User login
PARIS – Thirty-day outcomes of the first European, all-transfemoral-approach study of the latest-generation Sapien 3 heart valve for transcatheter aortic valve implantation in intermediate-risk elderly patients with severe aortic stenosis included a 1.0% mortality rate and a mere 2.3% rate of moderate paravalvular aortic regurgitation, with no severe aortic regurgitation and a mild aortic regurgitation rate of 26%.
These initial results from the Sapien 3 CE IR study are highly concordant with the impressive results of two U.S. studies using the Sapien 3 valve for transcatheter aortic valve implantation (TAVI) reported earlier this year at the American College of Cardiology meeting in San Diego; one study was of 1,076 intermediate–surgical risk patients and the other involved 583 high-risk patients.
“These results represent at least parity with the best reported surgical outcomes. If we step forward, these favorable results suggest that Sapien 3 TAVI may be expected to challenge surgical aortic valve replacement as the gold standard therapy in elderly patients with aortic stenosis,” Dr. Alec Vahanian declared in presenting the 30-day Sapien 3 CE IR study results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
At present, the Sapien 3 valve is approved in Europe for treatment of high-risk or inoperable patients with severe aortic stenosis, but not for intermediate-risk patients such as those in the Sapien 3 CE IR study. The valve remains investigational in the United States, although its manufacturer Edwards Lifesciences, has filed for Food and Drug Administration approval of the device in high-risk patients.
The European Sapien 3 CE IR study includes 101 patients, mean age 84.4 years, with a Society of Thoracic Surgeons (STS) risk score of 5.2%. All underwent TAVI via a transfemoral approach. Fifty-five percent did so under conscious sedation, in contrast to the U.S. trial in intermediate-risk patients, where fewer than 20% had conscious sedation.
To put the observed 30-day all-cause mortality rate of 1.0% in perspective, it’s the lowest seen in the 11 clinical trials performed over the years with the three generations of the Sapien valve. The mortality rate is in line with that found in the much larger U.S. trial in intermediate-risk patients, where the subgroup treated via a transfemoral approach had a 1.1% mortality rate, observed Dr. Vahanian, head of cardiology at Bichat University Hospital in Paris.
The technical procedural success rate in the European trial was 98%. There was no coronary obstruction, valve embolization, or annular rupture.
The 30-day overall stroke incidence was 4%, including a 2% incidence of disabling stroke.
The incidence of vascular complications was low: a major vascular complication rate of 2%, with life-threatening bleeding in 2% of patients. No acute MIs occurred within 30 days, the acute kidney injury rate was 2%, and new-onset atrial fibrillation occurred in 6.9% of patients. Four percent of patients required a new permanent pacemaker, a rate lower than in the U.S. study. There have been no cases of worsening heart failure.
Plus, patients feel a lot better: While 64% were New York Heart Association class III or IV at baseline, 90% were class I or II after 1 month, the cardiologist continued.
In terms of key hemodynamic outcomes, the valve area doubled after TAVI, while the mean gradient plunged from close to 50 mm Hg at baseline to 12 mm Hg 1 month post procedure.
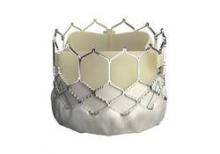
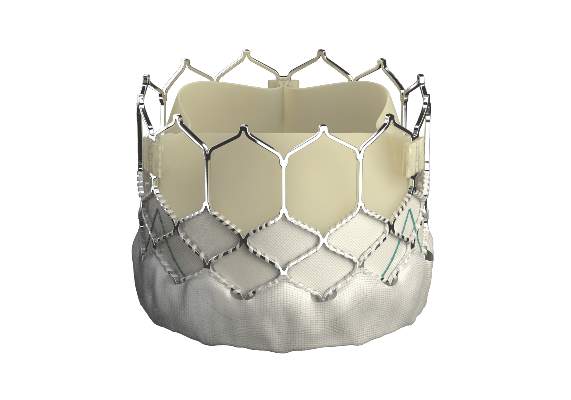
The Sapien 3 valve has the lowest profile of any heart valve. It is typically delivered through a 14-French expandable sheath. The device features a skirt of fabric at the bottom of the frame that’s designed to minimize paravalvular leak.
