User login
Mr. D, age 41, presents to the emergency department (ED) with altered mental status and suspected intoxication. His medical history includes alcohol use disorder and spinal injury. Upon initial examination, he is confused, disorganized, and agitated. He receives IM lorazepam 4 mg to manage his agitation. His laboratory workup includes a negative screening for blood alcohol, slightly elevated creatine kinase, and urine toxicology positive for barbiturates and opioids. During re-evaluation by the consulting psychiatrist the following morning, Mr. D is alert, oriented, and calm with an organized thought process. He does not appear to be in withdrawal from any substances and tells the psychiatrist that he takes butalbital/acetaminophen/caffeine/codeine as needed for migraines. Mr. D says that 3 days before he came to the ED, he also began taking a supplement called phenibut that he purchased online for “well-being and sleep.”
Natural substances have been used throughout history as medicinal agents, sacred substances in religious rituals, and for recreational purposes.1 Supplement use in the United States is prevalent, with 57.6% of adults age ≥20 reporting supplement use in the past 30 days.2 Between 2000 and 2017, US poison control centers recorded a 74.1% increase in calls involving exposure to natural psychoactive substances, mostly driven by cases involving marijuana in adults and adolescents.3 Like synthetic drugs, herbal supplements may have psychoactive properties, including sedative, stimulant, psychedelic, euphoric, or anticholinergic effects. The variety and unregulated nature of supplements makes managing patients who use supplements particularly challenging.
Why patients use supplements
People may use supplements to treat or prevent vitamin deficiencies (eg, vitamin D, iron, calcium). Other reasons may include for promoting wellness in various disease states, for weight loss, for recreational use or misuse, or for overall well-being. In the mental health realm, patients report using supplements to treat depression, anxiety, insomnia, memory, or for vague indications such as “mood support.”4,5
Patients may view supplements as appealing alternatives to prescription medications because they are widely accessible, may be purchased over-the-counter, are inexpensive, and represent a “natural” treatment option.6 For these reasons, they may also falsely perceive supplements as categorically safe.1 People with psychiatric diagnoses may choose such alternative treatments due to a history of adverse effects or treatment failure with traditional psychiatric medications, mistrust of the health care or pharmaceutical industry, or based on the recommendations of others.7
Regulation, safety, and efficacy of dietary supplements
In the US, dietary supplements are regulated more like food products than medications. Under the Dietary Supplement Health and Education Act of 1994, the FDA regulates the quality, safety, and labeling of supplements using Current Good Manufacturing Practice regulations.8 The Federal Trade Commission monitors advertisements and marketing. Despite some regulations, dietary supplements may be adulterated or contaminated, contain unknown or toxic ingredients, have inconsistent potencies, or be sold at toxic doses.9 Importantly, supplements are not required to be evaluated for clinical efficacy. As a result, it is not known if most supplements are effective in treating the conditions for which they are promoted, mainly due to a lack of financial incentive for manufacturers to conduct large, high-quality trials.5
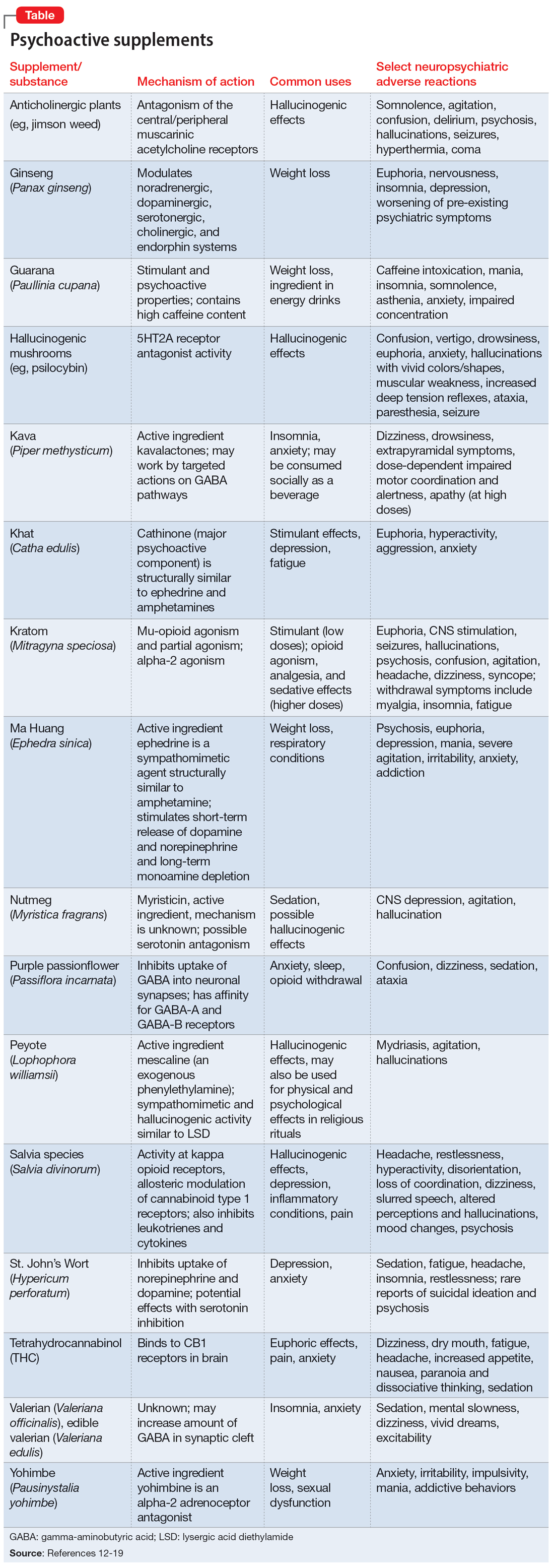
Further complicating matters is the inconsistent labeling of supplements or similar products that are easily obtainable via the internet. These products might be marketed as nutritional supplements or nootropics, which often are referred to as “cognitive enhancers” or “smart drugs.” New psychoactive substances (NPS) are drugs of misuse or abuse developed to imitate illicit drugs or controlled drug substances.10 They are sometimes referred to as “herbal highs” or “legal highs.”11 Supplements may also be labeled as performance- or image-enhancing agents and may include medications marketed to promote weight loss. This includes herbal substances (Table12-19) and medications associated with neuropsychiatric adverse effects that may be easily accessible online without a prescription.12,20
