6 comparing PRP to HA injections showed a higher mean improvement in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores in the PRP group compared to the HA group (44.7% vs 12.6%, respectively; P < .01).6 Six of 11 studies using the visual analog scale (VAS) for pain reported significantly less pain in the PRP group compared to the HA group (P < .05).6 The mean follow-up time was 11.1 months.6 Three of 6 studies reported improved subjective International Knee Documentation Committee (IKDC) scores (range from 0-100, with higher scores representing higher levels of function and lower levels of symptoms) in the PRP group compared to the HA group: 75.7 ± 15.1 vs 65.6 ± 16.9 (P = .004); 65.5 ± 3.6 vs 55.8 ± 3.8 (P = .01); and 60.8 ± 9.8 vs 48.4 ± 6.2 (P < .05).6 There was concern for moderate-to-high heterogeneity.6
Other systematic reviews and meta-analyses found similar efficacy of PRP for knee OA, including improved WOMAC scores and patient-reported outcomes (eg, pain, physical function, stiffness) compared to other injectable options.14,15 A systematic review of 14 RCTs (N = 1423) by Shen et al15 showed improved WOMAC scores at 3 months (mean differences [MD] = –14.53; 95% CI, –29.97 to –7.09; P < .001), 6 months
Despite a lack of consensus regarding the optimal preparation of PRP for knee OA, another recent RCT (N = 192) found significant improvement in mean subjective IKDC scores in the LR-PRP group (45.5 ± 15.5 to 60.7 ± 21.1; P < .0005) and the LP-PRP group (46.8 ± 15.8 to 62.9 ± 19.9; P < .0005), indicating efficacy regardless of PRP type.4
Continue to: Ankle osteoarthritis
Ankle osteoarthritis
❯ ❯ ❯ Additional research is needed
Ankle OA affects 3.4% of all adults and is more common in the younger population than knee or hip OA.16 An RCT (N = 100) investigating PRP vs placebo (saline) injections showed no statistically significant difference in American Orthopedic Foot and Ankle Society scores evaluating pain and function over 26 weeks (–2 points; 95% CI, –5 to 1; P = .16).16 Limitations to this study include its small sample size and the PRP formulation used. (The intervention group received 2 injections of 2 mL of PRP, and the platelet concentration was not reported.)16
Hip osteoarthritis
❯ ❯ ❯ Additional research is needed
Symptomatic hip OA occurs in 40% of adults older than 65 years, with a higher prevalence in women.18 Currently, corticosteroid injections are the only intra-articular therapy recommended by international guidelines for hip OA.19 A systematic review and meta-analysis comparing PRP to HA injections that included 4 RCTs (N = 303) showed a statistically significant reduction in VAS scores at 2 months in the PRP group compared to the HA group (weighted mean difference [WMD] = –0.376; 95% CI, –0.614 to –0.138; P = .002).18 However, there were no significant differences in VAS scores between the PRP and HA groups at 6 months (WMD = –0.141; 95% CI, –0.401 to 0.119; P = .289) and 12 months (WMD = –0.083; 95% CI, –0.343 to 0.117; P = .534). Likewise, no significant differences were found in WOMAC scores at 6 months (WMD = –2.841; 95% CI, –6.248 to 0.565; P = .102) and 12 months (WMD = –3.134; 95% CI, –6.624 to 0.356; P = .078) and Harris Hip Scores (HHS) at 6 months (WMD = 2.782; 95% CI, –6.639 to 12.203; P =.563) and 12 months (WMD = 0.706; 95% CI, –6.333 to 7.745; P = .844).18
A systematic review of 6 RCTs (N = 408) by Belk et al20 comparing PRP to HA for hip OA found similar short-term improvements in WOMAC scores (standardized mean differences [SMD] = 0.27; 95% CI, –0.05 to 0.59; P = .09), VAS scores (MD = 0.59; 95% CI, –0.741 to 1.92; P = .39), and HHS (MD = -0.81; 95% CI, –10.06 to 8.43; P = .93).The average follow-up time was 12.2 and 11.9 months for the PRP and HA groups, respectively.20
LR-PRP, which was used in 1 of the 6 RCTs, showed improvement in VAS scores and HHS from baseline, but no significant difference compared to HA at the latest follow-up.20 A pooled subanalysis of the 3 studies that used LP-PRP found no difference in WOMAC scores between the PRP and HA groups (SMD = 0.42; 95% CI, –0.01 to 0.86; P = .06).20 Future studies comparing the efficacy of intra-articular steroid vs PRP for hip OA would be beneficial.18
Continue to: Rotator cuff tendinopathy
Rotator cuff tendinopathy
❯ ❯ ❯ Consider PRP for short-term pain relief
Painful conditions of the rotator cuff include impingement syndrome, tendonitis, and partial and complete tears. A 2021 RCT (N = 58) by Dadgostar et al21 comparing PRP injection to corticosteroid therapy (methylprednisolone and lidocaine) for the treatment of rotator cuff tendinopathy showed significant improvement in VAS scores at 3 months in the PRP group compared to the corticosteroid group (6.66
Another RCT (N = 99) by Kwong et al22 comparing PRP to corticosteroids found similar short-term advantages of LP-PRP with an improved VAS score (–13.6 vs 0.4; P = .03), American Shoulder and Elbow Surgeons score (13.0 vs 2.9; P = .02), and Western Ontario Rotator Cuff Index score (16.8 vs 5.8; P = .03).However, there was no long-term benefit of PRP over corticosteroids found at 12 months.22
A 2021 systematic review and meta-analysis by Hamid et al23 that included 8 RCTs (N = 976) favored PRP over control (no injection, saline injections, and/or shoulder rehabilitation) with improved VAS scores at 12 months (SMD = –0.5; 95% CI, –0.7 to –0.2; P < .001).The evidence on functional outcome was mixed. Data pooled from 2 studies (n = 228) found better Shoulder Pain and Disability Index (SPADI) scores compared to controls at 3- and 6-month follow-ups. However, there were no significant differences in Disabilities of the Arm, Shoulder and Hand (DASH) scores between the 2 groups.23
Patellar tendinopathy
❯ ❯ ❯ Consider using PRP for return to sport
Patellar tendinopathy, a common MSK condition encountered in the primary care setting, has an overall prevalence of 22% in elite athletes at some point in their career.24 Nonsurgical management options include rest, ice, eccentric and isometric exercises, anti-inflammatory drugs, extracorporeal shock wave therapy (ESWT), and dry needling (DN).
A 2014 RCT (N = 23) evaluating DN vs PRP for patellar tendinopathy favored PRP with improved VAS scores (mean ± SD = 25.4 ± 23.2 points; P = .01 vs 5.2 ± 12.5 points; P = .20) at 12 weeks (P = .02). However, at ≥ 26 weeks, the improvement in pain and function scores was similar between the DN and PRP groups (33.2 ± 14.0 points; P = .001 vs 28.9 ± 25.2 points; P = .01). Notably, there was significantly more improvement in the PRP group at 12 weeks (P = .02) but not at 26 weeks (P = .66).25
Continue to: Another perspective study...
Another prospective study (N = 31) comparing PRP to physiotherapy showed a greater improvement in sport activity level reflected by the Tegner score in the PRP group (percentage improvement, 39 ± 22%) compared to control (20 ± 27%; P = .048) at 6 months.7
A recent RCT (N = 20) revealed improved VAS scores at 6 months with rehabilitation paired with either bone marrow mesenchymal stem cells (BM-MSC) or LP-PRP when compared with baseline (BM-MSC group: 4.23 ± 2.13 to 2.52 ± 2.37; P = .0621; LP-PRP group: 3.10 ± 1.20 to 1.13 ± 1.25; P = .0083). Pain was significantly reduced during sport play in both groups at 6 months when compared with baseline (BM-MSC group: 6.91 ± 1.11 to 3.06 ± 2.89, P = .0049; PRP group: 7.03 ± 1.42 to 1.94 ± 1.24, P = .0001).26
A 2019 systematic review and meta-analysis (N = 2530) demonstrated greater improvements in Victorian Institute of Sport Assessment scale for patellar tendinopathy (VISA-P) with multiple injections of PRP (38.7 points; 95% CI, 26.3-51.2 points) compared to single injections of PRP (24.3 points; 95% CI, 18.2-30.5 points), eccentric exercise (28.3 points; 95% CI, 18.9-37.8 points) and ESWT (27.4 points; 95% CI, 10.0-39.8 points) after 6 months.27 In contrast, an RCT (n = 57) comparing a single injection of LR-PRP or LP-PRP was no more effective than a single injection of saline for improvement in mean VISA-P scores (P > .05) at 1 year.28
Lateral epicondylitis
❯ ❯ ❯ Consider using PRP
Lateral epicondylitis (“tennis elbow”) is caused by overuse of the elbow extensors at the site of the lateral epicondyle. Chronic lateral epicondylosis involves tissue degeneration and microtrauma.Most cases of epicondylar tendinopathies are treated nonoperatively, with corticosteroid injections being a mainstay of treatment despite their short-term benefit29 and potential to deteriorate connective tissue over time. Recent studies suggest PRP therapy for epicondylitis and epicondylosis may increase long-term pain relief and improve function.
A 2017 systematic review and meta-analysis of 16 RCTs (N = 1018) concluded PRP was more efficacious than control injections (bupivacaine) for pain reduction in tendinopathies (effect size = 0.47; 95% CI, 0.22-0.72).30 In the review, lateral epicondylitis was evaluated in 12 studies and was most responsive to PRP (effect size = 0.57) when compared to control injection.30 In another systematic review (5 RCTs; 250 patients), corticosteroid injections improved pain within the first 6 weeks of treatment. However, PRP outperformed corticosteroid in VAS scores (21.3 ± 28.1 vs 42.4 ± 26.8) and DASH scores (17.6 ± 24.0 vs 36.5 ± 23.8) (P < .001) at 2 years.31
Continue to: A 2022 systematic review...
A 2022 systematic review and meta-analysis (26 studies; N = 1040) comparing scores at baseline vs 2 years post-PRP showed improvement in VAS scores (7.4 ± 1.30 vs 3.71 ± 2.35; P < .001), DASH scores (60.8 ± 12.5 vs 13.0 ± 18.5; P < .001), Patient-Rated Tennis Elbow Evaluation (55.6 ± 14.7 vs 48.8 ± 4.1; P < .001), and Mayo Clinic Performance Index (55.5 ± 6.1 vs 93.0 ± 6.7; P < .001).32
Regarding the therapeutic effects of different PRP types in lateral epicondylitis, a 2022 systematic review of 33 studies (N = 2420) found improved function and pain relief with LR-PRP and LP-PRP with no significant differences.33 Pretreatment VAS scores in the LR-PRP group, which ranged from 6.1 to 8.0, improved to 1.5 to 4.0 at 3 months and 0.6 to 3.3 after 1 year.33 Similarly, pretreatment VAS scores in the LP-PRP group, which ranged from 4.2 to 8.4, improved to 1.6 to 5.9 at 3 months and 0.7 to 2.7 after 1 year.34 DASH scores also improved in the LR-PRP and LP-PRP groups, with pretreatment scores (LR-PRP, 47.0 to 54.3; LP-PRP, 30.0 to 67.7) improving to 20.0 to 22.0 and 5.5 to 19.0, respectively, at 1 year.33
Achilles tendinopathy
❯ ❯ ❯ Do not use PRP; evidence is lacking
Achilles tendinopathy, caused by chronic overuse and overload resulting in microtrauma and poor tissue healing, typically occurs in the most poorly vascularized portion of the tendon and is common in runners. First-line treatments for Achilles tendinopathy include eccentric strength training and anti-inflammatory drugs.34,35 Corticosteroid injections are not recommended, given concern for degraded tendon tissue over time and worse function.34
A 2020 systematic review of 11 randomized and nonrandomized clinical trials (N = 406) found PRP improved Victorian Institute of Sports Assessment—Achilles (VISA-A) scores at 24 weeks compared to other nonsurgical treatment options (41.2 vs 70.12; P < .018).34 However, a higher-quality 2021 systematic review and meta-analysis of 4 RCTs (N = 170) comparing PRP injections with placebo showed no significant difference in VISA-A scores at 3 months (0.23; 95% CI, –0.45 to 0.91), 6 months (0.83; 95% CI, –0.26 to 1.92), and 12 months (0.83; 95% CI, –0.77 to 2.44).36 Therefore, further studies are warranted to evaluate the benefit of PRP injections for Achilles tendinopathy.
Conclusions
While high-quality studies support the use of PRP for knee OA and lateral epicondylitis, they have a moderate-to-high risk for bias. Several RCTs show that PRP provides superior short-term pain relief and range of motion compared to corticosteroids for rotator cuff tendinopathy. Multiple injections of PRP for patellar tendinopathy may accelerate return to sport and improve symptoms over the long term. However, current evidence does not support PRP therapy for Achilles tendinopathy. Given variability in PRP preparation, an accurate interpretation of the literature regarding its use in MSK conditions is recommended (TABLE4,6,7,14-18,20-23,25-28,30-34,36).
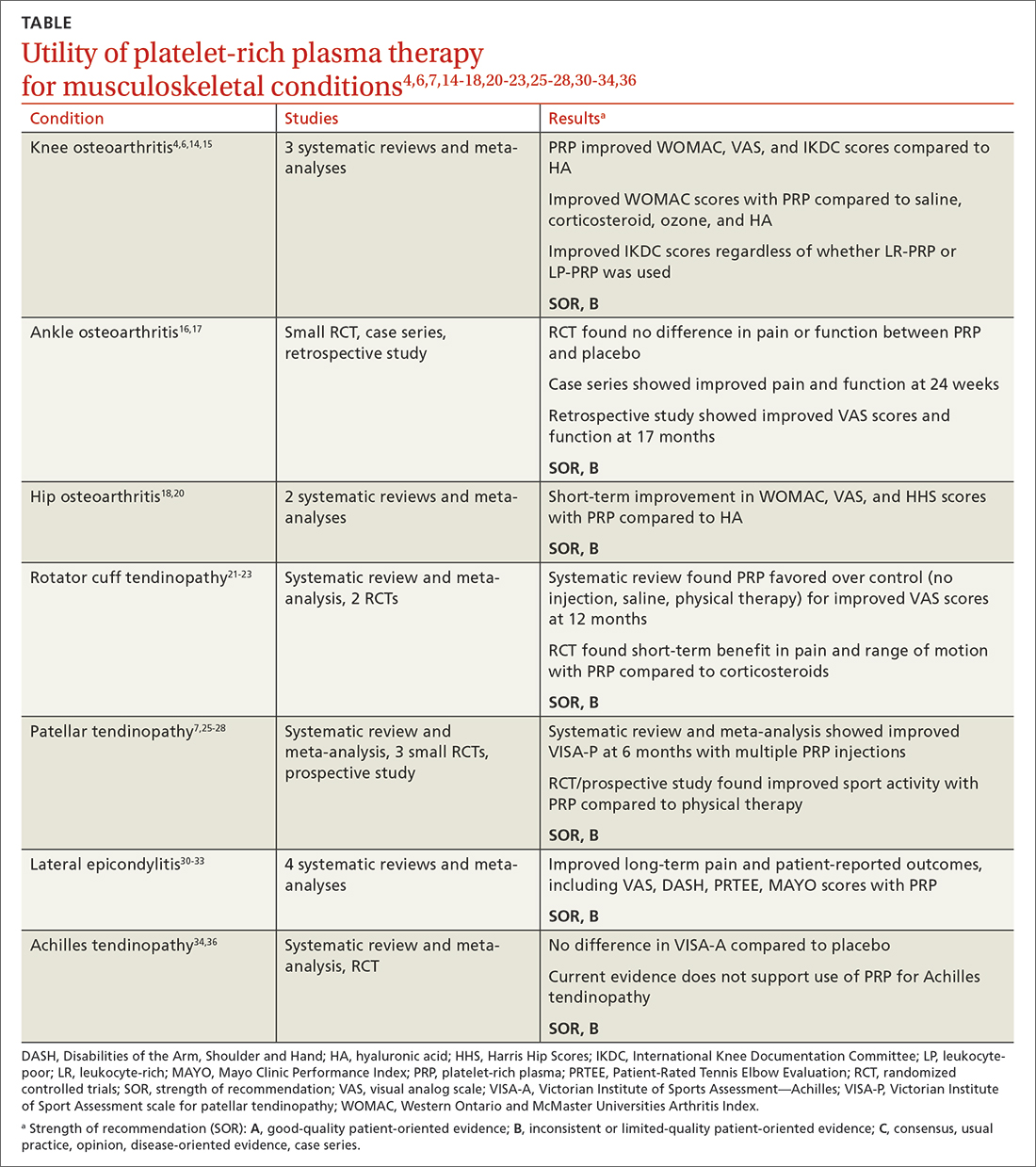
Continue to: Concerning the effectiveness of PRP...
Concerning the effectiveness of PRP, it is important to consider early publication bias. Although recent studies have shown its benefits,6,14,15,37 additional studies comparing PRP to placebo will help demonstrate its efficacy. Interestingly, a literature search by Bar-Or et al38 found intra-articular saline may have a therapeutic effect on knee OA and confound findings when used as a placebo.
Recognizing the presence or lack of clinically significant improvement in the literature is important. For example, while some recent studies have shown PRP exceeds the minimal clinically significant difference for knee OA and lateral epicondylitis, others have not.32,37 A 2021 systematic review of 11 clinical practice guidelines for the use of PRP in knee OA found that 9 were “uncertain or unable to make a recommendation” and 2 recommended against it.39
In its 2021 position statement for the responsible use of regenerative medicine, the American Medical Society for Sports Medicine includes guidance on integrating orthobiologics into clinical practice. The guideline emphasizes informed consent and provides an evidence-based rationale for using PRP in certain patient populations (lateral epicondylitis and younger patients with mild-to-moderate knee OA), recommending its use only after exhausting other conservative options.40 Patients should be referred to physicians with experience using PRP and image-guided procedures.
CORRESPONDENCE
Gregory D. Bentz Jr, MD, 3640 High Street Suite 3B, Portsmouth, VA 23707; bentzgd@evms.edu
1. Cecerska-Heryć E, Goszka M, Serwin N, et al. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2022;64:84-94. doi: 10.1016/j.cytogfr.2021.11.003
2. Le ADK, Enweze L, DeBaun MR, et al. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11:624-634. doi: 10.1007/s12178-018-9527-7
3. Everts P, Onishi K, Jayaram P, et al. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21:7794. doi: 10.3390/ijms21207794
4. Di Martino A, Boffa A, Andriolo L, et al. Leukocyte-rich versus leukocyte-poor platelet-rich plasma for the treatment of knee osteoarthritis: a double-blind randomized trial. Am J Sports Med. 2022;50:609-617. doi: 10.1177/03635465211064303
5. Mariani E, Pulsatelli L. Platelet concentrates in musculoskeletal medicine. Int J Mol Sci. 2020;21:1328. doi: 10.3390/ijms21041328
6. Belk JW, Kraeutler MJ, Houck DA, et al. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. 2021;49:249-260. doi: 10.1177/0363546520909397
7. Filardo G, Kon E, Della Villa S, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34:909-915. doi: 10.1007/s00264-009-0845-7
8. Kon E, Filardo G, Delcogliano M, et al. Platelet-rich plasma: new clinical application: a pilot study for treatment of jumper’s knee. Injury. 2009;40:598-603. doi: 10.1016/j.injury.2008.11.026
9. Kanchanatawan W, Arirachakaran A, Chaijenkij K, et al. Short-term outcomes of platelet-rich plasma injection for treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24:1665-1677. doi: 10.1007/s00167-015-3784-4
10. Cook J, Young M. Biologic therapies for tendon and muscle injury. UpToDate. Updated August 11, 2022. Accessed May 23, 2023. www.uptodate.com/contents/biologic-therapies-for-tendon-and-muscle-injury
11. Bendich I, Rubenstein WJ, Cole BJ, et al. What is the appropriate price for platelet-rich plasma injections for knee osteoarthritis? A cost-effectiveness analysis based on evidence from Level I randomized controlled trials. Arthroscopy. 2020;36:1983-1991.e1. doi: 10.1016/j.arthro.2020.02.004
12. Jones IA, Togashi RC, Thomas Vangsness C Jr. The economics and regulation of PRP in the evolving field of orthopedic biologics. Curr Rev Musculoskelet Med. 2018;11:558-565. doi: 10.1007/s12178-018-9514-z
13. Costa LAV, Lenza M, Irrgang JJ, et al. How does platelet-rich plasma compare clinically to other therapies in the treatment of knee osteoarthritis? A systematic review and meta-analysis. Am J Sports Med. 2023;51:1074-1086 doi: 10.1177/03635465211062243
14. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505. doi: 10.1016/j.arthro.2015.08.005
15. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12:16. doi: 10.1186/s13018-017-0521-3
16. Paget LDA, Reurink G, de Vos RJ, et al; PRIMA Study Group. Effect of platelet-rich plasma injections vs. placebo on ankle symptoms and function in patients with ankle osteoarthritis: a randomized clinical trial. JAMA. 2021;326:1595-1605. doi: 10.1001/jama.2021.16602
17. Evans A, Ibrahim M, Pope R, et al. Treating hand and foot osteoarthritis using a patient’s own blood: a systematic review and meta-analysis of platelet-rich plasma. J Orthop. 2020;18:226-236. doi: 10.1016/j.jor.2020.01.037
18. Ye Y, Zhou X, Mao S, et al. Platelet rich plasma versus hyaluronic acid in patients with hip osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surg. 2018;53:279-287. doi: 10.1016/j.ijsu.2018.03.078.
19. Berney M, McCarroll P, Glynn L, et al. Platelet-rich plasma injections for hip osteoarthritis: a review of the evidence. Ir J Med Sci. 2021;190:1021-1025. doi: 10.1007/s11845-020-02388-z
20. Belk JW, Houck DA, Littlefield CP, et al. Platelet-rich plasma versus hyaluronic acid for hip osteoarthritis yields similarly beneficial short-term clinical outcomes: a systematic review and meta-analysis of Level I and II randomized controlled trials. Arthroscopy. 2022;38:2035-2046. doi: 10.1016/j.arthro.2021.11.005
21. Dadgostar H, Fahimipour F, Pahlevan Sabagh A, et al. Corticosteroids or platelet-rich plasma injections for rotator cuff tendinopathy: a randomized clinical trial study. J Orthop Surg Res. 2021;16:333. doi: 10.1186/s13018-021-02470-x
22. Kwong CA, Woodmass JM, Gusnowski EM, et al. Platelet-rich plasma in patients with partial-thickness rotator cuff tears or tendinopathy leads to significantly improved short-term pain relief and function compared with corticosteroid injection: a double-blind randomized controlled trial. Arthroscopy. 2021;37:510-517. doi: 10.1016/j.arthro.2020.10.037
23. A Hamid MS, Sazlina SG. Platelet-rich plasma for rotator cuff tendinopathy: a systematic review and meta-analysis. PLoS One. 2021;16:e0251111. doi: 10.1371/journal.pone.0251111
24. Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33:561-567. doi: 10.1177/0363546504270454
25. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618. doi: 10.1177/0363546513518416.
26. Rodas G, Soler-Rich R, Rius-Tarruella J, et al. Effect of autologous expanded bone marrow mesenchymal stem cells or leukocyte-poor platelet-rich plasma in chronic patellar tendinopathy (with gap >3 mm): preliminary outcomes after 6 months of a double-blind, randomized, prospective study. Am J Sports Med. 2021;49:1492-1504. doi: 10.1177/0363546521998725
27. Andriolo L, Altamura SA, Reale D, et al. Nonsurgical treatments of patellar tendinopathy: multiple injections of platelet-rich plasma are a suitable option: a systematic review and meta-analysis. Am J Sports Med. 2019;47:1001-1018. doi: 10.1177/0363546518759674
28. Scott A, LaPrade RF, Harmon KG, et al. Platelet-rich plasma for patellar tendinopathy: a randomized controlled trial of leukocyte-rich PRP or leukocyte-poor PRP versus saline. Am J Sports Med. 2019;47:1654-1661. doi: 10.1177/0363546519837954
29. Kemp JA, Olson MA, Tao MA, et al. Platelet-rich plasma versus corticosteroid injection for the treatment of lateral epicondylitis: a systematic review of systematic reviews. Int J Sports Phys Ther. 2021;16:597-605. doi: 10.26603/001c.24148
30. Miller LE, Parrish WR, Roides B, et al. Efficacy of platelet-rich plasma injections for symptomatic tendinopathy: systematic review and meta-analysis of randomised injection-controlled trials. BMJ Open Sport Exerc Med. 2017;3:e000237. doi: 10.1136/bmjsem-2017- 000237
31. Ben-Nafa W, Munro W. The effect of corticosteroid versus platelet-rich plasma injection therapies for the management of lateral epicondylitis: a systematic review. SICOT J. 2018;4:11.
32. Niemiec P, Szyluk K, Jarosz A, et al. Effectiveness of platelet-rich plasma for lateral epicondylitis: a systematic review and meta-analysis based on achievement of minimal clinically important difference. Orthop J Sports Med. 2022;10:23259671221086920. doi: 10.1177/23259671221086920
33. Li S, Yang G, Zhang H, et al. A systematic review on the efficacy of different types of platelet-rich plasma in the management of lateral epicondylitis. J Shoulder Elbow Surg. 2022;311533-1544. doi: 10.1016/j.jse.2022.02.017.
34. Madhi MI, Yausep OE, Khamdan K, et al. The use of PRP in treatment of Achilles tendinopathy: a systematic review of literature. Study design: systematic review of literature. Ann Med Surg (Lond). 2020;55:320-326. doi: 10.1016/j.amsu.2020.04.042
35. Loppini M, Maffulli N. Conservative management of tendinopathy: an evidence-based approach. Muscles Ligaments Tendons J. 2012;1:134-137.
36. Nauwelaers AK, Van Oost L, Peers K. Evidence for the use of PRP in chronic midsubstance Achilles tendinopathy: a systematic review with meta-analysis. Foot Ankle Surg. 2021;27:486-495. doi: 10.1016/j.fas.2020.07.009
37. Dai WL, Zhou AG, Zhang H, et al. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33:659-670.e1. doi: 10.1016/j.arthro.2016.09.024
38. Bar-Or D, Rael LT, Brody EN. Use of saline as a placebo in intra-articular injections in osteoarthritis: potential contributions to nociceptive pain relief. Open Rheumatol J. 2017;11:16-22. doi: 10.2174/1874312901711010016
39. Phillips M, Bhandari M, Grant J, et al. A systematic review of current clinical practice guidelines on intra-articular hyaluronic acid, corticosteroid, and platelet-rich plasma injection for knee osteoarthritis: an international perspective. Orthop J Sports Med. 2021;9:23259671211030272. doi: 10.1177/23259671211030272
40. Finnoff JT, Awan TM, Borg-Stein J, et al. American Medical Society for Sports Medicine position statement: principles for the responsible use of regenerative medicine in sports medicine. Clin J Sport Med. 2021;31:530-541. doi: 10.1097/JSM.0000000000000973
Platelet-rich plasma (PRP) injections have become a popular treatment option in a variety of specialties including sports medicine, maxillofacial surgery, dermatology, cosmetology, and reproductive medicine.1 PRP is an autologous blood product derived from whole blood, using a centrifuge to isolate a concentrated layer of platelets. The a-granules in platelets release transforming growth factor b 1, vascular endothelial growth factor, platelet-derived growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor 1, and other mediatorsthat enhance the natural healing process.2

