User login
CHICAGO – Patients undergoing Roux-en-Y gastric bypass are twice as likely to need a reoperation as those having sleeve gastrectomy, according to ACS NSQIP data.
Reoperation among Roux-en-Y patients was associated with a 10-fold increase in mortality over sleeve gastrectomy (1.2% vs. 0.1%; P less than .01) and a 3-fold increase in length of stay (6 days vs. 2 days; P less than .01), Dr. Matthew Whealon reported at the American College of Surgeons Clinical Congress
The results are consistent with prior contemporary analyses using ACS National Surgical Quality Improvement Program (NSQIP) data reporting reoperation rates of 2.5%-5.1% for Roux-en-Y gastric bypass (RYGB) and 1.6%-3% for sleeve gastrectomy. Those analyses, however, did not include the reasons for reoperation, as these data were not available until the 2012 database release, he said.
With these data now in hand, lead author Dr. Mark Hanna and his fellow investigators at the University of California, Irvine, identified 36,757 adults in the 2012-2013 database who underwent RYGB (n = 19,597) or sleeve gastrectomy (n = 17,160) for morbid obesity and performed multivariate regression analyses to identify risk factors associated with reoperation.
In all, 518 RYGB patients and 231 sleeve gastrectomy patients required an unplanned return to the operating room (2.6% vs. 1.3%), Dr. Whealon said. The mean time from the index procedure to reoperation was 7.6 days and 7.1 days, respectively.
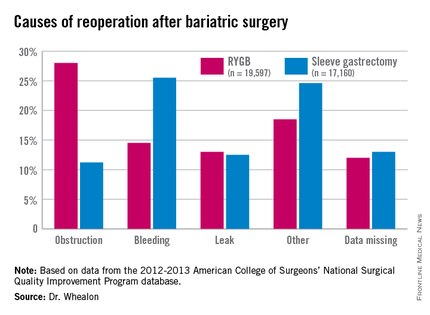
Obstruction was the biggest driver of reoperation following RYGB, accounting for 28% of reoperations. Other causes were bleeding (14.5%), leak (13%), and other unspecified reasons (18.5%), with data missing in 12%.
Bleeding was the most common indication for reoperation after sleeve gastrectomy (25.5%), followed by other unspecified reasons(24.6%), missing data (13%), leak (12.55%), and obstruction (11.2%), he said.
In adjusted multivariate analyses, factors that significantly increased the risk for reoperation were heart failure (adjusted odds ratio, 2.3), dependent functional status (aOR, 2.1), RYGB (aOR, 1.94), chronic obstructive pulmonary disease (aOR, 1.7), open operation (aOR, 1.6), and male sex (aOR, 1.1). The P values were less than .05 for all comparisons.
Factors not significant for reoperation included body mass index, age, smoking status, bleeding disorder, steroid use, dialysis, hypertension, diabetes, preoperative sepsis, emergent admission, elective operation, and preoperative weight loss.
While bariatric surgery remains a safe operation with low mortality and reoperation rates, additional studies are needed, because of the increased mortality associated with reoperation, to identify ways to mitigate these complications, Dr. Whealon said.
Limitations of the study were that ICD-9 codes for postoperative hemorrhage could not differentiate between intra-abdominal and gastrointestinal bleeding, the database is subject to coding errors, and missing data may have introduced bias into the study, he noted.
Discussant Dr. Matthew Goldblatt of the Medical College of Wisconsin in Milwaukee commented that use of the ACS MBSAQIP (Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program) database would have avoided some of the coding errors for reoperation.
He also questioned whether the average 7-day return to surgery interval reflects the use of endoscopy, as few surgeons would wait that long if, as the analysis suggests, a primary reason for reoperation was postoperative bleeding.
Endoscopy was included in the reoperations, Dr. Whealon said, but he could not speak to the exact percentage it comprised.
Finally, Dr. Goldblatt said, “the patients that you identified as being the highest risk for complication, as is often the case in these reviews, are really the ones most likely to gain the most from the procedure. … So how can people avoid operating on these patients when they are the ones that can get the most out of it?”
