User login
The Centers for Disease Control and Prevention (CDC) has published its recommendations for the use of influenza vaccines for the 2015-2016 influenza season.1 (See the CDC’s Web site at http://www.cdc.gov/flu/professionals/vaccination/index.htm.) This Practice Alert describes recent changes in vaccine products, discusses the timing of vaccination, reviews a new algorithm for deciding on the number of doses for children ages 6 months through 8 years, and raises issues to consider when thinking about specific products for individual patients.
Vaccine product modifications for 2015-2016
Influenza vaccines contain either 3 or 4 antigens (trivalent or quadrivalent) and either inactivated or modified live viruses (inactivated influenza vaccine [IIV]) or live attenuated influenza vaccine [LAIV]). These vaccines are produced in eggs, by cell cultures, or with recombinant technology. The vaccine products for this influenza season will contain antigens of one H1N1 virus, one H3N2 virus, and one B virus (trivalent products) or 2 B viruses (quadrivalent products).
The viruses selected for the vaccine are based on the most prevalent types in circulation globally, and vaccine effectiveness in the United States will be directly proportional to how well these strains match those circulating here during the influenza season. In the 2014-2015 influenza season, an antigenic drift in the circulating H3N2 virus rendered influenza vaccines only 23% effective in preventing laboratory-confirmed influenza.2
One product, Afluria, an IIV trivalent product, has been approved for administration using a needle-free jet injector in individuals ages 18 to 64 years.1 Additionally, intramuscular injection of Afluria is still available for those 18 years and older. All other IIV products are administered via needle and syringe. Vaccination with a jet injector achieves protection equivalent to needle injection, but its use is associated with higher rates of local reactions.
Flublok, another trivalent IIV product, is produced using a recombinant egg-free process. It was originally approved for individuals 18 to 49 years; there is now no upper age limit, providing an egg-free option for an expanded age group. An intradermal option, Fluzone, was a trivalent product last year and will be replaced by Fluzone Intradermal Quadrivalent this season.
A complete list of all influenza products and their respective patient-specific recommendations can be found on the CDC influenza Web site (http://www.cdc.gov/flu/professionals/vaccination/index.htm).
Timing of vaccine administration
Start providing influenza vaccine by the beginning of October and continue offering it throughout the influenza season to those who are unimmunized. In the past, the recommendation was to begin vaccination as soon as vaccine was available. But studies have shown that vaccine effectiveness declines after 6 months, especially among those over age 65 years, which can result in inadequate protection late in the influenza season.3,4 Administering the vaccine later in the fall may confer greater protection later in the season, but, as a strategy, it could also lead to missed opportunities to vaccinate.
Annual vaccination for the entire population is a public health challenge and the October start date is appropriate middle ground. However, children who need 2 doses should receive the first dose as soon as vaccine is available, and the second dose 4 weeks later.1
One or two doses in children?
Children ages 6 months through 8 years who are receiving influenza vaccine for the first or second time need 2 doses for maximum immune response. Past algorithms that aided in deciding which children needed 2 doses instead of one considered not only the number and timing of previous doses but also whether the product had contained pandemic H1N1 antigen. The algorithm for the coming year asks just one question: How many doses of influenza vaccine has the child received previously? This is without regard to when or to the specific products. If the answer is 2 or more doses (not necessarily given in the same season or even in consecutive seasons), only one dose is needed this season. If the answer is “one dose” or “none,” 2 doses are recommended this season, separated by at least 4 weeks.
Considerations for individual patients
The Advisory Committee on Immunization Practices (ACIP) recommends annual influenza vaccination for everyone ages 6 months and older who do not have a contraindication. It states no preference for any product for any age. Last year’s preference for LAIV over IIV for children through age 8 years has been changed; either LAIV or IIV is appropriate for this age group.1 Although quadrivalent vaccines offer some added protection with an additional B virus, do not delay vaccination if only a trivalent product is available.
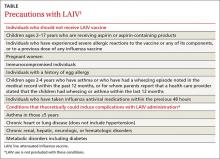
Use the LAIV only for individuals ages 2 years to 49 years who do not have a contraindication listed in the TABLE.1 There are other conditions that pose a theoretical increased risk of complications with the use of LAIV (TABLE), but they do not preclude the use of the vaccine. Additionally, anyone providing care for a severely immunosuppressed individual should avoid being vaccinated with LAIV or, if vaccinated with the live virus, avoid contact with the individual for 7 days following vaccination.
