User login
Venous thromboembolism (VTE) after shoulder arthroplasty (SA) is relatively uncommon. Reported rates of VTE development are highly variable, ranging from 0.2% to 13% (pulmonary embolism [PE], 0.2%-10.8%; deep venous thrombosis [DVT], 0.1%-13%).1-4 Sources of this variability include different methods of capturing cases (small clinical series vs large database studies, which capture mainly hospital readmissions), differences in defining or detecting VTE, and different patient populations (fracture vs osteoarthritis).1-3 Most studies have also tried to identify factors associated with increased risk for VTE. Risk factors associated with development of VTE after SA include history of VTE, advanced age, prolonged operating room time, higher body mass index (BMI), trauma, history of cancer, female sex, and raised Charlson Comorbidity Index (CCI).1-7 Limitations of clinical series include the smaller number of reporting institutions—a potential source of bias given regional variability.1,3,4,7 Limitations of large state or national databases include capturing only events coded during inpatient admission and capturing readmissions for complications at the same institution. This underreporting may lead to very conservative estimates of VTE incidence.2,5,6,8
In this study, we retrospectively identified all the SAs performed at a single institution over a 13-year period and evaluated the cases for development of VTE (DVT, PE). We hypothesized that the VTE rate would be lower than the very high rates reported by Hoxie and colleagues1 and Willis and colleagues4 but higher than those reported for large state or national databases.2,3 We also evaluated clotting risk factors, including many never analyzed before.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we searched our database for all SAs performed at our institution between January 1999 and May 2012 and identified cases in which symptomatic VTE developed within the first 90 days after surgery. Charts were reviewed for information on medical history, surgical procedure, and in-hospital and out-of-hospital care within the 90-day postoperative period. We recorded data on symptomatic VTE (DVT, PE) as documented by lower or upper extremity duplex ultrasonography (US) or chest computed tomography (CT) angiography. There had been no routine screening of patients; duplex US or CT angiography was performed only if a patient was clinically symptomatic (leg swelling, leg pain, shortness of breath, tachycardia, chest pain) for a potential DVT or PE. For a patient who had repeat SAs on the same shoulder or bilateral SAs at different times, only the first procedure was included in the analysis. Arthroplasties performed for fracture were excluded.
Study data were collected and managed with REDCap (Research Electronic Data Capture) tools hosted at the University of Utah School of Medicine.9 Continuous and discrete data collected on medical history and postoperative course included BMI, age at surgery, preoperative hemoglobin (Hb) and hematocrit (Hct) levels, days in hospital, days until out of bed and days until ambulation (both documented in nursing and physical therapy notes), postoperative Hb and Hct levels, and CCI. Categorical data included sex, diagnosis (primary osteoarthritis, rotator cuff arthropathy, rheumatoid arthritis, failed hemiarthroplasty [HA], failed total SA [TSA], others), attending surgeon, procedure (TSA, HA, reverse TSA, revision SA), anesthesia (general endotracheal anesthesia [GETA] alone, interscalene nerve block alone, GETA plus block), prophylactic use of aspirin after surgery, presence of various medical comorbidities (diabetes, hypertension, cardiac disease, clotting disorders, cancer), hormone replacement therapy, family history of a clotting disorder, and VTE consequences (cardiac events, death).
Statistical Analysis
Descriptive statistics were calculated to summarize aspects of the surgical procedures, the study cohort’s demographics and medical histories, and the incidence of VTE. Logistic regression analysis was performed to explore the association between development of VTE (DVT, PE) and potential risk factors. Unadjusted odds ratios (ORs) were estimated for the risk factors of age, BMI, revision SA, CCI, prophylactic use of aspirin after surgery, preoperative history of VTE, preoperative and postoperative Hb and Hct levels, diabetes, anesthesia (GETA with and without interscalene nerve block), family history of a clotting disorder, days until out of bed, hormone replacement therapy, race, discharge home or to rehabilitation, distance traveled for surgery, hypertension, cardiac disease, cement use, and history of cancer. In addition, ORs were adjusted for age, BMI, and revision SA. For all statistical tests, significance was set at P < .05. All analyses were performed with SAS Version 9.3 (SAS Institute).
Results
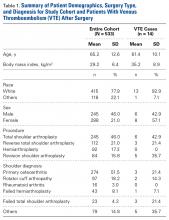
We identified 533 SAs: 245 anatomical TSAs, 112 reverse TSAs, 92 HAs, and 84 revision SAs. Three different surgeons performed the procedures, and no patients were lost to follow-up within the first 90 days after surgery. Although SAs were performed for various diagnoses, more than 50% (274) of the SAs were for primary osteoarthritis; 97 were performed for rotator cuff arthropathy, 16 for rheumatoid arthritis, 43 for failed HA, 23 for failed TSA, and 79 for other diagnoses.
Of the 533 patients, 288 were female and 245 were male. Mean age at surgery was 65.2 years (range, 16-93 years). Mean (SD) BMI was 29.2 (6.4) kg/m2. Mean (SD) preoperative Hb level was 13.7 (1.8) g/dL, and mean preoperative Hct level was 40.1% (4.8%). Mean (SD) length of hospital stay was 2.6 (1.5) days. Mean (SD) time before patients were out of bed was 1.1 (0.7) days. On postoperative day 1, mean Hb level was 11.1 (1.7) g/dL, and mean (SD) Hct level was 33.2% (4.8%). Mean (SD) CCI was 1.1 (0.9).
Anesthesia for the 533 patients consisted of GETA (209 patients, 39.0%), interscalene nerve block (2, 0.4%), or GETA with nerve block (314, 59.0%). After surgery, 125 patients (24.3%) received aspirin as prophylaxis. Diabetes was reported by 83 patients, hypertension by 286, cardiac disease by 74, a history of a clotting disorder by 2, a family history of a clotting disorder by 8, ongoing cancer by 4, a history of cancer by 67, and hormone replacement therapy by 104.
For the entire cohort of 533 patients, the symptomatic VTE rate was 2.6% (14 patients), the DVT rate was 0.9% (5), and the PE rate was 2.3% (12). Although VTE did not cause any deaths, there were 3 cardiac events.