When patients ask. Familiarity with the use of PRP to treat specific musculoskeletal (MSK) conditions is essential for family physicians who frequently are asked by patients about whether PRP is right for them. These patients may have experienced failure of medication therapy or declined surgical intervention, or may not be surgical candidates. This review details the evidence surrounding common intra-articular and extra-articular applications of PRP. But first, a word about how PRP is prepared, its contraindications, and costs.
Preparation and types of PRP
Although there are many commercial systems for preparing PRP, there is no consensus on the optimal formulation.2 Other terms for PRP, such as autologous concentrated platelets and super-concentrated platelets, are based on concentration of red blood cells, leukocytes, and fibrin.3 PRP therapies usually are categorized as leukocyte-rich PRP (LR-PRP) or leukocyte-poor PRP (LP-PRP), based on neutrophil concentrations that are above and below baseline.2 Leukocyte concentration is one of the most debated topics in PRP therapy.4
Common commercially available preparation systems produce platelet concentrations between 3 to 6 times the baseline platelet count.5 Although there is no universally agreed upon PRP formulation, studies have shown 2 centrifugation cycles (“double-spun” or “dual centrifugation”) that yield platelet concentrations between 1.8 to 1.9 times the baseline values significantly improve MSK conditions.6-8
For MSK purposes, PRP may be injected into intratendinous, peritendinous, and intra-articular spaces. Currently, there is no consensus regarding injection frequency. Many studies have incorporated single-injection protocols, while some have used 2 to 3 injections repeated over several weeks to months. PRP commonly is injected at point-of-care without requiring storage.
Contraindications. PRP has been shown to be safe, with most adverse effects attributed to local injection site pain, bleeding, swelling, and bruising.9
Contraindications to PRP include active malignancy or recent remission from malignancy with the exception of nonmetastatic skin tumors.10 PRP is not recommended for patients with an allergy to manufacturing components (eg, dimethyl sulfoxide), thrombocytopenia, nonsteroidal anti-inflammatory drug use within 2 weeks, active infection causing fever, and local infection at the injection site.10 Since local anesthetics may impair platelet function, they should not be given at the same injection site as PRP.10
Continue to: Cost
Cost. PRP is not covered by most insurance plans.11,12 The cost for PRP may range from $500 to $2500 for a single injection.12
Evidence-based summary by condition
Knee osteoarthritis
❯❯❯ Consider using PRP
Knee osteoarthritis (OA) is a common cause of pain and disability. Treatment options include physical therapy, pharmacotherapy, and surgery. PRP has gained popularity as a nonsurgical option. A recent meta-analysis by Costa et al13 of 40 studies with 3035 participants comparing intra-articular PRP with hyaluronic acid (HA), corticosteroid, and saline injections, found that PRP appears to be more effective or as effective as other nonsurgical modalities. However, due to study heterogeneity and high risk for bias, the authors could not recommend PRP for knee OA in clinical practice.13
Despite Costa et al’s findings, reproducible data have demonstrated the superiority of PRP over other nonsurgical treatment options for knee OA. A 2021 systematic review and meta-analysis of 18 randomized controlled trials (RCTs; N = 811) by Belk et al6 comparing PRP to HA injections showed a higher mean improvement in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores in the PRP group compared to the HA group (44.7% vs 12.6%, respectively; P < .01).6 Six of 11 studies using the visual analog scale (VAS) for pain reported significantly less pain in the PRP group compared to the HA group (P < .05).6 The mean follow-up time was 11.1 months.6 Three of 6 studies reported improved subjective International Knee Documentation Committee (IKDC) scores (range from 0-100, with higher scores representing higher levels of function and lower levels of symptoms) in the PRP group compared to the HA group: 75.7 ± 15.1 vs 65.6 ± 16.9 (P = .004); 65.5 ± 3.6 vs 55.8 ± 3.8 (P = .01); and 60.8 ± 9.8 vs 48.4 ± 6.2 (P < .05).6 There was concern for moderate-to-high heterogeneity.6
Other systematic reviews and meta-analyses found similar efficacy of PRP for knee OA, including improved WOMAC scores and patient-reported outcomes (eg, pain, physical function, stiffness) compared to other injectable options.14,15 A systematic review of 14 RCTs (N = 1423) by Shen et al15 showed improved WOMAC scores at 3 months (mean differences [MD] = –14.53; 95% CI, –29.97 to –7.09; P < .001), 6 months
Despite a lack of consensus regarding the optimal preparation of PRP for knee OA, another recent RCT (N = 192) found significant improvement in mean subjective IKDC scores in the LR-PRP group (45.5 ± 15.5 to 60.7 ± 21.1; P < .0005) and the LP-PRP group (46.8 ± 15.8 to 62.9 ± 19.9; P < .0005), indicating efficacy regardless of PRP type.4
Continue to: Ankle osteoarthritis
Ankle osteoarthritis
❯ ❯ ❯ Additional research is needed
Ankle OA affects 3.4% of all adults and is more common in the younger population than knee or hip OA.16 An RCT (N = 100) investigating PRP vs placebo (saline) injections showed no statistically significant difference in American Orthopedic Foot and Ankle Society scores evaluating pain and function over 26 weeks (–2 points; 95% CI, –5 to 1; P = .16).16 Limitations to this study include its small sample size and the PRP formulation used. (The intervention group received 2 injections of 2 mL of PRP, and the platelet concentration was not reported.)16
Hip osteoarthritis
❯ ❯ ❯ Additional research is needed
Symptomatic hip OA occurs in 40% of adults older than 65 years, with a higher prevalence in women.18 Currently, corticosteroid injections are the only intra-articular therapy recommended by international guidelines for hip OA.19 A systematic review and meta-analysis comparing PRP to HA injections that included 4 RCTs (N = 303) showed a statistically significant reduction in VAS scores at 2 months in the PRP group compared to the HA group (weighted mean difference [WMD] = –0.376; 95% CI, –0.614 to –0.138; P = .002).18 However, there were no significant differences in VAS scores between the PRP and HA groups at 6 months (WMD = –0.141; 95% CI, –0.401 to 0.119; P = .289) and 12 months (WMD = –0.083; 95% CI, –0.343 to 0.117; P = .534). Likewise, no significant differences were found in WOMAC scores at 6 months (WMD = –2.841; 95% CI, –6.248 to 0.565; P = .102) and 12 months (WMD = –3.134; 95% CI, –6.624 to 0.356; P = .078) and Harris Hip Scores (HHS) at 6 months (WMD = 2.782; 95% CI, –6.639 to 12.203; P =.563) and 12 months (WMD = 0.706; 95% CI, –6.333 to 7.745; P = .844).18
A systematic review of 6 RCTs (N = 408) by Belk et al20 comparing PRP to HA for hip OA found similar short-term improvements in WOMAC scores (standardized mean differences [SMD] = 0.27; 95% CI, –0.05 to 0.59; P = .09), VAS scores (MD = 0.59; 95% CI, –0.741 to 1.92; P = .39), and HHS (MD = -0.81; 95% CI, –10.06 to 8.43; P = .93).The average follow-up time was 12.2 and 11.9 months for the PRP and HA groups, respectively.20
LR-PRP, which was used in 1 of the 6 RCTs, showed improvement in VAS scores and HHS from baseline, but no significant difference compared to HA at the latest follow-up.20 A pooled subanalysis of the 3 studies that used LP-PRP found no difference in WOMAC scores between the PRP and HA groups (SMD = 0.42; 95% CI, –0.01 to 0.86; P = .06).20 Future studies comparing the efficacy of intra-articular steroid vs PRP for hip OA would be beneficial.18
Continue to: Rotator cuff tendinopathy
Rotator cuff tendinopathy
❯ ❯ ❯ Consider PRP for short-term pain relief
Painful conditions of the rotator cuff include impingement syndrome, tendonitis, and partial and complete tears. A 2021 RCT (N = 58) by Dadgostar et al21 comparing PRP injection to corticosteroid therapy (methylprednisolone and lidocaine) for the treatment of rotator cuff tendinopathy showed significant improvement in VAS scores at 3 months in the PRP group compared to the corticosteroid group (6.66
Another RCT (N = 99) by Kwong et al22 comparing PRP to corticosteroids found similar short-term advantages of LP-PRP with an improved VAS score (–13.6 vs 0.4; P = .03), American Shoulder and Elbow Surgeons score (13.0 vs 2.9; P = .02), and Western Ontario Rotator Cuff Index score (16.8 vs 5.8; P = .03).However, there was no long-term benefit of PRP over corticosteroids found at 12 months.22
A 2021 systematic review and meta-analysis by Hamid et al23 that included 8 RCTs (N = 976) favored PRP over control (no injection, saline injections, and/or shoulder rehabilitation) with improved VAS scores at 12 months (SMD = –0.5; 95% CI, –0.7 to –0.2; P < .001).The evidence on functional outcome was mixed. Data pooled from 2 studies (n = 228) found better Shoulder Pain and Disability Index (SPADI) scores compared to controls at 3- and 6-month follow-ups. However, there were no significant differences in Disabilities of the Arm, Shoulder and Hand (DASH) scores between the 2 groups.23
Patellar tendinopathy
❯ ❯ ❯ Consider using PRP for return to sport
Patellar tendinopathy, a common MSK condition encountered in the primary care setting, has an overall prevalence of 22% in elite athletes at some point in their career.24 Nonsurgical management options include rest, ice, eccentric and isometric exercises, anti-inflammatory drugs, extracorporeal shock wave therapy (ESWT), and dry needling (DN).
A 2014 RCT (N = 23) evaluating DN vs PRP for patellar tendinopathy favored PRP with improved VAS scores (mean ± SD = 25.4 ± 23.2 points; P = .01 vs 5.2 ± 12.5 points; P = .20) at 12 weeks (P = .02). However, at ≥ 26 weeks, the improvement in pain and function scores was similar between the DN and PRP groups (33.2 ± 14.0 points; P = .001 vs 28.9 ± 25.2 points; P = .01). Notably, there was significantly more improvement in the PRP group at 12 weeks (P = .02) but not at 26 weeks (P = .66).25
Continue to: Another perspective study...
Another prospective study (N = 31) comparing PRP to physiotherapy showed a greater improvement in sport activity level reflected by the Tegner score in the PRP group (percentage improvement, 39 ± 22%) compared to control (20 ± 27%; P = .048) at 6 months.7
A recent RCT (N = 20) revealed improved VAS scores at 6 months with rehabilitation paired with either bone marrow mesenchymal stem cells (BM-MSC) or LP-PRP when compared with baseline (BM-MSC group: 4.23 ± 2.13 to 2.52 ± 2.37; P = .0621; LP-PRP group: 3.10 ± 1.20 to 1.13 ± 1.25; P = .0083). Pain was significantly reduced during sport play in both groups at 6 months when compared with baseline (BM-MSC group: 6.91 ± 1.11 to 3.06 ± 2.89, P = .0049; PRP group: 7.03 ± 1.42 to 1.94 ± 1.24, P = .0001).26
A 2019 systematic review and meta-analysis (N = 2530) demonstrated greater improvements in Victorian Institute of Sport Assessment scale for patellar tendinopathy (VISA-P) with multiple injections of PRP (38.7 points; 95% CI, 26.3-51.2 points) compared to single injections of PRP (24.3 points; 95% CI, 18.2-30.5 points), eccentric exercise (28.3 points; 95% CI, 18.9-37.8 points) and ESWT (27.4 points; 95% CI, 10.0-39.8 points) after 6 months.27 In contrast, an RCT (n = 57) comparing a single injection of LR-PRP or LP-PRP was no more effective than a single injection of saline for improvement in mean VISA-P scores (P > .05) at 1 year.28
Lateral epicondylitis
❯ ❯ ❯ Consider using PRP
Lateral epicondylitis (“tennis elbow”) is caused by overuse of the elbow extensors at the site of the lateral epicondyle. Chronic lateral epicondylosis involves tissue degeneration and microtrauma.Most cases of epicondylar tendinopathies are treated nonoperatively, with corticosteroid injections being a mainstay of treatment despite their short-term benefit29 and potential to deteriorate connective tissue over time. Recent studies suggest PRP therapy for epicondylitis and epicondylosis may increase long-term pain relief and improve function.
A 2017 systematic review and meta-analysis of 16 RCTs (N = 1018) concluded PRP was more efficacious than control injections (bupivacaine) for pain reduction in tendinopathies (effect size = 0.47; 95% CI, 0.22-0.72).30 In the review, lateral epicondylitis was evaluated in 12 studies and was most responsive to PRP (effect size = 0.57) when compared to control injection.30 In another systematic review (5 RCTs; 250 patients), corticosteroid injections improved pain within the first 6 weeks of treatment. However, PRP outperformed corticosteroid in VAS scores (21.3 ± 28.1 vs 42.4 ± 26.8) and DASH scores (17.6 ± 24.0 vs 36.5 ± 23.8) (P < .001) at 2 years.31
Continue to: A 2022 systematic review...
A 2022 systematic review and meta-analysis (26 studies; N = 1040) comparing scores at baseline vs 2 years post-PRP showed improvement in VAS scores (7.4 ± 1.30 vs 3.71 ± 2.35; P < .001), DASH scores (60.8 ± 12.5 vs 13.0 ± 18.5; P < .001), Patient-Rated Tennis Elbow Evaluation (55.6 ± 14.7 vs 48.8 ± 4.1; P < .001), and Mayo Clinic Performance Index (55.5 ± 6.1 vs 93.0 ± 6.7; P < .001).32
Regarding the therapeutic effects of different PRP types in lateral epicondylitis, a 2022 systematic review of 33 studies (N = 2420) found improved function and pain relief with LR-PRP and LP-PRP with no significant differences.33 Pretreatment VAS scores in the LR-PRP group, which ranged from 6.1 to 8.0, improved to 1.5 to 4.0 at 3 months and 0.6 to 3.3 after 1 year.33 Similarly, pretreatment VAS scores in the LP-PRP group, which ranged from 4.2 to 8.4, improved to 1.6 to 5.9 at 3 months and 0.7 to 2.7 after 1 year.34 DASH scores also improved in the LR-PRP and LP-PRP groups, with pretreatment scores (LR-PRP, 47.0 to 54.3; LP-PRP, 30.0 to 67.7) improving to 20.0 to 22.0 and 5.5 to 19.0, respectively, at 1 year.33
Achilles tendinopathy
❯ ❯ ❯ Do not use PRP; evidence is lacking
Achilles tendinopathy, caused by chronic overuse and overload resulting in microtrauma and poor tissue healing, typically occurs in the most poorly vascularized portion of the tendon and is common in runners. First-line treatments for Achilles tendinopathy include eccentric strength training and anti-inflammatory drugs.34,35 Corticosteroid injections are not recommended, given concern for degraded tendon tissue over time and worse function.34
A 2020 systematic review of 11 randomized and nonrandomized clinical trials (N = 406) found PRP improved Victorian Institute of Sports Assessment—Achilles (VISA-A) scores at 24 weeks compared to other nonsurgical treatment options (41.2 vs 70.12; P < .018).34 However, a higher-quality 2021 systematic review and meta-analysis of 4 RCTs (N = 170) comparing PRP injections with placebo showed no significant difference in VISA-A scores at 3 months (0.23; 95% CI, –0.45 to 0.91), 6 months (0.83; 95% CI, –0.26 to 1.92), and 12 months (0.83; 95% CI, –0.77 to 2.44).36 Therefore, further studies are warranted to evaluate the benefit of PRP injections for Achilles tendinopathy.
Conclusions
While high-quality studies support the use of PRP for knee OA and lateral epicondylitis, they have a moderate-to-high risk for bias. Several RCTs show that PRP provides superior short-term pain relief and range of motion compared to corticosteroids for rotator cuff tendinopathy. Multiple injections of PRP for patellar tendinopathy may accelerate return to sport and improve symptoms over the long term. However, current evidence does not support PRP therapy for Achilles tendinopathy. Given variability in PRP preparation, an accurate interpretation of the literature regarding its use in MSK conditions is recommended (TABLE4,6,7,14-18,20-23,25-28,30-34,36).

Continue to: Concerning the effectiveness of PRP...
Concerning the effectiveness of PRP, it is important to consider early publication bias. Although recent studies have shown its benefits,6,14,15,37 additional studies comparing PRP to placebo will help demonstrate its efficacy. Interestingly, a literature search by Bar-Or et al38 found intra-articular saline may have a therapeutic effect on knee OA and confound findings when used as a placebo.
Recognizing the presence or lack of clinically significant improvement in the literature is important. For example, while some recent studies have shown PRP exceeds the minimal clinically significant difference for knee OA and lateral epicondylitis, others have not.32,37 A 2021 systematic review of 11 clinical practice guidelines for the use of PRP in knee OA found that 9 were “uncertain or unable to make a recommendation” and 2 recommended against it.39
In its 2021 position statement for the responsible use of regenerative medicine, the American Medical Society for Sports Medicine includes guidance on integrating orthobiologics into clinical practice. The guideline emphasizes informed consent and provides an evidence-based rationale for using PRP in certain patient populations (lateral epicondylitis and younger patients with mild-to-moderate knee OA), recommending its use only after exhausting other conservative options.40 Patients should be referred to physicians with experience using PRP and image-guided procedures.
CORRESPONDENCE
Gregory D. Bentz Jr, MD, 3640 High Street Suite 3B, Portsmouth, VA 23707; bentzgd@evms.edu
Platelet-rich plasma (PRP) injections have become a popular treatment option in a variety of specialties including sports medicine, maxillofacial surgery, dermatology, cosmetology, and reproductive medicine.1 PRP is an autologous blood product derived from whole blood, using a centrifuge to isolate a concentrated layer of platelets. The a-granules in platelets release transforming growth factor b 1, vascular endothelial growth factor, platelet-derived growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor 1, and other mediatorsthat enhance the natural healing process.2

When patients ask. Familiarity with the use of PRP to treat specific musculoskeletal (MSK) conditions is essential for family physicians who frequently are asked by patients about whether PRP is right for them. These patients may have experienced failure of medication therapy or declined surgical intervention, or may not be surgical candidates. This review details the evidence surrounding common intra-articular and extra-articular applications of PRP. But first, a word about how PRP is prepared, its contraindications, and costs.
Preparation and types of PRP
Although there are many commercial systems for preparing PRP, there is no consensus on the optimal formulation.2 Other terms for PRP, such as autologous concentrated platelets and super-concentrated platelets, are based on concentration of red blood cells, leukocytes, and fibrin.3 PRP therapies usually are categorized as leukocyte-rich PRP (LR-PRP) or leukocyte-poor PRP (LP-PRP), based on neutrophil concentrations that are above and below baseline.2 Leukocyte concentration is one of the most debated topics in PRP therapy.4
Common commercially available preparation systems produce platelet concentrations between 3 to 6 times the baseline platelet count.5 Although there is no universally agreed upon PRP formulation, studies have shown 2 centrifugation cycles (“double-spun” or “dual centrifugation”) that yield platelet concentrations between 1.8 to 1.9 times the baseline values significantly improve MSK conditions.6-8
For MSK purposes, PRP may be injected into intratendinous, peritendinous, and intra-articular spaces. Currently, there is no consensus regarding injection frequency. Many studies have incorporated single-injection protocols, while some have used 2 to 3 injections repeated over several weeks to months. PRP commonly is injected at point-of-care without requiring storage.
Contraindications. PRP has been shown to be safe, with most adverse effects attributed to local injection site pain, bleeding, swelling, and bruising.9
Contraindications to PRP include active malignancy or recent remission from malignancy with the exception of nonmetastatic skin tumors.10 PRP is not recommended for patients with an allergy to manufacturing components (eg, dimethyl sulfoxide), thrombocytopenia, nonsteroidal anti-inflammatory drug use within 2 weeks, active infection causing fever, and local infection at the injection site.10 Since local anesthetics may impair platelet function, they should not be given at the same injection site as PRP.10
Continue to: Cost
Cost. PRP is not covered by most insurance plans.11,12 The cost for PRP may range from $500 to $2500 for a single injection.12
Evidence-based summary by condition
Knee osteoarthritis
❯❯❯ Consider using PRP
Knee osteoarthritis (OA) is a common cause of pain and disability. Treatment options include physical therapy, pharmacotherapy, and surgery. PRP has gained popularity as a nonsurgical option. A recent meta-analysis by Costa et al13 of 40 studies with 3035 participants comparing intra-articular PRP with hyaluronic acid (HA), corticosteroid, and saline injections, found that PRP appears to be more effective or as effective as other nonsurgical modalities. However, due to study heterogeneity and high risk for bias, the authors could not recommend PRP for knee OA in clinical practice.13
Despite Costa et al’s findings, reproducible data have demonstrated the superiority of PRP over other nonsurgical treatment options for knee OA. A 2021 systematic review and meta-analysis of 18 randomized controlled trials (RCTs; N = 811) by Belk et al6 comparing PRP to HA injections showed a higher mean improvement in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores in the PRP group compared to the HA group (44.7% vs 12.6%, respectively; P < .01).6 Six of 11 studies using the visual analog scale (VAS) for pain reported significantly less pain in the PRP group compared to the HA group (P < .05).6 The mean follow-up time was 11.1 months.6 Three of 6 studies reported improved subjective International Knee Documentation Committee (IKDC) scores (range from 0-100, with higher scores representing higher levels of function and lower levels of symptoms) in the PRP group compared to the HA group: 75.7 ± 15.1 vs 65.6 ± 16.9 (P = .004); 65.5 ± 3.6 vs 55.8 ± 3.8 (P = .01); and 60.8 ± 9.8 vs 48.4 ± 6.2 (P < .05).6 There was concern for moderate-to-high heterogeneity.6
Other systematic reviews and meta-analyses found similar efficacy of PRP for knee OA, including improved WOMAC scores and patient-reported outcomes (eg, pain, physical function, stiffness) compared to other injectable options.14,15 A systematic review of 14 RCTs (N = 1423) by Shen et al15 showed improved WOMAC scores at 3 months (mean differences [MD] = –14.53; 95% CI, –29.97 to –7.09; P < .001), 6 months
Despite a lack of consensus regarding the optimal preparation of PRP for knee OA, another recent RCT (N = 192) found significant improvement in mean subjective IKDC scores in the LR-PRP group (45.5 ± 15.5 to 60.7 ± 21.1; P < .0005) and the LP-PRP group (46.8 ± 15.8 to 62.9 ± 19.9; P < .0005), indicating efficacy regardless of PRP type.4
Continue to: Ankle osteoarthritis
Ankle osteoarthritis
❯ ❯ ❯ Additional research is needed
Ankle OA affects 3.4% of all adults and is more common in the younger population than knee or hip OA.16 An RCT (N = 100) investigating PRP vs placebo (saline) injections showed no statistically significant difference in American Orthopedic Foot and Ankle Society scores evaluating pain and function over 26 weeks (–2 points; 95% CI, –5 to 1; P = .16).16 Limitations to this study include its small sample size and the PRP formulation used. (The intervention group received 2 injections of 2 mL of PRP, and the platelet concentration was not reported.)16
Hip osteoarthritis
❯ ❯ ❯ Additional research is needed
Symptomatic hip OA occurs in 40% of adults older than 65 years, with a higher prevalence in women.18 Currently, corticosteroid injections are the only intra-articular therapy recommended by international guidelines for hip OA.19 A systematic review and meta-analysis comparing PRP to HA injections that included 4 RCTs (N = 303) showed a statistically significant reduction in VAS scores at 2 months in the PRP group compared to the HA group (weighted mean difference [WMD] = –0.376; 95% CI, –0.614 to –0.138; P = .002).18 However, there were no significant differences in VAS scores between the PRP and HA groups at 6 months (WMD = –0.141; 95% CI, –0.401 to 0.119; P = .289) and 12 months (WMD = –0.083; 95% CI, –0.343 to 0.117; P = .534). Likewise, no significant differences were found in WOMAC scores at 6 months (WMD = –2.841; 95% CI, –6.248 to 0.565; P = .102) and 12 months (WMD = –3.134; 95% CI, –6.624 to 0.356; P = .078) and Harris Hip Scores (HHS) at 6 months (WMD = 2.782; 95% CI, –6.639 to 12.203; P =.563) and 12 months (WMD = 0.706; 95% CI, –6.333 to 7.745; P = .844).18
A systematic review of 6 RCTs (N = 408) by Belk et al20 comparing PRP to HA for hip OA found similar short-term improvements in WOMAC scores (standardized mean differences [SMD] = 0.27; 95% CI, –0.05 to 0.59; P = .09), VAS scores (MD = 0.59; 95% CI, –0.741 to 1.92; P = .39), and HHS (MD = -0.81; 95% CI, –10.06 to 8.43; P = .93).The average follow-up time was 12.2 and 11.9 months for the PRP and HA groups, respectively.20
LR-PRP, which was used in 1 of the 6 RCTs, showed improvement in VAS scores and HHS from baseline, but no significant difference compared to HA at the latest follow-up.20 A pooled subanalysis of the 3 studies that used LP-PRP found no difference in WOMAC scores between the PRP and HA groups (SMD = 0.42; 95% CI, –0.01 to 0.86; P = .06).20 Future studies comparing the efficacy of intra-articular steroid vs PRP for hip OA would be beneficial.18
Continue to: Rotator cuff tendinopathy
Rotator cuff tendinopathy
❯ ❯ ❯ Consider PRP for short-term pain relief
Painful conditions of the rotator cuff include impingement syndrome, tendonitis, and partial and complete tears. A 2021 RCT (N = 58) by Dadgostar et al21 comparing PRP injection to corticosteroid therapy (methylprednisolone and lidocaine) for the treatment of rotator cuff tendinopathy showed significant improvement in VAS scores at 3 months in the PRP group compared to the corticosteroid group (6.66
Another RCT (N = 99) by Kwong et al22 comparing PRP to corticosteroids found similar short-term advantages of LP-PRP with an improved VAS score (–13.6 vs 0.4; P = .03), American Shoulder and Elbow Surgeons score (13.0 vs 2.9; P = .02), and Western Ontario Rotator Cuff Index score (16.8 vs 5.8; P = .03).However, there was no long-term benefit of PRP over corticosteroids found at 12 months.22
A 2021 systematic review and meta-analysis by Hamid et al23 that included 8 RCTs (N = 976) favored PRP over control (no injection, saline injections, and/or shoulder rehabilitation) with improved VAS scores at 12 months (SMD = –0.5; 95% CI, –0.7 to –0.2; P < .001).The evidence on functional outcome was mixed. Data pooled from 2 studies (n = 228) found better Shoulder Pain and Disability Index (SPADI) scores compared to controls at 3- and 6-month follow-ups. However, there were no significant differences in Disabilities of the Arm, Shoulder and Hand (DASH) scores between the 2 groups.23
Patellar tendinopathy
❯ ❯ ❯ Consider using PRP for return to sport
Patellar tendinopathy, a common MSK condition encountered in the primary care setting, has an overall prevalence of 22% in elite athletes at some point in their career.24 Nonsurgical management options include rest, ice, eccentric and isometric exercises, anti-inflammatory drugs, extracorporeal shock wave therapy (ESWT), and dry needling (DN).
A 2014 RCT (N = 23) evaluating DN vs PRP for patellar tendinopathy favored PRP with improved VAS scores (mean ± SD = 25.4 ± 23.2 points; P = .01 vs 5.2 ± 12.5 points; P = .20) at 12 weeks (P = .02). However, at ≥ 26 weeks, the improvement in pain and function scores was similar between the DN and PRP groups (33.2 ± 14.0 points; P = .001 vs 28.9 ± 25.2 points; P = .01). Notably, there was significantly more improvement in the PRP group at 12 weeks (P = .02) but not at 26 weeks (P = .66).25
Continue to: Another perspective study...
Another prospective study (N = 31) comparing PRP to physiotherapy showed a greater improvement in sport activity level reflected by the Tegner score in the PRP group (percentage improvement, 39 ± 22%) compared to control (20 ± 27%; P = .048) at 6 months.7
A recent RCT (N = 20) revealed improved VAS scores at 6 months with rehabilitation paired with either bone marrow mesenchymal stem cells (BM-MSC) or LP-PRP when compared with baseline (BM-MSC group: 4.23 ± 2.13 to 2.52 ± 2.37; P = .0621; LP-PRP group: 3.10 ± 1.20 to 1.13 ± 1.25; P = .0083). Pain was significantly reduced during sport play in both groups at 6 months when compared with baseline (BM-MSC group: 6.91 ± 1.11 to 3.06 ± 2.89, P = .0049; PRP group: 7.03 ± 1.42 to 1.94 ± 1.24, P = .0001).26
A 2019 systematic review and meta-analysis (N = 2530) demonstrated greater improvements in Victorian Institute of Sport Assessment scale for patellar tendinopathy (VISA-P) with multiple injections of PRP (38.7 points; 95% CI, 26.3-51.2 points) compared to single injections of PRP (24.3 points; 95% CI, 18.2-30.5 points), eccentric exercise (28.3 points; 95% CI, 18.9-37.8 points) and ESWT (27.4 points; 95% CI, 10.0-39.8 points) after 6 months.27 In contrast, an RCT (n = 57) comparing a single injection of LR-PRP or LP-PRP was no more effective than a single injection of saline for improvement in mean VISA-P scores (P > .05) at 1 year.28
Lateral epicondylitis
❯ ❯ ❯ Consider using PRP
Lateral epicondylitis (“tennis elbow”) is caused by overuse of the elbow extensors at the site of the lateral epicondyle. Chronic lateral epicondylosis involves tissue degeneration and microtrauma.Most cases of epicondylar tendinopathies are treated nonoperatively, with corticosteroid injections being a mainstay of treatment despite their short-term benefit29 and potential to deteriorate connective tissue over time. Recent studies suggest PRP therapy for epicondylitis and epicondylosis may increase long-term pain relief and improve function.
A 2017 systematic review and meta-analysis of 16 RCTs (N = 1018) concluded PRP was more efficacious than control injections (bupivacaine) for pain reduction in tendinopathies (effect size = 0.47; 95% CI, 0.22-0.72).30 In the review, lateral epicondylitis was evaluated in 12 studies and was most responsive to PRP (effect size = 0.57) when compared to control injection.30 In another systematic review (5 RCTs; 250 patients), corticosteroid injections improved pain within the first 6 weeks of treatment. However, PRP outperformed corticosteroid in VAS scores (21.3 ± 28.1 vs 42.4 ± 26.8) and DASH scores (17.6 ± 24.0 vs 36.5 ± 23.8) (P < .001) at 2 years.31
Continue to: A 2022 systematic review...
A 2022 systematic review and meta-analysis (26 studies; N = 1040) comparing scores at baseline vs 2 years post-PRP showed improvement in VAS scores (7.4 ± 1.30 vs 3.71 ± 2.35; P < .001), DASH scores (60.8 ± 12.5 vs 13.0 ± 18.5; P < .001), Patient-Rated Tennis Elbow Evaluation (55.6 ± 14.7 vs 48.8 ± 4.1; P < .001), and Mayo Clinic Performance Index (55.5 ± 6.1 vs 93.0 ± 6.7; P < .001).32
Regarding the therapeutic effects of different PRP types in lateral epicondylitis, a 2022 systematic review of 33 studies (N = 2420) found improved function and pain relief with LR-PRP and LP-PRP with no significant differences.33 Pretreatment VAS scores in the LR-PRP group, which ranged from 6.1 to 8.0, improved to 1.5 to 4.0 at 3 months and 0.6 to 3.3 after 1 year.33 Similarly, pretreatment VAS scores in the LP-PRP group, which ranged from 4.2 to 8.4, improved to 1.6 to 5.9 at 3 months and 0.7 to 2.7 after 1 year.34 DASH scores also improved in the LR-PRP and LP-PRP groups, with pretreatment scores (LR-PRP, 47.0 to 54.3; LP-PRP, 30.0 to 67.7) improving to 20.0 to 22.0 and 5.5 to 19.0, respectively, at 1 year.33
Achilles tendinopathy
❯ ❯ ❯ Do not use PRP; evidence is lacking
Achilles tendinopathy, caused by chronic overuse and overload resulting in microtrauma and poor tissue healing, typically occurs in the most poorly vascularized portion of the tendon and is common in runners. First-line treatments for Achilles tendinopathy include eccentric strength training and anti-inflammatory drugs.34,35 Corticosteroid injections are not recommended, given concern for degraded tendon tissue over time and worse function.34
A 2020 systematic review of 11 randomized and nonrandomized clinical trials (N = 406) found PRP improved Victorian Institute of Sports Assessment—Achilles (VISA-A) scores at 24 weeks compared to other nonsurgical treatment options (41.2 vs 70.12; P < .018).34 However, a higher-quality 2021 systematic review and meta-analysis of 4 RCTs (N = 170) comparing PRP injections with placebo showed no significant difference in VISA-A scores at 3 months (0.23; 95% CI, –0.45 to 0.91), 6 months (0.83; 95% CI, –0.26 to 1.92), and 12 months (0.83; 95% CI, –0.77 to 2.44).36 Therefore, further studies are warranted to evaluate the benefit of PRP injections for Achilles tendinopathy.
Conclusions
While high-quality studies support the use of PRP for knee OA and lateral epicondylitis, they have a moderate-to-high risk for bias. Several RCTs show that PRP provides superior short-term pain relief and range of motion compared to corticosteroids for rotator cuff tendinopathy. Multiple injections of PRP for patellar tendinopathy may accelerate return to sport and improve symptoms over the long term. However, current evidence does not support PRP therapy for Achilles tendinopathy. Given variability in PRP preparation, an accurate interpretation of the literature regarding its use in MSK conditions is recommended (TABLE4,6,7,14-18,20-23,25-28,30-34,36).

