User login
American Heart Association (AHA): Scientific Sessions 2013
Anakinra showed benefits in diastolic heart failure
DALLAS – Interleukin-1 blockade resulted in significantly improved exercise capacity and a corresponding reduction in systemic inflammation in patients with heart failure with preserved ejection fraction in the small, pilot D-HART study.
"These results confirm the role of IL-1 in regulating cardiovascular function and provide the first evidence that an IL-1-targeted therapeutic strategy may be valuable in HFpEF [heart failure with preserved ejection fraction]," Benjamin W. Van Tassell, Pharm.D., declared at the American Heart Association scientific sessions.
The findings are welcome given that there are no approved therapies for HFpEF, which accounts for at least half of all cases of heart failure, noted Dr. Van Tassell of Virginia Commonwealth University, Richmond.
D-HART was a randomized, double-blind, placebo-controlled study involving 12 patients with symptomatic HFpEF and high levels of systemic inflammation as reflected in their elevated baseline high-sensitivity C-reactive protein levels. Patients were placed on 14 days of daily subcutaneous injections of the IL-1 antagonist anakinra (Kineret) at 100 mg or placebo, then crossed over to the other study arm. Half of the subjects had New York Heart Association (NYHA) class II heart failure, the other half class III.
Since heart failure is a syndrome of exercise intolerance secondary to impaired cardiac function, Dr. Van Tassell and his coinvestigators chose change in peak oxygen consumption (VO2) as the primary study endpoint. The median peak VO2 during the anakinra phase of the study was 16.3 mL/kg per minute, which was a 1.2–mL/kg per minute or 8% improvement over the peak value on placebo.
The improvement in peak VO2 correlated with a sharp reduction in high-sensitivity C-reactive protein (hsCRP), a surrogate marker for IL-1 activity. From a baseline of 7.5 mg/L, hsCRP levels plunged by 84% after 14 days on anakinra compared to 10% with placebo.
Ventilatory efficiency (VE) improved with IL-1 blockade as evidenced by a significant increase in the oxygen uptake efficiency slope. However, the VE/VCO2 slope – another measure of ventilatory efficiency – improved significantly with anakinra only in the five patients with above-median values at baseline. Their 12% improvement in this metric during IL-1 inhibition implies that maximum benefit occurs in patients with impaired baseline function, according to Dr. Van Tassell.
He added that these encouraging findings from D-HART provide a sound basis for larger confirmatory randomized trials evaluating IL-1 inhibition in patients with HFpEF. Anakinra is approved for the treatment of rheumatoid arthritis.
D-HART was supported by the American Heart Association and university funds. Dr. Van Tassell reported having no financial conflicts of interest.
DALLAS – Interleukin-1 blockade resulted in significantly improved exercise capacity and a corresponding reduction in systemic inflammation in patients with heart failure with preserved ejection fraction in the small, pilot D-HART study.
"These results confirm the role of IL-1 in regulating cardiovascular function and provide the first evidence that an IL-1-targeted therapeutic strategy may be valuable in HFpEF [heart failure with preserved ejection fraction]," Benjamin W. Van Tassell, Pharm.D., declared at the American Heart Association scientific sessions.
The findings are welcome given that there are no approved therapies for HFpEF, which accounts for at least half of all cases of heart failure, noted Dr. Van Tassell of Virginia Commonwealth University, Richmond.
D-HART was a randomized, double-blind, placebo-controlled study involving 12 patients with symptomatic HFpEF and high levels of systemic inflammation as reflected in their elevated baseline high-sensitivity C-reactive protein levels. Patients were placed on 14 days of daily subcutaneous injections of the IL-1 antagonist anakinra (Kineret) at 100 mg or placebo, then crossed over to the other study arm. Half of the subjects had New York Heart Association (NYHA) class II heart failure, the other half class III.
Since heart failure is a syndrome of exercise intolerance secondary to impaired cardiac function, Dr. Van Tassell and his coinvestigators chose change in peak oxygen consumption (VO2) as the primary study endpoint. The median peak VO2 during the anakinra phase of the study was 16.3 mL/kg per minute, which was a 1.2–mL/kg per minute or 8% improvement over the peak value on placebo.
The improvement in peak VO2 correlated with a sharp reduction in high-sensitivity C-reactive protein (hsCRP), a surrogate marker for IL-1 activity. From a baseline of 7.5 mg/L, hsCRP levels plunged by 84% after 14 days on anakinra compared to 10% with placebo.
Ventilatory efficiency (VE) improved with IL-1 blockade as evidenced by a significant increase in the oxygen uptake efficiency slope. However, the VE/VCO2 slope – another measure of ventilatory efficiency – improved significantly with anakinra only in the five patients with above-median values at baseline. Their 12% improvement in this metric during IL-1 inhibition implies that maximum benefit occurs in patients with impaired baseline function, according to Dr. Van Tassell.
He added that these encouraging findings from D-HART provide a sound basis for larger confirmatory randomized trials evaluating IL-1 inhibition in patients with HFpEF. Anakinra is approved for the treatment of rheumatoid arthritis.
D-HART was supported by the American Heart Association and university funds. Dr. Van Tassell reported having no financial conflicts of interest.
DALLAS – Interleukin-1 blockade resulted in significantly improved exercise capacity and a corresponding reduction in systemic inflammation in patients with heart failure with preserved ejection fraction in the small, pilot D-HART study.
"These results confirm the role of IL-1 in regulating cardiovascular function and provide the first evidence that an IL-1-targeted therapeutic strategy may be valuable in HFpEF [heart failure with preserved ejection fraction]," Benjamin W. Van Tassell, Pharm.D., declared at the American Heart Association scientific sessions.
The findings are welcome given that there are no approved therapies for HFpEF, which accounts for at least half of all cases of heart failure, noted Dr. Van Tassell of Virginia Commonwealth University, Richmond.
D-HART was a randomized, double-blind, placebo-controlled study involving 12 patients with symptomatic HFpEF and high levels of systemic inflammation as reflected in their elevated baseline high-sensitivity C-reactive protein levels. Patients were placed on 14 days of daily subcutaneous injections of the IL-1 antagonist anakinra (Kineret) at 100 mg or placebo, then crossed over to the other study arm. Half of the subjects had New York Heart Association (NYHA) class II heart failure, the other half class III.
Since heart failure is a syndrome of exercise intolerance secondary to impaired cardiac function, Dr. Van Tassell and his coinvestigators chose change in peak oxygen consumption (VO2) as the primary study endpoint. The median peak VO2 during the anakinra phase of the study was 16.3 mL/kg per minute, which was a 1.2–mL/kg per minute or 8% improvement over the peak value on placebo.
The improvement in peak VO2 correlated with a sharp reduction in high-sensitivity C-reactive protein (hsCRP), a surrogate marker for IL-1 activity. From a baseline of 7.5 mg/L, hsCRP levels plunged by 84% after 14 days on anakinra compared to 10% with placebo.
Ventilatory efficiency (VE) improved with IL-1 blockade as evidenced by a significant increase in the oxygen uptake efficiency slope. However, the VE/VCO2 slope – another measure of ventilatory efficiency – improved significantly with anakinra only in the five patients with above-median values at baseline. Their 12% improvement in this metric during IL-1 inhibition implies that maximum benefit occurs in patients with impaired baseline function, according to Dr. Van Tassell.
He added that these encouraging findings from D-HART provide a sound basis for larger confirmatory randomized trials evaluating IL-1 inhibition in patients with HFpEF. Anakinra is approved for the treatment of rheumatoid arthritis.
D-HART was supported by the American Heart Association and university funds. Dr. Van Tassell reported having no financial conflicts of interest.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: Patients with heart failure with preserved ejection fraction showed a significant 8% placebo-subtracted gain in peak oxygen consumption after 14 days of interleukin-1 inhibition via daily anakinra. This was accompanied by an 84% reduction in baseline elevated high-sensitivity C-reactive protein levels.
Data source: D-HART, a randomized, double-blind pilot study in which 12 patients with heart failure with preserved ejection fraction were placed on 14 days of daily subcutaneous injections of anakinra at 100 mg or placebo, then crossed to 14 days of the alternative.
Disclosures: D-HART was supported by the American Heart Association and university funds. Dr. Van Tassell reported having no financial conflicts of interest.
Healthy diet after diabetes diagnosis improves survival
DALLAS – Women who embraced a healthy diet after being diagnosed with type 2 diabetes had significantly lower rates of all-cause and cardiovascular mortality than did those with a bad diet, in a large observational study with up to 26 years of follow-up.
"It’s never too late to improve your diet," Dr. Hyun Joon Shin said in presenting his analysis of data from the Nurses’ Health Study at the American Heart Association scientific sessions.
The prospective observational Nurses’ Health Study began in 1976. It included 121,700 female American nurses aged 30-55 upon enrollment. Dr. Shin’s analysis included 8,354 women with no baseline cardiovascular disease, diabetes, or cancer who were diagnosed with type 2 diabetes during 1984-2006 and followed to June 2010.
During follow-up, there were 1,183 deaths in the cohort diagnosed with type 2 diabetes, including 491 deaths due to cardiovascular disease and 514 due to cancer.
Mortality risks were evaluated in relation to the extent to which the nurses adhered to a healthy, high-quality diet following their diagnosis of type 2 diabetes. The yardstick employed in evaluating eating patterns was the Alternative Healthy Eating Index–2010 (AHEI). The index, developed by researchers at Harvard University, scores patients’ consumption of various foods and nutrients known to be predictive of chronic disease risk, explained Dr. Shin of Baylor University Medical Center, Dallas.
There were 14.4 deaths per 1,000 persons per year in the top quintile in terms of adherence to the AHEI. A dose-response relationship was evident: The mortality rate was 28.8/1,000 persons per year among those in the bottom quintile, 23.1/1,000 in the second quintile, 19.3 in the third, and 20.7 in the fourth.
In a multivariate analysis adjusted for numerous potential confounders, including a participant’s prediabetic AHEI score, the diabetic nurses in the top quintile in terms of healthy eating had a 52% reduction in the risk of all-cause mortality compared with those in the lowest AHEI quintile. The healthiest eaters also had a 49% lower risk of cardiovascular mortality. However, their more modest 28% reduction in the risk of cancer-related death fell just short of statistical significance.
The AHEI, updated in 2010, awards points for increased consumption of whole grains, nuts, legumes, vegetables, fruits, fish or fish oil, and moderate alcohol intake. The index penalizes for consumption of red meat, processed meat products, trans fats, sodium, sugar-sweetened beverages, and fruit juices (J. Nutr. 2012;142:1009-18).
In examining the study findings more closely in terms of the impact of individual dietary components of the AHEI, Dr. Shin found nearly all of them had significant effects in multivariate analyses. For example, subjects in the top quintile for whole grain consumption had a 41% reduction in the risk of all-cause mortality compared with those in the bottom quintile, as well as a 33% reduction in the risk of cardiovascular mortality. Participants who drank the least amount of sugar-sweetened beverages had reductions in all-cause and cardiovascular mortality of 26% and 37%, compared with these rates for the quintile of biggest quaffers.
This was the first-ever postdiabetes dietary pattern analysis examining mortality in women with incident type 2 diabetes, according to Dr. Shin. The mechanism for the observed inverse relationship between diet quality and mortality is straightforward: A poor-quality diet is associated with increased risks of coronary heart disease, stroke, and weight gain and higher circulating levels of fasting insulin, inflammatory cytokines, and leptin, he said.
D. Shin’s study was supported by Baylor University research funding. He reported having no relevant financial conflicts.
DALLAS – Women who embraced a healthy diet after being diagnosed with type 2 diabetes had significantly lower rates of all-cause and cardiovascular mortality than did those with a bad diet, in a large observational study with up to 26 years of follow-up.
"It’s never too late to improve your diet," Dr. Hyun Joon Shin said in presenting his analysis of data from the Nurses’ Health Study at the American Heart Association scientific sessions.
The prospective observational Nurses’ Health Study began in 1976. It included 121,700 female American nurses aged 30-55 upon enrollment. Dr. Shin’s analysis included 8,354 women with no baseline cardiovascular disease, diabetes, or cancer who were diagnosed with type 2 diabetes during 1984-2006 and followed to June 2010.
During follow-up, there were 1,183 deaths in the cohort diagnosed with type 2 diabetes, including 491 deaths due to cardiovascular disease and 514 due to cancer.
Mortality risks were evaluated in relation to the extent to which the nurses adhered to a healthy, high-quality diet following their diagnosis of type 2 diabetes. The yardstick employed in evaluating eating patterns was the Alternative Healthy Eating Index–2010 (AHEI). The index, developed by researchers at Harvard University, scores patients’ consumption of various foods and nutrients known to be predictive of chronic disease risk, explained Dr. Shin of Baylor University Medical Center, Dallas.
There were 14.4 deaths per 1,000 persons per year in the top quintile in terms of adherence to the AHEI. A dose-response relationship was evident: The mortality rate was 28.8/1,000 persons per year among those in the bottom quintile, 23.1/1,000 in the second quintile, 19.3 in the third, and 20.7 in the fourth.
In a multivariate analysis adjusted for numerous potential confounders, including a participant’s prediabetic AHEI score, the diabetic nurses in the top quintile in terms of healthy eating had a 52% reduction in the risk of all-cause mortality compared with those in the lowest AHEI quintile. The healthiest eaters also had a 49% lower risk of cardiovascular mortality. However, their more modest 28% reduction in the risk of cancer-related death fell just short of statistical significance.
The AHEI, updated in 2010, awards points for increased consumption of whole grains, nuts, legumes, vegetables, fruits, fish or fish oil, and moderate alcohol intake. The index penalizes for consumption of red meat, processed meat products, trans fats, sodium, sugar-sweetened beverages, and fruit juices (J. Nutr. 2012;142:1009-18).
