User login
The US Food and Drug Administration’s (FDA) Oncologic Drugs Advisory Committee (ODAC) has unanimously recommended approval for the chimeric antigen receptor (CAR) T-cell therapy CTL019 (tisagenlecleucel).
The committee voted 10 to 0 in favor of approving CTL019 for the treatment of pediatric and young adult patients (ages 3 to 25) with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL).
The FDA will consider this vote as it reviews the biologics license application (BLA) for CTL019, but the agency is not obligated to follow the ODAC’s recommendation.
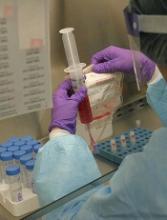
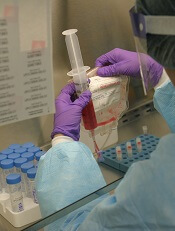
The BLA for CTL019 is supported by results from 3 trials.
This includes a pilot study, which was presented at the 2015 ASH Annual Meeting; the phase 2 ENSIGN trial, which was presented at the 2016 ASH Annual Meeting; and the phase 2 ELIANA study, which was recently presented at the 22nd Congress of the European Hematology Association (EHA).
ELIANA enrolled 88 patients with relapsed/refractory B-cell ALL, and 68 of them received CTL019.
Nine patients did not receive CTL019 due to death or adverse events, 7 patients were affected by manufacturing failures, and 4 patients were pending infusion at last follow-up.
Most of the infused patients (n=65) received lymphodepleting chemotherapy prior to CTL019 (single dose). The median dose was 3.0 × 106 (range, 0.2-5.4 × 106) transduced CTL019 cells/kg.
Sixty-three patients were evaluable for efficacy.
The overall response rate—complete response (CR) plus CR with incomplete hematologic recovery (CRi)—was 83% (52/63). All patients with CR/CRis were minimal residual disease-negative in the bone marrow.
Sixty-eight patients were evaluated for safety.
Serious adverse events occurred in 69% of patients. These included life-threatening cytokine release syndrome (CRS) and hemophagocytic lymphohistiocytosis, neurological events that occurred with CRS or after CRS was resolved, coagulopathies with CRS, and life-threatening infections.
Seventy-eight percent of patients had CRS—21% with grade 3 and 27% with grade 4 CRS. There were no deaths from CRS.
Forty-four percent of patients had neurological toxicities—15% grade 3 or higher. These included encephalopathy, delirium, hallucinations, somnolence, cognitive disorder, seizure, depressed level of consciousness, mental status changes, dysphagia, muscular weakness, and dysarthria.
Severe infectious complications occurred in 26% of patients, and 3 patients died of such complications.
Eleven patients died after receiving CTL019—7 due to ALL, 1 from cerebral hemorrhage, 1 from encephalitis, 1 from a respiratory tract infection, and 1 from systemic mycosis. ![]()
The US Food and Drug Administration’s (FDA) Oncologic Drugs Advisory Committee (ODAC) has unanimously recommended approval for the chimeric antigen receptor (CAR) T-cell therapy CTL019 (tisagenlecleucel).
The committee voted 10 to 0 in favor of approving CTL019 for the treatment of pediatric and young adult patients (ages 3 to 25) with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL).
The FDA will consider this vote as it reviews the biologics license application (BLA) for CTL019, but the agency is not obligated to follow the ODAC’s recommendation.
The BLA for CTL019 is supported by results from 3 trials.
This includes a pilot study, which was presented at the 2015 ASH Annual Meeting; the phase 2 ENSIGN trial, which was presented at the 2016 ASH Annual Meeting; and the phase 2 ELIANA study, which was recently presented at the 22nd Congress of the European Hematology Association (EHA).
ELIANA enrolled 88 patients with relapsed/refractory B-cell ALL, and 68 of them received CTL019.
Nine patients did not receive CTL019 due to death or adverse events, 7 patients were affected by manufacturing failures, and 4 patients were pending infusion at last follow-up.
