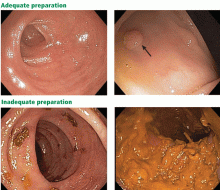
User login
Adequate bowel preparation depends on the right choice of bowel-cleansing agent. But with a myriad of products available, the right choice can be confusing to make.
This review discusses the currently recommended methods for bowel preparation before colonoscopy and suggests ways to solve common problems.
EARLY DETECTION IS KEY
Colorectal cancer is the third most common cancer in the United States and the second most common cause of cancer deaths. It largely can be prevented by detecting and removing adenomatous polyps, and survival rates are significantly better when it is diagnosed while still localized.5 Early detection, through widely applied screening programs that include colonoscopy, is thought to be playing a key role in the recent decline of colorectal cancer rates in developed countries.6
THREE TYPES OF AGENTS
Bowel preparation agents, for the most part, can be classified into one of three categories:
- Polyethylene glycol solutions, which work as high-volume gut lavage solutions
- Osmotic agents, such as sodium phosphate, magnesium citrate, lactulose, and mannitol, which draw extracellular fluid across the bowel wall and into the lumen
- Stimulants (castor oil, senna, sodium picosulfte, and bisacodyl), which work by increasing smooth muscle activity within the wall of the colon.
POLYETHYLENE GLYCOL SOLUTIONS
Bowel preparation in the past consisted of dietary restriction, stimulant laxatives, and enemas. 7,8 However, these were time-consuming (taking 48–72 hours), harsh, and not very effective for adequate visualization during colonoscopy.
In 1980, Davis et al9 developed an osmotically balanced, high-molecular weight, nonabsorbable polymer given in a dilute electrolyte solution. The osmotic effect of the polymer keeps the electrolyte solution in the colon. Since little fluid is exchanged across the colonic membrane, the potential for systemic electrolyte disturbance is limited.
Since then, these solutions have become some of the preferred bowel cleansing agents worldwide.7,8 They work as an oral lavage and hence need to be taken in high volume (typically 4 L) for bowel cleansing.
Advantages and disadvantages of polyethylene glycol solutions
Polyethylene glycol solutions are more effective and better tolerated than regimens of diet combined with cathartic agents, or high-volume balanced electrolyte solutions, or mannitol-based solutions.7 Since they are osmotically balanced and do not induce substantial shifts in fluid and electrolytes, they are safe for patients who have electrolyte imbalances, advanced liver disease, poorly compensated congestive heart failure, or renal failure.
These preparations are, however, contraindicated in patients who have allergies to polyethylene glycol compounds, gastric outlet obstruction, high-grade small-bowel obstruction, significant colonic obstruction, perforation, diverticulitis, or hemodynamic instability. In addition, they are classified by the US Food and Drug Administration (FDA) as pregnancy category C and have been associated (albeit rarely) with Mallory-Weiss tear, toxic colitis, pulmonary aspiration, hypothermia, cardiac arrhythmias, pancreatitis, and inappropriate antidiuretic hormone secretion.10,11
The main disadvantages of these solutions are the large volume of fluid (4 L) that patients must drink and their unpalatable taste, which is due to sodium sulfate. The large volume of ingestion is the main reason for nausea, bloating, cramping, and vomiting with these products, which affect patient compliance and the ultimate success of colonoscopy.
Commercially available polyethylene glycol solutions
Standard full-volume solutions (Colyte, GoLYTELY) have been widely studied and have the most evidence of safety and effectiveness. They are also inexpensive, and most insurance companies pay for them. However, about 5% to 15% of patients do not complete the preparation, because of poor palatability, large volume, or both.7
Sulfate-free and flavored solutions. To make polyethylene glycol solutions more tolerable, sulfate-free solutions have been developed. These are less salty, more palatable, and comparable to standard solutions in terms of effective colonic cleansing.12 Sulfate-free polyethylene glycol solutions commercially available in the United States are NuLytely (flavors: cherry, lemon-lime, orange, pineapple) and TriLyte (flavors: cherry, citrus-berry, lemon-lime, orange, pineapple).
Low-volume solutions have been developed in an attempt to increase acceptability and reduce volume-related adverse effects such as bloating. For example, HalfLytely (flavor: lemon-lime) consists of 2 L of polyethylene glycol solution packaged with two bisacodyl tablets. Stimulant laxatives such as bisacodyl and magnesium citrate effectively debulk the colon of solid stool and allow a lower volume of solution to be used.13,14
Also commercially available is a preparation that contains ascorbic acid (MoviPrep). Ascorbic acid acts as a flavoring and as a cathartic, also permitting a lower volume of fluid to be used.
Studies that compared full-volume and low-volume regimens (the latter including ascorbic acid, magnesium citrate, or bisacodyl) found the low-volume regimens to be as effective and more tolerable.14–18
Combining over-the-counter polyethylene glycol 3350 laxative powder (MiraLAX) and Gatorade or Crystal Light (or another clear liquid of choice) has also been shown to improve the taste and tolerability of the preparation. Although beneficial and commonly used in certain regions of the United States, this combination is not approved for bowel preparation and its use is considered off-label.
Increasing patient adherence to polyethylene glycol solutions
One way to increase tolerability and patient adherence is to split the dose so that the patient takes half the laxative prescription (polyethylene glycol or otherwise) the night before colonoscopy and the other half in the morning, usually about 4 to 5 hours before the scheduled time of the procedure.18,19
Split dosing not only improves patient acceptability, but also cleans the colon better.4 Traditional dosing, ie, drinking the entire volume of solution the night before, leaves a long interval between the end of the preparation process and the start of the procedure. Thick intestinal secretions empty out of the small intestine during that interval and obscure the cecum and ascending colon. With split dosing, the second dose is completed a few hours before the procedure, cleaning out the remaining intestinal secretions and obviating this problem.
Other measures that can make polyethylene glycol solutions more tolerable are:
- Chilling the solution
- Adding lemon slices or sugar-free flavor enhancers (such as Crystal Light) or lemon juice
- Giving the solution by nasogastric tube (at a rate of 1.2–1.8 L per hour) in patients with swallowing dysfunction or altered mental status
- Adding metoclopramide (Reglan) 5 to 10 mg orally to prevent or treat nausea
- Adding magnesium citrate (1 bottle, about 300 mL) in patients without renal insufficiency, or bisacodyl (two to four tablets of 5 mg each), so that the volume can be less15,16
- Stopping further ingestion of solution once the stool is watery and clear on the morning of the procedure (for patients who can clearly understand and follow bowel preparation instructions).17
SODIUM PHOSPHATE SOLUTIONS
Sodium phosphate is an osmotic laxative that draws water into the bowel lumen to promote colonic cleansing. Retention of water in the lumen of the colon stimulates peristalsis and bowel movements.
Advantages and disadvantages of sodium phosphate solutions
Sodium phosphate is widely used worldwide and has been found to be a very acceptable and effective bowel cleansing agent. A recent systematic review of 25 studies18 found that sodium phosphate was superior to polyethylene glycol in 14 studies, that there was no significant difference in 10 studies, and that only one study found polyethylene glycol to be better tolerated than sodium phosphate.18 Similarly, a meta-analysis19 found sodium phosphate to be more effective than polyethylene glycol in bowel cleansing (odds ratio 0.75; P = .0004); more easily completed by patients (odds ratio 0.16; P < .00001); and comparable in terms of adverse events (odds ratio 0.98; P = .81).19 However, most of the clinical trials excluded patients who had renal failure, ascites, or serious heart disease—the groups most at risk of significant adverse effects from sodium phosphate use. The main reasons sodium phosphate was better tolerated were better flavor and smaller volume (1.5–2 L compared with 4 L for polyethylene glycol).20–22
The main disadvantage of sodium phosphate is its potential to cause large fluid and electrolyte shifts. Its use has been associated with a variety of electrolyte abnormalities, including hyperphosphatemia, hypocalcemia, hypokalemia, increased plasma osmolality, hyponatremia, and, conversely, hypernatremia.7,8,23 Asymptomatic hyperphosphatemia alone can be seen in as many as 40% of healthy patients completing sodium phosphate preparations. It may be significant in patients with renal failure and can lead to acute phosphate nephropathy.
