User login
Peripheral intravenous catheters (PIVCs) are fundamental to the healthcare practitioners’ ability to provide vital intravenous fluids, medications, and blood products, and as a prophylactic measure prior to some procedures, making insertion of these devices the most common in-hospital invasive procedure in pediatrics.1,2 Despite the prevalence and ubiquity of PIVCs,1 successful insertion in pediatrics is problematic,3-5 and device dysfunction prior to completion of treatment is common.3,6 The inability to attain timely PIVC access and maintain postinsertion function has significant short- and long-term sequelae, including pain and anxiety for children and their parents,3,7 delays in treatment,3 prolonged hospitalization,8 and increased healthcare-associated costs.8-10
Approximately 50% of pediatric PIVC insertions are challenging, often requiring upwards of four insertion attempts, and a similar proportion fail prior to treatment completion.3,11 Exactly why PIVC insertion is difficult in children, and the mechanisms of failure, are unknown. It is likely to be multifaceted and related to factors concerning the patient (eg, comorbidities, age, gender, adiposity),11,12 provider (eg, insertion practice, care, and maintenance),3,13,14 device (eg, size, length, catheter-to-vein ratio),15,16 and therapy (eg, vessel irritation).11,13,17 Observational studies and randomized controlled trials (RCTs) in hospitalized pediatric patients report that the average PIVC dwell is approximately 48 hours, suggesting multiple PIVCs are required to complete a single admission.3,18
Conventionally, PIVC insertion involved physical assessment through palpation and visualization (landmark approach), and although postinsertion care varies among healthcare facilities, minimal requirements are a dressing over the insertion site and regular flushes to ensure device patency.1,3,19 Recently, clinicians have investigated insertion and management practices to improve PIVC outcomes. These can be grouped into techniques—the art of doing (the manner of performance, or the details, of any surgical operation, experiment, or mechanical act) and technologies—the application of scientific knowledge for practical purposes.20 Individual studies have examined the outcomes of new techniques and technologies; however, an overall estimation of their clinical significance or effect is unknown.11,18 Therefore, the aim of this review was to systematically search published studies, conduct a pooled analysis of findings, and report the success of various techniques and technologies to improve insertion success and reduce overall PIVC failure.
METHODS
Design
The protocol for this systematic review was prospectively registered with PROSPERO (CRD42020165288). This review followed Cochrane Collaboration systematic review methods21 and was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.22
Inclusion and Exclusion Criteria
Studies were eligible for inclusion if they met predefined criteria: (1) RCT design; (2) included standard-length PIVC; (3) participants aged 0 to 18 years, excluding preterm infants (less than 36 weeks’ gestation); (4) required PIVC insertion in an inpatient healthcare setting; and (5) reported PIVC insertion outcomes (described below). Studies were excluded if they were cluster or crossover RCTs, published before 2010, or not written in English.
Interventions
Interventions were PIVC insertion and management techniques, defined as “the manner of performance, or the details, of any surgical operation, experiment, or mechanical act” (eg, needle-tip positioning, vein selection [site of insertion], comfort measures, and flushing regimen), or technologies, defined as “the application of scientific knowledge for practical purpose” (eg, vessel visualization, catheter material, and catheter design), compared with current practice, defined as commonly known, practiced, or accepted (eg, landmark PIVC insertion).20
Primary and Secondary Outcomes
The primary outcome was first-time insertion success (one skin puncture to achieve PIVC insertion; can aspirate and flush PIVC without resistance).23 Secondary outcomes included: (1) overall PIVC insertion success23; (2) all-cause PIVC failure (cessation of PIVC function prior to treatment completion)6; (3) dwell time14; (4) PIVC insertion time; (5) insertion attempts23; (6) individual elements of failure (dislodgement, extravasation, infection, occlusion, pain, phlebitis, and thrombosis)6; and (7) patient/parent satisfaction. Some outcomes evaluated were author defined within each study (patient/parent satisfaction, pain score).
Systematic Search
A search of the Cochrane Library and Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health (CINAHL), US National Institutes of Health National Library of Medicine (PubMed), and Embase databases between 2010 to 2020 was undertaken on June 23, 2020, and updated March 4, 2021. Medical Subject Heading (MeSH) terms and relevant keywords and their variants were used in collaboration with a healthcare librarian (Appendix Table 1). Additional studies were identified through hand searches of bibliographies.19 Studies were included if two authors (TMK and JS) independently agreed they met the inclusion criteria.
Data Extraction
Two authors (TMK/JS) independently abstracted study data using a standardized form managed in Microsoft Excel.
Quality Assessment
Included studies were assessed by two authors (TMK and JS) for quality using the Cochrane risk of bias (RoB2) tool.21,24 The overall quality of evidence for each outcome was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE)25 approach. Individual RCTs began at high quality, downgraded by one level for “serious” or two levels for “very serious” study limitations, including high risk of bias, serious inconsistency, publication bias, or indirectness of evidence.
Data Analysis and Synthesis
Where two or more trials with evidence of study homogeneity (trial interventions and population) were identified, meta-analysis using RevMan 5 (version 5.4.1)26 with random effects was conducted. Descriptive statistics summarized study population, interventions, and results. For dichotomous outcomes, we calculated risk ratio (RR) plus 95% CI. For continuous outcomes, we planned to calculate the mean difference (MD) plus 95% CI and the standardized mean difference (SMD) (difference between experimental and control groups across trials) reported as the summary statistic.
Subgroup analyses, where possible, included: difficult intravenous access (DIVA), defined by study authors; age (0-3 years; >3 years up to 18 years); hospital setting during PIVC insertion (awake clinical environment vs awake emergency department vs asleep operating room setting); and by operator (bedside nurse, anesthesiologist).
RESULTS
Search Strategy
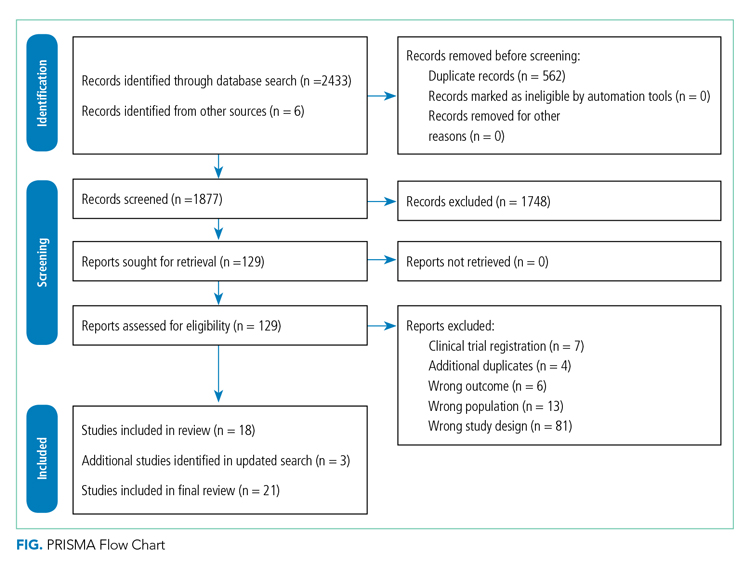
Figure 1 describes study selection in accordance with the PRISMA guidelines.22 We identified 1877 records, and 18 articles met the inclusion criteria. An additional 3 studies were identified in the updated search, totaling 21 studies included in the final review.
Study Characteristics
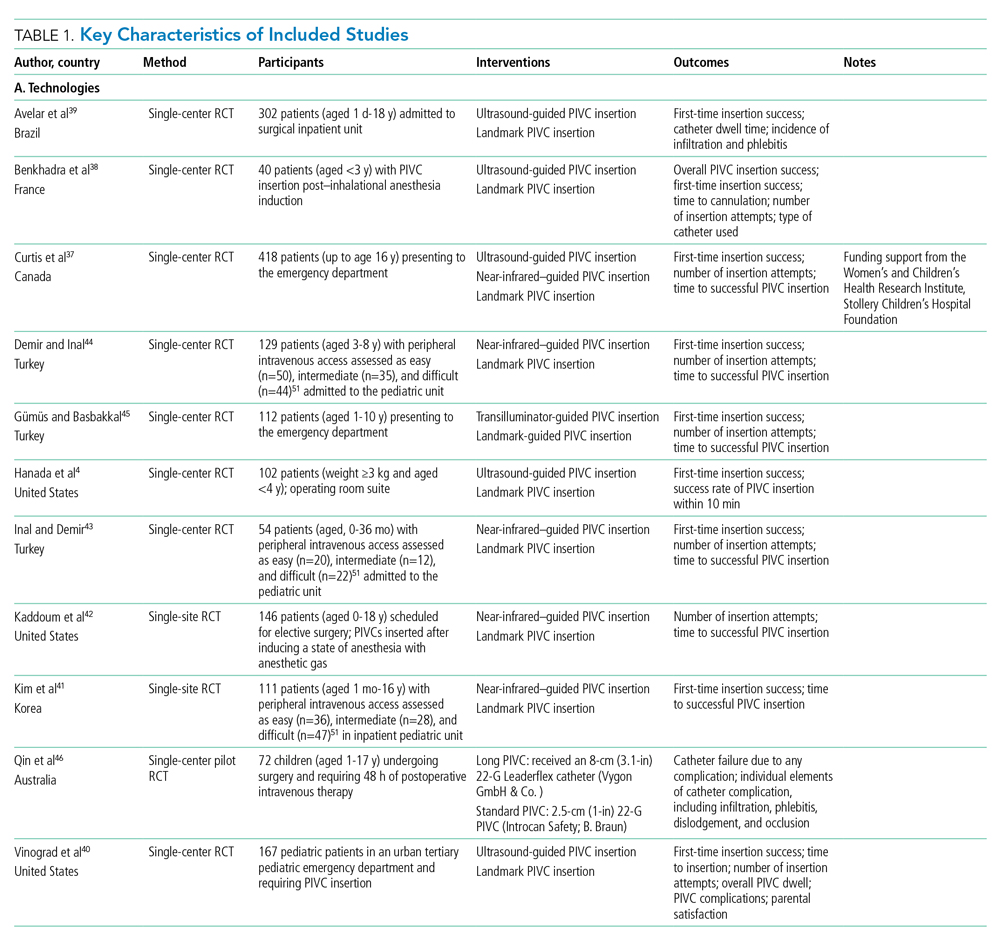
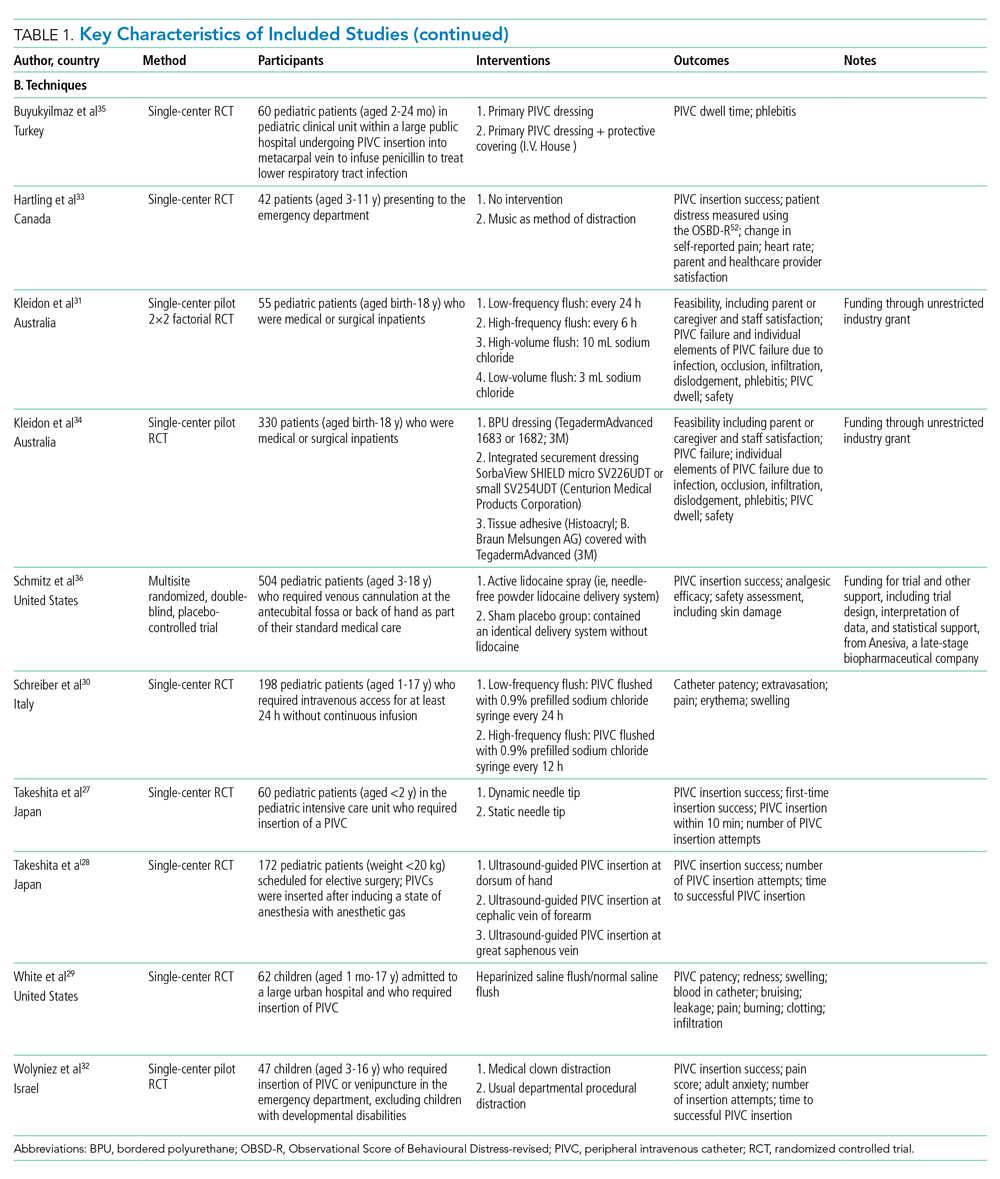
Collectively, 3237 patients and 3098 successful PIVC insertions were reported. In the included studies, 139 patients did not receive a PIVC owing to failed insertion. Ten studies examined techniques (needle-tip positioning,27 vein choice for PIVC insertion,28 flushing regimen,29-31 nonpharmacological32,33 dressing and securement,34,35 and pharmacological comfort measures36), and 11 studies examined technologies (vessel visualization including ultrasound,4,37-40 near-infrared [image of vein projected onto the skin],37,41-44 transillumination [transmission of light through the skin],45 and catheter design46). Table 1 outlines characteristics of included studies. Most trials were single center and conducted in an acute inpatient pediatric-specific setting4,27-34,36-41,44-46 or dedicated pediatric unit in a large public hospital35,43,44; one study was a multicenter trial.36 All trials described evidence of ethical review board approval and participant consent for trial participation.