Most of the infused patients (n=65) received lymphodepleting chemotherapy prior to CTL019 (single dose). The median dose was 3.0 × 106 (range, 0.2-5.4 × 106) transduced CTL019 cells/kg.
Sixty-three patients were evaluable for efficacy.
The overall response rate—complete response (CR) plus CR with incomplete hematologic recovery (CRi)—was 83% (52/63). All patients with CR/CRis were minimal residual disease-negative in the bone marrow.
Sixty-eight patients were evaluated for safety.
Serious adverse events occurred in 69% of patients. These included life-threatening cytokine release syndrome (CRS) and hemophagocytic lymphohistiocytosis, neurological events that occurred with CRS or after CRS was resolved, coagulopathies with CRS, and life-threatening infections.
Seventy-eight percent of patients had CRS—21% with grade 3 and 27% with grade 4 CRS. There were no deaths from CRS.
Forty-four percent of patients had neurological toxicities—15% grade 3 or higher. These included encephalopathy, delirium, hallucinations, somnolence, cognitive disorder, seizure, depressed level of consciousness, mental status changes, dysphagia, muscular weakness, and dysarthria.
Severe infectious complications occurred in 26% of patients, and 3 patients died of such complications.
Eleven patients died after receiving CTL019—7 due to ALL, 1 from cerebral hemorrhage, 1 from encephalitis, 1 from a respiratory tract infection, and 1 from systemic mycosis. ![]()
The US Food and Drug Administration’s (FDA) Oncologic Drugs Advisory Committee (ODAC) has unanimously recommended approval for the chimeric antigen receptor (CAR) T-cell therapy CTL019 (tisagenlecleucel).
The committee voted 10 to 0 in favor of approving CTL019 for the treatment of pediatric and young adult patients (ages 3 to 25) with relapsed or refractory B-cell acute lymphoblastic leukemia (ALL).
The FDA will consider this vote as it reviews the biologics license application (BLA) for CTL019, but the agency is not obligated to follow the ODAC’s recommendation.
The BLA for CTL019 is supported by results from 3 trials.
This includes a pilot study, which was presented at the 2015 ASH Annual Meeting; the phase 2 ENSIGN trial, which was presented at the 2016 ASH Annual Meeting; and the phase 2 ELIANA study, which was recently presented at the 22nd Congress of the European Hematology Association (EHA).
ELIANA enrolled 88 patients with relapsed/refractory B-cell ALL, and 68 of them received CTL019.
Nine patients did not receive CTL019 due to death or adverse events, 7 patients were affected by manufacturing failures, and 4 patients were pending infusion at last follow-up.
Most of the infused patients (n=65) received lymphodepleting chemotherapy prior to CTL019 (single dose). The median dose was 3.0 × 106 (range, 0.2-5.4 × 106) transduced CTL019 cells/kg.
Sixty-three patients were evaluable for efficacy.
The overall response rate—complete response (CR) plus CR with incomplete hematologic recovery (CRi)—was 83% (52/63). All patients with CR/CRis were minimal residual disease-negative in the bone marrow.
Sixty-eight patients were evaluated for safety.
Serious adverse events occurred in 69% of patients. These included life-threatening cytokine release syndrome (CRS) and hemophagocytic lymphohistiocytosis, neurological events that occurred with CRS or after CRS was resolved, coagulopathies with CRS, and life-threatening infections.
Seventy-eight percent of patients had CRS—21% with grade 3 and 27% with grade 4 CRS. There were no deaths from CRS.
Forty-four percent of patients had neurological toxicities—15% grade 3 or higher. These included encephalopathy, delirium, hallucinations, somnolence, cognitive disorder, seizure, depressed level of consciousness, mental status changes, dysphagia, muscular weakness, and dysarthria.
Severe infectious complications occurred in 26% of patients, and 3 patients died of such complications.
Eleven patients died after receiving CTL019—7 due to ALL, 1 from cerebral hemorrhage, 1 from encephalitis, 1 from a respiratory tract infection, and 1 from systemic mycosis. ![]()