Rare adverse events such as nephrocalcinosis with acute renal failure also have been reported, especially in patients taking angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers.23
The significant volume contraction and consequent dehydration seen in some patients using sodium phosphate may be decreased by encouraging patients to drink fluids liberally, especially before the day of the procedure and after the procedure.7
Recently, renal failure due to hyperphosphatemia (acute phosphate nephropathy) has been reported even in patients with normal kidney function.24 Because of the risk of inappropriate use or overdose associated with over-the counter sodium phosphate, the FDA recommended on December 11, 2008, that sodium phosphate products be available only by prescription when they are used for bowel cleansing.25 The C.B. Fleet Company voluntarily recalled its oral sodium phosphate products sold over the counter (Fleet Phospho-Soda and Fleet EZ-PREP). In addition, the FDA required a black box warning on the prescription oral sodium phosphate products Visicol and OsmoPrep, alerting consumers to the risk of acute phosphate nephropathy.25 According to the FDA, health professionals should use caution when prescribing Visicol or OsmoPrep for patients who may be at higher risk of kidney injury, such as:
- Patients over 55 years of age
- Patients who are dehydrated or who have kidney disease, acute colitis, or delayed bowel emptying
- Patients taking certain drugs that affect kidney function, such as diuretics, ACE inhibitors, angiotensin receptor blockers, and nonsteroidal anti-inflammatory drugs.16
Commercially available sodium phosphate products
Sodium phosphate products can still be prescribed, but they are no longer available over the counter in the United States. Patients should be screened to make sure they can safely take these products, and the doses should not exceed the maximum recommended.
Figure 2 shows a simplified algorithm for selecting the optimal bowel preparation agent for an individual patient.
OTHER BOWEL PREPARATION AGENTS AND ADJUNCTS
Magnesium citrate
Like sodium phosphate, magnesium citrate is a hyperosmotic agent that promotes bowel cleansing by increasing intraluminal fluid volume. Since magnesium is eliminated solely by the kidney, it should be used with extreme caution in patients with renal insufficiency or renal failure.
Adding magnesium citrate as an adjunct to polyethylene glycol has been shown to reduce the amount of polyethylene glycol solution required (2 L) for the same result.17
For patients who cannot tolerate polyethylene glycol, a reasonable alternative is magnesium citrate (1 bottle, around 300 mL) the evening before the procedure plus either bisacodyl tablets at the same time as the magnesium citrate or rectal pulsed irrigation immediately before the procedure.7
Saline laxatives that include sodium picosulfate and magnesium citrate in combination are available primarily in the United Kingdom for bowel preparation for colonoscopy. Sodium picosulfate acts locally in the colon as a stimulant laxative and by increasing the force of laxatives, whereas magnesium citrate acts as an osmotic laxative by retaining fluids in the colon to clear the colon and rectum of fecal contents. The combination has been found to have similar efficacy and tolerability as sodium phosphate but is not currently available in the United States.26
Enemas
Enemas are sufficient for flexible sigmoidoscopy, but when used alone they do not clean out the proximal colon enough for adequate visualization during colonoscopy. They are best used as adjuncts to other bowel preparation agents when patients present with poor distal colon preparation for colonoscopy.7,27 Enemas are also useful in washing out the distal segment of bowel in patients with a proximal stoma. The common types of enemas used are tap water, sodium biphosphate (Fleet), and mineral oil.
Tap water enemas distend the rectum and mimic the natural distention by the stool to allow the rectum to empty itself. Tap water (1 L) has fewer adverse effects than sodium biphosphate or mineral oil but is less effective.
Sodium biphosphate (Fleet) enemas draw fluid into the bowel by osmotic action, prompting contraction. One or two bottles are commonly used for bowel cleansing before sigmoidoscopy. However, as with oral sodium phosphate, sodium biphosphate enemas should be avoided in the elderly and in those with renal failure because of the risk of hyperphosphatemia and subsequent hypocalcemia.
In a head-to-head comparison,28 sodium biphosphate enema was found to provide significantly better bowel preparation than the sodium picosulfate-magnesium citrate combination (currently not available in the United States) for flexible sigmoidoscopy, being judged adequate or better in 93% of procedures as opposed to 74%.28
Oil-based enemas such as cottonseed oil plus docusate (Colace) and diatrizoate sodium (Hypaque) are powerful lubricant laxatives that work by slowing the absorption of water from the bowel, so that the stool is softer. However, they have a number of adverse effects, such as severe allergic reactions (including angioedema and anaphylaxis), muscle cramps, and sporadic seepage that can soil the patient's undergarments for up to 24 hours. Also, their safety in children less than 2 years of age and in pregnant and breastfeeding mothers is not established.
Oil-based enemas are usually reserved for short-term use in refractory constipation, especially to soften feces that has become hardened within the rectum (as in fecal impaction).27
Adjuncts
Diet. Dietary modifications alone, such as a clear liquid diet, are inadequate for colonoscopy, but they may be beneficial as adjuncts to other cleansing methods by decreasing the formation of solid residue. Clear liquids also help maintain adequate hydration during bowel preparation and are recommended with all bowel preparation regimens.
Hyperosmolar or stimulant laxatives. Bisacodyl (two to four tablets of 5 mg each), magnesium citrate (one bottle, about 300 mL), and low-dose senna (36 mg, about four 8.6-mg Sennakot tablets) have been used as adjuncts to low-volume polyethylene glycol solution, achieving results similar to those with full-volume polyethylene glycol. Depending on the type of study to be done, these agents are taken within 2 to 6 hours of starting the polyethylene glycol solution.
In contrast, the routine use of nonabsorbable carbohydrates such as mannitol and lactulose is not favored for bowel preparation, since the hydrogen gas produced by bacterial fermentation of the nonabsorbed carbohydrates increases the risk of explosion during electrosurgical procedures.29
Antiemetic agents. Metoclopramide (5–10 mg), a dopamine antagonist gastroprokinetic that sensitizes tissues to the action of acetylcholine, is commonly used to prevent nausea or vomiting associated with bowel preparation agents.7,30
Antifoaming agent. Simethicone (three tablets of 80 mg each, total dose 240 mg), an anti-flatulent, anti-gas agent, is prescribed by many gastroenterologists in an attempt to reduce bubbles during colonoscopy and improve visibility. It works by reducing the surface tension of air bubbles and causing small bubbles to coalesce into larger ones that pass more easily with belching or flatulence.
Nasogastric or orogastric tubes have been used to instill colonic preparations, especially for inpatients unable to drink polyethylene glycol solutions or for patients who are unresponsive or mechanically ventilated. This method can also be useful for rapid bowel cleansing (within 2 to 3 hours) for patients with lower gastrointestinal bleeding. However, routine use of a nasogastric tube solely for bowel preparation is discouraged as it can lead to severe complications, such as aspiration and trauma during insertion.7
OTHER CONSIDERATIONS
Patient education
The importance of patient education for successful bowel preparation cannot be overemphasized. Patients need to be informed about why they need to undergo colonoscopy, the importance of bowel preparation, the side effects of agents used, and the exact preparation instructions. An interactive educational tutorial about colonoscopy for patients is available at Medline Plus at http://www.nlm.nih.gov/medlineplus/tutorials/colonoscopy/htm/index.htm.
Role of hydration
A commonly held misconception is that patients taking 4 L of polyethylene glycol do not need additional hydration, since they are already ingesting such a large volume of fluid. Given that bowel preparations induce diarrhea and, in some instances, nausea and vomiting, all patients taking bowel preparations are at risk of dehydration.32 In fact, the fluid loss during bowel preparation may exceed 2 to 3 L. It is not surprising that many safety issues associated with bowel preparation agents are related to dehydration and its complications.
Hence, patients should be advised to consume at least 64 oz (approximately 2 L) of clear fluid on the day before the colonoscopy. According to the American Society of Anesthesiologists, clear liquids can be safely ingested up until 2 hours before receiving anesthesia.33 Patients should contact their physicians if they experience vomiting or cannot comply with clear liquid volume instructions prior to colonoscopy. Metoclopramide has been found useful in many cases of nausea or vomiting associated with bowel preparation agents.18 In addition, patients should also be reminded to keep drinking extra fluids after the procedure is completed to reduce the risk of dehydration and its complications (Table 2).
- Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc 2003; 58:76–79.
- Hendry PO, Jenkins JT, Diament RH. The impact of poor bowel preparation on colonoscopy: a prospective single centre study of 10,571 colonoscopies. Colorectal Dis 2007; 9:745–748.
- Burke CA, Church JM. Enhancing the quality of colonoscopy: the importance of bowel purgatives. Gastrointest Endosc 2007; 66:565–573.
- Rex DK, Imperiale TF, Latinovich DR, Bratcher LL. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol 2002; 97:1696–1700.
