User login
PCOS is a common problem, with a prevalence of 6% to 10% among women of reproductive age.1 Patients with PCOS often present with hirsutism, acne, female androgenetic alopecia, oligomenorrhea (also known as infrequent menstrual bleeding), amenorrhea, infertility, overweight, or obesity. In addition, many patients with PCOS have insulin resistance, dyslipidemia, metabolic syndrome, and an increased risk for developing type 2 diabetes mellitus (DM).2 A simplified approach to the diagnosis of PCOS will save health care resources by reducing the use of low-value diagnostic tests. A simplified approach to the treatment of PCOS will support patient medication adherence and improve health outcomes.
Simplify the diagnosis of PCOS
Simplify PCOS diagnosis by focusing on the core criteria of hyperandrogenism and oligo-ovulation. There are 3 major approaches to diagnosis:
- the 1990 National Institutes of Health (NIH) criteria3
- the 2003 Rotterdam criteria4,5
- the 2008 Androgen Excess and PCOS Society (AES) criteria.6
Using the 1990 NIH approach, the diagnosis of PCOS is made by the presence of 2 core criteria: hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea. In addition, other causes of hyperandrogenism should be excluded, including nonclassical adrenal hyperplasia (NCAH) due to 21-hydroxylase deficiency.3 Using the 1990 NIH criteria, PCOS can be diagnosed based on history (oligomenorrhea) and physical examination (assessment of the severity of hirsutism), but laboratory tests including total testosterone are often ordered.7
The Rotterdam approach to the diagnosis added a third criteria, the detection by ultrasonography of a multifollicular ovary and/or increased ovarian volume.4,5 Using the Rotterdam approach, PCOS is diagnosed in the presence of any 2 of the following 3 criteria: hyperandrogenism, oligo-ovulation, or ultrasound imaging showing the presence of a multifollicular ovary, identified by ≥ 12 antral follicles (2 to 9 mm in diameter) in each ovary or increased ovarian volume (> 10 mL).4,5
The Rotterdam approach using ovarian ultrasound as a criterion to diagnose PCOS is rife with serious problems, including:
- The number of small antral follicles in the normal ovary is age dependent, and many ovulatory and nonhirsute patients have ≥ 12 small antral follicles in each ovary.8,9
- There is no consensus on the number of small antral follicles needed to diagnose a multifollicular ovary, with recommendations to use thresholds of 124,5 or 20 follicles10 as the diagnostic cut-off.
- Accurate counting of the number of small ovarian follicles requires transvaginal ultrasound, which is not appropriate for many young adolescent patients.
- The process of counting ovarian follicles is operator-dependent.
- The high cost of ultrasound assessment of ovarian follicles (≥ $500 per examination).
The Rotterdam approach supports the diagnosis of PCOS in a patient with oligo-ovulation plus an ultrasound showing a multifollicular ovary in the absence of any clinical or laboratory evidence of hyperandrogenism.3,4,5 This approach to the diagnosis of PCOS is rejected by both the 1990 NIH3 and AES6 recommendations, which require the presence of hyperandrogenism as the sine qua non in the diagnosis of PCOS. I recommend against diagnosing PCOS in a non-hyperandrogenic patient with oligo-ovulation and a multifollicular ovary because other diagnoses are also possible, such as functional hypothalamic oligo-ovulation, especially in young patients. The Rotterdam approach also supports the diagnosis of PCOS in a patient with hyperandrogenism, an ultrasound showing a multifollicular ovary, and normal ovulation and menses.3,4 For most patients with normal, regular ovulation and menses, the testosterone concentration is normal and the only evidence of hyperandrogenism is hirsutism. Patients with normal, regular ovulation and menses plus hirsutism usually have idiopathic hirsutism. Idiopathic hirsutism is a problem caused by excessive 5-alpha-reductase activity in the hair pilosebaceous unit, which catalyzes the conversion of weak androgens into dihydrotestosterone, a potent intracellular androgen that stimulates terminal hair growth.11 In my opinion, the Rotterdam approach to diagnosing PCOS has created unnecessary confusion and complexity for both clinicians and patients. I believe we should simplify the diagnosis of PCOS and return to the 1990 NIH criteria.3
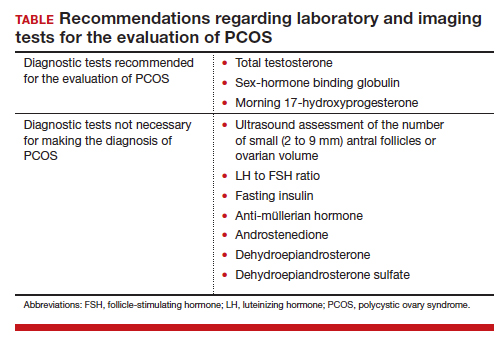
On occasion, a patient presents for a consultation and has already had an ovarian ultrasound to assess for a multifollicular ovary. I carefully read the report and, if a multifollicular ovary has been identified, I consider it as a secondary supporting finding of PCOS in my clinical assessment. But I do not base my diagnosis on the ultrasound finding. Patients often present with other laboratory tests that are secondary supporting findings of PCOS, which I carefully consider but do not use to make a diagnosis of PCOS. Secondary supporting laboratory findings consistent with PCOS include: 1) a markedly elevated anti-müllerian hormone (AMH) level,12 2) an elevated fasting insulin level,2,13 and 3) an elevated luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio.13,14 But it is not necessary to measure AMH, fasting insulin, LH, and FSH levels. To conserve health care resources, I recommend against measuring those analytes to diagnose PCOS.
Continue to: Simplify the core laboratory tests...
Simplify the core laboratory tests
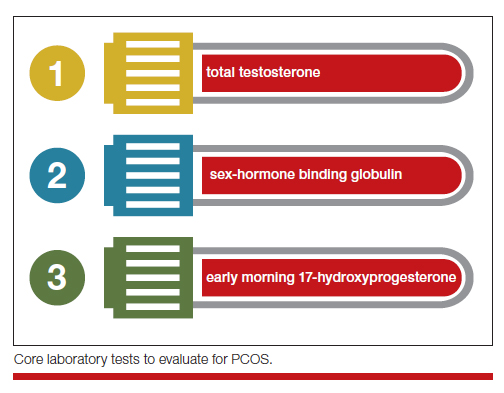
Simplify the testing used to support the diagnosis of PCOS by measuring total testosterone, sex-hormone binding globulin (SHBG) and early morning 17-hydroxyprogesterone (17-OH Prog).
The core criteria for diagnosis of PCOS are hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea or amenorrhea. Hyperandrogenism can be clinically diagnosed by assessing for the presence of hirsutism.7 Elevated levels of total testosterone, free testosterone, androstenedione, and/or dehydroepiandrosterone sulfate (DHEAS) suggest the presence of hyperandrogenism. In clinical practice, the laboratory approach to the diagnosis of hyperandrogenism can be simplified to the measurement of total testosterone, SHBG, and 17-OH Prog. By measuring total testosterone and SHBG, an estimate of free testosterone can be made. If the total testosterone is elevated, it is highly likely that the free testosterone is elevated. If the SHBG is abnormally low and the total testosterone level is in the upper limit of the normal range, the free testosterone is likely to be elevated.15 Using this approach, either an elevated total testosterone or an abnormally low SHBG indicate elevated free testosterone. For patients with hyperandrogenism and oligo-ovulation, an early morning (8 to 9 AM) 17-OH Prog level ≤ 2 ng/mL rules out the presence of NCAH due to a 21-hydroxylase deficiency.16 In my practice, the core laboratory tests I order when considering the diagnosis of PCOS are a total testosterone, SHBG, and 17-OH Prog.
