User login
Genetically corrected autologous skin grafts produced wound healing in a phase I trial involving four men with recessive dystrophic epidermolysis bullosa in what the investigators described as the first human trial of cutaneous gene therapy for this indication, according to a report published in JAMA.
The wound healing varied according to graft site and across the four patients, and generally declined over the course of 1 year of follow-up. Given that this study focused on safety outcomes and that the treatment’s safety profile was deemed “acceptable,” the Food and Drug Administration has permitted a phase IIA trial (NCT01263379) that is currently enrolling adolescents with the disease and will focus on clinical outcomes. Longer-term follow-up of these four patients will continue, and “controlled trials are needed with a broader range of patients to better understand the potential long-term efficacy of genetically corrected autologous epidermal grafts,” said Zurab Siprashvili, Ph.D., of Stanford (Calif.) University and associates.
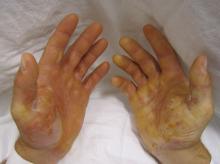
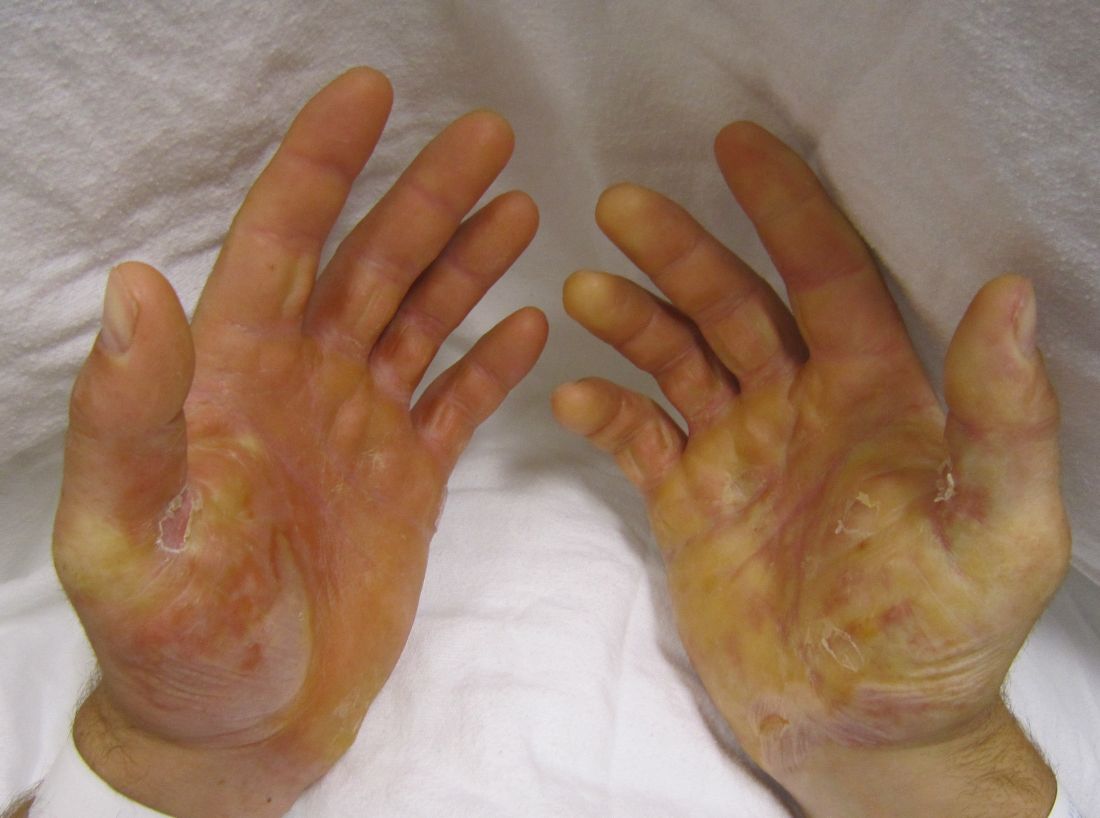
The investigators assessed whether grafting of type VII collagen gene-corrected autologous keratinocytes onto existing wounds would promote their healing. They first harvested and cultured keratinocytes from biopsies of the patients’ unwounded, unscarred skin and transduced these samples with COL7A1-containing retrovirus, producing eight gene-corrected grafts for each patient. All the biopsy sites healed completely, without complications.
The wound beds were prepared for engraftment by cauterization to minimize any retained epidermal stem cells. Then grafts were applied using dissolvable sutures to six wound sites on each patient; the sites had been selected for their accessibility, their potential to enhance the patients’ quality of life, and their ability to ease the period of immobilization after grafting.