Study Quality
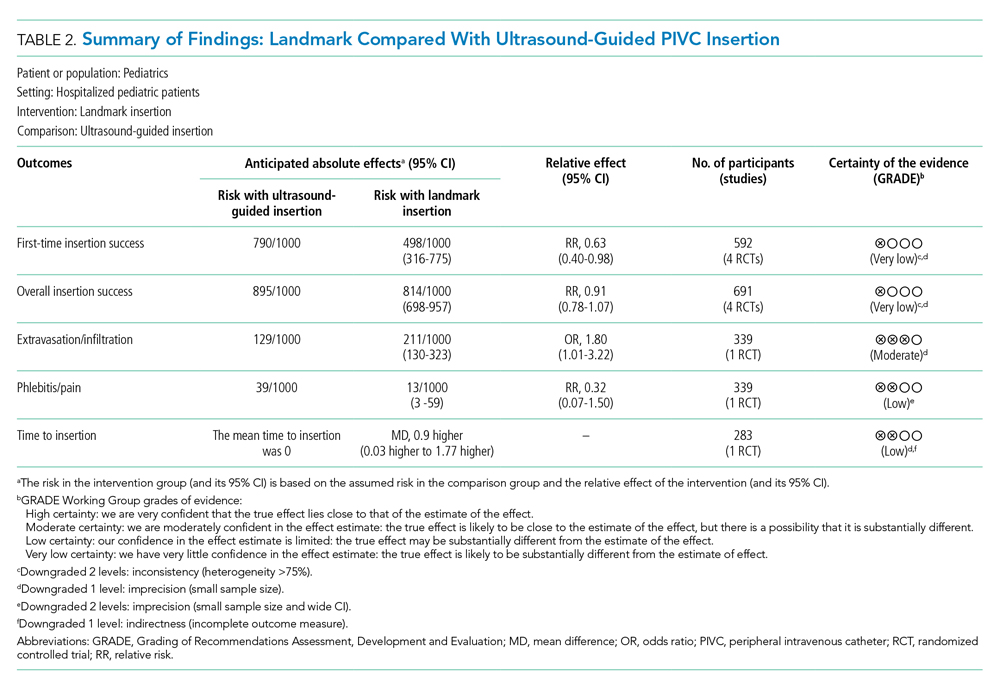
The certainty of evidence at the outcome level varied from moderate to very low. Table 2 and Table 3 outline the summary of findings for landmark insertion compared with ultrasound-guided and landmark insertion compared with near-infrared PIVC insertion, respectively. The remaining summary-of-findings comparisons that included more than one study or addressed clinically relevant questions can be found in Appendix Tables 2, 3, 4, 5, 6, 7, and 8. At the individual study level, most domains were assessed as low risk of bias (Appendix Figure 1).
Effectiveness of Interventions
Technology to Improve PIVC Outcomes
Landmark compared with ultrasound-guided PIVC insertion. Five studies compared PIVC insertion success outcomes when traditional landmark technique was used in comparison with ultrasound guidance (Appendix Figure 2). Four studies (592 patients)4,37,38,40 assessed the primary outcome of first-time insertion success. Appendix Figure 2.1 demonstrates PIVCs were 1.5 times more likely to be inserted on first attempt when ultrasound guidance was used compared with landmark insertion (RR, 1.60; 95% CI, 1.02-2.50). When examining only studies that included DIVA,4,38,40 the effect size increased and CIs tightened (RR, 1.87; 95% CI, 1.56-2.24). No evidence of effect was demonstrated when comparing this outcome in children aged 0 to 3 years (RR, 1.39; 95% CI, 0.88-2.18) or >3 years (RR, 0.72; 95% CI, 0.35-1.51. Two studies4,38 demonstrated that first-time insertion success with ultrasound (compared with landmark) was almost twice as likely (RR, 1.87; 95% CI, 1.44-2.42) after induction of anesthesia in contrast to no effect in studies undertaken in the emergency department37,40 (RR, 1.32; 95% CI, 0.68-2.56). One study39 (339 patients) reported the secondary outcomes of extravasation/infiltration and phlebitis. Extravasation/infiltration was nearly twice as likely with ultrasound compared with landmark insertion (RR, 1.80; 95% CI, 1.01-3.22); however, there was no evidence of effect related to phlebitis (RR, 0.32; 95% CI, 0.07-1.50).
Four studies4,38-40 compared the review’s secondary outcome of PIVC insertion success (Appendix Figure 2.2), with no evidence of an effect (RR, 1.10; 95% CI, 0.94-1.28). No improvement in overall insertion success was demonstrated in the following subgroup analyses: patients with DIVA (RR, 1.18; 95% CI, 0.95-1.47), children under 3 years of age (RR, 1.23; 95% CI, 0.90-1.68), and PIVCs inserted by anesthesiologists (RR, 1.25; 95% CI, 0.91-1.72). One study measured this outcome in children aged >3 years (RR, 1.13; 95% CI, 0.99-1.29) with no effect and in the emergency department (RR, 1.09; 95% CI, 1.00-1.20), where ultrasound guidance improved overall PIVC insertion success.
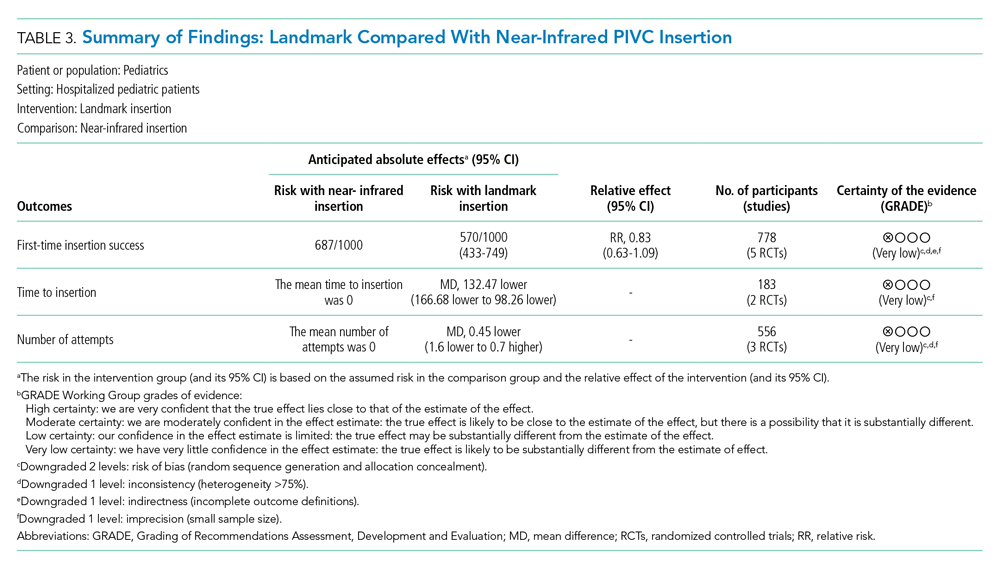
Landmark compared with near-infrared PIVC insertion. First-time insertion success (Appendix Figure 3.1) was reported in five studies37,41-44 and 778 patients with no evidence of effect (RR, 1.21; 95% CI, 0.91-1.59). Subgroup analysis by DIVA41-44 demonstrated first-time insertion success more than doubled with near-infrared technology compared with landmark (RR, 2.72; 95% CI, 1.02-7.24). Subgroup analysis by age did not demonstrate an effect in children younger than 3 years or children older than 3 years. Subgroup analysis by clinician inserting did not demonstrate an effect. Of the five studies reporting time to insertion,37,41-44 two41,42 reported median rather than mean, so could not be included in the analysis. Of the remaining three studies,37,43,44 near-infrared reduced PIVC time to insertion (Appendix Figure 3.2).
Four studies37,42-44 reported the number of attempts required for successful PIVC insertion where no difference was detected; however, subgroup analysis of patients with DIVA43,44 and insertion by bedside nurse43,44 demonstrated fewer PIVC insertion attempts and a reduction in insertion time, respectively, with the use of near-infrared technology (Appendix Figure 3.3).
Landmark compared with transillumination PIVC insertion. One study45 (112 participants) found a positive effect with the use of transillumination and first-time insertion success (RR, 1.29; 95% CI, 1.07-1.54), reduced time to insertion (MD, –9.70; 95% CI, –17.40 to –2.00), and fewer insertion attempts (MD, –0.24; 95% CI, –0.40 to –0.08) compared with landmark insertion.
Long PIVC compared with short PIVC. A single study46 demonstrated a 70% reduction in PIVC failure (RR, 0.29; 95% CI, 0.14-0.59) when long PIVCs were compared with standard PIVCs. Specifically, PIVC failure due to infiltration was reduced with the use of a long PIVC (RR, 0.08; 95% CI, 0.01-0.61). There was no difference in insertion success (RR, 1.00; 95% CI, 0.95-1.05) or phlebitis (RR, 1.00; 95% CI, 0.07-15.38).
Technique to Improve PIVC Outcomes
Static ultrasound-guided compared with dynamic needle-tip PIVC insertion. In a single study comparing variation in ultrasound-guided PIVC insertion technique27 (60 patients), dynamic needle-tip positioning improved first-time insertion success (RR, 1.44; 95% CI, 1.04-2.00) and overall PIVC insertion success (RR, 1.42; 95% CI, 1.06-1.91).
Variation in vein choice for successful PIVC insertion. Insertion of PIVC in the cephalic vein of the forearm improved insertion success in a single study28 of 172 patients compared with insertion in the dorsal vein of the hand (RR, 1.39; 95% CI, 1.15-1.69) and great saphenous vein (RR, 1.27; 95% CI, 1.08-1.49).
Variation in PIVC flush. Heparinized saline compared with 0.9% sodium chloride flush29 did not reduce infiltration (RR, 0.31; 95% CI, 0.03-2.84), occlusion (RR, 1.88; 95% CI, 0.18-19.63) during dwell, or hematoma (RR, 0.94; 95% CI, 0.06-14.33) at insertion.
Two studies30,31 (253 participants) compared PIVC flush frequency (daily compared with more frequent flush regimes). There was no reduction in overall PIVC failure, extravasation/infiltration, phlebitis, or occlusion during dwell (Appendix Figure 4.1-4.4). Additionally, no effect was demonstrated when a single study31 investigated volume of flush on extravasation/infiltration, dislodgement, phlebitis, or occlusion.
Variation in dressing and securement. One trial (330 participants)34 demonstrated that integrated securement and dressing (ISD) product reduced PIVC failure (RR, 0.65; 95% CI, 0.45-0.93) and occlusion (RR, 0.35; 95% CI, 0.13-0.94) compared with bordered polyurethane (BPU). There was no difference in the proportion of PIVC failure between BPU compared with tissue adhesive (TA) (RR, 0.74; 95% CI, 0.52-1.06). When comparing individual elements of PIVC failure, there was no evidence of effect between BPU and ISD in reducing infiltration (RR, 0.74; 95% CI, 0.43-1.27), dislodgement (RR, 0.49; 95% CI, 0.15-1.58), or phlebitis/pain (RR, 0.54; 95% CI, 0.21-1.39); similarly, the use of TA compared with BPU did not reduce failure due to infiltration (RR, 0.78; 95% CI, 0.45-1.33), dislodgement (RR, 0.37; 95% CI, 0.10-1.35), occlusion (RR, 0.91; 95% CI, 0.45-1.84), or phlebitis/pain (RR, 0.42; 95% CI, 0.17-1.05).