Additional laboratory tests may be warranted to assess the patient diagnosed with PCOS. For example, if the patient has amenorrhea due to anovulation, tests for prolactin, FSH, and thyroid-stimulating hormone levels are warranted to assess for the presence of a prolactinoma, primary ovarian insufficiency, or thyroid disease, respectively. If the patient has a body mass index (BMI) ≥ 25 kg/m2, a hemoglobin A1c concentration is warranted to assess for the presence of prediabetes or DM.2 Many patients with PCOS have dyslipidemia, manifested through low high-density lipoprotein cholesterol levels and elevated low-density lipoprotein cholesterol levels, and a lipid panel assessment may be indicated. Among patients with PCOS, the most common lipid abnormality is a low high-density lipoprotein cholesterol level.17
Simplify the treatment of PCOS
Simplify treatment by counseling about lifestyle changes and prescribing an estrogen-progestin contraceptive, spironolactone, and metformin.
Most patients with PCOS have dysfunction in reproductive, metabolic, and dermatologic systems. For patients who are overweight or obese, lifestyle changes, including diet and exercise, that result in a 5% to 10% decrease in weight can improve metabolic balance, reduce circulating androgens, and increase menstrual frequency.18 For patients with PCOS and weight issues, referral to nutrition counseling or a full-service weight loss program can be very beneficial. In addition to lifestyle changes, patients with PCOS benefit from treatment with estrogen-progestin medications, spironolactone, and metformin.
Combination estrogen-progestin medications will lower LH secretion, decrease ovarian androgen production, increase SHBG production, decrease free testosterone levels and, if given cyclically, cause regular withdrawal bleeding.19 Spironolactone is an antiandrogen, which blocks the intracellular action of dihydrotestosterone and improves hirsutism and acne. Spironolactone also modestly decreases circulating levels of testosterone and DHEAS.20 For patients with metabolic problems, including insulin resistance and obesity, weight loss and/or treatment with metformin can help improve metabolic balance, which may result in restoration of ovulatory menses.21,22 Metformin can be effective in restoring ovulatory menses in both obese and lean patients with PCOS.22 The most common dermatologic problem caused by PCOS are hirsutism and acne. Both combination estrogen-progestin medications and spironolactone are effective treatments for hirsutism and acne.23
Estrogen-progestin hormones, spironolactone, and metformin are low-cost medications for the treatment of PCOS. Additional high-cost options for treatment of PCOS in obese patients include bariatric surgery and glucagon-like peptide (GLP-1) agonist medications (liraglutide and exenatide). For patients with PCOS and a body mass index (BMI) ≥ 35 kg/m2, bariatric surgery often results in sufficient weight loss to resolve the patient’s hyperandrogenism and oligo-ovulation, restoring spontaneous ovulatory cycles.24 In a study of more than 1,000 patients with: PCOS; mean BMI, 44 kg/m2; mean age, 31 years who were followed post-bariatric surgery for 5 years, > 90% of patients reported reductions in hirsutism and resumption of regular menses.25 For patients with PCOS seeking fertility, bariatric surgery often results in spontaneous pregnancy and live birth.26 GLP-1 agonists, including liraglutide or exenatide with or without metformin are effective in reducing weight, decreasing androgen levels, and restoring ovulatory menses.27,28
In my practice, I often prescribe 2 or 3 core medications for a patient with PCOS: 1) combination estrogen-progestin used cyclically or continuously, 2) spironolactone, and 3) metformin.19 Any estrogen-progestin contraceptive will suppress LH and ovarian androgen production; however, in the treatment of patients with PCOS, I prefer to use an estrogen-progestin combination that does not contain the androgenic progestin levonorgestrel.29 For the treatment of PCOS, I prefer to use an estrogen-progestin contraceptive with a non-androgenic progestin such as drospirenone, desogestrel, or gestodene. I routinely prescribe spironolactone at a dose of 100 mg, once daily, a dose near the top of the dose-response curve. A daily dose ≤ 50 mg of spironolactone is subtherapeutic for the treatment of hirsutism. A daily dose of 200 mg of spironolactone may cause bothersome breakthrough bleeding. When prescribing metformin, I usually recommend the extended-release formulation, at a dose of 750 mg with dinner. If well tolerated, I will increase the dose to 1,500 mg with dinner. Most of my patients with PCOS are taking a combination of 2 medications, either an estrogen-progestin contraceptive plus spironolactone or an estrogen-progestin contraceptive plus metformin.19 Some of my patients are taking all 3 medications. All 3 medications are very low cost.
For patients with PCOS and anovulatory infertility, letrozole treatment often results in ovulatory cycles and pregnancy with live birth. In obese PCOS patients, compared with clomiphene, letrozole results in superior live birth rates.30 Unlike clomiphene, letrozole is not approved by the US Food and Drug Administration for the treatment of anovulatory infertility.
The diagnosis of PCOS is often delayed due to confusion about how to make the diagnosis.31 To simplify the diagnosis of PCOS and improve patient encounters for PCOS, I focus on 2 core criteria: hyperandrogenism and oligo-ovulation. I recommend against ordering ultrasound imaging to assess for the presence of a multifollicular ovary. To simplify the treatment of PCOS I frequently prescribe an estrogen-progestin contraceptive, spironolactone, and metformin. By simplifying the diagnosis and treatment of PCOS, ObGyns will reduce patient confusion, improve outcomes, and save health care resources. ●
PCOS and adolescent patients
It is difficult to diagnose polycystic ovary syndrome (PCOS) in adolescents because oligo-ovulation is a common physiological feature of adolescence. Based on consensus among experts, PCOS should not be diagnosed within the first 2 years following menarche because the prevalence of oligo-ovulation is common at this stage of pubertal development. Two years after menarche, if an adolescent has a cycle length that is routinely > 45 days, it is likely that the pattern will persist, suggesting the presence of oligo-ovulation. Hyperandrogenism can be diagnosed based on the presence of moderate to severe hirsutism and/or an elevated testosterone or abnormally low sex-hormone binding globulin (SHBG) concentration. Two years after menarche the presence of oligo-ovulation and hyperandrogenism establishes the diagnosis of PCOS.1
PCOS and thrombophilia or migraine with aura
For patients with PCOS and a Factor V Leiden allele, where an estrogen-progestin contraceptive is contraindicated because of an increased risk of a venous thrombus, I prescribe spironolactone plus a levonorgestrel-intrauterine device. A low-dose oral progestin also may be considered because it will modestly suppress LH and ovarian androgen production. Similarly for patients with migraine with aura, where an estrogen-progestin contraceptive is contraindicated because of an increased of stroke, spironolactone plus a levonorgesterel intrauterine device may be effective in the treatment of hirsutism.
