User login
On Aug. 29, 2019, the first commercial case utilizing the Sonata system to transcervically ablate symptomatic uterine fibroids under ultrasound guidance was performed at Stamford (Conn.) Hospital. This truly minimally invasive new treatment expands our options in the surgical management of uterine fibroids.
Uterine fibroids are the most common benign tumors of the reproductive tract. It has been estimated that nearly half of the 70%-80% of women who develop fibroids during their reproductive years are symptomatic. Given that some patients present with fertility concerns, it also has been estimated that at least one in three women with fibroids have symptoms such as heavy bleeding (menorrhagia) and bulk symptoms, pain (dyspareunia, dysmenorrhea, noncyclic pain), and increased urinary frequency.
Fibroids are the most common cause of hysterectomy in the United States, with 240,000 (40% of 600,000) performed annually, yet research shows that many women are interested in minimally invasive options and in uterine conservation. In a 2013 national survey published in the American Journal of Obstetrics and Gynecology, 79% of women expressed an interest in minimally invasive approaches for fibroid treatment, and over 50% reported a desire for uterine conservation.1
Both myomectomy and uterine artery embolization are uterine-sparing procedures. However, uterine artery embolization should not be performed in a woman interested in pregnancy. Moreover, there are reports of ovarian reserve issues when the procedure is performed in women in their later reproductive years.
Depending on the technique performed, women undergoing hysteroscopic myomectomy are at risk of fluid overload, hyponatremia, gas-related embolism, and postoperative adhesions. The suture requirements of a laparoscopic myomectomy make this approach an often-difficult one to master, even with robotic assistance. It also requires intubation and potentially places the patient at risk for bleeding and infection. Furthermore, long-term risks include adhesions and the need for C-section with pregnancy.
The impact of uterine fibroids on patients’ lives and their desire for uterine conservation has spurred growing interest in the use of radiofrequency (RF) energy to ablate uterine fibroids. In a 2018 systematic review of nonresective treatments for uterine fibroids published in the International Journal of Hyperthermia, investigators found that the pooled fibroid volume reductions at 6 months after RF ablation and uterine artery embolization were 70% and 54%, respectively.2