A comparison of protective covering35 (60 participants) did not demonstrate a significant improvement in PIVC dwell (RR, 0.83; 95% CI, 0.25-1.41).
Pharmacological and nonpharmacological interventions. A comparison of nonpharmacological comfort techniques, including music during insertion (one trial, 42 participants), did not improve first-time insertion success between the two groups (RR, 0.74; 95% CI, 0.53-1.03). Similarly, incorporation of a clown32 (47 patients) as method of distraction did not demonstrate an effect on PIVC insertion success (RR, 0.90; 95% CI, 0.77-1.06) or time to PIVC insertion (MD, –0.20; 95% CI, –1.74 to 1.34). In a double-blinded, placebo-controlled RCT36 of pharmacological techniques to reduce PIVC insertion-related pain (504 participants), no evidence of effect was established between the placebo control group and the active analgesia in overall PIVC insertion success (RR, 1.01; 95% CI, 0.97-1.04).
DISCUSSION
Despite their pervasiveness, PIVC insertion in children is problematic and premature device failure is common, yet effective strategies to overcome these challenges have not been systematically reviewed to date. This systematic review (including meta-analysis) examines techniques and technologies to improve PIVC insertion success and reduce overall failure. We demonstrated ultrasound-guided PIVC insertion significantly improved first-time insertion success in general pediatrics.
Analogous to a previous systematic review in adult patients (1660 patients, odds ratio, 2.49; 95% CI, 1.37-4.52; P = .003; I2, 69%),47 we confirm ultrasound improves first-time PIVC insertion success, most notably in pediatric patients with difficult intravenous access. However, widespread use of ultrasound-guided PIVC insertion is limited by operator skills, as it requires practice and dexterity, especially for DIVA patients.5,47 Healthcare facilities should prioritize teaching and training to support acquisition of this skill to reduce the deleterious effects of multiple insertion attempts, including vessel damage, delayed treatment, pain, and anxiety associated with needles.
Other vessel-visualization technologies (near-infrared and transillumination) did not improve PIVC insertion in generic pediatrics.5 However, they significantly improved first-time insertion, time to insertion, and number of insertion attempts in patients with DIVA and should be considered in the absence of ultrasound-proficient clinicians.
Although vessel-visualization technologies provide efficient PIVC insertion, complication-free PIVC dwell is equally important. Few studies examined both insertion outcomes and PIVC postinsertion outcomes (dwell time and complications during treatment). One study reported more postinsertion complications ( eg, infiltration) with ultrasound compared with landmark technique.39 Vessel-visualization tools should be used to assess the vein to guide PIVC choice. Pandurangadu et al15 reported increased PIVC failure when less than 65% of the catheter length resides within the vein; this was consistent with the single RCT46 included in this review that demonstrated reduced infiltration with long PIVCs compared with standard-length PIVCs. To reduce this knowledge practice gap, it is critical that clinicians continue to evaluate and publish findings of novel techniques to improve PIVC outcomes.
The review findings have important implications for future research, clinical practice, and policy. Unlike earlier reviews,48 vessel-visualization technologies, particularly ultrasound, improved PIVC insertion success; however, during-dwell outcomes were inconsistently reported, and future research should include these. In addition, while there is evidence to support these new technologies, adequate training and resources to ensure a sustained, skilled workforce to optimize PIVC insertion are necessary for successful implementation.
Our study had some limitations, including the methodological quality of included studies (small sample size and significant clinical and statistical heterogeneity). Subgroup analyses were undertaken to reduce the heterogeneity inherent in pediatric populations; however, future studies should stratify for patient (age, DIVA, indication for insertion) and setting (conscious/unconscious, emergent/nonemergent) factors. Incomplete or absent outcome definitions and varied reporting measures (eg, median vs mean) prevented calculation of the pooled incidence of catheter failure and dwell time.
Our review also has notable strengths. Two independent investigators performed a rigorous literature search. Only RCTs were included, ensuring the most robust methods to inform clinically important questions. The primary and secondary outcomes were derived from patient-centered outcomes.
CONCLUSION
This systematic review and meta-analysis describes the pooled incidence of PIVC insertion success and outcomes, including complication and failure in pediatric patients. PIVC insertion with ultrasound should be used to improve insertion success in generic pediatric patients, and any form of vessel-visualization technology (ultrasound, near-infrared, transillumination) should be considered for anticipated difficult insertions.
1. Ullman AJ, Takashima M, Kleidon T, Ray-Barruel G, Alexandrou E, Rickard CM. Global pediatric peripheral intravenous catheter practice and performance: a secondary analysis of 4206 catheters. J Pediatr Nurs. 2020;50:e18-e25. https://doi.org/10.1016/j.pedn.2019.09.023
2. Millington SJ, Hendin A, Shiloh AL, Koenig S. Better with ultrasound peripheral intravenous catheter insertion. Chest. 2020;157(2):369-375. https://doi.org/10.1016/j.chest.2019.04.139
3. Kleidon TM, Cattanach P, Mihala G, Ullman AJ. Implementation of a paediatric peripheral intravenous catheter care bundle: a quality improvement initiative. J Paediatr Child Health. 2019;55(10):1214-1223. https://doi.org/10.1111/jpc.14384
4. Hanada S, Van Winkle MT, Subramani S, Ueda K. Dynamic ultrasound-guided short-axis needle tip navigation technique vs. landmark technique for difficult saphenous vein access in children: a randomised study. Anaesthesia. 2017;72(12):1508-1515. https://doi.org/10.1111/anae.14082
5. Heinrichs J, Fritze Z, Klassen T, Curtis S. A systematic review and meta-analysis of new interventions for peripheral intravenous cannulation of children. Pediatr Emerg Care. 2013;29(7):858-866. https://doi.org/10.1097/PEC.0b013e3182999bcd
6. Indarwati F, Mathew S, Munday J, Keogh S. Incidence of peripheral intravenous catheter failure and complications in paediatric patients: systematic review and meta analysis. Int J Nurs Stud. 2020;102:103488. https://doi.org/10.1016/j.ijnurstu.2019.103488
7. Cooke M, Ullman AJ, Ray-Barruel G, Wallis M, Corley A, Rickard CM. Not “just” an intravenous line: consumer perspectives on peripheral intravenous cannulation (PIVC). An international cross-sectional survey of 25 countries. PLoS One. 2018;13(2):e0193436. https://doi.org/10.1371/journal.pone.0193436
8. Goff DA, Larsen P, Brinkley J, et al. Resource utilization and cost of inserting peripheral intravenous catheters in hospitalized children. Hosp Pediatr. 2013;3(3):185-191. https://doi.org/10.1542/hpeds.2012-0089
9. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Heath Policy. 2014;12(1):51-58. https://doi.org/10.1007/s40258-013-0077-2
10. Suliman M, Saleh W, Al-Shiekh H, Taan W, AlBashtawy M. The incidence of peripheral intravenous catheter phlebitis and risk factors among pediatric patients. J Pediatr Nurs. 2020;50:89-93. https://doi.org/10.1016/j.pedn.2019.11.006
11. Ben Abdelaziz R, Hafsi H, Hajji H, et al. Peripheral venous catheter complications in children: predisposing factors in a multicenter prospective cohort study. BMC Pediatr. 2017;17(1):208. https://doi.org/10.1186/s12887-017-0965-y
12. Reigart JR, Camberlain KH, Eldridge D, et al. Peripheral intravenous access in pediatric inpatients. Clin Pediatr (Phila). 2012;51(1):468-472. https://doi.org/10.1177/0009922811435164
13. Holder MR, Stutzman SE, Olson DM. Impact of ultrasound on short peripheral intravenous catheter placement on vein thrombosis risk. J Infus Nurs. 2017;40(3):176-182. https://doi.org/10.1097/NAN.0000000000000214
14. Marsh N, Webster J, Larsen E, et al. Expert versus generalist inserters for peripheral intravenous catheter insertion: a pilot randomised controlled trial. Trials. 2018;19(1):564. https://doi.org/10.1186/s13063-018-2946-3
15. Pandurangadu AV, Tucker J, Brackney AR, Bahl A. Ultrasound-guided intravenous catheter survival impacted by amount of catheter residing in the vein. Emerg Med J. 2018;35(9):550-555. https://doi.org/10.1136/emermed-2017-206803
16. Bahl A, Hijazi M, Chen NW, Lachapelle-Clavette L, Price J. Ultralong versus standard long peripheral intravenous catheters: a randomized controlled trial of ultrasonographically guided catheter survival. Ann Emerg Med. 2020;76(2):134-142. https://doi.org/10.1016/j.annemergmed.2019.11.013
17. Takahashi T, Murayama R, Abe-Doi M, et al. Preventing peripheral intravenous catheter failure by reducing mechanical irritation. Sci Rep. 2020;10(1):1550. https://doi.org/10.1038/s41598-019-56873-2
18. Vinograd AM, Zorc JJ, Dean AJ, Abbadessa MKF, Chen AE. First-attempt success, longevity, and complication rates of ultrasound-guided peripheral intravenous catheters in children. Pediatr Emerg Care. 2018;34(6):376-380. https://doi.org/10.1097/PEC.0000000000001063
19. Gorski LA, Hadaway L, Hagle ME, et al. Infusion Therapy Standards of Practice, 8th edition. J Infus Nurs. 2021;44(1S Suppl 1):S1-S224. https://doi.org/10.1097/NAN.0000000000000396
20. Stedman’s Medical Dictionary for the Health Professions and Nursing. 7th ed.Lippincott Williams & Wilkins; 2012.
21. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Cochrane; 2020. www.training.cochrane.org/handbook
22. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341. https://doi.org/10.1016/j.ijsu.2010.02.007
23. Stolz LA, Cappa AR, Minckler MR, et al. Prospective evaluation of the learning curve for ultrasound-guided peripheral intravenous catheter placement. J Vasc Access. 2016;17(4):366-370. https://doi.org/10.5301/jva.5000574
24. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898
25. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. https://doi.org/10.1136/bmj.328.7454.1490
26. Diaz-Hennessey S, O’Shea ER, King K. Virtual reality: augmenting the acute pain experience in children. Pediatr Nurs. 2019;45(3):122-127.
