User login
- Consider an oral contraceptive for women who would prefer less frequent menstrual periods (A).
- An intrauterine device may be appropriate for women with prior pelvic inflammatory disease, ectopic pregnancy, or an abnormal Papanicolaou (Pap) smear result, and for many adolescents (A).
- There are no medical contraindications to progestin-only emergency contraception (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
A 35-year-old woman with a family history of breast cancer (mother diagnosed with breast cancer at age 55) requests your help in choosing an appropriate method of contraception. She is a nonsmoker, has a body mass index of 25, and dislikes taking pills. Which options would you recommend to her? Are there any that you would rule out?
Helping your patient make the best choice requires that you be as up to date as possible. In this review, we discuss select new options in a clinically relevant manner. Specifically, we explore the newest oral contraceptives (OCs), including extended-cycle, continuous, and shortened hormone-free interval formulations. In addition, we review the latest data and updated recommendations for the contraceptive patch and ring, intrauterine devices (IUDs), implants, and emergency contraception (TABLE). We conclude by describing appropriate choices for the patient described above. (See “So what do you recommend?” on page 803.)
Oral contraceptives
Since OCs became available in the 1960s, the standard regimen has been 21 active pills followed by 7 placebo pills, simulating the average unassisted monthly menstrual cycle in which “menstrual” or withdrawal bleeding occurs. Clinicians have successfully lengthened intermenstrual intervals with OCs, without incurring additional risk, to control symptoms of endometriosis, premenstrual syndrome, and menstrual-withdrawal headaches, or to satisfy many patients’ preference for fewer menses per year.1,2
Any monophasic active OC can be used without a placebo interval to delay menses for extended periods. Until recently, such usage was off-label. Based on extensive safety and efficacy studies, however, the US Food and Drug Administration (FDA) has now approved several formulations for extended-cycle and continuous-cycle use.
Extended-cycle OCs: Fewer menses per year
Two FDA-approved extended-cycle OCs are available: Seasonale and Seasonique.3,4 Both products enable 4 scheduled menstrual intervals per year, as opposed to about 13 with 28-day cycles. Each regimen uses 84 consecutive pills of levonorgestrel 0.15 mg and ethinyl estradiol (EE) 0.03 mg, followed by 7 placebo pills (Seasonale) or 7 pills of EE 0.01 mg (Seasonique).
Other potential advantages. With Seasonique, the average length of menses is 3 days, which is shorter than the average unassisted menstrual period. Seasonique’s 7 additional low-dose estrogen pills may help decrease estrogen withdrawal symptoms, such as headaches in women with menstrual migraine and vasomotor instability in perimenopausal women. Though this effect has also been reported with other OCs containing low-dose estrogen during the traditional placebo week, specific supportive evidence is not yet available for these formulations.5,6
Disadvantages to address. With Seasonale and Seasonique, unscheduled spotting or bleeding has been reported—especially during initial use—at rates considerably higher than those associated with comparable traditional OCs.3,4 Effective counseling will help ensure patient compliance and satisfaction.
During the first cycle (days 1-91), about 65% of women taking either formulation reported ≥7 days of spotting, and 29% to 35% reported ≥20 days of spotting. By the 4th cycle (days 273-364), 39% to 42% of patients reported ≥7 days of spotting and 11% to 15% reported ≥20 days of spotting. For patients taking comparable progestin and EE doses in traditional monthly regimens, 38% reported ≥7 days of spotting and 6% reported ≥20 days of spotting during the first cycle. Thirty-nine percent and 4%, respectively, reported spotting during the fourth cycle.3,4
Continuous OC: Consistent hormonal milieu
Lybrel, the only FDA-approved OC for continuous use, contains levonorgestrel 90 mcg and EE 20 mcg; pills are taken daily throughout the year.7 Progestin and estrogen doses are lower than those found in many monthly OCs and in all extended-cycle formulations. A phase 3 trial of 2134 women reported the safety and efficacy of Lybrel to be comparable to cyclic OCs.8 Again, unscheduled bleeding and spotting rates were relatively high but decreased at pack 3 from 47% and 26%, respectively, to 21% and 20%, respectively, at pack 13. Predictably, amenorrhea rates increased from 27% to 59% between pack 3 and pack 13.
Shortened hormone-free interval OCs: Less breakthrough ovulation
The shortened hormone-free interval OC is an alternative for patients who want regular, but shorter, menstrual intervals. With 24 active and 4 placebo pills in each cycle, this regimen suppresses the pituitary/ovarian axis to a greater extent than traditional 21/7-day regimens and thus lowers the rate of breakthrough ovulation.9
Loestrin 24 Fe contains norethindrone 1 mg and EE 20 mcg; placebo pills contain 75 mg of ferrous fumarate.
Yaz contains the newer progestin drospirenone 3 mg and EE 20 mcg. Drospirenone, an analog of the antihypertensive spironolactone, was introduced in a 21/7 formulation, Yasmin, and proved to have beneficial effects on mood, water retention, and acne.10 Yaz, which contains a lower dose of EE than Yasmin, provides 3 additional days of antimineralocorticoid and antiandrogenic activity, and is indicated for the treatment of premenstrual dysphoric disorder, a more severe form of premenstrual syndrome. Drospirenone-containing OCs are contraindicated for patients with renal, adrenal, or hepatic impairment because of the progestin’s metabolism via these routes.
An established OC with a twist: Chewable pills
For patients unable to swallow OCs, a chewable formulation, Femcon Fe, is available.11 It is hormonally identical to Ovcon 35, a well-established OC containing norethindrone acetate 0.4 mg and EE 35 mcg. The 7 placebo pills contain ferrous fumarate 75 mg. The spearmint-flavored chewable pill (which can also be swallowed) must be taken with 8 ounces of water.
Noncontraceptive benefits of OCs—there are many
An extensive body of evidence supports the noncontraceptive health benefits of OCs. These include a decreased risk of:
- endometrial and ovarian cancer
- bone loss
- benign breast disease
- pelvic inflammatory disease
- ectopic pregnancy
- rheumatoid arthritis.
Women with symptoms of androgen excess, premenstrual mood disorders, or endometriosis pain have long benefited from treatment with OCs.10,12-14 Healthy perimenopausal women are excellent candidates for OCs to regulate menses and treat symptoms of estrogen deficiency. OCs with added estrogen during the menstrual interval or shortened hormone-free interval may be more effective in moderating the perimenopausal transition. However, specific evidence about these effects is not yet available for the newest OC formulations.
Risks of OCs have been reduced, but some remain
Many of the well-known risks and side effects of OCs have been minimized over the years as total doses of estrogen have decreased and less androgenic progestins have been incorporated into OC formulations. Nonetheless, OCs are contraindicated for women who have a history of venous thromboembolism (VTE) or coronary artery disease (or are at risk for these complications), are over the age of 35 and smoke, are pregnant or newly postpartum, or are immobilized after surgery.
OCs remain relatively contraindicated for women with a history of migraines and focal auras, due to the increased risk for ischemic stroke.15 Breast and other estrogen-dependent cancers as well as liver disease preclude the use of OCs.16 Additional studies using the newer formulations of OCs are needed to definitively determine their long-term risk compared with traditional monthly formulations.
Contraceptive patch: Improving compliance
The contraceptive patch Evra is applied weekly and releases norelgestromin 150 mcg and EE 20 mcg each day, providing an OC alternative that is less dependent on compliance. However, in November 2005, the FDA modified product labeling to inform providers and the public that, based on pharmacokinetic studies, patients using the patch were exposed to hormone levels about 60% higher than with OCs of similar dosage.17 Another FDA labeling change made in 2008 states that “it is not known whether there are changes in the risk of serious adverse events based on the differences in pharmacokinetic profiles of EE in women using [the patch] as compared with women using oral contraceptives containing 35 mcg of EE.”
What is the real risk of VTE? One case-control study reported that the rates of VTE events in patch users and OC users were 52 per 100,000 woman-years and 42 per 100,000 woman-years, respectively.18 Another large case-control study showed the odds ratio (OR) of VTE to be 2.4 (95% confidence interval [CI], 1.1-5.5) with the patch compared with OCs; data were corrected for high-risk factors.19 However, the absolute risks for the patch and OCs were 40 and 18 per 100,000 woman-years, respectively—both lower than the risk of VTE associated with pregnancy.
The risk of myocardial infarction. The OR for myocardial infarction among patch users in the same population was 1.8 (95% CI, 0.5-6.8), and there was no statistically significant increase in the rate of cerebrovascular accidents.19 Thus, it is reasonable to use the patch with caution in patients without cardiac risk factors and to limit total hormone dosage by not using the patch in an extended-cycle manner. Of note, the patch is reported to have decreased efficacy in patients weighing over 90 kg (198 lb).20
Vaginal ring: Fewer drug interactions
NuvaRing, the ethylene vinyl vaginal ring, releases etonorgestrel (ENG) 120 mcg and EE 15 mcg each day (a lower estrogen dose than is contained in OCs or the patch).21 The device is 5.4 cm in diameter and 4 mm thick. Patients insert the ring intravaginally, remove it 3 weeks later for menses, and insert a new ring 1 week later.
Continuous use regimen. A randomized controlled trial evaluating the frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring reported a reduction in bleeding, flow, and pelvic pain, and a high continuation rate. Most patients considered the bleeding profile with the continuous vaginal ring acceptable compared with the baseline 21/7 use.22 Each ring contains up to a 28-day supply of hormones.
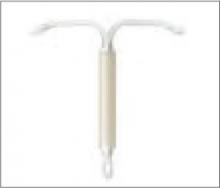
LNG-IUS (Mirena)
Fewer interactions. Transvaginal absorption of hormones with the vaginal ring avoids a first pass through the liver, thus decreasing many medication interactions. Irregular bleeding experienced with OCs or the patch may be effectively reduced with the steadily released, rapidly acting hormones in the ring.23
Intrauterine contraception
Intrauterine contraception is increasingly accepted by women who want long-term and effective contraception without having to comply with a particular regimen.
Copper IUD: Many contraindications are lifted
The nonhormonal IUD ParaGard T 380A is indicated for contraception for up to 10 years in women who are 16 years of age and older.24 This IUD’s active ingredient is the spermicidal copper wire wound around the short arms of the device. A recent meta-analysis reported an association between the use of a copper IUD and a decrease in the risk of endometrial cancer (OR=0.39; 95% CI, 0.29-0.51), though the mechanism for this association is unclear.25
In late 2005, the FDA broadened the use of copper IUDs to include women who are nulliparous; have a history of pelvic inflammatory disease (PID), sexually transmitted disease, or ectopic pregnancy; are in nonmonogamous relationships; or have a history of premenopausal breast cancer. This method also may be used by women with asymptomatic human immunodeficiency virus infection, Actinomyces infection, abnormal Papanicolaou (Pap) test results, or vaginitis. Furthermore, data support its use in adolescents who are at particularly high risk for unintended pregnancy.26
The copper IUD remains contra-indicated for patients with acute PID or current high-risk behavior for sexually transmitted infections, as well as for those who have mucopurulent cervicitis or have had postpartum endometritis within the past 3 months.24 Wilson’s disease is also a contraindication.