Dr. Whealon agreed that high-risk patients have the most to gain and suggested that “optimizing their comorbid conditions before operation will help reduce their risk.”
The authors reported having no conflicts of interest.
CHICAGO – Patients undergoing Roux-en-Y gastric bypass are twice as likely to need a reoperation as those having sleeve gastrectomy, according to ACS NSQIP data.
Reoperation among Roux-en-Y patients was associated with a 10-fold increase in mortality over sleeve gastrectomy (1.2% vs. 0.1%; P less than .01) and a 3-fold increase in length of stay (6 days vs. 2 days; P less than .01), Dr. Matthew Whealon reported at the American College of Surgeons Clinical Congress
The results are consistent with prior contemporary analyses using ACS National Surgical Quality Improvement Program (NSQIP) data reporting reoperation rates of 2.5%-5.1% for Roux-en-Y gastric bypass (RYGB) and 1.6%-3% for sleeve gastrectomy. Those analyses, however, did not include the reasons for reoperation, as these data were not available until the 2012 database release, he said.
With these data now in hand, lead author Dr. Mark Hanna and his fellow investigators at the University of California, Irvine, identified 36,757 adults in the 2012-2013 database who underwent RYGB (n = 19,597) or sleeve gastrectomy (n = 17,160) for morbid obesity and performed multivariate regression analyses to identify risk factors associated with reoperation.
In all, 518 RYGB patients and 231 sleeve gastrectomy patients required an unplanned return to the operating room (2.6% vs. 1.3%), Dr. Whealon said. The mean time from the index procedure to reoperation was 7.6 days and 7.1 days, respectively.
Obstruction was the biggest driver of reoperation following RYGB, accounting for 28% of reoperations. Other causes were bleeding (14.5%), leak (13%), and other unspecified reasons (18.5%), with data missing in 12%.
Bleeding was the most common indication for reoperation after sleeve gastrectomy (25.5%), followed by other unspecified reasons(24.6%), missing data (13%), leak (12.55%), and obstruction (11.2%), he said.
In adjusted multivariate analyses, factors that significantly increased the risk for reoperation were heart failure (adjusted odds ratio, 2.3), dependent functional status (aOR, 2.1), RYGB (aOR, 1.94), chronic obstructive pulmonary disease (aOR, 1.7), open operation (aOR, 1.6), and male sex (aOR, 1.1). The P values were less than .05 for all comparisons.
Factors not significant for reoperation included body mass index, age, smoking status, bleeding disorder, steroid use, dialysis, hypertension, diabetes, preoperative sepsis, emergent admission, elective operation, and preoperative weight loss.

While bariatric surgery remains a safe operation with low mortality and reoperation rates, additional studies are needed, because of the increased mortality associated with reoperation, to identify ways to mitigate these complications, Dr. Whealon said.
Limitations of the study were that ICD-9 codes for postoperative hemorrhage could not differentiate between intra-abdominal and gastrointestinal bleeding, the database is subject to coding errors, and missing data may have introduced bias into the study, he noted.
Discussant Dr. Matthew Goldblatt of the Medical College of Wisconsin in Milwaukee commented that use of the ACS MBSAQIP (Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program) database would have avoided some of the coding errors for reoperation.
He also questioned whether the average 7-day return to surgery interval reflects the use of endoscopy, as few surgeons would wait that long if, as the analysis suggests, a primary reason for reoperation was postoperative bleeding.
Endoscopy was included in the reoperations, Dr. Whealon said, but he could not speak to the exact percentage it comprised.
Finally, Dr. Goldblatt said, “the patients that you identified as being the highest risk for complication, as is often the case in these reviews, are really the ones most likely to gain the most from the procedure. … So how can people avoid operating on these patients when they are the ones that can get the most out of it?”
Dr. Whealon agreed that high-risk patients have the most to gain and suggested that “optimizing their comorbid conditions before operation will help reduce their risk.”
The authors reported having no conflicts of interest.
CHICAGO – Patients undergoing Roux-en-Y gastric bypass are twice as likely to need a reoperation as those having sleeve gastrectomy, according to ACS NSQIP data.