27. Takeshita J, Yoshida T, Nakajima Y, et al. Superiority of dynamic needle tip positioning for ultrasound-guided peripheral venous catheterization in patients younger than 2 years old: a randomized controlled trial. Pediatr Crit Care Med. 2019;20(9):e410-e414. https://doi.org/10.1097/PCC.0000000000002034
28. Takeshita J, Nakayama Y, Nakajima Y, et al. Optimal site for ultrasound-guided venous catheterisation in paediatric patients: an observational study to investigate predictors for catheterisation success and a randomised controlled study to determine the most successful site. Crit Care. 2015;19(1):15. https://doi.org/10.1186/s13054-014-0733-4
29. White ML, Crawley J, Rennie EA, Lewandowski LA. Examining the effectiveness of 2 solutions used to flush capped pediatric peripheral intravenous catheters. J Infus Nurs. 2011;34(4):260-270. https://doi.org/10.1097/NAN.0b013e31821da29a
30. Schreiber S, Zanchi C, Ronfani L, et al. Normal saline flushes performed once daily maintain peripheral intravenous catheter patency: a randomised controlled trial. Arch Dis Child. 2015;100(7):700-703. https://doi.org/10.1136/archdischild-2014-307478
31. Kleidon TM, Keogh S, Flynn J, Schults J, Mihala G, Rickard CM. Flushing of peripheral intravenous catheters: a pilot, factorial, randomised controlled trial of high versus low frequency and volume in paediatrics. J Paediatr Child Health. 2019;56(1):22-29. https://doi.org/10.1111/jpc.14482
32. Wolyniez I, Rimon A, Scolnik D, et al. The effect of a medical clown on pain during intravenous access in the pediatric emergency department: a randomized prospective pilot study. Clin Pediatr (Phila). 2013;52(12):1168-1172. https://doi.org/10.1177/0009922813502257
33. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department: a randomized clinical trial. JAMA Pediatr. 2013;167(9):826‐835. https://doi.org/10.1001/jamapediatrics.2013.200
34. Kleidon TM, Rickard CM, Gibson V, et al. Smile - secure my intravenous line effectively: a pilot randomised controlled trial of peripheral intravenous catheter securement in paediatrics. J Tissue Viability. 2020;29(2):82-90. https://doi.org/10.1016/j.jtv.2020.03.006
35. Büyükyilmaz F, Sahiner NC, Caglar S, Eren H. Effectiveness of an intravenous protection device in pediatric patients on catheter dwell time and phlebitis score. Asian Nurs Res (Korean Soc Nurs Sci). 2019;13(4):236-241. https://doi.org/10.1016/j.anr.2019.09.001
36. Schmitz ML, Zempsky WT, Meyer JM. Safety and efficacy of a needle-free powder lidocaine delivery system in pediatric patients undergoing venipuncture or peripheral venous cannulation: randomized double-blind COMFORT-004 trial. Clin Ther. 2015;37(8):1761-1772. https://doi.org/10.1016/j.clinthera.2015.05.515
37. Curtis SJ, Craig WR, Logue E, Vandermeer B, Hanson A, Klassen T. Ultrasound or near-infrared vascular imaging to guide peripheral intravenous catheterization in children: a pragmatic randomized controlled trial. CMAJ. 2015;187(8):563-570. https://doi.org/10.1503/cmaj.141012
38. Benkhadra M, Collignon M, Fournel I, et al. Ultrasound guidance allows faster peripheral IV cannulation in children under 3 years of age with difficult venous access: a prospective randomized study. Paediatr Anaesth. 2012;22(5):449-454. https://doi.org/10.1111/j.1460-9592.2012.03830.x
39. Avelar AFM, Peterlini MAS, da Luz Gonçalves Pedreira M. Ultrasonography-guided peripheral intravenous access in children: a randomized controlled trial. J Infus Nurs. 2015;38(5):320‐327. https://doi.org/10.1097/NAN.0000000000000126
40. Vinograd AM, Chen AE, Woodford AL, et al. Ultrasonographic guidance to improve first-attempt success in children with predicted difficult intravenous access in the emergency department: a randomized controlled trial. Ann Emerg Med. 2019;74(1):19-27. https://doi.org/10.1016/j.annemergmed.2019.02.019
41. Kim MJ, Park JM, Rhee N, et al. Efficacy of VeinViewer in pediatric peripheral intravenous access: a randomized controlled trial. Eur J Pediatr. 2012;171(7):1121-1125. https://doi.org/10.1007/s00431-012-1713-9
42. Kaddoum RN, Anghelescu DL, et al. A randomized controlled trial comparing the AccuVein AV300 device to standard insertion technique for intravenous cannulation of anesthetized children. Paediatr Anaesth. 2012;22(9):884-889. https://doi.org/10.1111/j.1460-9592.2012.03896.x
43. Inal S, Demir D. Impact of peripheral venous catheter placement with vein visualization device support on success rate and pain levels in pediatric patients aged 0 to 3 years. Pediatr Emerg Care. 2021;37(3):138-144. https://doi.org/10.1097/PEC.0000000000001493
44. Demir D, Inal S. Does the use of a vein visualization device for peripheral venous catheter placement increase success rate in pediatric patients? Pediatr Emerg Care. 2019;35(7):474-479. https://doi.org/10.1097/PEC.0000000000001007
45. Gümüs M, Basbakkal Z. Efficacy of Veinlite PEDI in pediatric peripheral intravenous access: a randomized controlled trial. Pediatr Emerg Care. 2021;37(3):145-149. https://doi.org/10.1097/PEC.0000000000001515
46. Qin KR, Ensor N, Barnes R, Englin A, Nataraja RM, Pacilli M. Standard versus long peripheral catheters for multiday IV therapy: a randomized controlled trial. Pediatrics. 2021;147(2): e2020000877. https://doi.org/10.1542/peds.2020-000877
47. van Loon FHJ, Buise MP, Claassen JJF, Dierick-van Daele ATM, Bouwman ARA. Comparison of ultrasound guidance with palpation and direct visualisation for peripheral vein cannulation in adult patients: a systematic review and meta-analysis. Br J Anaesth. 2018;121(2):358-366. https://doi.org/10.1016/j.bja.2018.04.047
48. Parker SIA, Benzies KM, Hayden KA. A systematic review: effectiveness of pediatric peripheral intravenous catheterization strategies. J Adv Nurs. 2017;73(7):1570-1582. https://doi.org/10.1111/jan.13211
Peripheral intravenous catheters (PIVCs) are fundamental to the healthcare practitioners’ ability to provide vital intravenous fluids, medications, and blood products, and as a prophylactic measure prior to some procedures, making insertion of these devices the most common in-hospital invasive procedure in pediatrics.1,2 Despite the prevalence and ubiquity of PIVCs,1 successful insertion in pediatrics is problematic,3-5 and device dysfunction prior to completion of treatment is common.3,6 The inability to attain timely PIVC access and maintain postinsertion function has significant short- and long-term sequelae, including pain and anxiety for children and their parents,3,7 delays in treatment,3 prolonged hospitalization,8 and increased healthcare-associated costs.8-10
Approximately 50% of pediatric PIVC insertions are challenging, often requiring upwards of four insertion attempts, and a similar proportion fail prior to treatment completion.3,11 Exactly why PIVC insertion is difficult in children, and the mechanisms of failure, are unknown. It is likely to be multifaceted and related to factors concerning the patient (eg, comorbidities, age, gender, adiposity),11,12 provider (eg, insertion practice, care, and maintenance),3,13,14 device (eg, size, length, catheter-to-vein ratio),15,16 and therapy (eg, vessel irritation).11,13,17 Observational studies and randomized controlled trials (RCTs) in hospitalized pediatric patients report that the average PIVC dwell is approximately 48 hours, suggesting multiple PIVCs are required to complete a single admission.3,18
Conventionally, PIVC insertion involved physical assessment through palpation and visualization (landmark approach), and although postinsertion care varies among healthcare facilities, minimal requirements are a dressing over the insertion site and regular flushes to ensure device patency.1,3,19 Recently, clinicians have investigated insertion and management practices to improve PIVC outcomes. These can be grouped into techniques—the art of doing (the manner of performance, or the details, of any surgical operation, experiment, or mechanical act) and technologies—the application of scientific knowledge for practical purposes.20 Individual studies have examined the outcomes of new techniques and technologies; however, an overall estimation of their clinical significance or effect is unknown.11,18 Therefore, the aim of this review was to systematically search published studies, conduct a pooled analysis of findings, and report the success of various techniques and technologies to improve insertion success and reduce overall PIVC failure.
METHODS
Design
The protocol for this systematic review was prospectively registered with PROSPERO (CRD42020165288). This review followed Cochrane Collaboration systematic review methods21 and was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.22
Inclusion and Exclusion Criteria
Studies were eligible for inclusion if they met predefined criteria: (1) RCT design; (2) included standard-length PIVC; (3) participants aged 0 to 18 years, excluding preterm infants (less than 36 weeks’ gestation); (4) required PIVC insertion in an inpatient healthcare setting; and (5) reported PIVC insertion outcomes (described below). Studies were excluded if they were cluster or crossover RCTs, published before 2010, or not written in English.
Interventions
Interventions were PIVC insertion and management techniques, defined as “the manner of performance, or the details, of any surgical operation, experiment, or mechanical act” (eg, needle-tip positioning, vein selection [site of insertion], comfort measures, and flushing regimen), or technologies, defined as “the application of scientific knowledge for practical purpose” (eg, vessel visualization, catheter material, and catheter design), compared with current practice, defined as commonly known, practiced, or accepted (eg, landmark PIVC insertion).20
Primary and Secondary Outcomes
The primary outcome was first-time insertion success (one skin puncture to achieve PIVC insertion; can aspirate and flush PIVC without resistance).23 Secondary outcomes included: (1) overall PIVC insertion success23; (2) all-cause PIVC failure (cessation of PIVC function prior to treatment completion)6; (3) dwell time14; (4) PIVC insertion time; (5) insertion attempts23; (6) individual elements of failure (dislodgement, extravasation, infection, occlusion, pain, phlebitis, and thrombosis)6; and (7) patient/parent satisfaction. Some outcomes evaluated were author defined within each study (patient/parent satisfaction, pain score).
Systematic Search
A search of the Cochrane Library and Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health (CINAHL), US National Institutes of Health National Library of Medicine (PubMed), and Embase databases between 2010 to 2020 was undertaken on June 23, 2020, and updated March 4, 2021. Medical Subject Heading (MeSH) terms and relevant keywords and their variants were used in collaboration with a healthcare librarian (Appendix Table 1). Additional studies were identified through hand searches of bibliographies.19 Studies were included if two authors (TMK and JS) independently agreed they met the inclusion criteria.
Data Extraction
Two authors (TMK/JS) independently abstracted study data using a standardized form managed in Microsoft Excel.
Quality Assessment
Included studies were assessed by two authors (TMK and JS) for quality using the Cochrane risk of bias (RoB2) tool.21,24 The overall quality of evidence for each outcome was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE)25 approach. Individual RCTs began at high quality, downgraded by one level for “serious” or two levels for “very serious” study limitations, including high risk of bias, serious inconsistency, publication bias, or indirectness of evidence.
Data Analysis and Synthesis
Where two or more trials with evidence of study homogeneity (trial interventions and population) were identified, meta-analysis using RevMan 5 (version 5.4.1)26 with random effects was conducted. Descriptive statistics summarized study population, interventions, and results. For dichotomous outcomes, we calculated risk ratio (RR) plus 95% CI. For continuous outcomes, we planned to calculate the mean difference (MD) plus 95% CI and the standardized mean difference (SMD) (difference between experimental and control groups across trials) reported as the summary statistic.
Subgroup analyses, where possible, included: difficult intravenous access (DIVA), defined by study authors; age (0-3 years; >3 years up to 18 years); hospital setting during PIVC insertion (awake clinical environment vs awake emergency department vs asleep operating room setting); and by operator (bedside nurse, anesthesiologist).
RESULTS
Search Strategy
Figure 1 describes study selection in accordance with the PRISMA guidelines.22 We identified 1877 records, and 18 articles met the inclusion criteria. An additional 3 studies were identified in the updated search, totaling 21 studies included in the final review.

Study Characteristics
Collectively, 3237 patients and 3098 successful PIVC insertions were reported. In the included studies, 139 patients did not receive a PIVC owing to failed insertion. Ten studies examined techniques (needle-tip positioning,27 vein choice for PIVC insertion,28 flushing regimen,29-31 nonpharmacological32,33 dressing and securement,34,35 and pharmacological comfort measures36), and 11 studies examined technologies (vessel visualization including ultrasound,4,37-40 near-infrared [image of vein projected onto the skin],37,41-44 transillumination [transmission of light through the skin],45 and catheter design46). Table 1 outlines characteristics of included studies. Most trials were single center and conducted in an acute inpatient pediatric-specific setting4,27-34,36-41,44-46 or dedicated pediatric unit in a large public hospital35,43,44; one study was a multicenter trial.36 All trials described evidence of ethical review board approval and participant consent for trial participation.


Study Quality
The certainty of evidence at the outcome level varied from moderate to very low. Table 2 and Table 3 outline the summary of findings for landmark insertion compared with ultrasound-guided and landmark insertion compared with near-infrared PIVC insertion, respectively. The remaining summary-of-findings comparisons that included more than one study or addressed clinically relevant questions can be found in Appendix Tables 2, 3, 4, 5, 6, 7, and 8. At the individual study level, most domains were assessed as low risk of bias (Appendix Figure 1).

Effectiveness of Interventions
Technology to Improve PIVC Outcomes
Landmark compared with ultrasound-guided PIVC insertion. Five studies compared PIVC insertion success outcomes when traditional landmark technique was used in comparison with ultrasound guidance (Appendix Figure 2). Four studies (592 patients)4,37,38,40 assessed the primary outcome of first-time insertion success. Appendix Figure 2.1 demonstrates PIVCs were 1.5 times more likely to be inserted on first attempt when ultrasound guidance was used compared with landmark insertion (RR, 1.60; 95% CI, 1.02-2.50). When examining only studies that included DIVA,4,38,40 the effect size increased and CIs tightened (RR, 1.87; 95% CI, 1.56-2.24). No evidence of effect was demonstrated when comparing this outcome in children aged 0 to 3 years (RR, 1.39; 95% CI, 0.88-2.18) or >3 years (RR, 0.72; 95% CI, 0.35-1.51. Two studies4,38 demonstrated that first-time insertion success with ultrasound (compared with landmark) was almost twice as likely (RR, 1.87; 95% CI, 1.44-2.42) after induction of anesthesia in contrast to no effect in studies undertaken in the emergency department37,40 (RR, 1.32; 95% CI, 0.68-2.56). One study39 (339 patients) reported the secondary outcomes of extravasation/infiltration and phlebitis. Extravasation/infiltration was nearly twice as likely with ultrasound compared with landmark insertion (RR, 1.80; 95% CI, 1.01-3.22); however, there was no evidence of effect related to phlebitis (RR, 0.32; 95% CI, 0.07-1.50).

Four studies4,38-40 compared the review’s secondary outcome of PIVC insertion success (Appendix Figure 2.2), with no evidence of an effect (RR, 1.10; 95% CI, 0.94-1.28). No improvement in overall insertion success was demonstrated in the following subgroup analyses: patients with DIVA (RR, 1.18; 95% CI, 0.95-1.47), children under 3 years of age (RR, 1.23; 95% CI, 0.90-1.68), and PIVCs inserted by anesthesiologists (RR, 1.25; 95% CI, 0.91-1.72). One study measured this outcome in children aged >3 years (RR, 1.13; 95% CI, 0.99-1.29) with no effect and in the emergency department (RR, 1.09; 95% CI, 1.00-1.20), where ultrasound guidance improved overall PIVC insertion success.