All 24 grafts were well tolerated, and no serious adverse events occurred during 1 year of follow-up, which represented approximately 10 epidermal turnover cycles. The most common adverse events – pruritus (three patients) and drainage (two patients) at the graft site – were mild. None of the patients showed systemic autoimmune symptoms or increased blistering outside of the grafted areas, and no clinical signs of malignancy developed.
The gene-corrected graft sites showed type VII collagen localization to anchoring fibrils at the dermal-epidermal junction, in contrast to control sites that showed no such localization or fibril formation. “Gene-corrected graft sites showed fully differentiated epidermis with spinous and granular layers, which were positive for epidermal markers keratin 14, keratin 1, and loricirin resembling normal skin,” Dr. Siprashvili and his associates wrote.
At 1-month follow-up, 20 of the 24 grafts showed 75% or greater wound healing, compared with baseline, and the other 4 grafts showed 50%-74% wound healing. At 3 months, 21 of the 24 graft sites showed 75% or greater wound healing while the remaining 3 grafts showed 50%-74% wound healing. At 6 months, 16 of 24 graft sites showed 75% or greater wound healing and 5 graft sites showed 50%-74% wound healing, but 3 graft sites showed extensive blisters or erosions and were considered graft failures. At 12 months, only 12 of the 24 graft sites showed 75% or greater wound healing.
This general decline in efficacy might be related to the relatively small number of stem cells available for transplantation in these patients, who had only small areas of unscarred skin for harvesting. Or it may be that uncorrected cells in the wound beds began competing with corrected cells within the graft and eventually overcame them, the researchers said.
This study was supported by the National Institutes of Health, the Epidermolysis Bullosa Medical Research Foundation, the Epidermolysis Bullosa Research Partnership, and the Palo Alto VA Medical Center. Dr. Siprashvili and some associates reported having U.S. patents pending; two associates also reported ties to Scioderm and Fibrocell.
Genetically corrected autologous skin grafts produced wound healing in a phase I trial involving four men with recessive dystrophic epidermolysis bullosa in what the investigators described as the first human trial of cutaneous gene therapy for this indication, according to a report published in JAMA.
The wound healing varied according to graft site and across the four patients, and generally declined over the course of 1 year of follow-up. Given that this study focused on safety outcomes and that the treatment’s safety profile was deemed “acceptable,” the Food and Drug Administration has permitted a phase IIA trial (NCT01263379) that is currently enrolling adolescents with the disease and will focus on clinical outcomes. Longer-term follow-up of these four patients will continue, and “controlled trials are needed with a broader range of patients to better understand the potential long-term efficacy of genetically corrected autologous epidermal grafts,” said Zurab Siprashvili, Ph.D., of Stanford (Calif.) University and associates.
The investigators assessed whether grafting of type VII collagen gene-corrected autologous keratinocytes onto existing wounds would promote their healing. They first harvested and cultured keratinocytes from biopsies of the patients’ unwounded, unscarred skin and transduced these samples with COL7A1-containing retrovirus, producing eight gene-corrected grafts for each patient. All the biopsy sites healed completely, without complications.
The wound beds were prepared for engraftment by cauterization to minimize any retained epidermal stem cells. Then grafts were applied using dissolvable sutures to six wound sites on each patient; the sites had been selected for their accessibility, their potential to enhance the patients’ quality of life, and their ability to ease the period of immobilization after grafting.
All 24 grafts were well tolerated, and no serious adverse events occurred during 1 year of follow-up, which represented approximately 10 epidermal turnover cycles. The most common adverse events – pruritus (three patients) and drainage (two patients) at the graft site – were mild. None of the patients showed systemic autoimmune symptoms or increased blistering outside of the grafted areas, and no clinical signs of malignancy developed.
The gene-corrected graft sites showed type VII collagen localization to anchoring fibrils at the dermal-epidermal junction, in contrast to control sites that showed no such localization or fibril formation. “Gene-corrected graft sites showed fully differentiated epidermis with spinous and granular layers, which were positive for epidermal markers keratin 14, keratin 1, and loricirin resembling normal skin,” Dr. Siprashvili and his associates wrote.
At 1-month follow-up, 20 of the 24 grafts showed 75% or greater wound healing, compared with baseline, and the other 4 grafts showed 50%-74% wound healing. At 3 months, 21 of the 24 graft sites showed 75% or greater wound healing while the remaining 3 grafts showed 50%-74% wound healing. At 6 months, 16 of 24 graft sites showed 75% or greater wound healing and 5 graft sites showed 50%-74% wound healing, but 3 graft sites showed extensive blisters or erosions and were considered graft failures. At 12 months, only 12 of the 24 graft sites showed 75% or greater wound healing.