Insertion tip. Misoprostol may be used to soften the nulliparous cervix for insertion.27
Levonorgestrel intrauterine system: An alternative to the copper IUD
The levonorgestrel intrauterine system (LNG IUS), sold under the brand name Mirena, is gaining tremendous popularity in the United States. Multiple mechanisms of action, including endometrial thinning, cervical mucus thickening, inhibition of sperm function, and intermittent ovulation suppression are responsible for the >99% efficacy of this 5-year contraceptive.
Irregular menses can be expected initially, and 20% of patients reported amenorrhea at 1 year of use. In the unlikely event that a woman becomes pregnant while using Mirena, evaluate for ectopic pregnancy, which occurs in about half of pregnancies in women using this system.
Noncontraceptive benefits. The system’s primary noncontraceptive benefit is the dramatic reduction of menstrual blood loss, reported to be up to 90%.28 This contraceptive has been used as a cost-effective alternative to hysterectomy and endometrial ablation.29,30 Mirena imparts a protective effect against PID, likely secondary to progestin-mediated cervical mucus thickening.31
Subdermal implant (Implanon)
It appears safe and expeditious to provide both counseling and intrauterine contraception insertion in one visit, provided pregnancy is excluded.32 Confirm normalcy of cervical cytology and screen for sexually transmitted disease, if indicated. Prophylactic antibiotics are unnecessary, as the risk of PID within 20 days of insertion is only 9.7 per 1000 woman- years.33 After 20 days, the risk declines to 1.4 per 1000 woman-years, the same as that of the general population.34
Subdermal implant: Easily reversible
In July 2006, the FDA approved Implanon, a subdermal contraceptive implant.35 It has been available worldwide since 1998. The 40 × 2 mm single-rod implant containing etonogestrel (ENG) 68 mg diffuses the hormone at a rate of 60 mcg/d immediately after insertion and then steadily at 30 mcg/d for up to 3 years.
Its primary mechanism of action is ovulation suppression, with no ovulation detected for 30 months in a study group of more than 17,000 women.35 Increased cervical mucus viscosity also contributes to its effectiveness.35 In a large clinical trial, no pregnancies were reported in more than 6100 cycles.36 However, this trial excluded women weighing more than 130% of their ideal body weight, so no data are available to support the effectiveness of Implanon in obese women.36
Benefits and risks. This implant does not cause a hypoestrogenic state and ovulation suppression is rapidly reversible, with ENG levels undetectable within 10 days of implant removal.35 Furthermore, this method has no reported deleterious effects on bone mineral density or lactation.37,38 When counseling women about the implant, emphasize its propensity to result in “irregular and unpredictable” bleeding. An average of 7 bleeding and 10 spotting days within a 90-day period has been reported. Most women had fewer bleeding/spotting days than they would without contraception, but unscheduled bleeding was the leading reason for method discontinuation (11%), followed by weight gain, emotional lability, acne, headache, and depression (each ≈1%-2%).35
Implant insertion. The device is inserted in the sulcus between the biceps and triceps muscles of the nondominant arm. It is crucial to place the implant subdermally, tenting the skin during insertion to prevent deep insertion. High-frequency ultrasound can be used to detect nonpalpable implants. The FDA has mandated 3 hours of training for clinicians before they can obtain the device.
Depot medroxyprogesterone: Tried and true alternative
The depot medroxyprogesterone acetate (DMPA) injection has been a mainstay of contraception for decades. Available under the brand name Depo-Provera, it’s an option for women in whom estrogen-containing contraceptives are contraindicated. Its convenience, reduced risk of anemia, and postpartum benefits are all well known, and we have thus limited our discussion of DMPA to the summary in the TABLE.
TABLE
How do these contraceptives compare?
| METHOD | ROUTE OF ADMINISTRATION | FREQUENCY OF ADMINISTRATION | FAILURES/YR WITH TYPICAL USE 33,35,51 | EXPECTED MENSTRUAL PATTERN | ADVERSE EFFECTS/CONTRAINDICATIONS |
|---|---|---|---|---|---|
| Cyclic OCs | Oral | Daily | 8% | Monthly menses, may have BTB initially | Hormonal adverse effects |
| Extended-cycle OCs (Seasonale, Seasonique) | Oral | Daily | 8% | Menses 4/yr, frequent BTB | Hormonal adverse effects, unscheduled bleeding |
| Continuous OCs (Lybrel) | Oral | Daily | 8% | No scheduled menses, frequent BTB | Hormonal adverse effects, unscheduled bleeding |
| Shortened hormone-free interval OCs (Loestrin 24 Fe, Yaz) | Oral | Daily | 8% | Shorter monthly menses | Hormonal adverse effects, unscheduled bleeding |
| Transdermal patch (Evra) | Patch applied to skin | New patch applied weekly for 3 wk; off for 1 wk | 8% | Monthly menses, may have BTB initially | Hormonal adverse effects, increased risk of VTE higher than OCs but lower than pregnancy; MI risk higher than comparable OCs, but use is reasonable if no cardiac risk factors |
| Vaginal ring (NuvaRing) | Ring inserted in vagina by patient | Ring inserted for 3 wk, removed for 1 wk | 8% | Monthly menses, may have BTB initially | Hormonal adverse effects |
| Copper IUD (ParaGard T 380A) | IUD inserted & removed by clinician | Every 10 yr | 0. 8% | Heavier menses, may have BTB | Menorrhagia. Contraindications: Acute PID or high risk for STI; postpartum endometritis within 3 mo; mucopurulent cervicitis; Wilson’s disease |
| Levonorgestrel IUS (LNG IUS, Mirena) | IUS inserted & removed by clinician | Every 5 yr | < 0.1% | Lighter, shorter menses or amenorrhea | Minimal hormonal adverse effects. Contraindications: Acute PID, history of or high risk for PID; postpartum endometritis within 3 mo; mucopurulent cervicitis |
| Subdermal implant (Implanon) | Inserted subdermally & removed by clinician | Every 3 yr | 0.3% | Irregular, unpredictable bleeding | Unscheduled bleeding, mood symptoms, headache, weight gain, acne |
| Depot medroxyprogesterone acetate (Depo-Provera) | IM injection | Every 3 mo | 3% | Irregular bleeding, amenorrhea | Unscheduled bleeding, reversible bone loss |
| BTB, breakthrough bleeding; IM, intramuscular; IUD, intrauterine device; IUS, intrauterine system; MI, myocardial infarction; OC, oral contraceptive; PID, pelvic inflammatory disease; STI, sexually transmitted infection; VTE, venous thromboembolism. | |||||
Emergency contraception: 2 pills, 12 hours apart
Plan B contains 2 tablets of levonorgestrel 0.75 mg, to be taken 12 hours apart as soon as possible after unprotected intercourse. However, taking both doses together is as effective as taking them separately, and doing so may improve compliance.39
How it works. Emergency contraception (EC) works by inhibiting or delaying the surge of luteinizing hormone and follicular rupture before ovulation. It does not affect implantation or corpus luteum function, and it poses no risk to an established pregnancy or embryo. EC is ineffective when administered after ovulation.40
In a World Health Organization (WHO) multicenter randomized trial, EC prevented 79% to 84% of pregnancies if taken 1 to 3 days after intercourse, and 60% to 63% of pregnancies if taken 4 to 5 days after intercourse.41 Plan B is available without a prescription for women ages 18 and older. It is important to screen for pregnancy before prescribing Plan B for younger patients.
Adverse effects include nausea and vomiting, occurring in 23% and 5% of patients, respectively. Intermenstrual bleeding occurs in 8% of patients taking progestin-only EC. Menses are expected within 21 days of EC administration, and the second cycle after EC should be of normal length.42 The authors of a WHO report concluded that “there are no medical conditions wherein risks outweigh benefits of EC.”43
Combination estrogen/progestin for EC is no more effective than progestin-only EC and results in higher rates of adverse effects, especially nausea and vomiting.44
IUD can also be used in an emergency
Insertion of a copper IUD provides EC as well as ongoing contraception. It is hormone free and can be used effectively for EC up to 5 days after sexual intercourse, then continued for primary contraception for up to 10 years.45 Its estimated failure rate was less than 0.1% in more than 8400 postcoital insertions.46 The IUD works by impairing fertilization and implantation and by altering sperm motility and integrity. With a copper IUD, additional primary contraception is unnecessary. The LNG IUS (Mirena) has not been studied as an alternative EC.
Some worry that EC’s availability will encourage unprotected sex
Some authorities have wondered if increased access to EC might paradoxically lead to more pregnancies by encouraging unprotected sex. Researchers are exploring this issue. One study reported that unfettered access to free EC resulted in an increase in EC use, and another study reported that patients with unrestricted EC access had inadequately protected sex more often than those in the control group.47,48
A systematic review of 23 articles studying the effect of increased access to EC confirmed an increase in EC use,49 but no statistically significant differences in pregnancy or abortion rates.
For patients like the 35-year-old woman discussed earlier, who do not like taking pills, there are many contraception options to choose from, including the patch, vaginal ring, chewable OC, IUD, depot medroxyprogesterone injection, and subdermal implant.
Hormonal contraception would not be an issue for this patient—even though she has a family history of breast cancer. Using hormonal contraception does not increase the risk of breast cancer for individuals with a family history of breast cancer in a first-or second-degree relative.50
Correspondence
Petra M. Casey, MD, Department of Obstetrics and Gynecology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; casey.petra@mayo.edu.
1. Andrist LC, Arias RD, Nucatola D, et al. Women’s and providers’ attitudes toward menstrual suppression with extended use of oral contraceptives. Contraception. 2004;70:359-363.
2. Glasier AF, Smith KB, van der Spuy ZM, et al. Amenorrhea associated with contraception—an international study on acceptability. Contraception. 2003;67:1-8.
3. Seasonale [package insert]. Pomona, NY: Duramed Pharmaceuticals, Inc; 2008.
4. Seasonique [package insert]. Pomona, NY: Duramed Pharmaceuticals, Inc; 2006.
5. Shortened pill-free interval delivered by new 20 mcg pill, Organon’s Mircette, schedule for US debut this summer Contraception Technol Update. 1998;19:85-87.
6. Casper RF, Dodin S, Reid RL. For the Study Investigators. The effect of 20 mcg ethinyl estradiol/1 mg norethindrone acetate (Minestrin), a low-dose oral contraceptive, on vaginal bleeding patterns, hot flashes and quality of life in symptomatic perimenopausal women. Menopause. 1997;4:139-147.
