User login
On July 17, the US Food and Drug Administration (FDA) approved what may be the most effective hormonal contraceptive ever developed, a single-rod implant that goes by the trade name Implanon. The implant contains 68 mg of etonogestrel (ENG), the active metabolite of desogestrel, in a membrane of ethylene vinyl acetate. In clinical trials involving 20,648 cycles of exposure, only 6 pregnancies occurred, for a cumulative Pearl Index of 0.38 per 100 woman-years.1
This article reviews:
- the 2 contraceptive mechanisms
- indications and patient selection
- pharmacology, safety, adverse effects
- patient satisfaction and discontinuation rates
- insertion and removal
- key points of patient counseling
Unlike Norplant, a multi-rod implant which garnered a million American users before it was removed from the market, the single-rod implant is easy to insert and remove. Before you can order the implant, you must complete a manufacturer-sponsored training program.
Which patients are suitable candidates?
Because the subdermal implant contains only progestin, provides up to 3 years of protection, and requires no daily, weekly, or even monthly action on the part of the user, it is well-suited for:
- Women who wish to or need to avoid estrogen
- Teens who find adherence to a contraceptive regimen difficult
- Healthy adult women who desire long-term protection
- Women who are breastfeeding
Pros and cons
Advantages include:
- Cost. A study2 of 15 contraceptive methods found the implant cost-effective compared to short-acting methods, provided it was used long-term. As of press time, the manufacturer had not released the price.
- Short fertility-recovery time.
- No serious cardiovascular effects.3
Drawbacks. Progestin-only contraceptives also have disadvantages:
- Implants require a minor surgical procedure by trained clinicians for insertion and removal.
- Cost-effectiveness depends on duration of use; early discontinuation negates this benefit.2
- The implant does not protect against sexually transmitted infections—this is a disadvantage of all contraceptive methods except condoms and, perhaps, other barrier methods.
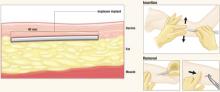
After Implanon is inserted just below the dermis 6 to 8 cm above the elbow crease on the inner aspect of the arm, it remains effective for up to 3 years. To remove it, make a 2-mm incision at the distal tip of the implant and push on the other end of the rod until it pops out.
The implant resides beneath the dermis but above the subcutaneous fat. It remains palpable but invisible, releasing about 60 μg of etonogestrel per day
Images: Rich Larocco
The Norplant experience
Research and development of progestin-only subdermal implants began more than 35 years ago, but early research involving very-low-dose implants found that they did not prevent ectopic pregnancies. This problem ended with Norplant, a 6-capsule implant using the potent progestin levonorgestrel (LNG).
Norplant was highly effective. Over 7 years of use, fewer than 1% of women became pregnant.4 Despite low pregnancy rates and few serious side effects, limitations in component supplies and negative media coverage on complications with removal led to its withdrawal in 2002, leaving no implant available in the US.5
The antiestrogenic effect
Long-acting, progestin-only contraceptives such as the new implant, the LNG-releasing intrauterine system (Mirena), and injectable methods (Depo-Provera) are safer than oral contraceptives (OCs) because they lack estrogen, which can provoke deep venous thrombosis.6,7
LNG, the gonane progestin used in Norplant, binds with high affinity to the progesterone, androgen, mineralocorticoid, and glucocorticoid receptors, but not to estrogen receptors. ENG, also known as 3-keto-desogestrel, demonstrates no estrogenic, anti-inflammatory, or mineralocorticoid activity, but has shown weak androgenic and anabolic activity, as well as strong antiestrogenic activity.
Unlike LNG, which binds mainly to sex hormone-binding globulin, ENG binds mainly to albumin, which is not affected by varying endogenous or exogenous estradiol levels. The safety of ENG has been demonstrated in studies of combined estrogen-progestin OCs and progestin-only OCs that use desogestrel as a component.
The progestin-only implant has 2 primary mechanisms:
- Inhibition of ovulation
- Restriction of sperm penetration of cervical mucus28
Ovulation is suppressed. LNG implants disrupt follicular growth and inhibit ovulation by exerting negative feedback on the hypothalamic–pituitary axis, causing a variety of changes, from anovulation to insufficient luteal function. A few women using LNG implants have quiescent ovaries, but most begin to ovulate as LNG blood concentrations gradually fall.29 The ENG implant suppresses ovulation by altering the hypothalamic–pituitary–ovarian axis and down-regulating the luteinizing hormone surge, which is required to support the production, growth, and maturation of ovarian follicles.11
Oocytes are not fertilized, even if follicles grow during use of the progestin implant. If the follicle ruptures, abnormalities of the ovulatory process prevent release of a viable egg.
Sperm cannot penetrate the cervical mucus. The antiestrogenic action of the progestin renders the cervical mucus viscous, scanty, and impenetrable by sperm.12
Contraceptive effects occur before fertilization
No signs of embryonic development have been found among implant users, indicating the implant lacks abortifacient properties.
Desogestrel in combination with ethinyl estradiol may slightly increase the attributable risk of deep venous thrombosis, but this response has not been shown without estrogen.
Design features
The single-rod design means little discomfort for patients at insertion or removal, an unobtrusive implant, and almost no scarring. Insertion and removal are predictably brief. In US and European trials, which began 10 years ago, average insertion time was 1 minute, and removal time was 3 minutes. In contrast, Norplant required up to 10 minutes to insert and 1 hour to remove.8
Because only 1 rod is implanted, there is no risk of dislocating previously placed capsules.9 Nor is it necessary, as it was with Norplant, to create channels under the skin, which made implants difficult to palpate after insertion.
Finally, ethylene vinyl acetate, the plastic from which Implanon is made, is less likely than Norplant’s Silastic to form a fibrous sheath that can prolong removal.10
Pharmacology of Implanon
Implanon is a single nonbiodegradable rod of 40% ethylene vinyl acetate and 60% ENG (40 mm×2 mm) covered with a membrane of rate-controlling ethylene vinyl acetate 0.06 mm thick.
Bioavailability
The 68 mg of ENG contained in the rod are initially absorbed by the body at a rate of 60 μg per day, slowly declining to 30 μg per day after 2 years.
Peak serum concentrations (266 pg/mL) of ENG are achieved within 1 day after insertion, effectively suppressing ovulation (which requires 90 pg/mL ENG or more).11,12
The steady release of ENG into the circulation avoids first-pass effects on the liver.
The bioavailability of ENG remains nearly 100% throughout 2 years of use. The elimination half-life of ENG is 25 hours, compared with 42 hours for Norplant’s LNG.
Rapid return to ovulation
After removal, serum ENG concentrations become undetectable within 1 week, and ovulation resumes in 94% of women within 3 to 6 weeks after the implant is removed.11,12
Efficacy and safety
Liver enzyme-inducing drugs lower ENG levels
Like other contraceptive steroids, serum levels of ENG are reduced in women taking liver enzyme-inducing drugs such as rifampin, griseofulvin, phenylbutazone, phenytoin, and carbamazepine, as well as anti-HIV protease inhibitors. Pregnancies were reported among Australian women using Implanon along with some of these antiepileptic drugs.13
Equal efficacy in obese women?
The efficacy of the single-rod implant was studied in clinical trials involving 20,648 cycles of use.1 Only 6 pregnancies occurred in this population—2 each in years 1, 2, and 3 of use. None of the women who weighed 154 lb (70 kg) or more became pregnant.12
However, questions remain as to whether the new implant will maintain its high efficacy in obese women, as it has not been studied in women weighing more than 130% of their ideal body weight. Serum levels of ENG are inversely related to body weight and diminish over time, but increased pregnancy rates in obese women have not been reported.