Continue to: Concerning the effectiveness of PRP...
Concerning the effectiveness of PRP, it is important to consider early publication bias. Although recent studies have shown its benefits,6,14,15,37 additional studies comparing PRP to placebo will help demonstrate its efficacy. Interestingly, a literature search by Bar-Or et al38 found intra-articular saline may have a therapeutic effect on knee OA and confound findings when used as a placebo.
Recognizing the presence or lack of clinically significant improvement in the literature is important. For example, while some recent studies have shown PRP exceeds the minimal clinically significant difference for knee OA and lateral epicondylitis, others have not.32,37 A 2021 systematic review of 11 clinical practice guidelines for the use of PRP in knee OA found that 9 were “uncertain or unable to make a recommendation” and 2 recommended against it.39
In its 2021 position statement for the responsible use of regenerative medicine, the American Medical Society for Sports Medicine includes guidance on integrating orthobiologics into clinical practice. The guideline emphasizes informed consent and provides an evidence-based rationale for using PRP in certain patient populations (lateral epicondylitis and younger patients with mild-to-moderate knee OA), recommending its use only after exhausting other conservative options.40 Patients should be referred to physicians with experience using PRP and image-guided procedures.
CORRESPONDENCE
Gregory D. Bentz Jr, MD, 3640 High Street Suite 3B, Portsmouth, VA 23707; bentzgd@evms.edu
1. Cecerska-Heryć E, Goszka M, Serwin N, et al. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2022;64:84-94. doi: 10.1016/j.cytogfr.2021.11.003
2. Le ADK, Enweze L, DeBaun MR, et al. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11:624-634. doi: 10.1007/s12178-018-9527-7
3. Everts P, Onishi K, Jayaram P, et al. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21:7794. doi: 10.3390/ijms21207794
4. Di Martino A, Boffa A, Andriolo L, et al. Leukocyte-rich versus leukocyte-poor platelet-rich plasma for the treatment of knee osteoarthritis: a double-blind randomized trial. Am J Sports Med. 2022;50:609-617. doi: 10.1177/03635465211064303
5. Mariani E, Pulsatelli L. Platelet concentrates in musculoskeletal medicine. Int J Mol Sci. 2020;21:1328. doi: 10.3390/ijms21041328
6. Belk JW, Kraeutler MJ, Houck DA, et al. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. 2021;49:249-260. doi: 10.1177/0363546520909397
7. Filardo G, Kon E, Della Villa S, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34:909-915. doi: 10.1007/s00264-009-0845-7
8. Kon E, Filardo G, Delcogliano M, et al. Platelet-rich plasma: new clinical application: a pilot study for treatment of jumper’s knee. Injury. 2009;40:598-603. doi: 10.1016/j.injury.2008.11.026
9. Kanchanatawan W, Arirachakaran A, Chaijenkij K, et al. Short-term outcomes of platelet-rich plasma injection for treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24:1665-1677. doi: 10.1007/s00167-015-3784-4
10. Cook J, Young M. Biologic therapies for tendon and muscle injury. UpToDate. Updated August 11, 2022. Accessed May 23, 2023. www.uptodate.com/contents/biologic-therapies-for-tendon-and-muscle-injury
11. Bendich I, Rubenstein WJ, Cole BJ, et al. What is the appropriate price for platelet-rich plasma injections for knee osteoarthritis? A cost-effectiveness analysis based on evidence from Level I randomized controlled trials. Arthroscopy. 2020;36:1983-1991.e1. doi: 10.1016/j.arthro.2020.02.004
12. Jones IA, Togashi RC, Thomas Vangsness C Jr. The economics and regulation of PRP in the evolving field of orthopedic biologics. Curr Rev Musculoskelet Med. 2018;11:558-565. doi: 10.1007/s12178-018-9514-z
13. Costa LAV, Lenza M, Irrgang JJ, et al. How does platelet-rich plasma compare clinically to other therapies in the treatment of knee osteoarthritis? A systematic review and meta-analysis. Am J Sports Med. 2023;51:1074-1086 doi: 10.1177/03635465211062243
14. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505. doi: 10.1016/j.arthro.2015.08.005
15. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12:16. doi: 10.1186/s13018-017-0521-3
16. Paget LDA, Reurink G, de Vos RJ, et al; PRIMA Study Group. Effect of platelet-rich plasma injections vs. placebo on ankle symptoms and function in patients with ankle osteoarthritis: a randomized clinical trial. JAMA. 2021;326:1595-1605. doi: 10.1001/jama.2021.16602
17. Evans A, Ibrahim M, Pope R, et al. Treating hand and foot osteoarthritis using a patient’s own blood: a systematic review and meta-analysis of platelet-rich plasma. J Orthop. 2020;18:226-236. doi: 10.1016/j.jor.2020.01.037
18. Ye Y, Zhou X, Mao S, et al. Platelet rich plasma versus hyaluronic acid in patients with hip osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surg. 2018;53:279-287. doi: 10.1016/j.ijsu.2018.03.078.
19. Berney M, McCarroll P, Glynn L, et al. Platelet-rich plasma injections for hip osteoarthritis: a review of the evidence. Ir J Med Sci. 2021;190:1021-1025. doi: 10.1007/s11845-020-02388-z
20. Belk JW, Houck DA, Littlefield CP, et al. Platelet-rich plasma versus hyaluronic acid for hip osteoarthritis yields similarly beneficial short-term clinical outcomes: a systematic review and meta-analysis of Level I and II randomized controlled trials. Arthroscopy. 2022;38:2035-2046. doi: 10.1016/j.arthro.2021.11.005
21. Dadgostar H, Fahimipour F, Pahlevan Sabagh A, et al. Corticosteroids or platelet-rich plasma injections for rotator cuff tendinopathy: a randomized clinical trial study. J Orthop Surg Res. 2021;16:333. doi: 10.1186/s13018-021-02470-x
22. Kwong CA, Woodmass JM, Gusnowski EM, et al. Platelet-rich plasma in patients with partial-thickness rotator cuff tears or tendinopathy leads to significantly improved short-term pain relief and function compared with corticosteroid injection: a double-blind randomized controlled trial. Arthroscopy. 2021;37:510-517. doi: 10.1016/j.arthro.2020.10.037
23. A Hamid MS, Sazlina SG. Platelet-rich plasma for rotator cuff tendinopathy: a systematic review and meta-analysis. PLoS One. 2021;16:e0251111. doi: 10.1371/journal.pone.0251111
24. Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33:561-567. doi: 10.1177/0363546504270454
25. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618. doi: 10.1177/0363546513518416.
26. Rodas G, Soler-Rich R, Rius-Tarruella J, et al. Effect of autologous expanded bone marrow mesenchymal stem cells or leukocyte-poor platelet-rich plasma in chronic patellar tendinopathy (with gap >3 mm): preliminary outcomes after 6 months of a double-blind, randomized, prospective study. Am J Sports Med. 2021;49:1492-1504. doi: 10.1177/0363546521998725
27. Andriolo L, Altamura SA, Reale D, et al. Nonsurgical treatments of patellar tendinopathy: multiple injections of platelet-rich plasma are a suitable option: a systematic review and meta-analysis. Am J Sports Med. 2019;47:1001-1018. doi: 10.1177/0363546518759674
28. Scott A, LaPrade RF, Harmon KG, et al. Platelet-rich plasma for patellar tendinopathy: a randomized controlled trial of leukocyte-rich PRP or leukocyte-poor PRP versus saline. Am J Sports Med. 2019;47:1654-1661. doi: 10.1177/0363546519837954
29. Kemp JA, Olson MA, Tao MA, et al. Platelet-rich plasma versus corticosteroid injection for the treatment of lateral epicondylitis: a systematic review of systematic reviews. Int J Sports Phys Ther. 2021;16:597-605. doi: 10.26603/001c.24148
30. Miller LE, Parrish WR, Roides B, et al. Efficacy of platelet-rich plasma injections for symptomatic tendinopathy: systematic review and meta-analysis of randomised injection-controlled trials. BMJ Open Sport Exerc Med. 2017;3:e000237. doi: 10.1136/bmjsem-2017- 000237
31. Ben-Nafa W, Munro W. The effect of corticosteroid versus platelet-rich plasma injection therapies for the management of lateral epicondylitis: a systematic review. SICOT J. 2018;4:11.
32. Niemiec P, Szyluk K, Jarosz A, et al. Effectiveness of platelet-rich plasma for lateral epicondylitis: a systematic review and meta-analysis based on achievement of minimal clinically important difference. Orthop J Sports Med. 2022;10:23259671221086920. doi: 10.1177/23259671221086920
33. Li S, Yang G, Zhang H, et al. A systematic review on the efficacy of different types of platelet-rich plasma in the management of lateral epicondylitis. J Shoulder Elbow Surg. 2022;311533-1544. doi: 10.1016/j.jse.2022.02.017.
34. Madhi MI, Yausep OE, Khamdan K, et al. The use of PRP in treatment of Achilles tendinopathy: a systematic review of literature. Study design: systematic review of literature. Ann Med Surg (Lond). 2020;55:320-326. doi: 10.1016/j.amsu.2020.04.042
35. Loppini M, Maffulli N. Conservative management of tendinopathy: an evidence-based approach. Muscles Ligaments Tendons J. 2012;1:134-137.
36. Nauwelaers AK, Van Oost L, Peers K. Evidence for the use of PRP in chronic midsubstance Achilles tendinopathy: a systematic review with meta-analysis. Foot Ankle Surg. 2021;27:486-495. doi: 10.1016/j.fas.2020.07.009
37. Dai WL, Zhou AG, Zhang H, et al. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33:659-670.e1. doi: 10.1016/j.arthro.2016.09.024
38. Bar-Or D, Rael LT, Brody EN. Use of saline as a placebo in intra-articular injections in osteoarthritis: potential contributions to nociceptive pain relief. Open Rheumatol J. 2017;11:16-22. doi: 10.2174/1874312901711010016
39. Phillips M, Bhandari M, Grant J, et al. A systematic review of current clinical practice guidelines on intra-articular hyaluronic acid, corticosteroid, and platelet-rich plasma injection for knee osteoarthritis: an international perspective. Orthop J Sports Med. 2021;9:23259671211030272. doi: 10.1177/23259671211030272
40. Finnoff JT, Awan TM, Borg-Stein J, et al. American Medical Society for Sports Medicine position statement: principles for the responsible use of regenerative medicine in sports medicine. Clin J Sport Med. 2021;31:530-541. doi: 10.1097/JSM.0000000000000973
1. Cecerska-Heryć E, Goszka M, Serwin N, et al. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2022;64:84-94. doi: 10.1016/j.cytogfr.2021.11.003
2. Le ADK, Enweze L, DeBaun MR, et al. Current clinical recommendations for use of platelet-rich plasma. Curr Rev Musculoskelet Med. 2018;11:624-634. doi: 10.1007/s12178-018-9527-7
3. Everts P, Onishi K, Jayaram P, et al. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21:7794. doi: 10.3390/ijms21207794
4. Di Martino A, Boffa A, Andriolo L, et al. Leukocyte-rich versus leukocyte-poor platelet-rich plasma for the treatment of knee osteoarthritis: a double-blind randomized trial. Am J Sports Med. 2022;50:609-617. doi: 10.1177/03635465211064303
5. Mariani E, Pulsatelli L. Platelet concentrates in musculoskeletal medicine. Int J Mol Sci. 2020;21:1328. doi: 10.3390/ijms21041328
6. Belk JW, Kraeutler MJ, Houck DA, et al. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med. 2021;49:249-260. doi: 10.1177/0363546520909397
7. Filardo G, Kon E, Della Villa S, et al. Use of platelet-rich plasma for the treatment of refractory jumper’s knee. Int Orthop. 2010;34:909-915. doi: 10.1007/s00264-009-0845-7
8. Kon E, Filardo G, Delcogliano M, et al. Platelet-rich plasma: new clinical application: a pilot study for treatment of jumper’s knee. Injury. 2009;40:598-603. doi: 10.1016/j.injury.2008.11.026
9. Kanchanatawan W, Arirachakaran A, Chaijenkij K, et al. Short-term outcomes of platelet-rich plasma injection for treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24:1665-1677. doi: 10.1007/s00167-015-3784-4
10. Cook J, Young M. Biologic therapies for tendon and muscle injury. UpToDate. Updated August 11, 2022. Accessed May 23, 2023. www.uptodate.com/contents/biologic-therapies-for-tendon-and-muscle-injury
11. Bendich I, Rubenstein WJ, Cole BJ, et al. What is the appropriate price for platelet-rich plasma injections for knee osteoarthritis? A cost-effectiveness analysis based on evidence from Level I randomized controlled trials. Arthroscopy. 2020;36:1983-1991.e1. doi: 10.1016/j.arthro.2020.02.004
12. Jones IA, Togashi RC, Thomas Vangsness C Jr. The economics and regulation of PRP in the evolving field of orthopedic biologics. Curr Rev Musculoskelet Med. 2018;11:558-565. doi: 10.1007/s12178-018-9514-z
13. Costa LAV, Lenza M, Irrgang JJ, et al. How does platelet-rich plasma compare clinically to other therapies in the treatment of knee osteoarthritis? A systematic review and meta-analysis. Am J Sports Med. 2023;51:1074-1086 doi: 10.1177/03635465211062243
14. Meheux CJ, McCulloch PC, Lintner DM, et al. Efficacy of intra-articular platelet-rich plasma injections in knee osteoarthritis: a systematic review. Arthroscopy. 2016;32:495-505. doi: 10.1016/j.arthro.2015.08.005
15. Shen L, Yuan T, Chen S, et al. The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Orthop Surg Res. 2017;12:16. doi: 10.1186/s13018-017-0521-3
16. Paget LDA, Reurink G, de Vos RJ, et al; PRIMA Study Group. Effect of platelet-rich plasma injections vs. placebo on ankle symptoms and function in patients with ankle osteoarthritis: a randomized clinical trial. JAMA. 2021;326:1595-1605. doi: 10.1001/jama.2021.16602
17. Evans A, Ibrahim M, Pope R, et al. Treating hand and foot osteoarthritis using a patient’s own blood: a systematic review and meta-analysis of platelet-rich plasma. J Orthop. 2020;18:226-236. doi: 10.1016/j.jor.2020.01.037
18. Ye Y, Zhou X, Mao S, et al. Platelet rich plasma versus hyaluronic acid in patients with hip osteoarthritis: a meta-analysis of randomized controlled trials. Int J Surg. 2018;53:279-287. doi: 10.1016/j.ijsu.2018.03.078.
19. Berney M, McCarroll P, Glynn L, et al. Platelet-rich plasma injections for hip osteoarthritis: a review of the evidence. Ir J Med Sci. 2021;190:1021-1025. doi: 10.1007/s11845-020-02388-z
20. Belk JW, Houck DA, Littlefield CP, et al. Platelet-rich plasma versus hyaluronic acid for hip osteoarthritis yields similarly beneficial short-term clinical outcomes: a systematic review and meta-analysis of Level I and II randomized controlled trials. Arthroscopy. 2022;38:2035-2046. doi: 10.1016/j.arthro.2021.11.005
21. Dadgostar H, Fahimipour F, Pahlevan Sabagh A, et al. Corticosteroids or platelet-rich plasma injections for rotator cuff tendinopathy: a randomized clinical trial study. J Orthop Surg Res. 2021;16:333. doi: 10.1186/s13018-021-02470-x
22. Kwong CA, Woodmass JM, Gusnowski EM, et al. Platelet-rich plasma in patients with partial-thickness rotator cuff tears or tendinopathy leads to significantly improved short-term pain relief and function compared with corticosteroid injection: a double-blind randomized controlled trial. Arthroscopy. 2021;37:510-517. doi: 10.1016/j.arthro.2020.10.037
23. A Hamid MS, Sazlina SG. Platelet-rich plasma for rotator cuff tendinopathy: a systematic review and meta-analysis. PLoS One. 2021;16:e0251111. doi: 10.1371/journal.pone.0251111
24. Lian OB, Engebretsen L, Bahr R. Prevalence of jumper’s knee among elite athletes from different sports: a cross-sectional study. Am J Sports Med. 2005;33:561-567. doi: 10.1177/0363546504270454
25. Dragoo JL, Wasterlain AS, Braun HJ, et al. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med. 2014;42:610-618. doi: 10.1177/0363546513518416.
26. Rodas G, Soler-Rich R, Rius-Tarruella J, et al. Effect of autologous expanded bone marrow mesenchymal stem cells or leukocyte-poor platelet-rich plasma in chronic patellar tendinopathy (with gap >3 mm): preliminary outcomes after 6 months of a double-blind, randomized, prospective study. Am J Sports Med. 2021;49:1492-1504. doi: 10.1177/0363546521998725
27. Andriolo L, Altamura SA, Reale D, et al. Nonsurgical treatments of patellar tendinopathy: multiple injections of platelet-rich plasma are a suitable option: a systematic review and meta-analysis. Am J Sports Med. 2019;47:1001-1018. doi: 10.1177/0363546518759674
28. Scott A, LaPrade RF, Harmon KG, et al. Platelet-rich plasma for patellar tendinopathy: a randomized controlled trial of leukocyte-rich PRP or leukocyte-poor PRP versus saline. Am J Sports Med. 2019;47:1654-1661. doi: 10.1177/0363546519837954
29. Kemp JA, Olson MA, Tao MA, et al. Platelet-rich plasma versus corticosteroid injection for the treatment of lateral epicondylitis: a systematic review of systematic reviews. Int J Sports Phys Ther. 2021;16:597-605. doi: 10.26603/001c.24148
30. Miller LE, Parrish WR, Roides B, et al. Efficacy of platelet-rich plasma injections for symptomatic tendinopathy: systematic review and meta-analysis of randomised injection-controlled trials. BMJ Open Sport Exerc Med. 2017;3:e000237. doi: 10.1136/bmjsem-2017- 000237
31. Ben-Nafa W, Munro W. The effect of corticosteroid versus platelet-rich plasma injection therapies for the management of lateral epicondylitis: a systematic review. SICOT J. 2018;4:11.
32. Niemiec P, Szyluk K, Jarosz A, et al. Effectiveness of platelet-rich plasma for lateral epicondylitis: a systematic review and meta-analysis based on achievement of minimal clinically important difference. Orthop J Sports Med. 2022;10:23259671221086920. doi: 10.1177/23259671221086920
33. Li S, Yang G, Zhang H, et al. A systematic review on the efficacy of different types of platelet-rich plasma in the management of lateral epicondylitis. J Shoulder Elbow Surg. 2022;311533-1544. doi: 10.1016/j.jse.2022.02.017.
34. Madhi MI, Yausep OE, Khamdan K, et al. The use of PRP in treatment of Achilles tendinopathy: a systematic review of literature. Study design: systematic review of literature. Ann Med Surg (Lond). 2020;55:320-326. doi: 10.1016/j.amsu.2020.04.042
35. Loppini M, Maffulli N. Conservative management of tendinopathy: an evidence-based approach. Muscles Ligaments Tendons J. 2012;1:134-137.
36. Nauwelaers AK, Van Oost L, Peers K. Evidence for the use of PRP in chronic midsubstance Achilles tendinopathy: a systematic review with meta-analysis. Foot Ankle Surg. 2021;27:486-495. doi: 10.1016/j.fas.2020.07.009
37. Dai WL, Zhou AG, Zhang H, et al. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33:659-670.e1. doi: 10.1016/j.arthro.2016.09.024
38. Bar-Or D, Rael LT, Brody EN. Use of saline as a placebo in intra-articular injections in osteoarthritis: potential contributions to nociceptive pain relief. Open Rheumatol J. 2017;11:16-22. doi: 10.2174/1874312901711010016
39. Phillips M, Bhandari M, Grant J, et al. A systematic review of current clinical practice guidelines on intra-articular hyaluronic acid, corticosteroid, and platelet-rich plasma injection for knee osteoarthritis: an international perspective. Orthop J Sports Med. 2021;9:23259671211030272. doi: 10.1177/23259671211030272
40. Finnoff JT, Awan TM, Borg-Stein J, et al. American Medical Society for Sports Medicine position statement: principles for the responsible use of regenerative medicine in sports medicine. Clin J Sport Med. 2021;31:530-541. doi: 10.1097/JSM.0000000000000973
PRACTICE RECOMMENDATIONS
› Consider plateletrich plasma (PRP) for conservative management of knee osteoarthritis and lateral epicondylitis. B
› Consider giving multiple injections of PRP for longterm pain relief and expedited return to sport in patellar tendinopathy. B
› Do not use PRP for Achilles tendinopathy due to a lack of clinical evidence. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Revisiting our approach to behavioral health referrals
Approximately 1 in 4 people ages 18 years and older and 1 in 3 people ages 18 to 25 years had a mental illness in the past year, according to the 2021 National Survey of Drug Use and Health.1 The survey also found that adults ages 18 to 25 years had the highest rate of serious mental illness but the lowest treatment rate compared to other adult age groups.1 Unfortunately, more than 60% of patients receiving mental health treatment fail to benefit to a clinically meaningful degree.2
However, there is growing evidence that referring patients to behavioral health practitioners (BHPs) with outcome-measured skills that meet the patient’s specific needs can have a dramatic and positive impact. There are 2 main steps to pairing patients with an appropriate BHP: (1) use of measurement-based care data that can be analyzed at the patient and therapist level, and (2) data-driven referrals that pair patients with BHPs based on such routine outcome monitoring data (paired-on outcome data).
Psychotherapy’s slow road toward measurement-based care
Routine outcome monitoring is the systematic measurement of symptoms and functioning during treatment. It serves multiple functions, including program evaluation and benchmarking of patient improvement rates. Moreover, routine outcome monitoring–derived feedback (based on repeated patient outcome measurements) can inform personalized and responsive care decisions throughout treatment.
For all intents and purposes,
- routinely administered symptom/functioning measure, ideally before each clinical encounter,
- practitioner review of these patient-level data,
- patient review of these data with their practitioner, and
- collaborative reevaluation of the person-specific treatment plan informed by these data.
CASE SCENARIO
Violeta W is a 33-year-old woman who presented to her family physician for her annual wellness exam. Prior to the exam, the medical assistant administered a Patient Health Questionnaire-9 (PHQ-9) to screen for depressive symptoms. Ms. W’s score was 20 out of 27, suggestive of depression. To further assess the severity of depressive symptoms and their effect on daily function, the physician reviewed responses to the questionnaire with her and discussed treatment options. Ms. W was most interested in trying a low-dose selective serotonin reuptake inhibitor (SSRI).
At her follow-up visit 4 weeks later, the medical assistant re-administered the PHQ-9. The physician then reviewed Ms. W’s responses with her and, based on Ms. W’s subjective report and objective symptoms (still a score of 20 out of 27 on the PHQ-9), increased her SSRI dose. At each subsequent visit, Ms. W completed a PHQ-9 and reviewed responses and depressive symptoms with her physician.
The value of measurement-based care in mental health care
A narrative review by Lewis et al3 of 21 randomized controlled clinical trials (RCTs) across a range of age groups (eg, adolescents, young adults, adults), disorders (eg, anxiety, mood), and settings (eg, outpatient, inpatient) found that in at least 9 review articles, measurement-based care was associated with significantly improved outcomes vs usual care (ie, treatment without routine outcome monitoring plus feedback). The average increase in treatment effect size was about 30% when treatment was accompanied by measurement-based care.3
Continue to: Moreover, a recent within-patient meta-analysis...
Moreover, a recent within-patient meta-analysis by de Jong et al4 shows that measurement-based care yields a small but significant increase in therapeutic outcomes (d = .15). Use of measurement-based care also is associated with improved communication between the patient and therapist.5 In pharmacotherapy practice, measurement-based care has been shown to predict rapid dose increases and changes in medication, when necessary; faster recovery rates; higher response rates to treatment3; and fewer dropouts.4
Perhaps one of the best-studied benefits of measurement-based mental health care is the ability to predict deterioration in care (ie, patients who are off-track in a way that practitioners often miss without the help of routine outcome monitoring data).6,7 Studies show that without a data-informed approach to care, some forms of psychotherapy or therapy with BHPs who are not sufficiently skilled in treating a given diagnosis increase symptoms or create significant harmful and iatrogenic effects.8-10 Conversely, the meta-analysis by de Jong et al4 found a lower percentage of deterioration in patients receiving measurement-based care. The difference in deterioration was significant: An average of 5.4% of patients in control conditions deteriorated compared to an average of 4.6% in feedback (measurement-based care) groups. There were even larger effect sizes when therapists received training in the feedback system.4
Routine outcome monitoring without a dialogue between patient and practitioner about the assessments (eg, ignoring complete measurement-based care requirements) may be inadequate. A recent review by Muir et al6 found no differences in patient outcomes when data were used solely for aggregate quality improvement activities, suggesting the need for practitioners to review results of routine outcome monitoring assessments with patients and use data to alter care when necessary.
Measurement-based care is believed to deliver benefits and reduce harm by enhancing and encouraging active patient involvement, improving patient understanding of symptoms, promoting better communication, and facilitating better care coordination.3 The benefits of measurement-based care can be enhanced with a comprehensive core routine outcome monitoring tool and the level of monitoring-generated information delivered for multiple stakeholders (eg, patient, therapist, clinic).11
A look at multidimensional assessment
The features of routine outcome monitoring tools vary significantly.12 Some measures assess single-symptom or problem domains (eg, PHQ-9 for depression or Generalized Anxiety Disorder-7 [GAD-7] scale for anxiety) or multiple dimensions (multidimensional routine outcome monitoring).Multidimensional routine outcome monitoring may have benefits over single-domain measures. Single-domain measures and the subscales or factors of more comprehensive multidimensional routine outcome monitoring assessments should possess adequate specificity and sensitivity.
Continue to: Some recent research findings...
Some recent research findings question the construct validity of brief single-domain measures of common presenting problems, such as depression and anxiety. For example, results from a factor analysis of the PHQ-9 and GAD-7 scale in patients with traumatic brain injury suggest these tools measure 1 psychological construct that includes depression and the cognitive components of anxiety (eg, worry)13—a finding consistent with those of other tools.14 Similarly, a larger study of 7763 BH patients found that a single factor accounted for most of the variance of the 2 combined measures, with no set of factors meeting the exacting standards used to develop multidimensional routine outcome monitoring.15 These findings suggest that the PHQ-9 and GAD-7 largely overlap and are not measuring different aspects of health as most practitioners believe (eg, depression and anxiety).
In commonly used assessments, multiple-factor analytic studies with high standards have supported the construct validity of domain-specific subscales, indicating that the various questions tap into different constructs of psychological health.14,16,17
Beyond multiple domain–specific indicators, multidimensional routine outcome measurements provide a global total score that minimizes Type I (false-positive conclusion) and Type II (false-negative conclusion) errors in tracking patient improvement or deterioration.18 As one would expect, multidimensional routine outcome monitoring generally includes more items than single-domain measures; however, this comes with a trade-off. If there are specificity and sensitivity concerns with an ultra-brief single-domain measure, an alternative to a core multidimensional routine outcome measurement is to aggregate a series of single-domain measures into a battery of patient self-reports. However, this approach may take longer for patients to complete since they would have to shift among the varying response sets and wording across the unique single-domain measures.
In addition, the standardization/normalization of multidimensional routine outcome monitoring likely makes interpretation easier than referring to norms and clinical severity cutoffs for many distinct measures. Furthermore, increased specificity enhances predictive power and allows BHPs to screen and track other conditions besides depression and anxiety. (It is worth noting that there are no known studies that have looked at the difference in time to administer or ease of interpretation of multidimensional routine outcome monitoring tools vs multiple single-domain measures.)
Two multidimensional routine outcome monitoring tools that cover a comprehensive series of discrete symptom and functional domains are the Treatment Outcome Package12 and Counseling Center Assessment of Psychological Symptoms.16 These tools, which include subscales beyond general depression and anxiety (eg, sleep, substance misuse, social conflict), take 7 to 10 minutes to complete and provide outcome results across 12 symptom and 8 functional dimensions. As an example, the Treatment Outcome Package has good psychometric qualities (eg, reliability, construct and concurrent validity) for adults,12 children,14,19 and adolescents,19 and can be administered through a secure online data collection portal. The Counseling Center Assessment of Psychological Symptoms has demonstrated high construct validity and good convergent validity.16 These assessments can be administered in paper or digital (eg, electronic medical record portal, smartphone) format.20
Continue to: CASE SCENARIO
CASE SCENARIO
Ms. W’s physician asked her to go online using her phone and answer the questions in the Treatment Outcome Package. Her results, which she viewed with her physician, were displayed in graph form (FIGURE). Her scores were represented in Z scores normalized to the general population, with “0” representing the general, nontreatment-seeking population average and positive scores representing the number of standard deviations (SDs) more severe than the general population average.
Although this assessment scored Ms. W’s clinically elevated depression as mild, it revealed abnormalities in 3 other domains. Sexual functioning issues represented the most abnormal domain at greater than 3 SDs (more severe than the general population), followed by poor life quality and school/work functioning.
After reviewing Ms. W’s report, her physician decided that pharmacologic management alone (for depression) was not the most appropriate treatment course. Therefore, her physician recommended psychotherapy in addition to the SSRI she was taking. Ms. W agreed to a customized referral for psychotherapy.
Data-driven referrals
When psychotherapy is chosen as a treatment, the individual BHP is an active component of that treatment. Consequently, it is essential to customize referrals to match a patient’s most elevated mental health concerns with a therapist who will most effectively treat those domains. It is rare for a BHP to be skilled in treating every mental health domain.9 Multiple studies have shown that BHPs have identifiable treatment skills in specific domains, which physicians should consider when making referrals.9,21,22 These studies demonstrate the utility of aggregating patient-level routine outcome monitoring data to better understand therapist-level (and ultimately clinic- and system-level) outcomes.
Additionally, recent research has tested this idea prospectively. An RCT funded by the Patient-Centered Outcome Research Institute and published in JAMA Psychiatry showed a significant and positive effect on patient outcomes (ie, reductions in general impairment, impairment involving a patient’s most elevated domain, and global distress) using paired-on outcome data matching vs as-usual matching protocols (eg, therapist self-defined areas of specialty).22 In the RCT, the most effective matching protocol was a combination of eliminating harm and matching the patient on their 3 most problematic domains (the highest match level). These patients ended care as healthy as the general population after 16 weeks of treatment. A random 1-year follow-up assessment from the original RCT showed that most patients who had been matched had maintained their improvement.23
Continue to: Therefore, a multidimensional routine outcome...
Therefore, a multidimensional routine outcome monitoring tool can be used to identify a BHP’s relative strengths and weaknesses across multiple outcome domains. Within a system of care, a sample of BHPs will possess varying outcome-domain profiles. When a new patient is seeking a referral to a BHP, these profiles (or domain-specific outcome track records) can be used to support paired-on outcome data matching. Specifically, a new patient completes the multidimensional routine outcome monitoring tool at pretreatment, and the results reveal the outcome domains on which the patient is most clinically severe. This pattern of domain-specific severity then can be used to pair the new patient with a BHP who has demonstrated success in addressing the same outcome domain(s). This approach matches a new patient to a BHP with established expertise based on routine outcome monitoring.
Retrospective and prospective studies have found that most BHPs have stable performance in their strengths and weaknesses.11,21 One study found that assessing BHP performance with their most recent 30 patients can reliably predict future performance with their next 30 patients.24 This predictability in a practitioner’s outcomes suggests report cards that are updated frequently can be utilized to make case assignments within BH or referrals to a specific BHP from primary care.
Making a paired-on outcome data–matched referral
Making customized BH referrals requires access to information about a practitioner’s previous routine outcome monitoring data per clinical domain (eg, suicidality, violence, quality of life) from their most recent patients. Previous research suggests that follow-up data from a minimum of 15 patients is necessary to make a reliable evaluation of a practitioner’s strengths and weaknesses (ie, effectiveness “report card”) per clinical domain.24
Few, if any, physicians have access to this level of updated outcome data from their referral network. To facilitate widespread use of paired-on outcome data matching, a new Web system (MatchedTherapists.com) will allow the general public and PCPs to access these grades. As a public service option, this site currently allows for a self-assessment using the Treatment Outcome Package. Pending versions will generate paired-on outcome data grades, and users will receive a list of local therapists available for in-person appointments as well as therapists available for virtual appointments. The paired-on outcome data grades are delivered in school-based letter grades. An “A+,” for example, represents the best matching grade. Users also will be able to sort and filter results for other criteria such as telemedicine, insurance, age, gender, and appointment availability. Currently, there are more than 77,000 therapists listed on the site nationwide. A basic listing is free.
CASE SCENARIO
After Ms. W took the multidimensional routine outcome assessment online, she received a list of therapists rank-ordered by paired-on outcome data grade, with the “A+” matches listed first. Three of the best-matched referrals accepted her insurance and were willing to see her through telemedicine. Therapists with available in-person appointments had a “B” grade. After discussing the options with her physician, Ms. W opted for telehealth counseling with the therapist whose profile she liked best. The therapist and PCP tracked her progress through routine outcome monitoring reporting until all her symptoms became subclinical.
Continue to: The future of a "referral bridge"
The future of a “referral bridge”
In this article, we present a solution to a common issue faced by mental health care patients: failure to benefit meaningfully from mental health treatment. Matching patients to specific BHPs based on effectiveness data regarding the therapist’s strengths and skills can improve patient outcomes and reduce harm. In addition, patients appear to value this approach. A Robert Wood Johnson Foundation–funded study demonstrated that patients value seeing practitioners who have a track record of successfully treating previous patients with similar issues.25,26 In many cases, patients indicated they would prioritize this matching process over other factors such as practitioners with a higher number of years of experience or the same demographic characteristics as the patient.25,26
These findings may represent a new area in the science of health care. Over the past century, major advances in diagnosis and treatment—the 2 primary pillars of health care—have turned the art of medicine into a science. However, the art of making referrals has not advanced commensurately, as there has been little attention focused on the “referral bridge” between these 2 pillars. As the studies reviewed in this paper demonstrate, a referral bridge deserves exploration in all fields of medicine.
CORRESPONDENCE
David R. Kraus, PhD, 1 Speen Street, Framingham, MA 01701; dkraus@outcomereferrals.com
1. HHS. 2021 National Survey of Drug Use and Health (NSDUH) Releases. Accessed March 29, 2023. www.samhsa.gov/data/release/2021-national-survey-drug-use-and-health-nsduh-releases
2. Barkham M, Lambert, MJ. The efficacy and effectiveness of psychological therapies. In: Barkham M, Lutz W, Castonguay LG, eds. Bergin and Garfield’s Handbook of Psychotherapy and Behavior Change: 50th Anniversary Edition. 7th ed. John Wiley & Sons, Inc; 2021:135-189.
3. Lewis CC, Boyd M, Puspitasari A, et al. Implementing measurement-based care in behavioral health: a review. JAMA Psychiatry. 2019;76:324-335. doi: 10.1001/jamapsychiatry.2018.3329
4. de Jong K, Conijn JM, Gallagher RAV, et al. Using progress feedback to improve outcomes and reduce drop-out, treatment duration, and deterioration: a multilevel meta-analysis. Clin Psychol Rev. 2021;85:102002. doi: 10.1016/j.cpr.2021.102002
5. Carlier IVE, Meuldijk D, Van Vliet IM, et al. Routine outcome monitoring and feedback on physical or mental health status: evidence and theory. J Eval Clin Pract. 2012;18:104-110. doi: 10.1111/j.1365-2753.2010.01543.x
6. Muir HJ, Coyne AE, Morrison NR, et al. Ethical implications of routine outcomes monitoring for patients, psychotherapists, and mental health care systems. Psychotherapy (Chic). 2019;56:459-469. doi: 10.1037/pst0000246
7. Hannan C, Lambert MJ, Harmon C, et al. A lab test and algorithms for identifying clients at risk for treatment failure. J Clin Psychol. 2005;61:155-163. doi: 10.1002/jclp.20108
8. Castonguay LG, Boswell JF, Constantino MJ, et al. Training implications of harmful effects of psychological treatments. Am Psychol. 2010;65:34-49. doi: 10.1037/a0017330
9. Kraus DR, Castonguay LG, Boswell JF, et al. Therapist effectiveness: implications for accountability and patient care. Psychother Res. 2011;21:267-276. doi: 10.1080/10503307.2011.563249
10. Lilienfeld SO. Psychological treatments that cause harm. Perspect Psychol Sci. 2007;2:53-70. doi: 10.1111/j.1745-6916.2007.00029.x
11. Boswell JF, Constantino MJ, Kraus DR, et al. The expanding relevance of routinely collected outcome data for mental health care decision making. Adm Policy Ment Health. 2016;43:482-491. doi: 10.1007/s10488-015-0649-6
12. Lyon AR, Lewis CC, Boyd MR, et al. Capabilities and characteristics of digital measurement feedback systems: results from a comprehensive review. Adm Policy Ment Health. 2016;43:441-466. doi: 10.1007/s10488-016-0719-4
13. Teymoori A, Gorbunova A, Haghish FE, et al. Factorial structure and validity of depression (PHQ-9) and anxiety (GAD-7) scales after traumatic brain injury. J Clin Med. 2020;9:873. doi: 10.3390/jcm9030873
14. Kraus DR, Seligman DA, Jordan JR. Validation of a behavioral health treatment outcome and assessment tool designed for naturalistic settings: the Treatment Outcome Package. J Clin Psychol. 2005;61:285‐314. doi: 10.1002/jclp.20084
15. Boothroyd L, Dagnan D, Muncer S. Psychometric analysis of the Generalized Anxiety Disorder Scale and the Patient Health Questionnaire using Mokken scaling and confirmatory factor analysis. Health Prim Care. 2018;2:1-4. doi: 10.15761/HPC.1000145
16. Locke BD, Buzolitz JS, Lei PW, et al. Development of the Counseling Center Assessment of Psychological Symptoms-62 (CCAPS-62). J Couns Psychol. 2011;58:97-109. doi: 10.1037/a0021282
17. Kraus DR, Boswell JF, Wright AGC, et al. Factor structure of the treatment outcome package for children. J Clin Psychol. 2010;66:627-640. doi: 10.1002/jclp.20675
18. McAleavey AA, Nordberg SS, Kraus D, et al. Errors in treatment outcome monitoring: implications for real-world psychotherapy. Can Psychol. 2010;53:105-114. doi: 10.1037/a0027833
19. Baxter EE, Alexander PC, Kraus DR, et al. Concurrent validation of the Treatment Outcome Package (TOP) for children and adolescents. J Child Fam Stud. 2016;25:2415-2422. doi: 10.1007/s10826-016-0419-4
20. Gual-Montolio P, Martínez-Borba V, Bretón-López JM, et al. How are information and communication technologies supporting routine outcome monitoring and measurement-based care in psychotherapy? A systematic review. Int J Environ Res Public Health. 2020;17:3170. doi: 10.3390/ijerph17093170
21. Kraus DR, Bentley JH, Alexander PC, et al. Predicting therapist effectiveness from their own practice-based evidence. J Consult Clin Psychol. 2016;84:473‐483. doi: 10.1037/ccp0000083
22. Constantino MJ, Boswell JF, Coyne AE, et al. Effect of matching therapists to patients vs assignment as usual on adult psychotherapy outcomes. A randomized clinical trial. JAMA Psychiatry. 2021;78:960-969. doi: 10.1001/jamapsychiatry.2021.1221
23. Constantino MJ, Boswell JF, Kraus DR, et al. Matching patients with therapists to improve mental health care. Patient-Centered Outcomes Research Institute (PCORI). 2021. Accessed March 1, 2023. www.pcori.org/research-results/2015/matching-patients-therapists-improve-mental-health-care
24. Institute of Medicine. Committee on Crossing the Quality Chasm: Adaptation to Mental Health and Addictive Disorders. Improving the Quality of Health Care for Mental and Substance-Use Conditions. National Academies Press; 2006. Accessed February 21, 2023. https://nap.nationalacademies.org/read/11470/chapter/1
25. Boswell JF, Constantino MJ, Oswald JM, et al. A multimethod study of mental health care patients’ attitudes toward clinician-level performance information. Psychiatr Serv. 2021;72:452-456. doi: 10.1176/appi.ps.202000366
26. Boswell JF, Constantino MJ, Oswald JM, et al. Mental health care consumers’ relative valuing of clinician performance information. J Consult Clin Psychol. 2018;86:301‐308. doi: 10.1037/ccp0000264
Approximately 1 in 4 people ages 18 years and older and 1 in 3 people ages 18 to 25 years had a mental illness in the past year, according to the 2021 National Survey of Drug Use and Health.1 The survey also found that adults ages 18 to 25 years had the highest rate of serious mental illness but the lowest treatment rate compared to other adult age groups.1 Unfortunately, more than 60% of patients receiving mental health treatment fail to benefit to a clinically meaningful degree.2
However, there is growing evidence that referring patients to behavioral health practitioners (BHPs) with outcome-measured skills that meet the patient’s specific needs can have a dramatic and positive impact. There are 2 main steps to pairing patients with an appropriate BHP: (1) use of measurement-based care data that can be analyzed at the patient and therapist level, and (2) data-driven referrals that pair patients with BHPs based on such routine outcome monitoring data (paired-on outcome data).
Psychotherapy’s slow road toward measurement-based care
Routine outcome monitoring is the systematic measurement of symptoms and functioning during treatment. It serves multiple functions, including program evaluation and benchmarking of patient improvement rates. Moreover, routine outcome monitoring–derived feedback (based on repeated patient outcome measurements) can inform personalized and responsive care decisions throughout treatment.
For all intents and purposes,
- routinely administered symptom/functioning measure, ideally before each clinical encounter,
- practitioner review of these patient-level data,
- patient review of these data with their practitioner, and
- collaborative reevaluation of the person-specific treatment plan informed by these data.
CASE SCENARIO
Violeta W is a 33-year-old woman who presented to her family physician for her annual wellness exam. Prior to the exam, the medical assistant administered a Patient Health Questionnaire-9 (PHQ-9) to screen for depressive symptoms. Ms. W’s score was 20 out of 27, suggestive of depression. To further assess the severity of depressive symptoms and their effect on daily function, the physician reviewed responses to the questionnaire with her and discussed treatment options. Ms. W was most interested in trying a low-dose selective serotonin reuptake inhibitor (SSRI).
At her follow-up visit 4 weeks later, the medical assistant re-administered the PHQ-9. The physician then reviewed Ms. W’s responses with her and, based on Ms. W’s subjective report and objective symptoms (still a score of 20 out of 27 on the PHQ-9), increased her SSRI dose. At each subsequent visit, Ms. W completed a PHQ-9 and reviewed responses and depressive symptoms with her physician.
The value of measurement-based care in mental health care
A narrative review by Lewis et al3 of 21 randomized controlled clinical trials (RCTs) across a range of age groups (eg, adolescents, young adults, adults), disorders (eg, anxiety, mood), and settings (eg, outpatient, inpatient) found that in at least 9 review articles, measurement-based care was associated with significantly improved outcomes vs usual care (ie, treatment without routine outcome monitoring plus feedback). The average increase in treatment effect size was about 30% when treatment was accompanied by measurement-based care.3
Continue to: Moreover, a recent within-patient meta-analysis...
Moreover, a recent within-patient meta-analysis by de Jong et al4 shows that measurement-based care yields a small but significant increase in therapeutic outcomes (d = .15). Use of measurement-based care also is associated with improved communication between the patient and therapist.5 In pharmacotherapy practice, measurement-based care has been shown to predict rapid dose increases and changes in medication, when necessary; faster recovery rates; higher response rates to treatment3; and fewer dropouts.4
Perhaps one of the best-studied benefits of measurement-based mental health care is the ability to predict deterioration in care (ie, patients who are off-track in a way that practitioners often miss without the help of routine outcome monitoring data).6,7 Studies show that without a data-informed approach to care, some forms of psychotherapy or therapy with BHPs who are not sufficiently skilled in treating a given diagnosis increase symptoms or create significant harmful and iatrogenic effects.8-10 Conversely, the meta-analysis by de Jong et al4 found a lower percentage of deterioration in patients receiving measurement-based care. The difference in deterioration was significant: An average of 5.4% of patients in control conditions deteriorated compared to an average of 4.6% in feedback (measurement-based care) groups. There were even larger effect sizes when therapists received training in the feedback system.4
Routine outcome monitoring without a dialogue between patient and practitioner about the assessments (eg, ignoring complete measurement-based care requirements) may be inadequate. A recent review by Muir et al6 found no differences in patient outcomes when data were used solely for aggregate quality improvement activities, suggesting the need for practitioners to review results of routine outcome monitoring assessments with patients and use data to alter care when necessary.
Measurement-based care is believed to deliver benefits and reduce harm by enhancing and encouraging active patient involvement, improving patient understanding of symptoms, promoting better communication, and facilitating better care coordination.3 The benefits of measurement-based care can be enhanced with a comprehensive core routine outcome monitoring tool and the level of monitoring-generated information delivered for multiple stakeholders (eg, patient, therapist, clinic).11
A look at multidimensional assessment
The features of routine outcome monitoring tools vary significantly.12 Some measures assess single-symptom or problem domains (eg, PHQ-9 for depression or Generalized Anxiety Disorder-7 [GAD-7] scale for anxiety) or multiple dimensions (multidimensional routine outcome monitoring).Multidimensional routine outcome monitoring may have benefits over single-domain measures. Single-domain measures and the subscales or factors of more comprehensive multidimensional routine outcome monitoring assessments should possess adequate specificity and sensitivity.
Continue to: Some recent research findings...
Some recent research findings question the construct validity of brief single-domain measures of common presenting problems, such as depression and anxiety. For example, results from a factor analysis of the PHQ-9 and GAD-7 scale in patients with traumatic brain injury suggest these tools measure 1 psychological construct that includes depression and the cognitive components of anxiety (eg, worry)13—a finding consistent with those of other tools.14 Similarly, a larger study of 7763 BH patients found that a single factor accounted for most of the variance of the 2 combined measures, with no set of factors meeting the exacting standards used to develop multidimensional routine outcome monitoring.15 These findings suggest that the PHQ-9 and GAD-7 largely overlap and are not measuring different aspects of health as most practitioners believe (eg, depression and anxiety).
In commonly used assessments, multiple-factor analytic studies with high standards have supported the construct validity of domain-specific subscales, indicating that the various questions tap into different constructs of psychological health.14,16,17
Beyond multiple domain–specific indicators, multidimensional routine outcome measurements provide a global total score that minimizes Type I (false-positive conclusion) and Type II (false-negative conclusion) errors in tracking patient improvement or deterioration.18 As one would expect, multidimensional routine outcome monitoring generally includes more items than single-domain measures; however, this comes with a trade-off. If there are specificity and sensitivity concerns with an ultra-brief single-domain measure, an alternative to a core multidimensional routine outcome measurement is to aggregate a series of single-domain measures into a battery of patient self-reports. However, this approach may take longer for patients to complete since they would have to shift among the varying response sets and wording across the unique single-domain measures.
In addition, the standardization/normalization of multidimensional routine outcome monitoring likely makes interpretation easier than referring to norms and clinical severity cutoffs for many distinct measures. Furthermore, increased specificity enhances predictive power and allows BHPs to screen and track other conditions besides depression and anxiety. (It is worth noting that there are no known studies that have looked at the difference in time to administer or ease of interpretation of multidimensional routine outcome monitoring tools vs multiple single-domain measures.)
Two multidimensional routine outcome monitoring tools that cover a comprehensive series of discrete symptom and functional domains are the Treatment Outcome Package12 and Counseling Center Assessment of Psychological Symptoms.16 These tools, which include subscales beyond general depression and anxiety (eg, sleep, substance misuse, social conflict), take 7 to 10 minutes to complete and provide outcome results across 12 symptom and 8 functional dimensions. As an example, the Treatment Outcome Package has good psychometric qualities (eg, reliability, construct and concurrent validity) for adults,12 children,14,19 and adolescents,19 and can be administered through a secure online data collection portal. The Counseling Center Assessment of Psychological Symptoms has demonstrated high construct validity and good convergent validity.16 These assessments can be administered in paper or digital (eg, electronic medical record portal, smartphone) format.20
Continue to: CASE SCENARIO
CASE SCENARIO
Ms. W’s physician asked her to go online using her phone and answer the questions in the Treatment Outcome Package. Her results, which she viewed with her physician, were displayed in graph form (FIGURE). Her scores were represented in Z scores normalized to the general population, with “0” representing the general, nontreatment-seeking population average and positive scores representing the number of standard deviations (SDs) more severe than the general population average.