7. Lybrel [package insert] Philadelphia, Pa: Wyeth Pharmaceuticals, Inc; 2007.
8. Archer DF, Jensen JT, Johnson JV, et al. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception. 2006;74:439-445.
9. Willis SA, Kuehl TJ, Spiekerman AM, et al. Greater inhibition of the pituitary: ovarian axis in oral contraceptive regimens with a shortened hormone-free interval. Contraception. 2006;74:100-103.
10. Parsey KS, Pong A. An open-label, multicenter study to evaluate Yasmin, a low-dose combination oral contraceptive containing drospirenone, a new progestogen. Contraception. 2000;61:105-111.
11. Femcon Fe [package insert]. Rockaway, NJ: Warner Chilcott (US), Inc; 2007.
12. Burkman R. The evolution of oral contraceptives: 40 years of continuous improvement. Clinician. 2002;20:3-30.
13. Vercellini P, Frontino G, De Giorgi O, et al. Continuous use of an oral contraceptive for endometriosis-associated recurrent dysmenorrhea that does not respond to a cyclic pill regimen. Fertil Steril. 2003;80:560-563.
14. Guido M, Romualdi D, Giuliani M. Drospirenone for the treatment of hirsute women with PCOS; a clinical endocrinological, metabolic pilot study. J Clin Endocrinol Metab. 2004;89:2817-2823.
15. Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischaemic stroke in young women. Br Med J. 1995;310:830-833.
16. Steinauer J, Autry AM. Extended cycle combined hormonal contraception. Obstet Gynecol Clin North Am. 2007;34:43-55, viii.
17. Ortho Evra [prescribing information]. Raritan, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2001.
18. Jick SS, Kaye JA, Russmann S, et al. Risk of non-fatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg/l of ethinyl estradiol. Contraception. 2006;73:223-228.
19. Cole JA, Norman H, Doherty M, et al. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol. 2007;109:339-346.
20. American College of Obstetricians and Gynecologists. ACOG practice bulletin. Use of hormonal contraceptives in women with coexisting medical conditions. No. 73, June 2006. Obstet Gynecol. 2006;107:1453-1472.
21. Mulders TM, Dieben TO. Use of the novel combined contraceptive vaginal ring NuvaRing for ovulation inhibition. Fertil Steril. 2001;75:865-870.
22. Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring. Obstet Gynecol. 2008;112:563-571.
23. Bjarnadottir RI, Tuppurainen M, Killick SR. Comparison of cycle control with a combined contraceptive vaginal ring and oral levonorgestrel/ethinyl estradiol. Am J Obstet Gynecol. 2002;186:389-395.
24. ParaGard T 380A [prescribing information]. Pomona, NY: Duramed Pharmaceuticals, Inc; 2006.
25. Beining RM, Dennis LK, Smith EM, et al. Meta-analysis of intrauterine device use and risk of endometrial cancer. Ann Epidem. 2008;18:492-499.
26. ACOG Committee Opinion. Intrauterine Device and Adolescents No. 392. Obstet Gynecol. 2007;110:1493-1495.
27. McNaught J. Adolescents and IUCDs—Not a contraindication. J Ped Adolesc Gyn. 2006;19:303-305.
28. Lethaby AE, Cooke I, Rees M. Progesterone/progestogen releasing intrauterine systems versus either placebo or any other medication for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.-
29. Hurskainen R, Teperi J, Rissanen P, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomised trial. Lancet. 2001;357:273-277.
30. Römer T. Prospective comparison study of levonorgestrel IUD versus Roller-Ball endometrial ablation in the management of refractory recurrent hypermenorrhea. Eur J Obstet Gynecol Reprod Biol. 2000;90:27-29.
31. Toivonen J, Luukkainen T, Allonen H. Protective effect of intrauterine release of levonorgestrel on pelvic infection: three years’ comparative experience of levonorgestrel- and copper-releasing intrauterine devices. Obstet Gynecol. 1991;77:261-264.
32. Ball CE. News and controversies in contraception. AudioDigest Obstet Gynecol Clin Updates. 2007;54:18.-
33. Grimes DA. Intrauterine device and upper genital tract infection. Lancet. 2000;356:1013-1019.
34. Farley TM, Rosenberg MJ, Rowe PJ, et al. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339:785-788.
35. Implanon [package insert]. Roseland, NJ: Organon USA Inc; 2007.
36. Funk S, Miller MM, Mishell DR, Jr, et al. The Implanon US Study Group. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception. 2005;71:319-326.
37. Beerthuizen R, van Beek A, Massai R, et al. Bone mineral density during long-term use of the progestagen contraceptive implant Implanon compared to a non-hormonal method of contraception. Hum Reprod. 2000;15:118-122.
38. Reinprayoon D, Taneepanichskul S, Bunyavejchevin S, et al. Effects of the etonogestrel-releasing contraceptive implant (Implanon) on parameters of breastfeeding compared to those of an intrauterine device. Contraception. 2000;62:239-246.
39. Ngai SW, Fan S, Li S, et al. A randomized trial to compare 24 h versus 12 h double dose regimen of levonorgestrel for emergency contraception. Hum Reprod. 2005;20:307-311.
40. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 69: emergency contraception. Obstet Gynecol. 2005;106:1443-1452.
41. Von Hertzen H, Piaggio G, Ding J. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. WHO Research Group on Post-Ovulatory Methods of Fertility Regulation. Lancet. 2002;360:1803-1810.
42. Raymond EG, Goldberg A, Trussell J, et al. Bleeding patterns after use of levonorgestrel emergency contraceptive pills. Contraception. 2006;73:376-381.
43. World Health Organization. Medical eligibility criteria for contraceptive use. 3rd ed. 2004. Available at: www.who.int/reproductive-health/publication/med/mec.pdf. Accessed February 7, 2008.
44. Task Force on Postovulatory Methods of Fertility Regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352:428-433.
45. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 59. Intrauterine device. Obstet Gynecol. 2005;105:223-232.
46. LaValleur J. Emergency contraception. Obstet Gynecol Clin North Am. 2000;27:817-839, vii.
47. Raymond EG, Stewart F, Weaver M, et al. Impact of increased access to emergency contraceptive pills: a randomized controlled trial. Obstet Gynecol. 2006;108:1098-1106.
48. Raymond EG, Weaver MA. Effect of an emergency contraceptive pill intervention on pregnancy risk behavior. Contraception. 2008;77:333-336.
49. Raymond EG, Trussell J, Polis CB. Population effect of increased access to emergency contraceptive pills: a systematic review. Obstet Gynecol. 2007;109:181-188.
50. Casey PM, Cerhan JR, Pruthi S. Oral contraceptive use and risk of breast cancer Mayo Clinic Proc. 2008;83:86-89.
51. Trussell J. Contraceptive efficacy. In: Hatcher RA, Trussell J, Stewart FH, et al, eds. Contraceptive Technology, 18th ed. New York, NY: Ardent Media, Inc; 2004:773-845.
- Consider an oral contraceptive for women who would prefer less frequent menstrual periods (A).
- An intrauterine device may be appropriate for women with prior pelvic inflammatory disease, ectopic pregnancy, or an abnormal Papanicolaou (Pap) smear result, and for many adolescents (A).
- There are no medical contraindications to progestin-only emergency contraception (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
A 35-year-old woman with a family history of breast cancer (mother diagnosed with breast cancer at age 55) requests your help in choosing an appropriate method of contraception. She is a nonsmoker, has a body mass index of 25, and dislikes taking pills. Which options would you recommend to her? Are there any that you would rule out?
Helping your patient make the best choice requires that you be as up to date as possible. In this review, we discuss select new options in a clinically relevant manner. Specifically, we explore the newest oral contraceptives (OCs), including extended-cycle, continuous, and shortened hormone-free interval formulations. In addition, we review the latest data and updated recommendations for the contraceptive patch and ring, intrauterine devices (IUDs), implants, and emergency contraception (TABLE). We conclude by describing appropriate choices for the patient described above. (See “So what do you recommend?” on page 803.)
Oral contraceptives
Since OCs became available in the 1960s, the standard regimen has been 21 active pills followed by 7 placebo pills, simulating the average unassisted monthly menstrual cycle in which “menstrual” or withdrawal bleeding occurs. Clinicians have successfully lengthened intermenstrual intervals with OCs, without incurring additional risk, to control symptoms of endometriosis, premenstrual syndrome, and menstrual-withdrawal headaches, or to satisfy many patients’ preference for fewer menses per year.1,2
Any monophasic active OC can be used without a placebo interval to delay menses for extended periods. Until recently, such usage was off-label. Based on extensive safety and efficacy studies, however, the US Food and Drug Administration (FDA) has now approved several formulations for extended-cycle and continuous-cycle use.
Extended-cycle OCs: Fewer menses per year
Two FDA-approved extended-cycle OCs are available: Seasonale and Seasonique.3,4 Both products enable 4 scheduled menstrual intervals per year, as opposed to about 13 with 28-day cycles. Each regimen uses 84 consecutive pills of levonorgestrel 0.15 mg and ethinyl estradiol (EE) 0.03 mg, followed by 7 placebo pills (Seasonale) or 7 pills of EE 0.01 mg (Seasonique).
Other potential advantages. With Seasonique, the average length of menses is 3 days, which is shorter than the average unassisted menstrual period. Seasonique’s 7 additional low-dose estrogen pills may help decrease estrogen withdrawal symptoms, such as headaches in women with menstrual migraine and vasomotor instability in perimenopausal women. Though this effect has also been reported with other OCs containing low-dose estrogen during the traditional placebo week, specific supportive evidence is not yet available for these formulations.5,6
Disadvantages to address. With Seasonale and Seasonique, unscheduled spotting or bleeding has been reported—especially during initial use—at rates considerably higher than those associated with comparable traditional OCs.3,4 Effective counseling will help ensure patient compliance and satisfaction.
During the first cycle (days 1-91), about 65% of women taking either formulation reported ≥7 days of spotting, and 29% to 35% reported ≥20 days of spotting. By the 4th cycle (days 273-364), 39% to 42% of patients reported ≥7 days of spotting and 11% to 15% reported ≥20 days of spotting. For patients taking comparable progestin and EE doses in traditional monthly regimens, 38% reported ≥7 days of spotting and 6% reported ≥20 days of spotting during the first cycle. Thirty-nine percent and 4%, respectively, reported spotting during the fourth cycle.3,4
Continuous OC: Consistent hormonal milieu
Lybrel, the only FDA-approved OC for continuous use, contains levonorgestrel 90 mcg and EE 20 mcg; pills are taken daily throughout the year.7 Progestin and estrogen doses are lower than those found in many monthly OCs and in all extended-cycle formulations. A phase 3 trial of 2134 women reported the safety and efficacy of Lybrel to be comparable to cyclic OCs.8 Again, unscheduled bleeding and spotting rates were relatively high but decreased at pack 3 from 47% and 26%, respectively, to 21% and 20%, respectively, at pack 13. Predictably, amenorrhea rates increased from 27% to 59% between pack 3 and pack 13.
