User login
The COVID-19 pandemic has resulted in notable morbidity and mortality worldwide. In December 2020, the US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA (mRNA) vaccines—produced by Pfizer-BioNTech and Moderna—for the prevention of COVID-19. Phase 3 trials of the vaccine developed by Moderna showed 94.1% efficacy at preventing COVID-19 after 2 doses.1
Common cutaneous adverse effects of the Moderna COVID-19 Vaccine include injection-site reactions, such as pain, induration, and erythema. Less frequently reported dermatologic adverse effects include diffuse bullous rash and hypersensitivity reactions.1 We report a case of reactivation of a BCG vaccination scar after the first dose of the Moderna COVID-19 Vaccine.
Case Report
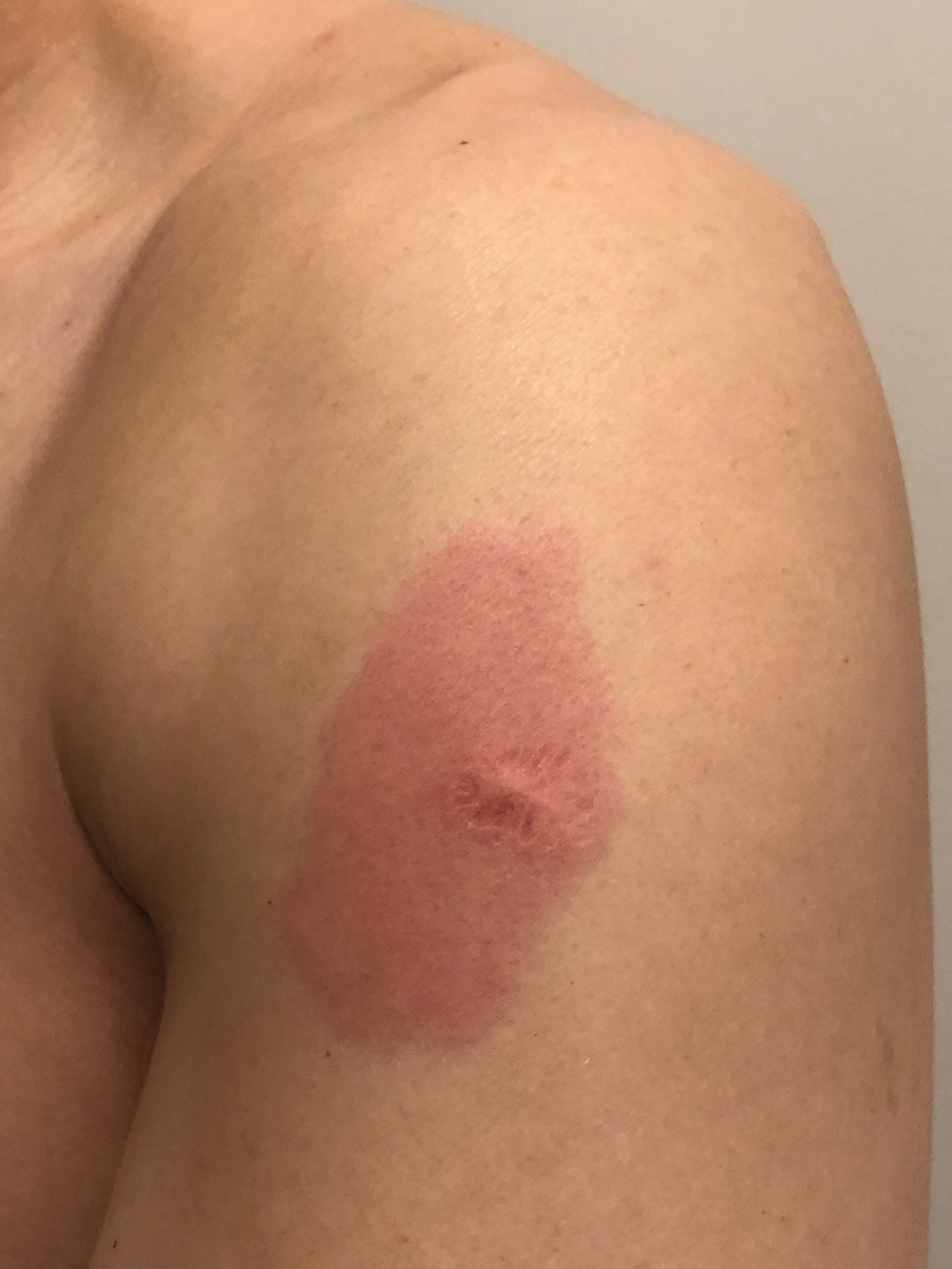
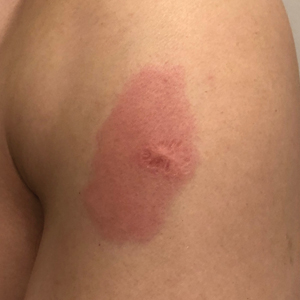
A 48-year-old Asian man who was otherwise healthy presented with erythema, induration, and mild pruritus on the deltoid muscle of the left arm, near the scar from an earlier BCG vaccine, which he received at approximately 5 years of age when living in Taiwan. The patient received the first dose of the Moderna COVID-19 Vaccine approximately 5 to 7 cm distant from the BCG vaccination scar. One to 2 days after inoculation, the patient endorsed tenderness at the site of COVID-19 vaccination but denied systemic symptoms. He had never been given a diagnosis of COVID-19. His SARS-CoV-2 antibody status was unknown.
Eight days later, the patient noticed a well-defined, erythematous, indurated plaque with mild itchiness overlying and around the BCG vaccination scar that did not involve the COVID-19 vaccination site. The following day, the redness and induration became worse (Figure).
The patient was otherwise well. Vital signs were normal; there was no lymphadenopathy. The rash resolved without treatment over the next 4 days.
Comment
