User login
Moderate to severe back pain
The history and findings in this case are suggestive of axial psoriatic arthritis (PsA).
Psoriasis is a complex, chronic, inflammatory, immune-mediated disease that is associated with significant morbidity, reduced quality of life, and increased mortality. Approximately 7.4 million adults in the United States have psoriasis; worldwide, approximately 2%-3% of the population is affected. Patients with psoriasis frequently have comorbidities; PsA, an inflammatory, seronegative musculoskeletal disease, is among the most common. It is estimated that 25%-30% of patients with psoriasis develop PsA.
PsA is a heterogeneous disease. Patients may present with nail and skin changes, peripheral arthritis, enthesitis, dactylitis, and axial spondyloarthritis (SpA), either alone or in combination. Men and women are equally affected by PsA, which typically develops when patients are age 30-50 years. Like psoriasis, PsA is associated with numerous comorbidities, including cardiovascular disease, metabolic syndrome, obesity, diabetes, depression, uveitis, and anxiety.
PsA is a potentially erosive disease. Structural damage and functional impairment occurs within 2 years of initial assessment in approximately 50% of patients; as the disease progresses, patients may experience irreversible joint damage and disability. Axial involvement occurs in 25%-70% of patients with PsA; exclusive axial involvement is uncommon, occurring in 5% of patients. Common symptoms of axial PsA include inflammatory back pain (eg, pain that improves with activity but worsens with rest, morning stiffness lasting longer than 30 minutes). Some patients with axial involvement may be asymptomatic. If untreated, cervical spinal mobility and lateral flexion significantly decline within 5 years in patients with axial PsA. In addition, sacroiliitis worsens over time; 37% and 52% of patients develop grade 2 or higher sacroiliitis within 5 and 10 years, respectively. This highlights the importance of early identification and treatment of patients with axial PsA.
The diagnosis of axial PsA is confirmed by physical examination and imaging. Axial PsA characteristics, including sacroiliitis and spondylitis, are distinguished by the development of syndesmophytes (ie, ossification of the annulus fibrosis). PsA can be differentiated from ankylosing spondylitis by the asymmetric and frequently unilateral presentation of sacroiliitis and syndesmophytes, which frequently presents as nonmarginal, bulky, asymmetric, and discontinuous skipping vertebral levels.
Plain radiography, CT, ultrasound, and MRI are all useful tools for evaluating patients with PsA. MRI and ultrasound may be more sensitive than plain radiography is for detecting early joint inflammation and damage as well as axial changes, including sacroiliitis; however, they are not required for a diagnosis of PsA.
The treatment of axial PsA is based on international guidelines developed by the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the Assessment of SpondyloArthritis International Society–European League Against Rheumatism. Treatment focuses on minimizing pain, stiffness, and fatigue; improving and preserving spinal flexibility and posture; enhancing functional capacity; and maintaining the ability to work, with a target of remission or minimal/low disease activity.
Medications for symptomatic relief include nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and sacroiliac joint injections with glucocorticoids for mild disease; however, long-term treatment with systemic glucocorticoids is not recommended. If patients remain symptomatic or if erosive disease or other indications of high disease activity is observed, guidelines recommend initiation of a TNF inhibitor. Disease-modifying antirheumatic drugs, such as methotrexate, are not routinely prescribed for patients with axial disease because they have not been shown to be effective.
If symptoms of axial PsA are not controlled by NSAIDs, tumor necrosis factor (TNF) inhibitors are recommended. However, interleukin 17A inhibitors may be used in preference to TNF inhibitors in patients with significant skin involvement. In the United States, adalimumab, certolizumab pegol, golimumab, and infliximab are recommended over etanercept for patients with axial SpA in the presence of concomitant inflammatory bowel disease (IBD) or recurrent uveitis (although there is no evidence for golimumab) because etanercept has contradictory results for uveitis and has not been shown to have efficacy in IBD.
If patients fail to respond to a first trial of a TNF inhibitor, trying a second TNF inhibitor before switching to a different class of biologic is recommended by US guidelines. A Janus kinase inhibitor (tofacitinib) may be considered for patients who do not respond to TNF inhibitors.
Nonpharmacologic therapies (ie, exercise, physical therapy, massage therapy, occupational therapy, acupuncture) are recommended for all patients with active PsA.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of axial psoriatic arthritis (PsA).
Psoriasis is a complex, chronic, inflammatory, immune-mediated disease that is associated with significant morbidity, reduced quality of life, and increased mortality. Approximately 7.4 million adults in the United States have psoriasis; worldwide, approximately 2%-3% of the population is affected. Patients with psoriasis frequently have comorbidities; PsA, an inflammatory, seronegative musculoskeletal disease, is among the most common. It is estimated that 25%-30% of patients with psoriasis develop PsA.