Potential for ectopic pregancy
Suspect ectopic pregnancy in the rare event that a woman becomes pregnant or experiences lower abdominal pain.1 The reason: Pregnancies in women using contraceptive implants are more likely to be ectopic than are pregnancies in the general population. Ovulation is possible in the third year of use, but intrauterine pregnancies are very rare.1
Limited metabolic effects
Published studies indicate that the metabolic effects of the ENG implant are unlikely to be clinically significant, including its effects on lipid and carbohydrate metabolism, liver function, hemostasis, blood pressure, and thyroid and adrenal function.14-17
Adverse event rates
Overall, implants, including the ENG implant, appear to be safe. The rate of adverse events is comparable to rates in nonusers18 (death, neoplastic disease, cardiovascular events, anemia, hypertension, bone-density changes, diabetes, gall bladder disease, thrombocytopenia, and pelvic inflammatory disease).
Lactation
In a study comparing 42 lactating mother–infant pairs using the ENG implant, compared with 38 pairs using intrauterine devices, there were no significant differences in milk volume, milk constituents, timing and amount of supplementary food, or infant growth rates.19
Because it contains no estrogen, Implanon is a good choice for immediate postpartum contraception.
Insertion and removal
Although the ENG implant is designed to facilitate rapid, simple insertion and removal, clinicians require training in Implanon-specific technique.20 Insertion takes an average of 1 to 2 minutes.21
The disposable trocar comes preloaded,22 and the needle tip has 2 cutting edges with different slopes. The extreme tip has a greater angle and is sharp to allow penetration through the skin. The second upper angle is smaller, and the corresponding edge is unsharpened to reduce the risk of placing the implant in muscle tissue.
Subdermal placement is imperative for efficacy and easy removal.
Insertion technique
Have the patient lie on the examination table with her nondominant arm flexed at the elbow and her hand next to her ear. Identify and mark the insertion site using a sterile marker, and apply any necessary local anesthetic. The insertion site should be 6 to 8 cm above the elbow crease on the inner aspect of the arm. Also mark the skin 6 to 8 cm proximal to the first mark; this serves as a guide during insertion.
Remove Implanon from its package and, with the shield still on the needle, confirm the presence of the implant (a white cylinder) within the needle tip. Remove the needle shield, holding the inserter upright to prevent the implant from falling out. Apply countertraction to the skin at the insertion point and, holding the inserter at an angle no greater than 20 degrees, insert the needle tip into the skin with the beveled side up. Lower the inserter to a horizontal position, lifting the skin with the needle tip without removing the tip from the subdermal connective tissue, and insert the needle to its full length.
Next, break the seal of the applicator by pressing the obturator support, and rotate the obturator 90% (in regard to the needle) in either direction. At this point, the insertion process is the opposite of an injection: Hold the obturator in place and retract the cannula.
After insertion, the implant may not be visible but must remain palpable.1
Timing the insertion. Placement should occur at one of the following times:
- For women who have not been using contraception or who have been using a nonhormonal method, insert the implant between days 1 and 5 of menses.
- For women changing from a combination or progestin-only OC, insert the implant any time during pill-taking.
- For women changing from injectable contraception, insert the implant on the date the next injection is scheduled.
- For women using the IUD, insertion can take place at any time.
Additional birth control for 7 days
Advise all women to use an additional barrier method of contraception for 7 days following insertion, unless insertion directly follows an abortion (in which case, no additional contraception is needed).
In all cases, exclude pregnancy before inserting the implant, although there is no evidence that hormonal contraceptives cause birth defects.
Removal technique
The ENG implant can be removed at any time at the woman’s discretion, but will remain effective for 3 years.
Removal requires a 2-mm incision at the distal tip of the implant. The other end of the rod is then pushed until the rod pops out of the incision.9
Mean removal time is 2.6 to 5.4 minutes.22
In rare cases, the ENG implant cannot be found when the time comes for removal, and special procedures, including sonographic determination of location and sonographically guided removal, are required.
Pain, swelling, redness, and hematoma have been reported during insertion and removal.
Because ovulation resumes rapidly following removal, women still desiring contraception should begin another method immediately or have a new rod inserted through the removal incision.
Tell patients to expect altered bleeding
Side effects associated with the ENG implant include menstrual irregularities:
- infrequent bleeding, 26.9%
- amenorrhea, 18.6%
- prolonged bleeding, 15.1%
- frequent bleeding, 7.4%
Other effects include weight gain, 20.7%; acne, 15.3%; breast pain, 9.1%; and headache, 8.5%.8,18
These symptoms rarely provoke discontinuation. Women using any progestin-only method will have changed bleeding patterns; it is estrogen, along with regular progestin withdrawal, that provides predictable uterine bleeding.23,24
A comparison of bleeding patterns in ENG implant users and LNG implant users found a lower mean number of bleeding/spotting days with the former (15.9–19.3 vs 19.4–21.6; P=.0169).25 Because total uterine blood loss is reduced, users of progestin-only contraceptives (as well as OCs) are less likely to be anemic.
The same comparison25 found that users of ENG implants had more variable bleeding patterns than users of LNG implants. Unfortunately, it is impossible to predict which patterns a woman is likely to experience.
The ENG implant reduced or eliminated menstrual pain in 88% of women who previously experienced dysmenorrhea; pain increased in only 2% of ENG implant users.18
Most women liked the implant
Despite side effects and the need for a physician to insert and remove the implants, most women are satisfied with the method, citing its long duration, convenience, and high efficacy.26,27
Discontinuation rates
Nevertheless, some women do choose to discontinue the method before 3 years are up. Discontinuation rates range from 30.2% in Europe and Canada to 0.9% in Southeast Asia.18,22 Bleeding irregularities are the most commonly cited reason for discontinuation, as they were for Norplant.
A meta-analysis of 13 studies published between 1989 and 1992 found that, among 1,716 women using the ENG implant, 5.3% discontinued in months 1 to 6, 6.4% discontinued in months 7 to 12, 4.1% discontinued in months 13 to 18, and 2.8% discontinued in months 19 to 24. Overall, 82% of women continued to use the ENG implant for up to 24 months.8
Lack of training, lack of counseling, lack of success
When the ENG implant was introduced in Australia in 2001, an unexpectedly high number of unintended pregnancies were reported: 100 pregnancies during the first 18 months.13 Almost universally, these events were traced to improper insertion by untrained clinicians, as well as poor patient selection, timing, and counseling.
In response, the Royal Australian College of General Practitioners developed policies for adequate documentation of proper insertion procedures and patient education. The problems disappeared.13
Preinsertion counseling and postinsertion follow-up are essential for continued use of implants because they increase the patient’s satisfaction with the method and help minimize costly removals.
What to advise patients30
- Strongly stress the likelihood of changes in bleeding patterns
- Lack of inherent protection against sexually transmitted infections, and importance of protective measures (though the implant likely reduces the risk of pelvic inflammatory disease)
- User-specific lifestyle and health advice, such as the need for supplemental contraception in some women during the first 7 days of use
- Pros and cons of implants compared with other methods
- The date when removal is indicated1
Consent form
The patient should sign a consent form, which should be included in her medical record.
Extensive, worldwide experience
Already the 6-capsule and 2-rod LNG implants and the single-rod ENG implant have been used successfully by millions of women. The benefits are high efficacy, long duration, absence of estrogen, ease of use, reversibility, and safety.
Dr. Darney reports that he is a consultant to Organon and a speaker for Barr Laboratories, Berlex, and Organon.
1. Implanon [package insert]. Roseland, NJ: Organon; 2006.
2. Trussell J, Leveque JA, Koenig JD, et al. The economic value of contraception: a comparison of 15 methods. Am J Public Health. 1995;85:494-503.
3. Diaz S, Pavez M, Cardenas H, Croxatto HB. Recovery of fertility and outcome of planned pregnancies after the removal of Norplant subdermal implants or copper-T IUDs. Contraception. 1987;35:569-579.
4. Sivin I, Mishell DR, Jr, Darney P, Wan L, Christ M. Levonorgestrel capsule implants in the United States: a 5-year study. Obstet Gynecol. 1998;92:337-344.
