User login
Researchers seek a way to predict cognitive deficits in children treated for ALL
Researchers are attempting to determine, early in the treatment process, which children with acute lymphoblastic leukemia (ALL) have an increased risk of neurocognitive deficits after chemotherapy.
The goal of the researchers’ project (5R01CA220568-02) is to determine if gene variants and biomarkers associated with oxidative stress, neuroinflammation, and folate physiology correlate with cognitive decline during and after chemotherapy. Ideally, certain variants and biomarkers will reveal patients who might benefit from interventions to prevent or even reverse cognitive deficits.
Peter D. Cole, MD, of Rutgers Cancer Institute, New Brunswick, N.J., and colleagues are conducting this research in patients from the DFCI-16-001 trial (NCT03020030). This multicenter, phase 3 study is enrolling patients (aged 1-21 years) with B- or T-cell ALL who then receive a multidrug chemotherapy regimen.
Dr. Cole and colleagues are analyzing a subset of patients from the trial, looking for relationships between chemotherapy-induced neurocognitive changes, gene variants, and changes in biomarkers detected in cerebrospinal fluid (CSF).
“We’re looking at a broad panel of target gene variants that are associated with either drug metabolism, defenses against oxidative stress, neuroinflammation, or folate physiology,” Dr. Cole said in an interview.
This includes variants Dr. Cole and colleagues identified in a previous, retrospective study of ALL survivors. The researchers found that survivors who were homozygous for NOS3 894T, had a variant SLCO2A1 G allele, or had at least one GSTP1 T allele were more likely to exhibit cognitive deficits (J Clin Oncol. 2015 Jul 1;33[19]:2205-11).
The researchers are also analyzing CSF samples, looking for changes in tau protein, homocysteine, homocysteic acid, the adenosylmethionine to adenosylhomocysteine ratio, and other biomarkers of oxidative stress, neuroinflammation, and folate physiology. The CSF is collected at five time points: the start of chemotherapy, day 18, the start of first consolidation, the end of first consolidation, and 7 weeks later in second consolidation.
Cognitive testing
While Dr. Cole is leading the genetic and biomarker analyses, Stephen A. Sands, PsyD, of Memorial Sloan Kettering Cancer Center in New York, is leading the cognitive testing.
The researchers are evaluating patients for cognitive decline using computerized tests from a company called Cogstate. The tests are designed to assess functions such as processing speed, attention, visual learning, and working memory. The tests are administered on an iPad and involve tasks like identifying features of playing cards and finding the correct way through a maze.
The patients – aged 3 years and older – undergo cognitive testing at six time points: baseline, which is any time between days 8 and 32 of induction (except within 72 hours after sedation or anesthesia); at first consolidation; the end of central nervous system therapy; 1 year into chemotherapy; the end of chemotherapy; and 1 year after chemotherapy ends.
In a prior study, Cogstate testing proved reliable for detecting neurocognitive changes in patients undergoing treatment for ALL (Support Care Cancer. 2017;25[2]:449-57). In the current study, the researchers are supplementing Cogstate test results with Wechsler IQ tests administered 1 year after patients complete chemotherapy.
Dr. Sands noted that Cogstate tests provide benefits over the Wechsler “paper-and-pencil” tests. One benefit is that Cogstate tests can be given more often without inducing practice effects (J Clin Exp Neuropsychol. 2006 Oct;28[7]:1095-112). Another is that Cogstate tests can be administered by anyone with a bachelor’s degree who has undergone the appropriate training, while Wechsler IQ tests must be given by psychologists.
Preliminary results
This research is ongoing, so it’s too early to announce any discoveries, but the study is moving along as planned.
“The preliminary data we have so far are demonstrating the validity of the study,” Dr. Cole said. “Things are going well. We’re able to do the cognitive testing and collect the samples that we need and ship them without losing the integrity of the samples.”
Dr. Sands noted that enrollment has been encouraging. As this is a substudy of DFCI-16-001, the researchers must obtain consent separately from the main study. Dr. Sands said about 89% of parents involved in the main study have agreed to enroll their children in the substudy.
Dr. Sands also said that early results from Cogstate testing have revealed patients who are experiencing cognitive decline during treatment. The researchers still have to determine if these results correlate with any biomarkers or gene variants.
Potential interventions
If the researchers can pinpoint patients at risk for cognitive deficits, the next step will be to investigate pharmacologic and behavioral interventions.
Dr. Cole said he is particularly interested in treatments that reduce oxidative stress, such as dextromethorphan and memantine. Dextromethorphan has been shown to resolve symptoms of methotrexate-induced neurotoxicity in patients (Pediatr Hematol Oncol. 2002 Jul-Aug;19[5]:319-27), and memantine reduced memory deficits in animals treated with methotrexate (Clin Cancer Res. 2013 Aug 15;19[16]:4446-54).
“Memantine hasn’t been used in kids with leukemia yet, but it’s something that I’d like to see brought to a clinical trial,” Dr. Cole said.
Dr. Sands pointed to other potential pharmacologic interventions, including the stimulants methylphenidate and modafinil. Both drugs have been shown to improve cognitive deficits in cancer survivors (J Clin Oncol. 2001 Mar 15;19[6]:1802-8; Cancer. 2009 Jun 15; 115[12]: 2605-16).
Computer-based cognitive training tools may be another option. One such tool, Lumosity, improved executive functions in a study of breast cancer survivors (Clin Breast Cancer. 2013 Aug;13[4]:299-306). Another tool, CogMed, improved working memory in survivors of brain tumors and ALL (Psychooncology. 2013 Aug; 22[8]: 1856-65).
Other behavioral interventions might include sleep hygiene and exercise. Sleep hygiene has been shown to improve cognitive function in childhood cancer survivors (Cancer. 2011 Jun 1;117[11]:2559-68), and a recent study revealed an association between exercise intolerance and negative neurocognitive outcomes in ALL survivors (Cancer. 2019 Oct 21. doi: 10.1002/cncr.32510).
“What we need to figure out is which children will respond to which interventions,” Dr. Sands said, adding that interventions will likely need to be combined.
“It’s not going to be one thing that will work for everybody,” he said. “It’s going to be: What packages of things will work for different people?”
Dr. Sands and Dr. Cole reported having no relevant financial disclosures.
Researchers are attempting to determine, early in the treatment process, which children with acute lymphoblastic leukemia (ALL) have an increased risk of neurocognitive deficits after chemotherapy.
The goal of the researchers’ project (5R01CA220568-02) is to determine if gene variants and biomarkers associated with oxidative stress, neuroinflammation, and folate physiology correlate with cognitive decline during and after chemotherapy. Ideally, certain variants and biomarkers will reveal patients who might benefit from interventions to prevent or even reverse cognitive deficits.
Peter D. Cole, MD, of Rutgers Cancer Institute, New Brunswick, N.J., and colleagues are conducting this research in patients from the DFCI-16-001 trial (NCT03020030). This multicenter, phase 3 study is enrolling patients (aged 1-21 years) with B- or T-cell ALL who then receive a multidrug chemotherapy regimen.
Dr. Cole and colleagues are analyzing a subset of patients from the trial, looking for relationships between chemotherapy-induced neurocognitive changes, gene variants, and changes in biomarkers detected in cerebrospinal fluid (CSF).
“We’re looking at a broad panel of target gene variants that are associated with either drug metabolism, defenses against oxidative stress, neuroinflammation, or folate physiology,” Dr. Cole said in an interview.
This includes variants Dr. Cole and colleagues identified in a previous, retrospective study of ALL survivors. The researchers found that survivors who were homozygous for NOS3 894T, had a variant SLCO2A1 G allele, or had at least one GSTP1 T allele were more likely to exhibit cognitive deficits (J Clin Oncol. 2015 Jul 1;33[19]:2205-11).
The researchers are also analyzing CSF samples, looking for changes in tau protein, homocysteine, homocysteic acid, the adenosylmethionine to adenosylhomocysteine ratio, and other biomarkers of oxidative stress, neuroinflammation, and folate physiology. The CSF is collected at five time points: the start of chemotherapy, day 18, the start of first consolidation, the end of first consolidation, and 7 weeks later in second consolidation.
Cognitive testing
While Dr. Cole is leading the genetic and biomarker analyses, Stephen A. Sands, PsyD, of Memorial Sloan Kettering Cancer Center in New York, is leading the cognitive testing.
The researchers are evaluating patients for cognitive decline using computerized tests from a company called Cogstate. The tests are designed to assess functions such as processing speed, attention, visual learning, and working memory. The tests are administered on an iPad and involve tasks like identifying features of playing cards and finding the correct way through a maze.
The patients – aged 3 years and older – undergo cognitive testing at six time points: baseline, which is any time between days 8 and 32 of induction (except within 72 hours after sedation or anesthesia); at first consolidation; the end of central nervous system therapy; 1 year into chemotherapy; the end of chemotherapy; and 1 year after chemotherapy ends.
In a prior study, Cogstate testing proved reliable for detecting neurocognitive changes in patients undergoing treatment for ALL (Support Care Cancer. 2017;25[2]:449-57). In the current study, the researchers are supplementing Cogstate test results with Wechsler IQ tests administered 1 year after patients complete chemotherapy.
Dr. Sands noted that Cogstate tests provide benefits over the Wechsler “paper-and-pencil” tests. One benefit is that Cogstate tests can be given more often without inducing practice effects (J Clin Exp Neuropsychol. 2006 Oct;28[7]:1095-112). Another is that Cogstate tests can be administered by anyone with a bachelor’s degree who has undergone the appropriate training, while Wechsler IQ tests must be given by psychologists.
Preliminary results
This research is ongoing, so it’s too early to announce any discoveries, but the study is moving along as planned.
“The preliminary data we have so far are demonstrating the validity of the study,” Dr. Cole said. “Things are going well. We’re able to do the cognitive testing and collect the samples that we need and ship them without losing the integrity of the samples.”
Dr. Sands noted that enrollment has been encouraging. As this is a substudy of DFCI-16-001, the researchers must obtain consent separately from the main study. Dr. Sands said about 89% of parents involved in the main study have agreed to enroll their children in the substudy.
Dr. Sands also said that early results from Cogstate testing have revealed patients who are experiencing cognitive decline during treatment. The researchers still have to determine if these results correlate with any biomarkers or gene variants.
Potential interventions
If the researchers can pinpoint patients at risk for cognitive deficits, the next step will be to investigate pharmacologic and behavioral interventions.
Dr. Cole said he is particularly interested in treatments that reduce oxidative stress, such as dextromethorphan and memantine. Dextromethorphan has been shown to resolve symptoms of methotrexate-induced neurotoxicity in patients (Pediatr Hematol Oncol. 2002 Jul-Aug;19[5]:319-27), and memantine reduced memory deficits in animals treated with methotrexate (Clin Cancer Res. 2013 Aug 15;19[16]:4446-54).
“Memantine hasn’t been used in kids with leukemia yet, but it’s something that I’d like to see brought to a clinical trial,” Dr. Cole said.
Dr. Sands pointed to other potential pharmacologic interventions, including the stimulants methylphenidate and modafinil. Both drugs have been shown to improve cognitive deficits in cancer survivors (J Clin Oncol. 2001 Mar 15;19[6]:1802-8; Cancer. 2009 Jun 15; 115[12]: 2605-16).
Computer-based cognitive training tools may be another option. One such tool, Lumosity, improved executive functions in a study of breast cancer survivors (Clin Breast Cancer. 2013 Aug;13[4]:299-306). Another tool, CogMed, improved working memory in survivors of brain tumors and ALL (Psychooncology. 2013 Aug; 22[8]: 1856-65).
Other behavioral interventions might include sleep hygiene and exercise. Sleep hygiene has been shown to improve cognitive function in childhood cancer survivors (Cancer. 2011 Jun 1;117[11]:2559-68), and a recent study revealed an association between exercise intolerance and negative neurocognitive outcomes in ALL survivors (Cancer. 2019 Oct 21. doi: 10.1002/cncr.32510).
“What we need to figure out is which children will respond to which interventions,” Dr. Sands said, adding that interventions will likely need to be combined.
“It’s not going to be one thing that will work for everybody,” he said. “It’s going to be: What packages of things will work for different people?”
Dr. Sands and Dr. Cole reported having no relevant financial disclosures.
Researchers are attempting to determine, early in the treatment process, which children with acute lymphoblastic leukemia (ALL) have an increased risk of neurocognitive deficits after chemotherapy.
The goal of the researchers’ project (5R01CA220568-02) is to determine if gene variants and biomarkers associated with oxidative stress, neuroinflammation, and folate physiology correlate with cognitive decline during and after chemotherapy. Ideally, certain variants and biomarkers will reveal patients who might benefit from interventions to prevent or even reverse cognitive deficits.
Peter D. Cole, MD, of Rutgers Cancer Institute, New Brunswick, N.J., and colleagues are conducting this research in patients from the DFCI-16-001 trial (NCT03020030). This multicenter, phase 3 study is enrolling patients (aged 1-21 years) with B- or T-cell ALL who then receive a multidrug chemotherapy regimen.
Dr. Cole and colleagues are analyzing a subset of patients from the trial, looking for relationships between chemotherapy-induced neurocognitive changes, gene variants, and changes in biomarkers detected in cerebrospinal fluid (CSF).
