User login
Potentially fatal neuroleptic malignant syndrome (NMS)—though less common than in the past—can happen with either conventional or atypical antipsychotics.1,2 To help you protect patients when prescribing antipsychotics or consulting with other clinicians about these drugs, this article discusses:
- risk factors and clinical features that warn of NMS onset
- differential diagnosis of disease states most often confused with NMS
- management recommendations, including supportive measures and specific interventions such as benzodiazepines, dopamine agonists, dantrolene, and electroconvulsive therapy (ECT).
WHY NMS REMAINS RELEVANT
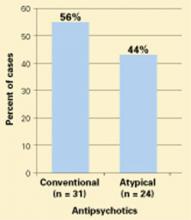
NMS remains a risk in susceptible patients receiving atypical antipsychotics, according to clinical reports and drug adverse event surveys (Figure).2
Moreover, NMS continues to be reported with conventional antipsychotics, which remain in widespread use. Patients who receive long-acting depot conventional antipsychotics are at risk for prolonged NMS episodes.
Figure 55 NMS cases reported with use of antipsychotics, 1998-2002
Probable or definite neuroleptic malignant syndrome cases associated with antipsychotic monotherapy reported to the Neuroleptic Malignant Syndrome Information Service.NMS in medical settings. Psychiatrists may be consulted when patients develop NMS while receiving conventional antipsychotics or other dopaminereceptor antagonists used in medical settings.3,4 Haloperidol remains the recommended drug of choice for treating agitation and delirium and continues to be the single most common trigger of NMS. Although often overlooked, antiemetics and sedatives with neuroleptic properties—such as prochlorperazine, metoclopramide, and promethazine—also have triggered NMS.
Other hyperthermic conditions. NMS is often considered in the differential diagnosis of patients who develop fever or encephalopathy while being treated with psychotropics. In these acute, complex, and often grave situations, psychiatrists may be consulted to recommend treatment for behavioral control or to distinguish NMS from other conditions.
NEWER VS. OLDER ANTIPSYCHOTICS
Has NMS incidence declined with the atypical agents? Probably, but providing proof is difficult:
- NMS is uncommon; its incidence in psychiatric patients treated with conventional antipsychotics is approximately 0.2%.5 To demonstrate reduced NMS incidence with atypicals, a very large sample of patients would be required to reach statistical significance.
- As doctors have used lower doses of conventional agents—which reduces the risk of NMS—any beneficial impact from atypicals has become more difficult to detect.5,6
- Reports of NMS frequency with atypicals may be inflated by bias in publishing adverse events with newer versus older agents.
- Patients switched to atypicals may represent a high-risk group that is intolerant or resistant to conventional antipsychotics.
So far, few unequivocal cases of NMS have been attributed to the use of quetiapine, ziprasidone, or aripiprazole, the most recently introduced atypicals. Moreover, case reports of NMS associated with clozapine, risperidone, or olanzapine2 are often difficult to interpret because of incomplete clinical details, varying diagnostic criteria, and concomitant use of more than one antipsychotic.
Milder NMS? Do the newer antipsychotics produce an “atypical” or milder form of NMS? Case reports indicate that extreme temperature elevations and extrapyramidal dysfunction are less frequent in NMS associated with atypical compared with conventional antipsychotics.2 However, case descriptions of NMS were heterogeneous even with conventional agents, and clinicians’ growing awareness of NMS even before atypicals were introduced allowed for earlier diagnosis of mild and partial NMS cases.7
CLINICAL FEATURES OF NMS
Regardless of drug selection, it is important to recognize early and mild signs of NMS. Any case can progress to a fulminant form that is more difficult to treat.
Patients at risk. NMS may be more likely to develop in patients with:5,6,8
- dehydration
- agitation
- low serum iron
- underlying brain damage
- catatonia.
Some patients may have genetic abnormalities in central dopamine systems that increase their susceptibility to NMS.6,9
Fifteen to 20% of patients who develop NMS have experienced a previous episode while taking antipsychotics, which is why taking a careful drug history is important.5,6 Although most often reported with therapeutic antipsychotic doses, NMS has been associated with rapid dose titration, especially when given parenterally.8
On the other hand, the practical value of these risk factors is often limited in individual cases and may lead one to overestimate NMS risk. NMS is rare and idiosyncratic. Risk factors may not outweigh antipsychotics’ benefits when these drugs are indicated for a patient with psychosis.
Incipient NMS. Identifying early signs of NMS may be impossible in fulminant cases, but patients with incipient NMS may show:
- unexpected mental status changes
- new-onset catatonia
- refractory extrapyramidal and bulbar signs such as rigidity, dysphagia, or dysarthria.5,7,10
Other clues to NMS onset include tachycardia, tachypnea, and elevated temperature or serum creatine phosphokinase (CPK). These signs, however, do not precede or progress to NMS in all cases. A high index of suspicion for NMS, tempered by sound clinical judgment, is called for when assessing all patients receiving antipsychotics.
Diagnostic criteria. Clinical signs of NMS as a fullblown hypermetabolic syndrome are distinctive and well described (Table 1).5,6,11,12 Elevated temperature is accompanied by profuse sweating. Extreme temperatures (>104° F), especially if prolonged or associated with hypoxia or hypotension, pose a high risk for brain damage, rhabdomyolysis, disseminated intravascular coagulation, multisystem organ failure, and death.
Muscle rigidity is a characteristic finding and may be accompanied by tremors, cogwheeling, myoclonus, or rhabdomyolysis. Changes in vital signs—such as tachycardia and tachypnea—are typical.
Mental status examination usually reveals catatonic signs of mutism and stupor, but delirium and coma also have been described. No laboratory findings are specific for NMS, but elevated white blood cell counts, low serum iron, metabolic acidosis, hypoxia, and elevated serum CPK and catecholamines have been reported.
Table 1
Common clinical features of NMS
| Signs and symptoms | Altered level of consciousness, catatonia, dysarthria, dysphagia, elevated temperature, labile blood pressure, muscle rigidity, mutism, myoclonus, tachycardia, tachypnea, tremor |
| Laboratory findings | Elevated catecholamines and serum creatine phosphokinase, hypoxia, leukocytosis, low serum iron, metabolic acidosis |
Resolution. If recognized promptly, NMS resolves within 1 to 2 weeks in two-thirds of patients after antipsychotics are discontinued. The average recovery time of 7 to 10 days may be prolonged in patients who were taking long-acting depot antipsychotics or in those with persistent residual catatonic symptoms.13
Risk of death. NMS remains potentially fatal, especially if high temperatures develop or episodes are prolonged. Causes of death include cardiorespiratory arrest, renal failure, pulmonary emboli, pneumonia, sepsis, disseminated intravascular coagulation, and multisystem organ failure.