- Levin B, Lieberman DA, McFarland B, et al; American Cancer Society Colorectal Cancer Advisory Group. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008; 134:1570–1595.
- Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer 2007; 110:2119–2152.
- Wexner SD, Beck DE, Baron TH, et al. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Dis Colon Rectum 2006; 49:792–809.
- Barkun A, Chiba N, Enns R, et al. Commonly used preparations for colonoscopy: efficacy, tolerability, and safety—a Canadian Association of Gastroenterology position paper. Can J Gastroenterol 2006; 20:699–710.
- Davis GR, Santa Ana CA, Morawski SG, Fordtran JS. Development of a lavage solution associated with minimal water and electrolyte absorption or secretion. Gastroenterology 1980; 78:991–995.
- Clark LE, Dipalma JA. Safety issues regarding colonic cleansing for diagnostic and surgical procedures. Drug Saf 2004; 27:1235–1242.
- Nelson DB, Barkun AN, Block KP, et al. Technology Status Evaluation report. Colonoscopy preparations. May 2001. Gastrointest Endosc 2001; 54:829–832.
- DiPalma JA, Marshall JB. Comparison of a new sulfate-free polyethylene glycol electrolyte lavage solution versus a standard solution for colonoscopy cleansing. Gastrointest Endosc 1990; 36:285–289.
- DiPalma JA, Wolff BG, Meagher A, Cleveland M. Comparison of reduced volume versus four liters sulfate-free electrolyte lavage solutions for colonoscopy colon cleansing. Am J Gastroenterol 2003; 98:2187–2191.
- Ell C, Fischbach W, Bronisch HJ, et al. Randomized trial of low-volume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am J Gastroenterol 2008; 103:883–893.
- Adams WJ, Meagher AP, Lubowski DZ, King DW. Bisacodyl reduces the volume of polyethylene glycol solution required for bowel preparation. Dis Colon Rectum 1994; 37:229–233.
- Ker TS. Comparison of reduced volume versus four-liter electrolyte lavage solutions for colon cleansing. Am Surg 2006; 72:909–911.
- Sharma VK, Steinberg EN, Vasudeva R, Howden CW. Randomized, controlled study of pretreatment with magnesium citrate on the quality of colonoscopy preparation with polyethylene glycol electrolyte lavage solution. Gastrointest Endosc 1997; 46:541–543.
- Belsey J, Epstein O, Heresbach D. Systematic review: oral bowel preparation for colonoscopy. Aliment Pharmacol Ther 2007; 25:373–384.
- Tan JJ, Tjandra JJ. Which is the optimal bowel preparation for colonoscopy—a meta-analysis. Colorectal Dis 2006; 8:247–258.
- Kastenberg D, Chasen R, Choudhary C, et al. Efficacy and safety of sodium phosphate tablets compared with PEG solution in colon cleansing: two identically designed, randomized, controlled, parallel group, multicenter phase III trials. Gastrointest Endosc 2001; 54:705–713.
- Balaban DH, Leavell BS, Oblinger MJ, Thompson WO, Bolton ND, Pambianco DJ. Low volume bowel preparation for colonoscopy: randomized, endoscopist-blinded trial of liquid sodium phosphate versus tablet sodium phosphate. Am J Gastroenterol 2003; 98:827–832.
- Rex DK. 10 Questions You Need to Ask About Colonoscopy. New York Times February 25, 2009. http://www.nytimes.com/2009/02/24/health/esn-colonoscopy-expert.html?_r=1. Accessed March 14, 2010.
- Makkar R, Shen B. What are the caveats to using sodium phosphate agents for bowel preparation? Cleve Clin J Med 2008; 75:173–176.
- Hookey LC, Depew WT, Vanner S. The safety profile of oral sodium phosphate for colonic cleansing before colonoscopy in adults. Gastrointestinal Endosc 2002; 56:895–902.
- US Food and Drug Administration (FDA). Oral Sodium Phosphate (OSP) Products for Bowel Cleansing (marketed as Visicol and OsmoPrep, and oral sodium phosphate products available without a prescription). FDA Alert. December 11, 2008. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm094900.htm. Accessed March 14, 2010.
- Hoy SM, Scott LJ, Wagstaff AJ. Sodium picosulfate/magnesium citrate: a review of its use as a colorectal cleanser. Drugs 2009; 69:123–136.
- Sohn N, Weinstein MA. Management of the poorly prepared colonoscopy patient: colonoscopic colon enemas as a preparation for colonoscopy. Dis Colon Rectum 2008; 51:462–466.
- Drew PJ, Hughes M, Hodson R, et al. The optimum bowel preparation for flexible sigmoidoscopy. Eur J Surg Oncol 1997; 23:315–316.
- Bigard MA, Gaucher P, Lassalle C. Fatal colonic explosion during colonoscopic polypectomy. Gastroenterology 1979; 77:1307–1310.
- Rhodes JB, Engstrom J, Stone KF. Metoclopramide reduces the distress associated with colon cleansing by an oral electrolyte overload. Gastrointest Endosc 1978; 24:162–163.
- Abuksis G, Mor M, Segal N, et al. A patient education program is cost-effective for preventing failure of endoscopic procedures in a gastroenterology department. Am J Gastroenterol 2001; 96:1786–1790.
- Dykes C, Cash BD. Key safety issues of bowel preparations for colonoscopy and importance of adequate hydration. Gastroenterol Nurs 2008; 31:30–35.
- Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: a report by the American Society of Anesthesiologist Task Force on Preoperative Fasting. Anesthesiology 1999; 90:896–905.
Adequate bowel preparation depends on the right choice of bowel-cleansing agent. But with a myriad of products available, the right choice can be confusing to make.
This review discusses the currently recommended methods for bowel preparation before colonoscopy and suggests ways to solve common problems.
EARLY DETECTION IS KEY
Colorectal cancer is the third most common cancer in the United States and the second most common cause of cancer deaths. It largely can be prevented by detecting and removing adenomatous polyps, and survival rates are significantly better when it is diagnosed while still localized.5 Early detection, through widely applied screening programs that include colonoscopy, is thought to be playing a key role in the recent decline of colorectal cancer rates in developed countries.6
THREE TYPES OF AGENTS
Bowel preparation agents, for the most part, can be classified into one of three categories:
- Polyethylene glycol solutions, which work as high-volume gut lavage solutions
- Osmotic agents, such as sodium phosphate, magnesium citrate, lactulose, and mannitol, which draw extracellular fluid across the bowel wall and into the lumen
- Stimulants (castor oil, senna, sodium picosulfte, and bisacodyl), which work by increasing smooth muscle activity within the wall of the colon.
POLYETHYLENE GLYCOL SOLUTIONS
Bowel preparation in the past consisted of dietary restriction, stimulant laxatives, and enemas. 7,8 However, these were time-consuming (taking 48–72 hours), harsh, and not very effective for adequate visualization during colonoscopy.
In 1980, Davis et al9 developed an osmotically balanced, high-molecular weight, nonabsorbable polymer given in a dilute electrolyte solution. The osmotic effect of the polymer keeps the electrolyte solution in the colon. Since little fluid is exchanged across the colonic membrane, the potential for systemic electrolyte disturbance is limited.
Since then, these solutions have become some of the preferred bowel cleansing agents worldwide.7,8 They work as an oral lavage and hence need to be taken in high volume (typically 4 L) for bowel cleansing.
Advantages and disadvantages of polyethylene glycol solutions
Polyethylene glycol solutions are more effective and better tolerated than regimens of diet combined with cathartic agents, or high-volume balanced electrolyte solutions, or mannitol-based solutions.7 Since they are osmotically balanced and do not induce substantial shifts in fluid and electrolytes, they are safe for patients who have electrolyte imbalances, advanced liver disease, poorly compensated congestive heart failure, or renal failure.
These preparations are, however, contraindicated in patients who have allergies to polyethylene glycol compounds, gastric outlet obstruction, high-grade small-bowel obstruction, significant colonic obstruction, perforation, diverticulitis, or hemodynamic instability. In addition, they are classified by the US Food and Drug Administration (FDA) as pregnancy category C and have been associated (albeit rarely) with Mallory-Weiss tear, toxic colitis, pulmonary aspiration, hypothermia, cardiac arrhythmias, pancreatitis, and inappropriate antidiuretic hormone secretion.10,11
The main disadvantages of these solutions are the large volume of fluid (4 L) that patients must drink and their unpalatable taste, which is due to sodium sulfate. The large volume of ingestion is the main reason for nausea, bloating, cramping, and vomiting with these products, which affect patient compliance and the ultimate success of colonoscopy.
