User login
Brain deposits of hyperphosphorylated tau are detectable in traumatic brain injury (TBI) patients 18-51 years after a single moderate to severe incident occurred, researchers reported Sept. 4 in Science Translational Medicine.
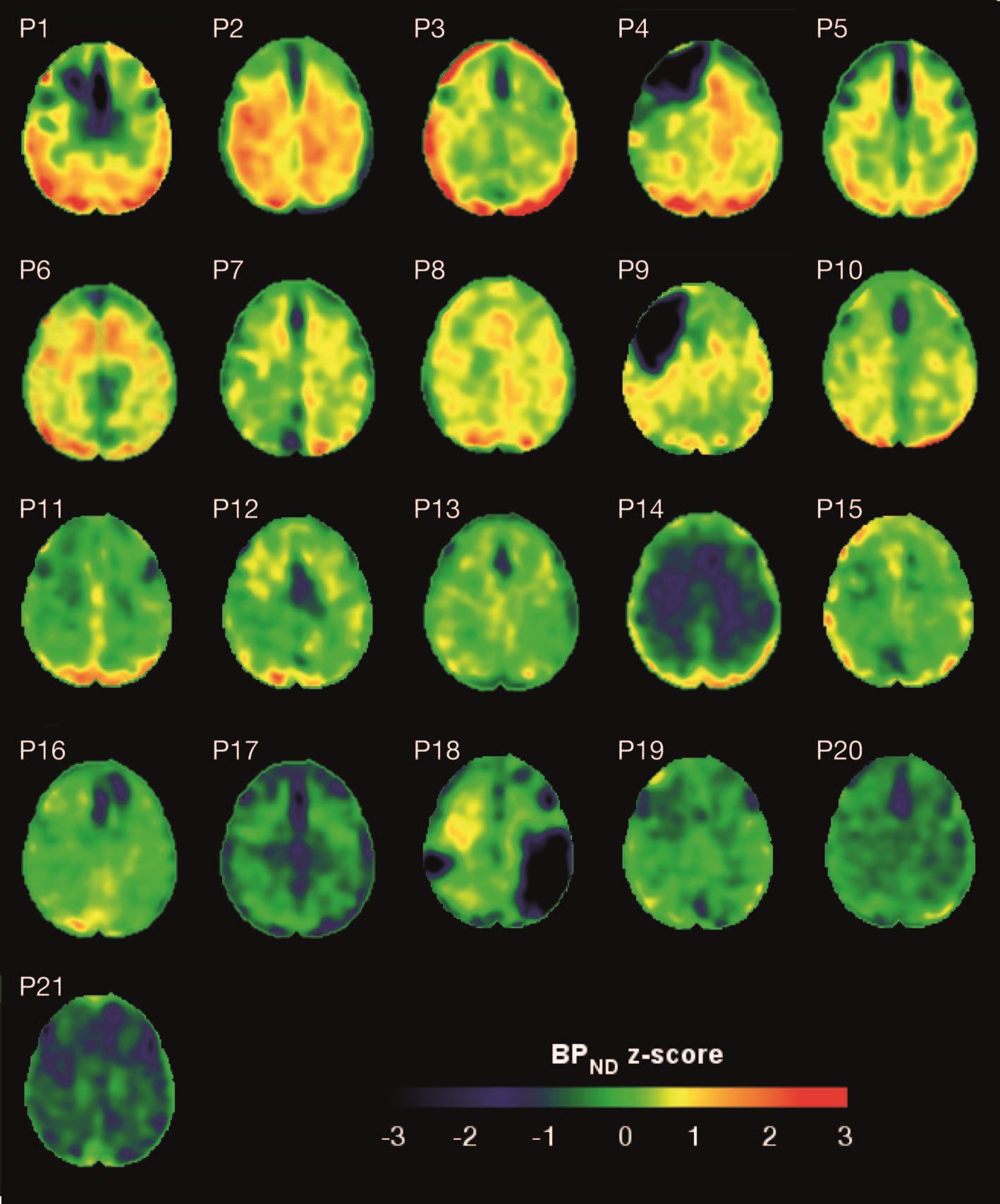
Imaging with the tau-specific PET radioligand flortaucipir showed that the protein was most apparent in the right occipital cortex, and was associated with changes in cognitive scores, tau and beta amyloid in cerebrospinal fluid (CSF), and white matter density, Nikos Gorgoraptis, PhD, of Imperial College London and his colleagues wrote.
“The ability to detect tau pathology in vivo after TBI has major potential implications for diagnosis and prognostication of clinical outcomes after TBI,” the researchers explained. “It is also likely to assist in patient selection and stratification for future treatment trials targeting tau.”
The cohort study comprised 21 subjects (median age, 49 years) who had experienced a single moderate to severe TBI a median of 32 years (range, 18-51 years) before enrollment. A control group comprised 11 noninjured adults who were matched for age and other demographic factors. Everyone underwent a PET scan with flortaucipir, brain MRI, CSF sampling, apolipoprotein E genotyping, and neuropsychological testing.
