User login
CASE Somnolent, confused, and hungry
Mr. G, age 14, presents to the emergency department (ED) for acute-onset hypersomnia that has gradually worsened over the course of a few days. Mr. G now sleeps most of the day, has altered mental status, and is experiencing emotional dysregulation with no clear etiology. His mother, who accompanies him to the ED, says that prior to the onset of these symptoms her son had been healthy. She notes that he has been eating more than usual, which she assumes is due to a growth spurt.
Mr. G’s symptoms began 4 days ago when he became increasingly fatigued, sleeping for 11 to 12 hours per day, with intermittent episodes of staring and unresponsiveness from which he rapidly returned to baseline. During the next 3 days, he became more confused and somnolent, and began to display bizarre behavior, including eating food out of the trash and attempting to microwave a full metal pot. He exhibited unexplained crying spells, calling out for his “mommy,” and saying he was “afraid I’m dying.”
During the 2 days before he came to our ED, Mr. G was seen at 2 other hospitals. Following extensive imaging and laboratory work-up, clinicians at these facilities attributed his symptoms to intoxication from an unknown substance. Mr. G has a history of marijuana use, and his mother reports that his friends had recently been using synthetic marijuana. However, no intoxicant was identified on urine or gas chromatography drug screening.
Mr. G’s history includes oppositional behavior, and a brief psychiatric hospitalization at age 5 for aggression. He has otherwise been healthy. His family history is significant for maternal substance use and anxiety disorders. In addition to sporadic cannabis use, Mr. G’s social history includes multiple recent family losses, previous foster care placement, and recent declining academic performance.
[polldaddy:10148168]
EVALUATION No red flags
On admission, Mr. G appears somnolent and displays disorganized speech, impulsivity, frequent disorientation, and intermittent agitation/anxiety; he sleeps 16 to 18 hours per day. Mr. G is admitted with a presumptive diagnosis of substance intoxication and transferred to the general pediatric inpatient unit. Upon arrival, he is found to be bradycardic (42 beats per minute), although afebrile with otherwise age-appropriate vitals. On exam, he is somnolent but arousable and follows simple commands.
Continue to: Mr. G undergoes a Monospot test...
Mr. G undergoes a Monospot test, which is positive, with subsequent evidence of a prior, but not active, Epstein-Barr virus (EBV) infection. He also has a mildly elevated CSF protein level, but subsequent CSF labs are negative for both infectious and non-infectious processes. An EEG reveals focal neuronal slowing.
During brief periods of wakefulness, Mr. G calls out to his mother and says, “I’m not going to make it to my birthday,” and “You’re going to have to let me go.” He occasionally becomes combative, pulling at IV lines and swearing at staff. His bradycardia resolves without intervention during his admission. On Day 8 of his hospitalization, Mr. G displays hypersexuality and makes sexually suggestive comments toward female staff members. He also experiences recurrence of hyperphagia.
On Day 10 of his stay on the pediatric unit, because of the emergence of hypersexuality and hyperphagia, along with a largely negative medical evaluation, Mr. G is transferred to the pediatric psychiatric unit for ongoing evaluation and management.
[polldaddy:10148172]
The authors’ observations
Given Mr. G’s rapid onset of confusion, hypersomnia, and emotional dysregulation, our differential diagnosis included delirium of unclear etiology, substance intoxication, autoimmune encephalitis, viral meningitis, heavy metal intoxication, primary psychotic disorder, and KLS. Mr. G underwent an extensive diagnostic evaluation, which was largely unremarkable (Table). He had a mildly elevated CSF protein level, but subsequent CSF labs were negative for both infectious and non-infectious processes. When Mr. G was transferred to the pediatric inpatient psychiatric unit on Day 10, the presumptive diagnosis was KLS.