The BCG vaccine is an intradermal live attenuated virus vaccine used to prevent certain forms of tuberculosis and potentially other Mycobacterium infections. Although the vaccine is not routinely administered in the United States, it is part of the vaccination schedule in most countries, administered most often to newborns and infants. Administration of the BCG vaccine commonly results in mild localized erythema, swelling, and pain at the injection site. Most inoculated patients also develop an ulcer that heals with the characteristic BCG vaccination scar.2,3
There is evidence that the BCG vaccine can enhance the innate immune system response and might decrease the rate of infection by unrelated pathogens, including viruses.4 Several epidemiologic studies have suggested that the BCG vaccine might offer some protection against COVID-19, possibly due to a resemblance of the amino acid sequences of BCG and SARS-CoV-2, which might provoke cross-reactive T cells.5,6 Further studies are underway to determine whether the BCG vaccine is truly protective against COVID-19.
BCG vaccination scar reactivation presents as redness, swelling, or ulceration at the BCG injection site months to years after inoculation. Although erythema and induration of the BCG scar are not included in the diagnostic criteria of Kawasaki disease, likely due to variable vaccine requirements in different countries, these findings are largely recognized as specific for Kawasaki disease and present in approximately half of affected patients who received the BCG vaccine.2
Heat Shock Proteins—Heat shock proteins (HSPs) are produced by cells in response to stressors. The proposed mechanism of BCG vaccination scar reactivation is a cross-reaction between human homologue HSP 63 and Mycobacterium HSP 65, leading to hyperactivity of the immune system against BCG.7 There also are reports of reactivation of a BCG vaccination scar from measles infection and influenza vaccination.2,8,9 Most prior reports of BCG vaccination scar reactivation have been in pediatric patients; our patient is an adult who received the BCG vaccine more than 40 years ago.