DIFFERENTIAL DIAGNOSIS
Differential diagnosis of NMS encompasses disorders that present with fever and encephalopathy.5,6,12 Primary brain disorders that resemble NMS include:9,14-16
- infections
- acute psychotic disorders that progress to malignant catatonia or delirious mania
- midbrain structural lesions
- seizures.
Also exclude hormonal and autoimmune disorders and environmental heatstroke (Table 2).17,18 Similar hyperthermic syndromes have been reported with other toxins and drugs, including malignant hyperthermia of anesthesia, serotonin syndrome, and dopamine agonist withdrawal in patients with Parkinson’s disease (Table 3).5,6,19
Table 2
7 disease states most often confused with NMS
| Infections |
| Malignant catatonia secondary to psychotic disorders |
| Benign extrapyramidal side effects |
| Agitated delirium from diverse causes |
| Environmental heatstroke |
| Serotonin syndrome |
| Withdrawal from dopamine agonists, other drugs, or alcohol |
| Source: Neuroleptic Malignant Syndrome Information Service hotline |
Table 3
Drugs that can cause NMS-like hyperthermic syndromes
| Anticholinergics Dopamine antagonists Hallucinogens Inhalational anesthetics Monoamine oxidase inhibitors Psychostimulants Salicylates | Serotonergic drugs Succinylcholine Withdrawal from: • Dopamine agonists • Alcohol • Sedative/hypnotics • Baclofen |
MANAGING NMS
The standard approach to managing patients with NMS includes four steps:
- recognize the diagnosis early
- exclude alternate causes of symptoms
- discontinue suspected triggering drugs
- provide supportive care to reduce temperatures, ensure fluid balance, and detect complications.5,6,20
Beyond supportive care, several specific therapies have been proposed based on theoretical mechanisms of NMS and meta-analyses of offlabel use in anecdotal clinical reports (Table 4). If benzodiazepines, dopamine agonists, or dantrolene are effective, taper slowly after recovery to prevent rebound symptoms.
Benzodiazepines. Given the concept of NMS as a form of catatonia, benzodiazepines have been used effectively in some cases.20,21 A trial of lorazepam, 1 to 2 mg parenterally, is a reasonable first step. Higher doses may be required, with adequate monitoring of respiratory status. Oral lorazepam can maintain the therapeutic effect.
Dopamine agonists. To reverse the parkinsonism and dopamine antagonist properties of antipsychotics, dopamine agonists such as bromocriptine or amantadine have been tried and have reduced NMS duration and mortality.20,22,23 Newer drugs such as ropinorole and pramipexole may also be useful. Dopaminergic drugs, however, can worsen psychosis and cause hypotension and emesis.
Dantrolene may reduce hyperthermia related to skeletal muscle hypermetabolism of any cause and has been effective in rapidly reducing extreme temperatures in some NMS cases.20,22-26 Dantrolene is given IV, 1 to 2.5 mg/kg every 6 hours. An oral form can be substituted if a response is obtained. Dantrolene can impair respiratory and hepatic function and should not be combined with calcium channel blockers.
ECT is increasingly recognized as an effective NMS treatment and should not be overlooked for patients:
Standard ECT is given, although nondepolarizing muscle relaxants instead of succinylcholine are used in patients with serious rhabdomyolysis to avoid the risk of hyperkalemia.20
Recommendation. Although these modalities offer a spectrum of therapeutic options, it is premature to recommend any single remedy over others or over supportive care alone because:
- randomized, controlled trials have not been conducted
- NMS episodes are heterogeneous in presentation and outcome
- the syndrome is often self-limited after antipsychotics are discontinued.
I recommend that you choose therapies empirically, based on the character, severity, and duration of symptoms in a given case.5,6,20
Table 4
How to treat neuroleptic malignant syndrome
| General measures | Diagnose early, discontinue antipsychotic, provide supportive care |
| Specific interventions under investigation | |
| Benzodiazepines | Parenteral lorazepam, 1 to 2 mg or higher; monitor respiratory status |
| Dopamine agonists | Bromocriptine, 2.5 mg every 8 hours or amantadine, 100 mg every 8 hours; monitor psychosis, blood pressure, nausea |
| Dantrolene | 1 to 2.5 mg/kg IV every 6 hours; monitor respiratory and hepatic function; avoid calcium channel blockers |
| ECT | Standard administration; avoid succinylcholine in patients with rhabdomyolysis |
REDUCING RISK OF RECURRENCE
Patients vary in susceptibility to recurrence after they recover from NMS, but the risk approaches 30% with future exposure to antipsychotics.5,6 You may be able to minimize recurrence risk by:
- reducing risk factors, such as dehydration
- considering alternatives to antipsychotics, such as treating bipolar disorder with lithium or ECT
- using atypical instead of conventional antipsychotics, starting with low dosages and titrating slowly.
Before you reintroduce antipsychotics, carefully document informed consent and your rationale for treatment decisions in the patient’s chart.
Related resources
- Neuroleptic Malignant Syndrome Information Service. Hotline for health professionals. (888) 667-8367. www.nmsis.org
- Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing, 2003.
- Caroff SN, Mann SC, Francis A, Fricchione GL. Catatonia: from psychopathology to neurobiology. Washington, DC: American Psychiatric Publishing (in press).
Drug brand names
- Amantadine • Symmetrel
- Aripiprazole • Abilify
- Bromocriptine • Parlodel
- Clozapine • Clozaril
- Dantrolene • Dantrium
- Haloperidol • Haldol
- Lorazepam • Ativan
- Metoclopramide • Reglan
- Olanzapine • Zyprexa
- Pramipexole • Mirapex
- Prochlorperazine • Compazine
- Promethazine • Phenergan
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ropinirole • Requip
- Ziprasidone • Geodon
Disclosure
Dr. Caroff receives research support from Janssen Pharmaceutica and Pfizer Inc., and is a consultant to Eli Lilly and Co. and Bristol-Myers Squibb Co.
1. Caroff SN, Mann SC, Campbell EC, Sullivan KA. Movement disorders associated with atypical antipsychotic drugs. J Clin Psychiatry 2002;63(suppl 4):12-19.