“We’re looking at a broad panel of target gene variants that are associated with either drug metabolism, defenses against oxidative stress, neuroinflammation, or folate physiology,” Dr. Cole said in an interview.
This includes variants Dr. Cole and colleagues identified in a previous, retrospective study of ALL survivors. The researchers found that survivors who were homozygous for NOS3 894T, had a variant SLCO2A1 G allele, or had at least one GSTP1 T allele were more likely to exhibit cognitive deficits (J Clin Oncol. 2015 Jul 1;33[19]:2205-11).
The researchers are also analyzing CSF samples, looking for changes in tau protein, homocysteine, homocysteic acid, the adenosylmethionine to adenosylhomocysteine ratio, and other biomarkers of oxidative stress, neuroinflammation, and folate physiology. The CSF is collected at five time points: the start of chemotherapy, day 18, the start of first consolidation, the end of first consolidation, and 7 weeks later in second consolidation.
Cognitive testing
While Dr. Cole is leading the genetic and biomarker analyses, Stephen A. Sands, PsyD, of Memorial Sloan Kettering Cancer Center in New York, is leading the cognitive testing.
The researchers are evaluating patients for cognitive decline using computerized tests from a company called Cogstate. The tests are designed to assess functions such as processing speed, attention, visual learning, and working memory. The tests are administered on an iPad and involve tasks like identifying features of playing cards and finding the correct way through a maze.
The patients – aged 3 years and older – undergo cognitive testing at six time points: baseline, which is any time between days 8 and 32 of induction (except within 72 hours after sedation or anesthesia); at first consolidation; the end of central nervous system therapy; 1 year into chemotherapy; the end of chemotherapy; and 1 year after chemotherapy ends.
In a prior study, Cogstate testing proved reliable for detecting neurocognitive changes in patients undergoing treatment for ALL (Support Care Cancer. 2017;25[2]:449-57). In the current study, the researchers are supplementing Cogstate test results with Wechsler IQ tests administered 1 year after patients complete chemotherapy.
Dr. Sands noted that Cogstate tests provide benefits over the Wechsler “paper-and-pencil” tests. One benefit is that Cogstate tests can be given more often without inducing practice effects (J Clin Exp Neuropsychol. 2006 Oct;28[7]:1095-112). Another is that Cogstate tests can be administered by anyone with a bachelor’s degree who has undergone the appropriate training, while Wechsler IQ tests must be given by psychologists.
Preliminary results
This research is ongoing, so it’s too early to announce any discoveries, but the study is moving along as planned.
“The preliminary data we have so far are demonstrating the validity of the study,” Dr. Cole said. “Things are going well. We’re able to do the cognitive testing and collect the samples that we need and ship them without losing the integrity of the samples.”
Dr. Sands noted that enrollment has been encouraging. As this is a substudy of DFCI-16-001, the researchers must obtain consent separately from the main study. Dr. Sands said about 89% of parents involved in the main study have agreed to enroll their children in the substudy.
Dr. Sands also said that early results from Cogstate testing have revealed patients who are experiencing cognitive decline during treatment. The researchers still have to determine if these results correlate with any biomarkers or gene variants.
Potential interventions
If the researchers can pinpoint patients at risk for cognitive deficits, the next step will be to investigate pharmacologic and behavioral interventions.
Dr. Cole said he is particularly interested in treatments that reduce oxidative stress, such as dextromethorphan and memantine. Dextromethorphan has been shown to resolve symptoms of methotrexate-induced neurotoxicity in patients (Pediatr Hematol Oncol. 2002 Jul-Aug;19[5]:319-27), and memantine reduced memory deficits in animals treated with methotrexate (Clin Cancer Res. 2013 Aug 15;19[16]:4446-54).
“Memantine hasn’t been used in kids with leukemia yet, but it’s something that I’d like to see brought to a clinical trial,” Dr. Cole said.
Dr. Sands pointed to other potential pharmacologic interventions, including the stimulants methylphenidate and modafinil. Both drugs have been shown to improve cognitive deficits in cancer survivors (J Clin Oncol. 2001 Mar 15;19[6]:1802-8; Cancer. 2009 Jun 15; 115[12]: 2605-16).
Computer-based cognitive training tools may be another option. One such tool, Lumosity, improved executive functions in a study of breast cancer survivors (Clin Breast Cancer. 2013 Aug;13[4]:299-306). Another tool, CogMed, improved working memory in survivors of brain tumors and ALL (Psychooncology. 2013 Aug; 22[8]: 1856-65).
Other behavioral interventions might include sleep hygiene and exercise. Sleep hygiene has been shown to improve cognitive function in childhood cancer survivors (Cancer. 2011 Jun 1;117[11]:2559-68), and a recent study revealed an association between exercise intolerance and negative neurocognitive outcomes in ALL survivors (Cancer. 2019 Oct 21. doi: 10.1002/cncr.32510).
“What we need to figure out is which children will respond to which interventions,” Dr. Sands said, adding that interventions will likely need to be combined.
“It’s not going to be one thing that will work for everybody,” he said. “It’s going to be: What packages of things will work for different people?”
Dr. Sands and Dr. Cole reported having no relevant financial disclosures.
Foundation launches direct-to-patient registry in multiple myeloma
The Multiple Myeloma Research Foundation (MMRF) recently launched its Direct-to-Patient registry, in what the organization’s leaders are describing as a “disruptive” step toward improving outcomes for patients with multiple myeloma.
The new registry is intended to build upon CoMMpass, a program started 8 years ago that now represents the largest genomic database of any type of cancer. Although CoMMpass includes data from about 1,150 patients with myeloma, it’s not enough information, according to the chief marketing and development officer at the MMRF, Anne Quinn Young.
“For a disease as heterogenous as myeloma is, we need more, particularly because we don’t have all the samples for later-stage disease,” Ms. Quinn Young said in an interview. “And even with the clinical data, given the patient population, both [in terms of] demographics and the nature of the disease, the numbers of patients still living after multiple relapses is rather small.”
In an earlier effort to gather more data, the MMRF first turned to other organizations for help, but this approach fell short because of scarcity of data, and in some cases, unwillingness to share. Steven Labkoff, MD, chief data officer at the MMRF, described this experience in an interview.
“When the MMRF was looking around for different data sources for myeloma data, it was always the claim that, ‘Sure, we have plenty of patients, we have plenty of data, and it’s rich and really complete.’ However, as we approached an array of organizations – big organizations – as we dug into the details and reviewed patient counts or data completeness, they either didn’t have a sufficient number of patients, they didn’t have sufficiently complete data for our needs, and in the case where some did have sufficient numbers and complete data sets, they simply weren’t in a position to share that data outside their institution,” Dr. Labkoff said.
Undeterred, the MMRF switched tactics to the current, patient-centric approach.
“We’re leveraging one aspect of the HIPAA legislation,” Dr. Labkoff said, referring to patients’ rights to request their own medical records and an institution’s legal obligation to provide those records.
In the short-term, the registry will collect three types of data: patient donated data (answers from a patient survey), electronic medical records abstracted from all relevant past providers, and genomic test results. Participating patients will have blood drawn at home by a phlebotomist for the genomic assay. Additional tubes of blood will be concurrently collected and biobanked. This will eventually allow for immune profiling, Dr. Labkoff said.
Future goals include a patient-reported outcomes module and the ability to link data with medical claims.
So far, 79 patients have participated in the pilot program, according to the MMRF. As the database builds, Ms. Quinn Young and Dr. Labkoff anticipate that it will yield answers to a variety of real-world questions.
Dr. Labkoff offered two examples. “Of all the patients who have been exposed to ‘name your drug,’ what were the costs of their therapy, and what were the outcomes?” he said. In addition, researchers will be able to query clinical trial inclusion criteria to search for data on a specific patient profile, such as patients with a 4:14 translocation, who have had a bone marrow transplant in the last 2 years, and have been exposed to a certain drug regimen.
Ms. Quinn Young noted that doctors may be able to use the database to reliably identify high-risk patients and guide agent selection. Common patient questions also will be addressed, she said, including best treatment regimens for certain types of patients.
“For patients who may have run out of all commercially available options, or for patients who are perhaps seen at a community center, where certainly this type of profiling is not standard, it’s opening up a whole new set of options for them,” Ms. Quinn Young said. “And if their physician doesn’t pursue those options, they have the report that they can use to seek a second opinion.”
The Direct-to-Patient registry is unique because it aims to empower patients in a way that hasn’t been done before, Ms. Quinn Young said. “We are committed ... ever since we conceived of this project, to giving results back to patients. That is disruptive because right now that doesn’t exist.”
But the cost of implementing the registry, which has an approximate budget of $20 million, stands in the way of a completely free flow of anonymized data. MMRF leaders are exploring different strategies to sustain funding for the program.
Another MMRF program, CoMMpass, uses a precompetitive consortium model, in which several pharmaceutical companies pay for a preview of the data 6 months in advance of nonprofit researchers. A similar model may be used with the Direct-to-Patient registry, but this has yet to be determined, according to Dr. Labkoff and Ms. Quinn Young.
For now, Ms. Quinn Young said she hopes that physicians will be receptive to the program. “[The short term goal is that] when patients come to their doctors asking about this, that there is support and open-mindedness,” she said.
Looking to the future, Dr. Labkoff described how the registry could accelerate myeloma research, ultimately toward a cure.
“It is generally accepted that it can take 17 years to get something – a therapy, a new drug, or a guideline – from the bench to the bedside,” he said. “It’s my hope that we can take next generation sequencing and the results of this registry and bend that curve, maybe ... to 10 [years], or very aggressively, to 7 or 5 [years], where doctors are able to use the information in these reports for the patients that have literally given themselves, and use this to help guide the choices of their therapy or the trials they apply for, to help them get a better outcome in general.”
The Direct-to-Patient registry is a collaborative effort between the MMRF and multiple organizations, including the health care technology company COTA, the Broad Institute of Harvard and MIT, Prometheus Research, Tempus, and the Dana Farber Cancer Institute.
The Multiple Myeloma Research Foundation (MMRF) recently launched its Direct-to-Patient registry, in what the organization’s leaders are describing as a “disruptive” step toward improving outcomes for patients with multiple myeloma.
The new registry is intended to build upon CoMMpass, a program started 8 years ago that now represents the largest genomic database of any type of cancer. Although CoMMpass includes data from about 1,150 patients with myeloma, it’s not enough information, according to the chief marketing and development officer at the MMRF, Anne Quinn Young.
“For a disease as heterogenous as myeloma is, we need more, particularly because we don’t have all the samples for later-stage disease,” Ms. Quinn Young said in an interview. “And even with the clinical data, given the patient population, both [in terms of] demographics and the nature of the disease, the numbers of patients still living after multiple relapses is rather small.”
In an earlier effort to gather more data, the MMRF first turned to other organizations for help, but this approach fell short because of scarcity of data, and in some cases, unwillingness to share. Steven Labkoff, MD, chief data officer at the MMRF, described this experience in an interview.
“When the MMRF was looking around for different data sources for myeloma data, it was always the claim that, ‘Sure, we have plenty of patients, we have plenty of data, and it’s rich and really complete.’ However, as we approached an array of organizations – big organizations – as we dug into the details and reviewed patient counts or data completeness, they either didn’t have a sufficient number of patients, they didn’t have sufficiently complete data for our needs, and in the case where some did have sufficient numbers and complete data sets, they simply weren’t in a position to share that data outside their institution,” Dr. Labkoff said.
Undeterred, the MMRF switched tactics to the current, patient-centric approach.
“We’re leveraging one aspect of the HIPAA legislation,” Dr. Labkoff said, referring to patients’ rights to request their own medical records and an institution’s legal obligation to provide those records.
In the short-term, the registry will collect three types of data: patient donated data (answers from a patient survey), electronic medical records abstracted from all relevant past providers, and genomic test results. Participating patients will have blood drawn at home by a phlebotomist for the genomic assay. Additional tubes of blood will be concurrently collected and biobanked. This will eventually allow for immune profiling, Dr. Labkoff said.
Future goals include a patient-reported outcomes module and the ability to link data with medical claims.
So far, 79 patients have participated in the pilot program, according to the MMRF. As the database builds, Ms. Quinn Young and Dr. Labkoff anticipate that it will yield answers to a variety of real-world questions.
Dr. Labkoff offered two examples. “Of all the patients who have been exposed to ‘name your drug,’ what were the costs of their therapy, and what were the outcomes?” he said. In addition, researchers will be able to query clinical trial inclusion criteria to search for data on a specific patient profile, such as patients with a 4:14 translocation, who have had a bone marrow transplant in the last 2 years, and have been exposed to a certain drug regimen.
Ms. Quinn Young noted that doctors may be able to use the database to reliably identify high-risk patients and guide agent selection. Common patient questions also will be addressed, she said, including best treatment regimens for certain types of patients.
“For patients who may have run out of all commercially available options, or for patients who are perhaps seen at a community center, where certainly this type of profiling is not standard, it’s opening up a whole new set of options for them,” Ms. Quinn Young said. “And if their physician doesn’t pursue those options, they have the report that they can use to seek a second opinion.”
The Direct-to-Patient registry is unique because it aims to empower patients in a way that hasn’t been done before, Ms. Quinn Young said. “We are committed ... ever since we conceived of this project, to giving results back to patients. That is disruptive because right now that doesn’t exist.”
