User login
MIAMI BEACH – The U.S. pivotal trial assessing renal denervation for blood pressure reduction began in October and a report on its final results won’t appear until next year, but excitement based on earlier study findings and the European clinical experience is already running high for this new approach to treating drug-resistant hypertension.
"Resistant hypertension is common, and is becoming more common despite a huge armamentarium of pharmacologic agents. The early literature [on renal denervation] is very impressive" for safety and efficacy, and the approach is plausibly "based on physiology," Dr. Michael R. Jaff said at ISET 2012, an international symposium on endovascular therapy. Moreover, on top of the expected antihypertensive effect of renal denervation, recent results from a pilot, randomized controlled study with 50 hypertensive patients suggested that the intervention could also help normalize glucose metabolism and reverse type 2 diabetes in some patients.
Renal denervation "could arguably be the most exciting advance in interventional vascular medicine," said Dr. Jaff, medical director of the vascular center at Massachusetts General Hospital in Boston. "People’s imaginations are running wild about the potential implications of this type of therapy for a whole host of patients. In the near term I’m incredibly bullish [about this treatment] and in the far term I’m even more enthusiastic."
In addition to the clinical efficacy that renal denervation so far has shown, it has the pluses of being relatively simple and easy to do, easy to learn, and fairly quick; the procedure involves modestly priced equipment and appears very safe as well, noted Dr. Horst Sievert, an interventional cardiologist who collaborated on one of the efficacy trials and now routinely performs renal denervations at the cardiovascular center of Saint Katharinen Hospital in Frankfurt, Germany.
The Symplicity Renal Denervation System, developed by Ardian, which is now owned by Medtronic, has been available for routine use in Europe and Australia since 2010. The U.S. pivotal trial, Symplicity HTN-3 (Renal Denervation in Patients With Uncontrolled Hypertension), plans to enroll 530 patients at 60 U.S. centers and should be completed by March of next year.
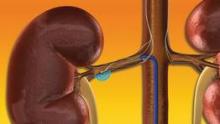
The procedure, which requires just a flexible catheter and radiofrequency generator, delivers four to eight low-power treatments along the length of both main renal arteries using a 6F sheath, targeting the renal nerves in the adventitia of the renal arteries and producing reduced sympathetic activity and renin release. According to Dr. Sievert, the typical procedure takes 45-60 minutes, and is successful in significantly reducing systolic and diastolic pressure in 75%-80% of drug-refractory patients. The worldwide case experience so far has led to few complications, no reported thrombus formations, no detrimental effects on renal blood flow or function, and no reported cases of severe hypotension following treatment, he said.
"I believe that renal denervation will become as important as PCI [percutaneous coronary intervention] or PTA [percutaneous transluminal angioplasty]," said Dr. Sievert, director of the cardiovascular center and also on the faculty of the University of Frankfurt.
"The risks are so few and the complication rate so low that it’s not worth figuring out which patients will respond and who won’t. I would just do the treatment and see if the patient responds," Dr. Sievert said at the meeting. No explanation exists as to why some patients don’t respond, and no marker exists to distinguish patients who will respond and those who won’t.
The largest systematic assessment of the antihypertensive impact of renal denervation came in a randomized trial, Symplicity HTN-2, with 106 patients, which was sponsored by Ardian and published in 2010. All patients had an entry systolic pressure of at least 160 mm Hg (or at least 150 mm Hg if they also had type 2 diabetes) despite current treatment with at least three antihypertensive medications. At 6 months after treatment, the 52 patients treated with denervation had an average blood pressure reduction of 32/12 mm Hg, compared with an average systolic pressure rise of 1 mm Hg and no average diastolic change in the 54 control patients (Lancet 2010;376:1903-9).