The first commercially available system utilizing RF frequency to shrink fibrosis – Acessa – involves laparoscopy, and thus requires abdominal incisions. In August 2018, the Sonata system (Gynesonics: Redwood, Calif.) received Food and Drug Administration clearance after having received European CE-Mark approval in 2010 (for the original device, the VizAblate) and in 2014 (for the next-generation device, the Sonata).
The technology
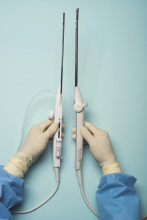
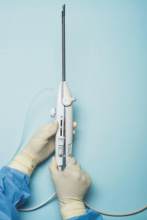
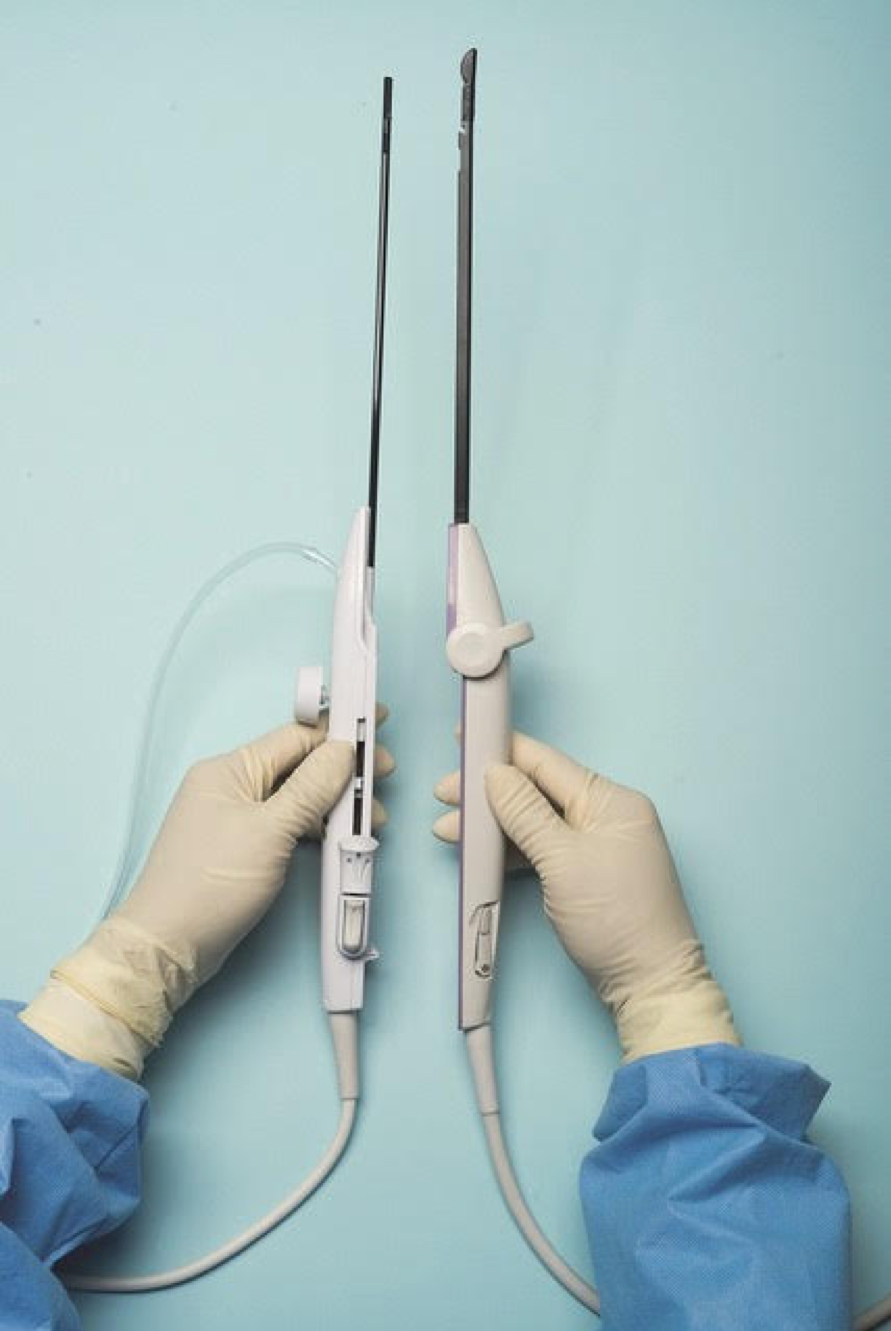
For a complete description of transcervical, intrauterine sonography–guided radiofrequency ablation of uterine fibroids, one can refer to the excellent outline by David Toub, MD, in Current Obstetrics and Gynecology Reports.3 Basically, the Sonata system allows for real-time, image-guided treatment through the use of a reusable intrauterine ultrasound (IUUS) probe, a single-use RF ablation (RFA) handpiece, and graphical guidance software for diagnosis and targeting.
Initially, the IUUS probe enables identification of fibroids from within the uterine cavity, then guides deployment of an introducer and needle electrode into the targeted fibroid(s). The probe image is curvilinear, penetrates more than 9 cm, and provides a 90-degree field of view.
The RFA handpiece contains the introducer and needle electrode array. It snaps together with the IUUS probe to form and integrate into a single treatment device that contains all controls needed to place and size the ablation. Mechanical stops and lockouts within the RFA handpiece further enhance proper localization and sizing of the ablation.
The system’s graphical guidance software, also known as the SMART Guide, is a real-time graphical overlay on the ultrasound display, which enables one to visually select deployment length, width, and position of the ablation guides. In so doing, the mechanical stops for the introducer and needle electrodes are determined prior to their insertion into the targeted fibroid(s). This was validated in more than 4,000 ablations in bovine muscle and human-extirpated uteri, as well as in vivo at time of laparotomy.
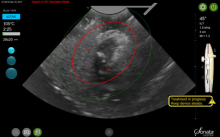
By displaying the ellipsoidal region where the ablation will take place (ablation zone) along with a surrounding ellipsoid (thermal safety border) where tissue temperature will be elevated, the SMART Guide provides a safer and more accurate understanding of the ablation than if it showed only the ablation zone.