But the cost of implementing the registry, which has an approximate budget of $20 million, stands in the way of a completely free flow of anonymized data. MMRF leaders are exploring different strategies to sustain funding for the program.
Another MMRF program, CoMMpass, uses a precompetitive consortium model, in which several pharmaceutical companies pay for a preview of the data 6 months in advance of nonprofit researchers. A similar model may be used with the Direct-to-Patient registry, but this has yet to be determined, according to Dr. Labkoff and Ms. Quinn Young.
For now, Ms. Quinn Young said she hopes that physicians will be receptive to the program. “[The short term goal is that] when patients come to their doctors asking about this, that there is support and open-mindedness,” she said.
Looking to the future, Dr. Labkoff described how the registry could accelerate myeloma research, ultimately toward a cure.
“It is generally accepted that it can take 17 years to get something – a therapy, a new drug, or a guideline – from the bench to the bedside,” he said. “It’s my hope that we can take next generation sequencing and the results of this registry and bend that curve, maybe ... to 10 [years], or very aggressively, to 7 or 5 [years], where doctors are able to use the information in these reports for the patients that have literally given themselves, and use this to help guide the choices of their therapy or the trials they apply for, to help them get a better outcome in general.”
The Direct-to-Patient registry is a collaborative effort between the MMRF and multiple organizations, including the health care technology company COTA, the Broad Institute of Harvard and MIT, Prometheus Research, Tempus, and the Dana Farber Cancer Institute.
The Multiple Myeloma Research Foundation (MMRF) recently launched its Direct-to-Patient registry, in what the organization’s leaders are describing as a “disruptive” step toward improving outcomes for patients with multiple myeloma.
The new registry is intended to build upon CoMMpass, a program started 8 years ago that now represents the largest genomic database of any type of cancer. Although CoMMpass includes data from about 1,150 patients with myeloma, it’s not enough information, according to the chief marketing and development officer at the MMRF, Anne Quinn Young.
“For a disease as heterogenous as myeloma is, we need more, particularly because we don’t have all the samples for later-stage disease,” Ms. Quinn Young said in an interview. “And even with the clinical data, given the patient population, both [in terms of] demographics and the nature of the disease, the numbers of patients still living after multiple relapses is rather small.”
In an earlier effort to gather more data, the MMRF first turned to other organizations for help, but this approach fell short because of scarcity of data, and in some cases, unwillingness to share. Steven Labkoff, MD, chief data officer at the MMRF, described this experience in an interview.
“When the MMRF was looking around for different data sources for myeloma data, it was always the claim that, ‘Sure, we have plenty of patients, we have plenty of data, and it’s rich and really complete.’ However, as we approached an array of organizations – big organizations – as we dug into the details and reviewed patient counts or data completeness, they either didn’t have a sufficient number of patients, they didn’t have sufficiently complete data for our needs, and in the case where some did have sufficient numbers and complete data sets, they simply weren’t in a position to share that data outside their institution,” Dr. Labkoff said.
Undeterred, the MMRF switched tactics to the current, patient-centric approach.
“We’re leveraging one aspect of the HIPAA legislation,” Dr. Labkoff said, referring to patients’ rights to request their own medical records and an institution’s legal obligation to provide those records.
In the short-term, the registry will collect three types of data: patient donated data (answers from a patient survey), electronic medical records abstracted from all relevant past providers, and genomic test results. Participating patients will have blood drawn at home by a phlebotomist for the genomic assay. Additional tubes of blood will be concurrently collected and biobanked. This will eventually allow for immune profiling, Dr. Labkoff said.
Future goals include a patient-reported outcomes module and the ability to link data with medical claims.
So far, 79 patients have participated in the pilot program, according to the MMRF. As the database builds, Ms. Quinn Young and Dr. Labkoff anticipate that it will yield answers to a variety of real-world questions.
Dr. Labkoff offered two examples. “Of all the patients who have been exposed to ‘name your drug,’ what were the costs of their therapy, and what were the outcomes?” he said. In addition, researchers will be able to query clinical trial inclusion criteria to search for data on a specific patient profile, such as patients with a 4:14 translocation, who have had a bone marrow transplant in the last 2 years, and have been exposed to a certain drug regimen.
Ms. Quinn Young noted that doctors may be able to use the database to reliably identify high-risk patients and guide agent selection. Common patient questions also will be addressed, she said, including best treatment regimens for certain types of patients.
“For patients who may have run out of all commercially available options, or for patients who are perhaps seen at a community center, where certainly this type of profiling is not standard, it’s opening up a whole new set of options for them,” Ms. Quinn Young said. “And if their physician doesn’t pursue those options, they have the report that they can use to seek a second opinion.”
The Direct-to-Patient registry is unique because it aims to empower patients in a way that hasn’t been done before, Ms. Quinn Young said. “We are committed ... ever since we conceived of this project, to giving results back to patients. That is disruptive because right now that doesn’t exist.”
But the cost of implementing the registry, which has an approximate budget of $20 million, stands in the way of a completely free flow of anonymized data. MMRF leaders are exploring different strategies to sustain funding for the program.
Another MMRF program, CoMMpass, uses a precompetitive consortium model, in which several pharmaceutical companies pay for a preview of the data 6 months in advance of nonprofit researchers. A similar model may be used with the Direct-to-Patient registry, but this has yet to be determined, according to Dr. Labkoff and Ms. Quinn Young.
For now, Ms. Quinn Young said she hopes that physicians will be receptive to the program. “[The short term goal is that] when patients come to their doctors asking about this, that there is support and open-mindedness,” she said.
Looking to the future, Dr. Labkoff described how the registry could accelerate myeloma research, ultimately toward a cure.
“It is generally accepted that it can take 17 years to get something – a therapy, a new drug, or a guideline – from the bench to the bedside,” he said. “It’s my hope that we can take next generation sequencing and the results of this registry and bend that curve, maybe ... to 10 [years], or very aggressively, to 7 or 5 [years], where doctors are able to use the information in these reports for the patients that have literally given themselves, and use this to help guide the choices of their therapy or the trials they apply for, to help them get a better outcome in general.”
The Direct-to-Patient registry is a collaborative effort between the MMRF and multiple organizations, including the health care technology company COTA, the Broad Institute of Harvard and MIT, Prometheus Research, Tempus, and the Dana Farber Cancer Institute.
Armored CAR T cells elicit responses in NHL patients
NATIONAL HARBOR, MD – An armored chimeric antigen receptor (CAR) T-cell therapy has demonstrated efficacy in vitro and in patients with relapsed or refractory non-Hodgkin lymphoma (NHL), according to findings presented at the annual meeting of the Society for Immunotherapy of Cancer.
ICTCAR014, a dominant negative PD-1 armored CAR T-cell therapy, proved more cytotoxic than traditional CAR T-cell therapy in vitro and produced responses in 12 of 13 NHL patients who received it.
Xiaobin Victor Lu, PhD, of Innovative Cellular Therapeutics, Shanghai, China, presented results with ICTCAR014 at the meeting.
Dr. Lu explained that ICTCAR014 consists of CD19-targeted CAR T cells genetically engineered to overexpress a PD-1 dominant negative protein with an altered intracellular signaling domain. The dominant negative protein can act as a “decoy receptor” to bind and block the PD-L1/2 inhibitory signal, thereby enhancing the efficacy of CAR T cells.
Innovative Cellular Therapeutics is developing ICTCAR014 because there is “some room to improve” with commercially available CAR T-cell products, Dr. Lu said. Specifically, tisagenlecleucel produced a 52% response rate in the JULIET trial (N Engl J Med. 2019;380:45-56), and axicabtagene ciloleucel produced an 82% response rate in the ZUMA-1 trial (N Engl J Med. 2017;377:2531-44).
There is also evidence to suggest that PD-1 blockade can modulate and “refuel” CAR T cells in relapsed/refractory NHL patients who fail or relapse after traditional anti-CD19 CAR T-cell therapy (Blood. 2017 Feb 23;129[8]:1039-41). This finding has prompted researchers to conduct trials of PD-1 inhibitors in combination with CAR T-cell therapies. But this combination approach may be expensive and cause more side effects than the armored CAR T-cell approach, Dr. Lu said.
In preclinical studies, Dr. Lu and colleagues found that ICTCAR014 was more effective than traditional anti-CD19 CAR T cells in killing Nalm6-PDL1 cells. In addition, the PD-1 dominant negative protein protected CAR T cells from exhaustion.
Dr. Lu also presented results in 13 NHL patients who have received ICTCAR014 in a phase 1 trial in China. Eleven patients had diffuse large B-cell lymphoma (DLBCL), and two had follicular lymphoma.
The objective response rate was 92.3% (12/13), which included five partial responses (38.5%) and seven complete responses (53.8%). Both follicular lymphoma patients and five DLBCL patients achieved a complete response. Five DLBCL patients achieved a partial response, and the remaining DLBCL patient did not respond.
Dr. Lu did not present safety data. However, he reported that there was no increased incidence of cytokine release syndrome or neurotoxicity in these patients, compared with patients receiving traditional CAR T-cell therapy.
Dr. Lu is employed by Innovative Cellular Therapeutics, which funded the research and is developing ICTCAR014.
SOURCE: Lu V et al. SITC 2019, Abstract O25.
NATIONAL HARBOR, MD – An armored chimeric antigen receptor (CAR) T-cell therapy has demonstrated efficacy in vitro and in patients with relapsed or refractory non-Hodgkin lymphoma (NHL), according to findings presented at the annual meeting of the Society for Immunotherapy of Cancer.
ICTCAR014, a dominant negative PD-1 armored CAR T-cell therapy, proved more cytotoxic than traditional CAR T-cell therapy in vitro and produced responses in 12 of 13 NHL patients who received it.
Xiaobin Victor Lu, PhD, of Innovative Cellular Therapeutics, Shanghai, China, presented results with ICTCAR014 at the meeting.
Dr. Lu explained that ICTCAR014 consists of CD19-targeted CAR T cells genetically engineered to overexpress a PD-1 dominant negative protein with an altered intracellular signaling domain. The dominant negative protein can act as a “decoy receptor” to bind and block the PD-L1/2 inhibitory signal, thereby enhancing the efficacy of CAR T cells.
Innovative Cellular Therapeutics is developing ICTCAR014 because there is “some room to improve” with commercially available CAR T-cell products, Dr. Lu said. Specifically, tisagenlecleucel produced a 52% response rate in the JULIET trial (N Engl J Med. 2019;380:45-56), and axicabtagene ciloleucel produced an 82% response rate in the ZUMA-1 trial (N Engl J Med. 2017;377:2531-44).
There is also evidence to suggest that PD-1 blockade can modulate and “refuel” CAR T cells in relapsed/refractory NHL patients who fail or relapse after traditional anti-CD19 CAR T-cell therapy (Blood. 2017 Feb 23;129[8]:1039-41). This finding has prompted researchers to conduct trials of PD-1 inhibitors in combination with CAR T-cell therapies. But this combination approach may be expensive and cause more side effects than the armored CAR T-cell approach, Dr. Lu said.
In preclinical studies, Dr. Lu and colleagues found that ICTCAR014 was more effective than traditional anti-CD19 CAR T cells in killing Nalm6-PDL1 cells. In addition, the PD-1 dominant negative protein protected CAR T cells from exhaustion.
Dr. Lu also presented results in 13 NHL patients who have received ICTCAR014 in a phase 1 trial in China. Eleven patients had diffuse large B-cell lymphoma (DLBCL), and two had follicular lymphoma.
The objective response rate was 92.3% (12/13), which included five partial responses (38.5%) and seven complete responses (53.8%). Both follicular lymphoma patients and five DLBCL patients achieved a complete response. Five DLBCL patients achieved a partial response, and the remaining DLBCL patient did not respond.
Dr. Lu did not present safety data. However, he reported that there was no increased incidence of cytokine release syndrome or neurotoxicity in these patients, compared with patients receiving traditional CAR T-cell therapy.
Dr. Lu is employed by Innovative Cellular Therapeutics, which funded the research and is developing ICTCAR014.
SOURCE: Lu V et al. SITC 2019, Abstract O25.
NATIONAL HARBOR, MD – An armored chimeric antigen receptor (CAR) T-cell therapy has demonstrated efficacy in vitro and in patients with relapsed or refractory non-Hodgkin lymphoma (NHL), according to findings presented at the annual meeting of the Society for Immunotherapy of Cancer.
ICTCAR014, a dominant negative PD-1 armored CAR T-cell therapy, proved more cytotoxic than traditional CAR T-cell therapy in vitro and produced responses in 12 of 13 NHL patients who received it.
Xiaobin Victor Lu, PhD, of Innovative Cellular Therapeutics, Shanghai, China, presented results with ICTCAR014 at the meeting.
Dr. Lu explained that ICTCAR014 consists of CD19-targeted CAR T cells genetically engineered to overexpress a PD-1 dominant negative protein with an altered intracellular signaling domain. The dominant negative protein can act as a “decoy receptor” to bind and block the PD-L1/2 inhibitory signal, thereby enhancing the efficacy of CAR T cells.