Androgen secreting tumors
Occasionally during the evaluation of a patient with hyperandrogenism and oligo-ovulation, measurement of total testosterone levels will reveal a value > 1.5 ng/mL. Most patients with PCOS have a total testosterone level ≤ 1.5 ng/mL. A total testosterone concentration > 1.5 ng/mL may be caused by ovarian stromal hyperthecosis or an androgen-producing tumor.2
Strongly-held patient perspectives on PCOS
At the first consultation visit, some patients are fearful and not receptive to a diagnosis of PCOS. If a clinician senses that the patient is not prepared to hear that they have PCOS, the clinician can be supportive of the patient’s perspective and focus on the patient’s chief health concerns, which may include abnormal cycle length, hirsutism, and/or overweight or obesity. During follow-up visits, as the patient builds trust with the clinician, the patient will be better prepared to discuss the diagnosis of PCOS. At the first consultation visit, some patients present with a strong belief that they have PCOS but have seen clinicians who conclude that they do not have PCOS. The diagnosis of PCOS is confusing because of competing diagnostic frameworks (NIH, Rotterdam, and AES). I avoid engaging in an argument with a patient who strongly believes that they have PCOS. In these situations, I focus on identifying the patient’s chief health concerns and discussing interventions to support their health goals.
References
1. Rosenfield RL. Perspectives on the international recommendations for the diagnosis and treatment of polycystic ovary syndrome in adolescence. J Pediatr Adolesc Gynecol. 2020;33:445-447.
2. Meczekalski B, Szeliga A, Maciejewska-Jeske M, et al. Hyperthecosis: an underestimated nontumorous cause of hyperandrogenism. Gynecol Endocrinol. 2021;37:677-682.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Polycystic Ovary Syndrome. Current Issues in Endocrinology and Metabolism. Dunaif A, Givens JR, Haseltine FP, Merriam GE (eds.). Blackwell Scientific Inc. Boston, Massachusetts; 1992:377.
- Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human Reprod. 2004;19:41-47.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Hatch R, Rosenfield RS, Kim MH, et al. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815-830.
- Johnstone EB, Rosen MP, Neril R, et al. The polycystic ovary post-Rotterdam: a common age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. 2010;95:4965-4972.
- Alsamarai S, Adams JM, Murphy MK, et al. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metab. 2009;94:4961-4970.
- Teede HJ, Misso ML, Costello MF, et al. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364-379.
- Serafini P, Lobo RA. Increased 5 alpha-reductase activity in idiopathic hirsutism. Fertil Steril. 1985;43:74-78.
- Pigny P, Jonard S, Robert Y, et al. Serum anti-Müllerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941-945.
- Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812-841.
- Kumar N, Agarwal H. Early clinical, biochemical and radiologic features in obese and non-obese young women with polycystic ovarian syndrome: a comparative study. Horm Metab Res. 2022;54:620-624.
- Lim SS, Norman RJ, Davies MJ, et al. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14:95-109.
- Nordenstrom A, Falhammar H. Management of endocrine disease: diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol. 2019;180:R127-145.
- Guo F, Gong Z, Fernando T, et al. The lipid profiles in different characteristics of women with PCOS and the interaction between dyslipidemia and metabolic disorder states: a retrospective study in Chinese population. Front Endocrinol. 2022;13:892125.
- Dietz de Loos ALP, Jiskoot G, Timman R, et al. Improvements in PCOS characteristics and phenotype severity during a randomized controlled lifestyle intervention. Reprod Biomed Online. 2021;43:298-309.
- Ezeh U, Huang A, Landay M, et al. Long-term response of hirsutism and other hyperandrogenic symptoms to combination therapy in polycystic ovary syndrome. J Women’s Health. 2018;27:892-902.
- Ashraf Ganie M, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Pasquali R, Gambineri A, Cavazza C, et al. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur J Endocrinol. 2011;164:53-60.
- Yang PK, Hsu CY, Chen MJ, et al. The efficacy of 24-month metformin for improving menses, hormones and metabolic profiles in polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103:890-899.
- Garg V, Choi J, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355.
- Hu L, Ma L, Ying T, et al. Efficacy of bariatric surgery in the treatment of women with obesity and polycystic ovary syndrome. J Clin Endocrinol Metab. 2022;107:e3217-3229.
- Bhandari M, Kosta S, Bhandari M, et al. Effects of bariatric surgery on people with obesity and polycystic ovary syndrome: a large single center study from India. Obes Surg. 2022;32:3305-3312.
- Benito E, Gomez-Martin JM, Vega-Pinero B, et al. Fertility and pregnancy outcomes in women with polycystic ovary syndrome following bariatric surgery. J Clin Endocrinol Metab. 2020;105:e3384-3391.
- Xing C, Li C, He B. Insulin sensitizers for improving the endocrine and metabolic profile in overweight women with PCOS. J Clin Endocrinol Metab. 2020;105:2950-2963.
- Elkind-Hirsch KE, Chappell N, Shaler D, et al. Liraglutide 3 mg on weight, body composition and hormonal and metabolic parameters in women with obesity and polycystic ovary syndrome: a randomized placebo-controlled-phase 3 study. Fertil Steril. 2022;118:371-381.
- Amiri M, Nahidi F, Bidhendi-Yarandi R, et al. A comparison of the effects of oral contraceptives on the clinical and biochemical manifestations of polycystic ovary syndrome: a crossover randomized controlled trial. Hum Reprod. 2020;35:175-186.
- Legro RS, Brzyski RG, Diamond NP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119-129.
- Gibson-Helm M, Teede H, Dunaif A, et al. Delayed diagnosis and lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102:604-612.
PCOS is a common problem, with a prevalence of 6% to 10% among women of reproductive age.1 Patients with PCOS often present with hirsutism, acne, female androgenetic alopecia, oligomenorrhea (also known as infrequent menstrual bleeding), amenorrhea, infertility, overweight, or obesity. In addition, many patients with PCOS have insulin resistance, dyslipidemia, metabolic syndrome, and an increased risk for developing type 2 diabetes mellitus (DM).2 A simplified approach to the diagnosis of PCOS will save health care resources by reducing the use of low-value diagnostic tests. A simplified approach to the treatment of PCOS will support patient medication adherence and improve health outcomes.
Simplify the diagnosis of PCOS
Simplify PCOS diagnosis by focusing on the core criteria of hyperandrogenism and oligo-ovulation. There are 3 major approaches to diagnosis:
- the 1990 National Institutes of Health (NIH) criteria3
- the 2003 Rotterdam criteria4,5
- the 2008 Androgen Excess and PCOS Society (AES) criteria.6
Using the 1990 NIH approach, the diagnosis of PCOS is made by the presence of 2 core criteria: hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea. In addition, other causes of hyperandrogenism should be excluded, including nonclassical adrenal hyperplasia (NCAH) due to 21-hydroxylase deficiency.3 Using the 1990 NIH criteria, PCOS can be diagnosed based on history (oligomenorrhea) and physical examination (assessment of the severity of hirsutism), but laboratory tests including total testosterone are often ordered.7
The Rotterdam approach to the diagnosis added a third criteria, the detection by ultrasonography of a multifollicular ovary and/or increased ovarian volume.4,5 Using the Rotterdam approach, PCOS is diagnosed in the presence of any 2 of the following 3 criteria: hyperandrogenism, oligo-ovulation, or ultrasound imaging showing the presence of a multifollicular ovary, identified by ≥ 12 antral follicles (2 to 9 mm in diameter) in each ovary or increased ovarian volume (> 10 mL).4,5
The Rotterdam approach using ovarian ultrasound as a criterion to diagnose PCOS is rife with serious problems, including:
- The number of small antral follicles in the normal ovary is age dependent, and many ovulatory and nonhirsute patients have ≥ 12 small antral follicles in each ovary.8,9
- There is no consensus on the number of small antral follicles needed to diagnose a multifollicular ovary, with recommendations to use thresholds of 124,5 or 20 follicles10 as the diagnostic cut-off.