The growing popularity of the internet and social media plays an important role in the availability of supplements and nonregulated substances and may contribute to misleading claims of efficacy and safety. While many herbal supplements are available in pharmacies or supplement stores, NPS are usually sold through anonymous, low-risk means either via traditional online vendors or the deep web (parts of the internet that are not indexed via search engines). Strategies to circumvent regulation and legislative control include labeling NPS as research chemicals, fertilizers, incense, bath salts, or other identifiers and marketing them as “not for human consumption.”21 Manufacturers frequently change the chemical structures of NPS, which allows these products to exist within a legal gray area due to the lag time between when a new compound hits the market and when it is categorized as a regulated substance.10
Continue to: Another category of "supplements"...
Another category of “supplements” includes medications that are not FDA-approved but are approved for therapeutic use in other countries and readily available in the US via online sources. Such medications include phenibut, a glutamic acid derivative that functions as a gamma-aminobutyric acid-B receptor agonist in the brain, spinal cord, and autonomic nervous system. Phenibut was developed in the Soviet Union in the 1960s, and outside of the US it is prescribed for anxiolysis and other psychiatric indications.22 In the US, phenibut may be used as a nootropic or as a dietary supplement to treat anxiety, sleep problems, and other psychiatric disorders.22 It may also be used recreationally to induce euphoria. Chronic phenibut use results in tolerance and abrupt discontinuation may mimic benzodiazepine withdrawal symptoms.13,22
Educating patients about supplements
One of the most critical steps in assessing a patient’s supplement use is to directly ask them about their use of herbal or over-the-counter products. Research has consistently shown that patients are unlikely to disclose supplement use unless they are specifically asked.23,24
Additional strategies include25,26:
- Approach patients without judgment; ask open-ended questions to determine their motivations for using supplements.
- Explain the difference between supplements medically necessary to treat vitamin deficiencies (eg, vitamin D, calcium, magnesium) and those without robust clinical evidence.
- Counsel patients that many supplements with psychoactive properties, if indicated, are generally meant to be used short-term and not as substitutes for prescription medications.
- Educate patients that supplements have limited evidence regarding their safety and efficacy, but like prescription medications, supplements may cause organ damage, adverse effects, and drug-drug interactions.
- Remind patients that commonly used nutritional supplements/dietary aids, including protein or workout supplements, may contain potentially harmful ingredients.
- Utilize evidence-based resources such as the Natural Medicines Comprehensive Database14 or the National Center for Complementary and Integrative Health (https://www.nccih.nih.gov) to review levels of evidence and educate patients.
- When toxicity or withdrawal is suspected, reach out to local poison control centers for guidance.
- For a patient with a potential supplement-related substance use disorder, urine drug screens may be of limited utility and evidence is often sparse; clinicians may need to rely on primary literature such as case reports to guide management.
- If patients wish to continue taking a supplement, recommend they purchase supplements from manufacturers that have achieved the US Pharmacopeia (USP) verification mark. Products with the USP mark undergo quality assurance measures to ensure the product contains the ingredients listed on the label in the declared potency and amounts, does not contain harmful levels of contaminants, will be metabolized in the body within a specified amount of time, and has been produced in keeping with FDA Current Good Manufacturing Practice regulations.
CASE CONTINUED
In the ED, the consulting psychiatry team discusses Mr. D’s use of phenibut with him, and asks if he uses any additional supplements or nonprescription medications. Mr. D discloses he has been anxious and having trouble sleeping, and a friend recommended phenibut as a safe, natural alternative to medication. The team explains to Mr. D that phenibut’s efficacy has not been studied in the US and that based on available evidence, it is likely unsafe. It may have serious adverse effects, drug-drug interactions, and is potentially addictive.