Innovative Cellular Therapeutics is developing ICTCAR014 because there is “some room to improve” with commercially available CAR T-cell products, Dr. Lu said. Specifically, tisagenlecleucel produced a 52% response rate in the JULIET trial (N Engl J Med. 2019;380:45-56), and axicabtagene ciloleucel produced an 82% response rate in the ZUMA-1 trial (N Engl J Med. 2017;377:2531-44).
There is also evidence to suggest that PD-1 blockade can modulate and “refuel” CAR T cells in relapsed/refractory NHL patients who fail or relapse after traditional anti-CD19 CAR T-cell therapy (Blood. 2017 Feb 23;129[8]:1039-41). This finding has prompted researchers to conduct trials of PD-1 inhibitors in combination with CAR T-cell therapies. But this combination approach may be expensive and cause more side effects than the armored CAR T-cell approach, Dr. Lu said.
In preclinical studies, Dr. Lu and colleagues found that ICTCAR014 was more effective than traditional anti-CD19 CAR T cells in killing Nalm6-PDL1 cells. In addition, the PD-1 dominant negative protein protected CAR T cells from exhaustion.
Dr. Lu also presented results in 13 NHL patients who have received ICTCAR014 in a phase 1 trial in China. Eleven patients had diffuse large B-cell lymphoma (DLBCL), and two had follicular lymphoma.
The objective response rate was 92.3% (12/13), which included five partial responses (38.5%) and seven complete responses (53.8%). Both follicular lymphoma patients and five DLBCL patients achieved a complete response. Five DLBCL patients achieved a partial response, and the remaining DLBCL patient did not respond.
Dr. Lu did not present safety data. However, he reported that there was no increased incidence of cytokine release syndrome or neurotoxicity in these patients, compared with patients receiving traditional CAR T-cell therapy.
Dr. Lu is employed by Innovative Cellular Therapeutics, which funded the research and is developing ICTCAR014.
SOURCE: Lu V et al. SITC 2019, Abstract O25.
REPORTING FROM SITC 2019
FDA approves anemia treatment for transfusion-dependent beta thalassemia patients
The Food and Drug Administration has approved the first treatment for anemia in adults with transfusion-dependent beta thalassemia.
Luspatercept-aamt (Reblozyl) is an erythroid maturation agent that reduced the transfusion burden for patients with beta thalassemia in the BELIEVE trial of 336 patients. In total, 21% of patients who received luspatercept-aamt achieved at least a 33% reduction in red blood cell transfusions, compared with 4.5% of patients who received placebo, according to the FDA.
Common side effects associated with luspatercept-aamt were headache, bone pain, arthralgia, fatigue, cough, abdominal pain, diarrhea, and dizziness. Patients taking the agent should be monitored for thrombosis, the FDA advised.
Celgene, which makes luspatercept-aamt, said the agent would be available about 1 week following the FDA approval.
The FDA is also evaluating luspatercept-aamt as an anemia treatment in adults with very-low– to intermediate-risk myelodysplastic syndromes who have ring sideroblasts and require red blood cell transfusions. The agency is expected to take action on that application in April 2020.
The Food and Drug Administration has approved the first treatment for anemia in adults with transfusion-dependent beta thalassemia.
Luspatercept-aamt (Reblozyl) is an erythroid maturation agent that reduced the transfusion burden for patients with beta thalassemia in the BELIEVE trial of 336 patients. In total, 21% of patients who received luspatercept-aamt achieved at least a 33% reduction in red blood cell transfusions, compared with 4.5% of patients who received placebo, according to the FDA.
Common side effects associated with luspatercept-aamt were headache, bone pain, arthralgia, fatigue, cough, abdominal pain, diarrhea, and dizziness. Patients taking the agent should be monitored for thrombosis, the FDA advised.
Celgene, which makes luspatercept-aamt, said the agent would be available about 1 week following the FDA approval.
The FDA is also evaluating luspatercept-aamt as an anemia treatment in adults with very-low– to intermediate-risk myelodysplastic syndromes who have ring sideroblasts and require red blood cell transfusions. The agency is expected to take action on that application in April 2020.
The Food and Drug Administration has approved the first treatment for anemia in adults with transfusion-dependent beta thalassemia.
Luspatercept-aamt (Reblozyl) is an erythroid maturation agent that reduced the transfusion burden for patients with beta thalassemia in the BELIEVE trial of 336 patients. In total, 21% of patients who received luspatercept-aamt achieved at least a 33% reduction in red blood cell transfusions, compared with 4.5% of patients who received placebo, according to the FDA.
Common side effects associated with luspatercept-aamt were headache, bone pain, arthralgia, fatigue, cough, abdominal pain, diarrhea, and dizziness. Patients taking the agent should be monitored for thrombosis, the FDA advised.
Celgene, which makes luspatercept-aamt, said the agent would be available about 1 week following the FDA approval.
The FDA is also evaluating luspatercept-aamt as an anemia treatment in adults with very-low– to intermediate-risk myelodysplastic syndromes who have ring sideroblasts and require red blood cell transfusions. The agency is expected to take action on that application in April 2020.
Survival ‘excellent’ after rituximab-bendamustine induction in transplant-eligible MCL
The combination of rituximab and bendamustine (RB) provided “excellent” survival with less toxicity, compared with a cytarabine-based induction regimen, in transplant-eligible patients with mantle cell lymphoma, according to a long-term follow-up report from randomized phase 2 trial.
The 5-year survival rates for RB were “provocatively similar” to what was achieved with the standard, intensive R-hyperCVAD regimen, investigators said in this update on the Southwest Oncology Group (SWOG) S1106 study.
By contrast, the R-hyperCVAD regimen was associated with more toxicity and higher failure rates for stem cell mobilization, according to the report’s lead author, Manali Kamdar, MD, of the University of Colorado, Denver, and coauthors.
“Overall, S1106 demonstrated that an outpatient-based, less intensive induction therapy of bendamustine plus rituximab is highly effective, safe, and durable in untreated transplant-eligible MCL patients,” Dr. Kamdar and her colleagues reported in Blood Advances.
The results have guided the design of an upcoming study, EA4181, in which patients with mantle cell lymphoma will be treated with an RB backbone plus cytarabine, the BTK inhibitor acalabrutinib, or both, according to the authors.
In the present study, S1106, patients with mantle cell lymphoma were randomized to receive RB or the R-hyperCVAD regimen, which consisted of rituximab with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone, alternating with high-dose cytarabine and methotrexate. Both regimens were followed by autologous hematopoietic stem cell transplant.
The stem cell mobilization failure rate was 29% in the R-hyperCVAD arm in an interim analysis conducted after 53 of a planned 160 patients had been enrolled, including 35 in the RB arm and 17 in the R-hyperCVAD arm, according to a report published in the British Journal of Haematology (2016 Dec 19. doi: 10.1111/bjh.14480). That analysis triggered a shutdown of the study, based on a rule stating that either arm would be deemed “unacceptably toxic” if the mobilization rate exceeded 10%.
Accordingly, R-hyperCVAD is “not an ideal platform” for future trials, the investigators said. At that time, the estimated 2-year progression-free survival (PFS) was 81% versus 82% for RB and R-hyperCVAD, respectively, while overall survival (OS) was 87% versus 88%.
With additional follow-up, the 5-year PFS is 66% and 62% in the RB and R-hyperCVAD arms, respectively, while 5-year OS is 80% and 74%, according to the investigators.
The RB regimen also results in “excellent” minimal residual disease (MRD) negativity, they added.
MRD status was evaluated in 12 paired pre- and postinduction therapy specimens, of which 2 pairs were from patients in the R-hyperCVAD arm, and 10 pairs were from patients in the RB arm.
In the R-hyperCVAD arm, both patients were MRD positive at baseline, and MRD negative after induction, according to the investigators. Similarly, 9 of 10 patients in the RB arm were MRD positive at baseline, and of those, 7 converted to MRD negative following induction.
The research was supported by the National Cancer Institute, and in part by Sequenta (Adaptive Biotechnologies). Dr. Kamdar reported being on the speakers bureau of Seattle Genetics and receiving consultancy fees from AstraZeneca, Celgene, and Genentech. Co-authors of the study provided disclosures related to Millennium Pharmaceuticals, Affimed, Seattle Genetics, Pharmacyclics, and Merck, among others.
SOURCE: Kamdar M et al. Blood Adv. 2019 Oct 22;3(20):3132-5.
The combination of rituximab and bendamustine (RB) provided “excellent” survival with less toxicity, compared with a cytarabine-based induction regimen, in transplant-eligible patients with mantle cell lymphoma, according to a long-term follow-up report from randomized phase 2 trial.
The 5-year survival rates for RB were “provocatively similar” to what was achieved with the standard, intensive R-hyperCVAD regimen, investigators said in this update on the Southwest Oncology Group (SWOG) S1106 study.
By contrast, the R-hyperCVAD regimen was associated with more toxicity and higher failure rates for stem cell mobilization, according to the report’s lead author, Manali Kamdar, MD, of the University of Colorado, Denver, and coauthors.
“Overall, S1106 demonstrated that an outpatient-based, less intensive induction therapy of bendamustine plus rituximab is highly effective, safe, and durable in untreated transplant-eligible MCL patients,” Dr. Kamdar and her colleagues reported in Blood Advances.
The results have guided the design of an upcoming study, EA4181, in which patients with mantle cell lymphoma will be treated with an RB backbone plus cytarabine, the BTK inhibitor acalabrutinib, or both, according to the authors.
In the present study, S1106, patients with mantle cell lymphoma were randomized to receive RB or the R-hyperCVAD regimen, which consisted of rituximab with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone, alternating with high-dose cytarabine and methotrexate. Both regimens were followed by autologous hematopoietic stem cell transplant.
The stem cell mobilization failure rate was 29% in the R-hyperCVAD arm in an interim analysis conducted after 53 of a planned 160 patients had been enrolled, including 35 in the RB arm and 17 in the R-hyperCVAD arm, according to a report published in the British Journal of Haematology (2016 Dec 19. doi: 10.1111/bjh.14480). That analysis triggered a shutdown of the study, based on a rule stating that either arm would be deemed “unacceptably toxic” if the mobilization rate exceeded 10%.
Accordingly, R-hyperCVAD is “not an ideal platform” for future trials, the investigators said. At that time, the estimated 2-year progression-free survival (PFS) was 81% versus 82% for RB and R-hyperCVAD, respectively, while overall survival (OS) was 87% versus 88%.
With additional follow-up, the 5-year PFS is 66% and 62% in the RB and R-hyperCVAD arms, respectively, while 5-year OS is 80% and 74%, according to the investigators.
The RB regimen also results in “excellent” minimal residual disease (MRD) negativity, they added.
MRD status was evaluated in 12 paired pre- and postinduction therapy specimens, of which 2 pairs were from patients in the R-hyperCVAD arm, and 10 pairs were from patients in the RB arm.
In the R-hyperCVAD arm, both patients were MRD positive at baseline, and MRD negative after induction, according to the investigators. Similarly, 9 of 10 patients in the RB arm were MRD positive at baseline, and of those, 7 converted to MRD negative following induction.
The research was supported by the National Cancer Institute, and in part by Sequenta (Adaptive Biotechnologies). Dr. Kamdar reported being on the speakers bureau of Seattle Genetics and receiving consultancy fees from AstraZeneca, Celgene, and Genentech. Co-authors of the study provided disclosures related to Millennium Pharmaceuticals, Affimed, Seattle Genetics, Pharmacyclics, and Merck, among others.
SOURCE: Kamdar M et al. Blood Adv. 2019 Oct 22;3(20):3132-5.
The combination of rituximab and bendamustine (RB) provided “excellent” survival with less toxicity, compared with a cytarabine-based induction regimen, in transplant-eligible patients with mantle cell lymphoma, according to a long-term follow-up report from randomized phase 2 trial.
The 5-year survival rates for RB were “provocatively similar” to what was achieved with the standard, intensive R-hyperCVAD regimen, investigators said in this update on the Southwest Oncology Group (SWOG) S1106 study.
By contrast, the R-hyperCVAD regimen was associated with more toxicity and higher failure rates for stem cell mobilization, according to the report’s lead author, Manali Kamdar, MD, of the University of Colorado, Denver, and coauthors.
“Overall, S1106 demonstrated that an outpatient-based, less intensive induction therapy of bendamustine plus rituximab is highly effective, safe, and durable in untreated transplant-eligible MCL patients,” Dr. Kamdar and her colleagues reported in Blood Advances.
The results have guided the design of an upcoming study, EA4181, in which patients with mantle cell lymphoma will be treated with an RB backbone plus cytarabine, the BTK inhibitor acalabrutinib, or both, according to the authors.
In the present study, S1106, patients with mantle cell lymphoma were randomized to receive RB or the R-hyperCVAD regimen, which consisted of rituximab with hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone, alternating with high-dose cytarabine and methotrexate. Both regimens were followed by autologous hematopoietic stem cell transplant.
The stem cell mobilization failure rate was 29% in the R-hyperCVAD arm in an interim analysis conducted after 53 of a planned 160 patients had been enrolled, including 35 in the RB arm and 17 in the R-hyperCVAD arm, according to a report published in the British Journal of Haematology (2016 Dec 19. doi: 10.1111/bjh.14480). That analysis triggered a shutdown of the study, based on a rule stating that either arm would be deemed “unacceptably toxic” if the mobilization rate exceeded 10%.
