User login
Ultrasound has classically been marketed and used as a diagnostic tool. Radiologists, emergency physicians, and sports physicians used ultrasound units to rapidly and appropriately diagnose numerous injuries and disorders, in a timely and cost effective manner. Part 11 and Part 22 of this series showed how to use ultrasound in the shoulder for diagnosis and how to code and get reimbursed for its use.Ultrasound can also be used to help guide procedures and interventions performed to treat patients. Currently, more physicians are beginning to recognize the utility of this modality as an aid to interventional procedures.
First-generation procedures use ultrasound to improve accuracy of joint, bursal, tendon, and muscular injections.3 Recent studies have shown a significant improvement in accuracy, outcomes, and patient satisfaction using ultrasound guidance for injections.3-12 Within the limitation of using a needle, second-generation procedures—hydrodissection of peripherally entrapped nerves, capsular distention, mechanical disruption of neovascularization, and needle fenestration or barbotage in chronic tendinopathy—try to simulate surgical objectives while minimizing tissue burden and other complications of surgery.3 More advanced procedures include needle fenestration/release of the carpal ligament in carpal tunnel syndrome and A1 pulley needle release in the setting of trigger finger.3 Innovative third-generation procedures involve the use of surgical tools such as hook blades under ultrasound guidance to perform surgical procedures. Surgeons are now improving already established percutaneous, arthroscopic, and open surgical procedures with ultrasound assistance.3 Aside from better guidance, reducing cost and improving surgeon comfort may be additional benefits of ultrasound assisted surgery.
Image-Guided Treatment Options
Prior to image guidance, palpation of surface anatomy helped physicians determine the anatomic placement of injections, incisions, or portals. Joints and bursas that do not have any inflammation or fluid can sometimes be difficult to identify or locate by palpation alone. Palpation-guided joint injections often miss their target and cause significant pain when the therapeutic agent is injected into a muscle, tendon, ligament, fat, or other tissue. Ultrasound-guided injections have proven to be more accurate and have better patient satisfaction when compared to blind injections.3-12
X-ray fluoroscopy has been the primary option for surgeons to assist in surgery. This is a natural modality for orthopedic surgeons; their primary use is for bone to help with fracture reduction and fixation as the bone, instrumentation, and fixation methods are usually radio-opaque. With the advancement in technology, many orthopedic surgeons are regularly using radiolucent fixation devices and working with soft tissue as opposed to bone. Fixation of tendons, ligaments, and muscles would be done using a large incision, palpation of the anatomy, then fixation or repair. Many surgeons began looking for ways to minimize the incisions. Turning to fluoroscopy, a traditional and well-used modality, was a natural progression. Guides and methods were developed to isolate insertions and drill placements. However, fluoroscopy is limited by its difficulty in changing planes and the large equipment required. Also, it is limited in its ability to image soft tissue.
Computed tomography (CT) scans and magnetic resonance imaging (MRI) are far better at imaging soft tissue but cannot be taken for use into the office or surgical suite. These modalities are also far more expensive and take up significant space.
Ultrasound Procedural Basics
Appropriate use of ultrasound still remains highly technician-dependent. Unlike other imaging modalities, ultrasound requires a higher skill level by the physician to implement the use of ultrasound and identification of pathology to treat these disease processes. However, this is no different from the use of arthroscopy or fluoroscopy to treat patients. Training is required, as well as an understanding of the ultrasound machine, anatomy, and sono-anatomy—identification of anatomy and pathology as shown by the ultrasound machine.2
In ultrasound, the long axis refers to looking at a structure along its length, as in longitudinal. The short axis refers to evaluating a structure in cross-section, transverse, or along its shortest length. “In plane” refers to performing a procedure where the needle or object being used enters the ultrasound field along the plane of the transducer, allowing visualization of the majority of the needle as it crosses tissue planes. “Out of plane” has the needle entering perpendicular to the plane of the transducer, showing the needle on the monitor as a bright, hyperechoic dot. Some studies have suggested that novice ultrasonographers should start in a long axis view and use the in plane technique when injecting, as doing so may decrease time to identify the target and improve mean imaging quality during needle advancement.13
Anisotropy is the property of being directionally dependent. The ultrasound beam needs to be perpendicular to the structure being imaged to give the optimal image. When the beam hits a longitudinal structure like a needle at an angle <90°, the linear structure might reflect most of the beam away from the transducer. So when using a needle to localize or inject a specific area, maintaining the probe as close to perpendicular as possible with the needle will give a better image. New technology exists to better visualize needles even at high acuity angles by using a multi-beam processing algorithm, which can significantly aid the physician without the need for specialized needles.
Despite better technology, advance planning is key to a successful procedure. Positioning the patient and ultrasound machine in a manner that is comfortable and makes the desired target accessible while being able to visualize the ultrasound monitor comes first. Identifying the target, mapping the needle trajectory using depth markings, and scanning for nerves, vessels, and other structures that may be damaged along the needle path comes next. Using the in plane ultrasound technique with color Doppler and the nerve contrast setting can ensure that the physician has placed the therapeutic agent to the proper location while avoiding any nerves, arteries, or veins. Marking the borders of the ultrasound probe and needle entry site can be helpful to return to the same area after sterile preparation is done. As in any procedure, sterile technique is paramount. Sterile technique considerations may include using sterile gloves and a probe cover with sterile gel, cleaning the area thoroughly, planning the needle entry point 3 cm to 5 cm away from the probe, and maintaining a dry and gel-free needle entry.14-15 The probe should be sterilized between patients to avoid cross-contamination; note that certain solutions like alcohol or ethyl chloride can damage the transducer.14-15 However, simple injections do not require such stringent standards when simple sterile technique is observed by cleaning and then never touching the cleaned area again except with the needle to avoid contamination. Also, ethyl chloride has been found to not contaminate a sterile site and can be used safely to anesthetize the skin.
