User login
› Recommend that women consider having a single mammogram at age 40 as a baseline so that breast density can be included in the assessment of risk. minor MRSA skin lesions in children with mupirocin. C
› Advise women with low breast density and no other significant risk factors that they are at lower than average risk for breast cancer and should consider this when discussing when to begin routine screening with their physician. C
› Recommend that women with a 2-fold increased risk for breast cancer begin regular screening in their 40s. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
“Doctor, when should I start having mammograms?” That’s a question you’re apt to hear again and again from women in their early 40s. It’s also a question with no easy answer.
While deaths from breast cancer are declining, it remains the most commonly diagnosed cancer among US women. In 2012, approximately 229,060 new cases of breast cancer were detected and an estimated 39,920 women died from breast cancer1—about 10% of them in their 40s.2
Based on these numbers alone, it would seem that every woman should begin regular screening at age 40. Yet there are many other issues to consider, namely the high rate of false positives, as well as the overdiagnosis and overtreatment associated with such screening. Further complicating matters is the fact that there is no consensus as to whether screening mammography should be recommended—and if so, how often—for women ages 40 to 49 years who are at average risk.
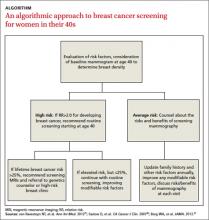
In light of this, we offer a risk-based strategy to mammography for younger women, which we’ve distilled into an ALGORITHM. But first, let’s look at the evidence and what the US Preventive Services Task Force (USPSTF) and major medical groups have to say.
To screen or not to screen? A look at the evidence
A decision to perform screening mammography in premenopausal women should be made by weighing benefits vs harms. Benefits include diagnosis of breast cancer when it’s in an early stage and a reduction in death. Meta-analyses have consistently shown that routine screening mammograms for women in their 40s can reduce mortality from breast cancer by 15% to 20%.3-5 As noted by Cochrane reviewers in a meta-analysis of 7 randomized controlled studies of breast cancer screening in younger women, a 15% relative risk (RR) reduction represents an absolute risk reduction of 0.05%.5
Potential harms include the financial cost; the screening regimen itself, which includes radiation exposure, pain, inconvenience, and anxiety; the ensuing diagnostic workup in the case of false positive results; and overdiagnosis—ie, detection of lowgrade cancer that would not have otherwise become clinically evident—and subsequent overtreatment.6 Diagnosis of ductal carcinoma in situ (DCIS) was rare before the advent of screening mammography. Now, DCIS accounts for 25% of all breast cancer diagnoses, and more than 90% of cases are detected only by imaging.6 A large epidemiologic review published in 2012 suggested that the increase in breast cancer survival over the last 30 years is due to improved treatment regimens, not early detection.7
Recommendations are equivocal
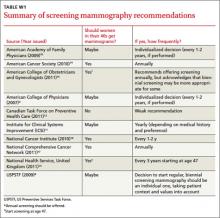
Groups like the USPSTF, the American College of Obstetricians and Gynecologists, and the American Cancer Society, among others (See TABLE W1,8-17 at the end of this article), recognize that women in their 40s may benefit from screening mammography. They generally acknowledge, however, that, the evidence is not strong enough to definitely recommend routine screening mammograms due to the higher risk of false positives and the lower overall incidence of breast cancer in this age group.
The USPSTF set off a firestorm in 2009 with its initial recommendation against routine screening for women in their 40s.8 Shortly after, the group issued an update to “clarify their ... intent,” stating that the decision to start regular screening mammography before age 50 should be an individual one based on patient values as well as an assessment of benefits and risks.8
False positives decline with age
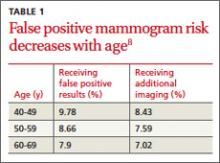
The risk of having a false positive result on a screening mammography decreases with increasing age, as the incidence of breast cancer rises (TABLE 1).8 More than 1900 women in their 40s need to undergo screening mammography in order to prevent just one death from breast cancer in 11 years of follow-up,8 with a direct cost of more than 20,000 visits for breast imaging and approximately 2000 false positive mammograms. In contrast, fewer than 400 women in their 60s would need to be screened in order to prevent one breast cancer death in 13 years of follow-up.18 A large prospective cohort study (N=169,456) found that women who started annual screening at age 40 had a 61% chance of receiving at least one false positive mammogram result over the course of 10 years; the chance of a false positive dropped to 41.6% with biennial screening.19
The impact of a false positive lingers. A cohort study that followed 454 women for 3 years after they received a false positive mammogram result found that it continued to have a negative psychological impact on them.20
A risk-based screening approach
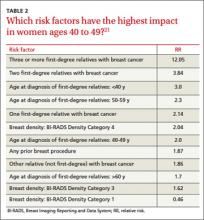
With no clear consensus on when to begin screening, primary care physicians and their patients would be wise to adopt a risk-based approach. Risk-based screening would focus efforts on women ages 40 to 49 who are more likely to benefit from screening mammography, which would represent a more effective use of resources.2 To implement such an approach, it is critical to know the magnitude of risk reduction that would tip the balance of benefits and harms in favor of early screening, and which risk factors are associated with such an elevated risk (TABLE 2).21
A recent comparative modeling study found that for women with a 2-fold increased risk for breast cancer, the benefits and risks of starting biennial screening at age 40 are about the same as that of women at average risk who start biennial screening at age 50. As biennial screening at age 50 is widely recommended, the results of this study suggest that ≥2-fold risk is a useful threshold in determining when to start mammography screening for women in their 40s.21
The traditional counseling of women about breast cancer risks focuses on parity and age of first delivery, breastfeeding, obesity, and alcohol use, in addition to family history. However, none of these has an RR >1.5.22
Two risk factors are associated with ≥2-fold RR for breast cancer:
• having one or more first-degree relatives with breast cancer
• having extremely dense breasts.
