User login
Staphylococcus lugdunensis (S lugdunensis) is a species of coagulase-negative Staphylococcus (CoNS) and a constituent of human skin flora. Unlike other strains of CoNS, however, S lugdunensis has gained notoriety for virulence that resembles Staphylococcus aureus (S aureus). S lugdunensis is now recognized as an important nosocomial pathogen and cause of prosthetic device infections, including vascular catheter infections. We present a case of persistent S lugdunensis bacteremia occurring in a patient on hemodialysis (HD) without any implanted prosthetic materials.
Case Presentation
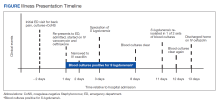
A 60-year-old man with a history of uncontrolled type 2 diabetes mellitus (T2DM) and end-stage renal disease on home HD via arteriovenous fistula (AVF) presented to the emergency department (ED) for evaluation of subacute progressive low back pain. His symptoms began abruptly 2 weeks prior to presentation without any identifiable trigger or trauma. His pain localized to the lower thoracic spine, radiating anteriorly into his abdomen. He reported tactile fever for several days before presentation but no chills, night sweats, paresthesia, weakness, or bowel/bladder incontinence. He had no recent surgeries, implanted hardware, or invasive procedures involving the spine. HD was performed 5 times a week at home with a family member cannulating his AVF via buttonhole technique. He initially sought evaluation in a community hospital several days prior, where he underwent magnetic resonance imaging (MRI) of the thoracic spine. He was discharged from the community ED with oral opioids prior to the MRI results. He presented to West Los Angeles Veterans Affairs Medical Center (WLAVAMC) ED when MRI results came back indicating abnormalities and he reported recalcitrant pain.
On arrival at WLAVAMC, the patient was afebrile with a heart rate of 107 bpm and blood pressure of 152/97 mm Hg. The remainder of his vital signs were normal. The physical examination revealed midline tenderness on palpation of the distal thoracic and proximal lumbar spine. Muscle strength was 4 of 5 in the bilateral hip flexors, though this was limited by pain. The remainder of his neurologic examination was nonfocal. The cardiac examination was unremarkable with no murmurs auscultated. His left upper extremity AVF had an audible bruit and palpable thrill. The skin examination was notable for acanthosis nigricans but no areas of skin erythema or induration and no obvious stigmata of infective endocarditis.
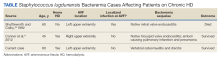
The initial laboratory workup was remarkable for a white blood cell (WBC) count of 10.0 × 103/µL with left shift, blood urea nitrogen level of 59 mg/dL, and creatinine level of 9.3 mg/dL. The patient’s erythrocyte sedimentation rate (ESR) was 45 mm/h (reference range, ≤ 20 mm/h) and C-reactive protein level was > 8.0 mg/L (reference range, ≤ 0.74 mg/L). Two months prior the hemoglobin A1c had been recorded at 9.9%.
Given his intractable low back pain and elevated inflammatory markers, the patient underwent an MRI of the thoracic and lumbar spine with contrast while in the ED. This MRI revealed abnormal marrow edema in the T11-T12 vertebrae with abnormal fluid signal in the T11-T12 disc space. Subjacent paravertebral edema also was noted. There was no well-defined fluid collection or abnormal signal in the spinal cord. Taken together, these findings were concerning for T11-T12 discitis with osteomyelitis.
Two sets of blood cultures were obtained, and empiric IV vancomycin and ceftriaxone were started. Interventional radiology was consulted for consideration of vertebral biopsy but deferred while awaiting blood culture data. Neurosurgery also was consulted and recommended nonoperative management given his nonfocal neurologic examination and imaging without evidence of abscess. Both sets of blood cultures collected on admission later grew methicillin-sensitive S lugdunensis, a species of CoNS. A transthoracic and later transesophageal echocardiogram did not show any valvular vegetations. The patient’s antibiotic regimen was narrowed to IV oxacillin based on susceptibility data. It was later discovered that both blood cultures obtained during his outside ED encounter were also growing S lugdunensis.
The patient’s S lugdunensis bacteremia persisted for the first 8 days of his admission despite appropriate dosing of oxacillin. During this time, the patient remained afebrile with stable vital signs and a normal WBC count. Positron emission tomography was obtained to evaluate for potential sources of his persistent bacteremia. Aside from tracer uptake in the T11-T12 vertebral bodies and intervertebral disc space, no other areas showed suspicious uptake. Neurosurgery reevaluated the patient and again recommended nonoperative management. Blood cultures cleared and based on recommendations from an infectious disease specialist, the patient was transitioned to IV cefazolin dosed 3 times weekly after HD, which was transitioned to an outpatient dialysis center. The patient continued taking cefazolin for 6 weeks with subsequent improvement in back pain and normalization of inflammatory markers at outpatient follow-up.
Discussion
CoNS are a major contributor to human skin flora, a common contaminant of blood cultures, and an important cause of nosocomial bloodstream infections.1,2 These species have a predilection for forming biofilms, making CoNS a major cause of prosthetic device infections.3 S lugdunensis is a CoNS species that was first described in 1988.4 In addition to foreign body–related infections, S lugdunensis has been implicated in bone/joint infections, native valve endocarditis, toxic shock syndrome, and brain abscesses.5-8 Infections due to S lugdunensis are notorious for their aggressive and fulminant courses. With its increased virulence that is atypical of other CoNS, S lugdunensis has understandably been likened more to S aureus.