Commercially available polyethylene glycol solutions
Standard full-volume solutions (Colyte, GoLYTELY) have been widely studied and have the most evidence of safety and effectiveness. They are also inexpensive, and most insurance companies pay for them. However, about 5% to 15% of patients do not complete the preparation, because of poor palatability, large volume, or both.7
Sulfate-free and flavored solutions. To make polyethylene glycol solutions more tolerable, sulfate-free solutions have been developed. These are less salty, more palatable, and comparable to standard solutions in terms of effective colonic cleansing.12 Sulfate-free polyethylene glycol solutions commercially available in the United States are NuLytely (flavors: cherry, lemon-lime, orange, pineapple) and TriLyte (flavors: cherry, citrus-berry, lemon-lime, orange, pineapple).
Low-volume solutions have been developed in an attempt to increase acceptability and reduce volume-related adverse effects such as bloating. For example, HalfLytely (flavor: lemon-lime) consists of 2 L of polyethylene glycol solution packaged with two bisacodyl tablets. Stimulant laxatives such as bisacodyl and magnesium citrate effectively debulk the colon of solid stool and allow a lower volume of solution to be used.13,14
Also commercially available is a preparation that contains ascorbic acid (MoviPrep). Ascorbic acid acts as a flavoring and as a cathartic, also permitting a lower volume of fluid to be used.
Studies that compared full-volume and low-volume regimens (the latter including ascorbic acid, magnesium citrate, or bisacodyl) found the low-volume regimens to be as effective and more tolerable.14–18
Combining over-the-counter polyethylene glycol 3350 laxative powder (MiraLAX) and Gatorade or Crystal Light (or another clear liquid of choice) has also been shown to improve the taste and tolerability of the preparation. Although beneficial and commonly used in certain regions of the United States, this combination is not approved for bowel preparation and its use is considered off-label.
Increasing patient adherence to polyethylene glycol solutions
One way to increase tolerability and patient adherence is to split the dose so that the patient takes half the laxative prescription (polyethylene glycol or otherwise) the night before colonoscopy and the other half in the morning, usually about 4 to 5 hours before the scheduled time of the procedure.18,19
Split dosing not only improves patient acceptability, but also cleans the colon better.4 Traditional dosing, ie, drinking the entire volume of solution the night before, leaves a long interval between the end of the preparation process and the start of the procedure. Thick intestinal secretions empty out of the small intestine during that interval and obscure the cecum and ascending colon. With split dosing, the second dose is completed a few hours before the procedure, cleaning out the remaining intestinal secretions and obviating this problem.
Other measures that can make polyethylene glycol solutions more tolerable are:
- Chilling the solution
- Adding lemon slices or sugar-free flavor enhancers (such as Crystal Light) or lemon juice
- Giving the solution by nasogastric tube (at a rate of 1.2–1.8 L per hour) in patients with swallowing dysfunction or altered mental status
- Adding metoclopramide (Reglan) 5 to 10 mg orally to prevent or treat nausea
- Adding magnesium citrate (1 bottle, about 300 mL) in patients without renal insufficiency, or bisacodyl (two to four tablets of 5 mg each), so that the volume can be less15,16
- Stopping further ingestion of solution once the stool is watery and clear on the morning of the procedure (for patients who can clearly understand and follow bowel preparation instructions).17
SODIUM PHOSPHATE SOLUTIONS
Sodium phosphate is an osmotic laxative that draws water into the bowel lumen to promote colonic cleansing. Retention of water in the lumen of the colon stimulates peristalsis and bowel movements.
Advantages and disadvantages of sodium phosphate solutions
Sodium phosphate is widely used worldwide and has been found to be a very acceptable and effective bowel cleansing agent. A recent systematic review of 25 studies18 found that sodium phosphate was superior to polyethylene glycol in 14 studies, that there was no significant difference in 10 studies, and that only one study found polyethylene glycol to be better tolerated than sodium phosphate.18 Similarly, a meta-analysis19 found sodium phosphate to be more effective than polyethylene glycol in bowel cleansing (odds ratio 0.75; P = .0004); more easily completed by patients (odds ratio 0.16; P < .00001); and comparable in terms of adverse events (odds ratio 0.98; P = .81).19 However, most of the clinical trials excluded patients who had renal failure, ascites, or serious heart disease—the groups most at risk of significant adverse effects from sodium phosphate use. The main reasons sodium phosphate was better tolerated were better flavor and smaller volume (1.5–2 L compared with 4 L for polyethylene glycol).20–22
The main disadvantage of sodium phosphate is its potential to cause large fluid and electrolyte shifts. Its use has been associated with a variety of electrolyte abnormalities, including hyperphosphatemia, hypocalcemia, hypokalemia, increased plasma osmolality, hyponatremia, and, conversely, hypernatremia.7,8,23 Asymptomatic hyperphosphatemia alone can be seen in as many as 40% of healthy patients completing sodium phosphate preparations. It may be significant in patients with renal failure and can lead to acute phosphate nephropathy.
Rare adverse events such as nephrocalcinosis with acute renal failure also have been reported, especially in patients taking angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers.23
The significant volume contraction and consequent dehydration seen in some patients using sodium phosphate may be decreased by encouraging patients to drink fluids liberally, especially before the day of the procedure and after the procedure.7
Recently, renal failure due to hyperphosphatemia (acute phosphate nephropathy) has been reported even in patients with normal kidney function.24 Because of the risk of inappropriate use or overdose associated with over-the counter sodium phosphate, the FDA recommended on December 11, 2008, that sodium phosphate products be available only by prescription when they are used for bowel cleansing.25 The C.B. Fleet Company voluntarily recalled its oral sodium phosphate products sold over the counter (Fleet Phospho-Soda and Fleet EZ-PREP). In addition, the FDA required a black box warning on the prescription oral sodium phosphate products Visicol and OsmoPrep, alerting consumers to the risk of acute phosphate nephropathy.25 According to the FDA, health professionals should use caution when prescribing Visicol or OsmoPrep for patients who may be at higher risk of kidney injury, such as:
- Patients over 55 years of age
- Patients who are dehydrated or who have kidney disease, acute colitis, or delayed bowel emptying
- Patients taking certain drugs that affect kidney function, such as diuretics, ACE inhibitors, angiotensin receptor blockers, and nonsteroidal anti-inflammatory drugs.16
Commercially available sodium phosphate products
Sodium phosphate products can still be prescribed, but they are no longer available over the counter in the United States. Patients should be screened to make sure they can safely take these products, and the doses should not exceed the maximum recommended.
Figure 2 shows a simplified algorithm for selecting the optimal bowel preparation agent for an individual patient.
OTHER BOWEL PREPARATION AGENTS AND ADJUNCTS
Magnesium citrate
Like sodium phosphate, magnesium citrate is a hyperosmotic agent that promotes bowel cleansing by increasing intraluminal fluid volume. Since magnesium is eliminated solely by the kidney, it should be used with extreme caution in patients with renal insufficiency or renal failure.
Adding magnesium citrate as an adjunct to polyethylene glycol has been shown to reduce the amount of polyethylene glycol solution required (2 L) for the same result.17
For patients who cannot tolerate polyethylene glycol, a reasonable alternative is magnesium citrate (1 bottle, around 300 mL) the evening before the procedure plus either bisacodyl tablets at the same time as the magnesium citrate or rectal pulsed irrigation immediately before the procedure.7
Saline laxatives that include sodium picosulfate and magnesium citrate in combination are available primarily in the United Kingdom for bowel preparation for colonoscopy. Sodium picosulfate acts locally in the colon as a stimulant laxative and by increasing the force of laxatives, whereas magnesium citrate acts as an osmotic laxative by retaining fluids in the colon to clear the colon and rectum of fecal contents. The combination has been found to have similar efficacy and tolerability as sodium phosphate but is not currently available in the United States.26
Enemas
Enemas are sufficient for flexible sigmoidoscopy, but when used alone they do not clean out the proximal colon enough for adequate visualization during colonoscopy. They are best used as adjuncts to other bowel preparation agents when patients present with poor distal colon preparation for colonoscopy.7,27 Enemas are also useful in washing out the distal segment of bowel in patients with a proximal stoma. The common types of enemas used are tap water, sodium biphosphate (Fleet), and mineral oil.