TBI subjects were grouped according to recovery status: good and disabled. Overall, they showed impairments on multiple cognitive domains (processing speed, executive function, motivation, inhibition, and verbal and visual memory), compared with controls. These findings were largely driven by the disabled group.
Eight TBI subjects had elevated tau binding greater than 2,000 voxels above the threshold of detection (equivalent to 16 cm3 of brain volume), and seven had an increase of 249-1,999 voxels above threshold. Tau binding in the remainder was similar to that in controls. Recovery status didn’t correlate with the tau-binding strength.
Overall, the tau-binding signal appeared most strongly in the right lateral occipital cortex, regardless of functional recovery status.
In TBI subjects, CSF total tau correlated significantly with flortaucipir uptake in cortical gray matter, but not white matter. CSF phosphorylated tau correlated with uptake in white matter, but not gray matter.
The investigators also examined fractional anisotropy, a measure of fiber density, axonal diameter, and myelination in white matter. In TBI subjects, there was more flortaucipir uptake in areas of decreased fractional anisotropy, including association, commissural, and projection tracts.
“Correlations were observed in the genu and body of the corpus callosum, as well as in several association tracts within the ipsilateral (right) hemisphere, including the cingulum bundle, inferior longitudinal fasciculus, uncinate fasciculus, and anterior thalamic radiation, but not in the contralateral hemisphere. Higher cortical flortaucipir [signal] was associated with reduced tissue density in remote white matter regions including the corpus callosum and right prefrontal white matter. The same analysis for gray matter density did not show an association.”
The increased tau signal in TBI subjects “is in keeping with a causative role for traumatic axonal injury in the pathophysiology of posttraumatic tau pathology,” the authors said. “Mechanical forces exerted at the time of head injury are thought to disrupt axonal organization, producing damage to microtubule structure and associated axonal tau. This damage may lead to hyperphosphorylation of tau, misfolding, and neurofibrillary tangle formation, which eventually causes neurodegeneration. Mechanical forces are maximal in points of geometric inflection such as the base of cortical sulci, where tau pathology is seen in chronic traumatic encephalopathy.”
These patterns suggest that tau imaging could provide valuable diagnostic information about the type of posttraumatic neurodegeneration, they said.
The work was supported by the Medical Research Council and UK Dementia Research Institute. None of the authors declared having any competing interests related to the current study. Some authors reported financial ties to pharmaceutical companies.
SOURCE: Gorgoraptis N et al. Sci Transl Med. 2019;11:eaaw1993. doi: 10.1126/scitranslmed.aaw1993.
Brain deposits of hyperphosphorylated tau are detectable in traumatic brain injury (TBI) patients 18-51 years after a single moderate to severe incident occurred, researchers reported Sept. 4 in Science Translational Medicine.
Imaging with the tau-specific PET radioligand flortaucipir showed that the protein was most apparent in the right occipital cortex, and was associated with changes in cognitive scores, tau and beta amyloid in cerebrospinal fluid (CSF), and white matter density, Nikos Gorgoraptis, PhD, of Imperial College London and his colleagues wrote.
“The ability to detect tau pathology in vivo after TBI has major potential implications for diagnosis and prognostication of clinical outcomes after TBI,” the researchers explained. “It is also likely to assist in patient selection and stratification for future treatment trials targeting tau.”
The cohort study comprised 21 subjects (median age, 49 years) who had experienced a single moderate to severe TBI a median of 32 years (range, 18-51 years) before enrollment. A control group comprised 11 noninjured adults who were matched for age and other demographic factors. Everyone underwent a PET scan with flortaucipir, brain MRI, CSF sampling, apolipoprotein E genotyping, and neuropsychological testing.
TBI subjects were grouped according to recovery status: good and disabled. Overall, they showed impairments on multiple cognitive domains (processing speed, executive function, motivation, inhibition, and verbal and visual memory), compared with controls. These findings were largely driven by the disabled group.
Eight TBI subjects had elevated tau binding greater than 2,000 voxels above the threshold of detection (equivalent to 16 cm3 of brain volume), and seven had an increase of 249-1,999 voxels above threshold. Tau binding in the remainder was similar to that in controls. Recovery status didn’t correlate with the tau-binding strength.
Overall, the tau-binding signal appeared most strongly in the right lateral occipital cortex, regardless of functional recovery status.
In TBI subjects, CSF total tau correlated significantly with flortaucipir uptake in cortical gray matter, but not white matter. CSF phosphorylated tau correlated with uptake in white matter, but not gray matter.
The investigators also examined fractional anisotropy, a measure of fiber density, axonal diameter, and myelination in white matter. In TBI subjects, there was more flortaucipir uptake in areas of decreased fractional anisotropy, including association, commissural, and projection tracts.