“I’m very impressed with the data you present. Fantastic!” declared session chair Dr. Carlos E. Ruiz of Lenox Hill Hospital in New York. “Obviously, this valve has raised the bar to a level that will be very hard for other valve technologies to emulate.”
Session cochair Dr. A. Pieter Kappetein, professor of cardiothoracic surgery at Erasmus University in Rotterdam, the Netherlands, posed a question: If you have patients with a mean age of 84, an STS score that would project a 30-day mortality of 5.2% with surgical aortic valve replacement, and yet you only have 1% mortality with Sapien 3 TAVI, why bother with a randomized study before widespread adoption of the less invasive procedure as the treatment of choice in intermediate-risk patients?
Dr. Vahanian replied that this is a time to accumulate evidence. The plan is to follow the study participants for 5 years, a long-term follow-up he views as essential when extending TAVI beyond a high-risk population with limited life expectancy to a less sick group of patients with a longer remaining lifetime. He added that a randomized trial of surgery vs. TAVI is coming in the near future, and the results will provide a solid basis for definitive new practice guidelines. In the meantime, individual patient management decisions are made by heart teams – and the heart teams are keeping up to date regarding the emerging impressive evidence favoring TAVI.
The Sapien 3 studies are sponsored by Edwards Lifesciences. Dr. Vahanian is a consultant to the company.
PARIS – Thirty-day outcomes of the first European, all-transfemoral-approach study of the latest-generation Sapien 3 heart valve for transcatheter aortic valve implantation in intermediate-risk elderly patients with severe aortic stenosis included a 1.0% mortality rate and a mere 2.3% rate of moderate paravalvular aortic regurgitation, with no severe aortic regurgitation and a mild aortic regurgitation rate of 26%.
These initial results from the Sapien 3 CE IR study are highly concordant with the impressive results of two U.S. studies using the Sapien 3 valve for transcatheter aortic valve implantation (TAVI) reported earlier this year at the American College of Cardiology meeting in San Diego; one study was of 1,076 intermediate–surgical risk patients and the other involved 583 high-risk patients.
“These results represent at least parity with the best reported surgical outcomes. If we step forward, these favorable results suggest that Sapien 3 TAVI may be expected to challenge surgical aortic valve replacement as the gold standard therapy in elderly patients with aortic stenosis,” Dr. Alec Vahanian declared in presenting the 30-day Sapien 3 CE IR study results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
At present, the Sapien 3 valve is approved in Europe for treatment of high-risk or inoperable patients with severe aortic stenosis, but not for intermediate-risk patients such as those in the Sapien 3 CE IR study. The valve remains investigational in the United States, although its manufacturer Edwards Lifesciences, has filed for Food and Drug Administration approval of the device in high-risk patients.
The European Sapien 3 CE IR study includes 101 patients, mean age 84.4 years, with a Society of Thoracic Surgeons (STS) risk score of 5.2%. All underwent TAVI via a transfemoral approach. Fifty-five percent did so under conscious sedation, in contrast to the U.S. trial in intermediate-risk patients, where fewer than 20% had conscious sedation.
To put the observed 30-day all-cause mortality rate of 1.0% in perspective, it’s the lowest seen in the 11 clinical trials performed over the years with the three generations of the Sapien valve. The mortality rate is in line with that found in the much larger U.S. trial in intermediate-risk patients, where the subgroup treated via a transfemoral approach had a 1.1% mortality rate, observed Dr. Vahanian, head of cardiology at Bichat University Hospital in Paris.
The technical procedural success rate in the European trial was 98%. There was no coronary obstruction, valve embolization, or annular rupture.