Discussion
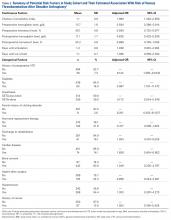
VTE after SA is rare. We report an overall VTE incidence of 2.6%, with DVT at 0.9% and PE at 2.3%. These rates are similar to those reported in clinical series and significantly higher than those reported for large institutional or national databases.2-7 Our results also support a previously reported trend: The ratio of PE to DVT for SA is significantly higher than historically reported ratios for lower extremity arthroplasty.2,6-8 We have identified many VTE risk factors: raised CCI, preoperative thrombotic event, lower preoperative Hb and Hct levels, lower postoperative Hb level, diabetes, use of GETA without interscalene nerve block, higher BMI, and revision SA. Results of other studies support 3 findings (higher BMI, raised CCI, preoperative thrombotic event); new findings include correlation with Hb and Hct levels, diabetes, type of anesthesia, and revision SA.6,7 Identification of these other factors may be useful in making treatment decisions in patients symptomatic after SA and in lowering the threshold for performing diagnostic tests in these patients at risk for VTE.
Reported rates of VTE after SA are highly variable, ranging from 0.2% to 13%.10 Our rationale for investigating VTE rates at a single institution was to estimate the rates that can be expected in a university-based practice and to determine whether these rates are high enough to warrant routine thromboprophylaxis. The rate variability seems to result in part from variability in the data sources. Most studies that have reported very low VTE rates typically used large state or national databases, which likely were subject to underreporting.
Lyman and colleagues6 found 0.5% DVT and 0.2% PE rates in a New York state hospital database, but only in-hospital immediate postoperative symptomatic complications were included; slightly delayed complications may have been missed. Farng and colleagues5 reported a 0.6% VTE rate, but only inpatient (immediate postoperative or readmission) events were included; all outpatient events were missed. Jameson and colleagues,2 using a national database that included only cases involving inpatient treatment, reported 0% DVT and 0.2% PE rates, again missing outpatient events, and relying on appropriate coding to capture events. Using electronic health records from a large healthcare system, Navarro and colleagues8 queried for VTE cases and reported 0.5% DVT and 0.5% PE rates. The inclusiveness of their data source for the outcome of interest was potentially improved relative to national or statewide databases—and the resulting data reported in their study should reflect that improvement. However, the authors relied on ICD–9 (International Classification of Diseases, Ninth Revision) coding to screen for VTE events and excluded patients with prior VTE, preoperative prophylaxis (enoxaparin or warfarin), or follow-up of <90 days. As patients with prior VTE are those most at risk (present study OR, 6-7), excluding them significantly reduces the overall incidence of clotting reported.
Only 4 studies specifically used information drawn directly from physicians’ clinic notes, vs data retrieved (using code-based queries) from databases.1,3,4,7 These studies may provide a better representation of the rate of VTE after SA, as they were not reliant on codes, included both inpatient and outpatient events, and were inclusive of outpatient follow-up of at least 3 months.
Three of the 4 studies used the Mayo Clinic Total Joint Registry.1,3,4 Hoxie and colleagues1 reported an 11% rate of PE after HA performed for fracture (we excluded SA for fracture). As several other investigators have reported an association between trauma and increased risk for VTE, postoperative anticoagulation should be considered in this patient population (though it was not the focus of the present study).6-8 Sperling and Cofield3 and Singh and colleagues7 reported on the risk for PE among SA patients at the Mayo Clinic. Sperling and Cofield3 included only those events that occurred within the first 7 days after surgery; Singh and colleagues7 included events out to 90 days after surgery. Sperling and Cofield3 reported a 0.17% PE rate; Singh and colleagues7 reported 0.6% PE and 0.1% DVT rates. Sperling and Cofield3 reported on 2885 SAs; Singh and colleagues7 reported on 4019 SAs from the same database. As it is unclear whether these 2 studies had complete information on all patients, underreporting may be an issue. Information was obtained through “clinic visits, medical records and/or standardized mailed and telephone-administered questionnaires.”7The fourth study, a prospective study of 100 patients by Willis and colleagues,4 had the best data on development of symptomatic PE after SA. The authors reported a 2% PE rate and a high (13%) DVT rate. Because US was not performed before the surgical procedures, the number of patients with new and existing DVT cases could not be determined. However, all PEs were new, and the 2% rate found there is similar to the 2.3% in our study. Therefore, we think these rates capture the data most accurately and avoid the underreporting that marks large databases.4Studies have identified various factors that increase the risk for VTE after SA. Singh and colleagues7 identified the risk factors of age over 70 years, female sex, higher BMI (25-29.9 kg/m2), CCI above 1, traumatic etiology, prior history of VTE, and HA. However, their use of univariate regression analysis may have confounded the effects—one factor may have become a surrogate for another (ie, trauma and HA, as most fractures treated with SA during the study period were treated with HA). Lyman and colleagues6 also found advanced age and trauma were associated with higher VTE risk, and reported prior history of cancer as a risk factor as well. Navarro and colleagues8 identified trauma as a risk factor, as in the other 2 studies.6,7 Our data support prior history of VTE, higher BMI, and raised CCI as increasing the risk for VTE.
Other factors identified in the present study are use of GETA without interscalene nerve block, lower preoperative and postoperative Hb levels, diabetes, and revision SA. Because of the limited number of events, only ORs with and without limited control of confounders were performed. Just as in the study by Singh and colleagues,7 uncontrolled confounding could have occurred. A nerve block may be protective, as less postoperative pain may allow patients quicker mobilization and therapy. Diabetes may be a surrogate for other medical comorbidities, as reflected by the higher overall risk with raised CCI. Lower preoperative and postoperative Hb levels were associated with clotting and may be representative of patients with poorer overall health and more complicated surgical procedures (eg, revision SA). In an earlier study, we found increased risk for transfusions in revision SA relative to primary SA.11 Lower preoperative Hb level correlated with development of VTE after lower extremity arthroplasty.12 Postoperative use of aspirin was not found to significantly reduce the incidence of clotting, though this finding may have resulted from lack of power. Therefore, from the present data, there is nothing to conclude about the efficacy of aspirin in preventing thrombosis.
