User login
The “Shark Tank” winning innovation at the American Gastroenterological Association (AGA) Tech Summit in Chicago this April has “life-altering” potential for ostomy patients, according to one of the judges, and eliminates the need for constant pouch wear.
The innovation is called Twistomy and it is designed to replace current ostomy-pouch systems that can cause leaks, odor, skin irritation, embarrassment, and social and emotional distress. The AGA Committee for GI Innovation and Technology (CGIT) organizes the annual Tech Summit.
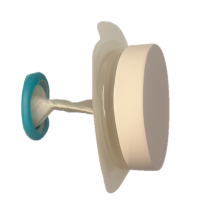
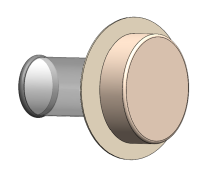
Twistomy’s winning design includes a flexible ring and sleeve, which are inserted into the stoma and secured on the outside with a set of rings that make up the housing unit attached to a standard wafer. The housing unit twists the sleeve closed, allowing the user to control fecal output. For evacuation, the user attaches a pouch, untwists the sleeve, evacuates cleanly and effectively, and then discards the pouch.
Twistomy cofounders Devon Horton, BS, senior bioengineer, and Lily Williams, BS, biomedical researcher and engineer, both work for the department of surgery at University of Colorado, Denver.
Horton said in an interview that when he was approached with the idea to create a better ostomy solution for a senior-year capstone project he was intrigued because the traditional ostomy system “has not changed in more than 70 years. It was crazy that no one had done anything to change that.”
The Twistomy team also won the Grand Prize this spring at the Emerging Medical Innovation Valuation Competition at the Design of Medical Devices Conference held at the University of Minnesota, Minneapolis.
Witnessing the Struggle as a CNA
Horton also works as a certified nursing assistant at an inpatient unit at University of Colorado Hospital and the ostomy patients he sees there every shift help drive his passion to find a better solution.
He hears the emotional stories of people who manage their ostomy daily.
“Many express feelings of depression and anxiety, feeling isolated with their severe inability to go out and do things because of the fear of the noise the stoma makes, or the crinkling of the plastic bag in a yoga class,” he said. “We want to help them regain that control of quality of life.”
They also hope to cut down on the ostomy management time. “Initial user testing [for Twistomy] was less than 75 seconds to insert and assemble,” he said. “I did an interview with a patient yesterday who said they probably spend an hour a day managing their ostomy,” including cleaning and replacing.
Horton and Williams have a patent on the device and currently use three-dimensional printing for the prototypes.
Williams said they are now conducting consumer discovery studies through the National Science Foundation and are interviewing 30 stakeholders — “anyone who has a relationship with an ostomy,” whether a colorectal surgeon, a gastrointestinal nurse, ostomy patients, or insurers.
Those interviews will help in refining the device so they can start consulting with manufacturers and work toward approval as a Class II medical device from the US Food and Drug Administration (FDA), Williams said.
Saving Healthcare Costs
Another potential benefit for Twistomy is its ability to cut healthcare costs, Horton said. Traditional ostomies are prone to leakage, which can lead to peristomal skin complications.
He pointed to a National Institutes of Health analysis that found that on average peristomal skin complications caused upwards of $80,000 more per ostomy patient in increased healthcare costs over a 3-month period than for those without the complications.
“With Twistomy, we are reducing leakage most likely to zero,” Horton said. “We set out to say if we could reduce [infections] by half or a little less than half, we can cut out those tens of thousands of dollars that insurance companies and payers are spending.”
Permanent and Temporary Ostomy Markets
He pointed out that not all ostomies are permanent ostomies, adding that the reversal rate “is about 65%.” Often those reversal surgeries cannot take place until peristomal skin complications have been healed.
“We’re not only hoping to market to the permanent stoma patients, but the patients with temporary stomas as well,” he said.
The team estimates it will need $4 million–$6 million in funding for manufacturing and consultation costs as well as costs involved in seeking FDA approval.
Horton and Williams project the housing unit cost will be $399 based on known out-of-pocket expenses for patients with ostomy care products and the unit would be replaced annually. Disposable elements would be an additional cost.