The 30-day overall stroke incidence was 4%, including a 2% incidence of disabling stroke.
The incidence of vascular complications was low: a major vascular complication rate of 2%, with life-threatening bleeding in 2% of patients. No acute MIs occurred within 30 days, the acute kidney injury rate was 2%, and new-onset atrial fibrillation occurred in 6.9% of patients. Four percent of patients required a new permanent pacemaker, a rate lower than in the U.S. study. There have been no cases of worsening heart failure.
Plus, patients feel a lot better: While 64% were New York Heart Association class III or IV at baseline, 90% were class I or II after 1 month, the cardiologist continued.
In terms of key hemodynamic outcomes, the valve area doubled after TAVI, while the mean gradient plunged from close to 50 mm Hg at baseline to 12 mm Hg 1 month post procedure.
The Sapien 3 valve has the lowest profile of any heart valve. It is typically delivered through a 14-French expandable sheath. The device features a skirt of fabric at the bottom of the frame that’s designed to minimize paravalvular leak.
“I’m very impressed with the data you present. Fantastic!” declared session chair Dr. Carlos E. Ruiz of Lenox Hill Hospital in New York. “Obviously, this valve has raised the bar to a level that will be very hard for other valve technologies to emulate.”
Session cochair Dr. A. Pieter Kappetein, professor of cardiothoracic surgery at Erasmus University in Rotterdam, the Netherlands, posed a question: If you have patients with a mean age of 84, an STS score that would project a 30-day mortality of 5.2% with surgical aortic valve replacement, and yet you only have 1% mortality with Sapien 3 TAVI, why bother with a randomized study before widespread adoption of the less invasive procedure as the treatment of choice in intermediate-risk patients?
Dr. Vahanian replied that this is a time to accumulate evidence. The plan is to follow the study participants for 5 years, a long-term follow-up he views as essential when extending TAVI beyond a high-risk population with limited life expectancy to a less sick group of patients with a longer remaining lifetime. He added that a randomized trial of surgery vs. TAVI is coming in the near future, and the results will provide a solid basis for definitive new practice guidelines. In the meantime, individual patient management decisions are made by heart teams – and the heart teams are keeping up to date regarding the emerging impressive evidence favoring TAVI.
The Sapien 3 studies are sponsored by Edwards Lifesciences. Dr. Vahanian is a consultant to the company.
PARIS – Thirty-day outcomes of the first European, all-transfemoral-approach study of the latest-generation Sapien 3 heart valve for transcatheter aortic valve implantation in intermediate-risk elderly patients with severe aortic stenosis included a 1.0% mortality rate and a mere 2.3% rate of moderate paravalvular aortic regurgitation, with no severe aortic regurgitation and a mild aortic regurgitation rate of 26%.
These initial results from the Sapien 3 CE IR study are highly concordant with the impressive results of two U.S. studies using the Sapien 3 valve for transcatheter aortic valve implantation (TAVI) reported earlier this year at the American College of Cardiology meeting in San Diego; one study was of 1,076 intermediate–surgical risk patients and the other involved 583 high-risk patients.
“These results represent at least parity with the best reported surgical outcomes. If we step forward, these favorable results suggest that Sapien 3 TAVI may be expected to challenge surgical aortic valve replacement as the gold standard therapy in elderly patients with aortic stenosis,” Dr. Alec Vahanian declared in presenting the 30-day Sapien 3 CE IR study results at the annual congress of the European Association of Percutaneous Cardiovascular Interventions.
At present, the Sapien 3 valve is approved in Europe for treatment of high-risk or inoperable patients with severe aortic stenosis, but not for intermediate-risk patients such as those in the Sapien 3 CE IR study. The valve remains investigational in the United States, although its manufacturer Edwards Lifesciences, has filed for Food and Drug Administration approval of the device in high-risk patients.