2. Caroff SN, Mann SC, Campbell EC. Atypical antipsychotics and neuroleptic malignant syndrome. Psychiatric Annals 2000;30(5):314-21.
3. Caroff SN, Rosenberg H, Mann SC, et al. Neuroleptic malignant syndrome in the perioperative setting. Am J Anesthesiology 2001;28(8):387-93.
4. Caroff SN, Rosenberg H, Mann SC, et al. Neuroleptic malignant syndrome in the critical care unit. Crit Care Med 2002;30(11):2609.-
5. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77(1):185-202.
6. Caroff SN. Neuroleptic malignant syndrome. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A (eds). Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing Inc., 2003;1-44.
7. Velamoor VR, Swamy GN, Parmar RS, et al. Management of suspected neuroleptic malignant syndrome. Can J Psychiatry 1995;40(9):545-50.
8. Keck PE, Jr, Pope HG, Jr, Cohen BM, et al. Risk factors for neuroleptic malignant syndrome. Arch Gen Psychiatry 1989;46:914-18.
9. Mann SC. Malignant catatonia. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A (eds). Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing, 2003;121-43.
10. Velamoor VR, Norman RMG, Caroff SN, et al. Progression of symptoms in neuroleptic malignant syndrome. J Nerv Ment Dis 1994;182(3):168-73.
11. Delay J, Pichot P, Lemperiere T, et al. Un neuroleptique majeur non-phenothiazine et non reserpinique, l’haloperidol, dans le traitement des psychoses. Annales Medico-Psychologique 1960;118(1):145-52.
12. Caroff SN. The neuroleptic malignant syndrome. J Clin Psychiatry 1980;41(3):79-83.
13. Caroff SN, Mann SC, Keck PE, Jr, Francis A. Residual catatonic state following neuroleptic malignant syndrome. J Clin Psychopharmacol 2000;20(2):257-9.
14. Caroff SN, Mann SC, McCarthy M, et al. Acute infectious encephalitis complicated by neuroleptic malignant syndrome. J Clin Psychopharmacol 1998;18(4):349-51.
15. Caroff SN, Mann SC, Gliatto MF, et al. Psychiatric manifestations of acute viral encephalitis. Psychiatric Annals 2001;31(3):193-204.
16. Caroff SN, Mann SC, Francis A, Fricchione GL (eds). Catatonia: from psychopathology to neurobiology. Washington, DC: American Psychiatric Publishing (in press).
17. Mann SC. Thermoregulatory mechanisms and antipsychotic drugrelated heatstroke. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A (eds). Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing, 2003;45-74.
18. Caroff SN, Mann SC, Campbell EC. Risk of fatal heatstroke after hospitalization. Psychiatric Serv 2000;51(7):938.-
19. Keck PE, Jr. Serotonin syndrome. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A (eds). Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing, 2003;75-92.
20. Davis JM, Caroff SN, Mann SC. Treatment of neuroleptic malignant syndrome. Psychiatric Annals 2000;30(5):325-31.
21. Francis A, Chandragiri S, Rizvi S, et al. Lorazepam a treatment for neuroleptic malignant syndrome? CNS Spectrums 2000;5(7):54-7.
22. Sakkas P, Davis JM, Hua J, Wang Z. Pharmacotherapy of neuroleptic malignant syndrome. Psychiatr Ann 1991;21:157-64.
23. Rosenberg MR, Green M. Neuroleptic malignant syndrome: review of response to therapy. Arch Intern Med 1989;149:1927-31.
24. Henderson A, Longdon P. Fulminant metoclopramide-induced neuroleptic malignant syndrome rapidly responsive to intravenous dantrolene. Aust N Z J Med 1991;21:742-3.
25. Yamawaki S, Morio M, Kazamatsuri G, et al. Clinical evaluation and effective usage of dantrolene sodium in neuroleptic malignant syndrome. Kiso to Rinsyou (Clinical Reports) 1993;27(3):1045-66.
26. Tsutsumi Y, Yamamoto K, Matsuura S, et al. The treatment of neuroleptic malignant syndrome using dantrolene sodium. Psychiatry Clin Neurosci 1998;52:433-8.
27. Nisijima K, Ishiguro T. Electroconvulsive therapy for the treatment of neuroleptic malignant syndrome: a report of five cases. J ECT 1999;15:158-63.
28. Troller JN, Sachdev PS. Electroconvulsive treatment of neuroleptic malignant syndrome: a review and report of cases. Aust N Z J Psychiatry 1999;33:650-9.
Potentially fatal neuroleptic malignant syndrome (NMS)—though less common than in the past—can happen with either conventional or atypical antipsychotics.1,2 To help you protect patients when prescribing antipsychotics or consulting with other clinicians about these drugs, this article discusses:
- risk factors and clinical features that warn of NMS onset
- differential diagnosis of disease states most often confused with NMS
- management recommendations, including supportive measures and specific interventions such as benzodiazepines, dopamine agonists, dantrolene, and electroconvulsive therapy (ECT).
WHY NMS REMAINS RELEVANT
NMS remains a risk in susceptible patients receiving atypical antipsychotics, according to clinical reports and drug adverse event surveys (Figure).2
Moreover, NMS continues to be reported with conventional antipsychotics, which remain in widespread use. Patients who receive long-acting depot conventional antipsychotics are at risk for prolonged NMS episodes.
Figure 55 NMS cases reported with use of antipsychotics, 1998-2002
Probable or definite neuroleptic malignant syndrome cases associated with antipsychotic monotherapy reported to the Neuroleptic Malignant Syndrome Information Service.NMS in medical settings. Psychiatrists may be consulted when patients develop NMS while receiving conventional antipsychotics or other dopaminereceptor antagonists used in medical settings.3,4 Haloperidol remains the recommended drug of choice for treating agitation and delirium and continues to be the single most common trigger of NMS. Although often overlooked, antiemetics and sedatives with neuroleptic properties—such as prochlorperazine, metoclopramide, and promethazine—also have triggered NMS.
Other hyperthermic conditions. NMS is often considered in the differential diagnosis of patients who develop fever or encephalopathy while being treated with psychotropics. In these acute, complex, and often grave situations, psychiatrists may be consulted to recommend treatment for behavioral control or to distinguish NMS from other conditions.
NEWER VS. OLDER ANTIPSYCHOTICS
Has NMS incidence declined with the atypical agents? Probably, but providing proof is difficult:
- NMS is uncommon; its incidence in psychiatric patients treated with conventional antipsychotics is approximately 0.2%.5 To demonstrate reduced NMS incidence with atypicals, a very large sample of patients would be required to reach statistical significance.