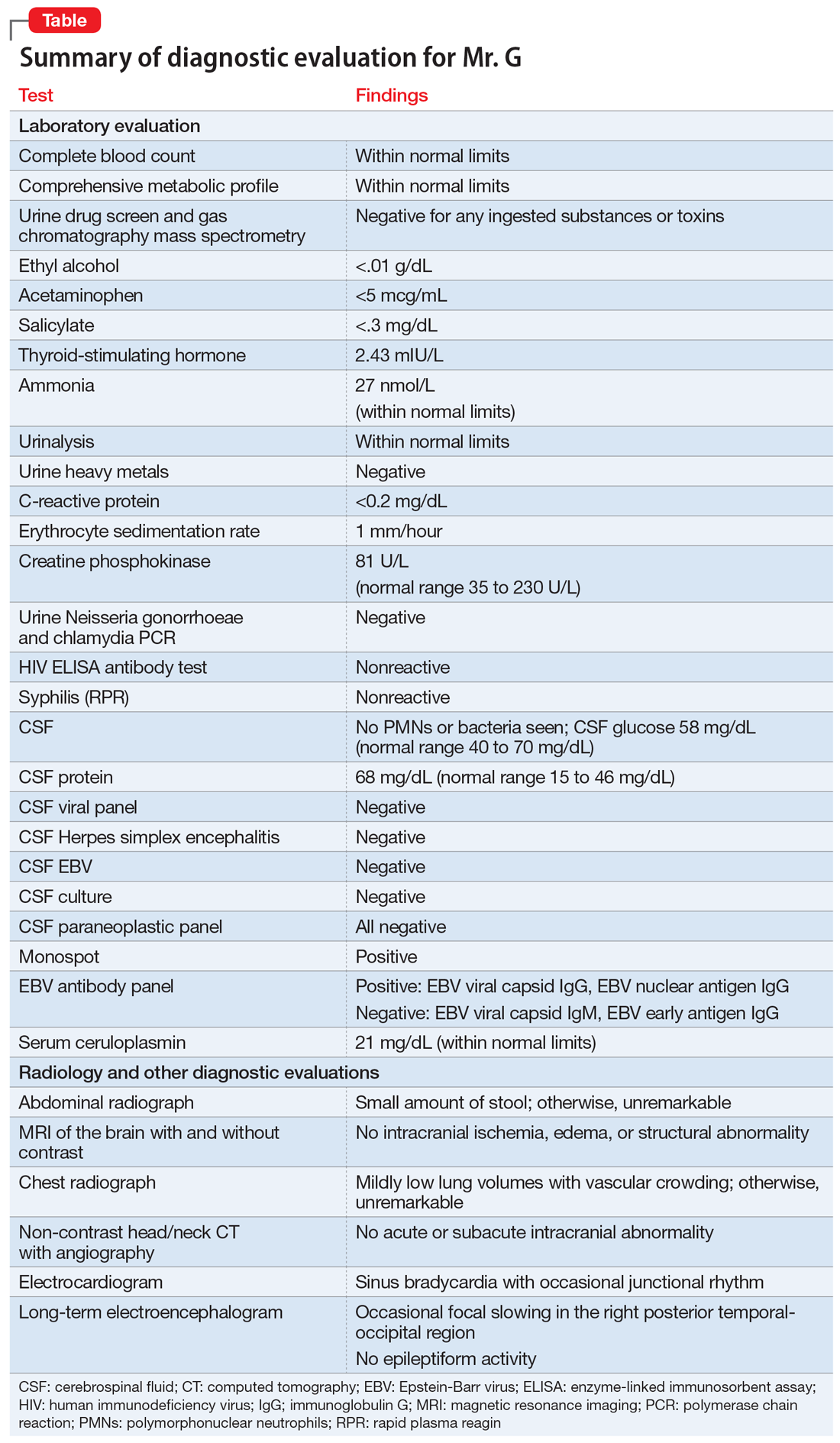
KLS is a rare neurologic disorder, with an incidence of 1 to 5 in 1 million and a 4:1 male-to-female predominance.1 It poses a diagnostic challenge due to its low prevalence and broad differential. The disorder typically presents in early adolescence and is characterized by episodes of severe hypersomnia with associated cognitive and/or behavioral disturbance2 (Box2-4). Bradycardia, as seen in Mr. G, and other forms of autonomic dysregulation have been reported in the literature, as has the focal neuronal slowing noted on Mr. G’s EEG.3

[polldaddy:10148174]
Continue to: TREATMENT Methylphenidate and a safety plan
TREATMENT Methylphenidate and a safety plan
On Day 11 of hospitalization, Mr. G is started on methylphenidate, 10 mg in the morning and 5 mg in the afternoon. After starting methylphenidate, he sustains more regular wakefulness, with improved thought organization, engagement, and fewer disruptive behaviors. He receives infrequent, as-needed doses of olanzapine, and by Day 14, he returns to his baseline behavior and cognition.