PsA is a heterogeneous disease. Patients may present with nail and skin changes, peripheral arthritis, enthesitis, dactylitis, and axial spondyloarthritis (SpA), either alone or in combination. Men and women are equally affected by PsA, which typically develops when patients are age 30-50 years. Like psoriasis, PsA is associated with numerous comorbidities, including cardiovascular disease, metabolic syndrome, obesity, diabetes, depression, uveitis, and anxiety.
PsA is a potentially erosive disease. Structural damage and functional impairment occurs within 2 years of initial assessment in approximately 50% of patients; as the disease progresses, patients may experience irreversible joint damage and disability. Axial involvement occurs in 25%-70% of patients with PsA; exclusive axial involvement is uncommon, occurring in 5% of patients. Common symptoms of axial PsA include inflammatory back pain (eg, pain that improves with activity but worsens with rest, morning stiffness lasting longer than 30 minutes). Some patients with axial involvement may be asymptomatic. If untreated, cervical spinal mobility and lateral flexion significantly decline within 5 years in patients with axial PsA. In addition, sacroiliitis worsens over time; 37% and 52% of patients develop grade 2 or higher sacroiliitis within 5 and 10 years, respectively. This highlights the importance of early identification and treatment of patients with axial PsA.
The diagnosis of axial PsA is confirmed by physical examination and imaging. Axial PsA characteristics, including sacroiliitis and spondylitis, are distinguished by the development of syndesmophytes (ie, ossification of the annulus fibrosis). PsA can be differentiated from ankylosing spondylitis by the asymmetric and frequently unilateral presentation of sacroiliitis and syndesmophytes, which frequently presents as nonmarginal, bulky, asymmetric, and discontinuous skipping vertebral levels.
Plain radiography, CT, ultrasound, and MRI are all useful tools for evaluating patients with PsA. MRI and ultrasound may be more sensitive than plain radiography is for detecting early joint inflammation and damage as well as axial changes, including sacroiliitis; however, they are not required for a diagnosis of PsA.
The treatment of axial PsA is based on international guidelines developed by the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the Assessment of SpondyloArthritis International Society–European League Against Rheumatism. Treatment focuses on minimizing pain, stiffness, and fatigue; improving and preserving spinal flexibility and posture; enhancing functional capacity; and maintaining the ability to work, with a target of remission or minimal/low disease activity.
Medications for symptomatic relief include nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and sacroiliac joint injections with glucocorticoids for mild disease; however, long-term treatment with systemic glucocorticoids is not recommended. If patients remain symptomatic or if erosive disease or other indications of high disease activity is observed, guidelines recommend initiation of a TNF inhibitor. Disease-modifying antirheumatic drugs, such as methotrexate, are not routinely prescribed for patients with axial disease because they have not been shown to be effective.
If symptoms of axial PsA are not controlled by NSAIDs, tumor necrosis factor (TNF) inhibitors are recommended. However, interleukin 17A inhibitors may be used in preference to TNF inhibitors in patients with significant skin involvement. In the United States, adalimumab, certolizumab pegol, golimumab, and infliximab are recommended over etanercept for patients with axial SpA in the presence of concomitant inflammatory bowel disease (IBD) or recurrent uveitis (although there is no evidence for golimumab) because etanercept has contradictory results for uveitis and has not been shown to have efficacy in IBD.
If patients fail to respond to a first trial of a TNF inhibitor, trying a second TNF inhibitor before switching to a different class of biologic is recommended by US guidelines. A Janus kinase inhibitor (tofacitinib) may be considered for patients who do not respond to TNF inhibitors.
Nonpharmacologic therapies (ie, exercise, physical therapy, massage therapy, occupational therapy, acupuncture) are recommended for all patients with active PsA.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of axial psoriatic arthritis (PsA).
Psoriasis is a complex, chronic, inflammatory, immune-mediated disease that is associated with significant morbidity, reduced quality of life, and increased mortality. Approximately 7.4 million adults in the United States have psoriasis; worldwide, approximately 2%-3% of the population is affected. Patients with psoriasis frequently have comorbidities; PsA, an inflammatory, seronegative musculoskeletal disease, is among the most common. It is estimated that 25%-30% of patients with psoriasis develop PsA.
PsA is a heterogeneous disease. Patients may present with nail and skin changes, peripheral arthritis, enthesitis, dactylitis, and axial spondyloarthritis (SpA), either alone or in combination. Men and women are equally affected by PsA, which typically develops when patients are age 30-50 years. Like psoriasis, PsA is associated with numerous comorbidities, including cardiovascular disease, metabolic syndrome, obesity, diabetes, depression, uveitis, and anxiety.