5. Kuiper H, Miller S, Martinez E, Loeb L, Darney PD. Urban adolescent females’ views on the implant and contraceptive decisionmaking: a double paradox. Fam Plann Perspect. 1997;29:167-172.
6. Fu H, Darroch JE, Haas T, Ranjit N. Contraceptive failure rates: new estimates from the 1995 National Survey of Family Growth. Fam Plann Perspect. 1999;31:56-63.
7. Meirik O, Farley TMM, Sivin I, for the International Collaborative Post-Marketing Surveillance of Norplant. Safety and efficacy of levonorgestrel implant, intrauterine device, and sterilization. Am J Obstet Gynecol. 2001;97:539.-
8. Croxatto HB, Urbancsek J, Massai R, Coelingh Bennink H, van Beek A. A multicentre efficacy and safety study of the single contraceptive implant Implanon. Implanon Study Group. Hum Reprod. 1999;14:976-981.
9. Zieman M, Klaisle C, Walker D, Bahisteri E, Darney P. Fingers versus instruments for removing levonorgestrel contraceptive implants (Norplant). J Gynecol Tech. 1997;3:213.-
10. Meckstroth K, Darney PD. Implantable contraception. Obstet Gynecol Clin North Am. 2000;27:781-815.
11. Markarainen L, van Beek A, Tuomiyaara L, Asplund B, Bennink HC. Ovarian function during the use of a single contraceptive implant (Implanon) compared with Norplant. Fertil Steril. 1998;69:714-721.
12. Croxatto HB, Mäkäräinen L. The pharmacodynamics and efficacy of Implanon. Contraception. 1998;58:91S-97S.
13. Harrison-Woolrych M, Hill R. Unintended pregnancies with the etonogestrel implant (Implanon): a case series from post-marketing experience in Australia. Contraception. 2005;71:306-308.
14. Mascarenhas L, van Beek A, Bennink H, Newton J. Twenty-four month comparison of apolipoproteins A-1, A-II and B in contraceptive implant users (Norplant and Implanon) in Birmingham, United Kingdom. Contraception. 1998;58:215-219.
15. Biswas A, Viegas OA, Bennink HJ, Korver T, Ratnam SS. Effect of Implanon use on selected parameters of thyroid and adrenal function. Contraception. 2000;62:247-251.
16. Biswas A, Viegas OA, Coeling Bennink JH, Korver T, Ratnam SS. Implanon contraceptive implants: effects on carbohydrate metabolism. Contraception. 2001;63:137-141.
17. Biswas A, Viegas OA, Roy AC. Effect of Implanon and Norplant subdermal contraceptive implants on serum lipids-a randomized comparative study. Contraception. 2003;68:189-193.
18. Rekers H, Affandi B. Implanon studies conducted in Indonesia. Contraception. 2004;70:433.-
19. Reinprayoon D, Taneepanichskul S, Bunyavejchevin S, et al. Effects of the etonogestrel-releasing contraceptive implant (Implanon) on parameters of breastfeeding compared to those of an intrauterine device. Contraception. 2000;62:239-246.
20. Bromham DR, Davey A, Gaffikin L, Ajello CA. Materials, methods and results of the Norplant training program. Br J Fam Plann. 1995;10:256.-
21. Mascarenhas L. Insertion and removal of Implanon: practical considerations. Eur J Contracept Reprod Health Care. 2000;5(suppl 2):29.-
22. Smith A, Reuter S. An assessment of the use of Implanon in three community services. J Fam Plann Reprod Health Care. 2002;28:193-196.
23. Darney PD, Taylor RN, Klaisle C, Bottles K, Zaloudek C. Serum concentrations of estradiol, progesterone, and levonorgestrel are not determinants of endometrial histology or abnormal bleeding in long-term Norplant implant users. Contraception. 1996;53:97-100.
24. Alvarez-Sanchez F, Brache V, Thevenin F, Cochon L, Faundes A. Hormonal treatment for bleeding irregularities in Norplant implant users. Am J Obstet Gynecol. 1996;174:919-922.
25. Zheng S-R, Zheng H-M, Qian S-Z, Sang G-W, Kaper RF. A randomized multicenter study comparing the efficacy and bleeding pattern of a single-rod (Implanon) and a six-capsule (Norplant) hormonal contraceptive implant. Contraception. 1999;60:1-8.
26. Darney PD, Atkinson E, Tanner S, et al. Acceptance and perceptions of Norplant among users in San Francisco, USA. Stud Fam Plann. 1990;21:152-160.
27. Darney PD, Callegari LS, Swift A, Atkinson ES, Robert AM. Condom practices of urban teens using Norplant contraceptive implants, oral contraceptives, and condoms for contraception. Am J Obstet Gynecol. 1999;180:929-937.
28. Shaaban MM, Salem HT, Abdullah KA. Influence of levonorgestrel contraceptive implants, Norplant, initiated early postpartum, upon lactation and infant growth. Contraception. 1985;32:623-635.
29. Brache V, Faundes A, Johansson E, Alvarez F. Anovulation, inadequate luteal phase, and poor sperm penetration in cervical mucus during prolonged use of Norplant implants. Contraception. 1985;31:261-273.
30. Funk S, Miller MM, Mishell DR, Jr, et al. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception. 2005;71:319-326.
On July 17, the US Food and Drug Administration (FDA) approved what may be the most effective hormonal contraceptive ever developed, a single-rod implant that goes by the trade name Implanon. The implant contains 68 mg of etonogestrel (ENG), the active metabolite of desogestrel, in a membrane of ethylene vinyl acetate. In clinical trials involving 20,648 cycles of exposure, only 6 pregnancies occurred, for a cumulative Pearl Index of 0.38 per 100 woman-years.1
This article reviews:
- the 2 contraceptive mechanisms
- indications and patient selection
- pharmacology, safety, adverse effects
- patient satisfaction and discontinuation rates
- insertion and removal
- key points of patient counseling
Unlike Norplant, a multi-rod implant which garnered a million American users before it was removed from the market, the single-rod implant is easy to insert and remove. Before you can order the implant, you must complete a manufacturer-sponsored training program.
Which patients are suitable candidates?
Because the subdermal implant contains only progestin, provides up to 3 years of protection, and requires no daily, weekly, or even monthly action on the part of the user, it is well-suited for:
- Women who wish to or need to avoid estrogen
- Teens who find adherence to a contraceptive regimen difficult
- Healthy adult women who desire long-term protection
- Women who are breastfeeding
Pros and cons
Advantages include:
- Cost. A study2 of 15 contraceptive methods found the implant cost-effective compared to short-acting methods, provided it was used long-term. As of press time, the manufacturer had not released the price.
- Short fertility-recovery time.
- No serious cardiovascular effects.3
Drawbacks. Progestin-only contraceptives also have disadvantages:
- Implants require a minor surgical procedure by trained clinicians for insertion and removal.
- Cost-effectiveness depends on duration of use; early discontinuation negates this benefit.2
- The implant does not protect against sexually transmitted infections—this is a disadvantage of all contraceptive methods except condoms and, perhaps, other barrier methods.
After Implanon is inserted just below the dermis 6 to 8 cm above the elbow crease on the inner aspect of the arm, it remains effective for up to 3 years. To remove it, make a 2-mm incision at the distal tip of the implant and push on the other end of the rod until it pops out.
The implant resides beneath the dermis but above the subcutaneous fat. It remains palpable but invisible, releasing about 60 μg of etonogestrel per day
Images: Rich Larocco
The Norplant experience
Research and development of progestin-only subdermal implants began more than 35 years ago, but early research involving very-low-dose implants found that they did not prevent ectopic pregnancies. This problem ended with Norplant, a 6-capsule implant using the potent progestin levonorgestrel (LNG).
