User login
Case Scenario
A 69-year-old man was transported to the ED via emergency medical services after a family member discovered him alone at home and confused. His wife stated that her husband had been sick with the flu and had been febrile for the previous several days. The patient’s blood pressure taken on the scene by the emergency medical technician was 80/40 mm Hg, and 1 L normal saline was infused during transport. Upon arrival to the ED, his vital signs were: temperature, 103.3°F; heart rate,130 beats/minute; BP, 90/48 mm Hg; and respiratory rate, 24 breaths/minute. Oxygen saturation was 92% on nasal canula. An electrocardiogram showed sinus tachycardia with nonspecific changes.
Based on the patient’s symptoms, the emergency physician (EP) suspected sepsis and ordered the appropriate laboratory studies and radiographic images. During evaluation, the patient’s systolic BP decreased to 70 from 80 mm Hg, and the EP ordered another fluid bolus and considered assessing the patient’s volume status.
Introduction
There is a long-standing debate as to the most accurate method of determining the volume status of a critically ill patient, as well as the physiological ability to respond to fluid therapy. In the assessment of a critically ill patient receiving volume replacement, a wide variability of assessment options are available; however, the current literature has yet to determine which method is the best. This article reviews multiple approaches to estimating the intravascular volume status of critically ill patients and the use of modalities to determine a patient’s physiological response to fluid therapy.
Basic Physiology
Central venous pressure (CVP) is the pressure in the thoracic vena cava adjacent to the right atrium. The heart functions as a two-sided pump; the right side pumps volume at low pressure and the left side pumps against systemic arterial pressure. The major determinant of the filling pressure of the right ventricle (RV) at the end of diastole is CVP, which is affected by the initial stretching of the ventricles before contraction (preload).
Frank-Starling Mechanism
The Frank-Starling mechanism describes the relationship between cardiac performance and intravascular volume. Stroke volume increases in response to an increase in preload volume. The increased volume of blood stretches the ventricular wall, causing the cardiac muscle to contract more forcefully. The change in volume (∆V) of blood divided by the change in pressure (∆P) is termed compliance
(∆V/∆P).
The venous system is the major reservoir within the vascular system and is markedly more compliant than the arterial system. Thus, CVP will increase with a decrease in venous compliance and/or an increase in the venous volume. These relationships can be quite dynamic depending on the disease state.
History of CVP Monitoring
The resuscitation of hemodynamically unstable patients historically stressed the use of intravenous (IV) fluid boluses. However, measuring the efficacy of this approach has been difficult. This issue was first addressed in the 1960s and 1970s when clinicians began to use central venous catheters (CVCs) to measure CVP as a surrogate measure of right atrial volume, which had been interpreted as a measure of the amount of blood returning to the heart. However, CVP measurements were static measurements of a dynamic filtration, and derivation of cardiac output required a long and complex calculation. The Swan-Ganz pulmonary artery catheter was the first catheter that enabled continuous monitoring and allowed clinicians to obtain cardiac index calculations at the bedside.1
The CVP is an approximation of the right atrial pressure and is an indicator of RV preload, which is a major determinant of RV filling pressure. Both RV preload and RV filling pressure correlate with intravascular volume. Lower CVP may occur with vasodilation or hypovolemia, which decreases the volume returning to the right atrium. This volume depletion creates a need for fluid replacement.
To illustrate this point, picture the body’s blood supply contained within a 6-L expandable tank. Vasodilation may expand the tank to a 9-L capacity, with a 3-L volume deficit. Similarly, blood loss from the 6-L tank may drain 3-L from the tank, leaving a 3-L deficit. Both mechanisms may cause a 3-L deficit, with the tank partially empty. Although it might make sense to replace the loss or “fill the tank in both scenarios,” fluid replacement may have risks. Overly aggressive fluid resuscitation may cause multiorgan dysfunction such as pulmonary edema, abdominal compartment syndrome, altered mental status, dilutional anemia, or dilutional coagulopathy. However, suboptimal fluid treatment may cause inadequate resuscitation that may be complicated by persistent hypotension, hypoperfusion, and end-organ damage and failure.
Up until the 1980s, it was believed that maintenance of normal hemodynamic parameters was the key to resuscitation of critically ill patients. Shoemaker et al2,3 then published several papers about increasing patient survival by “supranormalizing” cardiac indices. They recommended increasing cardiac index, oxygen transport, and CVP to higher than normal. High-risk surgical patients had placement of a pulmonary artery catheter and were randomized into three groups: (1) normalization of CVP; (2) pulmonary artery catheter monitoring and normalization of CVP; or (3) a pulmonary artery catheter protocol based on increasing normal cardiac indices to supranormal values. The time to intervention was greater than 6 hours. The study demonstrated no mortality difference among the CVP and pulmonary artery control groups, but did demonstrate a significant mortality reduction in the pulmonary artery catheter protocol group where the hemodynamic markers were kept at values higher (supranormalization group) than normal.
Early Goal-Directed Therapy
The intervention time of 6 hours was questioned in a study by Rivers et al,4 who suggested this delay was too long. In this study, early goal-directed therapy (EGDT) was compared to standard therapy in the ED in severe sepsis and septic shock. A CVP catheter was used within the right atrium, and critically ill patients were randomized into the following two groups: (1) CVC with continuous central venous oxygen saturation (ScvO2) measurements; and (2) the standard therapy group which was treated at the clinician’s discretion according to standard ED care with the exception of placement of a CVC without ScvO2 monitoring. Both groups had targeted goals of CVP, 8 to 12 mm Hg; mean arterial pressure, greater than 65 mm Hg; and urine output, greater than 0.5 mL/kg/h. Both groups received an equal volume of crystalloid fluids, which exceeded the commonly given amount of fluid to patients. The EDGT group received 4981± 2984 mL compared to the standard group which received 3499 ± 2438 mL. The EGDT-targeted supranormalization of ScvO2 employs dobutamine to achieve a goal of ScvO2 level greater than 70% and uses transfusion to achieve hematocrit level greater than 30%. The study showed 21% overall reduction in mortality in the EGDT group. Aggressive care and early recognition of disease seemed critical to patient survival. The study supported the measurement of CVP as a guide in fluid resuscitation in protocol-driven therapy during the initial 6 hours for patients who had severe sepsis and septic shock.4 The 2012 Surviving Sepsis Campaign guidelines for the treatment of severe sepsis and septic shock recommend maintaining CVP at 8 to 12 mm Hg for nonventilated patients and higher for ventilated patients.5
Since the publication of the EGDT study,4 the use of protocolized “bundle” therapy as a guide for resuscitation in severe sepsis and septic shock has been brought into question. The debate begs to answer which intervention within the bundle (CVP, transfusions, ScvO2, serial lactate, blood transfusions) results in a mortality benefit.
Between 2014 and 2015, three trials were published with the goal of determining which bundle intervention of EGDT was important in decreasing mortality. These three randomized worldwide trials, the so-called “trilogy of EDGT,” were the Protocol-based Care for Early Septic Shock (PROCESS),6 Australasian Resuscitation in Sepsis Evaluation (ARISE),7 and Protocolised Management in Sepsis (ProMISe).8 The results of all three trials were consistent. From a population standpoint, if the comprehensive processes are in place for the early detection of sepsis, aggressive IV fluid administration, early antibiotic administration, and serial lactate measurement; the subsequent algorithm-driven EGDT (as defined by Rivers et al4), including continuous central venous oxygenation and CVP monitoring, did not lead to an improvement in outcomes. Patients in the usual care group received central-line and arterial-line placement at a much higher rate than expected.
A number of potential reasons for differences in results from the original study by Rivers et al4 exist—eg, randomization occurred later, patients appeared to be less ill at baseline, all patients received antibiotics prior to randomization (Table 1). It is important to bear in mind that usual care, as defined in the “trilogy” may in fact not have been the “usual” care back in the mid-1990s when Rivers et al4 were conducting his EGDT. In addition, due to the influences of the original paper, the Surviving Sepsis Guidelines publications, improvement in EMS, critical care improvement, what Rivers et al4 termed usual care was really a modification of EDGT. One can, however, conclude from the trilogy is that placing a CVP or an ScvO2 catheter just for the purpose of chasing a CVP is no longer recommended.
Central Venous Pressure Measurement
A CVC must be placed in a sterile fashion with the tip of the catheter at the junction between the right atrium and superior vena cava. After the catheter has been properly secured and placement has been confirmed, a pressure transducer is connected from the most distal port of the CVC to the monitor. The use of CVP in the treatment of critically ill patients has logistical, mechanical, and placement issues that can complicate the clinical picture. Additionally, placement of a CVC is an invasive procedure with a set of complications that can compromise an already complex patient picture.9,10
The mechanical issues are numerous. The transducer is a water column that must be calibrated and set to zero at the level of the heart along the same plane of the right atrium (phlebostatic axis). The tip of the catheter inadvertently can be moved easily by health care workers, and a slight change in position may cause reading errors. The monitor must be recalibrated after the patient undergoes care by ancillary staff or is logrolled, moved, or repositioned in a way that affects the level of the heart. Some staff may not have adequate experience using the equipment. Misplacement of the catheter may cause erroneous and inaccurate measurements. The catheter tip must be in the right atrium, but using a catheter that is too long or short may have the catheter tip located in the superior vena cava, ventricle, or inferior vena cava (IVC). All these conditions will cause false reading of CVP.
Central Venous Pressure Interpretation
Normal CVP is 2 to 4 mm Hg, but interpretation of the value may vary. Low CVP typically indicates intravascular volume depletion and need for fluid replacement. However, caution is required with this approach. Depending on the cardiac compliance, some never have adequate volume with a low CVP and others with an elevated CVP may still augment cardiac output with additional fluid therapy (ie, a patient with hypertrophic Cardiomyopathy or advanced Pulmonary HTN).11,12
CVP as a trend may be more useful when compared to a single reading. Patients may vary on an individual basis, thereby making CVP a poor static marker. It should be used in the context of the patient’s clinical condition as it indicates the relationship between circulating blood volume and the capacity of the heart at a given time. As a trend, it is more sensitive to guide continued resuscitation efforts.13
Dynamic Techniques to Monitor Cardiac Output and Determine Fluid Responsiveness
Central venous pressure can be affected by anatomical and physiological factors such as valvular heart disease, right heart failure, poor lung compliance, or arrhythmias. In 2008, Marik et al14 performed a systematic review of 24 studies reviewing the benefits of CVP in the management of fluid therapy. In 2013, Marik et al14,15 repeated the meta-analysis of the literature which included 43 articles, and again concluded that there were no data to support the use of CVP to guide fluid therapy, and both papers conluded that CVP should not be used for fluid resuscitation. Static measures of fluid responsiveness such as CVP may not be the most appropriate measures, and may be less accurate physiologically than dynamic measures.