As with transabdominal or transvaginal sonography, the serosa will appear hyperechoic at the time of intrauterine ultrasound. By using the SMART Guide, the ablation is sized and positioned to encompass as much of the fibroid as possible while maintaining thermal energy within the uterine serosal margin. Once the desired ablation size has been selected, and safe placement of the needle electrodes is confirmed by rotating the IUUS probe in multiple planes, therapeutic RF energy is delivered to the fibroid; the fixed treatment cycle is dependent on ablation size.
The system will modulate power (up to 150W) to keep temperature at the tips of the needle electrode at 105° C. Moreover, the time of energy delivery at the temperature of 105° – 2-7 minutes – is automatically set based on ablation size, which is a continuum up to 4 cm wide and up to 5 cm long. Multiple ablations may be utilized in a particularly large fibroid.
Unlike hysteroscopic myomectomy, only a small amount of hypotonic solution is instilled within the uterine cavity to enhance acoustic coupling. Furthermore, the treatment device (RFA handpiece and IUUS probe) is only 8.3 mm in diameter. This requires Hegar dilatation of the cervix to 9.
The procedure
After administering anesthesia (regional or sedation), dispersive electrode pads are placed on the anterior thighs. After the cervix is dilated to Hegar dilatation of 9, the treatment device is inserted transcervically into the uterine cavity and the fibroid(s) are identified with the ultrasound probe. The physician plans and optimizes the ablation by sizing and aligning the graphical overlay targeting guide (the SMART Guide) over the live image. Once the size and location of the ablation are set, the trocar-tipped introducer is advanced into the fibroid. After ensuring the guide is within the serosal boundary, the needle electrodes are deployed.
A second visual safety check is completed, and the delivery of RF energy is initiated using a footswitch control. The time of energy delivery is determined based on the size of the desired ablation, up to 7 minutes for the largest ablation size (5 cm x 4 cm). The targeting and treatment steps are repeated as required to treat additional fibroids. Once the treatment is completed, the needle electrodes and introducer are retracted, and the treatment device removed.
Study results and the future
The 12-month safety and effectiveness data for ultrasound-guided transcervical ablation of uterine fibroids were reported in January 2019 in Obstetrics & Gynecology.4 Women enrolled in the prospective, multicenter, single-arm, interventional trial had 1-10 fibroids – the International Federation of Gynecology and Obstetrics (FIGO) types 1, 2, 3, 4, and 2-5 (pedunculated fibroids excluded) – with diameters of 1-5 centimeters. Patients also were required to have at least one fibroid indenting or impinging on the endometrial cavity (FIGO type 1, 2, 3, or 2-5).
Upon study entry, the pictorial assessment blood loss was required to be 150-500 cc. The study included 147 patients. Both coprimary endpoints were satisfied at 12 months; that is, 65% of patients experienced a 50% or greater reduction in menstrual bleeding, and 99% were free from surgical intervention at 1 year.
The mean pictorial blood loss decreased by 39%, 48%, and 51% at 3, 6, and 12 months respectively. Moreover, 95% of the study population experienced some reduction in menstrual bleeding at 12 months. There also were mean improvements in symptom severity and health-related quality-of-life parameters. Mean maximal fibroid volume reduction per patient was 62%.
More than half of the patients returned to normal activity within 1 day, 96% of patients reported symptom improvement at 12 months, and 97% expressed satisfaction with the procedure and results at 12 months. There were no device-related adverse events.
I am the lead author for the 2-year follow-up study utilizing transcervical RFA of symptomatic uterine fibroids, which currently is in press. Suffice it to say, the quality-of-life data, symptom improvement, and lower rate of surgical reintervention all are significant and compelling. Ultimately, I believe Sonata will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller disclosed that he is a consultant for Gynesonics and holds a stock option agreement with the company.
References
1. Am J Obstet Gynecol. 2013 Oct;209(4):319.e1-319.e20.
2. Int J Hyperthermia. 2019;36(1):295-301.
3. Curr Obstet Gynecol Rep. 2017; 6(1): 67-73.
4. Obstet Gynecol. 2019 Jan;133(1):13-22.
On Aug. 29, 2019, the first commercial case utilizing the Sonata system to transcervically ablate symptomatic uterine fibroids under ultrasound guidance was performed at Stamford (Conn.) Hospital. This truly minimally invasive new treatment expands our options in the surgical management of uterine fibroids.