Landmark compared with near-infrared PIVC insertion. First-time insertion success (Appendix Figure 3.1) was reported in five studies37,41-44 and 778 patients with no evidence of effect (RR, 1.21; 95% CI, 0.91-1.59). Subgroup analysis by DIVA41-44 demonstrated first-time insertion success more than doubled with near-infrared technology compared with landmark (RR, 2.72; 95% CI, 1.02-7.24). Subgroup analysis by age did not demonstrate an effect in children younger than 3 years or children older than 3 years. Subgroup analysis by clinician inserting did not demonstrate an effect. Of the five studies reporting time to insertion,37,41-44 two41,42 reported median rather than mean, so could not be included in the analysis. Of the remaining three studies,37,43,44 near-infrared reduced PIVC time to insertion (Appendix Figure 3.2).
Four studies37,42-44 reported the number of attempts required for successful PIVC insertion where no difference was detected; however, subgroup analysis of patients with DIVA43,44 and insertion by bedside nurse43,44 demonstrated fewer PIVC insertion attempts and a reduction in insertion time, respectively, with the use of near-infrared technology (Appendix Figure 3.3).
Landmark compared with transillumination PIVC insertion. One study45 (112 participants) found a positive effect with the use of transillumination and first-time insertion success (RR, 1.29; 95% CI, 1.07-1.54), reduced time to insertion (MD, –9.70; 95% CI, –17.40 to –2.00), and fewer insertion attempts (MD, –0.24; 95% CI, –0.40 to –0.08) compared with landmark insertion.
Long PIVC compared with short PIVC. A single study46 demonstrated a 70% reduction in PIVC failure (RR, 0.29; 95% CI, 0.14-0.59) when long PIVCs were compared with standard PIVCs. Specifically, PIVC failure due to infiltration was reduced with the use of a long PIVC (RR, 0.08; 95% CI, 0.01-0.61). There was no difference in insertion success (RR, 1.00; 95% CI, 0.95-1.05) or phlebitis (RR, 1.00; 95% CI, 0.07-15.38).
Technique to Improve PIVC Outcomes
Static ultrasound-guided compared with dynamic needle-tip PIVC insertion. In a single study comparing variation in ultrasound-guided PIVC insertion technique27 (60 patients), dynamic needle-tip positioning improved first-time insertion success (RR, 1.44; 95% CI, 1.04-2.00) and overall PIVC insertion success (RR, 1.42; 95% CI, 1.06-1.91).
Variation in vein choice for successful PIVC insertion. Insertion of PIVC in the cephalic vein of the forearm improved insertion success in a single study28 of 172 patients compared with insertion in the dorsal vein of the hand (RR, 1.39; 95% CI, 1.15-1.69) and great saphenous vein (RR, 1.27; 95% CI, 1.08-1.49).
Variation in PIVC flush. Heparinized saline compared with 0.9% sodium chloride flush29 did not reduce infiltration (RR, 0.31; 95% CI, 0.03-2.84), occlusion (RR, 1.88; 95% CI, 0.18-19.63) during dwell, or hematoma (RR, 0.94; 95% CI, 0.06-14.33) at insertion.
Two studies30,31 (253 participants) compared PIVC flush frequency (daily compared with more frequent flush regimes). There was no reduction in overall PIVC failure, extravasation/infiltration, phlebitis, or occlusion during dwell (Appendix Figure 4.1-4.4). Additionally, no effect was demonstrated when a single study31 investigated volume of flush on extravasation/infiltration, dislodgement, phlebitis, or occlusion.
Variation in dressing and securement. One trial (330 participants)34 demonstrated that integrated securement and dressing (ISD) product reduced PIVC failure (RR, 0.65; 95% CI, 0.45-0.93) and occlusion (RR, 0.35; 95% CI, 0.13-0.94) compared with bordered polyurethane (BPU). There was no difference in the proportion of PIVC failure between BPU compared with tissue adhesive (TA) (RR, 0.74; 95% CI, 0.52-1.06). When comparing individual elements of PIVC failure, there was no evidence of effect between BPU and ISD in reducing infiltration (RR, 0.74; 95% CI, 0.43-1.27), dislodgement (RR, 0.49; 95% CI, 0.15-1.58), or phlebitis/pain (RR, 0.54; 95% CI, 0.21-1.39); similarly, the use of TA compared with BPU did not reduce failure due to infiltration (RR, 0.78; 95% CI, 0.45-1.33), dislodgement (RR, 0.37; 95% CI, 0.10-1.35), occlusion (RR, 0.91; 95% CI, 0.45-1.84), or phlebitis/pain (RR, 0.42; 95% CI, 0.17-1.05).
A comparison of protective covering35 (60 participants) did not demonstrate a significant improvement in PIVC dwell (RR, 0.83; 95% CI, 0.25-1.41).
Pharmacological and nonpharmacological interventions. A comparison of nonpharmacological comfort techniques, including music during insertion (one trial, 42 participants), did not improve first-time insertion success between the two groups (RR, 0.74; 95% CI, 0.53-1.03). Similarly, incorporation of a clown32 (47 patients) as method of distraction did not demonstrate an effect on PIVC insertion success (RR, 0.90; 95% CI, 0.77-1.06) or time to PIVC insertion (MD, –0.20; 95% CI, –1.74 to 1.34). In a double-blinded, placebo-controlled RCT36 of pharmacological techniques to reduce PIVC insertion-related pain (504 participants), no evidence of effect was established between the placebo control group and the active analgesia in overall PIVC insertion success (RR, 1.01; 95% CI, 0.97-1.04).
DISCUSSION
Despite their pervasiveness, PIVC insertion in children is problematic and premature device failure is common, yet effective strategies to overcome these challenges have not been systematically reviewed to date. This systematic review (including meta-analysis) examines techniques and technologies to improve PIVC insertion success and reduce overall failure. We demonstrated ultrasound-guided PIVC insertion significantly improved first-time insertion success in general pediatrics.
Analogous to a previous systematic review in adult patients (1660 patients, odds ratio, 2.49; 95% CI, 1.37-4.52; P = .003; I2, 69%),47 we confirm ultrasound improves first-time PIVC insertion success, most notably in pediatric patients with difficult intravenous access. However, widespread use of ultrasound-guided PIVC insertion is limited by operator skills, as it requires practice and dexterity, especially for DIVA patients.5,47 Healthcare facilities should prioritize teaching and training to support acquisition of this skill to reduce the deleterious effects of multiple insertion attempts, including vessel damage, delayed treatment, pain, and anxiety associated with needles.
Other vessel-visualization technologies (near-infrared and transillumination) did not improve PIVC insertion in generic pediatrics.5 However, they significantly improved first-time insertion, time to insertion, and number of insertion attempts in patients with DIVA and should be considered in the absence of ultrasound-proficient clinicians.
Although vessel-visualization technologies provide efficient PIVC insertion, complication-free PIVC dwell is equally important. Few studies examined both insertion outcomes and PIVC postinsertion outcomes (dwell time and complications during treatment). One study reported more postinsertion complications ( eg, infiltration) with ultrasound compared with landmark technique.39 Vessel-visualization tools should be used to assess the vein to guide PIVC choice. Pandurangadu et al15 reported increased PIVC failure when less than 65% of the catheter length resides within the vein; this was consistent with the single RCT46 included in this review that demonstrated reduced infiltration with long PIVCs compared with standard-length PIVCs. To reduce this knowledge practice gap, it is critical that clinicians continue to evaluate and publish findings of novel techniques to improve PIVC outcomes.
The review findings have important implications for future research, clinical practice, and policy. Unlike earlier reviews,48 vessel-visualization technologies, particularly ultrasound, improved PIVC insertion success; however, during-dwell outcomes were inconsistently reported, and future research should include these. In addition, while there is evidence to support these new technologies, adequate training and resources to ensure a sustained, skilled workforce to optimize PIVC insertion are necessary for successful implementation.
Our study had some limitations, including the methodological quality of included studies (small sample size and significant clinical and statistical heterogeneity). Subgroup analyses were undertaken to reduce the heterogeneity inherent in pediatric populations; however, future studies should stratify for patient (age, DIVA, indication for insertion) and setting (conscious/unconscious, emergent/nonemergent) factors. Incomplete or absent outcome definitions and varied reporting measures (eg, median vs mean) prevented calculation of the pooled incidence of catheter failure and dwell time.
Our review also has notable strengths. Two independent investigators performed a rigorous literature search. Only RCTs were included, ensuring the most robust methods to inform clinically important questions. The primary and secondary outcomes were derived from patient-centered outcomes.
CONCLUSION
This systematic review and meta-analysis describes the pooled incidence of PIVC insertion success and outcomes, including complication and failure in pediatric patients. PIVC insertion with ultrasound should be used to improve insertion success in generic pediatric patients, and any form of vessel-visualization technology (ultrasound, near-infrared, transillumination) should be considered for anticipated difficult insertions.
Peripheral intravenous catheters (PIVCs) are fundamental to the healthcare practitioners’ ability to provide vital intravenous fluids, medications, and blood products, and as a prophylactic measure prior to some procedures, making insertion of these devices the most common in-hospital invasive procedure in pediatrics.1,2 Despite the prevalence and ubiquity of PIVCs,1 successful insertion in pediatrics is problematic,3-5 and device dysfunction prior to completion of treatment is common.3,6 The inability to attain timely PIVC access and maintain postinsertion function has significant short- and long-term sequelae, including pain and anxiety for children and their parents,3,7 delays in treatment,3 prolonged hospitalization,8 and increased healthcare-associated costs.8-10
Approximately 50% of pediatric PIVC insertions are challenging, often requiring upwards of four insertion attempts, and a similar proportion fail prior to treatment completion.3,11 Exactly why PIVC insertion is difficult in children, and the mechanisms of failure, are unknown. It is likely to be multifaceted and related to factors concerning the patient (eg, comorbidities, age, gender, adiposity),11,12 provider (eg, insertion practice, care, and maintenance),3,13,14 device (eg, size, length, catheter-to-vein ratio),15,16 and therapy (eg, vessel irritation).11,13,17 Observational studies and randomized controlled trials (RCTs) in hospitalized pediatric patients report that the average PIVC dwell is approximately 48 hours, suggesting multiple PIVCs are required to complete a single admission.3,18
Conventionally, PIVC insertion involved physical assessment through palpation and visualization (landmark approach), and although postinsertion care varies among healthcare facilities, minimal requirements are a dressing over the insertion site and regular flushes to ensure device patency.1,3,19 Recently, clinicians have investigated insertion and management practices to improve PIVC outcomes. These can be grouped into techniques—the art of doing (the manner of performance, or the details, of any surgical operation, experiment, or mechanical act) and technologies—the application of scientific knowledge for practical purposes.20 Individual studies have examined the outcomes of new techniques and technologies; however, an overall estimation of their clinical significance or effect is unknown.11,18 Therefore, the aim of this review was to systematically search published studies, conduct a pooled analysis of findings, and report the success of various techniques and technologies to improve insertion success and reduce overall PIVC failure.
METHODS
Design
The protocol for this systematic review was prospectively registered with PROSPERO (CRD42020165288). This review followed Cochrane Collaboration systematic review methods21 and was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.22
Inclusion and Exclusion Criteria
Studies were eligible for inclusion if they met predefined criteria: (1) RCT design; (2) included standard-length PIVC; (3) participants aged 0 to 18 years, excluding preterm infants (less than 36 weeks’ gestation); (4) required PIVC insertion in an inpatient healthcare setting; and (5) reported PIVC insertion outcomes (described below). Studies were excluded if they were cluster or crossover RCTs, published before 2010, or not written in English.
Interventions
Interventions were PIVC insertion and management techniques, defined as “the manner of performance, or the details, of any surgical operation, experiment, or mechanical act” (eg, needle-tip positioning, vein selection [site of insertion], comfort measures, and flushing regimen), or technologies, defined as “the application of scientific knowledge for practical purpose” (eg, vessel visualization, catheter material, and catheter design), compared with current practice, defined as commonly known, practiced, or accepted (eg, landmark PIVC insertion).20
Primary and Secondary Outcomes
The primary outcome was first-time insertion success (one skin puncture to achieve PIVC insertion; can aspirate and flush PIVC without resistance).23 Secondary outcomes included: (1) overall PIVC insertion success23; (2) all-cause PIVC failure (cessation of PIVC function prior to treatment completion)6; (3) dwell time14; (4) PIVC insertion time; (5) insertion attempts23; (6) individual elements of failure (dislodgement, extravasation, infection, occlusion, pain, phlebitis, and thrombosis)6; and (7) patient/parent satisfaction. Some outcomes evaluated were author defined within each study (patient/parent satisfaction, pain score).