Recommendations for use of influenza vaccines in those who say they are allergic to eggs remain unchanged from last year (FIGURE1). The amount of egg protein in influenza vaccines is very low and serious allergic reactions are rare. The availability of trivalent recombinant vaccine provides an egg-free option for those ages 18 and older.
Vaccines are not all we have to protect the public
Remember that while influenza vaccines are recommended and are the most effective intervention to prevent influenza morbidity and mortality, they are imperfect. Their effectiveness varies from year to year, and it wanes with time after administration. The proportion of the population vaccinated is also suboptimal, which makes other prevention interventions important to implement. These include good infection control practices in all health care facilities, social distancing of those who are infectious, infection control practices in homes with an infected person, vaccination of all health care workers, and judicious use of pre- and post-exposure chemoprevention when indicated. These have all been discussed in a previous Practice Alert.5
1. Grohskopf LA, Sokolow LZ, Olsen SJ, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2015-16 Influenza Season. MMWR Morb Mortal Wkly Rep. 2015;64:818-825.
2. Flannery B, Clippard J. End-of-season influenza vaccine effectiveness estimates for the 2014-15 season. Presented at: Meeting of the Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/flu-02-flannery.pdf. Accessed August 11, 2015.
3. Song JY, Cheong HJ, Hwang IS, et al. Long-term immunogenicity of influenza vaccine among the elderly: Risk factors for poor immune response and persistence. Vaccine. 2010;28:3929-3935.
4. Castilla J, Martinez-Baz I, Martinez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18(5). pii:20388.
5. Campos-Outcalt D. Influenza: Update for the 2013-2014 season. J Fam Prac. 2013;62:494-498.
The Centers for Disease Control and Prevention (CDC) has published its recommendations for the use of influenza vaccines for the 2015-2016 influenza season.1 (See the CDC’s Web site at http://www.cdc.gov/flu/professionals/vaccination/index.htm.) This Practice Alert describes recent changes in vaccine products, discusses the timing of vaccination, reviews a new algorithm for deciding on the number of doses for children ages 6 months through 8 years, and raises issues to consider when thinking about specific products for individual patients.
Vaccine product modifications for 2015-2016
Influenza vaccines contain either 3 or 4 antigens (trivalent or quadrivalent) and either inactivated or modified live viruses (inactivated influenza vaccine [IIV]) or live attenuated influenza vaccine [LAIV]). These vaccines are produced in eggs, by cell cultures, or with recombinant technology. The vaccine products for this influenza season will contain antigens of one H1N1 virus, one H3N2 virus, and one B virus (trivalent products) or 2 B viruses (quadrivalent products).
The viruses selected for the vaccine are based on the most prevalent types in circulation globally, and vaccine effectiveness in the United States will be directly proportional to how well these strains match those circulating here during the influenza season. In the 2014-2015 influenza season, an antigenic drift in the circulating H3N2 virus rendered influenza vaccines only 23% effective in preventing laboratory-confirmed influenza.2
One product, Afluria, an IIV trivalent product, has been approved for administration using a needle-free jet injector in individuals ages 18 to 64 years.1 Additionally, intramuscular injection of Afluria is still available for those 18 years and older. All other IIV products are administered via needle and syringe. Vaccination with a jet injector achieves protection equivalent to needle injection, but its use is associated with higher rates of local reactions.
Flublok, another trivalent IIV product, is produced using a recombinant egg-free process. It was originally approved for individuals 18 to 49 years; there is now no upper age limit, providing an egg-free option for an expanded age group. An intradermal option, Fluzone, was a trivalent product last year and will be replaced by Fluzone Intradermal Quadrivalent this season.
A complete list of all influenza products and their respective patient-specific recommendations can be found on the CDC influenza Web site (http://www.cdc.gov/flu/professionals/vaccination/index.htm).
Timing of vaccine administration
Start providing influenza vaccine by the beginning of October and continue offering it throughout the influenza season to those who are unimmunized. In the past, the recommendation was to begin vaccination as soon as vaccine was available. But studies have shown that vaccine effectiveness declines after 6 months, especially among those over age 65 years, which can result in inadequate protection late in the influenza season.3,4 Administering the vaccine later in the fall may confer greater protection later in the season, but, as a strategy, it could also lead to missed opportunities to vaccinate.
Annual vaccination for the entire population is a public health challenge and the October start date is appropriate middle ground. However, children who need 2 doses should receive the first dose as soon as vaccine is available, and the second dose 4 weeks later.1
One or two doses in children?