Although this assessment scored Ms. W’s clinically elevated depression as mild, it revealed abnormalities in 3 other domains. Sexual functioning issues represented the most abnormal domain at greater than 3 SDs (more severe than the general population), followed by poor life quality and school/work functioning.
After reviewing Ms. W’s report, her physician decided that pharmacologic management alone (for depression) was not the most appropriate treatment course. Therefore, her physician recommended psychotherapy in addition to the SSRI she was taking. Ms. W agreed to a customized referral for psychotherapy.
Data-driven referrals
When psychotherapy is chosen as a treatment, the individual BHP is an active component of that treatment. Consequently, it is essential to customize referrals to match a patient’s most elevated mental health concerns with a therapist who will most effectively treat those domains. It is rare for a BHP to be skilled in treating every mental health domain.9 Multiple studies have shown that BHPs have identifiable treatment skills in specific domains, which physicians should consider when making referrals.9,21,22 These studies demonstrate the utility of aggregating patient-level routine outcome monitoring data to better understand therapist-level (and ultimately clinic- and system-level) outcomes.
Additionally, recent research has tested this idea prospectively. An RCT funded by the Patient-Centered Outcome Research Institute and published in JAMA Psychiatry showed a significant and positive effect on patient outcomes (ie, reductions in general impairment, impairment involving a patient’s most elevated domain, and global distress) using paired-on outcome data matching vs as-usual matching protocols (eg, therapist self-defined areas of specialty).22 In the RCT, the most effective matching protocol was a combination of eliminating harm and matching the patient on their 3 most problematic domains (the highest match level). These patients ended care as healthy as the general population after 16 weeks of treatment. A random 1-year follow-up assessment from the original RCT showed that most patients who had been matched had maintained their improvement.23
Continue to: Therefore, a multidimensional routine outcome...
Therefore, a multidimensional routine outcome monitoring tool can be used to identify a BHP’s relative strengths and weaknesses across multiple outcome domains. Within a system of care, a sample of BHPs will possess varying outcome-domain profiles. When a new patient is seeking a referral to a BHP, these profiles (or domain-specific outcome track records) can be used to support paired-on outcome data matching. Specifically, a new patient completes the multidimensional routine outcome monitoring tool at pretreatment, and the results reveal the outcome domains on which the patient is most clinically severe. This pattern of domain-specific severity then can be used to pair the new patient with a BHP who has demonstrated success in addressing the same outcome domain(s). This approach matches a new patient to a BHP with established expertise based on routine outcome monitoring.
Retrospective and prospective studies have found that most BHPs have stable performance in their strengths and weaknesses.11,21 One study found that assessing BHP performance with their most recent 30 patients can reliably predict future performance with their next 30 patients.24 This predictability in a practitioner’s outcomes suggests report cards that are updated frequently can be utilized to make case assignments within BH or referrals to a specific BHP from primary care.
Making a paired-on outcome data–matched referral
Making customized BH referrals requires access to information about a practitioner’s previous routine outcome monitoring data per clinical domain (eg, suicidality, violence, quality of life) from their most recent patients. Previous research suggests that follow-up data from a minimum of 15 patients is necessary to make a reliable evaluation of a practitioner’s strengths and weaknesses (ie, effectiveness “report card”) per clinical domain.24
Few, if any, physicians have access to this level of updated outcome data from their referral network. To facilitate widespread use of paired-on outcome data matching, a new Web system (MatchedTherapists.com) will allow the general public and PCPs to access these grades. As a public service option, this site currently allows for a self-assessment using the Treatment Outcome Package. Pending versions will generate paired-on outcome data grades, and users will receive a list of local therapists available for in-person appointments as well as therapists available for virtual appointments. The paired-on outcome data grades are delivered in school-based letter grades. An “A+,” for example, represents the best matching grade. Users also will be able to sort and filter results for other criteria such as telemedicine, insurance, age, gender, and appointment availability. Currently, there are more than 77,000 therapists listed on the site nationwide. A basic listing is free.
CASE SCENARIO
After Ms. W took the multidimensional routine outcome assessment online, she received a list of therapists rank-ordered by paired-on outcome data grade, with the “A+” matches listed first. Three of the best-matched referrals accepted her insurance and were willing to see her through telemedicine. Therapists with available in-person appointments had a “B” grade. After discussing the options with her physician, Ms. W opted for telehealth counseling with the therapist whose profile she liked best. The therapist and PCP tracked her progress through routine outcome monitoring reporting until all her symptoms became subclinical.
Continue to: The future of a "referral bridge"
The future of a “referral bridge”
In this article, we present a solution to a common issue faced by mental health care patients: failure to benefit meaningfully from mental health treatment. Matching patients to specific BHPs based on effectiveness data regarding the therapist’s strengths and skills can improve patient outcomes and reduce harm. In addition, patients appear to value this approach. A Robert Wood Johnson Foundation–funded study demonstrated that patients value seeing practitioners who have a track record of successfully treating previous patients with similar issues.25,26 In many cases, patients indicated they would prioritize this matching process over other factors such as practitioners with a higher number of years of experience or the same demographic characteristics as the patient.25,26
These findings may represent a new area in the science of health care. Over the past century, major advances in diagnosis and treatment—the 2 primary pillars of health care—have turned the art of medicine into a science. However, the art of making referrals has not advanced commensurately, as there has been little attention focused on the “referral bridge” between these 2 pillars. As the studies reviewed in this paper demonstrate, a referral bridge deserves exploration in all fields of medicine.
CORRESPONDENCE
David R. Kraus, PhD, 1 Speen Street, Framingham, MA 01701; dkraus@outcomereferrals.com
Approximately 1 in 4 people ages 18 years and older and 1 in 3 people ages 18 to 25 years had a mental illness in the past year, according to the 2021 National Survey of Drug Use and Health.1 The survey also found that adults ages 18 to 25 years had the highest rate of serious mental illness but the lowest treatment rate compared to other adult age groups.1 Unfortunately, more than 60% of patients receiving mental health treatment fail to benefit to a clinically meaningful degree.2
However, there is growing evidence that referring patients to behavioral health practitioners (BHPs) with outcome-measured skills that meet the patient’s specific needs can have a dramatic and positive impact. There are 2 main steps to pairing patients with an appropriate BHP: (1) use of measurement-based care data that can be analyzed at the patient and therapist level, and (2) data-driven referrals that pair patients with BHPs based on such routine outcome monitoring data (paired-on outcome data).
Psychotherapy’s slow road toward measurement-based care
Routine outcome monitoring is the systematic measurement of symptoms and functioning during treatment. It serves multiple functions, including program evaluation and benchmarking of patient improvement rates. Moreover, routine outcome monitoring–derived feedback (based on repeated patient outcome measurements) can inform personalized and responsive care decisions throughout treatment.
For all intents and purposes,
- routinely administered symptom/functioning measure, ideally before each clinical encounter,
- practitioner review of these patient-level data,
- patient review of these data with their practitioner, and
- collaborative reevaluation of the person-specific treatment plan informed by these data.
CASE SCENARIO
Violeta W is a 33-year-old woman who presented to her family physician for her annual wellness exam. Prior to the exam, the medical assistant administered a Patient Health Questionnaire-9 (PHQ-9) to screen for depressive symptoms. Ms. W’s score was 20 out of 27, suggestive of depression. To further assess the severity of depressive symptoms and their effect on daily function, the physician reviewed responses to the questionnaire with her and discussed treatment options. Ms. W was most interested in trying a low-dose selective serotonin reuptake inhibitor (SSRI).
At her follow-up visit 4 weeks later, the medical assistant re-administered the PHQ-9. The physician then reviewed Ms. W’s responses with her and, based on Ms. W’s subjective report and objective symptoms (still a score of 20 out of 27 on the PHQ-9), increased her SSRI dose. At each subsequent visit, Ms. W completed a PHQ-9 and reviewed responses and depressive symptoms with her physician.
The value of measurement-based care in mental health care
A narrative review by Lewis et al3 of 21 randomized controlled clinical trials (RCTs) across a range of age groups (eg, adolescents, young adults, adults), disorders (eg, anxiety, mood), and settings (eg, outpatient, inpatient) found that in at least 9 review articles, measurement-based care was associated with significantly improved outcomes vs usual care (ie, treatment without routine outcome monitoring plus feedback). The average increase in treatment effect size was about 30% when treatment was accompanied by measurement-based care.3
Continue to: Moreover, a recent within-patient meta-analysis...
Moreover, a recent within-patient meta-analysis by de Jong et al4 shows that measurement-based care yields a small but significant increase in therapeutic outcomes (d = .15). Use of measurement-based care also is associated with improved communication between the patient and therapist.5 In pharmacotherapy practice, measurement-based care has been shown to predict rapid dose increases and changes in medication, when necessary; faster recovery rates; higher response rates to treatment3; and fewer dropouts.4
Perhaps one of the best-studied benefits of measurement-based mental health care is the ability to predict deterioration in care (ie, patients who are off-track in a way that practitioners often miss without the help of routine outcome monitoring data).6,7 Studies show that without a data-informed approach to care, some forms of psychotherapy or therapy with BHPs who are not sufficiently skilled in treating a given diagnosis increase symptoms or create significant harmful and iatrogenic effects.8-10 Conversely, the meta-analysis by de Jong et al4 found a lower percentage of deterioration in patients receiving measurement-based care. The difference in deterioration was significant: An average of 5.4% of patients in control conditions deteriorated compared to an average of 4.6% in feedback (measurement-based care) groups. There were even larger effect sizes when therapists received training in the feedback system.4
Routine outcome monitoring without a dialogue between patient and practitioner about the assessments (eg, ignoring complete measurement-based care requirements) may be inadequate. A recent review by Muir et al6 found no differences in patient outcomes when data were used solely for aggregate quality improvement activities, suggesting the need for practitioners to review results of routine outcome monitoring assessments with patients and use data to alter care when necessary.
Measurement-based care is believed to deliver benefits and reduce harm by enhancing and encouraging active patient involvement, improving patient understanding of symptoms, promoting better communication, and facilitating better care coordination.3 The benefits of measurement-based care can be enhanced with a comprehensive core routine outcome monitoring tool and the level of monitoring-generated information delivered for multiple stakeholders (eg, patient, therapist, clinic).11
A look at multidimensional assessment
The features of routine outcome monitoring tools vary significantly.12 Some measures assess single-symptom or problem domains (eg, PHQ-9 for depression or Generalized Anxiety Disorder-7 [GAD-7] scale for anxiety) or multiple dimensions (multidimensional routine outcome monitoring).Multidimensional routine outcome monitoring may have benefits over single-domain measures. Single-domain measures and the subscales or factors of more comprehensive multidimensional routine outcome monitoring assessments should possess adequate specificity and sensitivity.
Continue to: Some recent research findings...
Some recent research findings question the construct validity of brief single-domain measures of common presenting problems, such as depression and anxiety. For example, results from a factor analysis of the PHQ-9 and GAD-7 scale in patients with traumatic brain injury suggest these tools measure 1 psychological construct that includes depression and the cognitive components of anxiety (eg, worry)13—a finding consistent with those of other tools.14 Similarly, a larger study of 7763 BH patients found that a single factor accounted for most of the variance of the 2 combined measures, with no set of factors meeting the exacting standards used to develop multidimensional routine outcome monitoring.15 These findings suggest that the PHQ-9 and GAD-7 largely overlap and are not measuring different aspects of health as most practitioners believe (eg, depression and anxiety).
In commonly used assessments, multiple-factor analytic studies with high standards have supported the construct validity of domain-specific subscales, indicating that the various questions tap into different constructs of psychological health.14,16,17
Beyond multiple domain–specific indicators, multidimensional routine outcome measurements provide a global total score that minimizes Type I (false-positive conclusion) and Type II (false-negative conclusion) errors in tracking patient improvement or deterioration.18 As one would expect, multidimensional routine outcome monitoring generally includes more items than single-domain measures; however, this comes with a trade-off. If there are specificity and sensitivity concerns with an ultra-brief single-domain measure, an alternative to a core multidimensional routine outcome measurement is to aggregate a series of single-domain measures into a battery of patient self-reports. However, this approach may take longer for patients to complete since they would have to shift among the varying response sets and wording across the unique single-domain measures.
In addition, the standardization/normalization of multidimensional routine outcome monitoring likely makes interpretation easier than referring to norms and clinical severity cutoffs for many distinct measures. Furthermore, increased specificity enhances predictive power and allows BHPs to screen and track other conditions besides depression and anxiety. (It is worth noting that there are no known studies that have looked at the difference in time to administer or ease of interpretation of multidimensional routine outcome monitoring tools vs multiple single-domain measures.)
Two multidimensional routine outcome monitoring tools that cover a comprehensive series of discrete symptom and functional domains are the Treatment Outcome Package12 and Counseling Center Assessment of Psychological Symptoms.16 These tools, which include subscales beyond general depression and anxiety (eg, sleep, substance misuse, social conflict), take 7 to 10 minutes to complete and provide outcome results across 12 symptom and 8 functional dimensions. As an example, the Treatment Outcome Package has good psychometric qualities (eg, reliability, construct and concurrent validity) for adults,12 children,14,19 and adolescents,19 and can be administered through a secure online data collection portal. The Counseling Center Assessment of Psychological Symptoms has demonstrated high construct validity and good convergent validity.16 These assessments can be administered in paper or digital (eg, electronic medical record portal, smartphone) format.20
Continue to: CASE SCENARIO
CASE SCENARIO
Ms. W’s physician asked her to go online using her phone and answer the questions in the Treatment Outcome Package. Her results, which she viewed with her physician, were displayed in graph form (FIGURE). Her scores were represented in Z scores normalized to the general population, with “0” representing the general, nontreatment-seeking population average and positive scores representing the number of standard deviations (SDs) more severe than the general population average.