Shortened hormone-free interval OCs: Less breakthrough ovulation
The shortened hormone-free interval OC is an alternative for patients who want regular, but shorter, menstrual intervals. With 24 active and 4 placebo pills in each cycle, this regimen suppresses the pituitary/ovarian axis to a greater extent than traditional 21/7-day regimens and thus lowers the rate of breakthrough ovulation.9
Loestrin 24 Fe contains norethindrone 1 mg and EE 20 mcg; placebo pills contain 75 mg of ferrous fumarate.
Yaz contains the newer progestin drospirenone 3 mg and EE 20 mcg. Drospirenone, an analog of the antihypertensive spironolactone, was introduced in a 21/7 formulation, Yasmin, and proved to have beneficial effects on mood, water retention, and acne.10 Yaz, which contains a lower dose of EE than Yasmin, provides 3 additional days of antimineralocorticoid and antiandrogenic activity, and is indicated for the treatment of premenstrual dysphoric disorder, a more severe form of premenstrual syndrome. Drospirenone-containing OCs are contraindicated for patients with renal, adrenal, or hepatic impairment because of the progestin’s metabolism via these routes.
An established OC with a twist: Chewable pills
For patients unable to swallow OCs, a chewable formulation, Femcon Fe, is available.11 It is hormonally identical to Ovcon 35, a well-established OC containing norethindrone acetate 0.4 mg and EE 35 mcg. The 7 placebo pills contain ferrous fumarate 75 mg. The spearmint-flavored chewable pill (which can also be swallowed) must be taken with 8 ounces of water.
Noncontraceptive benefits of OCs—there are many
An extensive body of evidence supports the noncontraceptive health benefits of OCs. These include a decreased risk of:
- endometrial and ovarian cancer
- bone loss
- benign breast disease
- pelvic inflammatory disease
- ectopic pregnancy
- rheumatoid arthritis.
Women with symptoms of androgen excess, premenstrual mood disorders, or endometriosis pain have long benefited from treatment with OCs.10,12-14 Healthy perimenopausal women are excellent candidates for OCs to regulate menses and treat symptoms of estrogen deficiency. OCs with added estrogen during the menstrual interval or shortened hormone-free interval may be more effective in moderating the perimenopausal transition. However, specific evidence about these effects is not yet available for the newest OC formulations.
Risks of OCs have been reduced, but some remain
Many of the well-known risks and side effects of OCs have been minimized over the years as total doses of estrogen have decreased and less androgenic progestins have been incorporated into OC formulations. Nonetheless, OCs are contraindicated for women who have a history of venous thromboembolism (VTE) or coronary artery disease (or are at risk for these complications), are over the age of 35 and smoke, are pregnant or newly postpartum, or are immobilized after surgery.
OCs remain relatively contraindicated for women with a history of migraines and focal auras, due to the increased risk for ischemic stroke.15 Breast and other estrogen-dependent cancers as well as liver disease preclude the use of OCs.16 Additional studies using the newer formulations of OCs are needed to definitively determine their long-term risk compared with traditional monthly formulations.
Contraceptive patch: Improving compliance
The contraceptive patch Evra is applied weekly and releases norelgestromin 150 mcg and EE 20 mcg each day, providing an OC alternative that is less dependent on compliance. However, in November 2005, the FDA modified product labeling to inform providers and the public that, based on pharmacokinetic studies, patients using the patch were exposed to hormone levels about 60% higher than with OCs of similar dosage.17 Another FDA labeling change made in 2008 states that “it is not known whether there are changes in the risk of serious adverse events based on the differences in pharmacokinetic profiles of EE in women using [the patch] as compared with women using oral contraceptives containing 35 mcg of EE.”
What is the real risk of VTE? One case-control study reported that the rates of VTE events in patch users and OC users were 52 per 100,000 woman-years and 42 per 100,000 woman-years, respectively.18 Another large case-control study showed the odds ratio (OR) of VTE to be 2.4 (95% confidence interval [CI], 1.1-5.5) with the patch compared with OCs; data were corrected for high-risk factors.19 However, the absolute risks for the patch and OCs were 40 and 18 per 100,000 woman-years, respectively—both lower than the risk of VTE associated with pregnancy.
The risk of myocardial infarction. The OR for myocardial infarction among patch users in the same population was 1.8 (95% CI, 0.5-6.8), and there was no statistically significant increase in the rate of cerebrovascular accidents.19 Thus, it is reasonable to use the patch with caution in patients without cardiac risk factors and to limit total hormone dosage by not using the patch in an extended-cycle manner. Of note, the patch is reported to have decreased efficacy in patients weighing over 90 kg (198 lb).20
Vaginal ring: Fewer drug interactions
NuvaRing, the ethylene vinyl vaginal ring, releases etonorgestrel (ENG) 120 mcg and EE 15 mcg each day (a lower estrogen dose than is contained in OCs or the patch).21 The device is 5.4 cm in diameter and 4 mm thick. Patients insert the ring intravaginally, remove it 3 weeks later for menses, and insert a new ring 1 week later.
Continuous use regimen. A randomized controlled trial evaluating the frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring reported a reduction in bleeding, flow, and pelvic pain, and a high continuation rate. Most patients considered the bleeding profile with the continuous vaginal ring acceptable compared with the baseline 21/7 use.22 Each ring contains up to a 28-day supply of hormones.
LNG-IUS (Mirena)
Fewer interactions. Transvaginal absorption of hormones with the vaginal ring avoids a first pass through the liver, thus decreasing many medication interactions. Irregular bleeding experienced with OCs or the patch may be effectively reduced with the steadily released, rapidly acting hormones in the ring.23
Intrauterine contraception
Intrauterine contraception is increasingly accepted by women who want long-term and effective contraception without having to comply with a particular regimen.
Copper IUD: Many contraindications are lifted
The nonhormonal IUD ParaGard T 380A is indicated for contraception for up to 10 years in women who are 16 years of age and older.24 This IUD’s active ingredient is the spermicidal copper wire wound around the short arms of the device. A recent meta-analysis reported an association between the use of a copper IUD and a decrease in the risk of endometrial cancer (OR=0.39; 95% CI, 0.29-0.51), though the mechanism for this association is unclear.25
In late 2005, the FDA broadened the use of copper IUDs to include women who are nulliparous; have a history of pelvic inflammatory disease (PID), sexually transmitted disease, or ectopic pregnancy; are in nonmonogamous relationships; or have a history of premenopausal breast cancer. This method also may be used by women with asymptomatic human immunodeficiency virus infection, Actinomyces infection, abnormal Papanicolaou (Pap) test results, or vaginitis. Furthermore, data support its use in adolescents who are at particularly high risk for unintended pregnancy.26
The copper IUD remains contra-indicated for patients with acute PID or current high-risk behavior for sexually transmitted infections, as well as for those who have mucopurulent cervicitis or have had postpartum endometritis within the past 3 months.24 Wilson’s disease is also a contraindication.
Insertion tip. Misoprostol may be used to soften the nulliparous cervix for insertion.27
Levonorgestrel intrauterine system: An alternative to the copper IUD
The levonorgestrel intrauterine system (LNG IUS), sold under the brand name Mirena, is gaining tremendous popularity in the United States. Multiple mechanisms of action, including endometrial thinning, cervical mucus thickening, inhibition of sperm function, and intermittent ovulation suppression are responsible for the >99% efficacy of this 5-year contraceptive.
Irregular menses can be expected initially, and 20% of patients reported amenorrhea at 1 year of use. In the unlikely event that a woman becomes pregnant while using Mirena, evaluate for ectopic pregnancy, which occurs in about half of pregnancies in women using this system.
Noncontraceptive benefits. The system’s primary noncontraceptive benefit is the dramatic reduction of menstrual blood loss, reported to be up to 90%.28 This contraceptive has been used as a cost-effective alternative to hysterectomy and endometrial ablation.29,30 Mirena imparts a protective effect against PID, likely secondary to progestin-mediated cervical mucus thickening.31
Subdermal implant (Implanon)
It appears safe and expeditious to provide both counseling and intrauterine contraception insertion in one visit, provided pregnancy is excluded.32 Confirm normalcy of cervical cytology and screen for sexually transmitted disease, if indicated. Prophylactic antibiotics are unnecessary, as the risk of PID within 20 days of insertion is only 9.7 per 1000 woman- years.33 After 20 days, the risk declines to 1.4 per 1000 woman-years, the same as that of the general population.34
Subdermal implant: Easily reversible
In July 2006, the FDA approved Implanon, a subdermal contraceptive implant.35 It has been available worldwide since 1998. The 40 × 2 mm single-rod implant containing etonogestrel (ENG) 68 mg diffuses the hormone at a rate of 60 mcg/d immediately after insertion and then steadily at 30 mcg/d for up to 3 years.
Its primary mechanism of action is ovulation suppression, with no ovulation detected for 30 months in a study group of more than 17,000 women.35 Increased cervical mucus viscosity also contributes to its effectiveness.35 In a large clinical trial, no pregnancies were reported in more than 6100 cycles.36 However, this trial excluded women weighing more than 130% of their ideal body weight, so no data are available to support the effectiveness of Implanon in obese women.36
Benefits and risks. This implant does not cause a hypoestrogenic state and ovulation suppression is rapidly reversible, with ENG levels undetectable within 10 days of implant removal.35 Furthermore, this method has no reported deleterious effects on bone mineral density or lactation.37,38 When counseling women about the implant, emphasize its propensity to result in “irregular and unpredictable” bleeding. An average of 7 bleeding and 10 spotting days within a 90-day period has been reported. Most women had fewer bleeding/spotting days than they would without contraception, but unscheduled bleeding was the leading reason for method discontinuation (11%), followed by weight gain, emotional lability, acne, headache, and depression (each ≈1%-2%).35
Implant insertion. The device is inserted in the sulcus between the biceps and triceps muscles of the nondominant arm. It is crucial to place the implant subdermally, tenting the skin during insertion to prevent deep insertion. High-frequency ultrasound can be used to detect nonpalpable implants. The FDA has mandated 3 hours of training for clinicians before they can obtain the device.
Depot medroxyprogesterone: Tried and true alternative
The depot medroxyprogesterone acetate (DMPA) injection has been a mainstay of contraception for decades. Available under the brand name Depo-Provera, it’s an option for women in whom estrogen-containing contraceptives are contraindicated. Its convenience, reduced risk of anemia, and postpartum benefits are all well known, and we have thus limited our discussion of DMPA to the summary in the TABLE.
