User login
Perioperative hyperglycemia, defined as blood glucose levels ≥ 180 mg/dL in the immediate pre- and postoperative period, is associated with increased postoperative morbidity, including infections, preoperative interventions, and in-hospital mortality.1-3 Despite being identified as a barrier to optimal perioperative glycemic control, limited evidence is available on patient or health care practitioner (HCP) adherence to preoperative insulin protocols.4-6
Background
Despite mounting evidence of the advantages of maintaining perioperative glucose levels between 80 and 180 mg/dL, available guidelines vary in their recommendations for long-acting basal insulin dosing.7-10 The Society of Ambulatory Anesthesia suggests using 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery in patients without a history of nocturnal or morning hypoglycemia (category 2A evidence).9 However, the revised 2016 United Kingdom National Health Service consensus guideline recommends using 80% to 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery.7 The 2022 American Diabetes Association references an observational study of patients with type 2 DM (T2DM) treated with evening-only, long-acting glargine insulin, indicating that the optimal basal insulin dose on the evening before surgery is about 75% of the outpatient dose.5,10 However, in a randomized, prospective open trial of patients with DM treated with evening-only long-acting basal insulin, no significant difference was noted in the target day of surgery (DOS) glucose levels among different dosing strategies on the evening before surgery.6 Presently, the optimal dose of long-acting insulin analogs on the evening before surgery is unknown.
Additionally, little is known about the other factors that influence perioperative glycemic control. Several barriers to optimal perioperative care of patients with DM have been identified, including lack of prioritization by HCPs, lack of knowledge about current evidence-based recommendations, and lack of patient information and involvement.4 To determine the effect of patient adherence to preoperative medication instructions on postoperative outcome, a cross-sectional study assessed surgical patients admitted to the postanesthetic care unit (PACU) and found that only 70% of patients with insulin-treated DM took their medications preoperatively. Additionally, 23% of nonadherent patients who omitted their medications either did not understand or forgot preoperative medication management instructions. Preoperative DM medication omission was associated with higher rates of hyperglycemia in the PACU (23.8% vs 3.6%; P = .02).11 Importantly, to our knowledge, the extent of HCP adherence to DM management protocols and the subsequent effect on DOS hyperglycemia has not been examined until now.For patients with DM treated with an evening dose of long-acting basal insulin (ie, either once-daily long-acting basal insulin in the evening or twice-daily long-acting basal insulin, both morning and evening) presenting for elective noncardiac surgery, our aim was to decrease the rate of DOS hyperglycemia from 29% (our baseline) to 15% by intensifying the dose of insulin on the evening before surgery without increasing the rate of hypoglycemia. We also sought to determine the rates of HCP adherence to our insulin protocols as well as patients’ self-reported adherence to HCP instructions over the course of this quality improvement (QI) initiative.
Quality Improvement Program
Our surgical department consists of 11 surgical subspecialties that performed approximately 4400 noncardiac surgeries in 2019. All patients undergoing elective surgery are evaluated in the preoperative clinic, which is staffed by an anesthesiology professional (attending and resident physicians, nurse practitioners, and physician assistants) and internal medicine attending physicians. At the preoperative visit, each patient is evaluated by anesthesiology; medically complex patients may also be referred to an internal medicine professional for further risk stratification and optimization before surgery.
At the preoperative clinic visit, HCPs prepare written patient instructions for the preoperative management of medications, including glucose-lowering medications, based on a DM management protocol that was implemented in 2016 for the preoperative management of insulin, noninsulin injectable agents, and oral hyperglycemic agents. According to this protocol, patients with DM treated with evening long-acting basal insulin (eg, glargine insulin) are instructed to take 50% of their usual evening dose the evening before surgery. A preoperative clinic nurse reviews the final preoperative medication instructions with the patient at the end of the clinic visit. Patients are also instructed to avoid oral intake other than water and necessary medications after midnight before surgery regardless of the time of surgery. On the DOS, the patient’s blood glucose level is measured on arrival to the presurgical area.
Our QI initiative focused only on the dose of self-administered, long-acting basal insulin on the evening before surgery. The effect of the morning of surgery long-acting insulin dose on the DOS glucose levels largely depends on the timing of surgery, which is variable; therefore, we did not target this dose for our initiative. Patients receiving intermediate-acting neutral protamine Hagedorn (NPH) insulin were excluded because our protocol does not recommend a dose reduction for NPH insulin on the evening before surgery.
We developed a comprehensive driver diagram to help elucidate the different factors contributing to DOS hyperglycemia and to guide specific QI interventions.12 Some of the identified contributors to DOS hyperglycemia, such as the length of preoperative fasting and timing of surgery, are unpredictable and were deemed difficult to address preoperatively. Other contributors to DOS hyperglycemia, such as outpatient DM management, often require interventions over several months, which is well beyond the time usually allotted for preoperative evaluation and optimization. On the other hand, immediate preoperative insulin dosing directly affects DOS glycemic control; therefore, improvement of the preoperative insulin management protocol to optimize the dosage on the evening before surgery was considered to be an achievable QI goal with the potential for decreasing the rate of DOS hyperglycemia in patients presenting for elective noncardiac surgery.
We used the Model for Understanding Success in Quality (MUSIQ) as a framework to identify key contextual factors that may affect the success of our QI project.13 Limited resource availability and difficulty with dissemination of protocol changes in the preoperative clinic were determined to be potential barriers to the successful implementation of our QI initiative. Nonetheless, senior leadership support, microsystem QI culture, QI team skills, and physician involvement supported the implementation. The revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were followed for this study.14
Interventions
With stakeholder input from anesthesiology, internal medicine, endocrinology, and nursing, we designed an intervention to iteratively change the HCP protocol instructions for long-acting insulin dosing on the evening before surgery. In phase 1 of the study (October 1, 2018, to March 11, 2019), we obtained baseline data on the rates of DOS hyperglycemia (blood glucose ≥ 180 mg/dL) and hypoglycemia (blood glucose < 80 mg/dL), as well as patient and HCP adherence rates to our existing preoperative DM protocol. For phase 2 (March 12, 2019, to July 22, 2019), the preoperative DM management protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with hemoglobin A1c (HbA1c) levels > 8% from 50% of the usual outpatient dose to 100%. Finally, in phase 3 (July 23, 2019, to March 12, 2020), the protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with HbA1c levels ≤ 8% from 50% of the usual outpatient dose to 75% while sustaining the phase 2 change. Preoperative HCPs were informed of the protocol changes in person and were provided with electronic and hard copies of each new protocol.
Protocol
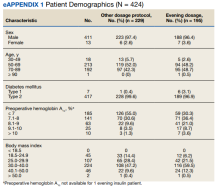
We used a prospective cohort design of 424 consecutive patients with DM who presented for preoperative evaluation for elective noncardiac surgery between October 1, 2018, and March 12, 2020. For the subset of 195 patients treated with an evening dose of long-acting basal insulin, we examined the effect of intensification of this preoperative basal insulin dose on DOS hyperglycemia and hypoglycemia, HCP adherence to iterative changes of the protocol, and patient adherence to HCP instructions on preoperative medication dosing. The QI project was concluded when elective surgeries were paused due to the COVID-19 pandemic.