In the denervation group, 84% of patients had at least a 10-mm Hg drop in their systolic pressure 6 months after treatment compared with 35% of the control patients having this response, and 39% of the denervation patients had their systolic pressure fall below 140 mm Hg at 6 months compared with 6% of control patients. A subsequent report on a total of 153 patients treated with renal denervation documented that the blood pressure reductions seen at 6 months persisted out to 2 years (Hypertension 2011;57:911-7).
Striking as this effect was, perhaps even more notable was a report last year from a separate pilot study that randomized 50 patients who met the same definition of refractory hypertension. In addition to showing similar blood pressure results in the denervated patients, the researchers on this study also looked at several measures of glucose metabolism. At 3 months after intervention, the 37 denervated patients had an average 9% cut in their fasting blood glucose level and an average 12% drop in their average fasting blood insulin levels, both statistically significant changes from baseline, compared with no significant change in the 13 control patients. The percent of patients with normal glucose tolerance rose by 11% in the denervated group, and dropped by 7% in the control group. The authors of the report concluded that renal sympathetic activity plays a role in insulin resistance (Circulation 2011;123:1940-6).
Future studies will likely examine the possible impact of renal denervation on heart failure and on obstructive sleep apnea, noted Dr. Jaff.
Dr. Jaff said that he has financial relationships with several cardiovascular device companies including Medtronic Hansen Medical and Medtronic Vascular. Dr. Sievert said that he has financial relationships with several cardiovascular device and pharmaceutical companies including Ardian and Medtronic.
MIAMI BEACH – The U.S. pivotal trial assessing renal denervation for blood pressure reduction began in October and a report on its final results won’t appear until next year, but excitement based on earlier study findings and the European clinical experience is already running high for this new approach to treating drug-resistant hypertension.
"Resistant hypertension is common, and is becoming more common despite a huge armamentarium of pharmacologic agents. The early literature [on renal denervation] is very impressive" for safety and efficacy, and the approach is plausibly "based on physiology," Dr. Michael R. Jaff said at ISET 2012, an international symposium on endovascular therapy. Moreover, on top of the expected antihypertensive effect of renal denervation, recent results from a pilot, randomized controlled study with 50 hypertensive patients suggested that the intervention could also help normalize glucose metabolism and reverse type 2 diabetes in some patients.
Renal denervation "could arguably be the most exciting advance in interventional vascular medicine," said Dr. Jaff, medical director of the vascular center at Massachusetts General Hospital in Boston. "People’s imaginations are running wild about the potential implications of this type of therapy for a whole host of patients. In the near term I’m incredibly bullish [about this treatment] and in the far term I’m even more enthusiastic."
In addition to the clinical efficacy that renal denervation so far has shown, it has the pluses of being relatively simple and easy to do, easy to learn, and fairly quick; the procedure involves modestly priced equipment and appears very safe as well, noted Dr. Horst Sievert, an interventional cardiologist who collaborated on one of the efficacy trials and now routinely performs renal denervations at the cardiovascular center of Saint Katharinen Hospital in Frankfurt, Germany.
The Symplicity Renal Denervation System, developed by Ardian, which is now owned by Medtronic, has been available for routine use in Europe and Australia since 2010. The U.S. pivotal trial, Symplicity HTN-3 (Renal Denervation in Patients With Uncontrolled Hypertension), plans to enroll 530 patients at 60 U.S. centers and should be completed by March of next year.
The procedure, which requires just a flexible catheter and radiofrequency generator, delivers four to eight low-power treatments along the length of both main renal arteries using a 6F sheath, targeting the renal nerves in the adventitia of the renal arteries and producing reduced sympathetic activity and renin release. According to Dr. Sievert, the typical procedure takes 45-60 minutes, and is successful in significantly reducing systolic and diastolic pressure in 75%-80% of drug-refractory patients. The worldwide case experience so far has led to few complications, no reported thrombus formations, no detrimental effects on renal blood flow or function, and no reported cases of severe hypotension following treatment, he said.