PsA is a potentially erosive disease. Structural damage and functional impairment occurs within 2 years of initial assessment in approximately 50% of patients; as the disease progresses, patients may experience irreversible joint damage and disability. Axial involvement occurs in 25%-70% of patients with PsA; exclusive axial involvement is uncommon, occurring in 5% of patients. Common symptoms of axial PsA include inflammatory back pain (eg, pain that improves with activity but worsens with rest, morning stiffness lasting longer than 30 minutes). Some patients with axial involvement may be asymptomatic. If untreated, cervical spinal mobility and lateral flexion significantly decline within 5 years in patients with axial PsA. In addition, sacroiliitis worsens over time; 37% and 52% of patients develop grade 2 or higher sacroiliitis within 5 and 10 years, respectively. This highlights the importance of early identification and treatment of patients with axial PsA.
The diagnosis of axial PsA is confirmed by physical examination and imaging. Axial PsA characteristics, including sacroiliitis and spondylitis, are distinguished by the development of syndesmophytes (ie, ossification of the annulus fibrosis). PsA can be differentiated from ankylosing spondylitis by the asymmetric and frequently unilateral presentation of sacroiliitis and syndesmophytes, which frequently presents as nonmarginal, bulky, asymmetric, and discontinuous skipping vertebral levels.
Plain radiography, CT, ultrasound, and MRI are all useful tools for evaluating patients with PsA. MRI and ultrasound may be more sensitive than plain radiography is for detecting early joint inflammation and damage as well as axial changes, including sacroiliitis; however, they are not required for a diagnosis of PsA.
The treatment of axial PsA is based on international guidelines developed by the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network, the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis and the Assessment of SpondyloArthritis International Society–European League Against Rheumatism. Treatment focuses on minimizing pain, stiffness, and fatigue; improving and preserving spinal flexibility and posture; enhancing functional capacity; and maintaining the ability to work, with a target of remission or minimal/low disease activity.
Medications for symptomatic relief include nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticoids, and sacroiliac joint injections with glucocorticoids for mild disease; however, long-term treatment with systemic glucocorticoids is not recommended. If patients remain symptomatic or if erosive disease or other indications of high disease activity is observed, guidelines recommend initiation of a TNF inhibitor. Disease-modifying antirheumatic drugs, such as methotrexate, are not routinely prescribed for patients with axial disease because they have not been shown to be effective.
If symptoms of axial PsA are not controlled by NSAIDs, tumor necrosis factor (TNF) inhibitors are recommended. However, interleukin 17A inhibitors may be used in preference to TNF inhibitors in patients with significant skin involvement. In the United States, adalimumab, certolizumab pegol, golimumab, and infliximab are recommended over etanercept for patients with axial SpA in the presence of concomitant inflammatory bowel disease (IBD) or recurrent uveitis (although there is no evidence for golimumab) because etanercept has contradictory results for uveitis and has not been shown to have efficacy in IBD.
If patients fail to respond to a first trial of a TNF inhibitor, trying a second TNF inhibitor before switching to a different class of biologic is recommended by US guidelines. A Janus kinase inhibitor (tofacitinib) may be considered for patients who do not respond to TNF inhibitors.
Nonpharmacologic therapies (ie, exercise, physical therapy, massage therapy, occupational therapy, acupuncture) are recommended for all patients with active PsA.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
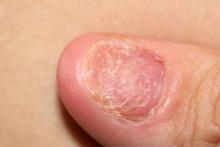
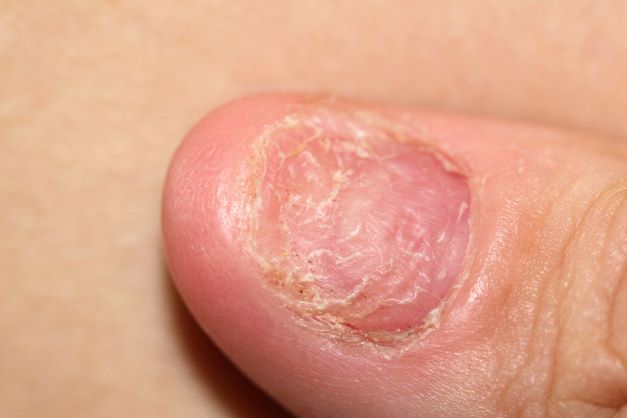
A 38-year-old nonsmoking woman presents with complaints of moderate to severe back pain of approximately 6 months' duration. She also reports morning back/neck stiffness that lasts for approximately 45 minutes and pain/stiffness in her wrists and fingers. The patient states that her back pain improves with exercise (walking and stretching) and worsens in the evening and during long periods of rest. On occasion, she is awakened during the early morning hours because of her back pain. The patient has a 15-year history of moderate to severe psoriasis and a history of irritable bowel disease (IBD). Current medications include cyclosporine 3 mg/d, topical roflumilast 0.3%/d, and loperamide 3 mg as needed. The patient is 5 ft 5 in and weighs 183 lb (BMI of 30.4).