In examining the study findings more closely in terms of the impact of individual dietary components of the AHEI, Dr. Shin found nearly all of them had significant effects in multivariate analyses. For example, subjects in the top quintile for whole grain consumption had a 41% reduction in the risk of all-cause mortality compared with those in the bottom quintile, as well as a 33% reduction in the risk of cardiovascular mortality. Participants who drank the least amount of sugar-sweetened beverages had reductions in all-cause and cardiovascular mortality of 26% and 37%, compared with these rates for the quintile of biggest quaffers.
This was the first-ever postdiabetes dietary pattern analysis examining mortality in women with incident type 2 diabetes, according to Dr. Shin. The mechanism for the observed inverse relationship between diet quality and mortality is straightforward: A poor-quality diet is associated with increased risks of coronary heart disease, stroke, and weight gain and higher circulating levels of fasting insulin, inflammatory cytokines, and leptin, he said.
D. Shin’s study was supported by Baylor University research funding. He reported having no relevant financial conflicts.
DALLAS – Women who embraced a healthy diet after being diagnosed with type 2 diabetes had significantly lower rates of all-cause and cardiovascular mortality than did those with a bad diet, in a large observational study with up to 26 years of follow-up.
"It’s never too late to improve your diet," Dr. Hyun Joon Shin said in presenting his analysis of data from the Nurses’ Health Study at the American Heart Association scientific sessions.
The prospective observational Nurses’ Health Study began in 1976. It included 121,700 female American nurses aged 30-55 upon enrollment. Dr. Shin’s analysis included 8,354 women with no baseline cardiovascular disease, diabetes, or cancer who were diagnosed with type 2 diabetes during 1984-2006 and followed to June 2010.
During follow-up, there were 1,183 deaths in the cohort diagnosed with type 2 diabetes, including 491 deaths due to cardiovascular disease and 514 due to cancer.
Mortality risks were evaluated in relation to the extent to which the nurses adhered to a healthy, high-quality diet following their diagnosis of type 2 diabetes. The yardstick employed in evaluating eating patterns was the Alternative Healthy Eating Index–2010 (AHEI). The index, developed by researchers at Harvard University, scores patients’ consumption of various foods and nutrients known to be predictive of chronic disease risk, explained Dr. Shin of Baylor University Medical Center, Dallas.
There were 14.4 deaths per 1,000 persons per year in the top quintile in terms of adherence to the AHEI. A dose-response relationship was evident: The mortality rate was 28.8/1,000 persons per year among those in the bottom quintile, 23.1/1,000 in the second quintile, 19.3 in the third, and 20.7 in the fourth.
In a multivariate analysis adjusted for numerous potential confounders, including a participant’s prediabetic AHEI score, the diabetic nurses in the top quintile in terms of healthy eating had a 52% reduction in the risk of all-cause mortality compared with those in the lowest AHEI quintile. The healthiest eaters also had a 49% lower risk of cardiovascular mortality. However, their more modest 28% reduction in the risk of cancer-related death fell just short of statistical significance.
The AHEI, updated in 2010, awards points for increased consumption of whole grains, nuts, legumes, vegetables, fruits, fish or fish oil, and moderate alcohol intake. The index penalizes for consumption of red meat, processed meat products, trans fats, sodium, sugar-sweetened beverages, and fruit juices (J. Nutr. 2012;142:1009-18).
In examining the study findings more closely in terms of the impact of individual dietary components of the AHEI, Dr. Shin found nearly all of them had significant effects in multivariate analyses. For example, subjects in the top quintile for whole grain consumption had a 41% reduction in the risk of all-cause mortality compared with those in the bottom quintile, as well as a 33% reduction in the risk of cardiovascular mortality. Participants who drank the least amount of sugar-sweetened beverages had reductions in all-cause and cardiovascular mortality of 26% and 37%, compared with these rates for the quintile of biggest quaffers.
This was the first-ever postdiabetes dietary pattern analysis examining mortality in women with incident type 2 diabetes, according to Dr. Shin. The mechanism for the observed inverse relationship between diet quality and mortality is straightforward: A poor-quality diet is associated with increased risks of coronary heart disease, stroke, and weight gain and higher circulating levels of fasting insulin, inflammatory cytokines, and leptin, he said.
D. Shin’s study was supported by Baylor University research funding. He reported having no relevant financial conflicts.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: Women who placed in the top quintile for a healthy eating pattern following their diagnosis of type 2 diabetes had all-cause and cardiovascular mortality rates 52% and 49% lower, respectively, than did women in the bottom quintile for healthy eating.
Data source: An analysis of long-term mortality trends in relation to eating habits in 8,354 women in the prospective Nurses’ Health Study who were diagnosed with type 2 diabetes and followed for a maximum of 26 years.
Disclosures: The study was supported by a Baylor University research grant. The presenter reported having no relevant financial conflicts.
Statin reduces MI risk in ischemic heart failure
DALLAS – Rosuvastatin appears to reduce the risk of acute myocardial infarction in patients with ischemic heart failure, according to a meta-analysis of two landmark statin trials.
This finding is at odds with current thinking as expressed in the new American Heart Association/American College of Cardiology (AHA/ACC) guidelines, which don’t specifically recommend statin therapy for heart failure patients because no large randomized trials have shown a significant reduction in cardiovascular events.
That’s probably because those studies relied on traditional statistical techniques of survival analysis rather than the less familiar method of competing risk analysis, which is better suited to detecting a statin benefit in heart failure patients who are simultaneously at risk for multiple types of events, including arrhythmias, cancer, infection, and renal failure, as well as ischemic events, Dr. Matthew Feinstein explained at the American Heart Association scientific sessions.
When he and his coinvestigators applied competing risk analysis in their meta-analysis of the randomized, placebo-controlled CORONA (N. Engl. J. Med. 2007; 357:2248-61) and GISSI-HF trials, they found after accounting for competing risks in close to 10,000 heart failure patients that those on 10 mg/day of rosuvastatin had a 17% reduction in the risk of myocardial infarction during follow-up, compared with those on placebo. This benefit fell just shy of statistical significance. However, when the researchers restricted the analysis to the nearly 7,100 subjects with ischemic heart failure, the resultant 19% reduction in MI risk with rosuvastatin did achieve significance.
While there was a consistent trend for a lower MI risk with rosuvastatin in all prespecified subgroups of patients with ischemic heart failure in the pooled analysis, this trend reached statistical significance in two subgroups: men, who were 22% less likely to have an MI on rosuvastatin than with placebo, and patients with a baseline low-density lipoprotein (LDL) cholesterol above 96 mg/dL, who had a 23% reduction in risk, reported Dr. Feinstein of Northwestern University, Chicago, and his colleagues.
"It’s likely that ischemic heart failure patients do benefit from statin therapy, contrary to current thinking, if they are likely to live long enough to derive benefit from statins in preventing atherothrombotic events," he concluded.
Session chair Dr. Lori Mosca was enthusiastic about the meta-analysis findings and their clinical implications. She also was strongly supportive of the application of competing risk analysis in this setting.
"That was actually a brilliant analysis. I really loved it. The lesson it teaches is that when we design a clinical trial where our nonischemic outcomes are more common than the ischemic outcomes that we’re trying to prevent, we really need to take a look at those competing risks. We might come to completely different conclusions if we do that," said Dr. Mosca, professor of medicine at Columbia University, New York, and director of preventive cardiology at New York-Presbyterian Hospital.
Other important take-home points from this pooled analysis of the CORONA and GISSI-HF trials are that despite all the confusion surrounding the new AHA/ACC prevention guidelines, LDL cholesterol levels still matter in terms of predicting outcomes, and that, as always, it’s important to look at gender interactions in looking at clinical trial outcomes, added Dr. Mosca, who is current chair of the AHA Expert Panel for the Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women.
Noting that it’s not always possible for a physician to tell whether the cause of a patient’s heart failure is ischemic or nonischemic, she asked Dr. Feinstein if he’d prescribe a statin if he merely suspected that the heart failure was ischemic, perhaps because the patient had evidence of underlying coronary disease.
Yes, he replied, provided the patient had no contraindications to statin therapy, was able to tolerate it, and had a sufficiently long life expectancy to be able to reap the benefits. He added a caveat: Heart failure patients typically have multiple comorbid conditions that could place them at risk with higher doses of statins.
"Rosuvastatin tends to be a somewhat less toxic and better tolerated statin, and 10 mg is a relatively low dose," Dr. Feinstein noted.
The meta-analysis was conducted free of commercial sponsorship. Dr. Feinstein reported having no financial conflicts of interest.
DALLAS – Rosuvastatin appears to reduce the risk of acute myocardial infarction in patients with ischemic heart failure, according to a meta-analysis of two landmark statin trials.
This finding is at odds with current thinking as expressed in the new American Heart Association/American College of Cardiology (AHA/ACC) guidelines, which don’t specifically recommend statin therapy for heart failure patients because no large randomized trials have shown a significant reduction in cardiovascular events.
That’s probably because those studies relied on traditional statistical techniques of survival analysis rather than the less familiar method of competing risk analysis, which is better suited to detecting a statin benefit in heart failure patients who are simultaneously at risk for multiple types of events, including arrhythmias, cancer, infection, and renal failure, as well as ischemic events, Dr. Matthew Feinstein explained at the American Heart Association scientific sessions.
When he and his coinvestigators applied competing risk analysis in their meta-analysis of the randomized, placebo-controlled CORONA (N. Engl. J. Med. 2007; 357:2248-61) and GISSI-HF trials, they found after accounting for competing risks in close to 10,000 heart failure patients that those on 10 mg/day of rosuvastatin had a 17% reduction in the risk of myocardial infarction during follow-up, compared with those on placebo. This benefit fell just shy of statistical significance. However, when the researchers restricted the analysis to the nearly 7,100 subjects with ischemic heart failure, the resultant 19% reduction in MI risk with rosuvastatin did achieve significance.
While there was a consistent trend for a lower MI risk with rosuvastatin in all prespecified subgroups of patients with ischemic heart failure in the pooled analysis, this trend reached statistical significance in two subgroups: men, who were 22% less likely to have an MI on rosuvastatin than with placebo, and patients with a baseline low-density lipoprotein (LDL) cholesterol above 96 mg/dL, who had a 23% reduction in risk, reported Dr. Feinstein of Northwestern University, Chicago, and his colleagues.
"It’s likely that ischemic heart failure patients do benefit from statin therapy, contrary to current thinking, if they are likely to live long enough to derive benefit from statins in preventing atherothrombotic events," he concluded.
Session chair Dr. Lori Mosca was enthusiastic about the meta-analysis findings and their clinical implications. She also was strongly supportive of the application of competing risk analysis in this setting.
"That was actually a brilliant analysis. I really loved it. The lesson it teaches is that when we design a clinical trial where our nonischemic outcomes are more common than the ischemic outcomes that we’re trying to prevent, we really need to take a look at those competing risks. We might come to completely different conclusions if we do that," said Dr. Mosca, professor of medicine at Columbia University, New York, and director of preventive cardiology at New York-Presbyterian Hospital.
Other important take-home points from this pooled analysis of the CORONA and GISSI-HF trials are that despite all the confusion surrounding the new AHA/ACC prevention guidelines, LDL cholesterol levels still matter in terms of predicting outcomes, and that, as always, it’s important to look at gender interactions in looking at clinical trial outcomes, added Dr. Mosca, who is current chair of the AHA Expert Panel for the Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women.
Noting that it’s not always possible for a physician to tell whether the cause of a patient’s heart failure is ischemic or nonischemic, she asked Dr. Feinstein if he’d prescribe a statin if he merely suspected that the heart failure was ischemic, perhaps because the patient had evidence of underlying coronary disease.
Yes, he replied, provided the patient had no contraindications to statin therapy, was able to tolerate it, and had a sufficiently long life expectancy to be able to reap the benefits. He added a caveat: Heart failure patients typically have multiple comorbid conditions that could place them at risk with higher doses of statins.
"Rosuvastatin tends to be a somewhat less toxic and better tolerated statin, and 10 mg is a relatively low dose," Dr. Feinstein noted.
The meta-analysis was conducted free of commercial sponsorship. Dr. Feinstein reported having no financial conflicts of interest.
DALLAS – Rosuvastatin appears to reduce the risk of acute myocardial infarction in patients with ischemic heart failure, according to a meta-analysis of two landmark statin trials.
This finding is at odds with current thinking as expressed in the new American Heart Association/American College of Cardiology (AHA/ACC) guidelines, which don’t specifically recommend statin therapy for heart failure patients because no large randomized trials have shown a significant reduction in cardiovascular events.
That’s probably because those studies relied on traditional statistical techniques of survival analysis rather than the less familiar method of competing risk analysis, which is better suited to detecting a statin benefit in heart failure patients who are simultaneously at risk for multiple types of events, including arrhythmias, cancer, infection, and renal failure, as well as ischemic events, Dr. Matthew Feinstein explained at the American Heart Association scientific sessions.
When he and his coinvestigators applied competing risk analysis in their meta-analysis of the randomized, placebo-controlled CORONA (N. Engl. J. Med. 2007; 357:2248-61) and GISSI-HF trials, they found after accounting for competing risks in close to 10,000 heart failure patients that those on 10 mg/day of rosuvastatin had a 17% reduction in the risk of myocardial infarction during follow-up, compared with those on placebo. This benefit fell just shy of statistical significance. However, when the researchers restricted the analysis to the nearly 7,100 subjects with ischemic heart failure, the resultant 19% reduction in MI risk with rosuvastatin did achieve significance.
While there was a consistent trend for a lower MI risk with rosuvastatin in all prespecified subgroups of patients with ischemic heart failure in the pooled analysis, this trend reached statistical significance in two subgroups: men, who were 22% less likely to have an MI on rosuvastatin than with placebo, and patients with a baseline low-density lipoprotein (LDL) cholesterol above 96 mg/dL, who had a 23% reduction in risk, reported Dr. Feinstein of Northwestern University, Chicago, and his colleagues.
"It’s likely that ischemic heart failure patients do benefit from statin therapy, contrary to current thinking, if they are likely to live long enough to derive benefit from statins in preventing atherothrombotic events," he concluded.