A prior breast biopsy is also associated with a high RR (1.87).21
Does your patient have dense breasts? A baseline mammogram is necessary to determine a woman’s breast density. The American College of Radiology developed BI-RADS (Breast Imaging Reporting and Data System) to standardize the reporting of density on mammograms.23 BI-RADS has 4 categories of breast density:
1. Breast tissue is almost entirely fatty. (Adipose tissue is radiolucent and makes the mammogram easier to read.)
2. There are scattered fibroglandular densities in the breast.
3. The breasts are heterogeneously dense.
4. The breasts are extremely dense.
When there is a discrepancy between the density of the left and right breasts, radiologists are instructed to use the higher density.23 Another method of documenting density assesses the percentage of the breast tissue that is dense as compared to fatty tissue.
Increased density (BI-RADS category 3 or 4) likely accounts for a sizeable proportion of nonfamilial breast cancers.24 In a large case control study (N=1112), density in ≥75% of the breast was associated with 26% of all breast cancers diagnosed in women under 56 years.25 While a number of other risk factors for breast cancer are related to breast density (nulliparity, positive family history of breast cancer, and hormone therapy), higher density is associated with large increased risks of breast cancer independent of the other factors.24
Initiate regular screening for women at high risk
Most high-risk women should have regular screening beginning at age 40. The American Cancer Society recommends screening with magnetic resonance imaging (MRI) as opposed to mammography for women with ≥20% lifetime risk of developing breast cancer.26
Adding an annual ultrasound to mammography may be another method of screening for high-risk women. A study of 2809 women with elevated breast cancer risk and dense breasts demonstrated that the addition of annual screening with either ultrasound or MRI detected an additional 3.7 cancers per 1000 women per year beyond mammography alone.27 In that study, however, there was a significant number of false positive results, as well.
MRI is not indicated for women with a 15% to 20% lifetime risk. These women will benefit from routine screening starting at age 40, as well as genetic counseling if they have a family history of breast cancer. Increased breast density can also make mammograms harder to read, and there is concern that density can mask an early cancer. In fact, multiple studies have refuted that claim.28 Breast density does tend to decrease with age, but the relationship between increased density and elevated risk of breast cancer persists through all age groups.
Get a baseline mammogram for those at lower risk
One approach to risk-based screening is to recommend that all women at average risk have an initial screening mammogram at age 40 to determine breast density and discuss other pertinent risk factors. If they are found to have BI-RADS density category 3 or 4, regular screening mammography throughout their 40s is a reasonable approach.
For those at low or average risk, things are less clear, and a discussion to determine the appropriate course of screening is needed. Some women with no family history of breast cancer will elect to wait until age 50 to start screening mammography; others may not be comfortable doing so. It is important to point out to patients with very low density (BI-RADS density category 1) breasts that their risk for breast cancer is very low (RR=0.46) and that waiting until age 50 to start regular screening mammography would be a reasonable decision.
1. Siegel R, Naishadham D, Jemal A. Cancer statistics. Cancer J Clin. 2012;62:10-29.
2. Brawley OW. Risk-based mammography screening: an effort to maximize the benefits and minimize the harms. Ann Intern Med. 2012;156:662-663.
3. Hendrick RE, Smith RA, Rutledge JH 3rd, et al. Benefit of screening mammography in women aged 40-49: a new meta-analysis of randomized controlled trials. J Natl Cancer Inst Monogr. 1997;22:87-92.