Ultrasound-Guided Procedures
Many injectable therapeutic options exist as interventions. Cortisone, hyaluronic acid, platelet-rich plasma (PRP), stem cells/bone marrow concentrate (BMC), amniotic fluid, prolotherapy, and saline are now commonly used.16-17 A meta-analysis of the literature assessing the accuracy of ultrasound-guided shoulder girdle injections vs a landmark-guided injection was done in 2015.18 It showed that for the acromioclavicular joint, accuracy was 93.6% vs 68.2% (P < .0001), based on single studies. The accuracy of ultrasound vs a landmark-guided injection was 65% vs 70% for the subacromial space (P > .05); 86.7% vs 26.7% for the biceps tendon sheath (P < .05); and 92.5% vs 72.5% for the glenohumeral joint (P = .025).18
With cortisone, injecting into muscle, ligament, or tendons could potentially harm the tissue or cause worsening of the disease process.19-20 With the advent of orthobiologics, injecting into these structures is now desirable, instead of a potential complication.19-20 Ultrasound has become even more important to the accurate delivery of these therapies to the disease locations. Multiple studies using leukocyte-poor PRP for osteoarthritis show significant differences in pain scores.21-23 Peerbooms and colleagues24,25 also showed that PRP reduced pain and increased function compared to cortisone injections for lateral epicondylitis in 1- and 2-year double-blind randomized controlled trials. Centeno and colleagues26 performed a prospective, multi-site registry study on 102 patients with symptomatic osteoarthritis and/or rotator cuff tears that were injected with bone marrow concentrate. There was a statistically significant improvement in Disabilities of the Arm, Shoulder and Hand (DASH) scores from 36.1 to 17.1 (P < .001) and numeric pain scores improved from 4.3 to 2.4 (P < .001).
By being able to see the pathology, like a hypoechoic region in a tendon, ligament, or muscle, the physician can reliably place the therapeutic agent into the precise location. Also, adjacent para-tendon or para-ligament injections allow for in-season athletes to get some relief from symptoms while allowing to return to play quickly; injections into muscle, ligament, or tendon can damage the structure and require days or weeks of rest, while para-tendon and para-ligament injections are far less painful.
Second-generation techniques have provided patients with great options that can help avoid surgery. Calcific tendonitis appears brightly hyperechoic on ultrasound and is easily identified. The physician can attempt to break up the calcium by fenestration or barbotage of the calcium. The same can be accomplished by injecting the density with PRP or stem cells. If the calcium is soft or “toothpaste-like,” the negative pressure will make it easy to aspirate it into the syringe. A 2-year, longitudinal prospective study of 121 patients demonstrated that visual analog score (VAS) pain scores and size of calcium significantly decreased with ultrasound-guided percutaneous needle lavage; 89% of patients were pain-free at 1-year follow-up.27 Moreover, a randomized controlled trial of 48 patients comparing needle lavage vs subacromial steroid injection showed statistically significant radiographic and clinically better outcomes with the needle lavage group at the 1-year mark.28
The Tenex procedure is a novel technique that uses ultrasonic energy to fenestrate diseased tendon tissue. It also can be used to break up calcific deposits. After the Tenex probe is guided to the diseased tendon/calcium, the TX-1 tip oscillates at the speed of sound, fenestrating/cutting through the tendon or calcium while lavaging the tendon with saline. Multiple prospective, noncontrolled studies done in common extensor, patellar, and rotator cuff tendinopathy have demonstrated good to excellent improvements in pain scores with the Tenex procedure.29-31
Ultrasound is extremely useful in the treatment of adhesive capsulitis.32 The posterior glenohumeral capsule can be distended using a large volume (60 cc) of saline to loosen adhesions in preparation for manipulation. Because the manipulation can be an extremely painful procedure, ultrasound can be used to perform an inter-scalene block for regional anesthesia prior to the procedure. In 2014, Park and colleagues33 performed a randomized prospective trial that showed that capsular distension followed by manipulation was more effective than cortisone injection alone for the treatment of adhesive capsulitis.Ultrasound guidance was found to be just as efficacious as fluoroscopy in a randomized controlled trial in 2014; the authors noted that ultrasound does not expose the patient or clinician to radiation and can be done in office.34
Currently, techniques to perform ultrasound-guided percutaneous tenotomies of the long head of the biceps tendon using hook blades are being studied.35
Ultrasound-Assisted Surgery
Ultrasound has been a boon to surgeons who perform minimally invasive procedures. It is far less cumbersome than classic fluoroscopy. Fluoroscopy requires the use of heavy lead aprons by the surgeons. Combining this with the impervious gowns and hot lights, the surgeons’ comfort level is severely sacrificed. When having to do many long surgeries in a row, this situation can take a toll on the surgeons’ endurance and strength. Improving the comfort of the surgeon is not the primary goal of surgery, but can significantly help our ability to do a better job.
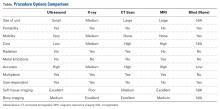
Ultrasound allows the surgeon to localize any superficial foreign objects, especially with radiolucent objects like fragments of glass. Small glass fragments or pieces of wood have always been extremely difficult to remove. X-rays cannot localize these objects, so getting a proper orientation is difficult. MRI and CT scans easily identify these types of foreign objects, but cannot be used intraoperatively (Figure 1A). Often, these objects cannot be felt and therefore require a large dissection. The objects may encapsulate and be easily confused with other soft tissues.
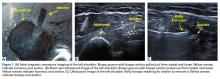
By using the ultrasound intraoperatively, the surgeon can identify the exact position of the biceps tendon (medial/lateral) and where it lies just below the groove and above the pectoralis major (superior/inferior) (Figure 2A).
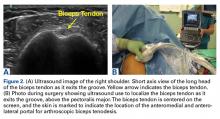
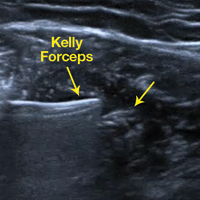
Reconstruction of ligaments is another ideal use of ultrasound. Surface anatomy cannot always tell the exact location of a ligament or tendon insertion. The best example of this is the anterolateral ligament (ALL). Identification of the lateral epicondyle of the femur and anatomic insertion of the ALL can be difficult in some patients. Ultrasound can be used to identify the origin and insertion of the ALL during surgery under sterile conditions (see page 418). A spinal needle can be placed under direct vision with an in-plane ultrasound guidance over the bony insertion (Figure 3A). A percutaneous incision is made.
This technique is also used by the senior author (AMH) to repair, reconstruct, or internally brace the medial collateral ligament, medial patellofemoral ligament, and lateral collateral ligament. This technique is ideally suited to superficial ligament and tendon reattachment, reconstruction, or internal bracing. The knee, ankle, and elbow superficial ligaments are especially amenable to this easy, percutaneous technique.
Conclusion
Ultrasound is quickly becoming a popular imaging modality due to its simplicity, portability, and cost efficiency. Its use as a diagnostic tool is widely known. As an adjunct for procedures and interventions, its advantages over larger, more expensive modalities such as fluoroscopy, CT, or MRI make it stand out. Ultrasound is not the perfect solution to all problems, but it is clearly a technology that is gaining traction. Ultrasound is another imaging modality and tool that physicians and surgeons can use to improve their patients’ treatment.
1. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 1: coding and reimbursement. Am J Orthop. 2016;45(3):176-182.
2. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016; 45(4):233-238.
3. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: Interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
4. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
5. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
6. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
7. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
8. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
9. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
10. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
11. Sethi P, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: A cadaveric study. Orthopedics. 2006;29(2):149-152.
12. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
13. Speer M, McLennan N, Nixon C. Novice learner in-plane ultrasound imaging: which visualization technique? Reg Anesth Pain Med. 2013;38(4):350-352.
14. Marhofer P, Schebesta K, Marhofer D. [Hygiene aspects in ultrasound-guided regional anesthesia]. Anaesthesist. 2016;65(7):492-498.
15. Sherman T, Ferguson J, Davis W, Russo M, Argintar E. Does the use of ultrasound affect contamination of musculoskeletal injection sites? Clin Orthop Relat Res. 2015;473(1):351-357.
16. Bashir J, Panero AJ, Sherman AL. The emerging use of platelet-rich plasma in musculoskeletal medicine. J Am Osteopath Assoc. 2015;115(1):23-31.
17. Royall NA, Farrin E, Bahner DP, Stanislaw PA. Ultrasound-assisted musculoskeletal procedures: A practical overview of current literature. World J Orthop. 2011;2(7):57-66.
18. Aly AR, Rajasekaran S, Ashworth N. Ultrasound-guided shoulder girdle injections are more accurate and more effective than landmark-guided injections: a systematic review and meta-analysis. Br J Sports Med. 2015;49(16):1042-1049.
19. Maman E, Yehuda C, Pritsch T, et al. Detrimental effect of repeated and single subacromial corticosteroid injections on the intact and injured rotator cuff: A biomechanical and imaging study in rats. Am J Sports Med. 2016;44(1):177-182.
20. Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015;23(1):1-5.
21. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-364.
22. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822-2827.
23. Spakova T, Rosocha J, Lacko M, Harvanova D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91(5):411-417.
24. Peerbooms JC, Sluimer J, Brujin DJ, Gosens T. Positive effects of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255-262.
25. Gosens T, Peerbooms JC, van Laar W, den Oudsten BL. Ongoing positive effects of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with a 2-year follow-up. Am J Sports Med. 2011;39(6):1200-1208.
26. Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. J Pain Res. 2015;8:269-276.
27. Del Castillo-Gonzalez F, Ramos-Alvarez JJ, Rodriguez-Fabian G, Gonzalez-Perez J, Calderon-Montero J. Treatment of the calcific tendinopathy of the rotator cuff by ultrasound-guided percutaneous needle lavage. Two years prospective study. Muscles Ligaments Tendons J. 2015;4(4):407-412.
28. De Witte PB, Selten JW, Navas A, et al. Calcific tendinitis of the rotator cuff: a randomized controlled trial of ultrasound-guided needling and lavage versus subacromial corticosteroids. Am J Sports Med. 2013;41(7):1665-1673.
29. Koh J, Mohan P, Morrey B, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
30. Elattrache N, Morrey B. Percutaneous ultrasonic tenotomy as a treatment for chronic patellar tendinopathy–Jumper’s knee. Oper Tech Orthop. 2013;23(2):98-103
31. Patel MM. A novel treatment for refractory plantar fasciitis. Am J Orthop. 2015;444(3):107-110.
32. Harris G, Bou-Haidar P, Harris C. Adhesive capsulitis: Review of imaging and treatment. J Med Imaging Radiat Oncol. 2013;57:633-643.
33. Park SW, Lee HS, Kim JH. The effectiveness of intensive mobilization techniques combined with capsular distention for adhesive capsulitis of the shoulder. J Phys Ther Sci. 2014;26(11):1776-1770.
34. Bae JH, Park YS, Chang HJ, et al. Randomized controlled trial for efficacy of capsular distension for adhesive capsulitis: Fluoroscopy-guided anterior versus ultrasonography-guided posterolateral approach. Ann Rehabil Med. 2014;38(3):360-368.
35. Aly AR, Rajasekaran S, Mohamed A, Beavis C, Obaid H. Feasibility of ultrasound-guided percutaneous tenotomy of long head of the biceps tendon–A pilot cadaveric study. J Clin Ultrasound. 2015;43(6):361-366.
Ultrasound has classically been marketed and used as a diagnostic tool. Radiologists, emergency physicians, and sports physicians used ultrasound units to rapidly and appropriately diagnose numerous injuries and disorders, in a timely and cost effective manner. Part 11 and Part 22 of this series showed how to use ultrasound in the shoulder for diagnosis and how to code and get reimbursed for its use.Ultrasound can also be used to help guide procedures and interventions performed to treat patients. Currently, more physicians are beginning to recognize the utility of this modality as an aid to interventional procedures.
First-generation procedures use ultrasound to improve accuracy of joint, bursal, tendon, and muscular injections.3 Recent studies have shown a significant improvement in accuracy, outcomes, and patient satisfaction using ultrasound guidance for injections.3-12 Within the limitation of using a needle, second-generation procedures—hydrodissection of peripherally entrapped nerves, capsular distention, mechanical disruption of neovascularization, and needle fenestration or barbotage in chronic tendinopathy—try to simulate surgical objectives while minimizing tissue burden and other complications of surgery.3 More advanced procedures include needle fenestration/release of the carpal ligament in carpal tunnel syndrome and A1 pulley needle release in the setting of trigger finger.3 Innovative third-generation procedures involve the use of surgical tools such as hook blades under ultrasound guidance to perform surgical procedures. Surgeons are now improving already established percutaneous, arthroscopic, and open surgical procedures with ultrasound assistance.3 Aside from better guidance, reducing cost and improving surgeon comfort may be additional benefits of ultrasound assisted surgery.