- As doctors have used lower doses of conventional agents—which reduces the risk of NMS—any beneficial impact from atypicals has become more difficult to detect.5,6
- Reports of NMS frequency with atypicals may be inflated by bias in publishing adverse events with newer versus older agents.
- Patients switched to atypicals may represent a high-risk group that is intolerant or resistant to conventional antipsychotics.
So far, few unequivocal cases of NMS have been attributed to the use of quetiapine, ziprasidone, or aripiprazole, the most recently introduced atypicals. Moreover, case reports of NMS associated with clozapine, risperidone, or olanzapine2 are often difficult to interpret because of incomplete clinical details, varying diagnostic criteria, and concomitant use of more than one antipsychotic.
Milder NMS? Do the newer antipsychotics produce an “atypical” or milder form of NMS? Case reports indicate that extreme temperature elevations and extrapyramidal dysfunction are less frequent in NMS associated with atypical compared with conventional antipsychotics.2 However, case descriptions of NMS were heterogeneous even with conventional agents, and clinicians’ growing awareness of NMS even before atypicals were introduced allowed for earlier diagnosis of mild and partial NMS cases.7
CLINICAL FEATURES OF NMS
Regardless of drug selection, it is important to recognize early and mild signs of NMS. Any case can progress to a fulminant form that is more difficult to treat.
Patients at risk. NMS may be more likely to develop in patients with:5,6,8
- dehydration
- agitation
- low serum iron
- underlying brain damage
- catatonia.
Some patients may have genetic abnormalities in central dopamine systems that increase their susceptibility to NMS.6,9
Fifteen to 20% of patients who develop NMS have experienced a previous episode while taking antipsychotics, which is why taking a careful drug history is important.5,6 Although most often reported with therapeutic antipsychotic doses, NMS has been associated with rapid dose titration, especially when given parenterally.8
On the other hand, the practical value of these risk factors is often limited in individual cases and may lead one to overestimate NMS risk. NMS is rare and idiosyncratic. Risk factors may not outweigh antipsychotics’ benefits when these drugs are indicated for a patient with psychosis.
Incipient NMS. Identifying early signs of NMS may be impossible in fulminant cases, but patients with incipient NMS may show:
- unexpected mental status changes
- new-onset catatonia
- refractory extrapyramidal and bulbar signs such as rigidity, dysphagia, or dysarthria.5,7,10
Other clues to NMS onset include tachycardia, tachypnea, and elevated temperature or serum creatine phosphokinase (CPK). These signs, however, do not precede or progress to NMS in all cases. A high index of suspicion for NMS, tempered by sound clinical judgment, is called for when assessing all patients receiving antipsychotics.
Diagnostic criteria. Clinical signs of NMS as a fullblown hypermetabolic syndrome are distinctive and well described (Table 1).5,6,11,12 Elevated temperature is accompanied by profuse sweating. Extreme temperatures (>104° F), especially if prolonged or associated with hypoxia or hypotension, pose a high risk for brain damage, rhabdomyolysis, disseminated intravascular coagulation, multisystem organ failure, and death.
Muscle rigidity is a characteristic finding and may be accompanied by tremors, cogwheeling, myoclonus, or rhabdomyolysis. Changes in vital signs—such as tachycardia and tachypnea—are typical.
Mental status examination usually reveals catatonic signs of mutism and stupor, but delirium and coma also have been described. No laboratory findings are specific for NMS, but elevated white blood cell counts, low serum iron, metabolic acidosis, hypoxia, and elevated serum CPK and catecholamines have been reported.
Table 1
Common clinical features of NMS
| Signs and symptoms | Altered level of consciousness, catatonia, dysarthria, dysphagia, elevated temperature, labile blood pressure, muscle rigidity, mutism, myoclonus, tachycardia, tachypnea, tremor |
| Laboratory findings | Elevated catecholamines and serum creatine phosphokinase, hypoxia, leukocytosis, low serum iron, metabolic acidosis |
Resolution. If recognized promptly, NMS resolves within 1 to 2 weeks in two-thirds of patients after antipsychotics are discontinued. The average recovery time of 7 to 10 days may be prolonged in patients who were taking long-acting depot antipsychotics or in those with persistent residual catatonic symptoms.13
Risk of death. NMS remains potentially fatal, especially if high temperatures develop or episodes are prolonged. Causes of death include cardiorespiratory arrest, renal failure, pulmonary emboli, pneumonia, sepsis, disseminated intravascular coagulation, and multisystem organ failure.
DIFFERENTIAL DIAGNOSIS
Differential diagnosis of NMS encompasses disorders that present with fever and encephalopathy.5,6,12 Primary brain disorders that resemble NMS include:9,14-16
- infections
- acute psychotic disorders that progress to malignant catatonia or delirious mania
- midbrain structural lesions
- seizures.
Also exclude hormonal and autoimmune disorders and environmental heatstroke (Table 2).17,18 Similar hyperthermic syndromes have been reported with other toxins and drugs, including malignant hyperthermia of anesthesia, serotonin syndrome, and dopamine agonist withdrawal in patients with Parkinson’s disease (Table 3).5,6,19
Table 2
7 disease states most often confused with NMS
| Infections |
| Malignant catatonia secondary to psychotic disorders |
| Benign extrapyramidal side effects |
| Agitated delirium from diverse causes |
| Environmental heatstroke |
| Serotonin syndrome |
| Withdrawal from dopamine agonists, other drugs, or alcohol |
| Source: Neuroleptic Malignant Syndrome Information Service hotline |
Table 3
Drugs that can cause NMS-like hyperthermic syndromes
| Anticholinergics Dopamine antagonists Hallucinogens Inhalational anesthetics Monoamine oxidase inhibitors Psychostimulants Salicylates | Serotonergic drugs Succinylcholine Withdrawal from: • Dopamine agonists • Alcohol • Sedative/hypnotics • Baclofen |
MANAGING NMS
The standard approach to managing patients with NMS includes four steps:
- recognize the diagnosis early
- exclude alternate causes of symptoms
- discontinue suspected triggering drugs
- provide supportive care to reduce temperatures, ensure fluid balance, and detect complications.5,6,20
Beyond supportive care, several specific therapies have been proposed based on theoretical mechanisms of NMS and meta-analyses of offlabel use in anecdotal clinical reports (Table 4). If benzodiazepines, dopamine agonists, or dantrolene are effective, taper slowly after recovery to prevent rebound symptoms.