Reoperation among Roux-en-Y patients was associated with a 10-fold increase in mortality over sleeve gastrectomy (1.2% vs. 0.1%; P less than .01) and a 3-fold increase in length of stay (6 days vs. 2 days; P less than .01), Dr. Matthew Whealon reported at the American College of Surgeons Clinical Congress
The results are consistent with prior contemporary analyses using ACS National Surgical Quality Improvement Program (NSQIP) data reporting reoperation rates of 2.5%-5.1% for Roux-en-Y gastric bypass (RYGB) and 1.6%-3% for sleeve gastrectomy. Those analyses, however, did not include the reasons for reoperation, as these data were not available until the 2012 database release, he said.
With these data now in hand, lead author Dr. Mark Hanna and his fellow investigators at the University of California, Irvine, identified 36,757 adults in the 2012-2013 database who underwent RYGB (n = 19,597) or sleeve gastrectomy (n = 17,160) for morbid obesity and performed multivariate regression analyses to identify risk factors associated with reoperation.
In all, 518 RYGB patients and 231 sleeve gastrectomy patients required an unplanned return to the operating room (2.6% vs. 1.3%), Dr. Whealon said. The mean time from the index procedure to reoperation was 7.6 days and 7.1 days, respectively.
Obstruction was the biggest driver of reoperation following RYGB, accounting for 28% of reoperations. Other causes were bleeding (14.5%), leak (13%), and other unspecified reasons (18.5%), with data missing in 12%.
Bleeding was the most common indication for reoperation after sleeve gastrectomy (25.5%), followed by other unspecified reasons(24.6%), missing data (13%), leak (12.55%), and obstruction (11.2%), he said.
In adjusted multivariate analyses, factors that significantly increased the risk for reoperation were heart failure (adjusted odds ratio, 2.3), dependent functional status (aOR, 2.1), RYGB (aOR, 1.94), chronic obstructive pulmonary disease (aOR, 1.7), open operation (aOR, 1.6), and male sex (aOR, 1.1). The P values were less than .05 for all comparisons.
Factors not significant for reoperation included body mass index, age, smoking status, bleeding disorder, steroid use, dialysis, hypertension, diabetes, preoperative sepsis, emergent admission, elective operation, and preoperative weight loss.

While bariatric surgery remains a safe operation with low mortality and reoperation rates, additional studies are needed, because of the increased mortality associated with reoperation, to identify ways to mitigate these complications, Dr. Whealon said.
Limitations of the study were that ICD-9 codes for postoperative hemorrhage could not differentiate between intra-abdominal and gastrointestinal bleeding, the database is subject to coding errors, and missing data may have introduced bias into the study, he noted.
Discussant Dr. Matthew Goldblatt of the Medical College of Wisconsin in Milwaukee commented that use of the ACS MBSAQIP (Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program) database would have avoided some of the coding errors for reoperation.
He also questioned whether the average 7-day return to surgery interval reflects the use of endoscopy, as few surgeons would wait that long if, as the analysis suggests, a primary reason for reoperation was postoperative bleeding.
Endoscopy was included in the reoperations, Dr. Whealon said, but he could not speak to the exact percentage it comprised.
Finally, Dr. Goldblatt said, “the patients that you identified as being the highest risk for complication, as is often the case in these reviews, are really the ones most likely to gain the most from the procedure. … So how can people avoid operating on these patients when they are the ones that can get the most out of it?”
Dr. Whealon agreed that high-risk patients have the most to gain and suggested that “optimizing their comorbid conditions before operation will help reduce their risk.”
The authors reported having no conflicts of interest.
AT THE ACS CLINICAL CONGRESS
Key clinical point: Patients undergoing Roux-en-Y gastric bypass were twice as likely to need a reoperation as with sleeve gastrectomy, and reoperation increased morbidity 10-fold.
Major finding: The reoperation rate for Roux-en-Y gastric bypass was 2.6% vs. 1.3% for sleeve gastrectomy.
Data source: An ACS NSQIP database analysis of 36,757 patients undergoing bariatric surgery.
Disclosures: The authors reported having no conflicts of interest.