Uterine fibroids are the most common benign tumors of the reproductive tract. It has been estimated that nearly half of the 70%-80% of women who develop fibroids during their reproductive years are symptomatic. Given that some patients present with fertility concerns, it also has been estimated that at least one in three women with fibroids have symptoms such as heavy bleeding (menorrhagia) and bulk symptoms, pain (dyspareunia, dysmenorrhea, noncyclic pain), and increased urinary frequency.
Fibroids are the most common cause of hysterectomy in the United States, with 240,000 (40% of 600,000) performed annually, yet research shows that many women are interested in minimally invasive options and in uterine conservation. In a 2013 national survey published in the American Journal of Obstetrics and Gynecology, 79% of women expressed an interest in minimally invasive approaches for fibroid treatment, and over 50% reported a desire for uterine conservation.1
Both myomectomy and uterine artery embolization are uterine-sparing procedures. However, uterine artery embolization should not be performed in a woman interested in pregnancy. Moreover, there are reports of ovarian reserve issues when the procedure is performed in women in their later reproductive years.
Depending on the technique performed, women undergoing hysteroscopic myomectomy are at risk of fluid overload, hyponatremia, gas-related embolism, and postoperative adhesions. The suture requirements of a laparoscopic myomectomy make this approach an often-difficult one to master, even with robotic assistance. It also requires intubation and potentially places the patient at risk for bleeding and infection. Furthermore, long-term risks include adhesions and the need for C-section with pregnancy.
The impact of uterine fibroids on patients’ lives and their desire for uterine conservation has spurred growing interest in the use of radiofrequency (RF) energy to ablate uterine fibroids. In a 2018 systematic review of nonresective treatments for uterine fibroids published in the International Journal of Hyperthermia, investigators found that the pooled fibroid volume reductions at 6 months after RF ablation and uterine artery embolization were 70% and 54%, respectively.2
The first commercially available system utilizing RF frequency to shrink fibrosis – Acessa – involves laparoscopy, and thus requires abdominal incisions. In August 2018, the Sonata system (Gynesonics: Redwood, Calif.) received Food and Drug Administration clearance after having received European CE-Mark approval in 2010 (for the original device, the VizAblate) and in 2014 (for the next-generation device, the Sonata).
The technology
For a complete description of transcervical, intrauterine sonography–guided radiofrequency ablation of uterine fibroids, one can refer to the excellent outline by David Toub, MD, in Current Obstetrics and Gynecology Reports.3 Basically, the Sonata system allows for real-time, image-guided treatment through the use of a reusable intrauterine ultrasound (IUUS) probe, a single-use RF ablation (RFA) handpiece, and graphical guidance software for diagnosis and targeting.
Initially, the IUUS probe enables identification of fibroids from within the uterine cavity, then guides deployment of an introducer and needle electrode into the targeted fibroid(s). The probe image is curvilinear, penetrates more than 9 cm, and provides a 90-degree field of view.
The RFA handpiece contains the introducer and needle electrode array. It snaps together with the IUUS probe to form and integrate into a single treatment device that contains all controls needed to place and size the ablation. Mechanical stops and lockouts within the RFA handpiece further enhance proper localization and sizing of the ablation.
The system’s graphical guidance software, also known as the SMART Guide, is a real-time graphical overlay on the ultrasound display, which enables one to visually select deployment length, width, and position of the ablation guides. In so doing, the mechanical stops for the introducer and needle electrodes are determined prior to their insertion into the targeted fibroid(s). This was validated in more than 4,000 ablations in bovine muscle and human-extirpated uteri, as well as in vivo at time of laparotomy.
By displaying the ellipsoidal region where the ablation will take place (ablation zone) along with a surrounding ellipsoid (thermal safety border) where tissue temperature will be elevated, the SMART Guide provides a safer and more accurate understanding of the ablation than if it showed only the ablation zone.
As with transabdominal or transvaginal sonography, the serosa will appear hyperechoic at the time of intrauterine ultrasound. By using the SMART Guide, the ablation is sized and positioned to encompass as much of the fibroid as possible while maintaining thermal energy within the uterine serosal margin. Once the desired ablation size has been selected, and safe placement of the needle electrodes is confirmed by rotating the IUUS probe in multiple planes, therapeutic RF energy is delivered to the fibroid; the fixed treatment cycle is dependent on ablation size.