Tap water enemas distend the rectum and mimic the natural distention by the stool to allow the rectum to empty itself. Tap water (1 L) has fewer adverse effects than sodium biphosphate or mineral oil but is less effective.
Sodium biphosphate (Fleet) enemas draw fluid into the bowel by osmotic action, prompting contraction. One or two bottles are commonly used for bowel cleansing before sigmoidoscopy. However, as with oral sodium phosphate, sodium biphosphate enemas should be avoided in the elderly and in those with renal failure because of the risk of hyperphosphatemia and subsequent hypocalcemia.
In a head-to-head comparison,28 sodium biphosphate enema was found to provide significantly better bowel preparation than the sodium picosulfate-magnesium citrate combination (currently not available in the United States) for flexible sigmoidoscopy, being judged adequate or better in 93% of procedures as opposed to 74%.28
Oil-based enemas such as cottonseed oil plus docusate (Colace) and diatrizoate sodium (Hypaque) are powerful lubricant laxatives that work by slowing the absorption of water from the bowel, so that the stool is softer. However, they have a number of adverse effects, such as severe allergic reactions (including angioedema and anaphylaxis), muscle cramps, and sporadic seepage that can soil the patient's undergarments for up to 24 hours. Also, their safety in children less than 2 years of age and in pregnant and breastfeeding mothers is not established.
Oil-based enemas are usually reserved for short-term use in refractory constipation, especially to soften feces that has become hardened within the rectum (as in fecal impaction).27
Adjuncts
Diet. Dietary modifications alone, such as a clear liquid diet, are inadequate for colonoscopy, but they may be beneficial as adjuncts to other cleansing methods by decreasing the formation of solid residue. Clear liquids also help maintain adequate hydration during bowel preparation and are recommended with all bowel preparation regimens.
Hyperosmolar or stimulant laxatives. Bisacodyl (two to four tablets of 5 mg each), magnesium citrate (one bottle, about 300 mL), and low-dose senna (36 mg, about four 8.6-mg Sennakot tablets) have been used as adjuncts to low-volume polyethylene glycol solution, achieving results similar to those with full-volume polyethylene glycol. Depending on the type of study to be done, these agents are taken within 2 to 6 hours of starting the polyethylene glycol solution.
In contrast, the routine use of nonabsorbable carbohydrates such as mannitol and lactulose is not favored for bowel preparation, since the hydrogen gas produced by bacterial fermentation of the nonabsorbed carbohydrates increases the risk of explosion during electrosurgical procedures.29
Antiemetic agents. Metoclopramide (5–10 mg), a dopamine antagonist gastroprokinetic that sensitizes tissues to the action of acetylcholine, is commonly used to prevent nausea or vomiting associated with bowel preparation agents.7,30
Antifoaming agent. Simethicone (three tablets of 80 mg each, total dose 240 mg), an anti-flatulent, anti-gas agent, is prescribed by many gastroenterologists in an attempt to reduce bubbles during colonoscopy and improve visibility. It works by reducing the surface tension of air bubbles and causing small bubbles to coalesce into larger ones that pass more easily with belching or flatulence.
Nasogastric or orogastric tubes have been used to instill colonic preparations, especially for inpatients unable to drink polyethylene glycol solutions or for patients who are unresponsive or mechanically ventilated. This method can also be useful for rapid bowel cleansing (within 2 to 3 hours) for patients with lower gastrointestinal bleeding. However, routine use of a nasogastric tube solely for bowel preparation is discouraged as it can lead to severe complications, such as aspiration and trauma during insertion.7
OTHER CONSIDERATIONS
Patient education
The importance of patient education for successful bowel preparation cannot be overemphasized. Patients need to be informed about why they need to undergo colonoscopy, the importance of bowel preparation, the side effects of agents used, and the exact preparation instructions. An interactive educational tutorial about colonoscopy for patients is available at Medline Plus at http://www.nlm.nih.gov/medlineplus/tutorials/colonoscopy/htm/index.htm.
Role of hydration
A commonly held misconception is that patients taking 4 L of polyethylene glycol do not need additional hydration, since they are already ingesting such a large volume of fluid. Given that bowel preparations induce diarrhea and, in some instances, nausea and vomiting, all patients taking bowel preparations are at risk of dehydration.32 In fact, the fluid loss during bowel preparation may exceed 2 to 3 L. It is not surprising that many safety issues associated with bowel preparation agents are related to dehydration and its complications.
Hence, patients should be advised to consume at least 64 oz (approximately 2 L) of clear fluid on the day before the colonoscopy. According to the American Society of Anesthesiologists, clear liquids can be safely ingested up until 2 hours before receiving anesthesia.33 Patients should contact their physicians if they experience vomiting or cannot comply with clear liquid volume instructions prior to colonoscopy. Metoclopramide has been found useful in many cases of nausea or vomiting associated with bowel preparation agents.18 In addition, patients should also be reminded to keep drinking extra fluids after the procedure is completed to reduce the risk of dehydration and its complications (Table 2).
Adequate bowel preparation depends on the right choice of bowel-cleansing agent. But with a myriad of products available, the right choice can be confusing to make.
This review discusses the currently recommended methods for bowel preparation before colonoscopy and suggests ways to solve common problems.
EARLY DETECTION IS KEY
Colorectal cancer is the third most common cancer in the United States and the second most common cause of cancer deaths. It largely can be prevented by detecting and removing adenomatous polyps, and survival rates are significantly better when it is diagnosed while still localized.5 Early detection, through widely applied screening programs that include colonoscopy, is thought to be playing a key role in the recent decline of colorectal cancer rates in developed countries.6
THREE TYPES OF AGENTS
Bowel preparation agents, for the most part, can be classified into one of three categories:
- Polyethylene glycol solutions, which work as high-volume gut lavage solutions
- Osmotic agents, such as sodium phosphate, magnesium citrate, lactulose, and mannitol, which draw extracellular fluid across the bowel wall and into the lumen
- Stimulants (castor oil, senna, sodium picosulfte, and bisacodyl), which work by increasing smooth muscle activity within the wall of the colon.
POLYETHYLENE GLYCOL SOLUTIONS
Bowel preparation in the past consisted of dietary restriction, stimulant laxatives, and enemas. 7,8 However, these were time-consuming (taking 48–72 hours), harsh, and not very effective for adequate visualization during colonoscopy.
In 1980, Davis et al9 developed an osmotically balanced, high-molecular weight, nonabsorbable polymer given in a dilute electrolyte solution. The osmotic effect of the polymer keeps the electrolyte solution in the colon. Since little fluid is exchanged across the colonic membrane, the potential for systemic electrolyte disturbance is limited.
Since then, these solutions have become some of the preferred bowel cleansing agents worldwide.7,8 They work as an oral lavage and hence need to be taken in high volume (typically 4 L) for bowel cleansing.
Advantages and disadvantages of polyethylene glycol solutions
Polyethylene glycol solutions are more effective and better tolerated than regimens of diet combined with cathartic agents, or high-volume balanced electrolyte solutions, or mannitol-based solutions.7 Since they are osmotically balanced and do not induce substantial shifts in fluid and electrolytes, they are safe for patients who have electrolyte imbalances, advanced liver disease, poorly compensated congestive heart failure, or renal failure.
These preparations are, however, contraindicated in patients who have allergies to polyethylene glycol compounds, gastric outlet obstruction, high-grade small-bowel obstruction, significant colonic obstruction, perforation, diverticulitis, or hemodynamic instability. In addition, they are classified by the US Food and Drug Administration (FDA) as pregnancy category C and have been associated (albeit rarely) with Mallory-Weiss tear, toxic colitis, pulmonary aspiration, hypothermia, cardiac arrhythmias, pancreatitis, and inappropriate antidiuretic hormone secretion.10,11
The main disadvantages of these solutions are the large volume of fluid (4 L) that patients must drink and their unpalatable taste, which is due to sodium sulfate. The large volume of ingestion is the main reason for nausea, bloating, cramping, and vomiting with these products, which affect patient compliance and the ultimate success of colonoscopy.
Commercially available polyethylene glycol solutions
Standard full-volume solutions (Colyte, GoLYTELY) have been widely studied and have the most evidence of safety and effectiveness. They are also inexpensive, and most insurance companies pay for them. However, about 5% to 15% of patients do not complete the preparation, because of poor palatability, large volume, or both.7
Sulfate-free and flavored solutions. To make polyethylene glycol solutions more tolerable, sulfate-free solutions have been developed. These are less salty, more palatable, and comparable to standard solutions in terms of effective colonic cleansing.12 Sulfate-free polyethylene glycol solutions commercially available in the United States are NuLytely (flavors: cherry, lemon-lime, orange, pineapple) and TriLyte (flavors: cherry, citrus-berry, lemon-lime, orange, pineapple).