Mechanism of Reactivation—The mechanism of BCG vaccination scar reactivation in our patient, who received the Moderna COVID-19 Vaccine, is unclear. Possible mechanisms include (1) release of HSP mediated by the COVID-19 vaccine, leading to an immune response at the BCG vaccine scar, or (2) another immune-mediated cross-reaction between BCG and the Moderna COVID-19 Vaccine mRNA nanoparticle or encoded spike protein antigen. It has been hypothesized that the BCG vaccine might offer some protection against COVID-19; this remains uncertain and is under further investigation.10 A recent retrospective cohort study showed that a BCG vaccination booster may decrease COVID-19 infection rates in higher-risk populations.11
Conclusion
We present a case of BCG vaccine scar reactivation occurring after a dose of the Moderna COVID-19 Vaccine, a likely underreported, self-limiting, cutaneous adverse effect of this mRNA vaccine.
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403-416. doi:10.1056/NEJMoa2035389
- Muthuvelu S, Lim KS, Huang L-Y, et al. Measles infection causing bacillus Calmette-Guérin reactivation: a case report. BMC Pediatr. 2019;19:251. doi:10.1186/s12887-019-1635-z
- Fatima S, Kumari A, Das G, et al. Tuberculosis vaccine: a journey from BCG to present. Life Sci. 2020;252:117594. doi:10.1016/j.lfs.2020.117594
- O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335-337. doi:10.1038/s41577-020-0337-y
- Brooks NA, Puri A, Garg S, et al. The association of coronavirus disease-19 mortality and prior bacille Calmette-Guérin vaccination: a robust ecological analysis using unsupervised machine learning. Sci Rep. 2021;11:774. doi:10.1038/s41598-020-80787-z
- Tomita Y, Sato R, Ikeda T, et al. BCG vaccine may generate cross-reactive T-cells against SARS-CoV-2: in silico analyses and a hypothesis. Vaccine. 2020;38:6352-6356. doi:10.1016/j.vaccine.2020.08.045
- Lim KYY, Chua MC, Tan NWH, et al. Reactivation of BCG inoculation site in a child with febrile exanthema of 3 days duration: an early indicator of incomplete Kawasaki disease. BMJ Case Rep. 2020;13:E239648. doi:10.1136/bcr-2020-239648
- Kondo M, Goto H, Yamamoto S. First case of redness and erosion at bacillus Calmette-Guérin inoculation site after vaccination against influenza. J Dermatol. 2016;43:1229-1231. doi:10.1111/1346-8138.13365
- Chavarri-Guerra Y, Soto-Pérez-de-Celis E. Erythema at the bacillus Calmette-Guerin scar after influenza vaccination. Rev Soc Bras Med Trop. 2019;53:E20190390. doi:10.1590/0037-8682-0390-2019
- Fu W, Ho P-C, Liu C-L, et al. Reconcile the debate over protective effects of BCG vaccine against COVID-19. Sci Rep. 2021;11:8356. doi:10.1038/s41598-021-87731-9
- Amirlak L, Haddad R, Hardy JD, et al. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum Vaccin Immunother. 2021;17:3913-3915. doi:10.1080/21645515.2021.1956228
The COVID-19 pandemic has resulted in notable morbidity and mortality worldwide. In December 2020, the US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA (mRNA) vaccines—produced by Pfizer-BioNTech and Moderna—for the prevention of COVID-19. Phase 3 trials of the vaccine developed by Moderna showed 94.1% efficacy at preventing COVID-19 after 2 doses.1
Common cutaneous adverse effects of the Moderna COVID-19 Vaccine include injection-site reactions, such as pain, induration, and erythema. Less frequently reported dermatologic adverse effects include diffuse bullous rash and hypersensitivity reactions.1 We report a case of reactivation of a BCG vaccination scar after the first dose of the Moderna COVID-19 Vaccine.
Case Report
A 48-year-old Asian man who was otherwise healthy presented with erythema, induration, and mild pruritus on the deltoid muscle of the left arm, near the scar from an earlier BCG vaccine, which he received at approximately 5 years of age when living in Taiwan. The patient received the first dose of the Moderna COVID-19 Vaccine approximately 5 to 7 cm distant from the BCG vaccination scar. One to 2 days after inoculation, the patient endorsed tenderness at the site of COVID-19 vaccination but denied systemic symptoms. He had never been given a diagnosis of COVID-19. His SARS-CoV-2 antibody status was unknown.