Dynamic measurements based on the Frank-Starling principle use the changes in the venous return (preload) and stroke volume as a marker of fluid responsiveness and may be more useful. There are several dynamic methods to assess fluid responsiveness. The first such method is the measurement of right atrial pressure. In a case series of 33 medical and surgical intensive care unit (ICU) patients who had pulmonary artery catheters, it was hypothesized that right atrial pressure predicted the response to fluid pressure as right atrial pressure should not decrease during spontaneous inspiration in patients who had a heart that was not volume responsive. Patients were classified as having a positive response test when right atrial pressure decreased ≥1 mm Hg during inspiration, or a negative response when right atrial pressure decreased <1 mm Hg. A positive response correlated with cardiac output increase of 250 mL/h.16
Evaluation of Pulse Pressure and Stroke Volume Variation
Pulse pressure variation (PPV), stroke volume variation (SVV), and variation of the amplitude of pulse oximeter plethysmographic waveform are highly predictive of fluid responsiveness in mechanically ventilated patients who have septic or hemorrhagic shock.17,18 The PPV is derived from the analysis of the arterial waveform, and SVV is derived from pulse contour analysis. The PPV uses the physiologic changes that occur during positive pressure ventilation. The delivery of a mechanical breath increases pleural pressure on inspiration, causing the following: (1) a decrease in RV preload because of decreased venous return; and (2) increase in RV afterload because of increased transpulmonary pressure. These changes lead to decreased RV stroke volume, which is at a minimal level at the end of inspiration. The inspiration reduction in RV ejection leads to a decrease in LV filling after a phase lag of two to three heart beats because of long pulmonary transit time. Thus, the LV preload reduction may induce a decrease in LV stroke volume, which is at its minimum volume during the inspiratory period of mechanical ventilation.18 The variation between the RV and LV stroke volume are greatest when the ventricles operate on the steep part of the Frank-Starling curve (rather than the flat portion). The PPV is calculated as the difference between maximum and minimum pulse pressures divided by the average of their sum, and multiplied by 100%. A variation in PPV of greater than 13% is highly predictive of volume responsiveness.19 The use of PPV is feasible in the ED because the only requirements include arterial access, measurement of the minimum and maximum pulse pressures during 30 seconds, and performance of the calculation. The PPV has been validated in different patient populations. However, the use of PPV is limited to a conventional volume control mode of ventilation and restricted to tidal volumes (TVs) over 7 mL/kg, this method of measurement was validated in patients receiving tidal volumes of at least 8 cc/kg ideal body weight—which may be higher than seen in contemporary clinical practice with more restrictive TV, ventilation strategies in patients with acute respiratory distress syndrome.20 Furthermore, patients must be ventilated passively, with heavy sedation or chemical paralysis to prevent spontaneous breathing. They must also have a normal heart rhythm. Most acute lung injury states are managed with lung protective strategy with TV of 4 to 6 mL/kg, PPV values obtained using lower TVs are less reliable and their use is not recommended.20-22
The Pleth Variation Index (PVI) is similar to PPV but is an automated measure of the dynamic change in the perfusion index that occurs during a respiratory cycle. Perfusion index is the ratio of nonpulsatile to pulsatile blood flow through the peripheral bed, measured noninvasively with a pulse oximeter probe. The PVI can predict positive fluid response in mechanically ventilated patients. However, PVI has the same limitations as PPV, the patient must be in sinus rhythm, and PVI cannot be used in patients who are breathing spontaneously.23
Ultrasonographic Assessment
Bedside ultrasonography is noninvasive, can be performed rapidly, and provides real-time clinically relevant data. There is much evidence that ultrasonography is effective in evaluating hemodynamic and volume status, and it may be used to assess fluid responsiveness during resuscitation.
As there is growing awareness of sepsis and fluid resuscitation, IVC measurements have grown in popularity as a noninvasive approach for such monitoring.24 IVC collapsibility in spontaneously breathing patients and caval index in mechanically ventilated patients can be determined rapidly at the bedside. There are two views that most easily allow access to measure the IVC: subxyphoid and right upper quadrant. Using the phased array probe or a curvilinear probe, the IVC can be seen traversing through the liver with the hepatic vein joining the IVC just before the diaphragm and emptying into the right atrium. Interrater reliability is often questioned when ultrasound is used. However, Fields et al25 were able to show that there was a high degree of interrater reliability among EPs when measuring IVC collapsibility.
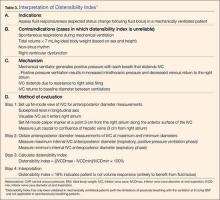
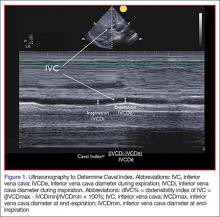
A systematic review by Zhang et al,26 showed change in IVC measured with point-of-care ultrasonography can reliably predict fluid responsiveness, particularly in patients that are mechanically ventilated. A caval index of 0.72 corresponds to CVP less than 7 cm water; a caval index of 1.23 corresponds to CVP 8 to 12 cm water; and a caval index of 1.59 corresponds to CVP greater than 13 cm water. The distensibility index is similar and calculated based on the IVC diameter at end-expiration (IVCDmax) and end-inspiration (IVCDmin).27 The ratio of (IVCDmax - IVCDmin)/IVCDmin is expressed as a percentage (dIVC%) in mechanically ventilated patients. A distensibility index less than 18% may indicate that the patient is not volume responsive (Tables 2 and 3; Figure 1).
Caval Index
A more widely utilized method for IVC evaluation is described by Nagdev et al.29 The caval index is calculated as the relative decrease in inferior vena cava diameter during one respiratory cycle. A caval index greater than or equal to 50% is strongly associated with a low CVP with 91% sensitivity and 94% specificity.29 It is important to remember, however, that IVC collapsibility is only useful at the extremes. Nevertheless, IVC measurement is limited by increased PEEP, increased TV, and increased intraabdominal pressure.
While IVC is the most commonly used vessel for sonographic volume status assessment, other vessels can also be used. Kent et al30 describe using the internal jugular vein as well as the femoral vein. Guarracino et al31 achieved similar results when using the internal jugular vein for distensibility index for assessing fluid responsiveness. When compared to the invasive CVP measurements, new CVP quantification methods could be used as a reliable approach for monitoring hemodynamic status.
Stroke Volume Variation
While not used regularly in the ED, respiratory changes in aortic blood velocity as measured by transesophageal echocardiography (TEE) may predict fluid responsiveness in mechanically ventilated patients.34 Peak aortic blood flow velocity variation is measured by TEE. Similarly, ventilator-induced variation in descending aortic blood flow measured by esophageal Doppler monitoring may predict fluid responsiveness.34 However peak aortic blood flow velocity measurements determined by TEE may have limited utility because TEE is an invasive procedure. Similarly, esophageal Doppler monitors can be used but are limited because of low predictive value and rare usage in the emergency setting.35
Passive Leg Raise
In spontaneously breathing patients, passive leg raising (PLR) has been studied as a substitute for volume challenge due to the ease of performing PLR at the bedside and absence of adverse events such as volume overload. When performing PLR, the patient starts in a semirecumbent position and is repositioned supine with the legs raised to 45°. Blood transferred to the heart during PLR increases cardiac preload and tests preload responsiveness. The maximum hemodynamic response to PLR occurs within one minute of performing the maneuver.36 The effects of PLR are assessed by the changes in cardiac output or stroke volume after PLR, which are extrapolated from aortic blood flow measured by esophageal Doppler, velocity time integral measured by transthoracic echocardiography, and femoral artery flow measured by arterial Doppler.36 These modalities may provide additional data points in the evaluation of fluid responsiveness but is out of the scope of this review.
Data in mechanically ventilated patients with esophageal Doppler and arterial access demonstrated that an increase in aortic blood flow by 10% with PLR predicted a positive fluid response with sensitivity 97% and specificity 94%.37 However, in the same study, the specificity in spontaneously breathing patients was markedly reduced (46%).37,38
Another study used a more conventional noninvasive measurement with transthoracic echocardiography to determine whether PLR could predict fluid responsiveness in hemodynamically unstable patients. In this study, a PLR-induced increase in stroke volume greater than or equal to 12.5% predicted an increase in stroke volume by greater than or equal to 15% after fluid administration with specificity 100% and sensitivity 77%.38 This study included patients on mechanical ventilation with active inspiration, patients without mechanical support, and patients with atrial fibrillation, enabling better generalization of results than previous studies.39
Bioreactance Technology
Cardiac output measurement using bioreactance technology is an alternative noninvasive method to measure cardiac output using only four surface electrodes. This technology is based on an analysis of relative phase shifts of an oscillating current that occurs when the current traverses the thoracic cavity. The bioreactance device (NICOM, Cheetah Medical, Tel Aviv, Israel) is comprised of a high frequency (75 kHz) sine wave generator and four dual electrode stickers that are used to establish electrical contact with the body. The cardiac output measured by bioreactance correlates well with values measured by thermodilution and pulse contour analysis.40 Performing PLR and determining its response using a bioreactance machine may be appropriate in the ED, in the ward, or at the initial presentation to the ICU because it is noninvasive and less labor intensive than other methods. In postoperative cardiac surgery patients, PLR-induced changes in cardiac output measured by bioreactance had sensitivity 88% and specificity 100%.40 In hemodynamically unstable patients, the results were more encouraging with a sensitivity of 94% and a specificity of 100% in predicting fluid responsiveness (defined as greater than10% increase in stroke volume index).41 However, in a group of critically ill patients (83% septic, 10% hypovolemic, and 7% cardiogenic), bioreactance coupled with PLR was unable to measure cardiac index compared with transpulmonary thermodilution, and bioreactance failed to predict fluid responsiveness.42 More research on bioreactance technology is needed, and its noninvasive evaluation of critically ill patients who need cardiac output monitoring and fluid therapy.
Conclusion
There are many tools available to estimate the volume status and fluid responsiveness of the critically ill patient. One of these tools, CVP measurement, must be used cautiously as an assessment of fluid responsiveness. It is important to understand the limitations of this technology. While other more advanced tools, such as ultrasonography to measure the IVC at the bedside and assess IVC variation or TEE to assess LV diastolic size and contractility during fluid resuscitation, may provide a better diagnostic picture, these tools/devices are not always available at most community hospitals.
The authors do not recommend placing a CVC simply to measure CVP; however, when a CVC or peripherally inserted central catheter is medically needed for treatment, the catheter can be used to trend CVP since the value of CVP is greatest as a trend to guide resuscitation. Other minimally invasive and noninvasive diagnostic tools currently are available, such as bedside ultrasound, and enable clinicians to assess volume responsiveness using dynamic procedures that challenge the Frank-Starling curve.4 These technologies have a useful place in resuscitation but each has its own limitations. With an understanding of the tools available, with their strengths and limitations, physicians can better individualize intravascular volume resuscitation.
Dr Farcy is the chairman of the department of emergency medicine, medical director of intensivists at Mount Sinai Medical Center, Miami Beach, Florida; and clinical assistant professor, Florida International University Medical School, Miami, Florida. Dr Jain is an assistant professor, director of critical care ultrasound, department of emergency medicine at the SUNY Downstate Medical Center, Kings County Hospital Center, Brooklyn, New York. Dr Dalley is the emergency-medicine residency program director at Mount Sinai Medical Center, Miami Beach, Florida.