Our findings can be placed in the context of the Virchow triad. Specifically, 3 categories of factors are thought to contribute to thrombosis: hypercoagulability, hemodynamic stasis, and endothelial injury. In grouping factors, we identified prior thrombotic event and obesity as increasing hypercoagulability; revision SA, more comorbidities, lower Hb and Hct levels, diabetes, and GETA as increasing hemodynamic stasis; and revision SA (longer operating room times) as leading to stasis. More comorbidities can be associated with delayed postoperative ambulation, and diabetes and lower Hb and Hct levels can be surrogates for more comorbidities. Surgery performed with the patient under GETA without interscalene nerve block can lead to higher levels of pain and less early mobility.
The present findings have made us more aware of patients at risk for VTE, and we have lowered our threshold for evaluating them for potential clots. Before this study, we used warfarin or enoxaparin for anticoagulation in patients with a history of VTE or active cancer. We are continuing this protocol, but not with other patients. Patients with many comorbidities, lower preoperative Hb level, revision SA, high BMI, or diabetes are carefully monitored for clots early in the postoperative course. Our new threshold for these high-risk patients is to order diagnostic testing, including duplex US or CT angiography. Now, even mild oxygen requirements or mild tachycardia within postoperative week 1 typically prompt a study in these patients. We hope this increased awareness will limit the potential negative consequences associated with development of VTE. Given the present data, we do not think the simple presence of increased comorbidities, lower preoperative Hb, revision SA, high BMI or diabetes should rule out performing SA; rather, it should increase surgeons’ postoperative vigilance in evaluating for potential clots.
Limitations of our study include its retrospective nature and reliance on clinic chart review. Patients were not directly questioned about venous thrombus at follow-up, so all events may not have been captured. Although retrospective review has its drawbacks, it allows for accurate identification of events, even uncoded events. Therefore, more events are likely to be captured with this technique than with large database analyses using only coding information. We tried to identify as many cases as possible by reviewing all outpatient records (orthopedic, nonorthopedic), inpatient records, radiologic studies, and scanned outside records. Another limitation is that having a small number of VTE events limited our ability to perform a multivariate analysis, and uncontrolled confounding likely resulted. Only a very large multi-institutional study can capture enough events to allow a multivariate analysis. A third limitation is that the small number of events may have underpowered the study. Having more patients would have allowed other potential factors to be identified as being significantly associated with VTE. Last, as the study captured only symptomatic VTE events, it may have underreported VTE events. Given our complete review of the medical records, however, most clinically significant events likely were captured.
Conclusion
VTE after SA is rare. In our single-institution study, the symptomatic DVT rate was 0.9%, and the symptomatic PE rate was 2.3%. Risk factors associated with clotting included prior VTE, higher BMI, lower preoperative and postoperative Hb levels, raised CCI, diabetes, use of GETA without interscalene nerve block, and revision SA. Risk factors can be used to identify patients who may benefit from a more scrutinized postoperative evaluation and from increased surgeon awareness of the potential for VTE development. Rates of VTE can be used to counsel SA patients regarding overall surgical risks.
Am J Orthop. 2016;45(6):E379-E385. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism after operative treatment of proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(6):782-783.
2. Jameson SS, James P, Howcroft DW, et al. Venous thromboembolic events are rare after shoulder surgery: analysis of a national database. J Shoulder Elbow Surg. 2011;20(5):764-770.
3. Sperling JW, Cofield RH. Pulmonary embolism following shoulder arthroplasty. J Bone Joint Surg Am. 2002;84(11):1939-1941.
4. Willis AA, Warren RF, Craig EV, et al. Deep vein thrombosis after reconstructive shoulder arthroplasty: a prospective observational study. J Shoulder Elbow Surg. 2009;18(1):100-106.
5. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563.
6. Lyman S, Sherman S, Carter TI, Bach PB, Mandl LA, Marx RG. Prevalence and risk factors for symptomatic thromboembolic events after shoulder arthroplasty. Clin Orthop Relat Res. 2006;(448):152-156.
7. Singh JA, Sperling JW, Cofield RH. Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum. 2012;41(5):689-697.
8. Navarro RA, Inacio MC, Burke MF, Costouros JG, Yian EH. Risk of thromboembolism in shoulder arthroplasty: effect of implant type and traumatic indication. Clin Orthop Relat Res. 2013;471(5):1576-1581.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
10. Saleh HE, Pennings AL, ElMaraghy AW. Venous thromboembolism after shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2013;22(10):1440-1448.
11. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
12. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341.
Venous thromboembolism (VTE) after shoulder arthroplasty (SA) is relatively uncommon. Reported rates of VTE development are highly variable, ranging from 0.2% to 13% (pulmonary embolism [PE], 0.2%-10.8%; deep venous thrombosis [DVT], 0.1%-13%).1-4 Sources of this variability include different methods of capturing cases (small clinical series vs large database studies, which capture mainly hospital readmissions), differences in defining or detecting VTE, and different patient populations (fracture vs osteoarthritis).1-3 Most studies have also tried to identify factors associated with increased risk for VTE. Risk factors associated with development of VTE after SA include history of VTE, advanced age, prolonged operating room time, higher body mass index (BMI), trauma, history of cancer, female sex, and raised Charlson Comorbidity Index (CCI).1-7 Limitations of clinical series include the smaller number of reporting institutions—a potential source of bias given regional variability.1,3,4,7 Limitations of large state or national databases include capturing only events coded during inpatient admission and capturing readmissions for complications at the same institution. This underreporting may lead to very conservative estimates of VTE incidence.2,5,6,8
In this study, we retrospectively identified all the SAs performed at a single institution over a 13-year period and evaluated the cases for development of VTE (DVT, PE). We hypothesized that the VTE rate would be lower than the very high rates reported by Hoxie and colleagues1 and Willis and colleagues4 but higher than those reported for large state or national databases.2,3 We also evaluated clotting risk factors, including many never analyzed before.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we searched our database for all SAs performed at our institution between January 1999 and May 2012 and identified cases in which symptomatic VTE developed within the first 90 days after surgery. Charts were reviewed for information on medical history, surgical procedure, and in-hospital and out-of-hospital care within the 90-day postoperative period. We recorded data on symptomatic VTE (DVT, PE) as documented by lower or upper extremity duplex ultrasonography (US) or chest computed tomography (CT) angiography. There had been no routine screening of patients; duplex US or CT angiography was performed only if a patient was clinically symptomatic (leg swelling, leg pain, shortness of breath, tachycardia, chest pain) for a potential DVT or PE. For a patient who had repeat SAs on the same shoulder or bilateral SAs at different times, only the first procedure was included in the analysis. Arthroplasties performed for fracture were excluded.