Physical examination reveals psoriatic plaques on the hands, elbows, and knees and nail dystrophy (onycholysis and pitting). Vital signs are within normal ranges. Pertinent laboratory findings include white blood count of 12,000 mcL (> 50% polymorphonuclear leukocytes), erythrocyte sedimentation rate of 19 mm/h, and c-reactive protein of 3 mg/dL. Rheumatoid factor, antinuclear antibody, and anti-citrullinated protein antibody antibody were negative.
PsA Pathophysiology and Etiology
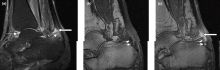
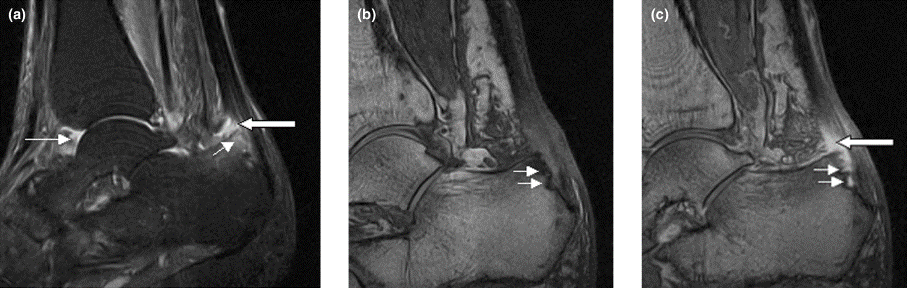
Right ankle pain and swelling
This patient's findings are consistent with a diagnosis of psoriatic enthesitis.
Enthesitis is a hallmark manifestation of psoriatic arthritis (PsA). Approximately 30% of patients with psoriasis are estimated to be affected by PsA, which belongs to the spondyloarthritis (SpA) family of inflammatory rheumatic diseases.
An enthesis is an attachment site of ligaments, tendons, and joint capsules to bone and is a key inflammatory target in SpA. It is a complex structure that dissipates biomechanical stress to preserve homeostasis. Entheses are anatomically and functionally integrated with bursa, fibrocartilage, and synovium in a synovial entheseal complex; biomechanical stress in this area may trigger inflammation. Enthesitis is an early manifestation of PsA that has been associated with radiographic peripheral/axial joint damage and severe disease, as well as reduced quality of life.
Enthesitis can be difficult to diagnose in clinical practice. Symptoms include tenderness, soreness, and pain at entheses on palpation, often without overt clinical evidence of inflammation. In contrast, dactylitis, another hallmark manifestation of PsA, can be recognized by swelling of an entire digit that is different from adjacent digits. Fibromyalgia frequently coexists with enthesitis, and it can be difficult to distinguish the two given the anatomic overlap between the tender points of fibromyalgia and many entheseal sites. Long-lasting morning stiffness and a sustained response to a course of steroids is more suggestive of enthesitis, whereas a higher number of somatoform symptoms is more suggestive of fibromyalgia.
Enthesitis is included in the Classification Criteria for Psoriatic Arthritis (CASPAR) as a hallmark of PsA. While it can be diagnosed clinically, imaging studies may be required, particularly in patients in whom symptoms may be difficult to discern. Evidence of enthesitis by conventional radiography includes bone cortex irregularities, erosions, entheseal soft tissue calcifications, and new bone formation; however, entheseal bone changes detected with conventional radiography appear relatively late in the disease process. Ultrasound is highly sensitive for assessing inflammation and can detect various features of enthesitis, such as increased thickness of tendon insertion, hypoechogenicity, erosions, enthesophytes, and subclinical enthesitis in people with PsA. MRI has the advantage of identifying perientheseal inflammation with adjacent bone marrow edema. Fat-suppressed MRI with or without gadolinium enhancement is a highly sensitive method for visualizing active enthesitis and can identify perientheseal inflammation with adjacent bone marrow edema.
Delayed treatment of PsA can result in irreversible joint damage and reduced quality of life; thus, patients with psoriasis should be closely monitored for early signs of its development, such as enthesitis. A thorough evaluation of the key clinical features of PsA (psoriasis, arthritis, enthesitis, dactylitis, and spondylitis), including evaluation of severity of each feature and impact on physical function and quality of life, is encouraged at each clinical encounter. Because patients may not understand the link between psoriasis and joint pain, specific probing questions can be helpful. Screening questionnaires to detect early signs and symptoms of PsA are available, such as the Psoriasis Epidemiology Screening Tool (PEST), Psoriatic Arthritis Screening and Evaluation (PASE) questionnaire, and Toronto Psoriatic Arthritis Screening (ToPAS) questionnaire. These and many others may be used to help dermatologists detect early signs and symptoms of PsA. Although these questionnaires all have limitations in sensitivity and specificity for the diagnosis of PsA, their use can still improve early diagnosis.