Norplant was highly effective. Over 7 years of use, fewer than 1% of women became pregnant.4 Despite low pregnancy rates and few serious side effects, limitations in component supplies and negative media coverage on complications with removal led to its withdrawal in 2002, leaving no implant available in the US.5
The antiestrogenic effect
Long-acting, progestin-only contraceptives such as the new implant, the LNG-releasing intrauterine system (Mirena), and injectable methods (Depo-Provera) are safer than oral contraceptives (OCs) because they lack estrogen, which can provoke deep venous thrombosis.6,7
LNG, the gonane progestin used in Norplant, binds with high affinity to the progesterone, androgen, mineralocorticoid, and glucocorticoid receptors, but not to estrogen receptors. ENG, also known as 3-keto-desogestrel, demonstrates no estrogenic, anti-inflammatory, or mineralocorticoid activity, but has shown weak androgenic and anabolic activity, as well as strong antiestrogenic activity.
Unlike LNG, which binds mainly to sex hormone-binding globulin, ENG binds mainly to albumin, which is not affected by varying endogenous or exogenous estradiol levels. The safety of ENG has been demonstrated in studies of combined estrogen-progestin OCs and progestin-only OCs that use desogestrel as a component.
The progestin-only implant has 2 primary mechanisms:
- Inhibition of ovulation
- Restriction of sperm penetration of cervical mucus28
Ovulation is suppressed. LNG implants disrupt follicular growth and inhibit ovulation by exerting negative feedback on the hypothalamic–pituitary axis, causing a variety of changes, from anovulation to insufficient luteal function. A few women using LNG implants have quiescent ovaries, but most begin to ovulate as LNG blood concentrations gradually fall.29 The ENG implant suppresses ovulation by altering the hypothalamic–pituitary–ovarian axis and down-regulating the luteinizing hormone surge, which is required to support the production, growth, and maturation of ovarian follicles.11
Oocytes are not fertilized, even if follicles grow during use of the progestin implant. If the follicle ruptures, abnormalities of the ovulatory process prevent release of a viable egg.
Sperm cannot penetrate the cervical mucus. The antiestrogenic action of the progestin renders the cervical mucus viscous, scanty, and impenetrable by sperm.12
Contraceptive effects occur before fertilization
No signs of embryonic development have been found among implant users, indicating the implant lacks abortifacient properties.
Desogestrel in combination with ethinyl estradiol may slightly increase the attributable risk of deep venous thrombosis, but this response has not been shown without estrogen.
Design features
The single-rod design means little discomfort for patients at insertion or removal, an unobtrusive implant, and almost no scarring. Insertion and removal are predictably brief. In US and European trials, which began 10 years ago, average insertion time was 1 minute, and removal time was 3 minutes. In contrast, Norplant required up to 10 minutes to insert and 1 hour to remove.8
Because only 1 rod is implanted, there is no risk of dislocating previously placed capsules.9 Nor is it necessary, as it was with Norplant, to create channels under the skin, which made implants difficult to palpate after insertion.
Finally, ethylene vinyl acetate, the plastic from which Implanon is made, is less likely than Norplant’s Silastic to form a fibrous sheath that can prolong removal.10
Pharmacology of Implanon
Implanon is a single nonbiodegradable rod of 40% ethylene vinyl acetate and 60% ENG (40 mm×2 mm) covered with a membrane of rate-controlling ethylene vinyl acetate 0.06 mm thick.
Bioavailability
The 68 mg of ENG contained in the rod are initially absorbed by the body at a rate of 60 μg per day, slowly declining to 30 μg per day after 2 years.
Peak serum concentrations (266 pg/mL) of ENG are achieved within 1 day after insertion, effectively suppressing ovulation (which requires 90 pg/mL ENG or more).11,12
The steady release of ENG into the circulation avoids first-pass effects on the liver.
The bioavailability of ENG remains nearly 100% throughout 2 years of use. The elimination half-life of ENG is 25 hours, compared with 42 hours for Norplant’s LNG.
Rapid return to ovulation
After removal, serum ENG concentrations become undetectable within 1 week, and ovulation resumes in 94% of women within 3 to 6 weeks after the implant is removed.11,12
Efficacy and safety
Liver enzyme-inducing drugs lower ENG levels
Like other contraceptive steroids, serum levels of ENG are reduced in women taking liver enzyme-inducing drugs such as rifampin, griseofulvin, phenylbutazone, phenytoin, and carbamazepine, as well as anti-HIV protease inhibitors. Pregnancies were reported among Australian women using Implanon along with some of these antiepileptic drugs.13
Equal efficacy in obese women?
The efficacy of the single-rod implant was studied in clinical trials involving 20,648 cycles of use.1 Only 6 pregnancies occurred in this population—2 each in years 1, 2, and 3 of use. None of the women who weighed 154 lb (70 kg) or more became pregnant.12
However, questions remain as to whether the new implant will maintain its high efficacy in obese women, as it has not been studied in women weighing more than 130% of their ideal body weight. Serum levels of ENG are inversely related to body weight and diminish over time, but increased pregnancy rates in obese women have not been reported.
Potential for ectopic pregancy
Suspect ectopic pregnancy in the rare event that a woman becomes pregnant or experiences lower abdominal pain.1 The reason: Pregnancies in women using contraceptive implants are more likely to be ectopic than are pregnancies in the general population. Ovulation is possible in the third year of use, but intrauterine pregnancies are very rare.1
Limited metabolic effects
Published studies indicate that the metabolic effects of the ENG implant are unlikely to be clinically significant, including its effects on lipid and carbohydrate metabolism, liver function, hemostasis, blood pressure, and thyroid and adrenal function.14-17
Adverse event rates
Overall, implants, including the ENG implant, appear to be safe. The rate of adverse events is comparable to rates in nonusers18 (death, neoplastic disease, cardiovascular events, anemia, hypertension, bone-density changes, diabetes, gall bladder disease, thrombocytopenia, and pelvic inflammatory disease).
Lactation
In a study comparing 42 lactating mother–infant pairs using the ENG implant, compared with 38 pairs using intrauterine devices, there were no significant differences in milk volume, milk constituents, timing and amount of supplementary food, or infant growth rates.19
Because it contains no estrogen, Implanon is a good choice for immediate postpartum contraception.
Insertion and removal
Although the ENG implant is designed to facilitate rapid, simple insertion and removal, clinicians require training in Implanon-specific technique.20 Insertion takes an average of 1 to 2 minutes.21
The disposable trocar comes preloaded,22 and the needle tip has 2 cutting edges with different slopes. The extreme tip has a greater angle and is sharp to allow penetration through the skin. The second upper angle is smaller, and the corresponding edge is unsharpened to reduce the risk of placing the implant in muscle tissue.
Subdermal placement is imperative for efficacy and easy removal.
Insertion technique
Have the patient lie on the examination table with her nondominant arm flexed at the elbow and her hand next to her ear. Identify and mark the insertion site using a sterile marker, and apply any necessary local anesthetic. The insertion site should be 6 to 8 cm above the elbow crease on the inner aspect of the arm. Also mark the skin 6 to 8 cm proximal to the first mark; this serves as a guide during insertion.
Remove Implanon from its package and, with the shield still on the needle, confirm the presence of the implant (a white cylinder) within the needle tip. Remove the needle shield, holding the inserter upright to prevent the implant from falling out. Apply countertraction to the skin at the insertion point and, holding the inserter at an angle no greater than 20 degrees, insert the needle tip into the skin with the beveled side up. Lower the inserter to a horizontal position, lifting the skin with the needle tip without removing the tip from the subdermal connective tissue, and insert the needle to its full length.
Next, break the seal of the applicator by pressing the obturator support, and rotate the obturator 90% (in regard to the needle) in either direction. At this point, the insertion process is the opposite of an injection: Hold the obturator in place and retract the cannula.