This general decline in efficacy might be related to the relatively small number of stem cells available for transplantation in these patients, who had only small areas of unscarred skin for harvesting. Or it may be that uncorrected cells in the wound beds began competing with corrected cells within the graft and eventually overcame them, the researchers said.
This study was supported by the National Institutes of Health, the Epidermolysis Bullosa Medical Research Foundation, the Epidermolysis Bullosa Research Partnership, and the Palo Alto VA Medical Center. Dr. Siprashvili and some associates reported having U.S. patents pending; two associates also reported ties to Scioderm and Fibrocell.
Genetically corrected autologous skin grafts produced wound healing in a phase I trial involving four men with recessive dystrophic epidermolysis bullosa in what the investigators described as the first human trial of cutaneous gene therapy for this indication, according to a report published in JAMA.
The wound healing varied according to graft site and across the four patients, and generally declined over the course of 1 year of follow-up. Given that this study focused on safety outcomes and that the treatment’s safety profile was deemed “acceptable,” the Food and Drug Administration has permitted a phase IIA trial (NCT01263379) that is currently enrolling adolescents with the disease and will focus on clinical outcomes. Longer-term follow-up of these four patients will continue, and “controlled trials are needed with a broader range of patients to better understand the potential long-term efficacy of genetically corrected autologous epidermal grafts,” said Zurab Siprashvili, Ph.D., of Stanford (Calif.) University and associates.
The investigators assessed whether grafting of type VII collagen gene-corrected autologous keratinocytes onto existing wounds would promote their healing. They first harvested and cultured keratinocytes from biopsies of the patients’ unwounded, unscarred skin and transduced these samples with COL7A1-containing retrovirus, producing eight gene-corrected grafts for each patient. All the biopsy sites healed completely, without complications.
The wound beds were prepared for engraftment by cauterization to minimize any retained epidermal stem cells. Then grafts were applied using dissolvable sutures to six wound sites on each patient; the sites had been selected for their accessibility, their potential to enhance the patients’ quality of life, and their ability to ease the period of immobilization after grafting.
All 24 grafts were well tolerated, and no serious adverse events occurred during 1 year of follow-up, which represented approximately 10 epidermal turnover cycles. The most common adverse events – pruritus (three patients) and drainage (two patients) at the graft site – were mild. None of the patients showed systemic autoimmune symptoms or increased blistering outside of the grafted areas, and no clinical signs of malignancy developed.
The gene-corrected graft sites showed type VII collagen localization to anchoring fibrils at the dermal-epidermal junction, in contrast to control sites that showed no such localization or fibril formation. “Gene-corrected graft sites showed fully differentiated epidermis with spinous and granular layers, which were positive for epidermal markers keratin 14, keratin 1, and loricirin resembling normal skin,” Dr. Siprashvili and his associates wrote.
At 1-month follow-up, 20 of the 24 grafts showed 75% or greater wound healing, compared with baseline, and the other 4 grafts showed 50%-74% wound healing. At 3 months, 21 of the 24 graft sites showed 75% or greater wound healing while the remaining 3 grafts showed 50%-74% wound healing. At 6 months, 16 of 24 graft sites showed 75% or greater wound healing and 5 graft sites showed 50%-74% wound healing, but 3 graft sites showed extensive blisters or erosions and were considered graft failures. At 12 months, only 12 of the 24 graft sites showed 75% or greater wound healing.
This general decline in efficacy might be related to the relatively small number of stem cells available for transplantation in these patients, who had only small areas of unscarred skin for harvesting. Or it may be that uncorrected cells in the wound beds began competing with corrected cells within the graft and eventually overcame them, the researchers said.
This study was supported by the National Institutes of Health, the Epidermolysis Bullosa Medical Research Foundation, the Epidermolysis Bullosa Research Partnership, and the Palo Alto VA Medical Center. Dr. Siprashvili and some associates reported having U.S. patents pending; two associates also reported ties to Scioderm and Fibrocell.
FROM JAMA
Key clinical point: Genetically corrected autologous skin grafts produced wound healing in a phase I study of four patients with recessive dystrophic epidermolysis bullosa.
Major finding: All 24 grafts were well tolerated, and no serious adverse events occurred during 1 year of follow-up; none of the patients showed systemic autoimmune symptoms or increased blistering outside of the grafted areas, and no clinical signs of malignancy developed.
Data source: A single-center open-label phase I study involving four men followed for 1 year.
Disclosures: This study was supported by the National Institutes of Health, the Epidermolysis Bullosa Medical Research Foundation, the Epidermolysis Bullosa Research Partnership, and the Palo Alto VA Medical Center. Dr. Siprashvili and some associates reported having U.S. patents pending; two associates also reported ties to Scioderm and Fibrocell.