A safety plan is created for the family to address worsening symptoms or future episodes. The safety plan is developed with Mr. G and input from his family. It is to be administered in all settings and we particularly emphasized using it in the school setting, where staff may not be familiar with KLS. The safety plan involves a description of KLS, its symptoms, the risks for hypersomnolence, hypersexuality, and psychotic symptoms or behavioral dysregulation. It stresses close supervision of Mr. G, not allowing him to be unsupervised or unchaperoned on school trips or other outings, and lethal means restriction. It outlines a detailed plan if Mr. G’s behavior decompensates or escalates, including a step-wise approach to engaging psychological interventions and mental health resources, and securing crisis services as needed.
On Day 15, he is discharged to home in stable condition with outpatient mental health follow-up and continues to take the prescribed methylphenidate.
The authors’ observations
Management of KLS is primarily supportive. Stimulants may help reduce hypersomnia, impulsivity, and inattention early in the disease course.1 However, in a systematic review, 89% of patients with KLS who received methylphenidate experienced worsening or no improvement, and 11% showed only partial improvement.2 Amantadine was more promising, with 29% of patients with KLS showing partial benefit and 12% showing significant benefit.2 Multiple other pharmacologic agents have been described with varying efficacy, including lithium, valproate, risperidone, bupropion, and immunoglobulins.2 Furthermore, lithium and valproate have been suggested to be helpful in preventing recurrences in some cases.6
The circumstances surrounding Mr. G’s symptom onset are unclear and may have been multifactorial. It is possible that his prior EBV infection was a trigger for this KLS-associated episode, as EBV is a known precipitant for KLS episodes.3 Mr. G’s history of cannabis use may also have served as an early trigger for KLS.3
This case highlights the importance of multidisciplinary collaboration in a diagnostically challenging case. It emphasizes the need for a broad differential and the importance of challenging a previous diagnosis in the face of mounting evidence to the contrary. In this case, the patient’s history of irritability, aggression, and cannabis use resulted in multiple clinicians misattributing his symptoms to substance use or a primary psychiatric disorder. However, given his symptom acuity, progression, and the lack of findings on diagnostic evaluation to explain his presentation, these initial diagnoses did not explain the severity, nature, or duration of his symptoms. Keeping KLS in the differential is particularly important for patients with a prior history of psychiatric illness or substance use, because these patients are at higher risk for misattribution of symptoms to pre-existing psychiatric illness. Evolution of symptoms, a negative diagnostic evaluation, and maintaining a broad differential resulted in eventually reaching the final diagnosis of KLS and development of a longitudinal management plan.
While further work must be done to clearly define the pharmacologic approach to acute management of KLS episodes, nonpharmacologic aspects of care must not be neglected. Behavioral planning, adjustment of the environment, engagement with schools/community supports, and family education are valuable tools for facilitating the patient’s de-escalation, avoiding unneeded polypharmacy, reducing anxieties, and safeguarding the patient from unnecessary harm.7 Clinicians can support their patients’ transitions back into the community by ensuring careful outpatient follow-up for symptom monitoring and by communicating with patients’ schools and employers.
OUTCOME Asymptomatic; no recurrence of symptoms
Forty-six days after his symptoms began and 31 days after hospital discharge, Mr. G is asymptomatic with no recurrence of symptoms.
