User login
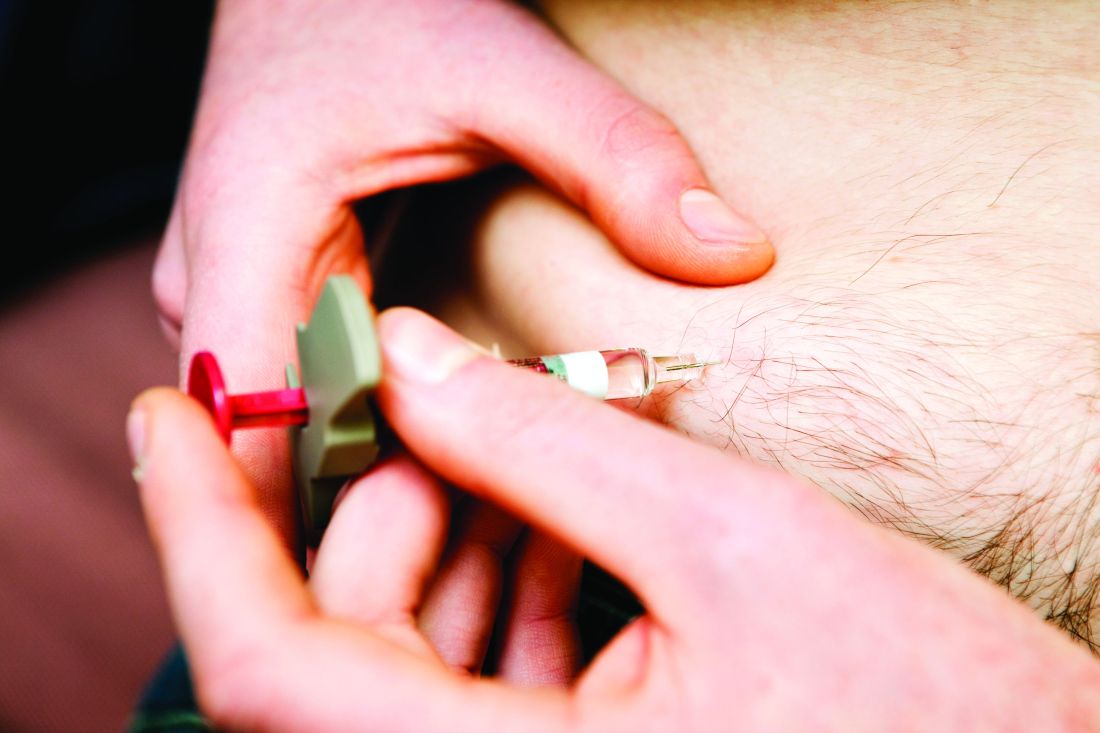
Rheumatology drugs, like most drugs, are seeing significant price hikes in recent years and, according to a new analysis, those hikes account for the majority of growth in spending.
From 2012 to 2016, annual spending on public-payer claims for 10 biologic disease-modifying antirheumatic drugs (bDMARDs) more than doubled (from $3.8 billion to $8.6 billion), with median drug price increases of 51% in the Medicare Part D prescription drug plans (mean, 54%) and 8% within Medicare Part B drugs administered within the physician office (mean, 21%).
“The prominent bDMARD drug cost increases observed in this study, which were substantially greater under Part D than Part B, even when accounting for rebates, represent a growing burden to taxpayers and beneficiaries,” first author Natalie McCormick, PhD, a research fellow at Massachusetts General Hospital, Boston, and colleagues wrote in Arthritis & Rheumatology.
“The magnitude of these increases is independent from any assessment of value,” the authors continued.
Dr. McCormick and colleagues highlighted the limited effect that rebates negotiated by pharmacy benefit managers (PBMs) for Part D have on patient out-of-pocket costs.
Negotiations “may not necessarily result in lower price increases since pharmacy benefit managers, who negotiate on behalf of Part D plans, retain a percentage of the negotiated rebates as compensation, and higher list prices can achieve higher rebates,” they stated. “While these rebates accrue to insurance plans and pharmacy benefit managers, they do not directly impact Part D beneficiaries’ out-of-pocket costs, which are driven by prerebate prices. Thus, many patients end up paying higher out-of-pocket costs when the list price increases, creating financial barriers to use and adherence.”
They further noted that, for most Part D drugs individually, “drug prices accounted for approximately 50% or more of the increase in spending. Adalimumab and etanercept, two of the oldest bDMARDs, were prescribed to the largest number of Part D beneficiaries (more than 47,000 in 2016) and had the biggest 5-year price increases: 84% and 88%, respectively (72% and 75% respectively, accounting for rebates).”