Children ages 6 months through 8 years who are receiving influenza vaccine for the first or second time need 2 doses for maximum immune response. Past algorithms that aided in deciding which children needed 2 doses instead of one considered not only the number and timing of previous doses but also whether the product had contained pandemic H1N1 antigen. The algorithm for the coming year asks just one question: How many doses of influenza vaccine has the child received previously? This is without regard to when or to the specific products. If the answer is 2 or more doses (not necessarily given in the same season or even in consecutive seasons), only one dose is needed this season. If the answer is “one dose” or “none,” 2 doses are recommended this season, separated by at least 4 weeks.
Considerations for individual patients
The Advisory Committee on Immunization Practices (ACIP) recommends annual influenza vaccination for everyone ages 6 months and older who do not have a contraindication. It states no preference for any product for any age. Last year’s preference for LAIV over IIV for children through age 8 years has been changed; either LAIV or IIV is appropriate for this age group.1 Although quadrivalent vaccines offer some added protection with an additional B virus, do not delay vaccination if only a trivalent product is available.
Use the LAIV only for individuals ages 2 years to 49 years who do not have a contraindication listed in the TABLE.1 There are other conditions that pose a theoretical increased risk of complications with the use of LAIV (TABLE), but they do not preclude the use of the vaccine. Additionally, anyone providing care for a severely immunosuppressed individual should avoid being vaccinated with LAIV or, if vaccinated with the live virus, avoid contact with the individual for 7 days following vaccination.
Recommendations for use of influenza vaccines in those who say they are allergic to eggs remain unchanged from last year (FIGURE1). The amount of egg protein in influenza vaccines is very low and serious allergic reactions are rare. The availability of trivalent recombinant vaccine provides an egg-free option for those ages 18 and older.
Vaccines are not all we have to protect the public
Remember that while influenza vaccines are recommended and are the most effective intervention to prevent influenza morbidity and mortality, they are imperfect. Their effectiveness varies from year to year, and it wanes with time after administration. The proportion of the population vaccinated is also suboptimal, which makes other prevention interventions important to implement. These include good infection control practices in all health care facilities, social distancing of those who are infectious, infection control practices in homes with an infected person, vaccination of all health care workers, and judicious use of pre- and post-exposure chemoprevention when indicated. These have all been discussed in a previous Practice Alert.5
The Centers for Disease Control and Prevention (CDC) has published its recommendations for the use of influenza vaccines for the 2015-2016 influenza season.1 (See the CDC’s Web site at http://www.cdc.gov/flu/professionals/vaccination/index.htm.) This Practice Alert describes recent changes in vaccine products, discusses the timing of vaccination, reviews a new algorithm for deciding on the number of doses for children ages 6 months through 8 years, and raises issues to consider when thinking about specific products for individual patients.
Vaccine product modifications for 2015-2016
Influenza vaccines contain either 3 or 4 antigens (trivalent or quadrivalent) and either inactivated or modified live viruses (inactivated influenza vaccine [IIV]) or live attenuated influenza vaccine [LAIV]). These vaccines are produced in eggs, by cell cultures, or with recombinant technology. The vaccine products for this influenza season will contain antigens of one H1N1 virus, one H3N2 virus, and one B virus (trivalent products) or 2 B viruses (quadrivalent products).
The viruses selected for the vaccine are based on the most prevalent types in circulation globally, and vaccine effectiveness in the United States will be directly proportional to how well these strains match those circulating here during the influenza season. In the 2014-2015 influenza season, an antigenic drift in the circulating H3N2 virus rendered influenza vaccines only 23% effective in preventing laboratory-confirmed influenza.2
One product, Afluria, an IIV trivalent product, has been approved for administration using a needle-free jet injector in individuals ages 18 to 64 years.1 Additionally, intramuscular injection of Afluria is still available for those 18 years and older. All other IIV products are administered via needle and syringe. Vaccination with a jet injector achieves protection equivalent to needle injection, but its use is associated with higher rates of local reactions.
Flublok, another trivalent IIV product, is produced using a recombinant egg-free process. It was originally approved for individuals 18 to 49 years; there is now no upper age limit, providing an egg-free option for an expanded age group. An intradermal option, Fluzone, was a trivalent product last year and will be replaced by Fluzone Intradermal Quadrivalent this season.
A complete list of all influenza products and their respective patient-specific recommendations can be found on the CDC influenza Web site (http://www.cdc.gov/flu/professionals/vaccination/index.htm).
Timing of vaccine administration
Start providing influenza vaccine by the beginning of October and continue offering it throughout the influenza season to those who are unimmunized. In the past, the recommendation was to begin vaccination as soon as vaccine was available. But studies have shown that vaccine effectiveness declines after 6 months, especially among those over age 65 years, which can result in inadequate protection late in the influenza season.3,4 Administering the vaccine later in the fall may confer greater protection later in the season, but, as a strategy, it could also lead to missed opportunities to vaccinate.