Bottom Line
Kleine-Levin syndrome (KLS) is a rare, often-overlooked condition that should be considered in the differential diagnosis for patients who present with hypersomnolence and altered mental status without a clear etiology. Rapid recognition of KLS can prevent misattribution of symptoms, unnecessary treatment, and missed opportunities for care.
1. Billiard M, Jaussent I, Dauvilliers Y, et al. Recurrent hypersomnia: a review of 339 cases. Sleep Med. 2011;15(4):247-257.
2. Arnulf I, Lin L, Gadoth N, et al. Kleine-Levin syndrome: a systematic study of 108 patients. Ann Neurol. 2008;63(4):482-493.
3. Arnulf I. Kleine-Levin syndrome: a systematic review of 186 cases in the literature. Brain. 2005;128(12):2763-2776.
4. Lisk R. Kleine-Levin syndrome. Pract Neurol. 2009;9(1);42-45.
5. de Araújo Lima TF, da Silva Behrens NS, Lopes E, et al. Kleine–Levin Syndrome: a case report. Sleep Sci. 2014;7(2):122-125.
6. Goldberg MA. The treatment of Kleine-Levin syndrome with lithium. Can J Psychiatry. 1983;28:491-493.
7. Gadoth N, Kesler A, Vainstein G, et al. Clinical and polysomnographic characteristics of 34 patients with Kleine-Levin syndrome. J Sleep Res. 2001;10(4):337-341.
CASE Somnolent, confused, and hungry
Mr. G, age 14, presents to the emergency department (ED) for acute-onset hypersomnia that has gradually worsened over the course of a few days. Mr. G now sleeps most of the day, has altered mental status, and is experiencing emotional dysregulation with no clear etiology. His mother, who accompanies him to the ED, says that prior to the onset of these symptoms her son had been healthy. She notes that he has been eating more than usual, which she assumes is due to a growth spurt.
Mr. G’s symptoms began 4 days ago when he became increasingly fatigued, sleeping for 11 to 12 hours per day, with intermittent episodes of staring and unresponsiveness from which he rapidly returned to baseline. During the next 3 days, he became more confused and somnolent, and began to display bizarre behavior, including eating food out of the trash and attempting to microwave a full metal pot. He exhibited unexplained crying spells, calling out for his “mommy,” and saying he was “afraid I’m dying.”
During the 2 days before he came to our ED, Mr. G was seen at 2 other hospitals. Following extensive imaging and laboratory work-up, clinicians at these facilities attributed his symptoms to intoxication from an unknown substance. Mr. G has a history of marijuana use, and his mother reports that his friends had recently been using synthetic marijuana. However, no intoxicant was identified on urine or gas chromatography drug screening.
Mr. G’s history includes oppositional behavior, and a brief psychiatric hospitalization at age 5 for aggression. He has otherwise been healthy. His family history is significant for maternal substance use and anxiety disorders. In addition to sporadic cannabis use, Mr. G’s social history includes multiple recent family losses, previous foster care placement, and recent declining academic performance.
[polldaddy:10148168]
EVALUATION No red flags
On admission, Mr. G appears somnolent and displays disorganized speech, impulsivity, frequent disorientation, and intermittent agitation/anxiety; he sleeps 16 to 18 hours per day. Mr. G is admitted with a presumptive diagnosis of substance intoxication and transferred to the general pediatric inpatient unit. Upon arrival, he is found to be bradycardic (42 beats per minute), although afebrile with otherwise age-appropriate vitals. On exam, he is somnolent but arousable and follows simple commands.
Continue to: Mr. G undergoes a Monospot test...
Mr. G undergoes a Monospot test, which is positive, with subsequent evidence of a prior, but not active, Epstein-Barr virus (EBV) infection. He also has a mildly elevated CSF protein level, but subsequent CSF labs are negative for both infectious and non-infectious processes. An EEG reveals focal neuronal slowing.
During brief periods of wakefulness, Mr. G calls out to his mother and says, “I’m not going to make it to my birthday,” and “You’re going to have to let me go.” He occasionally becomes combative, pulling at IV lines and swearing at staff. His bradycardia resolves without intervention during his admission. On Day 8 of his hospitalization, Mr. G displays hypersexuality and makes sexually suggestive comments toward female staff members. He also experiences recurrence of hyperphagia.