It is not surprising that the analysis found that prices are rising and are a driving force in spending.
“We know that high launch prices of a new drug is an important consideration for patient access,” Jeromie Ballreich, PhD, a health economist at Johns Hopkins University, Baltimore, said in an interview. “But we also know that a number of these drugs, particularly Humira and Enbrel, have been on the market for a while. They have experienced a tremendous price increase over the past 10 years plus. That’s exactly what the authors found. So that is really not surprising. Analysis of drug spending changes over the time [shows that] it is indeed price increases that really have been driving increases in drug spending.”
Angus Worthing, MD, a rheumatologist at Arthritis & Rheumatism Associates in Washington, D.C., and current chair of the American College of Rheumatology’s Government Affairs Committee, said that the faster price hikes in Part D stood out in the analysis and the role PBMs played in it.
“I was a little surprised at just how much more PBMs were responsible for higher inflation, compared to the part B system,” he said in an interview. “In the McCormick article, drug prices went up 45% after rebates in the Part D space, but only 21% in the Part B space, and we had seen hints that drug prices were going up faster, we just didn’t know just how much faster, so more than double the increase is more than I expected, and it is very concerning to look at that and realize that PBMs are more than doubling an already high inflation rate in drug prices.”
He suggested that part of the rise in Part D bDMARD prices observed in the study might be because of the application of step therapy in Part D.
“In Medicare specifically, step therapy is allowed in Part D but not in Part B – except in Medicare Advantage plans starting in 2019 – and this important difference between Part B and Part D explains how Part D rebates, linked with step therapy, may be the main reason that bDMARD prices rose more in Part D compared to the open formulary system in Medicare Part B,” Dr. Worthing wrote in an editorial on Dr. McCormick’s study.
The Pharmaceutical Care Management Association, the lobby group representing PBMs, declined an interview request, but challenged the analysis’ portrayal of the role of PBMs in an email. It pointed to a Government Accountability Organization report from July 2019 that found that Part D rebates helped offset spending by about 20% and that PBM compensation was primarily derived from administrative fees and not from maintaining a portion of rebates that the PBM negotiated on behalf of the plan.
Dr. Worthing said in an interview that these bDMARD price hikes are causing access issues, particularly in Medicare where beneficiaries cannot take advantage of manufacturer programs, such as discount cards and copay coupons, and potentially face coinsurance payments based on the list price and not the net-of-rebate price.
From a policy standpoint, he advocated for a cap on out-of-pocket spending for Medicare Part D drugs, which is a feature of legislation (H.R. 3, Lower Drug Costs Now Act of 2019) that is moving through the House of Representatives, most recently passing the House Ways and Means Committee in a party-line vote of 24-17, with 1 Democrat not voting.
Dr. Worthing’s editorial covers a few other policy options, including more financial transparency in the role PBMs play in the drug supply chain, the elimination of rebates in favor of a flat fee for PBMs or requiring all rebates on drugs to be passed through to Part D beneficiaries, penalties on manufacturers for price increases that exceed inflation, and usage of a price index based on what foreign countries pay for the same drug.
Many of the suggestions from Dr. Worthing’s editorial are in H.R. 3 and face an uphill battle to get through the Senate.
Dr. Worthing said that tackling the rebate issue by removing incentives for manufacturers to raise prices in order to offer higher rebates and changing step-therapy rules are things Congress could probably get accomplished as it heads into an election year, but there is a chance it could be even more comprehensive.
“With such an important issue like high drug prices, it probably benefits both parties, and everybody facing an election will be able to go back to their constituents and say, ‘Look we got something done. I was part of this,’ and there is a better chance they will do it if they hear about it from their constituents,” he said.
Dr. Ballreich was less optimistic that H.R. 3 is going to be able to survive.
“The interesting thing with this bill is that a number of pieces of it have been endorsed by President Trump, and the question is, [is] that a meaningful endorsement? Is the Trump administration going to want to use the this plan as a win for him, as a win for showing potential voters that he can work in a bipartisan way, [that] he could take on ‘big drug companies’ – all those successful soundbites? Can then Trump use his power to push this bill through the Senate? I think that is a question mark,” he said, noting that it could take a lot of political capital to counter the powerful industry lobby. Ultimately, he expects that, if drug pricing does make it to the White House, it will go through some changes from where H.R. 3 is right now.
“I don’t think it will pass in its current iteration,” he said.
Each author of Dr. McCormick’s report had funding from different sources, though the funders had no role in the design and conduct of the study. No other disclosures were made.