Systematic Search
A search of the Cochrane Library and Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health (CINAHL), US National Institutes of Health National Library of Medicine (PubMed), and Embase databases between 2010 to 2020 was undertaken on June 23, 2020, and updated March 4, 2021. Medical Subject Heading (MeSH) terms and relevant keywords and their variants were used in collaboration with a healthcare librarian (Appendix Table 1). Additional studies were identified through hand searches of bibliographies.19 Studies were included if two authors (TMK and JS) independently agreed they met the inclusion criteria.
Data Extraction
Two authors (TMK/JS) independently abstracted study data using a standardized form managed in Microsoft Excel.
Quality Assessment
Included studies were assessed by two authors (TMK and JS) for quality using the Cochrane risk of bias (RoB2) tool.21,24 The overall quality of evidence for each outcome was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE)25 approach. Individual RCTs began at high quality, downgraded by one level for “serious” or two levels for “very serious” study limitations, including high risk of bias, serious inconsistency, publication bias, or indirectness of evidence.
Data Analysis and Synthesis
Where two or more trials with evidence of study homogeneity (trial interventions and population) were identified, meta-analysis using RevMan 5 (version 5.4.1)26 with random effects was conducted. Descriptive statistics summarized study population, interventions, and results. For dichotomous outcomes, we calculated risk ratio (RR) plus 95% CI. For continuous outcomes, we planned to calculate the mean difference (MD) plus 95% CI and the standardized mean difference (SMD) (difference between experimental and control groups across trials) reported as the summary statistic.
Subgroup analyses, where possible, included: difficult intravenous access (DIVA), defined by study authors; age (0-3 years; >3 years up to 18 years); hospital setting during PIVC insertion (awake clinical environment vs awake emergency department vs asleep operating room setting); and by operator (bedside nurse, anesthesiologist).
RESULTS
Search Strategy
Figure 1 describes study selection in accordance with the PRISMA guidelines.22 We identified 1877 records, and 18 articles met the inclusion criteria. An additional 3 studies were identified in the updated search, totaling 21 studies included in the final review.

Study Characteristics
Collectively, 3237 patients and 3098 successful PIVC insertions were reported. In the included studies, 139 patients did not receive a PIVC owing to failed insertion. Ten studies examined techniques (needle-tip positioning,27 vein choice for PIVC insertion,28 flushing regimen,29-31 nonpharmacological32,33 dressing and securement,34,35 and pharmacological comfort measures36), and 11 studies examined technologies (vessel visualization including ultrasound,4,37-40 near-infrared [image of vein projected onto the skin],37,41-44 transillumination [transmission of light through the skin],45 and catheter design46). Table 1 outlines characteristics of included studies. Most trials were single center and conducted in an acute inpatient pediatric-specific setting4,27-34,36-41,44-46 or dedicated pediatric unit in a large public hospital35,43,44; one study was a multicenter trial.36 All trials described evidence of ethical review board approval and participant consent for trial participation.


Study Quality
The certainty of evidence at the outcome level varied from moderate to very low. Table 2 and Table 3 outline the summary of findings for landmark insertion compared with ultrasound-guided and landmark insertion compared with near-infrared PIVC insertion, respectively. The remaining summary-of-findings comparisons that included more than one study or addressed clinically relevant questions can be found in Appendix Tables 2, 3, 4, 5, 6, 7, and 8. At the individual study level, most domains were assessed as low risk of bias (Appendix Figure 1).

Effectiveness of Interventions
Technology to Improve PIVC Outcomes
Landmark compared with ultrasound-guided PIVC insertion. Five studies compared PIVC insertion success outcomes when traditional landmark technique was used in comparison with ultrasound guidance (Appendix Figure 2). Four studies (592 patients)4,37,38,40 assessed the primary outcome of first-time insertion success. Appendix Figure 2.1 demonstrates PIVCs were 1.5 times more likely to be inserted on first attempt when ultrasound guidance was used compared with landmark insertion (RR, 1.60; 95% CI, 1.02-2.50). When examining only studies that included DIVA,4,38,40 the effect size increased and CIs tightened (RR, 1.87; 95% CI, 1.56-2.24). No evidence of effect was demonstrated when comparing this outcome in children aged 0 to 3 years (RR, 1.39; 95% CI, 0.88-2.18) or >3 years (RR, 0.72; 95% CI, 0.35-1.51. Two studies4,38 demonstrated that first-time insertion success with ultrasound (compared with landmark) was almost twice as likely (RR, 1.87; 95% CI, 1.44-2.42) after induction of anesthesia in contrast to no effect in studies undertaken in the emergency department37,40 (RR, 1.32; 95% CI, 0.68-2.56). One study39 (339 patients) reported the secondary outcomes of extravasation/infiltration and phlebitis. Extravasation/infiltration was nearly twice as likely with ultrasound compared with landmark insertion (RR, 1.80; 95% CI, 1.01-3.22); however, there was no evidence of effect related to phlebitis (RR, 0.32; 95% CI, 0.07-1.50).

Four studies4,38-40 compared the review’s secondary outcome of PIVC insertion success (Appendix Figure 2.2), with no evidence of an effect (RR, 1.10; 95% CI, 0.94-1.28). No improvement in overall insertion success was demonstrated in the following subgroup analyses: patients with DIVA (RR, 1.18; 95% CI, 0.95-1.47), children under 3 years of age (RR, 1.23; 95% CI, 0.90-1.68), and PIVCs inserted by anesthesiologists (RR, 1.25; 95% CI, 0.91-1.72). One study measured this outcome in children aged >3 years (RR, 1.13; 95% CI, 0.99-1.29) with no effect and in the emergency department (RR, 1.09; 95% CI, 1.00-1.20), where ultrasound guidance improved overall PIVC insertion success.
Landmark compared with near-infrared PIVC insertion. First-time insertion success (Appendix Figure 3.1) was reported in five studies37,41-44 and 778 patients with no evidence of effect (RR, 1.21; 95% CI, 0.91-1.59). Subgroup analysis by DIVA41-44 demonstrated first-time insertion success more than doubled with near-infrared technology compared with landmark (RR, 2.72; 95% CI, 1.02-7.24). Subgroup analysis by age did not demonstrate an effect in children younger than 3 years or children older than 3 years. Subgroup analysis by clinician inserting did not demonstrate an effect. Of the five studies reporting time to insertion,37,41-44 two41,42 reported median rather than mean, so could not be included in the analysis. Of the remaining three studies,37,43,44 near-infrared reduced PIVC time to insertion (Appendix Figure 3.2).
Four studies37,42-44 reported the number of attempts required for successful PIVC insertion where no difference was detected; however, subgroup analysis of patients with DIVA43,44 and insertion by bedside nurse43,44 demonstrated fewer PIVC insertion attempts and a reduction in insertion time, respectively, with the use of near-infrared technology (Appendix Figure 3.3).
Landmark compared with transillumination PIVC insertion. One study45 (112 participants) found a positive effect with the use of transillumination and first-time insertion success (RR, 1.29; 95% CI, 1.07-1.54), reduced time to insertion (MD, –9.70; 95% CI, –17.40 to –2.00), and fewer insertion attempts (MD, –0.24; 95% CI, –0.40 to –0.08) compared with landmark insertion.
Long PIVC compared with short PIVC. A single study46 demonstrated a 70% reduction in PIVC failure (RR, 0.29; 95% CI, 0.14-0.59) when long PIVCs were compared with standard PIVCs. Specifically, PIVC failure due to infiltration was reduced with the use of a long PIVC (RR, 0.08; 95% CI, 0.01-0.61). There was no difference in insertion success (RR, 1.00; 95% CI, 0.95-1.05) or phlebitis (RR, 1.00; 95% CI, 0.07-15.38).
Technique to Improve PIVC Outcomes
Static ultrasound-guided compared with dynamic needle-tip PIVC insertion. In a single study comparing variation in ultrasound-guided PIVC insertion technique27 (60 patients), dynamic needle-tip positioning improved first-time insertion success (RR, 1.44; 95% CI, 1.04-2.00) and overall PIVC insertion success (RR, 1.42; 95% CI, 1.06-1.91).
Variation in vein choice for successful PIVC insertion. Insertion of PIVC in the cephalic vein of the forearm improved insertion success in a single study28 of 172 patients compared with insertion in the dorsal vein of the hand (RR, 1.39; 95% CI, 1.15-1.69) and great saphenous vein (RR, 1.27; 95% CI, 1.08-1.49).
Variation in PIVC flush. Heparinized saline compared with 0.9% sodium chloride flush29 did not reduce infiltration (RR, 0.31; 95% CI, 0.03-2.84), occlusion (RR, 1.88; 95% CI, 0.18-19.63) during dwell, or hematoma (RR, 0.94; 95% CI, 0.06-14.33) at insertion.
Two studies30,31 (253 participants) compared PIVC flush frequency (daily compared with more frequent flush regimes). There was no reduction in overall PIVC failure, extravasation/infiltration, phlebitis, or occlusion during dwell (Appendix Figure 4.1-4.4). Additionally, no effect was demonstrated when a single study31 investigated volume of flush on extravasation/infiltration, dislodgement, phlebitis, or occlusion.
Variation in dressing and securement. One trial (330 participants)34 demonstrated that integrated securement and dressing (ISD) product reduced PIVC failure (RR, 0.65; 95% CI, 0.45-0.93) and occlusion (RR, 0.35; 95% CI, 0.13-0.94) compared with bordered polyurethane (BPU). There was no difference in the proportion of PIVC failure between BPU compared with tissue adhesive (TA) (RR, 0.74; 95% CI, 0.52-1.06). When comparing individual elements of PIVC failure, there was no evidence of effect between BPU and ISD in reducing infiltration (RR, 0.74; 95% CI, 0.43-1.27), dislodgement (RR, 0.49; 95% CI, 0.15-1.58), or phlebitis/pain (RR, 0.54; 95% CI, 0.21-1.39); similarly, the use of TA compared with BPU did not reduce failure due to infiltration (RR, 0.78; 95% CI, 0.45-1.33), dislodgement (RR, 0.37; 95% CI, 0.10-1.35), occlusion (RR, 0.91; 95% CI, 0.45-1.84), or phlebitis/pain (RR, 0.42; 95% CI, 0.17-1.05).
A comparison of protective covering35 (60 participants) did not demonstrate a significant improvement in PIVC dwell (RR, 0.83; 95% CI, 0.25-1.41).
Pharmacological and nonpharmacological interventions. A comparison of nonpharmacological comfort techniques, including music during insertion (one trial, 42 participants), did not improve first-time insertion success between the two groups (RR, 0.74; 95% CI, 0.53-1.03). Similarly, incorporation of a clown32 (47 patients) as method of distraction did not demonstrate an effect on PIVC insertion success (RR, 0.90; 95% CI, 0.77-1.06) or time to PIVC insertion (MD, –0.20; 95% CI, –1.74 to 1.34). In a double-blinded, placebo-controlled RCT36 of pharmacological techniques to reduce PIVC insertion-related pain (504 participants), no evidence of effect was established between the placebo control group and the active analgesia in overall PIVC insertion success (RR, 1.01; 95% CI, 0.97-1.04).
DISCUSSION
Despite their pervasiveness, PIVC insertion in children is problematic and premature device failure is common, yet effective strategies to overcome these challenges have not been systematically reviewed to date. This systematic review (including meta-analysis) examines techniques and technologies to improve PIVC insertion success and reduce overall failure. We demonstrated ultrasound-guided PIVC insertion significantly improved first-time insertion success in general pediatrics.
Analogous to a previous systematic review in adult patients (1660 patients, odds ratio, 2.49; 95% CI, 1.37-4.52; P = .003; I2, 69%),47 we confirm ultrasound improves first-time PIVC insertion success, most notably in pediatric patients with difficult intravenous access. However, widespread use of ultrasound-guided PIVC insertion is limited by operator skills, as it requires practice and dexterity, especially for DIVA patients.5,47 Healthcare facilities should prioritize teaching and training to support acquisition of this skill to reduce the deleterious effects of multiple insertion attempts, including vessel damage, delayed treatment, pain, and anxiety associated with needles.
Other vessel-visualization technologies (near-infrared and transillumination) did not improve PIVC insertion in generic pediatrics.5 However, they significantly improved first-time insertion, time to insertion, and number of insertion attempts in patients with DIVA and should be considered in the absence of ultrasound-proficient clinicians.
Although vessel-visualization technologies provide efficient PIVC insertion, complication-free PIVC dwell is equally important. Few studies examined both insertion outcomes and PIVC postinsertion outcomes (dwell time and complications during treatment). One study reported more postinsertion complications ( eg, infiltration) with ultrasound compared with landmark technique.39 Vessel-visualization tools should be used to assess the vein to guide PIVC choice. Pandurangadu et al15 reported increased PIVC failure when less than 65% of the catheter length resides within the vein; this was consistent with the single RCT46 included in this review that demonstrated reduced infiltration with long PIVCs compared with standard-length PIVCs. To reduce this knowledge practice gap, it is critical that clinicians continue to evaluate and publish findings of novel techniques to improve PIVC outcomes.