Image-Guided Treatment Options
Prior to image guidance, palpation of surface anatomy helped physicians determine the anatomic placement of injections, incisions, or portals. Joints and bursas that do not have any inflammation or fluid can sometimes be difficult to identify or locate by palpation alone. Palpation-guided joint injections often miss their target and cause significant pain when the therapeutic agent is injected into a muscle, tendon, ligament, fat, or other tissue. Ultrasound-guided injections have proven to be more accurate and have better patient satisfaction when compared to blind injections.3-12
X-ray fluoroscopy has been the primary option for surgeons to assist in surgery. This is a natural modality for orthopedic surgeons; their primary use is for bone to help with fracture reduction and fixation as the bone, instrumentation, and fixation methods are usually radio-opaque. With the advancement in technology, many orthopedic surgeons are regularly using radiolucent fixation devices and working with soft tissue as opposed to bone. Fixation of tendons, ligaments, and muscles would be done using a large incision, palpation of the anatomy, then fixation or repair. Many surgeons began looking for ways to minimize the incisions. Turning to fluoroscopy, a traditional and well-used modality, was a natural progression. Guides and methods were developed to isolate insertions and drill placements. However, fluoroscopy is limited by its difficulty in changing planes and the large equipment required. Also, it is limited in its ability to image soft tissue.
Computed tomography (CT) scans and magnetic resonance imaging (MRI) are far better at imaging soft tissue but cannot be taken for use into the office or surgical suite. These modalities are also far more expensive and take up significant space.
Ultrasound Procedural Basics
Appropriate use of ultrasound still remains highly technician-dependent. Unlike other imaging modalities, ultrasound requires a higher skill level by the physician to implement the use of ultrasound and identification of pathology to treat these disease processes. However, this is no different from the use of arthroscopy or fluoroscopy to treat patients. Training is required, as well as an understanding of the ultrasound machine, anatomy, and sono-anatomy—identification of anatomy and pathology as shown by the ultrasound machine.2
In ultrasound, the long axis refers to looking at a structure along its length, as in longitudinal. The short axis refers to evaluating a structure in cross-section, transverse, or along its shortest length. “In plane” refers to performing a procedure where the needle or object being used enters the ultrasound field along the plane of the transducer, allowing visualization of the majority of the needle as it crosses tissue planes. “Out of plane” has the needle entering perpendicular to the plane of the transducer, showing the needle on the monitor as a bright, hyperechoic dot. Some studies have suggested that novice ultrasonographers should start in a long axis view and use the in plane technique when injecting, as doing so may decrease time to identify the target and improve mean imaging quality during needle advancement.13
Anisotropy is the property of being directionally dependent. The ultrasound beam needs to be perpendicular to the structure being imaged to give the optimal image. When the beam hits a longitudinal structure like a needle at an angle <90°, the linear structure might reflect most of the beam away from the transducer. So when using a needle to localize or inject a specific area, maintaining the probe as close to perpendicular as possible with the needle will give a better image. New technology exists to better visualize needles even at high acuity angles by using a multi-beam processing algorithm, which can significantly aid the physician without the need for specialized needles.
Despite better technology, advance planning is key to a successful procedure. Positioning the patient and ultrasound machine in a manner that is comfortable and makes the desired target accessible while being able to visualize the ultrasound monitor comes first. Identifying the target, mapping the needle trajectory using depth markings, and scanning for nerves, vessels, and other structures that may be damaged along the needle path comes next. Using the in plane ultrasound technique with color Doppler and the nerve contrast setting can ensure that the physician has placed the therapeutic agent to the proper location while avoiding any nerves, arteries, or veins. Marking the borders of the ultrasound probe and needle entry site can be helpful to return to the same area after sterile preparation is done. As in any procedure, sterile technique is paramount. Sterile technique considerations may include using sterile gloves and a probe cover with sterile gel, cleaning the area thoroughly, planning the needle entry point 3 cm to 5 cm away from the probe, and maintaining a dry and gel-free needle entry.14-15 The probe should be sterilized between patients to avoid cross-contamination; note that certain solutions like alcohol or ethyl chloride can damage the transducer.14-15 However, simple injections do not require such stringent standards when simple sterile technique is observed by cleaning and then never touching the cleaned area again except with the needle to avoid contamination. Also, ethyl chloride has been found to not contaminate a sterile site and can be used safely to anesthetize the skin.
Ultrasound-Guided Procedures
Many injectable therapeutic options exist as interventions. Cortisone, hyaluronic acid, platelet-rich plasma (PRP), stem cells/bone marrow concentrate (BMC), amniotic fluid, prolotherapy, and saline are now commonly used.16-17 A meta-analysis of the literature assessing the accuracy of ultrasound-guided shoulder girdle injections vs a landmark-guided injection was done in 2015.18 It showed that for the acromioclavicular joint, accuracy was 93.6% vs 68.2% (P < .0001), based on single studies. The accuracy of ultrasound vs a landmark-guided injection was 65% vs 70% for the subacromial space (P > .05); 86.7% vs 26.7% for the biceps tendon sheath (P < .05); and 92.5% vs 72.5% for the glenohumeral joint (P = .025).18
With cortisone, injecting into muscle, ligament, or tendons could potentially harm the tissue or cause worsening of the disease process.19-20 With the advent of orthobiologics, injecting into these structures is now desirable, instead of a potential complication.19-20 Ultrasound has become even more important to the accurate delivery of these therapies to the disease locations. Multiple studies using leukocyte-poor PRP for osteoarthritis show significant differences in pain scores.21-23 Peerbooms and colleagues24,25 also showed that PRP reduced pain and increased function compared to cortisone injections for lateral epicondylitis in 1- and 2-year double-blind randomized controlled trials. Centeno and colleagues26 performed a prospective, multi-site registry study on 102 patients with symptomatic osteoarthritis and/or rotator cuff tears that were injected with bone marrow concentrate. There was a statistically significant improvement in Disabilities of the Arm, Shoulder and Hand (DASH) scores from 36.1 to 17.1 (P < .001) and numeric pain scores improved from 4.3 to 2.4 (P < .001).
By being able to see the pathology, like a hypoechoic region in a tendon, ligament, or muscle, the physician can reliably place the therapeutic agent into the precise location. Also, adjacent para-tendon or para-ligament injections allow for in-season athletes to get some relief from symptoms while allowing to return to play quickly; injections into muscle, ligament, or tendon can damage the structure and require days or weeks of rest, while para-tendon and para-ligament injections are far less painful.