4. Kerlikowske K, Grady D, Ernster V. Benefit of mammography screening in women ages 40-49 years: current evidence from randomized controlled trials. Cancer. 1995;76:1679-1681.
5. Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2011;(1):CD001877.
6. Warner E. Breast-cancer screening. N Engl J Med. 2011;365:1025-1032.
7. Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998-2005.
8. Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727-737.
9. Qaseem A, Snow V, Sherif K, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening mammography for women 40 to 49 years of age: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007; 146:511-515.
10. American Academy of Family Physicians. AAFP screening recommendation. Breast cancer, mammography before age 50. Available at: http://www.aafp.org/online/en/home/clinical/exam/ae.html. Accessed September 25, 2012.
11. Institute for Clinical Systems Improvement (ICSI) Breast cancer screening recommendations. Available at http://www.icsi.org/breast_disease_diagnosis/diagnosis_of_breast_disease_2.html. Accessed September 25, 2012.
12. Canadian Task Force on Preventive Health Care. Screening for breast cancer, 2011. Available at: http://www.canadiantaskforce.ca/recommendations/2011_01_eng.html. Accessed September 25, 2012.
13. National Health Service,. Breast cancer screening. Available at: http://www.screening.nhs.uk/professionals. Accessed September 25, 2012.
14. American Cancer Society. Guidelines for the early detection of cancer, breast cancer screening. Available at: http://www.cancer.org/Healthy/FindCancerEarly/CancerScreeningGuidelines/american-cancer-society-guidelines-for-the-early-detection-ofcancer. Accessed October 1, 2012.
15. American College of Obstetricians and Gynecologists (ACOG). Breast cancer screening. Washington (DC): American College of Obstetricians and Gynecologists (ACOG); 2011 Aug. 11 p. (ACOG practice bulletin; no. 122).
16. National Cancer Institute. Breast cancer screening. Available at: http://www.cancer.gov/cancertopics/pdq/screening/breast/healthprofessional/page1. Accessed September 25, 2012.
17. National Comprehensive Cancer Institute. Guidelines for the detection of breast cancer. Available at: http://www.nccn.org. Accessed October 1, 2012.
18. Quanstrum KH, Hayward RA. Lessons from the mammography wars. N Engl J Med. 2010;363 :1076-1079.
19. Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
20. Brodersen J, Siersma VD. Long-term psychosocial consequences of false-positive screening mammography. Ann Fam Med. 2013;11:106-115.
21. van Ravesteyn NT, Miglioretti DL, Stout NK, et al. Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years: a comparative modeling study of risk. Ann Int Med. 2012;156:609-617.
22. Nelson HD, Zakher B, Cantor A, et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and metaanalysis. Ann Intern Med. 2012;156:635-648.
23. D’Orsi CJ, Bassett LW, Berg WA, et al. Breast Imaging Reporting and Data System: ACR Bi-RADS Mammography. 4th ed. Reston, VA: American College of Radiology; 2003.
24. Gierach GL, Ichikawa L, Kerikowske K, et al. Relationship between mammographic density and breast cancer death in the Breast Cancer Surveillance Consortium. J Natl Cancer Inst. 2012;104:1218-1227.
25. Boyd NJ, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236.
26. Saslow D, Boetets C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
27. Berg WA, Zhang A, Lehrer D, et al; ACRINN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404.
28. McCormark VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Epidemol Biomarkers Prev. 2006;15:1159-1169.
› Recommend that women consider having a single mammogram at age 40 as a baseline so that breast density can be included in the assessment of risk. minor MRSA skin lesions in children with mupirocin. C
› Advise women with low breast density and no other significant risk factors that they are at lower than average risk for breast cancer and should consider this when discussing when to begin routine screening with their physician. C
› Recommend that women with a 2-fold increased risk for breast cancer begin regular screening in their 40s. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
“Doctor, when should I start having mammograms?” That’s a question you’re apt to hear again and again from women in their early 40s. It’s also a question with no easy answer.
While deaths from breast cancer are declining, it remains the most commonly diagnosed cancer among US women. In 2012, approximately 229,060 new cases of breast cancer were detected and an estimated 39,920 women died from breast cancer1—about 10% of them in their 40s.2
Based on these numbers alone, it would seem that every woman should begin regular screening at age 40. Yet there are many other issues to consider, namely the high rate of false positives, as well as the overdiagnosis and overtreatment associated with such screening. Further complicating matters is the fact that there is no consensus as to whether screening mammography should be recommended—and if so, how often—for women ages 40 to 49 years who are at average risk.
In light of this, we offer a risk-based strategy to mammography for younger women, which we’ve distilled into an ALGORITHM. But first, let’s look at the evidence and what the US Preventive Services Task Force (USPSTF) and major medical groups have to say.