The review findings have important implications for future research, clinical practice, and policy. Unlike earlier reviews,48 vessel-visualization technologies, particularly ultrasound, improved PIVC insertion success; however, during-dwell outcomes were inconsistently reported, and future research should include these. In addition, while there is evidence to support these new technologies, adequate training and resources to ensure a sustained, skilled workforce to optimize PIVC insertion are necessary for successful implementation.
Our study had some limitations, including the methodological quality of included studies (small sample size and significant clinical and statistical heterogeneity). Subgroup analyses were undertaken to reduce the heterogeneity inherent in pediatric populations; however, future studies should stratify for patient (age, DIVA, indication for insertion) and setting (conscious/unconscious, emergent/nonemergent) factors. Incomplete or absent outcome definitions and varied reporting measures (eg, median vs mean) prevented calculation of the pooled incidence of catheter failure and dwell time.
Our review also has notable strengths. Two independent investigators performed a rigorous literature search. Only RCTs were included, ensuring the most robust methods to inform clinically important questions. The primary and secondary outcomes were derived from patient-centered outcomes.
CONCLUSION
This systematic review and meta-analysis describes the pooled incidence of PIVC insertion success and outcomes, including complication and failure in pediatric patients. PIVC insertion with ultrasound should be used to improve insertion success in generic pediatric patients, and any form of vessel-visualization technology (ultrasound, near-infrared, transillumination) should be considered for anticipated difficult insertions.
1. Ullman AJ, Takashima M, Kleidon T, Ray-Barruel G, Alexandrou E, Rickard CM. Global pediatric peripheral intravenous catheter practice and performance: a secondary analysis of 4206 catheters. J Pediatr Nurs. 2020;50:e18-e25. https://doi.org/10.1016/j.pedn.2019.09.023
2. Millington SJ, Hendin A, Shiloh AL, Koenig S. Better with ultrasound peripheral intravenous catheter insertion. Chest. 2020;157(2):369-375. https://doi.org/10.1016/j.chest.2019.04.139
3. Kleidon TM, Cattanach P, Mihala G, Ullman AJ. Implementation of a paediatric peripheral intravenous catheter care bundle: a quality improvement initiative. J Paediatr Child Health. 2019;55(10):1214-1223. https://doi.org/10.1111/jpc.14384
4. Hanada S, Van Winkle MT, Subramani S, Ueda K. Dynamic ultrasound-guided short-axis needle tip navigation technique vs. landmark technique for difficult saphenous vein access in children: a randomised study. Anaesthesia. 2017;72(12):1508-1515. https://doi.org/10.1111/anae.14082
5. Heinrichs J, Fritze Z, Klassen T, Curtis S. A systematic review and meta-analysis of new interventions for peripheral intravenous cannulation of children. Pediatr Emerg Care. 2013;29(7):858-866. https://doi.org/10.1097/PEC.0b013e3182999bcd
6. Indarwati F, Mathew S, Munday J, Keogh S. Incidence of peripheral intravenous catheter failure and complications in paediatric patients: systematic review and meta analysis. Int J Nurs Stud. 2020;102:103488. https://doi.org/10.1016/j.ijnurstu.2019.103488
7. Cooke M, Ullman AJ, Ray-Barruel G, Wallis M, Corley A, Rickard CM. Not “just” an intravenous line: consumer perspectives on peripheral intravenous cannulation (PIVC). An international cross-sectional survey of 25 countries. PLoS One. 2018;13(2):e0193436. https://doi.org/10.1371/journal.pone.0193436
8. Goff DA, Larsen P, Brinkley J, et al. Resource utilization and cost of inserting peripheral intravenous catheters in hospitalized children. Hosp Pediatr. 2013;3(3):185-191. https://doi.org/10.1542/hpeds.2012-0089
9. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Heath Policy. 2014;12(1):51-58. https://doi.org/10.1007/s40258-013-0077-2
10. Suliman M, Saleh W, Al-Shiekh H, Taan W, AlBashtawy M. The incidence of peripheral intravenous catheter phlebitis and risk factors among pediatric patients. J Pediatr Nurs. 2020;50:89-93. https://doi.org/10.1016/j.pedn.2019.11.006
11. Ben Abdelaziz R, Hafsi H, Hajji H, et al. Peripheral venous catheter complications in children: predisposing factors in a multicenter prospective cohort study. BMC Pediatr. 2017;17(1):208. https://doi.org/10.1186/s12887-017-0965-y
12. Reigart JR, Camberlain KH, Eldridge D, et al. Peripheral intravenous access in pediatric inpatients. Clin Pediatr (Phila). 2012;51(1):468-472. https://doi.org/10.1177/0009922811435164
13. Holder MR, Stutzman SE, Olson DM. Impact of ultrasound on short peripheral intravenous catheter placement on vein thrombosis risk. J Infus Nurs. 2017;40(3):176-182. https://doi.org/10.1097/NAN.0000000000000214
14. Marsh N, Webster J, Larsen E, et al. Expert versus generalist inserters for peripheral intravenous catheter insertion: a pilot randomised controlled trial. Trials. 2018;19(1):564. https://doi.org/10.1186/s13063-018-2946-3
15. Pandurangadu AV, Tucker J, Brackney AR, Bahl A. Ultrasound-guided intravenous catheter survival impacted by amount of catheter residing in the vein. Emerg Med J. 2018;35(9):550-555. https://doi.org/10.1136/emermed-2017-206803
16. Bahl A, Hijazi M, Chen NW, Lachapelle-Clavette L, Price J. Ultralong versus standard long peripheral intravenous catheters: a randomized controlled trial of ultrasonographically guided catheter survival. Ann Emerg Med. 2020;76(2):134-142. https://doi.org/10.1016/j.annemergmed.2019.11.013
17. Takahashi T, Murayama R, Abe-Doi M, et al. Preventing peripheral intravenous catheter failure by reducing mechanical irritation. Sci Rep. 2020;10(1):1550. https://doi.org/10.1038/s41598-019-56873-2
18. Vinograd AM, Zorc JJ, Dean AJ, Abbadessa MKF, Chen AE. First-attempt success, longevity, and complication rates of ultrasound-guided peripheral intravenous catheters in children. Pediatr Emerg Care. 2018;34(6):376-380. https://doi.org/10.1097/PEC.0000000000001063
19. Gorski LA, Hadaway L, Hagle ME, et al. Infusion Therapy Standards of Practice, 8th edition. J Infus Nurs. 2021;44(1S Suppl 1):S1-S224. https://doi.org/10.1097/NAN.0000000000000396
20. Stedman’s Medical Dictionary for the Health Professions and Nursing. 7th ed.Lippincott Williams & Wilkins; 2012.
21. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Cochrane; 2020. www.training.cochrane.org/handbook
22. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341. https://doi.org/10.1016/j.ijsu.2010.02.007
23. Stolz LA, Cappa AR, Minckler MR, et al. Prospective evaluation of the learning curve for ultrasound-guided peripheral intravenous catheter placement. J Vasc Access. 2016;17(4):366-370. https://doi.org/10.5301/jva.5000574
24. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898
25. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. https://doi.org/10.1136/bmj.328.7454.1490
26. Diaz-Hennessey S, O’Shea ER, King K. Virtual reality: augmenting the acute pain experience in children. Pediatr Nurs. 2019;45(3):122-127.
27. Takeshita J, Yoshida T, Nakajima Y, et al. Superiority of dynamic needle tip positioning for ultrasound-guided peripheral venous catheterization in patients younger than 2 years old: a randomized controlled trial. Pediatr Crit Care Med. 2019;20(9):e410-e414. https://doi.org/10.1097/PCC.0000000000002034
28. Takeshita J, Nakayama Y, Nakajima Y, et al. Optimal site for ultrasound-guided venous catheterisation in paediatric patients: an observational study to investigate predictors for catheterisation success and a randomised controlled study to determine the most successful site. Crit Care. 2015;19(1):15. https://doi.org/10.1186/s13054-014-0733-4
29. White ML, Crawley J, Rennie EA, Lewandowski LA. Examining the effectiveness of 2 solutions used to flush capped pediatric peripheral intravenous catheters. J Infus Nurs. 2011;34(4):260-270. https://doi.org/10.1097/NAN.0b013e31821da29a
30. Schreiber S, Zanchi C, Ronfani L, et al. Normal saline flushes performed once daily maintain peripheral intravenous catheter patency: a randomised controlled trial. Arch Dis Child. 2015;100(7):700-703. https://doi.org/10.1136/archdischild-2014-307478
31. Kleidon TM, Keogh S, Flynn J, Schults J, Mihala G, Rickard CM. Flushing of peripheral intravenous catheters: a pilot, factorial, randomised controlled trial of high versus low frequency and volume in paediatrics. J Paediatr Child Health. 2019;56(1):22-29. https://doi.org/10.1111/jpc.14482
32. Wolyniez I, Rimon A, Scolnik D, et al. The effect of a medical clown on pain during intravenous access in the pediatric emergency department: a randomized prospective pilot study. Clin Pediatr (Phila). 2013;52(12):1168-1172. https://doi.org/10.1177/0009922813502257
33. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department: a randomized clinical trial. JAMA Pediatr. 2013;167(9):826‐835. https://doi.org/10.1001/jamapediatrics.2013.200
34. Kleidon TM, Rickard CM, Gibson V, et al. Smile - secure my intravenous line effectively: a pilot randomised controlled trial of peripheral intravenous catheter securement in paediatrics. J Tissue Viability. 2020;29(2):82-90. https://doi.org/10.1016/j.jtv.2020.03.006
35. Büyükyilmaz F, Sahiner NC, Caglar S, Eren H. Effectiveness of an intravenous protection device in pediatric patients on catheter dwell time and phlebitis score. Asian Nurs Res (Korean Soc Nurs Sci). 2019;13(4):236-241. https://doi.org/10.1016/j.anr.2019.09.001
36. Schmitz ML, Zempsky WT, Meyer JM. Safety and efficacy of a needle-free powder lidocaine delivery system in pediatric patients undergoing venipuncture or peripheral venous cannulation: randomized double-blind COMFORT-004 trial. Clin Ther. 2015;37(8):1761-1772. https://doi.org/10.1016/j.clinthera.2015.05.515
37. Curtis SJ, Craig WR, Logue E, Vandermeer B, Hanson A, Klassen T. Ultrasound or near-infrared vascular imaging to guide peripheral intravenous catheterization in children: a pragmatic randomized controlled trial. CMAJ. 2015;187(8):563-570. https://doi.org/10.1503/cmaj.141012
38. Benkhadra M, Collignon M, Fournel I, et al. Ultrasound guidance allows faster peripheral IV cannulation in children under 3 years of age with difficult venous access: a prospective randomized study. Paediatr Anaesth. 2012;22(5):449-454. https://doi.org/10.1111/j.1460-9592.2012.03830.x
39. Avelar AFM, Peterlini MAS, da Luz Gonçalves Pedreira M. Ultrasonography-guided peripheral intravenous access in children: a randomized controlled trial. J Infus Nurs. 2015;38(5):320‐327. https://doi.org/10.1097/NAN.0000000000000126
40. Vinograd AM, Chen AE, Woodford AL, et al. Ultrasonographic guidance to improve first-attempt success in children with predicted difficult intravenous access in the emergency department: a randomized controlled trial. Ann Emerg Med. 2019;74(1):19-27. https://doi.org/10.1016/j.annemergmed.2019.02.019
41. Kim MJ, Park JM, Rhee N, et al. Efficacy of VeinViewer in pediatric peripheral intravenous access: a randomized controlled trial. Eur J Pediatr. 2012;171(7):1121-1125. https://doi.org/10.1007/s00431-012-1713-9
42. Kaddoum RN, Anghelescu DL, et al. A randomized controlled trial comparing the AccuVein AV300 device to standard insertion technique for intravenous cannulation of anesthetized children. Paediatr Anaesth. 2012;22(9):884-889. https://doi.org/10.1111/j.1460-9592.2012.03896.x
43. Inal S, Demir D. Impact of peripheral venous catheter placement with vein visualization device support on success rate and pain levels in pediatric patients aged 0 to 3 years. Pediatr Emerg Care. 2021;37(3):138-144. https://doi.org/10.1097/PEC.0000000000001493
44. Demir D, Inal S. Does the use of a vein visualization device for peripheral venous catheter placement increase success rate in pediatric patients? Pediatr Emerg Care. 2019;35(7):474-479. https://doi.org/10.1097/PEC.0000000000001007
45. Gümüs M, Basbakkal Z. Efficacy of Veinlite PEDI in pediatric peripheral intravenous access: a randomized controlled trial. Pediatr Emerg Care. 2021;37(3):145-149. https://doi.org/10.1097/PEC.0000000000001515