Accordingly, R-hyperCVAD is “not an ideal platform” for future trials, the investigators said. At that time, the estimated 2-year progression-free survival (PFS) was 81% versus 82% for RB and R-hyperCVAD, respectively, while overall survival (OS) was 87% versus 88%.
With additional follow-up, the 5-year PFS is 66% and 62% in the RB and R-hyperCVAD arms, respectively, while 5-year OS is 80% and 74%, according to the investigators.
The RB regimen also results in “excellent” minimal residual disease (MRD) negativity, they added.
MRD status was evaluated in 12 paired pre- and postinduction therapy specimens, of which 2 pairs were from patients in the R-hyperCVAD arm, and 10 pairs were from patients in the RB arm.
In the R-hyperCVAD arm, both patients were MRD positive at baseline, and MRD negative after induction, according to the investigators. Similarly, 9 of 10 patients in the RB arm were MRD positive at baseline, and of those, 7 converted to MRD negative following induction.
The research was supported by the National Cancer Institute, and in part by Sequenta (Adaptive Biotechnologies). Dr. Kamdar reported being on the speakers bureau of Seattle Genetics and receiving consultancy fees from AstraZeneca, Celgene, and Genentech. Co-authors of the study provided disclosures related to Millennium Pharmaceuticals, Affimed, Seattle Genetics, Pharmacyclics, and Merck, among others.
SOURCE: Kamdar M et al. Blood Adv. 2019 Oct 22;3(20):3132-5.
FROM BLOOD ADVANCES
Best practice alerts really can work
SAN ANTONIO – Clinicians don’t appear to mind too much when their red blood cell orders are flagged for review by a best practice alert system, and alert fatigue doesn’t seem to hamper patient blood management efforts, investigators in a single-center study reported.
At the Medical University of South Carolina, Charleston (MUSC), if clinicians order RBC transfusions for patients with hemoglobin levels over 7.0 g/dL or for patients who did not have a hemoglobin determination over the past 24 hours, they receive a best practice alert. They must acknowledge it and cancel the order, or override it and document a reason in the medical record.
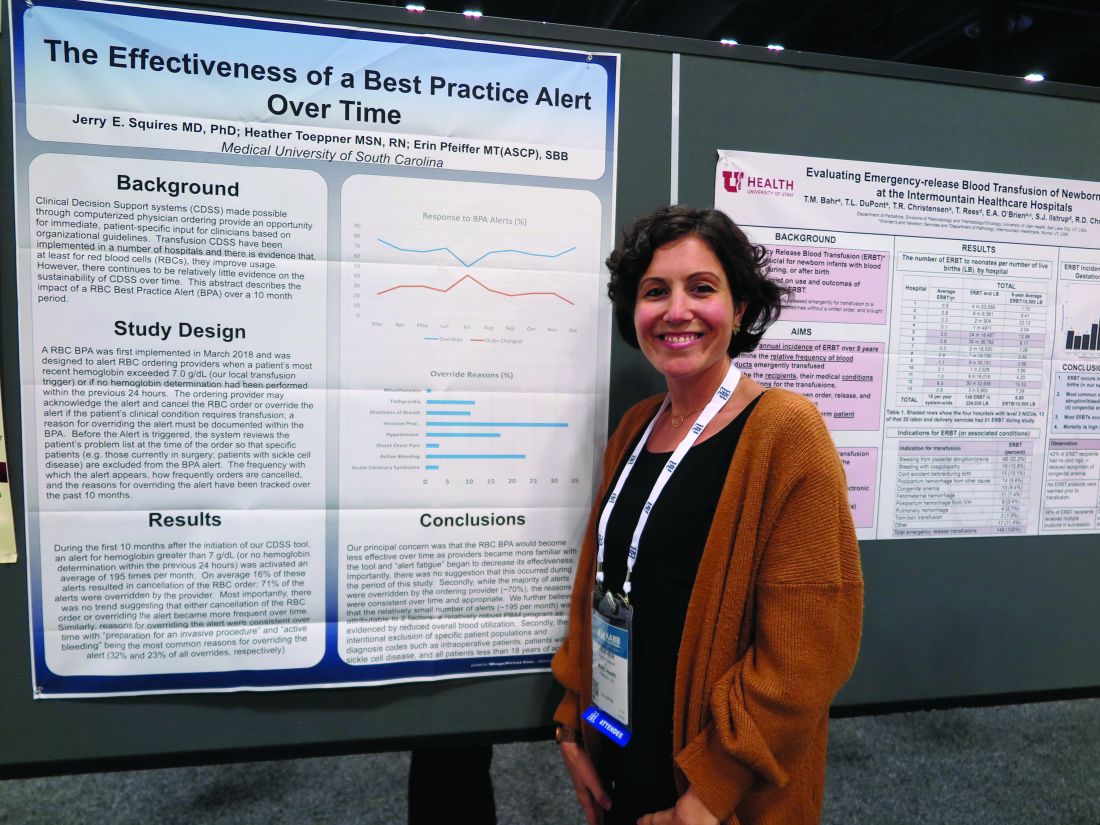
Although approximately 70% of alerts were overridden, the reasons for the overrides “were consistent over time and appropriate,” reported Jerry E. Squires, MD, PhD, and colleagues from MUSC in a poster presentation at the annual meeting of AABB, the group formerly known as the American Association of Blood Banks.
The goal of the study was to find out if the effectiveness of the alert was wearing out after months of active use by clinicians. “Is it true that they’re clicking too much and they’re inundated with other [best practice alerts], and are they even paying attention?” said coauthor Heather Toeppner, RN, also from MUSC, in an interview. “All in all, we found that the alert is making a lasting impression in our institution,” she said.
Transfusion clinical decision support systems that produce automated alerts for clinicians can improve usage and reduce waste of RBCs, but whether the effect is sustained over time was unknown, Ms. Toeppner said, prompting the investigators to study the effect of the RBC best practice alert over 10 months.
As noted, the alert is triggered when providers order RBCs for patients with hemoglobin levels over 7.0 g/dL or when there is no record of a hemoglobin test in the chart within the past 24 hours. Before the alert is triggered, however, the system reviews the record and excludes alerts for patients with specific conditions, such as concurrent surgery or sickle cell disease.
The authors found that the alert was triggered an average of 195 times per month over the 10 months studied. On average, 16% of the alerts resulted in a cancellation of the RBC order, and 71% of alerts were overridden.
“Most importantly, there was no trend suggesting that either cancellation of the RBC order or overriding the alert became more frequent over time,” the investigators wrote. “Similarly, reasons for overriding the alert were consistent over time, with ‘preparation for an invasive procedure’ and ‘active bleeding’ being the most common reasons for overriding the alert (32% and 23% of all overrides, respectively).”
Other common reasons for overrides included tachycardia, shortness of breath, hypotension, onset of chest pain, and acute coronary syndrome.
Interestingly, but perhaps not surprisingly, they found that overrides dropped sharply and changed orders rose by the same magnitude in July, when new residents started their rotations.
The investigators wrote that the relatively small number of alerts may be attributable to their institution’s robust patient blood management program and the intentional exclusion of orders for patients with specific diagnostic codes, including intraoperative patients, those with sickle cell disease, and all patients aged younger than 18 years.
The study was internally funded. The authors reported having no conflicts of interest.
SAN ANTONIO – Clinicians don’t appear to mind too much when their red blood cell orders are flagged for review by a best practice alert system, and alert fatigue doesn’t seem to hamper patient blood management efforts, investigators in a single-center study reported.
At the Medical University of South Carolina, Charleston (MUSC), if clinicians order RBC transfusions for patients with hemoglobin levels over 7.0 g/dL or for patients who did not have a hemoglobin determination over the past 24 hours, they receive a best practice alert. They must acknowledge it and cancel the order, or override it and document a reason in the medical record.
Although approximately 70% of alerts were overridden, the reasons for the overrides “were consistent over time and appropriate,” reported Jerry E. Squires, MD, PhD, and colleagues from MUSC in a poster presentation at the annual meeting of AABB, the group formerly known as the American Association of Blood Banks.
The goal of the study was to find out if the effectiveness of the alert was wearing out after months of active use by clinicians. “Is it true that they’re clicking too much and they’re inundated with other [best practice alerts], and are they even paying attention?” said coauthor Heather Toeppner, RN, also from MUSC, in an interview. “All in all, we found that the alert is making a lasting impression in our institution,” she said.
Transfusion clinical decision support systems that produce automated alerts for clinicians can improve usage and reduce waste of RBCs, but whether the effect is sustained over time was unknown, Ms. Toeppner said, prompting the investigators to study the effect of the RBC best practice alert over 10 months.
As noted, the alert is triggered when providers order RBCs for patients with hemoglobin levels over 7.0 g/dL or when there is no record of a hemoglobin test in the chart within the past 24 hours. Before the alert is triggered, however, the system reviews the record and excludes alerts for patients with specific conditions, such as concurrent surgery or sickle cell disease.
The authors found that the alert was triggered an average of 195 times per month over the 10 months studied. On average, 16% of the alerts resulted in a cancellation of the RBC order, and 71% of alerts were overridden.
“Most importantly, there was no trend suggesting that either cancellation of the RBC order or overriding the alert became more frequent over time,” the investigators wrote. “Similarly, reasons for overriding the alert were consistent over time, with ‘preparation for an invasive procedure’ and ‘active bleeding’ being the most common reasons for overriding the alert (32% and 23% of all overrides, respectively).”
Other common reasons for overrides included tachycardia, shortness of breath, hypotension, onset of chest pain, and acute coronary syndrome.
Interestingly, but perhaps not surprisingly, they found that overrides dropped sharply and changed orders rose by the same magnitude in July, when new residents started their rotations.
The investigators wrote that the relatively small number of alerts may be attributable to their institution’s robust patient blood management program and the intentional exclusion of orders for patients with specific diagnostic codes, including intraoperative patients, those with sickle cell disease, and all patients aged younger than 18 years.
The study was internally funded. The authors reported having no conflicts of interest.
SAN ANTONIO – Clinicians don’t appear to mind too much when their red blood cell orders are flagged for review by a best practice alert system, and alert fatigue doesn’t seem to hamper patient blood management efforts, investigators in a single-center study reported.
At the Medical University of South Carolina, Charleston (MUSC), if clinicians order RBC transfusions for patients with hemoglobin levels over 7.0 g/dL or for patients who did not have a hemoglobin determination over the past 24 hours, they receive a best practice alert. They must acknowledge it and cancel the order, or override it and document a reason in the medical record.
Although approximately 70% of alerts were overridden, the reasons for the overrides “were consistent over time and appropriate,” reported Jerry E. Squires, MD, PhD, and colleagues from MUSC in a poster presentation at the annual meeting of AABB, the group formerly known as the American Association of Blood Banks.
The goal of the study was to find out if the effectiveness of the alert was wearing out after months of active use by clinicians. “Is it true that they’re clicking too much and they’re inundated with other [best practice alerts], and are they even paying attention?” said coauthor Heather Toeppner, RN, also from MUSC, in an interview. “All in all, we found that the alert is making a lasting impression in our institution,” she said.
Transfusion clinical decision support systems that produce automated alerts for clinicians can improve usage and reduce waste of RBCs, but whether the effect is sustained over time was unknown, Ms. Toeppner said, prompting the investigators to study the effect of the RBC best practice alert over 10 months.
As noted, the alert is triggered when providers order RBCs for patients with hemoglobin levels over 7.0 g/dL or when there is no record of a hemoglobin test in the chart within the past 24 hours. Before the alert is triggered, however, the system reviews the record and excludes alerts for patients with specific conditions, such as concurrent surgery or sickle cell disease.
The authors found that the alert was triggered an average of 195 times per month over the 10 months studied. On average, 16% of the alerts resulted in a cancellation of the RBC order, and 71% of alerts were overridden.
“Most importantly, there was no trend suggesting that either cancellation of the RBC order or overriding the alert became more frequent over time,” the investigators wrote. “Similarly, reasons for overriding the alert were consistent over time, with ‘preparation for an invasive procedure’ and ‘active bleeding’ being the most common reasons for overriding the alert (32% and 23% of all overrides, respectively).”
Other common reasons for overrides included tachycardia, shortness of breath, hypotension, onset of chest pain, and acute coronary syndrome.
Interestingly, but perhaps not surprisingly, they found that overrides dropped sharply and changed orders rose by the same magnitude in July, when new residents started their rotations.
The investigators wrote that the relatively small number of alerts may be attributable to their institution’s robust patient blood management program and the intentional exclusion of orders for patients with specific diagnostic codes, including intraoperative patients, those with sickle cell disease, and all patients aged younger than 18 years.
The study was internally funded. The authors reported having no conflicts of interest.
REPORTING FROM AABB 2019
First NCCN guideline on hematopoietic cell transplantation focuses on GVHD
Recommendations for the diagnosis and management of acute and chronic graft-versus-host disease (GVHD) are the central focus of the first National Comprehensive Cancer Network (NCCN) guideline on hematopoietic cell transplantation.
The guideline presents detailed recommendations for the evaluation of hematopoietic cell transplant (HCT) recipients, and an extensive section on the diagnosis and workup of GVHD, including information on staging and grading of acute GVHD, grading of chronic GVHD, treatment response criteria, and suggested systemic therapies for steroid-refractory disease.