The treatment of PsA focuses on achieving the least amount of disease activity and inflammation possible; optimizing functional status, quality of life, and well-being; and preventing structural damage. Treatment decisions are based on the specific domains affected. Nonsteroidal anti-inflammatory drugs and corticosteroid injections are first-line treatments for enthesitis. Early use of tumor necrosis factor inhibitors (TNF) (adalimumab, certolizumab pegol, etanercept, infliximab, and golimumab) is recommended. Alternative biologic disease-modifying agents are indicated when these TNF inhibitors provide an inadequate response. They include ustekinumab (dual interleukin [IL]-12 and IL-23 inhibitor), secukinumab (IL-17A inhibitor), and apremilast (phosphodiesterase-4 inhibitor) and may be considered for patients with predominantly entheseal manifestations of PsA or dactylitis. Biological disease-modifying agents approved for PsA that have shown efficacy for enthesitis include ixekizumab (which targets IL-17A), abatacept (a T-cell inhibitor), guselkumab (monoclonal antibody), and ustekinumab (monoclonal antibody). Tofacitinib and upadacitinib, both oral Janus kinase inhibitors, may also be considered.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's findings are consistent with a diagnosis of psoriatic enthesitis.
Enthesitis is a hallmark manifestation of psoriatic arthritis (PsA). Approximately 30% of patients with psoriasis are estimated to be affected by PsA, which belongs to the spondyloarthritis (SpA) family of inflammatory rheumatic diseases.
An enthesis is an attachment site of ligaments, tendons, and joint capsules to bone and is a key inflammatory target in SpA. It is a complex structure that dissipates biomechanical stress to preserve homeostasis. Entheses are anatomically and functionally integrated with bursa, fibrocartilage, and synovium in a synovial entheseal complex; biomechanical stress in this area may trigger inflammation. Enthesitis is an early manifestation of PsA that has been associated with radiographic peripheral/axial joint damage and severe disease, as well as reduced quality of life.
Enthesitis can be difficult to diagnose in clinical practice. Symptoms include tenderness, soreness, and pain at entheses on palpation, often without overt clinical evidence of inflammation. In contrast, dactylitis, another hallmark manifestation of PsA, can be recognized by swelling of an entire digit that is different from adjacent digits. Fibromyalgia frequently coexists with enthesitis, and it can be difficult to distinguish the two given the anatomic overlap between the tender points of fibromyalgia and many entheseal sites. Long-lasting morning stiffness and a sustained response to a course of steroids is more suggestive of enthesitis, whereas a higher number of somatoform symptoms is more suggestive of fibromyalgia.
Enthesitis is included in the Classification Criteria for Psoriatic Arthritis (CASPAR) as a hallmark of PsA. While it can be diagnosed clinically, imaging studies may be required, particularly in patients in whom symptoms may be difficult to discern. Evidence of enthesitis by conventional radiography includes bone cortex irregularities, erosions, entheseal soft tissue calcifications, and new bone formation; however, entheseal bone changes detected with conventional radiography appear relatively late in the disease process. Ultrasound is highly sensitive for assessing inflammation and can detect various features of enthesitis, such as increased thickness of tendon insertion, hypoechogenicity, erosions, enthesophytes, and subclinical enthesitis in people with PsA. MRI has the advantage of identifying perientheseal inflammation with adjacent bone marrow edema. Fat-suppressed MRI with or without gadolinium enhancement is a highly sensitive method for visualizing active enthesitis and can identify perientheseal inflammation with adjacent bone marrow edema.
Delayed treatment of PsA can result in irreversible joint damage and reduced quality of life; thus, patients with psoriasis should be closely monitored for early signs of its development, such as enthesitis. A thorough evaluation of the key clinical features of PsA (psoriasis, arthritis, enthesitis, dactylitis, and spondylitis), including evaluation of severity of each feature and impact on physical function and quality of life, is encouraged at each clinical encounter. Because patients may not understand the link between psoriasis and joint pain, specific probing questions can be helpful. Screening questionnaires to detect early signs and symptoms of PsA are available, such as the Psoriasis Epidemiology Screening Tool (PEST), Psoriatic Arthritis Screening and Evaluation (PASE) questionnaire, and Toronto Psoriatic Arthritis Screening (ToPAS) questionnaire. These and many others may be used to help dermatologists detect early signs and symptoms of PsA. Although these questionnaires all have limitations in sensitivity and specificity for the diagnosis of PsA, their use can still improve early diagnosis.