Session chair Dr. Lori Mosca was enthusiastic about the meta-analysis findings and their clinical implications. She also was strongly supportive of the application of competing risk analysis in this setting.
"That was actually a brilliant analysis. I really loved it. The lesson it teaches is that when we design a clinical trial where our nonischemic outcomes are more common than the ischemic outcomes that we’re trying to prevent, we really need to take a look at those competing risks. We might come to completely different conclusions if we do that," said Dr. Mosca, professor of medicine at Columbia University, New York, and director of preventive cardiology at New York-Presbyterian Hospital.
Other important take-home points from this pooled analysis of the CORONA and GISSI-HF trials are that despite all the confusion surrounding the new AHA/ACC prevention guidelines, LDL cholesterol levels still matter in terms of predicting outcomes, and that, as always, it’s important to look at gender interactions in looking at clinical trial outcomes, added Dr. Mosca, who is current chair of the AHA Expert Panel for the Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women.
Noting that it’s not always possible for a physician to tell whether the cause of a patient’s heart failure is ischemic or nonischemic, she asked Dr. Feinstein if he’d prescribe a statin if he merely suspected that the heart failure was ischemic, perhaps because the patient had evidence of underlying coronary disease.
Yes, he replied, provided the patient had no contraindications to statin therapy, was able to tolerate it, and had a sufficiently long life expectancy to be able to reap the benefits. He added a caveat: Heart failure patients typically have multiple comorbid conditions that could place them at risk with higher doses of statins.
"Rosuvastatin tends to be a somewhat less toxic and better tolerated statin, and 10 mg is a relatively low dose," Dr. Feinstein noted.
The meta-analysis was conducted free of commercial sponsorship. Dr. Feinstein reported having no financial conflicts of interest.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: Rosuvastatin at 10 mg/day resulted in a significant 19% reduction in the risk of MI, compared with placebo, in patients with ischemic heart failure.
Data source: A post hoc meta-analysis of two landmark, randomized, placebo-controlled clinical trials – the CORONA and GISSI-HF trials – totaling nearly 10,000 subjects with heart failure followed prospectively for a median of 33 and 47 months, respectively.
Disclosures: The meta-analysis was conducted free of commercial sponsorship. Dr. Feinstein reported having no financial conflicts of interest.
Body fat and cardiovascular risk: Location, location, location!
DALLAS – It’s not obesity per se that affects cardiovascular risk, it’s where that excess body fat is stored, according to the results of a novel adipose tissue–imaging study.
Excess visceral adipose tissue is independently associated with increased risk of developing cardiovascular disease. In contrast, increased lower body subcutaneous adipose tissue – that is, fat around the hips and a big butt – actually seems to protect against cardiovascular disease, Dr. Ian J. Neeland reported at the American Heart Association scientific sessions.
He presented an analysis of 972 obese participants in the Dallas Heart Study with a mean age of 44 years at enrollment and no baseline cardiovascular disease. All underwent dual-energy x-ray absorptiometry and MRI assessment of their body fat distribution, focusing on the visceral, abdominal subcutaneous, and lower body subcutaneous adipose tissue depots. Participants were then followed prospectively for a median 8.1 years, during which 91 cardiovascular events occurred in 68 subjects.
The impetus for the adipose tissue imaging study was the researchers’ recognition that obesity is a heterogeneous disorder.
"Currently, we know that obesity is associated with incident cardiovascular disease at a general population level. However, body mass index alone is really an inadequate marker of risk among the obese. Many individuals with even high BMIs do not develop cardiovascular disease. Marked abdominal obesity is a stronger predictor of cardiovascular disease but still lacks the necessary specificity. So there’s really a clinical need for tools to differentiate obese individuals who will develop cardiovascular disease from those who will be free of cardiovascular disease for their lifetime," explained Dr. Neeland, a fellow in cardiovascular medicine at the University of Texas Southwestern Medical Center, Dallas.
In a multivariate analysis adjusted for age, sex, race, and the conventional cardiovascular risk factors, each 1–standard deviation increase in visceral adipose tissue was independently associated with a 24% increase in the risk of developing cardiovascular disease during follow-up. Dividing the study population into quartiles on the basis of their extent of visceral adipose tissue, the cumulative incidence of cardiovascular disease rose in stepwise fashion, with subjects in the lowest quartile having the least cardiovascular events and those in the top quartile having the most.
Having more lower body subcutaneous fat had the opposite effect. For every 1–standard deviation increase in the amount of fat at that location, the cardiovascular event risk dropped by 27%.
Participants’ amount of abdominal subcutaneous fat didn’t affect their cardiovascular event risk one way or another. Nor did BMI, waist circumference, waist-hip ratio, or the amount of liver fat on MRI show any significant association with cardiovascular disease risk.
"These results really underscore the biologic importance of body fat distribution with regard to cardiovascular disease risk in obesity and suggest a possible prognostic role for imaging-based assessment of body fat distribution in high-risk obese patients," according to Dr. Neeland.
One intriguing clinical implication of this study is that preventing accumulation of visceral adipose tissue may have benefit in terms of cardiovascular disease prevention even in the absence of meaningful weight loss. It’s possible that new drugs could be developed that lower cardiovascular risk in obese patients by changing their body fat distribution profile rather than lopping off pounds.
In the Dallas study, increased visceral abdominal tissue was consistently associated with a higher risk of cardiovascular disease across subgroups based upon age, race, sex, and BMI.
"Interestingly, those with increased visceral abdominal fat who were less than 40 years of age had greater risk for cardiovascular disease than [did] those over 40. This could suggest that visceral abdominal tissue has a greater impact on the young, in whom other cardiovascular risk factors have not yet accumulated over time," Dr. Neeland observed.
The cardiovascular event endpoint in the study was a composite of cardiovascular death, acute MI, stroke, heart failure, atrial fibrillation, or event-driven coronary or peripheral artery revascularization.
The Dallas Heart Study is funded by the Donald W. Reynolds Foundation. Dr. Neeland reported having no financial conflicts.
DALLAS – It’s not obesity per se that affects cardiovascular risk, it’s where that excess body fat is stored, according to the results of a novel adipose tissue–imaging study.
Excess visceral adipose tissue is independently associated with increased risk of developing cardiovascular disease. In contrast, increased lower body subcutaneous adipose tissue – that is, fat around the hips and a big butt – actually seems to protect against cardiovascular disease, Dr. Ian J. Neeland reported at the American Heart Association scientific sessions.
He presented an analysis of 972 obese participants in the Dallas Heart Study with a mean age of 44 years at enrollment and no baseline cardiovascular disease. All underwent dual-energy x-ray absorptiometry and MRI assessment of their body fat distribution, focusing on the visceral, abdominal subcutaneous, and lower body subcutaneous adipose tissue depots. Participants were then followed prospectively for a median 8.1 years, during which 91 cardiovascular events occurred in 68 subjects.
The impetus for the adipose tissue imaging study was the researchers’ recognition that obesity is a heterogeneous disorder.
"Currently, we know that obesity is associated with incident cardiovascular disease at a general population level. However, body mass index alone is really an inadequate marker of risk among the obese. Many individuals with even high BMIs do not develop cardiovascular disease. Marked abdominal obesity is a stronger predictor of cardiovascular disease but still lacks the necessary specificity. So there’s really a clinical need for tools to differentiate obese individuals who will develop cardiovascular disease from those who will be free of cardiovascular disease for their lifetime," explained Dr. Neeland, a fellow in cardiovascular medicine at the University of Texas Southwestern Medical Center, Dallas.
In a multivariate analysis adjusted for age, sex, race, and the conventional cardiovascular risk factors, each 1–standard deviation increase in visceral adipose tissue was independently associated with a 24% increase in the risk of developing cardiovascular disease during follow-up. Dividing the study population into quartiles on the basis of their extent of visceral adipose tissue, the cumulative incidence of cardiovascular disease rose in stepwise fashion, with subjects in the lowest quartile having the least cardiovascular events and those in the top quartile having the most.
Having more lower body subcutaneous fat had the opposite effect. For every 1–standard deviation increase in the amount of fat at that location, the cardiovascular event risk dropped by 27%.
Participants’ amount of abdominal subcutaneous fat didn’t affect their cardiovascular event risk one way or another. Nor did BMI, waist circumference, waist-hip ratio, or the amount of liver fat on MRI show any significant association with cardiovascular disease risk.
"These results really underscore the biologic importance of body fat distribution with regard to cardiovascular disease risk in obesity and suggest a possible prognostic role for imaging-based assessment of body fat distribution in high-risk obese patients," according to Dr. Neeland.
One intriguing clinical implication of this study is that preventing accumulation of visceral adipose tissue may have benefit in terms of cardiovascular disease prevention even in the absence of meaningful weight loss. It’s possible that new drugs could be developed that lower cardiovascular risk in obese patients by changing their body fat distribution profile rather than lopping off pounds.
In the Dallas study, increased visceral abdominal tissue was consistently associated with a higher risk of cardiovascular disease across subgroups based upon age, race, sex, and BMI.
"Interestingly, those with increased visceral abdominal fat who were less than 40 years of age had greater risk for cardiovascular disease than [did] those over 40. This could suggest that visceral abdominal tissue has a greater impact on the young, in whom other cardiovascular risk factors have not yet accumulated over time," Dr. Neeland observed.
The cardiovascular event endpoint in the study was a composite of cardiovascular death, acute MI, stroke, heart failure, atrial fibrillation, or event-driven coronary or peripheral artery revascularization.
The Dallas Heart Study is funded by the Donald W. Reynolds Foundation. Dr. Neeland reported having no financial conflicts.
DALLAS – It’s not obesity per se that affects cardiovascular risk, it’s where that excess body fat is stored, according to the results of a novel adipose tissue–imaging study.
Excess visceral adipose tissue is independently associated with increased risk of developing cardiovascular disease. In contrast, increased lower body subcutaneous adipose tissue – that is, fat around the hips and a big butt – actually seems to protect against cardiovascular disease, Dr. Ian J. Neeland reported at the American Heart Association scientific sessions.
He presented an analysis of 972 obese participants in the Dallas Heart Study with a mean age of 44 years at enrollment and no baseline cardiovascular disease. All underwent dual-energy x-ray absorptiometry and MRI assessment of their body fat distribution, focusing on the visceral, abdominal subcutaneous, and lower body subcutaneous adipose tissue depots. Participants were then followed prospectively for a median 8.1 years, during which 91 cardiovascular events occurred in 68 subjects.
The impetus for the adipose tissue imaging study was the researchers’ recognition that obesity is a heterogeneous disorder.
"Currently, we know that obesity is associated with incident cardiovascular disease at a general population level. However, body mass index alone is really an inadequate marker of risk among the obese. Many individuals with even high BMIs do not develop cardiovascular disease. Marked abdominal obesity is a stronger predictor of cardiovascular disease but still lacks the necessary specificity. So there’s really a clinical need for tools to differentiate obese individuals who will develop cardiovascular disease from those who will be free of cardiovascular disease for their lifetime," explained Dr. Neeland, a fellow in cardiovascular medicine at the University of Texas Southwestern Medical Center, Dallas.
In a multivariate analysis adjusted for age, sex, race, and the conventional cardiovascular risk factors, each 1–standard deviation increase in visceral adipose tissue was independently associated with a 24% increase in the risk of developing cardiovascular disease during follow-up. Dividing the study population into quartiles on the basis of their extent of visceral adipose tissue, the cumulative incidence of cardiovascular disease rose in stepwise fashion, with subjects in the lowest quartile having the least cardiovascular events and those in the top quartile having the most.
Having more lower body subcutaneous fat had the opposite effect. For every 1–standard deviation increase in the amount of fat at that location, the cardiovascular event risk dropped by 27%.
Participants’ amount of abdominal subcutaneous fat didn’t affect their cardiovascular event risk one way or another. Nor did BMI, waist circumference, waist-hip ratio, or the amount of liver fat on MRI show any significant association with cardiovascular disease risk.
"These results really underscore the biologic importance of body fat distribution with regard to cardiovascular disease risk in obesity and suggest a possible prognostic role for imaging-based assessment of body fat distribution in high-risk obese patients," according to Dr. Neeland.
One intriguing clinical implication of this study is that preventing accumulation of visceral adipose tissue may have benefit in terms of cardiovascular disease prevention even in the absence of meaningful weight loss. It’s possible that new drugs could be developed that lower cardiovascular risk in obese patients by changing their body fat distribution profile rather than lopping off pounds.
In the Dallas study, increased visceral abdominal tissue was consistently associated with a higher risk of cardiovascular disease across subgroups based upon age, race, sex, and BMI.
"Interestingly, those with increased visceral abdominal fat who were less than 40 years of age had greater risk for cardiovascular disease than [did] those over 40. This could suggest that visceral abdominal tissue has a greater impact on the young, in whom other cardiovascular risk factors have not yet accumulated over time," Dr. Neeland observed.
The cardiovascular event endpoint in the study was a composite of cardiovascular death, acute MI, stroke, heart failure, atrial fibrillation, or event-driven coronary or peripheral artery revascularization.
The Dallas Heart Study is funded by the Donald W. Reynolds Foundation. Dr. Neeland reported having no financial conflicts.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: Excess visceral adipose tissue in obese adults was independently associated with increased risk of subsequent cardiovascular disease, while excess lower body subcutaneous fat had a protective effect.
Data source: This report involved 972 obese participants in the prospective, single-center Dallas Heart Study, all without baseline cardiovascular disease who underwent baseline imaging of specific adipose tissue depots.
Disclosures: The Dallas Heart Study is funded by the Donald W. Reynolds Foundation. The presenter reported having no relevant financial interests.
Bromocriptine-QR slashes cardiovascular events in diabetics on metformin
DALLAS – The quick-release formulation of bromocriptine known as Cycloset shows strong potential as a novel approach for cardiovascular risk reduction in type 2 diabetic patients already on metformin, according to Dr. Bindu Chamarthi.