Mr. D says he was unaware of these risks and agrees to stop taking phenibut. The treatment team discharges him from the ED with a referral for outpatient psychiatric services to address his anxiety and insomnia.
Related Resources
- Tillman B. The hidden dangers of supplements: a case of substance-induced psychosis. Current Psychiatry. 2020; 19(7):e7-e8. doi:10.12788/cp.0018
- McQueen CE. Herb–drug interactions: caution patients when changing supplements. Current Psychiatry. 2017; 16(6):38-41.
Drug Brand Names
Butalbital/acetaminophen/caffeine/codeine • Fioricet with Codeine
1. Graziano S, Orsolini L, Rotolo MC, et al. Herbal highs: review on psychoactive effects and neuropharmacology. Curr Neuropharmacol. 2017;15(5):750-761.
2. Mishra S, Stierman B, Gahche JJ, et al. Dietary supplement use among adults: United States, 2017-2018. NCHS Data Brief. 2021;(399):1-8.
3. O’Neill-Dee C, Spiller HA, Casavant MJ, et al. Natural psychoactive substance-related exposures reported to United States poison control centers, 2000-2017. Clin Toxicol (Phila). 2020;58(8):813-820.
4. Gray DC, Rutledge CM. Herbal supplements in primary care: patient perceptions, motivations, and effects on use. Holist Nurs Pract. 2013;27(1):6-12.
5. Wu K, Messamore E. Reimagining roles of dietary supplements in psychiatric care. AMA J Ethics. 2022;24(5):E437-E442.
6. Snyder FJ, Dundas ML, Kirkpatrick C, et al. Use and safety perceptions regarding herbal supplements: a study of older persons in southeast Idaho. J Nutr Elder. 2009;28(1):81-95.
7. Schulz P, Hede V. Alternative and complementary approaches in psychiatry: beliefs versus evidence. Dialogues Clin Neurosci. 2018;20(3):207-214.
8. Dietary Supplement Health and Education Act of 1994, Pub L 103-417, 103rd Cong (1993-1994).
9. Starr RR. Too little, too late: ineffective regulation of dietary supplements in the United States. Am J Public Health. 2015;105(3):478-485.
10. New psychoactive substances. Alcohol and Drug Foundation. November 10, 2021. Updated November 28, 2022. Accessed January 25, 2023. https://adf.org.au/drug-facts/new-psychoactive-substances/
11. Shafi A, Berry AJ, Sumnall H, et al. New psychoactive substances: a review and updates. Ther Adv Psychopharmacol. 2020;10:2045125320967197.
12. Bersani FS, Coviello M, Imperatori C, et al. Adverse psychiatric effects associated with herbal weight-loss products. Biomed Res Int. 2015;2015:120679.
13. IBM Micromedex POISINDEX® System. IBM Watson Health. Accessed October 3, 2022. https://www.micromedexsolutions.com
14. Natural Medicines Comprehensive Database. Therapeutic Research Center. Accessed October 3, 2022. https://naturalmedicines.therapeuticresearch.com
15. Savage KM, Stough CK, Byrne GJ, et al. Kava for the treatment of generalised anxiety disorder (K-GAD): study protocol for a randomised controlled trial. Trials. 2015;16:493.
16. Swogger MT, Smith KE, Garcia-Romeu A, et al. Understanding kratom use: a guide for healthcare providers. Front Pharmacol. 2022;13:801855.
17. Modabbernia A, Akhondzadeh S. Saffron, passionflower, valerian and sage for mental health. Psychiatr Clin North Am. 2013;36(1):85-91.
18. Coffeen U, Pellicer F. Salvia divinorum: from recreational hallucinogenic use to analgesic and anti-inflammatory action. J Pain Res. 2019;12:1069-1076.
19. National Institutes of Health, Office of Dietary Supplements. Valerian Fact Sheet for Health Professionals. Updated March 15, 2013. Accessed January 25, 2023. https://ods.od.nih.gov/factsheets/Valerian-HealthProfessional
20. An H, Sohn H, Chung S. Phentermine, sibutramine and affective disorders. Clin Psychopharmacol Neurosci. 2013;11(1):7-12.
21. Miliano C, Margiani G, Fattore L, et al. Sales and advertising channels of new psychoactive substances (NPS): internet, social networks, and smartphone apps. Brain Sci. 2018;8(7):123.
22. Hardman MI, Sprung J, Weingarten TN. Acute phenibut withdrawal: a comprehensive literature review and illustrative case report. Bosn J Basic Med Sci. 2019;19(2):125-129.
23. Guzman JR, Paterniti DA, Liu Y, et al. Factors related to disclosure and nondisclosure of dietary supplements in primary care, integrative medicine, and naturopathic medicine. J Fam Med Dis Prev. 2019;5(4):10.23937/2469-5793/1510109.
24. Foley H, Steel A, Cramer H, et al. Disclosure of complementary medicine use to medical providers: a systematic review and meta-analysis. Sci Rep. 2019;9(1):1573.
25. Aldridge Young C. ‘No miracle cures’: counseling patients about dietary supplements. Pharmacy Today. 2014;February:35.