The treatment of PsA focuses on achieving the least amount of disease activity and inflammation possible; optimizing functional status, quality of life, and well-being; and preventing structural damage. Treatment decisions are based on the specific domains affected. Nonsteroidal anti-inflammatory drugs and corticosteroid injections are first-line treatments for enthesitis. Early use of tumor necrosis factor inhibitors (TNF) (adalimumab, certolizumab pegol, etanercept, infliximab, and golimumab) is recommended. Alternative biologic disease-modifying agents are indicated when these TNF inhibitors provide an inadequate response. They include ustekinumab (dual interleukin [IL]-12 and IL-23 inhibitor), secukinumab (IL-17A inhibitor), and apremilast (phosphodiesterase-4 inhibitor) and may be considered for patients with predominantly entheseal manifestations of PsA or dactylitis. Biological disease-modifying agents approved for PsA that have shown efficacy for enthesitis include ixekizumab (which targets IL-17A), abatacept (a T-cell inhibitor), guselkumab (monoclonal antibody), and ustekinumab (monoclonal antibody). Tofacitinib and upadacitinib, both oral Janus kinase inhibitors, may also be considered.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's findings are consistent with a diagnosis of psoriatic enthesitis.
Enthesitis is a hallmark manifestation of psoriatic arthritis (PsA). Approximately 30% of patients with psoriasis are estimated to be affected by PsA, which belongs to the spondyloarthritis (SpA) family of inflammatory rheumatic diseases.
An enthesis is an attachment site of ligaments, tendons, and joint capsules to bone and is a key inflammatory target in SpA. It is a complex structure that dissipates biomechanical stress to preserve homeostasis. Entheses are anatomically and functionally integrated with bursa, fibrocartilage, and synovium in a synovial entheseal complex; biomechanical stress in this area may trigger inflammation. Enthesitis is an early manifestation of PsA that has been associated with radiographic peripheral/axial joint damage and severe disease, as well as reduced quality of life.
Enthesitis can be difficult to diagnose in clinical practice. Symptoms include tenderness, soreness, and pain at entheses on palpation, often without overt clinical evidence of inflammation. In contrast, dactylitis, another hallmark manifestation of PsA, can be recognized by swelling of an entire digit that is different from adjacent digits. Fibromyalgia frequently coexists with enthesitis, and it can be difficult to distinguish the two given the anatomic overlap between the tender points of fibromyalgia and many entheseal sites. Long-lasting morning stiffness and a sustained response to a course of steroids is more suggestive of enthesitis, whereas a higher number of somatoform symptoms is more suggestive of fibromyalgia.
Enthesitis is included in the Classification Criteria for Psoriatic Arthritis (CASPAR) as a hallmark of PsA. While it can be diagnosed clinically, imaging studies may be required, particularly in patients in whom symptoms may be difficult to discern. Evidence of enthesitis by conventional radiography includes bone cortex irregularities, erosions, entheseal soft tissue calcifications, and new bone formation; however, entheseal bone changes detected with conventional radiography appear relatively late in the disease process. Ultrasound is highly sensitive for assessing inflammation and can detect various features of enthesitis, such as increased thickness of tendon insertion, hypoechogenicity, erosions, enthesophytes, and subclinical enthesitis in people with PsA. MRI has the advantage of identifying perientheseal inflammation with adjacent bone marrow edema. Fat-suppressed MRI with or without gadolinium enhancement is a highly sensitive method for visualizing active enthesitis and can identify perientheseal inflammation with adjacent bone marrow edema.
Delayed treatment of PsA can result in irreversible joint damage and reduced quality of life; thus, patients with psoriasis should be closely monitored for early signs of its development, such as enthesitis. A thorough evaluation of the key clinical features of PsA (psoriasis, arthritis, enthesitis, dactylitis, and spondylitis), including evaluation of severity of each feature and impact on physical function and quality of life, is encouraged at each clinical encounter. Because patients may not understand the link between psoriasis and joint pain, specific probing questions can be helpful. Screening questionnaires to detect early signs and symptoms of PsA are available, such as the Psoriasis Epidemiology Screening Tool (PEST), Psoriatic Arthritis Screening and Evaluation (PASE) questionnaire, and Toronto Psoriatic Arthritis Screening (ToPAS) questionnaire. These and many others may be used to help dermatologists detect early signs and symptoms of PsA. Although these questionnaires all have limitations in sensitivity and specificity for the diagnosis of PsA, their use can still improve early diagnosis.