TABLE
How do these contraceptives compare?
| METHOD | ROUTE OF ADMINISTRATION | FREQUENCY OF ADMINISTRATION | FAILURES/YR WITH TYPICAL USE 33,35,51 | EXPECTED MENSTRUAL PATTERN | ADVERSE EFFECTS/CONTRAINDICATIONS |
|---|---|---|---|---|---|
| Cyclic OCs | Oral | Daily | 8% | Monthly menses, may have BTB initially | Hormonal adverse effects |
| Extended-cycle OCs (Seasonale, Seasonique) | Oral | Daily | 8% | Menses 4/yr, frequent BTB | Hormonal adverse effects, unscheduled bleeding |
| Continuous OCs (Lybrel) | Oral | Daily | 8% | No scheduled menses, frequent BTB | Hormonal adverse effects, unscheduled bleeding |
| Shortened hormone-free interval OCs (Loestrin 24 Fe, Yaz) | Oral | Daily | 8% | Shorter monthly menses | Hormonal adverse effects, unscheduled bleeding |
| Transdermal patch (Evra) | Patch applied to skin | New patch applied weekly for 3 wk; off for 1 wk | 8% | Monthly menses, may have BTB initially | Hormonal adverse effects, increased risk of VTE higher than OCs but lower than pregnancy; MI risk higher than comparable OCs, but use is reasonable if no cardiac risk factors |
| Vaginal ring (NuvaRing) | Ring inserted in vagina by patient | Ring inserted for 3 wk, removed for 1 wk | 8% | Monthly menses, may have BTB initially | Hormonal adverse effects |
| Copper IUD (ParaGard T 380A) | IUD inserted & removed by clinician | Every 10 yr | 0. 8% | Heavier menses, may have BTB | Menorrhagia. Contraindications: Acute PID or high risk for STI; postpartum endometritis within 3 mo; mucopurulent cervicitis; Wilson’s disease |
| Levonorgestrel IUS (LNG IUS, Mirena) | IUS inserted & removed by clinician | Every 5 yr | < 0.1% | Lighter, shorter menses or amenorrhea | Minimal hormonal adverse effects. Contraindications: Acute PID, history of or high risk for PID; postpartum endometritis within 3 mo; mucopurulent cervicitis |
| Subdermal implant (Implanon) | Inserted subdermally & removed by clinician | Every 3 yr | 0.3% | Irregular, unpredictable bleeding | Unscheduled bleeding, mood symptoms, headache, weight gain, acne |
| Depot medroxyprogesterone acetate (Depo-Provera) | IM injection | Every 3 mo | 3% | Irregular bleeding, amenorrhea | Unscheduled bleeding, reversible bone loss |
| BTB, breakthrough bleeding; IM, intramuscular; IUD, intrauterine device; IUS, intrauterine system; MI, myocardial infarction; OC, oral contraceptive; PID, pelvic inflammatory disease; STI, sexually transmitted infection; VTE, venous thromboembolism. | |||||
Emergency contraception: 2 pills, 12 hours apart
Plan B contains 2 tablets of levonorgestrel 0.75 mg, to be taken 12 hours apart as soon as possible after unprotected intercourse. However, taking both doses together is as effective as taking them separately, and doing so may improve compliance.39
How it works. Emergency contraception (EC) works by inhibiting or delaying the surge of luteinizing hormone and follicular rupture before ovulation. It does not affect implantation or corpus luteum function, and it poses no risk to an established pregnancy or embryo. EC is ineffective when administered after ovulation.40
In a World Health Organization (WHO) multicenter randomized trial, EC prevented 79% to 84% of pregnancies if taken 1 to 3 days after intercourse, and 60% to 63% of pregnancies if taken 4 to 5 days after intercourse.41 Plan B is available without a prescription for women ages 18 and older. It is important to screen for pregnancy before prescribing Plan B for younger patients.
Adverse effects include nausea and vomiting, occurring in 23% and 5% of patients, respectively. Intermenstrual bleeding occurs in 8% of patients taking progestin-only EC. Menses are expected within 21 days of EC administration, and the second cycle after EC should be of normal length.42 The authors of a WHO report concluded that “there are no medical conditions wherein risks outweigh benefits of EC.”43
Combination estrogen/progestin for EC is no more effective than progestin-only EC and results in higher rates of adverse effects, especially nausea and vomiting.44
IUD can also be used in an emergency
Insertion of a copper IUD provides EC as well as ongoing contraception. It is hormone free and can be used effectively for EC up to 5 days after sexual intercourse, then continued for primary contraception for up to 10 years.45 Its estimated failure rate was less than 0.1% in more than 8400 postcoital insertions.46 The IUD works by impairing fertilization and implantation and by altering sperm motility and integrity. With a copper IUD, additional primary contraception is unnecessary. The LNG IUS (Mirena) has not been studied as an alternative EC.
Some worry that EC’s availability will encourage unprotected sex
Some authorities have wondered if increased access to EC might paradoxically lead to more pregnancies by encouraging unprotected sex. Researchers are exploring this issue. One study reported that unfettered access to free EC resulted in an increase in EC use, and another study reported that patients with unrestricted EC access had inadequately protected sex more often than those in the control group.47,48
A systematic review of 23 articles studying the effect of increased access to EC confirmed an increase in EC use,49 but no statistically significant differences in pregnancy or abortion rates.
For patients like the 35-year-old woman discussed earlier, who do not like taking pills, there are many contraception options to choose from, including the patch, vaginal ring, chewable OC, IUD, depot medroxyprogesterone injection, and subdermal implant.
Hormonal contraception would not be an issue for this patient—even though she has a family history of breast cancer. Using hormonal contraception does not increase the risk of breast cancer for individuals with a family history of breast cancer in a first-or second-degree relative.50
Correspondence
Petra M. Casey, MD, Department of Obstetrics and Gynecology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; casey.petra@mayo.edu.
- Consider an oral contraceptive for women who would prefer less frequent menstrual periods (A).
- An intrauterine device may be appropriate for women with prior pelvic inflammatory disease, ectopic pregnancy, or an abnormal Papanicolaou (Pap) smear result, and for many adolescents (A).
- There are no medical contraindications to progestin-only emergency contraception (A).
Strength of recommendation (SOR)
- Good-quality patient-oriented evidence
- Inconsistent or limited-quality patient-oriented evidence
- Consensus, usual practice, opinion, disease-oriented evidence, case series
A 35-year-old woman with a family history of breast cancer (mother diagnosed with breast cancer at age 55) requests your help in choosing an appropriate method of contraception. She is a nonsmoker, has a body mass index of 25, and dislikes taking pills. Which options would you recommend to her? Are there any that you would rule out?
Helping your patient make the best choice requires that you be as up to date as possible. In this review, we discuss select new options in a clinically relevant manner. Specifically, we explore the newest oral contraceptives (OCs), including extended-cycle, continuous, and shortened hormone-free interval formulations. In addition, we review the latest data and updated recommendations for the contraceptive patch and ring, intrauterine devices (IUDs), implants, and emergency contraception (TABLE). We conclude by describing appropriate choices for the patient described above. (See “So what do you recommend?” on page 803.)
Oral contraceptives
Since OCs became available in the 1960s, the standard regimen has been 21 active pills followed by 7 placebo pills, simulating the average unassisted monthly menstrual cycle in which “menstrual” or withdrawal bleeding occurs. Clinicians have successfully lengthened intermenstrual intervals with OCs, without incurring additional risk, to control symptoms of endometriosis, premenstrual syndrome, and menstrual-withdrawal headaches, or to satisfy many patients’ preference for fewer menses per year.1,2
Any monophasic active OC can be used without a placebo interval to delay menses for extended periods. Until recently, such usage was off-label. Based on extensive safety and efficacy studies, however, the US Food and Drug Administration (FDA) has now approved several formulations for extended-cycle and continuous-cycle use.
Extended-cycle OCs: Fewer menses per year
Two FDA-approved extended-cycle OCs are available: Seasonale and Seasonique.3,4 Both products enable 4 scheduled menstrual intervals per year, as opposed to about 13 with 28-day cycles. Each regimen uses 84 consecutive pills of levonorgestrel 0.15 mg and ethinyl estradiol (EE) 0.03 mg, followed by 7 placebo pills (Seasonale) or 7 pills of EE 0.01 mg (Seasonique).
Other potential advantages. With Seasonique, the average length of menses is 3 days, which is shorter than the average unassisted menstrual period. Seasonique’s 7 additional low-dose estrogen pills may help decrease estrogen withdrawal symptoms, such as headaches in women with menstrual migraine and vasomotor instability in perimenopausal women. Though this effect has also been reported with other OCs containing low-dose estrogen during the traditional placebo week, specific supportive evidence is not yet available for these formulations.5,6
Disadvantages to address. With Seasonale and Seasonique, unscheduled spotting or bleeding has been reported—especially during initial use—at rates considerably higher than those associated with comparable traditional OCs.3,4 Effective counseling will help ensure patient compliance and satisfaction.
During the first cycle (days 1-91), about 65% of women taking either formulation reported ≥7 days of spotting, and 29% to 35% reported ≥20 days of spotting. By the 4th cycle (days 273-364), 39% to 42% of patients reported ≥7 days of spotting and 11% to 15% reported ≥20 days of spotting. For patients taking comparable progestin and EE doses in traditional monthly regimens, 38% reported ≥7 days of spotting and 6% reported ≥20 days of spotting during the first cycle. Thirty-nine percent and 4%, respectively, reported spotting during the fourth cycle.3,4
Continuous OC: Consistent hormonal milieu
Lybrel, the only FDA-approved OC for continuous use, contains levonorgestrel 90 mcg and EE 20 mcg; pills are taken daily throughout the year.7 Progestin and estrogen doses are lower than those found in many monthly OCs and in all extended-cycle formulations. A phase 3 trial of 2134 women reported the safety and efficacy of Lybrel to be comparable to cyclic OCs.8 Again, unscheduled bleeding and spotting rates were relatively high but decreased at pack 3 from 47% and 26%, respectively, to 21% and 20%, respectively, at pack 13. Predictably, amenorrhea rates increased from 27% to 59% between pack 3 and pack 13.
Shortened hormone-free interval OCs: Less breakthrough ovulation
The shortened hormone-free interval OC is an alternative for patients who want regular, but shorter, menstrual intervals. With 24 active and 4 placebo pills in each cycle, this regimen suppresses the pituitary/ovarian axis to a greater extent than traditional 21/7-day regimens and thus lowers the rate of breakthrough ovulation.9
Loestrin 24 Fe contains norethindrone 1 mg and EE 20 mcg; placebo pills contain 75 mg of ferrous fumarate.