SOURCES: McCormick N et al. Arthritis Rheumatol. 2019 Oct 14. doi: 10.1002/ART.41138; Worthing A. Arthritis Rheumatol. 2019 Oct 14. doi: 10.1002/art.41135.
Rheumatology drugs, like most drugs, are seeing significant price hikes in recent years and, according to a new analysis, those hikes account for the majority of growth in spending.
From 2012 to 2016, annual spending on public-payer claims for 10 biologic disease-modifying antirheumatic drugs (bDMARDs) more than doubled (from $3.8 billion to $8.6 billion), with median drug price increases of 51% in the Medicare Part D prescription drug plans (mean, 54%) and 8% within Medicare Part B drugs administered within the physician office (mean, 21%).
“The prominent bDMARD drug cost increases observed in this study, which were substantially greater under Part D than Part B, even when accounting for rebates, represent a growing burden to taxpayers and beneficiaries,” first author Natalie McCormick, PhD, a research fellow at Massachusetts General Hospital, Boston, and colleagues wrote in Arthritis & Rheumatology.
“The magnitude of these increases is independent from any assessment of value,” the authors continued.
Dr. McCormick and colleagues highlighted the limited effect that rebates negotiated by pharmacy benefit managers (PBMs) for Part D have on patient out-of-pocket costs.
Negotiations “may not necessarily result in lower price increases since pharmacy benefit managers, who negotiate on behalf of Part D plans, retain a percentage of the negotiated rebates as compensation, and higher list prices can achieve higher rebates,” they stated. “While these rebates accrue to insurance plans and pharmacy benefit managers, they do not directly impact Part D beneficiaries’ out-of-pocket costs, which are driven by prerebate prices. Thus, many patients end up paying higher out-of-pocket costs when the list price increases, creating financial barriers to use and adherence.”
They further noted that, for most Part D drugs individually, “drug prices accounted for approximately 50% or more of the increase in spending. Adalimumab and etanercept, two of the oldest bDMARDs, were prescribed to the largest number of Part D beneficiaries (more than 47,000 in 2016) and had the biggest 5-year price increases: 84% and 88%, respectively (72% and 75% respectively, accounting for rebates).”
It is not surprising that the analysis found that prices are rising and are a driving force in spending.
“We know that high launch prices of a new drug is an important consideration for patient access,” Jeromie Ballreich, PhD, a health economist at Johns Hopkins University, Baltimore, said in an interview. “But we also know that a number of these drugs, particularly Humira and Enbrel, have been on the market for a while. They have experienced a tremendous price increase over the past 10 years plus. That’s exactly what the authors found. So that is really not surprising. Analysis of drug spending changes over the time [shows that] it is indeed price increases that really have been driving increases in drug spending.”
Angus Worthing, MD, a rheumatologist at Arthritis & Rheumatism Associates in Washington, D.C., and current chair of the American College of Rheumatology’s Government Affairs Committee, said that the faster price hikes in Part D stood out in the analysis and the role PBMs played in it.
“I was a little surprised at just how much more PBMs were responsible for higher inflation, compared to the part B system,” he said in an interview. “In the McCormick article, drug prices went up 45% after rebates in the Part D space, but only 21% in the Part B space, and we had seen hints that drug prices were going up faster, we just didn’t know just how much faster, so more than double the increase is more than I expected, and it is very concerning to look at that and realize that PBMs are more than doubling an already high inflation rate in drug prices.”
He suggested that part of the rise in Part D bDMARD prices observed in the study might be because of the application of step therapy in Part D.
“In Medicare specifically, step therapy is allowed in Part D but not in Part B – except in Medicare Advantage plans starting in 2019 – and this important difference between Part B and Part D explains how Part D rebates, linked with step therapy, may be the main reason that bDMARD prices rose more in Part D compared to the open formulary system in Medicare Part B,” Dr. Worthing wrote in an editorial on Dr. McCormick’s study.
The Pharmaceutical Care Management Association, the lobby group representing PBMs, declined an interview request, but challenged the analysis’ portrayal of the role of PBMs in an email. It pointed to a Government Accountability Organization report from July 2019 that found that Part D rebates helped offset spending by about 20% and that PBM compensation was primarily derived from administrative fees and not from maintaining a portion of rebates that the PBM negotiated on behalf of the plan.
Dr. Worthing said in an interview that these bDMARD price hikes are causing access issues, particularly in Medicare where beneficiaries cannot take advantage of manufacturer programs, such as discount cards and copay coupons, and potentially face coinsurance payments based on the list price and not the net-of-rebate price.