"I believe that renal denervation will become as important as PCI [percutaneous coronary intervention] or PTA [percutaneous transluminal angioplasty]," said Dr. Sievert, director of the cardiovascular center and also on the faculty of the University of Frankfurt.
"The risks are so few and the complication rate so low that it’s not worth figuring out which patients will respond and who won’t. I would just do the treatment and see if the patient responds," Dr. Sievert said at the meeting. No explanation exists as to why some patients don’t respond, and no marker exists to distinguish patients who will respond and those who won’t.
The largest systematic assessment of the antihypertensive impact of renal denervation came in a randomized trial, Symplicity HTN-2, with 106 patients, which was sponsored by Ardian and published in 2010. All patients had an entry systolic pressure of at least 160 mm Hg (or at least 150 mm Hg if they also had type 2 diabetes) despite current treatment with at least three antihypertensive medications. At 6 months after treatment, the 52 patients treated with denervation had an average blood pressure reduction of 32/12 mm Hg, compared with an average systolic pressure rise of 1 mm Hg and no average diastolic change in the 54 control patients (Lancet 2010;376:1903-9).
In the denervation group, 84% of patients had at least a 10-mm Hg drop in their systolic pressure 6 months after treatment compared with 35% of the control patients having this response, and 39% of the denervation patients had their systolic pressure fall below 140 mm Hg at 6 months compared with 6% of control patients. A subsequent report on a total of 153 patients treated with renal denervation documented that the blood pressure reductions seen at 6 months persisted out to 2 years (Hypertension 2011;57:911-7).
Striking as this effect was, perhaps even more notable was a report last year from a separate pilot study that randomized 50 patients who met the same definition of refractory hypertension. In addition to showing similar blood pressure results in the denervated patients, the researchers on this study also looked at several measures of glucose metabolism. At 3 months after intervention, the 37 denervated patients had an average 9% cut in their fasting blood glucose level and an average 12% drop in their average fasting blood insulin levels, both statistically significant changes from baseline, compared with no significant change in the 13 control patients. The percent of patients with normal glucose tolerance rose by 11% in the denervated group, and dropped by 7% in the control group. The authors of the report concluded that renal sympathetic activity plays a role in insulin resistance (Circulation 2011;123:1940-6).
Future studies will likely examine the possible impact of renal denervation on heart failure and on obstructive sleep apnea, noted Dr. Jaff.
Dr. Jaff said that he has financial relationships with several cardiovascular device companies including Medtronic Hansen Medical and Medtronic Vascular. Dr. Sievert said that he has financial relationships with several cardiovascular device and pharmaceutical companies including Ardian and Medtronic.
MIAMI BEACH – The U.S. pivotal trial assessing renal denervation for blood pressure reduction began in October and a report on its final results won’t appear until next year, but excitement based on earlier study findings and the European clinical experience is already running high for this new approach to treating drug-resistant hypertension.
"Resistant hypertension is common, and is becoming more common despite a huge armamentarium of pharmacologic agents. The early literature [on renal denervation] is very impressive" for safety and efficacy, and the approach is plausibly "based on physiology," Dr. Michael R. Jaff said at ISET 2012, an international symposium on endovascular therapy. Moreover, on top of the expected antihypertensive effect of renal denervation, recent results from a pilot, randomized controlled study with 50 hypertensive patients suggested that the intervention could also help normalize glucose metabolism and reverse type 2 diabetes in some patients.
Renal denervation "could arguably be the most exciting advance in interventional vascular medicine," said Dr. Jaff, medical director of the vascular center at Massachusetts General Hospital in Boston. "People’s imaginations are running wild about the potential implications of this type of therapy for a whole host of patients. In the near term I’m incredibly bullish [about this treatment] and in the far term I’m even more enthusiastic."
In addition to the clinical efficacy that renal denervation so far has shown, it has the pluses of being relatively simple and easy to do, easy to learn, and fairly quick; the procedure involves modestly priced equipment and appears very safe as well, noted Dr. Horst Sievert, an interventional cardiologist who collaborated on one of the efficacy trials and now routinely performs renal denervations at the cardiovascular center of Saint Katharinen Hospital in Frankfurt, Germany.