Yaz contains the newer progestin drospirenone 3 mg and EE 20 mcg. Drospirenone, an analog of the antihypertensive spironolactone, was introduced in a 21/7 formulation, Yasmin, and proved to have beneficial effects on mood, water retention, and acne.10 Yaz, which contains a lower dose of EE than Yasmin, provides 3 additional days of antimineralocorticoid and antiandrogenic activity, and is indicated for the treatment of premenstrual dysphoric disorder, a more severe form of premenstrual syndrome. Drospirenone-containing OCs are contraindicated for patients with renal, adrenal, or hepatic impairment because of the progestin’s metabolism via these routes.
An established OC with a twist: Chewable pills
For patients unable to swallow OCs, a chewable formulation, Femcon Fe, is available.11 It is hormonally identical to Ovcon 35, a well-established OC containing norethindrone acetate 0.4 mg and EE 35 mcg. The 7 placebo pills contain ferrous fumarate 75 mg. The spearmint-flavored chewable pill (which can also be swallowed) must be taken with 8 ounces of water.
Noncontraceptive benefits of OCs—there are many
An extensive body of evidence supports the noncontraceptive health benefits of OCs. These include a decreased risk of:
- endometrial and ovarian cancer
- bone loss
- benign breast disease
- pelvic inflammatory disease
- ectopic pregnancy
- rheumatoid arthritis.
Women with symptoms of androgen excess, premenstrual mood disorders, or endometriosis pain have long benefited from treatment with OCs.10,12-14 Healthy perimenopausal women are excellent candidates for OCs to regulate menses and treat symptoms of estrogen deficiency. OCs with added estrogen during the menstrual interval or shortened hormone-free interval may be more effective in moderating the perimenopausal transition. However, specific evidence about these effects is not yet available for the newest OC formulations.
Risks of OCs have been reduced, but some remain
Many of the well-known risks and side effects of OCs have been minimized over the years as total doses of estrogen have decreased and less androgenic progestins have been incorporated into OC formulations. Nonetheless, OCs are contraindicated for women who have a history of venous thromboembolism (VTE) or coronary artery disease (or are at risk for these complications), are over the age of 35 and smoke, are pregnant or newly postpartum, or are immobilized after surgery.
OCs remain relatively contraindicated for women with a history of migraines and focal auras, due to the increased risk for ischemic stroke.15 Breast and other estrogen-dependent cancers as well as liver disease preclude the use of OCs.16 Additional studies using the newer formulations of OCs are needed to definitively determine their long-term risk compared with traditional monthly formulations.
Contraceptive patch: Improving compliance
The contraceptive patch Evra is applied weekly and releases norelgestromin 150 mcg and EE 20 mcg each day, providing an OC alternative that is less dependent on compliance. However, in November 2005, the FDA modified product labeling to inform providers and the public that, based on pharmacokinetic studies, patients using the patch were exposed to hormone levels about 60% higher than with OCs of similar dosage.17 Another FDA labeling change made in 2008 states that “it is not known whether there are changes in the risk of serious adverse events based on the differences in pharmacokinetic profiles of EE in women using [the patch] as compared with women using oral contraceptives containing 35 mcg of EE.”
What is the real risk of VTE? One case-control study reported that the rates of VTE events in patch users and OC users were 52 per 100,000 woman-years and 42 per 100,000 woman-years, respectively.18 Another large case-control study showed the odds ratio (OR) of VTE to be 2.4 (95% confidence interval [CI], 1.1-5.5) with the patch compared with OCs; data were corrected for high-risk factors.19 However, the absolute risks for the patch and OCs were 40 and 18 per 100,000 woman-years, respectively—both lower than the risk of VTE associated with pregnancy.
The risk of myocardial infarction. The OR for myocardial infarction among patch users in the same population was 1.8 (95% CI, 0.5-6.8), and there was no statistically significant increase in the rate of cerebrovascular accidents.19 Thus, it is reasonable to use the patch with caution in patients without cardiac risk factors and to limit total hormone dosage by not using the patch in an extended-cycle manner. Of note, the patch is reported to have decreased efficacy in patients weighing over 90 kg (198 lb).20
Vaginal ring: Fewer drug interactions
NuvaRing, the ethylene vinyl vaginal ring, releases etonorgestrel (ENG) 120 mcg and EE 15 mcg each day (a lower estrogen dose than is contained in OCs or the patch).21 The device is 5.4 cm in diameter and 4 mm thick. Patients insert the ring intravaginally, remove it 3 weeks later for menses, and insert a new ring 1 week later.
Continuous use regimen. A randomized controlled trial evaluating the frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring reported a reduction in bleeding, flow, and pelvic pain, and a high continuation rate. Most patients considered the bleeding profile with the continuous vaginal ring acceptable compared with the baseline 21/7 use.22 Each ring contains up to a 28-day supply of hormones.
LNG-IUS (Mirena)
Fewer interactions. Transvaginal absorption of hormones with the vaginal ring avoids a first pass through the liver, thus decreasing many medication interactions. Irregular bleeding experienced with OCs or the patch may be effectively reduced with the steadily released, rapidly acting hormones in the ring.23
Intrauterine contraception
Intrauterine contraception is increasingly accepted by women who want long-term and effective contraception without having to comply with a particular regimen.
Copper IUD: Many contraindications are lifted
The nonhormonal IUD ParaGard T 380A is indicated for contraception for up to 10 years in women who are 16 years of age and older.24 This IUD’s active ingredient is the spermicidal copper wire wound around the short arms of the device. A recent meta-analysis reported an association between the use of a copper IUD and a decrease in the risk of endometrial cancer (OR=0.39; 95% CI, 0.29-0.51), though the mechanism for this association is unclear.25
In late 2005, the FDA broadened the use of copper IUDs to include women who are nulliparous; have a history of pelvic inflammatory disease (PID), sexually transmitted disease, or ectopic pregnancy; are in nonmonogamous relationships; or have a history of premenopausal breast cancer. This method also may be used by women with asymptomatic human immunodeficiency virus infection, Actinomyces infection, abnormal Papanicolaou (Pap) test results, or vaginitis. Furthermore, data support its use in adolescents who are at particularly high risk for unintended pregnancy.26
The copper IUD remains contra-indicated for patients with acute PID or current high-risk behavior for sexually transmitted infections, as well as for those who have mucopurulent cervicitis or have had postpartum endometritis within the past 3 months.24 Wilson’s disease is also a contraindication.
Insertion tip. Misoprostol may be used to soften the nulliparous cervix for insertion.27
Levonorgestrel intrauterine system: An alternative to the copper IUD
The levonorgestrel intrauterine system (LNG IUS), sold under the brand name Mirena, is gaining tremendous popularity in the United States. Multiple mechanisms of action, including endometrial thinning, cervical mucus thickening, inhibition of sperm function, and intermittent ovulation suppression are responsible for the >99% efficacy of this 5-year contraceptive.
Irregular menses can be expected initially, and 20% of patients reported amenorrhea at 1 year of use. In the unlikely event that a woman becomes pregnant while using Mirena, evaluate for ectopic pregnancy, which occurs in about half of pregnancies in women using this system.
Noncontraceptive benefits. The system’s primary noncontraceptive benefit is the dramatic reduction of menstrual blood loss, reported to be up to 90%.28 This contraceptive has been used as a cost-effective alternative to hysterectomy and endometrial ablation.29,30 Mirena imparts a protective effect against PID, likely secondary to progestin-mediated cervical mucus thickening.31
Subdermal implant (Implanon)
It appears safe and expeditious to provide both counseling and intrauterine contraception insertion in one visit, provided pregnancy is excluded.32 Confirm normalcy of cervical cytology and screen for sexually transmitted disease, if indicated. Prophylactic antibiotics are unnecessary, as the risk of PID within 20 days of insertion is only 9.7 per 1000 woman- years.33 After 20 days, the risk declines to 1.4 per 1000 woman-years, the same as that of the general population.34
Subdermal implant: Easily reversible
In July 2006, the FDA approved Implanon, a subdermal contraceptive implant.35 It has been available worldwide since 1998. The 40 × 2 mm single-rod implant containing etonogestrel (ENG) 68 mg diffuses the hormone at a rate of 60 mcg/d immediately after insertion and then steadily at 30 mcg/d for up to 3 years.
Its primary mechanism of action is ovulation suppression, with no ovulation detected for 30 months in a study group of more than 17,000 women.35 Increased cervical mucus viscosity also contributes to its effectiveness.35 In a large clinical trial, no pregnancies were reported in more than 6100 cycles.36 However, this trial excluded women weighing more than 130% of their ideal body weight, so no data are available to support the effectiveness of Implanon in obese women.36
Benefits and risks. This implant does not cause a hypoestrogenic state and ovulation suppression is rapidly reversible, with ENG levels undetectable within 10 days of implant removal.35 Furthermore, this method has no reported deleterious effects on bone mineral density or lactation.37,38 When counseling women about the implant, emphasize its propensity to result in “irregular and unpredictable” bleeding. An average of 7 bleeding and 10 spotting days within a 90-day period has been reported. Most women had fewer bleeding/spotting days than they would without contraception, but unscheduled bleeding was the leading reason for method discontinuation (11%), followed by weight gain, emotional lability, acne, headache, and depression (each ≈1%-2%).35
Implant insertion. The device is inserted in the sulcus between the biceps and triceps muscles of the nondominant arm. It is crucial to place the implant subdermally, tenting the skin during insertion to prevent deep insertion. High-frequency ultrasound can be used to detect nonpalpable implants. The FDA has mandated 3 hours of training for clinicians before they can obtain the device.
Depot medroxyprogesterone: Tried and true alternative
The depot medroxyprogesterone acetate (DMPA) injection has been a mainstay of contraception for decades. Available under the brand name Depo-Provera, it’s an option for women in whom estrogen-containing contraceptives are contraindicated. Its convenience, reduced risk of anemia, and postpartum benefits are all well known, and we have thus limited our discussion of DMPA to the summary in the TABLE.