Assuming insurance acceptance of the product, he said, “With about an 80/20 insurance coverage, typical for many patients, it would be about $100 in out-of-pocket expenses per month to use our device, which is around the lower end of what a lot of patients are spending out of pocket.”
One of the Tech Summit judges, Somaya Albhaisi, MD, a gastroenterology/hepatology fellow at University of Southern California, Los Angeles, said in an interview that the Shark Tank results were unanimous among the five judges and Twistomy also took the fan favorite vote.
She said the teams were judged on quality of pitch, potential clinical impact, and feasibility of business plan. Teams got 5-7 minutes to pitch and answered questions afterward.
“Deep Understanding” of Patient Need
“They combined smart engineering with deep understanding of patient need, which is restoring control, dignity, and quality of life for ostomy users while also reducing healthcare costs. It is rare to see a solution this scalable and impactful. It was a deeply empathetic solution overall.” She noted that nearly 1 million people in the United States currently use an ostomy.
Ostomy users’ quality of life is compromised, and they often have mental health challenges, Albhaisi said. This innovation appears to offer easy use, more dignity and control.
The other four Shark Tank finalists were:
- AI Lumen, which developed a retroview camera system, which attaches to the colonoscope and enhances imaging to detect hidden polyps that may evade conventional endoscopes.
- Amplified Sciences, which developed an ultrasensitive diagnostic platform that detects biomarker activities in minute volumes of fluid from pancreatic cystic lesions, helping to stratify patients into low risk or potential malignancy, reducing unneeded surgeries, costs, and comorbidities.
- KITE Endoscopic Innovations, which designed the Dynaflex TruCut needle to offer a simpler endoscopic ultrasound (EUS)–guided biopsy procedure with fewer needle passes, deeper insights into tumor pathology, and more tissue for geonomic analysis.
- MicroSteer, which designed a device to facilitate semiautomated endoscopic submucosal dissection (ESD) by decoupling the dissecting knife from the endoscope, enhancing safety and effectiveness during the procedure.
The Twistomy Team “Surprised Everyone”
The competitors’ scores were “very close,” one of the judges, Kevin Berliner, said in an interview. “The Twistomy team surprised everyone — the judges and the crowd — with their succinct, informative, and impactful pitch. That presentation disparity was the tiebreaker for me,” said Berliner, who works for Medtronic, a sponsor of the competition, in Chicago.
He said Horton and Williams were the youngest presenters and had the earliest stage pitch they judged, but they “outpresented other competitors in clarity, simplification, and storytelling.”
Also impressive was their description of their “commercially viable path to success” and their plan for the challenges ahead, he said.
Those challenges to get Twistomy to market center “on the ongoing changing climate we have with research funds lately,” Horton said. “We’re giving it an estimate of 3-5 years.”
Horton, Williams, Albhaisi, and Berliner reported no relevant financial relationships.
The “Shark Tank” winning innovation at the American Gastroenterological Association (AGA) Tech Summit in Chicago this April has “life-altering” potential for ostomy patients, according to one of the judges, and eliminates the need for constant pouch wear.
The innovation is called Twistomy and it is designed to replace current ostomy-pouch systems that can cause leaks, odor, skin irritation, embarrassment, and social and emotional distress. The AGA Committee for GI Innovation and Technology (CGIT) organizes the annual Tech Summit.
Twistomy’s winning design includes a flexible ring and sleeve, which are inserted into the stoma and secured on the outside with a set of rings that make up the housing unit attached to a standard wafer. The housing unit twists the sleeve closed, allowing the user to control fecal output. For evacuation, the user attaches a pouch, untwists the sleeve, evacuates cleanly and effectively, and then discards the pouch.
Twistomy cofounders Devon Horton, BS, senior bioengineer, and Lily Williams, BS, biomedical researcher and engineer, both work for the department of surgery at University of Colorado, Denver.
Horton said in an interview that when he was approached with the idea to create a better ostomy solution for a senior-year capstone project he was intrigued because the traditional ostomy system “has not changed in more than 70 years. It was crazy that no one had done anything to change that.”