A post-hoc analysis of 1,791 such patients who participated in the 1-year double-blind Cycloset Safety Trial showed a 53% reduction in cardiovascular events in those randomized to bromocriptine-QR, compared with placebo-treated controls, Dr. Chamarthi reported at the American Heart Association scientific sessions.
The composite cardiovascular endpoint comprising a first MI, stroke, coronary revascularization, or hospitalization for either angina or heart failure occurred in 1.3% of 1,208 bromocriptine-QR–treated patients, compared with 3.1% of 583 placebo-treated controls. All subjects were on metformin at baseline, were generally in good metabolic control, and had high rates of utilization of guideline-recommended cardioprotective medications, including statins and antihypertensive agents, noted Dr. Chamarthi, an endocrinologist at Brigham and Women’s Hospital, Boston.
This sharp reduction in cardiovascular events is particularly impressive in light of the increased risk of such events faced by patients with type 2 diabetes, along with the lack of conclusive evidence of cardiovascular benefit for any of the approved therapies for the disease other than metformin, she added.
Bromocriptine-QR is approved by the Food and Drug Administration for the treatment of type 2 diabetes, so it’s not surprising that the group assigned to the drug evidenced improved glycemic control. At the end of 52 weeks of treatment, they were 1.75-fold more likely than controls to be in good glycemic control, as defined by a hemoglobin A1c level of 7.0% or less.
Current evidence suggests that the development of obesity, insulin resistance, and diabetes is linked to a central hypodopaminergic state.
Bromocriptine-QR is a sympatholytic dopamine agonist approved in 2009. Taken once daily with breakfast, it provides a circadian-timed brief period of increased central dopaminergic activity. The result is improved postprandial blood glucose control without raising the insulin concentration.
How bromocriptine-QR achieved the observed reduction in cardiovascular events seen in this study remains unclear. One possibility is that the mechanism involves a reduction in elevated sympathetic nervous system drive to the vasculature, liver, and adipose tissue along with a reduction in hypothalamic-pituitary-adrenal axis activity, with resultant diminution of endothelial dysfunction. These benefits might flow from the drug’s ability to restore the daily morning peak in central circadian dopaminergic neuroendocrine activities that are disrupted in patients with type 2 diabetes. Further studies are planned, according to Dr. Chamarthi.
This study was sponsored by VeroScience, which markets bromocriptine-QR. Dr. Chamarthi is a consultant for the company.
DALLAS – The quick-release formulation of bromocriptine known as Cycloset shows strong potential as a novel approach for cardiovascular risk reduction in type 2 diabetic patients already on metformin, according to Dr. Bindu Chamarthi.
A post-hoc analysis of 1,791 such patients who participated in the 1-year double-blind Cycloset Safety Trial showed a 53% reduction in cardiovascular events in those randomized to bromocriptine-QR, compared with placebo-treated controls, Dr. Chamarthi reported at the American Heart Association scientific sessions.
The composite cardiovascular endpoint comprising a first MI, stroke, coronary revascularization, or hospitalization for either angina or heart failure occurred in 1.3% of 1,208 bromocriptine-QR–treated patients, compared with 3.1% of 583 placebo-treated controls. All subjects were on metformin at baseline, were generally in good metabolic control, and had high rates of utilization of guideline-recommended cardioprotective medications, including statins and antihypertensive agents, noted Dr. Chamarthi, an endocrinologist at Brigham and Women’s Hospital, Boston.
This sharp reduction in cardiovascular events is particularly impressive in light of the increased risk of such events faced by patients with type 2 diabetes, along with the lack of conclusive evidence of cardiovascular benefit for any of the approved therapies for the disease other than metformin, she added.
Bromocriptine-QR is approved by the Food and Drug Administration for the treatment of type 2 diabetes, so it’s not surprising that the group assigned to the drug evidenced improved glycemic control. At the end of 52 weeks of treatment, they were 1.75-fold more likely than controls to be in good glycemic control, as defined by a hemoglobin A1c level of 7.0% or less.
Current evidence suggests that the development of obesity, insulin resistance, and diabetes is linked to a central hypodopaminergic state.
Bromocriptine-QR is a sympatholytic dopamine agonist approved in 2009. Taken once daily with breakfast, it provides a circadian-timed brief period of increased central dopaminergic activity. The result is improved postprandial blood glucose control without raising the insulin concentration.
How bromocriptine-QR achieved the observed reduction in cardiovascular events seen in this study remains unclear. One possibility is that the mechanism involves a reduction in elevated sympathetic nervous system drive to the vasculature, liver, and adipose tissue along with a reduction in hypothalamic-pituitary-adrenal axis activity, with resultant diminution of endothelial dysfunction. These benefits might flow from the drug’s ability to restore the daily morning peak in central circadian dopaminergic neuroendocrine activities that are disrupted in patients with type 2 diabetes. Further studies are planned, according to Dr. Chamarthi.
This study was sponsored by VeroScience, which markets bromocriptine-QR. Dr. Chamarthi is a consultant for the company.
DALLAS – The quick-release formulation of bromocriptine known as Cycloset shows strong potential as a novel approach for cardiovascular risk reduction in type 2 diabetic patients already on metformin, according to Dr. Bindu Chamarthi.
A post-hoc analysis of 1,791 such patients who participated in the 1-year double-blind Cycloset Safety Trial showed a 53% reduction in cardiovascular events in those randomized to bromocriptine-QR, compared with placebo-treated controls, Dr. Chamarthi reported at the American Heart Association scientific sessions.
The composite cardiovascular endpoint comprising a first MI, stroke, coronary revascularization, or hospitalization for either angina or heart failure occurred in 1.3% of 1,208 bromocriptine-QR–treated patients, compared with 3.1% of 583 placebo-treated controls. All subjects were on metformin at baseline, were generally in good metabolic control, and had high rates of utilization of guideline-recommended cardioprotective medications, including statins and antihypertensive agents, noted Dr. Chamarthi, an endocrinologist at Brigham and Women’s Hospital, Boston.
This sharp reduction in cardiovascular events is particularly impressive in light of the increased risk of such events faced by patients with type 2 diabetes, along with the lack of conclusive evidence of cardiovascular benefit for any of the approved therapies for the disease other than metformin, she added.
Bromocriptine-QR is approved by the Food and Drug Administration for the treatment of type 2 diabetes, so it’s not surprising that the group assigned to the drug evidenced improved glycemic control. At the end of 52 weeks of treatment, they were 1.75-fold more likely than controls to be in good glycemic control, as defined by a hemoglobin A1c level of 7.0% or less.
Current evidence suggests that the development of obesity, insulin resistance, and diabetes is linked to a central hypodopaminergic state.
Bromocriptine-QR is a sympatholytic dopamine agonist approved in 2009. Taken once daily with breakfast, it provides a circadian-timed brief period of increased central dopaminergic activity. The result is improved postprandial blood glucose control without raising the insulin concentration.
How bromocriptine-QR achieved the observed reduction in cardiovascular events seen in this study remains unclear. One possibility is that the mechanism involves a reduction in elevated sympathetic nervous system drive to the vasculature, liver, and adipose tissue along with a reduction in hypothalamic-pituitary-adrenal axis activity, with resultant diminution of endothelial dysfunction. These benefits might flow from the drug’s ability to restore the daily morning peak in central circadian dopaminergic neuroendocrine activities that are disrupted in patients with type 2 diabetes. Further studies are planned, according to Dr. Chamarthi.
This study was sponsored by VeroScience, which markets bromocriptine-QR. Dr. Chamarthi is a consultant for the company.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: The 1-year composite cardiovascular event rate in type 2 diabetic patients on metformin at baseline was 1.3% in patients on quick-release bromocriptine and 3.1% in those taking placebo, for a 53% relative risk reduction.
Data source: A secondary post hoc analysis of the randomized, double-blind Cycloset Safety Trial, which was restricted to the 1,791-patient subgroup on metformin at enrollment.
Disclosures: The study was sponsored by VeroScience. The presenter is a consultant to the company, which markets bromocriptine-QR.
High dietary phosphorus linked to increased mortality
DALLAS – More than one-third of Americans have a high dietary phosphorus intake and an all-cause mortality rate that is more than double that of Americans with lower dietary phosphorus consumption, based on a large observational study.
High dietary phosphorus density – a value of at least 35 mg/kcal for the product of dietary phosphorus intake divided by total energy intake – was associated with a 2.27-fold increased risk of all-cause mortality and a 3.39-fold elevation in cardiovascular mortality, Dr. Alex R. Chang reported at the American Heart Association scientific sessions.
The findings came from an analysis of a nationally representative cohort comprising 8,686 adult participants in the Third National Health and Nutrition Examination Survey (NHANES III). Since this was an observational study, the strong association between high phosphorus intake and increased mortality doesn’t prove causality and there is no evidence that reducing phosphorus consumption would reduce mortality. But the study findings do raise public health concerns, given that 35% of Americans consume daily more than 1,400 mg of phosphorus.
That intake level, twice the Recommended Daily Allowance of 700 mg, was associated with a 2.23-fold increased risk of all-cause mortality in an NHANES III multivariate analysis adjusted for demographics, traditional cardiovascular risk factors, estimated glomerular filtration rate, vitamin D status, and total energy intake. Consumption below the 1,400 mg/day threshold was not associated with any increased mortality risk, according to Dr. Chang, a nephrologist at the Geisinger Health System in Danville, Pa.
During a median 14.7 years of follow-up in the study, there were 1,129 deaths, including 384 cardiovascular deaths.
Inorganic phosphates added to processed foods as preservatives are the chief sources of phosphorus in the U.S. diet. A recent study by researchers at Case Western Reserve University, Cleveland, determined that fully 44% of the 2,394 top-selling branded grocery products contain phosphorus additives. The additives were present in 72% of prepared frozen foods, 70% of dry food mixes, 65% of packaged meats, 57% of baked goods, 54% of soups, and 51% of yogurts. Phosphorus-added foods were not only widespread, they cost less than foods free of phosphorus additives (J. Ren. Nutr. 2013;23:265-70).
It’s noteworthy that the inorganic phosphates used in food additives are more bioavailable than the organic phosphorus found naturally in eggs, nuts, and other foods, Dr. Chang said.
Animal studies point to several possible mechanisms that might account for the observed association between high dietary phosphorus and increased mortality in NHANES III, including promotion of vascular calcification, endothelial dysfunction, and increased levels of fibroblast growth factor 23, which is a phosphaturic hormone whose overexpression is linked to left ventricular hypertrophy, progression of chronic kidney disease, and cardiovascular events.
Dr. Chang’s study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. He reported having no financial conflicts.
DALLAS – More than one-third of Americans have a high dietary phosphorus intake and an all-cause mortality rate that is more than double that of Americans with lower dietary phosphorus consumption, based on a large observational study.
High dietary phosphorus density – a value of at least 35 mg/kcal for the product of dietary phosphorus intake divided by total energy intake – was associated with a 2.27-fold increased risk of all-cause mortality and a 3.39-fold elevation in cardiovascular mortality, Dr. Alex R. Chang reported at the American Heart Association scientific sessions.
The findings came from an analysis of a nationally representative cohort comprising 8,686 adult participants in the Third National Health and Nutrition Examination Survey (NHANES III). Since this was an observational study, the strong association between high phosphorus intake and increased mortality doesn’t prove causality and there is no evidence that reducing phosphorus consumption would reduce mortality. But the study findings do raise public health concerns, given that 35% of Americans consume daily more than 1,400 mg of phosphorus.
That intake level, twice the Recommended Daily Allowance of 700 mg, was associated with a 2.23-fold increased risk of all-cause mortality in an NHANES III multivariate analysis adjusted for demographics, traditional cardiovascular risk factors, estimated glomerular filtration rate, vitamin D status, and total energy intake. Consumption below the 1,400 mg/day threshold was not associated with any increased mortality risk, according to Dr. Chang, a nephrologist at the Geisinger Health System in Danville, Pa.
During a median 14.7 years of follow-up in the study, there were 1,129 deaths, including 384 cardiovascular deaths.
Inorganic phosphates added to processed foods as preservatives are the chief sources of phosphorus in the U.S. diet. A recent study by researchers at Case Western Reserve University, Cleveland, determined that fully 44% of the 2,394 top-selling branded grocery products contain phosphorus additives. The additives were present in 72% of prepared frozen foods, 70% of dry food mixes, 65% of packaged meats, 57% of baked goods, 54% of soups, and 51% of yogurts. Phosphorus-added foods were not only widespread, they cost less than foods free of phosphorus additives (J. Ren. Nutr. 2013;23:265-70).
It’s noteworthy that the inorganic phosphates used in food additives are more bioavailable than the organic phosphorus found naturally in eggs, nuts, and other foods, Dr. Chang said.
Animal studies point to several possible mechanisms that might account for the observed association between high dietary phosphorus and increased mortality in NHANES III, including promotion of vascular calcification, endothelial dysfunction, and increased levels of fibroblast growth factor 23, which is a phosphaturic hormone whose overexpression is linked to left ventricular hypertrophy, progression of chronic kidney disease, and cardiovascular events.
Dr. Chang’s study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. He reported having no financial conflicts.
DALLAS – More than one-third of Americans have a high dietary phosphorus intake and an all-cause mortality rate that is more than double that of Americans with lower dietary phosphorus consumption, based on a large observational study.
High dietary phosphorus density – a value of at least 35 mg/kcal for the product of dietary phosphorus intake divided by total energy intake – was associated with a 2.27-fold increased risk of all-cause mortality and a 3.39-fold elevation in cardiovascular mortality, Dr. Alex R. Chang reported at the American Heart Association scientific sessions.
The findings came from an analysis of a nationally representative cohort comprising 8,686 adult participants in the Third National Health and Nutrition Examination Survey (NHANES III). Since this was an observational study, the strong association between high phosphorus intake and increased mortality doesn’t prove causality and there is no evidence that reducing phosphorus consumption would reduce mortality. But the study findings do raise public health concerns, given that 35% of Americans consume daily more than 1,400 mg of phosphorus.