- Accurate counting of the number of small ovarian follicles requires transvaginal ultrasound, which is not appropriate for many young adolescent patients.
- The process of counting ovarian follicles is operator-dependent.
- The high cost of ultrasound assessment of ovarian follicles (≥ $500 per examination).

The Rotterdam approach supports the diagnosis of PCOS in a patient with oligo-ovulation plus an ultrasound showing a multifollicular ovary in the absence of any clinical or laboratory evidence of hyperandrogenism.3,4,5 This approach to the diagnosis of PCOS is rejected by both the 1990 NIH3 and AES6 recommendations, which require the presence of hyperandrogenism as the sine qua non in the diagnosis of PCOS. I recommend against diagnosing PCOS in a non-hyperandrogenic patient with oligo-ovulation and a multifollicular ovary because other diagnoses are also possible, such as functional hypothalamic oligo-ovulation, especially in young patients. The Rotterdam approach also supports the diagnosis of PCOS in a patient with hyperandrogenism, an ultrasound showing a multifollicular ovary, and normal ovulation and menses.3,4 For most patients with normal, regular ovulation and menses, the testosterone concentration is normal and the only evidence of hyperandrogenism is hirsutism. Patients with normal, regular ovulation and menses plus hirsutism usually have idiopathic hirsutism. Idiopathic hirsutism is a problem caused by excessive 5-alpha-reductase activity in the hair pilosebaceous unit, which catalyzes the conversion of weak androgens into dihydrotestosterone, a potent intracellular androgen that stimulates terminal hair growth.11 In my opinion, the Rotterdam approach to diagnosing PCOS has created unnecessary confusion and complexity for both clinicians and patients. I believe we should simplify the diagnosis of PCOS and return to the 1990 NIH criteria.3
On occasion, a patient presents for a consultation and has already had an ovarian ultrasound to assess for a multifollicular ovary. I carefully read the report and, if a multifollicular ovary has been identified, I consider it as a secondary supporting finding of PCOS in my clinical assessment. But I do not base my diagnosis on the ultrasound finding. Patients often present with other laboratory tests that are secondary supporting findings of PCOS, which I carefully consider but do not use to make a diagnosis of PCOS. Secondary supporting laboratory findings consistent with PCOS include: 1) a markedly elevated anti-müllerian hormone (AMH) level,12 2) an elevated fasting insulin level,2,13 and 3) an elevated luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio.13,14 But it is not necessary to measure AMH, fasting insulin, LH, and FSH levels. To conserve health care resources, I recommend against measuring those analytes to diagnose PCOS.

Continue to: Simplify the core laboratory tests...
Simplify the core laboratory tests
Simplify the testing used to support the diagnosis of PCOS by measuring total testosterone, sex-hormone binding globulin (SHBG) and early morning 17-hydroxyprogesterone (17-OH Prog).
The core criteria for diagnosis of PCOS are hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea or amenorrhea. Hyperandrogenism can be clinically diagnosed by assessing for the presence of hirsutism.7 Elevated levels of total testosterone, free testosterone, androstenedione, and/or dehydroepiandrosterone sulfate (DHEAS) suggest the presence of hyperandrogenism. In clinical practice, the laboratory approach to the diagnosis of hyperandrogenism can be simplified to the measurement of total testosterone, SHBG, and 17-OH Prog. By measuring total testosterone and SHBG, an estimate of free testosterone can be made. If the total testosterone is elevated, it is highly likely that the free testosterone is elevated. If the SHBG is abnormally low and the total testosterone level is in the upper limit of the normal range, the free testosterone is likely to be elevated.15 Using this approach, either an elevated total testosterone or an abnormally low SHBG indicate elevated free testosterone. For patients with hyperandrogenism and oligo-ovulation, an early morning (8 to 9 AM) 17-OH Prog level ≤ 2 ng/mL rules out the presence of NCAH due to a 21-hydroxylase deficiency.16 In my practice, the core laboratory tests I order when considering the diagnosis of PCOS are a total testosterone, SHBG, and 17-OH Prog.
Additional laboratory tests may be warranted to assess the patient diagnosed with PCOS. For example, if the patient has amenorrhea due to anovulation, tests for prolactin, FSH, and thyroid-stimulating hormone levels are warranted to assess for the presence of a prolactinoma, primary ovarian insufficiency, or thyroid disease, respectively. If the patient has a body mass index (BMI) ≥ 25 kg/m2, a hemoglobin A1c concentration is warranted to assess for the presence of prediabetes or DM.2 Many patients with PCOS have dyslipidemia, manifested through low high-density lipoprotein cholesterol levels and elevated low-density lipoprotein cholesterol levels, and a lipid panel assessment may be indicated. Among patients with PCOS, the most common lipid abnormality is a low high-density lipoprotein cholesterol level.17
Simplify the treatment of PCOS
Simplify treatment by counseling about lifestyle changes and prescribing an estrogen-progestin contraceptive, spironolactone, and metformin.
Most patients with PCOS have dysfunction in reproductive, metabolic, and dermatologic systems. For patients who are overweight or obese, lifestyle changes, including diet and exercise, that result in a 5% to 10% decrease in weight can improve metabolic balance, reduce circulating androgens, and increase menstrual frequency.18 For patients with PCOS and weight issues, referral to nutrition counseling or a full-service weight loss program can be very beneficial. In addition to lifestyle changes, patients with PCOS benefit from treatment with estrogen-progestin medications, spironolactone, and metformin.