Prior cases have been reported of S lugdunensis bacteremia in patients using HD. However, the suspected source of bacteremia in these cases has generally been central venous catheters.9-12
Notably, our patient’s AVF was accessed using the buttonhole technique for his home HD sessions, which involves cannulating the same site along the fistula until an epithelialized track has formed from scar tissue. At later HD sessions, duller needles can then be used to cannulate this same track. In contrast, the rope-ladder technique involves cannulating a different site along the fistula until the entire length of the fistula has been used. Patients report higher levels of satisfaction with the buttonhole technique, citing decreased pain, decreased oozing, and the perception of easier cannulation by HD nurses.14 However, the buttonhole technique also appears to confer a higher risk of vascular access-related bloodstream infection when compared with the rope-ladder technique.13,15,16
The buttonhole technique is hypothesized to increase infection risk due to the repeated use of the same site for needle entry. Skin flora, including CoNS, may colonize the scab that forms after dialysis access. If proper sterilization techniques are not rigorously followed, the bacteria colonizing the scab and adjacent skin may be introduced into a patient’s bloodstream during needle puncture. Loss of skin integrity due to frequent cannulation of the same site may also contribute to this increased infection risk. It is relevant to recall that our patient received HD 5 times weekly using the buttonhole technique. The use of the buttonhole technique, frequency of his HD sessions, unclear sterilization methods, and immune dysfunction related to his uncontrolled T2DM and renal disease all likely contributed to our patient’s bacteremia.
Using topical mupirocin for prophylaxis at the intended buttonhole puncture site has shown promising results in decreasing rates of S aureus bacteremia.17 It is unclear whether this intervention also would be effective against S lugdunensis. Increasing rates of mupirocin resistance have been reported among S lugdunensis isolates in dialysis settings, but further research in this area is warranted.18
There are no established treatment guidelines for S lugdunensis infections. In vitro studies suggest that S lugdunensis is susceptible to a wide variety of antibiotics. The mecA gene is a major determinant of methicillin resistance that is commonly observed among CoNS but is uncommonly seen with S lugdunensis.5 In a study by Tan and colleagues of 106 S lugdunensis isolates, they found that only 5 (4.7%) were mecA positive.19
Vancomycin is generally reasonable for empiric antibiotic coverage of staphylococci while speciation is pending. However, if S lugdunensis is isolated, its favorable susceptibility pattern typically allows for de-escalation to an antistaphylococcal β-lactam, such as oxacillin or nafcillin. In cases of bloodstream infections caused by methicillin-sensitive S aureus, treatment with a β-lactam has demonstrated superiority over vancomycin due to the lower rates of treatment failure and mortality with β-lactams.20,21 It is unknown whether β-lactams is superior for treating bacteremia with methicillin-sensitive S lugdunensis.
Our patient’s isolate of S lugdunensis was pansensitive to all antibiotics tested, including penicillin. These susceptibility data were used to guide the de-escalation of his empiric vancomycin and ceftriaxone to oxacillin on hospital day 1.
Due to their virulence, bloodstream infections caused by S aureus and S lugdunensis often require more than timely antimicrobial treatment to ensure eradication. Consultation with an infectious disease specialist to manage patients with S aureus bacteremia has been proven to reduce mortality.25 A similar mortality benefit is seen when infectious disease specialists are consulted for S lugdunensis bacteremia.26 This mortality benefit is likely explained by S lugdunensis’ propensity to cause aggressive, metastatic infections. In such cases, infectious disease consultants may recommend additional imaging (eg, transthoracic echocardiogram) to evaluate for occult sources of infection, advocate for appropriate source control, and guide the selection of an appropriate antibiotic course to ensure resolution of the bacteremia.
Conclusions
S lugdunensis is an increasingly recognized cause of nosocomial bloodstream infections. Given the commonalities in virulence that S lugdunensis shares with S aureus, treatment of bacteremia caused by either species should follow similar management principles: prompt initiation of IV antistaphylococcal therapy, a thorough evaluation for the source(s) of bacteremia as well as metastatic complications, and consultation with an infectious disease specialist. This case report also highlights the importance of considering a patient’s AVF as a potential source for infection even in the absence of localized signs of infection. The buttonhole method of AVF cannulation was thought to be a major contributor to the development and persistence of our patient’s bacteremia. This risk should be discussed with patients using a shared decision-making approach when developing a dialysis treatment plan.