We created a standardized preoperative data collection form that included information on the most recent HbA1c, time, dose, and type of patient-administered insulin on the evening before surgery, and DOS blood glucose level. A preoperative clinic nurse completed the standardized preoperative data collection form. The HCP’s preoperative medication instructions and the preoperative data collection forms were gathered for review and data analysis.
The primary outcome was DOS hyperglycemia (blood glucose levels ≥ 180 mg/dL). We monitored the rate of DOS hypoglycemia (blood glucose levels < 80 mg/dL) as a balancing measure to ensure safety with long-acting basal insulin intensification. Although hypoglycemia is defined as a blood glucose level < 70 mg/dL, a target glucose range of 80 mg/dL to 180 mg/dL is recommended during the perioperative period.8 Therefore, we chose a more conservative definition of hypoglycemia (blood glucose levels < 80 mg/dL) to adhere to the recommended perioperative glucose target range.
Process measures included HCP adherence to each protocol change, which was assessed by comparing written preoperative patient instructions to the current protocol. Similarly, patient adherence to HCP-recommended long-acting basal insulin dosing was assessed by comparing written preoperative patient instructions to the patient’s self-reported time and dose of long-acting basal insulin on the evening before surgery. For any discrepancy between the HCP instructions and protocol or HCP-recommended dose and patient self-reported dose of long-acting basal insulin, a detailed chart review was performed to determine the etiology.
Statistical Analysis
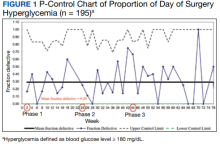
We used the statistical process p-control chart to assess the effect of iterative changes to the preoperative long-acting basal insulin protocol on DOS hyperglycemia. The proportion defective (rate of DOS hyperglycemia) was plotted against time to determine whether the observed variations in the rate of DOS hyperglycemia over time were attributable to random common causes or special causes because of our intervention. The lower control limit (LCL) and upper control limit (UCL) define the limits of expected outcome measures in a stable process prior to introducing changes and were set at 3 SDs from the mean to balance the likelihood of type I (false-positive) and type II (false-negative) errors. Because of the variable interval sample sizes, we used the CRITBINOM function of Microsoft Excel to calculate the exact UCL satisfying the 3 SD limits of 0.99865.15 The Shewhart rules (outliers, runs or shifts, trends, sawtooth) were used to analyze the p-control chart to identify special cause signals resulting from our interventions.16 We used the statistical process t-control chart to record the time (days) between the few occurrences of DOS hypoglycemia because cases of hypoglycemia were rare.
Ethical Consideration
The Human Research Protection Program, Associate Chief of Staff for Research and Development, and Quality, Safety, and Values department reviewed this project in accordance with the Veterans Health Administration Program Guide 1200.21 and determined that it was a nonresearch operations activity; thus, approval by an institutional review board was not needed. The authors declare no competing interests.
Patient Outcomes
We prospectively followed 424 consecutive patients with DM undergoing elective noncardiac surgery from the time of the preoperative clinic evaluation until DOS; 195 patients were on evening
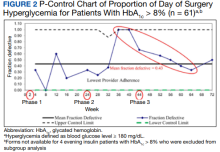
A subgroup analysis of DOS glucose levels in insulin-treated patients with preoperative HbA1c levels > 8% did not demonstrate a change in the rate of
Only 7 of 424 (1.7%) patients with DM and 4 of 195 (2.1%) patients treated with evening, long-acting basal insulin had marked hyperglycemia (DOS glucose levels ≥ 300 mg/dL). Only 1 patient who was not on outpatient insulin treatment had surgery canceled for hyperglycemia.
Overall, 89% of the HCPs followed the preoperative insulin protocol. HCP adherence to the protocol decreased to 77% after the phase 2 change, often related to deviations from the protocol or when a prior version was used. By the end of phase 3, HCP adherence returned to the baseline rate (88%). Patient adherence to medication instructions was not affected by protocol changes (86% throughout the study period). Prospective data collection was briefly interrupted between January 18, 2019, and March 5, 2019, while designing our phase 2 intervention. We were unable to track the total number of eligible patients during this time, but were able to identify 8 insulin-treated patients with DM who underwent elective noncardiac surgery and included their data in phase 1.
Discussion
The management and prevention of immediate perioperative hyperglycemia and glycemic variability have attracted attention as evidence has mounted for their association with postoperative morbidity and mortality.1,2,17 Available guidelines for preventing DOS hyperglycemia vary in their recommendations for preoperative insulin management.7-10 Notably, concerns about iatrogenic hypoglycemia often hinder efforts to lower rates of DOS hyperglycemia.4 We successfully implemented an iterative intensification protocol for preoperative long-acting basal insulin doses on the evening before surgery but did not observe a lower rate of hyperglycemia. Importantly, we also did not observe a higher rate of hypoglycemia on the DOS, as observed in a previous study.5
The observational study by Demma and colleagues found that patients receiving 75% of their evening, long-acting basal insulin dose were significantly more likely to achieve target blood glucose levels of 100 to 180 mg/dL than patients receiving no insulin at all (78% vs 0%; P = .001). However, no significant difference was noted when this group was compared with patients receiving 50% of their evening, long-acting basal insulin doses (78% vs 70%; P = .56). This is more clinically pertinent as it is generally accepted that the evening, long-acting insulin dose should not be entirely withheld on the evening before surgery.5
These findings are consistent with our observation that the rate of DOS hyperglycemia did not decrease with intensification of the evening, long-acting insulin dose from 50% to 100% of the prescribed dose in patients with HbA1c levels > 8% (phase 2) and 50% to 75% of the prescribed dose in patients with HbA1c levels ≤ 8% (phase 3). In the study by Demma and colleagues, few patients presented with preoperative hypoglycemia (2.7%) but all had received 100% of their evening, long-acting basal insulin dose, suggesting a significant increase in the rate of hypoglycemia compared with patients receiving lower doses of insulin (P = .01).5 However, long-term DM control as assessed by HbA1c level was available for < 10% of the patients, making it difficult to evaluate the effect of overall DM control on the results.5 In our study, preoperative HbA1c levels were available for 99.5% of the patients and only those with HbA1c levels > 8% received 100% of their evening, long-acting insulin dose on the evening before surgery. Notably, we did not observe a higher rate of hypoglycemia in this patient population, indicating that preoperative insulin dose intensification is safe for this subgroup.
Although HCP adherence to perioperative DM management protocols has been identified as a predominant barrier to the delivery of optimal perioperative DM care, prior studies of various preoperative insulin protocols to reduce perioperative hyperglycemia have not reported HCP adherence to their insulin protocols or its effect on DOS hyperglycemia.4-6 Additionally, patient adherence to HCP instructions is a key factor identified in our driver diagram that may influence DOS hyperglycemia, a hypothesis that is supported by a prior cross-sectional study showing an increased rate of hyperglycemia in the PACU with omission of preoperative DM medication.11 In our study, patient adherence to preoperative medication management instructions was higher than reported previously and remained consistently high regardless of protocol changes, which may explain why patient adherence did not affect the rate of DOS hyperglycemia.
Although not part of our study protocol, our preoperative HCPs routinely prepare written patient instructions for the preoperative management of medications for all patients, which likely explains higher patient adherence to instructions in our study than seen in the previous study where written instructions were only encouraged.11 However, HCP adherence to the protocol decreased after our phase 2 changes and was associated with a transient increase in DOS hyperglycemia rates. The DOS hyperglycemia rates returned to baseline levels with ongoing QI efforts and education to improve HCP adherence to protocol.
