User login
A novel device has shown a high rate of accuracy in predicting which patients with obstructive sleep apnea (OSA) will improve with oral appliance therapy, according to a study.
“At the present time CPAP is our go-to standard medical therapy [for treating OSA]. While it is a wonderful therapy, it has a very serious drawback, which is poor compliance, and that undercuts its long-term effectiveness in reducing the incidence of cardiovascular disease,” said John E. Remmers, MD, the principal investigator, in an interview.
Referring to the Sleep Apnea Cardiovascular Endpoints (SAVE) trial’s finding that continuous positive airway pressure (CPAP) did not reduce long-term cardiovascular incidents, he claimed that “these incidents are not being reduced by CPAP, because people don’t use it” (N Engl J Med. 2016 Sept 8;375[10]:919-31).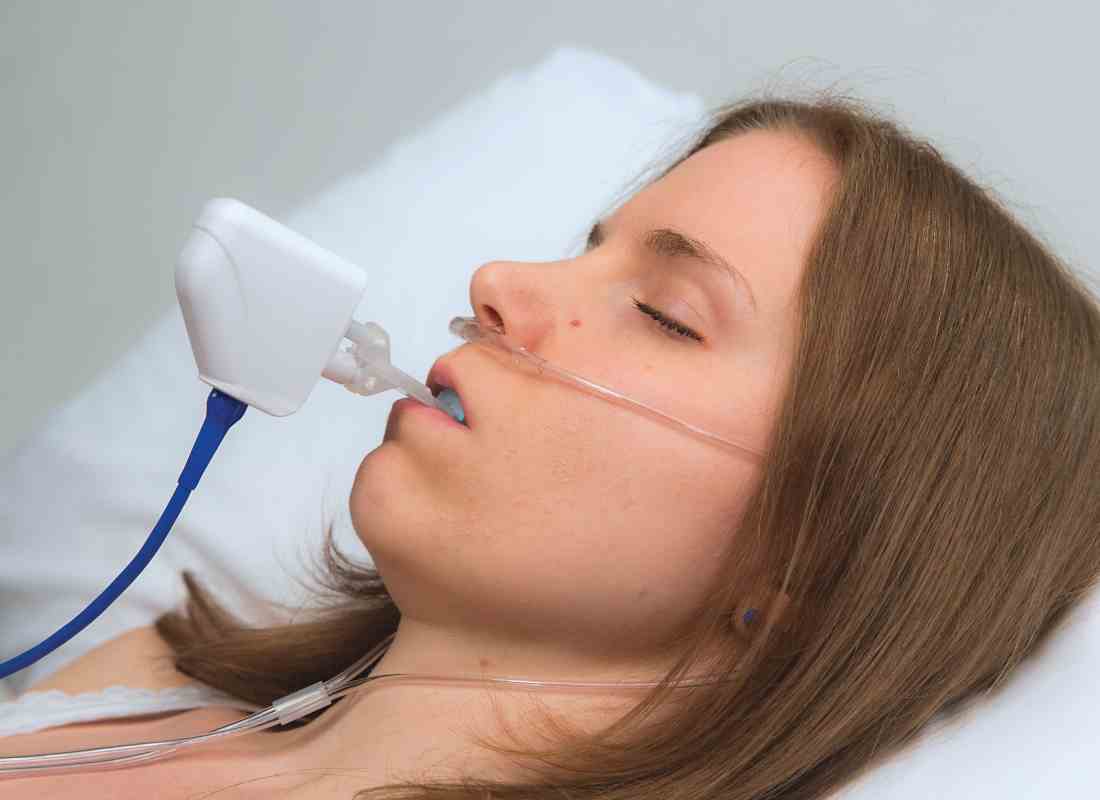
In Dr. Remmers’ new two-part study, 202 adults – primarily overweight, middle-aged men, diagnosed with moderate sleep apnea – were divided into two groups. The first included 149 people who were given a two-night, in-home, feedback controlled mandibular positioner (FCMP) test, using equipment manufactured by Zephyr Sleep Technologies. In this test, a custom-fit oral appliance is simulated using a temporary set of trays and impression material. The trays are connected to a small motor controlled by a little computer that sits on the stomach and moves the mandible when the patient has a problem breathing.
All patients received a custom oral appliance designed using data acquired from the test. The patients then wore the custom oral appliances while connected to a validated monitor as an outcomes study.
Finally, the researchers fed all of the data they collected from this first group of patients into a machine learning model. Then the second set of patients participated in the testing. Outcomes data on the appliance’s performance in each individual in the first group were used to create a classification system to predict therapeutic outcomes for the 53 patients in the second group. The patients in the second group then received their custom oral appliances, connected to the same type of monitor used by the first group.
Therapeutic success or failure was defined as having mean oxygen desaturation index values of less than or greater than 10 events/hour, respectively. The investigators determined that the test had an 85% sensitivity level with 93% specificity, a positive predictive value of 97%, and a negative predictive value of 72%. Of those who were predicted to respond to therapy, the mandibular protrusive position was efficacious in 86% of patients.