1. Huebner J, Goldmann DA. Coagulase-negative staphylococci: role as pathogens. Annu Rev Med. 1999;50(1):223-236. doi:10.1146/annurev.med.50.1.223
2. Beekmann SE, Diekema DJ, Doern GV. Determining the clinical significance of coagulase-negative staphylococci isolated from blood cultures. Infect Control Hosp Epidemiol. 2005;26(6):559-566. doi:10.1086/502584
3. Arrecubieta C, Toba FA, von Bayern M, et al. SdrF, a Staphylococcus epidermidis surface protein, contributes to the initiation of ventricular assist device driveline–related infections. PLoS Pathog. 2009;5(5):e1000411. doi.10.1371/journal.ppat.1000411
4. Freney J, Brun Y, Bes M, et al. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38(2):168-172. doi:10.1099/00207713-38-2-168
5. Frank KL, del Pozo JL, Patel R. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev. 2008;21(1):111-133. doi:10.1128/CMR.00036-07
6. Anguera I, Del Río A, Miró JM; Hospital Clinic Endocarditis Study Group. Staphylococcus lugdunensis infective endocarditis: description of 10 cases and analysis of native valve, prosthetic valve, and pacemaker lead endocarditis clinical profiles. Heart. 2005;91(2):e10. doi:10.1136/hrt.2004.040659
7. Pareja J, Gupta K, Koziel H. The toxic shock syndrome and Staphylococcus lugdunensis bacteremia. Ann Intern Med. 1998;128(7):603-604. doi:10.7326/0003-4819-128-7-199804010-00029
8. Woznowski M, Quack I, Bölke E, et al. Fulminant Staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 201;15(9):410-414. doi:10.1186/2047-783x-15-9-410
9. Mallappallil M, Salifu M, Woredekal Y, et al. Staphylococcus lugdunensis bacteremia in hemodialysis patients. Int J Microbiol Res. 2012;4(2):178-181. doi:10.9735/0975-5276.4.2.178-181
10. Shuttleworth R, Colby W. Staphylococcus lugdunensis endocarditis. J Clin Microbiol. 1992;30(8):5. doi:10.1128/jcm.30.8.1948-1952.1992
11. Conner RC, Byrnes TJ, Clough LA, Myers JP. Staphylococcus lugdunensis tricuspid valve endocarditis associated with home hemodialysis therapy: report of a case and review of the literature. Infect Dis Clin Pract. 2012;20(3):182-183. doi:1097/IPC.0b013e318245d4f1
12. Kamaraju S, Nelson K, Williams D, Ayenew W, Modi K. Staphylococcus lugdunensis pulmonary valve endocarditis in a patient on chronic hemodialysis. Am J Nephrol. 1999;19(5):605-608. doi:1097/IPC.0b013e318245d4f1
13. Lok C, Sontrop J, Faratro R, Chan C, Zimmerman DL. Frequent hemodialysis fistula infectious complications. Nephron Extra. 2014;4(3):159-167. doi:10.1159/000366477
14. Hashmi A, Cheema MQ, Moss AH. Hemodialysis patients’ experience with and attitudes toward the buttonhole technique for arteriovenous fistula cannulation. Clin Nephrol. 2010;74(5):346-350. doi:10.5414/cnp74346
15. Lyman M, Nguyen DB, Shugart A, Gruhler H, Lines C, Patel PR. Risk of vascular access infection associated with buttonhole cannulation of fistulas: data from the National Healthcare Safety Network. Am J Kidney Dis. 2020;76(1):82-89. doi:10.1053/j.ajkd.2019.11.006
16. MacRae JM, Ahmed SB, Atkar R, Hemmelgarn BR. A randomized trial comparing buttonhole with rope ladder needling in conventional hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(10):1632-1638. doi:10.2215/CJN.02730312
17. Nesrallah GE, Cuerden M, Wong JHS, Pierratos A. Staphylococcus aureus bacteremia and buttonhole cannulation: long-term safety and efficacy of mupirocin prophylaxis. Clin J Am Soc Nephrol. 2010;5(6):1047-1053. doi:10.2215/CJN.00280110
18. Ho PL, Liu MCJ, Chow KH, et al. Emergence of ileS2 -carrying, multidrug-resistant plasmids in Staphylococcus lugdunensis. Antimicrob Agents Chemother. 2016;60(10):6411-6414. doi:10.1128/AAC.00948-16
19. Tan TY, Ng SY, He J. Microbiological characteristics, presumptive identification, and antibiotic susceptibilities of Staphylococcus lugdunensis. J Clin Microbiol. 2008;46(7):2393-2395. doi:10.1128/JCM.00740-08
20. Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore). 2003;82(5):333-339. doi:10.1097/01.md.0000091184.93122.09
21. Shurland S, Zhan M, Bradham DD, Roghmann MC. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):273-279. doi:10.1086/512627
22. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115(9):674. doi:10.7326/0003-4819-115-9-674
23. Fowler VG, Karchmer AW, Tally FP, et al; S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653-665 . doi:10.1056/NEJMoa053783
24. Duhon B, Dallas S, Velasquez ST, Hand E. Staphylococcus lugdunensis bacteremia and endocarditis treated with cefazolin and rifampin. Am J Health Syst Pharm. 2015;72(13):1114-1118. doi:10.2146/ajhp140498
25. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. doi:10.1097/MD.0b013e3181b8fccb
26. Forsblom E, Högnäs E, Syrjänen J, Järvinen A. Infectious diseases specialist consultation in Staphylococcus lugdunensis bacteremia. PLoS ONE. 2021;16(10):e0258511. doi:10.1371/journal.pone.0258511
Staphylococcus lugdunensis (S lugdunensis) is a species of coagulase-negative Staphylococcus (CoNS) and a constituent of human skin flora. Unlike other strains of CoNS, however, S lugdunensis has gained notoriety for virulence that resembles Staphylococcus aureus (S aureus). S lugdunensis is now recognized as an important nosocomial pathogen and cause of prosthetic device infections, including vascular catheter infections. We present a case of persistent S lugdunensis bacteremia occurring in a patient on hemodialysis (HD) without any implanted prosthetic materials.
Case Presentation
A 60-year-old man with a history of uncontrolled type 2 diabetes mellitus (T2DM) and end-stage renal disease on home HD via arteriovenous fistula (AVF) presented to the emergency department (ED) for evaluation of subacute progressive low back pain. His symptoms began abruptly 2 weeks prior to presentation without any identifiable trigger or trauma. His pain localized to the lower thoracic spine, radiating anteriorly into his abdomen. He reported tactile fever for several days before presentation but no chills, night sweats, paresthesia, weakness, or bowel/bladder incontinence. He had no recent surgeries, implanted hardware, or invasive procedures involving the spine. HD was performed 5 times a week at home with a family member cannulating his AVF via buttonhole technique. He initially sought evaluation in a community hospital several days prior, where he underwent magnetic resonance imaging (MRI) of the thoracic spine. He was discharged from the community ED with oral opioids prior to the MRI results. He presented to West Los Angeles Veterans Affairs Medical Center (WLAVAMC) ED when MRI results came back indicating abnormalities and he reported recalcitrant pain.