To screen or not to screen? A look at the evidence
A decision to perform screening mammography in premenopausal women should be made by weighing benefits vs harms. Benefits include diagnosis of breast cancer when it’s in an early stage and a reduction in death. Meta-analyses have consistently shown that routine screening mammograms for women in their 40s can reduce mortality from breast cancer by 15% to 20%.3-5 As noted by Cochrane reviewers in a meta-analysis of 7 randomized controlled studies of breast cancer screening in younger women, a 15% relative risk (RR) reduction represents an absolute risk reduction of 0.05%.5
Potential harms include the financial cost; the screening regimen itself, which includes radiation exposure, pain, inconvenience, and anxiety; the ensuing diagnostic workup in the case of false positive results; and overdiagnosis—ie, detection of lowgrade cancer that would not have otherwise become clinically evident—and subsequent overtreatment.6 Diagnosis of ductal carcinoma in situ (DCIS) was rare before the advent of screening mammography. Now, DCIS accounts for 25% of all breast cancer diagnoses, and more than 90% of cases are detected only by imaging.6 A large epidemiologic review published in 2012 suggested that the increase in breast cancer survival over the last 30 years is due to improved treatment regimens, not early detection.7
Recommendations are equivocal
Groups like the USPSTF, the American College of Obstetricians and Gynecologists, and the American Cancer Society, among others (See TABLE W1,8-17 at the end of this article), recognize that women in their 40s may benefit from screening mammography. They generally acknowledge, however, that, the evidence is not strong enough to definitely recommend routine screening mammograms due to the higher risk of false positives and the lower overall incidence of breast cancer in this age group.
The USPSTF set off a firestorm in 2009 with its initial recommendation against routine screening for women in their 40s.8 Shortly after, the group issued an update to “clarify their ... intent,” stating that the decision to start regular screening mammography before age 50 should be an individual one based on patient values as well as an assessment of benefits and risks.8
False positives decline with age
The risk of having a false positive result on a screening mammography decreases with increasing age, as the incidence of breast cancer rises (TABLE 1).8 More than 1900 women in their 40s need to undergo screening mammography in order to prevent just one death from breast cancer in 11 years of follow-up,8 with a direct cost of more than 20,000 visits for breast imaging and approximately 2000 false positive mammograms. In contrast, fewer than 400 women in their 60s would need to be screened in order to prevent one breast cancer death in 13 years of follow-up.18 A large prospective cohort study (N=169,456) found that women who started annual screening at age 40 had a 61% chance of receiving at least one false positive mammogram result over the course of 10 years; the chance of a false positive dropped to 41.6% with biennial screening.19
The impact of a false positive lingers. A cohort study that followed 454 women for 3 years after they received a false positive mammogram result found that it continued to have a negative psychological impact on them.20
A risk-based screening approach
With no clear consensus on when to begin screening, primary care physicians and their patients would be wise to adopt a risk-based approach. Risk-based screening would focus efforts on women ages 40 to 49 who are more likely to benefit from screening mammography, which would represent a more effective use of resources.2 To implement such an approach, it is critical to know the magnitude of risk reduction that would tip the balance of benefits and harms in favor of early screening, and which risk factors are associated with such an elevated risk (TABLE 2).21
A recent comparative modeling study found that for women with a 2-fold increased risk for breast cancer, the benefits and risks of starting biennial screening at age 40 are about the same as that of women at average risk who start biennial screening at age 50. As biennial screening at age 50 is widely recommended, the results of this study suggest that ≥2-fold risk is a useful threshold in determining when to start mammography screening for women in their 40s.21
The traditional counseling of women about breast cancer risks focuses on parity and age of first delivery, breastfeeding, obesity, and alcohol use, in addition to family history. However, none of these has an RR >1.5.22
Two risk factors are associated with ≥2-fold RR for breast cancer:
• having one or more first-degree relatives with breast cancer
• having extremely dense breasts.
A prior breast biopsy is also associated with a high RR (1.87).21
Does your patient have dense breasts? A baseline mammogram is necessary to determine a woman’s breast density. The American College of Radiology developed BI-RADS (Breast Imaging Reporting and Data System) to standardize the reporting of density on mammograms.23 BI-RADS has 4 categories of breast density:
1. Breast tissue is almost entirely fatty. (Adipose tissue is radiolucent and makes the mammogram easier to read.)
2. There are scattered fibroglandular densities in the breast.
3. The breasts are heterogeneously dense.
4. The breasts are extremely dense.
When there is a discrepancy between the density of the left and right breasts, radiologists are instructed to use the higher density.23 Another method of documenting density assesses the percentage of the breast tissue that is dense as compared to fatty tissue.