26. United States Pharmacopeia. USP Verified Mark. Accessed January 25, 2023. https://www.usp.org/verification-services/verified-mark
Mr. D, age 41, presents to the emergency department (ED) with altered mental status and suspected intoxication. His medical history includes alcohol use disorder and spinal injury. Upon initial examination, he is confused, disorganized, and agitated. He receives IM lorazepam 4 mg to manage his agitation. His laboratory workup includes a negative screening for blood alcohol, slightly elevated creatine kinase, and urine toxicology positive for barbiturates and opioids. During re-evaluation by the consulting psychiatrist the following morning, Mr. D is alert, oriented, and calm with an organized thought process. He does not appear to be in withdrawal from any substances and tells the psychiatrist that he takes butalbital/acetaminophen/caffeine/codeine as needed for migraines. Mr. D says that 3 days before he came to the ED, he also began taking a supplement called phenibut that he purchased online for “well-being and sleep.”
Natural substances have been used throughout history as medicinal agents, sacred substances in religious rituals, and for recreational purposes.1 Supplement use in the United States is prevalent, with 57.6% of adults age ≥20 reporting supplement use in the past 30 days.2 Between 2000 and 2017, US poison control centers recorded a 74.1% increase in calls involving exposure to natural psychoactive substances, mostly driven by cases involving marijuana in adults and adolescents.3 Like synthetic drugs, herbal supplements may have psychoactive properties, including sedative, stimulant, psychedelic, euphoric, or anticholinergic effects. The variety and unregulated nature of supplements makes managing patients who use supplements particularly challenging.
Why patients use supplements
People may use supplements to treat or prevent vitamin deficiencies (eg, vitamin D, iron, calcium). Other reasons may include for promoting wellness in various disease states, for weight loss, for recreational use or misuse, or for overall well-being. In the mental health realm, patients report using supplements to treat depression, anxiety, insomnia, memory, or for vague indications such as “mood support.”4,5
Patients may view supplements as appealing alternatives to prescription medications because they are widely accessible, may be purchased over-the-counter, are inexpensive, and represent a “natural” treatment option.6 For these reasons, they may also falsely perceive supplements as categorically safe.1 People with psychiatric diagnoses may choose such alternative treatments due to a history of adverse effects or treatment failure with traditional psychiatric medications, mistrust of the health care or pharmaceutical industry, or based on the recommendations of others.7
Regulation, safety, and efficacy of dietary supplements
In the US, dietary supplements are regulated more like food products than medications. Under the Dietary Supplement Health and Education Act of 1994, the FDA regulates the quality, safety, and labeling of supplements using Current Good Manufacturing Practice regulations.8 The Federal Trade Commission monitors advertisements and marketing. Despite some regulations, dietary supplements may be adulterated or contaminated, contain unknown or toxic ingredients, have inconsistent potencies, or be sold at toxic doses.9 Importantly, supplements are not required to be evaluated for clinical efficacy. As a result, it is not known if most supplements are effective in treating the conditions for which they are promoted, mainly due to a lack of financial incentive for manufacturers to conduct large, high-quality trials.5
Further complicating matters is the inconsistent labeling of supplements or similar products that are easily obtainable via the internet. These products might be marketed as nutritional supplements or nootropics, which often are referred to as “cognitive enhancers” or “smart drugs.” New psychoactive substances (NPS) are drugs of misuse or abuse developed to imitate illicit drugs or controlled drug substances.10 They are sometimes referred to as “herbal highs” or “legal highs.”11 Supplements may also be labeled as performance- or image-enhancing agents and may include medications marketed to promote weight loss. This includes herbal substances (Table12-19) and medications associated with neuropsychiatric adverse effects that may be easily accessible online without a prescription.12,20

The growing popularity of the internet and social media plays an important role in the availability of supplements and nonregulated substances and may contribute to misleading claims of efficacy and safety. While many herbal supplements are available in pharmacies or supplement stores, NPS are usually sold through anonymous, low-risk means either via traditional online vendors or the deep web (parts of the internet that are not indexed via search engines). Strategies to circumvent regulation and legislative control include labeling NPS as research chemicals, fertilizers, incense, bath salts, or other identifiers and marketing them as “not for human consumption.”21 Manufacturers frequently change the chemical structures of NPS, which allows these products to exist within a legal gray area due to the lag time between when a new compound hits the market and when it is categorized as a regulated substance.10
Continue to: Another category of "supplements"...
Another category of “supplements” includes medications that are not FDA-approved but are approved for therapeutic use in other countries and readily available in the US via online sources. Such medications include phenibut, a glutamic acid derivative that functions as a gamma-aminobutyric acid-B receptor agonist in the brain, spinal cord, and autonomic nervous system. Phenibut was developed in the Soviet Union in the 1960s, and outside of the US it is prescribed for anxiolysis and other psychiatric indications.22 In the US, phenibut may be used as a nootropic or as a dietary supplement to treat anxiety, sleep problems, and other psychiatric disorders.22 It may also be used recreationally to induce euphoria. Chronic phenibut use results in tolerance and abrupt discontinuation may mimic benzodiazepine withdrawal symptoms.13,22
Educating patients about supplements
One of the most critical steps in assessing a patient’s supplement use is to directly ask them about their use of herbal or over-the-counter products. Research has consistently shown that patients are unlikely to disclose supplement use unless they are specifically asked.23,24
Additional strategies include25,26:
- Approach patients without judgment; ask open-ended questions to determine their motivations for using supplements.