The high rate of accuracy for predicting who will derive the most benefit from the appliance, along with the demonstrated preference for oral appliances compared to continuous positive airway pressure devices among patients, increases the clinical utility of the appliance, and expands options for clinical management of sleep apnea, according to the study authors (Clin Sleep Med. 2017;13[7]:871-80).
“Our test allows the physician to prescribe the therapy knowing it will get rid of sleep apnea, and it tells the dentist how far the mandible needs to be pulled out by the custom fit device,” Dr. Remmers explained.
Dentists will also benefit from the test, because it allows them to make an appliance that will not need to be adjusted and will have a higher success rate than the current 60% success rate that oral appliances have at treating sleep apnea, he noted.
“This opens up a new an alternative clinical avenue at a critical time, when we have just learned over the past few years that there are serious questions about the effectiveness of CPAP in the long term,” Dr. Remmers added. “[With oral appliance therapy] you have an opportunity for higher compliance, because people prefer the less obtrusive oral appliance therapy over CPAP, and they use it more than CPAP. ... Because our product says you don’t treat everybody, you only undertake oral appliance therapy for those who we know in advance will have a favorable outcome, it removes a major barrier to oral appliance therapy that has been the barrier for many years.”
Dr. Remmers noted that his test was not nearly as good at identifying people who would be failures as it was at identifying people who would be successes and that he is carrying out another trial with a similar device.
Some participants reported sore gums when using the device, but there were no long-lasting adverse events reported.
The mandibular positioner home test has not been approved or cleared for use by the Food and Drug Administration, but is currently being sold in Canada, according to Dr. Remmers.
Zephyr Sleep Technologies and Alberta Innovates Technology Futures sponsored the study. It is registered on clinicaltrials.gov as NCT03011762. All of the investigators, other than Nikola Vranjes, are employed or associated with Zephyr Sleep Technologies.
Whitney McKnight contributed to this report.
A novel device has shown a high rate of accuracy in predicting which patients with obstructive sleep apnea (OSA) will improve with oral appliance therapy, according to a study.
“At the present time CPAP is our go-to standard medical therapy [for treating OSA]. While it is a wonderful therapy, it has a very serious drawback, which is poor compliance, and that undercuts its long-term effectiveness in reducing the incidence of cardiovascular disease,” said John E. Remmers, MD, the principal investigator, in an interview.
Referring to the Sleep Apnea Cardiovascular Endpoints (SAVE) trial’s finding that continuous positive airway pressure (CPAP) did not reduce long-term cardiovascular incidents, he claimed that “these incidents are not being reduced by CPAP, because people don’t use it” (N Engl J Med. 2016 Sept 8;375[10]:919-31).
In Dr. Remmers’ new two-part study, 202 adults – primarily overweight, middle-aged men, diagnosed with moderate sleep apnea – were divided into two groups. The first included 149 people who were given a two-night, in-home, feedback controlled mandibular positioner (FCMP) test, using equipment manufactured by Zephyr Sleep Technologies. In this test, a custom-fit oral appliance is simulated using a temporary set of trays and impression material. The trays are connected to a small motor controlled by a little computer that sits on the stomach and moves the mandible when the patient has a problem breathing.
All patients received a custom oral appliance designed using data acquired from the test. The patients then wore the custom oral appliances while connected to a validated monitor as an outcomes study.
Finally, the researchers fed all of the data they collected from this first group of patients into a machine learning model. Then the second set of patients participated in the testing. Outcomes data on the appliance’s performance in each individual in the first group were used to create a classification system to predict therapeutic outcomes for the 53 patients in the second group. The patients in the second group then received their custom oral appliances, connected to the same type of monitor used by the first group.
Therapeutic success or failure was defined as having mean oxygen desaturation index values of less than or greater than 10 events/hour, respectively. The investigators determined that the test had an 85% sensitivity level with 93% specificity, a positive predictive value of 97%, and a negative predictive value of 72%. Of those who were predicted to respond to therapy, the mandibular protrusive position was efficacious in 86% of patients.
The high rate of accuracy for predicting who will derive the most benefit from the appliance, along with the demonstrated preference for oral appliances compared to continuous positive airway pressure devices among patients, increases the clinical utility of the appliance, and expands options for clinical management of sleep apnea, according to the study authors (Clin Sleep Med. 2017;13[7]:871-80).
“Our test allows the physician to prescribe the therapy knowing it will get rid of sleep apnea, and it tells the dentist how far the mandible needs to be pulled out by the custom fit device,” Dr. Remmers explained.
Dentists will also benefit from the test, because it allows them to make an appliance that will not need to be adjusted and will have a higher success rate than the current 60% success rate that oral appliances have at treating sleep apnea, he noted.