On arrival at WLAVAMC, the patient was afebrile with a heart rate of 107 bpm and blood pressure of 152/97 mm Hg. The remainder of his vital signs were normal. The physical examination revealed midline tenderness on palpation of the distal thoracic and proximal lumbar spine. Muscle strength was 4 of 5 in the bilateral hip flexors, though this was limited by pain. The remainder of his neurologic examination was nonfocal. The cardiac examination was unremarkable with no murmurs auscultated. His left upper extremity AVF had an audible bruit and palpable thrill. The skin examination was notable for acanthosis nigricans but no areas of skin erythema or induration and no obvious stigmata of infective endocarditis.
The initial laboratory workup was remarkable for a white blood cell (WBC) count of 10.0 × 103/µL with left shift, blood urea nitrogen level of 59 mg/dL, and creatinine level of 9.3 mg/dL. The patient’s erythrocyte sedimentation rate (ESR) was 45 mm/h (reference range, ≤ 20 mm/h) and C-reactive protein level was > 8.0 mg/L (reference range, ≤ 0.74 mg/L). Two months prior the hemoglobin A1c had been recorded at 9.9%.
Given his intractable low back pain and elevated inflammatory markers, the patient underwent an MRI of the thoracic and lumbar spine with contrast while in the ED. This MRI revealed abnormal marrow edema in the T11-T12 vertebrae with abnormal fluid signal in the T11-T12 disc space. Subjacent paravertebral edema also was noted. There was no well-defined fluid collection or abnormal signal in the spinal cord. Taken together, these findings were concerning for T11-T12 discitis with osteomyelitis.
Two sets of blood cultures were obtained, and empiric IV vancomycin and ceftriaxone were started. Interventional radiology was consulted for consideration of vertebral biopsy but deferred while awaiting blood culture data. Neurosurgery also was consulted and recommended nonoperative management given his nonfocal neurologic examination and imaging without evidence of abscess. Both sets of blood cultures collected on admission later grew methicillin-sensitive S lugdunensis, a species of CoNS. A transthoracic and later transesophageal echocardiogram did not show any valvular vegetations. The patient’s antibiotic regimen was narrowed to IV oxacillin based on susceptibility data. It was later discovered that both blood cultures obtained during his outside ED encounter were also growing S lugdunensis.
The patient’s S lugdunensis bacteremia persisted for the first 8 days of his admission despite appropriate dosing of oxacillin. During this time, the patient remained afebrile with stable vital signs and a normal WBC count. Positron emission tomography was obtained to evaluate for potential sources of his persistent bacteremia. Aside from tracer uptake in the T11-T12 vertebral bodies and intervertebral disc space, no other areas showed suspicious uptake. Neurosurgery reevaluated the patient and again recommended nonoperative management. Blood cultures cleared and based on recommendations from an infectious disease specialist, the patient was transitioned to IV cefazolin dosed 3 times weekly after HD, which was transitioned to an outpatient dialysis center. The patient continued taking cefazolin for 6 weeks with subsequent improvement in back pain and normalization of inflammatory markers at outpatient follow-up.
Discussion
CoNS are a major contributor to human skin flora, a common contaminant of blood cultures, and an important cause of nosocomial bloodstream infections.1,2 These species have a predilection for forming biofilms, making CoNS a major cause of prosthetic device infections.3 S lugdunensis is a CoNS species that was first described in 1988.4 In addition to foreign body–related infections, S lugdunensis has been implicated in bone/joint infections, native valve endocarditis, toxic shock syndrome, and brain abscesses.5-8 Infections due to S lugdunensis are notorious for their aggressive and fulminant courses. With its increased virulence that is atypical of other CoNS, S lugdunensis has understandably been likened more to S aureus.
Prior cases have been reported of S lugdunensis bacteremia in patients using HD. However, the suspected source of bacteremia in these cases has generally been central venous catheters.9-12
Notably, our patient’s AVF was accessed using the buttonhole technique for his home HD sessions, which involves cannulating the same site along the fistula until an epithelialized track has formed from scar tissue. At later HD sessions, duller needles can then be used to cannulate this same track. In contrast, the rope-ladder technique involves cannulating a different site along the fistula until the entire length of the fistula has been used. Patients report higher levels of satisfaction with the buttonhole technique, citing decreased pain, decreased oozing, and the perception of easier cannulation by HD nurses.14 However, the buttonhole technique also appears to confer a higher risk of vascular access-related bloodstream infection when compared with the rope-ladder technique.13,15,16
The buttonhole technique is hypothesized to increase infection risk due to the repeated use of the same site for needle entry. Skin flora, including CoNS, may colonize the scab that forms after dialysis access. If proper sterilization techniques are not rigorously followed, the bacteria colonizing the scab and adjacent skin may be introduced into a patient’s bloodstream during needle puncture. Loss of skin integrity due to frequent cannulation of the same site may also contribute to this increased infection risk. It is relevant to recall that our patient received HD 5 times weekly using the buttonhole technique. The use of the buttonhole technique, frequency of his HD sessions, unclear sterilization methods, and immune dysfunction related to his uncontrolled T2DM and renal disease all likely contributed to our patient’s bacteremia.
Using topical mupirocin for prophylaxis at the intended buttonhole puncture site has shown promising results in decreasing rates of S aureus bacteremia.17 It is unclear whether this intervention also would be effective against S lugdunensis. Increasing rates of mupirocin resistance have been reported among S lugdunensis isolates in dialysis settings, but further research in this area is warranted.18
There are no established treatment guidelines for S lugdunensis infections. In vitro studies suggest that S lugdunensis is susceptible to a wide variety of antibiotics. The mecA gene is a major determinant of methicillin resistance that is commonly observed among CoNS but is uncommonly seen with S lugdunensis.5 In a study by Tan and colleagues of 106 S lugdunensis isolates, they found that only 5 (4.7%) were mecA positive.19
Vancomycin is generally reasonable for empiric antibiotic coverage of staphylococci while speciation is pending. However, if S lugdunensis is isolated, its favorable susceptibility pattern typically allows for de-escalation to an antistaphylococcal β-lactam, such as oxacillin or nafcillin. In cases of bloodstream infections caused by methicillin-sensitive S aureus, treatment with a β-lactam has demonstrated superiority over vancomycin due to the lower rates of treatment failure and mortality with β-lactams.20,21 It is unknown whether β-lactams is superior for treating bacteremia with methicillin-sensitive S lugdunensis.