From a policy standpoint, he advocated for a cap on out-of-pocket spending for Medicare Part D drugs, which is a feature of legislation (H.R. 3, Lower Drug Costs Now Act of 2019) that is moving through the House of Representatives, most recently passing the House Ways and Means Committee in a party-line vote of 24-17, with 1 Democrat not voting.
Dr. Worthing’s editorial covers a few other policy options, including more financial transparency in the role PBMs play in the drug supply chain, the elimination of rebates in favor of a flat fee for PBMs or requiring all rebates on drugs to be passed through to Part D beneficiaries, penalties on manufacturers for price increases that exceed inflation, and usage of a price index based on what foreign countries pay for the same drug.
Many of the suggestions from Dr. Worthing’s editorial are in H.R. 3 and face an uphill battle to get through the Senate.
Dr. Worthing said that tackling the rebate issue by removing incentives for manufacturers to raise prices in order to offer higher rebates and changing step-therapy rules are things Congress could probably get accomplished as it heads into an election year, but there is a chance it could be even more comprehensive.
“With such an important issue like high drug prices, it probably benefits both parties, and everybody facing an election will be able to go back to their constituents and say, ‘Look we got something done. I was part of this,’ and there is a better chance they will do it if they hear about it from their constituents,” he said.
Dr. Ballreich was less optimistic that H.R. 3 is going to be able to survive.
“The interesting thing with this bill is that a number of pieces of it have been endorsed by President Trump, and the question is, [is] that a meaningful endorsement? Is the Trump administration going to want to use the this plan as a win for him, as a win for showing potential voters that he can work in a bipartisan way, [that] he could take on ‘big drug companies’ – all those successful soundbites? Can then Trump use his power to push this bill through the Senate? I think that is a question mark,” he said, noting that it could take a lot of political capital to counter the powerful industry lobby. Ultimately, he expects that, if drug pricing does make it to the White House, it will go through some changes from where H.R. 3 is right now.
“I don’t think it will pass in its current iteration,” he said.
Each author of Dr. McCormick’s report had funding from different sources, though the funders had no role in the design and conduct of the study. No other disclosures were made.
SOURCES: McCormick N et al. Arthritis Rheumatol. 2019 Oct 14. doi: 10.1002/ART.41138; Worthing A. Arthritis Rheumatol. 2019 Oct 14. doi: 10.1002/art.41135.
Rheumatology drugs, like most drugs, are seeing significant price hikes in recent years and, according to a new analysis, those hikes account for the majority of growth in spending.
From 2012 to 2016, annual spending on public-payer claims for 10 biologic disease-modifying antirheumatic drugs (bDMARDs) more than doubled (from $3.8 billion to $8.6 billion), with median drug price increases of 51% in the Medicare Part D prescription drug plans (mean, 54%) and 8% within Medicare Part B drugs administered within the physician office (mean, 21%).
“The prominent bDMARD drug cost increases observed in this study, which were substantially greater under Part D than Part B, even when accounting for rebates, represent a growing burden to taxpayers and beneficiaries,” first author Natalie McCormick, PhD, a research fellow at Massachusetts General Hospital, Boston, and colleagues wrote in Arthritis & Rheumatology.
“The magnitude of these increases is independent from any assessment of value,” the authors continued.
Dr. McCormick and colleagues highlighted the limited effect that rebates negotiated by pharmacy benefit managers (PBMs) for Part D have on patient out-of-pocket costs.
Negotiations “may not necessarily result in lower price increases since pharmacy benefit managers, who negotiate on behalf of Part D plans, retain a percentage of the negotiated rebates as compensation, and higher list prices can achieve higher rebates,” they stated. “While these rebates accrue to insurance plans and pharmacy benefit managers, they do not directly impact Part D beneficiaries’ out-of-pocket costs, which are driven by prerebate prices. Thus, many patients end up paying higher out-of-pocket costs when the list price increases, creating financial barriers to use and adherence.”
They further noted that, for most Part D drugs individually, “drug prices accounted for approximately 50% or more of the increase in spending. Adalimumab and etanercept, two of the oldest bDMARDs, were prescribed to the largest number of Part D beneficiaries (more than 47,000 in 2016) and had the biggest 5-year price increases: 84% and 88%, respectively (72% and 75% respectively, accounting for rebates).”
It is not surprising that the analysis found that prices are rising and are a driving force in spending.