Dr Scalea holds the Francis X. Kelly professorship in trauma surgery, is the distinguished professor in trauma, and director, program in trauma at the University of Maryland School of Medicine, Baltimore; he is also the physician in chief, shock trauma center, and system chief for critical care services at the University of Maryland Medical System, Baltimore.
- Pitfalls in Using Central Venous Pressure as a Marker of Fluid Responsiveness
- Chatterjee K. The Swan-Ganz catheters: past, present, and future. A viewpoint. Circulation. 2009;119(1):147-52.
- Shoemaker WC, Appel PL, Waxman K, Schwartz S, Chang P. Clinical trial of survivors’ cardiorespiratory patterns as therapeutic goals in critically ill postoperative patients. Crit Care Med. 1982;10(6):398-403.
- Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94(6):1176-1186.
- Rivers E, Nguyen B, Havstad S, et al; Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368-1377.
- Dellinger RP, Levy MM, Carlet JM, et al; International Surviving Sepsis Campaign Guidelines Committee; American Association of Critical-Care Nurses, American College of Chest Physicians, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296-327.
- ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of Protocol-based care for Early Septic Shock. N Engl J Med. 2014;370(18):1683-1693.
- Peake SL, Delaney A, Bellomo R; ARISE Investigators. Goal-directed resuscitation in septic shock. N Engl J Med. 2015; 372(2):190-191.
- Mouncey PR, Osborn TM, Power GS, et al; ProMISe Trial Investigators. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301-1311.
- Akmal AH, Hasan M, Mariam A. The incidence of complications of central venous catheters at an intensive care unit. Ann Thorac Med. 2007;2(2):61–63.
- Ruesch S, Walder B, Tramèr MR. Complications of central venous catheters: internal jugular versus subclavian access—a systematic review. Crit Care Med. 2002;30(2):454–460.
- Magder S. How to use central venous pressure measurements. Curr Opin Crit Care. 2005;11(3):264-270.
- Bafaqeeh F, Magder S. CVP and volume responsiveness of cardiac output. Am J Respir Crit Care Med. 2004;169:A344.
- Schummer W. Central venous pressure. Validity, informative value and correct measurement [in German]. Anaesthesist. 2009;58(5):499-505.
- Marik PE, Baram M, Vahid B. Does the central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134(1):172-178.
- Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41(7):1774-1781.
- Magder S, Georgiadis G, Cheong T. Respiratory variations in right atrial pressure predict the response to fluid challenge. J Crit Care. 1992;7(2):76-85.
- Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37(9):2642-2647.
- Reuter DA, Kirchner A, Felbinger TW, et al. Usefulness of left ventricular stroke volume variation to assess fluid responsiveness in patients with reduced cardiac function. Crit Care Med. 2003;31(5):1399-1404.
- Perel A, Pizov R, Cotev S. Systolic blood pressure variation is a sensitive indicator of hypovolemia in ventilated dogs subjected to graded hemorrhage. Anesthesiology. 1987;67(4):498-502.
- De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med. 2005;31(4):517-523.
- Charron C, Fessenmeyer C, Cosson C, et al. The influence of tidal volume on the dynamic variables of fluid responsiveness in critically ill patients. Anesth Analg. 2006;102(5):1511-1517.
- Galas F, Hajjar L, Polastri T, et al. Passive leg raising predicts fluid responsiveness after cardiac surgery. Crit Care. 2008;12(suppl 2):P89
- Cannesson M, Desebbe O, Rosamel P, et al. Pleth variability index to monitor the respiratory variations in the pulse oximeter plethysmographic waveform amplitude and predict fluid responsiveness in the operating theatre. Br J Anaesth. 2008;101(2):200-206.
- Coen D, Cortellaro F, Pasini S, et al. Towards a less invasive approach to the early goal-directed treatment of septic shock in the ED. Am J Emerg Med. 2014;32(6):563-568.
- Fields JM, Lee PA, Jenq KY, Mark DG, Panebianco NL, Dean AJ. The interrater reliability of inferior vena cava ultrasound by bedside clinician sonographers in emergency department patients. Acad Emerg Med. 2011;18(1):98-101.
- Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: systematic review and meta-analysis. Ultrasound Med Biol. 2014;40(5):845-853
- Sefidbakht S, Assadsangabi R, Abbasi HR, Nabavizadeh A. Sonographic measurement of the inferior vena cava as a predictor of shock in trauma patients. Emerg Radiol. 2007;14(3):181-185.
- Rudski LW, Lai WW, Afilalo J, et al. Guidelines of the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2013;23(7):658-713.
- Nagdev AD, Merchant RC, Tirado-Gonzalez A, Sisson CA, Murphy MC. Emergency department bedside ultrasonographic measurement of the caval index for noninvasive determination of low central venous pressure. Ann Emerg Med. 2010;55(3):290-295.
- Kent A, Patil P, Davila V, et al. Sonographic evaluation of intravascular volume status: Can internal jugular or femoral vein collapsibility be used in the absence of IVC visualization? Ann Thorac Med. 2015;10(1):44-49.
- Guarracino F, Ferro B, Forfori F, Bertini P, Magliacano L, Pinsky MR. Jugular vein distensibility predicts fluid responsiveness in septic patients. Crit Care. 2014;18(6):647.
- Chin JH, Jun IG, Lee J, Seo H, Hwang GS, Kim YK. Can stroke volume variation be an alternative to central venous pressure in patients undergoing kidney transplantation? Transplant Proc. 2014;46(10):3363-3366.
- Zhang Z, Lu B, Sheng X, Jin N. Accuracy of stroke volume variation in predicting fluid responsiveness: a systematic review and meta-analysis. J Anesth. 2011;25(6):904-916.
- Feissel M, Michard F, Mangin I, Ruyer O, Faller JP, Teboul JL. Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest. 2001;119(3):867-873.
- Davies SJ, Minhas S, Wilson RJ, Yates D, Howell SJ. Comparison of stroke volume and fluid responsiveness measurements in commonly used technologies for goal-directed therapy. J Clin Anesth. 2013;25(6):466-474.
- Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann Intensive Care. 2011;1(1):1.
- Monnet X, Rienzo M, Osman D, et al. Esophageal Doppler monitoring predicts fluid responsiveness in critically ill ventilated patients. Intensive Care Med. 2005;31(9):1195-1201.
- Monnet X, Rienzo M, Osman D, et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006;34(5):1402-1407.
- Lamia B, Ochagavia A, Monnet X, Chemla D, Richard C, Teboul JL. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007;33(7):1125-1132.
- Benomar B, Ouattara A, Estagnasie P, Brusset A, Squara P. Fluid responsiveness predicted by noninvasive bioreactance-based passive leg raise test. Intensive Care Med. 2010;36(11):1875-1881.
- Marik PE, Levitov A, Young A, Andrews L. The use of bioreactance and carotid Doppler to determine volume responsiveness and blood flow redistribution following passive leg raising in hemodynamically unstable patients. Chest. 2013;143(2):364-370.
- Kupersztych-Hagege E, Teboul JL, Artigas A, et al. Bioreactance is not reliable for estimating cardiac output and the effects of passive leg raising in critically ill patients. Br J Anaesth. 2013;111(6):961-966.
Case Scenario
A 69-year-old man was transported to the ED via emergency medical services after a family member discovered him alone at home and confused. His wife stated that her husband had been sick with the flu and had been febrile for the previous several days. The patient’s blood pressure taken on the scene by the emergency medical technician was 80/40 mm Hg, and 1 L normal saline was infused during transport. Upon arrival to the ED, his vital signs were: temperature, 103.3°F; heart rate,130 beats/minute; BP, 90/48 mm Hg; and respiratory rate, 24 breaths/minute. Oxygen saturation was 92% on nasal canula. An electrocardiogram showed sinus tachycardia with nonspecific changes.
Based on the patient’s symptoms, the emergency physician (EP) suspected sepsis and ordered the appropriate laboratory studies and radiographic images. During evaluation, the patient’s systolic BP decreased to 70 from 80 mm Hg, and the EP ordered another fluid bolus and considered assessing the patient’s volume status.
Introduction
There is a long-standing debate as to the most accurate method of determining the volume status of a critically ill patient, as well as the physiological ability to respond to fluid therapy. In the assessment of a critically ill patient receiving volume replacement, a wide variability of assessment options are available; however, the current literature has yet to determine which method is the best. This article reviews multiple approaches to estimating the intravascular volume status of critically ill patients and the use of modalities to determine a patient’s physiological response to fluid therapy.
Basic Physiology
Central venous pressure (CVP) is the pressure in the thoracic vena cava adjacent to the right atrium. The heart functions as a two-sided pump; the right side pumps volume at low pressure and the left side pumps against systemic arterial pressure. The major determinant of the filling pressure of the right ventricle (RV) at the end of diastole is CVP, which is affected by the initial stretching of the ventricles before contraction (preload).
Frank-Starling Mechanism
The Frank-Starling mechanism describes the relationship between cardiac performance and intravascular volume. Stroke volume increases in response to an increase in preload volume. The increased volume of blood stretches the ventricular wall, causing the cardiac muscle to contract more forcefully. The change in volume (∆V) of blood divided by the change in pressure (∆P) is termed compliance
(∆V/∆P).
The venous system is the major reservoir within the vascular system and is markedly more compliant than the arterial system. Thus, CVP will increase with a decrease in venous compliance and/or an increase in the venous volume. These relationships can be quite dynamic depending on the disease state.
History of CVP Monitoring
The resuscitation of hemodynamically unstable patients historically stressed the use of intravenous (IV) fluid boluses. However, measuring the efficacy of this approach has been difficult. This issue was first addressed in the 1960s and 1970s when clinicians began to use central venous catheters (CVCs) to measure CVP as a surrogate measure of right atrial volume, which had been interpreted as a measure of the amount of blood returning to the heart. However, CVP measurements were static measurements of a dynamic filtration, and derivation of cardiac output required a long and complex calculation. The Swan-Ganz pulmonary artery catheter was the first catheter that enabled continuous monitoring and allowed clinicians to obtain cardiac index calculations at the bedside.1
The CVP is an approximation of the right atrial pressure and is an indicator of RV preload, which is a major determinant of RV filling pressure. Both RV preload and RV filling pressure correlate with intravascular volume. Lower CVP may occur with vasodilation or hypovolemia, which decreases the volume returning to the right atrium. This volume depletion creates a need for fluid replacement.
To illustrate this point, picture the body’s blood supply contained within a 6-L expandable tank. Vasodilation may expand the tank to a 9-L capacity, with a 3-L volume deficit. Similarly, blood loss from the 6-L tank may drain 3-L from the tank, leaving a 3-L deficit. Both mechanisms may cause a 3-L deficit, with the tank partially empty. Although it might make sense to replace the loss or “fill the tank in both scenarios,” fluid replacement may have risks. Overly aggressive fluid resuscitation may cause multiorgan dysfunction such as pulmonary edema, abdominal compartment syndrome, altered mental status, dilutional anemia, or dilutional coagulopathy. However, suboptimal fluid treatment may cause inadequate resuscitation that may be complicated by persistent hypotension, hypoperfusion, and end-organ damage and failure.