Our patient’s isolate of S lugdunensis was pansensitive to all antibiotics tested, including penicillin. These susceptibility data were used to guide the de-escalation of his empiric vancomycin and ceftriaxone to oxacillin on hospital day 1.
Due to their virulence, bloodstream infections caused by S aureus and S lugdunensis often require more than timely antimicrobial treatment to ensure eradication. Consultation with an infectious disease specialist to manage patients with S aureus bacteremia has been proven to reduce mortality.25 A similar mortality benefit is seen when infectious disease specialists are consulted for S lugdunensis bacteremia.26 This mortality benefit is likely explained by S lugdunensis’ propensity to cause aggressive, metastatic infections. In such cases, infectious disease consultants may recommend additional imaging (eg, transthoracic echocardiogram) to evaluate for occult sources of infection, advocate for appropriate source control, and guide the selection of an appropriate antibiotic course to ensure resolution of the bacteremia.
Conclusions
S lugdunensis is an increasingly recognized cause of nosocomial bloodstream infections. Given the commonalities in virulence that S lugdunensis shares with S aureus, treatment of bacteremia caused by either species should follow similar management principles: prompt initiation of IV antistaphylococcal therapy, a thorough evaluation for the source(s) of bacteremia as well as metastatic complications, and consultation with an infectious disease specialist. This case report also highlights the importance of considering a patient’s AVF as a potential source for infection even in the absence of localized signs of infection. The buttonhole method of AVF cannulation was thought to be a major contributor to the development and persistence of our patient’s bacteremia. This risk should be discussed with patients using a shared decision-making approach when developing a dialysis treatment plan.
Staphylococcus lugdunensis (S lugdunensis) is a species of coagulase-negative Staphylococcus (CoNS) and a constituent of human skin flora. Unlike other strains of CoNS, however, S lugdunensis has gained notoriety for virulence that resembles Staphylococcus aureus (S aureus). S lugdunensis is now recognized as an important nosocomial pathogen and cause of prosthetic device infections, including vascular catheter infections. We present a case of persistent S lugdunensis bacteremia occurring in a patient on hemodialysis (HD) without any implanted prosthetic materials.
Case Presentation
A 60-year-old man with a history of uncontrolled type 2 diabetes mellitus (T2DM) and end-stage renal disease on home HD via arteriovenous fistula (AVF) presented to the emergency department (ED) for evaluation of subacute progressive low back pain. His symptoms began abruptly 2 weeks prior to presentation without any identifiable trigger or trauma. His pain localized to the lower thoracic spine, radiating anteriorly into his abdomen. He reported tactile fever for several days before presentation but no chills, night sweats, paresthesia, weakness, or bowel/bladder incontinence. He had no recent surgeries, implanted hardware, or invasive procedures involving the spine. HD was performed 5 times a week at home with a family member cannulating his AVF via buttonhole technique. He initially sought evaluation in a community hospital several days prior, where he underwent magnetic resonance imaging (MRI) of the thoracic spine. He was discharged from the community ED with oral opioids prior to the MRI results. He presented to West Los Angeles Veterans Affairs Medical Center (WLAVAMC) ED when MRI results came back indicating abnormalities and he reported recalcitrant pain.
On arrival at WLAVAMC, the patient was afebrile with a heart rate of 107 bpm and blood pressure of 152/97 mm Hg. The remainder of his vital signs were normal. The physical examination revealed midline tenderness on palpation of the distal thoracic and proximal lumbar spine. Muscle strength was 4 of 5 in the bilateral hip flexors, though this was limited by pain. The remainder of his neurologic examination was nonfocal. The cardiac examination was unremarkable with no murmurs auscultated. His left upper extremity AVF had an audible bruit and palpable thrill. The skin examination was notable for acanthosis nigricans but no areas of skin erythema or induration and no obvious stigmata of infective endocarditis.
The initial laboratory workup was remarkable for a white blood cell (WBC) count of 10.0 × 103/µL with left shift, blood urea nitrogen level of 59 mg/dL, and creatinine level of 9.3 mg/dL. The patient’s erythrocyte sedimentation rate (ESR) was 45 mm/h (reference range, ≤ 20 mm/h) and C-reactive protein level was > 8.0 mg/L (reference range, ≤ 0.74 mg/L). Two months prior the hemoglobin A1c had been recorded at 9.9%.
Given his intractable low back pain and elevated inflammatory markers, the patient underwent an MRI of the thoracic and lumbar spine with contrast while in the ED. This MRI revealed abnormal marrow edema in the T11-T12 vertebrae with abnormal fluid signal in the T11-T12 disc space. Subjacent paravertebral edema also was noted. There was no well-defined fluid collection or abnormal signal in the spinal cord. Taken together, these findings were concerning for T11-T12 discitis with osteomyelitis.
Two sets of blood cultures were obtained, and empiric IV vancomycin and ceftriaxone were started. Interventional radiology was consulted for consideration of vertebral biopsy but deferred while awaiting blood culture data. Neurosurgery also was consulted and recommended nonoperative management given his nonfocal neurologic examination and imaging without evidence of abscess. Both sets of blood cultures collected on admission later grew methicillin-sensitive S lugdunensis, a species of CoNS. A transthoracic and later transesophageal echocardiogram did not show any valvular vegetations. The patient’s antibiotic regimen was narrowed to IV oxacillin based on susceptibility data. It was later discovered that both blood cultures obtained during his outside ED encounter were also growing S lugdunensis.