Limitations
Our QI initiative had several limitations. Nearly all patients were male veterans with T2DM, and most were older (range, 50-89 years). This limits the generalizability to women, younger patients, and people with type 1 DM. Additionally, our data collection relied on completion and collection of the preoperative form by different HCPs, allowing for sampling bias if some patients with DM undergoing elective noncardiac surgery were missed. Furthermore, although we could verify HCP adherence to the preoperative DM management protocols by reviewing their written instructions, we relied on patients’ self-reported adherence to the preoperative instructions. Finally, we did not evaluate postoperative blood glucose levels because the effect of intraoperative factors such as fluid, insulin, and glucocorticoid administration on postoperative glucose levels are variable. To the best of our knowledge, no other major systematic changes occurred in the preoperative care of patients with DM during the study period.
Conclusions
The findings of our QI initiative suggest that HCP adherence to preoperative DM management protocols may be a key contributor to DOS hyperglycemia and that ensuring HCP adherence may be as important as preoperative insulin dose adjustments. To our knowledge, this is the first study to report rates of HCP adherence to preoperative DM management protocols and its effect on DOS hyperglycemia. We will focus future QI efforts on optimizing HCP adherence to preoperative DM management protocols at our institution.
Acknowledgments
We thank our endocrinology expert, Dr. Kristina Utzschneider, for her guidance in designing this improvement project and our academic research coach, Dr. Helene Starks, for her help in editing the manuscript.
1. van den Boom W, Schroeder RA, Manning MW, Setji TL, Fiestan GO, Dunson DB. Effect of A1c and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018;41(4):782-788. doi:10.2337/dc17-2232
2. Punthakee Z, Iglesias PP, Alonso-Coello P, et al. Association of preoperative glucose concentration with myocardial injury and death after non-cardiac surgery (GlucoVISION): a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6(10):790-797. doi:10.1016/S2213-8587(18)30205-5
3. Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8-14. doi:10.1097/SLA.0b013e31827b6bbc
4. Hommel I, van Gurp PJ, den Broeder AA, et al. Reactive rather than proactive diabetes management in the perioperative period. Horm Metab Res. 2017;49(7):527-533. doi:10.1055/s-0043-105501
5. Demma LJ, Carlson KT, Duggan EW, Morrow JG 3rd, Umpierrez G. Effect of basal insulin dosage on blood glucose concentration in ambulatory surgery patients with type 2 diabetes. J Clin Anesth. 2017;36:184-188. doi:10.1016/j.jclinane.2016.10.003
6. Rosenblatt SI, Dukatz T, Jahn R, et al. Insulin glargine dosing before next-day surgery: comparing three strategies. J Clin Anesth. 2012;24(8):610-617. doi:10.1016/j.jclinane.2012.02.010
7. Dhatariya K, Levy N, Flanagen D, et al; Joint British Diabetes Societies for Inpatient Care. Management of adults with diabetes undergoing surgery and elective procedures: improving standards. Summary. Published 2011. Revised March 2016. Accessed October 31, 2022. https://www.diabetes.org.uk/resources-s3/2017-09/Surgical%20guideline%202015%20-%20summary%20FINAL%20amended%20Mar%202016.pdf
8. American Diabetes Association. 15. Diabetes care in the hospital: standards of medical care in diabetes–2021. Diabetes Care. 2021;44(suppl 1):S211-S220. doi:10.2337/dc21-S015
9. Joshi GP, Chung F, Vann MA, et al; Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111(6):1378-1387. doi:10.1213/ANE.0b013e3181f9c288
10. American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: standards of medical care in diabetes–2022. Diabetes Care. 2021;45(suppl 1):S244-S253. doi:10.2337/dc22-S016
11. Notaras AP, Demetriou E, Galvin J, Ben-Menachem E. A cross-sectional study of preoperative medication adherence and early postoperative recovery. J Clin Anesth. 2016;35:129-135. doi:10.1016/j.jclinane.2016.07.007
12. Bennett B, Provost L. What’s your theory? Driver diagram serves as tool for building and testing theories for improvement. Quality Progress. 2015;48(7):36-43. Accessed August 31, 2022. http://www.apiweb.org/QP_whats-your-theory_201507.pdf
13. Kaplan HC, Provost LP, Froehle CM, Margolis PA. The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13-20. doi:10.1136/bmjqs-2011-000010
14. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
15. Duclos A, Voirin N. The p-control chart: a tool for care improvement. Int J Qual Health Care. 2010;22(5):402-407. doi:10.1093/intqhc/mzq037
16. Cheung YY, Jung B, Sohn JH, Ogrinc G. Quality initiatives: statistical control charts: simplifying the analysis of data for quality improvement. Radiographics. 2012;32(7):2113-2126. doi:10.1148/rg.327125713
17. Simha V, Shah P. Perioperative glucose control in patients with diabetes undergoing elective surgery. JAMA. 2019;321(4):399. doi:10.1001/jama.2018.20922
Perioperative hyperglycemia, defined as blood glucose levels ≥ 180 mg/dL in the immediate pre- and postoperative period, is associated with increased postoperative morbidity, including infections, preoperative interventions, and in-hospital mortality.1-3 Despite being identified as a barrier to optimal perioperative glycemic control, limited evidence is available on patient or health care practitioner (HCP) adherence to preoperative insulin protocols.4-6
Background
Despite mounting evidence of the advantages of maintaining perioperative glucose levels between 80 and 180 mg/dL, available guidelines vary in their recommendations for long-acting basal insulin dosing.7-10 The Society of Ambulatory Anesthesia suggests using 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery in patients without a history of nocturnal or morning hypoglycemia (category 2A evidence).9 However, the revised 2016 United Kingdom National Health Service consensus guideline recommends using 80% to 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery.7 The 2022 American Diabetes Association references an observational study of patients with type 2 DM (T2DM) treated with evening-only, long-acting glargine insulin, indicating that the optimal basal insulin dose on the evening before surgery is about 75% of the outpatient dose.5,10 However, in a randomized, prospective open trial of patients with DM treated with evening-only long-acting basal insulin, no significant difference was noted in the target day of surgery (DOS) glucose levels among different dosing strategies on the evening before surgery.6 Presently, the optimal dose of long-acting insulin analogs on the evening before surgery is unknown.
Additionally, little is known about the other factors that influence perioperative glycemic control. Several barriers to optimal perioperative care of patients with DM have been identified, including lack of prioritization by HCPs, lack of knowledge about current evidence-based recommendations, and lack of patient information and involvement.4 To determine the effect of patient adherence to preoperative medication instructions on postoperative outcome, a cross-sectional study assessed surgical patients admitted to the postanesthetic care unit (PACU) and found that only 70% of patients with insulin-treated DM took their medications preoperatively. Additionally, 23% of nonadherent patients who omitted their medications either did not understand or forgot preoperative medication management instructions. Preoperative DM medication omission was associated with higher rates of hyperglycemia in the PACU (23.8% vs 3.6%; P = .02).11 Importantly, to our knowledge, the extent of HCP adherence to DM management protocols and the subsequent effect on DOS hyperglycemia has not been examined until now.For patients with DM treated with an evening dose of long-acting basal insulin (ie, either once-daily long-acting basal insulin in the evening or twice-daily long-acting basal insulin, both morning and evening) presenting for elective noncardiac surgery, our aim was to decrease the rate of DOS hyperglycemia from 29% (our baseline) to 15% by intensifying the dose of insulin on the evening before surgery without increasing the rate of hypoglycemia. We also sought to determine the rates of HCP adherence to our insulin protocols as well as patients’ self-reported adherence to HCP instructions over the course of this quality improvement (QI) initiative.