Although this assessment scored Ms. W’s clinically elevated depression as mild, it revealed abnormalities in 3 other domains. Sexual functioning issues represented the most abnormal domain at greater than 3 SDs (more severe than the general population), followed by poor life quality and school/work functioning.
After reviewing Ms. W’s report, her physician decided that pharmacologic management alone (for depression) was not the most appropriate treatment course. Therefore, her physician recommended psychotherapy in addition to the SSRI she was taking. Ms. W agreed to a customized referral for psychotherapy.
Data-driven referrals
When psychotherapy is chosen as a treatment, the individual BHP is an active component of that treatment. Consequently, it is essential to customize referrals to match a patient’s most elevated mental health concerns with a therapist who will most effectively treat those domains. It is rare for a BHP to be skilled in treating every mental health domain.9 Multiple studies have shown that BHPs have identifiable treatment skills in specific domains, which physicians should consider when making referrals.9,21,22 These studies demonstrate the utility of aggregating patient-level routine outcome monitoring data to better understand therapist-level (and ultimately clinic- and system-level) outcomes.
Additionally, recent research has tested this idea prospectively. An RCT funded by the Patient-Centered Outcome Research Institute and published in JAMA Psychiatry showed a significant and positive effect on patient outcomes (ie, reductions in general impairment, impairment involving a patient’s most elevated domain, and global distress) using paired-on outcome data matching vs as-usual matching protocols (eg, therapist self-defined areas of specialty).22 In the RCT, the most effective matching protocol was a combination of eliminating harm and matching the patient on their 3 most problematic domains (the highest match level). These patients ended care as healthy as the general population after 16 weeks of treatment. A random 1-year follow-up assessment from the original RCT showed that most patients who had been matched had maintained their improvement.23
Continue to: Therefore, a multidimensional routine outcome...
Therefore, a multidimensional routine outcome monitoring tool can be used to identify a BHP’s relative strengths and weaknesses across multiple outcome domains. Within a system of care, a sample of BHPs will possess varying outcome-domain profiles. When a new patient is seeking a referral to a BHP, these profiles (or domain-specific outcome track records) can be used to support paired-on outcome data matching. Specifically, a new patient completes the multidimensional routine outcome monitoring tool at pretreatment, and the results reveal the outcome domains on which the patient is most clinically severe. This pattern of domain-specific severity then can be used to pair the new patient with a BHP who has demonstrated success in addressing the same outcome domain(s). This approach matches a new patient to a BHP with established expertise based on routine outcome monitoring.
Retrospective and prospective studies have found that most BHPs have stable performance in their strengths and weaknesses.11,21 One study found that assessing BHP performance with their most recent 30 patients can reliably predict future performance with their next 30 patients.24 This predictability in a practitioner’s outcomes suggests report cards that are updated frequently can be utilized to make case assignments within BH or referrals to a specific BHP from primary care.
Making a paired-on outcome data–matched referral
Making customized BH referrals requires access to information about a practitioner’s previous routine outcome monitoring data per clinical domain (eg, suicidality, violence, quality of life) from their most recent patients. Previous research suggests that follow-up data from a minimum of 15 patients is necessary to make a reliable evaluation of a practitioner’s strengths and weaknesses (ie, effectiveness “report card”) per clinical domain.24
Few, if any, physicians have access to this level of updated outcome data from their referral network. To facilitate widespread use of paired-on outcome data matching, a new Web system (MatchedTherapists.com) will allow the general public and PCPs to access these grades. As a public service option, this site currently allows for a self-assessment using the Treatment Outcome Package. Pending versions will generate paired-on outcome data grades, and users will receive a list of local therapists available for in-person appointments as well as therapists available for virtual appointments. The paired-on outcome data grades are delivered in school-based letter grades. An “A+,” for example, represents the best matching grade. Users also will be able to sort and filter results for other criteria such as telemedicine, insurance, age, gender, and appointment availability. Currently, there are more than 77,000 therapists listed on the site nationwide. A basic listing is free.
CASE SCENARIO
After Ms. W took the multidimensional routine outcome assessment online, she received a list of therapists rank-ordered by paired-on outcome data grade, with the “A+” matches listed first. Three of the best-matched referrals accepted her insurance and were willing to see her through telemedicine. Therapists with available in-person appointments had a “B” grade. After discussing the options with her physician, Ms. W opted for telehealth counseling with the therapist whose profile she liked best. The therapist and PCP tracked her progress through routine outcome monitoring reporting until all her symptoms became subclinical.
Continue to: The future of a "referral bridge"
The future of a “referral bridge”
In this article, we present a solution to a common issue faced by mental health care patients: failure to benefit meaningfully from mental health treatment. Matching patients to specific BHPs based on effectiveness data regarding the therapist’s strengths and skills can improve patient outcomes and reduce harm. In addition, patients appear to value this approach. A Robert Wood Johnson Foundation–funded study demonstrated that patients value seeing practitioners who have a track record of successfully treating previous patients with similar issues.25,26 In many cases, patients indicated they would prioritize this matching process over other factors such as practitioners with a higher number of years of experience or the same demographic characteristics as the patient.25,26
These findings may represent a new area in the science of health care. Over the past century, major advances in diagnosis and treatment—the 2 primary pillars of health care—have turned the art of medicine into a science. However, the art of making referrals has not advanced commensurately, as there has been little attention focused on the “referral bridge” between these 2 pillars. As the studies reviewed in this paper demonstrate, a referral bridge deserves exploration in all fields of medicine.
CORRESPONDENCE
David R. Kraus, PhD, 1 Speen Street, Framingham, MA 01701; dkraus@outcomereferrals.com
1. HHS. 2021 National Survey of Drug Use and Health (NSDUH) Releases. Accessed March 29, 2023. www.samhsa.gov/data/release/2021-national-survey-drug-use-and-health-nsduh-releases
2. Barkham M, Lambert, MJ. The efficacy and effectiveness of psychological therapies. In: Barkham M, Lutz W, Castonguay LG, eds. Bergin and Garfield’s Handbook of Psychotherapy and Behavior Change: 50th Anniversary Edition. 7th ed. John Wiley & Sons, Inc; 2021:135-189.
3. Lewis CC, Boyd M, Puspitasari A, et al. Implementing measurement-based care in behavioral health: a review. JAMA Psychiatry. 2019;76:324-335. doi: 10.1001/jamapsychiatry.2018.3329
4. de Jong K, Conijn JM, Gallagher RAV, et al. Using progress feedback to improve outcomes and reduce drop-out, treatment duration, and deterioration: a multilevel meta-analysis. Clin Psychol Rev. 2021;85:102002. doi: 10.1016/j.cpr.2021.102002
5. Carlier IVE, Meuldijk D, Van Vliet IM, et al. Routine outcome monitoring and feedback on physical or mental health status: evidence and theory. J Eval Clin Pract. 2012;18:104-110. doi: 10.1111/j.1365-2753.2010.01543.x
6. Muir HJ, Coyne AE, Morrison NR, et al. Ethical implications of routine outcomes monitoring for patients, psychotherapists, and mental health care systems. Psychotherapy (Chic). 2019;56:459-469. doi: 10.1037/pst0000246
7. Hannan C, Lambert MJ, Harmon C, et al. A lab test and algorithms for identifying clients at risk for treatment failure. J Clin Psychol. 2005;61:155-163. doi: 10.1002/jclp.20108
8. Castonguay LG, Boswell JF, Constantino MJ, et al. Training implications of harmful effects of psychological treatments. Am Psychol. 2010;65:34-49. doi: 10.1037/a0017330
9. Kraus DR, Castonguay LG, Boswell JF, et al. Therapist effectiveness: implications for accountability and patient care. Psychother Res. 2011;21:267-276. doi: 10.1080/10503307.2011.563249
10. Lilienfeld SO. Psychological treatments that cause harm. Perspect Psychol Sci. 2007;2:53-70. doi: 10.1111/j.1745-6916.2007.00029.x
11. Boswell JF, Constantino MJ, Kraus DR, et al. The expanding relevance of routinely collected outcome data for mental health care decision making. Adm Policy Ment Health. 2016;43:482-491. doi: 10.1007/s10488-015-0649-6
12. Lyon AR, Lewis CC, Boyd MR, et al. Capabilities and characteristics of digital measurement feedback systems: results from a comprehensive review. Adm Policy Ment Health. 2016;43:441-466. doi: 10.1007/s10488-016-0719-4
13. Teymoori A, Gorbunova A, Haghish FE, et al. Factorial structure and validity of depression (PHQ-9) and anxiety (GAD-7) scales after traumatic brain injury. J Clin Med. 2020;9:873. doi: 10.3390/jcm9030873
14. Kraus DR, Seligman DA, Jordan JR. Validation of a behavioral health treatment outcome and assessment tool designed for naturalistic settings: the Treatment Outcome Package. J Clin Psychol. 2005;61:285‐314. doi: 10.1002/jclp.20084
15. Boothroyd L, Dagnan D, Muncer S. Psychometric analysis of the Generalized Anxiety Disorder Scale and the Patient Health Questionnaire using Mokken scaling and confirmatory factor analysis. Health Prim Care. 2018;2:1-4. doi: 10.15761/HPC.1000145
16. Locke BD, Buzolitz JS, Lei PW, et al. Development of the Counseling Center Assessment of Psychological Symptoms-62 (CCAPS-62). J Couns Psychol. 2011;58:97-109. doi: 10.1037/a0021282
17. Kraus DR, Boswell JF, Wright AGC, et al. Factor structure of the treatment outcome package for children. J Clin Psychol. 2010;66:627-640. doi: 10.1002/jclp.20675
18. McAleavey AA, Nordberg SS, Kraus D, et al. Errors in treatment outcome monitoring: implications for real-world psychotherapy. Can Psychol. 2010;53:105-114. doi: 10.1037/a0027833
19. Baxter EE, Alexander PC, Kraus DR, et al. Concurrent validation of the Treatment Outcome Package (TOP) for children and adolescents. J Child Fam Stud. 2016;25:2415-2422. doi: 10.1007/s10826-016-0419-4
20. Gual-Montolio P, Martínez-Borba V, Bretón-López JM, et al. How are information and communication technologies supporting routine outcome monitoring and measurement-based care in psychotherapy? A systematic review. Int J Environ Res Public Health. 2020;17:3170. doi: 10.3390/ijerph17093170
21. Kraus DR, Bentley JH, Alexander PC, et al. Predicting therapist effectiveness from their own practice-based evidence. J Consult Clin Psychol. 2016;84:473‐483. doi: 10.1037/ccp0000083
22. Constantino MJ, Boswell JF, Coyne AE, et al. Effect of matching therapists to patients vs assignment as usual on adult psychotherapy outcomes. A randomized clinical trial. JAMA Psychiatry. 2021;78:960-969. doi: 10.1001/jamapsychiatry.2021.1221
23. Constantino MJ, Boswell JF, Kraus DR, et al. Matching patients with therapists to improve mental health care. Patient-Centered Outcomes Research Institute (PCORI). 2021. Accessed March 1, 2023. www.pcori.org/research-results/2015/matching-patients-therapists-improve-mental-health-care
24. Institute of Medicine. Committee on Crossing the Quality Chasm: Adaptation to Mental Health and Addictive Disorders. Improving the Quality of Health Care for Mental and Substance-Use Conditions. National Academies Press; 2006. Accessed February 21, 2023. https://nap.nationalacademies.org/read/11470/chapter/1
25. Boswell JF, Constantino MJ, Oswald JM, et al. A multimethod study of mental health care patients’ attitudes toward clinician-level performance information. Psychiatr Serv. 2021;72:452-456. doi: 10.1176/appi.ps.202000366
26. Boswell JF, Constantino MJ, Oswald JM, et al. Mental health care consumers’ relative valuing of clinician performance information. J Consult Clin Psychol. 2018;86:301‐308. doi: 10.1037/ccp0000264
1. HHS. 2021 National Survey of Drug Use and Health (NSDUH) Releases. Accessed March 29, 2023. www.samhsa.gov/data/release/2021-national-survey-drug-use-and-health-nsduh-releases
2. Barkham M, Lambert, MJ. The efficacy and effectiveness of psychological therapies. In: Barkham M, Lutz W, Castonguay LG, eds. Bergin and Garfield’s Handbook of Psychotherapy and Behavior Change: 50th Anniversary Edition. 7th ed. John Wiley & Sons, Inc; 2021:135-189.
3. Lewis CC, Boyd M, Puspitasari A, et al. Implementing measurement-based care in behavioral health: a review. JAMA Psychiatry. 2019;76:324-335. doi: 10.1001/jamapsychiatry.2018.3329
4. de Jong K, Conijn JM, Gallagher RAV, et al. Using progress feedback to improve outcomes and reduce drop-out, treatment duration, and deterioration: a multilevel meta-analysis. Clin Psychol Rev. 2021;85:102002. doi: 10.1016/j.cpr.2021.102002
5. Carlier IVE, Meuldijk D, Van Vliet IM, et al. Routine outcome monitoring and feedback on physical or mental health status: evidence and theory. J Eval Clin Pract. 2012;18:104-110. doi: 10.1111/j.1365-2753.2010.01543.x
6. Muir HJ, Coyne AE, Morrison NR, et al. Ethical implications of routine outcomes monitoring for patients, psychotherapists, and mental health care systems. Psychotherapy (Chic). 2019;56:459-469. doi: 10.1037/pst0000246
7. Hannan C, Lambert MJ, Harmon C, et al. A lab test and algorithms for identifying clients at risk for treatment failure. J Clin Psychol. 2005;61:155-163. doi: 10.1002/jclp.20108
8. Castonguay LG, Boswell JF, Constantino MJ, et al. Training implications of harmful effects of psychological treatments. Am Psychol. 2010;65:34-49. doi: 10.1037/a0017330
9. Kraus DR, Castonguay LG, Boswell JF, et al. Therapist effectiveness: implications for accountability and patient care. Psychother Res. 2011;21:267-276. doi: 10.1080/10503307.2011.563249
10. Lilienfeld SO. Psychological treatments that cause harm. Perspect Psychol Sci. 2007;2:53-70. doi: 10.1111/j.1745-6916.2007.00029.x
11. Boswell JF, Constantino MJ, Kraus DR, et al. The expanding relevance of routinely collected outcome data for mental health care decision making. Adm Policy Ment Health. 2016;43:482-491. doi: 10.1007/s10488-015-0649-6
12. Lyon AR, Lewis CC, Boyd MR, et al. Capabilities and characteristics of digital measurement feedback systems: results from a comprehensive review. Adm Policy Ment Health. 2016;43:441-466. doi: 10.1007/s10488-016-0719-4
13. Teymoori A, Gorbunova A, Haghish FE, et al. Factorial structure and validity of depression (PHQ-9) and anxiety (GAD-7) scales after traumatic brain injury. J Clin Med. 2020;9:873. doi: 10.3390/jcm9030873
14. Kraus DR, Seligman DA, Jordan JR. Validation of a behavioral health treatment outcome and assessment tool designed for naturalistic settings: the Treatment Outcome Package. J Clin Psychol. 2005;61:285‐314. doi: 10.1002/jclp.20084
15. Boothroyd L, Dagnan D, Muncer S. Psychometric analysis of the Generalized Anxiety Disorder Scale and the Patient Health Questionnaire using Mokken scaling and confirmatory factor analysis. Health Prim Care. 2018;2:1-4. doi: 10.15761/HPC.1000145
16. Locke BD, Buzolitz JS, Lei PW, et al. Development of the Counseling Center Assessment of Psychological Symptoms-62 (CCAPS-62). J Couns Psychol. 2011;58:97-109. doi: 10.1037/a0021282
17. Kraus DR, Boswell JF, Wright AGC, et al. Factor structure of the treatment outcome package for children. J Clin Psychol. 2010;66:627-640. doi: 10.1002/jclp.20675
18. McAleavey AA, Nordberg SS, Kraus D, et al. Errors in treatment outcome monitoring: implications for real-world psychotherapy. Can Psychol. 2010;53:105-114. doi: 10.1037/a0027833
19. Baxter EE, Alexander PC, Kraus DR, et al. Concurrent validation of the Treatment Outcome Package (TOP) for children and adolescents. J Child Fam Stud. 2016;25:2415-2422. doi: 10.1007/s10826-016-0419-4
20. Gual-Montolio P, Martínez-Borba V, Bretón-López JM, et al. How are information and communication technologies supporting routine outcome monitoring and measurement-based care in psychotherapy? A systematic review. Int J Environ Res Public Health. 2020;17:3170. doi: 10.3390/ijerph17093170
21. Kraus DR, Bentley JH, Alexander PC, et al. Predicting therapist effectiveness from their own practice-based evidence. J Consult Clin Psychol. 2016;84:473‐483. doi: 10.1037/ccp0000083
22. Constantino MJ, Boswell JF, Coyne AE, et al. Effect of matching therapists to patients vs assignment as usual on adult psychotherapy outcomes. A randomized clinical trial. JAMA Psychiatry. 2021;78:960-969. doi: 10.1001/jamapsychiatry.2021.1221
23. Constantino MJ, Boswell JF, Kraus DR, et al. Matching patients with therapists to improve mental health care. Patient-Centered Outcomes Research Institute (PCORI). 2021. Accessed March 1, 2023. www.pcori.org/research-results/2015/matching-patients-therapists-improve-mental-health-care
24. Institute of Medicine. Committee on Crossing the Quality Chasm: Adaptation to Mental Health and Addictive Disorders. Improving the Quality of Health Care for Mental and Substance-Use Conditions. National Academies Press; 2006. Accessed February 21, 2023. https://nap.nationalacademies.org/read/11470/chapter/1
25. Boswell JF, Constantino MJ, Oswald JM, et al. A multimethod study of mental health care patients’ attitudes toward clinician-level performance information. Psychiatr Serv. 2021;72:452-456. doi: 10.1176/appi.ps.202000366
26. Boswell JF, Constantino MJ, Oswald JM, et al. Mental health care consumers’ relative valuing of clinician performance information. J Consult Clin Psychol. 2018;86:301‐308. doi: 10.1037/ccp0000264
Subclinical hypothyroidism: Let the evidence be your guide
Subclinical hypothyroidism (SCH) is a biochemical state in which the thyroid-stimulating hormone (TSH) is elevated while the free thyroxine (T4) level is normal. Overt hypothyroidism is not diagnosed until the free T4 level is decreased, regardless of the degree of TSH elevation.
The overall prevalence of SCH in iodine-rich areas is 4% to 10%, with a risk for progression to overt hypothyroidism of between 2% and 6% annually.1 The prevalence of SCH varies depending on the TSH reference range used.1 The normal reference range for TSH varies depending on the laboratory and/or the reference population surveyed, with the range likely widening with increasing age.
SCH is most common among women, the elderly, and White individuals.2 The discovery of SCH is often incidental, given that usually it is detected by laboratory findings alone without associated symptoms of overt hypothyroidism.3
The not-so-significant role of symptoms in subclinical hypothyroidism
Symptoms associated with overt hypothyroidism include constipation, dry skin, fatigue, slow thinking, poor memory, muscle cramps, weakness, and cold intolerance. In SCH, these symptoms are inconsistent, with around 1 in 3 patients having no symptoms
One study reported that roughly 18% of euthyroid individuals, 22% of SCH patients, and 26% of those with overt hypothyroidism reported 4 or more symptoms classically thought to be related to hypothyroidism.4 A large Danish cohort study found that hypothyroid symptoms were no more common in patients with SCH than in euthyroid individuals in the general population.5 These studies question the validity of attributing symptoms to SCH.
Adverse health associations
Observational data suggest that SCH is associated with an increased risk for dyslipidemia, coronary heart disease, heart failure, and cardiovascular mortality, particularly in those with TSH levels ≥ 10 mIU/L.6,7 Such associations were not found for most adults with TSH levels between 5 and 10 mIU/L.8 There are also potential associations of SCH with obesity, nonalcoholic fatty liver disease, and nonalcoholic steatohepatitis.9,10 Despite thyroid studies being commonly ordered as part of a mental health evaluation, SCH has not been statistically associated with depressive symptoms.11,12
Caveats with laboratory testing
There are several issues to consider when performing a laboratory assessment of thyroid function. TSH levels fluctuate considerably during the day, as TSH secretion has a circadian rhythm. TSH values are 50% higher at night and in the early morning than during the rest of the day.13 TSH values also may rise in response to current illness or stress. Due to this biologic variability, repeat testing to confirm TSH levels is recommended if an initial test result is abnormal.14
Continue to: An exact reference range...
An exact reference range for TSH is not widely agreed upon—although most laboratories regard 4.0 to 5.0 mIU/L as the high-end cutoff for normal. Also, “normal” TSH levels appear to differ by age. Accordingly, some experts have recommended an age-based reference range for TSH levels,15 although this is not implemented widely by laboratories. A TSH level of 6.0 mIU/L (or even higher) may be more appropriate for adults older than 65 years.1
Biotin supplementation has been shown to cause spurious thyroid testing results (TSH, T3, T4) depending on the type of assay used. Therefore, supplements containing biotin should be withheld for several days before assessing thyroid function.16Patients with SCH are often categorized as having TSH levels between 4.5 and 10 mIU/L (around 90% of patients) or levels ≥ 10 mIU/L.8,17 If followed for 5 years, approximately 60% of patients with SCH and TSH levels between 4 and 10 mIU/L will normalize without intervention.18 Normalization is less common in patients with a TSH level greater than 10 mIU/L.18
The risk for progression to overt hypothyroidism also appears to be higher for those with certain risk factors. These include higher baseline TSH levels, presence of thyroid peroxidase antibodies (TPOAbs), or history of neck irradiation or radioactive iodine uptake.1 Other risk factors for eventual thyroid dysfunction include female sex, older age, goiter, and high iodine intake.13
Evidence for treatment varies
Guidelines for the treatment of SCH (TABLE 18,14,19,20) are founded on the condition’s risk for progression to overt hypothyroidism and its association with health consequences such as cardiovascular disease. Guidelines of the American Thyroid Association (ATA) and European Thyroid Association (ETA), and those of the United Kingdom–based National Institute for Health and Care Excellence (NICE), prioritize treatment for individuals with a TSH level > 10 mIU/La and for those with
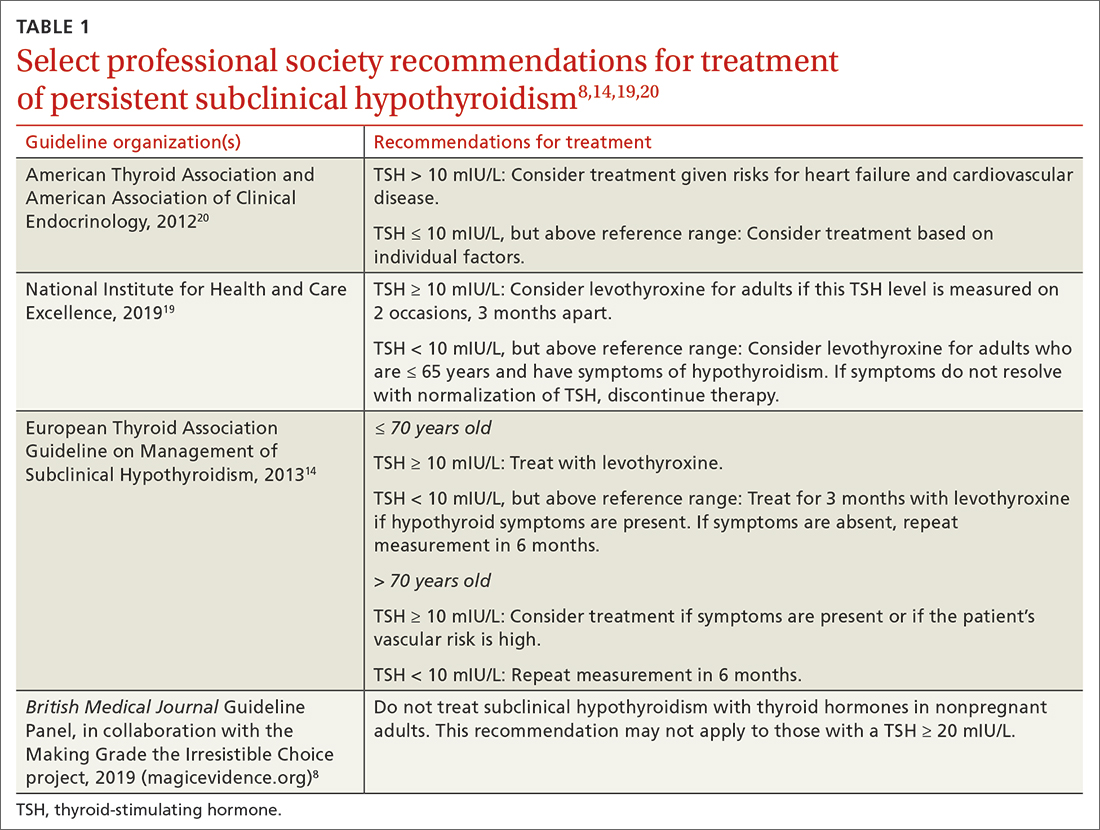
There are few large RCTs of treatment outcomes for SCH. A 2017 RCT (the Thyroid Hormone Replacement for Untreated Older Adults with Subclinical Hypothyroidism, or TRUST, trial) of 737 adults older than 65 years with SCH evaluated the ability of levothyroxine to normalize TSH values compared with placebo. At 1 year, there was no difference in hypothyroid symptoms or tiredness scale scores with levothyroxine treatment compared with placebo.21 This finding was consistent even in the subgroup with a higher baseline symptom burden.22
Continue to: Two small RCTs evaluated...
Two small RCTs evaluated treatment of SCH with depressive symptoms and cognitive function, neither finding benefit compared with placebo.12,23 A 2018 systematic review and meta-analysis of 21 studies and 2192 adults did not show a benefit to quality of life or thyroid-specific symptoms in those treated for SCH compared with controls.24
RCT support also is lacking for a reduction in cardiovascular mortality following treatment for SCH. A large population-level retrospective cohort from Denmark showed no difference in cardiovascular mortality or myocardial infarction in those treated for SCH compared with controls.25 Pooled results from 2 RCTs (for patients older than 65 years, and those older than 80 years) showed no change in risk for cardiovascular outcomes in older adults treated for SCH.26 Older adults treated for SCH in the TRUST trial showed no improvements in systolic or diastolic function on echocardiography.27 Two trials showed no difference in carotid intima-media thickness with treatment of SCH compared with placebo.28,29
While most of the RCT data come from older adults, a retrospective cohort study in the United Kingdom of younger (ages 40-70 years; n = 3093) and older (age > 70 years; n = 1642) patients showed a reduction in cardiovascular mortality among treated patients who were younger (hazard ratio [HR] = 0.61; 4.2% vs. 6.6%) but not those who were older (HR = 0.99; 12.7% vs. 10.7%).30 There is also evidence that thyroid size in those with goiter can be reduced with treatment of SCH.31
A measured approach to treating subclinical hypothyroidism
Consider several factors when deciding whether to treat SCH. For instance, RCT data suggest a lack of treatment benefit in relieving depression, improving cognition, or reducing general hypothyroid symptoms. Treatment of SCH in older adults does not appear to improve cardiovascular outcomes. The question of whether long-term treatment of SCH in younger patients reduces cardiovascular morbidity or mortality lacks answers from RCTs. Before diagnosing SCH or starting treatment, always confirm SCH with repeat testing in 2 to 3 months, as a high percentage of those with untreated SCH will have normal thyroid function on repeat testing.
In the event you and your patient elect to treat SCH, guidelines and trials generally support a low initial daily dose of 25 to 50 mcg of levothyroxine (T4), followed with dose changes every 4 to 8 weeks and a goal of normalizing TSH to within the lower half of the reference range (0.4-2.5 mIU/L).14 This is generally similar to published treatment goals for primary hypothyroidism and is based on studies suggesting the lower half of the reference range is normal for young, healthy, euthyroid individuals.32 Though full replacement doses (1.6-1.8 mcg/kg of ideal body weight) can be started for those who are elderly or who have ischemic heart disease or angina, this approach should be avoided in favor of low-dose initial therapy.33 Thyroid supplements are best absorbed when taken apart from food, calcium, or iron supplements. The ATA suggests taking thyroid medication 60 minutes before breakfast or at bedtime (3 or more hours after the evening meal).33
Continue to: Screening guidelines differ
Screening guidelines differ
Lacking population-level screening data from RCTs, most organizations do not recommend screening for thyroid dysfunction or they note insufficient evidence to make a screening recommendation (TABLE 217,19,20,34). In their most recent recommendation statement on the subject in 2015, the US Preventive Services Task Force (USPSTF) concluded the current evidence was insufficient to recommend for or against thyroid dysfunction screening in nonpregnant, asymptomatic adults.17 This differs from the ATA and the American Association of Clinical Endocrinology (AACE; formerly known as the American Association of Clinical Endocrinologists), which both recommend targeted screening for thyroid dysfunction based on symptoms or risk factors.20
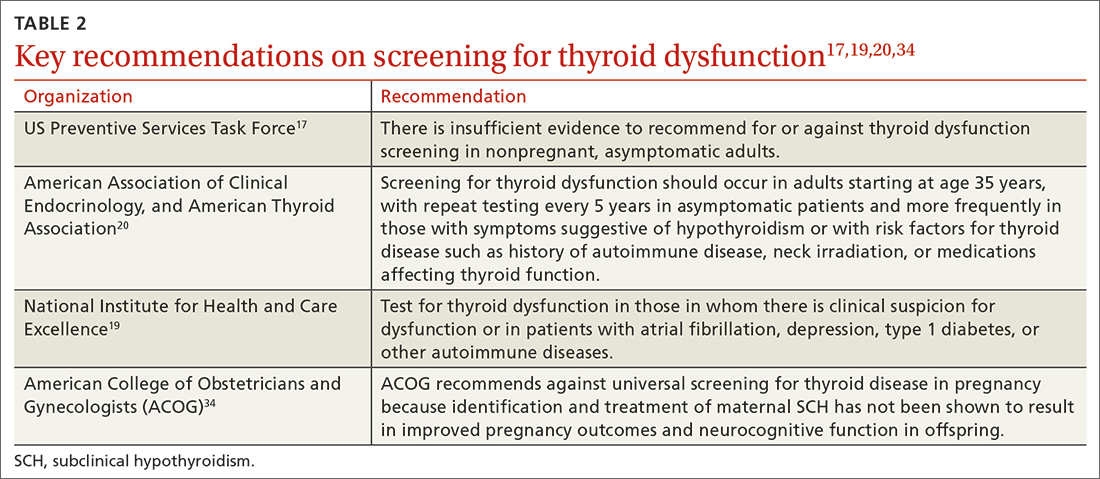
What about subclinical hypothyroidism in pregnancy?
Overt hypothyroidism is associated with adverse events during pregnancy and with subsequent neurodevelopmental complications in children, although the effects of SCH during pregnancy remain less certain. Concerns have been raised over the potential association of SCH with pregnancy loss, placental abruption, premature rupture of membranes, and neonatal death.35 Historically, the prevalence of SCH during pregnancy has ranged from 2% to 2.5%, but using lower trimester-based TSH reference ranges, the prevalence of SCH in pregnancy may be as high as 15%.35
Guided by a large RCT that failed to find benefit (pregnancy outcomes, neurodevelopmental outcomes in children) following treatment of SCH in pregnancy,36 the American College of Obstetricians and Gynecologists (ACOG) recommends against routine screening for thyroid disease in pregnancy.34 The ATA notes insufficient evidence to rec-ommend universal screening for thyroid dysfunction in pregnancy but recommends targeted screening of those with risk factors.37 Data are conflicting on the benefit of treating known or recently detected SCH on pregnancy outcomes including pregnancy loss.35,38 As such, the American Society of Reproductive Medicine and the ATA both generally recommend treatment of SCH in pregnant patients, particularly when the TSH is ≥ 4.0 mIU/L and TPOAbs are present.37,39
a The ATA, ETA, and NICE have slightly different recommendations when a TSH level = 10 mIU/L. ETA and NICE recommend prioritizing treatment for individuals with this level, while ATA recommends treatment when individual factors are also considered.
ACKNOWLEDGEMENT
The authors thank Family Medicine Medical Librarian Gwen Wilson, MLS, AHIP, for her assistance with literature searches.
CORRESPONDENCE
Nicholas LeFevre, MD, Family and Community Medicine, University of Missouri–Columbia School of Medicine, One Hospital Drive, M224 Medical Science Building, Columbia, MO 65212; nlefevre@health.missouri.edu
1. Reyes Domingo F, Avey MT, Doull M. Screening for thyroid dysfunction and treatment of screen-detected thyroid dysfunction in asymptomatic, community-dwelling adults: a systematic review. Syst Rev. 2019;8:260. doi: 10.1186/s13643-019-1181-7
2. Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379:1142-1154. doi: 10.1016/S0140-6736(11)60276-6
3. Bauer BS, Azcoaga-Lorenzo A, Agrawal U, et al. Management strategies for patients with subclinical hypothyroidism: a protocol for an umbrella review. Syst Rev. 2021;10:290. doi: 10.1186/s13643-021-01842-y
4. Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526-534. doi: 10.1001/archinte.160.4.526
5. Carlé A, Karmisholt JS, Knudsen N, et al. Does subclinical hypothyroidism add any symptoms? Evidence from a Danish population-based study. Am J Med. 2021;134:1115-1126.e1. doi: 10.1016/j.amjmed.2021.03.009
6. Gencer B, Collet TH, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126:1040-1049. doi: 10.1161/CIRCULATIONAHA.112.096024
7. Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365-1374. doi: 10.1001/jama.2010.1361
8. Bekkering GE, Agoritsas T, Lytvyn L, et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ. 2019;365:l2006. doi: 10.1136/bmj.l2006
9. Chung GE, Kim D, Kim W, et al. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J Hepatol. 2012;57:150-156. doi: 10.1016/j.jhep.2012.02.027
10. Kim D, Kim W, Joo SK, et al. Subclinical hypothyroidism and low-normal thyroid function are associated with nonalcoholic steatohepatitis and fibrosis. Clin Gastroenterol Hepatol. 2018;16:123-131.e1. doi: 10.1016/j.cgh.2017.08.014
11. Kim JS, Zhang Y, Chang Y, et al. Subclinical hypothyroidism and incident depression in young and middle-age adults. J Clin Endocrinol Metab. 2018;103:1827-1833. doi: 10.1210/jc.2017-01247
12. Jorde R, Waterloo K, Storhaug H, et al. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab. 2006;91:145-53. doi: 10.1210/jc.2005-1775
13. Azim S, Nasr C. Subclinical hypothyroidism: when to treat. Cleve Clin J Med. 2019;86:101-110. doi: 10.3949/ccjm.86a.17053
14. Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA Guideline: Management of subclinical hypothyroidism. Eur Thyroid J. 2013;2:215-228. doi: 10.1159/000356507
15. Cappola AR. The thyrotropin reference range should be changed in older patients. JAMA. 2019;322:1961-1962. doi: 10.1001/jama.2019.14728
16. Li D, Radulescu A, Shrestha RT, et al. Association of biotin ingestion with performance of hormone and nonhormone assays in healthy adults. JAMA. 2017;318:1150-1160.
17. LeFevre ML, USPSTF. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162:641-650. doi: 10.7326/M15-0483
18. Meyerovitch J, Rotman-Pikielni P, Sherf M, et al. Serum thyrotropin measurements in the community: five-year follow-up in a large network of primary care physicians. Arch Intern Med. 2007;167:1533-1538. doi: 10.1001/archinte.167.14.1533
19. NICE. Thyroid Disease: assessment and management (NICE guideline NG145). 2019. Accessed March 14, 2023. www.nice.org.uk/guidance/ng145/resources/thyroid-disease-assessment-and-management-pdf-66141781496773
20. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200-1235. doi: 10.1089/thy.2012.0205
21. Stott DJ, Rodondi N, Kearney PM, et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. 2017;376:2534-2544. doi: 10.1056/NEJMoa1603825
22. de Montmollin M, Feller M, Beglinger S, et al. L-thyroxine therapy for older adults with subclinical hypothyroidism and hypothyroid symptoms: secondary analysis of a randomized trial. Ann Intern Med. 2020;172:709-716. doi: 10.7326/M19-3193
23. Parle J, Roberts L, Wilson S, et al. A randomized controlled trial of the effect of thyroxine replacement on cognitive function in community-living elderly subjects with subclinical hypothyroidism: the Birmingham Elderly Thyroid study. J Clin Endocrinol Metab. 2010;95:3623-3632. doi: 10.1210/jc.2009-2571
24. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320:1349-1359. doi: 10.1001/jama.2018.13770
25. Andersen MN, Schjerning Olsen A-M, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PLoS One. 2015;10:e0129793. doi: 10.1371/journal.pone.0129793
26. Zijlstra LE, Jukema JW, Westendorp RG, et al. Levothyroxine treatment and cardiovascular outcomes in older people with subclinical hypothyroidism: pooled individual results of two randomised controlled trials. Front Endocrinol (Lausanne). 2021;12:674841. doi: 10.3389/fendo.2021.674841
27. Gencer B, Moutzouri E, Blum MR, et al. The impact of levothyroxine on cardiac function in older adults with mild subclinical hypothyroidism: a randomized clinical trial. Am J Med. 2020;133:848-856.e5. doi: 10.1016/j.amjmed.2020.01.018
28. Blum MR, Gencer B, Adam L, et al. Impact of thyroid hormone therapy on atherosclerosis in the elderly with subclinical hypothyroidism: a randomized trial. J Clin Endocrinol Metab. 2018;103:2988-2997. doi: 10.1210/jc.2018-00279
29. Aziz M, Kandimalla Y, Machavarapu A, et al. Effect of thyroxin treatment on carotid intima-media thickness (CIMT) reduction in patients with subclinical hypothyroidism (SCH): a meta-analysis of clinical trials. J Atheroscler Thromb. 2017;24:643-659. doi: 10.5551/jat.39917
30. Razvi S, Weaver JU, Butler TJ, et al. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med. 2012;172:811-817. doi: 10.1001/archinternmed.2012.1159
31. Romaldini JH, Biancalana MM, Figueiredo DI, et al. Effect of L-thyroxine administration on antithyroid antibody levels, lipid profile, and thyroid volume in patients with Hashimoto’s thyroiditis. Thyroid. 1996;6:183-188. doi: 10.1089/thy.1996.6.183
32. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76-131. doi: 10.1210/er.2006-0043
33. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751. doi: 10.1089/thy.2014.0028
34. ACOG. Thyroid disease in pregnancy: ACOG practice bulletin, Number 223. Obstet Gynecol. 2020;135:e261-e274. doi: 10.1097/AOG.0000000000003893
35. Maraka S, Ospina NM, O’Keeffe ET, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid. 2016;26:580-590. doi: 10.1089/thy.2015.0418
36. Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825. doi: 10.1056/NEJMoa1606205
37. Alexander EK, Pearce EN, Brent FA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017;27:315-389. doi: 10.1089/thy.2016.0457
38. Dong AC, Morgan J, Kane M, et al. Subclinical hypothyroidism and thyroid autoimmunity in recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. 2020;113:587-600.e1. doi: 10.1016/j.fertnstert.2019.11.003
39. Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104:545-553. doi: 10.1016/j.fertnstert.2015.05.028
Subclinical hypothyroidism (SCH) is a biochemical state in which the thyroid-stimulating hormone (TSH) is elevated while the free thyroxine (T4) level is normal. Overt hypothyroidism is not diagnosed until the free T4 level is decreased, regardless of the degree of TSH elevation.
The overall prevalence of SCH in iodine-rich areas is 4% to 10%, with a risk for progression to overt hypothyroidism of between 2% and 6% annually.1 The prevalence of SCH varies depending on the TSH reference range used.1 The normal reference range for TSH varies depending on the laboratory and/or the reference population surveyed, with the range likely widening with increasing age.
SCH is most common among women, the elderly, and White individuals.2 The discovery of SCH is often incidental, given that usually it is detected by laboratory findings alone without associated symptoms of overt hypothyroidism.3
The not-so-significant role of symptoms in subclinical hypothyroidism
Symptoms associated with overt hypothyroidism include constipation, dry skin, fatigue, slow thinking, poor memory, muscle cramps, weakness, and cold intolerance. In SCH, these symptoms are inconsistent, with around 1 in 3 patients having no symptoms
One study reported that roughly 18% of euthyroid individuals, 22% of SCH patients, and 26% of those with overt hypothyroidism reported 4 or more symptoms classically thought to be related to hypothyroidism.4 A large Danish cohort study found that hypothyroid symptoms were no more common in patients with SCH than in euthyroid individuals in the general population.5 These studies question the validity of attributing symptoms to SCH.
Adverse health associations
Observational data suggest that SCH is associated with an increased risk for dyslipidemia, coronary heart disease, heart failure, and cardiovascular mortality, particularly in those with TSH levels ≥ 10 mIU/L.6,7 Such associations were not found for most adults with TSH levels between 5 and 10 mIU/L.8 There are also potential associations of SCH with obesity, nonalcoholic fatty liver disease, and nonalcoholic steatohepatitis.9,10 Despite thyroid studies being commonly ordered as part of a mental health evaluation, SCH has not been statistically associated with depressive symptoms.11,12
Caveats with laboratory testing
There are several issues to consider when performing a laboratory assessment of thyroid function. TSH levels fluctuate considerably during the day, as TSH secretion has a circadian rhythm. TSH values are 50% higher at night and in the early morning than during the rest of the day.13 TSH values also may rise in response to current illness or stress. Due to this biologic variability, repeat testing to confirm TSH levels is recommended if an initial test result is abnormal.14
Continue to: An exact reference range...
An exact reference range for TSH is not widely agreed upon—although most laboratories regard 4.0 to 5.0 mIU/L as the high-end cutoff for normal. Also, “normal” TSH levels appear to differ by age. Accordingly, some experts have recommended an age-based reference range for TSH levels,15 although this is not implemented widely by laboratories. A TSH level of 6.0 mIU/L (or even higher) may be more appropriate for adults older than 65 years.1
Biotin supplementation has been shown to cause spurious thyroid testing results (TSH, T3, T4) depending on the type of assay used. Therefore, supplements containing biotin should be withheld for several days before assessing thyroid function.16Patients with SCH are often categorized as having TSH levels between 4.5 and 10 mIU/L (around 90% of patients) or levels ≥ 10 mIU/L.8,17 If followed for 5 years, approximately 60% of patients with SCH and TSH levels between 4 and 10 mIU/L will normalize without intervention.18 Normalization is less common in patients with a TSH level greater than 10 mIU/L.18
The risk for progression to overt hypothyroidism also appears to be higher for those with certain risk factors. These include higher baseline TSH levels, presence of thyroid peroxidase antibodies (TPOAbs), or history of neck irradiation or radioactive iodine uptake.1 Other risk factors for eventual thyroid dysfunction include female sex, older age, goiter, and high iodine intake.13
Evidence for treatment varies
Guidelines for the treatment of SCH (TABLE 18,14,19,20) are founded on the condition’s risk for progression to overt hypothyroidism and its association with health consequences such as cardiovascular disease. Guidelines of the American Thyroid Association (ATA) and European Thyroid Association (ETA), and those of the United Kingdom–based National Institute for Health and Care Excellence (NICE), prioritize treatment for individuals with a TSH level > 10 mIU/La and for those with