The Twistomy team also won the Grand Prize this spring at the Emerging Medical Innovation Valuation Competition at the Design of Medical Devices Conference held at the University of Minnesota, Minneapolis.
Witnessing the Struggle as a CNA
Horton also works as a certified nursing assistant at an inpatient unit at University of Colorado Hospital and the ostomy patients he sees there every shift help drive his passion to find a better solution.
He hears the emotional stories of people who manage their ostomy daily.
“Many express feelings of depression and anxiety, feeling isolated with their severe inability to go out and do things because of the fear of the noise the stoma makes, or the crinkling of the plastic bag in a yoga class,” he said. “We want to help them regain that control of quality of life.”
They also hope to cut down on the ostomy management time. “Initial user testing [for Twistomy] was less than 75 seconds to insert and assemble,” he said. “I did an interview with a patient yesterday who said they probably spend an hour a day managing their ostomy,” including cleaning and replacing.
Horton and Williams have a patent on the device and currently use three-dimensional printing for the prototypes.
Williams said they are now conducting consumer discovery studies through the National Science Foundation and are interviewing 30 stakeholders — “anyone who has a relationship with an ostomy,” whether a colorectal surgeon, a gastrointestinal nurse, ostomy patients, or insurers.
Those interviews will help in refining the device so they can start consulting with manufacturers and work toward approval as a Class II medical device from the US Food and Drug Administration (FDA), Williams said.
Saving Healthcare Costs
Another potential benefit for Twistomy is its ability to cut healthcare costs, Horton said. Traditional ostomies are prone to leakage, which can lead to peristomal skin complications.
He pointed to a National Institutes of Health analysis that found that on average peristomal skin complications caused upwards of $80,000 more per ostomy patient in increased healthcare costs over a 3-month period than for those without the complications.
“With Twistomy, we are reducing leakage most likely to zero,” Horton said. “We set out to say if we could reduce [infections] by half or a little less than half, we can cut out those tens of thousands of dollars that insurance companies and payers are spending.”
Permanent and Temporary Ostomy Markets
He pointed out that not all ostomies are permanent ostomies, adding that the reversal rate “is about 65%.” Often those reversal surgeries cannot take place until peristomal skin complications have been healed.
“We’re not only hoping to market to the permanent stoma patients, but the patients with temporary stomas as well,” he said.
The team estimates it will need $4 million–$6 million in funding for manufacturing and consultation costs as well as costs involved in seeking FDA approval.
Horton and Williams project the housing unit cost will be $399 based on known out-of-pocket expenses for patients with ostomy care products and the unit would be replaced annually. Disposable elements would be an additional cost.
Assuming insurance acceptance of the product, he said, “With about an 80/20 insurance coverage, typical for many patients, it would be about $100 in out-of-pocket expenses per month to use our device, which is around the lower end of what a lot of patients are spending out of pocket.”
One of the Tech Summit judges, Somaya Albhaisi, MD, a gastroenterology/hepatology fellow at University of Southern California, Los Angeles, said in an interview that the Shark Tank results were unanimous among the five judges and Twistomy also took the fan favorite vote.
She said the teams were judged on quality of pitch, potential clinical impact, and feasibility of business plan. Teams got 5-7 minutes to pitch and answered questions afterward.
“Deep Understanding” of Patient Need
“They combined smart engineering with deep understanding of patient need, which is restoring control, dignity, and quality of life for ostomy users while also reducing healthcare costs. It is rare to see a solution this scalable and impactful. It was a deeply empathetic solution overall.” She noted that nearly 1 million people in the United States currently use an ostomy.
Ostomy users’ quality of life is compromised, and they often have mental health challenges, Albhaisi said. This innovation appears to offer easy use, more dignity and control.
The other four Shark Tank finalists were:
- AI Lumen, which developed a retroview camera system, which attaches to the colonoscope and enhances imaging to detect hidden polyps that may evade conventional endoscopes.