That intake level, twice the Recommended Daily Allowance of 700 mg, was associated with a 2.23-fold increased risk of all-cause mortality in an NHANES III multivariate analysis adjusted for demographics, traditional cardiovascular risk factors, estimated glomerular filtration rate, vitamin D status, and total energy intake. Consumption below the 1,400 mg/day threshold was not associated with any increased mortality risk, according to Dr. Chang, a nephrologist at the Geisinger Health System in Danville, Pa.
During a median 14.7 years of follow-up in the study, there were 1,129 deaths, including 384 cardiovascular deaths.
Inorganic phosphates added to processed foods as preservatives are the chief sources of phosphorus in the U.S. diet. A recent study by researchers at Case Western Reserve University, Cleveland, determined that fully 44% of the 2,394 top-selling branded grocery products contain phosphorus additives. The additives were present in 72% of prepared frozen foods, 70% of dry food mixes, 65% of packaged meats, 57% of baked goods, 54% of soups, and 51% of yogurts. Phosphorus-added foods were not only widespread, they cost less than foods free of phosphorus additives (J. Ren. Nutr. 2013;23:265-70).
It’s noteworthy that the inorganic phosphates used in food additives are more bioavailable than the organic phosphorus found naturally in eggs, nuts, and other foods, Dr. Chang said.
Animal studies point to several possible mechanisms that might account for the observed association between high dietary phosphorus and increased mortality in NHANES III, including promotion of vascular calcification, endothelial dysfunction, and increased levels of fibroblast growth factor 23, which is a phosphaturic hormone whose overexpression is linked to left ventricular hypertrophy, progression of chronic kidney disease, and cardiovascular events.
Dr. Chang’s study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases. He reported having no financial conflicts.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: Of American adults, 35% have a dietary phosphorus intake in excess of 1,400 mg/day, which is associated with a 2.23-fold increased risk of all-cause mortality.
Data source: This study included 8,686 participants in the Third National Health and Nutrition Examination Survey with an average of nearly 15 years of follow-up.
Disclosures: The study was funded by the NIDDKD. The presenter reported having no relevant financial interests.
New-onset hypertension in pregnancy boosts post-delivery risk
DALLAS – Previously normotensive women who develop a hypertensive disorder during pregnancy are at more than double the risk of being diagnosed with prehypertension or hypertension within the first year after delivery, according to a large California study.
"Early postpartum screening and improved follow-up of women with hypertensive disorders first identified during pregnancy may be necessary to identify those at risk for future hypertension or prehypertension," Mary Helen Black, Ph.D., concluded in presenting the results of her population-based study at the American Heart Association scientific sessions.
The study was a retrospective review of the comprehensive electronic health records of 5,960 women who received their prenatal care and subsequently delivered a live singleton neonate at 20 weeks’ gestation or more at a single Kaiser Permanente Southern California medical center during 2006-2010. All of these women were specifically identified as being normotensive prior to their pregnancy and 6% of them developed a hypertensive disorder in pregnancy; 215 of the 358 affected women had preeclampsia or eclampsia.
During the first year after delivery, 63 women, or 1.1% of the total study population, developed frank hypertension. Another 902, or 15%, developed prehypertension during that first year. Prehypertension was defined as a modestly elevated blood pressure of at least 120/80 mm Hg on two consecutive ambulatory visits.
After adjustment for potential confounders including age, race, parity, prepregnancy body mass index, and smoking status, previously normotensive women with hypertension in pregnancy had an adjusted 2.4-fold increased risk of developing hypertension or prehypertension in the year after delivery, compared with women without any hypertensive disorder in pregnancy. Women who developed preeclampsia or eclampsia had a 2.5-fold increased risk, according to Dr. Black, a research scientist at Kaiser Permanente Southern California in Pasadena.
Protocols exist for monitoring and treatment of pregnant women identified as having chronic hypertension prior to pregnancy. However, the new Kaiser study is one of the first to look at the downstream impact of hypertensive disorders in pregnancy arising among women with no preexisting indication of hypertension. The plan is to follow these women further to learn how their clinical outcomes progress. Long-term Scandinavian registry studies have linked hypertensive disorders in pregnancy to increased risk of eventual type 2 diabetes, hypertension, and cardiovascular disease.
Dr. Black and her coinvestigators are planning a postpartum intervention study aimed at preventing onset of type 2 diabetes and cardiovascular disease in previously normotensive women with hypertensive disorders in pregnancy. Serious consideration is being given to intervening with the DASH diet and other nonpharmacologic interventions, she said.
While women with chronic hypertension prior to pregnancy are known to be at increased risk for development of preeclampsia and other hypertensive disorders of pregnancy as well as for gestational diabetes mellitus, the situation regarding women with prepregnancy prehypertension has been less clear. Dr. Black and coworkers shed new light on this issue in a separate study presented at the AHA meeting. She reported that women with prepregnancy prehypertension are also at increased risk for these pregnancy complications.
This study involved 2,156 Kaiser Permanente patients with prepregnancy prehypertension and 5,646 who were normotensive. A total of 8.4% of the women developed a hypertensive disorder during pregnancy, including 373 with preeclampsia/eclampsia. In addition, 1,877 women developed gestational diabetes mellitus.
Women with prehypertension prior to pregnancy were an adjusted 2.7-fold more likely to develop a hypertensive disorder in pregnancy, 2.2-fold more likely to have preeclampsia/eclampsia, and 1.2-fold more likely to develop gestational diabetes than women who were normotensive before pregnancy.
The risk of preeclampsia/eclampsia among women with prepregnancy prehypertension was significantly greater if they were at least 30 years old than if they were younger.
Both studies were funded by Kaiser Permanente. Dr. Black is an employee of the company.
DALLAS – Previously normotensive women who develop a hypertensive disorder during pregnancy are at more than double the risk of being diagnosed with prehypertension or hypertension within the first year after delivery, according to a large California study.
"Early postpartum screening and improved follow-up of women with hypertensive disorders first identified during pregnancy may be necessary to identify those at risk for future hypertension or prehypertension," Mary Helen Black, Ph.D., concluded in presenting the results of her population-based study at the American Heart Association scientific sessions.
The study was a retrospective review of the comprehensive electronic health records of 5,960 women who received their prenatal care and subsequently delivered a live singleton neonate at 20 weeks’ gestation or more at a single Kaiser Permanente Southern California medical center during 2006-2010. All of these women were specifically identified as being normotensive prior to their pregnancy and 6% of them developed a hypertensive disorder in pregnancy; 215 of the 358 affected women had preeclampsia or eclampsia.
During the first year after delivery, 63 women, or 1.1% of the total study population, developed frank hypertension. Another 902, or 15%, developed prehypertension during that first year. Prehypertension was defined as a modestly elevated blood pressure of at least 120/80 mm Hg on two consecutive ambulatory visits.
After adjustment for potential confounders including age, race, parity, prepregnancy body mass index, and smoking status, previously normotensive women with hypertension in pregnancy had an adjusted 2.4-fold increased risk of developing hypertension or prehypertension in the year after delivery, compared with women without any hypertensive disorder in pregnancy. Women who developed preeclampsia or eclampsia had a 2.5-fold increased risk, according to Dr. Black, a research scientist at Kaiser Permanente Southern California in Pasadena.
Protocols exist for monitoring and treatment of pregnant women identified as having chronic hypertension prior to pregnancy. However, the new Kaiser study is one of the first to look at the downstream impact of hypertensive disorders in pregnancy arising among women with no preexisting indication of hypertension. The plan is to follow these women further to learn how their clinical outcomes progress. Long-term Scandinavian registry studies have linked hypertensive disorders in pregnancy to increased risk of eventual type 2 diabetes, hypertension, and cardiovascular disease.
Dr. Black and her coinvestigators are planning a postpartum intervention study aimed at preventing onset of type 2 diabetes and cardiovascular disease in previously normotensive women with hypertensive disorders in pregnancy. Serious consideration is being given to intervening with the DASH diet and other nonpharmacologic interventions, she said.
While women with chronic hypertension prior to pregnancy are known to be at increased risk for development of preeclampsia and other hypertensive disorders of pregnancy as well as for gestational diabetes mellitus, the situation regarding women with prepregnancy prehypertension has been less clear. Dr. Black and coworkers shed new light on this issue in a separate study presented at the AHA meeting. She reported that women with prepregnancy prehypertension are also at increased risk for these pregnancy complications.
This study involved 2,156 Kaiser Permanente patients with prepregnancy prehypertension and 5,646 who were normotensive. A total of 8.4% of the women developed a hypertensive disorder during pregnancy, including 373 with preeclampsia/eclampsia. In addition, 1,877 women developed gestational diabetes mellitus.
Women with prehypertension prior to pregnancy were an adjusted 2.7-fold more likely to develop a hypertensive disorder in pregnancy, 2.2-fold more likely to have preeclampsia/eclampsia, and 1.2-fold more likely to develop gestational diabetes than women who were normotensive before pregnancy.
The risk of preeclampsia/eclampsia among women with prepregnancy prehypertension was significantly greater if they were at least 30 years old than if they were younger.
Both studies were funded by Kaiser Permanente. Dr. Black is an employee of the company.
DALLAS – Previously normotensive women who develop a hypertensive disorder during pregnancy are at more than double the risk of being diagnosed with prehypertension or hypertension within the first year after delivery, according to a large California study.
"Early postpartum screening and improved follow-up of women with hypertensive disorders first identified during pregnancy may be necessary to identify those at risk for future hypertension or prehypertension," Mary Helen Black, Ph.D., concluded in presenting the results of her population-based study at the American Heart Association scientific sessions.
The study was a retrospective review of the comprehensive electronic health records of 5,960 women who received their prenatal care and subsequently delivered a live singleton neonate at 20 weeks’ gestation or more at a single Kaiser Permanente Southern California medical center during 2006-2010. All of these women were specifically identified as being normotensive prior to their pregnancy and 6% of them developed a hypertensive disorder in pregnancy; 215 of the 358 affected women had preeclampsia or eclampsia.
During the first year after delivery, 63 women, or 1.1% of the total study population, developed frank hypertension. Another 902, or 15%, developed prehypertension during that first year. Prehypertension was defined as a modestly elevated blood pressure of at least 120/80 mm Hg on two consecutive ambulatory visits.
After adjustment for potential confounders including age, race, parity, prepregnancy body mass index, and smoking status, previously normotensive women with hypertension in pregnancy had an adjusted 2.4-fold increased risk of developing hypertension or prehypertension in the year after delivery, compared with women without any hypertensive disorder in pregnancy. Women who developed preeclampsia or eclampsia had a 2.5-fold increased risk, according to Dr. Black, a research scientist at Kaiser Permanente Southern California in Pasadena.
Protocols exist for monitoring and treatment of pregnant women identified as having chronic hypertension prior to pregnancy. However, the new Kaiser study is one of the first to look at the downstream impact of hypertensive disorders in pregnancy arising among women with no preexisting indication of hypertension. The plan is to follow these women further to learn how their clinical outcomes progress. Long-term Scandinavian registry studies have linked hypertensive disorders in pregnancy to increased risk of eventual type 2 diabetes, hypertension, and cardiovascular disease.
Dr. Black and her coinvestigators are planning a postpartum intervention study aimed at preventing onset of type 2 diabetes and cardiovascular disease in previously normotensive women with hypertensive disorders in pregnancy. Serious consideration is being given to intervening with the DASH diet and other nonpharmacologic interventions, she said.
While women with chronic hypertension prior to pregnancy are known to be at increased risk for development of preeclampsia and other hypertensive disorders of pregnancy as well as for gestational diabetes mellitus, the situation regarding women with prepregnancy prehypertension has been less clear. Dr. Black and coworkers shed new light on this issue in a separate study presented at the AHA meeting. She reported that women with prepregnancy prehypertension are also at increased risk for these pregnancy complications.
This study involved 2,156 Kaiser Permanente patients with prepregnancy prehypertension and 5,646 who were normotensive. A total of 8.4% of the women developed a hypertensive disorder during pregnancy, including 373 with preeclampsia/eclampsia. In addition, 1,877 women developed gestational diabetes mellitus.
Women with prehypertension prior to pregnancy were an adjusted 2.7-fold more likely to develop a hypertensive disorder in pregnancy, 2.2-fold more likely to have preeclampsia/eclampsia, and 1.2-fold more likely to develop gestational diabetes than women who were normotensive before pregnancy.
The risk of preeclampsia/eclampsia among women with prepregnancy prehypertension was significantly greater if they were at least 30 years old than if they were younger.
Both studies were funded by Kaiser Permanente. Dr. Black is an employee of the company.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: Women who were normotensive prepregnancy but developed a hypertensive disorder during pregnancy were at 2.4-fold increased risk of frank hypertension or prehypertension in their first year after delivery, compared with women who remained normotensive in pregnancy.
Data source: An observational study of 5,960 women who delivered a singleton neonate, 6% of whom developed a hypertensive disorder of pregnancy. All were known to be normotensive prior to pregnancy.
Disclosures: Dr. Black is an employee of Kaiser Permanente, which funded the study.
Heart failure exacerbation by saxagliptin called ‘real’
DALLAS – The glycemia-lowering drug saxagliptin led to a "real" excess of hospitalizations for heart failure in a 16,000-patient trial designed to examine the drug’s cardiovascular safety, but given saxagliptin’s overall safety in the study the signal of heart failure exacerbation should not rule out the drug’s use for most patients, even those with preexisting heart failure.
"Every drug has adverse effects. With these data, the calculation about saxagliptin can be made to a much more certain level. Clinicians need to weigh a patient’s risk factors, which drugs they can or cannot take, and a drug’s overall safety for ischemic events," said Dr. Benjamin M. Scirica while presenting a poster at the American Heart Association’s scientific sessions. "At this point, we have no definitive data to a priori say don’t give saxagliptin to patients with heart failure."