Up until the 1980s, it was believed that maintenance of normal hemodynamic parameters was the key to resuscitation of critically ill patients. Shoemaker et al2,3 then published several papers about increasing patient survival by “supranormalizing” cardiac indices. They recommended increasing cardiac index, oxygen transport, and CVP to higher than normal. High-risk surgical patients had placement of a pulmonary artery catheter and were randomized into three groups: (1) normalization of CVP; (2) pulmonary artery catheter monitoring and normalization of CVP; or (3) a pulmonary artery catheter protocol based on increasing normal cardiac indices to supranormal values. The time to intervention was greater than 6 hours. The study demonstrated no mortality difference among the CVP and pulmonary artery control groups, but did demonstrate a significant mortality reduction in the pulmonary artery catheter protocol group where the hemodynamic markers were kept at values higher (supranormalization group) than normal.
Early Goal-Directed Therapy
The intervention time of 6 hours was questioned in a study by Rivers et al,4 who suggested this delay was too long. In this study, early goal-directed therapy (EGDT) was compared to standard therapy in the ED in severe sepsis and septic shock. A CVP catheter was used within the right atrium, and critically ill patients were randomized into the following two groups: (1) CVC with continuous central venous oxygen saturation (ScvO2) measurements; and (2) the standard therapy group which was treated at the clinician’s discretion according to standard ED care with the exception of placement of a CVC without ScvO2 monitoring. Both groups had targeted goals of CVP, 8 to 12 mm Hg; mean arterial pressure, greater than 65 mm Hg; and urine output, greater than 0.5 mL/kg/h. Both groups received an equal volume of crystalloid fluids, which exceeded the commonly given amount of fluid to patients. The EDGT group received 4981± 2984 mL compared to the standard group which received 3499 ± 2438 mL. The EGDT-targeted supranormalization of ScvO2 employs dobutamine to achieve a goal of ScvO2 level greater than 70% and uses transfusion to achieve hematocrit level greater than 30%. The study showed 21% overall reduction in mortality in the EGDT group. Aggressive care and early recognition of disease seemed critical to patient survival. The study supported the measurement of CVP as a guide in fluid resuscitation in protocol-driven therapy during the initial 6 hours for patients who had severe sepsis and septic shock.4 The 2012 Surviving Sepsis Campaign guidelines for the treatment of severe sepsis and septic shock recommend maintaining CVP at 8 to 12 mm Hg for nonventilated patients and higher for ventilated patients.5
Since the publication of the EGDT study,4 the use of protocolized “bundle” therapy as a guide for resuscitation in severe sepsis and septic shock has been brought into question. The debate begs to answer which intervention within the bundle (CVP, transfusions, ScvO2, serial lactate, blood transfusions) results in a mortality benefit.
Between 2014 and 2015, three trials were published with the goal of determining which bundle intervention of EGDT was important in decreasing mortality. These three randomized worldwide trials, the so-called “trilogy of EDGT,” were the Protocol-based Care for Early Septic Shock (PROCESS),6 Australasian Resuscitation in Sepsis Evaluation (ARISE),7 and Protocolised Management in Sepsis (ProMISe).8 The results of all three trials were consistent. From a population standpoint, if the comprehensive processes are in place for the early detection of sepsis, aggressive IV fluid administration, early antibiotic administration, and serial lactate measurement; the subsequent algorithm-driven EGDT (as defined by Rivers et al4), including continuous central venous oxygenation and CVP monitoring, did not lead to an improvement in outcomes. Patients in the usual care group received central-line and arterial-line placement at a much higher rate than expected.
A number of potential reasons for differences in results from the original study by Rivers et al4 exist—eg, randomization occurred later, patients appeared to be less ill at baseline, all patients received antibiotics prior to randomization (Table 1). It is important to bear in mind that usual care, as defined in the “trilogy” may in fact not have been the “usual” care back in the mid-1990s when Rivers et al4 were conducting his EGDT. In addition, due to the influences of the original paper, the Surviving Sepsis Guidelines publications, improvement in EMS, critical care improvement, what Rivers et al4 termed usual care was really a modification of EDGT. One can, however, conclude from the trilogy is that placing a CVP or an ScvO2 catheter just for the purpose of chasing a CVP is no longer recommended.
Central Venous Pressure Measurement
A CVC must be placed in a sterile fashion with the tip of the catheter at the junction between the right atrium and superior vena cava. After the catheter has been properly secured and placement has been confirmed, a pressure transducer is connected from the most distal port of the CVC to the monitor. The use of CVP in the treatment of critically ill patients has logistical, mechanical, and placement issues that can complicate the clinical picture. Additionally, placement of a CVC is an invasive procedure with a set of complications that can compromise an already complex patient picture.9,10
The mechanical issues are numerous. The transducer is a water column that must be calibrated and set to zero at the level of the heart along the same plane of the right atrium (phlebostatic axis). The tip of the catheter inadvertently can be moved easily by health care workers, and a slight change in position may cause reading errors. The monitor must be recalibrated after the patient undergoes care by ancillary staff or is logrolled, moved, or repositioned in a way that affects the level of the heart. Some staff may not have adequate experience using the equipment. Misplacement of the catheter may cause erroneous and inaccurate measurements. The catheter tip must be in the right atrium, but using a catheter that is too long or short may have the catheter tip located in the superior vena cava, ventricle, or inferior vena cava (IVC). All these conditions will cause false reading of CVP.
Central Venous Pressure Interpretation
Normal CVP is 2 to 4 mm Hg, but interpretation of the value may vary. Low CVP typically indicates intravascular volume depletion and need for fluid replacement. However, caution is required with this approach. Depending on the cardiac compliance, some never have adequate volume with a low CVP and others with an elevated CVP may still augment cardiac output with additional fluid therapy (ie, a patient with hypertrophic Cardiomyopathy or advanced Pulmonary HTN).11,12
CVP as a trend may be more useful when compared to a single reading. Patients may vary on an individual basis, thereby making CVP a poor static marker. It should be used in the context of the patient’s clinical condition as it indicates the relationship between circulating blood volume and the capacity of the heart at a given time. As a trend, it is more sensitive to guide continued resuscitation efforts.13
Dynamic Techniques to Monitor Cardiac Output and Determine Fluid Responsiveness
Central venous pressure can be affected by anatomical and physiological factors such as valvular heart disease, right heart failure, poor lung compliance, or arrhythmias. In 2008, Marik et al14 performed a systematic review of 24 studies reviewing the benefits of CVP in the management of fluid therapy. In 2013, Marik et al14,15 repeated the meta-analysis of the literature which included 43 articles, and again concluded that there were no data to support the use of CVP to guide fluid therapy, and both papers conluded that CVP should not be used for fluid resuscitation. Static measures of fluid responsiveness such as CVP may not be the most appropriate measures, and may be less accurate physiologically than dynamic measures.
Dynamic measurements based on the Frank-Starling principle use the changes in the venous return (preload) and stroke volume as a marker of fluid responsiveness and may be more useful. There are several dynamic methods to assess fluid responsiveness. The first such method is the measurement of right atrial pressure. In a case series of 33 medical and surgical intensive care unit (ICU) patients who had pulmonary artery catheters, it was hypothesized that right atrial pressure predicted the response to fluid pressure as right atrial pressure should not decrease during spontaneous inspiration in patients who had a heart that was not volume responsive. Patients were classified as having a positive response test when right atrial pressure decreased ≥1 mm Hg during inspiration, or a negative response when right atrial pressure decreased <1 mm Hg. A positive response correlated with cardiac output increase of 250 mL/h.16
Evaluation of Pulse Pressure and Stroke Volume Variation
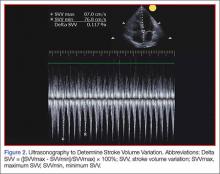
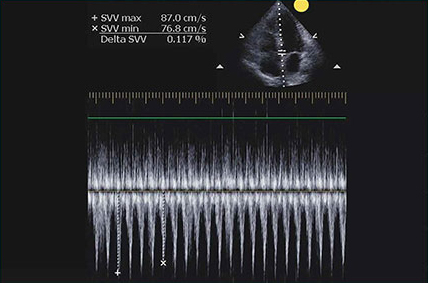
Pulse pressure variation (PPV), stroke volume variation (SVV), and variation of the amplitude of pulse oximeter plethysmographic waveform are highly predictive of fluid responsiveness in mechanically ventilated patients who have septic or hemorrhagic shock.17,18 The PPV is derived from the analysis of the arterial waveform, and SVV is derived from pulse contour analysis. The PPV uses the physiologic changes that occur during positive pressure ventilation. The delivery of a mechanical breath increases pleural pressure on inspiration, causing the following: (1) a decrease in RV preload because of decreased venous return; and (2) increase in RV afterload because of increased transpulmonary pressure. These changes lead to decreased RV stroke volume, which is at a minimal level at the end of inspiration. The inspiration reduction in RV ejection leads to a decrease in LV filling after a phase lag of two to three heart beats because of long pulmonary transit time. Thus, the LV preload reduction may induce a decrease in LV stroke volume, which is at its minimum volume during the inspiratory period of mechanical ventilation.18 The variation between the RV and LV stroke volume are greatest when the ventricles operate on the steep part of the Frank-Starling curve (rather than the flat portion). The PPV is calculated as the difference between maximum and minimum pulse pressures divided by the average of their sum, and multiplied by 100%. A variation in PPV of greater than 13% is highly predictive of volume responsiveness.19 The use of PPV is feasible in the ED because the only requirements include arterial access, measurement of the minimum and maximum pulse pressures during 30 seconds, and performance of the calculation. The PPV has been validated in different patient populations. However, the use of PPV is limited to a conventional volume control mode of ventilation and restricted to tidal volumes (TVs) over 7 mL/kg, this method of measurement was validated in patients receiving tidal volumes of at least 8 cc/kg ideal body weight—which may be higher than seen in contemporary clinical practice with more restrictive TV, ventilation strategies in patients with acute respiratory distress syndrome.20 Furthermore, patients must be ventilated passively, with heavy sedation or chemical paralysis to prevent spontaneous breathing. They must also have a normal heart rhythm. Most acute lung injury states are managed with lung protective strategy with TV of 4 to 6 mL/kg, PPV values obtained using lower TVs are less reliable and their use is not recommended.20-22
The Pleth Variation Index (PVI) is similar to PPV but is an automated measure of the dynamic change in the perfusion index that occurs during a respiratory cycle. Perfusion index is the ratio of nonpulsatile to pulsatile blood flow through the peripheral bed, measured noninvasively with a pulse oximeter probe. The PVI can predict positive fluid response in mechanically ventilated patients. However, PVI has the same limitations as PPV, the patient must be in sinus rhythm, and PVI cannot be used in patients who are breathing spontaneously.23
Ultrasonographic Assessment
Bedside ultrasonography is noninvasive, can be performed rapidly, and provides real-time clinically relevant data. There is much evidence that ultrasonography is effective in evaluating hemodynamic and volume status, and it may be used to assess fluid responsiveness during resuscitation.