The treatment of PsA focuses on achieving the least amount of disease activity and inflammation possible; optimizing functional status, quality of life, and well-being; and preventing structural damage. Treatment decisions are based on the specific domains affected. Nonsteroidal anti-inflammatory drugs and corticosteroid injections are first-line treatments for enthesitis. Early use of tumor necrosis factor inhibitors (TNF) (adalimumab, certolizumab pegol, etanercept, infliximab, and golimumab) is recommended. Alternative biologic disease-modifying agents are indicated when these TNF inhibitors provide an inadequate response. They include ustekinumab (dual interleukin [IL]-12 and IL-23 inhibitor), secukinumab (IL-17A inhibitor), and apremilast (phosphodiesterase-4 inhibitor) and may be considered for patients with predominantly entheseal manifestations of PsA or dactylitis. Biological disease-modifying agents approved for PsA that have shown efficacy for enthesitis include ixekizumab (which targets IL-17A), abatacept (a T-cell inhibitor), guselkumab (monoclonal antibody), and ustekinumab (monoclonal antibody). Tofacitinib and upadacitinib, both oral Janus kinase inhibitors, may also be considered.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 42-year-old woman with a 20-year history of plaque psoriasis presents with complaints of a 3-month history of pain, tenderness, and swelling in her right ankle and foot, of unknown origin. Physical examination reveals active psoriasis, with a Psoriasis Area and Severity Index (PASI) score of 6.7 and psoriatic nail dystrophy, including onycholysis, pitting, and hyperkeratosis. Tenderness and swelling are noted at the back of the heel. The patient denies any other complaints. Laboratory tests are normal, including negative rheumatoid factor and antinuclear factor. MRI reveals soft tissue and bone marrow edema below the Achilles insertion.
PsA Guidelines
Elbow tenderness and swollen joints
The diagnosis for this case is psoriatic arthritis (PsA). The 2018 American College of Rheumatology/National Psoriasis Foundation guidelines offer current treatment recommendations for this condition. For patients with active PsA who are treatment-naive, treatment recommendations are:
- Tumor necrosis factor (TNF) inhibitors are preferred over oral small molecules (OSMs), interleukin (IL)–17 inhibitors, or IL-12/23 inhibitors
- OSMs are recommended over IL-17 inhibitors or IL-12/23 inhibitors
- Methotrexate is recommended over nonsteroidal anti-inflammatory drugs
- IL-17 inhibitors are recommended over IL-12/23 inhibitors
Treatment recommendations for patients with active PsA despite the use of OSMs are:
- Switching to a TNF inhibitor over another OSM, IL-17 or IL-12/23 inhibitors, abatacept, or tofacitinib
- Switching to an IL-17 inhibitor over another OSM, IL-12/23 inhibitor, abatacept, or tofacitinib
- Switching to an IL-12/23 inhibitor over another OSM, abatacept, or tofacitinib.
International groups, such as the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the European League Against Rheumatism (EULAR), have published treatment recommendations as well. These address both PsA and, to a lesser extent, psoriasis. The GRAPPA recommendations consider six domains of involvement (peripheral arthritis, axial disease, enthesitis, dactylitis, skin psoriasis, and nail psoriasis) and use a grid approach to account for various levels of disease activity and severity. The EULAR recommendations use an algorithmic approach that focuses mainly on musculoskeletal manifestations, specifically peripheral arthritis; manifestations, such as dactylitis, enthesitis, and skin and nail involvement, are considered separately.
Psoriasis precedes the onset of PsA in 60%-80% of patients (sometimes by up to 20 years but usually by less than 10 years); but in as many as 15%-20% of patients, arthritis appears before psoriasis. On occasion, arthritis and psoriasis appear simultaneously.
Patients with PsA are typically seronegative for rheumatoid factor and antinuclear antibody; antinuclear antibody titers in persons with PsA do not differ from those of age- and sex-matched controls. C-reactive protein may be elevated but is often normal. Lack of C-reactive protein elevation, however, does not mean that systemic inflammation is absent but rather indicates that a different type of systemic inflammation may be at play for those patients.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The diagnosis for this case is psoriatic arthritis (PsA). The 2018 American College of Rheumatology/National Psoriasis Foundation guidelines offer current treatment recommendations for this condition. For patients with active PsA who are treatment-naive, treatment recommendations are:
- Tumor necrosis factor (TNF) inhibitors are preferred over oral small molecules (OSMs), interleukin (IL)–17 inhibitors, or IL-12/23 inhibitors
- OSMs are recommended over IL-17 inhibitors or IL-12/23 inhibitors
- Methotrexate is recommended over nonsteroidal anti-inflammatory drugs
- IL-17 inhibitors are recommended over IL-12/23 inhibitors
Treatment recommendations for patients with active PsA despite the use of OSMs are:
- Switching to a TNF inhibitor over another OSM, IL-17 or IL-12/23 inhibitors, abatacept, or tofacitinib
- Switching to an IL-17 inhibitor over another OSM, IL-12/23 inhibitor, abatacept, or tofacitinib
- Switching to an IL-12/23 inhibitor over another OSM, abatacept, or tofacitinib.