“We wanted to both build on the commonly used approach to stage and treat graft-versus-host disease, and make sure that this information is readily available for physicians-in-training and young physicians who are learning about transplants,” said guideline committee chair Ayman Saad, MB BCh, of The Ohio State University Comprehensive Cancer Center, James Cancer Hospital and Solove Research Institute in Columbus, Ohio.
In an interview, Dr. Saad emphasized that an important goal of the guidelines is to encourage general oncologists to recognize early signs of GVHD and refer potential candidates to transplant centers for further evaluation.
“We also urge oncologists who may be caring for patients after HCT to familiarize themselves with the varied manifestations of GVHD – a very common and significant posttransplant complication – and to consult with transplant providers to optimize their ongoing care. The guidelines explain how to diagnose and treat this condition in order to achieve the best possible outcomes,” guideline panel member Alison W. Loren, MD, director of blood and marrow transplantation at Abraham Cancer Center, University of Pennsylvania, Philadelphia, said in a statement.
The guideline includes links to other NCCN guidelines for diseases where HCT is a common therapeutic option, including leukemias, myeloid malignancies, lymphomas, central nervous system cancers, and testicular cancer.
The HCT guideline includes:
- Pretransplant recipient evaluation with recommendations for clinical assessment and imaging.
- Diagnosis and workup of GVHD, with separate algorithms for suspected acute or chronic GVHD.
- Specific interventions for management of acute GVHD with corticosteroids or other systemic agents.
- Chronic GVHD diagnosis by organ site and symptoms, with a severity scoring system.
- Chronic GVHD steroid response definitions and criteria.
- Suggested systemic agents for steroid-refractory GVHD.
One feature that is unusual for an NCCN guideline document is a page of photographs to assist clinicians in diagnosing range-of-motion abnormalities in the shoulder, elbow, hand, and ankle of patients with suspected or confirmed GVHD. Dr. Saad said that future iterations of the guideline will include additional photos to help clinicians develop a visual repertoire of potential GVHD signs.
Future versions will also include a discussion section and more comprehensive information on other common complications following HCT transplant, as well as management of posttransplant relapse.
Ideally, the guideline will help clinicians document and justify clinical decisions surrounding HCT and GVHD management in discussions with third-party payers, Dr. Saad said.
“Sometimes we struggle with payers when we want to use a certain modality to treat GVHD, and they respond ‘that’s not approved,’ or ‘that’s not a common indication,’ et cetera,” he said. “What we’re trying to put here are the commonly used therapies that most, but not all, experts agree on, and we can use this to negotiate with payers.”
He also emphasized that the guideline is meant to be instructive rather than prescriptive and is not meant to hinder innovations that may emerge from clinical trials.
“We would appreciate any feedback from non-NCCN centers as well as experts at NCCN centers, and we’ll be more than happy to address any concerns or criticisms they have,” he said.
Dr. Saad reported financial relationships with Actinium and Incysus Biomedical. Dr. Loren has previously reported having no disclosures.
Recommendations for the diagnosis and management of acute and chronic graft-versus-host disease (GVHD) are the central focus of the first National Comprehensive Cancer Network (NCCN) guideline on hematopoietic cell transplantation.
The guideline presents detailed recommendations for the evaluation of hematopoietic cell transplant (HCT) recipients, and an extensive section on the diagnosis and workup of GVHD, including information on staging and grading of acute GVHD, grading of chronic GVHD, treatment response criteria, and suggested systemic therapies for steroid-refractory disease.
“We wanted to both build on the commonly used approach to stage and treat graft-versus-host disease, and make sure that this information is readily available for physicians-in-training and young physicians who are learning about transplants,” said guideline committee chair Ayman Saad, MB BCh, of The Ohio State University Comprehensive Cancer Center, James Cancer Hospital and Solove Research Institute in Columbus, Ohio.
In an interview, Dr. Saad emphasized that an important goal of the guidelines is to encourage general oncologists to recognize early signs of GVHD and refer potential candidates to transplant centers for further evaluation.
“We also urge oncologists who may be caring for patients after HCT to familiarize themselves with the varied manifestations of GVHD – a very common and significant posttransplant complication – and to consult with transplant providers to optimize their ongoing care. The guidelines explain how to diagnose and treat this condition in order to achieve the best possible outcomes,” guideline panel member Alison W. Loren, MD, director of blood and marrow transplantation at Abraham Cancer Center, University of Pennsylvania, Philadelphia, said in a statement.
The guideline includes links to other NCCN guidelines for diseases where HCT is a common therapeutic option, including leukemias, myeloid malignancies, lymphomas, central nervous system cancers, and testicular cancer.
The HCT guideline includes:
- Pretransplant recipient evaluation with recommendations for clinical assessment and imaging.
- Diagnosis and workup of GVHD, with separate algorithms for suspected acute or chronic GVHD.
- Specific interventions for management of acute GVHD with corticosteroids or other systemic agents.
- Chronic GVHD diagnosis by organ site and symptoms, with a severity scoring system.
- Chronic GVHD steroid response definitions and criteria.
- Suggested systemic agents for steroid-refractory GVHD.
One feature that is unusual for an NCCN guideline document is a page of photographs to assist clinicians in diagnosing range-of-motion abnormalities in the shoulder, elbow, hand, and ankle of patients with suspected or confirmed GVHD. Dr. Saad said that future iterations of the guideline will include additional photos to help clinicians develop a visual repertoire of potential GVHD signs.
Future versions will also include a discussion section and more comprehensive information on other common complications following HCT transplant, as well as management of posttransplant relapse.
Ideally, the guideline will help clinicians document and justify clinical decisions surrounding HCT and GVHD management in discussions with third-party payers, Dr. Saad said.
“Sometimes we struggle with payers when we want to use a certain modality to treat GVHD, and they respond ‘that’s not approved,’ or ‘that’s not a common indication,’ et cetera,” he said. “What we’re trying to put here are the commonly used therapies that most, but not all, experts agree on, and we can use this to negotiate with payers.”
He also emphasized that the guideline is meant to be instructive rather than prescriptive and is not meant to hinder innovations that may emerge from clinical trials.
“We would appreciate any feedback from non-NCCN centers as well as experts at NCCN centers, and we’ll be more than happy to address any concerns or criticisms they have,” he said.
Dr. Saad reported financial relationships with Actinium and Incysus Biomedical. Dr. Loren has previously reported having no disclosures.
Recommendations for the diagnosis and management of acute and chronic graft-versus-host disease (GVHD) are the central focus of the first National Comprehensive Cancer Network (NCCN) guideline on hematopoietic cell transplantation.
The guideline presents detailed recommendations for the evaluation of hematopoietic cell transplant (HCT) recipients, and an extensive section on the diagnosis and workup of GVHD, including information on staging and grading of acute GVHD, grading of chronic GVHD, treatment response criteria, and suggested systemic therapies for steroid-refractory disease.
“We wanted to both build on the commonly used approach to stage and treat graft-versus-host disease, and make sure that this information is readily available for physicians-in-training and young physicians who are learning about transplants,” said guideline committee chair Ayman Saad, MB BCh, of The Ohio State University Comprehensive Cancer Center, James Cancer Hospital and Solove Research Institute in Columbus, Ohio.
In an interview, Dr. Saad emphasized that an important goal of the guidelines is to encourage general oncologists to recognize early signs of GVHD and refer potential candidates to transplant centers for further evaluation.
“We also urge oncologists who may be caring for patients after HCT to familiarize themselves with the varied manifestations of GVHD – a very common and significant posttransplant complication – and to consult with transplant providers to optimize their ongoing care. The guidelines explain how to diagnose and treat this condition in order to achieve the best possible outcomes,” guideline panel member Alison W. Loren, MD, director of blood and marrow transplantation at Abraham Cancer Center, University of Pennsylvania, Philadelphia, said in a statement.
The guideline includes links to other NCCN guidelines for diseases where HCT is a common therapeutic option, including leukemias, myeloid malignancies, lymphomas, central nervous system cancers, and testicular cancer.
The HCT guideline includes:
- Pretransplant recipient evaluation with recommendations for clinical assessment and imaging.
- Diagnosis and workup of GVHD, with separate algorithms for suspected acute or chronic GVHD.
- Specific interventions for management of acute GVHD with corticosteroids or other systemic agents.
- Chronic GVHD diagnosis by organ site and symptoms, with a severity scoring system.
- Chronic GVHD steroid response definitions and criteria.
- Suggested systemic agents for steroid-refractory GVHD.
One feature that is unusual for an NCCN guideline document is a page of photographs to assist clinicians in diagnosing range-of-motion abnormalities in the shoulder, elbow, hand, and ankle of patients with suspected or confirmed GVHD. Dr. Saad said that future iterations of the guideline will include additional photos to help clinicians develop a visual repertoire of potential GVHD signs.
Future versions will also include a discussion section and more comprehensive information on other common complications following HCT transplant, as well as management of posttransplant relapse.
Ideally, the guideline will help clinicians document and justify clinical decisions surrounding HCT and GVHD management in discussions with third-party payers, Dr. Saad said.
“Sometimes we struggle with payers when we want to use a certain modality to treat GVHD, and they respond ‘that’s not approved,’ or ‘that’s not a common indication,’ et cetera,” he said. “What we’re trying to put here are the commonly used therapies that most, but not all, experts agree on, and we can use this to negotiate with payers.”
He also emphasized that the guideline is meant to be instructive rather than prescriptive and is not meant to hinder innovations that may emerge from clinical trials.
“We would appreciate any feedback from non-NCCN centers as well as experts at NCCN centers, and we’ll be more than happy to address any concerns or criticisms they have,” he said.
Dr. Saad reported financial relationships with Actinium and Incysus Biomedical. Dr. Loren has previously reported having no disclosures.
Fibrinogen concentrate effective, safe for postop bleeding
SAN ANTONIO – Fibrinogen concentrate was noninferior to cryoprecipitate for controlling bleeding following cardiac surgery in the randomized FIBRES trial, Canadian investigators reported.
Among 827 patients undergoing cardiopulmonary bypass, there were no significant differences in the use of allogenenic transfusion products within 24 hours of surgery for patients assigned to receive fibrinogen concentrate for control of bleeding, compared with patients who received cryoprecipitate, reported Jeannie Callum, MD, from Sunnybrook Health Sciences Centre in Toronto, on behalf of coinvestigators in the FIBRES trial.
Fibrinogen concentrate, commonly used to control postoperative bleeding in Europe, was associated with numerically, but not statistically, lower incidence of both adverse events and serious adverse events than cryoprecipitate, the current standard of care in North America.
“Given its safety and logistical advantages, fibrinogen concentrate may be considered in bleeding patients with acquired hypofibrinogenemia,” Dr. Callum said at the annual meeting of the AABB, the group formerly known as the American Association of Blood Banks.
Results of the FIBRES trial were published simultaneously in JAMA (2019 Oct 21. doi: 10.1001/jama.2019.17312).
Acquired hypofibrinogenemia, defined as a fibrinogen level below the range of 1.5-2.0 g/L, is a major cause of excess bleeding after cardiac surgery. European guidelines on the management of bleeding following trauma or cardiac surgery recommend the use of either cryoprecipitate or fibrinogen concentrate to control excessive bleeding in patients with acquired hypofibrinogenemia, Dr. Callum noted.
Cryoprecipitate is a pooled plasma–derived product that contains fibrinogen, but also fibronectin, platelet microparticles, coagulation factors VIII and XIII, and von Willebrand factor.
Additionally, fibrinogen levels in cryoprecipitate can range from as low as 3 g/L to as high as 30 g/L, and the product is normally kept and shipped frozen, and is then thawed for use and pooled prior to administration, with a shelf life of just 4-6 hours.
In contrast, fibrinogen concentrates “are pathogen-reduced and purified; have standardized fibrinogen content (20 g/L); are lyophilized, allowing for easy storage, reconstitution, and administration; and have longer shelf life after reconstitution (up to 24 hours),” Dr. Callum and her colleagues reported.
Despite the North American preference for cryoprecipitate and the European preference for fibrinogen concentrate, there have been few studies directly comparing the two products, which prompted the FIBRES investigators to design a head-to-head trial.
The randomized trial was conducted in 11 Canadian hospitals with adults undergoing cardiac surgery with cardiopulmonary bypass for whom fibrinogen supplementation was ordered in accordance with accepted clinical standards.
Patients were randomly assigned to received either 4 g of fibrinogen concentrate or 10 units of cryoprecipitate for 24 hours, with all patients receiving additional cryoprecipitate as needed after the first day.
Of 15,412 cardiac patients treated at the participating sites, 827 patients met the trial criteria and were randomized. Because the trial met the prespecified stopping criterion for noninferiority of fibrinogen at the interim analysis, the trial was halted, leaving the 827 patients as the final analysis population.
The mean number of allogeneic blood component units administered – the primary outcome – was 16.3 units in the fibrinogen concentrate group and 17.0 units in the cryoprecipitate group (mean ratio, 0.96; P for noninferiority less than .001; P for superiority = .50).