Combination estrogen-progestin medications will lower LH secretion, decrease ovarian androgen production, increase SHBG production, decrease free testosterone levels and, if given cyclically, cause regular withdrawal bleeding.19 Spironolactone is an antiandrogen, which blocks the intracellular action of dihydrotestosterone and improves hirsutism and acne. Spironolactone also modestly decreases circulating levels of testosterone and DHEAS.20 For patients with metabolic problems, including insulin resistance and obesity, weight loss and/or treatment with metformin can help improve metabolic balance, which may result in restoration of ovulatory menses.21,22 Metformin can be effective in restoring ovulatory menses in both obese and lean patients with PCOS.22 The most common dermatologic problem caused by PCOS are hirsutism and acne. Both combination estrogen-progestin medications and spironolactone are effective treatments for hirsutism and acne.23
Estrogen-progestin hormones, spironolactone, and metformin are low-cost medications for the treatment of PCOS. Additional high-cost options for treatment of PCOS in obese patients include bariatric surgery and glucagon-like peptide (GLP-1) agonist medications (liraglutide and exenatide). For patients with PCOS and a body mass index (BMI) ≥ 35 kg/m2, bariatric surgery often results in sufficient weight loss to resolve the patient’s hyperandrogenism and oligo-ovulation, restoring spontaneous ovulatory cycles.24 In a study of more than 1,000 patients with: PCOS; mean BMI, 44 kg/m2; mean age, 31 years who were followed post-bariatric surgery for 5 years, > 90% of patients reported reductions in hirsutism and resumption of regular menses.25 For patients with PCOS seeking fertility, bariatric surgery often results in spontaneous pregnancy and live birth.26 GLP-1 agonists, including liraglutide or exenatide with or without metformin are effective in reducing weight, decreasing androgen levels, and restoring ovulatory menses.27,28
In my practice, I often prescribe 2 or 3 core medications for a patient with PCOS: 1) combination estrogen-progestin used cyclically or continuously, 2) spironolactone, and 3) metformin.19 Any estrogen-progestin contraceptive will suppress LH and ovarian androgen production; however, in the treatment of patients with PCOS, I prefer to use an estrogen-progestin combination that does not contain the androgenic progestin levonorgestrel.29 For the treatment of PCOS, I prefer to use an estrogen-progestin contraceptive with a non-androgenic progestin such as drospirenone, desogestrel, or gestodene. I routinely prescribe spironolactone at a dose of 100 mg, once daily, a dose near the top of the dose-response curve. A daily dose ≤ 50 mg of spironolactone is subtherapeutic for the treatment of hirsutism. A daily dose of 200 mg of spironolactone may cause bothersome breakthrough bleeding. When prescribing metformin, I usually recommend the extended-release formulation, at a dose of 750 mg with dinner. If well tolerated, I will increase the dose to 1,500 mg with dinner. Most of my patients with PCOS are taking a combination of 2 medications, either an estrogen-progestin contraceptive plus spironolactone or an estrogen-progestin contraceptive plus metformin.19 Some of my patients are taking all 3 medications. All 3 medications are very low cost.
For patients with PCOS and anovulatory infertility, letrozole treatment often results in ovulatory cycles and pregnancy with live birth. In obese PCOS patients, compared with clomiphene, letrozole results in superior live birth rates.30 Unlike clomiphene, letrozole is not approved by the US Food and Drug Administration for the treatment of anovulatory infertility.
The diagnosis of PCOS is often delayed due to confusion about how to make the diagnosis.31 To simplify the diagnosis of PCOS and improve patient encounters for PCOS, I focus on 2 core criteria: hyperandrogenism and oligo-ovulation. I recommend against ordering ultrasound imaging to assess for the presence of a multifollicular ovary. To simplify the treatment of PCOS I frequently prescribe an estrogen-progestin contraceptive, spironolactone, and metformin. By simplifying the diagnosis and treatment of PCOS, ObGyns will reduce patient confusion, improve outcomes, and save health care resources. ●
PCOS and adolescent patients
It is difficult to diagnose polycystic ovary syndrome (PCOS) in adolescents because oligo-ovulation is a common physiological feature of adolescence. Based on consensus among experts, PCOS should not be diagnosed within the first 2 years following menarche because the prevalence of oligo-ovulation is common at this stage of pubertal development. Two years after menarche, if an adolescent has a cycle length that is routinely > 45 days, it is likely that the pattern will persist, suggesting the presence of oligo-ovulation. Hyperandrogenism can be diagnosed based on the presence of moderate to severe hirsutism and/or an elevated testosterone or abnormally low sex-hormone binding globulin (SHBG) concentration. Two years after menarche the presence of oligo-ovulation and hyperandrogenism establishes the diagnosis of PCOS.1
PCOS and thrombophilia or migraine with aura
For patients with PCOS and a Factor V Leiden allele, where an estrogen-progestin contraceptive is contraindicated because of an increased risk of a venous thrombus, I prescribe spironolactone plus a levonorgestrel-intrauterine device. A low-dose oral progestin also may be considered because it will modestly suppress LH and ovarian androgen production. Similarly for patients with migraine with aura, where an estrogen-progestin contraceptive is contraindicated because of an increased of stroke, spironolactone plus a levonorgesterel intrauterine device may be effective in the treatment of hirsutism.
Androgen secreting tumors
Occasionally during the evaluation of a patient with hyperandrogenism and oligo-ovulation, measurement of total testosterone levels will reveal a value > 1.5 ng/mL. Most patients with PCOS have a total testosterone level ≤ 1.5 ng/mL. A total testosterone concentration > 1.5 ng/mL may be caused by ovarian stromal hyperthecosis or an androgen-producing tumor.2
Strongly-held patient perspectives on PCOS
At the first consultation visit, some patients are fearful and not receptive to a diagnosis of PCOS. If a clinician senses that the patient is not prepared to hear that they have PCOS, the clinician can be supportive of the patient’s perspective and focus on the patient’s chief health concerns, which may include abnormal cycle length, hirsutism, and/or overweight or obesity. During follow-up visits, as the patient builds trust with the clinician, the patient will be better prepared to discuss the diagnosis of PCOS. At the first consultation visit, some patients present with a strong belief that they have PCOS but have seen clinicians who conclude that they do not have PCOS. The diagnosis of PCOS is confusing because of competing diagnostic frameworks (NIH, Rotterdam, and AES). I avoid engaging in an argument with a patient who strongly believes that they have PCOS. In these situations, I focus on identifying the patient’s chief health concerns and discussing interventions to support their health goals.
References
1. Rosenfield RL. Perspectives on the international recommendations for the diagnosis and treatment of polycystic ovary syndrome in adolescence. J Pediatr Adolesc Gynecol. 2020;33:445-447.
2. Meczekalski B, Szeliga A, Maciejewska-Jeske M, et al. Hyperthecosis: an underestimated nontumorous cause of hyperandrogenism. Gynecol Endocrinol. 2021;37:677-682.
PCOS is a common problem, with a prevalence of 6% to 10% among women of reproductive age.1 Patients with PCOS often present with hirsutism, acne, female androgenetic alopecia, oligomenorrhea (also known as infrequent menstrual bleeding), amenorrhea, infertility, overweight, or obesity. In addition, many patients with PCOS have insulin resistance, dyslipidemia, metabolic syndrome, and an increased risk for developing type 2 diabetes mellitus (DM).2 A simplified approach to the diagnosis of PCOS will save health care resources by reducing the use of low-value diagnostic tests. A simplified approach to the treatment of PCOS will support patient medication adherence and improve health outcomes.