On Day 10 of his stay on the pediatric unit, because of the emergence of hypersexuality and hyperphagia, along with a largely negative medical evaluation, Mr. G is transferred to the pediatric psychiatric unit for ongoing evaluation and management.
[polldaddy:10148172]
The authors’ observations
Given Mr. G’s rapid onset of confusion, hypersomnia, and emotional dysregulation, our differential diagnosis included delirium of unclear etiology, substance intoxication, autoimmune encephalitis, viral meningitis, heavy metal intoxication, primary psychotic disorder, and KLS. Mr. G underwent an extensive diagnostic evaluation, which was largely unremarkable (Table). He had a mildly elevated CSF protein level, but subsequent CSF labs were negative for both infectious and non-infectious processes. When Mr. G was transferred to the pediatric inpatient psychiatric unit on Day 10, the presumptive diagnosis was KLS.

KLS is a rare neurologic disorder, with an incidence of 1 to 5 in 1 million and a 4:1 male-to-female predominance.1 It poses a diagnostic challenge due to its low prevalence and broad differential. The disorder typically presents in early adolescence and is characterized by episodes of severe hypersomnia with associated cognitive and/or behavioral disturbance2 (Box2-4). Bradycardia, as seen in Mr. G, and other forms of autonomic dysregulation have been reported in the literature, as has the focal neuronal slowing noted on Mr. G’s EEG.3

[polldaddy:10148174]
Continue to: TREATMENT Methylphenidate and a safety plan
TREATMENT Methylphenidate and a safety plan
On Day 11 of hospitalization, Mr. G is started on methylphenidate, 10 mg in the morning and 5 mg in the afternoon. After starting methylphenidate, he sustains more regular wakefulness, with improved thought organization, engagement, and fewer disruptive behaviors. He receives infrequent, as-needed doses of olanzapine, and by Day 14, he returns to his baseline behavior and cognition.
A safety plan is created for the family to address worsening symptoms or future episodes. The safety plan is developed with Mr. G and input from his family. It is to be administered in all settings and we particularly emphasized using it in the school setting, where staff may not be familiar with KLS. The safety plan involves a description of KLS, its symptoms, the risks for hypersomnolence, hypersexuality, and psychotic symptoms or behavioral dysregulation. It stresses close supervision of Mr. G, not allowing him to be unsupervised or unchaperoned on school trips or other outings, and lethal means restriction. It outlines a detailed plan if Mr. G’s behavior decompensates or escalates, including a step-wise approach to engaging psychological interventions and mental health resources, and securing crisis services as needed.
On Day 15, he is discharged to home in stable condition with outpatient mental health follow-up and continues to take the prescribed methylphenidate.
The authors’ observations
Management of KLS is primarily supportive. Stimulants may help reduce hypersomnia, impulsivity, and inattention early in the disease course.1 However, in a systematic review, 89% of patients with KLS who received methylphenidate experienced worsening or no improvement, and 11% showed only partial improvement.2 Amantadine was more promising, with 29% of patients with KLS showing partial benefit and 12% showing significant benefit.2 Multiple other pharmacologic agents have been described with varying efficacy, including lithium, valproate, risperidone, bupropion, and immunoglobulins.2 Furthermore, lithium and valproate have been suggested to be helpful in preventing recurrences in some cases.6
The circumstances surrounding Mr. G’s symptom onset are unclear and may have been multifactorial. It is possible that his prior EBV infection was a trigger for this KLS-associated episode, as EBV is a known precipitant for KLS episodes.3 Mr. G’s history of cannabis use may also have served as an early trigger for KLS.3
This case highlights the importance of multidisciplinary collaboration in a diagnostically challenging case. It emphasizes the need for a broad differential and the importance of challenging a previous diagnosis in the face of mounting evidence to the contrary. In this case, the patient’s history of irritability, aggression, and cannabis use resulted in multiple clinicians misattributing his symptoms to substance use or a primary psychiatric disorder. However, given his symptom acuity, progression, and the lack of findings on diagnostic evaluation to explain his presentation, these initial diagnoses did not explain the severity, nature, or duration of his symptoms. Keeping KLS in the differential is particularly important for patients with a prior history of psychiatric illness or substance use, because these patients are at higher risk for misattribution of symptoms to pre-existing psychiatric illness. Evolution of symptoms, a negative diagnostic evaluation, and maintaining a broad differential resulted in eventually reaching the final diagnosis of KLS and development of a longitudinal management plan.