Study data were collected and managed with REDCap (Research Electronic Data Capture) tools hosted at the University of Utah School of Medicine.9 Continuous and discrete data collected on medical history and postoperative course included BMI, age at surgery, preoperative hemoglobin (Hb) and hematocrit (Hct) levels, days in hospital, days until out of bed and days until ambulation (both documented in nursing and physical therapy notes), postoperative Hb and Hct levels, and CCI. Categorical data included sex, diagnosis (primary osteoarthritis, rotator cuff arthropathy, rheumatoid arthritis, failed hemiarthroplasty [HA], failed total SA [TSA], others), attending surgeon, procedure (TSA, HA, reverse TSA, revision SA), anesthesia (general endotracheal anesthesia [GETA] alone, interscalene nerve block alone, GETA plus block), prophylactic use of aspirin after surgery, presence of various medical comorbidities (diabetes, hypertension, cardiac disease, clotting disorders, cancer), hormone replacement therapy, family history of a clotting disorder, and VTE consequences (cardiac events, death).
Statistical Analysis
Descriptive statistics were calculated to summarize aspects of the surgical procedures, the study cohort’s demographics and medical histories, and the incidence of VTE. Logistic regression analysis was performed to explore the association between development of VTE (DVT, PE) and potential risk factors. Unadjusted odds ratios (ORs) were estimated for the risk factors of age, BMI, revision SA, CCI, prophylactic use of aspirin after surgery, preoperative history of VTE, preoperative and postoperative Hb and Hct levels, diabetes, anesthesia (GETA with and without interscalene nerve block), family history of a clotting disorder, days until out of bed, hormone replacement therapy, race, discharge home or to rehabilitation, distance traveled for surgery, hypertension, cardiac disease, cement use, and history of cancer. In addition, ORs were adjusted for age, BMI, and revision SA. For all statistical tests, significance was set at P < .05. All analyses were performed with SAS Version 9.3 (SAS Institute).
Results
We identified 533 SAs: 245 anatomical TSAs, 112 reverse TSAs, 92 HAs, and 84 revision SAs. Three different surgeons performed the procedures, and no patients were lost to follow-up within the first 90 days after surgery. Although SAs were performed for various diagnoses, more than 50% (274) of the SAs were for primary osteoarthritis; 97 were performed for rotator cuff arthropathy, 16 for rheumatoid arthritis, 43 for failed HA, 23 for failed TSA, and 79 for other diagnoses.
Of the 533 patients, 288 were female and 245 were male. Mean age at surgery was 65.2 years (range, 16-93 years). Mean (SD) BMI was 29.2 (6.4) kg/m2. Mean (SD) preoperative Hb level was 13.7 (1.8) g/dL, and mean preoperative Hct level was 40.1% (4.8%). Mean (SD) length of hospital stay was 2.6 (1.5) days. Mean (SD) time before patients were out of bed was 1.1 (0.7) days. On postoperative day 1, mean Hb level was 11.1 (1.7) g/dL, and mean (SD) Hct level was 33.2% (4.8%). Mean (SD) CCI was 1.1 (0.9).
Anesthesia for the 533 patients consisted of GETA (209 patients, 39.0%), interscalene nerve block (2, 0.4%), or GETA with nerve block (314, 59.0%). After surgery, 125 patients (24.3%) received aspirin as prophylaxis. Diabetes was reported by 83 patients, hypertension by 286, cardiac disease by 74, a history of a clotting disorder by 2, a family history of a clotting disorder by 8, ongoing cancer by 4, a history of cancer by 67, and hormone replacement therapy by 104.
For the entire cohort of 533 patients, the symptomatic VTE rate was 2.6% (14 patients), the DVT rate was 0.9% (5), and the PE rate was 2.3% (12). Although VTE did not cause any deaths, there were 3 cardiac events.
Discussion
VTE after SA is rare. We report an overall VTE incidence of 2.6%, with DVT at 0.9% and PE at 2.3%. These rates are similar to those reported in clinical series and significantly higher than those reported for large institutional or national databases.2-7 Our results also support a previously reported trend: The ratio of PE to DVT for SA is significantly higher than historically reported ratios for lower extremity arthroplasty.2,6-8 We have identified many VTE risk factors: raised CCI, preoperative thrombotic event, lower preoperative Hb and Hct levels, lower postoperative Hb level, diabetes, use of GETA without interscalene nerve block, higher BMI, and revision SA. Results of other studies support 3 findings (higher BMI, raised CCI, preoperative thrombotic event); new findings include correlation with Hb and Hct levels, diabetes, type of anesthesia, and revision SA.6,7 Identification of these other factors may be useful in making treatment decisions in patients symptomatic after SA and in lowering the threshold for performing diagnostic tests in these patients at risk for VTE.
Reported rates of VTE after SA are highly variable, ranging from 0.2% to 13%.10 Our rationale for investigating VTE rates at a single institution was to estimate the rates that can be expected in a university-based practice and to determine whether these rates are high enough to warrant routine thromboprophylaxis. The rate variability seems to result in part from variability in the data sources. Most studies that have reported very low VTE rates typically used large state or national databases, which likely were subject to underreporting.
Lyman and colleagues6 found 0.5% DVT and 0.2% PE rates in a New York state hospital database, but only in-hospital immediate postoperative symptomatic complications were included; slightly delayed complications may have been missed. Farng and colleagues5 reported a 0.6% VTE rate, but only inpatient (immediate postoperative or readmission) events were included; all outpatient events were missed. Jameson and colleagues,2 using a national database that included only cases involving inpatient treatment, reported 0% DVT and 0.2% PE rates, again missing outpatient events, and relying on appropriate coding to capture events. Using electronic health records from a large healthcare system, Navarro and colleagues8 queried for VTE cases and reported 0.5% DVT and 0.5% PE rates. The inclusiveness of their data source for the outcome of interest was potentially improved relative to national or statewide databases—and the resulting data reported in their study should reflect that improvement. However, the authors relied on ICD–9 (International Classification of Diseases, Ninth Revision) coding to screen for VTE events and excluded patients with prior VTE, preoperative prophylaxis (enoxaparin or warfarin), or follow-up of <90 days. As patients with prior VTE are those most at risk (present study OR, 6-7), excluding them significantly reduces the overall incidence of clotting reported.