As there is growing awareness of sepsis and fluid resuscitation, IVC measurements have grown in popularity as a noninvasive approach for such monitoring.24 IVC collapsibility in spontaneously breathing patients and caval index in mechanically ventilated patients can be determined rapidly at the bedside. There are two views that most easily allow access to measure the IVC: subxyphoid and right upper quadrant. Using the phased array probe or a curvilinear probe, the IVC can be seen traversing through the liver with the hepatic vein joining the IVC just before the diaphragm and emptying into the right atrium. Interrater reliability is often questioned when ultrasound is used. However, Fields et al25 were able to show that there was a high degree of interrater reliability among EPs when measuring IVC collapsibility.
A systematic review by Zhang et al,26 showed change in IVC measured with point-of-care ultrasonography can reliably predict fluid responsiveness, particularly in patients that are mechanically ventilated. A caval index of 0.72 corresponds to CVP less than 7 cm water; a caval index of 1.23 corresponds to CVP 8 to 12 cm water; and a caval index of 1.59 corresponds to CVP greater than 13 cm water. The distensibility index is similar and calculated based on the IVC diameter at end-expiration (IVCDmax) and end-inspiration (IVCDmin).27 The ratio of (IVCDmax - IVCDmin)/IVCDmin is expressed as a percentage (dIVC%) in mechanically ventilated patients. A distensibility index less than 18% may indicate that the patient is not volume responsive (Tables 2 and 3; Figure 1).
Caval Index
A more widely utilized method for IVC evaluation is described by Nagdev et al.29 The caval index is calculated as the relative decrease in inferior vena cava diameter during one respiratory cycle. A caval index greater than or equal to 50% is strongly associated with a low CVP with 91% sensitivity and 94% specificity.29 It is important to remember, however, that IVC collapsibility is only useful at the extremes. Nevertheless, IVC measurement is limited by increased PEEP, increased TV, and increased intraabdominal pressure.
While IVC is the most commonly used vessel for sonographic volume status assessment, other vessels can also be used. Kent et al30 describe using the internal jugular vein as well as the femoral vein. Guarracino et al31 achieved similar results when using the internal jugular vein for distensibility index for assessing fluid responsiveness. When compared to the invasive CVP measurements, new CVP quantification methods could be used as a reliable approach for monitoring hemodynamic status.
Stroke Volume Variation
While not used regularly in the ED, respiratory changes in aortic blood velocity as measured by transesophageal echocardiography (TEE) may predict fluid responsiveness in mechanically ventilated patients.34 Peak aortic blood flow velocity variation is measured by TEE. Similarly, ventilator-induced variation in descending aortic blood flow measured by esophageal Doppler monitoring may predict fluid responsiveness.34 However peak aortic blood flow velocity measurements determined by TEE may have limited utility because TEE is an invasive procedure. Similarly, esophageal Doppler monitors can be used but are limited because of low predictive value and rare usage in the emergency setting.35
Passive Leg Raise
In spontaneously breathing patients, passive leg raising (PLR) has been studied as a substitute for volume challenge due to the ease of performing PLR at the bedside and absence of adverse events such as volume overload. When performing PLR, the patient starts in a semirecumbent position and is repositioned supine with the legs raised to 45°. Blood transferred to the heart during PLR increases cardiac preload and tests preload responsiveness. The maximum hemodynamic response to PLR occurs within one minute of performing the maneuver.36 The effects of PLR are assessed by the changes in cardiac output or stroke volume after PLR, which are extrapolated from aortic blood flow measured by esophageal Doppler, velocity time integral measured by transthoracic echocardiography, and femoral artery flow measured by arterial Doppler.36 These modalities may provide additional data points in the evaluation of fluid responsiveness but is out of the scope of this review.
Data in mechanically ventilated patients with esophageal Doppler and arterial access demonstrated that an increase in aortic blood flow by 10% with PLR predicted a positive fluid response with sensitivity 97% and specificity 94%.37 However, in the same study, the specificity in spontaneously breathing patients was markedly reduced (46%).37,38
Another study used a more conventional noninvasive measurement with transthoracic echocardiography to determine whether PLR could predict fluid responsiveness in hemodynamically unstable patients. In this study, a PLR-induced increase in stroke volume greater than or equal to 12.5% predicted an increase in stroke volume by greater than or equal to 15% after fluid administration with specificity 100% and sensitivity 77%.38 This study included patients on mechanical ventilation with active inspiration, patients without mechanical support, and patients with atrial fibrillation, enabling better generalization of results than previous studies.39
Bioreactance Technology
Cardiac output measurement using bioreactance technology is an alternative noninvasive method to measure cardiac output using only four surface electrodes. This technology is based on an analysis of relative phase shifts of an oscillating current that occurs when the current traverses the thoracic cavity. The bioreactance device (NICOM, Cheetah Medical, Tel Aviv, Israel) is comprised of a high frequency (75 kHz) sine wave generator and four dual electrode stickers that are used to establish electrical contact with the body. The cardiac output measured by bioreactance correlates well with values measured by thermodilution and pulse contour analysis.40 Performing PLR and determining its response using a bioreactance machine may be appropriate in the ED, in the ward, or at the initial presentation to the ICU because it is noninvasive and less labor intensive than other methods. In postoperative cardiac surgery patients, PLR-induced changes in cardiac output measured by bioreactance had sensitivity 88% and specificity 100%.40 In hemodynamically unstable patients, the results were more encouraging with a sensitivity of 94% and a specificity of 100% in predicting fluid responsiveness (defined as greater than10% increase in stroke volume index).41 However, in a group of critically ill patients (83% septic, 10% hypovolemic, and 7% cardiogenic), bioreactance coupled with PLR was unable to measure cardiac index compared with transpulmonary thermodilution, and bioreactance failed to predict fluid responsiveness.42 More research on bioreactance technology is needed, and its noninvasive evaluation of critically ill patients who need cardiac output monitoring and fluid therapy.
Conclusion
There are many tools available to estimate the volume status and fluid responsiveness of the critically ill patient. One of these tools, CVP measurement, must be used cautiously as an assessment of fluid responsiveness. It is important to understand the limitations of this technology. While other more advanced tools, such as ultrasonography to measure the IVC at the bedside and assess IVC variation or TEE to assess LV diastolic size and contractility during fluid resuscitation, may provide a better diagnostic picture, these tools/devices are not always available at most community hospitals.
The authors do not recommend placing a CVC simply to measure CVP; however, when a CVC or peripherally inserted central catheter is medically needed for treatment, the catheter can be used to trend CVP since the value of CVP is greatest as a trend to guide resuscitation. Other minimally invasive and noninvasive diagnostic tools currently are available, such as bedside ultrasound, and enable clinicians to assess volume responsiveness using dynamic procedures that challenge the Frank-Starling curve.4 These technologies have a useful place in resuscitation but each has its own limitations. With an understanding of the tools available, with their strengths and limitations, physicians can better individualize intravascular volume resuscitation.
Dr Farcy is the chairman of the department of emergency medicine, medical director of intensivists at Mount Sinai Medical Center, Miami Beach, Florida; and clinical assistant professor, Florida International University Medical School, Miami, Florida. Dr Jain is an assistant professor, director of critical care ultrasound, department of emergency medicine at the SUNY Downstate Medical Center, Kings County Hospital Center, Brooklyn, New York. Dr Dalley is the emergency-medicine residency program director at Mount Sinai Medical Center, Miami Beach, Florida.
Dr Scalea holds the Francis X. Kelly professorship in trauma surgery, is the distinguished professor in trauma, and director, program in trauma at the University of Maryland School of Medicine, Baltimore; he is also the physician in chief, shock trauma center, and system chief for critical care services at the University of Maryland Medical System, Baltimore.
Case Scenario
A 69-year-old man was transported to the ED via emergency medical services after a family member discovered him alone at home and confused. His wife stated that her husband had been sick with the flu and had been febrile for the previous several days. The patient’s blood pressure taken on the scene by the emergency medical technician was 80/40 mm Hg, and 1 L normal saline was infused during transport. Upon arrival to the ED, his vital signs were: temperature, 103.3°F; heart rate,130 beats/minute; BP, 90/48 mm Hg; and respiratory rate, 24 breaths/minute. Oxygen saturation was 92% on nasal canula. An electrocardiogram showed sinus tachycardia with nonspecific changes.
Based on the patient’s symptoms, the emergency physician (EP) suspected sepsis and ordered the appropriate laboratory studies and radiographic images. During evaluation, the patient’s systolic BP decreased to 70 from 80 mm Hg, and the EP ordered another fluid bolus and considered assessing the patient’s volume status.
Introduction
There is a long-standing debate as to the most accurate method of determining the volume status of a critically ill patient, as well as the physiological ability to respond to fluid therapy. In the assessment of a critically ill patient receiving volume replacement, a wide variability of assessment options are available; however, the current literature has yet to determine which method is the best. This article reviews multiple approaches to estimating the intravascular volume status of critically ill patients and the use of modalities to determine a patient’s physiological response to fluid therapy.
Basic Physiology
Central venous pressure (CVP) is the pressure in the thoracic vena cava adjacent to the right atrium. The heart functions as a two-sided pump; the right side pumps volume at low pressure and the left side pumps against systemic arterial pressure. The major determinant of the filling pressure of the right ventricle (RV) at the end of diastole is CVP, which is affected by the initial stretching of the ventricles before contraction (preload).
Frank-Starling Mechanism
The Frank-Starling mechanism describes the relationship between cardiac performance and intravascular volume. Stroke volume increases in response to an increase in preload volume. The increased volume of blood stretches the ventricular wall, causing the cardiac muscle to contract more forcefully. The change in volume (∆V) of blood divided by the change in pressure (∆P) is termed compliance
(∆V/∆P).
The venous system is the major reservoir within the vascular system and is markedly more compliant than the arterial system. Thus, CVP will increase with a decrease in venous compliance and/or an increase in the venous volume. These relationships can be quite dynamic depending on the disease state.
History of CVP Monitoring
The resuscitation of hemodynamically unstable patients historically stressed the use of intravenous (IV) fluid boluses. However, measuring the efficacy of this approach has been difficult. This issue was first addressed in the 1960s and 1970s when clinicians began to use central venous catheters (CVCs) to measure CVP as a surrogate measure of right atrial volume, which had been interpreted as a measure of the amount of blood returning to the heart. However, CVP measurements were static measurements of a dynamic filtration, and derivation of cardiac output required a long and complex calculation. The Swan-Ganz pulmonary artery catheter was the first catheter that enabled continuous monitoring and allowed clinicians to obtain cardiac index calculations at the bedside.1
The CVP is an approximation of the right atrial pressure and is an indicator of RV preload, which is a major determinant of RV filling pressure. Both RV preload and RV filling pressure correlate with intravascular volume. Lower CVP may occur with vasodilation or hypovolemia, which decreases the volume returning to the right atrium. This volume depletion creates a need for fluid replacement.