Low-volume solutions have been developed in an attempt to increase acceptability and reduce volume-related adverse effects such as bloating. For example, HalfLytely (flavor: lemon-lime) consists of 2 L of polyethylene glycol solution packaged with two bisacodyl tablets. Stimulant laxatives such as bisacodyl and magnesium citrate effectively debulk the colon of solid stool and allow a lower volume of solution to be used.13,14
Also commercially available is a preparation that contains ascorbic acid (MoviPrep). Ascorbic acid acts as a flavoring and as a cathartic, also permitting a lower volume of fluid to be used.
Studies that compared full-volume and low-volume regimens (the latter including ascorbic acid, magnesium citrate, or bisacodyl) found the low-volume regimens to be as effective and more tolerable.14–18
Combining over-the-counter polyethylene glycol 3350 laxative powder (MiraLAX) and Gatorade or Crystal Light (or another clear liquid of choice) has also been shown to improve the taste and tolerability of the preparation. Although beneficial and commonly used in certain regions of the United States, this combination is not approved for bowel preparation and its use is considered off-label.
Increasing patient adherence to polyethylene glycol solutions
One way to increase tolerability and patient adherence is to split the dose so that the patient takes half the laxative prescription (polyethylene glycol or otherwise) the night before colonoscopy and the other half in the morning, usually about 4 to 5 hours before the scheduled time of the procedure.18,19
Split dosing not only improves patient acceptability, but also cleans the colon better.4 Traditional dosing, ie, drinking the entire volume of solution the night before, leaves a long interval between the end of the preparation process and the start of the procedure. Thick intestinal secretions empty out of the small intestine during that interval and obscure the cecum and ascending colon. With split dosing, the second dose is completed a few hours before the procedure, cleaning out the remaining intestinal secretions and obviating this problem.
Other measures that can make polyethylene glycol solutions more tolerable are:
- Chilling the solution
- Adding lemon slices or sugar-free flavor enhancers (such as Crystal Light) or lemon juice
- Giving the solution by nasogastric tube (at a rate of 1.2–1.8 L per hour) in patients with swallowing dysfunction or altered mental status
- Adding metoclopramide (Reglan) 5 to 10 mg orally to prevent or treat nausea
- Adding magnesium citrate (1 bottle, about 300 mL) in patients without renal insufficiency, or bisacodyl (two to four tablets of 5 mg each), so that the volume can be less15,16
- Stopping further ingestion of solution once the stool is watery and clear on the morning of the procedure (for patients who can clearly understand and follow bowel preparation instructions).17
SODIUM PHOSPHATE SOLUTIONS
Sodium phosphate is an osmotic laxative that draws water into the bowel lumen to promote colonic cleansing. Retention of water in the lumen of the colon stimulates peristalsis and bowel movements.
Advantages and disadvantages of sodium phosphate solutions
Sodium phosphate is widely used worldwide and has been found to be a very acceptable and effective bowel cleansing agent. A recent systematic review of 25 studies18 found that sodium phosphate was superior to polyethylene glycol in 14 studies, that there was no significant difference in 10 studies, and that only one study found polyethylene glycol to be better tolerated than sodium phosphate.18 Similarly, a meta-analysis19 found sodium phosphate to be more effective than polyethylene glycol in bowel cleansing (odds ratio 0.75; P = .0004); more easily completed by patients (odds ratio 0.16; P < .00001); and comparable in terms of adverse events (odds ratio 0.98; P = .81).19 However, most of the clinical trials excluded patients who had renal failure, ascites, or serious heart disease—the groups most at risk of significant adverse effects from sodium phosphate use. The main reasons sodium phosphate was better tolerated were better flavor and smaller volume (1.5–2 L compared with 4 L for polyethylene glycol).20–22
The main disadvantage of sodium phosphate is its potential to cause large fluid and electrolyte shifts. Its use has been associated with a variety of electrolyte abnormalities, including hyperphosphatemia, hypocalcemia, hypokalemia, increased plasma osmolality, hyponatremia, and, conversely, hypernatremia.7,8,23 Asymptomatic hyperphosphatemia alone can be seen in as many as 40% of healthy patients completing sodium phosphate preparations. It may be significant in patients with renal failure and can lead to acute phosphate nephropathy.
Rare adverse events such as nephrocalcinosis with acute renal failure also have been reported, especially in patients taking angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers.23
The significant volume contraction and consequent dehydration seen in some patients using sodium phosphate may be decreased by encouraging patients to drink fluids liberally, especially before the day of the procedure and after the procedure.7
Recently, renal failure due to hyperphosphatemia (acute phosphate nephropathy) has been reported even in patients with normal kidney function.24 Because of the risk of inappropriate use or overdose associated with over-the counter sodium phosphate, the FDA recommended on December 11, 2008, that sodium phosphate products be available only by prescription when they are used for bowel cleansing.25 The C.B. Fleet Company voluntarily recalled its oral sodium phosphate products sold over the counter (Fleet Phospho-Soda and Fleet EZ-PREP). In addition, the FDA required a black box warning on the prescription oral sodium phosphate products Visicol and OsmoPrep, alerting consumers to the risk of acute phosphate nephropathy.25 According to the FDA, health professionals should use caution when prescribing Visicol or OsmoPrep for patients who may be at higher risk of kidney injury, such as:
- Patients over 55 years of age
- Patients who are dehydrated or who have kidney disease, acute colitis, or delayed bowel emptying
- Patients taking certain drugs that affect kidney function, such as diuretics, ACE inhibitors, angiotensin receptor blockers, and nonsteroidal anti-inflammatory drugs.16
Commercially available sodium phosphate products
Sodium phosphate products can still be prescribed, but they are no longer available over the counter in the United States. Patients should be screened to make sure they can safely take these products, and the doses should not exceed the maximum recommended.
Figure 2 shows a simplified algorithm for selecting the optimal bowel preparation agent for an individual patient.
OTHER BOWEL PREPARATION AGENTS AND ADJUNCTS
Magnesium citrate
Like sodium phosphate, magnesium citrate is a hyperosmotic agent that promotes bowel cleansing by increasing intraluminal fluid volume. Since magnesium is eliminated solely by the kidney, it should be used with extreme caution in patients with renal insufficiency or renal failure.
Adding magnesium citrate as an adjunct to polyethylene glycol has been shown to reduce the amount of polyethylene glycol solution required (2 L) for the same result.17
For patients who cannot tolerate polyethylene glycol, a reasonable alternative is magnesium citrate (1 bottle, around 300 mL) the evening before the procedure plus either bisacodyl tablets at the same time as the magnesium citrate or rectal pulsed irrigation immediately before the procedure.7
Saline laxatives that include sodium picosulfate and magnesium citrate in combination are available primarily in the United Kingdom for bowel preparation for colonoscopy. Sodium picosulfate acts locally in the colon as a stimulant laxative and by increasing the force of laxatives, whereas magnesium citrate acts as an osmotic laxative by retaining fluids in the colon to clear the colon and rectum of fecal contents. The combination has been found to have similar efficacy and tolerability as sodium phosphate but is not currently available in the United States.26
Enemas
Enemas are sufficient for flexible sigmoidoscopy, but when used alone they do not clean out the proximal colon enough for adequate visualization during colonoscopy. They are best used as adjuncts to other bowel preparation agents when patients present with poor distal colon preparation for colonoscopy.7,27 Enemas are also useful in washing out the distal segment of bowel in patients with a proximal stoma. The common types of enemas used are tap water, sodium biphosphate (Fleet), and mineral oil.
Tap water enemas distend the rectum and mimic the natural distention by the stool to allow the rectum to empty itself. Tap water (1 L) has fewer adverse effects than sodium biphosphate or mineral oil but is less effective.
Sodium biphosphate (Fleet) enemas draw fluid into the bowel by osmotic action, prompting contraction. One or two bottles are commonly used for bowel cleansing before sigmoidoscopy. However, as with oral sodium phosphate, sodium biphosphate enemas should be avoided in the elderly and in those with renal failure because of the risk of hyperphosphatemia and subsequent hypocalcemia.