- Explain the difference between supplements medically necessary to treat vitamin deficiencies (eg, vitamin D, calcium, magnesium) and those without robust clinical evidence.
- Counsel patients that many supplements with psychoactive properties, if indicated, are generally meant to be used short-term and not as substitutes for prescription medications.
- Educate patients that supplements have limited evidence regarding their safety and efficacy, but like prescription medications, supplements may cause organ damage, adverse effects, and drug-drug interactions.
- Remind patients that commonly used nutritional supplements/dietary aids, including protein or workout supplements, may contain potentially harmful ingredients.
- Utilize evidence-based resources such as the Natural Medicines Comprehensive Database14 or the National Center for Complementary and Integrative Health (https://www.nccih.nih.gov) to review levels of evidence and educate patients.
- When toxicity or withdrawal is suspected, reach out to local poison control centers for guidance.
- For a patient with a potential supplement-related substance use disorder, urine drug screens may be of limited utility and evidence is often sparse; clinicians may need to rely on primary literature such as case reports to guide management.
- If patients wish to continue taking a supplement, recommend they purchase supplements from manufacturers that have achieved the US Pharmacopeia (USP) verification mark. Products with the USP mark undergo quality assurance measures to ensure the product contains the ingredients listed on the label in the declared potency and amounts, does not contain harmful levels of contaminants, will be metabolized in the body within a specified amount of time, and has been produced in keeping with FDA Current Good Manufacturing Practice regulations.
CASE CONTINUED
In the ED, the consulting psychiatry team discusses Mr. D’s use of phenibut with him, and asks if he uses any additional supplements or nonprescription medications. Mr. D discloses he has been anxious and having trouble sleeping, and a friend recommended phenibut as a safe, natural alternative to medication. The team explains to Mr. D that phenibut’s efficacy has not been studied in the US and that based on available evidence, it is likely unsafe. It may have serious adverse effects, drug-drug interactions, and is potentially addictive.
Mr. D says he was unaware of these risks and agrees to stop taking phenibut. The treatment team discharges him from the ED with a referral for outpatient psychiatric services to address his anxiety and insomnia.
Related Resources
- Tillman B. The hidden dangers of supplements: a case of substance-induced psychosis. Current Psychiatry. 2020; 19(7):e7-e8. doi:10.12788/cp.0018
- McQueen CE. Herb–drug interactions: caution patients when changing supplements. Current Psychiatry. 2017; 16(6):38-41.
Drug Brand Names
Butalbital/acetaminophen/caffeine/codeine • Fioricet with Codeine
Mr. D, age 41, presents to the emergency department (ED) with altered mental status and suspected intoxication. His medical history includes alcohol use disorder and spinal injury. Upon initial examination, he is confused, disorganized, and agitated. He receives IM lorazepam 4 mg to manage his agitation. His laboratory workup includes a negative screening for blood alcohol, slightly elevated creatine kinase, and urine toxicology positive for barbiturates and opioids. During re-evaluation by the consulting psychiatrist the following morning, Mr. D is alert, oriented, and calm with an organized thought process. He does not appear to be in withdrawal from any substances and tells the psychiatrist that he takes butalbital/acetaminophen/caffeine/codeine as needed for migraines. Mr. D says that 3 days before he came to the ED, he also began taking a supplement called phenibut that he purchased online for “well-being and sleep.”
Natural substances have been used throughout history as medicinal agents, sacred substances in religious rituals, and for recreational purposes.1 Supplement use in the United States is prevalent, with 57.6% of adults age ≥20 reporting supplement use in the past 30 days.2 Between 2000 and 2017, US poison control centers recorded a 74.1% increase in calls involving exposure to natural psychoactive substances, mostly driven by cases involving marijuana in adults and adolescents.3 Like synthetic drugs, herbal supplements may have psychoactive properties, including sedative, stimulant, psychedelic, euphoric, or anticholinergic effects. The variety and unregulated nature of supplements makes managing patients who use supplements particularly challenging.