Second-generation techniques have provided patients with great options that can help avoid surgery. Calcific tendonitis appears brightly hyperechoic on ultrasound and is easily identified. The physician can attempt to break up the calcium by fenestration or barbotage of the calcium. The same can be accomplished by injecting the density with PRP or stem cells. If the calcium is soft or “toothpaste-like,” the negative pressure will make it easy to aspirate it into the syringe. A 2-year, longitudinal prospective study of 121 patients demonstrated that visual analog score (VAS) pain scores and size of calcium significantly decreased with ultrasound-guided percutaneous needle lavage; 89% of patients were pain-free at 1-year follow-up.27 Moreover, a randomized controlled trial of 48 patients comparing needle lavage vs subacromial steroid injection showed statistically significant radiographic and clinically better outcomes with the needle lavage group at the 1-year mark.28
The Tenex procedure is a novel technique that uses ultrasonic energy to fenestrate diseased tendon tissue. It also can be used to break up calcific deposits. After the Tenex probe is guided to the diseased tendon/calcium, the TX-1 tip oscillates at the speed of sound, fenestrating/cutting through the tendon or calcium while lavaging the tendon with saline. Multiple prospective, noncontrolled studies done in common extensor, patellar, and rotator cuff tendinopathy have demonstrated good to excellent improvements in pain scores with the Tenex procedure.29-31
Ultrasound is extremely useful in the treatment of adhesive capsulitis.32 The posterior glenohumeral capsule can be distended using a large volume (60 cc) of saline to loosen adhesions in preparation for manipulation. Because the manipulation can be an extremely painful procedure, ultrasound can be used to perform an inter-scalene block for regional anesthesia prior to the procedure. In 2014, Park and colleagues33 performed a randomized prospective trial that showed that capsular distension followed by manipulation was more effective than cortisone injection alone for the treatment of adhesive capsulitis.Ultrasound guidance was found to be just as efficacious as fluoroscopy in a randomized controlled trial in 2014; the authors noted that ultrasound does not expose the patient or clinician to radiation and can be done in office.34
Currently, techniques to perform ultrasound-guided percutaneous tenotomies of the long head of the biceps tendon using hook blades are being studied.35
Ultrasound-Assisted Surgery
Ultrasound has been a boon to surgeons who perform minimally invasive procedures. It is far less cumbersome than classic fluoroscopy. Fluoroscopy requires the use of heavy lead aprons by the surgeons. Combining this with the impervious gowns and hot lights, the surgeons’ comfort level is severely sacrificed. When having to do many long surgeries in a row, this situation can take a toll on the surgeons’ endurance and strength. Improving the comfort of the surgeon is not the primary goal of surgery, but can significantly help our ability to do a better job.
Ultrasound allows the surgeon to localize any superficial foreign objects, especially with radiolucent objects like fragments of glass. Small glass fragments or pieces of wood have always been extremely difficult to remove. X-rays cannot localize these objects, so getting a proper orientation is difficult. MRI and CT scans easily identify these types of foreign objects, but cannot be used intraoperatively (Figure 1A). Often, these objects cannot be felt and therefore require a large dissection. The objects may encapsulate and be easily confused with other soft tissues.
By using the ultrasound intraoperatively, the surgeon can identify the exact position of the biceps tendon (medial/lateral) and where it lies just below the groove and above the pectoralis major (superior/inferior) (Figure 2A).
Reconstruction of ligaments is another ideal use of ultrasound. Surface anatomy cannot always tell the exact location of a ligament or tendon insertion. The best example of this is the anterolateral ligament (ALL). Identification of the lateral epicondyle of the femur and anatomic insertion of the ALL can be difficult in some patients. Ultrasound can be used to identify the origin and insertion of the ALL during surgery under sterile conditions (see page 418). A spinal needle can be placed under direct vision with an in-plane ultrasound guidance over the bony insertion (Figure 3A). A percutaneous incision is made.
This technique is also used by the senior author (AMH) to repair, reconstruct, or internally brace the medial collateral ligament, medial patellofemoral ligament, and lateral collateral ligament. This technique is ideally suited to superficial ligament and tendon reattachment, reconstruction, or internal bracing. The knee, ankle, and elbow superficial ligaments are especially amenable to this easy, percutaneous technique.
Conclusion
Ultrasound is quickly becoming a popular imaging modality due to its simplicity, portability, and cost efficiency. Its use as a diagnostic tool is widely known. As an adjunct for procedures and interventions, its advantages over larger, more expensive modalities such as fluoroscopy, CT, or MRI make it stand out. Ultrasound is not the perfect solution to all problems, but it is clearly a technology that is gaining traction. Ultrasound is another imaging modality and tool that physicians and surgeons can use to improve their patients’ treatment.
Ultrasound has classically been marketed and used as a diagnostic tool. Radiologists, emergency physicians, and sports physicians used ultrasound units to rapidly and appropriately diagnose numerous injuries and disorders, in a timely and cost effective manner. Part 11 and Part 22 of this series showed how to use ultrasound in the shoulder for diagnosis and how to code and get reimbursed for its use.Ultrasound can also be used to help guide procedures and interventions performed to treat patients. Currently, more physicians are beginning to recognize the utility of this modality as an aid to interventional procedures.
First-generation procedures use ultrasound to improve accuracy of joint, bursal, tendon, and muscular injections.3 Recent studies have shown a significant improvement in accuracy, outcomes, and patient satisfaction using ultrasound guidance for injections.3-12 Within the limitation of using a needle, second-generation procedures—hydrodissection of peripherally entrapped nerves, capsular distention, mechanical disruption of neovascularization, and needle fenestration or barbotage in chronic tendinopathy—try to simulate surgical objectives while minimizing tissue burden and other complications of surgery.3 More advanced procedures include needle fenestration/release of the carpal ligament in carpal tunnel syndrome and A1 pulley needle release in the setting of trigger finger.3 Innovative third-generation procedures involve the use of surgical tools such as hook blades under ultrasound guidance to perform surgical procedures. Surgeons are now improving already established percutaneous, arthroscopic, and open surgical procedures with ultrasound assistance.3 Aside from better guidance, reducing cost and improving surgeon comfort may be additional benefits of ultrasound assisted surgery.
Image-Guided Treatment Options
Prior to image guidance, palpation of surface anatomy helped physicians determine the anatomic placement of injections, incisions, or portals. Joints and bursas that do not have any inflammation or fluid can sometimes be difficult to identify or locate by palpation alone. Palpation-guided joint injections often miss their target and cause significant pain when the therapeutic agent is injected into a muscle, tendon, ligament, fat, or other tissue. Ultrasound-guided injections have proven to be more accurate and have better patient satisfaction when compared to blind injections.3-12
X-ray fluoroscopy has been the primary option for surgeons to assist in surgery. This is a natural modality for orthopedic surgeons; their primary use is for bone to help with fracture reduction and fixation as the bone, instrumentation, and fixation methods are usually radio-opaque. With the advancement in technology, many orthopedic surgeons are regularly using radiolucent fixation devices and working with soft tissue as opposed to bone. Fixation of tendons, ligaments, and muscles would be done using a large incision, palpation of the anatomy, then fixation or repair. Many surgeons began looking for ways to minimize the incisions. Turning to fluoroscopy, a traditional and well-used modality, was a natural progression. Guides and methods were developed to isolate insertions and drill placements. However, fluoroscopy is limited by its difficulty in changing planes and the large equipment required. Also, it is limited in its ability to image soft tissue.