In a head-to-head comparison,28 sodium biphosphate enema was found to provide significantly better bowel preparation than the sodium picosulfate-magnesium citrate combination (currently not available in the United States) for flexible sigmoidoscopy, being judged adequate or better in 93% of procedures as opposed to 74%.28
Oil-based enemas such as cottonseed oil plus docusate (Colace) and diatrizoate sodium (Hypaque) are powerful lubricant laxatives that work by slowing the absorption of water from the bowel, so that the stool is softer. However, they have a number of adverse effects, such as severe allergic reactions (including angioedema and anaphylaxis), muscle cramps, and sporadic seepage that can soil the patient's undergarments for up to 24 hours. Also, their safety in children less than 2 years of age and in pregnant and breastfeeding mothers is not established.
Oil-based enemas are usually reserved for short-term use in refractory constipation, especially to soften feces that has become hardened within the rectum (as in fecal impaction).27
Adjuncts
Diet. Dietary modifications alone, such as a clear liquid diet, are inadequate for colonoscopy, but they may be beneficial as adjuncts to other cleansing methods by decreasing the formation of solid residue. Clear liquids also help maintain adequate hydration during bowel preparation and are recommended with all bowel preparation regimens.
Hyperosmolar or stimulant laxatives. Bisacodyl (two to four tablets of 5 mg each), magnesium citrate (one bottle, about 300 mL), and low-dose senna (36 mg, about four 8.6-mg Sennakot tablets) have been used as adjuncts to low-volume polyethylene glycol solution, achieving results similar to those with full-volume polyethylene glycol. Depending on the type of study to be done, these agents are taken within 2 to 6 hours of starting the polyethylene glycol solution.
In contrast, the routine use of nonabsorbable carbohydrates such as mannitol and lactulose is not favored for bowel preparation, since the hydrogen gas produced by bacterial fermentation of the nonabsorbed carbohydrates increases the risk of explosion during electrosurgical procedures.29
Antiemetic agents. Metoclopramide (5–10 mg), a dopamine antagonist gastroprokinetic that sensitizes tissues to the action of acetylcholine, is commonly used to prevent nausea or vomiting associated with bowel preparation agents.7,30
Antifoaming agent. Simethicone (three tablets of 80 mg each, total dose 240 mg), an anti-flatulent, anti-gas agent, is prescribed by many gastroenterologists in an attempt to reduce bubbles during colonoscopy and improve visibility. It works by reducing the surface tension of air bubbles and causing small bubbles to coalesce into larger ones that pass more easily with belching or flatulence.
Nasogastric or orogastric tubes have been used to instill colonic preparations, especially for inpatients unable to drink polyethylene glycol solutions or for patients who are unresponsive or mechanically ventilated. This method can also be useful for rapid bowel cleansing (within 2 to 3 hours) for patients with lower gastrointestinal bleeding. However, routine use of a nasogastric tube solely for bowel preparation is discouraged as it can lead to severe complications, such as aspiration and trauma during insertion.7
OTHER CONSIDERATIONS
Patient education
The importance of patient education for successful bowel preparation cannot be overemphasized. Patients need to be informed about why they need to undergo colonoscopy, the importance of bowel preparation, the side effects of agents used, and the exact preparation instructions. An interactive educational tutorial about colonoscopy for patients is available at Medline Plus at http://www.nlm.nih.gov/medlineplus/tutorials/colonoscopy/htm/index.htm.
Role of hydration
A commonly held misconception is that patients taking 4 L of polyethylene glycol do not need additional hydration, since they are already ingesting such a large volume of fluid. Given that bowel preparations induce diarrhea and, in some instances, nausea and vomiting, all patients taking bowel preparations are at risk of dehydration.32 In fact, the fluid loss during bowel preparation may exceed 2 to 3 L. It is not surprising that many safety issues associated with bowel preparation agents are related to dehydration and its complications.
Hence, patients should be advised to consume at least 64 oz (approximately 2 L) of clear fluid on the day before the colonoscopy. According to the American Society of Anesthesiologists, clear liquids can be safely ingested up until 2 hours before receiving anesthesia.33 Patients should contact their physicians if they experience vomiting or cannot comply with clear liquid volume instructions prior to colonoscopy. Metoclopramide has been found useful in many cases of nausea or vomiting associated with bowel preparation agents.18 In addition, patients should also be reminded to keep drinking extra fluids after the procedure is completed to reduce the risk of dehydration and its complications (Table 2).
- Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc 2003; 58:76–79.
- Hendry PO, Jenkins JT, Diament RH. The impact of poor bowel preparation on colonoscopy: a prospective single centre study of 10,571 colonoscopies. Colorectal Dis 2007; 9:745–748.
- Burke CA, Church JM. Enhancing the quality of colonoscopy: the importance of bowel purgatives. Gastrointest Endosc 2007; 66:565–573.
- Rex DK, Imperiale TF, Latinovich DR, Bratcher LL. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol 2002; 97:1696–1700.
- Levin B, Lieberman DA, McFarland B, et al; American Cancer Society Colorectal Cancer Advisory Group. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008; 134:1570–1595.
- Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer 2007; 110:2119–2152.
- Wexner SD, Beck DE, Baron TH, et al. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Dis Colon Rectum 2006; 49:792–809.
- Barkun A, Chiba N, Enns R, et al. Commonly used preparations for colonoscopy: efficacy, tolerability, and safety—a Canadian Association of Gastroenterology position paper. Can J Gastroenterol 2006; 20:699–710.
- Davis GR, Santa Ana CA, Morawski SG, Fordtran JS. Development of a lavage solution associated with minimal water and electrolyte absorption or secretion. Gastroenterology 1980; 78:991–995.
- Clark LE, Dipalma JA. Safety issues regarding colonic cleansing for diagnostic and surgical procedures. Drug Saf 2004; 27:1235–1242.
- Nelson DB, Barkun AN, Block KP, et al. Technology Status Evaluation report. Colonoscopy preparations. May 2001. Gastrointest Endosc 2001; 54:829–832.
- DiPalma JA, Marshall JB. Comparison of a new sulfate-free polyethylene glycol electrolyte lavage solution versus a standard solution for colonoscopy cleansing. Gastrointest Endosc 1990; 36:285–289.
- DiPalma JA, Wolff BG, Meagher A, Cleveland M. Comparison of reduced volume versus four liters sulfate-free electrolyte lavage solutions for colonoscopy colon cleansing. Am J Gastroenterol 2003; 98:2187–2191.
- Ell C, Fischbach W, Bronisch HJ, et al. Randomized trial of low-volume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am J Gastroenterol 2008; 103:883–893.
- Adams WJ, Meagher AP, Lubowski DZ, King DW. Bisacodyl reduces the volume of polyethylene glycol solution required for bowel preparation. Dis Colon Rectum 1994; 37:229–233.
- Ker TS. Comparison of reduced volume versus four-liter electrolyte lavage solutions for colon cleansing. Am Surg 2006; 72:909–911.
- Sharma VK, Steinberg EN, Vasudeva R, Howden CW. Randomized, controlled study of pretreatment with magnesium citrate on the quality of colonoscopy preparation with polyethylene glycol electrolyte lavage solution. Gastrointest Endosc 1997; 46:541–543.
- Belsey J, Epstein O, Heresbach D. Systematic review: oral bowel preparation for colonoscopy. Aliment Pharmacol Ther 2007; 25:373–384.
- Tan JJ, Tjandra JJ. Which is the optimal bowel preparation for colonoscopy—a meta-analysis. Colorectal Dis 2006; 8:247–258.
- Kastenberg D, Chasen R, Choudhary C, et al. Efficacy and safety of sodium phosphate tablets compared with PEG solution in colon cleansing: two identically designed, randomized, controlled, parallel group, multicenter phase III trials. Gastrointest Endosc 2001; 54:705–713.
- Balaban DH, Leavell BS, Oblinger MJ, Thompson WO, Bolton ND, Pambianco DJ. Low volume bowel preparation for colonoscopy: randomized, endoscopist-blinded trial of liquid sodium phosphate versus tablet sodium phosphate. Am J Gastroenterol 2003; 98:827–832.
- Rex DK. 10 Questions You Need to Ask About Colonoscopy. New York Times February 25, 2009. http://www.nytimes.com/2009/02/24/health/esn-colonoscopy-expert.html?_r=1. Accessed March 14, 2010.
- Makkar R, Shen B. What are the caveats to using sodium phosphate agents for bowel preparation? Cleve Clin J Med 2008; 75:173–176.
- Hookey LC, Depew WT, Vanner S. The safety profile of oral sodium phosphate for colonic cleansing before colonoscopy in adults. Gastrointestinal Endosc 2002; 56:895–902.