Fibrinogen was also noninferior for the secondary outcomes of individual 24-hour and cumulative 7-day blood component transfusions, and in a post-hoc analysis of cumulative transfusions measured from product administration to 24 hours after termination of cardiopulmonary bypass. These endpoints should be interpreted with caution, however, because they were not corrected for type 1 error, the investigators noted.
Fibrinogen concentrate also appeared to be noninferior for all defined subgroups, except for patients who underwent nonelective procedures, which included all patients in critical state before surgery.
Adverse events (AEs) of any kind occurred in 66.7% of patients with fibrinogen concentrate vs. 72.7% of those on cryoprecipitate. Serious AEs occurred in 31.5% vs. 34.7%, respectively.
Thromboembolic events – stroke or transient ischemic attack, amaurosis fugax (temporary vision loss), myocardial infarction, deep-vein thrombosis, pulmonary embolism, other-vessel thrombosis, disseminated intravascular coagulation, or thrombophlebitis – occurred in 7% vs. 9.6%, respectively.
The investigators acknowledged that the study was limited by the inability to blind the clinical team to the product used, by the adult-only population, and by the likelihood of variable dosing in the cryoprecipitate group.
Advantages of fibrinogen concentrate over cryoprecipitate are that the former is pathogen reduced and is easier to deliver, the investigators said.
“One important consideration is the cost differential that currently favors cryoprecipitate, but this varies across regions, and the most recent economic analysis failed to include the costs of future emerging pathogens and did not include comprehensive activity-based costing,” the investigators wrote in JAMA.
The trial was sponsored by Octapharma AG, which also provided fibrinogen concentrate. Cryoprecipitate was provided by the Canadian Blood Services and Héma-Québec. Dr. Callum reported receiving grants from Canadian Blood Services, Octapharma, and CSL Behring during the conduct of the study. Multiple coauthors had similar disclosures.
SOURCE: Callum J et al. JAMA. 2019 Oct 21. doi:10.1001/jama.2019.17312.
SAN ANTONIO – Fibrinogen concentrate was noninferior to cryoprecipitate for controlling bleeding following cardiac surgery in the randomized FIBRES trial, Canadian investigators reported.
Among 827 patients undergoing cardiopulmonary bypass, there were no significant differences in the use of allogenenic transfusion products within 24 hours of surgery for patients assigned to receive fibrinogen concentrate for control of bleeding, compared with patients who received cryoprecipitate, reported Jeannie Callum, MD, from Sunnybrook Health Sciences Centre in Toronto, on behalf of coinvestigators in the FIBRES trial.
Fibrinogen concentrate, commonly used to control postoperative bleeding in Europe, was associated with numerically, but not statistically, lower incidence of both adverse events and serious adverse events than cryoprecipitate, the current standard of care in North America.
“Given its safety and logistical advantages, fibrinogen concentrate may be considered in bleeding patients with acquired hypofibrinogenemia,” Dr. Callum said at the annual meeting of the AABB, the group formerly known as the American Association of Blood Banks.
Results of the FIBRES trial were published simultaneously in JAMA (2019 Oct 21. doi: 10.1001/jama.2019.17312).
Acquired hypofibrinogenemia, defined as a fibrinogen level below the range of 1.5-2.0 g/L, is a major cause of excess bleeding after cardiac surgery. European guidelines on the management of bleeding following trauma or cardiac surgery recommend the use of either cryoprecipitate or fibrinogen concentrate to control excessive bleeding in patients with acquired hypofibrinogenemia, Dr. Callum noted.
Cryoprecipitate is a pooled plasma–derived product that contains fibrinogen, but also fibronectin, platelet microparticles, coagulation factors VIII and XIII, and von Willebrand factor.
Additionally, fibrinogen levels in cryoprecipitate can range from as low as 3 g/L to as high as 30 g/L, and the product is normally kept and shipped frozen, and is then thawed for use and pooled prior to administration, with a shelf life of just 4-6 hours.
In contrast, fibrinogen concentrates “are pathogen-reduced and purified; have standardized fibrinogen content (20 g/L); are lyophilized, allowing for easy storage, reconstitution, and administration; and have longer shelf life after reconstitution (up to 24 hours),” Dr. Callum and her colleagues reported.
Despite the North American preference for cryoprecipitate and the European preference for fibrinogen concentrate, there have been few studies directly comparing the two products, which prompted the FIBRES investigators to design a head-to-head trial.
The randomized trial was conducted in 11 Canadian hospitals with adults undergoing cardiac surgery with cardiopulmonary bypass for whom fibrinogen supplementation was ordered in accordance with accepted clinical standards.
Patients were randomly assigned to received either 4 g of fibrinogen concentrate or 10 units of cryoprecipitate for 24 hours, with all patients receiving additional cryoprecipitate as needed after the first day.
Of 15,412 cardiac patients treated at the participating sites, 827 patients met the trial criteria and were randomized. Because the trial met the prespecified stopping criterion for noninferiority of fibrinogen at the interim analysis, the trial was halted, leaving the 827 patients as the final analysis population.
The mean number of allogeneic blood component units administered – the primary outcome – was 16.3 units in the fibrinogen concentrate group and 17.0 units in the cryoprecipitate group (mean ratio, 0.96; P for noninferiority less than .001; P for superiority = .50).
Fibrinogen was also noninferior for the secondary outcomes of individual 24-hour and cumulative 7-day blood component transfusions, and in a post-hoc analysis of cumulative transfusions measured from product administration to 24 hours after termination of cardiopulmonary bypass. These endpoints should be interpreted with caution, however, because they were not corrected for type 1 error, the investigators noted.
Fibrinogen concentrate also appeared to be noninferior for all defined subgroups, except for patients who underwent nonelective procedures, which included all patients in critical state before surgery.
Adverse events (AEs) of any kind occurred in 66.7% of patients with fibrinogen concentrate vs. 72.7% of those on cryoprecipitate. Serious AEs occurred in 31.5% vs. 34.7%, respectively.
Thromboembolic events – stroke or transient ischemic attack, amaurosis fugax (temporary vision loss), myocardial infarction, deep-vein thrombosis, pulmonary embolism, other-vessel thrombosis, disseminated intravascular coagulation, or thrombophlebitis – occurred in 7% vs. 9.6%, respectively.
The investigators acknowledged that the study was limited by the inability to blind the clinical team to the product used, by the adult-only population, and by the likelihood of variable dosing in the cryoprecipitate group.
Advantages of fibrinogen concentrate over cryoprecipitate are that the former is pathogen reduced and is easier to deliver, the investigators said.
“One important consideration is the cost differential that currently favors cryoprecipitate, but this varies across regions, and the most recent economic analysis failed to include the costs of future emerging pathogens and did not include comprehensive activity-based costing,” the investigators wrote in JAMA.
The trial was sponsored by Octapharma AG, which also provided fibrinogen concentrate. Cryoprecipitate was provided by the Canadian Blood Services and Héma-Québec. Dr. Callum reported receiving grants from Canadian Blood Services, Octapharma, and CSL Behring during the conduct of the study. Multiple coauthors had similar disclosures.
SOURCE: Callum J et al. JAMA. 2019 Oct 21. doi:10.1001/jama.2019.17312.
SAN ANTONIO – Fibrinogen concentrate was noninferior to cryoprecipitate for controlling bleeding following cardiac surgery in the randomized FIBRES trial, Canadian investigators reported.
Among 827 patients undergoing cardiopulmonary bypass, there were no significant differences in the use of allogenenic transfusion products within 24 hours of surgery for patients assigned to receive fibrinogen concentrate for control of bleeding, compared with patients who received cryoprecipitate, reported Jeannie Callum, MD, from Sunnybrook Health Sciences Centre in Toronto, on behalf of coinvestigators in the FIBRES trial.
Fibrinogen concentrate, commonly used to control postoperative bleeding in Europe, was associated with numerically, but not statistically, lower incidence of both adverse events and serious adverse events than cryoprecipitate, the current standard of care in North America.
“Given its safety and logistical advantages, fibrinogen concentrate may be considered in bleeding patients with acquired hypofibrinogenemia,” Dr. Callum said at the annual meeting of the AABB, the group formerly known as the American Association of Blood Banks.
Results of the FIBRES trial were published simultaneously in JAMA (2019 Oct 21. doi: 10.1001/jama.2019.17312).
Acquired hypofibrinogenemia, defined as a fibrinogen level below the range of 1.5-2.0 g/L, is a major cause of excess bleeding after cardiac surgery. European guidelines on the management of bleeding following trauma or cardiac surgery recommend the use of either cryoprecipitate or fibrinogen concentrate to control excessive bleeding in patients with acquired hypofibrinogenemia, Dr. Callum noted.
Cryoprecipitate is a pooled plasma–derived product that contains fibrinogen, but also fibronectin, platelet microparticles, coagulation factors VIII and XIII, and von Willebrand factor.
Additionally, fibrinogen levels in cryoprecipitate can range from as low as 3 g/L to as high as 30 g/L, and the product is normally kept and shipped frozen, and is then thawed for use and pooled prior to administration, with a shelf life of just 4-6 hours.
In contrast, fibrinogen concentrates “are pathogen-reduced and purified; have standardized fibrinogen content (20 g/L); are lyophilized, allowing for easy storage, reconstitution, and administration; and have longer shelf life after reconstitution (up to 24 hours),” Dr. Callum and her colleagues reported.
Despite the North American preference for cryoprecipitate and the European preference for fibrinogen concentrate, there have been few studies directly comparing the two products, which prompted the FIBRES investigators to design a head-to-head trial.
The randomized trial was conducted in 11 Canadian hospitals with adults undergoing cardiac surgery with cardiopulmonary bypass for whom fibrinogen supplementation was ordered in accordance with accepted clinical standards.
Patients were randomly assigned to received either 4 g of fibrinogen concentrate or 10 units of cryoprecipitate for 24 hours, with all patients receiving additional cryoprecipitate as needed after the first day.
Of 15,412 cardiac patients treated at the participating sites, 827 patients met the trial criteria and were randomized. Because the trial met the prespecified stopping criterion for noninferiority of fibrinogen at the interim analysis, the trial was halted, leaving the 827 patients as the final analysis population.
The mean number of allogeneic blood component units administered – the primary outcome – was 16.3 units in the fibrinogen concentrate group and 17.0 units in the cryoprecipitate group (mean ratio, 0.96; P for noninferiority less than .001; P for superiority = .50).
Fibrinogen was also noninferior for the secondary outcomes of individual 24-hour and cumulative 7-day blood component transfusions, and in a post-hoc analysis of cumulative transfusions measured from product administration to 24 hours after termination of cardiopulmonary bypass. These endpoints should be interpreted with caution, however, because they were not corrected for type 1 error, the investigators noted.
Fibrinogen concentrate also appeared to be noninferior for all defined subgroups, except for patients who underwent nonelective procedures, which included all patients in critical state before surgery.
Adverse events (AEs) of any kind occurred in 66.7% of patients with fibrinogen concentrate vs. 72.7% of those on cryoprecipitate. Serious AEs occurred in 31.5% vs. 34.7%, respectively.
Thromboembolic events – stroke or transient ischemic attack, amaurosis fugax (temporary vision loss), myocardial infarction, deep-vein thrombosis, pulmonary embolism, other-vessel thrombosis, disseminated intravascular coagulation, or thrombophlebitis – occurred in 7% vs. 9.6%, respectively.
The investigators acknowledged that the study was limited by the inability to blind the clinical team to the product used, by the adult-only population, and by the likelihood of variable dosing in the cryoprecipitate group.
Advantages of fibrinogen concentrate over cryoprecipitate are that the former is pathogen reduced and is easier to deliver, the investigators said.
“One important consideration is the cost differential that currently favors cryoprecipitate, but this varies across regions, and the most recent economic analysis failed to include the costs of future emerging pathogens and did not include comprehensive activity-based costing,” the investigators wrote in JAMA.
The trial was sponsored by Octapharma AG, which also provided fibrinogen concentrate. Cryoprecipitate was provided by the Canadian Blood Services and Héma-Québec. Dr. Callum reported receiving grants from Canadian Blood Services, Octapharma, and CSL Behring during the conduct of the study. Multiple coauthors had similar disclosures.
SOURCE: Callum J et al. JAMA. 2019 Oct 21. doi:10.1001/jama.2019.17312.
REPORTING FROM AABB 2019
SEER analysis reveals medication adherence factors in newly diagnosed myeloma
Black race, polypharmacy, and increasing age were associated with poor adherence to lenalidomide in older patients with newly-diagnosed multiple myeloma, according to an analysis of Surveillance, Epidemiology, and End Results (SEER)–Medicare linked data.
The objective of the study was to examine factors affecting adherence in older adults who received lenalidomide.
Of 793 patients diagnosed and treated between 2007 and 2014, 302 (38%) had poor adherence to lenalidomide, reported Hira Mian, MD, of McMaster University, Hamilton, Ont., and colleagues. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
The researchers studied patients 65 years and older who had received at least two lenalidomide prescriptions in the first year following diagnosis. Only patients who filled a prescription for lenalidomide within 60 days of a myeloma diagnosis were included.
The median age of the patients was 73 years; 43% were aged 75 years or older. Most of the patients included in the analysis were white.
The medication possession ratio, defined as the “ratio of the number of days the patient had pills in their possession to the number of days in the observation period,” was used to evaluate adherence to therapy. A ratio of less than 90% was deemed poor adherence by the researchers.