- Amplified Sciences, which developed an ultrasensitive diagnostic platform that detects biomarker activities in minute volumes of fluid from pancreatic cystic lesions, helping to stratify patients into low risk or potential malignancy, reducing unneeded surgeries, costs, and comorbidities.
- KITE Endoscopic Innovations, which designed the Dynaflex TruCut needle to offer a simpler endoscopic ultrasound (EUS)–guided biopsy procedure with fewer needle passes, deeper insights into tumor pathology, and more tissue for geonomic analysis.
- MicroSteer, which designed a device to facilitate semiautomated endoscopic submucosal dissection (ESD) by decoupling the dissecting knife from the endoscope, enhancing safety and effectiveness during the procedure.
The Twistomy Team “Surprised Everyone”
The competitors’ scores were “very close,” one of the judges, Kevin Berliner, said in an interview. “The Twistomy team surprised everyone — the judges and the crowd — with their succinct, informative, and impactful pitch. That presentation disparity was the tiebreaker for me,” said Berliner, who works for Medtronic, a sponsor of the competition, in Chicago.
He said Horton and Williams were the youngest presenters and had the earliest stage pitch they judged, but they “outpresented other competitors in clarity, simplification, and storytelling.”
Also impressive was their description of their “commercially viable path to success” and their plan for the challenges ahead, he said.
Those challenges to get Twistomy to market center “on the ongoing changing climate we have with research funds lately,” Horton said. “We’re giving it an estimate of 3-5 years.”
Horton, Williams, Albhaisi, and Berliner reported no relevant financial relationships.
The “Shark Tank” winning innovation at the American Gastroenterological Association (AGA) Tech Summit in Chicago this April has “life-altering” potential for ostomy patients, according to one of the judges, and eliminates the need for constant pouch wear.
The innovation is called Twistomy and it is designed to replace current ostomy-pouch systems that can cause leaks, odor, skin irritation, embarrassment, and social and emotional distress. The AGA Committee for GI Innovation and Technology (CGIT) organizes the annual Tech Summit.
Twistomy’s winning design includes a flexible ring and sleeve, which are inserted into the stoma and secured on the outside with a set of rings that make up the housing unit attached to a standard wafer. The housing unit twists the sleeve closed, allowing the user to control fecal output. For evacuation, the user attaches a pouch, untwists the sleeve, evacuates cleanly and effectively, and then discards the pouch.
Twistomy cofounders Devon Horton, BS, senior bioengineer, and Lily Williams, BS, biomedical researcher and engineer, both work for the department of surgery at University of Colorado, Denver.
Horton said in an interview that when he was approached with the idea to create a better ostomy solution for a senior-year capstone project he was intrigued because the traditional ostomy system “has not changed in more than 70 years. It was crazy that no one had done anything to change that.”
The Twistomy team also won the Grand Prize this spring at the Emerging Medical Innovation Valuation Competition at the Design of Medical Devices Conference held at the University of Minnesota, Minneapolis.
Witnessing the Struggle as a CNA
Horton also works as a certified nursing assistant at an inpatient unit at University of Colorado Hospital and the ostomy patients he sees there every shift help drive his passion to find a better solution.
He hears the emotional stories of people who manage their ostomy daily.
“Many express feelings of depression and anxiety, feeling isolated with their severe inability to go out and do things because of the fear of the noise the stoma makes, or the crinkling of the plastic bag in a yoga class,” he said. “We want to help them regain that control of quality of life.”
They also hope to cut down on the ostomy management time. “Initial user testing [for Twistomy] was less than 75 seconds to insert and assemble,” he said. “I did an interview with a patient yesterday who said they probably spend an hour a day managing their ostomy,” including cleaning and replacing.
Horton and Williams have a patent on the device and currently use three-dimensional printing for the prototypes.
Williams said they are now conducting consumer discovery studies through the National Science Foundation and are interviewing 30 stakeholders — “anyone who has a relationship with an ostomy,” whether a colorectal surgeon, a gastrointestinal nurse, ostomy patients, or insurers.
Those interviews will help in refining the device so they can start consulting with manufacturers and work toward approval as a Class II medical device from the US Food and Drug Administration (FDA), Williams said.