Only 4 studies specifically used information drawn directly from physicians’ clinic notes, vs data retrieved (using code-based queries) from databases.1,3,4,7 These studies may provide a better representation of the rate of VTE after SA, as they were not reliant on codes, included both inpatient and outpatient events, and were inclusive of outpatient follow-up of at least 3 months.
Three of the 4 studies used the Mayo Clinic Total Joint Registry.1,3,4 Hoxie and colleagues1 reported an 11% rate of PE after HA performed for fracture (we excluded SA for fracture). As several other investigators have reported an association between trauma and increased risk for VTE, postoperative anticoagulation should be considered in this patient population (though it was not the focus of the present study).6-8 Sperling and Cofield3 and Singh and colleagues7 reported on the risk for PE among SA patients at the Mayo Clinic. Sperling and Cofield3 included only those events that occurred within the first 7 days after surgery; Singh and colleagues7 included events out to 90 days after surgery. Sperling and Cofield3 reported a 0.17% PE rate; Singh and colleagues7 reported 0.6% PE and 0.1% DVT rates. Sperling and Cofield3 reported on 2885 SAs; Singh and colleagues7 reported on 4019 SAs from the same database. As it is unclear whether these 2 studies had complete information on all patients, underreporting may be an issue. Information was obtained through “clinic visits, medical records and/or standardized mailed and telephone-administered questionnaires.”7The fourth study, a prospective study of 100 patients by Willis and colleagues,4 had the best data on development of symptomatic PE after SA. The authors reported a 2% PE rate and a high (13%) DVT rate. Because US was not performed before the surgical procedures, the number of patients with new and existing DVT cases could not be determined. However, all PEs were new, and the 2% rate found there is similar to the 2.3% in our study. Therefore, we think these rates capture the data most accurately and avoid the underreporting that marks large databases.4Studies have identified various factors that increase the risk for VTE after SA. Singh and colleagues7 identified the risk factors of age over 70 years, female sex, higher BMI (25-29.9 kg/m2), CCI above 1, traumatic etiology, prior history of VTE, and HA. However, their use of univariate regression analysis may have confounded the effects—one factor may have become a surrogate for another (ie, trauma and HA, as most fractures treated with SA during the study period were treated with HA). Lyman and colleagues6 also found advanced age and trauma were associated with higher VTE risk, and reported prior history of cancer as a risk factor as well. Navarro and colleagues8 identified trauma as a risk factor, as in the other 2 studies.6,7 Our data support prior history of VTE, higher BMI, and raised CCI as increasing the risk for VTE.
Other factors identified in the present study are use of GETA without interscalene nerve block, lower preoperative and postoperative Hb levels, diabetes, and revision SA. Because of the limited number of events, only ORs with and without limited control of confounders were performed. Just as in the study by Singh and colleagues,7 uncontrolled confounding could have occurred. A nerve block may be protective, as less postoperative pain may allow patients quicker mobilization and therapy. Diabetes may be a surrogate for other medical comorbidities, as reflected by the higher overall risk with raised CCI. Lower preoperative and postoperative Hb levels were associated with clotting and may be representative of patients with poorer overall health and more complicated surgical procedures (eg, revision SA). In an earlier study, we found increased risk for transfusions in revision SA relative to primary SA.11 Lower preoperative Hb level correlated with development of VTE after lower extremity arthroplasty.12 Postoperative use of aspirin was not found to significantly reduce the incidence of clotting, though this finding may have resulted from lack of power. Therefore, from the present data, there is nothing to conclude about the efficacy of aspirin in preventing thrombosis.
Our findings can be placed in the context of the Virchow triad. Specifically, 3 categories of factors are thought to contribute to thrombosis: hypercoagulability, hemodynamic stasis, and endothelial injury. In grouping factors, we identified prior thrombotic event and obesity as increasing hypercoagulability; revision SA, more comorbidities, lower Hb and Hct levels, diabetes, and GETA as increasing hemodynamic stasis; and revision SA (longer operating room times) as leading to stasis. More comorbidities can be associated with delayed postoperative ambulation, and diabetes and lower Hb and Hct levels can be surrogates for more comorbidities. Surgery performed with the patient under GETA without interscalene nerve block can lead to higher levels of pain and less early mobility.
The present findings have made us more aware of patients at risk for VTE, and we have lowered our threshold for evaluating them for potential clots. Before this study, we used warfarin or enoxaparin for anticoagulation in patients with a history of VTE or active cancer. We are continuing this protocol, but not with other patients. Patients with many comorbidities, lower preoperative Hb level, revision SA, high BMI, or diabetes are carefully monitored for clots early in the postoperative course. Our new threshold for these high-risk patients is to order diagnostic testing, including duplex US or CT angiography. Now, even mild oxygen requirements or mild tachycardia within postoperative week 1 typically prompt a study in these patients. We hope this increased awareness will limit the potential negative consequences associated with development of VTE. Given the present data, we do not think the simple presence of increased comorbidities, lower preoperative Hb, revision SA, high BMI or diabetes should rule out performing SA; rather, it should increase surgeons’ postoperative vigilance in evaluating for potential clots.
Limitations of our study include its retrospective nature and reliance on clinic chart review. Patients were not directly questioned about venous thrombus at follow-up, so all events may not have been captured. Although retrospective review has its drawbacks, it allows for accurate identification of events, even uncoded events. Therefore, more events are likely to be captured with this technique than with large database analyses using only coding information. We tried to identify as many cases as possible by reviewing all outpatient records (orthopedic, nonorthopedic), inpatient records, radiologic studies, and scanned outside records. Another limitation is that having a small number of VTE events limited our ability to perform a multivariate analysis, and uncontrolled confounding likely resulted. Only a very large multi-institutional study can capture enough events to allow a multivariate analysis. A third limitation is that the small number of events may have underpowered the study. Having more patients would have allowed other potential factors to be identified as being significantly associated with VTE. Last, as the study captured only symptomatic VTE events, it may have underreported VTE events. Given our complete review of the medical records, however, most clinically significant events likely were captured.