Quality Improvement Program
Our surgical department consists of 11 surgical subspecialties that performed approximately 4400 noncardiac surgeries in 2019. All patients undergoing elective surgery are evaluated in the preoperative clinic, which is staffed by an anesthesiology professional (attending and resident physicians, nurse practitioners, and physician assistants) and internal medicine attending physicians. At the preoperative visit, each patient is evaluated by anesthesiology; medically complex patients may also be referred to an internal medicine professional for further risk stratification and optimization before surgery.
At the preoperative clinic visit, HCPs prepare written patient instructions for the preoperative management of medications, including glucose-lowering medications, based on a DM management protocol that was implemented in 2016 for the preoperative management of insulin, noninsulin injectable agents, and oral hyperglycemic agents. According to this protocol, patients with DM treated with evening long-acting basal insulin (eg, glargine insulin) are instructed to take 50% of their usual evening dose the evening before surgery. A preoperative clinic nurse reviews the final preoperative medication instructions with the patient at the end of the clinic visit. Patients are also instructed to avoid oral intake other than water and necessary medications after midnight before surgery regardless of the time of surgery. On the DOS, the patient’s blood glucose level is measured on arrival to the presurgical area.
Our QI initiative focused only on the dose of self-administered, long-acting basal insulin on the evening before surgery. The effect of the morning of surgery long-acting insulin dose on the DOS glucose levels largely depends on the timing of surgery, which is variable; therefore, we did not target this dose for our initiative. Patients receiving intermediate-acting neutral protamine Hagedorn (NPH) insulin were excluded because our protocol does not recommend a dose reduction for NPH insulin on the evening before surgery.
We developed a comprehensive driver diagram to help elucidate the different factors contributing to DOS hyperglycemia and to guide specific QI interventions.12 Some of the identified contributors to DOS hyperglycemia, such as the length of preoperative fasting and timing of surgery, are unpredictable and were deemed difficult to address preoperatively. Other contributors to DOS hyperglycemia, such as outpatient DM management, often require interventions over several months, which is well beyond the time usually allotted for preoperative evaluation and optimization. On the other hand, immediate preoperative insulin dosing directly affects DOS glycemic control; therefore, improvement of the preoperative insulin management protocol to optimize the dosage on the evening before surgery was considered to be an achievable QI goal with the potential for decreasing the rate of DOS hyperglycemia in patients presenting for elective noncardiac surgery.
We used the Model for Understanding Success in Quality (MUSIQ) as a framework to identify key contextual factors that may affect the success of our QI project.13 Limited resource availability and difficulty with dissemination of protocol changes in the preoperative clinic were determined to be potential barriers to the successful implementation of our QI initiative. Nonetheless, senior leadership support, microsystem QI culture, QI team skills, and physician involvement supported the implementation. The revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were followed for this study.14
Interventions
With stakeholder input from anesthesiology, internal medicine, endocrinology, and nursing, we designed an intervention to iteratively change the HCP protocol instructions for long-acting insulin dosing on the evening before surgery. In phase 1 of the study (October 1, 2018, to March 11, 2019), we obtained baseline data on the rates of DOS hyperglycemia (blood glucose ≥ 180 mg/dL) and hypoglycemia (blood glucose < 80 mg/dL), as well as patient and HCP adherence rates to our existing preoperative DM protocol. For phase 2 (March 12, 2019, to July 22, 2019), the preoperative DM management protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with hemoglobin A1c (HbA1c) levels > 8% from 50% of the usual outpatient dose to 100%. Finally, in phase 3 (July 23, 2019, to March 12, 2020), the protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with HbA1c levels ≤ 8% from 50% of the usual outpatient dose to 75% while sustaining the phase 2 change. Preoperative HCPs were informed of the protocol changes in person and were provided with electronic and hard copies of each new protocol.
Protocol
We used a prospective cohort design of 424 consecutive patients with DM who presented for preoperative evaluation for elective noncardiac surgery between October 1, 2018, and March 12, 2020. For the subset of 195 patients treated with an evening dose of long-acting basal insulin, we examined the effect of intensification of this preoperative basal insulin dose on DOS hyperglycemia and hypoglycemia, HCP adherence to iterative changes of the protocol, and patient adherence to HCP instructions on preoperative medication dosing. The QI project was concluded when elective surgeries were paused due to the COVID-19 pandemic.
We created a standardized preoperative data collection form that included information on the most recent HbA1c, time, dose, and type of patient-administered insulin on the evening before surgery, and DOS blood glucose level. A preoperative clinic nurse completed the standardized preoperative data collection form. The HCP’s preoperative medication instructions and the preoperative data collection forms were gathered for review and data analysis.
The primary outcome was DOS hyperglycemia (blood glucose levels ≥ 180 mg/dL). We monitored the rate of DOS hypoglycemia (blood glucose levels < 80 mg/dL) as a balancing measure to ensure safety with long-acting basal insulin intensification. Although hypoglycemia is defined as a blood glucose level < 70 mg/dL, a target glucose range of 80 mg/dL to 180 mg/dL is recommended during the perioperative period.8 Therefore, we chose a more conservative definition of hypoglycemia (blood glucose levels < 80 mg/dL) to adhere to the recommended perioperative glucose target range.
Process measures included HCP adherence to each protocol change, which was assessed by comparing written preoperative patient instructions to the current protocol. Similarly, patient adherence to HCP-recommended long-acting basal insulin dosing was assessed by comparing written preoperative patient instructions to the patient’s self-reported time and dose of long-acting basal insulin on the evening before surgery. For any discrepancy between the HCP instructions and protocol or HCP-recommended dose and patient self-reported dose of long-acting basal insulin, a detailed chart review was performed to determine the etiology.
Statistical Analysis
We used the statistical process p-control chart to assess the effect of iterative changes to the preoperative long-acting basal insulin protocol on DOS hyperglycemia. The proportion defective (rate of DOS hyperglycemia) was plotted against time to determine whether the observed variations in the rate of DOS hyperglycemia over time were attributable to random common causes or special causes because of our intervention. The lower control limit (LCL) and upper control limit (UCL) define the limits of expected outcome measures in a stable process prior to introducing changes and were set at 3 SDs from the mean to balance the likelihood of type I (false-positive) and type II (false-negative) errors. Because of the variable interval sample sizes, we used the CRITBINOM function of Microsoft Excel to calculate the exact UCL satisfying the 3 SD limits of 0.99865.15 The Shewhart rules (outliers, runs or shifts, trends, sawtooth) were used to analyze the p-control chart to identify special cause signals resulting from our interventions.16 We used the statistical process t-control chart to record the time (days) between the few occurrences of DOS hypoglycemia because cases of hypoglycemia were rare.