Simplify the diagnosis of PCOS
Simplify PCOS diagnosis by focusing on the core criteria of hyperandrogenism and oligo-ovulation. There are 3 major approaches to diagnosis:
- the 1990 National Institutes of Health (NIH) criteria3
- the 2003 Rotterdam criteria4,5
- the 2008 Androgen Excess and PCOS Society (AES) criteria.6
Using the 1990 NIH approach, the diagnosis of PCOS is made by the presence of 2 core criteria: hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea. In addition, other causes of hyperandrogenism should be excluded, including nonclassical adrenal hyperplasia (NCAH) due to 21-hydroxylase deficiency.3 Using the 1990 NIH criteria, PCOS can be diagnosed based on history (oligomenorrhea) and physical examination (assessment of the severity of hirsutism), but laboratory tests including total testosterone are often ordered.7
The Rotterdam approach to the diagnosis added a third criteria, the detection by ultrasonography of a multifollicular ovary and/or increased ovarian volume.4,5 Using the Rotterdam approach, PCOS is diagnosed in the presence of any 2 of the following 3 criteria: hyperandrogenism, oligo-ovulation, or ultrasound imaging showing the presence of a multifollicular ovary, identified by ≥ 12 antral follicles (2 to 9 mm in diameter) in each ovary or increased ovarian volume (> 10 mL).4,5
The Rotterdam approach using ovarian ultrasound as a criterion to diagnose PCOS is rife with serious problems, including:
- The number of small antral follicles in the normal ovary is age dependent, and many ovulatory and nonhirsute patients have ≥ 12 small antral follicles in each ovary.8,9
- There is no consensus on the number of small antral follicles needed to diagnose a multifollicular ovary, with recommendations to use thresholds of 124,5 or 20 follicles10 as the diagnostic cut-off.
- Accurate counting of the number of small ovarian follicles requires transvaginal ultrasound, which is not appropriate for many young adolescent patients.
- The process of counting ovarian follicles is operator-dependent.
- The high cost of ultrasound assessment of ovarian follicles (≥ $500 per examination).

The Rotterdam approach supports the diagnosis of PCOS in a patient with oligo-ovulation plus an ultrasound showing a multifollicular ovary in the absence of any clinical or laboratory evidence of hyperandrogenism.3,4,5 This approach to the diagnosis of PCOS is rejected by both the 1990 NIH3 and AES6 recommendations, which require the presence of hyperandrogenism as the sine qua non in the diagnosis of PCOS. I recommend against diagnosing PCOS in a non-hyperandrogenic patient with oligo-ovulation and a multifollicular ovary because other diagnoses are also possible, such as functional hypothalamic oligo-ovulation, especially in young patients. The Rotterdam approach also supports the diagnosis of PCOS in a patient with hyperandrogenism, an ultrasound showing a multifollicular ovary, and normal ovulation and menses.3,4 For most patients with normal, regular ovulation and menses, the testosterone concentration is normal and the only evidence of hyperandrogenism is hirsutism. Patients with normal, regular ovulation and menses plus hirsutism usually have idiopathic hirsutism. Idiopathic hirsutism is a problem caused by excessive 5-alpha-reductase activity in the hair pilosebaceous unit, which catalyzes the conversion of weak androgens into dihydrotestosterone, a potent intracellular androgen that stimulates terminal hair growth.11 In my opinion, the Rotterdam approach to diagnosing PCOS has created unnecessary confusion and complexity for both clinicians and patients. I believe we should simplify the diagnosis of PCOS and return to the 1990 NIH criteria.3
On occasion, a patient presents for a consultation and has already had an ovarian ultrasound to assess for a multifollicular ovary. I carefully read the report and, if a multifollicular ovary has been identified, I consider it as a secondary supporting finding of PCOS in my clinical assessment. But I do not base my diagnosis on the ultrasound finding. Patients often present with other laboratory tests that are secondary supporting findings of PCOS, which I carefully consider but do not use to make a diagnosis of PCOS. Secondary supporting laboratory findings consistent with PCOS include: 1) a markedly elevated anti-müllerian hormone (AMH) level,12 2) an elevated fasting insulin level,2,13 and 3) an elevated luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio.13,14 But it is not necessary to measure AMH, fasting insulin, LH, and FSH levels. To conserve health care resources, I recommend against measuring those analytes to diagnose PCOS.

Continue to: Simplify the core laboratory tests...
Simplify the core laboratory tests
Simplify the testing used to support the diagnosis of PCOS by measuring total testosterone, sex-hormone binding globulin (SHBG) and early morning 17-hydroxyprogesterone (17-OH Prog).
The core criteria for diagnosis of PCOS are hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea or amenorrhea. Hyperandrogenism can be clinically diagnosed by assessing for the presence of hirsutism.7 Elevated levels of total testosterone, free testosterone, androstenedione, and/or dehydroepiandrosterone sulfate (DHEAS) suggest the presence of hyperandrogenism. In clinical practice, the laboratory approach to the diagnosis of hyperandrogenism can be simplified to the measurement of total testosterone, SHBG, and 17-OH Prog. By measuring total testosterone and SHBG, an estimate of free testosterone can be made. If the total testosterone is elevated, it is highly likely that the free testosterone is elevated. If the SHBG is abnormally low and the total testosterone level is in the upper limit of the normal range, the free testosterone is likely to be elevated.15 Using this approach, either an elevated total testosterone or an abnormally low SHBG indicate elevated free testosterone. For patients with hyperandrogenism and oligo-ovulation, an early morning (8 to 9 AM) 17-OH Prog level ≤ 2 ng/mL rules out the presence of NCAH due to a 21-hydroxylase deficiency.16 In my practice, the core laboratory tests I order when considering the diagnosis of PCOS are a total testosterone, SHBG, and 17-OH Prog.
Additional laboratory tests may be warranted to assess the patient diagnosed with PCOS. For example, if the patient has amenorrhea due to anovulation, tests for prolactin, FSH, and thyroid-stimulating hormone levels are warranted to assess for the presence of a prolactinoma, primary ovarian insufficiency, or thyroid disease, respectively. If the patient has a body mass index (BMI) ≥ 25 kg/m2, a hemoglobin A1c concentration is warranted to assess for the presence of prediabetes or DM.2 Many patients with PCOS have dyslipidemia, manifested through low high-density lipoprotein cholesterol levels and elevated low-density lipoprotein cholesterol levels, and a lipid panel assessment may be indicated. Among patients with PCOS, the most common lipid abnormality is a low high-density lipoprotein cholesterol level.17
Simplify the treatment of PCOS
Simplify treatment by counseling about lifestyle changes and prescribing an estrogen-progestin contraceptive, spironolactone, and metformin.
Most patients with PCOS have dysfunction in reproductive, metabolic, and dermatologic systems. For patients who are overweight or obese, lifestyle changes, including diet and exercise, that result in a 5% to 10% decrease in weight can improve metabolic balance, reduce circulating androgens, and increase menstrual frequency.18 For patients with PCOS and weight issues, referral to nutrition counseling or a full-service weight loss program can be very beneficial. In addition to lifestyle changes, patients with PCOS benefit from treatment with estrogen-progestin medications, spironolactone, and metformin.