Conclusion
VTE after SA is rare. In our single-institution study, the symptomatic DVT rate was 0.9%, and the symptomatic PE rate was 2.3%. Risk factors associated with clotting included prior VTE, higher BMI, lower preoperative and postoperative Hb levels, raised CCI, diabetes, use of GETA without interscalene nerve block, and revision SA. Risk factors can be used to identify patients who may benefit from a more scrutinized postoperative evaluation and from increased surgeon awareness of the potential for VTE development. Rates of VTE can be used to counsel SA patients regarding overall surgical risks.
Am J Orthop. 2016;45(6):E379-E385. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
Venous thromboembolism (VTE) after shoulder arthroplasty (SA) is relatively uncommon. Reported rates of VTE development are highly variable, ranging from 0.2% to 13% (pulmonary embolism [PE], 0.2%-10.8%; deep venous thrombosis [DVT], 0.1%-13%).1-4 Sources of this variability include different methods of capturing cases (small clinical series vs large database studies, which capture mainly hospital readmissions), differences in defining or detecting VTE, and different patient populations (fracture vs osteoarthritis).1-3 Most studies have also tried to identify factors associated with increased risk for VTE. Risk factors associated with development of VTE after SA include history of VTE, advanced age, prolonged operating room time, higher body mass index (BMI), trauma, history of cancer, female sex, and raised Charlson Comorbidity Index (CCI).1-7 Limitations of clinical series include the smaller number of reporting institutions—a potential source of bias given regional variability.1,3,4,7 Limitations of large state or national databases include capturing only events coded during inpatient admission and capturing readmissions for complications at the same institution. This underreporting may lead to very conservative estimates of VTE incidence.2,5,6,8
In this study, we retrospectively identified all the SAs performed at a single institution over a 13-year period and evaluated the cases for development of VTE (DVT, PE). We hypothesized that the VTE rate would be lower than the very high rates reported by Hoxie and colleagues1 and Willis and colleagues4 but higher than those reported for large state or national databases.2,3 We also evaluated clotting risk factors, including many never analyzed before.
Materials and Methods
After obtaining Institutional Review Board approval for this study, we searched our database for all SAs performed at our institution between January 1999 and May 2012 and identified cases in which symptomatic VTE developed within the first 90 days after surgery. Charts were reviewed for information on medical history, surgical procedure, and in-hospital and out-of-hospital care within the 90-day postoperative period. We recorded data on symptomatic VTE (DVT, PE) as documented by lower or upper extremity duplex ultrasonography (US) or chest computed tomography (CT) angiography. There had been no routine screening of patients; duplex US or CT angiography was performed only if a patient was clinically symptomatic (leg swelling, leg pain, shortness of breath, tachycardia, chest pain) for a potential DVT or PE. For a patient who had repeat SAs on the same shoulder or bilateral SAs at different times, only the first procedure was included in the analysis. Arthroplasties performed for fracture were excluded.
Study data were collected and managed with REDCap (Research Electronic Data Capture) tools hosted at the University of Utah School of Medicine.9 Continuous and discrete data collected on medical history and postoperative course included BMI, age at surgery, preoperative hemoglobin (Hb) and hematocrit (Hct) levels, days in hospital, days until out of bed and days until ambulation (both documented in nursing and physical therapy notes), postoperative Hb and Hct levels, and CCI. Categorical data included sex, diagnosis (primary osteoarthritis, rotator cuff arthropathy, rheumatoid arthritis, failed hemiarthroplasty [HA], failed total SA [TSA], others), attending surgeon, procedure (TSA, HA, reverse TSA, revision SA), anesthesia (general endotracheal anesthesia [GETA] alone, interscalene nerve block alone, GETA plus block), prophylactic use of aspirin after surgery, presence of various medical comorbidities (diabetes, hypertension, cardiac disease, clotting disorders, cancer), hormone replacement therapy, family history of a clotting disorder, and VTE consequences (cardiac events, death).
Statistical Analysis
Descriptive statistics were calculated to summarize aspects of the surgical procedures, the study cohort’s demographics and medical histories, and the incidence of VTE. Logistic regression analysis was performed to explore the association between development of VTE (DVT, PE) and potential risk factors. Unadjusted odds ratios (ORs) were estimated for the risk factors of age, BMI, revision SA, CCI, prophylactic use of aspirin after surgery, preoperative history of VTE, preoperative and postoperative Hb and Hct levels, diabetes, anesthesia (GETA with and without interscalene nerve block), family history of a clotting disorder, days until out of bed, hormone replacement therapy, race, discharge home or to rehabilitation, distance traveled for surgery, hypertension, cardiac disease, cement use, and history of cancer. In addition, ORs were adjusted for age, BMI, and revision SA. For all statistical tests, significance was set at P < .05. All analyses were performed with SAS Version 9.3 (SAS Institute).
Results
We identified 533 SAs: 245 anatomical TSAs, 112 reverse TSAs, 92 HAs, and 84 revision SAs. Three different surgeons performed the procedures, and no patients were lost to follow-up within the first 90 days after surgery. Although SAs were performed for various diagnoses, more than 50% (274) of the SAs were for primary osteoarthritis; 97 were performed for rotator cuff arthropathy, 16 for rheumatoid arthritis, 43 for failed HA, 23 for failed TSA, and 79 for other diagnoses.
Of the 533 patients, 288 were female and 245 were male. Mean age at surgery was 65.2 years (range, 16-93 years). Mean (SD) BMI was 29.2 (6.4) kg/m2. Mean (SD) preoperative Hb level was 13.7 (1.8) g/dL, and mean preoperative Hct level was 40.1% (4.8%). Mean (SD) length of hospital stay was 2.6 (1.5) days. Mean (SD) time before patients were out of bed was 1.1 (0.7) days. On postoperative day 1, mean Hb level was 11.1 (1.7) g/dL, and mean (SD) Hct level was 33.2% (4.8%). Mean (SD) CCI was 1.1 (0.9).
Anesthesia for the 533 patients consisted of GETA (209 patients, 39.0%), interscalene nerve block (2, 0.4%), or GETA with nerve block (314, 59.0%). After surgery, 125 patients (24.3%) received aspirin as prophylaxis. Diabetes was reported by 83 patients, hypertension by 286, cardiac disease by 74, a history of a clotting disorder by 2, a family history of a clotting disorder by 8, ongoing cancer by 4, a history of cancer by 67, and hormone replacement therapy by 104.
For the entire cohort of 533 patients, the symptomatic VTE rate was 2.6% (14 patients), the DVT rate was 0.9% (5), and the PE rate was 2.3% (12). Although VTE did not cause any deaths, there were 3 cardiac events.