Eight days later, the patient noticed a well-defined, erythematous, indurated plaque with mild itchiness overlying and around the BCG vaccination scar that did not involve the COVID-19 vaccination site. The following day, the redness and induration became worse (Figure).
The patient was otherwise well. Vital signs were normal; there was no lymphadenopathy. The rash resolved without treatment over the next 4 days.
Comment
The BCG vaccine is an intradermal live attenuated virus vaccine used to prevent certain forms of tuberculosis and potentially other Mycobacterium infections. Although the vaccine is not routinely administered in the United States, it is part of the vaccination schedule in most countries, administered most often to newborns and infants. Administration of the BCG vaccine commonly results in mild localized erythema, swelling, and pain at the injection site. Most inoculated patients also develop an ulcer that heals with the characteristic BCG vaccination scar.2,3
There is evidence that the BCG vaccine can enhance the innate immune system response and might decrease the rate of infection by unrelated pathogens, including viruses.4 Several epidemiologic studies have suggested that the BCG vaccine might offer some protection against COVID-19, possibly due to a resemblance of the amino acid sequences of BCG and SARS-CoV-2, which might provoke cross-reactive T cells.5,6 Further studies are underway to determine whether the BCG vaccine is truly protective against COVID-19.
BCG vaccination scar reactivation presents as redness, swelling, or ulceration at the BCG injection site months to years after inoculation. Although erythema and induration of the BCG scar are not included in the diagnostic criteria of Kawasaki disease, likely due to variable vaccine requirements in different countries, these findings are largely recognized as specific for Kawasaki disease and present in approximately half of affected patients who received the BCG vaccine.2
Heat Shock Proteins—Heat shock proteins (HSPs) are produced by cells in response to stressors. The proposed mechanism of BCG vaccination scar reactivation is a cross-reaction between human homologue HSP 63 and Mycobacterium HSP 65, leading to hyperactivity of the immune system against BCG.7 There also are reports of reactivation of a BCG vaccination scar from measles infection and influenza vaccination.2,8,9 Most prior reports of BCG vaccination scar reactivation have been in pediatric patients; our patient is an adult who received the BCG vaccine more than 40 years ago.
Mechanism of Reactivation—The mechanism of BCG vaccination scar reactivation in our patient, who received the Moderna COVID-19 Vaccine, is unclear. Possible mechanisms include (1) release of HSP mediated by the COVID-19 vaccine, leading to an immune response at the BCG vaccine scar, or (2) another immune-mediated cross-reaction between BCG and the Moderna COVID-19 Vaccine mRNA nanoparticle or encoded spike protein antigen. It has been hypothesized that the BCG vaccine might offer some protection against COVID-19; this remains uncertain and is under further investigation.10 A recent retrospective cohort study showed that a BCG vaccination booster may decrease COVID-19 infection rates in higher-risk populations.11
Conclusion
We present a case of BCG vaccine scar reactivation occurring after a dose of the Moderna COVID-19 Vaccine, a likely underreported, self-limiting, cutaneous adverse effect of this mRNA vaccine.
The COVID-19 pandemic has resulted in notable morbidity and mortality worldwide. In December 2020, the US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA (mRNA) vaccines—produced by Pfizer-BioNTech and Moderna—for the prevention of COVID-19. Phase 3 trials of the vaccine developed by Moderna showed 94.1% efficacy at preventing COVID-19 after 2 doses.1
Common cutaneous adverse effects of the Moderna COVID-19 Vaccine include injection-site reactions, such as pain, induration, and erythema. Less frequently reported dermatologic adverse effects include diffuse bullous rash and hypersensitivity reactions.1 We report a case of reactivation of a BCG vaccination scar after the first dose of the Moderna COVID-19 Vaccine.
Case Report
A 48-year-old Asian man who was otherwise healthy presented with erythema, induration, and mild pruritus on the deltoid muscle of the left arm, near the scar from an earlier BCG vaccine, which he received at approximately 5 years of age when living in Taiwan. The patient received the first dose of the Moderna COVID-19 Vaccine approximately 5 to 7 cm distant from the BCG vaccination scar. One to 2 days after inoculation, the patient endorsed tenderness at the site of COVID-19 vaccination but denied systemic symptoms. He had never been given a diagnosis of COVID-19. His SARS-CoV-2 antibody status was unknown.