Computed tomography (CT) scans and magnetic resonance imaging (MRI) are far better at imaging soft tissue but cannot be taken for use into the office or surgical suite. These modalities are also far more expensive and take up significant space.
Ultrasound Procedural Basics
Appropriate use of ultrasound still remains highly technician-dependent. Unlike other imaging modalities, ultrasound requires a higher skill level by the physician to implement the use of ultrasound and identification of pathology to treat these disease processes. However, this is no different from the use of arthroscopy or fluoroscopy to treat patients. Training is required, as well as an understanding of the ultrasound machine, anatomy, and sono-anatomy—identification of anatomy and pathology as shown by the ultrasound machine.2
In ultrasound, the long axis refers to looking at a structure along its length, as in longitudinal. The short axis refers to evaluating a structure in cross-section, transverse, or along its shortest length. “In plane” refers to performing a procedure where the needle or object being used enters the ultrasound field along the plane of the transducer, allowing visualization of the majority of the needle as it crosses tissue planes. “Out of plane” has the needle entering perpendicular to the plane of the transducer, showing the needle on the monitor as a bright, hyperechoic dot. Some studies have suggested that novice ultrasonographers should start in a long axis view and use the in plane technique when injecting, as doing so may decrease time to identify the target and improve mean imaging quality during needle advancement.13
Anisotropy is the property of being directionally dependent. The ultrasound beam needs to be perpendicular to the structure being imaged to give the optimal image. When the beam hits a longitudinal structure like a needle at an angle <90°, the linear structure might reflect most of the beam away from the transducer. So when using a needle to localize or inject a specific area, maintaining the probe as close to perpendicular as possible with the needle will give a better image. New technology exists to better visualize needles even at high acuity angles by using a multi-beam processing algorithm, which can significantly aid the physician without the need for specialized needles.
Despite better technology, advance planning is key to a successful procedure. Positioning the patient and ultrasound machine in a manner that is comfortable and makes the desired target accessible while being able to visualize the ultrasound monitor comes first. Identifying the target, mapping the needle trajectory using depth markings, and scanning for nerves, vessels, and other structures that may be damaged along the needle path comes next. Using the in plane ultrasound technique with color Doppler and the nerve contrast setting can ensure that the physician has placed the therapeutic agent to the proper location while avoiding any nerves, arteries, or veins. Marking the borders of the ultrasound probe and needle entry site can be helpful to return to the same area after sterile preparation is done. As in any procedure, sterile technique is paramount. Sterile technique considerations may include using sterile gloves and a probe cover with sterile gel, cleaning the area thoroughly, planning the needle entry point 3 cm to 5 cm away from the probe, and maintaining a dry and gel-free needle entry.14-15 The probe should be sterilized between patients to avoid cross-contamination; note that certain solutions like alcohol or ethyl chloride can damage the transducer.14-15 However, simple injections do not require such stringent standards when simple sterile technique is observed by cleaning and then never touching the cleaned area again except with the needle to avoid contamination. Also, ethyl chloride has been found to not contaminate a sterile site and can be used safely to anesthetize the skin.
Ultrasound-Guided Procedures
Many injectable therapeutic options exist as interventions. Cortisone, hyaluronic acid, platelet-rich plasma (PRP), stem cells/bone marrow concentrate (BMC), amniotic fluid, prolotherapy, and saline are now commonly used.16-17 A meta-analysis of the literature assessing the accuracy of ultrasound-guided shoulder girdle injections vs a landmark-guided injection was done in 2015.18 It showed that for the acromioclavicular joint, accuracy was 93.6% vs 68.2% (P < .0001), based on single studies. The accuracy of ultrasound vs a landmark-guided injection was 65% vs 70% for the subacromial space (P > .05); 86.7% vs 26.7% for the biceps tendon sheath (P < .05); and 92.5% vs 72.5% for the glenohumeral joint (P = .025).18
With cortisone, injecting into muscle, ligament, or tendons could potentially harm the tissue or cause worsening of the disease process.19-20 With the advent of orthobiologics, injecting into these structures is now desirable, instead of a potential complication.19-20 Ultrasound has become even more important to the accurate delivery of these therapies to the disease locations. Multiple studies using leukocyte-poor PRP for osteoarthritis show significant differences in pain scores.21-23 Peerbooms and colleagues24,25 also showed that PRP reduced pain and increased function compared to cortisone injections for lateral epicondylitis in 1- and 2-year double-blind randomized controlled trials. Centeno and colleagues26 performed a prospective, multi-site registry study on 102 patients with symptomatic osteoarthritis and/or rotator cuff tears that were injected with bone marrow concentrate. There was a statistically significant improvement in Disabilities of the Arm, Shoulder and Hand (DASH) scores from 36.1 to 17.1 (P < .001) and numeric pain scores improved from 4.3 to 2.4 (P < .001).
By being able to see the pathology, like a hypoechoic region in a tendon, ligament, or muscle, the physician can reliably place the therapeutic agent into the precise location. Also, adjacent para-tendon or para-ligament injections allow for in-season athletes to get some relief from symptoms while allowing to return to play quickly; injections into muscle, ligament, or tendon can damage the structure and require days or weeks of rest, while para-tendon and para-ligament injections are far less painful.