Benzodiazepines. Given the concept of NMS as a form of catatonia, benzodiazepines have been used effectively in some cases.20,21 A trial of lorazepam, 1 to 2 mg parenterally, is a reasonable first step. Higher doses may be required, with adequate monitoring of respiratory status. Oral lorazepam can maintain the therapeutic effect.
Dopamine agonists. To reverse the parkinsonism and dopamine antagonist properties of antipsychotics, dopamine agonists such as bromocriptine or amantadine have been tried and have reduced NMS duration and mortality.20,22,23 Newer drugs such as ropinorole and pramipexole may also be useful. Dopaminergic drugs, however, can worsen psychosis and cause hypotension and emesis.
Dantrolene may reduce hyperthermia related to skeletal muscle hypermetabolism of any cause and has been effective in rapidly reducing extreme temperatures in some NMS cases.20,22-26 Dantrolene is given IV, 1 to 2.5 mg/kg every 6 hours. An oral form can be substituted if a response is obtained. Dantrolene can impair respiratory and hepatic function and should not be combined with calcium channel blockers.
ECT is increasingly recognized as an effective NMS treatment and should not be overlooked for patients:
Standard ECT is given, although nondepolarizing muscle relaxants instead of succinylcholine are used in patients with serious rhabdomyolysis to avoid the risk of hyperkalemia.20
Recommendation. Although these modalities offer a spectrum of therapeutic options, it is premature to recommend any single remedy over others or over supportive care alone because:
- randomized, controlled trials have not been conducted
- NMS episodes are heterogeneous in presentation and outcome
- the syndrome is often self-limited after antipsychotics are discontinued.
I recommend that you choose therapies empirically, based on the character, severity, and duration of symptoms in a given case.5,6,20
Table 4
How to treat neuroleptic malignant syndrome
| General measures | Diagnose early, discontinue antipsychotic, provide supportive care |
| Specific interventions under investigation | |
| Benzodiazepines | Parenteral lorazepam, 1 to 2 mg or higher; monitor respiratory status |
| Dopamine agonists | Bromocriptine, 2.5 mg every 8 hours or amantadine, 100 mg every 8 hours; monitor psychosis, blood pressure, nausea |
| Dantrolene | 1 to 2.5 mg/kg IV every 6 hours; monitor respiratory and hepatic function; avoid calcium channel blockers |
| ECT | Standard administration; avoid succinylcholine in patients with rhabdomyolysis |
REDUCING RISK OF RECURRENCE
Patients vary in susceptibility to recurrence after they recover from NMS, but the risk approaches 30% with future exposure to antipsychotics.5,6 You may be able to minimize recurrence risk by:
- reducing risk factors, such as dehydration
- considering alternatives to antipsychotics, such as treating bipolar disorder with lithium or ECT
- using atypical instead of conventional antipsychotics, starting with low dosages and titrating slowly.
Before you reintroduce antipsychotics, carefully document informed consent and your rationale for treatment decisions in the patient’s chart.
Related resources
- Neuroleptic Malignant Syndrome Information Service. Hotline for health professionals. (888) 667-8367. www.nmsis.org
- Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing, 2003.
- Caroff SN, Mann SC, Francis A, Fricchione GL. Catatonia: from psychopathology to neurobiology. Washington, DC: American Psychiatric Publishing (in press).
Drug brand names
- Amantadine • Symmetrel
- Aripiprazole • Abilify
- Bromocriptine • Parlodel
- Clozapine • Clozaril
- Dantrolene • Dantrium
- Haloperidol • Haldol
- Lorazepam • Ativan
- Metoclopramide • Reglan
- Olanzapine • Zyprexa
- Pramipexole • Mirapex
- Prochlorperazine • Compazine
- Promethazine • Phenergan
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ropinirole • Requip
- Ziprasidone • Geodon
Disclosure
Dr. Caroff receives research support from Janssen Pharmaceutica and Pfizer Inc., and is a consultant to Eli Lilly and Co. and Bristol-Myers Squibb Co.
Potentially fatal neuroleptic malignant syndrome (NMS)—though less common than in the past—can happen with either conventional or atypical antipsychotics.1,2 To help you protect patients when prescribing antipsychotics or consulting with other clinicians about these drugs, this article discusses:
- risk factors and clinical features that warn of NMS onset
- differential diagnosis of disease states most often confused with NMS
- management recommendations, including supportive measures and specific interventions such as benzodiazepines, dopamine agonists, dantrolene, and electroconvulsive therapy (ECT).
WHY NMS REMAINS RELEVANT
NMS remains a risk in susceptible patients receiving atypical antipsychotics, according to clinical reports and drug adverse event surveys (Figure).2
Moreover, NMS continues to be reported with conventional antipsychotics, which remain in widespread use. Patients who receive long-acting depot conventional antipsychotics are at risk for prolonged NMS episodes.
Figure 55 NMS cases reported with use of antipsychotics, 1998-2002
Probable or definite neuroleptic malignant syndrome cases associated with antipsychotic monotherapy reported to the Neuroleptic Malignant Syndrome Information Service.NMS in medical settings. Psychiatrists may be consulted when patients develop NMS while receiving conventional antipsychotics or other dopaminereceptor antagonists used in medical settings.3,4 Haloperidol remains the recommended drug of choice for treating agitation and delirium and continues to be the single most common trigger of NMS. Although often overlooked, antiemetics and sedatives with neuroleptic properties—such as prochlorperazine, metoclopramide, and promethazine—also have triggered NMS.
Other hyperthermic conditions. NMS is often considered in the differential diagnosis of patients who develop fever or encephalopathy while being treated with psychotropics. In these acute, complex, and often grave situations, psychiatrists may be consulted to recommend treatment for behavioral control or to distinguish NMS from other conditions.
NEWER VS. OLDER ANTIPSYCHOTICS
Has NMS incidence declined with the atypical agents? Probably, but providing proof is difficult:
- NMS is uncommon; its incidence in psychiatric patients treated with conventional antipsychotics is approximately 0.2%.5 To demonstrate reduced NMS incidence with atypicals, a very large sample of patients would be required to reach statistical significance.
- As doctors have used lower doses of conventional agents—which reduces the risk of NMS—any beneficial impact from atypicals has become more difficult to detect.5,6
- Reports of NMS frequency with atypicals may be inflated by bias in publishing adverse events with newer versus older agents.
- Patients switched to atypicals may represent a high-risk group that is intolerant or resistant to conventional antipsychotics.