Discussion
VTE after SA is rare. We report an overall VTE incidence of 2.6%, with DVT at 0.9% and PE at 2.3%. These rates are similar to those reported in clinical series and significantly higher than those reported for large institutional or national databases.2-7 Our results also support a previously reported trend: The ratio of PE to DVT for SA is significantly higher than historically reported ratios for lower extremity arthroplasty.2,6-8 We have identified many VTE risk factors: raised CCI, preoperative thrombotic event, lower preoperative Hb and Hct levels, lower postoperative Hb level, diabetes, use of GETA without interscalene nerve block, higher BMI, and revision SA. Results of other studies support 3 findings (higher BMI, raised CCI, preoperative thrombotic event); new findings include correlation with Hb and Hct levels, diabetes, type of anesthesia, and revision SA.6,7 Identification of these other factors may be useful in making treatment decisions in patients symptomatic after SA and in lowering the threshold for performing diagnostic tests in these patients at risk for VTE.
Reported rates of VTE after SA are highly variable, ranging from 0.2% to 13%.10 Our rationale for investigating VTE rates at a single institution was to estimate the rates that can be expected in a university-based practice and to determine whether these rates are high enough to warrant routine thromboprophylaxis. The rate variability seems to result in part from variability in the data sources. Most studies that have reported very low VTE rates typically used large state or national databases, which likely were subject to underreporting.
Lyman and colleagues6 found 0.5% DVT and 0.2% PE rates in a New York state hospital database, but only in-hospital immediate postoperative symptomatic complications were included; slightly delayed complications may have been missed. Farng and colleagues5 reported a 0.6% VTE rate, but only inpatient (immediate postoperative or readmission) events were included; all outpatient events were missed. Jameson and colleagues,2 using a national database that included only cases involving inpatient treatment, reported 0% DVT and 0.2% PE rates, again missing outpatient events, and relying on appropriate coding to capture events. Using electronic health records from a large healthcare system, Navarro and colleagues8 queried for VTE cases and reported 0.5% DVT and 0.5% PE rates. The inclusiveness of their data source for the outcome of interest was potentially improved relative to national or statewide databases—and the resulting data reported in their study should reflect that improvement. However, the authors relied on ICD–9 (International Classification of Diseases, Ninth Revision) coding to screen for VTE events and excluded patients with prior VTE, preoperative prophylaxis (enoxaparin or warfarin), or follow-up of <90 days. As patients with prior VTE are those most at risk (present study OR, 6-7), excluding them significantly reduces the overall incidence of clotting reported.
Only 4 studies specifically used information drawn directly from physicians’ clinic notes, vs data retrieved (using code-based queries) from databases.1,3,4,7 These studies may provide a better representation of the rate of VTE after SA, as they were not reliant on codes, included both inpatient and outpatient events, and were inclusive of outpatient follow-up of at least 3 months.
Three of the 4 studies used the Mayo Clinic Total Joint Registry.1,3,4 Hoxie and colleagues1 reported an 11% rate of PE after HA performed for fracture (we excluded SA for fracture). As several other investigators have reported an association between trauma and increased risk for VTE, postoperative anticoagulation should be considered in this patient population (though it was not the focus of the present study).6-8 Sperling and Cofield3 and Singh and colleagues7 reported on the risk for PE among SA patients at the Mayo Clinic. Sperling and Cofield3 included only those events that occurred within the first 7 days after surgery; Singh and colleagues7 included events out to 90 days after surgery. Sperling and Cofield3 reported a 0.17% PE rate; Singh and colleagues7 reported 0.6% PE and 0.1% DVT rates. Sperling and Cofield3 reported on 2885 SAs; Singh and colleagues7 reported on 4019 SAs from the same database. As it is unclear whether these 2 studies had complete information on all patients, underreporting may be an issue. Information was obtained through “clinic visits, medical records and/or standardized mailed and telephone-administered questionnaires.”7The fourth study, a prospective study of 100 patients by Willis and colleagues,4 had the best data on development of symptomatic PE after SA. The authors reported a 2% PE rate and a high (13%) DVT rate. Because US was not performed before the surgical procedures, the number of patients with new and existing DVT cases could not be determined. However, all PEs were new, and the 2% rate found there is similar to the 2.3% in our study. Therefore, we think these rates capture the data most accurately and avoid the underreporting that marks large databases.4Studies have identified various factors that increase the risk for VTE after SA. Singh and colleagues7 identified the risk factors of age over 70 years, female sex, higher BMI (25-29.9 kg/m2), CCI above 1, traumatic etiology, prior history of VTE, and HA. However, their use of univariate regression analysis may have confounded the effects—one factor may have become a surrogate for another (ie, trauma and HA, as most fractures treated with SA during the study period were treated with HA). Lyman and colleagues6 also found advanced age and trauma were associated with higher VTE risk, and reported prior history of cancer as a risk factor as well. Navarro and colleagues8 identified trauma as a risk factor, as in the other 2 studies.6,7 Our data support prior history of VTE, higher BMI, and raised CCI as increasing the risk for VTE.
Other factors identified in the present study are use of GETA without interscalene nerve block, lower preoperative and postoperative Hb levels, diabetes, and revision SA. Because of the limited number of events, only ORs with and without limited control of confounders were performed. Just as in the study by Singh and colleagues,7 uncontrolled confounding could have occurred. A nerve block may be protective, as less postoperative pain may allow patients quicker mobilization and therapy. Diabetes may be a surrogate for other medical comorbidities, as reflected by the higher overall risk with raised CCI. Lower preoperative and postoperative Hb levels were associated with clotting and may be representative of patients with poorer overall health and more complicated surgical procedures (eg, revision SA). In an earlier study, we found increased risk for transfusions in revision SA relative to primary SA.11 Lower preoperative Hb level correlated with development of VTE after lower extremity arthroplasty.12 Postoperative use of aspirin was not found to significantly reduce the incidence of clotting, though this finding may have resulted from lack of power. Therefore, from the present data, there is nothing to conclude about the efficacy of aspirin in preventing thrombosis.