While further work must be done to clearly define the pharmacologic approach to acute management of KLS episodes, nonpharmacologic aspects of care must not be neglected. Behavioral planning, adjustment of the environment, engagement with schools/community supports, and family education are valuable tools for facilitating the patient’s de-escalation, avoiding unneeded polypharmacy, reducing anxieties, and safeguarding the patient from unnecessary harm.7 Clinicians can support their patients’ transitions back into the community by ensuring careful outpatient follow-up for symptom monitoring and by communicating with patients’ schools and employers.
OUTCOME Asymptomatic; no recurrence of symptoms
Forty-six days after his symptoms began and 31 days after hospital discharge, Mr. G is asymptomatic with no recurrence of symptoms.
Bottom Line
Kleine-Levin syndrome (KLS) is a rare, often-overlooked condition that should be considered in the differential diagnosis for patients who present with hypersomnolence and altered mental status without a clear etiology. Rapid recognition of KLS can prevent misattribution of symptoms, unnecessary treatment, and missed opportunities for care.
CASE Somnolent, confused, and hungry
Mr. G, age 14, presents to the emergency department (ED) for acute-onset hypersomnia that has gradually worsened over the course of a few days. Mr. G now sleeps most of the day, has altered mental status, and is experiencing emotional dysregulation with no clear etiology. His mother, who accompanies him to the ED, says that prior to the onset of these symptoms her son had been healthy. She notes that he has been eating more than usual, which she assumes is due to a growth spurt.
Mr. G’s symptoms began 4 days ago when he became increasingly fatigued, sleeping for 11 to 12 hours per day, with intermittent episodes of staring and unresponsiveness from which he rapidly returned to baseline. During the next 3 days, he became more confused and somnolent, and began to display bizarre behavior, including eating food out of the trash and attempting to microwave a full metal pot. He exhibited unexplained crying spells, calling out for his “mommy,” and saying he was “afraid I’m dying.”
During the 2 days before he came to our ED, Mr. G was seen at 2 other hospitals. Following extensive imaging and laboratory work-up, clinicians at these facilities attributed his symptoms to intoxication from an unknown substance. Mr. G has a history of marijuana use, and his mother reports that his friends had recently been using synthetic marijuana. However, no intoxicant was identified on urine or gas chromatography drug screening.
Mr. G’s history includes oppositional behavior, and a brief psychiatric hospitalization at age 5 for aggression. He has otherwise been healthy. His family history is significant for maternal substance use and anxiety disorders. In addition to sporadic cannabis use, Mr. G’s social history includes multiple recent family losses, previous foster care placement, and recent declining academic performance.
[polldaddy:10148168]
EVALUATION No red flags
On admission, Mr. G appears somnolent and displays disorganized speech, impulsivity, frequent disorientation, and intermittent agitation/anxiety; he sleeps 16 to 18 hours per day. Mr. G is admitted with a presumptive diagnosis of substance intoxication and transferred to the general pediatric inpatient unit. Upon arrival, he is found to be bradycardic (42 beats per minute), although afebrile with otherwise age-appropriate vitals. On exam, he is somnolent but arousable and follows simple commands.
Continue to: Mr. G undergoes a Monospot test...
Mr. G undergoes a Monospot test, which is positive, with subsequent evidence of a prior, but not active, Epstein-Barr virus (EBV) infection. He also has a mildly elevated CSF protein level, but subsequent CSF labs are negative for both infectious and non-infectious processes. An EEG reveals focal neuronal slowing.