There are few large RCTs of treatment outcomes for SCH. A 2017 RCT (the Thyroid Hormone Replacement for Untreated Older Adults with Subclinical Hypothyroidism, or TRUST, trial) of 737 adults older than 65 years with SCH evaluated the ability of levothyroxine to normalize TSH values compared with placebo. At 1 year, there was no difference in hypothyroid symptoms or tiredness scale scores with levothyroxine treatment compared with placebo.21 This finding was consistent even in the subgroup with a higher baseline symptom burden.22
Continue to: Two small RCTs evaluated...
Two small RCTs evaluated treatment of SCH with depressive symptoms and cognitive function, neither finding benefit compared with placebo.12,23 A 2018 systematic review and meta-analysis of 21 studies and 2192 adults did not show a benefit to quality of life or thyroid-specific symptoms in those treated for SCH compared with controls.24
RCT support also is lacking for a reduction in cardiovascular mortality following treatment for SCH. A large population-level retrospective cohort from Denmark showed no difference in cardiovascular mortality or myocardial infarction in those treated for SCH compared with controls.25 Pooled results from 2 RCTs (for patients older than 65 years, and those older than 80 years) showed no change in risk for cardiovascular outcomes in older adults treated for SCH.26 Older adults treated for SCH in the TRUST trial showed no improvements in systolic or diastolic function on echocardiography.27 Two trials showed no difference in carotid intima-media thickness with treatment of SCH compared with placebo.28,29
While most of the RCT data come from older adults, a retrospective cohort study in the United Kingdom of younger (ages 40-70 years; n = 3093) and older (age > 70 years; n = 1642) patients showed a reduction in cardiovascular mortality among treated patients who were younger (hazard ratio [HR] = 0.61; 4.2% vs. 6.6%) but not those who were older (HR = 0.99; 12.7% vs. 10.7%).30 There is also evidence that thyroid size in those with goiter can be reduced with treatment of SCH.31
A measured approach to treating subclinical hypothyroidism
Consider several factors when deciding whether to treat SCH. For instance, RCT data suggest a lack of treatment benefit in relieving depression, improving cognition, or reducing general hypothyroid symptoms. Treatment of SCH in older adults does not appear to improve cardiovascular outcomes. The question of whether long-term treatment of SCH in younger patients reduces cardiovascular morbidity or mortality lacks answers from RCTs. Before diagnosing SCH or starting treatment, always confirm SCH with repeat testing in 2 to 3 months, as a high percentage of those with untreated SCH will have normal thyroid function on repeat testing.
In the event you and your patient elect to treat SCH, guidelines and trials generally support a low initial daily dose of 25 to 50 mcg of levothyroxine (T4), followed with dose changes every 4 to 8 weeks and a goal of normalizing TSH to within the lower half of the reference range (0.4-2.5 mIU/L).14 This is generally similar to published treatment goals for primary hypothyroidism and is based on studies suggesting the lower half of the reference range is normal for young, healthy, euthyroid individuals.32 Though full replacement doses (1.6-1.8 mcg/kg of ideal body weight) can be started for those who are elderly or who have ischemic heart disease or angina, this approach should be avoided in favor of low-dose initial therapy.33 Thyroid supplements are best absorbed when taken apart from food, calcium, or iron supplements. The ATA suggests taking thyroid medication 60 minutes before breakfast or at bedtime (3 or more hours after the evening meal).33
Continue to: Screening guidelines differ
Screening guidelines differ
Lacking population-level screening data from RCTs, most organizations do not recommend screening for thyroid dysfunction or they note insufficient evidence to make a screening recommendation (TABLE 217,19,20,34). In their most recent recommendation statement on the subject in 2015, the US Preventive Services Task Force (USPSTF) concluded the current evidence was insufficient to recommend for or against thyroid dysfunction screening in nonpregnant, asymptomatic adults.17 This differs from the ATA and the American Association of Clinical Endocrinology (AACE; formerly known as the American Association of Clinical Endocrinologists), which both recommend targeted screening for thyroid dysfunction based on symptoms or risk factors.20

What about subclinical hypothyroidism in pregnancy?
Overt hypothyroidism is associated with adverse events during pregnancy and with subsequent neurodevelopmental complications in children, although the effects of SCH during pregnancy remain less certain. Concerns have been raised over the potential association of SCH with pregnancy loss, placental abruption, premature rupture of membranes, and neonatal death.35 Historically, the prevalence of SCH during pregnancy has ranged from 2% to 2.5%, but using lower trimester-based TSH reference ranges, the prevalence of SCH in pregnancy may be as high as 15%.35
Guided by a large RCT that failed to find benefit (pregnancy outcomes, neurodevelopmental outcomes in children) following treatment of SCH in pregnancy,36 the American College of Obstetricians and Gynecologists (ACOG) recommends against routine screening for thyroid disease in pregnancy.34 The ATA notes insufficient evidence to rec-ommend universal screening for thyroid dysfunction in pregnancy but recommends targeted screening of those with risk factors.37 Data are conflicting on the benefit of treating known or recently detected SCH on pregnancy outcomes including pregnancy loss.35,38 As such, the American Society of Reproductive Medicine and the ATA both generally recommend treatment of SCH in pregnant patients, particularly when the TSH is ≥ 4.0 mIU/L and TPOAbs are present.37,39
a The ATA, ETA, and NICE have slightly different recommendations when a TSH level = 10 mIU/L. ETA and NICE recommend prioritizing treatment for individuals with this level, while ATA recommends treatment when individual factors are also considered.
ACKNOWLEDGEMENT
The authors thank Family Medicine Medical Librarian Gwen Wilson, MLS, AHIP, for her assistance with literature searches.
CORRESPONDENCE
Nicholas LeFevre, MD, Family and Community Medicine, University of Missouri–Columbia School of Medicine, One Hospital Drive, M224 Medical Science Building, Columbia, MO 65212; nlefevre@health.missouri.edu
Subclinical hypothyroidism (SCH) is a biochemical state in which the thyroid-stimulating hormone (TSH) is elevated while the free thyroxine (T4) level is normal. Overt hypothyroidism is not diagnosed until the free T4 level is decreased, regardless of the degree of TSH elevation.
The overall prevalence of SCH in iodine-rich areas is 4% to 10%, with a risk for progression to overt hypothyroidism of between 2% and 6% annually.1 The prevalence of SCH varies depending on the TSH reference range used.1 The normal reference range for TSH varies depending on the laboratory and/or the reference population surveyed, with the range likely widening with increasing age.
SCH is most common among women, the elderly, and White individuals.2 The discovery of SCH is often incidental, given that usually it is detected by laboratory findings alone without associated symptoms of overt hypothyroidism.3
The not-so-significant role of symptoms in subclinical hypothyroidism
Symptoms associated with overt hypothyroidism include constipation, dry skin, fatigue, slow thinking, poor memory, muscle cramps, weakness, and cold intolerance. In SCH, these symptoms are inconsistent, with around 1 in 3 patients having no symptoms
One study reported that roughly 18% of euthyroid individuals, 22% of SCH patients, and 26% of those with overt hypothyroidism reported 4 or more symptoms classically thought to be related to hypothyroidism.4 A large Danish cohort study found that hypothyroid symptoms were no more common in patients with SCH than in euthyroid individuals in the general population.5 These studies question the validity of attributing symptoms to SCH.
Adverse health associations
Observational data suggest that SCH is associated with an increased risk for dyslipidemia, coronary heart disease, heart failure, and cardiovascular mortality, particularly in those with TSH levels ≥ 10 mIU/L.6,7 Such associations were not found for most adults with TSH levels between 5 and 10 mIU/L.8 There are also potential associations of SCH with obesity, nonalcoholic fatty liver disease, and nonalcoholic steatohepatitis.9,10 Despite thyroid studies being commonly ordered as part of a mental health evaluation, SCH has not been statistically associated with depressive symptoms.11,12
Caveats with laboratory testing
There are several issues to consider when performing a laboratory assessment of thyroid function. TSH levels fluctuate considerably during the day, as TSH secretion has a circadian rhythm. TSH values are 50% higher at night and in the early morning than during the rest of the day.13 TSH values also may rise in response to current illness or stress. Due to this biologic variability, repeat testing to confirm TSH levels is recommended if an initial test result is abnormal.14
Continue to: An exact reference range...
An exact reference range for TSH is not widely agreed upon—although most laboratories regard 4.0 to 5.0 mIU/L as the high-end cutoff for normal. Also, “normal” TSH levels appear to differ by age. Accordingly, some experts have recommended an age-based reference range for TSH levels,15 although this is not implemented widely by laboratories. A TSH level of 6.0 mIU/L (or even higher) may be more appropriate for adults older than 65 years.1
Biotin supplementation has been shown to cause spurious thyroid testing results (TSH, T3, T4) depending on the type of assay used. Therefore, supplements containing biotin should be withheld for several days before assessing thyroid function.16Patients with SCH are often categorized as having TSH levels between 4.5 and 10 mIU/L (around 90% of patients) or levels ≥ 10 mIU/L.8,17 If followed for 5 years, approximately 60% of patients with SCH and TSH levels between 4 and 10 mIU/L will normalize without intervention.18 Normalization is less common in patients with a TSH level greater than 10 mIU/L.18
The risk for progression to overt hypothyroidism also appears to be higher for those with certain risk factors. These include higher baseline TSH levels, presence of thyroid peroxidase antibodies (TPOAbs), or history of neck irradiation or radioactive iodine uptake.1 Other risk factors for eventual thyroid dysfunction include female sex, older age, goiter, and high iodine intake.13
Evidence for treatment varies
Guidelines for the treatment of SCH (TABLE 18,14,19,20) are founded on the condition’s risk for progression to overt hypothyroidism and its association with health consequences such as cardiovascular disease. Guidelines of the American Thyroid Association (ATA) and European Thyroid Association (ETA), and those of the United Kingdom–based National Institute for Health and Care Excellence (NICE), prioritize treatment for individuals with a TSH level > 10 mIU/La and for those with

There are few large RCTs of treatment outcomes for SCH. A 2017 RCT (the Thyroid Hormone Replacement for Untreated Older Adults with Subclinical Hypothyroidism, or TRUST, trial) of 737 adults older than 65 years with SCH evaluated the ability of levothyroxine to normalize TSH values compared with placebo. At 1 year, there was no difference in hypothyroid symptoms or tiredness scale scores with levothyroxine treatment compared with placebo.21 This finding was consistent even in the subgroup with a higher baseline symptom burden.22
Continue to: Two small RCTs evaluated...
Two small RCTs evaluated treatment of SCH with depressive symptoms and cognitive function, neither finding benefit compared with placebo.12,23 A 2018 systematic review and meta-analysis of 21 studies and 2192 adults did not show a benefit to quality of life or thyroid-specific symptoms in those treated for SCH compared with controls.24
RCT support also is lacking for a reduction in cardiovascular mortality following treatment for SCH. A large population-level retrospective cohort from Denmark showed no difference in cardiovascular mortality or myocardial infarction in those treated for SCH compared with controls.25 Pooled results from 2 RCTs (for patients older than 65 years, and those older than 80 years) showed no change in risk for cardiovascular outcomes in older adults treated for SCH.26 Older adults treated for SCH in the TRUST trial showed no improvements in systolic or diastolic function on echocardiography.27 Two trials showed no difference in carotid intima-media thickness with treatment of SCH compared with placebo.28,29
While most of the RCT data come from older adults, a retrospective cohort study in the United Kingdom of younger (ages 40-70 years; n = 3093) and older (age > 70 years; n = 1642) patients showed a reduction in cardiovascular mortality among treated patients who were younger (hazard ratio [HR] = 0.61; 4.2% vs. 6.6%) but not those who were older (HR = 0.99; 12.7% vs. 10.7%).30 There is also evidence that thyroid size in those with goiter can be reduced with treatment of SCH.31
A measured approach to treating subclinical hypothyroidism
Consider several factors when deciding whether to treat SCH. For instance, RCT data suggest a lack of treatment benefit in relieving depression, improving cognition, or reducing general hypothyroid symptoms. Treatment of SCH in older adults does not appear to improve cardiovascular outcomes. The question of whether long-term treatment of SCH in younger patients reduces cardiovascular morbidity or mortality lacks answers from RCTs. Before diagnosing SCH or starting treatment, always confirm SCH with repeat testing in 2 to 3 months, as a high percentage of those with untreated SCH will have normal thyroid function on repeat testing.
In the event you and your patient elect to treat SCH, guidelines and trials generally support a low initial daily dose of 25 to 50 mcg of levothyroxine (T4), followed with dose changes every 4 to 8 weeks and a goal of normalizing TSH to within the lower half of the reference range (0.4-2.5 mIU/L).14 This is generally similar to published treatment goals for primary hypothyroidism and is based on studies suggesting the lower half of the reference range is normal for young, healthy, euthyroid individuals.32 Though full replacement doses (1.6-1.8 mcg/kg of ideal body weight) can be started for those who are elderly or who have ischemic heart disease or angina, this approach should be avoided in favor of low-dose initial therapy.33 Thyroid supplements are best absorbed when taken apart from food, calcium, or iron supplements. The ATA suggests taking thyroid medication 60 minutes before breakfast or at bedtime (3 or more hours after the evening meal).33
Continue to: Screening guidelines differ
Screening guidelines differ
Lacking population-level screening data from RCTs, most organizations do not recommend screening for thyroid dysfunction or they note insufficient evidence to make a screening recommendation (TABLE 217,19,20,34). In their most recent recommendation statement on the subject in 2015, the US Preventive Services Task Force (USPSTF) concluded the current evidence was insufficient to recommend for or against thyroid dysfunction screening in nonpregnant, asymptomatic adults.17 This differs from the ATA and the American Association of Clinical Endocrinology (AACE; formerly known as the American Association of Clinical Endocrinologists), which both recommend targeted screening for thyroid dysfunction based on symptoms or risk factors.20

What about subclinical hypothyroidism in pregnancy?
Overt hypothyroidism is associated with adverse events during pregnancy and with subsequent neurodevelopmental complications in children, although the effects of SCH during pregnancy remain less certain. Concerns have been raised over the potential association of SCH with pregnancy loss, placental abruption, premature rupture of membranes, and neonatal death.35 Historically, the prevalence of SCH during pregnancy has ranged from 2% to 2.5%, but using lower trimester-based TSH reference ranges, the prevalence of SCH in pregnancy may be as high as 15%.35
Guided by a large RCT that failed to find benefit (pregnancy outcomes, neurodevelopmental outcomes in children) following treatment of SCH in pregnancy,36 the American College of Obstetricians and Gynecologists (ACOG) recommends against routine screening for thyroid disease in pregnancy.34 The ATA notes insufficient evidence to rec-ommend universal screening for thyroid dysfunction in pregnancy but recommends targeted screening of those with risk factors.37 Data are conflicting on the benefit of treating known or recently detected SCH on pregnancy outcomes including pregnancy loss.35,38 As such, the American Society of Reproductive Medicine and the ATA both generally recommend treatment of SCH in pregnant patients, particularly when the TSH is ≥ 4.0 mIU/L and TPOAbs are present.37,39
a The ATA, ETA, and NICE have slightly different recommendations when a TSH level = 10 mIU/L. ETA and NICE recommend prioritizing treatment for individuals with this level, while ATA recommends treatment when individual factors are also considered.
ACKNOWLEDGEMENT
The authors thank Family Medicine Medical Librarian Gwen Wilson, MLS, AHIP, for her assistance with literature searches.
CORRESPONDENCE
Nicholas LeFevre, MD, Family and Community Medicine, University of Missouri–Columbia School of Medicine, One Hospital Drive, M224 Medical Science Building, Columbia, MO 65212; nlefevre@health.missouri.edu
1. Reyes Domingo F, Avey MT, Doull M. Screening for thyroid dysfunction and treatment of screen-detected thyroid dysfunction in asymptomatic, community-dwelling adults: a systematic review. Syst Rev. 2019;8:260. doi: 10.1186/s13643-019-1181-7
2. Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379:1142-1154. doi: 10.1016/S0140-6736(11)60276-6
3. Bauer BS, Azcoaga-Lorenzo A, Agrawal U, et al. Management strategies for patients with subclinical hypothyroidism: a protocol for an umbrella review. Syst Rev. 2021;10:290. doi: 10.1186/s13643-021-01842-y
4. Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526-534. doi: 10.1001/archinte.160.4.526
5. Carlé A, Karmisholt JS, Knudsen N, et al. Does subclinical hypothyroidism add any symptoms? Evidence from a Danish population-based study. Am J Med. 2021;134:1115-1126.e1. doi: 10.1016/j.amjmed.2021.03.009
6. Gencer B, Collet TH, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126:1040-1049. doi: 10.1161/CIRCULATIONAHA.112.096024
7. Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365-1374. doi: 10.1001/jama.2010.1361
8. Bekkering GE, Agoritsas T, Lytvyn L, et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ. 2019;365:l2006. doi: 10.1136/bmj.l2006
9. Chung GE, Kim D, Kim W, et al. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J Hepatol. 2012;57:150-156. doi: 10.1016/j.jhep.2012.02.027
10. Kim D, Kim W, Joo SK, et al. Subclinical hypothyroidism and low-normal thyroid function are associated with nonalcoholic steatohepatitis and fibrosis. Clin Gastroenterol Hepatol. 2018;16:123-131.e1. doi: 10.1016/j.cgh.2017.08.014
11. Kim JS, Zhang Y, Chang Y, et al. Subclinical hypothyroidism and incident depression in young and middle-age adults. J Clin Endocrinol Metab. 2018;103:1827-1833. doi: 10.1210/jc.2017-01247
12. Jorde R, Waterloo K, Storhaug H, et al. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab. 2006;91:145-53. doi: 10.1210/jc.2005-1775
13. Azim S, Nasr C. Subclinical hypothyroidism: when to treat. Cleve Clin J Med. 2019;86:101-110. doi: 10.3949/ccjm.86a.17053
14. Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA Guideline: Management of subclinical hypothyroidism. Eur Thyroid J. 2013;2:215-228. doi: 10.1159/000356507
15. Cappola AR. The thyrotropin reference range should be changed in older patients. JAMA. 2019;322:1961-1962. doi: 10.1001/jama.2019.14728
16. Li D, Radulescu A, Shrestha RT, et al. Association of biotin ingestion with performance of hormone and nonhormone assays in healthy adults. JAMA. 2017;318:1150-1160.
17. LeFevre ML, USPSTF. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162:641-650. doi: 10.7326/M15-0483
18. Meyerovitch J, Rotman-Pikielni P, Sherf M, et al. Serum thyrotropin measurements in the community: five-year follow-up in a large network of primary care physicians. Arch Intern Med. 2007;167:1533-1538. doi: 10.1001/archinte.167.14.1533
19. NICE. Thyroid Disease: assessment and management (NICE guideline NG145). 2019. Accessed March 14, 2023. www.nice.org.uk/guidance/ng145/resources/thyroid-disease-assessment-and-management-pdf-66141781496773
20. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200-1235. doi: 10.1089/thy.2012.0205
21. Stott DJ, Rodondi N, Kearney PM, et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. 2017;376:2534-2544. doi: 10.1056/NEJMoa1603825
22. de Montmollin M, Feller M, Beglinger S, et al. L-thyroxine therapy for older adults with subclinical hypothyroidism and hypothyroid symptoms: secondary analysis of a randomized trial. Ann Intern Med. 2020;172:709-716. doi: 10.7326/M19-3193
23. Parle J, Roberts L, Wilson S, et al. A randomized controlled trial of the effect of thyroxine replacement on cognitive function in community-living elderly subjects with subclinical hypothyroidism: the Birmingham Elderly Thyroid study. J Clin Endocrinol Metab. 2010;95:3623-3632. doi: 10.1210/jc.2009-2571
24. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320:1349-1359. doi: 10.1001/jama.2018.13770
25. Andersen MN, Schjerning Olsen A-M, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PLoS One. 2015;10:e0129793. doi: 10.1371/journal.pone.0129793
26. Zijlstra LE, Jukema JW, Westendorp RG, et al. Levothyroxine treatment and cardiovascular outcomes in older people with subclinical hypothyroidism: pooled individual results of two randomised controlled trials. Front Endocrinol (Lausanne). 2021;12:674841. doi: 10.3389/fendo.2021.674841
27. Gencer B, Moutzouri E, Blum MR, et al. The impact of levothyroxine on cardiac function in older adults with mild subclinical hypothyroidism: a randomized clinical trial. Am J Med. 2020;133:848-856.e5. doi: 10.1016/j.amjmed.2020.01.018
28. Blum MR, Gencer B, Adam L, et al. Impact of thyroid hormone therapy on atherosclerosis in the elderly with subclinical hypothyroidism: a randomized trial. J Clin Endocrinol Metab. 2018;103:2988-2997. doi: 10.1210/jc.2018-00279
29. Aziz M, Kandimalla Y, Machavarapu A, et al. Effect of thyroxin treatment on carotid intima-media thickness (CIMT) reduction in patients with subclinical hypothyroidism (SCH): a meta-analysis of clinical trials. J Atheroscler Thromb. 2017;24:643-659. doi: 10.5551/jat.39917
30. Razvi S, Weaver JU, Butler TJ, et al. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med. 2012;172:811-817. doi: 10.1001/archinternmed.2012.1159
31. Romaldini JH, Biancalana MM, Figueiredo DI, et al. Effect of L-thyroxine administration on antithyroid antibody levels, lipid profile, and thyroid volume in patients with Hashimoto’s thyroiditis. Thyroid. 1996;6:183-188. doi: 10.1089/thy.1996.6.183
32. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76-131. doi: 10.1210/er.2006-0043
33. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751. doi: 10.1089/thy.2014.0028
34. ACOG. Thyroid disease in pregnancy: ACOG practice bulletin, Number 223. Obstet Gynecol. 2020;135:e261-e274. doi: 10.1097/AOG.0000000000003893
35. Maraka S, Ospina NM, O’Keeffe ET, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid. 2016;26:580-590. doi: 10.1089/thy.2015.0418
36. Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825. doi: 10.1056/NEJMoa1606205
37. Alexander EK, Pearce EN, Brent FA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017;27:315-389. doi: 10.1089/thy.2016.0457
38. Dong AC, Morgan J, Kane M, et al. Subclinical hypothyroidism and thyroid autoimmunity in recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. 2020;113:587-600.e1. doi: 10.1016/j.fertnstert.2019.11.003
39. Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104:545-553. doi: 10.1016/j.fertnstert.2015.05.028
1. Reyes Domingo F, Avey MT, Doull M. Screening for thyroid dysfunction and treatment of screen-detected thyroid dysfunction in asymptomatic, community-dwelling adults: a systematic review. Syst Rev. 2019;8:260. doi: 10.1186/s13643-019-1181-7
2. Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379:1142-1154. doi: 10.1016/S0140-6736(11)60276-6
3. Bauer BS, Azcoaga-Lorenzo A, Agrawal U, et al. Management strategies for patients with subclinical hypothyroidism: a protocol for an umbrella review. Syst Rev. 2021;10:290. doi: 10.1186/s13643-021-01842-y
4. Canaris GJ, Manowitz NR, Mayor G, et al. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526-534. doi: 10.1001/archinte.160.4.526
5. Carlé A, Karmisholt JS, Knudsen N, et al. Does subclinical hypothyroidism add any symptoms? Evidence from a Danish population-based study. Am J Med. 2021;134:1115-1126.e1. doi: 10.1016/j.amjmed.2021.03.009
6. Gencer B, Collet TH, Virgini V, et al. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts. Circulation. 2012;126:1040-1049. doi: 10.1161/CIRCULATIONAHA.112.096024
7. Rodondi N, den Elzen WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365-1374. doi: 10.1001/jama.2010.1361
8. Bekkering GE, Agoritsas T, Lytvyn L, et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ. 2019;365:l2006. doi: 10.1136/bmj.l2006
9. Chung GE, Kim D, Kim W, et al. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J Hepatol. 2012;57:150-156. doi: 10.1016/j.jhep.2012.02.027
10. Kim D, Kim W, Joo SK, et al. Subclinical hypothyroidism and low-normal thyroid function are associated with nonalcoholic steatohepatitis and fibrosis. Clin Gastroenterol Hepatol. 2018;16:123-131.e1. doi: 10.1016/j.cgh.2017.08.014
11. Kim JS, Zhang Y, Chang Y, et al. Subclinical hypothyroidism and incident depression in young and middle-age adults. J Clin Endocrinol Metab. 2018;103:1827-1833. doi: 10.1210/jc.2017-01247
12. Jorde R, Waterloo K, Storhaug H, et al. Neuropsychological function and symptoms in subjects with subclinical hypothyroidism and the effect of thyroxine treatment. J Clin Endocrinol Metab. 2006;91:145-53. doi: 10.1210/jc.2005-1775
13. Azim S, Nasr C. Subclinical hypothyroidism: when to treat. Cleve Clin J Med. 2019;86:101-110. doi: 10.3949/ccjm.86a.17053
14. Pearce SH, Brabant G, Duntas LH, et al. 2013 ETA Guideline: Management of subclinical hypothyroidism. Eur Thyroid J. 2013;2:215-228. doi: 10.1159/000356507
15. Cappola AR. The thyrotropin reference range should be changed in older patients. JAMA. 2019;322:1961-1962. doi: 10.1001/jama.2019.14728
16. Li D, Radulescu A, Shrestha RT, et al. Association of biotin ingestion with performance of hormone and nonhormone assays in healthy adults. JAMA. 2017;318:1150-1160.
17. LeFevre ML, USPSTF. Screening for thyroid dysfunction: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162:641-650. doi: 10.7326/M15-0483
18. Meyerovitch J, Rotman-Pikielni P, Sherf M, et al. Serum thyrotropin measurements in the community: five-year follow-up in a large network of primary care physicians. Arch Intern Med. 2007;167:1533-1538. doi: 10.1001/archinte.167.14.1533
19. NICE. Thyroid Disease: assessment and management (NICE guideline NG145). 2019. Accessed March 14, 2023. www.nice.org.uk/guidance/ng145/resources/thyroid-disease-assessment-and-management-pdf-66141781496773
20. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid. 2012;22:1200-1235. doi: 10.1089/thy.2012.0205
21. Stott DJ, Rodondi N, Kearney PM, et al. Thyroid hormone therapy for older adults with subclinical hypothyroidism. N Engl J Med. 2017;376:2534-2544. doi: 10.1056/NEJMoa1603825
22. de Montmollin M, Feller M, Beglinger S, et al. L-thyroxine therapy for older adults with subclinical hypothyroidism and hypothyroid symptoms: secondary analysis of a randomized trial. Ann Intern Med. 2020;172:709-716. doi: 10.7326/M19-3193
23. Parle J, Roberts L, Wilson S, et al. A randomized controlled trial of the effect of thyroxine replacement on cognitive function in community-living elderly subjects with subclinical hypothyroidism: the Birmingham Elderly Thyroid study. J Clin Endocrinol Metab. 2010;95:3623-3632. doi: 10.1210/jc.2009-2571
24. Feller M, Snel M, Moutzouri E, et al. Association of thyroid hormone therapy with quality of life and thyroid-related symptoms in patients with subclinical hypothyroidism: a systematic review and meta-analysis. JAMA. 2018;320:1349-1359. doi: 10.1001/jama.2018.13770
25. Andersen MN, Schjerning Olsen A-M, Madsen JC, et al. Levothyroxine substitution in patients with subclinical hypothyroidism and the risk of myocardial infarction and mortality. PLoS One. 2015;10:e0129793. doi: 10.1371/journal.pone.0129793
26. Zijlstra LE, Jukema JW, Westendorp RG, et al. Levothyroxine treatment and cardiovascular outcomes in older people with subclinical hypothyroidism: pooled individual results of two randomised controlled trials. Front Endocrinol (Lausanne). 2021;12:674841. doi: 10.3389/fendo.2021.674841
27. Gencer B, Moutzouri E, Blum MR, et al. The impact of levothyroxine on cardiac function in older adults with mild subclinical hypothyroidism: a randomized clinical trial. Am J Med. 2020;133:848-856.e5. doi: 10.1016/j.amjmed.2020.01.018
28. Blum MR, Gencer B, Adam L, et al. Impact of thyroid hormone therapy on atherosclerosis in the elderly with subclinical hypothyroidism: a randomized trial. J Clin Endocrinol Metab. 2018;103:2988-2997. doi: 10.1210/jc.2018-00279
29. Aziz M, Kandimalla Y, Machavarapu A, et al. Effect of thyroxin treatment on carotid intima-media thickness (CIMT) reduction in patients with subclinical hypothyroidism (SCH): a meta-analysis of clinical trials. J Atheroscler Thromb. 2017;24:643-659. doi: 10.5551/jat.39917
30. Razvi S, Weaver JU, Butler TJ, et al. Levothyroxine treatment of subclinical hypothyroidism, fatal and nonfatal cardiovascular events, and mortality. Arch Intern Med. 2012;172:811-817. doi: 10.1001/archinternmed.2012.1159
31. Romaldini JH, Biancalana MM, Figueiredo DI, et al. Effect of L-thyroxine administration on antithyroid antibody levels, lipid profile, and thyroid volume in patients with Hashimoto’s thyroiditis. Thyroid. 1996;6:183-188. doi: 10.1089/thy.1996.6.183
32. Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocr Rev. 2008;29:76-131. doi: 10.1210/er.2006-0043
33. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24:1670-1751. doi: 10.1089/thy.2014.0028
34. ACOG. Thyroid disease in pregnancy: ACOG practice bulletin, Number 223. Obstet Gynecol. 2020;135:e261-e274. doi: 10.1097/AOG.0000000000003893
35. Maraka S, Ospina NM, O’Keeffe ET, et al. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid. 2016;26:580-590. doi: 10.1089/thy.2015.0418
36. Casey BM, Thom EA, Peaceman AM, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med. 2017;376:815-825. doi: 10.1056/NEJMoa1606205
37. Alexander EK, Pearce EN, Brent FA, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017;27:315-389. doi: 10.1089/thy.2016.0457
38. Dong AC, Morgan J, Kane M, et al. Subclinical hypothyroidism and thyroid autoimmunity in recurrent pregnancy loss: a systematic review and meta-analysis. Fertil Steril. 2020;113:587-600.e1. doi: 10.1016/j.fertnstert.2019.11.003
39. Practice Committee of the American Society for Reproductive Medicine. Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015;104:545-553. doi: 10.1016/j.fertnstert.2015.05.028
PRACTICE RECOMMENDATIONS
› Do not routinely screen for subclinical or overt hypothyroidism in asymptomatic nonpregnant adults. B
› Consider treatment of known or screening-detected subclinical hypothyroidism (SCH) in patients who are pregnant or trying to conceive. C
› Consider treating SCH in younger adults whose thyroidstimulating hormone level is ≥ 10 mIU/L. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Physician wellness: Managing stress and preventing burnout
Meet Dr. A and Dr. M
Dr. A is a 50-year-old family physician who provides prenatal care in a busy practice. She sees patients in eight 4-hour clinic sessions per week and is on inpatient call 1 week out of every 2 months. Dr. A has become disillusioned with her practice. She typically works until 7
Dr. M is a single, 32-year-old family physician working at an academic medical center. Dr. M is unhappy in his job, is trying to grow his practice, and views himself as having little impact or autonomy. He finds himself lost while navigating the electronic health record (EHR) and struggles to be efficient in the clinic. Dr. M has multiple administrative responsibilities that require him to work evenings and weekends. Debt from medical school loans also motivates him to moonlight several weekends per month. Over the past few months, Dr. M has become frustrated and discouraged, making his depression more difficult to manage. He feels drained by the time he arrives home, where he lives alone. He has stopped exercising, socializing with friends, and dating. Dr. M often wonders if he is in the wrong profession.
Defining burnout, stress, and wellness
Dr. A and Dr. M are experiencing symptoms of burnout, common to physicians and other health care professionals. Recent studies showed an increase in burnout during the COVID-19 pandemic.1,2 In a survey using the Maslach Burnout Inventory (MBI), approximately 44% of physicians reported at least one symptom of burnout.3 After adjusting for age, gender, relationship status, and hours worked per week, physicians were found to be at greater risk for burnout than nonphysician workers.3 The latest Medscape physician burnout survey found an increase in burnout among US physicians from 42% in 2021 to 47% in 2022 during the COVID-19 pandemic.1 Rates of burnout were even higher among family physicians and other frontline (eg, emergency, infectious disease, and critical care) physicians.1
Burnout has 3 key dimensions: (1) overwhelming exhaustion; (2) feelings of cynicism and detachment from the job; and (3) a sense of ineffectiveness and lack of accomplishment.4 The MBI is considered the standard tool for research in the field of burnout and has been repeatedly assessed for reliability and validity.4 The original MBI includes such items as: “I feel emotionally drained from my work,” “I feel like I’m working too hard on my job,” and “I worry that this job is hardening me emotionally.”5
According to the World Health Organization, burnout is an occupational phenomenon associated with chronic work-related stress that is not successfully managed.6 This definition emphasizes work stress as the cause of burnout, thus highlighting the importance of addressing the work environment.7 Physician burnout can affect physician health and wellness and the quality of patient care.8-13 Because of the cost of burnout to individuals and the health care system, it is important to understand stressors that can lead to physician burnout.
Stress has been described as “physical, mental, or emotional strain or tension … when a person perceives that demands exceed the personal and social resources the individual is able to mobilize.”14 Work-related sources of stress affecting practicing physicians include long workdays, multiple bureaucratic tasks, lack of autonomy/control, and complex patients.1,15
The COVID-19 pandemic is a stressor that increased physicians’ exposure to patient suffering and deaths and physicians’ vulnerability to disease at work.16 Physicians taking care of patients with COVID-19 risk infection and the possibility of infecting others.Online health records are another source of stress for many physicians.17,18 Access to online health records on personal devices can blur the line between work and home. For each hour of direct patient contact, a physician spends an additional 2 hours interacting with an EHR.19 Among family physicians and other primary care physicians, increased EHR interaction outside clinic hours has been associated with decreased workplace satisfaction and increased rates of burnout.11,19,20 Time spent on non-patient-facing clinical tasks, such as peer-to-peer reviews and billing queries, contributes more to burnout than clinic time alone.17
Continue to: These and other organizational factors...
These and other organizational factors contribute to the stress experienced by physicians. Many describe themselves as feeling consumed by their work. At the beginning of the COVID-19 pandemic, physicians (and the rest of the health care team) had to quickly learn how to conduct virtual office visits. Clerical responsibilities increased as patients relied more on patient portals and telephone calls to receive care.
Who is predisposed to burnout? Although burnout is a work-related syndrome, studies have shown an increase in burnout associated with individual (ie, personal) factors. For example, female physicians have been shown to have higher rates of burnout compared with male physicians.1,3 The stress of balancing the demands of the profession can begin during medical school and residency, with younger physicians having nearly twice the risk for stress-related symptoms when compared with older colleagues.15,20-23 Having a child younger than 21 years old, and other personal factors related to balancing family and life demands, increases the likelihood of burnout.11,21,22
Physicians with certain personality types and predispositions are at increased risk for burnout.23-25 For example, neuroticism on the Big Five Personality Inventory (one of the most well-known of the psychology inventories) is associated with an increased risk for burnout. Neuroticism may manifest as sadness or related emotional dysregulation (eg, irritability, anxiety).26 Other traits measured by the Big Five Personality Inventory include extraversion, agreeableness, conscientiousness, and openness to experience.26
A history of depression is also associated with an increased risk for burnout.27 Although depression and burnout are separate conditions, a 2016 study found significant overlap between the two.27 Physicians in this study who were depressed were more likely to experience burnout symptoms (87.5%); however, only 26.2% of physicians experiencing burnout were diagnosed as having depression.27 Rates of depression are higher among physicians when compared with nonphysicians, yet physicians are less likely to seek help due to fear of stigma and potential licensing concerns.28,29 Because of this, when physicians experience depressive symptoms, they may respond by working harder rather than seeking professional counseling or emotional support. They might believe that “asking for help is a sign of weakness,” thus sacrificing their wellness.
Wellness encompasses a sense of thriving characterized by thoughts and feelings of contentment, joy, and fulfillment—and the absence of severe distress.30 Wellness is a multifaceted condition that includes physical, psychological, and social aspects of an individual’s personal and professional life. Individuals experience a sense of wellness when they nurture their physical selves, minds, and relationships. People experience a sense of wellness when they balance their schedules, eat well, and maintain physical activity. Making time to enjoy family and friends also contributes to wellness.
Continue to: The culture of medicine often rewards...
The culture of medicine often rewards physician attitudes and behaviors that detract from wellness.31 Physicians internalize the culture of medicine that promotes perfectionism and downplays personal vulnerability.32 Physicians are reluctant to protect and preserve their wellness, believing self-sacrifice makes them good doctors. Physicians may spend countless hours counseling patients on the importance of wellness, but then work when ill or neglect their personal health needs and self-care—potentially decreasing their resilience and increasing the risk for burnout.31
Two paths to managing stress and preventing burnout
Patel and colleagues distinguish between 2 burnout intervention categories: (1) those that focus on individual physicians and (2) those that focus on the organizational environment.33 We find these distinctions useful and offer strategies for enhancing individual physician wellness (TABLE 134-41). Similar to West and colleagues,11 we offer strategies for addressing organizational sources of stress (TABLE 242-48). The following text describes these burnout intervention categories, emphasizing increasing self-care and changes that enable physicians to adapt effectively.