Combination estrogen-progestin medications will lower LH secretion, decrease ovarian androgen production, increase SHBG production, decrease free testosterone levels and, if given cyclically, cause regular withdrawal bleeding.19 Spironolactone is an antiandrogen, which blocks the intracellular action of dihydrotestosterone and improves hirsutism and acne. Spironolactone also modestly decreases circulating levels of testosterone and DHEAS.20 For patients with metabolic problems, including insulin resistance and obesity, weight loss and/or treatment with metformin can help improve metabolic balance, which may result in restoration of ovulatory menses.21,22 Metformin can be effective in restoring ovulatory menses in both obese and lean patients with PCOS.22 The most common dermatologic problem caused by PCOS are hirsutism and acne. Both combination estrogen-progestin medications and spironolactone are effective treatments for hirsutism and acne.23
Estrogen-progestin hormones, spironolactone, and metformin are low-cost medications for the treatment of PCOS. Additional high-cost options for treatment of PCOS in obese patients include bariatric surgery and glucagon-like peptide (GLP-1) agonist medications (liraglutide and exenatide). For patients with PCOS and a body mass index (BMI) ≥ 35 kg/m2, bariatric surgery often results in sufficient weight loss to resolve the patient’s hyperandrogenism and oligo-ovulation, restoring spontaneous ovulatory cycles.24 In a study of more than 1,000 patients with: PCOS; mean BMI, 44 kg/m2; mean age, 31 years who were followed post-bariatric surgery for 5 years, > 90% of patients reported reductions in hirsutism and resumption of regular menses.25 For patients with PCOS seeking fertility, bariatric surgery often results in spontaneous pregnancy and live birth.26 GLP-1 agonists, including liraglutide or exenatide with or without metformin are effective in reducing weight, decreasing androgen levels, and restoring ovulatory menses.27,28
In my practice, I often prescribe 2 or 3 core medications for a patient with PCOS: 1) combination estrogen-progestin used cyclically or continuously, 2) spironolactone, and 3) metformin.19 Any estrogen-progestin contraceptive will suppress LH and ovarian androgen production; however, in the treatment of patients with PCOS, I prefer to use an estrogen-progestin combination that does not contain the androgenic progestin levonorgestrel.29 For the treatment of PCOS, I prefer to use an estrogen-progestin contraceptive with a non-androgenic progestin such as drospirenone, desogestrel, or gestodene. I routinely prescribe spironolactone at a dose of 100 mg, once daily, a dose near the top of the dose-response curve. A daily dose ≤ 50 mg of spironolactone is subtherapeutic for the treatment of hirsutism. A daily dose of 200 mg of spironolactone may cause bothersome breakthrough bleeding. When prescribing metformin, I usually recommend the extended-release formulation, at a dose of 750 mg with dinner. If well tolerated, I will increase the dose to 1,500 mg with dinner. Most of my patients with PCOS are taking a combination of 2 medications, either an estrogen-progestin contraceptive plus spironolactone or an estrogen-progestin contraceptive plus metformin.19 Some of my patients are taking all 3 medications. All 3 medications are very low cost.
For patients with PCOS and anovulatory infertility, letrozole treatment often results in ovulatory cycles and pregnancy with live birth. In obese PCOS patients, compared with clomiphene, letrozole results in superior live birth rates.30 Unlike clomiphene, letrozole is not approved by the US Food and Drug Administration for the treatment of anovulatory infertility.
The diagnosis of PCOS is often delayed due to confusion about how to make the diagnosis.31 To simplify the diagnosis of PCOS and improve patient encounters for PCOS, I focus on 2 core criteria: hyperandrogenism and oligo-ovulation. I recommend against ordering ultrasound imaging to assess for the presence of a multifollicular ovary. To simplify the treatment of PCOS I frequently prescribe an estrogen-progestin contraceptive, spironolactone, and metformin. By simplifying the diagnosis and treatment of PCOS, ObGyns will reduce patient confusion, improve outcomes, and save health care resources. ●
PCOS and adolescent patients
It is difficult to diagnose polycystic ovary syndrome (PCOS) in adolescents because oligo-ovulation is a common physiological feature of adolescence. Based on consensus among experts, PCOS should not be diagnosed within the first 2 years following menarche because the prevalence of oligo-ovulation is common at this stage of pubertal development. Two years after menarche, if an adolescent has a cycle length that is routinely > 45 days, it is likely that the pattern will persist, suggesting the presence of oligo-ovulation. Hyperandrogenism can be diagnosed based on the presence of moderate to severe hirsutism and/or an elevated testosterone or abnormally low sex-hormone binding globulin (SHBG) concentration. Two years after menarche the presence of oligo-ovulation and hyperandrogenism establishes the diagnosis of PCOS.1
PCOS and thrombophilia or migraine with aura
For patients with PCOS and a Factor V Leiden allele, where an estrogen-progestin contraceptive is contraindicated because of an increased risk of a venous thrombus, I prescribe spironolactone plus a levonorgestrel-intrauterine device. A low-dose oral progestin also may be considered because it will modestly suppress LH and ovarian androgen production. Similarly for patients with migraine with aura, where an estrogen-progestin contraceptive is contraindicated because of an increased of stroke, spironolactone plus a levonorgesterel intrauterine device may be effective in the treatment of hirsutism.
Androgen secreting tumors
Occasionally during the evaluation of a patient with hyperandrogenism and oligo-ovulation, measurement of total testosterone levels will reveal a value > 1.5 ng/mL. Most patients with PCOS have a total testosterone level ≤ 1.5 ng/mL. A total testosterone concentration > 1.5 ng/mL may be caused by ovarian stromal hyperthecosis or an androgen-producing tumor.2
Strongly-held patient perspectives on PCOS
At the first consultation visit, some patients are fearful and not receptive to a diagnosis of PCOS. If a clinician senses that the patient is not prepared to hear that they have PCOS, the clinician can be supportive of the patient’s perspective and focus on the patient’s chief health concerns, which may include abnormal cycle length, hirsutism, and/or overweight or obesity. During follow-up visits, as the patient builds trust with the clinician, the patient will be better prepared to discuss the diagnosis of PCOS. At the first consultation visit, some patients present with a strong belief that they have PCOS but have seen clinicians who conclude that they do not have PCOS. The diagnosis of PCOS is confusing because of competing diagnostic frameworks (NIH, Rotterdam, and AES). I avoid engaging in an argument with a patient who strongly believes that they have PCOS. In these situations, I focus on identifying the patient’s chief health concerns and discussing interventions to support their health goals.
References
1. Rosenfield RL. Perspectives on the international recommendations for the diagnosis and treatment of polycystic ovary syndrome in adolescence. J Pediatr Adolesc Gynecol. 2020;33:445-447.
2. Meczekalski B, Szeliga A, Maciejewska-Jeske M, et al. Hyperthecosis: an underestimated nontumorous cause of hyperandrogenism. Gynecol Endocrinol. 2021;37:677-682.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Polycystic Ovary Syndrome. Current Issues in Endocrinology and Metabolism. Dunaif A, Givens JR, Haseltine FP, Merriam GE (eds.). Blackwell Scientific Inc. Boston, Massachusetts; 1992:377.
- Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human Reprod. 2004;19:41-47.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Hatch R, Rosenfield RS, Kim MH, et al. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815-830.
- Johnstone EB, Rosen MP, Neril R, et al. The polycystic ovary post-Rotterdam: a common age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. 2010;95:4965-4972.
- Alsamarai S, Adams JM, Murphy MK, et al. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metab. 2009;94:4961-4970.
- Teede HJ, Misso ML, Costello MF, et al. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364-379.