So far, few unequivocal cases of NMS have been attributed to the use of quetiapine, ziprasidone, or aripiprazole, the most recently introduced atypicals. Moreover, case reports of NMS associated with clozapine, risperidone, or olanzapine2 are often difficult to interpret because of incomplete clinical details, varying diagnostic criteria, and concomitant use of more than one antipsychotic.
Milder NMS? Do the newer antipsychotics produce an “atypical” or milder form of NMS? Case reports indicate that extreme temperature elevations and extrapyramidal dysfunction are less frequent in NMS associated with atypical compared with conventional antipsychotics.2 However, case descriptions of NMS were heterogeneous even with conventional agents, and clinicians’ growing awareness of NMS even before atypicals were introduced allowed for earlier diagnosis of mild and partial NMS cases.7
CLINICAL FEATURES OF NMS
Regardless of drug selection, it is important to recognize early and mild signs of NMS. Any case can progress to a fulminant form that is more difficult to treat.
Patients at risk. NMS may be more likely to develop in patients with:5,6,8
- dehydration
- agitation
- low serum iron
- underlying brain damage
- catatonia.
Some patients may have genetic abnormalities in central dopamine systems that increase their susceptibility to NMS.6,9
Fifteen to 20% of patients who develop NMS have experienced a previous episode while taking antipsychotics, which is why taking a careful drug history is important.5,6 Although most often reported with therapeutic antipsychotic doses, NMS has been associated with rapid dose titration, especially when given parenterally.8
On the other hand, the practical value of these risk factors is often limited in individual cases and may lead one to overestimate NMS risk. NMS is rare and idiosyncratic. Risk factors may not outweigh antipsychotics’ benefits when these drugs are indicated for a patient with psychosis.
Incipient NMS. Identifying early signs of NMS may be impossible in fulminant cases, but patients with incipient NMS may show:
- unexpected mental status changes
- new-onset catatonia
- refractory extrapyramidal and bulbar signs such as rigidity, dysphagia, or dysarthria.5,7,10
Other clues to NMS onset include tachycardia, tachypnea, and elevated temperature or serum creatine phosphokinase (CPK). These signs, however, do not precede or progress to NMS in all cases. A high index of suspicion for NMS, tempered by sound clinical judgment, is called for when assessing all patients receiving antipsychotics.
Diagnostic criteria. Clinical signs of NMS as a fullblown hypermetabolic syndrome are distinctive and well described (Table 1).5,6,11,12 Elevated temperature is accompanied by profuse sweating. Extreme temperatures (>104° F), especially if prolonged or associated with hypoxia or hypotension, pose a high risk for brain damage, rhabdomyolysis, disseminated intravascular coagulation, multisystem organ failure, and death.
Muscle rigidity is a characteristic finding and may be accompanied by tremors, cogwheeling, myoclonus, or rhabdomyolysis. Changes in vital signs—such as tachycardia and tachypnea—are typical.
Mental status examination usually reveals catatonic signs of mutism and stupor, but delirium and coma also have been described. No laboratory findings are specific for NMS, but elevated white blood cell counts, low serum iron, metabolic acidosis, hypoxia, and elevated serum CPK and catecholamines have been reported.
Table 1
Common clinical features of NMS
| Signs and symptoms | Altered level of consciousness, catatonia, dysarthria, dysphagia, elevated temperature, labile blood pressure, muscle rigidity, mutism, myoclonus, tachycardia, tachypnea, tremor |
| Laboratory findings | Elevated catecholamines and serum creatine phosphokinase, hypoxia, leukocytosis, low serum iron, metabolic acidosis |
Resolution. If recognized promptly, NMS resolves within 1 to 2 weeks in two-thirds of patients after antipsychotics are discontinued. The average recovery time of 7 to 10 days may be prolonged in patients who were taking long-acting depot antipsychotics or in those with persistent residual catatonic symptoms.13
Risk of death. NMS remains potentially fatal, especially if high temperatures develop or episodes are prolonged. Causes of death include cardiorespiratory arrest, renal failure, pulmonary emboli, pneumonia, sepsis, disseminated intravascular coagulation, and multisystem organ failure.
DIFFERENTIAL DIAGNOSIS
Differential diagnosis of NMS encompasses disorders that present with fever and encephalopathy.5,6,12 Primary brain disorders that resemble NMS include:9,14-16
- infections
- acute psychotic disorders that progress to malignant catatonia or delirious mania
- midbrain structural lesions
- seizures.
Also exclude hormonal and autoimmune disorders and environmental heatstroke (Table 2).17,18 Similar hyperthermic syndromes have been reported with other toxins and drugs, including malignant hyperthermia of anesthesia, serotonin syndrome, and dopamine agonist withdrawal in patients with Parkinson’s disease (Table 3).5,6,19
Table 2
7 disease states most often confused with NMS
| Infections |
| Malignant catatonia secondary to psychotic disorders |
| Benign extrapyramidal side effects |
| Agitated delirium from diverse causes |
| Environmental heatstroke |
| Serotonin syndrome |
| Withdrawal from dopamine agonists, other drugs, or alcohol |
| Source: Neuroleptic Malignant Syndrome Information Service hotline |
Table 3
Drugs that can cause NMS-like hyperthermic syndromes
| Anticholinergics Dopamine antagonists Hallucinogens Inhalational anesthetics Monoamine oxidase inhibitors Psychostimulants Salicylates | Serotonergic drugs Succinylcholine Withdrawal from: • Dopamine agonists • Alcohol • Sedative/hypnotics • Baclofen |
MANAGING NMS
The standard approach to managing patients with NMS includes four steps:
- recognize the diagnosis early
- exclude alternate causes of symptoms
- discontinue suspected triggering drugs
- provide supportive care to reduce temperatures, ensure fluid balance, and detect complications.5,6,20
Beyond supportive care, several specific therapies have been proposed based on theoretical mechanisms of NMS and meta-analyses of offlabel use in anecdotal clinical reports (Table 4). If benzodiazepines, dopamine agonists, or dantrolene are effective, taper slowly after recovery to prevent rebound symptoms.
Benzodiazepines. Given the concept of NMS as a form of catatonia, benzodiazepines have been used effectively in some cases.20,21 A trial of lorazepam, 1 to 2 mg parenterally, is a reasonable first step. Higher doses may be required, with adequate monitoring of respiratory status. Oral lorazepam can maintain the therapeutic effect.