After insertion, the implant may not be visible but must remain palpable.1
Timing the insertion. Placement should occur at one of the following times:
- For women who have not been using contraception or who have been using a nonhormonal method, insert the implant between days 1 and 5 of menses.
- For women changing from a combination or progestin-only OC, insert the implant any time during pill-taking.
- For women changing from injectable contraception, insert the implant on the date the next injection is scheduled.
- For women using the IUD, insertion can take place at any time.
Additional birth control for 7 days
Advise all women to use an additional barrier method of contraception for 7 days following insertion, unless insertion directly follows an abortion (in which case, no additional contraception is needed).
In all cases, exclude pregnancy before inserting the implant, although there is no evidence that hormonal contraceptives cause birth defects.
Removal technique
The ENG implant can be removed at any time at the woman’s discretion, but will remain effective for 3 years.
Removal requires a 2-mm incision at the distal tip of the implant. The other end of the rod is then pushed until the rod pops out of the incision.9
Mean removal time is 2.6 to 5.4 minutes.22
In rare cases, the ENG implant cannot be found when the time comes for removal, and special procedures, including sonographic determination of location and sonographically guided removal, are required.
Pain, swelling, redness, and hematoma have been reported during insertion and removal.
Because ovulation resumes rapidly following removal, women still desiring contraception should begin another method immediately or have a new rod inserted through the removal incision.
Tell patients to expect altered bleeding
Side effects associated with the ENG implant include menstrual irregularities:
- infrequent bleeding, 26.9%
- amenorrhea, 18.6%
- prolonged bleeding, 15.1%
- frequent bleeding, 7.4%
Other effects include weight gain, 20.7%; acne, 15.3%; breast pain, 9.1%; and headache, 8.5%.8,18
These symptoms rarely provoke discontinuation. Women using any progestin-only method will have changed bleeding patterns; it is estrogen, along with regular progestin withdrawal, that provides predictable uterine bleeding.23,24
A comparison of bleeding patterns in ENG implant users and LNG implant users found a lower mean number of bleeding/spotting days with the former (15.9–19.3 vs 19.4–21.6; P=.0169).25 Because total uterine blood loss is reduced, users of progestin-only contraceptives (as well as OCs) are less likely to be anemic.
The same comparison25 found that users of ENG implants had more variable bleeding patterns than users of LNG implants. Unfortunately, it is impossible to predict which patterns a woman is likely to experience.
The ENG implant reduced or eliminated menstrual pain in 88% of women who previously experienced dysmenorrhea; pain increased in only 2% of ENG implant users.18
Most women liked the implant
Despite side effects and the need for a physician to insert and remove the implants, most women are satisfied with the method, citing its long duration, convenience, and high efficacy.26,27
Discontinuation rates
Nevertheless, some women do choose to discontinue the method before 3 years are up. Discontinuation rates range from 30.2% in Europe and Canada to 0.9% in Southeast Asia.18,22 Bleeding irregularities are the most commonly cited reason for discontinuation, as they were for Norplant.
A meta-analysis of 13 studies published between 1989 and 1992 found that, among 1,716 women using the ENG implant, 5.3% discontinued in months 1 to 6, 6.4% discontinued in months 7 to 12, 4.1% discontinued in months 13 to 18, and 2.8% discontinued in months 19 to 24. Overall, 82% of women continued to use the ENG implant for up to 24 months.8
Lack of training, lack of counseling, lack of success
When the ENG implant was introduced in Australia in 2001, an unexpectedly high number of unintended pregnancies were reported: 100 pregnancies during the first 18 months.13 Almost universally, these events were traced to improper insertion by untrained clinicians, as well as poor patient selection, timing, and counseling.
In response, the Royal Australian College of General Practitioners developed policies for adequate documentation of proper insertion procedures and patient education. The problems disappeared.13
Preinsertion counseling and postinsertion follow-up are essential for continued use of implants because they increase the patient’s satisfaction with the method and help minimize costly removals.
What to advise patients30
- Strongly stress the likelihood of changes in bleeding patterns
- Lack of inherent protection against sexually transmitted infections, and importance of protective measures (though the implant likely reduces the risk of pelvic inflammatory disease)
- User-specific lifestyle and health advice, such as the need for supplemental contraception in some women during the first 7 days of use
- Pros and cons of implants compared with other methods
- The date when removal is indicated1
Consent form
The patient should sign a consent form, which should be included in her medical record.
Extensive, worldwide experience
Already the 6-capsule and 2-rod LNG implants and the single-rod ENG implant have been used successfully by millions of women. The benefits are high efficacy, long duration, absence of estrogen, ease of use, reversibility, and safety.
Dr. Darney reports that he is a consultant to Organon and a speaker for Barr Laboratories, Berlex, and Organon.
On July 17, the US Food and Drug Administration (FDA) approved what may be the most effective hormonal contraceptive ever developed, a single-rod implant that goes by the trade name Implanon. The implant contains 68 mg of etonogestrel (ENG), the active metabolite of desogestrel, in a membrane of ethylene vinyl acetate. In clinical trials involving 20,648 cycles of exposure, only 6 pregnancies occurred, for a cumulative Pearl Index of 0.38 per 100 woman-years.1
This article reviews:
- the 2 contraceptive mechanisms
- indications and patient selection
- pharmacology, safety, adverse effects
- patient satisfaction and discontinuation rates
- insertion and removal
- key points of patient counseling
Unlike Norplant, a multi-rod implant which garnered a million American users before it was removed from the market, the single-rod implant is easy to insert and remove. Before you can order the implant, you must complete a manufacturer-sponsored training program.
Which patients are suitable candidates?
Because the subdermal implant contains only progestin, provides up to 3 years of protection, and requires no daily, weekly, or even monthly action on the part of the user, it is well-suited for:
- Women who wish to or need to avoid estrogen
- Teens who find adherence to a contraceptive regimen difficult
- Healthy adult women who desire long-term protection
- Women who are breastfeeding
Pros and cons
Advantages include:
- Cost. A study2 of 15 contraceptive methods found the implant cost-effective compared to short-acting methods, provided it was used long-term. As of press time, the manufacturer had not released the price.
- Short fertility-recovery time.
- No serious cardiovascular effects.3
Drawbacks. Progestin-only contraceptives also have disadvantages:
- Implants require a minor surgical procedure by trained clinicians for insertion and removal.
- Cost-effectiveness depends on duration of use; early discontinuation negates this benefit.2
- The implant does not protect against sexually transmitted infections—this is a disadvantage of all contraceptive methods except condoms and, perhaps, other barrier methods.
After Implanon is inserted just below the dermis 6 to 8 cm above the elbow crease on the inner aspect of the arm, it remains effective for up to 3 years. To remove it, make a 2-mm incision at the distal tip of the implant and push on the other end of the rod until it pops out.
The implant resides beneath the dermis but above the subcutaneous fat. It remains palpable but invisible, releasing about 60 μg of etonogestrel per day
Images: Rich Larocco
The Norplant experience
Research and development of progestin-only subdermal implants began more than 35 years ago, but early research involving very-low-dose implants found that they did not prevent ectopic pregnancies. This problem ended with Norplant, a 6-capsule implant using the potent progestin levonorgestrel (LNG).
Norplant was highly effective. Over 7 years of use, fewer than 1% of women became pregnant.4 Despite low pregnancy rates and few serious side effects, limitations in component supplies and negative media coverage on complications with removal led to its withdrawal in 2002, leaving no implant available in the US.5
The antiestrogenic effect
Long-acting, progestin-only contraceptives such as the new implant, the LNG-releasing intrauterine system (Mirena), and injectable methods (Depo-Provera) are safer than oral contraceptives (OCs) because they lack estrogen, which can provoke deep venous thrombosis.6,7
LNG, the gonane progestin used in Norplant, binds with high affinity to the progesterone, androgen, mineralocorticoid, and glucocorticoid receptors, but not to estrogen receptors. ENG, also known as 3-keto-desogestrel, demonstrates no estrogenic, anti-inflammatory, or mineralocorticoid activity, but has shown weak androgenic and anabolic activity, as well as strong antiestrogenic activity.