Increased density (BI-RADS category 3 or 4) likely accounts for a sizeable proportion of nonfamilial breast cancers.24 In a large case control study (N=1112), density in ≥75% of the breast was associated with 26% of all breast cancers diagnosed in women under 56 years.25 While a number of other risk factors for breast cancer are related to breast density (nulliparity, positive family history of breast cancer, and hormone therapy), higher density is associated with large increased risks of breast cancer independent of the other factors.24
Initiate regular screening for women at high risk
Most high-risk women should have regular screening beginning at age 40. The American Cancer Society recommends screening with magnetic resonance imaging (MRI) as opposed to mammography for women with ≥20% lifetime risk of developing breast cancer.26
Adding an annual ultrasound to mammography may be another method of screening for high-risk women. A study of 2809 women with elevated breast cancer risk and dense breasts demonstrated that the addition of annual screening with either ultrasound or MRI detected an additional 3.7 cancers per 1000 women per year beyond mammography alone.27 In that study, however, there was a significant number of false positive results, as well.
MRI is not indicated for women with a 15% to 20% lifetime risk. These women will benefit from routine screening starting at age 40, as well as genetic counseling if they have a family history of breast cancer. Increased breast density can also make mammograms harder to read, and there is concern that density can mask an early cancer. In fact, multiple studies have refuted that claim.28 Breast density does tend to decrease with age, but the relationship between increased density and elevated risk of breast cancer persists through all age groups.
Get a baseline mammogram for those at lower risk
One approach to risk-based screening is to recommend that all women at average risk have an initial screening mammogram at age 40 to determine breast density and discuss other pertinent risk factors. If they are found to have BI-RADS density category 3 or 4, regular screening mammography throughout their 40s is a reasonable approach.
For those at low or average risk, things are less clear, and a discussion to determine the appropriate course of screening is needed. Some women with no family history of breast cancer will elect to wait until age 50 to start screening mammography; others may not be comfortable doing so. It is important to point out to patients with very low density (BI-RADS density category 1) breasts that their risk for breast cancer is very low (RR=0.46) and that waiting until age 50 to start regular screening mammography would be a reasonable decision.
› Recommend that women consider having a single mammogram at age 40 as a baseline so that breast density can be included in the assessment of risk. minor MRSA skin lesions in children with mupirocin. C
› Advise women with low breast density and no other significant risk factors that they are at lower than average risk for breast cancer and should consider this when discussing when to begin routine screening with their physician. C
› Recommend that women with a 2-fold increased risk for breast cancer begin regular screening in their 40s. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
“Doctor, when should I start having mammograms?” That’s a question you’re apt to hear again and again from women in their early 40s. It’s also a question with no easy answer.
While deaths from breast cancer are declining, it remains the most commonly diagnosed cancer among US women. In 2012, approximately 229,060 new cases of breast cancer were detected and an estimated 39,920 women died from breast cancer1—about 10% of them in their 40s.2
Based on these numbers alone, it would seem that every woman should begin regular screening at age 40. Yet there are many other issues to consider, namely the high rate of false positives, as well as the overdiagnosis and overtreatment associated with such screening. Further complicating matters is the fact that there is no consensus as to whether screening mammography should be recommended—and if so, how often—for women ages 40 to 49 years who are at average risk.
In light of this, we offer a risk-based strategy to mammography for younger women, which we’ve distilled into an ALGORITHM. But first, let’s look at the evidence and what the US Preventive Services Task Force (USPSTF) and major medical groups have to say.
To screen or not to screen? A look at the evidence
A decision to perform screening mammography in premenopausal women should be made by weighing benefits vs harms. Benefits include diagnosis of breast cancer when it’s in an early stage and a reduction in death. Meta-analyses have consistently shown that routine screening mammograms for women in their 40s can reduce mortality from breast cancer by 15% to 20%.3-5 As noted by Cochrane reviewers in a meta-analysis of 7 randomized controlled studies of breast cancer screening in younger women, a 15% relative risk (RR) reduction represents an absolute risk reduction of 0.05%.5
Potential harms include the financial cost; the screening regimen itself, which includes radiation exposure, pain, inconvenience, and anxiety; the ensuing diagnostic workup in the case of false positive results; and overdiagnosis—ie, detection of lowgrade cancer that would not have otherwise become clinically evident—and subsequent overtreatment.6 Diagnosis of ductal carcinoma in situ (DCIS) was rare before the advent of screening mammography. Now, DCIS accounts for 25% of all breast cancer diagnoses, and more than 90% of cases are detected only by imaging.6 A large epidemiologic review published in 2012 suggested that the increase in breast cancer survival over the last 30 years is due to improved treatment regimens, not early detection.7
Recommendations are equivocal
Groups like the USPSTF, the American College of Obstetricians and Gynecologists, and the American Cancer Society, among others (See TABLE W1,8-17 at the end of this article), recognize that women in their 40s may benefit from screening mammography. They generally acknowledge, however, that, the evidence is not strong enough to definitely recommend routine screening mammograms due to the higher risk of false positives and the lower overall incidence of breast cancer in this age group.