- US Food and Drug Administration (FDA). Oral Sodium Phosphate (OSP) Products for Bowel Cleansing (marketed as Visicol and OsmoPrep, and oral sodium phosphate products available without a prescription). FDA Alert. December 11, 2008. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm094900.htm. Accessed March 14, 2010.
- Hoy SM, Scott LJ, Wagstaff AJ. Sodium picosulfate/magnesium citrate: a review of its use as a colorectal cleanser. Drugs 2009; 69:123–136.
- Sohn N, Weinstein MA. Management of the poorly prepared colonoscopy patient: colonoscopic colon enemas as a preparation for colonoscopy. Dis Colon Rectum 2008; 51:462–466.
- Drew PJ, Hughes M, Hodson R, et al. The optimum bowel preparation for flexible sigmoidoscopy. Eur J Surg Oncol 1997; 23:315–316.
- Bigard MA, Gaucher P, Lassalle C. Fatal colonic explosion during colonoscopic polypectomy. Gastroenterology 1979; 77:1307–1310.
- Rhodes JB, Engstrom J, Stone KF. Metoclopramide reduces the distress associated with colon cleansing by an oral electrolyte overload. Gastrointest Endosc 1978; 24:162–163.
- Abuksis G, Mor M, Segal N, et al. A patient education program is cost-effective for preventing failure of endoscopic procedures in a gastroenterology department. Am J Gastroenterol 2001; 96:1786–1790.
- Dykes C, Cash BD. Key safety issues of bowel preparations for colonoscopy and importance of adequate hydration. Gastroenterol Nurs 2008; 31:30–35.
- Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: a report by the American Society of Anesthesiologist Task Force on Preoperative Fasting. Anesthesiology 1999; 90:896–905.
- Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc 2003; 58:76–79.
- Hendry PO, Jenkins JT, Diament RH. The impact of poor bowel preparation on colonoscopy: a prospective single centre study of 10,571 colonoscopies. Colorectal Dis 2007; 9:745–748.
- Burke CA, Church JM. Enhancing the quality of colonoscopy: the importance of bowel purgatives. Gastrointest Endosc 2007; 66:565–573.
- Rex DK, Imperiale TF, Latinovich DR, Bratcher LL. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol 2002; 97:1696–1700.
- Levin B, Lieberman DA, McFarland B, et al; American Cancer Society Colorectal Cancer Advisory Group. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology 2008; 134:1570–1595.
- Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer 2007; 110:2119–2152.
- Wexner SD, Beck DE, Baron TH, et al. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Dis Colon Rectum 2006; 49:792–809.
- Barkun A, Chiba N, Enns R, et al. Commonly used preparations for colonoscopy: efficacy, tolerability, and safety—a Canadian Association of Gastroenterology position paper. Can J Gastroenterol 2006; 20:699–710.
- Davis GR, Santa Ana CA, Morawski SG, Fordtran JS. Development of a lavage solution associated with minimal water and electrolyte absorption or secretion. Gastroenterology 1980; 78:991–995.
- Clark LE, Dipalma JA. Safety issues regarding colonic cleansing for diagnostic and surgical procedures. Drug Saf 2004; 27:1235–1242.
- Nelson DB, Barkun AN, Block KP, et al. Technology Status Evaluation report. Colonoscopy preparations. May 2001. Gastrointest Endosc 2001; 54:829–832.
- DiPalma JA, Marshall JB. Comparison of a new sulfate-free polyethylene glycol electrolyte lavage solution versus a standard solution for colonoscopy cleansing. Gastrointest Endosc 1990; 36:285–289.
- DiPalma JA, Wolff BG, Meagher A, Cleveland M. Comparison of reduced volume versus four liters sulfate-free electrolyte lavage solutions for colonoscopy colon cleansing. Am J Gastroenterol 2003; 98:2187–2191.
- Ell C, Fischbach W, Bronisch HJ, et al. Randomized trial of low-volume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am J Gastroenterol 2008; 103:883–893.
- Adams WJ, Meagher AP, Lubowski DZ, King DW. Bisacodyl reduces the volume of polyethylene glycol solution required for bowel preparation. Dis Colon Rectum 1994; 37:229–233.
- Ker TS. Comparison of reduced volume versus four-liter electrolyte lavage solutions for colon cleansing. Am Surg 2006; 72:909–911.
- Sharma VK, Steinberg EN, Vasudeva R, Howden CW. Randomized, controlled study of pretreatment with magnesium citrate on the quality of colonoscopy preparation with polyethylene glycol electrolyte lavage solution. Gastrointest Endosc 1997; 46:541–543.
- Belsey J, Epstein O, Heresbach D. Systematic review: oral bowel preparation for colonoscopy. Aliment Pharmacol Ther 2007; 25:373–384.
- Tan JJ, Tjandra JJ. Which is the optimal bowel preparation for colonoscopy—a meta-analysis. Colorectal Dis 2006; 8:247–258.
- Kastenberg D, Chasen R, Choudhary C, et al. Efficacy and safety of sodium phosphate tablets compared with PEG solution in colon cleansing: two identically designed, randomized, controlled, parallel group, multicenter phase III trials. Gastrointest Endosc 2001; 54:705–713.
- Balaban DH, Leavell BS, Oblinger MJ, Thompson WO, Bolton ND, Pambianco DJ. Low volume bowel preparation for colonoscopy: randomized, endoscopist-blinded trial of liquid sodium phosphate versus tablet sodium phosphate. Am J Gastroenterol 2003; 98:827–832.
- Rex DK. 10 Questions You Need to Ask About Colonoscopy. New York Times February 25, 2009. http://www.nytimes.com/2009/02/24/health/esn-colonoscopy-expert.html?_r=1. Accessed March 14, 2010.
- Makkar R, Shen B. What are the caveats to using sodium phosphate agents for bowel preparation? Cleve Clin J Med 2008; 75:173–176.
- Hookey LC, Depew WT, Vanner S. The safety profile of oral sodium phosphate for colonic cleansing before colonoscopy in adults. Gastrointestinal Endosc 2002; 56:895–902.
- US Food and Drug Administration (FDA). Oral Sodium Phosphate (OSP) Products for Bowel Cleansing (marketed as Visicol and OsmoPrep, and oral sodium phosphate products available without a prescription). FDA Alert. December 11, 2008. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm094900.htm. Accessed March 14, 2010.
- Hoy SM, Scott LJ, Wagstaff AJ. Sodium picosulfate/magnesium citrate: a review of its use as a colorectal cleanser. Drugs 2009; 69:123–136.
- Sohn N, Weinstein MA. Management of the poorly prepared colonoscopy patient: colonoscopic colon enemas as a preparation for colonoscopy. Dis Colon Rectum 2008; 51:462–466.
- Drew PJ, Hughes M, Hodson R, et al. The optimum bowel preparation for flexible sigmoidoscopy. Eur J Surg Oncol 1997; 23:315–316.
- Bigard MA, Gaucher P, Lassalle C. Fatal colonic explosion during colonoscopic polypectomy. Gastroenterology 1979; 77:1307–1310.
- Rhodes JB, Engstrom J, Stone KF. Metoclopramide reduces the distress associated with colon cleansing by an oral electrolyte overload. Gastrointest Endosc 1978; 24:162–163.
- Abuksis G, Mor M, Segal N, et al. A patient education program is cost-effective for preventing failure of endoscopic procedures in a gastroenterology department. Am J Gastroenterol 2001; 96:1786–1790.
- Dykes C, Cash BD. Key safety issues of bowel preparations for colonoscopy and importance of adequate hydration. Gastroenterol Nurs 2008; 31:30–35.
- Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures: a report by the American Society of Anesthesiologist Task Force on Preoperative Fasting. Anesthesiology 1999; 90:896–905.
KEY POINTS
- Polyethylene glycol solutions are fast, effective, and preferred for cleansing the colon.
- Use of split dosing, a low-volume solution, or both can increase patient acceptability without compromising efficacy.
- Sodium phosphate can be prescribed for patients who cannot tolerate polyethylene glycol solutions, provided they are not at risk of electrolyte or fluid imbalances.
- Enemas, bisacodyl, magnesium citrate, and metoclopramide (Reglan) can be useful as adjuncts to polyethylene glycol but by themselves are inadequate for cleansing the entire colon.
- Educating patients about bowel preparation instructions, including correct dosing and adequate hydration, helps reduce the risk of adverse events and serious adverse events.