Ethical Consideration
The Human Research Protection Program, Associate Chief of Staff for Research and Development, and Quality, Safety, and Values department reviewed this project in accordance with the Veterans Health Administration Program Guide 1200.21 and determined that it was a nonresearch operations activity; thus, approval by an institutional review board was not needed. The authors declare no competing interests.
Patient Outcomes
We prospectively followed 424 consecutive patients with DM undergoing elective noncardiac surgery from the time of the preoperative clinic evaluation until DOS; 195 patients were on evening
A subgroup analysis of DOS glucose levels in insulin-treated patients with preoperative HbA1c levels > 8% did not demonstrate a change in the rate of
Only 7 of 424 (1.7%) patients with DM and 4 of 195 (2.1%) patients treated with evening, long-acting basal insulin had marked hyperglycemia (DOS glucose levels ≥ 300 mg/dL). Only 1 patient who was not on outpatient insulin treatment had surgery canceled for hyperglycemia.
Overall, 89% of the HCPs followed the preoperative insulin protocol. HCP adherence to the protocol decreased to 77% after the phase 2 change, often related to deviations from the protocol or when a prior version was used. By the end of phase 3, HCP adherence returned to the baseline rate (88%). Patient adherence to medication instructions was not affected by protocol changes (86% throughout the study period). Prospective data collection was briefly interrupted between January 18, 2019, and March 5, 2019, while designing our phase 2 intervention. We were unable to track the total number of eligible patients during this time, but were able to identify 8 insulin-treated patients with DM who underwent elective noncardiac surgery and included their data in phase 1.
Discussion
The management and prevention of immediate perioperative hyperglycemia and glycemic variability have attracted attention as evidence has mounted for their association with postoperative morbidity and mortality.1,2,17 Available guidelines for preventing DOS hyperglycemia vary in their recommendations for preoperative insulin management.7-10 Notably, concerns about iatrogenic hypoglycemia often hinder efforts to lower rates of DOS hyperglycemia.4 We successfully implemented an iterative intensification protocol for preoperative long-acting basal insulin doses on the evening before surgery but did not observe a lower rate of hyperglycemia. Importantly, we also did not observe a higher rate of hypoglycemia on the DOS, as observed in a previous study.5
The observational study by Demma and colleagues found that patients receiving 75% of their evening, long-acting basal insulin dose were significantly more likely to achieve target blood glucose levels of 100 to 180 mg/dL than patients receiving no insulin at all (78% vs 0%; P = .001). However, no significant difference was noted when this group was compared with patients receiving 50% of their evening, long-acting basal insulin doses (78% vs 70%; P = .56). This is more clinically pertinent as it is generally accepted that the evening, long-acting insulin dose should not be entirely withheld on the evening before surgery.5
These findings are consistent with our observation that the rate of DOS hyperglycemia did not decrease with intensification of the evening, long-acting insulin dose from 50% to 100% of the prescribed dose in patients with HbA1c levels > 8% (phase 2) and 50% to 75% of the prescribed dose in patients with HbA1c levels ≤ 8% (phase 3). In the study by Demma and colleagues, few patients presented with preoperative hypoglycemia (2.7%) but all had received 100% of their evening, long-acting basal insulin dose, suggesting a significant increase in the rate of hypoglycemia compared with patients receiving lower doses of insulin (P = .01).5 However, long-term DM control as assessed by HbA1c level was available for < 10% of the patients, making it difficult to evaluate the effect of overall DM control on the results.5 In our study, preoperative HbA1c levels were available for 99.5% of the patients and only those with HbA1c levels > 8% received 100% of their evening, long-acting insulin dose on the evening before surgery. Notably, we did not observe a higher rate of hypoglycemia in this patient population, indicating that preoperative insulin dose intensification is safe for this subgroup.
Although HCP adherence to perioperative DM management protocols has been identified as a predominant barrier to the delivery of optimal perioperative DM care, prior studies of various preoperative insulin protocols to reduce perioperative hyperglycemia have not reported HCP adherence to their insulin protocols or its effect on DOS hyperglycemia.4-6 Additionally, patient adherence to HCP instructions is a key factor identified in our driver diagram that may influence DOS hyperglycemia, a hypothesis that is supported by a prior cross-sectional study showing an increased rate of hyperglycemia in the PACU with omission of preoperative DM medication.11 In our study, patient adherence to preoperative medication management instructions was higher than reported previously and remained consistently high regardless of protocol changes, which may explain why patient adherence did not affect the rate of DOS hyperglycemia.
Although not part of our study protocol, our preoperative HCPs routinely prepare written patient instructions for the preoperative management of medications for all patients, which likely explains higher patient adherence to instructions in our study than seen in the previous study where written instructions were only encouraged.11 However, HCP adherence to the protocol decreased after our phase 2 changes and was associated with a transient increase in DOS hyperglycemia rates. The DOS hyperglycemia rates returned to baseline levels with ongoing QI efforts and education to improve HCP adherence to protocol.
Limitations
Our QI initiative had several limitations. Nearly all patients were male veterans with T2DM, and most were older (range, 50-89 years). This limits the generalizability to women, younger patients, and people with type 1 DM. Additionally, our data collection relied on completion and collection of the preoperative form by different HCPs, allowing for sampling bias if some patients with DM undergoing elective noncardiac surgery were missed. Furthermore, although we could verify HCP adherence to the preoperative DM management protocols by reviewing their written instructions, we relied on patients’ self-reported adherence to the preoperative instructions. Finally, we did not evaluate postoperative blood glucose levels because the effect of intraoperative factors such as fluid, insulin, and glucocorticoid administration on postoperative glucose levels are variable. To the best of our knowledge, no other major systematic changes occurred in the preoperative care of patients with DM during the study period.
Conclusions
The findings of our QI initiative suggest that HCP adherence to preoperative DM management protocols may be a key contributor to DOS hyperglycemia and that ensuring HCP adherence may be as important as preoperative insulin dose adjustments. To our knowledge, this is the first study to report rates of HCP adherence to preoperative DM management protocols and its effect on DOS hyperglycemia. We will focus future QI efforts on optimizing HCP adherence to preoperative DM management protocols at our institution.
Acknowledgments
We thank our endocrinology expert, Dr. Kristina Utzschneider, for her guidance in designing this improvement project and our academic research coach, Dr. Helene Starks, for her help in editing the manuscript.
Perioperative hyperglycemia, defined as blood glucose levels ≥ 180 mg/dL in the immediate pre- and postoperative period, is associated with increased postoperative morbidity, including infections, preoperative interventions, and in-hospital mortality.1-3 Despite being identified as a barrier to optimal perioperative glycemic control, limited evidence is available on patient or health care practitioner (HCP) adherence to preoperative insulin protocols.4-6
Background
Despite mounting evidence of the advantages of maintaining perioperative glucose levels between 80 and 180 mg/dL, available guidelines vary in their recommendations for long-acting basal insulin dosing.7-10 The Society of Ambulatory Anesthesia suggests using 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery in patients without a history of nocturnal or morning hypoglycemia (category 2A evidence).9 However, the revised 2016 United Kingdom National Health Service consensus guideline recommends using 80% to 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery.7 The 2022 American Diabetes Association references an observational study of patients with type 2 DM (T2DM) treated with evening-only, long-acting glargine insulin, indicating that the optimal basal insulin dose on the evening before surgery is about 75% of the outpatient dose.5,10 However, in a randomized, prospective open trial of patients with DM treated with evening-only long-acting basal insulin, no significant difference was noted in the target day of surgery (DOS) glucose levels among different dosing strategies on the evening before surgery.6 Presently, the optimal dose of long-acting insulin analogs on the evening before surgery is unknown.