Our findings can be placed in the context of the Virchow triad. Specifically, 3 categories of factors are thought to contribute to thrombosis: hypercoagulability, hemodynamic stasis, and endothelial injury. In grouping factors, we identified prior thrombotic event and obesity as increasing hypercoagulability; revision SA, more comorbidities, lower Hb and Hct levels, diabetes, and GETA as increasing hemodynamic stasis; and revision SA (longer operating room times) as leading to stasis. More comorbidities can be associated with delayed postoperative ambulation, and diabetes and lower Hb and Hct levels can be surrogates for more comorbidities. Surgery performed with the patient under GETA without interscalene nerve block can lead to higher levels of pain and less early mobility.
The present findings have made us more aware of patients at risk for VTE, and we have lowered our threshold for evaluating them for potential clots. Before this study, we used warfarin or enoxaparin for anticoagulation in patients with a history of VTE or active cancer. We are continuing this protocol, but not with other patients. Patients with many comorbidities, lower preoperative Hb level, revision SA, high BMI, or diabetes are carefully monitored for clots early in the postoperative course. Our new threshold for these high-risk patients is to order diagnostic testing, including duplex US or CT angiography. Now, even mild oxygen requirements or mild tachycardia within postoperative week 1 typically prompt a study in these patients. We hope this increased awareness will limit the potential negative consequences associated with development of VTE. Given the present data, we do not think the simple presence of increased comorbidities, lower preoperative Hb, revision SA, high BMI or diabetes should rule out performing SA; rather, it should increase surgeons’ postoperative vigilance in evaluating for potential clots.
Limitations of our study include its retrospective nature and reliance on clinic chart review. Patients were not directly questioned about venous thrombus at follow-up, so all events may not have been captured. Although retrospective review has its drawbacks, it allows for accurate identification of events, even uncoded events. Therefore, more events are likely to be captured with this technique than with large database analyses using only coding information. We tried to identify as many cases as possible by reviewing all outpatient records (orthopedic, nonorthopedic), inpatient records, radiologic studies, and scanned outside records. Another limitation is that having a small number of VTE events limited our ability to perform a multivariate analysis, and uncontrolled confounding likely resulted. Only a very large multi-institutional study can capture enough events to allow a multivariate analysis. A third limitation is that the small number of events may have underpowered the study. Having more patients would have allowed other potential factors to be identified as being significantly associated with VTE. Last, as the study captured only symptomatic VTE events, it may have underreported VTE events. Given our complete review of the medical records, however, most clinically significant events likely were captured.
Conclusion
VTE after SA is rare. In our single-institution study, the symptomatic DVT rate was 0.9%, and the symptomatic PE rate was 2.3%. Risk factors associated with clotting included prior VTE, higher BMI, lower preoperative and postoperative Hb levels, raised CCI, diabetes, use of GETA without interscalene nerve block, and revision SA. Risk factors can be used to identify patients who may benefit from a more scrutinized postoperative evaluation and from increased surgeon awareness of the potential for VTE development. Rates of VTE can be used to counsel SA patients regarding overall surgical risks.
Am J Orthop. 2016;45(6):E379-E385. Copyright Frontline Medical Communications Inc. 2016. All rights reserved.
1. Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism after operative treatment of proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(6):782-783.
2. Jameson SS, James P, Howcroft DW, et al. Venous thromboembolic events are rare after shoulder surgery: analysis of a national database. J Shoulder Elbow Surg. 2011;20(5):764-770.
3. Sperling JW, Cofield RH. Pulmonary embolism following shoulder arthroplasty. J Bone Joint Surg Am. 2002;84(11):1939-1941.
4. Willis AA, Warren RF, Craig EV, et al. Deep vein thrombosis after reconstructive shoulder arthroplasty: a prospective observational study. J Shoulder Elbow Surg. 2009;18(1):100-106.
5. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563.
6. Lyman S, Sherman S, Carter TI, Bach PB, Mandl LA, Marx RG. Prevalence and risk factors for symptomatic thromboembolic events after shoulder arthroplasty. Clin Orthop Relat Res. 2006;(448):152-156.
7. Singh JA, Sperling JW, Cofield RH. Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum. 2012;41(5):689-697.
8. Navarro RA, Inacio MC, Burke MF, Costouros JG, Yian EH. Risk of thromboembolism in shoulder arthroplasty: effect of implant type and traumatic indication. Clin Orthop Relat Res. 2013;471(5):1576-1581.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
10. Saleh HE, Pennings AL, ElMaraghy AW. Venous thromboembolism after shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2013;22(10):1440-1448.
11. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
12. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341.
1. Hoxie SC, Sperling JW, Cofield RH. Pulmonary embolism after operative treatment of proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(6):782-783.
2. Jameson SS, James P, Howcroft DW, et al. Venous thromboembolic events are rare after shoulder surgery: analysis of a national database. J Shoulder Elbow Surg. 2011;20(5):764-770.
3. Sperling JW, Cofield RH. Pulmonary embolism following shoulder arthroplasty. J Bone Joint Surg Am. 2002;84(11):1939-1941.
4. Willis AA, Warren RF, Craig EV, et al. Deep vein thrombosis after reconstructive shoulder arthroplasty: a prospective observational study. J Shoulder Elbow Surg. 2009;18(1):100-106.
5. Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011;20(4):557-563.
6. Lyman S, Sherman S, Carter TI, Bach PB, Mandl LA, Marx RG. Prevalence and risk factors for symptomatic thromboembolic events after shoulder arthroplasty. Clin Orthop Relat Res. 2006;(448):152-156.
7. Singh JA, Sperling JW, Cofield RH. Cardiopulmonary complications after primary shoulder arthroplasty: a cohort study. Semin Arthritis Rheum. 2012;41(5):689-697.
8. Navarro RA, Inacio MC, Burke MF, Costouros JG, Yian EH. Risk of thromboembolism in shoulder arthroplasty: effect of implant type and traumatic indication. Clin Orthop Relat Res. 2013;471(5):1576-1581.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
10. Saleh HE, Pennings AL, ElMaraghy AW. Venous thromboembolism after shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg. 2013;22(10):1440-1448.
11. Hardy JC, Hung M, Snow BJ, et al. Blood transfusion associated with shoulder arthroplasty. J Shoulder Elbow Surg. 2013;22(2):233-239.
12. Gangireddy C, Rectenwald JR, Upchurch GR, et al. Risk factors and clinical impact of postoperative symptomatic venous thromboembolism. J Vasc Surg. 2007;45(2):335-341.