Dopamine agonists. To reverse the parkinsonism and dopamine antagonist properties of antipsychotics, dopamine agonists such as bromocriptine or amantadine have been tried and have reduced NMS duration and mortality.20,22,23 Newer drugs such as ropinorole and pramipexole may also be useful. Dopaminergic drugs, however, can worsen psychosis and cause hypotension and emesis.
Dantrolene may reduce hyperthermia related to skeletal muscle hypermetabolism of any cause and has been effective in rapidly reducing extreme temperatures in some NMS cases.20,22-26 Dantrolene is given IV, 1 to 2.5 mg/kg every 6 hours. An oral form can be substituted if a response is obtained. Dantrolene can impair respiratory and hepatic function and should not be combined with calcium channel blockers.
ECT is increasingly recognized as an effective NMS treatment and should not be overlooked for patients:
Standard ECT is given, although nondepolarizing muscle relaxants instead of succinylcholine are used in patients with serious rhabdomyolysis to avoid the risk of hyperkalemia.20
Recommendation. Although these modalities offer a spectrum of therapeutic options, it is premature to recommend any single remedy over others or over supportive care alone because:
- randomized, controlled trials have not been conducted
- NMS episodes are heterogeneous in presentation and outcome
- the syndrome is often self-limited after antipsychotics are discontinued.
I recommend that you choose therapies empirically, based on the character, severity, and duration of symptoms in a given case.5,6,20
Table 4
How to treat neuroleptic malignant syndrome
| General measures | Diagnose early, discontinue antipsychotic, provide supportive care |
| Specific interventions under investigation | |
| Benzodiazepines | Parenteral lorazepam, 1 to 2 mg or higher; monitor respiratory status |
| Dopamine agonists | Bromocriptine, 2.5 mg every 8 hours or amantadine, 100 mg every 8 hours; monitor psychosis, blood pressure, nausea |
| Dantrolene | 1 to 2.5 mg/kg IV every 6 hours; monitor respiratory and hepatic function; avoid calcium channel blockers |
| ECT | Standard administration; avoid succinylcholine in patients with rhabdomyolysis |
REDUCING RISK OF RECURRENCE
Patients vary in susceptibility to recurrence after they recover from NMS, but the risk approaches 30% with future exposure to antipsychotics.5,6 You may be able to minimize recurrence risk by:
- reducing risk factors, such as dehydration
- considering alternatives to antipsychotics, such as treating bipolar disorder with lithium or ECT
- using atypical instead of conventional antipsychotics, starting with low dosages and titrating slowly.
Before you reintroduce antipsychotics, carefully document informed consent and your rationale for treatment decisions in the patient’s chart.
Related resources
- Neuroleptic Malignant Syndrome Information Service. Hotline for health professionals. (888) 667-8367. www.nmsis.org
- Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing, 2003.
- Caroff SN, Mann SC, Francis A, Fricchione GL. Catatonia: from psychopathology to neurobiology. Washington, DC: American Psychiatric Publishing (in press).
Drug brand names
- Amantadine • Symmetrel
- Aripiprazole • Abilify
- Bromocriptine • Parlodel
- Clozapine • Clozaril
- Dantrolene • Dantrium
- Haloperidol • Haldol
- Lorazepam • Ativan
- Metoclopramide • Reglan
- Olanzapine • Zyprexa
- Pramipexole • Mirapex
- Prochlorperazine • Compazine
- Promethazine • Phenergan
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ropinirole • Requip
- Ziprasidone • Geodon
Disclosure
Dr. Caroff receives research support from Janssen Pharmaceutica and Pfizer Inc., and is a consultant to Eli Lilly and Co. and Bristol-Myers Squibb Co.
1. Caroff SN, Mann SC, Campbell EC, Sullivan KA. Movement disorders associated with atypical antipsychotic drugs. J Clin Psychiatry 2002;63(suppl 4):12-19.
2. Caroff SN, Mann SC, Campbell EC. Atypical antipsychotics and neuroleptic malignant syndrome. Psychiatric Annals 2000;30(5):314-21.
3. Caroff SN, Rosenberg H, Mann SC, et al. Neuroleptic malignant syndrome in the perioperative setting. Am J Anesthesiology 2001;28(8):387-93.
4. Caroff SN, Rosenberg H, Mann SC, et al. Neuroleptic malignant syndrome in the critical care unit. Crit Care Med 2002;30(11):2609.-
5. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77(1):185-202.
6. Caroff SN. Neuroleptic malignant syndrome. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A (eds). Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing Inc., 2003;1-44.
7. Velamoor VR, Swamy GN, Parmar RS, et al. Management of suspected neuroleptic malignant syndrome. Can J Psychiatry 1995;40(9):545-50.
8. Keck PE, Jr, Pope HG, Jr, Cohen BM, et al. Risk factors for neuroleptic malignant syndrome. Arch Gen Psychiatry 1989;46:914-18.
9. Mann SC. Malignant catatonia. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A (eds). Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing, 2003;121-43.
10. Velamoor VR, Norman RMG, Caroff SN, et al. Progression of symptoms in neuroleptic malignant syndrome. J Nerv Ment Dis 1994;182(3):168-73.
11. Delay J, Pichot P, Lemperiere T, et al. Un neuroleptique majeur non-phenothiazine et non reserpinique, l’haloperidol, dans le traitement des psychoses. Annales Medico-Psychologique 1960;118(1):145-52.
12. Caroff SN. The neuroleptic malignant syndrome. J Clin Psychiatry 1980;41(3):79-83.
13. Caroff SN, Mann SC, Keck PE, Jr, Francis A. Residual catatonic state following neuroleptic malignant syndrome. J Clin Psychopharmacol 2000;20(2):257-9.
14. Caroff SN, Mann SC, McCarthy M, et al. Acute infectious encephalitis complicated by neuroleptic malignant syndrome. J Clin Psychopharmacol 1998;18(4):349-51.
15. Caroff SN, Mann SC, Gliatto MF, et al. Psychiatric manifestations of acute viral encephalitis. Psychiatric Annals 2001;31(3):193-204.
16. Caroff SN, Mann SC, Francis A, Fricchione GL (eds). Catatonia: from psychopathology to neurobiology. Washington, DC: American Psychiatric Publishing (in press).
17. Mann SC. Thermoregulatory mechanisms and antipsychotic drugrelated heatstroke. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A (eds). Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing, 2003;45-74.
18. Caroff SN, Mann SC, Campbell EC. Risk of fatal heatstroke after hospitalization. Psychiatric Serv 2000;51(7):938.-
19. Keck PE, Jr. Serotonin syndrome. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A (eds). Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing, 2003;75-92.