Eight days later, the patient noticed a well-defined, erythematous, indurated plaque with mild itchiness overlying and around the BCG vaccination scar that did not involve the COVID-19 vaccination site. The following day, the redness and induration became worse (Figure).
The patient was otherwise well. Vital signs were normal; there was no lymphadenopathy. The rash resolved without treatment over the next 4 days.
Comment
The BCG vaccine is an intradermal live attenuated virus vaccine used to prevent certain forms of tuberculosis and potentially other Mycobacterium infections. Although the vaccine is not routinely administered in the United States, it is part of the vaccination schedule in most countries, administered most often to newborns and infants. Administration of the BCG vaccine commonly results in mild localized erythema, swelling, and pain at the injection site. Most inoculated patients also develop an ulcer that heals with the characteristic BCG vaccination scar.2,3
There is evidence that the BCG vaccine can enhance the innate immune system response and might decrease the rate of infection by unrelated pathogens, including viruses.4 Several epidemiologic studies have suggested that the BCG vaccine might offer some protection against COVID-19, possibly due to a resemblance of the amino acid sequences of BCG and SARS-CoV-2, which might provoke cross-reactive T cells.5,6 Further studies are underway to determine whether the BCG vaccine is truly protective against COVID-19.
BCG vaccination scar reactivation presents as redness, swelling, or ulceration at the BCG injection site months to years after inoculation. Although erythema and induration of the BCG scar are not included in the diagnostic criteria of Kawasaki disease, likely due to variable vaccine requirements in different countries, these findings are largely recognized as specific for Kawasaki disease and present in approximately half of affected patients who received the BCG vaccine.2
Heat Shock Proteins—Heat shock proteins (HSPs) are produced by cells in response to stressors. The proposed mechanism of BCG vaccination scar reactivation is a cross-reaction between human homologue HSP 63 and Mycobacterium HSP 65, leading to hyperactivity of the immune system against BCG.7 There also are reports of reactivation of a BCG vaccination scar from measles infection and influenza vaccination.2,8,9 Most prior reports of BCG vaccination scar reactivation have been in pediatric patients; our patient is an adult who received the BCG vaccine more than 40 years ago.
Mechanism of Reactivation—The mechanism of BCG vaccination scar reactivation in our patient, who received the Moderna COVID-19 Vaccine, is unclear. Possible mechanisms include (1) release of HSP mediated by the COVID-19 vaccine, leading to an immune response at the BCG vaccine scar, or (2) another immune-mediated cross-reaction between BCG and the Moderna COVID-19 Vaccine mRNA nanoparticle or encoded spike protein antigen. It has been hypothesized that the BCG vaccine might offer some protection against COVID-19; this remains uncertain and is under further investigation.10 A recent retrospective cohort study showed that a BCG vaccination booster may decrease COVID-19 infection rates in higher-risk populations.11
Conclusion
We present a case of BCG vaccine scar reactivation occurring after a dose of the Moderna COVID-19 Vaccine, a likely underreported, self-limiting, cutaneous adverse effect of this mRNA vaccine.