Why patients use supplements
People may use supplements to treat or prevent vitamin deficiencies (eg, vitamin D, iron, calcium). Other reasons may include for promoting wellness in various disease states, for weight loss, for recreational use or misuse, or for overall well-being. In the mental health realm, patients report using supplements to treat depression, anxiety, insomnia, memory, or for vague indications such as “mood support.”4,5
Patients may view supplements as appealing alternatives to prescription medications because they are widely accessible, may be purchased over-the-counter, are inexpensive, and represent a “natural” treatment option.6 For these reasons, they may also falsely perceive supplements as categorically safe.1 People with psychiatric diagnoses may choose such alternative treatments due to a history of adverse effects or treatment failure with traditional psychiatric medications, mistrust of the health care or pharmaceutical industry, or based on the recommendations of others.7
Regulation, safety, and efficacy of dietary supplements
In the US, dietary supplements are regulated more like food products than medications. Under the Dietary Supplement Health and Education Act of 1994, the FDA regulates the quality, safety, and labeling of supplements using Current Good Manufacturing Practice regulations.8 The Federal Trade Commission monitors advertisements and marketing. Despite some regulations, dietary supplements may be adulterated or contaminated, contain unknown or toxic ingredients, have inconsistent potencies, or be sold at toxic doses.9 Importantly, supplements are not required to be evaluated for clinical efficacy. As a result, it is not known if most supplements are effective in treating the conditions for which they are promoted, mainly due to a lack of financial incentive for manufacturers to conduct large, high-quality trials.5
Further complicating matters is the inconsistent labeling of supplements or similar products that are easily obtainable via the internet. These products might be marketed as nutritional supplements or nootropics, which often are referred to as “cognitive enhancers” or “smart drugs.” New psychoactive substances (NPS) are drugs of misuse or abuse developed to imitate illicit drugs or controlled drug substances.10 They are sometimes referred to as “herbal highs” or “legal highs.”11 Supplements may also be labeled as performance- or image-enhancing agents and may include medications marketed to promote weight loss. This includes herbal substances (Table12-19) and medications associated with neuropsychiatric adverse effects that may be easily accessible online without a prescription.12,20

The growing popularity of the internet and social media plays an important role in the availability of supplements and nonregulated substances and may contribute to misleading claims of efficacy and safety. While many herbal supplements are available in pharmacies or supplement stores, NPS are usually sold through anonymous, low-risk means either via traditional online vendors or the deep web (parts of the internet that are not indexed via search engines). Strategies to circumvent regulation and legislative control include labeling NPS as research chemicals, fertilizers, incense, bath salts, or other identifiers and marketing them as “not for human consumption.”21 Manufacturers frequently change the chemical structures of NPS, which allows these products to exist within a legal gray area due to the lag time between when a new compound hits the market and when it is categorized as a regulated substance.10
Continue to: Another category of "supplements"...
Another category of “supplements” includes medications that are not FDA-approved but are approved for therapeutic use in other countries and readily available in the US via online sources. Such medications include phenibut, a glutamic acid derivative that functions as a gamma-aminobutyric acid-B receptor agonist in the brain, spinal cord, and autonomic nervous system. Phenibut was developed in the Soviet Union in the 1960s, and outside of the US it is prescribed for anxiolysis and other psychiatric indications.22 In the US, phenibut may be used as a nootropic or as a dietary supplement to treat anxiety, sleep problems, and other psychiatric disorders.22 It may also be used recreationally to induce euphoria. Chronic phenibut use results in tolerance and abrupt discontinuation may mimic benzodiazepine withdrawal symptoms.13,22
Educating patients about supplements
One of the most critical steps in assessing a patient’s supplement use is to directly ask them about their use of herbal or over-the-counter products. Research has consistently shown that patients are unlikely to disclose supplement use unless they are specifically asked.23,24
Additional strategies include25,26:
- Approach patients without judgment; ask open-ended questions to determine their motivations for using supplements.
- Explain the difference between supplements medically necessary to treat vitamin deficiencies (eg, vitamin D, calcium, magnesium) and those without robust clinical evidence.
- Counsel patients that many supplements with psychoactive properties, if indicated, are generally meant to be used short-term and not as substitutes for prescription medications.
- Educate patients that supplements have limited evidence regarding their safety and efficacy, but like prescription medications, supplements may cause organ damage, adverse effects, and drug-drug interactions.
- Remind patients that commonly used nutritional supplements/dietary aids, including protein or workout supplements, may contain potentially harmful ingredients.
- Utilize evidence-based resources such as the Natural Medicines Comprehensive Database14 or the National Center for Complementary and Integrative Health (https://www.nccih.nih.gov) to review levels of evidence and educate patients.
- When toxicity or withdrawal is suspected, reach out to local poison control centers for guidance.
- For a patient with a potential supplement-related substance use disorder, urine drug screens may be of limited utility and evidence is often sparse; clinicians may need to rely on primary literature such as case reports to guide management.
- If patients wish to continue taking a supplement, recommend they purchase supplements from manufacturers that have achieved the US Pharmacopeia (USP) verification mark. Products with the USP mark undergo quality assurance measures to ensure the product contains the ingredients listed on the label in the declared potency and amounts, does not contain harmful levels of contaminants, will be metabolized in the body within a specified amount of time, and has been produced in keeping with FDA Current Good Manufacturing Practice regulations.
CASE CONTINUED
In the ED, the consulting psychiatry team discusses Mr. D’s use of phenibut with him, and asks if he uses any additional supplements or nonprescription medications. Mr. D discloses he has been anxious and having trouble sleeping, and a friend recommended phenibut as a safe, natural alternative to medication. The team explains to Mr. D that phenibut’s efficacy has not been studied in the US and that based on available evidence, it is likely unsafe. It may have serious adverse effects, drug-drug interactions, and is potentially addictive.