20. Davis JM, Caroff SN, Mann SC. Treatment of neuroleptic malignant syndrome. Psychiatric Annals 2000;30(5):325-31.
21. Francis A, Chandragiri S, Rizvi S, et al. Lorazepam a treatment for neuroleptic malignant syndrome? CNS Spectrums 2000;5(7):54-7.
22. Sakkas P, Davis JM, Hua J, Wang Z. Pharmacotherapy of neuroleptic malignant syndrome. Psychiatr Ann 1991;21:157-64.
23. Rosenberg MR, Green M. Neuroleptic malignant syndrome: review of response to therapy. Arch Intern Med 1989;149:1927-31.
24. Henderson A, Longdon P. Fulminant metoclopramide-induced neuroleptic malignant syndrome rapidly responsive to intravenous dantrolene. Aust N Z J Med 1991;21:742-3.
25. Yamawaki S, Morio M, Kazamatsuri G, et al. Clinical evaluation and effective usage of dantrolene sodium in neuroleptic malignant syndrome. Kiso to Rinsyou (Clinical Reports) 1993;27(3):1045-66.
26. Tsutsumi Y, Yamamoto K, Matsuura S, et al. The treatment of neuroleptic malignant syndrome using dantrolene sodium. Psychiatry Clin Neurosci 1998;52:433-8.
27. Nisijima K, Ishiguro T. Electroconvulsive therapy for the treatment of neuroleptic malignant syndrome: a report of five cases. J ECT 1999;15:158-63.
28. Troller JN, Sachdev PS. Electroconvulsive treatment of neuroleptic malignant syndrome: a review and report of cases. Aust N Z J Psychiatry 1999;33:650-9.
1. Caroff SN, Mann SC, Campbell EC, Sullivan KA. Movement disorders associated with atypical antipsychotic drugs. J Clin Psychiatry 2002;63(suppl 4):12-19.
2. Caroff SN, Mann SC, Campbell EC. Atypical antipsychotics and neuroleptic malignant syndrome. Psychiatric Annals 2000;30(5):314-21.
3. Caroff SN, Rosenberg H, Mann SC, et al. Neuroleptic malignant syndrome in the perioperative setting. Am J Anesthesiology 2001;28(8):387-93.
4. Caroff SN, Rosenberg H, Mann SC, et al. Neuroleptic malignant syndrome in the critical care unit. Crit Care Med 2002;30(11):2609.-
5. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am 1993;77(1):185-202.
6. Caroff SN. Neuroleptic malignant syndrome. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A (eds). Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing Inc., 2003;1-44.
7. Velamoor VR, Swamy GN, Parmar RS, et al. Management of suspected neuroleptic malignant syndrome. Can J Psychiatry 1995;40(9):545-50.
8. Keck PE, Jr, Pope HG, Jr, Cohen BM, et al. Risk factors for neuroleptic malignant syndrome. Arch Gen Psychiatry 1989;46:914-18.
9. Mann SC. Malignant catatonia. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A (eds). Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing, 2003;121-43.
10. Velamoor VR, Norman RMG, Caroff SN, et al. Progression of symptoms in neuroleptic malignant syndrome. J Nerv Ment Dis 1994;182(3):168-73.
11. Delay J, Pichot P, Lemperiere T, et al. Un neuroleptique majeur non-phenothiazine et non reserpinique, l’haloperidol, dans le traitement des psychoses. Annales Medico-Psychologique 1960;118(1):145-52.
12. Caroff SN. The neuroleptic malignant syndrome. J Clin Psychiatry 1980;41(3):79-83.
13. Caroff SN, Mann SC, Keck PE, Jr, Francis A. Residual catatonic state following neuroleptic malignant syndrome. J Clin Psychopharmacol 2000;20(2):257-9.
14. Caroff SN, Mann SC, McCarthy M, et al. Acute infectious encephalitis complicated by neuroleptic malignant syndrome. J Clin Psychopharmacol 1998;18(4):349-51.
15. Caroff SN, Mann SC, Gliatto MF, et al. Psychiatric manifestations of acute viral encephalitis. Psychiatric Annals 2001;31(3):193-204.
16. Caroff SN, Mann SC, Francis A, Fricchione GL (eds). Catatonia: from psychopathology to neurobiology. Washington, DC: American Psychiatric Publishing (in press).
17. Mann SC. Thermoregulatory mechanisms and antipsychotic drugrelated heatstroke. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A (eds). Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing, 2003;45-74.
18. Caroff SN, Mann SC, Campbell EC. Risk of fatal heatstroke after hospitalization. Psychiatric Serv 2000;51(7):938.-
19. Keck PE, Jr. Serotonin syndrome. In: Mann SC, Caroff SN, Keck PE Jr, Lazarus A (eds). Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing, 2003;75-92.
20. Davis JM, Caroff SN, Mann SC. Treatment of neuroleptic malignant syndrome. Psychiatric Annals 2000;30(5):325-31.
21. Francis A, Chandragiri S, Rizvi S, et al. Lorazepam a treatment for neuroleptic malignant syndrome? CNS Spectrums 2000;5(7):54-7.
22. Sakkas P, Davis JM, Hua J, Wang Z. Pharmacotherapy of neuroleptic malignant syndrome. Psychiatr Ann 1991;21:157-64.
23. Rosenberg MR, Green M. Neuroleptic malignant syndrome: review of response to therapy. Arch Intern Med 1989;149:1927-31.
24. Henderson A, Longdon P. Fulminant metoclopramide-induced neuroleptic malignant syndrome rapidly responsive to intravenous dantrolene. Aust N Z J Med 1991;21:742-3.
25. Yamawaki S, Morio M, Kazamatsuri G, et al. Clinical evaluation and effective usage of dantrolene sodium in neuroleptic malignant syndrome. Kiso to Rinsyou (Clinical Reports) 1993;27(3):1045-66.
26. Tsutsumi Y, Yamamoto K, Matsuura S, et al. The treatment of neuroleptic malignant syndrome using dantrolene sodium. Psychiatry Clin Neurosci 1998;52:433-8.
27. Nisijima K, Ishiguro T. Electroconvulsive therapy for the treatment of neuroleptic malignant syndrome: a report of five cases. J ECT 1999;15:158-63.
28. Troller JN, Sachdev PS. Electroconvulsive treatment of neuroleptic malignant syndrome: a review and report of cases. Aust N Z J Psychiatry 1999;33:650-9.