The system will modulate power (up to 150W) to keep temperature at the tips of the needle electrode at 105° C. Moreover, the time of energy delivery at the temperature of 105° – 2-7 minutes – is automatically set based on ablation size, which is a continuum up to 4 cm wide and up to 5 cm long. Multiple ablations may be utilized in a particularly large fibroid.
Unlike hysteroscopic myomectomy, only a small amount of hypotonic solution is instilled within the uterine cavity to enhance acoustic coupling. Furthermore, the treatment device (RFA handpiece and IUUS probe) is only 8.3 mm in diameter. This requires Hegar dilatation of the cervix to 9.
The procedure
After administering anesthesia (regional or sedation), dispersive electrode pads are placed on the anterior thighs. After the cervix is dilated to Hegar dilatation of 9, the treatment device is inserted transcervically into the uterine cavity and the fibroid(s) are identified with the ultrasound probe. The physician plans and optimizes the ablation by sizing and aligning the graphical overlay targeting guide (the SMART Guide) over the live image. Once the size and location of the ablation are set, the trocar-tipped introducer is advanced into the fibroid. After ensuring the guide is within the serosal boundary, the needle electrodes are deployed.
A second visual safety check is completed, and the delivery of RF energy is initiated using a footswitch control. The time of energy delivery is determined based on the size of the desired ablation, up to 7 minutes for the largest ablation size (5 cm x 4 cm). The targeting and treatment steps are repeated as required to treat additional fibroids. Once the treatment is completed, the needle electrodes and introducer are retracted, and the treatment device removed.
Study results and the future
The 12-month safety and effectiveness data for ultrasound-guided transcervical ablation of uterine fibroids were reported in January 2019 in Obstetrics & Gynecology.4 Women enrolled in the prospective, multicenter, single-arm, interventional trial had 1-10 fibroids – the International Federation of Gynecology and Obstetrics (FIGO) types 1, 2, 3, 4, and 2-5 (pedunculated fibroids excluded) – with diameters of 1-5 centimeters. Patients also were required to have at least one fibroid indenting or impinging on the endometrial cavity (FIGO type 1, 2, 3, or 2-5).
Upon study entry, the pictorial assessment blood loss was required to be 150-500 cc. The study included 147 patients. Both coprimary endpoints were satisfied at 12 months; that is, 65% of patients experienced a 50% or greater reduction in menstrual bleeding, and 99% were free from surgical intervention at 1 year.
The mean pictorial blood loss decreased by 39%, 48%, and 51% at 3, 6, and 12 months respectively. Moreover, 95% of the study population experienced some reduction in menstrual bleeding at 12 months. There also were mean improvements in symptom severity and health-related quality-of-life parameters. Mean maximal fibroid volume reduction per patient was 62%.
More than half of the patients returned to normal activity within 1 day, 96% of patients reported symptom improvement at 12 months, and 97% expressed satisfaction with the procedure and results at 12 months. There were no device-related adverse events.
I am the lead author for the 2-year follow-up study utilizing transcervical RFA of symptomatic uterine fibroids, which currently is in press. Suffice it to say, the quality-of-life data, symptom improvement, and lower rate of surgical reintervention all are significant and compelling. Ultimately, I believe Sonata will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller disclosed that he is a consultant for Gynesonics and holds a stock option agreement with the company.
References
1. Am J Obstet Gynecol. 2013 Oct;209(4):319.e1-319.e20.
2. Int J Hyperthermia. 2019;36(1):295-301.
3. Curr Obstet Gynecol Rep. 2017; 6(1): 67-73.
4. Obstet Gynecol. 2019 Jan;133(1):13-22.
On Aug. 29, 2019, the first commercial case utilizing the Sonata system to transcervically ablate symptomatic uterine fibroids under ultrasound guidance was performed at Stamford (Conn.) Hospital. This truly minimally invasive new treatment expands our options in the surgical management of uterine fibroids.