To illustrate this point, picture the body’s blood supply contained within a 6-L expandable tank. Vasodilation may expand the tank to a 9-L capacity, with a 3-L volume deficit. Similarly, blood loss from the 6-L tank may drain 3-L from the tank, leaving a 3-L deficit. Both mechanisms may cause a 3-L deficit, with the tank partially empty. Although it might make sense to replace the loss or “fill the tank in both scenarios,” fluid replacement may have risks. Overly aggressive fluid resuscitation may cause multiorgan dysfunction such as pulmonary edema, abdominal compartment syndrome, altered mental status, dilutional anemia, or dilutional coagulopathy. However, suboptimal fluid treatment may cause inadequate resuscitation that may be complicated by persistent hypotension, hypoperfusion, and end-organ damage and failure.
Up until the 1980s, it was believed that maintenance of normal hemodynamic parameters was the key to resuscitation of critically ill patients. Shoemaker et al2,3 then published several papers about increasing patient survival by “supranormalizing” cardiac indices. They recommended increasing cardiac index, oxygen transport, and CVP to higher than normal. High-risk surgical patients had placement of a pulmonary artery catheter and were randomized into three groups: (1) normalization of CVP; (2) pulmonary artery catheter monitoring and normalization of CVP; or (3) a pulmonary artery catheter protocol based on increasing normal cardiac indices to supranormal values. The time to intervention was greater than 6 hours. The study demonstrated no mortality difference among the CVP and pulmonary artery control groups, but did demonstrate a significant mortality reduction in the pulmonary artery catheter protocol group where the hemodynamic markers were kept at values higher (supranormalization group) than normal.
Early Goal-Directed Therapy
The intervention time of 6 hours was questioned in a study by Rivers et al,4 who suggested this delay was too long. In this study, early goal-directed therapy (EGDT) was compared to standard therapy in the ED in severe sepsis and septic shock. A CVP catheter was used within the right atrium, and critically ill patients were randomized into the following two groups: (1) CVC with continuous central venous oxygen saturation (ScvO2) measurements; and (2) the standard therapy group which was treated at the clinician’s discretion according to standard ED care with the exception of placement of a CVC without ScvO2 monitoring. Both groups had targeted goals of CVP, 8 to 12 mm Hg; mean arterial pressure, greater than 65 mm Hg; and urine output, greater than 0.5 mL/kg/h. Both groups received an equal volume of crystalloid fluids, which exceeded the commonly given amount of fluid to patients. The EDGT group received 4981± 2984 mL compared to the standard group which received 3499 ± 2438 mL. The EGDT-targeted supranormalization of ScvO2 employs dobutamine to achieve a goal of ScvO2 level greater than 70% and uses transfusion to achieve hematocrit level greater than 30%. The study showed 21% overall reduction in mortality in the EGDT group. Aggressive care and early recognition of disease seemed critical to patient survival. The study supported the measurement of CVP as a guide in fluid resuscitation in protocol-driven therapy during the initial 6 hours for patients who had severe sepsis and septic shock.4 The 2012 Surviving Sepsis Campaign guidelines for the treatment of severe sepsis and septic shock recommend maintaining CVP at 8 to 12 mm Hg for nonventilated patients and higher for ventilated patients.5
Since the publication of the EGDT study,4 the use of protocolized “bundle” therapy as a guide for resuscitation in severe sepsis and septic shock has been brought into question. The debate begs to answer which intervention within the bundle (CVP, transfusions, ScvO2, serial lactate, blood transfusions) results in a mortality benefit.
Between 2014 and 2015, three trials were published with the goal of determining which bundle intervention of EGDT was important in decreasing mortality. These three randomized worldwide trials, the so-called “trilogy of EDGT,” were the Protocol-based Care for Early Septic Shock (PROCESS),6 Australasian Resuscitation in Sepsis Evaluation (ARISE),7 and Protocolised Management in Sepsis (ProMISe).8 The results of all three trials were consistent. From a population standpoint, if the comprehensive processes are in place for the early detection of sepsis, aggressive IV fluid administration, early antibiotic administration, and serial lactate measurement; the subsequent algorithm-driven EGDT (as defined by Rivers et al4), including continuous central venous oxygenation and CVP monitoring, did not lead to an improvement in outcomes. Patients in the usual care group received central-line and arterial-line placement at a much higher rate than expected.
A number of potential reasons for differences in results from the original study by Rivers et al4 exist—eg, randomization occurred later, patients appeared to be less ill at baseline, all patients received antibiotics prior to randomization (Table 1). It is important to bear in mind that usual care, as defined in the “trilogy” may in fact not have been the “usual” care back in the mid-1990s when Rivers et al4 were conducting his EGDT. In addition, due to the influences of the original paper, the Surviving Sepsis Guidelines publications, improvement in EMS, critical care improvement, what Rivers et al4 termed usual care was really a modification of EDGT. One can, however, conclude from the trilogy is that placing a CVP or an ScvO2 catheter just for the purpose of chasing a CVP is no longer recommended.
Central Venous Pressure Measurement
A CVC must be placed in a sterile fashion with the tip of the catheter at the junction between the right atrium and superior vena cava. After the catheter has been properly secured and placement has been confirmed, a pressure transducer is connected from the most distal port of the CVC to the monitor. The use of CVP in the treatment of critically ill patients has logistical, mechanical, and placement issues that can complicate the clinical picture. Additionally, placement of a CVC is an invasive procedure with a set of complications that can compromise an already complex patient picture.9,10
The mechanical issues are numerous. The transducer is a water column that must be calibrated and set to zero at the level of the heart along the same plane of the right atrium (phlebostatic axis). The tip of the catheter inadvertently can be moved easily by health care workers, and a slight change in position may cause reading errors. The monitor must be recalibrated after the patient undergoes care by ancillary staff or is logrolled, moved, or repositioned in a way that affects the level of the heart. Some staff may not have adequate experience using the equipment. Misplacement of the catheter may cause erroneous and inaccurate measurements. The catheter tip must be in the right atrium, but using a catheter that is too long or short may have the catheter tip located in the superior vena cava, ventricle, or inferior vena cava (IVC). All these conditions will cause false reading of CVP.
Central Venous Pressure Interpretation
Normal CVP is 2 to 4 mm Hg, but interpretation of the value may vary. Low CVP typically indicates intravascular volume depletion and need for fluid replacement. However, caution is required with this approach. Depending on the cardiac compliance, some never have adequate volume with a low CVP and others with an elevated CVP may still augment cardiac output with additional fluid therapy (ie, a patient with hypertrophic Cardiomyopathy or advanced Pulmonary HTN).11,12
CVP as a trend may be more useful when compared to a single reading. Patients may vary on an individual basis, thereby making CVP a poor static marker. It should be used in the context of the patient’s clinical condition as it indicates the relationship between circulating blood volume and the capacity of the heart at a given time. As a trend, it is more sensitive to guide continued resuscitation efforts.13
Dynamic Techniques to Monitor Cardiac Output and Determine Fluid Responsiveness
Central venous pressure can be affected by anatomical and physiological factors such as valvular heart disease, right heart failure, poor lung compliance, or arrhythmias. In 2008, Marik et al14 performed a systematic review of 24 studies reviewing the benefits of CVP in the management of fluid therapy. In 2013, Marik et al14,15 repeated the meta-analysis of the literature which included 43 articles, and again concluded that there were no data to support the use of CVP to guide fluid therapy, and both papers conluded that CVP should not be used for fluid resuscitation. Static measures of fluid responsiveness such as CVP may not be the most appropriate measures, and may be less accurate physiologically than dynamic measures.
Dynamic measurements based on the Frank-Starling principle use the changes in the venous return (preload) and stroke volume as a marker of fluid responsiveness and may be more useful. There are several dynamic methods to assess fluid responsiveness. The first such method is the measurement of right atrial pressure. In a case series of 33 medical and surgical intensive care unit (ICU) patients who had pulmonary artery catheters, it was hypothesized that right atrial pressure predicted the response to fluid pressure as right atrial pressure should not decrease during spontaneous inspiration in patients who had a heart that was not volume responsive. Patients were classified as having a positive response test when right atrial pressure decreased ≥1 mm Hg during inspiration, or a negative response when right atrial pressure decreased <1 mm Hg. A positive response correlated with cardiac output increase of 250 mL/h.16
Evaluation of Pulse Pressure and Stroke Volume Variation
Pulse pressure variation (PPV), stroke volume variation (SVV), and variation of the amplitude of pulse oximeter plethysmographic waveform are highly predictive of fluid responsiveness in mechanically ventilated patients who have septic or hemorrhagic shock.17,18 The PPV is derived from the analysis of the arterial waveform, and SVV is derived from pulse contour analysis. The PPV uses the physiologic changes that occur during positive pressure ventilation. The delivery of a mechanical breath increases pleural pressure on inspiration, causing the following: (1) a decrease in RV preload because of decreased venous return; and (2) increase in RV afterload because of increased transpulmonary pressure. These changes lead to decreased RV stroke volume, which is at a minimal level at the end of inspiration. The inspiration reduction in RV ejection leads to a decrease in LV filling after a phase lag of two to three heart beats because of long pulmonary transit time. Thus, the LV preload reduction may induce a decrease in LV stroke volume, which is at its minimum volume during the inspiratory period of mechanical ventilation.18 The variation between the RV and LV stroke volume are greatest when the ventricles operate on the steep part of the Frank-Starling curve (rather than the flat portion). The PPV is calculated as the difference between maximum and minimum pulse pressures divided by the average of their sum, and multiplied by 100%. A variation in PPV of greater than 13% is highly predictive of volume responsiveness.19 The use of PPV is feasible in the ED because the only requirements include arterial access, measurement of the minimum and maximum pulse pressures during 30 seconds, and performance of the calculation. The PPV has been validated in different patient populations. However, the use of PPV is limited to a conventional volume control mode of ventilation and restricted to tidal volumes (TVs) over 7 mL/kg, this method of measurement was validated in patients receiving tidal volumes of at least 8 cc/kg ideal body weight—which may be higher than seen in contemporary clinical practice with more restrictive TV, ventilation strategies in patients with acute respiratory distress syndrome.20 Furthermore, patients must be ventilated passively, with heavy sedation or chemical paralysis to prevent spontaneous breathing. They must also have a normal heart rhythm. Most acute lung injury states are managed with lung protective strategy with TV of 4 to 6 mL/kg, PPV values obtained using lower TVs are less reliable and their use is not recommended.20-22
The Pleth Variation Index (PVI) is similar to PPV but is an automated measure of the dynamic change in the perfusion index that occurs during a respiratory cycle. Perfusion index is the ratio of nonpulsatile to pulsatile blood flow through the peripheral bed, measured noninvasively with a pulse oximeter probe. The PVI can predict positive fluid response in mechanically ventilated patients. However, PVI has the same limitations as PPV, the patient must be in sinus rhythm, and PVI cannot be used in patients who are breathing spontaneously.23
Ultrasonographic Assessment
Bedside ultrasonography is noninvasive, can be performed rapidly, and provides real-time clinically relevant data. There is much evidence that ultrasonography is effective in evaluating hemodynamic and volume status, and it may be used to assess fluid responsiveness during resuscitation.