International groups, such as the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the European League Against Rheumatism (EULAR), have published treatment recommendations as well. These address both PsA and, to a lesser extent, psoriasis. The GRAPPA recommendations consider six domains of involvement (peripheral arthritis, axial disease, enthesitis, dactylitis, skin psoriasis, and nail psoriasis) and use a grid approach to account for various levels of disease activity and severity. The EULAR recommendations use an algorithmic approach that focuses mainly on musculoskeletal manifestations, specifically peripheral arthritis; manifestations, such as dactylitis, enthesitis, and skin and nail involvement, are considered separately.
Psoriasis precedes the onset of PsA in 60%-80% of patients (sometimes by up to 20 years but usually by less than 10 years); but in as many as 15%-20% of patients, arthritis appears before psoriasis. On occasion, arthritis and psoriasis appear simultaneously.
Patients with PsA are typically seronegative for rheumatoid factor and antinuclear antibody; antinuclear antibody titers in persons with PsA do not differ from those of age- and sex-matched controls. C-reactive protein may be elevated but is often normal. Lack of C-reactive protein elevation, however, does not mean that systemic inflammation is absent but rather indicates that a different type of systemic inflammation may be at play for those patients.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The diagnosis for this case is psoriatic arthritis (PsA). The 2018 American College of Rheumatology/National Psoriasis Foundation guidelines offer current treatment recommendations for this condition. For patients with active PsA who are treatment-naive, treatment recommendations are:
- Tumor necrosis factor (TNF) inhibitors are preferred over oral small molecules (OSMs), interleukin (IL)–17 inhibitors, or IL-12/23 inhibitors
- OSMs are recommended over IL-17 inhibitors or IL-12/23 inhibitors
- Methotrexate is recommended over nonsteroidal anti-inflammatory drugs
- IL-17 inhibitors are recommended over IL-12/23 inhibitors
Treatment recommendations for patients with active PsA despite the use of OSMs are:
- Switching to a TNF inhibitor over another OSM, IL-17 or IL-12/23 inhibitors, abatacept, or tofacitinib
- Switching to an IL-17 inhibitor over another OSM, IL-12/23 inhibitor, abatacept, or tofacitinib
- Switching to an IL-12/23 inhibitor over another OSM, abatacept, or tofacitinib.
International groups, such as the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) and the European League Against Rheumatism (EULAR), have published treatment recommendations as well. These address both PsA and, to a lesser extent, psoriasis. The GRAPPA recommendations consider six domains of involvement (peripheral arthritis, axial disease, enthesitis, dactylitis, skin psoriasis, and nail psoriasis) and use a grid approach to account for various levels of disease activity and severity. The EULAR recommendations use an algorithmic approach that focuses mainly on musculoskeletal manifestations, specifically peripheral arthritis; manifestations, such as dactylitis, enthesitis, and skin and nail involvement, are considered separately.
Psoriasis precedes the onset of PsA in 60%-80% of patients (sometimes by up to 20 years but usually by less than 10 years); but in as many as 15%-20% of patients, arthritis appears before psoriasis. On occasion, arthritis and psoriasis appear simultaneously.
Patients with PsA are typically seronegative for rheumatoid factor and antinuclear antibody; antinuclear antibody titers in persons with PsA do not differ from those of age- and sex-matched controls. C-reactive protein may be elevated but is often normal. Lack of C-reactive protein elevation, however, does not mean that systemic inflammation is absent but rather indicates that a different type of systemic inflammation may be at play for those patients.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 45-year-old man presents with complaints of intermittent joint aches to the point that he can no longer golf and has trouble with his handwriting. He has a 6-year history of scalp psoriasis that he has controlled with a salicylic acid shampoo. On physical examination, he has tenderness over both elbows and in his metacarpophalangeal and proximal interphalangeal joints on both hands. Swollen joints are noted in the proximal and distal joints of the right hand. His fingernails show uniform pitting. Neurologic examination shows no sensory deficits or hyperesthesia. Motor examination is unremarkable, as are chest and abdominal findings. Blood pressure is 128/80 mm Hg. Radiographic findings showed periarticular soft-tissue swelling of the distal interphalangeal joints of the right second and fourth fingers and left thumb, although no significant bony abnormalities were observed. There is asymmetric narrowing of the joint space in the interphalangeal joints. Laboratory findings reveal an erythrocyte sedimentation rate of 35 mm/h, negative for rheumatoid factor, negative for antinuclear antibody, and C-reactive protein level of 9 mg/dL.