- Serafini P, Lobo RA. Increased 5 alpha-reductase activity in idiopathic hirsutism. Fertil Steril. 1985;43:74-78.
- Pigny P, Jonard S, Robert Y, et al. Serum anti-Müllerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941-945.
- Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812-841.
- Kumar N, Agarwal H. Early clinical, biochemical and radiologic features in obese and non-obese young women with polycystic ovarian syndrome: a comparative study. Horm Metab Res. 2022;54:620-624.
- Lim SS, Norman RJ, Davies MJ, et al. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14:95-109.
- Nordenstrom A, Falhammar H. Management of endocrine disease: diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol. 2019;180:R127-145.
- Guo F, Gong Z, Fernando T, et al. The lipid profiles in different characteristics of women with PCOS and the interaction between dyslipidemia and metabolic disorder states: a retrospective study in Chinese population. Front Endocrinol. 2022;13:892125.
- Dietz de Loos ALP, Jiskoot G, Timman R, et al. Improvements in PCOS characteristics and phenotype severity during a randomized controlled lifestyle intervention. Reprod Biomed Online. 2021;43:298-309.
- Ezeh U, Huang A, Landay M, et al. Long-term response of hirsutism and other hyperandrogenic symptoms to combination therapy in polycystic ovary syndrome. J Women’s Health. 2018;27:892-902.
- Ashraf Ganie M, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Pasquali R, Gambineri A, Cavazza C, et al. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur J Endocrinol. 2011;164:53-60.
- Yang PK, Hsu CY, Chen MJ, et al. The efficacy of 24-month metformin for improving menses, hormones and metabolic profiles in polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103:890-899.
- Garg V, Choi J, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355.
- Hu L, Ma L, Ying T, et al. Efficacy of bariatric surgery in the treatment of women with obesity and polycystic ovary syndrome. J Clin Endocrinol Metab. 2022;107:e3217-3229.
- Bhandari M, Kosta S, Bhandari M, et al. Effects of bariatric surgery on people with obesity and polycystic ovary syndrome: a large single center study from India. Obes Surg. 2022;32:3305-3312.
- Benito E, Gomez-Martin JM, Vega-Pinero B, et al. Fertility and pregnancy outcomes in women with polycystic ovary syndrome following bariatric surgery. J Clin Endocrinol Metab. 2020;105:e3384-3391.
- Xing C, Li C, He B. Insulin sensitizers for improving the endocrine and metabolic profile in overweight women with PCOS. J Clin Endocrinol Metab. 2020;105:2950-2963.
- Elkind-Hirsch KE, Chappell N, Shaler D, et al. Liraglutide 3 mg on weight, body composition and hormonal and metabolic parameters in women with obesity and polycystic ovary syndrome: a randomized placebo-controlled-phase 3 study. Fertil Steril. 2022;118:371-381.
- Amiri M, Nahidi F, Bidhendi-Yarandi R, et al. A comparison of the effects of oral contraceptives on the clinical and biochemical manifestations of polycystic ovary syndrome: a crossover randomized controlled trial. Hum Reprod. 2020;35:175-186.
- Legro RS, Brzyski RG, Diamond NP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119-129.
- Gibson-Helm M, Teede H, Dunaif A, et al. Delayed diagnosis and lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102:604-612.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Polycystic Ovary Syndrome. Current Issues in Endocrinology and Metabolism. Dunaif A, Givens JR, Haseltine FP, Merriam GE (eds.). Blackwell Scientific Inc. Boston, Massachusetts; 1992:377.
- Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human Reprod. 2004;19:41-47.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Hatch R, Rosenfield RS, Kim MH, et al. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815-830.
- Johnstone EB, Rosen MP, Neril R, et al. The polycystic ovary post-Rotterdam: a common age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. 2010;95:4965-4972.
- Alsamarai S, Adams JM, Murphy MK, et al. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metab. 2009;94:4961-4970.
- Teede HJ, Misso ML, Costello MF, et al. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364-379.
- Serafini P, Lobo RA. Increased 5 alpha-reductase activity in idiopathic hirsutism. Fertil Steril. 1985;43:74-78.
- Pigny P, Jonard S, Robert Y, et al. Serum anti-Müllerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941-945.
- Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812-841.
- Kumar N, Agarwal H. Early clinical, biochemical and radiologic features in obese and non-obese young women with polycystic ovarian syndrome: a comparative study. Horm Metab Res. 2022;54:620-624.
- Lim SS, Norman RJ, Davies MJ, et al. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14:95-109.
- Nordenstrom A, Falhammar H. Management of endocrine disease: diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol. 2019;180:R127-145.
- Guo F, Gong Z, Fernando T, et al. The lipid profiles in different characteristics of women with PCOS and the interaction between dyslipidemia and metabolic disorder states: a retrospective study in Chinese population. Front Endocrinol. 2022;13:892125.
- Dietz de Loos ALP, Jiskoot G, Timman R, et al. Improvements in PCOS characteristics and phenotype severity during a randomized controlled lifestyle intervention. Reprod Biomed Online. 2021;43:298-309.
- Ezeh U, Huang A, Landay M, et al. Long-term response of hirsutism and other hyperandrogenic symptoms to combination therapy in polycystic ovary syndrome. J Women’s Health. 2018;27:892-902.
- Ashraf Ganie M, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Pasquali R, Gambineri A, Cavazza C, et al. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur J Endocrinol. 2011;164:53-60.
- Yang PK, Hsu CY, Chen MJ, et al. The efficacy of 24-month metformin for improving menses, hormones and metabolic profiles in polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103:890-899.
- Garg V, Choi J, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355.
- Hu L, Ma L, Ying T, et al. Efficacy of bariatric surgery in the treatment of women with obesity and polycystic ovary syndrome. J Clin Endocrinol Metab. 2022;107:e3217-3229.
- Bhandari M, Kosta S, Bhandari M, et al. Effects of bariatric surgery on people with obesity and polycystic ovary syndrome: a large single center study from India. Obes Surg. 2022;32:3305-3312.
- Benito E, Gomez-Martin JM, Vega-Pinero B, et al. Fertility and pregnancy outcomes in women with polycystic ovary syndrome following bariatric surgery. J Clin Endocrinol Metab. 2020;105:e3384-3391.
- Xing C, Li C, He B. Insulin sensitizers for improving the endocrine and metabolic profile in overweight women with PCOS. J Clin Endocrinol Metab. 2020;105:2950-2963.
- Elkind-Hirsch KE, Chappell N, Shaler D, et al. Liraglutide 3 mg on weight, body composition and hormonal and metabolic parameters in women with obesity and polycystic ovary syndrome: a randomized placebo-controlled-phase 3 study. Fertil Steril. 2022;118:371-381.
- Amiri M, Nahidi F, Bidhendi-Yarandi R, et al. A comparison of the effects of oral contraceptives on the clinical and biochemical manifestations of polycystic ovary syndrome: a crossover randomized controlled trial. Hum Reprod. 2020;35:175-186.
- Legro RS, Brzyski RG, Diamond NP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119-129.
- Gibson-Helm M, Teede H, Dunaif A, et al. Delayed diagnosis and lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102:604-612.