Second-generation techniques have provided patients with great options that can help avoid surgery. Calcific tendonitis appears brightly hyperechoic on ultrasound and is easily identified. The physician can attempt to break up the calcium by fenestration or barbotage of the calcium. The same can be accomplished by injecting the density with PRP or stem cells. If the calcium is soft or “toothpaste-like,” the negative pressure will make it easy to aspirate it into the syringe. A 2-year, longitudinal prospective study of 121 patients demonstrated that visual analog score (VAS) pain scores and size of calcium significantly decreased with ultrasound-guided percutaneous needle lavage; 89% of patients were pain-free at 1-year follow-up.27 Moreover, a randomized controlled trial of 48 patients comparing needle lavage vs subacromial steroid injection showed statistically significant radiographic and clinically better outcomes with the needle lavage group at the 1-year mark.28
The Tenex procedure is a novel technique that uses ultrasonic energy to fenestrate diseased tendon tissue. It also can be used to break up calcific deposits. After the Tenex probe is guided to the diseased tendon/calcium, the TX-1 tip oscillates at the speed of sound, fenestrating/cutting through the tendon or calcium while lavaging the tendon with saline. Multiple prospective, noncontrolled studies done in common extensor, patellar, and rotator cuff tendinopathy have demonstrated good to excellent improvements in pain scores with the Tenex procedure.29-31
Ultrasound is extremely useful in the treatment of adhesive capsulitis.32 The posterior glenohumeral capsule can be distended using a large volume (60 cc) of saline to loosen adhesions in preparation for manipulation. Because the manipulation can be an extremely painful procedure, ultrasound can be used to perform an inter-scalene block for regional anesthesia prior to the procedure. In 2014, Park and colleagues33 performed a randomized prospective trial that showed that capsular distension followed by manipulation was more effective than cortisone injection alone for the treatment of adhesive capsulitis.Ultrasound guidance was found to be just as efficacious as fluoroscopy in a randomized controlled trial in 2014; the authors noted that ultrasound does not expose the patient or clinician to radiation and can be done in office.34
Currently, techniques to perform ultrasound-guided percutaneous tenotomies of the long head of the biceps tendon using hook blades are being studied.35
Ultrasound-Assisted Surgery
Ultrasound has been a boon to surgeons who perform minimally invasive procedures. It is far less cumbersome than classic fluoroscopy. Fluoroscopy requires the use of heavy lead aprons by the surgeons. Combining this with the impervious gowns and hot lights, the surgeons’ comfort level is severely sacrificed. When having to do many long surgeries in a row, this situation can take a toll on the surgeons’ endurance and strength. Improving the comfort of the surgeon is not the primary goal of surgery, but can significantly help our ability to do a better job.
Ultrasound allows the surgeon to localize any superficial foreign objects, especially with radiolucent objects like fragments of glass. Small glass fragments or pieces of wood have always been extremely difficult to remove. X-rays cannot localize these objects, so getting a proper orientation is difficult. MRI and CT scans easily identify these types of foreign objects, but cannot be used intraoperatively (Figure 1A). Often, these objects cannot be felt and therefore require a large dissection. The objects may encapsulate and be easily confused with other soft tissues.
By using the ultrasound intraoperatively, the surgeon can identify the exact position of the biceps tendon (medial/lateral) and where it lies just below the groove and above the pectoralis major (superior/inferior) (Figure 2A).
Reconstruction of ligaments is another ideal use of ultrasound. Surface anatomy cannot always tell the exact location of a ligament or tendon insertion. The best example of this is the anterolateral ligament (ALL). Identification of the lateral epicondyle of the femur and anatomic insertion of the ALL can be difficult in some patients. Ultrasound can be used to identify the origin and insertion of the ALL during surgery under sterile conditions (see page 418). A spinal needle can be placed under direct vision with an in-plane ultrasound guidance over the bony insertion (Figure 3A). A percutaneous incision is made.
This technique is also used by the senior author (AMH) to repair, reconstruct, or internally brace the medial collateral ligament, medial patellofemoral ligament, and lateral collateral ligament. This technique is ideally suited to superficial ligament and tendon reattachment, reconstruction, or internal bracing. The knee, ankle, and elbow superficial ligaments are especially amenable to this easy, percutaneous technique.
Conclusion
Ultrasound is quickly becoming a popular imaging modality due to its simplicity, portability, and cost efficiency. Its use as a diagnostic tool is widely known. As an adjunct for procedures and interventions, its advantages over larger, more expensive modalities such as fluoroscopy, CT, or MRI make it stand out. Ultrasound is not the perfect solution to all problems, but it is clearly a technology that is gaining traction. Ultrasound is another imaging modality and tool that physicians and surgeons can use to improve their patients’ treatment.
1. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 1: coding and reimbursement. Am J Orthop. 2016;45(3):176-182.
2. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016; 45(4):233-238.
3. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: Interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
4. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
5. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
6. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
7. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
8. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
9. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
10. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
11. Sethi P, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: A cadaveric study. Orthopedics. 2006;29(2):149-152.
12. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
13. Speer M, McLennan N, Nixon C. Novice learner in-plane ultrasound imaging: which visualization technique? Reg Anesth Pain Med. 2013;38(4):350-352.
14. Marhofer P, Schebesta K, Marhofer D. [Hygiene aspects in ultrasound-guided regional anesthesia]. Anaesthesist. 2016;65(7):492-498.
15. Sherman T, Ferguson J, Davis W, Russo M, Argintar E. Does the use of ultrasound affect contamination of musculoskeletal injection sites? Clin Orthop Relat Res. 2015;473(1):351-357.
16. Bashir J, Panero AJ, Sherman AL. The emerging use of platelet-rich plasma in musculoskeletal medicine. J Am Osteopath Assoc. 2015;115(1):23-31.
17. Royall NA, Farrin E, Bahner DP, Stanislaw PA. Ultrasound-assisted musculoskeletal procedures: A practical overview of current literature. World J Orthop. 2011;2(7):57-66.
18. Aly AR, Rajasekaran S, Ashworth N. Ultrasound-guided shoulder girdle injections are more accurate and more effective than landmark-guided injections: a systematic review and meta-analysis. Br J Sports Med. 2015;49(16):1042-1049.
19. Maman E, Yehuda C, Pritsch T, et al. Detrimental effect of repeated and single subacromial corticosteroid injections on the intact and injured rotator cuff: A biomechanical and imaging study in rats. Am J Sports Med. 2016;44(1):177-182.
20. Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015;23(1):1-5.
21. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-364.
22. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822-2827.
23. Spakova T, Rosocha J, Lacko M, Harvanova D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91(5):411-417.
24. Peerbooms JC, Sluimer J, Brujin DJ, Gosens T. Positive effects of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255-262.
25. Gosens T, Peerbooms JC, van Laar W, den Oudsten BL. Ongoing positive effects of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with a 2-year follow-up. Am J Sports Med. 2011;39(6):1200-1208.
26. Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. J Pain Res. 2015;8:269-276.
27. Del Castillo-Gonzalez F, Ramos-Alvarez JJ, Rodriguez-Fabian G, Gonzalez-Perez J, Calderon-Montero J. Treatment of the calcific tendinopathy of the rotator cuff by ultrasound-guided percutaneous needle lavage. Two years prospective study. Muscles Ligaments Tendons J. 2015;4(4):407-412.