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403-416. doi:10.1056/NEJMoa2035389
- Muthuvelu S, Lim KS, Huang L-Y, et al. Measles infection causing bacillus Calmette-Guérin reactivation: a case report. BMC Pediatr. 2019;19:251. doi:10.1186/s12887-019-1635-z
- Fatima S, Kumari A, Das G, et al. Tuberculosis vaccine: a journey from BCG to present. Life Sci. 2020;252:117594. doi:10.1016/j.lfs.2020.117594
- O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335-337. doi:10.1038/s41577-020-0337-y
- Brooks NA, Puri A, Garg S, et al. The association of coronavirus disease-19 mortality and prior bacille Calmette-Guérin vaccination: a robust ecological analysis using unsupervised machine learning. Sci Rep. 2021;11:774. doi:10.1038/s41598-020-80787-z
- Tomita Y, Sato R, Ikeda T, et al. BCG vaccine may generate cross-reactive T-cells against SARS-CoV-2: in silico analyses and a hypothesis. Vaccine. 2020;38:6352-6356. doi:10.1016/j.vaccine.2020.08.045
- Lim KYY, Chua MC, Tan NWH, et al. Reactivation of BCG inoculation site in a child with febrile exanthema of 3 days duration: an early indicator of incomplete Kawasaki disease. BMJ Case Rep. 2020;13:E239648. doi:10.1136/bcr-2020-239648
- Kondo M, Goto H, Yamamoto S. First case of redness and erosion at bacillus Calmette-Guérin inoculation site after vaccination against influenza. J Dermatol. 2016;43:1229-1231. doi:10.1111/1346-8138.13365
- Chavarri-Guerra Y, Soto-Pérez-de-Celis E. Erythema at the bacillus Calmette-Guerin scar after influenza vaccination. Rev Soc Bras Med Trop. 2019;53:E20190390. doi:10.1590/0037-8682-0390-2019
- Fu W, Ho P-C, Liu C-L, et al. Reconcile the debate over protective effects of BCG vaccine against COVID-19. Sci Rep. 2021;11:8356. doi:10.1038/s41598-021-87731-9
- Amirlak L, Haddad R, Hardy JD, et al. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum Vaccin Immunother. 2021;17:3913-3915. doi:10.1080/21645515.2021.1956228
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403-416. doi:10.1056/NEJMoa2035389
- Muthuvelu S, Lim KS, Huang L-Y, et al. Measles infection causing bacillus Calmette-Guérin reactivation: a case report. BMC Pediatr. 2019;19:251. doi:10.1186/s12887-019-1635-z
- Fatima S, Kumari A, Das G, et al. Tuberculosis vaccine: a journey from BCG to present. Life Sci. 2020;252:117594. doi:10.1016/j.lfs.2020.117594
- O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335-337. doi:10.1038/s41577-020-0337-y
- Brooks NA, Puri A, Garg S, et al. The association of coronavirus disease-19 mortality and prior bacille Calmette-Guérin vaccination: a robust ecological analysis using unsupervised machine learning. Sci Rep. 2021;11:774. doi:10.1038/s41598-020-80787-z
- Tomita Y, Sato R, Ikeda T, et al. BCG vaccine may generate cross-reactive T-cells against SARS-CoV-2: in silico analyses and a hypothesis. Vaccine. 2020;38:6352-6356. doi:10.1016/j.vaccine.2020.08.045
- Lim KYY, Chua MC, Tan NWH, et al. Reactivation of BCG inoculation site in a child with febrile exanthema of 3 days duration: an early indicator of incomplete Kawasaki disease. BMJ Case Rep. 2020;13:E239648. doi:10.1136/bcr-2020-239648
- Kondo M, Goto H, Yamamoto S. First case of redness and erosion at bacillus Calmette-Guérin inoculation site after vaccination against influenza. J Dermatol. 2016;43:1229-1231. doi:10.1111/1346-8138.13365
- Chavarri-Guerra Y, Soto-Pérez-de-Celis E. Erythema at the bacillus Calmette-Guerin scar after influenza vaccination. Rev Soc Bras Med Trop. 2019;53:E20190390. doi:10.1590/0037-8682-0390-2019
- Fu W, Ho P-C, Liu C-L, et al. Reconcile the debate over protective effects of BCG vaccine against COVID-19. Sci Rep. 2021;11:8356. doi:10.1038/s41598-021-87731-9
- Amirlak L, Haddad R, Hardy JD, et al. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum Vaccin Immunother. 2021;17:3913-3915. doi:10.1080/21645515.2021.1956228
Practice Points
- BCG vaccination scar reactivation is a potential benign, self-limited reaction in patients who receive the Moderna COVID-19 Vaccine.
- Symptoms of BCG vaccination scar reactivation, which is seen more commonly in children with Kawasaki disease, include redness, swelling, and ulceration.