The European Sapien 3 CE IR study includes 101 patients, mean age 84.4 years, with a Society of Thoracic Surgeons (STS) risk score of 5.2%. All underwent TAVI via a transfemoral approach. Fifty-five percent did so under conscious sedation, in contrast to the U.S. trial in intermediate-risk patients, where fewer than 20% had conscious sedation.
To put the observed 30-day all-cause mortality rate of 1.0% in perspective, it’s the lowest seen in the 11 clinical trials performed over the years with the three generations of the Sapien valve. The mortality rate is in line with that found in the much larger U.S. trial in intermediate-risk patients, where the subgroup treated via a transfemoral approach had a 1.1% mortality rate, observed Dr. Vahanian, head of cardiology at Bichat University Hospital in Paris.
The technical procedural success rate in the European trial was 98%. There was no coronary obstruction, valve embolization, or annular rupture.
The 30-day overall stroke incidence was 4%, including a 2% incidence of disabling stroke.
The incidence of vascular complications was low: a major vascular complication rate of 2%, with life-threatening bleeding in 2% of patients. No acute MIs occurred within 30 days, the acute kidney injury rate was 2%, and new-onset atrial fibrillation occurred in 6.9% of patients. Four percent of patients required a new permanent pacemaker, a rate lower than in the U.S. study. There have been no cases of worsening heart failure.
Plus, patients feel a lot better: While 64% were New York Heart Association class III or IV at baseline, 90% were class I or II after 1 month, the cardiologist continued.
In terms of key hemodynamic outcomes, the valve area doubled after TAVI, while the mean gradient plunged from close to 50 mm Hg at baseline to 12 mm Hg 1 month post procedure.
The Sapien 3 valve has the lowest profile of any heart valve. It is typically delivered through a 14-French expandable sheath. The device features a skirt of fabric at the bottom of the frame that’s designed to minimize paravalvular leak.
“I’m very impressed with the data you present. Fantastic!” declared session chair Dr. Carlos E. Ruiz of Lenox Hill Hospital in New York. “Obviously, this valve has raised the bar to a level that will be very hard for other valve technologies to emulate.”
Session cochair Dr. A. Pieter Kappetein, professor of cardiothoracic surgery at Erasmus University in Rotterdam, the Netherlands, posed a question: If you have patients with a mean age of 84, an STS score that would project a 30-day mortality of 5.2% with surgical aortic valve replacement, and yet you only have 1% mortality with Sapien 3 TAVI, why bother with a randomized study before widespread adoption of the less invasive procedure as the treatment of choice in intermediate-risk patients?
Dr. Vahanian replied that this is a time to accumulate evidence. The plan is to follow the study participants for 5 years, a long-term follow-up he views as essential when extending TAVI beyond a high-risk population with limited life expectancy to a less sick group of patients with a longer remaining lifetime. He added that a randomized trial of surgery vs. TAVI is coming in the near future, and the results will provide a solid basis for definitive new practice guidelines. In the meantime, individual patient management decisions are made by heart teams – and the heart teams are keeping up to date regarding the emerging impressive evidence favoring TAVI.
The Sapien 3 studies are sponsored by Edwards Lifesciences. Dr. Vahanian is a consultant to the company.
AT EUROPCR 2015
Key clinical point: Short-term outcomes following transcatheter aortic valve implantation using the Sapien 3 valve in patients with severe aortic stenosis at intermediate surgical risk are the best ever reported with TAVI or surgical valve replacement.
Major finding: Key 30-day outcomes after TAVI using the Sapien 3 valve via a transfemoral approach in intermediate–surgical risk patients with severe aortic stenosis included 1% overall mortality, a 2% rate of disabling stroke, and no severe paravalvular aortic regurgitation.
Data source: The European Sapien 3 CE IR study is a nonrandomized study of 101 intermediate–surgical risk octogenarians with severe aortic stenosis who underwent TAVI with the Sapien 3 valve via a transfemoral approach.
Disclosures: The European Sapien 3 CE IR study is sponsored by Edwards Lifesciences. The presenter is a consultant to the company.