During brief periods of wakefulness, Mr. G calls out to his mother and says, “I’m not going to make it to my birthday,” and “You’re going to have to let me go.” He occasionally becomes combative, pulling at IV lines and swearing at staff. His bradycardia resolves without intervention during his admission. On Day 8 of his hospitalization, Mr. G displays hypersexuality and makes sexually suggestive comments toward female staff members. He also experiences recurrence of hyperphagia.
On Day 10 of his stay on the pediatric unit, because of the emergence of hypersexuality and hyperphagia, along with a largely negative medical evaluation, Mr. G is transferred to the pediatric psychiatric unit for ongoing evaluation and management.
[polldaddy:10148172]
The authors’ observations
Given Mr. G’s rapid onset of confusion, hypersomnia, and emotional dysregulation, our differential diagnosis included delirium of unclear etiology, substance intoxication, autoimmune encephalitis, viral meningitis, heavy metal intoxication, primary psychotic disorder, and KLS. Mr. G underwent an extensive diagnostic evaluation, which was largely unremarkable (Table). He had a mildly elevated CSF protein level, but subsequent CSF labs were negative for both infectious and non-infectious processes. When Mr. G was transferred to the pediatric inpatient psychiatric unit on Day 10, the presumptive diagnosis was KLS.

KLS is a rare neurologic disorder, with an incidence of 1 to 5 in 1 million and a 4:1 male-to-female predominance.1 It poses a diagnostic challenge due to its low prevalence and broad differential. The disorder typically presents in early adolescence and is characterized by episodes of severe hypersomnia with associated cognitive and/or behavioral disturbance2 (Box2-4). Bradycardia, as seen in Mr. G, and other forms of autonomic dysregulation have been reported in the literature, as has the focal neuronal slowing noted on Mr. G’s EEG.3

[polldaddy:10148174]
Continue to: TREATMENT Methylphenidate and a safety plan
TREATMENT Methylphenidate and a safety plan
On Day 11 of hospitalization, Mr. G is started on methylphenidate, 10 mg in the morning and 5 mg in the afternoon. After starting methylphenidate, he sustains more regular wakefulness, with improved thought organization, engagement, and fewer disruptive behaviors. He receives infrequent, as-needed doses of olanzapine, and by Day 14, he returns to his baseline behavior and cognition.
A safety plan is created for the family to address worsening symptoms or future episodes. The safety plan is developed with Mr. G and input from his family. It is to be administered in all settings and we particularly emphasized using it in the school setting, where staff may not be familiar with KLS. The safety plan involves a description of KLS, its symptoms, the risks for hypersomnolence, hypersexuality, and psychotic symptoms or behavioral dysregulation. It stresses close supervision of Mr. G, not allowing him to be unsupervised or unchaperoned on school trips or other outings, and lethal means restriction. It outlines a detailed plan if Mr. G’s behavior decompensates or escalates, including a step-wise approach to engaging psychological interventions and mental health resources, and securing crisis services as needed.
On Day 15, he is discharged to home in stable condition with outpatient mental health follow-up and continues to take the prescribed methylphenidate.
The authors’ observations
Management of KLS is primarily supportive. Stimulants may help reduce hypersomnia, impulsivity, and inattention early in the disease course.1 However, in a systematic review, 89% of patients with KLS who received methylphenidate experienced worsening or no improvement, and 11% showed only partial improvement.2 Amantadine was more promising, with 29% of patients with KLS showing partial benefit and 12% showing significant benefit.2 Multiple other pharmacologic agents have been described with varying efficacy, including lithium, valproate, risperidone, bupropion, and immunoglobulins.2 Furthermore, lithium and valproate have been suggested to be helpful in preventing recurrences in some cases.6
The circumstances surrounding Mr. G’s symptom onset are unclear and may have been multifactorial. It is possible that his prior EBV infection was a trigger for this KLS-associated episode, as EBV is a known precipitant for KLS episodes.3 Mr. G’s history of cannabis use may also have served as an early trigger for KLS.3
This case highlights the importance of multidisciplinary collaboration in a diagnostically challenging case. It emphasizes the need for a broad differential and the importance of challenging a previous diagnosis in the face of mounting evidence to the contrary. In this case, the patient’s history of irritability, aggression, and cannabis use resulted in multiple clinicians misattributing his symptoms to substance use or a primary psychiatric disorder. However, given his symptom acuity, progression, and the lack of findings on diagnostic evaluation to explain his presentation, these initial diagnoses did not explain the severity, nature, or duration of his symptoms. Keeping KLS in the differential is particularly important for patients with a prior history of psychiatric illness or substance use, because these patients are at higher risk for misattribution of symptoms to pre-existing psychiatric illness. Evolution of symptoms, a negative diagnostic evaluation, and maintaining a broad differential resulted in eventually reaching the final diagnosis of KLS and development of a longitudinal management plan.