This follow-up analysis of data collected in the SAVOR-TIMI 53 (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus – Thrombolysis in Myocardial Infarction) trial occurred because prior reports on the study’s outcomes had shown a small but statistically significant excess incidence of hospitalization for heart failure among the 8,280 patients treated with saxagliptin, compared with the 8,212 patients randomized to placebo.
Researchers ran the SAVOR-TIMI 53 trial in patients with type 2 diabetes and either established cardiovascular disease or multiple risk factors to explicitly test the cardiovascular safety of saxagliptin (Onglyza), a selective dipeptidyl peptidase 4 (DPP-4) inhibitor, following guidance from the Food and Drug Administration in 2009 (N. Engl. J. Med. 2013;369:1317-26). The primary outcome from the study showed no statistically significant difference in the combined rate of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke between the saxagliptin and placebo arms during a median 2.1 years of follow-up. The results also showed saxagliptin met the FDA’s recommended safety criterion for cardiovascular safety in a drug meant to treat type 2 diabetes.
More detailed analyses showed a 0.7% absolute increased risk for hospitalization for heart failure in the saxagliptin-treated patients, compared with the placebo group (a 27% increase in relative risk), and that the largest number of increased hospitalizations occurred among the quartile of patients enrolled in the trial with baseline blood levels of NT–pro-brain natriuretic peptide of at least 333 pg/mL. Further analysis reported for the first time by Dr. Scirica in his poster showed that the greatest excess rate of hospitalization for heart failure occurred during the first 6 months of treatment. After the first 6 months, hospitalization rates for this reason were virtually identical in the two treatment arms of the study, said Dr. Scirica, a cardiologist at Brigham and Women’s Hospital and Harvard Medical School in Boston.
His analysis also compared the impact of saxagliptin on hospitalization for heart failure with the same effect of alogliptin, another DPP-4 inhibitor, in results from the EXAMINE (Examination of Cardiovascular Outcomes With Alogliptin vs. Standard of Care) trial (N. Engl. J. Med. 2013;369:1327-35). In EXAMINE the 2,701 patients who received alogliptin (Nesina) had an absolute 0.6% higher rate of hospitalizations for heart failure (an 18% relative increase) that was not statistically significant. EXAMINE also showed no significantly different rate between alogliptin and placebo treatment for the study’s primary, combined cardiovascular endpoint during a median 1.5 years of treatment in patients with type 2 diabetes and acute coronary syndrome.
"One could look at the alogliptin results and say there was no signal [for increased hospitalization for heart failure], or that there was a signal, although not statistically significant. The only way to know for sure is to see what the next trial shows," said Dr. Deepak L. Bhatt, professor of medicine at Harvard Medical School, a cardiologist at Brigham and Women’s Hospital, and senior investigator for SAVOR-TIMI 53. The TECOS (Sitagliptin Cardiovascular Outcome Study) study using sitagliptin (Januvia), a third DPP-4 inhibitor, is slated to finish at the end of 2014. "Whether this is a drug or class effect still very much has yet to be seen," said Dr. Scirica. The mechanism also remains elusive for the time being.
"The most important message is that future trials [of diabetes drugs] need to prospectively look at heart failure rates, adjudicate heart failure events, and really look for any heart failure effect," Dr. Bhatt said.
SAVOR-TIMI 53 was sponsored by Astra Zeneca and Bristol-Myers Squibb. Dr. Scirica said that he has received honoraria as a consultant or adviser to Gilead, Eisai, Lexicon, Arena, St. Jude’s Medical, Decision Resources, and Boston Clinical research Institute, and that he has received research grants from six other drug companies. Dr. Bhatt said that he has received research grants from six other drug or device companies.
On Twitter @mitchelzoler
DALLAS – The glycemia-lowering drug saxagliptin led to a "real" excess of hospitalizations for heart failure in a 16,000-patient trial designed to examine the drug’s cardiovascular safety, but given saxagliptin’s overall safety in the study the signal of heart failure exacerbation should not rule out the drug’s use for most patients, even those with preexisting heart failure.
"Every drug has adverse effects. With these data, the calculation about saxagliptin can be made to a much more certain level. Clinicians need to weigh a patient’s risk factors, which drugs they can or cannot take, and a drug’s overall safety for ischemic events," said Dr. Benjamin M. Scirica while presenting a poster at the American Heart Association’s scientific sessions. "At this point, we have no definitive data to a priori say don’t give saxagliptin to patients with heart failure."
This follow-up analysis of data collected in the SAVOR-TIMI 53 (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus – Thrombolysis in Myocardial Infarction) trial occurred because prior reports on the study’s outcomes had shown a small but statistically significant excess incidence of hospitalization for heart failure among the 8,280 patients treated with saxagliptin, compared with the 8,212 patients randomized to placebo.
Researchers ran the SAVOR-TIMI 53 trial in patients with type 2 diabetes and either established cardiovascular disease or multiple risk factors to explicitly test the cardiovascular safety of saxagliptin (Onglyza), a selective dipeptidyl peptidase 4 (DPP-4) inhibitor, following guidance from the Food and Drug Administration in 2009 (N. Engl. J. Med. 2013;369:1317-26). The primary outcome from the study showed no statistically significant difference in the combined rate of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke between the saxagliptin and placebo arms during a median 2.1 years of follow-up. The results also showed saxagliptin met the FDA’s recommended safety criterion for cardiovascular safety in a drug meant to treat type 2 diabetes.
More detailed analyses showed a 0.7% absolute increased risk for hospitalization for heart failure in the saxagliptin-treated patients, compared with the placebo group (a 27% increase in relative risk), and that the largest number of increased hospitalizations occurred among the quartile of patients enrolled in the trial with baseline blood levels of NT–pro-brain natriuretic peptide of at least 333 pg/mL. Further analysis reported for the first time by Dr. Scirica in his poster showed that the greatest excess rate of hospitalization for heart failure occurred during the first 6 months of treatment. After the first 6 months, hospitalization rates for this reason were virtually identical in the two treatment arms of the study, said Dr. Scirica, a cardiologist at Brigham and Women’s Hospital and Harvard Medical School in Boston.
His analysis also compared the impact of saxagliptin on hospitalization for heart failure with the same effect of alogliptin, another DPP-4 inhibitor, in results from the EXAMINE (Examination of Cardiovascular Outcomes With Alogliptin vs. Standard of Care) trial (N. Engl. J. Med. 2013;369:1327-35). In EXAMINE the 2,701 patients who received alogliptin (Nesina) had an absolute 0.6% higher rate of hospitalizations for heart failure (an 18% relative increase) that was not statistically significant. EXAMINE also showed no significantly different rate between alogliptin and placebo treatment for the study’s primary, combined cardiovascular endpoint during a median 1.5 years of treatment in patients with type 2 diabetes and acute coronary syndrome.
"One could look at the alogliptin results and say there was no signal [for increased hospitalization for heart failure], or that there was a signal, although not statistically significant. The only way to know for sure is to see what the next trial shows," said Dr. Deepak L. Bhatt, professor of medicine at Harvard Medical School, a cardiologist at Brigham and Women’s Hospital, and senior investigator for SAVOR-TIMI 53. The TECOS (Sitagliptin Cardiovascular Outcome Study) study using sitagliptin (Januvia), a third DPP-4 inhibitor, is slated to finish at the end of 2014. "Whether this is a drug or class effect still very much has yet to be seen," said Dr. Scirica. The mechanism also remains elusive for the time being.
"The most important message is that future trials [of diabetes drugs] need to prospectively look at heart failure rates, adjudicate heart failure events, and really look for any heart failure effect," Dr. Bhatt said.
SAVOR-TIMI 53 was sponsored by Astra Zeneca and Bristol-Myers Squibb. Dr. Scirica said that he has received honoraria as a consultant or adviser to Gilead, Eisai, Lexicon, Arena, St. Jude’s Medical, Decision Resources, and Boston Clinical research Institute, and that he has received research grants from six other drug companies. Dr. Bhatt said that he has received research grants from six other drug or device companies.
On Twitter @mitchelzoler
DALLAS – The glycemia-lowering drug saxagliptin led to a "real" excess of hospitalizations for heart failure in a 16,000-patient trial designed to examine the drug’s cardiovascular safety, but given saxagliptin’s overall safety in the study the signal of heart failure exacerbation should not rule out the drug’s use for most patients, even those with preexisting heart failure.
"Every drug has adverse effects. With these data, the calculation about saxagliptin can be made to a much more certain level. Clinicians need to weigh a patient’s risk factors, which drugs they can or cannot take, and a drug’s overall safety for ischemic events," said Dr. Benjamin M. Scirica while presenting a poster at the American Heart Association’s scientific sessions. "At this point, we have no definitive data to a priori say don’t give saxagliptin to patients with heart failure."
This follow-up analysis of data collected in the SAVOR-TIMI 53 (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus – Thrombolysis in Myocardial Infarction) trial occurred because prior reports on the study’s outcomes had shown a small but statistically significant excess incidence of hospitalization for heart failure among the 8,280 patients treated with saxagliptin, compared with the 8,212 patients randomized to placebo.
Researchers ran the SAVOR-TIMI 53 trial in patients with type 2 diabetes and either established cardiovascular disease or multiple risk factors to explicitly test the cardiovascular safety of saxagliptin (Onglyza), a selective dipeptidyl peptidase 4 (DPP-4) inhibitor, following guidance from the Food and Drug Administration in 2009 (N. Engl. J. Med. 2013;369:1317-26). The primary outcome from the study showed no statistically significant difference in the combined rate of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke between the saxagliptin and placebo arms during a median 2.1 years of follow-up. The results also showed saxagliptin met the FDA’s recommended safety criterion for cardiovascular safety in a drug meant to treat type 2 diabetes.
More detailed analyses showed a 0.7% absolute increased risk for hospitalization for heart failure in the saxagliptin-treated patients, compared with the placebo group (a 27% increase in relative risk), and that the largest number of increased hospitalizations occurred among the quartile of patients enrolled in the trial with baseline blood levels of NT–pro-brain natriuretic peptide of at least 333 pg/mL. Further analysis reported for the first time by Dr. Scirica in his poster showed that the greatest excess rate of hospitalization for heart failure occurred during the first 6 months of treatment. After the first 6 months, hospitalization rates for this reason were virtually identical in the two treatment arms of the study, said Dr. Scirica, a cardiologist at Brigham and Women’s Hospital and Harvard Medical School in Boston.
His analysis also compared the impact of saxagliptin on hospitalization for heart failure with the same effect of alogliptin, another DPP-4 inhibitor, in results from the EXAMINE (Examination of Cardiovascular Outcomes With Alogliptin vs. Standard of Care) trial (N. Engl. J. Med. 2013;369:1327-35). In EXAMINE the 2,701 patients who received alogliptin (Nesina) had an absolute 0.6% higher rate of hospitalizations for heart failure (an 18% relative increase) that was not statistically significant. EXAMINE also showed no significantly different rate between alogliptin and placebo treatment for the study’s primary, combined cardiovascular endpoint during a median 1.5 years of treatment in patients with type 2 diabetes and acute coronary syndrome.
"One could look at the alogliptin results and say there was no signal [for increased hospitalization for heart failure], or that there was a signal, although not statistically significant. The only way to know for sure is to see what the next trial shows," said Dr. Deepak L. Bhatt, professor of medicine at Harvard Medical School, a cardiologist at Brigham and Women’s Hospital, and senior investigator for SAVOR-TIMI 53. The TECOS (Sitagliptin Cardiovascular Outcome Study) study using sitagliptin (Januvia), a third DPP-4 inhibitor, is slated to finish at the end of 2014. "Whether this is a drug or class effect still very much has yet to be seen," said Dr. Scirica. The mechanism also remains elusive for the time being.
"The most important message is that future trials [of diabetes drugs] need to prospectively look at heart failure rates, adjudicate heart failure events, and really look for any heart failure effect," Dr. Bhatt said.
SAVOR-TIMI 53 was sponsored by Astra Zeneca and Bristol-Myers Squibb. Dr. Scirica said that he has received honoraria as a consultant or adviser to Gilead, Eisai, Lexicon, Arena, St. Jude’s Medical, Decision Resources, and Boston Clinical research Institute, and that he has received research grants from six other drug companies. Dr. Bhatt said that he has received research grants from six other drug or device companies.
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Major finding: Treatment with saxagliptin led to a "real" 0.7% increased rate of hospitalization for heart failure, compared with patients on placebo.
Data source: The SAVOR-TIMI 53 study, which randomized 16,492 patients with type 2 diabetes to treatment with saxagliptin or placebo at 788 sites in 26 countries.
Disclosures: SAVOR-TIMI 53 was sponsored by AstraZeneca and Bristol-Myers Squibb. Dr. Scirica said that he has received honoraria as a consultant or adviser to Gilead, Eisai, Lexicon, Arena, St. Jude’s Medical, Decision Resources, and Boston Clinical Research Institute, and that he has received research grants from six other drug companies. Dr. Bhatt said that he has received research grants from six other drug or device companies.
Dopamine, nesiritide ineffective in acute heart failure with renal dysfunction
DALLAS – Neither low-dose dopamine nor low-dose nesiritide enhanced decongestion or improved renal function in patients with acute heart failure and kidney dysfunction in the randomized ROSE AHF trial.
Thus, the major unmet need for drugs that accomplish these benefits continues. There remains no Food and Drug Administration–approved therapy for enhancing renal function in acute heart failure, Dr. Horng H. Chen observed in presenting the ROSE AHF results at the American Heart Association scientific sessions.
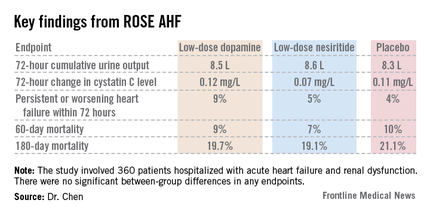
The need for such therapies is great. Most patients with heart failure eventually develop renal dysfunction. If this dysfunction is moderate or severe, patients may experience inadequate decongestion in response to diuretic therapy along with worsening renal dysfunction, both linked to poor clinical outcomes.