Unlike LNG, which binds mainly to sex hormone-binding globulin, ENG binds mainly to albumin, which is not affected by varying endogenous or exogenous estradiol levels. The safety of ENG has been demonstrated in studies of combined estrogen-progestin OCs and progestin-only OCs that use desogestrel as a component.
The progestin-only implant has 2 primary mechanisms:
- Inhibition of ovulation
- Restriction of sperm penetration of cervical mucus28
Ovulation is suppressed. LNG implants disrupt follicular growth and inhibit ovulation by exerting negative feedback on the hypothalamic–pituitary axis, causing a variety of changes, from anovulation to insufficient luteal function. A few women using LNG implants have quiescent ovaries, but most begin to ovulate as LNG blood concentrations gradually fall.29 The ENG implant suppresses ovulation by altering the hypothalamic–pituitary–ovarian axis and down-regulating the luteinizing hormone surge, which is required to support the production, growth, and maturation of ovarian follicles.11
Oocytes are not fertilized, even if follicles grow during use of the progestin implant. If the follicle ruptures, abnormalities of the ovulatory process prevent release of a viable egg.
Sperm cannot penetrate the cervical mucus. The antiestrogenic action of the progestin renders the cervical mucus viscous, scanty, and impenetrable by sperm.12
Contraceptive effects occur before fertilization
No signs of embryonic development have been found among implant users, indicating the implant lacks abortifacient properties.
Desogestrel in combination with ethinyl estradiol may slightly increase the attributable risk of deep venous thrombosis, but this response has not been shown without estrogen.
Design features
The single-rod design means little discomfort for patients at insertion or removal, an unobtrusive implant, and almost no scarring. Insertion and removal are predictably brief. In US and European trials, which began 10 years ago, average insertion time was 1 minute, and removal time was 3 minutes. In contrast, Norplant required up to 10 minutes to insert and 1 hour to remove.8
Because only 1 rod is implanted, there is no risk of dislocating previously placed capsules.9 Nor is it necessary, as it was with Norplant, to create channels under the skin, which made implants difficult to palpate after insertion.
Finally, ethylene vinyl acetate, the plastic from which Implanon is made, is less likely than Norplant’s Silastic to form a fibrous sheath that can prolong removal.10
Pharmacology of Implanon
Implanon is a single nonbiodegradable rod of 40% ethylene vinyl acetate and 60% ENG (40 mm×2 mm) covered with a membrane of rate-controlling ethylene vinyl acetate 0.06 mm thick.
Bioavailability
The 68 mg of ENG contained in the rod are initially absorbed by the body at a rate of 60 μg per day, slowly declining to 30 μg per day after 2 years.
Peak serum concentrations (266 pg/mL) of ENG are achieved within 1 day after insertion, effectively suppressing ovulation (which requires 90 pg/mL ENG or more).11,12
The steady release of ENG into the circulation avoids first-pass effects on the liver.
The bioavailability of ENG remains nearly 100% throughout 2 years of use. The elimination half-life of ENG is 25 hours, compared with 42 hours for Norplant’s LNG.
Rapid return to ovulation
After removal, serum ENG concentrations become undetectable within 1 week, and ovulation resumes in 94% of women within 3 to 6 weeks after the implant is removed.11,12
Efficacy and safety
Liver enzyme-inducing drugs lower ENG levels
Like other contraceptive steroids, serum levels of ENG are reduced in women taking liver enzyme-inducing drugs such as rifampin, griseofulvin, phenylbutazone, phenytoin, and carbamazepine, as well as anti-HIV protease inhibitors. Pregnancies were reported among Australian women using Implanon along with some of these antiepileptic drugs.13
Equal efficacy in obese women?
The efficacy of the single-rod implant was studied in clinical trials involving 20,648 cycles of use.1 Only 6 pregnancies occurred in this population—2 each in years 1, 2, and 3 of use. None of the women who weighed 154 lb (70 kg) or more became pregnant.12
However, questions remain as to whether the new implant will maintain its high efficacy in obese women, as it has not been studied in women weighing more than 130% of their ideal body weight. Serum levels of ENG are inversely related to body weight and diminish over time, but increased pregnancy rates in obese women have not been reported.
Potential for ectopic pregancy
Suspect ectopic pregnancy in the rare event that a woman becomes pregnant or experiences lower abdominal pain.1 The reason: Pregnancies in women using contraceptive implants are more likely to be ectopic than are pregnancies in the general population. Ovulation is possible in the third year of use, but intrauterine pregnancies are very rare.1
Limited metabolic effects
Published studies indicate that the metabolic effects of the ENG implant are unlikely to be clinically significant, including its effects on lipid and carbohydrate metabolism, liver function, hemostasis, blood pressure, and thyroid and adrenal function.14-17
Adverse event rates
Overall, implants, including the ENG implant, appear to be safe. The rate of adverse events is comparable to rates in nonusers18 (death, neoplastic disease, cardiovascular events, anemia, hypertension, bone-density changes, diabetes, gall bladder disease, thrombocytopenia, and pelvic inflammatory disease).
Lactation
In a study comparing 42 lactating mother–infant pairs using the ENG implant, compared with 38 pairs using intrauterine devices, there were no significant differences in milk volume, milk constituents, timing and amount of supplementary food, or infant growth rates.19
Because it contains no estrogen, Implanon is a good choice for immediate postpartum contraception.
Insertion and removal
Although the ENG implant is designed to facilitate rapid, simple insertion and removal, clinicians require training in Implanon-specific technique.20 Insertion takes an average of 1 to 2 minutes.21
The disposable trocar comes preloaded,22 and the needle tip has 2 cutting edges with different slopes. The extreme tip has a greater angle and is sharp to allow penetration through the skin. The second upper angle is smaller, and the corresponding edge is unsharpened to reduce the risk of placing the implant in muscle tissue.
Subdermal placement is imperative for efficacy and easy removal.
Insertion technique
Have the patient lie on the examination table with her nondominant arm flexed at the elbow and her hand next to her ear. Identify and mark the insertion site using a sterile marker, and apply any necessary local anesthetic. The insertion site should be 6 to 8 cm above the elbow crease on the inner aspect of the arm. Also mark the skin 6 to 8 cm proximal to the first mark; this serves as a guide during insertion.
Remove Implanon from its package and, with the shield still on the needle, confirm the presence of the implant (a white cylinder) within the needle tip. Remove the needle shield, holding the inserter upright to prevent the implant from falling out. Apply countertraction to the skin at the insertion point and, holding the inserter at an angle no greater than 20 degrees, insert the needle tip into the skin with the beveled side up. Lower the inserter to a horizontal position, lifting the skin with the needle tip without removing the tip from the subdermal connective tissue, and insert the needle to its full length.
Next, break the seal of the applicator by pressing the obturator support, and rotate the obturator 90% (in regard to the needle) in either direction. At this point, the insertion process is the opposite of an injection: Hold the obturator in place and retract the cannula.
After insertion, the implant may not be visible but must remain palpable.1
Timing the insertion. Placement should occur at one of the following times:
- For women who have not been using contraception or who have been using a nonhormonal method, insert the implant between days 1 and 5 of menses.
- For women changing from a combination or progestin-only OC, insert the implant any time during pill-taking.
- For women changing from injectable contraception, insert the implant on the date the next injection is scheduled.
- For women using the IUD, insertion can take place at any time.
Additional birth control for 7 days
Advise all women to use an additional barrier method of contraception for 7 days following insertion, unless insertion directly follows an abortion (in which case, no additional contraception is needed).
In all cases, exclude pregnancy before inserting the implant, although there is no evidence that hormonal contraceptives cause birth defects.