The recommendations outlined in this article are based on published stress and burnout literature, as well as the experiences of the authors. However, the number of randomized controlled studies of interventions aimed at reducing physician stress and burnout is limited. In addition, strategies proposed to reduce burnout in other professions may not address the unique stressors physicians encounter. Hence, our recommendations are limited. We have included interventions that seem optimal for individual physicians and the organizations that employ them.
Individual strategies target physical, psychological, and social wellness
Physician wellness strategies are divided into 3 categories: physical, psychological, and social wellness. Most strategies to improve physical wellness are widely known, evidence based, and recommended to patients by physicians.34-36 For example, most physicians advise their patients to eat healthy balanced meals, avoid unhealthy foods and beverages, maintain a healthy body weight, get daily exercise and adequate sleep, avoid excessive alcohol use, and abstain from tobacco use. However, discrepancies between physicians’ advice to patients and their own behaviors are common. Simply stated, physicians are well advised to follow their own advice regarding physical self-care.
CBT and mindfulness are key to psychological wellness. Recommendations for enhancing psychological wellness are primarily derived from cognitive behavioral therapy (CBT) and mindfulness principles and practices.37,38 CBT has been called the “gold standard” of psychotherapy, based on the breadth of research demonstrating that “no other form of psychotherapy has been shown to be systematically superior to CBT.”39
Continue to: CBT is based on the premise...
CBT is based on the premise that individuals’ thoughts and beliefs largely determine how they feel (emotions) and act (behaviors). Certain thoughts lead to positive feelings and effective behaviors, while others lead to negative feelings and less effective behaviors. For example, when a physician has self-critical or helpless thoughts (eg, “I’m just no good at managing my life”), they are more likely to feel unhappy and abandon problem-solving. In contrast, when a physician has self-affirming or hopeful thoughts (eg, “This is difficult, but I have the personal resources to succeed”), they are more likely to feel confident and act to solve problems.
Physicians vacillate between these thoughts and beliefs, and their emotions and behaviors follow accordingly. When hyper-focused on “the hassles of medicine,” physicians feel defeated, depressed, and anxious about their work. In contrast, when physicians recognize and challenge problematic thoughts and focus on what they love about medicine, they feel good and interact with patients and coworkers in positive and self-reinforcing ways.
Mindfulness can help reduce psychological stress and increase personal fulfillment. Mindfulness is characterized as being in the present moment, fully accepting “what is,” and having a sense of gratitude and compassion for self and others.40 In practice, mindfulness involves being intentional.
Dahl and colleagues41 describe a framework for human flourishing that includes 4 core dimensions of well-being (awareness, insight, connection, and purpose) that are all closely linked to mindful, intentional living. Based on their work, it is apparent that those who maintain a “heightened and flexible attentiveness” to their thoughts and feelings are likely to benefit by experiencing “improved mental health and psychological well-being.”41
However, the utility of CBT and mindfulness practices depends on receptivity to psychological interventions. Individuals who are not receptive may be hesitant to use these practices or likely will not benefit from them. Given these limitations of behavioral interventions, it would be helpful if more attention were paid to preventing and managing physician stress and burnout, especially through research focused on organizational changes.
Continue to: Supportive relationships are powerful
Supportive relationships are powerful. Finally, to enhance social wellness, it would be difficult to overstate the potential benefits of positive, supportive, close relationships.42 However, the demands of a career in medicine, starting in medical school, have the potential for inhibiting (rather than enhancing) close relationships.
Placing value on relationships with friends and family members is essential. As Dr. M began experiencing burnout, he felt increasingly lonely, yet he isolated himself from those who cared about him. Dr. A felt lonely at home, even though she was surrounded by family. Physicians are often reluctant to initiate vulnerable communication with others, believing “no one wants to hear about my problems.” However, by realizing the need for help and asking friends and family for emotional support, physicians can improve their wellness. Fostering supportive relationships can help provide the resilience needed to address organizational stressors.
Tackling organizational challenges
Long hours and pressure to see large numbers of patients (production demands) are a challenge across practice settings. Limiting work hours has been effective in improving the well-being of physician trainees but has had an inconsistent effect on burnout.43,44
Organizations can offer flexible scheduling, and physicians considering limiting work hours may switch to part-time status or shift work. However, decreasing work hours may have the unintended consequence of increased stress as some physicians feel pressure to do more in less time.45 Therefore, it’s important to set clear boundaries around work time and when and where work tasks are completed (eg, home vs office).
How we use technology matters. Given technology’s ever-increasing role in medicine, organizations must identify and use the most efficient, effective technology for managing clerical processes. When physicians participate in these decisions and share their experiences, technology is likely to be more user-friendly and impose less stress.46
Continue to: If technology contributes to stress...
If technology contributes to stress by being too complex or impractical, it’s important to identify individuals in the workplace (eg, IT support or “super-users”) to help address these challenges. Organizations can implement multidisciplinary teams to address EHR challenges and decrease physician stress and burnout by training support staff to assist with clerical duties, allowing physicians to focus on patient care.47,48 Such organizational-directed interventions will be most successful when physicians are included in the decision-making process.47
Take on leadership roles to influence change. Leadership may be formal (involving a title and authority) or informal (leading by example). Health care organizations that are committed to the well-being of physicians will make the effort to improve the systems in which physicians work. Physicians working in organizations that are reluctant to change have several choices: implement individual strategies, take on leadership roles to influence change, or reconsider their fit for the organization. Physicians in solo practice might consider joining others in solo practices to share systems (call, phone triage, technical resources, etc) to implement some of these interventions.
Dr. A and Dr. M implement new wellness strategies
Dr. A and Dr. M have recently committed to addressing stressors in their lives and improving their wellness. Dr. A has become more assertive at work, highlighting her need for additional resources to function effectively. In response, her practice has hired scribes to assist in documenting visits. This success has inspired Dr. A to pay attention to her lifestyle choices. Gradually, she has begun to exercise and engage in healthy eating.
Dr. M has begun to utilize resources at his medical center to improve his EHR efficiency and patient flow. He has taken steps to address his financial concerns, developing a budget and spending judiciously. He practices mindfulness and ensures that he gets at least 7 hours of sleep per night, improving his mental and physical health. By doing so, he has more energy to connect with friends, exercise, and date.
CORRESPONDENCE
Margaret L. Smith, MD, MPH, MHSA, KUMC, Family Medicine and Community Health, 3901 Rainbow Boulevard – Mailstop 4010, Kansas City, KS 66160; msmith33@kumc.edu
1. Kane L. Physician burnout & depression report: stress, anxiety, and anger. Medscape. January 21, 2022. Accessed February 23, 2023. www.medscape.com/slideshow/2022-lifestyle-burnout-6014664
2. Lockwood L, Patel N, Bukelis I. 45.5 Physician burnout and the COVID-19 pandemic: the silent epidemic. J Am Acad Child Adolesc Psychiatry. 2021;60:S242. doi: 10.1016/j.jaac.2021.09.354
3. Shanafelt TD, West CP, Sinsky C, et al. Changes in burnout and satisfaction with work-life integration in physicians and the general US working population between 2011 and 2017. Mayo Clin Proc. 2019;94:1681-1694. doi: 10.1016/j.mayocp.2018.10.023
4. Maslach C, Leiter MP. Understanding the burnout experience: recent research and its implications for psychiatry. World Psychiatry. 2016;15:103-111. doi: 10.1002/wps.20311
5. Maslach C, Jackson SE. The measurement of experienced burnout. J Organ Behav. 1981;2:99-113. doi: 10.1002/job.4030020205
6. World Health Organization. Burn-out an “occupational phenomenon”: International Classification of Diseases. May 28, 2019. Accessed February 23, 2023. www.who.int/news/item/28-05-2019-burn-out-an-occupational-phenomenon-international-classification-of-diseases
7. Berg S. WHO adds burnout to ICD-11. What it means for physicians. American Medical Association. July 23, 2019. Accessed February 23, 2023. www.ama-assn.org/practice-management/physician-health/who-adds-burnout-icd-11-what-it-means-physicians
8. Brown SD, Goske MJ, Johnson CM. Beyond substance abuse: stress, burnout, and depression as causes of physician impairment and disruptive behavior. J Am Coll Radiol. 2009;6:479-485. doi: 10.1016/j.jacr.2008.11.029
9. Williams ES, Rathert C, Buttigieg SC. The personal and professional consequences of physician burnout: a systematic review of the literature. Med Care Res Rev. 2020;77:371-386. doi: 10.1177/ 1077558719856787
10. Yates SW. Physician Stress and Burnout. Am J Med. 2020;133:160-164. doi: 10.1016/j.amjmed.2019.08.034
11. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529. doi: 10.1111/joim.12752
12. Firth-Cozens J, Greenhalgh J. Doctors’ perceptions of the links between stress and lowered clinical care. Soc Sci Med. 1997;44:1017-1022. doi: 10.1016/s0277-9536(96)00227-4
13. Dewa CS, Loong D, Bonato S, et al. The relationship between physician burnout and quality of healthcare in terms of safety and acceptability: a systematic review. BMJ Open. 2017;7:e015141. doi: 10.1136/bmjopen-2016-015141
14. American Institute of Stress. What is stress? April 29, 2022. Accessed February 23, 2023. www.stress.org/daily-life
15. Regehr C, Glancy D, Pitts A, et al. Interventions to reduce the consequences of stress in physicians: a review and meta-analysis. J Nerv Ment Dis. 2014;202:353-359. doi: 10.1097/NMD. 0000000000000130
16. Fitzpatrick K, Patterson R, Morley K, et al. Physician wellness during a pandemic. West J Emerg Med. 2020;21:83-87. doi: 10.5811/westjem.2020.7.48472
17. Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91:836-848. doi: 10.1016/j.mayocp.2016.05.007
18. Arndt BG, Beasley JW, Watkinson MD, et al. Tethered to the EHR: primary care physician workload assessment using EHR event log data and time-motion observations. Ann Fam Med. 2017;15:419-426. doi: 10.1370/afm.2121
19. Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165:753-760. doi: 10.7326/M16-0961
20. Robertson SL, Robinson MD, Reid A. Electronic health record effects on work-life balance and burnout within the I3 Population Collaborative. J Grad Med Educ. 2017;9:479-484. doi: 10.4300/JGME-D-16-00123.1
21. Fares J, Al Tabosh H, Saadeddin Z, et al. Stress, burnout and coping strategies in preclinical medical students. N Am J Med Sci. 2016;8:75-81. doi: 10.4103/1947-2714.177299
22. Patel RS, Bachu R, Adikey A, et al. Factors related to physician burnout and its consequences: a review. Behav Sci (Basel). 2018; 8:98. doi: 10.3390/bs8110098
23. Shanafelt TD, Sloan JA, Habermann TM. The well-being of physicians. Am J Med. 2003;114:513-519. doi: 10.1016/s0002-9343(03)00117-7
24. Drummond D. Physician burnout: its origin, symptoms, and five main causes. Fam Pract Manag. 2015;22:42-47.
25. Brown PA, Slater M, Lofters A. Personality and burnout among primary care physicians: an international study. Psychol Res Behav Manag. 2019;12:169-177. doi: 10.2147/PRBM.S195633.
26. John OP, Donahue EM, Kentle RL. The Big Five Inventory – Versions 4A and 54. Institute of Personality and Social Research, University of California; 1991.
27. Wurm W, Vogel K, Holl A, et al. Depression-burnout overlap in physicians. PLoS One. 2016;11:e0149913. doi: 10.1371/journal.pone.0149913
28. Mehta SS, Edwards ML. Suffering in silence: Mental health stigma and physicians’ licensing fears. Am J Psychiatry Resid J. 2018;13:2-4.
29. Adam AR, Golu FT. Prevalence of depression among physicians: A comprehensive meta-analysis. Ro Med J. 2021;68:327-337. doi: 10.37897/RMJ.2021.3.1
30. Brady KJS, Trockel MT, Khan CT, et al. What do we mean by physician wellness? A systematic review of its definition and measurement. Acad Psychiatry. 2018;42:94-108. doi: 10.1007/s40596-017-0781-6
31. Shanafelt TD, Schein E, Minor LB, et al. Healing the professional culture of medicine. Mayo Clin Proc. 2019;94:1556-1566. doi: 10.1016/j.mayocp.2019.03.026
32. Horan S, Flaxman PE, Stride CB. The perfect recovery? Interactive influence of perfectionism and spillover work tasks on changes in exhaustion and mood around a vacation. J Occup Health Psychol. 2021;26:86-107. doi: 10.1037/ocp0000208
33. Patel RS, Sekhri S, Bhimanadham NN, et al. A review on strategies to manage physician burnout. Cureus. 2019;11:e4805. doi: 10.7759/cureus.4805
34. US Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd edition. US Department of Health and Human Services; 2018.
35. Kim ES, Chen Y, Nakamura JS, et al. Sense of purpose in life and subsequent physical, behavioral, and psychosocial health: an outcome-wide approach. Am J Health Promot. 2022;36:137-147. doi: 10.1177/08901171211038545
36. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383-388. doi: 10.1016/j.sleh.2017.07.013
37. Fordham B, Sugavanam T, Edwards K, et al. The evidence for cognitive behavioural therapy in any condition, population or context: a meta-review of systematic reviews and panoramic meta-analysis. Psychol Med. 2021;51:21-29. doi: 10.1017/S0033291720005292
38. Goldberg SB, Tucker RP, Greene PA, et al. Mindfulness-based interventions for psychiatric disorders: a systematic review and meta-analysis. Clin Psychol Rev. 2018;59:52-60. doi: 10.1016/j.cpr.2017.10.011
39. David D, Cristea I, Hofmann SG. Why cognitive behavioral therapy is the current gold standard of psychotherapy. Front Psychiatry. 2018;9:4. doi: 10.3389/fpsyt.2018.00004
40. Fendel JC, Bürkle JJ, Göritz AS. Mindfulness-based interventions to reduce burnout and stress in physicians: a systematic review and meta-analysis. Acad Med. 2021;96:751-764. doi: 10.1097/ACM.0000000000003936
41. Dahl CJ, Wilson-Mendenhall CD, Davidson RJ. The plasticity of well-being: a training-based framework for the cultivation of human flourishing. Proc Natl Acad Sci USA. 2020;117:32197-32206. doi: 10.1073/pnas.2014859117
42. Holt-Lunstad J. Why social relationships are important for physical health: a systems approach to understanding and modifying risk and protection. Annu Rev Psychol. 2018;69:437-458. doi: 10.1146/annurev-psych-122216-011902
43. Desai SV, Asch DA, Bellini LM, et al. Education outcomes in a duty-hour flexibility trial in internal medicine. N Engl J Med. 2018; 378:1494-1508. doi: 10.1056/NEJMoa1800965
44. Shea JA, Bellini LM, Dinges DF, et al. Impact of protected sleep period for internal medicine interns on overnight call on depression, burnout, and empathy. J Grad Med Educ. 2014;6:256-263. doi: 10.4300/JGME-D-13-00241.1
45. Morrow G, Burford B, Carter M, et al. Have restricted working hours reduced junior doctors’ experience of fatigue? A focus group and telephone interview study. BMJ Open. 2014;4:e004222. doi: 10.1136/bmjopen-2013-004222
46. Shanafelt TD, Noseworthy JH. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92:129-146. doi: 10.1016/j.mayocp.2016.10.004
47. Sequeira L, Almilaji K, Strudwick G, et al. EHR “SWAT” teams: a physician engagement initiative to improve Electronic Health Record (EHR) experiences and mitigate possible causes of EHR-related burnout. JAMA Open. 2021;4:1-7. doi: 10.1093/jamiaopen/ooab018
48. Smith PC, Lyon C, English AF, et al. Practice transformation under the University of Colorado’s primary care redesign model. Ann Fam Med. 2019;17:S24-S32. doi: 10.1370/afm.2424
Meet Dr. A and Dr. M
Dr. A is a 50-year-old family physician who provides prenatal care in a busy practice. She sees patients in eight 4-hour clinic sessions per week and is on inpatient call 1 week out of every 2 months. Dr. A has become disillusioned with her practice. She typically works until 7
Dr. M is a single, 32-year-old family physician working at an academic medical center. Dr. M is unhappy in his job, is trying to grow his practice, and views himself as having little impact or autonomy. He finds himself lost while navigating the electronic health record (EHR) and struggles to be efficient in the clinic. Dr. M has multiple administrative responsibilities that require him to work evenings and weekends. Debt from medical school loans also motivates him to moonlight several weekends per month. Over the past few months, Dr. M has become frustrated and discouraged, making his depression more difficult to manage. He feels drained by the time he arrives home, where he lives alone. He has stopped exercising, socializing with friends, and dating. Dr. M often wonders if he is in the wrong profession.
Defining burnout, stress, and wellness
Dr. A and Dr. M are experiencing symptoms of burnout, common to physicians and other health care professionals. Recent studies showed an increase in burnout during the COVID-19 pandemic.1,2 In a survey using the Maslach Burnout Inventory (MBI), approximately 44% of physicians reported at least one symptom of burnout.3 After adjusting for age, gender, relationship status, and hours worked per week, physicians were found to be at greater risk for burnout than nonphysician workers.3 The latest Medscape physician burnout survey found an increase in burnout among US physicians from 42% in 2021 to 47% in 2022 during the COVID-19 pandemic.1 Rates of burnout were even higher among family physicians and other frontline (eg, emergency, infectious disease, and critical care) physicians.1
Burnout has 3 key dimensions: (1) overwhelming exhaustion; (2) feelings of cynicism and detachment from the job; and (3) a sense of ineffectiveness and lack of accomplishment.4 The MBI is considered the standard tool for research in the field of burnout and has been repeatedly assessed for reliability and validity.4 The original MBI includes such items as: “I feel emotionally drained from my work,” “I feel like I’m working too hard on my job,” and “I worry that this job is hardening me emotionally.”5
According to the World Health Organization, burnout is an occupational phenomenon associated with chronic work-related stress that is not successfully managed.6 This definition emphasizes work stress as the cause of burnout, thus highlighting the importance of addressing the work environment.7 Physician burnout can affect physician health and wellness and the quality of patient care.8-13 Because of the cost of burnout to individuals and the health care system, it is important to understand stressors that can lead to physician burnout.
Stress has been described as “physical, mental, or emotional strain or tension … when a person perceives that demands exceed the personal and social resources the individual is able to mobilize.”14 Work-related sources of stress affecting practicing physicians include long workdays, multiple bureaucratic tasks, lack of autonomy/control, and complex patients.1,15
The COVID-19 pandemic is a stressor that increased physicians’ exposure to patient suffering and deaths and physicians’ vulnerability to disease at work.16 Physicians taking care of patients with COVID-19 risk infection and the possibility of infecting others.Online health records are another source of stress for many physicians.17,18 Access to online health records on personal devices can blur the line between work and home. For each hour of direct patient contact, a physician spends an additional 2 hours interacting with an EHR.19 Among family physicians and other primary care physicians, increased EHR interaction outside clinic hours has been associated with decreased workplace satisfaction and increased rates of burnout.11,19,20 Time spent on non-patient-facing clinical tasks, such as peer-to-peer reviews and billing queries, contributes more to burnout than clinic time alone.17
Continue to: These and other organizational factors...
These and other organizational factors contribute to the stress experienced by physicians. Many describe themselves as feeling consumed by their work. At the beginning of the COVID-19 pandemic, physicians (and the rest of the health care team) had to quickly learn how to conduct virtual office visits. Clerical responsibilities increased as patients relied more on patient portals and telephone calls to receive care.
Who is predisposed to burnout? Although burnout is a work-related syndrome, studies have shown an increase in burnout associated with individual (ie, personal) factors. For example, female physicians have been shown to have higher rates of burnout compared with male physicians.1,3 The stress of balancing the demands of the profession can begin during medical school and residency, with younger physicians having nearly twice the risk for stress-related symptoms when compared with older colleagues.15,20-23 Having a child younger than 21 years old, and other personal factors related to balancing family and life demands, increases the likelihood of burnout.11,21,22
Physicians with certain personality types and predispositions are at increased risk for burnout.23-25 For example, neuroticism on the Big Five Personality Inventory (one of the most well-known of the psychology inventories) is associated with an increased risk for burnout. Neuroticism may manifest as sadness or related emotional dysregulation (eg, irritability, anxiety).26 Other traits measured by the Big Five Personality Inventory include extraversion, agreeableness, conscientiousness, and openness to experience.26
A history of depression is also associated with an increased risk for burnout.27 Although depression and burnout are separate conditions, a 2016 study found significant overlap between the two.27 Physicians in this study who were depressed were more likely to experience burnout symptoms (87.5%); however, only 26.2% of physicians experiencing burnout were diagnosed as having depression.27 Rates of depression are higher among physicians when compared with nonphysicians, yet physicians are less likely to seek help due to fear of stigma and potential licensing concerns.28,29 Because of this, when physicians experience depressive symptoms, they may respond by working harder rather than seeking professional counseling or emotional support. They might believe that “asking for help is a sign of weakness,” thus sacrificing their wellness.
Wellness encompasses a sense of thriving characterized by thoughts and feelings of contentment, joy, and fulfillment—and the absence of severe distress.30 Wellness is a multifaceted condition that includes physical, psychological, and social aspects of an individual’s personal and professional life. Individuals experience a sense of wellness when they nurture their physical selves, minds, and relationships. People experience a sense of wellness when they balance their schedules, eat well, and maintain physical activity. Making time to enjoy family and friends also contributes to wellness.
Continue to: The culture of medicine often rewards...
The culture of medicine often rewards physician attitudes and behaviors that detract from wellness.31 Physicians internalize the culture of medicine that promotes perfectionism and downplays personal vulnerability.32 Physicians are reluctant to protect and preserve their wellness, believing self-sacrifice makes them good doctors. Physicians may spend countless hours counseling patients on the importance of wellness, but then work when ill or neglect their personal health needs and self-care—potentially decreasing their resilience and increasing the risk for burnout.31

Two paths to managing stress and preventing burnout
Patel and colleagues distinguish between 2 burnout intervention categories: (1) those that focus on individual physicians and (2) those that focus on the organizational environment.33 We find these distinctions useful and offer strategies for enhancing individual physician wellness (TABLE 134-41). Similar to West and colleagues,11 we offer strategies for addressing organizational sources of stress (TABLE 242-48). The following text describes these burnout intervention categories, emphasizing increasing self-care and changes that enable physicians to adapt effectively.