Additionally, little is known about the other factors that influence perioperative glycemic control. Several barriers to optimal perioperative care of patients with DM have been identified, including lack of prioritization by HCPs, lack of knowledge about current evidence-based recommendations, and lack of patient information and involvement.4 To determine the effect of patient adherence to preoperative medication instructions on postoperative outcome, a cross-sectional study assessed surgical patients admitted to the postanesthetic care unit (PACU) and found that only 70% of patients with insulin-treated DM took their medications preoperatively. Additionally, 23% of nonadherent patients who omitted their medications either did not understand or forgot preoperative medication management instructions. Preoperative DM medication omission was associated with higher rates of hyperglycemia in the PACU (23.8% vs 3.6%; P = .02).11 Importantly, to our knowledge, the extent of HCP adherence to DM management protocols and the subsequent effect on DOS hyperglycemia has not been examined until now.For patients with DM treated with an evening dose of long-acting basal insulin (ie, either once-daily long-acting basal insulin in the evening or twice-daily long-acting basal insulin, both morning and evening) presenting for elective noncardiac surgery, our aim was to decrease the rate of DOS hyperglycemia from 29% (our baseline) to 15% by intensifying the dose of insulin on the evening before surgery without increasing the rate of hypoglycemia. We also sought to determine the rates of HCP adherence to our insulin protocols as well as patients’ self-reported adherence to HCP instructions over the course of this quality improvement (QI) initiative.
Quality Improvement Program
Our surgical department consists of 11 surgical subspecialties that performed approximately 4400 noncardiac surgeries in 2019. All patients undergoing elective surgery are evaluated in the preoperative clinic, which is staffed by an anesthesiology professional (attending and resident physicians, nurse practitioners, and physician assistants) and internal medicine attending physicians. At the preoperative visit, each patient is evaluated by anesthesiology; medically complex patients may also be referred to an internal medicine professional for further risk stratification and optimization before surgery.
At the preoperative clinic visit, HCPs prepare written patient instructions for the preoperative management of medications, including glucose-lowering medications, based on a DM management protocol that was implemented in 2016 for the preoperative management of insulin, noninsulin injectable agents, and oral hyperglycemic agents. According to this protocol, patients with DM treated with evening long-acting basal insulin (eg, glargine insulin) are instructed to take 50% of their usual evening dose the evening before surgery. A preoperative clinic nurse reviews the final preoperative medication instructions with the patient at the end of the clinic visit. Patients are also instructed to avoid oral intake other than water and necessary medications after midnight before surgery regardless of the time of surgery. On the DOS, the patient’s blood glucose level is measured on arrival to the presurgical area.
Our QI initiative focused only on the dose of self-administered, long-acting basal insulin on the evening before surgery. The effect of the morning of surgery long-acting insulin dose on the DOS glucose levels largely depends on the timing of surgery, which is variable; therefore, we did not target this dose for our initiative. Patients receiving intermediate-acting neutral protamine Hagedorn (NPH) insulin were excluded because our protocol does not recommend a dose reduction for NPH insulin on the evening before surgery.
We developed a comprehensive driver diagram to help elucidate the different factors contributing to DOS hyperglycemia and to guide specific QI interventions.12 Some of the identified contributors to DOS hyperglycemia, such as the length of preoperative fasting and timing of surgery, are unpredictable and were deemed difficult to address preoperatively. Other contributors to DOS hyperglycemia, such as outpatient DM management, often require interventions over several months, which is well beyond the time usually allotted for preoperative evaluation and optimization. On the other hand, immediate preoperative insulin dosing directly affects DOS glycemic control; therefore, improvement of the preoperative insulin management protocol to optimize the dosage on the evening before surgery was considered to be an achievable QI goal with the potential for decreasing the rate of DOS hyperglycemia in patients presenting for elective noncardiac surgery.
We used the Model for Understanding Success in Quality (MUSIQ) as a framework to identify key contextual factors that may affect the success of our QI project.13 Limited resource availability and difficulty with dissemination of protocol changes in the preoperative clinic were determined to be potential barriers to the successful implementation of our QI initiative. Nonetheless, senior leadership support, microsystem QI culture, QI team skills, and physician involvement supported the implementation. The revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were followed for this study.14
Interventions
With stakeholder input from anesthesiology, internal medicine, endocrinology, and nursing, we designed an intervention to iteratively change the HCP protocol instructions for long-acting insulin dosing on the evening before surgery. In phase 1 of the study (October 1, 2018, to March 11, 2019), we obtained baseline data on the rates of DOS hyperglycemia (blood glucose ≥ 180 mg/dL) and hypoglycemia (blood glucose < 80 mg/dL), as well as patient and HCP adherence rates to our existing preoperative DM protocol. For phase 2 (March 12, 2019, to July 22, 2019), the preoperative DM management protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with hemoglobin A1c (HbA1c) levels > 8% from 50% of the usual outpatient dose to 100%. Finally, in phase 3 (July 23, 2019, to March 12, 2020), the protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with HbA1c levels ≤ 8% from 50% of the usual outpatient dose to 75% while sustaining the phase 2 change. Preoperative HCPs were informed of the protocol changes in person and were provided with electronic and hard copies of each new protocol.
Protocol
We used a prospective cohort design of 424 consecutive patients with DM who presented for preoperative evaluation for elective noncardiac surgery between October 1, 2018, and March 12, 2020. For the subset of 195 patients treated with an evening dose of long-acting basal insulin, we examined the effect of intensification of this preoperative basal insulin dose on DOS hyperglycemia and hypoglycemia, HCP adherence to iterative changes of the protocol, and patient adherence to HCP instructions on preoperative medication dosing. The QI project was concluded when elective surgeries were paused due to the COVID-19 pandemic.
We created a standardized preoperative data collection form that included information on the most recent HbA1c, time, dose, and type of patient-administered insulin on the evening before surgery, and DOS blood glucose level. A preoperative clinic nurse completed the standardized preoperative data collection form. The HCP’s preoperative medication instructions and the preoperative data collection forms were gathered for review and data analysis.
The primary outcome was DOS hyperglycemia (blood glucose levels ≥ 180 mg/dL). We monitored the rate of DOS hypoglycemia (blood glucose levels < 80 mg/dL) as a balancing measure to ensure safety with long-acting basal insulin intensification. Although hypoglycemia is defined as a blood glucose level < 70 mg/dL, a target glucose range of 80 mg/dL to 180 mg/dL is recommended during the perioperative period.8 Therefore, we chose a more conservative definition of hypoglycemia (blood glucose levels < 80 mg/dL) to adhere to the recommended perioperative glucose target range.