TABLE
How do these contraceptives compare?
| METHOD | ROUTE OF ADMINISTRATION | FREQUENCY OF ADMINISTRATION | FAILURES/YR WITH TYPICAL USE 33,35,51 | EXPECTED MENSTRUAL PATTERN | ADVERSE EFFECTS/CONTRAINDICATIONS |
|---|---|---|---|---|---|
| Cyclic OCs | Oral | Daily | 8% | Monthly menses, may have BTB initially | Hormonal adverse effects |
| Extended-cycle OCs (Seasonale, Seasonique) | Oral | Daily | 8% | Menses 4/yr, frequent BTB | Hormonal adverse effects, unscheduled bleeding |
| Continuous OCs (Lybrel) | Oral | Daily | 8% | No scheduled menses, frequent BTB | Hormonal adverse effects, unscheduled bleeding |
| Shortened hormone-free interval OCs (Loestrin 24 Fe, Yaz) | Oral | Daily | 8% | Shorter monthly menses | Hormonal adverse effects, unscheduled bleeding |
| Transdermal patch (Evra) | Patch applied to skin | New patch applied weekly for 3 wk; off for 1 wk | 8% | Monthly menses, may have BTB initially | Hormonal adverse effects, increased risk of VTE higher than OCs but lower than pregnancy; MI risk higher than comparable OCs, but use is reasonable if no cardiac risk factors |
| Vaginal ring (NuvaRing) | Ring inserted in vagina by patient | Ring inserted for 3 wk, removed for 1 wk | 8% | Monthly menses, may have BTB initially | Hormonal adverse effects |
| Copper IUD (ParaGard T 380A) | IUD inserted & removed by clinician | Every 10 yr | 0. 8% | Heavier menses, may have BTB | Menorrhagia. Contraindications: Acute PID or high risk for STI; postpartum endometritis within 3 mo; mucopurulent cervicitis; Wilson’s disease |
| Levonorgestrel IUS (LNG IUS, Mirena) | IUS inserted & removed by clinician | Every 5 yr | < 0.1% | Lighter, shorter menses or amenorrhea | Minimal hormonal adverse effects. Contraindications: Acute PID, history of or high risk for PID; postpartum endometritis within 3 mo; mucopurulent cervicitis |
| Subdermal implant (Implanon) | Inserted subdermally & removed by clinician | Every 3 yr | 0.3% | Irregular, unpredictable bleeding | Unscheduled bleeding, mood symptoms, headache, weight gain, acne |
| Depot medroxyprogesterone acetate (Depo-Provera) | IM injection | Every 3 mo | 3% | Irregular bleeding, amenorrhea | Unscheduled bleeding, reversible bone loss |
| BTB, breakthrough bleeding; IM, intramuscular; IUD, intrauterine device; IUS, intrauterine system; MI, myocardial infarction; OC, oral contraceptive; PID, pelvic inflammatory disease; STI, sexually transmitted infection; VTE, venous thromboembolism. | |||||
Emergency contraception: 2 pills, 12 hours apart
Plan B contains 2 tablets of levonorgestrel 0.75 mg, to be taken 12 hours apart as soon as possible after unprotected intercourse. However, taking both doses together is as effective as taking them separately, and doing so may improve compliance.39
How it works. Emergency contraception (EC) works by inhibiting or delaying the surge of luteinizing hormone and follicular rupture before ovulation. It does not affect implantation or corpus luteum function, and it poses no risk to an established pregnancy or embryo. EC is ineffective when administered after ovulation.40
In a World Health Organization (WHO) multicenter randomized trial, EC prevented 79% to 84% of pregnancies if taken 1 to 3 days after intercourse, and 60% to 63% of pregnancies if taken 4 to 5 days after intercourse.41 Plan B is available without a prescription for women ages 18 and older. It is important to screen for pregnancy before prescribing Plan B for younger patients.
Adverse effects include nausea and vomiting, occurring in 23% and 5% of patients, respectively. Intermenstrual bleeding occurs in 8% of patients taking progestin-only EC. Menses are expected within 21 days of EC administration, and the second cycle after EC should be of normal length.42 The authors of a WHO report concluded that “there are no medical conditions wherein risks outweigh benefits of EC.”43
Combination estrogen/progestin for EC is no more effective than progestin-only EC and results in higher rates of adverse effects, especially nausea and vomiting.44
IUD can also be used in an emergency
Insertion of a copper IUD provides EC as well as ongoing contraception. It is hormone free and can be used effectively for EC up to 5 days after sexual intercourse, then continued for primary contraception for up to 10 years.45 Its estimated failure rate was less than 0.1% in more than 8400 postcoital insertions.46 The IUD works by impairing fertilization and implantation and by altering sperm motility and integrity. With a copper IUD, additional primary contraception is unnecessary. The LNG IUS (Mirena) has not been studied as an alternative EC.
Some worry that EC’s availability will encourage unprotected sex
Some authorities have wondered if increased access to EC might paradoxically lead to more pregnancies by encouraging unprotected sex. Researchers are exploring this issue. One study reported that unfettered access to free EC resulted in an increase in EC use, and another study reported that patients with unrestricted EC access had inadequately protected sex more often than those in the control group.47,48
A systematic review of 23 articles studying the effect of increased access to EC confirmed an increase in EC use,49 but no statistically significant differences in pregnancy or abortion rates.
For patients like the 35-year-old woman discussed earlier, who do not like taking pills, there are many contraception options to choose from, including the patch, vaginal ring, chewable OC, IUD, depot medroxyprogesterone injection, and subdermal implant.
Hormonal contraception would not be an issue for this patient—even though she has a family history of breast cancer. Using hormonal contraception does not increase the risk of breast cancer for individuals with a family history of breast cancer in a first-or second-degree relative.50
Correspondence
Petra M. Casey, MD, Department of Obstetrics and Gynecology, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; casey.petra@mayo.edu.
1. Andrist LC, Arias RD, Nucatola D, et al. Women’s and providers’ attitudes toward menstrual suppression with extended use of oral contraceptives. Contraception. 2004;70:359-363.
2. Glasier AF, Smith KB, van der Spuy ZM, et al. Amenorrhea associated with contraception—an international study on acceptability. Contraception. 2003;67:1-8.
3. Seasonale [package insert]. Pomona, NY: Duramed Pharmaceuticals, Inc; 2008.
4. Seasonique [package insert]. Pomona, NY: Duramed Pharmaceuticals, Inc; 2006.
5. Shortened pill-free interval delivered by new 20 mcg pill, Organon’s Mircette, schedule for US debut this summer Contraception Technol Update. 1998;19:85-87.
6. Casper RF, Dodin S, Reid RL. For the Study Investigators. The effect of 20 mcg ethinyl estradiol/1 mg norethindrone acetate (Minestrin), a low-dose oral contraceptive, on vaginal bleeding patterns, hot flashes and quality of life in symptomatic perimenopausal women. Menopause. 1997;4:139-147.
7. Lybrel [package insert] Philadelphia, Pa: Wyeth Pharmaceuticals, Inc; 2007.
8. Archer DF, Jensen JT, Johnson JV, et al. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception. 2006;74:439-445.
9. Willis SA, Kuehl TJ, Spiekerman AM, et al. Greater inhibition of the pituitary: ovarian axis in oral contraceptive regimens with a shortened hormone-free interval. Contraception. 2006;74:100-103.
10. Parsey KS, Pong A. An open-label, multicenter study to evaluate Yasmin, a low-dose combination oral contraceptive containing drospirenone, a new progestogen. Contraception. 2000;61:105-111.
11. Femcon Fe [package insert]. Rockaway, NJ: Warner Chilcott (US), Inc; 2007.
12. Burkman R. The evolution of oral contraceptives: 40 years of continuous improvement. Clinician. 2002;20:3-30.
13. Vercellini P, Frontino G, De Giorgi O, et al. Continuous use of an oral contraceptive for endometriosis-associated recurrent dysmenorrhea that does not respond to a cyclic pill regimen. Fertil Steril. 2003;80:560-563.
14. Guido M, Romualdi D, Giuliani M. Drospirenone for the treatment of hirsute women with PCOS; a clinical endocrinological, metabolic pilot study. J Clin Endocrinol Metab. 2004;89:2817-2823.
15. Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischaemic stroke in young women. Br Med J. 1995;310:830-833.
16. Steinauer J, Autry AM. Extended cycle combined hormonal contraception. Obstet Gynecol Clin North Am. 2007;34:43-55, viii.
17. Ortho Evra [prescribing information]. Raritan, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2001.
18. Jick SS, Kaye JA, Russmann S, et al. Risk of non-fatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg/l of ethinyl estradiol. Contraception. 2006;73:223-228.
19. Cole JA, Norman H, Doherty M, et al. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol. 2007;109:339-346.
20. American College of Obstetricians and Gynecologists. ACOG practice bulletin. Use of hormonal contraceptives in women with coexisting medical conditions. No. 73, June 2006. Obstet Gynecol. 2006;107:1453-1472.
21. Mulders TM, Dieben TO. Use of the novel combined contraceptive vaginal ring NuvaRing for ovulation inhibition. Fertil Steril. 2001;75:865-870.
22. Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring. Obstet Gynecol. 2008;112:563-571.
23. Bjarnadottir RI, Tuppurainen M, Killick SR. Comparison of cycle control with a combined contraceptive vaginal ring and oral levonorgestrel/ethinyl estradiol. Am J Obstet Gynecol. 2002;186:389-395.
24. ParaGard T 380A [prescribing information]. Pomona, NY: Duramed Pharmaceuticals, Inc; 2006.
25. Beining RM, Dennis LK, Smith EM, et al. Meta-analysis of intrauterine device use and risk of endometrial cancer. Ann Epidem. 2008;18:492-499.
26. ACOG Committee Opinion. Intrauterine Device and Adolescents No. 392. Obstet Gynecol. 2007;110:1493-1495.
27. McNaught J. Adolescents and IUCDs—Not a contraindication. J Ped Adolesc Gyn. 2006;19:303-305.
28. Lethaby AE, Cooke I, Rees M. Progesterone/progestogen releasing intrauterine systems versus either placebo or any other medication for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.-
29. Hurskainen R, Teperi J, Rissanen P, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomised trial. Lancet. 2001;357:273-277.
30. Römer T. Prospective comparison study of levonorgestrel IUD versus Roller-Ball endometrial ablation in the management of refractory recurrent hypermenorrhea. Eur J Obstet Gynecol Reprod Biol. 2000;90:27-29.
31. Toivonen J, Luukkainen T, Allonen H. Protective effect of intrauterine release of levonorgestrel on pelvic infection: three years’ comparative experience of levonorgestrel- and copper-releasing intrauterine devices. Obstet Gynecol. 1991;77:261-264.
32. Ball CE. News and controversies in contraception. AudioDigest Obstet Gynecol Clin Updates. 2007;54:18.-
33. Grimes DA. Intrauterine device and upper genital tract infection. Lancet. 2000;356:1013-1019.
34. Farley TM, Rosenberg MJ, Rowe PJ, et al. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339:785-788.
35. Implanon [package insert]. Roseland, NJ: Organon USA Inc; 2007.
36. Funk S, Miller MM, Mishell DR, Jr, et al. The Implanon US Study Group. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception. 2005;71:319-326.
37. Beerthuizen R, van Beek A, Massai R, et al. Bone mineral density during long-term use of the progestagen contraceptive implant Implanon compared to a non-hormonal method of contraception. Hum Reprod. 2000;15:118-122.