After analysis, the researchers found that black race (adjusted odds ratio, 1.72; P = .022), polypharmacy (aOR, 1.04 per drug; P = .008), and increasing age (aOR, 1.03 per year; P = .024) were all significantly associated with poor adherence to lenalidomide.
The mean medication possession ratio among study patients was 89.5%. Overall, 38% of patients in the study had poor adherence to lenalidomide, while just 7% of patients in the study had a medication possession ratio of 100%, indicating “perfect adherence.”
There was a trend toward inferior overall survival among patients with poor adherence to lenalidomide, but it was not statistically significant (hazard ratio 1.10, 95% confidence interval, 0.88-1.38).
“Our study emphasizes the need for both better clinical monitoring of adherence and for future prospective studies in accurately understanding the rates and predictors of adherence while simultaneously developing strategies for improving adherence for patients that are at high risk of nonadherence,” the researchers wrote.
The National Institutes of Health funded the study. No conflicts of interest were reported.
SOURCE: Mian H et al. Clin Lymphoma Myeloma Leuk. 2019 Oct 9. doi: 10.1016/j.clml.2019.09.618.
Black race, polypharmacy, and increasing age were associated with poor adherence to lenalidomide in older patients with newly-diagnosed multiple myeloma, according to an analysis of Surveillance, Epidemiology, and End Results (SEER)–Medicare linked data.
The objective of the study was to examine factors affecting adherence in older adults who received lenalidomide.
Of 793 patients diagnosed and treated between 2007 and 2014, 302 (38%) had poor adherence to lenalidomide, reported Hira Mian, MD, of McMaster University, Hamilton, Ont., and colleagues. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
The researchers studied patients 65 years and older who had received at least two lenalidomide prescriptions in the first year following diagnosis. Only patients who filled a prescription for lenalidomide within 60 days of a myeloma diagnosis were included.
The median age of the patients was 73 years; 43% were aged 75 years or older. Most of the patients included in the analysis were white.
The medication possession ratio, defined as the “ratio of the number of days the patient had pills in their possession to the number of days in the observation period,” was used to evaluate adherence to therapy. A ratio of less than 90% was deemed poor adherence by the researchers.
After analysis, the researchers found that black race (adjusted odds ratio, 1.72; P = .022), polypharmacy (aOR, 1.04 per drug; P = .008), and increasing age (aOR, 1.03 per year; P = .024) were all significantly associated with poor adherence to lenalidomide.
The mean medication possession ratio among study patients was 89.5%. Overall, 38% of patients in the study had poor adherence to lenalidomide, while just 7% of patients in the study had a medication possession ratio of 100%, indicating “perfect adherence.”
There was a trend toward inferior overall survival among patients with poor adherence to lenalidomide, but it was not statistically significant (hazard ratio 1.10, 95% confidence interval, 0.88-1.38).
“Our study emphasizes the need for both better clinical monitoring of adherence and for future prospective studies in accurately understanding the rates and predictors of adherence while simultaneously developing strategies for improving adherence for patients that are at high risk of nonadherence,” the researchers wrote.
The National Institutes of Health funded the study. No conflicts of interest were reported.
SOURCE: Mian H et al. Clin Lymphoma Myeloma Leuk. 2019 Oct 9. doi: 10.1016/j.clml.2019.09.618.
Black race, polypharmacy, and increasing age were associated with poor adherence to lenalidomide in older patients with newly-diagnosed multiple myeloma, according to an analysis of Surveillance, Epidemiology, and End Results (SEER)–Medicare linked data.
The objective of the study was to examine factors affecting adherence in older adults who received lenalidomide.
Of 793 patients diagnosed and treated between 2007 and 2014, 302 (38%) had poor adherence to lenalidomide, reported Hira Mian, MD, of McMaster University, Hamilton, Ont., and colleagues. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
The researchers studied patients 65 years and older who had received at least two lenalidomide prescriptions in the first year following diagnosis. Only patients who filled a prescription for lenalidomide within 60 days of a myeloma diagnosis were included.
The median age of the patients was 73 years; 43% were aged 75 years or older. Most of the patients included in the analysis were white.
The medication possession ratio, defined as the “ratio of the number of days the patient had pills in their possession to the number of days in the observation period,” was used to evaluate adherence to therapy. A ratio of less than 90% was deemed poor adherence by the researchers.
After analysis, the researchers found that black race (adjusted odds ratio, 1.72; P = .022), polypharmacy (aOR, 1.04 per drug; P = .008), and increasing age (aOR, 1.03 per year; P = .024) were all significantly associated with poor adherence to lenalidomide.
The mean medication possession ratio among study patients was 89.5%. Overall, 38% of patients in the study had poor adherence to lenalidomide, while just 7% of patients in the study had a medication possession ratio of 100%, indicating “perfect adherence.”
There was a trend toward inferior overall survival among patients with poor adherence to lenalidomide, but it was not statistically significant (hazard ratio 1.10, 95% confidence interval, 0.88-1.38).
“Our study emphasizes the need for both better clinical monitoring of adherence and for future prospective studies in accurately understanding the rates and predictors of adherence while simultaneously developing strategies for improving adherence for patients that are at high risk of nonadherence,” the researchers wrote.
The National Institutes of Health funded the study. No conflicts of interest were reported.
SOURCE: Mian H et al. Clin Lymphoma Myeloma Leuk. 2019 Oct 9. doi: 10.1016/j.clml.2019.09.618.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Hematopoietic cell transplant offers realistic cure in secondary AML
yielding significantly better survival outcomes, according to findings from an observational study.
Although secondary AML has been identified as an independent predictor of poor prognosis, it is not included in current risk classifications that provide the basis of deciding when to perform HCT.
Christer Nilsson, MD, of Karolinska Institute, Stockholm, and colleagues, used two nationwide Swedish registries – the Swedish AML Registry and the Swedish Cancer Registry – to characterize how often HCT is performed in these patients and to evaluate its impact in a real-world setting. The registries include all patients with AML diagnosed between 1997 and 2013.
Their findings are in Biology of Blood and Marrow Transplantation.
The analysis included 3,337 adult patients with AML who were intensively treated and did not have acute promyelocytic leukemia. More than three-quarters of the patients had de novo AML and the remainder had secondary AML that was either therapy related or developed after an antecedent myeloid disease. In total, 100 patients with secondary AML underwent HCT while in first complete remission.
In terms of crude survival at 5 years after diagnosis, patients with secondary AML who did not undergo HCT did very poorly. The survival rate was 0% in those with AML preceded by myeloproliferative neoplasm (MPN-AML), 2% in patients with AML preceded by myelodysplastic syndrome (MDS-AML), and 4% in patients with therapy-related AML (t-AML). In contrast, the 5-year overall survival in patients who underwent HCT at any time point or disease stage was 32% for patients with MPN-AML, 18% for patients with MDS-AML, and 25% for patients t-AML.
These crude survival figures suggest that “HCT is the sole realistic curable treatment option for [secondary] AML,” the researchers wrote.
The researchers also performed a propensity score matching analysis of HCT versus chemotherapy consolidation in patients with secondary AML who had been in first complete remission for more than 90 days. The model matched 45 patients who underwent HCT with 66 patients treated with chemotherapy consolidation. The projected 5-year overall survival was 48% in the HCT group, compared with 20% in the consolidation group (P = .01). Similarly, 5-year relapse-free survival was also higher in the HCT group, compared with the consolidation group (43% vs. 21%, P = .02).
“Ideally, the role of transplantation in [secondary] AML should be evaluated in a prospective randomized trial, minimizing the risk of any bias,” the researchers wrote. “However, such a trial is lacking and most likely will never be performed.”
The researchers concluded that HCT should be considered for all patients with secondary AML who are eligible and fit for transplantation.
The study was supported by the Swedish Cancer Foundation, Swedish Research Council, Stockholm County Council, Gothenberg Medical Society, and Assar Gabrielsson Foundation. The researchers reported having no conflicts of interest.
SOURCE: Nilson C et al. Biol Blood Marrow Tranplant. 2019;25:1770-8.
yielding significantly better survival outcomes, according to findings from an observational study.
Although secondary AML has been identified as an independent predictor of poor prognosis, it is not included in current risk classifications that provide the basis of deciding when to perform HCT.
Christer Nilsson, MD, of Karolinska Institute, Stockholm, and colleagues, used two nationwide Swedish registries – the Swedish AML Registry and the Swedish Cancer Registry – to characterize how often HCT is performed in these patients and to evaluate its impact in a real-world setting. The registries include all patients with AML diagnosed between 1997 and 2013.
Their findings are in Biology of Blood and Marrow Transplantation.
The analysis included 3,337 adult patients with AML who were intensively treated and did not have acute promyelocytic leukemia. More than three-quarters of the patients had de novo AML and the remainder had secondary AML that was either therapy related or developed after an antecedent myeloid disease. In total, 100 patients with secondary AML underwent HCT while in first complete remission.
In terms of crude survival at 5 years after diagnosis, patients with secondary AML who did not undergo HCT did very poorly. The survival rate was 0% in those with AML preceded by myeloproliferative neoplasm (MPN-AML), 2% in patients with AML preceded by myelodysplastic syndrome (MDS-AML), and 4% in patients with therapy-related AML (t-AML). In contrast, the 5-year overall survival in patients who underwent HCT at any time point or disease stage was 32% for patients with MPN-AML, 18% for patients with MDS-AML, and 25% for patients t-AML.
These crude survival figures suggest that “HCT is the sole realistic curable treatment option for [secondary] AML,” the researchers wrote.
The researchers also performed a propensity score matching analysis of HCT versus chemotherapy consolidation in patients with secondary AML who had been in first complete remission for more than 90 days. The model matched 45 patients who underwent HCT with 66 patients treated with chemotherapy consolidation. The projected 5-year overall survival was 48% in the HCT group, compared with 20% in the consolidation group (P = .01). Similarly, 5-year relapse-free survival was also higher in the HCT group, compared with the consolidation group (43% vs. 21%, P = .02).
“Ideally, the role of transplantation in [secondary] AML should be evaluated in a prospective randomized trial, minimizing the risk of any bias,” the researchers wrote. “However, such a trial is lacking and most likely will never be performed.”
The researchers concluded that HCT should be considered for all patients with secondary AML who are eligible and fit for transplantation.
The study was supported by the Swedish Cancer Foundation, Swedish Research Council, Stockholm County Council, Gothenberg Medical Society, and Assar Gabrielsson Foundation. The researchers reported having no conflicts of interest.
SOURCE: Nilson C et al. Biol Blood Marrow Tranplant. 2019;25:1770-8.
yielding significantly better survival outcomes, according to findings from an observational study.
Although secondary AML has been identified as an independent predictor of poor prognosis, it is not included in current risk classifications that provide the basis of deciding when to perform HCT.
Christer Nilsson, MD, of Karolinska Institute, Stockholm, and colleagues, used two nationwide Swedish registries – the Swedish AML Registry and the Swedish Cancer Registry – to characterize how often HCT is performed in these patients and to evaluate its impact in a real-world setting. The registries include all patients with AML diagnosed between 1997 and 2013.
Their findings are in Biology of Blood and Marrow Transplantation.
The analysis included 3,337 adult patients with AML who were intensively treated and did not have acute promyelocytic leukemia. More than three-quarters of the patients had de novo AML and the remainder had secondary AML that was either therapy related or developed after an antecedent myeloid disease. In total, 100 patients with secondary AML underwent HCT while in first complete remission.
In terms of crude survival at 5 years after diagnosis, patients with secondary AML who did not undergo HCT did very poorly. The survival rate was 0% in those with AML preceded by myeloproliferative neoplasm (MPN-AML), 2% in patients with AML preceded by myelodysplastic syndrome (MDS-AML), and 4% in patients with therapy-related AML (t-AML). In contrast, the 5-year overall survival in patients who underwent HCT at any time point or disease stage was 32% for patients with MPN-AML, 18% for patients with MDS-AML, and 25% for patients t-AML.
These crude survival figures suggest that “HCT is the sole realistic curable treatment option for [secondary] AML,” the researchers wrote.
The researchers also performed a propensity score matching analysis of HCT versus chemotherapy consolidation in patients with secondary AML who had been in first complete remission for more than 90 days. The model matched 45 patients who underwent HCT with 66 patients treated with chemotherapy consolidation. The projected 5-year overall survival was 48% in the HCT group, compared with 20% in the consolidation group (P = .01). Similarly, 5-year relapse-free survival was also higher in the HCT group, compared with the consolidation group (43% vs. 21%, P = .02).
“Ideally, the role of transplantation in [secondary] AML should be evaluated in a prospective randomized trial, minimizing the risk of any bias,” the researchers wrote. “However, such a trial is lacking and most likely will never be performed.”
The researchers concluded that HCT should be considered for all patients with secondary AML who are eligible and fit for transplantation.
The study was supported by the Swedish Cancer Foundation, Swedish Research Council, Stockholm County Council, Gothenberg Medical Society, and Assar Gabrielsson Foundation. The researchers reported having no conflicts of interest.
SOURCE: Nilson C et al. Biol Blood Marrow Tranplant. 2019;25:1770-8.
FROM BIOLOGY OF BLOOD AND MARROW TRANSPLANTATION