Uterine fibroids are the most common benign tumors of the reproductive tract. It has been estimated that nearly half of the 70%-80% of women who develop fibroids during their reproductive years are symptomatic. Given that some patients present with fertility concerns, it also has been estimated that at least one in three women with fibroids have symptoms such as heavy bleeding (menorrhagia) and bulk symptoms, pain (dyspareunia, dysmenorrhea, noncyclic pain), and increased urinary frequency.
Fibroids are the most common cause of hysterectomy in the United States, with 240,000 (40% of 600,000) performed annually, yet research shows that many women are interested in minimally invasive options and in uterine conservation. In a 2013 national survey published in the American Journal of Obstetrics and Gynecology, 79% of women expressed an interest in minimally invasive approaches for fibroid treatment, and over 50% reported a desire for uterine conservation.1
Both myomectomy and uterine artery embolization are uterine-sparing procedures. However, uterine artery embolization should not be performed in a woman interested in pregnancy. Moreover, there are reports of ovarian reserve issues when the procedure is performed in women in their later reproductive years.
Depending on the technique performed, women undergoing hysteroscopic myomectomy are at risk of fluid overload, hyponatremia, gas-related embolism, and postoperative adhesions. The suture requirements of a laparoscopic myomectomy make this approach an often-difficult one to master, even with robotic assistance. It also requires intubation and potentially places the patient at risk for bleeding and infection. Furthermore, long-term risks include adhesions and the need for C-section with pregnancy.
The impact of uterine fibroids on patients’ lives and their desire for uterine conservation has spurred growing interest in the use of radiofrequency (RF) energy to ablate uterine fibroids. In a 2018 systematic review of nonresective treatments for uterine fibroids published in the International Journal of Hyperthermia, investigators found that the pooled fibroid volume reductions at 6 months after RF ablation and uterine artery embolization were 70% and 54%, respectively.2
The first commercially available system utilizing RF frequency to shrink fibrosis – Acessa – involves laparoscopy, and thus requires abdominal incisions. In August 2018, the Sonata system (Gynesonics: Redwood, Calif.) received Food and Drug Administration clearance after having received European CE-Mark approval in 2010 (for the original device, the VizAblate) and in 2014 (for the next-generation device, the Sonata).
The technology
For a complete description of transcervical, intrauterine sonography–guided radiofrequency ablation of uterine fibroids, one can refer to the excellent outline by David Toub, MD, in Current Obstetrics and Gynecology Reports.3 Basically, the Sonata system allows for real-time, image-guided treatment through the use of a reusable intrauterine ultrasound (IUUS) probe, a single-use RF ablation (RFA) handpiece, and graphical guidance software for diagnosis and targeting.
Initially, the IUUS probe enables identification of fibroids from within the uterine cavity, then guides deployment of an introducer and needle electrode into the targeted fibroid(s). The probe image is curvilinear, penetrates more than 9 cm, and provides a 90-degree field of view.
The RFA handpiece contains the introducer and needle electrode array. It snaps together with the IUUS probe to form and integrate into a single treatment device that contains all controls needed to place and size the ablation. Mechanical stops and lockouts within the RFA handpiece further enhance proper localization and sizing of the ablation.
The system’s graphical guidance software, also known as the SMART Guide, is a real-time graphical overlay on the ultrasound display, which enables one to visually select deployment length, width, and position of the ablation guides. In so doing, the mechanical stops for the introducer and needle electrodes are determined prior to their insertion into the targeted fibroid(s). This was validated in more than 4,000 ablations in bovine muscle and human-extirpated uteri, as well as in vivo at time of laparotomy.
By displaying the ellipsoidal region where the ablation will take place (ablation zone) along with a surrounding ellipsoid (thermal safety border) where tissue temperature will be elevated, the SMART Guide provides a safer and more accurate understanding of the ablation than if it showed only the ablation zone.
As with transabdominal or transvaginal sonography, the serosa will appear hyperechoic at the time of intrauterine ultrasound. By using the SMART Guide, the ablation is sized and positioned to encompass as much of the fibroid as possible while maintaining thermal energy within the uterine serosal margin. Once the desired ablation size has been selected, and safe placement of the needle electrodes is confirmed by rotating the IUUS probe in multiple planes, therapeutic RF energy is delivered to the fibroid; the fixed treatment cycle is dependent on ablation size.