Removal technique
The ENG implant can be removed at any time at the woman’s discretion, but will remain effective for 3 years.
Removal requires a 2-mm incision at the distal tip of the implant. The other end of the rod is then pushed until the rod pops out of the incision.9
Mean removal time is 2.6 to 5.4 minutes.22
In rare cases, the ENG implant cannot be found when the time comes for removal, and special procedures, including sonographic determination of location and sonographically guided removal, are required.
Pain, swelling, redness, and hematoma have been reported during insertion and removal.
Because ovulation resumes rapidly following removal, women still desiring contraception should begin another method immediately or have a new rod inserted through the removal incision.
Tell patients to expect altered bleeding
Side effects associated with the ENG implant include menstrual irregularities:
- infrequent bleeding, 26.9%
- amenorrhea, 18.6%
- prolonged bleeding, 15.1%
- frequent bleeding, 7.4%
Other effects include weight gain, 20.7%; acne, 15.3%; breast pain, 9.1%; and headache, 8.5%.8,18
These symptoms rarely provoke discontinuation. Women using any progestin-only method will have changed bleeding patterns; it is estrogen, along with regular progestin withdrawal, that provides predictable uterine bleeding.23,24
A comparison of bleeding patterns in ENG implant users and LNG implant users found a lower mean number of bleeding/spotting days with the former (15.9–19.3 vs 19.4–21.6; P=.0169).25 Because total uterine blood loss is reduced, users of progestin-only contraceptives (as well as OCs) are less likely to be anemic.
The same comparison25 found that users of ENG implants had more variable bleeding patterns than users of LNG implants. Unfortunately, it is impossible to predict which patterns a woman is likely to experience.
The ENG implant reduced or eliminated menstrual pain in 88% of women who previously experienced dysmenorrhea; pain increased in only 2% of ENG implant users.18
Most women liked the implant
Despite side effects and the need for a physician to insert and remove the implants, most women are satisfied with the method, citing its long duration, convenience, and high efficacy.26,27
Discontinuation rates
Nevertheless, some women do choose to discontinue the method before 3 years are up. Discontinuation rates range from 30.2% in Europe and Canada to 0.9% in Southeast Asia.18,22 Bleeding irregularities are the most commonly cited reason for discontinuation, as they were for Norplant.
A meta-analysis of 13 studies published between 1989 and 1992 found that, among 1,716 women using the ENG implant, 5.3% discontinued in months 1 to 6, 6.4% discontinued in months 7 to 12, 4.1% discontinued in months 13 to 18, and 2.8% discontinued in months 19 to 24. Overall, 82% of women continued to use the ENG implant for up to 24 months.8
Lack of training, lack of counseling, lack of success
When the ENG implant was introduced in Australia in 2001, an unexpectedly high number of unintended pregnancies were reported: 100 pregnancies during the first 18 months.13 Almost universally, these events were traced to improper insertion by untrained clinicians, as well as poor patient selection, timing, and counseling.
In response, the Royal Australian College of General Practitioners developed policies for adequate documentation of proper insertion procedures and patient education. The problems disappeared.13
Preinsertion counseling and postinsertion follow-up are essential for continued use of implants because they increase the patient’s satisfaction with the method and help minimize costly removals.
What to advise patients30
- Strongly stress the likelihood of changes in bleeding patterns
- Lack of inherent protection against sexually transmitted infections, and importance of protective measures (though the implant likely reduces the risk of pelvic inflammatory disease)
- User-specific lifestyle and health advice, such as the need for supplemental contraception in some women during the first 7 days of use
- Pros and cons of implants compared with other methods
- The date when removal is indicated1
Consent form
The patient should sign a consent form, which should be included in her medical record.
Extensive, worldwide experience
Already the 6-capsule and 2-rod LNG implants and the single-rod ENG implant have been used successfully by millions of women. The benefits are high efficacy, long duration, absence of estrogen, ease of use, reversibility, and safety.
Dr. Darney reports that he is a consultant to Organon and a speaker for Barr Laboratories, Berlex, and Organon.
1. Implanon [package insert]. Roseland, NJ: Organon; 2006.
2. Trussell J, Leveque JA, Koenig JD, et al. The economic value of contraception: a comparison of 15 methods. Am J Public Health. 1995;85:494-503.
3. Diaz S, Pavez M, Cardenas H, Croxatto HB. Recovery of fertility and outcome of planned pregnancies after the removal of Norplant subdermal implants or copper-T IUDs. Contraception. 1987;35:569-579.
4. Sivin I, Mishell DR, Jr, Darney P, Wan L, Christ M. Levonorgestrel capsule implants in the United States: a 5-year study. Obstet Gynecol. 1998;92:337-344.
5. Kuiper H, Miller S, Martinez E, Loeb L, Darney PD. Urban adolescent females’ views on the implant and contraceptive decisionmaking: a double paradox. Fam Plann Perspect. 1997;29:167-172.
6. Fu H, Darroch JE, Haas T, Ranjit N. Contraceptive failure rates: new estimates from the 1995 National Survey of Family Growth. Fam Plann Perspect. 1999;31:56-63.
7. Meirik O, Farley TMM, Sivin I, for the International Collaborative Post-Marketing Surveillance of Norplant. Safety and efficacy of levonorgestrel implant, intrauterine device, and sterilization. Am J Obstet Gynecol. 2001;97:539.-
8. Croxatto HB, Urbancsek J, Massai R, Coelingh Bennink H, van Beek A. A multicentre efficacy and safety study of the single contraceptive implant Implanon. Implanon Study Group. Hum Reprod. 1999;14:976-981.
9. Zieman M, Klaisle C, Walker D, Bahisteri E, Darney P. Fingers versus instruments for removing levonorgestrel contraceptive implants (Norplant). J Gynecol Tech. 1997;3:213.-
10. Meckstroth K, Darney PD. Implantable contraception. Obstet Gynecol Clin North Am. 2000;27:781-815.
11. Markarainen L, van Beek A, Tuomiyaara L, Asplund B, Bennink HC. Ovarian function during the use of a single contraceptive implant (Implanon) compared with Norplant. Fertil Steril. 1998;69:714-721.
12. Croxatto HB, Mäkäräinen L. The pharmacodynamics and efficacy of Implanon. Contraception. 1998;58:91S-97S.
13. Harrison-Woolrych M, Hill R. Unintended pregnancies with the etonogestrel implant (Implanon): a case series from post-marketing experience in Australia. Contraception. 2005;71:306-308.
14. Mascarenhas L, van Beek A, Bennink H, Newton J. Twenty-four month comparison of apolipoproteins A-1, A-II and B in contraceptive implant users (Norplant and Implanon) in Birmingham, United Kingdom. Contraception. 1998;58:215-219.
15. Biswas A, Viegas OA, Bennink HJ, Korver T, Ratnam SS. Effect of Implanon use on selected parameters of thyroid and adrenal function. Contraception. 2000;62:247-251.
16. Biswas A, Viegas OA, Coeling Bennink JH, Korver T, Ratnam SS. Implanon contraceptive implants: effects on carbohydrate metabolism. Contraception. 2001;63:137-141.
17. Biswas A, Viegas OA, Roy AC. Effect of Implanon and Norplant subdermal contraceptive implants on serum lipids-a randomized comparative study. Contraception. 2003;68:189-193.
18. Rekers H, Affandi B. Implanon studies conducted in Indonesia. Contraception. 2004;70:433.-
19. Reinprayoon D, Taneepanichskul S, Bunyavejchevin S, et al. Effects of the etonogestrel-releasing contraceptive implant (Implanon) on parameters of breastfeeding compared to those of an intrauterine device. Contraception. 2000;62:239-246.
20. Bromham DR, Davey A, Gaffikin L, Ajello CA. Materials, methods and results of the Norplant training program. Br J Fam Plann. 1995;10:256.-
21. Mascarenhas L. Insertion and removal of Implanon: practical considerations. Eur J Contracept Reprod Health Care. 2000;5(suppl 2):29.-
22. Smith A, Reuter S. An assessment of the use of Implanon in three community services. J Fam Plann Reprod Health Care. 2002;28:193-196.