46. Qin KR, Ensor N, Barnes R, Englin A, Nataraja RM, Pacilli M. Standard versus long peripheral catheters for multiday IV therapy: a randomized controlled trial. Pediatrics. 2021;147(2): e2020000877. https://doi.org/10.1542/peds.2020-000877
47. van Loon FHJ, Buise MP, Claassen JJF, Dierick-van Daele ATM, Bouwman ARA. Comparison of ultrasound guidance with palpation and direct visualisation for peripheral vein cannulation in adult patients: a systematic review and meta-analysis. Br J Anaesth. 2018;121(2):358-366. https://doi.org/10.1016/j.bja.2018.04.047
48. Parker SIA, Benzies KM, Hayden KA. A systematic review: effectiveness of pediatric peripheral intravenous catheterization strategies. J Adv Nurs. 2017;73(7):1570-1582. https://doi.org/10.1111/jan.13211
1. Ullman AJ, Takashima M, Kleidon T, Ray-Barruel G, Alexandrou E, Rickard CM. Global pediatric peripheral intravenous catheter practice and performance: a secondary analysis of 4206 catheters. J Pediatr Nurs. 2020;50:e18-e25. https://doi.org/10.1016/j.pedn.2019.09.023
2. Millington SJ, Hendin A, Shiloh AL, Koenig S. Better with ultrasound peripheral intravenous catheter insertion. Chest. 2020;157(2):369-375. https://doi.org/10.1016/j.chest.2019.04.139
3. Kleidon TM, Cattanach P, Mihala G, Ullman AJ. Implementation of a paediatric peripheral intravenous catheter care bundle: a quality improvement initiative. J Paediatr Child Health. 2019;55(10):1214-1223. https://doi.org/10.1111/jpc.14384
4. Hanada S, Van Winkle MT, Subramani S, Ueda K. Dynamic ultrasound-guided short-axis needle tip navigation technique vs. landmark technique for difficult saphenous vein access in children: a randomised study. Anaesthesia. 2017;72(12):1508-1515. https://doi.org/10.1111/anae.14082
5. Heinrichs J, Fritze Z, Klassen T, Curtis S. A systematic review and meta-analysis of new interventions for peripheral intravenous cannulation of children. Pediatr Emerg Care. 2013;29(7):858-866. https://doi.org/10.1097/PEC.0b013e3182999bcd
6. Indarwati F, Mathew S, Munday J, Keogh S. Incidence of peripheral intravenous catheter failure and complications in paediatric patients: systematic review and meta analysis. Int J Nurs Stud. 2020;102:103488. https://doi.org/10.1016/j.ijnurstu.2019.103488
7. Cooke M, Ullman AJ, Ray-Barruel G, Wallis M, Corley A, Rickard CM. Not “just” an intravenous line: consumer perspectives on peripheral intravenous cannulation (PIVC). An international cross-sectional survey of 25 countries. PLoS One. 2018;13(2):e0193436. https://doi.org/10.1371/journal.pone.0193436
8. Goff DA, Larsen P, Brinkley J, et al. Resource utilization and cost of inserting peripheral intravenous catheters in hospitalized children. Hosp Pediatr. 2013;3(3):185-191. https://doi.org/10.1542/hpeds.2012-0089
9. Tuffaha HW, Rickard CM, Webster J, et al. Cost-effectiveness analysis of clinically indicated versus routine replacement of peripheral intravenous catheters. Appl Health Econ Heath Policy. 2014;12(1):51-58. https://doi.org/10.1007/s40258-013-0077-2
10. Suliman M, Saleh W, Al-Shiekh H, Taan W, AlBashtawy M. The incidence of peripheral intravenous catheter phlebitis and risk factors among pediatric patients. J Pediatr Nurs. 2020;50:89-93. https://doi.org/10.1016/j.pedn.2019.11.006
11. Ben Abdelaziz R, Hafsi H, Hajji H, et al. Peripheral venous catheter complications in children: predisposing factors in a multicenter prospective cohort study. BMC Pediatr. 2017;17(1):208. https://doi.org/10.1186/s12887-017-0965-y
12. Reigart JR, Camberlain KH, Eldridge D, et al. Peripheral intravenous access in pediatric inpatients. Clin Pediatr (Phila). 2012;51(1):468-472. https://doi.org/10.1177/0009922811435164
13. Holder MR, Stutzman SE, Olson DM. Impact of ultrasound on short peripheral intravenous catheter placement on vein thrombosis risk. J Infus Nurs. 2017;40(3):176-182. https://doi.org/10.1097/NAN.0000000000000214
14. Marsh N, Webster J, Larsen E, et al. Expert versus generalist inserters for peripheral intravenous catheter insertion: a pilot randomised controlled trial. Trials. 2018;19(1):564. https://doi.org/10.1186/s13063-018-2946-3
15. Pandurangadu AV, Tucker J, Brackney AR, Bahl A. Ultrasound-guided intravenous catheter survival impacted by amount of catheter residing in the vein. Emerg Med J. 2018;35(9):550-555. https://doi.org/10.1136/emermed-2017-206803
16. Bahl A, Hijazi M, Chen NW, Lachapelle-Clavette L, Price J. Ultralong versus standard long peripheral intravenous catheters: a randomized controlled trial of ultrasonographically guided catheter survival. Ann Emerg Med. 2020;76(2):134-142. https://doi.org/10.1016/j.annemergmed.2019.11.013
17. Takahashi T, Murayama R, Abe-Doi M, et al. Preventing peripheral intravenous catheter failure by reducing mechanical irritation. Sci Rep. 2020;10(1):1550. https://doi.org/10.1038/s41598-019-56873-2
18. Vinograd AM, Zorc JJ, Dean AJ, Abbadessa MKF, Chen AE. First-attempt success, longevity, and complication rates of ultrasound-guided peripheral intravenous catheters in children. Pediatr Emerg Care. 2018;34(6):376-380. https://doi.org/10.1097/PEC.0000000000001063
19. Gorski LA, Hadaway L, Hagle ME, et al. Infusion Therapy Standards of Practice, 8th edition. J Infus Nurs. 2021;44(1S Suppl 1):S1-S224. https://doi.org/10.1097/NAN.0000000000000396
20. Stedman’s Medical Dictionary for the Health Professions and Nursing. 7th ed.Lippincott Williams & Wilkins; 2012.
21. Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Cochrane; 2020. www.training.cochrane.org/handbook
22. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341. https://doi.org/10.1016/j.ijsu.2010.02.007
23. Stolz LA, Cappa AR, Minckler MR, et al. Prospective evaluation of the learning curve for ultrasound-guided peripheral intravenous catheter placement. J Vasc Access. 2016;17(4):366-370. https://doi.org/10.5301/jva.5000574
24. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898
25. Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. https://doi.org/10.1136/bmj.328.7454.1490
26. Diaz-Hennessey S, O’Shea ER, King K. Virtual reality: augmenting the acute pain experience in children. Pediatr Nurs. 2019;45(3):122-127.
27. Takeshita J, Yoshida T, Nakajima Y, et al. Superiority of dynamic needle tip positioning for ultrasound-guided peripheral venous catheterization in patients younger than 2 years old: a randomized controlled trial. Pediatr Crit Care Med. 2019;20(9):e410-e414. https://doi.org/10.1097/PCC.0000000000002034
28. Takeshita J, Nakayama Y, Nakajima Y, et al. Optimal site for ultrasound-guided venous catheterisation in paediatric patients: an observational study to investigate predictors for catheterisation success and a randomised controlled study to determine the most successful site. Crit Care. 2015;19(1):15. https://doi.org/10.1186/s13054-014-0733-4
29. White ML, Crawley J, Rennie EA, Lewandowski LA. Examining the effectiveness of 2 solutions used to flush capped pediatric peripheral intravenous catheters. J Infus Nurs. 2011;34(4):260-270. https://doi.org/10.1097/NAN.0b013e31821da29a
30. Schreiber S, Zanchi C, Ronfani L, et al. Normal saline flushes performed once daily maintain peripheral intravenous catheter patency: a randomised controlled trial. Arch Dis Child. 2015;100(7):700-703. https://doi.org/10.1136/archdischild-2014-307478
31. Kleidon TM, Keogh S, Flynn J, Schults J, Mihala G, Rickard CM. Flushing of peripheral intravenous catheters: a pilot, factorial, randomised controlled trial of high versus low frequency and volume in paediatrics. J Paediatr Child Health. 2019;56(1):22-29. https://doi.org/10.1111/jpc.14482
32. Wolyniez I, Rimon A, Scolnik D, et al. The effect of a medical clown on pain during intravenous access in the pediatric emergency department: a randomized prospective pilot study. Clin Pediatr (Phila). 2013;52(12):1168-1172. https://doi.org/10.1177/0009922813502257
33. Hartling L, Newton AS, Liang Y, et al. Music to reduce pain and distress in the pediatric emergency department: a randomized clinical trial. JAMA Pediatr. 2013;167(9):826‐835. https://doi.org/10.1001/jamapediatrics.2013.200
34. Kleidon TM, Rickard CM, Gibson V, et al. Smile - secure my intravenous line effectively: a pilot randomised controlled trial of peripheral intravenous catheter securement in paediatrics. J Tissue Viability. 2020;29(2):82-90. https://doi.org/10.1016/j.jtv.2020.03.006
35. Büyükyilmaz F, Sahiner NC, Caglar S, Eren H. Effectiveness of an intravenous protection device in pediatric patients on catheter dwell time and phlebitis score. Asian Nurs Res (Korean Soc Nurs Sci). 2019;13(4):236-241. https://doi.org/10.1016/j.anr.2019.09.001
36. Schmitz ML, Zempsky WT, Meyer JM. Safety and efficacy of a needle-free powder lidocaine delivery system in pediatric patients undergoing venipuncture or peripheral venous cannulation: randomized double-blind COMFORT-004 trial. Clin Ther. 2015;37(8):1761-1772. https://doi.org/10.1016/j.clinthera.2015.05.515
37. Curtis SJ, Craig WR, Logue E, Vandermeer B, Hanson A, Klassen T. Ultrasound or near-infrared vascular imaging to guide peripheral intravenous catheterization in children: a pragmatic randomized controlled trial. CMAJ. 2015;187(8):563-570. https://doi.org/10.1503/cmaj.141012
38. Benkhadra M, Collignon M, Fournel I, et al. Ultrasound guidance allows faster peripheral IV cannulation in children under 3 years of age with difficult venous access: a prospective randomized study. Paediatr Anaesth. 2012;22(5):449-454. https://doi.org/10.1111/j.1460-9592.2012.03830.x
39. Avelar AFM, Peterlini MAS, da Luz Gonçalves Pedreira M. Ultrasonography-guided peripheral intravenous access in children: a randomized controlled trial. J Infus Nurs. 2015;38(5):320‐327. https://doi.org/10.1097/NAN.0000000000000126
40. Vinograd AM, Chen AE, Woodford AL, et al. Ultrasonographic guidance to improve first-attempt success in children with predicted difficult intravenous access in the emergency department: a randomized controlled trial. Ann Emerg Med. 2019;74(1):19-27. https://doi.org/10.1016/j.annemergmed.2019.02.019
41. Kim MJ, Park JM, Rhee N, et al. Efficacy of VeinViewer in pediatric peripheral intravenous access: a randomized controlled trial. Eur J Pediatr. 2012;171(7):1121-1125. https://doi.org/10.1007/s00431-012-1713-9
42. Kaddoum RN, Anghelescu DL, et al. A randomized controlled trial comparing the AccuVein AV300 device to standard insertion technique for intravenous cannulation of anesthetized children. Paediatr Anaesth. 2012;22(9):884-889. https://doi.org/10.1111/j.1460-9592.2012.03896.x
43. Inal S, Demir D. Impact of peripheral venous catheter placement with vein visualization device support on success rate and pain levels in pediatric patients aged 0 to 3 years. Pediatr Emerg Care. 2021;37(3):138-144. https://doi.org/10.1097/PEC.0000000000001493
44. Demir D, Inal S. Does the use of a vein visualization device for peripheral venous catheter placement increase success rate in pediatric patients? Pediatr Emerg Care. 2019;35(7):474-479. https://doi.org/10.1097/PEC.0000000000001007
45. Gümüs M, Basbakkal Z. Efficacy of Veinlite PEDI in pediatric peripheral intravenous access: a randomized controlled trial. Pediatr Emerg Care. 2021;37(3):145-149. https://doi.org/10.1097/PEC.0000000000001515
46. Qin KR, Ensor N, Barnes R, Englin A, Nataraja RM, Pacilli M. Standard versus long peripheral catheters for multiday IV therapy: a randomized controlled trial. Pediatrics. 2021;147(2): e2020000877. https://doi.org/10.1542/peds.2020-000877
47. van Loon FHJ, Buise MP, Claassen JJF, Dierick-van Daele ATM, Bouwman ARA. Comparison of ultrasound guidance with palpation and direct visualisation for peripheral vein cannulation in adult patients: a systematic review and meta-analysis. Br J Anaesth. 2018;121(2):358-366. https://doi.org/10.1016/j.bja.2018.04.047
48. Parker SIA, Benzies KM, Hayden KA. A systematic review: effectiveness of pediatric peripheral intravenous catheterization strategies. J Adv Nurs. 2017;73(7):1570-1582. https://doi.org/10.1111/jan.13211
© 2021 Society of Hospital Medicine