As there is growing awareness of sepsis and fluid resuscitation, IVC measurements have grown in popularity as a noninvasive approach for such monitoring.24 IVC collapsibility in spontaneously breathing patients and caval index in mechanically ventilated patients can be determined rapidly at the bedside. There are two views that most easily allow access to measure the IVC: subxyphoid and right upper quadrant. Using the phased array probe or a curvilinear probe, the IVC can be seen traversing through the liver with the hepatic vein joining the IVC just before the diaphragm and emptying into the right atrium. Interrater reliability is often questioned when ultrasound is used. However, Fields et al25 were able to show that there was a high degree of interrater reliability among EPs when measuring IVC collapsibility.
A systematic review by Zhang et al,26 showed change in IVC measured with point-of-care ultrasonography can reliably predict fluid responsiveness, particularly in patients that are mechanically ventilated. A caval index of 0.72 corresponds to CVP less than 7 cm water; a caval index of 1.23 corresponds to CVP 8 to 12 cm water; and a caval index of 1.59 corresponds to CVP greater than 13 cm water. The distensibility index is similar and calculated based on the IVC diameter at end-expiration (IVCDmax) and end-inspiration (IVCDmin).27 The ratio of (IVCDmax - IVCDmin)/IVCDmin is expressed as a percentage (dIVC%) in mechanically ventilated patients. A distensibility index less than 18% may indicate that the patient is not volume responsive (Tables 2 and 3; Figure 1).
Caval Index
A more widely utilized method for IVC evaluation is described by Nagdev et al.29 The caval index is calculated as the relative decrease in inferior vena cava diameter during one respiratory cycle. A caval index greater than or equal to 50% is strongly associated with a low CVP with 91% sensitivity and 94% specificity.29 It is important to remember, however, that IVC collapsibility is only useful at the extremes. Nevertheless, IVC measurement is limited by increased PEEP, increased TV, and increased intraabdominal pressure.
While IVC is the most commonly used vessel for sonographic volume status assessment, other vessels can also be used. Kent et al30 describe using the internal jugular vein as well as the femoral vein. Guarracino et al31 achieved similar results when using the internal jugular vein for distensibility index for assessing fluid responsiveness. When compared to the invasive CVP measurements, new CVP quantification methods could be used as a reliable approach for monitoring hemodynamic status.
Stroke Volume Variation
While not used regularly in the ED, respiratory changes in aortic blood velocity as measured by transesophageal echocardiography (TEE) may predict fluid responsiveness in mechanically ventilated patients.34 Peak aortic blood flow velocity variation is measured by TEE. Similarly, ventilator-induced variation in descending aortic blood flow measured by esophageal Doppler monitoring may predict fluid responsiveness.34 However peak aortic blood flow velocity measurements determined by TEE may have limited utility because TEE is an invasive procedure. Similarly, esophageal Doppler monitors can be used but are limited because of low predictive value and rare usage in the emergency setting.35
Passive Leg Raise
In spontaneously breathing patients, passive leg raising (PLR) has been studied as a substitute for volume challenge due to the ease of performing PLR at the bedside and absence of adverse events such as volume overload. When performing PLR, the patient starts in a semirecumbent position and is repositioned supine with the legs raised to 45°. Blood transferred to the heart during PLR increases cardiac preload and tests preload responsiveness. The maximum hemodynamic response to PLR occurs within one minute of performing the maneuver.36 The effects of PLR are assessed by the changes in cardiac output or stroke volume after PLR, which are extrapolated from aortic blood flow measured by esophageal Doppler, velocity time integral measured by transthoracic echocardiography, and femoral artery flow measured by arterial Doppler.36 These modalities may provide additional data points in the evaluation of fluid responsiveness but is out of the scope of this review.
Data in mechanically ventilated patients with esophageal Doppler and arterial access demonstrated that an increase in aortic blood flow by 10% with PLR predicted a positive fluid response with sensitivity 97% and specificity 94%.37 However, in the same study, the specificity in spontaneously breathing patients was markedly reduced (46%).37,38
Another study used a more conventional noninvasive measurement with transthoracic echocardiography to determine whether PLR could predict fluid responsiveness in hemodynamically unstable patients. In this study, a PLR-induced increase in stroke volume greater than or equal to 12.5% predicted an increase in stroke volume by greater than or equal to 15% after fluid administration with specificity 100% and sensitivity 77%.38 This study included patients on mechanical ventilation with active inspiration, patients without mechanical support, and patients with atrial fibrillation, enabling better generalization of results than previous studies.39
Bioreactance Technology
Cardiac output measurement using bioreactance technology is an alternative noninvasive method to measure cardiac output using only four surface electrodes. This technology is based on an analysis of relative phase shifts of an oscillating current that occurs when the current traverses the thoracic cavity. The bioreactance device (NICOM, Cheetah Medical, Tel Aviv, Israel) is comprised of a high frequency (75 kHz) sine wave generator and four dual electrode stickers that are used to establish electrical contact with the body. The cardiac output measured by bioreactance correlates well with values measured by thermodilution and pulse contour analysis.40 Performing PLR and determining its response using a bioreactance machine may be appropriate in the ED, in the ward, or at the initial presentation to the ICU because it is noninvasive and less labor intensive than other methods. In postoperative cardiac surgery patients, PLR-induced changes in cardiac output measured by bioreactance had sensitivity 88% and specificity 100%.40 In hemodynamically unstable patients, the results were more encouraging with a sensitivity of 94% and a specificity of 100% in predicting fluid responsiveness (defined as greater than10% increase in stroke volume index).41 However, in a group of critically ill patients (83% septic, 10% hypovolemic, and 7% cardiogenic), bioreactance coupled with PLR was unable to measure cardiac index compared with transpulmonary thermodilution, and bioreactance failed to predict fluid responsiveness.42 More research on bioreactance technology is needed, and its noninvasive evaluation of critically ill patients who need cardiac output monitoring and fluid therapy.
Conclusion
There are many tools available to estimate the volume status and fluid responsiveness of the critically ill patient. One of these tools, CVP measurement, must be used cautiously as an assessment of fluid responsiveness. It is important to understand the limitations of this technology. While other more advanced tools, such as ultrasonography to measure the IVC at the bedside and assess IVC variation or TEE to assess LV diastolic size and contractility during fluid resuscitation, may provide a better diagnostic picture, these tools/devices are not always available at most community hospitals.
The authors do not recommend placing a CVC simply to measure CVP; however, when a CVC or peripherally inserted central catheter is medically needed for treatment, the catheter can be used to trend CVP since the value of CVP is greatest as a trend to guide resuscitation. Other minimally invasive and noninvasive diagnostic tools currently are available, such as bedside ultrasound, and enable clinicians to assess volume responsiveness using dynamic procedures that challenge the Frank-Starling curve.4 These technologies have a useful place in resuscitation but each has its own limitations. With an understanding of the tools available, with their strengths and limitations, physicians can better individualize intravascular volume resuscitation.
Dr Farcy is the chairman of the department of emergency medicine, medical director of intensivists at Mount Sinai Medical Center, Miami Beach, Florida; and clinical assistant professor, Florida International University Medical School, Miami, Florida. Dr Jain is an assistant professor, director of critical care ultrasound, department of emergency medicine at the SUNY Downstate Medical Center, Kings County Hospital Center, Brooklyn, New York. Dr Dalley is the emergency-medicine residency program director at Mount Sinai Medical Center, Miami Beach, Florida.
Dr Scalea holds the Francis X. Kelly professorship in trauma surgery, is the distinguished professor in trauma, and director, program in trauma at the University of Maryland School of Medicine, Baltimore; he is also the physician in chief, shock trauma center, and system chief for critical care services at the University of Maryland Medical System, Baltimore.
- Pitfalls in Using Central Venous Pressure as a Marker of Fluid Responsiveness
- Chatterjee K. The Swan-Ganz catheters: past, present, and future. A viewpoint. Circulation. 2009;119(1):147-52.
- Shoemaker WC, Appel PL, Waxman K, Schwartz S, Chang P. Clinical trial of survivors’ cardiorespiratory patterns as therapeutic goals in critically ill postoperative patients. Crit Care Med. 1982;10(6):398-403.
- Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94(6):1176-1186.
- Rivers E, Nguyen B, Havstad S, et al; Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368-1377.
- Dellinger RP, Levy MM, Carlet JM, et al; International Surviving Sepsis Campaign Guidelines Committee; American Association of Critical-Care Nurses, American College of Chest Physicians, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296-327.
- ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of Protocol-based care for Early Septic Shock. N Engl J Med. 2014;370(18):1683-1693.
- Peake SL, Delaney A, Bellomo R; ARISE Investigators. Goal-directed resuscitation in septic shock. N Engl J Med. 2015; 372(2):190-191.
- Mouncey PR, Osborn TM, Power GS, et al; ProMISe Trial Investigators. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301-1311.
- Akmal AH, Hasan M, Mariam A. The incidence of complications of central venous catheters at an intensive care unit. Ann Thorac Med. 2007;2(2):61–63.
- Ruesch S, Walder B, Tramèr MR. Complications of central venous catheters: internal jugular versus subclavian access—a systematic review. Crit Care Med. 2002;30(2):454–460.
- Magder S. How to use central venous pressure measurements. Curr Opin Crit Care. 2005;11(3):264-270.
- Bafaqeeh F, Magder S. CVP and volume responsiveness of cardiac output. Am J Respir Crit Care Med. 2004;169:A344.
- Schummer W. Central venous pressure. Validity, informative value and correct measurement [in German]. Anaesthesist. 2009;58(5):499-505.
- Marik PE, Baram M, Vahid B. Does the central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134(1):172-178.
- Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41(7):1774-1781.
- Magder S, Georgiadis G, Cheong T. Respiratory variations in right atrial pressure predict the response to fluid challenge. J Crit Care. 1992;7(2):76-85.
- Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37(9):2642-2647.
- Reuter DA, Kirchner A, Felbinger TW, et al. Usefulness of left ventricular stroke volume variation to assess fluid responsiveness in patients with reduced cardiac function. Crit Care Med. 2003;31(5):1399-1404.
- Perel A, Pizov R, Cotev S. Systolic blood pressure variation is a sensitive indicator of hypovolemia in ventilated dogs subjected to graded hemorrhage. Anesthesiology. 1987;67(4):498-502.
- De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med. 2005;31(4):517-523.
- Charron C, Fessenmeyer C, Cosson C, et al. The influence of tidal volume on the dynamic variables of fluid responsiveness in critically ill patients. Anesth Analg. 2006;102(5):1511-1517.
- Galas F, Hajjar L, Polastri T, et al. Passive leg raising predicts fluid responsiveness after cardiac surgery. Crit Care. 2008;12(suppl 2):P89
- Cannesson M, Desebbe O, Rosamel P, et al. Pleth variability index to monitor the respiratory variations in the pulse oximeter plethysmographic waveform amplitude and predict fluid responsiveness in the operating theatre. Br J Anaesth. 2008;101(2):200-206.