The recommendations outlined in this article are based on published stress and burnout literature, as well as the experiences of the authors. However, the number of randomized controlled studies of interventions aimed at reducing physician stress and burnout is limited. In addition, strategies proposed to reduce burnout in other professions may not address the unique stressors physicians encounter. Hence, our recommendations are limited. We have included interventions that seem optimal for individual physicians and the organizations that employ them.
Individual strategies target physical, psychological, and social wellness
Physician wellness strategies are divided into 3 categories: physical, psychological, and social wellness. Most strategies to improve physical wellness are widely known, evidence based, and recommended to patients by physicians.34-36 For example, most physicians advise their patients to eat healthy balanced meals, avoid unhealthy foods and beverages, maintain a healthy body weight, get daily exercise and adequate sleep, avoid excessive alcohol use, and abstain from tobacco use. However, discrepancies between physicians’ advice to patients and their own behaviors are common. Simply stated, physicians are well advised to follow their own advice regarding physical self-care.
CBT and mindfulness are key to psychological wellness. Recommendations for enhancing psychological wellness are primarily derived from cognitive behavioral therapy (CBT) and mindfulness principles and practices.37,38 CBT has been called the “gold standard” of psychotherapy, based on the breadth of research demonstrating that “no other form of psychotherapy has been shown to be systematically superior to CBT.”39
Continue to: CBT is based on the premise...
CBT is based on the premise that individuals’ thoughts and beliefs largely determine how they feel (emotions) and act (behaviors). Certain thoughts lead to positive feelings and effective behaviors, while others lead to negative feelings and less effective behaviors. For example, when a physician has self-critical or helpless thoughts (eg, “I’m just no good at managing my life”), they are more likely to feel unhappy and abandon problem-solving. In contrast, when a physician has self-affirming or hopeful thoughts (eg, “This is difficult, but I have the personal resources to succeed”), they are more likely to feel confident and act to solve problems.
Physicians vacillate between these thoughts and beliefs, and their emotions and behaviors follow accordingly. When hyper-focused on “the hassles of medicine,” physicians feel defeated, depressed, and anxious about their work. In contrast, when physicians recognize and challenge problematic thoughts and focus on what they love about medicine, they feel good and interact with patients and coworkers in positive and self-reinforcing ways.
Mindfulness can help reduce psychological stress and increase personal fulfillment. Mindfulness is characterized as being in the present moment, fully accepting “what is,” and having a sense of gratitude and compassion for self and others.40 In practice, mindfulness involves being intentional.
Dahl and colleagues41 describe a framework for human flourishing that includes 4 core dimensions of well-being (awareness, insight, connection, and purpose) that are all closely linked to mindful, intentional living. Based on their work, it is apparent that those who maintain a “heightened and flexible attentiveness” to their thoughts and feelings are likely to benefit by experiencing “improved mental health and psychological well-being.”41
However, the utility of CBT and mindfulness practices depends on receptivity to psychological interventions. Individuals who are not receptive may be hesitant to use these practices or likely will not benefit from them. Given these limitations of behavioral interventions, it would be helpful if more attention were paid to preventing and managing physician stress and burnout, especially through research focused on organizational changes.
Continue to: Supportive relationships are powerful
Supportive relationships are powerful. Finally, to enhance social wellness, it would be difficult to overstate the potential benefits of positive, supportive, close relationships.42 However, the demands of a career in medicine, starting in medical school, have the potential for inhibiting (rather than enhancing) close relationships.
Placing value on relationships with friends and family members is essential. As Dr. M began experiencing burnout, he felt increasingly lonely, yet he isolated himself from those who cared about him. Dr. A felt lonely at home, even though she was surrounded by family. Physicians are often reluctant to initiate vulnerable communication with others, believing “no one wants to hear about my problems.” However, by realizing the need for help and asking friends and family for emotional support, physicians can improve their wellness. Fostering supportive relationships can help provide the resilience needed to address organizational stressors.
Tackling organizational challenges
Long hours and pressure to see large numbers of patients (production demands) are a challenge across practice settings. Limiting work hours has been effective in improving the well-being of physician trainees but has had an inconsistent effect on burnout.43,44
Organizations can offer flexible scheduling, and physicians considering limiting work hours may switch to part-time status or shift work. However, decreasing work hours may have the unintended consequence of increased stress as some physicians feel pressure to do more in less time.45 Therefore, it’s important to set clear boundaries around work time and when and where work tasks are completed (eg, home vs office).
How we use technology matters. Given technology’s ever-increasing role in medicine, organizations must identify and use the most efficient, effective technology for managing clerical processes. When physicians participate in these decisions and share their experiences, technology is likely to be more user-friendly and impose less stress.46
Continue to: If technology contributes to stress...
If technology contributes to stress by being too complex or impractical, it’s important to identify individuals in the workplace (eg, IT support or “super-users”) to help address these challenges. Organizations can implement multidisciplinary teams to address EHR challenges and decrease physician stress and burnout by training support staff to assist with clerical duties, allowing physicians to focus on patient care.47,48 Such organizational-directed interventions will be most successful when physicians are included in the decision-making process.47
Take on leadership roles to influence change. Leadership may be formal (involving a title and authority) or informal (leading by example). Health care organizations that are committed to the well-being of physicians will make the effort to improve the systems in which physicians work. Physicians working in organizations that are reluctant to change have several choices: implement individual strategies, take on leadership roles to influence change, or reconsider their fit for the organization. Physicians in solo practice might consider joining others in solo practices to share systems (call, phone triage, technical resources, etc) to implement some of these interventions.
Dr. A and Dr. M implement new wellness strategies
Dr. A and Dr. M have recently committed to addressing stressors in their lives and improving their wellness. Dr. A has become more assertive at work, highlighting her need for additional resources to function effectively. In response, her practice has hired scribes to assist in documenting visits. This success has inspired Dr. A to pay attention to her lifestyle choices. Gradually, she has begun to exercise and engage in healthy eating.
Dr. M has begun to utilize resources at his medical center to improve his EHR efficiency and patient flow. He has taken steps to address his financial concerns, developing a budget and spending judiciously. He practices mindfulness and ensures that he gets at least 7 hours of sleep per night, improving his mental and physical health. By doing so, he has more energy to connect with friends, exercise, and date.
CORRESPONDENCE
Margaret L. Smith, MD, MPH, MHSA, KUMC, Family Medicine and Community Health, 3901 Rainbow Boulevard – Mailstop 4010, Kansas City, KS 66160; msmith33@kumc.edu
Meet Dr. A and Dr. M
Dr. A is a 50-year-old family physician who provides prenatal care in a busy practice. She sees patients in eight 4-hour clinic sessions per week and is on inpatient call 1 week out of every 2 months. Dr. A has become disillusioned with her practice. She typically works until 7
Dr. M is a single, 32-year-old family physician working at an academic medical center. Dr. M is unhappy in his job, is trying to grow his practice, and views himself as having little impact or autonomy. He finds himself lost while navigating the electronic health record (EHR) and struggles to be efficient in the clinic. Dr. M has multiple administrative responsibilities that require him to work evenings and weekends. Debt from medical school loans also motivates him to moonlight several weekends per month. Over the past few months, Dr. M has become frustrated and discouraged, making his depression more difficult to manage. He feels drained by the time he arrives home, where he lives alone. He has stopped exercising, socializing with friends, and dating. Dr. M often wonders if he is in the wrong profession.
Defining burnout, stress, and wellness
Dr. A and Dr. M are experiencing symptoms of burnout, common to physicians and other health care professionals. Recent studies showed an increase in burnout during the COVID-19 pandemic.1,2 In a survey using the Maslach Burnout Inventory (MBI), approximately 44% of physicians reported at least one symptom of burnout.3 After adjusting for age, gender, relationship status, and hours worked per week, physicians were found to be at greater risk for burnout than nonphysician workers.3 The latest Medscape physician burnout survey found an increase in burnout among US physicians from 42% in 2021 to 47% in 2022 during the COVID-19 pandemic.1 Rates of burnout were even higher among family physicians and other frontline (eg, emergency, infectious disease, and critical care) physicians.1
Burnout has 3 key dimensions: (1) overwhelming exhaustion; (2) feelings of cynicism and detachment from the job; and (3) a sense of ineffectiveness and lack of accomplishment.4 The MBI is considered the standard tool for research in the field of burnout and has been repeatedly assessed for reliability and validity.4 The original MBI includes such items as: “I feel emotionally drained from my work,” “I feel like I’m working too hard on my job,” and “I worry that this job is hardening me emotionally.”5
According to the World Health Organization, burnout is an occupational phenomenon associated with chronic work-related stress that is not successfully managed.6 This definition emphasizes work stress as the cause of burnout, thus highlighting the importance of addressing the work environment.7 Physician burnout can affect physician health and wellness and the quality of patient care.8-13 Because of the cost of burnout to individuals and the health care system, it is important to understand stressors that can lead to physician burnout.
Stress has been described as “physical, mental, or emotional strain or tension … when a person perceives that demands exceed the personal and social resources the individual is able to mobilize.”14 Work-related sources of stress affecting practicing physicians include long workdays, multiple bureaucratic tasks, lack of autonomy/control, and complex patients.1,15
The COVID-19 pandemic is a stressor that increased physicians’ exposure to patient suffering and deaths and physicians’ vulnerability to disease at work.16 Physicians taking care of patients with COVID-19 risk infection and the possibility of infecting others.Online health records are another source of stress for many physicians.17,18 Access to online health records on personal devices can blur the line between work and home. For each hour of direct patient contact, a physician spends an additional 2 hours interacting with an EHR.19 Among family physicians and other primary care physicians, increased EHR interaction outside clinic hours has been associated with decreased workplace satisfaction and increased rates of burnout.11,19,20 Time spent on non-patient-facing clinical tasks, such as peer-to-peer reviews and billing queries, contributes more to burnout than clinic time alone.17
Continue to: These and other organizational factors...
These and other organizational factors contribute to the stress experienced by physicians. Many describe themselves as feeling consumed by their work. At the beginning of the COVID-19 pandemic, physicians (and the rest of the health care team) had to quickly learn how to conduct virtual office visits. Clerical responsibilities increased as patients relied more on patient portals and telephone calls to receive care.
Who is predisposed to burnout? Although burnout is a work-related syndrome, studies have shown an increase in burnout associated with individual (ie, personal) factors. For example, female physicians have been shown to have higher rates of burnout compared with male physicians.1,3 The stress of balancing the demands of the profession can begin during medical school and residency, with younger physicians having nearly twice the risk for stress-related symptoms when compared with older colleagues.15,20-23 Having a child younger than 21 years old, and other personal factors related to balancing family and life demands, increases the likelihood of burnout.11,21,22
Physicians with certain personality types and predispositions are at increased risk for burnout.23-25 For example, neuroticism on the Big Five Personality Inventory (one of the most well-known of the psychology inventories) is associated with an increased risk for burnout. Neuroticism may manifest as sadness or related emotional dysregulation (eg, irritability, anxiety).26 Other traits measured by the Big Five Personality Inventory include extraversion, agreeableness, conscientiousness, and openness to experience.26
A history of depression is also associated with an increased risk for burnout.27 Although depression and burnout are separate conditions, a 2016 study found significant overlap between the two.27 Physicians in this study who were depressed were more likely to experience burnout symptoms (87.5%); however, only 26.2% of physicians experiencing burnout were diagnosed as having depression.27 Rates of depression are higher among physicians when compared with nonphysicians, yet physicians are less likely to seek help due to fear of stigma and potential licensing concerns.28,29 Because of this, when physicians experience depressive symptoms, they may respond by working harder rather than seeking professional counseling or emotional support. They might believe that “asking for help is a sign of weakness,” thus sacrificing their wellness.
Wellness encompasses a sense of thriving characterized by thoughts and feelings of contentment, joy, and fulfillment—and the absence of severe distress.30 Wellness is a multifaceted condition that includes physical, psychological, and social aspects of an individual’s personal and professional life. Individuals experience a sense of wellness when they nurture their physical selves, minds, and relationships. People experience a sense of wellness when they balance their schedules, eat well, and maintain physical activity. Making time to enjoy family and friends also contributes to wellness.
Continue to: The culture of medicine often rewards...
The culture of medicine often rewards physician attitudes and behaviors that detract from wellness.31 Physicians internalize the culture of medicine that promotes perfectionism and downplays personal vulnerability.32 Physicians are reluctant to protect and preserve their wellness, believing self-sacrifice makes them good doctors. Physicians may spend countless hours counseling patients on the importance of wellness, but then work when ill or neglect their personal health needs and self-care—potentially decreasing their resilience and increasing the risk for burnout.31

Two paths to managing stress and preventing burnout
Patel and colleagues distinguish between 2 burnout intervention categories: (1) those that focus on individual physicians and (2) those that focus on the organizational environment.33 We find these distinctions useful and offer strategies for enhancing individual physician wellness (TABLE 134-41). Similar to West and colleagues,11 we offer strategies for addressing organizational sources of stress (TABLE 242-48). The following text describes these burnout intervention categories, emphasizing increasing self-care and changes that enable physicians to adapt effectively.

The recommendations outlined in this article are based on published stress and burnout literature, as well as the experiences of the authors. However, the number of randomized controlled studies of interventions aimed at reducing physician stress and burnout is limited. In addition, strategies proposed to reduce burnout in other professions may not address the unique stressors physicians encounter. Hence, our recommendations are limited. We have included interventions that seem optimal for individual physicians and the organizations that employ them.
Individual strategies target physical, psychological, and social wellness
Physician wellness strategies are divided into 3 categories: physical, psychological, and social wellness. Most strategies to improve physical wellness are widely known, evidence based, and recommended to patients by physicians.34-36 For example, most physicians advise their patients to eat healthy balanced meals, avoid unhealthy foods and beverages, maintain a healthy body weight, get daily exercise and adequate sleep, avoid excessive alcohol use, and abstain from tobacco use. However, discrepancies between physicians’ advice to patients and their own behaviors are common. Simply stated, physicians are well advised to follow their own advice regarding physical self-care.
CBT and mindfulness are key to psychological wellness. Recommendations for enhancing psychological wellness are primarily derived from cognitive behavioral therapy (CBT) and mindfulness principles and practices.37,38 CBT has been called the “gold standard” of psychotherapy, based on the breadth of research demonstrating that “no other form of psychotherapy has been shown to be systematically superior to CBT.”39
Continue to: CBT is based on the premise...
CBT is based on the premise that individuals’ thoughts and beliefs largely determine how they feel (emotions) and act (behaviors). Certain thoughts lead to positive feelings and effective behaviors, while others lead to negative feelings and less effective behaviors. For example, when a physician has self-critical or helpless thoughts (eg, “I’m just no good at managing my life”), they are more likely to feel unhappy and abandon problem-solving. In contrast, when a physician has self-affirming or hopeful thoughts (eg, “This is difficult, but I have the personal resources to succeed”), they are more likely to feel confident and act to solve problems.
Physicians vacillate between these thoughts and beliefs, and their emotions and behaviors follow accordingly. When hyper-focused on “the hassles of medicine,” physicians feel defeated, depressed, and anxious about their work. In contrast, when physicians recognize and challenge problematic thoughts and focus on what they love about medicine, they feel good and interact with patients and coworkers in positive and self-reinforcing ways.
Mindfulness can help reduce psychological stress and increase personal fulfillment. Mindfulness is characterized as being in the present moment, fully accepting “what is,” and having a sense of gratitude and compassion for self and others.40 In practice, mindfulness involves being intentional.
Dahl and colleagues41 describe a framework for human flourishing that includes 4 core dimensions of well-being (awareness, insight, connection, and purpose) that are all closely linked to mindful, intentional living. Based on their work, it is apparent that those who maintain a “heightened and flexible attentiveness” to their thoughts and feelings are likely to benefit by experiencing “improved mental health and psychological well-being.”41
However, the utility of CBT and mindfulness practices depends on receptivity to psychological interventions. Individuals who are not receptive may be hesitant to use these practices or likely will not benefit from them. Given these limitations of behavioral interventions, it would be helpful if more attention were paid to preventing and managing physician stress and burnout, especially through research focused on organizational changes.
Continue to: Supportive relationships are powerful
Supportive relationships are powerful. Finally, to enhance social wellness, it would be difficult to overstate the potential benefits of positive, supportive, close relationships.42 However, the demands of a career in medicine, starting in medical school, have the potential for inhibiting (rather than enhancing) close relationships.
Placing value on relationships with friends and family members is essential. As Dr. M began experiencing burnout, he felt increasingly lonely, yet he isolated himself from those who cared about him. Dr. A felt lonely at home, even though she was surrounded by family. Physicians are often reluctant to initiate vulnerable communication with others, believing “no one wants to hear about my problems.” However, by realizing the need for help and asking friends and family for emotional support, physicians can improve their wellness. Fostering supportive relationships can help provide the resilience needed to address organizational stressors.
Tackling organizational challenges
Long hours and pressure to see large numbers of patients (production demands) are a challenge across practice settings. Limiting work hours has been effective in improving the well-being of physician trainees but has had an inconsistent effect on burnout.43,44
Organizations can offer flexible scheduling, and physicians considering limiting work hours may switch to part-time status or shift work. However, decreasing work hours may have the unintended consequence of increased stress as some physicians feel pressure to do more in less time.45 Therefore, it’s important to set clear boundaries around work time and when and where work tasks are completed (eg, home vs office).
How we use technology matters. Given technology’s ever-increasing role in medicine, organizations must identify and use the most efficient, effective technology for managing clerical processes. When physicians participate in these decisions and share their experiences, technology is likely to be more user-friendly and impose less stress.46
Continue to: If technology contributes to stress...
If technology contributes to stress by being too complex or impractical, it’s important to identify individuals in the workplace (eg, IT support or “super-users”) to help address these challenges. Organizations can implement multidisciplinary teams to address EHR challenges and decrease physician stress and burnout by training support staff to assist with clerical duties, allowing physicians to focus on patient care.47,48 Such organizational-directed interventions will be most successful when physicians are included in the decision-making process.47
Take on leadership roles to influence change. Leadership may be formal (involving a title and authority) or informal (leading by example). Health care organizations that are committed to the well-being of physicians will make the effort to improve the systems in which physicians work. Physicians working in organizations that are reluctant to change have several choices: implement individual strategies, take on leadership roles to influence change, or reconsider their fit for the organization. Physicians in solo practice might consider joining others in solo practices to share systems (call, phone triage, technical resources, etc) to implement some of these interventions.
Dr. A and Dr. M implement new wellness strategies
Dr. A and Dr. M have recently committed to addressing stressors in their lives and improving their wellness. Dr. A has become more assertive at work, highlighting her need for additional resources to function effectively. In response, her practice has hired scribes to assist in documenting visits. This success has inspired Dr. A to pay attention to her lifestyle choices. Gradually, she has begun to exercise and engage in healthy eating.
Dr. M has begun to utilize resources at his medical center to improve his EHR efficiency and patient flow. He has taken steps to address his financial concerns, developing a budget and spending judiciously. He practices mindfulness and ensures that he gets at least 7 hours of sleep per night, improving his mental and physical health. By doing so, he has more energy to connect with friends, exercise, and date.
CORRESPONDENCE
Margaret L. Smith, MD, MPH, MHSA, KUMC, Family Medicine and Community Health, 3901 Rainbow Boulevard – Mailstop 4010, Kansas City, KS 66160; msmith33@kumc.edu
1. Kane L. Physician burnout & depression report: stress, anxiety, and anger. Medscape. January 21, 2022. Accessed February 23, 2023. www.medscape.com/slideshow/2022-lifestyle-burnout-6014664
2. Lockwood L, Patel N, Bukelis I. 45.5 Physician burnout and the COVID-19 pandemic: the silent epidemic. J Am Acad Child Adolesc Psychiatry. 2021;60:S242. doi: 10.1016/j.jaac.2021.09.354
3. Shanafelt TD, West CP, Sinsky C, et al. Changes in burnout and satisfaction with work-life integration in physicians and the general US working population between 2011 and 2017. Mayo Clin Proc. 2019;94:1681-1694. doi: 10.1016/j.mayocp.2018.10.023
4. Maslach C, Leiter MP. Understanding the burnout experience: recent research and its implications for psychiatry. World Psychiatry. 2016;15:103-111. doi: 10.1002/wps.20311
5. Maslach C, Jackson SE. The measurement of experienced burnout. J Organ Behav. 1981;2:99-113. doi: 10.1002/job.4030020205
6. World Health Organization. Burn-out an “occupational phenomenon”: International Classification of Diseases. May 28, 2019. Accessed February 23, 2023. www.who.int/news/item/28-05-2019-burn-out-an-occupational-phenomenon-international-classification-of-diseases
7. Berg S. WHO adds burnout to ICD-11. What it means for physicians. American Medical Association. July 23, 2019. Accessed February 23, 2023. www.ama-assn.org/practice-management/physician-health/who-adds-burnout-icd-11-what-it-means-physicians
8. Brown SD, Goske MJ, Johnson CM. Beyond substance abuse: stress, burnout, and depression as causes of physician impairment and disruptive behavior. J Am Coll Radiol. 2009;6:479-485. doi: 10.1016/j.jacr.2008.11.029
9. Williams ES, Rathert C, Buttigieg SC. The personal and professional consequences of physician burnout: a systematic review of the literature. Med Care Res Rev. 2020;77:371-386. doi: 10.1177/ 1077558719856787
10. Yates SW. Physician Stress and Burnout. Am J Med. 2020;133:160-164. doi: 10.1016/j.amjmed.2019.08.034
11. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529. doi: 10.1111/joim.12752
12. Firth-Cozens J, Greenhalgh J. Doctors’ perceptions of the links between stress and lowered clinical care. Soc Sci Med. 1997;44:1017-1022. doi: 10.1016/s0277-9536(96)00227-4
13. Dewa CS, Loong D, Bonato S, et al. The relationship between physician burnout and quality of healthcare in terms of safety and acceptability: a systematic review. BMJ Open. 2017;7:e015141. doi: 10.1136/bmjopen-2016-015141
14. American Institute of Stress. What is stress? April 29, 2022. Accessed February 23, 2023. www.stress.org/daily-life
15. Regehr C, Glancy D, Pitts A, et al. Interventions to reduce the consequences of stress in physicians: a review and meta-analysis. J Nerv Ment Dis. 2014;202:353-359. doi: 10.1097/NMD. 0000000000000130
16. Fitzpatrick K, Patterson R, Morley K, et al. Physician wellness during a pandemic. West J Emerg Med. 2020;21:83-87. doi: 10.5811/westjem.2020.7.48472
17. Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91:836-848. doi: 10.1016/j.mayocp.2016.05.007
18. Arndt BG, Beasley JW, Watkinson MD, et al. Tethered to the EHR: primary care physician workload assessment using EHR event log data and time-motion observations. Ann Fam Med. 2017;15:419-426. doi: 10.1370/afm.2121
19. Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165:753-760. doi: 10.7326/M16-0961
20. Robertson SL, Robinson MD, Reid A. Electronic health record effects on work-life balance and burnout within the I3 Population Collaborative. J Grad Med Educ. 2017;9:479-484. doi: 10.4300/JGME-D-16-00123.1
21. Fares J, Al Tabosh H, Saadeddin Z, et al. Stress, burnout and coping strategies in preclinical medical students. N Am J Med Sci. 2016;8:75-81. doi: 10.4103/1947-2714.177299
22. Patel RS, Bachu R, Adikey A, et al. Factors related to physician burnout and its consequences: a review. Behav Sci (Basel). 2018; 8:98. doi: 10.3390/bs8110098
23. Shanafelt TD, Sloan JA, Habermann TM. The well-being of physicians. Am J Med. 2003;114:513-519. doi: 10.1016/s0002-9343(03)00117-7
24. Drummond D. Physician burnout: its origin, symptoms, and five main causes. Fam Pract Manag. 2015;22:42-47.
25. Brown PA, Slater M, Lofters A. Personality and burnout among primary care physicians: an international study. Psychol Res Behav Manag. 2019;12:169-177. doi: 10.2147/PRBM.S195633.
26. John OP, Donahue EM, Kentle RL. The Big Five Inventory – Versions 4A and 54. Institute of Personality and Social Research, University of California; 1991.
27. Wurm W, Vogel K, Holl A, et al. Depression-burnout overlap in physicians. PLoS One. 2016;11:e0149913. doi: 10.1371/journal.pone.0149913
28. Mehta SS, Edwards ML. Suffering in silence: Mental health stigma and physicians’ licensing fears. Am J Psychiatry Resid J. 2018;13:2-4.
29. Adam AR, Golu FT. Prevalence of depression among physicians: A comprehensive meta-analysis. Ro Med J. 2021;68:327-337. doi: 10.37897/RMJ.2021.3.1
30. Brady KJS, Trockel MT, Khan CT, et al. What do we mean by physician wellness? A systematic review of its definition and measurement. Acad Psychiatry. 2018;42:94-108. doi: 10.1007/s40596-017-0781-6
31. Shanafelt TD, Schein E, Minor LB, et al. Healing the professional culture of medicine. Mayo Clin Proc. 2019;94:1556-1566. doi: 10.1016/j.mayocp.2019.03.026
32. Horan S, Flaxman PE, Stride CB. The perfect recovery? Interactive influence of perfectionism and spillover work tasks on changes in exhaustion and mood around a vacation. J Occup Health Psychol. 2021;26:86-107. doi: 10.1037/ocp0000208
33. Patel RS, Sekhri S, Bhimanadham NN, et al. A review on strategies to manage physician burnout. Cureus. 2019;11:e4805. doi: 10.7759/cureus.4805
34. US Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd edition. US Department of Health and Human Services; 2018.
35. Kim ES, Chen Y, Nakamura JS, et al. Sense of purpose in life and subsequent physical, behavioral, and psychosocial health: an outcome-wide approach. Am J Health Promot. 2022;36:137-147. doi: 10.1177/08901171211038545
36. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383-388. doi: 10.1016/j.sleh.2017.07.013
37. Fordham B, Sugavanam T, Edwards K, et al. The evidence for cognitive behavioural therapy in any condition, population or context: a meta-review of systematic reviews and panoramic meta-analysis. Psychol Med. 2021;51:21-29. doi: 10.1017/S0033291720005292
38. Goldberg SB, Tucker RP, Greene PA, et al. Mindfulness-based interventions for psychiatric disorders: a systematic review and meta-analysis. Clin Psychol Rev. 2018;59:52-60. doi: 10.1016/j.cpr.2017.10.011
39. David D, Cristea I, Hofmann SG. Why cognitive behavioral therapy is the current gold standard of psychotherapy. Front Psychiatry. 2018;9:4. doi: 10.3389/fpsyt.2018.00004
40. Fendel JC, Bürkle JJ, Göritz AS. Mindfulness-based interventions to reduce burnout and stress in physicians: a systematic review and meta-analysis. Acad Med. 2021;96:751-764. doi: 10.1097/ACM.0000000000003936
41. Dahl CJ, Wilson-Mendenhall CD, Davidson RJ. The plasticity of well-being: a training-based framework for the cultivation of human flourishing. Proc Natl Acad Sci USA. 2020;117:32197-32206. doi: 10.1073/pnas.2014859117
42. Holt-Lunstad J. Why social relationships are important for physical health: a systems approach to understanding and modifying risk and protection. Annu Rev Psychol. 2018;69:437-458. doi: 10.1146/annurev-psych-122216-011902
43. Desai SV, Asch DA, Bellini LM, et al. Education outcomes in a duty-hour flexibility trial in internal medicine. N Engl J Med. 2018; 378:1494-1508. doi: 10.1056/NEJMoa1800965
44. Shea JA, Bellini LM, Dinges DF, et al. Impact of protected sleep period for internal medicine interns on overnight call on depression, burnout, and empathy. J Grad Med Educ. 2014;6:256-263. doi: 10.4300/JGME-D-13-00241.1
45. Morrow G, Burford B, Carter M, et al. Have restricted working hours reduced junior doctors’ experience of fatigue? A focus group and telephone interview study. BMJ Open. 2014;4:e004222. doi: 10.1136/bmjopen-2013-004222
46. Shanafelt TD, Noseworthy JH. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92:129-146. doi: 10.1016/j.mayocp.2016.10.004
47. Sequeira L, Almilaji K, Strudwick G, et al. EHR “SWAT” teams: a physician engagement initiative to improve Electronic Health Record (EHR) experiences and mitigate possible causes of EHR-related burnout. JAMA Open. 2021;4:1-7. doi: 10.1093/jamiaopen/ooab018
48. Smith PC, Lyon C, English AF, et al. Practice transformation under the University of Colorado’s primary care redesign model. Ann Fam Med. 2019;17:S24-S32. doi: 10.1370/afm.2424
1. Kane L. Physician burnout & depression report: stress, anxiety, and anger. Medscape. January 21, 2022. Accessed February 23, 2023. www.medscape.com/slideshow/2022-lifestyle-burnout-6014664
2. Lockwood L, Patel N, Bukelis I. 45.5 Physician burnout and the COVID-19 pandemic: the silent epidemic. J Am Acad Child Adolesc Psychiatry. 2021;60:S242. doi: 10.1016/j.jaac.2021.09.354
3. Shanafelt TD, West CP, Sinsky C, et al. Changes in burnout and satisfaction with work-life integration in physicians and the general US working population between 2011 and 2017. Mayo Clin Proc. 2019;94:1681-1694. doi: 10.1016/j.mayocp.2018.10.023
4. Maslach C, Leiter MP. Understanding the burnout experience: recent research and its implications for psychiatry. World Psychiatry. 2016;15:103-111. doi: 10.1002/wps.20311
5. Maslach C, Jackson SE. The measurement of experienced burnout. J Organ Behav. 1981;2:99-113. doi: 10.1002/job.4030020205
6. World Health Organization. Burn-out an “occupational phenomenon”: International Classification of Diseases. May 28, 2019. Accessed February 23, 2023. www.who.int/news/item/28-05-2019-burn-out-an-occupational-phenomenon-international-classification-of-diseases
7. Berg S. WHO adds burnout to ICD-11. What it means for physicians. American Medical Association. July 23, 2019. Accessed February 23, 2023. www.ama-assn.org/practice-management/physician-health/who-adds-burnout-icd-11-what-it-means-physicians
8. Brown SD, Goske MJ, Johnson CM. Beyond substance abuse: stress, burnout, and depression as causes of physician impairment and disruptive behavior. J Am Coll Radiol. 2009;6:479-485. doi: 10.1016/j.jacr.2008.11.029
9. Williams ES, Rathert C, Buttigieg SC. The personal and professional consequences of physician burnout: a systematic review of the literature. Med Care Res Rev. 2020;77:371-386. doi: 10.1177/ 1077558719856787
10. Yates SW. Physician Stress and Burnout. Am J Med. 2020;133:160-164. doi: 10.1016/j.amjmed.2019.08.034
11. West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529. doi: 10.1111/joim.12752
12. Firth-Cozens J, Greenhalgh J. Doctors’ perceptions of the links between stress and lowered clinical care. Soc Sci Med. 1997;44:1017-1022. doi: 10.1016/s0277-9536(96)00227-4
13. Dewa CS, Loong D, Bonato S, et al. The relationship between physician burnout and quality of healthcare in terms of safety and acceptability: a systematic review. BMJ Open. 2017;7:e015141. doi: 10.1136/bmjopen-2016-015141
14. American Institute of Stress. What is stress? April 29, 2022. Accessed February 23, 2023. www.stress.org/daily-life
15. Regehr C, Glancy D, Pitts A, et al. Interventions to reduce the consequences of stress in physicians: a review and meta-analysis. J Nerv Ment Dis. 2014;202:353-359. doi: 10.1097/NMD. 0000000000000130
16. Fitzpatrick K, Patterson R, Morley K, et al. Physician wellness during a pandemic. West J Emerg Med. 2020;21:83-87. doi: 10.5811/westjem.2020.7.48472
17. Shanafelt TD, Dyrbye LN, Sinsky C, et al. Relationship between clerical burden and characteristics of the electronic environment with physician burnout and professional satisfaction. Mayo Clin Proc. 2016;91:836-848. doi: 10.1016/j.mayocp.2016.05.007
18. Arndt BG, Beasley JW, Watkinson MD, et al. Tethered to the EHR: primary care physician workload assessment using EHR event log data and time-motion observations. Ann Fam Med. 2017;15:419-426. doi: 10.1370/afm.2121
19. Sinsky C, Colligan L, Li L, et al. Allocation of physician time in ambulatory practice: a time and motion study in 4 specialties. Ann Intern Med. 2016;165:753-760. doi: 10.7326/M16-0961
20. Robertson SL, Robinson MD, Reid A. Electronic health record effects on work-life balance and burnout within the I3 Population Collaborative. J Grad Med Educ. 2017;9:479-484. doi: 10.4300/JGME-D-16-00123.1
21. Fares J, Al Tabosh H, Saadeddin Z, et al. Stress, burnout and coping strategies in preclinical medical students. N Am J Med Sci. 2016;8:75-81. doi: 10.4103/1947-2714.177299
22. Patel RS, Bachu R, Adikey A, et al. Factors related to physician burnout and its consequences: a review. Behav Sci (Basel). 2018; 8:98. doi: 10.3390/bs8110098
23. Shanafelt TD, Sloan JA, Habermann TM. The well-being of physicians. Am J Med. 2003;114:513-519. doi: 10.1016/s0002-9343(03)00117-7
24. Drummond D. Physician burnout: its origin, symptoms, and five main causes. Fam Pract Manag. 2015;22:42-47.
25. Brown PA, Slater M, Lofters A. Personality and burnout among primary care physicians: an international study. Psychol Res Behav Manag. 2019;12:169-177. doi: 10.2147/PRBM.S195633.
26. John OP, Donahue EM, Kentle RL. The Big Five Inventory – Versions 4A and 54. Institute of Personality and Social Research, University of California; 1991.
27. Wurm W, Vogel K, Holl A, et al. Depression-burnout overlap in physicians. PLoS One. 2016;11:e0149913. doi: 10.1371/journal.pone.0149913
28. Mehta SS, Edwards ML. Suffering in silence: Mental health stigma and physicians’ licensing fears. Am J Psychiatry Resid J. 2018;13:2-4.
29. Adam AR, Golu FT. Prevalence of depression among physicians: A comprehensive meta-analysis. Ro Med J. 2021;68:327-337. doi: 10.37897/RMJ.2021.3.1
30. Brady KJS, Trockel MT, Khan CT, et al. What do we mean by physician wellness? A systematic review of its definition and measurement. Acad Psychiatry. 2018;42:94-108. doi: 10.1007/s40596-017-0781-6
31. Shanafelt TD, Schein E, Minor LB, et al. Healing the professional culture of medicine. Mayo Clin Proc. 2019;94:1556-1566. doi: 10.1016/j.mayocp.2019.03.026
32. Horan S, Flaxman PE, Stride CB. The perfect recovery? Interactive influence of perfectionism and spillover work tasks on changes in exhaustion and mood around a vacation. J Occup Health Psychol. 2021;26:86-107. doi: 10.1037/ocp0000208
33. Patel RS, Sekhri S, Bhimanadham NN, et al. A review on strategies to manage physician burnout. Cureus. 2019;11:e4805. doi: 10.7759/cureus.4805
34. US Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd edition. US Department of Health and Human Services; 2018.
35. Kim ES, Chen Y, Nakamura JS, et al. Sense of purpose in life and subsequent physical, behavioral, and psychosocial health: an outcome-wide approach. Am J Health Promot. 2022;36:137-147. doi: 10.1177/08901171211038545
36. Ogilvie RP, Patel SR. The epidemiology of sleep and obesity. Sleep Health. 2017;3:383-388. doi: 10.1016/j.sleh.2017.07.013
37. Fordham B, Sugavanam T, Edwards K, et al. The evidence for cognitive behavioural therapy in any condition, population or context: a meta-review of systematic reviews and panoramic meta-analysis. Psychol Med. 2021;51:21-29. doi: 10.1017/S0033291720005292
38. Goldberg SB, Tucker RP, Greene PA, et al. Mindfulness-based interventions for psychiatric disorders: a systematic review and meta-analysis. Clin Psychol Rev. 2018;59:52-60. doi: 10.1016/j.cpr.2017.10.011
39. David D, Cristea I, Hofmann SG. Why cognitive behavioral therapy is the current gold standard of psychotherapy. Front Psychiatry. 2018;9:4. doi: 10.3389/fpsyt.2018.00004
40. Fendel JC, Bürkle JJ, Göritz AS. Mindfulness-based interventions to reduce burnout and stress in physicians: a systematic review and meta-analysis. Acad Med. 2021;96:751-764. doi: 10.1097/ACM.0000000000003936
41. Dahl CJ, Wilson-Mendenhall CD, Davidson RJ. The plasticity of well-being: a training-based framework for the cultivation of human flourishing. Proc Natl Acad Sci USA. 2020;117:32197-32206. doi: 10.1073/pnas.2014859117
42. Holt-Lunstad J. Why social relationships are important for physical health: a systems approach to understanding and modifying risk and protection. Annu Rev Psychol. 2018;69:437-458. doi: 10.1146/annurev-psych-122216-011902
43. Desai SV, Asch DA, Bellini LM, et al. Education outcomes in a duty-hour flexibility trial in internal medicine. N Engl J Med. 2018; 378:1494-1508. doi: 10.1056/NEJMoa1800965
44. Shea JA, Bellini LM, Dinges DF, et al. Impact of protected sleep period for internal medicine interns on overnight call on depression, burnout, and empathy. J Grad Med Educ. 2014;6:256-263. doi: 10.4300/JGME-D-13-00241.1
45. Morrow G, Burford B, Carter M, et al. Have restricted working hours reduced junior doctors’ experience of fatigue? A focus group and telephone interview study. BMJ Open. 2014;4:e004222. doi: 10.1136/bmjopen-2013-004222
46. Shanafelt TD, Noseworthy JH. Executive leadership and physician well-being: nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92:129-146. doi: 10.1016/j.mayocp.2016.10.004
47. Sequeira L, Almilaji K, Strudwick G, et al. EHR “SWAT” teams: a physician engagement initiative to improve Electronic Health Record (EHR) experiences and mitigate possible causes of EHR-related burnout. JAMA Open. 2021;4:1-7. doi: 10.1093/jamiaopen/ooab018
48. Smith PC, Lyon C, English AF, et al. Practice transformation under the University of Colorado’s primary care redesign model. Ann Fam Med. 2019;17:S24-S32. doi: 10.1370/afm.2424
PRACTICE RECOMMENDATIONS
› Serve as a leader and positively influence the systems (ie, organizations, institutions, offices) in which you practice as a way to address organizational stress. C
› Establish and maintain positive, supportive, and close relationships with friends, family, and colleagues to improve social wellness. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series