The USPSTF set off a firestorm in 2009 with its initial recommendation against routine screening for women in their 40s.8 Shortly after, the group issued an update to “clarify their ... intent,” stating that the decision to start regular screening mammography before age 50 should be an individual one based on patient values as well as an assessment of benefits and risks.8
False positives decline with age
The risk of having a false positive result on a screening mammography decreases with increasing age, as the incidence of breast cancer rises (TABLE 1).8 More than 1900 women in their 40s need to undergo screening mammography in order to prevent just one death from breast cancer in 11 years of follow-up,8 with a direct cost of more than 20,000 visits for breast imaging and approximately 2000 false positive mammograms. In contrast, fewer than 400 women in their 60s would need to be screened in order to prevent one breast cancer death in 13 years of follow-up.18 A large prospective cohort study (N=169,456) found that women who started annual screening at age 40 had a 61% chance of receiving at least one false positive mammogram result over the course of 10 years; the chance of a false positive dropped to 41.6% with biennial screening.19
The impact of a false positive lingers. A cohort study that followed 454 women for 3 years after they received a false positive mammogram result found that it continued to have a negative psychological impact on them.20
A risk-based screening approach
With no clear consensus on when to begin screening, primary care physicians and their patients would be wise to adopt a risk-based approach. Risk-based screening would focus efforts on women ages 40 to 49 who are more likely to benefit from screening mammography, which would represent a more effective use of resources.2 To implement such an approach, it is critical to know the magnitude of risk reduction that would tip the balance of benefits and harms in favor of early screening, and which risk factors are associated with such an elevated risk (TABLE 2).21
A recent comparative modeling study found that for women with a 2-fold increased risk for breast cancer, the benefits and risks of starting biennial screening at age 40 are about the same as that of women at average risk who start biennial screening at age 50. As biennial screening at age 50 is widely recommended, the results of this study suggest that ≥2-fold risk is a useful threshold in determining when to start mammography screening for women in their 40s.21
The traditional counseling of women about breast cancer risks focuses on parity and age of first delivery, breastfeeding, obesity, and alcohol use, in addition to family history. However, none of these has an RR >1.5.22
Two risk factors are associated with ≥2-fold RR for breast cancer:
• having one or more first-degree relatives with breast cancer
• having extremely dense breasts.
A prior breast biopsy is also associated with a high RR (1.87).21
Does your patient have dense breasts? A baseline mammogram is necessary to determine a woman’s breast density. The American College of Radiology developed BI-RADS (Breast Imaging Reporting and Data System) to standardize the reporting of density on mammograms.23 BI-RADS has 4 categories of breast density:
1. Breast tissue is almost entirely fatty. (Adipose tissue is radiolucent and makes the mammogram easier to read.)
2. There are scattered fibroglandular densities in the breast.
3. The breasts are heterogeneously dense.
4. The breasts are extremely dense.
When there is a discrepancy between the density of the left and right breasts, radiologists are instructed to use the higher density.23 Another method of documenting density assesses the percentage of the breast tissue that is dense as compared to fatty tissue.
Increased density (BI-RADS category 3 or 4) likely accounts for a sizeable proportion of nonfamilial breast cancers.24 In a large case control study (N=1112), density in ≥75% of the breast was associated with 26% of all breast cancers diagnosed in women under 56 years.25 While a number of other risk factors for breast cancer are related to breast density (nulliparity, positive family history of breast cancer, and hormone therapy), higher density is associated with large increased risks of breast cancer independent of the other factors.24
Initiate regular screening for women at high risk
Most high-risk women should have regular screening beginning at age 40. The American Cancer Society recommends screening with magnetic resonance imaging (MRI) as opposed to mammography for women with ≥20% lifetime risk of developing breast cancer.26
Adding an annual ultrasound to mammography may be another method of screening for high-risk women. A study of 2809 women with elevated breast cancer risk and dense breasts demonstrated that the addition of annual screening with either ultrasound or MRI detected an additional 3.7 cancers per 1000 women per year beyond mammography alone.27 In that study, however, there was a significant number of false positive results, as well.