23. Darney PD, Taylor RN, Klaisle C, Bottles K, Zaloudek C. Serum concentrations of estradiol, progesterone, and levonorgestrel are not determinants of endometrial histology or abnormal bleeding in long-term Norplant implant users. Contraception. 1996;53:97-100.
24. Alvarez-Sanchez F, Brache V, Thevenin F, Cochon L, Faundes A. Hormonal treatment for bleeding irregularities in Norplant implant users. Am J Obstet Gynecol. 1996;174:919-922.
25. Zheng S-R, Zheng H-M, Qian S-Z, Sang G-W, Kaper RF. A randomized multicenter study comparing the efficacy and bleeding pattern of a single-rod (Implanon) and a six-capsule (Norplant) hormonal contraceptive implant. Contraception. 1999;60:1-8.
26. Darney PD, Atkinson E, Tanner S, et al. Acceptance and perceptions of Norplant among users in San Francisco, USA. Stud Fam Plann. 1990;21:152-160.
27. Darney PD, Callegari LS, Swift A, Atkinson ES, Robert AM. Condom practices of urban teens using Norplant contraceptive implants, oral contraceptives, and condoms for contraception. Am J Obstet Gynecol. 1999;180:929-937.
28. Shaaban MM, Salem HT, Abdullah KA. Influence of levonorgestrel contraceptive implants, Norplant, initiated early postpartum, upon lactation and infant growth. Contraception. 1985;32:623-635.
29. Brache V, Faundes A, Johansson E, Alvarez F. Anovulation, inadequate luteal phase, and poor sperm penetration in cervical mucus during prolonged use of Norplant implants. Contraception. 1985;31:261-273.
30. Funk S, Miller MM, Mishell DR, Jr, et al. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception. 2005;71:319-326.
1. Implanon [package insert]. Roseland, NJ: Organon; 2006.
2. Trussell J, Leveque JA, Koenig JD, et al. The economic value of contraception: a comparison of 15 methods. Am J Public Health. 1995;85:494-503.
3. Diaz S, Pavez M, Cardenas H, Croxatto HB. Recovery of fertility and outcome of planned pregnancies after the removal of Norplant subdermal implants or copper-T IUDs. Contraception. 1987;35:569-579.
4. Sivin I, Mishell DR, Jr, Darney P, Wan L, Christ M. Levonorgestrel capsule implants in the United States: a 5-year study. Obstet Gynecol. 1998;92:337-344.
5. Kuiper H, Miller S, Martinez E, Loeb L, Darney PD. Urban adolescent females’ views on the implant and contraceptive decisionmaking: a double paradox. Fam Plann Perspect. 1997;29:167-172.
6. Fu H, Darroch JE, Haas T, Ranjit N. Contraceptive failure rates: new estimates from the 1995 National Survey of Family Growth. Fam Plann Perspect. 1999;31:56-63.
7. Meirik O, Farley TMM, Sivin I, for the International Collaborative Post-Marketing Surveillance of Norplant. Safety and efficacy of levonorgestrel implant, intrauterine device, and sterilization. Am J Obstet Gynecol. 2001;97:539.-
8. Croxatto HB, Urbancsek J, Massai R, Coelingh Bennink H, van Beek A. A multicentre efficacy and safety study of the single contraceptive implant Implanon. Implanon Study Group. Hum Reprod. 1999;14:976-981.
9. Zieman M, Klaisle C, Walker D, Bahisteri E, Darney P. Fingers versus instruments for removing levonorgestrel contraceptive implants (Norplant). J Gynecol Tech. 1997;3:213.-
10. Meckstroth K, Darney PD. Implantable contraception. Obstet Gynecol Clin North Am. 2000;27:781-815.
11. Markarainen L, van Beek A, Tuomiyaara L, Asplund B, Bennink HC. Ovarian function during the use of a single contraceptive implant (Implanon) compared with Norplant. Fertil Steril. 1998;69:714-721.
12. Croxatto HB, Mäkäräinen L. The pharmacodynamics and efficacy of Implanon. Contraception. 1998;58:91S-97S.
13. Harrison-Woolrych M, Hill R. Unintended pregnancies with the etonogestrel implant (Implanon): a case series from post-marketing experience in Australia. Contraception. 2005;71:306-308.
14. Mascarenhas L, van Beek A, Bennink H, Newton J. Twenty-four month comparison of apolipoproteins A-1, A-II and B in contraceptive implant users (Norplant and Implanon) in Birmingham, United Kingdom. Contraception. 1998;58:215-219.
15. Biswas A, Viegas OA, Bennink HJ, Korver T, Ratnam SS. Effect of Implanon use on selected parameters of thyroid and adrenal function. Contraception. 2000;62:247-251.
16. Biswas A, Viegas OA, Coeling Bennink JH, Korver T, Ratnam SS. Implanon contraceptive implants: effects on carbohydrate metabolism. Contraception. 2001;63:137-141.
17. Biswas A, Viegas OA, Roy AC. Effect of Implanon and Norplant subdermal contraceptive implants on serum lipids-a randomized comparative study. Contraception. 2003;68:189-193.
18. Rekers H, Affandi B. Implanon studies conducted in Indonesia. Contraception. 2004;70:433.-
19. Reinprayoon D, Taneepanichskul S, Bunyavejchevin S, et al. Effects of the etonogestrel-releasing contraceptive implant (Implanon) on parameters of breastfeeding compared to those of an intrauterine device. Contraception. 2000;62:239-246.
20. Bromham DR, Davey A, Gaffikin L, Ajello CA. Materials, methods and results of the Norplant training program. Br J Fam Plann. 1995;10:256.-
21. Mascarenhas L. Insertion and removal of Implanon: practical considerations. Eur J Contracept Reprod Health Care. 2000;5(suppl 2):29.-
22. Smith A, Reuter S. An assessment of the use of Implanon in three community services. J Fam Plann Reprod Health Care. 2002;28:193-196.
23. Darney PD, Taylor RN, Klaisle C, Bottles K, Zaloudek C. Serum concentrations of estradiol, progesterone, and levonorgestrel are not determinants of endometrial histology or abnormal bleeding in long-term Norplant implant users. Contraception. 1996;53:97-100.
24. Alvarez-Sanchez F, Brache V, Thevenin F, Cochon L, Faundes A. Hormonal treatment for bleeding irregularities in Norplant implant users. Am J Obstet Gynecol. 1996;174:919-922.
25. Zheng S-R, Zheng H-M, Qian S-Z, Sang G-W, Kaper RF. A randomized multicenter study comparing the efficacy and bleeding pattern of a single-rod (Implanon) and a six-capsule (Norplant) hormonal contraceptive implant. Contraception. 1999;60:1-8.
26. Darney PD, Atkinson E, Tanner S, et al. Acceptance and perceptions of Norplant among users in San Francisco, USA. Stud Fam Plann. 1990;21:152-160.
27. Darney PD, Callegari LS, Swift A, Atkinson ES, Robert AM. Condom practices of urban teens using Norplant contraceptive implants, oral contraceptives, and condoms for contraception. Am J Obstet Gynecol. 1999;180:929-937.
28. Shaaban MM, Salem HT, Abdullah KA. Influence of levonorgestrel contraceptive implants, Norplant, initiated early postpartum, upon lactation and infant growth. Contraception. 1985;32:623-635.
29. Brache V, Faundes A, Johansson E, Alvarez F. Anovulation, inadequate luteal phase, and poor sperm penetration in cervical mucus during prolonged use of Norplant implants. Contraception. 1985;31:261-273.
30. Funk S, Miller MM, Mishell DR, Jr, et al. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception. 2005;71:319-326.