Mr. D says he was unaware of these risks and agrees to stop taking phenibut. The treatment team discharges him from the ED with a referral for outpatient psychiatric services to address his anxiety and insomnia.
Related Resources
- Tillman B. The hidden dangers of supplements: a case of substance-induced psychosis. Current Psychiatry. 2020; 19(7):e7-e8. doi:10.12788/cp.0018
- McQueen CE. Herb–drug interactions: caution patients when changing supplements. Current Psychiatry. 2017; 16(6):38-41.
Drug Brand Names
Butalbital/acetaminophen/caffeine/codeine • Fioricet with Codeine
1. Graziano S, Orsolini L, Rotolo MC, et al. Herbal highs: review on psychoactive effects and neuropharmacology. Curr Neuropharmacol. 2017;15(5):750-761.
2. Mishra S, Stierman B, Gahche JJ, et al. Dietary supplement use among adults: United States, 2017-2018. NCHS Data Brief. 2021;(399):1-8.
3. O’Neill-Dee C, Spiller HA, Casavant MJ, et al. Natural psychoactive substance-related exposures reported to United States poison control centers, 2000-2017. Clin Toxicol (Phila). 2020;58(8):813-820.
4. Gray DC, Rutledge CM. Herbal supplements in primary care: patient perceptions, motivations, and effects on use. Holist Nurs Pract. 2013;27(1):6-12.
5. Wu K, Messamore E. Reimagining roles of dietary supplements in psychiatric care. AMA J Ethics. 2022;24(5):E437-E442.
6. Snyder FJ, Dundas ML, Kirkpatrick C, et al. Use and safety perceptions regarding herbal supplements: a study of older persons in southeast Idaho. J Nutr Elder. 2009;28(1):81-95.
7. Schulz P, Hede V. Alternative and complementary approaches in psychiatry: beliefs versus evidence. Dialogues Clin Neurosci. 2018;20(3):207-214.
8. Dietary Supplement Health and Education Act of 1994, Pub L 103-417, 103rd Cong (1993-1994).
9. Starr RR. Too little, too late: ineffective regulation of dietary supplements in the United States. Am J Public Health. 2015;105(3):478-485.
10. New psychoactive substances. Alcohol and Drug Foundation. November 10, 2021. Updated November 28, 2022. Accessed January 25, 2023. https://adf.org.au/drug-facts/new-psychoactive-substances/
11. Shafi A, Berry AJ, Sumnall H, et al. New psychoactive substances: a review and updates. Ther Adv Psychopharmacol. 2020;10:2045125320967197.
12. Bersani FS, Coviello M, Imperatori C, et al. Adverse psychiatric effects associated with herbal weight-loss products. Biomed Res Int. 2015;2015:120679.
13. IBM Micromedex POISINDEX® System. IBM Watson Health. Accessed October 3, 2022. https://www.micromedexsolutions.com
14. Natural Medicines Comprehensive Database. Therapeutic Research Center. Accessed October 3, 2022. https://naturalmedicines.therapeuticresearch.com
15. Savage KM, Stough CK, Byrne GJ, et al. Kava for the treatment of generalised anxiety disorder (K-GAD): study protocol for a randomised controlled trial. Trials. 2015;16:493.
16. Swogger MT, Smith KE, Garcia-Romeu A, et al. Understanding kratom use: a guide for healthcare providers. Front Pharmacol. 2022;13:801855.
17. Modabbernia A, Akhondzadeh S. Saffron, passionflower, valerian and sage for mental health. Psychiatr Clin North Am. 2013;36(1):85-91.
18. Coffeen U, Pellicer F. Salvia divinorum: from recreational hallucinogenic use to analgesic and anti-inflammatory action. J Pain Res. 2019;12:1069-1076.
19. National Institutes of Health, Office of Dietary Supplements. Valerian Fact Sheet for Health Professionals. Updated March 15, 2013. Accessed January 25, 2023. https://ods.od.nih.gov/factsheets/Valerian-HealthProfessional
20. An H, Sohn H, Chung S. Phentermine, sibutramine and affective disorders. Clin Psychopharmacol Neurosci. 2013;11(1):7-12.
21. Miliano C, Margiani G, Fattore L, et al. Sales and advertising channels of new psychoactive substances (NPS): internet, social networks, and smartphone apps. Brain Sci. 2018;8(7):123.
22. Hardman MI, Sprung J, Weingarten TN. Acute phenibut withdrawal: a comprehensive literature review and illustrative case report. Bosn J Basic Med Sci. 2019;19(2):125-129.
23. Guzman JR, Paterniti DA, Liu Y, et al. Factors related to disclosure and nondisclosure of dietary supplements in primary care, integrative medicine, and naturopathic medicine. J Fam Med Dis Prev. 2019;5(4):10.23937/2469-5793/1510109.