The patient’s S lugdunensis bacteremia persisted for the first 8 days of his admission despite appropriate dosing of oxacillin. During this time, the patient remained afebrile with stable vital signs and a normal WBC count. Positron emission tomography was obtained to evaluate for potential sources of his persistent bacteremia. Aside from tracer uptake in the T11-T12 vertebral bodies and intervertebral disc space, no other areas showed suspicious uptake. Neurosurgery reevaluated the patient and again recommended nonoperative management. Blood cultures cleared and based on recommendations from an infectious disease specialist, the patient was transitioned to IV cefazolin dosed 3 times weekly after HD, which was transitioned to an outpatient dialysis center. The patient continued taking cefazolin for 6 weeks with subsequent improvement in back pain and normalization of inflammatory markers at outpatient follow-up.
Discussion
CoNS are a major contributor to human skin flora, a common contaminant of blood cultures, and an important cause of nosocomial bloodstream infections.1,2 These species have a predilection for forming biofilms, making CoNS a major cause of prosthetic device infections.3 S lugdunensis is a CoNS species that was first described in 1988.4 In addition to foreign body–related infections, S lugdunensis has been implicated in bone/joint infections, native valve endocarditis, toxic shock syndrome, and brain abscesses.5-8 Infections due to S lugdunensis are notorious for their aggressive and fulminant courses. With its increased virulence that is atypical of other CoNS, S lugdunensis has understandably been likened more to S aureus.
Prior cases have been reported of S lugdunensis bacteremia in patients using HD. However, the suspected source of bacteremia in these cases has generally been central venous catheters.9-12
Notably, our patient’s AVF was accessed using the buttonhole technique for his home HD sessions, which involves cannulating the same site along the fistula until an epithelialized track has formed from scar tissue. At later HD sessions, duller needles can then be used to cannulate this same track. In contrast, the rope-ladder technique involves cannulating a different site along the fistula until the entire length of the fistula has been used. Patients report higher levels of satisfaction with the buttonhole technique, citing decreased pain, decreased oozing, and the perception of easier cannulation by HD nurses.14 However, the buttonhole technique also appears to confer a higher risk of vascular access-related bloodstream infection when compared with the rope-ladder technique.13,15,16
The buttonhole technique is hypothesized to increase infection risk due to the repeated use of the same site for needle entry. Skin flora, including CoNS, may colonize the scab that forms after dialysis access. If proper sterilization techniques are not rigorously followed, the bacteria colonizing the scab and adjacent skin may be introduced into a patient’s bloodstream during needle puncture. Loss of skin integrity due to frequent cannulation of the same site may also contribute to this increased infection risk. It is relevant to recall that our patient received HD 5 times weekly using the buttonhole technique. The use of the buttonhole technique, frequency of his HD sessions, unclear sterilization methods, and immune dysfunction related to his uncontrolled T2DM and renal disease all likely contributed to our patient’s bacteremia.
Using topical mupirocin for prophylaxis at the intended buttonhole puncture site has shown promising results in decreasing rates of S aureus bacteremia.17 It is unclear whether this intervention also would be effective against S lugdunensis. Increasing rates of mupirocin resistance have been reported among S lugdunensis isolates in dialysis settings, but further research in this area is warranted.18
There are no established treatment guidelines for S lugdunensis infections. In vitro studies suggest that S lugdunensis is susceptible to a wide variety of antibiotics. The mecA gene is a major determinant of methicillin resistance that is commonly observed among CoNS but is uncommonly seen with S lugdunensis.5 In a study by Tan and colleagues of 106 S lugdunensis isolates, they found that only 5 (4.7%) were mecA positive.19
Vancomycin is generally reasonable for empiric antibiotic coverage of staphylococci while speciation is pending. However, if S lugdunensis is isolated, its favorable susceptibility pattern typically allows for de-escalation to an antistaphylococcal β-lactam, such as oxacillin or nafcillin. In cases of bloodstream infections caused by methicillin-sensitive S aureus, treatment with a β-lactam has demonstrated superiority over vancomycin due to the lower rates of treatment failure and mortality with β-lactams.20,21 It is unknown whether β-lactams is superior for treating bacteremia with methicillin-sensitive S lugdunensis.
Our patient’s isolate of S lugdunensis was pansensitive to all antibiotics tested, including penicillin. These susceptibility data were used to guide the de-escalation of his empiric vancomycin and ceftriaxone to oxacillin on hospital day 1.
Due to their virulence, bloodstream infections caused by S aureus and S lugdunensis often require more than timely antimicrobial treatment to ensure eradication. Consultation with an infectious disease specialist to manage patients with S aureus bacteremia has been proven to reduce mortality.25 A similar mortality benefit is seen when infectious disease specialists are consulted for S lugdunensis bacteremia.26 This mortality benefit is likely explained by S lugdunensis’ propensity to cause aggressive, metastatic infections. In such cases, infectious disease consultants may recommend additional imaging (eg, transthoracic echocardiogram) to evaluate for occult sources of infection, advocate for appropriate source control, and guide the selection of an appropriate antibiotic course to ensure resolution of the bacteremia.
Conclusions
S lugdunensis is an increasingly recognized cause of nosocomial bloodstream infections. Given the commonalities in virulence that S lugdunensis shares with S aureus, treatment of bacteremia caused by either species should follow similar management principles: prompt initiation of IV antistaphylococcal therapy, a thorough evaluation for the source(s) of bacteremia as well as metastatic complications, and consultation with an infectious disease specialist. This case report also highlights the importance of considering a patient’s AVF as a potential source for infection even in the absence of localized signs of infection. The buttonhole method of AVF cannulation was thought to be a major contributor to the development and persistence of our patient’s bacteremia. This risk should be discussed with patients using a shared decision-making approach when developing a dialysis treatment plan.