“This opens up a new an alternative clinical avenue at a critical time, when we have just learned over the past few years that there are serious questions about the effectiveness of CPAP in the long term,” Dr. Remmers added. “[With oral appliance therapy] you have an opportunity for higher compliance, because people prefer the less obtrusive oral appliance therapy over CPAP, and they use it more than CPAP. ... Because our product says you don’t treat everybody, you only undertake oral appliance therapy for those who we know in advance will have a favorable outcome, it removes a major barrier to oral appliance therapy that has been the barrier for many years.”
Dr. Remmers noted that his test was not nearly as good at identifying people who would be failures as it was at identifying people who would be successes and that he is carrying out another trial with a similar device.
Some participants reported sore gums when using the device, but there were no long-lasting adverse events reported.
The mandibular positioner home test has not been approved or cleared for use by the Food and Drug Administration, but is currently being sold in Canada, according to Dr. Remmers.
Zephyr Sleep Technologies and Alberta Innovates Technology Futures sponsored the study. It is registered on clinicaltrials.gov as NCT03011762. All of the investigators, other than Nikola Vranjes, are employed or associated with Zephyr Sleep Technologies.
Whitney McKnight contributed to this report.
A novel device has shown a high rate of accuracy in predicting which patients with obstructive sleep apnea (OSA) will improve with oral appliance therapy, according to a study.
“At the present time CPAP is our go-to standard medical therapy [for treating OSA]. While it is a wonderful therapy, it has a very serious drawback, which is poor compliance, and that undercuts its long-term effectiveness in reducing the incidence of cardiovascular disease,” said John E. Remmers, MD, the principal investigator, in an interview.
Referring to the Sleep Apnea Cardiovascular Endpoints (SAVE) trial’s finding that continuous positive airway pressure (CPAP) did not reduce long-term cardiovascular incidents, he claimed that “these incidents are not being reduced by CPAP, because people don’t use it” (N Engl J Med. 2016 Sept 8;375[10]:919-31).
In Dr. Remmers’ new two-part study, 202 adults – primarily overweight, middle-aged men, diagnosed with moderate sleep apnea – were divided into two groups. The first included 149 people who were given a two-night, in-home, feedback controlled mandibular positioner (FCMP) test, using equipment manufactured by Zephyr Sleep Technologies. In this test, a custom-fit oral appliance is simulated using a temporary set of trays and impression material. The trays are connected to a small motor controlled by a little computer that sits on the stomach and moves the mandible when the patient has a problem breathing.
All patients received a custom oral appliance designed using data acquired from the test. The patients then wore the custom oral appliances while connected to a validated monitor as an outcomes study.
Finally, the researchers fed all of the data they collected from this first group of patients into a machine learning model. Then the second set of patients participated in the testing. Outcomes data on the appliance’s performance in each individual in the first group were used to create a classification system to predict therapeutic outcomes for the 53 patients in the second group. The patients in the second group then received their custom oral appliances, connected to the same type of monitor used by the first group.
Therapeutic success or failure was defined as having mean oxygen desaturation index values of less than or greater than 10 events/hour, respectively. The investigators determined that the test had an 85% sensitivity level with 93% specificity, a positive predictive value of 97%, and a negative predictive value of 72%. Of those who were predicted to respond to therapy, the mandibular protrusive position was efficacious in 86% of patients.
The high rate of accuracy for predicting who will derive the most benefit from the appliance, along with the demonstrated preference for oral appliances compared to continuous positive airway pressure devices among patients, increases the clinical utility of the appliance, and expands options for clinical management of sleep apnea, according to the study authors (Clin Sleep Med. 2017;13[7]:871-80).
“Our test allows the physician to prescribe the therapy knowing it will get rid of sleep apnea, and it tells the dentist how far the mandible needs to be pulled out by the custom fit device,” Dr. Remmers explained.
Dentists will also benefit from the test, because it allows them to make an appliance that will not need to be adjusted and will have a higher success rate than the current 60% success rate that oral appliances have at treating sleep apnea, he noted.
“This opens up a new an alternative clinical avenue at a critical time, when we have just learned over the past few years that there are serious questions about the effectiveness of CPAP in the long term,” Dr. Remmers added. “[With oral appliance therapy] you have an opportunity for higher compliance, because people prefer the less obtrusive oral appliance therapy over CPAP, and they use it more than CPAP. ... Because our product says you don’t treat everybody, you only undertake oral appliance therapy for those who we know in advance will have a favorable outcome, it removes a major barrier to oral appliance therapy that has been the barrier for many years.”
Dr. Remmers noted that his test was not nearly as good at identifying people who would be failures as it was at identifying people who would be successes and that he is carrying out another trial with a similar device.
Some participants reported sore gums when using the device, but there were no long-lasting adverse events reported.
The mandibular positioner home test has not been approved or cleared for use by the Food and Drug Administration, but is currently being sold in Canada, according to Dr. Remmers.
Zephyr Sleep Technologies and Alberta Innovates Technology Futures sponsored the study. It is registered on clinicaltrials.gov as NCT03011762. All of the investigators, other than Nikola Vranjes, are employed or associated with Zephyr Sleep Technologies.
Whitney McKnight contributed to this report.