24. Foley H, Steel A, Cramer H, et al. Disclosure of complementary medicine use to medical providers: a systematic review and meta-analysis. Sci Rep. 2019;9(1):1573.
25. Aldridge Young C. ‘No miracle cures’: counseling patients about dietary supplements. Pharmacy Today. 2014;February:35.
26. United States Pharmacopeia. USP Verified Mark. Accessed January 25, 2023. https://www.usp.org/verification-services/verified-mark
1. Graziano S, Orsolini L, Rotolo MC, et al. Herbal highs: review on psychoactive effects and neuropharmacology. Curr Neuropharmacol. 2017;15(5):750-761.
2. Mishra S, Stierman B, Gahche JJ, et al. Dietary supplement use among adults: United States, 2017-2018. NCHS Data Brief. 2021;(399):1-8.
3. O’Neill-Dee C, Spiller HA, Casavant MJ, et al. Natural psychoactive substance-related exposures reported to United States poison control centers, 2000-2017. Clin Toxicol (Phila). 2020;58(8):813-820.
4. Gray DC, Rutledge CM. Herbal supplements in primary care: patient perceptions, motivations, and effects on use. Holist Nurs Pract. 2013;27(1):6-12.
5. Wu K, Messamore E. Reimagining roles of dietary supplements in psychiatric care. AMA J Ethics. 2022;24(5):E437-E442.
6. Snyder FJ, Dundas ML, Kirkpatrick C, et al. Use and safety perceptions regarding herbal supplements: a study of older persons in southeast Idaho. J Nutr Elder. 2009;28(1):81-95.
7. Schulz P, Hede V. Alternative and complementary approaches in psychiatry: beliefs versus evidence. Dialogues Clin Neurosci. 2018;20(3):207-214.
8. Dietary Supplement Health and Education Act of 1994, Pub L 103-417, 103rd Cong (1993-1994).
9. Starr RR. Too little, too late: ineffective regulation of dietary supplements in the United States. Am J Public Health. 2015;105(3):478-485.
10. New psychoactive substances. Alcohol and Drug Foundation. November 10, 2021. Updated November 28, 2022. Accessed January 25, 2023. https://adf.org.au/drug-facts/new-psychoactive-substances/
11. Shafi A, Berry AJ, Sumnall H, et al. New psychoactive substances: a review and updates. Ther Adv Psychopharmacol. 2020;10:2045125320967197.
12. Bersani FS, Coviello M, Imperatori C, et al. Adverse psychiatric effects associated with herbal weight-loss products. Biomed Res Int. 2015;2015:120679.
13. IBM Micromedex POISINDEX® System. IBM Watson Health. Accessed October 3, 2022. https://www.micromedexsolutions.com
14. Natural Medicines Comprehensive Database. Therapeutic Research Center. Accessed October 3, 2022. https://naturalmedicines.therapeuticresearch.com
15. Savage KM, Stough CK, Byrne GJ, et al. Kava for the treatment of generalised anxiety disorder (K-GAD): study protocol for a randomised controlled trial. Trials. 2015;16:493.
16. Swogger MT, Smith KE, Garcia-Romeu A, et al. Understanding kratom use: a guide for healthcare providers. Front Pharmacol. 2022;13:801855.
17. Modabbernia A, Akhondzadeh S. Saffron, passionflower, valerian and sage for mental health. Psychiatr Clin North Am. 2013;36(1):85-91.
18. Coffeen U, Pellicer F. Salvia divinorum: from recreational hallucinogenic use to analgesic and anti-inflammatory action. J Pain Res. 2019;12:1069-1076.
19. National Institutes of Health, Office of Dietary Supplements. Valerian Fact Sheet for Health Professionals. Updated March 15, 2013. Accessed January 25, 2023. https://ods.od.nih.gov/factsheets/Valerian-HealthProfessional
20. An H, Sohn H, Chung S. Phentermine, sibutramine and affective disorders. Clin Psychopharmacol Neurosci. 2013;11(1):7-12.
21. Miliano C, Margiani G, Fattore L, et al. Sales and advertising channels of new psychoactive substances (NPS): internet, social networks, and smartphone apps. Brain Sci. 2018;8(7):123.
22. Hardman MI, Sprung J, Weingarten TN. Acute phenibut withdrawal: a comprehensive literature review and illustrative case report. Bosn J Basic Med Sci. 2019;19(2):125-129.
23. Guzman JR, Paterniti DA, Liu Y, et al. Factors related to disclosure and nondisclosure of dietary supplements in primary care, integrative medicine, and naturopathic medicine. J Fam Med Dis Prev. 2019;5(4):10.23937/2469-5793/1510109.
24. Foley H, Steel A, Cramer H, et al. Disclosure of complementary medicine use to medical providers: a systematic review and meta-analysis. Sci Rep. 2019;9(1):1573.
25. Aldridge Young C. ‘No miracle cures’: counseling patients about dietary supplements. Pharmacy Today. 2014;February:35.
26. United States Pharmacopeia. USP Verified Mark. Accessed January 25, 2023. https://www.usp.org/verification-services/verified-mark