Annual vaccination for the entire population is a public health challenge and the October start date is appropriate middle ground. However, children who need 2 doses should receive the first dose as soon as vaccine is available, and the second dose 4 weeks later.1
One or two doses in children?
Children ages 6 months through 8 years who are receiving influenza vaccine for the first or second time need 2 doses for maximum immune response. Past algorithms that aided in deciding which children needed 2 doses instead of one considered not only the number and timing of previous doses but also whether the product had contained pandemic H1N1 antigen. The algorithm for the coming year asks just one question: How many doses of influenza vaccine has the child received previously? This is without regard to when or to the specific products. If the answer is 2 or more doses (not necessarily given in the same season or even in consecutive seasons), only one dose is needed this season. If the answer is “one dose” or “none,” 2 doses are recommended this season, separated by at least 4 weeks.
Considerations for individual patients
The Advisory Committee on Immunization Practices (ACIP) recommends annual influenza vaccination for everyone ages 6 months and older who do not have a contraindication. It states no preference for any product for any age. Last year’s preference for LAIV over IIV for children through age 8 years has been changed; either LAIV or IIV is appropriate for this age group.1 Although quadrivalent vaccines offer some added protection with an additional B virus, do not delay vaccination if only a trivalent product is available.
Use the LAIV only for individuals ages 2 years to 49 years who do not have a contraindication listed in the TABLE.1 There are other conditions that pose a theoretical increased risk of complications with the use of LAIV (TABLE), but they do not preclude the use of the vaccine. Additionally, anyone providing care for a severely immunosuppressed individual should avoid being vaccinated with LAIV or, if vaccinated with the live virus, avoid contact with the individual for 7 days following vaccination.
Recommendations for use of influenza vaccines in those who say they are allergic to eggs remain unchanged from last year (FIGURE1). The amount of egg protein in influenza vaccines is very low and serious allergic reactions are rare. The availability of trivalent recombinant vaccine provides an egg-free option for those ages 18 and older.
Vaccines are not all we have to protect the public
Remember that while influenza vaccines are recommended and are the most effective intervention to prevent influenza morbidity and mortality, they are imperfect. Their effectiveness varies from year to year, and it wanes with time after administration. The proportion of the population vaccinated is also suboptimal, which makes other prevention interventions important to implement. These include good infection control practices in all health care facilities, social distancing of those who are infectious, infection control practices in homes with an infected person, vaccination of all health care workers, and judicious use of pre- and post-exposure chemoprevention when indicated. These have all been discussed in a previous Practice Alert.5
1. Grohskopf LA, Sokolow LZ, Olsen SJ, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2015-16 Influenza Season. MMWR Morb Mortal Wkly Rep. 2015;64:818-825.
2. Flannery B, Clippard J. End-of-season influenza vaccine effectiveness estimates for the 2014-15 season. Presented at: Meeting of the Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/flu-02-flannery.pdf. Accessed August 11, 2015.
3. Song JY, Cheong HJ, Hwang IS, et al. Long-term immunogenicity of influenza vaccine among the elderly: Risk factors for poor immune response and persistence. Vaccine. 2010;28:3929-3935.
4. Castilla J, Martinez-Baz I, Martinez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18(5). pii:20388.
5. Campos-Outcalt D. Influenza: Update for the 2013-2014 season. J Fam Prac. 2013;62:494-498.
1. Grohskopf LA, Sokolow LZ, Olsen SJ, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2015-16 Influenza Season. MMWR Morb Mortal Wkly Rep. 2015;64:818-825.
2. Flannery B, Clippard J. End-of-season influenza vaccine effectiveness estimates for the 2014-15 season. Presented at: Meeting of the Advisory Committee on Immunization Practices; June 24, 2015; Atlanta, GA. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2015-06/flu-02-flannery.pdf. Accessed August 11, 2015.
3. Song JY, Cheong HJ, Hwang IS, et al. Long-term immunogenicity of influenza vaccine among the elderly: Risk factors for poor immune response and persistence. Vaccine. 2010;28:3929-3935.
4. Castilla J, Martinez-Baz I, Martinez-Artola V, et al. Decline in influenza vaccine effectiveness with time after vaccination, Navarre, Spain, season 2011/12. Euro Surveill. 2013;18(5). pii:20388.
5. Campos-Outcalt D. Influenza: Update for the 2013-2014 season. J Fam Prac. 2013;62:494-498.