- Coen D, Cortellaro F, Pasini S, et al. Towards a less invasive approach to the early goal-directed treatment of septic shock in the ED. Am J Emerg Med. 2014;32(6):563-568.
- Fields JM, Lee PA, Jenq KY, Mark DG, Panebianco NL, Dean AJ. The interrater reliability of inferior vena cava ultrasound by bedside clinician sonographers in emergency department patients. Acad Emerg Med. 2011;18(1):98-101.
- Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: systematic review and meta-analysis. Ultrasound Med Biol. 2014;40(5):845-853
- Sefidbakht S, Assadsangabi R, Abbasi HR, Nabavizadeh A. Sonographic measurement of the inferior vena cava as a predictor of shock in trauma patients. Emerg Radiol. 2007;14(3):181-185.
- Rudski LW, Lai WW, Afilalo J, et al. Guidelines of the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2013;23(7):658-713.
- Nagdev AD, Merchant RC, Tirado-Gonzalez A, Sisson CA, Murphy MC. Emergency department bedside ultrasonographic measurement of the caval index for noninvasive determination of low central venous pressure. Ann Emerg Med. 2010;55(3):290-295.
- Kent A, Patil P, Davila V, et al. Sonographic evaluation of intravascular volume status: Can internal jugular or femoral vein collapsibility be used in the absence of IVC visualization? Ann Thorac Med. 2015;10(1):44-49.
- Guarracino F, Ferro B, Forfori F, Bertini P, Magliacano L, Pinsky MR. Jugular vein distensibility predicts fluid responsiveness in septic patients. Crit Care. 2014;18(6):647.
- Chin JH, Jun IG, Lee J, Seo H, Hwang GS, Kim YK. Can stroke volume variation be an alternative to central venous pressure in patients undergoing kidney transplantation? Transplant Proc. 2014;46(10):3363-3366.
- Zhang Z, Lu B, Sheng X, Jin N. Accuracy of stroke volume variation in predicting fluid responsiveness: a systematic review and meta-analysis. J Anesth. 2011;25(6):904-916.
- Feissel M, Michard F, Mangin I, Ruyer O, Faller JP, Teboul JL. Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest. 2001;119(3):867-873.
- Davies SJ, Minhas S, Wilson RJ, Yates D, Howell SJ. Comparison of stroke volume and fluid responsiveness measurements in commonly used technologies for goal-directed therapy. J Clin Anesth. 2013;25(6):466-474.
- Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann Intensive Care. 2011;1(1):1.
- Monnet X, Rienzo M, Osman D, et al. Esophageal Doppler monitoring predicts fluid responsiveness in critically ill ventilated patients. Intensive Care Med. 2005;31(9):1195-1201.
- Monnet X, Rienzo M, Osman D, et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006;34(5):1402-1407.
- Lamia B, Ochagavia A, Monnet X, Chemla D, Richard C, Teboul JL. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007;33(7):1125-1132.
- Benomar B, Ouattara A, Estagnasie P, Brusset A, Squara P. Fluid responsiveness predicted by noninvasive bioreactance-based passive leg raise test. Intensive Care Med. 2010;36(11):1875-1881.
- Marik PE, Levitov A, Young A, Andrews L. The use of bioreactance and carotid Doppler to determine volume responsiveness and blood flow redistribution following passive leg raising in hemodynamically unstable patients. Chest. 2013;143(2):364-370.
- Kupersztych-Hagege E, Teboul JL, Artigas A, et al. Bioreactance is not reliable for estimating cardiac output and the effects of passive leg raising in critically ill patients. Br J Anaesth. 2013;111(6):961-966.
- Pitfalls in Using Central Venous Pressure as a Marker of Fluid Responsiveness
- Chatterjee K. The Swan-Ganz catheters: past, present, and future. A viewpoint. Circulation. 2009;119(1):147-52.
- Shoemaker WC, Appel PL, Waxman K, Schwartz S, Chang P. Clinical trial of survivors’ cardiorespiratory patterns as therapeutic goals in critically ill postoperative patients. Crit Care Med. 1982;10(6):398-403.
- Shoemaker WC, Appel PL, Kram HB, Waxman K, Lee TS. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest. 1988;94(6):1176-1186.
- Rivers E, Nguyen B, Havstad S, et al; Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368-1377.
- Dellinger RP, Levy MM, Carlet JM, et al; International Surviving Sepsis Campaign Guidelines Committee; American Association of Critical-Care Nurses, American College of Chest Physicians, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296-327.
- ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, et al. A randomized trial of Protocol-based care for Early Septic Shock. N Engl J Med. 2014;370(18):1683-1693.
- Peake SL, Delaney A, Bellomo R; ARISE Investigators. Goal-directed resuscitation in septic shock. N Engl J Med. 2015; 372(2):190-191.
- Mouncey PR, Osborn TM, Power GS, et al; ProMISe Trial Investigators. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301-1311.
- Akmal AH, Hasan M, Mariam A. The incidence of complications of central venous catheters at an intensive care unit. Ann Thorac Med. 2007;2(2):61–63.
- Ruesch S, Walder B, Tramèr MR. Complications of central venous catheters: internal jugular versus subclavian access—a systematic review. Crit Care Med. 2002;30(2):454–460.
- Magder S. How to use central venous pressure measurements. Curr Opin Crit Care. 2005;11(3):264-270.
- Bafaqeeh F, Magder S. CVP and volume responsiveness of cardiac output. Am J Respir Crit Care Med. 2004;169:A344.
- Schummer W. Central venous pressure. Validity, informative value and correct measurement [in German]. Anaesthesist. 2009;58(5):499-505.
- Marik PE, Baram M, Vahid B. Does the central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134(1):172-178.
- Marik PE, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41(7):1774-1781.
- Magder S, Georgiadis G, Cheong T. Respiratory variations in right atrial pressure predict the response to fluid challenge. J Crit Care. 1992;7(2):76-85.
- Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37(9):2642-2647.
- Reuter DA, Kirchner A, Felbinger TW, et al. Usefulness of left ventricular stroke volume variation to assess fluid responsiveness in patients with reduced cardiac function. Crit Care Med. 2003;31(5):1399-1404.
- Perel A, Pizov R, Cotev S. Systolic blood pressure variation is a sensitive indicator of hypovolemia in ventilated dogs subjected to graded hemorrhage. Anesthesiology. 1987;67(4):498-502.
- De Backer D, Heenen S, Piagnerelli M, Koch M, Vincent JL. Pulse pressure variations to predict fluid responsiveness: influence of tidal volume. Intensive Care Med. 2005;31(4):517-523.
- Charron C, Fessenmeyer C, Cosson C, et al. The influence of tidal volume on the dynamic variables of fluid responsiveness in critically ill patients. Anesth Analg. 2006;102(5):1511-1517.
- Galas F, Hajjar L, Polastri T, et al. Passive leg raising predicts fluid responsiveness after cardiac surgery. Crit Care. 2008;12(suppl 2):P89
- Cannesson M, Desebbe O, Rosamel P, et al. Pleth variability index to monitor the respiratory variations in the pulse oximeter plethysmographic waveform amplitude and predict fluid responsiveness in the operating theatre. Br J Anaesth. 2008;101(2):200-206.
- Coen D, Cortellaro F, Pasini S, et al. Towards a less invasive approach to the early goal-directed treatment of septic shock in the ED. Am J Emerg Med. 2014;32(6):563-568.
- Fields JM, Lee PA, Jenq KY, Mark DG, Panebianco NL, Dean AJ. The interrater reliability of inferior vena cava ultrasound by bedside clinician sonographers in emergency department patients. Acad Emerg Med. 2011;18(1):98-101.
- Zhang Z, Xu X, Ye S, Xu L. Ultrasonographic measurement of the respiratory variation in the inferior vena cava diameter is predictive of fluid responsiveness in critically ill patients: systematic review and meta-analysis. Ultrasound Med Biol. 2014;40(5):845-853
- Sefidbakht S, Assadsangabi R, Abbasi HR, Nabavizadeh A. Sonographic measurement of the inferior vena cava as a predictor of shock in trauma patients. Emerg Radiol. 2007;14(3):181-185.
- Rudski LW, Lai WW, Afilalo J, et al. Guidelines of the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2013;23(7):658-713.
- Nagdev AD, Merchant RC, Tirado-Gonzalez A, Sisson CA, Murphy MC. Emergency department bedside ultrasonographic measurement of the caval index for noninvasive determination of low central venous pressure. Ann Emerg Med. 2010;55(3):290-295.
- Kent A, Patil P, Davila V, et al. Sonographic evaluation of intravascular volume status: Can internal jugular or femoral vein collapsibility be used in the absence of IVC visualization? Ann Thorac Med. 2015;10(1):44-49.
- Guarracino F, Ferro B, Forfori F, Bertini P, Magliacano L, Pinsky MR. Jugular vein distensibility predicts fluid responsiveness in septic patients. Crit Care. 2014;18(6):647.
- Chin JH, Jun IG, Lee J, Seo H, Hwang GS, Kim YK. Can stroke volume variation be an alternative to central venous pressure in patients undergoing kidney transplantation? Transplant Proc. 2014;46(10):3363-3366.
- Zhang Z, Lu B, Sheng X, Jin N. Accuracy of stroke volume variation in predicting fluid responsiveness: a systematic review and meta-analysis. J Anesth. 2011;25(6):904-916.
- Feissel M, Michard F, Mangin I, Ruyer O, Faller JP, Teboul JL. Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest. 2001;119(3):867-873.
- Davies SJ, Minhas S, Wilson RJ, Yates D, Howell SJ. Comparison of stroke volume and fluid responsiveness measurements in commonly used technologies for goal-directed therapy. J Clin Anesth. 2013;25(6):466-474.
- Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Ann Intensive Care. 2011;1(1):1.
- Monnet X, Rienzo M, Osman D, et al. Esophageal Doppler monitoring predicts fluid responsiveness in critically ill ventilated patients. Intensive Care Med. 2005;31(9):1195-1201.
- Monnet X, Rienzo M, Osman D, et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006;34(5):1402-1407.
- Lamia B, Ochagavia A, Monnet X, Chemla D, Richard C, Teboul JL. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007;33(7):1125-1132.
- Benomar B, Ouattara A, Estagnasie P, Brusset A, Squara P. Fluid responsiveness predicted by noninvasive bioreactance-based passive leg raise test. Intensive Care Med. 2010;36(11):1875-1881.
- Marik PE, Levitov A, Young A, Andrews L. The use of bioreactance and carotid Doppler to determine volume responsiveness and blood flow redistribution following passive leg raising in hemodynamically unstable patients. Chest. 2013;143(2):364-370.
- Kupersztych-Hagege E, Teboul JL, Artigas A, et al. Bioreactance is not reliable for estimating cardiac output and the effects of passive leg raising in critically ill patients. Br J Anaesth. 2013;111(6):961-966.