1. Huebner J, Goldmann DA. Coagulase-negative staphylococci: role as pathogens. Annu Rev Med. 1999;50(1):223-236. doi:10.1146/annurev.med.50.1.223
2. Beekmann SE, Diekema DJ, Doern GV. Determining the clinical significance of coagulase-negative staphylococci isolated from blood cultures. Infect Control Hosp Epidemiol. 2005;26(6):559-566. doi:10.1086/502584
3. Arrecubieta C, Toba FA, von Bayern M, et al. SdrF, a Staphylococcus epidermidis surface protein, contributes to the initiation of ventricular assist device driveline–related infections. PLoS Pathog. 2009;5(5):e1000411. doi.10.1371/journal.ppat.1000411
4. Freney J, Brun Y, Bes M, et al. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38(2):168-172. doi:10.1099/00207713-38-2-168
5. Frank KL, del Pozo JL, Patel R. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev. 2008;21(1):111-133. doi:10.1128/CMR.00036-07
6. Anguera I, Del Río A, Miró JM; Hospital Clinic Endocarditis Study Group. Staphylococcus lugdunensis infective endocarditis: description of 10 cases and analysis of native valve, prosthetic valve, and pacemaker lead endocarditis clinical profiles. Heart. 2005;91(2):e10. doi:10.1136/hrt.2004.040659
7. Pareja J, Gupta K, Koziel H. The toxic shock syndrome and Staphylococcus lugdunensis bacteremia. Ann Intern Med. 1998;128(7):603-604. doi:10.7326/0003-4819-128-7-199804010-00029
8. Woznowski M, Quack I, Bölke E, et al. Fulminant Staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 201;15(9):410-414. doi:10.1186/2047-783x-15-9-410
9. Mallappallil M, Salifu M, Woredekal Y, et al. Staphylococcus lugdunensis bacteremia in hemodialysis patients. Int J Microbiol Res. 2012;4(2):178-181. doi:10.9735/0975-5276.4.2.178-181
10. Shuttleworth R, Colby W. Staphylococcus lugdunensis endocarditis. J Clin Microbiol. 1992;30(8):5. doi:10.1128/jcm.30.8.1948-1952.1992
11. Conner RC, Byrnes TJ, Clough LA, Myers JP. Staphylococcus lugdunensis tricuspid valve endocarditis associated with home hemodialysis therapy: report of a case and review of the literature. Infect Dis Clin Pract. 2012;20(3):182-183. doi:1097/IPC.0b013e318245d4f1
12. Kamaraju S, Nelson K, Williams D, Ayenew W, Modi K. Staphylococcus lugdunensis pulmonary valve endocarditis in a patient on chronic hemodialysis. Am J Nephrol. 1999;19(5):605-608. doi:1097/IPC.0b013e318245d4f1
13. Lok C, Sontrop J, Faratro R, Chan C, Zimmerman DL. Frequent hemodialysis fistula infectious complications. Nephron Extra. 2014;4(3):159-167. doi:10.1159/000366477
14. Hashmi A, Cheema MQ, Moss AH. Hemodialysis patients’ experience with and attitudes toward the buttonhole technique for arteriovenous fistula cannulation. Clin Nephrol. 2010;74(5):346-350. doi:10.5414/cnp74346
15. Lyman M, Nguyen DB, Shugart A, Gruhler H, Lines C, Patel PR. Risk of vascular access infection associated with buttonhole cannulation of fistulas: data from the National Healthcare Safety Network. Am J Kidney Dis. 2020;76(1):82-89. doi:10.1053/j.ajkd.2019.11.006
16. MacRae JM, Ahmed SB, Atkar R, Hemmelgarn BR. A randomized trial comparing buttonhole with rope ladder needling in conventional hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(10):1632-1638. doi:10.2215/CJN.02730312
17. Nesrallah GE, Cuerden M, Wong JHS, Pierratos A. Staphylococcus aureus bacteremia and buttonhole cannulation: long-term safety and efficacy of mupirocin prophylaxis. Clin J Am Soc Nephrol. 2010;5(6):1047-1053. doi:10.2215/CJN.00280110
18. Ho PL, Liu MCJ, Chow KH, et al. Emergence of ileS2 -carrying, multidrug-resistant plasmids in Staphylococcus lugdunensis. Antimicrob Agents Chemother. 2016;60(10):6411-6414. doi:10.1128/AAC.00948-16
19. Tan TY, Ng SY, He J. Microbiological characteristics, presumptive identification, and antibiotic susceptibilities of Staphylococcus lugdunensis. J Clin Microbiol. 2008;46(7):2393-2395. doi:10.1128/JCM.00740-08
20. Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore). 2003;82(5):333-339. doi:10.1097/01.md.0000091184.93122.09
21. Shurland S, Zhan M, Bradham DD, Roghmann MC. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):273-279. doi:10.1086/512627
22. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115(9):674. doi:10.7326/0003-4819-115-9-674
23. Fowler VG, Karchmer AW, Tally FP, et al; S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653-665 . doi:10.1056/NEJMoa053783
24. Duhon B, Dallas S, Velasquez ST, Hand E. Staphylococcus lugdunensis bacteremia and endocarditis treated with cefazolin and rifampin. Am J Health Syst Pharm. 2015;72(13):1114-1118. doi:10.2146/ajhp140498
25. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. doi:10.1097/MD.0b013e3181b8fccb
26. Forsblom E, Högnäs E, Syrjänen J, Järvinen A. Infectious diseases specialist consultation in Staphylococcus lugdunensis bacteremia. PLoS ONE. 2021;16(10):e0258511. doi:10.1371/journal.pone.0258511