The Symplicity Renal Denervation System, developed by Ardian, which is now owned by Medtronic, has been available for routine use in Europe and Australia since 2010. The U.S. pivotal trial, Symplicity HTN-3 (Renal Denervation in Patients With Uncontrolled Hypertension), plans to enroll 530 patients at 60 U.S. centers and should be completed by March of next year.
The procedure, which requires just a flexible catheter and radiofrequency generator, delivers four to eight low-power treatments along the length of both main renal arteries using a 6F sheath, targeting the renal nerves in the adventitia of the renal arteries and producing reduced sympathetic activity and renin release. According to Dr. Sievert, the typical procedure takes 45-60 minutes, and is successful in significantly reducing systolic and diastolic pressure in 75%-80% of drug-refractory patients. The worldwide case experience so far has led to few complications, no reported thrombus formations, no detrimental effects on renal blood flow or function, and no reported cases of severe hypotension following treatment, he said.
"I believe that renal denervation will become as important as PCI [percutaneous coronary intervention] or PTA [percutaneous transluminal angioplasty]," said Dr. Sievert, director of the cardiovascular center and also on the faculty of the University of Frankfurt.
"The risks are so few and the complication rate so low that it’s not worth figuring out which patients will respond and who won’t. I would just do the treatment and see if the patient responds," Dr. Sievert said at the meeting. No explanation exists as to why some patients don’t respond, and no marker exists to distinguish patients who will respond and those who won’t.
The largest systematic assessment of the antihypertensive impact of renal denervation came in a randomized trial, Symplicity HTN-2, with 106 patients, which was sponsored by Ardian and published in 2010. All patients had an entry systolic pressure of at least 160 mm Hg (or at least 150 mm Hg if they also had type 2 diabetes) despite current treatment with at least three antihypertensive medications. At 6 months after treatment, the 52 patients treated with denervation had an average blood pressure reduction of 32/12 mm Hg, compared with an average systolic pressure rise of 1 mm Hg and no average diastolic change in the 54 control patients (Lancet 2010;376:1903-9).
In the denervation group, 84% of patients had at least a 10-mm Hg drop in their systolic pressure 6 months after treatment compared with 35% of the control patients having this response, and 39% of the denervation patients had their systolic pressure fall below 140 mm Hg at 6 months compared with 6% of control patients. A subsequent report on a total of 153 patients treated with renal denervation documented that the blood pressure reductions seen at 6 months persisted out to 2 years (Hypertension 2011;57:911-7).
Striking as this effect was, perhaps even more notable was a report last year from a separate pilot study that randomized 50 patients who met the same definition of refractory hypertension. In addition to showing similar blood pressure results in the denervated patients, the researchers on this study also looked at several measures of glucose metabolism. At 3 months after intervention, the 37 denervated patients had an average 9% cut in their fasting blood glucose level and an average 12% drop in their average fasting blood insulin levels, both statistically significant changes from baseline, compared with no significant change in the 13 control patients. The percent of patients with normal glucose tolerance rose by 11% in the denervated group, and dropped by 7% in the control group. The authors of the report concluded that renal sympathetic activity plays a role in insulin resistance (Circulation 2011;123:1940-6).
Future studies will likely examine the possible impact of renal denervation on heart failure and on obstructive sleep apnea, noted Dr. Jaff.
Dr. Jaff said that he has financial relationships with several cardiovascular device companies including Medtronic Hansen Medical and Medtronic Vascular. Dr. Sievert said that he has financial relationships with several cardiovascular device and pharmaceutical companies including Ardian and Medtronic.
EXPERT ANALYSIS FROM ISET 2012, AN INTERNATIONAL SYMPOSIUM ON ENDOVASCULAR THERAPY