Prior small, single-center studies with various methodologic shortcomings had suggested possible benefit for dopamine or nesiritide in low doses thought to be renal specific. The ROSE AHF (Renal Optimization Strategies Evaluation in Acute Heart Failure) trial was conducted to provide definitive evidence to guide practice, explained Dr. Chen, professor of medicine at the Mayo Clinic, Rochester, Minn.
ROSE AHF was a randomized, double-blind, placebo-controlled clinical trial involving 360 patients hospitalized with AHF and renal dysfunction at 26 U.S. and Canadian centers. The study was carried out by the National Heart, Lung, and Blood Institute (NHLBI) Heart Failure Clinical Research Network. Patients were randomized within 24 hours of admission to low-dose dopamine at 2 mcg/kg per minute, low-dose nesiritide at 0.005 mcg/kg per minute, or placebo.
The coprimary endpoints were 72-hour cumulative urine volume as a measure of decongestion and 72-hour change in serum cystatin C as an indicator of renal function.
Interestingly, the two drugs clearly were not truly renal specific, even in these low doses widely considered to be so, Dr. Chen noted. This lack of renal specificity was evident from the side effect profile. Significant tachycardia occurred in 7.2% of the low-dose dopamine group, none on nesiritide, and 10.4% of placebo-treated controls. In contrast, significant hypotension occurred in 0.9% of patients on dopamine, 18.8% on nesiritide, and 10.4% on placebo.
A trend toward a differential treatment response depending upon whether a patient had heart failure with a reduced versus preserved ejection fraction was evident. Patients with diastolic heart failure tended to do worse with dopamine than placebo in terms of 72-hour urine output, while those with systolic heart failure tended to do better with nesiritide than placebo. However, the trial was insufficiently powered to draw definitive conclusions on this score.
"One of our take-away messages when we looked at the data was that acute heart failure is broadly defined and includes a very diverse group of patients. With the suggestion that there may be a differential response based on ejection fraction, as we proceed with future heart failure studies we may consider targeting subsets of patients. Some therapies may benefit patients with reduced ejection fraction, others will benefit patients with preserved ejection fraction. We might want to power our studies to look at these subgroups," according to Dr. Chen.
Noting that current American Heart Association/American College of Cardiology guidelines for AHF management state that low-dose dopamine "may be considered" to improve diuresis and preserve renal function during diuretic therapy, the cardiologist said "the only conclusion we can make from our study is they should not be used routinely."
He added a caveat, however: "In my own practice, if I have a patient who is hypotensive and who hasn’t responded to increased doses of diuretics, I still may consider using low-dose dopamine at that stage" because of the reassuringly low 0.9% rate of hypotension in ROSE AHF.
Discussant Dr. Marco Metra of the University of Brescia (Italy) noted that low doses of dopamine and nesiritide are widely used in the United States and Europe. Recent data suggest nesiritide is used in 5%-10% of American patients with AHF and low-dose dopamine in 3%-5%.
"Surely this trial should reduce the use of these drugs in patients with acute heart failure. They are given routinely too often in some places," he said.
Discussant Marc Pfeffer called ROSE AHF "yet another example of shedding the light of the randomized controlled trial on things that we assume to be true but actually aren’t. And we probably spend more time on rounds talking about [improving decongestion in patients with AHF and renal dysfunction] than anything else," added Dr. Pfeffer, professor of medicine at Harvard University, Boston.
The ROSE AHF trial was sponsored by the NHLBI. Dr. Chen reported receiving research grants from Scios and royalties from Niles Therapeutics, Anexon, and UpToDate. Dr. Metra is a paid consultant to a handful of pharmaceutical companies.
DALLAS – Neither low-dose dopamine nor low-dose nesiritide enhanced decongestion or improved renal function in patients with acute heart failure and kidney dysfunction in the randomized ROSE AHF trial.
Thus, the major unmet need for drugs that accomplish these benefits continues. There remains no Food and Drug Administration–approved therapy for enhancing renal function in acute heart failure, Dr. Horng H. Chen observed in presenting the ROSE AHF results at the American Heart Association scientific sessions.

The need for such therapies is great. Most patients with heart failure eventually develop renal dysfunction. If this dysfunction is moderate or severe, patients may experience inadequate decongestion in response to diuretic therapy along with worsening renal dysfunction, both linked to poor clinical outcomes.
Prior small, single-center studies with various methodologic shortcomings had suggested possible benefit for dopamine or nesiritide in low doses thought to be renal specific. The ROSE AHF (Renal Optimization Strategies Evaluation in Acute Heart Failure) trial was conducted to provide definitive evidence to guide practice, explained Dr. Chen, professor of medicine at the Mayo Clinic, Rochester, Minn.
ROSE AHF was a randomized, double-blind, placebo-controlled clinical trial involving 360 patients hospitalized with AHF and renal dysfunction at 26 U.S. and Canadian centers. The study was carried out by the National Heart, Lung, and Blood Institute (NHLBI) Heart Failure Clinical Research Network. Patients were randomized within 24 hours of admission to low-dose dopamine at 2 mcg/kg per minute, low-dose nesiritide at 0.005 mcg/kg per minute, or placebo.
The coprimary endpoints were 72-hour cumulative urine volume as a measure of decongestion and 72-hour change in serum cystatin C as an indicator of renal function.
Interestingly, the two drugs clearly were not truly renal specific, even in these low doses widely considered to be so, Dr. Chen noted. This lack of renal specificity was evident from the side effect profile. Significant tachycardia occurred in 7.2% of the low-dose dopamine group, none on nesiritide, and 10.4% of placebo-treated controls. In contrast, significant hypotension occurred in 0.9% of patients on dopamine, 18.8% on nesiritide, and 10.4% on placebo.
A trend toward a differential treatment response depending upon whether a patient had heart failure with a reduced versus preserved ejection fraction was evident. Patients with diastolic heart failure tended to do worse with dopamine than placebo in terms of 72-hour urine output, while those with systolic heart failure tended to do better with nesiritide than placebo. However, the trial was insufficiently powered to draw definitive conclusions on this score.
"One of our take-away messages when we looked at the data was that acute heart failure is broadly defined and includes a very diverse group of patients. With the suggestion that there may be a differential response based on ejection fraction, as we proceed with future heart failure studies we may consider targeting subsets of patients. Some therapies may benefit patients with reduced ejection fraction, others will benefit patients with preserved ejection fraction. We might want to power our studies to look at these subgroups," according to Dr. Chen.
Noting that current American Heart Association/American College of Cardiology guidelines for AHF management state that low-dose dopamine "may be considered" to improve diuresis and preserve renal function during diuretic therapy, the cardiologist said "the only conclusion we can make from our study is they should not be used routinely."
He added a caveat, however: "In my own practice, if I have a patient who is hypotensive and who hasn’t responded to increased doses of diuretics, I still may consider using low-dose dopamine at that stage" because of the reassuringly low 0.9% rate of hypotension in ROSE AHF.
Discussant Dr. Marco Metra of the University of Brescia (Italy) noted that low doses of dopamine and nesiritide are widely used in the United States and Europe. Recent data suggest nesiritide is used in 5%-10% of American patients with AHF and low-dose dopamine in 3%-5%.
"Surely this trial should reduce the use of these drugs in patients with acute heart failure. They are given routinely too often in some places," he said.
Discussant Marc Pfeffer called ROSE AHF "yet another example of shedding the light of the randomized controlled trial on things that we assume to be true but actually aren’t. And we probably spend more time on rounds talking about [improving decongestion in patients with AHF and renal dysfunction] than anything else," added Dr. Pfeffer, professor of medicine at Harvard University, Boston.
The ROSE AHF trial was sponsored by the NHLBI. Dr. Chen reported receiving research grants from Scios and royalties from Niles Therapeutics, Anexon, and UpToDate. Dr. Metra is a paid consultant to a handful of pharmaceutical companies.
DALLAS – Neither low-dose dopamine nor low-dose nesiritide enhanced decongestion or improved renal function in patients with acute heart failure and kidney dysfunction in the randomized ROSE AHF trial.
Thus, the major unmet need for drugs that accomplish these benefits continues. There remains no Food and Drug Administration–approved therapy for enhancing renal function in acute heart failure, Dr. Horng H. Chen observed in presenting the ROSE AHF results at the American Heart Association scientific sessions.

The need for such therapies is great. Most patients with heart failure eventually develop renal dysfunction. If this dysfunction is moderate or severe, patients may experience inadequate decongestion in response to diuretic therapy along with worsening renal dysfunction, both linked to poor clinical outcomes.
Prior small, single-center studies with various methodologic shortcomings had suggested possible benefit for dopamine or nesiritide in low doses thought to be renal specific. The ROSE AHF (Renal Optimization Strategies Evaluation in Acute Heart Failure) trial was conducted to provide definitive evidence to guide practice, explained Dr. Chen, professor of medicine at the Mayo Clinic, Rochester, Minn.
ROSE AHF was a randomized, double-blind, placebo-controlled clinical trial involving 360 patients hospitalized with AHF and renal dysfunction at 26 U.S. and Canadian centers. The study was carried out by the National Heart, Lung, and Blood Institute (NHLBI) Heart Failure Clinical Research Network. Patients were randomized within 24 hours of admission to low-dose dopamine at 2 mcg/kg per minute, low-dose nesiritide at 0.005 mcg/kg per minute, or placebo.
The coprimary endpoints were 72-hour cumulative urine volume as a measure of decongestion and 72-hour change in serum cystatin C as an indicator of renal function.
Interestingly, the two drugs clearly were not truly renal specific, even in these low doses widely considered to be so, Dr. Chen noted. This lack of renal specificity was evident from the side effect profile. Significant tachycardia occurred in 7.2% of the low-dose dopamine group, none on nesiritide, and 10.4% of placebo-treated controls. In contrast, significant hypotension occurred in 0.9% of patients on dopamine, 18.8% on nesiritide, and 10.4% on placebo.
A trend toward a differential treatment response depending upon whether a patient had heart failure with a reduced versus preserved ejection fraction was evident. Patients with diastolic heart failure tended to do worse with dopamine than placebo in terms of 72-hour urine output, while those with systolic heart failure tended to do better with nesiritide than placebo. However, the trial was insufficiently powered to draw definitive conclusions on this score.
"One of our take-away messages when we looked at the data was that acute heart failure is broadly defined and includes a very diverse group of patients. With the suggestion that there may be a differential response based on ejection fraction, as we proceed with future heart failure studies we may consider targeting subsets of patients. Some therapies may benefit patients with reduced ejection fraction, others will benefit patients with preserved ejection fraction. We might want to power our studies to look at these subgroups," according to Dr. Chen.
Noting that current American Heart Association/American College of Cardiology guidelines for AHF management state that low-dose dopamine "may be considered" to improve diuresis and preserve renal function during diuretic therapy, the cardiologist said "the only conclusion we can make from our study is they should not be used routinely."
He added a caveat, however: "In my own practice, if I have a patient who is hypotensive and who hasn’t responded to increased doses of diuretics, I still may consider using low-dose dopamine at that stage" because of the reassuringly low 0.9% rate of hypotension in ROSE AHF.
Discussant Dr. Marco Metra of the University of Brescia (Italy) noted that low doses of dopamine and nesiritide are widely used in the United States and Europe. Recent data suggest nesiritide is used in 5%-10% of American patients with AHF and low-dose dopamine in 3%-5%.
"Surely this trial should reduce the use of these drugs in patients with acute heart failure. They are given routinely too often in some places," he said.
Discussant Marc Pfeffer called ROSE AHF "yet another example of shedding the light of the randomized controlled trial on things that we assume to be true but actually aren’t. And we probably spend more time on rounds talking about [improving decongestion in patients with AHF and renal dysfunction] than anything else," added Dr. Pfeffer, professor of medicine at Harvard University, Boston.
The ROSE AHF trial was sponsored by the NHLBI. Dr. Chen reported receiving research grants from Scios and royalties from Niles Therapeutics, Anexon, and UpToDate. Dr. Metra is a paid consultant to a handful of pharmaceutical companies.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: Neither low-dose dopamine nor low-dose nesiritide improved decongestion or boosted renal function when added to diuretic therapy in patients hospitalized with acute heart failure and kidney dysfunction.
Data source: ROSE AHF, a multicenter, randomized, double-blind, placebo-controlled clinical trial of 360 patients with acute heart failure and renal dysfunction.
Disclosures. The trial was sponsored by the National Heart, Lung, and Blood Institute. Dr. Chen reported receiving research grants from Scios and royalties from Niles Therapeutics, Anexon, and UpToDate. Dr. Metra is a paid consultant to a handful of pharmaceutical companies.
Chelation plus vitamins halved cardiovascular events in diabetics
DALLAS – An EDTA-based chelation regimen for secondary prevention of cardiovascular events in patients with a history of myocardial infarction got a boost from a new TACT trial analysis showing additive benefit when chelation was accompanied by high-dose oral multivitamins.
"The benefit of chelation plus vitamins compared to placebo plus placebo is statistically significant and of a magnitude sufficient to be clinically important, with a number needed to treat of 12 to prevent one primary event over 5 years," Dr. Gervasio A. Lamas said in presenting the latest TACT (Trial to Assess Chelation Therapy) results at the American Heart Association scientific sessions.
Moreover, the benefit of chelation plus multivitamins was magnified in the more than 600 TACT participants with diabetes. The number needed to treat in that group was an impressively low 5.5, added Dr. Lamas, chairman of medicine at Mount Sinai Medical Center, Miami Beach, and chief of the Columbia University division of cardiology at Mount Sinai Medical Center.
Since 1956, EDTA (ethylenediaminetetraacetic acid) chelation has been utilized in complementary and alternative medical (CAM) practice to treat atherosclerotic disease, despite an absence of any supporting evidence. The TACT trial, sponsored by the National Institutes of Health, was conducted in order to put the CAM regimen to the test.