While further work must be done to clearly define the pharmacologic approach to acute management of KLS episodes, nonpharmacologic aspects of care must not be neglected. Behavioral planning, adjustment of the environment, engagement with schools/community supports, and family education are valuable tools for facilitating the patient’s de-escalation, avoiding unneeded polypharmacy, reducing anxieties, and safeguarding the patient from unnecessary harm.7 Clinicians can support their patients’ transitions back into the community by ensuring careful outpatient follow-up for symptom monitoring and by communicating with patients’ schools and employers.
OUTCOME Asymptomatic; no recurrence of symptoms
Forty-six days after his symptoms began and 31 days after hospital discharge, Mr. G is asymptomatic with no recurrence of symptoms.
Bottom Line
Kleine-Levin syndrome (KLS) is a rare, often-overlooked condition that should be considered in the differential diagnosis for patients who present with hypersomnolence and altered mental status without a clear etiology. Rapid recognition of KLS can prevent misattribution of symptoms, unnecessary treatment, and missed opportunities for care.
1. Billiard M, Jaussent I, Dauvilliers Y, et al. Recurrent hypersomnia: a review of 339 cases. Sleep Med. 2011;15(4):247-257.
2. Arnulf I, Lin L, Gadoth N, et al. Kleine-Levin syndrome: a systematic study of 108 patients. Ann Neurol. 2008;63(4):482-493.
3. Arnulf I. Kleine-Levin syndrome: a systematic review of 186 cases in the literature. Brain. 2005;128(12):2763-2776.
4. Lisk R. Kleine-Levin syndrome. Pract Neurol. 2009;9(1);42-45.
5. de Araújo Lima TF, da Silva Behrens NS, Lopes E, et al. Kleine–Levin Syndrome: a case report. Sleep Sci. 2014;7(2):122-125.
6. Goldberg MA. The treatment of Kleine-Levin syndrome with lithium. Can J Psychiatry. 1983;28:491-493.
7. Gadoth N, Kesler A, Vainstein G, et al. Clinical and polysomnographic characteristics of 34 patients with Kleine-Levin syndrome. J Sleep Res. 2001;10(4):337-341.
1. Billiard M, Jaussent I, Dauvilliers Y, et al. Recurrent hypersomnia: a review of 339 cases. Sleep Med. 2011;15(4):247-257.
2. Arnulf I, Lin L, Gadoth N, et al. Kleine-Levin syndrome: a systematic study of 108 patients. Ann Neurol. 2008;63(4):482-493.
3. Arnulf I. Kleine-Levin syndrome: a systematic review of 186 cases in the literature. Brain. 2005;128(12):2763-2776.
4. Lisk R. Kleine-Levin syndrome. Pract Neurol. 2009;9(1);42-45.
5. de Araújo Lima TF, da Silva Behrens NS, Lopes E, et al. Kleine–Levin Syndrome: a case report. Sleep Sci. 2014;7(2):122-125.
6. Goldberg MA. The treatment of Kleine-Levin syndrome with lithium. Can J Psychiatry. 1983;28:491-493.
7. Gadoth N, Kesler A, Vainstein G, et al. Clinical and polysomnographic characteristics of 34 patients with Kleine-Levin syndrome. J Sleep Res. 2001;10(4):337-341.