“Correlations were observed in the genu and body of the corpus callosum, as well as in several association tracts within the ipsilateral (right) hemisphere, including the cingulum bundle, inferior longitudinal fasciculus, uncinate fasciculus, and anterior thalamic radiation, but not in the contralateral hemisphere. Higher cortical flortaucipir [signal] was associated with reduced tissue density in remote white matter regions including the corpus callosum and right prefrontal white matter. The same analysis for gray matter density did not show an association.”
The increased tau signal in TBI subjects “is in keeping with a causative role for traumatic axonal injury in the pathophysiology of posttraumatic tau pathology,” the authors said. “Mechanical forces exerted at the time of head injury are thought to disrupt axonal organization, producing damage to microtubule structure and associated axonal tau. This damage may lead to hyperphosphorylation of tau, misfolding, and neurofibrillary tangle formation, which eventually causes neurodegeneration. Mechanical forces are maximal in points of geometric inflection such as the base of cortical sulci, where tau pathology is seen in chronic traumatic encephalopathy.”
These patterns suggest that tau imaging could provide valuable diagnostic information about the type of posttraumatic neurodegeneration, they said.
The work was supported by the Medical Research Council and UK Dementia Research Institute. None of the authors declared having any competing interests related to the current study. Some authors reported financial ties to pharmaceutical companies.
SOURCE: Gorgoraptis N et al. Sci Transl Med. 2019;11:eaaw1993. doi: 10.1126/scitranslmed.aaw1993.
Brain deposits of hyperphosphorylated tau are detectable in traumatic brain injury (TBI) patients 18-51 years after a single moderate to severe incident occurred, researchers reported Sept. 4 in Science Translational Medicine.
Imaging with the tau-specific PET radioligand flortaucipir showed that the protein was most apparent in the right occipital cortex, and was associated with changes in cognitive scores, tau and beta amyloid in cerebrospinal fluid (CSF), and white matter density, Nikos Gorgoraptis, PhD, of Imperial College London and his colleagues wrote.
“The ability to detect tau pathology in vivo after TBI has major potential implications for diagnosis and prognostication of clinical outcomes after TBI,” the researchers explained. “It is also likely to assist in patient selection and stratification for future treatment trials targeting tau.”
The cohort study comprised 21 subjects (median age, 49 years) who had experienced a single moderate to severe TBI a median of 32 years (range, 18-51 years) before enrollment. A control group comprised 11 noninjured adults who were matched for age and other demographic factors. Everyone underwent a PET scan with flortaucipir, brain MRI, CSF sampling, apolipoprotein E genotyping, and neuropsychological testing.
TBI subjects were grouped according to recovery status: good and disabled. Overall, they showed impairments on multiple cognitive domains (processing speed, executive function, motivation, inhibition, and verbal and visual memory), compared with controls. These findings were largely driven by the disabled group.
Eight TBI subjects had elevated tau binding greater than 2,000 voxels above the threshold of detection (equivalent to 16 cm3 of brain volume), and seven had an increase of 249-1,999 voxels above threshold. Tau binding in the remainder was similar to that in controls. Recovery status didn’t correlate with the tau-binding strength.
Overall, the tau-binding signal appeared most strongly in the right lateral occipital cortex, regardless of functional recovery status.
In TBI subjects, CSF total tau correlated significantly with flortaucipir uptake in cortical gray matter, but not white matter. CSF phosphorylated tau correlated with uptake in white matter, but not gray matter.
The investigators also examined fractional anisotropy, a measure of fiber density, axonal diameter, and myelination in white matter. In TBI subjects, there was more flortaucipir uptake in areas of decreased fractional anisotropy, including association, commissural, and projection tracts.
“Correlations were observed in the genu and body of the corpus callosum, as well as in several association tracts within the ipsilateral (right) hemisphere, including the cingulum bundle, inferior longitudinal fasciculus, uncinate fasciculus, and anterior thalamic radiation, but not in the contralateral hemisphere. Higher cortical flortaucipir [signal] was associated with reduced tissue density in remote white matter regions including the corpus callosum and right prefrontal white matter. The same analysis for gray matter density did not show an association.”
The increased tau signal in TBI subjects “is in keeping with a causative role for traumatic axonal injury in the pathophysiology of posttraumatic tau pathology,” the authors said. “Mechanical forces exerted at the time of head injury are thought to disrupt axonal organization, producing damage to microtubule structure and associated axonal tau. This damage may lead to hyperphosphorylation of tau, misfolding, and neurofibrillary tangle formation, which eventually causes neurodegeneration. Mechanical forces are maximal in points of geometric inflection such as the base of cortical sulci, where tau pathology is seen in chronic traumatic encephalopathy.”
These patterns suggest that tau imaging could provide valuable diagnostic information about the type of posttraumatic neurodegeneration, they said.
The work was supported by the Medical Research Council and UK Dementia Research Institute. None of the authors declared having any competing interests related to the current study. Some authors reported financial ties to pharmaceutical companies.
SOURCE: Gorgoraptis N et al. Sci Transl Med. 2019;11:eaaw1993. doi: 10.1126/scitranslmed.aaw1993.
FROM SCIENCE TRANSLATIONAL MEDICINE