TACT was a rigorously conducted, randomized, double-blind, two-by-two factorial design trial in which 1,708 stable patients with a previous MI at 134 North American sites were placed on the intravenous chelation regimen or placebo and high-dose oral multivitamins or placebo. It was an arduous regimen designed to replicate what’s being used in CAM practice. The chelation regimen consisted of 30 weekly 500-cc intravenous infusions followed by another 10 infusions at 2- to 8-week intervals. Patients randomized to the multivitamin arm took 6 capsules per day.
"The capsules are large. They’re a bear to take," according to the cardiologist.
Nevertheless, 77% of patients completed 30 infusions and 65% completed all 40. And more than three-quarters of patients took the vitamins for at least a year, and half of participants did so for at least 3 years. All participants had high rates of guideline-recommended preventive medications usage.
The primary composite endpoint was the 5-year rate of all-cause mortality, MI, stroke, coronary revascularization, or hospitalization for angina. As previously reported, chelation therapy resulted in a statistically significant 18% reduction in the primary endpoint relative to placebo (JAMA 2013;309:1241-50), while high-dose multivitamin therapy led to a nonsignificant 11% reduction (Ann. Intern. Med., in press).
At the Dallas AHA meeting, Dr. Lamas focused on the results in the 421 subjects randomized to both chelation and multivitamins as compared to the 437 patients on double placebo. The primary composite endpoint occurred in 26% of patients on double active therapy and 32% on double placebo, representing a 26% reduction in relative risk. Thus, the addition of multivitamin therapy increased the magnitude of risk reduction in patients on chelation therapy from 18% to 26%.
The prespecified secondary ‘hard’ endpoint, a composite of cardiovascular death, recurrent MI, or stroke, occurred in 9% of participants on chelation plus multivitamins versus 13% of those on dual placebo, for a 34% reduction in risk.
The results of active therapy were even more impressive in patients with diabetes. They had a 41% reduction in risk of the primary composite endpoint with chelation alone compared with placebo, with a 5-year incidence of 25%, compared with 38% in controls. Notably, diabetic patients’ all-cause mortality with chelation therapy was 10%, compared with 16% with placebo, a 43% reduction in relative risk, while their rate of the secondary composite hard endpoint was 11%, versus 17% in controls.
The diabetic subgroup assigned to dual active therapy with both chelation and multivitamins fared even better, with a 51% decrease in the primary composite endpoint compared with controls on double placebo.
"A lot more work needs to be done on this before we can bring it to the bedside, but this is certainly suggestive data," Dr. Lamas said in summary.
Specific details on the chelation and multivitamin components of the regimen are available in an earlier publication (Am. Heart J. 2012;163:7-12).
Asked about the proposed mechanism of benefit of this CAM therapy, the cardiologist was quick to reply, "The simple answer is we do not really know what is happening."
Plausible hypotheses abound, though. One of the leading ones has to do with the fact that EDTA is a superb metal chelator.
"Heavy metals are associated very well in epidemiologic data with cardiovascular events; in particular, lead, cadmium, arsenic, sometimes mercury, and others that have less evidence, like tungsten and antimony. They’re all in our environment. Any of us who are of an age to have been exposed to leaded gasoline have lead in our bones. If we get an infusion of EDTA, we’ll have lead in our urine. It’s just the way it is. And as you get older and become osteoporotic, that lead starts getting released," Dr. Lamas explained.
In addition, in diabetic patients the formation of advanced glycation endpoints requires catalytic activity by metals in order to create oxygen species and cross linkage, he continued.
The TACT trial was sponsored by the National Center for Complementary and Alternative Medicine and the National Heart, Lung, and Blood Institute. Dr. Lamas reported having no relevant financial interests.
DALLAS – An EDTA-based chelation regimen for secondary prevention of cardiovascular events in patients with a history of myocardial infarction got a boost from a new TACT trial analysis showing additive benefit when chelation was accompanied by high-dose oral multivitamins.
"The benefit of chelation plus vitamins compared to placebo plus placebo is statistically significant and of a magnitude sufficient to be clinically important, with a number needed to treat of 12 to prevent one primary event over 5 years," Dr. Gervasio A. Lamas said in presenting the latest TACT (Trial to Assess Chelation Therapy) results at the American Heart Association scientific sessions.
Moreover, the benefit of chelation plus multivitamins was magnified in the more than 600 TACT participants with diabetes. The number needed to treat in that group was an impressively low 5.5, added Dr. Lamas, chairman of medicine at Mount Sinai Medical Center, Miami Beach, and chief of the Columbia University division of cardiology at Mount Sinai Medical Center.
Since 1956, EDTA (ethylenediaminetetraacetic acid) chelation has been utilized in complementary and alternative medical (CAM) practice to treat atherosclerotic disease, despite an absence of any supporting evidence. The TACT trial, sponsored by the National Institutes of Health, was conducted in order to put the CAM regimen to the test.
TACT was a rigorously conducted, randomized, double-blind, two-by-two factorial design trial in which 1,708 stable patients with a previous MI at 134 North American sites were placed on the intravenous chelation regimen or placebo and high-dose oral multivitamins or placebo. It was an arduous regimen designed to replicate what’s being used in CAM practice. The chelation regimen consisted of 30 weekly 500-cc intravenous infusions followed by another 10 infusions at 2- to 8-week intervals. Patients randomized to the multivitamin arm took 6 capsules per day.
"The capsules are large. They’re a bear to take," according to the cardiologist.
Nevertheless, 77% of patients completed 30 infusions and 65% completed all 40. And more than three-quarters of patients took the vitamins for at least a year, and half of participants did so for at least 3 years. All participants had high rates of guideline-recommended preventive medications usage.
The primary composite endpoint was the 5-year rate of all-cause mortality, MI, stroke, coronary revascularization, or hospitalization for angina. As previously reported, chelation therapy resulted in a statistically significant 18% reduction in the primary endpoint relative to placebo (JAMA 2013;309:1241-50), while high-dose multivitamin therapy led to a nonsignificant 11% reduction (Ann. Intern. Med., in press).
At the Dallas AHA meeting, Dr. Lamas focused on the results in the 421 subjects randomized to both chelation and multivitamins as compared to the 437 patients on double placebo. The primary composite endpoint occurred in 26% of patients on double active therapy and 32% on double placebo, representing a 26% reduction in relative risk. Thus, the addition of multivitamin therapy increased the magnitude of risk reduction in patients on chelation therapy from 18% to 26%.
The prespecified secondary ‘hard’ endpoint, a composite of cardiovascular death, recurrent MI, or stroke, occurred in 9% of participants on chelation plus multivitamins versus 13% of those on dual placebo, for a 34% reduction in risk.
The results of active therapy were even more impressive in patients with diabetes. They had a 41% reduction in risk of the primary composite endpoint with chelation alone compared with placebo, with a 5-year incidence of 25%, compared with 38% in controls. Notably, diabetic patients’ all-cause mortality with chelation therapy was 10%, compared with 16% with placebo, a 43% reduction in relative risk, while their rate of the secondary composite hard endpoint was 11%, versus 17% in controls.
The diabetic subgroup assigned to dual active therapy with both chelation and multivitamins fared even better, with a 51% decrease in the primary composite endpoint compared with controls on double placebo.
"A lot more work needs to be done on this before we can bring it to the bedside, but this is certainly suggestive data," Dr. Lamas said in summary.
Specific details on the chelation and multivitamin components of the regimen are available in an earlier publication (Am. Heart J. 2012;163:7-12).
Asked about the proposed mechanism of benefit of this CAM therapy, the cardiologist was quick to reply, "The simple answer is we do not really know what is happening."
Plausible hypotheses abound, though. One of the leading ones has to do with the fact that EDTA is a superb metal chelator.
"Heavy metals are associated very well in epidemiologic data with cardiovascular events; in particular, lead, cadmium, arsenic, sometimes mercury, and others that have less evidence, like tungsten and antimony. They’re all in our environment. Any of us who are of an age to have been exposed to leaded gasoline have lead in our bones. If we get an infusion of EDTA, we’ll have lead in our urine. It’s just the way it is. And as you get older and become osteoporotic, that lead starts getting released," Dr. Lamas explained.
In addition, in diabetic patients the formation of advanced glycation endpoints requires catalytic activity by metals in order to create oxygen species and cross linkage, he continued.
The TACT trial was sponsored by the National Center for Complementary and Alternative Medicine and the National Heart, Lung, and Blood Institute. Dr. Lamas reported having no relevant financial interests.
DALLAS – An EDTA-based chelation regimen for secondary prevention of cardiovascular events in patients with a history of myocardial infarction got a boost from a new TACT trial analysis showing additive benefit when chelation was accompanied by high-dose oral multivitamins.
"The benefit of chelation plus vitamins compared to placebo plus placebo is statistically significant and of a magnitude sufficient to be clinically important, with a number needed to treat of 12 to prevent one primary event over 5 years," Dr. Gervasio A. Lamas said in presenting the latest TACT (Trial to Assess Chelation Therapy) results at the American Heart Association scientific sessions.
Moreover, the benefit of chelation plus multivitamins was magnified in the more than 600 TACT participants with diabetes. The number needed to treat in that group was an impressively low 5.5, added Dr. Lamas, chairman of medicine at Mount Sinai Medical Center, Miami Beach, and chief of the Columbia University division of cardiology at Mount Sinai Medical Center.
Since 1956, EDTA (ethylenediaminetetraacetic acid) chelation has been utilized in complementary and alternative medical (CAM) practice to treat atherosclerotic disease, despite an absence of any supporting evidence. The TACT trial, sponsored by the National Institutes of Health, was conducted in order to put the CAM regimen to the test.
TACT was a rigorously conducted, randomized, double-blind, two-by-two factorial design trial in which 1,708 stable patients with a previous MI at 134 North American sites were placed on the intravenous chelation regimen or placebo and high-dose oral multivitamins or placebo. It was an arduous regimen designed to replicate what’s being used in CAM practice. The chelation regimen consisted of 30 weekly 500-cc intravenous infusions followed by another 10 infusions at 2- to 8-week intervals. Patients randomized to the multivitamin arm took 6 capsules per day.
"The capsules are large. They’re a bear to take," according to the cardiologist.
Nevertheless, 77% of patients completed 30 infusions and 65% completed all 40. And more than three-quarters of patients took the vitamins for at least a year, and half of participants did so for at least 3 years. All participants had high rates of guideline-recommended preventive medications usage.
The primary composite endpoint was the 5-year rate of all-cause mortality, MI, stroke, coronary revascularization, or hospitalization for angina. As previously reported, chelation therapy resulted in a statistically significant 18% reduction in the primary endpoint relative to placebo (JAMA 2013;309:1241-50), while high-dose multivitamin therapy led to a nonsignificant 11% reduction (Ann. Intern. Med., in press).
At the Dallas AHA meeting, Dr. Lamas focused on the results in the 421 subjects randomized to both chelation and multivitamins as compared to the 437 patients on double placebo. The primary composite endpoint occurred in 26% of patients on double active therapy and 32% on double placebo, representing a 26% reduction in relative risk. Thus, the addition of multivitamin therapy increased the magnitude of risk reduction in patients on chelation therapy from 18% to 26%.
The prespecified secondary ‘hard’ endpoint, a composite of cardiovascular death, recurrent MI, or stroke, occurred in 9% of participants on chelation plus multivitamins versus 13% of those on dual placebo, for a 34% reduction in risk.
The results of active therapy were even more impressive in patients with diabetes. They had a 41% reduction in risk of the primary composite endpoint with chelation alone compared with placebo, with a 5-year incidence of 25%, compared with 38% in controls. Notably, diabetic patients’ all-cause mortality with chelation therapy was 10%, compared with 16% with placebo, a 43% reduction in relative risk, while their rate of the secondary composite hard endpoint was 11%, versus 17% in controls.
The diabetic subgroup assigned to dual active therapy with both chelation and multivitamins fared even better, with a 51% decrease in the primary composite endpoint compared with controls on double placebo.
"A lot more work needs to be done on this before we can bring it to the bedside, but this is certainly suggestive data," Dr. Lamas said in summary.
Specific details on the chelation and multivitamin components of the regimen are available in an earlier publication (Am. Heart J. 2012;163:7-12).
Asked about the proposed mechanism of benefit of this CAM therapy, the cardiologist was quick to reply, "The simple answer is we do not really know what is happening."
Plausible hypotheses abound, though. One of the leading ones has to do with the fact that EDTA is a superb metal chelator.
"Heavy metals are associated very well in epidemiologic data with cardiovascular events; in particular, lead, cadmium, arsenic, sometimes mercury, and others that have less evidence, like tungsten and antimony. They’re all in our environment. Any of us who are of an age to have been exposed to leaded gasoline have lead in our bones. If we get an infusion of EDTA, we’ll have lead in our urine. It’s just the way it is. And as you get older and become osteoporotic, that lead starts getting released," Dr. Lamas explained.
In addition, in diabetic patients the formation of advanced glycation endpoints requires catalytic activity by metals in order to create oxygen species and cross linkage, he continued.
The TACT trial was sponsored by the National Center for Complementary and Alternative Medicine and the National Heart, Lung, and Blood Institute. Dr. Lamas reported having no relevant financial interests.
AT THE AHA SCIENTIFIC SESSIONS
Major finding: A mere 5.5 diabetic patients with a history of MI needed to be treated with a regimen of EDTA chelation plus high-dose multivitamins in order to prevent 1 additional cardiovascular event over 5 years in a large randomized trial.
Data source: The TACT trial was a double-blind, placebo-controlled, multicenter study with a two-by-two factorial design, involving 1,708 patients with a history of MI.
Disclosures. The TACT trial was sponsored by the National Center for Complementary and Alternative Medicine and the National Heart, Lung, and Blood Institute. Dr. Lamas reported having no relevant financial interests.
