Process measures included HCP adherence to each protocol change, which was assessed by comparing written preoperative patient instructions to the current protocol. Similarly, patient adherence to HCP-recommended long-acting basal insulin dosing was assessed by comparing written preoperative patient instructions to the patient’s self-reported time and dose of long-acting basal insulin on the evening before surgery. For any discrepancy between the HCP instructions and protocol or HCP-recommended dose and patient self-reported dose of long-acting basal insulin, a detailed chart review was performed to determine the etiology.
Statistical Analysis
We used the statistical process p-control chart to assess the effect of iterative changes to the preoperative long-acting basal insulin protocol on DOS hyperglycemia. The proportion defective (rate of DOS hyperglycemia) was plotted against time to determine whether the observed variations in the rate of DOS hyperglycemia over time were attributable to random common causes or special causes because of our intervention. The lower control limit (LCL) and upper control limit (UCL) define the limits of expected outcome measures in a stable process prior to introducing changes and were set at 3 SDs from the mean to balance the likelihood of type I (false-positive) and type II (false-negative) errors. Because of the variable interval sample sizes, we used the CRITBINOM function of Microsoft Excel to calculate the exact UCL satisfying the 3 SD limits of 0.99865.15 The Shewhart rules (outliers, runs or shifts, trends, sawtooth) were used to analyze the p-control chart to identify special cause signals resulting from our interventions.16 We used the statistical process t-control chart to record the time (days) between the few occurrences of DOS hypoglycemia because cases of hypoglycemia were rare.
Ethical Consideration
The Human Research Protection Program, Associate Chief of Staff for Research and Development, and Quality, Safety, and Values department reviewed this project in accordance with the Veterans Health Administration Program Guide 1200.21 and determined that it was a nonresearch operations activity; thus, approval by an institutional review board was not needed. The authors declare no competing interests.
Patient Outcomes
We prospectively followed 424 consecutive patients with DM undergoing elective noncardiac surgery from the time of the preoperative clinic evaluation until DOS; 195 patients were on evening
A subgroup analysis of DOS glucose levels in insulin-treated patients with preoperative HbA1c levels > 8% did not demonstrate a change in the rate of
Only 7 of 424 (1.7%) patients with DM and 4 of 195 (2.1%) patients treated with evening, long-acting basal insulin had marked hyperglycemia (DOS glucose levels ≥ 300 mg/dL). Only 1 patient who was not on outpatient insulin treatment had surgery canceled for hyperglycemia.
Overall, 89% of the HCPs followed the preoperative insulin protocol. HCP adherence to the protocol decreased to 77% after the phase 2 change, often related to deviations from the protocol or when a prior version was used. By the end of phase 3, HCP adherence returned to the baseline rate (88%). Patient adherence to medication instructions was not affected by protocol changes (86% throughout the study period). Prospective data collection was briefly interrupted between January 18, 2019, and March 5, 2019, while designing our phase 2 intervention. We were unable to track the total number of eligible patients during this time, but were able to identify 8 insulin-treated patients with DM who underwent elective noncardiac surgery and included their data in phase 1.
Discussion
The management and prevention of immediate perioperative hyperglycemia and glycemic variability have attracted attention as evidence has mounted for their association with postoperative morbidity and mortality.1,2,17 Available guidelines for preventing DOS hyperglycemia vary in their recommendations for preoperative insulin management.7-10 Notably, concerns about iatrogenic hypoglycemia often hinder efforts to lower rates of DOS hyperglycemia.4 We successfully implemented an iterative intensification protocol for preoperative long-acting basal insulin doses on the evening before surgery but did not observe a lower rate of hyperglycemia. Importantly, we also did not observe a higher rate of hypoglycemia on the DOS, as observed in a previous study.5
The observational study by Demma and colleagues found that patients receiving 75% of their evening, long-acting basal insulin dose were significantly more likely to achieve target blood glucose levels of 100 to 180 mg/dL than patients receiving no insulin at all (78% vs 0%; P = .001). However, no significant difference was noted when this group was compared with patients receiving 50% of their evening, long-acting basal insulin doses (78% vs 70%; P = .56). This is more clinically pertinent as it is generally accepted that the evening, long-acting insulin dose should not be entirely withheld on the evening before surgery.5
These findings are consistent with our observation that the rate of DOS hyperglycemia did not decrease with intensification of the evening, long-acting insulin dose from 50% to 100% of the prescribed dose in patients with HbA1c levels > 8% (phase 2) and 50% to 75% of the prescribed dose in patients with HbA1c levels ≤ 8% (phase 3). In the study by Demma and colleagues, few patients presented with preoperative hypoglycemia (2.7%) but all had received 100% of their evening, long-acting basal insulin dose, suggesting a significant increase in the rate of hypoglycemia compared with patients receiving lower doses of insulin (P = .01).5 However, long-term DM control as assessed by HbA1c level was available for < 10% of the patients, making it difficult to evaluate the effect of overall DM control on the results.5 In our study, preoperative HbA1c levels were available for 99.5% of the patients and only those with HbA1c levels > 8% received 100% of their evening, long-acting insulin dose on the evening before surgery. Notably, we did not observe a higher rate of hypoglycemia in this patient population, indicating that preoperative insulin dose intensification is safe for this subgroup.
Although HCP adherence to perioperative DM management protocols has been identified as a predominant barrier to the delivery of optimal perioperative DM care, prior studies of various preoperative insulin protocols to reduce perioperative hyperglycemia have not reported HCP adherence to their insulin protocols or its effect on DOS hyperglycemia.4-6 Additionally, patient adherence to HCP instructions is a key factor identified in our driver diagram that may influence DOS hyperglycemia, a hypothesis that is supported by a prior cross-sectional study showing an increased rate of hyperglycemia in the PACU with omission of preoperative DM medication.11 In our study, patient adherence to preoperative medication management instructions was higher than reported previously and remained consistently high regardless of protocol changes, which may explain why patient adherence did not affect the rate of DOS hyperglycemia.
Although not part of our study protocol, our preoperative HCPs routinely prepare written patient instructions for the preoperative management of medications for all patients, which likely explains higher patient adherence to instructions in our study than seen in the previous study where written instructions were only encouraged.11 However, HCP adherence to the protocol decreased after our phase 2 changes and was associated with a transient increase in DOS hyperglycemia rates. The DOS hyperglycemia rates returned to baseline levels with ongoing QI efforts and education to improve HCP adherence to protocol.
Limitations
Our QI initiative had several limitations. Nearly all patients were male veterans with T2DM, and most were older (range, 50-89 years). This limits the generalizability to women, younger patients, and people with type 1 DM. Additionally, our data collection relied on completion and collection of the preoperative form by different HCPs, allowing for sampling bias if some patients with DM undergoing elective noncardiac surgery were missed. Furthermore, although we could verify HCP adherence to the preoperative DM management protocols by reviewing their written instructions, we relied on patients’ self-reported adherence to the preoperative instructions. Finally, we did not evaluate postoperative blood glucose levels because the effect of intraoperative factors such as fluid, insulin, and glucocorticoid administration on postoperative glucose levels are variable. To the best of our knowledge, no other major systematic changes occurred in the preoperative care of patients with DM during the study period.
Conclusions
The findings of our QI initiative suggest that HCP adherence to preoperative DM management protocols may be a key contributor to DOS hyperglycemia and that ensuring HCP adherence may be as important as preoperative insulin dose adjustments. To our knowledge, this is the first study to report rates of HCP adherence to preoperative DM management protocols and its effect on DOS hyperglycemia. We will focus future QI efforts on optimizing HCP adherence to preoperative DM management protocols at our institution.