MRI is not indicated for women with a 15% to 20% lifetime risk. These women will benefit from routine screening starting at age 40, as well as genetic counseling if they have a family history of breast cancer. Increased breast density can also make mammograms harder to read, and there is concern that density can mask an early cancer. In fact, multiple studies have refuted that claim.28 Breast density does tend to decrease with age, but the relationship between increased density and elevated risk of breast cancer persists through all age groups.
Get a baseline mammogram for those at lower risk
One approach to risk-based screening is to recommend that all women at average risk have an initial screening mammogram at age 40 to determine breast density and discuss other pertinent risk factors. If they are found to have BI-RADS density category 3 or 4, regular screening mammography throughout their 40s is a reasonable approach.
For those at low or average risk, things are less clear, and a discussion to determine the appropriate course of screening is needed. Some women with no family history of breast cancer will elect to wait until age 50 to start screening mammography; others may not be comfortable doing so. It is important to point out to patients with very low density (BI-RADS density category 1) breasts that their risk for breast cancer is very low (RR=0.46) and that waiting until age 50 to start regular screening mammography would be a reasonable decision.
1. Siegel R, Naishadham D, Jemal A. Cancer statistics. Cancer J Clin. 2012;62:10-29.
2. Brawley OW. Risk-based mammography screening: an effort to maximize the benefits and minimize the harms. Ann Intern Med. 2012;156:662-663.
3. Hendrick RE, Smith RA, Rutledge JH 3rd, et al. Benefit of screening mammography in women aged 40-49: a new meta-analysis of randomized controlled trials. J Natl Cancer Inst Monogr. 1997;22:87-92.
4. Kerlikowske K, Grady D, Ernster V. Benefit of mammography screening in women ages 40-49 years: current evidence from randomized controlled trials. Cancer. 1995;76:1679-1681.
5. Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2011;(1):CD001877.
6. Warner E. Breast-cancer screening. N Engl J Med. 2011;365:1025-1032.
7. Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998-2005.
8. Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727-737.
9. Qaseem A, Snow V, Sherif K, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening mammography for women 40 to 49 years of age: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007; 146:511-515.
10. American Academy of Family Physicians. AAFP screening recommendation. Breast cancer, mammography before age 50. Available at: http://www.aafp.org/online/en/home/clinical/exam/ae.html. Accessed September 25, 2012.
11. Institute for Clinical Systems Improvement (ICSI) Breast cancer screening recommendations. Available at http://www.icsi.org/breast_disease_diagnosis/diagnosis_of_breast_disease_2.html. Accessed September 25, 2012.
12. Canadian Task Force on Preventive Health Care. Screening for breast cancer, 2011. Available at: http://www.canadiantaskforce.ca/recommendations/2011_01_eng.html. Accessed September 25, 2012.
13. National Health Service,. Breast cancer screening. Available at: http://www.screening.nhs.uk/professionals. Accessed September 25, 2012.
14. American Cancer Society. Guidelines for the early detection of cancer, breast cancer screening. Available at: http://www.cancer.org/Healthy/FindCancerEarly/CancerScreeningGuidelines/american-cancer-society-guidelines-for-the-early-detection-ofcancer. Accessed October 1, 2012.
15. American College of Obstetricians and Gynecologists (ACOG). Breast cancer screening. Washington (DC): American College of Obstetricians and Gynecologists (ACOG); 2011 Aug. 11 p. (ACOG practice bulletin; no. 122).
16. National Cancer Institute. Breast cancer screening. Available at: http://www.cancer.gov/cancertopics/pdq/screening/breast/healthprofessional/page1. Accessed September 25, 2012.
17. National Comprehensive Cancer Institute. Guidelines for the detection of breast cancer. Available at: http://www.nccn.org. Accessed October 1, 2012.
18. Quanstrum KH, Hayward RA. Lessons from the mammography wars. N Engl J Med. 2010;363 :1076-1079.
19. Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
20. Brodersen J, Siersma VD. Long-term psychosocial consequences of false-positive screening mammography. Ann Fam Med. 2013;11:106-115.
21. van Ravesteyn NT, Miglioretti DL, Stout NK, et al. Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years: a comparative modeling study of risk. Ann Int Med. 2012;156:609-617.
22. Nelson HD, Zakher B, Cantor A, et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and metaanalysis. Ann Intern Med. 2012;156:635-648.
23. D’Orsi CJ, Bassett LW, Berg WA, et al. Breast Imaging Reporting and Data System: ACR Bi-RADS Mammography. 4th ed. Reston, VA: American College of Radiology; 2003.
24. Gierach GL, Ichikawa L, Kerikowske K, et al. Relationship between mammographic density and breast cancer death in the Breast Cancer Surveillance Consortium. J Natl Cancer Inst. 2012;104:1218-1227.