Saving Healthcare Costs
Another potential benefit for Twistomy is its ability to cut healthcare costs, Horton said. Traditional ostomies are prone to leakage, which can lead to peristomal skin complications.
He pointed to a National Institutes of Health analysis that found that on average peristomal skin complications caused upwards of $80,000 more per ostomy patient in increased healthcare costs over a 3-month period than for those without the complications.
“With Twistomy, we are reducing leakage most likely to zero,” Horton said. “We set out to say if we could reduce [infections] by half or a little less than half, we can cut out those tens of thousands of dollars that insurance companies and payers are spending.”
Permanent and Temporary Ostomy Markets
He pointed out that not all ostomies are permanent ostomies, adding that the reversal rate “is about 65%.” Often those reversal surgeries cannot take place until peristomal skin complications have been healed.
“We’re not only hoping to market to the permanent stoma patients, but the patients with temporary stomas as well,” he said.
The team estimates it will need $4 million–$6 million in funding for manufacturing and consultation costs as well as costs involved in seeking FDA approval.
Horton and Williams project the housing unit cost will be $399 based on known out-of-pocket expenses for patients with ostomy care products and the unit would be replaced annually. Disposable elements would be an additional cost.
Assuming insurance acceptance of the product, he said, “With about an 80/20 insurance coverage, typical for many patients, it would be about $100 in out-of-pocket expenses per month to use our device, which is around the lower end of what a lot of patients are spending out of pocket.”
One of the Tech Summit judges, Somaya Albhaisi, MD, a gastroenterology/hepatology fellow at University of Southern California, Los Angeles, said in an interview that the Shark Tank results were unanimous among the five judges and Twistomy also took the fan favorite vote.
She said the teams were judged on quality of pitch, potential clinical impact, and feasibility of business plan. Teams got 5-7 minutes to pitch and answered questions afterward.
“Deep Understanding” of Patient Need
“They combined smart engineering with deep understanding of patient need, which is restoring control, dignity, and quality of life for ostomy users while also reducing healthcare costs. It is rare to see a solution this scalable and impactful. It was a deeply empathetic solution overall.” She noted that nearly 1 million people in the United States currently use an ostomy.
Ostomy users’ quality of life is compromised, and they often have mental health challenges, Albhaisi said. This innovation appears to offer easy use, more dignity and control.
The other four Shark Tank finalists were:
- AI Lumen, which developed a retroview camera system, which attaches to the colonoscope and enhances imaging to detect hidden polyps that may evade conventional endoscopes.
- Amplified Sciences, which developed an ultrasensitive diagnostic platform that detects biomarker activities in minute volumes of fluid from pancreatic cystic lesions, helping to stratify patients into low risk or potential malignancy, reducing unneeded surgeries, costs, and comorbidities.
- KITE Endoscopic Innovations, which designed the Dynaflex TruCut needle to offer a simpler endoscopic ultrasound (EUS)–guided biopsy procedure with fewer needle passes, deeper insights into tumor pathology, and more tissue for geonomic analysis.
- MicroSteer, which designed a device to facilitate semiautomated endoscopic submucosal dissection (ESD) by decoupling the dissecting knife from the endoscope, enhancing safety and effectiveness during the procedure.
The Twistomy Team “Surprised Everyone”
The competitors’ scores were “very close,” one of the judges, Kevin Berliner, said in an interview. “The Twistomy team surprised everyone — the judges and the crowd — with their succinct, informative, and impactful pitch. That presentation disparity was the tiebreaker for me,” said Berliner, who works for Medtronic, a sponsor of the competition, in Chicago.
He said Horton and Williams were the youngest presenters and had the earliest stage pitch they judged, but they “outpresented other competitors in clarity, simplification, and storytelling.”
Also impressive was their description of their “commercially viable path to success” and their plan for the challenges ahead, he said.
Those challenges to get Twistomy to market center “on the ongoing changing climate we have with research funds lately,” Horton said. “We’re giving it an estimate of 3-5 years.”
Horton, Williams, Albhaisi, and Berliner reported no relevant financial relationships.