1. Huebner J, Goldmann DA. Coagulase-negative staphylococci: role as pathogens. Annu Rev Med. 1999;50(1):223-236. doi:10.1146/annurev.med.50.1.223
2. Beekmann SE, Diekema DJ, Doern GV. Determining the clinical significance of coagulase-negative staphylococci isolated from blood cultures. Infect Control Hosp Epidemiol. 2005;26(6):559-566. doi:10.1086/502584
3. Arrecubieta C, Toba FA, von Bayern M, et al. SdrF, a Staphylococcus epidermidis surface protein, contributes to the initiation of ventricular assist device driveline–related infections. PLoS Pathog. 2009;5(5):e1000411. doi.10.1371/journal.ppat.1000411
4. Freney J, Brun Y, Bes M, et al. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38(2):168-172. doi:10.1099/00207713-38-2-168
5. Frank KL, del Pozo JL, Patel R. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev. 2008;21(1):111-133. doi:10.1128/CMR.00036-07
6. Anguera I, Del Río A, Miró JM; Hospital Clinic Endocarditis Study Group. Staphylococcus lugdunensis infective endocarditis: description of 10 cases and analysis of native valve, prosthetic valve, and pacemaker lead endocarditis clinical profiles. Heart. 2005;91(2):e10. doi:10.1136/hrt.2004.040659
7. Pareja J, Gupta K, Koziel H. The toxic shock syndrome and Staphylococcus lugdunensis bacteremia. Ann Intern Med. 1998;128(7):603-604. doi:10.7326/0003-4819-128-7-199804010-00029
8. Woznowski M, Quack I, Bölke E, et al. Fulminant Staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 201;15(9):410-414. doi:10.1186/2047-783x-15-9-410
9. Mallappallil M, Salifu M, Woredekal Y, et al. Staphylococcus lugdunensis bacteremia in hemodialysis patients. Int J Microbiol Res. 2012;4(2):178-181. doi:10.9735/0975-5276.4.2.178-181
10. Shuttleworth R, Colby W. Staphylococcus lugdunensis endocarditis. J Clin Microbiol. 1992;30(8):5. doi:10.1128/jcm.30.8.1948-1952.1992
11. Conner RC, Byrnes TJ, Clough LA, Myers JP. Staphylococcus lugdunensis tricuspid valve endocarditis associated with home hemodialysis therapy: report of a case and review of the literature. Infect Dis Clin Pract. 2012;20(3):182-183. doi:1097/IPC.0b013e318245d4f1
12. Kamaraju S, Nelson K, Williams D, Ayenew W, Modi K. Staphylococcus lugdunensis pulmonary valve endocarditis in a patient on chronic hemodialysis. Am J Nephrol. 1999;19(5):605-608. doi:1097/IPC.0b013e318245d4f1
13. Lok C, Sontrop J, Faratro R, Chan C, Zimmerman DL. Frequent hemodialysis fistula infectious complications. Nephron Extra. 2014;4(3):159-167. doi:10.1159/000366477
14. Hashmi A, Cheema MQ, Moss AH. Hemodialysis patients’ experience with and attitudes toward the buttonhole technique for arteriovenous fistula cannulation. Clin Nephrol. 2010;74(5):346-350. doi:10.5414/cnp74346
15. Lyman M, Nguyen DB, Shugart A, Gruhler H, Lines C, Patel PR. Risk of vascular access infection associated with buttonhole cannulation of fistulas: data from the National Healthcare Safety Network. Am J Kidney Dis. 2020;76(1):82-89. doi:10.1053/j.ajkd.2019.11.006
16. MacRae JM, Ahmed SB, Atkar R, Hemmelgarn BR. A randomized trial comparing buttonhole with rope ladder needling in conventional hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(10):1632-1638. doi:10.2215/CJN.02730312
17. Nesrallah GE, Cuerden M, Wong JHS, Pierratos A. Staphylococcus aureus bacteremia and buttonhole cannulation: long-term safety and efficacy of mupirocin prophylaxis. Clin J Am Soc Nephrol. 2010;5(6):1047-1053. doi:10.2215/CJN.00280110
18. Ho PL, Liu MCJ, Chow KH, et al. Emergence of ileS2 -carrying, multidrug-resistant plasmids in Staphylococcus lugdunensis. Antimicrob Agents Chemother. 2016;60(10):6411-6414. doi:10.1128/AAC.00948-16
19. Tan TY, Ng SY, He J. Microbiological characteristics, presumptive identification, and antibiotic susceptibilities of Staphylococcus lugdunensis. J Clin Microbiol. 2008;46(7):2393-2395. doi:10.1128/JCM.00740-08
20. Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore). 2003;82(5):333-339. doi:10.1097/01.md.0000091184.93122.09
21. Shurland S, Zhan M, Bradham DD, Roghmann MC. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):273-279. doi:10.1086/512627
22. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115(9):674. doi:10.7326/0003-4819-115-9-674
23. Fowler VG, Karchmer AW, Tally FP, et al; S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653-665 . doi:10.1056/NEJMoa053783
24. Duhon B, Dallas S, Velasquez ST, Hand E. Staphylococcus lugdunensis bacteremia and endocarditis treated with cefazolin and rifampin. Am J Health Syst Pharm. 2015;72(13):1114-1118. doi:10.2146/ajhp140498
25. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. doi:10.1097/MD.0b013e3181b8fccb
26. Forsblom E, Högnäs E, Syrjänen J, Järvinen A. Infectious diseases specialist consultation in Staphylococcus lugdunensis bacteremia. PLoS ONE. 2021;16(10):e0258511. doi:10.1371/journal.pone.0258511