The system will modulate power (up to 150W) to keep temperature at the tips of the needle electrode at 105° C. Moreover, the time of energy delivery at the temperature of 105° – 2-7 minutes – is automatically set based on ablation size, which is a continuum up to 4 cm wide and up to 5 cm long. Multiple ablations may be utilized in a particularly large fibroid.
Unlike hysteroscopic myomectomy, only a small amount of hypotonic solution is instilled within the uterine cavity to enhance acoustic coupling. Furthermore, the treatment device (RFA handpiece and IUUS probe) is only 8.3 mm in diameter. This requires Hegar dilatation of the cervix to 9.
The procedure
After administering anesthesia (regional or sedation), dispersive electrode pads are placed on the anterior thighs. After the cervix is dilated to Hegar dilatation of 9, the treatment device is inserted transcervically into the uterine cavity and the fibroid(s) are identified with the ultrasound probe. The physician plans and optimizes the ablation by sizing and aligning the graphical overlay targeting guide (the SMART Guide) over the live image. Once the size and location of the ablation are set, the trocar-tipped introducer is advanced into the fibroid. After ensuring the guide is within the serosal boundary, the needle electrodes are deployed.
A second visual safety check is completed, and the delivery of RF energy is initiated using a footswitch control. The time of energy delivery is determined based on the size of the desired ablation, up to 7 minutes for the largest ablation size (5 cm x 4 cm). The targeting and treatment steps are repeated as required to treat additional fibroids. Once the treatment is completed, the needle electrodes and introducer are retracted, and the treatment device removed.
Study results and the future
The 12-month safety and effectiveness data for ultrasound-guided transcervical ablation of uterine fibroids were reported in January 2019 in Obstetrics & Gynecology.4 Women enrolled in the prospective, multicenter, single-arm, interventional trial had 1-10 fibroids – the International Federation of Gynecology and Obstetrics (FIGO) types 1, 2, 3, 4, and 2-5 (pedunculated fibroids excluded) – with diameters of 1-5 centimeters. Patients also were required to have at least one fibroid indenting or impinging on the endometrial cavity (FIGO type 1, 2, 3, or 2-5).
Upon study entry, the pictorial assessment blood loss was required to be 150-500 cc. The study included 147 patients. Both coprimary endpoints were satisfied at 12 months; that is, 65% of patients experienced a 50% or greater reduction in menstrual bleeding, and 99% were free from surgical intervention at 1 year.
The mean pictorial blood loss decreased by 39%, 48%, and 51% at 3, 6, and 12 months respectively. Moreover, 95% of the study population experienced some reduction in menstrual bleeding at 12 months. There also were mean improvements in symptom severity and health-related quality-of-life parameters. Mean maximal fibroid volume reduction per patient was 62%.
More than half of the patients returned to normal activity within 1 day, 96% of patients reported symptom improvement at 12 months, and 97% expressed satisfaction with the procedure and results at 12 months. There were no device-related adverse events.
I am the lead author for the 2-year follow-up study utilizing transcervical RFA of symptomatic uterine fibroids, which currently is in press. Suffice it to say, the quality-of-life data, symptom improvement, and lower rate of surgical reintervention all are significant and compelling. Ultimately, I believe Sonata will not only be a treatment of choice in the appropriate patient presenting with heavy menstrual flow or bulk symptoms secondary to uterine fibroids, but will prove to be beneficial in women with impinging or deep submucosal fibroids and implantation failure.
Dr. Miller is a clinical associate professor at the University of Illinois in Chicago and past president of the AAGL. He is a reproductive endocrinologist and minimally invasive gynecologic surgeon in metropolitan Chicago and the director of minimally invasive gynecologic surgery at Advocate Lutheran General Hospital, Park Ridge, Ill. Dr. Miller disclosed that he is a consultant for Gynesonics and holds a stock option agreement with the company.
References
1. Am J Obstet Gynecol. 2013 Oct;209(4):319.e1-319.e20.
2. Int J Hyperthermia. 2019;36(1):295-301.
3. Curr Obstet Gynecol Rep. 2017; 6(1): 67-73.
4. Obstet Gynecol. 2019 Jan;133(1):13-22.