25. Boyd NJ, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236.
26. Saslow D, Boetets C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
27. Berg WA, Zhang A, Lehrer D, et al; ACRINN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404.
28. McCormark VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Epidemol Biomarkers Prev. 2006;15:1159-1169.
1. Siegel R, Naishadham D, Jemal A. Cancer statistics. Cancer J Clin. 2012;62:10-29.
2. Brawley OW. Risk-based mammography screening: an effort to maximize the benefits and minimize the harms. Ann Intern Med. 2012;156:662-663.
3. Hendrick RE, Smith RA, Rutledge JH 3rd, et al. Benefit of screening mammography in women aged 40-49: a new meta-analysis of randomized controlled trials. J Natl Cancer Inst Monogr. 1997;22:87-92.
4. Kerlikowske K, Grady D, Ernster V. Benefit of mammography screening in women ages 40-49 years: current evidence from randomized controlled trials. Cancer. 1995;76:1679-1681.
5. Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2011;(1):CD001877.
6. Warner E. Breast-cancer screening. N Engl J Med. 2011;365:1025-1032.
7. Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012;367:1998-2005.
8. Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the US Preventive Services Task Force. Ann Intern Med. 2009;151:727-737.
9. Qaseem A, Snow V, Sherif K, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Screening mammography for women 40 to 49 years of age: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007; 146:511-515.
10. American Academy of Family Physicians. AAFP screening recommendation. Breast cancer, mammography before age 50. Available at: http://www.aafp.org/online/en/home/clinical/exam/ae.html. Accessed September 25, 2012.
11. Institute for Clinical Systems Improvement (ICSI) Breast cancer screening recommendations. Available at http://www.icsi.org/breast_disease_diagnosis/diagnosis_of_breast_disease_2.html. Accessed September 25, 2012.
12. Canadian Task Force on Preventive Health Care. Screening for breast cancer, 2011. Available at: http://www.canadiantaskforce.ca/recommendations/2011_01_eng.html. Accessed September 25, 2012.
13. National Health Service,. Breast cancer screening. Available at: http://www.screening.nhs.uk/professionals. Accessed September 25, 2012.
14. American Cancer Society. Guidelines for the early detection of cancer, breast cancer screening. Available at: http://www.cancer.org/Healthy/FindCancerEarly/CancerScreeningGuidelines/american-cancer-society-guidelines-for-the-early-detection-ofcancer. Accessed October 1, 2012.
15. American College of Obstetricians and Gynecologists (ACOG). Breast cancer screening. Washington (DC): American College of Obstetricians and Gynecologists (ACOG); 2011 Aug. 11 p. (ACOG practice bulletin; no. 122).
16. National Cancer Institute. Breast cancer screening. Available at: http://www.cancer.gov/cancertopics/pdq/screening/breast/healthprofessional/page1. Accessed September 25, 2012.
17. National Comprehensive Cancer Institute. Guidelines for the detection of breast cancer. Available at: http://www.nccn.org. Accessed October 1, 2012.
18. Quanstrum KH, Hayward RA. Lessons from the mammography wars. N Engl J Med. 2010;363 :1076-1079.
19. Hubbard RA, Kerlikowske K, Flowers CI, et al. Cumulative probability of false-positive recall or biopsy recommendation after 10 years of screening mammography: a cohort study. Ann Intern Med. 2011;155:481-492.
20. Brodersen J, Siersma VD. Long-term psychosocial consequences of false-positive screening mammography. Ann Fam Med. 2013;11:106-115.
21. van Ravesteyn NT, Miglioretti DL, Stout NK, et al. Tipping the balance of benefits and harms to favor screening mammography starting at age 40 years: a comparative modeling study of risk. Ann Int Med. 2012;156:609-617.
22. Nelson HD, Zakher B, Cantor A, et al. Risk factors for breast cancer for women aged 40 to 49 years: a systematic review and metaanalysis. Ann Intern Med. 2012;156:635-648.
23. D’Orsi CJ, Bassett LW, Berg WA, et al. Breast Imaging Reporting and Data System: ACR Bi-RADS Mammography. 4th ed. Reston, VA: American College of Radiology; 2003.
24. Gierach GL, Ichikawa L, Kerikowske K, et al. Relationship between mammographic density and breast cancer death in the Breast Cancer Surveillance Consortium. J Natl Cancer Inst. 2012;104:1218-1227.
25. Boyd NJ, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227-236.
26. Saslow D, Boetets C, Burke W, et al; American Cancer Society Breast Cancer Advisory Group. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75-89.
27. Berg WA, Zhang A, Lehrer D, et al; ACRINN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404.
28. McCormark VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Epidemol Biomarkers Prev. 2006;15:1159-1169.