38. Reinprayoon D, Taneepanichskul S, Bunyavejchevin S, et al. Effects of the etonogestrel-releasing contraceptive implant (Implanon) on parameters of breastfeeding compared to those of an intrauterine device. Contraception. 2000;62:239-246.
39. Ngai SW, Fan S, Li S, et al. A randomized trial to compare 24 h versus 12 h double dose regimen of levonorgestrel for emergency contraception. Hum Reprod. 2005;20:307-311.
40. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 69: emergency contraception. Obstet Gynecol. 2005;106:1443-1452.
41. Von Hertzen H, Piaggio G, Ding J. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. WHO Research Group on Post-Ovulatory Methods of Fertility Regulation. Lancet. 2002;360:1803-1810.
42. Raymond EG, Goldberg A, Trussell J, et al. Bleeding patterns after use of levonorgestrel emergency contraceptive pills. Contraception. 2006;73:376-381.
43. World Health Organization. Medical eligibility criteria for contraceptive use. 3rd ed. 2004. Available at: www.who.int/reproductive-health/publication/med/mec.pdf. Accessed February 7, 2008.
44. Task Force on Postovulatory Methods of Fertility Regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352:428-433.
45. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 59. Intrauterine device. Obstet Gynecol. 2005;105:223-232.
46. LaValleur J. Emergency contraception. Obstet Gynecol Clin North Am. 2000;27:817-839, vii.
47. Raymond EG, Stewart F, Weaver M, et al. Impact of increased access to emergency contraceptive pills: a randomized controlled trial. Obstet Gynecol. 2006;108:1098-1106.
48. Raymond EG, Weaver MA. Effect of an emergency contraceptive pill intervention on pregnancy risk behavior. Contraception. 2008;77:333-336.
49. Raymond EG, Trussell J, Polis CB. Population effect of increased access to emergency contraceptive pills: a systematic review. Obstet Gynecol. 2007;109:181-188.
50. Casey PM, Cerhan JR, Pruthi S. Oral contraceptive use and risk of breast cancer Mayo Clinic Proc. 2008;83:86-89.
51. Trussell J. Contraceptive efficacy. In: Hatcher RA, Trussell J, Stewart FH, et al, eds. Contraceptive Technology, 18th ed. New York, NY: Ardent Media, Inc; 2004:773-845.
1. Andrist LC, Arias RD, Nucatola D, et al. Women’s and providers’ attitudes toward menstrual suppression with extended use of oral contraceptives. Contraception. 2004;70:359-363.
2. Glasier AF, Smith KB, van der Spuy ZM, et al. Amenorrhea associated with contraception—an international study on acceptability. Contraception. 2003;67:1-8.
3. Seasonale [package insert]. Pomona, NY: Duramed Pharmaceuticals, Inc; 2008.
4. Seasonique [package insert]. Pomona, NY: Duramed Pharmaceuticals, Inc; 2006.
5. Shortened pill-free interval delivered by new 20 mcg pill, Organon’s Mircette, schedule for US debut this summer Contraception Technol Update. 1998;19:85-87.
6. Casper RF, Dodin S, Reid RL. For the Study Investigators. The effect of 20 mcg ethinyl estradiol/1 mg norethindrone acetate (Minestrin), a low-dose oral contraceptive, on vaginal bleeding patterns, hot flashes and quality of life in symptomatic perimenopausal women. Menopause. 1997;4:139-147.
7. Lybrel [package insert] Philadelphia, Pa: Wyeth Pharmaceuticals, Inc; 2007.
8. Archer DF, Jensen JT, Johnson JV, et al. Evaluation of a continuous regimen of levonorgestrel/ethinyl estradiol: phase 3 study results. Contraception. 2006;74:439-445.
9. Willis SA, Kuehl TJ, Spiekerman AM, et al. Greater inhibition of the pituitary: ovarian axis in oral contraceptive regimens with a shortened hormone-free interval. Contraception. 2006;74:100-103.
10. Parsey KS, Pong A. An open-label, multicenter study to evaluate Yasmin, a low-dose combination oral contraceptive containing drospirenone, a new progestogen. Contraception. 2000;61:105-111.
11. Femcon Fe [package insert]. Rockaway, NJ: Warner Chilcott (US), Inc; 2007.
12. Burkman R. The evolution of oral contraceptives: 40 years of continuous improvement. Clinician. 2002;20:3-30.
13. Vercellini P, Frontino G, De Giorgi O, et al. Continuous use of an oral contraceptive for endometriosis-associated recurrent dysmenorrhea that does not respond to a cyclic pill regimen. Fertil Steril. 2003;80:560-563.
14. Guido M, Romualdi D, Giuliani M. Drospirenone for the treatment of hirsute women with PCOS; a clinical endocrinological, metabolic pilot study. J Clin Endocrinol Metab. 2004;89:2817-2823.
15. Tzourio C, Tehindrazanarivelo A, Iglesias S, et al. Case-control study of migraine and risk of ischaemic stroke in young women. Br Med J. 1995;310:830-833.
16. Steinauer J, Autry AM. Extended cycle combined hormonal contraception. Obstet Gynecol Clin North Am. 2007;34:43-55, viii.
17. Ortho Evra [prescribing information]. Raritan, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc; 2001.
18. Jick SS, Kaye JA, Russmann S, et al. Risk of non-fatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg/l of ethinyl estradiol. Contraception. 2006;73:223-228.
19. Cole JA, Norman H, Doherty M, et al. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol. 2007;109:339-346.
20. American College of Obstetricians and Gynecologists. ACOG practice bulletin. Use of hormonal contraceptives in women with coexisting medical conditions. No. 73, June 2006. Obstet Gynecol. 2006;107:1453-1472.
21. Mulders TM, Dieben TO. Use of the novel combined contraceptive vaginal ring NuvaRing for ovulation inhibition. Fertil Steril. 2001;75:865-870.
22. Sulak PJ, Smith V, Coffee A, et al. Frequency and management of breakthrough bleeding with continuous use of the transvaginal contraceptive ring. Obstet Gynecol. 2008;112:563-571.
23. Bjarnadottir RI, Tuppurainen M, Killick SR. Comparison of cycle control with a combined contraceptive vaginal ring and oral levonorgestrel/ethinyl estradiol. Am J Obstet Gynecol. 2002;186:389-395.
24. ParaGard T 380A [prescribing information]. Pomona, NY: Duramed Pharmaceuticals, Inc; 2006.
25. Beining RM, Dennis LK, Smith EM, et al. Meta-analysis of intrauterine device use and risk of endometrial cancer. Ann Epidem. 2008;18:492-499.
26. ACOG Committee Opinion. Intrauterine Device and Adolescents No. 392. Obstet Gynecol. 2007;110:1493-1495.
27. McNaught J. Adolescents and IUCDs—Not a contraindication. J Ped Adolesc Gyn. 2006;19:303-305.
28. Lethaby AE, Cooke I, Rees M. Progesterone/progestogen releasing intrauterine systems versus either placebo or any other medication for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;(4):CD002126.-
29. Hurskainen R, Teperi J, Rissanen P, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomised trial. Lancet. 2001;357:273-277.
30. Römer T. Prospective comparison study of levonorgestrel IUD versus Roller-Ball endometrial ablation in the management of refractory recurrent hypermenorrhea. Eur J Obstet Gynecol Reprod Biol. 2000;90:27-29.
31. Toivonen J, Luukkainen T, Allonen H. Protective effect of intrauterine release of levonorgestrel on pelvic infection: three years’ comparative experience of levonorgestrel- and copper-releasing intrauterine devices. Obstet Gynecol. 1991;77:261-264.
32. Ball CE. News and controversies in contraception. AudioDigest Obstet Gynecol Clin Updates. 2007;54:18.-
33. Grimes DA. Intrauterine device and upper genital tract infection. Lancet. 2000;356:1013-1019.
34. Farley TM, Rosenberg MJ, Rowe PJ, et al. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet. 1992;339:785-788.
35. Implanon [package insert]. Roseland, NJ: Organon USA Inc; 2007.
36. Funk S, Miller MM, Mishell DR, Jr, et al. The Implanon US Study Group. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception. 2005;71:319-326.
37. Beerthuizen R, van Beek A, Massai R, et al. Bone mineral density during long-term use of the progestagen contraceptive implant Implanon compared to a non-hormonal method of contraception. Hum Reprod. 2000;15:118-122.
38. Reinprayoon D, Taneepanichskul S, Bunyavejchevin S, et al. Effects of the etonogestrel-releasing contraceptive implant (Implanon) on parameters of breastfeeding compared to those of an intrauterine device. Contraception. 2000;62:239-246.
39. Ngai SW, Fan S, Li S, et al. A randomized trial to compare 24 h versus 12 h double dose regimen of levonorgestrel for emergency contraception. Hum Reprod. 2005;20:307-311.
40. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 69: emergency contraception. Obstet Gynecol. 2005;106:1443-1452.
41. Von Hertzen H, Piaggio G, Ding J. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. WHO Research Group on Post-Ovulatory Methods of Fertility Regulation. Lancet. 2002;360:1803-1810.
42. Raymond EG, Goldberg A, Trussell J, et al. Bleeding patterns after use of levonorgestrel emergency contraceptive pills. Contraception. 2006;73:376-381.
43. World Health Organization. Medical eligibility criteria for contraceptive use. 3rd ed. 2004. Available at: www.who.int/reproductive-health/publication/med/mec.pdf. Accessed February 7, 2008.
44. Task Force on Postovulatory Methods of Fertility Regulation. Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Lancet. 1998;352:428-433.
45. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 59. Intrauterine device. Obstet Gynecol. 2005;105:223-232.
46. LaValleur J. Emergency contraception. Obstet Gynecol Clin North Am. 2000;27:817-839, vii.
47. Raymond EG, Stewart F, Weaver M, et al. Impact of increased access to emergency contraceptive pills: a randomized controlled trial. Obstet Gynecol. 2006;108:1098-1106.
48. Raymond EG, Weaver MA. Effect of an emergency contraceptive pill intervention on pregnancy risk behavior. Contraception. 2008;77:333-336.
49. Raymond EG, Trussell J, Polis CB. Population effect of increased access to emergency contraceptive pills: a systematic review. Obstet Gynecol. 2007;109:181-188.
50. Casey PM, Cerhan JR, Pruthi S. Oral contraceptive use and risk of breast cancer Mayo Clinic Proc. 2008;83:86-89.
51. Trussell J. Contraceptive efficacy. In: Hatcher RA, Trussell J, Stewart FH, et al, eds. Contraceptive Technology, 18th ed. New York, NY: Ardent Media, Inc; 2004:773-845.