“We know that high launch prices of a new drug is an important consideration for patient access,” Jeromie Ballreich, PhD, a health economist at Johns Hopkins University, Baltimore, said in an interview. “But we also know that a number of these drugs, particularly Humira and Enbrel, have been on the market for a while. They have experienced a tremendous price increase over the past 10 years plus. That’s exactly what the authors found. So that is really not surprising. Analysis of drug spending changes over the time [shows that] it is indeed price increases that really have been driving increases in drug spending.”
Angus Worthing, MD, a rheumatologist at Arthritis & Rheumatism Associates in Washington, D.C., and current chair of the American College of Rheumatology’s Government Affairs Committee, said that the faster price hikes in Part D stood out in the analysis and the role PBMs played in it.
“I was a little surprised at just how much more PBMs were responsible for higher inflation, compared to the part B system,” he said in an interview. “In the McCormick article, drug prices went up 45% after rebates in the Part D space, but only 21% in the Part B space, and we had seen hints that drug prices were going up faster, we just didn’t know just how much faster, so more than double the increase is more than I expected, and it is very concerning to look at that and realize that PBMs are more than doubling an already high inflation rate in drug prices.”
He suggested that part of the rise in Part D bDMARD prices observed in the study might be because of the application of step therapy in Part D.
“In Medicare specifically, step therapy is allowed in Part D but not in Part B – except in Medicare Advantage plans starting in 2019 – and this important difference between Part B and Part D explains how Part D rebates, linked with step therapy, may be the main reason that bDMARD prices rose more in Part D compared to the open formulary system in Medicare Part B,” Dr. Worthing wrote in an editorial on Dr. McCormick’s study.
The Pharmaceutical Care Management Association, the lobby group representing PBMs, declined an interview request, but challenged the analysis’ portrayal of the role of PBMs in an email. It pointed to a Government Accountability Organization report from July 2019 that found that Part D rebates helped offset spending by about 20% and that PBM compensation was primarily derived from administrative fees and not from maintaining a portion of rebates that the PBM negotiated on behalf of the plan.
Dr. Worthing said in an interview that these bDMARD price hikes are causing access issues, particularly in Medicare where beneficiaries cannot take advantage of manufacturer programs, such as discount cards and copay coupons, and potentially face coinsurance payments based on the list price and not the net-of-rebate price.
From a policy standpoint, he advocated for a cap on out-of-pocket spending for Medicare Part D drugs, which is a feature of legislation (H.R. 3, Lower Drug Costs Now Act of 2019) that is moving through the House of Representatives, most recently passing the House Ways and Means Committee in a party-line vote of 24-17, with 1 Democrat not voting.
Dr. Worthing’s editorial covers a few other policy options, including more financial transparency in the role PBMs play in the drug supply chain, the elimination of rebates in favor of a flat fee for PBMs or requiring all rebates on drugs to be passed through to Part D beneficiaries, penalties on manufacturers for price increases that exceed inflation, and usage of a price index based on what foreign countries pay for the same drug.
Many of the suggestions from Dr. Worthing’s editorial are in H.R. 3 and face an uphill battle to get through the Senate.
Dr. Worthing said that tackling the rebate issue by removing incentives for manufacturers to raise prices in order to offer higher rebates and changing step-therapy rules are things Congress could probably get accomplished as it heads into an election year, but there is a chance it could be even more comprehensive.
“With such an important issue like high drug prices, it probably benefits both parties, and everybody facing an election will be able to go back to their constituents and say, ‘Look we got something done. I was part of this,’ and there is a better chance they will do it if they hear about it from their constituents,” he said.
Dr. Ballreich was less optimistic that H.R. 3 is going to be able to survive.
“The interesting thing with this bill is that a number of pieces of it have been endorsed by President Trump, and the question is, [is] that a meaningful endorsement? Is the Trump administration going to want to use the this plan as a win for him, as a win for showing potential voters that he can work in a bipartisan way, [that] he could take on ‘big drug companies’ – all those successful soundbites? Can then Trump use his power to push this bill through the Senate? I think that is a question mark,” he said, noting that it could take a lot of political capital to counter the powerful industry lobby. Ultimately, he expects that, if drug pricing does make it to the White House, it will go through some changes from where H.R. 3 is right now.
“I don’t think it will pass in its current iteration,” he said.
Each author of Dr. McCormick’s report had funding from different sources, though the funders had no role in the design and conduct of the study. No other disclosures were made.
SOURCES: McCormick N et al. Arthritis Rheumatol. 2019 Oct 14. doi: 10.1002/ART.41138; Worthing A. Arthritis Rheumatol. 2019 Oct 14. doi: 10.1002/art.41135.
FROM ARTHRITIS & RHEUMATOLOGY