28. De Witte PB, Selten JW, Navas A, et al. Calcific tendinitis of the rotator cuff: a randomized controlled trial of ultrasound-guided needling and lavage versus subacromial corticosteroids. Am J Sports Med. 2013;41(7):1665-1673.
29. Koh J, Mohan P, Morrey B, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
30. Elattrache N, Morrey B. Percutaneous ultrasonic tenotomy as a treatment for chronic patellar tendinopathy–Jumper’s knee. Oper Tech Orthop. 2013;23(2):98-103
31. Patel MM. A novel treatment for refractory plantar fasciitis. Am J Orthop. 2015;444(3):107-110.
32. Harris G, Bou-Haidar P, Harris C. Adhesive capsulitis: Review of imaging and treatment. J Med Imaging Radiat Oncol. 2013;57:633-643.
33. Park SW, Lee HS, Kim JH. The effectiveness of intensive mobilization techniques combined with capsular distention for adhesive capsulitis of the shoulder. J Phys Ther Sci. 2014;26(11):1776-1770.
34. Bae JH, Park YS, Chang HJ, et al. Randomized controlled trial for efficacy of capsular distension for adhesive capsulitis: Fluoroscopy-guided anterior versus ultrasonography-guided posterolateral approach. Ann Rehabil Med. 2014;38(3):360-368.
35. Aly AR, Rajasekaran S, Mohamed A, Beavis C, Obaid H. Feasibility of ultrasound-guided percutaneous tenotomy of long head of the biceps tendon–A pilot cadaveric study. J Clin Ultrasound. 2015;43(6):361-366.
1. Hirahara AM, Panero AJ. A guide to ultrasound of the shoulder, part 1: coding and reimbursement. Am J Orthop. 2016;45(3):176-182.
2. Panero AJ, Hirahara AM. A guide to ultrasound of the shoulder, part 2: the diagnostic evaluation. Am J Orthop. 2016; 45(4):233-238.
3. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: Interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
4. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
5. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
6. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
7. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
8. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
9. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
10. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
11. Sethi P, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: A cadaveric study. Orthopedics. 2006;29(2):149-152.
12. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
13. Speer M, McLennan N, Nixon C. Novice learner in-plane ultrasound imaging: which visualization technique? Reg Anesth Pain Med. 2013;38(4):350-352.
14. Marhofer P, Schebesta K, Marhofer D. [Hygiene aspects in ultrasound-guided regional anesthesia]. Anaesthesist. 2016;65(7):492-498.
15. Sherman T, Ferguson J, Davis W, Russo M, Argintar E. Does the use of ultrasound affect contamination of musculoskeletal injection sites? Clin Orthop Relat Res. 2015;473(1):351-357.
16. Bashir J, Panero AJ, Sherman AL. The emerging use of platelet-rich plasma in musculoskeletal medicine. J Am Osteopath Assoc. 2015;115(1):23-31.
17. Royall NA, Farrin E, Bahner DP, Stanislaw PA. Ultrasound-assisted musculoskeletal procedures: A practical overview of current literature. World J Orthop. 2011;2(7):57-66.
18. Aly AR, Rajasekaran S, Ashworth N. Ultrasound-guided shoulder girdle injections are more accurate and more effective than landmark-guided injections: a systematic review and meta-analysis. Br J Sports Med. 2015;49(16):1042-1049.
19. Maman E, Yehuda C, Pritsch T, et al. Detrimental effect of repeated and single subacromial corticosteroid injections on the intact and injured rotator cuff: A biomechanical and imaging study in rats. Am J Sports Med. 2016;44(1):177-182.
20. Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015;23(1):1-5.
21. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41(2):356-364.
22. Cerza F, Carni S, Carcangiu A, et al. Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med. 2012;40(12):2822-2827.
23. Spakova T, Rosocha J, Lacko M, Harvanova D, Gharaibeh A. Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am J Phys Med Rehabil. 2012;91(5):411-417.
24. Peerbooms JC, Sluimer J, Brujin DJ, Gosens T. Positive effects of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med. 2010;38(2):255-262.
25. Gosens T, Peerbooms JC, van Laar W, den Oudsten BL. Ongoing positive effects of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with a 2-year follow-up. Am J Sports Med. 2011;39(6):1200-1208.
26. Centeno CJ, Al-Sayegh H, Bashir J, Goodyear S, Freeman MD. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. J Pain Res. 2015;8:269-276.
27. Del Castillo-Gonzalez F, Ramos-Alvarez JJ, Rodriguez-Fabian G, Gonzalez-Perez J, Calderon-Montero J. Treatment of the calcific tendinopathy of the rotator cuff by ultrasound-guided percutaneous needle lavage. Two years prospective study. Muscles Ligaments Tendons J. 2015;4(4):407-412.
28. De Witte PB, Selten JW, Navas A, et al. Calcific tendinitis of the rotator cuff: a randomized controlled trial of ultrasound-guided needling and lavage versus subacromial corticosteroids. Am J Sports Med. 2013;41(7):1665-1673.
29. Koh J, Mohan P, Morrey B, et al. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy: early clinical experience with a novel device for minimally invasive percutaneous microresection. Am J Sports Med. 2013;41(3):636-644.
30. Elattrache N, Morrey B. Percutaneous ultrasonic tenotomy as a treatment for chronic patellar tendinopathy–Jumper’s knee. Oper Tech Orthop. 2013;23(2):98-103
31. Patel MM. A novel treatment for refractory plantar fasciitis. Am J Orthop. 2015;444(3):107-110.
32. Harris G, Bou-Haidar P, Harris C. Adhesive capsulitis: Review of imaging and treatment. J Med Imaging Radiat Oncol. 2013;57:633-643.
33. Park SW, Lee HS, Kim JH. The effectiveness of intensive mobilization techniques combined with capsular distention for adhesive capsulitis of the shoulder. J Phys Ther Sci. 2014;26(11):1776-1770.
34. Bae JH, Park YS, Chang HJ, et al. Randomized controlled trial for efficacy of capsular distension for adhesive capsulitis: Fluoroscopy-guided anterior versus ultrasonography-guided posterolateral approach. Ann Rehabil Med. 2014;38(3):360-368.
35. Aly AR, Rajasekaran S, Mohamed A, Beavis C, Obaid H. Feasibility of ultrasound-guided percutaneous tenotomy of long head of the biceps tendon–A pilot cadaveric study. J Clin Ultrasound. 2015;43(6):361-366.