Acknowledgments
We thank our endocrinology expert, Dr. Kristina Utzschneider, for her guidance in designing this improvement project and our academic research coach, Dr. Helene Starks, for her help in editing the manuscript.
1. van den Boom W, Schroeder RA, Manning MW, Setji TL, Fiestan GO, Dunson DB. Effect of A1c and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018;41(4):782-788. doi:10.2337/dc17-2232
2. Punthakee Z, Iglesias PP, Alonso-Coello P, et al. Association of preoperative glucose concentration with myocardial injury and death after non-cardiac surgery (GlucoVISION): a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6(10):790-797. doi:10.1016/S2213-8587(18)30205-5
3. Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8-14. doi:10.1097/SLA.0b013e31827b6bbc
4. Hommel I, van Gurp PJ, den Broeder AA, et al. Reactive rather than proactive diabetes management in the perioperative period. Horm Metab Res. 2017;49(7):527-533. doi:10.1055/s-0043-105501
5. Demma LJ, Carlson KT, Duggan EW, Morrow JG 3rd, Umpierrez G. Effect of basal insulin dosage on blood glucose concentration in ambulatory surgery patients with type 2 diabetes. J Clin Anesth. 2017;36:184-188. doi:10.1016/j.jclinane.2016.10.003
6. Rosenblatt SI, Dukatz T, Jahn R, et al. Insulin glargine dosing before next-day surgery: comparing three strategies. J Clin Anesth. 2012;24(8):610-617. doi:10.1016/j.jclinane.2012.02.010
7. Dhatariya K, Levy N, Flanagen D, et al; Joint British Diabetes Societies for Inpatient Care. Management of adults with diabetes undergoing surgery and elective procedures: improving standards. Summary. Published 2011. Revised March 2016. Accessed October 31, 2022. https://www.diabetes.org.uk/resources-s3/2017-09/Surgical%20guideline%202015%20-%20summary%20FINAL%20amended%20Mar%202016.pdf
8. American Diabetes Association. 15. Diabetes care in the hospital: standards of medical care in diabetes–2021. Diabetes Care. 2021;44(suppl 1):S211-S220. doi:10.2337/dc21-S015
9. Joshi GP, Chung F, Vann MA, et al; Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111(6):1378-1387. doi:10.1213/ANE.0b013e3181f9c288
10. American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: standards of medical care in diabetes–2022. Diabetes Care. 2021;45(suppl 1):S244-S253. doi:10.2337/dc22-S016
11. Notaras AP, Demetriou E, Galvin J, Ben-Menachem E. A cross-sectional study of preoperative medication adherence and early postoperative recovery. J Clin Anesth. 2016;35:129-135. doi:10.1016/j.jclinane.2016.07.007
12. Bennett B, Provost L. What’s your theory? Driver diagram serves as tool for building and testing theories for improvement. Quality Progress. 2015;48(7):36-43. Accessed August 31, 2022. http://www.apiweb.org/QP_whats-your-theory_201507.pdf
13. Kaplan HC, Provost LP, Froehle CM, Margolis PA. The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13-20. doi:10.1136/bmjqs-2011-000010
14. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
15. Duclos A, Voirin N. The p-control chart: a tool for care improvement. Int J Qual Health Care. 2010;22(5):402-407. doi:10.1093/intqhc/mzq037
16. Cheung YY, Jung B, Sohn JH, Ogrinc G. Quality initiatives: statistical control charts: simplifying the analysis of data for quality improvement. Radiographics. 2012;32(7):2113-2126. doi:10.1148/rg.327125713
17. Simha V, Shah P. Perioperative glucose control in patients with diabetes undergoing elective surgery. JAMA. 2019;321(4):399. doi:10.1001/jama.2018.20922
1. van den Boom W, Schroeder RA, Manning MW, Setji TL, Fiestan GO, Dunson DB. Effect of A1c and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018;41(4):782-788. doi:10.2337/dc17-2232
2. Punthakee Z, Iglesias PP, Alonso-Coello P, et al. Association of preoperative glucose concentration with myocardial injury and death after non-cardiac surgery (GlucoVISION): a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6(10):790-797. doi:10.1016/S2213-8587(18)30205-5
3. Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8-14. doi:10.1097/SLA.0b013e31827b6bbc
4. Hommel I, van Gurp PJ, den Broeder AA, et al. Reactive rather than proactive diabetes management in the perioperative period. Horm Metab Res. 2017;49(7):527-533. doi:10.1055/s-0043-105501
5. Demma LJ, Carlson KT, Duggan EW, Morrow JG 3rd, Umpierrez G. Effect of basal insulin dosage on blood glucose concentration in ambulatory surgery patients with type 2 diabetes. J Clin Anesth. 2017;36:184-188. doi:10.1016/j.jclinane.2016.10.003
6. Rosenblatt SI, Dukatz T, Jahn R, et al. Insulin glargine dosing before next-day surgery: comparing three strategies. J Clin Anesth. 2012;24(8):610-617. doi:10.1016/j.jclinane.2012.02.010
7. Dhatariya K, Levy N, Flanagen D, et al; Joint British Diabetes Societies for Inpatient Care. Management of adults with diabetes undergoing surgery and elective procedures: improving standards. Summary. Published 2011. Revised March 2016. Accessed October 31, 2022. https://www.diabetes.org.uk/resources-s3/2017-09/Surgical%20guideline%202015%20-%20summary%20FINAL%20amended%20Mar%202016.pdf
8. American Diabetes Association. 15. Diabetes care in the hospital: standards of medical care in diabetes–2021. Diabetes Care. 2021;44(suppl 1):S211-S220. doi:10.2337/dc21-S015
9. Joshi GP, Chung F, Vann MA, et al; Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111(6):1378-1387. doi:10.1213/ANE.0b013e3181f9c288
10. American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: standards of medical care in diabetes–2022. Diabetes Care. 2021;45(suppl 1):S244-S253. doi:10.2337/dc22-S016
11. Notaras AP, Demetriou E, Galvin J, Ben-Menachem E. A cross-sectional study of preoperative medication adherence and early postoperative recovery. J Clin Anesth. 2016;35:129-135. doi:10.1016/j.jclinane.2016.07.007
12. Bennett B, Provost L. What’s your theory? Driver diagram serves as tool for building and testing theories for improvement. Quality Progress. 2015;48(7):36-43. Accessed August 31, 2022. http://www.apiweb.org/QP_whats-your-theory_201507.pdf
13. Kaplan HC, Provost LP, Froehle CM, Margolis PA. The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13-20. doi:10.1136/bmjqs-2011-000010
14. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
15. Duclos A, Voirin N. The p-control chart: a tool for care improvement. Int J Qual Health Care. 2010;22(5):402-407. doi:10.1093/intqhc/mzq037
16. Cheung YY, Jung B, Sohn JH, Ogrinc G. Quality initiatives: statistical control charts: simplifying the analysis of data for quality improvement. Radiographics. 2012;32(7):2113-2126. doi:10.1148/rg.327125713
17. Simha V, Shah P. Perioperative glucose control in patients with diabetes undergoing elective surgery. JAMA. 2019;321(4):399. doi:10.1001/jama.2018.20922